Abstract
Background:
Erythropoietin (EPO) has antiapoptotic and tissue-protective effects, but previous clinical studies using high-dose EPO have not shown cardioprotective effects, probably because of platelet activation and a lack of knowledge regarding the optimal dose. In contrast, a small pilot study using low-dose EPO has shown improvement in left ventricular function without adverse cardiovascular events.
Methods and Results:
We performed a multicenter (25 hospitals), prospective, randomized, double-blind, placebo-controlled, dose-finding study to clarify the efficacy and safety of low-dose EPO in patients with ST-segment elevation myocardial infarction (STEMI) under the Evaluation System of Investigational Medical Care of the Ministry of Health, Labor and Welfare of Japan. In total, 198 STEMI patients with low left ventricular ejection fraction (LVEF <50%) were randomly assigned to receive intravenous administration of EPO (6,000 or 12,000 IU) or placebo within 6 h of successful percutaneous coronary intervention. At 6 months, there was no significant dose-response relationship in LVEF improvement among the 3 groups tested (EPO 12,000 IU: 5.4±9.3%, EPO 6,000 IU: 7.3±7.7%, Placebo: 8.1±8.3%, P=0.862). Low-dose EPO also did not improve cardiac function, as evaluated by 99 mTc-MIBI SPECT or NT-proBNP at 6 months and did not increase adverse events.
Conclusions:
Administration of low-dose EPO did not improve LVEF at 6 months in STEMI patients (UMIN000005721).
Early reperfusion therapy with percutaneous coronary intervention (PCI) has improved the clinical outcomes of patients with acute myocardial infarction (AMI).1
Nevertheless, deaths associated with AMI remain high, and heart failure (HF) can develop because of left ventricular (LV) remodeling in the chronic stage after AMI.1
To date, there are no therapeutic agents available to decrease infarct size or improve cardiac function in the clinical setting.2,3
Therefore, cardioprotective therapy against AMI remains one of the most important unmet medical needs.4
Erythropoietin (EPO), a hematopoietic hormone, has antiapoptotic and tissue-protective effects.5
In animal models, we and others have demonstrated that intravenous administration of EPO associated with reperfusion therapy decreases myocardial infarct size, prevents cardiac remodeling and enhances neovascularization.6–8
Several clinical studies have been performed to clarify the cardioprotective effects of EPO in patients with AMI. However, administration of high-dose EPO (60,000–99,000 IU) has not been found to improve LV ejection fraction (LVEF) or to decrease infarct size.9–11
Platelet activation by high-dose EPO12
and the lack of a known optimal dose of EPO for limiting infarct size may have resulted in these negative outcomes.13
In contrast, in the EPO-AMI-I study, a multicenter, prospective, randomized, single-blind study, performed to clarify the effects of the low-dose (12,000 IU) of EPO on the improvement of LVEF at 6 months in 36 patients with ST-segment elevation MI (STEMI),14
low-dose EPO was associated with improved LV function without major cardiovascular events (MCE). Furthermore, a retrospective analysis of EPO-AMI-I revealed that administration of low-dose EPO was highly associated with improved LV function in patients with low LVEF (<50%).14
Therefore, we performed EPO-AMI-II, a prospective, randomized, double-blind, placebo-controlled, dose-finding study (6,000 IU and 12,000 IU), multicenter study in first STEMI patients to clarify the efficacy and safety of low-dose EPO in STEMI patients with low LVEF (<50%).
Methods
Study Design
The protocol for EPO-AMI-II was approved by both the Evaluation System of Investigational Medical Care of the Ministry of Health, Labor and Welfare of Japan and the Japanese governmental health insurance system. It was also approved by the ethics committees of the participating hospitals and the study was performed in accordance with the principles of the Declaration of Helsinki. Informed consent was obtained before the enrollment of all patients. The study was registered at the UMIN Clinical Trials Registry as UMIN000005721.
Patients
We prospectively enrolled patients who were ≥20 and ≤80 years of age, with a first STEMI and successful reperfusion by PCI within 12 h of symptom onset. According to a subanalysis of EPO-AMI-I, patients with low LVEF (<50% at enrollment, measured by echocardiography or left ventriculography) were enrolled because they were likely to benefit most from low-dose EPO.14
Exclusion criteria included residual significant stenosis requiring revascularization, impaired reperfusion (TIMI flow grade ≤1), Killip class III or IV or cardiogenic shock at admission, advanced renal or hepatic dysfunction (creatinine ≥2 mg/dL or total bilirubin ≥3 mg/dL), blood pressure ≥140/90 mmHg after PCI, hematocrit ≥54% at admission, atrial fibrillation after PCI, malignant hypertension, previous EPO administration, blood transfusion in the previous 3 months, cancer in the previous 5 years, severe infection, contraindications for aspirin or thienopyridine derivatives, pregnancy and breastfeeding.
Study Protocol
Standard antiplatelet treatments for AMI were administered prior to or at the time of primary PCI. The enrolled patients were randomly divided into 3 groups (epoetin-β 12,000 or 6,000 IU) or placebo. EPO or placebo was diluted in 10 mL of saline and administered intravenously over the course of 1 min within 6 h of PCI. The double-blind administration was ensured by a subject identification code unknown to the physicians, nurses or patients. Drug or placebo was prepared under medical supervision according to instructions contained in predefined packages provided by the EPO-AMI-II organization. Standard treatments, including β-blockade, lipid-lowering therapy, and angiotensin-converting enzyme inhibition or angiotensin-II receptor blockade, were additionally prescribed. Patients were followed for 6 months after enrollment. EPO and placebo were provided by Chugai Pharmaceutical Co. Ltd. (Tokyo, Japan).
