I. Introduction
1. Preface to Revision
In March 2018, the revised version of the guidelines regarding infective endocarditis (IE) was published in Japanese from the Japanese Society of Cardiology (the Guidelines for Prevention and Treatment of Infective Endocarditis (JCS2017)).1,2
On the revision of the guidelines we reorganized the Guideline Writing Group. In addition to the Japanese Circulation Society, the Japanese Association for Thoracic Surgery, the Japanese Society of Pediatric Cardiology and Cardiac Surgery, and the Japanese College of Cardiology as previous members, new members, namely, the Japanese Society of Echocardiography, the Japanese Society for Cardiovascular Surgery, the Japanese Society for Adult Congenital Heart Disease, the Japan Stroke Society, the Japanese Association for Infectious Diseases, the Japanese Society of Chemotherapy, dentists specializing in IE, a visiting chief researcher of EBM Medical Information Division (Minds) of the Japan Council for Quality Health Care, joined the team. Furthermore, Japan Medical Library Association also joined to cooperate with the aim of developing clinical questions (CQs) of good quality and the responses to them.
Major points of updating from the previous revision are shown below.
1) Description of advancement of imaging techniques and bacteriological examination in the diagnosis of IE was added.
2) Indication of early surgery for IE, and the timing of surgery after the occurrence of neurological complications were discussed on the basis of accumulated evidence.
3) Many issues discussed about prevention of IE were reconsidered, and the opinions at the present time point were displayed.
4) New chapters were created to discuss device infection, right-sided IE, IE during pregnancy, non-bacterial thrombotic endocarditis (NBTE), and IE in elderly people.
This English version is a translated, abbreviated form of the Japanese version; the sections are selected based on importance, novelty, and difference from the existing guidelines. Thus, some parts were omitted from the English version.
We hope the guidelines will help not only cardiologists but also physicians in other fields and dentists lead to achievement of prevention of IE and treatment of good quality. Please remember that these guidelines intend to provide just one policy for helping diagnosis and decision of treatment strategy, and are not designed to deny the discretion of physicians.
2. About Recommendations
For the present guidelines, systematic review was conducted focusing on five CQs (Table 3). For each CQ, systemically collected articles were evaluated with respect to the ability to support recommendations, and the strength of recommendation was determined according to the rule of unanimity. To discriminate the recommendations based on systematic review and other recommendations, the former was presented using the method of Minds 2014 (strong recommendation, weak recommendation, and strength of body of evidence from A to D) to evaluate the body of evidence,3
and the latter was presented using the conventional method (Class I to III, level of evidence A to C).
Table 3.
Clinical Questions Examined in Present Guidelines and Recommendations
| |
Evaluation by systematic review |
Strength of
recommendation |
Strength of body
of evidence |
| CQ1 |
Is brain MRI useful for patients without neurological symptoms who have or are suspected to have IE?
(In “Chapter V. 3. 2. Neurological Complications”, [Related section] “Chapter V. 3. 2. b. Methods of Diagnosis of Neurological
Complications”, Table 14) |
It is proposed to obtain brain MRI (including DWI, FLAIR images, T2*WI, and MRA) at an
early timing in the patients without neurological symptoms who have or are suspected to
have IE |
2 (weak) |
C (weak) |
| CQ2 |
Should early surgery be conducted if a large vegetation is present?
(In “Chapter VI. 2. Indications of Surgical Treatment and Timing of Operation”, [Related section] “Chapter VI. 2. a. General Remarks
on Indications of Surgical Treatment”, Table 16) |
Surgery at the earliest timing possible is recommended for the patients with IE in the native
valve (aortic valve or mitral valve) who have a vegetation of 10 mm or larger accompanying
severe valve dysfunction |
1 (strong) |
B (moderate) |
| CQ3 |
Should surgery of IE be conducted at an early timing when neurological complications have occurred?
(In “Chapter VI. 2. Indications of Surgical Treatment and Timing of Operation”, [Related section] “Chapter VI. 2. a. General Remarks
on Indications of Surgical Treatment”, Table 16) |
(1) Surgery of IE is recommended not to be postponed if it is indicated, even when
concurrent cerebral infarction is present*
*Except for the cases with concurrent coma, herniation, or cerebral hemorrhage, as
well as major central lesions |
1 (strong) |
B (moderate) |
(2) If new intracranial hemorrhage* is observed, it is proposed to wait 4 weeks to conduct
open heart surgery if the hemodynamic condition is stable
*Except for cerebral microbleeds |
2 (weak) |
C (weak) |
| CQ4 |
Is antibiotic prophylaxis necessary for prevention of IE in dental procedures for the patients with high-risk heart diseases?
(In “Chapter VIII 3. 2 Dental Diseases”, [Related section] “Chapter VIII. 1. General Remarks on Prevention of IE”, Table 17) |
(1) Antibiotic prophylaxis is recommended before dental procedures inducing bacteremia,
such as tooth extraction, in adult highest-risk patients |
1 (strong) |
B (moderate) |
(2) Antibiotic prophylaxis is proposed before dental procedures inducing bacteremia,
such as tooth extraction, in adult moderate-risk patients |
2 (weak) |
C (weak) |
| CQ5 |
Is antibiotic prophylaxis necessary for prevention of IE in dental procedures for pediatric/congenital heart diseases?
(In “Chapter IX 1. 5 Prevention”, [Related section] “Chapter IX. 1. 2 Risks According to Underlying Heart Disease”, Table 22) |
(1) Antibiotic prophylaxis is recommended before dental procedures inducing bacteremia,
such as tooth extraction, in highest-risk patients with pediatric/adult congenital heart
disease |
1 (strong) |
C (weak) |
(2) Antibiotic prophylaxis is proposed before dental procedures inducing bacteremia, such
as tooth extraction, in moderate-risk patients with pediatric/adult congenital heart
disease |
2 (weak) |
C (weak) |
IE, infective endocarditis; MRI, magnetic resonance imaging; DWI, diffusion weighted image; FLAIR, fluid attenuated inversion recovery; MRA, magnetic resonance angiography; T2*WI, T2*-weighted image.
The strength of recommendation and the strength of body of evidence for 5 CQs are expressed as follows.3
■ Strength of Recommendation
“1”: Strongly recommended.
“2”: Weakly recommended (proposed).
■ Strength of Body of Evidence
A (strong): Strongly confident of the estimate of effect
B (moderate): Moderately confident of the estimate of effect
C (weak): Limited confidence of the estimate of effect
D (very weak): Very little confident of the estimate of effect
Class of recommendation and level of evidence for other parts are expressed in
Table 1
and
Table 2.
Table 1.
Class of Recommendation
| Class I |
There is evidence and/or general agreement that a given procedure or treatment is effective and/or useful |
| Class II |
There is no consistent evidence and/or general agreement that a given procedure or treatment is effective and/or useful |
| Class IIa |
Weight of evidence and opinion is in favor of usefulness and/or effectiveness |
| Class IIb |
Usefulness or effectiveness is not fully established by evidence or opinion |
| Class III |
There is evidence and/or general agreement that the procedure or treatment is not effective and/or useful or may even be harmful |
Table 2.
Level of Evidence
| Level A |
Demonstrated with multiple randomized, controlled studies or meta-analyses |
| Level B |
Demonstrated with a single randomized intervention clinical study or non- randomized, non-intervention studies |
| Level C |
Only consensus opinion of experts and/or small-scale clinical studies (including retrospective studies and registration) |
II. General Remarks
1. What Is IE?
IE is a systemic septic disease accompanying generation of vegetation containing bacterial aggregation on the valve, endocardium, and intima of large vessels, and showing various clinical symptoms such as bacteremia, vascular embolization, and cardiac disorders. Abnormal blood flow associated with valvular disease, congenital heart disease, or prosthetic valve replacement causes non-bacterial thrombotic endocarditis (NBTE) which is considered important precursors for IE. When transient bacteremia occurs in a patient with NBTE after dental or other procedures, bacteria adhere to the site of NBTE and grow to generate vegetation.
While IE often occurs in the patients with some underlying heart diseases, it may occur in the patients without a history of heart diseases. The predisposing event is unclear in many cases. It is important to keep the possibility of IE in mind when examining the patients with fever or embolism of unknown origin.
The diagnosis of IE is made on the basis of clinical symptoms associated with sepsis, identification of causative microorganisms in blood, and confirmation of destruction of the intracardiac structure associated with infection, including vegetation. Therefore, the major criteria in Duke criteria for diagnosis of IE are comprised of blood culture and echocardiography.7,8
However, revision of the diagnostic criteria may become necessary in the future because identification of causative microorganisms by gene analysis, usefulness of computed tomography (CT) in evaluation of the intracardiac structure, and usefulness of positron emission tomography (PET), which visualizes inflammation itself, have come to be known.
2. Team Medicine
A team covering a wide range of areas (IE team) is required in the clinical management of IE presenting in various clinical aspects and necessitating advanced expertise.4–6
It is not easy to constantly involve a group of specialists in actual clinical settings. However, at least close discussion with the specialists from the relevant areas should be continued.
3. Timing of Referral to Specialized Hospital
Not all IE patients visit a specialized hospital with an IE team. Therefore, when an institution finds it difficult to control a patient at their institution, they should consult another institution providing team medicine, or should refer the patient to such an institution for the purpose of transfer.
III. Diagnosis
1. Diagnostic Criteria for IE
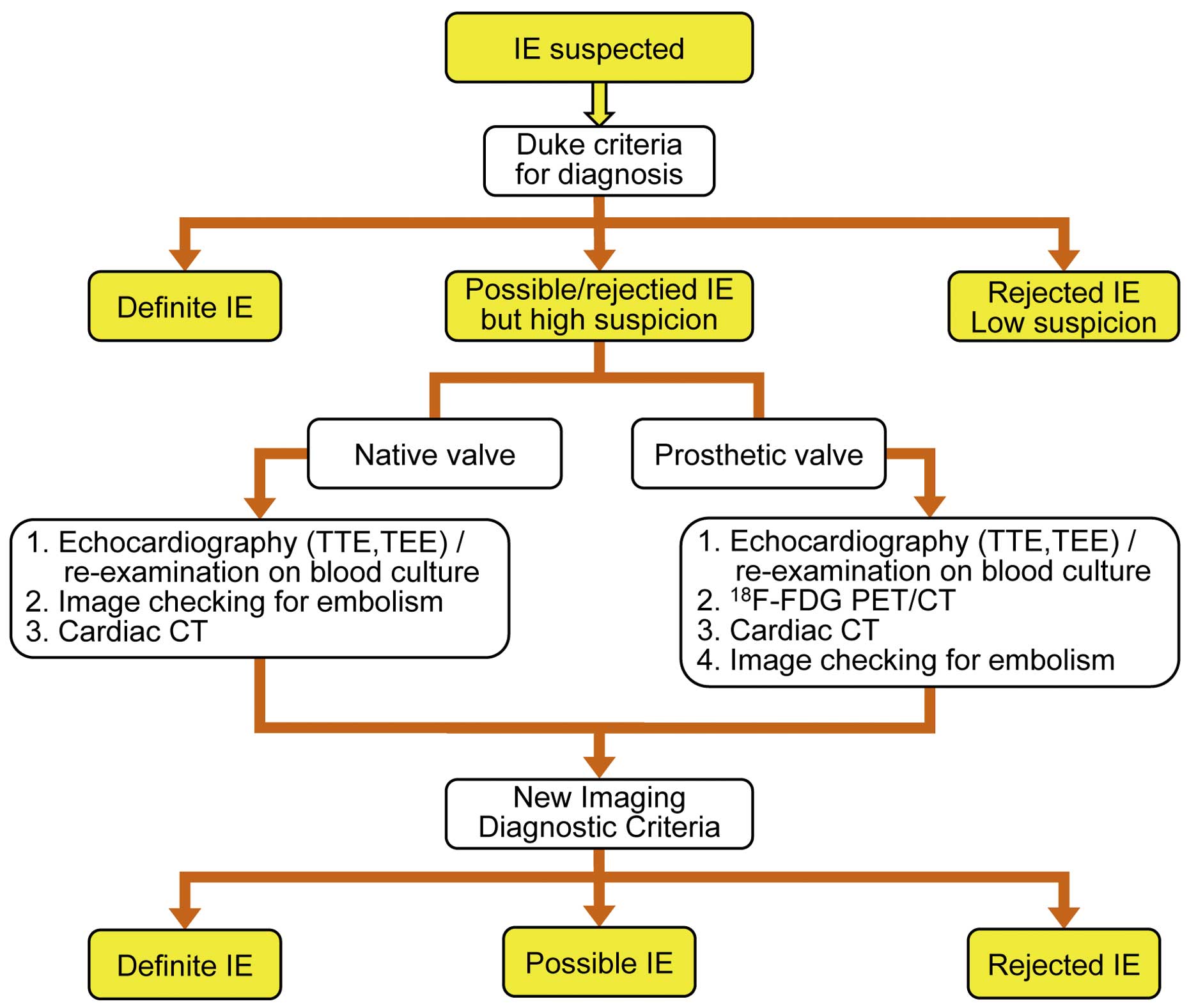
Duke criteria (modified) are helpful for the diagnosis of IE (Table 4).7,8
While the diagnostic sensitivity of Duke criteria is approximately 80%, the sensitivity in the early stage of disease is even lower. In particular, it becomes particularly low in cases with abscess formation, cases after prosthetic valve replacement, and cases after pacemaker implantation.9,10
Recently, the usefulness of CT and 18F-fluorodeoxyglucose (18F-FDG) PET/CT in depicting annular abscess in cases of IE following prosthetic valve replacement has been reported. Since 18F-FDG PET/CT is useful in searching systemic inflammation, it has been reported to be useful in detecting the remote lesion causing IE.
Table 5
and
Figure 1
show the criteria of diagnosis according to the guidelines of the European Society of Cardiology (ESC).
Table 4.
Diagnostic Criteria for IE (Modified Duke Criteria)
8
| Definite infective endocarditis |
| Pathologic criteria |
(1) Microorganisms demonstrated by culture or histologic examination of a vegetation, a vegetation that has embolized, or an intracardiac
abscess specimen; or |
| (2) Pathologic lesions; vegetation or intracardiac abscess confirmed by histologic examination showing active endocarditis |
| Clinical criteria |
| (1) 2 major criteria; or |
| (2) 1 major criterion and 3 minor criteria; or |
| (3) 5 minor criteria |
| Possible infective endocarditis |
| (1) 1 major criterion and 1 minor criterion; or |
| (2) 3 minor criteria |
| Rejected |
| (1) Firm alternate diagnosis explaining evidence of infective endocarditis; or |
| (2) Resolution of infective endocarditis syndrome with antibiotic therapy for ≤4 days; or |
| (3) No pathologic evidence of infective endocarditis at surgery or autopsy, with antibiotic therapy for ≤4 days; or |
| (4) Does not meet criteria for possible infective endocarditis, as above |
| Definition of criteria |
| Major criteria |
| Blood culture positive for IE |
| Typical microorganisms consistent with IE from 2 separate blood cultures: |
| Viridans streptococci, Streptococcus bovis, HACEK group, Staphylococcus aureus; or |
| Community-acquired enterococci, in the absence of a primary focus; or |
| Microorganisms consistent with IE from persistently positive blood cultures, defined as follows: |
| At least 2 positive cultures of blood samples drawn >12 h apart; or |
| All of 3 or a majority of >4 separate cultures of blood (with first and last sample drawn at least 1 h apart) |
| Single positive blood culture for Coxiella burnetii or antiphase I IgG antibody titer 11:800 |
| Evidence of endocardial involvement |
Echocardiogram positive for IE (TEE recommended in patients with prosthetic valves, rated at least “possible IE” by clinical criteria, or
complicated IE [paravalvular abscess]; TTE as first test in other patients), defined as follows : |
Oscillating intracardiac mass on valve or supporting structures, in the path of regurgitant jets, or on implanted material in the absence of
an alternative anatomic explanation; or |
| Abscess; or |
| New partial dehiscence of prosthetic valve |
| New valvular regurgitation (worsening or changing of pre-existing murmur not sufficient) |
| Minor criteria |
| (1) Predisposition, predisposing heart condition or injection drug use |
| (2) Fever, temperature >38℃ |
(3) Vascular phenomena, major arterial emboli, septic pulmonary infarcts, mycotic aneurysm, intracranial hemorrhage, conjunctival
hemorrhages, and Janeway’s lesions |
| (4) Immunologic phenomena: glomerulonephritis, Osler’s nodes, Roth’s spots, and rheumatoid factor |
(5) Microbiological evidence: positive blood culture but does not meet a major criterion as noted above or serological evidence of active
infection with organism consistent with IE |
Table 5.
New Imaging Diagnostic Criteria (ESC Guidelines 2015)
4
| Imaging positive for IE |
| a. Echocardiogram positive for IE: |
| • Vegetation; |
| • Abscess, pseudoaneurysm, intracardiac; |
| • Valvular perforation or aneurysm; |
| • New partial dehiscence of prosthetic valve |
b. Abnormal activity around the site of prosthetic valve implantation detected by 18F-FDG PET/CT (only if the prosthesis was implanted for
>3 months) or radiolabelled leukocytes SPECT/CT |
| c. Definite paravalvular lesions by cardiac CT |
| ESC guidelines incorporates these new imaging diagnostic criteria as major criteria in addition to modified Duke criteria (Table 4). |
This section is omitted from the English version.
3. Microbiological Examination
3.1 Blood Culture
Blood culture is very important in the diagnosis of IE in the same way as sputum culture for pneumonia. IE may be suspected on the basis of positive results in blood cultures, and IE is observed in approximately 5 to 30% of the patients with bacteremia caused by
Staphylococcus aureus.11,12
Isolation of the causative microorganisms on blood culture will enable bacterial identification and sensitivity testing. The positive rate on blood culture becomes 90% or higher if blood specimens are collected before antibiotic treatments, but the positive rate on blood culture may decrease dramatically for some bacterial species if antibiotics have already been administered.13,14
At least three sets of specimens should be submitted for blood culture. Submission of specimens for multiple times increases the volume of blood to be subjected to culture, and may increase detection sensitivity. The interval for collection of blood specimens has not been established, although there have been some recommendations such as collection of every 30 minutes, the interval of 1 hour between the first and last collection, or 6 hours or more.15
No difference in detection rate has been observed for arterial blood and venous blood, and it is unnecessary to collect blood specimens in the presence of high fever. Blood collection via catheters should be avoided in order to avoid contamination during blood collection. Even before the causative microorganisms determined, antibiotic treatment should be started in the emergency cases showing sepsis, and at least two sets of blood specimens should be collected within 1 hour.
Conversely, antibiotic treatment may be transiently suspended in cases with a subacute course. The appropriate duration of suspension is believed to be 2 or 3 days. However, antibiotics should not be discontinued in the patients with an unstable cardiorespiratory condition due to heart failure, patients with progressive infection foci (such as annular abscess), and patients who have embolism or are at high risk of embolism. Suspension of antibiotics must be avoided also in the IE patients with prosthetic valve.
For the patients with positive blood cultures, blood cultures should be repeated within several days after the start of treatment (approximately 3 days, or 48 to 72 hours after the start of treatment) to check the effect of the treatment.16
Although it is unnecessary to discontinue antibiotic treatment before collection of specimens, it is reasonable to collect specimens immediately before administration of antibiotics when the blood concentration of antibiotics is low. Blood culture should be repeated until negative results are obtained. Once the result has turned negative, additional blood culture is unnecessary unless changes in symptoms are observed.
3.2 Other Test Methods
a. Serologic Diagnosis and Polymerase Chain Reaction (PCR)
Microorganisms which are difficult to culture by usual blood culture, such as
Bartonella
and
Coxiella burnetii, may cause IE. Concerning
Bartonella,
Bartonella quintana, which is known for trench fever, and
Bartonella henselae, which is known as causative microorganism for cat scratch disease, may cause IE. Although the number is small, there have been case reports.17,18
However, the percentage of
Bartonella
in the cases of IE is less than 1%, and routine antibody tests are unnecessary.
The positive rate on blood culture is also low in the cases of IE caused by fungi. Especially as to filamentous fungus such as
Aspergillus, the positive rate is less than 10%. Although blood β-glucan and blood aspergillus antigen are useful, they are used for auxiliary diagnosis (even if the β-glucan level is high, differentiation of
Candida
and other fungi including
Aspergillus
is difficult, and cautions are required for non-specific reactions).
b. PCR for Extracted (Surgical) Specimens
The valve tissues obtained at the time of surgery can be subjected to culture and histological examination. While PCR and sequencing using 16S RNA amplification can be outsourced in Japan, they are not covered by insurance, and they are not conducted as routine tests in laboratories. The cases in which PCR is considered useful are the following: the cases with negative blood cultures, the cases in which blood culture is positive only once, and rare microorganism or normal bacterial flora on the skin are suspected to be causative microorganisms.19
However, contamination at the time of extraction of specimens may cause false positive results. Therefore, the clinical course and the results of blood cultures should also be taken into consideration when making judgement.20
Detection by PCR becomes difficult after formalin fixation of specimens.
4. Echocardiography
Echocardiography plays the most important role in the diagnosis, treatment, follow-up and estimation of prognosis of IE. When IE is suspected, it should be performed in all cases, including the cases with negative blood cultures.21,22
a. Positive Criteria
The major items related to echocardiographic findings on Duke criteria for diagnosis (Table 4) include (1) vegetation, (2) abscess or pseudoaneurysm, (3) new partial dehiscence of prosthetic valve, and (4) emergence of new valvular regurgitation (exacerbation of existing murmur alone is insufficient).
b. Significance of Vegetation
Vegetation is defined as periodically vibrating mass echo adhering to the endocardium mainly around the valve, or intracardiac device. When findings suggestive of vegetation are observed, the size, shape, adhesion site, and mobility should be monitored.
Examination of the changes in the size and mobility of vegetation after treatment is useful for evaluation of the effects of antibiotics. However, observation of the vegetation echo after treatment does not always mean recurrence.
c. Accuracy of Diagnosis
The sensitivity of transthoracic echocardiography (TTE) in detecting vegetation is approximately 70% for native valves, and approximately 50% for prosthetic valves. The sensitivity of transesophageal echocardiography (TEE) in detecting vegetation is 90% or more for both native valves and prosthetic valves. Both TTE and TEE show high specificity of approximately 90% in detecting vegetation. On the other hand, the sensitivity in detecting perivalvular abscess was low at 30 to 50% for TTE, and it varied from 50% to 90% among reports in TEE. Both TTE and TEE showed high specificity of 90% or more in detecting perivalvular abscess. The detection rate of positive findings for IE was low in cases with poor images, cases showing small vegetation (<3 mm), prosthetic valve, and valvular changes (including prolapse, thickening, and calcification), and cases with placement of devices such as pacemakers.
d. Indication of TTE and TEE (Table 6)
Table 6.
Recommendations of Echocardiography in IE and Level of Evidence
| |
Class of
recommendation |
Level of
evidence |
| TTE for all cases of suspected IE |
I |
B |
| TEE in the cases in which IE is suspected and adequate images cannot be obtained with TTE |
I |
B |
| TEE in the cases in which IE is suspected, and prosthetic valve or any other device has been placed |
I |
B |
Re-examination after 3 to 7 days in the cases in which IE is clinically suspected in spite of the
negative result on the first echocardiography |
I |
C |
| Echocardiography for the cases of staphylococcal bacteremia |
IIa |
B |
| TEE in the cases with positive result on TTE (except for the cases with IE only in the right cardiac valve) |
IIa |
C |
| Follow-up echocardiography after the onset of new complications |
I |
B |
| Follow-up echocardiography for evaluating therapeutic effects |
I |
C |
| Follow-up echocardiography for evaluating the onset of asymptomatic intracardiac complications |
IIa |
B |
| TTE at the end of treatment |
I |
C |
IE, infective endocarditis; TTE, transthoracic echocardiography; TEE, transesophageal echocardiography.
While TTE is inferior to TEE in terms of sensitivity and specificity, it is noninvasive and can be performed repeatedly. Moreover, it is superior to TEE in evaluation of cardiac functions and hemodynamics using Doppler method. Therefore, it should be performed as soon as possible in all cases of suspected IE.21,22
TEE should be performed when TTE cannot be used for diagnosis because of poor images, when IE is clinically suspected in spite of the negative result on TTE,21,23–25
and when IE is suspected in the cases with prosthetic valve or the cases with insertion of other devices.21,24,26
It is recommended to conduct TEE even in the cases with positive results on TTE for the purpose of evaluating the presence or absence of intracardiac complications.27
Since TEE may fail to detect abnormalities in the early stage, the test should be performed again 3 to 7 days later if IE is clinically suspected.
Since IE is highly likely in the cases of staphylococcal bacteremia, TTE or TEE should proactively be performed in these cases.11,28
e. Timing of Follow-up Echocardiography
Follow-up echocardiography should be conducted after 3 to 7 days when IE is clinically suspected even if the results on TTE and TEE are negative, or for the purpose of evaluating the effects of antibiotics or onset of intracardiac complications in the cases with the established diagnosis of IE. If staphylococci are the causative microorganism, follow-up should be conducted after an even shorter interval. Follow-up echocardiography should also be conducted if any changes have occurred in clinical findings.
f. Echocardiography at the End of Treatment
Echocardiography should always be conducted at the end of treatment to obtain follow-up basic data after completion of treatment. The shape of valves, condition of residual vegetation, and extent of regurgitation should be evaluated.
5. Other Imaging Diagnosis (Table 7)
Table 7.
Recommendations of Imaging Diagnosis Other Than Echocardiography and Level of Evidence in Diagnosis of IE and Its Complications
| |
Class of
recommendation |
Level of
evidence |
It should be considered to conduct CT to detect vegetation or perivalvular abnormality, to diagnose coronary
arterial disease, and to search systemic embolism in the patients who have or who are suspected to have
IE in the native valve or IE in the prosthetic valve (it is preferred to use contrast, if possible) |
IIa |
C |
It is recommended to conduct MRI to diagnose cerebrovascular diseases in the patients who
have or who are suspected to have IE in the native valve or IE in the prosthetic valve |
I (IIa*) |
C |
It may be considered to conduct MRI to diagnose systemic complications such as vegetation, perivalvular
abscess, and osteomyelitis in the patients who have or who are suspected to have IE in the native valve
or IE in the prosthetic valve |
IIb |
C |
It may be considered to conduct gallium scintigraphy in the patients who are suspected to have IE if definite
diagnosis cannot be made by other methods |
IIb |
C |
In the patients who are suspected to have IE, especially the patients with implantation of a prosthetic valve
or any devices, 18F-FDG PET/CT should be considered if definite diagnosis cannot be made by other
methods (IE is not covered by insurance in Japan) |
IIa |
C |
In the patients who are suspected to have IE, labeled leukocyte scintigraphy should be considered at a facility
with the capability to conduct it, if definite diagnosis cannot be made by other methods |
IIa |
C |
*If neurological symptoms are absent, class of recommendation is IIa. See “Chapter V. 3. 2. b. Methods of Diagnosis of Neurological Complications” and “CQ 1 Is brain MRI useful for patients without neurological symptoms who have or are suspected to have IE?”. IE, infective endocarditis; 18F-FDG, 18F-fluorodeoxyglucose.
Imaging diagnostic technology other than echocardiography has been used for the diagnosis of IE and its complications. See “Chapter III. 1. Diagnostic Criteria for IE” for the procedure of diagnosis of IE.
a. CT
Reduction of the scan time and improvement of temporal resolution can be achieved by increasing the number of detectors in multidetector-row CT (MDCT) and shortening the gantry rotation time, and it has become easier to obtain favorable images in the field of cardiology.
