2019 Volume 83 Issue 12 Pages 2466-2478
2019 Volume 83 Issue 12 Pages 2466-2478
Background: The use of bilateral internal thoracic artery (BITA) grafting concomitant with other cardiac operations is regarded as a risky strategy and the long-term advantages of BITA use remain unproven.
Methods and Results: Pooled results from 3 series of patients (totaling 1,123 patients; mean age, 71.3 years; mean EuroSCORE II, 7.4%) undergoing combined coronary surgery using BITA were reviewed. Predictors of immediate and long-term adverse outcomes were identified by multivariable analyses. In-hospital and 30-day mortality was 7.9% and 6.3%, respectively. Diabetes on insulin (P=0.045), severe renal impairment (P<0.0001), extracardiac arteriopathy (P=0.0058), New York Heart Association class III−IV (P=0.017), recent myocardial infarction (P=0.0009), left ventricular dysfunction (P=0.0054), pulmonary hypertension (P=0.0016), active infective endocarditis (P=0.0011), and prolonged cross-clamp time (P=0.04) were predictors of in-hospital death. Multiple transfusions (27.3%), prolonged mechanical ventilation or reintubation (16.7%), acute kidney injury (11.5%), and sternal wound infections (10.4%) were relevant postoperative complications. Any neurological dysfunction occurred in 5.4% of cases. Median follow-up was 4.2 years. Female sex, chronic dialysis, extracardiac arteriopathy, and left ventricular dysfunction were predictors of both cardiac/cerebrovascular death and major adverse cardiac/cerebrovascular events (MACCE). The 10-year adjusted survival free of cardiac/cerebrovascular death, cerebrovascular accident after discharge, and MACCE was 84.2%, 94.8% and 54.6%, respectively.
Conclusions: BITA grafting concomitant with other cardiac operations may be performed with satisfactory results. Long-term outcomes mostly depend on sex, preoperative comorbidities, and baseline cardiac function.
The use of bilateral internal thoracic artery (BITA) for multiple coronary artery bypass grafting (CABG) in addition to other cardiac operations (combined CABG) is mostly regarded as a risky strategy. The increased length of surgery, as well as the increased risk of bleeding and sternal complications from BITA harvest1–15 could outweigh the potential benefits of reduced manipulation of the ascending aorta, either natural or prosthetic.6,7,10,16,17 Consequently, there are no large studies exploring the outcomes of surgery that combines use of BITA with procedures on cardiac valves, the left ventricle, and/or thoracic aorta. The only available evidence stems from anecdotal cases of this strategy being adopted to overcome contingent issues.18,19 In addition, the long-term benefits that may be derived from BITA use are still unproven.15
Editorial p ????
Therefore, we reviewed pooled results from 3 large series of patients who underwent BITA grafting concomitant with other cardiac operations at 3 European centers with extensive experience in BITA use. The aims of this retrospective study were to describe the immediate and long-term outcomes of these patients, and to identify independent predictors of in-hospital death, postoperative complications, and adverse long-term outcomes.
The study population consisted of a pooled series of consecutive subjects who underwent combined CABG using BITA at 1 of 3 European cardiac surgery units: (1) the Department of Thoracic and Cardiovascular Surgery of the University Hospital Jean-Minjoz of Besançon, France (series 1: 346 patients, period of surgery, February 28, 2000–April 13, 2018); (2) the Department of Cardio-Thoracic Surgery of the University Hospital Henry-Mondor, Créteil, Paris, France (series 2: 251 patients, period of surgery, January 2, 2013–April 11, 2018); and (3) the Cardio-Thoracic and Vascular Department of the University Hospital of Trieste, Italy (series 3: 526 patients, period of surgery, February 15, 1999–April 30, 2018).
Baseline characteristics of the patients, surgical findings and operative data, as well as relevant details pertaining to the hospital course of patients and follow-up period were retrospectively collected from patient files in institutional and national databases.
In addition, in the Cardio-Thoracic and Vascular Department of Trieste, the survival of patients who underwent combined CABG using BITA (series 3) was compared with a consecutive series of patients with multivessel coronary artery disease who underwent combined CABG (≥2 coronary anastomoses) using single ITA (SITA) and saphenous vein during the same period. Because a very small number of patients underwent SITA grafting in the 2 French centers during the corresponding study periods, those subjects were not considered for analysis.
DefinitionsUnless otherwise stated, the definitions and cutoff values of preoperative variables were those used for the European System for Cardiac Operative Risk Evaluation II (EuroSCORE II).20 The risk profile of each patient was established preoperatively according to EuroSCORE II. Internationally agreed definitions of complications after cardiac surgery were adopted, as validated and published in the literature (Supplementary Methods).21–25
Permanent neurological dysfunction, prolonged (>48 h) mechanical ventilation or reintubation, low cardiac output requiring mechanical support (intra-aortic balloon pump [IABP] and/or extracorporeal membrane oxygenator), acute kidney injury requiring renal replacement therapy, mesenteric ischemia or infarction, mediastinal re-entry through re-sternotomy for bleeding or tamponade, deep sternal wound infection (SWI), sepsis, multiorgan failure, and multiple blood transfusions (need for transfusion of ≥3 packs of red blood cells) were defined as major postoperative complications.
We recorded all major adverse cardiac and cerebrovascular events (MACCE) after hospital discharge, namely sudden death, recurrent angina, myocardial infarction (MI), congestive heart failure (CHF), percutaneous coronary intervention (PCI), repeat cardiac operation, pulmonary embolism, or cerebrovascular accident.
Surgical TechniquesAll operations were performed through a median sternotomy with cardiopulmonary bypass and cross-clamping of the aorta. Different cardioplegic solutions and strategies were adopted over the years in the 3 series of patients. Both ITAs were dissected as skeletonized conduits, and the ITA harvesting technique did not change in the 3 centers during the study period (Supplementary Methods).5,7–13,17,26,27
All coronary anastomoses on the inferolateral cardiac wall were performed before any other surgical procedure that involved cardiac valves, the left ventricle, or thoracic aorta. Cardiac tumor removal and operations for arrhythmias were included in the study. Patients undergoing concomitant carotid thromboendoarterectomy were ruled out of the study.
Follow-upIn each center, post-discharge surveillance of surgical wounds was performed for every patient in a specifically dedicated surgical outpatient clinic. All patients having surgical site complications were referred to this outpatient clinic, at any time following hospital discharge. Details pertaining to the patients and their disease during the follow-up period were recorded in a computerized data registry.8,13,24
Clinical follow-up was obtained by the following sequential procedure: telephone contact with the patient, or the patient’s family; if they could not be contacted, telephone contact with the general practitioner, referring cardiologist or other specialists listed in the patient’s medical file; finally, consultation of the national death registry or the town halls of the place of birth to obtain data regarding the vital status (dead or alive at the cutoff date). Information on long-term survival of patients or cause of death (where applicable), as well as data regarding hospital readmission for CHF or any other cause during the follow-up period was recorded. Readmission data were obtained from the hospital medical informatics systems and patients’ medical files.
The cutoff date for collecting data was fixed at April 30, 2018.
