Abbreviations
| AIVR |
accelerated idioventricular rhythm |
| ACT |
activated coagulation time |
| APTT |
activated partial thromboplastin time |
| AAD |
acute aortic dissection |
| ACS |
acute coronary syndrome |
| AKI |
acute kidney injury |
| AMI |
acute myocardial infarction |
| ADP |
adenosine diphosphate |
| ATP |
adenosine triphosphate |
| ACC |
American College of Cardiology |
| AHA |
American Heart Association |
| ACE |
angiotensin converting enzyme |
| ARB |
angiotensin II receptor blocker |
| ALPM |
anterolateral papillary muscle |
| ANP |
atrial natriuretic peptide |
| AV |
atrioventricular |
| AED |
automated external defibrillator |
| BMS |
bare metal stent |
| BLS |
basic life support |
| BiPAP |
biphasic positive airway pressure |
| BMI |
body mass index |
| BNP |
brain natriuretic peptide |
| CCB |
calcium channel blocker |
| CANVAS |
CANagliflozin cardioVascular Assessment Study |
| CI |
cardiac index |
| CMR |
cardiac magnetic resonance |
| CVD |
cardiovascular disease |
| CKD |
chronic kidney disease |
| CT |
computed tomography |
| CPAP |
continuous positive airway pressure |
| CIN |
contrast-induced nephropathy |
| CAG |
coronary angiography |
| CABG |
coronary artery bypass grafting |
| CAD |
coronary artery disease |
| CE |
coronary artery embolism |
| CCU |
coronary care unit |
| CK |
creatine kinase |
| CK-MB |
creatine kinase MB |
| CRP |
C-reactive protein |
| DPC |
the Diagnosis Procedure Combination |
| DPP-4 |
dipeptidyl peptidase 4 |
| DOAC |
direct oral anticoagulants |
| DHA |
docosahexaenoic acid |
| DES |
drug-eluting stent |
| DAPT |
dual antiplatelet therapy |
| EPA/AA |
eicosapentaenoic / arachidonic acids ratio |
| EPA |
eicosapentanoic acid |
| ECG |
electrocardiogram |
| ECC |
emergency cardiovascular care |
| EMS |
emergency medical service |
| ER |
emergency room |
| eGFR |
estimated glomerular filtration rate |
| ESC |
European Society of Cardiology |
| ELIXA |
Evaluation of LIXisenatide in Acute Coronary Syndrome |
| EXAMINE |
EXamination of CArdiovascular OutcoMes with AlogliptIN versus Standard of CarE in
Patients with Type 2 Diabetes Mellitus and Acute Coronary Syndrome |
| EXSCEL |
Exenatide Study of Cardiovascular Event Lowering |
| FH |
familial hypercholesterolemia |
| FIELD |
Fenofibrate Intervention and Event Lowering in Diabetes |
| FDG |
fluorodeoxyglucose |
| FFR |
fractional flow reserve |
| FOURIER |
Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk |
| GIB |
gastrointestinal bleeding |
| GLAGOV |
Global Assessment of Plaque Regression With a PCSK9 Antibody as Measured by Intravascular Ultrasound |
| GRACE |
Global Registry of Acute Coronary Events |
| GLP-1 |
glucagon-like peptide 1 |
| GIP |
glucose-dependent insulinotropic peptide |
| H-FABP |
heart type fatty acid-binding protein |
| HbA1c |
Hemoglobin A1c |
| HIT |
heparin-induced thrombocytopenia |
| HDL-C |
high-density lipoprotein cholesterol |
| hANP |
human atrial natriuretic polypeptide |
| ICU-AW |
ICU acquired weakness |
| ICD |
implantable cardioverter defibrillator |
| IMPROVE-IT |
IMProved Reduction of Outcomes: Vytorin Efficacy International Trial |
| ICU |
intensive care unit |
| IABP |
intra-aortic balloon pumping |
| IVUS |
Intravascular ultrasound |
| JCS |
the Japanese Circulation Society |
| JSICM |
the Japanese Society of Intensive Care Medicine |
| KPNC |
Kaiser Permanente Northern California |
| LGE |
late gadolinium enhancement |
| LAD |
left anterior descending coronary artery |
| LCX |
left circumflex coronary artery |
| LV |
left ventricular |
| LVAD |
left ventricular assist device |
| LVEF |
left ventricular ejection fraction |
| LVFWR |
left ventricular free wall rupture |
| LEADER |
Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results |
| LDL-C |
low-density lipoprotein cholesterol |
| MACE |
major adverse cardiovascular event |
| MVO |
microvascular obstruction |
| MINOCA |
myocardial infarction with non-obstructive coronary arteries |
| NAC |
N-acetyl cysteine |
| NCD |
the National Clinical Database |
| NMES |
neuro muscular electrical stimulation |
| NYHA |
New York Heart Association |
| NPPV |
non-invasive positive pressure ventilation |
| NSAIDs |
non-steroidal anti- inflammatory drugs |
| NSTE-ACS |
non-ST-segment elevation ACS |
| NSTEMI |
non-ST-segment elevation myocardial infarction |
| NT-proBNP |
N-terminal pro B-type natriuretic peptide |
| NNT |
number needed to treat |
| OGTT |
oral glucose tolerance test |
| PM |
papillary muscle |
| PMR |
papillary muscle rupture |
| PCPS |
percutaneous cardiopulmonary support |
| PCI |
percutaneous coronary intervention |
| PAD |
peripheral arterial disease |
| PDE |
phosphodiesterase |
| PRECISE-IVUS |
Plaque Regression With Cholesterol Absorption Inhibitor or Synthesis Inhibitor
Evaluated by Intravascular Ultrasound |
| PEEP |
positive end-expiratory pressure |
| PET |
positron emission tomography |
| PMPM |
posteromedian papillary muscle |
| PVC |
premature ventricular contractions |
| PSV |
pressure support ventilation |
| PACIFIC |
Prevention of AtherothrombotiC Incidents Following Ischemic Coronary attack |
| PCSK9 |
proprotein convertase subtilisin/kexin type 9 |
| PROactive |
PROspective pioglitAzone Clinical Trial In macroVascular Events |
| PPI |
proton-pump inhibitor |
| pro-UK |
prourokinase |
| PCWP |
pulmonary capillary wedge pressure |
| QOL |
quality of life |
| QI |
quality indicator |
| RCT |
randomized clinical trial / randomized controlled trial |
| REAL-CAD |
Randomized Evaluation of Aggressive or Moderate Lipid Lowering
Therapy With Pitavastatin in Coronary Artery Disease |
| RIC |
remote ischemic conditioning |
| RCA |
right coronary artery |
| RV |
right ventricular |
| SAVOR-TIMI53 |
Saxagliptin Assessment of Vascular Outcomes Recorded in patients with diabetes
mellitus – Thrombolysis in Myocardial Infarction-53 |
| SCr |
serum creatinine |
| SPECT |
single photon emission computed tomography |
| SGLT-2 |
sodium glucose co-transporter 2 |
| SCAD |
spontaneous coronary artery dissection |
| STEMI |
ST-segment elevation myocardial infarction |
| STOP-NIDDM |
Study to Prevent NIDDM |
| TIMI |
Thrombolysis in Myocardial Infarction |
| tPA |
tissue plasminogen activator |
| TECOS |
Trial Evaluating Cardiovascular Outcomes with Sitagliptin |
| SUSTAIN-6 |
Trial to Evaluate Cardiovascular and Other Long-term Outcomes
with Semaglutide in Subjects with Type 2 Diabetes |
| TAPT |
triple antiplatelet therapy |
| UFH |
unfractionated heparin |
| UKPDS |
United Kingdom Prospective Diabetes Study |
| UA |
unstable angina |
| VA-ECMO |
veno-arterial extracorporeal membrane oxygenation |
| VF |
ventricular fibrillation |
| VSP |
ventricular septal perforation |
| VSR |
ventricular septal rupture |
| VT |
ventricular tachycardia |
| WCD |
wearable cardioverter defibrillator |
| 95% CI |
95% confidence interval |
I. Introduction
1. Regarding the Revision
Acute coronary syndromes (ACS) are a comprehensive disease concept characterized by acute myocardial ischemia caused by disruption of coronary artery plaque and consequent thrombosis-induced severe coronary artery stenosis or occlusion, leading to unstable angina (UA), acute myocardial infarction (AMI) or sudden cardiac death. However, these diagnoses cannot be established without evaluation of the time course of myocardial markers and were thus unsuitable for rapid diagnosis and establishment of the treatment policy in the emergency room. In addition, the prognosis of ACS with coronary artery thrombosis is considerably different from that of stable coronary artery disease (CAD) with organic stenosis of the coronary arteries. In 1992, Fuster et al. proposed that unstable angina pectoris and AMI caused by coronary artery thrombosis are the same disease states and should therefore be included in the category of ACS. The initial diagnosis and decision of management in the clinical setting thus changed considerably after introduction of the concept of ACS based on the underlying mechanism. In recent years, the introduction of cardiac troponin, which can detect even minor myocardial damage unable to be detected by creatine kinase (CK) or CK-MB, has also contributed substantially to the clinical diagnosis and risk stratification of ACS. Five years have passed, since the guidelines for the management of patients with ST-elevation acute myocardial infarction (Chairperson: Kazuo Kimura) was issued by the Japanese Circulation Society (JCS). However, revision of this guideline independently of the guidelines for management of acute coronary syndrome without persistent ST segment elevation (Chairperson: Takeshi Kimura) may not match the present conditions. Although the short-term outcomes of patients with ACS have improved, the long-term outcomes, which can be negatively affected by heart failure, must be further improved. Guidelines for secondary prevention of myocardial infarction (Chairperson: Hisao Ogawa) were issued 7 years ago, and remarkable progress has been made in this field. We therefore comprehensively integrated the 3 guidelines consisting of the guidelines for the management of patients with ST-elevation acute myocardial infarction, the guidelines for management of acute coronary syndrome without persistent ST segment elevation, and the guidelines for secondary prevention of myocardial infarction into “JCS 2018 guideline on diagnosis and treatment of acute coronary syndrome.” At present, ACS guideline including ST-segment elevation acute myocardial infarction (STEMI) and non-ST-segment elevation acute coronary syndrome (NSTE-ACS) is not available as American College of Cardiology (ACC) /American Heart Association (AHA) guidelines or European Society of Cardiology (ESC) guidelines. We therefore attempted to avoid shortcomings and duplication of the contents from the stage of chapter preparation. However, similar descriptions were found in parts of the manuscript. In the present guidelines, recommendation levels and evidence levels were stated similarly to the conventional Japanese Circulation Society guidelines, ACC/AHA guidelines, and ESC guidelines. In addition, class III recommendations were classified as “no benefit” and “harm” in accordance with the ACC/AHA guidelines. The present guidelines were designed to be used as a reference in Asian countries, not only in Japan, and new efforts were incorporated during the preparation. Guidelines for diagnosis and treatment should be reviewed by all members of group meetings to avoid biased opinions and ways of thinking. However, owing to the extensive contents and many committee members involved in consolidation of the 3 guidelines, it is difficult to hold the meetings of the committee members many times over a long period. Working groups consisting of members who wanted to participate were therefore established to discuss manuscript revisions in a training camp format and hold multiple mail discussions. In particular, we extensively discussed classifications and evidence levels for which opinions were divided, spending as much time on discussion as possible. Guidelines are prepared on the basis of a critical appraisal of evidence obtained from large clinical trials and observation studies of patients with the diseases covered by the guidelines and are designed to standardize and improve the quality of medical treatment. Guideline recommendations are designed for average patients with disease. Guidelines should not be followed uniformly in a medical care setting. Patients for whom the guidelines are indicated as well as differences among individual patients should be considered, and it is important to indicate treatment best suited to the individual patient. The results of diagnosis and treatment performed according to the guidelines as well as problems should be clarified, and improved guidelines should be prepared after several years to contribute to enhancing the levels of diagnosis and treatment provided to patients.
2. Classifications and Evidence Levels
Unapproved techniques, treatments, and drugs not yet approved in Japan or for which adequate evidence supporting effectiveness and usefulness is available from foreign countries or for which expert opinion is largely consistent are appropriately listed in the present guidelines (Tables 1,2). In addition, indications, uses and dosage not approved by health insurance in Japan were also stated if necessary. The health insurance indications are as of December 2018.
Table 1.
Class of Recommendation (COR)
| I |
There is evidence and/or general agreement that a given procedure or treatment is effective and/or useful. |
| II |
There is conflicting evidence and/or a divergence of opinion about the efficacy/usefulness of a given
procedure or treatment. |
| IIa |
There is a high probability of efficacy/usefulness based on evidence and opinion. |
| IIb |
Effectiveness/usefulness is not well established based on evidence and opinion. |
| III |
There is evidence and/or general agreement that the procedure or treatment is not effective and/or useful,
or may even be harmful. |
III:
No benefit |
There is evidence and/or general agreement that the procedure or treatment is not effective and/or useful. |
III:
Harm |
There is evidence and/or general agreement that the procedure or treatment is harmful. |
Table 2.
Level of Evidence (LOE)
| A |
Demonstrated by multiple randomized clinical trials or meta-analysis. |
| B |
Demonstrated by a single randomized clinical trial or large non-randomized studies. |
| C |
Consensus from expert opinion and/or small clinical trials (including retrospective studies and case series). |
The abbreviations used in the guidelines are listed on Pages 1086–1087.
II. Concept and Epidemiology
1. Concept and Definition
ACS is the clinical spectrum of unstable ischemic heart disease, in which myocardial ischemia/necrosis is caused by rapid narrowing/obstruction of coronary artery as a consequence of atheromatous plaque disruption and thrombogenesis.1,2
In early stage atherosclerosis, intima thickening occurs, due to infiltration and accumulation of macrophages and lipids.3
During atherosclerotic plaque formation, the vessel wall may expand and preserve the vessel lumen (positive remodeling). As the plaque progresses, the lumen becomes narrowed and effort angina may develop. Inflammation plays an important role in the development and progression of atherosclerosis. In some lesions, accumulated lipid components may develop a necrotic core that is rich in inflammatory cells and cholesterol crystal. Fibroatheroma has necrotic core and, if its fibrous cap becomes thin, is prone to rupture (vulnerable plaque). It is generally believed that rupture of vulnerable plaque followed by thrombogenesis is the leading cause of ACS.4
On the other hand, pathological studies have revealed that some patients have intracoronary thrombus without plaque rupture. It is recognized that erosion is one of the mechanisms that leads to thrombus without rupture, and, although not so frequent, calcified nodules are another. ACS may develop from less severe stenotic lesions that don’t cause effort angina.5
It should be emphasized that UA, AMI and sudden cardiac death caused by myocardial ischemia are cardiac events caused by thrombogenesis that are distinct from plaque progression in effort angina, and are addressed together as ACS.
AMI is subdivided into STEMI and non-ST-segment elevation myocardial infarction (NSTEMI), because of the difference in initial stratification of diagnosis and treatment (Figure 1). UA and AMI are clinically differentiated by elevation of cardiac biomarkers. However, it is often difficult to distinguish between UA and NSTEMI at presentation. During initial evaluation, they are managed together as NSTE-ACS.
STEMI is ACS with persistent ST-segment elevation or new left bundle-branch block.
Electrocardiographic ST-segment elevation on electrocardiogram (ECG) generally reflects transmural ischemia caused by acute thrombotic occlusion of a coronary artery. Necrosis occurs first in the subendocardial myocardium and then, with longer durations of coronary occlusion, involves progressively more of the transmural ischemic zone myocardium.6
Reperfusion therapy salvages ischemic myocardium by restoring coronary blood flow. After pioneering studies by Fletcher et al. and Chazov et al.,7,8
Rentrop et al. reported 5 cases of STEMI who underwent reperfusion therapy with intracoronary nitroglycerin and streptokinase infusion in 1979.9
Since then, several studies have demonstrated significant reduction in mortality with intravenous streptokinase in the late 1980 s.10,11
In recent years, percutaneous coronary intervention (PCI) with coronary stent implantation has become widely applied and the prognosis of STEMI has dramatically improved. Because prompt restoration of coronary blood flow is essential to maximize benefits of reperfusion therapy, a strategy to minimize time from onset to reperfusion is of primary importance for patients with STEMI.
Patients with NSTE-ACS have persistent or transient ST-segment depression, T-wave abnormalities or no electrocardiographic changes at presentation. Because patients with NSTE-ACS generally have residual blood flow through a non-obstructive coronary lesion or sufficient collaterals, the management strategy for NSTE-ACS should be clearly distinguished from STEMI. The spectrum of NSTE-ACS is wide and variable, from patients without elevation of cardiac biomarkers to those with hemodynamic collapse due to left main trunk disease. During initial evaluation, prompt diagnosis and risk stratification should be appropriately provided to select the treatment strategy. Because it is often difficult to distinguish between UA and NSTEMI at presentation, they are managed together as NSTE-ACS. By the time of hospital discharge, the final diagnosis is given according to elevation of cardiac biomarkers.12
In the past, elevation of CK/CK-MB was used for clinical diagnosis of MI. In 2000, the ESC and ACC proposed a new definition of MI, a universal definition, in which cardiac troponin was adopted as the preferred cardiac biomarker.13
Because cardiac troponin has higher sensitivity and specificity over CK/CK-MB, numerous patients who were formerly diagnosed as unstable angina by CK/CK-MB criteria are now diagnosed as NSTEMI. The Japanese Registry of Acute Myocardial Infarction Diagnosed by Universal Definition (J-MINUET) is a prospective registry that enrolled 3,283 Japanese patients with AMI diagnosed by universal definition (type 1 and type 2).14
Nearly half of the patients with NSTEMI did not have elevation of CK/CK-MB, and these NSTEMI patients without CK/CK-MB elevation had favorable short-term outcome as compared to those with CK/CK-MB elevation. Of note, however, long-term outcome after the recovery period for these NSTEMI patients without CK/CK-MB elevation was as poor as NSTEMI patients with CK/CK-MB elevation and worse than those with STEMI. These findings indicate the clinical rationale of a universal definition of myocardial infarction in Japanese patients, and this guideline recommends cardiac troponin as the preferred cardiac biomarker for diagnosis of AMI. Needless to say, symptoms, ECG and imaging findings of myocardial ischemia are also required. Because cardiac troponin may be elevated in some other conditions, including aging, renal dysfunction and congestive heart failure, cardiac troponin should be measured serially to detect its rise and/or fall. The term reinfarction is applied to AMI occurring within 28 days after the index episode of AMI. If the myocardial infarction occurs after 28 days following the index episode, it is referred to as recurrent myocardial infarction. Although the universal definition classified myocardial infarction into 5 types,15
this guideline mainly deals with spontaneous myocardial infarction (type 1) which is caused by thrombogenesis related to atherosclerotic plaque rupture, erosion and so on, and refers to myocardial infarction secondary to an ischemic imbalance (type 2) as in the section of
VIII. Conditions Requiring Special Consideration.
2. Epidemiology
ACS refers to a spectrum of clinical presentations, including AMI, UA, and sudden cardiac death, and is often associated with rupture of an atherosclerotic plaque and partial or complete thrombosis of the infarct-related artery. This section mainly describes AMI, for which abundant epidemiologic evidence is available.
2.1 Coronary Risk Factors
Many epidemiological studies including NIPPON DATA (National Integrated Project for Prospective Observation of Non-communicable Disease And Trends in the Aged) demonstrated that the risk factors for AMI in Japanese are hypertension, diabetes mellitus, smoking, family history, and hypercholesterolemia, similar to those of the Western population.16–18
In the 2000 s, the prevalence of hypertension was about 50% in Japanese AMI patients and was equivalent to that of the Western population, whereas the prevalence of diabetes and smoking was higher in Japanese patients.19,20
Recently, in the general Japanese population, blood pressure control among hypertensive individuals has improved significantly and the smoking rate has decreased, whereas the prevalence of impaired glucose tolerance, dyslipidemia, and metabolic disorder have increased steeply, as demonstrated by research in Hisayama.21
Careful observation of future changes in the prevalence of risk factors for AMI in Japanese and trends is important.
2.2 Epidemiology
The data from the 30-year MIYAGI-AMI Registry Study, one of the regional epidemiological studies, demonstrated that the overall age-adjusted incidence of AMI (/100,000 persons/year) markedly increased by about 4-fold, from 7.4 in 1979 to 27.0 in 2008.22
However, the current incidence of AMI in Japan is still lower than that in North America and Europe; the incidence of AMI for males (/100,000 persons/year) is 824 in Finland, 823 in United Kingdom, 605 in Canada, 508 in the United States, 314 in France, and 270 in Italy.23
The incidence of AMI showed a male predominance, as demonstrated in the Takashima AMI registry (100.7 in males vs. 35.7 in females in 1999–2001)24
and the Niigata and Nagaoka study (41.9 in males vs. 5.3 in females in 1994–1996).25
Moreover, in the above-mentioned Miyagi AMI Registry, the 3-fold higher incidence of AMI in males remains largely unchanged throughout the last three decades.22
Furthermore, it was reported that the mean age of onset of AMI was older in female patients than in male patients, with an age difference of 10 years, which is similar to results of overseas studies such as the Framingham Heart Study.26
This may be due to the cardiovascular protective effects of female hormones, namely estrogen, which are evident before menopause but rapidly decrease thereafter.
In Western countries, it was previously reported that the age-adjusted incidence of AMI decreased from the late 1980 s to the 2000 s.26,27
This trend was especially evident in STEMI patients.27
The decline in the incidence of AMI may be attributed to the reduction in the prevalence of coronary risk factors and the increase in the prevalence of the pre-critical use of cardioprotective drugs, including angiotensin converting enzyme (ACE) inhibitors/angiotensin receptor blockers (ARBs), b-blockers, and statins.26,27
However, a recent report from the Miyagi AMI Registry Study demonstrated that, during the last 30 years in Japan, the age-adjusted incidence of AMI significantly increased in the first decade (1985–1994), but has remained unchanged in the last 2 decades (1995–2004 and 2005–2014).28
The same trend was noted for male patients, whereas age-adjusted AMI incidence significantly decreased in female patients in the last decade (2005–2014).28
Similarly, it has been recently reported that the incidence of AMI was steadily decreasing in the Kumamoto Prefecture in both sexes between 2004 and 2011.29
In contrast, while the frequency of AMI in the elderly aged 70 years and older has declined from 2005 to 2014 in the Miyagi AMI Registry Study, AMI incidence continues to increase in younger patients aged ≤59 years of both sexes in the past 3 decades.28
Indeed, it was previously reported that the increased prevalence of dyslipidemia in younger AMI patients related to dietary habits and westernized lifestyle was responsible, at least in part, for the increased incidence of AMI in this population.28
Additionally, smoking rates in young people are high, with a rate of ∼50% in males and 30% in females. These results suggest that more strict control of coronary risk factors is needed in young populations to reduce the future occurrence of AMI in Japan. In other Asian countries, Taiwan and Korea, increasing trends in the age-adjusted incidence of AMI have been reported together with westernization of diet and lifestyle and social aging.30,31
However, the latest study in Korea found that the age-adjusted incidence of AMI has declined since 2006, which could be due to, at least in part, the preventive care projects for chronic illnesses and cardio-cerebrovascular diseases since 2000 s in Korea.32
This Korean experience may provide useful information in promoting effective preventative measures in Japan.
2.3 Prognosis
The 30-day mortality from AMI in Japan was reported to be 7.1% in the OACIS Study33
and 9.4% in the HIJAMI Study.20
In the J-MINUET Study,34
which is a registry of Japanese patients hospitalized for AMI diagnosed by the new universal definition, there was no significant difference in hospital mortality between STEMI and NSTEMI with CK elevation (7.1% vs. 7.8%), whereas the mortality rate was significantly lower in NSTEMI without CK elevation (1.7%) than in STEMI or NSTEMI with CK elevation.34
Tokyo CCU Network reported that hospital mortality was significantly higher in STEMI patients than NSTEMI patients (7.7 vs. 5.1%) and treatment of dyslipidemia with statins was associated with lower risk in both STEMI and NSTEMI patients based on Killip classification.35
In Western countries, hospital mortality of AMI decreased between the 1980 s and late 2000 s, along with improvement in critical care for AMI (e.g., reperfusion therapy).36
The same trend in reduced hospital mortality of AMI was also noted in Asian countries, Taiwan and Korea, between the late 1990 s and early 2000 s.31
Meanwhile, during the last 30 years in Japan, although in-hospital cardiac mortality of AMI progressively decreased during the 1st and 2nd decades (1985–1994 and 1995–2004), no further improvement was noted in the last decade (2005–2014) irrespective of sex.28
It is important to note that hospital mortality of AMI continues to be higher in female patients than in male patients today.28
It is generally considered that the poorer outcome of female AMI patients could be caused by multiple factors, including higher age, longer time from onset to admission, poorer condition on admission such as coexisting heart failure, and lower rate of performing primary PCI.28,37
A number of western studies have shown that patients with NSTEMI have a worse long-term prognosis compared with those with STEMI. In the GRACE registry, the 6-month post-discharge mortality rate was 3.6% and 6.2% in patients with STEMI and those with NSTEMI, respectively.38
Similarly, the J-MINUET study recently reported that long-term outcome of NSTEMI patients was worse than STEMI patients, a consistent finding with Western countries.14
This could be explained by the fact that NSTEMI patients have more comorbid factors and more extensive CAD than STEMI patients.14
Furthermore, a recent report from the French National Registry survey demonstrated that chronic-phase mortality has consistently declined in STEMI patients between 1995 and 2015, whereas chronic-phase mortality reached a plateau in NSTEMI patients after 2010.39
From now, a national registry for ACS will be launched in Japan and is expected to produce evidence in the Japanese population.
III. Prehospital Care
1. Emergency Medical Dispatchers
Patients with possible ischemic symptoms need to call medical dispatch number 1-1-9 and be transported to hospital, rather than visiting the hospital themselves.40,41
ACS, especially STEMI, has the risk of sudden cardiac death, and it is important to promote the importance of calling emergency medical services (EMS) in the early phase after onset (regarding symptoms of ACS, please refer to chapter
IV 1.2.1 Chest Pain). Patients prescribed with sublingual nitroglycerin tablets can take 1 tablet every 3 to 5 minutes for ongoing symptoms (total of 3 doses), only if they remain hemodynamically stable. However, if nitroglycerin does not relieve ischemic chest pain, patients should immediately call EMS.42
2. Initial Management of Physician on Scene
Physicians who first treat patients with suspected ACS on scene assess vital signs and perform a physical examination. They should record and interpret the 12-lead ECG, start initial management (please refer to chapter
IV 2. Initial Therapy) and call EMS urgently. Interpretation of computer-assisted ECG in acute phase of STEMI does not always have high diagnostic performance, and should not be used alone to rule out STEMI.43
Patients with suspected STEMI should be transported to a primary PCI capable center,44–47
and in this situation, the physician should report the vital signs and ECG findings to the cardiologist at the primary PCI capable center. See “chapter
V 2. Fibrinolysis
” regarding the indication for fibrinolysis for patients with STEMI who arrive at non-primary PCI capable center.
3. Management of Emergency Medical Service Personnel
EMS personnel rapidly assess vital signs, monitor the ECG, and take precautions against cardiac arrest. Monitoring of pulse oximeter is also recommended. When patients prescribed with sublingual nitroglycerin tablets remain hemodynamically stable, EMS personnel permit administration of nitroglycerin if requested by patients. In the present state of affairs in Japan, only a physician can administrate nitroglycerin or aspirin if a doctor car or medical helicopter has been dispatched to the scene. Further investigation is needed to confirm the benefit of prehospital administration of nitroglycerin and aspirin by EMS personnel. If available, prehospital 12-lead ECG acquisition in patients with suspected STEMI and notifying the destination hospital are recommended.48
Some studies have shown the benefit of prehospital 12-lead ECG acquisition with destination hospital notification in reducing first-medical contact-to-reperfusion time, door-to-device time, and door-to-needle time compared with no ECG in patients with STEMI.49–57
It has also been reported that the benefit of prehospital 12-lead ECG acquisition with destination hospital notification is a 32% relative reduction in 30-day mortality compared with no ECG in patients with STEMI treated with primary PCI.48
Prehospital 12-lead ECG acquisition with destination hospital notification by EMS personnel can activate the cardiac catheterization laboratory earlier, call the catheterization team earlier, reduce the time from onset to reperfusion, and finally, is expected to improve prognosis in patients with STEMI. However, prehospital 12-lead ECG is not currently widely available in Japan. Thus, raising awareness through participation by cardiologists in regional medical control organizations, for example, is needed in order to promote utilization and familiarity.
4. Emergency Medical System
In the management of ACS, early diagnosis and rapid treatment are important. The systematic approach for ACS includes primary prevention, recognition of patients, medical therapy, surgical treatment, and rehabilitation, as well as secondary prevention and cooperation with medical institutions. In the treatment strategy for STEMI, it is important to reduce time from onset to reperfusion. The goal of treatment in STEMI are to achieve reperfusion within 120 minutes from onset, which means initiating therapy within 30 minutes from first contact with medical personnel (including EMS personnel) for fibrinolysis, and catheter treatment within 90 minutes from first contact with medical personnel for PCI. Furthermore, when a STEMI patient attends a facility where reperfusion therapy cannot be administered, the goal has been set to keep door-in-door-out time to within 30 minutes.58
In order to achieve earlier reperfusion, it is important to establish an integrated medical system for the treatment of STEMI involving regional medical administration, medical control organizations, emergency medical transport, medical (doctor) associations and specialized health facilities. Regarding prehospital care in patients with ACS, please refer to the JRC (Japan Resuscitation Council) resuscitation guidelines 2015.59
IV. Initial Diagnosis and Treatment
1. Initial Patient Evaluation
1.1 Triage
Since it is well established that reperfusion therapy early after the onset of STEMI results in better prognosis, it is important to diagnose and treat STEMI as early as possible. The disease state should be assessed in accordance with the prespecified procedure and initial treatment should be started immediately. It is important to check vital signs, perform continuous ECG monitoring, collect a brief and accurate medical history, record 12-lead ECG, and perform laboratory tests within 10 minutes of hospital arrival.60,61
Regarding reperfusion therapy for STEMI, it is ideal to administer a fibrinolytic agent within 30 minutes of hospital arrival when fibrinolysis is selected, and to inflate the first balloon within 90 minutes of first medical contact (including contact with ambulance attendants) when PCI is selected.51,61,62
For NSTE-ACS, invasive strategy is recommended for moderate- to high-risk patients, but the appropriate timing remains unclear. Timely invasive strategy should be considered after assessing risk based on the thrombolysis in myocardial infarction (TIMI) risk score,63,64
and/or Global Registry of Acute Coronary Events (GRACE) score,65
etc (Figure 2). A meta-analysis of 8 clinical studies involving 5,324 patients showed that, compared with elective invasive strategy, early invasive strategy did not reduce the mortality rate or the incidence of non-fatal myocardial infarction, but may reduce the mortality rate in high-risk patients, including patients with positive cardiac enzymes, diabetes mellitus, a GRACE risk score of >140, and aged 75 years or older.66

Medical history is very important information in the diagnosis of ACS and should be collected thoroughly and quickly, since treatment protocol differs depending on diagnosis. Particular attention should be paid to chest pain in terms of site, description, trigger, duration, changes over time, and associated symptoms, etc. In parallel, information on past medical history, coronary risk factors, and family history should be collected to differentiate ACS from other diseases as quickly as possible.67
1.2.1 Chest Pain
Chest pain associated with ACS is often described as precordial or retrosternal heaviness, pressure, tightness, choking sensation, or burning sensation, but the complaint is sometimes simply discomfort. It should be noted that chest pain may radiate to the jaw, neck, shoulder, epigastrium, back, and/or arm, and symptoms are sometimes localized in these regions without involving the chest. Stabbing pain, pricking pain, and pain on palpation are mostly non-anginal and unlikely to be affected by respiration, cough, or postural change. Meanwhile, since serious ACS occurs often with atypical or minimal symptoms, ACS cannot be excluded based only on symptoms. Atypical symptoms also often occur in elderly patients, diabetic patients, and women.68–70
In addition, the elderly may complain of shortness of breath as a symptom of myocardial ischemia71
and present only with general malaise, anorexia, syncope, or depressed level of consciousness.
Symptoms of myocardial infarction persist for at least 20 minutes, often for several hours. While approximately half of patients have intense pain requiring morphine hydrochloride, the intensity of symptoms is not always consistent with severity of ACS. Common associated symptoms are cold sweat in men and nausea, vomiting, and dyspnea in women.68,69
Radiation to the jaw, neck, shoulder, back, and arm is more frequently reported in women.68,69,72,73
① The duration of chest pain associated with angina or UA is mostly a few minutes, at most 15 to 20 minutes. Chest pain persisting for 20 seconds or less is unlikely to be anginal pain. Chest pain persisting for 20 minutes or more is likely to be associated with AMI when described as ACS.
② Chest pain occurs not only during physical activity, including walking hastily, walking upstairs, and lifting or carrying a heavy weight, but also at rest. Chest pain is also triggered by mental excitement or meals. Chest pain occurs more frequently in the early morning due to lower threshold. Angina at rest often occurs during nocturnal sleep or in the early morning.
③ Angina at rest, new-onset angina, and angina with changing pattern should be distinguished from one another based on the mode of onset of chest pain and its change over time. While a new single episode of chest pain indicates new-onset angina, frequent recurrence of chest pain indicates angina with changing pattern, and both should be treated aggressively.
④ Chest pain that resolves within 1 to 5 minutes after rest or use of nitroglycerin is often angina.
⑤ When chest pain is accompanied by dyspnea or loss of consciousness, this indicates higher severity, and myocardial infarction should be considered. When chest pain is accompanied by pyrexia, infection, including pneumonia, pleurisy, and pericarditis, should be considered.
⑥ When a patient with a history of ischemic heart disease presents with symptoms that are similar to or more intense than those of ischemic heart disease, ACS is most likely.
The HEART score has been proposed as a score to specify the acute phase risk (all-cause mortality within 6 weeks, myocardial infarction, coronary revascularization) in patients with low-risk chest pain who do not fulfil the criteria for ACS74
(https://www.mdcalc.com/heart-score-major-cardiac-events). The score is an acronym for History, ECG, Age, Risk factors, and initial Troponin, and the score is calculated from these components. The safety of early hospital discharge based on the HEART score has been demonstrated, and the utility of the HEART score in cost reduction including frequency of examinations as well as the superiority of the HEART score for identification of low-risk patients compared to the TIMI and GRACE risk scores have been reported.75–77
1.2.2 Past History
It is important to collect past medical history. Questions should be asked as to whether the patient has experienced similar symptoms, has a history of myocardial infarction, has undergone coronary angiography (CAG), coronary angioplasty, or coronary artery bypass, has cerebrovascular or peripheral vascular disease, and has been diagnosed or treated by another physician. For patients with any previous disease, a more appropriate treatment can be selected by considering detailed information on previous diagnosis and treatments given.
1.2.3 Family History
A family history of CAD (especially early onset at less than 55 years of age for men and at less than 65 years of age for women) is important.
1.2.4 Coronary Risk Factors
Every effort should be made to collect information on coronary risk factors. ACS is more probable when at least 3 risk factors (age, smoking, dyslipidemia, diabetes mellitus, hypertension, family history, and renal impairment) are present, in addition to symptoms suggestive of ACS.
1.3 Physical Findings
Careful examination of physical findings is important not only to diagnose ACS, but also to determine the presence or absence of complications, differentiate ACS from other diseases with chest pain, select treatment, and determine the arterial access site. In particular, the intensity of symptoms of AMI varies individually, and patients with intense symptoms appear anguished and often cannot move because of the pain. In addition, patients should be assessed for previous stroke and dementia based on brief examination of neurological findings. If there is pulmonary edema as a complication, dyspnea, orthopnea, cough, and/or foamy bloody sputum are observed. Patients with shock have a pale face, cold wet skin, blue patchy spots, and cyanosis of the lips and nail bed. Cerebral circulatory disorder due to reduced cardiac output may result in depressed level of consciousness, including confusional state. Inferior AMI with right ventricular (RV) infarction may be accompanied by signs of right heart failure, including jugular venous distention, hepatomegaly, and lower leg edema. Finally, it should be determined whether any finding such as vascular bruit in the carotid artery, abdominal aorta, or femoral artery, anemia, or abdominal aneurysm precludes reperfusion therapy, including urgent cardiac catheterization.
1.3.1 Vital Signs
Blood pressure is usually normal in the absence of complications, but intense anxiety or excitement may result in sympathetic activation and a consequent transient increase in blood pressure. In general, inferior AMI is characterized by findings suggestive of parasympathicotonia such as bradycardia due to Bezold-Jarisch reflex, whereas anterior infarction is characterized by findings reflecting sympathicotonia such as tachycardia. Hypotension of ≤90 mmHg for more than 30 minutes or ≥30 mmHg decrease in systolic blood pressure from baseline level is diagnosed as shock. According to a scientific statement from the AHA on the management of cardiogenic shock,78
cardiogenic shock is classified into 4 groups based on intravascular volume (wet or dry) and peripheral circulation (cold or warm). While two thirds of cardiogenic shock related to AMI is the classic “cold and wet” type, the SHOCK trial reported that 28% of patients had “cold and dry” cardiogenic shock.79,80
Palpable pulses in extremities are also important to ensure arterial access for urgent cardiac catheterization.
1.3.2 Auscultatory Findings
a. Heart Sounds and Cardiac Murmur
Third heart sound detected by auscultation, which reflects severe left ventricular (LV) dysfunction with increased LV filling pressure, is used to determine the Killip class (Table 3).81
Systolic murmur in the course of severe ACS indicates LV enlargement, papillary muscle dysfunction, mitral regurgitation due to ruptured chordae tendineae or papillary muscle, or ventricular septal perforation. Mitral regurgitation due to ruptured chordae tendineae or papillary muscle is most pronounced in the cardiac apex, heard as notable holosystolic murmur sometimes with thrill, and accompanied by deteriorating hemodynamics. Systolic murmur due to ventricular septal perforation is similarly described, but is often most pronounced in the fourth intercostal left sternal border. Pericardial rub is rare immediately after onset; instead, it is heard during inspiration 2 to 3 days after the onset of extensive transmural myocardial infarction. Since aortic stenosis may have symptoms in common with angina, patients should be checked for ejection systolic murmur.
Table 3.
Killip Classification: Severity Classification Based on Physical Findings
| Class I |
No pump failure |
No rales over the lung field and no third heart sound |
| Class II |
Mild to moderate heart failure |
Rales over less than 50% of the lung fields or third heart sound |
| Class III |
Severe heart failure, pulmonary edema |
Rales over 50% or more of the lung fields |
| Class IV |
Cardiogenic shock |
Blood pressure <90 mmHg, decreased urine output, cyanosis,
cold wet skin, and consciousness disturbance |
(Source: Prepared based on Killip T. 196781)
Moist rales result from leakage of body fluid into the alveoli and/or airways under reduced LV compliance. In auscultation of the lung fields, the presence or absence and extent of moist rales are important and should be assessed together with the aforementioned third heart sound to determine the severity of heart failure based on Killip class (Table 3).81
Attention should be paid to respiratory rate, depth and speed of respiration, comfortable breathing position, and moist rales, especially dorsal moist rales.
1.4 Differentiation of Diseases
Since it has been reported that, of all patients transported to an emergency department due to acute chest pain, STEMI accounts for 5% to 10%, NSTEMI for 15% to 20%, UA for 10%, other cardiac diseases for 15%,82
and non-cardiac diseases for 50%,82–87
ACS should be differentiated from the other diseases based on medical history and physical findings (Table 4). It is important to understand the trigger and the extent of the chest pain, as well as to area(s) to which the chest pain radiates, through a medical interview. The presence of other clinical symptoms such as cold-like symptoms and pyrexia may facilitate differential diagnosis of ACS. ECG, chest x-ray, biochemistry, and echocardiography are useful and essential for differential diagnosis.
Table 4.
Differentiation of Diseases With Acute Chest Pain
Cardiac
disease |
Pulmonary
disease |
Macrovascular
disease |
Digestive
disease |
Orthopedic
disease |
Other |
Myocarditis,
cardiomyopathy |
Acute pulmonary
thromboembolism |
Acute aortic
dissection |
Reflux esophagitis |
Skeletal muscle disorder |
Anxiety neurosis |
| Tachyarrhythmia |
Pneumothorax |
Symptomatic aortic
aneurysm |
Esophageal spasm |
Chest trauma |
Herpes zoster |
| Acute heart failure |
Bronchitis, pneumonia |
Stroke |
Peptic ulcer, gastritis |
Muscle disorder/myositis |
Anemia |
Hypertensive
emergency |
Pleurisy |
|
Pancreatitis |
Costochondritis |
Pyrexia |
| Aortic stenosis |
|
|
Cholecystitis,
gallstone |
Cervical spine pathology |
Hyperthyroidism |
| Takotsubo syndrome |
|
|
Peptic ulcer, gastritis |
Intercostal neuralgia |
Increased blood
viscosity |
| Coronary spasm |
|
|
|
|
|
| Cardiac trauma |
|
|
|
|
|
(Source: Prepared base on Ibanez B, et al. 2018166)
The most common non-cardiac diseases associated with chest pain are digestive diseases. Reflux esophagitis, which is characterized by heartburn-like burning sensation, is aggravated in the supine position after meals and alleviated by antacids. Esophageal spasm involves pain that arises in the posterior surface of sternum and radiates to the neck and back. Esophageal spasm is neither exertional nor of a certain duration, and is triggered by eating or drinking and often resolves after drinking water. While nitrates and Calcium antagonists may be effective, few specific examinations are available to support a diagnosis. When prandial upper abdominal pain is accompanied by tenderness, peptic ulcer, gallstone, and cholecystitis should be considered.
Chest pain is also attributable to respiratory (pulmonary) diseases, including pulmonary thromboembolism, pleurisy, pneumothorax, and pneumonia, and other common diseases to consider are skin and skeletal diseases, including herpes zoster, intercostal neuralgia, and rib fracture, as well as psychogenic cardiac neurosis.
1.4.2 Fatal Diseases With Chest Pain Requiring Prompt Differentiation
Prompt differentiation is important for acute pulmonary thromboembolism and acute aortic dissection (AAD). Acute pulmonary thromboembolism often involves precordial and back symptoms as seen in AMI, but is accompanied by dyspnea and tachypnea, and sometimes by shock and loss of consciousness in severe cases. Patients when walking for the first time after postoperative rest in bed or who have an underlying disease such as deep vein thrombosis, abnormal coagulation, or malignant tumor are more vulnerable. AAD often involves more intense and severe pain than myocardial infarction. Tearing sharp pain that suddenly radiates to the back, sometimes accompanied by dyspnea and loss of consciousness, and spreads to the lumbar region and rarely to the lower extremities occurs with progression of dissection. Patients should be carefully checked for any difference (>15 mmHg) in blood pressure between different extremities and for aortic regurgitation murmur. Stanford-A AAD affects the coronary ostium and may be complicated by STEMI (approximately 5% of Stanford-A cases, especially involving the right coronary artery (RCA) ostium). While it is important to judge a disease based on characteristic physical findings, it should be noted that these findings cannot always be used to establish a diagnosis. Definitive diagnosis often requires computed tomography (CT), pulmonary perfusion scintigraphy, and CAG.
1.4.3 Other
Other pathological conditions that induce myocardial ischemia include (1) diseases associated with increased oxygen demand and (2) those associated with decreased oxygen supply. It should be noted that symptoms similar to those of angina occur in these pathological conditions without CAD. In addition, stable angina may be destabilized when complicated by these pathologies.
(1) Diseases associated with increased oxygen demand: high temperature, hyperthyroidism, poorly controlled hypertension, and persistent tachyarrhythmia
(2) Diseases associated with decreased oxygen supply: anemia, pulmonary disease, and increased blood viscosity
1.5 ECG (Table 5)
Table 5.
Recommendations and Evidence Level of ECG in the Diagnosis of ACS
| |
COR |
LOE |
In patients with symptoms suggestive of ACS, recording of 12-lead ECG should be performed within
10 minutes of the patient’s arrival.88 |
I |
C |
If AMI is clinically strongly suspected but the initial ECG is not diagnostic, serial recording of 12-lead
ECGs at 5- to 10-minute intervals should be performed. |
I |
C |
If the possibility of ACS cannot be clinically excluded and the initial ECG is not diagnostic, serial
recording of 12-lead ECGs should be performed.92 |
I |
C |
| ECG monitoring should be performed as soon as possible in patients with STEMI.98,99 |
I |
B |
In patients with inferior STEMI, ECG recording with an additional right precordial lead (lead V4R)
should be performed.102–104 |
I |
B |
If AMI is clinically suspected but the initial ECG is not diagnostic, ECG recording with additional
posterior leads (leads V7–V9) should be considered.105,106 |
IIa |
C |
Abbreviations: ACS, acute coronary syndrome; ECG, electrocardiogram; STEMI, ST-segment elevation myocardial infarction; AMI, acute myocardial infarction.
ACS is an emergency cardiovascular disease with a risk of cardiac events soon after its onset; therefore, prompt and precise diagnosis and treatment are essential. Although various diagnostic techniques have been developed, the 12-lead ECG is simple, readily available, non-invasive, and inexpensive, making it the most important initial examination for the diagnosis of ACS. The 12-lead ECG plays a central role in diagnostic and triage pathways for ACS and provides important prognostic information.
It is recommended to record a 12-lead ECG within 10 minutes of the patient’s arrival.88,89
ACS is classified according to the presence or absence of ST-segment elevation (STEMI or NSTE-ACS). Reperfusion therapy must be initiated as soon as possible in patients with STEMI, and an optimal treatment strategy based on early risk stratification is needed in those with NSTE-ACS.
It should be appreciated that a normal ECG does not exclude the possibility of ACS.90
ECG must be interpreted considering the presence or absence of symptoms at the time of presentation and time elapsed before recording ECG from symptom onset. Some patients with ACS may have an initially normal ECG because they have no anginal attack at presentation or because the ECG is performed very early after symptom onset. In patients where there is clinical suspicion of ACS in whom initial ECG shows no diagnostic ST-T changes, it is important to repeat the ECG or compare with a previous ECG, which can enhance the accuracy of the diagnosis of ACS.91,92
1.5.2 ECG Criteria for ST-Segment Elevation
ST-segment elevation on ECG can represent transmural myocardial ischemia,15
and identify patients who will benefit from reperfusion therapy. In patients with transmural ischemia, ST-segment elevation is present in leads facing the site of ischemia. However, ST-segment elevation can be also observed as a normal finding. In healthy individuals, the magnitude of ST-segment elevation differs according to age, sex, and lead, and ST levels are generally highest in leads V2–3 and higher in males than in females.93
According to the universal definition of myocardial infarction,15
ECG findings suggestive of acute myocardial ischemia are defined as new ST-segment elevation in at least two contiguous leads; ST-segment elevation in leads V2-3 of at least 2.0 mm (0.2 mV) in men aged 40 years and above, at least 2.5 mm (0.25 mV) in men under 40 years of age, or at least 1.5 mm (0.15 mV) in women of any age, and ST-segment elevation in leads other than V2–3 of at least 1.0 mm (0.1 mV). These criteria are applied in the absence of LV hypertrophy or left bundle branch block. The J point is used to determine ST level. ST-segment elevation is also seen in diseases or conditions other than STEMI. It is important to comprehensively make the diagnosis of STEMI considering the medical history, clinical features, and other diagnostic test results.94–96
*In standard 12-lead ECG display, the precordial leads are displayed in their anatomically contiguous order, which makes it easy to understand the positional relationships between the precordial leads and the heart. However, the limb leads are not displayed in their anatomically contiguous order. For the limb leads to be displayed in an anatomically contiguous manner from the left superior-based to right inferior, the display should be aVL, I, −aVR (i.e., the inverse lead of aVR), II, aVF, and III. In this configuration, lead −aVR (+30°) bridges the gap between lead I (0°) and lead II (+60°) and faces the apical and inferolateral regions. This display is known as the ‘Cabrera sequence’. The Cabrera sequence makes it easy to understand the positional relationships between the limb leads and the heart,15,97
and lead grouping such as inferior leads (II, III, and aVF) or lateral leads (I, aVL).
1.5.3 STEMI
This guideline is applied to patients with new onset left bundle branch brock who have chest pain or those with true posterior AMI who have no significant ST-segment elevation as well as those with ST-segment elevation as mentioned above.
ECG monitoring should be performed as soon as possible in patients with STEMI to detect life-threatening arrhythmias, which often occur in the acute phase of STEMI.98,99
Early reperfusion therapy helps to achieve more myocardial salvage. However, in the very acute phase of STEMI, ECG diagnosis is difficult because the ECG does not yet show ST-segment elevation. In this phase, one should confirm whether hyperacute T waves, which may be seen before ST-segment elevation development, are present or not.15
In patients with anterior STEMI, the more proximal the occlusion, the more extensive is the area at risk. ST-segment depression in inferior leads, ST-segment elevation in lead aVR, and complete right bundle branch block have been shown to be suggestive of the left anterior descending coronary artery (LAD) occlusion proximal to the first septal branch. Especially ST-segment depression in inferior leads is very useful; whereas ST-segment elevation in lead aVR and complete right bundle branch block are shown to have high specificities, but low sensitivities.100,101
In patients with inferior STEMI, those with right ventricular (RV) infarction have a poor prognosis. In addition, administration of nitrates must be avoided in those with RV infarction. RV infarction during inferior STEMI can be accurately diagnosed by ST-segment elevation ≥1.0 mm (0.1 mV) in right precordial lead, especially lead V4R.102–104
In patients with inferior STEMI, ECG should be recorded with an additional right precordial lead (lead V4R) to identify concomitant RV infarction. However, ST-segment elevation in right precordial leads has been reported to be short lived, disappearing within 10 hours after the onset of symptoms in half of patients with inferior AMI and RV infarction.104
ECG diagnosis is often difficult in patients with true posterior AMI caused by occlusion of the left circumflex coronary artery (LCX) because there are no leads facing the LV posterior wall in the standard 12-lead ECG. ST-segment elevation ≥0.5 mm (0.05 mV) in ≥2 contiguous posterior chest leads (lead V7–9) is considered to be diagnostic of posterior AMI.105,106
Even in cases without ST-segment elevation on standard 12-lead ECG, the presence of ST-segment elevation in posterior chest leads is an indication for emergent coronary angiography (CAG) to perform timely reperfusion therapy. It has been reported that ST-segment elevation is present solely in posterior chest leads in about 4% of all patients with AMI.105
If the initial ECG is not diagnostic of STEMI but there is a high clinical suspicion for STEMI, ECG recording with additional posterior leads (leads V7–9) is indicated to exclude true posterior AMI.
ECG can provide useful information about not only the diagnosis of STEMI, but also the culprit artery, the extent of area at risk, and the degree of myocardial damage and prognosis.100,107,108
ECG diagnosis is more difficult in patients with secondary ST-T changes such as ventricular pacing, Wolff-Parkinson-White syndrome, or bundle branch block. In these patients, it is important to comprehensively make the diagnosis of STEMI considering the medical history, clinical features, and other diagnostic test results. In addition, comparison with a previous ECG may be helpful to make the diagnosis of STEMI in this setting. Sgarbossa et al.109
reported the ECG criteria, based on simple ST-T change, for the diagnosis of AMI in patients with left bundle branch block, which are concordant ST-segment elevation ≥1.0 mm (0.1 mV) in leads with a positive QRS complex, concordant ST-segment depression ≥1.0 mm (0.1 mV) in leads V1–3, and discordant ST-segment elevation ≥5.0 mm (0.5 mV) in leads with a negative QRS complex. In particular, concordant ST-segment elevation ≥1.0 mm (0.1 mV) in leads with a positive QRS complex has been reported to be strongly suggestive of STEMI in left bundle branch block. However, these Sgarbossa criteria have been reported to have limited utility in clinical practice because of their low sensitivity.110,111
It is likely that, in patients with left bundle branch block, ECG diagnosis of AMI is possible in limited patients with profound ST-T change. Importantly, AMI patients with left bundle branch block have a worse clinical profile and poorer prognosis.112
Therefore, clinical suspicion of AMI in the presence of left bundle branch block is an indication for emergent CAG to perform timely reperfusion therapy.113
1.5.4 NSTE-ACS
ST-segment changes are considered the most important electrocardiographic feature during acute myocardial ischemia. The changes of T waves, QRS complex, and U waves, and the occurrence of arrhythmias are also useful for the diagnosis of myocardial ischemia. Electrocardiographic findings of myocardial ischemia are described as follows (These findings can be also applied in STEMI).
a. ST-Segment Depression
The presence of acute ischemic changes on admission ECG has been associated with a higher risk of cardiac events; ST-segment depression is an especially strong predictor of poor outcomes in patients with NSTE-ACS.114,115
The presence of even minimal [0.5 mm (0.05 mV)] ST-segment depression has been shown to be independently associated with adverse outcomes.114,115
Furthermore, the degree, extent, and serial changes of ST-segment depression, not only its presence or absence, can facilitate early risk stratification in patients with NSTE-ACS.114
ST-segment elevation is present in leads facing the site of ischemia. Therefore, the culprit artery can be predicted on the basis of the leads showing ST-segment elevation during ischemic attacks. However, in many patients with non-transmural (subendocardial) ischemia, ST-segment depression occurs in leads V4–6 (mainly in lead V5) independently of the culprit artery, for which the underlying mechanism remains unclear. It is thus difficult to predict the culprit artery on the basis of leads with ST-segment depression, but increased cumulative ST-segment depression, an increased number of leads with ST-segment depression on admission, and prolonged ST-segment depression after admission have been shown to be associated with worse clinical outcomes in patients with NSTE-ACS.114,116
In clinical practice, clinicians have used an “11-lead” ECG, neglecting lead aVR. However, lead aVR has a unique position because the positive pole is oriented to the right upper side of the heart. In NSTE-ACS, lead aVR looks into the left ventricular cavity from the right shoulder. Lead aVR is therefore referred to as a “cavity lead,” and ST-segment elevation in this lead might reflect global subendocardial ischemia. ST-segment elevation in lead aVR is highly suggestive of severe ischemia due to left main or multi-vessel disease,114,117–119
which would most likely require urgent coronary artery bypass grafting (CABG).
ST-segment depression can be caused not only by subendocardial ischemia but also by reciprocal changes of ST-segment elevation in the opposite lead. In the interpretation of ST-segment depression on ECG, one should confirm whether ST-segment elevation is present in the opposite lead. In patients with true posterior AMI due to the LCX occlusion, standard 12-lead ECG often shows no ST-segment elevation, but shows precordial ST-segment depression as reciprocal changes of ST-segment elevation in the posterior wall. True posterior AMI should thus be considered in the differential diagnosis of NSTE-ACS. It has been shown that in patients with true posterior AMI, ST-segment depression is more marked in leads V1–3,120
whereas in patients with subendocardial ischemia, ST-segment depression is more apparent in leads V4–6. These different patterns of ST-segment depression in precordial leads may be helpful to differentiate these 2 conditions; however, recording of leads V7–9 is necessary to make the diagnosis of posterior AMI.
b. Negative T Waves
In patients with NSTE-ACS, negative T waves are common ECG changes, as well as ST-segment depression, and are associated with a relatively benign prognosis as compared with ST-segment depression.121
However, it is reported that patients with negative T waves in ≥6 leads have a poor prognosis.115
Negative T waves can actually be preceded by a transient ST-segment elevation that resolves by the time ECG is recorded. Therefore, the culprit artery can be predicted on the basis of the distribution of negative T waves. In patients with NSTE-ACS patients, negative T waves in precordial leads suggest severe ischemia of the LV anterior wall due to LAD disease.122
However, this electrocardiographic finding is also frequently observed in patients with severe acute pulmonary thromboembolism or takotsubo syndrome. Acute pulmonary thromboembolism and takotsubo syndrome should be included in the differential diagnosis of ACS in patients who have precordial negative T waves at initial presentation.114,123
c. Negative U Waves
The appearance of negative U waves during anginal attack or exercise is known to be a highly specific marker for severe myocardial ischemia in the perfusion territory of the culprit artery. One should confirm whether negative U waves, very small waves following T waves, are present or not during the ischemic attack. Negative U waves distributed primarily around leads V3–5 are highly predictive of significant narrowing of the LAD.124
However, negative U waves appear in patients with other conditions such as elevated blood pressure or aortic regurgitation without myocardial ischemia. Prominent positive U waves distributed primarily around leads V2–3 have also been suggested as reciprocal changes of negative U waves due to posterior wall ischemia.
d. QRS Complex
Myocardial ischemia has been reported to result in slow conduction velocity in ischemic areas. The decreased conduction velocity associated with myocardial ischemia is manifested as QRS prolongation on the surface ECG. QRS prolongation has been shown to be more sensitive than ST-segment changes for the detection of myocardial ischemia. A prolonged QRS duration has also been shown to be a useful predictor of severe CAD such as left main and/or multi-vessel disease.114,125
The presence of abnormal Q waves is helpful to make the diagnosis of prior myocardial infarction.15
However, an isolated Q wave in lead III or a QS complex in lead V1 is seen even in healthy subject.15
Therefore, it is necessary to comprehensively make the diagnosis of myocardial infarction considering clinical features and other diagnostic test results.
e. Differential Diagnosis
ST-T changes are often observed in patients with vasospastic angina, AAD, acute pulmonary thromboembolism, takotsubo syndrome, fulminant myocarditis, or acute pericarditis.15,94–96,114,123,126,127
Also, various circumstances including ventricular hypertrophy, intraventricular conduction disturbance, cardiomyopathy, metabolic disturbance, electrolyte abnormalities, medications such as digitalis, and so on, influence ST-T changes. It is important not to confuse other causes of ST-T changes with ACS by considering clinical features and other diagnostic test results.
1.6 Cardiac Biomarkers (Table 6)
Table 6.
Recommendations and Evidence Level of Biomarkers in the Diagnosis of ACS
| |
COR |
LOE |
Cardiac troponins* should be measured to stratify the early risk for patients with chest
symptoms suggestive of ACS.171,178–184 |
I |
C |
| Biochemistry should be performed immediately.184 |
I |
C |
Cardiac troponin levels should be assessed with the time of arrival as the onset time
for patients with unknown time of onset.171,180,185 |
I |
A |
Measurement of CK-MB or myoglobin is not recommended to diagnose ACS when
cardiac troponins can be measured.186–192 |
III: No
benefit |
A |
Abbreviations: ACS, acute coronary syndrome; CK-MB, creatine kinase MB. *Cardiac troponins: troponin I and troponin T.
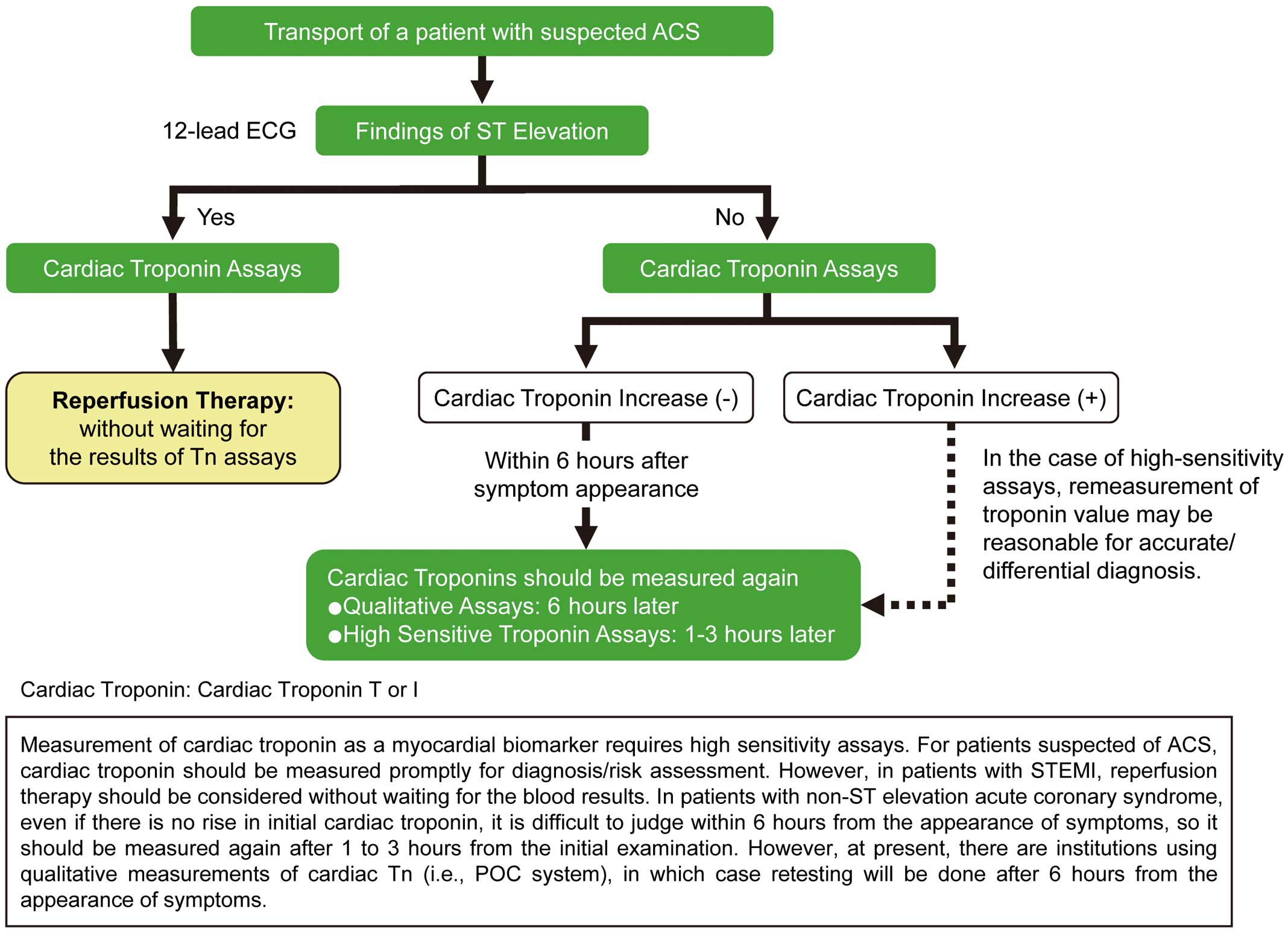
To make a clinical diagnosis of AMI, transient increases in biochemical markers reflecting myocardial necrosis are essential, and either prolonged chest pain or ECG findings suggestive of ischemia are also required. However, increases in cardiac biomarkers are often overlooked immediately after onset. For patients with STEMI diagnosed by ECG or symptoms, reperfusion therapy should be started as early as possible without delay to wait for biochemical marker results (Figure 3).128
Regarding biochemical markers, blood release of cardiac enzymes, including CK, CK-MB, myoglobin, GOT, and LDH, as well as myocardial proteins in AMI has been conventionally known and widely used to diagnose and assess the severity of AMI. During the course from ischemia to myocardial necrosis, the myocardial cell membrane is initially injured, releasing cytoplasmic soluble fraction markers (CK, CK-MB, myoglobin, and heart-type fatty acid-binding protein [H-FABP]) into the circulation. Severe prolonged ischemia involves myofibrillar degradation, resulting in release of cardiac myofibrillar proteins including cardiac troponin T, cardiac troponin I, and myosin light chains. In STEMI, cardiac troponin T, which partly (approximately 6%) exists as a soluble fraction in the cytoplasm, has a bimodal release profile with release from the cytoplasm during early ischemia (first peak at 12 to 18 hours of onset) and release due to myofibrillar necrosis (second peak at 90 to 120 hours of onset), contrary to cardiac troponin I, which has a monomodal release profile.129
CK is the most common traditional marker of myocardial necrosis130,131
and has been widely used for diagnosis and prognostic prediction of myocardial infarction.131
CK-MB, which is myocardium-specific, is of high significance in the assessment of myocardial disorder in view of its ratio to total CK. When the skeletal muscle is damaged by shock, direct currents, etc., both total CK and CK-MB increase; however, skeletal muscle damage can be differentiated from myocardial infarction in that the proportion of CK-MB does not exceed 5%. In STEMI, CK-MB begins to increase within 3 to 8 hours, peaks at 10 to 24 hours, and normalizes 3 to 6 days after onset. The maximum blood CK level, which reflects the amount of myocardial necrosis, is observed earlier with higher levels in patients treated with early reperfusion therapy. However, CK, CK-MB, myoglobin, and other biochemical markers are less sensitive than cardiac troponins, requiring more severe tissue damage for the test to be positive. In contrast, cardiac troponins are highly myocardium-specific and never increase in healthy individuals. An increase in cardiac troponin is defined as >99% of the upper limit of normal, and cardiac troponins can be reliably used to detect myocardial micro-injury involving no increase in CK. In the assessment of ACS based on biochemical markers, simplicity of measurement and quick availability of results (preferably within 30 minutes) are important. Commercially available kits can generate results 10 to 12 minutes after blood collection at bedside (point of care system), and are useful for quick qualitative and quantitative assays of cardiac troponin T. Compared with conventional cardiac troponin assays, more sensitive cardiac troponin assays have been shown to be more accurate and useful for diagnosis of ACS within 2 hours of onset.132–134
A higher cardiac troponin level at arrival indicates higher mortality risk.135–138
The measurement of cardiac troponin in patients with suspected ACS is shown in
Figure 3. However, it should be noted that cardiac troponins increase in non-ischemic myocardial injury, including heart failure, renal failure, myocarditis, acute pulmonary thromboembolism, and sepsis.139
In addition, ACS due to coronary spasm cannot be excluded even in cardiac troponin-negative patients.
Measurement of cardiac troponin as a myocardial biomarker requires high sensitive assays. For patients suspected of ACS, cardiac troponin should be measured promptly for diagnosis/risk assessment. However, in patients with STEMI, reperfusion therapy should be considered without waiting for the blood results. In patients with NSTE-ACS, even if there is no rise in initial cardiac troponin, it is difficult to judge within 6 hours from the appearance of symptoms, so it should be measured again after 1 to 3 hours from the initial examination. However, at present, there are institutions using qualitative measurements of cardiac troponin (i.e., POC system), in which case retesting will be done after 6 hours from the appearance of symptoms.
1.7 Diagnostic Imaging
1.7.1 Chest x-ray (Table 7)
Table 7.
Recommendations and Evidence Level of Chest x-ray in the Diagnosis of ACS
| |
COR |
LOE |
Chest x-ray should be performed for patients with signs or symptoms of cardiac disease (congestive
heart failure, cardiac valvular disease, or ischemic heart disease), pericardial disease, or aortic disease
(acute aortic dissection). |
I |
C |
Chest x-ray should be considered for patients with signs or symptoms of pulmonary/pleural disease or
mediastinal disease. |
IIa |
C |
| Chest x-ray may be considered for all patients with chest pain. |
IIb |
C |
In diagnosis of ACS, chest x-ray is important for making a differential diagnosis and assessing the severity. Patients should be checked for enlargement of the cardiac silhouette, pulmonary congestion, pulmonary edema, and pleural effusion. Enlarged cardiac silhouette reflects LV volume overload associated with previous myocardial infarction, acute left heart failure, pericardial effusion, or aortic or MR. Chest x-ray is used alone to differentiate only a few diseases with chest pain, but is useful for morphological diagnosis of rib disease, airway disease, pulmonary/pleural disease, mediastinal disease, cardiac/pericardial disease, and pulmonary/systemic vascular disease. Diseases that require urgent diagnosis and treatment are acute aortic dissection and acute pulmonary thromboembolism. Ascending aortic dissection is sometimes complicated by AMI due to involvement of a coronary artery, which can make the diagnosis difficult. When chest x-ray reveals an enlarged or double shadow of the superior mediastinum or shifted intimal calcification in the aortic wall, acute aortic dissection should be suspected and differentiated by ultrasonography and contrast computed tomography (CT). When chest x-ray reveals disruption or blockage of the pulmonary artery or focal ischemia, acute pulmonary thromboembolism should be suspected, and ultrasonography and contrast CT should be performed. Acute pulmonary thromboembolism should also be suspected when there are no abnormal chest x-ray findings despite dyspnea or hypoxemia. In chest x-ray assessment, imaging posture and conditions should always be confirmed. In emergency or severe patients, x-rays are often taken in the recumbent position using a portable machine without sufficient cessation of inspiration. It should be kept in mind that chest x-ray findings may be underestimated or overestimated in these conditions.
1.7.2 Echocardiography (Table 8)
Table 8.
Recommendations and Evidence Level of Echocardiography in the Diagnosis of ACS
| |
COR |
LOE |
Echocardiography should be performed to assess the regional wall motion or global LV function for
diagnosis and differentiation. |
I |
C |
| Mechanical complication and LV mural thrombus should be diagnosed by echocardiography. |
I |
C |
| Inferior infarction possibly complicated by RV infarction should be diagnosed by echocardiography. |
I |
C |
Stress echocardiography should be performed for patients in whom ACS cannot be excluded despite lack
of recurrent chest pain, ECG changes, or elevated cardiac troponin. |
IIa |
C |
When chest symptoms are present, echocardiography should be considered for patients with suspected
ACS without ECG abnormality. |
IIa |
C |
Echocardiography should be considered to assess LV function for patients with evidence of ACS in whom
neither coronary angiography nor left ventriculography will be performed. |
IIa |
C |
Abbreviations: LV, left ventricular; RV, right ventricular; ACS, acute coronary syndrome; ECG, electrocardiogram.
Echocardiography is beneficial in the care of patients with chest pain in that it can be repeated in an emergency room (ER) to make a diagnosis on site. Echocardiography can be used for diagnosis of ACS to deduce the culprit coronary lesion, identify the extent and severity of myocardial ischemia, assess LV function, and detect mechanical complications. Echocardiography is also useful to differentiate diseases with chest pain other than myocardial ischemia, including acute aortic dissection, acute pulmonary thromboembolism, pericarditis, aortic stenosis, hypertrophic cardiomyopathy, and fulminant myocarditis.
The culprit coronary artery can be deduced from the site and extent of abnormal wall motion.140
In a study by Horowitz et al, in patients with a clinical diagnosis of myocardial infarction, the diagnostic sensitivity of abnormal LV regional wall motion by echocardiography for myocardial infarction was 94% for, compared with 45% for ECG and 52% for a cardiac biomarker (CK-MB) immediately after onset.141
UA can be diagnosed by abnormal LV wall motion that persists after resolution of chest symptoms or ST-T changes. When urgent CAG reveals multi-vessel disease with chronic total occlusion, the target of reperfusion therapy may be determined based on the wall motion and thickness on echocardiogram. Hypotension immediately after onset of myocardial infarction is primarily attributed to vagotonia, as seen in patients with AMI; in these patients, cardiogenic shock can be excluded when echocardiography shows good anterolateral wall motion without extensive RV infarction or mechanical complication. LV free wall rupture is the most serious mechanical complication and is often rapidly fatal. RV early diastolic collapse with pericardial effusion (echo free space) indicates cardiac tamponade even if effusion is not discernible. Ventricular septal perforration (VSP) can be localized by color Doppler echocardiography as shunt flow. Papillary muscle ischemia with dysfunction or rupture results in mitral regurgitation. In particular, papillary muscle rupture rapidly results in serious heart failure due to mitral regurgitation. The ruptured papillary muscle attached to the chordae tendineae is found as a mobile hyperechoic mass on cross-sectional imaging. Echocardiography is also useful to differentiate ACS from other cardiovascular diseases with chest pain, including acute aortic dissection (ascending or abdominal aortic intimal flap, aortic regurgitation, and pericardial effusion), acute pulmonary thromboembolism (right atrial or ventricular enlargement and LV compression), and acute pericarditis (pericardial effusion without abnormal regional wall motion). For patients with a definitive diagnosis of STEMI, however, reperfusion therapy should not be delayed to perform echocardiography. Non-invasive stress echocardiography should be performed for patients in whom ACS cannot be excluded despite lack of recurrent chest pain, ECG changes, or abnormal cardiac troponin. Gibler et al. reported that 82.1% of emergency patients were safely discharged according to an algorithm using exercise echocardiography.142
Bholasingh et al. evaluated the utility of dobutamine stress echocardiography in low-risk emergency patients with chest pain who had no typical ECG changes and were negative for cardiac troponin T, reporting that the subsequent incidence of cardiovascular events was significantly lower in patients negative for dobutamine stress echocardiography.143
Kang et al. reported that the diagnostic sensitivity and specificity of myocardial contrast echocardiography were 93% and 63% for AMI, and 59% and 96% for unstable angina, respectively, in patients complaining of chest pain on exertion or at rest without evidence of ST-segment elevation or abnormal Q waves on ECG. Myocardial contrast echocardiography was more effective in diagnosing ACS than ECG, cardiac troponins, or abnormal LV wall motion alone.144
Tong et al. reported that myocardial contrast echocardiography in an emergency room predicted the short- and long-term prognosis of patients with chest pain quicker than abnormal biomarkers could be detected.145
1.8 Risk Assessment at Initial Diagnosis (Table 9)
Table 9.
Recommendations and Evidence Level Regarding Risk Assessment at the Time of Initial Diagnosis
| |
COR |
LOE |
Risk assessment should be performed for diagnosis or short-term prognostic assessment using clinical
course, symptoms, vital signs, physical findings, ECG, and biomarkers.60,167,193–195 |
I |
A |
| Risk assessment using risk scores (GRACE, TIMI, etc.) should be performed.64,159,162,196–201 |
I |
A |
| Using a risk score (GRACE, TIMI, etc.) to determine treatment strategy should be considered.64,159,162,197–202 |
IIa |
B |
Abbreviations: ECG, electrocardiogram.
Note: For patients with unknown time of onset, cardiac troponin levels should be assessed with the time of arrival as the onset time.
When patients with chest pain arrive at a hospital, available information (e.g., age, current medical history, past medical history, physical findings, 12-lead ECG, laboratory findings) should be used to determine whether ACS is highly suspected or not. Patients are classified into 4 categories according to the probability of ACS: non-cardiac disease, chronic stable angina, possible ACS, and definite ACS. When definite or probable ACS is diagnosed, it is important for prognostic improvement to stratify the short-term prognosis (cardiac death and non-fatal cardiac event) and promptly treat appropriately. While it is sometimes necessary to determine treatment protocol without waiting for cardiac enzyme results, which are essential for clinical diagnosis of myocardial infarction, it is important to assess the risk quickly based on available information and provide information on predictable prognosis to patients and their families.
1.8.1 Risk Assessment Based on Medical History and Physical Findings
Classification of UA accounting for severity, clinical presentation, and treatment status was proposed by Braunwald in 1989 (Table 10).145
There have been many reports that this classification is useful in predicting the short-term prognosis147,148
and contributes to decision of treatment strategy.105,149
In addition, the classification has been shown to correlate with the severity of coronary angiographic lesions150–152
and complications of PCI. Class II (subacute), Class III (acute), Class B (primary UA), and Class C (post-infarction angina) are considered moderate to high risk. Braunwald et al. pointed out that angina at rest persisting for 20 minutes or more, pulmonary edema complicated by ischemia, angina with third heart sounds or rales, and angina with hypotension had poor short-term prognosis.
Table 10.
Classification of UA
| [Severity] |
Class I: New-onset severe angina or angina with changing pattern
• Angina that occurred in the past 2 months
• At least 3 attacks of angina daily or angina on exertion with changing pattern defined as attacks caused by light exercise.
No angina at rest is observed.
Class II: Subacute angina at rest
• At least 1 attack of angina at rest in the past month, but no attack in 48 hours
Class III: Acute angina at rest
• At least 1 attack of angina at rest in 48 hours |
| [Clinical Presentation] |
Class A: Secondary unstable angina (triggered by non-cardiac factors, including anemia, pyrexia, hypotension, and tachycardia)
Class B: Primary unstable angina (without non-cardiac factors as listed for Class A)
Class C: Post-infarction unstable angina (unstable angina within 2 weeks after the onset of myocardial infarction) |
| [Treatment Status] |
1) Untreated or undergoing minimal treatment for angina
2) Undergoing usual treatment for stable angina (usual doses of β-blockers, long-acting nitrates, or Ca antagonists)
3) Undergoing maximal treatment with antianginal medication, including intravenous nitroglycerin |
Abbreviations: UA, unstable angina. (Source: Prepared based on Braunwald E. 1989146)
The TIMI risk score (https://www.mdcalc.com/timi-risk-score-ua-nstemi), which is often used for risk assessment in patients with NSTE-ACS, is calculated from 7 factors: age (≥65 years); at least 3 coronary risk factors (family history, hypertension, hypercholesterolemia, diabetes mellitus, and smoking); known significant (≥50%) coronary stenosis; ST changes ≥0.5 mm on ECG; at least 2 episodes of angina in 24 hours; aspirin use in the past 7 days; and increased cardiac markers; therefore, most of these factors can be assessed immediately after transport of patients (Table 11). As the score increases, the incidence of major cardiovascular complications in the following 2 weeks increases synergistically.64,153
Since treatment strategy for NSTE-ACS differs depending on the risk, early risk assessment is more important.
Table 11.
TIMI Risk Score for Predicting the Prognosis of NSTE-ACS
| Age ≥65 years |
No 0 |
Yes +1 |
≥3 CAD risk factors
Hypertension, hypercholesterolemia, diabetes >mellitus, family history of CAD,
or current smoker |
No 0 |
Yes +1 |
| Known CAD (stenosis ≥50%) |
No 0 |
Yes +1 |
| Aspirin use in past 7 days |
No 0 |
Yes +1 |
| ≥2 episodes of angina in 24 hours |
No 0 |
Yes +1 |
| ST changes ≥0.5 mm on ECG |
No 0 |
Yes +1 |
| Positive cardiac marker |
No 0 |
Yes +1 |
Abbreviations: CAD, coronary artery disease; ECG, electrocardiogram. (Source: Prepared based on Antman EM, et al. 200064)
Factors influencing the prognosis of STEMI include age, Killip class, time to reperfusion, cardiac arrest, heart rate (tachycardia), systolic blood pressure (hypotension), infarction site (anterior), previous myocardial infarction, diabetes mellitus, smoking status, renal function, sex, and low body weight.37,154–156
Killip classification (Table 3) is convenient in assessment of the severity made primarily based on auscultatory findings and is useful for prediction of the prognosis.81
Cardiogenic shock, classified as Killip Class IV, is the most common cause of in-hospital mortality, with a mortality rate as high as 40% to 70%. However, it has been shown that advances in treatment, primarily early reperfusion therapy, contribute to improvement in survival for patients with shock.63,157,158
Cardiogenic shock associated with myocardial infarction is generally defined as hypotension (<90 mmHg) persisting for at least 30 minutes with signs of peripheral circulatory failure despite adequate LV filling pressure. However, decreased tissue perfusion with blood pressure of 90 mmHg or higher is considered as pre-shock and treatment should be the same as for shock.
The GRACE ACS risk model (https://www.mdcalc.com/grace-acs-risk-mortality-calculator), which is used for overall risk assessment in patients with ACS, including STEMI and NSTE-ACS, is designed to calculate the probability of death and probability of death or myocardial infarction at admission and at 6 months by weighting 8 risk factors: age, heart rate, systolic blood pressure, initial serum creatinine, Killip class, hospitalization due to cardiac arrest, positive cardiac biomarker, and ST-segment deviation (Table 12). This model can be used to stratify the risk (low risk, moderate risk, or high risk) for STEMI and NSTE-ACS separately, and predict the hospital mortality and prognosis at 6 months after being discharged alive for each risk group.65,159
However, no organization in Asia, including Japan, participated in the GRACE study. According to data from the Osaka Acute Coronary Insufficiency Study, the long-term (median, 3.9 years) mortality rate was 2.0%, 6.3%, 11.8%, and 16.8% in patients alive at discharge with GRACE scores of <100, 101 to 120, 121 to 140, and ≥141, respectively.160
Table 12.
GRACE ACS Risk Model
| |
Score |
| Age (years) |
<40 |
0 |
| 40–49 |
18 |
| 50–59 |
36 |
| 60–69 |
55 |
| 70–79 |
73 |
| ≥80 |
91 |
| Heart rate (bpm) |
<70 |
0 |
| 70–89 |
7 |
| 90–109 |
13 |
| 110–149 |
23 |
| 150–199 |
36 |
| ≥200 |
46 |
| Systolic blood pressure (mmHg) |
<80 |
63 |
| 80–99 |
58 |
| 100–119 |
47 |
| 120–139 |
37 |
| 140–159 |
26 |
| 160–199 |
11 |
| ≥200 |
0 |
| Initial serum creatinine (mg/dL) |
0–0.39 |
2 |
| 0.4–0.79 |
5 |
| 0.8–1.19 |
8 |
| 1.2–1.59 |
11 |
| 1.6–1.99 |
14 |
| 2–3.99 |
23 |
| ≥4.0 |
31 |
| Killip class |
Class I |
0 |
| Class II |
21 |
| Class III |
43 |
| Class IV |
64 |
| Hospitalization due to cardiac arrest |
43 |
| Positive cardiac marker |
15 |
| ST-segment deviation |
30 |
(Source: Prepared based on Eagle KA, et al. 2004,159 Granger CB, et al. 2003197)
The TIMI risk score was the first risk score and has been validated most extensively. The score is easy to use in an ER, since all factors in this risk score can be readily assessed based on medical history and examinations in an ER. However, it was reported that the prediction accuracy of this score was lower than that of the GRACE risk score.161
The GRACE risk score is relatively complex to calculate,159,162
but its clinical utility has been verified extensively. The TIMI and GRACE risk scores, which can be calculated through websites on the Internet, are easy to use in emergency care.
1.8.2 Risk Assessment n Based on ECG
The 12-lead ECG plays a central role in diagnostic and triage pathways for ACS and provides important prognostic information.
