Abbreviations
| ACE |
angiotensin converting enzyme |
| BAL |
bronchoalveolar lavage |
| CRT |
cardiac resynchronization therapy |
| CT |
computed tomography |
| 18F-FDG |
fluorine-18 fluorodeoxyglucose |
| 67Ga |
gallium-67 |
| HRCT |
high resolution computed tomography |
| ICD |
implantable cardioverter defibrillator |
| MRI |
magnetic resonance imaging |
| PET |
positron emission tomography |
| sIL-2R |
soluble interleukin 2 recepter |
| SPECT |
single photon emission computed tomography |
I. Introduction
1. Background of the Guidelines
Sarcoidosis is a systemic granulomatous disease of unknown cause. Sarcoidosis involving the heart, i.e., cardiac sarcoidosis, affects the prognosis of patients significantly as it may lead to life-threatening arrhythmias or severe heart failure, and may cause sudden death. The prevalence of cardiac sarcoidosis is higher in Japan than Western countries. Since immunosuppressive therapy using corticosteroids is expected to delay the progression of cardiac involvement, an accurate and early diagnosis is essential.
In the clinical setting, a diagnosis of cardiac sarcoidosis is typically made when cardiac symptoms develop in patients who have been diagnosed with sarcoidosis involving organs other than the heart, or when patients with myocardial disease or arrhythmias of unknown cause are examined in detail. Making a diagnosis of cardiac sarcoidosis is not easy in many cases. In fact, it is difficult to differentiate it from dilated cardiomyopathy,1–3,224
chronic myocarditis and giant cell myocarditis.4
Some patients are not diagnosed with cardiac sarcoidosis before histological examination of myocardium obtained at autopsy, heart transplantation or left ventricular restoration reveals it.
In Japan, according to the “Guidelines for the diagnosis of cardiac sarcoidosis” published in 1992,5
a diagnosis could be made only when epithelioid granulomas are found in at least one organ. Some patients were not diagnosed even when clinical findings were strongly suggestive of cardiac sarcoidosis. Since the guidelines listed non-specific ECG findings such as ST-T changes and left ventricular hypertrophy as ECG findings suggestive of sarcoidosis, patients with hypertensive heart disease might be misdiagnosed with cardiac sarcoidosis. In 2006, the Japan Society of Sarcoidosis and Other Granulomatous Disorders and the Japanese College of Cardiology collaborated with other academic societies to revise the guidelines, and published the “Diagnostic Standard and Guideline for Sarcoidosis - 2006.”6
In the “Guidelines for the Diagnosis of Cardiac Involvement in Patients with Sarcoidosis” in the 2006 version and “Diagnostic Guidelines for Cardiac Manifestations of Cardiac Sarcoidosis” in the “Guidelines for Diagnosis and Treatment of Myocarditis (JCS 2009)” proposed by the Japanese Circulation Society7,8
(1) pathohistological criteria were updated, and (2) clinical findings that are typical or frequently observed in patients with cardiac sarcoidosis are weighed and included in the major criteria for diagnosis. These revisions have raised awareness of cardiac sarcoidosis among physicians and helped them more accurately diagnose patients who were previously overlooked. In Japan, patients with cardiac sarcoidosis have been treated according to the section “Treatment of Cardiac Sarcoidosis” in “Views on the Treatment of Sarcoidosis - 2003”9
prepared by the Japan Society of Sarcoidosis and Other Granulomatous Disorders, the Japanese College of Cardiology and other groups.
Recent advancements in imaging techniques such as 18F-FDG PET, cardiac MRI, cardiac CT and echocardiography as well as accumulated clinical experience have enabled physicians to suspect cardiac sarcoidosis earlier than before. Appropriate criteria for early diagnosis are becoming increasingly more important. Also, it has been revealed that some patients have isolated cardiac sarcoidosis with no involvement of other organs. New, non-steroidal pharmacological treatments using immunosuppressants and novel approaches have been developed. Non-pharmacological approaches such as catheter ablation for severe ventricular arrhythmias and CRT for severe heart failure have also been advanced. We therefore decided to revise the guidelines for the diagnosis and treatment of cardiac sarcoidosis.
The Ministry of Health, Labour and Welfare (MHLW) has included sarcoidosis in its list of intractable diseases for which the MHLW conducts research projects. As one of the focused intractable diseases, the government has implemented measures to (1) promote research and studies, (2) build systems for diagnosis and treatment in medical institutions, (3) encourage local healthcare/welfare systems to collaborate each other, (4) improve welfare policies for patients to improve their quality of life (QOL), and (5) reduce financial burden of patients with sarcoidosis. This indicates that the Japanese government places a special emphasis on the diagnosis and treatment of sarcoidosis (See the Japanese Intractable Diseases Information Center http://www.nanbyou.or.jp/).
The present guidelines are based on a comprehensive review of evidence on the diagnosis and treatment of sarcoidosis, especially cardiac sarcoidosis.
2. Basic Principles for the Preparation of the Guidelines
The present guidelines are structured in a way similar to other JCS guidelines. In the body text and tables, evidence is categorized according to the following classifications.
Level of evidence and grade of recommendation
(1) Level of Evidence
Level 1: Data derived from systematic reviews or meta-analyses of multiple randomized clinical studies
Level 2: Data derived from one or more randomized clinical studies
Level 3: Data derived from non-randomized studies
Level 4a: Data derived from analytical epidemiological studies (cohort studies)
Level 4b: Data derived from analytical epidemiological studies (case-control studies or cross-sectional studies)
Level 5: Data derived from descriptive studies (case reports or case series)
Level 6: Reports of expert committees and opinions of experts.
The level of evidence is classified according to the type of study design.
When more than one literature with different levels of evidence is available, the highest level of evidence is indicated.
(2) Grade of Recommendation
Grade A: Strongly recommended and supported by strong evidence
Grade B: Recommended with moderately strong supporting evidence
Grade C1: Recommended despite no strong supporting evidence
Grade C2: Not recommended because of the absence of strong supporting evidence.
Grade D: Not recommended as evidence indicates that the treatment is ineffective or even harmful.
The grade of recommendation is determined through a comprehensive assessment of (1) the level of evidence, (2) the number of evidence sources and the distribution of numbers of evidence sources by evidence level, (3) the magnitude of clinical efficacy, (4) clinical applicability (e.g., physicians’ capabilities, local characteristics, medical resources, and health insurance systems), and (5) evidence on harmful effects and costs.
Some recommendations are classified as follows in order to ensure consistency with guidelines published by other relevant academic groups.
(3) Classification of Recommendations
Class I: There is evidence and/or general agreement that a given procedure or treatment is useful and effective
Class II: There is conflicting evidence and/or a divergence of opinion about the usefulness/efficacy of a given procedure or treatment
Class IIa: Weight of evidence and data and opinion is in favor of usefulness and/or effectiveness.
Class IIb: Usefulness/efficacy is less well established by evidence/opinion.
Class III: There is evidence and/or general agreement that the procedure/treatment is not useful/effective, and in some cases may be harmful.
The present guidelines basically reflect treatments and procedures that are currently available and covered by the National Health Insurance (NHI) in Japan. Those not covered by the NHI are described as such. Diagnostics and treatments that are currently under research and are expected to become clinically available in the near future are described as topics or future prospects. This guideline document outlines of cardiac sarcoidosis and describes how to diagnose and treat cardiac sarcoidosis. In the questions and answers (Q&A) section at the end of the guideline document, the relevant chapter is referenced for each Q&A.
II. Outline of Sarcoidosis
1. Epidemiology of Sarcoidosis
Sarcoidosis is slightly more prevalent in women, and develops mainly in adults under 40 years of age with a peak at 20 s.10
However, in Japan and Scandinavian countries the incidence also peaks at 50 s, showing a biphasic pattern.11,12
According to a recent report from Japan,13
the incidence of sarcoidosis is increasing among older people. The incidence in women is changing from a biphasic to a monophasic pattern where the incidence peaks among middle-aged and older people, while the incidence in men is changing from a monophasic pattern with a peak at young adults to a biphasic pattern. A population-based study in the United States has reported that the annual prevalence of sarcoidosis is 5.9 in 100,000 men and 6.3 in 100,000 women.14
A report from the United States14
has described that the lifetime risk of sarcoidosis (accumulated incidence of sarcoidosis) is 0.85% for Caucasian Americans and 2.4% for African Americans. Another report from the United States15
has described that the prevalence of sarcoidosis is 10.9/100,000 for Caucasian Americans and 35.5/100,000 for African Americans. Both reports indicate that sarcoidosis is more common among African Americans. Sarcoidosis is more prevalent in Scandinavian countries such as Sweden and Denmark than many other countries.11
The prevalence of sarcoidosis differs between races and geographical areas, and is generally higher in Northern countries than in Southern countries. In Japan, the prevalence of sarcoidosis is estimated to range from 7.5 to 9.3 patients in 100,000 people, and the annual incidence is about 1 in 100,000 people.16
The severity and affected organs also differ between races. It has been reported that sarcoidosis is generally more severe among black patients than in white patients, and that cardiac and eye involvement of sarcoidosis is more common in Japanese17
(Evidence level 4a).
It is not fully understood how often sarcoidosis involves the heart. It has been reported that about 5% of patients with sarcoidosis have cardiac signs/symptoms, but cardiac findings are more often found in autopsy.18,19
In Western countries, no gender difference has been reported in the prevalence of cardiac involvement, and cardiac sarcoidosis is listed as a risk factor of sudden death among young individuals.20
In Japan, cardiac sarcoidosis is more common in middle-aged or older women,21
which indicates a clear racial difference. However, no peak is found in the incidence of cardiac sarcoidosis in Japanese men. Physicians should be aware that cardiac sarcoidosis is not rare among young men in Japan21
(Evidence level 4a).
2. Etiology and Pathophysiology of Sarcoidosis
2.1 Etiology and Pathophysiology
The following sections describe how sarcoidosis develops from the viewpoints of causal factors, predispositions and pathogenesis of granuloma.
2.1.1 Causal Factors
From 1970 to 1990, researchers in Japan tried to isolate causative organisms from lymph nodes obtained from patients with sarcoidosis, and reported that the concentration of
Propionibacterium acnes, a commensal bacterium in the human skin microbiome, in lymph nodes was higher in patients than controls,22
and the concentrations of DNA of
P. acnes
and
Propionibacterium granulosum
were higher in patients than controls23
(Evidence level 4b). It has also been reported that
P.acnes
can induce pulmonary granuloma in mice.24
However, it is still unclear whether these
Propionibacterium
species are the true cause of sarcoidosis because antimicrobial agents that were evaluated in the early years were likely to be ineffective,25
and epithelioid granuloma may have a specific feature that allows
P. acnes
to survive and proliferate.26
Moller et al at Johns Hopkins University have detected antibodies against
Mycobacterium tuberculosis
catalase-peroxidase (mKatG) in sera obtained from patients with sarcoidosis, and mkatG DNA in biopsy tissues in 39% of patients.27
Many of these sarcoidosis tissues contained DNA that encodes ribosomal RNA (rRNA) of
M. tuberculosis. Based on these findings, Moller et al suggested that mKatG is a target of the host immune response in some patients with sarcoidosis (Evidence level 5). They have also suggested that sarcoidosis is triggered by the host’s T helper type 1 (Th1) immune response to promote the production of serum amyloid A protein that binds to Toll-like receptor 2 (TLR-2) and thereby induce the production of tumor necrosis factor α (TNF-α), and interleukin 18 (IL-18), which induces granuloma formation.28
However, DNA of
M. tuberculosis
has been detected only at low copy numbers in some lymph node samples from patients with sarcoidosis,23
and only 3.3% of patients with sarcoidosis had increased interferon-gamma (IFN-γ) production resulting from immune response to early secretory antigen target 6 (ESAT-6) and culture filtrate protein 10 (CFP-10), which are specific to
M. tuberculosis.29
It is not convincing enough to conclude that mKatG or mycobacteria induces sarcoidosis.
2.1.2 Predispositions
Familial clustering of sarcoidosis has been reported in a limited number of families, and the relative risk (odds ratio) of sarcoidosis in the family members of a patient with sarcoidosis has been reported to be 8.1 in Japanese people and 18.0 in American Caucasian30,31
(Evidence level 4b).
As type IV hypersensitivity reactions to exogenous antigens are considered to play an important role in the pathogenesis of sarcoidosis, researchers have investigated possible relationships between the disease and human leukocyte antigen (HLA) class II antigens. A study in Japan32
has reported that the frequency of HLA alleles such as DRB1*1101, DRB1*1201, DRB1*1401, and DRB1*0802 is significantly higher in patients with sarcoidosis than people without it, and these HLA-DR molecules have a common sequence of amino acid (Tyr-Ser-Thr) in the 10th through 12th amino acid residues of the β-chain. The relationships between sarcoidosis and the DRB1*1201 and DRB1*1401 alleles have also been reported in Caucasian populations,33,34
indicating that there truly is a relationship between sarcoidosis and HLA-DR alleles (Evidence level 4b). It has been reported that the HLA-DRB1*0301 allele is frequently found in Scandinavian patients with sarcoidosis, and those with this allele tend to have a favorable clinical course35
(Evidence level 4b).
There has been reported that susceptibility to sarcoidosis may be associated with the V64I mutation in the C-C motif chemokine receptor 2 (CCR2),36
and polymorphisms of the gene encoding nucleotide-binding oligomerization domain-containing protein 1 (NOD1) which recognizes intracellular pathogens and activates cells through nuclear factor kappa B (NF-κB).37
A genome-wide linkage analysis has revealed that a single nucleotide polymorphism (rs2076530) in the butyrophilin-like 2 (BTNL2) gene is linked to susceptibility to sarcoidosis.38
The researchers assumed that a splice variant of BTNL2 does not fully transmit an inhibitory signal to T cells, and thereby promote the onset of sarcoidosis. This finding was not replicated in other populations,34,39
suggesting a possibility that the association between sarcoidosis and
BTNL2
may be a result of linkage disequilibrium with its nearby locus
HLA-DRB1.
Genome-wide association studies that compared the frequency of SNPs between patients with sarcoidosis and controls have revealed the annexin A11 (ANXA11) gene,40
RAB23,41
CCDC88B,42
and
OS943
in Caucasian populations, and
XAF-144
in African Americans may confer susceptibility to sarcoidosis.
2.1.3 Pathophysiology
This section describes the pathogenesis of granulomas in sarcoidosis through the recruitment and activation of macrophages or lymphocytes, granuloma development and fibrosis.
Patients with sarcoidosis have been reported to have an increased expression of mRNA of C-C motif chemokine ligand 5 (CCL5, also called as RANTES) by bronchoalveolar lavage (BAL) cells,45
as well as the increased antigen-presentation capacity, accessory cell function to T cells and production of cytokines such as IL-1β, IL-15, TNF-α, granulocyte macrophage colony stimulating factor (GM-CSF) of alveolar macrophages.46–48
IFN-γ plays an important role in activating macrophages and other cells of the immune system49,50
(Evidence level 4b), and IL-12 and IL-18 are involved in its production51,52
(Evidence level 4b). It has been reported that patients with sarcoidosis show an increase in C-X-C motif chemokine ligand 10 (CXCL10, also called as IP-10) level in BAL fluid, and this level correlates with the number of memory CD4+
cells,53
which indicates the accumulation of CD4+
T cells in sarcoidosis lesions.
Recently, Th17 was identified as a new T cell subset. A study has described patients with sarcoidosis have an increased number of cells that produce both INF-γ and IL-17 in peripheral blood,54
while another study has reported that IL-17 production in response to the putative antigen is lower in patients with sarcoidosis than in controls.55
The significance of IL-17 in sarcoidosis remains unclear. Hypergammaglobulinemia, which reflects B cell activation often observed in patients with sarcoidosis, has been suggested to be caused by increased blood levels of B cell-activating factor from the TNF family (BAFF).56
Chronic granulomas in sarcoidosis may become fibrous. It has been suggested that platelet derived growth factor-B (PDGF-B), insulin-like growth factor 1 (IGF1) and insulin-like growth factor binding protein-related protein 2 (IGFBP-rP2) play roles in this process.57–59
2.2 Topics on P. acnes as a Cause of Sarcoidosis
P. acnes
is drawing attention as a cause of sarcoidosis.
P. acnes
is the only microorganism that has been isolated from sarcoidosis lesions.22,60
P. acnes
DNA has been detected at high levels in sarcoidosis lesions,23,61
and the accumulation of
P. acnes
genomes in and around sarcoid granulomas has been reported.62
Immunohistochemistry using an anti-Propionibacterium acnes
monoclonal antibody (PAB antibody) can detect
P. acnes
in sarcoidosis granulomas readily and specifically,63
which is found as different-sized small round bodies in epithelioid cells and giant cells. Immunohistochemistry with PAB antibody is increasingly used in the diagnosis of sarcoidosis.
In patients with cardiac sarcoidosis, PAB antibody-positive cells are found in granulomas and their surrounding tissues infiltrated with inflammatory cells (Figure 1). The sensitivity and specificity of the presence of
P. acnes
in the diagnosis of cardiac sarcoidosis are 83% and 100% in surgically operated myocardial biopsy and 77% and 100% in endomyocardial biopsy, which indicates a possibility that a diagnosis of sarcoidosis may be made by immunostaining tissue sections containing inflammatory foci even if granuloma is not confirmed with endomyocardial biopsy in patients suspected to have cardiac sarcoidosis.

According to a hypothesis that
P. acnes
is involved in the etiology of sarcoidosis, intracellular proliferation of cell-wall-deficient (L form)
P.acnes
during latent infection may trigger the onset of granulomas.64
Granulomas are considered to develop in people with an allergic disposition to
P. acnes. In patients with cardiac sarcoidosis, intracellular proliferation of
P.acnes
may not only lead to granuloma formation but also trigger new latent infection in neighboring tissues. Inflammation may continue to recur until this latent infection is eliminated completely. Recurrent infections cause granulomatous inflammation that damages the myocardium. Postinflammatory fibrosis and recurrent inflammation promote the expansion of sarcoidosis lesions (Figure 2). Accordingly, immunosuppressive treatment including corticosteroids and prophylactic antimicrobial therapy may prevent recurrent inflammation and the expansion of sarcoidosis lesions.
3. Diagnosis of Sarcoidosis
According to the diagnostic standard called “histological diagnosis group,” sarcoidosis is diagnosed histologically when histological examination demonstrates the presence of epithelioid granulomas and granulomas due to other causes can be ruled out. As sarcoidosis is a specified intractable disease where the national and local governments in Japan cover its healthcare expenses, the government needed another diagnostic standard based on clinical findings for people who cannot undergo histological examination. In 1976, the MHLW created the criteria called “clinical diagnosis group” for people suspected to have sarcoidosis but cannot undergo biopsy.65
The Japan Society of Sarcoidosis and other Granulomatous Disorders revised its diagnostic criteria in 2006,6
while the MHLW’s criteria had not been revised. In 2013, the Japan Society of Sarcoidosis and Other Granulomatous Disorders and the MHLW’s diffuse pulmonary disease study group decided to revise these criteria for the diagnosis of sarcoidosis, and published the revised criteria and severity classification in January 201566,67
(Evidence level 6, Recommendation grade C1). In January 2015, the Act on Medical Care for Patients with Rare/Intractable Diseases was newly implemented, and the diagnostic standard of sarcoidosis as a designated intractable disease was revised as described in the following sections. The definition of the histological diagnosis group was revised to require that the presence of epithelioid granulomas should be demonstrated and granulomas due to other causes should be ruled out. The revised criteria also require detailed examination to observe for characteristic laboratory findings and systemic disorder. Since histological diagnosis using biopsy samples may not be made for some patients who have characteristic clinical findings in the eyes, heart, and respiratory tract, the clinical diagnosis group criteria was also revised to include the following criterion: When the patient shows clinical findings strongly suggestive of sarcoidosis in at least two organs of the respiratory tract, the eyes, and the heart, and has at least two of the five characteristic laboratory findings of sarcoidosis. There is no evidence to indicate the accuracy of the criteria for the clinical diagnosis group. The present clinical criteria for diagnosis are stricter than the conventional clinical criteria, but they are expected to be reserved for patients who have no characteristic findings in other organs from which biopsy samples can be readily obtained. Although clinical findings in organs other than the eyes, heart and respiratory tract are not included in the diagnostic criteria of sarcoidosis, the present guidelines describe them as they are important to indications of the disease.
3.1 Diagnostic Standard of Sarcoidosis
Histological Diagnosis Group
Sarcoidosis is diagnosed histologically when biopsy specimens demonstrate non-caseating epithelioid granulomas in some organs of the body, and when granulomas due to other causes and local sarcoid reactions can be ruled out. The patient should also be carefully examined for characteristic laboratory findings and systemic disorder.
Clinical Diagnosis Group
Sarcoidosis is diagnosed clinically when clinical findings are strongly suggestive of sarcoidosis in at least two organs of the respiratory tract, the eyes, and the heart, and when at least two of the five characteristic laboratory findings of sarcoidosis (Table 1) are present, although epithelioid granulomas are not found.
Table 1.
Characteristic Laboratory Findings of Sarcoidosis
| (1) Bilateral hilar lymphadenopathy |
| (2) High serum angiotensin-converting enzyme (ACE) activity or elevated serum lysozyme levels |
| (3) High serum soluble interleukin-2 receptor (sIL-2R) levels |
| (4) Significant tracer accumulation in 67Ga citrate scintigraphy or 18F-FDG PET |
| (5) A high percentage of lymphocytes with a CD4/CD8 ratio of >3.5 in BAL fluid |
A diagnosis of sarcoidosis is established when at least two of the above five characteristic findings are observed.
Adapted from Japan Society of Sarcoidosis and Other Granulomatous Disorders. 2015.67
Note
(1) As skin specimens are easy to obtain, patients with skin lesions should undergo skin biopsy whenever possible in order to make a correct diagnosis. Minimal skin lesions may be found only by dermatologists.
(2) In some patients with manifestations suggestive of sarcoidosis involving the nervous systems or other organs, biopsy specimens may not be obtained with ease. However, they should undergo systemic examination and relevant tests and then undergo histological examination whenever possible to confirm the diagnosis.
(3) Patients in the clinical diagnosis group require careful differentiation from other diseases causing similar clinical manifestations.
(4) The National Health Insurance in Japan does not cover serum lysozyme assay or the use of sIL-2R assay for the diagnosis of sarcoidosis. Also, it does not cover the use of 18F-FDG PET for the diagnosis of sarcoidosis in general, while it does cover its use for the diagnosis of cardiac sarcoidosis.
How to Diagnose Sarcoidosis
Sarcoidosis is expected to be diagnosed as outlined in
Figure 3.67
Sarcoidosis is known to cause diverse manifestations. Some patients are asymptomatic when it was found during health checkups, while other patients have manifestations involving many organs and generalized symptoms. In Japan where respiratory, eye and cardiovascular complaints are particularly common among patients with sarcoidosis, physicians typically order examinations specific to sarcoidosis when patients present with signs and symptoms strongly suggestive of sarcoidosis involving the respiratory tract, eyes or heart. Patients presenting with abnormal findings in other organs may be diagnosed as sarcoidosis when biopsies reveal non-caseating epithelioid granulomas, and detailed systemic examinations are performed to confirm the diagnosis. In both cases, histopathological examinations should be ordered whenever possible, and detailed examinations should be performed to rule out other possible conditions.

3.2 Clinical Findings Suggestive of Sarcoidosis Involving Different Organs
This section describes clinical findings characteristic for sarcoidosis involving the respiratory tract, eyes and skin and other organ dysfunctions associated with sarcoidosis-related diseases according to the “Diagnostic Standard and Guideline for Sarcoidosis - 2015”.67
The diagnosis of sarcoidosis should be based on histological examination, but patients with clinical findings strongly suggestive of sarcoidosis involving the respiratory tract, eyes or heart may be regarded to have lesions involving the relevant organ even when they are not confirmed histologically.
a. Clinical Findings Defining Respiratory Involvement
Sarcoidosis of the respiratory system may cause lesions in the alveoli (alveolitis), bronchial vessels, hilar lymph nodes, mediastinal lymph nodes, trachea and bronchi, and pleura.
Clinical findings strongly suggestive of respiratory tract involvement of sarcoidosis are defined as the presence of (1) or (2) of
Table 2
(See section “4. Pulmonary Lesions of Sarcoidosis” for details).
Table 2.
Respiratory Manifestations of Sarcoidosis
| (1) Bilateral hilar lymphadenopathy is observed. |
(2) CT/HRCT images show thickened interstitium surrounding the bronchial vascular bundles and multiple nodular shadows
along lymphatic vessels. Multiple nodular shadows along lymphatic vessels may be found in the central and/or peripheral parts
of lobules along the pleura, interlobular septa, and bronchopulmonary arteries. |
Adapted from the Japan Society of Sarcoidosis and Other Granulomatous Disorders. 2015.67
b. Clinical Findings Defining Eye Involvement
Clinical findings strongly suggestive of eye involvement of sarcoidosis are defined as the presence of at least two of the six clinical findings listed in
Table 3.
Table 3.
Ocular Manifestations of Sarcoidosis
| (1) Granulomatous anterior uveitis (mutton-fat keratic precipitate/iris nodules) |
| (2) Trabecular meshwork nodules and/or tent-shaped peripheral anterior synechia |
| (3) A mass of vitreous opacities (snowball or string-of-pearls type opacities) |
| (4) Retinal perivasculitis (mainly periphlebitis) with perivascular nodules |
| (5) Multiple candle-wax type chorioretinal exudates and nodules and/or laser photocoagulation spots-like chorioretinal atrophy |
| (6) Optic disc nodule(s)/granuloma(s) and/or solitary choroidal nodule |
Other possible ocular manifestations: Corneal xerosis, episcleritis/scleritis, lacrimal swelling, swelling of eyelids, and facial palsy. Adapted from the Japan Society of Sarcoidosis and Other Granulomatous Disorders. 2015.67
c. Clinical Findings Defining Cardiac Involvement
See Section “3. Diagnostic Guidelines” in Chapter III, for clinical findings defining cardiac involvement.
d. Clinical Findings Defining Skin Involvement
Table 4
lists clinical findings that define skin involvement of sarcoidosis.
Table 4.
Dermal Manifestations of Sarcoidosis
| (1) Skin sarcoids (specific lesions) |
i. Nodular form; ii. Plaque form; iii. Diffuse infiltrative form; iv. Subcutaneous form; v. Other forms (Lichenoid type, nodular
erythema-like, Ichthyosis type, or other rare forms.) |
| (2) Infiltrated scars |
(The patient should show clinical findings strongly suggestive of skin lesions, and the presence of granuloma should be
confirmed histologically.) |
Adapted from the Japan Society of Sarcoidosis and Other Granulomatous Disorders. 2015.67
Note
Erythema nodosum, which is not specific to sarcoidosis, may develop without nodules in some patients, but the prevalence of this finding is low in Japan.
e. Clinical Findings Strongly Suggestive of Sarcoidosis of Organs Other Than the Respiratory Tract, Eyes, Heart and Skin.
Clinical findings strongly suggestive of sarcoidosis in organs other than the respiratory tract, eyes, heart and skin include imaging findings on CT, MRT, ultrasonography, endoscopy, 67Ga scintigraphy, and 18F-FDG PET, among others. In patients with clinical findings strongly suggestive of sarcoidosis in organs other than the respiratory tract, eyes, heart and skin, the presence of epithelioid granulomas in at least one organ must be demonstrated to confirm the diagnosis of sarcoidosis (Table 5).
Table 5.
Findings of Other Organ Involvement
| (1) Nerve lesions |
| i. CNS lesions |
| a. Granulomatous lesions in the parenchyma |
| a-1. Localized mass lesions; a-2. Diffuse sporadic granulomatous lesions; a-3. Spinal lesions |
| b. Meningeal lesions |
| b-1. Meningitis/meningoencephalitis; b-2. Hypertrophic granulomatous pachymeningitis |
| c. Hydrocephalus |
| d. Vascular lesions |
| d-1. Vasculitis; d-2. Periventricular white matter lesions; d-3. Sinus thrombosis |
| e. Encephalitis |
| ii. Peripheral nerves |
| a. Cranial nerve paralysis |
a-1. Facial palsy; a-2. Glossopharyngeal/vagus nerve disorder; a-3. Auditory nerve disorder; a-4. Optic nerve disorder;
a-5. Trigeminal nerve disorder; a-6. Olfactory nerve disorder; a-7. Other cranial nerve disorders |
| b. Spinal nerves paralysis |
b-1. Mononeuritis multiplex; b-2. Polyneuritis (including small fiber neuropathy); b-3. Single nerve paralysis; b-4.
Other disorders: e.g., Radiculopathy and cauda equina syndrome |
| (2) Hepatic lesions: Hepatomegaly, multifocal nodules |
| (3) Splenic lesions: Splenomegaly, hypersplenism, multifocal nodules |
| (4) Gastrointestinal lesions: Ulcer, mucosal hypertrophy, raised lesions |
(5) Renal lesions: Renal mass, renal lesions associated with calcium metabolism disorder, tubulointerstitial nephritis,
granulomatous nephritis, glomerulonephritis, renal vasculitis |
| (6) Extrathoracic lymph node lesions: Superficial lymphadenopathy, intraabdominal lymphadenopathy, etc. |
| (7) Exocrine lesions: Parotid gland enlargement, submaxillary gland enlargement, lacrimal gland enlargement |
| (8) Upper respiratory tract lesions: Nasal cavity lesions, mass in the upper respiratory tract |
| (9) Bone lesions: Lacy trabecular patterns, osteolytic lesions, round cystic radiolucent area in the bone |
| (10) Muscle lesions |
| i. Acute to subacute myositis-like lesions |
| ii. Chronic myopathy |
| iii. Nodular myopathy |
| (11) Joint lesions: Joint swelling and deformity |
| (12) Genital organ lesions: Mass in the uterus, testicles, epididymis or spermatic cord. |
(13) Other lesions: Bone marrow lesions, pancreatic lesions, biliary/gallbladder lesions, peritoneal lesions, mammary lesions,
thyroid lesions, etc. |
Adapted from the Japan Society of Sarcoidosis and Other Granulomatous Disorders. 2015.67
Note
Sarcoidosis may be associated with the following related conditions as well as organ disorders that result from these related conditions. These conditions are not “clinical findings strongly suggestive of organ involvement of sarcoidosis” but are important conditions associated with sarcoidosis.
(1) Abnormal calcium metabolism (hypercalcemia, hypercalciuria, nephrolithiasis and urinary calculus)
(2) Neurological disorders underlined in Table 5
(3) Kidney disorders underlined in Table 5
3.3 Exclusion Criteria
Possible causes other than sarcoidosis should be ruled out according to the following exclusion criteria.
(1) Rule out systemic diseases of known causes or other types of systemic diseases: e.g., malignant lymphoma, other lymphoproliferative disorders, cancer (lymphangiosis carcinomatosa), tuberculosis, non-tuberculous granulomatous infections (e.g., non-tuberculous mycobacterial infection and mycosis), BehÇet’s disease, amyloidosis, granulomatosis with polyangiitis, Wegener’s granulomatosis, and IgG4-related disease
(2) Rule out sarcoid reaction to foreign substances or cancer
(3) Rule out other types of pulmonary granuloma: e.g., beryllium lung disease, pneumoconiosis, and hypersensitivity pneumonitis
(4) Rule out giant cell myocarditis
(5) Rule out uveitis of known cause: e.g., herpes uveitis, HTLV-1 uveitis, and Posner-Schlossman syndrome
(6) Rule out other skin granuloma: e.g., granuloma annulare, annular elastolytic giant cell granuloma, necrobiosis lipoidica, Melkersson-Rosenthal syndrome, lupus miliaris disseminatus faciei, and rosacea.
(7) Rule out other types of hepatic granulomas: e.g., primary biliary cirrhosis
3.4 Important Points in the Diagnosis and Follow-up Evaluation of Sarcoidosis
Since sarcoidosis is a systemic disease that can affect multiple organs simultaneously or consecutively, physicians should carefully review the patient’s clinical history, and observe the patient periodically for organ involvements of sarcoidosis. In Japan, patients with typical clinical findings that consist with the diagnostic standard or sarcoidosis in the relevant organ may apply to the national patient support program as those who meet the definition of the clinical diagnosis group even when histopathological examination is difficult to conduct. However, these patients need detailed examination to rule out other possible diseases. Patients who are suspected to have sarcoidosis but do not meet the above-mentioned criteria and who do not have to start treatment immediately should be classified as suspected cases and be observed periodically. For patients who are strongly suspected to have cardiac sarcoidosis or central nervous system (CNS) sarcoidosis and are at risk of life-threatening conditions, a therapeutic diagnosis may be made to start treatment.
4. Pulmonary Lesions of Sarcoidosis
Since it has been reported that pulmonary involvement is present in more than 90% of patients with sarcoidosis,68,69
chest imaging findings are important information in the diagnosis of sarcoidosis (Evidence level 4b, Recommendation grade A). A typical finding of sarcoidosis on plain chest X-ray is bilateral hilar lymphadenopathy (BHL) (Figures 4,5). Intrathoracic lymphadenopathy is the most common X-ray finding among patients with pulmonary sarcoidosis. In addition to BHL, lymphadenopathy may also be found in upper mediastinal lymph nodes. Lymphadenopathy usually occurs bilaterally, and unilateral lymphadenopathy accounts for less than 5% in patients with pulmonary sarcoidosis. Typical pulmonary imaging findings are fine nodular and patchy ground-glass opacities, pale infiltrates, and cyst formation that distribute along the lymphatic vessels. Lesions are found more commonly in the upper lung fields. Patients with progressive pulmonary sarcoidosis show diverse imaging findings including reticular shadows due to fibrosis, and reduced total lung capacity. Pulmonary sarcoidosis is classified into five stages according to plain chest X-ray findings. This staging can predict prognosis, but does not always reflect disease activity or the severity of pulmonary dysfunction10,70
(Table 6).71
Chest X-ray findings of pulmonary sarcoidosis are classified into the following five stages: Stage 0: No intrathoracic involvement is found. Stage I: Lymphadenopathy including BHL is found with no lung involvement. However, lung biopsy often reveals granulomas. Stage II: BHL and lung involvement are found. Stage III: Lung involvement without BHL is found. Stage IV: Lung fibrosis and tissue damage are found.

Table 6.
Staging Classification of Pulmonary Sarcoidosis
| Stage |
Chest X-ray |
Prevalence
(%) |
Spontaneous
cure rate (%) |
| 0 |
Normal chest X-ray findings |
5 to 15 |
– |
| I |
Bilateral hilar lymphadenopathy |
45 to 65 |
50 to 90 |
| II |
Bilateral hilar lymphadenopathy+lung opacity |
30 to 40 |
30 to 70 |
| III |
Lung opacity only (without bilateral hilar lymphadenopathy) |
10 to 15 |
10 to 20 |
| IV |
Lung fibrosis |
5 |
0 |
Adapted from Fujimoto K. 201371
with modification.
Since pulmonary involvement in sarcoidosis is mainly characterized with fine nodular shadows (about 0.2 mm in diameter) that distribute along the lymphatic vessels, HRCT should be performed to examine the lesions in detail72–75
(Evidence level 5, Recommendation grade A). In addition to BHL, HRCT typically reveals very fine to fine nodular shadows along the peribronchovascular sheaths, interlobular septa and pleural surface, tracheal wall thickening, ground-glass opacities, and mass-like lesions associated with cavities and cysts. In advanced cases, HRCT may reveal fibrosis and traction bronchodilation. In rare cases, nodular shadows or mass-like shadows with large coalescent parenchymal nodules described as the “sarcoid galaxy sign” may be found. In some patients, HRCT reveals mosaic attenuation caused by air trapping due to airway stenosis by granulomas and cystic shadows. HRCT findings are mainly observed in the upper and middle lobes. In the “Diagnostic Standard and Guideline for Sarcoidosis - 2006”, the presence of (1) BHL or (2) one or more findings in
Table 76
are listed as imaging findings strongly suggestive of pulmonary involvement of sarcoidosis.
Table 7.
