2018 Volume 82 Issue 5 Pages 1309-1318
2018 Volume 82 Issue 5 Pages 1309-1318
Background: In long-term left ventricular assist device (LVAD) therapy, recurrent bleeding events may justify cessation of anticoagulation therapy (AT). However, data about THE safety and risks of AT cessation in LVAD patients are scarce.
Methods and Results: Between 2010 and 2015, 128 patients received a HeartMate II (HMII). Following recurrent bleeding events, we ceased vitamin K antagonist (VKA) therapy in 13 patients (10%) (no-VKA group). To characterize the hemostatic profile, we performed von Willebrand factor (vWF), platelet function (PF), and other hemostatic tests in all HMII patients. The incidence of pump thrombosis (PT), ischemic stroke (IS) and bleeding events in this HMII population was 4.7 %, 6.2% and 36.7%, respectively. Median survival without VKA was 435 days. No cases of PT and only 1 of IS occurred after AT discontinuation. All patients had impaired PF and acquired von Willebrand syndrome (AvWS). However, the vWF collagen-binding activity to antigen ratio before and after VKA cessation was significantly lower in the no-VKA group compared with the HMII population (0.60±0.12 vs. 0.73±0.14, P=0.006). The thrombin-antithrombin III complex (TAT) value was significantly higher in the no-VKA group (P=0.0005).
Conclusions: We experienced good results with AT cessation in specific HMII patients. The simultaneous onset of AvWS and high TAT values could explain at least in part the low thromboembolic rate in HMII patients without VKA.
Although continuous-flow left ventricular assist devices (CF-LVADs) show good durability and improved patient survival, bleeding and thromboembolic events remain the most common postoperative complications. With the growing use of CF-LVADs as destination therapy (DT), bleeding rates are increasing.1–3 The current anticoagulation recommendation for HeartMate II (HMII) is vitamin K antagonist (VKA) treatment with an international normalized ratio (INR) target of 2.5±0.5, together with daily antiplatelet therapy (APT) using acetylsalicylic acid (ASA: 81–325 mg).4 Considering the risk of thromboembolic events, the cessation of anticoagulation therapy (AT) may be the only viable option in patients with recurrent bleeding events during CF-LVAD support as DT.5,6 There is limited information regarding the hemostatic profile of HMII patients who survive despite cessation of VKA and APT without the development of thromboembolism.
Editorial p 1245
Therefore, the present study assessed the safety of VKA therapy cessation in HMII patients by evaluating recurrent bleeding events and investigating a panel of coagulation assays.
This was a single-center combined retrospective and prospective observational study of HMII patients in whom VKA treatment and APT therapy were ceased for at least 30 days because of recurrent bleeding events. All patients who received HMII implantation from May 2010 until May 2015 were included and their data were analyzed. The institutional ethics committee waived the requirement of informed consent for retrospective data analysis. The prospective analysis was approved by the local ethics committee (EK 151/09). Written informed consent was given by each patient for the collection of blood samples and clinical data. Data were collected from the electronic database of our clinic, and included patient demographics, medical history, laboratory assessments, adverse events (AEs), and rehospitalization. All LVAD patients are routinely followed up during which the device parameter (flow [L/min], power [Watts], pulse index (PI) and revolutions per minute [rpm]) are controlled and documented by the LVAD coordinator. In addition, all LVAD patients document their device parameters daily and send the document each month to the LVAD coordinator. All patients were routinely followed up in the outpatient clinic until April 2017.
Laboratory TestsBesides the routine laboratory assessments, fibrinogen, plasma free hemoglobin (fHb), and D-dimer levels were routinely measured in all patients. In addition, as part of the routine laboratory evaluation of LVAD patients, von Willebrand (vWF) analysis, including measurement of vWF collagen-binding activity (vWF:CBA) and the vWF antigen (vWF:Ag) ratio and multimer analysis were performed, starting 1 month after implantation and then at 3-month intervals until transplantation or therapy end. Thrombin-antithrombin complex (TAT) as an indicator of the activation of the procoagulant pathway was measured in all 13 patients for whom AT was ceased and in 50 HMII patients who were treated with VKA and ASA. Pre-AT cessation TAT data were only available in 6 of the 13 patients, for whom AT was then ceased.
Platelet function was evaluated with light transmission aggregometry (LTA) and PFA-100, at 3 and 6 months after implantation. Further coagulation analysis was carried out in patients for whom AT was ceased and who survived until April 2016. Coagulation was assessed in whole blood by performing extrinsic- and intrinsic-activated thromboelastometry (EXTEM and INTEM, respectively). Thrombin generation in plasma was measured using the calibrated automated thrombogram (CAT; Thrombinoscope, Maastricht, The Netherlands). For detailed information about blood sampling and hemostatic analysis, please refer to Supplementary File 1.