Endpoints
The primary endpoint was LVEF improvement between the acute (4–7 days) and chronic stages (6 months). The secondary endpoints were the efficacy and safety of EPO treatment. Efficacy was evaluated on the basis of indexes of cardiac function measured by 99 mTc-MIBI SPECT, such as LV end-diastolic volume (LVEDV), LV end-systolic volume (LVESD), LVEDV index, LVESV index, summed motion score (SMS), uptake at rest, summed rest score (SRS), summed difference score (SDS), defect size at rest, and defect size with stress and ischemia. The survival rate, MCE (defined as cardiac death, stroke, non-fatal MI, admission for worsening of HF, admission for unstable angina (UAP), revascularization, and onset of HF symptoms) and NT-proBNP at 6 months were also analyzed to evaluate efficacy. Safety was evaluated on the basis of the incidence of adverse events, clinical laboratory tests and vital signs.
Quantification of LV Function and Infarct Size
We performed ECG-gated 99 mTc-MIBI SPECT (600–740 MBq, FUJIFILM RI Pharma Co., Ltd, Tokyo, Japan) 4–7 days and 6 months after administration of EPO or placebo. ECG-gated SPECT image acquisition was performed 60 min after 99 mTc-MIBI injection. With ECG gating, the SPECT data divided into 16 equal intervals were analyzed by Quantitative Gated SPECT software (Cedars-Sinai Medical Center, Los Angeles, CA, USA).15
A small heart, defined on the basis of an end-systolic volume <20 mL, was excluded from the analysis of RF, LVESV and LVESVI because underestimation of LVESV and overestimation of EF would probably be caused by inappropriate delineation of the left ventricle.16
Adenosine stress tests were also performed at 6 months with non-gated 99 mTc-MIBI SPECT. Adenosine (Adenoscan; Daiichi Sankyo, Tokyo, Japan) was administered at a rate of 0.72 mg/kg for 6 min. The 99 mTc-MIBI was injected 3 min after the start of adenosine infusion. Uptake and defect size were calculated by Quantitative Perfusion SPECT software (Cedars-Sinai Medical Center).17
Uptake was assessed by application of a 17-segment model of the left ventricle according to the standard myocardial segmentation of the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association and was expressed as the mean uptake count in these segments.18
Defect size was expressed as the percentage of defects at less than the threshold of 60% of peak counts in the entire left ventricle. SPECT data were analyzed in a blinded fashion with the assistance of nuclear medicine special radiological technologists at MICRON Co., Ltd. (Tokyo, Japan) and the Japan Academic Research Forum (Osaka, Japan). Finally, the analyzed data were evaluated by an independent radioisotope (RI) assessment committee.
Clinical and Laboratory Measures
Blood pressure and heart rate were monitored at regular intervals until patient discharge and were recorded at baseline, 1 h, 4, 7 and 35 days and 6 months after administration of EPO or placebo. Hematologic tests were measured at baseline, 4, 7 and 35 days and 6 months. Reticulocytes were measured at baseline and 7 days, because we had confirmed in EPO-AMI-I that they peaked 7 days after EPO administration and decreased thereafter.14
NT-proBNP was measured at 6 months. Adverse events were recorded during hospitalization and up to 6 months thereafter.
Sample Size Calculation and Interim Analysis
According to the results of EPO-AMI-I, we estimated the difference in LVEF improvement between the EPO (12,000 IU) and placebo group to be 4.42%, the common standard deviation to be 14.33%, and the effect size to be 0.31. To demonstrate a treatment difference with a power of 0.85 and a 1-sided type 1 error of 0.025, 190 patients per group were required. By taking into account 2 interim analyses and several patients dropping out, the recruited sample size was determined to be 200 patients per group (i.e., 600 patients in total).
We designed 2 formal interim analyses for the primary endpoint and safety: when 198 and 396 enrolled patients, respectively, completed 6-months of follow-up. An independent data safety monitoring board (DSMB) evaluated the primary endpoint according to the rules determined by the Lan-DeMets method with the O’Brien-Fleming error spending function. Asymmetric stopping boundaries were planned, in which early termination of the study was to be recommended with evidence of either overwhelming benefit (1-sided favoring EPO) or futility (1-sided against EPO). There was no statistical rule used for terminating this study because of safety concerns. The DSMB recommendations were based on the clinical assessment of the frequency of severe adverse events, their nature and relation to the investigational treatment.
Statistical Analysis
Summary statistics of quantitative continuous variables are presented as the mean±standard deviation (SD). NT-proBNP is presented as both mean±SD and median (interquartile range [IQR]), owing to heteroscedasticity. Categorical data are presented as absolute frequencies. Data were analyzed on the basis of the intention-to-treat principle. For LVEF improvement, the null hypothesis that all treatment groups had the same mean LVEF improvement was tested against the alternative hypothesis that the mean LVEF improvement increased in the order of placebo, EPO (6,000 IU) and EPO (12,000 IU). This test was performed by the linear contrast test with coefficient (−1, 0, 1) and the t-statistic. The primary endpoint was further analyzed within the subgroups of the location of culprit lesion (left anterior descending coronary artery (LAD) vs. non-LAD) and sex. All other 99 mTc-MIBI SPECT data were compared by Jonckheere-Terpstra test. Cardiovascular events and NT-proBNP were also compared by Jonckheere-Terpstra test. The proportion of patients with ischemia was compared by the Cochran-Armitage test. Survival rates were analyzed by the Kaplan-Meier method and compared by log-rank test. Reticulocyte counts were compared by one-way ANOVA. All data were evaluated by a linear contrast test based on a 1-sided significance level of 0.025, except for major cardiovascular or adverse events, which were evaluated on the basis of a 2-sided significance level of 0.05.
Results
First Interim Analysis
From December 2011 to February 2015, a total of 198 patients were enrolled at 25 hospitals. After the 6-month follow-up of the final randomized patient, the first interim analysis was performed. The DSMB recommended study termination because the results of the analysis met the futility stop criterion. Therefore, the principal investigators decided to terminate the study and performed the final analysis.