Chest X-ray, CT/HRCT and Bronchoscopic Findings of Pulmonary Sarcoidosis
| 1. Chest X-ray findings |
| (1) Diffuse lung opacities, mainly fine nodular or patchy opacities, are observed mainly in the upper lung fields. |
| (2) Irregular opacities and thickening of the area surrounding the bronchial vascular bundles. |
| (3) When advanced, fibrosis and shrinking of the lung develop mainly in the upper lungs. |
| 2. CT/HRCT findings |
(1) Fine nodular shadows in lung fields and thickened interstitium surrounding the bronchial vascular bundles are often found. Fine nodular
shadows with focal shrinking reflect the distribution of lymph components and are found both in the central and peripheral parts of
lobules along the pleura, interlobular septa, and bronchopulmonary arteries. |
(2) Nodular shadows, mass shadows, or evenly-distributed shadows may be observed in rare cases. Pleural fluid is rare. Advanced fibrous
lesions are observed as evenly-distributed shadows indicating tissue shrinking associated with traction bronchodilation rather than as
typical honeycomb lungs. |
| 3. Bronchoscopy findings |
| (1) Network formation |
| (2) Nodules |
| (3) Bronchostenosis |
Adapted from Committee for revision of the diagnostic standard for sarcoidosis. 2006.6
5.1 Introduction
Sarcoidosis, a systemic granulomatous inflammatory disease, generally does not cause significant symptoms and may resolve spontaneously, but may lead to serious life-threatening conditions when it involves the heart and lungs. Prolonged involvement of the eyes, nerves, skin and kidneys among other organs may not affect the life expectancy of patients, but does reduce their QOL significantly. Appropriate intervention should also be made.
It is well known that corticosteroids are effective at least in the short-term control of granulomatous inflammation, and there is no doubt that corticosteroid therapy should be initiated without delay for patients with acute exacerbation of sarcoidosis. The most important point in the treatment of sarcoidosis is to consider the indications of treatment, types and doses of drugs used, and length of treatment for diverse types of conditions that are often asymptomatic and may improve spontaneously. Treatment benefits must overweigh the burden imposed by the treatment. However, its clinical course varies and lasts long, it is difficult to assess the efficacy of treatments appropriately. A limited number of large-scale prospective clinical studies in sarcoidosis have been conducted, but these studies focused on patients with pulmonary sarcoidosis.
Corticosteroids work as a double-edged sword as they may quickly relieve granulomatous inflammation but also suppress lymphocytes that remove the pathogen. Adverse drug reactions (ADRs) to corticosteroid therapy may sometimes lower the QOL of patients significantly, which may cause corticosteroid phobia. The diverse clinical course of sarcoidosis, the dual nature of corticosteroids, and ADRs of corticosteroids and patients’ fear of corticosteroids make it difficult to standardize corticosteroid therapy for patients with sarcoidosis.
5.2 Indications of Corticosteroids for Patients With Pulmonary Involvement of Sarcoidosis
Large-scale prospective randomised clinical trials to investigate the efficacy of systemic corticosteroid therapy in sarcoidosis have only been conducted in patients with pulmonary sarcoidosis. The Cochrane review on corticosteroids for pulmonary sarcoidosis have summarized that the results fits with the currently held view that patients with stage I disease (bilateral hilar lymphadenopathy alone) do not need treatment with oral steroids, but that those with interstitial lung disease (stage II and III) may show an improvement in chest X-ray and in global scores when treated with oral steroids.
Gibson et al77
(Evidence level 3) and Pietinalho et al78
(Evidence level 2) have reported that patients receiving corticosteroid therapy, including those with relatively mild symptoms, showed better pulmonary function and chest X-ray findings over the subsequent 5 years than patients not receiving corticosteroids. The editorial article79
on the report by Pietinalho et al discussed these two articles and described that corticosteroid therapy should be initiated early when no significant symptoms present. Gibson described the results of corticosteroid therapy as “a small but definite long-term advantage.”
However, a slightly better outcome at 5 years in terms of the percentages of patients in whom abnormal chest X-ray findings disappeared and pulmonary function improved is not a strong basis supporting corticosteroid therapy for relatively asymptomatic patients with pulmonary sarcoidosis. Corticosteroid therapy should be advised only when expected benefits are greater than potential harms. The above-mentioned two reports indicated a slight superiority of the corticosteroid group over the control group in terms of chest X-ray findings and pulmonary functions at 5 years, which does not guarantee better QOL and life expectancy at 5 years or thereafter. Several reports have pointed out that intensive corticosteroid therapy in relatively asymptomatic patients may deteriorate prognoses.80–82
Recent reports from Western countries have described that drug treatment is not indicated for relatively asymptomatic patients without pulmonary dysfunction83,84
(Evidence level 6). Judson has described that corticosteroids are not indicated for asymptomatic patients with forced vital capacity (FVC) 70% or more.85
In response to a question whether asymptomatic patients with intact respiratory function should be treated corticosteroids, an increasing number of researchers and physicians answer that corticosteroid therapy is not a positive option even though clinical evidence on the risk and benefits of corticosteroids in this patient population has not yet been accumulated sufficiently. This represents how difficult it is to conduct prospective clinical research in patients with sarcoidosis, a disease that progresses over decades.
5.3 Treatment Indications Considering Fibrosis of Granulomas
The section “treatment procedures for patients with pulmonary sarcoidosis” in “Views on the Treatment of Sarcoidosis - 2003”9
prepared by the Japan Society of Sarcoidosis and Other Granulomatous Disorders in 2003 describes that asymptomatic or mildly symptomatic patients with stage II or III pulmonary sarcoidosis should be left untreated and monitored carefully, and corticosteroid therapy should be considered according to their individual clinical conditions for those with progression of chest X ray findings.
“Patients with chronic granulomatous disease with no or mild fibrosis (granuloma type lesions)” tend to maintain intact pulmonary function, are expected to at least partially improve without particular treatment, respond well to corticosteroid therapy, and often lead to clinical remission even though the disease may sometimes flare up. Accordingly, patients diagnosed as having granuloma type lesions may be treated with short-term, low-dose corticosteroid therapy to wait for spontaneous improvement.
The main factor that reduces life expectancy in patients with pulmonary involvement of sarcoidosis is fibrosis secondary to granulomatous disease. Patients who have a poor outcome due to respiratory failure, pulmonary hypertension and lung infection among other conditions are almost as limited as those with advanced lung fibrosis. Accordingly, it is important to identify patients with “diffuse fibrosis of granulomatous lesions (diffuse fibrosis type lesions)” based on slight changes in chest X-ray findings at an early stage and intervene appropriately. However, no data have been reported which types of chest X-ray findings indicate the risk of diffuse fibrosis. Further reports are awaited.
5.4 Dose and Duration of Corticosteroid Therapy
There has been a consensus where corticosteroid therapy for patients with pulmonary sarcoidosis should be initiated at 0.5 mg/kg/day (20 to 40 mg/day) of prednisolone and then reduced thereafter9,10
(Evidence level 6, Recommendation grade C1). However, it is unknown whether the dose is optimal or not. Considering a report describing that low-dose prednisolone therapy at doses of 5 to 10 mg/day is effective for patients complicated with diabetes mellitus or infectious diseases,86
corticosteroid therapy at lower doses for a shorter period of time than those previous reported would be effective for patients with granuloma-type lesions.
As “if the regimen is discontinued while the antigen is still present, the patient will relapse”85
corticosteroid therapy for pulmonary sarcoidosis may last for decades. In the United States where severe pulmonary sarcoidosis is more prevalent than in Japan, those who are maintained on ≤15 mg/day of prednisolone are considered successful cases, while in Japan the target maintenance dose is ≤5 mg/day.
5.5 Treatment Indications for Extrapulmonary Manifestations of Sarcoidosis
Only a limited number or reports have described indications of corticosteroid therapy for extrapulmonary manifestations of sarcoidosis. In many cases extrapulmonary manifestations are treated to improve QOL rather than prolong the life of patients. Treatment is often initiated after symptoms develop, and corticosteroids are administered when required.
However, cardiac involvement is a life-threatening condition where corticosteroid therapy is recommended regardless of whether symptoms are present or not21,87
(Evidence level 4b, Recommendation grade C1). Nerve involvement and ocular involvement, which may significantly reduce QOL if they remain untreated, are indicated for corticosteroid therapy even if patients are asymptomatic. However, there is no evidence on the optimal dose and duration of corticosteroid therapy for these conditions. Corticosteroids are used at the discretion of physicians.
5.6 Drug Treatment Other Than Corticosteroids
Evidence on the efficacy of methotrexate and azathioprine, which are used as immunosuppressants, has been accumulated in many countries88–91
(Evidence level 5, Recommendation grade C1). Patients who cannot discontinue corticosteroid therapy for a long period of time should also receive these drugs to achieve the steroid-sparing effect. Low-dose methotrexate therapy is commonly used for patients with rheumatoid arthritis, and regimens combining low-dose corticosteroids and low-dose methotrexate are expected to be beneficial in the treatment of sarcoidosis90
(Evidence level 3). Methotrexate monotherapy is known to be somewhat effective,91
and may be considered for patients who want to avoid or are contraindicated for corticosteroids. (Note: Currently, methotrexate therapy in sarcoidosis is not covered by the National Health Insurance in Japan).
Antimicrobial drugs have been used for the treatment of sarcoidosis. Chloroquine hydrochloride, an antimalarial drug, has been used widely for the treatment of sarcoidosis in the United States even though its efficacy has not been proven yet. As some patients, especially those with skin lesions, respond well to tetracyclines,92,93
these drugs may be prescribed to patients who accept the treatment after detailed assessment and explanation by the physician (Evidence level 5).
5.7 Treatment for General Symptoms
Patients with sarcoidosis often complain of non-organ-specific general symptoms such as pain, fatigue and shortness of breath, which often represent their most annoying symptoms.94,95
No sufficient treatment has been established to manage these symptoms, but Chinese herbal medicine96
and high-dose corticosteroids97
are effective in some patients.
5.8 Conclusion
As described in this section, no sufficient evidence has been accumulated on the indications, types, duration, doses and endpoints of treatments that can be used to develop guidelines for the treatment of sarcoidosis. Further findings are needed.
III. Diagnosis of Cardiac Sarcoidosis
1. Pathology
As in sarcoidosis affecting other organs, cardiac sarcoidosis is characterized histologically by the presence of multinucleated giant cells in non-caseating epithelioid granulomas. The presence of giant cell is an important finding to differentiate cardiac sarcoidosis from other cardiac diseases (Evidence level 4a, Recommendation grade A).
It is often difficult to make a definitive diagnosis of cardiac sarcoidosis because it manifests diverse pathological conditions and no diagnostic measures other than endomyocardial biopsy can identify the presence of cardiac sarcoidosis. Although pathological findings of cardiac sarcoidosis are highly specific, endomyocardial biopsy samples often show fibrosis and lymphocytic infiltration only without epithelioid granulomas or giant cells. Only about 20% of biopsy samples obtained from patients with cardiac sarcoidosis show positive findings.
1.1 Endomyocardial Biopsy
The diagnostic rate achieved with endomyocardial biopsy in cardiac sarcoidosis is as low as about 20% due to frequent errors in obtaining positive biopsy samples.98,99
Accordingly, cardiac sarcoidosis cannot be ruled out even if endomyocardial biopsy shows a negative result.100
1.2 Macro-Pathological Findings of Cardiac Sarcoidosis
Valantine et al reviewed cases of cardiac sarcoidosis and pointed out that the basal portion of the ventricular septum is the most common location affected by cardiac sarcoidosis.101
Since then, a basal thining of the ventricular septum found in echocardiography is attracting attention as a finding typical of cardiac sarcoidosis. A thin ventricular septum is often observed in postmortem examinations of patients with cardiac sarcoidosis (Figure 6A), and is associated with enlarged ventricles (Figure 6B).

Cardiac sarcoidosis affects any parts of the myocardial wall including the papillary muscles. Lesions have been found in the basal portion of the ventricular septum, atrial wall and cardiac conducting system, and some patients had predominant involvement of the epicardium102
(Figures 6B,7). When cardiac involvement manifests clinically, granulomas have already started to become fibrous in many patients. The borders between lesions and non-lesions are clear, and fibrous granulomas can be seen as patchy or linear lesions (Figures 7,8). When fibrosis progresses, ventricular aneurysm or dilated cardiomyopathy-like ventricular enlargement develop103
(Figures 6,8).
1.3 Histological Findings in Cardiac Sarcoidosis
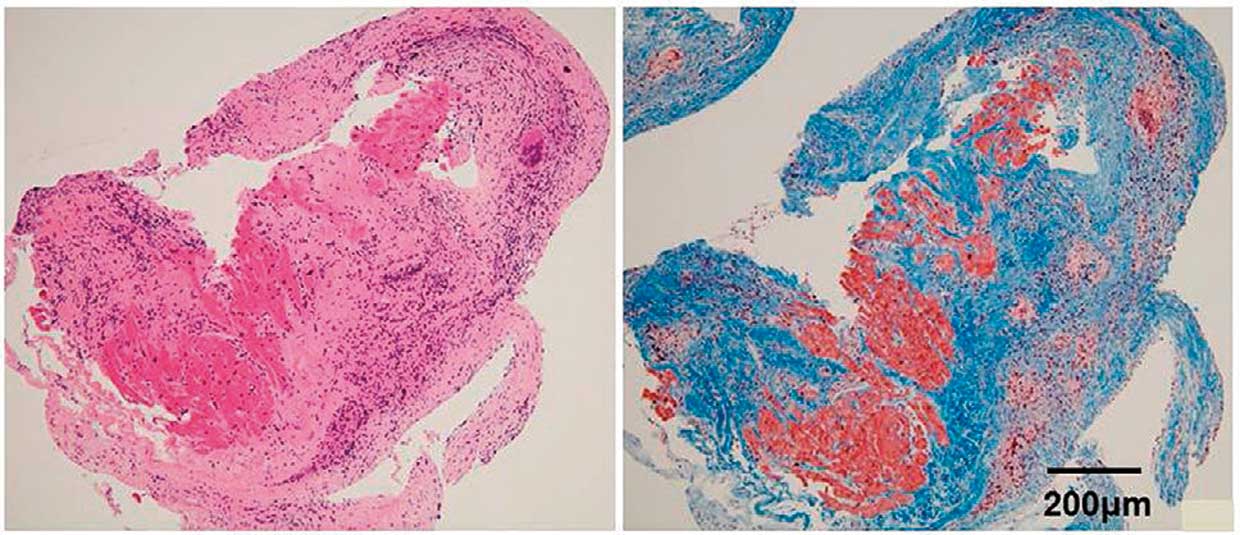
As in sarcoidosis in other organs, cardiac sarcoidosis is characterized histologically by the presence of multinucleated giant cells in non-caseating epithelioid granulomas (Figure 9).17
Granulomas consist of epithelioid cells, multinucleated giant cells, macrophages, lymphocytes and plasma cells among others. As described earlier, granuloma typical of sarcoidosis can be detected only in about 20% of patients undergoing myocardial biopsy,98,99
and many biopsy samples contain giant cells and epithelioid cells only. When granuloma is not detected, serial sections should be examined for the presence of giant cells. Caseating necrosis suggestive of tuberculosis is not present in patients with cardiac sarcoidosis. Giant cells often appear as Langhans-type multinucleated giant cells with nuclei arranged peripherally (Figure 10), but may appear as foreign-body-type or Touton-type multinucleated giant cells (Figures 11,12). Giant cells may also contain cytoplasmic inclusions such as asteroid bodies (Figure 13A) and Schaumann bodies (Figure 13B).104
These cytoplasmic inclusions are useful in the diagnosis of sarcoidosis. The degree of lymphocytic infiltration in tissues adjacent to granulomas relates to the stage (activity) of sarcoidosis. Generally, CD4 lymphocytes accumulate predominantly in granulomas, while CD8 lymphocytes are found sporadically at the periphery of granulomas (Figure 14). At the scarring stage, fibrosis becomes prominent with little lymphocyte infiltration, and granulomas tend to disappear over time. In some lesions only giant cells are found (Figure 15). The presence of giant cells in myocardial biopsy samples is strongly suggestive of cardiac sarcoidosis. The borders between lesions and non-lesions are often clear, and myocardial cells in non-lesions are intact in some cases (Figure 16).







Giant cell myocarditis (Figure 17), an important condition that should be differentiated from cardiac sarcoidosis, is characterized by necrosis of myocardial cells, which is absent in cardiac sarcoidosis.105
Eosinophilic infiltration is also rare in cardiac sarcoidosis. Giant cells found in cases of giant cell myocarditis are derived from macrophages and cardiac myocytes, while both epithelioid cells and giant cells found in cases of cardiac sarcoidosis are derived from macrophages (Figure 18). In cardiac sarcoidosis, activated T lymphocytes differentiate into Th1 cells, which produce Th1 cytokines such as IL-2 and IFN-γ that mediate migration, activation and differentiation of monocytes and macrophages, and eventually lead to granuloma formation. In order to rule out other conditions causing epithelioid granulomas, mycosis and tuberculosis, Grocott staining for fungal infection and Ziehl-Neelsen staining for acid-fast microorganisms should be conducted whenever necessary. Since fibrosis becomes prominent (Figure 19) and only slight lymphocyte infiltration is present at the scarring stage, serial sections of biopsy specimens should be prepared to increase the accuracy of diagnosis (Figure 20). Lymph node biopsy may be needed in some cases.

2.1 Clinical Manifestations
Sarcoidosis is a systemic inflammatory disease that causes clinical symptoms in 60 to 70% of patients. However, symptoms are not specific. Sarcoidosis is often diagnosed after abnormal findings are detected at chest X-ray or ECG during routine health checkups or screening tests to evaluate non-specific symptoms.6
a. Clinical Manifestation of Systemic Sarcoidosis
About a third of patients with sarcoidosis complain of non-specific symptoms of systemic inflammation such as fever (mild fever in many cases), general malaise, night sweats and decreased weight.10
Sarcoidosis is an important possible cause of fever of unknown origin, but these non-specific symptoms are not common among Japanese patients with sarcoidosis.106
Sarcoidosis may affect the lungs and hilar lymph nodes as well as the skin, eyes, muscles, bones, heart and central nervous system, and may cause organ-specific signs and symptoms. It has been reported that the incidence of extrapulmonary lesions differs among races, and that the heart and eyes are more often affected in Japanese patients than in other races.106
However, many Japanese patients with cardiac or eye involvement of sarcoidosis are asymptomatic, and are often diagnosed at routine health checkups or screening tests to evaluate non-specific symptoms.
b. Clinical Manifestations of Cardiac Sarcoidosis
Cardiac lesions are found at autopsy in about 25% of patients with sarcoidosis, while imaging studies reveal asymptomatic cardiac involvement in 3.7 to 54.9% of the patients with extracardiac sarcoidosis, depending on the type of imaging study used, of patients with cardiac sarcoidosis.107
Patients with mild cardiac involvement are often asymptomatic, but symptoms occur when lesions affect the conduction system or the cardiac function of the heart. When sarcoidosis affects the cardiac conduction system, bundle branch block and atrioventricular block may develop. Atrioventricular block may cause mild bradycardia causing palpitations and dizziness or may even cause severe bradycardia leading to syncopes or sudden death. Myocardial inflammation and fibrosis due to sarcoidosis may cause ventricular arrhythmias such as premature ventricular contractions, ventricular tachycardia and ventricular fibrillation. Premature ventricular contractions may cause palpitations and shortness of breath. Sustained ventricular tachycardia and ventricular fibrillation may lead to sudden death (See Section “2.4 Electrocardiogram”).
As cardiac involvement progresses, myocardial function deteriorates further to cause systolic and/or diastolic dysfunction, causing heart failure. Symptoms of heart failure include those associated with fluid retention and those associated with lower cardiac output. Fluid retention causes edema, cough and dyspnea. As dyspnea and cough are common symptoms of respiratory diseases, it is important to differentiate whether they are caused by pulmonary or cardiac lesions when such symptoms develop or worsen in patients with sarcoidosis mainly affecting the respiratory tract. Decreased cardiac output may cause oliguria, general malaise, insomnia, depression and dizziness, and may result in syncope, confusion, and decreased level of consciousness in severe cases. Clinical manifestations of cardiac sarcoidosis may include those associated with cardiac conduction disturbance and those associated with myocardial impairment, depending on the location and severity of granulomatous inflammation.
These symptoms are not specific to cardiac sarcoidosis, but physicians should always consider this as a possible cause of cardiac symptoms. Mehta et al have reported the prevalence of symptoms and the results of cardiac MRI and 18F-FDG PET scanning in 62 patients diagnosed as sarcoidosis (mainly pulmonary sarcoidosis) based on pathological examination of non-cardiac organs.108
They described that the prevalence of cardiac symptoms (e.g., palpitations and presyncope) was significantly higher in patients with cardiac involvement than those without it (46% vs. 5%), and concluded that cardiac symptoms are an important indicator of cardiac involvement in patients diagnosed with non-cardiac sarcoidosis (Evidence level 4a, Recommendation grade B).
2.2 Blood and Urine Tests (Biomarkers)
No biological materials currently being investigated as potential biomarkers of sarcoidosis are highly specific in determining the progression of cardiac sarcoidosis or detecting isolated cardiac involvement of sarcoidosis. However, these markers are useful as screening tests for myocardial injuries to detect abnormalities without using imaging modalities such as MRI, echocardiography and 18F-FDG PET. Further studies are expected to reveal which types of biomarkers would be more useful in assessing treatment efficacy and prognosis in patients with cardiac sarcoidosis or isolated cardiac sarcoidosis.
2.2.1 Biomarkers in Screening for Cardiac Involvement in Patients Diagnosed With Systemic Sarcoidosis
a. Angiotensin Converting Enzyme (ACE) Activity
In a study in Japanese patients diagnosed with cardiac sarcoidosis, serum ACE activity was high in 21.8% of patients.109
In another study in 516 patients with sarcoidosis with histologically-proven epithelioid granulomas, serum ACE activity and serum lysozyme activity were high in 49.8% and 51.7% of the patients, respectively.110
It has been also reported that serum ACE activity was high in 62.3% of 106 patients with sarcoidosis-associated uveitis and 18% of 100 patients suspected to have the same condition.111
Conversely, a study has described that serum ACE activity was significantly lower in patients diagnosed with cardiac sarcoidosis than those with sarcoidosis but without cardiac involvement.112
Further studies are needed to conclude whether serum ACE activity relate to the occurrence or severity of cardiac involvement in sarcoidosis (Evidence level 4b, Recommendation grade C1).
b. Atrial Natriuretic Peptide (ANP) and Brain Natriuretic Peptide (BNP)
In a comparison between patients with sarcoidosis with and without cardiac involvement, plasma levels of atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP) were higher in those with cardiac involvement than those without it, and all patients with a plasma BNP level of >100 pg/mL had cardiac involvement.113
However, BNP may not be an accurate marker to differentiate cardiac sarcoidosis from systemic sarcoidosis complicated with cardiac hypertrophy, cardiac dysfunction, atrial fibrillation, renal dysfunction, or conditions associated with aging. A report described that plasma levels of N-terminal pro brain natriuretic peptide (NT-proBNP) were higher in patients with sarcoidosis with cardiac involvement than those without it112
(Evidence level 4b, Recommendation grade C1).
c. High-Sensitivity Cardiac Troponin T (hs-cTnT) and High-Sensitivity Cardiac Troponin I (hs-cTnI)
In a study in patients with cardiac sarcoidosis to assess the usefulness of high-sensitivity cardiac troponin T (hs-cTnT) as a marker of the activity of cardiac involvement as compared with BNP, ACE, lysozyme, cardiac MRI, 67Ga scintigraphy and 18F-FDG PET, hs-cTnT was comparable with and superior to BNP in terms of sensitivity (87.5% vs 87.5%) and specificity (75% vs 50%).114
This study also reported hs-cTnT levels decreased after corticosteroid therapy in some patients, and concluded that hs-cTnT is a good marker of the activity of cardiac sarcoidosis (Evidence level 4b, Recommendation grade C1). In another study in patients with cardiac sarcoidosis where serum levels of high-sensitivity cardiac troponin I (hs-cTnI) were determined in serial samples before and after corticosteroid therapy, hs-cTnI levels decreased as cardiac function improved115
(Evidence level 5).
Also, in a study where hs-cTnT and hs-cTnI levels in patients with cardiac sarcoidosis were determined before and after corticosteroid therapy, patients with elevated hs-cTnT/I levels had significantly lower left ventricular ejection fraction and tended to have more cardiac accidents than those with normal hs-cTnT/I levels.116
These findings indicated that hs-cTnT or hs-cTnI may be used as an index of disease activity and treatment response in patients with cardiac sarcoidosis (Evidence level 4b, Recommendation grade C1). (Note: cardiac troponin test in sarcoidosis is not covered by the National Health Insurance in Japan.)
d. Urinary 8-Hydroxy-2’-Deoxyguanosine (8-OHdG)
In a study to assess the relationship between urinary 8-hydroxy-2’-deoxyguanosine (8-OHdG) levels and 18F-FDG PET findings as makers of disease activity in patients with cardiac sarcoidosis, urinary-8-OHdG was a powerful predictor of disease activity with a sensitivity of 88.2% and a specificity of 92.9%.117
In patients who responded well to corticosteroid therapy, urinary 8-OHdG levels decreased as 18F-FDG PET findings improved (Evidence level 4b).
e. Soluble Interleukin-2 Receptors (sIL-2R)
Studies have reported that serum levels of soluble interleukin-2 receptors (sIL-2R) are a useful marker of T cell activity in patients with sarcoidosis.118,119
A large-scale study in the Netherlands where serum levels of CRP, ACE, amyloid and sIL-2R were compared between patients with sarcoidosis who are receiving and not receiving treatment has reported that sIL-2R appears to be a useful marker of the severity of respiratory involvement in sarcoidosis, especially in untreated patients.120
No evidence is available as to whether sIL-2R levels reflect the severity of cardiac involvement. (Note: Currently, sIL-2R tests in sarcoidosis is not covered by the National Health Insurance in Japan).
2.2.2 Biomarkers in Diagnosing Isolated Cardiac Sarcoidosis
a. ACE and Lysozyme
In a study in 89 patients with an implantable pacemaker who have not been diagnosed as sarcoidosis affecting non-cardiac organs, screening using biomarkers including ACE and lysozyme detected cardiac sarcoidosis in 10 patients among those who could be followed up.121
In this study, serum levels of ACE and lysozyme were high in all patients diagnosed as cardiac sarcoidosis, suggesting their usefulness in the diagnosis of cardiac sarcoidosis. Of note, two of the 10 patients had positive findings of myocardial biopsy. This means that 2 (2.2%) of the 89 patients with atrioventricular block had histologically-proven isolated cardiac sarcoidosis (Evidence level 4b, Recommendation grade C1).
b. Th1-Related Cytokines
A study investigated the expression of cytokines in myocardial tissues obtained through left ventricular restoration or other cardiac surgeries from patients with histologically proven cardiac sarcoidosis as compared with control patients with idiopathic dilated cardiomyopathy.122
Patients with cardiac sarcoidosis had atrioventricular block more frequently and had smaller end-diastolic left ventricular diameters than patients with dilated cardiomyopathy. A significantly higher mRNA expression of Th1-related cytokines such as IL-1α, IL-2, IL-12 p40 and INF-γ was observed in patients with cardiac sarcoidosis. However, this study did not assess for the presence of sarcoidosis in other organs. Further studies should be conducted to assess whether Th1-related cytokines can be used as markers of isolated cardiac sarcoidosis (Evidence level 4b).
c. Myeloid-Related Protein 8/14 Complex (MRP8/14, Also Known as S100A8/A9)
In a study to measure serum levels of myeloid-related protein 8/14 complex (MRP8/14) in healthy volunteers, patients with idiopathic dilated cardiomyopathy, patients with histologically proven sarcoidosis without cardiac involvement, and patients with histologically proven cardiac sarcoidosis, serum MRP8/14 levels were significantly higher in patients with cardiac sarcoidosis than in other groups123
(Evidence level 4b).
2.3 Chest X-ray and Chest CT
Patients who are found to have pulmonary lesions of sarcoidosis (See Section “4. Pulmonary Lesions of Sarcoidosis” in Chapter II) should be examined for cardiac involvement.
Patients with cardiac sarcoidosis do not show any specific cardiac shadows or may show even only normal findings when they have no pulmonary lesions of sarcoidosis. If they have cardiac dysfunction causing ventricular enlargement or heart failure, enlarged cardiac shadow and pulmonary congestion are found on chest X-ray. Enlarged cardiac shadow is also found in patients with pericardial effusion or ventricular aneurysm.
Autopsy and detailed examination of patients with cardiac sarcoidosis often reveal epithelioid granulomas in non-cardiac organs and hilar or mediastinal lymph nodes.124
Hilar or mediastinal lymphadenopathy is an important finding of chest X-ray or chest CT, and is commonly observed in patients with cardiac sarcoidosis. A study has reported that these findings were found in about 50 to 60% of patients.109
Otsuka et al retrospectively analyzed CT images obtained from 20 patients with idiopathic dilated cardiomyopathy and 8 patients with cardiac sarcoidosis who had findings of dilated cardiomyopathy but did not have hilar lymphadenopathy on chest X-ray, and reported that mediastinal lymphadenopathy was found on chest CT specifically in patients with cardiac sarcoidosis.2
This finding indicates that it is important to examine for mediastinal lymphadenopathy in addition to hilar lymphadenopathy in the diagnosis of cardiac sarcoidosis (Evidence level 4b, Recommendation grade B).
2.4 Electrocardiogram (ECG)
Common ECG findings related to cardiac sarcoidosis include right bundle branch block and atrioventricular block due to dysfunction of specialized cardiac muscles in the cardiac conduction system, axis deviation, abnormal Q waves, ST changes, and ventricular arrhythmias reflecting damaged ordinary cardiac muscles of the left ventricle5,125–127
(Figure 21). These abnormal ECG findings are observed in more than 90% of patients diagnosed with cardiac sarcoidosis, but a few percentage of patients present with no abnormal ECG findings.128,129
ECG is essential for patients with sarcoidosis who need long-term follow-up, since a considerable number of patients are diagnosed with cardiac sarcoidosis based on ECG changes several years after the onset of non-cardiac sarcoisosis128,130
(Evidence level 4b, Recommendation grade B).
a. Right Bundle Branch Block
Right bundle branch block is a typical finding of cardiac sarcoidosis that is in about 12 to 66% of patients.126,128,131,132
Although this is not a finding specific to this condition, cardiac sarcoidosis should be suspected when this is observed in patients with non-cardiac sarcoidosis during follow-up or patients with dilated cardiomyopathy.5
b. Complete Atrioventricular Block and Advanced Atrioventricular Block
Complete or advanced atrioventricular block is found as the initial manifestation of cardiac sarcoidosis in 23% to 77.4% of patients109,125,126,128,131–134
(Evidence level 4b). About 50% of patients present with left ventricular dysfunction, but the presence of complete or advanced atrioventricular block does not correlate with the progression of left ventricular systolic dysfunction.128,131
Spontaneous improvement of complete atrioventricular block has been reported in some patients with cardiac sarcoidosis several years after pacemaker implantation.135
On the other hand, it has been reported that 11.2% to 20% of patients who underwent pacemaker implantation for the treatment of idiopathic complete atrioventricular block were diagnosed with cardiac sarcoidosis based on myocardial biopsy.98,136
c. Axis Deviation, Abnormal Q Waves, and ST Changes
Patients may present with axis deviation, abnormal Q waves and ST changes that reflect myocardial damage, although they are not specific to cardiac sarcoidosis5,126,128,137
(Evidence level 4b). Since cardiac involvement of sarcoidosis is characterized pathologically by scattered lesions that do not align with coronary blood flow,137
the location of leads showing abnormal ECG findings and their magnitudes may differ.