AT After HMII ImplantationWhen chest tube drainage was <50 mL/h, continuous infusion of heparin was initiated within the 24-h postoperative period in order to achieve an activated partial thromboplastin time (aPTT) of 50–60 s. For platelet inhibition, ASA (100 mg/day) was added to the medication regimen on postoperative day (POD) 1. At POD 3, VKA (Phenprocoumon, Phenpro-ratiopharm® 3 mg) was administered orally with a target INR of 1.8–2.2.
Patient GroupsAT was ceased in patients who had more than 4 bleeding events or hemorrhagic stroke (HS) and 1 further bleeding event within a period of 6 months during HMII support, and they resisted ICU readmission, and transfusion of blood products. After stabilization, all patients underwent repeated endoscopic diagnostic tests, such as upper and lower endoscopy or capsule endoscopy or computer tomography with angiography, and no definitive source of bleeding, which would have enabled intervention, could be identified.
Patients then received only ASA (100 mg/day) without VKA. This definition was a consensus among experts from cardiac surgery, cardiology and gastroenterology based on our experience with HMII and other LVAD devices during the past decade in our institution.
These 13 patients formed the no-VKA group. The rest of the population HMII (115 patients) formed the VKA group. After termination of AT, we frequently performed transoesophageal echocardiography, CT angiography, laboratory tests, and HMII-VAD device reading to detect thromboembolic disorders or events.
AE MonitoringEach patient’s AE history was evaluated during hospital stay and at every outpatient visit. AEs were defined according to the 2013 Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) Version 2.2 definitions for ischemic stroke (IS), HS, transient ischemic attack (TIA), pump thrombosis (PT), and bleeding.7
Statistical AnalysisContinuous variables are presented as mean±standard deviation (SD) or median with interquartile range [IQR] as appropriate, and categorical variables are described in absolute numbers and percentages. Owing to non-normal distribution of data, continuous variables were analyzed using the Wilcoxon signed-rank test for paired or repeated measurements and the Mann-Whitney U test for unpaired measurements. Categorical variables were analyzed using the chi-square test or, if appropriate, Fisher’s exact test. To compensate for the small sample number (n=6) of available TAT measurements in the no-VKA group prior to AT cessation and to perform a comparison with the VKA group, we performed propensity matching. Propensity scores were calculated for each patient using multivariate logistic regression based on the following preoperative covariates: age, sex, body mass index (BMI), INTERMACS level. The 6 no-VKA patients were then paired with 6 patients in the no-VKA group to compare TAT values before AT cessation.
The 6 no-VKA patients were also paired with 6 patients in the no-VKA group to compare TAT values before AT cessation between groups.
Events per patient-years were analyzed using Poisson regression. Kaplan-Meier analyses were performed to determine survival, freedom from AEs and to evaluate time-related outcomes using the log-rank test. All data analyses were performed using SPSS 23 (IBM Corp., Armonk, NY, USA). P<0.05 was considered statistically significant.
The study included 128 patients, with a median follow-up of 562 [IQR: 28, 1021] days. An overview of patient demographics is presented in Table 1. AT was ceased in 13 patients (10%) (no-VKA group), and these patients were older than the rest of the HMII population (VKA group) (68±7 vs. 61±9 years, P=0.003). Among these patients, 84% were treated as DT. In the no-VKA group, 100% of the patients had ischemic heart failure, 61% had INTERMACS IV at implantation, and 46% had a history of atrial fibrillation.