The presence of Q waves on the presentation ECG was reported to reflect a more advanced stage of infarct evolution. This ECG marker has been reported to be associated with poor clinical outcomes in patients with STEMI treated with PCI as well as fibrinolysis.163,164
QRS score is a quantitative index of myocardial damage calculated not only by the number of Q waves but also by increased Q wave width and decreased R wave amplitude and width.165
QRS score may be a more accurate indicator of the stages of infarct evolution than the mere presence or absence of Q waves. It has been shown that higher QRS score on the presentation ECG is associated with a larger infarct size, and higher long-term mortality in 2,607 patients with STEMI undergoing primary PCI.108
In patients with anterior STEMI, the more proximal the occlusion, the more extensive the area at risk. ST-segment depression in inferior leads, ST-segment elevation in lead aVR, and complete right bundle branch block have been shown to be suggestive of LAD occlusion proximal to the first septal branch. In particular, ST-segment depression in inferior leads is very useful, whereas ST-segment elevation in lead aVR and complete right bundle branch block are shown to have high specificities, but low sensitivities.100,101
In patients with inferior STEMI, those with RV infarction have a poor prognosis. RV infarction during inferior STEMI can be accurately diagnosed by ST-segment elevation ≥1.0 mm (0.1 mV) in the right precordial lead, especially lead V4R.104
However, ST-segment elevation in right precordial leads has been reported to be short lived, disappearing within 10 hours after onset of symptoms in half of patients with inferior AMI and RV involvement.104
ECG diagnosis is often difficult in patients with bundle branch block. Patients with right or left bundle branch block, especially the latter, have a worse clinical profile and poorer prognosis. and therefore, a clinical suspicion of AMI in the presence of bundle branch block is an indication for emergent CAG to perform timely reperfusion therapy.166
In patients with NSTE-ACS, ST-segment depression ≥0.5 mm (0.05 mV) is a strong predictor of poor outcomes.114
The degree, extent, and serial changes of ST-segment depression, not only its presence or absence, can facilitate early risk stratification in patients with NSTE-ACS.114
ST-segment elevation in lead aVR with extensive ST-segment depression is highly suggestive of severe ischemia due to left main or multi-vessel disease.114
In patients with NSTE-ACS, negative T waves are associated with a relatively benign prognosis as compared with ST-segment depression.121
However, it is reported that patients with negative T waves in ≥6 leads have a poor prognosis.115
In addition, negative T waves in precordial leads suggest severe ischemia of the LV anterior wall due to LAD disease.122
QRS prolongation has been shown to be more sensitive than ST-segment changes for the detection of myocardial ischemia. A prolonged QRS duration is associated with severe ischemia in patients with ACS.114
1.8.3 Risk Assessment Based on Cardiac Biomarker (Table 13)
Table 13.
Recommendations and Evidence Level of Risk Assessment by Examination of Blood Biochemistry
| |
COR |
LOE |
Increase and increment in cardiac troponin levels should be used in short- and long-term
prognostic prediction.134,183,203,204 |
I |
B |
Cardiac troponin levels measured 72 to 96 hours after the onset of AMI may be used as measures of
infarction size.192,204 |
IIb |
B |
Measurement of BNP or NT-proBNP may be considered for risk assessment in patients with
suspected ACS.205–210 |
IIb |
B |
Abbreviations: BNP, brain natriuretic peptide; NT-proBNP, N-terminal pro B-type natriuretic peptide; AMI, acute myocardial infarction; ACS, acute coronary syndrome.
It has been reported that the short-term mortality rate of patients seen in an emergency department due to chest pain increased linearly from 1.0% to 7.5% according to the increment in cardiac troponin I despite lack of ST elevation on ECG and normal CK-MB,167–169
and the increase in cardiac troponin T is the most useful factor for 30-day prognostic prediction in patients with ST-T change on ECG and increased serum CK-MB in addition to chest pain.170
It has been reported that the measurement of highly sensitive cardiac troponins is useful in early diagnosis of not only STEMI, but also NSTEMI, with a higher level associated with a higher mortality rate.171
Blood C-reactive protein (CRP) is a marker that reflects acute inflammation, and it has been reported that the incidence of early cardiac events was 3 times higher in UA patients with a CRP level ≥0.3 mg/dL than in those with a CRP level <0.3 mg/dL. CRP attracted attention as a marker of unstable atheroma in coronary artery sclerosis,172
and increases in measures of acute inflammatory reaction in UA were considered to indicate persistent instability or possible recurrence even in asymptomatic patients.173
In the CANTOS study, which evaluated the efficacy of an anti-inflammatory IL-1β inhibitor in previous myocardial infarction patients with a highly sensitive CRP level of ≥2 mg/L, the mortality rates from myocardial infarction, stroke, and cardiovascular disease in the 4-year observation period decreased with decreases in CRP, again highlighting CRP as a useful biomarker. The 2014 ACC/AHA Guidelines for Management of NSTE-ACS stated that B-type natriuretic peptide (BNP) is a new biomarker that may provide prognostic information (Class IIb, level B).175
In addition, hyperglycemia is a strong predictor of mortality and heart failure in non-diabetic patients,176
and renal impairment affects the short- and long-term prognosis.177
Since serum creatinine, which is affected by age and sex, is a limited measure of renal function, creatinine clearance and estimated glomerular filtration rate (eGFR) are used.
For ACS, diagnosis, severity assessment, and prognostic prediction can be made based on medical history, brief medical examination, and other examinations, and it is important to collect medical history and obtain physical examination findings quickly and accurately. However, not a few patients with ACS have atypical or no symptoms. In a US study involving more than 430,000 patients with AMI, 33% had no chest pain at presentation, and the chest pain-free group had a higher proportion of elderly patients (74 years vs. 67 years), female patients (49% vs. 38%), diabetic patients (33% vs. 25%), and patients with history of heart failure (26% vs. 12%) than the chest pain group.116
AMI patients with no chest pain tend to have a longer time to hospital presentation and a delayed diagnosis. As a consequence, fewer patients receive appropriate treatment or reperfusion therapy, with a 2.21 times higher hospital mortality, requiring caution.
Coronary care unit (CCU) care is essential for patients considered to be high risk at initial diagnosis, and moderate-risk patients should be managed accordingly. Low-risk patients may be managed on an outpatient basis. Since assessment only at arrival may fail to detect all high-risk patients, treatment protocol should be determined through symptom monitoring, ECG, and assessment of cardiac markers over time, with change in treatment strategy considered if reassessment reveals increased risk.
2. Initial Therapy
2.1 Oxygen (Table 14)
Table 14.
Recommendations and Evidence Level of Oxygen Administration in Initial Treatment
| General, early stage treatment |
COR |
LOE |
| Oxygen is indicated in patients with hypoxemia (oxygen saturation <90%) or signs of heart failure. |
I |
C |
| Routine oxygen is not recommended in patients with oxygen saturation ≥90%.211–214 |
III: No
benefit |
A |
Guidelines for the management of patients with ST-elevation acute myocardial infarction (JCS 2013) recommend administering oxygen to all STEMI patients for 6 hours after hospital arrival (Class IIa, Evidence level C). That said, the efficacy of routine oxygen administration for AMI patients without hypoxemia was refuted by recent studies.211–214
While oxygen should be given when there are signs of hypoxemia, heart failure, or shock, routine oxygen administration is not recommended. Oxygen saturation should be monitored immediately upon hospital arrival to assess the need for oxygen. Oxygen is not contraindicated when the level of oxygen saturation is unclear. If there is a risk of a hypoxic state occurring after ACS onset, administer oxygen. Further, severe hypoxemia due to a variety of causes can be managed with a ventilator by tracheal intubation. Noninvasive positive-pressure ventilation is reported to be effective for acute cardiogenic pulmonary edema, though a consensus has not been reached regarding its safety and efficacy in AMI patients.
2.2 Nitrates (Table 15)
Table 15.
Recommendations and Evidence Level of Nitric Acid Administration in Initial Treatment
| |
COR |
LOE |
Nitroglycerin sublingually or via oral spray is indicated in patients with chest symptoms due to
myocardial ischemia. |
I |
C |
Nitrates should not be administered in patients with inferior AMI complicated by RV infarction
and those with systolic blood pressure <90 mmHg (or decreased by ≥30 mmHg from baseline),
severe bradycardia (<50 bpm), tachycardia (>100 bpm). |
III:
Harm |
C |
| Nitrates should not be administered for 24 hours after taking erectile dysfunction medication.218 |
III:
Harm |
B |
Abbreviations: AMI, acute myocardial infarction; RV, right ventricular.
Nitrates have the pharmacological action of dilating the venous system, arterial system, and coronary arteries. Dilation of peripheral veins reduces LV preload and volume, and dilation of peripheral arteries reduces blood pressure and afterload, which lowers myocardial oxygen consumption. These drugs are also widely used for dilation of coronary and bypassed arteries, to improve blood flow to ischemic myocardium, and to prevent and reverse coronary vasospasm.
Many studies have found nitrates to be effective for pump failure and postinfarction angina in the acute stage of myocardial infarction. They have also become widely used due to their ability to shrink infarcted areas, prevent remodeling of the LV myocardium, and reduce mortality rates. That said, several large clinical trials in Europe and the United States (ESPRIM,215
GISSI-3,216
ISIS-4217) failed to confirm the ability of nitrates to reduce mortality and refuted their effectiveness. Therefore, while nitrates have been shown to improve prognosis by reducing blood pressure, caution must be exercised so that the use of other drugs with antihypertensive action (β-blockers, ACE inhibitors, etc.) is not hindered.
Patients with ischemic chest discomfort can be given nitroglycerin sublingually or via oral spray. If the symptoms do not markedly improve after administration of 1 nitroglycerin tablet, call an ambulance. Intravenous nitroglycerin is indicated for chest discomfort, to control hypertension and treat pulmonary congestion. Nitrates are indicated during the first 24 to 48 hours in patients experiencing repeated ischemic attacks. However, administration should be avoided in patients with systolic blood pressure <90 mmHg, a decrease of ≥30 mmHg from normal blood pressure, severe bradycardia (<50/bpm), tachycardia (>100/bpm), or suspected acute inferior and RV infarction. Caution is warranted in elderly or dehydrated patients, as nitrates can excessively lower blood pressure. Nitrates are contraindicated within 24 hours after taking erectile dysfunction medication (Viagra®, others), as excessively lowering blood pressure can induce myocardial ischemia or shock.218
2.3 Analgesics (Table 16)
Table 16.
Recommendation and Evidence Level of Analgesia Administration in Initial Treatment
| |
COR |
LOE |
| Morphine hydrochloride is recommended when chest symptoms persist despite treatment with nitrates. |
I |
C |
Persistent chest pain can increase myocardial oxygen consumption, expand the infarct area, and induce arrhythmia. Therefore, prompt analgesia or sedation should be provided. Regardless of whether nitrates were used, morphine hydrochloride can be effective for persistent pain. Further, because morphine hydrochloride is a vasodilator, it is effective for pulmonary congestion, but therefore should not be administered to patients who may have reduced circulating blood volume. If morphine causes blood pressure to decline, elevate the legs to provide fluid loading, but proceed with caution as this can exacerbate pulmonary congestion. Morphine hydrochloride 2–4 mg is given intravenously; if the effect is insufficient, an additional 2–8 mg can be given every 5–15 minutes. However, monitor the respiratory status, fluctuations in blood pressure, and side effects such as vomiting. Use with caution, particularly in inferior AMI, as vagotonia tends to reduce blood pressure in association with vomiting. Intravenous administration of buprenorphine (0.1–0.2 mg) is effective for chest symptoms and diazepam (2.5–5.0 mg) for sedation, but be cautious of respiratory depression.
2.4 Antiplatelet Agents (Table 17)
Table 17.
Recommendation and Evidence Level of Antiplatelet Drug Administration in Initial Treatment
| |
COR |
LOE |
Aspirin (162–200 mg, chewed) is recommended in patients in whom ACS is clinically strongly
suspected.219–225 |
I |
A |
| Thienopyridine antiplatelet drug is recommended when aspirin is contraindicated. |
I |
C |
Aspirin should not be administered to patients with severe blood disorders, aspirin-induced asthma,
or hypersensitivity to aspirin. |
III:
Harm |
C |
Abbreviation: ACS, acute coronary syndrome.
Many trials have shown aspirin to be useful for improving the prognosis of AMI. The large ISIS-2 trial10
found that aspirin alone reduced vascular-related mortality by 23±4%, compared to that when combined with thrombolytic therapy. Administration after the acute stage has also been shown to reduce vascular-related mortality. Studies have shown that the sooner aspirin is administered, the greater the improvement in mortality rate.10,219–225
Therefore, except in patients with severe blood disorders, aspirin-induced asthma, or hypersensitivity to aspirin, aspirin should be given as soon as possible. Even outside hospital, patients can chew 162–200 mg of aspirin to obtain a rapid effect. Aspirin should not be used in patients known to be hypersensitive, and caution is needed in patients with blood diseases or severe liver disorders. Further, while bleeding tendency associated with increased hemorrhagic complications of cardiovascular surgery has been reported, an increase in the reoperation rate was not observed.226
Patients with a history of upper gastrointestinal bleeding who take low-dose aspirin have been found to have higher rates of gastrointestinal complications, including peptic ulcer and bleeding.227
Helicobacter pylori
eradication or proton-pump inhibitor (PPI) administration is effective for preventing recurrence, though guidelines state that bacterial eradication combined with PPI is more effective than eradication alone.228
Administering clopidogrel to STEMI patients who have undergone PCI has been shown to reduce cardiovascular mortality, nonfatal myocardial infarctions, and total mortality, with only a small increase in major bleeding.229,230
In combination with aspirin, loading STEMI patients with 300 mg of clopidogrel prior to PCI, then starting 75 mg/day the next day, has been shown to reduce the risk of cardiovascular events. See chapter
VII section 3.1
for these guidelines on the use of antithrombotic agents during primary PCI.
Clopidogrel has also been shown to be effective in patients who have undergone fibrinolytic therapy and patients who have not undergone reperfusion therapy. Administration of clopidogrel to NSTEMI patients was found to reduce the risk of vascular-related events (cardiovascular death, myocardial infarction, and stroke) compared to that in a control group that received a placebo.231
V. STEMI
1. Primary PCI
1.1 Indications for Primary PCI (Table 18)
Table 18.
Recommendations and Evidence Level of Primary PCI in STEMI
| Indication for primary PCI |
COR |
LOE |
Primary PCI (including stent implantation) should be performed promptly in STEMI patients
within 12 hours after symptom onset.232–234 |
I |
A |
| DES should be used when performing primary PCI.246,247 |
I |
A |
FIbrinolysis should be considered when symptom onset is within 3 hours and the time delay to
perform primary PCI exceeds 1 hour.243 |
I |
B |
Primary PCI should be considered if there is clinical or ECG evidence of ongoing ischemia
within 12 to 24 hours after symptom onset.235,236 |
IIa |
B |
Primary PCI may be considered in patients who have no symptoms and are hemodynamically
and electrophysiologically stable within 12 to 24 hours after symptom onset.235 |
IIb |
B |
Routine primary PCI of non-infarct related artery is not recommended in patients who
are hemodynamically stable.237–239 |
III: No
benefit |
B |
Primary PCI is not recommended in asymptomatic patients ≥24 hours after symptom onset who
are hemodynamically and electrophysiologically stable.245 |
III: No
benefit |
A |
Primary PCI should not be performed in centers that do not meet the standards stipulated by
Ordinance of the Ministry of Health, Labor and Welfare or by unexperienced operators. |
III:
Harm |
C |
Abbreviations: PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction; DES, drug eluting stent; ECG, electrocardiogram.
Reperfusion therapy for STEMI is now widely accepted as an acute treatment of myocardial infarction and its efficacy has been established in patients within 12 hours of symptom onset. It is of importance to restore coronary blood flow promptly and securely without any complications. In treating STEMI, it is important for improved prognosis to establish TIMI 3 reperfusion as soon as possible with fibrinolysis or PCI. PCI is preferred for treating STEMI in current clinical practice in Japan. The use of PCI as the first line for reperfusion without preceding fibrinolysis is called primary PCI.
In principal, PCI should be performed by accredited, skilled operators, certified by the board of a PCI-related Society in experienced centers.
Prognosis of patients with STEMI depends on the time required to establish reperfusion of the infarct-related culprit artery after symptom onset. Primary PCI is considered appropriate reperfusion therapy when it is performed in patients with STEMI within 12 hours of symptom onset by a skilled team and reperfusion is achieved within 90 minutes from the arrival of the patient at the medical institution. A meta-analysis of 23 randomized controlled trials published in the
Lancet
in 2003 reported that primary PCI was superior to fibrinolysis in improving the prognosis of patients with STEMI.232
Primary PCI therefore has become a standard of care.
There are however some circumstances, such as in remote areas and outer islands, when primary PCI is not available as first choice and the use of fibrinolysis is adequate due to long distances to PCI-capable medical centers. In these circumstances, it is recommended to transfer the patient to a PCI-capable medical center after fibrinolysis.240–243
It is crucial in treating STEMI to shorten the total ischemic time, the time from symptom onset to reperfusion. Door to balloon time is widely used as an index of early reperfusion in primary PCI for STEMI and PCI-capable medical centers aim for door to balloon time shorter than 90 minutes. The CREDO-Kyoto AMI Registry, a large-scale observational study of AMI in Japan, however, revealed that long-term clinical outcomes were not significantly different between patients who had a door to balloon time shorter than 90 minutes and those who did not.244
On the other hand, treatment outcomes were favorable in patients who obtained reperfusion early, with a total ischemic time shorter than 3 hours, and got worse as total ischemic time got longer. Of note, a door to balloon time shorter than 90 minutes was associated with favorable long-term clinical outcomes only in patients who presented early to medical institutions, that is, within 2 hours of symptom onset. These results indicate the importance of reduction in total ischemic time, which includes door to balloon time. A door to device time shorter than 90 minutes is a minimum acceptable time, but not a target time. The goal should be to make the time from the diagnosis of STEMI to wire crossing of the lesion shorter than 60 minutes, considering the fact that a shorter total ischemic time to recanalization is associated with more favorable prognosis.
In contrast, primary PCI provides limited benefits in patients with STEMI after 12 hours of symptom onset, who are hemodynamically and electrophysiologically stable and asymptomatic.235,245
Primary PCI with bare metal stents (BMS) is associated with lower incidence of revascularization although it is not associated with a lower mortality rate compared with plain old balloon angioplasty in treating STEMI.246,247
In recent years, drug-eluting stents (DES) have been frequently used in the treatment of STEMI. Many clinical studies have reported that DES and BMS are equivalent with respect to the incidence of death and myocardial infarction, and DES are superior to BMS with respect to the rate of repeat revascularization.248,249
The use of DES is, therefore, more strongly considered when patient characteristics and/or lesion characteristics are potentially associated with a high risk of restenosis. However, bleeding risk due to oral dual antiplatelet therapy (DAPT) and a need for an invasive procedure, e.g. an operation, within several days, should be taken into account before using DES.
With regard to the timing of stent implantation for the infarct-related culprit lesion, a treatment strategy called “deferred” or “delayed” stent implantation was proposed, in which stent implantation was withheld after the initial angioplasty has stabilized the blood flow through the infarct-related culprit lesion. This strategy might reduce the risk of thromboembolism and thereby improve clinical outcomes.
The DANAMI 3-DEFER trial,250
a study that assessed whether delayed stent implantation reduces the risk of impaired myocardial blood flow and improves the clinical course of patients with STEMI compared to conventional PCI, showed no between-group differences in all-cause mortality, heart failure hospitalization, or recurrence of nonfatal myocardial infarction, but confirmed a significantly higher rate of unscheduled target vessel revascularization in the deferred stent group. Routinely delaying stent implantation in patients with STEMI as a treatment strategy is thus considered to have no benefit.
1.2 Primary PCI Without On-Site Cardiac Surgery (Table 19)
Table 19.
Recommendation and Evidence Level of Primary PCI When There Is No Emergency Cardiac Surgery Facility
| Primary PCI without on-site cardiac surgery |
COR |
LOE |
Primary PCI should be considered only when emergency transfer of patients to a facility with cardiac
surgery is available with appropriate hemodynamic assistance (Only in cases primary PCI can be
performed without delay).251 |
IIa |
B |
Abbreviation: PCI, percutaneous coronary intervention.
The performance of primary PCI without on-site cardiac surgery is acceptable only when a skilled operating physician performs primary PCI for STEMI requiring emergency treatment. Off-site cardiac surgical backup is mandatory.
1.3 Primary PCI in Patients With Cardiogenic Shock (Table 20)
Table 20.
Recommendations and Evidence Level of Primary PCI for Patients With Cardiogenic Shock
| Primary PCI in patients with cardiogenic shock |
COR |
LOE |
| Primary PCI should be performed in patients with cardiogenic shock under <75 years of age.158 |
I |
A |
| Primary PCI should be considered in patients with cardiogenic shock ≥75 years of age.252–254 |
IIa |
B |
Abbreviation: PCI, percutaneous coronary intervention.
Emergency revascularization significantly decreased mortality at 6 months and 1 year after the procedure in patients with STEMI complicated by cardiogenic shock and was especially effective in patients under 75 years of age.158
Even in patients who are 75 years of age or older, emergent revascularization improved survival rate if their functional status was good.252–254
Primary PCI is strongly recommended in patients with cardiogenic shock, because prompt revascularization is critical. According to data collected in Japan, however, primary PCI does not improve prognosis in elderly patients presenting with cardiogenic shock after cardiac arrest.255
1.4 Primary PCI in Patients Following Cardiac Arrest and Return of Spontaneous Circulation (Table 21)
Table 21.
Recommendation and Evidence Level of Primary PCI for Patients After Cardiac Arrest With Return of Spontaneous Circulation
| Primary PCI in patients following cardiac arrest and return of spontaneous circulation |
COR |
LOE |
Emergent angiography (and PCI if indicated) should be performed in patients with resuscitated
cardiac arrest with ST-segment elevation or new onset of complete left bundle branch block.256–260 |
I |
B |
Emergent angiography (and PCI if indicated) should be considered in patients with resuscitated
cardiac arrest without diagnostic ST-segment elevation but with a high suspicion of ongoing
myocardial ischemia.256–259,261 |
IIa |
C |
Abbreviation: PCI, percutaneous coronary intervention.
In Japan, approximately 100 thousand people suddenly die outside medical institutions every year, and substantial proportion of these deaths is to ACS. Measures and actions against cardiac arrest attributed to ACS are therefore important issues in emergency care.
Primary PCI in patients with ST-segment elevation that is confirmed on 12-lead ECG following cardiac arrest and the return of spontaneous circulation is recommended from the viewpoint of improvement in prognosis and neurologic outcome.260
In patients without ST-segment elevation, emergent CAG should be considered, taking into account the neurologic sequelae, and PCI, if suitable, should be performed when non-cardiogenic causes are excluded and progressive myocardial ischemia is strongly suspected.256–259,261
It is frequently difficult to diagnose myocardial ischemia accurately from electrocardiography in patients following cardiac arrest and the return of spontaneous circulation. The absence of ST-segment elevation, therefore, does not necessarily exclude acute coronary artery occlusion. The use of emergent CAG in preparation for coronary revascularization should be appropriately determined based on the overall clinical findings in patients following cardiac arrest and the return of spontaneous circulation.
1.5 Thrombus Aspiration (Table 22)
Table 22.
Recommendations and Evidence Level of Thrombectomy at the Time of Primary PCI
| Thrombus aspiration |
COR |
LOE |
Selective or bailout manual aspiration thrombectomy may be considered for patients undergoing
primary PCI.262–264 |
IIb |
C |
| Routine manual aspiration thrombectomy in primary PCI is not recommended.262–264 |
III: No
benefit |
A |
Abbreviation: PCI, percutaneous coronary intervention.
The performance of thrombus aspiration prior to primary PCI may reduce the amount of distally scattered plaque fragments and thrombi and thereby contribute to reduced occurrence of no-reflow phenomenon and improve cardiac function. Multiple clinical studies have suggested that thrombus aspiration may provide more favorable reperfusion and improved prognosis.265
Based on these results, thrombus aspiration was classified as a Class IIa recommendation in both the 2013 AHA/ACC Guidelines and the 2013 JCS Guidelines.
A meta-analysis of 17 studies including the TOTAL study262
and the TASTE study,263
however, showed no effects of thrombus aspiration on death, reinfarction, stent thrombosis, or repeat revascularization.264
The risk of stroke, though not statistically significant, tended to be slightly higher in the thrombus aspiration arm. In addition, unlike in the previous studies, thrombus aspiration showed no effectiveness in treating a large amount of thrombus in the TOTAL study and the TASTE study. Based on these results, there were changes in the 2015 ACC/AHA/SCAI Guidelines such that routine thrombus aspiration was classified as a Class III recommendation and selective or bailout thrombus aspiration was classified as a Class IIb recommendation.
Several randomized clinical studies showed no clear benefit of distal protection devices in reducing infarct area or improving prognosis.266
In the Japanese trial, for the patients who had attenuated plaques of at least 5 mm long visible on intravascular ultrasound, PCI with distal filter protection and thrombus aspiration reduced the incidence of no reflow during PCI.267
1.6 Access Route in Performing Primary PCI (Table 23)
Table 23.
Recommendation and Evidence Level Regarding Access Route for Primary PCI
| Access route in performing primary PCI |
COR |
LOE |
For operators experienced in radial artery approach, radial artery approach should be performed
rather than femoral artery approach.268–271 |
I |
A |
Abbreviation: PCI, percutaneous coronary intervention.
Several studies have recently reported favorable results of radial artery access in PCI for ACS patients performed by operators experienced in radial artery access.
Radial artery access was associated with reduced risks of hemorrhage, vascular complications, the need for transfusion, and death in all of the studies including the MATRIX study,268
RIVAL study269
enrolling patients with ACS, and RIFLE-STEACS study270
enrolling patients with STEMI.
In Japan, radial artery access in STEMI patients without cardiogenic shock was reportedly associated with reduced time to reperfusion, puncture site complications, and 30-day mortality.271
Radial artery access in STEMI patients with cardiogenic shock was associated with reduced vascular complications, but not with reduced mortality.
The use of the radial artery is not appropriate in a dialysis patient, and caution should be paid in the use of the radial artery in a chronic kidney disease (CKD) patient with the possibility of future dialysis.
1.7 Antithrombotic Therapy in Primary PCI
1.7.1 Antiplatelet Therapy
a. Aspirin, Thienopyridine Antiplatelet Drugs (Tables 24,25)
Table 24.
Recommendations and Evidence Level of Aspirin Administration at the Time of Primary PCI
| Aspirin |
COR |
LOE |
Aspirin 162–325 mg should be administered before primary PCI (Chewing is necessary for
enteric-coated formulation).272–274 |
I |
A |
| Aspirin 81–162 mg/day should be continued indefinitely after PCI if not contraindicated.275–277 |
I |
A |
Abbreviation: PCI, percutaneous coronary intervention.
Table 25.
Recommendations and Evidence Level of Thienopyridine Antiplatelet Drug Administration at the Time of Primary PCI
| Thienopyridine antiplatelet drugs |
COR |
LOE |
| Clopidogrel 300 mg should be administered before primary PCI and 75 mg/day should be continued.281 |
I |
A |
| Prasugrel 20 mg should be administered before primary PCI and 3.75 mg/day should be continued.282,283 |
I |
A |
Cilostazol may be considered in patients with contraindication to taking aspirin or thienopyridine antiplatelet
drugs.278 |
IIb |
C |
Abbreviation: PCI, percutaneous coronary intervention.
Stents are usually implanted in primary PCI. Appropriate administration of antiplatelet drugs is important for preventing stent thrombosis. The release of adenosine diphosphate (ADP) from platelets in response to stimuli from the surrounding environment plays an important role in thrombogenesis. ADP released from platelets causes platelet aggregation through binding to the P2Y12
ADP receptor on the cell membrane of platelets. Prasugrel and clopidogrel are called thienopyridine antiplatelet drugs and inhibit the binding of ADP to the P2Y12
ADP receptor, thereby suppressing platelet aggregation and thrombogenesis. The start of the administration of a thienopyridine antiplatelet agent in combination with aspirin (DAPT) before stent implantation for the prevention of stent thrombosis has been proven to suppress the occurrence of stent thrombosis and has become standard care after stent implantation.279
Aspirin suppresses platelet aggregation through blockage of the production of thromboxane A2
by inhibiting cyclooxygenase.
Antiplatelet drugs should be adequately acting at the time of stent implantation. This is because stent thrombosis following stent implantation is likely to develop within 24 hours after the procedure. The research on the current status of PCI in patients with AMI in Japan revealed that stent thrombosis mostly developed within 10 days, especially within 1 day, after stent implantation.280
Emergent PCI in patients with ACS must often be performed before antiplatelet drugs adequately exert their effects. The pathological condition of ACS in which thrombi are present at the lesion also increases the risk of stent thrombosis. In order to reduce the risk of stent thrombosis, the loading doses of aspirin and ADP P2Y12
receptor antagonists should be administered in preparation for coronary revascularization with PCI when the conduct of emergency catheterization is decided upon. For facilitating aspirin absorption, it is recommended that patients chew aspirin at a dose of 162 to 325 mg.
In the past, ticlopidine at a dose of approximately 200 mg/day was administered. The use of ticlopidine was, however, reportedly associated with, though infrequently, side effects, such as leukopenia, severe liver dysfunction, or thrombotic thrombocytopenic purpura. Clopidogrel is widely used at present. The administration of clopidogrel at a loading dose of 300 mg before PCI promptly exerts its effects and is highly effective in preventing stent thrombosis. Following stent implantation, it is recommended to administer clopidogrel 75 mg/day and concomitant aspirin orally. Administration of these drugs is recommended for 1 month in cases of bare metal stents and for at least 6 months to 1 year in cases of drug-eluting stents.281
Prasugrel is a third generation thienopyridine antiplatelet drug. Compared with clopidogrel, prasugrel has a simple metabolic pathway, exerts its effects promptly, and is less affected by CYP2C19 polymorphism and there is hence little difference in efficacy between individuals. In the TRITON-TIMI 38 trial, a large clinical trial conducted in the United States and Europe to obtain approvals, there were fewer thrombotic events in the prasugrel group than in the clopidogrel group.282
In the United States and Europe, a loading dose of 60 mg and a maintenance dose of 10 mg are approved for prasugrel. In Japan, a loading dose of 20 mg and a maintenance dose of 3.75 mg are approved for prasugrel, which is one-third of the doses approved in the United States and Europe.283
Prasugrel, which promptly exerts its antiplatelet effects, has an important role in preventing stent thrombosis in patients with ACS.
In patients who underwent primary PCI, cilostazol, an antiplatelet drug that inhibits phosphodiesterase III, was as effective as ticlopidine.278
However, conversely, it was associated with frequent stent thrombosis when compared with ticlopidine after stent implantation.284
b. Other Antiplatelet Drugs
One of the antiplatelet drugs that is unavailable in Japan is platelet membrane glycoprotein IIb/IIIa inhibitor, because it failed to demonstrate efficacy. It is a potent platelet aggregation inhibitor, which inhibits the binding of fibrinogen and thereby inhibits platelet aggregation. In countries other than Japan, a large clinical study demonstrated that platelet membrane glycoprotein IIb/IIIa inhibitor was effective in stabilizing angina pectoris in the short term and improving the early outcomes of revascularization in patients with unstable angina on aspirin and heparin. The use of the inhibitor, however, has not been approved in Japan, because no clinical studies conducted in Japan have demonstrated efficacy.285
c. Duration of DAPT
First generation DESs had a problem that the DESs were associated with a higher rate of stent thrombosis beyond 1 year after implantation compared with BMSs and therefore required prolonged DAPT. The development of second generation and third generation DESs since 2009 has decreased the incidence of stent thrombosis and has shortened the duration of DAPT. There is no definite conclusion on the duration of DAPT to prevent stent thrombosis. It is a matter of a trade-off between thrombosis prophylaxis and hemorrhagic complications, because prolonged DAPT would increase the incidence of hemorrhagic complications.
Patients with atrial fibrillation who are on oral anticoagulants need to continue to take anticoagulants even after they suffered from ACS. There is a concern about bleeding risk when patients who require long-term oral anticoagulation therapy, such as warfarin therapy, start to receive antiplatelet therapy additionally in preparation for PCI. With regard to this concern, the WOEST study was conducted.286
It showed that the risks of bleeding as well as all-cause mortality and cardiovascular events were lower in the group receiving anticoagulants plus clopidogrel only than in the group receiving anticoagulants plus the dual antiplatelets of aspirin and clopidogrel. On the other hand, there was no difference in the risk of stent thrombosis between the groups. No optimal antithrombotic therapy has been established in patients on oral anticoagulants after PCI. It is, however, desirable to reduce the duration of concomitant administration of 3 antithrombotic agents consisting of warfarin, aspirin, and clopidogrel as much as possible. For details, refer to chapter
VII. Evaluation and Management During Hospitalization (3. Pharmacological Therapy, 3.1 Antithrombotic Therapy).
1.7.2 Anticoagulant Therapy
a. Unfractionated Heparin (Table 26)
Table 26.
Recommendations and Evidence Level of Unfractionated Heparin Administration at the Time of Primary PCI
| Unfractionated heparin |
COR |
LOE |
70–100 IU/kg intravenous bolus of UFH adjusted to obtain ACT ≥250 seconds should be administered in
addition to aspirin during primary PCI.287,288 |
I |
C |
| Measurements of platelet count should be performed for prediction and diagnosis of HIT. |
I |
C |
Additional use of UFH under APTT monitoring should be considered in patients treated with tPA,
pro-UK or mutant tPA. |
IIa |
C |
Abbreviations: UFH, unfractionated heparin; PCI, percutaneous coronary intervention; HIT, heparin-induced thrombocytopenia; APTT, activated partial thromboplastin time; pro-UK, prourokinase; tPA, tissue plasminogen activator.
There is established evidence from the pre-reperfusion era that unfractionated heparin (UFH) is effective in treatment of patients with ACS. Now, PCI is usually performed in the acute phase and UFH is generally used during PCI. In Japan, there is no study that has assessed the dose of UFH during primary PCI. The 2013 ACC/AHA Guidelines recommend a bolus injection of intravenous UFH at a dose of 70 to 100 units/kg and maintenance of activated coagulation time (ACT) at 250 seconds or longer.58
A small study involving patients with ACS confirmed no efficacy of treatment with heparin alone, and concomitant administration of aspirin and heparin is therefore recommended.288
The anticoagulation effect of heparin differs largely between individuals. ACT or activated partial thromboplastin time (APTT) should therefore be monitored. Sudden discontinuation of heparin may activate thrombin and thereby cause thrombogenicity.289,290
Tapering is recommended before discontinuation of heparin therapy.
Heparin-induced thrombocytopenia (HIT) develops at a rate of approximately 3%.294
Patients with a platelet count of less than 100,000 should be treated with caution. HIT is classified into type I and type II. Type I HIT is caused by a non-immune mechanism. In Type II HIT, heparin-dependent antibodies are produced. Type I HIT causes a transient thrombocytopenia (a decrease by 10 to 20%) attributed to the physical and biological characteristics of heparin itself, which occurs within the first few days of heparin administration and resolves even if heparin therapy is continued. In Type II HIT, a fall in platelet count to less than 50% of baseline level occurs after 5 to 14 days of commencement of heparin due mainly to the production of anti-PF4/heparin complex antibodies (HIT antibodies). Type II HIT may be complicated by serious arteriovenous thrombosis. Treatment with heparin needs to be promptly switched to anticoagulation therapy with argatroban. It is reported that low molecular weight heparin, compared with unfractionated heparin, is similarly or more effective in increasing reperfusion rate and decreasing the rates of reinfarction and mortality292–295
and does not increase the risk of hemorrhagic complications in patients with STEMI.296,297
In Japan, the use of low molecular weight heparin is not approved for PCI in patients with STEMI.
b. Antithrombin Agent (Argatroban) (Table 27)
Table 27.
Recommendation and Evidence Level of Antithrombin (Argatroban) Administration at the Time of Primary PCI
| Antithrombin agent (argatroban) |
COR |
LOE |
| Argatroban should be administered in patients with HIT.299 |
I |
B |
Abbreviation: HIT, heparin-induced thrombocytopenia.
Argatroban is the only antithrombin agent that can be used intravenously instead of UFH in Japan. In the United States, argatroban has been shown to be as efficacious as UFH when used concomitantly with fibrinolysis and is also used for PCI in patients with ACS complicated by HIT.298,299
In Japan, argatroban was initially indicated for the prevention of thrombosis in patients with HIT and was additionally approved in 2011 as an anticoagulant for PCI in patients with or at risk of HIT.
Argatroban is intravenously administered at a dose of 0.1 mg/kg for 3 to 5 minutes when starting PCI and is continued at a dose of approximately 6 μg/kg/minute until 4 hours after the procedure. ACT is measured at approximately 10 minutes after the start of administration. The continuous dose is adjusted to maintain an ACT between 250 and 450 seconds until 4 hours after the procedure.300
Whether argatroban needs to be continued beyond 4 hours after the procedure is determined depending on patient condition. When continuing, reduce the dose to 0.7 μg/kg/minute, measure APTT as required, and adjust the dose to maintain APTT at approximately 1.5–3 times the baseline level (discontinue administration temporary if a measured APTT value is more than 3 times the baseline level). The above-mentioned continuous doses are only rough estimates and thus need to be properly adjusted with appropriate monitoring, considering bleeding risk. This agent is metabolized in the liver and therefore requires dose reduction in patients with hepatic dysfunction (if continuous administration is needed beyond 4 hours after the procedure in patients with liver dysfunction, the dose should be decreased to 0.2 μg/kg/minute).
1.8 Coronary Revascularization for Residual Lesions
1.8.1 Indication for and Timing of Coronary Revascularization
It is not rare that patients with STEMI have multivessel disease. Approximately 50% of patients with STEMI reportedly have multivessel disease.301,302
Prognosis is worse in patients with STEMI when they have multivessel disease. It is therefore important to consider coronary revascularization for residual CAD. There is, however, no clear evidence for an appropriate timing of revascularization for residual coronary artery lesion and an appropriate timing of cardiac stress test to determine the indication for revascularization in patients with STEMI.
1.8.2 PCI of Residual Lesions (Table 28)
Table 28.
Recommendations and Evidence Level of PCI Targeting Residual Lesions
| PCI of non-infarct related artery |
COR |
LOE |
PCI of non-infarct related artery should be performed during the same hospitalization in patients
with multivessel disease who have ongoing ischemic symptoms.303,305 |
I |
B |
Staged PCI of non-infarct related artery should be considered in patients with multivessel disease
during the same hospitalization.301,305–311 |
IIa |
A |
Abbreviation: PCI, percutaneous coronary intervention.
Previous research on PCI for residual CAD varied in terms of randomization/non-randomization and the timing of PCI. This may have contributed to the inconsistency of previous research, although performing primary PCI of the infarct-related artery only and staged PCI for residual non-infarct-related artery later was considered to reduce the incidence of adverse events.237–239,311a–311d
There are few randomized trials that may resolve the discrepancy between these arguments. In one randomized trial, 214 subjects with STEMI and multivessel disease were assigned to either of the following 3 arms: arm 1 consisted of subjects who underwent revascularization of the infarct-related culprit coronary lesion only; arm 2 consisted of subjects who underwent simultaneous revascularization for the infarct-related culprit coronary lesion and residual CAD, and arm 3 consisted of subjects who underwent revascularization with staged PCI for residual CAD. The rate of major cardiovascular events during the mean observation period of 2.5 years was significantly higher in the “revascularization of the infarct-related culprit coronary lesion only” arm.301
Following this trial, 5 randomized trials were conducted to compare PCI of the infarct-related culprit lesion only to complete revascularization: the PRAMI trial (n=465, observation period: 23 months),302
Compare-Acute trial (n=885, observation period: 12 months),303
and TRANSLATE-ACS Observational trial (n=6,601, observation period: 12 months),304
in which PCI for residual CAD was simultaneously performed with index PCI; the DANAMI-3-PRIMULTI trial (n=627, observation period: 27 months),305
in which staged PCI was performed during hospitalization; and the CvLPRIT trial (n=296, observation period: 12 months),306
in which immediate PCI or staged PCI was performed during hospitalization. Residual coronary artery lesion with angiographic stenosis of 50% or greater and that with stenosis of 70% or greater were considered indications for PCI in the PRAMI trial and in the CvLPRIT trial, respectively. Fractional flow reserve (FFR) was used to determine indications for PCI in the other 2 trials. The rate of major events was significantly lower in the complete revascularization group in each trial, although composite endpoints differed between trials. There was no significant difference between groups in all-cause mortality in all trials. The rate of repeat revascularization was lower in the complete revascularization group in the PRAMI, DANAMI-3-PRIMULTI, and Compare-Acute trials. In the TRANSLATE-ACS Observational trial, readmission rate within 6 weeks was lower in the complete revascularization group, but there were no between-group differences in the incidence of readmission or angina pectoris within 1 year. In addition, 3 meta-analyses demonstrated no effects of PCI for residual lesion on the rate of death or myocardial infarction.307–309
In Japan, no randomized controlled studies on PCI for residual lesion have been conducted. There is however a report on the analyses of an observational study, the CREDO-Kyoto AMI Registry.310
This observational study compared the following 2 groups: a group of patients with STEMI who received staged PCI of lesions in non-infarct-related arteries remaining after primary PCI (Staged PCI group); and the other group of patients with STEMI who received treatment of the infarct-related culprit lesion only (Culprit-only PCI group). All-cause mortality during the observation period of 5 years was significantly lower and prognosis was better in the Staged PCI group (9.5% vs. 16.0%; P<0.001; HR: 0.69; 95% CI: 0.50–0.96; P=0.03).
It is often difficult to determine the timing of PCI for lesions in non-infarct-related arteries in AMI patients with multivessel disease complicated by cardiogenic shock. The CULPRIT-SHOCK trial randomized 706 AMI patients with multivessel disease complicated by cardiogenic shock to either of the following groups and assessed the composite endpoint of death or severe renal failure requiring renal replacement therapy within 30 days of randomization: a group of patients that simultaneously underwent PCI of the culprit lesion and all coronary arterial lesions with stenoses of 70% or greater (n=355); and the other group of patients that first underwent PCI of the culprit lesion only and then staged PCI if non-invasive stress test or FFR indicated PCI (n=351).311
The results showed that the risk of the composite endpoint of death or severe renal failure leading to renal replacement therapy within 30 days after randomization was lower in the “culprit lesion only PCI plus optional staged PCI” group than in the “simultaneous residual multi-lesion PCI” group.
Simultaneous PCI of severe stenoses in non-infarct-related arteries perfusing viable myocardium in acute phase may reduce the hibernation of those areas and the occurrence of ischemic attack, thereby promoting early recovery of cardiac function. On the other hand, a prolonged time, increased complexity of the procedure and consequently increased amount of contrast media may cause contrast nephropathy. There is also another idea that PCI of residual lesions should be performed after the stabilization of plaques unless ischemic attack occurs, since simultaneous PCI in the acute phase when plaques are fragile may cause side branch occlusion or distal occlusion. Clear evidence is lacking to support the use of simultaneous PCI in emergency patients with cardiogenic shock. It is fundamental to determine a treatment strategy based on the advantages and disadvantages to individual patients and estimation of the success rate of each procedure.
2. Fibrinolysis
2.1 Indications for Fibrinolysis (Table 29)
Table 29.
Recommendations and Evidence Level of Fibrinolysis in STEMI
| Indications for fibrinolysis |
COR |
LOE |
Fibrinolysis should be administered in patients within 12 hours of symptom onset if primary PCI cannot
be performed within 120 minutes from first medical contact.10,313–318 |
I |
A |
When PCI is not available, fibrinolysis should be considered in patients with STEMI if there is clinical
or ECG evidence of ongoing ischemia within 12 to 24 hours of symptom onset and if myocardial
ischemia is extensive or the patient is hemodynamically unstable. |
IIa |
C |
| A half-dose of fibrinolytic agent should be considered in patients 75 years of age or older.320 |
IIa |
B |
Fibrinolysis should not be administered in patients without ST-segment elevation (excluding posterior
wall infarction).3,117,322–324 |
III:
Harm |
B |
Abbreviations: PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction; ECG, electrocardiogram.
In Japan, where there are many PCI-capable medical centers compared to foreign countries, fibrinolysis is performed in less than 10% of patients with STEMI who receive reperfusion therapy.22,312
Fibrinolysis has an established effect in patients with STEMI or myocardial infarction with bundle branch block. The earlier the reperfusion with fibrinolysis in patients within 12 hours of symptom onset, the lower the mortality and rate of complications.10,313–318
The use of fibrinolysis should be considered in STEMI patients who cannot be promptly transferred to a PCI-capable medical center. Fibrinolysis is recommended in STEMI patients within 12 hours of symptom onset when they cannot be treated with primary PCI within 2 hours of the diagnosis. The effect of fibrinolysis decreases as time from onset increases. The use of primary PCI should be considered especially more than 3 hours after onset.319–321
2.2 Contraindications to Fibrinolysis
Fibrinolysis is not contraindicated in patients after brief and successful cardiopulmonary resuscitation. Fibrinolysis will not be effective or may even increase bleeding risk in patients with repeat cardiac arrest. Fibrinolysis increases bleeding risk in patients after prolonged successful cardiopulmonary resuscitation and is therefore relatively contraindicated in such patients.
Absolute and relative contraindications to fibrinolysis are listed below.
Absolute Contraindications:
1. Previous intracranial hemorrhage
2. Cerebral infarction within the past 6 months
3. Intracranial neoplasm or arteriovenous malformation
4. Recent major trauma, surgery, or head trauma
5. Gastrointestinal bleeding within the past month
6. Active bleeding
7. Known or suspected aortic dissection
Relative Contraindications:
1. Previous cerebrovascular disorder not included in absolute contraindications
2. Ongoing anticoagulant therapy
3. Pregnancy or within 1 month postpartum
4. Uncontrolled severe hypertension (blood pressure ≥180/110 mmHg)
5. Advanced liver disease
6. Active peptic ulcer
7. Prolonged cardiopulmonary resuscitation
2.3 PCI Following Fibrinolysis (Table 30)
Table 30.
Recommendations and Evidence Level of PCI After Fibrinolysis
| PCI following fibrinolysis |
COR |
LOE |
PCI should be performed in patients with significant stenosis suitable for PCI in the infarct-related
artery and cardiogenic shock or acute severe heart failure.252 |
I |
B |
PCI before discharge should be performed in patients with significant stenosis suitable for PCI
in the infarct-related artery and myocardial ischemia.325,326 |
I |
C |
PCI should be considered in patients with evidence of failed reperfusion or reocclusion after
fibrinolysis.327–330 |
IIa |
B |
PCI for significant stenosis in the infarct-related artery may be considered in stable patients after
24 hours after symptom onset as one of the invasive strategies.245,325,326,335–340 |
IIb |
B |
PCI for significant stenosis in the infarct-related artery is not recommended in stable patients
within the first 2 to 3 hours after fibrinolysis.240,241,331–334 |
III: No
benefit |
B |
Abbreviation: PCI, percutaneous coronary intervention.
Approaches to PCI after fibrinolysis have traditionally been classified into facilitated PCI and rescue PCI. Facilitated PCI is defined as planned PCI that is performed shortly after initiating fibrinolysis in preparation for PCI. Rescue PCI is defined as PCI that is performed after failed fibrinolysis or following fibrinolysis for some reasons. The distinction between these 2 approaches is currently considered of no importance in selecting treatment options for STEMI. This guideline describes PCI following fibrinolysis without distinguishing between them. The success of recanalization should be verified in patients after fibrinolysis when primary PCI is not available.
Systematic PCI, in which patients are transferred to PCI-capable medical centers after fibrinolysis and undergo angiography and PCI, is reportedly associated with favorable treatment outcomes compared to fibrinolysis alone, although fibrinolysis is recently performed less frequently in medical centers where PCI is unavailable.240,241
Some reports demonstrate no differences in mortality rate between systematic PCI and primary PCI. It is thus important to transfer patients to PCI-capable medical centers after performing fibrinolysis in order to assess whether such patients are at high risk and have indications for systematic PCI.240–243
3. Emergent CABG (Table 31)
Table 31.
Recommendation and Evidence Level of Emergent CABG in STEMI
| |
COR |
LOE |
For patients with failed PCI or technical difficulty, persistent ischemic episodes and hemodynamic
instability refractory to medical treatment (cardiogenic shock or life-threatening arrhythmias due to
myocardial ischemia), emergent CABG should be discussed in the heart team.252,341,342,355–358 |
I |
C |
Abbreviations: CABG, coronary artery bypass grafting; PCI, percutaneous coronary intervention.
Prompt reperfusion is the priority in treating STEMI patients. Since PCI can be performed faster than CABG in many cases, there are few instances where CABG is selected as the reperfusion therapy. Emergent CABG is indicated, if distal site of the infarct-related artery is considered to be suitable for CABG, the lesion is anatomically inappropriate for PCI, PCI is unsuccessful, or there is a complication such as coronary artery perforation during PCI. However, with recent advances in PCI techniques, there are few cases in which emergent CABG is required.341–343
The in-hospital mortality rate of emergent CABG is higher than that of urgent and elective CABG,341,344,350
and increases as the patient’s condition deteriorates.351
Although there is no definite consensus on the optimal timing for CABG after onset of STEMI, patients who underwent emergent CABG in the acute period within 48 hours had poor outcomes, and there are reports that outcomes are the same as for elective CABG performed at a later time.350,352
However, CABG aiming for early reperfusion should be performed emergently. On the other hand, good outcomes have been reported for early CABG in hemodynamically stable STEMI patients, and the CABG indication criteria for NSTEMI or stable angina can also be applied.353,354
For patients requiring urgent surgical repair of mechanical complications after STEMI including VSP, left ventricular free wall rupture (LVFWR) or papillary muscle rupture (PMR) with acute severe mitral regurgitation, consider performing CABG at the same time.358–360
4. Assessment of Reperfusion
In treatment with fibrinolysis, patency of the infarct-related artery has been assessed by non-invasive markers, including symptom relief, hemodynamic/electrical stabilization, and resolution of ST-segment elevation with ECG.361
If successful reperfusion is achieved, significant increases in serum concentrations of CK and CK-MB fraction are observed to 5–10 times higher than baseline at 6–90 minutes after initiation of fibrinolysis.362
If those findings are not observed, failure to achieve successful reperfusion of the infarct-related artery can be predicted. Some reports also demonstrated the usefulness of transthoracic Doppler echocardiography for evaluation of distal LAD reperfusion.363
In patients undergoing primary PCI, coronary flow can be assessed by CAG. TIMI flow grade classifies antegrade coronary flow,364
and no reflow and delayed flow with TIMI flow grade ≤2 are associated with poorer prognosis. TIMI frame count, which is obtained by counting the number of cineframes from the first frame in which dye fully enters the artery to the last frame in which dye first enters the distal landmark branch, can assess coronary flow more objectively.365
Furthermore, only recovery of flow in epicardial coronary artery is not enough in STEMI patients, but it is also important to obtain recovery of microcirculation in myocardium. In fact, a previous report demonstrated that about a quarter of cases showed a residual contrast defect in the at-risk area on myocardial contrast echocardiography, even after obtaining TIMI 3 flow. This finding is considered to be myocardial no reflow phenomenon, which is associated with poor functional recovery of the postischemic myocardium.366
Myocardial blush grade, which assesses myocardial perfusion by myocardial blush or contrast density, is well correlated with findings of myocardial contrast echocardiography, and is considered as an independent prognostic factor from coronary flow.367,368
Another way to assess microcirculation is measurement of coronary flow velocity pattern using Doppler guidewire. Microvascular injury is determined by the presence of systolic flow reversal, and rapid deceleration of diastolic flow, and the assessment could predict clinical outcomes and recovery of LV function.369
Assessment of ST-segment elevation resolution on ECG is a useful non-invasive tool to estimate coronary microvascular dysfunction and obstruction. Poor ST-segment elevation resolution has been shown to be associated with microvascular dysfunction, larger infarct size, and worse clinical outcomes, compared to complete ST-segment elevation resolution.370,371
Cardiac magnetic resonance with delayed enhancement is also a useful tool to detect microvascular dysfunction and obstruction.372
5. Adjuncts to Reperfusion Therapy
In this section, adjunctive treatments to reperfusion therapy are described, and refer to other sections about antithrombotic agents.
5.1 Carperitide (Table 32)
Table 32.
Recommendation and Evidence Level of Carperitide Administration as Adjunctive Therapy
| |
COR |
LOE |
The administration of intravenous carperitide may be considered for patients undergoing primary
PCI within 12 hours after symptom onset.373–375 |
IIb |
B |
Abbreviation: PCI, percutaneous coronary intervention.
Carperitide (Atrial natriuretic peptide) causes vasodilation and diuresis, and has been widely used for acute heart failure. For acute myocardial infarction patients, it has been reported that carperitide improves cardiac sympathetic nerve activity, inhibits the renin angiotensin aldosterone system, and prevents LV remodeling.373,374
J-WIND-ANP assessed the effect of carperitide on infarct size and cardiovascular outcome in 569 acute myocardial infarction patients undergoing reperfusion treatment.375
In the results, a reduction of 14.7% in infarct size, an increase in 5.1% left ventricular ejection fraction (LVEF) at chronic phase, and a reduction in findings of reperfusion injury (including ventricular arrhythmia, ST re-elevation, and worsening chest pain at reperfusion), were observed in the carperitide group when compared to the control group. In addition, the rates of cardiac death and hospitalization due to heart failure were lower in carperitide group, suggesting the effectiveness of carperitide on long-term prognosis.
Initiation of intravenous carperitide at 0.025 µg/kg/min before reperfusion and continuing for 3 days may be considered. However, much surveillance is necessary for complications such as a decrease in blood pressure when using carperitide.
5.2 Nicorandil (Table 33)
Table 33.
Recommendation and Evidence Level of Nicorandil Administration as Adjunctive Therapy
| |
COR |
LOE |
The administration of intravenous nicorandil may be considered for patients undergoing primary PCI
within 12 hours after symptom onset to improve coronary microvascular circulation.376–383 |
IIb |
B |
Abbreviation: PCI, percutaneous coronary intervention.
Nicorandil is a hybrid compound of an ATP-sensitive potassium channel opener and a nitric oxide donor. Prior studies have reported that nicorandil as an adjunct to primary PCI improves microvascular circulation and cardiac function in the chronic phase. Although mainly small-scale, a number of studies including Japanese studies have been published,376–379
and meta-analyses have also demonstrated the efficacy.380,381
Ishii et al. compared outcomes among 185 patients who received administration of 12 mg intravenous nicorandil for 30 minutes initiated before primary PCI and 183 patients with placebo. As the result, the nicorandil group had a better TIMI frame count and ST resolution, indicating the effectiveness for improving microvascular dysfunction. Furthermore, the effects of nicorandil on long-term prognosis were demonstrated with lower incidences of cardiovascular death and hospitalization due to heart failure during follow-up (median 2.4 years).377
On the other hand, J-WIND-KAPT did not show any effect of nicorandil on infarct size or prognosis when comparing 276 patients who received bolus intravenous administration of 0.067 mg/kg nicorandil followed by continuous administration of 1.67 µg/kg/min for 24 hours, with 269 patients who received the same dose of placebo.375
The fact that the dose of nicorandil used in the latter trial was only about one-third of the former may be the reason for the difference in results between the two trials.377
Superiority of a higher dose of nicorandil before reperfusion has been also reported,382
therefore, it is important to use sufficient dose before reperfusion. In the Korean acute myocardial infarction registry (nicorandil was used for 1,313 STEMI patients among 6,370 patients), and an improvement effect of nicorandil on prognosis was observed among STEMI patients, but not among NSTEMI patients.383
5.3 Remote Ischemic Conditioning
Remote ischemic conditioning (RIC) is the concept that reversible episodes of ischemia and reperfusion in one vascular bed, tissue, or organ can bring tolerance to ischemia and reperfusion injury in another remote organ.384
Recently, many randomized trials, applying this phenomenon in a clinical setting, have demonstrated this effect as inhibition of reperfusion injury in STEMI patients.385–392
Generally, RIC is performed as about 4 cycles of 5-minute inflation to 200 mmHg and 5-minutes deflation using an upper-arm blood pressure cuff, before reperfusion. Although further high-quality trials are desirable, meta-analyses have demonstrated the effect of RIC prior to primary PCI, including myocardial salvage, reduction in infarct size and cardiovascular events.393
Unfortunately, there remains little data available in Japan, and to perform RIC before reperfusion requires establishing a cooperative system among ambulance transport and emergency room teams. However, RIC is a non-invasive, safe and low-cost therapy and is expected to be applied in clinical practice in the near future.
5.4 Others
Various other adjunctive treatments have been attempted to order to inhibit reperfusion injury in clinical trials, based on the results of animal experiments. However, most of them have failed to demonstrate clinical effects. Some of the treatments have been suggested to be possibly useful, but little data are available in Japan. Therefore, the treatments are listed just for information in this section.
Post-conditioning is a phenomenon that alternating ischemia and reperfusion several times immediately after reperfusion can reduce infarct size.394
Several small studies have reported this effect, but recent larger trials failed to demonstrate the effect.387,395,396
Adenosine has been reported to have microvascular dilatation, anti-inflammation, and pre-conditioning effects, but its clinical benefit is still controversial. A recent meta-analysis reported that these effects including inhibition of microvascular dysfunction and reduction in developing heart failure were observed only with intracoronary administration of adenosine.397
Some reports have shown that use of Glucagon like peptide-1 receptor agonist before reperfusion can reduce infarct size in STEMI patients.398
A trial performed in Japan reported that the administration of edaravone prior to primary PCI inhibited reperfusion injury,399
however, there remains no further available data.
Erythropoetin and cyclosporine A had been expected to reduce infarct size, inhibit reperfusion injury, and improve prognosis, but currently those effects are considered negative.400,401
Hypothermia has been reported to be effective for reducing infarct size in STEMI patients, however, at present its clinical benefit is considered to be limited.402
VI. NSTE-ACS
1. Risk Assessment (Table 34)
Table 34.
Recommendations and Evidence Level Regarding Risk Stratification in NSTE-ACS
| |
COR |
LOE |
Assessment of the risk based on symptoms, ECG, and cardiac biomarkers should be performed
in all patients with suspected ACS.179,182 |
I |
A |
| The initial treatment strategy should be decided according to the stratification of early risk.403 |
I |
B |
| Patients assessed to be high risk in the emergency department should be managed in CCU.404 |
I |
B |
Serial evaluation of ECG and cardiac biomarkers should be performed in patients with highly
suspected ACS.179,182 |
I |
B |
| Use of risk scores such as TIMI and GRACE should be considered.196–198 |
IIa |
B |
Abbreviations: ECG, electrocardiogram; ACS, acute coronary syndrome; CCU, coronary care unit.
Risk assessment of individual patients for adverse events is fundamental in considering the treatment strategy for NSTE-ACS patients to improve their short- and long-term outcomes.
All patients suspected of ACS are divided into the following 4 categories according to the likelihood of ACS; Non-cardiac disease, chronic stable angina pectoris, possible ACS, and definite ACS. In case of possible/definite ACS, risk stratification for short-term mortality (as well as non-fatal cardiac adverse events) should be done. Assessment using Braunwald’s classification (Table 10) is well established for estimating short-term mortality, angiographic severity, and complications of PCI.147,148,151,152
See details on risk scores in chapter
IV 1.8.1.
Decision making based on risk stratification should be done within 12 hours after arrival, and is useful for deciding the needs for admission, intensive care in CCU or intensive care unit (ICU), and emergency coronary revascularization.403
Moderate and high risk patients should be managed in CCU, while low risk patients may be observed in the outpatient clinic.404
Risk assessment using ECG and cardiac biomarkers should be continued over time and treatment strategy should be changed in cases where risk changes over time.179,182
GRACE, TIMI, and PURSUIT risk scores, which were originally developed to stratify long-term risk for prognosis, are also useful to evaluate initial risk assessment.64,196–198
See details on risk scores in chapter
IV 1.8.1.
Bleeding is an important predictor of prognosis in patients with NSTE-ACS. ACS patients as compared with stable CAD patients often suffer from bleeding related to use of antithrombotic agents.405
Risk factors related to bleeding are female sex, older age, impaired renal function, high white blood cell count, anemia, STEMI or NSTEMI, heparin use, and GP IIb/IIIa inhibitors use.406
The CRUSADE bleeding risk score consists of the following factors; (1) hematocrit level on admission, (2) creatinine clearance, (3) heart rate, (4) female sex, (5) heart failure, (6) history of vascular disease, (7) diabetes mellitus, and (8) systolic blood pressure.407
2. Conservative Strategy and Invasive Strategy
2.1 Treatment Strategy (Table 35)
Table 35.
Recommendations and Evidence Level of Treatment Strategies Targeting NSTE-ACS
| |
COR |
LOE |
| Early invasive strategy should be recommended in high-risk* patients.64,151,197,410,411 |
I |
A |
Moderate/high-risk patients who initially presented to a non-PCI-capable hospital should be
transferred immediately to a PCI-capable center. |
I |
C |
Early invasive strategy may be considered for high-risk patients stabilized by medical therapy, and
delayed invasive strategy may be considered for low risk patients.422 |
IIb |
B |
Early invasive strategy should not be recommended for patients with severe comorbidities (such as
respiratory failure and malignancy) in whom there is no expected benefit from coronary revascularization. |
III:
Harm |
C |
| Early invasive strategy should not be recommended for patients with extremely low likelihood of ACS. |
III:
Harm |
C |
Abbreviations: PCI, percutaneous coronary intervention; ACS, acute coronary syndrome.
*High-risk indicates patients with ongoing or recurrent chest pain refractory to medical treatment, congestive heart failure, hemodynamic instability, life-threatening arrhythmias or cardiac arrest, mechanical complications (acute mitral regurgitation, etc.), transient ST-segment elevation, dynamic ST-T change, rise and fall in cardiac troponin compatible with AMI, and/or GRACE risk score >140.
Treatment strategy for patients with suspected ACS is divided into 2 strategies according to timing of CAG and coronary revascularization. Conservative strategy prioritizes medical treatment and invasive treatment is not performed routinely unless patients have ongoing or recurrent chest pain or hemodynamic instability. This strategy has advantages to be able to stabilize the culprit lesion with statins and antithrombotic agents and to avoid unnecessary invasive procedures with inherent procedural risk. In invasive strategy, CAG, followed by PCI, is routinely performed for all patients.
The results of clinical trials comparing conservative strategy and early invasive strategy have changed over time.
In the era of balloon angioplasty, the TIMI IIIB trial reported that early invasive strategy was superior to conservative strategy in terms of hospital stay and re-admission rate in high-risk patients, although the risk for major adverse cardiac events was neutral between the two groups.408
The DANAMI trial, which enrolled patients with prior myocardial infarction and myocardial ischemia, showed significantly lower incidence of adverse cardiac events (death, myocardial infarction, re-admission for UA) in early invasive treatment than in conservative treatment.326
However, invasive treatment strategy was associated with significantly higher in-hospital and one-year higher cardiac adverse events in the VANQWISH trial.409
The advantage of early invasive over conservative strategy was demonstrated in the FRISC-II, TACTICS-TIMI18, and RITA3 trials conducted in the era of PCI using stents.
In the FRISC-II trial, the cumulative incidence of major adverse cardiac events at 6 months was significantly lower in the early invasive strategy group than in the conservative group (9.4% vs. 12.1%).151
Early invasive strategy was associated with lower major adverse cardiac events than conservative strategy in the TACTICS-TIMI 18, in which all patients were administered the GP IIb/IIIa antagonist tirofiban with a mean interval of 22 hours before coronary angiography (15.9% vs. 19.4%).410
In the ICTUS trial, however, there was no significant difference between early invasive and selective invasive strategies in NSTEMI patients with high troponin level, although early invasive strategy significantly reduced re-admission.411
In several trials such as TACTICS-TIMI 18 and FRISC II trials, the advantages of early invasive therapy were observed only in high-risk patients with moderate to high TIMI risk score, high troponin level, or ST change.151,410
Majority of trials reporting no advantage with invasive strategy over conservative strategy were conducted before the introduction of stents,412,413
and important factors were varied among trials such as revascularization rate, the rate of stent use, mortality of surgery, and the rate of GP IIb/IIIa inhibitors. In the era of stents, the advantages of early invasive strategy over conservative strategy were demonstrated despite of a non-statistically significant higher risk of early complications such as CK elevation.411,414–416
Optimal antiplatelet therapy facilitates the benefits of early revascularization. The CURRENT-OASIS 7 trial evaluated the efficacy and safety of a loading dose of clopidogrel, which was introduced in Japan in 2009, in 25,086 ACS patients. In this study, a 600 mg loading regimen was superior to a 300 mg loading regimen in terms of stent thrombosis (0.7% vs. 1.3%, P=0.0001), and 30-day major adverse cardiovascular events (MACEs) (cardiovascular death/non-fatal myocardial infarction/stroke) (3.9% vs. 4.5%, P=0.035).274,417
However, a 600 mg loading regimen was associated with higher risk of bleeding (1.6% vs. 1.1%, P=0.009). Newer P2Y12
Inhibitors had a lower risk for cardiovascular events than clopidgrel, but were associated with higher risk of bleeding in the TRITON-TIMI 38 (prasugrel) and PLATO (ticagrelor) trials.282,418
2.2 Timing of Invasive Strategy
Invasive strategy is divided into 3 categories according to the timing of CAG and coronary revascularization. Early invasive strategy has the potential to reduce the risk of ischemic events in the time course of ACS, while preceding antiplatelet therapy may reduce the risk of complications related to thrombosis at the time of revascularization.
2.2.1 Immediate Invasive Strategy (Within 2 Hours)
Very high-risk patients indicated in
Table 36
are recommended to receive immediate invasive strategy, because this category of patients have poor prognosis without optimal treatment. This category of patients was generally excluded from randomized controlled trials. If patients present to non-PCI capable hospitals, immediate transfer is highly recommended.
Table 36.
Selection of Treatment Strategy and Timing in NSTE-ACS
| Risk |
Treatment strategy |
|
| High |
Immediate invasive strategy (within 2 h) |
Recurrent or ongoing chest pain refractory to medical treatment
Heart failure
Hemodynamic instability
Life-threatening arrhythmias or cardiac arrest
Mechanical complication (acute mitral regurgitation etc.)
Transient ST-segment elevation, or recurrent dynamic ST-T changes |
| Early invasive strategy (within 24 h) |
Rise and fall in cardiac troponin compatible with myocardial infarction
New ECG changes (dynamic ST-T changes)
GRACE risk score >140 |
| Moderate |
Delayed invasive strategy (within 72 h) |
Diabetes mellitus
Renal insufficiency (GFR <60 mL/min/1.73 m2)
LV dysfunction (LVEF <40%)
Early post-infarction angina
Prior coronary revascularization (PCI, CABG)
GRACE risk score: 109–140 |
| Low |
Early conservative strategy |
None of the above-mentioned factors, clinically suitable for conservative strategy
GRACE risk score <109 |
Abbreviations: ECG, electrocardiogram; GFR, glomerular filtration rate; LV, left ventricular; LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention; CABG, coronary artery bypass grafting. (Source: Prepared based on Antman EM, et al. 200064)
The ABOARD (Angioplasty to Blunt the Rise of Troponin in Acute Coronary Syndromes) trials compared immediate intervention (median 70 minutes) with delayed intervention (next day: median 21 hours). The primary endpoint of the peak troponin value during hospitalization, as well as the key secondary endpoint comprising death, myocardial infarction, or urgent revascularization at 1-month follow-up, were not significantly different between the 2 groups.419
Therefore, the need to perform immediate CAG is not necessarily clear. However, it is reasonable to perform emergent coronary angiography followed by PCI if needed in patients who have congestive heart failure as a complication, ongoing chest pain, a large area of ischemia as indicated by ECG findings, hemodynamic instability, and/or life threatening arrhythmias.
Survivors of out-of-hospital cardiac arrest without ST-segment elevation need to be managed with an individualized approach. Conscious survivors are recommended to receive immediate CAG, while comatose survivors are recommended to receive CAG after surveillance for other causes of cardiac arrest.261
2.2.2 Early Invasive Strategy (Within 24 Hours)
Early invasive strategy is defined as the treatment strategy in which CAG is performed within 24 hours after admission. Early invasive strategy is recommended if patients fulfill at least one high-risk criteria indicated in
Table 36. These patients should be transferred to PCI capable centers immediately if initially presenting to non-PCI capable centers.
The CRUSADE study reported that the incidence of in-hospital adverse events was not significantly different between patients presenting on weekdays and those on weekends, although time to catheterization was significantly delayed in patients presenting on weekends than those on weekdays (median 46.3 vs. 23.4 hours, P<0.0001).420
The ISAR-COOL trial evaluated the efficacy of antithrombotic pretreatment using unfractionated heparin, aspirin, clopidogrel, and tirofiban for 3 to 5 days with early intervention with a composite primary endpoint of 30-day incidence of large nonfatal myocardial infarction and all-cause death.421
In this trial, the primary endpoint was reached in 11.6% in the antithrombotic treatment group and 5.9% in the early intervention group (P=0.04). The merit of early intervention over antithrombotic pretreatment was attributed to the higher incidence of events before intervention in the antithrombotic pretreatment group.
In the TIMACS trial, 3,031 ACS patients without ST-segment elevation were randomly assigned to undergo either routine early intervention (coronary angiography within 24 hours after randomization) or delayed intervention (coronary angiography beyond 36 hours after randomization).422
Although the primary endpoint of a composite of death, myocardial infarction or stroke at 6 months was not significantly different between the 2 groups (9.6% vs. 11.3%, P=0.15), a composite of death, myocardial infarction or refractory ischemia at 6 months was significantly lower in the early intervention group than in the delayed intervention group (9.5% vs. 12.9%, P=0.003). The VERDICT study assigned 2,147 patients with NSTE-ACS within 12 hours of onset to coronary revascularization within 12 hours (median 4.7 hours) or between 48–72 hours (median 61.6 hours) and compared the long-term outcomes (over 4.3 years).423
There was no difference between the groups in the main composite endpoint (all-cause death, nonfatal myocardial infarction, hospitalization due to refractory myocardial ischemia, and/or heart failure), but in patients with a GRACE score >140, the prognosis was better in the group who received treatment within 12 hours.
Based on the results above, the benefit of earlier revascularization in patients with GRACE score >140 is evident and delays in CAG and coronary revascularization are undesirable.
2.2.3 Delayed Invasive Strategy (Within 72 Hours)
Delayed invasive strategy with CAG within 72 hours after admission is recommended if patients do not suffer from recurrence of symptoms and fulfill at least one of the moderate risk criteria indicated in
Table 36. Those patients should undergo CAG within 72 hours even in needing transfer.
2.3 Conservative Strategy
Patients with low risk and without recurrence of symptoms are considered as candidates for conservative strategy. Non-invasive evaluation for ischemia including imaging is recommended before CAG.
3. Coronary Revascularization
3.1 Selection of Revascularization Strategy (Table 37)
Table 37.
Recommendation and Evidence Level Regarding the Selection Method of Coronary Artery Revascularization in NSTE-ACS
| |
COR |
LOE |
| The revascularization strategy (PCI or CABG) should be discussed in the heart team as needed. |
I |
C |
Abbreviations: PCI, percutaneous coronary intervention; CABG, coronary artery bypass grafting.
Regarding the choice between PCI and CABG, it is important to judge whether patients need to have an emergency procedure or not. Patients with hemodynamic instability or ongoing chest pain despite of medical treatment need emergency coronary revascularization predominantly by PCI.158
CABG may be considered if patients without a need for immediate revascularization have a lesion anatomically unsuitable for PCI or a left main and/or proximal LAD lesion.
The criteria for selection of coronary revascularization in the early invasive strategy for NSTE-ACS is basically the same as that in stable CAD because the outcome of early CABG for NSTEMI/UA was reported to be almost same as that of elective CABG. Consulting the heart team is recommended for selection of PCI or CABG for severe CAD (left main disease, multi-vessel disease with proximal LAD lesion, and poor LV function).
In emergent or early PCI for NSTE-ACS patients with multivessel disease, culprit-only PCI is usually recommended, because a single culprit lesion can usually be identified based on ECG or angiographic findings. However, multivessel PCI may be considered in hemodynamically stable patients, in whom the first target lesion could be successfully treated with an acceptable amount of contrast and procedural time, although there is no consensus about the optimal strategy for multivessel PCI.424–427
3.2 Fibrinolysis (Table 38)
Table 38.
Recommendation and Evidence Level of Fibrinolysis in NSTE-ACS
| |
COR |
LOE |
| Fibrinolysis for NSTE-ACS patients should not be performed.408,428 |
III:
Harm |
A |
Abbreviation: NSTE-ACS, non-ST-segment elevation acute coronary syndrome.
Fibrinolysis for NSTE-ACS should not be performed. Fibrinolysis does not have any mortality benefit, and has increased bleeding complications and myocardial infarction in both UNASEM and TIMI IIIB trials.408,428
3.3 PCI (Table 39)
Table 39.
Recommendations and Evidence Level Regarding PCI in NSTE-ACS
| |
COR |
LOE |
For operators experienced in radial artery approach, radial artery approach should
be performed rather than femoral artery approach in emergent or early PCI.268,269 |
I |
A |
| DES should be used in emergent or early PCI.441,442 |
I |
A |
| Multivessel PCI may be considered at the time of emergent or early PCI.424–427 |
IIb |
B |
| Routine manual aspiration thrombectomy is not recommended.262,263 |
III: No
Benefit |
A |
Abbreviations: PCI, percutaneous coronary intervention; DES, drug eluting stent.
In the early 1990 s, the outcome of emergent PCI for ACS patients was worse than that of elective PCI for stable CAD.429,430
Coronary stent implantation could stabilize a ruptured plaque and or dissection caused by balloon dilatation, resulting in the improvement of clinical outcome in ACS patients who received emergent PCI.412,430a,431,432
Early invasive strategy using PCI was reported to improve clinical outcomes in many recent clinical trials.421,422,425
The advantage of early PCI over delayed PCI is particularly clear in moderate- to high-risk patients.433
Radial artery approach is recommended as the preferred vascular access site in ACS patients based on the results of clinical trials reporting the advantage of radial artery over femoral artery approach.268,269
In the Minimizing Adverse Hemorrhagic Events by TRansradial Access Site and Systemic Implementation of angioX (MATRIX) trial comparing radial access with femoral access in 8404 ACS patients, radial access was superior to femoral access in 30-day MACE (8.8% vs. 10.3%, P=0.03), mortality (1.6% vs. 2.2%, P=0.045), and major bleeding (1.6% vs. 2.3%, P=0.01).268
Previous clinical studies consistently reporting DES use relative to BMS use showed comparable risk for stent thrombosis and lower risk for target lesion revascularization in ACS patients.248,434–439
Use of DES as compared with BMS was reported to have lower risk of death or myocardial infarction in high-risk NSTE-ACS patients in the CRUSADE registry.440
Newer generation DESs were reported to have lower risk of stent thrombosis than first generation DESs.441
In the Norwegian Coronary Stent Trial (NORSTENT) in which 9,013 patients were randomly assigned to either newer generation DESs or BMS (including 43.5% of NSTE-ACS patients), the primary endpoint of a composite of death or myocardial infarction was not significantly different between the two groups, but the newer generation DESs group showed significantly lower risks for repeat coronary revascularization (16.5% vs. 19.8% at 6 years, P<0.001) and definite stent thrombosis (0.8% vs. 1.2% at 6 years, P=0.0498).442
Routine thrombectomy is not recommended in PCI for NSTE-ACS. There is no clear evidence for the benefit of thrombectomy in NSTE-ACS as in the STEMI clinical trials.262,263,443,444
However, selective thrombectomy or distal protection may be considered if large thrombus is visible on angiography.445,446
3.4 CABG (Table 40)
Table 40.
Recommendations and Evidence Level of CABG in NSTE-ACS
| |
COR |
LOE |
For patients with failed PCI or technical difficulty, persistent ischemic attacks and hemodynamic
instability refractory to medical treatment (cardiogenic shock or life-threatening arrhythmias due to
myocardial ischemia), emergent CABG should be discussed in the heart team. |
I |
C |
For patients with frequent ischemic attacks refractory to medical treatment and a large risk area (severe
stenosis in the left main trunk or proximal LAD), early CABG should be discussed in the heart team. |
I |
C |
Abbreviations: PCI, percutaneous coronary intervention; CABG, coronary artery bypass grafting; LAD, left anterior descending artery.
Clinical outcome after early CABG in patients with UA is almost comparable to that after elective CABG.447
However, PCI may be preferred to CABG in patients with a large risk area and hemodynamic instability, especially in those with a culprit lesion in left main trunk, because reperfusion can be achieved more rapidly by PCI than by CABG.350,448–452
Emergent CABG within several hours of symptom onset is targeted towards patients with failed PCI or technical difficulty, persistent ischemic attacks and hemodynamic instability refractory to medical treatment (cardiogenic shock or life-threatening arrhythmias due to myocardial ischemia).
In cases where chest pain attacks occur frequently despite adequate pharmacotherapy and the risk area is large (severe stenosis in the left main trunk or proximal LAD, etc.), early CABG should be discussed in the heart team considering its efficacy, safety as well as the institute’s facilities. Even in this situation, CABG may be preferred to be performed several days after initial treatment to stabilize hemodynamic status and perform adequate pre-operative examinations.350
Multiple factors such as renal insufficiency, advanced age, and cerebrovascular disorder increase operative risk in emergency CABG for ACS patients in addition to ongoing myocardial ischemia in extensive risk area and heart failure.449,450
Regarding procedural aspects, an internal thoracic artery graft can be expected to have excellent long-term patency, and use of the internal thoracic artery does not increased operative risk even in emergent CABG if hemodynamic status is stable.453–455
VII. Evaluation and Management During Hospitalization
1. The Role of CCU (Table 41)
Table 41.
Recommendations and Evidence Level Regarding CCU Admission
| Role of CCU |
COR |
LOE |
CCU should provide comprehensive treatment to patients with AMI immediately after symptom onset,
while monitoring their ECG and vital signs.462 |
I |
B |
| CCU should treat and manage patients with pump failure monitoring using Swan-Ganz catheter.463 |
I |
B |
Abbreviations: CCU, coronary care unit; AMI, acute myocardial infarction; ECG, electrocardiogram.
The likelihood of a diagnosis and its short-term risk are two important considerations when deciding whether to hospitalize patients with suspected ACS. Clinicians should consider admitting patients diagnosed with ACS and those with relatively high short-term risk to a CCU (an ICU specialized in the treatment of CAD), in order to strictly monitor and manage their condition in the early stages of hospitalization. Disease management soon after initial symptom onset in a CCU or equivalent facility is particularly recommended for patients with AMI. In addition to the Braunwald classification (Table 10),146,412,414
a wide variety of risk scores to evaluate short-term risk in ACS have been proposed.64,86,196,197
Risk scores can be calculated based on patients’ disease history and physical findings, as well as test results obtained during ER management, which should be applied in all cases of suspected ACS. Of these, the TIMI risk score (Table 11) was the first proposed and most thoroughly validated. It can easily be determined from a patient’s medical history and ER tests, making it easy to use in an emergency outpatient setting. However, one study has reported that its predictive accuracy is inferior to those of the GRACE and PURSUIT risk scores.161
While the calculation of the GRACE score (Table 12) is rather complex, online calculators (e.g., https://www.mdcalc.com/grace-acs-risk-mortality-calculator) make it easy to utilize, even in emergency care settings, and its clinical utility has been proven in many investigations.159,162
These risk scores are valuable tools when making decisions on where to first provide medical care (i.e., the CCU, a general ward, or a different outpatient unit), which treatment strategy (or strategies) to adopt, and (especially) whether revascularization is necessary.
One of the roles of a CCU is to gather more information to refine the assessed risk, including detailed physical findings and medical history immediately after admission to the unit, as well as ECG findings and cardiac biomarker levels upon arrival at the hospital (admission). Age, systolic blood pressure, heart rate, Killip class, and infarct site are all important prognostic (predictive) factors.154
Other prognostic factors include past history of myocardial infarction, cerebrovascular disease, or peripheral arterial disease; comorbidity with diabetes, hypertension, or CKD; and smoking habit. In addition, elevation of cardiac biomarkers (e.g., cardiac troponins T and I, BNP, and N-terminal prohormone of BNP [NTproBNP]) upon admission and ST-segment elevation in the early recovery phase are useful indicators when performing early-stage risk assessments of STEMI.456,457
Cardiac troponin elevation upon admission predicts both short- and long-term mortality, independent of whether PCI is performed.458,459
Many patients with STEMI undergo primary PCI all across Japan, and the hospital mortality rate has decreased remarkably in the last 20 years.460
Clinicians must be mindful of bleeding, contrast-induced nephropathy (CIN), and other sequelae that may develop after CAG or PCI.
1.2 The Importance of CCUs
Dr. Hughes Day is widely known to have established the world’s first CCU in 1962 at Bethany Hospital in Kansas, USA.461
CCUs were also set up at Philadelphia’s Presbyterian Hospital and Canada’s Toronto General Hospital at about the same time. Leading up to their establishment, the medical field saw the development of electrical defibrillators, artificial pacemakers, and ECG monitoring systems. At the time, CCUs were intended to treat life-threatening arrhythmias in the immediate period after AMI, and ECG monitoring, electrical defibrillation, cardiac pacing, and other techniques remarkably reduced mortality rates after AMI.462
Later, the advent of the Swan-Ganz catheter would lead to significant progress in therapies for pump failure,463
while the introduction of early reperfusion therapy has reduced rates of mortality and a wide variety of complications.464,465
These developments mean that CCUs today have many roles to play in the treatment of ACS beyond the management of life-threatening arrhythmias, including the monitoring and treatment of unstable hemodynamics, heart failure, and new complications after ischemia-reperfusion therapy.
Despite the name “coronary care unit,” it is nowadays rare to find CCUs whose scope is limited to ACS alone. Instead, most are instituted as so-called
intensive cardiac care units, whose major goals are the housing and treatment of patients with severe cardiovascular diseases (e.g., heart failure, arrhythmia, myocarditis, acute aortic dissection, and acute pulmonary thromboembolism) as soon as possible. Since a CCU also works in cooperation with other medical institutions and emergency services in its community, functional intrahospital collaboration with emergency medicine departments is also of critical importance. Acute-phase cardiac rehabilitation is another important feature of CCUs, ensuring that admitted patients have plenty of bed rest to quickly yet safely stabilize their condition until they recover enough to be managed in general wards.
1.3 CCU Standards
The Japanese Society of Intensive Care Medicine (JSICM)’s
CCU Establishment Guidelines466
were proposed as “a single guideline for creating the ideal CCU.” They defined CCU as an intensive care ward in which all beds are used to treat “patients with serious conditions in the area of cardiovascular medicine.” They advised that CCUs should have dedicated physicians working full-time in the unit, including at least one qualified physician to assume an educational role in cardiovascular emergencies, such as an
intensive care specialist
(certified by JSICM) or cardiovascular specialist (certified by the JCS). The guidelines also recommend a cardiac surgeon and anesthesiologist working within the same hospital. Moreover, at least one nurse for every two patients in the unit should be on duty at all times, and a system should be put in place where that ratio can be increased to one nurse for every 1.5 patients if needed. High expertise is demanded from other professionals involved in the activities of the unit, and they should work inside the hospital or even inside the CCU itself. At present, there are no insurance reimbursement criteria that specify what qualifies as a CCU, and many hospitals dedicate a fraction of the beds in their general ICU wards to CCU patients. Intensive care for patients with acute heart disease requires quite sophisticated monitoring, differing in many respects from intensive care for other patients. In reality, the specific composition and structure of a given CCU depends on the conditions and limitations of its associated hospital.