On contrast-enhanced CT, vegetation is visualized as a low-density nodule adhering to the valve or blood vessel. When the height is low, it is visualized as the thickening of the valve. Diagnosis is difficult when the movement of vegetation is fast or vegetation is small.29,30
TEE is better for the diagnosis of vegetation, and addition of CT information does not improve the diagnostic ability. However, addition of CT improves the ability to diagnose perivalvular abnormality.31
The features of CT in diagnosis of IE include the following.29–33
1) It is capable of visualizing vegetation, and the size shows good correlation with TEE, but diagnosis of small vegetation is difficult.
2) It shows good ability to detect perivalvular abnormalities such as abscess. If IE is suspected after prosthetic valve replacement, additional information can be expected.
3) If vegetation with a risk of embolism is observed in the aortic valve or the aortic wall, it can be used for preoperative examination of the coronary artery.
4) It can be used for the search of embolism in the whole body.
b. MRI
Magnetic resonance imaging (MRI) shows good ability in diagnosis of cerebrovascular diseases. It is recommended to conduct MRI, if possible, even in the patients without neurological symptoms (See “[CQ 1] Is brain MRI useful for patients without neurological symptoms who have or are suspected to have IE?”). It is also useful for diagnosis of osteomyelitis in the spine, etc. However, since it is inferior to CT in terms of spatial resolution and the scan time is longer, the situation in which it is used for the diagnosis of vegetation and perivalvular abscess is limited.29
c. Gallium Scintigraphy/CT
Sensitivity of gallium scintigraphy for unidentified fever has been suggested to be 30% or lower. Gallium scintigraphy may be useful when definite diagnosis cannot be made by other methods. However, diagnostic accuracy for IE has not been established.34,35
d. 18F-FDG PET/CT
Although insurance coverage of 18F-FDG PET and 18F-FDG PET/CT for heart diseases has been approved for viability assessment of the myocardium in patients with heart failure caused by ischemic heart disease, and diagnosis of inflammation sites in cardiac sarcoidosis, the use for IE and unidentified fever is not covered by Japanese medical insurance. However, improvement of diagnostic ability after addition of 18F-FDG PET/CT has been reported in patients with unidentified fever and patients with implantation of a prosthetic valve or devices.36,37
e. Labeled Leukocyte Scintigraphy
It is not used very frequently in Japan because the labeling procedure is complicated. Concerning diagnostic ability of labeled leukocyte scintigraphy for IE, sensitivity of 90% and specificity of 100% have been reported.38
6. Risk Evaluation at Admission
This section is omitted from the English version.
IV. Medical Treatment
1. Antimicrobial Treatment: Policy and General Principles
In the treatment of IE, the choice and treatment period of the antibiotics recommended in the existing guidelines and present guidelines are mainly based on the type of causative microorganisms, antibiotic susceptibility results, and the type of the valve (native valve or prosthetic valve). Isolation and identification of causative microorganisms is very important. Moreover, the factors related to the treatment results are the duration before diagnosis, immune status of hosts, causative microorganisms, severity of valvular regurgitation, progression of lesions (such as annular abscess), concurrent embolism, and organ dysfunction such as heart failure and renal failure, as well as surgical treatment and its timing. Multidisciplinary treatment provided through cooperation of not only cardiologists but also specialists from multiple fields (IE team) is required.
As to antimicrobial treatment, the roles of infectious disease specialists and pharmacists are important. In addition to the choice of antibiotics for bacteria with decreased susceptibility or multidrug-resistance and fungi, they should take pharmacokinetics (PK) and pharmacodynamics (PD) into consideration. They also play important roles in modifying and changing antibiotics against adverse reactions.
a. General Principles and PK/PD
When designing administration of antimicrobials, it is important to consider PK/PD parameters in order to avoid the emergence of resistant strain and to ensure efficacy. Administration design based on therapeutic drug monitoring (TDM) should be employed for vancomycin, teicoplanin, and aminoglycosides15,39
(Table 8). It is necessary to administer over 1 hour for vancomycin and at least 30 minutes for teicoplanin (particular attention should be paid for the initial loading dose) in order to avoid red man syndrome (condition in which erythema and itching appear in the face and neck).
Table 8.
Relationship Between Recommended Method of Use of Drugs for Therapeutic Drug Monitoring and Method of Use Approved in Japan
| |
Gentamicin |
Vancomycin |
Teicoplanin |
Dosage regimen
(when renal functions are normal) |
3 mg/kg/day in 1–3 doses. |
15–20 mg/kg per dose, twice |
Day 1: 10 mg/kg, twice
Day 2: 10 mg/kg, once or twice
Day 3: 10 mg/kg, twice |
Timing of blood sampling
(when renal functions are normal) |
Day 2 after the start of
administration |
Day 3 after the start of
administration |
(3) to 4 days after the start of
administration |
Timing of blood
sampling |
Peak
concentration |
1 hour after the start of drip infusion
(30 minutes after completion of
30-minute administration) |
Not determined in routine
examination |
Not determined |
Trough
concentration |
Within 30 minutes before administration |
Target blood
concentration |
Peak
concentration |
3–5 μg/mL
(when divided into 2 or 3 doses*) |
– |
– |
Trough
concentration |
Lower than 1 μg/mL |
Adjusted to 15 to 20 μg/mL by
TDM aiming at the initial
concentration of 10 to 15 μg/mL |
20–30 μg/mL |
Dosage regimen in package
insert |
3 mg/kg/day in 1–3 doses |
2 g/day in 2–4 doses |
Day 1: 800 mg/day in 2 doses.
Day 2, 3: 400 mg in one dose.
Day 4~: dose adjusted according to
renal functions. |
*The recommended target level is not set for once daily administration. TDM, therapeutic drug monitoring.
Duration of treatment should be the period recommended in the present guidelines. The necessary treatment period starts on the first day when negative blood cultures are obtained.
c. Relationship Between the Recommended Dose of Antibiotics and the Doses Approved in Japan
In IE treatment, antibiotics are often used at higher doses. Since aminoglycosides are used aiming at synergetic effect, they are used at stipulated doses.
d. New Antimicrobials (Daptomycin and Linezolid)
Daptomycin and linezolid are anti-methicillin-resistant
Staphylococcus aureus
(MRSA) drugs. Non-inferiority of daptomycin to vancomycin in the treatment of IE has been reported in a comparative study, and daptomycin is positioned as a drug of the first choice.40
Although linezolid has been revealed to be effective in the treatment of IE, it is not approved for IE treatment in Japan, and it is positioned as an alternative therapy.41
Attention should be paid to adverse reactions, such as thrombocytopenia in long-term administration.
e. Treatment of Infection Foci as Portal of Entry and Remote Site Infection
Treatment is necessary for the infection foci which has become the portal of entry of the causative microorganisms of IE, as well as the remote lesions developed from IE. The treatment method recommended as standard for each infection should be followed. Surgical approach should also be made as needed. The same applies to dental lesions.
2. Empirical Treatment
When antibiotic treatment is started before the blood culture results are obtained, the following points should be kept in mind in selecting antibiotics: (1) acute onset or subacute onset, (2) community-acquired infection or nosocomial or healthcare-associated infection, (3) severity (such as APACHE II score and presence/absence of septic shock), (4) native valve or prosthetic valve (or post-operative period), (5) clinical effects of antibiotics if they have already been administered, (6) coverage of most common causative microorganisms estimated based on age, patients’ medical background (such as dialysis), history of MRSA colonization, and so on. In particular,
Staphylococcus aureus
(especially those with methicillin resistance) is very important. When one considers to defer antibiotics for several days, the patients’ cardiopulmonary condition, presence or absence of annular abscess / intracardiac abscess, and embolism (size of vegetation ≥10 mm is a risk) should be checked.
a. Recent Trends in Causative Microorganisms (Table 9)
Table 9.
Factors Associated With IE and Frequent Isolates
| Associated area and item |
Frequent isolate |
| Pediatric |
Staphylococcus aureus, VGS, CNS, enterococci, and Streptococcus pneumoniae
|
| Native valve |
VGS, Staphylococcus aureus, CNS, enterococci, and other streptococci |
| Prosthetic valve |
CNS, Staphylococcus aureus, VGS, enterococci, and Streptococcus gallolytics (bovis)
|
| Congenital heart disease |
VGS, Staphylococcus aureus, CNS, Streptococcus gallolytics (bovis), and enterococci |
| Healthcareassociated |
Staphylococcus aureus, enterococci, VGS, CNS, and Streptococcus gallolytics (bovis)
|
| Dialysis |
Staphylococcus aureus, CNS, enterococci, VGS, and Pseudomonas aeruginosa
|
| Drug injection |
Staphylococcus aureus, VGS, CNS, enterococci, and Candida albicans
|
IE, infective endocarditis; VGS, viridans group streptococci; CNS, coagulase negative staphylococci.
The top three causative microorganisms are viridans group streptococci (VGS), staphylococci, and enterococci. While VGS is predominant in Japan,42
staphylococci have also been reported as predominant.43,44
MRSA represents 7.5% of all cases of IE.42
Enterococci account for approximately 10%, and are common in elderly people (mean age of approximately 70 years).45,46
b. Native Valve IE (Table 9 and Table 10)
Table 10.
Recommendations of Empiric Treatment or Blood Culture* Negative IE
| |
Antibiotics |
Dose |
Class of
recommendation |
Level of
evidence |
Remarks |
Native
valve |
Sulbactam/ampicillin |
3 g, 3–4 times daily |
IIb |
C |
When MRSA is unlikely
When following a subacute clinical
course |
| +Ceftriaxone |
2 g, once daily |
| Daptomycin |
8–10 mg/kg per dose, once
daily |
IIb |
C |
In the case of penicillin allergy |
| +Ceftriaxone |
+2 g, once daily |
| Daptomycin |
8–10 mg/kg/day, once daily |
IIb |
C |
MRSA is considered |
+Sulbactam/ampicillin, or
Panipenem/betamipron |
3 g, 3–4 times daily
0.5 g, 3–4 times daily |
| Vancomycin |
1 g, twice daily, or 15 mg/kg,
twice daily |
IIb |
C |
In the case of penicillin allergy
Enterococcus is also considered
Precautions are required in the
patients with decreased renal
functions and elderly patients |
| +Gentamicin |
2 to 3 mg/kg, once daily |
Prosthetic
valve |
Daptomycin |
8–10 mg/kg, once daily |
IIb |
C |
Ceftriaxone can be replaced with
sulbactam/ampicillin |
| +Ceftriaxone |
2 g, once daily |
| Daptomycin |
8–10 mg/kg, once daily |
IIb |
C |
MRSA is considered |
| +Panipenem/betamipron |
0.5 g, 3–4 times daily |
| Vancomycin |
1 g, twice daily, or 15 mg/kg,
twice daily |
IIb |
C |
Gentamicin can be administered at
1 mg/kg, 2 to 3 times daily
Precautions are required in the
patients with decreased renal
functions and elderly patients |
| +Gentamicin |
2–3 mg/kg, once daily |
*Targeted therapy should be conducted after the causative microorganisms has been identified. IE, infective endocarditis.
For community-acquired IE, VGS, staphylococci and enterococci should be covered. A choice of anti-MRSA drugs should be considered in cases of healthcare-associated onset or the cases with a history of MRSA colonization. When the date of onset is relatively clear, the clinical course is acute and the patient’s condition is rather severe, staphylococci and β-hemolytic streptococci are likely to be the causes, while VGS and enterococci can still be the causes.
c. Prosthetic Valve/Intracardiac Device IE (Table 9
and
Table 10)
Staphylococci are causative microorganisms in 40% or more of the cases of prosthetic valve IE.47
Early-onset IE within 2 months after valve surgery is attributable to staphylococci in a majority of cases. Coagulase negative staphylococci (CNS) are more predominant than
Staphylococcus aureus. CNS in many of such cases are methicillin-resistant.48,49
The causative microorganisms in the cases of onset more than 1 year after the operation are similar to those for IE in the native valve. Major causative microorganisms of IE associated with intracardiac devices are bacterial flora of the skin, and are staphylococci in 80% or more.50
The choice of empiric antibiotics is similar to that for methicillin-resistant staphylococci.51
d. Culture-Negative IE
The following three reasons are plausible explanations for negative blood cultures. (1) The causative microorganisms are
Coxiella,
Bartonella, and other bacteria which are difficult to culture, (2) nutritionally variant streptococci, HACEK (Haemophilus aphrophilus,
Haemophilus paraphrophilus Aggregatibacter actinomycetemcomitans,
Cardiobacterium hominis,
Eikenella corrodens,
Kingella kingae), fungi and so on are likely to be the cause, and (3) antibiotics have been administered before blood culture.14,52
In Japan, (3) is believed to be the main reason.20
3. Targeted Therapy
3.1 Streptococci
a. Penicillin-Susceptible Streptococci (Table 11 and Table 12)
Table 11.
Recommendations of Targeted Therapy for Native Valve IE
| Antibiotics |
Dosage |
Period
(weeks) |
Class of
recommendation |
Level of
evidence |
Remarks |
| 1) Penicillin G-susceptible (MIC ≤0.12 μg/mL) streptococci (VGS, Streptococcus gallolytics, and other streptococci) |
| Penicillin G |
24 million unit/day* in 6
doses, or continuously |
4 |
I |
B |
|
| Ampicillin |
8–12 g/day in 4–6 doses,
or continuously |
4 |
I |
B |
|
| Ceftriaxone |
2 g, once daily |
4 |
I |
B |
Patients allergic to penicillin, elderly patients,
and patients with decreased renal functions |
| Penicillin G |
24 million unit/day* in 6
doses, or continuously |
2 |
I |
B |
See the text for short-term treatment with
Gentamicin |
| +Gentamicin |
3 mg/kg, once daily |
2 |
| Vancomycin |
1 g, twice daily, or
15 mg/kg, twice daily |
4 |
I |
C |
Patients allergic to β-lactams. See the text for
dosage regimen and TDM |
| 2) Streptococci non-susceptible to penicillin G (MIC ≥0.25 μg/mL)** |
| Penicillin G |
24 million unit/day* in 6
doses, or continuously |
4 |
I |
B |
Gentamicin can be administered at the dose of
1 mg/kg per dose, 2 to 3 times daily
Not recommended in the cases of MIC
>1.0 μg/mL for penicillin G |
| +Gentamicin |
2–3 mg/kg, once daily |
2 |
| Ampicillin |
8–12 g/day in 4–6 doses,
or continuously |
4–6** |
I |
B |
Gentamicin can be administered at the dose of
1 mg/kg per dose, 2 to 3 times daily |
| +Gentamicin |
2–3 mg/kg, once daily |
2–6** |
| Vancomycin |
1 g, twice daily, or
15 mg/kg, twice daily |
4 |
I |
C |
Patients allergic to penicillin |
| 3) Enterococci |
| Ampicillin |
8–12 g/day in 4–6 doses,
or continuously |
4–6 |
I |
B |
Gentamicin can be administered at the dose of
1 mg/kg per dose, 2 to 3 times daily. See the
text for administration period of gentamicin |
| +Gentamicin |
2–3 mg/kg, once daily |
4 (2)–6 |
| Ampicillin |
8–12 g/day in 4–6 doses,
or continuously |
6 |
IIa |
B |
Should not be used for elderly patients,
patients with decreased renal functions, and
Enterococcus faecium |
| +Ceftriaxone |
2 g, twice daily |
6 |
| Vancomycin |
1 g, twice daily, or
15 mg/kg, twice daily |
4–6 |
I |
B |
Patients allergic to β-lactams. Not allowed for
the strains highly resistant to gentamicin |
| +Gentamicin |
2–3 mg/kg, once daily |
4–6 |
| 4) Methicillin-susceptible staphylococci |
| Cefazolin |
2 g, 3 times daily |
4–6 |
I |
B |
Cefazolin can be replaced with
sulbactam/ampicillin |
Daptomycin±
β-lactams, etc. |
8–10 mg/kg, once daily |
4–6 |
IIa |
C |
Patients allergic to β-lactams. See the text for
doses and concomitant therapy |
| Vancomycin |
1 g, twice daily, or
15 mg/kg, twice daily |
4–6 |
IIa |
C |
Patients allergic to β-lactams |
| 5) Methicillin-resistant staphylococci |
Daptomycin±
β-lactams, etc. |
8–10 mg/kg, once daily |
4–6 |
I |
B |
See the text for doses and concomitant therapy |
| Vancomycin |
1 g, twice daily, or
15 mg/kg, twice daily |
4–6 |
I |
B |
Adjusted to 15 to 20 μg/mL by TDM aiming at
the initial concentration of 10 to 15 μg/mL
Teicoplanin can be used (TDM is necessary) |
*Doses should be adjusted according to the body weight and renal functions. 12–30 million unit/day, 30 million at maximum. **Infectious disease specialists should be consulted, including the administration with GM, in the cases non-susceptible to penicillin G, especially the cases with MIC >1.0 μg/mL. IE, infective endocarditis; VGS, viridans group streptococci; TDM, Therapeutic drug monitoring.
Table 12.
Recommendations of Targeted Therapy for Prosthetic Valve IE
| Antibiotics |
Dosage |
Period
(weeks) |
Class of
recommendation |
Level of
evidence |
Remarks |
| 1) Streptococci (VGS, Streptococcus gallolytics, and other streptococci) |
| Penicillin G |
24 million unit/day* in 6
doses, or continuously |
6 |
IIa |
B |
Monotherapy is permitted for the cases
susceptible to penicillin G (MIC ≤0.12 μg/mL)
Gentamicin can be administered at the dose of
1 mg/kg, 2–3 times daily |
| ±Gentamicin |
2–3 mg/kg, once daily |
2–6 |
| Ampicillin |
8–12 g/day in 4–6 doses,
or continuously |
6 |
IIa |
B |
Gentamicin can be administered at the dose of
1 mg/kg, 2–3 times daily |
| ±Gentamicin |
2–3 mg/kg, once daily |
2–6 |
| Ampicillin |
8–12 g/day in 4–6 doses,
or continuously |
6 |
IIb |
C |
Elderly patients and patients with decreased
renal functions |
| +Ceftriaxone |
2 g, twice daily |
6 |
| Vancomycin |
1 g, twice daily, or
15 mg/kg, twice daily |
6 |
IIa |
C |
Patients allergic to β-lactams |
| 2) Enterococci |
| Ampicillin |
8–12 g/day in 4–6 doses,
or continuously |
6 |
I |
B |
Not allowed for the strains highly resistant to
gentamicin
Gentamicin can be administered at the dose of
1 mg/kg, 2–3 times daily |
| +Gentamicin |
2–3 mg/kg, once daily |
6 |
| Ampicillin |
8–12 g/day in 4–6 doses,
or continuously |
6 |
IIa |
C |
Should not be used for Enterococcus faecium |
| +Ceftriaxone |
2 g, twice daily |
6 |
| Vancomycin |
1 g, twice daily, or
15 mg/kg, twice daily |
6 |
IIb |
C |
When the patient cannot tolerate β-lactams
Not allowed for the strains highly resistant to
gentamicin
Gentamicin can be administered at the dose of
1 mg/kg, 2–3 times daily |
| +Gentamicin |
2–3 mg/kg, once daily |
6 |
| 3) Methicillin-sensitive staphylococci |
| Cefazolin |
2 g, three times daily |
6–8 |
I |
C |
Cefazolin can be replaced with sulbactam/
ampicillin
Gentamicin can be administered at the dose of
1 mg/kg, 2–3 times daily
See the text for the effects of rifampicin |
| +Gentamicin |
2–3 mg/kg, once daily |
2** |
| ±Rifampicin |
450–600 mg/day in 1–2
doses |
6–8 |
Daptomycin±
β-lactams, etc. |
8–10 mg/kg, once daily |
6–8 |
IIa |
C |
See the text for doses and combination therapy |
| Vancomycin |
1 g, twice daily, or
15 mg/kg, twice daily |
6–8 |
IIa |
C |
Patients allergic to β-lactams
Gentamicin can be administered at the dose of
1 mg/kg, 2–3 times daily |
| +Gentamicin |
2–3 mg/kg, once daily |
2** |
| ±Rifampicin |
450–600 mg/day in 1–2
doses |
6–8 |
| 4) Methicillin-resistant staphylococci |
Daptomycin+
β-lactams, etc. |
8–10 mg/kg, once daily |
6–8 |
I |
C |
See the text for doses and concomitant therapy |
| Vancomycin |
1 g, twice daily, or
15 mg/kg, twice daily |
6–8 |
I |
C |
Adjusted to 15 to 20 μg/mL by TDM aiming at
the initial concentration of 10 to 15 μg/mL
Teicoplanin can be used (TDM is necessary)
See the text for aminoglycosides (including
arbekacin)
Gentamicin can be administered at the dose of
1 mg/kg per dose, 2 to 3 times daily |
| +Gentamicin |
2–3 mg/kg, once daily |
2** |
| ±Rifampicin |
450–600 mg/day in 1–2
doses |
6–8 |
*Doses should be adjusted according to the body weight and renal functions. 12 million unit to 30 million unit/day at maximum. **Some opinion recommends concomitant administration of gentamicin for more than 2 weeks. IE, infective endocarditis; VGS, viridans group streptococci.
Among streptococci, VGS as an oral streptococcus is detected frequently, and is a main causative microorganism for the community-acquired IE in the native valve and IE in the prosthetic valve more than 1 year after an operation. For penicillin G, isolates with minimum inhibitory concentration (MIC) ≤0.12 μg/mL is considered susceptible. Most of VGS,
Streptococcus gallolytics
(Streptococcus bovis), and other streptococci show good susceptibility to penicillin G. Since penicillin G shows a short half-life in blood (30 minutes, when renal functions are normal), it is administered every 4 hours53
or is administered continuously. Ampicillin can also be a choice of antibiotics. If the patient does not have immediate-type allergy to penicillin, cefazolin or ceftriaxone can be alternative of choice. If the patient cannot tolerate β-lactams including penicillin, vancomycin or teicoplanin should be selected. Some experts recommend addition of gentamicin for 2 weeks in the treatment regimen for prosthetic valve IE.15
b. Penicillin Non-Susceptible Streptococci (Table 11
and
Table 12)
In evaluation of susceptibility to penicillin, VGS is regarded to be non-sensitive (moderate resistance or resistance) when MIC of penicillin G is ≥0.25 μg/mL and MIC of ampicillin is ≥0.5 μg/mL. Consultation with infectious disease specialists is recommended about the combined use of gentamicin and so on. For non-susceptible strains, penicillin G or ampicillin should be administered in combination with gentamicin for 2 to 4 weeks (4 to 6 weeks for prosthetic valve).54
In the cases susceptible to ceftriaxone, gentamicin-including regimen is reasonable. In the cases non-susceptible to ceftriaxone, carbapenems can be selected.55,56
If the patients are intolerant to β-lactams, combination of vancomycin or teicoplanin with gentamicin can be administered. Daptomycin has not been studied sufficiently. If administration of aminoglycosides is difficult in patients with renal dysfunction, monotherapy with vancomycin or teicoplanin with TDM, or combination of ampicillin and ceftriaxone can be considered.
c. Other Streptococci
Streptococcus pyogenes
and
Streptococcus agalactiae
are highly pathogenic, and follow a relatively acute clinical course similar to that of staphylococci. Clinical symptoms are severe, and the mortality rate is also high (20% or higher).57
Since susceptibility to penicillin is almost constantly good, penicillin G, ampicillin, or ceftriaxone is selected choice, while some experts recommend combination with gentamicin (Table 11).4
3.2 Enterococci (Table 11
and
Table 12)
For enterococci, species identification and susceptibility tests should be performed.
Enterococcus faecalis
accounts for 90% or more of enterococci causing IE, and shows favorable susceptibility to penicillin. However, enterococci are resistant to many drugs including β-lactams, and long-term treatment is necessary. Since combination with gentamicin, which is used as the standard treatment, accompanies a problem of renal dysfunction, long-term administration of gentamicin is difficult, particularly in elderly patients.
In the treatment of enterococcal IE, ampicillin or vancomycin should be administered in combination with gentamicin (cases with MIC ≤500 μg/mL). For vancomycin, TDM should be conducted. The trough level should be 15 μg/mL according to that for MRSA (Table 8). The daily dose of gentamicin should be administered once daily or divided into 2 to 3 doses. The treatment results do not show differences even when gentamicin is administered once daily, and renal toxicity is less.58,59
Concerning the duration of gentamicin administration, some reports suggest that the treatment results do not differ even when the duration is 2 weeks.59,60
However, 2 weeks treatment should be avoided when IE is in the prosthetic valve, the vegetation size is large, and the patient is immunocompromised. The combination of ampicillin and ceftriaxone is also selected when renal dysfunction (creatinine clearance <50 mL/min) is present or when the strain shows high resistance to gentamicin (MIC >500 μg/mL).61–63
Ampicillin and ceftriaxone combination should not be used for
Enterococcus faecium.
Some researchers suggest that daptomycin does not exhibit sufficient efficacy as a monotherapy.64
At present, its use is limited to the cases with vancomycin resistant enterococci (VRE) or salvage use in the same way as linezolid. For VRE, monotherapy with linezolid, or combination of daptomycin with ampicillin or gentamicin should be used.
3.3 Staphylococci
Staphylococci account for one-third of the cases of IE.42
The clinical course is acute, occasionally shows sudden changes, and is likely to become severe. Valve destruction and perivalvular progression are rapid, and remote lesions are frequent. Therefore, treatment with appropriate antibiotics and decision of operation without delay are required.
a. Staphylococcus Aureus (Table 11
and
Table 12)
Staphylococcus aureus
is associated with in-hospital mortality in IE. The mortality rate from IE caused by
S. aureus
is 20% or more,65,66
and that in cases of IE in the prosthetic valve is even higher (47.5%).67
MRSA accounts for 7.5% of all cases in which the pathogen is identified,42
and associated with age (elderly patients), and prosthesis-associated or healthcare-associated infection.68,69
The mortality rate in the cases caused by MRSA has been reported to exceed 60%.68,69
Cefazolin is the drug of first choice for methicillin-sensitive
S. aureus
(MSSA) in Japan. Daptomycin,70
vancomycin, or teicoplanin should be used in cases intolerant of β-lactams because of allergy, etc. Addition of gentamicin is not recommended for staphylococcal native valve IE because of the nephrotoxity risk.71
The recommended duration of treatment is 4 to 6 weeks after negative conversion of blood culture. In cases accompanied by brain abscess or meningitis, other drugs rather than cefazolin should be selected, because delivery of cefazolin to the central nervous system is poor. Panipenem/betamipron, meropenem, or vancomycin should be considered as in the treatment of meningitis.72
Some experts recommend treatment with 3 drugs including gentamicin and rifampicin for IE in the prosthetic valve,4,15,73
but the level of evidence is not sufficiently high.
For MRSA, daptomycin or vancomycin should be selected as the drug of first choice. While the indication of daptomycin in Japanese health insurance is limited to right-sided IE, it is also used for left-sided IE.74
The duration of administration should be 4 to 6 weeks after negative conversion of blood culture. The duration should be 6 weeks or longer, approximately 8 weeks, for prosthetic valve IE. When daptomycin is selected, administration should be started at 8 to 10 mg/kg per dose, once daily. Better efficacy has been observed at higher doses (8 to 10 mg/kg) than once daily administration of 6 mg/kg per dose,75
and some experts recommend a dose of ≥10 mg/kg.70
The usefulness of administration of daptomycin in combination with β-lactams, aminoglycosides, rifampicin, fosfomycin, or sulfamethoxazole/trimethoprim has been suggested in some experimental and clinical studies.76–80
Combination therapy should be considered under consultation with infectious disease specialists. In particular, combination therapy is recommended for prosthetic valve IE. The choices of additional antibiotics are the following: β-lactams such as panipenem/betamipron 2.0 to 3.0 g/day and sulbactam/ampicillin 9 g (or ampicillin 6 g)/day, gentamicin at 2 to 3 mg/kg/day, rifampicin at 450 to 600 mg/day, fosfomycin at 6.0 g/day, and sulfamethoxazole/trimethoprim such as trimethoprim at 5 to 8 mg/kg/day. As adverse reactions to daptomycin, attention should be paid to elevation of the blood creatine kinase (CK) level, eosinophilia, and eosinophilic pneumonia.