Patients’ Enrollment and Baseline Characteristics
Of the 198 patients, 68 were assigned to the EPO 12,000 IU group, 70 to the EPO 6,000 IU group and 60 to the placebo group (Figure 1). One patient (EPO 6,000 IU) was excluded from the safety evaluation because the study drug was not administered within 6 h after PCI; 10 patients were excluded from the efficacy evaluation because 7 (EPO 12,000 IU: 4, EPO 6,000 IU: 1, Placebo: 2) were lost to follow-up, 2 (EPO 6,000 IU: 1, Placebo: 1) withdrew their consent, and 1 (EPO 6,000) died of non-cardiac cause. Additionally, 47 patients were excluded from the primary endpoint evaluation because 14 (EPO 12,000 IU: 4, EPO 6,000 IU: 6, Placebo: 4) had small hearts, 11 (EPO 12,000 IU: 4, EPO 6,000 IU: 5, Placebo: 2) had poor image quality, 18 (EPO 12,000 IU: 4, EPO 6,000 IU: 1, Placebo: 2) deviated from the schedule, 2 (EPO 12,000 IU: 1, Placebo: 1) were mistakenly administered thallium instead of technetium and 2 (EPO 6,000 IU: 1, Placebo: 1) had adverse events. The baseline characteristics are shown in
Table 1.
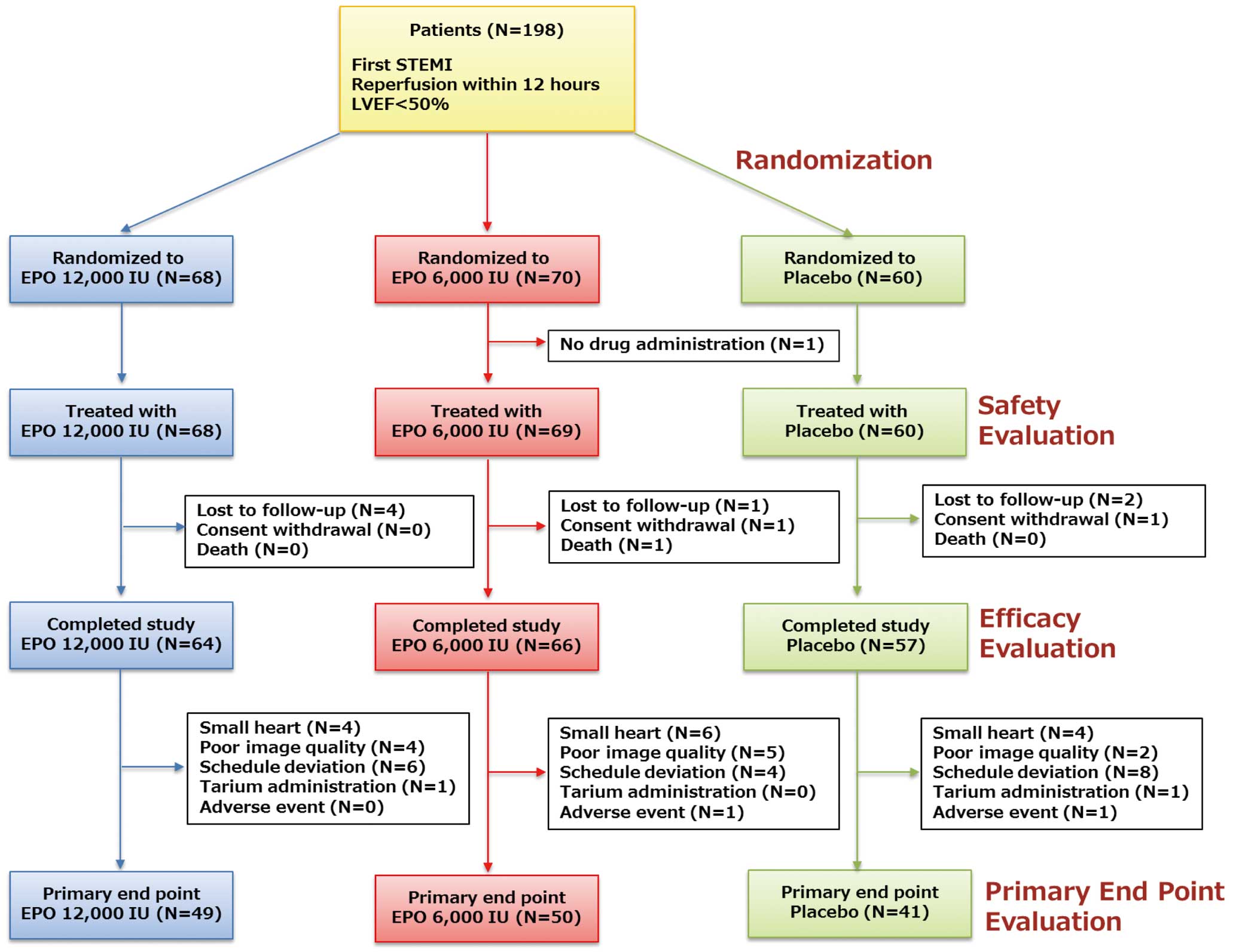
Table 1.
