2018 Volume 82 Issue 10 Pages 2602-2608
2018 Volume 82 Issue 10 Pages 2602-2608
Background: The addition of a proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor to statin therapy reduces the rate of cardiovascular events. This study examined the cost-effectiveness of PCSK9 inhibitor+statin compared with standard therapy (statin monotherapy) in the treatment of triple-vessel coronary artery disease (CAD) in Japan.
Methods and Results: A Markov model was applied to assess the costs and benefits associated with PCSK9 inhibitor+statin over a projected 30-year period from the perspective of a public healthcare payer in Japan. The incremental cost-effectiveness ratio (ICER), expressed as the quality-adjusted life-years (QALYs), was estimated. The effects on survival and numbers of events were based on the FOURIER trial and the CREDO Kyoto registry. The ICER of PCSK9 inhibitor+statin over standard therapy was 13.5 million (95% confidence interval 7.6–23.5 million) Japanese Yen (JPY) per QALY gained for triple-vessel CAD. The probability of the cost-effectiveness of PCSK9 inhibitor+statin vs. standard therapy was 0.0008% at a cost-effectiveness threshold of 5 million JPY. In patients with poorly controlled familial hypercholesterolemia (FH) with triple-vessel CAD, the ICER was 3.4 million JPY per QALY gained.
Conclusions: PCSK9 inhibitor plus statin did not show good cost-effectiveness for triple-vessel CAD; however, it showed good cost-effectiveness for patients with triple-vessel CAD and poorly controlled FH in Japan.
High serum levels of low-density lipoprotein-cholesterol (LDL-C) contribute to the development of coronary artery disease (CAD) and the risk of cardiovascular events,1 but a reduction in LDL-C concentration has been shown to decrease the rate of cardiovascular events.1 The 2017 Japanese Atherosclerosis Society guidelines recommend a reduction of the LDL-C level to 70 mg/dL in patients with acute coronary syndrome (ACS), for secondary prevention of familial hypercholesterolemia (FH), and in patients with diabetes mellitus (DM) with additional risk factors.2 Statins are first-line drugs that significantly reduce serum LDL-C concentrations, but other drugs that effectively lower LDL-C concentrations include ezetimibe and eicosapentaenoic acid (EPA). However, not all patients respond sufficiently to these treatments.3 In such patients, treatment instead consists of a proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor.4 PCSK9 is an enzyme that plays an important role in lipid metabolism by modulating the density of LDL-C receptors in multiple organs. Genetically, loss-of-function alleles in PCSK9 increase the number of LDL receptors, leading to a decrease in LDL-C concentration and a reduction in the risk of myocardial infarction.4 Evolocumab, a human monoclonal antibody that binds human PCSK9 and interferes with its function, has been shown to reduce LDL-C concentration by approximately 60%.5 The Further Cardiovascular Outcome Research with PCSK9 Inhibition in Subjects with Elevated Risk (FOURIER) trial showed a significant reduction of cardiovascular events with evolocumab,5 but despite its demonstrated efficacy in patients with cardiovascular disease, its high cost has led to cost-effectiveness analyses in several countries.6–11 Most of those studies concluded that the use of a PCSK9 inhibitor was not cost-effective; however, the cost-effectiveness may differ between countries because of differences in the cost of the drug. For example, evolocumab costs 22,948 Japanese Yen (JPY), equivalent to US$205, in Japan, but US$500 in the USA.9 In addition, the cost-effectiveness of a drug differs between low- and high-risk patients, with better cost-effectiveness generally achieved more often in the latter group. Patients with triple-vessel CAD constitute a high-risk group, so this study was performed to evaluate the cost-effectiveness of PCSK9 inhibitor+statin in Japanese patients with triple-vessel CAD.
