2018 Volume 82 Issue 10 Pages 2535-2541
2018 Volume 82 Issue 10 Pages 2535-2541
Background: This study aimed to evaluate the early and late clinical outcomes after repeat aortic valve replacement (AVR) for subaortic pannus in patients with mechanical valves.
Methods and Results: Between 2001 and 2017, 51 patients (median age, 59 years; 42 women) with prosthetic aortic stenosis (AS) caused by pannus ingrowth underwent repeat AVR because of acute malfunction of monoleaflet valve (3 patients), severe prosthetic AS (30 patients), and moderate prosthetic AS at the time of tricuspid or mitral valve surgery (18 patients). The median follow-up duration was 100 (interquartile range, 64–138) months. Double valve replacement was performed in 45 (88%) patients. Median time interval from previous operation was 161 (interquartile range, 121–194) months. The explanted mechanical AV was monoleaflet and bileaflet in 16 (31%) and 34 (67%) patients, respectively. Concomitant procedures included 16 mitral valve replacements (14 repeat) and 36 tricuspid valve surgeries (15 replacements, 21 repairs). No hospital deaths or cases of heart block occurred. Overall survival and event-free survival rates at 10 years were 88% and 51%, respectively. Late complications included recurrent prosthetic AS (4 patients), new paravalvular leakage of the mitral valve (5 patients), and severe tricuspid regurgitation (2 patients).
Conclusions: Although repeat AVR for subaortic pannus had acceptable early and late survival, recurrent prosthetic AS was frequently observed during late follow-up.
Although malfunction of mechanical valves in the aortic position has been extremely rare after the introduction of bileaflet mechanical valves, prosthetic aortic stenosis (AS) caused by subaortic pannus is not uncommon during long-term follow-up after mechanical aortic valve replacement (AVR).1–7 Recently, multislice computed tomography (CT) and 3D transesophageal echocardiography were found to have an important role in clear visualization of subaortic pannus in patients with mechanical AVR.8–11 However, serial cardiac echocardiography is still the main diagnostic method to detect subaortic pannus. Previously, we reported that an aortic transprosthetic mean pressure gradient (TMPG) increment >15 mmHg during follow-up correlated with subaortic pannus and was associated with adverse events after AVR with the Medtronic-Hall valve.12 Additionally, we reported that simultaneous AVR and mitral valve replacement (i.e., double valve replacement [DVR]) with a mechanical valve was associated with increased aortic TMPG compared with isolated AVR.12–14 In those studies, increased aortic TMPG was also associated with late significant tricuspid regurgitation (TR). Therefore, concomitant repeat AVR for subaortic pannus formation was frequently performed at the time of tricuspid valve surgery for severe TR.15,16
The purpose of this study was to evaluate the early and late clinical outcomes after repeat AVR for pannus formation in patients with mechanical AVs and to analyze the postoperative hemodynamic changes in recurrent prosthetic AS.
The study group comprised 51 consecutive patients who between January 2001 and August 2017 underwent repeat AVR because of pannus formation after mechanical AV implantation (median age, 58.7 years, interquartile range [IQR], 48.7–63.1 years; 42 [82.4%] women); 38 patients (74.5%) had atrial fibrillation. Previous operation was performed in the Samsung Medical Center in 20 patients (39%) and in other hospitals in 31 (61%) patients. The underlying pathology of the AV was rheumatic heart disease in 48 (94%), bicuspid aortic stenosis in 2, and endocarditis in 1 patient. During the same study period, 686 AVR and 377 DVR procedures with mechanical valves were performed in the Samsung Medical Center. The detailed patient characteristics are described in Table 1. This study was approved by the institutional review board, and the informed consent requirement was waived (IRB File no. 2017-03-114).