Baseline Characteristics of Patients in EPO-AMI-II Study
| Characteristics |
EPO 12,000 IU
(n=68) |
EPO 6,000 IU
(n=69) |
Placebo
(n=60) |
| Male |
58 (85.3) |
59 (85.5) |
52 (86.7) |
| Age (years) |
61.3±10.7 |
61.4±11.6 |
60.2±10.7 |
| BMI (kg/m2) |
24.8±4.2 |
24.0±3.5 |
24.1±3.2 |
| Smoking status |
| Current |
39 (57.4) |
27 (39.1) |
32 (53.3) |
| Past |
16 (23.5) |
23 (33.3) |
18 (30.0) |
| Clinical history |
| Cerebral infarction |
3 (4.4) |
3 (4.3) |
2 (3.3) |
| Angina |
5 (7.4) |
4 (5.8) |
4 (6.7) |
| HF |
1 (1.5) |
0 (0) |
0 (0) |
| Hypertension |
46 (67.6) |
40 (58.0) |
32 (53.3) |
| Hyperlipidemia |
39 (57.4) |
43 (62.3) |
36 (60.0) |
| Diabetes |
17 (25.0) |
12 (17.4) |
16 (26.7) |
| Initial TIMI flow grade |
| 0 |
53 (77.9) |
50 (72.5) |
46 (76.7) |
| 1 |
5 (7.4) |
9 (13.0) |
2 (3.3) |
| 2 |
8 (11.8) |
10 (14.5) |
9 (15.0) |
| 3 |
2 (2.9) |
0 (0) |
3 (5.0) |
| Final TIMI flow grade |
| 2 |
4 (5.9) |
3 (4.3) |
0 (0) |
| 3 |
64 (94.1) |
66 (95.7) |
60 (100) |
| Stent (%) |
67 (98.5) |
65 (94.2) |
58 (96.7) |
| Time from symptom onset to admission (min) |
157±105 |
183±155 |
169±145 |
| Time from door to balloon (min) |
57±28 |
65±39 |
60±30 |
| Time from reperfusion to drug administration (min) |
181±75 |
195±98 |
189±71 |
| eGFR (mL/min/1.73 m2) |
83±21 |
88±23 |
85±22 |
| Culprit lesion location |
| RCA |
14 (20.6) |
5 (7.2) |
11 (18.3) |
| LAD |
51 (75.0) |
60 (87.0) |
45 (75.0) |
| LCX |
3 (4.4) |
4 (5.8) |
4 (6.7) |
| Killip class |
| I |
65 (95.6) |
61 (88.4) |
56 (93.3) |
| II |
3 (4.4) |
8 (11.6) |
4 (6.7) |
| EF at admission (%) |
42.6±6.1 |
42.5±5.2 |
42.4±6.2 |
| Max CPK (IU/L) |
3,946±2,721 |
3,559±2,641 |
3,531±2,534 |
| Max CPK-MB (IU/L) |
331±225 |
313±233 |
300±183 |
| Medication at 35 days |
| Aspirin |
67 (100) |
67 (98.5) |
58 (100) |
| Thienopyridine |
65 (97.0) |
64 (94.1) |
53 (91.4) |
| ACEI/ARB |
57 (85.1) |
59 (86.8) |
47 (81.0) |
| β-blocker |
56 (83.6) |
57 (83.8) |
46 (79.3) |
| CCB |
10 (14.9) |
8 (11.8) |
5 (8.6) |
| Statins |
63 (94.0) |
65 (95.6) |
50 (86.2) |
Data are presented as n (%) or mean±SD. ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin-II receptor blocker; BMI, body mass index; CCB, calcium-channel blocker; CPK, creatine kinase; EF, ejection fraction; eGFR, estimated glomerular filtration rate; EPO, erythropoietin; HF, heart failure; IU, international units; LAD, left anterior descending artery; LCX, left circumflex coronary artery; RCA, right coronary artery; TIMI, Thrombosis in Myocardial Infarction.
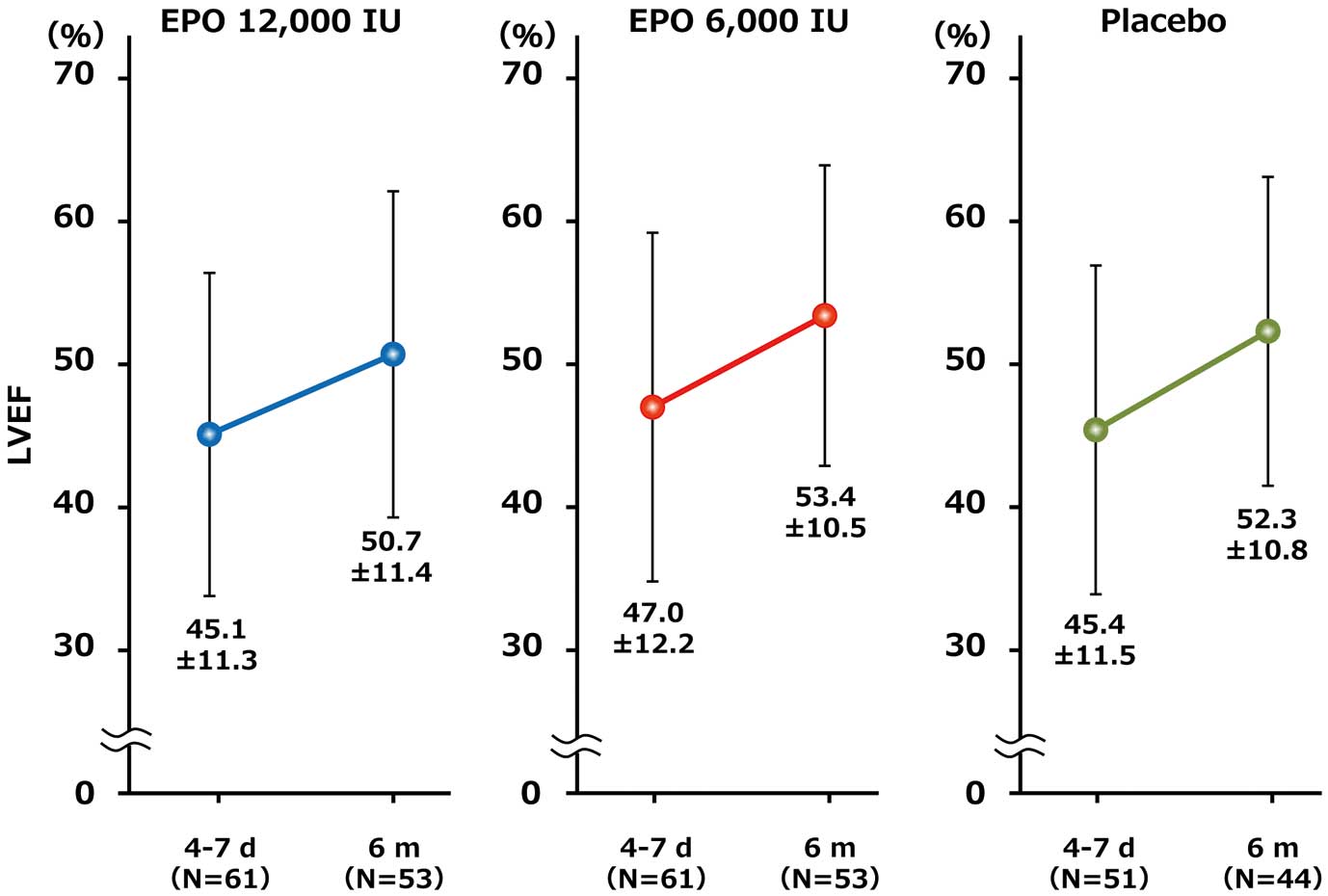
At 6 months, the mean (±SD) LVEF increased in all groups from baseline (EPO 12,000 IU: from 45.1±11.3% to 50.7±11.4%; EPO 6,000 IU: from 47.0±12.2% to 53.4±10.5%; Placebo: from 45.4±11.5% to 52.3±10.8%), as shown in
Figure 2. However, there was no significant dose–response relationship in the LVEF improvement at 6 months (∆LVEF) among the 3 groups (EPO 12,000 IU: 5.4±9.3%; EPO 6,000 IU: 7.3±7.7%; Placebo: 8.1±8.3%, P=0.862) (Table 2).
