2018 Volume 82 Issue 3 Pages 791-797
2018 Volume 82 Issue 3 Pages 791-797
Background: The CRUSADE, ACTION and ACUITY-HORIZONS bleeding scores have been derived using Caucasian patients, and little is known about which has the better predictive ability in Chinese patients, especially for patients with STEMI.
Methods and Results: We retrospectively analyzed 2,208 consecutive STEMI patients undergoing primary PCI (PPCI). Major bleeding events were defined according to Bleeding Academic Research Consortium criteria (type 3 or 5). Predictive ability of the 3 scores was assessed using logistic regression and AUC. Unadjusted HR for 1-year death were determined on Cox proportional hazard modeling. The major bleeding rate was 2.4%. The AUC of the CRUSADE, ACTION and ACUTIY-HORIZONS models was 0.88 (95% CI: 0.84–0.92), 0.90 (95% CI: 0.87–0.94), and 0.78 (95% CI: 0.87–0.94). The calibration of the ACUTIY-HORIZONS model was not acceptable overall, or in the subgroup of access site (P<0.05). In the high-risk category, 1-year mortality was approximately 4–7-fold greater than in the low-risk category (CRUSADE: HR, 7.27; 95% CI: 3.30–16.02, P<0.001; ACTION: HR, 7.13; 95% CI: 2.19–15.41, P<0.001; ACUITY-HORIZONS: HR, 4.06; 95% CI: 1.62–10.16; P=0.003).
Conclusions: The CRUSADE and ACTION scores have greater predictive ability for in-hospital major bleeding than the ACUITY-HORIZONS risk score in Chinese STEMI patients undergoing PPCI. Mortality would increase with the transition from low- to high-risk category in 1 year.
The treatment of ST-segment elevation myocardial infarction (STEMI) traditionally was focused on potent anti-thrombotic agents and primary percutaneous coronary intervention (PCI).1 These strategies reduce recurrent ischemic events, at the expense of an increase in major bleeding, which is associated with worse outcomes.2–4 Given the lower body weight of Asian patients and the different genetic background, increased bleeding is viewed as a potential threat and has received more attention,5–7 thus predictive risk modeling is an important measure for management of acute coronary syndrome (ACS) in East Asia.
There are 3 main bleeding risk scores: the Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcome with Early Implementation of the American College of Cardiology/American Heart Association Guidelines (CRUSADE) risk score,8 the Acute Coronary Treatment and Intervention Outcomes Network Registry-Get With the Guidelines (ACTION Registry-GWTG) risk score,9 and the Acute catheterization and urgent intervention triage strategy and The Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction (ACUITY-HORIZONS) bleeding risk score.10 Despite these scores offering quantitative methods for assessing bleeding risk in a broader spectrum of ACS, none of them was developed specially for STEMI patients. Moreover, they were not homogeneous with regard to clinical subjects and variables, and used different post-admission treatments to predict major bleeding according to their own definition. It is noteworthy that almost all of the participants were Caucasian, and few studies exist on the scores’ application in East Asian patients with STEMI.
We therefore validated the performance of the 3 scores in Chinese STEMI patients (representative of an East Asian population) undergoing primary PCI, with the aim to (1) obtain evidence for which score is accurate and simple for predicting in-hospital major bleeding in STEMI patients, and assess them with regard to vascular access site and glycoprotein IIb/IIIa inhibitors (GPI) use; and (2) explore the association between bleeding risk model and 1-year mortality.
We consecutively selected patients with STEMI who underwent successful primary PCI between October 2013 and January 2016 from the database of Beijing Anzhen Hospital, Capital Medical University, China. Patients who underwent coronary artery bypass graft (CABG), conservative therapy, fibrinolytic therapy, and non-primary PCI were excluded.
A loading dose of 300 mg aspirin followed by 100 mg once daily, and 300–600 mg clopidogrel followed by 75 mg once daily, or 180 mg ticagrelor followed by 90 mg twice daily were given. The use of unfractionated heparin and low-molecular-weight heparin was weight-adjusted as per standard recommendations. GPI (tirofiban) use, access site, choice of stents and other devices were left to the operator’s discretion. Concomitant treatment was according to the relevant practice guidelines.1
Data CollectionData on clinical characteristics, anti-thrombotic therapy, biochemistry and electrocardiography, bleeding risk score and in-hospital complications were collected and recorded by physicians using a standardized case report form. One-year mortality was followed up via telephone interview.