| Baseline characteristics | |
| Age, years, median (IQR) | 58.7 (48.7–63.1) |
| Sex, female, n (%) | 42 (82) |
| Body surface area, m2, mean±SD | 1.57±0.13 |
| NYHA class III or IV, n (%) | 16 (31) |
| Emergency operation, n (%) | 3 (6) |
| Comorbidity, n (%) | |
| Atrial fibrillation | 38 (75) |
| Hypertension | 7 (14) |
| Diabetes mellitus | 6 (12) |
| History of stroke | 10 (20) |
| Coronary artery disease | 1 (2) |
| No. of previous surgeries, n (%) | |
| 1 | 44 (86) |
| 2 | 5 (10) |
| 3 | 2 (4) |
| Status of implanted mechanical valve, n (%) | |
| Double valve replacement | 45 (88) |
| Aortic valve replacement | 6 (12) |
| Interval from previous operation, months, median (IQR) | 161 (121–194) |
| Hospital where previous operation was performed, n (%) | |
| Study hospital | 20 (39) |
| Other hospital | 31 (61) |
| Preoperative laboratory and echocardiographic data | |
| Hemoglobin, g/dL, mean±SD | 11.0±2.1 |
| Bilirubin, mg/dL, mean±SD | 1.11±0.77 |
| Creatinine, mg/dL, mean±SD | 1.09±1.14 |
| LVEF, %, mean±SD | 61.3±8.50 |
| Aortic TMPG, n (%) | |
| ≥40 mmHg | 30 (59) |
| <40 mmHg | 21 (41) |
| Mean aortic TMPG, mm Hg, mean±SD | 43.2±13.4 |
| Mitral regurgitation, n | |
| Native mitral valve | |
| Moderate | 1 |
| Severe | 1 |
| Prosthetic mitral valve | |
| Severe | 5 |
| Moderate | 2 |
| Tricuspid regurgitation, n | |
| Severe | 19 |
| Moderate | 7 |
AVR, aortic valve replacement; IQR, interquartile range; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; SD, standard deviation; TMPG, transprosthetic mean pressure gradient.
Operative indications of repeat AVR were: (1) acute prosthetic AV malfunction; (2) severe prosthetic AS with aortic TMPG ≥40 mmHg; and (3) monoleaflet AV or moderate prosthetic AS (serial aortic TMPG increment ≥15 mmHg) at the time of tricuspid or mitral valve surgery. In all patients, repeat AVR was performed and subaortic pannus was identified.
Follow-upData were collected from a computerized hospital database. Additional follow-up data were obtained through telephone interviews with patients or their family members. The national registry that maintains mortality and survival data for the Republic of Korea was searched for patients lost to hospital follow-up. Follow-up closed on August 31, 2017. Among the 45 late surviving patients, 37 (80.4%) were monitored at the Samsung Medical Center and 4 (8.7%) were followed at other hospitals. The survival data for the remaining 4 patients were obtained from the national registry. The survival follow-up was 100% complete, with median follow-up of 99.9 (IQR range, 64–138) months. Standard guidelines were used to define morbidity and mortality.17 2D Doppler echocardiography was performed before discharge and at 1, 3, 5, and >7 years after the surgery. Echocardiographic criteria of recurrent prosthetic AS were 2 consecutive increments of aortic TMPG ≥15 mmHg at late follow-up compared with aortic TMPG at discharge or at 1 year. Non-severe prosthetic AS was defined as aortic TMPG <40 mmHg.
A normal-functioning prosthetic stenosis showed normal leaflet motion of the mechanical valve without abnormal regurgitant flow on echocardiography, regardless of moderate or severe prosthetic stenosis.
Statistical AnalysisData were statistically analyzed using STATA version 10 (2007, Stata Statistical Software: release 10; StataCorp LP, College Station, TX, USA). Distribution of general characteristics, preoperative laboratory data, echocardiographic data, operative data, and previously implanted mechanical mitral valve data were analyzed using mean with standard deviation for normally distributed continuous data. Median with IQR was used for other continuous variables. Student’s paired t test was used to compare continuous variables. Kaplan-Meier and Cox hazard modeling was calculated for overall and event-free survival rates. Analyzed predictors and risk factors were regarded as significant in accordance with the ratio formula with P<0.05.
