2019 Volume 83 Issue 8 Pages 1726-1736
2019 Volume 83 Issue 8 Pages 1726-1736
Background: Withaferin A (WFA), an anticancer constituent of the plant Withania somnifera, inhibits tumor growth in association with apoptosis induction. However, the potential role of WFA in the cardiovascular system is little-studied and controversial.
Methods and Results: Two different doses of WFA were tested to determine their cardioprotective effects in myocardial ischemia/reperfusion (MI/R) injury through evaluation of cardiofunction in wild-type and AMP-activated protein kinase domain negative (AMPK-DN) gentransgenic mice. Surprisingly, cardioprotective effects (improved cardiac function and reduced infarct size) were observed with low-dose WFA (1 mg/kg) delivery but not high-dose (5 mg/kg). Mechanistically, low-dose WFA attenuated myocardial apoptosis. It decreased MI/R-induced activation of caspase 9, the indicator of the intrinsic mitochondrial pathway, but not caspase 8. It also upregulated the level of AMP-activated protein kinase (AMPK) phosphorylation and increased the MI/R inhibited ratio of Bcl2/Bax. In AMPK-deficient mice, WFA did not ameliorate MI/R-induced cardiac dysfunction, attenuate infarct size, or restore the Bcl2/Bax (B-cell lymphoma2/Mcl-2-like protein 4) ratio.
Conclusions: These results demonstrated for the first time that low-dose WFA is cardioprotective via upregulation of the anti-apoptotic mitochondrial pathway in an AMPK-dependent manner.
Withaferin A (WFA), a steroidal lactone purified from Withania somnifera, is one of the most active components found in the Indian winter cherry, which is an important medicinal plant in the Ayurvedic and Uniai systems of medicine. Prospective studies have reported that WFA has antitumor and immunomodulatory activities.1–4 Experimental studies have demonstrated that WFA inhibits nuclear factor κ-B (NF-κB)-regulated gene expression,5–7 and activates the MAPK family in multiple experimental models,8–10 displaying anti-inflammatory and antioxidant properties when tested on human cancer cells in vitro. Pharmacological levels of WFA triggered anticancer effects specific to several breast cancer cell lines.11 Although these results suggest that WFA is associated with a positive treatment effect in promoting apoptosis in cancer cells, other researchers have reported that it is responsible for an antiapoptotic effect in brain cells;12 these discrepant roles of WFA in vivo have been not addressed completely and are even unclear in the cardiovascular system.
Myocardial infarction (MI) is a leading cause of death and a major health problem worldwide.13 Despite WFA possessing positive effects in oncology, the possible worrisome manifestation of toxicity in the cardiovascular system is a concern. Recent in vitro studies have revealed antiapoptotic effects of WFA on cardiomyocytes in the regulation of Akt activation.14 However, WFA exerts potent antitumor activity in vivo at doses that are different from those previously reported for antiangiogenic activity.15,16 Because this discrepancy remains unexplained, there is a great need for clarification of WFA’s influence in the cardiovascular system in vivo in order to explore a safer therapeutic strategy for cancer patients with cardiovascular complications by determining the therapeutic effects and toxicity of WFA.
Reducing inflammation and oxidative stress reduces ischemia reperfusion-induced cardiomyocyte apoptosis. Clarifying the mechanism by which WFA acts upon the cardiovascular system will contribute to our understanding of its biological function and provide insight into a new, potential therapeutic modality. Because necrosis and apoptosis appear to be ongoing during ischemia, while apoptosis is boosted by the reperfusion event,17 we explored an ischemia/reperfusion (I/R) model to determine the effects of WFA on the cardiovascular apoptosis in vivo and sought to investigate the potential underlying the cellular and molecular mechanisms of phenomena observed in an animal model.
The aims of the current study were to: (1) determine whether WFA modulates myocardial apoptosis in mice subjected to myocardial I/R (MI/R); (2) determine whether WFA exerts regulatory effects in an AMP-activated protein kinase (AMPK)-dependent fashion; and (3) to dissect the molecular mechanisms underlying WFA’s cardioprotective role.