Table 2.
LVEF Improvement at 6 Months (ΔLVEF) in EPO-AMI-II Study
| |
EPO 12,000 IU
(n=49) |
EPO 6,000 IU
(n=50) |
Placebo
(n=41) |
P value |
| ΔLVEF |
5.4±9.3 |
7.3±7.7 |
8.1±8.3 |
0.862 |
Data are presented as the mean±SD. LV, left ventricular. Other abbreviations as in Table 1.
We also found that ∆LVEF did not differ between patients with LAD culprit lesions (n=156) and those with non-LAD ones (n=41) (LAD: 7.3% vs. non-LAD 5.9%, P=0.56). Furthermore, we found that ∆LVEF in men (n=169) was significantly superior to that in women (n=28) (men: 7.2% vs. women 4.1%, P=0.02).
Efficacy of EPO Towards Cardiac Function and Ischemia by 99 mTc-MIBI SPECT
There was no significant dose–response relationship in the differences between 4–7-day and 6-month evaluations for LVEDV, LVESV, LVEDVI, LVESVI, SMS, uptake at rest, SRS and defect size at rest among the 3 groups (Table 3). There was also no significant dose-response relationship in SDS, defect size with stress and ischemia at 6 months.
Table 3.
Cardiac Function and Ischemia by
99 mTc-MIBI SPECT in EPO-AMI-II Study
| |
EPO 12,000 IU |
EPO 6,000 IU |
Placebo |
P value |
| LVEDV (mL) |
| 4–7 days |
122.4±36.3 |
111.5±31.0 |
121.9±40.4 |
– |
| 6 months |
119.6±41.3 |
109.6±30.5 |
114.5±38.7 |
– |
| ΔLVEDV |
−2.4±27.3 |
−5.4±21.2 |
−7.8±21.2 |
0.856 |
| LVESV (mL) |
| 4–7 days |
69.8±31.3 |
61.4±28.7 |
69.0±32.8 |
– |
| 6 months |
62.6±37.6 |
52.7±26.1 |
57.3±30.2 |
– |
| ΔLVESV |
−6.8±25.5 |
−11.4±18.3 |
−13.3±16.8 |
0.929 |
| LVEDVI (mL/m2) |
| 4–7 days |
70.6±18.8 |
65.6±16.1 |
73.9±21.3 |
– |
| 6 months |
70.3±25.0 |
63.8±14.7 |
65.8±21.8 |
– |
| ΔLVEDI |
0.5±17.2 |
−3.2±12.8 |
−6.8±13.1 |
0.976 |
| LVESVI (mL/m2) |
| 4–7 days |
40.0±17.1 |
35.9±15.9 |
42.1±18.6 |
– |
| 6 months |
37.0±23.1 |
30.6±11.6 |
33.1±17.8 |
– |
| ΔLVESVI |
−2.0±15.4 |
−7.0±10.8 |
−9.3±10.2 |
0.989 |
| SMS |
| 4–7 days |
19.1±13.2 |
19.9±14.8 |
20.1±13.3 |
– |
| 6 months |
11.7±11.6 |
11.3±10.9 |
12.3±11.9 |
– |
| ΔSMS |
−7.9±9.9 |
−8.8±9.1 |
−8.1±9.6 |
0.451 |
| Uptake at rest (%) |
| 4–7 days |
62.9±8.8 |
63.6±8.6 |
64.2±7.9 |
– |
| 6 months |
65.5±7.2 |
66.6±6.8 |
66.9±6.0 |
– |
| Δuptake at rest |
3.6±4.6 |
2.9±4.6 |
3.2±4.4 |
0.336 |
| SRS |
| 4–7 days |
18.2±11.9 |
16.8±11.1 |
18.0±10.6 |
– |
| 6 months |
11.8±9.1 |
10.0±8.5 |
10.6±8.0 |
– |
| ΔSRS |
−7.0±6.5 |
−6.6±5.8 |
−7.7±6.3 |
0.729 |
| SDS |
| 6 months |
1.4±1.5 |
1.5±1.9 |
1.4±1.6 |
0.504 |
| Defect size at rest (%) |
| 4–7 days |
31.0±19.5 |
29.3±19.1 |
31.5±18.4 |
– |
| 6 months |
20.6±16.5 |
17.6±15.2 |
19.1±15.5 |
– |
| Δdefect size at rest |
−11.1±9.9 |
−11.5±10.0 |
−13.1±10.7 |
0.838 |
| Defect size at stress (%) |
| 6 months |
22.7±16.1 |
19.4±15.2 |
21.3±15.3 |
0.668 |
| Patients with ischemia |
| 6 months |
6/57 (10.5%) |
2/58 (3.4%) |
6/48 (12.5%) |
0.606 |
Data are mean±SD. LVEDV, LV end-diastolic volume; LVEDVI, LV end-diastolic volume index; LVESV, LV end-systolic volume; LVESVI, LV end-systolic volume index; SDS, summed difference score; SMS, summed motion score; SRS, summed rest score. Other abbreviations as in Tables 1,2.
