A 62-year-old man with chronic atrial fibrillation was admitted to hospital with dyspnea on effort. He had a medical history of mucopolysaccharidosis type II (MPS II), also termed Hunter syndrome, and had the typical findings of short stature, enlarged head, broad nose, thickened lips, and macroglossia. He had deficient iduronate 2-sulfatase enzyme activity in white cells. He had been receiving weekly i.v. enzymatic replacement therapy for the last 9 years. He was 153 cm tall, weighed 74 kg, and had normal intelligence. He did not have any risk factors for atherosclerosis, such as smoking, diabetes mellitus, hypertension, and family history. Chest radiography indicated cardiomegaly with slight congestion. Electrocardiography showed atrial fibrillation with complete right bundle branch block. Transthoracic echocardiography indicated a thickened, partly calcified, and restricted mitral valve (MV) and severely calcified aortic valve (AV). The mean pressure gradient and area of the MV were 8 mmHg and 1.2 cm2, respectively, resulting in severe mitral stenosis (MS). Those of the AV were 45 mmHg and 0.8 cm2, respectively, resulting in severe aortic stenosis (AS). Left ventricular (LV) ejection fraction was 60%. The patient also had moderate MV regurgitation, severe tricuspid valve regurgitation, and secondary pulmonary hypertension, with an estimated systolic pulmonary artery pressure of 69 mmHg. Owing to the heart failure and the slowly progressive nature of his MPS II phenotype, we decided on triple valve surgery. The operation was performed through a median sternotomy. On aortotomy the AV was a tricuspid valve with abundant calcification at the whole cusps and aortic annulus (Figure 1A,B). The gross appearance was identical to that of senile calcified AS although the patient was relatively young for development of valvular deformity. Commissural fusion, a characteristic feature of rheumatic valve disease, was not prominent (Figure 1A,B). Appearance of the MV was unique, with each segment of the posterior leaflet (P1, P2, and P3) being thickened and curled up toward the LV side. The protruded scalloped posterior leaflet of the MV was prominent. The posterior mitral leaflet had a shiny and thickened appearance (Figure 1C). Unlike the AV, severe calcification was identified only at the P3 and P3 annulus. Both the clear and rough zones of the anterior and posterior leaflets, as well as the subvalvular apparatus, were extremely thickened, but commissural fusion was not apparent. On the basis of the aforementioned observation, the MV pathology seemed to be neither rheumatic nor calcified MS, the latter being attributed instead to calcification of the mitral leaflet in addition to the annulus.
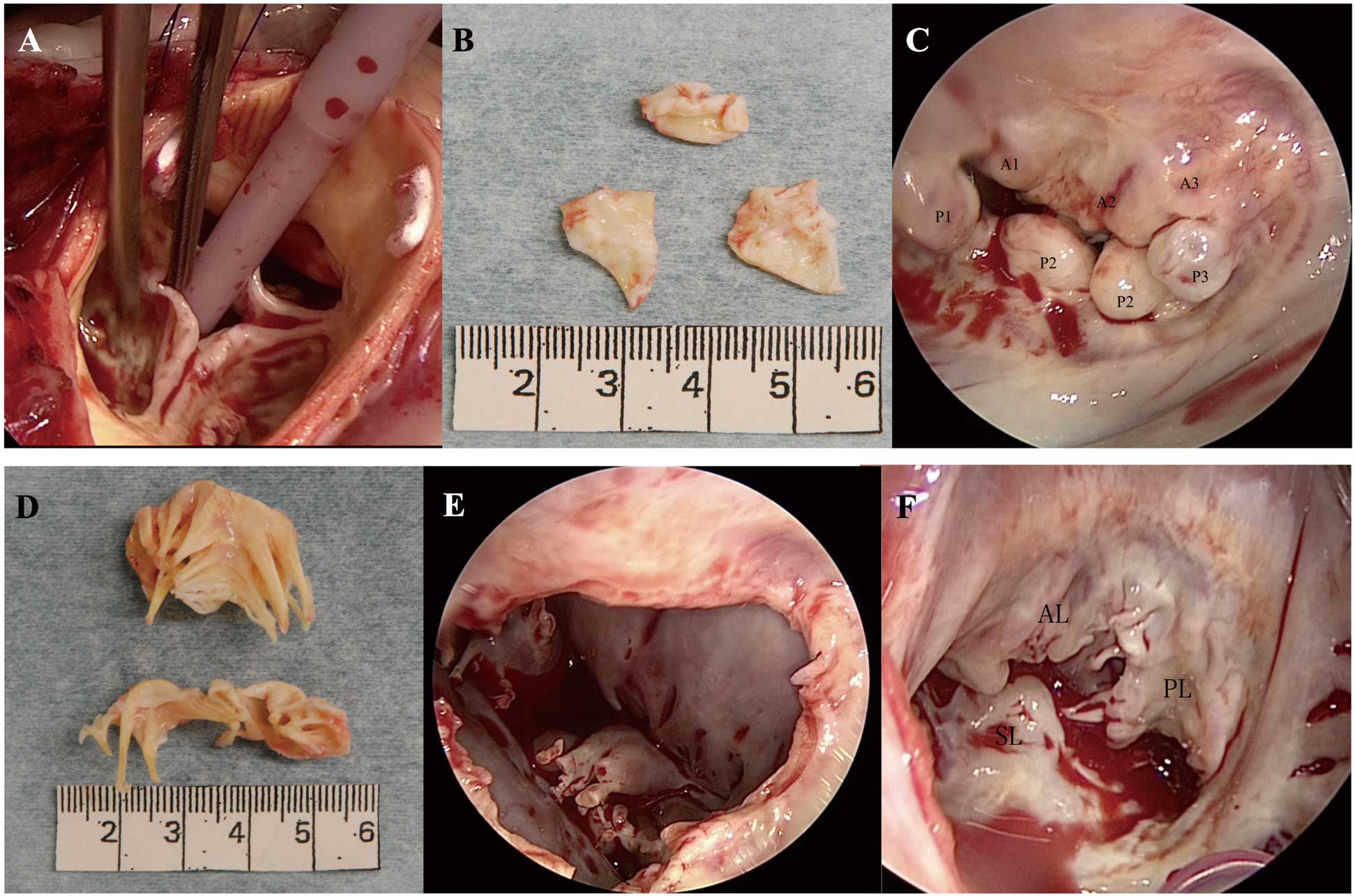
We removed the MV from the mitral annulus and performed MV replacement with a 27-mm mechanical valve (St. Jude Medical, St. Paul, MN, USA). The tricuspid valve was slightly thickened in both the clear and rough zones, without a thickened subvalvular apparatus. Tricuspid annular dilatation was seen at the anterior and posterior leaflets, as seen in the secondary tricuspid regurgitation (Figure 1F). We then performed tricuspid annuloplasty using a 28-mm Physio tricuspid ring (Edwards Lifesciences, Irvine, CA, USA) and AV replacement