The study was performed in accordance with the Declaration of Helsinki. Approval to conduct the study, as well as to contact the patients and their families, was given by the local ethics committee of each participating center; based on the retrospective data retrieval; the need for individual written consent was waived.
Statistical AnalysisDiscrete variables are reported in accordance with internationally agreed categories, and continuous variables are categorized in quartiles. Values are number of patients with the corresponding percentage of in-hospital deaths, neurological complications, mediastinal re-entry for tamponade or bleeding, SWIs, and a composite endpoint comprising in-hospital death and any major complications post-surgery. Rates of these complications according to baseline patient characteristics, surgical findings and operative data, as well as other adverse events early after surgery, were performed using the Chi-square statistic. Backward stepwise logistic regression was used to identify independent predictors of each complication. All variables with a P-value <0.1 by univariable analysis were included in the multivariable model. For each variable, the odds ratio (OR) and the corresponding 95% confidence interval (95% CI) were calculated. Cox proportional hazards regression (method, backward stepwise) was used to identify independent predictors of all-cause death, cardiac or cerebrovascular death, MACCE, PCI or repeat cardiac operation, and hospital readmission for CHF during the follow-up period. For each variable, the proportional hazards assumption was verified with the Schoenfeld residuals test. Nonparametric curves for freedom from all-cause death according to each variable with a P-value <0.1 at the Cox proportional hazards regression, as well as for type of surgical procedure (surgical target) and EuroSCORE II, were prepared using the Kaplan-Meier method. Nonparametric estimates and curves for freedom from all-cause death, cardiac or cerebrovascular death, MACCE, PCI or reoperation, as well as from hospital readmission for CHF during the follow-up period were prepared (Kaplan-Meier method), and adjusted (Cox regression) for EuroSCORE II or definite covariates (Supplementary Methods). A single survival curve at the mean EuroSCORE II or at the mean of all covariates in the model was displayed. In addition, nonparametric estimates and curves for freedom from cerebrovascular accident during the follow-up period were prepared using the Kaplan-Meier method, and adjusted (Cox regression) for EuroSCORE II or the following covariates: age, estimated glomerular filtration rate (eGFR) by the Cockcroft-Gault formula, extracardiac arteriopathy, and surgery on the thoracic aorta. Finally, a comparison of freedom from all-cause death, before and after adjusting for EuroSCORE II or definite covariates (Supplementary Methods), of patients (only those from Trieste) undergoing combined CABG using either BITA or SITA was performed. P<0.05 was considered significant. Data analysis was performed using the SPSS software for Windows, version 13.0 (SPSS, Inc., Chicago, IL, USA).
In-hospital outcomes of the 1,123 consecutive patients (mean age, 71.3±8.8 years, females, 19.6%) undergoing cardiac operations at the 3 participating centers were reviewed. Baseline characteristics of the patients, surgical findings and operative data are listed in Table 1 and Table 2. The mean expected operative risk by EuroSCORE II was 7.4%±8.7% (5.2%, 5.7% and 9.8% for each center).
| Variables / Categories | n | % |
|---|---|---|
| Series | ||
| 1 | 346 | 0.308 |
| 2 | 251 | 0.224 |
| 3 | 526 | 0.468 |
| Period of surgery | ||
| [Feb 15, 1999–Aug 1 st, 2007] | 252 | 0.224 |
| [Aug 13, 2007–Jul 31, 2012] | 252 | 0.224 |
| [Aug 3, 2012–Oct 12, 2015] | 352 | 0.313 |
| [Oct 21, 2015–Apr 30, 2018] | 267 | 0.238 |
| Age (years) | ||
| [36–66] | 292 | 0.260 |
| [67–72] | 282 | 0.251 |
| [73–77] | 272 | 0.242 |
| [78–94] | 277 | 0.247 |
| Missing | 0 | 0.000 |
| Sex | ||
| Male | 903 | 0.804 |
| Female | 220 | 0.196 |
| Missing | 0 | 0.000 |
| Hypertension on treatment | ||
| No | 272 | 0.242 |
| Yes | 848 | 0.755 |
| Missing | 3 | 0.003 |
| Current smoking | ||
| No | 502 | 0.447 |
| Yes | 204 | 0.182 |
| Missing | 417 | 0.371 |
| Anemia | ||
| No | 531 | 0.473 |
| Yes | 525 | 0.467 |
| Missing | 67 | 0.060 |
| Body mass index (kg/m2) | ||
| [16.9–24.2] | 295 | 0.263 |
| [24.3–26.6] | 269 | 0.240 |
| [26.7–29.3] | 274 | 0.244 |
| [29.4–48.4] | 279 | 0.248 |
| Missing | 6 | 0.005 |
| Glycaemia on admission (mmol/L) | ||
| [1.0–4.7] | 268 | 0.239 |
| [4.8–5.5] | 238 | 0.212 |
| [5.6–6.6] | 240 | 0.214 |
| [6.7–25.2] | 247 | 0.220 |
| Missing | 130 | 0.116 |
| Diabetes | ||
| No | 743 | 0.662 |
| Orally treated | 296 | 0.264 |
| On insulin | 84 | 0.075 |
| Missing | 0 | 0.000 |
| Chronic lung disease | ||
| No | 1,011 | 0.900 |
| Yes | 99 | 0.088 |
| Missing | 13 | 0.012 |
| Renal impairment | ||
| No (eGFR‡ >85 mL/min) | 293 | 0.261 |
| Moderate (eGFR‡ 50–85 mL/min) | 591 | 0.526 |
| Severe (eGFR‡ ≤50 mL/min) | 217 | 0.193 |
| Dialysis (regardless of eGFR‡) | 22 | 0.020 |
| Missing | 0 | 0.000 |
| Extracardiac arteriopathy | ||
| No | 830 | 0.739 |
| Yes | 281 | 0.250 |
| Missing | 12 | 0.011 |
| Prior CV accident | ||
| No | 607 | 0.541 |
| Yes | 52 | 0.046 |
| Missing | 462 | 0.411 |
| Poor mobility | ||
| No | 737 | 0.656 |
| Yes | 16 | 0.014 |
| Missing | 368 | 0.328 |
| Cardiac rhythm | ||
| Sinus | 978 | 0.871 |
| AF or pacemaker-induced | 138 | 0.123 |
| Missing | 7 | 0.006 |
| NYHA class | ||
| I | 212 | 0.189 |
| II | 439 | 0.391 |
| III | 356 | 0.317 |
| IV | 106 | 0.094 |
| Missing | 10 | 0.009 |
| Unstable angina | ||
| No | 888 | 0.791 |
| Yes | 226 | 0.201 |
| Missing | 9 | 0.008 |
| Recent MI | ||
| No | 1,042 | 0.928 |
| Yes | 78 | 0.069 |
| Missing | 3 | 0.003 |
| Left ventricular function | ||
| Good (LVEF >50%) | 636 | 0.566 |
| Moderate (LVEF 31–50%) | 381 | 0.339 |
| Poor (LVEF 21–30%) | 73 | 0.065 |
| Very poor (LVEF ≤20%) | 30 | 0.027 |
| Missing | 3 | 0.003 |
| Pulmonary hypertension | ||
| No | 771 | 0.687 |
| Moderate (PAP systolic 31–54 mmHg) | 251 | 0.224 |
| Severe (PAP systolic >54 mmHg) | 88 | 0.078 |
| Missing | 13 | 0.012 |
| Critical preoperative state | ||
| No | 1,006 | 0.896 |
| Yes | 115 | 0.102 |
| Missing | 2 | 0.002 |
| Preoperative IABP | ||
| No | 1,053 | 0.938 |
| Yes | 68 | 0.061 |
| Missing | 2 | 0.002 |
| Active infective endocarditis | ||
| No | 1,090 | 0.971 |
| Yes | 18 | 0.016 |
| Missing | 15 | 0.013 |
| Prior cardiac operation | ||
| No | 1,035 | 0.922 |
| Yes | 75 | 0.067 |
| Missing | 13 | 0.012 |
| Surgical priority | ||
| Elective | 516 | 0.459 |
| Non-elective | 607 | 0.541 |
| Missing | 0 | 0.000 |
| Expected operative risk (by EuroSCORE II) (%)§ | ||
| [0.006–0.025] | 283 | 0.252 |
| [0.026–0.044] | 286 | 0.255 |
| [0.045–0.083] | 275 | 0.245 |
| [0.084–0.773] | 276 | 0.246 |
| Missing | 3 | 0.003 |
| Overall | 1,123 | 1.000 |
*Discrete variables reported in accordance with internationally agreed categories; continuous variables categorized in quartiles (with the range in brackets). Values are number of patients with the corresponding percentage. †See Definitions. ‡The creatinine clearance rate, calculated by the Cockcroft-Gault formula, was used to approximate the GFR. §Nashef et al.20 AF, atrial fibrillation; CV, cerebrovascular; eGFR, estimated glomerular filtration rate; EuroSCORE, European System for Cardiac Operative Risk Evaluation; IABP, intra-aortic balloon pump; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NYHA, New York Heart Association; PAP, pulmonary artery pressure.