The previously implanted mechanical valve was DVR in 45 (88.2%) and AVR in 6 (11.8%) patients. Incidence of subaortic pannus after mechanical DVR was significantly higher than that of isolated mechanical AVR in patients operated at Samsung Medical Center (DVR: 4.2% [16/377] vs. AVR: 0.6% [4 /686], P<0.001). The median time interval from previous operation was 161 (IQR range, 121–194) months. The main indications for reoperative cardiac surgery were cardiogenic shock because of acute malfunction of mechanical AV in 3 patients, severe prosthetic AS in 28, severe TR with severe prosthetic AS in 9, and severe TR in 6 patients (Table 2); 3 patients underwent emergency operation associated with aortic regurgitation caused by acute malfunction of Medtronic-Hall valve, and 1 patient required extracorporeal membrane oxygenation for cardiac arrest in the emergency room. The explanted mechanical AV was bileaflet in 34 (66.7%), monoleaflet in 16 (31.4%) and ball valve in 1 (2%) patient. The mean preoperative aortic TMPG was 43.2±13.4 mmHg; 19 patients (37.3%) had aortic TMPG <40 mmHg. All serial preoperative aortic TMPG data was available for the 11 patients who underwent previous operation in Samsung Medical Center. The average preoperative aortic TMPG before repeat AVR was 15.4±4.8, 20.8±5.5, 24.9±6.5, and 31.9±5.2 mmHg at discharge, 1-year, 5-year, and 7-year postoperative follow-up, respectively. Preoperative aortic TMPG at 7 years was significantly increased compared with the TMPG at discharge after the previous operation (P<0.001).
| Main indications for reoperation, n (%) | |
| Acute malfunction of prosthetic AV | 3 (6) |
| Severe prosthetic AS | 28 (55) |
| Severe TR | 6 (12) |
| Severe TR with severe prosthetic AS | 9 (18) |
| PVL of MV with severe prosthetic AS | 3 (6) |
| PVL of MV | 2 (4) |
| Malfunction of prosthetic MV | 1 (2) |
| Concomitant operations, n (%) | |
| TVR | 15 (29) |
| TV repair | 21 (41) |
| MVR | |
| Primary MVR | 2 (4) |
| Repeat MVR | 14 (27) |
| Aortic root widening | 5 (10) |
| Maze procedure | 3 (6) |
| Ascending aorta replacement | 1 (2) |
| TMPR | 23 (45) |
| Aortic cross-clamp time, min, mean±SD | 152±68 |
| Pump time, min, mean±SD | 215±81 |
AS, aortic stenosis; MV, mitral valve; MVR, mitral valve replacement; PVL, paravalvular leakage; SD, standard deviation; TMPR, transaortic mitral pannus removal; TR, tricuspid regurgitation; TV, tricuspid valve; TVR, tricuspid valve replacement. Other abbreviations as in Table 1.
In all patients repeat AVR was performed for prosthetic AS caused by subaortic pannus. Supra-aortic pannus was minimal on the sewing cuff without obstructive findings in all patients. Occasionally, a small amount of fresh thrombus was identified between the lower surface of the mechanical AV and the subaortic pannus (Figure 1). Of the newly implanted AVs, 3 were tissue valves and 48 were mechanical valves (Table 3). As concomitant procedures, repeat mitral valve replacement (MVR) was performed in 14 patients (7 paravalvular leakage, 6 pannus, 1 thrombus with pannus) and primary MVR was performed in 2 patients with rheumatic progress after previous MVR. In addition, 15 tricuspid valve replacements (TVRs), including 11 cases of previous tricuspid annuloplasty, and 21 tricuspid valve repairs, including 5 cases of previous tricuspid annuloplasty, were performed. Protruding pannus on the ventricular side of mechanical mitral valves was removed through the aorta in 23 patients. Details of the concomitant procedures are shown in Table 2.
