2019 Volume 83 Issue 5 Pages 991-999
2019 Volume 83 Issue 5 Pages 991-999
Background: Practice-based investigations on direct oral anticoagulant (DOAC) treatment for non-valvular atrial fibrillation (NVAF) have shown that off-label under-dosing is increasingly becoming an issue. Here, we investigate the significance of drug monitoring to prevent undesirable under-dosing with DOAC.
Methods and Results: In 255 outpatients with NVAF undergoing treatment with rivaroxaban or apixaban we estimated the cut-offs for bleeding events using drug plasma concentration (PC) data 3 h after drug treatment, that is, at the peak level. Furthermore, we evaluated the appropriateness of labeled and off-label dosing implemented for 348 patients using the obtainable PC threshold. A total of 73 off-label under-dose users of rivaroxaban (37% of all users and 63% of lower dose users) had acceptable peak PC (155–400 ng/mL). Additionally, 46 off-label under-dose users of apixaban (31% of all users and 55% of lower dose users) received appropriate doses according to peak PC threshold (90−386.4 ng/mL). These off-label under-dose users reported no bleeding or thromboembolic events during follow-up.
Conclusions: Anticoagulation monitoring enables personalized and appropriate off-label under-dosing in NVAF patients on rivaroxaban or apixaban through the measurement of peak PC during DOAC use.
Direct oral anticoagulants (DOAC) provide a similar degree of anti-thrombotic effect with less bleeding compared with vitamin K antagonists, and are a good treatment option for patients with non-valvular atrial fibrillation (NVAF) with a favorable risk-benefit profile.1 Bleeding episodes necessitating discontinuation of drug usage, however, have been reported in NVAF patients taking dabigatran and rivaroxaban.2,3 In contrast, apixaban reportedly has a lower bleeding risk and higher higher treatment compliance rates.4
Recent practice-based investigations regarding DOAC treatment have identified instances in which off-label DOAC have been associated with an increased risk of adverse events.5,6 A rivaroxaban post-marketing surveillance report on Japanese patients with NVAF showed that ≥50% of patients prescribed a lower dose had creatinine clearance (CrCl) ≥50 mL/min, and were judged as “off-label” lower dose users.7 Another study on the efficacy and safety of rivaroxaban for Japanese patients with NVAF also found that 30% of patients prescribed a lower dose (10 mg use) were off-label users, but the study found no differences in the rates of symptomatic stroke and systemic embolism events between these off-label under-dose users and standard-dose (15 mg rivaroxaban official dose) users (0.9%/year vs. 0.8%/year).8 Thus, it may be hasty to conclude that all off-label under-dose use is undesirable. The 2018 European Heart Rhythm Association practical guide on DOAC use for patients with NVAF stated that anti-factor Xa (FXa) chromogenic assays are available to measure the plasma concentration (PC) of FXa inhibitors using validated calibrators.9 Currently, reports concerning the assessment of the anticoagulant effects of DOAC are insufficient.10–12 We designed this study with 2 goals in mind: first, to confirm the attainable PC according to the doses given in patients with NVAF who received 2 FXa inhibitors, rivaroxaban or apixaban, and examine the threshold PC for the prediction of bleeding events; and second, to prospectively evaluate the appropriateness of labeled and off-labeled under-dosing for the initial and additional patients using the obtainable PC threshold.
The initial observational study was conducted at the Hokusetsu General Hospital from January 2014 to June 2016 on 255 outpatients (168 men and 87 women; age range, 45–90 years) with NVAF, who were undergoing treatment with the F-Xa inhibitors, rivaroxaban or apixaban. Of the 255 patients, 136 received rivaroxaban and 119 received apixaban. The second prospective study for dose adjustment was performed from July 2016 to March 2018 for 348 patients (242 patiens from the initial 255 and an additional 106 patients; 219 men and 129 women, ranging in age from 40–89 years) with NVAF using these 2 DOAC. Of the 348 patients, 200 received rivaroxaban and 148 received apixaban.
To monitor the anticoagulant activity of the DOAC, PC was first measured at a steady state 1 month after the initiation of treatment. PC was re-measured within a few months for patients with a higher or lower first measurement compared with the previous dose selection data13,14 and DOAC PC study.15 We also measured PC intermittently during 6–24 months of continuous DOAC treatment to determine the reproducibility of the obtained values, and recorded the higher values if differences were observed. Blood samples for PC measurement were primarily collected 3 h after drug treatment (at peak) and, if possible, immediately beforehand (trough).
The CrCl was calculated using the Cockcroft-Gault formula adjusted for age, body weight (BW), serum creatinine, and gender. We calculated CHADS2 score immediately before DOAC treatment initiation based on the presence of heart failure, hypertension, age ≥75 years, and a history of stroke.
Although the Japanese standard dose for rivaroxaban is 15 mg once daily (o.d.; labeled 15 mg), the recommended lower dose of 10 mg o.d. (labeled 10 mg) is used for patients with CrCl <50 mL/min, based on pharmacokinetic data.16 Additionally, 5 mg twice daily (b.i.d.) is used as the standard apixaban dose (labeled 5 mg b.i.d.), but 2.5 mg b.i.d. is the recommended lower dose (labeled 2.5 mg b.i.d.) for patients meeting ≥2 of the following dose reduction criteria: serum creatinine ≥1.5 mg/dL, BW ≤60 kg, and age ≥80 years. We selected the DOAC doses for most patients based on the aforementioned dose recommendations and reduction criteria. Given, however, that the DOAC dose at the time of treatment initiation was delegated to the first prescribers, some patients began treatment with off-label lower doses (i.e., they were under-dosed) due to bleeding concerns.