Adult male wild-type (WT) mice and adult male AMPK-DN mice [dominant negative α2-subunit (D157A) of AMPK] and respective male littermate controls were used in this study. Generation, breeding, phenotype characteristics, and genotyping of AMPK-DN mice (80% inhibition of cardiac AMPK activity) have been previously described in detail.18 All experiments were performed in adherence with the National Institutes of Health Guidelines on the Use of Laboratory Animals and were approved by the Thomas Jefferson University Committee on Animal Care and Shanxi Medical University Committee on Animal Care.
Myocardial I/RLow-dose (1 mg/kg) or high-dose (5 mg/kg) WFA was administered by abdominal injection 90 min prior to surgery. Mice were anesthetized with 2% isoflurane. The MI/R surgical procedure was performed as described previously.19 Briefly, the left anterior descending coronary artery was bound and 30 min later, the myocardium was reperfused for 3 h (all assays except cardiac function and infarct size) or 24 h (cardiac function and infarct size). Sham-operated control mice (Sham MI/R) underwent the same surgical procedure, except the suture placed under the left coronary artery was not tied. All assays utilized tissue from the I/R area and the area-at-risk (AAR), identified by Evans blue negative staining.
Determination of Myocardial Apoptosis and Myocardial Infarct SizeMyocardial apoptosis was determined by terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) staining and caspase-3 activity, as described in our previous study.19,20 Mice hearts were fixed and sectioned along the long axis (6 μm in thickness). TUNEL staining of the sections was performed with the In-Situ Cell Death Detection Kit (Roche).
Apoptotic cells with green fluorescence were counted in a blinded fashion using Image J to assess the apoptotic index (TUNEL-positive cardiomyocytes). More than 1,000 cells per heart were counted. Myocardial infarct size was assessed by the Evans blue-2,3,5-triphenyl tetrazolium chloride (TTC) double-staining method.19
Caspase-3 activity assay was performed as previously described.21 Briefly, cardiac tissues were harvested using caspase lysis buffer, and the lysate samples (50 µg) were used to carry out the fluorometric assay per the manufacturer’s instructions of a fluorometric kit (R&D System, Minneapolis, MN, USA). The fluorescence emission of 7-amino-4-trifluoromethyl-coumarin (AFC), released on proteolytic cleavage of the fluorogenic substrate DEVD-AFC by active caspase-3, was measured using SpectraMax-Plus microplate spectrophotometer (Molecular Devices, Sunnyvale, CA, USA) with a fixed wavelength (excitation wavelength, 400 nm; emission wavelength, 505 nm). Caspase-3 activity was expressed as nmol AFC/h/mg protein.
Determination of Cardiac FunctionTransthoracic echocardiographic analysis was performed with a 12-MHz probe in a blinded manner while the mice were anesthetized with isoflurane (2% maintenance). M-mode echocardiography was carried out in the parasternal short-axis in mice 24 h after reperfusion to assess heart rate, LV ejection fraction (LVEF) and fractional shortening.
Hemodynamic analysis was conducted in a blinded manner as described previously.19,22 Briefly, a 1.4-Fr micromanometer-tipped catheter (Millar Inc., Houston, TX, USA) was inserted into the right carotid artery and advanced into the LV. Steady-state LV maximum (+dP/dTmax) and minimum (−dP/dTmax) was recorded in closed-chest mode via PowerLab DAQ system (Millar Inc.).
Primary Neonatal Cardiomyocyte Culture and siRNA DeliveryNeonatal rat ventricular myocytes (NRVMs) were isolated from 1–2-day-old Sprague-Dawley rats (Envigo RMS, Indianapolis, IN, USA) by enzymatic digestion method as previously described.23 Fibroblasts were removed by preplating for 2 h. NRVMs were cultured overnight in F10 medium (Mediatech, Manassas, VA, USA) containing 10% horse serum, 5% fetal bovine serum (FBS), and 1% penicillin/streptomycin/amphotericin B solution (Invitrogen) at 37℃ in a humidified incubator with 5% CO2. The following day, medium was replaced with F-10 medium containing 1% FBS.