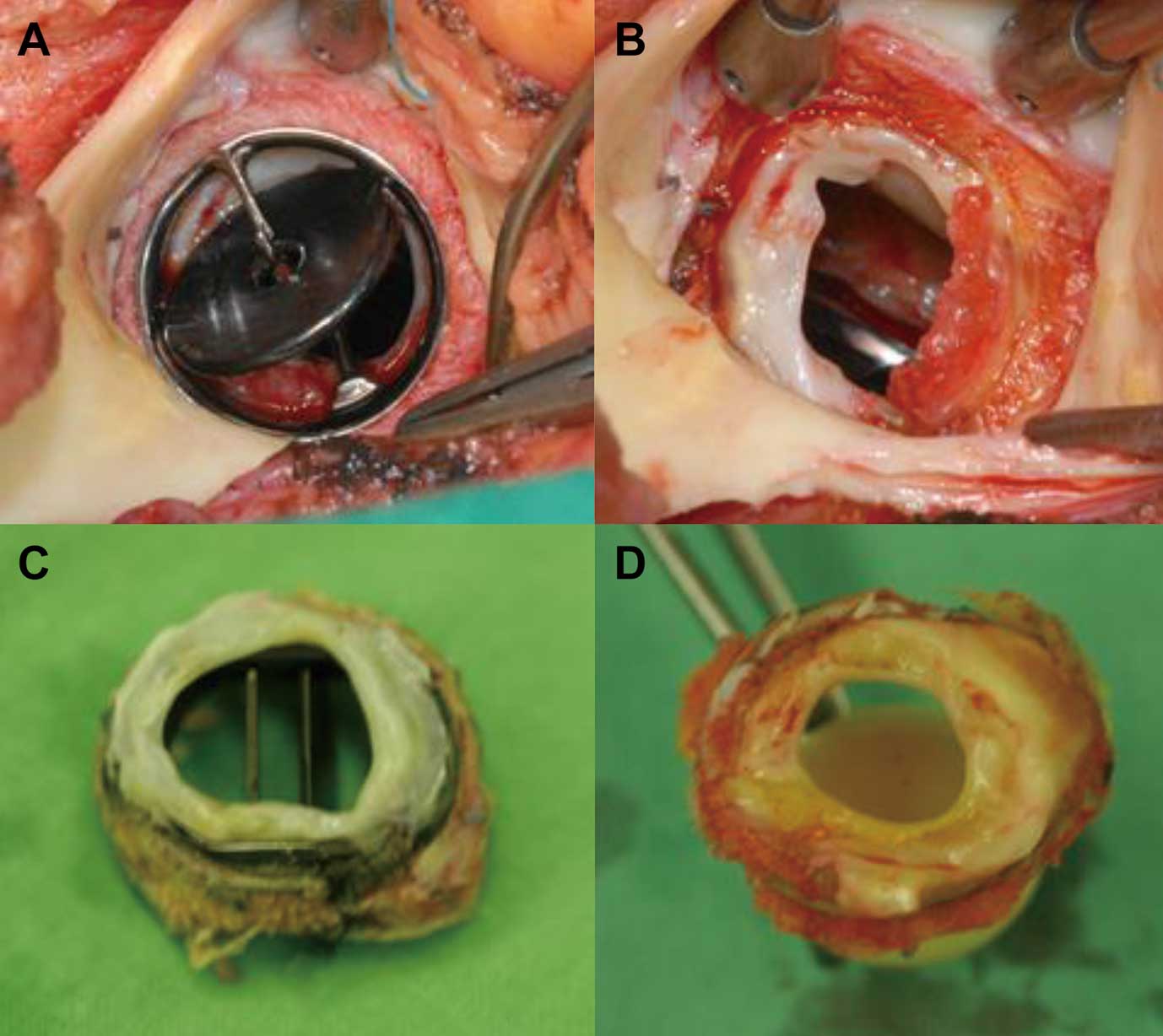
(A,B) Surgical view of subaortic pannus in a mechanical valve: A fresh thrombus is located between the lower surface of the aortic mechanical valve and the subaortic pannus. (C,D) Circular subaortic pannus in a bileaflet valve and ball valve, respectively.