Clinical Endpoint and DefinitionsThe primary endpoint was major bleeding events defined according to Bleeding Academic Research Consortium (BARC) criteria (type 3 or 5) during hospitalization: type 3a, overt bleeding plus hemoglobin drop 3–5 g/dL, or any transfusion with overt bleeding; type 3b, overt bleeding plus hemoglobin drop >5 g/dL; cardiac tamponade, bleeding requiring surgical intervention or i.v. vasoactive drugs; type 3c, intracranial hemorrhage (does not include microbleeds or hemorrhagic transformation; does include intraspinal), subcategories confirmed by autopsy, imaging, or lumbar puncture; intraocular bleed compromising vision type 5a, probable fatal bleeding (no autopsy or imaging evidence but clinical suspicion); type 5b, definite fatal bleeding (overt bleeding or autopsy or imaging confirmation).11
Statistical AnalysisContinuous data are described as median (interquartile range, IQR) and were compared using Student’s t-test or Wilcoxon rank-sum test. Categorical data are described as n (%) and were compared using the chi-squared test. Risk scores were calculated for each patient from the corresponding prognostic variable scores (Tables S1–S3). Both CRUSADE and ACTION models were constructed to assign patients into 5 risk strata (very low; low; moderate; high; and very high). In the ACUITY-HORIZONS, patients were stratified into 4 risk categories for bleeding (low; moderate; high; and very high). In the present study, patients were categorized into 3 bleeding risk strata for all scores by combining the very high- and high-risk categories into a high-risk category, and the very low- and low-risk categories into a low-risk category.
Both calibration and discrimination of the models were assessed with respect to the total patient group. Risk model calibration was assessed using the Hosmer-Lemeshow goodness-of-fit test.12 Discrimination was evaluated on receiver operating characteristic (ROC) curves and expressed as c-statistics (>0.70 has acceptable discriminatory capacity),13 and the area under the curve (AUC) was calculated to assess the predictive accuracy of these models. The non-parametric method described by DeLong et al14 was used to compare the predictive ability of the 3 risk models.
Unadjusted HR for 1-year death were determined on Cox proportional hazard modeling. Adjusted HR were estimated from multivariable Cox models selected in a stepwise process with backward and exit thresholds set to 0.10. Two-sided P<0.05 was considered to indicate a statistically significant difference. All statistical analyses were performed using SPSS version 23 (IBM, USA).
Initially, this cohort study involved 2,484 patients. Patients who underwent CABG (n=92), conservative therapy (n=58), fibrinolytic therapy (n=73) and non-primary PCI (n=53) were excluded from this study (n=276). Thus, 2,208 patients constituted the final study group, and follow up was carried out successfully in all of these patients.
Of the 2,208 patients with STEMI who underwent PCI, 53 (2.4%) developed BARC type 3/5 bleeding complication during hospitalization. Patients with major bleeding were mostly elderly and female with low body weight and a frequent history of peptic ulcer, diabetes mellitus and cancer, as well as worse renal function, higher bleeding risk scores, and unstable hemodynamic status during hospitalization (Table 1). The most prevalent cause of major bleeding complications was gastrointestinal bleeding (47/53, 90.6%), followed by intracranial hemorrhage (5/53, 9.4%) and angiography site-related bleeding due to the femoral approach (1/53, 1.9%).