All patients signed written informed consent forms, and the Hokusetsu General Hospital ethics review committee approved this study.
Drug PCWe measured PC under a steady state ≥4 weeks after the initiation of rivaroxaban or apixaban using the anti-Xa-derived indirect method with the Biophen® DiXaI kit (Hyphen Biomed, Neuville Sur Oise, France). This kit utilizes a chromogenic method based on the inhibition of a constant and excess quantity of factor Xa by the drug being assayed, with a calibrator and control for each drug.17,18
To examine the reproducibility of the DiXaI kit, we evaluated intra-individual variations in the correlation between the first and the second PC measurements in ≤6 months in 60 patients.
Bleeding and Thromboembolic EventsAccording to the J-ROCKET AF Study, major bleeding was defined as clinically overt bleeding associated with a fall ≥2.0 g/dL in the hemoglobin level, need for a transfusion of ≥2 units packed red cells or whole blood, involvement of a critical site, or a fatal outcome.19 Non-major clinically relevant bleeding was defined as clinically overt bleeding that did not meet the criteria for major bleeding, but that required medical intervention, unscheduled consultation with a physician, or temporary discontinuation of study treatment, pain, or impairment of daily activities.19 Thromboembolic events were also defined according to the J-ROCKET AF Study definitions.19 In the present study, all patients were followed up at the present hospital or by the general physician. For patients who had bleeding or neurologic symptoms, blood samples were collected and the next course of treatment, such as drug interruption or further therapy, was decided. We also recorded the outcome of follow-up by reviewing outpatient charts or by phone call to the general physician and/or the patients. All data collection was stopped on 31 March 2018.
Dose AdjustmentFor patients with peak PC above the threshold for bleeding and/or bleeding events, off-labeled dosing was initiated to avoid further bleeding events after obtaining consent for dose adjustment from the Hokusetsu General Hospital ethics review committee and from the patients.
Statistical AnalysisStatistical analysis was performed based on a 5% level of significance. We used the Wilcoxon 2-sample test to compare the continuous variables, and a simple linear regression and correlation test, Stat Mate version 5 (ATMS, Tokyo, Japan), to analyze the correlation coefficients. In addition, we performed logistic regression analyses using SAS version 9.3 (SAS Institute, USA) to determine the risk factors related to bleeding events. Data are expressed as mean±SD.
Table 1 lists the baseline data for the 136 (rivaroxaban) and 119 (apixaban) patients. Apixaban users were older than the rivaroxaban users. The lower-dose users for both DOAC were older, but had lower BW and CrCl than the standard-dose users. In addition, CHADS2 score was higher for the lower-dose users than for the standard-dose users of apixaban. Single or dual antiplatelet drugs were frequently used together with a lower apixaban dose.
| Rivaroxaban (n=136) | Apixaban (n=119) | |||||
|---|---|---|---|---|---|---|
| 15 mg o.d. (n=90) |
10 mg o.d. (n=46)† |
P-value 15 mg vs. 10 mg |
5 mg b.i.d. (n=58) |
2.5 mg b.i.d. (n=61)‡ |
P-value 5 mg vs. 2.5 mg |
|
| Age (years) | 64±9 | 74±7 | <0.001 | 73±6 | 80±6 | <0.001 |
| M/F | 65/25 | 27/19 | 38/20 | 45/16 | ||
| BW (kg) | 69±15 | 58±10 | <0.01 | 63±12 | 57±10 | <0.01 |
| CrCl (mL/min) | 85±29 | 51±10 | <0.001 | 65±20 | 46±12 | <0.001 |
| CHADS2 score | 1.5±0.7 | 1.7±0.8 | NS | 1.7±1.0 | 2.1±0.9 | <0.05 |
| 0–1 | 53 | 23 | 30 | 11 | ||
| 2 | 32 | 17 | 20 | 36 | ||
| ≥3 | 5 | 6 | 8 | 14 | ||
| HT (+/−) | 56/34 | 33/13 | 38/20 | 45/16 | ||
| APT use (%) | 10 | 17.4 | 12.1 | 31.1 | ||
| Single/dual | 9/0 | 8/0 | 7/0 | 16/3 | ||
| Observation term (months) | 14.0±6.7 | 13.9±6.7 | 16.6±7.3 | 14.5±4.8 | ||
Data given as mean±SD. †In 46, 27 had CrCl 50–66 mL/min on rivaroxaban 10-mg o.d. use. ‡In 61, 35 had no or only one DR criterion in apixaban 2.5-mg b.i.d. use. APT, antiplatelet therapy (single, 1 of aspirin, clopidogrel or cilostazol; dual, aspirin plus clopidogrel or cilostazol); b.i.d., twice daily; BW, body weight; CrCl, creatinine clearance; DR, dose reduction; HT, hypertension; o.d., once daily.
Of the 46 patients prescribed a lower rivaroxaban dose, 27 (59%) had CrCl 50–70 mL/min and thus, were off-label dose users. Of the 61 patients prescribed lower apixaban doses, 35 (57%) were off-label dose users.
DOAC Dose and PCPeak PC for rivaroxaban (Figure 1) was higher for the standard-dose users (Japanese dose, 15 mg o.d.; n=90; 5th–95th percentile peak PC, 219–544 ng/mL) than for the lower-dose users (Japanese dose, 10 mg o.d.; n=46; 5th–95th percentile peak PC, 155–440 ng/mL). The trough PC, however, did not differ between the 2 rivaroxaban groups.

