2019 Volume 83 Issue 1 Pages 156-163
2019 Volume 83 Issue 1 Pages 156-163
Background: The independent role of uric acid (UA) as a risk factor for atrial fibrillation (AF) has not been fully elucidated.
Methods and Results: We studied 111,566 subjects (53,416 men; 58,150 women) who underwent annual health check-ups. We divided them by sex into tertile of baseline UA. To investigate the predictive power of UA for new-onset AF, we performed Cox proportional hazard analysis including UA tertiles, body mass index, creatinine, smoking and drinking status, and presence of hypertension, diabetes, and dyslipidemia. During 4.1 years, 467 men (0.87%) and 180 women (0.31%) had AF (P<0.001). Cut-off points for tertiles of UA were as follows: women, ≤3.9, 4.0–4.8, and ≥4.9 mg/dL; men, ≤5.4, 5.5–6.4, and ≥6.5 mg/dL. Hazard ratio (HR) for third to first tertile was 1.74 (95% CI: 1.15–2.70; P=0.008), whereas there were no differences between tertiles in men. Rate of new-onset AF was significantly higher in the group with initially increased UA (ΔUA ≥0.3 mg/dL) than that with unchanged UA (ΔUA, −0.2 or +0.2 mg/dL) in the third tertile of baseline UA in both sexes.
Conclusions: Higher baseline UA was significantly associated with higher AF incidence in women. Initial increase in UA was significantly associated with AF incidence when baseline UA was ≥6.5 mg/dL in men, and ≥4.9 mg/dL in women.
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia and its prevalence in the Japanese general population is 1.6%.1 The incidence of AF is expected to rise further because life expectancy is increasing globally. AF is of great concern because it contributes to cardiac mortality and morbidity through clinical consequences, such as heart failure and stroke.2 A better understanding of the risk factors for the onset of AF is important for developing preventive strategies. Several clinical risk factors for the development of AF have been confirmed, including age,3 male sex,4 hypertension,5 congestive heart failure,4 chronic kidney disease,6 diabetes mellitus,7 and habitual alcohol drinking.8,9 Although many studies have reported an association between hyperuricemia and cardiovascular diseases, such as heart failure,10 coronary artery disease (CAD),11 and cardiovascular events,12 the concept of uric acid (UA) as a risk factor for AF has only recently been developed. The impact of UA on AF onset, however, remains unclear. UA as a risk factor for AF is complicated and multifactorial, because there are many confounding factors between UA and AF, such as race, sex, and any other risk factors.
Editorial p 27
Our cross-sectional study in a large number of Japanese subjects undergoing health check-ups identified an independent association between UA and the prevalence of AF.13 We could not, however, clarify the causal relationship between serum UA and AF, or determine the predictive value of UA for the incidence of AF because of its cross-sectional design. Moreover, to our knowledge, research on the Japanese population has not demonstrated a relationship between baseline UA and new-onset AF. We therefore investigated this association by analyzing the stored examination data of a large number of Japanese subjects who underwent annual medical health check-ups.
A total of 118,387 consecutive subjects who underwent annual health check-ups at least twice at JA Kagoshima Kouseiren Medical Health Care Center between 1 April 2005 and 31 March 2012 were enrolled in this study. Subjects who presented with AF at their first health check-up, were receiving medical treatment for hyperuricemia, and had no serum UA data were excluded. Subjects with heart failure, CAD, or hyperthyroidism were excluded, because these diseases influence the prevalence of AF. Subjects with cancer, which influences serum UA level, were also excluded.
The study conformed to the Declaration of Helsinki and was approved by the institutional ethics committees of the Graduate School of Medical and Dental Sciences, Kagoshima University, and JA Kagoshima Kouseiren Hospital.
Data CollectionData on the medical history of hypertension, diabetes mellitus, dyslipidemia, medication, cigarette smoking, and alcohol drinking were obtained via self-administered questionnaires. Body mass index (BMI) was calculated for each subject using body weight (kg) and height (m). Blood pressure was measured using a mercury sphygmomanometer after subjects sat quietly for 5 min. Twelve-lead surface electrocardiogram (ECG) was performed. Blood samples were obtained with the patient in the overnight fasting state. Serum UA concentration was determined using a commercial kit (Aqua-auto Kainos UA-II Test Kit, Kainos Laboratories, Tokyo, Japan) and an automated biochemical analyzer (BioMajesty, JEOL, Tokyo, Japan). Other biochemistry parameters, including triglyceride, low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C), and creatinine were measured using standard laboratory procedures.
