2019 Volume 83 Issue 2 Pages 304-312
2019 Volume 83 Issue 2 Pages 304-312
Background: The Substrate and Trigger Ablation for Reduction of Atrial Fibrillation Trial Part II (STAR-AF2) emphasized the importance of circumferential pulmonary vein isolation (CPVI) during AF ablation.
Methods and Results: This study involved 2,297 consecutive patients (mean age, 58±11 years; 73.1% male, 70.1% paroxysmal AF) undergoing AF ablation from 2009 to 2017. We investigated the ablation lesion set, ablation time, catheter type, and clinical outcomes. Over the 9 years, the extra-pulmonary vein (PV) left atrial (LA) ablation rate (76.8% to 19.4%, P<0.001 for trend) and ablation time (P<0.001 for trend) decreased dramatically, whereas the 1-year recurrence rates decreased (21.8% to 14.1%, P=0.04 for trend). In persistent AF patients, the extra-PV LA ablation rate (91.4% to 55.3%, P<0.001) and ablation time (P<0.001) decreased after the STAR-AF2 report, but the 1-year recurrence rates remained similar (22.1% to 17.9%, P=0.281). A mesh-type flexible tip (MFT) catheter with a moderately increased radiofrequency power was used since 2012, and the MFT catheter was independently associated with a lower clinical recurrence compared to other irrigated-tip catheters (HR, 0.670; 95% CI: 0.559–0.803, P<0.001; log rank P=0.002) without increasing the procedure-related complications (OR, 1.434; 95% CI: 0.937–2.194, P=0.097).
Conclusions: Over the 9 years the extra-PV LA ablation and 1-year recurrence rates in the AF ablation cohort decreased, in part due to improved catheter technology.
Over the past decade, catheter ablation of atrial fibrillation (AF) has been shown to maintain sinus rhythm more effectively than anti-arrhythmic drugs (AAD).1 It was also shown that triggers in the pulmonary veins (PV) were important in the initiation of AF,2 and circumferential PV isolation subsequently became the basic ablation strategy in all AF ablation procedures. There has been a high recurrence rate, however, in patients with persistent and long-standing persistent AF who underwent circumferential PV isolation (CPVI) alone. To improve the clinical outcome, a substrate modification was introduced, such as additional linear and complex fractionated atrial electrogram (CFAE) ablation. A recently published randomized control trial, the Substrate and Trigger Ablation for Reduction of Atrial Fibrillation Trial Part II (STAR-AF2), found that an extra-PV ablation does not have an added benefit compared with CPVI alone.3 With the change in the ablation strategy, various ablation catheters have been developed to improve the efficacy and safety of AF ablation. It is well known that radiofrequency (RF) power delivery and optimal electrode-tissue contact are important for improving the efficacy of AF ablation.1 To achieve better clinical and rhythm outcomes, the catheter technology has continuously been evolving, but it is also necessary to perform a long-term evaluation. The Yonsei AF cohort has enrolled and followed more than 2,200 patients after a de novo AF catheter ablation during the past 9 years, and has performed relatively strict rhythm monitoring based on the 2012 Heart Rhythm Society (HRS)/European Heart Rhythm Association (EHRA)/European Cardiac Arrhythmia Society (ECAS) Expert Consensus Statement guidelines. The purposes of this study were to review the characteristics of this patient population, procedure methods and instruments, and efficacy and safety of AF ablation over the 9 years, and to examine the differences between that before and after the STAR-AF2 report.
This study protocol adhered to the principles of the Declaration of Helsinki and was approved by the Institutional Review Board of the Yonsei University Health System. All patients provided written informed consent for inclusion in the Yonsei AF ablation Cohort Database (ClinicalTrials.gov identifier: NCT02138695). This study included 2,297 consecutive patients (mean age, 58±11 years; 73.1% male, 70.1% paroxysmal AF) who underwent de novo ablation between March 2009 and December 2017. We performed a serial rhythm follow-up based on the 2012 HRS/EHRA/ECAS Expert Consensus Statement guidelines. The exclusion criteria were as follows: (1) permanent AF refractory to electrical cardioversion; (2) AF with valvular disease ≥grade 2; and (3) a previous repeated ablation procedure. Echocardiography was performed in all patents to obtain the left atrial (LA) diameter, left ventricular ejection fraction (LVEF), peak transmitral flow velocity (E), and tissue Doppler imaging of the peak septal mitral annular velocity (Em) according to the American Society of Echocardiography guidelines.
Electrophysiological MappingThe intracardiac electrograms were recorded using the Prucka CardioLabTM Electrophysiology system (General Electric Medical Systems, Milwaukee, WI, USA), and RF catheter ablation (RFCA) was performed in all patients using 3-D electroanatomical mapping (NavX; St. Jude Medical, Minnetonka, MN, USA) merged with 3-D spiral computed tomography (CT). Double trans-septal punctures were performed and multi-view pulmonary venograms were obtained. After securing a trans-septal access, a circumferential PV-mapping catheter (Lasso; Biosense-Webster, Diamond Bar, CA, USA) was advanced to the PV using a long sheath (Schwartz SL1, St. Jude Medical). Systemic anticoagulation with i.v. heparin was achieved to maintain an activated clotting time of 350–400 s during the procedure. For electroanatomical mapping, the 3-D geometry of both the LA and PV was generated using the NavX system and then merged with 3-D spiral CT.