| Variables / Categories | n | % |
|---|---|---|
| Coronary artery disease | ||
| Isolated left main | 30 | 0.027 |
| 1-vessel | 67 | 0.060 |
| 2-vessel | 326 | 0.290 |
| 3-vessel | 698 | 0.622 |
| Missing | 2 | 0.002 |
| No. of coronary anastomoses | ||
| 2 | 399 | 0.355 |
| 3 | 408 | 0.363 |
| 4 | 212 | 0.189 |
| 5 | 78 | 0.069 |
| ≥6 | 24 | 0.021 |
| Missing | 2 | 0.002 |
| Use of vein grafts (with aortic anastomosis) | ||
| No | 744 | 0.663 |
| Yes | 377 | 0.336 |
| Missing | 2 | 0.002 |
| Valve surgery | ||
| No | 104 | 0.093 |
| Aortic±tricuspid | 683 | 0.608 |
| Mitral±tricuspid | 287 | 0.256 |
| Aortic+mitral±tricuspid | 41 | 0.037 |
| Tricuspid | 8 | 0.007 |
| Missing | 0 | 0.000 |
| Surgery on thoracic aorta | ||
| No | 1,030 | 0.917 |
| Yes | 93 | 0.083 |
| Missing | 0 | 0.000 |
| Left ventricular surgery | ||
| No | 1,101 | 0.980 |
| Reshaping | 15 | 0.013 |
| Post-infarct rupture repair | 4 | 0.004 |
| Thrombectomy | 3 | 0.003 |
| Missing | 0 | 0.000 |
| Other concomitant surgery‡ | ||
| No | 1,078 | 0.960 |
| Yes | 45 | 0.040 |
| Missing | 0 | 0.000 |
| No. of procedures | ||
| 2 | 973 | 0.866 |
| 3 or more | 150 | 0.134 |
| Missing | 0 | 0.000 |
| Cardioplegia | ||
| Multidose cold blood | 438 | 0.390 |
| Multidose tepid blood | 106 | 0.094 |
| Multidose warm blood | 83 | 0.074 |
| Custodiol-HTK solution | 277 | 0.247 |
| St. Thomas’ Hospital solution No. 2 | 142 | 0.126 |
| Missing | 77 | 0.069 |
| Cardiopulmonary bypass time (min) | ||
| [30–102] | 288 | 0.256 |
| [103–130] | 276 | 0.246 |
| [131–171] | 275 | 0.245 |
| [172–540] | 280 | 0.249 |
| Missing | 4 | 0.004 |
| Aortic cross-clamp time (min) | ||
| [20–84] | 278 | 0.248 |
| [85–107] | 278 | 0.248 |
| [108–140] | 287 | 0.256 |
| [141–397] | 269 | 0.240 |
| Missing | 11 | 0.010 |
| Length of surgery (min) | ||
| [129–301] | 288 | 0.256 |
| [302–339] | 276 | 0.246 |
| [340–386] | 279 | 0.248 |
| [387–897] | 278 | 0.248 |
| Missing | 2 | 0.002 |
| Use of chlorhexidine-alcohol (because of iodine allergy) | ||
| No | 944 | 0.841 |
| Yes | 50 | 0.045 |
| Missing | 129 | 0.115 |
| Overall | 1,123 | 1.000 |
*Discrete variables reported in accordance with internationally agreed categories; continuous variables categorized in quartiles (with the range in brackets). Values are number of patients with the corresponding percentage. †See Definitions. ‡Cardiac tumor removal and operations for arrhythmias. HTK, histidine-tryptophan-ketoglutarate.
A total of 71 (6.3%) patients died in hospital within 30 days after surgery, and a further 18 patients died in hospital more than 30 days after surgery, yielding an overall in-hospital mortality rate of 7.9% (6.3%, 9.2% and 9.6% for each center). Multiple transfusions (27.3%), prolonged mechanical ventilation or reintubation (16.7%), acute kidney injury (11.5%), and superficial or deep SWI (10.4%) were the most frequent postoperative complications. Temporary or permanent neurological dysfunction occurred in 5.4% of cases. At least 1 major postoperative complication occurred in 31.8% of patients (25.1%, 25.7% and 41.4% for each center). The mean length of intensive care unit and in-hospital stay was 6.4±13.4 days and 16.6±18.6 days, respectively (Table 3).