2. General Early-Phase Treatments (Table 42)
Table 42.
Recommendations and Evidence Level Regarding Early Treatment in CCU
| General early-phase treatment |
COR |
LOE |
Sufficient sleep should be promoted with sleep medication, anxiolytics should be administered, and a
psychiatrist or professional counselor should interview the patient.481,482 |
I |
C |
Explanations of the patients’ condition and guidance on lifestyle after discharge should be promptly
provided to the patients and their families to help prevent/treat anxiety and depression.483 |
I |
C |
| Oxygen is indicated in patients with hypoxemia (oxygen saturation <90%) or signs of heart failure.211,470 |
I |
C |
| Routine oxygen is not recommended in patients with oxygen saturation ≥90%.211–214,470 |
III: No
benefit |
A |
Early mobilization and early rehabilitation should be considered when early reperfusion is successful and
patients do not have complications.471 |
IIa |
C |
When patients are admitted to the hospital, measurement HbA1c level is recommended to diagnose
diabetes mellitus.474–477 |
I |
A |
Management of high blood glucose level with continuous insulin infusion may be considered in the
acute phase with the goal of avoiding hypoglycemia and keeping blood glucose within the target range
(<180 mg/dL).479,480 |
IIb |
A |
Abbreviation: AMI, acute myocardial infarction.
Patients in the CCU should be treated while connected to continuous monitoring equipment that can track their status over time. ‘Status” here refers to not only vital signs (heart rate, blood pressure, breathing rate, temperature) but also other metrics such as arterial oxygen saturation, urine output, and central venous pressure. When patients have pump failure resistant to control, physicians should also continuously monitor pulmonary capillary wedge pressure (PCWP), mixed venous oxygen saturation, and cardiac index (CI). In addition, longitudinal measurements of cardiac troponins, CK, and other cardiac biomarkers can be used to predict how effective reperfusion therapy will be, as well as the formation process of the infarct. Patients should have their cardiac biomarkers retested between 6 and 12 hours after symptom onset if the results are initially negative in the first 6 hours. Regardless of these findings, reperfusion therapy must be performed early in the course of treatment when patients need it.467–469
Moreover, clinicians must not forget to elaborate on a patient’s initial clinical history and physical findings hastily obtained by the emergency department after he or she has been admitted to the CCU.
2.1 Oxygen Inhalation
In recent reports, the effectiveness of oxygen administration to patients without hypoxemia has been refuted;211–214,470
oxygen should only be administered if there are signs of hypoxemia, heart failure, or shock, and routine administration of oxygen is not recommended.
2.2 Rest
Blood pressure control is essential in patients with ACS with marked and significant elevation in cardiac biomarker levels as this may indicate that the cardiac muscle has weakened due to myocardial necrosis. To avoid putting excessive strain on the heart, patients should rest as comfortably as possible in the initial period after symptom onset. The length of the rest period should be decided on an individual basis, according to whether reperfusion is successful, peak level of cardiac biomarker, or changes of ECG findings. Recently, early mobilization and early rehabilitation programs have been recommended when early reperfusion is successful and patients do not have complications.471
In specific terms, a patient should stay in bed and rest on his or her first day in the CCU. On the second day, the staff should have the patient stand by the bedside and check if there are changes in ECG findings or vital signs. After two days, once serum CK levels have peaked, the patient should begin walking to the toilet and sink with staff assistance and supervision.
2.3 Food and Glycemic Control
Patients should fast immediately after PCI. After a few hours, they should begin drinking water with assistance from the medical staff; if this step is unproblematic, they should then begin eating meals again. Neither hot food/drinks nor cold drinks are believed to adversely affect patients with AMI.472
In addition, the severity of AMI correlates with reduced glucose tolerance in the acute phase.473
Acute stress hyperglycemia is a predictor of poor outcomes after AMI (independent of diabetes status),474,475
even with early reperfusion.476
Elevated blood glucose level in the acute phase of AMI does not necessarily imply a history of diabetes; it is triggered by acute stressors such as ischemia, LV dysfunction, and pain stimuli.477
However, persistent hyperglycemia provokes harmful physiological responses, including oxidative stress, endothelial dysfunction, and hypercoagulability. The DIGAMI-1 Study reported that acute insulin therapy to manage blood glucose level reduced mortality rates following AMI.478
In contrast, the NICE-SUGAR study revealed a different conclusion: intensive glucose control actually increases the risk of hypoglycemia and cardiovascular accidents; specifically, severe hypoglycemia and mortality were both significantly more common in patients treated with intensive glucose control (target range, 81–108 mg/dL) than with conventional control (<180 mg/dL).478a
Avoiding hypoglycemia is thus an essential requirement of acute glucose control in AMI. Notably, by resolving ischemia and alleviating symptoms, early reperfusion therapy is quite likely to improve stress hyperglycemia. When combined with the hypoglycemic action of insulin, early reperfusion therapy can rapidly reduce blood glucose level, triggering hypoglycemia. Based on these findings, clinicians should aim to maintain the blood glucose level between 90 and 180 mg/dL if a patient presents with hyperglycemia, while frequently monitoring their levels during the hospital stay. A diabetes specialist should be consulted whenever possible if a patient is started on a continuous insulin infusion.
2.4 Urination and Defecation
Except when cumulative urine collection is absolutely necessary, do not insert indwelling catheters because they can potentially cause urethral damage and infections.481
Heart rate increases during defecation,482
which can trigger reattack or cardiac rupture: consider facilitating smooth bowel movements using magnesium oxide or other laxatives.
2.5 Sedation, Sleep, and Psychotherapy
Clinicians must bear in mind that anxiety or depressive states can be induced in patients by a number of CCU-related triggers, including the strict observation conditions, sounds of monitoring equipment, forced sedation, insufficient sleep, restraints imposed by intravenous lines, pain due to inserted Foley catheters, and insufficient explanation of their condition.483
In many cases, these triggers can be effectively mitigated by sleep medication, anxiolytics, and interviews by a psychiatrist or professional counselor.484
Clinicians must also be vigilant about delirium, dementia, and drug-induced psychological effects (e.g., due to lidocaine, β-blockers, or digitalis). To do so, the patient’s living conditions before admission must be ascertained.
2.6 Family Interviews and Patient Education
To prevent and treat anxiety and depression, it is important to provide patients and their families with prompt explanations of their condition and guidance on lifestyle after discharge.485
In particular, recovery is greatly influenced by how well a patient’s spouse understands his or her condition.486
During the recovery period in the CCU, spouses should be educated about not only their husband or wife’s condition, treatment contents, and prognosis, but also triggering factors (type A behavior pattern, genetic predisposition, diet and other lifestyle factors, stressors, etc.).
2.7 CCU Stay Length
The average time that patients with uncomplicated AMI were treated successfully by reperfusion therapy stay in CCUs is decreasing with each passing year.487
It is common for such patients to be transferred to a general ward the day after admission, if not the same day. Consider housing low-risk patients who underwent successful PCI directly in a step-down unit that can perform basic monitoring if the hospital has one. However, it is necessary for patients with severe arrhythmias or risk of cardiac rupture to stay in a general ward capable of providing safe conditions for acute cardiac rehabilitation.
3. Pharmacological Therapy
3.1 Antithrombotic Therapy (Table 43)
Table 43.
Recommendations and Evidence Level Regarding Antithrombotic Administration During Hospital Admission
| |
COR |
LOE |
| Aspirin (162–200 mg) should be administered before PCI in aspirin naive patients.295,488 |
I |
A |
A loading dose (clopidogrel 300 mg and prasugrel 20 mg) should be administered before PCI to
patients not taking thienopyridine antiplatelet drug, followed by a maintenance dose (clopidogrel
75 mg/day and prasugrel 3.75 mg/day).281,283,489 |
I |
A |
DAPT with aspirin plus a thienopyridine antiplatelet drug should be administered to patients
receiving PCI.281,283,289 |
I |
A |
| Clopidogrel (75 mg/day) should be administered to patients intolerant to aspirin. |
I |
C |
Concomitant anticoagulation should be administered to patients with left atrial or left ventricular
thrombus.494 |
I |
B |
Ticagrelor should be considered in patients for whom DAPT is indicated but thienopyridine antiplatelet
drug is not suitable.490,491 |
IIa |
B |
Dual antithrombotic regimen with an oral anticoagulant and clopidogrel (without aspirin) should be
considered at discharge in patients on anticoagulation after PCI.286,492,493 |
IIa |
B |
Abbreviations: PCI, percutaneous coronary intervention; DAPT, dual antiplatelet therapy.
Aspirin (81–162 mg/day) has been shown to reduce the rates of short- and long-term cardiovascular events.295,488
In primary PCI for STEMI, DAPT with 300 mg loading and 75 mg maintenance dose of clopidogrel on top of aspirin reduced cardiovascular event risk.281
In the COMMIT trial, DAPT with aspirin plus clopidogrel reduced the cardiovascular composite endpoint of short term mortality, MI, and stroke without increasing bleeding risk.489
Prasugrel achieves a faster and more consistent degree of P2Y12
inhibition compared to clopidogrel. In PRASFIT-ACS, a Japanese clinical trial comparing prasugrel vs. clopidogrel, 20 mg loading/3.75 mg maintenance dose of prasugrel plus aspirin was associated with a numerically lower rate of cardiovascular events as compared with 300 mg loading/3.75 mg maintenance dose of clopidogrel plus aspirin in ACS patients receiving PCI.283
In the TRITON trial,282
which adopted 60 mg loading and 10 mg maintenance doses of prasugrel, prasugrel was associated with an increaseed risk of bleeding; however, there was no significant difference in bleeding events between prasugrel and clopidogrel in PRASFIT-ACS trial with Japanese doses. In NSTEMI patients in whom coronary anatomy is not known, use of prasugrel is not recommended.283
In Japan, ticagrelor is approved for patients for whom DAPT is indicated but other P2Y12
inhibitors are not suitable. The PLATO trial compared ticagrelor plus aspirin vs. clopidogrel plus aspirin in patients with STEMI/NSTEMI. DAPT with ticagrelor plus aspirin was associated with reduced risk of cardiovascular events, all-cause mortality, and stent thrombosis, with similar risk of major bleeding as compared with DAPT with clopidogrel plus aspirin.418
However, in the PHILO trial done in Asian (mostly Japanese) patients, ticagrelor was associated with numerically higher thrombotic and bleeding rate.490
In an elderly society such as Japan, ACS is often complicated by atrial fibrillation.491
In ACS patients with atrial fibrillation, anticoagulation plus DAPT (triple therapy: TAPT) is often administered. However, TAPT is associated with a significantly higher risk of bleeding. In recent clinical trials including WOEST, PIONEER AF-PCI, and RE-DUAL PCI, dual antithrombotic therapy with anticoagulation plus clopidogrel was associated with significantly reduced bleeding events while not increasing thrombotic events.286,492,493
These clinical trials suggest TAPT could be worse than the dual antithrombotic regimen using an anticoagulant agent plus clopidogrel.
In patients on anticoagulation, dual antithrombotic regimen with an oral anticoagulant and clopidogrel (without aspirin) after PCI should be considered at discharge. Since there are no data to support optimal duration/dosing of antithrombotic agents regarding TAPT, clinicians should evaluate thrombotic and bleeding risk in each patient to individualize the medication.
In patients with severe renal dysfunction, warfarin is contraindicated. The Japanese guidelines for hemodialysis recommend not to prescribe warfarin in patients on hemodialysis. The guidelines also state that keeping PT-INR <2.0 is desirable if the benefit of anticoagulation outweighs the harm.
3.2 β-Blockers (Table 44)
Table 44.
Recommendations and Evidence Level of β-Blocker Administration During Hospital Admission
| |
COR |
LOE |
Oral β-blockers should be administered to patients with clinical signs of heart failure or LVEF
≤40%, gradually increasing from a low dose in early phase.501–503 |
I |
A |
| Oral β-blockers should be considered in patients if not contraindicated.496,497,504–506 |
IIa |
A |
Intravenous β-blockers should not be administered to patients with hypotension, acute heart
failure or severe bradycardia such as AV block.498 |
III:
Harm |
B |
Abbreviations: LVEF, left ventricular ejection fraction; AV, atrioventricular.
β-blockers can decrease heart rate, myocardial contraction and blood pressure, and as a result of these, myocardial oxygen consumption is reduced. Consequently it is expected that the progression of myocardial necrosis decreases and patients’ prognosis improves if β-blockers are administered in the early phase of NSTE-ACS and STEMI, the time when myocardial ischemia develops. In fact, the efficacy of β-blockers was proven in a clinical study more than a half century ago.495
However, the use of β-blockers is still controversial and its recommendation in guidelines changes frequently because the efficacy of the drug was refuted in several clinical studies done later. The discordance in the guidelines is partly because of difference in drug indication between NSTE-ACS and STEMI, and between conservative or fibrinolysis days and the PCI era. Furthermore, lack of original randomized controlled trials in Japan and unavailability of metoprolol in Japan, which has much evidence in other countries, make it difficult to formulate original Japanese guidelines for use of β-blockers in Japanese patients with ACS.
Regarding NSTE-ACS, even overseas there is limited evidence available for the effectiveness of β-blockers. In the CRUSADE registry, the effects of early (within 24 hours from onset) administration of β-blockers in 72,054 NSTE-ACS patients from 509 institutes in the United States were studied.496
As a result, hospital mortality was significantly reduced by 34%. From a retrospective study done in England, early (within 4 hours from admission) oral administration of low-dose bisoprolol (1.25–2.5 mg) significantly improved the rates of arrhythmias and cardiac death as compared to delayed (within 5 to 24 hours) administration.497
The effects of intravenous metoprolol followed by oral administration in patients with STEMI were examined in the COMMIT study with a cohort comprised of almost 90% STEMI patients. As a result, although early intravenous administration of β-blockers in STEMI patients without heart failure reduced reinfarction and ventricular fibrillation, cardiogenic shock immediately after drug administration was increased.498
In the METO-CARD study, with only a limited number of participants, the effects of prereperfusion intravenous administration of β-blockers in patients with STEMI in the era of primary PCI were studied.499
Intravenous administration of metoprolol in anterior STEMI patients without heart failure symptoms reduced infarction size, and consequently increased LVEF without any adverse events within 24 hours after administration. On the other hand, the EARLY-BAMI study with similar concept to the METO-CARD study failed to show reduced infarction size.500
Furthermore, the effects of early administration of β-blockers are not same between studies limited by primary PCI as the reperfusion method. In addition to the variation in effects, as mentioned before, since metoprolol is not available in Japan, studies of metoprolol are not applicable to our daily practice in Japan. In some studies, the authors speculate that the reason why metoprolol increased adverse events is its relatively long half-life of 3-4 hours.
3.3 Renin-Angiotensin-Aldosterone Inhibitors (Table 45)
Table 45.
Recommendations and Evidence Level of RAAS Inhibitor Administration During Hospital Admission
| |
COR |
LOE |
ACE inhibitors should be administered within 24 hours from onset in high-risk patients
with reduced LV function (LVEF ≤40%) or heart failure.217 |
I |
A |
ARB should be administered instead of ACE inhibitors in patients with reduced LV function
or heart failure, particularly in those with ACE inhibitors intolerance.508,511 |
I |
A |
Mineralocorticoid receptor antagonist should be added to ACE inhibitors and β-blocker
in patients with reduced LV function (LVEF ≤40%), heart failure or diabetes, and without
renal failure or hyperpotassemia.512 |
I |
A |
| Administration of ACE inhibitors should be considered if not contraindicated.513,514 |
IIa |
A |
Abbreviations: ACE, angiotensin converting enzyme; LV, left ventricular; LVEF, left ventricular ejection fraction; ARB, angiotensin II receptor blocker.
Administration of ACE inhibitors is recommended in patients with reduced LV function (LVEF ≤40%) or symptomatic heart failure particularly in its early phase after ACS onset.217
Meta-analysis by the ACE Inhibitor Myocardial Infarction Collaborative Group revealed that administration of ACE inhibitors within 0 to 36 hours from ACS onset significantly reduced 30-day mortality.507
The effect is sizable, particularly within a week after ACS onset. Furthermore, the effect is more pronounced in high-risk patients with Killip class 2 or 3 and heart rate on admission ≥100 bpm or in those with anterior myocardial infarction.
The VALIANT study examined the effects of ARB administered in the early phase of ACS.508
Although any additional effects of ARB over ACE inhibitors are not clear, valsartan adding on standard therapy as an alternative to ACE inhibitors prevented cardiovascular events similar to ACE inhibitors.
The effects of the mineralocorticoid receptor inhibitor eplerenone were examined in the REMINDER study in STEMI patients without heart failure symptoms.509
As a result, NT-proBNP was decreased by additional administration of eplerenone. With a similar method, the ALBATROSS study revealed that single injection of potassium canrenoate 200 mg followed by oral administration of spironolactone 25 mg reduced mortality by up to 80% in patients with STEMI.510
3.4 Nitrates, Nicorandil (Table 46)
Table 46.
Recommendations and Evidence Level of Nitric Acid and Nicorandil Administration During Hospital Admission
| |
COR |
LOE |
| Sublingual nitroglycerin is indicated in patients with ongoing chest pain. |
I |
C |
Intravenous nitroglycerin should be administered to patients with ongoing chest pain,
hypertension or heart failure. |
I |
C |
| Intravenous nicorandil may be considered in STEMI patients undergoing primary PCI.381 |
IIb |
B |
| Nitrates should not be administered for 24 hours after taking erectile dysfunction medication.517 |
III:
Harm |
B |
Nitrates should not be administered in patients with inferior AMI complicated by RV infarction and
those with systolic blood pressure <90 mmHg (or decreased by ≥30 mmHg from baseline),
severe bradycardia (<50 bpm) or tachycardia (>100 bpm). |
III:
Harm |
C |
Abbreviations: STEMI, ST-segment elevation myocardial infarction; PCI, percutaneous coronary intervention; AMI, acute myocardial infarction; RV, right ventricular.
Nitrates can improve myocardial ischemia not only by endothelium-independent coronary dilatation but also by the inhibitory effects of myocardial oxygen consumption due to pre- and afterload reduction of the left ventricle. On the other hand, nicorandil, which is a combination drug of both nitrate and adenosine triphosphate (ATP)-sensitive potassium-channel opener improves microvascular circulation by dilatation of vascular smooth muscle cells and nitrate-like effects.
In ISIS-4, a prospective randomized control trial of nitrate in 58,050 ACS patients within 24 hours after onset, orally administered long-acting isosorbide mononitrate did not improve mortality at 5 weeks.217
In a similar trial for nicorandil in Japanese STEMI patients, single bolus injection of nicorandil 12 mg significantly decreased cardiovascular events for 2.4 years.377
On the other hand, nicorandil via drip infusion at 0.1 mg/kg/hr for 24 hours after bolus injection of 0.067 mg/kg did not decrease infarct size (∑CK) nor improve prognosis in Japanese patients with ACS in the J-WIND-KATP study.375
A meta-analysis including the above-mentioned 2 studies revealed that nicorandil administered during reperfusion therapy for AMI was effective for improvement of microcirculation and LV function.381
3.5 Calcium Channel Blockers (CCBs) (Table 47)
Table 47.
Recommendations and Evidence Level of Calcium Antagonists During Hospital Admission
| |
COR |
LOE |
Long-acting CCBs should be administered in patients with coronary spasm as the apparent
cause or with vasospastic angina to prevent ischemic attacks.522 |
I |
B |
| Routine administration of CCBs is not recommended in patients without coronary spasm.518 |
III: No
benefit |
A |
| Short-acting dihydropyridine CCB should not be administered in the acute phase after onset.523 |
III:
Harm |
A |
Diltiazem or verapamil should not be administered in patients with reduced LV function or
AV block.524,525 |
III:
Harm |
C |
Abbreviations: CCB, calcium channel blocker; LV, left ventricular; AV, atrioventricular.
A meta-analysis of western populations with UA revealed that calcium channel blockers (CCBs) did not inhibit the onset of myocardial infarction.518
It was reported that Japanese patients with myocardial infarction have 3 times the rate of coronary spasm based on challenge tests as compared to western patients in the early phase after onset.519
In this way, since coronary spasm could play an important role in the basic pathogenesis of myocardial infarction, it is possible that CCBs have more potential for secondary prevention in Japanese than western populations. In fact, in 2 prospective randomized controlled studies in Japanese patients, CCBs were proven to have a similar protective effect for cardiovascular events as compared with β-blockers.520,521
However, short-acting dihydropyridine CCBs may induce myocardial ischemia due to sympathetic nervous system activation, tachycardia and hypotension.
3.6 Lipid-Lowering Therapy (Table 48)
Table 48.
Recommendations and Evidence Level Regarding Lipid Lowering Therapy in the Acute Phase
| |
COR |
LOE |
| The maximum tolerable dose of a strong statin should be administered.526–537 |
I |
A |
A family history interview and physical examination including the Achilles tendon should be
performed to diagnose FH.539–541 |
I |
A |
Abbreviation: FH, familial hypercholesterolemia.
Many large-scale clinical trials have demonstrated that low-density lipoprotein cholesterol (LDL-C)-lowering therapies with statins reduce cardiovascular events in patients with AMI, and a body of evidence for lowering LDL-C has been established.526–528
In PROVE IT-TIMI 22 the benefits of atorvastatin therapy were evident within 30 days of randomization. The MIRACL trial demonstrated an early benefit from treating ACS patients intensively with statins vs. placebo. Given these trials, the early benefit from treating ACS patients intensively with statins has been demonstrated in Western populations. Intravascular ultrasound studies have also reported that intensive LDL-C lowering therapy with maximum tolerable doses of statins leads to regression of coronary atherosclerotic plaques, and that the reduction in plaque volume is positively correlated with the decrease in LDL-C.529,542
In Japan, prospective clinical trials for ACS have been performed, which investigated not only cardiovascular events as a primary endpoint but also changes in coronary plaque.530,531
Furthermore, the relationship between plaque regression and mid- to long-term cardiovascular events was investigated in observational studies, in which short-term plaque regression was demonstrated to be a predictor of future better cardiovascular outcomes.532–537
Extended-ESTABLISH trial demonstrated that early atorvastatin treatment improved long-term outcomes for patients with ACS. Given this result, early administration of the maximum tolerated dose of strong statin medication (atorvastatin, pitavastatin, rosuvastatin) is also recommended in Japanese ACS patients.
The target of LDL-C in post-ACS management is set at <70 mg/dL, which is described in chapter
9 Section 2.6.1. Intensive LDL-C lowering with statins reduced cardiovascular events even in patients with low baseline cholesterol <80 mg/dL to similar extent to those with higher levels. Additionally, statin treatment was associated with improved outcomes in Korean patients with AMI and LDL-C levels below 70 mg/dL.537,538
Based on these findings, statin therapy is recommended regardless of the LDL-C level prior to statin treatment. However, there is no contemporary evidence regarding the lower limit.538
3.6.2 Latent Familial Hypercholesterolemia (FH)
Familial hypercholesterolemia (FH) is a highly prevalent autosomal dominant genetic disorder presenting with three major signs: high LDL cholesterolemia, tendon xanthoma/skin xanthoma, and premature coronary artery disease.543
Patients with FH have an increased risk of developing premature atherosclerotic diseases due to sustained high levels of LDL-C from birth. Heterozygous FH is estimated to affect one in 200–500 people in Japan and is the most frequent genetic disorder, and therefore is often encountered in daily practice. It has also been reported that the prevalence of FH in patients with ACS is approximately 10 times greater than in the general population.539
An observational study in Japanese patients with AMI reported that 2 in 7 patients with Achilles tendon thickening (≥9 mm) met all three diagnostic criteria for adult heterozygous FH.540
During screening for FH in ACS patients, it is important to note the level of LDL-C is lower during the acute phase of ACS16
than the daily baseline level, and that Achilles tendon thickening is not obvious in young individuals. Therefore, early and careful screening for FH is necessary, especially in patients with ACS.
3.6.3 Management of LDL-C and Considerations for Non-Statin LDL-C-Lowering Medications During the Acute Phase of ACS
The maximum tolerable dose of a strong statin is recommended from the acute phase of ACS, but it is difficult to establish a target level of LDL-C in the acute phase of ACS. In patients who do not achieve the target level of LCL-C in the chronic phase (<70 mg/dL/ 50% reduction), even when FH is suspected and the maximum statin dose is given, consider using ezetimibe or proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor. Ezetimibe or PCSK9 inhibitors are also considered in statin-intolerant patients who have statin-induced disorders such as hepatic dysfunction, renal dysfunction, and myolysis.
4. Assisted Circulation
In Japan, the treatment strategies for hemodynamically unstable patients have been changing in response to the approval of a novel cardiac assist device targeting patients with ACS after a long interval. The outcomes of large-scale, prospective, randomized, clinical trials have led to the modification of the latest western guidelines pertaining to the use of cardiac assist devices. In the guidelines for the management of patients with ST-elevation acute myocardial infarction (JCS 2013), cardiac assist devices were written throughout the different chapters based on each pathological condition. Considering the current clinical demand, we recognized the need to revise the new guideline and include an independent subsection titled Assisted Circulation within a chapter titled Evaluation and Management of ACS Patients. Furthermore, due to the features of ACS, cardiac assist devices should be installed without delay in hemodynamically unstable patients during transportation or in the emergency room. Accordingly, the use of cardiac assist devices in the emergency room is addressed in this guideline.
Currently available devices in Japan include the intra-aortic balloon pumping (IABP), the IMPELLA®
peripheral ventricular assist device (Abiomed, Danvers, MA, USA), veno-arterial extracorporeal cardiopulmonary resuscitation (VA-ECMO [PCPS]), and the left ventricular assist device (LVAD). Although the first three systems are percutaneously inserted by a physician, LVAD requires a surgical procedure. However, in the clinical setting, the majority of interventional centers treating a large number of ACS patients are not equipped to install devices due to restrictions on medical resources. This means that confronting the big problems of ACS treatment in our country requires discussion of optimal distribution of medical resources considering the grand-design of ACS treatment. The four categories of cardiac assist devices are individually summarized in the following subsections.
4.1 IABP (Table 49)
Table 49.
Recommendations and Evidence Level of IABP Usage
| |
COR |
LOE |
| IABP is recommended in patients with cardiogenic shock due to mechanical complications. |
I |
C |
| IABP should be considered in patients with treatment resistant cardiogenic shock.546,548 |
IIa |
C |
IABP should be considered in patients with persistent myocardial ischemia after
reperfusion therapy. |
IIa |
C |
| Routine IABP is not recommended in patients with cardiogenic shock.546,547 |
III: No
benefit |
B |
Abbreviarion: IABP, intra-aortic balloon pumping.
The main mechanism of the IABP is the augmentation of coronary blood flow through inflation of an intra-aortic balloon during the cardiac diastolic phase.544
This effect is enhanced during conditions of decreased microvascular coronary flow such as cardiogenic shock or prolonged systemic hypotension.545
On the other hand, using rapid balloon deflation during the systolic phase, cardiac output increases through decreased after-load.544
Based on these approaches, IABP may increase the supply of oxygen to the myocardium. In addition, decreasing the demand for oxygen in the myocardium is widely used for the treatment of ischemic heart disease. For a long time, this device has played an important role in the treatment of ACS patients with persistent or recurrent ischemia, hypotension due to reduced cardiac function, and cardiogenic shock. Advantages of IABP include insertion and removal of the device by interventional cardiologists, the relatively low complication rates at vascular access sites, low device cost, and availability in the majority of interventional sites.
However, several large-scale, randomized clinical trials have failed to demonstrate the actual clinical utility of this device. Therefore, the effectiveness of IABP remains controversial, especially in western countries.
According to meta-analyses of small randomized clinical trials,546
the routine use of IABP was ineffective in the treatment of STEMI patients. Furthermore, the CRISP-AMI study failed to demonstrate reduction in infarct size through the routine use of IABP in anterior AMI patients without cardiogenic shock.547
Accordingly, considering the potential risk of bleeding, the routine use of IABP is not recommended in the treatment of ACS patients. Meta-analyses of previous cohort studies investigating the effectiveness of IABP in patients with cardiogenic shock have suggested improvement in survival.546
However, the effects of potential confounding factors (e.g., age or disease severity) should be taken into consideration. Finally, a large, prospective, randomized trial involving a total of 600 STEMI patients with cardiogenic shock and absence of mechanical complications (IABP-SHOCK II study) was conducted.548
This study failed to demonstrate superiority of IABP vs. medication treatment in terms of 30-day mortality (39.7% vs. 41.3%, respectively P=not significant). Furthermore, a meta-analysis of 12 randomized clinical trials including the IABP-SHOCK II study concluded that IABP did not improve 30-day mortality in AMI patients.549
Based on these findings, the latest United States and European treatment guidelines downgraded the recommendation for the use of IABP in the treatment of cardiogenic shock.58,166,175
Several inherent problems associated with this device have been emphasized in the aforementioned randomized clinical trials. For example, the majority of shock patients (87%) are treated with IABP following primary PCI. In the IABP-SHOCK II trial, it was suggested that earlier introduction of IABP may be preferable. Furthermore, a significant crossover of treatments was observed. For example, control patients (8.5%) in the CRISP-AMI study were treated with IABP, while 10% were treated with IABP and 7.4% were treated with LVAD in the IABP-SHOCK II study, which may have influenced the outcomes of these studies. Thus, it is evident that clinical trials investigating the use of cardiac assist devices in STEMI patients have inherent limitations regarding their effectiveness. This may be attributed to difficulty in avoiding of changes to allocated treatment due to prioritization of prolonging the patient’s life, difficulty in judgment by intention-to-treat analyses, and significant variation in the severity of the conditions. A sub-analysis of the data from the CRISP-AMI study demonstrated improvement in 6-month mortality in patients with larger infarct size or ischemic area.550
In addition, analysis of data from a Japanese database including more than 14,000 STEMI patients showed that hospital mortality of patients with cardiogenic shock (n=486) was significantly higher in those treated with IABP compared with those not treated with IABP.551
This guideline aims to establish a standard of care for ACS considering the clinical setting in Japan. The important issue remains whether the assisted circulation recommended in this guideline can be truly available in the majority of interventional sites that accommodate ACS patients. Despite the great expectations for effectiveness in ACS patients, the newly developed cardiac assist device IMPELLA®
has important limitations in clinical evidence and utilization in Japan. Despite the data of the IABP-SHOCK II study, the utilization rates of IABP varied significantly between sites in the United States.552
Accordingly, the guideline committee decided the recommendation level of IABP in this guideline considering the characteristic of the majority of primary PCI centers of medium and small case volume in our country. However, it is necessary to carefully monitor the developing trends in the guidelines of western countries.
4.2 IMPELLA
This is a flow assist device placed in the left ventricle, moving blood from the left ventricle to the aorta through the use of a driving motor. Use of this catheter-based device leads to improvement in cardiac function through reduction in the LV pre-load and increase in coronary blood flow. In an acute setting, this device may be inserted rapidly and minimally invasively. IMPELLA®
is suitable for ACS and may be simultaneously used during revascularization because most ACS patients are treated with catheter intervention. According to small registry studies, the use of IMPELLA®
may be effective in patients suffering from cardiogenic shock.553
However, the superiority of IMPELLA®
over IABP has not been demonstrated in randomized clinical trials.554,555
Of note, these randomized clinical trials have small sample sizes and important limitations in patient selection, which does not negate the device’s effectiveness when implanted in optimal patients. At present, concrete recommendations for the use of IMPELLA®
have been avoided due to the lack of strong clinical evidence. The device has only been used recently in Japan. Thus, its applicability and effectiveness should be monitored closely. Further large-scale clinical trials involving optimal patients are warranted to objectively evaluate the effectiveness and safety of this device.
4.3 Venoarterial (VA)-Extracorporeal Membrane Oxygenation (ECMO) (Table 50)
Table 50.
Recommendations and Evidence Level of VA-ECMO Application
| |
COR |
LOE |
| VA-ECMO should be considered in patients with cardiogenic shock refractory to medical treatment.558,559 |
IIa |
C |
VA-ECMO may be considered until surgery in patients with progressive circulatory failure due to mechanical
complications.311,562 |
IIb |
C |
VA-ECMO may be considered in patients with severely impaired cardiac function after recovery from
cardiopulmonary arrest.566 |
IIb |
C |
Abbreviation: VA-ECMO, veno-arterial extracorporeal membrane oxygenation.
ECMO involves membrane oxygenation combined with a centrifugal pump. This system is easily inserted by a cardiologist. The outlet port is inserted in the femoral artery and the inlet port is positioned in the right atrium via the femoral vein. Blood is removed from the right atrium using a centrifugal pump and delivered to the femoral artery following oxygenation using a membrane oxygenator. Although in Japan VA-ECMO is also known as percutaneous cardiopulmonary support (PCPS) to align with the designation of western countries, the ACS committee has decided to describe PCPS as VA-ECMO in this guideline. Venovenous (VV)-ECMO should be distinguished from VA-ECMO. Although VA-ECMO is regularly used in Asian countries, its usage in Western countries is uncommon due to the availability of IMPELLA.556
In 2013, the PCPS study group reported based on a questionnaire that VA-ECMO was used in 7,697 patients within a period of three years.557
Among those, 3,298 cases were acute cardiopulmonary failure and 2,364 were other emergency conditions. Although the data for ACS were unclear, VA-ECMO may have been used in approximately 1,700 patients because 75% of patients in the SAVE-J study were diagnosed with ACS. Therefore, it was estimated that VA-ECMO was used in a total of 600 cases of ACS per year. Previous VA-ECMO studies were observational rather than randomized. VA-ECMO is used in the setting of acute circulatory failure and there is little data involving patients with ACS. In previous studies, the onset-to-reperfusion time and reperfusion success were also shown to be independent predictors in patients treated with VA-ECMO.558
It is thought that the efficacy of the cardio-supportive system was affected by the severity of cardiogenic shock. A previous retrospective, observational, single-center study suggested that VA-ECMO was effective for moderate shock but ineffective for severe cardiogenic shock.559
Moreover, another observational study demonstrated that the efficacy of VA-ECMO was determined by age and cardiac index.560
Unlike non-CAD such as fulminant myocarditis where restoration of cardiac function is highly likely, VA-ECMO may have been less efficacious in patients with ACS because the myocardial damage is fixed except for the area of salvage by coronary intervention.
In addition, early coronary reperfusion using PCI in ACS patients needing cardiopulmonary support requires more rapid and complete reperfusion. Therefore, an interventional cardiologist must work based on “time is muscle,” especially in patients who require a cardiopulmonary support system, to shorten door-to-device and onset-to-recanalization time.558
4.3.1 Indication for Cardiopulmonary Support System in the Emergency Room
The indication for the cardiopulmonary support system in patients with ACS in emergency room is as follows: 1) cardiopulmonary arrest on arrival and absence of response after ACLS; 2) fatal arrhythmia with cardiogenic shock combined with resistance to catecholamines without circulatory collapse. Moreover, circulatory failure due to mechanical complication can also be considered an indication for the cardiopulmonary support system. Although systemic oxygenation is maintained, ventricle septal perforation causes left-to-right ventricular shunting and decreases forward flow, leading to progression of metabolic acidosis. VA-ECMO is considered to be particularly indicated in patients with ventricle septal perforation because of the expected removal of blood from the right ventricle with volume overload.561,562
The main mechanical complication is rapid progression of circulatory failure, and it is not uncommon to use the cardiopulmonary support system while confirming the diagnosis. The selection between VA-ECMO or IABP depends on the condition of the patient. Use of VA-ECMO is preferred in cases of rhythm failure causing complete failure or catecholamine refractory cardiogenic shock. If there is relatively more time to spare, IABP may be preferred followed by coronary revascularization and VA-ECMO depending on the condition of the patient. However, following the introduction of IMPELLA®, this treatment approach was modified. The reasons for the modification are as follows:544
there is no requirement for VA-ECMO to support oxygenation because of the absence of respiratory insufficiency;545
VA-ECMO is not a physiological support because it increases afterload, whereas IMPELLA®
can provide physiological support;3
lack of evidence for IABP. The circumstances of cardiopulmonary support systems has reached a transition period. This was suggested by the low frequency of VA-ECMO usage in the CULPRIT-SHOCK study (limited to 20% of patients) and the high frequency of IMPELLA®
usage.311
The JCS guideline working group recommends referring to the JRC guideline for cardiopulmonary support systems for the treatment of patients with ACS and cardiopulmonary arrest. There are several references regarding coronary intervention during extracorporeal cardiopulmonary resuscitation.563–565
The JRC guideline includes low-level evidence for the use of the cardiopulmonary support system in patients with low cardiac function following recovery from cardiopulmonary arrest.566
4.3.2 Weaning From VA-ECMO
Following the recovery of cardiac function, weaning from VA-ECMO is attempted. Weaning from VA-ECMO is when supported flow is decreased to 1.0 L/min and there is maintenance of cardiac function and circulation index during an on/off test. In general, blood pressure, heart rate, LV function, LVEF, cardiac index, end tidal CO2, and urine output are all assessed prior to weaning from VA-ECMO. However, there is a lack of prospective studies and the level of evidence is currently low. Previous studies showed that base excess567
and APACHE II scores568
were good indices for weaning from VA-ECMO. However, these studies were retrospective and characterized by small sample sizes. Additional multicenter prospective studies are warranted to investigate weaning from VA-ECMO.
4.4 LVAD (Table 51)
Table 51.
Recommendation and Evidence Level of LVAD Application
| |
COR |
LOE |
Extracorporeal LVAD may be considered in patients with cardiogenic shock with left main or multivessel
disease for whom weaning from IABP, VA-ECMO or IMPELLA® is not possible.566,567 |
IIb |
C |
Abbreviations: LVAD, left ventricular assist device; IABP, intra-aortic balloon pumping; VA-ECMO, veno-arterial extracorporeal membrane oxygenation.
Currently, LVAD is used in a limited the number of patients with ACS. Among patients with ACS, LVAD is used in those with severe CAD (e.g., left main and multivessel disease). Under such conditions, there are quite a few patients treated with IABP or VA-ECMO prior to introducing the LVAD system. Generally, LVAD is considered in patients with difficulty to wean from VA-ECMO or IABP following the acute phase. However, the use of LVAD is limited by the occurrence of complications (e.g., multiple organ failure) observed in such cases. The LVAD system is introduced after overcoming multiple organ failure and infection. In a small, retrospective, observational study, the rate of “bridge-to-LVAD” was 37% of patients with left main-ACS treated with VA-ECMO. This study also showed that severe circulatory failure and heart failure were independent predictors of mortality (≥Killip class III).569
The main LVAD system involves the removal of blood from the left ventricle or atrium and blood supply to the aorta via a thoracotomy. LVAD is classified into two systems, termed extracorporeal and implantable. Ordinarily, because use of LVAD is revised over time, the extracorporeal type is used. For details of the indication for LVAD, please see the “Implantable Artificial Heart Treatment Guidelines 2013 for Severe Heart Failure” of the Society.570
5. Management of Arrhythmias
5.1 Ventricular Arrhythmias
5.1.1 Ventricular Fibrillation (VF), Ventricular Tachycardia (VT) (Table 52)
Table 52.
Recommendations and Evidence Level Regarding Ventricular Arrhythmia Treatment
| Recommendations |
COR |
LOE |
Immediate electrical cardioversion should be performed for sustained VT and VF, and should
be performed under sedation in patients with hemodynamically stable VT.571,596
|
I |
B |
Intravenous amiodarone or nifekalant is recommended for treatment of recurrent, refractory,
and hemodynamically unstable VT or polymorphic sustained VT or VF.579–585 |
I |
B |
| Immediate PCI and CABG is recommended for the treatment of myocardial ischemia.258,578 |
I |
C |
Electrolyte imbalances (especially hypokalemia and hypomagnesemia) should be corrected
in patients with VT or VF.166,577 |
I |
C |
| Overdrive pacing should be considered for recurrent VT.589 |
IIa |
C |
Radiofrequency catheter ablation should be considered in patients with recurrent VT or VF
(electrical storm) despite of complete revascularization and optimal medical therapy.590,591 |
IIa |
C |
Lidocaine may be considered in patients with recurrent, refractory, hemodynamically unstable VT
or polymorphic sustained VT, if amiodarone and nifekalant are not effective or applicable.166,597 |
IIb |
B |
Abbreviations: VT, ventricular tachycardia; VF, ventricular fibrillation; PCI, percutaneous coronary intervention; CABG, coronary artery bypass grafting.
Ventricular tachycardia (VT) and ventricular fibrillation (VF) often develop in the early phase of ACS, which are responsible for out-of-hospital death. The incidence of death due to VT and VF has declined over recent decades, most probably due to the uptake of reperfusion strategies, ICU management, and the immediate electrical cardioversion.571
The retrospective analysis of GUSTOIIB and GUSTOIII trial demonstrated that VT or VF was documented in 1,126 (5.9%) of 19,190 patients with AMI.572
According to the APEEXAMI study in the PCI era, VT or VF was detected in 329 (5.7%) of 5,745 patients with STEMI, of which 282 patients developed VT/VF within 48 hours after onset of STEMI.99
A meta-analysis of 57,158 STEMI patients showed that ST-segment elevation on admission ECG, early onset, smoking, male gender, absence of history of angina, low heart rate, atrioventricular block, and hypokalemia are independently associated with primary VF.573
The prognostic value of early VT/VF within the first 48 hours of ACS is still controversial. Recent studies suggested that it was associated with in-hospital death but not associated with long-term prognosis.574,575
a. Prevention
No reports have demonstrated that prophylactic administration of antiarrhythmic drugs improves prognosis considering in-hospital death, as well as mortality at 30 days and 60 days. Therefore, prophylactic antiarrhythmic drug therapy is not recommended for patients with suspected ACS both in and out of hospital. β-blocker therapy is recommended if there are no contraindications, since it has demonstrated a significant reduction in the incidence of primary VF.576
AHA/ACC/ESC guidelines recommend use of β-blockers in the acute phase of ACS unless contraindicated.58,166
Correction of electrolyte imbalances (K >4.0 mEq/L, Mg >2.0 mg/dL) is recommended, because it predisposes to VT/VF.577
b. Treatment
Urgent revascularization is critical as ischemia often triggers VT/VF.578
Immediate electrical cardioversion is necessary for VF or pulseless VT. If there is no sufficient control after repetitive electrical cardioversion, intravenous administration of amiodarone should be considered. The recommended initial dose of amiodarone is 300 mg or 5 mg/kg body-weight. As compared with lidocaine and placebo, amiodarone leads to higher rates of survival at hospital admission in patients with shock-resistant VF/VT.579,580
However, it does not increase survival rates to hospital discharge. In case where amiodarone is contraindicated, lidocaine may be considered, although no studies have shown an increase in rate of return of spontaneous circulation.
Similar to amiodarone, nifekalant showed higher rates of survival at hospital admission in patients with shock-resistant VF/VT compared with lidocaine.579,580
However, it did not lead to an increase in the rate of survival at hospital discharge or discharge without neurological sequelae. Nifekalant is a pure potassium channel blocker without negative inotropic effect, which has been developed in Japan. It can be considered in cases of recurrent VT/VF after cardioversion, as it was reported to be effective for recurrent VT/VF.581,582
Intravenous β-blocker therapy,586,587
IABP devices, VA-ECMO, and urgent PCI should be considered in patients with recurrent VF or pulseless VT despite antiarrhythmic drug therapy. Electrical cardioversion under sedation is indicated for hemodynamically stable VT.
Some patients may develop an electrical storm despite complete revascularization. β-blocker therapy and deep sedation may be effective for suppression of VT.588
Furthermore, overdrive pacing or radiofrequency catheter ablation may help to control VT in some cases.589–591
5.1.2 Premature Ventricular Contractions (PVC)
Accelerated Idioventricular Rhythm (AIVR) and Accelerated Junctional Rhythm (Table 53)
Table 53.
Recommendations and Evidence Level Regarding Antiarrhythmic Treatment
| |
COR |
LOE |
| Prophylactic treatment for sustained VT/VF with antiarrhythmic drugs is not recommended.598,599 |
III: No
benefit |
B |
| Antiarrhythmic drugs should not be administered for hemodynamically irrelevant PVC.166,600 |
III:
Harm |
B |
Abbreviations: VT, ventricular tachycardia; VF, ventricular fibrillation; PVC, premature ventricular contraction.
Premature ventricular contractions (PVC), accelerated idioventricular rhythm (AIVR) and accelerated junctional rhythm are very frequent in the acute phase of ACS. Their value as predictors of VT/VF is questionable and no specific therapy is required.
5.1.3 Ventricular Arrhythmias Over 48 Hours After Onset of ACS (Table 54)
Table 54.
Recommendation and Evidence Level of ICD Application
| |
COR |
LOE |
ICD implantation should be performed for patients with sustained VT/VF over 48 hours after
onset of AMI, which are not related to acute ischemia or other reversible causes.592–594 |
I |
B |
Abbreviations: ICD, implantable cardioverter defibrillator; VT, ventricular tachycardia; VF, ventricular fibrillation; AMI, acute myocardial infarction.
Implantable Cardioverter Defibrillator (ICD) implantation is indicated for patients with VT/VF over 48 hours after onset of ACS.592–594
Patients with non-sustained VT should be re-evaluated for ICD implantation 40 days or later after ACS onset under optimal medical therapy. Although no antiarrhythmic drugs are required for PVC, increase the dose of β-blockers and consider correction of electrolyte imbalances.
5.1.4 Wearable Cardioverter Defibrillator (WCD) for Primary Prevention of Sudden Cardiac Death (Table 55)
Table 55.
Recommendation and Evidence Level of WCD Application
| |
COR |
LOE |
| WCD may be considered for patients with LVEF ≤35% and NYHA class II or III within 40 days after AMI onset.595 |
IIb |
C |
Abbreviations: WCD, wearable cardioverter defibrillator; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; AMI, acute myocardial infarction.
In general, ICD is not indicated for ACS patients without VT/VF, within 40 days after onset of ACS. These patients should be re-evaluated at 6-12 weeks after onset of ACS.166
However, a wearable cardioverter defibrillator (WCD) may be considered for patients with high risk of sudden cardiac death. A statement by the Japanese heart rhythm society suggests that WCD may be considered for patients with LVEF ≤35% and New York Heart Association (NYHA) class II or III within 40 days after ACS onset.595
5.2 Supraventricular Arrhythmias (Table 56)
Table 56.
Recommendations and Evidence Level Regarding Supraventricular Arrhythmia Treatment
| |
COR |
LOE |
Synchronized cardioversion should be performed in patients with atrial fibrillation or flutter,
where the heart rate cannot be fully controlled using pharmacotherapy and is associated
with hemodynamic deterioration or refractory ischemia.601–604 |
I |
C |
Unfractionated heparin should be administered to patients with atrial fibrillation or flutter
unless antithrombotic therapy is contraindicated.605 |
I |
C |
| β-blockers should be administered to patients with rapid atrial fibrillation or flutter.606,607 |
I |
C |
Intravenous administration of β-blockers should be considered to reduce the rapid ventricular
response due to atrial fibrillation/flutter in patients without bronchospasm or AV block.608,609 |
IIa |
C |
Intravenous administration of non-dihydropyridine CCBs should be considered to reduce the
rapid ventricular response due to atrial fibrillation/flutter in patients without reduced LV function
or AV block.610 |
IIa |
C |
Intravenous administration of amiodarone should be considered to reduce the rapid ventricular
response due to atrial fibrillation/flutter and improve LV function.611–614 |
IIa |
C |
Intravenous administration of digoxin should be considered to reduce the rapid ventricular
response and improve LV function in patients with atrial fibrillation/flutter with severe LV
dysfunction or heart failure.615 |
IIa |
C |
Class Ic antiarrhythmic agents should not be administered to patients with atrial fibrillation
or flutter.607,616 |
III:
Harm |
B |
Abbreviations: AV, atrioventricular; CCB, calcium channel blocker; LV, left ventricular.
Synchronized cardioversion is recommended in atrial fibrillation or flutter accompanied by hemodynamic deterioration or refractory ischemia. The initial shock should be 200 J if monophasic and 120–200 J if biphasic; incrementally increase the energy of subsequent shocks if defibrillation fails.601–604
It is recommended to stabilize the heart rate using pharmacotherapy in cases of atrial fibrillation that are unresponsive to cardioversion, or immediately recur after cardioversion, and those without complications of hemodynamic deterioration or heart failure.
β-blockers are the preferred medications in atrial fibrillation, as long as serious chronic obstructive pulmonary disease and allergies are absent.606,607
Propranolol, landiolol, or esmolol should be administered intravenously to gain control in the acute phase (propranolol, 2–10 mg, not to exceed 1 mg/min; landiolol, 1μg/kg/min as the initial dose, 1–10 μg/kg/min thereafter; esmolol, single 0.1 mL/kg or 1 mg/kg dose for 30 s). Administration should be discontinued if blood pressure drops below 100 mmHg or the heart rate is below 50 bpm. Intravenous administration of β-blockers during the acute stages of an AMI was reported to stabilize heart rate and reduce the oxygen demand of the cardiac muscle.608,609
If β-blockers are contraindicated, consider intravenous administration of CCBs, such as verapamil or diltiazem. However, clinicians must be vigilant of the risk of exacerbating heart failure due to the negative inotropic effects of CCBs.610
Atrial fibrillation may be treated with intravenous amiodarone: the drug acts as a CCB and suppresses sympathetic nervous activity, thereby inhibiting atrioventricular (AV) conductivity. Intravenous amiodarone is unlikely to restore sinus rhythm but is expected to control heart rate effectively.611
No studies have evaluated the efficacy of amiodarone in ACS complicated by atrial fibrillation. However, studies have demonstrated its efficacy in stabilizing heart rate in cases of heart failure612
and treating patients with atrial tachyarrhythmias persisting even after conventional therapy.613
In the 2014 AHA Guidelines, amiodarone is classified as a Class IIa treatment for heart rate control in cases of AMI complicated by atrial fibrillation.614
Unfortunately, intravenous amiodarone is not covered by health insurance in Japan.
Digoxin is an alternative therapy for AMI cases complicated by severe LV dysfunction or heart failure.615
Physicians must be careful of overdosing digoxin in patients with hypokalemia or impaired renal function, as well as elderly individuals. However, the effects of digoxin take an hour or more to appear, and do not reach peak until after more than 6 hours. The effects of digoxin are reduced in sympathetic hyperactivity.
AV-node reentry paroxysmal supraventricular tachycardia is characterized by a significantly increased heart rate; recommended treatments are as follows:617
1) Vagus nerve stimulation (e.g., Valsalva maneuver)
2) Intravenous adenosine triphosphate (ATP, 10 mg for 1–2 s; if persistent, 20 mg after 1–2 min, followed by repeated 20 mg doses if necessary)
3) Synchronized cardioversion, if persistent.
5.3 Bradyarrhythmias (Tables 57,58)
Table 57.
Recommendations and Evidence Level Regarding Medication Therapy for Bradyarrhythmia
| |
COR |
LOE |
| Administration of intravenous atropine should be considered to treat symptomatic bradycardia.59 |
IIa |
C |
| Administration of intravenous epinephrine or dopamine may be considered to treat symptomatic bradycardia.59 |
IIb |
C |
Table 58.
Recommendations and Evidence Level of Temporary Pacing for Bradyarrhythmia
| Bradyarrhythmia indications for temporary pacing |
COR |
LOE |
Temporary pacing should be performed for the following types of bradyarrhythmia:59
① Complete AV block
② Symptomatic bradycardia non-responsive to pharmacotherapy
③ Mobitz II second-degree AV block complicated by bifascicular block or new bundle branch block |
I |
C |
Temporary pacing should be considered for the following types of bradyarrhythmia:618
1) Mobitz II second-degree AV block (unless ③ applies)
2) Bifascicular block or new bundle branch block associated with impaired AV nodal conduction
(unless ③ applies) |
IIa |
C |
Abbreviation: AV, atrioventricular.
Many kinds of bradyarrhythmia can arise in ACS as a result of an impaired conduction system, including sinus bradycardia, AV block, and bundle branch block. However, bradyarrhythmias are relatively uncommon, affecting only 0.3–18% of patients with ACS; symptomatic bradyarrhythmia as an indication for pacemaker has an even lower incidence. Sinus bradycardia in ACS is often complicated by an inferior AMI. Increased parasympathetic activity is considered to be another contributing factor.619
In ACS cases, sinus bradycardia is usually temporary and asymptomatic. Potential symptoms and signs include dizziness, shortness of breath, fainting, chest pain, and hypotension; however, even if they appear, most patients will recover when administered with intravenous atropine (0.5–1 mg, max. rate of 0.04 mg/kg every 5 minutes). If that is ineffective, administer intravenous adrenaline (2–10 μg/min) or dopamine (2–10 μg/kg/min), and consider transcutaneous pacing.59,618
Some ACS cases strongly suspected to later progress to complete AV block require temporary pacing, even in the absence of bradycardia or associated symptoms at the initial phase. Transcutaneous pacing and intravenous atropine are recommended therapies for symptomatic AV block.59,618
In many cases, although the AV block is observed early in the course of inferior AMI, normal sinus rhythm is promptly restored by reperfusion therapy alone.620
Permanent pacing is not indicated in all patients with ACS, even if they required temporary pacing during the acute stage. Moreover, pacing has not been shown to have any benefit in treating cardiac arrest.621–623
Cardiac arrest should be handled by starting cardiopulmonary resuscitation and administering adrenaline and vasopressin.
Indications for Permanent Pacemaker Implantation
Whether permanent pacing is indicated for AV block treatment depends on whether abnormal conduction is present in the His-Purkinje system or more distal cardiac conduction system. Indications are not necessarily determined by the presence or absence of symptoms. If initial treatment is expected to completely eliminate AV block, or pacemaker implantation is thought to have no effect on long-term prognosis, there is no need to immediately perform permanent pacemaker implantation. Cases of spontaneous recovery of AV nodal conduction more than one week following an AV block have been reported. The long-term prognosis of patients with the complication of AV block is not dependent on the severity of the AV block but rather the extent of myocardial damage and specific characteristics of the abnormal intraventricular conduction.624
The indication for permanent pacemaker in sinus node dysfunction is independent of myocardial infarction comorbidity. Sinus node dysfunction is usually transient when it appears within an hour after inferior AMI onset or after reperfusion of the RCA. Even if temporary pacing is required, clinicians should avoid implanting a permanent pacemaker.
Treat both AV block and sinus node dysfunction according to the “Guidelines for Non-Pharmacotherapy of Cardiac Arrhythmias” (JCS 2018).625
6. Heart Failure Evaluation and Treatment
6.1 Hemodynamic Evaluation
Hear failure is defined as a clinical syndrome characterized by typical symptoms (e.g. breathlessness, ankle swelling and fatigue) and signs (e.g. elevated jugular venous pressure, pulmonary congestion and peripheral edema) caused by a structural and/or functional cardiac abnormality, resulting in a reduced cardiac output and/or elevated intracardiac pressures.626
In STEMI, rapid loss of viable myocardium results in systolic and diastolic ventricular dysfunction. A loss of 20% or more of LV myocardium causes heart failure symptoms, and 40% or more loss may result in cardiogenic shock.627
Small infarction may result in heart failure when prior myocardial infarction is present. Extension of infarct area during clinical course may cause delayed heart failure development even if the patient does not initially show such symptoms.
Killip classification assesses the severity of pump failure of AMI using clinical findings including auscultation. Killip classification is widely used in daily clinical practice81,628
(Table 3). The mortality rate of patients with Killip class IV remains still high despite progressing PCI.20,629,630
According to Tokyo CCU network 2012, the in-hospital mortality rate was 32.8% for males and 39.8% for females. Early reperfusion therapy reduced the mortality rate in patients with cardiogenic shock.157,631
Pulmonary artery catheterization accurately assesses the severity of hemodynamic parameters.632
Forrester et al proposed the use of pulmonary capillary wedge pressure and cardiac index to categorize patients with AMI into 4 hemodynamic subsets. To characterize the relation between clinical and hemodynamic state in AMI, 200 patients with AMI were evaluated with clinical and hemodynamic criteria.633
Subset I: PCWP ≤18 mmHg, CI >2.2 L/min/m2
No pump failure; intravenous nitrates, ACE inhibitors, ARBs, and β-blockers may be considered in patients without heart failure
Subset II: PCWP >18 mmHg, CI >2.2 L/min/m2
Pulmonary congestion; Vasodilators and loop diuretics are recommended in patients with pulmonary edema.
Subset III: PCWP ≤18 mmHg, CI ≤2.2 L/min/m2
Peripheral hypoperfusion; Fluid infusions are recommended, and inotropic agents may be considered in patients with the presence of RV dysfunction. Temporary pacing is indicated in cases of bradycardia.
Subset IV: PCWP >18 mmHg, CI ≤2.2 L/min/m2
Pulmonary congestion and peripheral hypoperfusion; Inotropes and/or mechanical support such as IABP and/or VA-ECMO are often needed to maintain systolic blood pressure >90 mmHg.
Nohria et al. demonstrated that patients were classified by the physical examination into four profiles: profile A, patients with no evidence of congestion or hypoperfusion (dry-warm); profile B, congestion with adequate perfusion (wet-warm); profile C, congestion and hypoperfusion (wet-cold); and profile L, hypoperfusion without congestion (dry-cold).626,634
The Nohria-Stevenson classification is analogous to the Forrester classification.
The severity of pump failure can be evaluated by blood pressure, heart rate, urine volume, cardiac auscultatory findings, and chest radiographic finding in most AMI patients without significant complications.635
Swan-Ganz catheter may not be reliable in measuring pressures especially when the tip position is not optimal. Major complications of Swan-Ganz catheter include ventricular arrhythmias, bundle branch block, pulmonary hemorrhage, infection, and thrombus formation. In 2005, a meta-analysis showed that the use of Swan-Ganz catheter in critically ill patients did not improve survival or decrease the length of hospital stay.636
Therefore, current indication of Swan-Ganz catheter is relatively limited. Swan-Ganz catheter should be considered when the benefit of information obtained by Swan-Ganz catheter outweighs the risks.
Invasive monitoring with a radial artery line is recommended in patients with hypotension and cardiogenic shock. Swan-Ganz catheter or arterial lines preferably should not be placed >5 days in the same access site to prevent infection.637
6.2 Pulmonary Congestion, Acute Pulmonary Edema (Table 59)
Table 59.
Recommendations and Evidence Level Regarding Treatment of Pulmonary Congestion and Acute Pulmonary Edema
| |
COR |
LOE |
ACE inihibitors should be administered to patients with LVEF ≤40% in the absence of
contraindications including hypotension, renal dysfunction, or bilateral renal artery stenosis.508,658 |
I |
A |
Mineralocorticoid antagonist should be administered to patients with LVEF ≤40% in the
absence of severe renal failure or hyperkalemia.512 |
I |
A |
| Diuretics are recommended for patients with pulmonary congestion with fluid overload.645,660 |
I |
C |
| Nitrate is recommended for patients without hypotension.645,660 |
I |
C |
Oxygen (including NPPV) is recommended for patients with pulmonary congestion with
oxygen saturation <90%.640–643 |
I |
C |
Echocardiography is recommended for patients with heart failure to evaluate LV function and
mechanical complications.660 |
I |
C |
| Morphine may be considered in patients with pulmonary congestion.646,659 |
IIb |
B |
Inotropic/vasopressor agents may be considered in patients with pulmonary congestion with
hypotension (systolic blood pressure <90 mmHg or decreased by ≥30 mmHg from baseline).660 |
IIb |
C |
| IABP may be considered in patients with pulmonary congestion resistant to pharmacotherapy.654 |
IIb |
C |
Abbreviations: ACE, angiotensin converting enzyme; LVEF, left ventricular ejection fraction; NPPV, noninvasive positive pressure ventilation; LV, left ventricular; IABP, intra-aortic balloon pumping.
Early reperfusion is an important first step in the treatment of STEMI. Primary PCI is more effective than fibrinolysis in patients with severe heart failure.638,639
Oxygen therapy is recommended to treat tissue hypoxia. Oxygen can be adjusted according to oxygen saturation monitoring. Non-invasive positive pressure ventilation (NPPV) is effective in patients with pulmonary congestion with oxygen saturation <90%.640–643
Pressure support ventilation (PSV), positive end-expiratory pressure (PEEP), biphasic positive airway pressure (BiPAP), and, continuous positive airway pressure (CPAP) are recommended. Endotracheal intubation may be required in patients unable to keep adequate blood oxygenation.644
Pharmacological therapy to reduce cardiac preload includes morphine, nitrates, and diuretics.645
Morphine (3–5 mg intravenous) has analgesic effects as well as vasodilation and negative chronotropic effects.646
Nitrates (nitroglycerine, isosorbide) and nicorandil reduce preload by venodilation in lower dose. They reduce afterload by dilatation of the arteries, and also increase cardiac index in higher dose. They are used to maintain systolic pressure of 90–100 mmHg; however, higher blood pressure target may be applied for elderly or hypertensive patients.
Loop diuretics are first choice of diuretic therapy. Caution should be exercised when using diuretics to hypotensive patients with dehydration, or elderly patients. On the other hand, higher doses may be required for patients with renal impairment or patients already on diuretics. Electrolyte correction such as sodium, potassium, and magnesium is required using diuretics.
Tolvaptan, a vasopressin receptor 2 antagonist, may be considered in patients who are refractory to other diuretics, especially in patients with hyponatremia.647
Carperitide, an atrial natriuretic peptide (ANP), is approved for the treatment of heart failure in Japan. In addition to its vasodilator and diuretic effect, it suppresses the renin-angiotensin-aldosterone system and sympathetic nervous system, both of which may exert cardiac and renal protection effects.648–650
6.3 Low Cardiac Output State
Cold extremities, cyanosis, decreased urine output, and deteriorated mental status are observed in the absence of hypotension in patients with low cardiac output.651
These symptoms are often seen in patients with low cardiac output due to mechanical complications. RV infarction should also be ruled out in low cardiac output patients. In patients with RV infarction, adequate fluid resuscitation and intravenous inotropic agents (e.g., dobutamine 1–5 g/kg/min) are indicated. IABP should be considered in patients with hemodynamic instability. Revascularization by PCI or CABG improves myocardial ischemia to achieve hemodynamic stability. IABP should be considered for mechanical complications including VSP, PMR, and LVFWR, because of relative ineffectiveness of medical therapy.
6.4 Cardiogenic Shock (Table 60)
Table 60.
Recommendations and Evidence Level Regarding Treatment of Cardiogenic Shock
| |
COR |
LOE |
| Emergent CABG is recommended, if PCI is inappropriate or unsuitable for revascularization.158,252 |
I |
B |
| Invasive arterial pressure monitoring is recommended.660,661 |
I |
C |
| Echocardiography is recommended to evaluate LV function and mechanical complications.660,661 |
I |
C |
| Heart Team should discuss the patients with mechanical complications.660,661 |
I |
C |
| Oxygen and respiratory support is recommended.640–643 |
I |
C |
| IABP should be used in patients with cardiogenic shock due to mechanical complications.626,660 |
I |
C |
| VA-ECMO should be considered in patients with treatment-resistant cardiogenic shock. |
IIa |
C |
| Complete revascularization by PCI or CABG should be considered.158,252 |
IIa |
C |
| Hemodynamic assessment with Swan-Ganz catheter may be considered.636 |
IIb |
B |
| Ultrafiltration may be considered for patients resistant to diuretics.662,663 |
IIb |
B |
| Inotropic/vasopressor agents may be considered.661,664 |
IIb |
C |
| Routine IABP is not recommended in patients with cardiogenic shock.548,665,666 |
III: No
benefit |
B |
Abbreviations; CABG, coronary artery bypass grafting; PCI, percutaneous coronary intervention; LV, left ventricular; IABP, intra-aortic balloon pumping; VA-ECMO, veno-arterial extracorporeal membrane oxygenation.
Cardiogenic shock remains the leading cause of death in patients hospitalized with AMI. Cardiogenic shock often results from LV dysfunction due to extensive infarction. Transthoracic echocardiography is useful to evaluate LV function and mechanical complications for patients with cardiogenic shock.652
Cardiogenic shock is defined as (1) persistent hypotension (systolic blood pressure <90 mmHg or decreased by ≥30 mmHg from baseline), (2) diuresis <20 mL/h, (3) deteriorated consciousness, (4) sweating, cool hands and feet. Immediate search for the cause and treatments should be initiated. Hypovolemia is present in 10∼15% of patients with cardiogenic shock; and pulmonary congestion on chest x-ray is not seen in 30% of the patients. Therefore, invasive monitoring with an arterial line is recommended to use inotropic/vasopressor agents and diuretics.80
According to the SHOCK Trial Registry, only 9% patients were in shock at arrival. Nevertheless, about half of patients (47%) developed cardiogenic shock within 6 hours of AMI onset and 74% of patients would eventually develop cardiogenic shock by 24 hours.653
Possible explanations may include (1) the diagnosis of pre-shock or severe heart failure may be missed at arrival, (2) agent-induced hypotension may cause iatrogenic hypotension. It is important for these patients to be adequately assessed for hemodynamic deterioration, and for the need for revascularization therapy.
The first step in patients with cardiogenic shock is intravenous dopamine 5–15 g/kg/min in case of hypotension and intravenous dobutamine 2–15 ug/kg/min with keeping blood pressure. Norepinephrine (0.03∼0.3 g/kg/min) can be added to dopamine/dobutamine to maintain systolic blood pressure >90 mmHg. IABP and VA-ECMO should be considered in patients with hemodynamic instability/cardiogenic shock.654,655
Dopamine is a precursor of noradrenaline, which changes its mechanism of action by dosing. Lower dose (<2 g/kg/min) acts on peripheral dopamine receptor to increase renal blood flow. Mid-range dose (>2 g/kg/min) dopamine acts on β receptor to increase myocardial contractility. Higher dose (>5 g/kg/min) exerts α receptor activation to constrict blood vessels. Dobutamine increases myocardial contractility through β1 receptor. Low output state con be treated with phosphodiesterase (PDE) inhibitors (milrinone, olprinone) or an adenylate cyclase stimulator.
Primary PCI has been the first choice of treatment in patients with STEMI. Early revascularization is particularly important in patients with AMI complicated by cardiogenic shock. In a Japanese multi-center registry of 3,113 STEMI patients from 1996 to 1998, the mortality rate of 126 cardiogenic shock patients was 59%.656
In the SHOCK Trial, patients with AMI and cardiogenic shock were randomly assigned to an early revascularization group (n=152), which mandated revascularization within 6 hours of randomization, and an initial medical stabilization group (n=150). The 30-day mortality did not differ between early revascularization group and initial medical stabilization group (46.7% vs. 56.0%, NS), but early revascularization resulted in improved 1-year survival (46.7% vs. 33.6%, P<0.03). In patients younger than 75 years, the 30-day mortality was lower in early revascularization group; therefore, early revascularization is of clinical importance in severe heart failure or cardiogenic shock patients.158,252,656
The details of primary PCI are stated in chapter
V section 1.3 ‘Primary PCI in Patients With Cardiogenic Shock’
in this guideline. In case when primary PCI is not available, or in case of longer transfer time to PCI institute, fibrinolysis may also be indicated.657
In patients who are already on β-blockers and/or ACE inhibitors, these agents can be withheld until hemodynamic stabilization. However, these medications should be restarted from lower doses once the congestion is resolved.
7. RV Infarction
RV infarction is often silent and only 10–15% of patients develop clinically evident hemodynamic manifestations on presentation.102,667
The right ventricle is a volume dependent chamber and, therefore, any significant insult can lead to severe hemodynamic compromise. The most common culprit vessel for causing RV infarction is occlusion of the RV branch or acute marginal branch of RCA. ST-segment elevation in leads V1 and V3R–V6R confirms RV infarction; ST-segment elevation in lead V4R in particular is highly sensitive and specific for RV infarction. Signs and symptoms can include some of the common manifestations of RV infarction such as hypotension, shock, elevated jugular venous pressure, Kussmaul’s sign, and pulsus paradoxus.102
Infarcted RV is stiff, dilated and dyskinetic, resulting in decrease in RV compliance, reduced filling and decreased RV stroke volume. These results decrease LV filling and cardiac output despite normal LV contractility. The LV compliance is further decreased by increased intrapericardial pressure as a result of RV dilatation.
Table 61
shows the diagnostic criteria for RV infarction.668,669
Table 61.
Diagnostic Criteria of RVI
| Autopsy |
|
Major
criteria |
• ST-segment elevation (at least 0.1 mV) in lead V4R on ECG
• RV akinesis or dyskinesis on echocardiography
• Average right atrial pressure of ≥10 mmHg, and (Average PCWP-average right atrial pressure) ≤5 mmHg
• Noncompliant right atrial waveform
• Pulsus alternans or early rising of pulmonary artery pressure |
Minor
criteria |
• Inferior AMI
• RV dilatation on echocardiography
• Average right atrial pressure of ≥6 mmHg (at rest)
• Kussmaul sign
• Accumulation of 99mTc pyrophosphate in the right ventricle |
Definitive
diagnosis |
1. Autopsy diagnosis
2. Clinical diagnosis
• 2 or more major criteria
• 1 major and 2 or more minor criteria (without overlapping echocardiography and average right atrial pressure criteria)
• 4 or more minor criteria |
Abbreviations: ECG, electrocardiogram; RV, right ventricular; AMI, acute myocardial infarction. (Modified reprint after permission granted Goto Y. 1988668)
The features of RV infarction treatment are based on its pathophysiology, which include optimization of oxygen supply and demand, optimization of ventricular preload, parenteral inotropic support for persistent hemodynamic compromise, restoration of physiologic rhythm, and early reperfusion. Invasive hemodynamic measurements by Swan-Ganz catheter provide reliable information about the extent and severity of right heart involvement. Diagnosis of RV infarction can be confirmed when the right atrial pressure exceeds 10 mmHg with pulmonary artery wedge pressure of 1–5 mmHg, and when the ratio of right atrial pressure to pulmonary capillary wedge pressure exceeds 0.8 (normal value <0.6).
Isotonic saline or low molecular weight dextran is appropriate if a patient has clear lungs, hypotension and a low jugular venous pressure, indicating low cardiac output, to increase RV filling which in turn will increase filling of the under filled LV and increase cardiac output, keeping pulmonary artery wedge pressure at 15 mmHg. Caution should be paid to avoid excessive volume administration above and beyond that documented to augment output, resulting in further decrease in RV pump performance and inducing severe systemic venous congestion. For those who experience no response to an initial trial of fluids (500–1,000 mL), catecholamine and mechanical support, such as use of IABP and RV assist device, may be appropriate.670
We should avoid agents that can decrease preload such as nitrates, opioids, and diuretics. Bradyarrhythmias may precipitate severe hemodynamic compromise in patients with RV infarction. AV sequential pacing may be necessary for increasing the cardiac output and reversing the shock associated with AV dyssynchrony in RV infarction.671
In patients with atrial fibrillation, prompt cardioversion and restoration of AV synchrony should be considered at the earliest sign of hemodynamic compromise. Early revascularization is the gold standard treatment, as it can clearly improve the clinical outcome and prevent AV block.672,673
8. Diagnosis and Treatment of Mechanical Complications After AMI (Table 62)
Table 62.
Recommendations and Evidence Level Regarding Treatment of Mechanical Complications
| |
COR |
LOE |
Mechanical complications after AMI should be rapidly diagnosed with echocardiography,
and cardiac surgeons should be consulted without delay for emergent surgical treatment. |
I |
C |
| IABP is recommended in patients with cardiogenic shock due to mechanical complications. |
I |
C |
Abbreviations: AMI, acute myocardial infarction; IABP, intra-aortic balloon pumping.
Mechanical complications after AMI indicate LV free wall rupture (LVFWR), ventricular septal rupture or perforation (VSR or VSP), or papillary muscle rupture (PMR). Tear and rupture of weakened myocardium due to infarction causes these mechanical complications. Sudden onset of circulatory deterioration often occurs due to cardiac tamponade by LVFWR and acute change of intra-cardiac blood stream by VSR and PMR, and the mortality rate of these three mechanical complications after AMI remains very high. In particular, LVFWR is reported to be the third most frequent cause of hospital mortality, after cardiogenic shock and congestive heart failure, in STEMI patients, although the frequency of occurrence is very low.674
① Incidence Rate
The APEX-AMI registry report in 2010 showed that mechanical complications after AMI occurred in 52 patients (0.91%) out of 5,745 STEMI patients: 0.52% in LVFWR, 0.17% in VSP, and 0.26% in PMR.675
Historically, the frequency of mechanical complications has been decreasing as the treatment for AMI has progressed from pre-reperfusion therapy and thrombolysis to PCI and stent implantation.676
② Period of Occurrence and Risk Factors
Previously, it was said that mechanical complications typically occur 2 or 3 days to 1 week after onset of AMI, but there are recent reports that mechanical complications have often occurred within 24 hours after onset of AMI.358
The risk factors for mechanical complications are delayed hospital admission (later than 24 hours after the onset of AMI), physical activity that raises blood pressure after onset, older age, female sex, first AMI, etc.677
On the contrary, reperfusion therapy, as well as use of β-blockers, ACE inhibitors, and aspirin, are reported to decrease the risk of mechanical complications after AMI.676
③ Diagnosis
Every mechanical complication after AMI requires emergent treatment due to acute hemodynamic deterioration. Echocardiography is extremely useful for bedside diagnosis. The diagnosis is confirmed on echocardiography by observing cardiac tamponade in LVFWR, left-to-right shunt blood flow between left and right ventricle in VSR, and severe mitral valve regurgitation associated with mitral valve prolapse and ruptured papillary muscle in PMR.
④ Treatment
Although surgical treatment is the only way to successfully treat mechanical complications after AMI, previous reports have demonstrated that surgical results are still not satisfactory. Acute circulatory deterioration negatively and strongly influenced the surgical results for mechanical complications. Therefore, early surgical intervention with maintenance of circulation by initiation of VA-ECMO and IABP for acute hemodynamic deterioration and prevention of systemic deterioration is considered to improve the surgical results.
8.1 Left Ventricular Free Wall Rupture (LVFWR)
① Condition
In the 1980 s, LVFWR occurred in up to 10% of AMI patients who died, which was higher than that of VSP (1–2%) and that of PMR (0.5–5%). However, the spread of PCI has decreased the occurrence rate of LVFWR, which is currently below 1% of patients with transmural AMI, within 7 days after AMI onset.166
LVFWR is classified into a blow-out (abrupt) type, where the infarcted portion suddenly ruptures causing circulatory collapse and an oozing (gradual) type, where the infarcted portion bleeds gradually. Risk factors for LVFWR are reported to be older age, female sex, hypertension, delayed reperfusion of an occluded coronary artery, and a first AMI.
② Diagnosis
Transthoracic echocardiogram should be carried out urgently when acute circulatory deterioration occurs after AMI.678
When the circulation is maintained, computed tomographic scans can also be useful. Pericardial effusion, cardiac tamponade, and blood with pericardiocentesis can confirm the diagnosis of LVFWR.
③ Treatment
The survival rate from LVFWR without surgery is quite low, and emergent surgery is the only effective treatment. The priority of treatment is recovery and maintenance of circulation, and therefore, initiation of VA-ECMO and IABP support is necessary in some cases for resuscitation. Even when circulation is maintained, the support of IABP prevents dilatation of the rupture site and rupture of suture lines after surgery by reducing afterload and therefore LV wall stress.
In addition to the initiation of mechanical circulatory assist, pericardial drainage is important. Percutaneous pericardial drainage is sometimes ineffective when the pericardial effusion becomes clotted, and blood clots have to be removed by urgent open-chest surgery.
In the oozing type, the sutureless technique is reported to be effective because bleeding has already stopped by the time of surgery in most cases.679–683
Because the sutureless technique can be performed as an off-pump procedure, adequate hemostasis can also be achieved without any bleeding tendency. In the blow-out type, it is necessary to perform direct closure or infarctectomy and patch closure.679,681,684
Suturing the weakened infarcted wall carries the risk of re-rupture, and therefore suturing the non-infarct LV wall is important.
④ Treatment Results
The mortality rate of LVFWR remains at approximately 80%, and the mortality rate of the blow-out type has remained quite high despite progress in diagnostic techniques and surgical procedures.359,680,685
On the other hand, the survival rate of the oozing type is good. According to the investigation in 2017 by the Japanese association of coronary artery surgery, the mortality rate was 66.6% in blow-out type, 20.2% in oozing type, and 35.1% in total in patients who could undergo surgery. Regarding salvage from LVFWR, recovery and maintenance of circulation by urgent pericardial drainage, rapid initiation of mechanical circulatory support such as VA-ECMO and IABP, and transferring patients to an operating room are the key to life-saving treatment.
8.2 Ventricular Septal Rupture or Perforation (VSR or VSP)
① Condition
The first report on VSR is quite old. Latham described the pathology of VSR in 1847.686
The rate of VSR has been decreasing because of aggressive coronary revascularization such as PCI. The rate of occurrence was 1 to 2% in the pre-thrombolytic era, 0.4 to 0.6% in the thrombolytic era, and is 0.17 to 0.31% at present.675,687–689
Although the rate of occurrence has decreased, the mortality rate remains high (41% to 80%).690
The data from which this information was collected, about one hundred and fifty thousand patients between 1990 and 2007, revealed that the rate of in-hospital death and one-year death was 41% and 60%, respectively, between 1990 and 1992, and 44% and 56%, respectively, between 2005 and 2007. The data also suggested that the benefit of surgical treatment for VSR was only for improvement of hospital mortality and not effective for 30-day survival or 1-year survival, and worsening systemic condition from sudden severe circulatory deterioration due to VSR strongly affected the low one-year survival rate.685
LAD supplies blood to the anterior apical ventricular septum, while the RCA and sometimes a septal branch of the dominant LCX supplies blood to the posterior and basal ventricular septum.690
Anterior AMI due to occlusion of LAD causes perforation near the apex, and posterior and inferior AMI due to occlusion of RCA and dominant LCX cause perforation near the basal ventricular septum. The occurrence rate of antero-apical VSR is reported to be identical to the occurrence rate of posterior basal VSR.
VSR tends to occur in patients who have one-vessel occlusion with lack of any collateral blood flow, which is similar to other mechanical complications.358,691,692
Left-to-right shunt flow is produced by VSR, and the amount of shunt flow, as well as the width of AMI, RV infarction, and myocardial stunning, influence the circulatory condition, and there is a range of hemodynamic conditions from severe circulatory deterioration to relatively stabilized circulation.693
② Diagnosis
The evaluation methods of VSR are basically auscultation, echocardiogram, and cardiac catheterization including left ventriculogram. A Levine 2-4 pan-systolic murmur is heard at left sternal border on auscultation. Transthoracic echocardiography is a rapid and less invasive method for evaluation of mechanical complications than left ventriculogram. The diagnosis of VSR is confirmed by shunt flow from LV to RV with the color doppler method. Left ventriculogram shows left-to-right shunt blood flow in left anterior oblique views in VSR. Moreover, O2
step up between the right atrium and pulmonary artery measured by a Swan-Ganz catheter strongly raises the suspicion of VSR, and the pulmonary blood flow/systemic blood flow ratio can be calculated from this.
③ Treatment
Blockage of shunt flow between LV and RV is the fundamental treatment for VSR. In sub-analysis of the GUSTO-I trial published in 2000, death rates of 47% in the surgical treatment group and 94% in the medical treatment group were reported.
There are two types of surgical procedure for VSR; Daggett procedure (patch closure) and David procedure (infarct exclusion).694,695
Many modified procedures to solve problems with these two procedures have been reported, and improvement in surgical results can be expected. According to data of JACAS in 2017, the infarct exclusion method was more frequently utilized for anterior VSR than the patch closure technique, and patch closure technique was frequently utilized for posterior and inferior VSR.696
④ Treatment Results
According to data of JACAS in 2017, the surgical mortality for VSR has been approximately 20 to 30% since 2000, and the mortality rate in 2017 was 23.2% in total.696
The mortality rate was 21.2% for anterior VSR, 23.8% for the patch closure technique, and 22.9% for the infarct exclusion technique. The mortality rate for posterior and inferior VSR (27.1%) was slightly higher than anterior VSP.
According to the STS (Society of Thoracic Surgeons) database on 2,876 patients between 1999 and 2010, the surgical mortality rate was 42.9% in total, 13.2% in elective surgery, 56.0% in emergent surgery, and 80.5% in salvage surgery, which suggested that the surgical results in patients who required surgery in the early phase after onset due to circulatory deterioration was extremely bad. Many papers reported that the surgical mortality was negatively related to the duration from onset of VSR to surgery.697
There is a paper that demonstrated that the surgical mortality was higher than 60% in patients whose surgery was performed within 24 hours from onset, 54% within a week, 18% at 7 days or later from the onset.698
The unsatisfactory results of early surgery might be caused by residual shunt flow due to rupture of sutures in weakened infarcted cardiac wall. Therefore, in patients who have relatively stabilized circulation with or without mechanical circulatory support, it might be reasonable to postpone VSR surgery until one or two weeks later to allow for recovery of weakened infarcted myocardium. In the guidelines for heart failure established by ESC, short term mechanical circulatory support for patients with severe circulatory deterioration is Class IIa.699
Some papers have reported on the feasibility of improving surgical results for VSR by postponing surgery or bridging to heart transplantation with strong circulatory support such as a LV assist device and waiting for weakened infarcted myocardium to recover.700,705
Moreover, percutaneous VSR closure has also been reported as a catheter intervention.706,707
Complete closure of the shunt blood flow remains difficult at this moment. However, this procedure can be expected to bridge to a surgical VSR closure due to decreasing the shunt flow.708
8.3 Papillary Muscle Rupture (PMR)
① Condition
As mentioned above, the occurrence of PMR has been decreasing with the spread of reperfusion therapy after AMI, and the occurrence rate was reported to be less than 1%.709,710
The risks of PMR are delayed admission and treatment, older age, hypertension, and inferior AMI compared to patients without PMR. There are reports that 70 to 80% of PMR develops within 5 days of AMI onset.709,710
The papillary muscles (PM) are usually divided into the anterolateral PM (ALPM) and posteromedian PM (PMPM), and the ALPM is often perfused from LAD and LCX, while the PMPM is often perfused only from RCA. Therefore, the incidence of posteromedian PMR (90%) is considered to be higher than that of anterolateral PMR.711–713
Rupture of both PM has also been reported.679
PMR can occur in patients with non-transmural AMI. Although many papers have reported that the incidence of PMR in patients with STEMI is identical to that in patients with NSTEMI, some papers have reported that 27% of PMR occurs in patients with STEMI, and 73% in patients with NSTEMI.48,49
PMR is divided into a complete rupture type and a partial rupture type, depending on the pattern of papillary muscle rupture. The partial rupture type is further subdivided into a type in which one of the multiple heads of papillary muscle is divided and a type in which the papillary muscle partially breaks and is likely to later progress to complete rupture. The incidence of complete rupture ranged from around 30% to over 90%, and there is a wide range of reports.713–715
Cardiogenic shock was observed at the onset of PMR in 70 to 90% of patients.713
Because the range of prolapse in complete PMR is wider than that in partial PMR, patients with complete PMR can easily develop shock at the onset, and it was reported that IABP was necessary in 58% of patients with a partial PMR and in 80% of patients with complete PMR.715
② Diagnosis
The diagnosis of PMR can be suspected when mitral regurgitation murmur can be heard in patients with cardiogenic shock after AMI.715
Transthoracic echocardiography is the most useful diagnostic tool.715
“Frail leaflet” is a strong finding for diagnosis of PMR because the diagnostic sensitivity is 88% to 93%. The four major signs on echocardiography for diagnosis are tissue in the left ventricle not related to LV motion, deviation of valve leaflets, mitral regurgitation, and hyperkinetic LV wall motion in non-infarct zone.716
Chordal rupture has a whip-like appearance (slim tip), which is different from PMR (PMR has some PM tissue at the tip of the chorda).716
When it is difficult to diagnose with transthoracic echocardiography, transesophageal echocardiography is useful.717
③ Treatment
Since the mortality rate of PMR before mitral valve replacement had become possible was 80%, surgical treatment is the first choice for PMR. Mitral valve replacement is selected in most cases because definitive control of mitral regurgitation is the priority.679
However, mitral valve plasty can be feasible in patients who have a small prolapse due to partial PMR or who have residual healthy PM: Fixation of ruptured PM to healthy PM or artificial chorda implantation to healthy PM.718
A recent paper reported percutaneous mitral valve clip for PMR.719
④ Treatment Results
Progress in surgical treatment has improved the surgical mortality from 30% to 9%.679,713–715
As with other mechanical complications, proper management of acute circulatory deterioration at the onset of the disease and rapid transfer to an operating room are most important to improve surgical results.