Trough and peak plasma concentration (PC) in standard dose users and lower dose users of (Left) rivaroxaban and (Right) apixaban. Bottom of box, 25th percentile; horizontal line, 50th percentile (median, indicated by number); top of box, 75th percentile; whiskers, maximum and minimum non-outliers, respectively. bid, twice daily; od, once daily.
Peak PC for apixaban (Figure 1) was higher for the standard dose users (5 mg b.i.d.; n=58; 5th–95th percentile peak PC, 125–436 ng/mL) than for the lower-dose users (2.5 mg b.i.d.; n=61; 5th–95th percentile peak PC, 90–322 ng/mL), but the trough PC did not differ between the 2 apixaban groups.
On examining the reproducibility of the DiXaI kit, we found good correlations between the 2 measurements (for rivaroxaban, r=0.70, P<0.001; and for apixaban, r=0.74 m P<0.001).
Predictive Indexes Related to Bleeding EventsMost patients with bleeding events had blood sampling at the peak time on routine visits, but a few with major bleeding may have had sampling delays of approximately 1 h.
Of the 136 patients who received rivaroxaban, major bleeding events were observed in 9 and non-major clinically relevant bleeding events in 8 (Supplementary Table 1). On analysis, excluding trough PC, only peak PC was independently associated with the occurrence of bleeding events in rivaroxaban users (n=136; P<0.001, Supplementary Table 2). Of note, co-medication with antiplatelet drugs was not related to bleeding events. We obtained similar results from evaluating only the 100 patients with available trough PC. We also performed receiver operating characteristic (ROC) analysis of peak PC to predict major and non-major bleeding events with each rivaroxaban dose using the data for the 17 patients with bleeding events. We calculated a 400.0-ng/mL cut-off peak PC (Figure 2) and a 0.9085 area under the curve (AUC) to predict bleeding in all rivaroxaban users, with 100% sensitivity (95% CI: 0.79–1.0), 75% specificity (95% CI: 0.65–0.84), 41% positive predictive value (PPV), and 100% negative predictive value (NPV).
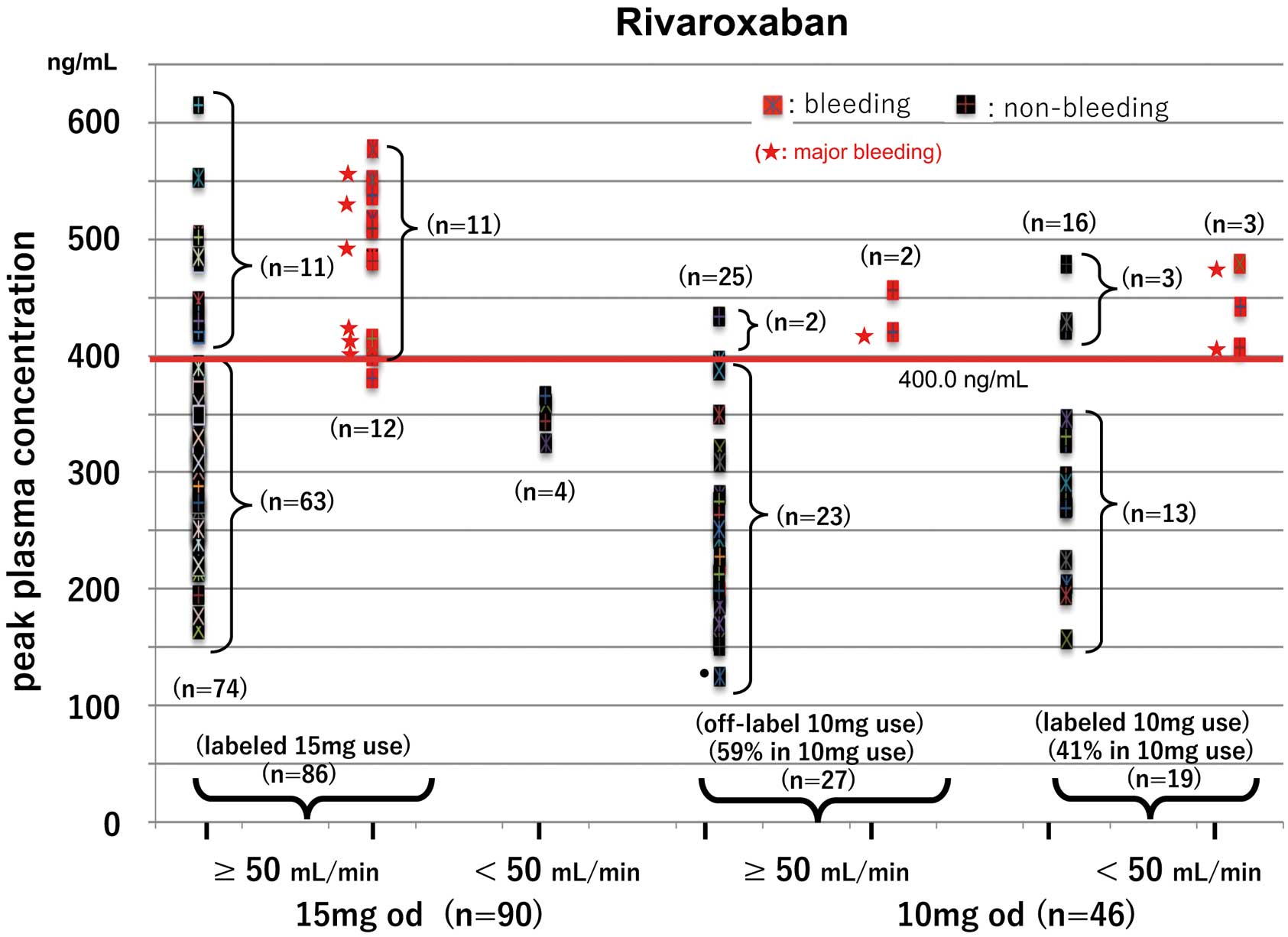
Peak plasma concentration (PC) according to creatinine clearance (CrCl; 50 mL/min being the dividing level for dose reduction) in (Left) 15-mg once daily (od) and (Right) 10-mg od rivaroxaban users. Of the 86 patients who received labeled 15-mg doses, 12 developed bleeding events but 11 had no bleeding events despite peak PC ≥400.0 ng/mL (cut-off). Of the 27 off-label 10-mg users, 2 developed bleeding events despite off-label use and 2 had PC ≥400.0 ng/mL despite no bleeding events. Of the 19 labeled 10-mg users, 3 with PC above the cut-off had bleeding events.