| Variable | VKA (n=115) |
No-VKA (n=13) |
P value |
|---|---|---|---|
| Preoperative data | |||
| Age, years | 60.7±8.7 | 68.3±7.0 | 0.003 |
| Female, n (%) | 18 (15.6) | 1 (7.7) | 0.690 |
| BMI, kg/m2 | 26.7±3.8 | 24.5±3.9 | 0.037 |
| Prior cardiac surgery, n (%) | 21 (18.3) | 1 (7.7) | 0.461 |
| Prior PCI | 28 (24.3) | 4 (30.8) | 0.735 |
| ICM, n (%) | 87 (75.6) | 13 (100) | 0.070 |
| DCM, n (%) | 28 (24.4) | 0 | 0.070 |
| DM | 34 (29.6) | 5 (38.5) | 0.533 |
| PAD | 26 (22.6) | 5 (38.5) | 0.302 |
| HT | 79 (68.7) | 11 (84.1) | 0.342 |
| Dialysis | 8 (6.9) | 0 | 1.000 |
| CVA, n (%) | 6 (5.2) | 0 | 1.000 |
| COPD, n (%) | 31 (26.9) | 3 (23.7) | 1.000 |
| PHT | 56 (48.7) | 6 (46.1) | 1.000 |
| DT | 87 (75.6) | 11 (84.6) | 0.731 |
| BTT | 28 (24.4) | 2 (15.4) | 0.731 |
| INTERMACS | |||
| I | 18 (15.6) | 2 (15.4) | 1.000 |
| II | 20 (17.4) | 1 (7.7) | 0.692 |
| III | 21 (18.3) | 2 (15.4) | 1.000 |
| IV | 56 (48.7) | 8 (61.5) | 0.560 |
| Laboratory data | |||
| Hb, g/dL | 12.3±2.3 | 12.1±2.1 | 0.732 |
| Platelet count, /nL | 220.3±90.3 | 229.6±84.3 | 0.783 |
| LDH, U/L | 332.3±415 | 229.1±67 | 0.323 |
| AST, U/L | 60.8±86.3 | 40.3±35.8 | 0.277 |
| ALT, U/L | 60.9±105.4 | 32.9±26.4 | 0.042 |
| Creatinine, mg/dL | 1.1±1.5 | 1.1±0.3 | 0.855 |
| Pre-op ECMO | 18 (15.6) | 2 (15.4) | 1.000 |
| Peri- and postoperative data | |||
| LVAD alone | 55 (47.8) | 3 (23.1) | 0.140 |
| LVAD+CABG | 36 (31.1) | 7 (53.8) | 0.125 |
| LVAD+TVR | 16 (13.9) | 2 (15.4) | 1.000 |
| LVAD+AVR | 8 (6.9) | 1 (7.7) | 1.000 |
| CBP time, min | 141±65 | 143±65 | 0.711 |
| PRBCs, Units | 3.7±3.4 | 3.5±2.6 | 0.995 |
| FFPs, Units | 3.2±2.8 | 3.2±2.2 | 0.630 |
| PC, Units | 1.8±1.2 | 1.8±1.7 | 0.918 |
| PRBCs 30 POD, Units | 6.7±6.1 | 6.9±6.7 | 0.978 |
| FFPs 30 POD, Units | 2.5±3.6 | 1.7±2.4 | 0.641 |
| PC 30 POD, Units | 0.7±1.2 | 0.5±0.7 | 0.991 |
| Pneumonia, n (%) | 43 (37.4) | 5 (38.5) | 1.000 |
| Dialysis | 30 (26.1) | 4 (30.8) | 0.744 |
| ICU stay, days | 12.7±14.2 | 15.1±21.9 | 0.932 |
| Hospital LOS, days | 25.8±41.1 | 37.4±32.9 | 0.052 |
aHT, arterial hypertension; ALT, alanine aminotransferase; AST, aspartate aminotransferase; AVR, aortic valve replacement; BMI, body mass index; BTT, bridge to transplantation; CABG, coronary artery bypass graft; CBP, cardiopulmonary bypass; COPD, chronic obstructive pulmonary disease; CVA, cardiovascular accident; DCM, dilative cardiomyopathy; DM, diabetes mellitus; DT, destination therapy; ECMO, extracorporeal membrane oxygenation; FFP, fresh frozen plasma; Hb, hemoglobin; IABP, intra-aortic balloon pump; ICM, ischemic cardiomyopathy; ICU, intensive care unit; LDH, lactate dehydrogenase; LOS, length of stay; LVAD, left ventricular assist device; Op, operation; PAD, peripheral arterial disease; PC, platelet concentrate; PCI, percutaneous coronary intervention; PHT, pulmonary hypertension; PRBCs, packed red blood cells; TVR, tricuspid valve replacement.
All HMII Patients The cumulative risk of PT was 2.3%, 3.1% and 4.7% at 1, 3 and 4 years, respectively (0.03 events per patient-year [eppy]); of IS it was 3.9%, 5.5%, and 6.2% at 1, 3 and 4 years, respectively (0.04 eppy); of HS was 2.3%, 3.1% and 3.1% at 1, 3and 4 years, respectively (0.02 eppy). The incidence of all gastrointestinal bleeding (GIB) was 25.8%, 35.1% and 36.7% at 1, 3 and 4 years, respectively (0.65 eppy). The distribution of bleeding events was as follows: 81.4% GIB; 10.9% epistaxis; bleeding from the urinary tract 4.6% and 3.1% HS. Eppy of all AEs in both groups are presented in Table 2.