| Name of valve | Size, mm (n) |
|---|---|
| Explanted valve | |
| Medtronic-Hall | 20 (10), 22 (3) |
| Omniscience | 19 (3) |
| Starr-Edwards | 21 (1) |
| Carbomedics | 19 (1), 21 (6) |
| Duromedics | 19 (1), 21 (2) |
| Edwards Tekna | 19 (1) |
| ATS | 19 (3), 21 (5) |
| St. Jude Standard | 19 (6), 21 (1) |
| Sorin | 19 (1) |
| Not available (bileaflet) | (7) |
| Implanted valve | |
| Sorin Overline | 18 (13), 20 (8), 22 (1) |
| St. Jude Regent | 19 (9), 21 (6) |
| On-X | 19 (7), 21 (2) |
| Medtronic-Hall | 20 (1), 22 (1) |
| Edwards Perimount | 19 (1), 21 (1) |
| Hancock | 21 (1) |
Abbreviations as in Table 1.
No cases of 30-day or in-hospital death occurred. No pacemakers were implanted for complete heart block. The median stay in the intensive care unit and hospital was 2 (IQR, 1.5–5) days and 17 (IQR, 13–24) days, respectively. The aortic TMPG after repeat AVR significantly decreased from 43.2±13.4 to 14.2±5.3 mmHg at discharge (P<0.001). The aortic TMPG in patients with moderate prosthetic AS was also significantly decreased from 31.2±6.9 to 13.9±6.4 mmHg (P<0.001).
Late OutcomesLate complications included recurrent prosthetic AS in 4 patients, new paravalvular leakage of mitral valve in 5, prosthetic valve endocarditis in 3, severe TR in 2, reoperation in 2, cerebral hemorrhage in 3, and bowel infarction in 1 patient. Of the 4 patients with late recurrent prosthetic AS, 1 died from heart failure associated with paravalvular leakage of the mitral valve, 1 underwent cardiac transplantation for heart failure from paravalvular leakage of the mitral valve and severe prosthetic AS, and the remaining 2 patients are currently being followed up. Of the 3 patients with prosthetic valve endocarditis, 1 was managed surgically and 2 received medical treatment; all 3 survived. Two cases of late severe TR occurred in patients with repeat tricuspid annuloplasty. There were 6 late deaths: heart failure in 3 patients, hepatocellular carcinoma in 1, sepsis in 1, and unknown etiology in 1 patient. Overall survival, freedom from cardiac-related death, event-free survival and freedom from recurrent prosthetic AS at 10 years were 87.6%, 90.3%, 50.8% and 86.7%, respectively (Figure 2).