9. Prevention of Acute Kidney Injury (AKI) (Including CIN) After CAG and PCI (Table 63)
Table 63.
Recommendations and Evidence Level Regarding Prevention of AKI (Including CIN) After CAG and PCI
| |
COR |
LOE |
| Avoiding unnecessary angiography and use of the possible minimum contrast volume are recommended.723–725 |
I |
B |
Use of intravenous volume expansion with isotonic sodium chloride is recommended before and after PCI
in patients with decreased renal dysfunction.721 |
I |
B |
Use of intravenous volume expansion with sodium bicarbonate solutions before CAG may be considered
in patients with decreased renal dysfunction.733 |
IIb |
C |
| Hemodialysis or hemofiltration to prevent CIN in patients with CKD is not recommended.721,734–736 |
III: No
benefit |
B |
| Diuretics, especially loop diuretics, should not be administered in patients without pulmonary congestion.730 |
III:
Harm |
B |
| NSAIDs should not be administered within 24 hours before and after using contrast medium.721,731,732 |
III:
Harm |
C |
Abbreviations: PCI, percutaneous coronary intervention; CAG, coronary angiography; CIN, contrast-induced nephropathy; CKD, chronic kidney disease; NSAIDs, nonsteroidal anti-inflammatory drugs.
In clinical settings, various mechanisms such as CIN, cholesterol embolism, and decreased renal blood flow due to cardiogenic shock may be causes for acute kidney injury (AKI) after PCI in patients with ACS. Therefore, it is sometimes difficult to explain precisely the specific etiology of AKI particularly in patients with high risk and a large ischemic area, as well as unstable hemodynamic conditions, following primary PCI.
As an assessment of AKI, the RIFLE (Risk, Injury, Failure, Loss of kidney function and End stage kidney disease) classification by the Acute Dialysis Quality Initiative (ADQI) in 2004, AKIN (Acute Kidney Injury Network) classification in 2007, and Kidney Disease: Improving Global Outcomes (KDIGO) classification in 2012 have been used as the international diagnostic criteria of AKI. In ACS cases, apart from use of contrast medium, shock status frequently induces AKI due to renal hemodynamic changes by low cardiac output and venous congestion. Also, the immune-inflammatory response, acidosis, and acute hyperglycemia are related to the onset of AKI.733a
CIN is one of the crucial factors for AKI in ACS cases undergoing CAG and PCI. Interventional cardiologists should pay careful attention to the occurrence of CIN in both elective and emergency cases.
CIN has been defined as i) an increase in serum creatinine (SCr) by ≥0.5 mg/dL within 72 hours; or ii) an increase in SCr by more than 25%, as reported in 1999 by the European Society of Urogenital Radiology.720
This definition has been also published in the “Guidelines on use of iodine contrast medium in patients with kidney injury”, revised edition (2008)721
by the Japan Society of Nephrology, Japan Radiological Society and Japanese Circulation Society.
The definition of AKI has been applied to that of CIN because CIN occurs during the acute phase. However, this has been controversial and much has been discussed about whether the definition of AKI can be fully adopted to that of CIN. AKI grades by KDIGO, but not the RIFLE or AKIN classifications, have been used for grading severity of CIN (Table 64).722
Table 64.
Severity of CIN (Staging)
| Stage |
Serum creatinine |
Urine output |
| 1 |
1.5–1.9 times baseline
or
≥0.3 mg/dl increase |
<0.5 mL/kg/h for 6–12 hours |
| 2 |
2.0–2.9 times baseline |
<0.5 mL/kg/h for ≥12 hours |
| 3 |
3.0 times baseline
or
Increase in serum creatinine to ≥4.0 mg/dL
or
Initiation of renal replacement therapy
or
eGFR <35 mL/min/1.73 m2 in patients under 18 years of age |
<0.3 mL/kg/h for ≥24 hours
or
Anuria for ≥12 hours |
Abbreviation: eGFR, estimated glomerular filtration rate. (Adapted from Japanese Society of Nephrology. 2018721)
Close cooperation with nephrologists is required for patients with AKI caused by unstable hemodynamics, and optimal medical treatment, hydration, hemofiltration and dialysis should be considered.
The volume of contrast media is closely related to the onset of CIN, and the use of a smaller volume of contrast media reduces the risk for developing CIN.723–725
Also, presence of CKD itself is a risk for CIN in patients requiring PCI.721
In addition, renal impairment is frequently seen in patients undergoing emergency PCI compared to elective PCI. Pre-existing renal functional impairment is important and the risk for CIN is clearly increasing as patients develop worse renal function.726
Other risk factors for developing CIN particularly in ACS cases include anterior AMI, reperfusion time ≥6 hours from the onset, large volume of contrast media, anemia, hemodynamic instability, use of IABP and so on.727,728
A report has suggested that unstable hemodynamic condition is related to development of CIN regardless of the presence or absence of CKD.729
Therefore, it is essential to fully inform the risk of renal impairment in ACS cases with low cardiac function and unstable hemodynamic who require emergency CAG and PCI. In addition, it is important to minimize contrast volume and optimize hydration without developing heart failure.
In the clinical settings, simple risk scores for prediction of CIN proposed by Brown and Mehran are occasionally used. However, there have been no data prospectively evaluating these scores yet, therefore current Japanese guidelines by JSN, JRS and JCS Joint Working Group721
does not recommend use of such risk scores.
Until now, there has been no specific reason to perform hemodialysis and hemofiltration for prevention of CIN. In the guidelines on the use of iodinated contrast media in patients with kidney disease by the JSN, JRS and JCS Joint Working Group,721
hemodialysis and hemofiltration for preventing CIN is positioned as Grade D, which means that a given treatment or procedure is not recommended because scientific evidence indicating the inefficacy or harm of the treatment/procedure is available. It is also positioned as Class III in the guidelines. However, there have been limited data on efficacies of hemodialysis and hemofiltration for preventing CIN in patients with ACS.
Studies have reported that diuretics, particularly loop diuretics, have no preventive effects for CIN.721
On the contrary, they may increase the occurrence of CIN.730
Therefore, use of diuretics in emergency PCI should be limited in patients with pulmonary congestion. Although use of non-steroidal anti- inflammatory drugs (NSAIDs) is considered to be a risk factor for CIN due to decreased renal flow resulting in renal impairment, there has been no evidence indicating a causal association between NSAIDs and CIN.731
However, it is suggested that patients receiving NSAIDs regularly should discontinue them 24 hours before, and not renew treatment until 24 hours after contrast radiography, based on the recommendation of SCAI732
and the guidelines on the use of iodinated contrast media in patients with kidney disease by the JSN, JRS and JCS Joint Working Group.721
Studies have suggested that intravenous volume expansion with isotonic sodium chloride before and after angiography and using the lowest possible dose of contrast medium in cardiac catheterization are favorable regarding prevention of CIN. However, there has not been sufficient consideration in patients with ACS. In general, the evidence based methods of using isotonic sodium chloride and sodium bicarbonate solutions to prevent CIN are listed below.721
1. Administer physiological saline intravenously at 1 mg/kg/h 6 hours before and 6∼12 hours after the contrast examination.
2. Administer sodium bicarbonate solution (1.26%, 152 mEq/L) at 3 mL/kg/h for 1 hour before and at 1 mL/kg/h for 4∼6 hours after the contrast examination.
However, in patients who need emergent CAG immediately after arrival, such as those with STEMI, there is no conclusive evidence on the efficacy of short-term intravenous hydration before the contrast examination.
Although sodium bicarbonate-based hydration decreases the risk for developing CIN and its effect may be similar to be saline hydration,733
we position sodium bicarbonate-based hydration as Class IIb because of the limited number of enrolled subjects. In spite of the small number of published reports, currently available evidence does not support the conclusion that human atrial natriuretic polypeptide (hANP) or N-acetyl cysteine (NAC) is effective for the prevention of CIN. Therefore, we have not given classifications for hANP and NAC. Also, the volume of fluids infused should be adjusted according to the cardiac function and general condition of the patient.
In most emergency cases, PCI is immediately performed after CAG, and therefore, the volume of contrast media may significantly increase when complications such as slow flow and no-reflow phenomena occur, compared to elective cases. Careful attention should be paid in this regard.
10. Bleeding Complication (Table 65)
Table 65.
Recommendation and Evidence Level Regarding Prevention of Hemorrhagic Complications
| |
COR |
LOE |
| PPI is indicated in addition to aspirin administration alone or DAPT in high gastrointestinal bleeding-risk patients.739 |
I |
B |
Abbreviations: PPI, proton pump inhibitor; DAPT, dual antiplatelet therapy.
The disadvantages of CAG are procedural complications, increasing unnecessary PCI, and growth of medical cost. The mortality related to CAG is less than 0.2% and procedural complications, such as stroke, myocardial infarction and bleeding, occur in less than 0.5%.737
According to the Ministry of Health and Welfare longevity science research, catheter-related complications are observed in 5.4% of patients (<60 years) and 9.1% of elderly patients (>70 years).738
Severe ischemic and bleeding complications, and vascular access complications such as large hematoma and pseudoaneurysm, are more common among elderly patients. Contraindications to CAG are febrile disease, abnormality of blood coagulation and the fibrinolytic system, serious contrast media allergy, and severe renal dysfunction.
Bleeding has recently emerged as an important outcome in the management of ACS, which is relatively frequent compared with stable CAD.405
Triple therapy with oral anticoagulation plus DAPT is the standard of care after PCI for patients with atrial fibrillation, but this therapy is associated with a high risk of bleeding. Bleeding has important implications in terms of prolonged hospitalization, prognosis, outcomes, and costs. In particular, gastrointestinal bleeding (GIB) is a serious condition in ACS and is independently associated with mortality and ischemic complications.739
Patients who experienced GIB had significantly higher rates of stent thrombosis compared to patients without GIB. Gastric protection with a PPI is recommended for patients with a history of GIB and is appropriate for patients with multiple risk factors for bleeding. Non-CABG-related major bleeding within 30 days had 6 independent baseline predictors (female sex, advanced age, elevated serum creatinine and white blood cell count, anemia, NSTEMI, or STEMI) and 1 treatment-related variable (use of heparin+a glycoprotein IIb/IIIa inhibitor).406
There has been a report of calculating bleeding risk based on a score incorporating
① STEMI, NSTEMI,
② cardiogenic shock,
③ female,
④ past history of heart failure,
⑤ past history of PCI,
⑥ New York Heart Association (NYHA) IV heart failure,
⑦ past history of vascular disease,
⑧ age,
⑨ estimated glomerular filtration rate (eGFR).740
For operators experienced in radial artery approach, radial artery approach should be performed rather than femoral artery approach.741
Radial vs. femoral access for coronary intervention (RIVAL) trial was investigated in a randomised, parallel group, multicenter trial.269
In patients with ACS undergoing PCI, radial artery approach did not reduce the primary outcome of death, myocardial infarction or stroke at 30 days compared with femoral access. However, radial artery approach significantly reduced vascular access complications, large hematoma and pseudoaneurysm, compared with femoral artery approach, with similar PCI success rates, and was more commonly preferred by patients for subsequent procedures. The choice to perform femoral artery approach will be made depending on the experience and expertise of the operator.
11. Other Complications
11.1 Reinfarction and Post-Infarction Angina (Table 66)
Table 66.
Recommendation and Evidence Level Regarding Treatment of Reinfarction and Post-Infarction Angina
| |
COR |
LOE |
| Intensifying medical treatment and performing CAG (and PCI if indicated) based on risk evaluation are recommended. |
I |
C |
Abbreviations: CAG, coronary angiography; PCI, percutaneous coronary intervention.
Medical treatment centered on nitric acid medicine and β-blockers should be intensified when chest pain, which was similar to the pain of onset, happens again in the acute phase. The rationale is that the pain may have been caused by ischemia. We should assess ECG changes and cardiac biomarkers when considering the diagnosis.742
In case of rising of cardiac biomarkers once again, reinfarction may be diagnosed. It is difficult to diagnose reinfarction until 24 hours from onset because the ECG and cardiac biomarkers have changed over time and are influenced by whether reperfusion was performed and size of infarction. We need to consider clinical findings including ECG, cardiac biomarkers, and echocardiography. Stent thrombosis (acute and subacute) should be suspected if a patient who underwent primary PCI feels chest pain from myocardial ischemia. Although the rate of reinfarction caused by stent thrombosis is lower in the DES era than in the BMS era, because of improvements in antiplatelet agents and DES, there are several severe cases where revascularization must be achieved rapidly.743,744
11.2 Acute Pericarditis
Many cases of acute pericarditis have occurred after a few days of AMI and some cases continued for a few weeks. Patients with pericarditis had large infarct, which frequently causes heart failure.745,746
The symptoms are stronger upon inspiration and in the supine position, and pericarditis is definite if pericardial rub is heard on auscultation. It is necessary to perform auscultation frequently because the time of performing auscultation is often short. We should consider oozing rupture when pericardial effusion has increased in case of Q wave infarction and no-reperfusion if the symptoms are not typical.747
First line treatment is aspirin (0.33–1.5 g/one time, 1.0–4.5 g/day). We could use NSAIDs for the purpose of reducing pain, but should not use NSAIDs for a long time because it can possibly lead to enlargement of the infarction and thinning of the infarction wall. In particular, ibuprofen reduces the effect of antiplatelet function, so should not be used.748
Corticosteroids should not be used in association with thinning of infarction wall and rupture as a rule.749,750
Although some reports showed efficiency of colchicine for preventing pericarditis recurrence, colchicine is currently not covered by insurance in Japan.751–753
11.3 Stroke
It has been reported that the rate of stroke is 0.75–1.2% with myocardial infarction.754,755,756
The mortality rate was high at 40%.2
History of cerebral infarction, hypertension, older age, LV dysfunction, and atrial fibrillation are all risk factors for stroke, with atrial fibrillation the most important risk factor.754,757,758
LV thrombus was observed in LV dysfunction and Killip class III and IV.759
Warfarin should be administered in patients who had LV thrombus and a broad area of asynergy with antiplatelet therapy, with the target of PT-INR at approximately 2. The duration of warfarin therapy should be considered in conjunction with original disease. Generally, the period of warfarin therapy for patients with LV thrombus is at least three months. Carotid artery ultrasonography should then be performed to evaluate whether there is carotid stenosis requiring surgical management.760
11.4 Deep Vein Thrombosis and Pulmonary Thromboembolism
Anticoagulation therapy was necessary when patients with heart failure require prolonged hospitalization or immobility.761
The guidelines to prevent deep vein thrombosis and method of risk assessment are presented in the Guidelines for Diagnosis, Treatment and Prevention of Pulmonary Thromboembolism and Deep Vein Thrombosis (JCS 2017).761a
12. Assessment of Infarct Size
Infarct size is an important prognostic factor because it is correlated with mortality in the acute and chronic phases and with re-hospitalization for heart failure in patients with ACS.762,763
Assessment of cardiac viability of infarcted areas also provides important information on ventricular remodeling and whether revascularization is indicated.
12.1 Myocardial Biomarkers
12.1.1 Myocardial Biomarkers and Infarct Size (Table 67)
Table 67.
Recommendation and Evidence Level of Myocardial Biomarkers in Assessment of Myocardial Infarction
| |
COR |
LOE |
| Cardiac troponin levels measured 72 to 96 hours after the onset of AMI may be used as measures of infarct size.204,767–769 |
IIb |
B |
Abbreviation: AMI, acute myocardial infarction.
Infarct size has conventionally been quantified based on peak CK or CK-MB values or total amount of CK or CK-MB released over time, which is calculated from the area under curve for release dynamics.764–766
Recently, cardiac troponin I and troponin T have also been used for such quantification.766
It has been reported that peak cardiac troponin T values767
and cardiac troponin T values measured at 72 to 96 hours,768,769
as well as cardiac troponin I values measured at 72 hours after infarction are correlated with infarct size.204
On the other hand, no studies have indicated whether high-sensitivity cardiac troponin I or high-sensitivity cardiac troponin T values, which are useful for early diagnosis of ACS, are correlated with infarct size.
12.2 Nuclear Cardiology
Two types of myocardial perfusion agents, 201Tl and 99 mTc (consisting of 99 mTc sestamib and 99 mTc tetrofosmin) are used in nuclear cardiology. These agents have similar diagnostic accuracy for infarct size estimation and cardiac viability assessment.770
The image of the left ventricle is divided into 17 or 20 segments, and the degree of perfusion abnormality is evaluated on a five-point scale with semiquantitative visual interpretation. Higher total scores indicate larger infarcts. Percent uptake is an index of cardiac viability that is calculated by comparing the accumulation in a lesion vs. a normal area. In general, cardiac viability is considered to be maintained when % uptake at rest is over 50–60%.770
Nuclear cardiology can diagnose wall motion recovery after revascularization with a sensitivity of approximately 80% and a specificity of approximately 60%.771
Myocardial perfusion imaging during the acute phase of infarction shows markedly less blood flow in the infarct area than in the remaining viable myocardial area. In addition, cardiac viability tends to be underestimated with imaging modalities during the acute phase. Regarding assessment of cardiac viability using18
F-fluorodeoxyglucose (18
F-FDG) positron emission tomography (PET), during the acute phase, FDG accumulation is observed both in the infarct site (reflecting inflammatory cell infiltration) and in the remaining visible myocardium (reflecting glucose metabolism). Therefore, accurate cardiac viability assessment during the acute phase is considered to be difficult. Details about nuclear cardiology are described in the “Guidelines for Clinical Use of Cardiac Nuclear Medicine” (JCS 2010).
12.3 Cardiac Magnetic Resonance (CMR) (Table 68)
Table 68.
Recommendations and Evidence Level of CMR in the Assessment of Myocardial Infarction
| |
COR |
LOE |
Cine-CMR should be considered in patients for whom echocardiography does not provide sufficient assessment
of infarct size and wall motion.780–783 |
IIa |
C |
| Gadolinium-enhanced LGE-CMR should be considered for visualizing myocardial infarction lesions.165,784–787 |
IIa |
C |
Abbreviations: CMR, cardiac magnetic resonance; LGE, late gadolinium enhancement.
Cine MRI, T2-weighted imaging, late gadolinium enhancement (LGE), and T1 and T2 mapping are cardiac magnetic resonance (CMR) techniques used in the assessment of ACS. The biggest advantage of cine CMR is that it can provide quantitative evaluation of wall motion, wall thickness, and volume of the left and right ventricles as well as LV function in a non-invasive manner. Cine MRI has high reproducibility and requires no radiation or contrast media exposure.772,773
12.3.1 Identification of Infarcts and Quantification of Infarct Size
In LGE-CMR imaging, positive images (i.e., white enhanced) correspond to both acute infarcts and lesions from previous myocardial infarction. Infarct size measured using LGE-CMR is strongly consistent with pathological findings. In AMI, LGE-CMR can accurately evaluate the presence, location, transmurality, and size of infarcts.774–777
For localization of myocardial infarction, LGE-CMR has the advantage of being able to diagnose small and subendocardial infarcts because of its high spatial resolution, which are difficult infarcts to diagnose using single photon emission computed tomography (SPECT). A study that directly compared LGE-CMR with SPECT showed that LGE-CMR has significantly higher diagnostic performance for all assessments of myocardial infarction.774
Furthermore, LGE-CMR is also superior for diagnosing RV infarction.776
In addition, it has been reported that infarct size measured using LGE-CMR is a better indicator of prognosis than conventional indicators of prognosis such as LV volume and LVEF.763,778
A meta-analysis of LGE-CMR performed within 1 month of the onset of AMI showed that the interquartile range with the largest infarct size is strongly associated with all-cause mortality and hospitalization for heart failure within 1 year.779
Nevertheless, when LGE-CMR is used for infarct size assessment, it should be noted that LGE-CMR overestimates infarct size during the acute phase (within 1 month) after the onset of AMI because areas at risk around the infarct are also enhanced as a result of myocardial edema. Therefore, for infarct size quantification and cardiac viability assessment (see below), LGE-CMR should be performed during the chronic phase, 1 month or more after the onset of AMI.
In LGE-CMR, an infarct that is not enhanced in an island-like shape but rather as a dark shadow in the center of the lesion is called a microvascular obstruction (MO or MVO). MVO is seen in perfusion MRI and LGE-MRI. MVO is classified into early MVO, which is defined as prolonged hypoenhancement seen 1 to 2 minutes after contrast injection during perfusion MRI, and late MVO, which is defined as prolonged hypoenhancement seen 10 to 15 minutes after contrast injection during LGE-MRI.780
MVO is considered to correspond to hemorrhage in a myocardial infarct because it has a low signal in T2* (T2 star) images. MVO is attributable to reperfusion injury.781,782
MVOs tend to occur in association with large infarcts when reperfusion therapy is delayed. One study indicated that the presence of MVO increases the occurrence of LV remodeling and cardiac events.783
12.3.2 Assessment of Myocardial Viability
LGE-CMR is also superior for myocardial viability assessment after myocardial infarction. On an LGE-CMR image, necrotic or irreversibly injured areas of the myocardium associated with infarction have high signal, while reversibly injured areas are not enhanced.784,785
Infarcts visualized with LGE-CMR correspond to infarcts visualized using18
F-FDG PET.786
Wall motion of a region with LGE of less than 50% of the wall thickness is expected to improve with revascularization via PCI, or CABG. On the other hand, a region with LGE of 50% or more of the wall thickness is considered to be nonviable; as such, improvement in wall motion is unlikely.785
A study showed that wall motion improved after revascularization when the depth of LGE was less than 50% of the wall thickness, even in myocardium with a thinned wall measuring less than 5.5 mm.787
12.4 Electrocardiography
In the era of non-reperfusion therapy, abnormal Q waves and coronary T waves were the representative electrocardiographic findings of previous myocardial infarction. Infarct sites were suspected based on leads with abnormal Q waves. QRS scores calculated from quantitative values such as the depth and width of an abnormal Q wave and the number of leads with abnormal Q waves were used as an indicator of infarct size.165
Today, however, it should be noted that reperfusion therapy is commonly used and that patients with and without reperfusion therapy have different ECG findings over time. Since ECG findings are also affected by time from AMI onset, it is difficult to estimate infarct size based on ECG findings. Thus, infarct size is usually estimated based on other testing methods.
13. Cardiac Rehabilitation
13.1 Inpatient Cardiac Rehabilitation
The acute phase of cardiac rehabilitation (called Phase I) is between ICU or CCU and the general ward. In cases of severe clinical condition, patients require super acute phase cardiac rehabilitation in ICU or CCU. After the acute phase patient completes the early recovery phase of cardiac rehabilitation (called Early Phase II) in the cardiac rehabilitation room, cardiac rehabilitation staff conduct pre-discharge coordination (Figure 4).788
In acute phase cardiac rehabilitation, additional medical fees are applicable for early phase cardiac rehabilitation and early ambulation in ICU/CCU. In this case, early cardiac rehabilitation has been recommended.
13.2 Super-Acute Phase Cardiac Rehabilitation (Table 69)
Table 69.
Recommendation and Evidence Level of Super-Acute Cardiac Rehabilitation
| Super-acute phase cardiac rehabilitation |
COR |
LOE |
| For early ambulation, super-acute phase cardiac rehabilitation should be considered.790–792 |
IIa |
B |
Although the survival rate of patients with severe diseases on artificial respiration management in ICU is improving, long term outcomes post ICU such as physical function and quality of life (QOL) have been evaluated. ICU acquired weakness (ICU-AW) contributed to poor QOL after discharge.
Patients with ACS on a life support device such as artificial respirator and IABP may develop ICU-AW. Pulmonary rehabilitation, preventive position management, reclining position and super-acute phase cardiac rehabilitation should begin within 3 days after admission.790
An example of the cardiac rehabilitation program in the CCU at the National Cerebral and Cardiovascular Center is shown as follows.791
Cardiac rehabilitation in CCU is not yet well established and creation of applicable early cardiac rehabilitation for severe heart failure patients is needed. Tamaki has made inclusion criteria (Table 70), minimizing circulatory influence and providing a step-wise progress sheet (Figure 5) that can check the physical condition of patients. Criteria for cessation are progression of neurological symptoms (consciousness disturbance, paresis etc.), heart rate less than 50 bpm or more than 130 bpm and 20 bpm increase from rest heart rate, systolic blood pressure less than 80 mmHg or more than 140 mmHg, 10 mmHg decrease from rest systolic blood pressure on resistance training day, new onset arrhythmia, respiratory rate more than 35/minute, oxygen satuation less than 90%, discoordination with artificial respirator, Borg scale of dyspnea or fatigue >13, complaint (orthostatic hypotension: dizziness, fainting, palpitation, headache etc.), cardiac low output symptom (cold sweating, general fatigue, cyanosis etc.), new onset myocardial ischemia, chest pain, and ECG changes. In addition, low intensity resistance training is available for patients with severe deconditioning. Specifically, in a supine position, a rubber band (such as Seraband®) is tied to the bed rails with a little clearance above the chest height, and the patient breathes in while moving the rubber band onto their head and then returns to the original position while breathing out (Silvester method). Then the rubber band is placed under the knees under tension, and is kicked out with the foot extended. Perform 3 sets of 5 repetitions, break for 1 min, and advance step by step to 3 sets of 7 repetitions, 3 sec per repetition, break for 1 min.792
Table 70.
Inclusion Criteria for Early Phase Cardiac Rehabilitation in CCU (Example of the National Cerebral and Cardiovascular Center)
1. Improvement from acute unstable hemodynamic state Main disease is healed or improving
2. No worsening heart failure
Remarkable decrease in urine volume, increased edema and body weight,
increased pulmonary congestion or CTR on chest x-ray
3. No dose increases of cardiovascular agonists within 12 hours
4. No active myocardial ischemia
Peak CK/CK-MB
No significant ST change ≥1 mm within 12 hours
5. No treatment that limits activities
Without VA-ECMO, during hypothermia therapy etc.
End seat in case of CHDF
6. Patient is awake and cooperative
RASS 0 ~ −1 (SAT is cleared)
RASS −2 ~ −5: only ROM training and H-up 90°
7. No progression of neurological symptoms (consciousness disturbance, paresis etc.) within 24 hours
8. No fever of more than 38℃
9. Resting heart rate more than 50bpm, less than 120 bpm
10. No shock with systolic blood pressure more than 80 mmHg, systolic blood pressure less than 140 mmHg
11. No new onset arrhythmias that need new antiarrhythmic drugs
12. Resting respiratory rate of more than 10times/min, less than 30 times/min
13. Synchronized with artificial respirator
14. PaO2 more than 60 mmHg, SpO2 more than 90%
15. FiO2 of less than 60%, PEEP less than 10 cmH2O |
Abbreviations: CK, creatine kinase; CK-MB, creatine kinase MB; VA-ECMO, veno-arterial extracorporeal membrane oxygenation; RASS, Richmond Agitation-Sedation Scale (sedation assessment scale); SAT, Spontaneous Awakening Trial (awaking test without sedation); H-up, Head up; PEEP, positive end-expiratory pressure. (Adapted from Tamaki Y, et al. 2016791)

If voluntary movement is impossible because of disturbed consciousness or sedation, neuro muscular electrical stimulation (NMES) may be attempted. NMES can prevent muscle atrophy and increase muscle strength. It is indicated for prevention of ICU-AW. Furthermore, NMES was reported to improve vascular endothelial function in AMI patients without complication.793
For coronary intervention, stent with anti-thrombotic effect is available and exercise therapy may produce additive effect.
13.3 Acute Phase Cardiac Rehabilitation Program (Table 71)
Table 71.
Recommendation and Evidence Level of Acute Rehabilitation
| Acute phase cardiac rehabilitation |
COR |
LOE |
| Acute phase cardiac rehabilitation should be performed using the clinical pathway.794,795 |
I |
A |
In patients with AMI, mobilization is permitted if the CK peak is recognized after PCI. Essential acute phase rehabilitation program is performed separately over a 1 week course / 2 weeks course / 3 weeks course according to criteria as follows.794
| 1 week program |
| ① Peak CK/CK-MB <1,000/100 IU/L |
| ② Killip class I~II |
| ③ No prior myocardial infarction |
| ④ PCI successful |
| ⑤ LVEF ≥30% |
| 2 week program |
| All of ②~⑤ is fulfilled |
| 3 week program |
| Except for 1 week / 2 week program |
An example of the AMI clinical pathway from National Cerebral and Cardiovascular Center is provided in
Table 72. The decision of the rehabilitation goal is important in the AMI clinical pathway. It can progress according to the criteria for evaluating exercise stress testing (Table 73).795
Table 72.
A 14-Day Clinical Pathway for Patients With AMI (Example of the National Cerebral and Cardiovascular Center)
| Day |
Goals |
Patient care/stress
test/rehabilitation |
Activities |
Meals |
Excretion |
Hygiene |
Day 1
after
PCI |
Prevent complications
of AMI and catheter-
related complications |
• Remove the tourniquet,
disinfect wounds
• Allow use of a
bedside commode |
After removing the
tourniquet, allow the
patient to move freely
on the bed |
• Regular diets for
patients with
cardiovascular
diseases
(1,600 kcal/day,
salt 6 g/day) |
• Urine: Urinary
catheter
• Stool: Bedside
commode |
• Face washing:
on the bed
• Body cleaning:
Bed-bath
A caretaker assists
bed-bathing of the
feet and back |
| Day 2 |
Prevent complications
of AMI and catheter-
related complications |
Remove the urinary
catheter |
Allow the patient to
move freely in the
room |
|
|
• Face washing:
on the bed
• Body cleaning:
Bed-bath
A caretaker assists
bed-bathing of the
feet and back |
| Day 3 |
Prevent complications
of AMI |
Remove the venous
access device |
Allow the patient to
walk to the toilet |
|
Urine and stool:
Use the toilet |
| Day 4 |
No myocardial
ischemia |
• When the patient
can pass a 200 m
walking test,
conduct 200 m
walking sessions 3
times a day
• Ask a dietitian to
provide nutrition
education |
Allow the patient to
walk around the ward
within a 200 m radius
of the room |
• Regular diets for
patients with
cardiovascular
diseases
(1,600 kcal/day,
salt 6 g/day)
• Allow drinking
water freely |
• Face washing:
Use the washstand
• Bed-bath: A
caretaker assists
bed-bathing of
the back |
| Day 5 |
• No myocardial
ischemia
• The patient can
take his/her drugs
as prescribed
• The patient can
access information
about important
points for daily
living after
discharge |
• Order a cardiac
rehabilitation
program
• Confirm when the
cardiac rehabilitation
program begins |
| Day 6 |
• Conduct an entry
test in the cardiac
rehabilitation room
• If the patient has
not attended the
cardiac rehabilitation,
conduct a 500 m
waiking test |
When patient passes
a submaximal stress
test, allow bathing
and walking freely in
the hospital |
• Face washing:
Use the washstand
• Provide bed-
bathing when
requested by the patient |
| Day 7 |
Day 8
Day 9
Day 10 |
• No myocardial
ischemia
• The patient can
understand important
points for daily
living after
discharge |
Conduct exercise
sessions at the
cardiac rehabilitation
room (If the patient
has not attended the
program, conduct a
Master’s single test
or allow having a bath
as a trial) |
• Face washing:
Use the washstand
• Allow bathing
when the patient
wants to bathe |
| Day 11 |
• No ischemia at
submaximal exercise
• The patient can list
important points for
daily living after
discharge |
| Day 12 |
| Day 13 |
| Day 14 |
Discharge |
Table 73.
Criteria for Evaluating the Results of an Exercise Stress Test Prior to Commencing Acute-Phase Rehabilitation in Patients With AMI
Acute-phase rehabilitation can be introduced when the patient does not exhibit any of the following:
1. Symptoms such as chest pain, dyspnea, and palpitation
2. An increase in heart rate to ≥120 bpm or by ≥40 bpm
3. Development of potentially dangerous cardiac arrhythmias
4. Ischemic ST depression of ≥1 mm, or significant ST elevation
5. A change in systolic blood pressure by ≥20 mmHg during the period before the patient was allowed to
use a bedside commode
(The criteria for blood pressure are not used for patients 2 weeks after the onset of acute myocardial infarction.) |
Patients who failed the exercise stress test should receive appropriate drug treatment or other measures, and undergo the same test on the next day.
(Adapted from JCS Working Group. 2012795)
Table 74.
Recommendations and Evidence Level Regarding Early Rehabilitation and Risk Management
| |
COR |
LOE |
Early phase cardiac rehabilitation should be performed for prevention of orthostatic hypotension
by bed rest and thrombotic events.796 |
I |
A |
| Acute phase cardiac rehabilitation is recommended for avoiding the risk of cardiac rupture.797 |
I |
B |
Exercise therapy accompanied with blood pressure elevation should not be performed for
patients at high risk of cardiac rupture in acute phase cardiac rehabilitation.798
|
III:
Harm |
B |
Upon mobilization, it is a problem that disappearance of the vasomotor reflex induces orthostatic hypotension and reflex tachycardia. With bed rest, 5% blood volume is lost in 24 hours, 10% after 6 days and 20% after 14 days. Because the decrease in serum is remarkable, thrombogenesis is promoted by high blood viscosity.
This is preventable by a few hours of chair sitting 2 or 3 times per day.796
However, the incidence of cardiac rupture decreased in the reperfusion era, its mortality rate remains high. For details, refer to
VII. 8. Diagnosis and Treatment of Mechanical Complications After AMI. Diagnosis and treatment of mechanical complications after AMI. Patients in whom reperfusion therapy was delayed or reperfusion therapy was not performed are considered to be at high risk of cardiac rupture. They should refrain from aggressive exercise therapy with blood pressure elevation within 9 days from onset for prevention of cardiac rupture.797
Bathing is a daily habit for Japanese people, so patients hope to shower/ bathe their body with towels. However, bathing is limited because of reports of cardiac arrest during bathing. Itoh et al. showed no bradycardia due to hydrostatic pressure during 38℃ bathing in AMI patients. They also reported that direct vasodilation by hyperthermia induced a decrease in blood pressure and no change of hemodynamic state and induced arrhythmia during bathtub drainage. In these circumferences, bathing may be safe because of no excessive vagal activation, possible improvement of hemodynamics and decrease in afterload.798
Kimura et al. reported that oxygen consumption during showering with nurse support was significantly less than that of a 200 m walk and there were no changes in heart rate and blood pressure.799
However, these reports were from a small sample size, so monitoring of symptoms, heart rate and blood pressure is necessary for safety during the first shower/bath.
13.5 Implementation/Significance of Exercise Stress Testing (Table 75)
Table 75.
Recommendation and Evidence Level of Exercise Stress Test
| |
COR |
LOE |
Exercise stress testing should be performed to evaluate prognosis, physical activities and
additional treatment in acute phase cardiac rehabilitation.800 |
I |
A |
In the ACC/AHA 2002 Guideline Update for Exercise Testing,800
exercise stress testing is recommended before discharge or immediately after discharge to evaluate prognosis, prescription for physical activities and additional treatment (medical therapy, PCI etc.) in acute phase cardiac rehabilitation. With the exception of special cases, it is recommended that exercise stress is performed at a submaximal level within 4–6 days from onset of AMI and symptom-limited at 14–21 days from onset of AMI. Mortality of AMI has decreased because of improvement in therapy including PCI. But it is known that patients have poor prognosis if emergent PCI and exercise stress testing are not available. In addition, patients with ST-segment depression at low intensity exercise load, exercise tolerance less than 5 METs, and exercise-induced decrease in blood pressure also have poor prognosis.800
13.6 Participation Rate of Cardiac Rehabilitation (Table 76)
Table 76.
Recommendation and Evidence Level Regarding Introduction of Cardiac Rehabilitation
| |
COR |
LOE |
Physician should recommend cardiac rehabilitation to AMI patients to enhance the participation
rate of outpatient cardiac rehabilitation.801,804 |
I |
A |
Abbreviation: AMI, acute myocardial infarction.
There is poor data on the efficacy of inpatient cardiac rehabilitation on long-term outcomes, but the data is stronger for secondary prevention. Cardiac rehabilitation immediately after discharge improves physical function, management of cardiovascular risk factors, mental and social health and rehospitalization rates, meaning that improving participation rates is significant.801
A Kyoto University study about quality indicators of cardiac rehabilitation after ACS showed referral for cardiac rehabilitation by physicians was low (32%) and the continuation rate was also low (38%).802
Despite the efficacy of cardiac rehabilitation, only 14–35% of AMI patients attend cardiac rehabilitation. The reasons why patients could not attend were accessibility to program, payment, female gender, elderly people and minorities.803
In elderly patients with CAD, referral from physicians succeeded for induction of cardiac rehabilitation.804
Thomas et al. mentioned that a cardiac rehabilitation/secondary prevention program was a systemic and effective care model to improve delivery of therapy for secondary prevention and improve prognosis of patients. But patient participation was inadequate because there were barriers in place for patients themselves, program providers and systems. In order to support its improvement, a system for induction, more referrals from physicians and accountability for induction are necessary.801
Campkin et al. found that external (safety, accessibility, and social support networks), internal (physical, cognitive and emotional domains), and cultural factors influence exercise initiation (Figure 6). In particular, cognitive and social domains were the most frequently cited factors influencing participation in exercise programs.805
It is reported that the participation rate by automatic referral was higher than usual referral at discharge (50% vs. 32%). It is important to make an easier system of referral for cardiac rehabilitation.806
VIII. Conditions Requiring Special Consideration
1. The Elderly (Table 77)
Table 77.
Recommendation and Evidence Level Regarding Treatment of Elderly ACS Patients
| |
COR |
LOE |
| Age and frailty should be considered when
deciding upon invasive strategies.809,811,812 |
IIa |
B |
The population over 65 years old in Japan had reached 25% in 2013, will reach 30% in 2025, and 39.9% in 2060. The population over 75 years old had already reached 15.6 million (12.3%) in 2013, and will reach 23.36 million in 2060. The elderly are divided into three groups, the young-old are 65–74 years old, the old-old are 75–89 years old, and the very elderly are over 90 years old. Each group has different medical characteristics. There are very few problems in daily life for the young-old and they can continue social activities. On the other hand, the number of old-old and very old patients who have many illnesses has increased remarkably. The concept of frailty has appeared which refers to weakness with stress, living dysfunction and requiring care. Furthermore, it includes not only physical problems such as muscle weakness but also social problems such as failure of cognitive function, depressive state, staying alone, and economic hardship. We should consider the elderly`s background such as frailty in case of ACS. In Japan, various observational studies on ACS have been performed. In Miyagi prefecture, the trend of an increasing number of patients over 80 years old with ACS over 30 years was recognized.28
The elderly do not develop the typical symptoms of ACS compared to the young. Because of this, cardiac biomarkers such as high-sensitivity cardiac troponin are very important markers for early diagnosis.807
However, we should consider pseudo positive cardiac troponin in elderly in cases that are not ACS.808
The mortality of the elderly in ACS is decreasing,809
and we should not always choose the conservative strategy simply for the reason being elderly. The strategy of early CAG and PCI should be selected in elderly patients with ACS. The After Eighty study showed that greater reduction of myocardial infarction and emergent coronary revascularization in the invasive strategy group compared with conservative strategy group810
lead to significant differences in death in hospital, myocardial infarction, and prognosis at one year in patients over 75 years old, demonstrating that the invasive strategy was better than the conservative strategy.811
Studies have reported that the invasive strategy is more effective for the elderly patients with STEMI than fibrinolysis.812
In relation to the different types of coronary stents in the elderly, an randomized controlled trial (RCT) conducted to assess the safety of DES in stable CAD and ACS indicated that DES was significantly better for the primary endpoint (all cause of death, myocardial infarction, stroke, target lesion revascularization) than BMS.813
Further, as the elderly have many contraindications to medications, there is inadequate medical treatment.814
In particular, it is important for the elderly patients to be careful with usage because of bleeding risk with antithrombotic drugs.815
We should make a decision about invasive or non-invasive treatments after considering carefully the age and frailty.
2. Women
The number of female ACS patients in Miyagi prefecture is decreasing gradually in people over 60 years old.28
Some features of female ACS are a higher age than males and multiple risk factors such as CKD, diabetes, hypertension, and hyperlipidemia. Therefore, female ACS patients have worse clinical outcomes than males.155,816–818
In ACS, women have often atypical symptoms such as shortness of breath and fatigue,816
and therefore, it is important that ECG and blood tests are aggressively performed in cases of atypical symptoms. The J-PCI registry showed the features of elderly female ACS had more hemorrhagic events.819
For this point, it was considered that women had overdosage of antithrombotic drugs relative to their body weight. Regarding prognosis, the number of females dying from cardiovascular disease has decreased in the United States since 2000.820
Although there were no significant change in in-hospital mortality from 2005 to 2014 in the AMI registry of Miyagi prefecture, in-hospital mortality in women was higher than that in men. On the other hand, other studies have reported no differences in in-hospital mortality after treatment of ACS and no difference in outcomes at one year between men and women.818,820
3. Coronary Spasm
Sudden excessive coronary vasoconstriction, i.e., coronary spasm, produces a transient reduction in blood flow, resulting in myocardial ischemia (supply ischemia/primary angina), AMI and sudden cardiac death. Emergent CAG reveals extremely mild organic stenosis in 3–18% of patients with AMI, as well as patients with complete coronary occlusion that exhibits recanalization after administration of nitrates alone. These patients constitute an intriguing subgroup referred to as myocardial infarction with non-obstructive coronary arteries (MINOCA).821,822
Coronary spasm was documented in 30–50% of the patients tested by acetylcholine.823,824
It has been suggested that coronary spasm is a cause of rupture of vulnerable plaques. Investigations of coronary lesions in autopsies have demonstrated that spasm causes endothelial cell derangement and fibrous cap rupture, resulting in the protrusion of the plaque content exposed to the vascular lumen, where thrombi are produced.825
In addition, coronary spasm is accompanied by hypercoagulation,826
decreased fibrinolytic activity,827
and activation of platelets and adhesion molecules,828
resulting in a thrombophilic state in ACS.
Prevention of coronary spasm is also important, particularly in Japanese, in whom the prevalence of coronary spasm is 3-fold higher than in Western countries.519
CCBs that suppress Ca2+
inflow into vascular smooth muscle cells are highly effective in preventing coronary spasm, and are deemed drugs of first choice for the treatment of vasospastic angina (Class I evidence in Guidelines for Diagnosis and Treatment of Patients with Vasospastic Angina [Coronary Spastic Angina] (JCS 2013)
Figure 7).829
4. Others
4.1 Acute Aortic Dissection
Incidence of coronary involvement in type A AAD has been reported to range from 3 to 15%. The leading culprit lesion is RCA.830–833
Underlying mechanisms are as follows; i) bulging of the dissected false lumen producing occlusion of the coronary artery lumen, ii) a retrograde extension of the dissection into the coronary arterial wall resulting in obstruction, iii) disruption or detachment of the coronary artery from the aortic root and iv) dynamic obstruction of the coronary orifice by flail intimal flap.832–834
Generally, type A AAD is the indication for emergency surgical treatment. It is reported that approximately one quarter of patients with AAD presented with aortic regurgitation, significant hypertension, shock or ischemic changes in ECG.830,835
In patients with type A AAD, ischemic changes in ECG lead to delays in diagnosis and increase the rate of hospital mortality.835,836
In ACS patients, we must differentiate between the usual form ACS and ACS secondary to type A AAD. Patients with AAD often complain of migratory back pain compared to those with ACS. Physiologically, the difference between right and left blood pressure is an important clinical sign. It is also important to assess for ascending aortic dilatation, intimal flap, aortic regurgitation or pericardial effusion by using echocardiography. In addition, increasing serum D-dimer and mediastinal widening on chest x-ray are also findings suspicious for AAD.837
Definitive diagnosis is made by detecting pseudo-lumen by contrast-enhanced CT. For this life-threating disease, we should quickly diagnose AAD and perform treatment in cooperation with the surgical team.
4.2 Spontaneous Coronary Artery Dissection
Spontaneous coronary artery dissection (SCAD) is a rare disease that causes ACS. It is reported that SCAD usually occurs in young females aged 50 years and less with an estimated prevalence ranging from 0.07% to 1.1% in ACS patients overall.838,839
An observational study of SCAD in Japan reported that the prevalence of SCAD was overall 0.31% in 20,195 AMI patients, with mean age of 46 years and 94% female.840
One of the important characteristics of SCAD patients is to have few atherosclerotic risk factors.840,841
An association between fibromuscular dysplasia and SCAD is reported, and one study of SCAD showed that 86% of SCAD patients had fibromuscular dysplasia in at least one non-coronary territory.842
Other precipitating factors reported are pregnancy, postmenopausal hormone therapy and emotional stress.840,841
Traditional findings of pathognomonic contrast dye staining by angiography in the arterial wall with multiple radiolucent lumens are seen in 25–43% of SCAD patients, and 55–70% of SCAD patients have diffuse smooth narrowing without intimal flap. In the latter type, medial dissection or intramural hematoma without atherosclerotic changes detected by intra-coronary imaging device is useful to diagnose SCAD.838,840,843
Regarding management of SCAD in the acute phase, the rate of SCAD recurrence does not differ between revascularization and conservative therapy.840,844
On the other hand, SCAD arteries heal spontaneously in most cases. Thus, an overall conservative approach is preferred on the basis of expert opinions derived from observational data. Nevertheless, a small proportion of patients should be considered for revascularization, including those with ongoing or recurrent ischemia, hemodynamic instability, ventricular arrhythmias, or left main dissection. Recently, some observational studies showed that the rate of SCAD recurrence is high. A Japanese study reported that the 5-year rate of MACEs and SCAD recurrence is 38% and 27%, respectively. In particular, 42% of those occurred during first 30 days.840
It is also reported that β-blocker treatment may reduce SCAD recurrence.843
4.3 Coronary Artery Embolism
Coronary artery embolism (CE) is classified as type 2 in the universal definition of myocardial infarction,845
which is not related to atherosclerotic plaque rupture. In a 1978 autopsy-based study of 55 AMI patients, Prizel et al. reported that underlying diseases predisposing to CE included valvular heart disease (40%), cardiomyopathy (29%), coronary atherosclerosis (16%), and atrial fibrillation (24%).845
There has been no systematic study after this autopsy-based study, however, in 2015, the observational study based on new criteria for diagnosis of CE was reported from Japan (Table 78).846
Table 78.
Proposed NCVC Criteria for the Clinical Diagnosis of Coronary Artery Embolism
| Major criteria |
• Angiographic evidence of coronary artery embolism (CE) and thrombosis without atherosclerotic components
• Concomitant coronary artery embolization at multiple sites*
• Concomitant systemic embolization without left ventricular thrombus attributable to AMI |
| Minor criteria |
• <25% stenosis on CAG, except for the culprit lesion
• Evidence of an embolic source based on transthoracic echocardiography, transesophageal echocardiography,
computed tomography, or MRI
• Presence of embolic risk factors: atrial fibrillation, cardiomyopathy, rheumatic valve disease, prosthetic heart valve,
patent foramen ovale, atrial septal defect, history of cardiac surgery, infective endocarditis, or hypercoagulable state |
| Exclusion criteria |
• Pathological evidence of atherosclerotic thrombus
• History of coronary revascularization
• Coronary artery ectasia
• Plaque disruption or erosion detected by intravascular ultrasound or optic coherence tomography in the proximal
part of the culprit lesion |
Abbreviations: NCVC, National Cerebral and Cardiovascular Center; AMI, acute myocardial infarction; CAG, coronary angiography.
*Indicate multiple vessels within 1 coronary artery territory or multiple vessels in the coronary tree.
Definite CE: two or more major criteria, one major criterion plus ≥2 minor criteria, or ≥3 minor criteria.
Probable CE: one major criterion plus 1 minor criterion, or two minor criteria.
(Adapted from Shibata T, et al. 2015846)
In Japan, the prevalence of CE was overall 2.9% in AMI patients, with mean age of 66 years. The most common underlying disease was atrial fibrillation (73%). Cardiomyopathy was the next most common cause (25%), followed by valvular heart disease (15%). Notable causes of CE were paradoxical embolism via an atrial septal defect attributable to deep vein thrombosis (4%), malignancy (10%), and septic emboli attributable to infective endocarditis (4%). Patients with concomitant systemic embolization were 23%, and an intracardiac embolic source was detected in 31%. It is notable that recurrent CE occurred exclusively in trial fibrillation patients with inadequate international normalized ratio.846
Regarding clinical outcome, the 5-year rate of all cause death (28%) and cardiac death (17.5%) were significantly higher compared to atherosclerotic AMI.846
Moreover, 5-year recurrence myocardial infarction was 8.7%, and all patients with recurrence myocardial infarction have atrial fibrillation.
Regarding revascularization, there have been few systematic studies. In a retrospective, single-center study,846
58% of patients underwent PCI, of which 97% had thrombus aspiration as the initial PCI strategy and myocardial reperfusion was achieved in 66%. The remaining 42% were treated conservatively because of far distal occlusion or small diameter of the vessel with the culprit lesion.
IX. Secondary Prevention
1. General Therapy
Lifestyle interventions after discharge are generally accepted to be effective for secondary prevention, and are written about smoking cessation and obesity and weight management in this section. Regarding other interventional factors including diet, alcohol intake, exercise and blood pressure control, please refer to the Japan Atherosclerosis Society (JAS) Guidelines for Prevention of Atherosclerotic Cardiovascular Diseases 2017.846a
1.1 Approach to Quitting Smoking (Table 79)
Table 79.
Recommendations and Evidence Level Regarding Smoking Cessation in Secondary Prevention
| |
COR |
LOE |
| Obtaining the smoking history is recommended. |
I |
A |
Providing an explanation of the risk of smoking and smoke exposure, supporting the smoking cessation and
providing the necessary guidance to the patient’s family and colleagues is recommended.848,853–855,858,859,862–866 |
I |
A |
Smoking is one of the major risk factors for atherosclerosis disease. Epidemiological studies worldwide have shown that smoking is implicated in the development and onset of CAD.847,848
As for the mechanism, multiple factors including endothelial dysfunction, oxidative stress, platelet aggregation, fibrinolytic system activation and inflammation are involved.849–852
Even with light smoking, the risk of myocardial infarction increases,853,854
and it does not depend on the type of cigarette.855
Recently, a newer type of cigarette is increasingly used, but the influence on health is unclear.856
Some studies report that the new type of cigarette raises the sympathetic activity, increasing the oxidative stress and risk of cardiovascular events.857
Patients with atherosclerosis should aim to quit smoking altogether, not only because of rising recurrence of cardiovascular events, but also because of onset of cancer and chronic lung disease.
Smoke exposure certainly increases the risk of cardiovascular events855,858,859
and decrease in smoke exposure reduces the risk of cardiovascular events too.860,861
The revised law about smoke exposure is clinically significance. It will be a social problem in future to thoroughly prevent smoke exposure at the workplace and the home.
A study from Japan reports smoking cessation inhibits the onset of cardiovascular events at a relatively early stage.862
It has been shown that the relative risk of recurrence of myocardial infarction is 0.57 for patients who achieved smoking cessation after ACS compared to patients who continue smoking.863
The instructions to quit smoking by a doctor raise the rate of smoke cessation,864
but it is unclear whether familiar support raises the rate of smoke cessation.865
Patients and their families must be instructed about the importance of smoking cessation. The guidelines of Japan and outside Japan have five recommended steps “5A approach” to smoking cessation (Table 80).866
There are smoking cessation products (nicotine patches, gum and varenicline) useful for the nicotine dependence as the treatment of smoking cessation. An overseas observational study reports that the nicotine replacement therapy does not increase cardiovascular events for one year after ACS.867
On the other hand, the benefit of varenicline was demonstrated for the rate of smoke cessation achievement, but was reported to increase the rate of cardiovascular events.868
It is clear that smoking cessation products are useful for smoking cessation, but now there is little evidence to indicate the relationship between smoking cessation products and cardiovascular events. At a minimum, the clinical significance of smoking cessation should be explained to patients. It is important to establish the relationship between doctors and patients for achievement of continuous smoke cessation.
Table 80.
“5A Approach” to Smoking Cessation
| Ask |
Ask about smoking status at every medical consultation |
| Advise |
Advise smokers to quit |
| Assess |
Assess motivation |
| Assist |
Assist in quitting |
| Arrange |
Arrange for follow-up |
(Adapted from Fiore M, et al. 2000866 with modification)
Table 81.
Recommendations and Evidence Level Regarding Body Weight Management in Secondary Prevention
| |
COR |
LOE |
Maintaining a body weight for patients with normal body weight (18.5≤BMI<25), but
aiming to reduce body weight by 3% over 3–6 months for obesity, is recommended.847,861 |
I |
B |
Measuring waist circumference to diagnosis metabolic syndrome, treating blood pressure,
lipids, and impaired glucose tolerance, and providing guidance to quit smoking and
exercise are recommended in non-obese patients.847,854–857 |
I |
C |
| Interventions aiming at low weight (BMI <18.5) is not recommended.850,853 |
III: No
benefit |
B |
Abbreviation: BMI, body mass index.
In Japan, people with body mass index (BMI) 22 had the least risk of morbidity based on the last epidemiological study. Because of this, BMI=22 was defined as normal weight, with BMI ≥25 as obesity. In people with obesity, symptomatic obesity was defined as obesity with health problems due to obesity or high risk patient with accumulation of excess visceral fat.869
Many epidemiological studies show the relationship between obesity and formation and progress of atherosclerosis or cardiovascular events. The Asia Cohort Consortium registered more than 1.12 million people in east Asia including Japan, and south Asia shows a trend of more obesity patients with high BMI dying from cardiovascular death. In this study, the relationship between BMI and mortality was inversely correlated. This was known as the obesity paradox.870,871
In the reports in Japan and the world about coronary heart diseases including ACS, the relationship between BMI and cardiovascular events was not linear. It was clear that underweight was a poor prognostic factor.872–875
In this study, the mortality of cardiovascular death was lowest with BMI of 20–24 in East Asia. Both in underweight (below 15 BMI) and obesity (over 27.5 of BMI) the mortality of cardiovascular death was higher, and this relationship shows a U shape. Comparing age, in the group over 53 years of age (HR 1.17; 95% CI 1.10–1.25) the relationship was stronger than in under 53 of age (HR 1.38; 95% CI 1.20–1.58). There was no difference between East Asia and Europe and America in this trend. There are many reports that in the middle age, obesity with high BMI is an important risk factor for cardiovascular events but, in older age, high BMI was not a factor of mortality.
It was considered that low BMI in older aged patients was the factor of mortality because of a combination of frailty, sarcopenia, liver failure, renal failure, and malignant tumors. On the other hand, in non-older aged patients, metabolic syndrome was considered as a concept of accumulation of visceral fat in addition to BMI. The concept was born from the fact that cardiovascular risk was getting higher by overlapping minor risk factors. Epidemiological studies and meta-analyses show that metabolic syndrome is a strong risk factor for cardiovascular events.876–878
It was reported that not only accumulation of single risk factors but also lowering the adiponectin conveyed high risk for cardiovascular events.879
The statement advocated the definition of metabolic syndrome as with three of five criteria (obesity, high triglyceride, low high-density lipoprotein cholesterol [HDL-C], hypertension, hyperglycemia). It was not necessary that metabolic syndrome include accumulation of visceral fat.880
In Japan, the waist circumference is used in all screening for accumulation of visceral fat and metabolic syndrome. In the guideline of obesity in Japan, lowering the body weight and waist circumference is directed by health guidance to decrease risk factors such as elevated blood glucose level, blood pressure and lipids. This shows the importance of treating the obesity.869
In particularly, among patients from young to middle aged with ACS, the rate of smoking history was high, and they already had applicable factors of metabolic syndrome. Health guidance which leads to reduction in body weight or waist circumference by 3% over 3-6 months should be provided.869,881
2. Pharmacotherapy
2.1 Antithrombotics (Table 82)
Table 82.
Recommendations and Evidence Level Regarding Antithrombotic Administration in Secondary Prevention
| |
COR |
LOE |
| Indefinite use of aspirin (81–162 mg/day) is recommended for patients without contraindication.295,488 |
I |
A |
Concomitant use of warfarin is recommended for patients with LV thrombus, atrial thrombus,
severe heart failure, LV aneurysm, or mechanical prosthetic valve.494 |
I |
B |
Six to 12 months of DAPT with aspirin (81–162 mg/day) plus clopidogrel (75 mg/day) or prasugrel
(3.75 mg/day) is recommended after stent implantation.281,283,489,890 |
I |
A |
| Clopidogrel (75 mg/day) is recommended for patients intolerant to aspirin. |
I |
C |
DAPT shorter than 3 months should be considered for patients with high bleeding risk after DES
implantation.889 |
IIa |
B |
Longer-term DAPT after stent implantation may be considered in patients with low bleeding risk
and high thrombotic risk.897 |
IIb |
B |
Combination therapy of aspirin and ticagrelor (120 mg/day) may be considered in patients with
prior myocardial infarction.891 |
IIb |
B |
Long-term triple antithrombotic therapy (i.e., ananticoagulant and dual antiplatelet agents) should not
be administered in high bleeding risk patients undergoing PCI with atrial fibrillation.286,492,493,885 |
III:
Harm |
B |
Abbreviations: LV, left ventricular; DAPT, dual antiplatelet therapy; DES, drug eluting stent; PCI, percutaneous coronary intervention.
Approximately 90% of ACS patients receive PCI in Japan.491
Antiplatelet therapy after coronary stent implantation is thought to be mandatory. Aspirin 81-162 mg/day plus a P12Y12
receptor inhibitor agent is the most commonly used regimen.281,489
Generally, Japanese patients are at higher risk for bleeding and at lower risk for thrombotic events as compared with Caucasians. Therefore, it is of importance to determine the rates of bleeding and thrombotic events in the Japanese population. Recent studies have shown that high thrombotic risk Japanese patients are also at high risk for bleeding.24,25
Very late stent thrombosis after first generation DES has been an issue. In the j-Cypher registry, discontinuation of thienopyridine antiplatelet drug 6 months after stenting was not associated with increased adverse cardiac events for up to 2 years, while discontinuation of thienopyridine antiplatelet drug within 6 months after stenting can be a risk for stent thrombosis.882
In addition, continuation of P2Y12
receptor inhibitors beyond 1 year did not reduce the risk of very late stent thrombosis or MACEs for up to 5-year follow-up.883
Newer generation DESs are associated with even lower risk of thrombosis, therefore, the required period of DAPT is becoming shorter.884
In ACC/AHA and ESC guidelines, 12-month DAPT (aspirin plus ticagrelor/prasugrel/clopidogrel) is recommended for ACS patients with low bleeding risk. For ACS patients with high bleeding risk, 6-month DAPT is recommended.885,886
For stable CAD, 6-month DAPT with aspirin plus clopidogrel is recommended for patients with low bleeding risk, and 3-month DAPT may be considered for high bleeding risk patients. A recent SENIOR study has shown that biodegradable polymer-based DES was noninferior to BMS with 6-month DAPT in elderly patients with ACS.813
The DAPT STEMI trial has shown that 6-month DAPT was noninferior to 12-month DAPT with respect to adverse cardiac events in STEMI patients receiving DES.887
The NIPPON trial and STOPDAPT trial have shown that there was no increase in stent thrombosis or MACEs with shorter DAPT.888,889
On the other hand, SMART-DATE suggested an increase in myocardial infarction with 6-month DAPT as compared with 12-month DAPT in ACS patients receiving DES, which did not show noninferiority of 6-month DAPT.890
With these results taken together, we defined 6–12 months as the standard DAPT period. Of note, the STOPDAPT-2 trial, which is comparing 1-month DAPT vs. 12-month DAPT after everolimus eluting stent implantation, is underway in Japan.
The PEGASUS-TIMI 54 trial has shown that DAPT with aspirin plus ticagrelor (90 mg twice daily or 60 mg twice daily) reduced cardiac events in high-risk patients with prior myocardial infarction. In the trial, DAPT was associated with increased risk of nonfatal bleeding.891
DAPT with aspirin plus ticagrelor 60 mg twice daily is approved in Japan.
Several Japanese studies have shown no benefit of DAPT beyond 1 year after stenting even after stratifying risk factors such as diabetes, history of myocardial infarction, or complex lesion characteristics.892,893
Longer use of DAPT was reported to be associated with an increased risk of moderate or severe bleeding.894
In ACS patients with high bleeding risk, longer DAPT should be avoided. Even shorter DAPT (less than 3 months) may be considered.
Risk scoring systems to evaluate thrombotic and bleeding risk are being developed. PRECISE-DAPT score, which is calculated at the time of PCI, seeks to predict the bleeding risk of DAPT. PRECISE-DAPT score is calculated with age, history of bleeding, white blood cell count, hemoglobin, and creatinine clearance.895
DAPT score, which is calculated in the chronic phase of antiplatelet therapy, is calculated with history of smoking, diabetes, myocardial infarction, history of revascularization or myocardial infarction, paclitaxel eluting stent implantation, smaller stent diameter, heart failure or low LVEF, saphenous vein graft lesion, and age. In the DAPT study, DAPT reduced myocardial infarction/stent thrombosis without increasing bleeding events in patients with higher DAPT scores (Table 83).896
Limited data are available to evaluate the applicability of these scoring systems to the Japanese population; however, clinicians should evaluate thrombotic and bleeding risk in each patient to individualize the medication.
Table 83.
Risk Scores Validated for DAPT Duration Decision-Making
| |
PRECISE-DAPT score |
DAPT score |
| Time of use |
At the time of coronary stenting |
After 12 months of uneventful DAPT |
DAPT duration
strategies assessed |
Short DAPT (3–6 months) vs.
Standard/long DAPT (12–24 months) |
Standard DAPT (12 months) vs.
Long DAPT (30 months) |
| Score calculation* |
 |
Age
≥75
65 to <75
<65
Cigarette smoking
Diabetes mellitus
MI at presentation
Prior PCI or prior MI
Paclitaxel-eluting stent
Stent diameter <3 mm
CHF or LVEF <30%
Vein graft stent |
−2 pt
−1 pt
0 pt
+1 pt
+1 pt
+1 pt
+1 pt
+1 pt
+1 pt
+2 pt
+2 pt |
| Score range |
0 to 100 points |
−2 to 10 points |
Decision making
cut-off suggested |
Score ≥25 → Short DAPT
Score <25 → Standard/long DAPT |
Score ≥2 → Long DAPT
Score <2 → Standard DAPT |
| Calculator |
www.precisedaptscore.com |
www.daptstudy.org |
*For the PRECISE-DAPT score use the score nomogram: mark patient’s value for each of the five clinical variables of the score and draw a vertical line to the ‘Point’ axis to determine the number of points obtained for each clinical variable. Then summate the points obtained for each clinical variable to the total score. A practical case example for score calculation is provided in Web Figure 1 of the Web Addenda.
For the DAPT score summate positive points for each value and subtract values for age to the total score.
Abbreviations: DAPT, dual antiplatelet therapy; Hb, hemoglobin; WBC, white blood cell; CrCl, creatinine clearance; MI, myocardial infarction; PCI, percutaneous coronary intervention; CHF, congestive heart failure; LVEF, left ventricular ejection fraction. (Adapted from Valgimigli M, et al. 2018885)
Anticoagulation is indicated for patients with atrial fibrillation.607
In a Japanese registry, 5% of the ACS patients are complicated by atrial fibrillation.491
Immediately after stenting, Triple therapy (DAPT with aspirin plus a P2Y12
inhibitor and an anticoagulant agent) are often prescribed. However, triple antithrombotic therapy is reported to significantly increase the risk of severe bleeding. Current ESC guidelines recommend 6-month triple therapy when thrombotic risk is high, and 1-month or no triple therapy (direct oral anticoagulants (DOAC) with aspirin plus clopidogrel) when bleeding risk is high for patients receiving coronary stent.885
Recent clinical trials have shown that DAPT with DOAC plus clopidogrel significantly reduced bleeding events without an increase in thrombotic events or revascularization.492,493
In those DOAC trials, ACS comprised a significant portion of patients, therefore, DOAC plus clopidogrel is a realistic option immediately after stenting for ACS patients as well. There are no data to support optimal duration/dosing of triple antithrombotic therapy. Prasugrel with a maintenance dose of 10 mg/day is associated with a significantly increased risk of bleeding when used with anticoagulation. However, prasugrel with a maintenance dose of 3.75 mg/day, which is the approved dose in Japan, has no data to support or dissuade its use. In the long-term follow-up (e.g., beyond 1 year) after stenting, there are limited data to support a regimen of oral anticoagulant alone or oral anticoagulant plus an antiplatelet agent.
2.2 β-Blockers (Table 84)
Table 84.
Recommendations and Evidence Level of β-Blockers in Secondary Prevention
| |
COR |
LOE |
Long-term oral administration of β-blockers is recommended for patients with clinical signs of
heart failure or LVEF ≤40%.501–503 |
I |
A |
Routine administration of β-blockers may be considered for patients with normal LV function and
those without clinical signs of heart failure.520,900 |
IIb |
C |
β-blockers should not be administered in patients with hypotension, acute heart failure and
severe bradycardia such as AV block.503 |
III:
Harm |
B |
| β-blocker alone should not be administered in patients with coronary spasm as a basic etiology.520 |
III:
Harm |
B |
| Initial high-dose β-blockers should not be administered in patients with heart failure.520 |
III:
Harm |
B |
Abbreviations: LVEF, left ventricular ejection fraction; LV, left ventricular; AV, atrioventricular.
It is well established by meta-analysis that long-term oral administration of β-blockers is effective for patients after ACS onset. However, each study included into the meta-analysis was done in the prereperfusion era.898
Therefore, the effects of β-blockers are based on studies in patients with LV systolic failure. In fact, the CAPRICORN study, which included patients with fibrinolysis or primary PCI reperfusion therapy and LVEF ≤40% (46% of the all study patients), revealed that carvedilol inhibited all-cause mortality.502
On the other hand, in a French nation-wide registry for ACS cases without heart failure, use of β-blockers after 30 days from onset did not reduce all-cause mortality.506
A similar result was shown by a larger observational study with a longer follow-up period.899
In the Japanese randomized controlled trial CAPITAL-RCT (observation period: median 3.9 years), the efficacy of carvedilol administration in STEMI patients with preserved LV function (LVEF of 40% or more) after primary PCI was investigated. There was no significant difference in the rate of major endpoints (total deaths, myocardial infarction, ACS, hospitalization due to heart failure) in the carvedilol group and non-administered group (6.8% vs. 7.9%).900
From the above-mentioned results, although it seems to be reasonable that administration of β-blockers in chronic phases of ACS should be limited to patients with reduced systolic LV function (LVEF ≤40%) and to those without contraindications (acute decompensated heart failure, unstable hemodynamics and advanced AV block), there were no differences between CCBs and β-blockers for cardiovascular death and reinfarction rates in general patients with prior myocardial infarction in the JBCMI study for Japanese patients over an average follow-up period of 455 days.520
Relatively better results for β-blockers in JBCMI would be related to the fact that primary PCI was done in more than 80% of the study population. This issue is under discussion.901
2.3 Renin-Angiotensin-Aldosterone Inhibitors (RAAI) (Table 85)
Table 85.
Recommendations and Evidence Level of RAAS Inhibitor Administration in Secondary Prevention
| |
COR |
LOE |
Long-term oral administration of ACE inhibitors is recommended for patients with reduced LV function
(LVEF ≤40%) or clinical signs of heart failure.217 |
I |
A |
Long-term oral administration of ACE inhibitors is recommended for high-risk patients with
hypertension or diabetes and with normal LV function.513,514 |
I |
B |
Long-term oral administration of ARBs is recommended for patients with reduced LV function
(LVEF ≤40%) or clinical signs of heart failure in cases of ACE inhibitors intolerance.508 |
I |
A |
A mineralocorticoid receptor antagonist should be administered in patients with reduced LV function
(LVEF ≤40%), heart failure or diabetes already treated with ACE inhibitors and β-blockers if there
is no renal failure or hyperkalemia.512 |
I |
A |
Long-term oral administration of ACE inhbitors should be considered for all patients without
contraindication.905 |
IIa |
A |
Long-term oral administration of ARB added onto ACE inhibitors may be considered for patients who have
reduced LV function or heart failure symptoms without concerns for deterioration in renal function.907 |
IIb |
B |
Long-term oral administration of ARB may be considered regardless of LV dysfunction or heart
failure symptoms.908 |
IIb |
B |
Abbreviations: ACE, angiotensin converting enzyme; LV, left ventriculrar; ARB, angiotensin II receptor blocker; LVEF, left ventricular ejection fraction.
It is also well established that administration of inhibitors of renin angiotensin aldosterone system is effective for prevention of cardiovascular events in patients with reduced LV function. Furthermore, from the fact that angiotensin II has proatherogenic effects,902
it is possible that inhibitors of renin angiotensin aldosterone system prevents recurrence of ACS. The HOPE study that examined the effects of ramipril in patients with preserved LV function and no previous history of heart failure revealed that all-cause mortality decreased by 16% and half of the study population had previous myocardial infarction.514
In the same way, perindopril reduced cardiovascular events by 20% in the EUROPA study.513
On the other hand, other ACE inhibitors, such as trandolapril903
and quinapril904
had no effect in regards to cardiovascular prevention. Therefore, the effects of ACE inhibitors in chronic-phase ACS patients without LV dysfunction may not be class effects but drug effects.905
Effects of ARB on secondary prevention of ACS were examined in the OPTIMAAL511
and VALIANT508
studies. Losartan did not inhibit cardiovascular events in the OPTIMAAL study. On the contrary, valsartan proved to be non-inferior to captopril in the VALIANT study, and therefore the drug could be substituted to ACE inhibitors. Furthermore, although the combination of ACE inhibitors and ARB did not improve the primary endpoint, the combination therapy had preventative effects for ACS recurrence and admission due to worsening of heart failure in a post hoc analysis of the limited patients with LV dysfunction. In the ONTARGET study906
that is comprised of patients with prior myocardial infarction in a half of the study populations, telmisartan had a similar effect to ramipril. In a substudy of the CHARM study of same study population, candesartan reduced cardiovascular events.907
In the 4C study, a Japanese study with ARB where 40% of the study populations had history of prior myocardial infarction, an average dose of 6.1 mg candesartan reduced a combined endpoint of cardiovascular death, non-fatal myocardial infarction, UA and admission due to heart failure worsening.908
In the EPHESUS trial, addition of the selective aldosterone antagonist eplerenone to optimal medication in AMI with both LV dysfunction and heart failure resulted in significantly reduced mortality and complications.512
2.4 Nitrates, Nicorandil (Table 86)
Table 86.
Recommendations and Evidence Level of Nitric Acid and Nicorandil Administration in Secondary Prevention
| |
COR |
LOE |
Administration of long-acting nitrates is recommended for patients with vasospastic angina or
those whose etiology is definitely coronary spasm to prevent ischemic events.909 |
I |
C |
Administration of nicorandil should be considered for prior myocardial infarction patients with
angina attacks.914 |
IIa |
B |
Most of the randomized controlled trials for chronic effects of nitrates after ACS onset were done before the era of reperfusion therapy; therefore, no new evidence is available in regards to nitrates. Recently drug effects were also confirmed from registry studies with some statistical methods to adjust background characteristics. In such registry studies in Japan, nitrates reduced cardiovascular events in the JCAD909
study, but in the HIJAMI910
and JACSS911
studies.
In a substudy of the IONA study done in European countries for stable angina, nicorandil reduced cardiovascular events in patients with prior myocardial infarction.914
On the other hand, use of nicorandil was a significant predictor for cardiovascular event in a small study done in Japan for STEMI.913
2.5 CCBs (Table 87)
Table 87.
Recommendations and Evidence Level of Ca Antagonist Administration in Secondary Prevention
| |
COR |
LOE |
Administration of long-acting CCB is recommended for patients with vasospastic angina or
those whose etiology is definitely coronary spasm to prevent ischemic events.520 |
I |
B |
Administration of long-acting CCB as an alternative to β-blockers should be considered to
prevent ischemic events regardless of vasospastic angina.521 |
IIa |
B |
Administration of long-acting CCB as an additional drug of standard medical therapy or an
alternative to ACE inhibitors may be considered to prevent ischemic events.915 |
IIb |
B |
Diltiazem or verapamil should not be administered in patients complicated with heart failure or
AV block.524,525 |
III:
Harm |
C |
Abbreviations: CCB, calcium-channel blocker; ACE, angiotensin converting enzyme; AV, atrioventricular.
According to the result of a meta-analysis in the era of short-acting CCBs,518
the drugs could not prevent recurrence of myocardial infarction. In the CAMELOT study with the long-acting CCB amlodipine,518
40% of the study populations had a history of prior myocardial infarction. In the study, amlodipine 10 mg significantly inhibited cardiovascular events as compared with enalapril 10 mg or placebo.
In the JBCMI study, which was a Japanese study including 1,090 patients with AMI,520
the effects of CCBs were compared with β-blockers. The rate of cardiovascular events was not different between CCBs and β-blockers during an average follow-up period of 455 days. Furthermore, in the study, UA related to coronary spasm increased significantly in patients treated with β-blockers. Same results were proved in another prospective randomized trial done with a smaller number of Japanese participants.521
In this study, patients with coronary spasm, 41% of the recruited myocardial infarction patients, were excluded from the study population. Therefore, the effects of CCBs were same with those of β-blockers even in patients without coronary spasm.
2.6 Lipid-Lowering Therapy (Table 88)
Table 88.
Recommendations and Evidence Level of Medication Administration to Treat Disorders of Lipid Metabolism in Secondary Prevention
| |
COR |
LOE |
| The maximum tolerable dose of a strong statin should be administered.542,921,922,924,926–930 |
I |
A |
PCKS9 inhibitors should be considered in patients with FH who do not achieve LDL-C goal of
<70 mg/dL with the maximum tolerable dose of statin.927,932–934 |
IIa |
B |
Ezetimibe should be considered in high-risk patients who do not achieve LDL-C goal of <70 mg/dL
with the maximum tolerable dose of statin.542,926,931,991–993 |
IIa |
B |
PCSK9 inhibitors may be considered in high-risk patients who do not achieve LDL-C goal of
<70 mg/dL with the maximum tolerable dose of statin.927,935–937 |
IIb |
B |
| Fibrates may be considered in patients with hypertriglyceridemia and low HDL-C levels.994 |
IIb |
C |
| Combination of EPA and statins may be considered.916,916a |
IIb |
B |
Abbreviations: PCSK9, Proprotein convertase subtilisin/kexin type 9; FH, familial hypercholesterolemia; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; EPA, eicosapentanoic acid.
Previous observational and epidemiological studies showed that patients with ACS had worse clinical outcomes than those with stable CAD. The incidence of cardiovascular events in the PACIFIC study, which was the prospective multicenter ACS registry in Japan, was more than twice the incidence in all patients with CAD (more than 35/1,000 per year491
vs. 4.5 to 15/1,000 per year,916,917,918
within one year after ACS onset. This result indicated that ACS patients had higher risk among patients with CAD. Systematic reviews and meta-analyses of randomized controlled trials showed that the achieved absolute LDL-C level correlated with the absolute rate of MACEs. Thus, aggressive lowering of LDL-C is necessary in Japanese ACS patients. In Japan, the target of LDL-C level for secondary prevention was previously less than 100 mg/dL;919,920
however, the 2017 Japan Atherosclerosis Society Guidelines specify the target goal in patients with ACS to be 70 mg/dL.921,922
The 2013 ACC/AHA guidelines recommend high-dose statin without a target value,923
and the ESC/EAS guidelines recommend the goal of LDL-C <70 mg/dL.924
These two recommendations are not necessarily mutually exclusive. The REAL-CAD study, the first large-scale clinical trial in Japan including 13,054 participants, showed that high-dose pitavastatin (4 mg/day) significantly reduced MACEs (cardiovascular death, non-fatal myocardial infarction, non-fatal ischemic stroke, or UA requiring emergency hospitalization) compared to low-dose pitavastatin (1 mg/day) (event rate: 4.3% vs. 5.4%, 95% CI 0.69–0.95, number needed to treat [NNT] 63).925
Moreover, the result for the primary endpoint was consistent across several prespecified subgroups, including age, sex, or LDL-C level before intervention. In the REAL-CAD study, 24% of participants had a history of ACS within 1 year. Based on the results of the REAL-CAD study and previous intravascular ultrasound trial, which showed the coronary plaque regression using high-dose statin, the maximum tolerable dose of strong statin (atorvastatin, rosuvastatin, and pitavastatin) is recommended as the first-line therapy for ACS patients regardless of pre-intervention LDL-C levels. However, it should be noted that there is not enough evidence for the recommendation of target value of LDL-C in the special population who had been excluded from previous large-scale clinical trials (≥80 years old, hemodialysis, active stage of heart failure). When LDL-C <70 mg/dL cannot be achieved with the maximum tolerable dose of statin, other lipid-lowering agents on top of statin therapy are considered based on evidence from Western countries.
In addition, with LDL-C <70 mg/dL as the target, the IMPROVE-IT trial926
and FOURIER trial,927
and ODYSSEY OUTCOMES trial928
demonstrated a reduction in events, and the GLAGOV trial929
and PRECISE-IVUS trial542
showed plaque regression. If the population at increased risk of ACS is identified in Japan through risk stratification, it is necessary to lower the LDL-C target goal.
2.6.2 LDL-C Lowering Drugs
a. Statins
Numerous trials have demonstrated that statin treatment improves clinical outcomes.921,922
The clinical benefits observed with statin therapy appear to be greater than what might be expected from changes in LDL-C alone, suggesting that the beneficial effects of statins, a so-called “pleiotropic effect”, might extend beyond the LDL-C lowering effects. Otherwise, the beneficial effects of LDL-C lowering of 1 mmol/L (38.7 mg/dL) on the incidence of cardiovascular events are not different between statin and non-statin agents.930
However, there are no prospective clinical trials comparing cardiovascular events between statins and non-statin drugs with LDL-C as the target goal. Furthermore, statins have the strongest unequivocal evidence for reducing cardiovascular risk of all lipid-lowering drugs. Therefore, the maximum tolerable dose of strong statin (atorvastatin, rosuvastatin, and pitavastatin) is recommended at the first-line therapy.
b. Ezetimibe
Ezetimibe reduces the absorption of cholesterol from the intestine. When added to statins, ezetimibe reduces LDL-C levels by an additional 35 to 50%, on average. A meta-analysis demonstrated that the combination of statin and ezetimibe reduced cardiovascular events in high-risk patients, such as those with ACS, peripheral arterial disease, and FH.931
In the PRECISE-IVUS trial, the combination of atorvastatin/ezetimibe achieved lower levels of LDL-C than atorvastatin alone, which resulted in greater coronary plaque regression.542
Moreover, the IMPROVE-IT trial, that included 18,144 ACS patients, showed that the combination of simvastatin/ezetimibe therapy achieved significantly lower levels of LDL-C compared to simvastatin alone (55 mg/dL vs. 70 mg/dL), with 6.4% relative risk reduction of cardiovascular events.926
Since NNT of the result was 50 in 7 years, assessment is challenging in the Japanese population, in whom the incidence of cardiovascular events is lower than the Western population. Subgroup analyses showed a 14% relative risk reduction with NNT of 18 in diabetic patients, a 19% relative risk reduction with NNT of 16 if at least 3 of 9 TIMI risk factors were present,931
and a 14% relative risk reduction with NNT of 11 in patients with a history of CABG. On the other hand, HIJ-PROPER study931a
examined the effect of pitavastatin plus ezetimibe in Japanese ACS patients with dyslipidaemia. The combination of pitavastatin/ezetimibe therapy was effective for the reduction of cardiovascular events only in patients with higher cholesterol absorption, but not in all study patients. Based on these findings, the combination of statin /ezetimibe therapy may be preferred to above-mentioned high-risk patients.
c. PCSK9 inhibitor
Monoclonal antibodies to PCSK9 induced an approximately 50–60% reduction in LDL-C levels in patients who are being treated with statins, as well as those with statin intolerance or FH.927,932,933,934
The GLAGOV trial assessed the effects of PCSK9 inhibition with evolocumab on progression of coronary atherosclerosis, which was measured by serial intravascular ultrasound measurements.929
The FOURIER trial, a large-scale clinical trial investigating the effects of evolocumab on cardiovascular events in 27,654 patients with atherosclerotic cardiovascular disease, showed an approximately 60 % reduction in LDL-C levels and a 15% relative risk reduction with NNT of 50.927
In subgroup analyses of the FOURIER trial, a PCSK9 inhibitor showed a 17% relative risk reduction with NNT of 37 in the diabetes group,935
21% relative risk reduction with NNT of 29 in peripheral arterial disease,936
and a 30% relative risk reduction with NNT of 29 in multivessel disease.937
Furthermore, the ODYSSEY OUTCOMES trial was published in 2018 as an event trial in ACS patients using a PCSK9 inhibitor, showing that alirocumab significantly reduced the rate of primary endpoints and all-cause mortality, both by 15%.928
The median follow-up period was 2.2 years and NNT was 49. In this study with high dose statin administration (atorvastatin 40 mg or more, rosuvastatin 20 mg or more), an inhibitory effect on the event rate was observed only in the subgroup with LDL-C value of 100 mg/dL or more before administration of the PCSK9 inhibitor. Therefore, even if statins are prescribed, administration of PCSK9 inhibitor to high-risk patients who do not achieve an LDL-C of 70 mg/dL should be considered. However, it is not currently at the stage of recommendation for use in all ACS patients because there has not yet been plaque regression or event trials in Japanese ACS patients. In FH patients, it is difficult to achieve the target level for management (LDL-C <70 mg/dL, 50% decrease from previous level) with statin treatment alone, and since they are at high risk for developing future events, the combination of PCSK9 inhibitors is considered in the early stage of ACS.