| Overall (n=2,208) |
Major bleeding (n=53) |
No major bleeding (n=2,155) |
P-value | |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Age (years) | 56.0 (10.7) | 64.2 (7.9) | 55.8 (10.6) | 0.019 |
| Female | 332 (15.0) | 14 (26.4) | 318 (14.8) | 0.030 |
| Weight (kg) | 75.3 (11.5) | 70.1 (8.1) | 75.4 (11.6) | 0.001 |
| Hypertension | 1,056 (47.8) | 27 (50.9) | 1,029 (47.7) | 0.678 |
| Diabetes mellitus | 643 (29.1) | 28 (52.8) | 615 (28.5) | <0.001 |
| Dyslipidemia | 786 (35.6) | 4 (7.5) | 782 (36.3) | <0.001 |
| CKD | 65 (2.9) | 14 (26.4) | 51 (2.4) | <0.001 |
| Previous PCI | 150 (6.8) | 8 (15.1) | 142 (6.6) | 0.031 |
| Previous CABG | 9 (0.4) | 0 (0) | 9 (0.4) | 1.000 |
| Previous MI | 40 (1.8) | 0 (0) | 40 (1.9) | 0.624 |
| Previous ischemic stroke | 101 (4.6) | 8 (15.1) | 93 (4.3) | 0.001 |
| PAD | 32 (1.4) | 0 (0) | 32 (1.5) | 0.755 |
| Cancer | 34 (1.5) | 6 (11.3) | 28 (1.3) | <0.001 |
| Previous GI ulcer | 130 (5.9) | 8 (15.1) | 122 (5.7) | 0.010 |
| On-admission data | ||||
| Killip class | ||||
| I | 1,781 (80.7) | 14 (26.4) | 1,767 (82.0) | <0.001 |
| II | 293 (13.3) | 9 (17.0) | 284 (13.2) | |
| III | 74 (3.4) | 14 (26.4) | 60 (2.8) | |
| IV | 60 (2.7) | 16 (30.2) | 44 (2.0) | |
| SBP (mmHg) | 121.5 (14.8) | 112.3 (19.1) | 121.8 (14.6) | 0.010 |
| Heart rate (beats/min) | 72.3 (11.5) | 85.2 (17.5) | 72.0 (11.2) | <0.001 |
| Hemoglobin (g/dL) | 142.4 (15.5) | 133.0 (21.8) | 142.6 (15.3) | 0.002 |
| Hematocrit (%) | 41.4 (4.1) | 39.0 (6.1) | 41.1 (4.0) | 0.005 |
| Anemia† | 334 (15.1) | 16 (30.2) | 318 (14.8) | 0.002 |
| Serum creatinine (mg/dL) | 0.9 (0.4) | 1.1 (0.6) | 0.9 (0.4) | 0.042 |
| Creatinine clearance (ml/min)‡ | 98.5 (32.1) | 73.2 (21.6) | 99.1 (32.0) | <0.001 |
| Leukocytes (g/L) | 7.1 (3.9) | 8.2 (4.4) | 6.9 (3.7) | 0.003 |
| Mean CRUSADE score | 19.5 (12.0) | 37.7 (10.6) | 19.0 (11.6) | <0.001 |
| Mean ACTION score | 27.4 (5.5) | 39.3 (8.5) | 27.1 (5.1) | <0.001 |
| Mean ACUITY-HORIZONS score | 12.6 (5.3) | 17.3 (4.3) | 12.4 (5.2) | <0.001 |
| In-hospital management | ||||
| Aspirin | 2,206 (99.9) | 53 (100) | 2,153 (99.9) | 1.000 |
| P2Y12 inhibitors | ||||
| Clopidogrel | 1,201 (54.4) | 28 (52.8) | 1,154 (53.4) | 0.722 |
| Ticagrelor | 1,007 (45.6) | 25 (47.2) | 1,001 (46.5) | |
| UFH | 2,186 (99.0) | 53 (100) | 2,133 (99.0) | 1.000 |
| LMFH | 1,515 (68.6) | 42 (79.2) | 1,473 (68.4) | <0.001 |
| Bivalirudin | 22 (1.0) | 0 (0) | 22 (1.0) | 1.000 |
| Tirofiban | 722 (32.7) | 18 (34.0) | 704 (32.7) | 0.843 |
| IABP | 52 (2.4) | 20 (37.7) | 32 (1.5) | <0.001 |
| Vascular access site | ||||
| Radial | 2,032 (92.0) | 32 (60.4) | 2,000 (92.8) | <0.001 |
| Femoral | 176 (8.0) | 21 (39.6) | 155 (7.2) | <0.001 |
| In-hospital clinical course | ||||
| VF | 67 (3.0) | 19 (35.8) | 48 (2.2) | <0.001 |
| Cardiac shock | 62 (2.8) | 22 (41.5) | 40 (1.9) | <0.001 |
| AV block | 10 (0.5) | 0 (0) | 10 (0.5) | 1.000 |
| In-hospital mortality | 25 (1.1) | 8 (15.1) | 17 (0.8) | <0.001 |
Data given as n (%) or median (IQR). †Men, hemoglobin <13 g/dL; women, hemoglobin <12 g/dL. ‡Estimated using the Cockcroft-Gault formula. AV, atrioventricular; CABG, coronary artery bypass graft; CKD, chronic kidney disease; ACTION, Acute Coronary Treatment and Intervention Outcomes Network; ACUITY-HORIZONS, Acute catheterization and urgent intervention triage strategy and The Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction; CRUSADE, Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcome with Early Implementation of the American College of Cardiology/American Heart Association Guidelines; GI, gastrointestinal; GPI, glycoprotein IIb/IIIa inhibitor; IABP, intra-aortic balloon pump; LMFH, low molecular weight heparin; MI, myocardial infarction; PAD, peripheral artery disease; PCI, percutaneous coronary intervention; SBP, systolic blood pressure; STEMI, ST-segment elevation myocardial infarction; UFH, unfractionated heparin; VF, ventricular fibrillation.