RFCAWe used an open irrigated-tip catheter (Celcius; Johnson & Johnson, Diamond Bar, CA, USA; NaviStar ThermoCool, Biosense-Webster; ThermoCool SF, Biosense-Webster; ThermoCool SmartTouch, Biosense-Webster; and TactiCath, St. Jude Medical) for RFCA. From 2012, we also started to use a mesh-type flexible tip (MFT) catheter (Coolflex; St. Jude Medical 30–35 W, 47℃; and FlexAbility, St. Jude Medical). All patients underwent de novo procedures with CPVI and the majority of patients (91.6%) underwent cavotricuspid isthmus block. For the extra-PV LA ablation, we conducted additional linear ablation including that of a roof line, posterior inferior line (posterior box lesion), and anterior line, especially in patients with persistent AF. A left lateral isthmus ablation, right atrial ablation, and CFAE ablation were performed in a minority of patients at the operator’s discretion (Supplementary Table 1). We defined extra-PV LA ablation as an additional linear ablation with or without CFAE ablation following the CPVI. The procedure ended when there was no immediate recurrence of AF in 10 min after cardioversion under an isoproterenol infusion (5–10 μg/min).
Post-Ablation Management and Follow-upAfter RFCA the patients visited the outpatient clinic at 1, 3, 6, and 12 months and every 6 months thereafter or whenever they had symptoms. Electrocardiography (ECG) was performed at every visit. Twenty-four-hour Holter monitoring was performed at 3, 6, and 12 months and then every 6 months according to the 2012 HRS/EHRA/ECAS Expert consensus statement guidelines. One-year recurrence of AF was defined as recurrent episodes of atrial tachycardia or AF lasting ≥30 s in the 3–12 months after the AF ablation.
Statistical AnalysisContinuous variables are reported as mean±SD and were analyzed using Student’s t-test for comparison between two groups. Categorical variables are reported as n (%) and were analyzed using chi-squared test and Fisher’s exact test. The trends in the categorical variables including the type of AF, sex, congestive heart failure, hypertension, diabetes, previous history of a stroke or transient ischemic attack, previous history of myocardial infarction (MI) or peripheral vascular disease, extra-PV LA ablation, post-ablation AAD use, 1-year recurrence rate, and complication rate were assessed on Cochran-Armitage trend analysis. The trends in the continuous variables including age, CHA2DS2VASC score, LA diameter, LVEF, E/Em, procedure time, and ablation time were analyzed using the ANOVA contrast test. Freedom from AF/atrial tachyarrhythmia (AF/AT) recurrence after AF ablation was compared across groups on Kaplan-Meier analysis, using the log-rank test for statistical significance. Univariate and multivariate cox regression analyses were performed to determine the predictors of AF/AT recurrence after AF ablation. Propensity score matching was carried out to reduce the selection bias in before-after comparisons with regard to STAR-AF2, and MFT catheter vs. other irrigated tip catheters. Logistic regression analysis was also used to investigate the risk factors for procedure-related complications. Statistical significance was considered for two-sided P<0.05. All statistical analysis was performed using SPSS for Windows (version 23.0; SPSS, Chicago, IL, USA), XLSTAT 2014 (Paris, France) and R (3.1.0, R Foundation for Statistical Computing, Boston, MA, USA).
The baseline characteristics of 2,297 enrolled patients who underwent ablations between 2009 and 2017 are listed in Table 1. Patient age (56±11 to 59±11 years, P<0.001 for trend), the proportion of female patients (17.9% to 30.8%, P=0.013 for trend), and CHA2DS2VASc score (1.1±1.1 to 1.6±1.4, P<0.001 for trend) gradually increased over the 9 years. The proportion of patients with congestive heart failure (2.7% to 11.7%, P<0.001 for trend) also increased.