d. Ventricular Arrhythmia
Severe ventricular arrhythmias such as ventricular tachycardia and ventricular fibrillation are relatively common, and are observed in 23% to 36% of patients newly diagnosed as having cardiac sarcoidosis109,126,131,132,138,139
(Evidence level 4b). Many patients with cardiac sarcoidosis-related ventricular arrhythmias show thinning of the left ventricular septum or generalized left ventricular wall, causing left ventricular systolic dysfunction.131
However, ventricular arrhythmias may suddenly develop regardless of cardiac function, which can lead to sudden cardiac death in some patients. Ventricular arrhythmias in patients with cardiac sarcoidosis is considered to be caused by a micro-reentrant pathway that results from damaged myocardium.126,140
Some patients may have more than one focus of ventricular tachycardia, which may cause intractable arrhythmic storm.139,141
2.5 Echocardiography
Among various imaging techniques used in the diagnosis of cardiac sarcoidosis,142–144
echocardiography is particularly useful as a non-invasive test to detect morphological and functional abnormalities of the heart. Echocardiography is used to screen for cardiac sarcoidosis in patients with abnormal ECG findings or arrhythmias of unknown cause or to follow up patients with cardiac sarcoidosis7,8
(Evidence level 4a, Recommendation grade B), and reveals diverse findings in different stages of the disease (Figure 22).145
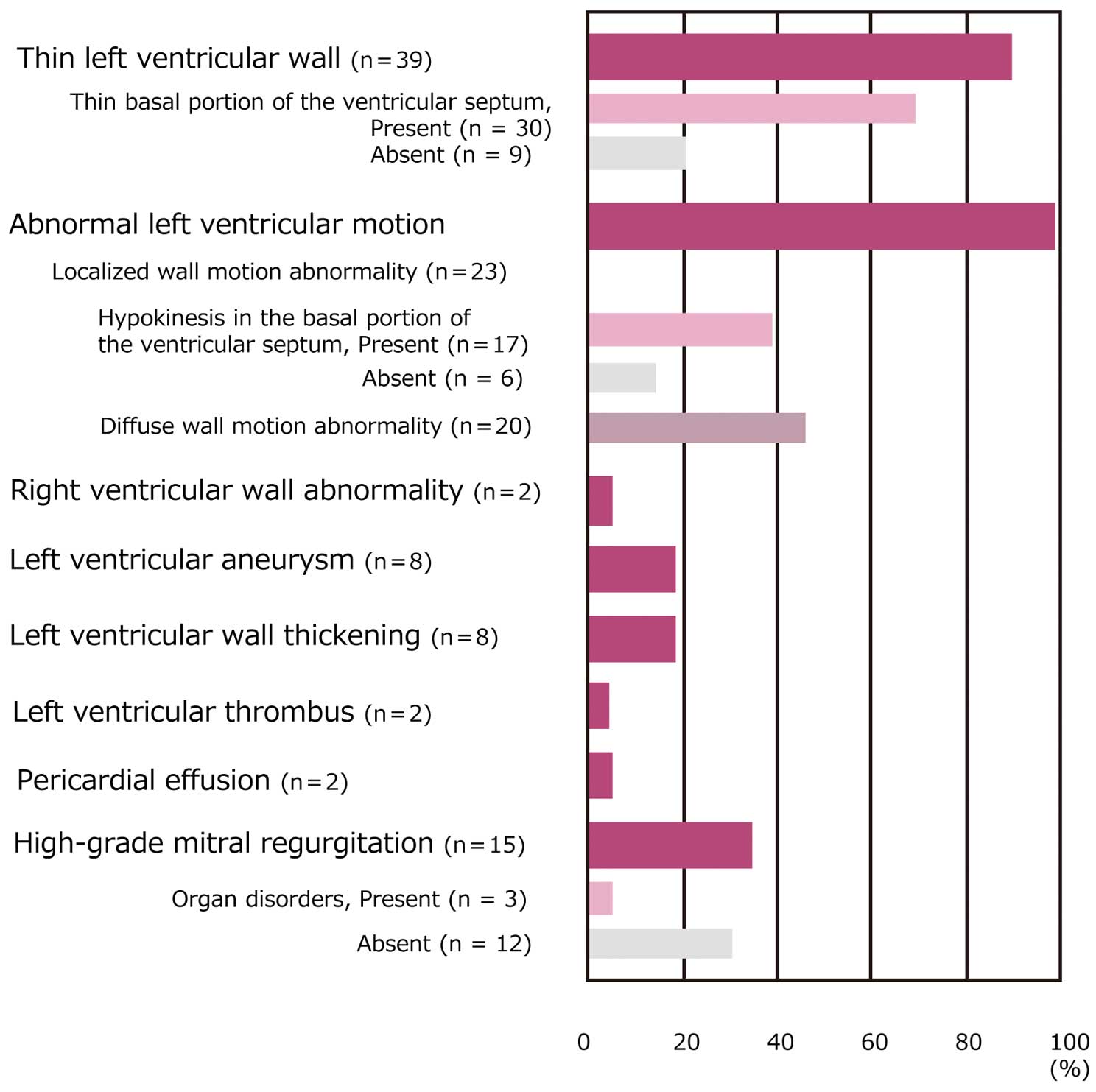
a. Ventricular Wall Thinning and Thickening
A thin basal portion of the ventricular septum is a highly specific finding in the diagnosis of cardiac sarcoidosis (Figure 23). Ventricular septal thinning, which is defined as ≤4 mm thick at 10 mm from the aortic annulus in the left ventricular long axis view, has 100% specificity and 12.6% sensitivity in the diagnosis of cardiac sarcoidosis. Another definition of ventricular septal thinning, which is defined as a ratio between the wall thickness at the same point and thickness of intact septum of ≤0.6 has 99% specificity and 35.4% sensitivity146
(Evidence level 4b). Lesions invading the basal portion of the ventricular septum may lead to atrioventricular block. Some patients with cardiac sarcoidosis showed findings mimicking hypertrophic cardiomyopathy due to substantial ventricular wall thickening147,148
(Evidence level 5), and others showed transient thickening of right ventricular free wall.149
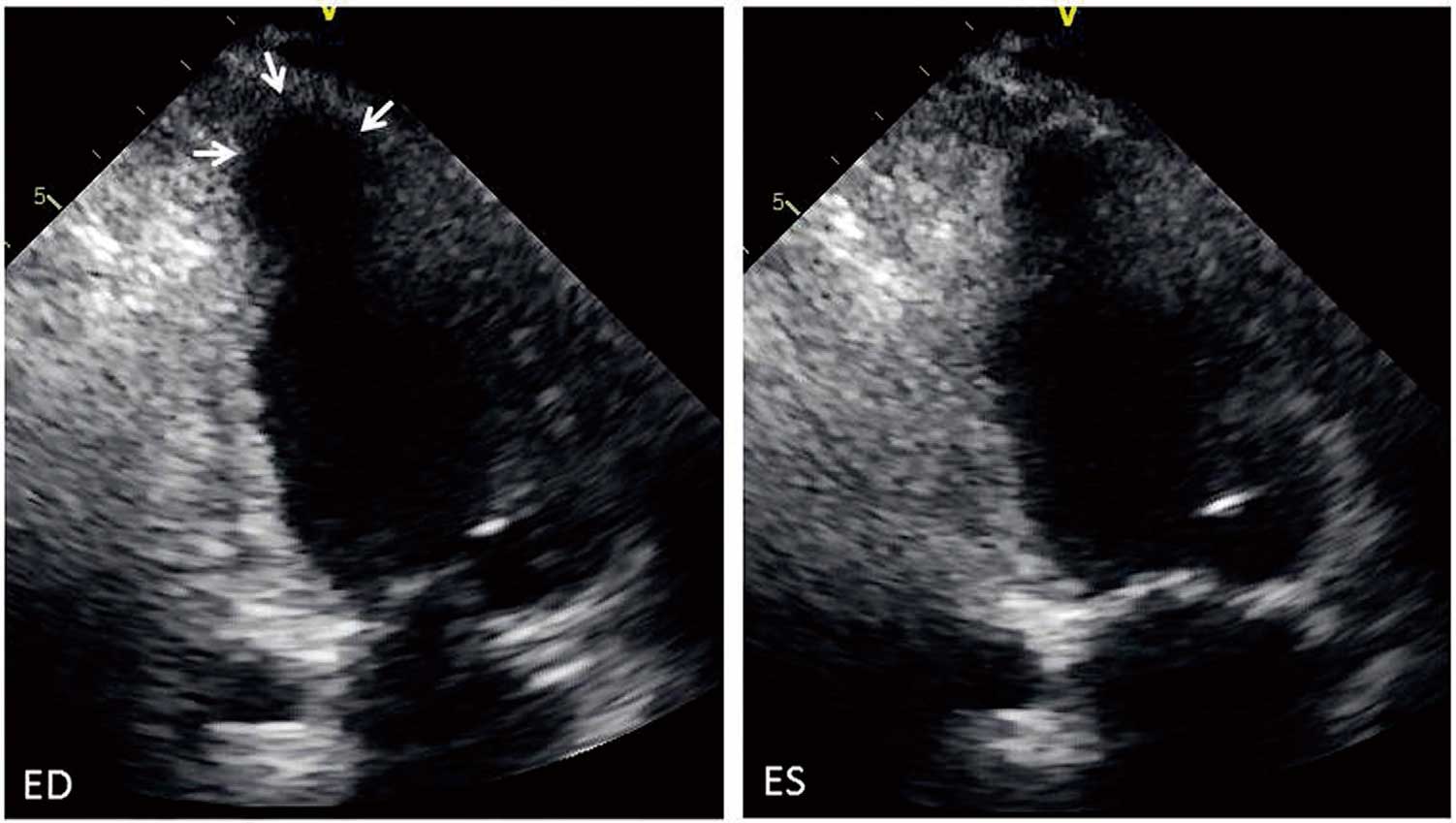
b. Localized Wall Motion Abnormalities and Diffuse Wall Hypokinesis
Cardiac sarcoidosis should be suspected in patients with localized wall motion abnormalities that cannot be explained by coronary status. In rare cases, coronary involvement may cause coronary spasm and vasculitis, leading to abnormal coronary perfusion and asynergy.150
As abnormalities may occur only in the right ventricle in some cases, right ventricular wall motion should also be observed.151
Cardiac involvement may commonly occur in the ventricular septum (especially upper septum), left ventricular posterolateral wall, left ventricular free wall including papillary muscles and right ventricular free wall, and rarely affects the atrial wall102
(Evidence level 4b).
Some patients present with findings mimicking dilated cardiomyopathy due to diffuse left ventricular myocardial infiltration. Cardiomegaly is an important prognostic factor21
(Evidence level 4b). The presence of abnormal wall thickness and uneven wall motion abnormalities suggests the presence of cardiac sarcoidosis.1
An uneven distribution of wall motion abnormalities in cardiac sarcoidosis is considered to reflect scattered epithelioid granulomas and fibrous scars. Some patients have right ventricular enlargement (Figure 24).
Patients with diffuse wall hypokinesia have enlarged cardiac chamber and decreased left ventricular ejection fraction, and may also have intracardiac thrombus. Left ventricular inflow velocity pattern shows abnormal relaxation, pseudo-normalization or restriction.
c. Ventricular Aneurysm
Ventricular aneurysm is found in 8% to 10% of patients with cardiac sarcoidosis, and may cause intractable ventricular arrhythmias (Figure 25). The aneurysm wall mainly consists of fibrous tissue, and contains no or few epithelioid granulomas.102
Corticosteroids may promote myocardial fibrosis and modify the course of aneurysm formation.
d. Pericardial Effusion
Substantial pericardial effusion due to pericardial involvement is found in 2% to 8% of patients with cardiac sarcoidosis.102
Some patients may have epithelioid granulomas in the epicardial myocardium and may also have serous effusion. Pericardial effusion may cause cardiac tamponade.152
e. Valvular Regurgitation
Systolic dysfunction and functional mitral regurgitation due to cardiomegaly may be observed (Figure 26). Infiltration of the papillary muscles or valves may play a role, but is not the main cause of valvular regurgitation.102
Papillary muscle dysfunction may cause mitral valve prolapse. In patients with valvular regurgitation complicated with atrial fibrillation develops, annulus dilation occurs and mitral or tricuspid regurgitation worsens. Severely calcified mitral annulus associated with hypercalcemia may present in some patients.102
f. Others
In patients with severe pulmonary sarcoidosis, prolonged pulmonary hypertension may show findings of cor pulmonale such as right ventricular enlargement, hypertrophy or dysfunction. It has been reported that some patients required differential diagnosis between sarcoid nodules and cardiac tumors.
g. New Technologies in the Evaluation of Cardiac Sarcoidosis
In a study, two-dimensional echocardiography revealed typical findings of cardiac sarcoidosis in 14% of patients with systemic sarcoidosis, and echocardiography was not sensitive for diagnosis of cardiac sarcoidosis.153
It has been reported that left ventricular global longitudinal strain reduced before the reduction of conventional measures such as left ventricular ejection fraction, and is useful as an independent prognostic factor for cardiac events or progression of cardiac sarcoidosis.154
2.6 Cardiac Magnetic Resonance Imaging (MRI)
Cardiac MRI, which provides images with high spatial, temporal and tissue resolution with no exposure to radiation, is considered useful in the diagnosis of cardiac sarcoidosis.155
Cardiac MRI may detect histopathological changes in patients with cardiac sarcoidosis, and may be useful in screening patients with non-cardiac sarcoidosis for cardiac involvement.
Cardiac MRI can (1) assess the function and anatomy of the heart such as cardiac function and the presence/absence of localized ventricular aneurysm and thin or thick ventricular septum156
(2) obtain histopathological information to assess for myocardial edema or fibrosis through T2-weighted black blood imaging with short TI inversion recovery (STIR) or early and late gadolinium enhancement images.
Table 8
outlines imaging protocols recommended by working groups of the Society for Cardiovascular Magnetic Resonance and the European Society of Cardiology.157,158
Typically, patients undergo cine steady-state free precession (SSFP) image, T2-weighted STIR imaging for the evaluation of myocardial edema, early gadolinium enhancement (EGE) (1 to 3 minutes after gadolinium contrast injection), and late gadolinium enhancement (LGE) (from 10 minutes after gadolinium contrast injection). Usually, about 1,000 images are recorded during a 60-minute cardiac MRI scan.
Table 8.
Preferable Imaging Module in Cardiac Sarcoidosis
| Module |
Imaging
sequences |
Imaging cross
sections |
Slice
thickness/gap |
Remarks |
Anatomical/morphological
information |
T1W BB
(TSE / FSE) |
Axial |
8 to 10 mm |
Examine from the aortic arch to the
infradiaphragmatic region. |
Information on left ventricular
function and wall motion |
Cine SSFP |
SA, VLA, HLA,
3-ch. view |
6 to 10 mm/4 to 0 mm
(slice interval=10 mm) |
Time resolution ≥45 msec |
Right ventricular function and
wall motion |
Cine SSFP |
SA, VLA, HLA,
3-ch. view |
6 to 8 mm/0 mm
(gapless) |
Time resolution ≥45 msec |
| Myocardial edema |
T2W BB (TSE / FSE
with STIR) |
SA, VLA, HLA |
≥10 mm |
Obtain images before contrast
injection. |
Pathological/histopathological
information |
EGE
3D-segmental
IR GRE |
3D: SA |
Same as Cine |
1 to 3 minutes after contrast
injection at 0.1 mmol/kg
(TI >400 msec) |
LGE
2D-/3D-segmental
IR GRE |
2D: SA
3D: SA, VLA,
HLA |
Same as Cine |
10 minutes after contrast injection
at 0.1 mmol/kg (Determine TI
according to TI Scout or LLS) |
Axial, transverse axial image; BB, black blood; EGE, early gadolinium enhanced; GRE, gradient echo; HLA, horizontal long axis (left-ventricular four-chamber view); IR, inversion recovery; LGE, late gadolinium enhancement; LLS, look-locker sequence; SSFP, steady-state free precession; SA, left ventricular short axis; STIR, short TI inversion recovery; TI, inversion time; TSE/FSE, turbo spin echo/fast spin echo; T1W, T1-weighted; T2W, T2-weighted; VLA, vertical long axis (left ventricular two-chamber view); 3-ch. view, left ventricular three-chamber view. Source: Prepared based on Kramer CM, et al. 2008.157
and Herzog B, et al. 2013.158
Table 9
outlines what should be assessed in cardiac MRI.157,158
Images obtained through multiple imaging protocols should be assessed comprehensively to review information on cardiac function and anatomy, histopathological findings and extracardiac lesions.
Table 9.
Points to Examine in MRI for Cardiac Sarcoidosis
| 1. Information on cardiac function and morphology: Evaluate mainly with cine SSFP images |
| • Use cine images to evaluate left ventricular function (EDV/ESV/EF) and, if necessary, right ventricular function. |
| • Evaluate left ventricular regional wall motion and, if necessary, right ventricular wall motion. |
• Evaluate for basal thinning of the ventricular septum, regional ventricular aneurysm, and myocardial thinning
inconsistent with coronary blood flow. |
| • Presence/absence of myocardial hypertrophy. |
| 2. Pathological information: Use T2-weighted STIR images and early and late enhancement images |
| • Changes due to myocardial edema: Use T2-weighted STIR images and early enhancement images. |
| • Late enhancement of left ventricular myocardium: Edema (pale) and fibrosis (clear). |
| • Contrast patterns: e.g., mainly in the epicardium, all layers, intermediate layer, or diffuse. |
| • Late enhancement of right ventricular myocardium: Preferably, a 3.0T instrument should be used. |
| 3. Extracardiac findings |
| • Hilar lymphadenopathy. |
| • Lung lesions. |
| • Hepatic lesions, etc. |
EDV, left ventricular end-diastolic volume; EF, left ventricular ejection fraction; ESV, left ventricular end-systolic volume.
Source: Prepared based on Kramer CM, et al. 2008.157
and Herzog B, et al. 2013.158
a. Cine MRI (Cine SSFP): Evaluation of Cardiac Anatomy and Function (Evidence level 4b, Recommendation grade B)
Cine MRI can image any imaging cross section. In the evaluation of cardiac sarcoidosis, cine MRI is usually performed with a temporal resolution of ≤45 msec to record images from the left-ventricular long axis two-chamber view, left-ventricular short axis view and left-ventricular four-chamber view. When necessary, images from the left-ventricular long-axis three chamber view and right-ventricular long-axis view should also be recorded.
Cine MRI can be used to detect typical morphological abnormalities in cardiac sarcoidosis such as localized myocardial thickening (Figure 27①a,②a, Figure 28①a, Figure 29①a,②a), ventricular aneurysm, diffuse myocardial thinning and left ventricular dilatation (dilated cardiomyopathy-like pattern).
b. T2-Weighted STIR in the Evaluation for Myocardial Edema (Evidence level 4b, Recommendation grade B)
T2-weighted short-tau inversion recovery (STIR), also called as triple IR, is a technique to delineate edema (water retention) as hyper-intense signal area by combining T2-weighted images, black blood pulse sequences that suppress blood flow signals, and STIR sequences that non-selectively suppress fat signals. The left-ventricular short axis view is commonly used, but the left-ventricular two-chamber view or left-ventricular four-chamber view should also be used to assess for apical myocardial edema. Currently, T2-weighted STIR images are assessed by visual inspection or comparison with late-enhanced images described below. Further studies should be conducted to investigate a possible relationship between T2-weighted STIR images and the activity of cardiac sarcoidosis (Figure 27①b,②b,③,④, Figure 28①b,②,③).
c. Gadolinium-Enhanced MRI in Histopathological Evaluation of Myocardium (Evidence level 4a, Recommendation grade A)
Currently, early gadolinium enhanced MRI (1 to 3 minutes after gadolinium contrast injection;
Figure 28①c, Figure 29①c,②c) and late gadolinium enhancement MRI (from 10 minutes after gadolinium contrast injection;
Figure 27①c,②c, Figure 28①d, Figure 29①d,②d). It has been reported that in patients with cardiac sarcoidosis, late enhancement was observed (1) relatively frequently in the septum near the base of the heart and in the lateral wall but in other area at lower frequencies and (2) in epicardial myocardium and in all layers of the heart,159–162
consisting with the distribution of lesions of cardiac sarcoidosis.18
(3) Cardiac sarcoidosis is also characterized by clear late enhancement, but some cases showed only modest late enhancement in the middle layer of the myocardium, which indicates that late enhancement associated with early-phase cardiac sarcoidosis may differ depending on the site of inflammation. Reports have described that (4) the area of late enhancement relates to the severity of left ventricular enlargement and left ventricular dysfunction (dilated cardiomyopathy-like pattern).163,164
It has been pointed out that late enhancement may predict the risk of cardiac accident in patients with cardiac sarcoidosis,143,165–168
multicenter studies should be conducted to confirm the hypothesis. Early enhancement (Figure 28①c, Figure 29①c,②c) is assessed for images obtained within 1 to 3 minutes after contrast injection with an inversion time (TI) of around 400 msec. However, no findings possibly related to cardiac sarcoidosis have been obtained. Further studies should be conducted to examine this matter.
d. Cardiac MRI in the Diagnosis of Isolated Cardiac Sarcoidosis (Evidence level 4b, Recommendation grade B)
The present document includes guidelines for the diagnosis of isolated cardiac sarcoidosis according to the histological diagnosis group criteria and the clinical diagnosis group criteria in Section “3.2 Isolated Cardiac Sarcoidosis”. One condition in the clinical diagnosis group criteria is late enhancement in cardiac MRI. Typical findings of late enhancement in cardiac sarcoidosis are described in the previous section. It is important to differentiate cardiac sarcoidosis from coronary artery diseases and myocardial diseases such as dilated cardiomyopathy. Findings of cardiac MRI should be evaluated together with 18F-FDG PET169,170
to assess disease activity171–174
(Figure 27③d,③e, Figure 28②a,②b).
e. Cardiac MRI in the Evaluation of Treatment Efficacy (Evidence level 4a, Recommendation grade C1)
It has been reported that change in cardiac MRI findings (e.g., normalization of myocardial hypertrophy, a decrease in late enhancement area, changes in signal intensity and disappearance of high-intensity signal in T2-weighted STIR images) were observed after starting treatment with corticosteroids and immunosuppressive drugs,175,176
but no consensus achieved regarding the usefulness of cardiac MRI as a measure to assess the appropriateness or efficacy of treatment. Further case series assessment and multicenter studies should be conducted to compare cardiac MRI with conventional measures of disease activities (biomarkers in blood and 67Ga SPECT) and 18F-FDG PET.
f. New Promising Technologies
As of Spring 2015, some MRI devices can perform non-contrast and contrast T1 mapping to determine absolute T1 values of myocardium or myocardial extracellular volume fraction using the modified look-locker inversion recovery (MOLLI) sequence,177
or measure myocardial strain that reflects the deformation of the myocardium,178
and these techniques are being increasingly used in the clinical setting. Further reports and studies on the usefulness of these techniques are awaited. Disease activity assessment using T2 mapping techniques179
is also expected to be useful, and future studies are needed.
2.7 Nuclear Imaging
a. 18F-FDG PET (Evidence level 4a, Recommendation grade A)
18F-fluorodeoxyglucose (18F-FDG) is a glucose analogue that is taken up by cells that have increased glucose use such as brain cells, myocytes, cancer cells, and inflammatory cells, and positron emission tomography (PET) delineates areas of high 18F-FDG uptake. The high 18F-FDG uptake in sarcoidosis lesions reflects infiltration of inflammatory cells such as macrophages.180,181
In order to detect lesion of cardiac sarcoidosis accurately, it is important to delineate lesions only by avoiding physiological 18F-FDG uptake by myocardium. Accordingly, detailed preparation conditions have been developed to decrease blood glucose level and increase free fatty acid levels to better delineate cardiac lesions from intact myocardium by modifying myocardial glucose uptake via the glucose fatty acid cycle.182
Specifically, it is recommended that patients should (1) fast for 12 hours before examination, (2) eat low-carbohydrate meal containing less than 5 g carbohydrate,183
and (3) receive heparin injection immediately before examination (to elevate free fatty acid levels in the blood).184
A recent report has described that longer fasting (18 hours) is more effective.185
The standardized protocol including the above-mentioned preparation conditions is summarized in the “Recommendations for 18F-FDG PET imaging for cardiac sarcoidosis” proposed by the Japanese Society of Nuclear Cardiology169,170
(Figure 30)169
(Evidence level 4a, Recommendation grade A). 18F-FDG PET images should be reviewed as follows: First, whole body scan image, such as maximum intensity projection (MIP) and transaxial view, should be examined for abnormal tracer accumulations in lymph nodes or other organs to search for systemic sarcoidosis. Next, chest images should be examined for abnormal tracer accumulation in the heart to search for cardiac sarcoidosis. Among typical cardiac 18F-FDG PET images listed in
Figure 31, photos C and D represent abnormal tracer accumulations indicative of cardiac involvement in the left ventricular myocardium. The ventricular septum and other frequently affected parts should also be examined carefully. Quantitative analysis using the standardized uptake value (SUV) provides useful information. A comparison of 18F-FDG PET images with myocardial blood flow SPECT (or PET) images that delineate myocardial injuries (perfusion defect) is very useful as well186–189
(Evidence level 4b, Recommendation grade A). 18F-FDG accumulation in or around perfusion defects strongly indicate the presence of cardiac lesions.

The following precautions have been suggested to ensure accurate diagnosis. (1) Some patients with isolated cardiac sarcoidosis may show no abnormal tracer accumulations in other organs.136,190
(2) Other conditions that cause abnormal tracer accumulation in myocardium such as ischemic heart disease,191
hypertrophic cardiomyopathy, and inflammatory myocarditis192
other than sarcoidosis should be ruled out. (3) A small number of healthy people may show localized tracer accumulations in the lateral heart wall or other parts of the heart.193–195
(4) Tracer accumulation in intact myocardium may not be inhibited in patients with severe heart failure, which may make diagnosis of cardiac sarcoidosis difficult.196,197
In a meta-analysis202
of seven recent studies,108,184,189,198–201 18F-FDG PET for detecting cardiac involvement had a sensitivity of 89% (range: 79% to 100% in the seven studies) and a specificity of 78% (38% to 100%) in 164 patients with sarcoidosis, of whom 50% had cardiac sarcoidosis. This finding indicates that 18F-FDG PET is highly sensitive for the detection of cardiac sarcoidosis, and may be useful to rule out the condition (Evidence level 4a, Recommendation grade A). However, the specificity of 18F-FDG PET differs substantially among institutions. Further studies should be conducted to address this issue. In the diagnosis and treatment of cardiac sarcoidosis, 18F-FDG PET is expected to be used in (1) assessment of disease severity and prognosis,203,204
(2) disease staging by comparing with imaging findings of myocardial injury (perfusion defects), (3) assessment of indications for and efficacy of immunosuppressive treatment using corticosteroids or other drugs,205,206
and (4) detecting flare-ups.205
b. Gallium-67 (67Ga) Citrate Scintigraphy (Evidence level 4a, Recommendation grade B)
Gallium scintigraphy using gallium-67 (67Ga) citrate has been reported to be effective in delineating not only malignant tumors but also inflammatory lesions, and has been used to detect active lesions and evaluating the efficacy of corticosteroid therapy in sarcoidosis since 1970 s.207
Typically, 48 to 74 hours after an intravenous injection of 67Ga citrate at a dose of 74 to 111 MBq, whole-body planar images are recorded. As the 67Ga tracer does not physiologically accumulate in the heart, accumulation of the tracer in the heart indicates the presence of cardiac lesions (i.e., cardiac sarcoidosis). However, the possibility of myocarditis, infective pericarditis and heart tumors should be excluded before the diagnosis of cardiac sarcoidosis. Because of its high specificity in cardiac sarcoidosis, 67Ga citrate scintigraphy has been used as a major criterion in the criteria for diagnosis of sarcoidosis. However, this technique is not sensitive and may overlook some patients.208
In order to overcome this situation, SPECT images are obtained in addition to conventional planar images.209–211
SPECT imaging, which provides superior spatial resolution in comparison of planer images, can delineate small lesions, differentiate lesions from tracer accumulation in bones, and provide data for comparison with myocardial blood flow SPECT. More recently, new techniques such as 67Ga SPECT scanning integrated with CT scanning using technetium-99 m (99 mTc)-labeled agents that can trace myocardial perfusion and hybrid SPECT/CT system are being investigated.212,213
67Ga-planar images have a specificity of 80% to 100% and a sensitivity of as low as 0% to 36%.208
In a recent multicenter study in 66 patients, the sensitivity was 48.5%.109
On the other hand, recent studies have reported that 67Ga SPECT images have a specificity of 54% to 100% and a sensitivity of 64% to 77%,186,201,212,213
showing an improvement in sensitivity (Evidence level 4b). These findings indicate that 67Ga SPECT images may help increase sensitivity of diagnosing cardiac sarcoidosis, but many studies have pointed out its lower sensitivity as compared with 18F-FDG PET.109,186,189,201
Gallium scintigraphy, a feasible and available technique for many medical institutions, has been used widely, but physicians must be aware of its limitations in the diagnosis of cardiac sarcoidosis (Evidence level 4a, Recommendation grade B).
c. Myocardial Perfusion Scintigraphy
Myocardial perfusion scintigraphy is a technique that can detect (1) localized myocardial injury at rest imaging, and (2) reduction of regional myocardial perfusion reserve at stress and rest imaging. Tracers used in this technique include thallium-201 chloride (Tl), technetium-99 m (99 mTc) methoxy isobutyl isonitrile (MIBI), and 99 mTc-tetrofosmin (99 mTcTF). Many studies of myocardial perfusion scintigraphy in cardiac sarcoidosis published in 1970 s to 1990 s used Tl.209–211,214–216
Recently, studies using 99 mTc-MIBI and 99 mTcTF are conducted to obtain evidence in current practice.217–219
Resting myocardial perfusion scintigraphy can detect localized perfusion defects that reflect regional myocardial injuries in cardiac sarcoidosis.214
Perfusion defects that are inconsistent with coronary artery territories, or occur in the ventricular septum which is often affected by sarcoidosis, are important findings to suspect cardiac sarcoidosis. Myocardial perfusion scintigraphy is generally used in combination with the above-mentioned inflammatory imaging techniques such as 67Ga scintigraphy and 18F-FDG PET to contribute to better diagnosis of cardiac sarcoidosis186,188,209,211,217
(Evidence level 4a, Recommendation grade B). In a study that compared Tl with a 99 mTc-labeled tracer (99 mTc-sestamibi), the latter was better in sensitivity for the diagnosis of myocardial sarcoidosis (46% vs. 65%).218
Studies have reported that reverse redistribution, a perfusion detect that develops or becomes more evident at rest imaging as compared with exercise or dipyridamole imaging, develops frequently in patients with cardiac sarcoidosis.209,216,218–221
A report has pointed out that reversible microvascular constriction in coronary arteriole surrounding granulomas is the most likely explanation of this phenomenon.216
However, further studies should be carried out to assess its diagnostic value.
2.8 Cardiac Catheterization
2.8.1 Contrast Left Ventriculography and Coronary Angiography
Sarcoidosis involving the coronary arteries are very rare,222,223
but coronary lesions as well as other non-ischemic myocardial diseases should be ruled out before the diagnosis of cardiac sarcoidosis. Like in cases of dilated cardiomyopathy and secondary cardiomyopathies, coronary angiography is indicated for patients with angina in whom treatment for myocardial ischemia may improve the condition (Evidence level 2, Recommendation grade A), and patients who have findings suggestive of myocardial ischemia do not have angina (Evidence level 3, Recommendation grade B). Accordingly, patients without symptoms or ECG findings suggestive of myocardial ischemia should first undergo non-invasive examinations such as exercise ECG, stress scintigraphy and coronary CT.224–228
As cardiac resynchronization therapy should be considered for patients with advanced heart failure not responding to drug treatment, it is important during coronary angiography to evaluate the venous phase extensively in addition to the arterial phase to specify the optimal location to place the left ventricular pacing lead.224
During contrast left ventriculography, left ventricular volume and regional wall motion should be evaluated in a manner similar to that for patients with dilated cardiomyopathy and secondary cardiomyopathies.224
It has been reported that patients with cardiac sarcoidosis may show regional wall motion abnormalities that cannot be explained by coronary status20
(Evidence level 4b), and left ventricular aneurysm develops in 8% to 10% of patients.229
Accordingly, echocardiography, cardiac CT, cardiac MRI and contrast left ventriculography may be used in the diagnosis of cardiac sarcoidosis (Figure 32).190,230–234
Left ventricular aneurysm without coronary artery disease should be examined to rule out hypertrophic cardiomyopathy, Chagas disease, arrhythmogenic right ventricular cardiomyopathy, chronic myocarditis, glycogen storage disease and idiopathic left ventricular aneurysm.235
Left ventricular aneurysm in cardiac sarcoidosis is an important finding as it has been reported that the presence of ventricular aneurysm predicts poor clinical outcome in cardiac sarcoidosis.236

2.8.2 Myocardial Biopsy
Percutaneous transvenous endomyocardial biopsy was developed by Konno and Sakakibara in 1962,237
and has been used worldwide. As epithelioid granulomas in cardiac sarcoidosis develop sporadically in the myocardium, it has been reported that only 20% of patients with epithelioid granulomas can be correctly diagnosed with an endomyocardial biopsy because biopsy samples do not contain granulomatous tissues.99
The positive detection rate in endomyocardial/myocardial biopsy is much lower in patients with normal heart function. A correct histological diagnosis is difficult to make in patients at an early stage of cardiac sarcoidosis.238,239
The “Guidelines for Management of Dilated Cardiomyopathy and Secondary Cardiomyopathy” proposed by the Japanese Circulation Society and other guidelines recommend that myocardial biopsy should be performed within 2 weeks after the onset of heart failure in patients with normal systolic function and no cardiomegaly (Evidence level 2, Recommendation grade A).224,240
Myocardial biopsy should also be considered for patients findings mimicking dilated cardiomyopathy associated with ventricular aneurysm of unknown cause (Evidence level 2, Recommendation grade B).241
As in any other invasive percutaneous techniques, endomyocardial biopsy has a risk of complications such as ventricular perforation, bundle branch block, cerebral embolism, vascular injury, and chordal rupture causing worsening of valvular regurgitation. The most serious complication associated with endomyocardial biopsy is cardiac tamponade due to ventricular wall perforation. In 1998, a national survey report has described that perforation occurred in 0.7% and death occurred in 0.05% in a total of 19,964 cases of percutaneous intravenous cardiac biopsy.242
When echocardiographically proven pericardial effusion, hypotension and tachycardia develop in association with a catheter-based procedure, echocardiographically-guided or open pericardiocentesis should be performed.243
Mortality of left ventricular wall perforation is 12.9% and that of right ventricular wall perforation is 5.2%.
However, histological diagnosis is important in cardiac sarcoidosis. A recent study has reported that electroanatomic voltage mapping improves the diagnostic yield of myocardial biopsy.244
Histological assessment of myocardial biopsy samples should be conducted whenever possible to understand the concept of cardiac sarcoidosis and create more effective treatment strategies (Evidence level 4b, Recommendation grade B).
a. Gross Pathological Findings of Cardiac Sarcoidosis
It is often difficult to differentiate cardiac sarcoidosis from other types of dilated cardiomyopathy based only on gross pathological findings. However, some cases of cardiac sarcoidosis may have yellowish or grayish white patchy granuloma lesions in the myocardium, or substantial thinning of the anterior half of the ventricular septum due to scarring (See Section “1. Pathology”).245
b. Histopathological Findings of Cardiac Sarcoidosis
In cardiac sarcoidosis, different levels of lymphocyte infiltrations may be found in myocardial stroma. Cases of significant cell infiltration should be examined to rule out chronic myocarditis.7,8
Samples containing multinucleated giant cells should be examined to differentiate between granulomatous inflammatory diseases including sarcoidosis, giant cell myocarditis, and non-specific foreign body reactions. It should be noted that some patients with cardiac sarcoidosis may not have typical granulomatous lesions but may only have scar-like fibrosis resulting from inflammation.245
Since sarcoidosis may show different types of histological feature, it is relatively easy to make a pathological diagnosis in patients with typical findings but may be difficult in some cases. Physicians should be aware of the importance of comprehensive clinical-radiological-pathological (CRP) diagnosis246
(Evidence level 5, Recommendation grade C1).
c. Diagnosis of Cardiac Sarcoidosis Using Immunostaining
In addition to commonly used staining procedures such as hematoxylin-eosin (HE) staining, immunostaining procedures using antibodies to macrophages and other inflammatory cells,
P. acnes, and tenascin C (an extracellular matrix protein) are used in diagnosing pulmonary sarcoidosis or differentiating it from other diseases63,247
(Evidence level 4b, Recommendation grade C1).
2.8.3 Electrophysiological Testing
Ventricular tachycardia is the second common ECG sign of cardiac sarcoidosis next to atrioventricular block, and is found in about 23% of patients.248
It is important to assess for ventricular tachycardia, which affects the prognosis of patients significantly, in order to determine optimal treatment strategies. Atrial arrhythmia is found in about 19% of patients with cardiac sarcoidosis.
a. Purpose of Electrophysiological Testing
Electrophysiological testing is performed to assess the prognosis of patients with cardiac sarcoidosis by evaluating the inducibility of ventricular arrhythmia. This test is performed to assess the risk of sudden death in asymptomatic patients or patients presenting with atrioventricular block. In patients presenting with ventricular tachycardia, this test is usually performed during ablation for the treatment of ventricular tachycardia to investigate its mechanisms (Evidence level 4a, Recommendation grade B). Ventricular tachycardia associated with cardiac sarcoidosis is reentry ventricular tachycardia in most patients,249
but some patients with cardiac conduction disorder have been found to have non-reentry ventricular tachycardia originating from Purkinje fiber.250
It has been reported that the inducibility of ventricular tachycardia does not always relate to the activity of sarcoidosis assessed with 67Ga scintigraphy, and that patients complicated with ventricular tachycardia tend to have poorer left ventricular function than those complicated with atrioventricular block.248,251
It also has been indicated that patients with late gadolinium enhancement in cardiac MRI have higher risk of ventricular tachycardia and sudden death,165
and that implantable cardioverter-defibrillators (ICDs) may reduce the risk of sudden death in these patients.252
Electrophysiological testing may also be used to assess the efficacy of drug treatment. A report described that patients with cardiac sarcoidosis with ventricular tachycardia underwent electrophysiological testing to assess the inducibility of ventricular tachycardia before and after drug treatment including corticosteroids to determine the timing when drug treatment may be terminated.253
However, it is difficult to assess the efficacy of treatment as the condition may recur even when no abnormal findings were found in pharmaceutical stress test.141
b. Prognosis Assessment Based on Electrophysiological Findings
It has been reported that patients suspected to have cardiac sarcoidosis in whom sustained ventricular arrhythmias was induced in electrophysiological testing have higher risk of sudden death and ICD therapies (relative hazard 4.47).254
In patients presenting without spontaneous sustained ventricular arrhythmias, those in whom ventricular arrhythmias were induced during electrophysiological testing have a 6.97-fold higher risk of sudden death and ICD therapies as compared those in whom no ventricular arrythmias were induced. It is also reported that more than 50% of patients with spontaneous and inducible ventricular tachycardia needed ICD therapies within 1 year after ICD implantation.
A considerable percentage of patients with sarcoidosis who have no cardiac symptoms or abnormal ECG findings are diagnosed as have cardiac sarcoidosis based on the findings of imaging studies such as cardiac MRI and 18F-FDG PET. A report has described that prognosis assessment is quite important in this patient population.255
In this study, about 10% of patients assessed were inducible for sustained ventricular arrhythmias during electrophysiological testing and received an ICD. During the follow-up period for about 5.5 years, ventricular tachycardia or death developed in 75% of patients with inducible ventricular arrhythmia and in 1.5% of patients without it (Figure 33).255
Average left ventricular ejection fraction was 36.4% in patients with inducible ventricular arrhythmia, and 55.8% in patients without it. Accordingly, electrophysiological testing may be performed in patients with a left ventricular ejection fraction of >35% to assess the risk of sudden cardiac death (Figure 34) (Evidence level 4a, Recommendation grade C1).