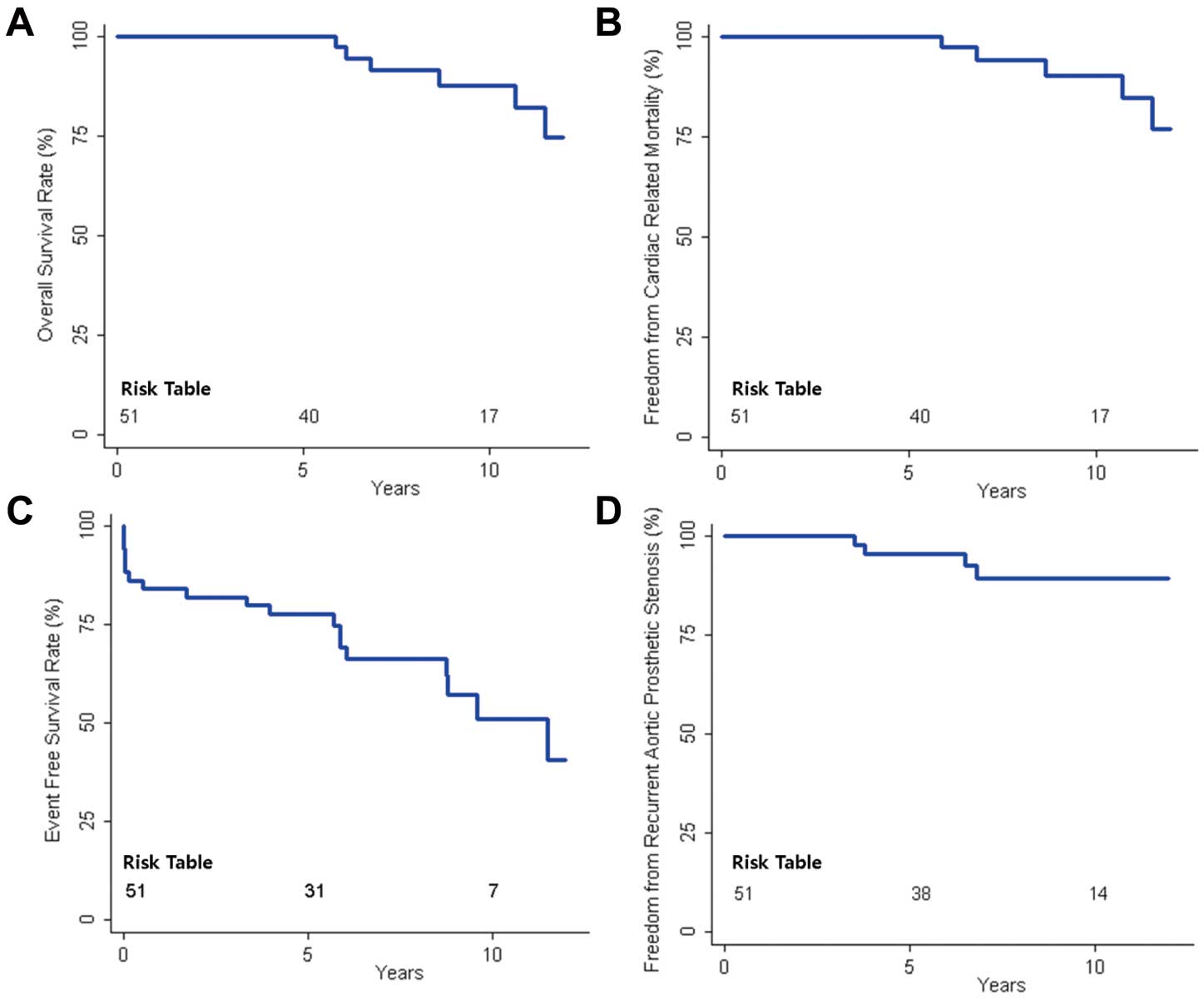
Kaplan-Meier survival curve after repeat aortic valve replacement for subaortic pannus in a mechanical aortic valve. (A) overall survival, (B) freedom from cardiac-related death, (C) event-free survival and (D) freedom from recurrent prosthetic aortic stenosis (increment of aortic transprosthetic mean pressure gradient ≥15 mmHg).
The main findings of this study were: (1) prosthetic AS caused by subaortic pannus was common in patients after DVR; (2) subaortic pannus was intraoperatively identified in all patients with an increment of aortic TMPG >15 mmHg or with moderate prosthetic AS; (3) repeat AVR for pannus ingrowth had acceptable early and late survival rates; and (4) recurrent prosthetic AS was frequent during follow-up.
Rizzoli et al showed an increasing occurrence of pannus formation with time after implantation of any mechanical valve.2 Pannus formation is reportedly more common in the aortic position than in the mitral position (70% vs. 21%).4 The time interval to significant pannus formation varies widely between 3 and 30 years according to associated risk factors and type of mechanical valve.2–8,10 Girard et al reported that the incidence of previous DVR was 31% in 49 patients with reoperation for mechanical AV obstruction.3 In that series, the cause of mechanical AV obstruction was pannus in 25 (53%), mismatch in 19 (39%), and thrombus in 4 (8%) patients. The incidence of rheumatic heart disease was only 45%. However, the reported incidence of previous DVR in patients with severe prosthetic AS was very high (85-92%) in Asian countries in which rheumatic heart disease was the most common etiology of valve disease in young patients.7,8,11 Our previous studies showed that DVR with a mechanical valve was associated with increased aortic TMPG compared with isolated AVR.12,13 These observations may explain the high incidence of subaortic pannus formation after DVR.