| Adverse events |
All HMII patients (n=128) |
VKA group (n=115) |
No-VKA before cessation (n=13) |
P values VKA vs. no-VKA before cessation |
P values VKA vs. no-VKA after cessation |
No-VKA after cessation (n=13) |
No-VKA before cessation (n=13) |
P values within no-VKA before vs. after |
|---|---|---|---|---|---|---|---|---|
| IS events, n (eppy) |
8 (0.036) | 8 (0.039) | 0 | 0.467 | 0.467 | 1 (0.045) | 0 | 0.999 |
| TIA events, n (eppy) |
11 (0.050) | 7 (0.039) | 1 (0.051) | 0.739 | 0.325 | 2 (0.091) | 1 (0.051) | 0.700 |
| HS events, n (eppy) |
4 (0.018) | 2 (0.011) | 2 (0.10) | 0.006 | 0.814 | 0 | 2 (0.10) | 0.2184 |
| GIB events, n (eppy) |
143 (0.65) | 73 (0.40) | 69 (3.58) | <0.001 | 0.011 | 2 (0.091) | 69 (3.58) | <0.001 |
| PT events, n (eppy) |
6 (0.027) | 6 (0.022) | 0 | 0.540 | 0.497 | 0 | 0 | – |
| Mean INR at event |
1.98±0.65 | 2.1±0.7 | 2.2±0.6 | 0.964 | 0.001 | 1.1±0.2 | 2.2±0.6 | 0.001 |
eppy, events per patient-year; GIB, gastrointestinal bleeding; HS, hemorrhagic stroke; INR, international normalized ratio; IS, ischemic stroke; PT, pump thrombosis; TIA, transitory ischemic attack.
Source of GIB All patients suffering GIB underwent either upper and lower endoscopy or capsule endoscopy or CT angiography. According to the results of the tests, the source of GIB was no active bleeding site detected in 31%; definitive arteriovenous malformation (AVM) in 37%; suspected AVM in 14%; ulcer in 10%; gastric angioectasia in 4%; and 4% diverticulosis.
Cessation of AT and OutcomesThe median duration of HMII support before AT cessation was 394 [IQR: 127, 861] days. After AT was ceased, during the follow-up period none of the patients in the no-VKA group had PT or HS; 2 patients had a TIA at 4 and 13 months, respectively, after AT cessation and 1 patient had IS at 9 months post-AT cessation; 1 patient had 2 GIB events during the first year after AT cessation. On comparing the rate of GIB before and after AT cessation (Table 2), the bleeding rate was significantly lower after AT cessation than before cessation (0.09 eppy vs. 3.58 eppy, P<0.001). The mean INR after AT cessation during follow-up was 1.0±0.2. There was no significant difference in the rates of IS, PT and TIA within the no-VKA group before and after cessation (Table 2).
Rates of HS and GIB in the no-VKA group (0.10 and 3.58, respectively) before AT cessation were significantly higher than in the VKA group (P=0.006 and P<0.001, respectively) (Table 2).
The median time until the first bleeding event after discharge that led to rehospitalization was 8.5 [IQR: 5, 19] months in the VKA group and 4 [IQR: 2.5, 9.5] months in the no-VKA group. Figure 1 shows the overall freedom from GIB, HS, IS, PT and TIA in the no-VKA group after AT cessation and Figure 2 demonstrates the overall survival free of AEs in the VKA group after HMII implantation. At 4 years after AT cessation, freedom from GIB was 92.3% and 51.2%, freedom from HS was 100% and 95.4, freedom from PT was 100% and 83.4%, freedom from IS was 87.5% and 85.6%, and freedom from TIA was 79.5% and 82.3% in the no-VKA (after AT cessation) and VKA groups, respectively (Figures 1,2).

Cumulative freedom from adverse events in the no-VKA group after anticoagulation cessation. (A) Ischemic stroke, (B) transitory ischemic attack, (C) pump thrombosis, (D) gastrointestinal bleeding, and (E) hemorrhagic stroke. VKA, vitamin K antagonist.

Cumulative freedom from adverse events in the VKA group after HMII implantation. (A) Ischemic stroke, (B) transitory ischemic attack, (C) pump thrombosis, (D) gastrointestinal bleeding, and (E) hemorrhagic stroke. HMII, HeartMate II; VKA, vitamin K antagonist.
Comparing the TAT values from the no-VKA group after cessation with those from 50 patients in the VKA group, we found that patients in the no-VKA group had significantly higher values (16.9±10.6 vs. 8.4±3.9, P=0.0005). TAT values were above the laboratory reference value (<4.0 µg/L) in both groups (Figure 3). TAT values were available for 6 patients in the no-VKA group during the pre-cessation period and on comparing the pre-AT cessation TAT values with values from 6 matched patients in the VKA group, we found that patients in the no-VKA group already had significantly higher values before AT cessation than patients in the VKA group (21.1±6.2 vs. 11.1±7.1, P=0.031).