2.9 Future Prospects: Genetic Assessment
(See also Section 2 “Etiology and Pathophysiology of Sarcoidosis” in Chapter II)
Cardiac sarcoidosis is considered a multifactorial disorder involving immune dysfunction driven by complex networks of cytokines and chemokines. Genetic studies have been conducted to identify genetic factors possibly associated with sarcoidosis. Further studies are expected to reveal key factors for more accurate diagnosis and successful treatment.
a. HLA Genes and Susceptibility to Sarcoidosis Manifestation
The human leukocyte antigen (HLA) gene is one of the most polymorphic genes in the human genome. Among the HLA genes which are essential for recognizing non-self,
HLA-DRB1
alleles in the HLA class II region are known to be strongly associated with sarcoidosis.256–258
However, HLA-DQB1*0501 is prevalent in Japanese patients with cardiac sarcoidosis but is not prevalent in Western patients. HLA alleles differ substantially among races. For example, the DRB1*401–DQB1*301 haplotype was found to be significantly protective in UK cohort (OR 0.54,
P=0.008) but a risk factor for sarcoidosis manifestation in Japanese patients (OR 7.49,
P=0.03).256
This finding indicates unique HLA associations with sarcoidosis in Japanese, which suggests that different HLA alleles and haplotypes associate with sarcoidosis in different races, organs, and disease types (Evidence level 4b).
b. Other Susceptibility Factors
A genome-wide association study (GWAS) in Germany has reported that a splice variant of the BTNL2 gene encoding butyrophilin-like protein 2, which was identified as a T-cell activation inhibitor, is associated with sarcoidosis manifestation.38
A GWAS project in Japanese has revealed a strong association between HLA class II region on chromosome 6 with sarcoidosis susceptibility at P=1.1×10−16
and also an association between
BTNL2
and sarcoidosis.259
Since, association between BTNL2 gene polymorphisms and sarcoidosis susceptibility has been found in African American and Greek patients, it is highly likely that HLA class II region is associated with susceptibility to sarcoidosis, and that
HLA-DRB1
and
BTNL2
are sarcoidosis susceptibility genes in all races.38,260
Studies also indicate that annexin A11 (ANXA11) gene and
XAF1
may also play a role as sarcoidosis susceptibility genes to contribute to the modification of immune cell activity.40,261
TNF-α is known as a cytokine involved in the activation of macrophages, and anti-TNF-α antibodies have been suggested to be effective in the treatment of uveitis and other conditions associated with sarcoidosis. The allele A of TNF-α-308G/A polymorphism has been suggested to increase the risk of sarcoidosis in Asian people and Caucasian people, and be associated particularly with the susceptibility to cardiac sarcoidosis. It has been reported that responsiveness to TNF inhibitors differs substantially among the three genotypes (GG, GA and AA) of this polymorphism.262
This difference should be considered when anti-TNF-α antibody therapy is tried for the treatment of cardiac sarcoidosis (Evidence level 5).
c. Assessment of the Effects of Th17-IL-23 on Immune Cell Dysfunction
Th17 cells were identified as an independent T cell subset different from the conventional T cell subsets Th1 and Th2, and were found to play an important role in T-cell dependent immune responses.263
Studies have suggested that Th17 cells in tissues or peripheral blood may be used as a marker of active sarcoidosis, and is attracting attention as a new marker or treatment target.264,265
Detailed genetic analyses such as genome-wide association studies and protein complex studies have revealed complex associations between genetic variants and sarcoidosis which may explain different prevalence of organ involvement among races. In the future, the patient’s genotype will be considered in the diagnosis to make better treatment strategies for individual patients.
3. Diagnostic Guidelines
3.1 Cardiac Sarcoidosis
Up until now, three guidelines for the treatment of cardiac sarcoidosis have been reported internationally. The first one is the guidelines created in Japan in 1992 and revised in 2006 (Evidence level 6).6–8
The second one is the guideline document published by the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) in 1999 as the sarcoidosis assessment instrument developed by the steering committee of A Case Control Etiologic Study of Sarcoidosis (ACCESS)266
(Evidence level 4b) and revised in 2014.267
In this document, a diagnosis of cardiac sarcoidosis is confirmed by the presence of histologically-proven granulomatous inflammation of the heart. The third one is the guideline document proposed by the Heart Rhythm Society (HRS) in the United States in 2014107
(Evidence level 6) and is basically consistent with the concept of the ACCESS assessment instrument. The clinical diagnosis group criteria in this guideline is expected to be applied to patients with histologically-proven sarcoidosis in organs other than the heart.
Making a diagnosis of cardiac sarcoidosis is not easy in many cases. In fact, it is difficult to differentiate it from dilated cardiomyopathy,1–3,224
chronic myocarditis and giant cell myocarditis,4
and myocarditis associated with systemic diseases. Some patients are not diagnosed with cardiac sarcoidosis before histological examination of myocardium obtained at autopsy, heart transplant, or left ventricular restoration reveals it. The “Guidelines for the diagnosis of cardiac sarcoidosis” proposed by Hiraga et al in 1992 were prepared based on cases of cardiac granulomas found in myocardial biopsy or autopsy.5
However, as the guidelines requests physicians to histologically prove the presence of epithelioid granuloma in some organs, some patients with clinical manifestations strongly suggestive of sarcoidosis may not be diagnosed as having the disease. To address this issue, the Japan Society of Sarcoidosis and Other Granulomatous Disorders and the Japanese College of Cardiology collaborated with other academic societies to revise the guidelines in 2006, and published the “Diagnostic Standard and Guideline for Sarcoidosis- 2006”.6
In the 2006 version of the “Guidelines for the Diagnosis of Cardiac Involvement in Patients with Sarcoidosis” and the “Diagnostic Guidelines for Cardiac Manifestations of Cardiac Sarcoidosis” in the “Guidelines for Diagnosis and Treatment of Myocarditis (JCS 2009)”,7,8
clinical findings that are typical or frequently observed in patients with cardiac sarcoidosis are weighed and included in the major criteria for diagnosis.
As described in Section “3. Diagnosis of Sarcoidosis” in Chapter II, sarcoidosis is a condition of unknown cause that leads non-caseating epithelioid granulomas with high Th1 responses in different organs. In the diagnosis of sarcoidosis, it is important to confirm the presence of (1) non-caseating epithelioid granulomas, (2) organ-specific clinical findings, and (3) laboratory findings specific to sarcoidosis. Accordingly, in the diagnosis of cardiac sarcoidosis, it is important to diagnose the presence of cardiac involvement (cardiac sarcoidosis) in patients diagnosed as having sarcoidosis in other organs according to the clinical diagnosis group or clinical diagnosis group criteria. On the contrary, patients with findings strongly suggestive of cardiac sarcoidosis should be examined carefully for involvement in other organs.267
In 2014, the “Diagnostic criteria for sarcoidosis” was revised by the Japan Society of Sarcoidosis and Other Granulomatous Disorders and the MHLW’s diffuse pulmonary disease study group to allow patients in whom non-caseating epithelioid granulomas in the myocardium were found through endomyocardial biopsy or cardiac surgery and other possible diagnoses were clearly ruled out to be diagnosed as having cardiac sarcoidosis on the basis of the histological diagnosis group criteria.67,268
The clinical diagnosis group criteria for sarcoidosis were also revised to emphasize the presence of lesions in the respiratory tract, eyes and heart in the clinical diagnosis of sarcoidosis (see Section “3.1 Diagnostic Standard of Sarcoidosis” in Chapter II).
Recent advancements of imaging techniques such as 18F-FDG PET, cardiac MRI, and echocardiography and accumulated clinical experience have helped physicians diagnose cardiac sarcoidosis more correctly. The WASOG and the HRS have proposed international guidelines for the diagnosis of sarcoidosis. Cases of isolated cardiac sarcoidosis with no involvement in organs other than the heart have been reported, and physicians have shared the clinical importance of this condition. Considering these situations, in 2014 and 2015, the Guideline Writing Group reviewed the “Guidelines for the Diagnosis of Cardiac Involvement in Patients with Sarcoidosis” in the “Diagnostic Standard and Guideline for Sarcoidosis - 2006” and proposed here the “Diagnostic guidelines for cardiac sarcoidosis” (Table 10,
Evidence level 6).
Table 10.
Diagnostic Guidelines for Cardiac Sarcoidosis
| Clinical findings defining cardiac involvement |
Cardiac findings should be assessed based on the major criteria and the minor criteria. Clinical findings that satisfy the following 1) or 2)
strongly suggest the presence of cardiac involvement. |
(See subsection c. “Clinical findings defining cardiac involvement” in the section II.3.2 “Clinical findings suggestive of sarcoidosis involving
different organs”) |
| 1) Two or more of the five major criteria (a) to (e) are satisfied |
| 2) One in the five major criteria (a) to (e) and two or more of the three minor criteria (f) to (h) are satisfied. |
| Criteria for cardiac involvement |
| 1. Major criteria |
(a) High-grade atrioventricular block (including complete atrioventricular block) or fatal ventricular arrhythmia (e.g., sustained ventricular tachycardia,
and ventricular fibrillation) |
(b) Basal thinning of the ventricular septum or abnormal ventricular wall anatomy (ventricular aneurysm, thinning of the middle or upper
ventricular septum, regional ventricular wall thickening) |
| (c) Left ventricular contractile dysfunction (left ventricular ejection fraction less than 50%) or focal ventricular wall asynergy |
| (d) 67Ga citrate scintigraphy or 18F-FDG PET reveals abnormally high tracer accumulation in the heart |
| (e) Gadolinium-enhanced MRI reveals delayed contrast enhancement of the myocardium |
| 2. Minor criteria |
(f) Abnormal ECG findings: Ventricular arrhythmias (nonsustained ventricular tachycardia, multifocal or frequent premature ventricular contractions),
bundle branch block, axis deviation, or abnormal Q waves |
| (g) Perfusion defects on myocardial perfusion scintigraphy (SPECT) |
| (h) Endomyocardial biopsy: Monocyte infiltration and moderate or severe myocardial interstitial fibrosis |
| Diagnostic guidelines for cardiac sarcoidosis |
| 1) Histological diagnosis group (those with positive myocardial biopsy findings) |
Cardiac sarcoidosis is diagnosed histologically when endomyocardial biopsy or surgical specimens demonstrate non-caseating epithelioid
granulomas (See Note (6)). |
| 2) Clinical diagnosis group (those with negative myocardial biopsy findings or those not undergoing myocardial biopsy) |
The patient is clinically diagnosed as cardiac sarcoidosis (1) when epithelioid granulomas are found in organs other than the heart, and
clinical findings strongly suggestive of the above-mentioned cardiac involvement are present; or (2) when the patient shows clinical findings
strongly suggestive of pulmonary or ophthalmic sarcoidosis; at least 2 of the five characteristic laboratory findings of sarcoidosis (Table 1)
(see section 3.1. “Diagnostic standard of sarcoidosis” in Chapter II); and clinical findings strongly suggest the above-mentioned cardiac
involvement. |
| Note |
(1) When differentiation from ischemic heart disease should be made, coronary examination (coronary angiography, coronary CT, or cardiac
magnetic resonance) should be performed. |
(2) As cardiac involvement of sarcoidosis may be found several years after the diagnosis of extracardiac sarcoidosis, patients should be
followed up with periodic ECG and echocardiography. |
| (3) Some patients may suffer from isolated cardiac sarcoidosis. |
(4) As nonspecific (physiological) tracer uptake in the myocardium may occur, 18F-FDG PET imaging should be conducted with optimal
imaging conditions. 18F-FDG PET imaging should be conducted according to “the Recommendations for 18F-fluorodeoxyglucose positron
emission tomography imaging for cardiac sarcoidosis”169,170 proposed by the Japanese Society of Nuclear Cardiology. |
(5) As endomyocardial biopsy has a low sensitivity in detecting non-caseating epithelioid granulomas, several biopsy specimens should be
collected to ensure that an accurate diagnosis is made. |
(6) When endomyocardial biopsy or surgical specimens demonstrate non-caseating epithelioid granulomas in the myocardium, and when
granulomas due to other causes and local sarcoid reactions can be ruled out, patients are categorized into the histological diagnosis
group (see section 3.1. “Diagnostic Standard of Sarcoidosis” in Chapter II). |
(7) Physicians should be aware that in Japan the National Health Insurance currently covers the use of 18F-FDG PET scan for “patients with
cardiac sarcoidosis in whom inflammation sites must be located.” |
This new guideline document describes criteria for “histological diagnosis (patients with a positive myocardial biopsy)” and “clinical diagnosis (patients with a negative myocardial biopsy or patients not undergoing biopsy)”. The Guideline Writing Group also newly created the “Diagnostic guidelines for isolated cardiac sarcoidosis” (Table 11) and a flowchart for the diagnosis of cardiac sarcoidosis (Figures 35,36).
Table 11.
Diagnostic Guidelines for Isolated Cardiac Sarcoidosis
| Prerequisite |
1. No clinical findings characteristics of sarcoidosis are observed in any organs other than the heart (The patient should be examined in
detail for respiratory, ophthalmic, and skin involvements of sarcoidosis. When the patient is symptomatic, other etiologies that can affect
the corresponding organs must be ruled out.). |
| 2. 67Ga scintigraphy or 18F-FDG PET reveals no abnormal tracer accumulation in any organs other than the heart. |
3. A chest CT scan reveals no shadow along the lymphatic tracts in the lungs or no hilar and mediastinal lymphadenopathy (minor axis
>10 mm). |
| 1) Histological diagnosis group |
Isolated cardiac sarcoidosis is diagnosed histologically when endomyocardial biopsy or surgical specimens demonstrate non-caseating
epithelioid granulomas. |
| 2) Clinical diagnosis group |
Isolated cardiac sarcoidosis is diagnosed clinically when the criterion (d) and at least three other criteria of the major criteria (a) to e) are
satisfied (Table 10). |
| Note |
(1) When the patient meets at least four criteria for cardiac involvement other than the criterion (d), or when the patient meets the criteria (b)
and (d) plus one of the remaining criteria, the patient should be suspected to have isolated cardiac sarcoidosis. |
(2) When the patient is strongly suspected to have isolated cardiac sarcoidosis that may pose a risk of death according to clinical diagnosis,
he/she may be treated empirically with corticosteroids or immunosuppressive drugs before a confirmatory diagnosis is made. |
(3) Coronary artery disease and other inflammatory myocardial diseases (e.g., chronic myocarditis, giant cell myocarditis, and myocarditis
associated with systemic disorders) should be ruled out. |
(4) As nonspecific (physiological) 18F-FDG uptake in the myocardium may occur, 18F-FDG PET imaging should be conducted with optimal
imaging conditions. 18F-FDG PET imaging should be conducted according to “the Recommendations for 18F-fluorodeoxyglucose positron
emission tomography imaging for cardiac sarcoidosis”169,170
proposed by the Japanese Society of Nuclear Cardiology. |
(5) As endomyocardial biopsy has a low sensitivity in detecting non-caseating epithelioid granulomas, several biopsy specimens should be
collected whenever possible to make histological diagnosis, which helps the physician plan the optimal treatment strategy reliably. |
(6) Physicians should be aware that in Japan the National Health Insurance currently covers the use of 18F-FDG PET scan for “patients with
cardiac sarcoidosis in whom inflammation sites must be located.” |
(7) Cases of isolated cardiac sarcoidosis that are categorized into the clinical diagnosis group are consistent with those “suspected to have
sarcoidosis” in the diagnostic standard for sarcoidosis (see section 3.1. “Diagnostic Standard of Sarcoidosis” in Chapter II). |

The key changes from the 2006 version are as follows. The reader should refer to the individual sections for details.
1) Considering the importance of fatal ventricular arrhythmia (e.g., sustained ventricular tachycardia and ventricular fibrillation),139,269,270
these conditions were included in the major criteria where high-grade atrioventricular block is listed.
2) Abnormal ventricular wall anatomy (ventricular aneurysm, thinning of the middle or upper ventricular septum, regional ventricular wall thickening), which is considered clinically significant in diagnosing cardiac involvement, was moved from the minor criteria to the major criteria where basal thinning of the ventricular septum is listed.
3) Abnormally high tracer accumulation in the heart in 18F-FDG PET is considered an important finding that reflects the activity of inflammation in cardiac sarcoidosis198,200,201
and was moved from the remarks to the major criteria.
4) The late-gadolinium enhancement (LGE) of the myocardium in gadolinium-enhanced MRI was moved from the minor criteria to the major criteria as it is considered an important index of tissue damage and fibrosis in cardiac sarcoidosis.143,163,271,272
5) Remarks are added to describe important points and a supplemental explanation.
3.2 Isolated Cardiac Sarcoidosis
As a systemic disorder, physicians had to confirm lesions in at least two different organs (including lymph nodes) to make a diagnosis of the disease according to the criteria published in Japan in 2006.6
However, there have been reported cases of non-caseating epithelioid cell granuloma and/or monocyte infiltration found in myocardial biopsy, and cases of clinical findings consistent with cardiac sarcoidosis in patients with no involvement other than the heart.134,136,171,190,273–283
These cases are referred to as isolated cardiac sarcoidosis. Isobe et al described that this condition may develop as (1) the initial lesion of sarcoidosis with no involvement in other organs (2) a possible type of sarcoidosis affecting the heart only, or (3) the detection of findings consistent with sarcoidosis only in the heart without findings in other organs because inflammation is too modest to detect or typical findings cannot be detected for other reasons.171
It has been estimated that cases of isolated cardiac sarcoidosis account for 5 to 15% of patients who are confirmed or suspected to have cardiac sarcoidosis.134,283
However, the following three points should be considered to interpret these estimations: (1) As isolated cardiac sarcoidosis does not satisfy the criteria for the diagnosis of sarcoidosis in 2006, physicians might not report these patients as cases of sarcoidosis. (2) Non-caseating epithelioid granuloma may not be proven in many myocardial biopsy samples obtained from patients with cardiac sarcoidosis. In a study of patients with relatively severe cardiac sarcoidosis who meet the conventional diagnostic standard, cardiac sarcoidosis is histologically diagnosed only in 10 to 17%.99
In other studies in patients suspected to have isolated cardiac sarcoidosis, the corresponding percentage was as low as 10%.273,274
(3) This patient population may include those presented with cardiac sarcoidosis who have not been evaluated for sarcoidosis in other organs. The above items (1) and (2) may cause underestimation and (3) may cause overestimation of the prevalence of isolated cardiac sarcoidosis.
Reports have described that cardiac involvement leads to about two-thirds of deaths in patients who died from sarcoidosis in Japan.17,284
In a multicenter retrospective survey in patients with cardiac sarcoidosis in Japan, corticosteroid therapy is considered to improve long-term prognosis more in patients with better left ventricular systolic function than those with poorer function.21
These findings suggest earlier treatment intervention after the detection of cardiac involvement may contribute to a better prognosis of patients with sarcoidosis. As isolated cardiac sarcoidosis is considered not to differ from cardiac sarcoidosis affecting other organs as well in terms of pathophysiology and prognosis,171
it is therefore important to start appropriate treatment without delay. In this guideline, we propose the “Diagnostic guidelines for isolated cardiac sarcoidosis” (Table 11). As a prerequisite, patients should be confirmed not to have clinical findings of sarcoidosis affecting other organs. Myocardial biopsy is important in considering treatment strategies. The diagnosis of isolated cardiac sarcoidosis may be confirmed when non-caseating epithelioid granulomas are found in myocardial tissues (Evidence level 5, Recommendation grade B). Patients with clinical findings suggestive of isolated cardiac sarcoidosis in whom myocardial biopsy is impossible or did not reveal the presence of granulomas in myocardial tissues should be evaluated according to the “Diagnostic guidelines for isolated cardiac sarcoidosis” (Evidence level 6, Recommendation grade C1). Patients who meet at least 4 items including (d) in the five major criteria (a) to (e) in the cardiac findings in the “Diagnostic guidelines for cardiac sarcoidosis” (Table 10) should be diagnosed as isolated cardiac sarcoidosis according to the clinical diagnosis group criteria.
Case reports on isolated cardiac sarcoidosis are still limited in number, and only a little evidence is available. Some cases may not be differentiated clearly from other types of inflammatory myocardial diseases or cardiomyopathies according to this guideline document. Physicians should carefully monitor over time and observe for other clinical findings to make a diagnosis.
3.3 Diagnostic Procedures (Flowcharts)
a. Follow-up Evaluation of Patients Diagnosed With Sarcoidosis Affecting Non-Cardiac Organs (e.g., Lungs, Skin, or Eyes) (Figure 35)107,285,286
Basically, patients should be followed up periodically through standard 12-lead ECG, which is an economical, convenient, and repeatable procedure. Echocardiography and Holter ECG may also be used in combination with 12-lead ECG as these procedures may detect abnormalities in patients with normal 12-lead ECG findings. When the 12-lead ECG detects arrhythmias such as atrioventricular block, bundle branch block, axis deviation, and premature ventricular contractions during follow-up, the patient should be consulted by a cardiologist and should undergo detailed examinations such as 18F-FDG PET, 67Ga scintigraphy and cardiac MRI in addition to echocardiography and Holter ECG. Findings of 18F-FDG PET and 67Ga scintigraphy should be assessed in comparison with findings of myocardial blood flow SPECT.169,170
Patients with abnormal findings on these imaging studies should be considered for cardiac catheterization to rule out coronary artery diseases, evaluate the disease condition, and obtain endomyocardial biopsy samples. Patients who have minor abnormal ECG findings without apparent abnormalities on imaging or invasive studies should be observed carefully and periodically through echocardiography, Holter ECG, or other appropriate methods.
Physicians should follow-up patients with cardiac sarcoidosis carefully as cardiac function may deteriorate rapidly within months or a year in some patients.287,288
b. How to Examine for Cardiac Sarcoidosis During Evaluation of Patients Presenting With Myocardial Diseases or Arrhythmias of Unknown Cause107,286 (Figure 36)
Cardiac sarcoidosis should be suspected for middle-aged or elderly women who have complete atrioventricular block or bifascicular block (e.g., right bundle branch block plus left axis deviation), basal thinning of the ventricular septum, co-presence of ventricular wall thinning and thickening and/or uneven wall motion (e.g., localized ventricular aneurysm).1,98
Imaging studies such as 18F-FDG PET, 67Ga scintigraphy, cardiac MRI, and myocardial blood flow SPECT or endomyocardial biopsy should be considered for such patients, but they should first undergo systemic evaluation for the presence of sarcoidosis in other organs.
Attending physicians (1) should consult with respiratory physicians, ophthalmologists, and dermatologists to evaluate for extracardiac lesions and (2) should evaluate whether the patient has at least 2 of the five laboratory findings typical for sarcoidosis (Table 1, Figure 36) (see Section “3.1 Diagnostic Standard of Sarcoidosis” in Chapter II). Specifically, chest CT should be conducted to evaluate for hilar or mediastinal lymphadenopathy, and serum levels of ACE, lysozyme, and sIL-2R should be determined. In 18F-FDG PET or 67Ga scintigraphy, whole-body images but not limited to chest images should be obtained as tracer accumulations may be found in the hilar or mediastinal lymph nodes, skin, subcutaneous nodules, skeletal muscle or lymph nodes. Tracer accumulations at sites where biopsy samples can be obtained will help physicians to make a histological diagnosis of sarcoidosis. Respiratory physicians should also be consulted to consider bronchoalveolar lavage fluid examination and transbronchial lung biopsy. Patients diagnosed with sarcoidosis should then be evaluated according to the flowchart for patients with sarcoidosis based on extracardiac lesions (Figure 35). When they meet the “Diagnostic guidelines for cardiac sarcoidosis,” the diagnosis of cardiac sarcoidosis is confirmed. Patients who were not diagnosed with sarcoidosis in other organs should be evaluated according to the “Diagnostic guidelines for isolated cardiac sarcoidosis”. It should be noted that some patients may not meet the prerequisite in the “Diagnostic guidelines for isolated cardiac sarcoidosis” but may be suspected to have cardiac sarcoidosis as they meet some of the criteria in the “Diagnostic guidelines for cardiac sarcoidosis”. Examples of such patients include those who have eye lesions but do not show typical laboratory findings and those who have no extracardiac lesions but show typical laboratory findings such as accumulations of 67Ga or 18F-FDG in the mediastinum. Since these patients may have cardiac sarcoidosis, appropriate treatment according to their disease conditions and careful follow-up are necessary.
Physicians should also note that the diagnostic criteria for cardiac sarcoidosis and the definition of isolated cardiac sarcoidosis in Japan differ from those in Western countries. In Western countries, diagnosis should be made histologically, while in Japan, diagnosis based only on clinical findings is allowed.136,171
Kandolin et al do not consider the presence/absence of mediastinal lymph node lesions as a prerequisite for isolated cardiac sarcoidosis and handle patients with positive mediastinal lymph node biopsy as cases of isolated cardiac sarcoidosis. Western reports have described that endomyocardial biopsy guided with imaging studies such as 18F-FDG PET and cardiac MRI136
or intracardiac potentials107
may improve the detection rate of biopsy.
IV. Treatment of Cardiac Sarcoidosis
1. Pharmacotherapy
1.1 Immunosuppressive Therapy
Pharmacotherapy for cardiac sarcoidosis mainly consists of immunosuppressants that are used for controlling inflammation and thereby improve clinical symptoms.289
Corticosteroids are widely used as first-line immunosuppressants for patients with cardiac sarcoidosis.290
The “Views on the treatment of sarcoidosis - 2003” published in 20039
describe that corticosteroid therapy should be considered for patients with cardiac sarcoidosis who have high-grade atrioventricular block, ventricular arrhythmias, or cardiac dysfunction (Evidence level 6, Recommendation grade C1). Although no placebo-controlled, prospective studies have demonstrated that corticosteroids may improve prognosis of cardiac sarcoidosis, clinical experience in patients showing improvement in clinical findings after starting corticosteroids has suggested their benefits.87,290
Reports have described that prognosis was better when corticosteroid therapy for cardiac sarcoidosis was initiated before rather than after the occurrence of cardiac dysfunction21,291
(Evidence level 4b, Recommendation grade C1). However, further evaluation should be undertaken to determine whether corticosteroid therapy should be introduced for patients with only mild manifestations of cardiac sarcoidosis such as abnormal ECG findings or localized late enhancement in cardiac MRI.166
On the other hand, corticosteroid therapy is considered less effective for patients who have severe cardiac dysfunction but do not show abnormal accumulation of 67Ga or 18F-FDG in the myocardium, but a report has suggested that corticosteroids therapy should be initiated regardless of whether myocardial tracer accumulation is observed or not.292
There are no established protocols regarding how to initiate, taper, or maintain prednisolone therapy in patients with cardiac sarcoidosis.290
According to the “Views on the Treatment of Sarcoidosis - 2003,” oral corticosteroid therapy for sarcoidosis should be started with the initial dose of 30 mg daily (0.5 mg/kg daily) as or 60 mg every other day (1.0 mg/kg every other day) prednisolone equivalent for the first 4 weeks. Then the dose should be reduced by 5 mg daily or 10 mg every other day at intervals of 2 to 4 weeks to maintain at 5 to 10 mg daily or 10 to 20 mg every other day (Evidence level 6, Recommendation grade C1) (Figure 37).9
Although a retrospective study in patients with cardiac sarcoidosis has reported that the prognosis did not differ between patients receiving prednisolone at an initial dose of 30 mg/day and ≥40 mg/day,21
some patients with active and rapidly progressing cardiac sarcoidosis have started corticosteroid therapy at high initial doses or underwent corticosteroid pulse therapy.293,294
Since there are no reliable markers or imaging modalities that reflect the activity of cardiac sarcoidosis,295
corticosteroid doses are reduced automatically. It is not easy to assess the progression and flare of cardiac involvement of sarcoidosis, but physicians determine the doses of corticosteroids for individual patients based on imaging studies such as 67Ga scintigraphy and 18F-FDG PET and clinical findings. Some patients may discontinue corticosteroid therapy, but many patients continue corticosteroid therapy for a long period of time (Evidence level 6, Recommendation grade C1).

Second-line immunosuppressive therapy for patients with extracardiac involvement of sarcoidosis such as those with intractable pulmonary sarcoidosis has been tried using cyclophosphamide,296
cyclosporine,297
azathioprine,298
methotrexate,299
thalidomide,300
hydroxychloroquine,301
pentoxifylline,302
and mycophenolic acid,303
among others (Evidence level 5). These drugs may be used as monotherapy or in combination with corticosteroids in patients who do not respond well to corticosteroids or cannot use them or increase their dose due to adverse drug reactions,290,304
but the use of these second-line drugs in cardiac sarcoidosis has only been described in case reports. Low-dose methotrexate is most commonly used for this purpose in Japan (Evidence level 5, Recommendation grade C1) and is expected to have a steroid-sparing effect or alleviate the severity of adverse drug reactions to corticosteroids.91
However, physicians should carefully observe for adverse drug reactions such as decreased white blood cell count, hepatic dysfunction and interstitial pneumonia. The recommended dose of methotrexate in Western countries is 10 to 20 mg/week,290
but methotrexate is often administered at doses of 5 to 8 mg/week in Japan.91,305
A report has described that cardiac function in patients with cardiac sarcoidosis was better maintained in patients who received methotrexate in combination with prednisolone than patients who started treatment with prednisolone monotherapy.305
Further studies are needed.
Recently, infliximab, an anti-TNF-α antibody that relieves inflammation by blocking TNF-α in the blood, inhibiting TNF-α to bind to receptors, and destroying TNF-α-producing cells has been tried as the third treatment option for pulmonary sarcoidosis in Western countries306
(Evidence level 5). Infliximab may be used for patients who do not respond to corticosteroids and at least one second-line therapy.290
Case reports have described the effectiveness of infliximab in patients with cardiac sarcoidosis.307,308
1.2 Treatment of Heart Failure
a. Basic Policies for Heart Failure Management
No clinical research has focused on the management of cardiac failure in patients with cardiac sarcoidosis. Accordingly, treatment should consist of immunosuppressive therapy for the treatment of cardiac sarcoidosis and basic heart failure management regardless of the stage of heart failure (Evidence level 6, Recommendation grade C1). Patients with heart failure associated with cardiac sarcoidosis should receive pharmacological treatment generally recommended based on evidence for patients with left ventricular systolic dysfunction, such as ACE inhibitors, angiotensin II receptor blockers, beta-blockers, and aldosterone antagonists, as well as immunosuppressants, for the treatment of sarcoidosis.309
Additional drugs should be given to patients with signs of congestions and lower cardiac output. Vasodilators and diuretics are used for the treatment of congestion, and inotropic drugs such as catecholamines are used for the treatment of low cardiac output. Patients who do not respond well to these drugs may need mechanical circulatory support. Antiarrhythmic drugs and pacemakers should be used for patients with arrhythmias that cause unstable cardiac rhythm or heart rate and may affect hemodynamics. Patients with hypoxemia due to pulmonary congestion should be treated with oxygenation and artificial respiration.310
b. Heart Failure Management Based on Disease Stages and Types
As indicated in the ACCF/AHA heart failure classification system,311
appropriate treatment to prevent disease progression is important for the treatment of heart failure in patients with cardiac sarcoidosis. Corticosteroids are less effective in patients with advanced heart dysfunction.21
Also, once myocardial damage in sarcoidosis occurs, left ventricular remodeling continues to progress even when the inflammation caused by sarcoidosis is alleviated. Heart failure should be treated according to the guidelines309–311
for the treatment of heart failure in general.
Left ventricular systolic dysfunction is the most common type of abnormal heart pumping function in patients with cardiac sarcoidosis.224
Patients with activated myocardial inflammation may show edematous wall thickening, which is similar to those observed in hypertrophic cardiomyopathy.156
However, no manifestations of heart failure due to diastolic dysfunction are found in many cases, and treatment of the underlying cardiac sarcoidosis is the main component of clinical management for patients with heart failure associated with cardiac sarcoidosis. Cases of cardiac sarcoidosis affecting the right ventricle with or without left ventricular involvement have been reported. In some such cases, differentiation from arrhythmogenic right ventricular cardiomyopathy is important.312
Patients with right and left ventricular involvement need more complex management such as careful fluid management and biventricular circulatory support.310
1.3 Treatment of Arrhythmias
<Indications for corticosteroid therapy for the treatment of tachyarrhythmia and bradyarrhythmia associated with cardiac sarcoidosis>
Class I
Patients with cardiac involvement demonstrated by positive 67Ga scintigraphy or 18F-FDG PET findings (Evidence level 4a, Recommendation grade A)
Class IIa
Patients without cardiac involvement demonstrated by positive 67Ga scintigraphy or 18F-FDG PET findings (Evidence level 4b, Recommendation grade C1) |
<Indications for antiarrhythmic drugs for the treatment of tachyarrhythmia associated with cardiac sarcoidosis>
Class I
None
Class IIa
(1) Beta blockers (Evidence level 6, Recommendation grade C1)
(2) Amiodarone, sotalol, and class Ib* antiarrhythmic drugs for the treatment of uncontrollable ventricular tachycardia (Evidence level 6, Recommendation grade C1)
Class IIb
(1) Amiodarone, sotalol, and verapamil for the treatment of controllable ventricular tachycardia (Evidence level 6, Recommendation grade C2)
(2) Class Ia* and Ic* antiarrhythmic drugs for the treatment of uncontrollable ventricular tachycardia (Evidence level 6, Recommendation grade C2)
Class III
Class Ia* and Ic* antiarrhythmic drugs for the treatment of controllable ventricular tachycardia (Evidence level 6, Recommendation grade D) |
*Vaughan Williams Classification
Atrioventricular block is the most frequent initial manifestation of arrhythmia associated with cardiac sarcoidosis,6
and fatal ventricular arrhythmia may often develop. Patients with advanced cardiac sarcoidosis may have cardiac dysfunction resulting from loss of cardiac myocytes, which may cause secondary tachyarrhythmia. Accordingly, antiarrhythmic drugs should be selected based on the remaining cardiac function and activity of cardiac sarcoidosis as well as the indications for non-pharmacological treatments such as pacemakers and ICD (Evidence level 6, Recommendation grade C1).
As it has been reported that corticosteroids may improve atrioventricular conduction in patients with atrioventricular block associated with cardiac sarcoidosis,87,248,313
the activity of inflammation should be evaluated whenever possible. Since tachyarrhythmia often develops in patients with poor cardiac function and corticosteroid therapy is important in maintaining cardiac function,292
corticosteroids should also be considered for patients without findings of inflammation. Ventricular arrhythmia may often recur even after successful ablation,270,314
patients should continue antiarrhythmic treatment.249,250,315
As arrhythmias associated with cardiac sarcoidosis are often originated from scar tissues or result from reentry,138,316
physicians may consider beta blockers (Evidence level 6, Recommendation grade C1) and drugs that prolong repolarization (e.g., amiodarone and sotalol) (Evidence level 6, Recommendation grade C1) for the treatment of arrhythmia associated with cardiac sarcoidosis but should try beta blockers to control heart failure as well (Evidence level 6, Recommendation grade C1). Calcium-channel blockers (verapamil) are not recommended (Evidence level 6, Recommendation grade C2). Sodium-channel blockers, especially Vaughan-Williams class Ia* and Ic* antiarrhythmic drugs, are not recommended for patients with poor cardiac function as they have proarrhythmic properties.317
The antiarrhythmic effects of corticosteroids are very limited in patients with severe cardiac dysfunction.318
2. Non-Drug Therapy
2.1 Permanent Pacing
<Indications for permanent pacing for the treatment of atrioventricular block>
Class I
(1) Patients with second-degree, advanced or third-degree atrioventricular block with symptoms of bradycardia (Evidence level 6, Recommendation grade B).
(2) Patients with advanced or third-degree atrioventricular block who experience profound bradycardia or long-lasting ventricular
arrest during wakefulness (Evidence level 6, Recommendation grade B).
Class IIa
(1) Asymptomatic, persistent third-degree atrioventricular block (Evidence level 6, Recommendation grade C1)
(2) Asymptomatic, second-degree or advanced atrioventricular block with at least one of the following conditions (Evidence level 6, Recommendation grade B):
① When the block occurs at or below the bundle of His;
② when the block is associated with progressive cardiomegaly due to bradycardia;
③ when exercise or atropine sulfate does not change or impair atrioventricular conduction.
(3) Patients who have symptoms that are likely due to bradycardia, who have first-degree atrioventricular block without other
causes, and in whom the block occurs at or below the bundle of His (Evidence level 6, Recommendation grade B).
Class IIb
Patients with first-degree atrioventricular block and heart failure in whom hemodynamics may be improved by setting the optimal
atrioventricular interval (Evidence level 6, Recommendation grade C2). |
<Treatment guidelines for permanent pacemaker implantation in patients with cardiac sarcoidosis>
(1) It is advisable that physicians refer to the “Guidelines for Non-Pharmacotherapy of Cardiac Arrhythmia (JCS 2011)” published
by the Japanese Circulation Society319,320 to determine whether permanent pacemaker implantation is indicated for their patients
with cardiac sarcoidosis (Evidence level 6, Recommendation grade A).
(2) Biventricular cardiac pacing rather than right ventricular pacing should be selected for patients with poor cardiac function with
a LVEF ejection fraction of ≤35% (Evidence level 6, Recommendation grade B).