This series included 3 patients with acute catastrophic malfunction of the monoleaflet valve (Medtronic-Hall) after DVR; 2 of them had cardiogenic shock. All patients underwent emergency operation with 1 having extracorporeal membrane oxygenation (ECMO) support, 1 having intra-aortic balloon pump support, and 1 being prepared for ECMO. Another patient with cardiac arrest caused by acute malfunction of Medtronic-Hall valve in DVR had ECMO support in the catheterization room, but emergency operation was not performed because of brain damage. Monoleaflet mechanical valves are still used in some countries because of the lower cost.18 However, monoleaflet AVs should not be used in DVR because of the sudden catastrophic events caused by subaortic pannus.
Although acute malfunction of bileaflet AVs is rare, critical situations can occur, with intermittent chest pain or pulmonary edema caused by acute aortic regurgitation with the valve in the fixed open position.18–22 Diagnosis can be made with careful history taking, transthoracic echocardiography, and fluoroscopy; other time-consuming diagnostic methods (transesophageal echocardiography, cardiac CT, coronary angiography) only delay emergency operation and decrease the chance of recovery. Emergency repeat AVR should be performed under preparation for ECMO. The most important factor in the diagnosis of this complication is awareness of acute mechanical valve malfunction caused by subaortic pannus in any mechanical AV. Therefore, it is essential to evaluate the patient’s risk factors for subaortic pannus formation, such as young age and female sex, DVR, monoleaflet AV, small-sized AV, serial increment of aortic TMPG, and more than 10 years since the previous operation. Although suture technique may have an effect on the incidence of subaortic pannus, it was very difficult for us to analyze this risk because of the small number of patients and incomplete previous operative data.
Significant prosthetic stenosis in mechanical valve implantation is not well defined. The recent proposed definition of possible structural valve degeneration for aortic stenosis after transcatheter AVR is an increase in the TMPG by >10 mmHg with concomitant decrease in effective orifice area by >0.3 cm2, and clinically relevant structural valve degeneration is defined as an increase in TMPG by ≥20 mmHg with concomitant decrease in effective orifice area by >0.6 cm2 during follow-up.23 In our study, 2 consecutive increments of aortic TMPG ≥15 mmHg or severe prosthetic AS ≥40 mmHg was considered indicative of significant subaortic pannus. With increasing use of cardiac CT, subaortic pannus can be clearly identified even in patients with moderate prosthetic AS. Han et al10 retrospectively reviewed 88 patients with a prosthetic AV who underwent cardiac CT. In 17 patients with subaortic pannus on CT, aortic TMPG was significantly higher than in 71 patients without pannus (28.1±19.8 vs. 14.0±6.5 mmHg, P=0.004). Furthermore, cardiac CT has an important role in decision making for repeat AVR in symptomatic patients with moderate prosthetic AS and low-flow/low-gradient phenomenon,8 and for visual confirmation of subaortic pannus in patients without previous serial echocardiographic data. Additionally, mechanical mitral valve pannus formation, which is frequently associated with subaortic pannus, can be detected during routine preoperative CT for repeat cardiac surgery.
In this study, repeat AVR was performed in 18 patients with moderate prosthetic AS in a normally functioning AV. Initial indication for repeat AVR in moderate prosthetic AS with a normally functioning prosthetic valve was serial increments of aortic TMPG ≥15 mmHg during follow-up echocardiography or a monoleaflet AV. Subaortic pannus was confirmed during reoperation in all patients. Subsequently, exploratory aortotomy to identify subaortic stenosis in patients with moderate prosthetic AS in a bileaflet AV has become a routine procedure. The decision regarding repeat AVR should be made based on patient life expectancy, surgeon’s experience, and additional risk factors associated with mitral valve and tricuspid valve surgery. However, we prefer repeat AVR in patients with moderate prosthetic AS for the following reasons: (1) to prevent catastrophic, sudden monoleaflet valve malfunction, (2) to improve the hemodynamic function of the new mechanical valve, (3) to prevent late progress of TR, (4) to reduce more complicated reoperations associated with high early deaths; and (5) repeat AVR with concomitant procedures does not increase the early mortality and morbidity in our experience. In our previous studies, postoperative aortic TMPG was an independent predictor of late TR after reoperative tricuspid valve surgery and DVR.14,16 Recently, Lee et al also reported that subaortic pannus was a risk factor for TR progression after DVR.24 Therefore, we believe that repeat AVR for moderate prosthetic AS using a new mechanical valve may prevent late TR and improve right heart failure at the time of tricuspid surgery.