| Overall | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | P-value | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. patients | 2,297 | 112 | 245 | 282 | 306 | 271 | 231 | 241 | 284 | 325 | NA |
| Paroxysmal AF | 1,610 (70.1) |
74 (66.1) |
179 (73.1) |
185 (65.6) |
216 (70.6) |
179 (66.1) |
181 (78.4) |
163 (67.6) |
203 (71.5) |
230 (70.8) |
0.379 |
| Age (years) | 58±11 | 56±11 | 56±11 | 58±11 | 58±11 | 58±11 | 60±12 | 59±10 | 59±11 | 59±11 | <0.001 |
| Male | 1,678 (73.1) |
92 (82.1) |
183 (74.7) |
214 (75.9) |
228 (74.5) |
189 (69.7) |
166 (71.9) |
174 (72.2) |
207 (72.9) |
225 (69.2) |
0.013 |
| CHA2DS2VASC score | 1.6±1.5 | 1.1±1.1 | 1.4±1.4 | 1.5±1.4 | 1.7±1.5 | 1.6±1.6 | 1.8±1.6 | 1.8±1.6 | 1.6±1.4 | 1.6±1.4 | <0.001 |
| Congestive heart failure | 226 (9.8) |
3 (2.7) |
5 (2.0) |
14 (5.0) |
27 (8.8) |
32 (11.8) |
37 (16.0) |
27 (11.2) |
43 (15.1) |
38 (11.7) |
<0.001 |
| Hypertension | 1,058 (46.1) |
47 (42) |
111 (45.3) |
136 (48.2) |
155 (50.7) |
124 (45.8) |
103 (44.6) |
116 (48.1) |
120 (42.3) |
146 (44.9) |
0.467 |
| Diabetes | 346 (15.1) |
14 (12.6) |
26 (10.6) |
47 (16.7) |
51 (16.7) |
34 (12.5) |
31 (13.4) |
45 (18.7) |
45 (15.8) |
53 (16.3) |
0.120 |
| Previous Stroke/TIA | 277 (12.1) |
5 (4.5) |
34 (13.9) |
27 (9.6) |
42 (13.7) |
32 (11.8) |
30 (13) |
43 (17.8) |
30 (10.6) |
34 (10.5) |
0.500 |
| Previous MI and peripheral vascular disease |
52 (2.3) |
0 (0) |
4 (1.6) |
8 (2.8) |
12 (3.9) |
6 (2.2) |
3 (1.3) |
8 (3.3) |
6 (2.1) |
5 (1.5) |
0.938 |
| LA diameter (mm) | 41±6 | 41±6 | 42±6 | 42±6 | 42±6 | 42±6 | 41±7 | 41±6 | 42±6 | 40±6 | 0.627 |
| LVEF (%) | 63±8 | 63±7 | 64±7 | 63±9 | 63±9 | 63±8 | 63±9 | 63±8 | 63±9 | 64±9 | 0.462 |
| E/Em | 10.3±4.6 | 9.8±3.2 | 10.4±4.6 | 10.4±5.1 | 10.5±5.3 | 10.7±4.7 | 10.2±4.0 | 10.2±4.0 | 10.3±5.3 | 9.8±3.6 | 0.737 |
| Extra-PV LA ablation | 843 (36.9) |
86 (76.8) |
170 (70.2) |
112 (40.4) |
92 (30.3) |
105 (38.7) |
78 (34.1) |
85 (35.6) |
52 (18.3) |
63 (19.4) |
<0.001 |
| Procedure time (min) | 183.5± 53.2 |
225.4± 63.7 |
189.0± 39.5 |
178.3± 37.3 |
193.5± 49.4 |
193.1± 49.0 |
188.6± 58.2 |
188.7± 63.0 |
172.4± 53.6 |
154.1± 48.3 |
<0.001 |
| Ablation time (min) | 80.2± 27.3 |
100.5± 32.6 |
85.9± 23.8 |
80.3± 24.5 |
87.9± 26.2 |
80.2± 24.1 |
81.4± 27.0 |
79.0± 32.0 |
75.6± 25.9 |
66.0± 23.1 |
<0.001 |
| 1-year recurrence† | |||||||||||
| Overall | 315 (16.3) |
24/110 (21.8) |
46/245 (18.8) |
53/279 (19.0) |
47/302 (15.6) |
30/263 (11.4) |
37/219 (16.9) |
39/237 (16.5) |
39/277 (14.1) |
NA | 0.040 |
| Off AAD | 147 (9.8) |
13/93 (14) |
27/210 (12.9) |
26/221 (11.8) |
14/225 (6.2) |
20/219 (9.1) |
13/155 (8.4) |
22/196 (11.2) |
12/185 (6.5) |
NA | 0.040 |
| Overall complication | 101 (4.4) |
9 (8) |
11 (4.5) |
12 (4.3) |
12 (3.9) |
13 (4.8) |
7 (3.0) |
8 (3.3) |
13 (4.6) |
16 (4.9) |
0.554 |
| Major complication | 58 (2.5) |
4 (3.6) |
5 (2.0) |
8 (2.8) |
6 (2) |
6 (2.2) |
2 (0.9) |
5 (2.1) |
9 (3.2) |
13 (4.0) |
0.342 |
Data given as n (%) or mean±SD. †1-year recurrence after AF ablations performed from 2009 to 2016. AAD, anti-arrhythmic drug; AF, atrial fibrillation; E/Em, early diastolic mitral inflow velocity/early diastolic mitral annular velocity; LA, left atrium; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NA, not applicable; PV, pulmonary vein; TIA, transient ischemic attack.