Editorial p 2481
An economic model was developed to evaluate the cost and effectiveness of PCSK9 inhibitor+statin therapy in patients with triple-vessel CAD. The model evaluated quality-adjusted life-years (QALYs) and the costs of PCSK9 inhibitor+statin vs. standard therapy (statin monotherapy) over a 30-year time horizon. The efficacy of PCSK9 inhibitor+statin was compared with that of standard therapy for the treatment of cardiovascular disease. The included parameters were derived from the FOURIER trial and the Coronary Revascularization Demonstrating Outcome Study in Kyoto (CREDO Kyoto).12 The FOURIER study, a randomized, double-blind, placebo-controlled trial of PCSK9 inhibitor in more than 27,000 patients with a history of cardiovascular disease.5 showed a 27% reduction in myocardial infarction, a 21% reduction in stroke, but no reduction in deaths in PCSK9-inhibitor-treated patients. In the CREDO Kyoto registry, more than 25,000 patients undergoing first-time coronary revascularization by either percutaneous coronary intervention or coronary artery bypass graft were registered and then followed-up for 5–10 years in multiple centers in Japan.12 In the present study, outcomes were calculated as the incremental cost-effectiveness ratio (ICER) per QALY gained. The analyses were performed according to the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement and Japanese guidelines.13,14
ModelA Markov model with Monte Carlo simulations was developed to estimate the efficacy of PCSK9 inhibitor+statin therapy (Figure 1). The model had a yearly cycle, and in every cycle the patient was assessed as stable, suffering from CAD, or from stroke, or having died. Accordingly, 6 situations were defined: baseline, new CAD, post-CAD, new stroke, post-stroke, and death. All patients started at baseline and were then reclassified according to the results of follow-up, evaluated on an annual basis. If a baseline patient was stable, he or she remained at baseline. If a patient had experienced CAD, then he or she was categorized as new CAD. If a new CAD patient was stable, he or she was considered to be post-CAD.

Markov model of the effects of proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor on cardiovascular disease. Patients were classified into 1 of 6 health conditions: baseline, new coronary artery disease (CAD), post-CAD, new stroke, post-stroke, death. The model applies to each yearly cycle. Each year, patients could be moved in the direction of any arrow.
The study population reflected that of the CREDO Kyoto registry, with an average age of 69 years; 70% were male, and the rates of DM and hypertension were 49% and 82%, respectively. The average serum LDL-C level was 118 mg/dL.15 PCSK9 inhibitor therapy+statin consisted of 140 mg of evolocumab administered every 2 weeks by self-injection plus a strong statin. Standard therapy consisted of the strong statin administered according to the Japanese guideline.
Time HorizonThe time horizon in our models was 30 years. The study population had an average age of 69 years. We estimated that a 30-year evaluation would be sufficient to calculate the cost-effectiveness of the 2 treatments. However, in the scenario analysis the time horizon was changed to 5, 10, 20, and 40 years.
Mortality and HospitalizationTable 1 shows the annual rates of morbidity and mortality for the 2 treatment groups, estimated based on the CREDO Kyoto registry, FOURIER trial, and other studies.1,5,12,16–21 Based on CREDO Kyoto and other studies.1,5,12 the annual mortality rate in patients in the <65, 65–75, 75–85, 85–90, 90–95, >95 years age groups who received standard therapy was estimated at 1.7%, 3.2%, 6.8%, 13.6%, 21.8%, and 32.0%, respectively. The annual CAD rate of patients in the <65, 65–75, 75–85, and >85 years age groups was 11%, 11%, 11%, and 9%, respectively.5,12,17,18,20 The estimated stroke rate increased with increasing age. PCSK9 inhibitor therapy was 20% superior to standard therapy in CAD prevention, 25% superior to standard therapy in stroke prevention, and 5% superior to standard therapy for all-cause death.1
| Value (range for probabilistic sensitivity analyses) |
References | |
|---|---|---|
| Parameter | ||
| Death | 1.7–32% (age dependent) | 1, 5, 12 |
| Stroke | 1–2.8% (age dependent) | 5, 12, 16, 17 |
| CAD | 9–11% (age dependent) | 5, 12, 17, 18, 20 |
| Intervention effect | ||
| Death | 0.95 | 1, 5, 21 |
| Stroke | 0.8 | 1, 5, 21 |
| CAD | 0.75 | 1, 5, 21 |
| Follow-up cost | 420,000 JPY (280,000–560,000) | 28 |
| PCSK9 inhibitor treatment | 600,000 JPY | Estimated |
| New CAD cost | 2,900,000 JPY (2,000,000–3,700,000) | 29 |
| New stroke cost | 1,150,000 JPY (640,000–1,600,000) | 29 |
| Post-stroke cost | 550,000 JPY (470,000–640,000) | 28 |
| Death cost | 1,900,000 JPY (1,100,000–2,800,000) | 29 |
| Utility | ||
| Baseline | 0.89 (0.61–0.99) | 26, 27 |
| New CAD | 0.8 (0.56–0.95) | 24, 26 |
| Post-CAD | 0.85 (0.63–0.98) | 23, 26 |
| New stroke | 0.52 (0.22–0.80) | 22, 25 |
| Post-stroke | 0.65 (0.35–0.90) | 22, 25 |
CAD, coronary artery disease; JPY, Japanese yen; PCSK9, proprotein convertase subtilisin/kexin type 9.