Concomitant procedures at the time of repeat AVR for subaortic pannus were quite common because of multi-valve disease. In Girard’s series in which the incidence of rheumatic disease was 45%, mitral valve surgery was required in 12 (24%) and tricuspid valve surgery in 9 (18%) patients.3 Oh et al reported an incidence of concomitant tricuspid valve surgery of 67% and of mitral valve surgery of 27% in a series of 33 cases with reoperation for subaortic pannus.7 In our study, 16 MVRs (32%: 14 repeat, 2 primary) and 36 tricuspid valve surgeries (72%: 15 TVRs, 21 tricuspid valve repairs) were performed. Additionally, we routinely inspect for pannus growth on the ventricular side of prosthetic mitral valves at the time of a repeat AVR. In our series, transaortic mitral pannus removal was performed in 23 patients to improve the hemodynamics of the mechanical mitral valve and to prevent future mitral reoperation and TR progression. Recently, we reported our experience with transaortic mitral pannus removal during repeat cardiac surgery.25 Subaortic pannus removal without repeat AVR is not recommended, because of incomplete hemodynamic improvement and the possibility of early recurrent subaortic stenosis.7
The 10-year overall survival was 88%; however, the event-free survival at 10 years was only 51% because of recurrent prosthetic AS, paravalvular leakage of the mitral valve, prosthetic endocarditis, and TR progression after tricuspid valve repair. Therefore, frequent echocardiographic examinations are required for early detection of late complications. The Kaplan-Meier curve of overall survival for tricuspid valve surgery showed no difference in patients with TVR compared with tricuspid valve repair (P=0.991). TVR should be considered in patients with severe TR after previous tricuspid valve repair, because 2 cases of late severe TR occurred in patients with repeat tricuspid valve repair.
The follow-up echocardiography showed that aortic TMPG was significantly increased again after repeat AVR in 4 patients. Recently, every effort is made to reduce the possibility of late pannus formation. All subaortic pannus tissue is removed, including from the conduction area and surrounding fibrotic tissue in the left ventricular outflow tract. Subaortic myectomy may be considered in patients with a protruding ventricular septum. Although the aortic root widening procedure in repeat AVR is very difficult to perform in patients with previous DVR, small pericardial patch widening may allow implantation of a 1 size bigger mechanical AV and can delay the process of pannus ingrowth from surrounding tissue. Aggressive root widening procedure at the time of primary DVR in young female patients may be considered to prevent future development of subaortic pannus.26
Study LimitationsThis retrospective single-center study was performed in Korea, where multiple valve disease caused by rheumatic disease was prevalent 2–3 decades ago. Serial preoperative echocardiographic data for diagnosis of subaortic pannus were available in a limited number of patients, because a large portion of the patients had undergone previous operation at other hospitals. In this study, serial increment of aortic TMPG ≥15 mmHg was used as a criterion of recurrent prosthetic AS. Other serial echocardiographic data (effective orifice area, Doppler velocity index) were not available. We did not routinely perform cine-fluoroscopy during follow-up. Prospective measurement of serial opening and closing angles on cine-fluoroscopy may provide additional information on abnormal function in a prosthetic valve leaflet. Although cardiac CT is a routine preoperative procedure, specific imaging to evaluate subaortic pannus was available in few patients. The good early clinical outcomes in our series may be attributed to the young age of the patients, fewer cases of coronary disease, good left ventricular function, mostly single-surgeon experience (84%), and intensive postoperative follow-up by the cardiac surgeon.
In conclusion, subaortic pannus formation in mechanical AVs is not uncommon after mechanical DVR. Although repeat AVR for subaortic pannus showed good early and late survival, recurrent prosthetic AS is frequently observed in late follow-up. Therefore, surgical procedures to prevent subaortic pannus should be considered at the time of initial and repeat AVR.
The authors have nothing to disclose with regard to commercial support. No potential conflicts exist, and no funding was provided.