Ratio of von Willebrand factor (vWF) collagen-binding activity (CBA) to vWF antigen (Ag) and thrombin-antithrombin (TAT) III complex. The dotted line indicates the normal cutoff values. (A) Comparison between the vitamin K antagonist (VKA) and no-VKA groups before anticoagulation cessation. (B) Comparison between VKA and no-VKA groups after anticoagulation cessation. (C) Comparison within the no-VKA group before and after anticoagulation cessation. (D) Comparison of TAT III complex between the no-VKA group (after cessation) and 50 patients from the VKA group. (E) Comparison of TAT III complex between 6 patients matched from the no-VKA group (before cessation) and VKA group.
The fHb, D-dimer, fibrinogen, and lactate dehydrogenase (LDH) levels did not differ significantly within the no-VKA group on comparing 12 values (measured within 9 months) before AT cessation to 12 values (within 6–9 months) after AT cessation (Figure 4I–L). The mean fibrinogen levels during the 3–9 months after AT cessation did not differ significantly between the no-VKA and VKA groups (3.3±0.2 vs. 3.4±0.3 g/L, P=0.357, respectively). Additionally, there were no significant differences between the VKA and no-VKA groups in the 12-month values of LDH, D-dimer, and fHb before and after AT cessation (Figure 4).
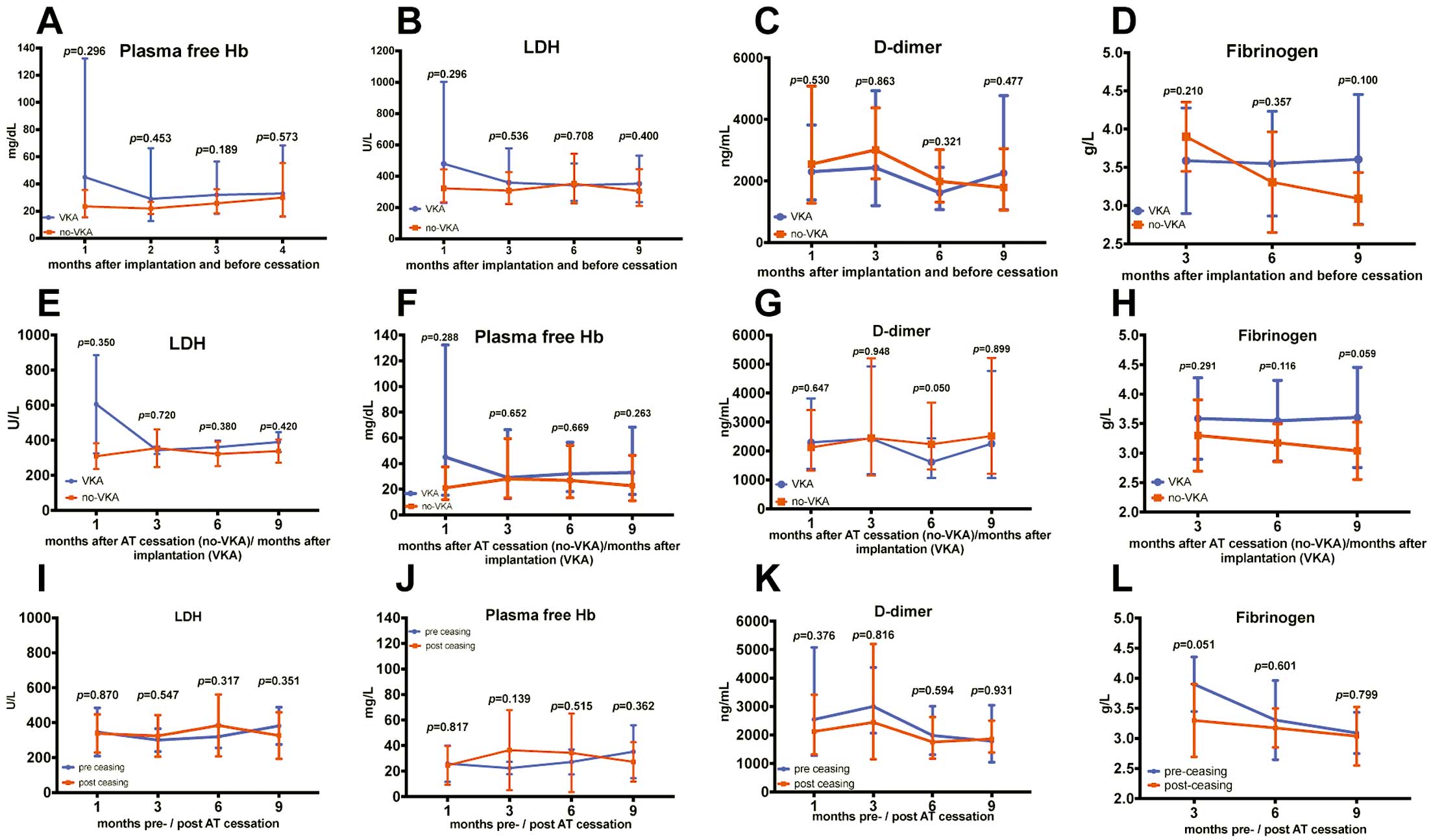
Comparison of lactate dehydrogenase (LDH), free plasma hemoglobin (fHb), fibrinogen, and D-dimer levels between groups and within the no-VKA group. (A–C) Comparing LDH, fHb, and D-dimer levels at 4 different time points (1, 3, 6 and 9 months after implantation and before anticoagulation therapy (AT) cessation) between the no-VKA and VKA groups. (D) Comparing fibrinogen at 3 different time points (3, 6 and 9 months after implantation and before AT cessation) between the no-VKA and VKA groups. (E–H) Comparison between the no-VKA (Time axis after AT cessation) and VKA group (Time axis after implantation). (I–L) Comparison within the no-VKA group before and after AT cessation.
Loss of high-molecular-weight multimers (HMWM) of vWF was detected 3 months after implantation with western blot analysis in all HMII patients, indicating the occurrence of acquired vWF syndrome (AvWS) after HMII implantation (Figures S1,S2 demonstrate the loss of HMWM in western blotting). Patients in the no-VKA group had significantly lower vWF:CBA to vWF:Ag ratio (vWF ratio) before AT cessation when compared with the rest of the HMII patients (cutoff 0.7) (0.60±0.12 vs. 0.73±0.14, P=0.006). The vWF:ratio was also significantly lower in the no-VKA group after AT cessation than in the VKA group (0.57±0.11 vs. 0.73±0.14, P=0.006, respectively). No significant change was detected on comparing the vWF ratio before and after AT cessation within the no-VKA group (Figure 3).