There was no significant dose-response relationship in plasma NT-proBNP levels at 6 months (EPO 12,000 IU: 201 pg/mL [110–391]; EPO 6,000 IU: 224 pg/mL [96–399]; Placebo: 144 pg/mL [102–254], P=0.909) (Table 4).
Table 4.
Plasma NT-proBNP Levels at 6 Months in EPO-AMI-II Study
| |
EPO 12,000 IU
(n=48) |
EPO 6,000 IU
(n=56) |
Placebo
(n=41) |
P value |
| NT-proBNP (pg/mL) |
201 (110–391) |
224 (96–399) |
144 (102–254) |
0.909 |
Data are presented as the median (IQR). EPO, erythropoietin; T-proBNP, N-terminal proB-type natriuretic peptide.
There were no cardiac deaths, and 1 non-cardiac death occurred in the EPO 6,000 IU group. There were no significant differences in survival rate among the 3 groups (P=0.526) (Figure 3).
Major Cardiovascular Events and Adverse Events
Major cardiovascular events and adverse events during the 6 months after enrollment are shown in
Table 5
and
Table S1, respectively. For MCE, there were no significant differences among the 3 groups (EPO 12,000 IU: 4 patients (5.9%) [1 stroke, 1 admission for worsening of HF, 1 admission for UAP and 1 onset of HF symptoms]; EPO 6,000 IU: 4 patients (5.8%) [1 stroke, 1 non-fatal MI, 2 admissions for UAP and 2 onset of HF symptoms]; Placebo: 4 patients (6.7%) [1 admission for worsening of HF, 1 for revascularization and 2 for onset of HF symptoms], P=0.877). Additionally, there were also no significant differences in the incidence non-cardiac death, intracardiac thrombi, endomyocarditis, Dressler syndrome, ventricular tachycardia, atrial fibrillation/flutter and sick sinus syndrome. No other venous embolic and thrombotic events were confirmed during the 6 months after EPO administration among groups.
Table 5.
Major Cardiovascular Events During 6 Months in EPO-AMI-II Study
| |
EPO 12,000 IU
(n=68) |
EPO 6,000 IU
(n=69) |
Placebo
(n=60) |
P value |
| Major cardiovascular events |
4 (5.9) |
4 (5.8) |
4 (6.7) |
0.877 |
| Cardiac death |
0 (0) |
0 (0) |
0 (0) |
– |
| Stroke |
1 (1.5) |
1 (1.4) |
0 (0) |
0.423 |
| Non-fatal MI |
0 (0) |
1 (1.4) |
0 (0) |
0.941 |
| Admission for worsening of HF |
1 (1.5) |
0 (0) |
1 (1.7) |
0.958 |
| Admission for UAP |
1 (1.5) |
2 (2.9) |
0 (0) |
0.541 |
| Revascularization |
0 (0) |
0 (0) |
1 (1.7) |
0.201 |
| Onset of HF symptoms |
1 (1.5) |
1 (1.4) |
2 (3.3) |
0.481 |
Data are presented as n (%). MI, myocardial infarction; UAP, unstable angina pectoris. Other abbreviations as in Table 1.
Reticulocyte counts tended to increase from baseline to 7 days after EPO administration (EPO 12,000 IU: 25.3±30.6×103
cells/μL; EPO 6,000 IU: 20.5±25.1×103
cells/μL; Placebo: 9.8±29.0×103
cells/μL, P=0.032) (Table S2A). There were no increases with EPO administration in red blood cell numbers, hemoglobin, hematocrit, platelets, systolic or diastolic pressure or heart rate (Tables S2B,S3).
Discussion
In STEMI patients with low LVEF (<50%), a single administration of low-dose EPO (epoetin-β 12,000 or 6,000 IU) within 6 h after successful PCI did not improve LVEF, cardiac function or NT-proBNP at 6 months. However, administration of low-dose EPO did not increase MCE.
Previous proof-of-concept studies using high-dose EPO have reported negative results in patients with STEMI.9–11
In contrast, low-dose EPO was deemed likely to be cardioprotective in a small clinical study (EPO-AMI-I).14
Potential mechanisms explaining the dose-dependent discrepancy of EPO in cardioprotection might be attributable to platelet activation and the existence of an unknown lower optimal dose for limiting infarct size.12,13
Because EPO has structural similarity to thrombopoietin,12
high doses increase platelet production and reactivity, thus leading to an increased risk of thrombosis and cardiovascular events. Additionally, the dose-response curve analyzing the bioactivity of cytokines does not appear to follow a sigmoid function. Positive intracellular signals of cytokine receptors, mediated by serial chain reactions of protein tyrosine kinases, are typically interfered with by automatic circuit reactions of protein tyrosine phosphatases, such as SHP1, to avoid overstimulation of growth and inflammation.19
Thus, high-dose EPO might have less cardioprotective activity in rat and mouse coronary ischemia/reperfusion models.