2.6.3 Management of Residual Risks
a. Triglycerides and Fibrates
Domestic and foreign epidemiological and observational studies have demonstrated that hypertriglyceridemia is a risk factor for CAD. High triglyceride levels (≥200 mg/dL) were significantly associated with worse clinical outcomes, even in patients who have achieved LDL-C levels less than 70 mg/dL with statin treatment,938–941
and hypertriglyceridemia is considered a residual risk. However, there is no evidence that triglyceride-lowering therapy is associated with better clinical outcomes. The subgroup analysis of the FIELD study showed that fenofibrate reduced the composite of cardiovascular events in patients with low HDL-C level. Thus, fibrates may be considered in patients with hypertriglyceridemia and low HDL-C level.
b. HDL-C
Although it has been shown in previous epidemiological studies that low HDL-C levels were associated with increased risk of CAD, interventions that increase HDL-C did not show clinical benefit.942,943
An observational study reported that patients with HDL-C level ≤40 mg/dL had a higher risk than those with higher HDL-C levels even in patients who achieved LDL-C <70 mg/dL with statin treatment.944
Thus, low HDL-C is regarded as a residual risk, similar to hypertriglyceridemia. However, the efficacy of a cholesteryl ester transfer protein inhibitor could not be demonstrated in a large-scale clinical trial, and there are no effective drug interventions at the present time.
c. N-3 Polyunsaturated Fatty Acids
Previous reports in Japan showed that supplementation and diet with N-3 polyunsaturated fatty acids, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), reduced cardiovascular events.945–947
On the other hand, despite many negative reports in Europe and the Unites States,948–950
the REDUCE-IT trial was the first to demonstrate event reduction.916a
The REDUCE-IT trial is a large scale clinical trial that examined the combined effect of statins and icosapent ethyl 4 g/day in 8,179 individuals, targeting high risk primary prevention and secondary prevention patients. There was a significant decrease in the rate of primary endpoints (cardiovascular death, non-fatal myocardial infarction, non-fatal stroke, coronary revascularization, or UA) by 25%, and NNT was 21, with overall significant decreases in all major endpoints except all-cause mortality. Further, statistical significance was demonstrated with secondary prevention in the group with triglyceride level of 150 mg/dL or more. However, it should be noted that this was a European and North American report, and that effectiveness was demonstrated with an unprecedented dose of icosapent ethyl in patients with icosapent ethyl before intervention of 26.1 μg/mL, lower than in Japanese people. The JELIS study conducted in Japan in the 1990 s951
was a large-scale clinical trial that evaluated the effect of EPA. Compared to the control group, the EPA group had a significantly lower incidence of cardiovascular events. In the subgroup analysis for secondary prevention,916
the EPA group had 23% lower cardiovascular events compared to the control group. Moreover, an observational study that showed that a high eicosapentaenoic/arachidonic acids ratio (EPA/AA ratio) was associated with better clinical outcomes.952
A meta-analysis of randomized controlled trials indicated that only n-3 polyunsaturated fatty acids and statins reduced both cardiac deaths and all-cause deaths.953
However, these studies were conducted in the 2000 s.
2.7 Treatment Strategy for Patients With Diabetes Mellitus (Table 89)
Table 89.
Recommendations and Evidence Level Regarding Diabetes Treatment in Secondary Prevention
| |
COR |
LOE |
Maintenance of well-controlled blood glucose, body weight, blood pressure, and serum lipid profiles is
recommended in diabetic patients.962,963 |
I |
A |
| Early blood glucose management with a goal HbA1c of <7.0% (NGSP) is recommended.961 |
I |
B |
| OGTT should be considered in patients without a history of diabetes.477 |
IIa |
A |
| Metformin should be considered in obese diabetic patients.965 |
IIa |
B |
| SGLT2 inhibitor which reduce cardiovascular events should be considered in diabetic patients.966–969,971,972 |
IIa |
B |
| Pioglitazone should be considered in diabetic patients without heart failure.977–979 |
IIa |
B |
| GLP-1 receptor agonists may be considered in diabetic patients.973–976 |
IIb |
B |
Abbreviations: OGTT, oral glucose tolerance test; SGLT2, sodium glucose cotransporter-2; GLP-1, glucagon-like peptide-1.
Diabetes mellitus is a significant risk factor for cardiovascular disease (CVD). Epidemiological and observational studies have reported that the incidence and mortality of CAD are 2–3 times greater in diabetic patients than in the normal population.954,955,956,957,958
Moreover, mid/long-term occurrence of cardiovascular events is higher in ACS patients with diabetes compared to non-diabetic patients.959–961
According to the UKPDS80 trial, early intensive blood glucose management is effective in preventing complications in diabetic patients.961
It has also been reported that patients with impaired glucose tolerance had a higher risk of CVD compared to the normal population. Hence, it is important to evaluate glucose tolerance with oral glucose tolerance tests even when fasting blood glucose is <126 mg/dL and Hemoglobin A1c (HbA1c) is <6.5%. Moreover, multifactorial intervention for coronary risk factors such as blood pressure, lipid disorders, smoking, and obesity have a potential benefit.962,963
According to the Japanese guideline for diabetes,964
the glycemic goal of HbA1c <7.0% is recommended to reduce diabetic complications. Moreover, it is important to determine the target glycemic goal individually according to the individual patient, considering factors including age, organ dysfunction, risk of hypoglycemia, and social and familial support system. In general, patients with diabetes should be instructed to improve their lifestyle primarily through diet and exercise therapy, and drug therapy is started when the glycemic goal is not achieved. Medications for secondary prevention of CAD should be determined with consideration of the evidence for cardiovascular events, characteristics of the drugs, and patient factors.
2.7.2 Hypoglycemic Agents
a. Metformin
Metformin is used as first-line therapy in Western countries and is one of the first-line therapies among other oral hypoglycemic agents in Japan. With the increase in approved dose to 2,250 mg/day in Japan, its frequency of use has been increasing. During 10 years of post-trial monitoring of UKPDS (UKPDS80), patients with early metformin had lower risk for any diabetes-related disease, myocardial infarction, and all-cause death compared to those with conventional therapy.961
There are few reports that metformin monotherapy reduced cardiovascular events in patients with CAD.965
However, due to its metabolic effects and cost-effectiveness, metformin is considered for obese diabetic patients after ACS.
b. SGLT2 Inhibitors
Sodium glucose co-transport 2 (SGLT2) inhibitors reduce glucose reabsorption in proximal tubule, producing a reduction in blood glucose without stimulating insulin release. Since it was shown in a large-scale clinical trial to reduce cardiovascular events and have renal protective effects, it is highly important in secondary prevention after myocardial infarction. In the EMPA-REG OUTCOME trial, empagliflozin showed a 14% relative risk reduction for 3-point MACE (cardiovascular death, non-fatal myocardial infarction, and non-fatal stroke) and a 38% relative risk reduction for cardiovascular death compared to placebo.966,967,968,969
In the CANVAS trial, canagliflozin showed a 14% relative risk reduction in 3-point MACE at a dose that exceeds the approved dose in Japan.970
The subgroup analysis of the secondary prevention group had a trend for lower risk of cardiovascular events.971
A subgroup analysis in Asians, including Japanese patients, showed that empagliflozin significantly reduced 3-point MACE, suggesting its potential efficacy in Asians.972
Furthermore, many participants with history of myocardial infarction in addition to heart failure were enrolled. Thus, SGLT2 inhibitor is considered in diabetic patients with CAD.
c. GLP-1–Receptor Agonists
GLP-1–receptor agonists are a class of parenteral glucose-lowering drugs that activate the receptor for the endogenous incretin GLP-1. These drugs reduce blood glucose levels by inhibiting the secretion of glucagon, promoting the release of insulin in response to hyperglycemia. In the LEADER trial, liraglutide, given at twice the approved dose in Japan, produced a 13% relative risk reduction in 3-point MACE (cardiovascular death, non-fatal myocardial infarction, non-fatal stroke), a 22% reduction in cardiovascular deaths, and a 15% reduction in all-cause deaths.973
The SUSTAIN-6 trial showed a significant reduction in 3-point MACE with semaglutide, a long-acting GLP-1 receptor agonist, compared to a placebo group.974
On the other hand, the ELIXA trial using lixisenatide975
and the EXSCEL trial using once-weekly exenatide976
did not demonstrate significant reduction in cardiovascular events compared to placebo. Since there are inconsistent results on the type of GLP-1 receptor agonists and difference in the approved dose of liraglutide in Japan, it is unclear whether the evidence from Western countries can be applied directly to Japanese patients. However, there is evidence that, under certain circumstances, this drug suppresses cardiovascular events. It is selected based on the opinion of a diabetes specialist.
d. Pioglitazone
Pioglitazone is a thiazolidine drug, an oral hypoglycemic agent that improves insulin resistance and has the potential for reduction of cardiovascular events in patients with prior CAD. Although the PROactive trial did not show a reduction in the primary endpoint, pioglitazone reduced the composite of all-cause deaths, non-fatal myocardial infarction, and stroke.977
A meta-analysis also reported that pioglitazone had a lower risk of cardiovascular events compared to the control group (placebo, sulfonylurea, biguanide, or insulin).978
Moreover, in type 2 diabetic patients with CAD, pioglitazone significantly reduced plaque progression on intravascular ultrasound compared to glimepiride during 18-month observation.979
It has been reported in an epidemiological study that pioglitazone might be associated with bladder cancer. For this reason, the package insert specifies pioglitazone to not be used during bladder cancer treatment and to be used with careful consideration and upon thorough explanation in patients with a history of bladder cancer. Recently, a subsequent prospective observational study (KPNC study) rejected the association between the incidence of bladder cancer and pioglitazone use, but there are currently diverse opinions on this subject.980–982
e. α-Glucosidase Inhibitor
The STOP-NIDDM trial demonstrated that acarbose, an α-glucosidase inhibitor that improves postprandial hyperglycemia by delaying carbohydrate absorption, reduced new onset of diabetes and cardiovascular events in patients with the impaired glucose tolerance.983,984
A meta-analysis also showed that acarbose reduced the risk of myocardial infarction in patients with type 2 diabetes.985
There are no recent clinical trials that have showed the efficacy for the secondary prevention. Thus, α-glucosidase inhibitor may be considered in patients with early stage diabetes or impaired glucose tolerance.
f. DPP-4 Inhibitors
Dipeptidyl peptidase 4 (DPP-4) inhibitors decrease blood glucose concentration by increasing the active levels of incretins, GLP-1 and glucose-dependent insulinotropic peptide. These drugs are well tolerated with neutral weight effects and a lower incidence of hypoglycemia and gastrointestinal adverse events compared to placebo, and are widely used in Japan. However, there is no robust evidence that has demonstrated the beneficial effect of these drugs for cardiovascular events. The EXAMINE trial (alogliptin)986
and the TECOS trial (sitagliptin)987
investigated the clinical outcomes in patients with ACS and CAD, respectively. However, these drugs did not reduce adverse cardiovascular events. In addition, the SAVOR-TIMI 53 trial showed that saxagliptin was associated with hospitalization for heart failure.988
However, these large-scale trials were designed to validate their non-inferiority and safety and did not disprove the efficacy of DPP-4 inhibitors.
2.8 Vaccination (Table 90)
Table 90.
Recommendations and Evidence Level of Vaccination in Secondary Prevention
| |
COR |
LOE |
Seasonal influenza vaccination is recommended in patients with a history of myocardial infarction
within one year.989 |
I |
B |
| Pneumococcal vaccination should be considered in elderly patients and high-risk patients.990 |
IIa |
C |
A meta-analysis showed that the influenza vaccine in high-risk patients with a history of myocardial infarction within one year was associated with a lower risk of composite cardiovascular events.989
There is little evidence on pneumococcal vaccination in patients with CAD, but the prevention of pneumonia may be beneficial in elderly patients and those with chronic heart disease.990
3. Cardiac Rehabilitation
3.1 The Role and Present Status of Outpatient Cardiac Rehabilitation
A worldwide survey showed that 7 million patients suffer from AMI every year995
and the mortality rate per year is approximately 10%.996
The JROAD study in Japan in 2016 registered 70 thousand AMI cases per year and the mortality rate in hospitals was about 8%. 20% of survivors experienced cardiovascular events within 1 year and 50% of patients with prior myocardial infarction suffered from major coronary events.997
Secondary prevention after myocardial infarction reduces the risk of cardiovascular events and disabilities. Evidence-based interventions include optimal medical therapy with antiplatelet drugs and statins, blood pressure, lipid, and blood sugar control, and optimization of lifestyle (exercise, diet, smoking).19
Outpatient cardiac rehabilitation plays a role to practice these interventions. It consists of late recovery phase (late phase II) and maintenance phase (phase III) (Figure 8).998

The participation rate in outpatient cardiac rehabilitation in the world is low in elderly patients and high after CABG.999
A study from England between 2003 and 2004 showed that the participation rate of AMI patients was 25% and lower than post CABG (66%), the same as post PCI (24%).1000
Participation rates around the world are not satisfactory (approximately 30%), but the rate in Japan is especially low compared to abroad despite high participation rates in hospital (58%).1001
3.2 Evidence for Outpatient Cardiac Rehabilitation (Table 91)
Table 91.
Recommendation and Evidence Level of Outpatient Cardiac Rehabilitation in Secondary Prevention
| Cardiac rehabilitation |
COR |
LOE |
| Outpatient cardiac rehabilitation should be performed.166,1002,1003 |
I |
A |
Guidelines for the management of STEMI was published by ESC in 2017. All patients should participate in cardiac rehabilitation considering age, prior physical activities and handicaps (Class I Evidence level A),166
and it is desirable that the cardiac rehabilitation program include exercise training, coronary risk modification, education, stress management and psychological support. Exercise therapy reduces the mortality rate of patients with CAD by 22%.166
Cardiac rehabilitation influences not only direct physical effects but also coronary risk factors and lifestyle.1002
Because of short hospital stay of ACS patients, it is an advantage to recognize achievement of evidence-based therapy through participation in outpatient cardiac rehabilitation. The recommended duration of outpatient cardiac rehabilitation is 8–24 weeks.1003,1005
3.2.1 Prognosis (Mortality Rate, Cardiovascular Events)
The 2005 French FAST-MI hospital registry divided patients according to AMI type: STEMI (n=1,523) and NSTEMI (n=1,371).1006
Patients referred to cardiac rehabilitation were younger and more had presented with STEMI than non-referred patients. Deaths occurred among patients referred to cardiac rehabilitation (14.7%) and among non-referred patients (25.9%) (P<0.001). After multivariable adjustment, the association between cardiac rehabilitation and mortality remained significant (HR 0.76; 95% CI 0.60–0.96). Patients referred to cardiac rehabilitation had post-AMI drug treatment that was closer to the recommendations (β-blocker, antiplatelet agent, statin and renin-angiotensin system inhibitor).1006
Suzuki et al. reported that the total mortality of patients who had exercise guidance at beginning of observation after multivariable adjustment was significantly lower than patients who had no exercise guidance. (HR 0.73; 95% CI 0.55–0.96) in Japanese Coronary Artery Disease (JCAD) 3 year follow up study.1007
Kamakura et al. showed low risk AMI patients (meet with all of young age/successful reperfusion/preserved LVEF/without heart failure) had morbidity of coronary risk factors of more than 3 factors compared to other patients. (49% vs. 39%, P<0.05), however, low risk AMI patients improved exercise tolerance and their coronary risk factors profile through active participation in outpatient cardiac rehabilitation compared to inactive patients.1008
Takaya et al. reported that active participation in outpatient cardiac rehabilitation in AMI with CKD significantly improved renal function (eGFR) accompanied with exercise tolerance/BNP/heart rate response.1009
DES apparently has a restenosis prevention effect, but it is impossible to reduce long term total mortality and prevent cardiovascular events.1010
Kurose et al. revealed that the group who participated in cardiac rehabilitation had smaller plaque area and volume of distal site from target lesions at follow up than the group did not participate in cardiac rehabilitation in ACS patients. The participating group also significantly increased peak V̇O2, peak watt at 6 months. Both groups had the same statin use, LDL-C level, LDL/HDL ratio between at the beginning and at follow up, but high-sensitivity CRP in the participating group tended to lower than in the non-participation group. This study has limits because evaluation was done by quantitative coronary angiography, but not intravascular ultrasound, data of exercise tolerance was not available in non-participation group at 6 months and it was not a randomized study. However, it indicated the possibility to suppress progression of coronary plaque by reduction in vascular inflammation through vascular endothelial function and decrease of inflammatory cytokines induced by exercise.1011
Tóth-Zsámboki et al. reported that platelet aggregation parameters and platelet derived growth factor significantly decreased in the group (84 patients) who participated in 3 months of multidisciplinary cardiac rehabilitation (including diet, physical activities, stress management, life style modification) compared to the group (51 patients) who participated in single diet education and group exercise training in ACS patients under optimal DAPT. It was suggested that cardiac rehabilitation had a new pleiotropic effect as non-pharmacologic therapy.1012
On other hand, CROS (the Cardiac Rehabilitation Outcome Study) explored the effects of cardiac rehabilitation in the era of acute phase reperfusion therapy and statins treatment on prognosis. In ACS patients, the mortality rate was significantly reduced in a cohort study, but only one RCT met the CROS inclusion criteria and revealed a neutral result.1013
The GOSPEL study showed that participation in cardiac rehabilitation significantly reduced total mortality by a propensity score-matched analysis of 1,438 patients who had PCI, but it did not reduce cardiac death, AMI onset or revascularization rate.1014
The RAMI study which included 1,813 AMI patients was done as a multicenter randomized controlled study in England. This study reported that the group who participated in comprehensive cardiac rehabilitation (20 hours exercise/6–8 weeks) was the same as the usual group in mortality rate after 2 years and 7–9 years, as well as cardiac events and QOL score.1015
This study had limitations because there was no difference in daily physical activity and no data of exercise tolerance between two groups. However, these two studies raised issues of cardiac rehabilitation in PCI era. The above points are consistent with the fact that there are few reports of multicenter studies on the effectiveness of cardiac rehabilitation in Japan and that evidence is insufficient, as identified by Goto,1016
and further study is needed in the future.
3.2.2 Psychological Issue/QOL
In general, AMI patients frequently have anxiety and it was reported they had 3 times as much morbidity from depression compared to general population. It is important to assess depression using the PHQ (Patient Health Questionnaire)-9 and to support psychological issues for AMI patients. Seki reported that 6 month outpatient cardiac rehabilitation improved muscle strength of limbs, significantly improved SF-36 scales and the anxiety index, but did not improve the depression index in a randomized trial of elderly patients with CAD.1017,1018
Concerning depression, intervention by clinical psychologists is beneficial using cognitive behavioral therapy etc.
Permission of hot and soak up bathing may fulfill wishes of patients and improve quality of life (QOL). Tanaka et al. studied 42℃ and soak up to shoulder bathing for cardiac patients. In the 10 cases, there were changes of double product (DP) in the individual, but no arrhythmia or ischemic ECG change.1019
Furthermore, they showed DP during bathing at any time was lower than DP at anaerobic threshold. Usual bathing at recovery phase cannot be limited in terms of QOL, keeping general precautions for bathing.
Patient tends to think sexual activity is vigorous exercise and fear reattack and sudden death. Hasegawa suggests that in patients with mild/moderate illness it is safe to engage in sexual activity at home because the strength required is the same as climbing stairs. However, sexual activity at exhaustion, after overeating or drinking alcohol, in circumstances of hot, cold or excitement is dangerous and extramarital sex should be prohibited.1020
Return to social activities is important issue, but ratio of return to work in younger females is lower than that in males of same age. Decision to return to work depends on LV function, complete revascularization, presence of arrhythmia and job description. For avoiding deconditioning induced by long rest, mild and moderate physical activities should be continued as possible.1021
There was little evidence about traveling abroad by airplane and the situation is different depending on trip duration, presence of accompanying persons and anxiety for travelling. Patients with LVEF more than 40% have little risk, but patients with LVEF less than 40%, residual myocardial ischemia and arrhythmia may best avoid travelling by airplane until their clinical condition becomes stable.1022
3.2.3 Safety
A national survey in Japan about the safety of cardiac rehabilitation conducted by the Ministry of Health, Labor, and Welfare revealed that recovery phase cardiac rehabilitation in Japan was safe, especially in an institution where formal cardiac rehabilitation was done according to exercise prescription based on exercise stress testing.1023
In addition, there were orthopedic disturbances in 0.3% and injuries in 0.1%, which needed change to or cessation of exercise therapy.
Onishi et al. reported that elderly CAD patients who attended 6 months of outpatient cardiac rehabilitation had a lower incidence of cardiac events (cardiovascular deaths, ACS, revascularization, hospitalization for heart failure, stroke) than patients who did not attend, with matched backgrounds.1024
Soga et al. reported that exercise training starting from the day following elective PCI at an intensity of Borg scale 13 (of range 6–20) was safe, without increase in stent thrombosis in 30 days or cardiovascular events.1025
They also showed that exercise training reduced unplanned visits for angina without increasing stent thrombosis in the prospective observational study of 3,672 patients.1026
3.2.4 Effects on Coronary Risk Factors
In 215 AMI cases (average age 65±11 years, 178 males), the proportion of patients who achieved control of BMI and smoking cessation was significantly higher in the group who participated in 4 months of recovery phase cardiac rehabilitation (123 cases) than in the group who did not participate (92 cases) (both P<0.05).1027
According to the OASIS (Organization to Assess Strategies in Acute Ischemic Syndromes) study, the rate of cardiovascular events at 6 months was lower in non-smoking patients than in smoking patients. Adherence to drug therapy was also better, for example, 96.1% in anti-platelet drugs, 78.9% in statins, 72.4% in ACE inhibitor/ARB, but on the other hand, adherence to exercise therapy or diet therapy was lower. Non-adherence to both was 28.5%, adherence to exercise or diet therapy only was 41.6%, and adherence to both was 29.9%.863
In the BLITZ-4 study, drug adherence at 6 months was 90%, while blood pressure <140/90 mmHg, LDL-C <100 mg/dL, HbA1c <7%, and smoking persistence were 74%, 76%, 45%, and 27%, which was not enough. Inadequate fish intake decreased from 73% to 55%, inadequate intake of fruit and vegetables from 32% to 23%, and insufficient exercise from 74% to 59%. At multivariable analysis, referral to cardiac rehabilitation was associated with a lower risk of insufficient physical exercise and persistent smoking and inadequate intake of fruit and vegetables.1028
In summary, medical professionals should be engaged to promote adherence by showing why patients need to take medications with established evidence not for only adherence. In these words, activities of pharmacists are anticipated.1029
The CHOICE (Choice of Health Options In prevention of Cardiovascular Events) trial evaluated 114 ACS survivors who did not access cardiac rehabilitation in a single center randomized controlled manner. The intervention group participated in telephone support, encompassing mandatory cholesterol lowering and tailored preferential risk modification. The control group participated in conventional care but no centrally coordinated secondary prevention. At 12 months, the intervention group had significantly better risk factor levels than controls for mean total cholesterol (156 vs. 183 mg/dL, P=0.001), systolic blood pressure (132 vs. 144 mmHg, P=0.001), BMI (28.9 vs. 31.2, P=0.025) and physical activity, as well as a better knowledge of risk factor targets.1030
From the above considerations, it shows that secondary prevention is beneficial for the management of risk factors despite exercise therapy, and that intervention by comprehensive cardiac rehabilitation is important.
3.2.5 Exercise Prescription
The Cardiac Rehabilitation Standard Program for Acute Myocardial Infarction (2013) from the Japanese Association of Cardiac Rehabilitation In the Recovery Phase of Myocardial Infarction made by the Japanese Association of Cardiac Rehabilitation Standard Cardiac Rehabilitation Program Writing Committee shows as follows.1031
1. Define the frequency, intensity, duration, and mode of aerobic exercise.
a) Frequency: 3–7 days/week, every day if possible
b) Intensity: 40–60% of maximal oxygen uptake, 55–69% of maximal heart rate, 40–60% of heart rate reserve (Karvonen: k=0.4–0.6), heart rate of the anaerobic metabolic threshold or intensity 1 minute before the anaerobic metabolic threshold
c) Duration: 20–60 minutes
d) Mode: Walking, aerobics, cycling, jogging, light swimming, etc.
2. Define the frequency, intensity, duration, and mode of resistance training.
a) Frequency: 2–3 times/week
b) Intensity: Upper limb exercise: 30–40% of 1RM, lower limb exercise: 50–60% of 1RM, 1 set consists of 10 to 15 times Repeatable load, moderate fatigue, Borg Scale: 11–13, Upper limit is set at “a little hard”
c) Duration: 1 to 3 sets of 8–10 kinds of exercises involving upper and lower limbs
d) Mode: rubber band, weights on ankles and wrists, dumbbells, free weights, pulley, weight machine, etc.
3. Start the exercise program during hospitalization, and participate in the program at the outpatient department for approximately 3 months (2 to 5 months) after discharge.
4. Receive supervised exercise training for 30 to 90 days after onset according to the degree of risk, and subsequently receive non-supervised exercise therapy. Finally, patients will be able to manage the exercise program by themselves.
3.2.6 Maintenance Phase
In Japan, group athletic therapy was introduced for patients with ischemic heart disease in 1982.1032
As a result, it was reported to improve perfusion on myocardial scintigraphy, reduce blood pressure, improve lipid profile, reduce arrhythmia and reduce depression. Exercise tolerance was associated with activities of NK cells (r=0.415, P=0.05), so that improvement in immune function is expected.1033
NPO corporation of Japan Heart Club was established to promote cardiac rehabilitation for primary/secondary prevention of CVD. Main activities include enlightening of proper knowledge about exercise therapy and its usefulness, and training of exercise therapy instructors for the general population and patients with chronic heart disease and lifestyle-related disease. Further, JHC manages Medix Clubs nationwide with reference to the maintenance phase cardiac rehabilitation system in Germany.1034
Exercise therapy at Medix Clubs is conducted by registered cardiac rehabilitation instructors and the quality of exercise therapy is secured.
In addition, Sunamura et al. reported that only 39% of 1,497 patients who received PCI in the acute phase were referred for cardiac rehabilitation, but the completion rate at 6 months was as good as 80%.1035
Also, Arakawa et al. indicated that the participation rate in cardiac rehabilitation was low (19%) despite of induction of community cooperation clinical pathways.1001
Matsuzawa et al. showed implementation rate of cardiac rehabilitation in recovery phase rehabilitation hospitals was 7.5%, and half the reason why they could not perform cardiac rehabilitation was the absence of cardiovascular specialists and staff experienced in cardiac rehabilitation. Relation systems between acute phase hospitals and recovery phase hospitals, as well as enhanced education of staff concerning cardiac rehabilitation, would be future issues.1036
All ACS patients are eligible for cardiac rehabilitation, however the system is not yet ready for participation by all patients.801
Recently, some outpatient clinics who provide cardiac rehabilitation have obtained a qualification. Furthermore, new models of cardiac rehabilitation using home-based cardiac rehabilitation with a system by internet or mobile communications is required. Health exercise instructors are expected to be active in coaching patients to maintain the fun of exercise.
4. Regional Clinical Alliance
There are two important issues for the effective secondary prevention of ACS:1037
to explain the need and methods for secondary prevention to patients and their families, and to get the understanding until their discharge,1038
to encourage them to participate in a valid care system after discharge.1037–1039
The patients should continue to have sufficient treatment for a long time, mostly in the framework of the so-called regional clinical alliance, which is implemented by the primary care physician. A multi-disciplinary care system with a regional framework is desirable because lifestyle improvement and modification are the most important. Thus, a close and collaborative interaction between a cardiovascular physician or a medical team in a specialized hospital and a local primary care system is critical. Currently, in most facilities and regional areas, they are being implemented in the form of a regional clinical alliance path. It is also useful to incorporate the idea of a disease management program into the regional framework. Disease management programs are structured treatment plans that aim to maintain and improve the quality of life and to improve the prognosis including re-hospitalization over the mid to long term. They include a comprehensive and planned medical evaluation, patient education, and lifestyle guidance before and after discharge from the hospital by a multi-disciplinary team.
X. Measurement and Assessment of the Quality of Diagnosis and Treatment
Quality measures for medical care have attracted attention from society only recently. It was reported that treatment outcomes and the number of operations varied among institutions worldwide after the 1980 s. In addition, multiple medical errors, such as patient misidentification, occurred in Japan in the late 1990 s, prompting the assessment of medical care quality, which began to receive public attention. In the context of the complicated presentation of CAD and advanced high-cost technologies, such as PCI, ensuring the quality of medical care has become an important policy issue in Japan. Recently, an objective assessment was attempted using a quality indicator (QI) obtained from the parameterization of actual compliance with evidence-based standard medical care for each field of CVD. Since a practical QI was suggested in the field of ACS) (Table 92), methods to monitor actual compliance have been explored in Japan.1040,1041
Table 92.
Specific Examples of QI Items for ACS Proposed in Western Countries
| ① Implementation of drug therapies recommended in the guidelines |
• Aspirin administration on arrival
• Prescription of an ACE inhibitors or ARB for patients with LV dysfunction
• Prescription of aspirin or a P2Y12 inhibitor at discharge
• Prescription of β-blockers at discharge
• Prescription of a strong statin at discharge
• No inappropriate use of NSAIDs |
| ② Satisfaction of QI items which have wide consensus |
• Time to primary PCI (Door-to-Device time): STEMI patients
• Risk assessment using a risk score (GRACE, TIMI, etc.): NSTE-ACS patients
• Evaluation of LV function (LVEF) during hospitalization
• Cardiac rehabilitation requests |
| ③ Participation in regional networks or nationally based registration systems |
• Participation in a network of medical institutions meeting the following requirements
• Presentation of a telephone number for emergency triage
• Ability to perform ECG diagnosis before arrival to hospital and make a decision to transfer to a PCI institution
• Ability to commence preparation of the catheter room before arrival to hospital
• Participation in the registry and programs for QI evaluation
• Carrying out aggregation of systemic patient satisfaction data |
Abbreviations: ACE, angiotensin converting enzyme; ARB, angiotensin II receptor blocker; LV, left ventricular; NSAIDs, nonsteroidal anti-inflammatory drugs; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction; NSTE-ACS, non-ST-elevation acute coronary syndrome; LVEF, left ventricular ejection fraction; ECG, electrocardiogram.
Specifically, whether the drug therapy recommended in the guideline is performed (Table 92-①), whether it satisfies the QI item which has wide consensus (Table 92-②), and whether there is participation in regional networks or nationally based registration (case, procedure record) systems (Table 92-③), etc. should be evaluated.
1. Approach by the PCI Registry (NCD)
The National Clinical Database (NCD) was established by ten academic societies affiliated with the Japan Surgical Society in 2010. In January 2011, registration of all surgeries performed in the NCD was initiated. The data for PCI have also been registered since 2013 (Japanese Association of Cardiovascular Intervention and Therapeutics [CVIT] J-PCI registry).1042
The NCD has been providing affiliated institutions with various feedback based on the analysis of registered data for improving the level of medical care. An example of NCD feedback is a comparison of patient characteristics as well as performance in clinical departments, which allows clinical departments in affiliated institutions to understand the performance of their institutions. The following seven items are details regarding PCI as of 2018.
• The proportion of ACS patients.
• The proportion of emergent or urgent PCI.
• Door-to-Device time among patients that underwent primary PCI for STEMI.
• The proportion of patients with loading of an antiplatelet agent prior to PCI.
• The proportion of patients that underwent PCI via radial artery access.
• The proportion of patients that underwent ischemia evaluation prior to elective PCI.
• The proportion of patients that underwent PCI for non-proximal lesions.
2. Approach Using the DPC Data (JROAD)
The Diagnosis Procedure Combination (DPC) system, introduced in Japan in 2003, is designed and maintained based on data reflecting actual medical care. Using these characteristics, JCS has developed an assessment method for the quality of medical care by collecting detailed data from medical institutions that have adopted the DPC system (The Japanese Registry of All Cardiac and Vascular Diseases: JROAD) since 2014.1043,1044
Due to the measures implemented to enhance the quality of medical care, proposals for improving the quality of cardiovascular medical care were provided to the members of society, the society, and the social insurance system based on the obtained data.
XI. Recommendations for Social Activities Required to Prevent ACS and Improve Prognosis
In order to improve the prognosis of ACS, we need to work on two issues separately; (1) enhancement of the emergency cardiovascular treatment (Emergency Cardiovascular Care: ECC) system including early transport and emergency catheter treatment, (2) primary and secondary prevention for ACS.
1. Enhancement of ECC System
1.1 Raising Awareness and Education About ACS for Local Residents
It is necessary to build an education system in local communities organized in each region by the medical institution, the administration, and the fire emergency system. We have to increase public interest in ACS or myocardial infarction and the knowledge of such diseases in local residents. There must be a foundation for constructing a chain of survival (rapid reporting, rapid cardiopulmonary resuscitation, rapid defibrillation, and advanced life support) in every region.
1.2 Installation of AEDs and Spread of BLS
Local communities should promote the further installation of automated external defibrillators (AEDs) and increase the activities for spreading basic life support (BLS) for out-of-hospital cardiac arrest. They should also promote the PAD (public access defibrillation) program using AEDs widely.1045
1.3 Education for Patients and Their Families
A variety of meetings and lectures should be held in local areas for patients and their families to learn the first three components of the chain of survival. Attending physicians and primary care physicians must play a significant role there.
1.4 Emergency and Medical Care System for ACS in Local Communities
The organization at the local-community level and the smooth operation are critical in emergent medical care systems for ACS. The hospital, the medical association, the regional administration, and the fire emergency system in a body must cooperate with each other and support the system. For resolution of the regional gaps, it is necessary for us to construct an attentive system according to each region and continue to adapt it to changing conditions.
2. Primary and Secondary Prevention for ACS
In addition to management of classical coronary risk factors such as diabetes mellitus, hypertension, dyslipidemia, obesity, and smoking, lifestyle improvement and modification including the regular exercise are key targets in the primary and secondary prevention of ACS. It is necessary for healthy general people, patients and their families, and the primary care system to raise awareness, and so-called Community Education should be performed thoroughly. Regarding the primary prevention, how we should extend the specific health checkups and specific health guidance in the current social health system to prevent the onset of ACS is important and we have to develop, validate and practice the method for evaluating its usefulness. For secondary prevention, a close partnership between a cardiovascular physician or a medical team in a specialized hospital and a regional primary care system is a challenge. Under the circumstances, the spread of a regional clinical alliance pathway or disease management program is a key. However, there are a variety of secondary prevention programs, and it has been estimated that participation in or access to the overall secondary prevention program is not necessarily high.999,1046
There is little verification for these in our country, and in order to practice a highly useful secondary prevention program, there must be further research and activities, such as connecting data to practice.1047
XII. Future Challenges: Based on Differences Compared to European and North American Clinical Practice Guidelines
Caring for patients with ACS requires active disease management and consideration of each patient’s risk. In many clinical practice guidelines in the Unites States and European countries, an example of such active disease management and consideration of risk profile is the treatment preferences for patients with NSTE-ACS; conservative therapy is recommended for low-risk patients and early reperfusion therapy via PCI is recommended for high-risk patients. However, there are numerous institutions that offer PCI in Japan (the number of the Japanese Association of Cardiovascular Intervention and Therapeutics [CVIT]-authorized institutions is 600 or more, as of 2018), which may lead to PCI being performed earlier in lower-risk patients.1048
Hence, patient risk stratification for appropriate treatment is still controversial in Japan.
In addition, there are major differences in the initial triaging of ACS according to troponin levels between Japan, the Unites States, and European countries. In the Unites States and European countries, a considerable amount of responsibility for overlooking ACS is imposed on physicians and hospitals. Therefore, a verified detailed algorithm is commonly prescribed in the Unites States and European guidelines.138,1049
In Japan, focusing on the initial triage of ACS is relatively rare because access to good medical institutions is typically ensured, among other reasons. Since it is uncertain whether such a medical care system will be sustainable in the future, it seems necessary to establish medical care pathways based on this system while monitoring the health care policy trend in terms of the distribution of medical resources.
The characteristics of patients with ACS in Japan are fundamentally different from those in the Unites States and European countries, as revealed by previous studies. In Japan, aging is progressing more rapidly than other countries. It is known that the mean age of patients with ACS who undergo PCI in Japan is also higher compared to the Unites States, as seen in a comparison of registries.1050
Therefore, even in patients considered as low-risk for nephropathy or diabetes, the general risk for these diseases increases due to aging. In Europe and the Unites States, clinical trials have been conducted to address these problems.810
In Japan, consensus has not yet been reached regarding treating patients with ACS based on differences in patient characteristics. It is thus necessary to actively search for new types of assessments. It is increasingly important to establish evidence from this perspective, in conjunction with the advantages of the use of machine learning and electronic media.
Further understanding the proper usage of antiplatelet agents and anticoagulant agents has become a global issue, especially in the medical treatment of ACS. In the past several years, various antiplatelet agents were introduced in Japan, similar to other countries. The results of the clinical trials that gained approval were various and some agents were approved, but only at a dose specific for the Japanese population.1051
In addition to antiplatelet agents and anticoagulant agents, β-blockers and statins have demonstrated similar differences. In particular, a high-intensity statin dose has become common globally. Japan is also at a major turning point, considering these facts. Further examination of the effects and safety of such agents in clinical settings in Japan is required and appropriate verification is needed.
Finally, as the incidences of hemorrhagic complications and acute nephrosis associated with treatment are higher in the East Asian population, preventive measures need to be taken in Japan.1050,1052
PCI can be performed on various types of patients, considering the current wide range of choices of assistive circulation device, also available for severe cases. Thus, the necessity for PCI is increasing. There are data indicating that perioperative complications are directly reflected in hospitalization costs,1053
and further progress is expected in this field in the future.
Appendix 1 JCS Joint Working Group
Chair:
• Kazuo Kimura, Division of Cardiology, Yokohama City University Medical Center
Members and Collaborators:
• Junya Ako, Department of Cardiovascular Medicine, Kitasato University School of Medicine
• Hirokuni Arai, Department of Cardiovascular Surgery, Tokyo Medical and Dental University
• Nobuhisa Hagiwara, Department of Cardiology, Tokyo Women’s Medical University
• Atsushi Hirayama, Division of Cardiology, Department of Medicine, Nihon University School of Medicine
• Masaharu Ishihara, Division of Cardiovascular Medicine, Department of Internal Medicine, Hyogo College of Medicine
• Hideki Ishii, Department of Cardiology, Nagoya University Graduate School of Medicine
• Takeshi Kimura, Department of Cardiovascular Medicine, Kyoto University Graduate School of Medicine
• Shun Kohsaka, Department of Cardiology, Keio University School of Medicine
• Katsumi Miyauchi, Cardiovascular Medicine, Juntendo Tokyo Koto Geriatric Medical Center
• Shunichi Miyazaki, Division of Cardiology, Department of Medicine, Kindai University Faculty of Medicine
• Yoshihiro Morino, Division of Cardiology, Iwate Medical University
• Yoshihisa Nakagawa, Department of Cardiovascular Medicine, Shiga University of Medical Science
• Koichi Nakao, Division of Cardiology, Cardiovascular Center, Saiseikai Kumamoto Hospital
• Hideki Origuchi, Department of Internal Medicine, Japan Community Health Care Organization Kyushu Hospital
• Wataru Shimizu, Department of Cardiovascular Medicine, Nippon Medical School
• Hiroaki Shimokawa, Department of Cardiovascular Medicine, Tohoku University Graduate School of Medicine
• Yoshio Tahara, Department of Cardiovascular Medicine, National Cerebral and Cardiovascular Center
• Hirofumi Takemura, Department of Thoracic, Cardiovascular and General Surgery, Kanazawa University
• Kenichi Tsujita, Department of Cardiovascular Medicine, Kumamoto University Graduate School of Medical Science
• Satoshi Yasuda, Department of Cardiovascular Medicine, National Cerebral and Cardiovascular Center
• Hideaki Yoshino, Division of Cardiology, Second Department of Internal Medicine, Kyorin University School of Medicine
Collaborators:
• Hirohisa Endo, Department of Cardiovascular Medicine, Juntendo University Hospital
• Daisuke Fukamachi, Department of Cardiology, Nihon University Itabashi Hospital
• Kenji Iino, Department of Thoracic, Cardiovascular and General Surgery, Kanazawa University
• Tomonori Itoh, Department of Medical Education, Iwate Medical University
• Yoshitaka Iwanaga, Division of Cardiology, Department of Medicine, Kindai University Faculty of Medicine
• Ken Kongoji, Division of Cardiology, Second Department of Internal Medicine, Kyorin University School of Medicine
• Masami Kosuge, Division of Cardiology, Yokohama City University Medical Center
• Tomohiro Mizuno, Department of Cardiovascular Surgery, Tokyo Medical and Dental University, Gradiate School of Medical and Dental Science
• Takahiro Nakashima, Department of Cardiovascular Medicine, National Cerebral and Cardiovascular Center
• Teruo Noguchi, Department of Cardiovascular Medicine, National Cerebral and Cardiovascular Center
• Kenji Sakamoto, Department of Cardiovascular Medicine, Kumamoto University Graduate School of Medical Sciences
• Tomohiro Sakamoto, Division of Cardiology, Cardiovascular Center, Saiseikai Kumamoto Hospital
• Takao Shimohama, Department of Cardiovascular Medicine, Kitasato University School of Medicine
• Hiroki Shiomi, Department of Cardiovascular Medicine, Kyoto University Hospital
• Atsushi Suzuki, Department of Cardiology, Tokyo Women’s Medical University
• Jun Takahashi, Department of Cardiovascular Medicine, Tohoku University Hospital
• Ichiro Takeuchi, Department of Emergency Medicine, Yokohama City University Medical Center
• Toshihiro Tamura, Department of Cardiology, Tenri Hospital
• Akihito Tanaka, Department of Cardiology, Nagoya University Hospital
• Keiji Uchida, Division of Cardiovascular Surgery, Yokohama City University Medical Center
• Junichi Yamaguchi, Department of Cardiology, Tokyo Women’s Medical University
• Kenji Yodogawa, Department of Cardiovascular Medicine, Nippon Medical School
Independent Assessment Committee
• Takashi Akasaka, Department of Cardiovascular Medicine, Wakayama Medical University
• Kazuo Haze, Department of Cardiology, Kashiwara Municipal Hospital
• Hisao Ogawa, National Cerebral and Cardiovascular Center
• Hiroyuki Tsutsui, Department of Cardiovascular Medicine, Faculty of Medical Science, Kyushu University Graduate School of Medical Science
• Tsutomu Yamazaki, Innovation & Research Center, International University of Health and Welfare
(The affiliations of the members are as of March, 2019)
Appendix 2 Disclosure of Potential Conflicts of Interest (COI): JCS 2018 Guideline on Diagnosis and Treatment of Acute Coronary Syndrome
| Author |
Employer/
leadership
position
(private
company) |
Stakeholder |
Patent royalty |
Honorarium |
Payment for
manuscripts |
Research grant |
Scholarship
(educational) grant |
Endowed chair |
Other
rewards |
Potential
COI of the
marital
partner,
first-degree
family
members, or
those who
share
income and
property |
Research grant |
Scholarship
(educational)
grant |
Kimura
Kazuo |
|
|
|
AstraZeneca K.K.
Daiichi Sankyo
Company, Limited
Boehringer Ingelheim
Japan, Inc.
Sanofi K.K.
Bristol-Myers Squibb |
|
Otsuka
Pharmaceutical
Co., Ltd.
Japan Medical
Association
Sanofi K.K.
Japan Agency for
Medical
Research and
Development
Bayer Yakuhin,
Ltd.
Research Institute
for Production
Development |
MSD K.K.
Bayer Yakuhin, Ltd.
Pfizer Japan Inc.
Ono Pharmaceutical
Co., Ltd.
Daiichi Sankyo
Company, Limited
Takeda Pharmaceutical
Company Limited |
|
|
|
|
|
| Junya Ako |
|
|
|
MSD K.K.
Astellas Pharma Inc.
Amgen Astellas
BioPharma K.K.
AstraZeneca K.K.
ABBOTT JAPAN CO.,
LTD.
Abbott Vascular Japan
Co., Ltd.
Eli Lilly and Company
Sanofi K.K.
TERUMO
CORPORATION
Bayer Yakuhin, Ltd.
Bristol-Myers Squibb
Kowa Pharmaceutical
Co., Ltd.
Ono Pharmaceutical
Co., Ltd.
Daiichi Sankyo
Company, Limited
Mitsubishi Tanabe
Pharma Corporation
Boehringer Ingelheim
Japan, Inc.
Takeda Pharmaceutical
Company Limited |
|
|
Astellas Pharma Inc.
Abbott Vascular Japan
Co., Ltd.
Nipro Corporation
Bayer Yakuhin, Ltd.
Pfizer Japan Inc.
Kowa Pharmaceutical
Co., Ltd.
Mochida
Pharmaceutical
Co.,Ltd.
Ono Pharmaceutical
Co., Ltd.
Otsuka Pharmaceutical
Co., Ltd.
Daiichi Sankyo
Company, Limited
ASAHI INTECC CO.,
LTD.
Mitsubishi Tanabe
Pharma Corporation
Boehringer Ingelheim
Japan, Inc.
Takeda Pharmaceutical
Company Limited |
|
|
|
|
|
Hirokuni
Arai |
|
|
Sumitomo
Bakelite
Co., Ltd. |
|
|
|
|
|
|
|
|
|
| Hideki Ishii |
|
|
|
Astellas Pharma Inc.
MSD K.K.
Chugai Pharmaceutical
Co.,Ltd.
Bayer Yakuhin, Ltd.
AstraZeneca K.K.
Otsuka Pharmaceutical
Co., Ltd.
Daiichi Sankyo
Company, Limited |
|
|
|
|
|
|
|
|
Masaharu
Ishihara |
|
|
|
Amgen Astellas
BioPharma K.K.
MSD K.K.
Sanofi K.K.
Bayer Yakuhin, Ltd.
AstraZeneca K.K.
Daiichi Sankyo
Company, Limited |
|
Amgen Astellas
BioPharma K.K. |
MSD
Astellas Pharma Inc.
Abbott Vascular Japan
Co., Ltd.
Eisai Co., Ltd.
MID
Sanofi K.K.
TERUMO
CORPORATION
Nipro Corporation
Bayer Yakuhin, Ltd.
Pfizer Japan Inc.
FUKUDA LIFE
TECH CHUGOKU
K.K.
Bristol-Myers Squibb
Boston Scientific
Corporation
Shionogi & Co., Ltd.
Kowa Pharmaceutical
Co., Ltd.
Ono Pharmaceutical
Co., Ltd.
Otsuka Pharmaceutical
Co., Ltd.
Daiichi Sankyo
Company, Limited
Teijin Pharma Limited
Mitsubishi Tanabe
Pharma Corporation
Japan Lifeline Co.,Ltd. |
Abbott Medical
Japan Co., Ltd.
Medtronic Japan
Co., Ltd.
Boehringer Ingelheim
Japan, Inc. |
|
|
|
|
Takeshi
Kimura |
|
|
|
Sanofi K.K.
Boehringer Ingelheim
Japan, Inc.
Kowa Pharmaceutical
Co., Ltd.
Bristol-Myers Squibb
Abbott Vascular Japan
Co., Ltd.
Daiichi Sankyo
Company, Limited |
|
EP - CRSU Co.,
Ltd.
IQVIA Japan.
Otsuka
Pharmaceutical
Co., Ltd.
EPS Corporation |
MID
Sumitomo Dainippon
Pharma Co., Ltd.
Otsuka Pharmaceutical
Co., Ltd.
Boehringer Ingelheim
Japan, Inc.
Daiichi Sankyo
Company, Limited
Mitsubishi Tanabe
Pharma Corporation
Takeda Pharmaceutical
Company Limited
Boston Scientific
Corporation
Astellas Pharma Inc. |
|
|
|
|
|
Shun
Kohsaka |
|
|
|
Bristol-Myers Squibb
Bayer Yakuhin, Ltd.
AstraZeneca K.K.
Pfizer Japan Inc. |
|
Bayer Yakuhin,
Ltd.
Daiichi Sankyo
Company,
Limited |
|
|
|
|
|
|
Wataru
Shimizu |
|
|
|
Daiichi Sankyo
Company, Limited
Bayer Yakuhin, Ltd.
Bristol-Myers Squibb
Pfizer Japan Inc.
Ono Pharmaceutical
Co., Ltd.
Bristol-Myers Squibb
Boehringer Ingelheim
Japan, Inc. |
|
|
Daiichi Sankyo
Company, Limited
Bayer Yakuhin, Ltd.
Bristol-Myers Squibb
Pfizer Japan Inc.
Ono Pharmaceutical
Co., Ltd.
Novartis Pharma K.K.
Otsuka Pharmaceutical
Co., Ltd.
Mitsubishi Tanabe
Pharma Corporation
Boehringer Ingelheim
Japan, Inc.
Eisai Co., Ltd.
Mitsubishi Tanabe
Pharma Corporation
Astellas Pharma Inc.
St. Jude Medical Japan
Co., Ltd. |
|
|
|
|
|
Hiroaki
Shimokawa |
|
|
|
Bayer Yakuhin, Ltd.
Daiichi Sankyo
Company, Limited
Boehringer Ingelheim
Japan, Inc.
Kyowa Hakko Kirin
Co., Ltd. |
|
Bayer Yakuhin,
Ltd. |
MSD K.K.
Astellas Pharma Inc.
Eisai Co., Ltd.
Sanofi K.K.
Bayer Yakuhin, Ltd.
BIOTRONIK Japan,
Inc.
Pfizer Japan Inc.
Bristol-Myers Squibb
Shionogi & Co., Ltd.
Kowa Pharmaceutical
Co., Ltd.
Otsuka Pharmaceutical
Co., Ltd.
Sumitomo Dainippon
Pharma Co., Ltd.
Daiichi Sankyo
Company, Limited
Teijin Pharma Limited
Mitsubishi Tanabe
Pharma Corporation
Nippon Shinyaku Co.,
Ltd.
Takeda Pharmaceutical
Company Limited |
MSD K.K.
Astellas Pharma Inc.
AstraZeneca K.K.
Abbott Medical
Japan Co., Ltd.
Abbott Vascular
Japan Co., Ltd.
GlaxoSmithKline
K.K.
ZEON MEDICAL
INC.
St. Jude Medical
Japan Co., Ltd.
Tesco
TERUMO
CORPORATION
Novartis Pharma
K.K.
Bayer Yakuhin, Ltd.
Asahi Kasei Pharma
Corp.
Shionogi & Co., Ltd.
Kyowa Hakko Kirin
Co., Ltd.
Kowa
Pharmaceutical
Co., Ltd.
Mochida
Pharmaceutical
Co.,Ltd.
Ono Pharmaceutical
Co., Ltd.
Otsuka
Pharmaceutical
Co., Ltd.
Sumitomo
Dainippon Pharma
Co., Ltd.
Daiichi Sankyo
Company, Limited
Chugai
Pharmaceutical
Co.,Ltd.
Mitsubishi Tanabe
Pharma
Corporation
Boehringer Ingelheim
Japan, Inc.
Medtronic Japan
Co., Ltd.
Japan Lifeline
Co.,Ltd.
Nihon Kohden Corp.
Nippon Shinyaku
Co., Ltd.
Hitachi, Ltd.
Takeda
Pharmaceutical
Company Limited |
|
|
|
|
Yoshio
Tahara |
|
|
|
|
|
|
|
|
|
|
|
Takeda
Pharmaceutical
Company
Limited |
Kenichi
Tsujita |
|
|
|
MSD K.K.
Amgen Astellas
BioPharma K.K.
Sanofi K.K.
Bayer Yakuhin, Ltd.
Kowa Pharmaceutical
Co., Ltd.
Otsuka Pharmaceutical
Co., Ltd.
Daiichi Sankyo
Company, Limited
Takeda Pharmaceutical
Company Limited |
|
AstraZeneca K.K.
Sugi Bee Garden
Co.,LTD
Japan Medical
Device
Technology Co.,
Ltd.
Linical Co.,Ltd.
TEIJIN HOME
HEALTHCARE
LIMITED |
Cardinal Health Japan
MSD K.K.
ITI Co., LTD
Astellas Pharma Inc.
Abbott Vascular Japan
Co., Ltd.
Eisai Co., Ltd.
KANEKA MEDIX
CORP.
Goodman Co.,LTD.
Sanofi K.K.
GM MEDICAL
CO.,LTD
St. Jude Medical Japan
Co., Ltd.
TERUMO
CORPORATION
Novartis Pharma K.K.
Bayer Yakuhin, Ltd.
Pfizer Japan Inc.
Fides-one,Inc.
Bristol-Myers Squibb
Boston Scientific
Corporation
Shionogi & Co., Ltd.
Otsuka Pharmaceutical
Co., Ltd.
Daiichi Sankyo
Company, Limited
Chugai Pharmaceutical
Co.,Ltd.
Mitsubishi Tanabe
Pharma Corporation
Boehringer Ingelheim
Japan, Inc.
Medtronic Japan Co.,
Ltd.
Japan Lifeline Co.,Ltd.
Takeda Pharmaceutical
Company Limited |
|
|
|
|
|
| Koichi Nakao |
|
|
|
Edwards Lifesciences
Corporation
Sanofi K.K.
Bayer Yakuhin, Ltd.
Daiichi Sankyo
Company, Limited
Boehringer Ingelheim
Japan, Inc. |
|
|
|
|
|
|
|
|
Yoshihisa
Nakagawa |
|
|
|
Daiichi Sankyo
Company, Limited
Abbott Vascular Japan
Co., Ltd.
Bayer Yakuhin, Ltd.
Boston Scientific
Corporation
Sanofi K.K.
Bristol-Myers Squibb
Kowa Pharmaceutical
Co., Ltd. |
|
|
|
|
|
|
|
|
| Nobuhisa
Hagiwara |
|
|
|
Boehringer Ingelheim
Japan, Inc.
Bristol-Myers Squibb
Bayer Yakuhin, Ltd. |
|
|
AEGERION
PHARMACEUTICALS
Otsuka Pharmaceutical
Co., Ltd.
Astellas Pharma Inc.
Bayer Yakuhin, Ltd.
Boehringer Ingelheim
Japan, Inc.
Daiichi Sankyo
Company, Limited
Takeda Pharmaceutical
Company Limited
Toray Industries, Inc.
Pfizer Japan Inc. |
|
|
|
|
|
Atsushi
Hirayama |
|
|
|
Amgen Astellas
BioPharma K.K.
Astellas Pharma Inc.
AstraZeneca K.K.
Sanofi K.K.
TOA EIYO LTD.
Bayer Yakuhin, Ltd.
Bristol-Myers Squibb
Sumitomo Dainippon
Pharma Co., Ltd.
Daiichi Sankyo
Company, Limited
Boehringer Ingelheim
Japan, Inc. |
|
|
|
Active Medical
Co.,Ltd.
Abbott Medical
Japan Co., Ltd.
St. Jude Medical
Japan Co., Ltd.
FUKUDA LIFE
TECH
CHUGOKU K.K.
HOKUSHIN
MEDICAL
Co.,Ltd
Boston Scientific
Corporation
KURIBARA
MEDICAL
Instruments
Otsuka
Pharmaceutical
Co., Ltd.
Medtronic Japan
Co., Ltd.
Japan Lifeline
Co.,Ltd. |
|
|
Daiichi Sankyo
Company,
Limited
Bristol-Myers
Squibb
Bayer Yakuhin,
Ltd. |
|
| Katsumi
Miyauchi |
|
|
|
Amgen Astellas
BioPharma K.K.
Astellas Pharma Inc.
Bayer HealthCare
Boehringer Ingelheim
Japan, Inc.
Bristol-Myers Squibb
MSD K.K.
Sanofi K.K.
Bayer Yakuhin, Ltd.
Bristol-Myers Squibb
Daiichi Sankyo
Company, Limited
Takeda Pharmaceutical
Company Limited |
|
|
|
|
|
|
|
|
Shunichi
Miyazaki |
|
|
|
|
|
|
MSD K.K.
Bayer Yakuhin, Ltd.
Otsuka Pharmaceutical
Co., Ltd.
Daiichi Sankyo
Company, Limited
Boehringer Ingelheim
Japan, Inc.
Takeda Pharmaceutical
Company Limited |
|
|
|
|
|
Yoshihiro
Morino |
|
|
|
Astellas Pharma Inc.
ABBOTT JAPAN CO.,
LTD.
Edwards Lifesciences
Corporation
Sanofi K.K.
Bayer Yakuhin, Ltd.
Boehringer Ingelheim
Japan, Inc.
Boston Scientific
Corporation
Daiichi Sankyo
Company, Limited
Japan Lifeline Co.,Ltd. |
|
|
|
|
|
|
|
TOSAY
Medical.Co.,
Ltd. |
Satoshi
Yasuda |
|
|
|
Bayer Yakuhin, Ltd.
Bristol-Myers Squibb
Takeda Pharmaceutical
Company Limited
Sanofi K.K.
AstraZeneca K.K.
Daiichi Sankyo
Company, Limited |
|
IQVIA Japan
JSR
Actelion
Pharmaceuticals
Japan Ltd.
Abbott Vascular
Japan Co., Ltd.
TERUMO
CORPORATION
Bayer Yakuhin,
Ltd.
Takeda
Pharmaceutical
Company
Limited |
Bayer Yakuhin, Ltd.
Takeda Pharmaceutical
Company Limited
Daiichi Sankyo
Company, Limited |
|
|
|
|
|
Hideaki
Yoshino |
|
|
|
|
|
|
Takeda Pharmaceutical
Company Limited |
|
|
|
|
|
Tomonori
Itoh |
|
|
|
Actelion
Pharmaceuticals
Japan Ltd.
AstraZeneca K.K.
Abbott Vascular Japan
Co., Ltd.
TOA EIYO LTD.
Bayer Yakuhin, Ltd.
Bristol-Myers Squibb
Kowa Pharmaceutical
Co., Ltd.
Sanwa Kagaku
Kenkyusho Co., Ltd.
Otsuka Pharmaceutical
Co., Ltd.
Daiichi Sankyo
Company, Limited
Mitsubishi Tanabe
Pharma Corporation
Boehringer Ingelheim
Japan, Inc.
Takeda Pharmaceutical
Company Limited |
|
|
|
|
|
|
|
|
Hirohisa
Endo |
|
|
|
|
|
|
|
|
|
|
Daiichi Sankyo
Company,
Limited |
Daiichi Sankyo
Company,
Limited
Takeda
Pharmaceutical
Company
Limited
MSD K.K.
Mitsubishi
Tanabe
Pharma
Corporation
Astellas Pharma
Inc.
Abbott Vascular
Japan Co.,
Ltd. |
Masami
Kosuge |
|
|
|
Daiichi Sankyo
Company, Limited |
|
|
|
|
|
|
|
|
Kenji
Sakamoto |
|
|
|
|
|
|
|
|
|
|
Daiichi Sankyo
Company,
Limited |
|
Tomohiro
Sakamoto |
|
|
|
AstraZeneca K.K.
Edwards Lifesciences
Corporation
Orbusneich Medical
K.K.
Sanofi K.K.
Bayer Yakuhin, Ltd.
Otsuka Pharmaceutical
Co., Ltd.
Daiichi Sankyo
Company, Limited
Medtronic Japan Co.,
Ltd.
Takeda Pharmaceutical
Company Limited |
|
|
|
|
|
|
|
|
Hiroki
Shiomi |
|
|
|
|
|
|
|
|
|
|
EP - CRSU Co.,
Ltd.
IQVIA Japan.
Otsuka
Pharmaceutical
Co., Ltd. |
Astellas Pharma
Inc.
MID
Boston Scientific
Corporation
Otsuka
Pharmaceutical
Co., Ltd.
Sumitomo
Dainippon
Pharma Co.,
Ltd.
Daiichi Sankyo
Company,
Limited
Mitsubishi
Tanabe
Pharma
Corporation
Boehringer
Ingelheim
Japan, Inc.
Takeda
Pharmaceutical
Company
Limited |
Takao
Shimohama |
|
|
|
Boehringer Ingelheim
Japan, Inc.
Abbott Vascular Japan
Co., Ltd. |
|
|
|
|
|
|
|
|
Jun
Takahashi |
|
|
|
|
|
|
|
|
|
|
Bayer Yakuhin,
Ltd. |
MSD K.K.
Astellas Pharma
Inc.
Eisai Co., Ltd.
Pfizer Japan Inc.
Boston Scientific
Corporation
Shionogi & Co.,
Ltd.
Otsuka
Pharmaceutical
Co., Ltd.
Sumitomo
Dainippon
Pharma Co.,
Ltd.
Daiichi Sankyo
Company,
Limited
Teijin Pharma
Limited
Mitsubishi
Tanabe
Pharma
Corporation
Nippon
Shinyaku Co.,
Ltd.
Takeda
Pharmaceutical
Company
Limited |
Takahiro
Nakashima |
|
|
|
|
|
|
|
|
|
|
Takeda
Pharmaceutical
Company
Limited |
|
Daisuke
Fukamachi |
|
|
|
Sanofi K.K. |
|
Daiichi Sankyo
Company,
Limited. |
|
|
|
|
|
|
Junichi
Yamaguchi |
|
|
|
|
|
|
|
Abbott Vascular
Japan Co., Ltd.
Boston Scientific
Corporation
Medtronic Japan
Co., Ltd.
TERUMO
CORPORATION |
|
|
|
AEGERION
PHARMACEUTICALS
Daiichi Sankyo
Company,
Limited
Takeda
Pharmaceutical
Company
Limited
Boehringer
Ingelheim
Japan, Inc.
Astellas Pharma
Inc.
Toray Industries,
Inc. |
Takashi
Akasaka |
|
|
|
Amgen Astellas
BioPharma K.K.
Abbott Vascular Japan
Co., Ltd.
St. Jude Medical Japan
Co., Ltd.
Daiichi Sankyo
Company, Limited
Boehringer Ingelheim
Japan, Inc. |
|
Daiichi Sankyo
Company,
Limited
Infraredx |
ACIST Medical
Systems.
Astellas Pharma Inc.
St. Jude Medical Japan
Co., Ltd.
Novartis Pharma K.K.
HeartFlow® Japan
G.K.
Bayer Yakuhin, Ltd.
Pfizer Japan Inc.
Daiichi Sankyo
Company, Limited |
Abbott Vascular
Japan Co., Ltd.
TERUMO
CORPORATION
Goodman Co.,LTD.
St. Jude Medical
Japan Co., Ltd.
Boston Scientific
Corporation |
|
|
|
|
Hiroyuki
Tsutsui |
|
|
|
MSD K.K.
Astellas Pharma Inc.
Novartis Pharma K.K.
Bayer Yakuhin, Ltd.
Pfizer Japan Inc.
Bristol-Myers Squibb
Otsuka Pharmaceutical
Co., Ltd.
Daiichi Sankyo
Company, Limited
Mitsubishi Tanabe
Pharma Corporation
Boehringer Ingelheim
Japan, Inc.
Takeda Pharmaceutical
Company Limited |
Nippon
Rinsho
Medical
Review
Co., Ltd |
IQVIA Japan.
Actelion
Pharmaceuticals
Japan Ltd.
Daiichi Sankyo
Company,
Limited
Mitsubishi Tanabe
Pharma
Corporation
Japan Tobacco
Inc.
Boehringer
Ingelheim Japan,
Inc. |
MSD K.K.
Astellas Pharma Inc.
Novartis Pharma K.K.
Daiichi Sankyo
Company, Limited
Teijin Pharma Limited
Mitsubishi Tanabe
Pharma Corporation
Takeda Pharmaceutical
Company Limited |
|
|
|
|
|
| Kazuo Haze |
|
|
|
Eisai Co., Ltd.
Astellas Pharma Inc. |
|
|
|
|
|
|
|
|
Tsutomu
Yamazaki |
|
|
|
Takeda Pharmaceutical
Company Limited
AstraZeneca K.K.
Shionogi & Co., Ltd.
Sumitomo Dainippon
Pharma Co., Ltd. |
|
|
|
MSD |
|
|
|
|
Companies are listed only by name.
The following members and collaborators have no relevant COIs.
Member: Hideki Origuchi, none
Member: Hirofumi Takemura, none
Collaborator: Kenji Iino, none
Collaborator: Yoshitaka Iwanaga, none
Collaborator: Keiji Uchida, none
Collaborator: Ken Kongoji, none
Collaborator: Atsushi Suzuki, none
Collaborator: Ichiro Takeuchi, none
Collaborator: Akihito Tanaka, none
Collaborator: Toshihiro Tamura, none
Collaborator: Teruo Noguchi, none
Collaborator: Tomohiro Mizuno, none
Collaborator: Kenji Yodogawa, none
Independent Assessment Committee: Hisao Ogawa
References
- 1.
Davies MJ, Thomas AC. Plaque fissuring: The cause of acute myocardial infarction, sudden ischaemic death, and crescendo angina. Br Heart J 1985; 53: 363–373. PMID: 3885978
- 2.
Fuster V, Badimon L, Badimon JJ, et al. The pathogenesis of coronary artery disease and the acute coronary syndromes. N Engl J Med 1992; 326: 242–250. PMID: 1727977
- 3.
Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation 2002; 105: 1135–1143. PMID: 11877368
- 4.
Virmani R, Kolodgie FD, Burke AP, et al. Lessons from sudden coronary death: A comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol 2000; 20: 1262–1275. PMID: 10807742
- 5.
Little WC, Constantinescu M, Applegate RJ, et al. Can coronary angiography predict the site of a subsequent myocardial infarction in patients with mild-to-moderate coronary artery disease? Circulation 1988; 78: 1157–1166. PMID: 3180375
- 6.
Reimer KA, Lowe JE, Rasmussen MM, et al. The wavefront phenomenon of ischemic cell death. 1. Myocardial infarct size vs duration of coronary occlusion in dogs. Circulation 1977; 56: 786–794. PMID: 912839
- 7.
Chazov EI, Matveeva LS, Mazaev AV, et al. Intracoronary administration of fibrinolysin in acute myocardial infarct. Ter Arkh 1976; 48: 8–19. PMID: 136054
- 8.
Fletcher AP, Sherry S, Alkjaersig N, et al. The maintenance of a sustained thrombolytic state in man. II. Clinical observations on patients with myocardial infarction and other thromboembolic disorders. J Clin Invest 1959; 38: 1111–1119. PMID: 13664786
- 9.
Rentrop KP, Blanke H, Karsch KR, et al. Acute myocardial infarction: Intracoronary application of nitroglycerin and streptokinase. Clin Cardiol 1979; 2: 354–363. PMID: 121799
- 10.
ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2. Lancet 1988; 332: 349–360. PMID: 2899772
- 11.
Gruppo Italiano per lo Studio della Streptochinasi nell’Infarto Miocardico (GISSI). Effectiveness of intravenous thrombolytic treatment in acute myocardial infarction. Lancet 1986; 327: 397–402. PMID: 2868337
- 12.
Braunwald E, Antman EM, Beasley JW, et al. ACC/AHA guidelines for the management of patients with unstable angina and non-ST-segment elevation myocardial infarction. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on the Management of Patients With Unstable Angina). J Am Coll Cardiol 2000; 36: 970–1062. PMID: 10987629
- 13.
Alpert JS, Thygesen K, Antman E, et al. Myocardial infarction redefined: A consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol 2000; 36: 959–969. PMID: 10987628
- 14.
Ishihara M, Nakao K, Ozaki Y, et al. J-MINUET Investigators. Long-term outcomes of non-ST-elevation myocardial infarction without creatine kinase elevation: The J-MINUET Study. Circ J 2017; 81: 958–965. PMID: 28320999
- 15.
Thygesen K, Alpert JS, Jaffe AS, et al. ESC Scientific Document Group. Fourth universal definition of myocardial infarction (2018). Eur Heart J 2019; 40: 237–269. PMID: 30165617
- 16.
Nakamura Y, Yamamoto T, Okamura T, et al. NIPPON DATA 80 Research Group. Combined cardiovascular risk factors and outcome: NIPPON DATA80, 1980–1994. Circ J 2006; 70: 960–964. PMID: 16864925
- 17.
Okayama A, Kadowaki T, Okamura T, et al. NIPPON DATA80 Research Group. Age-specific effects of systolic and diastolic blood pressures on mortality due to cardiovascular diseases among Japanese men (NIPPON DATA80). J Hypertens 2006; 24: 459–462. PMID: 16467648
- 18.
Kadota A, Hozawa A, Okamura T, et al. NIPPON DATA Research Group. Relationship between metabolic risk factor clustering and cardiovascular mortality stratified by high blood glucose and obesity: NIPPON DATA90, 1990–2000. Diabetes Care 2007; 30: 1533–1538. PMID: 17363755
- 19.
Yusuf S, Hawken S, Ounpuu S, et al. INTERHEART Study Investigators. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): Case-control study. Lancet 2004; 364: 937–952. PMID: 15364185
- 20.
Kasanuki H, Honda T, Haze K, et al. HIJAMI Investigators. A large-scale prospective cohort study on the current status of therapeutic modalities for acute myocardial infarction in Japan: Rationale and initial results of the HIJAMI Registry. Am Heart J 2005; 150: 411–418. PMID: 16169317
- 21.
Hata J, Ninomiya T, Hirakawa Y, et al. Secular trends in cardiovascular disease and its risk factors in Japanese: Half-century data from the Hisayama Study (1961–2009). Circulation 2013; 128: 1198–1205. PMID: 23902756
- 22.
Takii T, Yasuda S, Takahashi J, et al. MIYAGI-AMI Study Investigators. Trends in acute myocardial infarction incidence and mortality over 30 years in Japan: Report from the MIYAGI-AMI Registry Study. Circ J 2010; 74: 93–100. PMID: 19942783
- 23.
Tunstall-Pedoe H, Kuulasmaa K, Amouyel P, et al. Myocardial infarction and coronary deaths in the World Health Organization MONICA Project. Registration procedures, event rates, and case-fatality rates in 38 populations from 21 countries in four continents. Circulation 1994; 90: 583–612. PMID: 8026046
- 24.
Rumana N, Kita Y, Turin TC, et al. Trend of increase in the incidence of acute myocardial infarction in a Japanese population: Takashima AMI Registry, 1990–2001. Am J Epidemiol 2008; 167: 1358–1364. PMID: 18381360
- 25.
Tanabe N, Saito R, Sato T, et al. Event rates of acute myocardial infarction and coronary deaths in Niigata and Nagaoka cities in Japan. Circ J 2003; 67: 40–45. PMID: 12520150
- 26.
Lerner DJ, Kannel WB. Patterns of coronary heart disease morbidity and mortality in the sexes: A 26-year follow-up of the Framingham population. Am Heart J 1986; 111: 383–390. PMID: 3946178
- 27.
Yeh RW, Sidney S, Chandra M, et al. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med 2010; 362: 2155–2165. PMID: 20558366
- 28.
Cui Y, Hao K, Takahashi J, et al. Age-specific trends in the incidence and in-hospital mortality of acute myocardial infarction over 30 years in Japan: Report from the Miyagi AMI Registry Study. Circ J 2017; 81: 520–528. PMID: 28154296
- 29.
Kojima S, Matsui K, Ogawa H. Kumamoto Acute Coronary Events (KACE) Study Group. Temporal trends in hospitalization for acute myocardial infarction between 2004 and 2011 in Kumamoto, Japan. Circ J 2013; 77: 2841–2843. PMID: 24067325
- 30.
Hong JS, Kang HC, Lee SH, et al. Long-term trend in the incidence of acute myocardial infarction in Korea: 1997–2007. Korean Circ J 2009; 39: 467–476. PMID: 19997542
- 31.
Lee CH, Cheng CL, Yang YH, et al. Trends in the incidence and management of acute myocardial infarction from 1999 to 2008: Get with the guidelines performance measures in Taiwan. J Am Heart Assoc 2014; 3: e001066. PMID: 25112555
- 32.
Kim RB, Kim BG, Kim YM, et al. Trends in the incidence of hospitalized acute myocardial infarction and stroke in Korea, 2006–2010. J Korean Med Sci 2013; 28: 16–24. PMID: 23341707
- 33.
Kinjo K, Sato H, Sato H, et al. Osaka Acute Coronary Insufficiency Study (OACIS) Group. Prognostic significance of atrial fibrillation/atrial flutter in patients with acute myocardial infarction treated with percutaneous coronary intervention. Am J Cardiol 2003; 92: 1150–1154. PMID: 14609587
- 34.
Ishihara M, Fujino M, Ogawa H, et al. J-MINUET investigators. Clinical Presentation, Management and Outcome of Japanese Patients With Acute Myocardial Infarction in the Troponin Era: Japanese Registry of Acute Myocardial Infarction Diagnosed by Universal Definition (J-MINUET). Circ J 2015; 79: 1255–1262. PMID: 25912696
- 35.
Miyachi H, Takagi A, Miyauchi K, et al. Current characteristics and management of ST elevation and non-ST elevation myocardial infarction in the Tokyo metropolitan area: From the Tokyo CCU network registered cohort. Heart Vessels 2016; 31: 1740–1751. PMID: 26758733
- 36.
Schmidt M, Jacobsen JB, Lash TL, et al. 25 year trends in first time hospitalisation for acute myocardial infarction, subsequent short and long term mortality, and the prognostic impact of sex and comorbidity: A Danish nationwide cohort study. BMJ 2012; 344: e356. PMID: 22279115
- 37.
Kosuge M, Kimura K, Kojima S, et al. Japanese Acute Coronary Syndrome Study (JACSS) Investigators. Sex differences in early mortality of patients undergoing primary stenting for acute myocardial infarction. Circ J 2006; 70: 217–221. PMID: 16501282
- 38.
Goldberg RJ, Currie K, White K, et al. Six-month outcomes in a multinational registry of patients hospitalized with an acute coronary syndrome (the Global Registry of Acute Coronary Events [GRACE]). Am J Cardiol 2004; 93: 288–293. PMID: 14759376
- 39.
Puymirat E, Simon T, Cayla G, et al. USIK, USIC 2000, and FAST-MI investigators. Acute myocardial infarction: Changes in patient characteristics, management, and 6-month outcomes over a period of 20 years in the FAST-MI program (French Registry of Acute ST-Elevation or Non-ST-Elevation Myocardial Infarction) 1995 to 2015. Circulation 2017; 136: 1908–1919. PMID: 28844989
- 40.
Canto JG, Zalenski RJ, Ornato JP, et al. National Registry of Myocardial Infarction 2 Investigators. Use of emergency medical services in acute myocardial infarction and subsequent quality of care: Observations from the National Registry of Myocardial Infarction 2. Circulation 2002; 106: 3018–3023. PMID: 12473545
- 41.
Hutchings CB, Mann NC, Daya M, et al. Rapid Early Action for Coronary Treatment Study. Am Heart J 2004; 147: 35–41. PMID: 14691416
- 42.
Antman EM, Anbe DT, Armstrong PW, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction--executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1999 Guidelines for the Management of Patients With Acute Myocardial Infarction). Circulation 2004; 110: 588–636. PMID: 15289388
- 43.
Clark EN, Sejersten M, Clemmensen P, et al. Automated electrocardiogram interpretation programs versus cardiologists’ triage decision making based on teletransmitted data in patients with suspected acute coronary syndrome. Am J Cardiol 2010; 106: 1696–1702. PMID: 21126612
- 44.
Widimský P, Budesínský T, Vorác D, et al. ‘PRAGUE’ Study Group Investigators. Long distance transport for primary angioplasty vs immediate thrombolysis in acute myocardial infarction: Final results of the randomized national multicentre trial--PRAGUE-2. Eur Heart J 2003; 24: 94–104. PMID: 12559941
- 45.
Andersen HR, Nielsen TT, Rasmussen K, et al. DANAMI-2 Investigators. A comparison of coronary angioplasty with fibrinolytic therapy in acute myocardial infarction. N Engl J Med 2003; 349: 733–742. PMID: 12930925
- 46.
Grines CL, Westerhausen DR, Grines LL, et al. Air PAMI Study Group. A randomized trial of transfer for primary angioplasty versus on-site thrombolysis in patients with high-risk myocardial infarction: The air primary angioplasty in myocardial infarction study. J Am Coll Cardiol 2002; 39: 1713–1719. PMID: 12039480
- 47.
Widimský P, Groch L, Zelízko M, et al. Multicentre randomized trial comparing transport to primary angioplasty vs immediate thrombolysis vs combined strategy for patients with acute myocardial infarction presenting to a community hospital without a catheterization laboratory. The PRAGUE study. Eur Heart J 2000; 21: 823–831. PMID: 10781354
- 48.
Welsford M, Nikolaou NI, Beygui F, et al. Acute Coronary Syndrome Chapter Collaborators. Part 5: Acute coronary syndromes: 2015 International consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Circulation 2015; 132 Suppl: S146–S176. PMID: 26472852
- 49.
Kawakami S, Tahara Y, Noguchi T, et al. Time to reperfusion in ST-segment elevation myocardial infarction patients with vs. without pre-hospital mobile telemedicine 12-lead electrocardiogram transmission. Circ J 2016; 80: 1624–1633. PMID: 27250917
- 50.
Carstensen S, Nelson GC, Hansen PS, et al. Field triage to primary angioplasty combined with emergency department bypass reduces treatment delays and is associated with improved outcome. Eur Heart J 2007; 28: 2313–2319. PMID: 17670756
- 51.
Sørensen JT, Terkelsen CJ, Nørgaard BL, et al. Urban and rural implementation of pre-hospital diagnosis and direct referral for primary percutaneous coronary intervention in patients with acute ST-elevation myocardial infarction. Eur Heart J 2011; 32: 430–436. PMID: 21138933
- 52.
Brown JP, Mahmud E, Dunford JV, et al. Effect of prehospital 12-lead electrocardiogram on activation of the cardiac catheterization laboratory and door-to-balloon time in ST-segment elevation acute myocardial infarction. Am J Cardiol 2008; 101: 158–161. PMID: 18178399
- 53.
Canto JG, Rogers WJ, Bowlby LJ, et al. The prehospital electrocardiogram in acute myocardial infarction: Is its full potential being realized? National Registry of Myocardial Infarction 2 Investigators. J Am Coll Cardiol 1997; 29: 498–505. PMID: 9060884
- 54.
Terkelsen CJ, Lassen JF, Nørgaard BL, et al. Reduction of treatment delay in patients with ST-elevation myocardial infarction: Impact of pre-hospital diagnosis and direct referral to primary percutanous coronary intervention. Eur Heart J 2005; 26: 770–777. PMID: 15684279
- 55.
Chan AW, Kornder J, Elliott H, et al. Improved survival associated with pre-hospital triage strategy in a large regional ST-segment elevation myocardial infarction program. JACC Cardiovasc Interv 2012; 5: 1239–1246. PMID: 23257372
- 56.
Dhruva VN, Abdelhadi SI, Anis A, et al. ST-Segment Analysis Using Wireless Technology in Acute Myocardial Infarction (STAT-MI) trial. J Am Coll Cardiol 2007; 50: 509–513. PMID: 17678733
- 57.
Diercks DB, Kontos MC, Chen AY, et al. Utilization and impact of pre-hospital electrocardiograms for patients with acute ST-segment elevation myocardial infarction: Data from the NCDR (National Cardiovascular Data Registry) ACTION (Acute Coronary Treatment and Intervention Outcomes Network) Registry. J Am Coll Cardiol 2009; 53: 161–166. PMID: 19130984
- 58.
O’Gara PT, Kushner FG, Ascheim DD, et al. American College of Emergency Physicians, Society for Cardiovascular Angiography and Interventions. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 61: e78–e140. PMID: 23256914
- 59.
Tahara Y, Kikuchi K, Nonogi H, et al. Acute coronary syndrome. [Article in Japanese] In: Japan Resuscitation Council, Supervisor. JRC resuscitation guidelines 2015. Igaku-Shoin 2016: 292–344.
- 60.
Diercks DB, Peacock WF, Hiestand BC, et al. Frequency and consequences of recording an electrocardiogram > minutes after arrival in an emergency room in non-ST-segment elevation acute coronary syndromes (from the CRUSADE Initiative). Am J Cardiol 2006; 97: 437–442. PMID: 16461033
- 61.
Rokos IC, French WJ, Koenig WJ, et al. Integration of pre-hospital electrocardiograms and ST-elevation myocardial infarction receiving center (SRC) networks: Impact on Door-to-Balloon times across 10 independent regions. JACC Cardiovasc Interv 2009; 2: 339–346. PMID: 19463447
- 62.
Le May MR, So DY, Dionne R, et al. A citywide protocol for primary PCI in ST-segment elevation myocardial infarction. N Engl J Med 2008; 358: 231–240. PMID: 18199862
- 63.
Morrow DA, Antman EM, Charlesworth A, et al. TIMI risk score for ST-elevation myocardial infarction: A convenient, bedside, clinical score for risk assessment at presentation: An intravenous nPA for treatment of infarcting myocardium early II trial substudy. Circulation 2000; 102: 2031–2037. PMID: 11044416
- 64.
Antman EM, Cohen M, Bernink PJ, et al. The TIMI risk score for unstable angina/non-ST elevation MI: A method for prognostication and therapeutic decision making. JAMA 2000; 284: 835–842. PMID: 10938172
- 65.
Fox KA, Dabbous OH, Goldberg RJ, et al. Prediction of risk of death and myocardial infarction in the six months after presentation with acute coronary syndrome: Prospective multinational observational study (GRACE). BMJ 2006; 333: 1091. PMID: 17032691
- 66.
Jobs A, Mehta SR, Montalescot G, et al. Optimal timing of an invasive strategy in patients with non-ST-elevation acute coronary syndrome: A meta-analysis of randomised trials. Lancet 2017; 390: 737–746. PMID: 28778541
- 67.