Platelet FunctionLTA with adenosine diphosphate (ADP) 10 µmol/L, adrenalin 50 µmol/L, and arachidonic acid as agonists and PFA-100 tests demonstrated impaired platelet function in all HMII patients (Figure 5). The measured values did not show any significant changes within the no-VKA group on comparing values from at least one test before and one after AT cessation (Figure 5). On comparing the LTA and PFA-100 tests between the no-VKA group before cessation and the VKA group, only activation involving collagen/ADP differed significantly between the groups (VKA vs. no-VKA: 268±47.5 vs. 220.5±59.8 s, P=0.040) (Figure 5). This can be explained by the lower vWF:CBA ratio in the no-VKA group.

Comparison of light transmission platelet aggregometry and PFA-100 tests between groups and within the no-VKA group. The dotted line marks the cutoff value. (A–E) Comparison between no-VKA and VKA groups before anticoagulation cessation. (F–J) Comparison within the no-VKA group before and after anticoagulation cessation. ADP, adenosine diphosphate; Epi, epinephrine; VKA, vitamin K antagonist.
Of the patients in the no-VKA group, 5 who were on HMII support without AT until April 2017 underwent further analysis with TEM. We could not detect any specific characteristics or significant changes on comparing the findings to the cutoff values of a healthy population. A detailed overview of the measured parameters and results is presented in Supplementary File 1.
Echocardiographic FindingsTransthoracic echocardiography (TTE) was performed in all LVAD patients at hospital discharge and at each outpatient visit. The following parameters were routinely measured: left ventricular (LV) systolic diameter, LV diastolic diameter, LV sphericity index (SI) (SI was calculated as the ratio of the major (in 4-chamber view) to the minor axis (in parasternal view) of LV dimensions), and tricuspid annular plane systolic excursion (TAPSE) and aortic valve (AV) opening status was also evaluated. Table S1 shows the last available TTE parameters measured in the no-VKA group before AT cessation and the TTE parameters 3 months after AT cessation. No significant changes could be detected in LV diameter (LV inner diameter in diastole pre- vs. post-AT cessation: 5.6±1.1 vs. 5.5±0.9, P=0.272), or SI (SI pre vs. SI post-AT cessation: 0.65±0.10 vs. 0.64±0.10, P=0.468), which could raise the suspicion of inflow-cannula (IC) obstruction. In 9 patients, the AV was opening prior to AT cessation and no changes in AV opening status were detected after AT cessation.