When vancomycin is selected, designing before administration and TDM should be conducted. The target blood trough level should be approximately 15 to 20 μg/mL39
(Table 8).
The MIC of vancomycin ≤2 μg/mL means susceptible. However, if the MIC of vancomycin to the isolated MRSA is >1 μg/mL, the efficacy may be compromised even though target trough level is obtained. Therefore, efficacy evaluation should be conducted carefully for IE patients on the basis of confirmation of negative conversion of blood culture and the clinical course.
Combination of vancomycin and gentamicin is not recommended for native valve IE, because of the risk of renal toxicity. Three-drug combination therapy with vancomycin, gentamicin and rifampicin (6 weeks) is recommended as the standard therapy for staphylococcal prosthetic valve IE.4,15,73
However, the addition of rifampicin is not based on sufficient evidence. Moreover, since rifampicin requires precautions for hepatic toxicity and drug interactions, and rifampicin resistance is easy to occur within a short period, consultation with pharmacists and infectious disease specialists is recommended.
Teicoplanin and linezolid are the drugs of second choice.81–83
Teicoplanin is characterized by a quite long half-life in blood of approximately 50 hours, and loading dose is necessary for the blood concentration to reach a steady state at an early timing.39
The target trough level should be 20 μg/mL or higher (not exceeding 30 μg/mL). Linezolid is not a drug approved for IE in Japan, and administration for a duration longer than 2 weeks is related to thrombocytopenia.41
However, efficacy in the treatment of IE has been observed in the case series with prosthetic valve, and strains with low susceptibility to vancomycin, cases intolerant of vancomycin, and cases of unsuccessful treatment.83,84
Because linezolid shows favorable organ distribution, the cases accompanied by meningitis or brain abscess, and the cases accompanied by pneumonia are believed to be good indications. Infectious disease specialists should be consulted for combination therapy with other anti-MRSA drugs.
Concerning treatment with antibiotics other than anti-MRSA, combination therapy with imipenem/cilastatin and fosfomycin, which has been found to be useful in a multi-center study,85
and combination of sulfamethoxazole/trimethoprim and clindamycin have been considered in some cases, although they were intended for salvage therapy.86
b. Coagulase-Negative Staphylococci (CNS) (Table 11
and
Table 12)
CNS accounts for approximately 10% of all cases of IE,42
and is frequently detected as a causative microorganism in the prosthetic valve IE at a relatively early timing after valve replacement surgery. Although it is often regarded as less virulent than
S. aureus, the in-hospital mortality rate of IE caused by CNS is almost the same as that caused by
S. aureus. In particular, the mortality rate in methicillin-resistant cases is high (40%).48,49
The percentage of the cases that required surgical treatment was even higher than in the cases caused by
S. aureus.
Antibiotic treatment of IE caused by CNS should be conducted in the same way as that for
S. aureus. The combination therapy including rifampicin for IE in the prosthetic valve is based on a study of IE caused by CNS.
3.4 Gram-Negative Bacteria (Including HACEK)
HACEK is a group of gram-negative bacilli (Haemophilus aphrophilus,
Haemophilus paraphrophilus,
Aggregatibacter actinomycetemcomitans,
Cardiobacterium hominis,
Eikenella corrodens, and
Kingella kingae) accounting for about 1% of IE.42
Although isolation from blood cultures is rare, their association with IE is strongly suspected. The mean age of the patients with IE caused by HACEK is approximately 10 years younger than that of all IE patients. Most cases are of community-acquired infection, and the prognosis is relatively good.
HACEK organisms show good susceptibility to the third-generation and fourth-generation cephems (Table 13). Most strains are susceptible to ampicillin,87
but some are β-lactamase producing. Therefore, the susceptibility of isolated strains should be confirmed.88
In cases intolerant with β-lactams, quinolones such as ciprofloxacin and levofloxacin also become choices89
(Table 13).
Table 13.
Recommendations of Antibiotics in IE Caused by HACEK and Level of Evidence
| Antibiotics |
Dosage |
Period
(weeks) |
Class of
recommendation |
Level of
evidence |
Remarks |
| Ceftriaxone |
2 g, once daily |
4 |
IIa |
B |
Administration for 6 weeks for IE in the
prosthetic valve (class of recommendation IIb
and level of evidence C) |
| Sulbactam/ampicillin* |
3 g, 3–4 times daily |
4 |
IIb |
B |
| Ciprofloxacin, or |
300 mg, twice daily |
4 |
IIb |
C |
| Levofloxacin |
500 mg, once daily |
*Ampicillin can be administered in sensitive cases. IE, infective endocarditis.
IE caused by gram-negative bacteria other than HACEK is rare, accounting for only several percents.42
Among them,
Enterobacteriaceae
such as
Escherichia coli
and
Klebsiella pneumoniae
account for a majority, while
Pseudomonas aeruginosa
is common next to
Escherichia coli. Antibiotics should be selected from the third- and fourth-generation cephems, carbapenems, and quinolones according to the susceptibility results of isolates, and should be administered for up to approximately 6 weeks. Recommended treatment is combination of β-lactams and amikacin or gentamicin as the treatment of refractory gram-negative bacterial infection, but there has been no established treatment method, including the duration of aminoglycosides administration. Treatment is often difficult with antibiotics alone, and early surgery should be considered. However, the mortality rate exceeds 20% in spite of surgical treatment.90
3.5 Fungi
The incidence of fungal IE is rare but refractory, and the mortality rate is extremely high (30 to 50%).91
Fungal IE is common in patients with prosthetic valve. Most cases of fungal IE are caused by
Candida, and the cases caused by filamentous fungi such as
Aspergillus
are rare.
In the cases of fungal IE, it is difficult to control infection with medical treatment only, and some researchers recommend surgery within 1 week (native valve IE) or within several days (prosthetic valve IE).92
However, surgical treatment does not necessarily improve the survival rate,93
and the cases of IE in the prosthetic valve that could be controlled with antifungal drugs have been reported.94–96
As the drug of first choice for antifungal treatment, amphotericin-B lipid preparation, candins (micafungin and caspofungin), or voriconazole should be selected. However, combined administration of 2 drugs can be considered from the beginning of treatment97
(for example, amphotericin-B+candin). Infectious disease specialists should be consulted.
After surgery, treatment with antifungal drugs should be added for 6 to 8 weeks.93
The cases in which infection could be controlled with medical treatment alone should be treated for several months or more than 1 year (or lifelong) with oral azoles.97
4. Efficacy Evaluation and Duration of Antibiotic Treatment
Efficacy should be evaluated at approximately 72 hours (48 to 72 hours) after the start of treatment with antibiotics under careful monitoring.16
Overall judgement should be made basically on the basis of vital signs, as well as subjective symptoms such as pyrexia, dyspnea, malaise, and anorexia, physical findings (changes in cardiac murmur, edema in limbs, and symptoms of embolus), test data, and imaging findings (echocardiography, chest radiography, head and body CT/MRI, etc.). Negative conversion on blood culture is mandatory in the cases with positive blood cultures before initiation of treatment. In cases caused by
S. aureus
and cases in an unstable condition such as heart failure, immediate judgement for surgery is necessary. Cooperation with several specialists and judgement for early surgery are required at any time after the start of treatment (See “Chapter II. 2. Team Medicine”).
One of the clinical parameters for efficacy evaluation is body temperature. Among the patients who have received appropriate treatment with antibiotics, 70 to 75% of the patients get afebrile within 1 week, but it tends to take time when mucocutaneous findings (such as petechia and Janeway lesion) are present, the size of vegetation is large, embolism is present in large vessels, and diagnosis has taken many days. The number of days required for becoming afebrile is 2 to 4 days on average for VGS and enterococci, and approximately 7 to 10 days for
S. aureus.98,99
Pyrexia persists in spite of antibiotic treatment due to several reasons: progression of infection into the annular region, intracardiac abscess, formation of pulmonary embolism and other remote lesions, heart failure, and drug-induced fever. Uncontrolled infection is an important indication for early surgery, and may necessitate repeating blood culture and evaluation on echocardiography. If fever recurs, drug-induced fever is the most common cause (common around week 3 or week 4, and observed in approximately 30% of patients). Infection foci inside and outside of the heart still can be a cause for recurrent fever.99
In addition, complications such as catheter-related bloodstream infection, urinary tract infection, and pneumonia should be considered. As laboratory test findings, the white blood cell count, C-reactive protein (CRP), and other inflammation markers can ancillary be referred to for prediction of prognosis or estimation of complications.100
The duration of antibiotic treatment recommended in the present guidelines is not always based on sufficient comparative studies. While careful monitoring of the course is necessary even after completion of the scheduled treatment, routine blood culture is unnecessary if there are no findings suggestive of recurrence.
V. Evaluation and Management of Complications
1. Heart Failure
This section is omitted from the English version.
2. Uncontrolled Infection and Perivalvular Infection
This section is omitted from the English version.
3. Embolism
3.1 Evaluating the Risk of Embolism
This section is omitted from the English version.
3.2 Neurological Complications
a. Frequency and Types of Neurological Complications
Symptomatic neurological complications are observed in 10 to 35% of IE patients.101–104
When asymptomatic cases are included, 65 to 80% of the patients show one or more neurological complications.105–108
For the patients who are suspected to have IE, examination with brain MRI or contrast-enhanced CT is recommended even when obvious focal neurological symptoms are absent (see
Table 14
and “[CQ 1] Is brain MRI useful for patients without neurological symptoms who have or are suspected to have IE?”).
Table 14.
Recommendations for Diagnosis of Neurological Complications of Infective Endocarditis and Level of Evidence
| |
Class of
recommendation |
Level of
evidence |
When cerebral hemorrhage or subarachnoid hemorrhage is observed in a patient who is suspected to
have IE, mycotic aneurysm should be searched by cerebrovascular imaging (cerebral angiography, CTA,
or MRA) |
I |
C |
For the patients who are suspected to have IE, precise examination of neurological complications using
brain MRI should be considered, even when obvious focal neurological symptoms are not observed (see
“CQ 1 Is brain MRI useful for the patients without neurological symptoms who have or are suspected to
have IE?”) |
IIa |
C |
When MRI cannot be obtained in the patients who are suspected to have IE or when the systemic condition
of the patients is unstable, head plain CT may be considered, and three-dimensional CT angiography and
head contrast-enhanced CT may be added, if necessary |
IIb |
C |
| As imaging methods of MRI, DWI, FLAIR images, T2*WI or SWI, and MRA may be considered |
IIb |
C |
IE, infective endocarditis; CTA, computed tomography angiography; MRA, magnetic resonance angiography; DWI, diffusion weighted image; FLAIR, fluid attenuated inversion recovery; SWI, susceptibility-weighted imaging; T2*WI, T2*-weighted image.
The most common types of neurological complications are cerebral infarction and transient ischemic attack. Cerebral hemorrhage, subarachnoid hemorrhage, mycotic aneurysm, brain abscess, encephalomeningitis, toxic/metabolic encephalopathy, and epilepsy are also seen.102,106,109
Cerebral infarction, including asymptomatic cases, is observed in approximately 50% of IE patients,106,108
and cerebral hemorrhage and subarachnoid hemorrhage are observed in 5 to 10% of the patients.106,108,110
In studies using brain MRI-T2*-weighted images (T2*WI), cerebral microbleeds have been observed in a large percentage of cases (approximately 60%), in addition to the above findings.107,110,111
The percentage of the cases accompanied by mycotic aneurysm has been reported to be approximately 4 to 9%.112,113
When cerebral hemorrhage or subarachnoid hemorrhage is observed, the percentage of the cases accompanied by mycotic aneurysm increases to 22%.112
Mycotic aneurysm is often observed distal to the middle cerebral artery, and approximately 25% of the cases have multiple aneurysms.114
When cerebral hemorrhage or subarachnoid hemorrhage is observed in a patient who is suspected to have IE, it is strongly recommended to search mycotic aneurysm by cerebrovascular imaging (cerebral angiography, computed tomography angiography [CTA], or magnetic resonance angiography [MRA]) (Table 14).
b. Methods of Diagnosis of Neurological Complications (Table 14)
Brain MRI is most useful for the diagnosis of neurological complications accompanying IE. When MRI cannot be obtained, head plain CT should be obtained, and three-dimensional CT angiography and head contrast-enhanced CT should be added, if necessary (class of recommendation IIb, level of evidence C).
As imaging methods of MRI, diffusion weighted image (DWI), fluid attenuated inversion recovery (FLAIR) image, T2*WI or susceptibility-weighted image (SWI), and MRA are recommended (class of recommendation IIb, level of evidence C).
Since mycotic aneurysm often develops on the peripheral side of the middle cerebral artery, a larger field of view should be selected to include the entire middle cerebral artery (M3 or distal) when MRA is obtained. In the cases with cerebral hemorrhage or subarachnoid hemorrhage, cerebral angiography or three-dimensional CT angiography should be considered. In patients with IE, cerebral microbleeds are observed frequently on T2*WI and SWI, and association with mycotic aneurysm is suspected.115,116
Brain abscess shows marked high signals on DWI of MRI, and is depicted as a low-density area on CT. Characteristic capsular ring-enhancing effect is observed on contrast MRI and contrast-enhanced CT.
When headache, disturbance of consciousness, or meningeal irritation symptoms are present and meningitis or subarachnoid hemorrhage is suspected, cerebrospinal fluid examination by lumbar puncture should be conducted.
CQ 1 Is brain MRI useful for patients without neurological symptoms who have or are suspected to have IE?
Answer:
It is proposed to obtain brain MRI (including DWI, FLAIR images, T2*WI, and MRA) at an early timing in the patients without neurological symptoms who have or are suspected to have IE.
Strength of recommendation
2: Weakly recommended (proposed)
Strength of body of evidence
C (weak)
[Related section] “Chapter V. 2. b. Methods of Diagnosis of Neurological Complications”, Table 14
Commentary:
As described in detail in the previous section, various neurological complications are seen in patients with IE (See “Chapter V. 2. a. Frequency and Types of Neurological Complications”). Several studies have shown that, even in the IE patients without obvious neurological symptoms, screening tests using MRI show neurological complications in 40 to 80% of them.104,105,107,108,117–121
As compared with CT, MRI is able to detect small infarctions at a higher rate when DWI and FLAIR images are used. Moreover, when T2*WI or SWI is used, cerebral microbleeds can be detected.117,122
Cerebral microbleeds have been used as an indicator for diseases of cerebral small vessels such as hypertensive cerebral small vessel disease and amyloid angiopathy, but cerebral microbleeds are also observed at a high rate in IE patients,111
and its association with cerebral hemorrhage after open heart surgery123,124
and mycotic aneurysm120
is suspected. Magnetic resonance angiography is useful for identification of occluded vessels and mycotic aneurysm. Since mycotic aneurysm is often formed in the distal part of cerebral artery, an area including the distal part should be visualized on MRA, if possible (see “Chapter V. 2. b. Methods of Diagnosis of Neurological Complications”).
There has been no clear evidence regarding whether or not the patients’ prognosis is improved when brain MRI is obtained at an early timing in the patients without central nervous symptoms who have or are suspected to have IE.122
On the other hand, in a prospective observational study in which efficacy of obtaining brain MRI at an early timing in the patients who are suspected to have IE was examined, the accuracy of diagnosis of IE was improved in 32% of the patients when MRI was obtained at an early timing (improved from “possible” to “definite” in 26% and from “excluded” to “possible” in 6%), and the treatment strategy, including the timing of surgery and the antibiotics to be used, were changed on the basis of the results of MRI in 18% of the patients.106,125
One paper suggests that cardiac surgery should be waited for at least 2 weeks because the risk of exacerbation of perioperative mortality and neurological symptoms is high in the patients with neurological complications.103
However, recent observational studies have reported that cardiac surgery is conducted at earlier timings and perioperative mortality is low in patients with asymptomatic neurological complications,126
that, among neurological complications, only cerebral hemorrhage and moderate to severe cerebral infarction are associated with poor prognosis,101
and that the incidence of postoperative hemorrhagic infarction is very low even if cerebral infarction is present as comorbidity.118
When asymptomatic neurological complications are observed, treatment strategy should be determined on the basis of overall judgement of the patient’s systemic condition, degree of cardiac functions and valvular destruction, status of infection control, risk of recurrence of neurological complications, and risk of perioperative complications.
There has been no study on the cost-effectiveness of brain MRI in the patients without neurological symptoms who have or are suspected to have IE. While MRI is more expensive than CT, latent costs for CT associated with adverse effects by radiation exposure or contrast medium need to be taken into consideration.
In addition to the above considerations, the prevalence of brain MRI in Japan and safety of brain MRI were also taken into consideration to generate the recommendation for this CQ. Thus, it is proposed to obtain brain MRI (including DWI, FLAIR images, T2*WI, and MRA) at the earliest timing in patients without neurological symptoms who have or are suspected to have IE.
c. Treatment of Neurological Complications (Figure 2 and Table 15)
Table 15.
Recommendations for Treatment of Neurological Complications of Infective Endocarditis and Level of Evidence
| |
Class of
recommendation |
Level of
evidence |
Antiplatelet therapy and anticoagulant therapy should be discontinued immediately if IE is accompanied by
intracranial hemorrhage (except for cerebral microbleeds) |
I |
B |
| Surgical or endovascular treatment should be considered for ruptured mycotic aneurysm (see Figure 2) |
I |
C |
| It is not recommended to newly start antiplatelet therapy aiming at prevention of embolism in IE patients |
III |
B |
| Intravenous thrombolytic therapy is not recommended for acute cerebral infarction accompanying IE |
III |
B |
| It is not recommended to newly start anticoagulant therapy aiming at prevention of embolism in IE patients |
III |
C |
IE, infective endocarditis.
The types of neurological complications include cerebral infarction, transient ischemic attack, cerebral hemorrhage, mycotic aneurysm, meningitis, brain abscess, and epileptic seizure. The most important treatment to prevent the onset and recurrence of neurological complications is believed to start appropriate antibiotic treatment at an early timing. Open heart surgery for the patients who have cerebral infarction or intracranial hemorrhage as comorbidity is stated separately (See “[CQ 3] Should surgery of IE be conducted at an early timing when neurological complications have occurred?”).
i. Antithrombotic Therapy for Neurological Complications
When intracranial hemorrhage (except for cerebral microbleeds) has occurred as comorbidity, antiplatelet therapy and anticoagulant therapy should be discontinued immediately (Table 15). On the other hand, consensus has not been established at present regarding whether or not anticoagulant therapy should be continued for the patients who have been receiving anticoagulant therapy when ischemic cerebrovascular disease occurs.4
Therefore, the decision whether anticoagulant therapy is continued or not should carefully be made considering risks of both the options.
On the other hand, it is not recommended to newly start antiplatelet therapy in IE patients because a possibility to increase the risk of hemorrhagic complications has been suggested127
(Table 15). Although evidence regarding anticoagulant therapy is limited, it is not recommended to newly start anticoagulant therapy because it is also likely to increase the risk of hemorrhagic complications (Table 15).
ii. Acute Reperfusion Therapy for Neurological Complications
Intravenous thrombolytic therapy for acute cerebral infarction accompanying IE is not recommended because the incidence of intracranial hemorrhage after treatment is very high (approximately 20%)128
(Table 15). For acute major artery occlusion by vegetation, endovascular thrombectomy is suggested to be safe and effective even in patients receiving anticoagulant therapy.129
iii. Treatment for Intracerebral Hemorrhage and Hemorrhagic Infarction
When symptomatic intracerebral hemorrhage or hemorrhagic infarction exhibiting mass effect is observed, discontinuation or suspension of anticoagulant therapy should be considered. In some cases, craniotomy for removal of hematoma or decompressive craniectomy should be considered.130
iv. Coil Embolization/Surgical Treatment for Mycotic Aneurysm (Figure 2 and Table 15)
Although evidence based on a randomized controlled trial is not available regarding treatment methods,
Figure 2
summarizes treatment algorithm for mycotic aneurysm. While endovascular treatment has been conducted more often, surgical treatment may be preferred when intracerebral hemorrhage is present or revascularization is necessary.131,132
In selection of treatment methods, judgement should be made on the basis of thorough examination of the systemic condition for each patient.
3.3 Other Embolism
This section is omitted from the English version.
4. Renal Dysfunction
This section is omitted from the English version.
5. Disseminated Intravascular Coagulation
This section is omitted from the English version.
VI. Surgical Treatment
1. Evaluation of Surgical Risk and Preoperative Assessment
a. Evaluation of Surgical Risk
Surgical risk can be evaluated using JapanSCORE,133,134
STS score,135
and EuroSCORE.136
Operative mortality is high in patients requiring emergency surgery, cardiogenic/septic shock, infection by fungi or multidrug-resistant bacterium such as MRSA, prosthetic valve endocarditis, paravalvular abscess, neurological complications, renal failure, diabetes mellitus, and so forth.
b. Preoperative Assessment: Evaluation of Cerebral Vessels, Coronary Arteries, and Other Organs
Preoperative assessment of intracranial lesions is essential because IE accompanies neurological complications at high rates.4,73,101,103,118,123,124,137–142
The diagnosis of neurological complications is described in detail in “Chapter V. Evaluation and Management of Complications”. Patients with suspected myocardial ischemia require cardiac CT or coronary angiography.143
Optimal modality should be selected based on patient’s clinical condition. Cardiac CT can simultaneously evaluate coronary arteries and aortic root infection.32,33,144,145
Coronary angiography precisely evaluates stenosis with calcification or with tachycardia, but it carries the risk of catheter manipulation-related embolism, especially in patients with aortic valve IE. Assessment of embolism and abscess in the spleen or kidneys is possible to be performed with minimal contrast medium by additional late-phase systemic CT following cardiac CT.
2. Indications of Surgical Treatment and Timing of Operation
a. General Remarks on Indications of Surgical Treatment
In the treatment of IE, surgical treatment should be always kept in mind as an option. Early surgical treatment should be considered when there are possibilities of progressing heart failure, destruction of intracardiac structure, refractory infection, and embolism. The team should discuss the treatment strategy for each patient because the appropriate timing of surgery and postoperative results are varied according to the type of causative microorganisms, comorbidities and so on. Early surgery can be classified as emergency surgery (within 24 hours), urgent surgery (within several days), and elective surgery (1 to 2 weeks after antibiotic treatment).
Table 16
shows the indications of early surgery. In the cases not meeting the indications of early surgery, the indication and timing of surgery should be determined as usual valvular heart diseases. However, since the severity of valvular disease/bacteremia and possibility of embolism change from time to time in the cases of IE, careful monitoring is required to avoid missing the optimal timing of surgery.
Table 16.
Recommendations of Early Surgery for IE and Level of Evidence
| Situation |
Indication/recommendation*1 |
Timing |
Class of
recommendation |
Level of
evidence |
| Heart failure |
Refractory pulmonary edema and cardiogenic shock due to acute
severe valve dysfunction or fistula formation |
Emergency |
I |
B |
Heart failure due to severe valve dysfunction or rapidly worsening
paravalvular leakage |
Urgent |
I |
B |
Uncontrolled
infection |
Annular abscess, pseudoaneurysm formation, fistula formation,
increasing size of vegetation, and atrioventricular conduction
disturbance |
Urgent |
I |
B |
Persistent infection*2 exists despite no presence of other infection
foci. Persistent infection includes (1) positive blood culture obtained
at 2 to 3 days after appropriate antibiotic treatment or (2) sustained
fever for 3 to 5 days or more after appropriate antibiotic treatment |
Urgent |
IIa |
B |
| Infection by fungi or highly multidrug-resistant bacteria |
Urgent/elective |
I |
C |
Prosthetic valve endocarditis due to resistant staphylococci or
non-HACEK Gram-negative bacteria |
Urgent/elective |
IIa |
C |
| Recurrent prosthetic valve endocarditis |
Urgent/elective |
IIa |
C |
Prevention of
embolism |
One or more embolisms after the initiation of appropriate antibiotic
treatment and non-disappearing (size >10 mm) or growing vegetation |
Urgent |
I |
B |
Native valve endocarditis with mobile vegetation exceeding 10 mm in
size and severe valve dysfunction*3 |
Urgent |
IIa |
B |
| Isolated huge vegetation exceeding 30 mm in size |
Urgent |
IIa |
B |
| Mobile vegetation exceeding 10 mm in size*4 |
Urgent |
IIb |
C |
Timing of surgery
in patients with
neurological
complications*5 |
Surgery should not be postponed in patients with non-hemorrhagic
cerebral infarction when surgical treatment is appropriate
Note: Except for cases with coma, brain herniation, or huge major
central lesions |
– |
IIa |
B |
Surgery should be postponed at least 4 weeks when new
intracranial hemorrhage is detected.
Note: Except for cases involving cerebral microbleeds |
– |
IIa |
B |
*1: Unless otherwise specified, the statements apply to both native valve endocarditis and prosthetic valve endocarditis.
*2: Infectious condition should be comprehensively evaluated on the basis of negative conversion of blood culture in addition to the degree of lowering of body temperature or inflammatory markers such as the white blood cell count and CRP value.
*3: Early surgery is recommended, especially when the risk of surgery is low (See “CQ 2 Should early surgery be conducted if a large vegetation is present?”).
*4: Especially in cases with prosthetic valve endocarditis, anterior leaflet of mitral valve involvement, or the presence of other relative surgical indications.
*5: See “CQ 3 Should surgery of IE be conducted at an early timing when neurological complications have occurred?”
IE, infective endocarditis.
See “[CQ 3] Should surgery of IE be conducted at an early timing when neurological complications have occurred?” as well as
Figure 3
and
Table 16
for the timing of surgery in the cases in which neurological complications occur in the patients for whom early surgery is indicated.
b. Congestive Heart Failure (Table 16)
Congestive heart failure is triggered by regurgitation mainly caused by valvular destruction, but it can also be caused by intracardiac shunt, and valvular obstruction caused by vegetation. Heart failure of NYHA III to IV can become an indication of emergency surgery.146–149
Heart failure of NYHA II accompanied by severe valvular regurgitation also becomes an indication of early surgery if echocardiography suggests elevation of the left ventricular end-diastolic pressure and pulmonary hypertension.148–152
Even the cases accompanying severe valvular regurgitation can be treated with conservative treatment with antibiotics and management of heart failure, if there are no apparent signs of heart failure and there are no other reasons for surgical indication, such as large vegetation. In such cases, surgical indication should be determined as usual valvular heart diseases.
c. Uncontrolled Infection (Table 16)
Uncontrolled infection is an indication for urgent surgery. Uncontrolled infection is defined as continuing positive blood culture and/or sustained infection findings such as fever, elevated white blood cell count, and high CRP level despite appropriate antibiotic treatment for a certain period (approximately 3 to 5 days).16,99
Mortality is reported to be doubled if blood culture obtained at 48 to 72 hours after the initiation of antibiotics remains positive,16
and surgery should be performed without delay.