We successfully conducted CPVI in all patients (100%) and achieved bidirectional block of the cavotricuspid isthmus unless the patient had an atrioventricular nodal conduction problem. The extra-PV LA ablation rate had decreased remarkably (76.8% to 19.4%, P<0.001 for trend, Figure 1A). Accordingly, the procedure time (P<0.001 for trend) and ablation time (P<0.001 for trend) have also been decreasing (Figure 1A). The extra-PV LA lesion set and its bidirectional block rate are summarized in Supplementary Table 1. Despite the decreasing trend in the extra-PV LA ablation rate, the 1-year recurrence rate has significantly decreased (overall, 21.8% to 14.1%, P=0.040 for trend; off AAD 14.0% to 6.5%, P=0.040 for trend; Figure 1B).
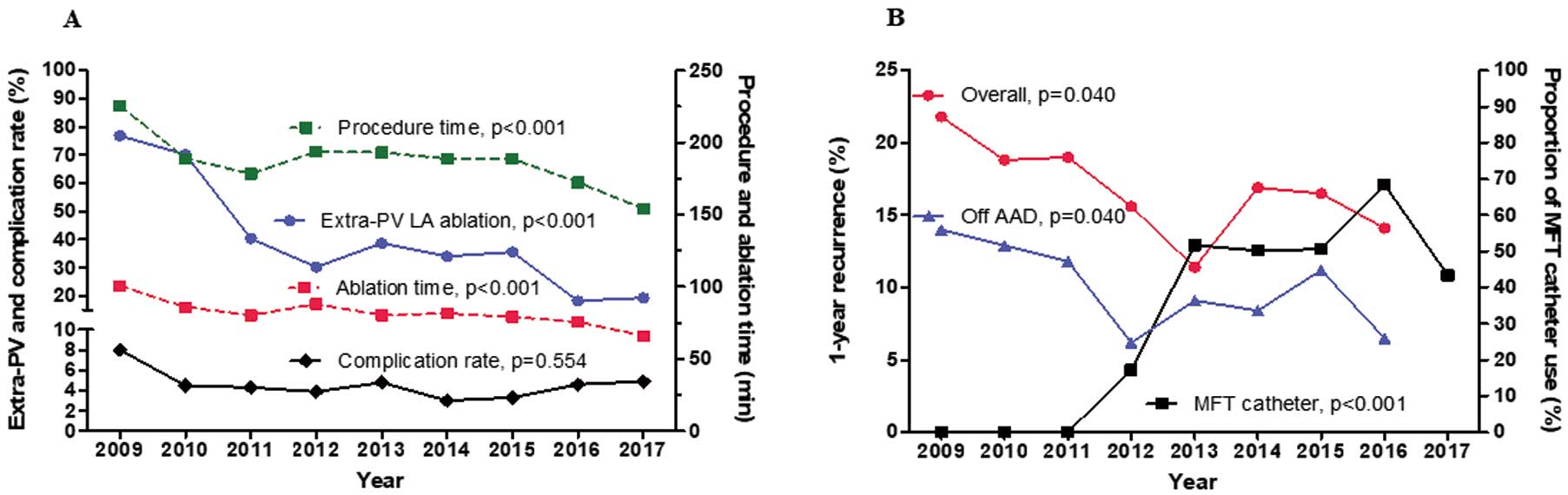
Trends in (A) ablation time, procedure time, extra-pulmonary vein (extra-PV) ablation, and complication rate over the past 9 years, and (B) 1-year recurrence rate after atrial fibrillation (AF) ablation and the use of a mesh-type flexible tip (MFT) catheter over the past 8 years. AAD, anti-arrhythmic drugs; LA, left atrial.
As shown in Figure 2A,D, the rhythm outcome was worse in patients with persistent AF than in those with paroxysmal AF (log rank P<0.001). For the 687 patients with persistent AF, we compared the periods before and after the STAR-AF2 publication (May 2015; Table 2). The extra-PV LA ablation rate (91.4% to 55.3%, P<0.001), procedure time (P<0.001), and ablation time (P<0.001) decreased significantly after the STAR-AF2 report, but the procedure-related complication rate did not significantly differ (P=0.427). The clinical recurrence rate of AF/AT before did not significantly differ from that after the STAR-AF2 report (log rank P=0.336; Figure 2B,E). Because the proportion of patients with congestive heart failure (P=0.029) and previous MI/peripheral artery disease (P=0.037) was higher in the post-STAR-AF2 period, we conducted propensity score matching for age, sex, LA diameter, and CHA2DS2VASC score and its components. Even after the propensity score matching, the overall AF/AT recurrence (log rank P=0.275; Figure 2C,F) and procedure-related complication rate (P=0.457) before did not significantly differ from that after the STAR-AF2 report in the patients with persistent AF.