Device ParametersFigure 6 demonstrates the trends in the HMII parameters for the no-VKA group at 4 months pre-AT cessation and 6 months post-AT cessation (each control represents the mean value of 28–30 measurements during 1 month on HMII support) and Table S2 includes detailed data of the mean values of the measured device parameters.

Trends of HMII parameters within the no-VKA group pre- and post-AT cessation. (A) Revolutions per minute, (B) flow, (C) pulse index, (D) power. Control=mean value of 28 or 30 measurements within 1 month of HeartMate II support. Controls on the left side of the dotted vertical line were done before anticoagulation therapy (AT) cessation and those on the right side were done after AT cessation. DF, degrees of freedom; F ratio, computed by dividing the MS value by another MS value; the MS value for the denominator depends on the experimental design; MS, mean square; Pat., patient; SS, sum-of-squares.
Only the PI changed significantly over time, as presented in the ANOVA results in Figure 6, not only on comparing pre- and post-AT cessation values, but even on comparing values within the same period. Taking into account that the PI is very dependent on volume status, these findings are not surprising. No significant changes could be detected in any of the other measured parameters, especially no increase in the power values or decrease in the flow values, as indirect signs of PT or IC obstruction.
Survival and MortalityThe mean survival duration of all HMII patients was 20.8±21.1 months. Over the study period, 12 (9.3%) patients underwent heart transplantation, and 64 (50%) patients died. Of the 128 patients, 12 (9.3%) died before discharge after LVAD implantation.
In the no-VKA group, 8 patients had a mean survival of 14.1±12.6 months on HMII support without AT. Overall survival without AT in the no-VKA group was 19.8±15 months (5 patients still on HMII support without AT). The causes of death in the 8 patients were not related to thromboembolic events: 3 patients died of multi-organ failure as a result of pneumonic septic shock; 2 patients had septic shock caused by recurrent driveline infection; 3 patients had right heart failure with decompensation.
Despite improved survival with CF-LVADs, recurrent bleeding events, especially GIB, may justify AT cessation. Some patients can tolerate AT cessation without increased risk of thromboembolic events, as presented in our study of a reasonable number of patients from a single center who had AT ceased after HMII implantation following recurrent GIB. Our data indicated that patients with a HMII had increased thrombin generation as indicated by high TAT values, which has been confirmed by Spanier et al.8 HMII patients in our study who did not receive VKA because of recurrent bleeding events but survived without increased thromboembolic rate seemed to have a balance between bleeding risk, because of a low vWF:CBA/vWF:Ag ratio, and the thromboembolic risk, represented by high TAT values indicating activated procoagulant pathways. These findings are similar to those of Spanier et al,8,9 who described a phenomenon of “compensated coagulopathy” underlying the apparent auto-anticoagulation in recipients of textured-surface LVADs. They also found low thromboembolic rates in LVAD patients with simultaneous increased thrombin generation and fibrinolysis. All of the present HMII patients in whom TAT was measured had higher values than the laboratory cutoff value (<4 µg/L), but the patients in the no-VKA group had significantly higher TAT values than the rest of this HMII population. Our comparison might not be appropriate because the 115 HMII patients were receiving a VKA, which in turn inhibits thrombin among other coagulation factors. This will lead to lower TAT values in the HMII patients who received VKA. TAT values were available in 6 patients in the no-VKA group before their VKA was ceased. However, TAT values in the no-VKA group before and after AT cessation were significantly higher than the values in the other HMII patients. These findings demonstrate that the balance between the procoagulant pathway and the bleeding risk from AvWS could explain, at least in part, the low thromboembolic rate in our no-VKA group.
Many factors contribute to the high incidence of bleeding after CF-LVAD implantation. AvWS is among the most discussed factor, and it results from mechanical destruction and proteolysis of the HMWM of vWF, induced by shear stress.10–12 In accordance with Heilmann et al,10 our entire HMII population showed loss of the HMWM of vWF, indicating AvWS; however, not all patients showed bleeding. The 13 patients with the most number of recurrent bleeding events, which necessitated AT cessation, had significantly lower vWF:CBA/vWF:Ag ratio than the rest of our HMII patients. These findings are consistent with those of Klovaite et al,13 who found that all HMII patients with a low vWF:CBA/vWF:Ag ratio (<0.7) showed bleeding and that patients with a very low vWF ratio value (<0.6) showed recurrent major bleeding events. In addition to AvWS, platelet dysfunction was present in all our CF-LVAD patients because of the high shear forces induced by the pump; this might contribute to the high bleeding incidence after CF-LVAD implantation.14 In concordance with results from Steinlechner et al14 and Baghai et al,15 our LTA and PFA-100 tests demonstrated impaired platelet function in all HMII patients; however, we identified no significant changes during the time course of AT cessation or on comparing the values before and after AT cessation.