Patients with positive conversion of blood culture after confirming the absence of other infection foci are diagnosed as recurrent infection, and early surgery should be considered. Fungi, gram-negative bacteria,153
or MRSA, which is less likely to respond to antibiotic treatment, often follows a clinical course of uncontrolled infection. Annular abscess, pseudoaneurysm formation, increasing size of vegetation, or heart block (complete atrioventricular block and left bundle branch block) are all characteristic of uncontrolled infection.27,150,152,154,155
The incidence of paravalvular abscess is higher in prosthetic valve endocarditis than in native valve endocarditis (55 to 78% vs. 10 to 32%).156–161
In native valve endocarditis, aortic valve endocarditis is associated with a high rate of paravalvular abscess. In prosthetic valve endocarditis, the incidence of paravalvular abscess is high in endocarditis at the mitral position as well.
d. Infectious Embolism (Table 16)
Embolism, especially cerebral embolism, dramatically exacerbates the patients’ activities of daily living, even if it does not directly endanger life. Generally, the use of appropriate antibiotics will decrease the risk of embolism.101
However, it has been reported that the risk of embolism does not decrease even after antibiotic treatment in the cases of giant vegetation larger than 30 mm.101
If embolic events repeat even after the start of appropriate antibiotic treatment, the risk of subsequent embolic events should be high, and early surgery is reasonably indicated.4
Vegetation adhering to anterior mitral leaflet also accompanies a high risk of embolism.73
CQ 2 Should early surgery be conducted if a large vegetation is present?
Answer:
Surgery at the earliest timing possible is recommended for the patients with IE in the native valve (aortic valve or mitral valve) who have a vegetation of 10 mm or larger accompanying severe valve dysfunction.
Strength of recommendation
1: Strongly recommended
Strength of body of evidence
B (moderate)
[Related section] “Chapter VI. 2. a. General Remarks on Indications of Surgical Treatment”,
Table 16
Commentary:
In the observational studies, early surgery of IE has been associated with better prognosis.162–164
The usefulness of early surgery has been reported in Asian countries and Japan.165,166
The indication of early surgery for IE cases accompanying heart failure, embolism which occurs repeatedly in spite of sufficient antibiotic treatment, uncontrollable local infection (abscess, pseudoaneurysm, and fistula), and drug-resistant causative microorganisms such as fungi has been generally established (see “Chapter VI. 2. a. General Remarks on Indications of Surgical Treatment”). On the other hand, implementation of early surgery in all cases of IE accompanies concerns about the risk of surgery-associated death and the risk of recurrence. Therefore, identification of the subgroup to be treated by early surgery has been needed.165
In addition to mortality due to heart failure and other complications, IE may cause serious disabilities due to cerebral infarction caused by embolism of vegetation. A large vegetation of 10 mm or larger in size has been reported to be a risk factor of recurrent embolism.27,167
The risk of embolism after the onset of IE is high in the early stage after onset, and appropriate antibiotic treatment is known to reduce the risks. However, it has been suggested that the risk of embolism does not decrease in cases with huge vegetation, as well as the cases in which vegetation grows in size during antibiotic treatment.101
Therefore, surgical indication has been discussed for prevention of embolism in cases with a large vegetation. In observational studies, the usefulness of early surgery for a large vegetation has been reported in Western countries and Japan,149,168
while some reports have concerned that the incidences of early death, recurrence of IE, and valve dysfunction was high in early surgery.169,170
In 2012, the only randomized study at present regarding surgical indication of IE was reported.171
Inclusion criteria were as follows: patients aged 18 years or older who have left-sided native valve IE accompanying a vegetation larger than 10 mm, who also have severe valve dysfunction (valvular regurgitation in most cases), and for whom emergency surgery is not indicated (moderate-to-severe heart failure, atrioventricular conduction block, abscess, destructive penetrating lesions, and IE caused by fungi). Patients aged 80 years or older, who had severe complications such as large cerebral infarction accompanying the risk of hemorrhagic changes and cancer were excluded. Patients with prosthetic valve IE and right-sided IE were also excluded. The patients were divided into the group to be surgically treated within 48 hours after randomization (37 patients) and the group receiving conventional treatment (39 patients). In both groups, appropriate antibiotics were administered. In the conventional treatment group, 30 patients (77%) were surgically treated (27 patients during the index hospitalization and 3 patients in the late phase), and 8 patients received semi-emergency surgery (6 to 10 days after randomization). Therefore, this study could be regarded to test the usefulness of urgent surgery for the patients with a large vegetation accompanying valve dysfunction.
Significant differences were observed in primary composite endpoints (in-hospital death, embolic events within 6 weeks after randomization) between the early surgery group and the conventional treatment group (hazard ratio 0.10 [95% confidence interval, 0.01–0.82, P=0.03]). While no difference was seen in the total number of deaths within 6 months (3% versus 5%, hazard ratio 0.51 [95% confidence interval 0.05–5.66, P=0.59]), differences were seen in the presence or absence of embolism observed within 6 weeks (0% versus 21%, P=0.005). In both groups, no patients were re-hospitalized because of embolic events and heart failure during the follow-up period. Moreover, there were few cases of recurrence of IE within 6 months: no significant differences were observed between the groups.
In the above report, randomization was conducted, but concealment of randomization was not performed. Blinding was not conducted, but systemic differences in other treatments are deemed to be unlikely. Embolic events were confirmed on images at the time of periodic visits to the hospital, and detection bias is thought to be small. Image inspection was conducted only when symptoms were present, and asymptomatic embolism was not evaluated. The necessary number of patients was calculated, and intention to treat analysis was conducted.
The frequencies of causative microorganisms were typical, with VGS accounting for 30% of the cases, other streptococci 30%, and
Staphylococcus aureus
10%. The cases of cerebral infarction represented 30%. On the other hand, the patients were young (between 40 and 49 years), were relatively low risk, and were treated at the hospitals coping with a large number of surgical cases. A report suggested that the cases satisfying the conditions of this study was only 11.3% of the actual cases of left-sided native valve IE undermining validity of the results.172
This cohort was followed up for 7 years. Although no difference was seen in total mortality, composite endpoints (total death, embolism, and recurrence of IE) were significantly better in the early surgery group (8.1%) than the conventional treatment group (30.8%).173
On the basis of these results, the present guidelines recommend urgent surgery for the patients with IE in the native valve (aortic valve or mitral valve) who have a vegetation of 10 mm or larger accompanying severe valve dysfunction. However, the risk of surgery should be evaluated carefully. Moreover, prosthetic valve IE should not be included in the targets of this indication.
CQ 3 Should surgery of IE be conducted at an early timing when neurological complications have occurred?
Answer:
(1) Surgery of IE is recommended not to be postponed if it is indicated, even when concurrent cerebral infarction is present.*
*Except for the cases with concurrent coma, herniation, or cerebral hemorrhage, as well as major central lesions.
Strength of recommendation
1: Strongly recommended.
Strength of body of evidence
B (moderate)
(2) If new intracranial hemorrhage* is observed, it is proposed to wait 4 weeks to conduct open heart surgery if the hemodynamic condition is stable.
*Except for cerebral microbleeds.
Strength of recommendation
2: Weakly recommended (proposed).
Strength of body of evidence
C (weak)
[Related section] “Chapter VI. 2. a. General Remarks on Indications of Surgical Treatment”,
Table 16
Commentary:
When neurological complications occur during the course of IE, it is often difficult to decide the timing of surgery. Because of the use of cardiopulmonary bypass, which may induce perioperative hypotension and necessitate the use of a large dose of heparin, there are risks of exacerbation of neurological complications such as cerebral ischemia and cerebral hemorrhage.103,174
A multi-center retrospective study in Japan reported that, among the 181 patients who received surgery for IE, the rates of nosocomial death and exacerbation of neurological complications were high in the patients who underwent surgery in the early stage after the onset of neurological complications.103
Therefore, it has been recommended to wait 4 weeks before surgery for IE accompanied by neurological complications. However, patients who show exacerbation of the condition while waiting for surgery are often observed. Furthermore, these early studies were retrospective observational studies, without risk stratification, and did not examine the impacts of the presence or absence of symptoms, the size of infarction lesion, and the size of vegetation.103,174
In recent years, findings about early surgery in cases with neurological complications have been accumulated, and it has been revealed that the prognosis is not as bad as initially anticipated even if early surgery is conducted in cases accompanied by cerebral infarction.105,137–140,175–178
When risks were stratified in a prospective multi-center registration study, neither in-hospital mortality nor one-year mortality showed significant exacerbation when early surgery was conducted within 7 days after onset as compared with the cases in which surgery was conducted after that period.139
Surgical mortality is high in the cases of cerebral infarction in the middle cerebral arterial region and cerebral infarction accompanied by cerebral hemorrhage, meningitis, and brain abscess; however, early cardiac surgery in the cases of cerebral infarction without these complications showed no significant differences in the incidences of perioperative neurological complications and mortality, as compared with the cases without cerebral infarction.137
Several reports have showed that exacerbation of neurological complications after early surgery is rare and recovery is likely if any exacerbation occurs in the cases of cerebral infarction of 15 to 20 mm, in the cases without hemorrhage, and in the cases of asymptomatic or transient ischemic attack.105,140,175–178
Magnetic resonance imaging is effective for discovery of asymptomatic cerebral infarction, and its use is recommended.104
According to a study in Japan, hemorrhagic changes were rare in the group of IE in which cerebral infarction was detected by MRI and early surgery was conducted.118
Only observational studies had been available regarding the usefulness of early surgery for IE, irrespective of the presence or absence of neurological complications. Kang et al. conducted prospective investigation of the usefulness of early surgery conducted within 48 hours for IE accompanied by a vegetation of 10 mm or larger and significant valve dysfunction. As a result, they reported that no significant differences were seen in the mortality, but the number of the cases of embolic events in particular was significantly small in the early surgery group.171
Since approximately 30% of these patients had cerebral infarction as comorbidity, these results seem to support the feasibility of early surgery in the cases of non-severe cerebral infarction.
Considering these results, we concluded that it is not appropriate to postpone surgery in the cases with neurological complications, unless neurological findings are severe (such as coma and brain herniation), if there are indications of early surgery such as heart failure, uncontrolled infection, abscess and huge vegetation accompanying high risks of embolism.
On the other hand, cerebral hemorrhage should be considered separately from cerebral embolism because it has a possibility of exacerbation irrespective of the timing of surgery after onset. Favorable surgical results without postoperative exacerbation of cerebral hemorrhage has been reported when the hemorrhagic focus is 20 to 30 mm or smaller in some reports,140,179
but others reported early surgery within 7 days after onset was associated with exacerbation of neurological complications, such as enlargement of hemorrhagic focus.138
Many reports recommend to wait approximately 4 weeks if cerebral hemorrhage accompanies,101,103,179,180
but preceding clipping or craniotomy may enable cardiac surgery to be performed earlier in cases of mycotic aneurysm181
(see “Chapter V. 3. 2. c. Treatment of Neurological Complications”). The risks and conditions of cardiac lesions and cerebral lesions in individual cases should be examined carefully before surgery is performed.
Figure 3
shows the treatment algorithm for the cases with neurological complications.
e. Prosthetic Valve Endocarditis (Table 16)
Prosthetic valve endocarditis should be treated with the potential for surgery.148–150,182–189
Indication for surgery is considered on the basis of three conditions, i.e., heart failure, uncontrolled infection, and risk of embolism,148,150,169
similar to native valve endocarditis. Heart failure due to rapidly worsening paravalvular regurgitation or prosthetic valve dysfunction is an indication for urgent surgery.147,148,150–152,154–156,160
Emergent or urgent surgery is required in patients with cardiogenic shock, fistula formation, or fluctuation of a prosthetic heart valve. Surgery for such patients improves their prognosis.148,150
3. Surgical Treatment and Postoperative Management
The objective of surgery for IE is complete elimination of infection foci and tissue reconstruction.4
The infection recurrence rate after prosthetic valve replacement is comparable for mechanical valves and bioprosthetic valves.190
When annular abscess is formed, small cavities can be closed after dissection of infected tissues, but large cavities necessitate drainage into the pericardium or blood flow.4
a. Mitral Valve IE
In cases of mitral valve IE, resection and reconstruction of infection foci can be achieved by mitral valve replacement if infection is confined to the valve or a part of subvalvular tissues. However, when durability of prosthetic mitral valves and complications are taken into consideration, mitral valvuloplasty is preferred in many cases. Patch repair with autologous pericardium or heterogeneous pericardium can be used for the lesion of valve perforation, and repair with artificial chordae tendineae can be performed for ruptured chordae tendineae. In the cases of more extensive valve destruction, it is important to judge feasibility of valve repair with residual tissues after dissection of infected tissues. On the other hand, valve replacement and reconstruction of the periannular region are necessary in severe cases in which infection reaches the periannular region to form abscess or the normal anatomic structure has been disrupted. The missing part should be reconstructed with autologous or heterogeneous pericardium after complete resection and dissection of infected tissues.
b. Aortic Valve IE
Replacement is effective when infection is confined to the valve.191,192
When annular abscess is formed, patch formation after dissection can be conducted if the abscess cavity is small, but drainage into the pericardium should be performed if the abscess cavity is large. In such cases, replacement of the root becomes necessary. When extensive abscess accompanies, simultaneous replacement of the mitral valve can become an option. When the root replacement is performed, no significant differences are observed in the results in the cases using allografts, and the cases using prosthetic valves (bioprosthetic valve and mechanical valve) with Dacron grafts.193
Favorable results of Ross surgery have been reported by facilities with good experience.194,195
c. Postoperative Management
The differences from postoperative management for usual valvular heart disease are that exacerbation or new perioperative neurological complications can be observed, that the incidence of perivalvular regurgitation is high, and that infection as a cause of these complications may persist. The use of a bioprosthetic valve or valvuloplasty not needing anticoagulant therapy should be considered in the patients who have or who may have neurological complications.
VII. Follow-up After Discharge
This section is omitted from the English version.
VIII. Prevention
1. General Remarks on Prevention of IE
The incidence of IE in general population is reported to be 3 to 7/100,000 person-year.73
It has become common to classify the patients with high risk of IE as the highest-risk group who is very likely to lead to serious outcome, including death, once developed IE, and as the moderate-risk group for others.196–198
Western guidelines are less willing to recommend antibiotic prophylaxis before dental procedures because bacteremia can be induced by daily activities including tooth brushing, and the incidence of IE after dental procedures is extremely low. In particular, the guidelines released by the National Institute for Health and Care Excellence (NICE) in 2008 stated that antibiotic prophylaxis is unnecessary in all kinds of invasive dental procedures (however, the expression was modified in 2016 to that “it is not always unnecessary”).199
The present guidelines recommend antibiotic prophylaxis for the high-risk patients with IE (both highest-risk and moderate-risk) (see
Table 17
and “[CQ 4] Is antibiotic prophylaxis necessary for prevention of IE in dental procedures for the patients with high-risk heart diseases?”). The appropriateness of these recommendations should be continuously verified in epidemiological studies.
Table 17.
Risks of Infective Endocarditis in Adults According to Underlying Heart Disease, Recommendations of Antibiotic Prophylaxis During Dental and Oral Surgical Procedures, and Level of Evidence
| Risk of IE |
Class of
recommendation |
Level of
evidence |
| 1. Highest risk: high incidence, morbidity and mortality of infective endocarditis |
• Patients after prosthetic valve replacement (bioprosthetic/mechanical valve), or patients with annular ring
• Patients with a previous episode of IE
• Patients with complex, cyanotic congenital heart disease (single ventricle, complete transposition of great
arteries, tetralogy of Fallot)
• Patients underwent shunting between systemic and pulmonary circulation |
I |
B |
| 2. Moderate risk: lower morbidity and mortality despite high incidence of infective endocarditis |
• Most of congenital heart diseases*1
• Acquired valvular heart diseases*2
• Hypertrophic cardiomyopathy with obstruction
• Mitral valve prolapse with regurgitation |
IIa |
C |
• Patients with intracardiac devices (pacemaker, implantable cardioverter defibrillator)
• Patients with a long-term central venous catheter |
IIb |
C |
See “CQ 4 Is antibiotic prophylaxis necessary for prevention of IE in dental procedures for the patients with high-risk heart diseases?” for details about evaluation of evidence.
*1: Except for simple atrial septal defect (ostium secundum type).
*2: The risk of IE is low in mitral valve stenosis without regurgitation.
IE, infective endocarditis.
Since the level of risk may differ among the diseases classified as moderate-risk of IE, the IE risk of each disease is described for each section in “Chapter VIII. 2. What Types of Patients With Heart Disease Are Likely to Develop IE?” Antibiotic prophylaxis was examined mainly in relation to dental procedures. Other risks in treatment are also stated in “Chapter VIII. 3. Procedures, Treatment, and Background for Risks of IE and Prevention”. Concerning the route of entrance of causative microorganisms for IE, invasion via skin is known to be common.200
It is important to pay attention to the diseases causing decreases in the skin barrier functions, and to keep the skin clean during procedures. The conditions and the methods of prophylaxis are described in each section of “Chapter VIII. 3. Procedures, Treatment, and Background for Risks of IE and Prevention”.
2. What Types of Patients With Heart Disease Are Likely to Develop IE?
a. Patients With Prosthetic Valve or Previous IE
This section is omitted from the English version.
b. Adult Congenital Heart Disease
This section is omitted from the English version.
c. Aortic Valvular Disease
This section is omitted from the English version.
d. Mitral Valvular Disease / Mitral Valve Prolapse
This section is omitted from the English version.
e. Right-Sided Valvular Disease
This section is omitted from the English version.
f. Hypertrophic Cardiomyopathy
This section is omitted from the English version.
g. After Device Implantation
Infection associated with implantable cardiac devices tends to increase in recent years as treatment with devices, such as pacemaker, increases (see “Chapter IX. 2. IE on Cardiac Devices”). According to a cross-sectional study, the incidence of IE in patients with device implantation is estimated to be 40 events/100,000 person-year, which is higher than the incidence in the general population. In the present guidelines, patients after device implantation are classified as being at moderate risk of IE. However, antibiotic prophylaxis before dental procedures, and gastrointestinal and urogenital treatment in the patients with device implantation is not recommended in the guidelines of American Dental Society, American Heart Association (AHA), American College of Cardiology (ACC), and European Society of Cardiology (ESC).4,201–203
h. Others
This section is omitted from the English version.
3. Procedures, Treatment, and Background for Risks of IE and Prevention
3.1 Introduction
The procedures for which antibiotic prophylaxis is recommended include invasive dental procedures including scaling, tonsillectomy/adenoidectomy, and implantation of pacemaker and implantable cardioverter defibrillator (ICD).196
Antibiotics should also be administered before invasive treatment of local infection.196
Antibiotic prophylaxis is also recommended before surgical implantation of prosthetic valve and other cardiovascular prostheses, and transurethral resection of the prostate (TUR-P) in the patients with prosthetic valve.204,205
However, it is not recommended for bronchoscopy/laryngoscopy, endotracheal intubation, tympanostomy tube insertion, TEE, gastrointestinal endoscopy, urethral catheterization, transurethral endoscopy, and central venous catheterization.196
Antibiotic prophylaxis is recommended only in the cases with a history of IE for sclerotherapy for esophageal varices, dilatation of esophageal stenosis, colonoscopic test/treatment, biliary tract surgery, urethral dilatation, vaginal delivery and therapeutic abortion, cardiac catheterization, and skin incision.196
These are summarized in
Table 18.
Table 18.
Recommendations of Antibiotic Prophylaxis for High-Risk Patients of Infective Endocarditis and Level of Evidence
| Antibiotic prophylaxis |
Situation |
Class of
recommendation |
Level of
evidence |
Antibiotic prophylaxis is
strongly recommended |
• Dental and oral surgery: All invasive dental procedures causing bleeding and
bacteremia (oral surgery such as tooth extraction, periodontal surgery, dental
implant surgery, dental scaling, infected root canal treatment etc.)
• Otolaryngologic field: Tonsillectomy and adenoidectomy
• Cardiovascular field: Implantation of pacemaker and implantable cardioverter
defibrillator |
I |
B |
Antibiotic prophylaxis is
preferable |
• Invasive procedures for local infection foci: Abscess drainage and endoscopic
examination and treatment for infection foci (including biliary obstruction)
• Cardiovascular field: Implantation of prosthetic valve and other cardiovascular
prostheses
• Transurethral prostatectomy: Especially patients with prosthetic valve |
IIa |
C |
Antibiotic prophylaxis can
be performed. However,
antibiotic prophylaxis is
recommended for the
patients with a history of IE |
• Gastrointestinal field: Sclerotherapy for esophageal varices, dilatation of
esophageal stenosis, mucosal biopsy or polypectomy by colonoscopy and
proctoscopy, and biliary tract surgery
• Urogenital/reproductive field: Urethral dilatation, vaginal delivery/vaginal
hysterectomy, intrauterine curettage, therapeutic abortion/induced abortion,
and insertion and removal of intrauterine devices
• Cardiovascular field: Cardiac catheterization/percutaneous intravascular
catheter intervention
• Skin incision associated with surgery (especially in the patients with atopic
dermatitis) |
IIb |
C |
Antibiotic prophylaxis is
not recommended |
• Dental and oral surgery: Local anesthesia from uninfected site, orthodontic
procedures, dental pulpectomy
• Respiratory field: Bronchoscopy/ laryngoscopy, endotracheal intubation
(transnasal/oral)
• Otolaryngologic field: Tube insertion for tympanic perforation
• Gastrointestinal field: Transesophageal echocardiography, gastrointestinal
endoscopy (including biopsy)
• Urogenital field: urethral catheterization and transurethral endoscopy
(cystourethroscopy and pyeloureteroscopy)
• Cardiovascular field: Central venous catheterization |
III |
B |
IE, infective endocarditis.
The two major dental diseases are dental caries and periodontal diseases, and are believed to be caused by respective causative oral bacterial species (Figures 4,5). Caries is known to be induced mainly by a type of Gram-positive facultative bacteria,
Streptococcus mutans,206
and periodontal diseases are known to be induced by mixed infection with several Gram-negative obligatory anaerobes.207
Figure 4
schematically shows progression of caries (healthy, and C1 to C4). When dental plaque containing
Streptococcus mutans
adheres to the surface of the crown, caries begins to develop. When enamel and dentin decay along with progression of caries, tissues called pulp cavity, which are composed of dental pulp (nerves) and capillary vessels, are exposed. Under such a condition, oral bacteria can enter blood flow anytime, and dental pulp infection occurs.
Figure 5
schematically shows progression of periodontal diseases (healthy, gingivitis, and periodontitis). Periodontal disease can be divided mainly into gingivitis and periodontitis. When gingivitis progresses from a healthy state, hemorrhage is induced by stimulation with tooth brush, etc., and oral bacteria become able to enter blood flow. When inflammation progresses even more, ulcers are formed, and oral bacteria become able to enter blood flow easily anytime.
Tooth extraction, oral surgical treatment accompanying bleeding, dental implantation, and removal of dental calculous (scaling) are recognized as the dental procedures causing bacteremia.208
On the other hand, chewing and tooth brushing in daily life may cause bleeding, and bacteremia may be induced.209
When a state susceptible to bacteremia is generated, an environment for the IE-causing microorganisms present in the oral cavity to enter blood flow is established. Most of the causative microorganisms originating from the oral cavity are considered to be oral streptococci.210,211
As major bacteria, mitis group streptococci, such as
Streptococcus sanguinis, are known.212
However, it is extremely rare that major causative microorganisms for periodontal disease are isolated from blood of the patients with IE.213
According to a questionnaire survey of general dental practitioners conducted in Japan, only a half of the responders answered that they used antibiotics for the purpose of preventing IE.214
Only a half of them mentioned guidelines as the help to be referred to in deciding antibiotic prophylaxis. It is necessary to extensively distribute the guidelines among dentists in the future.214
b. Doses of Antibiotics
It has long been suggested that IE can be induced by bacteremia resulting from dental procedures.215
The incidence of bacteremia associated with dental procedures is almost 100% for tooth extraction, etc., and it is also high for scaling.208
It can also be induced by chewing and tooth brushing (Table 19).208,209,216–220
Table 19.
Incidence of Bacteremia Caused by Oral Procedures
| Oral procedure |
Incidence (%) |
| Tooth extraction |
18–100 |
| Wisdom tooth extraction |
55 |
| Dental scaling |
8–79 |
| Periodontal surgery |
36–88 |
| Infectious root canal treatment |
42 |
| Rubber dam insertion |
29 |
| Tooth brushing |
23 |
| Chewing |
38 |
(Data summarized from Heimdahl A, et al. 1990,208 Roberts GI, et al. 1992,209 Lockhart PB, et al. 2008,216 Hall G, et al. 1999,217 Debelian GJ, et al. 1995,218 Everett ED, et al. 1977,219 and Guntheroth WG. 1984.220)
The incidence of bacteremia after tooth extraction is known to decrease by antibiotic prophylaxis.216
On the other hand, in a model of IE induced by tooth extraction in rats, antibiotics had no impacts on bacteremia after tooth extraction but inhibited the onset of IE. The target of action of antibiotics is assumed to be not only suppression of the bacteremia but also inhibiting adhering ability of bacteria and bacterial growth after adhesion.221
The MIC90
of amoxicillin against oral streptococci isolated from dental infection222
was 0.25 μg/mL in the anginosus group, and 0.5 μg/mL in the mitis group. On the other hand, the MIC90
of clindamycin for oral streptococci isolated from dental infection was 64 μg/mL, but it was 0.125 μg/mL or lower for 80% of strains. The MIC90
of a macrolide, azithromycin, was high (128 μg/mL). The cumulative curve of drug sensitivity was biphasic, and highly resistant strains were observed.
In antibiotic prophylaxis for oral streptococci, penicillins (amoxicillin, ampicillin, and penicillin V) are the drugs of first choice from antibiotic susceptibility. In cases of allergy to β-lactams (penicillins and cephems), macrolides (clarithromycin or azithromycin) or clindamycin are alternative.
Concerning the choice of antibiotics, AHA recommends single administration of oral medication in order to prescribe by dentists easier.197
They recommend 2 g oral amoxicillin, because a blood concentration by oral route is comparable to that by intravenous injection and the effect can be maintained over a long period. In animal experiments, re-growth of the bacteria adhering to the vegetation occurs after 6 to 9 hours. Therefore, it is necessary to maintain the blood concentration of antibiotics for long period.223
In the present guidelines, single oral administration of amoxicillin 2 g within 1 hour before surgery is recommended (Table 20). Clindamycin, clarithromycin, and azithromycin are recommended in the cases of allergic to β-lactams, and ampicillin, cefazolin, ceftriaxone, and clindamycin are recommended if oral medication is unable to take (Table 20). If the dose of amoxicillin is reduced from 2 g for some reasons, additional administration of amoxicillin should be considered 5 to 6 hours after the first administration for a pharmacological reason to suppress growth of the bacteria adhered to the valve.
Table 20.
Recommended Prophylactic Antibiotics Before Dental Procedures (Adults)
| Route |
Allergic to β-lactam
antibiotics |
Antibiotics |
Dose |
Frequency of
administration |
Remarks |
| Oral |
Absent |
Amoxicillin |
2 g*1,*2 |
Single |
1 hour before treatment |
| Present |
Clindamycin |
600 mg |
Single |
1 hour before treatment |
| Azithromycin |
500 mg |
| Clarithromycin |
400 mg |
Unable to take
oral medication |
Absent |
Ampicillin |
1–2 g |
Single |
Intravenous injection or intramuscular injection
within 30 minutes after the start of surgery, or
intravenous drip infusion over 30 minutes or
more from the start of surgery |
| Cefazolin |
1 g |
| Ceftriaxone |
1 g |
Intravenous injection within 30 minutes after the
start of surgery, or intravenous drip infusion over
30 minutes or more from the start of surgery |
| Present |
Clindamycin |
600 mg |
Single |
Intravenous injection within 30 minutes after the
start of surgery, or intravenous drug infusion
over 30 minutes or more from the start of
surgery |
*1: Or 30 mg/kg body weight.
*2: If the dose of amoxicillin is decreased from 2 g for some reasons, additional administration of 500 mg of amoxicillin should be considered 5 to 6 hours after first dose.