| Variables / Categories | n | % |
|---|---|---|
| In-hospital death | ||
| No | 1,034 | 0.921 |
| Within POD 30 | 71 | 0.063 |
| >POD 30 | 18 | 0.016 |
| Missing | 0 | 0.000 |
| Neurological complications | ||
| No | 1,059 | 0.943 |
| Temporary | 35 | 0.031 |
| Permanent | 26 | 0.023 |
| Missing | 3 | 0.003 |
| Prolonged (>48 h) mechanical ventilation or reintubation | ||
| No | 934 | 0.832 |
| Yes | 187 | 0.167 |
| Missing | 2 | 0.002 |
| Lower airway infection | ||
| No | 1,006 | 0.896 |
| Yes | 109 | 0.097 |
| Missing | 8 | 0.007 |
| AF, new-onset | ||
| No | 715 | 0.637 |
| Yes | 263 | 0.234 |
| Not applicable‡ | 138 | 0.123 |
| Missing | 7 | 0.006 |
| Low cardiac output | ||
| No | 607 | 0.541 |
| Inotropes | 394 | 0.351 |
| IABP±inotropes | 87 | 0.077 |
| ECMO±IABP | 13 | 0.012 |
| Missing | 22 | 0.020 |
| Use of norepinephrine | ||
| No | 397 | 0.354 |
| Yes | 704 | 0.627 |
| Missing | 22 | 0.020 |
| Acute kidney injury | ||
| No | 991 | 0.882 |
| IV diuretics | 76 | 0.068 |
| RRT | 53 | 0.047 |
| Missing | 3 | 0.003 |
| Mesenteric ischemia or infarction | ||
| No | 1,091 | 0.972 |
| Yes | 30 | 0.027 |
| Missing | 2 | 0.002 |
| Mediastinal re-entry for bleeding or tamponade | ||
| No | 1,009 | 0.898 |
| Re-sternotomy | 102 | 0.091 |
| Subxyphoid window | 9 | 0.008 |
| Missing | 3 | 0.003 |
| Sternal wound infection | ||
| No | 1,003 | 0.893 |
| Superficial | 46 | 0.041 |
| Deep | 71 | 0.063 |
| Missing | 3 | 0.003 |
| Sepsis | ||
| No | 1,062 | 0.946 |
| Yes | 48 | 0.043 |
| Missing | 13 | 0.012 |
| Multiorgan failure | ||
| No | 1,026 | 0.914 |
| Yes | 94 | 0.084 |
| Missing | 3 | 0.003 |
| No. of RBCs transfused | ||
| 0 | 476 | 0.429 |
| 1–2 | 338 | 0.301 |
| ≥3 | 307 | 0.273 |
| Missing | 2 | 0.002 |
| Any major complications | ||
| No | 764 | 0.680 |
| Yes | 357 | 0.318 |
| Missing | 2 | 0.002 |
| Length of ICU stay (days) | ||
| [0–2] | 345 | 0.307 |
| [3–4] | 338 | 0.301 |
| [5–6] | 196 | 0.175 |
| [7–195] | 241 | 0.215 |
| Missing | 3 | 0.003 |
| Length of hospital stay (days) | ||
| [0–8] | 281 | 0.250 |
| [9–12] | 332 | 0.296 |
| [13–17] | 243 | 0.216 |
| [18–217] | 266 | 0.237 |
| Missing | 1 | 0.001 |
| Overall | 1,123 | 1.000 |
*Discrete variables reported in accordance with internationally agreed categories; continuous variables categorized in quartiles (with the range in brackets). Values are number of patients with the corresponding percentage. †See Definitions. ‡Preoperative AF or rhythm pacemaker-induced. ECMO, extracorporeal membrane oxygenator; ICU, intensive care unit; IV, intravenous; POD, postoperative day; RBCs, packed red blood cells; RRT, renal replacement therapy. Other abbreviations as in Table 1.
The results of the univariable analysis of the risk factors for in-hospital mortality and postoperative complications are shown in Supplementary Tables 1–3. According to the multivariable analyses (Supplementary Tables 4–8), diabetes on insulin (OR, 2.00, P=0.045), eGFR ≤50 mL/min or dialysis (OR, 3.11, P<0.0001), extracardiac arteriopathy (OR, 2.00, P=0.0058), New York Heart Association (NYHA) class III–IV (OR, 1.80, P=0.017), recent MI (OR, 3.16, P=0.0009), left ventricular ejection fraction (LVEF) ≤50% (OR, 2.01, P=0.0054), systolic pulmonary artery pressure >54 mmHg (OR, 2.80, P=0.0016), active infective endocarditis (OR, 6.31, P=0.0011), and aortic cross-clamp time >140 min (OR, 1.68, P=0.04) were predictors of in-hospital death (area under receiver-operating characteristic curve [aROC], 0.770); dialysis (OR, 3.61, P=0.024), extracardiac arteriopathy (OR, 1.99, P=0.016), and critical preoperative state (OR, 3.67, P<0.0001) were predictors of neurological complications (aROC, 0.751); surgery performed between August 13, 2007 and July 31, 2012 (OR, 1.81, P=0.012), diabetes on insulin (OR, 2.20, P=0.023), preoperative use of IABP (OR, 2.02, P=0.045), ≥3 procedures (OR, 2.13, P=0.0056), and length of surgery >386 min (OR, 1.85, P=0.013) were predictors of mediastinal re-entry for bleeding or tamponade (aROC, 0.625); female sex (OR, 1.90, P=0.0075), body mass index >26.6 kg/m2 (OR, 2.08, P=0.0009), diabetes on insulin (OR, 3.85, P<0.0001), postoperative neurological complications (OR, 4.00, P<0.0001), and mediastinal re-entry through re-sternotomy (OR, 6.21, P<0.0001) were predictors of SWIs (aROC, 0.776); diabetes on insulin (OR, 2.09, P=0.0061), chronic lung disease (OR, 1.63, P=0.047), eGFR ≤50 mL/min or dialysis (OR, 1.79, P=0.0009), extracardiac arteriopathy (OR, 1.57, P=0.0074), recent MI (OR, 1.89, P=0.022), preoperative use of IABP (OR, 21.3, P<0.0001), non-elective surgical priority (OR, 1.43, P=0.022), use of Custodiol®-histidine-tryptophan-ketoglutarate (HTK) solution (OR, 1.54, P=0.011), and length of surgery >386 min (OR, 1.86, P=0.0035) were predictors of in-hospital death and any major complication (aROC, 0.743).
Late OutcomesFollow-up was complete for 1,123 (100%) patients. During the follow-up period (median, 4.2 years, range, 0–18.1 years, cumulative, 5,641.7 patient-years), there were 314 deaths (55.7/1,000 patient-years); 215 patients experienced at least 1 MACCE, 77 patients had at least 1 hospital readmission for CHF, and 24 underwent PCI or repeat cardiac operation; cerebrovascular accident, MI, or pulmonary embolism occurred in 26, 15 and 6 cases, respectively; and finally, 74 patients suffered from recurrent angina and 47 from dyspnea.
According to the Cox proportional hazards regression (Table 4), extracardiac arteriopathy and reduced LVEF were concomitant predictors of all-cause death, cardiac or cerebrovascular death, MACCE, and hospital readmission for CHF; female sex, extracardiac arteriopathy, chronic dialysis, and reduced LVEF were common predictors of cardiac or cerebrovascular death and MACCE (Supplementary Figures 1A,1B,2).