Kaplan-Meier estimates of (A–C) freedom from atrial fibrillation/atrial tachyarrhythmia (AF/AT) recurrence and (D–F) freedom from AF/AT recurrence off anti-arrhythmic drugs (AAD) according to (A,D) AF type; (B,E) before vs. after the Substrate and Trigger Ablation for Reduction of Atrial Fibrillation Trial Part II (STAR-AF2) report; and (C,F) before vs. after the STAR-AF2 report after propensity score matching for age, sex, AF type, left atrial diameter, and CHA2DS2VASc score and its components.
| Overall population | Propensity-matched population | |||||
|---|---|---|---|---|---|---|
| Before STAR-AF2 (n=449) |
After STAR-AF2 (n=238) |
P-value | Before STAR-AF2 (n=206) |
After STAR-AF2 (n=206) |
P-value | |
| Patient characteristics | ||||||
| Age (years) | 58±11 | 59±10 | 0.501 | 59±10 | 58±10 | 0.823 |
| Male | 344 (76.6) | 183 (76.9) | 0.935 | 162 (78.6) | 158 (76.7) | 0.636 |
| CHA2DS2VASC score | 1.7±1.5 | 1.8±1.6 | 0.299 | 1.7±1.4 | 1.7±1.5 | 0.787 |
| Congestive heart failure | 65 (14.5) | 50 (21) | 0.029 | 28 (13.6) | 31 (15.0) | 0.673 |
| Hypertension | 221 (49.2) | 109 (45.8) | 0.393 | 96 (46.6) | 96 (46.6) | >0.999 |
| Diabetes | 81 (18.1) | 50 (21) | 0.353 | 40 (19.4) | 42 (20.4) | 0.805 |
| Previous stroke/TIA | 59 (13.1) | 36 (15.1) | 0.473 | 32 (15.5) | 30 (14.6) | 0.783 |
| Previous MI/PVD | 6 (1.3) | 9 (3.8) | 0.037 | 3 (1.5) | 3 (1.5) | >0.999 |
| LA diameter (mm) | 45±6 | 44±6 | 0.266 | 45±6 | 44±5 | 0.782 |
| LVEF (%) | 61±9 | 61±9 | 0.932 | 62±8 | 62±8 | 0.745 |
| E/Em | 10.7±4.3 | 10.5±4.2 | 0.562 | 10.7±4.3 | 10.2±3.7 | 0.229 |
| Procedure pattern | ||||||
| Procedure time (min) | 222.6±52.0 | 191.5±60.0 | <0.001 | 221.6±49.7 | 191.4±61.2 | <0.001 |
| Ablation time (min) | 102.6±26.5 | 87.2±30.4 | <0.001 | 101.9±26.0 | 87.1±30.9 | <0.001 |
| Extra-PV LA ablation | 406 (91.4) | 131 (55.3) | <0.001 | 182 (89.7) | 110 (53.7) | <0.001 |
| Use of MFT catheter | 120 (26.7) | 176 (73.9) | <0.001 | 47 (22.8) | 155 (75.2) | <0.001 |
| 1-year recurrence† | ||||||
| Overall | 98/443 (22.1) | 25/140 (17.9) | 0.281 | 49//204 (24.0) | 23/122 (18.9) | 0.276 |
| Off AAD | 42/314 (13.4) | 13/98 (13.3) | 0.978 | 21/147 (14.3) | 12/86 (14.0) | 0.944 |
| Procedure-related Complications | ||||||
| Overall complications | 23 (5.1) | 9 (3.8) | 0.427 | 10 (4.9) | 7 (3.4) | 0.457 |
| Major complications | 12 (2.7) | 6 (2.5) | 0.909 | 6 (2.9) | 4 (2.0) | 0.522 |
Data given as n (%) or mean±SD. †1-year recurrence after AF ablation performed between 2009 and 2016. MFT, mesh-type flexible tip; PVD, peripheral vascular disease; STAR-AF2, Substrate and Trigger Ablation for Reduction of Atrial Fibrillation Trial Part II. Other abbreviations as in Table 1.
Of the total study population, the patients with extra-PV LA ablation had significantly higher proportions of male sex, persistent AF and congestive heart failure, larger LA, lower LVEF, and higher E/Em than those with CPVI alone (Supplementary Table 2). In the persistent AF group, LA size was larger (P=0.012) and MFT catheter was less frequently utilized (P=0.002) in patients who underwent extra-PV LA ablation than those with CPVI alone (Supplementary Table 2).
Role of the MFT CatheterWe have been using an MFT catheter with a moderately high RF power (30–35 W) since 2012, and the prevalence of its use has increased from 17.3% in 2012 to 43.4% in 2017 (P<0.001 for trend). The 1-year recurrence rates show a decreasing trend over this period (P=0.040 for trend, Figure 1B). Contact force-sensing catheters were used in a small proportion of patients since 2015 (n=131, 5.7%) and were included in the other catheter group. Despite some differences in the baseline characteristics between the MFT catheter group and other catheter group (Table 3), the long-term AF/AT recurrence rate was significantly lower in the MFT catheter group than in the other catheter group (log rank P=0.002; Figure 3A,B). The procedure-related complication rate was higher with the MFT catheter (5.7% vs. 3.7%, P=0.029), but the major complication rate did not significantly differ compared with the other catheters (3.1% vs. 2.2%, P=0.198, Table 3).