(3) Immunosuppressive drugs are expected to be effective in the treatment of conduction disturbance in some patients with cardiac
sarcoidosis, but it is difficult to predict whether individual patients will respond to the treatment or not. It is desirable that patients
who are indicated for permanent pacemaker implantation undergo pacemaker implantation before starting immunosuppressive
therapy (Evidence level 6, Recommendation grade B).
(4) Patients who are indicated for pacing should be considered for ICD implantation to prevent sudden death (Evidence level 6, Recommendation grade C2). |
Evidence level and recommendation grade are based on the classification scales in the reference articles 319 and 320.
Atrioventricular block is a common complication found in 26 to 67% of patients with cardiac sarcoidosis,285
and is caused by basal thinning or granuloma formation of the ventricular septum, or ischemia due to the involvement of the atrioventricular nodal artery.102
Indications for permanent pacemaker implantation should basically be considered according to the “Guidelines for Non-Pharmacotherapy of Cardiac Arrhythmias (JCS 2011)” proposed by the Japanese Circulation Society319,320
based on the cardiac function of individual patients. Also, CRT should be considered for patients who have a LVEF of ≤35%, are planned to undergo pacemaker implantation for the management of bradycardia, and are expected to frequently depend on ventricular pacing. In the BLOCK HF study, CRT significantly reduced the mortality and incidence of heart failure events.321
CRT should be strongly considered for patients with atrioventricular block and poor cardiac function.
Although many studies have reported that corticosteroid therapy is effective in the treatment of atrioventricular block in patients with cardiac sarcoidosis,149,313,322
no randomized clinical studies of immunosuppressive drugs were conducted in this patient population. Considering the difficulty in predicting the efficacy of treatment, the risk of recurrence, and the risk of device infection during treatment with oral corticosteroids, corticosteroid therapy should be initiated after device implantation.
ICD is indicated for patients with ventricular arrhythmia. Patients with atrioventricular block should be observed carefully since fatal ventricular arrhythmias have developed in patients with cardiac sarcoidosis before ICD implantation.323
ICD as the primary prevention strategy for atrioventricular block in patients with cardiac sarcoidosis has not been evaluated in randomized clinical studies. Further studies are awaited.
2.2 Implantable Cardioverter Defibrillator (ICD)
a. Risk Assessment for Sudden Death
Patients with cardiac sarcoidosis are at risk of sudden death due to ventricular arrhythmia,140,324
and ICD is indicated for those with a high risk of sudden death. It has been reported that patients with a history of life-threatening arrhythmias are at risk of recurrent arrhythmias and that the incidence of life-threatening arrhythmias is high among patients with poor cardiac function with no history of serious arrhythmias.325–327
ICD implantation is recommended for patients with a LVEF of ≤35% even under appropriate pharmacotherapy.328,329
Also, care should be taken for patients with modestly mild ventricular dysfunction with a LVEF of ≤36 to ≤49% when they have right ventricular dysfunction (right ventricular ejection fraction (RVEF): <40%). Reports have described the benefits of electrophysiological testing in risk assessment for sudden death in patients with cardiac sarcoidosis.254,255
It has been reported that the risk of death, sudden death, or appropriate ICD discharge is high in patients with late enhancement in cardiac MRI,143,330
but some reports have pointed out that imaging findings are not useful in risk assessment.108
An association between positive findings on 18F-FDG PET and the risk of death or sustained ventricular tachycardia has been reported,204
but this has not been investigated in larger populations.
b. Indications of ICD
<Indications of ICD for patients with cardiac sarcoidosis>
Class I
(1) Patients with a history of cardiac arrest or sustained ventricular tachycardia (Evidence level 4a, Recommendation grade B).
(2) Patients with a LVEF of ≤35%, and either (a) who have non-sustained ventricular tachycardia (ICD is not covered by the NHI
in Japan) or (b) in whom sustained ventricular tachycardia or ventricular fibrillation was induced during electrophysiological
testing** (Evidence level 4a, Recommendation grade C1).
Class IIa
(1) Patients with a LVEF of ≤35% even with optimal pharmacotherapy, including immunosuppressive therapy, (ICD is not covered
by the NHI in Japan) (Evidence level 4a, Recommendation grade C1).
(2) Patients with syncope of unknown cause, and either (a) who have non-sustained ventricular tachycardia (ICD is not covered
by the NHI in Japan) or (b) in whom sustained ventricular tachycardia or ventricular fibrillation was induced during
electrophysiological testing** (Evidence level 4a, Recommendation grade C1).
(3) Patients with a LVEF of <50%, who are indicated for pacemaker implantation, and either (a) who have non-sustained
ventricular tachycardia (ICD is not covered by the NHI in Japan) or (b) in whom sustained ventricular tachycardia or ventricular
fibrillation was induced during electrophysiological testing** (Evidence level 4a, Recommendation grade C1).
(4) Patients with a LVEF of <50%, and either (a) late enhancement in cardiac MRI, (b) positive findings of 18F-FDG PET or (c) 67Ga-
scintigraphy, and in whom sustained ventricular tachycardia or ventricular fibrillation was induced during electrophysiological
testing** (Evidence level 4a, Recommendation grade C1). |
**Sustained ventricular tachycardia is defined as having a duration of ≥30 seconds. Induction of VT/VF by 3 consecutive extrastimuli at a coupling interval of <220 msec is handled as a non-specific reaction.
NHI coverage: According to the rule issued on April 1, 1996, in Japan, the National Health Insurance covers the use of implantable cardioverter defibrillator (ICD) therapy based on the confirmation of spontaneous or induced hemodynamically compromising ventricular tachycardia or fibrillation. It should be noted that this rule is not consistent with the classification of recommendations in the present guideline document.
Indications of ICD should be determined based on whether the patient has had life-threatening arrhythmias, and, if absent, (1) left ventricular dysfunction, (2) nonsustained ventricular tachycardia, and (3) inducibility of arrhythmias during electrophysiological testing (Figure 38). The above descriptions are similar to those described in the HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis published by the Heart Rhythm Society,107
but there are differences in terms of severity classification of cardiac dysfunction and indications of ICD in patients indicated for pacemaker implantation. However, physicians should be aware that the risk of life-threatening arrhythmias is high among patients with atrioventricular block who were waiting for pacemaker implantation.323,325
Such patients should be monitored periodically.
2.3 Cardiac Resynchronization Therapy (Defibrillator) [CRT(D)]
<Indications of CRT for patients with cardiac sarcoidosis>
Class I
Patients with NYHA class III or ambulatory class IV chronic heart failure despite optimal drug therapy, with a LVEF of ≤35%, a
QRS interval of ≥120 msec, and sinus rhythm (Evidence level 6, Recommendation grade B).
Class IIa
(1) Patients with NYHA class III or ambulatory class IV chronic heart failure despite optimal drug therapy, a LVEF of ≤35%, a
QRS interval of ≥120 msec, and atrial fibrillation (Evidence level 6, Recommendation grade B).
(2) Patients with NYHA class III or ambulatory class IV chronic heart failure despite optimal drug therapy, a LVEF of ≤35%, and
who are using or plan to use a permanent pacemaker for the treatment of bradycardia and require or are expected to
require ventricular pacing frequently (Evidence level 6, Recommendation grade B).
Class IIb
Patients with NYHA class II chronic heart failure despite optimal drug therapy, a LVEF of ≤35%, and who plan to use a
permanent pacemaker for the treatment of bradycardia and are expected to require ventricular pacing frequently
(Evidence level 6, Recommendation grade C2).
Class III
(1) Asymptomatic patients with low LVEF who are not indicated for pacemaker implantation for the treatment of bradycardia
(Evidence level 6, Recommendation grade C2).
(2) Patients with restricted physical activity due to chronic diseases other than heart failure or those with a life expectancy of
12 months or less (Evidence level 6, Recommendation grade C2). |
<Indications of CRT-D in patients with cardiac sarcoidosis>
Class I
Patients with NYHA class III or ambulatory class IV chronic heart failure despite optimal drug therapy, a LVEF of ≤35%, a QRS
interval of ≥120 msec, and sinus rhythm, and who are indicated for ICD (Evidence level 6, Recommendation grade A).
Class IIa
(1) Patients with NYHA class III or ambulatory class IV chronic heart failure despite optimal drug therapy, a LVEF of ≤35%, a
QRS interval of ≥120 msec, and atrial fibrillation, and who are indicated for ICD (Evidence level 6, Recommendation grade B).
(2) Patients with NYHA class II chronic heart failure despite optimal drug therapy, a LVEF of ≤30%, a QRS interval of ≥150
msec, and sinus rhythm, and who are indicated for ICD (Evidence level 6, Recommendation grade B).
(3) Patients with NYHA class III or ambulatory class IV chronic heart failure despite optimal drug therapy, a LVEF of ≤35%, and
who are using or plan to use ICD and require or are expected to require ventricular pacing frequently (Evidence level 6, Recommendation grade B).
Class IIb
Patients with NYHA class II chronic heart failure despite optimal drug therapy, a LVEF of ≤35%, and who plan to use ICD and
are expected to require ventricular pacing frequently (Evidence level 6, Recommendation grade C2).
Class III
(1) Asymptomatic patients with low LVEF who are not indicated for ICD (Evidence level 6, Recommendation grade C2).
(2) Patients with restricted physical activity due to chronic diseases other than heart failure or those with a life expectancy of 12
months or less (Evidence level 6, Recommendation grade C2). |
In patients with heart failure due to cardiac sarcoidosis, dyssynchrony often occurs between an atrium and a ventricle, between the left and right ventricles, or in a ventricle in addition to intraventricular conduction disturbance. CRT is used to treat these conditions. Large-scale studies331–333
have revealed that CRT may prevent the progression of heart failure and improve the prognosis of patients. LVEF and the QRS interval of body surface ECG are important measures to predict the efficacy of CRT. In Japan, a wide QRS interval is defined as a QRS interval of ≥120 msec.319,320
Reports on the benefits of CRT in patients with cardiac sarcoidosis are limited. Accordingly, indications of CRT for patients with cardiac sarcoidosis are described based on the “Guidelines for Non-Pharmacotherapy of Cardiac Arrhythmias” proposed by the Japanese Circulation Society.319,320
The “HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis” proposed by the Heart Rhythm Society in 2014107
also recommends physicians to apply the guidance on device implantation to patients with cardiac sarcoidosis.
2.4 Catheter Ablation
a. Mechanism of Ventricular Tachycardia
Ventricular tachycardia develops as the initial sign of cardiac sarcoidosis in about 23% of patients248
and may lead to sudden death in some patients. Reentry is the most common mechanism of ventricular tachycardia in patients with cardiac sarcoidosis, but triggered activity and abnormal automaticity have also been reported.138,318,334
Ventricular tachycardia arising from the Purkinje fiber develops through triggered activity and is common among patients with conduction disorder.250
It is considered that there is no relationship between arrhythmogenesis and disease activity evaluated by 67Ga scintigraphy in cardiac sarcoidosis.248
b. Characteristics of Arrhythmogenic Substrates
It is important to identify arrhythmogenic substrates through late enhancement cardiac MRI before catheter ablation and 3D-mapping system during the procedure. In many patients, right ventricular scarring is confluent while left ventricular scarring is patchy.249
Epicardial scarring that requires epicardial ablation is found in about 30% of patients.249
Patients without endocardial or epicardial scarring are considered to have scarring deep inside the myocardium.
c. Indications of Ablation
<Indications of catheter ablation for patients with cardiac sarcoidosis> (Evidence level 5, Recommendation grade B)
Class I
(1) Patients with ventricular tachycardia uncontrollable with corticosteroids or antiarrhythmic drugs
(2) Patients with paroxysmal ventricular tachycardia who cannot take antiarrhythmic drugs
(3) Patients with or without ICD who experience storms of ventricular tachycardia. |
Catheter ablation is indicated for patients in whom ventricular tachycardia cannot be controlled with corticosteroids or antiarrhythmic drugs, patients who cannot tolerate drug treatment, and patients who experience storms of ventricular tachycardia. Ventricular tachycardia due to triggered activity or abnormal automaticity may be controlled with corticosteroid therapy.318,334
d. Acute-Phase Results (Table 12)249,250,270,335,336
Table 12.
Results of Ablation Therapy for the Treatment of Ventricular Tachycardia Associated With Cardiac Sarcoidosis
| Reports |
No. of
patients |
Mean
LVEF (%) |
No.of VT
events |
Complete elimination
(cases) |
Decreased
(cases) |
No.of recurrent
cases |
Follow-up
period |
| Koplan BA, et al.270 |
8 |
34 |
32 |
2/8 |
4/8 |
6/8 |
6 to 84 months |
| Jefic D, et al.335 |
9 |
42 |
44 |
5/9 |
3/9 |
4/9 |
19.8 months |
| Dechering DG, et al.336 |
8 |
36 |
|
5/8 |
|
|
6 months |
| Kumar S, et al.249 |
21 |
36 |
99 |
9/21 |
10/21 |
18/21 |
1 to 9.5 years |
| Naruse Y, et al.250 |
14 |
40 |
37 |
8/14 |
|
6/14 |
24 to 46 months |
LVEF, left ventricular ejection fraction; VT, ventricular tachycardia.
During catheter ablation, abnormalities other than symptomatic ventricular tachycardia are often found, and about 50% of patients require more than one ablation procedure. The most common reasons for unsuccessful ablation include ventricular tachycardia arising from the ventricular septum, diffuse right ventricular scarring with more than one reentry circuit, and tachycardia arising adjacent to a coronary artery or the His bundle. Kumar et al have reported that a single ablation procedure was enough to ablate all arrhythmogenic substrates of ventricular tachycardia in about 40% of patients, and more than one ablation procedure was needed in about 30% of patients to control ventricular tachycardia. Ablation terminated ventricular tachycardia storms not responding to pharmacotherapy in 71% of patients.249
Patients with ventricular tachycardia not responding to ablation, corticosteroids, and antiarrhythmic drugs are at a high risk of death and often require heart transplantation.
e. Long-Term Results
Many patients have more than one arrhythmogenic substrate causing ventricular tachycardia, and the incidence of recurrent arrhythmia after catheter ablation relates to the number of arrhythmogenic substrates where ventricular arrhythmia was induced.
Since ventricular arrhythmia may recur even in patients with successful ablation, the progression of cardiac sarcoidosis cannot be predicted, and the fibrosis of myocardium may continue, patients should undergo ICD implantation even after successful ablation (Evidence level 5, Recommendation grade B).
3. Surgical Treatment
3.1 Left Ventricular Restoration and Mitral Valvuloplasty
a. Feasibility of Surgical Treatment
In patients with cardiac sarcoidosis, granulomas lead to myocardial degeneration and then fibrosis. Myocardial injury may develop in any part of the heart. Some patients may have focal myocardial injuries, and many patients often have multifocal myocardial injuries.102
Focal injuries may progress in different patterns in different patients. Some patients may have left ventricular aneurysm, and other patients may show dilated cardiomyopathy-like findings associated with diffuse myocardial injuries. Patients with focal injuries may undergo resection and plasty to improve the shape and function of the left ventricle. As left ventricular dilation may lead to tethering the mitral valve and cause functional mitral valve insufficiency, mitral valvuloplasty should also be considered. Late gadolinium enhancement MRI and 18F-FDG PET have helped physicians identify whether lesions are focal or diffuse. Ventricular function may improve when appropriate procedures of left ventricular restoration are selected according to the location of myocardial lesions and conducted in combination with mitral or tricuspid valvuloplasty.
b. Results of Surgical Treatment
Case reports have described the results of surgical treatment in patients with left ventricular aneurysm associated with cardiac sarcoidosis232
and patients suspected to have sarcoidosis because of their history of atrioventricular block.337,338
In a report on 384 patients undergoing surgery for the treatment of non-ischemic cardiomyopathy,339
14 patients (3.6%), including 4 patients diagnosed as cardiac sarcoidosis before surgery, who underwent left ventricular restoration for the treatment of cardiac sarcoidosis had NYHA class IV severe heart failure not responding to medical treatment. Procedures of left ventricular restoration were selected according to the location of myocardial injuries and used in combination with mitral and/or tricuspid valvuloplasty. Among 12 patients who underwent planned surgery, 2 patients (17%) died. All 10 survived patients started corticosteroid therapy after surgery. Six of the 10 patients showed an improvement of heart failure to NYHA class II.339
Three-year and five-year survival rates were 65% and 52%, which were higher than those (50% and 37%)1
in patients receiving conventional medical therapy.
c. Limitations and Indications of Surgical Treatment
In patients with diffuse myocardial injuries in whom viable myocardium is limited, conventional surgical treatment cannot improve left ventricular function, and heart transplantation is an option. It has been reported that patients with cardiac sarcoidosis undergoing heart transplantation have acceptable long-term outcomes340
or better than the majority of heart transplant recipients.341
On the other hand, Akashi et al at Columbia University Medical Center have reported that among 825 patients undergoing heart transplantation, 14 patients (1.7%) had cardiac sarcoidosis and 2 of them (14%) died in the hospital. The 1 and 5-year survival rates were 78.5% and 52.4%, showing a post-transplant outcome.342
Akashi et al discussed that patients who received a diagnosis of cardiac sarcoidosis prior to heart transplant were more prone to be in a systemic pro-inflammatory condition, which might be related to the poor outcome in this patient population. Accordingly, patients with cardiac sarcoidosis should be assessed for whether they are indicated for left ventricular restoration or mitral valvuloplasty before considering heart transplantation.
d. Precautions for the Management of Patients After Surgery
Patients with cardiac sarcoidosis are always at a risk of the exacerbation of remaining lesions or the development of new lesions. Although many patients with cardiac sarcoidosis respond well to corticosteroid therapy, patients may be complicated with infectious diseases or have flares of cardiac sarcoidosis after reducing corticosteroid doses. Patients after surgery must be closely monitored for a long time and continue treatment. The recurrence of cardiac sarcoidosis after heart transplantation has been reported.343
Patients after heart surgery should be carefully observed for exacerbation or recurrence of cardiac sarcoidosis.
3.2 Artificial Heart and Heart Transplantation
a. Cardiac Sarcoidosis in Heart Transplantation
Heart transplantation is a possible treatment option for patients with cardiac sarcoidosis in Japan as well as in other countries341,344
(Evidence Level 5, Recommendation grade C1). In a report in the United States in 2013, among 1,069 patients undergoing heart transplantation, 19 patients had cardiac sarcoidosis.340
In Japan, a total of 222 patients underwent heart transplantation as of December 2014, and 3 of them had cardiac sarcoidosis (Figure 39).345
The percentage of patients with cardiac sarcoidosis is similar in the above report from the United States. The indication of heart transplantation and the use of a ventricular assist device (VAD) during the waiting period should be considered for patients with cardiac sarcoidosis.
b. Indications of Heart Transplantation and the Use of Ventricular Assist Devices (VADs)
Indications of heart transplantation and the use of VADs for patients with cardiac sarcoidosis are similar to those for other types of patients (Table 13),346
but there are some precautions specific to cardiac sarcoidosis. In general, the indication of heart transplantation is considered based on:
Table 13.
Heart Transplant Recipient Selection Criteria
| I. |
Are there any other options to save the life of the patient? |
| II. |
How long can the patient live if he/she does not undergo heart transplantation? |
| III. |
Can the patient mentally and physically endure periodic (and sometimes emergent) examinations after transplantation and
follow immunosuppressive regimens prescribed based on the results of examinations? |
| IV. |
Does the patient understand the necessity of transplantation and desire it? Do his/her family members support the patient? |
Adapted from the Heart Transplantation Committee of the Japanese Circulation Society. 2013346
1) Whether the patient has irreversible heart dysfunction with decreased exercise tolerance;
2) Whether the patient can physically tolerate heart transplantation; and
3) Whether the patient has a social environment encouraging heart transplantation.
VADs are indicated for patients who need prolonged circulatory support for the treatment of cardiogenic circulatory failure and patients in whom circulation cannot be maintained with aortic balloon pumping or percutaneous cardiopulmonary support, and who do not meet any of the exclusion criteria.
In Japan, patients who are classified into Level 1 to 3 according to the criteria used in the Japanese Registry for Mechanically Assisted Circulatory Support (J-MACS) that was developed based on the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) are indicated (Table 14).347,348
The detailed criteria for the indication of VADs have been proposed by the Japan VAD Council, a group of academic associations related to VAD use, and are described in the “Guidelines for Device Therapy: Implantable Left Ventricular Assist Device for Patients with Severe Heart Failure”349
proposed by the Japanese Circulation Society and the Japanese Society for Cardiovascular Surgery (Table 15).350
Table 14.
INTERMACS Profile and J-MACS Levels
| |
INTERMACS |
J-MACS |
| 1 |
Critical cardiogenic shock |
Critical cardiogenic shock |
| 2 |
Progressive decline |
Progressive decline |
| 3 |
Stable but inotrope dependent |
Stable but inotrope dependent |
| 4 |
Resting symptoms |
Resting symptoms |
| 5 |
Exertion intolerant |
Exertion intolerant |
| 6 |
Exertion limited |
Exertion limited |
| 7 |
Advanced NYHA III |
Stable condition |
Source: Prepared based on INTERMACS.347
and the Pharmaceuticals and Medical Devices Agency.348
Table 15.
Practice Standard for the Use of Ventricular Assist Devices (Draft Published on November 16, 2010)
Eligible
patients |
Disease conditions |
Patients with end-stage severe heart failure that satisfies the criteria for heart transplantation who have
underlying conditions such as dilated or hypertrophic cardiomyopathy, dilated phase of hypertrophic
cardiomyopathy, ischemic myocardial disease, valvular disorder, congenital heart disease, and
post-myocarditis cardiomyopathy. |
Eligibility
criteria |
Cardiac function |
NYHA Class III to IV (with a history of NYHA Class IV condition). |
| Stage |
D (Patients with severe structural heart disease who have apparent symptoms of heart failure despite
maximal medical therapy). |
| Drug treatment |
Maximal drug treatment using digitalis, diuretics, ACE inhibitors, angiotensin II receptor blockers,
nitrates, and beta-blockers has been given. |
Inotropic drugs and
assisted circulation |
Drug treatment with dobutamine, dopamine, epinephrine, norepinephrine and/or phosphodiesterase III
inhibitors, or intra-aortic balloon pumping or external ventricular assist devices are necessary. |
| Age |
Preferably patients ≤65 years of age (those over 65 years with relatively good physical conditions may
also be included). |
| Body surface area |
See the specification of the system to be used. |
| Hemodynamics |
Stage D, a history of NYHA Class IV condition. |
| Conditions |
Patients in whom other treatments cannot prolong life or whose QOL is severely affected, and in whom
the treatment is expected to improve QOL, help them receive home-based treatment for a long period
of time and return to daily activities. |
Understanding of
treatment |
Patients who understand the limitations of ventricular assist devices and the risk of complications, and
whose family members understand these situations and can support them. |
Exclusion
criteria |
Infections |
Patients with severe infections. |
Respiratory
diseases |
Patients with severe chronic obstructive pulmonary disease. |
| Patients with severe pulmonary hypertension. |
| Patients within 30 days after the onset of pulmonary arterial embolism developed. |
Cardiovascular
diseases |
Patients immediately after open-heart surgery (about 2 weeks). |
| Patients with untreatable abdominal aortic aneurysm or severe peripheral vascular diseases. |
| Patients with thoracic aortic aneurysm, ventricular aneurysm, or interventricular septum rupture. |
| Patients with moderate or severe aortic valve insufficiency. |
| Serious calcification of the thoracic aorta. |
Nervous system
disorders |
Severe CNS disorder. |
| History of drug or alcohol addiction. |
Patients with psychiatric/nervous system disorder who are considered not to be able to follow or
understand the protocol. |
Other organ
disorders |
Patients with severe hepatic disorder. |
Patients with severe bleeding tendency, those with advanced chronic renal failure, those on hemodialysis
for the treatment of chronic renal failure, those with malignant diseases such as cancer with poor life
expectancy, those with systemic diseases such as collagen disorders, or those with insulin-dependent
severe diabetes mellitus. |
| Pregnancy |
During pregnancy. |
| Others |
Other patients for whom the institutional review committee considered inappropriate for the treatment
for reasons such as severe obesity or refusal of blood transfusion. |
Adapted from the Japanese Association for Clinical Ventricular Assist Systems. 2011.350
c. Precautions for the Application of Heart Implantation for Patients With Cardiac Sarcoidosis
Corticosteroids are used as the main component of the drug treatment of cardiac sarcoidosis. It has been reported that corticosteroid therapy may improve exercise tolerance even in patines with NYHA Class III severe heart failure not responding to other types of drugs.171
Accordingly, the indication of heart transplantation should be considered after corticosteroid therapy is tried. For patients who are considered to have limited viable myocardium that may respond to corticosteroids, physicians should describe the reasons for such consideration and provide findings of imaging in the application form for heart transplantation. When the patient has systemic sarcoidosis at the time of application for heart transplantation, experts should reach a consensus that non-cardiac sarcoidosis will not become a factor that decides the prognosis of the patient for at least 5 years.
As cardiac sarcoidosis is difficult to diagnose, this condition may be first diagnosed based on the examination of myocardial samples obtained at the time of implantation of a left ventricular support device.351
Corticosteroids are administered to patients who are using external VAD to examine whether they should be included on the waiting list for heart transplantation but are often not administered to patients with VAD implantation because the risk of infection outweighs the beneficial effects of corticosteroids on cardiac function. It is thus advisable that a diagnosis of cardiac sarcoidosis should be made before VAD implantation whenever possible. However, patients in Japan wait for donor hearts for a long period of time after VAD implantation. During the waiting period, cardiac sarcoidosis, especially right ventricular involvement of the disease, may progress, but it is unknown whether corticosteroid therapy should be conducted or not.
d. Outcome of Heart Transplantation
According to Western reports, the outcome of heart transplantation in patients with cardiac sarcoidosis is comparable to other patient populations.352
A report from the United States in 2013 has described that there were no significant differences between patients with cardiac sarcoidosis and other patients in first-year freedom from any treated rejection, 5-year survival, 5-year freedom from chronic coronary artery disease, and 5-year freedom from nonfatal major adverse cardiac events.340
Further evaluation should be made to determine whether similar outcomes can be observed in patients in Japan who wait for donors for a long period of time.
Patients should be monitored carefully because the recurrence of cardiac sarcoidosis in a heart transplant recipient has been reported.343
However, this case is a recurrence of cardiac involvement of systemic sarcoidosis. Another report has described that no recurrence of sarcoidosis has been observed in patients with isolated cardiac sarcoidosis.353
Further findings are awaited.
4. Treatment Algorithm
It has been estimated that two out of three patients with sarcoidosis die from cardiac involvement (cardiac sarcoidosis). The presence or absence of cardiac involvement may determine the prognosis of patients with sarcoidosis. In general, immunosuppressive therapy (corticosteroid therapy) is effective in the treatment of cardiac involvement. Patients diagnosed with cardiac sarcoidosis should basically be treated with corticosteroids. Patients should also receive appropriate treatment for high-grade atrioventricular block, severe ventricular arrhythmia, heart failure, or other conditions.
Figure 40
describes the treatment algorithm for patients with cardiac sarcoidosis (Evidence level 6, Recommendation grade B). This algorithm includes surgical options such as mitral valvuloplasty or the treatment of mitral regurgitation due to cardiac remodeling and VADs. Some treatments should typically be given to all patients regardless of the severity of cardiac pump dysfunction, and other treatment should be modified according to the severity. The reader should refer to the relevant section for details.

V. Conclusion
1. Future Challenges
The etiology and pathogenesis of cardiac sarcoidosis have not been fully clarified. However, cardiac sarcoidosis develops more frequently in Japan than in Western countries and is an important complication that defines the prognosis of patients with sarcoidosis. Early diagnosis and treatment are important, but it is difficult to confirm the diagnosis of cardiac sarcoidosis in patients with no histological diagnosis of sarcoidosis affecting other organs because of the absence of disease-specific serological markers and the low sensitivity of endomyocardial biopsy.
The recent advancements of imaging techniques such as cardiac MRI and 18F-FDG PET have helped physicians identify patients suspected to have cardiac sarcoidosis earlier than before. However, even though patients have become suspected in an early phase of cardiac sarcoidosis, confirmatory diagnosis and treatment intervention for such patients are still slow.
There are three challenges that physicians face when addressing patients with cardiac sarcoidosis: (1) Etiologies (causes) are unclear; (2) Histological assessment is difficult to conduct for the heart; and (3) No treatment strategies for each stage have been established or prognosis for patients receiving currently available treatments is unknown. Further studies and data that may clarify these problems are awaited.
1.1 Etiologies (Causes)
Different etiologies have been suggested for cardiac sarcoidosis. The mechanism underlying the possible involvement of
P. acnes
on cardiac sarcoidosis as described in Section “2.2 Topics on P. acnes as a Cause of Sarcoidosis” in Chapter II has been discussed in Japan for years and is noteworthy.61,63,354
With recently advanced imaging modalities, future studies on the effect of
P. acnes
on cardiac sarcoidosis are expected to provide more data on the relationships between
P. acnes
and the diagnosis, treatment, and prognosis of cardiac sarcoidosis.
1.2 Histological Examinations
The rapid advancement of imaging techniques has made it possible to diagnose systemic sarcoidosis and determine its activity in the early phase of the disease. With the new imaging techniques, more patients are diagnosed with isolated cardiac sarcoidosis, i.e., the presence of sarcoidosis lesions without any other organ involvements.171
However, since cardiac sarcoidosis often causes scattered, uneven inflammation that affects only some layers of the myocardium, and endomyocardial biopsy samples may not contain sarcoidosis lesions in many cases, it is difficult to perform a histological assessment of the heart to confirm a diagnosis of isolated cardiac sarcoidosis in many patients. Pathological diagnosis will remain important, but the following approaches should be considered to facilitate early diagnosis and treatment intervention from the clinical point of view.
1) Approaches to Improve the Accuracy of Histological Diagnosis Based on Endomyocardial Biopsy
Hybrid diagnostic methods using imaging modalities such as cardiac MRI, PET/CT, and electrophysiological mapping277
to assist endomyocardial biopsy are expected to enable the accurate identification of areas to be sampled and thereby increase the accuracy of histopathological examination.
2) Supportive Diagnostic Methods Other Than Histopathological Examination Based on Endomyocardial Biopsy
If
P.acnes
infection and delayed allergic reactions to
P. acnes
are involved deeply in the onset of cardiac sarcoidosis as suggested by researchers,355
cardiac myocytes in endomyocardial biopsy samples obtained during active cardiac sarcoidosis should indicate positive reactions to
P. acnes
in immunostaining methods more frequently than samples obtained from patients with other cardiac diseases causing left ventricular dysfunction (e.g., dilated cardiomyopathy and inflammatory heart diseases). If this can be proven, immunological test results may support the diagnosis or assessment of cardiac sarcoidosis even when non-caseating epithelioid granuloma is not found in biopsy samples and histopathological diagnosis is difficult.
1.3 Treatment Intervention and Efficacy Assessment
As described earlier, the initiation of treatment intervention is also slow in patients with cardiac sarcoidosis because histopathological assessment is essential to confirm the diagnosis. Also, patients receiving standard treatment strategies consisting of immunosuppressive therapy mainly with corticosteroids and standard treatments for heart failure and arrhythmias are still at a high risk of cardiovascular events. If bacterial infection and allergic reactions against the causative organisms play an important role in the etiology of cardiac sarcoidosis as researchers point out, combination therapy using corticosteroids and antimicrobial drugs would be effective. However, no studies have been conducted to investigate the optimal timing and efficacy of such treatments. Future studies should address these points. The present guidelines and other guidelines recommend ICD implantation for patients with cardiac sarcoidosis who have high-grade atrioventricular block but do not have cardiac dysfunction or life-threatening arrhythmias such as ventricular fibrillation and ventricular tachycardia.107,323
When it becomes possible for cardiac sarcoidosis to be diagnosed earlier, the number of patients with such a condition will increase. Prospective studies in this patient population should be conducted to assess the effect of treatments recommended in this guideline document on their prognosis. Further findings should be accumulated.
2. Summary
The present guideline document was prepared to reflect the rapidly advanced understanding of and treatment for sarcoidosis. In the clinical practice, physicians should correctly grasp the disease condition of individual patients. As emphasized in each section of this document, clinical and physical findings are especially important. Cardiac sarcoidosis often manifests with non-specific cardiac symptoms, and some patients have not been diagnosed with sarcoidosis affecting other organs. Physicians should always consider the possibility of cardiac sarcoidosis when examining patients presenting with cardiac signs and symptoms. It is also important that physicians use current and appropriate treatment strategies that can address the disease condition of individual patients and assess them periodically for efficacy and to modify treatment strategies flexibly.
As the pathological understanding of cardiac sarcoidosis has advanced remarkably, and new technologies have been developed, we decided to greatly revise the guidelines for this disease. Among the recent advancements in the understanding of cardiac sarcoidosis, the pathophysiology of isolated cardiac sarcoidosis has been understood remarkably. Although only limited evidence is available for this rare disease, a nation-wide survey by the Japanese Heart Failure Society has revealed that there are more than a few patients who experience this condition in Japan. This could be considered a new type of sarcoidosis. According to the advancement of drug and non-drug treatment of heart failure and arrhythmias, treatment strategies for cardiac sarcoidosis have also changed. This guideline document described renewed treatment strategies as well. We hope that future clinical research will reveal which treatments are suitable for individual conditions and help physicians select optimal treatments for their patients. For clinicians, the important things are making a diagnosis of cardiac sarcoidosis at the early stage of disease and taking a multidisciplinary approach to treat the whole body of patients rather than just to control the heart disease. A multidisciplinary approach is an important factor for the treatment of cardiac sarcoidosis, as well as other conditions.
Finally, the reader should be aware that this guideline document provides information on standard diagnostic and treatment methods, and physicians and patients are fully responsible for making decisions and selecting treatment options for individual patients. Physicians should carefully assess patients for individual pathophysiological futures and modify standard treatments whenever necessary. We hope that this guideline document will be fully utilized in clinical practice to benefit patients.
VI. Q&A
Q1: What is the prevalence of sarcoidosis?
(Section “1. Epidemiology of Sarcoidosis” in Chapter II)
A:
In Japan, it is estimated that 7.5 to 9.3 people per 100,000 people have sarcoidosis. It is unclear how many patients with sarcoidosis have cardiac involvement. It has been reported that about 5% of patients with sarcoidosis have cardiac signs/symptoms, but cardiac findings are more often found in autopsy. The prevalence and type of sarcoidosis differ between races. It is said that Japanese patients tend to have cardiac involvement and eye involvement more frequently than Western patients.
Q2: Has the etiology of sarcoidosis been specified?
(Section “2. Etiology and Pathophysiology of Sarcoidosis” in Chapter II)
A:
No. Some researchers have hypothesized that sarcoidosis is caused by
P.acnes
and others by
M. tuberculosis. More evidence has been accumulated for the involvement of
P.acnes
in sarcoidosis, which is the leading hypothesis for the cause of sarcoidosis. However, the efficacy of antimicrobial drugs in the treatment of sarcoidosis has not been fully demonstrated, and it may also be likely that granulomas facilitate the proliferation of
P.acnes. No causal relationship between
P.acnes
and sarcoidosis has been demonstrated.
Q3: Are there any predispositions for the development of sarcoidosis?
(Section “2. Etiology and Pathophysiology of Sarcoidosis” in Chapter II)
A:
Epidemiological survey has revealed familial clustering of sarcoidosis. It appears that sarcoidosis may run in families, but the incidence in family members is low. Repeatable correlations have been reported between some HLA antigens and sarcoidosis. In Japanese, correlations with DRB1*1101, DRB1*1201, DRB1*1401, DRB1*0802, and DRB3*0101 have been reported.
Q4: How is sarcoidosis diagnosed?
(Section “3. Diagnosis of Sarcoidosis” in Chapter II)
A:
In January 2015, the Act on Medical Care for Patients with Rare/Intractable Diseases was newly implemented, and the diagnostic criteria of sarcoidosis as a designated intractable disease was revised. Diagnostic criteria include the histological diagnosis group and the clinical diagnosis group. The histological diagnosis requests to histologically prove the presence of epithelioid granuloma in some organs and rule out other granulomatous conditions. The clinical diagnosis group criteria require physicians to perform appropriate laboratory examinations to detect findings typical for sarcoidosis and assess for sarcoidosis affecting other organs.