The cardiovascular risk factors were defined as follows: hypertension, systolic blood pressure (SBP) ≥140 mmHg and/or diastolic blood pressure (DBP) ≥90 mmHg or current use of antihypertensive medications; diabetes mellitus, glycosylated hemoglobin ≥6.5% or active treatment with oral hypoglycemic agents or insulin; and dyslipidemia, serum triglycerides ≥150 mg/dL, serum LDL-C ≥140 mg/dL, or serum HDL-C <40 mmHg or use of lipid-lowering agents. Cigarette smoking and alcohol drinking status were classified as follows: smoker, smoking currently; non-smoker, never smoker or smoker in the past; chance drinker, drinking on ≤10 days per month; usual drinker, drinking on >10 days per month.
Baseline subject characteristics were obtained from data of the first health check-ups. Initial change of UA was defined as the difference between the first and second examinations.
Definition of AFAF was diagnosed using 12-lead surface ECG. Subjects diagnosed with AF after the first health check-up were also assigned to the AF group.
Statistical AnalysisBecause serum UA differs substantially between the sexes, and a significant effect modification of the association between UA and incident AF by sex was expected, analysis was performed for all subjects and followed with stratified analysis by sex. Continuous variables, including age, BMI, blood pressure, serum creatinine, and UA concentration, are expressed as mean±SD. Categorical variables, including presence of cardiovascular risk factors, and smoking or drinking habit, are expressed as number of subjects and proportion (percentage). Differences between men and women for continuous and categorical variables were analyzed using Student’s unpaired t-test and chi-squared test, respectively. We performed Cox proportional hazard analysis to clarify predictors of new-onset AF. Next, we divided all subjects into UA tertile groups, and performed univariate and multivariate Cox proportional hazard analysis including UA as an ordinal variable. The adjusted hazard ratio (HR) was adjusted for clinical covariates, including age, BMI, creatinine level, smoking and drinking status, and presence of hypertension, diabetes, and dyslipidemia. Finally, subjects were excluded who had initiation of UA-lowering medication during their first and second examinations, and the remaining subjects were divided into tertiles according to the change in UA from baseline to the second visit. Multivariate Cox proportional hazard analysis was then performed on the subjects divided by change in UA, and on subjects stratified into tertiles by baseline UA (adjusted for clinical covariates at baseline) to investigate the effects of initial change in UA on the onset of future AF.
All statistical analysis was performed using JMP Pro version 11 (SAS Institute, Cary, NC, USA) for Windows. P<0.05 was considered significant.
Of the 118,387 study participants, 568 were excluded because their serum UA data were missing; 4939 because of coexistent diseases meeting the exclusion criteria (2,957 were receiving medical treatment for hyperuricemia, and 299 had heart failure; 867, CAD; 225, hyperthyroidism; and 591, malignant cancer), and 1,314 because they presented with AF at baseline. Finally, we analyzed a total of 111,566 subjects.
Baseline subject clinical characteristics are listed in Table 1. There were 53,416 men (47.9%; mean age, 52.6±15.7 years) and 58,150 women (52.1%, mean age, 54.8±15.3 years). Average baseline serum UA was significantly higher in men than in women (5.9±1.3 and 4.4±1.0 mg/dL, respectively; P<0.001).