| Overall population | Propensity-matched population | |||||
|---|---|---|---|---|---|---|
| MFT catheter (n=769) |
Other catheters (n=1,526) |
P-value | MFT catheter (n=679) |
Other catheters (n=679) |
P-value | |
| Patient characteristics | ||||||
| Age (years) | 60±11 | 57±11 | <0.001 | 59±11 | 59±11 | 0.753 |
| Male | 545 (70.9) | 1,132 (74.2) | 0.092 | 493 (72.6) | 483 (71.1) | 0.546 |
| Paroxysmal AF | 473 (61.5) | 1,135 (74.4) | <0.001 | 447 (65.8) | 456 (67.2) | 0.605 |
| CHA2DS2VASC score | 2.0±1.7 | 1.4±1.3 | <0.001 | 1.8±1.5 | 1.8±1.5 | 0.914 |
| Congestive heart failure | 141 (18.3) | 85 (5.6) | <0.001 | 70 (10.3) | 71 (10.5) | 0.929 |
| Hypertension | 358 (46.6) | 700 (45.9) | 0.757 | 316 (46.5) | 313 (46.1) | 0.870 |
| Diabetes | 136 (17.7) | 210 (13.8) | 0.013 | 111 (16.3) | 110 (16.2) | 0.941 |
| Previous Stroke/TIA | 133 (17.3) | 144 (9.4) | <0.001 | 102 (15.0) | 101 (14.9) | 0.939 |
| Previous MI/PVD | 24 (3.1) | 28 (1.8) | 0.051 | 17 (2.5) | 16 (2.4) | 0.860 |
| LA diameter (mm) | 42±6 | 41±6 | <0.001 | 42±6 | 42±6 | 0.968 |
| LVEF (%) | 62±9 | 63±8 | 0.007 | 63±8 | 63±9 | 0.243 |
| E/Em | 10.6±4.7 | 10.1±4.5 | 0.015 | 10.2±4.1 | 10.4±4.5 | 0.386 |
| Procedure pattern | ||||||
| Procedure time (min) | 199±48 | 175.6±53.9 | <0.001 | 197.7±46.9 | 178.6±56.0 | <0.001 |
| Ablation time (min) | 88.7±23.4 | 76±28.1 | <0.001 | 88.1±23.1 | 77.2±28.7 | <0.001 |
| Extra-PV LA ablation | 293 (38.1) | 550 (36.4) | 0.436 | 237 (34.9) | 296 (44.2) | <0.001 |
| 1-year recurrence† | ||||||
| Overall | 71/611 (11.6) | 243/1,319 (18.4) | <0.001 | 61/539 (11.3) | 107/581 (18.4) | 0.001 |
| Off AAD | 35/476 (7.4) | 112/1,027 (10.9) | 0.031 | 31/425 (7.3) | 45/443 (10.2) | 0.136 |
| Procedure-related complication | ||||||
| Overall complication | 44 (5.7) | 57 (3.7) | 0.029 | 38 (5.6) | 22 (3.2) | 0.036 |
| Major complication | 24 (3.1) | 34 (2.2) | 0.198 | 21 (3.1) | 15 (2.2) | 0.315 |
Data given as n (%) or mean±SD. †1-year recurrence after AF ablation performed between 2009 and 2016. Abbreviations as in Tables 1,2.

Kaplan-Meier estimates of (A,C) freedom from AF/AT recurrence in the overall patient group and (B,D) freedom from AF/AT recurrence off AAD after AF ablation according to catheter type and (C,D) after propensity score matching for age, sex, AF type, left atrial diameter, and CHA2DS2VASc score and its components. Abbreviations as in Figures 1,2.
After propensity score matching for age, sex, AF type, LA diameter, and CHA2DS2-VASc score and its components, the 1-year recurrence rate (P=0.001, Table 3) and long-term AF/AT recurrence (log rank P=0.002; Figure 3C,D) remained lower in the MFT catheter group than in the other catheter group. Although the major complication rate did not differ (P=0.315), the overall complication rate was higher in the MFT catheter group than in the other catheter group (P=0.036, Table 3).
In patients who underwent CPVI alone, patients with MFT catheter had better rhythm outcome than those with other catheters on Kaplan-Meier analysis (log rank P=0.001, Supplementary Figure A,B). Considering propensity score matching, including extra-PV LA ablation and other factors as noted here, the 1-year recurrence rate (P=0.003; Supplementary Table 3) and long-term AF/AT recurrence (log rank P=0.012; Supplementary Figure C,D) remained lower in the MFT catheter group than in the other catheter group. Of the 195 patients who underwent repeat procedure, no PV potential was found at redo mapping in 21/49 (42.9%) after MFT catheter ablation and in 41/146 (28.1%) after utilizing other ablation catheters (P=0.055).