Of the 119 patients treated with apixaban, 5 of those who received 5 mg b.i.d. and 1 (PC, 470 ng/mL) of those who received 2.5 mg b.i.d. developed bleeding events (Supplementary Table 1). On logistic regression analysis there were no independent predictors of bleeding events in apixaban users (Supplementary Table 2). On ROC analysis of the peak PC to predict bleeding events in all apixaban users, however, the cut-off peak PC was 386.4 ng/mL (Figure 3), and AUC was 0.9846, with 100% sensitivity (95% CI: 0.57–1.0), 98% specificity (95% CI: 0.899–0.997), 83% PPV, and 100% NPV for these users.

Peak plasma concentration (PC) data in 5- and 2.5-mg twice daily (bid) users of apixaban according to the number of dose reduction (DR) criteria satisfied (0, 1 or ≥2). Of the 58 patients receiving labeled 5-mg bid apixaban, 5 with bleeding events and 1 without bleeding events had peak PC ≥386.4 ng/mL (the cut-off). Of the 61 patients receiving 2.5-mg bid apixaban, 35 were off-label users but of these 29 had acceptable peak PC. Of the 26 patients who received labeled 2.5-mg bid apixaban, 25 had potentially acceptable PC without bleeding events.
From these data, we defined the acceptable PC as that higher than the 5th percentile peak PC and lower than the peak PC cut-off for bleeding for each drug dose. Most of the patients were followed up at the present hospital or by the general physician for continued treatment, but DOAC was discontinued for 3 patients because NVAF disappeared in 2 and contact was lost with 1.
Peak PC According to Dose Reduction CriteriaFigure 2 shows the distribution of peak PC in the rivaroxaban users, classified into 2 groups according to CrCl, with 50 mL/min being the threshold for dose reduction. A total of 86 patients taking the 15-mg dose had CrCl ≥50 mL/min (termed the labeled 15-mg users). Of the 12 labeled 15-mg users who had bleeding events, 11 had peak PC above the cut-off (i.e., at 400.0 ng/mL), while 11 had PC above the cut-off despite no bleeding events. The remaining 63 had acceptable peak PC (155–400 ng/mL; 155 ng/mL being the 5th percentile peak PC in the lower dose use of rivaroxaban) and no thromboembolic events occurred in these patients. Also, of the 27 off-label 10-mg users of rivaroxaban, 2 had bleeding events despite off-label dosing, and 2 had peak PC above the cut-off (i.e., at 400.0 ng/mL) despite no bleeding events. Of the remaining 23 patients, 21 had acceptable PC, but 2 had peak PC <155 ng/mL (upon re-evaluation, 1 had acceptable PC again and 1 discontinued the medication). Of the 19 labeled 10-mg rivaroxaban users, 13 had acceptable PC and 3 had PC above the cut-off despite no bleeding events. The remaining 3 patients, however, had bleeding events despite taking the labeled 10-mg dose.
Of the 58 patients who received the labeled 5-mg b.i.d. dose of apixaban, 5 had bleeding events and had peak PC ≥386.4 ng/mL (Figure 3). Of the 53 patients who did not have bleeding events with the labeled 5-mg b.i.d. apixaban, 52 (98%) had acceptable peak PC (90–386.4 ng/mL; 90 ng/mL, the 5th percentile peak PC in the lower dose use of apixaban) although only one (2%) had peak PC above the cut-off. In contrast, of the 61 patients receiving 2.5 mg b.i.d. apixaban, 35 were off-label 2.5-mg b.i.d. users (9 did not meet the dose reduction criteria, while 26 satisfied only one). Of the 26 patients who received labeled 2.5 mg b.i.d., 25 had acceptable PC without any bleeding events but the remaining patient was switched to warfarin.
Off-Label Under-Dosing and Bleeding Events and/or High PCOf the rivaroxaban or apixaban users who developed bleeding events with the standard doses, the dosing was reduced to each lower dose. Users of rivaroxaban 10 mg or apixaban 2.5 mg b.i.d. who developed bleeding events were switched to apixaban or warfarin, respectively. These dose reductions were performed during the initial observational study to avoid further bleeding events after obtaining the consent for off-label use.