Table 1 shows the utility value for the key parameters evaluated in the study. Data from various previous trials were used to define quality of life (QOL).22–27 The QOL was 0.8 in new CAD patients and 0.52 in new stroke patients. Following the Japanese guidelines, the utility values were annually reduced by 2%.14
CostsAn economic estimation from the perspective of a public healthcare payer in Japan was performed as well. The costs included those for drugs, procedures, and hospitalization, but not for transportation or family care. Evolocumab (140 mg) costs 22,948 JPY in Japan. Its annual cost was estimated to be 600,000 JPY, including drug costs and self-injection guidance fees. The cost data and their sources are shown in Table 1.28,29 The follow-up and complication costs were estimated from previous studies. The costs were annually reduced by 2%.14
Scenario AnalysisSeveral analyses were performed to evaluate various scenarios. In the 1st scenario, the size of the atherosclerotic cardiovascular disease population was estimated using data from the FOURIER trial. The event rate from the FOURIER trial was used on the triple-vessel CAD population because it was much lower than that from the CREDO Kyoto trial. The 2nd scenario involved short-term evolocumab use for ACS. We estimated only 1 year of evolocumab use, started immediately after the diagnosis of ACS. The 3rd scenario involved patients with DM and triple-vessel CAD, who were estimated to be a high-risk population. The 4th scenario involved patients with triple-vessel CAD with poorly controlled FH (LDL-C level >200 mg/dL). Based on a prior study, we assumed that the incidence of cardiovascular events was doubled in patients with FH.30 In addition, in patients with poorly controlled FH, PCSK9 inhibitor strongly reduced LDL-C, and the suppression effect of cardiovascular events was assumed to be proportional to the absolute value of the LDL-C decrease.1 The 5th scenario involved patients with FH with triple-vessel CAD who were undergoing apheresis. The annual cost of LDL-C apheresis was assumed to be 2.4 million JPY, and the possibility of stopping apheresis with PCSK9 inhibitor therapy was assumed to be 60%.31,32 The 6th scenario compared ezetimibe+statin vs. PCSK9 inhibitor+statin. We calculated the additional annual cost of ezetimibe as 73,000 JPY. Assuming that the LDL-C-lowering effect of ezetimibe is 20%, it was assumed that cardiovascular events would decrease in proportion to the decrease in LDL-C.33 The 7th scenario examined the different effects of evolocumab on mortality. The mortality reduction effects of evolocumab were raised from 0% to 20%.
Sensitivity AnalysisNumerous sensitivity analyses were performed to evaluate the effects of uncertainty in the parameters on the results. Threshold analyses were conducted to evaluate the limits of each parameter, with a cost-effectiveness cutoff value of 5 million JPY per QALY gained, based on previous reports.34 One-way deterministic sensitivity analyses were used to assess the effects of several parameters; the results are shown in tornado diagrams (Figure 2). The degree of uncertainty for the input parameters was estimated probabilistically. The distribution of the parameters was selected according to the type of parameter and the results of previous studies.6–11 The outcomes of the 100,000 simulations are presented as the ICER per QALY gained. Cost-effectiveness acceptability curves were plotted to evaluate the probability that PCSK9 inhibitor+statin was more cost-effective than standard therapy. Our models were developed using commercial software (TreeAge Pro 2018; TreeAge, Williamstown, MA, USA).