with a 20-mm ATS mechanical valve (Medtronic ATS Medical, Minneapolis, MN, USA). The patient was discharged 14 days postoperatively and was doing well 14 months after surgery. On histopathology of the removed AV (Figure 2A,B) and MV (Figure 2D,E) abundant granules within foamy cells were noted, which were positive on periodic acid-Schiff and Alcian blue staining. In addition, fibrous thickening with hyalinization and calcification was observed in the AV and MV. On immunohistostaining, CD68-positive macrophages were noted in the AV and MV (Figure 2C,F). On the basis of the above-mentioned observations, the histopathological changes of both the AV and MV were due to the polysaccharide accumulation mixed with degenerative calcification.

MPS II consists of 2 types: a rapidly progressive (severe) type and a slowly progressive type. The life expectancy for the severe type is 10–20 years, whereas patients with the slowly progressive type typically survive until their fifth or sixth decade.1
All the previously described patients who underwent valve operations had the severe type.2–5
Of these patients, only 3 had a successful MV replacement for MS,2–4
and 1 had a successful AV replacement.5
Patient age ranged from 10 to 33 years. The present patient was the oldest reported patient to have undergone valve surgery,1–4
therefore we considered categorizing him as having the slowly progressive phenotypic subtype. Few reports have described valve surgery in patients with the slowly progressive phenotypic subtype; thus, the macroscopic findings of the triple valves in patients with this phenotype are unclear.
We have herein described an elderly patient with progressive AS and MS secondary to Hunter syndrome with concomitant tricuspid regurgitation who was treated with triple valve operation. The macroscopic features of shiny round leaflet segments of the MV seemed to be specific to MPS II with the slowly progressive phenotype, while the macroscopic degenerative change disproportionate to the patient’s age was more prominent in AV. The tricuspid valve also developed organic changes. Histopathology also confirmed that AV and MV were complicated with both MPS-related and degenerative changes.
Disclosures
The authors declare no conflicts of interest.
References
- 1.
Jones SA, Almássy Z, Beck M, Burt K, Clarke JT, Giugliani R, et al. Mortality and cause of death in mucopolysaccharidosis type II: A historical review based on data from the Hunter Outcome Survey (HOS). J Inherit Metab Dis 2009; 32: 534–543.
- 2.
Wraith JE, Scarpa M, Beck M, Bodamer OA, De Meirleir L, Guffon N, et al. Mucopolysaccharidosis type II (Hunter syndrome): A clinical review and recommendations for treatment in the era of enzyme replacement therapy. Eur J Pediatr 2008; 167: 267–277.
- 3.
Bhattacharya K, Gibson SC, Pathi VL. Mitral valve replacement for mitral stenosis secondary to Hunter’s syndrome. Ann Thorac Surg 2005; 80: 1911–1912.
- 4.
Lee SH, Kim J, Choi JH, Yun KW, Sohn CB, Han DC, et al. Severe mitral stenosis secondary to Hunter’s syndrome. Circulation 2013; 128: 1269–1270.
- 5.
Sato Y, Fujiwara M, Kobayashi H, Ida H. Massive accumulation of glycosaminoglycans in the aortic valve of a patient with Hunter syndrome during enzyme replacement therapy. Pediatr Cardiol 2013; 34: 2077–2079.