Rubini Gimenez M, Reiter M, Twerenbold R, et al. Sex-specific chest pain characteristics in the early diagnosis of acute myocardial infarction. JAMA Intern Med 2014; 174: 241–249. PMID: 24275751
- 68.
Kosuge M, Kimura K, Ishikawa T, et al. Differences between men and women in terms of clinical features of ST-segment elevation acute myocardial infarction. Circ J 2006; 70: 222–226. PMID: 16501283
- 69.
Patel H, Rosengren A, Ekman I. Symptoms in acute coronary syndromes: Does sex make a difference? Am Heart J 2004; 148: 27–33. PMID: 15215788
- 70.
Maynard C, Litwin PE, Martin JS, et al. Gender differences in the treatment and outcome of acute myocardial infarction: Results from the myocardial infarction triage and intervention registry. Arch Intern Med 1992; 152: 972–976. PMID: 1580724
- 71.
Weaver WD, Litwin PE, Martin JS, et al. The MITI Project Group. Effect of age on use of thrombolytic therapy and mortality in acute myocardial infarction. J Am Coll Cardiol 1991; 18: 657–662. PMID: 1869726
- 72.
Kannel WB. Prevalence and clinical aspects of unrecognized myocardial infarction and sudden unexpected death. Circulation 1987; 75: II4–II5. PMID: 3493089
- 73.
Grimm RH Jr, Tillinghast S, Daniels K, et al. Unrecognized myocardial infarction: Experience in the Multiple Risk Factor Intervention Trial (MRFIT). Circulation 1987; 75: II6–II8. PMID: 3815790
- 74.
Six AJ, Backus BE, Kelder JC. Chest pain in the emergency room: Value of the HEART score. Neth Heart J 2008; 16: 191–196. PMID: 18665203
- 75.
Backus BE, Six AJ, Kelder JC, et al. Chest pain in the emergency room: A multicenter validation of the HEART score. Crit Pathw Cardiol 2010; 9: 164–169. PMID: 20802272
- 76.
Poldervaart JM, Langedijk M, Backus BE, et al. Comparison of the GRACE, HEART and TIMI score to predict major adverse cardiac events in chest pain patients at the emergency department. Int J Cardiol 2017; 227: 656–661. PMID: 27810290
- 77.
Mahler SA, Riley RF, Hiestand BC, et al. The HEART Pathway randomized trial: Identifying emergency department patients with acute chest pain for early discharge. Circ Cardiovasc Qual Outcomes 2015; 8: 195–203. PMID: 25737484
- 78.
van Diepen S, Katz JN, Albert NM, et al. American Heart Association Council on clinical cardiology, council on cardiovascular and stroke nursing, council on quality of care and outcomes research, and mission: Lifeline. Contemporary management of cardiogenic shock: A scientific statement from the American Heart Association. Circulation 2017; 136: e232–e268. PMID: 28923988
- 79.
Freis ED, Schnaper HW, Johnson RL, et al. Hemodynamic alterations in acute myocardial infarction. I. Cardiac output, mean arterial pressure, total peripheral resistance, central and total blood volumes, venous pressure and average circulation time. J Clin Invest 1952; 31: 131–140. PMID: 14907892
- 80.
Menon V, White H, LeJemtel T, et al. The clinical profile of patients with suspected cardiogenic shock due to predominant left ventricular failure: A report from the SHOCK Trial Registry. J Am Coll Cardiol 2000; 36 Suppl: 1071–1076. PMID: 10985707
- 81.
Killip T 3rd, Kimball JT. Treatment of myocardial infarction in a coronary care unit: A two year experience with 250 patients. Am J Cardiol 1967; 20: 457–464. PMID: 6059183
- 82.
Cullen L, Mueller C, Parsonage WA, et al. Validation of high-sensitivity troponin I in a 2-hour diagnostic strategy to assess 30-day outcomes in emergency department patients with possible acute coronary syndrome. J Am Coll Cardiol 2013; 62: 1242–1249. PMID: 23583250
- 83.
Möckel M, Searle J, Hamm C, et al. Early discharge using single cardiac troponin and copeptin testing in patients with suspected acute coronary syndrome (ACS): A randomized, controlled clinical process study. Eur Heart J 2015; 36: 369–376. PMID: 24786301
- 84.
Body R, Carley S, McDowell G, et al. Rapid exclusion of acute myocardial infarction in patients with undetectable troponin using a high-sensitivity assay. J Am Coll Cardiol 2011; 58: 1332–1339. PMID: 21920261
- 85.
Bandstein N, Ljung R, Johansson M, et al. Undetectable high-sensitivity cardiac troponin T level in the emergency department and risk of myocardial infarction. J Am Coll Cardiol 2014; 63: 2569–2578. PMID: 24694529
- 86.
Than M, Cullen L, Reid CM, et al. A 2-h diagnostic protocol to assess patients with chest pain symptoms in the Asia-Pacific region (ASPECT): A prospective observational validation study. Lancet 2011; 377: 1077–1084. PMID: 21435709
- 87.
Than M, Cullen L, Aldous S, et al. 2-Hour accelerated diagnostic protocol to assess patients with chest pain symptoms using contemporary troponins as the only biomarker: The ADAPT trial. J Am Coll Cardiol 2012; 59: 2091–2098. PMID: 22578923
- 88.
National Heart Attack Alert Program Coordinating Committee, 60 Minutes to Treatment Working Group. Emergency department: Rapid identification and treatment of patients with acute myocardial infarction. Ann Emerg Med 1994; 23: 311–329. PMID: 8304613
- 89.
Antman EM, Anbe DT, Armstrong PW, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of Patients with Acute Myocardial Infarction). Circulation 2004; 110: e82–e292. PMID: 15339869
- 90.
Welch RD, Zalenski RJ, Frederick PD, et al. National Registry of Myocardial Infarction 2 and 3 Investigators. Prognostic value of a normal or nonspecific initial electrocardiogram in acute myocardial infarction. JAMA 2001; 286: 1977–1984. PMID: 11667934
- 91.
Patel DJ, Holdright DR, Knight CJ, et al. Early continuous ST segment monitoring in unstable angina: Prognostic value additional to the clinical characteristics and the admission electrocardiogram. Heart 1996; 75: 222–228. PMID: 8800982
- 92.
Jernberg T, Lindahl B, Wallentin L. The combination of a continuous 12-lead ECG and troponin T: A valuable tool for risk stratification during the first 6 hours in patients with chest pain and a non-diagnostic ECG. Eur Heart J 2000; 21: 1464–1472. PMID: 10952839
- 93.
Surawicz B, Parikh SR. Prevalence of male and female patterns of early ventricular repolarization in the normal ECG of males and females from childhood to old age. J Am Coll Cardiol 2002; 40: 1870–1876. PMID: 12446073
- 94.
Wang K, Asinger RW, Marriott HJ. ST-segment elevation in conditions other than acute myocardial infarction. N Engl J Med 2003; 349: 2128–2135. PMID: 14645641
- 95.
Kosuge M, Ebina T, Hibi K, et al. Simple and accurate electrocardiographic criteria to differentiate takotsubo cardiomyopathy from anterior acute myocardial infarction. J Am Coll Cardiol 2010; 55: 2514–2516. PMID: 20510222
- 96.
Kosuge M, Kimura K, Uchida K, et al. Clinical implications of electrocardiograms for patients with type A acute aortic dissection. Circ J 2017; 81: 1254–1260. PMID: 28529261
- 97.
Kosuge M, Kimura K. Implications of using the cabrera sequence for diagnosing acute coronary syndrome. Circ J 2016; 80: 1087–1096. PMID: 27019984
- 98.
O’Doherty M, Tayler DI, Quinn E, et al. Five hundred patients with myocardial infarction monitored within one hour of symptoms. Br Med J (Clin Res Ed) 1983; 286: 1405–1408. PMID: 6404481
- 99.
Mehta RH, Starr AZ, Lopes RD, et al. APEX AMI Investigators. Incidence of and outcomes associated with ventricular tachycardia or fibrillation in patients undergoing primary percutaneous coronary intervention. JAMA 2009; 301: 1779–1789. PMID: 19417195
- 100.
Kosuge M, Kimura K. Clinical implications of electrocardiograms for patients with anterior wall ST-segment elevation acute myocardial infarction in the interventional era. Circ J 2012; 76: 32–40. PMID: 22139364
- 101.
Engelen DJ, Gorgels AP, Cheriex EC, et al. Value of the electrocardiogram in localizing the occlusion site in the left anterior descending coronary artery in acute anterior myocardial infarction. J Am Coll Cardiol 1999; 34: 389–395. PMID: 10440150
- 102.
Zehender M, Kasper W, Kauder E, et al. Right ventricular infarction as an independent predictor of prognosis after acute inferior myocardial infarction. N Engl J Med 1993; 328: 981–988. PMID: 8450875
- 103.
Klein HO, Tordjman T, Ninio R, et al. The early recognition of right ventricular infarction: Diagnostic accuracy of the electrocardiographic V4R lead. Circulation 1983; 67: 558–565. PMID: 6821897
- 104.
Braat SH, Brugada P, de Zwaan C, et al. Value of electrocardiogram in diagnosing right ventricular involvement in patients with an acute inferior wall myocardial infarction. Br Heart J 1983; 49: 368–372. PMID: 6299315
- 105.
Matetzky S, Freimark D, Feinberg MS, et al. Acute myocardial infarction with isolated ST-segment elevation in posterior chest leads V7–9: “hidden”; ST-segment elevations revealing acute posterior infarction. J Am Coll Cardiol 1999; 34: 748–753. PMID: 10483956
- 106.
Agarwal JB, Khaw K, Aurignac F, et al. Importance of posterior chest leads in patients with suspected myocardial infarction, but nondiagnostic, routine 12-lead electrocardiogram. Am J Cardiol 1999; 83: 323–326. PMID: 10072216
- 107.
Kosuge M, Ebina T, Hibi K, et al. Implications of ST-segment elevation in leads V5 and V6 in patients with reperfused inferior wall acute myocardial infarction. Am J Cardiol 2012; 109: 314–319. PMID: 22078965
- 108.
Shiomi H, Kosuge M, Morimoto T, et al. CREDO-Kyoto AMI Investigators. QRS score at presentation electrocardiogram is correlated with infarct size and mortality in ST-segment elevation myocardial infarction patients undergoing primary percutaneous coronary intervention. Circ J 2017; 81: 1129–1136. PMID: 28381693
- 109.
Sgarbossa EB, Pinski SL, Barbagelata A, et al. GUSTO-1 (Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries) Investigators. Electrocardiographic diagnosis of evolving acute myocardial infarction in the presence of left bundle-branch block. N Engl J Med 1996; 334: 481–487. PMID: 8559200
- 110.
Shlipak MG, Lyons WL, Go AS, et al. Should the electrocardiogram be used to guide therapy for patients with left bundle-branch block and suspected myocardial infarction? JAMA 1999; 281: 714–719. PMID: 10052441
- 111.
Jain S, Ting HT, Bell M, et al. Utility of left bundle branch block as a diagnostic criterion for acute myocardial infarction. Am J Cardiol 2011; 107: 1111–1116. PMID: 21296327
- 112.
Erne P, Iglesias JF, Urban P, et al. Left bundle-branch block in patients with acute myocardial infarction: Presentation, treatment, and trends in outcome from 1997 to 2016 in routine clinical practice. Am Heart J 2017; 184: 106–113. PMID: 28224924
- 113.
Lopes RD, Siha H, Fu Y, et al. Diagnosing acute myocardial infarction in patients with left bundle branch block. Am J Cardiol 2011; 108: 782–788. PMID: 21726838
- 114.
Kosuge M, Kimura K. Clinical implications of electrocardiograms for patients with non-ST-segment elevation acute coronary syndromes in the interventional era. Circ J 2009; 73: 798–805. PMID: 19346660
- 115.
Damman P, Holmvang L, Tijssen JG, et al. Usefulness of the admission electrocardiogram to predict long-term outcomes after non-ST-elevation acute coronary syndrome (from the FRISC II, ICTUS, and RITA-3 [FIR] Trials). Am J Cardiol 2012; 109: 6–12. PMID: 21944677
- 116.
Kosuge M, Kimura K, Ishikawa T, et al. Clinical implications of persistent ST segment depression after admission in patients with non-ST segment elevation acute coronary syndrome. Heart 2005; 91: 95–96. PMID: 15604347
- 117.
Barrabés JA, Figueras J, Moure C, et al. Prognostic value of lead aVR in patients with a first non-ST-segment elevation acute myocardial infarction. Circulation 2003; 108: 814–819. PMID: 12885742
- 118.
Kosuge M, Ebina T, Hibi K, et al. An early and simple predictor of severe left main and/or three-vessel disease in patients with non-ST-segment elevation acute coronary syndrome. Am J Cardiol 2011; 107: 495–500. PMID: 21184992
- 119.
D’Ascenzo F, Presutti DG, Picardi E, et al. Prevalence and non-invasive predictors of left main or three-vessel coronary disease: Evidence from a collaborative international meta-analysis including 22 740 patients. Heart 2012; 98: 914–919. PMID: 22626899
- 120.
Vives-Borrás M, Moustafa AH, Álvarez-García J, et al. Clinical and prognostic value of the electrocardiogram in patients with acute occlusion of the left circumflex coronary artery. Am J Cardiol 2017; 120: 1487–1494. PMID: 28842146
- 121.
Savonitto S, Ardissino D, Granger CB, et al. Prognostic value of the admission electrocardiogram in acute coronary syndromes. JAMA 1999; 281: 707–713. PMID: 10052440
- 122.
Kannan L, Figueredo VM. Wellens’ syndrome. N Engl J Med 2015; 372: 66. PMID: 25551527
- 123.
Kosuge M, Ebina T, Hibi K, et al. Differences in negative T waves among acute coronary syndrome, acute pulmonary embolism, and Takotsubo cardiomyopathy. Eur Heart J Acute Cardiovasc Care 2012; 1: 349–357. PMID: 24062927
- 124.
Miwa K, Miyagi Y, Fujita M, et al. Transient terminal U wave inversion as a more specific marker for myocardial ischemia. Am Heart J 1993; 125: 981–986. PMID: 8465770
- 125.
Kosuge M, Ebina T, Hibi K, et al. Early, accurate, non-invasive predictors of left main or 3-vessel disease in patients with non-ST-segment elevation acute coronary syndrome. Circ J 2009; 73: 1105–1110. PMID: 19359810
- 126.
Aoyama N, Izumi T, Hiramori K, et al. Japanese Investigators of Fulminant Myocarditis. National survey of fulminant myocarditis in Japan: Therapeutic guidelines and long-term prognosis of using percutaneous cardiopulmonary support for fulminant myocarditis (special report from a scientific committee). Circ J 2002; 66: 133–144. PMID: 11999637
- 127.
Kosuge M, Uchida K, Imoto K, et al. Prognostic value of ST-segment elevation in lead aVR in patients with type A acute aortic dissection. J Am Coll Cardiol 2015; 65: 2570–2571. PMID: 26065996
- 128.
Luepker RV, Apple FS, Christenson RH, et al. Case definitions for acute coronary heart disease in epidemiology and clinical research studies: A statement from the AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; and the National Heart, Lung, and Blood Institute. Circulation 2003; 108: 2543–2549. PMID: 14610011
- 129.
Katus HA, Remppis A, Looser S, et al. Enzyme linked immuno assay of cardiac troponin T for the detection of acute myocardial infarction in patients. J Mol Cell Cardiol 1989; 21: 1349–1353. PMID: 2632816
- 130.
Ahumada G, Roberts R, Sobel BE. Evaluation of myocardial infarction with enzymatic indices. Prog Cardiovasc Dis 1976; 18: 405–420. PMID: 775531
- 131.
Lee TH, Goldman L. Serum enzyme assays in the diagnosis of acute myocardial infarction. Recommendations based on a quantitative analysis. Ann Intern Med 1986; 105: 221–233. PMID: 3524337
- 132.
Reichlin T, Hochholzer W, Bassetti S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med 2009; 361: 858–867. PMID: 19710484
- 133.
Keller T, Zeller T, Peetz D, et al. Sensitive troponin I assay in early diagnosis of acute myocardial infarction. N Engl J Med 2009; 361: 868–877. PMID: 19710485
- 134.
Bonaca M, Scirica B, Sabatine M, et al. Prospective evaluation of the prognostic implications of improved assay performance with a sensitive assay for cardiac troponin I. J Am Coll Cardiol 2010; 55: 2118–2124. PMID: 20447535
- 135.
Thygesen K, Mair J, Giannitsis E, et al. Study Group on Biomarkers in Cardiology of ESC Working Group on Acute Cardiac Care. How to use high-sensitivity cardiac troponins in acute cardiac care. Eur Heart J 2012; 33: 2252–2257. PMID: 22723599
- 136.
Mueller C. Biomarkers and acute coronary syndromes: An update. Eur Heart J 2014; 35: 552–556. PMID: 24357507
- 137.
Reichlin T, Twerenbold R, Reiter M, et al. Introduction of high-sensitivity troponin assays: Impact on myocardial infarction incidence and prognosis. Am J Med 2012; 125: 1205–1213.e1. PMID: 23164485
- 138.
Reichlin T, Schindler C, Drexler B, et al. One-hour rule-out and rule-in of acute myocardial infarction using high-sensitivity cardiac troponin T. Arch Intern Med 2012; 172: 1211–1218. PMID: 22892889
- 139.
Kociol RD, Pang PS, Gheorghiade M, et al. Troponin elevation in heart failure prevalence, mechanisms, and clinical implications. J Am Coll Cardiol 2010; 56: 1071–1078. PMID: 20863950
- 140.
Raskovalova T, Twerenbold R, Collinson PO, et al. Diagnostic accuracy of combined cardiac troponin and copeptin assessment for early rule-out of myocardial infarction: A systematic review and meta-analysis. Eur Heart J Acute Cardiovasc Care 2014; 3: 18–27. PMID: 24562800
- 141.
Horowitz RS, Morganroth J, Parrotto C, et al. Immediate diagnosis of acute myocardial infarction by two-dimensional echocardiography. Circulation 1982; 65: 323–329. PMID: 7053890
- 142.
Gibler WB, Runyon JP, Levy RC, et al. A rapid diagnostic and treatment center for patients with chest pain in the emergency department. Ann Emerg Med 1995; 25: 1–8. PMID: 7802357
- 143.
Bholasingh R, Cornel JH, Kamp O, et al. Prognostic value of predischarge dobutamine stress echocardiography in chest pain patients with a negative cardiac troponin T. J Am Coll Cardiol 2003; 41: 596–602. PMID: 12598071
- 144.
Kang DH, Kang SJ, Song JM, et al. Efficacy of myocardial contrast echocardiography in the diagnosis and risk stratification of acute coronary syndrome. Am J Cardiol 2005; 96: 1498–1502. PMID: 16310429
- 145.
Tong KL, Kaul S, Wang XQ, et al. Myocardial contrast echocardiography versus Thrombolysis In Myocardial Infarction score in patients presenting to the emergency department with chest pain and a nondiagnostic electrocardiogram. J Am Coll Cardiol 2005; 46: 920–927. PMID: 16139144
- 146.
Braunwald E. Unstable angina: A classification. Circulation 1989; 80: 410–414. PMID: 2752565
- 147.
Hamm CW, Braunwald E. A classification of unstable angina revisited. Circulation 2000; 102: 118–122. PMID: 10880424
- 148.
Angioi M, Danchin N, Alla F, et al. Long-term outcome in patients treated by intracoronary stenting with ticlopidine and aspirin, and deleterious prognostic role of unstable angina pectoris. Am J Cardiol 2000; 85: 1065–1070. PMID: 10781753
- 149.
Chia BL, Tan HC, Yip JW, et al. Electrocardiographic patterns in posterior chest leads (V7, V8, V9) in normal subjects. Am J Cardiol 2000; 85: 911–912. PMID: 10758941
- 150.
Cannon CP, McCabe CH, Stone PH, et al. The electrocardiogram predicts one-year outcome of patients with unstable angina and non-Q wave myocardial infarction: Results of the TIMI III registry ECG ancillary study. J Am Coll Cardiol 1997; 30: 133–140. PMID: 9207634
- 151.
FRagmin and Fast Revascularisation during InStability in Coronary artery disease Investigators. Invasive compared with non-invasive treatment in unstable coronary-artery disease: FRISC II prospective randomised multicentre study. Lancet 1999; 354: 708–715. PMID: 10475181
- 152.
Mathew V, Farkouh M, Grill DE, et al. Clinical risk stratification correlates with the angiographic extent of coronary artery disease in unstable angina. J Am Coll Cardiol 2001; 37: 2053–2058. PMID: 11419887
- 153.
Sabatine MS, Morrow DA, Giugliano RP, et al. Implications of upstream glycoprotein IIb/IIIa inhibition and coronary artery stenting in the invasive management of unstable angina/non-ST-elevation myocardial infarction: A comparison of the Thrombolysis In Myocardial Infarction (TIMI) IIIB trial and the Treat angina with Aggrastat and determine Cost of Therapy with Invasive or Conservative Strategy (TACTICS)-TIMI 18 trial. Circulation 2004; 109: 874–880. PMID: 14757697
- 154.
Lee KL, Woodlief LH, Topol EJ, et al. GUSTO-I Investigators. Predictors of 30-day mortality in the era of reperfusion for acute myocardial infarction. Results from an international trial of 41,021 patients. Circulation 1995; 91: 1659–1668. PMID: 7882472
- 155.
Mehilli J, Kastrati A, Dirschinger J, et al. Sex-based analysis of outcome in patients with acute myocardial infarction treated predominantly with percutaneous coronary intervention. JAMA 2002; 287: 210–215. PMID: 11779263
- 156.
Mehta RH, O’neill WW, Harjai KJ, et al. Primary Angioplasty in Myocardial Infarction (PAMI) and the Controlled Abciximab and Device Investigation to Lower Late Angioplasty Complications (CADILLAC) Investigators. Prediction of one-year mortality among 30-day survivors after primary percutaneous coronary interventions. Am J Cardiol 2006; 97: 817–822. PMID: 16516582
- 157.
Jeger RV, Harkness SM, Ramanathan K, et al. SHOCK Investigators. Emergency revascularization in patients with cardiogenic shock on admission: A report from the SHOCK trial and registry. Eur Heart J 2006; 27: 664–670. PMID: 16423873
- 158.
Hochman JS, Sleeper LA, Webb JG, et al. SHOCK Investigators. Should we emergently revascularize occluded coronaries for cardiogenic shock: Early revascularization in acute myocardial infarction complicated by cardiogenic shock. N Engl J Med 1999; 341: 625–634. PMID: 10460813
- 159.
Eagle KA, Lim MJ, Dabbous OH, et al. GRACE Investigators. A validated prediction model for all forms of acute coronary syndrome: Estimating the risk of 6-month postdischarge death in an international registry. JAMA 2004; 291: 2727–2733. PMID: 15187054
- 160.
Nakatani D, Sakata Y, Suna S, et al. Osaka Acute Coronary Insufficiency Study (OACIS) Investigators. Impact of beta blockade therapy on long-term mortality after ST-segment elevation acute myocardial infarction in the percutaneous coronary intervention era. Am J Cardiol 2013; 111: 457–464. PMID: 23228922
- 161.
Yan AT, Yan RT, Tan M, et al. Risk scores for risk stratification in acute coronary syndromes: Useful but simpler is not necessarily better. Eur Heart J 2007; 28: 1072–1078. PMID: 17437970
- 162.
Abu-Assi E, Ferreira-González I, Ribera A, et al. “Do GRACE (Global Registry of Acute Coronary events) risk scores still maintain their performance for predicting mortality in the era of contemporary management of acute coronary syndromes?” Am Heart J 2010; 160: 826–834.e3. PMID: 21095268
- 163.
Wong CK, Gao W, Raffel OC, et al. HERO-2 Investigators. Initial Q waves accompanying ST-segment elevation at presentation of acute myocardial infarction and 30-day mortality in patients given streptokinase therapy: An analysis from HERO-2. Lancet 2006; 367: 2061–2067. PMID: 16798389
- 164.
Armstrong PW, Fu Y, Westerhout CM, et al. Baseline Q-wave surpasses time from symptom onset as a prognostic marker in ST-segment elevation myocardial infarction patients treated with primary percutaneous coronary intervention. J Am Coll Cardiol 2009; 53: 1503–1509. PMID: 19389560
- 165.
Selvester RH, Wagner GS, Hindman NB. The Selvester QRS scoring system for estimating myocardial infarct size: The development and application of the system. Arch Intern Med 1985; 145: 1877–1881. PMID: 4037949
- 166.
Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2018; 39: 119–177. PMID: 28886621
- 167.
Antman EM, Tanasijevic MJ, Thompson B, et al. Cardiac-specific troponin I levels to predict the risk of mortality in patients with acute coronary syndromes. N Engl J Med 1996; 335: 1342–1349. PMID: 8857017
- 168.
Stubbs P, Collinson P, Moseley D, et al. Prospective study of the role of cardiac troponin T in patients admitted with unstable angina. BMJ 1996; 313: 262–264. PMID: 8704534
- 169.
Newby LK, Christenson RH, Ohman EM, et al. The GUSTO-IIa Investigators. Value of serial troponin T measures for early and late risk stratification in patients with acute coronary syndromes. Circulation 1998; 98: 1853–1859. PMID: 9799204
- 170.
Ohman EM, Armstrong PW, Christenson RH, et al. Cardiac troponin T levels for risk stratification in acute myocardial ischemia: GUSTO IIA Investigators. N Engl J Med 1996; 335: 1333–1341. PMID: 8857016
- 171.
Giannitsis E, Becker M, Kurz K, et al. High-sensitivity cardiac troponin T for early prediction of evolving non-ST-segment elevation myocardial infarction in patients with suspected acute coronary syndrome and negative troponin results on admission. Clin Chem 2010; 56: 642–650. PMID: 20167697
- 172.
Liuzzo G, Biasucci LM, Gallimore JR, et al. The prognostic value of C-reactive protein and serum amyloid a protein in severe unstable angina. N Engl J Med 1994; 331: 417–424. PMID: 7880233
- 173.
Biasucci LM, Liuzzo G, Grillo RL, et al. Elevated levels of C-reactive protein at discharge in patients with unstable angina predict recurrent instability. Circulation 1999; 99: 855–860. PMID: 10027805
- 174.
Ridker PM, Everett BM, Thuren T, et al. CANTOS Trial Group. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017; 377: 1119–1131. PMID: 28845751
- 175.
Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC Guideline for the Management of Patients with Non-ST-Elevation Acute Coronary Syndromes: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 64: e139–e228. PMID: 25260718
- 176.
Foo K, Cooper J, Deaner A, et al. A single serum glucose measurement predicts adverse outcomes across the whole range of acute coronary syndromes. Heart 2003; 89: 512–516. PMID: 12695455
- 177.
Wison S, Foo K, Cunningham J, et al. Renal function and risk stratification in acute coronary syndromes. Am J Cardiol 2003; 91: 1051–1054. PMID: 12714145
- 178.
Thygesen K, Alpert JS, Jaffe AS, et al. Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction. Third universal definition of myocardial infarction. Circulation 2012; 126: 2020–2035. PMID: 22923432
- 179.
Keller T, Zeller T, Ojeda F, et al. Serial changes in highly sensitive troponin I assay and early diagnosis of myocardial infarction. JAMA 2011; 306: 2684–2693. PMID: 22203537
- 180.
Eggers KM, Jaffe AS, Venge P, et al. Clinical implications of the change of cardiac troponin I levels in patients with acute chest pain: An evaluation with respect to the Universal Definition of Myocardial Infarction. Clin Chim Acta 2011; 412: 91–97. PMID: 20869357
- 181.
Lindahl B, Venge P, James S. The new high-sensitivity cardiac troponin T assay improves risk assessment in acute coronary syndromes. Am Heart J 2010; 160: 224–229. PMID: 20691825
- 182.
Reichlin T, Irfan A, Twerenbold R, et al. Utility of absolute and relative changes in cardiac troponin concentrations in the early diagnosis of acute myocardial infarction. Circulation 2011; 124: 136–145. PMID: 21709058
- 183.
Apple FS, Smith SW, Pearce LA, et al. Delta changes for optimizing clinical specificity and 60-day risk of adverse events in patients presenting with symptoms suggestive of acute coronary syndrome utilizing the ADVIA Centaur TnI-Ultra assay. Clin Biochem 2012; 45: 711–713. PMID: 22465126
- 184.
Thygesen K, Alpert JS, Jaffe AS, et al. The Writing Group on behalf of the Joint ESC/ACCF/AHA/WHF Task Force for the universal definition of myocardial infarction third universal definition of myocardial infarction. Eur Heart J 2012; 33: 2551–2567. PMID: 22922414
- 185.
Santaló M, Martin A, Velilla J, et al. Using high-sensitivity troponin T: The importance of the proper gold standard. Am J Med 2013; 126: 709–717. PMID: 23764266
- 186.
Kontos MC, de Lemos JA, Ou FS, et al. Troponin-positive, MB-negative patients with non-ST-elevation myocardial infarction: An undertreated but high-risk patient group: Results from the National Cardiovascular Data Registry Acute Coronary Treatment and Intervention Outcomes Network-Get With The Guidelines (NCDR ACTION-GWTG) Registry. Am Heart J 2010; 160: 819–825. PMID: 21095267
- 187.
Aviles RJ, Wright RS, Aviles JM, et al. Long-term prognosis of patients with clinical unstable angina pectoris without elevation of creatine kinase but with elevation of cardiac troponin i levels. Am J Cardiol 2002; 90: 875–878. PMID: 12372578
- 188.
Eggers KM, Oldgren J, Nordenskjöld A, et al. Diagnostic value of serial measurement of cardiac markers in patients with chest pain: Limited value of adding myoglobin to troponin I for exclusion of myocardial infarction. Am Heart J 2004; 148: 574–581. PMID: 15459585
- 189.
Volz KA, McGillicuddy DC, Horowitz GL, et al. Creatine kinase-MB does not add additional benefit to a negative troponin in the evaluation of chest pain. Am J Emerg Med 2012; 30: 188–190. PMID: 21129891
- 190.
Newby LK, Roe MT, Chen AY, et al. CRUSADE Investigators. Frequency and clinical implications of discordant creatine kinase-MB and troponin measurements in acute coronary syndromes. J Am Coll Cardiol 2006; 47: 312–318. PMID: 16412853
- 191.
Kavsak PA, MacRae AR, Newman AM, et al. Effects of contemporary troponin assay sensitivity on the utility of the early markers myoglobin and CKMB isoforms in evaluating patients with possible acute myocardial infarction. Clin Chim Acta 2007; 380: 213–216. PMID: 17306781
- 192.
Giannitsis E, Steen H, Kurz K, et al. Cardiac magnetic resonance imaging study for quantification of infarct size comparing directly serial versus single time-point measurements of cardiac troponin T. J Am Coll Cardiol 2008; 51: 307–314. PMID: 18206741
- 193.
Akkerhuis KM, Klootwijk PA, Lindeboom W, et al. Recurrent ischaemia during continuous multilead ST-segment monitoring identifies patients with acute coronary syndromes at high risk of adverse cardiac events: Meta-analysis of three studies involving 995 patients. Eur Heart J 2001; 22: 1997–2006. PMID: 11603907
- 194.
Hamm CW, Goldmann BU, Heeschen C, et al. Emergency room triage of patients with acute chest pain by means of rapid testing for cardiac troponin T or troponin I. N Engl J Med 1997; 337: 1648–1653. PMID: 9385123
- 195.
Scirica BM, Morrow DA, Budaj A, et al. Ischemia detected on continuous electrocardiography after acute coronary syndrome: Observations from the MERLIN-TIMI 36 (Metabolic Efficiency With Ranolazine for Less Ischemia in Non-ST-Elevation Acute Coronary Syndrome-Thrombolysis In Myocardial Infarction 36) trial. J Am Coll Cardiol 2009; 53: 1411–1421. PMID: 19371824
- 196.
Boersma E, Pieper KS, Steyerberg EW, et al. The PURSUIT Investigators. Predictors of outcome in patients with acute coronary syndromes without persistent ST-segment elevation: Results from an international trial of 9461 patients. Circulation 2000; 101: 2557–2567. PMID: 10840005
- 197.
Granger CB, Goldberg RJ, Dabbous O, et al. Global Registry of Acute Coronary Events Investigators. Predictors of hospital mortality in the global registry of acute coronary events. Arch Intern Med 2003; 163: 2345–2353. PMID: 14581255
- 198.
Pollack CV Jr, Sites FD, Shofer FS, et al. Application of the TIMI risk score for unstable angina and non-ST elevation acute coronary syndrome to an unselected emergency department chest pain population. Acad Emerg Med 2006; 13: 13–18. PMID: 16365321
- 199.
Go J, Narmi A, Sype J, et al. Impact of renal dysfunction on the prognostic value of the TIMI risk score in patients with non-ST elevation acute coronary syndrome. Coron Artery Dis 2011; 22: 411–415. PMID: 21691204
- 200.
Huynh T, Nasmith J, Luong TM, et al. Complementary prognostic values of ST segment deviation and Thrombolysis In Myocardial Infarction (TIMI) risk score in non-ST elevation acute coronary syndromes: Insights from the Platelet Receptor Inhibition in Ischemic Syndrome Management in Patients Limited by Unstable Signs and Symptoms (PRISM-PLUS) study. Can J Cardiol 2009; 25: e417–e421. PMID: 19960136
- 201.
Meune C, Drexler B, Haaf P, et al. The GRACE score’s performance in predicting in-hospital and 1-year outcome in the era of high-sensitivity cardiac troponin assays and B-type natriuretic peptide. Heart 2011; 97: 1479–1483. PMID: 21444339
- 202.
Eggers KM, Kempf T, Venge P, et al. Improving long-term risk prediction in patients with acute chest pain: The Global Registry of Acute Coronary Events (GRACE) risk score is enhanced by selected nonnecrosis biomarkers. Am Heart J 2010; 160: 88–94. PMID: 20598977
- 203.
Apple FS, Pearce LA, Smith SW, et al. Role of monitoring changes in sensitive cardiac troponin I assay results for early diagnosis of myocardial infarction and prediction of risk of adverse events. Clin Chem 2009; 55: 930–937. PMID: 19299542
- 204.
Younger JF, Plein S, Barth J, et al. Troponin-I concentration 72 h after myocardial infarction correlates with infarct size and presence of microvascular obstruction. Heart 2007; 93: 1547–1551. PMID: 17540686
- 205.
Haaf P, Reichlin T, Corson N, et al. B-type natriuretic peptide in the early diagnosis and risk stratification of acute chest pain. Am J Med 2011; 124: 444–452. PMID: 21531234
- 206.
Haaf P, Balmelli C, Reichlin T, et al. N-terminal pro B-type natriuretic peptide in the early evaluation of suspected acute myocardial infarction. Am J Med 2011; 124: 731–739. PMID: 21787902
- 207.
Brown AM, Sease KL, Robey JL, et al. The impact of B-type natriuretic peptide in addition to troponin I, creatine kinase-MB, and myoglobin on the risk stratification of emergency department chest pain patients with potential acute coronary syndrome. Ann Emerg Med 2007; 49: 153–163. PMID: 17084941
- 208.
Heeschen C, Hamm CW, Mitrovic V, et al. Platelet Receptor Inhibition in Ischemic Syndrome Management (PRISM) Investigators. N-terminal pro-B-type natriuretic peptide levels for dynamic risk stratification of patients with acute coronary syndromes. Circulation 2004; 110: 3206–3212. PMID: 15533869
- 209.
Morrow DA, de Lemos JA, Sabatine MS, et al. Evaluation of B-type natriuretic peptide for risk assessment in unstable angina/non-ST-elevation myocardial infarction: B-type natriuretic peptide and prognosis in TACTICS-TIMI 18. J Am Coll Cardiol 2003; 41: 1264–1272. PMID: 12706919
- 210.
James SK, Lindbäck J, Tilly J, et al. Troponin-T and N-terminal pro-B-type natriuretic peptide predict mortality benefit from coronary revascularization in acute coronary syndromes: A GUSTO-IV substudy. J Am Coll Cardiol 2006; 48: 1146–1154. PMID: 16978997
- 211.
Hofmann R, James SK, Jernberg T, et al. DETO2X–SWEDEHEART Investigators. Oxygen therapy in suspected acute myocardial infarction. N Engl J Med 2017; 377: 1240–1249. PMID: 28844200
- 212.
Sepehrvand N, James SK, Stub D, et al. Effects of supplemental oxygen therapy in patients with suspected acute myocardial infarction: A meta-analysis of randomised clinical trials. Heart 2018; 104: 1691–1698. PMID: 29599378
- 213.
Hofmann R, Witt N, Lagerqvist B, et al. DETO2X-SWEDEHEART Investigators. Oxygen therapy in ST-elevation myocardial infarction. Eur Heart J 2018; 39: 2730–2739. PMID: 29912429
- 214.
Jernberg T, Lindahl B, Alfredsson J, et al. DETO2X-SWEDEHEART Investigators. Long-term effects of oxygen therapy on death or hospitalization for heart failure in patients with suspected acute myocardial infarction. Circulation 2018; 138: 2754–2762. PMID: 30767504
- 215.
European Study of Prevention of Infarct with Molsidomine (ESPRIM) Group. The ESPRIM trial: Short-term treatment of acute myocardial infarction with molsidomine. Lancet 1994; 344: 91–97. PMID: 7912393
- 216.
Gruppo Italiano per lo Studio della Sopravvivenza nell’infarto Miocardico. GISSI-3: Effects of lisinopril and transdermal glyceryl trinitrate singly and together on 6-week mortality and ventricular function after acute myocardial infarction. Lancet 1994; 343: 1115–1122. PMID: 7910229
- 217.
ISIS-4 (Fourth International Study of Infarct Survival) Collaborative Group. ISIS-4: A randomised factorial trial assessing early oral captopril, oral mononitrate, and intravenous magnesium sulphate in 58,050 patients with suspected acute myocardial infarction. Lancet 1995; 345: 669–685. PMID: 7661937
- 218.
Webb DJ, Muirhead GJ, Wulff M, et al. Sildenafil citrate potentiates the hypotensive effects of nitric oxide donor drugs in male patients with stable angina. J Am Coll Cardiol 2000; 36: 25–31. PMID: 10898408
- 219.
Every NR, Parsons LS, Hlatky M, et al. Myocardial Infarction Triage and Intervention Investigators. A comparison of thrombolytic therapy with primary coronary angioplasty for acute myocardial infarction. N Engl J Med 1996; 335: 1253–1260. PMID: 8857004
- 220.
Freimark D, Matetzky S, Leor J, et al. Timing of aspirin administration as a determinant of survival of patients with acute myocardial infarction treated with thrombolysis. Am J Cardiol 2002; 89: 381–385. PMID: 11835915
- 221.
Barbash I, Freimark D, Gottlieb S, et al. Israeli working group on intensive cardiac care, Israel heart society. Outcome of myocardial infarction in patients treated with aspirin is enhanced by pre-hospital administration. Cardiology 2002; 98: 141–147. PMID: 12417813
- 222.
Special Writing Group. Fuster V, Dyken ML, Vokonas PS, et al. Aspirin as a therapeutic agent in cardiovascular disease. Circulation 1993; 87: 659–675. PMID: 8425313
- 223.
Becker RC. Antiplatelet therapy in coronary heart disease. Emerging strategies for the treatment and prevention of acute myocardial infarction. Arch Pathol Lab Med 1993; 117: 89–96. PMID: 8418769
- 224.
Juul-Möller S, Edvardsson N, Jahnmatz B, et al. The Swedish Angina Pectoris Aspirin Trial (SAPAT) Group. Double-blind trial of aspirin in primary prevention of myocardial infarction in patients with stable chronic angina pectoris. Lancet 1992; 340: 1421–1425. PMID: 1360557
- 225.
Yasue H, Ogawa H, Tanaka H, et al. Japanese Antiplatelets Myocardial Infarction Study (JAMIS) Investigators. Effects of aspirin and trapidil on cardiovascular events after acute myocardial infarction. Am J Cardiol 1999; 83: 1308–1313. PMID: 10235086
- 226.
Chesebro JH, Clements IP, Fuster V, et al. A platelet-inhibitor-drug trial in coronary-artery bypass operations: Benefit of perioperative dipyridamole and aspirin therapy on early postoperative vein-graft patency. N Engl J Med 1982; 307: 73–78. PMID: 7045659
- 227.
Chan FK, Chung SC, Suen BY, et al. Preventing recurrent upper gastrointestinal bleeding in patients with Helicobacter pylori infection who are taking low-dose aspirin or naproxen. N Engl J Med 2001; 344: 967–973. PMID: 11274623
- 228.
The Japanese Society of Gastroenterology. Evidence-based Clinical Practice Guidelines for Peptic Ulcer 2015 (2nd Edition). [Article in Japanese] Nankodo, 2015.
- 229.
Lev EI, Kornowski R, Vaknin-Assa H, et al. Effect of clopidogrel pretreatment on angiographic and clinical outcomes in patients undergoing primary percutaneous coronary intervention for ST-elevation acute myocardial infarction. Am J Cardiol 2008; 101: 435–439. PMID: 18312753
- 230.
Vlaar PJ, Svilaas T, Damman K, et al. Impact of pretreatment with clopidogrel on initial patency and outcome in patients treated with primary percutaneous coronary intervention for ST-segment elevation myocardial infarction: A systematic review. Circulation 2008; 118: 1828–1836. PMID: 18852370
- 231.
Yusuf S, Zhao F, Mehta SR, et al. Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 2001; 345: 494–502. PMID: 11519503
- 232.
Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: A quantitative review of 23 randomised trials. Lancet 2003; 361: 13–20. PMID: 12517460
- 233.
Zijlstra F, Hoorntje JC, de Boer MJ, et al. Long-term benefit of primary angioplasty as compared with thrombolytic therapy for acute myocardial infarction. N Engl J Med 1999; 341: 1413–1419. PMID: 10547403
- 234.
Global Use of Strategies to Open Occluded Coronary Arteries in Acute Coronary Syndromes (GUSTO IIb) Angioplasty Substudy Investigators. A clinical trial comparing primary coronary angioplasty with tissue plasminogen activator for acute myocardial infarction. N Engl J Med 1997; 336: 1621–1628. PMID: 9173270
- 235.
Schömig A, Mehilli J, Antoniucci D, et al. Beyond 12 hours Reperfusion AlternatiVe Evaluation (BRAVE-2) Trial Investigators. Mechanical reperfusion in patients with acute myocardial infarction presenting more than 12 hours from symptom onset: A randomized controlled trial. JAMA 2005; 293: 2865–2872. PMID: 15956631
- 236.
Gierlotka M, Gasior M, Wilczek K, et al. Reperfusion by primary percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction within 12 to 24 hours of the onset of symptoms (from a prospective national observational study [PL-ACS]). Am J Cardiol 2011; 107: 501–508. PMID: 21195380
- 237.
Hannan EL, Samadashvili Z, Walford G, et al. Culprit vessel percutaneous coronary intervention versus multivessel and staged percutaneous coronary intervention for ST-segment elevation myocardial infarction patients with multivessel disease. JACC Cardiovasc Interv 2010; 3: 22–31. PMID: 20129564
- 238.
Toma M, Buller CE, Westerhout CM, et al. APEX-AMI Investigators. Non-culprit coronary artery percutaneous coronary intervention during acute ST-segment elevation myocardial infarction: Insights from the APEX-AMI trial. Eur Heart J 2010; 31: 1701–1707. PMID: 20530505
- 239.
Vlaar PJ, Mahmoud KD, Holmes DR, et al. Culprit vessel only versus multivessel and staged percutaneous coronary intervention for multivessel disease in patients presenting with ST-segment elevation myocardial infarction: A pairwise and network meta-analysis. J Am Coll Cardiol 2011; 58: 692–703. PMID: 21816304
- 240.
Cantor WJ, Fitchett D, Borgundvaag B, et al. TRANSFER-AMI Trial Investigators. Routine early angioplasty after fibrinolysis for acute myocardial infarction. N Engl J Med 2009; 360: 2705–2718. PMID: 19553646
- 241.
Di Mario C, Dudek D, Piscione F, et al. CARESS-in-AMI Investigators. Immediate angioplasty versus standard therapy with rescue angioplasty after thrombolysis in the Combined Abciximab REteplase Stent Study in Acute Myocardial Infarction (CARESS-in-AMI): An open, prospective, randomised, multicentre trial. Lancet 2008; 371: 559–568. PMID: 18280326
- 242.
Fernández-Avilés F, Alonso JJ, Peña G, et al. GRACIA-2 (Groupo de Análisis de Cardiopatía Isquémica Aguda) Investigators. Primary angioplasty vs. early routine post-fibrinolysis angioplasty for acute myocardial infarction with ST-segment elevation: The GRACIA-2 non-inferiority, randomized, controlled trial. Eur Heart J 2007; 28: 949–960. PMID: 17244641
- 243.
Armstrong PW. WEST Steering Committee. A comparison of pharmacologic therapy with/without timely coronary intervention vs. primary percutaneous intervention early after ST-elevation myocardial infarction: The WEST (Which Early ST-elevation myocardial infarction Therapy) study. Eur Heart J 2006; 27: 1530–1538. PMID: 16757491
- 244.
Shiomi H, Nakagawa Y, Morimoto T, et al. CREDO-Kyoto AMI investigators. Association of onset to balloon and door to balloon time with long term clinical outcome in patients with ST elevation acute myocardial infarction having primary percutaneous coronary intervention: Observational study. BMJ 2012; 344: e3257. PMID: 22623632
- 245.
Ioannidis JP, Katritsis DG. Percutaneous coronary intervention for late reperfusion after myocardial infarction in stable patients. Am Heart J 2007; 154: 1065–1071. PMID: 18035076
- 246.
Nordmann AJ, Hengstler P, Harr T, et al. Clinical outcomes of primary stenting versus balloon angioplasty in patients with myocardial infarction: A meta-analysis of randomized controlled trials. Am J Med 2004; 116: 253–262. PMID: 14969654
- 247.
Zhu MM, Feit A, Chadow H, et al. Primary stent implantation compared with primary balloon angioplasty for acute myocardial infarction: A meta-analysis of randomized clinical trials. Am J Cardiol 2001; 88: 297–301. PMID: 11472712
- 248.
Stone GW, Lansky AJ, Pocock SJ, et al. HORIZONS-AMI Trial Investigators. Paclitaxel-eluting stents versus bare-metal stents in acute myocardial infarction. N Engl J Med 2009; 360: 1946–1959. PMID: 19420364
- 249.
Kastrati A, Dibra A, Spaulding C, et al. Meta-analysis of randomized trials on drug-eluting stents vs. bare-metal stents in patients with acute myocardial infarction. Eur Heart J 2007; 28: 2706–2713. PMID: 17901079
- 250.
Kelbæk H, Høfsten DE, Køber L, et al. Deferred versus conventional stent implantation in patients with ST-segment elevation myocardial infarction (DANAMI 3-DEFER): An open-label, randomised controlled trial. Lancet 2016; 387: 2199–2206. PMID: 27053444
- 251.
Singh M, Holmes DR, Dehmer GJ, et al. Percutaneous coronary intervention at centers with and without on-site surgery: A meta-analysis. JAMA 2011; 306: 2487–2494. PMID: 22166608
- 252.
Hochman JS, Sleeper LA, White HD, et al. SHOCK Investigators. One-year survival following early revascularization for cardiogenic shock. JAMA 2001; 285: 190–192. PMID: 11176812
- 253.
Dzavik V, Sleeper LA, Cocke TP, et al. SHOCK Investigators. Early revascularization is associated with improved survival in elderly patients with acute myocardial infarction complicated by cardiogenic shock: A report from the SHOCK Trial Registry. Eur Heart J 2003; 24: 828–837. PMID: 12727150
- 254.
Dauerman HL, Goldberg RJ, Malinski M, et al. Outcomes and early revascularization for patients > or = 65 years of age with cardiogenic shock. Am J Cardiol 2001; 87: 844–848. PMID: 11274938
- 255.
Numasawa Y, Sawano M, Miyata H, et al. Outcomes after percutaneous coronary intervention of acute coronary syndrome complicated with cardiopulmonary arrest (from a Japanese Multicenter Registry). Am J Cardiol 2017; 119: 1173–1178. PMID: 28236456
- 256.
Garot P, Lefevre T, Eltchaninoff H, et al. Six-month outcome of emergency percutaneous coronary intervention in resuscitated patients after cardiac arrest complicating ST-elevation myocardial infarction. Circulation 2007; 115: 1354–1362. PMID: 17353440
- 257.
Kern KB, Rahman O. Emergent percutaneous coronary intervention for resuscitated victims of out-of-hospital cardiac arrest. Catheter Cardiovasc Interv 2010; 75: 616–624. PMID: 20049976
- 258.
Spaulding CM, Joly LM, Rosenberg A, et al. Immediate coronary angiography in survivors of out-of-hospital cardiac arrest. N Engl J Med 1997; 336: 1629–1633. PMID: 9171064
- 259.
Kern KB. Optimal treatment of patients surviving out-of-hospital cardiac arrest. JACC Cardiovasc Interv 2012; 5: 597–605. PMID: 22721654
- 260.
Garcia-Tejada J, Jurado-Román A, Rodríguez J, et al. Post-resuscitation electrocardiograms, acute coronary findings and in-hospital prognosis of survivors of out-of-hospital cardiac arrest. Resuscitation 2014; 85: 1245–1250. PMID: 24929199
- 261.
Noc M, Fajadet J, Lassen JF, et al. European Association for Percutaneous Cardiovascular Interventions (EAPCI),Stent for Life (SFL) Group. Invasive coronary treatment strategies for out-of-hospital cardiac arrest: A consensus statement from the European association for percutaneous cardiovascular interventions (EAPCI)/stent for life (SFL) groups. EuroIntervention 2014; 10: 31–37. PMID: 24832635
- 262.
Jolly SS, Cairns JA, Yusuf S, et al. TOTAL Investigators. Randomized trial of primary PCI with or without routine manual thrombectomy. N Engl J Med 2015; 372: 1389–1398. PMID: 25853743
- 263.
Fröbert O, Lagerqvist B, Olivecrona GK, et al. TASTE Trial. Thrombus aspiration during ST-segment elevation myocardial infarction. N Engl J Med 2013; 369: 1587–1597. PMID: 23991656
- 264.
Elgendy IY, Huo T, Bhatt DL, et al. Is Aspiration thrombectomy beneficial in patients undergoing primary percutaneous coronary intervention? Meta-analysis of randomized trials. Circ Cardiovasc Interv 2015; 8: e002258. PMID: 26175531
- 265.
Ikari Y, Sakurada M, Kozuma K, et al. VAMPIRE Investigators. Upfront thrombus aspiration in primary coronary intervention for patients with ST-segment elevation acute myocardial infarction: Report of the VAMPIRE (VAcuuM asPIration thrombus REmoval) trial. JACC Cardiovasc Interv 2008; 1: 424–431. PMID: 19463340
- 266.
Stone GW, Webb J, Cox DA, et al. Enhanced Myocardial Efficacy and Recovery by Aspiration of Liberated Debris (EMERALD) Investigators. Distal microcirculatory protection during percutaneous coronary intervention in acute ST-segment elevation myocardial infarction: A randomized controlled trial. JAMA 2005; 293: 1063–1072. PMID: 15741528
- 267.
Hibi K, Kozuma K, Sonoda S, et al. VAMPIRE 3 Investigators. A randomized study of distal filter protection versus conventional treatment during percutaneous coronary intervention in patients with attenuated plaque identified by intravascular ultrasound. JACC Cardiovasc Interv 2018; 11: 1545–1555. PMID: 30077678
- 268.
Valgimigli M, Gagnor A, Calabró P, et al. MATRIX Investigators. Radial versus femoral access in patients with acute coronary syndromes undergoing invasive management: A randomised multicentre trial. Lancet 2015; 385: 2465–2476. PMID: 25791214
- 269.
Jolly SS, Yusuf S, Cairns J, et al. RIVAL trial group. Radial versus femoral access for coronary angiography and intervention in patients with acute coronary syndromes (RIVAL): A randomised, parallel group, multicentre trial. Lancet 2011; 377: 1409–1420. PMID: 21470671
- 270.
Romagnoli E, Biondi-Zoccai G, Sciahbasi A, et al. Radial versus femoral randomized investigation in ST-segment elevation acute coronary syndrome: The RIFLE-STEACS (Radial Versus Femoral Randomized Investigation in ST-Elevation Acute Coronary Syndrome) study. J Am Coll Cardiol 2012; 60: 2481–2489. PMID: 22858390
- 271.
Fujii T, Masuda N, Ijichi T, et al. Transradial intervention for patients with ST elevation myocardial infarction with or without cardiogenic shock. Catheter Cardiovasc Interv 2014; 83: E1–E7. PMID: 23441063
- 272.
Jolly SS, Pogue J, Haladyn K, et al. Effects of aspirin dose on ischaemic events and bleeding after percutaneous coronary intervention: Insights from the PCI-CURE study. Eur Heart J 2009; 30: 900–907. PMID: 18819961
- 273.
Barnathan ES, Schwartz JS, Taylor L, et al. Aspirin and dipyridamole in the prevention of acute coronary thrombosis complicating coronary angioplasty. Circulation 1987; 76: 125–134. PMID: 2954724
- 274.
Mehta SR, Bassand JP, Chrolavicius S, et al. CURRENT-OASIS 7 Investigators. Dose comparisons of clopidogrel and aspirin in acute coronary syndromes. N Engl J Med 2010; 363: 930–942. PMID: 20818903
- 275.
Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002; 324: 71–86. PMID: 11786451
- 276.
Schömig A, Neumann FJ, Kastrati A, et al. A randomized comparison of antiplatelet and anticoagulant therapy after the placement of coronary-artery stents. N Engl J Med 1996; 334: 1084–1089. PMID: 8598866
- 277.
Smith SC, Benjamin EJ, Bonow RO, et al. AHA/ACCF Secondary Prevention and Risk Reduction Therapy for Patients with Coronary and other Atherosclerotic Vascular Disease: 2011 update Circulation 2011; 124: 2458–2473. PMID: 22052934
- 278.
Ochiai M, Eto K, Takeshita S, et al. Impact of cilostazol on clinical and angiographic outcome after primary stenting for acute myocardial infarction. Am J Cardiol 1999; 84: 1074–1076. PMID: 10569666
- 279.
Leon MB, Baim DS, Popma JJ, et al. A clinical trial comparing three antithrombotic-drug regimens after coronary-artery stenting: Stent Anticoagulation Restenosis Study Investigators. N Engl J Med 1998; 339: 1665–1671. PMID: 9834303
- 280.
Nakamura M, Yamagishi M, Ueno T, et al. Current antiplatelet therapy for Japanese patients with ST elevation acute myocardial infarction: J-AMI registry. Cardiovasc Interv Ther 2013; 28: 162–169. PMID: 23233418
- 281.
Sabatine MS, Cannon CP, Gibson CM, et al. Clopidogrel as Adjunctive Reperfusion Therapy (CLARITY)-Thrombolysis in Myocardial Infarction (TIMI) 28 Investigators. Effect of clopidogrel pretreatment before percutaneous coronary intervention in patients with ST-elevation myocardial infarction treated with fibrinolytics: The PCI-CLARITY study. JAMA 2005; 294: 1224–1232. PMID: 16143698
- 282.
Wiviott SD, Braunwald E, McCabe CH, et al. TRITON-TIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007; 357: 2001–2015. PMID: 17982182
- 283.
Saito S, Isshiki T, Kimura T, et al. Efficacy and safety of adjusted-dose prasugrel compared with clopidogrel in Japanese patients with acute coronary syndrome: The PRASFIT-ACS study. Circ J 2014; 78: 1684–1692. PMID: 24759796
- 284.
Takeyasu N, Watanabe S, Noguchi Y, et al. Randomized comparison of cilostazol vs ticlopidine for antiplatelet therapy after coronary stenting. Circ J 2005; 69: 780–785. PMID: 15988102
- 285.
Nakagawa Y, Nobuyoshi M, Yamaguchi T, et al. Efficacy of abciximab for patients undergoing balloon angioplasty: Data from Japanese evaluation of c7E3 Fab for elective and primary PCI organization in randomized trial (JEPPORT). Circ J 2009; 73: 145–151. PMID: 19023152
- 286.
Dewilde WJ, Oirbans T, Verheugt FW, et al. WOEST study investigators. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: An open-label, randomised, controlled trial. Lancet 2013; 381: 1107–1115. PMID: 23415013
- 287.
O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013; 127: e362–e425. PMID: 23247304
- 288.
The RISC Group. Risk of myocardial infarction and death during treatment with low dose aspirin and intravenous heparin in men with unstable coronary artery disease. Lancet 1990; 336: 827–830. PMID: 1976875
- 289.
Granger CB, Miller JM, Bovill EG, et al. Rebound increase in thrombin generation and activity after cessation of intravenous heparin in patients with acute coronary syndromes. Circulation 1995; 91: 1929–1935. PMID: 7895349
- 290.
Théroux P, Waters D, Lam J, et al. Reactivation of unstable angina after the discontinuation of heparin. N Engl J Med 1992; 327: 141–145. PMID: 1608405
- 291.
Warkentin TE, Levine MN, Hirsh J, et al. Heparin-induced thrombocytopenia in patients treated with low-molecular-weight heparin or unfractionated heparin. N Engl J Med 1995; 332: 1330–1335. PMID: 7715641
- 292.
Eikelboom JW, Quinlan DJ, Mehta SR, et al. Unfractionated and low-molecular-weight heparin as adjuncts to thrombolysis in aspirin-treated patients with ST-elevation acute myocardial infarction: A meta-analysis of the randomized trials. Circulation 2005; 112: 3855–3867. PMID: 16344381
- 293.
Sabatine MS, Morrow DA, Montalescot G, et al. Clopidogrel as Adjunctive Reperfusion Therapy (CLARITY)-Thrombolysis in Myocardial Infarction (TIMI) 28 Investigators. Angiographic and clinical outcomes in patients receiving low-molecular-weight heparin versus unfractionated heparin in ST-elevation myocardial infarction treated with fibrinolytics in the CLARITY-TIMI 28 Trial. Circulation 2005; 112: 3846–3854. PMID: 16291601
- 294.
Kereiakes DJ, Montalescot G, Antman EM, et al. Low-molecular-weight heparin therapy for non-ST-elevation acute coronary syndromes and during percutaneous coronary intervention: An expert consensus. Am Heart J 2002; 144: 615–624. PMID: 12360156
- 295.
FRagmin Fast Revascularisation during In Stability in Coronary artery disease (FRISC II) Investigators. Long-term low-molecular-mass heparin in unstable coronary-artery disease: FRISC II prospective randomised multicentre study. Lancet 1999; 354: 701–707. PMID: 10475180
- 296.
Silvain J, Beygui F, Barthélémy O, et al. Efficacy and safety of enoxaparin versus unfractionated heparin during percutaneous coronary intervention: Systematic review and meta-analysis. BMJ 2012; 344: e553. PMID: 22306479
- 297.
Brieger D, Collet JP, Silvain J, et al. Heparin or enoxaparin anticoagulation for primary percutaneous coronary intervention. Catheter Cardiovasc Interv 2011; 77: 182–190. PMID: 20578166
- 298.
Cruz-Gonzalez I, Sanchez-Ledesma M, Baron SJ, et al. Efficacy and safety of argatroban with or without glycoprotein IIb/IIIa inhibitor in patients with heparin induced thrombocytopenia undergoing percutaneous coronary intervention for acute coronary syndrome. J Thromb Thrombolysis 2008; 25: 214–218. PMID: 17632689
- 299.
Jang IK, Brown DF, Giugliano RP, et al. A multicenter, randomized study of argatroban versus heparin as adjunct to tissue plasminogen activator (TPA) in acute myocardial infarction: Myocardial infarction with novastan and TPA (MINT) study. J Am Coll Cardiol 1999; 33: 1879–1885. PMID: 10362188
- 300.
Matsuo T. Please teach us about the diagnosis and treatment of heparin-induced thrombocytopenia (HIT). Thrombosis and Circulation 2005; 13: 170–173.
- 301.
Politi L, Sgura F, Rossi R, et al. A randomised trial of target-vessel versus multi-vessel revascularisation in ST-elevation myocardial infarction: Major adverse cardiac events during long-term follow-up. Heart 2010; 96: 662–667. PMID: 19778920
- 302.
Wald DS, Morris JK, Wald NJ, et al. PRAMI Investigators. Randomized trial of preventive angioplasty in myocardial infarction. N Engl J Med 2013; 369: 1115–1123. PMID: 23991625
- 303.
Smits PC, Abdel-Wahab M, Neumann FJ, et al. Compare-Acute Investigators. Fractional flow reserve-guided multivessel angioplasty in myocardial infarction. N Engl J Med 2017; 376: 1234–1244. PMID: 28317428
- 304.
Ibrahim H, Sharma PK, Cohen DJ, et al. Multivessel versus culprit vessel-only percutaneous coronary intervention among patients with acute myocardial infarction: Insights from the TRANSLATE-ACS observational study. J Am Heart Assoc 2017; 6: e006343. PMID: 28982673
- 305.
Engstrøm T, Kelbæk H, Helqvist S, et al. DANAMI-3—PRIMULTI Investigators. Complete revascularisation versus treatment of the culprit lesion only in patients with ST-segment elevation myocardial infarction and multivessel disease (DANAMI-3—PRIMULTI): An open-label, randomised controlled trial. Lancet 2015; 386: 665–671. PMID: 26347918
- 306.
Gershlick AH, Khan JN, Kelly DJ, et al. Randomized trial of complete versus lesion-only revascularization in patients undergoing primary percutaneous coronary intervention for STEMI and multivessel disease: The CvLPRIT trial. J Am Coll Cardiol 2015; 65: 963–972. PMID: 25766941
- 307.
Moreno R, Mehta SR. Nonculprit vessel intervention: Let’s COMPLETE the evidence. Rev Esp Cardiol (Engl Ed) 2017; 70: 418–420. PMID: 28139391
- 308.
Bangalore S, Toklu B, Wetterslev J. Complete versus culprit-only revascularization for ST-segment-elevation myocardial infarction and multivessel disease: A meta-analysis and trial sequential analysis of randomized trials. Circ Cardiovasc Interv 2015; 8: e002142. PMID: 25873730
- 309.
Elgendy IY, Mahmoud AN, Kumbhani DJ, et al. Complete or culprit-only revascularization for patients with multivessel coronary artery disease undergoing percutaneous coronary intervention: A pairwise and network meta-analysis of randomized trials. JACC Cardiovasc Interv 2017; 10: 315–324. PMID: 28231899
- 310.
Toyota T, Shiomi H, Taniguchi T, et al. CREDO-Kyoto AMI Registry Investigators. Culprit vessel-only vs. staged multivessel percutaneous coronary intervention strategies in patients with multivessel coronary artery disease undergoing primary percutaneous coronary intervention for ST-segment elevation myocardial infarction. Circ J 2016; 80: 371–378. PMID: 26597353
- 311.
Thiele H, Akin I, Sandri M, et al. CULPRIT-SHOCK Investigators. PCI strategies in patients with acute myocardial infarction and cardiogenic shock. N Engl J Med 2017; 377: 2419–2432. PMID: 29083953
- 311a.
Kornowski R, Mehran R, Dangas G, et al. HORIZONS-AMI Trial Investigators. Prognostic impact of staged versus “one-time” multivessel percutaneous intervention in acute myocardial infarction: Analysis from the HORIZONS-AMI (harmonizing outcomes with revascularization and stents in acute myocardial infarction) trial. J Am Coll Cardiol 2011; 58: 704–711. PMID: 21816305
- 311b.
Qarawani D, Nahir M, Abboud M, et al. Culprit only versus complete coronary revascularization during primary PCI. Int J Cardiol 2008; 123: 288–292. PMID: 17428557
- 311c.
Corpus RA, House JA, Marso SP, et al. Multivessel percutaneous coronary intervention in patients with multivessel disease and acute myocardial infarction. Am Heart J 2004; 148: 493-500. PMID: 15389238
- 311d.
Rigattieri S, Biondi-Zoccai G, Silvestri P, et al. Management of multivessel coronary disease after ST elevation myocardial infarction treated by primary angioplasty. J Interv Cardiol 2008; 21: 1–7. PMID: 18086133
- 312.
Ogawa H, Kojima S. Modern state of acute myocardial infarction in the interventional era: Observational case-control study--Japanese acute coronary syndrome study (JACSS). J Cardiol 2009; 54: 1–9. PMID: 19632514
- 313.
Fibrinolytic Therapy Trialists’ (FTT) Collaborative Group. Indications for fibrinolytic therapy in suspected acute myocardial infarction: Collaborative overview of early mortality and major morbidity results from all randomised trials of more than 1000 patients. Lancet 1994; 343: 311–322. PMID: 7905143
- 314.
AIMS Trial Study Group. Effect of intravenous APSAC on mortality after acute myocardial infarction: Preliminary report of a placebo-controlled clinical trial. Lancet 1988; 331: 545–549. PMID: 2894490
- 315.
EMERAS (Estudio Multicéntrico Estreptoquinasa Repúblicas de América del Sur) Collaborative Group. Randomised trial of late thrombolysis in patients with suspected acute myocardial infarction. Lancet 1993; 342: 767–772. PMID: 8103875
- 316.
LATE Study Group. Late Assessment of Thrombolytic Efficacy (LATE) study with alteplase 6–24 hours after onset of acute myocardial infarction. Lancet 1993; 342: 759–766. PMID: 8103874
- 317.
Rossi P, Bolognese L. Urochinasi per via Sistemica nell’Infarto Miocardico (USIM) Collaborative Group. Comparison of intravenous urokinase plus heparin versus heparin alone in acute myocardial infarction. Am J Cardiol 1991; 68: 585–592. PMID: 1877476
- 318.
Bonnefoy E, Steg PG, Boutitie F, et al. CAPTIM Investigators. Comparison of primary angioplasty and pre-hospital fibrinolysis in acute myocardial infarction (CAPTIM) trial: A 5-year follow-up. Eur Heart J 2009; 30: 1598–1606. PMID: 19429632
- 319.
Pinto DS, Frederick PD, Chakrabarti AK, et al. National Registry of Myocardial Infarction Investigators. Benefit of transferring ST-segment-elevation myocardial infarction patients for percutaneous coronary intervention compared with administration of onsite fibrinolytic declines as delays increase. Circulation 2011; 124: 2512–2521. PMID: 22064592
- 320.
Armstrong PW, Gershlick AH, Goldstein P, et al. STREAM Investigative Team. Fibrinolysis or primary PCI in ST-segment elevation myocardial infarction. N Engl J Med 2013; 368: 1379–1387. PMID: 23473396
- 321.
The I. S.A.M. Study Group. A prospective trial of intravenous streptokinase in acute myocardial infarction (I.S.A.M.). Mortality, morbidity, and infarct size at 21 days. N Engl J Med 1986; 314: 1465–1471. PMID: 2871492
- 322.
de Winter RJ, Verouden NJ, Wellens HJ, et al. Interventional Cardiology Group of the Academic Medical Center. A new ECG sign of proximal LAD occlusion. N Engl J Med 2008; 359: 2071–2073. PMID: 18987380
- 323.
Jong GP, Ma T, Chou P, et al. Reciprocal changes in 12-lead electrocardiography can predict left main coronary artery lesion in patients with acute myocardial infarction. Int Heart J 2006; 47: 13–20. PMID: 16479036
- 324.
The TIMI IIIA Investigators. Early effects of tissue-type plasminogen activator added to conventional therapy on the culprit coronary lesion in patients presenting with ischemic cardiac pain at rest: Results of the Thrombolysis in Myocardial Ischemia (TIMI IIIA) Trial. Circulation 1993; 87: 38–52. PMID: 8419023
- 325.
Erne P, Schoenenberger AW, Burckhardt D, et al. Effects of percutaneous coronary interventions in silent ischemia after myocardial infarction: The SWISSI II randomized controlled trial. JAMA 2007; 297: 1985–1991. PMID: 17488963
- 326.
Madsen JK, Grande P, Saunamäki K, et al. Danish multicenter randomized study of invasive versus conservative treatment in patients with inducible ischemia after thrombolysis in acute myocardial infarction (DANAMI): DANish trial in Acute Myocardial Infarction. Circulation 1997; 96: 748–755. PMID: 9264478
- 327.
Gibson CM, Murphy SA, Rizzo MJ, et al. Thrombolysis In Myocardial Infarction (TIMI) Study Group. Relationship between TIMI frame count and clinical outcomes after thrombolytic administration. Circulation 1999; 99: 1945–1950. PMID: 10208996
- 328.
Gibson CM, Cannon CP, Murphy SA, et al. TIMI Study Group. Relationship of the TIMI myocardial perfusion grades, flow grades, frame count, and percutaneous coronary intervention to long-term outcomes after thrombolytic administration in acute myocardial infarction. Circulation 2002; 105: 1909–1913. PMID: 11997276
- 329.
Sutton AG, Campbell PG, Graham R, et al. A randomized trial of rescue angioplasty versus a conservative approach for failed fibrinolysis in ST-segment elevation myocardial infarction: The Middlesbrough Early Revascularization to Limit INfarction (MERLIN) trial. J Am Coll Cardiol 2004; 44: 287–296. PMID: 15261920
- 330.
Sutton AG, Campbell PG, Price DJ, et al. Failure of thrombolysis by streptokinase: Detection with a simple electrocardiographic method. Heart 2000; 84: 149–156. PMID: 10908249
- 331.
Bøhmer E, Hoffmann P, Abdelnoor M, et al. Efficacy and safety of immediate angioplasty versus ischemia-guided management after thrombolysis in acute myocardial infarction in areas with very long transfer distances results of the NORDISTEMI (NORwegian study on DIstrict treatment of ST-elevation myocardial infarction). J Am Coll Cardiol 2010; 55: 102–110. PMID: 19747792
- 332.
Borgia F, Goodman SG, Halvorsen S, et al. Early routine percutaneous coronary intervention after fibrinolysis vs. standard therapy in ST-segment elevation myocardial infarction: A meta-analysis. Eur Heart J 2010; 31: 2156–2169. PMID: 20601393
- 333.
Fernandez-Avilés F, Alonso JJ, Castro-Beiras A, et al. GRACIA (Grupo de Análisis de la Cardiopatía Isquémica Aguda) Group. Routine invasive strategy within 24 hours of thrombolysis versus ischaemia-guided conservative approach for acute myocardial infarction with ST-segment elevation (GRACIA-1): A randomised controlled trial. Lancet 2004; 364: 1045–1053. PMID: 15380963
- 334.
White HD. Systems of care: Need for hub-and-spoke systems for both primary and systematic percutaneous coronary intervention after fibrinolysis. Circulation 2008; 118: 219–222. PMID: 18625904
- 335.
Hochman JS, Lamas GA, Buller CE, et al. Occluded Artery Trial Investigators. Coronary intervention for persistent occlusion after myocardial infarction. N Engl J Med 2006; 355: 2395–2407. PMID: 17105759
- 336.
Gibson CM, Karha J, Murphy SA, et al. TIMI Study Group. Early and long-term clinical outcomes associated with reinfarction following fibrinolytic administration in the Thrombolysis in Myocardial Infarction trials. J Am Coll Cardiol 2003; 42: 7–16. PMID: 12849652
- 337.
D’Souza SP, Mamas MA, Fraser DG, et al. Routine early coronary angioplasty versus ischaemia-guided angioplasty after thrombolysis in acute ST-elevation myocardial infarction: A meta-analysis. Eur Heart J 2011; 32: 972–982. PMID: 21036776
- 338.
Gupta M, Chang WC, Van de Werf F, et al. ASSENT II Investigators. International differences in in-hospital revascularization and outcomes following acute myocardial infarction: A multilevel analysis of patients in ASSENT-2. Eur Heart J 2003; 24: 1640–1650. PMID: 14499226
- 339.
Steg PG, Thuaire C, Himbert D, et al. DECOPI Investigators. DECOPI (DEsobstruction COronaire en Post-Infarctus): A randomized multi-centre trial of occluded artery angioplasty after acute myocardial infarction. Eur Heart J 2004; 25: 2187–2194. PMID: 15589635
- 340.
Wilson SH, Bell MR, Rihal CS, et al. Infarct artery reocclusion after primary angioplasty, stent placement, and thrombolytic therapy for acute myocardial infarction. Am Heart J 2001; 141: 704–710. PMID: 11320356
- 341.
Kjaergard H, Nielsen PH, Andreasen JJ, et al. Coronary artery bypass grafting within 30 days after treatment of acute myocardial infarctions with angioplasty or fibrinolysis: A surgical substudy of DANAMI-2. Scand Cardiovasc J 2004; 38: 143–146. PMID: 15223711
- 342.
Stone GW, Brodie BR, Griffin JJ, et al. Role of cardiac surgery in the hospital phase management of patients treated with primary angioplasty for acute myocardial infarction. Am J Cardiol 2000; 85: 1292–1296. PMID: 10831942
- 343.
Pi Y, Roe MT, Holmes DN, et al. Utilization, Characteristics, and in-hospital outcomes of coronary artery bypass grafting in patients with ST-segment-elevation myocardial infarction: Results from the National Cardiovascular Data Registry Acute Coronary Treatment and Intervention Outcomes Network Registry-Get With The Guidelines. Circ Cardiovasc Qual Outcomes 2017; 10: e003490. PMID: 28794118
- 344.
Filizcan U, Kurc E, Cetemen S, et al. Mortality predictors in ST-elevated myocardial infarction patients undergoing coronary artery bypass grafting. Angiology 2011; 62: 68–73. PMID: 20462895
- 345.
Creswell LL, Moulton MJ, Cox JL, et al. Revascularization after acute myocardial infarction. Ann Thorac Surg 1995; 60: 19–26. PMID: 7598589
- 346.
Kamohara K, Yoshikai M, Yunoki J, et al. Surgical revascularization for acute coronary syndrome: Comparative surgical and long-term results. Jpn J Thorac Cardiovasc Surg 2006; 54: 95–102. PMID: 16613226
- 347.
Kjaergard HK, Nielsen PH, Andreasen JJ, et al. Coronary artery bypass grafting within the first year after treatment of large acute myocardial infarctions with angioplasty or fibrinolysis. Scand Cardiovasc J 2006; 40: 25–28. PMID: 16448994
- 348.
Lee DC, Oz MC, Weinberg AD, et al. Optimal timing of revascularization: Transmural versus nontransmural acute myocardial infarction. Ann Thorac Surg 2001; 71: 1197–1204. PMID: 11308159
- 349.
Wasvary H, Shannon F, Bassett J, et al. Timing of coronary artery bypass grafting after acute myocardial infarction. Am Surg 1997; 63: 710–715. PMID: 9247439
- 350.
Weiss ES, Chang DD, Joyce DL, et al. Optimal timing of coronary artery bypass after acute myocardial infarction: A review of California discharge data. J Thorac Cardiovasc Surg 2008; 135: 503–511, e3. PMID: 18329460
- 351.
Lee JH, Murrell HK, Strony J, et al. Risk analysis of coronary bypass surgery after acute myocardial infarction. Surgery 1997; 122: 675–681. PMID: 9347842
- 352.
Lee DC, Oz MC, Weinberg AD, et al. Appropriate timing of surgical intervention after transmural acute myocardial infarction. J Thorac Cardiovasc Surg 2003; 125: 115–120. PMID: 12538993
- 353.
Grothusen C, Friedrich C, Loehr J, et al. Outcome of stable patients with acute myocardial infarction and coronary artery bypass surgery within 48 hours: A single-center, retrospective experience. J Am Heart Assoc 2017; 6: e005498. PMID: 28974496
- 354.
Khan AN, Sabbagh S, Ittaman S, et al. Outcome of early revascularization surgery in patients with ST-elevation myocardial infarction. J Interv Cardiol 2015; 28: 14–23. PMID: 25664508
- 355.
Donatelli F, Benussi S, Triggiani M, et al. Surgical treatment for life-threatening acute myocardial infarction: A prospective protocol. Eur J Cardiothorac Surg 1997; 11: 228–233. PMID: 9080148
- 356.
Mehta RH, Lopes RD, Ballotta A, et al. Percutaneous coronary intervention or coronary artery bypass surgery for cardiogenic shock and multivessel coronary artery disease? Am Heart J 2010; 159: 141–147. PMID: 20102880
- 357.
White HD, Assmann SF, Sanborn TA, et al. Comparison of percutaneous coronary intervention and coronary artery bypass grafting after acute myocardial infarction complicated by cardiogenic shock: Results from the Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock (SHOCK) trial. Circulation 2005; 112: 1992–2001. PMID: 16186436
- 358.
Menon V, Webb JG, Hillis LD, et al. Outcome and profile of ventricular septal rupture with cardiogenic shock after myocardial infarction: A report from the SHOCK Trial Registry. J Am Coll Cardiol 2000; 36: 1110–1116. PMID: 10985713
- 359.
Slater J, Brown RJ, Antonelli TA, et al. Cardiogenic shock due to cardiac free-wall rupture or tamponade after acute myocardial infarction: A report from the SHOCK Trial Registry. J Am Coll Cardiol 2000; 36: 1117–1122. PMID: 10985714
- 360.
Thompson CR, Buller CE, Sleeper LA, et al. Cardiogenic shock due to acute severe mitral regurgitation complicating acute myocardial infarction: A report from the SHOCK Trial Registry. J Am Coll Cardiol 2000; 36: 1104–1109. PMID: 10985712
- 361.
Hohnloser SH, Zabel M, Kasper W, et al. Assessment of coronary artery patency after thrombolytic therapy: Accurate prediction utilizing the combined analysis of three noninvasive markers. J Am Coll Cardiol 1991; 18: 44–49. PMID: 2050939
- 362.
Stewart JT, French JK, Théroux P, et al. Early noninvasive identification of failed reperfusion after intravenous thrombolytic therapy in acute myocardial infarction. J Am Coll Cardiol 1998; 31: 1499–1505. PMID: 9626826
- 363.
Lee S, Otsuji Y, Minagoe S, et al. Correlation between distal left anterior descending artery flow velocity by transthoracic Doppler echocardiography and corrected TIMI frame count before mechanical reperfusion in patients with anterior acute myocardial infarction. Circ J 2005; 69: 1022–1028. PMID: 16127180
- 364.
The TIMI Study Group. The Thrombolysis in Myocardial Infarction (TIMI) trial: Phase I findings. N Engl J Med 1985; 312: 932–936. PMID: 4038784
- 365.
Gibson CM, Cannon CP, Daley WL, et al. TIMI 4 Study Group. TIMI frame count: A quantitative method of assessing coronary artery flow. Circulation 1996; 93: 879–888. PMID: 8598078
- 366.
Ito H, Tomooka T, Sakai N, et al. Lack of myocardial perfusion immediately after successful thrombolysis: A predictor of poor recovery of left ventricular function in anterior myocardial infarction. Circulation 1992; 85: 1699–1705. PMID: 1572028
- 367.
van’t Hof AW, Liem A, Suryapranata H, et al. Zwolle Myocardial Infarction Study Group. Angiographic assessment of myocardial reperfusion in patients treated with primary angioplasty for acute myocardial infarction: Myocardial blush grade. Circulation 1998; 97: 2302–2306. PMID: 9639373
- 368.
Gibson CM, Cannon CP, Murphy SA, et al. TIMI (Thrombolysis In Myocardial Infarction) Study Group. Relationship of TIMI myocardial perfusion grade to mortality after administration of thrombolytic drugs. Circulation 2000; 101: 125–130. PMID: 10637197
- 369.
Yamamuro A, Akasaka T, Tamita K, et al. Coronary flow velocity pattern immediately after percutaneous coronary intervention as a predictor of complications and in-hospital survival after acute myocardial infarction. Circulation 2002; 106: 3051–3056. PMID: 12473550
- 370.
de Lemos JA, Braunwald E. ST segment resolution as a tool for assessing the efficacy of reperfusion therapy. J Am Coll Cardiol 2001; 38: 1283–1294. PMID: 11691496
- 371.
Buller CE, Fu Y, Mahaffey KW, et al. ST-segment recovery and outcome after primary percutaneous coronary intervention for ST-elevation myocardial infarction: Insights from the Assessment of Pexelizumab in Acute Myocardial Infarction (APEX-AMI) trial. Circulation 2008; 118: 1335–1346. PMID: 18779444
- 372.
Niccoli G, Scalone G, Lerman A, et al. Coronary microvascular obstruction in acute myocardial infarction. Eur Heart J 2016; 37: 1024–1033. PMID: 26364289
- 373.
Hayashi M, Tsutamoto T, Wada A, et al. Intravenous atrial natriuretic peptide prevents left ventricular remodeling in patients with first anterior acute myocardial infarction. J Am Coll Cardiol 2001; 37: 1820–1826. PMID: 11401117
- 374.
Kasama S, Toyama T, Hatori T, et al. Effects of intravenous atrial natriuretic peptide on cardiac sympathetic nerve activity and left ventricular remodeling in patients with first anterior acute myocardial infarction. J Am Coll Cardiol 2007; 49: 667–674. PMID: 17291931
- 375.
Kitakaze M, Asakura M, Kim J, et al. J-WIND investigators. Human atrial natriuretic peptide and nicorandil as adjuncts to reperfusion treatment for acute myocardial infarction (J-WIND): Two randomised trials. Lancet 2007; 370: 1483–1493. PMID: 17964349
- 376.
Ito H, Taniyama Y, Iwakura K, et al. Intravenous nicorandil can preserve microvascular integrity and myocardial viability in patients with reperfused anterior wall myocardial infarction. J Am Coll Cardiol 1999; 33: 654–660. PMID: 10080465
- 377.
Ishii H, Ichimiya S, Kanashiro M, et al. Impact of a single intravenous administration of nicorandil before reperfusion in patients with ST-segment-elevation myocardial infarction. Circulation 2005; 112: 1284–1288. PMID: 16116055
- 378.
Sakata Y, Kodama K, Komamura K, et al. Salutary effect of adjunctive intracoronary nicorandil administration on restoration of myocardial blood flow and functional improvement in patients with acute myocardial infarction. Am Heart J 1997; 133: 616–621. PMID: 9200388
- 379.
Ito N, Nanto S, Doi Y, et al. High index of microcirculatory resistance level after successful primary percutaneous coronary intervention can be improved by intracoronary administration of nicorandil. Circ J 2010; 74: 909–915. PMID: 20234097
- 380.
Wu M, Huang Z, Xie H, et al. Nicorandil in patients with acute myocardial infarction undergoing primary percutaneous coronary intervention: A systematic review and meta-analysis. PLoS One 2013; 8: e78231. PMID: 24167609
- 381.