Tornado plot showing the results of a deterministic one-way analysis. PCSK9 inhibitor plus statin vs. standard therapy for triple-vessel CAD. Abbreviations as in Figure 1.
The main results are shown in Table 2. Over the 30-year time horizon, PCSK9 inhibitor+statin and standard therapy had expected costs of 13.2 million JPY and 7.2 million JPY, respectively. The expected QALYs of PCSK9 inhibitor+statin therapy and standard therapy were 8.95 and 8.47, respectively. The ICER of PCSK9 inhibitor+statin compared with standard therapy was 13.5 million (95% confidence interval [CI] 7.6–23.5 million) JPY per QALY gained. To achieve a value threshold of 5 million JPY per QALY gained, a value-based price of annual PCSK9 inhibitor therapy would be 184,000 JPY, which is 70% lower than the present price.
| Arm | PCSK9 inhibitor | Standard therapy |
|---|---|---|
| Cost | ¥13,224,836 (11,688,951–14,850,846) | ¥7,174,489 (5,836,639–8,680,578) |
| QALY | 8.95 (7.29–9.96) | 8.47 (6.95–9.43) |
| ICER/QALY | ¥13,454,814 (7,560,865–23,549,313) | |
| VBP | ¥183,913 |
ICER, incremental cost-effectiveness ratio; PCSK9, proprotein convertase subtilisin/kexin type 9; QALY, quality-adjusted life-year; VBP, value-based price, defined as the estimated net annual price needed to achieve an ICER of 5,000,000 per QALY.
The results of the scenario analyses are presented in Table 3. The ICER for PCSK9 inhibitor+statin over standard therapy was 21.7 million JPY per QALY gained, using the FOURIER event rate estimate. The ICER for PCSK9 inhibitor+statin over standard therapy was 7.9 million JPY per QALY gained based on short-term PCSK9 inhibitor use by ACS patients. In patients with poorly controlled FH and triple-vessel CAD, the ICER for PCSK9 inhibitor+statin over standard therapy was 3.4 million JPY per QALY gained. In patients with FH with triple-vessel CAD undergoing LDL apheresis, the cost of PCSK9 inhibitor+statin was lower than that of apheresis therapy (19.5 vs. 23.3 million JPY), and the effect of PCSK9 inhibitor+statin was higher than that of apheresis therapy (8.96 vs. 8.48 QALY). In the comparison of ezetimibe plus statin, the ICER of PCSK9 inhibitor+statin was 21.6 million JPY per QALY gained for patients with triple-vessel CAD. With regard to the different effects of evolocumab on mortality estimates, the ICER for PCSK9 inhibitor+statin over standard therapy was 28.6 million per QALY gained in the case of no reduction in mortality. The ICERs for PCSK9 inhibitor+statin over standard therapy were 9.4 million JPY and 6.0 million JPY per QALY gained for reductions in mortality of 10% and 20%, respectively. For 85-year-old patients, the ICER for PCSK9 inhibitor+statin over standard therapy was 14.0 million JPY per QALY gained. The results with different time horizons are shown in Table 3. The ICER for PCSK9 inhibitor+statin over statin monotherapy based on a 5-year estimate was 53.0 million, and that with a 40-year estimate 13.3 million JPY per QALY gained.