Q5: Can chest CT be used in the staging of pulmonary lesions of sarcoidosis?
(Section “4. Pulmonary Lesions of Sarcoidosis” in Chapter II)
A:
Currently, the staging of pulmonary lesions of sarcoidosis is based on chest X-ray findings. However, HRCT can provide information that suggests the presence of additional lesions (e.g., lung field lesions and mediastinal lymphadenopathy) that are not used in the staging based on chest X-ray findings. In the future, findings with these two methods should be compared to establish disease staging criteria based on HRCT.
Q6: What kind of treatment is indicated for asymptomatic patients with pulmonary lesions of sarcoidosis (Stage II or III)?
(Section “5. Treatment of Sarcoidosis” in Chapter II)
A:
Asymptomatic patients should basically be monitored for 3 to 6 months before starting treatment. If respiratory function decreases or pulmonary fibrosis progresses during the observation period or these changes are expected to occur near the future, corticosteroid therapy should be considered. Corticosteroid therapy is not necessary for patients in whom fibrosis is not likely to occur according to the findings of patchy opacities.
Q7: Please describe how to add methotrexate to corticosteroid therapy.
(Section “5. Treatment of Sarcoidosis” in Chapter II)
A:
Methotrexate is not necessary for patients who are expected to reduce their corticosteroid dose smoothly and discontinue it. When more than 6 months of corticosteroid therapy with prednisolone at a dose of 10 to 15 mg/day is expected, the addition of low-dose methotrexate should be considered.
Q8: When myocardial biopsy in a patient reveals granulomas without giant cells, can the patient be diagnosed as having cardiac sarcoidosis?
(Section “1. Pathology” in Chapter III)
A:
It is highly likely that the patient has cardiac sarcoidosis, but please examine again with deeper cuts or serial sections. Pathologically, such cases are handled as cases suspected to have cardiac sarcoidosis.
Q9: How many biopsy samples should be obtained during myocardial biopsy?
(Section “1. Pathology” in Chapter III)
A:
It is desirable that more than one sample be obtained whenever possible to increase the positive rate.
Q10: What are common symptoms of cardiac sarcoidosis?
(Section “2.1 Clinical Manifestations” in Chapter III)
A:
Cardiac sarcoidosis may induce conduction system disorder, myocardial damages, and pump dysfunction, which may lead to arrhythmias, sudden death, or heart failure.
Q11: Are there biomarkers that indicate the presence of cardiac involvement in patients diagnosed with sarcoidosis?
(Section “2.2 Blood and Urine Tests (Biomarkers)” in Chapter III)
A:
High-sensitivity cardiac troponin T and I, as well as BNP, may be useful in the diagnosis of cardiac lesions associated with sarcoidosis or the assessment of treatment efficacy. However, it should be noted that these biomarkers are not specific to sarcoidosis.
Q12: What are important ECG findings in cardiac sarcoidosis?
(Section “2.4 Electrocardiogram (ECG)” in Chapter III)
A:
Cardiac sarcoidosis may cause diverse ECG abnormalities and arrhythmias. Among them, conduction disorders such as high-grade atrioventricular block and bundle branch block and life-threatening ventricular arrhythmias are especially important. It is important to observe patients with non-cardiac sarcoidosis carefully for the occurrence of ECG abnormalities. All patients with sarcoidosis should undergo periodic ECG follow-up.
Q13: Does a basal thinning of the ventricular septum in echocardiography indicate the presence of cardiac sarcoidosis? Can we make a diagnosis of cardiac sarcoidosis based on this finding? At which point should we measure the thickness of ventricular septum?
(Section “2.5 Echocardiography” in Chapter III)
A:
A basal thinning of the ventricular septum is a poorly sensitive but highly specific finding of cardiac sarcoidosis. Patients with this typical finding should be suspected to have cardiac sarcoidosis.
However, echocardiography findings are not enough to diagnose cardiac sarcoidosis. Results of other examinations should be considered to confirm the diagnosis. According to available study findings, a thin ventricular septum, which is defined as ≤4 mm thick at 10 mm from the aortic annulus in the left ventricular long axis view, has 12.6% sensitivity and 100% specificity in the diagnosis of cardiac sarcoidosis. In many patients, thinning begins immediately below the aortic annulus.
Q14: Can patients with chronic kidney disease receive gadolinium contrast agents?
(Section “2.6 Cardiac Magnetic Resonance Imaging (MRI)” in Chapter III)
A:
Patients with an eGFR of <30 mL/min/1.73 m2
and patients on hemodialysis are generally contraindicated for gadolinium contrast agents as they may increase the risk of nephrogenic systemic fibrosis. Patients with an eGFR of <45 mL/min/1.73 m2
may receive gadolinium contrast agents when (1) fluid therapy is conducted before examination to help wash out the contrast after examination; (2) no examination is conducted during the acute phase (e.g., immediately after surgery or invasive examination, during infection, and exacerbation or renal dysfunction); (3) structurally stable contrast agents are used; and (4) a minimum necessary volume of the contrast is given.
Q15: Can cardiac MRI be conducted for patients using implantable devices in whom conventional, MRI-unsafe devices have been replaced with MRI-safe devices?
(Section “2.6 Cardiac Magnetic Resonance Imaging (MRI)” in Chapter III)
A:
As of 2015, implantable devices and electrodes must be MRI-safe in patients undergoing cardiac MRI. Patients using conventional, MRI-unsafe implantable devices or electrodes cannot undergo cardiac MRI. Even when both the device and electrodes are MRI-safe, (1) different MRI instruments require different conditions and (2) medical institutions must conform to the criteria for medical institutions. Please consult with the manufacturer for details.
Q16: Please list precautions for 18F-FDG PET.
(Section “a.18F-FDG PET” of “2.7 Nuclear Imaging” in Chapter III)
A:
As the 18F-FDG tracer physiologically accumulates in the heart, imaging conditions must be followed strictly to prevent false-positive findings. Currently, it is recommended that patients should (1) fast for 12 hours before examination, (2) eat a low-carbohydrate meal in the previous evening before examination, and (3) receive heparin injection immediately before examination (to elevate free fatty acid levels in the blood).
Q17: Do any diseases other than cardiac sarcoidosis cause positive cardiac findings of 18F-FDG PET?
(Section “a. 18F-FDG PET” of “2.7 Nuclear Imaging” in Chapter III)
A:
Abnormal tracer accumulation in the heart may be observed in patients with ischemic heart disease, hypertrophic cardiomyopathy, myocarditis, (metastatic) tumors, and heart failure, among other diseases. These conditions should be ruled out to make a diagnosis of cardiac sarcoidosis.
Q18: What are the benefits of 67Ga scintigraphy?
(Section “b. Gallium-67 (67Ga) Citrate Scintigraphy” of “2.7 Nuclear Imaging” in Chapter III)
A:
It has been reported that 67Ga scintigraphy is a poorly sensitive but highly specific examination in diagnosing cardiac sarcoidosis. The advancement of imaging techniques such as SPECT has improved the diagnostic performance of 67Ga scintigraphy.
Q19: What are the benefits of myocardial perfusion scintigraphy?
(Section “c. Myocardial Perfusion Scintigraphy” of “2.7 Nuclear Imaging” in Chapter III)
A:
Myocardial perfusion scintigraphy is not highly specific in the diagnosis of cardiac sarcoidosis. However, perfusion defects that are inconsistent with coronary blood flow or occur in the ventricular septum and/or the base of the anterior wall, which are often affected by sarcoidosis, are important findings to suspect cardiac sarcoidosis. Myocardial perfusion scintigraphy is used with 67Ga scintigraphy or 18F-FDG PET to improve the diagnostic performance of imaging studies.
Q20: What is the significance of contrast left ventriculography?
(Section “2.8.1 Contrast Left Ventriculography and Coronary Angiography” in Chapter III)
A:
As patients with cardiac sarcoidosis may show localized wall motion abnormalities that cannot be explained by coronary status or ventricular aneurysms, contrast left ventriculography is useful in the diagnosis of cardiac sarcoidosis.
Q21: Is myocardial biopsy necessary to diagnose cardiac sarcoidosis?
(Section “2.8.2 Myocardial Biopsy” in Chapter III)
A:
As granulomas in patients with cardiac sarcoidosis develop sporadically in the myocardium, only a small percentage of patients can be correctly diagnosed with endomyocardial biopsy.
However, histological confirmation of cardiac sarcoidosis will help physicians build more appropriate treatment strategies. It is desirable that physicians perform myocardial biopsy for patients suspected to have cardiac sarcoidosis according to the precautions for reducing the risk of complications.
Q22: What types of patients need electrophysiological testing?
(Section “2.8.3 Electrophysiological Testing” in Chapter III)
A:
Electrophysiological testing is conducted to assess the risk of sudden death due to ventricular arrhythmia when a low LVEF, positive findings of late enhancement cardiac MRI, and/or abnormal findings of 18F-FDG PET or 67Ga scintigraphy are observed. Patients in whom sustained ventricular tachycardia or ventricular fibrillation was induced during electrophysiological testing should undergo ICD implantation. Patients with syncope of unknown cause should also undergo electrophysiological testing to investigate whether it is caused by conduction disturbance (deceleration) or ventricular tachycardia (acceleration). Also, patients who are indicated for permanent cardiac pacing for the treatment of atrioventricular block or other conditions should undergo electrophysiological testing to assess the risk of ventricular tachycardia when a low LVEF, abnormal cardiac MRI findings, or inflammation is observed. If ventricular tachycardia is induced during electrophysiological testing, the patient requires an ICD rather than an implantable pacemaker.
Q23: Can HLA typing help physicians predict whether patients are susceptible to sarcoidosis or respond well to treatment?
(Section “2.9 Future Prospects: Genetic Assessment” in Chapter III)
A:
Those who have HLA types that have been known to increase the risk of sarcoidosis account for only a small percentage of patients with sarcoidosis. Currently, HLA typing has not been established as a measure for risk assessment of this disease. Recently, researchers have indicated that some factors affecting non-HLA related immune response are involved in the susceptibility of sarcoidosis. In the future, HLA typing and other factors will be utilized to develop genetic diagnosis and better treatment strategies.
Q24: Can patients with “clinical findings strongly suggestive of cardiac involvement” be diagnosed as having cardiac sarcoidosis?
(Section “3. Diagnostic Guidelines” in Chapter III)
A:
No. Such findings are not enough to make a diagnosis of cardiac sarcoidosis. A diagnosis can be made when the patient meets the “Cardiac sarcoidosis,” described in Section 3.1 of Chapter III. Caution should be exercised not to make a diagnosis based only on the presence of “clinical findings strongly suggestive of cardiac involvement.” Patients without findings of sarcoidosis in other organs than the heart should be examined according to the “Diagnostic procedures for patients presenting with cardiac complaints who are suspected to have cardiac sarcoidosis” (Figure 36), described in Section 3.3 of Chapter III, as well as evaluated for the possibility of isolated cardiac sarcoidosis according to the “Isolated cardiac sarcoidosis” described in Section 3.2 of Chapter III.
Q25: Are patients diagnosed with cardiac sarcoidosis indicated for corticosteroids and other drugs even if they are asymptomatic?
A:
It is considered that patients diagnosed with cardiac sarcoidosis are indicated for corticosteroid therapy even if they have no severe symptoms. However, patients should be assessed individually based on their conditions, such as cardiac function and the severity of adverse drug reactions.
Q26: Should patients continue oral corticosteroid therapy for a long period of time?
(Section “1.1 Immunosuppressive Therapy” in Chapter IV)
A:
It is difficult to determine the disease activity of cardiac sarcoidosis. No outcome measures or imaging techniques have been established. Physicians should assess disease activity comprehensively based on their clinical findings, laboratory data, and imaging findings.
As flares or disease progression may occur during tapering of oral corticosteroids, many patients maintain oral corticosteroid therapy at a [prednisolone] dose of 5 to 10 mg/day for a long period of time.
Q27: What should physicians do for patients with flares during oral corticosteroid therapy for the treatment of cardiac sarcoidosis?
(Section “1.1 Immunosuppressive Therapy” in Chapter IV)
A:
Basically, the corticosteroid dose should be increased to the initial dose. If it is difficult to increase the dose due to the occurrence of adverse drug reactions or other reasons, the additional use of other immunosuppressants should be considered.
Q28: Should immunosuppressive therapy be strengthened without delay when left ventricular systolic function decreases continuously?
(Section “1.2 Treatment of Heart Failure”
in Chapter IV)
A:
Left ventricular remodeling and other pathologic processes associated with heart failure may continue progressing even when cardiac inflammation is controlled. Patients should, therefore, be treated for heart failure according to the guidelines for the treatment of heart failure before assessment for the disease activity of cardiac sarcoidosis. Immunosuppressive therapy should then be adjusted if necessary.
Q29: What kind of antiarrhythmic drugs should be used for the treatment of tachyarrhythmia associated with cardiac sarcoidosis?
(Section “1.3 Treatment of Arrhythmias” in Chapter IV)
A:
Beta blockers and amiodarone are options. It is very important to control arrhythmias associated with cardiac sarcoidosis because the outcomes of patients highly depend on whether arrhythmias can be controlled well or not. Drug therapy should be considered after considering whether the patient is indicated for non-drug therapy such as pacing and implantable cardioverter defibrillator therapy.
Q30: When the patient has high-grade atrioventricular block and is indicated for pacing, which should be done first, corticosteroid therapy or pacemaker implantation?
(Section “2.1 Permanent Pacing” in Chapter IV)
A:
Corticosteroid therapy is expected to be somewhat effective in improving atrioventricular conduction. However, it is difficult to predict whether individual patients will respond to the treatment or not. Considering the recurrent nature of sarcoidosis, it is desirable that patients who are indicated for permanent pacemaker implantation undergo pacemaker implantation before starting corticosteroid therapy. Also, oral corticosteroids may increase the risk of perioperative infection. Patients who require implantable devices such as pacemakers should start corticosteroid therapy after the completion of device implantation.
Q31: What should be done to treat patients with non-sustained ventricular tachycardia with a LVEF of ≥50%?
(Section “2.2 Implantable Cardioverter Defibrillator (ICD)” in Chapter IV)
A:
In patients with relatively preserved cardiac function, the presence of non-sustained ventricular tachycardia is not indicated for ICD. However, it is highly likely that the risk of life-threatening arrhythmia will increase as the disease progresses or when the disease activity is high. Patients who require corticosteroid therapy should be carefully followed up to assess cardiac function and examine for arrhythmogenic substrates using electrophysiological testing whenever necessary.
Q32: Who is indicated for catheter ablation in the treatment of cardiac sarcoidosis?
(Section “2.4 Catheter Ablation” in Chapter IV)
A:
Patients with ventricular tachycardia not responding to corticosteroids or antiarrhythmic drugs, those who cannot take these drugs, and those with intractable arrhythmic storm are indicated for catheter ablation.
Q33: Can patients with cardiac sarcoidosis undergo heart transplantation?
(Section “3.2 Artificial Heart and Heart Transplantation” in Chapter IV)
A:
Heart transplantation and ventricular assist devices should be considered for patients with end-stage heart failure associated with cardiac sarcoidosis. Because cardiac sarcoidosis is a systemic inflammatory disease, individual patients should be carefully examined to determine whether such a procedure is appropriate or not for them.
Appendix 1. JCS Joint Working Group
Chair:
• Fumio Terasaki, Medical Education Center / Department of Cardiology, Osaka Medical College
Members and Collaborators:
• Toshihisa Anzai, Department of Cardiovascular Medicine, National Cerebral and Cardiovascular Center
• Arata Azuma, Department of Pulmonary Medicine, Nippon Medical School
• Yoshinobu Eishi, Department of Human Pathology, Graduate School of Medical and Dental Science, Tokyo Medical and Dental University
• Taiko Horii, Department of Cardiovascular Surgery, Kagawa University School of Medicine
• Takayuki Inomata, Department of Cardiology, Kitasato University Kitasato Institute Hospital
• Hatsue Ishibashi-Ueda, Department of Pathology and Biobank, National Cerebral and Cardiovascular Center
• Yoshio Ishida, Department of Internal Medicine, Kaizuka City Hospital
• Nobukazu Ishizaka, Department of Internal Medicine (III) / Department of Cardiology, Osaka Medical College
• Mitsuaki Isobe, Department of Cardiovascular Medicine, Graduate School of Medical and Dental Science, Tokyo Medical and Dental University
• Masafumi Kitakaze, Department of Clinical Medicine and Development, National Cerebral and Cardiovascular Center
• Kengo Kusano, Department of Cardiovascular Medicine, National Cerebral and Cardiovascular Center
• Takatomo Nakajima, Division of Cardiology, Saitama Cardiovascular and Respiratory Center
• Satoshi Nakatani, Division of Functional Diagnostics, Department of Health Sciences, Osaka University Graduate School of Medicine
• Yasushi Sakata, Department of Cardiovascular Medicine, Osaka University Graduate School of Medicine
• Noriharu Shijubo, Department of Respiratory Medicine, JR Sapporo Hospital
• Akihito Tsuchida, Department of Cardiology, JR Sapporo Hospital
• Hiroyuki Tsutsui, Department of Cardiovascular Medicine, Kyushu University Graduate School of Medical Sciences
• Etsuro Yamaguchi, Department of Respiratory Medicine and Allergology, Aichi Medical University School of Medicine
• Tetsuo Yamaguchi, Shinjuku Kaijou Building Clinic
• Yoshikazu Yazaki, Department of Cardiovascular Medicine, Saku Central Hospital
Collaborators:
• Masahiko Goya, Department of Cardiology, Tokyo Medical and Dental University
• Takuya Hasegawa, Department of Cardiovascular Medicine, National Cerebral and Cardiovascular Center
• Tomomi Ide, Department of Cardiovascular Medicine, Faculty of Medical Sciences, Kyushu University
• Yasuchika Kato, Department of Cardiology, Fujita Health University
• Hideaki Morita, Department of Cardiology, Osaka Medical College
• Toshiyuki Nagai, Department of Cardiovascular Medicine, National Cerebral and Cardiovascular Center
• Hiroshi Nakamura, Department of General Medicine, Hiroshima-Nishi Medical Center
• Takashi Noda, Department of Cardiovascular Medicine, National Cerebral and Cardiovascular Center
• Hideo Okamura, Department of Cardiovascular Medicine, National Cerebral and Cardiovascular Center
• Mamoru Sakakibara, Department of Cardiovascular Medicine, Hokkaido University Graduate School of Medicine
• Kyoko Soejima, Department of Cardiology, Kyorin University Faculty of Medicine
Independent Assessment Committee
• Yasuki Kihara, Department of Cardiovascular Medicine, Graduate School of Biomedical and Health Sciences, Hiroshima University
• Shin-ichiro Morimoto, Department of Cardiology, Fujita Health University
• Tohru Ohe, Department of Cardiology, Sakakibara Heart Institute of Okayama
• Yoshihiko Saito, Department of Cardiorenal Medicine and Metabolic Disease, Nara Medical University
• Yukihiko Sugiyama, Division of Pulmonary Medicine, Department of Medicine, Jichi Medical University
• Akira Yamashina, Department of Cardiology, Tokyo Medical University
(The affiliations of the members are as of November, 2016)
Appendix 2. Disclosure of Potential Conflicts of Interest (COI): JCS 2016 Guideline on Diagnosis and Treatment of Cardiac Sarcoidosis
| Author |
Employer/
leadership
position
(private
company) |
Stakeholder |
Patent
royalty |
Honorarium |
Payment for
manuscripts |
Research grant |
Scholarship (educational)
grant/endowed chair |
Other
rewards |
Potential COI
of the marital
partner, first-
degree family
members, or
those who share
income and
property |
Member:
Arata Azuma |
|
|
|
Boehringer Ingelheim Japan,
Inc. |
|
|
|
|
|
Member:
Toshihisa
Anzai |
|
|
|
Otsuka Pharmaceutical Co., Ltd.
Teijin Pharma Limited. |
|
Bristol-Myers Squibb |
|
|
|
Member:
Nobukazu
Ishizaka |
|
|
|
|
|
Sanwa Kagaku
Kenkyusho Co., Ltd. |
|
|
|
Member:
Mitsuaki Isobe |
|
|
|
Daiichi Sankyo Company,
Limited.
Mitsubishi Tanabe Pharma
Corporation
Otsuka Pharmaceutical Co., Ltd. |
|
|
Daiichi Sankyo Company,
Limited.
Mitsubishi Tanabe Pharma
Corporation
Otsuka Pharmaceutical Co.,
Ltd.
Ono Pharmaceutical Co.,
Ltd.
Teijin Pharma Limited. |
|
|
Member:
Takayuki
Inomata |
|
|
|
Daiichi Sankyo Company,
Limited.
Otsuka Pharmaceutical Co., Ltd.
Toa Eiyo Ltd.
Medtronic |
Ono Pharmaceutical
Co., Ltd. |
|
|
|
|
Member:
Masafumi
Kitakaze |
|
|
|
Takeda Pharmaceutical
Company Limited.
Daiichi Sankyo Company,
Limited.
Mitsubishi Tanabe Pharma
Corporation |
|
Takeda Pharmaceutical
Company Limited. |
|
|
|
Member:
Kengo
Kusano |
|
|
|
|
|
Medtronic |
|
|
|
Member:
Yasushi
Sakata |
|
|
|
Otsuka Pharmaceutical Co., Ltd.
Ono Pharmaceutical Co., Ltd.
Daiichi Sankyo Company,
Limited.
Mitsubishi Tanabe Pharma
Corporation
Boehringer Ingelheim Japan,
Inc. |
|
The Japanese
Circulation Society
Kanazawa Memorial
Cardiac Research
Promotion
Foundation |
Sanwa Kagaku Kenkyusho
Co., Ltd.
Kawachi General Hospita
Amagasaki Chuo Hospital
Edwards Lifesciences
Corporation
FUJIFILM Toyama
Chemical Co., Ltd.
Abbott Vascular Japan Co.,
Ltd.
Medtronic
Otsuka Pharmaceutical Co.,
Ltd.
BIOTRONIK Japan, Inc.
Boston Scientific
Corporation
SOUSEI Hospital
Nippon Shinyaku Co., Ltd.
Boehringer Ingelheim Japan,
Inc.
Sumitomo Dainippon
Pharma Co., Ltd. |
|
|
Member:
Hiroyuki
Tsutsui |
|
|
|
MSD K.K.
Otsuka Pharmaceutical Co., Ltd.
Ono Pharmaceutical Co., Ltd.
Daiichi Sankyo Company,
Limited.
Takeda Pharmaceutical
Company Limited.
Mitsubishi Tanabe Pharma
Corporation
Teijin Pharma Limited.
Boehringer Ingelheim Japan,
Inc.
Bayer Yakuhin, Ltd.
Pfizer Japan Inc.
Bristol-Myers Squibb |
IGAKU-SHOIN
Ltd.
Medical Review Co.,
Ltd. |
|
Novartis Pharma K.K.
Daiichi Sankyo Company,
Limited.
Astellas Pharma Inc.
Takeda Pharmaceutical
Company Limited. |
|
|
Member:
Satoshi
Nakatani |
|
|
|
Edwards Lifesciences
Corporation |
|
|
|
|
|
Collaborator:
Tomomi Ide |
|
|
|
|
|
|
|
|
Higashida
Clinic |
Collaborator:
Hideo
Okamura |
|
|
|
|
|
|
Japanese Heart Rhythm
Society
Medtronic |
|
|
Collaborator:
Masahiko
Goya |
|
|
|
Medtronic |
|
|
|
|
|
Collaborator:
Kyoko
Soejima |
|
|
|
St. Jude Medical Japan Co., Ltd.
Medtronic
Boehringer Ingelheim Japan,
Inc. |
|
|
Daiichi Sankyo Company,
Limited.
Boehringer Ingelheim Japan,
Inc. |
|
|
Collaborator:
Takashi Noda |
|
|
|
Medtronic |
|
Medtronic |
|
|
|
Companies are listed only by name.
The following members and collaborators have no relevant COIs.
Chair: Fumio Terasaki, none
Member: Yoshio Ishida, none
Member: Hatsue Ishibashi-Ueda, none
Member: Yoshinobu Eishi, none
Member: Noriharu Shijubo, none
Member: Akihito Tsuchida, none
Member: Takatomo Nakajima, none
Member: Taiko Horii, none
Member: Yoshikazu Yazaki, none
Member: Etsuro Yamaguchi, none
Member: Tetsuo Yamaguchi, none
Collaborator: Yasuchika Kato, none
Collaborator: Mamoru Sakakibara, none
Collaborator: Toshiyuki Nagai, none
Collaborator: Hiroshi Nakamura, none
Collaborator: Takuya Hasegawa, none
Collaborator: Hideaki Morita, none
References
- 1.
Yazaki Y, Isobe M, Hiramitsu S, et al. Comparison of clinical features and prognosis of cardiac sarcoidosis and idiopathic dilated cardiomyopathy. Am J Cardiol 1998; 82: 537–540. PMID: 9723651
- 2.
Otsuka K, Terasaki F, Eishi Y, et al. Cardiac sarcoidosis underlies idiopathic dilated cardiomyopathy: Importance of mediastinal lymphadenopathy in differential diagnosis. Circ J 2007; 71: 1937–1941. PMID: 18037750
- 3.
Terasaki F, Kitaura Y. Current status of cardiac sarcoidosis with dilated cardiomyopathy - with special reference to patients who underwent left ventriculoplasty (Batista operation). The Japanese Journal of Sarcoidosis and Other Granulomatous Disorders 2004; 24: 21–30. [In Japanese]
- 4.
Okura Y, Dec GW, Hare JM, et al. A clinical and histopathologic comparison of cardiac sarcoidosis and idiopathic giant cell myocarditis. J Am Coll Cardiol 2003; 41: 322–329 PMID: 125358295.
- 5.
Hiraga Y, Iwai K, Hiroe M, et al. Guidelines for the diagnosis of cardiac sarcoidosis 1992 - Preparation process. The 1993 report of the MHLW-funded study group on diffuse pulmonary diseases; 23–24. [In Japanese]
- 6.
The Japan Society of Sarcoidosis and Other Granulomatous Disorders, the Diffuse Pulmonary Study Group of the Health and Labor Sciences Research Grant-supported Rare/Intractable Disease Project, et al. Committee for revision of the diagnostic standard for sarcoidosis. Diagnostic standard and guideline for sarcoidosis - 2006. The Japanese Journal of Sarcoidosis and Other Granulomatous Disorders 2007; 27: 89–102. [In Japanese]
- 7.
The Japanese Circulation Society. Guidelines for diagnosis and treatment of myocarditis (JCS 2009). http://www.j-circ.or.jp/guideline/pdf/JCS2009_izumi_h.pdf [In Japanese]
- 8.
JCS Joint Working Group. Guidelines for diagnosis and treatment of myocarditis (JCS 2009): Digest version. Circ J 2011; 75: 734–743.
- 9.
The Japan Society of Sarcoidosis and Other Granulomatous Disorders, the Japanese Respiratory Society, the Japanese College of Cardiology, et al. Sarcoidosis Treatment Guideline Preparation Committee. Views on the treatment of sarcoidosis - 2003. The Japanese Journal of Sarcoidosis and Other Granulomatous Disorders 2003; 23: 105–114. [In Japanese]
- 10.
American Thoracic Society (ATS), the European Respiratory Society (ERS), the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG). Statement on sarcoidosis. Am J Respir Crit Care Med 1999; 160: 736–755. PMID: 10430755
- 11.
Milman N, Selroos O. Pulmonary sarcoidosis in the Nordic countries 1950–1982: Epidemiology and clinical picture. Sarcoidosis 1990; 7: 50–57. PMID: 2345819
- 12.
Iwai K, Sekiguti M, Hosoda Y, et al. Racial difference in cardiac sarcoidosis incidence observed at autopsy. Sarcoidosis 1994; 11: 26–31. PMID: 8036339
- 13.
Sawahata M, Sugiyama Y, Nakamura Y, et al. Age-related and historical changes in the clinical characteristics of sarcoidosis in Japan. Respir Med 2015; 109: 272–278. PMID: 25613109
- 14.
Henke CE, Henke G, Elveback LR, et al. The epidemiology of sarcoidosis in Rochester, Minnesota: A population-based study of incidence and survival. Am J Epidemiol 1986; 123: 840–845. PMID: 3962966
- 15.
Rybicki BA, Major M, Popovich J Jr, et al. Racial differences in sarcoidosis incidence: A 5-year study in a health maintenance organization. Am J Epidemiol 1997; 145: 234–241. PMID: 9012596
- 16.
Morimoto Y, Azuma A, Abe S, et al. Epidemiology of sarcoidosis in Japan. The Japanese Journal of Sarcoidosis and Other Granulomatous Disorders 2007; 27: 103–108. [In Japanese]
- 17.
Iwai K, Tachibana T, Takemura T, et al. Pathological studies on sarcoidosis autopsy. I. Epidemiological features of 320 cases in Japan. Acta Pathol Jpn 1993; 43: 372–376. PMID: 8372682
- 18.
Matsui Y, Iwai K, Tachibana T, et al. Clinicopathological study of fatal myocardial sarcoidosis. Ann N Y Acad Sci 1976; 278: 455–469. PMID: 1067031
- 19.
Iwai K, Takemura T, Kitaichi M, et al. Pathological studies on sarcoidosis autopsy. II. Early change, mode of progression and death pattern. Acta Pathol Jpn 1993; 43: 377–385. PMID: 8372683
- 20.
Silverman KJ, Hutchins GM, Bulkley BH. Cardiac sarcoid: A clinicopathologic study of 84 unselected patients with systemic sarcoidosis. Circulation 1978; 58: 1204–1211. PMID: 709777
- 21.
Yazaki Y, Isobe M, Hiroe M, et al. Central Japan Heart Study Group. Prognostic determinants of long-term survival in Japanese patients with cardiac sarcoidosis treated with prednisone. Am J Cardiol 2001; 88: 1006–1010. PMID: 11703997
- 22.
Homma JY, Abe C, Chosa H, et al. Bacteriological investigation on biopsy specimens from patients with sarcoidosis. Jpn J Exp Med 1978; 48: 251–255. PMID: 713130
- 23.
Eishi Y, Suga M, Ishige I, et al. Quantitative analysis of mycobacterial and propionibacterial DNA in lymph nodes of Japanese and European patients with sarcoidosis. J Clin Microbiol 2002; 40: 198–204. PMID: 11773116
- 24.
Minami J, Eishi Y, Ishige Y, et al. Pulmonary granulomas caused experimentally in mice by a recombinant trigger-factor protein of Propionibacterium acnes. J Med Dent Sci 2003; 50: 265–274. PMID: 15074354
- 25.
Hiraga Y, Hashimoto T, Saito N, et al. Double-blind comparative study on antibiotic (L-Keflex) in sarcoidosis. The Japanese Journal of Sarcoidosis and Other Granulomatous Disorders 1987; 7: 103–105. [In Japanese]
- 26.
Harada K, Tsuneyama K, Sudo Y, et al. Molecular identification of bacterial 16S ribosomal RNA gene in liver tissue of primary biliary cirrhosis: Is Propionibacterium acnes involved in granuloma formation? Hepatology 2001; 33: 530–536. PMID: 11230731
- 27.
Song Z, Marzilli L, Greenlee BM, et al. Mycobacterial catalase-peroxidase is a tissue antigen and target of the adaptive immune response in systemic sarcoidosis. J Exp Med 2005; 201: 755–767. PMID: 15753209
- 28.
Chen ES, Moller DR. Etiologies of Sarcoidosis. Clin Rev Allergy Immunol 2015; 49: 6–18. PMID: 25771769
- 29.
Inui N, Suda T, Chida K. Use of the QuantiFERON-TB Gold test in Japanese patients with sarcoidosis. Respir Med 2008; 102: 313–315. PMID: 17980570
- 30.
Kataoka M, Nakata Y, Hiramatsu J, et al. Familial occurrence of sarcoidosis in Japan and analysis of genetic influence. The Japanese Journal of Sarcoidosis and Other Granulomatous Disorders 2000; 20: 21–26. [In Japanese]
- 31.
Rybicki BA, Iannuzzi MC, Frederick MM, et al. ACCESS Research Group. Familial aggregation of sarcoidosis: A case-control etiologic study of sarcoidosis (ACCESS). Am J Respir Crit Care Med 2001; 164: 2085–2091. PMID: 11739139
- 32.
Ishihara M, Ohno S, Ishida T, et al. Molecular genetic studies of HLA class II alleles in sarcoidosis. Tissue Antigens 1994; 43: 238–241. PMID: 8085259
- 33.
Foley PJ, McGrath DS, Puscinska E, et al. Human leukocyte antigen-DRB1 position 11 residues are a common protective marker for sarcoidosis. Am J Respir Cell Mol Biol 2001; 25: 272–277. PMID: 11588003
- 34.
Spagnolo P, Sato H, Grutters JC, et al. Analysis of BTNL2 genetic polymorphisms in British and Dutch patients with sarcoidosis. Tissue Antigens 2007; 70: 219–227. PMID: 17661910
- 35.
Berlin M, Fogdell-Hahn A, Olerup O, et al. HLA-DR predicts the prognosis in Scandinavian patients with pulmonary sarcoidosis. Am J Respir Crit Care Med 1997; 156: 1601–1605. PMID: 9372682
- 36.
Hizawa N, Yamaguchi E, Furuya K, et al. The role of the C-C chemokine receptor 2 gene polymorphism V64I (CCR2-64I) in sarcoidosis in a Japanese population. Am J Respir Crit Care Med 1999; 159: 2021–2023. PMID: 10351956
- 37.
Tanabe T, Ishige I, Suzuki Y, et al. Sarcoidosis and NOD1 variation with impaired recognition of intracellular Propionibacterium acnes. Biochim Biophys Acta 2006; 1762: 794–801. PMID: 16935475
- 38.
Valentonyte R, Hampe J, Huse K, et al. Sarcoidosis is associated with a truncating splice site mutation in BTNL2. Nat Genet 2005; 37: 357–364. PMID: 15735647
- 39.
Rybicki BA, Maliarik MJ, Poisson LM, et al. Sarcoidosis and granuloma genes: A family-based study in African-Americans. Eur Respir J 2004; 24: 251–257. PMID: 15332393
- 40.
Hofmann S, Franke A, Fischer A, et al. Genome-wide association study identifies ANXA11 as a new susceptibility locus for sarcoidosis. Nat Genet 2008; 40: 1103–1106. PMID: 19165924
- 41.
Hofmann S, Fischer A, Till A, et al. GenPhenReSa Consortium. A genome-wide association study reveals evidence of association with sarcoidosis at 6p12.1. Eur Respir J 2011; 38: 1127–1135. PMID: 21540310
- 42.
Fischer A, Schmid B, Ellinghaus D, et al. A novel sarcoidosis risk locus for Europeans on chromosome 11q13.1. Am J Respir Crit Care Med 2012; 186: 877–885. PMID: 22837380
- 43.
Hofmann S, Fischer A, Nothnagel M, et al. Genome-wide association analysis reveals 12q13.3-q14.1 as new risk locus for sarcoidosis. Eur Respir J 2013; 41: 888–900. PMID: 22936702
- 44.
Levin AM, Iannuzzi MC, Montgomery CG, et al. Admixture fine-mapping in African Americans implicates XAF1 as a possible sarcoidosis risk gene. PLoS One 2014; 9: e92646. PMID: 24663488
- 45.
Kodama N, Yamaguchi E, Hizawa N, et al. Expression of RANTES by bronchoalveolar lavage cells in nonsmoking patients with interstitial lung diseases. Am J Respir Cell Mol Biol 1998; 18: 526–531. PMID: 9533940
- 46.
Yamaguchi E, Okazaki N, Tsuneta Y, et al. Interleukins in pulmonary sarcoidosis. Dissociative correlations of lung interleukins 1 and 2 with the intensity of alveolitis. Am Rev Respir Dis 1988; 138: 645–651. PMID: 3264477
- 47.
Yamaguchi E, Itoh A, Furuya K, et al. Release of tumor necrosis factor-alpha from human alveolar macrophages is decreased in smokers. Chest 1993; 103: 479–483. PMID: 8432140
- 48.