The major bleeding rates across the quintiles of the 3 score categories were as follows: CRUSADE score low risk, 0.7%; moderate risk, 9.4%; high risk, 12.5%; ACTION score low risk, 0.5%; moderate risk, 6%; high risk, 31.4%; ACUITY-HORIZONS score low risk, 0.1%; moderate risk, 1.9%; and high risk, 5.7% (Figure 1). Major bleeding rate had a consistent gradient for each risk stratification (P<0.001). The AUC of the CRUSADE, ACTION and ACUTIY-HORIZONS models were 0.88 (95% CI: 0.84–0.92), 0.90 (95% CI: 0.87–0.94), and 0.78 (95% CI: 0.87–0.94), respectively, but the calibration of the ACUTIY-HORIZONS models was not acceptable in the STEMI cohort (P<0.05; Table 2; Figure 2).
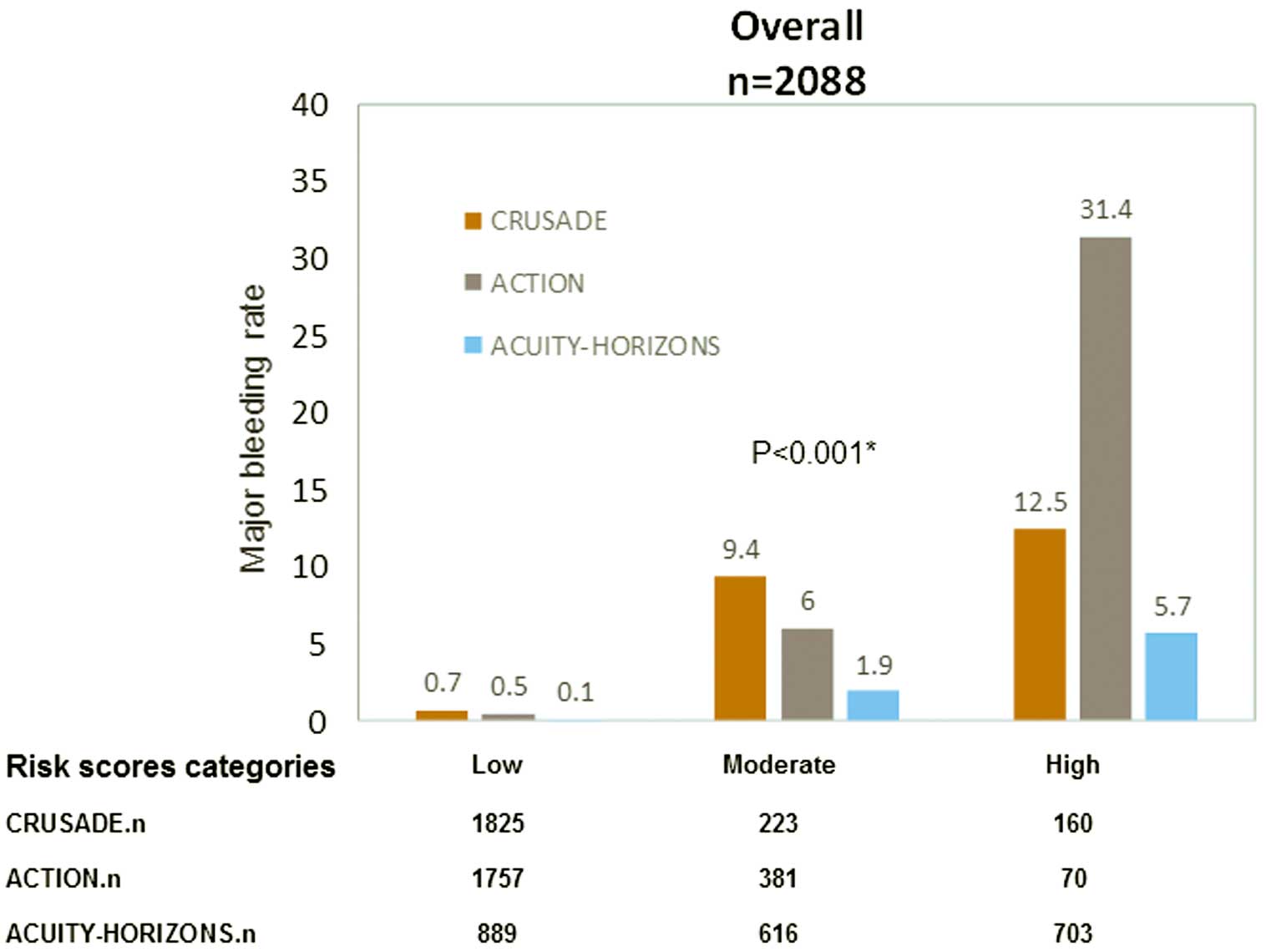
Major bleeding rate vs. risk category for the CRUSADE, ACTION, and ACUITY-HORIZONS bleeding risk scores, in the overall ST-segment elevation myocardial infarction patient group. *Comparison of risk category between the 3 score systems.