| ICER/QALY | |
|---|---|
| FOURIER population | ¥21,662,010 (11,710,510–462,197,21) |
| Short-term PCSK9 inhibitor for ACS | ¥7,938,767 (0–32,606,759) |
| DM with triple-vessel CAD | ¥15,547,035 (8,140,064–33,476,133) |
| FH with triple-vessel CAD | ¥3,379,410 (1,693,765–5,522,242) |
| FH with triple-vessel CAD on LDL apheresis | Dominant (less cost and better QALY) |
| Ezetimibe plus statin vs. PCSK9 inhibitor plus statin | ¥21,606,204 (11,847,489–36,564,983) |
| Mortality reduction | |
| 0% | ¥28,612,393 (12,302,629–83,393,537) |
| 10% | ¥9,447,226 (5,800,480–14,869,986) |
| 20% | ¥5,958,334 (3,986,964–8,560,536) |
| Starting age (years) | |
| 75 | ¥12,511,353 (7,205,254–22,154,796) |
| 80 | ¥13,332,303 (7,682,744–23,991,803) |
| 85 | ¥13,990,900 (8,034,235–25,918,021) |
| Time horizon (years) | |
| 5 | ¥53,029,624 (0–207,389,488) |
| 10 | ¥26,154,979 (11,614,148–71,271,516) |
| 20 | ¥14,568,379 (8,171,019–27,324,532) |
| 40 | ¥13,273,865 (7,719,237–23,207,734) |
ACS, acute coronary syndrome; DM, diabetes mellitus; FH, familial hypercholesterolemia; FOURIER, Further Cardiovascular Outcome Research with PCSK9 Inhibition in Subjects with Elevated Risk; ICER, incremental cost-effectiveness ratio. Other abbreviations as in Tables 1,2.
Figure 2 shows the differences in the cost-effectiveness ratios based on the results of the one-way sensitivity analyses. Change in the follow-up cost of PCSK9 inhibitor+statin resulted in the largest change in the ICER; therefore, this model was the most sensitive to the follow-up cost of PCSK9 inhibitor+statin. Changes in the cost incurred by new stroke and post-stroke resulted in little change in the ICER, indicating that the cost of either condition was not sensitive to change.
Probabilistic Sensitivity AnalysisFigure 3 shows the distribution of the projected differences in the costs and QALY of PCSK9 inhibitor+statin vs. standard therapy and Figure 4 shows the cost-effectiveness acceptability curves for PCSK9 inhibitor+statin. With a cost-effectiveness threshold of 5 million JPY per QALY gained, the cost-effectiveness probability of PCSK9 inhibitor+statin was 0.0008%.

Cost-effectiveness plots. The plots were generated for PCSK9 inhibitor plus statin in triple-vessel CAD from 100,000 simulations with a decision-analytic model. Ellipse shows the 95% confidence intervals, and the solid line shows the willingness to pay, with a slope of 5 million Japanese Yen (JPY) per quality-adjusted life-year (QALY) gain. The location of the ellipse above the solid line means that the incremental cost-effectiveness ratio (ICER) of PCSK9 inhibitor plus statin over standard therapy was >5 million JPY per QALY gain in more than 95% of the simulation cases. Abbreviations as in Figure 1.
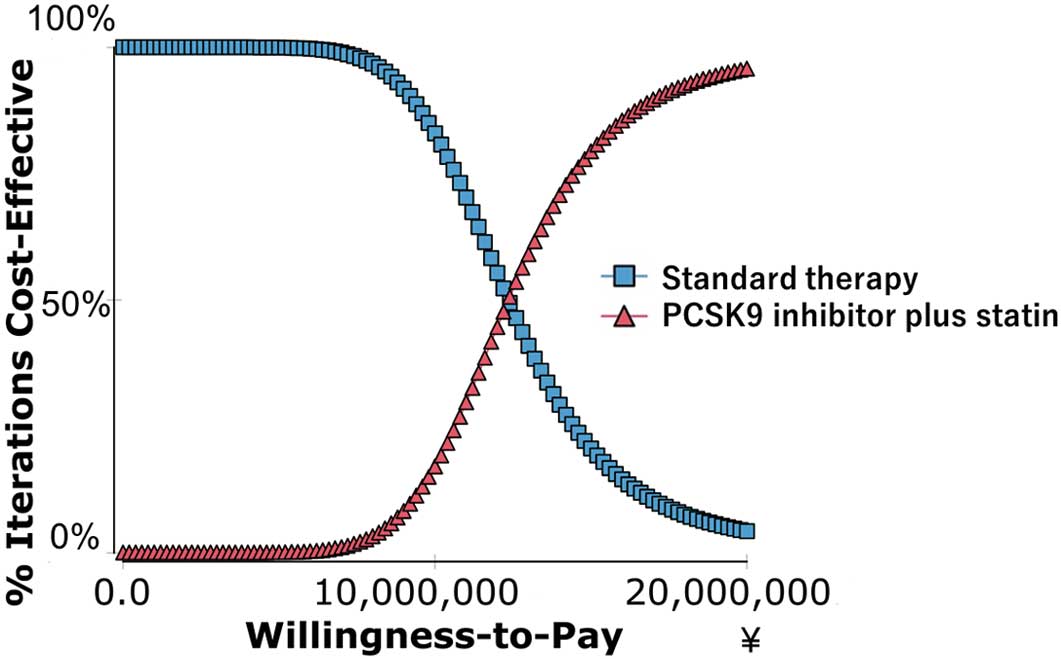
Cost-effective acceptability curve of PCSK9 inhibitor plus statin over standard therapy. PCSK9, proprotein convertase subtilisin/kexin type 9.