| All (n=111,566) |
Men (n=53,416) |
Women (n=58,150) |
P-value (men vs. women) |
|
|---|---|---|---|---|
| Age (years) | 53.8±15.6 | 52.6±15.7 | 54.8±15.3 | <0.001 |
| BMI (kg/m2) | 23.1±3.4 | 23.5±3.2 | 22.7±3.4 | <0.001 |
| Smoker | 19,470 (17.5) | 15,309 (29.2) | 4,161 (7.3) | <0.001 |
| Usual alcohol drinker | 26,634 (23.9) | 21,730 (41.4) | 4,904 (8.6) | <0.001 |
| Hypertension | 35,805 (32.1) | 18,283 (34.2) | 17,522 (30.1) | <0.001 |
| SBP (mmHg) | 125.3±19.7 | 127.7±18.8 | 124.6±20.4 | <0.001 |
| DBP (mmHg) | 76.0±11.4 | 78.4±11.3 | 74.7±11.0 | <0.001 |
| Diabetes | 3,973 (3.6) | 2,488 (4.7) | 1,485 (2.6) | <0.001 |
| HbA1c (%) | 5.4±0.7 | 5.4±0.8 | 5.3±0.6 | <0.001 |
| Dyslipidemia | 52,457 (47.0) | 26,368 (49.3) | 26,089 (44.9) | <0.001 |
| Total cholesterol (mg/dL) | 207.9±36.7 | 203.6±35.3 | 211.7±35.5 | <0.001 |
| Triglyceride (mg/dL) | 85 [60, 125] | 100 [70, 150] | 79 [58, 111] | <0.001 |
| LDL-C (mg/dL) | 124.8±34.9 | 120.7±33.5 | 129.0±32.3 | <0.001 |
| HDL-C (mg/dL) | 58.6±18.5 | 56.9±14.5 | 64.2±14.5 | <0.001 |
| Creatinine (mg/dL) | 0.72±0.21 | 0.82±0.22 | 0.61±0.13 | <0.001 |
| Uric acid (mg/dL) | 5.1±1.4 | 5.9±1.3 | 4.4±1.0 | <0.001 |
Data given as mean±SD or n (%). BMI, body mass index; DBP, diastolic blood pressure; HbA1c, glycosylated hemoglobin; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; SBP, systolic blood pressure.
During the median observation period of 4.1 years (433,522 person-years), 647 subjects had AF: 467 men (0.87%) and 180 women (0.31%; P<0.001). The overall rate of AF was 149 cases per 100,000 person-years. Table 2 lists the results of univariate and multivariate Cox proportional hazard analysis to investigate the predictors of new-onset AF. On multivariate analysis for all subjects, UA level was a significant predictor of incident AF (HR, 1.20; P<0.001). We performed stratified analysis by sex, because a significant effect modification of the association between UA level and incident AF by sex was found (P for interaction, <0.001). On multivariate analysis, age (HR, 1.07; P<0.001), BMI (HR, 1.08; P<0.001), and presence of hypertension (HR, 1.33; P=0.005) were significant positive predictors, and presence of dyslipidemia (HR, 0.73; P=0.002) was a significant negative predictor, while serum UA was not a significant predictor of new-onset AF in men. In contrast, in women, age (HR, 1.11; P<0.001), usual alcohol drinking (HR, 2.21; P=0.005), presence of hypertension (HR, 1.69; P=0.002), and serum UA level (HR, 1.17; P=0.041) were significant positive predictors, and presence of dyslipidemia (HR, 0.59; P<0.001) was a significant negative predictor of new-onset AF.
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| All | ||||
| Age (years) | 1.08 (1.07–1.09) | <0.001 | 1.08 (1.07–1.09) | <0.001 |
| BMI (kg/m2) | 1.07 (1.04–1.09) | <0.001 | 1.06 (1.03–1.09) | <0.001 |
| Smoker | 0.83 (0.66–1.03) | 0.089 | 1.57 (1.23–1.98) | <0.001 |