| Variables | All-cause death | Cardiac or CV death | MACCE | PCI or reoperation | Readmission due to CHF | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | |
| Series 3 | 0.77 | 0.60–1.00 | 0.053 | – | – | – | 1.44 | 1.05–1.98 | 0.023 | – | – | – | – | – | – |
| Age | 1.08 | 1.06–1.10 | <0.0001 | 1.03 | 1.00–1.06 | 0.024 | – | – | – | – | – | – | – | – | – |
| Female | – | – | – | 1.78 | 1.14–2.81 | 0.012 | 1.74 | 1.27–2.38 | 0.001 | – | – | – | 2.01 | 1.13–3.57 | 0.018 |
| Hypertension on treatment | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Diabetes orally treated | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Diabetes on insulin | 1.67 | 1.13–2.47 | 0.01 | 1.73 | 0.94–3.16 | 0.078 | – | – | – | 3.12 | 0.88–11.1 | 0.08 | – | – | – |
| Chronic lung disease | – | – | – | 1.68 | 0.99–2.85 | 0.056 | – | – | – | – | – | – | – | – | – |
| eGFR† | – | – | – | 0.98 | 0.97–0.99 | 0.002 | 0.99 | 0.99–1.00 | 0.052 | – | – | – | – | – | – |
| Chronic dialysis | 7.41 | 3.76–14.6 | <0.0001 | 3.61 | 1.25–10.4 | 0.018 | 2.58 | 1.06–6.27 | 0.038 | – | – | – | – | – | – |
| Extracardiac arteriopathy | 1.72 | 1.35–2.17 | <0.0001 | 1.74 | 1.16–2.61 | 0.008 | 1.64 | 1.24–2.16 | 0.001 | – | – | – | 1.89 | 1.12–3.18 | 0.017 |
| NYHA class III–IV | 1.43 | 1.13–1.81 | 0.003 | – | – | – | – | – | – | – | – | – | – | – | – |
| Recent MI | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| LVEF | 0.98 | 0.97–0.99 | <0.0001 | 0.96 | 0.95–0.98 | <0.0001 | 0.98 | 0.97–0.99 | <0.0001 | – | – | – | 0.98 | 0.96–0.99 | 0.007 |
| PAP systolic >54 mmHg | 1.47 | 1.00–2.16 | 0.049 | 1.92 | 1.09–3.36 | 0.023 | 1.51 | 0.95–2.41 | 0.080 | – | – | – | – | – | – |
| Critical preoperative state | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Active infective endocarditis | – | – | – | 6.12 | 2.09–17.9 | 0.001 | 3.34 | 1.00–11.1 | 0.051 | 18.3 | 2.14–155.9 | 0.008 | 7.83 | 1.06–57.7 | 0.044 |
| Prior cardiac operation | 4.29 | 1.36–13.5 | 0.013 | – | – | – | – | – | – | – | – | – | 7.18 | 0.97–53.0 | 0.054 |
| Salvage surgical priority | 6.07 | 1.75–21.0 | 0.005 | – | – | – | 3.48 | 0.83–14.5 | 0.088 | – | – | – | – | – | – |
| ≥3 cor. anastomoses | 0.76 | 0.58–1.00 | 0.051 | – | – | – | – | – | – | – | – | – | – | – | – |
| Aortic+mitral valve surgery | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 3 procedures | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Surgery on thoracic aorta | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Cardiopulmonary bypass time | – | – | – | 1.91 | 1.29–2.82 | 0.001 | – | – | – | – | – | – | 2.27 | 1.38–3.74 | 0.001 |
| Aortic cross-clamp time | 1.56 | 1.19–2.05 | 0.001 | – | – | – | 1.55 | 1.17–2.05 | 0.002 | 4.47 | 1.91–10.5 | 0.001 | – | – | – |
*See Definitions. †The creatinine clearance rate, calculated by Cockcroft-Gault formula, was used to approximate GFR. CHF, congestive heart failure; CI, confidence interval; HR, hazard ratio; MACCE, major adverse and cerebrovascular events; PCI, percutaneous coronary intervention. Other abbreviations as in Table 1.
The 10-year nonparametric estimates of freedom from all-cause death, cardiac or cerebrovascular death, MACCE, PCI or reoperation, and hospital readmission for CHF were 48.7% (95% CI, 46.4–51.0%), 78.8% (95% CI, 76.7–80.9%), 53.5% (95% CI, 50.9–56.1%), 96.6% (95% CI, 95.7–97.5%) and 84.3% (95% CI, 82.2–86.4%), respectively. The 10-year nonparametric estimates of EuroSCORE II-adjusted freedom from all-cause death, cardiac or cerebrovascular death, MACCE, PCI or reoperation, and hospital readmission for CHF were 49.1%, 79.4%, 53.8%, 96.5% and 83.6%, respectively. The 10-year nonparametric estimates of covariates-adjusted freedom from all-cause death, cardiac or cerebrovascular death, MACCE, PCI or reoperation, and hospital readmission for CHF were 50.3%, 84.2%, 54.6%, 97.1% and 85.7%, respectively (Figures 1–3).
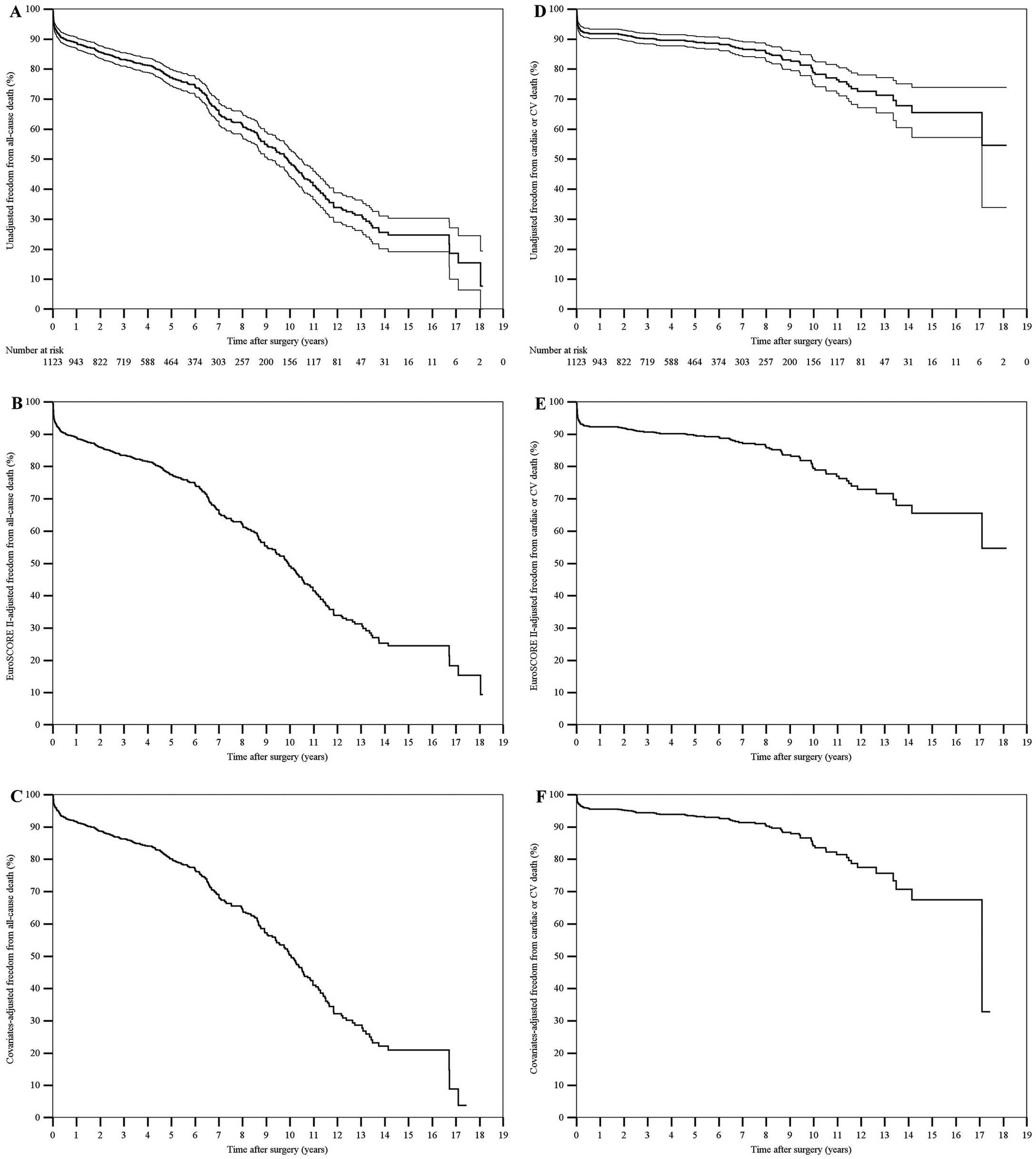
(A,D) Unadjusted, (B,E) EuroSCORE II-adjusted, and (C,F) covariates-adjusted curves (Kaplan-Meier method) of freedom from (A–C) all-cause death and (D–F) cardiac or cerebrovascular (CV) death after surgery. Unadjusted estimates are displayed with 95% confidence intervals, whereas the single survival curve at the mean EuroSCORE II or at the mean of all covariates in the model is displayed for adjusted estimates. The number of patients at risk is reported. EuroSCORE II, European System for Cardiac Operative Risk Evaluation II.