Contrary to our speculation, beneficial efficacy of low-dose EPO was not observed in EPO-AMI-II. There was no dose-response relationship in LVEF improvement among the 3 groups, and the LVEF improvement in the placebo group was somewhat higher than that in the EPO 12,000 and 6,000 IU treatment groups. Additionally, there was no dose-response relationship in cardiac function, as evaluated by 99 mTc-MIBI SPECT and NT-proBNP. Because reperfusion injury starts in the very early phase of recanalization of an infarct-related artery, drugs to prevent reperfusion injury should be administered as soon as possible after reperfusion.20
Although the administration of EPO within 24 h after successful PCI was effective for cardioprotection in EPO-AMI-I,14
this late administration of EPO would have contributed to the negative results in EPO-AMI-II. Additionally, patients with low LVEF (<50%) were enrolled in the present study according to a subanalysis of EPO-AMI-I. In reality, the culprit lesion was most often located in the LAD, and the severity of AMI was sufficient to evaluate the efficacy of low-dose EPO. However, we cannot deny the possibility that other confounders, such as coronary collateralization to the area at risk, that were not controlled for in EPO-AMI-II might have contributed to the different results from those in EPO-AMI-I.21
The primary endpoint was further analyzed within the subgroups of the location of the culprit lesion (LAD vs. non-LAD) and sex. Although LV improvement did not differ between patients with LAD culprit lesions (n=156) and those with non-LAD ones (n=41), we found that LV improvement in men (n=169) was significantly superior to that in women (n=28). We need caution when we consider these results, because the numbers in the subgroups were very different.
Increases in major cardiovascular events and adverse events were not observed in this study or in EPO-AMI-I.14
Although we did not perform special examination to determine venous embolic and thrombotic events, none were confirmed during the 6 months after EPO administration under routine medical care. In the REVEAL study, subanalysis showed that high-dose EPO (60,000 IU) was associated with a higher incidence of severe adverse events, although those authors noted that the analysis was based on a very small number of events.9
In contrast, in the HEBE III study, subanalysis revealed that EPO treatment showed a trend towards a decrease in enzymatic infarct size, and the incidence of the combined endpoint (cardiovascular death, MI, in-stent thrombosis, UAP and HF) was significantly decreased.10
In the REVIVAL study, EPO (33,000 IU×3) treatment showed a trend towards an increased rate of severe adverse effects.11
A meta-analysis showed that the administration of EPO appeared to be safe for patients with acute STEMI. In the present study, reticulocyte counts tended to increase from baseline to 7 days with EPO administration, owing to hematopoietic effects. However, there were no differences in red blood cell numbers, hemoglobin, hematocrit, platelets or vital signs. These results indicated that administration of low-dose EPO in STEMI patients was safe.
Study Limitations
This study was terminated at the first interim analysis, so it was not powered for clinical events, and the previous statements should be carefully interpreted. However, it is unlikely that positive findings were missed because of insufficient power.
Conclusions
In STEMI patients with low LVEF (<50%), we compared the effect of intravenous administration of low-dose EPO (12,000 or 6,000 IU) or placebo within 6 h after successful PCI. Low-dose EPO did not increase LVEF improvement 6 months after AMI.
Acknowledgments
We sincerely thank Dr. Akira Myoi (Medical Center for Translational Research Osaka University Hospital, Osaka, Japan) and Dr. Yoichi Yamamoto (Center for Clinical Investigation and Research, Osaka University Hospital, Osaka, Japan) for their advice on conducting the EPO-AMI-II study. We thank Yukako Kurokawa (Department of Cardiovascular Medicine, Osaka University Graduate School of Medicine, Osaka, Japan) for excellent assistance with the manuscript.
Funding Sources
Grants-in-Aid from the Ministry of Health, Labor and Welfare of Japan, Grants-in-Aid from Japan Agency for Medical Research and Development, and a Japanese Circulation Society Grant for Translational Research 2010.
Appendix
Principal Investigators:
Aizawa Y, Komuro I, Minamino T.
Office Secretariat:
Higo S.
Steering Committee:
Ozawa T, Kato K, Ito H, Kobayashi N, Suzuki H, Tobaru T, Toba K, Minamino T, Higo S.
Data Management:
Sawa A (Department of Medical Innovation, Osaka University Hospital, Osaka, Japan), Takahara S (Department of Medical Innovation, Osaka University Hospital, Osaka, Japan), Yamada T (Department of Medical Innovation, Osaka University Hospital, Osaka, Japan).
Statistical Analysis:
Yamamoto K (Department of Medical Innovation, Osaka University Hospital, Osaka, Japan (currently, Department of Medical Statistics, Osaka City University Graduate School of Medicine, Osaka).
RI Assessment Committee:
Ishida Y (Kaizuka City Hospital, Osaka, Japan), Yoshimura N (Department of Radiology, Niigata University Medical and Dental Hospital, Niigata, Japan), Araki R.
Data and Safety Monitoring Board:
Fujio Y, Hasegawa S (Department of Cardiology, JCHO Osaka Hospital, Osaka, Japan), Seki Y (Department of Internal Medicine (Hematology), Niigata Prefectural Shibata Hospital, Niigata, Japan), Sugimoto T (Department of Mathematical Sciences, Hirosaki University Graduate School of Science and Technology, Aomori, Japan).
EPO-AMI-II Investigators:
Niigata University Medical and Dental Hospital, Niigata: Minamino T; Tachikawa General Hospital, Niigata: Toba T; Dokkyo University Hospital, Tochigi: Ishimitsu T; Osaka University Hospital, Osaka: Higo S; Osaka Police Hospital, Osaka: Yasumura Y; Osaka General Medical Center, Osaka, Japan; Yamada T; Osaka National Hospital, Osaka: Ueda Y; Osaka Rosai Hospital, Osaka: Nishino M; Okayama University Hospital, Oakayama: Ito H; Showa University Fujigaoka Hospital, Kanagawa: Suzuki H; Kanto Rosai Hospital, Kanagawa: Namiki A; Sakakibara Memorial Hospital, Tokyo: Tobaru T; Kokura Memorial Hospital, Fukuoka: Ando K; National Cerebral and Cardiovascular Center, Osaka: Yasuda S; Osaka Saiseikai Senri Hospital, Osaka: Doi Y; Showa University Hospital, Tokyo: Koba S; Nippon Medical School Hospital, Tokyo: Yasutake M; St. Marianna University Hospital, Kanagawa: Akashi Y; Shonan Kamakura General Hospital, Kanagawa: Saito S; Nozaki Tokushukai Hospital, Osaka, Japan (Currently, Department of Internal Medicine, Kawasaki Medical School General Medical Center, Okayama); Okutsu M; Higashi-Osaka City General Hospital, Osaka: Kijima Y; Chiba-Nishi General Hospital, Chiba: Yoshida T; Nippon Medical School Musashikosugi Hospital, Kanagawa: Sato N; Chiba Emergency Medical Center, Chiba: Sakai Y; Edogawa Hospital, Tokyo: Ohira Y.