| Risk group | Risk score | Hosmer-Lemeshow test | c-statistic (95% CI) |
|
|---|---|---|---|---|
| χ2 | P-value | |||
| Overall (n=2,088) | CRUSADE | 12.9 | 0.116 | 0.88 (0.84–0.92) |
| ACTION | 11.3 | 0.186 | 0.90 (0.87–0.94) | |
| ACUITY-HORIZONS | 16.7 | 0.010 | 0.78 (0.73–0.82) | |
| Vascular access site | ||||
| Radial (n=2,032) | CRUSADE | 7.2 | 0.513 | 0.88 (0.83–0.92) |
| ACTION | 10.3 | 0.244 | 0.90 (0.86–0.94) | |
| ACUITY-HORIZONS | 21.2 | 0.003 | 0.82 (0.77–0.87) | |
| Femoral (n=176) | CRUSADE | 13.8 | 0.055 | 0.85 (0.77–0.93) |
| ACTION | 6.9 | 0.344 | 0.89 (0.83–0.95) | |
| ACUITY-HORIZONS | 17.5 | 0.015 | 0.71 (0.63–0.80) | |
| GPI use | ||||
| Yes (n=722) | CRUSADE | 6.1 | 0.633 | 0.90 (0.87–0.94) |
| ACTION | 11.1 | 0.133 | 0.91 (0.86–0.97) | |
| ACUITY-HORIZONS | 14.3 | 0.075 | 0.74 (0.65–0.83) | |
| No (n=1,486) | CRUSADE | 6.6 | 0.468 | 0.86 (0.81–092) |
| ACTION | 17.3 | 0.068 | 0.90 (0.86–0.94) | |
| ACUITY-HORIZONS | 8.0 | 0.438 | 0.80 (0.75–0.85) | |
Abbreviations as in Table 1.