We could not characterize the hemostatic profile of HMII patients in whom AT can be safely discontinued. These findings are consistent with those of Pereira et al,6 who performed diverse hemostatic tests on HMII patients who survived without VKA and thromboembolic events. However, we demonstrated that HMII patients who had a low vWF:CBA/vWF:Ag ratio and experienced recurrent bleeding events benefited from ASA and/or only VKA cessation without an increased risk of thromboembolic events and had a very low bleeding rate. These findings are consistent with those of Kamdar et al,5 who discontinued VKA in 14 of 213 HMII patients and found no additional adverse thromboembolic, hemolytic, or neurologic events after warfarin cessation in these patients.
Despite controversies regarding anticoagulation regimens in HMII patients,16,17 our departmental protocol for HMII patients has not changed from 2010 until now, with an INR target range of 1.8–2.2 and daily ASA 100 mg. Despite the low INR target, we demonstrated no increase in the rate of thromboembolic events. The incidence of both PT and IS was lower than the incidences reported by Starling et al,18 Stulak et al19 and McIlvennan et al,3 and comparable to the incidence reported in the PREVENtion study.4
Data on the safety, risks, and hemostatic profile of HMII patients in whom AT can be safely discontinued are scarce. The TRACE study20,21 evaluated the benefits and risks of reduced APT and/or AT in HMII patients, but did not include any hemostatic analysis. Netuka et al21 found in the European arm of the TRACE study that managing HMII patient with a reduced INR target of 2.3 without antiplatelet therapy was safe and reduced the risk of bleeding without increasing the thromboembolic risk. At the other hand the US arm of the TRACE study20 included HMII patients in whom VKA (28%) or VKA and ASA (34%) were completely discontinued and they found that despite AT cessation the bleeding risk might persist and there was a higher risk of DT. The lower thromboembolic rate in our HMII population could not be explained by our anticoagulation regimen or by the hemostatic analysis. Important factors besides hemostatic changes in LVAD patients that can contribute to PT are the surgical technique and the angle of the IC, as described by Taghavi et al.22 Data on the IC angle and outflow cannula were not available in our study.
There is still a lack of experience on balancing the risks and benefits associated with reduced AT in patients with CF-LVADs. Hence, the hemostatic profile and characteristics of patients in whom AT can be safely reduced or even discontinued are still unknown.
Study LimitationsOur study was limited by the usual shortcomings of a small cohort, single-center study. Owing to the absence of randomization and the partly retrospective nature of our study, our data were subjected to potential bias with regard to patient selection and data acquisition; therefore, caution should be taken when interpreting our results. Bleeding complications in LVAD patients are multifactorial, and as a result we cannot exclude the possibility that unmeasured confounders influenced our results. An important limitation to the study was the self-defined criteria for cessation of AT, which was determined by a consensus group of experts from cardiac surgery, cardiology and gastroenterology in our institution. However, currently no internationally accepted consensus or guidelines exist, and, given the rare number of cases of patients with LVAD implantation and cessation of AT, may justify such a retrospective analysis, which has relevance for many clinicians with comparable problems and limited experience in this field.
Another limitation was the relatively small number and the heterogeneity of the patients, which may limit generalization of the results.
We experienced good results with AT cessation in specific HMII patients. The simultaneous onset of AvWS and high TAT values could explain at least in part the low thromboembolic rate in these HMII patients without VKA. Despite a reduced INR target (1.8–2.2), no increase in the thromboembolic rate was noted in our HMII patients. Further studies in a larger number of patients are needed to identify the hemostatic profile of patients who would benefit from anticoagulation cessation and those who would not.
This study was funded by departmental sources.
None declared.
Supplementary File 1
Supplementary Methods
Figure S1. Thrombin generation.
Figure S2. Thromboelastometry.
Figure S3. Exemplary results from western blot analysis of HMII patients, demonstrating the loss of high-molecular-weight multimers of von Willebrand factor.
Table S1. Comparison of last available echocardiographic parameters in the no-VKA group pre-AT cessation with the 3 months post-AT cessation parameters
Table S2. Comparison of device parameters 4 months pre- and 4 months post-AT cessation
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-17-0897