Transient siRNA transfections were conducted using scramble siRNA or siRNA AMPKα2 (final concentration 50 nmol/L) and TransMessenger transfection reagent (Qiagen) per 35-mm dish of NRVMs. Experiments were conducted on cells 48 h after transfection.
Simulated I/R (SI/R)Prepared NRVMs were placed in a 5% CO2 incubator at 37℃. NRVMs were subjected to SI/R as modified in our published study.21 In brief, glucose-free culture medium was first gassed for 5 min with a hypoxic gas mixture (95% N2–5% CO2). Normal culture medium was quickly replaced with the hypoxic-hypoglycemic medium containing either vehicle or WFA (500 nmol/L), and NRVMs were placed in a HERAcell VIOS hypoxia (1% O2–5% CO2–94% N2) incubator (Thermo Scientific, Waltham, MA, USA). After 4 h of hypoxic-hypoglycemic culture, the hypoxic-hypoglycemic medium was replaced with normal culture medium (containing the same concentration of vehicle or WFA). Cells were then incubated under normoxic conditions in a CO2 incubator for an additional 6 h.
Western Blot AnalysisCardiac tissue (30 mg) was extracted with cell lysis buffer and quantified using the modified Lowry method (Bio-Rad Laboratories, USA). Next, 50 µg protein were separated on SDS-PAGE gels, transferred to PVDF membranes, and incubated with primary antibodies against cleaved caspase-3, caspase-3, cleaved caspase-9, caspase-9, cleaved caspase-8, caspase-8, phospho-AMPK, AMPK, phospho-ERK, ERK, phospho-JNK, JNK, phospho-p38, p38, phospho-AKT, AKT, BCL2, BAX, p62 and LC3I/II (Cell Signaling Technology, Danvers, MA, USA) and horseradish peroxidase-conjugated secondary antibody. The blot was developed with a Supersignal Chemiluminescence detection kit (Pierce, Rockford, IL, USA) and observed with a ChemiDocTM MP Imaging System (Bio-Rad).
Messenger RNA (mRNA) Extraction and Real-Time qPCRHeart total RNA was isolated using TRIzol reagent (Invitrogen) per manufacturer’s protocol. Briefly, 1 μg total RNA was first reversely transcribed to cDNA by PrimeScriptTM RT Master Mix (Takara). Real-time PCR reactions were performed via the PowerUpTM SYBRTM Green Master Mix (Thermo Fisher Scientific) on a QuantStudio 5 applied biosystems (Thermo Fisher Scientific). Primers used in the study are listed in the online supplement (Supplementary Table). The level of β-actin mRNA in each sample served as the internal control. Relative quantities were analyzed by the 2−∆∆Ct method. All reactions were executed and analyzed by Gene Expression Analysis software for iCycler iQ® Real-Time qPCR Detection System (Bio-Rad).
Statistical AnalysisAll data are reported as mean±SEM. Data were analyzed by 1- and 2-way analysis of variance (ANOVA) followed by the Tukey post hoc test for multiple comparisons. For all statistical tests, P<0.05 was considered statistically significant. All statistical analyses were performed via GraphPad Prism 7.0.
Previous studies have demonstrated that WFA’s pro-apoptotic properties in cancer are related to the dosage.1,24 Furthermore, at nanomolar doses, WFA preserves potent anti-invasive activity but limits the cytotoxic activity in cancer cells.8 Hence, we chose 1 mg/kg (equivalent to 500 nmol/L)25 and a higher dose (5 mg/kg)26 as the research doses to investigate WFA’s effect on apoptotic activity in the cardiovascular system as a method of identifying the properties of WFA in preventing cardiomyocyte damage. To clarify the effects of WFA on apoptosis during MI/R, we subjected mice to WFA pretreatment at different concentrations. Low-dose WFA (1 mg/kg) improved cardiac function, as evidenced by increased LVEF (Figure 1A,B) and increased dP/dtmax and dP/dtmin (Figure 1C), as well as reduced infarct size (Figure 1D). But high-dose WFA (5 mg/kg) showed negative modulatory effects on dP/dtmax and dP/dtmin compared with MI/R (Figure 1). Meanwhile, cardiac tissue weight (ventricular weight/body weight, mg/g) was evaluated after MI/R and there was no significant change in any of the groups (Sham MI/R: 3.41±0.26; WFA-0 mg MI/R: 3.54±0.56; WFA-1 mg MI/R: 3.73±0.38; WFA-5 mg MI/R: 3.74±0.28). These results provided direct evidence that low-dose WFA in vivo effectively protected cardiac function from MI/R injury. Low-dose WFA was administered in all subsequent experiments to dissect the responsible mechanistic signaling.