Supplementary Files
Supplementary File 1
Table S1.
Adverse events during 6 months
Table S2.
(A) Reticulocyte counts, (B) laboratory tests
Table S3.
Vital signs
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-17-0889
References
- 1.
Roe MT, Messenger JC, Weintraub WS, Cannon CP, Fonarow GC, Dai D, et al. Treatments, trends, and outcomes of acute myocardial infarction and percutaneous coronary intervention. J Am Coll Cardiol 2010; 56: 254–263.
- 2.
Kloner RA, Hale SL, Dai W, Shi J. Cardioprotection: Where to from here? Cardiovasc Drugs Ther 2017; 31: 53–61.
- 3.
Rossello X, Yellon DM. Cardioprotection: The disconnect between bench and bedside. Circulation 2016; 134: 574–575.
- 4.
Hausenloy DJ, Garcia-Dorado D, Erik Bøtker H, Davidson SM, Downey J, Engel FB, et al. Novel targets and future strategies for acute cardioprotection: Position Paper of the European Society of Cardiology Working Group on Cellular Biology of the Heart. Cardiovasc Res 2017 113: 564–585.
- 5.
Sanchis-Gomar F, Garcia-Gimenez JL, Pareja-Galeano H, Romagnoli M, Perez-Quilis C, Lippi G. Erythropoietin and the heart: Physiological effects and the therapeutic perspective. Int J Cardiol 2014; 171: 116–125.
- 6.
Parsa CJ, Matsumoto A, Kim J, Riel RU, Pascal LS, Walton GB, et al. A novel protective effect of erythropoietin in the infarcted heart. J Clin Invest 2003; 112: 999–1007.
- 7.
Hirata A, Minamino T, Asanuma H, Sanada S, Fujita M, Tsukamoto O, et al. Erythropoietin just before reperfusion reduces both lethal arrhythmias and infarct size via the phosphatidylinositol-3 kinase-dependent pathway in canine hearts. Cardiovasc Drugs Ther 2005; 19: 33–40.
- 8.
Hirata A, Minamino T, Asanuma H, Fujita M, Wakeno M, Myoishi M, et al. Erythropoietin enhances neovascularization of ischemic myocardium and improves left ventricular dysfunction after myocardial infarction in dogs. J Am Coll Cardiol 2006; 48: 176–184.
- 9.
Najjar SS, Rao SV, Melloni C, Raman SV, Povsic TJ, Melton L, et al. Intravenous erythropoietin in patients with ST-segment elevation myocardial infarction: REVEAL: A randomized controlled trial. JAMA 2011; 305: 1863–1872.
- 10.
Voors AA, Belonje AM, Zijlstra F, Hillege HL, Anker SD, Slart RH, et al. A single dose of erythropoietin in ST-elevation myocardial infarction. Eur Heart J 2010; 31: 2593–2600.
- 11.
Ott I, Schulz S, Mehilli J, Fichtner S, Hadamitzky M, Hoppe K, et al. Erythropoietin in patients with acute ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention: A randomized, double-blind trial. Circ Cardiovasc Interv 2010; 3: 408–413.
- 12.
Stohlawetz PJ, Dzirlo L, Hergovich N, Lackner E, Mensik C, Eichler HG, et al. Effects of erythropoietin on platelet reactivity and thrombopoiesis in humans. Blood 2000; 95: 2983–2989.
- 13.
Baker JE, Kozik D, Hsu AK, Fu X, Tweddell JS, Gross GJ. Darbepoetin alfa protects the rat heart against infarction: Dose-response, phase of action, and mechanisms. J Cardiovasc Pharmacol 2007; 49: 337–345.
- 14.
Ozawa T, Toba K, Suzuki H, Kato K, Iso Y, Akutsu Y, et al. Single-dose intravenous administration of recombinant human erythropoietin is a promising treatment for patients with acute myocardial infarction: Randomized controlled pilot trial of EPO/AMI-1 study. Circ J 2010; 74: 1415–1423.
- 15.
Germano G, Kiat H, Kavanagh PB, Moriel M, Mazzanti M, Su HT, et al. Automatic quantification of ejection fraction from gated myocardial perfusion SPECT. J Nucl Med 1995; 36: 2138–2147.
- 16.
Nakajima K, Okuda K, Nyström K, Richter J, Minarik D, Wakabayashi H, et al. Improved quantification of small hearts for gated myocardial perfusion imaging. Eur J Nucl Med Mol Imaging 2013; 40: 1163–1170.
- 17.
Germano G, Kavanagh PB, Waechter P, Areeda J, Van Kriekinge S, Sharir T, et al. A new algorithm for the quantification of myocardial perfusion SPECT. I: Technical principles and reproducibility. J Nucl Med 2000; 41: 712–719.
- 18.
Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 2002; 105: 539–542.
- 19.
Bittorf T, Seiler J, Zhang Z, Jaster R, Brock J. SHP1 protein tyrosine phosphatase negatively modulates erythroid differentiation and suppression of apoptosis in J2E erythroleukemic cells. Biol Chem 1999; 380: 1201–1209.
- 20.
Sharma V, Bell RM, Yellon DM. Targeting reperfusion injury in acute myocardial infarction: A review of reperfusion injury pharmacotherapy. Expert Opin Pharmacother 2012; 13: 1153–1175.
- 21.
Hausenloy DJ, Baxter G, Bell R, Bøtker HE, Davidson SM, Downey J, et al. Translating novel strategies for cardioprotection: The Hatter Workshop Recommendations. Basic Res Cardiol 2010; 105: 677–686.