Factors Affecting Efficacy and Safety of AF Catheter AblationTo determine the factors affecting the clinical recurrence of AF, we conducted a multivariate Cox regression analysis. Age (hazard ratio [HR], 0.992; 95% CI: 0.985–0.999; P=0.028), paroxysmal AF at procedure (HR, 0.767; 95% CI: 0.626–0.941; P=0.011), LA diameter (HR, 1.016; 95% CI: 1.002–1.030; P=0.021), ablation time (HR, 1.004; 95% CI: 1.001–1.008; P=0.007), use of an MFT catheter (HR, 0.670; 95% CI: 0.559–0.803; P<0.001), and post-ablation AAD use (HR, 4.092; 95% CI: 3.498–4.787; P<0.001) were independently associated with clinical recurrence after AF ablation (Table 4). In terms of the procedure-related complications, female sex (odds ratio [OR], 2.050; 95% CI: 1.270–3.309; P=0.003) and diastolic dysfunction measured on E/Em (OR, 1.040; 95% CI: 1.000–1.080; P=0.049) were independently associated with complications on multivariate logistic regression analysis (Table 5).
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Age | 0.997 (0.990–1.004) | 0.351 | 0.992 (0.985–0.999) | 0.028 |
| Male | 0.927 (0.787–1.091) | 0.362 | 0.894 (0.754–1.060) | 0.198 |
| Paroxysmal AF at procedure | 0.549 (0.472–0.637) | <0.001 | 0.767 (0.626–0.941) | 0.011 |
| CHA2DS2VASc score | 1.005 (0.957–1.055) | 0.833 | ||
| Congestive heart failure | 1.132 (0.884–1.450) | 0.324 | ||
| Hypertension | 1.034 (0.892–1.199) | 0.654 | ||
| Diabetes | 0.947 (0.768–1.168) | 0.612 | ||
| Previous Stroke/TIA | 1.065 (0.855–1.326) | 0.574 | ||
| Previous MI/PVD | 0.892 (0.525–1.514) | 0.671 | ||
| LA diameter | 1.040 (1.027–1.052) | <0.001 | 1.016 (1.002–1.030) | 0.021 |
| LVEF | 0.993 (0.984–1.001) | 0.085 | ||
| E/Em | 0.998 (0.982–1.015) | 0.858 | ||
| Extra-PV LA ablation | 1.606 (1.385–1.862) | <0.001 | 1.066 (0.873–1.302) | 0.530 |
| Ablation time | 1.007 (1.004–1.009) | <0.001 | 1.004 (1.001–1.008) | 0.007 |
| Use of MFT catheter | 0.757 (0.636–0.902) | 0.002 | 0.670 (0.559–0.803) | <0.001 |
| Post-ablation AAD use | 4.265 (3.666–4.961) | <0.001 | 4.092 (3.498–4.787) | <0.001 |
†Cox regression analysis. AT, atrial tachyarrhythmia. Other abbreviations as in Tables 1,2.
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Age | 1.029 (1.010–1.049) | 0.003 | 1.022 (0.996–1.049) | 0.095 |
| Female | 1.997 (1.329–3.000) | 0.001 | 2.050 (1.270–3.309) | 0.003 |
| Paroxysmal AF during the procedure | 0.913 (0.594–1.402) | 0.677 | ||
| CHA2DS2VASc score | 1.175 (1.040–1.328) | 0.009 | 0.855 (0.685–1.067) | 0.166 |
| Congestive heart failure | 1.259 (0.678–2.339) | 0.466 | ||
| Hypertension | 1.544 (1.033–2.309) | 0.034 | 1.631 (0.987–2.695) | 0.056 |
| Diabetes | 0.980 (0.559–1.717) | 0.944 | ||
| Previous Stroke/TIA | 1.079 (0.594–1.959) | 0.803 | ||
| Previous MI/PVD | 1.339 (0.410–4.371) | 0.629 | ||
| LA diameter | 0.984 (0.953–1.017) | 0.336 | ||
| LVEF | 1.002 (0.994–1.010) | 0.701 | ||
| E/Em | 1.053 (1.020–1.086) | 0.001 | 1.040 (1.000–1.080) | 0.049 |
| Extra-PV LA ablation | 0.960 (0.632–1.458) | 0.848 | ||
| Ablation time | 1.001 (0.994–1.008) | 0.816 | ||
| Use of MFT catheter | 1.563 (1.044–2.339) | 0.030 | 1.434 (0.937–2.194) | 0.097 |
†Logistic regression analysis. Abbreviations as in Tables 1,2,4.
During the 9-year study period for AF catheter ablation at the present institution, empirical extra-PV LA ablation decreased and annual 1-year recurrence rate improved. In the persistent AF group, reduction in extra-PV LA ablation did not affect the rhythm outcome of AF ablation. The use of an MFT catheter with moderately increased RF power was independently associated with a better rhythm outcome, and female sex was independently associated with higher complication rate after AF ablation.
STAR-AF2 and Extra-PV LA AblationCPVI is recognized as a cornerstone of AF ablation. In addition to blocking the PV trigger, which is the main mechanism of AF,2 wide CPVI also reduces the atrial critical mass, which is one of the AF maintenance mechanisms,4 and contributes to autonomic neural modulation by ablating the ganglionate plexi located on the epicardial side of the PV antrum.5 Nonetheless, the recurrence rate after AF ablation is still high after CPVI, especially in the case of persistent AF with significant atria structural remodeling.1 For this reason, many operators have performed empirical extra-PV foci ablation such as linear ablation,6 CFAE-guided ablation,7 rotor ablation,8 and/or extra-PV trigger ablation in addition to the CPVI.9 In a significant proportion of cases, however, AF recurrence is caused not only by extra-PV foci, but also by electrical reconnections of the CPVI.10 In 2015, the STAR-AF2 investigators reported that extra-PV LA ablation with an additional linear ablation or CFAE ablation had no additional clinical benefit as compared to that with PVI alone in the patients with persistent AF.3 Nonetheless, a recurrence rate of 50% in 1 year did not seem to be a satisfactory outcome.