The second prospective study for dose adjustment was performed on 348 patients (242 patients from the initial 255 and an additional 106 patients) using the 2 DOAC. Of the rivaroxaban or apixaban users who did not experience bleeding events, despite peak PC above each cut-off for bleeding events (rivaroxaban, 400 ng/mL; apixaban, 386.4 ng/mL), labeled rivaroxaban 15 mg or labeled apixaban 5 mg b.i.d. was switched to the respective off-label lower dose, and labeled rivaroxaban 10 mg was switched to apixaban 2.5 mg b.i.d. These dose adjustments were performed to avoid bleeding after obtaining the same consent. Figure 4 shows the change in peak PC according to dose reduction from each standard dose to each off-label lower dose in patients with peak PC greater than each cut-off and/or bleeding events who were receiving rivaroxaban or apixaban (rivaroxaban, n=23 in the initial study and n=4 in the second study; apixaban, 6 and 1, respectively). In contrast, because 6 patients using off-label apixaban 2.5 mg b.i.d. were under-dosed, as confirmed with PC data (data not shown), their doses were increased to 5 mg b.i.d.
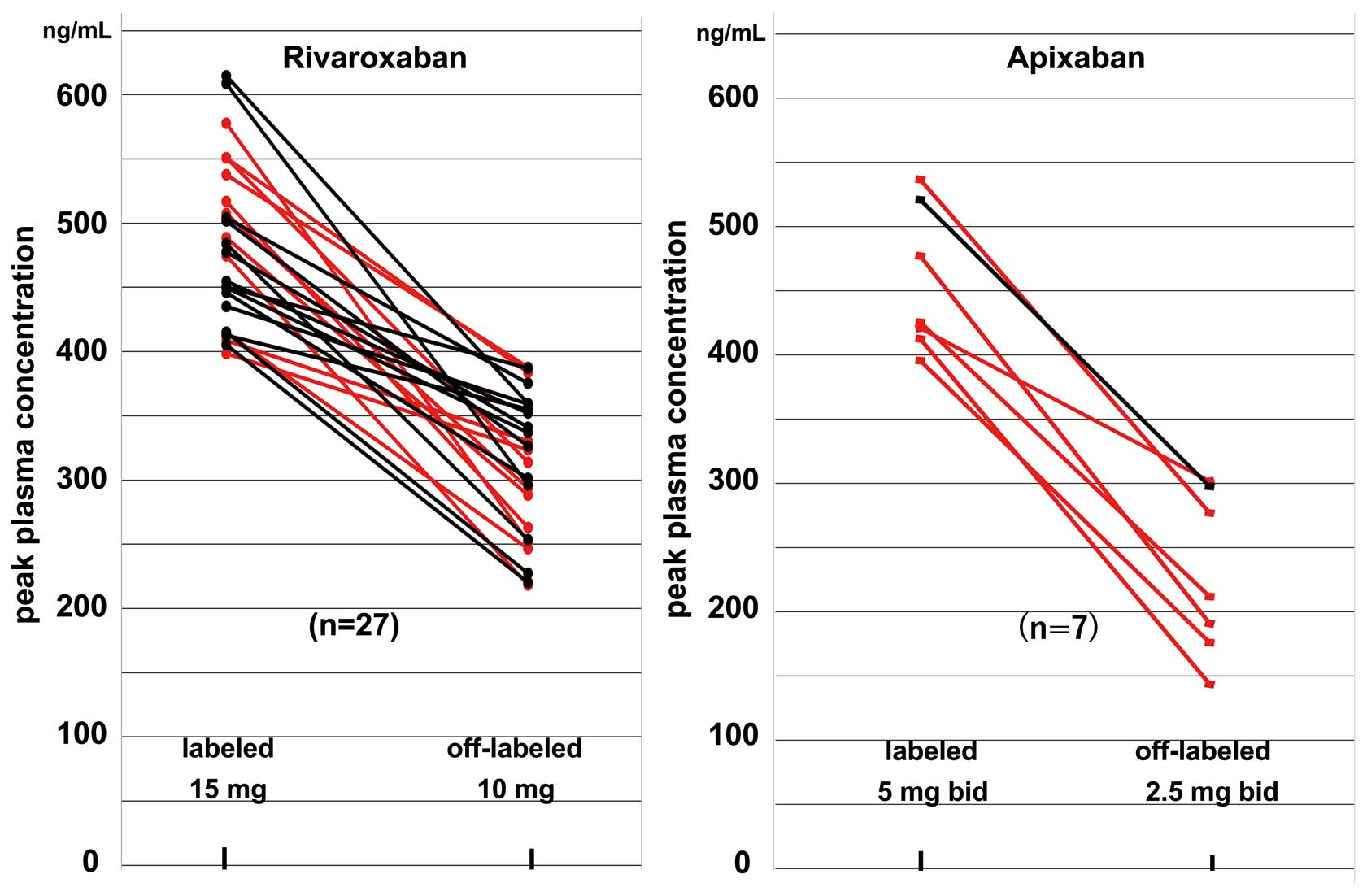
Change in peak plasma concentration (PC) after dose reduction from labeled standard dose to the off-label lower dose in patients with peak PC greater than the cut-off (black) and/or bleeding events (red) under rivaroxaban or apixaban use. Dose reduction was performed in the 27 patients receiving rivaroxaban (23 in the initial study and 4 in the second 1) and in 7 receiving apixaban (6 and 1, respectively).