| Usual alcohol drinker | 1.83 (1.56–2.15) | <0.001 | 1.43 (1.20–1.71) | <0.001 |
| Hypertension | 3.09 (2.64–3.61) | <0.001 | 1.18 (1.09–1.67) | <0.001 |
| Diabetes | 1.92 (1.37–2.62) | <0.001 | 1.27 (0.90–1.75) | 0.167 |
| Dyslipidemia | 0.78 (0.66–0.91) | 0.002 | 0.66 (0.56–0.78) | <0.001 |
| Creatinine (mg/dL) | 1.35 (1.21–1.45) | <0.001 | 1.32 (1.05–1.50) | 0.022 |
| Uric acid (mg/dL) | 1.27 (1.21–1.34) | <0.001 | 1.20 (1.13–1.28) | <0.001 |
| Men | ||||
| Age (years) | 1.07 (1.06–1.08) | <0.001 | 1.07 (1.06–1.08) | <0.001 |
| BMI (kg/m2) | 1.05 (1.02–1.08) | <0.001 | 1.08 (1.05–1.12) | <0.001 |
| Smoker | 0.56 (0.44–0.70) | <0.001 | 1.28 (0.99–1.63) | 0.061 |
| Usual alcohol drinker | 1.22 (1.01–1.46) | 0.034 | 1.04 (0.86–1.25) | 0.710 |
| Hypertension | 2.71 (2.25–3.25) | <0.001 | 1.33 (1.09–1.62) | 0.005 |
| Diabetes | 1.63 (1.11–2.31) | 0.014 | 1.14 (0.78–1.63) | 0.482 |
| Dyslipidemia | 0.71 (0.59–0.85) | <0.001 | 0.73 (0.60–0.88) | 0.002 |
| Creatinine (mg/dL) | 1.17 (0.90–1.34) | 0.198 | 1.07 (0.64–1.38) | 0.757 |
| Uric acid (mg/dL) | 1.04 (0.96–1.11) | 0.344 | 1.08 (0.97–1.18) | 0.101 |
| Women | ||||
| Age (years) | 1.11 (1.10–1.13) | <0.001 | 1.11 (1.09–1.13) | <0.001 |
| BMI (kg/m2) | 1.06 (1.02–1.10) | 0.004 | 1.02 (0.97–1.06) | 0.531 |
| Smoker | 0.75 (0.32–1.49) | 0.443 | 1.50 (0.57–3.23) | 0.377 |
| Usual alcohol drinker | 1.63 (0.98–2.56) | 0.059 | 2.21 (1.28–3.59) | 0.005 |
| Hypertension | 3.84 (2.85–5.21) | <0.001 | 1.69 (1.22–2.36) | 0.002 |
| Diabetes | 1.96 (0.89–3.73) | 0.091 | 1.02 (0.43–2.02) | 0.962 |
| Dyslipidemia | 0.85 (0.63–1.14) | 0.277 | 0.59 (0.43–0.80) | <0.001 |
| Creatinine (mg/dL) | 1.46 (0.87–1.79) | 0.115 | 0.86 (0.24–1.69) | 0.793 |
| Uric acid (mg/dL) | 1.38 (1.20–1.56) | <0.001 | 1.17 (1.01–1.37) | 0.041 |
BMI, body mass index.
The cut-off points for tertiles of UA were as follows: men, ≤5.4, 5.5–6.4, and ≥6.5 mg/dL; women, ≤3.9, 4.0–4.8, and ≥4.9 mg/dL. In men, the rate of new-onset AF was lowest in the second tertile (0.83%), and there were no significant differences between the 3 tertiles. In women, the rate of new-onset AF was lowest in the first tertile (0.20%) and increased significantly as UA tertile increased (second tertile, 0.31%; third tertile, 0.49%).
The Cox proportional hazard analysis investigating the predictors of new-onset AF is given in Figure 1. In men, the HR of the first and third tertiles, with the second tertile defined as a reference, were not statistically significance on non-adjusted and adjusted analyses. In women, on non-adjusted analysis the HR of the second and third tertiles, with the first tertile defined as a reference, were significantly high (second tertile, HR, 1.51; 95% CI: 1.01–2.30; P=0.049; third tertile, HR, 2.51; 95% CI: 1.71–3.77; P<0.001). On adjusted analysis the HR of the third tertile using the first tertile as a reference was significantly high (HR, 1.74; 95% CI: 1.15–2.70; P=0.008).