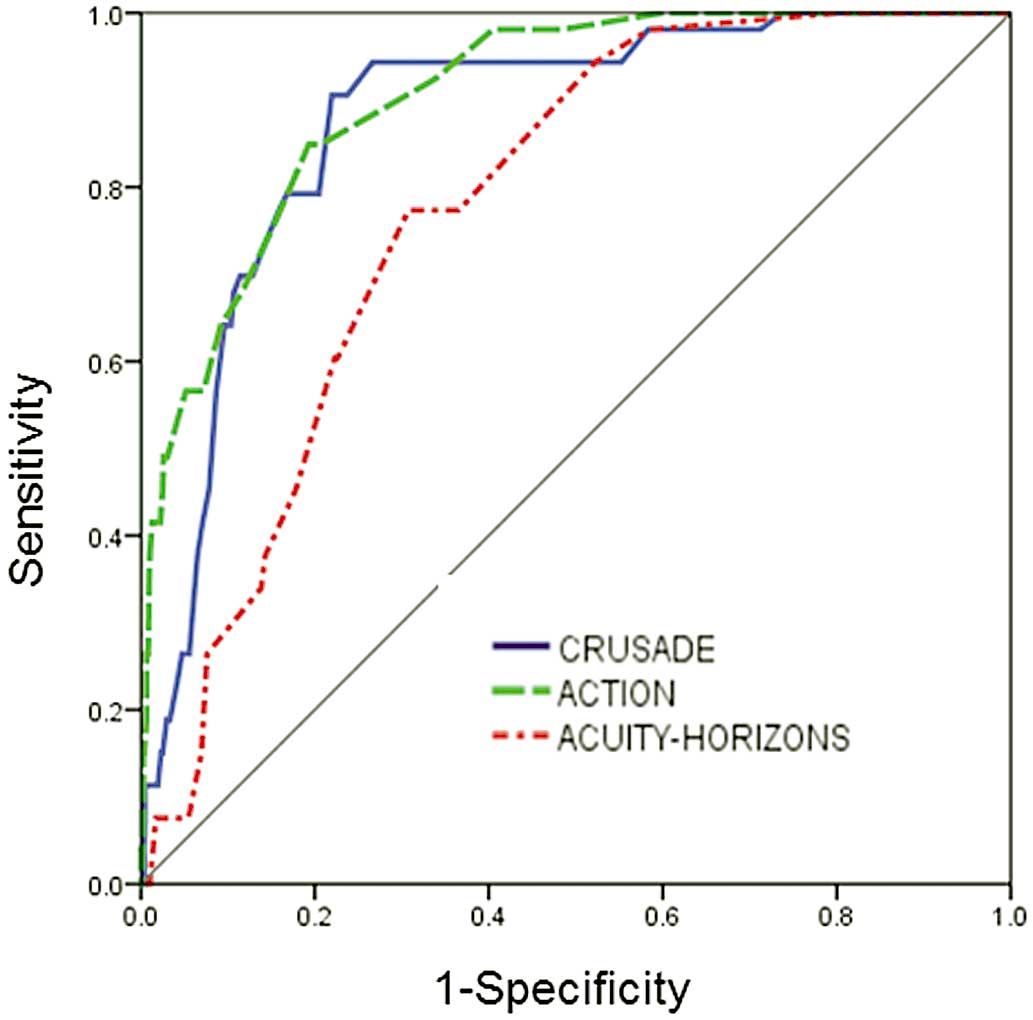
Receiver operating characteristics curves for major bleeding prediction in ST-segment elevation myocardial infarction patients.
The performance of the CRUSADE, ACTION and ACUTIY-HORIZONS bleeding risk scores across treatment subgroups was confirmed (Table 2). For patients receiving GPI (n=722), the AUC were 0.90 (95% CI: 0.87–0.94), 0.91 (95% CI: 0.86–0.97), and 0.74 (95% CI: 0.65–0.83), respectively. Major bleeding rate was higher in each model’s high-risk group for patients receiving GPI compared with those who did not receive GPI: CRUSADE, 16% vs. 10.9%; ACTION, 40% vs. 25%; and ACUITY-HORIZONS, 7.0% vs. 5.1% (P<0.001).
Good performance was noted both in patients undergoing radial angiography (n=2032, 92%) and femoral angiography (n=176, 8%). AUC for the CRUSADE, ACTION and ACUTIY-HORIZONS bleeding risk models for radial angiography were 0.88 (95% CI: 0.83–0.92), 0.90 (95% CI: 0.86–0.94), and 0.82 (95% CI: 0.77–0.87), respectively. For femoral angiography the AUC were 0.85 (95% CI: 0.77–0.93), 0.89 (95% CI: 0.83–0.95), and 0.71 (95% CI: 0.63–0.80), respectively. The bleeding rate in risk groups for the radial approach were all lower than for femoral approach: low risk, CRUSADE 0.3% vs. 5%; ACTION 0.3% vs. 2.5%; ACUITY-HORIZONS 0.1% vs. 0%; moderate risk, CRUSADE 8.8% vs. 25%; ACTION 5.4% vs. 14.3%; ACUITY-HORIZONS 0.5% vs. 18.4%; high risk, CRUSADE 6.1% vs. 42.9%; ACTION 18.2% vs. 53.8%; ACUITY-HORIZONS 4.4% vs. 19.7% (P<0.001). The calibration of the ACUTIY-HORIZONS models was not acceptable in either the radial group or the femoral group (P<0.05; Table 2).