Figure 5 shows the distribution of the peak PC for rivaroxaban users (n=200) after the aforementioned dose adjustment. All rivaroxaban users had peak PC <400 ng/mL with labeled 15-mg, off-label 10-mg, and labeled 10-mg use. Peak PC was above the 5th percentile in the lower dose users (155 ng/mL). Of these labeled and off-label patients, none had bleeding or thromboembolic events during the 6–21 months (mean±SD, 17±6 months) of follow-up. Of the 163 rivaroxaban users with the indication for the labeled 15-mg dose, 73 (45%) had acceptable PC despite use of off-label 10 mg. In other words, 73 off-label users of rivaroxaban had appropriate dosing based on the assessment of PC, comprising 63% (73/110) of the lower dose users and 37% (73/200) of all rivaroxaban users. Age increased while BW and Cr-CL decreased according to the dose reduction. There were significant differences between these 3 dose groups, especially between the labeled 15-mg and off-label 10-mg users (Table 2), but there were no differences in CHADS2 score, hypertension, antiplatelet drug use, or trough PC.
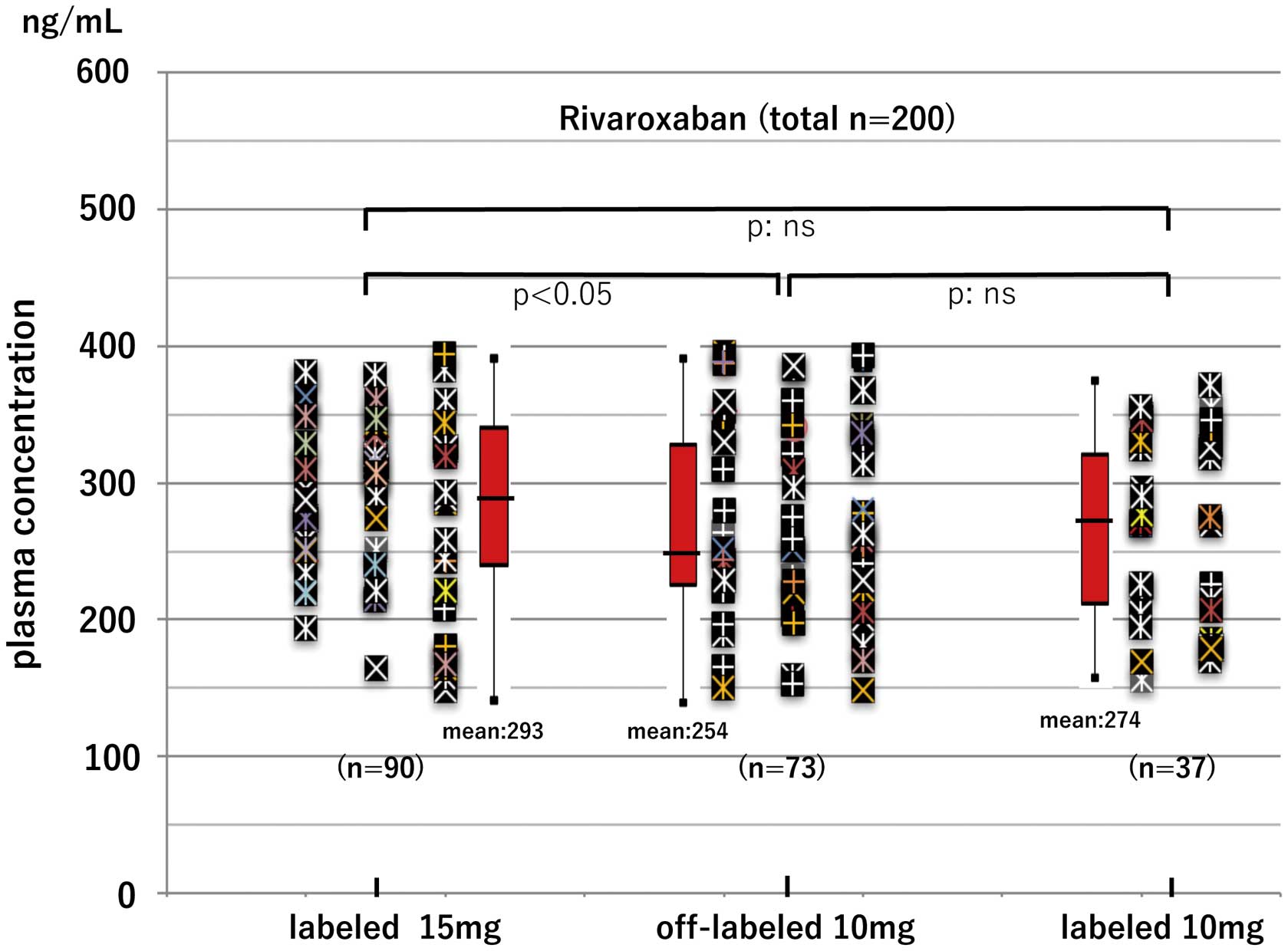
Peak plasma concentration (PC) according to dosing schedule in rivaroxaban users. PC cut-off to predict bleeding, 400.00 ng/mL. No patients had bleeding or thromboembolic events during the 6−60 months of follow-up. Of 163 rivaroxaban users with the indication for labeled 15-mg dose, 73 (45%) had acceptable PC despite off-label 10-mg use.