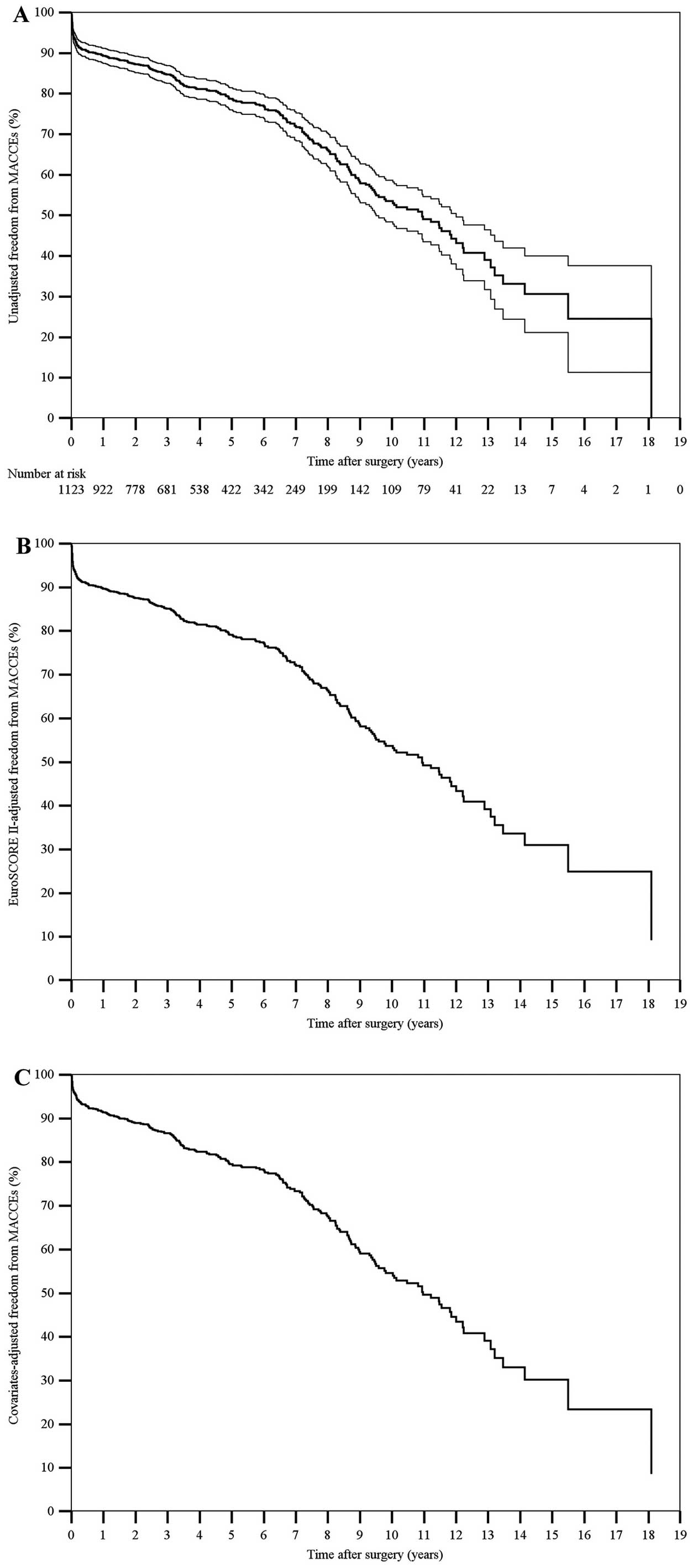
(A) Unadjusted, (B) EuroSCORE II-adjusted, and (C) covariates-adjusted curves (Kaplan-Meier method) of freedom from MACCE after surgery. Unadjusted estimates are displayed with 95% confidence intervals, whereas the single survival curve at the mean EuroSCORE II or at the mean of all covariates in the model is displayed for adjusted estimates. The number of patients at risk is reported. EuroSCORE II, European System for Cardiac Operative Risk Evaluation II; MACCE, major adverse cardiac and cerebrovascular events.