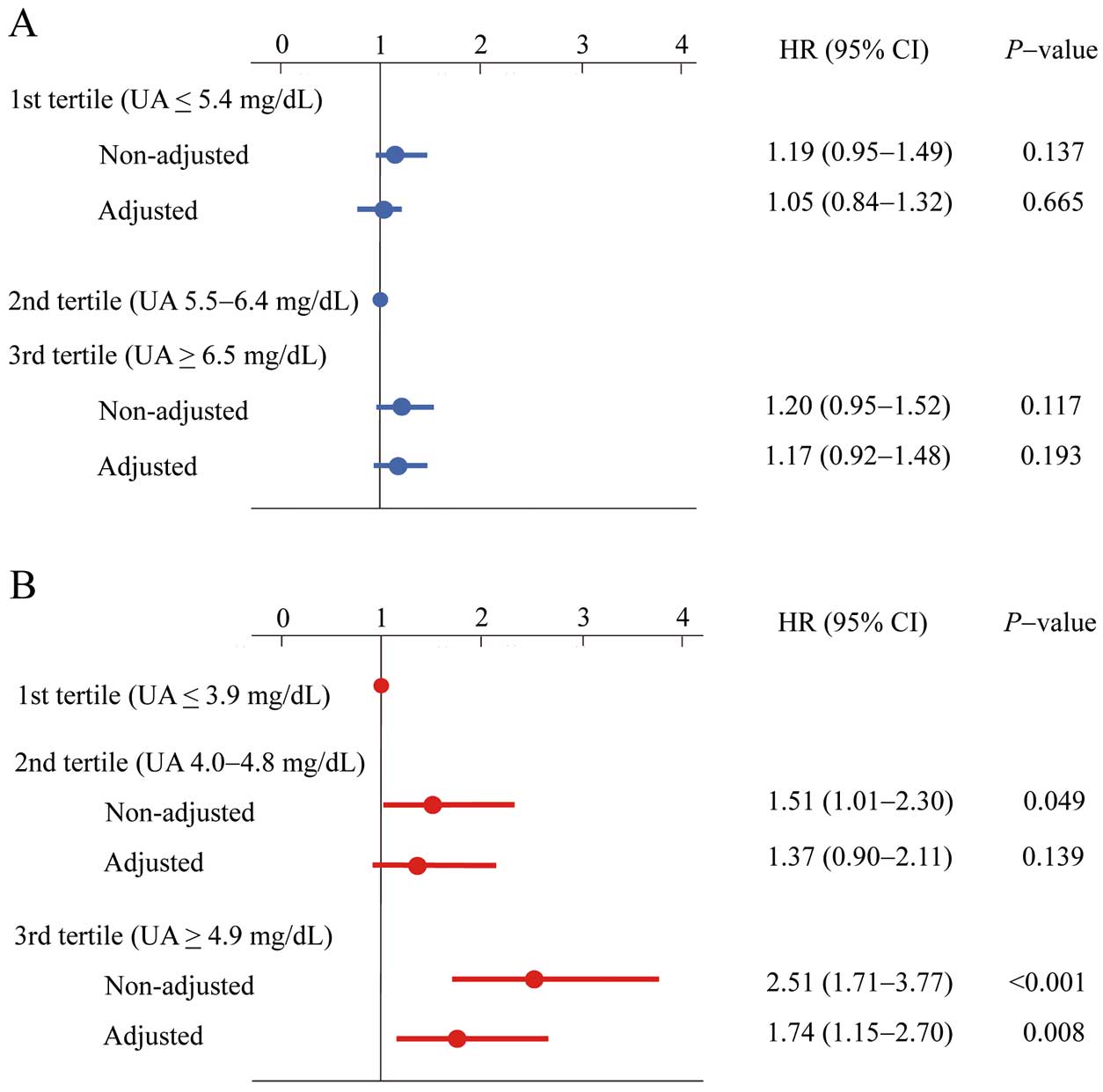
Hazard ratio (HR) for new-onset atrial fibrillation according to serum uric acid (UA) tertile in (A) men and (B) women. The adjusted HRs were adjusted for clinical covariates, including age, body mass index, creatinine level, smoking and drinking status, and presence of hypertension, diabetes mellitus, and dyslipidemia.
We excluded 238 subjects who had initiation of UA-lowering drugs during their first and second examinations. The remaining 111,328 subjects were analyzed for the correlation between initial change in UA and future onset of AF. The median period from baseline to second visit was 1.0 year (IQR, 1.0–2.0 years). Mean UA change was +0.1±0.7 mg/dL, and the tertile points were −0.2 mg/mL and 0.3 mg/mL. Therefore, the tertiles were defined as follows: decreased, UA decreased ≥0.3 mg/dL; unchanged, UA decreased ≤0.2 mg/dL or increased ≤0.2 mg/dL; and increased, UA increased ≥0.3 mg/dL. Figure 2 shows the results of multivariate Cox proportional analysis regarding the differences in incidence of AF based on initial change in UA. In all subjects, the rate of new-onset AF in the group with increased UA was not significantly higher than that in the group with unchanged UA in both men and women. Also, the incidence of AF in the group with decreased UA was not significantly different compared with the group with unchanged UA in both men and women (men, Figure 2A; women, Figure 2B).