Answer:
(1) Antibiotic prophylaxis is recommended before dental procedures inducing bacteremia, such as tooth extraction, in adult highest-risk patients.*1
Strength of recommendation
1: Strongly recommended
Strength of body of evidence
(moderate)
(2) Antibiotic prophylaxis is proposed before dental procedures inducing bacteremia, such as tooth extraction, in adult moderate-risk patients.*2
Strength of recommendation
2: Weakly recommended (proposed).
Strength of body of evidence
C (weak)
*1Highest-risk group (patients susceptible to infection whose condition becomes severe easily) includes 1) the cases after prosthetic valve surgery, 2) history of IE, 3) unrepaired cyanosis type congenital heart disease including the cases using palliative anastomosis and artificial blood vessels, 4) within 6 months after the repair of congenital heart disease using artificial materials regardless of surgery or catheterization, 5) cases accompanying residual lesions at the repair site in spite of repair with patch or artificial materials, and 6) aortic coarctation.
*2Moderate-risk group (patients whose condition is not always serious but who are likely to show endocarditis) includes congenital heart diseases other than highest- and low-risk, hypertrophic obstructive cardiomyopathy, and mitral valve prolapse accompanying valvular regurgitation.
[Related section] “Chapter VIII. 1. General Remarks on Prevention of IE”,
Table 18
Commentary:
Antibiotic prophylaxis for IE in dental procedures has been recommended in guidelines since 1950s,197
but there is no definite evidence for efficacy of antibiotic prophylaxis. Cochrane Review in 2013 also concluded that no evidence was observed for efficacy of antibiotic prophylaxis.224
Concerns about the allergic reaction induced by antibiotic prophylaxis and emergence of resistant bacteria have also been mentioned. Reflecting these concerns, antibiotic prophylaxis in dental procedures has been reconsidered in the United States and Europe.197–199,225
As described in a different section, there are heart diseases with higher risk of IE than usual population. Among the patients with high risks of IE, it has been accepted to differentiate the patients who are likely to have a serious outcome, including death, after the onset of IE (highest-risk group) from other patients (moderate-risk group).196–198
The guidelines issued in France in 2002,198
in the United States in 2007,197
and in Europe in 2009225
did not recommend antibiotic prophylaxis for the moderate-risk group but continuously recommended it for the highest-risk group. On the other hand, the recommendations by NICE in the United Kingdom in 2008 advised that antibiotic prophylaxis is not recommended for all patients including the highest-risk group.199
Under such circumstances, the previous guidelines in Japan continued to recommend antibiotic prophylaxis.196
In the present revision, a new recommendation has been generated on the basis of review of documents issued after revision of various guidelines in the 2000s.
The articles searched by systematic review and used for the present guidelines can be divided into three groups. The first is case control studies conducted before revision of the guidelines, which examined the presence or absence of antibiotic prophylaxis in the patients with and without IE.226–228
The second group is reports on the changes in the incidence of IE in foreign countries before and after revision of the guidelines.66,229–238
The third one is others, which includes a survey on the awareness of medical doctors and dentists about the guidelines,239
details about the cases in which IE occurred in spite of antibiotic prophylaxis,240
incidences of adverse reactions including allergy to antibiotics,241
and estimated data on IE which is believed to have been prevented by antibiotic prophylaxis and their economic analyses.242,243
Three articles are listed as case control studies.226–228
Some reports found association between the onset of IE and antibiotic prophylaxis,227
while other reports did not find any association.226,228
There are some limitations because of the method of subject registration or retrospective study design, and it is difficult to draw conclusions regarding antibiotic prophylaxis from these studies.
Articles of the second group, i.e., articles on the changes in the incidence of IE before and after changes of various guidelines, can be further divided into two subgroups. One is the studies conducted before and after discontinuation of antibiotic prophylaxis in all patients with heart diseases in the United Kingdom,230,231
and the other is the studies conducted in the United States and Europe other than the United Kingdom, in which antibiotic prophylaxis was continued in the highest-risk group and was discontinued in the moderate-risk group.66,229,232–238
In the former type, the changes of the number of prescriptions of antibiotics before and after the guidelines were recorded because of the insurance system in which the advice from NICE is linked with payment of medical expenses. For the latter, no clear data are available regarding to which extent the changes in the guidelines were complied with.
After the changes of the guidelines, the number of cases of antibiotic prophylaxis decreased dramatically in the United Kingdom.230,231
While no change was observed in the incidence of IE at first,231
a slight, but statistically significant increase in the incidence of IE was observed as of 5 years after the changes of the guidelines.230
Because of the nature of observational studies, it is impossible to demonstrate the causal relationship between the decrease of antibiotic prophylaxis and the increase in the incidence of IE. Moreover, it needs to be kept in mind that spontaneous increases of IE over time regardless of the changes of the guidelines have been reported. However, temporal agreement was observed between the changes of the guidelines and the increase in the incidence of IE. Moreover, increases in the number of IE were observed not only in the highest-risk group but also in the groups with moderate or lower risk.230
The results from the United States and Europe other than the United Kingdom showed no consistent tendency in the increase or decrease in the incidence of IE before and after changes of the guidelines.66,229,232–238,244
For all of these results, the data regarding whether or not the amount of antibiotics prescribed decreased after revision of the guidelines are missing. Articles on the increase of IE before and after revision of the guidelines include reports on the increase of IE induced by oral streptococci, and a causal relationship with the presence or absence of antibiotic prophylaxis in dental procedures is suggested.232
On the other hand, another article suggests that IE induced by streptococci has not increased or has rather decreased, and thus consensus has not been achieved.235,244
The difference from the report from the United Kingdom may support the policy to continue antibiotic prophylaxis limited to the highest-risk group. However, it is also possible that the changes of the guidelines did not sufficiently modify the behaviors of physicians and dentists in the fields.245
In the United Kingdom, the awareness of the guidelines was high but the compliance rate was not as high as the awareness, and many physicians were thought to have continued antibiotic prophylaxis in spite of the new guidelines.239
This may be a reason why the increase of IE after the release of the guidelines remained small.
Antibiotic prophylaxis may not be able to prevent IE in some cases.240
Moreover, an estimation shows that antibiotic prophylaxis can prevent IE in only a very small number of cases.242
However, since IE causes a serious outcome once it has occurred, the effect on the patients’ life and economic impact are great even if the number of cases of successful prevention is very small.243
On the other hand, adverse events caused by antibiotic prophylaxis for IE have been extremely rare.241
On the basis of the above considerations, the present guidelines recommend antibiotic prophylaxis for the highest-risk group. Moreover, considering the results showing that IE increased also in the patients at moderate or lower risk after discontinuation of antibiotic prophylaxis in the United Kingdom, and that IE induced by streptococci, which is resident bacterial flora in the oral cavity, increased in the United States and Europe other than the United Kingdom, the present guidelines also suggest antibiotic prophylaxis for the moderate-risk group. The number of patients with heart diseases classified into the moderate-risk group is huge, and cost-effectiveness of conducting antibiotic prophylaxis in all patients has not been established. However, once IE occurs, it may lead to hospitalization, surgery, cerebral infarction or death, and the impact on individual patients is tremendous. Although the possibility of leading to a serious outcome after the onset of IE is not very large in the moderate-risk group, it is advisable to decide to prescribe antibiotic prophylaxis for each patient on the basis of thorough discussion with the patient. Adequate measures for preventing IE other than antibiotic prophylaxis, including maintenance of oral hygiene, and education for early detection are also important. We hope that this recommendation further improves the awareness of patients and health professionals about IE and its prevention.
3.3 Skin Disease
This section is omitted from the English version.
3.4 Steroid Usage
This section is omitted from the English version.
3.5 Pneumonia and Other Remote Site Infection
This section is omitted from the English version.
3.6 Chronic Central Venous Catheter
This section is omitted from the English version.
3.7 Catheterization and Intracardiac Device Implantation
This section is omitted from the English version.
3.8 Prevention Before Respiratory, Esophageal, Gastrointestinal, Genitourinary Procedures
This section is omitted from the English version.
3.9 Cardiac Surgery
This section is omitted from the English version.
3.10 Education on IE in High-Risk Patients and Education on Measures for Coping With Pyrexia
Moderate-risk or highest-risk patients should be instructed to voluntarily report to medical personnel that they have a risk of IE. Attending physicians need to notify to the patients the name of the underlying heart disease, presence or absence of intracardiac prostheses or pacemaker, and necessity of antibiotic prophylaxis. It is recommended to distribute a document like the one shown in
Table 21
to patients.
Table 21.
Example of Document to be Distributed to High-Risk Patients for IE
You have a heart disease which is prone to infective endocarditis (a condition in which bacteria growth on the valves and inner
membranes inside the heart, and induce high fever, heart failure, brain stroke, and so on) |
| Therefore, |
1. Appropriate prevention is necessary when you have your tooth extracted or pyorrhea incision. Be sure to notify it to your dentist
and ask for appropriate prophylactic treatment
2. If you leave pyorrhea or dental caries reaching the tooth root untreated, you easily get infective endocarditis. You should
consult dentists for regular check-up of your oral condition
3. Keep your mouth clean by regular tooth brushing and care of the gums, and receive appropriate guidance from dentists
4. Some procedures and surgery may cause infective endocarditis. Before the procedure or surgery, please inform your physician
that you are prone to infective endocarditis
5. Do not take oral antibiotic drugs carelessly when you have high fever, when you cannot identify the cause of the fever, or when
the fever cannot be resolved immediately. Consult with your cardiologist in such cases |
IE, infective endocarditis.
Moderate-risk or highest-risk patients should be instructed to consult a medical institution if pyrexia persists for 4 days or longer. The usefulness of oral antibiotics in the case of pyrexia is unclear, and it may lower the detection rate on blood culture.
Daily oral and skin hygiene control is important for prevention of infection. It is also necessary to encourage them to visit dentists so that they can receive information regarding appropriate oral hygiene control. Since IE in the patients with atopic dermatitis becomes severe,246
consultation with dermatologists should also be considered.
IX. Management of Specific Situations
1. Congenital Heart Disease and Pediatric IE
1.1 General Remarks on Congenital Heart Disease and Pediatric IE
While the incidence of IE in general pediatric population is 0.34 to 0.64 per 100,000 person-year, which is lower than that in adults. However, the incidence becomes higher, 41 per 100,000 person-year, in the population with congenital heart disease. The percentage of the patients with congenital heart disease in the entire pediatric patients with IE is 30 to 80%.247–249
The risks of IE in pediatric patients are (1) presence of congenital heart disease, (2) abnormal hemodynamics sustained after the surgical repair of congenital heart disease, and the presence of prostheses used in surgery, such as prosthetic valve and patch, (3) catheter placement in surgery, (4) decreased immune defense mechanism, and (5) susceptibility to infection with
Staphylococcus aureus.197,250
The reasons for high risks of IE in patients with congenital heart disease are that they are susceptible to intimal damage caused by disturbed blood flow and jet lesion, and that bacteria are likely to adhere to the surface of prostheses used in surgical operation. Among disease groups, the higher incidence of IE is found in cases with heterotaxy syndrome especially in asplenia (right isomelism) which is prone to
Streptococcus pneumoniae
infection.248,249,251–255
The most common causative microorganisms in pediatric cases is
Staphylococcus aureus, followed by VGS and CNS in this order. Beyond that
Staphylococcus aureus
infection is common, the condition tends to become severe in pediatric age. Infection of placed catheter and nosocomial infection may also be caused by gram-negative bacteria and fungi.4,197,256
1.2 Risks According to Underlying Heart Disease
In the AHA Guidelines,197
the group in which complications are likely to occur and the mortality rate is high is defined as the highest risk, and antibiotic prophylaxis is recommended only for this group. On the other hand, “Management and Prevention of Infective Endocarditis in Congenital Heart Disease and Pediatric Cardiac Disease” issued by the Japanese Society of Pediatric Cardiology and Cardiac Surgery250
classified the risks for each underlying heart disease into three groups, highest-risk, moderate-risk, and low-risk. Highest-risk is a group in which the condition is likely to occur and is likely to become severe, moderate-risk is a group in which the condition is likely to occur but the risk of becoming severe is low, and low-risk is a group in which the risk is almost the same as in the cases without the disease. Thus, antibiotic prophylaxis is recommended in the highest-risk and moderate-risk groups.250
This is because many of the patients with IE occurred after dental procedures were in the moderate-risk group according to the national survey in Japan.
In this revision, the risk classification of the Japanese Society of Pediatric Cardiology and Cardiac Surgery was followed, and antibiotic prophylaxis in dental procedures was strongly recommended for the highest-risk group, and weakly recommended for the moderate-risk group (Table 22) (Also see “[CQ 5] Is antibiotic prophylaxis necessary for prevention of IE in dental procedures for pediatric/congenital heart diseases?”). Bicuspid aortic valve theoretically has a risk of IE if mild eccentric regurgitation is present even if stenosis is absent.
Table 22.
Risks of Infective Endocarditis in Pediatric/Congenital Heart Disease According to Underlying Heart Disease, Recommendations of Antibiotic Prophylaxis During Dental and Oral Surgical Procedures, and Level of Evidence
| Risk of infective endocarditis |
Class of
recommendation |
Level of
evidence |
| 1. Highest risk: high incidence, morbidity and mortality of infective endocarditis |
• After prosthetic valve surgery
• History of IE
• Unrepaired cyanosis type congenital heart disease including palliative anastomosis and use of artificial
blood vessels
• Within 6 months after the repair of congenital heart disease using artificial materials regardless of surgery
or catheterization
• Cases accompanying residual lesions at the repair site in spite of repair with patch or artificial materials
• Aortic coarctation |
I |
B |
| 2. Moderate risk: lower morbidity and mortality despite high incidence of infective endocarditis |
• Congenital heart diseases except for those in the highest-risk group and the low-risk group (including
bicuspid aortic valve)
• Hypertrophic cardiomyopathy with obstruction
• Mitral valve prolapse with regurgitation |
IIa |
C |
| 3. Low risk: No particular risk of infection, and almost the same risk of infection as ordinary people |
• Isolated atrial septal defect of ostium secundum type
• Repaired ventricular septal defect or patent ductus arteriosus 6 months after surgery and without residual
shunt
• After coronary artery bypass surgery
• Mitral valve prolapse without valvular regurgitation
• Physiological, functional or innocent cardiac murmur
• History of Kawasaki disease without valve dysfunction |
III |
C |
See “CQ 5 Is antibiotic prophylaxis necessary for prevention of IE in dental procedures for pediatric/congenital heart diseases?” for details about evaluation of evidence. IE, infective endocarditis.
In the national survey of the Japanese Society of Pediatric Cardiology and Cardiac Surgery, 170 pediatric patients with IE included 23 patients who did not show underlying heart disease (14%).250,251
Therefore, IE should be considered in differential diagnosis in the cases of pyrexia of unknown causes even if underlying heart disease is absent.
1.3 Diagnosis
a. General Remarks on Diagnosis of Congenital Heart Disease and Pediatric IE
The modified Duke criteria for diagnosis (Table 4) is widely used in clinical settings because the sensitivity of diagnosis exceeds 70% even in pediatric cases although the early diagnostic accuracy is low.250,257
In imaging for diagnosis, not only TEE but also MRI, CT, and PET should be considered as useful modalities for pediatric cases.4,257,258
It is also a key to improve the accuracy and the detection rate of blood culture in pediatric cases, by setting the proper timing, method, frequency, number of specimens of blood sampling as well as the storing method during sample transportation until conduct of the culture test.
b. Symptoms
This section is omitted from the English version.
c. Blood Culture
This section is omitted from the English version.
d. Echocardiography
This section is omitted from the English version.
e. Other Imaging Diagnosis
This section is omitted from the English version.
f. Evaluation of Complications (Heart Failure, Perivalvular Abscess, Embolism and Intracranial Complications)
This section is omitted from the English version.
1.4 Treatment
a. Medical Treatment
The recommendations for adults should be basically followed in the choice of antibiotics in pediatric cases. Recommendations of antibiotic treatment in pediatric cases are shown in
Table 23.
Table 23.
Recommendations of Antibiotics in Pediatric Infective Endocarditis and Level of Evidence
Causative
microorganisms |
Antibiotics |
In the case of
allergy to penicillin |
Administration
period (weeks) |
Class of
recommendation |
Level of
evidence |
Viridans group streptococci,
Streptococcus bovis,
enterococci (if sensitive) |
PenicillinG±Gentamicin |
Vancomycin |
PenicillinG: 4–6
Gentamicin: 2
Vancomycin: 4–6 |
I |
B |
| Ampicillin±Gentamicin |
Vancomycin |
Ampicillin: 4
Vancomycin: 4–6 |
I |
C |
Staphylococcus aureus
(methicillin-sensitive) |
Cefazolin±Gentamicin |
Vancomycin |
Cefazolin: 6–8
Gentamicin: 2
Vancomycin: 6–8 |
IIa |
C |
Staphylococcus aureus
(methicillin-resistant) |
Vancomycin
(or Teicoplanin)±
Gentamicin |
|
Vancomycin: 6–8
Gentamicin: 2
Teicoplanin: 6–8 |
I |
B |
| Daptomycin |
|
Daptomycin: 6–8 |
IIb |
C |
Gram-negative bacteria,
Enterobacteriaceae |
Broad-spectrum cephems
(cefepime, cefotaxime,
ceftazidime, ceftriaxone)
+gentamicin |
|
Broad-spectrum
cephems: ≥6 |
IIb |
C |
| Haemophilus (HACEK) |
Ceftriaxone |
|
Ceftriaxone: 4–6 |
I |
C |
| Ampicillin+gentamicin |
|
Ampicillin: 4–6
Gentamicin: 2 |
I |
C |
Blood culture negative
(postoperative cases) |
Vancomycin±gentamicin |
|
Vancomycin: 6–8
Gentamicin: 2 |
IIb |
C |
Blood culture negative
(non-postoperative cases) |
Cefazolin+gentamicin
±penicillin G |
|
Cefazolin: 6–8
Gentamicin: 2
Penicillin G: 6–8 |
IIb |
C |
| Fungi |
Lipoid amphotericin B |
|
Lipoid amphotericin B:
8 |
I |
C |
Daily dose for children with normal renal functions
Ampicillin: 50 mg/kg per dose, 4 to 6 times daily
Gentamicin*: 1 to 2.5 mg/kg per dose, 3 times daily
Sulbactam/ampicillin: 50 mg/kg per dose, 4 to 6 times daily
Cefazolin: 25 mg/kg per dose, 4 times daily
Cefepime: 50 mg/kg per dose, 3 times daily
Cefotaxime: 50 mg/kg per dose, 4 times daily
Ceftazidime: 50 mg/kg per dose, 3 times daily
Ceftriaxone: 50 mg/kg per dose, twice daily
Daptomycin: 6 mg/kg per dose, once daily (in the case of MRSA, 6 to 10/kg per dose, once daily)
Teicoplanin**: 10 mg/kg is administered three times at 12-hour intervals, followed by intravenous drip infusion of 10 mg/kg, once daily, over 30 minutes or more. Neonates are treated with intravenous drip infusion at the dose of 16 mg/kg only at the first dose, followed by drip infusion at the dose of 8 mg/kg/ over 30 minutes or more.
Vancomycin*: 15 to 20 mg/kg per dose, 4 times daily (younger than 13 years), intravenous drip infusion over 1 hour
Penicillin G: 50,000 unit/kg per dose, 4 to 6 times daily
Rifampicin: 8 to 10 mg/kg per dose, twice daily
Lipoid amphotericin B: 2.5 to 5 mg/kg per dose, once daily
*Blood concentrations should be determined periodically for gentamicin and vancomycin (TDM: Therapeutic drug monitoring), and the doses and administration should be planned.
**Since the half-life of teicoplanin is long (approximately 50 hours), TDM should aim at the peak of approximately 40 μg/mL and the trough of approximately 20 μg/mL (25 μg/mL, if possible).
IE, infective endocarditis.
For IE caused by streptococci, the guidelines of the Japanese Society of Pediatric Cardiology and Cardiac Surgery250
recommends concomitant administration of penicillin G and aminoglycoside if the strain is non-sensitive to penicillin G (benzylpenicillin). The statement of AHA257
recommends administration of penicillin G alone or ceftriaxone alone to avoid adverse reactions to aminoglycosides in the cases sensitive to penicillin.
Although IE caused by enterococci is not common in pediatric patients, concomitant administration of penicillin and gentamicin is recommended in the case of strains sensitive to penicillin.257
Involvement of specialists of infection is desired in cases of resistant strains.
As antibiotic treatment for staphylococci, the AHA Statement recommends penicillin G for the strains sensitive to penicillin G, penicillinase-resistant penicillin (oxacillin and cloxacillin) for resistant strains, vancomycin for methicillin-resistant strains (so-called MRSA), and daptomycin for vancomycin-resistant strains. The guidelines of the Japanese Society of Pediatric Cardiology and Cardiac Surgery recommends cefazolin for methicillin-sensitive strains, and vancomycin for methicillin-resistant strains. Among the cases of MRSA infection, concomitant administration of anti-MRSA drug and other antibiotics such as rifampicin should be considered for IE in the prosthetic valve and the cases refractory to early treatment.41
For antibiotic treatment in the cases negative on blood culture, the recommendations of the Japanese Society of Pediatric Cardiology and Cardiac Surgery should be followed. The AHA Statement recommends concomitant administration of sulbactam/ampicillin and gentamicin instead of cefazolin for non-postoperative cases of IE negative on blood culture.
b. Surgical Treatment
This section is omitted from the English version.
1.5 Prevention
a. Treatment Necessitating Prophylactic Measures and Current Status
The dental procedures necessitating prophylactic measures in the field of pediatrics are basically the same as the dental procedures for adults, although there are some unique points.250
Generally, the dental procedure which is most likely to induce bacteremia is tooth extraction. However, extraction of a primary tooth, which accompanies absorption of the root of the tooth, is not so invasive as extraction of a permanent tooth. Moreover, although bleeding may be observed when a primary tooth falls off, bacteremia is not considered in usual cases.259
In March 2017, a questionnaire survey was conducted among all members with cooperation of the Japanese Society of Pediatric Dentistry.260
The results suggested that the dental procedure which may become a risk of IE is recognized on the basis of the degree of bacteremia, which is assumed to be induced by each treatment.
b. Recommended Prophylactic Measures and Recurrent Status
Table 24
shows the type and dose of antibiotics used in antibiotic prophylaxis for pediatric patients.259,261
The guidelines state that amoxicillin should be selected for the cases in which oral administration is possible, and that the drug should be orally taken at the dose of 50 mg per kg body weight (2 g at maximum) 1 hour before the procedure. How the antibiotics are used is discussed in detail in the section for adults.
Table 24.
Recommended Antibiotic Prophylaxis Before Dental Procedures (Children)
| Route |
Allergy to β-lactam
antibiotics |
Antibiotics |
Dose |
Frequency of
administration |
Remarks |
Oral administration
is possible |
Absent |
Amoxicillin |
50 mg/kg
(2 g at maximum) |
Single |
1 hour before treatment |
| Present |
Clindamycin |
20 mg/kg
(600 mg at maximum) |
Single |
1 hour before treatment |
| Azithromycin |
15 mg/kg
(500 mg at maximum) |
| Clarithromycin |
15 mg/kg
(400 mg at maximum) |
Oral administration
is impossible |
Absent |
Ampicillin |
50 mg/kg
(2 g at maximum) |
Single |
Intravenous injection or intramuscular
injection within 30 minutes after the
start of surgery, or intravenous drug
infusion over 30 minutes or more
from the start of surgery |
| Cefazolin |
50 mg/kg
(1 g at maximum) |
| Ceftriaxone |
50 mg/kg
(1 g at maximum) |
Intravenous injection within 30
minutes after the start of surgery, or
intravenous drug infusion over 30
minutes or more from the start of
surgery |
| Present |
Clindamycin |
20 mg/kg
(600 mg at maximum) |
Single |
Intravenous injection within 30
minutes after the start of surgery, or
intravenous drug infusion over 30
minutes or more from the start of
surgery |
The prevalence of neonatal heart diseases in Japan is reported to be approximately 1 out of 100 patients,262
and general dental practitioners have many chances to encounter pediatric patients with a risk of IE. Further educational activities about the guidelines are important in order to enable all pediatric patients with a risk of IE to receive dental procedures with appropriate prevention of IE.
CQ 5 Is antibiotic prophylaxis necessary for prevention of IE in dental procedures for pediatric/congenital heart diseases?
Answer:
(1) Antibiotic prophylaxis is recommended before dental procedures inducing bacteremia, such as tooth extraction, in highest-risk patients with pediatric/adult congenital heart disease.*1
Strength of recommendation
1: Strongly recommended
Strength of body of evidence
C (weak)
(2) Antibiotic prophylaxis is proposed before dental procedures inducing bacteremia, such as tooth extraction, in moderate-risk patients with pediatric/adult congenital heart disease.*2
Strength of recommendation
2: Weakly recommended (proposed).
Strength of body of evidence
C (weak)
*1The highest-risk group (patients susceptible to infection whose condition becomes severe easily) includes the patients who have received bioprosthetic valve, prosthetic valve replacement including bioprosthetic valve and allogeneic valve, patients with a history of IE, complex, cyanotic congenital heart disease, and patients created shunts between systemic and pulmonary circulation.
*2Moderate-risk group (patients whose condition is not always serious but who are likely to show endocarditis) includes most congenital heart diseases, acquired valvular diseases, hypertrophic obstructive cardiomyopathy, and mitral valve prolapse accompanying valvular regurgitation
[Related section] “Chapter IX. 1. 2 Risks According to Underlying Heart Disease”,
Table 22
Commentary:
There have been no randomized studies to conclude this question. Moreover, it is difficult to expect that a meaningful randomized study is conducted in the future because the incidence of the condition is low. After revision of the AHA Guidelines in 2007, antibiotic prophylaxis for IE was completely discontinued or was changed to the policy to administer the drugs only to highest-risk patients.197,199
Recently, the late-phase outcomes in adults after revision of the AHA Guidelines in 2007 have been reported. As described in the section for adults, the number of cases of IE increased in the United Kingdom after revision of the guidelines.230
On the other hand, the risk of serious adverse reactions caused by a single dose of antibiotic prophylaxis is extremely low.241
Considering these results, the present guidelines recommend antibiotic prophylaxis in dental procedures for adult highest-risk patients, and weakly recommends it to moderate-risk patients.
Concerning the changes of the incidence of IE after modification of the guidelines, the present systematic review found only two reports each from the United States and Canada regarding the study focused on pediatric/congenital heart disease.237,263
Moreover, the results did not demonstrate increases of IE after modification of the guidelines. However, these are not considered to demonstrate the uselessness of antibiotic prophylaxis for pediatric/congenital heart disease. According to a study using the database of the Canadian Institute for Health Information, inpatients with IE of the age younger than 18 years accounts for only 2.4% of all inpatients with IE, and there have been no reports of statistical analysis focused on the changes in the number of inpatients with IE aged younger than 18 years.237
A study from the pediatric database in the United States incorporated only the data until the third year after modification of the guidelines, and it is considered to be too early to draw any conclusions.263
Even after the modification of the AHA Guidelines, changes in the behaviors for antibiotic prophylaxis in actual clinical settings were not marked.264
Because congenital heart disease is common among pediatric patients with IE and is likely to become serious, prophylaxis is believed to be more important than in adults.265
Moreover, considering the reports that antibiotic prophylaxis in pediatric patients reduces bacteremia in tooth extraction,266–271
it has been decided to continue recommendation of antibiotic prophylaxis for the pediatric patients except for those in the low-risk group. It is strongly recommended for the highest-risk group, and weakly recommended for the moderate-risk group.