Iwakura K, Ito H, Okamura A, et al. Nicorandil treatment in patients with acute myocardial infarction: A meta-analysis. Circ J 2009; 73: 925–931. PMID: 19325192
- 382.
Suematsu Y, Murasato Y, Miura S, et al. Safety and feasibility of high-dose administration of nicorandil before reperfusion therapy in acute myocardial infarction. Cardiovasc Interv Ther 2013; 28: 352–361. PMID: 23625617
- 383.
Yang MJ, Lee HC, Lee HW, et al. Effect of nicorandil on clinical outcomes in patients with ST-segment elevation and non-ST-segment elevation myocardial infarction: Based on the Korea Acute Myocardial Infarction Registry (KAMIR). Int J Cardiol 2013; 168: 4868–4869. PMID: 23899744
- 384.
Heusch G, Bøtker HE, Przyklenk K, et al. Remote ischemic conditioning. J Am Coll Cardiol 2015; 65: 177–195. PMID: 25593060
- 385.
Bøtker HE, Kharbanda R, Schmidt MR, et al. Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: A randomised trial. Lancet 2010; 375: 727–734. PMID: 20189026
- 386.
Sloth AD, Schmidt MR, Munk K, et al. CONDI Investigators. Improved long-term clinical outcomes in patients with ST-elevation myocardial infarction undergoing remote ischaemic conditioning as an adjunct to primary percutaneous coronary intervention. Eur Heart J 2014; 35: 168–175. PMID: 24031025
- 387.
Eitel I, Stiermaier T, Rommel KP, et al. Cardioprotection by combined intrahospital remote ischaemic perconditioning and postconditioning in ST-elevation myocardial infarction: The randomized LIPSIA CONDITIONING trial. Eur Heart J 2015; 36: 3049–3057. PMID: 26385956
- 388.
Munk K, Andersen NH, Schmidt MR, et al. Remote ischemic conditioning in patients with myocardial infarction treated with primary angioplasty: Impact on left ventricular function assessed by comprehensive echocardiography and gated single-photon emission CT. Circ Cardiovasc Imaging 2010; 3: 656–662. PMID: 20826592
- 389.
Rentoukas I, Giannopoulos G, Kaoukis A, et al. Cardioprotective role of remote ischemic periconditioning in primary percutaneous coronary intervention: Enhancement by opioid action. JACC Cardiovasc Interv 2010; 3: 49–55. PMID: 20129568
- 390.
Crimi G, Pica S, Raineri C, et al. Remote ischemic post-conditioning of the lower limb during primary percutaneous coronary intervention safely reduces enzymatic infarct size in anterior myocardial infarction: A randomized controlled trial. JACC Cardiovasc Interv 2013; 6: 1055–1063. PMID: 24156966
- 391.
White SK, Frohlich GM, Sado DM, et al. Remote ischemic conditioning reduces myocardial infarct size and edema in patients with ST-segment elevation myocardial infarction. JACC Cardiovasc Interv 2015; 8: 178–188. PMID: 25240548
- 392.
Yellon DM, Ackbarkhan AK, Balgobin V, et al. Remote ischemic conditioning reduces myocardial infarct size in STEMI patients treated by thrombolysis. J Am Coll Cardiol 2015; 65: 2764–2765. PMID: 26112203
- 393.
McLeod SL, Iansavichene A, Cheskes S. Remote ischemic perconditioning to reduce reperfusion injury during acute ST-segment-elevation myocardial infarction: A systematic review and meta-analysis. J Am Heart Assoc 2017; 6: e005522. PMID: 28515120
- 394.
Yang XM, Proctor JB, Cui L, et al. Multiple, brief coronary occlusions during early reperfusion protect rabbit hearts by targeting cell signaling pathways. J Am Coll Cardiol 2004; 44: 1103–1110. PMID: 15337225
- 395.
Hahn JY, Song YB, Kim EK, et al. Ischemic postconditioning during primary percutaneous coronary intervention: The effects of postconditioning on myocardial reperfusion in patients with ST-segment elevation myocardial infarction (POST) randomized trial. Circulation 2013; 128: 1889–1896. PMID: 24068776
- 396.
Limalanathan S, Andersen GØ, Kløw NE, et al. Effect of ischemic postconditioning on infarct size in patients with ST-elevation myocardial infarction treated by primary PCI results of the POSTEMI (POstconditioning in ST-Elevation Myocardial Infarction) randomized trial. J Am Heart Assoc 2014; 3: e000679. PMID: 24760962
- 397.
Bulluck H, Sirker A, Loke YK, et al. Clinical benefit of adenosine as an adjunct to reperfusion in ST-elevation myocardial infarction patients: An updated meta-analysis of randomized controlled trials. Int J Cardiol 2016; 202: 228–237. PMID: 26402450
- 398.
Lønborg J, Kelbæk H, Vejlstrup N, et al. Exenatide reduces final infarct size in patients with ST-segment-elevation myocardial infarction and short-duration of ischemia. Circ Cardiovasc Interv 2012; 5: 288–295. PMID: 22496084
- 399.
Tsujita K, Shimomura H, Kaikita K, et al. Long-term efficacy of edaravone in patients with acute myocardial infarction. Circ J 2006; 70: 832–837. PMID: 16799234
- 400.
Gao D, Ning N, Niu X, et al. Erythropoietin treatment in patients with acute myocardial infarction: A meta-analysis of randomized controlled trials. Am Heart J 2012; 164: 715–727.e1. PMID: 23137502
- 401.
Upadhaya S, Madala S, Baniya R, et al. Impact of cyclosporine A use in the prevention of reperfusion injury in acute myocardial infarction: A meta-analysis. Cardiol J 2017; 24: 43–50. PMID: 27734457
- 402.
Villablanca PA, Rao G, Briceno DF, et al. Therapeutic hypothermia in ST elevation myocardial infarction: A systematic review and meta-analysis of randomised control trials. Heart 2016; 102: 712–719. PMID: 26864673
- 403.
Stone PH, Thompson B, Zaret BL, et al. Factors associated with failure of medical therapy in patients with unstable angina and non-Q wave myocardial infarction: A TIMI-IIIB database study. Eur Heart J 1999; 20: 1084–1093. PMID: 10413638
- 404.
Farkouh ME, Smars PA, Reeder GS, et al. Chest Pain Evaluation in the Emergency Room (CHEER) Investigators. A clinical trial of a chest-pain observation unit for patients with unstable angina. N Engl J Med 1998; 339: 1882–1888. PMID: 9862943
- 405.
Steg PG, Huber K, Andreotti F, et al. Bleeding in acute coronary syndromes and percutaneous coronary interventions: Position paper by the Working Group on Thrombosis of the European Society of Cardiology. Eur Heart J 2011; 32: 1854–1864. PMID: 21715717
- 406.
Mehran R, Pocock SJ, Nikolsky E, et al. A risk score to predict bleeding in patients with acute coronary syndromes. J Am Coll Cardiol 2010; 55: 2556–2566. PMID: 20513595
- 407.
Subherwal S, Bach RG, Chen AY, et al. Baseline risk of major bleeding in non-ST-segment-elevation myocardial infarction: The CRUSADE (Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA Guidelines) Bleeding Score. Circulation 2009; 119: 1873–1882. PMID: 19332461
- 408.
The TIMI IIIB Investigators. Effects of tissue plasminogen activator and a comparison of early invasive and conservative strategies in unstable angina and non-Q-wave myocardial infarction. Results of the TIMI IIIB Trial. Thrombolysis in myocardial ischemia. Circulation 1994; 89: 1545–1556. PMID: 8149520
- 409.
Boden WE, O’Rourke RA, Crawford MH, et al. Veterans Affairs Non-Q-Wave Infarction Strategies in Hospital (VANQWISH) Trial Investigators. Outcomes in patients with acute non-Q-wave myocardial infarction randomly assigned to an invasive as compared with a conservative management strategy. N Engl J Med 1998; 338: 1785–1792. PMID: 9632444
- 410.
Cannon CP, Weintraub WS, Demopoulos LA, et al. TACTICS (Treat Angina with Aggrastat and Determine Cost of Therapy with an Invasive or Conservative Strategy)--Thrombolysis in Myocardial Infarction 18 Investigators. Comparison of early invasive and conservative strategies in patients with unstable coronary syndromes treated with the glycoprotein IIb/IIIa inhibitor tirofiban. N Engl J Med 2001; 344: 1879–1887. PMID: 11419424
- 411.
de Winter RJ, Windhausen F, Cornel JH, et al. Invasive versus Conservative Treatment in Unstable Coronary Syndromes (ICTUS) Investigators. Early invasive versus selectively invasive management for acute coronary syndromes. N Engl J Med 2005; 353: 1095–1104. PMID: 16162880
- 412.
van Domburg RT, van Miltenburg-van Zijl AJ, Veerhoek RJ, et al. Unstable angina: Good long-term outcome after a complicated early course. J Am Coll Cardiol 1998; 31: 1534–1539. PMID: 9626831
- 413.
Williams DO, Braunwald E, Thompson B, et al. Results of percutaneous transluminal coronary angioplasty in unstable angina and non-Q-wave myocardial infarction: Observations from the TIMI IIIB Trial. Circulation 1996; 94: 2749–2755. PMID: 8941099
- 414.
van Miltenburg-van Zijl AJ, Simoons ML, Veerhoek RJ, et al. Incidence and follow-up of Braunwald subgroups in unstable angina pectoris. J Am Coll Cardiol 1995; 25: 1286–1292. PMID: 7722122
- 415.
Cannon CP, Weintraub WS, Demopoulos LA, et al. TACTICS-TIMI 18 Investigators. Invasive versus conservative strategies in unstable angina and non-Q-wave myocardial infarction following treatment with tirofiban: Rationale and study design of the international TACTICS-TIMI 18 Trial. Am J Cardiol 1998; 82: 731–736. PMID: 9761082
- 416.
Kleiman NS, Lincoff AM, Flaker GC, et al. PURSUIT Investigators. Early percutaneous coronary intervention, platelet inhibition with eptifibatide, and clinical outcomes in patients with acute coronary syndromes. Circulation 2000; 101: 751–757. PMID: 10683348
- 417.
Mehta SR, Tanguay JF, Eikelboom JW, et al. CURRENT-OASIS 7 trial investigators. Double-dose versus standard-dose clopidogrel and high-dose versus low-dose aspirin in individuals undergoing percutaneous coronary intervention for acute coronary syndromes (CURRENT-OASIS 7): A randomised factorial trial. Lancet 2010; 376: 1233–1243. PMID: 20817281
- 418.
Wallentin L, Becker RC, Budaj A, et al. PLATO Investigators. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009; 361: 1045–1057. PMID: 19717846
- 419.
Montalescot G, Cayla G, Collet JP, et al. ABOARD Investigators. Immediate vs delayed intervention for acute coronary syndromes: A randomized clinical trial. JAMA 2009; 302: 947–954. PMID: 19724041
- 420.
Ryan JW, Peterson ED, Chen AY, et al. CRUSADE Investigators. Optimal timing of intervention in non-ST-segment elevation acute coronary syndromes: Insights from the CRUSADE (Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA guidelines) Registry. Circulation 2005; 112: 3049–3057. PMID: 16275863
- 421.
Neumann FJ, Kastrati A, Pogatsa-Murray G, et al. Evaluation of prolonged antithrombotic pretreatment (“cooling-off” strategy) before intervention in patients with unstable coronary syndromes: A randomized controlled trial. JAMA 2003; 290: 1593–1599. PMID: 14506118
- 422.
Mehta SR, Granger CB, Boden WE, et al. TIMACS Investigators. Early versus delayed invasive intervention in acute coronary syndromes. N Engl J Med 2009; 360: 2165–2175. PMID: 19458363
- 423.
Kofoed KF, Kelbæk H, Hansen PR, et al. Early versus standard care invasive examination and treatment of patients with non-ST-segment elevation acute coronary syndrome: VERDICT randomized controlled trial. Circulation 2018; 138: 2741–2750. PMID: 30565996
- 424.
Bangalore S, Faxon DP. Coronary intervention in patients with acute coronary syndrome: Does every culprit lesion require revascularization? Curr Cardiol Rep 2010; 12: 330–337. PMID: 20425159
- 425.
Brener SJ, Milford-Beland S, Roe MT, et al. American College of Cardiology National Cardiovascular Database Registry. Culprit-only or multivessel revascularization in patients with acute coronary syndromes: An American College of Cardiology National Cardiovascular Database Registry report. Am Heart J 2008; 155: 140–146. PMID: 18082505
- 426.
Shishehbor MH, Lauer MS, Singh IM, et al. In unstable angina or non-ST-segment acute coronary syndrome, should patients with multivessel coronary artery disease undergo multivessel or culprit-only stenting? J Am Coll Cardiol 2007; 49: 849–854. PMID: 17320742
- 427.
Sardella G, Lucisano L, Garbo R, et al. Single-staged compared with multi-staged PCI in multivessel NSTEMI patients: The SMILE Trial. J Am Coll Cardiol 2016; 67: 264–272. PMID: 26796390
- 428.
Bär FW, Verheugt FW, Col J, et al. Thrombolysis in patients with unstable angina improves the angiographic but not the clinical outcome: Results of UNASEM, a multicenter, randomized, placebo-controlled, clinical trial with anistreplase. Circulation 1992; 86: 131–137. PMID: 1617766
- 429.
Singh M, Holmes DR, Garratt KN, et al. Changing outcome of percutaneous intervention in patients with unstable angina. J Am Coll Cardiol 1999; 33 Suppl: 31A.
- 430.
Myler RK, Shaw RE, Stertzer SH, et al. Unstable angina and coronary angioplasty. Circulation 1990; 82: II88–II95. PMID: 2203565
- 430a.
Giugliano RP, Lloyd-Jones DM, Camargo CA, et al. Association of unstable angina guideline care with improved survival. Arch Intern Med 2000; 160: 1775–1780. PMID: 10871970
- 431.
Serruys PW, van Hout B, Bonnier H, et al. Randomised comparison of implantation of heparin-coated stents with balloon angioplasty in selected patients with coronary artery disease (Benestent II). Lancet 1998; 352: 673–681. PMID: 9728982
- 432.
Marzocchi A, Piovaccari G, Marrozzini C, et al. Results of coronary stenting for unstable versus stable angina pectoris. Am J Cardiol 1997; 79: 1314–1318. PMID: 9165149
- 433.
Fox KA, Clayton TC, Damman P, et al. FIR Collaboration. Long-term outcome of a routine versus selective invasive strategy in patients with non-ST-segment elevation acute coronary syndrome a meta-analysis of individual patient data. J Am Coll Cardiol 2010; 55: 2435–2445. PMID: 20359842
- 434.
Lemos PA, Lee CH, Degertekin M, et al. Early outcome after sirolimus-eluting stent implantation in patients with acute coronary syndromes: Insights from the Rapamycin-Eluting Stent Evaluated At Rotterdam Cardiology Hospital (RESEARCH) registry. J Am Coll Cardiol 2003; 41: 2093–2099. PMID: 12798587
- 435.
Valgimigli M, Campo G, Arcozzi C, et al. Two-year clinical follow-up after sirolimus-eluting versus bare-metal stent implantation assisted by systematic glycoprotein IIb/IIIa Inhibitor Infusion in patients with myocardial infarction: Results from the STRATEGY study. J Am Coll Cardiol 2007; 50: 138–145. PMID: 17616297
- 436.
Spaulding C, Henry P, Teiger E, et al. TYPHOON Investigators. Sirolimus-eluting versus uncoated stents in acute myocardial infarction. N Engl J Med 2006; 355: 1093–1104. PMID: 16971716
- 437.
Menichelli M, Parma A, Pucci E, et al. Randomized trial of Sirolimus-Eluting Stent versus bare-metal stent in Acute Myocardial Infarction (SESAMI). J Am Coll Cardiol 2007; 49: 1924–1930. PMID: 17498576
- 438.
van der Hoeven BL, Liem SS, Jukema JW, et al. Sirolimus-eluting stents versus bare-metal stents in patients with ST-segment elevation myocardial infarction: 9-month angiographic and intravascular ultrasound results and 12-month clinical outcome results from the MISSION! Intervention Study. J Am Coll Cardiol 2008; 51: 618–626. PMID: 18261680
- 439.
De Luca G, Valgimigli M, Spaulding C, et al. Short and long-term benefits of sirolimus-eluting stent in ST-segment elevation myocardial infarction: A meta-analysis of randomized trials. J Thromb Thrombolysis 2009; 28: 200–210. PMID: 19190859
- 440.
Kandzari DE, Roe MT, Ohman EM, et al. Frequency, predictors, and outcomes of drug-eluting stent utilization in patients with high-risk non-ST-segment elevation acute coronary syndromes. Am J Cardiol 2005; 96: 750–755. PMID: 16169352
- 441.
Palmerini T, Biondi-Zoccai G, Della Riva D, et al. Stent thrombosis with drug-eluting and bare-metal stents: Evidence from a comprehensive network meta-analysis. Lancet 2012; 379: 1393–1402. PMID: 22445239
- 442.
Bønaa KH, Mannsverk J, Wiseth R, et al. NORSTENT Investigators. Drug-eluting or bare-metal stents for coronary artery disease. N Engl J Med 2016; 375: 1242–1252. PMID: 27572953
- 443.
Vlaar PJ, Diercks GF, Svilaas T, et al. The feasibility and safety of routine thrombus aspiration in patients with non-ST-elevation myocardial infarction. Catheter Cardiovasc Interv 2008; 72: 937–942. PMID: 19021272
- 444.
V Korn H, Ohlow M, Donev S, et al. Export aspiration system in patients with acute coronary syndrome and visible thrombus provides no substantial benefit. Catheter Cardiovasc Interv 2007; 70: 35–42. PMID: 17585384
- 445.
Ben-Dor I, Pichard AD, Waksman R. Combined mechanical and pharmacological approach to a thrombus-containing lesion. Catheter Cardiovasc Interv 2010; 75: 972–976. PMID: 20146325
- 446.
Limbruno U, Ebert AG, Galli M. Filters to prevent distal embolization during coronary artery stenting: The risk of mousetrap. J Invasive Cardiol 2006; 18: E131–E133. PMID: 16723746
- 447.
Hirose H, Amano A, Yoshida S, et al. Surgical management of unstable patients in the evolving phase of acute myocardial infarction. Ann Thorac Surg 2000; 69: 425–428. PMID: 10735675
- 448.
Montalescot G, Brieger D, Eagle KA, et al. GRACE Investigators. Unprotected left main revascularization in patients with acute coronary syndromes. Eur Heart J 2009; 30: 2308–2317. PMID: 19720640
- 449.
Lazar HL, Jacobs AK, Aldea GS, et al. Factors influencing mortality after emergency coronary artery bypass grafting for failed percutaneous transluminal coronary angioplasty. Ann Thorac Surg 1997; 64: 1747–1752. PMID: 9436566
- 450.
Kaul TK, Fields BL, Riggins SL, et al. Coronary artery bypass grafting within 30 days of an acute myocardial infarction. Ann Thorac Surg 1995; 59: 1169–1176. PMID: 7733715
- 451.
Hochman JS, Boland J, Sleeper LA, et al. SHOCK Registry Investigators. Current spectrum of cardiogenic shock and effect of early revascularization on mortality: Results of an International Registry. Circulation 1995; 91: 873–881. PMID: 7828316
- 452.
Rao V, Ivanov J, Weisel RD, et al. Predictors of low cardiac output syndrome after coronary artery bypass. J Thorac Cardiovasc Surg 1996; 112: 38–51. PMID: 8691884
- 453.
Nollert G, Amend J, Reichart B. Use of the internal mammary artery as a graft in emergency coronary artery bypass grafting after failed PTCA. Thorac Cardiovasc Surg 1995; 43: 142–147. PMID: 7570565
- 454.
Zeff RH, Kongtahworn C, Iannone LA, et al. Internal mammary artery versus saphenous vein graft to the left anterior descending coronary artery: Prospective randomized study with 10-year follow-up. Ann Thorac Surg 1988; 45: 533–536. PMID: 3259128
- 455.
Cameron A, Davis KB, Green G, et al. Coronary bypass surgery with internal-thoracic-artery grafts: Effects on survival over a 15-year period. N Engl J Med 1996; 334: 216–219. PMID: 8531997
- 456.
Björklund E, Jernberg T, Johanson P, et al. ASSENT-2 and ASSENT-PLUS Study Groups. Admission N-terminal pro-brain natriuretic peptide and its interaction with admission troponin T and ST segment resolution for early risk stratification in ST elevation myocardial infarction. Heart 2006; 92: 735–740. PMID: 16251228
- 457.
Mega JL, Morrow DA, De Lemos JA, et al. B-type natriuretic peptide at presentation and prognosis in patients with ST-segment elevation myocardial infarction: An ENTIRE-TIMI-23 substudy. J Am Coll Cardiol 2004; 44: 335–339. PMID: 15261928
- 458.
Giannitsis E, Lehrke S, Wiegand UK, et al. Risk stratification in patients with inferior acute myocardial infarction treated by percutaneous coronary interventions: The role of admission troponin T. Circulation 2000; 102: 2038–2044. PMID: 11044417
- 459.
Matetzky S, Sharir T, Domingo M, et al. Elevated troponin I level on admission is associated with adverse outcome of primary angioplasty in acute myocardial infarction. Circulation 2000; 102: 1611–1616. PMID: 11015336
- 460.
Hao K, Yasuda S, Takii T, et al. MIYAGI-AMI Study Investigators. Urbanization, life style changes and the incidence/in-hospital mortality of acute myocardial infarction in Japan: Report from the MIYAGI-AMI Registry Study. Circ J 2012; 76: 1136–1144. PMID: 22343268
- 461.
Day HW. An intensive coronary care area. Dis Chest 1963; 44: 423–426. PMID: 14072745
- 462.
Lee TH, Goldman L. The coronary care unit turns 25: Historical trends and future directions. Ann Intern Med 1988; 108: 887–894. PMID: 3285749
- 463.
Swan HJ, Ganz W. Measurement of right atrial and pulmonary arterial pressures and cardiac output: Clinical application of hemodynamic monitoring. Adv Intern Med 1982; 27: 453–473. PMID: 7041550
- 464.
Ishida K, Kanamasa K, Hayashi T, et al. Recent decline in hospital mortality in acute myocardial infarction. Shinzo 2002; 34: 533–542.
- 465.
Lieu TA, Gurley RJ, Lundstrom RJ, et al. Primary angioplasty and thrombolysis for acute myocardial infarction: An evidence summary. J Am Coll Cardiol 1996; 27: 737–750. PMID: 8606291
- 466.
JSICM Investigative Committee for Standards for Establishing Intensive Care Units. CCU Establishment Guidelines. J Jpn Soc Intensive Care Med 2004; 11: 259–267.
- 467.
Tanaka K, Seino Y, Ohbayashi K, et al. Cardiac emergency triage and therapeutic decisions using whole blood rapid troponin T test for patients with suspicious acute coronary syndrome. Jpn Circ J 2001; 65: 424–428. PMID: 11348047
- 468.
Gust R, Gust A, Böttiger BW, et al. Bedside troponin T testing is not useful for early out-of-hospital diagnosis of myocardial infarction. Acta Anaesthesiol Scand 1998; 42: 414–417. PMID: 9563859
- 469.
Schuchert A, Hamm C, Scholz J, et al. Prehospital testing for troponin T in patients with suspected acute myocardial infarction. Am Heart J 1999; 138: 45–48. PMID: 10385762
- 470.
Stub D, Smith K, Bernard S, et al. AVOID Investigators. Air versus oxygen in ST-segment-elevation myocardial infarction. Circulation 2015; 131: 2143–2150. PMID: 26002889
- 471.
Herkner H, Thoennissen J, Nikfardjam M, et al. Short versus prolonged bed rest after uncomplicated acute myocardial infarction: A systematic review and meta-analysis. J Clin Epidemiol 2003; 56: 775–781. PMID: 12954470
- 472.
Cohen IM, Alpert JS, Francis GS, et al. Safety of hot and cold liquids in patients with acute myocardial infarction. Chest 1977; 71: 450–452. PMID: 322966
- 473.
Tanaka K. Pathogenesis of impaired glucose tolerance and its relationship to hemodynamics in patients with acute myocardial infarction. [Article in Japanese] J Nippon Med Sch 1985; 52: 49–57.
- 474.
Dungan KM, Braithwaite SS, Preiser JC. Stress hyperglycaemia. Lancet 2009; 373: 1798–1807. PMID: 19465235
- 475.
Capes SE, Hunt D, Malmberg K, et al. Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: A systematic overview. Lancet 2000; 355: 773–778. PMID: 10711923
- 476.
Singh K, Hibbert B, Singh B, et al. Meta-analysis of admission hyperglycaemia in acute myocardial infarction patients treated with primary angioplasty: A cause or a marker of mortality? Eur Heart J Cardiovasc Pharmacother 2015; 1: 220–228. PMID: 27532445
- 477.
Norhammar A, Tenerz A, Nilsson G, et al. Glucose metabolism in patients with acute myocardial infarction and no previous diagnosis of diabetes mellitus: A prospective study. Lancet 2002; 359: 2140–2144. PMID: 12090978
- 478.
Malmberg K, Rydén L, Efendic S, et al. Randomized trial of insulin-glucose infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardial infarction (DIGAMI study): Effects on mortality at 1 year. J Am Coll Cardiol 1995; 26: 57–65. PMID: 7797776
- 478a.
NICE-SUGAR Study Investigators, Finfer S, Chittock DR, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med 2009; 360: 1283–1297. PMID: 19318384
- 479.
de Mulder M, Umans VA, Cornel JH, et al. Intensive glucose regulation in hyperglycemic acute coronary syndrome: Results of the randomized BIOMarker study to identify the acute risk of a coronary syndrome-2 (BIOMArCS-2) glucose trial. JAMA Intern Med 2013; 173: 1896–1904. PMID: 24018647
- 480.
Svensson AM, McGuire DK, Abrahamsson P, et al. Association between hyper- and hypoglycaemia and 2 year all-cause mortality risk in diabetic patients with acute coronary events. Eur Heart J 2005; 26: 1255–1261. PMID: 15821004
- 481.
Reilly L, Sullivan P, Ninni S, et al. Reducing foley catheter device days in an intensive care unit: Using the evidence to change practice. AACN Adv Crit Care 2006; 17: 272–283. PMID: 16931923
- 482.
Singman H, Kinsella E, Goldberg E. Electrocardiographic changes in coronary care unit patients during defecation. Vasc Surg 1975; 9: 54–57. PMID: 1124593
- 483.
Stern TA. Psychiatric management of acute myocardial infarction in the coronary care unit. Am J Cardiol 1987; 60: J59–J67. PMID: 3321971
- 484.
Cassem NH, Hackett TP. Psychiatric consultation in a coronary care unit. Ann Intern Med 1971; 75: 9–14. PMID: 5091570
- 485.
Broadbent E, Petrie KJ, Ellis CJ, et al. Patients with acute myocardial infarction have an inaccurate understanding of their risk of a future cardiac event. Intern Med J 2006; 36: 643–647. PMID: 16958641
- 486.
Arefjord K, Hallaråker E, Havik OE, et al. Illness understanding, causal attributions and emotional reactions in wives of myocardial infarction patients. Psychol Psychother 2002; 75: 101–114. PMID: 12006203
- 487.
Mizuno Y. Do variations of types of disease, age and gender have effects on the number of days for CCU care and mortality? [Article in Japanese] Japanese Journal of Intensive Care Medicine 2003; 27: 554–557.
- 488.
Baigent C, Collins R, Appleby P, et al. The ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. ISIS-2: 10 year survival among patients with suspected acute myocardial infarction in randomised comparison of intravenous streptokinase, oral aspirin, both, or neither. BMJ 1998; 316: 1337–1343. PMID: 9563981
- 489.
Chen ZM, Jiang LX, Chen YP, et al. COMMIT (ClOpidogrel and Metoprolol in Myocardial Infarction Trial) collaborative group. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: Randomised placebo-controlled trial. Lancet 2005; 366: 1607–1621. PMID: 16271642
- 490.
Goto S, Huang CH, Park SJ, et al. Ticagrelor vs. clopidogrel in Japanese, Korean and Taiwanese patients with acute coronary syndrome: Randomized, double-blind, phase III PHILO study. Circ J 2015; 79: 2452–2460. PMID: 26376600
- 491.
Daida H, Miyauchi K, Ogawa H, et al. PACIFIC investigators. Management and two-year long-term clinical outcome of acute coronary syndrome in Japan: Prevention of atherothrombotic incidents following ischemic coronary attack (PACIFIC) registry. Circ J 2013; 77: 934–943. PMID: 23502993
- 492.
Gibson CM, Mehran R, Bode C, et al. Prevention of Bleeding in Patients with Atrial Fibrillation Undergoing PCI. N Engl J Med 2016; 375: 2423–2434. PMID: 27959713
- 493.
Cannon CP, Bhatt DL, Oldgren J, et al. RE-DUAL PCI Steering Committee and Investigators. Dual Antithrombotic Therapy with Dabigatran after PCI in Atrial Fibrillation. N Engl J Med 2017; 377: 1513–1524. PMID: 28844193
- 494.
Delewi R, Zijlstra F, Piek JJ. Left ventricular thrombus formation after acute myocardial infarction. Heart 2012; 98: 1743–1749. PMID: 23151669
- 495.
Sommers HM, Jennings RB. Ventricular fibrillation and myocardial necrosis after transient ischemia. Effect of treatment with oxygen, procainamide, reserpine, and propranolol. Arch Intern Med 1972; 129: 780–789. PMID: 5025900
- 496.
Miller CD, Roe MT, Mulgund J, et al. Impact of acute beta-blocker therapy for patients with non-ST-segment elevation myocardial infarction. Am J Med 2007; 120: 685–692. PMID: 17679127
- 497.
Maclean E, Zheng S, Nabeebaccus A, et al. Effect of early bisoprolol administration on ventricular arrhythmia and cardiac death in patients with non-ST elevation myocardial infarction. Heart Asia 2015; 7: 46–51. PMID: 27326220
- 498.
Chen ZM, Pan HC, Chen YP, et al. COMMIT (ClOpidogrel and Metoprolol in Myocardial Infarction Trial) collaborative group. Early intravenous then oral metoprolol in 45,852 patients with acute myocardial infarction: Randomised placebo-controlled trial. Lancet 2005; 366: 1622–1632. PMID: 16271643
- 499.
Ibanez B, Macaya C, Sánchez-Brunete V, et al. Effect of early metoprolol on infarct size in ST-segment-elevation myocardial infarction patients undergoing primary percutaneous coronary intervention: The Effect of Metoprolol in Cardioprotection During an Acute Myocardial Infarction (METOCARD-CNIC) trial. Circulation 2013; 128: 1495–1503. PMID: 24002794
- 500.
Roolvink V, Ibáñez B, Ottervanger JP, et al. EARLY-BAMI Investigators. Early intravenous beta-blockers in patients with ST-segment elevation myocardial infarction before primary percutaneous coronary intervention. J Am Coll Cardiol 2016; 67: 2705–2715. PMID: 27050189
- 501.
Dargie HJ. Effect of carvedilol on outcome after myocardial infarction in patients with left-ventricular dysfunction: The CAPRICORN randomised trial. Lancet 2001; 357: 1385–1390. PMID: 11356434
- 502.
CIBIS-II Investigators and Committees. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): A randomised trial. Lancet 1999; 353: 9–13. PMID: 10023943
- 503.
Packer M, Coats AJ, Fowler MB, et al. Carvedilol Prospective Randomized Cumulative Survival Study Group. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med 2001; 344: 1651–1658. PMID: 11386263
- 504.
Bangalore S, Makani H, Radford M, et al. Clinical outcomes with β-blockers for myocardial infarction: A meta-analysis of randomized trials. Am J Med 2014; 127: 939–953. PMID: 24927909
- 505.
Huang BT, Huang FY, Zuo ZL, et al. Meta-analysis of relation between oral β-blocker therapy and outcomes in patients with acute myocardial infarction who underwent percutaneous coronary intervention. Am J Cardiol 2015; 115: 1529–1538. PMID: 25862157
- 506.
Puymirat E, Riant E, Aissaoui N, et al. β blockers and mortality after myocardial infarction in patients without heart failure: Multicentre prospective cohort study. BMJ 2016; 354: i4801. PMID: 27650822
- 507.
ACE Inhibitor Myocardial Infarction Collaborative Group. Indications for ACE inhibitors in the early treatment of acute myocardial infarction: Systematic overview of individual data from 100,000 patients in randomized trials. Circulation 1998; 97: 2202–2212. PMID: 9631869
- 508.
Pfeffer MA, McMurray JJ, Velazquez EJ, et al. Valsartan in Acute Myocardial Infarction Trial Investigators. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med 2003; 349: 1893–1906. PMID: 14610160
- 509.
Montalescot G, Pitt B, Lopez de Sa E, et al. REMINDER Investigators. Early eplerenone treatment in patients with acute ST-elevation myocardial infarction without heart failure: The Randomized Double-Blind Reminder Study. Eur Heart J 2014; 35: 2295–2302. PMID: 24780614
- 510.
Beygui F, Cayla G, Roule V, et al. ALBATROSS Investigators. Early aldosterone blockade in acute myocardial infarction: The ALBATROSS randomized clinical trial. J Am Coll Cardiol 2016; 67: 1917–1927. PMID: 27102506
- 511.
Dickstein K, Kjekshus J. OPTIMAAL Steering Committee of the OPTIMAAL Study Group. Effects of losartan and captopril on mortality and morbidity in high-risk patients after acute myocardial infarction: The OPTIMAAL randomised trial. Optimal Trial in Myocardial Infarction with Angiotensin II Antagonist Losartan. Lancet 2002; 360: 752–760. PMID: 12241832
- 512.
Pitt B, Remme W, Zannad F, et al. Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 2003; 348: 1309–1321. PMID: 12668699
- 513.
Fox KM. EURopean trial On reduction of cardiac events with Perindopril in stable coronary Artery disease Investigators. Efficacy of perindopril in reduction of cardiovascular events among patients with stable coronary artery disease: Randomised, double-blind, placebo-controlled, multicentre trial (the EUROPA study). Lancet 2003; 362: 782–788. PMID: 13678872
- 514.
Yusuf S, Sleight P, Pogue J, et al. Heart Outcomes Prevention Evaluation Study Investigators. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N Engl J Med 2000; 342: 145–153. PMID: 10639539
- 515.
Bach RG, Cannon CP, Weintraub WS, et al. The effect of routine, early invasive management on outcome for elderly patients with non-ST-segment elevation acute coronary syndromes. Ann Intern Med 2004; 141: 186–195. PMID: 15289215
- 516.
Tinetti ME, Bogardus ST Jr, Agostini JV. Potential pitfalls of disease-specific guidelines for patients with multiple conditions. N Engl J Med 2004; 351: 2870–2874. PMID: 15625341
- 517.
Webb DJ, Freestone S, Allen MJ, et al. Sildenafil citrate and blood-pressure-lowering drugs: Results of drug interaction studies with an organic nitrate and a calcium antagonist. Am J Cardiol 1999; 83 Suppl: 21C–28C. PMID: 10078539
- 518.
Held PH, Yusuf S, Furberg CD. Calcium channel blockers in acute myocardial infarction and unstable angina: An overview. BMJ 1989; 299: 1187–1192. PMID: 2513047
- 519.
Pristipino C, Beltrame JF, Finocchiaro ML, et al. Major racial differences in coronary constrictor response between japanese and caucasians with recent myocardial infarction. Circulation 2000; 101: 1102–1108. PMID: 10715255
- 520.
Japanese beta-Blockers and Calcium Antagonists Myocardial Infarction (JBCMI) Investigators. Comparison of the effects of beta blockers and calcium antagonists on cardiovascular events after acute myocardial infarction in Japanese subjects. Am J Cardiol 2004; 93: 969–973. PMID: 15081437
- 521.
Nakagomi A, Kodani E, Takano H, et al. Secondary preventive effects of a calcium antagonist for ischemic heart attack: Randomized parallel comparison with β-blockers. Circ J 2011; 75: 1696–1705. PMID: 21576828
- 522.
Slavich M, Patel RS. Coronary artery spasm: Current knowledge and residual uncertainties. Int J Cardiol Heart Vasc 2016; 10: 47–53. PMID: 28616515
- 523.
Furberg CD, Psaty BM, Meyer JV. Nifedipine. Dose-related increase in mortality in patients with coronary heart disease. Circulation 1995; 92: 1326–1331. PMID: 7648682
- 524.
Goldstein RE, Boccuzzi SJ, Cruess D, et al. The Adverse Experience Committee; and the Multicenter Diltiazem Postinfarction Research Group. Diltiazem increases late-onset congestive heart failure in postinfarction patients with early reduction in ejection fraction. Circulation 1991; 83: 52–60. PMID: 1984898
- 525.
Zeltser D, Justo D, Halkin A, et al. Drug-induced atrioventricular block: Prognosis after discontinuation of the culprit drug. J Am Coll Cardiol 2004; 44: 105–108. PMID: 15234417
- 526.
Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med 1998; 339: 1349–1357. PMID: 9841303
- 527.
Sacks FM, Pfeffer MA, Moye LA, et al. Cholesterol and Recurrent Events Trial investigators. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med 1996; 335: 1001–1009. PMID: 8801446
- 528.
Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: The Scandinavian Simvastatin Survival Study (4S). Lancet 1994; 344: 1383–1389. PMID: 7968073
- 529.
Nissen SE, Nicholls SJ, Sipahi I, et al. ASTEROID Investigators. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: The ASTEROID trial. JAMA 2006; 295: 1556–1565. PMID: 16533939
- 530.
Hiro T, Kimura T, Morimoto T, et al. JAPAN-ACS Investigators. Effect of intensive statin therapy on regression of coronary atherosclerosis in patients with acute coronary syndrome: A multicenter randomized trial evaluated by volumetric intravascular ultrasound using pitavastatin versus atorvastatin (JAPAN-ACS [Japan assessment of pitavastatin and atorvastatin in acute coronary syndrome] study). J Am Coll Cardiol 2009; 54: 293–302. PMID: 19608026
- 531.
Okazaki S, Yokoyama T, Miyauchi K, et al. Early statin treatment in patients with acute coronary syndrome: Demonstration of the beneficial effect on atherosclerotic lesions by serial volumetric intravascular ultrasound analysis during half a year after coronary event: The ESTABLISH Study. Circulation 2004; 110: 1061–1068. PMID: 15326073
- 532.
Dohi T, Miyauchi K, Okazaki S, et al. Early intensive statin treatment for six months improves long-term clinical outcomes in patients with acute coronary syndrome (Extended-ESTABLISH trial): A follow-up study. Atherosclerosis 2010; 210: 497–502. PMID: 20036363
- 533.
Sato H, Kinjo K, Ito H, et al. Osaka Acute Coronary Insufficiency Study (OACIS)-LIPID Study Investigators. Effect of early use of low-dose pravastatin on major adverse cardiac events in patients with acute myocardial infarction: The OACIS-LIPID Study. Circ J 2008; 72: 17–22. PMID: 18159093
- 534.
Sakamoto T, Kojima S, Ogawa H, et al. Multicenter Study for Aggressive Lipid-Lowering Strategy by HMG-CoA Reductase Inhibitors in Patients With Acute Myocardial Infarction Investigators. Effects of early statin treatment on symptomatic heart failure and ischemic events after acute myocardial infarction in Japanese. Am J Cardiol 2006; 97: 1165–1171. PMID: 16616020
- 535.
de Lemos JA, Blazing MA, Wiviott SD, et al. A to Z Investigators. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: Phase Z of the A to Z trial. JAMA 2004; 292: 1307–1316. PMID: 15337732
- 536.
Cannon CP, Braunwald E, McCabe CH, et al. Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004; 350: 1495–1504. PMID: 15007110
- 537.
Schwartz GG, Olsson AG, Ezekowitz MD, et al. Myocardial Ischemia Reduction with Aggressive Cholesterol Lowering (MIRACL) Study Investigators. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: The MIRACL study: A randomized controlled trial. JAMA 2001; 285: 1711–1718. PMID: 11277825
- 538.
Lee KH, Jeong MH, Kim HM, et al. KAMIR (Korea Acute Myocardial Infarction Registry) Investigators. Benefit of early statin therapy in patients with acute myocardial infarction who have extremely low low-density lipoprotein cholesterol. J Am Coll Cardiol 2011; 58: 1664–1671. PMID: 21982310
- 539.
Gencer B, Nanchen D. Identifying familial hypercholesterolemia in acute coronary syndrome. Curr Opin Lipidol 2016; 27: 375–381. PMID: 27092769
- 540.
Ohmura H, Fukushima Y, Mizuno A, et al. Research Committee on Primary Hyperlipidemia of the Ministry of Health and Welfare of Japan. Estimated prevalence of heterozygous familial hypercholesterolemia in patients with acute coronary syndrome. Int Heart J 2017; 58: 88–94. PMID: 28123161
- 541.
Rosenson RS. Myocardial injury: The acute phase response and lipoprotein metabolism. J Am Coll Cardiol 1993; 22: 933–940. PMID: 7689082
- 542.
Tsujita K, Sugiyama S, Sumida H, et al. PRECISE–IVUS Investigators. Impact of dual lipid-lowering strategy with ezetimibe and atorvastatin on coronary plaque regression in patients with percutaneous coronary intervention: The multicenter randomized controlled PRECISE-IVUS trial. J Am Coll Cardiol 2015; 66: 495–507. PMID: 26227186
- 543.
Japan Atherosclerosis Society. Guidelines for the management of familial hypercholesterolemia 2017. [Article in Japanese] http://www.j-athero.org/publications/pdf/JAS_FH_GL2017.pdf
- 544.
Scheidt S, Wilner G, Mueller H, et al. Intra-aortic balloon counterpulsation in cardiogenic shock. Report of a co-operative clinical trial. N Engl J Med 1973; 288: 979–984. PMID: 4696253
- 545.
De Silva K, Lumley M, Kailey B, et al. Coronary and microvascular physiology during intra-aortic balloon counterpulsation. JACC Cardiovasc Interv 2014; 7: 631–640. PMID: 24726295
- 546.
Sjauw KD, Engström AE, Vis MM, et al. A systematic review and meta-analysis of intra-aortic balloon pump therapy in ST-elevation myocardial infarction: Should we change the guidelines? Eur Heart J 2009; 30: 459–468. PMID: 19168529
- 547.
Patel MR, Smalling RW, Thiele H, et al. Intra-aortic balloon counterpulsation and infarct size in patients with acute anterior myocardial infarction without shock: The CRISP AMI randomized trial. JAMA 2011; 306: 1329–1337. PMID: 21878431
- 548.
Thiele H, Zeymer U, Neumann FJ, et al. IABP-SHOCK II Trial Investigators. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med 2012; 367: 1287–1296. PMID: 22920912
- 549.
Ahmad Y, Sen S, Shun-Shin MJ, et al. Intra-aortic balloon pump therapy for acute myocardial infarction: A Meta-analysis. JAMA Intern Med 2015; 175: 931–939. PMID: 25822657
- 550.
van Nunen LX, van’t Veer M, Schampaert S, et al. Intra-aortic balloon counterpulsation reduces mortality in large anterior myocardial infarction complicated by persistent ischaemia: A CRISP-AMI substudy. EuroIntervention 2015; 11: 286–292. PMID: 25254356
- 551.
Inohara T, Miyata H, Ueda I, et al. Use of intra-aortic balloon pump in a Japanese multicenter percutaneous coronary intervention registry. JAMA Intern Med 2015; 175: 1980–1982. PMID: 26523549
- 552.
Sandhu A, McCoy LA, Negi SI, et al. Use of mechanical circulatory support in patients undergoing percutaneous coronary intervention: Insights from the National Cardiovascular Data Registry. Circulation 2015; 132: 1243–1251. PMID: 26286905
- 553.
Basir MB, Schreiber T, Dixon S, et al. Feasibility of early mechanical circulatory support in acute myocardial infarction complicated by cardiogenic shock: The Detroit cardiogenic shock initiative. Catheter Cardiovasc Interv 2018; 91: 454–461. PMID: 29266676
- 554.
Thiele H, Jobs A, Ouweneel DM, et al. Percutaneous short-term active mechanical support devices in cardiogenic shock: A systematic review and collaborative meta-analysis of randomized trials. Eur Heart J 2017; 38: 3523–3531. PMID: 29020341
- 555.
Ouweneel DM, Eriksen E, Sjauw KD, et al. Percutaneous mechanical circulatory support versus intra-aortic balloon pump in cardiogenic shock after acute myocardial infarction. J Am Coll Cardiol 2017; 69: 278–287. PMID: 27810347
- 556.
Shah M, Patnaik S, Patel B, et al. Trends in mechanical circulatory support use and hospital mortality among patients with acute myocardial infarction and non-infarction related cardiogenic shock in the United States. Clin Res Cardiol 2018; 107: 287–303. PMID: 29134345
- 557.
Japanese society of Percutaneous Cardio-Pulmonary Support and Extracorporeal Membrane Oxygenation. Aggregated results of PCPS study group questionnaire. [Article in Japanese] http://www2.convention.co.jp/pcps/pdf/enquete.pdf
- 558.
Yonezu K, Sakakura K, Watanabe Y, et al. Determinants of survival and favorable neurologic outcomes in ischemic heart disease treated by veno-arterial extracorporeal membrane oxygenation. Heart Vessels 2018; 33: 25–32. PMID: 28776067
- 559.
Aiba T, Nonogi H, Itoh T, et al. Appropriate indications for the use of a percutaneous cardiopulmonary support system in cases with cardiogenic shock complicating acute myocardial infarction. Jpn Circ J 2001; 65: 145–149. PMID: 11266185
- 560.
Garan AR, Eckhardt C, Takeda K, et al. Predictors of survival and ability to wean from short-term mechanical circulatory support device following acute myocardial infarction complicated by cardiogenic shock. Eur Heart J Acute Cardiovasc Care 2018; 7: 755–765. PMID: 29094607
- 561.
Mitsui N, Koyama T, Marui A, et al. Experience with emergency cardiac surgery following institution of percutaneous cardiopulmonary support. Artif Organs 1999; 23: 496–499. PMID: 10392272
- 562.
Okada H, Nishida M, Murakami M, et al. Surgical treatment for complications of acute myocardial infarction. Jpn J Thorac Cardiovasc Surg 2005; 53: 74–77. PMID: 15782567
- 563.
Sakamoto T, Morimura N, Nagao K, et al. SAVE-J Study Group. Extracorporeal cardiopulmonary resuscitation versus conventional cardiopulmonary resuscitation in adults with out-of-hospital cardiac arrest: A prospective observational study. Resuscitation 2014; 85: 762–768. PMID: 24530251
- 564.
Kagawa E, Dote K, Kato M, et al. Should we emergently revascularize occluded coronaries for cardiac arrest?: Rapid-response extracorporeal membrane oxygenation and intra-arrest percutaneous coronary intervention. Circulation 2012; 126: 1605–1613. PMID: 22899771
- 565.
Kuroki N, Abe D, Iwama T, et al. Association between delay to coronary reperfusion and outcome in patients with acute coronary syndrome undergoing extracorporeal cardiopulmonary resuscitation. Resuscitation 2017; 114: 1–6. PMID: 28215592
- 566.
Japan Resuscitation Council. Chapter 2, Adult Advanced Life Support (ALS). [Article in Japanese] In: JRC Resuscitation Guidelines 2015, Online Version. 2015: 117.
- 567.
Morisawa D, Higuchi Y, Iwakura K, et al. Predictive factors for successful weaning from percutaneous cardiopulmonary support in patients with cardiogenic shock complicating acute myocardial infarction. J Cardiol 2012; 60: 350–354. PMID: 22819038
- 568.
Oshima K, Morishita Y, Hinohara H, et al. Factors for weaning from a percutaneous cardiopulmonary support system (PCPS) in patients with severe cardiac failure: A comparative study in weaned and nonweaned patients. Int Heart J 2006; 47: 575–584. PMID: 16960412
- 569.
Yamauchi T, Masai T, Takeda K, et al. Percutaneous cardiopulmonary support after acute myocardial infarction at the left main trunk. Ann Thorac Cardiovasc Surg 2009; 15: 93–97. PMID: 19471222
- 570.
Japanese Circulation Society and Japanese Society for Cardiovascular Surgery Joint Guidelines. Cardiovascular disease guidelines series 2013 edition: Guidelines for device therapy: Implantable left ventricular assist device for patients with severe heart failure 2013. [Article in Japanese]
- 571.
Priori SG, Blomström-Lundqvist C, Mazzanti A, et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J 2015; 36: 2793–2867. PMID: 26320108
- 572.
Piccini JP, Schulte PJ, Pieper KS, et al. Antiarrhythmic drug therapy for sustained ventricular arrhythmias complicating acute myocardial infarction. Crit Care Med 2011; 39: 78–83. PMID: 20959785
- 573.
Gheeraert PJ, De Buyzere ML, Taeymans YM, et al. Risk factors for primary ventricular fibrillation during acute myocardial infarction: A systematic review and meta-analysis. Eur Heart J 2006; 27: 2499–2510. PMID: 16952926
- 574.
Liang JJ, Fender EA, Cha YM, et al. Long-term outcomes in survivors of early ventricular arrhythmias after acute ST-elevation and non-ST-elevation myocardial infarction treated with percutaneous coronary intervention. Am J Cardiol 2016; 117: 709–713. PMID: 26796195
- 575.
Masuda M, Nakatani D, Hikoso S, et al. OACIS investigators. Clinical impact of ventricular tachycardia and/or fibrillation during the acute phase of acute myocardial infarction on in-hospital and 5-year mortality rates in the percutaneous coronary intervention era. Circ J 2016; 80: 1539–1547. PMID: 27238618
- 576.
Hjalmarson A. Effects of beta blockade on sudden cardiac death during acute myocardial infarction and the postinfarction period. Am J Cardiol 1997; 80: 35J–39J. PMID: 9375948
- 577.
Nordrehaug JE, Johannessen KA, von der Lippe G. Serum potassium concentration as a risk factor of ventricular arrhythmias early in acute myocardial infarction. Circulation 1985; 71: 645–649. PMID: 3971535
- 578.
Dumas F, Cariou A, Manzo-Silberman S, et al. Immediate percutaneous coronary intervention is associated with better survival after out-of-hospital cardiac arrest: Insights from the PROCAT (Parisian Region Out of hospital Cardiac ArresT) registry. Circ Cardiovasc Interv 2010; 3: 200–207. PMID: 20484098
- 579.
Dorian P, Cass D, Schwartz B, et al. Amiodarone as compared with lidocaine for shock-resistant ventricular fibrillation. N Engl J Med 2002; 346: 884–890. PMID: 11907287
- 580.
Kudenchuk PJ, Cobb LA, Copass MK, et al. Amiodarone for resuscitation after out-of-hospital cardiac arrest due to ventricular fibrillation. N Engl J Med 1999; 341: 871–878. PMID: 10486418
- 581.
Ando J, Kakishita M, Sakai K, et al. Efficacy of nifekalant hydrochloride in the treatment of fatal ventricular arrhythmia in patients with ischemic heart disease. Int Heart J 2005; 46: 647–656. PMID: 16157956
- 582.
Katoh T, Mitamura H, Matsuda N, et al. Emergency treatment with nifekalant, a novel class III anti-arrhythmic agent, for life-threatening refractory ventricular tachyarrhythmias: Post-marketing special investigation. Circ J 2005; 69: 1237–1243. PMID: 16195624
- 583.
Shiga T, Tanaka K, Kato R, et al. Refractory VT/VF, Prospective Evaluation to Differentiate Lidocaine Efficacy from Nifekalant (RELIEF) Study Investigators. Nifekalant versus lidocaine for in-hospital shock-resistant ventricular fibrillation or tachycardia. Resuscitation 2010; 81: 47–52. PMID: 19913983
- 584.
Tahara Y, Kimura K, Kosuge M, et al. Comparison of nifekalant and lidocaine for the treatment of shock-refractory ventricular fibrillation. Circ J 2006; 70: 442–446. PMID: 16565562
- 585.
Amino M, Inokuchi S, Nagao K, et al. SOS-KANTO 2012 Study Group. Nifekalant hydrochloride and amiodarone hydrochloride result in similar improvements for 24-hour survival in cardiopulmonary arrest patients: The SOS-KANTO 2012 Study. J Cardiovasc Pharmacol 2015; 66: 600–609. PMID: 26317166
- 586.
Nademanee K, Taylor R, Bailey WE, et al. Treating electrical storm: Sympathetic blockade versus advanced cardiac life support-guided therapy. Circulation 2000; 102: 742–747. PMID: 10942741
- 587.
Miwa Y, Ikeda T, Mera H, et al. Effects of landiolol, an ultra-short-acting beta1-selective blocker, on electrical storm refractory to class III antiarrhythmic drugs. Circ J 2010; 74: 856–863. PMID: 20339194
- 588.
Burjorjee JE, Milne B. Propofol for electrical storm: A case report of cardioversion and suppression of ventricular tachycardia by propofol. Can J Anaesth 2002; 49: 973–977. PMID: 12419728
- 589.
Kurisu S, Inoue I, Kawagoe T, et al. Temporary overdriving pacing as an adjunct to antiarrhythmic drug therapy for electrical storm in acute myocardial infarction. Circ J 2005; 69: 613–616. PMID: 15849451
- 590.
Bänsch D, Oyang F, Antz M, et al. Successful catheter ablation of electrical storm after myocardial infarction. Circulation 2003; 108: 3011–3016. PMID: 14662718
- 591.
Hayashi M, Kobayashi Y, Iwasaki YK, et al. Novel mechanism of postinfarction ventricular tachycardia originating in surviving left posterior Purkinje fibers. Heart Rhythm 2006; 3: 908–918. PMID: 16876739
- 592.
Wever EF, Hauer RN, van Capelle FL, et al. Randomized study of implantable defibrillator as first-choice therapy versus conventional strategy in postinfarct sudden death survivors. Circulation 1995; 91: 2195–2203. PMID: 7697849
- 593.
Siebels J, Kuck KH. Implantable cardioverter defibrillator compared with antiarrhythmic drug treatment in cardiac arrest survivors (the Cardiac Arrest Study Hamburg). Am Heart J 1994; 127: 1139–1144. PMID: 8160593
- 594.
Connolly SJ, Hallstrom AP, Cappato R, et al. Meta-analysis of the implantable cardioverter defibrillator secondary prevention trials. AVID, CASH and CIDS studies. Antiarrhythmics vs Implantable Defibrillator study. Cardiac Arrest Study Hamburg. Canadian Implantable Defibrillator Study. Eur Heart J 2000; 21: 2071–2078. PMID: 11102258
- 595.
Japanese Heart Rhythm Society. Statement on the clinical use of Wearable Cardioverter Defibrillators (WCD). [Article in Japanese] http://new.jhrs.or.jp/pdf/guideline/statement201709_02.pdf
- 596.
Zafari AM, Zarter SK, Heggen V, et al. A program encouraging early defibrillation results in improved in-hospital resuscitation efficacy. J Am Coll Cardiol 2004; 44: 846–852. PMID: 15312869
- 597.
Gorenek B, Blomström Lundqvist C, Brugada Terradellas J, et al. Cardiac arrhythmias in acute coronary syndromes: Position paper from the joint EHRA, ACCA, and EAPCI task force. Europace 2014; 16: 1655–1673. PMID: 25172845
- 598.
Hine LK, Laird N, Hewitt P, et al. Meta-analytic evidence against prophylactic use of lidocaine in acute myocardial infarction. Arch Intern Med 1989; 149: 2694–2698. PMID: 2688587
- 599.
Huikuri HV, Castellanos A, Myerburg RJ. Sudden death due to cardiac arrhythmias. N Engl J Med 2001; 345: 1473–1482. PMID: 11794197
- 600.
Echt DS, Liebson PR, Mitchell LB, et al. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N Engl J Med 1991; 324: 781–788. PMID: 1900101
- 601.
Crijns HJ, Weijs B, Fairley AM, et al. Contemporary real life cardioversion of atrial fibrillation: Results from the multinational RHYTHM-AF study. Int J Cardiol 2014; 172: 588–594. PMID: 24556445
- 602.
Coll-Vinent B, Sala X, Fernández C, et al. Sedation for cardioversion in the emergency department: Analysis of effectiveness in four protocols. Ann Emerg Med 2003; 42: 767–772. PMID: 14634601
- 603.
del Arco C, Martín A, Laguna P, et al. Investigators in the Spanish Atrial Fibrillation in Emergency Medicine Study Group (GEFAUR). Analysis of current management of atrial fibrillation in the acute setting: GEFAUR-1 study. Ann Emerg Med 2005; 46: 424–430. PMID: 16271674
- 604.
Scheuermeyer FX, Grafstein E, Heilbron B, et al. Emergency department management and 1-year outcomes of patients with atrial flutter. Ann Emerg Med 2011; 57: 564–571.e2. PMID: 21257230
- 605.
You JJ, Singer DE, Howard PA, et al. Antithrombotic therapy for atrial fibrillation: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141 Suppl: e531S–e575S. PMID: 22315271
- 606.
Nikolaidou T, Channer KS. Chronic atrial fibrillation: A systematic review of medical heart rate control management. Postgrad Med J 2009; 85: 303–312. PMID: 19528305
- 607.
Kirchhof P, Benussi S, Kotecha D, et al. ESC Scientific Document Group. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016; 37: 2893–2962. PMID: 27567408
- 608.
Hanada K, Higuma T, Nishizaki F, et al. Randomized study on the efficacy and safety of landiolol, an ultra-short-acting β1-adrenergic blocker, in patients with acute myocardial infarction undergoing primary percutaneous coronary intervention. Circ J 2012; 76: 439–445. PMID: 22156314
- 609.
Kiyokuni M, Konishi M, Sakamaki K, et al. Beneficial effect of early infusion of landiolol, a very short-acting beta-1 adrenergic receptor blocker, on reperfusion status in acute myocardial infarction. Int J Cardiol 2016; 221: 321–326. PMID: 27404699
- 610.
Moss AJ, Oakes D, Benhorin J, et al. Multicenter Diltiazem Post-Infarction Research Group. The interaction between diltiazem and left ventricular function after myocardial infarction. Circulation 1989; 80 Suppl: IV102–IV106. PMID: 2688978
- 611.
Camm AJ, Capucci A, Hohnloser SH, et al. AVRO Investigators. A randomized active-controlled study comparing the efficacy and safety of vernakalant to amiodarone in recent-onset atrial fibrillation. J Am Coll Cardiol 2011; 57: 313–321. PMID: 21232669
- 612.
Deedwania PC, Singh BN, Ellenbogen K, et al. The Department of Veterans Affairs CHF-STAT Investigators. Spontaneous conversion and maintenance of sinus rhythm by amiodarone in patients with heart failure and atrial fibrillation: Observations from the veterans affairs congestive heart failure survival trial of antiarrhythmic therapy (CHF-STAT). Circulation 1998; 98: 2574–2579. PMID: 9843465
- 613.
Clemo HF, Wood MA, Gilligan DM, et al. Intravenous amiodarone for acute heart rate control in the critically ill patient with atrial tachyarrhythmias. Am J Cardiol 1998; 81: 594–598. PMID: 9514456
- 614.
January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014; 64: e1–e76. PMID: 24685669
- 615.
Ziff OJ, Lane DA, Samra M, et al. Safety and efficacy of digoxin: Systematic review and meta-analysis of observational and controlled trial data. BMJ 2015; 351: h4451. PMID: 26321114
- 616.
The Cardiac Arrhythmia Suppression Trial (CAST) Investigators. Preliminary report: Effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. N Engl J Med 1989; 321: 406–412. PMID: 2473403
- 617.
Page RL, Joglar JA, Caldwell MA, et al. 2015 ACC/AHA/HRS Guideline for the Management of Adult Patients With Supraventricular Tachycardia: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2016; 67: e27–e115. PMID: 26409259
- 618.
Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 130: e344–e426. PMID: 25249585
- 619.
Rotman M, Wagner GS, Wallace AG. Bradyarrhythmias in acute myocardial infarction. Circulation 1972; 45: 703–722. PMID: 4551931
- 620.
Kimura K, Kosuge M, Ishikawa T, et al. Comparison of results of early reperfusion in patients with inferior wall acute myocardial infarction with and without complete atrioventricular block. Am J Cardiol 1999; 84: 731–733. PMID: 10498146
- 621.
Barthell E, Troiano P, Olson D, et al. Prehospital external cardiac pacing: A prospective, controlled clinical trial. Ann Emerg Med 1988; 17: 1221–1226. PMID: 3056132
- 622.
Cummins RO, Graves JR, Larsen MP, et al. Out-of-hospital transcutaneous pacing by emergency medical technicians in patients with asystolic cardiac arrest. N Engl J Med 1993; 328: 1377–1382. PMID: 8474514
- 623.
White JD, Brown CG. Immediate transthoracic pacing for cardiac asystole in an emergency department setting. Am J Emerg Med 1985; 3: 125–128. PMID: 3882099
- 624.
Hindman MC, Wagner GS, JaRo M, et al. The clinical significance of bundle branch block complicating acute myocardial infarction. 2. Indications for temporary and permanent pacemaker insertion. Circulation 1978; 58: 689–699. PMID: 688580
- 625.
JCS Joint Working Group. Guidelines for non-pharmacotherapy of cardiac arrhythmias (JCS 2018). [Article in Japanese]
- 626.
Japanese Circulation Society and Japanese Heart Failure Society Joint Guidelines. Guidelines for diagnosis and treatment of acute and chronic heart failure (JCS 2017/JHFS 2017). [Article in Japanese] http://www.j-circ.or.jp/guideline/pdf/JCS2017_tsutsui_h.pdf
- 627.
Page DL, Caulfield JB, Kastor JA, et al. Myocardial changes associated with cardiogenic shock. N Engl J Med 1971; 285: 133–137. PMID: 5087702
- 628.
Werns SW, Bates ER. The enduring value of Killip classification. Am Heart J 1999; 137: 213–215. PMID: 9924153
- 629.
Shiba N, Watanabe J, Shinozaki T, et al. Poor prognosis of Japanese patients with chronic heart failure following myocardial infarction: Comparison with nonischemic cardiomyopathy. Circ J 2005; 69: 143–149. PMID: 15671603
- 630.
Kajimoto K, Minami Y, Sato N, et al. Investigators of the Acute Decompensated Heart Failure Syndromes (ATTEND) Registry. Etiology of heart failure and outcomes in patients hospitalized for acute decompensated heart failure with preserved or reduced ejection fraction. Am J Cardiol 2016; 118: 1881–1887. PMID: 27720439
- 631.
Ushigome R, Sakata Y, Nochioka K, et al. CHART-2 Investigators. Temporal trends in clinical characteristics, management and prognosis of patients with symptomatic heart failure in Japan: Report from the CHART Studies. Circ J 2015; 79: 2396–2407. PMID: 26356834
- 632.
Miura T, Matsuzaki M. Evaluation of heart function and the severity of heart failure at the bedside. [Article in Japanese] J Jpn Soc Intern Med 1994; 83: 31–36.
- 633.
Forrester JS, Diamond GA, Swan HJ. Correlative classification of clinical and hemodynamic function after acute myocardial infarction. Am J Cardiol 1977; 39: 137–145. PMID: 835473
- 634.
Nohria A, Tsang SW, Fang JC, et al. Clinical assessment identifies hemodynamic profiles that predict outcomes in patients admitted with heart failure. J Am Coll Cardiol 2003; 41: 1797–1804. PMID: 12767667
- 635.
Mueller HS, Chatterjee K, Davis KB, et al. ACC expert consensus document. Present use of bedside right heart catheterization in patients with cardiac disease: American College of Cardiology. J Am Coll Cardiol 1998; 32: 840–864. PMID: 9741535
- 636.
Shah MR, Hasselblad V, Stevenson LW, et al. Impact of the pulmonary artery catheter in critically ill patients: Meta-analysis of randomized clinical trials. JAMA 2005; 294: 1664–1670. PMID: 16204666
- 637.
O’Grady NP, Alexander M, Dellinger EP, et al. Guidelines for the prevention of intravascular catheter-related infections: Centers for disease control and prevention. MMWR Recomm Rep 2002; 51: 1–29. PMID: 12233868
- 638.
Hochman JS, Jaber WA, Bates ER, et al. Angioplasty versus thrombolytics for patients presenting with congestive heart failure: GUSTO IIb substudy findings. J Am Coll Cardiol 1998; 31 Suppl: 210–211.
- 639.
DeGeare VS, Boura JA, Grines LL, et al. Predictive value of the Killip classification in patients undergoing primary percutaneous coronary intervention for acute myocardial infarction. Am J Cardiol 2001; 87: 1035–1038. PMID: 11348598
- 640.
Bersten AD, Holt AW, Vedig AE, et al. Treatment of severe cardiogenic pulmonary edema with continuous positive airway pressure delivered by face mask. N Engl J Med 1991; 325: 1825–1830. PMID: 1961221
- 641.
Takeda S, Nejima J, Takano T, et al. Effect of nasal continuous positive airway pressure on pulmonary edema complicating acute myocardial infarction. Jpn Circ J 1998; 62: 553–558. PMID: 9741730
- 642.
Masip J, Betbesé AJ, Páez J, et al. Non-invasive pressure support ventilation versus conventional oxygen therapy in acute cardiogenic pulmonary oedema: A randomised trial. Lancet 2000; 356: 2126–2132. PMID: 11191538
- 643.
Vital FM, Saconato H, Ladeira MT, et al. Non-invasive positive pressure ventilation (CPAP or bilevel NPPV) for cardiogenic pulmonary edema. Cochrane Database Syst Rev 2008: CD005351. PMID: 18646124 PMID: 18646124
- 644.
Tanaka K. Treatment of acute heart failure: Practical management of respiration. Shinzo 2011; 43: 432–435.
- 645.
Guidelines 2000 for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Part 7: The era of reperfusion: Section 1: Acute coronary syndromes (acute myocardial infarction). The American Heart Association in collaboration with the International Liaison Committee on Resuscitation. Circulation 2000; 102 Suppl: I172–I203. PMID: 10966673
- 646.
Zelis R, Kinney EL, Flaim SF, et al. Morphine: Its use in pulmonary edema. Cardiovasc Rev Rep 1981; 2: 257–267.
- 647.
Semba H, Kinugawa K, Takeda N, et al. Preliminary report of tolvaptan treatment in Japanese patients with heart failure. Int Heart J 2012; 53: 72–74. PMID: 22398679
- 648.
Kikuchi M, Nakamura M, Suzuki T, et al. Usefulness of carperitide for the treatment of refractory heart failure due to severe acute myocardial infarction. Jpn Heart J 2001; 42: 271–280. PMID: 11605765
- 649.
Yokoyama H. Effect of atrial natriuretic peptide on acute heart failure with renal impairment. [Article in Japanese] Shinzo 2004; 36: 61–65.
- 650.
Asakura M, Jiyoong K, Minamino T, et al. J-WIND Investigators. Rationale and design of a large-scale trial using atrial natriuretic peptide (ANP) as an adjunct to percutaneous coronary intervention for ST-segment elevation acute myocardial infarction: Japan-Working groups of acute myocardial infarction for the reduction of Necrotic Damage by ANP (J-WIND-ANP). Circ J 2004; 68: 95–100. PMID: 14745141
- 651.
Menon V, Slater JN, White HD, et al. Acute myocardial infarction complicated by systemic hypoperfusion without hypotension: Report of the SHOCK trial registry. Am J Med 2000; 108: 374–380. PMID: 10759093
- 652.
Hochman JS, Buller CE, Sleeper LA, et al. Cardiogenic shock complicating acute myocardial infarction--etiologies, management and outcome: A report from the SHOCK Trial Registry. SHould we emergently revascularize Occluded Coronaries for cardiogenic shocK? J Am Coll Cardiol 2000; 36: 1063–1070. PMID: 10985706
- 653.
Webb JG, Sleeper LA, Buller CE, et al. Implications of the timing of onset of cardiogenic shock after acute myocardial infarction: A report from the SHOCK Trial Registry. SHould we emergently
revascularize Occluded Coronaries for cardiogenic shocK? J Am Coll Cardiol 2000; 36: 1084–1090. PMID: 10985709
- 654.
Chen EW, Canto JG, Parsons LS, et al. Investigators in the National Registry of Myocardial Infarction (NRMI) 2. Relation between hospital intra-aortic balloon counterpulsation volume and mortality in acute myocardial infarction complicated by cardiogenic shock. Circulation 2003; 108: 951–957. PMID: 12912817
- 655.
Hausmann H, Potapov EV, Koster A, et al. Prognosis after the implantation of an intra-aortic balloon pump in cardiac surgery calculated with a new score. Circulation 2002; 106 Suppl: I203–I206. PMID: 12354734
- 656.
Tobaru T, Sumiyoshi T, Haze K, et al. In-hospital prognosis of cardiogenic shock cases complicated by acute myocardial infarction in the age of recanalization therapy: Multi-center collaborative research. [Article in Japanese] Jpn Circ J 2000; 64 Suppl: 241.
- 657.
Barron HV, Every NR, Parsons LS, et al. Investigators in the National Registry of Myocardial Infarction 2. The use of intra-aortic balloon counterpulsation in patients with cardiogenic shock complicating acute myocardial infarction: Data from the National Registry of Myocardial Infarction 2. Am Heart J 2001; 141: 933–939. PMID: 11376306
- 658.
McAlister FA, Stewart S, Ferrua S, et al. Multidisciplinary strategies for the management of heart failure patients at high risk for admission: A systematic review of randomized trials. J Am Coll Cardiol 2004; 44: 810–819. PMID: 15312864
- 659.
Iakobishvili Z, Cohen E, Garty M, et al. Heart Failure Survey in Isarel (HFSIS) Investigators. Use of intravenous morphine for acute decompensated heart failure in patients with and without acute coronary syndromes. Acute Card Care 2011; 13: 76–80. PMID: 21627393
- 660.
Mebazaa A, Tolppanen H, Mueller C, et al. Acute heart failure and cardiogenic shock: A multidisciplinary practical guidance. Intensive Care Med 2016; 42: 147–163. PMID: 26370690
- 661.
Levy B, Bastien O, Karim B, et al. Experts’ recommendations for the management of adult patients with cardiogenic shock. Ann Intensive Care 2015; 5: 52. PMID: 26152849
- 662.
Bart BA, Goldsmith SR, Lee KL, et al. Heart Failure Clinical Research Network. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med 2012; 367: 2296–2304. PMID: 23131078
- 663.
Costanzo MR, Saltzberg MT, Jessup M, et al. Ultrafiltration Versus Intravenous Diuretics for Patients Hospitalized for Acute Decompensated Heart Failure (UNLOAD) Investigators. Ultrafiltration is associated with fewer rehospitalizations than continuous diuretic infusion in patients with decompensated heart failure: Results from UNLOAD. J Card Fail 2010; 16: 277–284. PMID: 20350693
- 664.
Levy B, Perez P, Perny J, et al. Comparison of norepinephrine-dobutamine to epinephrine for hemodynamics, lactate metabolism, and organ function variables in cardiogenic shock: A prospective, randomized pilot study. Crit Care Med 2011; 39: 450–455. PMID: 21037469
- 665.
Buerke M, Prondzinsky R, Lemm H, et al. Intra-aortic balloon counterpulsation in the treatment of infarction-related cardiogenic shock: Review of the current evidence. Artif Organs 2012; 36: 505–511. PMID: 22607158
- 666.
Thiele H, Zeymer U, Neumann FJ, et al. Intraaortic Balloon Pump in cardiogenic shock II (IABP-SHOCK II) trial investigators. Intra-aortic balloon counterpulsation in acute myocardial infarction complicated by cardiogenic shock (IABP-SHOCK II): Final 12 month results of a randomised, open-label trial. Lancet 2013; 382: 1638–1645. PMID: 24011548
- 667.
Berger PB, Ryan TJ. Inferior myocardial infarction. High-risk subgroups. Circulation 1990; 81: 401–411. PMID: 2404629
- 668.
Goto Y. Right ventricular infarct. [Article in Japanese] In: Hiramori K, Saito M, Haze K, et al. editors. Intensive care treatment of coronary artery disease. Nankodo, 1988: 115–122.
- 669.
Robalino BD, Whitlow PL, Underwood DA, et al. Electrocardiographic manifestations of right ventricular infarction. Am Heart J 1989; 118: 138–144. PMID: 2662727
- 670.
Namana V, Gupta SS, Abbasi AA, et al. Right ventricular infarction. Cardiovasc Revasc Med 2018; 19: 43–50. PMID: 28822687
- 671.
Braat SH, de Zwaan C, Brugada P, et al. Right ventricular involvement with acute inferior wall myocardial infarction identifies high risk of developing atrioventricular nodal conduction disturbances. Am Heart J 1984; 107: 1183–1187. PMID: 6326559
- 672.
Bowers TR, O’Neill WW, Grines C, et al. Effect of reperfusion on biventricular function and survival after right ventricular infarction. N Engl J Med 1998; 338: 933–940. PMID: 9521980
- 673.
Braat SH, Ramentol M, Halders S, et al. Reperfusion with streptokinase of an occluded right coronary artery: Effects on early and late right and left ventricular ejection fraction. Am Heart J 1987; 113: 257–260. PMID: 3544753
- 674.
French JK, Williams BF, Hart HH, et al. Prospective evaluation of eligibility for thrombolytic therapy in acute myocardial infarction. BMJ 1996; 312: 1637–1641. PMID: 8664716
- 675.
French JK, Hellkamp AS, Armstrong PW, et al. Mechanical complications after percutaneous coronary intervention in ST-elevation myocardial infarction (from APEX-AMI). Am J Cardiol 2010; 105: 59–63. PMID: 20102891
- 676.
Figueras J, Alcalde O, Barrabés JA, et al. Changes in hospital mortality rates in 425 patients with acute ST-elevation myocardial infarction and cardiac rupture over a 30-year period. Circulation 2008; 118: 2783–2789. PMID: 19064683
- 677.
Figueras J, Cortadellas J, Calvo F, et al. Relevance of delayed hospital admission on development of cardiac rupture during acute myocardial infarction: Study in 225 patients with free wall, septal or papillary muscle rupture. J Am Coll Cardiol 1998; 32: 135–139. PMID: 9669261
- 678.
Gueret P, Khalife K, Jobic Y, et al. Study Investigators. Echocardiographic assessment of the incidence of mechanical complications during the early phase of myocardial infarction in the reperfusion era: A French multicentre prospective registry. Arch Cardiovasc Dis 2008; 101: 41–47. PMID: 18391872
- 679.
Iemura J, Oku H, Otaki M, et al. Surgical strategy for left ventricular free wall rupture after acute myocardial infarction. Ann Thorac Surg 2001; 71: 201–204. PMID: 11216746
- 680.
Sakaguchi G, Komiya T, Tamura N, et al. Surgical treatment for postinfarction left ventricular free wall rupture. Ann Thorac Surg 2008; 85: 1344–1346. PMID: 18355523
- 681.
Okada K, Yamashita T, Matsumori M, et al. Surgical treatment for rupture of left ventricular free wall after acute myocardial infarction. Interact Cardiovasc Thorac Surg 2005; 4: 203–206. PMID: 17670393
- 682.
Alamanni F, Fumero A, Parolari A, et al. Sutureless double-patch-and-glue technique for repair of subacute left ventricular wall rupture after myocardial infarction. J Thorac Cardiovasc Surg 2001; 122: 836–837. PMID: 11581629
- 683.
Aoyagi S, Tayama K, Otsuka H, et al. Sutureless repair for left ventricular free wall rupture after acute myocardial infarction. J Card Surg 2014; 29: 178–180. PMID: 24428225
- 684.
Prêtre R, Benedikt P, Turina MI. Experience with postinfarction left ventricular free wall rupture. Ann Thorac Surg 2000; 69: 1342–1345. PMID: 10881802
- 685.
López-Sendón J, Gurfinkel EP, Lopez de Sa E, et al. Global Registry of Acute Coronary Events (GRACE) Investigators. Factors related to heart rupture in acute coronary syndromes in the Global Registry of Acute Coronary Events. Eur Heart J 2010; 31: 1449–1456. PMID: 20231153
- 686.
Latham PM. Lectures on Subjects Connected With Clinical Medicine: Comprising Diseases of the Heart, Volume 2. Langman, Recs, Orme, Brown, Green and Langman, 1847: 163–164.
- 687.
The GUSTO investigators. An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. N Engl J Med 1993; 329: 673–682. PMID: 8204123
- 688.
Birnbaum Y, Fishbein MC, Blanche C, et al. Ventricular septal rupture after acute myocardial infarction. N Engl J Med 2002; 347: 1426–1432. PMID: 12409546
- 689.
Crenshaw BS, Granger CB, Birnbaum Y, et al. GUSTO-I (Global Utilization of Streptokinase and TPA for Occluded Coronary Arteries) Trial Investigators. Risk factors, angiographic patterns, and outcomes in patients with ventricular septal defect complicating acute myocardial infarction. Circulation 2000; 101: 27–32. PMID: 10618300
- 690.
Moreyra AE, Huang MS, Wilson AC, et al. MIDAS Study Group (MIDAS 13). Trends in incidence and mortality rates of ventricular septal rupture during acute myocardial infarction. Am J Cardiol 2010; 106: 1095–1100. PMID: 20920645
- 691.
Prêtre R, Rickli H, Ye Q, et al. Frequency of collateral blood flow in the infarct-related coronary artery in rupture of the ventricular septum after acute myocardial infarction. Am J Cardiol 2000; 85: 497–499. PMID: 10728959
- 692.
Skehan JD, Carey C, Norrell MS, et al. Patterns of coronary artery disease in post-infarction ventricular septal rupture. Br Heart J 1989; 62: 268–272. PMID: 2803872
- 693.
Jones BM, Kapadia SR, Smedira NG, et al. Ventricular septal rupture complicating acute myocardial infarction: A contemporary review. Eur Heart J 2014; 35: 2060–2068. PMID: 24970335
- 694.
Daggett WM, Guyton RA, Mundth ED, et al. Surgery for post-myocardial infarct ventricular septal defect. Ann Surg 1977; 186: 260–271. PMID: 302110
- 695.
David TE, Dale L, Sun Z. Postinfarction ventricular septal rupture: Repair by endocardial patch with infarct exclusion. J Thorac Cardiovasc Surg 1995; 110: 1315–1322. PMID: 7475183
- 696.
The Survey Committee of Japanese Association for Coronary Artery Surgery (JACAS). The outcome from the annual national questionnaire in 2017. http://www.jacas.org/enquete/2017.html
- 697.
Arnaoutakis GJ, Zhao Y, George TJ, et al. Surgical repair of ventricular septal defect after myocardial infarction: Outcomes from the Society of Thoracic Surgeons National Database. Ann Thorac Surg 2012; 94: 436–443. PMID: 22626761
- 698.
Papalexopoulou N, Young CP, Attia RQ. What is the best timing of surgery in patients with post-infarct ventricular septal rupture? Interact Cardiovasc Thorac Surg 2013; 16: 193–196. PMID: 23143273
- 699.
McMurray JJ, Adamopoulos S, Anker SD, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 2012; 33: 1787–1847. PMID: 22611136
- 700.
Tsai MT, Wu HY, Chan SH, et al. Extracorporeal membrane oxygenation as a bridge to definite surgery in recurrent postinfarction ventricular septal defect. ASAIO J 2012; 58: 88–89. PMID: 22210655
- 701.
Neragi-Miandoab S, Michler RE, Goldstein D, et al. Extracorporeal membrane oxygenation as a temporizing approach in a patient with shock, myocardial infarct, and a large ventricle septal defect; successful repair after six days. J Card Surg 2013; 28: 193–195. PMID: 23350887
- 702.
La Torre MW, Centofanti P, Attisani M, et al. Posterior ventricular septal defect in presence of cardiogenic shock: Early implantation of the impella recover LP 5.0 as a bridge to surgery. Tex Heart Inst J 2011; 38: 42–49. PMID: 21423467
- 703.
Pitsis AA, Kelpis TG, Visouli AN, et al. Left ventricular assist device as a bridge to surgery in postinfarction ventricular septal defect. J Thorac Cardiovasc Surg 2008; 135: 951–952. PMID: 18374789
- 704.
Conradi L, Treede H, Brickwedel J, et al. Use of initial biventricular mechanical support in a case of postinfarction ventricular septal rupture as a bridge to surgery. Ann Thorac Surg 2009; 87: e37–e39. PMID: 19379852
- 705.
Samuels LE, Entwistle JC 3rd, Holmes EC, et al. Mechanical support of the unrepaired postinfarction ventricular septal defect with the Abiomed BVS 5000 ventricular assist device. J Thorac Cardiovasc Surg 2003; 126: 2100–2101. PMID: 14688739
- 706.
Thiele H, Kaulfersch C, Daehnert I, et al. Immediate primary transcatheter closure of postinfarction ventricular septal defects. Eur Heart J 2009; 30: 81–88. PMID: 19036747
- 707.
Assenza GE, McElhinney DB, Valente AM, et al. Transcatheter closure of post-myocardial infarction ventricular septal rupture. Circ Cardiovasc Interv 2013; 6: 59–67. PMID: 23339839
- 708.
Attia R, Blauth C. Which patients might be suitable for a septal occluder device closure of postinfarction ventricular septal rupture rather than immediate surgery? Interact Cardiovasc Thorac Surg 2010; 11: 626–629. PMID: 20621996
- 709.
Barbour DJ, Roberts WC. Rupture of a left ventricular papillary muscle during acute myocardial infarction: Analysis of 22 necropsy patients. J Am Coll Cardiol 1986; 8: 558–565. PMID: 3745700
- 710.
Vlodaver Z, Edwards JE. Rupture of ventricular septum or papillary muscle complicating myocardial infarction. Circulation 1977; 55: 815–822. PMID: 849638
- 711.
Voci P, Bilotta F, Caretta Q, et al. Papillary muscle perfusion pattern: A hypothesis for ischemic papillary muscle dysfunction. Circulation 1995; 91: 1714–1718. PMID: 7882478
- 712.
Rusu MC, Petrescu CI, Niculescu V, et al. Considerations on the left papillary muscles microangioarchitecture. Rom J Morphol Embryol 2006; 47: 63–66. PMID: 16838060
- 713.
Russo A, Suri RM, Grigioni F, et al. Clinical outcome after surgical correction of mitral regurgitation due to papillary muscle rupture. Circulation 2008; 118: 1528–1534. PMID: 18809799
- 714.