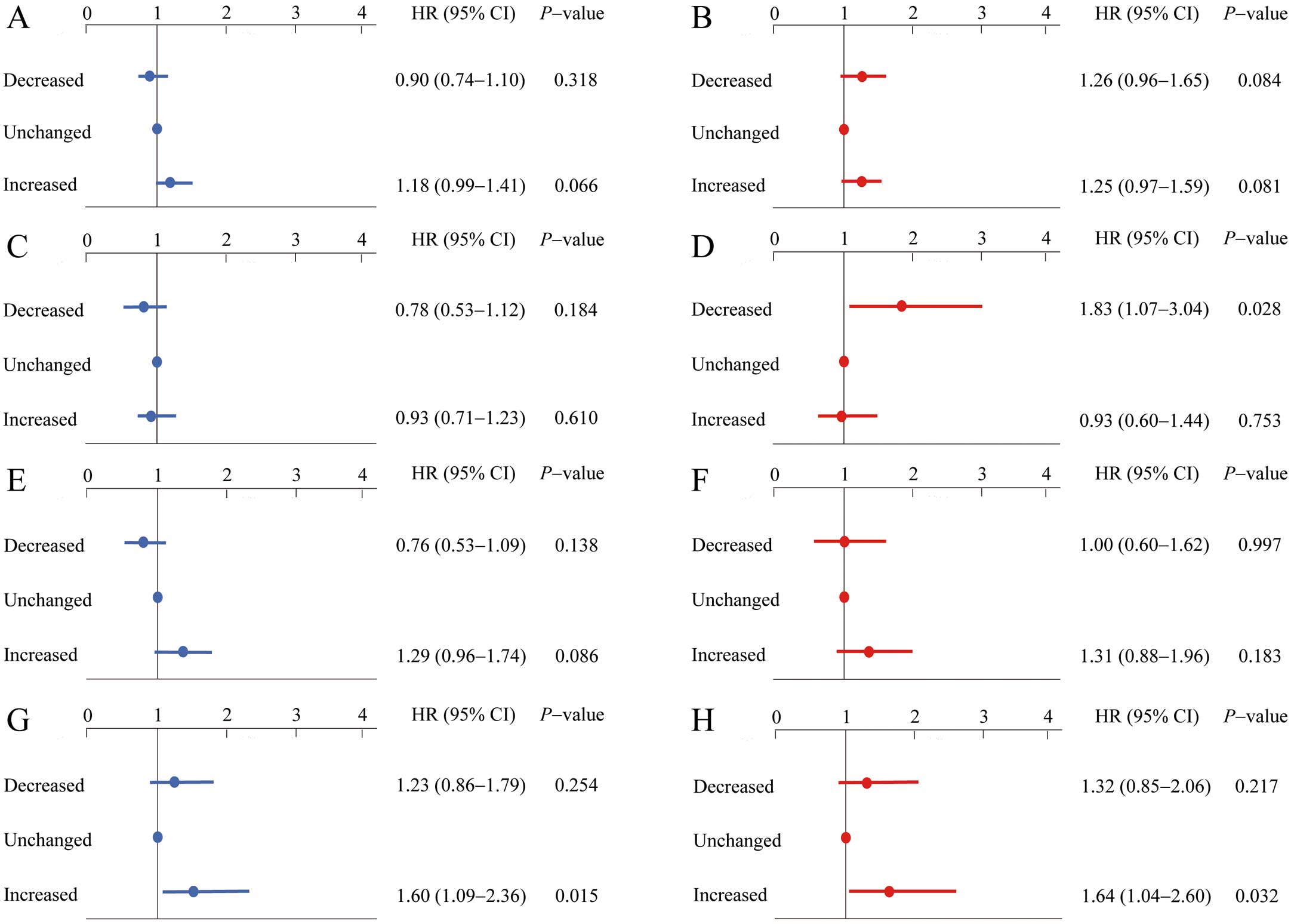
Multivariate hazard ratio (HR) for new-onset atrial fibrillation according to change in uric acid (UA) from baseline to second examination (decreased, UA decreased ≥0.3 mg/dL; unchanged, UA decreased ≤0.2 mg/dL or increased ≤0.2 mg/dL; and increased, UA increased ≥0.3 mg/dL) in (A) men and (B) women, and for (C) first tertile of baseline UA in men (UA ≤5.4 mg/dL); (D) first tertile in women (UA ≤3.9 mg/dL); (E) second tertile in men (UA 5.5–6.4 mg/dL); (F) second tertile in women (UA 4.0–4.8 mg/dL); (G) third tertile in men (UA ≥6.5 mg/dL); and (H) third tertile in women (UA ≥4.9 mg/dL). The HR was adjusted for clinical covariates at baseline, including age, body mass index, creatinine, smoking and drinking status, and presence of hypertension, diabetes, and dyslipidemia.