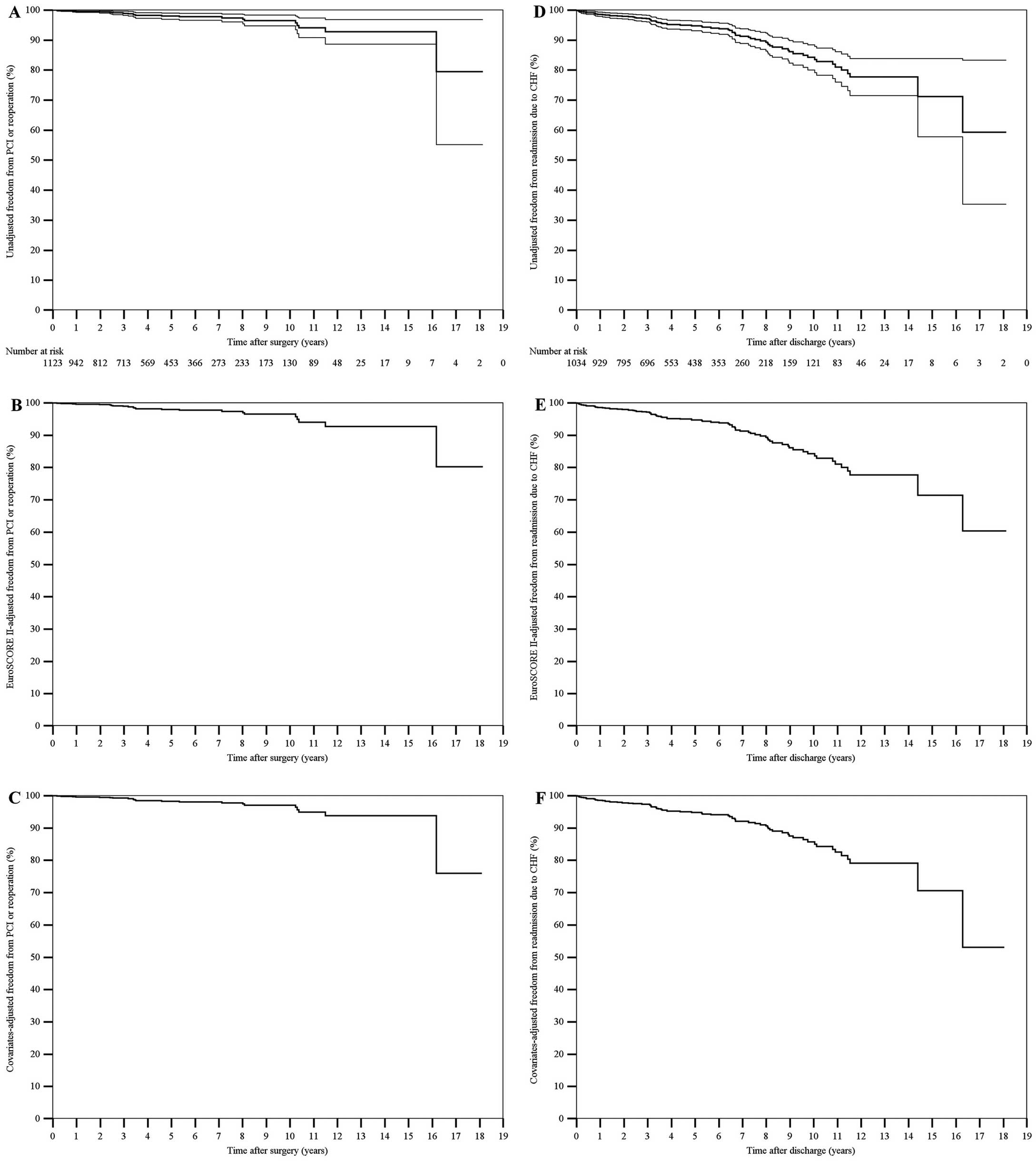
(A,D) Unadjusted, (B,E) EuroSCORE II-adjusted, and (C,F) covariates-adjusted curves (Kaplan-Meier method) of freedom from (A–C) PCI or reoperation after surgery and (D–F) hospital readmission for CHF. Unadjusted estimates are displayed with 95% confidence intervals, whereas the single survival curve at the mean EuroSCORE II or at the mean of all covariates in the model is displayed for adjusted estimates. The number of patients at risk is reported. EuroSCORE II, European System for Cardiac Operative Risk Evaluation II; CHF, congestive heart failure; PCI, percutaneous coronary intervention.
The 10-year nonparametric estimates of unadjusted, EuroSCORE II-adjusted, and covariates-adjusted freedom from cerebrovascular accident after hospital discharge were 95% (95% CI, 93.7–96.2%), 95.1% and 94.8%, respectively Figure 4). Nonparametric estimates of freedom from any neurological complication (including the in-hospital course) according to the use (or not) of vein grafts (with aortic anastomosis) in patients undergoing BITA grafting plus mitral valve surgery showed that there were improved results in the absence of aortic manipulation for the proximal venous anastomosis (hazard ratio [HR], 6.09, 95% CI, 1.63–22.8, log-rank test, P=0.051; Supplementary Figure 1C).

(A) Unadjusted, (B) EuroSCORE II-adjusted, and (C) covariates-adjusted curves (Kaplan-Meier method) of freedom from cerebrovascular (CV) accident after hospital discharge. Unadjusted estimates are displayed with 95% confidence intervals, whereas the single survival curve at the mean EuroSCORE II or at the mean of all covariates in the model is displayed for adjusted estimates. The number of patients at risk is reported. EuroSCORE II, European System for Cardiac Operative Risk Evaluation II.
Survival of 526 patients undergoing combined CABG using BITA (mean age, 70±8.1 years, females, 18.1%) was compared with that of 688 combined CABG patients using SITA (mean age, 71.7±8.1 years, females, 32%). Despite significant differences in the baseline patient characteristics, surgical findings and operative data between the 2 series (Supplementary Table 9), there was no significant difference in the in-hospital mortality rates (6.3% vs. 7.3%, P=0.572), as expected by EuroSCORE II (mean, 9.8±10.6% vs. 9.6±11.2%, P=0.217).
During the follow-up period (median, 4.9 vs. 5.0 years, P=0.086), the 10-year nonparametric estimates of unadjusted freedom from all-cause death was 52.6% (95% CI, 49.67–55.6%) for the combined BITA patients, and 40.3% (95% CI, 37.9–42.7%) for the combined SITA patients (HR, 0.77, 95% CI, 0.65–0.91, log-rank test, P=0.003; Supplementary Figure 1D). Although this survival difference was significant even for EuroSCORE II-adjusted estimates (HR, 0.78, 95% CI, 0.66–0.93, log-rank test, P=0.005), it was not after adjusting for multiple covariates (HR, 0.92, 95% CI, 0.75–1.12, log-rank test, P=0.391).
The present retrospective study is the first to explore immediate and long-term outcomes of a large series of patients undergoing BITA grafting concomitant with other cardiac operations. The effect of BITA use on outcome of combined CABG has not been investigated before to the best of our knowledge, so we reviewed the pooled results of 3 case series from 3 European centers.
The most important finding of the study was that using BITA grafts in addition to other cardiac operations did not increase in-hospital mortality rates. In fact, the 30-day (6.3%) and in-hospital (7.9%) mortality rates found in this study corresponded exactly to the mean expected operative risk by EuroSCORE II (7.4%), even though the original sample of patients in whom EuroSCORE II was derived presumably included very few patients undergoing either isolated or combined CABG with BITA.20 Furthermore, the mortality rates observed in our study were comparable with those reported in relevant studies in the literature exploring outcomes after complex cardiac operations (primarily CABG plus mitral valve surgery) where CABG was performed using saphenous vein grafts with or without 1 (usually the left) ITA; in-hospital mortality rate ranged between 3.9% and 18% in those studies.28–38 Insulin-treated diabetes, severe renal impairment, extracardiac arteriopathy, NYHA class III–IV, recent MI, left ventricular dysfunction, pulmonary hypertension, active infective endocarditis, and prolonged aortic cross-clamp time were found to be independent predictors of in-hospital death, although the accuracy of prediction of this multivariable model was weak (aROC, 0.770).