Effects of different in vivo doses of WFA on cardiac function after MI/R induced cardiac injury. (A) Representative echocardiogram recordings, (B) LVEF. (C) ±dP/dt max. (D) Myocardial infarct size (expressed as % of area-at-risk, AAR) after left anterior descending artery occlusion for 30 min and reperfusion for 24 h. (Top) Representative photographs of heart sections: black-staining indicates the nonischemic region; red-staining shows I/R but not the infarcted region. (Bottom) Summary of myocardial infarct size expressed as a percent of the total ischemic reperfused area. n=9–12 mice/group. *P<0.05 vs. Sham, #P<0.05 vs. vehicle treatment MI/R group. LVEF, left ventricular ejection fraction; MI/R, myocardial ischemia/reperfusion; WFA, withaferin A.
Apoptosis is a major cause of cardiomyocyte death after MI/R. To investigate whether WFA reduced infarct size in association with mitigation of cardiomyocyte apoptosis, TUNEL staining and caspase-3 activity in MI/R cardiac tissue were determined. We found that MI/R-induced cardiomyocyte apoptosis was markedly decreased in vivo in animals that received low-dose WFA (1 mg/kg), as evidenced by decreased TUNEL staining (Figure 2A) and caspase-3 activity (−29±4%) (Figure 2B).

Antiapoptotic effect of WFA (1 mg/kg). (A, Left) Representative TUNEL staining. Total nuclei (DAPI staining, blue) and TUNEL-positive nuclei (green) were counted. (Right) Index of apoptosis (number of positively attained myocytes/total number of myocytes×100%) was calculated. (B, Left) Representative photograph of Western blot and density analysis for caspase-3. (Right) Caspase-3 activation. (C) Representative photograph of Western blot and density analysis for cleaved caspase-9 and caspase-9. (D) Representative photograph of Western blot and density analysis for cleaved caspase-8 and caspase-8. n=5–7 mice/group. *P<0.05 vs. Sham, ##P<0.01 vs. vehicle. MI/R, myocardial ischemia/reperfusion; WFA, withaferin A.
Two apoptotic pathways exist: the extrinsic pathway (death receptor pathway), and the intrinsic pathway (mitochondrial pathway).27 To gain further insight into the signaling regulatory significance in WFA-induced cardioprotection, we next attempted to determine the apoptotic pathway affected by WFA. We found in cardiac tissues obtained from nonischemic control animals that WFA treatment alone did not activate caspase 3, 8, or 9. However, WFA decreased cleaved caspase-9 (the indicator of the mitochondrial pathway) levels augmented by MI/R (−28±9%, Figure 2C), but did not affect the levels of cleaved caspase-8 (the indicator of the death receptor pathway) upregulated by MI/R (Figure 2D), suggesting WFA possesses antiapoptotic cardioprotective properties associated with the mitochondrial cell death cascade pathway.
We further explored the mitochondrial-related signaling pathway in 2 groups based on function: antiapoptosis (Bcl-xL, Bcl-2, Mcl-1) and proapoptosis (Bax, Bak, BID, Bim).28 We found that WFA significantly increased both the mRNA level and protein expression of Bcl2 (Figure 3A,C). Meanwhile, WFA reduced the ratio of Bcl2/Bax 1.54±0.32 fold compared with MI/R (Figure 3C, Lower panel), suggesting Bcl-2 is the key antiapoptotic mediator in the mechanism of WFA’s cardioprotection.