The results of the present study indicated that PCSK9 inhibitor+statin was not cost-effective for the treatment of triple-vessel CAD patients in Japan. The ICER of PCSK9 inhibitor+statin over standard therapy was much higher than 5 million JPY per QALY, and the probability according to the cost-effectiveness acceptability curve of PCSK9 inhibitor+statin was 0.0008%. Although PCSK 9 inhibitor+statin yielded a better QALY than standard therapy, the cost exceeded its effect. We also examined patients with ACS and those with DM and triple-vessel CAD. The ICER was >5 million JPY per QALY, and PCSK9 inhibitor+statin was not cost-effective for these high-risk groups with an LDL-C level of 118 mg/dL. Regardless of whether LDL apheresis was performed, patients with triple-vessel CAD and FH with extremely high LDL-C levels (200 mg/dL) had an ICER of <5 million JPY, which was cost-effective.
In the scenario analysis, PCSK9 inhibitor+statin was cost-effective for patients with FH and triple-vessel CAD. The preventive effect of LDL-C-lowering drugs on cardiovascular events is reportedly proportional to the decrease in LDL-C. In the present study, in patients with higher LDL-C levels, PCSK9 inhibitors had a greater effect on LDL-C and the number of cardiovascular events was less, thus improving the cost-effectiveness of PCSK9 inhibitors. In this scenario analysis, assuming that the frequency of cardiovascular events doubled in patients with FH and the event suppression effect of PCSK9 inhibitors doubled with a LDL-C level >200 mg/dL, the ICER of PCSK9 inhibitors was <5 million JPY per QALY. PCSK9 inhibitor therapy could be cost-effective for patients with a high risk of cardiovascular events and a very high LDL-C level, such as in patients with FH. However, the cost-effectiveness of PCSK9 inhibitor was poor compared with that of ezetimibe. Although not analyzed in the present study, the cost-effectiveness of ezetimibe plus statin compared with statin was reportedly good in the UK.35 Additionally, for secondary prevention of CAD, the cost-effectiveness of EPA plus statin compared with statin was reportedly good in Japan.36
The scenario analysis indicated that a reduction in mortality of ~20% would result in good cost-effectiveness of PCSK9 inhibitor+statin. Based on the results of the FOURIER trial, PCSK9 inhibitor therapy is less likely to achieve a 20% improvement in the mortality rate. Indeed, the FOURIER trial showed no mortality reduction with PCSK9 inhibitor, probably because the follow-up period in that study was too short to detect a reduction. In the Randomized Evaluation of Aggressive or moderate Lipid lowering therapy with pitavastatin in Coronary Artery Disease (REAL-CAD) trial, strong statin treatment reduced total mortality by 19% during a 4-year follow-up.37 A meta-analysis of statins showed that mortality decreased by 9.5% when LDL-C decreased by 38.7 mg/dL; additionally, in the FOURIER study in which LDL-C decreased by 50 mg/dL, mortality was expected to decrease by 12.5%.1,5 Longer observation periods might result in a mortality reduction, which is consistent with the findings for other LDL-C-lowering drugs. We hypothesized a 5% mortality reduction in our basal case analysis. As a result, the addition of PCSK9 inhibitor would not be cost-effective in Japan without a decrease in the drug’s cost. Practically, the cost of evolocumab is much less in Japan than in the USA, but threshold analysis indicated that if the annual cost of, for example, evolocumab was <183,913 JPY, implying a 70% reduction of the current cost, a PCSK9 inhibitor should achieve cost-effectiveness with a cutoff of 5 million JPY per QALY.