Similar to 2 other comparable studies,31,35 almost one-third of patients in the present study suffered from at least 1 major complication, primarily multiple transfusions, prolonged mechanical ventilation, acute kidney injury or SWI. Consistently, there was a prolonged mean intensive care unit stay. As expected, there was a relatively high incidence of complications directly or indirectly related to BITA harvest and use, such as SWIs (and SWI-related sepsis), multiple transfusions and mediastinal re-entry for bleeding or tamponade, as well as prolonged mechanical ventilation or reintubation. Phrenic nerve palsy occurred very exceptionally and was never permanent, although it may have contributed to the prolonged mechanical ventilation in some cases. With respect to other reported experiences of isolated CABG with BITA5–13,15,17,27,39,40 or combined CABG using either vein grafts alone or vein grafts plus 1 ITA,28–38 the incidence of all SWIs of our study (10.4%) was slightly higher and did not differ significantly between the participating centers (9.2%, 10.6% and 11%). Nevertheless, SWI was confirmed to be a severe postoperative complication in the present study, where it was found to be associated with in-hospital death (P=0.0026) and almost all postoperative complications except low cardiac output and mesenteric ischemia. Some recognized risk factors for sternal complications following isolated BITA grafting, such as female sex, high body mass index, diabetes on insulin, and mediastinal re-entry through re-sternotomy,8,13 were independent predictors of SWIs even in the BITA patients of our study. Even the occurrence of postoperative neurological complications was associated with an increased risk of SWIs in this study, maybe because of prolonged mechanical ventilation, forced reduced mobility, and poor nutritional status of the patients with neurological dysfunction. Overall, the accuracy of prediction of this multivariable model for SWIs was weak (aROC, 0.776). As expected, extracardiac arteriopathy (including carotid artery disease), and chronic dialysis, which is frequently associated with aortic calcifications,8,10,13 as well as critical preoperative state (by EuroSCORE II), which includes ventricular tachycardia, ventricular fibrillation, aborted sudden death, and preoperative cardiac massage among the definition criteria,20 were specific predictors of neurological complications in the study patients (aROC, 0.751). It should be emphasized that the rate of permanent neurological complications early after surgery of this experience (2.3%) compared favorably with the rate of stroke (4.2%) in a similar study of 479 patients undergoing CABG concomitant with valve operations.31 Surgery performed between August 13, 2007 and July 31, 2012, diabetes on insulin, preoperative use of IABP, ≥3 procedures, and prolonged operative times were predictors of mediastinal re-entry for bleeding or tamponade. However, the accuracy of prediction of this model, which included a time variable and was therefore most likely affected by contingent issues, was poor (aROC, 0.625). Finally, diabetes on insulin, severe renal impairment, extracardiac arteriopathy, and recent MI were common predictors of both in-hospital death (as reported above) and the combined endpoint consisting of in-hospital death and major postoperative complications. Chronic lung disease, preoperative IABP, non-elective surgical priority, use of Custodiol-HTK solution, and prolonged duration of surgery were further predictors of this combined endpoint, realizing an overall predictive model with weak accuracy of prediction (aROC, 0.743). The result concerning Custodiol-HTK solution agreed substantially with the conclusions of a recent comparative study by the present authors that explored immediate outcomes of 1,164 propensity-matched pairs who underwent cardiac operations other than isolated CABG and received either single-dose Custodiol-HTK or multidose cold blood cardioplegia.26 According to that analysis, the optimal cutoff value of cross-clamping time for in-hospital death in the Custodiol-HTK cohort was 26 min shorter than for the multidose cold blood group, although there was no significant difference in in-hospital mortality rates between the 2 options of myocardial protection.
Long-term outcomes were comparable with those of other similar studies in the literature,28–38 but, as expected, poorer than those for isolated BITA grafting.5–13,15,17,27,39,40 In this series of patients with an average age of more than 70 years, approximately 50% of subjects were alive or free from MACCE at 10 years after surgery; approximately 85% of patients were free from cardiac or cerebrovascular death or from hospital readmission for CHF; approximately 97% of patients were free from PCI or cardiac reoperation; finally, approximately 95% of patients were free from cerebrovascular accident after hospital discharge. Although female sex, extracardiac arteriopathy, chronic dialysis, and poor systolic function before surgery were risk factors for both cardiac or cerebrovascular death and MACCE, active infective endocarditis and prolonged myocardial ischemia during surgery were the sole predictors of repeat operation or PCI. No details pertaining to the type of operation were found to be related to late outcomes. As to concerns of early and late neurological dysfunction following operation, based on the results of the subanalysis that was carried out only in patients undergoing BITA grafting plus mitral valve surgery, improved results occurred in the absence of aortic manipulation for the proximal venous anastomosis.
In fact, a comparison of patients undergoing combined CABG using BITA or SITA was also performed. However, because of too few patients from the 2 French centers undergoing SITA grafting during the study period, this comparison involved only the BITA or SITA patients from Trieste. Despite significant differences in baseline patient characteristics, surgical findings and operative data between the 2 series (suggesting some type of patient selection), there were significant differences neither as expected operative risk (by EuroSCORE II, P=0.217) nor in-hospital death (P=0.572). Both unadjusted and EuroSCORE II-adjusted estimates of survival during the follow-up period for combined BITA patients were higher than for combined SITA patients (P=0.003 and 0.005, respectively); however, this survival difference was not significant after adjusting estimates for multiple covariates (P=0.391).
Study LimitationsThis study suffered from some limitations that deserve to be noted. The retrospective nature of the study, and the fact that it was performed on patients operated on at 3 different centers (albeit all having large experience in BITA use) and over a period spanning ∼20 years could have affected the results. Although we chose this period in order to assemble as many patients as possible, we are aware that changes in surgical techniques, the medical and pharmacological environment, and differences in practices between centers during this period could have had a confounding effect. We are also well aware that there could have been some differences between the participating centers in terms of the data available in their files and databases. Nonetheless, no significant differences were found between centers in terms of in-hospital mortality rates and long-term survival. Besides, the surgical technique for ITA harvesting was the same in the 3 centers and did not change significantly during the study period time. A further limitation was that, in several cases, it was impossible to distinguish between cardiac and cerebrovascular death during the follow-up period. In addition, some multivariable analyses of the study could be depotentiated by the limited number of events that occurred during follow-up. Finally, because for many years in all 3 centers in the study both ITAs were harvested for most patients undergoing isolated or combined CABG, it was impossible to set up a comparative study between conventional combined CABG and combined CABG using BITA, without comparing non-contemporary cohorts of patients (a comparison between 2 reasonably contemporary cohorts of patients undergoing combined CABG using either BITA or SITA was possible only for 1 center). Therefore, the results of this study should be interpreted with caution, and warrant further confirmation by means of randomized controlled trials.
According to the results of this multicenter retrospective study, the use of BITA for combined CABG does not increase in-hospital mortality with respect to both the expected operative risk calculated by EuroSCORE II and relevant studies in the literature exploring outcomes of complex cardiac operations where CABG was performed using vein grafts with or without 1 ITA. Although almost one-third of patients in the present study suffered from at least 1 major complication (mainly multiple transfusions, prolonged mechanical ventilation, acute kidney injury or SWI), long-term outcomes seemed encouraging, and mostly depended on sex and preoperative comorbidities such as chronic dialysis and extracardiac arteriopathy, as well as on baseline cardiac function. There was a low rate of neurological complications both early after surgery and during the follow-up period; this fact might derive, at least partially, from reduced perioperative manipulation of the aorta, and be related to BITA use.
The authors declare that they have no conflicts of interest.
None.
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-19-0696