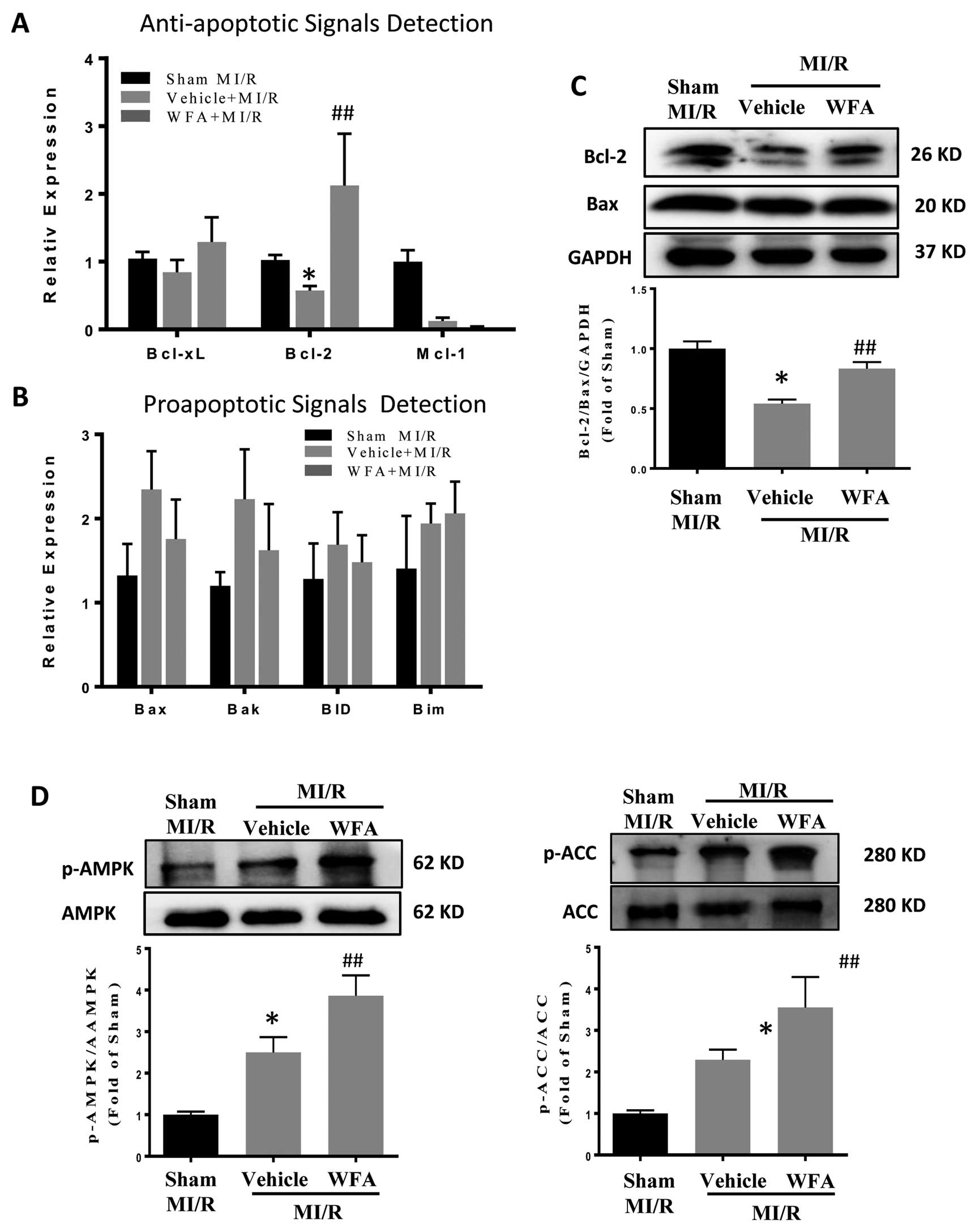
Effect of WFA (1 mg/kg) on apoptotic relative molecules after MI/R injury. (A) Real-time qPCR (RT-PCR) detected mRNA levels of antiapoptotic molecules. (B) RT-PCR detected mRNA levels of proapoptotic molecules. (C, Top) Bcl-2 and Bax protein content determined by Western blot. (Bottom) Bcl-2/Bax ratio. (D) Activation of AMPK and ACC in cardiac tissue measured after MI/R injury with and without WFA administration. n=5–7 mice/group. *P<0.05 vs. Sham, ##P<0.01 vs. vehicle. AMPK, AMP-activated protein kinase; MI/R, myocardial ischemia/reperfusion; WFA, withaferin A.