Itoh A, Yamaguchi E, Kuzumaki N, et al. Expression of granulocyte-macrophage colony-stimulating factor mRNA by inflammatory cells in the sarcoid lung. Am J Respir Cell Mol Biol 1990; 3: 245–249. PMID: 2202340
- 49.
Robinson BW, McLemore TL, Crystal RG. Gamma interferon is spontaneously released by alveolar macrophages and lung T lymphocytes in patients with pulmonary sarcoidosis. J Clin Invest 1985; 75: 1488–1495. PMID: 3923038
- 50.
Hancock WW, Kobzik L, Colby AJ, et al. Detection of lymphokines and lymphokine receptors in pulmonary sarcoidosis. Immunohistologic evidence that inflammatory macrophages express IL-2 receptors. Am J Pathol 1986; 123: 1–8. PMID: 3083688
- 51.
Shigehara K, Shijubo N, Ohmichi M, et al. Increased circulating interleukin-12 (IL-12) p40 in pulmonary sarcoidosis. Clin Exp Immunol 2003; 132: 152–157. PMID: 12653850
- 52.
Tanaka H, Miyazaki N, Oashi K, et al. IL-18 might reflect disease activity in mild and moderate asthma exacerbation. J Allergy Clin Immunol 2001; 107: 331–336. PMID: 11174201
- 53.
Agostini C, Cassatella M, Zambello R, et al. Involvement of the IP-10 chemokine in sarcoid granulomatous reactions. J Immunol 1998; 161: 6413–6420. PMID: 9834133
- 54.
Ten Berge B, Paats MS, Bergen IM, et al. Increased IL-17A expression in granulomas and in circulating memory T cells in sarcoidosis. Rheumatology (Oxford) 2012; 51: 37–46. PMID: 22075064
- 55.
Furusawa H, Suzuki Y, Miyazaki Y, et al. Th1 and Th17 immune responses to viable Propionibacterium acnes in patients with sarcoidosis. Respir Investig 2012; 50: 104–109. PMID: 23021769
- 56.
Saussine A, Tazi A, Feuillet S, et al. Active chronic sarcoidosis is characterized by increased transitional blood B cells, increased IL-10-producing regulatory B cells and high BAFF levels. PLoS One 2012; 7: e43588. PMID: 22927996
- 57.
Homma S, Nagaoka I, Abe H, et al. Localization of platelet-derived growth factor and insulin-like growth factor I in the fibrotic lung. Am J Respir Crit Care Med 1995; 152: 2084–2089. PMID: 8520779
- 58.
Ishioka S, Saito T, Hiyama K, et al. Increased expression of tumor necrosis factor-alpha, interleukin-6, platelet-derived growth factor-B and granulocyte-macrophage colony-stimulating factor mRNA in cells of bronchoalveolar lavage fluids from patients with sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 1996; 13: 139–145. PMID: 8893383
- 59.
Allen JT, Knight RA, Bloor CA, et al. Enhanced insulin-like growth factor binding protein-related protein 2 (Connective tissue growth factor) expression in patients with idiopathic pulmonary fibrosis and pulmonary sarcoidosis. Am J Respir Cell Mol Biol 1999; 21: 693–700. PMID: 10572066
- 60.
Abe C, Iwai K, Mikami R, et al. Frequent isolation of Propionibacterium acnes from sarcoidosis lymph nodes. Zentralbl Bakteriol Mikrobiol Hyg A 1984; 256: 541–547. PMID: 6377763
- 61.
Ishige I, Usui Y, Takemura T, et al. Quantitative PCR of mycobacterial and propionibacterial DNA in lymph nodes of Japanese patients with sarcoidosis. Lancet 1999; 354: 120–123. PMID: 10408488
- 62.
Yamada T, Eishi Y, Ikeda S, et al. In situ localization of Propionibacterium acnes DNA in lymph nodes from sarcoidosis patients by signal amplification with catalysed reporter deposition. J Pathol 2002; 198: 541–547. PMID: 12434425
- 63.
Negi M, Takemura T, Guzman J, et al. Localization of Propionibacterium acnes in granulomas supports a possible etiologic link between sarcoidosis and the bacterium. Mod Pathol 2012; 25: 1284–1297. PMID: 22596102
- 64.
Eishi Y. Etiologic aspect of sarcoidosis as an allergic endogenous infection caused by Propionibacterium acnes. Biomed Res Int 2013; 2013: 935289. PMID: 23844371
- 65.
MHW-funded study group on diffuse pulmonary diseases. Sarcoidosis. Diagnosis and treatment strategies for rare/intractable diseases (Supervised by the Disease Control Division, the Health Service Bureau, the Ministry of Health and Welfare). 1997; 62–65. [In Japanese]
- 66.
MHLW-funded study group on diffuse pulmonary diseases. Sarcoidosis. Textbook for rare/intractable disease specialist (Supervised by the Disease Control Division, the Health Service Bureau, the Ministry of Health, Labour and Welfare). 2015. [In Japanese]
- 67.
The Japan Society of Sarcoidosis and Other Granulomatous Disorders. Diagnostic standard and guideline for sarcoidosis – 2015. http://www.jssog.com/www/top/shindan/shindan2-1new.html [In Japanese]
- 68.
Baughman RP, Teirstein AS, Judson MA, et al. Case Control Etiologic Study of Sarcoidosis (ACCESS) research group. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med 2001; 164: 1885–1889. PMID: 11734441
- 69.
Baughman RP. Pulmonary sarcoidosis. Clin Chest Med 2004; 25: 521–530, vi. PMID: 15331189
- 70.
King TE Jr. Clinical manifestations and diagnosis of pulmonary sarcoidosis. UpToDate 2016. http://www.uptodate.com/contents/clinical-manifestations-and-diagnosis-of-pulmonary-sarcoidosis
- 71.
Fujimoto K. Thoracic diagnostic imaging of sarcoidosis. The Japanese Journal of Sarcoidosis and Other Granulomatous Disorders 2013; 33: 31–34. [In Japanese]
- 72.
Hours S, Nunes H, Kambouchner M, et al. Pulmonary cavitary sarcoidosis: Clinico-radiologic characteristics and natural history of a rare form of sarcoidosis. Medicine (Baltimore) 2008; 87: 142–151. PMID: 18520323
- 73.
Koyama T, Ueda H, Togashi K, et al. Radiologic manifestations of sarcoidosis in various organs. Radiographics 2004; 24: 87–104. PMID: 14730039
- 74.
Malaisamy S, Dalal B, Bimenyuy C, et al. The clinical and radiologic features of nodular pulmonary sarcoidosis. Lung 2009; 187: 9–15. PMID: 18843518
- 75.
Sileo C, Epaud R, Mahloul M, et al. Sarcoidosis in children: HRCT findings and correlation with pulmonary function tests. Pediatr Pulmonol 2014; 49: 1223–1233. PMID: 24339447
- 76.
Paramothayan NS, Lasserson TJ, Jones PW. Corticosteroids for pulmonary sarcoidosis. Cochrane Database Syst Rev 2005; CD001114. PMID: 15846612
- 77.
Gibson GJ, Prescott RJ, Muers MF, et al. British Thoracic Society Sarcoidosis study: Effects of long term corticosteroid treatment. Thorax 1996; 51: 238–247. PMID: 8779124
- 78.
Pietinalho A, Tukiainen P, Haahtela T, et al. Finnish Pulmonary Sarcoidosis Study Group. Early treatment of stage II sarcoidosis improves 5-year pulmonary function. Chest 2002; 121: 24–31. PMID: 11796428
- 79.
Miller A. Of time and experience: Sarcoidosis revisited. Chest 2002; 121: 3–5. PMID: 11796421
- 80.
Izumi T. Are corticosteroids harmful to sarcoidosis: A conclusion drawn from a retrospective study on the chest radiographic prognosis of 185 asymptomatic patients with pulmonary sarcoidosis followed up for more than 10 years. Sarcoidosis 1994; 11(Suppl 1): 119–122.
- 81.
Gottlieb JE, Israel HL, Steiner RM, et al. Outcome in sarcoidosis. The relationship of relapse to corticosteroid therapy. Chest 1997; 111: 623–631. PMID: 9118698
- 82.
Rizzato G, Montemurro L, Colombo P. The late follow-up of chronic sarcoid patients previously treated with corticosteroids. Sarcoidosis Vasc Diffuse Lung Dis 1998; 15: 52–58. PMID: 9572002
- 83.
Baughman RP, Culver DA, Judson MA. A concise review of pulmonary sarcoidosis. Am J Respir Crit Care Med 2011; 183: 573–581. PMID: 21037016
- 84.
Johns CJ, Michele TM. The clinical management of sarcoidosis. A 50-year experience at the Johns Hopkins Hospital. Medicine (Baltimore) 1999; 78: 65–111. PMID: 10195091
- 85.
Judson MA. The treatment of pulmonary sarcoidosis. Respir Med 2012; 106: 1351–1361. PMID: 22495110
- 86.
Shijubo N, Ito M, Ichimura S, et al. A case of pulmonary sarcoidosis with bronchovascular bundle lesions and cavity improved with low dose corticosteroid therapy. The Japanese Journal of Sarcoidosis and Other Granulomatous Disorders 2013; 33: 123–126. [In Japanese]
- 87.
Kato Y, Morimoto S, Uemura A, et al. Efficacy of corticosteroids in sarcoidosis presenting with atrioventricular block. Sarcoidosis Vasc Diffuse Lung Dis 2003; 20: 133–137. PMID: 12870723
- 88.
Cremers JP, Drent M, Bast A, et al. Multinational evidence-based World Association of Sarcoidosis and Other Granulomatous Disorders recommendations for the use of methotrexate in sarcoidosis: Integrating systematic literature research and expert opinion of sarcoidologists worldwide. Curr Opin Pulm Med 2013; 19: 545–561. PMID: 23880702
- 89.
Vorselaars AD, Wuyts WA, Vorselaars VM, et al. Methotrexate vs azathioprine in second-line therapy of sarcoidosis. Chest 2013; 144: 805–812. PMID: 23538719
- 90.
Nagai S, Yokomatsu T, Tanizawa K, et al. Treatment with methotrexate and low-dose corticosteroids in sarcoidosis patients with cardiac lesions. Intern Med 2014; 53: 427–433. PMID: 24583430
- 91.
Isshiki T, Yamaguchi T, Yamada Y, et al. Usefulness of low-dose methotrexate monotherapy for treating sarcoidosis. Intern Med 2013; 52: 2727–2732. PMID: 24334575
- 92.
Bachelez H, Senet P, Cadranel J, et al. The use of tetracyclines for the treatment of sarcoidosis. Arch Dermatol 2001; 137: 69–73. PMID: 11176663
- 93.
Yamaguchi T, Yamaguchi Y, Suzuki M, et al. Treatment of sarcoidosis by the use of doxycycline. The Japanese Journal of Sarcoidosis and Other Granulomatous Disorders 2014; 34: 31–33. [In Japanese]
- 94.
Michielsen HJ, Drent M, Peros-Golubicic T, et al. Fatigue is associated with quality of life in sarcoidosis patients. Chest 2006; 130: 989–994. PMID: 17035429
- 95.
Hoitsma E, De Vries J, van Santen-Hoeufft M, et al. Impact of pain in a Dutch sarcoidosis patient population. Sarcoidosis Vasc Diffuse Lung Dis 2003; 20: 33–39. PMID: 12737278
- 96.
Murai M, Yamaguchi T, Mitsuma T, et al. A case of sarcoidosis accompanying systemic symptoms successfully treated with kampo medications. Annals of the Japanese Respiratory Society 2012; 1: 9–13. [In Japanese]
- 97.
Saito H, Yamaguchi T, Adachi Y, et al. Neurological symptoms of sarcoidosis-induced small fiber neuropathy effectively relieved with high-dose steroid pulse therapy. Intern Med 2015; 54: 1281–1286. PMID: 25986271
- 98.
Yoshida Y, Morimoto S, Hiramitsu S, et al. Incidence of cardiac sarcoidosis in Japanese patients with high-degree atrioventricular block. Am Heart J 1997; 134: 382–386. PMID: 9327691
- 99.
Uemura A, Morimoto S, Hiramitsu S, et al. Histologic diagnostic rate of cardiac sarcoidosis: Evaluation of endomyocardial biopsies. Am Heart J 1999; 138: 299–302. PMID: 10426842
- 100.
From AM, Maleszewski JJ, Rihal CS. Current status of endomyocardial biopsy. Mayo Clin Proc 2011; 86: 1095–1102. PMID: 22033254
- 101.
Valantine H, McKenna WJ, Nihoyannopoulos P, et al. Sarcoidosis: A pattern of clinical and morphological presentation. Br Heart J 1987; 57: 256–263. PMID: 3566984
- 102.
Roberts WC, McAllister HA Jr, Ferrans VJ. Sarcoidosis of the heart. A clinicopathologic study of 35 necropsy patients (group I) and review of 78 previously described necropsy patients (group II). Am J Med 1977; 63: 86–108. PMID: 327806
- 103.
Seward JB, Casaclang-Verzosa G. Infiltrative cardiovascular diseases: Cardiomyopathies that look alike. J Am Coll Cardiol 2010; 55: 1769–1779. PMID: 20413025
- 104.
Lagana SM, Parwani AV, Nichols LC. Cardiac sarcoidosis: A pathology-focused review. Arch Pathol Lab Med 2010; 134: 1039–1046. PMID: 20586635
- 105.
Blauwet LA, Cooper LT. Idiopathic giant cell myocarditis and cardiac sarcoidosis. Heart Fail Rev 2013; 18: 733–746. PMID: 23111533
- 106.
Ando M, Oritsu M, Kitaichi M, et al. ATS/ERS/WASOG statement on sarcoidosis. In: The Japan Society of Sarcoidosis and Other Granulomatous Disorders, editor. Supervised by Ando M and Yotsumoto H. Sarcoidosis and other granulomatous disorders. Kokuseido Shuppan, 2006; 295–329. [In Japanese]
- 107.
Birnie DH, Sauer WH, Bogun F, et al. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm 2014; 11: 1305–1323. PMID: 24819193
- 108.
Mehta D, Lubitz SA, Frankel Z, et al. Cardiac involvement in patients with sarcoidosis: Diagnostic and prognostic value of outpatient testing. Chest 2008; 133: 1426–1435. PMID: 18339784
- 109.
Kato Y, Morimoto S. Analysis of clinical manifestations of cardiac sarcoidosis - A multicentre study: Preliminary report. The Japanese Journal of Sarcoidosis and Other Granulomatous Disorders 2010; 30: 73–76. [In Japanese]
- 110.
Shijubo N, Ichimura S, Ito M, et al. Analysis of several examinations in 516 histologically proven sarcoidosis patients. The Japanese Journal of Sarcoidosis and Other Granulomatous Disorders 2007; 27: 29–35. [In Japanese]
- 111.
Kiyotake R, Okinami S, Soma M, et al. Evaluation of revised diagnosis criteria for sarcoidosis. The Japanese Journal of Dermatology 2010; 114: 678–682. [In Japanese]
- 112.
Handa T, Nagai S, Ueda S, et al. Significance of plasma NT-proBNP levels as a biomarker in the assessment of cardiac involvement and pulmonary hypertension in patients with sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2010; 27: 27–35. PMID: 21086902
- 113.
Yasutake H, Seino Y, Kashiwagi M, et al. Detection of cardiac sarcoidosis using cardiac markers and myocardial integrated backscatter. Int J Cardiol 2005; 102: 259–268. PMID: 15982494
- 114.
Baba Y, Kubo T, Kitaoka H, et al. Usefulness of high-sensitive cardiac troponin T for evaluating the activity of cardiac sarcoidosis. Int Heart J 2012; 53: 287–292. PMID: 23038089
- 115.
Tanada Y, Sato Y, Sawa T, et al. Serial measurement of high-sensitivity cardiac troponin I and N-terminal proB-type natriuretic peptide in a patient presenting with cardiac sarcoidosis. Intern Med 2012; 51: 3379–3381. PMID: 23257523
- 116.
Kandolin R, Lehtonen J, Airaksinen J, et al. Usefulness of cardiac troponins as markers of early treatment response in cardiac sarcoidosis. Am J Cardiol 2015; 116: 960–964. PMID: 26209113
- 117.
Kobayashi S, Myoren T, Oda S, et al. Urinary 8-hydroxy-2´-deoxyguanosine as a novel biomarker of inflammatory activity in patients with cardiac sarcoidosis. Int J Cardiol 2015; 190: 319–328. PMID: 25935620
- 118.
Semenzato G, Cipriani A, Trentin L, et al. High serum levels of soluble interleukin-2 receptors in sarcoidosis. Sarcoidosis 1987; 4: 25–27. PMID: 3108983
- 119.
Grutters JC, Fellrath JM, Mulder L, et al. Serum soluble interleukin-2 receptor measurement in patients with sarcoidosis: A clinical evaluation. Chest 2003; 124: 186–195. PMID: 12853522
- 120.
Rothkrantz-Kos S, van Dieijen-Visser MP, Mulder PG, et al. Potential usefulness of inflammatory markers to monitor respiratory functional impairment in sarcoidosis. Clin Chem 2003; 49: 1510–1517. PMID: 12928233
- 121.
Yoshida Y, Morimoto S, Hiramitsu S, et al. Incidence of cardiac sarcoidosis in patients with high-degree atrioventricular block. SHINZO 1995; 10: 874–879. [In Japanese]
- 122.
Terasaki F, Ukimura A, Tsukada B, et al. Enhanced expression of type 1 helper T-cell cytokines in the myocardium of active cardiac sarcoidosis. Circ J 2008; 72: 1303–1307. PMID: 18654018
- 123.
Terasaki F, Fujita M, Shimomura H, et al. Enhanced expression of myeloid-related protein complex (MRP8/14) in macrophages and multinucleated giant cells in granulomas of patients with active cardiac sarcoidosis. Circ J 2007; 71: 1545–1550. PMID: 17895549
- 124.
Padilla ML. Cardiac sarcoidosis. In: Baughman RP, editor. Sarcoidosis (Lung Biology in Health and Disease vol.210). Taylor & Francis 2006.
- 125.
Tsuchida A. Clinical picture of cardiac sarcoidosis. Respiration and Circulation 2006; 54: 925–931. [In Japanese]
- 126.
Sekhri V, Sanal S, Delorenzo LJ, et al. Cardiac sarcoidosis: A comprehensive review. Arch Med Sci 2011; 7: 546–554. PMID: 22291785
- 127.
Oritsu M. Diagnostic criteria and procedures. In: The Japan Society of Sarcoidosis and Other Granulomatous Disorders, editor. Supervised by Ando M and Yotsumoto H. Sarcoidosis and other granulomatous disorders. Kokuseido Shuppan, 2006; 136–143. [In Japanese]
- 128.
Tsuchida A, Nanba M, Endo T, et al. Incidence of cardiac complications in sarcoidosis patients with no abnormalities on electrocardiograms. Circ J 2004; 68(Suppl 1): 626.
- 129.
Kato Y, Morimoto S, Hiramitsu S, et al. Two cases in which cardiac sarcoidosis was strongly suspected despite not fulfilling the diagnostic guideline. The Japanese Journal of Sarcoidosis and Other Granulomatous Disorders 1999; 19: 91–96. [In Japanese]
- 130.
Tsuchida A, Hasegawa T, Sakamoto J, et al. A case of cardiac sarcoidosis developed ten years after diagnosis of sarcoidosis. The Japanese Journal of Sarcoidosis and Other Granulomatous Disorders 2008; 28: 81–85. [In Japanese]
- 131.
Yazaki Y, Sekiguchi M, Nunoda S, et al. Recent clinical profile of cardiac sarcoidosis cases in Japan. Sarcoidosis 1992; 9(Suppl): 399–400.
- 132.
Uemura A, Morimoto S. Heart. In: Japan Society of Sarcoidosis and Other Granulomatous Disorders, editor. Ando M, Yotsumoto H, supervisors. Sarcoidosis and other granulomatous disorders. Kokuseido Shuppan, 2006; 72–76. [In Japanese]
- 133.
Kato Y, Morimoto S. Atrioventricular block in cardiac sarcoidosis. The Japanese Journal of Sarcoidosis and Other Granulomatous Disorders 2011; 31: 66–72. [In Japanese]
- 134.
Morimoto S, Kato Y, Kitakaze M, et al. Clinical picture of sarcoidosis in 134 patients: A multi-center study. J Cardiol 2014; (Suppl): O–209. [In Japanese]
- 135.
Tsuchida A, Hasegawa T, Sakamoto J, et al. Two cases of cardiac sarcoidosis with complete atrioventricular block naturally improved several years after implanting pacemaker. The Japanese Journal of Sarcoidosis and Other Granulomatous Disorders 2010; 30: 15–20. [In Japanese]
- 136.
Kandolin R, Lehtonen J, Graner M, et al. Diagnosing isolated cardiac sarcoidosis. J Intern Med 2011; 270: 461–468. PMID: 21535250
- 137.
Swanton RH. Sarcoidosis of the heart. Eur Heart J 1988; 9 Suppl G: 169–174. PMID: 3042416
- 138.
Furushima H, Chinushi M, Sugiura H, et al. Ventricular tachyarrhythmia associated with cardiac sarcoidosis: Its mechanisms and outcome. Clin Cardiol 2004; 27: 217–222. PMID: 15119697
- 139.
Uusimaa P, Ylitalo K, Anttonen O, et al. Ventricular tachyarrhythmia as a primary presentation of sarcoidosis. Europace 2008; 10: 760–766. PMID: 18456644
- 140.
Kusano K. Non-pharmacological therapy for cardiac sarcoidosis - Treatment of arrhythmias in cardiac sarcoidosis. Journal of Clinical and Experimental Medicine 2013; 247: 177–181. [In Japanese]
- 141.
Winters SL, Cohen M, Greenberg S, et al. Sustained ventricular tachycardia associated with sarcoidosis: Assessment of the underlying cardiac anatomy and the prospective utility of programmed ventricular stimulation, drug therapy and an implantable antitachycardia device. J Am Coll Cardiol 1991; 18: 937–943. PMID: 1894867
- 142.
Perry A, Vuitch F. Causes of death in patients with sarcoidosis. A morphologic study of 38 autopsies with clinicopathologic correlations. Arch Pathol Lab Med 1995; 119: 167–172. PMID: 7848065
- 143.
Patel MR, Cawley PJ, Heitner JF, et al. Detection of myocardial damage in patients with sarcoidosis. Circulation 2009; 120: 1969–1977. PMID: 19884472
- 144.
Sharma S. Cardiac imaging in myocardial sarcoidosis and other cardiomyopathies. Curr Opin Pulm Med 2009; 15: 507–512. PMID: 19542892
- 145.
Yamano T, Nakatani S. Cardiac Sarcoidosis: What can we know from Echocardiography? J Echocardiogr 2007; 5: 1–10.
- 146.
Kato Y, Morimoto S. Diagnosis and treatment of cardiac sarcoidosis. The Japanese Journal of Sarcoidosis and Other Granulomatous Disorders 2008; 28: 15–24. [In Japanese]
- 147.
Yazaki Y, Isobe M, Hayasaka M, et al. Cardiac sarcoidosis mimicking hypertrophic cardiomyopathy: Clinical utility of radionuclide imaging for differential diagnosis. Jpn Circ J 1998; 62: 465–468. PMID: 9652326
- 148.
Matsumori A, Hara M, Nagai S, et al. Hypertrophic cardiomyopathy as a manifestation of cardiac sarcoidosis. Jpn Circ J 2000; 64: 679–683. PMID: 10981852
- 149.
Okamura H, Goto Y, Terashima M, et al. Images in cardiovascular medicine. Reversible right ventricular hypertrophy due to cardiac sarcoidosis. Circulation 2005; 111: e383–e384. PMID: 15956140
- 150.
Lam CS, Tolep KA, Metke MP, et al. Coronary sarcoidosis presenting as acute coronary syndrome. Clin Cardiol 2009; 32: E68–E71. PMID: 19330817
- 151.
Shiraishi J, Tatsumi T, Shimoo K, et al. Cardiac sarcoidosis mimicking right ventricular dysplasia. Circ J 2003; 67: 169–171. PMID: 12548003
- 152.
Rubinstein I, Baum GL, Hiss Y. Cardiac tamponade as the presenting symptom of sarcoidosis. Am Heart J 1985; 109: 1387–1388. PMID: 4003247
- 153.
Burstow DJ, Tajik AJ, Bailey KR, et al. Two-dimensional echocardiographic findings in systemic sarcoidosis. Am J Cardiol 1989; 63: 478–482. PMID: 2916434
- 154.
Joyce E, Ninaber MK, Katsanos S, et al. Subclinical left ventricular dysfunction by echocardiographic speckle-tracking strain analysis relates to outcome in sarcoidosis. Eur J Heart Fail 2015; 17: 51–62. PMID: 25431267
- 155.
The Japanese Circulation Society. A Web report on the Japanese registry of all cardiac and vascular diseases (JROAD) in 2013 (conducted and published in 2014). http://www.j-circ.or.jp/jittai_chosa/jittai_chosa2013web.pdf [In Japanese]
- 156.
Nureki S, Miyazaki E, Nishio S, et al. Interventricular septal thickening as an early manifestation of cardiac sarcoidosis. Int Heart J 2014; 55: 181–183. PMID: 24632961
- 157.
Kramer CM, Barkhausen J, Flamm SD, et al. Society for Cardiovascular Magnetic Resonance Board of Trustees Task Force on Standardized Protocols. Standardized cardiovascular magnetic resonance imaging (CMR) protocols, society for cardiovascular magnetic resonance: Board of trustees task force on standardized protocols. J Cardiovasc Magn Reson 2008; 10: 35. PMID: 18605997
- 158.
Herzog B, Greenwood J, Plein S. Cardiovascular Magnetic Resonance Pocket Guide 2013. ESC working group, 2013.
- 159.
Smedema JP, Snoep G, van Kroonenburgh MP, et al. Evaluation of the accuracy of gadolinium-enhanced cardiovascular magnetic resonance in the diagnosis of cardiac sarcoidosis. J Am Coll Cardiol 2005; 45: 1683–1690. PMID: 15893188
- 160.
Tadamura E, Yamamuro M, Kubo S, et al. Effectiveness of delayed enhanced MRI for identification of cardiac sarcoidosis: Comparison with radionuclide imaging. AJR Am J Roentgenol 2005; 185: 110–115. PMID: 15972409
- 161.
Watanabe E, Kimura F, Nakajima T, et al. Late gadolinium enhancement in cardiac sarcoidosis: Characteristic magnetic resonance findings and relationship with left ventricular function. J Thorac Imaging 2013; 28: 60–66. PMID: 23249970
- 162.
Yang Y, Safka K, Graham JJ, et al. Correlation of late gadolinium enhancement MRI and quantitative T2 measurement in cardiac sarcoidosis. J Magn Reson Imaging 2014; 39: 609–616. PMID: 23720077
- 163.
Ichinose A, Otani H, Oikawa M, et al. MRI of cardiac sarcoidosis: Basal and subepicardial localization of myocardial lesions and their effect on left ventricular function. AJR Am J Roentgenol 2008; 191: 862–869. PMID: 18716120
- 164.
Donsky AS, Escobar J, Capehart J, et al. Heart transplantation for undiagnosed cardiac sarcoidosis. Am J Cardiol 2002; 89: 1447–1450. PMID: 12062749
- 165.
Greulich S, Deluigi CC, Gloekler S, et al. CMR imaging predicts death and other adverse events in suspected cardiac sarcoidosis. JACC Cardiovasc Imaging 2013; 6: 501–511. PMID: 23498675
- 166.
Nagai T, Kohsaka S, Okuda S, et al. Incidence and prognostic significance of myocardial late gadolinium enhancement in patients with sarcoidosis without cardiac manifestation. Chest 2014; 146: 1064–1072. PMID: 24853830
- 167.
Ise T, Hasegawa T, Morita Y, et al. Extensive late gadolinium enhancement on cardiovascular magnetic resonance predicts adverse outcomes and lack of improvement in LV function after steroid therapy in cardiac sarcoidosis. Heart 2014; 100: 1165–1172. PMID: 24829369
- 168.
Shafee MA, Fukuda K, Wakayama Y, et al. Delayed enhancement on cardiac magnetic resonance imaging is a poor prognostic factor in patients with cardiac sarcoidosis. J Cardiol 2012; 60: 448–453. PMID: 22944174
- 169.
The Japanese Society of Nuclear Cardiology. Recommendation for 18F FDG PET imaging for cardiac sarcoidosis. Journal of the Japanese Society of Nuclear Cardiology 2013; 15: 35–47. [In Japanese]
- 170.
Ishida Y, Yoshinaga K, Miyagawa M, et al. Recommendations for (18) F-fluorodeoxyglucose positron emission tomography imaging for cardiac sarcoidosis: Japanese Society of Nuclear Cardiology recommendations. Ann Nucl Med 2014; 28: 393–403. PMID: 24464391
- 171.
Isobe M, Tezuka D. Isolated cardiac sarcoidosis: Clinical characteristics, diagnosis and treatment. Int J Cardiol 2015; 182: 132–140. PMID: 25577749
- 172.
Schneider S, Batrice A, Rischpler C, et al. Utility of multimodal cardiac imaging with PET/MRI in cardiac sarcoidosis: Implications for diagnosis, monitoring and treatment. Eur Heart J 2014; 35: 312. PMID: 23975480
- 173.
White JA, Rajchl M, Butler J, et al. Active cardiac sarcoidosis: First clinical experience of simultaneous positron emission tomography--magnetic resonance imaging for the diagnosis of cardiac disease. Circulation 2013; 127: e639–e641. PMID: 23733970
- 174.
Yazaki Y. The diagnostic dilemma of isolated cardiac sarcoidosis. Intern Med 2013; 52: 1–2. PMID: 23291666
- 175.
Miyazaki S, Funabashi N, Nagai T, et al. Cardiac sarcoidosis complicated with atrioventricular block and wall thinning, edema and fibrosis in left ventricle: Confirmed recovery to normal sinus rhythm and visualization of edema improvement by administration of predonisolone. Int J Cardiol 2011; 150: e4–e10. PMID: 19540005
- 176.
Shimada T, Shimada K, Sakane T, et al. Diagnosis of cardiac sarcoidosis and evaluation of the effects of steroid therapy by gadolinium-DTPA-enhanced magnetic resonance imaging. Am J Med 2001; 110: 520–527. PMID: 11343665
- 177.
Moon JC, Messroghli DR, Kellman P, et al; Society for Cardiovascular Magnetic Resonance Imaging; Cardiovascular Magnetic Resonance Working Group of the European Society of Cardiology. Myocardial T1 mapping and extracellular volume quantification: A Society for Cardiovascular Magnetic Resonance (SCMR) and CMR Working Group of the European Society of Cardiology consensus statement. J Cardiovasc Magn Reson 2013; 15: 92. PMID: 24124732
- 178.
Nakano S, Kimura F, Osman N, et al. Improved myocardial strain measured by strain-encoded magnetic resonance imaging in a patient with cardiac sarcoidosis. Can J Cardiol 2013; 29: 1531.e9–e11. PMID: 23642331
- 179.
Hamlin SA, Henry TS, Little BP, et al. Mapping the future of cardiac MR imaging: Case-based review of T1 and T2 mapping techniques. Radiographics 2014; 34: 1594–1611. PMID: 25310419
- 180.
Pellegrino D, Bonab AA, Dragotakes SC, et al. Inflammation and infection: Imaging properties of 18F-FDG-labeled white blood cells versus 18F-FDG. J Nucl Med 2005; 46: 1522–1530. PMID: 16157536
- 181.
Koiwa H, Tsujino I, Ohira H, et al. Images in cardiovascular medicine: Imaging of cardiac sarcoid lesions using fasting cardiac 18F-fluorodeoxyglucose positron emission tomography: An autopsy case. Circulation 2010; 122: 535–536. PMID: 20679583
- 182.
Nuutila P, Koivisto VA, Knuuti J, et al. Glucose-free fatty acid cycle operates in human heart and skeletal muscle in vivo. J Clin Invest 1992; 89: 1767–1774. PMID: 1601987
- 183.
Ohira H, Tsujino I, Yoshinaga K. 18F-Fluoro-2-deoxyglucose positron emission tomography in cardiac sarcoidosis. Eur J Nucl Med Mol Imaging 2011; 38: 1773–1783. PMID: 21559980
- 184.
Nishiyama Y, Yamamoto Y, Fukunaga K, et al. Comparative evaluation of 18F-FDG PET and 67Ga scintigraphy in patients with sarcoidosis. J Nucl Med 2006; 47: 1571–1576. PMID: 17015889
- 185.
Morooka M, Moroi M, Uno K, et al. Long fasting is effective in inhibiting physiological myocardial 18F-FDG uptake and for evaluating active lesions of cardiac sarcoidosis. EJNMMI Res 2014; 4: 1. PMID: 24382020
- 186.
Ishida Y, Kiso K, Ueda H. Diagnosis of cardiac sarcoidosis by F-18 FDG PET - Usefulness and limitation. The Japanese Journal of Sarcoidosis and Other Granulomatous Disorders 2010; 30: 77–80. [In Japanese]
- 187.
Chen S, Bokhari S. Diagnosis of cardiac sarcoidosis through mismatched defects seen on N-13 NH3/F-18 FDG cardiac PET. Clin Nucl Med 2011; 36: 1156–1157. PMID: 22064101
- 188.
Brancato SC, Arrighi JA. Fasting FDG PET compared to MPI SPECT in cardiac sarcoidosis. J Nucl Cardiol 2011; 18: 371–374. PMID: 21318450
- 189.
Yamagishi H, Shirai N, Takagi M, et al. Identification of cardiac sarcoidosis with 13N-NH3/18F-FDG PET. J Nucl Med 2003; 44: 1030–1036. PMID: 12843216
- 190.
Kosuge H, Noda M, Kakuta T, et al. Left ventricular apical aneurysm in cardiac sarcoidosis. Jpn Heart J 2001; 42: 265–269. PMID: 11384087
- 191.
Egashira T, Makino S, Kunitomi A, et al. Necessity for rule out coronary artery disease with the positive findings of 18F-FDG-PET in case of systemic sarcoidosis. Int J Cardiol 2014; 172: e401–e402. PMID: 24461987
- 192.
Tung R, Bauer B, Schelbert H, et al. Incidence of abnormal positron emission tomography in patients with unexplained cardiomyopathy and ventricular arrhythmias: The potential role of occult inflammation in arrhythmogenesis. Heart Rhythm 2015; 12: 2488–2498. PMID: 26272522
- 193.
Berry JJ, Baker JA, Pieper KS, et al. The effect of metabolic milieu on cardiac PET imaging using fluorine-18-deoxyglucose and nitrogen-13-ammonia in normal volunteers. J Nucl Med 1991; 32: 1518–1525. PMID: 1869972
- 194.
Bartlett ML, Bacharach SL, Voipio-Pulkki LM, et al. Artifactual in homogeneities in myocardial PET and SPECT scans in normal subjects. J Nucl Med 1995; 36: 188–195. PMID: 7830111
- 195.
Gropler RJ, Siegel BA, Lee KJ, et al. Nonuniformity in myocardial accumulation of fluorine-18-fluorodeoxyglucose in normal fasted humans. J Nucl Med 1990; 31: 1749–1756. PMID: 2230987
- 196.
Ishida Y, Yasumura Y, Fukuchi K, et al. Increased myocardial glucose utilization in the fasting state as a metabolic indicator of severity of heart failure: A study by F-18 FDG PET. In: Nagara T, Tsukamoto E, Kuge Y, et al. editors. Positron emission tomography in the millennium. Elsevier Science 2000; 121–126.
- 197.
Dávila-Román VG, Vedala G, Herrero P, et al. Altered myocardial fatty acid and glucose metabolism in idiopathic dilated cardiomyopathy. J Am Coll Cardiol 2002; 40: 271–277. PMID: 12106931
- 198.
Langah R, Spicer K, Gebregziabher M, et al. Effectiveness of prolonged fasting 18f-FDG PET-CT in the detection of cardiac sarcoidosis. J Nucl Cardiol 2009; 16: 801–810. PMID: 19548047
- 199.
Ohira H, Tsujino I, Ishimaru S, et al. Myocardial imaging with 18F-fluoro-2-deoxyglucose positron emission tomography and magnetic resonance imaging in sarcoidosis. Eur J Nucl Med Mol Imaging 2008; 35: 933–941. PMID: 18084757
- 200.