The C-statistic for the ACUITY-HORIZONS model was significantly lower than that of the CRUSADE and ACTION scores for the prediction of in-hospital major bleeding in STEMI patients (compared with CRUSADE, z=4.21, P=0.04; compared with ACTION, z=5.43, P=0.02). No differences were observed when the CRUSADE and ACTION models were compared with each other (z=0.33, P=0.71; Table 3).
| Comparison | z† | P-value |
|---|---|---|
| CRUSADE vs. ACTION | 0.33 | 0.71 |
| CRUSADE vs. ACUITY-HORIZONS | 4.21 | 0.04 |
| ACTION vs. ACUITY-HORIZONS | 5.43 | 0.02 |
†Null-hypothesis result. Abbreviations as in Table 1.
In each model, the transition from the low- to high-risk category carried a significantly increased risk of 1-year mortality. The magnitude of the effect of moderate risk category in the CRUSADE and ACTION models on 1-year mortality was approximately 2.6-fold greater than that of the low risk category (CRUSADE: HR, 2.59; 95% CI: 1.41–7.10; P=0.044; ACTION: HR, 2.71; 95% CI: 1.07–6.89; P=0.036; ACUITY-HORIZONS: HR, 1.45; 95% CI: 0.47–4.49; P=0.521, respectively). In the high-risk category, 1-year mortality was approximately 4–7-fold greater than in the low-risk category (CRUSADE: HR, 7.27; 95% CI: 3.30–16.02; P<0.001; ACTION: HR, 7.13; 95% CI: 2.19–15.41; P<0.001; ACUITY-HORIZONS: HR, 4.06; 95% CI: 1.62–10.16; P=0.003, respectively; Table 4).
| Risk score | Low risk | Moderate risk | High risk | ||
|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | ||
| CRUSADE | Ref. | 2.59 (1.41–7.10) | 0.044 | 7.27 (3.30–16.02) | <0.001 |
| ACTION | Ref. | 2.71 (1.07–6.89) | 0.036 | 7.13 (2.19–15.41) | <0.001 |
| ACUITY-HORIZONS | Ref. | 1.45 (0.47–4.49) | 0.521 | 4.06 (1.62–10.16) | 0.003 |
CI, confidence interval; HR, hazard ratio. Other abbreviations as in Table 1.
The main results of the present analysis are as follows: (1) using neutral BARC criteria, the CRUSADE and ACTION models had good calibration and discrimination abilities with regard to major bleeding in Chinese STEMI patients, but the calibration of the ACUTIY-HORIZONS model was not acceptable in the overall group or with regard to access site; and (2) in each model, the transition from low- to high-risk category carried a significantly increased risk for 1-year mortality.
In the modern era of risk-tailored and personalized cardiovascular treatment, studies on risk prediction models have been inclined to use risk models constructed for different ethnicities and clinical settings. Assessing the models in an independent cohort before use would lower the likelihood of over or underestimating the real risks.