Furthermore, the association between initial change in UA and new-onset AF in each tertile was analyzed according to baseline UA. In men, the rate of new-onset AF was significantly higher in the group with increased UA than in the group with unchanged UA in the third tertile (HR, 1.60; 95% CI: 1.09–2.36, P=0.015; Figure 2G); and in women, the rate of new-onset AF was significantly higher in the third tertile (HR, 1.64; 95% CI: 1.04–2.60, P= 0.032, Figure 2H) in women.
We examined the association between baseline UA and new-onset AF in 111,566 Japanese general subjects who underwent health check-ups at least twice. Furthermore, we investigated the relationship between the incidence of AF and change in UA from baseline. The main findings were as follows: (1) higher baseline serum UA was significantly associated with higher rate of new-onset AF in women, but not in men; and (2) the initial increase in UA was significantly associated with incidence of AF when baseline UA was ≥6.5 mg/dL in men, and ≥4.9 mg/dL in women.
Hyperuricemia and Incidence of AFThe first study of the independent association between UA level and AF was reported in 2010,14 and this relationship has been confirmed on cross-sectional reports,15,16 prospective cohort studies,17 and 2 recent meta-analyses.18 These studies also have suggested that the relationship between UA and AF differed according to race, sex, and the presence or absence of other clinical coexisting diseases. Although several cross-sectional studies have shown the association between UA and AF in a Japanese population, to our knowledge this is the first time that UA level has been shown to be independently associated with incidence of AF in women in a longitudinal analysis of a large Japanese general population.
Despite the substantial progress in the understanding of AF pathophysiology, the precise mechanisms by which hyperuricemia leads to occurrence and continuation of AF are not completely understood. Serum UA is the end product of purine degradation catalyzed by xanthine oxidase (XO), which has been reported to be correlated with oxidative stress19 and elevation of systemic inflammatory markers. Thus, serum UA level is considered to be a marker of tissue oxidative stress and inflammation.20 In contrast, electrical and structural remodeling of the left atrium is an important process in the pathogenesis of AF.21–23 Onset and persistence of AF has been associated with oxidative stress24 and inflammation.25 In addition, intracellular accumulation of UA by activation of UA transporters, which are expressed not only in renal tubular cells but also in vascular smooth muscle and endothelial cells,26,27 has been reported to contribute to enhanced Kv1.5 protein expression and shorten the atrial action potential duration, resulting in the initiation or sustainment of AF.28
UA and Incidence of AF: Sex DifferencesSeveral studies have demonstrated that hyperuricemia is associated more strongly with cardiovascular events and mortality in women compared with men. In the LIFE study, the association between serum UA and cardiovascular events in hypertensive patients was stronger in women than in men with or without adjustment of the Framingham risk score.29 In the PIUMA study, the rate of total cardiovascular and fatal cardiovascular events, and all-cause death significantly increased at UA >4.6 mg/dL in women, compared with >6.2 mg/dL in men, in untreated subjects with essential hypertension.30 Furthermore, increased UA is also associated with a greater risk of AF in women.29 Although serum UA level was significantly associated with AF prevalence in male and female patients visiting a cardiovascular hospital in a non-adjusted analysis, the effect of serum UA level on AF after adjustment using the well-known cardiovascular risk factors remained independent in women, but not in men.15 In the present study we also showed that high UA is associated with the incidence of AF only in women. Although the mechanism underlying this sex difference remains unknown, it may be due to differences in the sex hormones. The level of UA in men is commonly higher than in women. This may be due to the role of estrogen in UA regulation. Estrogen decreases the number of active renal UA transporters, resulting in smaller UA reabsorption and reduced serum UA.31 In contrast, estrogen is known to play a cardioprotective role.32 In women, high UA may not only affect cardiovascular disease by itself,33 but may represent a lack of estrogen protection. Further studies are warranted to elucidate the underlying mechanism of sex-related differences in the association between serum UA level and incidence of AF.
The subjects with hypouricemia, with UA <2.0 mg/dL, may have different characteristics related to AF. In the present study, 423 subjects had UA <2.0 mg/dL (0.38%), and no subjects had new-onset AF during the observation period. As for the analysis for the whole population, on multivariate analysis for those without hypouricemia, serum UA level was a significant predictor in women (HR of thir-first tertile, 1.70; 95% CI: 1.11–2.63; P=0.013) but not in men.