2. IE on Cardiac Devices
Along with the development of various cardiac implantable electronic devices (CIEDs) and the increasing number of patients with CIED, infection of CIED continues to increase every year.272,273
Since CIED infection can often become serious and fatal,274
early diagnosis and appropriate decision of the treatment strategy are important.
a. Definition
Device-related infection can be classified into local device infection and cardiac device-related IE (CDRIE).4
Local device infection is the infection localized to the pocket of the cardiac device, and local clinical findings of inflammation are present at the device implantation site.275
On the other hand, CDRIE is a condition in which infection reaches the leads or endocardium including the cardiac valve leaflets.
b. Pathophysiology
The main route of CIED infection is the pocket infection during the process of implantation surgery, or percutaneous infection via the part where the device or the electrodes are exposed to skin surface. When inflammation reaches the cardiac chamber from the pocket infection via the intravascular portion of the electrode, IE occurs. The pocket infection or infection in the intravascular portion of the electrode can also occur in a secondary manner as a result of the bacteremia from the distant infected foci. Vegetation induced by IE can occur at any sites in the superior vena cava, tricuspid valve, right atrial wall, and right ventricular wall from the insertion vein. Septic pulmonary embolism is a frequent complication in association with the formation of vegetation.276,277
but it is often asymptomatic.278
The most common causative microorganisms is staphylococci in CDRIE.279,280
Polymicrobial infection is also sometimes identified as causative microorganisms.280–282
c. Diagnosis
The most important thing is to suspect CIED infection first and perform necessary examinations when unexplained fever is observed in the patients with CIED. On the other hand, fever is often blunted in CDRIE, particularly in elderly patients. Thus, attention should also be paid to laboratory tests including the white blood cell count and increases of CRP. It should be noted that CDRIE may develop with respiratory symptoms or chest pain induced by septic pulmonary embolism.
Similarly to other cases of IE, diagnosis of CDRIE is based mainly on blood culture and echocardiography. Echocardiography is useful for evaluation of vegetation adhered to the electrodes and in the cardiac chambers. While TEE is considered to be superior to TTE in diagnosing CDRIE,282–285
better images may be obtained by TTE in some cases. Therefore, it is recommended to combine TTE and TEE for evaluation of vegetation. On the other hand, in some cases, vegetation may not be detected because of artifacts even if these two methods are used, or vegetation may not be evaluated adequately because it is located along the lead course.286–288
Intracardiac echocardiography is effective in such cases. If vegetation cannot be ruled out by these echocardiographic tests, labeled leukocyte scintigraphy289
and 18F-FDG PET/CT are reported to be useful for the diagnosis in CDRIE suspected cases.37,289,290
Positive culture of intravascular electrode may support the diagnosis of CDRIE; however, the possibility of a pseudo-positive culture result cannot be ruled out, due to contamination from the skin or the wound site during the removal procedure of the lead. Using aforementioned imaging modalities enables evaluation of the location of inflammation in the lead, cardiac chamber or septic pulmonary embolism.37,290,291
d. Treatment Strategy
The essential treatment strategy in CDRIE is continuous antibiotic therapy and complete removal of CIED including the leads. Antibiotic therapy should be started after blood culture and before removal of the device, and should be continued for at least 2 weeks, or for 4 to 6 weeks, if necessary, after removal of the device.4
Table 25
shows the indication of CIED removal. Complete removal of CIED including the leads is recommended for all patients diagnosed as CDRIE (class of recommendation I). Medical treatment alone in CDRIE without removal of CIED is associated with high risk of recurrent IE and poor prognosis.292–294
On the other hand, removal of CIED by open heart surgery involves a high risk of perioperative mortality.281
Thus, percutaneous lead extraction with limited risks is recommended in the removal of infected CIED295–297
(Table 26). In cases more than 1 year after device implantation, percutaneous lead extraction by simple manual traction may be difficult because of strong adhesion of the lead to veins or myocardium or adhesion of multiple leads. In such cases, percutaneous extraction using excimer laser may be effective.298–300
Since percutaneous extraction of CIED may induce complications such as postoperative cardiac tamponade, it should be performed at experienced centers where sufficient surgical back-up is available in the cases of emergency295
(Table 26).
Table 25.
Recommendations for Device Removal in CIEDs Infection
| |
Class of
recommendation |
Level of
evidence |
| Complete removal of the device is recommended in patients diagnosed as definite CDRIE |
I |
C |
| Complete removal of the device is recommended in patients diagnosed as device local infection |
I |
C |
Complete removal of the device should be considered in patients in whom CIEDs infection is strongly
suspected and without other apparent sources of infection |
IIa |
C |
Complete removal of the device may be considered in patients in whom IE infection of native valve or
prosthetic valve was diagnosed but CEIDs infection is not definitive |
IIb |
C |
CIEDs, cardiac implantable electronic devices; CDRIE, cardiac device-associated IE.
Table 26.
Recommendations for Method of Device Removal for CIEDs Infection and Level of Evidence
| |
Class of
recommendation |
Level of
evidence |
Percutaneous extraction of the device including the lead is recommended in patients diagnosed as
CDRIE (including the cases with vegetation of 10 mm or larger) |
I |
B |
Percutaneous extraction of the device including the lead is recommended in patients diagnosed as
device local infection |
I |
C |
Percutaneous extraction of the device should be performed at experienced centers where sufficient
surgical back-up is available |
I |
B |
Surgical removal of the device should be considered in patients diagnosed as CDRIE if percutaneous
extraction of the device is expected to be difficult or impossible |
IIa |
C |
Surgical removal of the device should be considered in patients diagnosed as CDRIE if concomitant
surgical repair is required for destruction of valves or cardiac chambers |
IIa |
C |
Surgical removal of the device may be considered in patients diagnosed as CDRIE with large vegetation
of 20 mm or larger one |
IIb |
C |
CIEDs, cardiac implantable electronic devices; CDRIE, cardiac device-associated IE.
Surgical removal of CIED should be considered if percutaneous extraction is expected to be difficult or impossible. It also should be considered if concomitant surgical repair is required for destruction of valves or cardiac chambers. Since the patients having large vegetation are at a high risk of pulmonary embolism at percutaneous extraction,276,301
surgical resection of vegetation may be considered at the same time with surgical removal of CIED. On the other hand, septic pulmonary embolism is clinically asymptomatic in many cases,278
and clinical evidence is limited regarding the specific size of vegetation requiring surgery. Therefore, the present guidelines recommend surgical removal as Class IIb in the cases with a vegetation of 20 mm or larger and recommended percutaneous extraction as Class I in the cases with a vegetation of smaller than 20 mm according to the guidelines of ESC.4
However, surgical risk greatly depends on the individual condition of patients. It is essential to determine the method of device removal considering the procedural risks in each case.
e. Timing of Re-Implantation of Device
Past history of CIED infection is associated with a high risk of recurrent device infection after re-implantation of the device. Therefore, clinical indication of CIED re-implantation should be carefully evaluated after removal of the device275,296
(Table 27). Re-implantation should be performed at a timing when adequate infection control is obtained by antibiotic therapy. The device should be re-implanted on the contralateral side of the site of device infection. Surgical device implantation using the epicardial lead may be useful for preventing recurrence of infection. Leadless pacemaker is one of the options in the cases in which the risk of recurrence of pocket infection is high and VVI mode is indicated.301,302
Temporal pacing can become a risk factor for subsequent infection of re-implanted device303
and should be avoided if possible.
Table 27.
Recommendations for Re-Placement of CIEDs After Device Removal and Level of Evidence
| |
Class of
recommendation |
Level of
evidence |
| Clinical indication of the device implantation should be carefully re-evaluated after removal of the device |
I |
C |
If re-implantation of the device is considered to be necessary, antibiotic therapy should be continued
and device implantation should be conducted after confirming that blood culture is negative and findings
of inflammation have subsided |
IIa |
C |
In patients requiring re-implantation of the pacemaker, the temporal pacing on the contralateral side may
be considered until re-implantation |
IIb |
C |
| Routine temporal pacing is not recommended after removal of the infected device |
III |
C |
CIEDs, cardiac implantable electronic devices.
This section is omitted from the English version.
4. IE in Pregnancy
While IE associated with pregnancy is extremely rare, the mortality rate of pregnant women and fetuses is very high.4
The risk factors for IE in pregnancy are maternal congenital heart disease and rheumatic heart disease. In Western countries, intravenous drug user is listed as a risk factor.304
Guidelines in Japan and abroad recommend antibiotic prophylaxis before delivery for pregnant women at high risk for IE.305,306
See “Guidelines for Indication and Management of Pregnancy and Delivery in Women with Heat Disease”306
of The Japanese Circulation Society for specific prophylactic methods.
5. Non-Bacterial Thrombotic Endocarditis
This section is omitted from the English version.
6. IE in Elderly Patients
IE in elderly patients tends to increase.307
It is slightly more dominant in men, and infection with
Staphylococcus aureus
and healthcare-associated IE are common.307
The intestinal and urinary tract infections are likely to become the portal of entry of causative microorganisms.308,309
Since symptoms are atypical, the thresholds for blood culture and echocardiography could be set low. Vegetation is often small, and concurrent embolism is less frequent.310
The lesion is often difficult to visualize on echocardiography because of calcification of the valve. Since it has been reported that vegetation is small in IE in elderly patients and abscess is likely to occur concomitantly, TEE may be more useful than TTE.308
Contrast CT is often difficult because of poor renal functions, and abdominal ultrasonography and brain MRI may be needed instead.310
The incidence of adverse reactions to antibiotics is high because of renal dysfunction.311
Renal dysfunction and cognitive impairment may occur concomitantly, and surgery is not conducted in many cases in spite of surgical indication. The in-hospital prognosis is comparable in elderly patients and non-elderly patients, but the prognosis after discharge from the hospital is worse in elderly patients because complications are more common.308,309,311
Acknowledgments
We would like to thank the members of the Independent Assessment Committee, Professors Makoto Akaishi (Department of Cardiology, Tokai University Tokyo Hospital), Takashi Akasaka (Department of Cardiovascular Medicine, Wakayama Medical University), Yutaka Otsuji (Second Department of Internal Medicine, University of Occupational and Environmental Health, School of Medicine), Takeshi Kimura (Department of Cardiovascular Medicine, Kyoto University), Junjiro Kobayashi (Department of Cardiac Surgery, National Cerebral and Cardiovascular Center), and Kazuo Tanemoto (Department of Cardiovascular Surgery, Kawasaki Medical School).
Appendix 1 JCS Joint Working Group
Chair:
• Satoshi Nakatani, Division of Health Sciences, Osaka University Graduate School of Medicine
Members:
• Kyomi Ashihara, Department of Cardiology, Tokyo Women’s Medical University
• Masao Daimon, Department of Clinical Laboratory/Cardiology, the University of Tokyo
• Kiyoyuki Eishi, Division of Cardiovascular Surgery, Nagasaki University Graduate School of Biomedical Sciences
• Masahiro Higashi, Department of Radiology, National Hospital Organization, Osaka National Hospital
• Shiro Iwanaga, Department of Cardiology, Saitama Medical University International Medical Center
• Chisato Izumi, Department of Cardiovascular Medicine, National Cerebral and Cardiovascular Center
• Toshimi Kimura, Department of Pharmacy, Tokyo Women’s Medical University Hospital
• Kotaro Mitsutake, Department of Infectious Diseases and Infection Control, Saitama Medical University International Medical Center
• Tomoaki Murakami, Department of Cardiology, Chiba Children’s Hospital
• Kazuhiko Nakano, Division of Oral Infection and Disease Control, Osaka University Graduate School of Dentistry
• Hiroyuki Nakase, Department of Neurosurgery, Nara Medical University
• Takahiro Ohara, Division of Community Medicine, Tohoku Medical and Pharmaceutical University
• Yutaka Okita, Cardio-Aortic Center, Takatsuki General Hospital
• Kazunori Toyoda, Department of Cerebrovascular Medicine, National Cerebral and Cardiovascular Center
• Satoshi Yasukochi, Heart Center, Nagano Children’s Hospital
Collaborators:
• Katsuhito Fujiu, Department of Cardiovascular Medicine, the University of Tokyo
• Takashi Miura, Division of Cardiovascular Surgery, Nagasaki University Graduate School of Biomedical Sciences
• Toshio Morizane, Japan Council for Quality Health Care
• Ichiro Nakagawa, Department of Neurosurgery, Nara Medical University
• Ryota Nomura, Division of Oral Infection and Disease Control, Osaka University Graduate School of Dentistry
• Shuhei Okazaki, Department of Neurology, National Cerebral and Cardiovascular Center
• Haruo Sakamoto, Department of Oral and Maxicillofacial Surgery, Tokai University Hachioji Hospital
• Hiroshi Tanaka, Department of Surgery, Division of Cardiovascular Surgery, Kobe University
Independent Assessment Committee:
• Makoto Akaishi, Department of Cardiology, Tokai University Tokyo Hospital
• Takashi Akasaka, Department of Cardiovascular Medicine, Wakayama Medical University
• Takeshi Kimura, Department of Cardiovascular Medicine, Kyoto University
• Junjiro Kobayashi, Department of Cardiac Surgery, National Cerebral and Cardiovascular Center
• Yutaka Otsuji, Second Department of Internal Medicine, University of Occupational and Environmental Health, School of Medicine
• Kazuo Tanemoto, Department of Cardiovascular Surgery, Kawasaki Medical School
(The affiliations of the members are as of January, 2019)
Appendix 2 Disclosure of Potential Conflicts of Interest (COI): JCS 2017 Guideline on Prevention and Treatment of Infective Endocarditis in Japan
| Author |
Employment or
leading position
(Private company) |
Shareholder |
Royalty
of patent
right |
Remuneration |
Payment
for a
manuscript |
Provision
of research
fund |
Scholarship donation /
Endowed Chair |
Other
rewards |
Declaration
regarding spouse,
relative in the first
degree, or other
persons sharing
income/property |
Team
leader:
Satoshi
Nakatani |
|
|
|
Edwards
Lifesciences |
|
|
|
|
|
Team
member:
Yutaka
Okita |
JMS
Edwards
Lifesciences
TERUMO
CORPORATION
Medtronic Japan
Japan Lifeline |
|
|
St. Jude Medical |
|
|
Century Medical, Inc.
CSL Behring
Senko
Medical Instrument |
|
|
Team
member:
Toshimi
Kimura |
|
|
|
Meiji Seika
Pharma |
|
|
|
|
|
Team
member:
Kazunori
Toyoda |
|
|
|
Daiichi Sankyo
Bayer Yakuhin
Bristol-Myers
Squibb
Boehringer
Ingelheim
Takeda
Pharmaceutical
Company |
|
|
|
|
|
Team
member:
Kotaro
Mitsutake |
|
|
|
MSD |
|
|
|
|
|
Team
member:
Satoshi
Yasukochi |
|
|
|
Actelion
Pharmaceuticals
Japan |
|
|
|
|
|
Cooperator:
Katsuhito
Fujiu |
|
|
|
Daiichi Sankyo
Medtronic Japan |
|
|
Medtronic Japan
Abbott Japan
Boston Scientific
Japan
Japan Lifeline
BIOTRONIK Japan
NTT Docomo |
|
|
Notation of corporation is omitted. Nothing special to be mentioned for team members and cooperators other than those mentioned above.
Team member: Kyomi Ashihara, Absent
Team member: Chisato Izumi, Absent
Team member: Shiro Iwanaga, Absent
Team member: Kiyoyuki Eishi, Absent
Team member: Takahiro Ohara, Absent
Team member: Masao Daimon, Absent
Team member: Hiroyuki Nakase, Absent
Team member: Kazuhiko Nakano, Absent
Team member: Masahiro Higashi, Absent
Team member: Tomoaki Murakami, Absent
Cooperator: Shuhei Okazaki, Absent
Cooperator: Haruo Sakamoto, Absent
Cooperator: Hiroshi Tanaka, Absent
Cooperator: Ichiro Nakagawa, Absent
Cooperator: Ryota Nomura, Absent
Cooperator: Takashi Miura, Absent
Cooperator: Toshio Morizane, Absent
References
- 1.
2016–2017 Working Groups. Guidelines for prevention and treatment of infective endocarditis (JCS 2017): Original version. http://www.j-circ.or.jp/guideline/pdf/JCS2017_nakatani_h.pdf (in Japanese).
- 2.
2016–2017 Working Groups. Guidelines for prevention and treatment of infective endocarditis (JCS 2017): Digest version. http://www.j-circ.or.jp/guideline/pdf/JCS2017_nakatani_d.pdf (in Japanese).
- 3.
Minds Guideline Center, Japan Council for Quality Health Care (2014). Minds Handbook for Clinical Practice Guideline Development 2014. http://minds4.jcqhc.or.jp/minds/guideline/pdf/MindsHB2014.pdf
- 4.
Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 2015; 36: 3075–3128. PMID: 26320109
- 5.
Botelho-Nevers E, Thuny F, Casalta JP, et al. Dramatic reduction in infective endocarditis-related mortality with a management-based approach. Arch Intern Med 2009; 169: 1290–1298. PMID: 19636030
- 6.
Chirillo F, Scotton P, Rocco F, et al. Impact of a multidisciplinary management strategy on the outcome of patients with native valve infective endocarditis. Am J Cardiol 2013; 112: 1171–1176. PMID: 23831163
- 7.
Durack DT, Lukes AS, Bright DK. New criteria for diagnosis of infective endocarditis: Utilization of specific echocardiographic findings. Duke Endocarditis Service. Am J Med 1994; 96: 200–209. PMID: 8154507
- 8.
Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 2000; 30: 633–638. PMID: 10770721
- 9.
Hill EE, Herijgers P, Claus P, et al. Abscess in infective endocarditis: The value of transesophageal echocardiography and outcome: A 5-year study. Am Heart J 2007; 154: 923–928. PMID: 17967599
- 10.
Vieira ML, Grinberg M, Pomerantzeff PM, et al. Repeated echocardiographic examinations of patients with suspected infective endocarditis. Heart 2004; 90: 1020–1024. PMID: 15310690
- 11.
Rasmussen RV, Høst U, Arpi M, et al. Prevalence of infective endocarditis in patients with Staphylococcus aureus bacteraemia: The value of screening with echocardiography. Eur J Echocardiogr 2011; 12: 414–420. PMID: 21685200
- 12.
Chang FY, MacDonald BB, Peacock JE, et al. A prospective multicenter study of Staphylococcus aureus bacteremia: Incidence of endocarditis, risk factors for mortality, and clinical impact of methicillin resistance. Medicine (Baltimore) 2003; 82: 322–332. PMID: 14530781
- 13.
Washington JA. The microbiological diagnosis of infective endocarditis. J Antimicrob Chemother 1987; 20(Suppl): 29–39. PMID: 3316162
- 14.
Pazin GJ, Saul S, Thompson ME. Blood culture positivity: Suppression by outpatient antibiotic therapy in patients with bacterial endocarditis. Arch Intern Med 1982; 142: 263–268. PMID: 7059254
- 15.
Gould FK, Denning DW, Elliott TS, et al. Guidelines for the diagnosis and antibiotic treatment of endocarditis in adults: A report of the Working Party of the British Society for Antimicrobial Chemotherapy. J Antimicrob Chemother 2012; 67: 269–289. PMID: 22086858
- 16.
López J, Sevilla T, Vilacosta I, et al. Prognostic role of persistent positive blood cultures after initiation of antibiotic therapy in left-sided infective endocarditis. Eur Heart J 2013; 34: 1749–1754. PMID: 23144047
- 17.
Miyazaki S, Nakajima N, Tamura H, et al. Infective endocarditis due to Bartonella quintana. J Jpn Soc Int Med 2009; 98: 1112–1113 (in Japanese).
- 18.
Tengan T, Yokoyama J, Nakasu A, et al. Culture-negative endocarditis caused by Bartonella henselae. Jpn J Cardiovasc Surg 2012; 41: 46–48 (in Japanese).
- 19.
Miyazato A, Mitsutake K. Molecular identification of pathogens from excised heart valves of infective endocarditis cases. Med Res Arch 2017; 5. http://journals.ke-i.org/index.php/mra/article/view/1332
- 20.
Miyazato A, Ohkusu K, Tabata M, et al. Comparative molecular and microbiological diagnosis of 19 infective endocarditis cases in which causative microbes were identified by PCR-based DNA sequencing from the excised heart valves. J Infect Chemother 2012; 18: 318–323. PMID: 22045162
- 21.
Habib G, Badano L, Tribouilloy C, et al. European Association of Echocardiography. Recommendations for the practice of echocardiography in infective endocarditis. Eur J Echocardiogr 2010; 11: 202–219. PMID: 20223755
- 22.
Douglas PS, Garcia MJ, Haines DE, et al. ACCF/ASE/AHA/ASNC/HFSA/HRS/SCAI/SCCM/SCCT/SCMR 2011 Appropriate Use Criteria for Echocardiography. A Report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Society of Echocardiography, American Heart Association, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Critical Care Medicine, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance American College of Chest Physicians. J Am Soc Echocardiogr 2011; 24: 229–267. PMID: 21338862
- 23.
Daniel WG, Mügge A, Martin RP, et al. Improvement in the diagnosis of abscesses associated with endocarditis by transesophageal echocardiography. N Engl J Med 1991; 324: 795–800. PMID: 1997851
- 24.
Pedersen WR, Walker M, Olson JD, et al. Value of transesophageal echocardiography as an adjunct to transthoracic echocardiography in evaluation of native and prosthetic valve endocarditis. Chest 1991; 100: 351–356. PMID: 1864104
- 25.
Reynolds HR, Jagen MA, Tunick PA, et al. Sensitivity of transthoracic versus transesophageal echocardiography for the detection of native valve vegetations in the modern era. J Am Soc Echocardiogr 2003; 16: 67–70. PMID: 12514637
- 26.
Daniel WG, Mügge A, Grote J, et al. Comparison of transthoracic and transesophageal echocardiography for detection of abnormalities of prosthetic and bioprosthetic valves in the mitral and aortic positions. Am J Cardiol 1993; 71: 210–215. PMID: 8421985
- 27.
Vilacosta I, Graupner C, San Román JA, et al. Risk of embolization after institution of antibiotic therapy for infective endocarditis. J Am Coll Cardiol 2002; 39: 1489–1495. PMID: 11985912
- 28.
Incani A, Hair C, Purnell P, et al. Staphylococcus aureus bacteraemia: Evaluation of the role of transoesophageal echocardiography in identifying clinically unsuspected endocarditis. Eur J Clin Microbiol Infect Dis 2013; 32: 1003–1008. PMID: 23417650
- 29.
Bruun NE, Habib G, Thuny F, et al. Cardiac imaging in infectious endocarditis. Eur Heart J 2014; 35: 624–632. PMID: 23900698
- 30.
Grob A, Thuny F, Villacampa C, et al. Cardiac multidetector computed tomography in infective endocarditis: A pictorial essay. Insights Imaging 2014; 5: 559–570. PMID: 25225108
- 31.
Habets J, Tanis W, Reitsma JB, et al. Are novel non-invasive imaging techniques needed in patients with suspected prosthetic heart valve endocarditis? A systematic review and meta-analysis. Eur Radiol 2015; 25: 2125–2133. PMID: 25680715
- 32.
Gahide G, Bommart S, Demaria R, et al. Preoperative evaluation in aortic endocarditis: Findings on cardiac CT. AJR Am J Roentgenol 2010; 194: 574–578. PMID: 20173130
- 33.
Feuchtner GM, Stolzmann P, Dichtl W, et al. Multislice computed tomography in infective endocarditis: Comparison with transesophageal echocardiography and intraoperative findings. J Am Coll Cardiol 2009; 53: 436–444. PMID: 19179202
- 34.
Wiseman J, Rouleau J, Rigo P, et al. Gallium-67 myocardial imaging for the detection of bacterial endocarditis. Radiology 1976; 120: 135–138. PMID: 935435
- 35.
Melvin ET, Berger M, Lutzker LG, et al. Noninvasive methods for detection of valve vegetations in infective endocarditis. Am J Cardiol 1981; 47: 271–278. PMID: 7468477
- 36.
Tokmak H, Ergonul O, Demirkol O, et al. Diagnostic contribution of 18F-FDG-PET/CT in fever of unknown origin. Int J Infect Dis 2014; 19: 53–58. PMID: 24295559
- 37.
Pizzi MN, Roque A, Fernández-Hidalgo N, et al. Improving the Diagnosis of Infective Endocarditis in Prosthetic Valves and Intracardiac Devices With 18F-Fluordeoxyglucose Positron Emission Tomography/Computed Tomography Angiography: Initial Results at an Infective Endocarditis Referral Center. Circulation 2015; 132: 1113–1126. PMID: 26276890
- 38.
Erba PA, Conti U, Lazzeri E, et al. Added value of 99 mTc-HMPAO-labeled leukocyte SPECT/CT in the characterization and management of patients with infectious endocarditis. J Nucl Med 2012; 53: 1235–1243. PMID: 22787109
- 39.
Committee of Practice Guidelines for TDM of Antimicrobial Agents, Japanese Society of Chemotherapy, and Sectional Committee of Practice Guidelines for TDM, Antimicrobial Agents, The Japanese Society of Therapeutic Drug Monitoring. Revised guidelines for therapeutic drug monitoring of antimicrobials. Tokyo, 2016 (in Japanese).
- 40.
Fowler VG, Boucher HW, Corey GR, et al. S. aureus Endocarditis and Bacteremia Study Group. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N Engl J Med 2006; 355: 653–665. PMID: 16914701
- 41.
Editorial team members of treatment guidelines of MRSA infections revision 2017, the Japanese Society of Chemotherapy and Japanese Association for Infectious Disease. Practical Guidelines for the Management and Treatment of Infections caused by MRSA, the 3rd Edition. Tokyo, 2017 (in Japanese).
- 42.
Nakatani S, Mitsutake K, Ohara T, et al. CADRE Investigators. Recent picture of infective endocarditis in Japan: Lessons from Cardiac Disease Registration (CADRE-IE). Circ J 2013; 77: 1558–1564. PMID: 23524445
- 43.
Odaka Y, Obata A, Watanabe K, et al. Characteristics of the patients with infective endocarditis in last 10 years in our hospital. Heart 2013; 45: 1234–1238 (in Japanese).
- 44.
Hase R, Otsuka Y, Yoshida K, et al. Profile of infective endocarditis at a tertiary-care hospital in Japan over a 14-year period: Characteristics, outcome and predictors for in-hospital mortality. Int J Infect Dis 2015; 33: 62–66. PMID: 25576825
- 45.
Anderson DJ, Olaison L, McDonald JR, et al. Enterococcal prosthetic valve infective endocarditis: Report of 45 episodes from the International Collaboration on Endocarditis-merged database. Eur J Clin Microbiol Infect Dis 2005; 24: 665–670. PMID: 16244853
- 46.
McDonald JR, Olaison L, Anderson DJ, et al. Enterococcal endocarditis: 107 cases from the international collaboration on endocarditis merged database. Am J Med 2005; 118: 759–766. PMID: 15989910
- 47.
Murdoch DR, Corey GR, Hoen B, et al. International Collaboration on Endocarditis-Prospective Cohort Study (ICE-PCS) Investigators. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st Century: The International Collaboration on Endocarditis-Prospective Cohort Study. Arch Intern Med 2009; 169: 463–473. PMID: 19273776
- 48.
Chu VH, Woods CW, Miro JM, et al. International Collaboration on Endocarditis-Prospective Cohort Study Group. Emergence of coagulase-negative staphylococci as a cause of native valve endocarditis. Clin Infect Dis 2008; 46: 232–242. PMID: 18171255
- 49.
Chu VH, Miro JM, Hoen B, et al. Coagulase-negative staphylococcal prosthetic valve endocarditis–a contemporary update based on the International Collaboration on Endocarditis: Prospective cohort study. Heart 2009; 95: 570–576. PMID: 18952633
- 50.