| Rivaroxaban (n=200) | Apixaban (n=148) | |||||||
|---|---|---|---|---|---|---|---|---|
| Labeled 15 mg (A) (n=90) |
Off-label 10 mg (B) (n=73) |
Labeled 10 mg (C) (n=37) |
P-value | Labeled 5 mg b.i.d. (A) (n=64) |
Off-label 2.5 mg b.i.d. (B) (n=46) |
Labeled 2.5 mg b.i.d. (C) (n=38) |
P-value | |
| Age (years) | 65±10 | 69±91 | 78±72,3 | 1P<0.05 | 72±6 | 77±51 | 84±42,3 | 1P<0.01 |
| 2,3P<0.001 | 2,3P<0.001 | |||||||
| BW (kg) | 71±15 | 63±121 | 54±102,3 | 1P<0.001 | 64±11 | 60±111 | 51±72,3 | 1P<0.05 |
| 2,3P<0.001 | 2,3P<0.001 | |||||||
| Baseline CrCl (mL/min) |
87±30 | 69±181 | 40±92,3 | 1,2,3P<0.001 | 66±20 | 48±101 | 40±132,3 | 1,2P<0.001 |
| DR criteria | DR criteria | 3P<0.01 | ||||||
| 0, n=30 | 0, n=9 | |||||||
| 1, n=34 | 1, n=37 | |||||||
| CHADS2 score | NS | |||||||
| 0–1 | 51 (57) | 40 (55) | 14 (38) | 28 (44) | 12 (26)1 | 4 (10)2 | 1P<0.02 | |
| 2 | 35 (39) | 28 (38) | 13 (35) | 27 (42) | 21 (46) | 22 (58) | 2P<0.01 | |
| 3–5 | 4 (4) | 5 (7) | 10 (27) | 9 (14) | 13 (28) | 12 (32) | ||
| Hypertension | NS | NS | ||||||
| + | 67 (74) | 63 (86) | 27 (73) | 51 (80) | 36 (78) | 26 (68) | ||
| − | 23 (26) | 10 (14) | 10 (27) | 13 (20) | 10 (22) | 12 (32) | ||
| APT | NS | |||||||
| + | 8 (9) | 9 (12) | 5 (14) | 9 (14) | 14 (30)1 | 12 (32)2 | 1,2P<0.05 | |
| − | 82 (91) | 64 (88) | 32 (86) | 55 (86) | 32 (70) | 26 (68) | ||
| Trough PC (ng/mL) |
27±21 (n=56) |
30±21 (n=39) |
39±30 (n=14) |
NS | 100±43 (n=35) |
79±37 (n=23) |
97±57 (n=21) |
NS |
Data given as mean±SD or n (%). P value in comparison, 1: (A) vs (B), 2: (A) vs (C), 3: (B) vs (C). PC, plasma concentration. Other abbreviations as in Table 1.
Figure 6 shows the distribution of peak PC in apixaban users (n=148) after the aforementioned dose adjustments. All apixaban users had PC <386.4 ng/mL (the cut-off for bleeding events) with labeled 5-mg b.i.d., off-label 2.5-mg b.i.d., and labeled 2.5-mg b.i.d. use. Peak PC was above the 5th percentile in the lower dose users (90 ng/mL). These labeled and off-label patients did not have bleeding or thromboembolic events under careful observation during the 6–21 months (mean±SD, 17±5 months) of follow-up after dose reduction. Therefore, of 110 patients with the indication for labeled 5-mg b.i.d. dose, 46 (42%) had acceptable PC despite off-label 2.5 mg b.i.d. In other words, 46 off-label users of apixaban had appropriate dosing according to the assessment of PC, comprising 55% (46/84) of the lower dose users and 31% (46/148) of all apixaban users. The baseline data for labeled 5-mg b.i.d., off-label 2.5-mg b.i.d., and labeled 2.5-mg b.i.d. apixaban users are given in Table 2. The age increased while the BW and Cr-CL decreased with dose reduction. There were significant differences in age and CrCl and no differences in BW between labeled 5-mg b.i.d. and off-label 2.5-mg b.i.d. users, but there were no differences in CHADS2 score, hypertension, antiplatelet drug use, or trough PCs between labeled 5-mg b.i.d. and off-label 2.5-mg b.i.d. users.

Peak plasma concentration (PC) according to dosing schedule in apixaban users. PC cut-off to predict bleeding, 386.4 ng/mL. No patients had bleeding or thromboembolic events under careful observation during the 6−59 months of follow-up. Therefore, of 110 patients with indications for the labeled 5-mg twice daily (b.i.d.) dose, 46 (42%) had acceptable PC despite off-label 2.5 mg b.i.d. use.
To our knowledge, this is the first evaluation of the efficacy and safety of off-label under-dose DOAC use coupled with the monitoring of peak DOAC PC with anti-FXa chromogenic assay. The dose adjustment used these data revealed that 73 off-labeled users of rivaroxaban, comprising 37% of all users and 63% of lower dose users, had acceptable and suitable peak PC and did not experience bleeding or thromboembolic events. Also, 46 off-label under-dose users of apixaban, comprising 31% of all users and 55% of lower dose users, had appropriate doses and none had problematic events. Therefore, continuous use of these 2 DOAC may lead to over-dosing even for patients following the official dose adjustment guidelines. Careful monitoring is therefore necessary, and should be performed at least once using laboratory parameters, such as PC or anti-FXa activity, to reduce the bleeding risk owing to over-dosing and to establish an appropriate and personalized dosing to reduce the thromboembolism risk due to under-dosing or poor adherence. A recent report from the European Heart Rhythm Association recommended the measurement of PC rather than anti-FXa activity to quantitatively assess the concentration of a DOAC, and indicated the expected peak and trough PC levels in patients using DOAC.9
As suggested by several meta-analyses, rivaroxaban is likely to cause bleeding events that may be related to the drug profile.20 Thus, maintaining a higher peak PC is necessary to sustain sufficient levels over long periods after once-a-day treatment.21 Conversely, apixaban treatment showed a lower risk of major bleeding.22 In the present study both drugs caused bleeding events at markedly high peak PC. The incidences were slightly lower in apixaban users, possibly due to the maintenance of suitable PC with twice-a-day treatment, but direct differences between the 2 drugs were not evaluated.