Previous studies also concluded that PCSK9 inhibitor use was not cost-effective. In studies performed in the USA, ICERs of $141,000 and $268,000 per QALY were reported for atherosclerotic cardiovascular disease.6,9 A Norwegian study showed an ICER of €128,000 per QALY for secondary prevention in high-risk patients.10 A study performed in Spain that was sponsored by a pharmaceutical company showed good cost-effectiveness; however, the event reduction effects were overestimated in that study, with an especially high estimated rate of heart failure reduction. Unlike the Spanish study, we created a model from the FOURIER trial data and we consider that the results of the FOURIER trial regarding the effects of PCSK9 inhibitors were more accurate. However, although PCSK9 inhibitor suppressed cardiovascular events in the FOURIER study, the mortality reduction did not reach the level assumed to date. The other reason why the cost-effectiveness of PCSK9 inhibitors is not good in Japan is the lower ICER thresholds in Japan than in the USA. Despite this lack of cost-effectiveness, we would never recommend that PCSK9 inhibitors not be added to statin monotherapy. PCSK9 inhibitors exert potent protective effects on cardiovascular diseases. In particular, PCSK9 inhibitors remain important drugs for patients with statin intolerance or for those in whom statins cannot sufficiently lower the LDL-C levels, especially in patients with FH.
This study had several strengths. First, it was based on CREDO Kyoto, a large Japanese registry. Therefore, the patient population, event rate, and mortality rate were consistent with those in Japanese patients with CAD, and our data are therefore likely to be applicable to clinical practice in Japan. Second, this study was not an industry-based investigation and had a low degree of bias. Bell et al reported that industry-based studies tend to produce good results for the sponsoring company.38 Another strength of the investigation was the use of data from the FOURIER trial, whereas previous studies mainly used meta-analysis data of statin use rather than data relevant to PCSK9 inhibitor use. As the FOURIER trial was a large randomized trial designed to determine the effects of PCSK9 inhibitor, as compared with previous investigations, our study based on the FOURIER trial could estimate the effects of PCSK9 inhibitors more accurately.
This study also had several limitations, the first of which involved a number of assumptions in our data set, including event rate, cost, and QOL. Nevertheless, our one-way sensitivity analysis revealed that stroke cost and stroke utility had little effect on the ICER for the PCSK9 inhibitor. Scenario analyses showed the same results, independent of various mortality reduction rates and starting ages. Therefore, we assumed that our results were robust despite these assumptions. The second limitation was related to patient selection. In our analysis, we compared PCSK9 inhibitor+statin and statin monotherapy in triple-vessel CAD patients. We attempted to create a model of patients with FH from scratch, but because of the lack of epidemiological and clinical research in Japan, it was difficult to create a reasonable model to evaluate the cost-effectiveness of PCSK9 inhibitors. Therefore, we added an analysis of patients with FH as a scenario analysis. The third limitation was the type of drug used. The focus of our analysis was evolocumab, but an analysis of the PCSK9 inhibitor alirocumab may have yielded different results regarding cost-effectiveness. However, a randomized trial with alirocumab showed almost the same effects as evolocumab39 and the annual cost of alirocumab is the same as that of evolocumab; thus, the cost-effectiveness of the 2 drugs would be similar. The 4th limitation was that the device and drug treatments were different from those in the era of the CREDO Kyoto registry. Cost-effectiveness analysis using data from the REAL-CAD study conducted in Japan in recent years could have different results.
The addition of PCSK9 inhibitor to statin monotherapy was not cost-effective for the treatment of patients with triple-vessel CAD in Japan, but showed good cost-effectiveness for triple-vessel CAD patients with poorly controlled FH.
We thank the Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
This study did not receive grants from any funding agency in the public, commercial, or not-for-profit sectors.
The authors have no conflicts of interest to declare.