Okumura W, Iwasaki T, Toyama T, et al. Usefulness of fasting 18F-FDG PET in identification of cardiac sarcoidosis. J Nucl Med 2004; 45: 1989–1998. PMID: 15585472
- 201.
Ishimaru S, Tsujino I, Takei T, et al. Focal uptake on 18F-fluoro-2-deoxyglucose positron emission tomography images indicates cardiac involvement of sarcoidosis. Eur Heart J 2005; 26: 1538–1543. PMID: 15809286
- 202.
Youssef G, Leung E, Mylonas I, et al. The use of 18F-FDG PET in the diagnosis of cardiac sarcoidosis: A systematic review and metaanalysis including the Ontario experience. J Nucl Med 2012; 53: 241–248. PMID: 22228794
- 203.
Mc Ardle BA, Birnie DH, Klein R, et al. Is there an association between clinical presentation and the location and extent of myocardial involvement of cardiac sarcoidosis as assessed by 18F-fluorodeoxyglucose positron emission tomography? Circ Cardiovasc Imaging 2013; 6: 617–626. PMID: 23884290
- 204.
Blankstein R, Osborne M, Naya M, et al. Cardiac positron emission tomography enhances prognostic assessments of patients with suspected cardiac sarcoidosis. J Am Coll Cardiol 2014; 63: 329–336. PMID: 24140661
- 205.
Ishida Y. Treatment of cardiac sarcoidosis and nuclear imaging. In: Clinical applications of cardiac nuclear imaging - Clinical cases and explanations. Medical Review, 2010; 111–113. [In Japanese]
- 206.
Osborne MT, Hulten EA, Singh A, et al. Reduction in 18F-fluorodeoxyglucose uptake on serial cardiac positron emission tomography is associated with improved left ventricular ejection fraction in patients with cardiac sarcoidosis. J Nucl Cardiol 2014; 21: 166–174. PMID: 24307261
- 207.
Lynch JP 3rd, Hwang J, Bradfield J, et al. Cardiac involvement in sarcoidosis: Evolving concepts in diagnosis and treatment. Semin Respir Crit Care Med 2014; 35: 372–390. PMID: 25007089
- 208.
Schatka I, Bengel FM. Advanced imaging of cardiac sarcoidosis. J Nucl Med 2014; 55: 99–106. PMID: 24232870
- 209.
Okayama K, Kurata C, Tawarahara K, et al. Diagnostic and prognostic value of myocardial scintigraphy with thallium-201 and gallium-67 in cardiac sarcoidosis. Chest 1995; 107: 330–334. PMID: 7842756
- 210.
Taki J, Nakajima K, Bunko H, et al. Cardiac sarcoidosis demonstrated by Tl-201 and Ga-67 SPECT imaging. Clin Nucl Med 1990; 15: 636–639. PMID: 2208885
- 211.
Tawarahara K, Kurata C, Okayama K, et al. Thallium-201 and gallium 67 single photon emission computed tomographic imaging in cardiac sarcoidosis. Am Heart J 1992; 124: 1383–1384. PMID: 1442515
- 212.
Momose M, Kadoya M, Koshikawa M, et al. Usefulness of 67Ga SPECT and integrated low-dose CT scanning (SPECT/CT) in the diagnosis of cardiac sarcoidosis. Ann Nucl Med 2007; 21: 545–551. PMID: 18092130
- 213.
Kiso K, Hashimura K, Ishida Y, et al. Clinical Utility of Hybrid SPECT/CT System for the Diagnosis of Cardiac Sarcoidosis. Circ J 2010; 74(Suppl I): 293–294.
- 214.
Bulkley BH, Rouleau JR, Whitaker JQ, et al. The use of 201thallium for myocardial perfusion imaging in sarcoid heart disease. Chest 1977; 72: 27–32. PMID: 872650
- 215.
Kinney EL, Jackson GL, Reeves WC, et al. Thallium-scan myocardial defects and echocardiographic abnormalities in patients with sarcoidosis without clinical cardiac dysfunction: An analysis of 44 patients. Am J Med 1980; 68: 497–503. PMID: 7369231
- 216.
Hirose Y, Ishida Y, Hayashida K, et al. Myocardial involvement in patients with sarcoidosis: An analysis of 75 patients. Clin Nucl Med 1994; 19: 522–526. PMID: 8062473
- 217.
Forman MB, Sandler MP, Sacks GA, et al. Radionuclide imaging in myocardial sarcoidosis: Demonstration of myocardial uptake of technetium pyrophosphate99 m and gallium. Chest 1983; 83: 578–580. PMID: 6297857
- 218.
Le Guludec D, Menad F, Faraggi M, et al. Myocardial sarcoidosis. Clinical value of technetium-99 m sestamibi tomoscintigraphy. Chest 1994; 106: 1675–1682. PMID: 7988183
- 219.
Eguchi M, Tsuchihashi K, Hotta D, et al. Technetium-99 m sestamibi/tetrofosmin myocardial perfusion scanning in cardiac and noncardiac sarcoidosis. Cardiology 2000; 94: 193–199. PMID: 11279326
- 220.
Tellier P, Paycha F, Antony I, et al. Reversibility by dipyridamole of thallium-201 myocardial scan defects in patients with sarcoidosis. Am J Med 1988; 85: 189–193. PMID: 3400694
- 221.
Tellier P, Valeyre D, Nitenberg A, et al. Cardiac sarcoidosis: Reversion of myocardial perfusion abnormalities by dipyridamole. Eur J Nucl Med 1985; 11: 201–204. PMID: 4076228
- 222.
Butany J, Bahl NE, Morales K, et al. The intricacies of cardiac sarcoidosis: A case report involving the coronary arteries and a review of the literature. Cardiovasc Pathol 2006; 15: 222–227. PMID: 16844554
- 223.
Barton JH, Tavora F, Farb A, et al. Unusual cardiovascular manifestations of sarcoidosis, a report of three cases: Coronary artery aneurysm with myocardial infarction, symptomatic mitral valvular disease, and sudden death from ruptured splenic artery. Cardiovasc Pathol 2010; 19: e119–e123. PMID: 19502084
- 224.
The Japanese Circulation Society. Guidelines for management of dilated cardiomyopathy and secondary cardiomyopathy (JCS 2011). http://www.j-circ.or.jp/guideline/pdf/JCS2011_tomoike_h.pdf [In Japanese]
- 225.
Fox KF, Cowie MR, Wood DA, et al. Coronary artery disease as the cause of incident heart failure in the population. Eur Heart J 2001; 22: 228–236. PMID: 11161934
- 226.
Arques S, Ambrosi P, Gelisse R, et al. Prevalence of angiographic coronary artery disease in patients hospitalized for acute diastolic heart failure without clinical and electrocardiographic evidence of myocardial ischemia on admission. Am J Cardiol 2004; 94: 133–135. PMID: 15219526
- 227.
Kurtz CE, Gerber Y, Weston SA, et al. Use of ejection fraction tests and coronary angiography in patients with heart failure. Mayo Clin Proc 2006; 81: 906–913. PMID: 16835970
- 228.
Bluemke DA, Achenbach S, Budoff M, et al. Noninvasive coronary artery imaging: Magnetic resonance angiography and multidetector computed tomography angiography: A scientific statement from the american heart association committee on cardiovascular imaging and intervention of the council on cardiovascular radiology and intervention, and the councils on clinical cardiology and cardiovascular disease in the young. Circulation 2008; 118: 586–606. PMID: 18586979
- 229.
Lynch JP 3rd, Sharma OP, Baughman RP. Extrapulmonary sarcoidosis. Semin Respir Infect 1998; 13: 229–254. PMID: 9764954
- 230.
Sato T, Kanzaki H, Ishida Y, et al. Second left ventricular aneurysm newly developed in a patient with untreated cardiac sarcoidosis. Circ J 2010; 74: 2477–2478. PMID: 20890051
- 231.
Candell Riera J, Bardají Ruiz A, Sangristá Sauleda J, et al. [Left ventricular aneurysm in sarcoidosis: Its detection by isotopic ventriculography]. Rev Esp Cardiol 1986; 39: 151–153. PMID: 3726242
- 232.
Miyahara S, Mukohara N, Morimoto N, et al. Left ventricular restoration for cardiac sarcoidosis: Report of two cases. Surg Today 2014; 44: 568–571. PMID: 23271666
- 233.
Altay H, Altin C, Coner A, et al. Normal coronary artery patient presenting with left ventricular aneurysm. Case Rep Med 2011; 2011: 183050. PMID: 21845194
- 234.
Marks A, Anderson MH, Harrison NK. Ventricular aneurysm secondary to sarcoid disease. Heart 2004; 90: 694. PMID: 15145887
- 235.
Toda G, Iliev II, Kawahara F, et al. Left ventricular aneurysm without coronary artery disease, incidence and clinical features: Clinical analysis of 11 cases. Intern Med 2000; 39: 531–536. PMID: 10888207
- 236.
Miyazawa K, Yoshikawa T, Takamisawa I, et al. Presence of ventricular aneurysm predicts poor clinical outcomes in patients with cardiac sarcoidosis. Int J Cardiol 2014; 177: 720–722. PMID: 25456577
- 237.
Sakakibara S, Konno S. Endomyocardial biopsy. Jpn Heart J 1962; 3: 537–543. PMID: 13990927
- 238.
Ardehali H, Howard DL, Hariri A, et al. A positive endomyocardial biopsy result for sarcoid is associated with poor prognosis in patients with initially unexplained cardiomyopathy. Am Heart J 2005; 150: 459–463. PMID: 16169324
- 239.
Morimoto S, Kato S, Hiramitsu S, et al. Narrowing of the left ventricular cavity associated with transient ventricular wall thickening reduces stroke volume in patients with acute myocarditis. Circ J 2003; 67: 490–494. PMID: 12808264
- 240.
Cooper LT, Baughman KL, Feldman AM, et al. The role of endomyocardial biopsy in the management of cardiovascular disease: A scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. Endorsed by the Heart Failure Society of America and the Heart Failure Association of the European Society of Cardiology. J Am Coll Cardiol 2007; 50: 1914–1931. PMID: 17980265
- 241.
Falk HR, Hershberger ER. The dilated, restrictive, and infiltrative cardiomyopathies. In: Mann LD, Zipes PD, Libby P, et al, editors. Braunwald’s Heart Disease 10th edn. Elservier Saunders 2015. 1551–1573.
- 242.
Hiramitsu S, Hiroe M, Morimoto S, et al. National survey of the use of endomyocardial biopsy in Japan. Jpn Circ J 1998; 62: 909–912. PMID: 9890204
- 243.
Tsang TS, Freeman WK, Barnes ME, et al. Rescue echocardiographically guided pericardiocentesis for cardiac perforation complicating catheter-based procedures. The Mayo Clinic experience. J Am Coll Cardiol 1998; 32: 1345–1350. PMID: 809946
- 244.
Casella M, Pizzamiglio F, Dello Russo A, et al. Feasibility of combined unipolar and bipolar voltage maps to improve sensitivity of endomyocardial biopsy. Circ Arrhythm Electrophysiol 2015; 8: 625–632. PMID: 25829163
- 245.
Morimoto S, Uemura A, Hiramitsu S. Revised guidelines for the diagnosis of cardiac sarcoidosis. Respiration and Circulation 2006; 54: 955–961. [In Japanese]
- 246.
Tanaka T. Pathology of sarcoidosis and granulomatous lung disease. The Japanese Journal of Sarcoidosis and Other Granulomatous Disorders 2013; 33: 23–26. [In Japanese]
- 247.
Kul S, Ozcelik HK, Uyarel H, et al. Diagnostic value of strain echocardiography, galectin-3, and tenascin-C levels for the identification of patients with pulmonary and cardiac sarcoidosis. Lung 2014; 192: 533–542. PMID: 24777587
- 248.
Banba K, Kusano KF, Nakamura K, et al. Relationship between arrhythmogenesis and disease activity in cardiac sarcoidosis. Heart Rhythm 2007; 4: 1292–1299. PMID: 17905334
- 249.
Kumar S, Barbhaiya C, Nagashima K, et al. Ventricular tachycardia in cardiac sarcoidosis: Characterization of ventricular substrate and outcomes of catheter ablation. Circ Arrhythm Electrophysiol 2015; 8: 87–93. PMID: 25527825
- 250.
Naruse Y, Sekiguchi Y, Nogami A, et al. Systematic treatment approach to ventricular tachycardia in cardiac sarcoidosis. Circ Arrhythm Electrophysiol 2014; 7: 407–413. PMID: 24837644
- 251.
Mezaki T, Chinushi M, Washizuka T, et al. Discrepancy between inducibility of ventricular tachycardia and activity of cardiac sarcoidosis: Requirement of defibrillator implantation for the inactive stage of cardiac sarcoidosis. Intern Med 2001; 40: 731–735. PMID: 11518112
- 252.
Nadel J, Lancefield T, Voskoboinik A, et al. Late gadolinium enhancement identified with cardiac magnetic resonance imaging in sarcoidosis patients is associated with long-term ventricular arrhythmia and sudden cardiac death. Eur Heart J Cardiovasc Imaging 2015; 16: 634–641. PMID: 25617029
- 253.
Huang PL, Brooks R, Carpenter C, et al. Antiarrhythmic therapy guided by programmed electrical stimulation in cardiac sarcoidosis with ventricular tachycardia. Am Heart J 1991; 121: 599–601. PMID: 1990769
- 254.
Aizer A, Stern EH, Gomes JA, et al. Usefulness of programmed ventricular stimulation in predicting future arrhythmic events in patients with cardiac sarcoidosis. Am J Cardiol 2005; 96: 276–282. PMID: 16018857
- 255.
Mehta D, Mori N, Goldbarg SH, et al. Primary prevention of sudden cardiac death in silent cardiac sarcoidosis: Role of programmed ventricular stimulation. Circ Arrhythm Electrophysiol 2011; 4: 43–48. PMID: 21193539
- 256.
Sato H, Woodhead FA, Ahmad T, et al. Sarcoidosis HLA class II genotyping distinguishes differences of clinical phenotype across ethnic groups. Hum Mol Genet 2010; 19: 4100–4111. PMID: 20685690
- 257.
Pasturenzi L, Martinetti M, Cuccia M, et al. HLA class I, II, and III polymorphism in Italian patients with sarcoidosis: The Pavia-Padova Sarcoidosis Study Group. Chest 1993; 104: 1170–1175. PMID: 8404186
- 258.
Gardner J, Kennedy HG, Hamblin A, et al. HLA associations in sarcoidosis: A study of two ethnic groups. Thorax 1984; 39: 19–22. PMID: 6582657
- 259.
Ishihara M. Study of susceptibility genes for sarcoidosis. The Japanese Journal of Sarcoidosis and Other Granulomatous Disorders 2013; 33: 11–17. [In Japanese]
- 260.
Rybicki BA, Walewski JL, Maliarik MJ, et al. ACCESS Research Group. The BTNL2 gene and sarcoidosis susceptibility in African Americans and Whites. Am J Hum Genet 2005; 77: 491–499. PMID: 16080124
- 261.
Levin AM, Iannuzzi MC, Montgomery CG, et al. Association of ANXA11 genetic variation with sarcoidosis in African Americans and European Americans. Genes Immun 2013; 14: 13–18. PMID: 23151485
- 262.
Wijnen PA, Cremers JP, Nelemans PJ, et al. Association of the TNF-α G-308A polymorphism with TNF-inhibitor response in sarcoidosis. Eur Respir J 2014; 43: 1730–1739. PMID: 24558177
- 263.
Langrish CL, Chen Y, Blumenschein WM, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med 2005; 201: 233–240. PMID: 15657292
- 264.
Facco M, Cabrelle A, Teramo A, et al. Sarcoidosis is a Th1/Th17 multisystem disorder. Thorax 2011; 66: 144–150. PMID: 21139119
- 265.
Huang H, Lu Z, Jiang C, et al. Imbalance between Th17 and regulatory T-Cells in sarcoidosis. Int J Mol Sci 2013; 14: 21463–21473. PMID: 24177566
- 266.
Judson MA, Baughman RP, Teirstein AS, et al. Defining organ involvement in sarcoidosis: The ACCESS proposed instrument. ACCESS Research Group. A Case Control Etiologic Study of Sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 1999; 16: 75–86. PMID: 10207945
- 267.
Judson MA, Costabel U, Drent M, et al. The WASOG Sarcoidosis Organ Assessment Instrument: An update of a previous clinical tool. Sarcoidosis Vasc Diffuse Lung Dis 2014; 31: 19–27. PMID: 24751450
- 268.
The Ministry of Health, Labour and Welfare. Intractable diseases designated as of January 1, 2015 (new diseases). http://www.mhlw.go.jp/stf/seisakunitsuite/bunya/0000062437.html [In Japanese]
- 269.
Nery PB, Mc Ardle BA, Redpath CJ, et al. Prevalence of cardiac sarcoidosis in patients presenting with monomorphic ventricular tachycardia. Pacing Clin Electrophysiol 2014; 37: 364–374. PMID: 24102263
- 270.
Koplan BA, Soejima K, Baughman K, et al. Refractory ventricular tachycardia secondary to cardiac sarcoid: Electrophysiologic characteristics, mapping, and ablation. Heart Rhythm 2006; 3: 924–929. PMID: 16876741
- 271.
Patel AR, Klein MR, Chandra S, et al. Myocardial damage in patients with sarcoidosis and preserved left ventricular systolic function: An observational study. Eur J Heart Fail 2011; 13: 1231–1237. PMID: 21810833
- 272.
Cummings KW, Bhalla S, Javidan-Nejad C, et al. A pattern-based approach to assessment of delayed enhancement in nonischemic cardiomyopathy at MR imaging. Radiographics 2009; 29: 89–103. PMID: 19168838
- 273.
Kato Y. Solitary cardiac involvement of sarcoidosis. The Japanese Journal of Sarcoidosis and Other Granulomatous Disorders 2012; 32: 51–54. [In Japanese]
- 274.
Tezuka D, Terashima M, Kato Y, et al. Clinical characteristics of definite or suspected isolated cardiac sarcoidosis: Application of cardiac magnetic resonance imaging and 18F-Fluoro-2-deoxyglucose positron-emission tomography/computerized tomography. J Card Fail 2015; 21: 313–322. PMID: 25512195
- 275.
Brown ML, Reeder G, Unni KK, et al. Intraoperative diagnosis of isolated cardiac sarcoid. Heart Lung Circ 2007; 16: 315–317. PMID: 17254847
- 276.
Sugizaki Y, Tanaka H, Imanishi J, et al. Isolated primary cardiac sarcoidosis presenting as acute heart failure. Intern Med 2013; 52: 71–74. PMID: 23291676
- 277.
Nery PB, Keren A, Healey J, et al. Isolated cardiac sarcoidosis: Establishing the diagnosis with electroanatomic mapping-guided endomyocardial biopsy. Can J Cardiol 2013; 29: 1015.e1–e3. PMID: 23246240
- 278.
Meyer T, Lauschke J, Ruppert V, et al. Isolated cardiac sarcoidosis associated with the expression of a splice variant coding for a truncated BTNL2 protein. Cardiology 2008; 109: 117–121. PMID: 17703092
- 279.
Galati G, Leone O, Rapezzi C. The difficult diagnosis of isolated cardiac sarcoidosis: Usefulness of an integrated MRI and PET approach. Heart 2014; 100: 89–90. PMID: 23838000
- 280.
Tsai JH, Chou NK, Wang SS, et al. Isolated cardiac sarcoidosis: Case experience in heart transplantation. J Formos Med Assoc 2013; 112: 499–500. PMID: 24016616
- 281.
Kandolin R, Lehtonen J, Kupari M. Cardiac sarcoidosis and giant cell myocarditis as causes of atrioventricular block in young and middle-aged adults. Circ Arrhythm Electrophysiol 2011; 4: 303–309. PMID: 21427276
- 282.
Kandolin R, Lehtonen J, Airaksinen J, et al. Cardiac sarcoidosis: Epidemiology, characteristics, and outcome over 25 years in a nationwide study. Circulation 2015; 131: 624–632. PMID: 25527698
- 283.
Tsuchida A, Clinical characteristics of patients newly diagnosed as cardiac sarcoidosis - Five-year experience. Abstract of the 62nd annual scientific session of the Japanese College Cardiology 2014: P-075. [In Japanese]
- 284.
Sugie T, Hashimoto N, Iwai K. Clinical and autopsy studies on prognosis of sarcoidosis. Nihon Rinsho 1994; 52: 1567–1570. [In Japanese]
- 285.
Kim JS, Judson MA, Donnino R, et al. Cardiac sarcoidosis. Am Heart J 2009; 157: 9–21. PMID: 19081391
- 286.
Yazaki Y. Diagnosis of cardiac sarcoidosis in the era of multimodality cardiac imaging. SHINZO 1995; 47: 1046–1049. [In Japanese]
- 287.
Terasaki F, Ishizaka N. Deterioration of cardiac function during the progression of cardiac sarcoidosis: Diagnosis and treatment. Intern Med 2014; 53: 1595–1605. PMID: 25088870
- 288.
Terasaki F, Ishizaka N. Reversal of cardiac remodeling after treatment of IgG4 related cholangitis - Possibility of IgG4-related heart disease? Int J Cardiol 2016; 223: 477–478. PMID: 27544609
- 289.
Grutters JC, van den Bosch JM. Corticosteroid treatment in sarcoidosis. Eur Respir J 2006; 28: 627–636. PMID: 16946094
- 290.
Bussinguer M, Danielian A, Sharma OP. Cardiac sarcoidosis: Diagnosis and management. Curr Treat Options Cardiovasc Med 2012; 14: 652–664. PMID: 22983661
- 291.
Chiu CZ, Nakatani S, Zhang G, et al. Prevention of left ventricular remodeling by long-term corticosteroid therapy in patients with cardiac sarcoidosis. Am J Cardiol 2005; 95: 143–146. PMID: 15619415
- 292.
Nagai T, Nagano N, Sugano Y, et al. Effect of corticosteroid therapy on long-term clinical outcome and left ventricular function in patients with cardiac sarcoidosis. Circ J 2015; 79: 1593–1600. PMID: 25877828
- 293.
Chapelon-Abric C, de Zuttere D, Duhaut P, et al. Cardiac sarcoidosis: A retrospective study of 41 cases. Medicine (Baltimore) 2004; 83: 315–334. PMID: 15525844
- 294.
Hiramitsu S, Morimoto S, Uemura A, et al. National survey on status of steroid therapy for cardiac sarcoidosis in Japan. Sarcoidosis Vasc Diffuse Lung Dis 2005; 22: 210–213. PMID: 16315784
- 295.
Yazaki Y. How should we evaluate the activity of myocardial inflammation and guide corticosteroid treatment in patients with cardiac sarcoidosis? Circ J 2015; 79: 1450–1452. PMID: 26063083
- 296.
Demeter SL. Myocardial sarcoidosis unresponsive to steroids. Treatment with cyclophosphamide. Chest 1988; 94: 202–203. PMID: 3383636
- 297.
Wyser CP, van Schalkwyk EM, Alheit B, et al. Treatment of progressive pulmonary sarcoidosis with cyclosporin A: A randomized controlled trial. Am J Respir Crit Care Med 1997; 156: 1371–1376. PMID: 9372647
- 298.
Müller-Quernheim J, Kienast K, Held M, et al. Treatment of chronic sarcoidosis with an azathioprine/prednisolone regimen. Eur Respir J 1999; 14: 1117–1122. PMID: 10596700
- 299.
Baughman RP, Lower EE. A clinical approach to the use of methotrexate for sarcoidosis. Thorax 1999; 54: 742–746. PMID: 10413729
- 300.
Carlesimo M, Giustini S, Rossi A, et al. Treatment of cutaneous and pulmonary sarcoidosis with thalidomide. J Am Acad Dermatol 1995; 32: 866–869. PMID: 7722046
- 301.
Baltzan M, Mehta S, Kirkham TH, et al. Randomized trial of prolonged chloroquine therapy in advanced pulmonary sarcoidosis. Am J Respir Crit Care Med 1999; 160: 192–197. PMID: 10390399
- 302.
Zabel P, Entzian P, Dalhoff K, et al. Pentoxifylline in treatment of sarcoidosis. Am J Respir Crit Care Med 1997; 155: 1665–1669. PMID: 9154873
- 303.
Kikuchi N, Nunoda S, Serizawa N, et al. Combination therapy with corticosteroid and mycophenolate mofetil in a case of refractory cardiac sarcoidosis. J Cardiol Cases 2016; 13: 125–128.
- 304.
Vorselaars AD, Cremers JP, Grutters JC, et al. Cytotoxic agents in sarcoidosis: Which one should we choose? Curr Opin Pulm Med 2014; 20: 479–487. PMID: 25046427
- 305.
Nagai S, Yokomatsu T, Tanizawa K, et al. Treatment with methotrexate and low-dose corticosteroids in sarcoidosis patients with cardiac lesions. Intern Med 2014; 53: 2761. PMID: 25447669
- 306.
Rossman MD, Newman LS, Baughman RP, et al. A double-blinded, randomized, placebo-controlled trial of infliximab in subjects with active pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2006; 23: 201–208. PMID: 18038919
- 307.
Uthman I, Touma Z, Khoury M. Cardiac sarcoidosis responding to monotherapy with infliximab. Clin Rheumatol 2007; 26: 2001–2003. PMID: 17394036
- 308.
Barnabe C, McMeekin J, Howarth A, et al. Successful treatment of cardiac sarcoidosis with infliximab. J Rheumatol 2008; 35: 1686–1687. PMID: 18671332
- 309.
The Japanese Circulation Society. Guidelines for treatment of chronic heart failure (JCS 2010). http://www.j-circ.or.jp/guideline/pdf/JCS2010_matsuzaki_h.pdf [In Japanese]
- 310.
The Japanese Circulation Society. Guidelines for treatment of chronic heart failure (JCS 2011). http://www.j-circ.or.jp/guideline/pdf/JCS2011_izumi_h.pdf [In Japanese]
- 311.
Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: Executive summary: A report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013; 128: 1810–1852. PMID: 23741057
- 312.
Terasaki F, Ishizaka N. Cardiac sarcoidosis and arrhythmogenic right ventricular cardiomyopathy: Potential differential diagnoses for arrhythmogenic ventricular cardiomyopathy. Intern Med 2016; 55: 1041–1042. PMID: 27150851
- 313.
Yodogawa K, Seino Y, Shiomura R, et al. Recovery of atrioventricular block following steroid therapy in patients with cardiac sarcoidosis. J Cardiol 2013; 62: 320–325. PMID: 24016620
- 314.
Tokuda M, Tedrow UB, Kojodjojo P, et al. Catheter ablation of ventricular tachycardia in nonischemic heart disease. Circ Arrhythm Electrophysiol 2012; 5: 992–1000. PMID: 22942218
- 315.
Kusano KF, Satomi K. Diagnosis and treatment of cardiac sarcoidosis. Heart 2016; 102: 184–190. PMID: 26643814
- 316.
Noda T, Suyama K, Shimizu W, et al. Ventricular tachycardia with figure eight pattern originating from the right ventricle in a patient with cardiac sarcoidosis. Pacing Clin Electrophysiol 2004; 27: 561–562. PMID: 15078419
- 317.
Echt DS, Liebson PR, Mitchell LB, et al. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N Engl J Med 1991; 324: 781–788. PMID: 1900101
- 318.
Yodogawa K, Seino Y, Ohara T, et al. Effect of corticosteroid therapy on ventricular arrhythmias in patients with cardiac sarcoidosis. Ann Noninvasive Electrocardiol 2011; 16: 140–147. PMID: 21496164
- 319.
The Japanese Circulation Society. Guidelines for non-pharmacotherapy of cardiac arrhythmias (JCS 2011). http://www.j-circ.or.jp/guideline/pdf/JCS2011_okumura_h.pdf [In Japanese]
- 320.
JCS Joint Working Group. Guidelines for non-pharmacotherapy of cardiac arrhythmias (JCS 2011): Digest version. Circ J 2013; 77: 249–274. PMID: 23165786
- 321.
Curtis AB, Worley SJ, Adamson PB, et al. Biventricular versus Right Ventricular Pacing in Heart Failure Patients with Atrioventricular Block (BLOCK HF) Trial Investigators. Biventricular pacing for atrioventricular block and systolic dysfunction. N Engl J Med 2013; 368: 1585–1593. PMID: 23614585
- 322.
Sadek MM, Yung D, Birnie DH, et al. Corticosteroid therapy for cardiac sarcoidosis: A systematic review. Can J Cardiol 2013; 29: 1034–1041. PMID: 23623644
- 323.
Takaya Y, Kusano KF, Nakamura K, et al. Outcomes in patients with high-degree atrioventricular block as the initial manifestation of cardiac sarcoidosis. Am J Cardiol 2015; 115: 505–509. PMID: 25529542
- 324.
Sekiguchi M, Hiroe M, Take M, et al. Clinical and histopathological profile of sarcoidosis of the heart and acute idiopathic myocarditis. Concepts through a study employing endomyocardial biopsy. II. Myocarditis
. Jpn Circ J 1980; 44: 264–273. PMID: 6154812
- 325.
Betensky BP, Tschabrunn CM, Zado ES, et al. Long-term follow-up of patients with cardiac sarcoidosis and implantable cardioverter-defibrillators. Heart Rhythm 2012; 9: 884–891. PMID: 22338670
- 326.
Kron J, Sauer W, Schuller J, et al. Efficacy and safety of implantable cardiac defibrillators for treatment of ventricular arrhythmias in patients with cardiac sarcoidosis. Europace 2013; 15: 347–354. PMID: 23002195
- 327.
Schuller JL, Zipse M, Crawford T, et al. Implantable cardioverter defibrillator therapy in patients with cardiac sarcoidosis. J Cardiovasc Electrophysiol 2012; 23: 925–929. PMID: 22812589
- 328.
Epstein AE, DiMarco JP, Ellenbogen KA, et al. American College of Cardiology Foundation, American Heart Association Task Force on Practice Guidelines, Heart Rhythm Society. 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation 2013; 127: e283–e352. PMID: 23255456
- 329.
Epstein AE, Dimarco JP, Ellenbogen KA, et al. American College of Cardiology/American Heart Association Task Force on Practice, American Association for Thoracic Surgery, Society of Thoracic Surgeons. ACC/AHA/HRS 2008 guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: Executive summary. Heart Rhythm 2008; 5: 934–955. PMID: 18534377
- 330.
Crawford T, Mueller G, Sarsam S, et al. Magnetic resonance imaging for identifying patients with cardiac sarcoidosis and preserved or mildly reduced left ventricular function at risk of ventricular arrhythmias. Circ Arrhythm Electrophysiol 2014; 7: 1109–1115. PMID: 25266311
- 331.
Abraham WT, Fisher WG, Smith AL, et al. MIRACLE Study Group. Cardiac resynchronization in chronic heart failure. N Engl J Med 2002; 346: 1845–1853. PMID: 12063368
- 332.
Bristow MR, Saxon LA, Boehmer J, et al. Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) Investigators. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med 2004; 350: 2140–2150. PMID: 15152059
- 333.
Cleland JG, Daubert JC, Erdmann E, et al. Cardiac Resynchronization-Heart Failure (CARE-HF) Study Investigators. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med 2005; 352: 1539–1549. PMID: 15753115
- 334.
Stees CS, Khoo MS, Lowery CM, et al. Ventricular tachycardia storm successfully treated with immunosuppression and catheter ablation in a patient with cardiac sarcoidosis. J Cardiovasc Electrophysiol 2011; 22: 210–213. PMID: 20561106
- 335.
Jefic D, Joel B, Good E, et al. Role of radiofrequency catheter ablation of ventricular tachycardia in cardiac sarcoidosis: Report from a multicenter registry. Heart Rhythm 2009; 6: 189–195. PMID: 19187909
- 336.
Dechering DG, Kochhäuser S, Wasmer K, et al. Electrophysiological characteristics of ventricular tachyarrhythmias in cardiac sarcoidosis versus arrhythmogenic right ventricular cardiomyopathy. Heart Rhythm 2013; 10: 158–164. PMID: 23070261
- 337.
Daimon M, Sasaki T, Woo E, et al. Successful surgical treatment for dilated cardiomyopathy with cardiac sarcoidosis. Ann Thorac Surg 2007; 84: e12–e13. PMID: 17643597
- 338.
Shimamoto T, Nishina T, Marui A, et al. Dual left ventricular restorations in a patient with cardiac sarcoidosis. J Thorac Cardiovasc Surg 2009; 137: 1286–1288. PMID: 19380011
- 339.
Hirota M, Yoshida M, Hoshino J, et al. Sublocalization of cardiac involvement in sarcoidosis and surgical exclusion in patients with congestive heart failure. Ann Thorac Surg 2015; 100: 81–87. PMID: 25986102
- 340.
Perkel D, Czer LS, Morrissey RP, et al. Heart transplantation for endstage heart failure due to cardiac sarcoidosis. Transplant Proc 2013; 45: 2384–2386. PMID: 23953552
- 341.
Zaidi AR, Zaidi A, Vaitkus PT. Outcome of heart transplantation in patients with sarcoid cardiomyopathy. J Heart Lung Transplant 2007; 26: 714–717. PMID: 17613402
- 342.
Akashi H, Kato TS, Takayama H, et al. Outcome of patients with cardiac sarcoidosis undergoing cardiac transplantation: Single-center retrospective analysis. J Cardiol 2012; 60: 407–410. PMID: 22890069
- 343.
Yager JE, Hernandez AF, Steenbergen C, et al. Recurrence of cardiac sarcoidosis in a heart transplant recipient. J Heart Lung Transplant 2005; 24: 1988–1990. PMID: 16297811
- 344.
Milman N, Andersen CB, Mortensen SA, et al. Cardiac sarcoidosis and heart transplantation: A report of four consecutive patients. Sarcoidosis Vasc Diffuse Lung Dis 2008; 25: 51–59. PMID: 19070261
- 345. Meeting materials for the Heart Transplantation Committee of the Japanese Circulation Society. [In Japanese]
- 346.
The Heart Transplantation Committee of the Japanese Circulation Society. Criteria for heart transplant recipients. http://www.j-circ.or.jp/hearttp/HTRecCriteria.html [In Japanese]
- 347. INTERMACS website. http://www.uab.edu/medicine/intermacs/ [In Japanese]
- 348.
The Pharmaceuticals and Medical Devices Agency. Development of data collection and evaluation systems on the use of mobile medical device tracking. http://www.pmda.go.jp/safety/surveillance-analysis/0009.html [In Japanese]
- 349.
The Japanese Circulation Society, the Japanese Society for Cardiovascular Surgery. Guidelines for device therapy: Implantable left ventricular assist device for patients with severe heart failure (JCS/JSCVS2013). http://www.j-circ.or.jp/guideline/pdf/JCS2013_kyo_h.pdf [In Japanese]
- 350.
The Japanese Association for Clinical Ventricular Assist Systems. Practice standard for the use of ventricular assist devices (Draft published on November 16, 2010). http://www.jacvas.com/application/2/standard/ [In Japanese]
- 351.
Roberts WC, Vowels TJ, Ko JM, et al. Cardiac transplantation for cardiac sarcoidosis with initial diagnosis by examination of the left ventricular apical “core” excised for insertion of a left ventricular assist device for severe chronic heart failure. Am J Cardiol 2009; 103: 110–114. PMID: 19101239
- 352.
DePasquale EC, Nasir K, Jacoby DL. Outcomes of adults with restrictive cardiomyopathy after heart transplantation. J Heart Lung Transplant 2012; 31: 1269–1275. PMID: 23079066
- 353.
Chang TI, Chi NH, Chou NK, et al. Isolated cardiac sarcoidosis in heart transplantation. Transplant Proc 2012; 44: 903–906. PMID: 22564580
- 354.
Eishi Y. Etiology of sarcoidosis-in relation to infection, especially
Propionibacterium acnes. Propionibacterium acnes. The Japanese Journal of Sarcoidosis and Other Granulomatous Disorders 2011; 31: 81–83. [In Japanese]
- 355.
Eishi Y. Pathogenesis of cardiac sarcoidosis according to an etiologic hypothesis as caused by
Propionibacterium acnes. Propionibacterium acnes. The Japanese Journal of Sarcoidosis and Other Granulomatous Disorders 2010; 30: 86–88. [In Japanese]