In a study on the efficacy and safety between the standard and off-label use of rivaroxaban in Japanese NVAF patients, the primary efficacy endpoint of symptomatic stroke and systematic embolism was not different between these 2 types of users.8 The present study also suggests that low-dose rivaroxaban treatment may be effective in preventing bleeding events in some off-label users. Therefore, determination of the rivaroxaban dose based only on CrCl level might not be accurate. Similar to the present findings, 2 recent reports showed that peak anti-Xa activity appeared to be a useful predictor of bleeding events in NVAF patients: in rivaroxaban users by Sakaguchi et al,12 and in rivaroxaban and apixaban users by Wada et al.23 Although we consider their proposal important, their cut-off peak anti-Xa activity level for predicting bleeding events markedly overlapped between the patients with and without bleeding events, reducing the clinical significance of their results. It is possible that this peak anti-Xa activity cut-off may also aid in the identification of patients with acceptable anticoagulation despite off-label dosing, before the occurrence of bleeding events. Moreover, for some patients receiving the standard rivaroxaban dose, off-label use as per physician discretion may be suitable for bleeding prevention. Currently, trough PC is reported to be useful for monitoring bleeding events in edoxaban and dabigatran users,24,25 because these drugs have a lower protein-binding capacity than rivaroxaban and apixaban, resulting in drug accumulation.26 Therefore, rivaroxaban and apixaban may have different drug profiles for the assessment of bleeding events.
A secondary analysis of a randomized clinical trial (ARISTOTLE) showed that the standard 5-mg b.i.d. dose of apixaban was effective, safe, and appropriate for patients meeting only one of the dose reduction criteria.27 In the present study, however, all patients receiving the 2.5-mg b.i.d. apixaban dose, except for 1 (with a bleeding event), had suitable peak PC below the cut-off and above the 5th percentile peak PC level in the lower dose use (90 ng/mL) despite off-label use. Therefore, determining the appropriate apixaban dose solely based on the number of dose reduction criteria met may not be accurate, as indicated in Figure 3.
Although only a few reports have published PC measurements in patients taking DOAC, the maximum blood concentration (Cmax) was 248.73 ng/mL in Japanese NVAF patients with normal CrCl after 15-mg rivaroxaban, and 248.82 ng/mL (5th–95th percentiles, 180–420 ng/mL) in non-Japanese NVAF patients with normal renal function after 20-mg doses.13,26 In addition, in Japanese NVAF patients receiving 5 mg b.i.d., the Cmax was 250.53±82.35 ng/mL and 244.55±84.27 ng/mL after 2 and 4 h, respectively.14 A recent practice guide highlighted the expected peak and trough PC for the users of 4 DOAC.9 Most of the present patients who received rivaroxaban had peak PC 200–400 ng/mL; therefore, these data suggest that they were controlled based on PC evaluation. In addition, most of the present patients who received apixaban had peak PC 200–300 ng/mL, suggesting control with anticoagulant activity based on PC evaluation. Several patients treated with rivaroxaban or apixaban, however, had peak PC above the cut-off despite not experiencing any bleeding events. We also reduced their doses but they did not have thromboembolic events on the off-label DOAC doses. These higher peak PC may lead to a greater risk of bleeding and may be an indication for dose reduction. Therefore, PC measurement may be used to improve the risk-benefit ratio in these 2 types of DOAC users.
Study LimitationsThis study was performed at a single institution with a sample size ≤200 per drug. PC was measured once during treatment initiation and a few times thereafter. On deciding the acceptable peak PC range for each DOAC, we used the cut-off for bleeding as the upper limit, and the 5th percentile peak PC in the lower dose use of each DOAC as the lower limit. But because some off-label users were contained in the lower dose users in each DOAC, the lower limit may be underestimated. Therefore, the suitability of off-label dosing may vary in the patients with peak PC near the lower limit. Few reports have been carried out on the suitable PC for these 2 F-Xa inhibitors.9 The peak time in the present study was defined as 3 h after treatment, based on published recommendations.9 Given, however, that inter-individual variation was present in the peak PC data, collection of blood samples after 3 h may not reflect the actual peak time in some patients. In the present study, the Biophen DiXaI kit was used for the chromogenic anti-Xa assay. Given that only a few kits currently apply a chromogenic assay similar to that mentioned here, the cut-off PC to predict bleeding events may differ, depending on the quality of the kit used. Also, PC measurement is effective for the prevention of bleeding, but not sufficient for evaluating anticoagulant activity. Therefore, future studies should include a structured evaluation of the anticoagulant activity of DOAC using a multicenter approach. Currently, off-label dosing has been increasing despite evidence-based dosing guidelines. Dose reductions were implemented in this study for patients without bleeding events based on high PC data. Therefore, another study is needed to evaluate whether dose reduction with confirmation of anticoagulant activity is safe and can prevent bleeding events.
To prevent bleeding complications or thromboembolic events in NVAF patients receiving rivaroxaban or apixaban treatment, dose adjustments should be based not only on renal function, age, and BW, but also on the anticoagulant activity, which should be monitored at least once by measuring the PC or anti-Xa activity. In addition, for patients with off-label DOAC dosing, lower-than-indicated doses may help prevent bleeding events.
We gratefully acknowledge the support of Sysmex Corporation (Kobe, Japan) with regards to the introduction of the Biophen® DiXaI kit.
The authors declare no conflicts of interest.
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-18-1282