AMPK activation is an essential event in the cardiomyocyte adaptive response, modulating glucose and fatty acid metabolism, mitochondrial function, autophagy, and apoptosis.29 To identify whether AMPK was involved in WFA’s cardioprotective role, we analyzed its activation after MI/R with or without WFA administration. We found that WFA further increased the levels of phosphorylated AMPK and phosphorylated ACC (direct downstream of AMPK activation), suggesting AMPK confers a cardioprotective effect against MI/R injury (Figure 3D).
Effect of AMPK Deficiency on Cardioprotective Effects of WFATo obtain more evidence to support a causative role of AMPK in WFA’s cardioprotective effect, 2 additional experiments were performed. In the first series of experiments, AMPK-DN (AMPK dominant negative, AMPK activity deficient) mice were subjected to MI/R. WFA administration 90 min prior to MI/R did not augment cardiac function in the AMPK-DN mice, evidenced by echocardiographyic (LVEF, Figure 4A,B) and hemodynamic analyses (±dP/dt, Figure 4C). Moreover, WFA did not decrease MI/R-induced infarct size in the AMPK-DN mice (Figure 4D). Taken together, these results demonstrated that the cardioprotection conferred by WFA is AMPK dependent. In the second series of experiments investigating apoptosis, WFA administration did not inhibit MI/R-induced apoptosis in the AMPK-DN mice, as determined by TUNEL staining (Figure 4E) and cleaved/total caspase 3 levels (Figure 4F). Consistent with the results from the infarct size experiment, WFA did not decrease myocardial apoptosis in AMPK-DN mice compared with controls. In addition, cardiac tissue weight (ventricular weight/body weight, mg/g) was evaluated after MI/R surgery and showed no obvious change among all groups (Sham MI/R: 3.70±0.29; MI/R: 3.86±0.40; WFA MI/R: 3.93±0.45).

AMPK deficiency blocks the cardioprotective effect of WFA (1 mg/kg) after MI/R. (A) Representative echocardiography recording. (B) LVEF. (C) ±dP/dt max values. (D, Top) myocardial infarct size by TTC stain. Black-stained portion is nonischemic region; red-stained portion is the I/R but not infarcted region. (Bottom) Bar graph of infarct size analysis expressed as a percentage of AAR after left anterior descending artery occlusion for 30 min and reperfusion for 24 h. Effect of WFA treatment on myocardial apoptosis determined by TUNEL staining (E), cleaved caspase 3 (F, Left) and caspase activity (F, Right). n=15–17 mice/group. *P<0.05 vs. Sham. AMPK-DN, cardiac myocyte-specific AMPK-α2 subunit mutant transgenic mice; TTC, Evans blue-2,3,5-triphenyl tetrazolium chloride. Other abbreviations as in Figures 1,3.
After demonstrating that WFA’s antiapoptotic effect was AMPK-dependent, we tested a hypothesis that AMPK deficiency may block the regulatory effect on the mitochondrial pathway, affecting the cardioprotective role of WFA against MI/R injury. In AMPK-DN mice, WFA failed to reduce the levels of cleaved caspase-9 (Figure 5A) and cleaved caspase-8 (Figure 5B) compared with the control. Both real-time qPCR (mRNA level) and western blot (protein level) assays revealed that WFA exerts little effect on the Bcl2/Bax ratio (Figure 5C,D).

Effect of WFA (1 mg/kg) on the mitochondrial antiapoptotic pathway in AMPK-deficient mice after MI/R. (A) Cleaved caspase-9 detected by Western blot. (B) cleaved caspase-8 detected by Western blot. (C) RT-PCR analysis detected anti- and proapoptotic pathways. (D) Bcl-1/Bax protein ratio. n=7–8 mice/group. *P<0.05 vs. Sham. Abbreviations as in Figures 1,3.