Kishon Y, Oh JK, Schaff HV, et al. Mitral valve operation in postinfarction rupture of a papillary muscle: Immediate results and long-term follow-up of 22 patients. Mayo Clin Proc 1992; 67: 1023–1030. PMID: 1434862
- 715.
Ternus BW, Mankad S, Edwards WD, et al. Clinical presentation and echocardiographic diagnosis of postinfarction papillary muscle rupture: A review of 22 cases. Echocardiography 2017; 34: 973–977. PMID: 28560714
- 716.
Kerut EK, Hanawalt C, Everson C. Echo features of posteromedial papillary muscle rupture without papillary muscle prolapse into the left atrium. Echocardiography 2011; 28: 1046–1048. PMID: 21827548
- 717.
Havins J, Lick S, Boor P, et al. Real time three-dimensional transesophageal echocardiography in partial posteromedial papillary muscle rupture. Echocardiography 2013; 30: E179–E181. PMID: 23488568
- 718.
Patil NP, Falconieri F, Bahrami T. Mitral repair for decompensated postinfarction papillary muscle rupture. Ann Thorac Surg 2016; 101: 2393. PMID: 27211959
- 719.
Bilge M, Alemdar R, Yasar AS. Successful percutaneous mitral valve repair with the MitraClip system of acute mitral regurgitation due to papillary muscle rupture as complication of acute myocardial infarction. Catheter Cardiovasc Interv 2014; 83: E137–E140. PMID: 23592592
- 720.
Morcos SK, Thomsen HS, Webb JA. Contrast Media Safety Committee, European Society of Urogenital Radiology (ESUR). Contrast-media-induced nephrotoxicity: A consensus report. Eur Radiol 1999; 9: 1602–1613. PMID: 10525875
- 721.
Japanese Society of Nephrology, Japan Radiological Society, and Japanese Circulation Society. 2018 Guidelines on use of iodinated contrast agents in patients with renal impairment. [Article in Japanese] TOKYO IGAKUSHA, 2018.
- 722.
KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int Suppl 2012; 2: 1–138.
- 723.
McCullough PA. Contrast-induced acute kidney injury. J Am Coll Cardiol 2008; 51: 1419–1428. PMID: 18402894
- 724.
Brown JR, Robb JF, Block CA, et al. Does safe dosing of iodinated contrast prevent contrast-induced acute kidney injury? Circ Cardiovasc Interv 2010; 3: 346–350. PMID: 20587788
- 725.
Ogata N, Ikari Y, Nanasato M, et al. Safety margin of minimized contrast volume during percutaneous coronary intervention in patients with chronic kidney disease. Cardiovasc Interv Ther 2014; 29: 209–215. PMID: 24474044
- 726.
Chong E, Poh KK, Liang S, et al. Comparison of risks and clinical predictors of contrast-induced nephropathy in patients undergoing emergency versus nonemergency percutaneous coronary interventions. J Interv Cardiol 2010; 23: 451–459. PMID: 20796168
- 727.
Marenzi G, Lauri G, Assanelli E, et al. Contrast-induced nephropathy in patients undergoing primary angioplasty for acute myocardial infarction. J Am Coll Cardiol 2004; 44: 1780–1785. PMID: 15519007
- 728.
Abe D, Sato A, Hoshi T, et al. Clinical predictors of contrast-induced acute kidney injury in patients undergoing emergency versus elective percutaneous coronary intervention. Circ J 2014; 78: 85–91. PMID: 24107362
- 729.
Dangas G, Iakovou I, Nikolsky E, et al. Contrast-induced nephropathy after percutaneous coronary interventions in relation to chronic kidney disease and hemodynamic variables. Am J Cardiol 2005; 95: 13–19. PMID: 15619387
- 730.
Solomon R, Werner C, Mann D, et al. Effects of saline, mannitol, and furosemide on acute decreases in renal function induced by radiocontrast agents. N Engl J Med 1994; 331: 1416–1420. PMID: 7969280
- 731.
Schneider V, Lévesque LE, Zhang B, et al. Association of selective and conventional nonsteroidal antiinflammatory drugs with acute renal failure: A population-based, nested case-control analysis. Am J Epidemiol 2006; 164: 881–889. PMID: 17005625
- 732.
Schweiger MJ, Chambers CE, Davidson CJ, et al. Prevention of contrast induced nephropathy: Recommendations for the high risk patient undergoing cardiovascular procedures. Catheter Cardiovasc Interv 2007; 69: 135–140. PMID: 17139671
- 733.
Maioli M, Toso A, Leoncini M, et al. Sodium bicarbonate versus saline for the prevention of contrast-induced nephropathy in patients with renal dysfunction undergoing coronary angiography or intervention. J Am Coll Cardiol 2008; 52: 599–604. PMID: 18702961
- 733a.
Marenzi G, Cosentino N, Bartorelli AL. Acute kidney injury in patients with acute coronary syndromes. Heart 2015; 101: 1778–1785. PMID: 26243789
- 734.
Frank H, Werner D, Lorusso V, et al. Simultaneous hemodialysis during coronary angiography fails to prevent radiocontrast-induced nephropathy in chronic renal failure. Clin Nephrol 2003; 60: 176–182. PMID: 14524580
- 735.
Reinecke H, Fobker M, Wellmann J, et al. A randomized controlled trial comparing hydration therapy to additional hemodialysis or N-acetylcysteine for the prevention of contrast medium-induced nephropathy: The Dialysis-versus-Diuresis (DVD) Trial. Clin Res Cardiol 2007; 96: 130–139. PMID: 17180572
- 736.
Cruz DN, Goh CY, Marenzi G, et al. Renal replacement therapies for prevention of radiocontrast-induced nephropathy: A systematic review. Am J Med 2012; 125: 66–78.e3. PMID: 22195531
- 737.
Kennedy JW. Complications associated with cardiac catheterization and angiography. Cathet Cardiovasc Diagn 1982; 8: 5–11. PMID: 7060118
- 738.
Sumiyoshi T, Hosoda S, Oka T, et al. Japanese acute myocardial infarction in the elderly study (JAMIES) group.
Effect of interventional therapy on the outcome of acute myocardial infarction in the elderly: A multicenter collaborative study in Japan. In: Kawashima Y, Omote T, Lakatta EG, editors. Cardiovascular disease in the elderly. Churchill Livingstone, 1996: 33–46.
- 739.
Nikolsky E, Stone GW, Kirtane AJ, et al. Gastrointestinal bleeding in patients with acute coronary syndromes: Incidence, predictors, and clinical implications: Analysis from the ACUITY (Acute Catheterization and Urgent Intervention Triage Strategy) trial. J Am Coll Cardiol 2009; 54: 1293–1302. PMID: 19778672
- 740.
Mehta SK, Frutkin AD, Lindsey JB, et al. National Cardiovascular Data Registry. Bleeding in patients undergoing percutaneous coronary intervention: The development of a clinical risk algorithm from the National Cardiovascular Data Registry. Circ Cardiovasc Interv 2009; 2: 222–229. PMID: 20031719
- 741.
Andò G, Capodanno D. Radial versus femoral access in invasively managed patients with acute coronary syndrome: A systematic review and meta-analysis. Ann Intern Med 2015; 163: 932–940. PMID: 26551857
- 742.
Oliva PB, Hammill SC. The clinical distinction between regional postinfarction pericarditis and other causes of postinfarction chest pain: Ancillary observations regarding the effect of lytic therapy upon the frequency of postinfarction pericarditis, postinfarction angina, and reinfarction. Clin Cardiol 1994; 17: 471–478. PMID: 8001310
- 743.
Sabaté M, Brugaletta S, Cequier A, et al. Clinical outcomes in patients with ST-segment elevation myocardial infarction treated with everolimus-eluting stents versus bare-metal stents (EXAMINATION): 5-year results of a randomised trial. Lancet 2016; 387: 357–366. PMID: 26520230
- 744.
Isshiki T, Kimura T, Ogawa H, et al. PRASFIT-Elective Investigators. Prasugrel, a third-generation P2Y12 receptor antagonist, in patients with coronary artery disease undergoing elective percutaneous coronary intervention. Circ J 2014; 78: 2926–2934. PMID: 25342212
- 745.
Tofler GH, Muller JE, Stone PH, et al. MILIS Study Group Pericarditis in acute myocardial infarction: Characterization and clinical significance. Am Heart J 1989; 117: 86–92. PMID: 2643287
- 746.
Wall TC, Califf RM, Harrelson-Woodlief L, et al. The TAMI Study Group. Usefulness of a pericardial friction rub after thrombolytic therapy during acute myocardial infarction in predicting amount of myocardial damage. Am J Cardiol 1990; 66: 1418–1421. PMID: 2123603
- 747.
Tanaka K, Sato N, Yasutake M, et al. Clinical course, timing of rupture and relationship with coronary recanalization therapy in 77 patients with ventricular free wall rupture following acute myocardial infarction. J Nippon Med Sch 2002; 69: 481–488. PMID: 12382012
- 748.
Catella-Lawson F, Reilly MP, Kapoor SC, et al. Cyclooxygenase inhibitors and the antiplatelet effects of aspirin. N Engl J Med 2001; 345: 1809–1817. PMID: 11752357
- 749.
Bulkley BH, Roberts WC. Steroid therapy during acute myocardial infarction: A cause of delayed healing and of ventricular aneurysm. Am J Med 1974; 56: 244–250. PMID: 4812079
- 750.
Kloner RA, Fishbein MC, Lew H, et al. Mummification of the infarcted myocardium by high dose corticosteroids. Circulation 1978; 57: 56–63. PMID: 618398
- 751.
Maisch B, Ristić AD. Practical aspects of the management of pericardial disease. Heart 2003; 89: 1096–1103. PMID: 12923044
- 752.
Imazio M, Bobbio M, Cecchi E, et al. Colchicine as first-choice therapy for recurrent pericarditis: Results of the CORE (COlchicine for REcurrent pericarditis) trial. Arch Intern Med 2005; 165: 1987–1991. PMID: 16186468
- 753.
Imazio M, Brucato A, Cemin R, et al. ICAP Investigators. A randomized trial of colchicine for acute pericarditis. N Engl J Med 2013; 369: 1522–1528. PMID: 23992557
- 754.
Alter DA, Tu JV, Austin PC, et al. Waiting times, revascularization modality, and outcomes after acute myocardial infarction at hospitals with and without on-site revascularization facilities in Canada. J Am Coll Cardiol 2003; 42: 410–419. PMID: 12906964
- 755.
Tanne D, Gottlieb S, Hod H, et al. Secondary Prevention Reinfarction Israeli Nifedipine Trial (SPRINT) and Israeli Thrombolytic Survey Groups. Incidence and mortality from early stroke associated with acute myocardial infarction in the prethrombolytic and thrombolytic eras. J Am Coll Cardiol 1997; 30: 1484–1490. PMID: 9362406
- 756.
Mooe T, Eriksson P, Stegmayr B. Ischemic stroke after acute myocardial infarction: A population-based study. Stroke 1997; 28: 762–767. PMID: 9099193
- 757.
Mahaffey KW, Granger CB, Sloan MA, et al. GUSTO-I Investigators. Risk factors for in-hospital nonhemorrhagic stroke in patients with acute myocardial infarction treated with thrombolysis: Results from GUSTO-I. Circulation 1998; 97: 757–764. PMID: 9498539
- 758.
Loh E, Sutton MS, Wun CC, et al. Ventricular dysfunction and the risk of stroke after myocardial infarction. N Engl J Med 1997; 336: 251–257. PMID: 8995087
- 759.
Spirito P, Bellotti P, Chiarella F, et al. Prognostic significance and natural history of left ventricular thrombi in patients with acute anterior myocardial infarction: A two-dimensional echocardiographic study. Circulation 1985; 72: 774–780. PMID: 4028378
- 760.
Barnett HJ, Taylor DW, Eliasziw M, et al. North American Symptomatic Carotid Endarterectomy Trial Collaborators. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. N Engl J Med 1998; 339: 1415–1425. PMID: 9811916
- 761.
Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in nonsurgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141 Suppl: e195S–e226S. PMID: 22315261
- 761a.
JCS Working Group. Guidelines for diagnosis, treatment and prevention of pulmonary thromboembolism and deep vein thrombosis (JCS 2017). [Article in Japanese] http://www.j-circ.or.jp/guideline/pdf/JCS2017_ito_h.pdf
- 762.
Halkin A, Stone GW, Grines CL, et al. Prognostic implications of creatine kinase elevation after primary percutaneous coronary intervention for acute myocardial infarction. J Am Coll Cardiol 2006; 47: 951–961. PMID: 16516077
- 763.
Kelle S, Roes SD, Klein C, et al. Prognostic value of myocardial infarct size and contractile reserve using magnetic resonance imaging. J Am Coll Cardiol 2009; 54: 1770–1777. PMID: 19874990
- 764.
Dohi T, Maehara A, Brener SJ, et al. Utility of peak creatine kinase-MB measurements in predicting myocardial infarct size, left ventricular dysfunction, and outcome after first anterior wall acute myocardial infarction (from the INFUSE-AMI trial). Am J Cardiol 2015; 115: 563–570. PMID: 25586335
- 765.
Gibbons RJ, Valeti US, Araoz PA, et al. The quantification of infarct size. J Am Coll Cardiol 2004; 44: 1533–1542. PMID: 15489082
- 766.
Chia S, Senatore F, Raffel OC, et al. Utility of cardiac biomarkers in predicting infarct size, left ventricular function, and clinical outcome after primary percutaneous coronary intervention for ST-segment elevation myocardial infarction. JACC Cardiovasc Interv 2008; 1: 415–423. PMID: 19463339
- 767.
Byrne RA, Ndrepepa G, Braun S, et al. Peak cardiac troponin-T level, scintigraphic myocardial infarct size and one-year prognosis in patients undergoing primary percutaneous coronary intervention for acute myocardial infarction. Am J Cardiol 2010; 106: 1212–1217. PMID: 21029815
- 768.
Steen H, Giannitsis E, Futterer S, et al. Cardiac troponin T at 96 hours after acute myocardial infarction correlates with infarct size and cardiac function. J Am Coll Cardiol 2006; 48: 2192–2194. PMID: 17161244
- 769.
Licka M, Zimmermann R, Zehelein J, et al. Troponin T concentrations 72 hours after myocardial infarction as a serological estimate of infarct size. Heart 2002; 87: 520–524. PMID: 12010931
- 770.
Kapur A, Latus KA, Davies G, et al. A comparison of three radionuclide myocardial perfusion tracers in clinical practice: The ROBUST study. Eur J Nucl Med Mol Imaging 2002; 29: 1608–1616. PMID: 12458395
- 771.
Bax JJ, Wijns W, Cornel JH, et al. Accuracy of currently available techniques for prediction of functional recovery after revascularization in patients with left ventricular dysfunction due to chronic coronary artery disease: Comparison of pooled data. J Am Coll Cardiol 1997; 30: 1451–1460. PMID: 9362401
- 772.
Moon JC, Lorenz CH, Francis JM, et al. Breath-hold FLASH and FISP cardiovascular MR imaging: Left ventricular volume differences and reproducibility. Radiology 2002; 223: 789–797. PMID: 12034951
- 773.
Barkhausen J, Ruehm SG, Goyen M, et al. MR evaluation of ventricular function: True fast imaging with steady-state precession versus fast low-angle shot cine MR imaging: Feasibility study. Radiology 2001; 219: 264–269. PMID: 11274568
- 774.
Wagner A, Mahrholdt H, Holly TA, et al. Contrast-enhanced MRI and routine single photon emission computed tomography (SPECT) perfusion imaging for detection of subendocardial myocardial infarcts: An imaging study. Lancet 2003; 361: 374–379. PMID: 12573373
- 775.
Kim RJ, Albert TS, Wible JH, et al. Gadoversetamide Myocardial Infarction Imaging Investigators. Performance of delayed-enhancement magnetic resonance imaging with gadoversetamide contrast for the detection and assessment of myocardial infarction: An international, multicenter, double-blinded, randomized trial. Circulation 2008; 117: 629–637. PMID: 18212288
- 776.
Kumar A, Abdel-Aty H, Kriedemann I, et al. Contrast-enhanced cardiovascular magnetic resonance imaging of right ventricular infarction. J Am Coll Cardiol 2006; 48: 1969–1976. PMID: 17112986
- 777.
Ingkanisorn WP, Rhoads KL, Aletras AH, et al. Gadolinium delayed enhancement cardiovascular magnetic resonance correlates with clinical measures of myocardial infarction. J Am Coll Cardiol 2004; 43: 2253–2259. PMID: 15193689
- 778.
Eitel I, de Waha S, Wöhrle J, et al. Comprehensive prognosis assessment by CMR imaging after ST-segment elevation myocardial infarction. J Am Coll Cardiol 2014; 64: 1217–1226. PMID: 25236513
- 779.
Stone GW, Selker HP, Thiele H, et al. Relationship between infarct size and outcomes following primary PCI: Patient-level analysis from 10 randomized trials. J Am Coll Cardiol 2016; 67: 1674–1683. PMID: 27056772
- 780.
Hombach V, Grebe O, Merkle N, et al. Sequelae of acute myocardial infarction regarding cardiac structure and function and their prognostic significance as assessed by magnetic resonance imaging. Eur Heart J 2005; 26: 549–557. PMID: 15713695
- 781.
O’Regan DP, Ariff B, Neuwirth C, et al. Assessment of severe reperfusion injury with T2* cardiac MRI in patients with acute myocardial infarction. Heart 2010; 96: 1885–1891. PMID: 20965977
- 782.
Mather AN, Fairbairn TA, Ball SG, et al. Reperfusion haemorrhage as determined by cardiovascular MRI is a predictor of adverse left ventricular remodelling and markers of late arrhythmic risk. Heart 2011; 97: 453–459. PMID: 21051455
- 783.
de Waha S, Patel MR, Granger CB, et al. Relationship between microvascular obstruction and adverse events following primary percutaneous coronary intervention for ST-segment elevation myocardial infarction: An individual patient data pooled analysis from seven randomized trials. Eur Heart J 2017; 38: 3502–3510. PMID: 29020248
- 784.
Fieno DS, Kim RJ, Chen EL, et al. Contrast-enhanced magnetic resonance imaging of myocardium at risk: Distinction between reversible and irreversible injury throughout infarct healing. J Am Coll Cardiol 2000; 36: 1985–1991. PMID: 11092675
- 785.
Kim RJ, Wu E, Rafael A, et al. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med 2000; 343: 1445–1453. PMID: 11078769
- 786.
Klein C, Nekolla SG, Bengel FM, et al. Assessment of myocardial viability with contrast-enhanced magnetic resonance imaging: Comparison with positron emission tomography. Circulation 2002; 105: 162–167. PMID: 11790695
- 787.
Shah DJ, Kim HW, James O, et al. Prevalence of regional myocardial thinning and relationship with myocardial scarring in patients with coronary artery disease. JAMA 2013; 309: 909–918. PMID: 23462787
- 788.
Origuchi H. Cardiac rehabilitation and exercise training after myocardial infarction. [Article in Japanese] In: Fukui T, Takagi M, editors. Today’s therapy 2017. Igaku-Shoin, 2017: 427–429.
- 789.
Ministry of Health, Labour and Welfare, Health Insurance Bureau. 7. Rehabilitation. [Article in Japanese] https://www.mhlw.go.jp/file/06-Seisakujouhou-12400000-Hokenkyoku/0000114819.pdf
- 790.
Horibe T. Respiratory physical therapy and approach to disuse syndrome. [Article in Japanese] Sogo Rehabilitation 2017; 45: 605–615.
- 791.
Tamaki Y, Konishi H, Horiike S, et al. Introduction of early cardiac rehabilitation program for severe heart failure patients in cardiac intensive care unit. [Article in Japanese] Journal of Japanese Cardiac Rehabilitation 2016; 22: 71–76.
- 792.
Tamaki Y, Kawakami S, Goto Y, et al. Special issue: Cardiac rehabilitation: From perioperative management to improvement of long-term prognosis 5. Experiences of early cardiac rehabilitation for severe heart failure patients in intensive care unit. [Article in Japanese] Circ Cont 2017; 38: 107–112.
- 793.
Tanaka S, Masuda T, Kamiya K, et al. A Single session of neuromuscular electrical stimulation enhances vascular endothelial function and peripheral blood circulation in patients with acute myocardial infarction. Int Heart J 2016; 57: 676–681. PMID: 27818472
- 794.
Yamasaki M, Makita S, Majima M, et al. Change of complications and hospital stays by modified cardiac rehabilitation program. [Article in Japanese] Journal of Japanese Cardiac Rehabilitation 2004; 9: 71–74.
- 795.
JCS Joint Working Group. Guidelines for rehabilitation in patients with cardiovascular disease (JCS 2012). [Article in Japanese] http://www.j-circ.or.jp/guideline/pdf/JCS2012_nohara_h.pdf
- 796.
Makita S. Super-acute phase cardiac rehabilitation in CCU·ICU. [Article in Japanese] J Clin Rehabil 2014; 23: 745–750.
- 797.
JCS Joint Working Group. Guidelines for the management of patients with ST-elevation acute myocardial infarction (JCS 2013). [Article in Japanese] http://www.j-circ.or.jp/guideline/pdf/JCS2013_kimura_h.pdf
- 798.
Itoh M, Kojima S, Sakamoto T, et al. Safety and efficacy of graded water immersion at indifferent temperature during Early Phase period in patients with acute myocardial infarction (AMI). [Article in Japanese] Journal of Japanese Cardiac Rehabilitation 2012; 17: 76–81.
- 799.
Kimura M, Yamada M, Osawa R, et al. Study about early induction of showering after acute myocardial infarction. [Article in Japanese] Journal of Japanese Cardiac Rehabilitation 2001; 6: 105–107.
- 800.
Committee Members, Gibbons RJ, Balady GJ, Bricker JT, et al. ACC/AHA 2002 guideline update for exercise testing: Summary article: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines). Circulation 2002; 106: 1883–1892. PMID: 12356646
- 801.
Thomas RJ, Denna T. The role of cardiac rehabilitation following acute coronary syndromes. Curr Cardiol Rep 2014; 16: 534. PMID: 25135346
- 802.
Ohtera S, Kanazawa N, Ozasa N, et al. Proposal of quality indicators for cardiac rehabilitation after acute coronary syndrome in Japan: A modified Delphi method and practice test. BMJ Open 2017; 7: e013036. PMID: 28132004
- 803.
Suaya JA, Shepard DS, Normand SL, et al. Use of cardiac rehabilitation by Medicare beneficiaries after myocardial infarction or coronary bypass surgery. Circulation 2007; 116: 1653–1662. PMID: 17893274
- 804.
Ades PA, Waldmann ML, McCann WJ, et al. Predictors of cardiac rehabilitation participation in older coronary patients. Arch Intern Med 1992; 152: 1033–1035. PMID: 1580707
- 805.
Campkin LM, Boyd JM, Campbell DJT. Coronary artery disease patient perspectives on exercise participation. J Cardiopulm Rehabil Prev 2017; 37: 305–314. PMID: 28858031
- 806.
Grace SL, Scholey P, Suskin N, et al. A prospective comparison of cardiac rehabilitation enrollment following automatic vs usual referral. J Rehabil Med 2007; 39: 239–245. PMID: 17468793
- 807.
Singh M, Peterson ED, Roe MT, et al. Trends in the association between age and in-hospital mortality after percutaneous coronary intervention: National Cardiovascular Data Registry experience. Circ Cardiovasc Interv 2009; 2: 20–26. PMID: 20031689
- 808.
Gore MO, Seliger SL, Defilippi CR, et al. Age- and sex-dependent upper reference limits for the high-sensitivity cardiac troponin T assay. J Am Coll Cardiol 2014; 63: 1441–1448. PMID: 24530665
- 809.
Reiter M, Twerenbold R, Reichlin T, et al. Early diagnosis of acute myocardial infarction in the elderly using more sensitive cardiac troponin assays. Eur Heart J 2011; 32: 1379–1389. PMID: 21362702
- 810.
Tegn N, Abdelnoor M, Aaberge L, et al. After Eighty study investigators. Invasive versus conservative strategy in patients aged 80 years or older with non-ST-elevation myocardial infarction or unstable angina pectoris (After Eighty study): An open-label randomised controlled trial. Lancet 2016; 387: 1057–1065. PMID: 26794722
- 811.
Bauer T, Koeth O, Jünger C, et al. Acute Coronary Syndromes Registry (ACOS) Investigators. Effect of an invasive strategy on in-hospital outcome in elderly patients with non-ST-elevation myocardial infarction. Eur Heart J 2007; 28: 2873–2878. PMID: 17982163
- 812.
Bueno H, Betriu A, Heras M, et al. TRIANA Investigators. Primary angioplasty vs. fibrinolysis in very old patients with acute myocardial infarction: TRIANA (TRatamiento del Infarto Agudo de miocardio eN Ancianos) randomized trial and pooled analysis with previous studies. Eur Heart J 2011; 32: 51–60. PMID: 20971744
- 813.
Varenne O, Cook S, Sideris G, et al. SENIOR investigators. Drug-eluting stents in elderly patients with coronary artery disease (SENIOR): A randomised single-blind trial. Lancet 2018; 391: 41–50. PMID: 29102362
- 814.
Skolnick AH, Alexander KP, Chen AY, et al. Characteristics, management, and outcomes of 5,557 patients age ≥90 years with acute coronary syndromes: Results from the CRUSADE Initiative. J Am Coll Cardiol 2007; 49: 1790–1797. PMID: 17466230
- 815.
Andreotti F, Rocca B, Husted S, et al. ESC Thrombosis Working Group. Antithrombotic therapy in the elderly: Expert position paper of the European Society of Cardiology Working Group on Thrombosis. Eur Heart J 2015; 36: 3238–3249. PMID: 26163482
- 816.
Mackay MH, Ratner PA, Johnson JL, et al. Gender differences in symptoms of myocardial ischaemia. Eur Heart J 2011; 32: 3107–3114. PMID: 21920968
- 817.
Poon S, Goodman SG, Yan RT, et al. Bridging the gender gap: Insights from a contemporary analysis of sex-related differences in the treatment and outcomes of patients with acute coronary syndromes. Am Heart J 2012; 163: 66–73. PMID: 22172438
- 818.
Alfredsson J, Lindbäck J, Wallentin L, et al. Similar outcome with an invasive strategy in men and women with non-ST-elevation acute coronary syndromes: From the Swedish Web-System for Enhancement and Development of Evidence-Based Care in Heart Disease Evaluated According to Recommended Therapies (SWEDEHEART). Eur Heart J 2011; 32: 3128–3136. PMID: 21911338
- 819.
Numasawa Y, Inohara T, Ishii H, et al. Comparison of outcomes of women versus men with non-ST-elevation acute coronary syndromes undergoing percutaneous coronary intervention (from the Japanese Nationwide Registry). Am J Cardiol 2017; 119: 826–831. PMID: 28040190
- 820.
Mehta LS, Beckie TM, DeVon HA, et al. American Heart Association Cardiovascular Disease in Women and Special Populations Committee of the Council on Clinical Cardiology, Council on Epidemiology and Prevention, Council on Cardiovascular and Stroke Nursing, and Council on Quality of Care and Outcomes Research. Acute myocardial infarction in women: A scientific statement from the American Heart Association. Circulation 2016; 133: 916–947. PMID: 26811316
- 821.
Vincent GM, Anderson JL, Marshall HW. Coronary spasm producing coronary thrombosis and myocardial infarction. N Engl J Med 1983; 309: 220–223. PMID: 6866037
- 822.
Bugiardini R, Bairey Merz CN. Angina with “normal” coronary arteries: A changing philosophy. JAMA 2005; 293: 477–484. PMID: 15671433
- 823.
Ong P, Athanasiadis A, Hill S, et al. Coronary artery spasm as a frequent cause of acute coronary syndrome: The CASPAR (Coronary Artery Spasm in Patients With Acute Coronary Syndrome) Study. J Am Coll Cardiol 2008; 52: 523–527. PMID: 18687244
- 824.
Nakayama N, Kaikita K, Fukunaga T, et al. Clinical features and prognosis of patients with coronary spasm-induced non-ST-segment elevation acute coronary syndrome. J Am Heart Assoc 2014; 3: e000795. PMID: 24811613
- 825.
Lin CS, Penha PD, Zak FG, et al. Morphodynamic interpretation of acute coronary thrombosis, with special reference to volcano-like eruption of atheromatous plaque caused by coronary artery spasm. Angiology 1988; 39: 535–547. PMID: 3377273
- 826.
Oshima S, Yasue H, Ogawa H, et al. Fibrinopeptide A is released into the coronary circulation after coronary spasm. Circulation 1990; 82: 2222–2225. PMID: 2146992
- 827.
Misumi I, Ogawa H, Masuda T, et al. Increased plasma plasminogen activator inhibitor activity after coronary spasm. Int J Cardiol 1993; 41: 21–29. PMID: 8225669
- 828.
Kaikita K, Ogawa H, Yasue H, et al. Soluble P-selectin is released into the coronary circulation after coronary spasm. Circulation 1995; 92: 1726–1730. PMID: 7545553
- 829.
JCS Joint Working Group. Guidelines for diagnosis and treatment of patients with vasospastic angina (coronary spastic angina) (JCS 2013): Digest version. Circ J 2014; 78: 2779–2801. PMID: 25273915
- 830.
Hagan PG, Nienaber CA, Isselbacher EM, et al. The International Registry of Acute Aortic Dissection (IRAD): New insights into an old disease. JAMA 2000; 283: 897–903. PMID: 10685714
- 831.
Klompas M. Does this patient have an acute thoracic aortic dissection? JAMA 2002; 287: 2262–2272. PMID: 11980527
- 832.
Neri E, Toscano T, Papalia U, et al. Proximal aortic dissection with coronary malperfusion: Presentation, management, and outcome. J Thorac Cardiovasc Surg 2001; 121: 552–560. PMID: 11241091
- 833.
Jánosi RA, Buck T, Erbel R. Mechanism of coronary malperfusion due to type-a aortic dissection. Herz 2009; 34: 478. PMID: 19784566
- 834.
Massetti M, Neri E, Babatasi G, et al. Flap suffocation: An uncommon mechanism of coronary malperfusion in acute type A dissection. J Thorac Cardiovasc Surg 2003; 125: 1548–1550. PMID: 12830085
- 835.
Biagini E, Lofiego C, Ferlito M, et al. Frequency, determinants, and clinical relevance of acute coronary syndrome-like electrocardiographic findings in patients with acute aortic syndrome. Am J Cardiol 2007; 100: 1013–1019. PMID: 17826389
- 836.
Harris KM, Strauss CE, Eagle KA, et al. International Registry of Acute Aortic Dissection (IRAD) Investigators. Correlates of delayed recognition and treatment of acute type A aortic dissection: The International Registry of Acute Aortic Dissection (IRAD). Circulation 2011; 124: 1911–1918. PMID: 21969019
- 837.
Hazui H, Fukumoto H, Negoro N, et al. Simple and useful tests for discriminating between acute aortic dissection of the ascending aorta and acute myocardial infarction in the emergency setting. Circ J 2005; 69: 677–682. PMID: 15914945
- 838.
Saw J. Coronary angiogram classification of spontaneous coronary artery dissection. Catheter Cardiovasc Interv 2014; 84: 1115–1122. PMID: 24227590
- 839.
Mortensen KH, Thuesen L, Kristensen IB, et al. Spontaneous coronary artery dissection: A Western Denmark Heart Registry study. Catheter Cardiovasc Interv 2009; 74: 710–717. PMID: 19496145
- 840.
Nakashima T, Noguchi T, Haruta S, et al. Prognostic impact of spontaneous coronary artery dissection in young female patients with acute myocardial infarction: A report from the Angina Pectoris-Myocardial Infarction Multicenter Investigators in Japan. Int J Cardiol 2016; 207: 341–348. PMID: 26820364
- 841.
Tweet MS, Hayes SN, Pitta SR, et al. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation 2012; 126: 579–588. PMID: 22800851
- 842.
Persu A, Van der Niepen P, Touzé E, et al. Working Group “Hypertension and the Kidney” of the European Society of Hypertension and the European Fibromuscular Dysplasia Initiative. Revisiting fibromuscular dysplasia: Rationale of the European fibromuscular dysplasia initiative. Hypertension 2016; 68: 832–839. PMID: 27504007
- 843.
Saw J, Humphries K, Aymong E, et al. Spontaneous coronary artery dissection: Clinical outcomes and risk of recurrence. J Am Coll Cardiol 2017; 70: 1148–1158. PMID: 28838364
- 844.
Tweet MS, Eleid MF, Best PJ, et al. Spontaneous coronary artery dissection: Revascularization versus conservative therapy. Circ Cardiovasc Interv 2014; 7: 777–786. PMID: 25406203
- 845.
Prizel KR, Hutchins GM, Bulkley BH. Coronary artery embolism and myocardial infarction. Ann Intern Med 1978; 88: 155–161. PMID: 626443
- 846.
Shibata T, Kawakami S, Noguchi T, et al. Prevalence, clinical features, and prognosis of acute myocardial infarction attributable to coronary artery embolism. Circulation 2015; 132: 241–250. PMID: 26216084
- 846a.
Japan Atherosclerosis Society. Japan Atherosclerosis Society (JAS) guidelines for prevention of atherosclerotic cardiovascular diseases 2017. [Article in Japanese]
- 847.
Baba S, Iso H, Mannami T, et al. JPHC Study Group. Cigarette smoking and risk of coronary heart disease incidence among middle-aged Japanese men and women: The JPHC Study Cohort I. Eur J Cardiovasc Prev Rehabil 2006; 13: 207–213. PMID: 16575274
- 848.
Prescott E, Hippe M, Schnohr P, et al. Smoking and risk of myocardial infarction in women and men: Longitudinal population study. BMJ 1998; 316: 1043–1047. PMID: 9552903
- 849.
Csordas A, Bernhard D. The biology behind the atherothrombotic effects of cigarette smoke. Nat Rev Cardiol 2013; 10: 219–230. PMID: 23380975
- 850.
Lau PP, Li L, Merched AJ, et al. Nicotine induces proinflammatory responses in macrophages and the aorta leading to acceleration of atherosclerosis in low-density lipoprotein receptor−/− mice. Arterioscler Thromb Vasc Biol 2006; 26: 143–149. PMID: 16254210
- 851.
Miyazaki T, Shimada K, Mokuno H, et al. Adipocyte derived plasma protein, adiponectin, is associated with smoking status in patients with coronary artery disease. Heart 2003; 89: 663. PMID: 12748229
- 852.
Hung J, Lam JY, Lacoste L, et al. Cigarette smoking acutely increases platelet thrombus formation in patients with coronary artery disease taking aspirin. Circulation 1995; 92: 2432–2436. PMID: 7586342
- 853.
U.S. Department of Health and Human Services. The health consequences of smoking—50 years of progress: A report of the Surgeon General. US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2014: 17.
- 854.
Prescott E, Scharling H, Osler M, et al. Importance of light smoking and inhalation habits on risk of myocardial infarction and all cause mortality: A 22 year follow up of 12 149 men and women in The Copenhagen City Heart Study. J Epidemiol Community Health 2002; 56: 702–706. PMID: 12177089
- 855.
He J, Vupputuri S, Allen K, et al. Passive smoking and the risk of coronary heart disease: A meta-analysis of epidemiologic studies. N Engl J Med 1999; 340: 920–926. PMID: 10089185
- 856.
Benowitz NL, Fraiman JB. Cardiovascular effects of electronic cigarettes. Nat Rev Cardiol 2017; 14: 447–456. PMID: 28332500
- 857.
Moheimani RS, Bhetraratana M, Yin F, et al. Increased cardiac sympathetic activity and oxidative stress in habitual electronic cigarette users: Implications for cardiovascular risk. JAMA Cardiol 2017; 2: 278–284. PMID: 28146259
- 858.
Oono IP, Mackay DF, Pell JP. Meta-analysis of the association between secondhand smoke exposure and stroke. J Public Health (Oxf) 2011; 33: 496–502. PMID: 21422014
- 859.
Barnoya J, Glantz SA. Cardiovascular effects of secondhand smoke: Nearly as large as smoking. Circulation 2005; 111: 2684–2698. PMID: 15911719
- 860.
Tan CE, Glantz SA. Association between smoke-free legislation and hospitalizations for cardiac, cerebrovascular, and respiratory diseases: A meta-analysis. Circulation 2012; 126: 2177–2183. PMID: 23109514
- 861.
Pell JP, Haw S, Cobbe S, et al. Smoke-free legislation and hospitalizations for acute coronary syndrome. N Engl J Med 2008; 359: 482–491. PMID: 18669427
- 862.
Iso H, Date C, Yamamoto A, et al. JACC Study Group. Smoking cessation and mortality from cardiovascular disease among Japanese men and women: The JACC Study. Am J Epidemiol 2005; 161: 170–179. PMID: 15632267
- 863.
Chow CK, Jolly S, Rao-Melacini P, et al. Association of diet, exercise, and smoking modification with risk of early cardiovascular events after acute coronary syndromes. Circulation 2010; 121: 750–758. PMID: 20124123
- 864.
Stead LF, Bergson G, Lancaster T. Physician advice for smoking cessation. Cochrane Database Syst Rev 2008: CD000165. PMID: 18425860
- 865.
Hubbard G, Gorely T, Ozakinci G, et al. A systematic review and narrative summary of family-based smoking cessation interventions to help adults quit smoking. BMC Fam Pract 2016; 17: 73. PMID: 27342987
- 866.
Fiore M, Bailey W, Cohen S, et al. Treating tobacco use and dependence.
Clinical practice guideline. US Department of Health and Human Services, 2000.
- 867.
Woolf KJ, Zabad MN, Post JM, et al. Effect of nicotine replacement therapy on cardiovascular outcomes after acute coronary syndromes. Am J Cardiol 2012; 110: 968–970. PMID: 22727182
- 868.
Gershon AS, Campitelli MA, Hawken S, et al. Cardiovascular and neuropsychiatric events after varenicline use for smoking cessation. Am J Respir Crit Care Med 2018; 197: 913–922. PMID: 29260881
- 869.
Japan Society for the Study of Obesity Guidelines Prevention Committee. 2016 Obesity Clinical Practice Guideline. [Article in Japanese] Life Science Publishing, 2016.
- 870.
The Global BMI Mortality Collaboration. Body-mass index and all-cause mortality: Individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet 2016; 388: 776–786. PMID: 27423262
- 871.
Aune D, Sen A, Prasad M, et al. BMI and all cause mortality: Systematic review and non-linear dose-response meta-analysis of 230 cohort studies with 3.74 million deaths among 30.3 million participants. BMJ 2016; 353: i2156. PMID: 27146380
- 872.
Lamelas P, Schwalm JD, Quazi I, et al. Effect of body mass index on clinical events after acute coronary syndromes. Am J Cardiol 2017; 120: 1453–1459. PMID: 28916239
- 873.
Hioki H, Miura T, Motoki H, et al. SHINANO registry investigators. Lean body mass index prognostic value for cardiovascular events in patients with coronary artery disease. Heart Asia 2015; 7: 12–18. PMID: 26345318
- 874.
Niedziela J, Hudzik B, Niedziela N, et al. The obesity paradox in acute coronary syndrome: A meta-analysis. Springer, 2014. PMID: 25354991 PMID: 25354991
- 875.
Herrmann J, Gersh BJ, Goldfinger JZ, et al. Body mass index and acute and long-term outcomes after acute myocardial infarction (from the Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction Trial). Am J Cardiol 2014; 114: 9–16. PMID: 24846807
- 876.
Mottillo S, Filion KB, Genest J, et al. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol 2010; 56: 1113–1132. PMID: 20863953
- 877.
Malik S, Wong ND, Franklin SS, et al. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation 2004; 110: 1245–1250. PMID: 15326067
- 878.
Lakka HM, Laaksonen DE, Lakka TA, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA 2002; 288: 2709–2716. PMID: 12460094
- 879.
Wu ZJ, Cheng YJ, Gu WJ, et al. Adiponectin is associated with increased mortality in patients with already established cardiovascular disease: A systematic review and meta-analysis. Metabolism 2014; 63: 1157–1166. PMID: 24933398
- 880.
Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009; 120: 1640–1645. PMID: 19805654
- 881.
Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol 2014; 63: 2985–3023. PMID: 24239920
- 882.
Kimura T, Morimoto T, Nakagawa Y, et al. j-Cypher Registry Investigators. Antiplatelet therapy and stent thrombosis after sirolimus-eluting stent implantation. Circulation 2009; 119: 987–995. PMID: 19204304
- 883.
Kimura T, Morimoto T, Nakagawa Y, et al. Antiplatelet therapy and long-term clinical outcome after sirolimus-eluting stent implantation: 5-year outcome of the j-Cypher registry. Cardiovasc Interv Ther 2012; 27: 181–188. PMID: 22695921
- 884.
Sabate M, Cequier A, Iñiguez A, et al. Everolimus-eluting stent versus bare-metal stent in ST-segment elevation myocardial infarction (EXAMINATION): 1 year results of a randomised controlled trial. Lancet 2012; 380: 1482–1490. PMID: 22951305
- 885.
Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2018; 39: 213–260. PMID: 28886622
- 886.
Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines: An Update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention, 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease, 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction, 2014 AHA/ACC Guideline for the Management of Patients With Non-ST-Elevation Acute Coronary Syndromes, and 2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery. Circulation 2016; 134: e123–e155. PMID: 27026020
- 887.
Kedhi E, Fabris E, van der Ent M, et al. A prospective, randomized, open-label trial of 6-month versus 12-month dual antiplatelet therapy after drug-eluting stent implantation in ST-elevation myocardial infarction: Rationale and design of the “DAPT-STEMI trial”. Am Heart J 2017; 188: 11–17. PMID: 28577666
- 888.
Nakamura M, Iijima R, Ako J, et al. NIPPON Investigators. Dual antiplatelet therapy for 6 versus 18 months after biodegradable polymer drug-eluting stent implantation. JACC Cardiovasc Interv 2017; 10: 1189–1198. PMID: 28641838
- 889.
Natsuaki M, Morimoto T, Yamamoto E, et al. One-year outcome of a prospective trial stopping dual antiplatelet therapy at 3 months after everolimus-eluting cobalt-chromium stent implantation: ShortT and OPtimal duration of Dual AntiPlatelet Therapy after everolimus-eluting cobalt-chromium stent (STOPDAPT) trial. Cardiovasc Interv Ther 2016; 31: 196–209. PMID: 26518420
- 890.
Hahn JY, Song YB, Oh JH, et al. SMART-DATE investigators. 6-month versus 12-month or longer dual antiplatelet therapy after percutaneous coronary intervention in patients with acute coronary syndrome (SMART-DATE): A randomised, open-label, non-inferiority trial. Lancet 2018; 391: 1274–1284. PMID: 29544699
- 891.
Bonaca MP, Bhatt DL, Cohen M, et al. PEGASUS-TIMI 54 Steering Committee and Investigators. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med 2015; 372: 1791–1800. PMID: 25773268
- 892.
Harjai KJ, Shenoy C, Orshaw P, et al. Dual antiplatelet therapy for more than 12 months after percutaneous coronary intervention: Insights from the Guthrie PCI Registry. Heart 2009; 95: 1579–1586. PMID: 19549619
- 893.
Park SJ, Park DW, Kim YH, et al. Duration of dual antiplatelet therapy after implantation of drug-eluting stents. N Engl J Med 2010; 362: 1374–1382. PMID: 20231231
- 894.
Tada T, Natsuaki M, Morimoto T, et al. CREDO-Kyoto PCI/CABG Registry Cohort-2 Investigators. Duration of dual antiplatelet therapy and long-term clinical outcome after coronary drug-eluting stent implantation: Landmark analyses from the CREDO-Kyoto PCI/CABG Registry Cohort-2. Circ Cardiovasc Interv 2012; 5: 381–391. PMID: 22619260
- 895.
Costa F, van Klaveren D, James S, et al. PRECISE-DAPT Study Investigators. Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE-DAPT) score: A pooled analysis of individual-patient datasets from clinical trials. Lancet 2017; 389: 1025–1034. PMID: 28290994
- 896.
Lewis TT. Trauma and Posttraumatic Stress Disorder: Emerging Risk Factors for Cardiovascular Disease in Women? Circulation 2015; 132: 227–229. PMID: 26124184
- 897.
Mauri L, Kereiakes DJ, Yeh RW, et al. DAPT Study Investigators. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med 2014; 371: 2155–2166. PMID: 25399658
- 898.
Freemantle N, Cleland J, Young P, et al. β Blockade after myocardial infarction: Systematic review and meta regression analysis. BMJ 1999; 318: 1730–1737. PMID: 10381708
- 899.
Dondo TB, Hall M, West RM, et al. β-Blockers and Mortality After Acute Myocardial Infarction in Patients Without Heart Failure or Ventricular Dysfunction. J Am Coll Cardiol 2017; 69: 2710–2720. PMID: 28571635
- 900.
Watanabe H, Ozasa N, Morimoto T, et al. CAPITAL-RCT investigators. Long-term use of carvedilol in patients with ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention. PLoS One 2018; 13: e0199347. PMID: 30153268
- 901.
Noble S, Roffi M. Routine beta-blocker administration following acute myocardial infarction: Why still an unsolved issue? J Thorac Dis 2017; 9: 4191–4194. PMID: 29268468
- 902.
Werner N, Nickenig G. AT1 receptors in atherosclerosis:
Biological effects including growth, angiogenesis, and apotosis. Eur Heart J 2003; 5 Suppl: A9–A13.
- 903.
Braunwald E, Domanski MJ, Fowler SE, et al. PEACE Trial Investigators. Angiotensin-converting-enzyme inhibition in stable coronary artery disease. N Engl J Med 2004; 351: 2058–2068. PMID: 15531767
- 904.
Pitt B, O’Neill B, Feldman R, et al. QUIET Study Group. The QUinapril Ischemic Event Trial (QUIET): Evaluation of chronic ACE inhibitor therapy in patients with ischemic heart disease and preserved left ventricular function. Am J Cardiol 2001; 87: 1058–1063. PMID: 11348602
- 905.
Pilote L, Abrahamowicz M, Rodrigues E, et al. Mortality rates in elderly patients who take different angiotensin-converting enzyme inhibitors after acute myocardial infarction: A class effect? Ann Intern Med 2004; 141: 102–112. PMID: 15262665
- 906.
ONTARGET Investigators. Yusuf S, Teo KK, Pogue J, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 2008; 358: 1547–1559. PMID: 18378520
- 907.
Demers C, McMurray JJ, Swedberg K, et al. CHARM Investigators. Impact of candesartan on nonfatal myocardial infarction and cardiovascular death in patients with heart failure. JAMA 2005; 294: 1794–1798. PMID: 16219883
- 908.
Sakamoto T, Ogawa H, Nakao K, et al. 4C (Candesartan for Prevention of Cardiovascular Events after CYPHERTM or TAXUSTM Coronary Stenting) study investigators. Impact of candesartan on cardiovascular events after drug-eluting stent implantation in patients with coronary artery disease: The 4C trial. J Cardiol 2016; 67: 371–377. PMID: 26254019
- 909.
Kohro T, Hayashi D, Okada Y, et al. JCAD Investigators. Effects of medication on cardiovascular events in the Japanese coronary artery disease (JCAD) study. Circ J 2007; 71: 1835–1840. PMID: 18037732
- 910.
Yamauchi T, Hagiwara N, Kasanuki H, et al. Long-term nitrate use in acute myocardial infarction (the Heart Institute of Japan, Department of Cardiology nitrate evaluation program). Cardiovasc Drugs Ther 2008; 22: 177–184. PMID: 18288597
- 911.
Kojima S, Matsui K, Sakamoto T, et al. Japanese Acute Coronary Syndrome Study (JACSS) Investigators. Long-term nitrate therapy after acute myocardial infarction does not improve or aggravate prognosis. Circ J 2007; 71: 301–307. PMID: 17322625
- 912.
Horinaka S, Yabe A, Yagi H, et al. JCAD Study Investigators. Effects of nicorandil on cardiovascular events in patients with coronary artery disease in the Japanese Coronary Artery Disease (JCAD) study. Circ J 2010; 74: 503–509. PMID: 20081320
- 913.
Kasama S, Toyama T, Sumino H, et al. Prognostic value of cardiac sympathetic nerve activity evaluated by [123I]m-iodobenzylguanidine imaging in patients with ST-segment elevation myocardial infarction. Heart 2011; 97: 20–26. PMID: 21062772
- 914.
IONA Study Group. Impact of nicorandil in angina: Subgroup analyses. Heart 2004; 90: 1427–1430. PMID: 15547020
- 915.
Nissen SE, Tuzcu EM, Libby P, et al. CAMELOT Investigators. Effect of antihypertensive agents on cardiovascular events in patients with coronary disease and normal blood pressure: The CAMELOT study: A randomized controlled trial. JAMA 2004; 292: 2217–2225. PMID: 15536108
- 916.
Matsuzaki M, Yokoyama M, Saito Y, et al. JELIS Investigators. Incremental effects of eicosapentaenoic acid on cardiovascular events in statin-treated patients with coronary artery disease. Circ J 2009; 73: 1283–1290. PMID: 19423946
- 916a.
Bhatt DL, Steg PG, Miller M, et al; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med 2019; 380: 11–22. PMID: 30415628
- 917.
Furukawa Y, Taniguchi R, Ehara N, et al. CREDO-Kyoto Investigators. Better survival with statin administration after revascularization therapy in Japanese patients with coronary artery disease: Perspectives from the CREDO-Kyoto registry. Circ J 2008; 72: 1937–1945. PMID: 18948669
- 918.
Mabuchi H, Kita T, Matsuzaki M, et al. J-LIT Study Group. Japan Lipid Intervention Trial. Large scale cohort study of the relationship between serum cholesterol concentration and coronary events with low-dose simvastatin therapy in Japanese patients with hypercholesterolemia and coronary heart disease: Secondary prevention cohort study of the Japan Lipid Intervention Trial (J-LIT). Circ J 2002; 66: 1096–1100. PMID: 12499612
- 919.
Naito R, Miyauchi K, Konishi H, et al. Appropriate level of low-density lipoprotein cholesterol for secondary prevention of coronary artery disease. J Atheroscler Thromb 2016; 23: 413–421. PMID: 26558399
- 920.
Natsuaki M, Furukawa Y, Morimoto T, et al. CREDO-Kyoto PCI/CABG registry cohort-2 investigators. Intensity of statin therapy, achieved low-density lipoprotein cholesterol levels and cardiovascular outcomes in Japanese patients after coronary revascularization: Perspectives from the CREDO-Kyoto registry cohort-2. Circ J 2012; 76: 1369–1379. PMID: 22447012
- 921.
Mihaylova B, Emberson J, Blackwell L, et al. Cholesterol Treatment Trialists’ (CTT) Collaborators. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: Meta-analysis of individual data from 27 randomised trials. Lancet 2012; 380: 581–590. PMID: 22607822
- 922.
Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: A meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010; 376: 1670–1681. PMID: 21067804
- 923.
Stone NJ, Robinson JG, Lichtenstein AH, et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 63: 2889–2934. PMID: 24239923
- 924.
Piepoli MF, Hoes AW, Agewall S, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J 2016; 37: 2315–2381. PMID: 27222591
- 925.
Taguchi I, Iimuro S, Iwata H, et al. High-dose versus low-dose pitavastatin in Japanese patients with stable coronary artery disease (REAL-CAD): A Randomized Superiority Trial. Circulation 2018; 137: 1997–2009. PMID: 29735587
- 926.
Cannon CP, Blazing MA, Giugliano RP, et al. IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 2015; 372: 2387–2397. PMID: 26039521
- 927.
Sabatine MS, Giugliano RP, Keech AC, et al. FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017; 376: 1713–1722. PMID: 28304224
- 928.
Schwartz GG, Steg PG, Szarek M, et al. ODYSSEY OUTCOMES Committees and Investigators. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med 2018; 379: 2097–2107. PMID: 30403574
- 929.
Nicholls SJ, Puri R, Anderson T, et al. Effect of evolocumab on progression of coronary disease in statin-treated patients: The GLAGOV Randomized Clinical Trial. JAMA 2016; 316: 2373–2384. PMID: 27846344
- 930.
Silverman MG, Ference BA, Im K, et al. Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: A systematic review and meta-analysis. JAMA 2016; 316: 1289–1297. PMID: 27673306
- 931.
Thomopoulos C, Skalis G, Michalopoulou H, et al. Effect of low-density lipoprotein cholesterol lowering by ezetimibe/simvastatin on outcome incidence: Overview, meta-analyses, and meta-regression analyses of randomized trials. Clin Cardiol 2015; 38: 763–769. PMID: 26282344
- 931a.
Hagiwara N, Kawada -Watanabe E, Koyanagi R, et al. Low-densitiy lipoprotein cholesterol targeting with pitavastatin+ezetimibe for patients with acute coronary syndrome and dyslipidemia: The HIJ-PROPER study, a prospective, open-label, randomized trial. Eur Haert J 2017; 38: 2264–2276. PMID: 28430910
- 932.
Zhang XL, Zhu QQ, Zhu L, et al. Safety and efficacy of anti-PCSK9 antibodies: A meta-analysis of 25 randomized, controlled trials. BMC Med 2015; 13: 123. PMID: 26099511
- 933.
Sabatine MS, Giugliano RP, Wiviott SD, et al. Open-Label Study of Long-Term Evaluation against LDL Cholesterol (OSLER) Investigators. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med 2015; 372: 1500–1509. PMID: 25773607
- 934.
Robinson JG, Farnier M, Krempf M, et al. ODYSSEY LONG TERM Investigators. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med 2015; 372: 1489–1499. PMID: 25773378
- 935.
Sabatine MS, Leiter LA, Wiviott SD, et al. Cardiovascular safety and efficacy of the PCSK9 inhibitor evolocumab in patients with and without diabetes and the effect of evolocumab on glycaemia and risk of new-onset diabetes: A prespecified analysis of the FOURIER randomised controlled trial. Lancet Diabetes Endocrinol 2017; 5: 941–950. PMID: 28927706
- 936.
Bonaca MP, Nault P, Giugliano RP, et al. Low-density lipoprotein cholesterol lowering with evolocumab and outcomes in patients with peripheral artery disease: Insights from the FOURIER Trial (Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk). Circulation 2018; 137: 338–350. PMID: 29133605
- 937.
Sabatine MS, De Ferrari GM, Giugliano RP, et al. Clinical benefit of evolocumab by severity and extent of coronary artery disease. Circulation 2018; 138: 756–766. PMID: 29626068
- 938.
Nordestgaard BG, Varbo A. Triglycerides and cardiovascular disease. Lancet 2014; 384: 626–635. PMID: 25131982
- 939.
Iso H, Imano H, Yamagishi K, et al. CIRCS Investigators. Fasting and non-fasting triglycerides and risk of ischemic cardiovascular disease in Japanese men and women: The Circulatory Risk in Communities Study (CIRCS). Atherosclerosis 2014; 237: 361–368. PMID: 25443874
- 940.
Sarwar N, Danesh J, Eiriksdottir G, et al. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studies. Circulation 2007; 115: 450–458. PMID: 17190864
- 941.
Patel A, Barzi F, Jamrozik K, et al. Asia Pacific Cohort Studies Collaboration. Serum triglycerides as a risk factor for cardiovascular diseases in the Asia-Pacific region. Circulation 2004; 110: 2678–2686. PMID: 15492305
- 942.
Hirata T, Sugiyama D, Nagasawa SY, et al. Evidence for Cardiovascular Prevention from Observational Cohorts in Japan (EPOCH-JAPAN) Research Group. A pooled analysis of the association of isolated low levels of high-density lipoprotein cholesterol with cardiovascular mortality in Japan. Eur J Epidemiol 2017; 32: 547–557. PMID: 27709448
- 943.
Bartlett J, Predazzi IM, Williams SM, et al. Is isolated low high-density lipoprotein cholesterol a cardiovascular disease risk factor? New insights from the Framingham Offspring Study. Circ Cardiovasc Qual Outcomes 2016; 9: 206–212. PMID: 27166203
- 944.
Barter P, Gotto AM, LaRosa JC, et al. Treating to New Targets Investigators. HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N Engl J Med 2007; 357: 1301–1310. PMID: 17898099
- 945.
Miyagawa N, Miura K, Okuda N, et al. NIPPON DATA80 Research Group. Long-chain n-3 polyunsaturated fatty acids intake and cardiovascular disease mortality risk in Japanese: A 24-year follow-up of NIPPON DATA80. Atherosclerosis 2014; 232: 384–389. PMID: 24468152
- 946.
Yamagishi K, Iso H, Date C, et al. Japan Collaborative Cohort Study for Evaluation of Cancer Risk Study Group. Fish, omega-3 polyunsaturated fatty acids, and mortality from cardiovascular diseases in a nationwide community-based cohort of Japanese men and women the JACC (Japan Collaborative Cohort Study for Evaluation of Cancer Risk) Study. J Am Coll Cardiol 2008; 52: 988–996. PMID: 18786479
- 947.
Iso H, Kobayashi M, Ishihara J, et al. JPHC Study Group. Intake of fish and n3 fatty acids and risk of coronary heart disease among Japanese: The Japan Public Health Center-Based (JPHC) Study Cohort I. Circulation 2006; 113: 195–202. PMID: 16401768
- 948.
Rizos EC, Ntzani EE, Bika E, et al. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: A systematic review and meta-analysis. JAMA 2012; 308: 1024–1033. PMID: 22968891
- 949.
The ORIGIN Trial Investigators. n-3 fatty acids and cardiovascular outcomes in patients with dysglycemia. N Engl J Med 2012; 367: 309–318. PMID: 22686415
- 950.
Kromhout D, Giltay EJ, Geleijnse JM, et al. Alpha Omega Trial Group. n-3 fatty acids and cardiovascular events after myocardial infarction. N Engl J Med 2010; 363: 2015–2026. PMID: 20929341
- 951.
Yokoyama M, Origasa H, Matsuzaki M, et al. Japan EPA lipid intervention study (JELIS) Investigators. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): A randomised open-label, blinded endpoint analysis. Lancet 2007; 369: 1090–1098. PMID: 17398308
- 952.
Domei T, Yokoi H, Kuramitsu S, et al. Ratio of serum n-3 to n-6 polyunsaturated fatty acids and the incidence of major adverse cardiac events in patients undergoing percutaneous coronary intervention. Circ J 2012; 76: 423–429. PMID: 22156311
- 953.
Studer M, Briel M, Leimenstoll B, et al. Effect of different antilipidemic agents and diets on mortality: A systematic review. Arch Intern Med 2005; 165: 725–730. PMID: 15824290
- 954.
Yokoyama H, Matsushima M, Kawai K, et al. Japan Diabetes Clinical Data Management Study Group. Low incidence of cardiovascular events in Japanese patients with Type 2 diabetes in primary care settings: A prospective cohort study (JDDM 20). Diabet Med 2011; 28: 1221–1228. PMID: 21658121
- 955.
Sone H, Tanaka S, Tanaka S, et al. Japan Diabetes Complications Study Group. Serum level of triglycerides is a potent risk factor comparable to LDL cholesterol for coronary heart disease in Japanese patients with type 2 diabetes: Subanalysis of the Japan Diabetes Complications Study (JDCS). J Clin Endocrinol Metab 2011; 96: 3448–3456. PMID: 21865372
- 956.
Saito I, Kokubo Y, Yamagishi K, et al. Diabetes and the risk of coronary heart disease in the general Japanese population: The Japan Public Health Center-based prospective (JPHC) study. Atherosclerosis 2011; 216: 187–191. PMID: 21300356
- 957.
Kadowaki S, Okamura T, Hozawa A, et al. NIPPON DATA Research Group. Relationship of elevated casual blood glucose level with coronary heart disease, cardiovascular disease and all-cause mortality in a representative sample of the Japanese population. NIPPON DATA80. Diabetologia 2008; 51: 575–582. PMID: 18197396
- 958.
Fujishima M, Kiyohara Y, Kato I, et al. Diabetes and cardiovascular disease in a prospective population survey in Japan: The Hisayama Study. Diabetes 1996; 45 Suppl: S14–S16. PMID: 8674881
- 959.
Takara A, Ogawa H, Endoh Y, et al. Long-term prognosis of diabetic patients with acute myocardial infarction in the era of acute revascularization. Cardiovasc Diabetol 2010; 9: 1. PMID: 20047694
- 960.
Donahoe SM, Stewart GC, McCabe CH, et al. Diabetes and mortality following acute coronary syndromes. JAMA 2007; 298: 765–775. PMID: 17699010
- 961.
Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359: 1577–1589. PMID: 18784090
- 962.
Ueki K, Sasako T, Okazaki Y, et al. J-DOIT3 Study Group. Effect of an intensified multifactorial intervention on cardiovascular outcomes and mortality in type 2 diabetes (J-DOIT3): An open-label, randomised controlled trial. Lancet Diabetes Endocrinol 2017; 5: 951–964. PMID: 29079252
- 963.
Gaede P, Lund-Andersen H, Parving HH, et al. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 2008; 358: 580–591. PMID: 18256393
- 964.
Japan Diabetes Society. Practice Guideline for the Treatment of Diabetes 2016. [Article in Japanese] Nankodo, 2016.
- 965.
Hong J, Zhang Y, Lai S, et al. SPREAD-DIMCAD Investigators. Effects of metformin versus glipizide on cardiovascular outcomes in patients with type 2 diabetes and coronary artery disease. Diabetes Care 2013; 36: 1304–1311. PMID: 23230096
- 966.
Wanner C, Lachin JM, Inzucchi SE, et al. EMPA-REG OUTCOME Investigators. Empagliflozin and clinical outcomes in patients with type 2 diabetes mellitus, established cardiovascular disease, and chronic kidney disease. Circulation 2018; 137: 119–129. PMID: 28904068
- 967.
Wanner C, Inzucchi SE, Lachin JM, et al. EMPA-REG OUTCOME Investigators. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 2016; 375: 323–334. PMID: 27299675
- 968.
Fitchett D, Zinman B, Wanner C, et al. EMPA-REG OUTCOME® trial investigators. Heart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: Results of the EMPA-REG OUTCOME® trial. Eur Heart J 2016; 37: 1526–1534. PMID: 26819227
- 969.
Zinman B, Wanner C, Lachin JM, et al. EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373: 2117–2128. PMID: 26378978
- 970.
Neal B, Perkovic V, Mahaffey KW, et al. CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017; 377: 644–657. PMID: 28605608
- 971.
Mahaffey KW, Neal B, Perkovic V, et al. CANVAS Program Collaborative Group. Canagliflozin for primary and secondary prevention of cardiovascular events: Results from the CANVAS Program (Canagliflozin Cardiovascular Assessment Study). Circulation 2018; 137: 323–334. PMID: 29133604
- 972.
Kaku K, Lee J, Mattheus M, et al. EMPA-REG OUTCOME® Investigators. Empagliflozin and cardiovascular outcomes in Asian patients with type 2 diabetes and established cardiovascular disease: Results from EMPA-REG OUTCOME®. Circ J 2017; 81: 227–234. PMID: 28025462
- 973.
Marso SP, Daniels GH, Brown-Frandsen K, et al. LEADER Steering Committee. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016; 375: 311–322. PMID: 27295427
- 974.
Marso SP, Bain SC, Consoli A, et al. SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016; 375: 1834–1844. PMID: 27633186
- 975.
Pfeffer MA, Claggett B, Diaz R, et al. ELIXA Investigators. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med 2015; 373: 2247–2257. PMID: 26630143
- 976.
Holman RR, Bethel MA, Mentz RJ, et al. EXSCEL Study Group. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2017; 377: 1228–1239. PMID: 28910237
- 977.
Dormandy JA, Charbonnel B, Eckland DJ, et al. PROactive Investigators. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): A randomised controlled trial. Lancet 2005; 366: 1279–1289. PMID: 16214598
- 978.
Lincoff AM, Wolski K, Nicholls SJ, et al. Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: A meta-analysis of randomized trials. JAMA 2007; 298: 1180–1188. PMID: 17848652
- 979.
Nissen SE, Nicholls SJ, Wolski K, et al. PERISCOPE Investigators. Comparison of pioglitazone vs glimepiride on progression of coronary atherosclerosis in patients with type 2 diabetes: The PERISCOPE randomized controlled trial. JAMA 2008; 299: 1561–1573. PMID: 18378631
- 980.
Tuccori M, Filion KB, Yin H, et al. Pioglitazone use and risk of bladder cancer: Population based cohort study. BMJ 2016; 352: i1541. PMID: 27029385
- 981.
Lewis JD, Habel LA, Quesenberry CP, et al. Pioglitazone use and risk of bladder cancer and other common cancers in persons with diabetes. JAMA 2015; 314: 265–277. PMID: 26197187
- 982.
Levin D, Bell S, Sund R, et al. Scottish Diabetes Research Network Epidemiology Group, Diabetes and Cancer Research Consortium. Pioglitazone and bladder cancer risk: A multipopulation pooled, cumulative exposure analysis. Diabetologia 2015; 58: 493–504. PMID: 25481707
- 983.
Chiasson JL, Josse RG, Gomis R, et al. STOP-NIDDM Trial Research Group. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: The STOP-NIDDM trial. JAMA 2003; 290: 486–494. PMID: 12876091
- 984.
Chiasson JL, Josse RG, Gomis R, et al. STOP-NIDDM Trail Research Group. Acarbose for prevention of type 2 diabetes mellitus: The STOP-NIDDM randomised trial. Lancet 2002; 359: 2072–2077. PMID: 12086760
- 985.
Hanefeld M, Cagatay M, Petrowitsch T, et al. Acarbose reduces the risk for myocardial infarction in type 2 diabetic patients: Meta-analysis of seven long-term studies. Eur Heart J 2004; 25: 10–16. PMID: 14683737
- 986.
White WB, Cannon CP, Heller SR, et al. EXAMINE Investigators. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 2013; 369: 1327–1335. PMID: 23992602
- 987.
Green JB, Bethel MA, Armstrong PW, et al. TECOS Study Group. Effect of Sitagliptin on Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med 2015; 373: 232–242. PMID: 26052984
- 988.
Scirica BM, Bhatt DL, Braunwald E, et al. SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013; 369: 1317–1326. PMID: 23992601
- 989.
Udell JA, Zawi R, Bhatt DL, et al. Association between influenza vaccination and cardiovascular outcomes in high-risk patients: A meta-analysis. JAMA 2013; 310: 1711–1720. PMID: 24150467
- 990.
Ahmed MB, Patel K, Fonarow GC, et al. Higher risk for incident heart failure and cardiovascular mortality among community-dwelling octogenarians without pneumococcal vaccination. ESC Heart Fail 2016; 3: 11–17. PMID: 27668089
- 991.
Giugliano RP, Cannon CP, Blazing MA, et al. IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial) Investigators. Benefit of adding ezetimibe to statin therapy on cardiovascular outcomes and safety in patients with versus without diabetes mellitus: Results from IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial). Circulation 2018; 137: 1571–1582. PMID: 29263150
- 992.
Bohula EA, Morrow DA, Giugliano RP, et al. Atherothrombotic risk stratification and ezetimibe for secondary prevention. J Am Coll Cardiol 2017; 69: 911–921. PMID: 28231942
- 993.
Eisen A, Cannon CP, Blazing MA, et al. IMPROVE-IT (IMProved Reduction of Outcomes: Vytorin Efficacy International Trial) Investigators. The benefit of adding ezetimibe to statin therapy in patients with prior coronary artery bypass graft surgery and acute coronary syndrome in the IMPROVE-IT trial. Eur Heart J 2016; 37: 3576–3584. PMID: 27569841
- 994.
Keech A, Simes RJ, Barter P, et al. FIELD study investigators. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): Randomised controlled trial. Lancet 2005; 366: 1849–1861. PMID: 16310551
- 995.
White HD, Chew DP. Acute myocardial infarction. Lancet 2008; 372: 570–584. PMID: 18707987
- 996.
Pedersen F, Butrymovich V, Kelbæk H, et al. Short- and long-term cause of death in patients treated with primary PCI for STEMI. J Am Coll Cardiol 2014; 64: 2101–2108. PMID: 25457398
- 997.
Piepoli MF, Corrà U, Dendale P, et al. Challenges in secondary prevention after acute myocardial infarction: A call for action. Eur J Prev Cardiol 2016; 23: 1994–2006. PMID: 27600690
- 998.
Fukui T, Takagi M, Komuro I, total editors. Today’s Therapy 2017. [Article in Japanese] Igaku-Shoin, 2017.
- 999.
Goto Y. Current state of cardiac rehabilitation in Japan. Prog Cardiovasc Dis 2014; 56: 557–562. PMID: 24607022
- 1000.
Bethell H, Lewin R, Evans J, et al. Outpatient cardiac rehabilitation attendance in England: Variability by region and clinical characteristics. J Cardiopulm Rehabil Prev 2008; 28: 386–391. PMID: 19008693
- 1001.
Arakawa T, Kumasaka L, Nakanishi M, et al. Regional clinical alliance path and cardiac rehabilitation after hospital discharge for acute myocardial infarction patients in Japan: A nationwide survey. Circ J 2016; 80: 1750–1755. PMID: 27357332
- 1002.
Anderson L, Oldridge N, Thompson DR, et al. Exercise-based cardiac rehabilitation for coronary heart disease: Cochrane systematic review and meta-analysis. J Am Coll Cardiol 2016; 67: 1–12. PMID: 26764059
- 1003.
Taylor RS, Brown A, Ebrahim S, et al. Exercise-based rehabilitation for patients with coronary heart disease: Systematic review and meta-analysis of randomized controlled trials. Am J Med 2004; 116: 682–692. PMID: 15121495
- 1004.
Dalal HM, Zawada A, Jolly K, et al. Home based versus centre based cardiac rehabilitation: Cochrane systematic review and meta-analysis. BMJ 2010; 340: b5631. PMID: 20085991
- 1005.
EACPR Committee for Science Guidelines. Corrà U, Piepoli MF, Carré F, et al. Secondary prevention through cardiac rehabilitation: Physical activity counselling and exercise training: Key components of the position paper from the Cardiac Rehabilitation Section of the European Association of Cardiovascular Prevention and Rehabilitation. Eur Heart J 2010; 31: 1967–1974. PMID: 20643803
- 1006.
Pouche M, Ruidavets JB, Ferrières J, et al. Cardiac rehabilitation and 5-year mortality after acute coronary syndromes: The 2005 French FAST-MI study. Arch Cardiovasc Dis 2016; 109: 178–187. PMID: 26711546
- 1007.
Suzuki T, Kohro T, Hayashi D, et al. Frequency and impact of lifestyle modification in patients with coronary artery disease: The Japanese Coronary Artery Disease (JCAD) study. Am Heart J 2012; 163: 268–273. PMID: 22305846
- 1008.
Kamakura T, Kawakami R, Nakanishi M, et al. Efficacy of out-patient cardiac rehabilitation in low prognostic risk patients after acute myocardial infarction in primary intervention era. Circ J 2011; 75: 315–321. PMID: 21173494
- 1009.
Takaya Y, Kumasaka R, Arakawa T, et al. Impact of cardiac rehabilitation on renal function in patients with and without chronic kidney disease after acute myocardial infarction. Circ J 2014; 78: 377–384. PMID: 24225305
- 1010.
Stone GW, Moses JW, Ellis SG, et al. Safety and efficacy of sirolimus- and paclitaxel-eluting coronary stents. N Engl J Med 2007; 356: 998–1008. PMID: 17296824
- 1011.
Kurose S, Iwasaka J, Kimura Y, et al. Effect of cardiac rehabilitation on mild stenotic lesion in acute coronary syndrome patients. [Article in Japanese] Shinzo 2014; 46: 32–39.
- 1012.
Tóth-Zsámboki E, Horváth Z, Hajtman L, et al. Cardiac rehabilitation programme as a non-pharmacological platelet inhibitory tool in acute coronary syndrome survivors. Eur J Prev Cardiol 2017; 24: 1148–1156. PMID: 28438028
- 1013.
Rauch B, Davos CH, Doherty P, et al. ‘Cardiac Rehabilitation Section’, European Association of Preventive Cardiology (EAPC), in cooperation with the Institute of Medical Biometry and Informatics (IMBI), Department of Medical Biometry, University of Heidelberg, and the Cochrane Metabolic and Endocrine Disorders Group, Institute of General Practice, Heinrich-Heine University, Düsseldorf, Germany. The prognostic effect of cardiac rehabilitation in the era of acute revascularisation and statin therapy: A systematic review and meta-analysis of randomized and non-randomized studies: The Cardiac Rehabilitation Outcome Study (CROS). Eur J Prev Cardiol 2016; 23: 1914–1939. PMID: 27777324
- 1014.
Goel K, Lennon RJ, Tilbury RT, et al. Impact of cardiac rehabilitation on mortality and cardiovascular events after percutaneous coronary intervention in the community. Circulation 2011; 123: 2344–2352. PMID: 21576654
- 1015.
West RR, Jones DA, Henderson AH. Rehabilitation after myocardial infarction trial (RAMIT): Multi-centre randomised controlled trial of comprehensive cardiac rehabilitation in patients following acute myocardial infarction. Heart 2012; 98: 637–644. PMID: 22194152
- 1016.
Goto Y. Keynote lecture: Evidence of cardiac rehabilitation in Japan. [Article in Japanese] Journal of Japanese Cardiac Rehabilitation 2014; 19: 26–29.
- 1017.
Seki E, Watanabe Y, Sunayama S, et al. Effects of phase III cardiac rehabilitation programs on health-related quality of life in elderly patients with coronary artery disease: Juntendo Cardiac Rehabilitation Program (J-CARP). Circ J 2003; 67: 73–77. PMID: 12520156
- 1018.
Seki E, Watanabe Y, Shimada K, et al. Effects of a phase III cardiac rehabilitation program on physical status and lipid profiles in elderly patients with coronary artery disease: Juntendo Cardiac Rehabilitation Program (J-CARP). Circ J 2008; 72: 1230–1234. PMID: 18654005
- 1019.
Tanaka N, Nakane E, Ebihara K, et al. Effects to cardiac load by hot and soak up to shoulder bathing for improvement of QOL of cardiac patients. [Article in Japanese] Journal of Japanese Cardiac Rehabilitation 2004; 9: 144–148.
- 1020.
Hasegawa T. How to manage daily life: There are able and impossible activities. [Article in Japanese] Journal of Japanese Cardiac Rehabilitation 1999; 4: 26–28.
- 1021.
Dreyer RP, Xu X, Zhang W, et al. Return to work after acute myocardial infarction: Comparison between young women and men. Circ Cardiovasc Qual Outcomes 2016; 9 Suppl: S45–S52. PMID: 26908859
- 1022.
Smith D, Toff W, Joy M, et al. Fitness to fly for passengers with cardiovascular disease. Heart 2010; 96 Suppl: ii1–ii16. PMID: 20644218
- 1023.
Saito M, Ueshima K, Saito M, et al. Japanese Cardiac Rehabilitation Survey Investigators. Safety of exercise-based cardiac rehabilitation and exercise testing for cardiac patients in Japan: A nationwide survey. Circ J 2014; 78: 1646–1653. PMID: 24837707
- 1024.
Onishi T, Shimada K, Sato H, et al. Effects of phase III cardiac rehabilitation on mortality and cardiovascular events in elderly patients with stable coronary artery disease. Circ J 2010; 74: 709–714. PMID: 20208382
- 1025.
Soga Y, Yokoi H, Ando K, et al. Safety of early exercise training after elective coronary stenting in patients with stable coronary artery disease. Eur J Cardiovasc Prev Rehabil 2010; 17: 230–234. PMID: 20051870
- 1026.
Soga Y, Yokoi H, Amemiya K, et al. Safety and efficacy of exercise training after coronary stenting in patients with stable coronary artery disease. [Article in Japanese] Circ J 2011; 75: 2379–2386. PMID: 21799272
- 1027.
Kochi K, Kato M, Kawase S, et al. Effects of cardiac rehabilitation on achievement of coronary risk factors goal in patients with acute myocardial infarction. [Article in Japanese] Journal of Japanese Cardiac Rehabilitation 2016; 22: 269–273.
- 1028.
Urbinati S, Olivari Z, Gonzini L, et al. BLITZ-4 Investigators. Secondary prevention after acute myocardial infarction: Drug adherence, treatment goals, and predictors of health lifestyle habits. The BLITZ-4 Registry. Eur J Prev Cardiol 2015; 22: 1548–1556. PMID: 25452625
- 1029.
Piepoli MF, Corrà U, Dendale P, et al. Challenges in secondary prevention after acute myocardial infarction: A call for action. Eur Heart J Acute Cardiovasc Care 2017; 6: 299–310. PMID: 28608759
- 1030.
Redfern J, Briffa T, Ellis E, et al. Choice of secondary prevention improves risk factors after acute coronary syndrome: 1-year follow-up of the CHOICE (Choice of Health Options In prevention of Cardiovascular Events) randomised controlled trial. Heart 2009; 95: 468–475. PMID: 18801781
- 1031.
Japanese Association of Cardiac Rehabilitation Standard Cardiac Rehabilitation Program Writing Committee. Cardiac Rehabilitation Standard Program for Acute Myocardial Infarction (2013) from the Japanese Association of Cardiac Rehabilitation In the Recovery Phase of Myocardial Infarction. [Article in Japanese] http://www.jacr.jp/web/pdf/program2013.pdf
- 1032.
Nohara R, Kambara H, Mohiuddin IH, et al. Cardiac sports rehabilitation for patients with ischemic heart disease. Jpn Circ J 1990; 54: 1443–1450. PMID: 2287049
- 1033.
Makita S. Sport as cardiac rehabilitation. [Article in Japanese] Shinzo 2016; 48: 142–146.
- 1034.
JAPAN HEART CLUB. [in Japanese] http://npo-jhc.org/
- 1035.
Sunamura M, Ter Hoeve N, Geleijnse ML, et al. Cardiac rehabilitation in patients who underwent primary percutaneous coronary intervention for acute myocardial infarction: Determinants of programme participation and completion. Neth Heart J 2017; 25: 618–628. PMID: 28917025
- 1036.
Morisawa T, Iwata K, Ueno K, et al. A study of national survey on cardiac rehabilitation in convalescent rehabilitation hospitals: Based on results of national survey. [Article in Japanese] Rigakuryouhogaku 2016; 43: 10–17.
- 1037.
Coleman EA, Parry C, Chalmers S, et al. The care transitions intervention: Results of a randomized controlled trial. Arch Intern Med 2006; 166: 1822–1828. PMID: 17000937
- 1038.
Hesselink G, Schoonhoven L, Barach P, et al. Improving patient handovers from hospital to primary care: A systematic review. Ann Intern Med 2012; 157: 417–428. PMID: 22986379
- 1039.
Clark AM, Hartling L, Vandermeer B, et al. Meta-analysis: Secondary prevention programs for patients with coronary artery disease. Ann Intern Med 2005; 143: 659–672. PMID: 16263889
- 1040.
Schiele F, Gale CP, Bonnefoy E, et al. Quality indicators for acute myocardial infarction: A position paper of the Acute Cardiovascular Care Association. Eur Heart J Acute Cardiovasc Care 2017; 6: 34–59. PMID: 27574334
- 1041.
Jneid H, Addison D, Bhatt DL, et al. 2017 AHA/ACC clinical performance and quality measures for adults with ST-elevation and non-ST-elevation myocardial infarction: A report of the American College of Cardiology/American Heart Association Task Force on performance measures. J Am Coll Cardiol 2017; 70: 2048–2090. PMID: 28943066
- 1042.
Inohara T, Kohsaka S, Yamaji K, et al. J-PCI Registry Investigators. Impact of institutional and operator volume on short-term outcomes of percutaneous coronary intervention: A report from the Japanese Nationwide Registry. JACC Cardiovasc Interv 2017; 10: 918–927. PMID: 28473114
- 1043.
Yasuda S, Nakao K, Nishimura K, et al. on the behalf of JROAD Investigators. The current status of cardiovascular medicine in Japan: Analysis of a large number of health records from a Nationwide Claim-Based Database, JROAD-DPC. Circ J 2016; 80: 2327–2335. PMID: 27725417
- 1044.
Yasuda S, Miyamoto Y, Ogawa H. Current status of cardiovascular medicine in the Aging Society of Japan. Circulation 2018; 138: 965–967. PMID: 30354536
- 1045.
Kitamura T, Iwami T, Kawamura T, et al. Implementation Working Group for the All-Japan Utstein Registry of the Fire and Disaster Management Agency. Nationwide public-access defibrillation in Japan. N Engl J Med 2010; 362: 994–1004. PMID: 20237345
- 1046.
Piepoli MF, Corrà U, Abreu A, et al. Cardiac Rehabilitation Section of the European Association for Cardiovascular Prevention & Rehabilitation of the ESC. Challenges in secondary prevention of cardiovascular diseases: A review of the current practice. Int J Cardiol 2015; 180: 114–119. PMID: 25438230
- 1047.
Redfern J, Maiorana A, Neubeck L, et al. Achieving coordinated secondary prevention of coronary heart disease for all in need (SPAN). Int J Cardiol 2011; 146: 1–3. PMID: 20826024
- 1048.
Ikemura N, Sawano M, Ueda I, et al. Consequence of reimbursement policy alteration for urgent PCI in Japan. Lancet 2018; 391: 2208–2209. PMID: 29893216
- 1049.
Shiozaki M, Inoue K, Suwa S, et al. Utility of the 0-hour/1-hour high-sensitivity cardiac troponin T algorithm in Asian patients with suspected non-ST elevation myocardial infarction. Int J Cardiol 2017; 249: 32–35. PMID: 28986063
- 1050.
Kohsaka S, Miyata H, Ueda I, et al. JCD-KiCS and NCDR. An international comparison of patients undergoing percutaneous coronary intervention: A collaborative study of the National Cardiovascular Data Registry (NCDR) and Japan Cardiovascular Database-Keio interhospital Cardiovascular Studies (JCD-KiCS). Am Heart J 2015; 170: 1077–1085. PMID: 26678628
- 1051.
Levine GN, Jeong YH, Goto S, et al. Expert consensus document: World Heart Federation expert consensus statement on antiplatelet therapy in East Asian patients with ACS or undergoing PCI. Nat Rev Cardiol 2014; 11: 597–606. PMID: 25154978
- 1052.
Inohara T, Kohsaka S, Miyata H, et al. Performance and Validation of the U.S. NCDR Acute Kidney Injury Prediction Model in Japan. J Am Coll Cardiol 2016; 67: 1715–1722. PMID: 27056778
- 1053.
Inohara T, Numasawa Y, Higashi T, et al. Predictors of high cost after percutaneous coronary intervention: A review from Japanese multicenter registry overviewing the influence of procedural complications. Am Heart J 2017; 194: 61–72. PMID: 29223436