In the present study, the incidence of new-onset AF increased significantly in the tertile of UA ≥4.9 mg/dL. In our previous cross-sectional study, the AF prevalence increased at serum UA 5.0 mg/dL in women,13 which was almost identical to the current results. Regarding the previous studies, the cut-off for UA in women indicating increased risk of cardiovascular diseases was 4.6 mg/dL for cardiovascular events in patients with untreated essential hypertension,30 5.0 mg/dL for cardiovascular death in patients with type 2 diabetes mellitus,34 and 5.1 mg/dL for AF in outpatients of a cardiovascular hospital.15 Hypouricemia was defined as serum UA >7.0 mg/dL, based on the limit of serum solubility in both sexes. Considering the results of these studies, the goal of UA level regarding prevention of cardiovascular diseases may be set lower in women.
Changes in UA and Incidence of AFIn many epidemiological and clinical studies, a relationship between UA and risk of cardiovascular disease is claimed, and the interest is shifting to whether cardiovascular disease can be reduced by lowering UA. High-dose allopurinol greatly reduced vascular tissue oxidative stress and improved different measures of vascular/endothelial dysfunction in 60 optimally treated patients with CAD.35 Allopurinol reduced brain natriuretic peptide level, which was the best available surrogate marker for prognosis in 50 patients with congestive heart failure.36 In addition, large-scale prospective intervention studies to analyze the preventive effect of febuxostat on cardiorenovascular events currently are in progress.37,38
To the best of our knowledge, however, no study has demonstrated the effects of change in UA on the rate of developing cardiovascular diseases. Therefore, we additionally examined the relationship between change in serum UA from baseline and the incidence of AF. As a result, an initial increase in UA was significantly harmful for subjects whose baseline UA was >6.5 mg/dL in men, and >4.9 mg/dL in women. These results, in which both high baseline UA and increase of UA adversely affect AF incidence, are reasonable.
In contrast, AF incidence did not decrease in subjects with decreased UA, compared with those whose UA level remained unchanged. These results are inconsistent with those of the aforementioned UA-lowering interventional study with XO inhibitors.35,36 The effect of UA reduction on cardiovascular risk may vary depending on the method, in particular, with or without XO inhibitor use. Various mechanisms elevating serum UA have been reported, including increased production of and decreased excretion of UA. The former includes excessive consumption of dietary purines39 or fructose,40 and de novo synthesis of purines related to hypertriglyceridemia,41 whereas the latter includes reduced glomerular filtration due to chronic kidney disease,42 and decreased excretion due to obesity-related hyperinsulinemia.43 Even when UA decreases to the same extent, the XO activity reduction may differ between the therapies. If XO activity also contributes to the increased risk of cardiovascular disease, in addition to UA itself, then this may explain the difference in those results. Comparison of the effects of UA lowering by lifestyle improvement and by use of XO inhibitors or uricosuric agents may provide important clues for resolving the association between hypouricemia and cardiovascular disease.
Study LimitationsThe present study has several limitations. First, the data were not collected prospectively; therefore, the results should be verified in further prospective observational studies. Second, although we studied a large number of subjects, they were limited to participants of health check-ups at a single facility in Japan. Third, we should consider the measurement error of UA. The coefficient of variance, however, which is the indicator of measurement error, was <3.0%, suggesting that the definition of UA ≥0.3 mg/dL as increased or decreased is statistically acceptable. Fourth, we might have underestimated the prevalence of AF by failing to identify paroxysmal or persistent AF without symptoms, given that AF was diagnosed on 12-lead ECG or self-reported medical history. Fifth, we did not consider the existence of a respiratory problem such as chronic obstructive pulmonary disease, which is a risk factor for AF. Finally, we did not include data on medication, such as diuretics or renin-angiotensin system inhibitors, that may affect serum UA level and AF prevalence, or on menopause, which may influence serum UA level in women. Therefore, further research that includes these data should be performed.
In a large Japanese population of subjects undergoing health check-ups, higher baseline serum UA was significantly associated with higher rate of AF onset in women. Initial increase in UA is significantly associated with incidence of future AF when baseline UA is ≥6.5 mg/dL in men, and ≥4.9 mg/dL in women.
We thank the medical staff at Kagoshima Kouseiren Medical Health Care Center for support in data collection.
The authors declare no conflicts of interest.