In this single-center cohort registry study, use of extra-PV ablation was significantly lower in both paroxysmal and persistent AF, but the 1-year recurrence rate somewhat improved after the publication of the STAR-AF2 report. This might be because the time required for extra-PV foci mapping and ablation was concentrated on the CPVI, and there were no PV potential reconnections at all in approximately 37% of patients who underwent repeat ablation procedures in that cohort.10 Therefore, it is necessary to study a more selective mapping and ablation of extra-PV triggers, rather than an empirical extra-PV LA ablation.11
Learning in the Past 9 YearsOver the past 9 years we learned that AF is a chronic progressive disease and not curable by catheter ablation. Although the development of efficient ablation catheters and sophisticated mapping systems has improved the outcome of AF ablation, the long-term recurrence rate is still close to 50%. Nonetheless, we were able to evaluate the present results because we performed consistent and steady rhythm monitoring based on the 2012 HRS/EHRA/ECAS Expert consensus statement guidelines.
Of the prognostic factors associated with the patient characteristics, young age and female sex,12 high pericardial fat volume,13 being overweight,14 longer PR interval,15 and high LA pressure16 were factors associated with a higher clinical recurrence rate after AF ablation. In particular, the outcome of catheter ablation in patients with long-standing persistent AF was affected by the pre-ablation external cardioversion energy17 or specific genetic factors, such as the ZFHX3 genetic trait.18
Regarding the intra-procedural factors, CPVI alone proved sufficient in patients with paroxysmal AF,19 and appropriate parasympathetic modulation after AF ablation measured using heart rate variability (HRV)5 and absence of post-ablation extra-PV triggers9 were related to a good rhythm outcome after AF ablation. In the persistent AF group, recurrence was lower in those with posterior wall isolation in de novo ablation.20 For the patients who improved from persistent AF to paroxysmal AF, however, after using AAD, additional linear ablation after CPVI did not affect outcome.21 For the patients with longstanding persistent AF, additional CFAE ablation after CPVI plus linear ablation did not improve the rhythm outcome.22 In this study, the rate of procedure-related complications was higher in the female patients than in male patients.
An MFT catheter, which is designed to maintain high irrigation flow at the catheter-tissue contact surface, seems to be more efficient in generating a large transmural lesion.23,24 Moreover, we used moderately increased RF power (35 W on the anterior side of the PV antrum) with MFT catheter. That is why the MFT catheter was more effective for rhythm control than other catheters.
Future DirectionsStudies on the sex-specific energy titration and outcomes of cryoballoon ablation, which requires a shorter procedure time and has a larger lesion size than RF ablation, are warranted. It is clear that earlier AF intervention guarantees a better outcome. A careful post-cardioversion extra-PV trigger mapping-based ablation, rather than empirical extra-PV ablation, might be a reasonable approach. The development of more efficient and safer energy sources and of patient selection based on precision medicine are also needed.
Study LimitationsThis study was an observational cohort study from a single center that included highly selected patients referred for AF ablation. For this reason, despite propensity score matching, this study might have a selection bias for the patient baseline characteristics. Given the long-term study period of 9 years, some patients were lost to follow-up. Although we maintained a consistent rhythm follow-up protocol, 10.8% (247/2,297) of included patients either died or were missed over the 9 years of follow-up. Because we defined extra-PV LA ablation as additional linear ablation of the LA or a CFAE ablation, this study could not show the effect of other ablation strategies such as right atrial ablation. Although CPVI is appropriate for paroxysmal AF ablation, we conducted additional linear ablation in some patients with paroxysmal AF in the early 2010 s to determine the appropriate lesion set for paroxysmal AF by several clinical trials.5,19,25
During the past 9 years of performing AF catheter ablation in the Yonsei AF ablation cohort, the use of empirical extra-PV LA ablation has decreased and the annual 1-year recurrence rate has improved. Use of an MFT catheter with a moderately increased RF power was independently associated with better rhythm outcome, and female sex was independently associated with higher complication rate after AF ablation. It is necessary to investigate more selective mapping and ablation of extra-PV triggers, rather than empirical extra-PV LA ablation.
This work was supported by a grant (HI18C0070) from the Korea Health 21 R&D Project, Ministry of Health and Welfare, and a grant (NRF-2017R1A2B4003983) from the Basic Science Research Program run by the National Research Foundation of Korea (NRF), which is funded by the Ministry of Science, ICT, and Future Planning (MSIP). We thank Mr. John Martin for his linguistic assistance.
The authors declare no conflicts of interest.
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-18-0928