To examine the functionality of WFA in cultured ventricular cardiomyocytes, WFA (500 nmol/L) was administered followed by SI/R. When assessing the cells incubated with WFA, the SI/R-upregulated cleaved caspase-3 was abolished but abolition of the effect was blocked in the absence of AMPKα2, confirming that WFA’s antiapoptotic effect was AMPK-dependent in NRVMs (Supplementary Figure 1A). Meanwhile, we measured cleaved caspase-8, cleaved caspase-9 and Bcl2 levels to determine the effects of WFA on the mitochondrial cascade pathway of apoptosis after SI/R. WFA failed to downregulate SI/R-induced cleaved caspase-8 upregulation whether AMPK was present or absent (Supplementary Figure 1B); however, WFA effectively downregulated the level of cleaved caspase-9 and this downregulation was hindered in the absence of AMPKα2 (Supplementary Figure 1C). Bcl-2/Bax analysis revealed that upregulation of the Bcl-2/Bax ratio was responsible for WFA’s antiapoptotic regulation (Supplementary Figure 1D). In support of the NRVM experiments, WFA’s cardioprotective role is AMPK dependent and acts by means of the mitochondrial regulatory pathway.
The observation that cardiomyocytes retained the antiapoptotic effect of WFA was somewhat surprising because it opposes previously published results demonstrating the proapoptotic role of WFA in cancer cells via MAPK activation.10,30 One possible explanation for this discrepancy is that our WFA treatment approach failed to activate MAPK family members, and we therefore directly investigated this possibility. We found that treatment with low-dose WFA did not further activate p38 (Figure 6A,B), but decreased p-JNK (Figure 6C,D), and increased p-ERK (Figure 6E,F) in both WT and AMPK-DN mice, which is the opposite regulatory effect compared with cancer cells.10 On the basis of these studies, WFA inhibits cardiomyocytes apoptosis at nanomolar doses that have a cardioprotective effect. Taken together, the results demonstrated that WFA conferred cardiofunction against MI/R in an AMPK-dependent manner, not MAPK-dependent manner.

MAPK signalsing activation intact in AMPK-deficient mice after WFA (1 mg/kg) administration followed by MI/R. (A) Phosphorylation levels of p38 in WT mice. (B) Phosphorylation levels of p38 in AMPK-DN mice. (C) Upregulated level of phosphorylated JNK by WFA administration in WT mice followed by MI/R. (D) Phosphorylation level of JNK in AMPK-DN mice. (E) Phosphorylation level of ERK1/2 in WT mice. (F) Phosphorylation level of ERK1/2 in AMPK-DN mice. n=7–8 mice/group. *P<0.05 vs. Sham, #P<0.05, ##P<0.01 vs. vehicle. MAPK, mitogen-activated protein kinase; WT, wild-type. Other abbreviations as in Figures 1,3,4.
WFA exerts antitumorigenic activity against various cancer types,31,32 and we report the discovery of a differential effect of WFA on MI/R injury. Firstly, we demonstrated that micromolar concentrations of WFA (1 mg/kg) reduced apoptotic cell death via the intrinsic apoptotic pathway (summarized in Figure 7). Secondly, we demonstrated that the involved signaling mechanism includes upregulation of protein Bcl-2 and sequential activation of the mitochondrial antiapoptotic process.

Schematic showing the role of WFA in cardioprotective signaling. (Blue panel) Extrinsic apoptosis-related pathway that may be activated by MI/R. (Yellow panel) Intrinsic apoptotic pathway regulated by WFA in MI/R. Abbreviations as in Figures 1,3,6.