Carrasco F, Anguita M, Ruiz M, et al. Clinical features and changes in epidemiology of infective endocarditis on pacemaker devices over a 27-year period (1987–2013). Europace 2016; 18: 836–841. PMID: 26705558
- 51.
Le KY, Sohail MR, Friedman PA, et al. Mayo Cardiovascular Infections Study Group. Clinical features and outcomes of cardiovascular implantable electronic device infections due to staphylococcal species. Am J Cardiol 2012; 110: 1143–1149. PMID: 22762715
- 52.
Tunkel AR, Kaye D. Endocarditis with negative blood cultures. N Engl J Med 1992; 326: 1215–1217. PMID: 1557096
- 53.
Sandoe JA, Patel PA, Baig MW, et al. What is the effect of penicillin dosing interval on outcomes in streptococcal infective endocarditis? J Antimicrob Chemother 2013; 68: 2660–2663. PMID: 23766487
- 54.
Knoll B, Tleyjeh IM, Steckelberg JM, et al. Infective endocarditis due to penicillin-resistant viridans group streptococci. Clin Infect Dis 2007; 44: 1585–1592. PMID: 17516402
- 55.
Donabedian H, Freimer EH. Pathogenesis and treatment of endocarditis. Am J Med 1985; 78: 127–133. PMID: 3890533
- 56.
Oguni A, Kawasaki S, Hyogo M, et al. A case of lnfective endocarditis caused by the penicillin-resistant streptococcus milleri group successfully treated by panipenem/betamipron. Matsushita Medical Journal 1997; 35: 89–94 (in Japanese).
- 57.
El Rafei A, DeSimone DC, DeSimone CV, et al. Beta-haemolytic streptococcal endocarditis: Clinical presentation, management and outcomes. Infect Dis (Lond) 2016; 48: 373–378. PMID: 26950685
- 58.
Buchholtz K, Larsen CT, Schaadt B, et al. Once versus twice daily gentamicin dosing for infective endocarditis: A randomized clinical trial. Cardiology 2011; 119: 65–71. PMID: 21846985
- 59.
Dahl A, Rasmussen RV, Bundgaard H, et al. Enterococcus faecalis infective endocarditis: A pilot study of the relationship between duration of gentamicin treatment and outcome. Circulation 2013; 127: 1810–1817. PMID: 23543002
- 60.
Olaison L, Schadewitz K. Swedish Society of Infectious Diseases Quality Assurance Study Group for Endocarditis. Enterococcal endocarditis in Sweden, 1995–1999: Can shorter therapy with aminoglycosides be used? Clin Infect Dis 2002; 34: 159–166. PMID: 11740702
- 61.
Gavaldà J, Len O, Miró JM, et al. Brief communication: Treatment of Enterococcus faecalis endocarditis with ampicillin plus ceftriaxone. Ann Intern Med 2007; 146: 574–579. PMID: 17438316
- 62.
Fernández-Hidalgo N, Almirante B, Gavaldà J, et al. Ampicillin plus ceftriaxone is as effective as ampicillin plus gentamicin for treating enterococcus faecalis infective endocarditis. Clin Infect Dis 2013; 56: 1261–1268. PMID: 23392394
- 63.
Pericas JM, Cervera C, del Rio A, et al. Hospital Clinic Endocarditis Study Group. Changes in the treatment of Enterococcus faecalis infective endocarditis in Spain in the last 15 years: From ampicillin plus gentamicin to ampicillin plus ceftriaxone. Clin Microbiol Infect 2014; 20: O1075–O1083. PMID: 25040215
- 64.
Cerón I, Muñoz P, Marín M, et al. Group for the Management of Infective Endocarditis of the Gregorio Marañón Hospital (GAME). Efficacy of daptomycin in the treatment of enterococcal endocarditis: A 5 year comparison with conventional therapy. J Antimicrob Chemother 2014; 69: 1669–1674. PMID: 24532682
- 65.
Selton-Suty C, Célard M, Le Moing V, et al. AEPEI Study Group. Preeminence of Staphylococcus aureus in infective endocarditis: A 1-year population-based survey. Clin Infect Dis 2012; 54: 1230–1239. PMID: 22492317
- 66.
Keller K, von Bardeleben RS, Ostad MA, et al. Temporal trends in the prevalence of infective endocarditis in Germany between 2005 and 2014. Am J Cardiol 2017; 119: 317–322. PMID: 27816113
- 67.
Chirouze C, Cabell CH, Fowler VG, et al. International Collaboration on Endocarditis Study Group. Prognostic factors in 61 cases of Staphylococcus aureus prosthetic valve infective endocarditis from the International Collaboration on Endocarditis merged database. Clin Infect Dis 2004; 38: 1323–1327. PMID: 15127349
- 68.
Rogers BA, Drake AK, Spelman D. Methicillin resistant Staphylococcus aureus endocarditis in an Australian tertiary hospital: 1991–2006. Heart Lung Circ 2009; 18: 208–213. PMID: 19119075
- 69.
Takayama Y, Okamoto R, Sunakawa K. Definite infective endocarditis: Clinical and microbiological features of 155 episodes in one Japanese university hospital. J Formos Med Assoc 2010; 109: 788–799. PMID: 21126651
- 70.
Gudiol F, Aguado JM, Almirante B, et al. Diagnosis and treatment of bacteremia and endocarditis due to Staphylococcus aureus. A clinical guideline from the Spanish Society of Clinical Microbiology and Infectious Diseases (SEIMC). Enferm Infecc Microbiol Clin 2015; 33: 625.e1–625.e23. PMID: 25937457
- 71.
Cosgrove SE, Vigliani GA, Fowler VG, et al. Initial low-dose gentamicin for Staphylococcus aureus bacteremia and endocarditis is nephrotoxic. Clin Infect Dis 2009; 48: 713–721. PMID: 19207079
- 72.
Japanese Society of Neurology, Japanese Society of Neurological Therapeutics, Japanese Society for Neuroinfectious Diseases. Practical Guideline for Bacterial Meningitis. http://www.neuroinfection.jp/pdf/guideline101.pdf (in Japanese)
- 73.
Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: Diagnosis, antimicrobial therapy, and management of complications: A scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132: 1435–1486. PMID: 26373316
- 74.
Carugati M, Bayer AS, Miró JM, et al. International Collaboration on Endocarditis. High-dose daptomycin therapy for left-sided infective endocarditis: A prospective study from the international collaboration on endocarditis. Antimicrob Agents Chemother 2013; 57: 6213–6222. PMID: 24080644
- 75.
Kullar R, Casapao AM, Davis SL, et al. A multicentre evaluation of the effectiveness and safety of high-dose daptomycin for the treatment of infective endocarditis. J Antimicrob Chemother 2013; 68: 2921–2926. PMID: 23928022
- 76.
Mehta S, Singh C, Plata KB, et al. β-Lactams increase the antibacterial activity of daptomycin against clinical methicillin-resistant Staphylococcus aureus strains and prevent selection of daptomycin-resistant derivatives. Antimicrob Agents Chemother 2012; 56: 6192–6200. PMID: 22985884
- 77.
Dhand A, Bayer AS, Pogliano J, et al. Use of antistaphylococcal beta-lactams to increase daptomycin activity in eradicating persistent bacteremia due to methicillin-resistant Staphylococcus aureus: Role of enhanced daptomycin binding. Clin Infect Dis 2011; 53: 158–163. PMID: 21690622
- 78.
LaPlante KL, Woodmansee S. Activities of daptomycin and vancomycin alone and in combination with rifampin and gentamicin against biofilm-forming methicillin-resistant Staphylococcus aureus isolates in an experimental model of endocarditis. Antimicrob Agents Chemother 2009; 53: 3880–3886. PMID: 19564363
- 79.
Miró JM, Entenza JM, Del Río A, et al. Hospital Clinic Experimental Endocarditis Study Group. High-dose daptomycin plus fosfomycin is safe and effective in treating methicillin-susceptible and methicillin-resistant Staphylococcus aureus endocarditis. Antimicrob Agents Chemother 2012; 56: 4511–4515. PMID: 22644033
- 80.
Claeys KC, Smith JR, Casapao AM, et al. Impact of the combination of daptomycin and trimethoprim-sulfamethoxazole on clinical outcomes in methicillin-resistant Staphylococcus aureus infections. Antimicrob Agents Chemother 2015; 59: 1969–1976. PMID: 25605354
- 81.
Huang JH, Hsu RB. Treatment of infective endocarditis caused by methicillin-resistant Staphylococcus aureus: Teicoplanin versus vancomycin in a retrospective study. Scand J Infect Dis 2008; 40: 462–467. PMID: 18584532
- 82.
To K, Miyake N, Nagasaki Y, et al. Successful combination therapy with vancomycin and arbekacin against infective endocarditis caused by MRSA. Jpn J Antibiot 2011; 64: 389–394. PMID: 22686009
- 83.
Falagas ME, Manta KG, Ntziora F, et al. Linezolid for the treatment of patients with endocarditis: A systematic review of the published evidence. J Antimicrob Chemother 2006; 58: 273–280. PMID: 16735427
- 84.
Muñoz P, Rodríguez-Creixéms M, Moreno M, et al. GAME Study Group. Linezolid therapy for infective endocarditis. Clin Microbiol Infect 2007; 13: 211–215. PMID: 17328738
- 85.
del Río A, Gasch O, Moreno A, et al. FOSIMI Investigators. Efficacy and safety of fosfomycin plus imipenem as rescue therapy for complicated bacteremia and endocarditis due to methicillin-resistant Staphylococcus aureus: A multicenter clinical trial. Clin Infect Dis 2014; 59: 1105–1112. PMID: 25048851
- 86.
Casalta JP, Zaratzian C, Hubert S, et al. Treatment of Staphylococcus aureus endocarditis with high doses of trimethoprim/sulfamethoxazole and clindamycin-Preliminary report. Int J Antimicrob Agents 2013; 42: 190–191. PMID: 23796892
- 87.
Chambers ST, Murdoch D, Morris A, et al. International Collaboration on Endocarditis Prospective Cohort Study Investigators. HACEK infective endocarditis: Characteristics and outcomes from a large, multi-national cohort. PLoS One 2013; 8: e63181. PMID: 23690995
- 88.
Coburn B, Toye B, Rawte P, et al. Antimicrobial susceptibilities of clinical isolates of HACEK organisms. Antimicrob Agents Chemother 2013; 57: 1989–1991. PMID: 23403420
- 89.
Cunha BA, Brahmbhatt K, Raza M. Haemophilus parainfluenzae aortic prosthetic valve endocarditis (PVE) successfully treated with oral levofloxacin. Heart Lung 2015; 44: 317–320. PMID: 25998992
- 90.
Morpeth S, Murdoch D, Cabell CH, et al. International Collaboration on Endocarditis Prospective Cohort Study (ICE-PCS) Investigators. Non-HACEK gram-negative bacillus endocarditis. Ann Intern Med 2007; 147: 829–835. PMID: 18087053
- 91.
Baddley JW, Benjamin DK, Patel M, et al. International Collaboration on Endocarditis-Prospective Cohort Study Group (ICE-PCS). Candida infective endocarditis. Eur J Clin Microbiol Infect Dis 2008; 27: 519–529. PMID: 18283504
- 92.
Cornely OA, Bassetti M, Calandra T, et al. ESCMID Fungal Infection Study Group. ESCMID guideline for the diagnosis and management of Candida diseases 2012: Non-neutropenic adult patients. Clin Microbiol Infect 2012; 18 Suppl 7: 19–37. PMID: 23137135
- 93.
Steinbach WJ, Perfect JR, Cabell CH, et al. A meta-analysis of medical versus surgical therapy for Candida endocarditis. J Infect 2005; 51: 230–247. PMID: 16230221
- 94.
Lye DC, Hughes A, O’Brien D, et al. Candida glabrata prosthetic valve endocarditis treated successfully with fluconazole plus caspofungin without surgery: A case report and literature review. Eur J Clin Microbiol Infect Dis 2005; 24: 753–755. PMID: 16283214
- 95.
Rajendram R, Alp NJ, Mitchell AR, et al. Candida prosthetic valve endocarditis cured by caspofungin therapy without valve replacement. Clin Infect Dis 2005; 40: e72–e74. PMID: 15825018
- 96.
Noguchi M, Takai H, Eishi K, et al. Prosthetic valve endocarditis due to Candida albicans treated successfully with medical treatment alone. Jpn J Thorac Cardiovasc Surg 2004; 52: 318–321. PMID: 15242089
- 97.
Pasha AK, Lee JZ, Low SW, et al. Fungal Endocarditis: Update on Diagnosis and Management. Am J Med 2016; 129: 1037–1043. PMID: 27267285
- 98.
Olaison L, Hogevik H, Alestig K. Fever, C-reactive protein, and other acute-phase reactants during treatment of infective endocarditis. Arch Intern Med 1997; 157: 885–892. PMID: 9129548
- 99.
Lederman MM, Sprague L, Wallis RS, et al. Duration of fever during treatment of infective endocarditis. Medicine (Baltimore) 1992; 71: 52–57. PMID: 1549059
- 100.
Cornelissen CG, Frechen DA, Schreiner K, et al. Inflammatory parameters and prediction of prognosis in infective endocarditis. BMC Infect Dis 2013; 13: 272. PMID: 23767848
- 101.
García-Cabrera E, Fernández-Hidalgo N, Almirante B, et al. Group for the Study of Cardiovascular Infections of the Andalusian Society of Infectious Diseases. Neurological complications of infective endocarditis: Risk factors, outcome, and impact of cardiac surgery: A multicenter observational study. Circulation 2013; 127: 2272–2284. PMID: 23648777
- 102.
Heiro M, Nikoskelainen J, Engblom E, et al. Neurologic manifestations of infective endocarditis: A 17-year experience in a teaching hospital in Finland. Arch Intern Med 2000; 160: 2781–2787. PMID: 11025788
- 103.
Eishi K, Kawazoe K, Kuriyama Y, et al. Surgical management of infective endocarditis associated with cerebral complications. Multi-center retrospective study in Japan. J Thorac Cardiovasc Surg 1995; 110: 1745–1755. PMID: 8523887
- 104.
Snygg-Martin U, Gustafsson L, Rosengren L, et al. Cerebrovascular complications in patients with left-sided infective endocarditis are common: A prospective study using magnetic resonance imaging and neurochemical brain damage markers. Clin Infect Dis 2008; 47: 23–30. PMID: 18491965
- 105.
Cooper HA, Thompson EC, Laureno R, et al. Subclinical brain embolization in left-sided infective endocarditis: Results from the evaluation by MRI of the brains of patients with left-sided intracardiac solid masses (EMBOLISM) pilot study. Circulation 2009; 120: 585–591. PMID: 19652096
- 106.
Duval X, Iung B, Klein I, et al. IMAGE (Resonance Magnetic Imaging at the Acute Phase of Endocarditis) Study Group. Effect of early cerebral magnetic resonance imaging on clinical decisions in infective endocarditis: A prospective study. Ann Intern Med 2010; 152: 497–504, W175. PMID: 20404380
- 107.
Hess A, Klein I, Iung B, et al. Brain MRI findings in neurologically asymptomatic patients with infective endocarditis. AJNR Am J Neuroradiol 2013; 34: 1579–1584. PMID: 23639563
- 108.
Okazaki S, Yoshioka D, Sakaguchi M, et al. Acute ischemic brain lesions in infective endocarditis: Incidence, related factors, and postoperative outcome. Cerebrovasc Dis 2013; 35: 155–162. PMID: 23446361
- 109.
Novy E, Sonneville R, Mazighi M, et al. Neurological complications of infective endocarditis: New breakthroughs in diagnosis and management. Med Mal Infect 2013; 43: 443–450. PMID: 24215865
- 110.
Behrouz R. Preoperative cerebrovascular evaluation in patients with infective endocarditis. Clin Cardiol 2015; 38: 439–442. PMID: 25872491
- 111.
Klein I, Iung B, Labreuche J, et al. IMAGE Study Group. Cerebral microbleeds are frequent in infective endocarditis: A case-control study. Stroke 2009; 40: 3461–3465. PMID: 19762695
- 112.
Hui FK, Bain M, Obuchowski NA, et al. Mycotic aneurysm detection rates with cerebral angiography in patients with infective endocarditis. J Neurointerv Surg 2015; 7: 449–452. PMID: 24778139
- 113.
Monteleone PP, Shrestha NK, Jacob J, et al. Clinical utility of cerebral angiography in the preoperative assessment of endocarditis. Vasc Med 2014; 19: 500–506. PMID: 25362111
- 114.
Ducruet AF, Hickman ZL, Zacharia BE, et al. Intracranial infectious aneurysms: A comprehensive review. Neurosurg Rev 2010; 33: 37–46. PMID: 19838745
- 115.
Subramaniam S, Puetz V, Dzialowski I, et al. Cerebral microhemorrhages in a patient with mycotic aneurysm: Relevance of T2-GRE imaging in SBE. Neurology 2006; 67: 1697. PMID: 17101912
- 116.
Klein I, Iung B, Wolff M, et al. Silent T2* cerebral microbleeds: A potential new imaging clue in infective endocarditis. Neurology 2007; 68: 2043–2043. PMID: 17548558
- 117.
Champey J, Pavese P, Bouvaist H, et al. Cerebral imaging in infectious endocarditis: A clinical study. Infect Dis (Lond) 2016; 48: 235–240. PMID: 26567595
- 118.
Yoshioka D, Sakaguchi T, Yamauchi T, et al. Impact of early surgical treatment on postoperative neurologic outcome for active infective endocarditis complicated by cerebral infarction. Ann Thorac Surg 2012; 94: 489–495; discussion 496. PMID: 22704800
- 119.
Grabowski M, Hryniewiecki T, Janas J, et al. Clinically overt and silentcerebral embolism in the course of infective endocarditis. J Neurol 2011; 258: 1133–1139. PMID: 21222127
- 120.
Kin H, Yoshioka K, Kawazoe K, et al. Management of infectious endocarditis with mycotic aneurysm evaluated by brain magnetic resonance imaging. Eur J Cardiothorac Surg 2013; 44: 924–930. PMID: 23475590
- 121.
Takagi Y, Higuchi Y, Kondo H, et al. The importance of preoperative magnetic resonance imaging in valve surgery for active infective endocarditis. Gen Thorac Cardiovasc Surg 2011; 59: 467–471. PMID: 21751105
- 122.
Champey J, Pavese P, Bouvaist H, et al. Value of brain MRI in infective endocarditis: A narrative literature review. Eur J Clin Microbiol Infect Dis 2016; 35: 159–168. PMID: 26585337
- 123.
Ohira S, Doi K, Kawajiri H, et al. Prediction of early postoperative cerebral hemorrhage in infective endocarditis patients using magnetic resonance imaging. Gen Thorac Cardiovasc Surg 2014; 62: 608–613. PMID: 24913631
- 124.
Okazaki S, Sakaguchi M, Hyun B, et al. Cerebral microbleeds predict impending intracranial hemorrhage in infective endocarditis. Cerebrovasc Dis 2011; 32: 483–488. PMID: 22057098
- 125.
Iung B, Klein I, Mourvillier B, et al. Respective effects of early cerebral and abdominal magnetic resonance imaging on clinical decisions in infective endocarditis. Eur Heart J Cardiovasc Imaging 2012; 13: 703–710. PMID: 22334638
- 126.
Selton-Suty C, Delahaye F, Tattevin P, et al. AEPEI (Association pour l’Etude et la Prévention de l’Endocardite Infectieuse). Symptomatic and asymptomatic neurological complications of infective endocarditis: Impact on surgical management and prognosis. PLoS One 2016; 11: e0158522. PMID: 27400273
- 127.
Chan KL, Dumesnil JG, Cujec B, et al. Investigators of the Multicenter Aspirin Study in Infective Endocarditis. A randomized trial of aspirin on the risk of embolic events in patients with infective endocarditis. J Am Coll Cardiol 2003; 42: 775–780. PMID: 12957419
- 128.
Asaithambi G, Adil MM, Qureshi AI. Thrombolysis for ischemic stroke associated with infective endocarditis: Results from the nationwide inpatient sample. Stroke 2013; 44: 2917–2919. PMID: 23943218
- 129.
Scharf EL, Chakraborty T, Rabinstein A, et al. Endovascular management of cerebral septic embolism: Three recent cases and review of the literature. J Neurointerv Surg 2017; 9: 463–465. PMID: 27899517
- 130.
Mylonakis E, Calderwood SB. Infective endocarditis in adults. N Engl J Med 2001; 345: 1318–1330. PMID: 11794152
- 131.
Gross BA, Puri AS. Endovascular treatment of infectious intracranial aneurysms. Neurosurg Rev 2013; 36: 11–19; discussion 19. PMID: 22892702
- 132.
Kannoth S, Thomas SV. Intracranial microbial aneurysm (infectious aneurysm): Current options for diagnosis and management. Neurocrit Care 2009; 11: 120–129. PMID: 19322683
- 133.
Motomura N, Miyata H, Tsukihara H, et al. Japan Cardiovascular Surgery Database Organization. Risk model of valve surgery in Japan using the Japan Adult Cardiovascular Surgery Database. J Heart Valve Dis 2010; 19: 684–691. PMID: 21214090
- 134.
Miyata H, Motomura N, Tsukihara H, et al. Japan Cardiovascular Surgery Database. Risk models including high-risk cardiovascular procedures: Clinical predictors of mortality and morbidity. Eur J Cardiothorac Surg 2011; 39: 667–674. PMID: 21050770
- 135.
Gaca JG, Sheng S, Daneshmand MA, et al. Outcomes for endocarditis surgery in North America: A simplified risk scoring system. J Thorac Cardiovasc Surg 2011; 141: 98–106.e1–2. PMID: 21168017
- 136.
Nashef SA, Roques F, Sharples LD, et al. EuroSCORE II. Eur J Cardiothorac Surg 2012; 41: 734–744; discussion 744–745. PMID: 22378855
- 137.
Ruttmann E, Willeit J, Ulmer H, et al. Neurological outcome of septic cardioembolic stroke after infective endocarditis. Stroke 2006; 37: 2094–2099. PMID: 16794213
- 138.
Okita Y, Minakata K, Yasuno S, et al. Optimal timing of surgery for active infective endocarditis with cerebral complications: A Japanese multicentre study. Eur J Cardiothorac Surg 2016; 50: 374–382. PMID: 26968761
- 139.
Barsic B, Dickerman S, Krajinovic V, et al. International Collaboration on Endocarditis–Prospective Cohort Study Investigators. Influence of the timing of cardiac surgery on the outcome of patients with infective endocarditis and stroke. Clin Infect Dis 2013; 56: 209–217. PMID: 23074311
- 140.
Hosono M, Sasaki Y, Hirai H, et al. Considerations in timing of surgical intervention for infective endocarditis with cerebrovascular complications. J Heart Valve Dis 2010; 19: 321–325. PMID: 20583394
- 141.
Miura T, Hamawaki M, Hazama S, et al. Outcome of surgical management for active mitral native valve infective endocarditis: A collective review of 57 patients. Gen Thorac Cardiovasc Surg 2014; 62: 488–498. PMID: 24522750
- 142.
Piper C, Wiemer M, Schulte HD, et al. Stroke is not a contraindication for urgent valve replacement in acute infective endocarditis. J Heart Valve Dis 2001; 10: 703–711. PMID: 11767174
- 143.
Hekimian G, Kim M, Passefort S, et al. Preoperative use and safety of coronary angiography for acute aortic valve infective endocarditis. Heart 2010; 96: 696–700. PMID: 20424151
- 144.
Fagman E, Perrotta S, Bech-Hanssen O, et al. ECG-gated computed tomography: A new role for patients with suspected aortic prosthetic valve endocarditis. Eur Radiol 2012; 22: 2407–2414. PMID: 22622348
- 145.
Huang JS, Ho AS, Ahmed A, et al. Borne identity: CT imaging of vascular infections. Emerg Radiol 2011; 18: 335–343. PMID: 21424803
- 146.
Nadji G, Rusinaru D, Rémadi JP, et al. Heart failure in left-sided native valve infective endocarditis: Characteristics, prognosis, and results of surgical treatment. Eur J Heart Fail 2009; 11: 668–675. PMID: 19553397
- 147.
Anguera I, Miro JM, Vilacosta I, et al. Aorto-cavitary Fistula in Endocarditis Working Group. Aorto-cavitary fistulous tract formation in infective endocarditis: Clinical and echocardiographic features of 76 cases and risk factors for mortality. Eur Heart J 2005; 26: 288–297. PMID: 15618052
- 148.
Kiefer T, Park L, Tribouilloy C, et al. Association between valvular surgery and mortality among patients with infective endocarditis complicated by heart failure. JAMA 2011; 306: 2239–2247. PMID: 22110106
- 149.
Thuny F, Beurtheret S, Mancini J, et al. The timing of surgery influences mortality and morbidity in adults with severe complicated infective endocarditis: A propensity analysis. Eur Heart J 2011; 32: 2027–2033. PMID: 19329497
- 150.
Lalani T, Chu VH, Park LP, et al. International Collaboration on Endocarditis–Prospective Cohort Study Investigators. In-hospital and 1-year mortality in patients undergoing early surgery for prosthetic valve endocarditis. JAMA Intern Med 2013; 173: 1495–1504. PMID: 23857547
- 151.
López J, Sevilla T, Vilacosta I, et al. Clinical significance of congestive heart failure in prosthetic valve endocarditis. A multicenter study with 257 patients. Rev Esp Cardiol (Engl Ed) 2013; 66: 384–390. PMID: 24775821
- 152.
Habib G, Tribouilloy C, Thuny F, et al. Prosthetic valve endocarditis: Who needs surgery? A multicentre study of 104 cases. Heart 2005; 91: 954–959. PMID: 5958370
- 153.
Revilla A, López J, Vilacosta I, et al. Clinical and prognostic profile of patients with infective endocarditis who need urgent surgery. Eur Heart J 2007; 28: 65–71. PMID: 17032690
- 154.
Anguera I, Miro JM, San Roman JA, et al. Aorto-Cavitary Fistula in Endocarditis Working Group. Periannular complications in infective endocarditis involving prosthetic aortic valves. Am J Cardiol 2006; 98: 1261–1268. PMID: 17056343
- 155.
Aksoy O, Sexton DJ, Wang A, et al. Early surgery in patients with infective endocarditis: A propensity score analysis. Clin Infect Dis 2007; 44: 364–372. PMID: 17205442
- 156.
Graupner C, Vilacosta I, SanRomán J, et al. Periannular extension of infective endocarditis. J Am Coll Cardiol 2002; 39: 1204–1211. PMID: 11923047
- 157.
Leung DY, Cranney GB, Hopkins AP, et al. Role of transoesophageal echocardiography in the diagnosis and management of aortic root abscess. Br Heart J 1994; 72: 175–181. PMID: 7917692
- 158.
Anguera I, Miro JM, Evangelista A, et al. Aorto-Cavitary Fistula in Endocarditis Working Group. Periannular complications in infective endocarditis involving native aortic valves. Am J Cardiol 2006; 98: 1254–1260. PMID: 17056342
- 159.
Blumberg EA, Karalis DA, Chandrasekaran K, et al. Endocarditis-associated paravalvular abscesses: Do clinical parameters predict the presence of abscess? Chest 1995; 107: 898–903. PMID: 7705150
- 160.
Arnett EN, Roberts WC. Prosthetic valve endocarditis: Clinicopathologic analysis of 22 necropsy patients with comparison observations in 74 necropsy patients with active infective endocarditis involving natural left-sided cardiac valves. Am J Cardiol 1976; 38: 281–292. PMID: 989258
- 161.
Sabik JF, Lytle BW, Blackstone EH, et al. Aortic root replacement with cryopreserved allograft for prosthetic valve endocarditis. Ann Thorac Surg 2002; 74: 650–659; discussion 659. PMID: 12238819
- 162.