STEMI constitutes a high-risk clinical setting and requires more aggressive pharmacological treatment and invasive strategies, which are related to an increased risk of bleeding complications. Given the strong association between bleeding and subsequent mortality, bleeding prediction models are important for risk stratification and to determine treatment. Particularly in East Asian patients, compared with Caucasian patients, there is an increased susceptibility to bleeding because of the lower body weight, differences in genetic background, risk factors and disease patterns,15–17 and little clinical research exists on the application of these risk score systems. In the present STEMI cohort study, the in-hospital major bleeding rate was 2.4%, similar to previous studies with a reported range of 1–6%.18–20 The incidence rates of bleeding according to the neutral BARC criteria in the low-risk category were lower than the corresponding rates in the 3 original models. This may have been due to the different subject baseline characteristics. The present patients had lower baseline heart rate and systolic blood pressure (SBP), and lower prevalence of anemia and peripheral arteriopathy than Western patients.21 Another reason was the lower rate of iatrogenic artery access injury. Ariza-Sole et al reported that in a STEMI cohort, the access puncture site in coronary intervention was the femoral artery in 41.2%,22 and the femoral access site was associated with major bleeding in approximately 40% of all bleeds.23 In the present study, however, radial artery access was used in 90%, indicating that the radial artery is superior in reducing bleeding events, and could improve subsequent adverse outcomes in patients undergoing PCI.24
In the present study, the c-statistics of the CRUSADE, ACTION and ACUTIY-HORIZONS models were 0.88, 0.90, and 0.78, respectively, greater than in the original studies.8–10 This may be attributed to the differences in patient background, bleeding definitions, and genetic background. First, STEMI patients usually have a lower prevalence of comorbidities than non-ST-elevation ACS (NSTE-ACS) patients, and different timing of in-hospital treatment and procedures,25 thus potentially leading to a different overall bleeding risk. Second, the BARC criteria type 3 and 5 were used as the definitions of major bleeding, which provide more accurate prediction of bleeding events in STEMI patients than Thrombolysis In Myocardial Infarction (TIMI), Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries (GUSTO), and International Society on Thrombosis and Haemostasis (ISTH) bleeding classifications.26 In contrast, most validation studies used TIMI or GUSTO definitions. Third, increasing data suggest that East Asian patients have different thrombogenicity, platelet P2Y12-receptor inhibition, and propensity for bleeding complications compared with Caucasian patients.27 Genetic polymorphisms might partially account for the underlying mechanism of differing bleeding risk with ethnicity.28
In addition, we found that the CRUSADE and ACTION risk scores had greater predictive value for major bleeding than the ACUITY-HORIZONS risk model. This difference might be due to the different variables used in each model. The CRUSADE and ACTION models have similar baseline variables, such as heart rate, SBP, heart failure, diabetes, and vascular disease, but the ACUITY-HORIZONS risk model did not include these variables, and instead used the factor treatment with anti-thrombotic regimen, especially bivalirudin, which was rarely used in this study.
In modern East Asian society, the association between aging and the increasing prevalence of coronary artery disease (CAD) risk factors, has become prominent.29,30 STEMI is the most serious manifestation of CAD, and ischemia and bleeding events result in substantial morbidity and mortality. On analysis of the present risk models, we found that the transition from low- to high-risk category carried a significantly increased risk of 1-year mortality. The risk of bleeding was related to mortality, and different bleeding risk strata predict different rates of subsequent 1-year death. This observation and conclusion could be taken into consideration to optimize choice of anti-thrombotic agents during hospitalization, and improve individual survival in East Asian society.
Study LimitationsThe potential limitations of this study were as follows: first, this was a single-center retrospective study, although patient management was relatively homogenous. Second, we did not compare the 3 models using their own major bleeding definitions, but instead used neutral BARC criteria. Third, only 1% of patients used bivalirudin, which was proved to be associated with fewer bleeding complications. Finally, the performance of the ACUITY-HORIZONS score was assessed with regard to in-hospital bleeding events although it was originally designed to predict 30-day bleeding.
The CRUSADE, ACTION scores had a greater prediction ability for in-hospital major bleeding than the ACUITY-HORIZONS risk score in Chinese STEMI patients undergoing primary PCI. Mortality would increase with the transition from low- to high-risk category in 1 year. This study would help clinicians identify the accuracy of these risk stratifications and the association of risk category with mortality.
The authors thank Meng-Ge Zhou (Beijing Institute of Heart, Lung and Blood Vessel Diseases, Beijing Anzhen Hospital, Capital Medical University) for technical assistance with data cleaning and statistical analysis.
This study was supported by grants from the National High Technology Research and Development Program of China (2015AA020102) and Beijing Municipal Science & Technology Commission (Z141107002514014).
The author declare no conflicts of interest.
Supplementary File 1
Table S1. CRUSADE score system algorithms
Table S2. ACTION score system algorithms
Table S3. ACUITY-HORIZONS score system algorithms
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-17-0760