Many studies have demonstrated the discrepancy of WFA’s effect on apoptosis because of its differential affinity towards various target proteins or receptors, affecting its intracellular concentration and consequent regulation of apoptosis.32,33 The antiapoptotic effect of WFA relates to its subcytotoxic dose of administration. Our in vivo study demonstrated that low-dose WFA (1 mg/kg, equivalent to 500 nmol/L in mice) protected against MI/R injury, but a higher dose (5 mg/kg, equivalent to 2.5µmol/L in mice) was not protective, and even to some extent further deteriorated cardiac function, suggesting low and high doses of WFA may have opposing effects on apoptotic cell death. Meanwhile, WFA exhibited weak cytotoxic and antiapoptotic activities at 500 nmol/L and potent proapoptotic activity at higher doses. Our results are consistent with oncologic studies demonstrating WFA’s weak cytotoxic and apoptotic activities in breast cancer and lung cancer at doses less than 500 nmol/L,25 but inhibition of glioma cell proliferation in a dose-dependent fashion in vivo and in vitro.34 Because AMPK is the key regulator in cellular energy homeostasis and multiple biological processes in cell growth and survival, we investigated WFA’s effect on AMPK activation to better understand the underlying mechanism of WFA’s cardioprotective role. Consistent with the in vivo results, different doses of WFA activated AMPK phosphorylation in neonatal cardiomyocytes in a dose-dependent manner, but when the WFA dose reached 500 nmol/L (1 mg/kg), the phosphorylated AMPK level extended to the platform (Supplementary Figure 3A), suggesting that the biological effect on cardiomyocytes of a high dose of WFA may extend beyond AMPK activation. Although how the differential dosage of WFA shows discrepant biological functions in cardiomyocytes still remains unclear, understanding the specific function of WFA on AMPK complexes may be an important focus of future research in this field.
Two main apoptotic pathways exist: the extrinsic/death receptor pathway and the intrinsic/ mitochondrial pathway.27,35 Our data suggested that MI/R injury activates both apoptosis pathways, but that WFA effectively inhibits the intrinsic apoptotic pathway, not the extrinsic pathway. To gain a detailed understanding of the regulatory mechanism underlying WFA’s intrinsic pathway signaling, we screened pro- and antiapoptotic proteins relevant to mitochondria. The ratio of Bcl-2/Bax constitutes a critical indicator of cellular apoptotic status. MI/R decreases Bcl-2 expression (and the ratio of Bcl-2/Bax), activating mitochondrial apoptosis. Our results suggested WFA may affect Bcl-2 levels (and the ratio of Bcl-2/Bax), selectively inhibiting mitochondrial apoptosis, and thus preserving cardiac function. The Bcl-2 family consists of essential antiapoptotic molecules governing mitochondrial membrane permeability.36,37 By regulating Bcl-2 levels, WFA is an important antiapoptotic regulator. Additionally, we determined the effect of WFA on autophagy in the MI/R mice model: it had no regulatory effect on the autophagosome formation and degradation. Though autophagy is pivotal in the regulation of cardiomyocyte repair and reconstruction,38 WFA does not influence autophagy (Supplementary Figure 2).
The MAPK family (ERK, p38, and JNK) functions as integrators of cell growth, survival, and apoptosis in a wide range of cell types; the ERK pathway plays an especially important role in promoting cell growth and proliferation in many mammalian cell types.39–41 Our results indicated that, although WFA increased the activity of ERK and inhibited JNK, the changes induced by WFA were not markedly different between the WT and AMPK-DN mice. We hypothesize that WFA-induced AMPK activity is the key mechanism mediating cardioprotection against MI/R, and furthermore, this process is MAPK-independent. AMPK is a highly conserved serine/threonine protein kinase. A number of stresses (glucose deprivation, ischemia, hypoxia, and oxidative stress) can activate AMPK.42–44 Our study verified that WFA activates AMPK significantly, and suppresses the mitochondrial apoptosis pathway, protecting against MI/R injury in an AMPK-dependent manner.
In conclusion, our results demonstrated that low-dose WFA (1 mg/kg) ameliorated MI/R injury in mice. WFA induced AMPK activation, a significant regulator of molecular signaling, in a way that influenced mitochondrial apoptosis post-MI injury. Our work defines a molecular mechanism by which WFA protects against myocardial apoptosis, and may have therapeutic applications in cancer patients enduring cardiovascular system disorders.
This work was supported by the following grants: American Diabetes Association 1-17-IBS-297 (Y. Wang); American Diabetes Association 1-15-BS-122 (X. Ma); Natural Science Foundation of China 81670278; Innovative Talents of Higher Learning Institutions of Shanxi; Shanxi 1331 Project Key Subjects Construction. The funders had no role in study design, data collection, analysis, decision to publish, or manuscript preparation.
None.
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-18-1391