Lalani T, Cabell CH, Benjamin DK, et al. International Collaboration on Endocarditis-Prospective Cohort Study (ICE-PCS) Investigators. Analysis of the impact of early surgery on in-hospital mortality of native valve endocarditis: Use of propensity score and instrumental variable methods to adjust for treatment-selection bias. Circulation 2010; 121: 1005–1013. PMID: 20159831
- 163.
Liang F, Song B, Liu R, et al. Optimal timing for early surgery in infective endocarditis: A meta-analysis. Interact Cardiovasc Thorac Surg 2016; 22: 336–345. PMID: 26678152
- 164.
Nadji G, Goissen T, Brahim A, et al. Impact of early surgery on 6-month outcome in acute infective endocarditis. Int J Cardiol 2008; 129: 227–232. PMID: 17999936
- 165.
Ohara T, Nakatani S, Kokubo Y, et al. CADRE investigators. Clinical predictors of in-hospital death and early surgery for infective endocarditis: Results of CArdiac Disease REgistration (CADRE), a nation-wide survey in Japan. Int J Cardiol 2013; 167: 2688–2694. PMID: 22805554
- 166.
Kim DH, Kang DH, Lee MZ, et al. Impact of early surgery on embolic events in patients with infective endocarditis. Circulation 2010; 122: S17–S22. PMID: 20837909
- 167.
Thuny F, Di Salvo G, Disalvo G, et al. Risk of embolism and death in infective endocarditis: Prognostic value of echocardiography: A prospective multicenter study. Circulation 2005; 112: 69–75. PMID: 15983252
- 168.
Sato Y, Satokawa H, Takase S, et al. Efficacy of Early Surgical Strategy for Active Infective Endocarditis. Kyobu Geka 2015; 68: 930–935 (in Japanese). PMID: 26469260
- 169.
Bannay A, Hoen B, Duval X, et al. AEPEI Study Group. The impact of valve surgery on short- and long-term mortality in left-sided infective endocarditis: Do differences in methodological approaches explain previous conflicting results? Eur Heart J 2011; 32: 2003–2015. PMID: 19208650
- 170.
Desch S, Freund A, de Waha S, et al. Outcome in patients with left-sided native-valve infective endocarditis and isolated large vegetations. Clin Cardiol 2014; 37: 626–633. PMID: 25156579
- 171.
Kang DH, Kim YJ, Kim SH, et al. Early surgery versus conventional treatment for infective endocarditis. N Engl J Med 2012; 366: 2466–2473. PMID: 22738096
- 172.
Amin Kashef M, Atreya AR, Hernandez-Montfort J, et al. Not so EASE-Y: How often do hospitalized infective endocarditis patients meet criteria for early surgery? J Heart Valve Dis 2016; 25: 72–74. PMID: 27989088
- 173.
Kang DH, Lee S, Kim YJ, et al. Long-term results of early surgery versus conventional treatment for infective endocarditis trial. Korean Circ J 2016; 46: 846–850. PMID: 27826345
- 174.
Gillinov AM, Shah RV, Curtis WE, et al. Valve replacement in patients with endocarditis and acute neurologic deficit. Ann Thorac Surg 1996; 61: 1125–1129; discussion 1130. PMID: 8607669
- 175.
Thuny F, Avierinos JF, Tribouilloy C, et al. Impact of cerebrovascular complications on mortality and neurologic outcome during infective endocarditis: A prospective multicentre study. Eur Heart J 2007; 28: 1155–1161. PMID: 17363448
- 176.
Ting W, Silverman N, Levitsky S. Valve replacement in patients with endocarditis and cerebral septic emboli. Ann Thorac Surg 1991; 51: 18–21; discussion 22. PMID: 1985568
- 177.
Misfeld M, Girrbach F, Etz CD, et al. Surgery for infective endocarditis complicated by cerebral embolism: A consecutive series of 375 patients. J Thorac Cardiovasc Surg 2014; 147: 1837–1844. PMID: 24837722
- 178.
Sorabella RA, Han SM, Grbic M, et al. Early operation for endocarditis complicated by preoperative cerebral emboli is not associated with worsened outcomes. Ann Thorac Surg 2015; 100: 501–508. PMID: 26116483
- 179.
Shang E, Forrest GN, Chizmar T, et al. Mitral valve infective endocarditis: Benefit of early operation and aggressive use of repair. Ann Thorac Surg 2009; 87: 1728–1733; discussion 1734. PMID: 19463586
- 180.
Wilbring M, Irmscher L, Alexiou K, et al. The impact of preoperative neurological events in patients suffering from native infective valve endocarditis. Interact Cardiovasc Thorac Surg 2014; 18: 740–747. PMID: 24595248
- 181.
Fukuda W, Daitoku K, Minakawa M, et al. Management of infective endocarditis with cerebral complications. Ann Thorac Cardiovasc Surg 2014; 20: 229–236. PMID: 23558229
- 182.
Sohail MR, Martin KR, Wilson WR, et al. Medical versus surgical management of Staphylococcus aureus prosthetic valve endocarditis. Am J Med 2006; 119: 147–154. PMID: 16443417
- 183.
Hill EE, Herregods MC, Vanderschueren S, et al. Management of prosthetic valve infective endocarditis. Am J Cardiol 2008; 101: 1174–1178. PMID: 18394454
- 184.
Castillo JC, Anguita MP, Torres F, et al. Long-term prognosis of early and late prosthetic valve endocarditis. Am J Cardiol 2004; 93: 1185–1187. PMID: 15110221
- 185.
Delahaye F, Célard M, Roth O, et al. Indications and optimal timing for surgery in infective endocarditis. Heart 2004; 90: 618–620. PMID: 15145858
- 186.
Attaran S, Chukwuemeka A, Punjabi PP, et al. Do all patients with prosthetic valve endocarditis need surgery? Interact Cardiovasc Thorac Surg 2012; 15: 1057–1061. PMID: 22922449
- 187.
Truninger K, Attenhofer Jost CH, Seifert B, et al. Long term follow up of prosthetic valve endocarditis: What characteristics identify patients who were treated successfully with antibiotics alone? Heart 1999; 82: 714–720. PMID: 10573500
- 188.
Chirouze C, Alla F, Fowler VG, et al. ICE Prospective Investigators. Impact of early valve surgery on outcome of Staphylococcus aureus prosthetic valve infective endocarditis: Analysis in the International Collaboration of Endocarditis-Prospective Cohort Study. Clin Infect Dis 2015; 60: 741–749. PMID: 25389255
- 189.
Karchmer AW, Bayer AS. Editorial commentary: Surgical therapy for Staphylococcus aureus prosthetic valve endocarditis: Proceed with caution (Caveat Emptor). Clin Infect Dis 2015; 60: 750–752. PMID: 25389250
- 190.
Edwards MB, Ratnatunga CP, Dore CJ, et al. Thirty-day mortality and long-term survival following surgery for prosthetic endocarditis: A study from the UK heart valve registry. Eur J Cardiothorac Surg 1998; 14: 156–164. PMID: 9755001
- 191.
David TE. Aortic valve repair for active infective endocarditis. Eur J Cardiothorac Surg 2012; 42: 127–128. PMID: 22345288
- 192.
Mayer K, Aicher D, Feldner S, et al. Repair versus replacement of the aortic valve in active infective endocarditis. Eur J Cardiothorac Surg 2012; 42: 122–127. PMID: 22430178
- 193.
Okada K, Okita Y. Surgical treatment for aortic periannular abscess/pseudoaneurysm caused by infective endocarditis. Gen Thorac Cardiovasc Surg 2013; 61: 175–181. PMID: 23161575
- 194.
Prat A, Fabre OH, Vincentelli A, et al. Ross operation and mitral homograft for aortic and tricuspid valve endocarditis. Ann Thorac Surg 1998; 65: 1450–1452. PMID: 9594888
- 195.
Schmidtke C, Dahmen G, Sievers HH. Subcoronary Ross procedure in patients with active endocarditis. Ann Thorac Surg 2007; 83: 36–39. PMID: 17184627
- 196.
Guidelines for Diagnosis and Treatment of Cardiovascular Diseases (2007 Joint Working Groups Report). Guidelines for the prevention and treatment of infective endocarditis (JCS 2008). http://www.j-circ.or.jp/guideline/pdf/JCS2008_miyatake_h.pdf (in Japanese)
- 197.
Wilson W, Taubert KA, Gewitz M, et al. American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee. Prevention of infective endocarditis: guidelines from the American Heart Association: A guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation 2007; 116: 1736–1754. PMID: 17446442
- 198.
Danchin N, Duval X, Leport C. Prophylaxis of infective endocarditis: French recommendations 2002. Heart 2005; 91: 715–718. PMID: 15894758
- 199.
National Institute for Health and Clinical Excellence. Prophylaxis against infective endocarditis: Antimicrobial prophylaxis against infective endocarditis in adults and children undergoing interventional procedures. NICE Guidance 2008. https://www.nice.org.uk/guidance/cg64
- 200.
Delahaye F, M’Hammedi A, Guerpillon B, et al. Systematic search for present and potential portals of entry for infective endocarditis. J Am Coll Cardiol 2016; 67: 151–158. PMID: 26791061
- 201.
Baddour LM, Epstein AE, Erickson CC, et al. A summary of the update on cardiovascular implantable electronic device infections and their management: A scientific statement from the American Heart Association. J Am Dent Assoc 2011; 142: 159–165. PMID: 21282681
- 202.
Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC Guideline for the management of patients with valvular heart Disease: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2017; 135: e1159–e1195. PMID: 28298458
- 203.
Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129: e521–e643. PMID: 24589853
- 204.
Sullivan NM, Sutter VL, Mims MM, et al. Clinical aspects of bacteremia after manipulation of the genitourinary tract. J Infect Dis 1973; 127: 49–55. PMID: 4683102
- 205.
Dyson C, Barnes RA, Harrison GA. Infective endocarditis: An epidemiological review of 128 episodes. J Infect 1999; 38: 87–93. PMID: 10342647
- 206.
Selwitz RH, Ismail AI, Pitts NB. Dental caries. Lancet 2007; 369: 51–59. PMID: 17208642
- 207.
Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet 2005; 366: 1809–1820. PMID: 16298220
- 208.
Heimdahl A, Hall G, Hedberg M, et al. Detection and quantitation by lysis-filtration of bacteremia after different oral surgical procedures. J Clin Microbiol 1990; 28: 2205–2209. PMID: 2229342
- 209.
Roberts GJ, Gardner P, Simmons NA. Optimum sampling time for detection of dental bacteraemia in children. Int J Cardiol 1992; 35: 311–315. PMID: 1612793
- 210.
Moreillon P, Que YA. Infective endocarditis. Lancet 2004; 363: 139–149. PMID: 14726169
- 211.
Nakatani S, Mitsutake K, Hozumi T, et al. Committee on Guideline for Prevention and Management of Infective Endocarditis, Japanese Circulation Society. Current characteristics of infective endocarditis in Japan: An analysis of 848 cases in 2000 and 2001. Circ J 2003; 67: 901–905. PMID: 14578594
- 212.
Nakano K, Nomura R, Ooshima T. Streptococcus mutans and cardiovascular diseases. Jpn Dent Sci Rev 2008; 44: 29–37.
- 213.
Sharara SL, Tayyar R, Kanafani ZA, et al. HACEK endocarditis: A review. Expert Rev Anti Infect Ther 2016; 14: 539–545. PMID: 27124204
- 214.
Nomura R, Kokomoto K, Ohara T, et al. Current knowledge among Japanese experienced general dentists regarding prevention of infective endocarditis. Odontology 2018; 106: 297–305. PMID: 29435864
- 215.
Okell CC, Elliott SD. Bacteremia and oral sepsis with special reference to the aetiology of subacute endocarditis. Lancet 1935; 2: 869–872.
- 216.
Lockhart PB, Brennan MT, Sasser HC, et al. Bacteremia associated with toothbrushing and dental extraction. Circulation 2008; 117: 3118–3125. PMID: 18541739
- 217.
Hall G, Heimdahl A, Nord CE. Bacteremia after oral surgery and antibiotic prophylaxis for endocarditis. Clin Infect Dis 1999; 29: 1–8. PMID: 10433559
- 218.
Debelian GJ, Olsen I, Tronstad L. Bacteremia in conjunction with endodontic therapy. Endod Dent Traumatol 1995; 11: 142–149. PMID: 7641631
- 219.
Everett ED, Hirschmann JV. Transient bacteremia and endocarditis prophylaxis: A review. Medicine (Baltimore) 1977; 56: 61–77. PMID: 834137
- 220.
Guntheroth WG. How important are dental procedures as a cause of infective endocarditis? Am J Cardiol 1984; 54: 797–801. PMID: 6486031
- 221.
Malinverni R, Overholser CD, Bille J, et al. Antibiotic prophylaxis of experimental endocarditis after dental extractions. Circulation 1988; 77: 182–187. PMID: 3335066
- 222.
Japanese Association for Infectious Disease/Japanese Society of Chemotherapy; JAID/JSC Committee for Developing Treatment Guide and Guidelines for Clinical Management of Infectious Disease; Odontogenic Infection Working Group. The 2016 JAID/JSC guidelines for clinical management of infectious disease - Odontogenic infections. J Infect Chemother 2018; 24: 320–324. PMID: 29503229
- 223.
McGowan DA, Nair S, MacFarlane TW, et al. Prophylaxis of experimental endocarditis in rabbits using one or two doses of amoxycillin. Br Dent J 1983; 155: 88–90. PMID: 6577902
- 224.
Glenny AM, Oliver R, Roberts GJ, et al. Antibiotics for the prophylaxis of bacterial endocarditis in dentistry. Cochrane Database Syst Rev 2013: CD003813. PMID: 24108511
- 225.
Habib G, Hoen B, Tornos P, et al. Guidelines on the prevention, diagnosis, and treatment of infective endocarditis (new version 2009): The Task Force on the Prevention, Diagnosis, and Treatment of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and the International Society of Chemotherapy (ISC) for Infection and Cancer. Eur Heart J 2009; 30: 2369–2413. PMID: 19713420
- 226.
Strom BL, Abrutyn E, Berlin JA, et al. Dental and cardiac risk factors for infective endocarditis. A population-based, case-control study. Ann Intern Med 1998; 129: 761–769. PMID: 9841581
- 227.
Imperiale TF, Horwitz RI. Does prophylaxis prevent postdental infective endocarditis? A controlled evaluation of protective efficacy. Am J Med 1990; 88: 131–136. PMID: 2301438
- 228.
Van der Meer JT, Van Wijk W, Thompson J, et al. Efficacy of antibiotic prophylaxis for prevention of native-valve endocarditis. Lancet 1992; 339: 135–139. PMID: 1346008
- 229.
Duval X, Delahaye F, Alla F, et al. AEPEI Study Group. Temporal trends in infective endocarditis in the context of prophylaxis guideline modifications: Three successive population-based surveys. J Am Coll Cardiol 2012; 59: 1968–1976. PMID: 22624837
- 230.
Dayer MJ, Jones S, Prendergast B, et al. Incidence of infective endocarditis in England, 2000–13: A secular trend, interrupted time-series analysis. Lancet 2015; 385: 1219–1228. PMID: 25467569
- 231.
Thornhill MH, Dayer MJ, Forde JM, et al. Impact of the NICE guideline recommending cessation of antibiotic prophylaxis for prevention of infective endocarditis: Before and after study. BMJ 2011; 342: d2392. PMID: 21540258
- 232.
Pant S, Patel NJ, Deshmukh A, et al. Trends in infective endocarditis incidence, microbiology, and valve replacement in the United States from 2000 to 2011. J Am Coll Cardiol 2015; 65: 2070–2076. PMID: 25975469
- 233.
Bikdeli B, Wang Y, Kim N, et al. Trends in hospitalization rates and outcomes of endocarditis among Medicare beneficiaries. J Am Coll Cardiol 2013; 62: 2217–2226. PMID: 23994421
- 234.
Cahill TJ, Harrison JL, Jewell P, et al. Antibiotic prophylaxis for infective endocarditis: A systematic review and meta-analysis. Heart 2017; 103: 937–944. PMID: 28213367
- 235.
DeSimone DC, Tleyjeh IM, Correa de Sa DD, et al. Incidence of infective endocarditis due to viridans group streptococci before and after the 2007 American Heart Association’s prevention guidelines: An extended evaluation of the Olmsted County, Minnesota, population and nationwide inpatient sample. Mayo Clin Proc 2015; 90: 874–881. PMID: 26141329
- 236.
Erichsen P, Gislason GH, Bruun NE. The increasing incidence of infective endocarditis in Denmark, 1994–2011. Eur J Intern Med 2016; 35: 95–99. PMID: 27339641
- 237.
Mackie AS, Liu W, Savu A, et al. Infective endocarditis hospitalizations before and after the 2007 American Heart Association Prophylaxis Guidelines. Can J Cardiol 2016; 32: 942–948. PMID: 26868840
- 238.
van den Brink FS, Swaans MJ, Hoogendijk MG, et al. Increased incidence of infective endocarditis after the 2009 European Society of Cardiology guideline update: A nationwide study in the Netherlands. Eur Heart J Qual Care Clin Outcomes 2017; 3: 141–147. PMID: 28927175
- 239.
Dayer MJ, Chambers JB, Prendergast B, et al. NICE guidance on antibiotic prophylaxis to prevent infective endocarditis: A survey of clinicians’ attitudes. QJM 2013; 106: 237–243. PMID: 23286921
- 240.
Durack DT, Kaplan EL, Bisno AL. Apparent failures of endocarditis prophylaxis. Analysis of 52 cases submitted to a national registry. JAMA 1983; 250: 2318–2322. PMID: 6632128
- 241.
Thornhill MH, Dayer MJ, Prendergast B, et al. Incidence and nature of adverse reactions to antibiotics used as endocarditis prophylaxis. J Antimicrob Chemother 2015; 70: 2382–2388. PMID: 25925595
- 242.
Duval X, Alla F, Hoen B, et al. Estimated risk of endocarditis in adults with predisposing cardiac conditions undergoing dental procedures with or without antibiotic prophylaxis. Clin Infect Dis 2006; 42: e102–e107. PMID: 16705565
- 243.
Franklin M, Wailoo A, Dayer MJ, et al. The cost-effectiveness of antibiotic prophylaxis for patients at risk of infective endocarditis. Circulation 2016; 134: 1568–1578. PMID: 27840334
- 244.
Toyoda N, Chikwe J, Itagaki S, et al. Trends in Infective Endocarditis in California and New York State, 1998–2013. JAMA 2017; 317: 1652–1660. PMID: 28444279
- 245.
Lockhart PB, Hanson NB, Ristic H, et al. Acceptance among and impact on dental practitioners and patients of American Heart Association recommendations for antibiotic prophylaxis. J Am Dent Assoc 2013; 144: 1030–1035. PMID: 23989842
- 246.
Fukunaga N, Okada Y, Konishi Y, et al. Pay attention to valvular disease in the presence of atopic dermatitis. Circ J 2013; 77: 1862–1866. PMID: 23535217
- 247.
Knirsch W, Nadal D. Infective endocarditis in congenital heart disease. Eur J Pediatr 2011; 170: 1111–1127. PMID: 21773669
- 248.
Rushani D, Kaufman JS, Ionescu-Ittu R, et al. Infective endocarditis in children with congenital heart disease: Cumulative incidence and predictors. Circulation 2013; 128: 1412–1419. PMID: 24060942
- 249.
Day MD, Gauvreau K, Shulman S, et al. Characteristics of children hospitalized with infective endocarditis. Circulation 2009; 119: 865–870. PMID: 19188508
- 250.
Nakazawa M, Ishiwada T, Ichida F, et al. Management and Prevention of Infective Endocarditis in Congenital Heart Disease and Pediatric Cardiac Disease. Pediatric Cardiology and Cardiac Surgery 2012; 28: 6–39 (in Japanese).
- 251.
Niwa K, Nakazawa M, Tateno S, et al. Infective endocarditis in congenital heart disease: Japanese national collaboration study. Heart 2005; 91: 795–800. PMID: 15894782
- 252.
Verheugt CL, Uiterwaal CS, van der Velde ET, et al. Turning 18 with congenital heart disease: Prediction of infective endocarditis based on a large population. Eur Heart J 2011; 32: 1926–1934. PMID: 21217144
- 253.
Morris CD, Reller MD, Menashe VD. Thirty-year incidence of infective endocarditis after surgery for congenital heart defect. JAMA 1998; 279: 599–603. PMID: 9486754
- 254.
Di Filippo S, Delahaye F, Semiond B, et al. Current patterns of infective endocarditis in congenital heart disease. Heart 2006; 92: 1490–1495. PMID: 16818488
- 255.
Michel PL, Acar J. Native cardiac disease predisposing to infective endocarditis. Eur Heart J 1995; 16 Suppl: 2–6. PMID: 7671919
- 256.
Ware AL, Tani LY, Weng HY, et al. Resource utilization and outcomes of infective endocarditis in children. J Pediatr 2014; 165: 807–812.e1. PMID: 25064162
- 257.
Baltimore RS, Gewitz M, Baddour LM, et al. Infective endocarditis in childhood: 2015 Update: A scientific statement from the American Heart Association. Circulation 2015; 132: 1487–1515. PMID: 26373317
- 258.
Habib G. Infective endocarditis; epidemiology, diagnosis, imaging, therapy, and prevention. Springer International Publishing 2016.
- 259.
Dajani AS, Taubert KA, Wilson W, et al. Prevention of bacterial endocarditis: Recommendations by the American Heart Association. JAMA 1997; 277: 1794–1801. PMID: 9178793
- 260.
Kokomoto K, Nomura R, Ohara T, et al. Current knowledge among pediatric dentistry specialists in Japan regarding prevention of infective endocarditis. Pediatric Dental Journal 2018; 28: 110–117.
- 261.
Dajani AS, Bawdon RE, Berry MC. Oral amoxicillin as prophylaxis for endocarditis: What is the optimal dose? Clin Infect Dis 1994; 18: 157–160. PMID: 8161620
- 262.
Nakazawa M, Seguchi M, Takao A. The occurrence of newborn heart disease in Japan. The Journal of the Japan Pediatric Society 1986; 90: 2578–2587 (in Japanese).
- 263.
Pasquali SK, He X, Mohamad Z, et al. Trends in endocarditis hospitalizations at US children’s hospitals: Impact of the 2007 American Heart Association Antibiotic Prophylaxis Guidelines. Am Heart J 2012; 163: 894–899. PMID: 22607869
- 264.
Grattan MJ, Power A, Fruitman DS, et al. The impact of infective endocarditis prophylaxis recommendations on the practices of pediatric and adult congenital cardiologists. Can J Cardiol 2015; 31: 1497.e23–1497.e28. PMID: 26319967
- 265.
Nakazawa M, Niwa K, Yoshinaga M, et al. The national survey on prevention and treatments for infective endocarditis in children and adult congenital heart disease (the second report). Pediatric Cardiology and Cardiac Surgery 2004; 20: 668–673 (in Japanese).
- 266.
Coulter WA, Coffey A, Saunders ID, et al. Bacteremia in children following dental extraction. J Dent Res 1990; 69: 1691–1695. PMID: 2212215
- 267.
Hess J, Holloway Y, Dankert J. Incidence of postextraction bacteremia under penicillin cover in children with cardiac disease. Pediatrics 1983; 71: 554–558. PMID: 6550789
- 268.
Hess J, Holloway Y, Dankert J. Penicillin prophylaxis in children with cardiac disease: Postextraction bacteremia and penicillin-resistant strains of viridans streptococci. J Infect Dis 1983; 147: 133–136. PMID: 6549771
- 269.
Lockhart PB, Brennan MT, Kent ML, et al. Impact of amoxicillin prophylaxis on the incidence, nature, and duration of bacteremia in children after intubation and dental procedures. Circulation 2004; 109: 2878–2884. PMID: 15173031
- 270.
Lucas V, Roberts GJ. Odontogenic bacteremia following tooth cleaning procedures in children. Pediatr Dent 2000; 22: 96–100. PMID: 10769852
- 271.
Roberts GJ, Holzel HS, Sury MR, et al. Dental bacteremia in children. Pediatr Cardiol 1997; 18: 24–27. PMID: 8960488
- 272.
Ambrosioni J, Hernandez-Meneses M, Téllez A, et al. Hospital Clinic Infective Endocarditis Investigators. The changing epidemiology of infective endocarditis in the twenty-first century. Curr Infect Dis Rep 2017; 19: 21. PMID: 28401448
- 273.
Yuan SM. Right-sided infective endocarditis: Recent epidemiologic changes. Int J Clin Exp Med 2014; 7: 199–218. PMID: 24482708
- 274.
Sridhar AR, Lavu M, Yarlagadda V, et al. Cardiac implantable electronic device-related infection and extraction trends in the U.S. Pacing Clin Electrophysiol 2017; 40: 286–293. PMID: 28084622
- 275.
Sohail MR, Uslan DZ, Khan AH, et al. Management and outcome of permanent pacemaker and implantable cardioverter-defibrillator infections. J Am Coll Cardiol 2007; 49: 1851–1859. PMID: 17481444
- 276.
Meier-Ewert HK, Gray ME, John RM. Endocardial pacemaker or defibrillator leads with infected vegetations: A single-center experience and consequences of transvenous extraction. Am Heart J 2003; 146: 339–344. PMID: 12891205
- 277.
Klug D, Lacroix D, Savoye C, et al. Systemic infection related to endocarditis on pacemaker leads: Clinical presentation and management. Circulation 1997; 95: 2098–2107. PMID: 9133520
- 278.
Ye R, Zhao L, Wang C, et al. Clinical characteristics of septic pulmonary embolism in adults: A systematic review. Respir Med 2014; 108: 1–8. PMID: 24183289
- 279.
Villamil Cajoto I, Rodríguez Framil M, Van den Eynde Collado A, et al. Permanent transvenous pacemaker infections: An analysis of 59 cases. Eur J Intern Med 2007; 18: 484–488. PMID: 17822660
- 280.
Bongiorni MG, Tascini C, Tagliaferri E, et al. Microbiology of cardiac implantable electronic device infections. Europace 2012; 14: 1334–1339. PMID: 22399202
- 281.
del Río A, Anguera I, Miró JM, et al. Hospital Clínic Endocarditis Study Group. Surgical treatment of pacemaker and defibrillator lead endocarditis: The impact of electrode lead extraction on outcome. Chest 2003; 124: 1451–1459. PMID: 14555579
- 282.
Cacoub P, Leprince P, Nataf P, et al. Pacemaker infective endocarditis. Am J Cardiol 1998; 82: 480–484. PMID: 9723637
- 283.
Vilacosta I, Sarriá C, San Román JA, et al. Usefulness of transesophageal echocardiography for diagnosis of infected transvenous permanent pacemakers. Circulation 1994; 89: 2684–2687. PMID: 8205682
- 284.
Victor F, De Place C, Camus C, et al. Pacemaker lead infection: Echocardiographic features, management, and outcome. Heart 1999; 81: 82–87. PMID: 10220550
- 285.
Golzio PG, Fanelli AL, Vinci M, et al. Lead vegetations in patients with local and systemic cardiac device infections: Prevalence, risk factors, and therapeutic effects. Europace 2013; 15: 89–100. PMID: 22968846
- 286.
Narducci ML, Pelargonio G, Russo E, et al. Usefulness of intracardiac echocardiography for the diagnosis of cardiovascular implantable electronic device-related endocarditis. J Am Coll Cardiol 2013; 61: 1398–1405. PMID: 23500279
- 287.
Bongiorni MG, Di Cori A, Soldati E, et al. Intracardiac echocardiography in patients with pacing and defibrillating leads: A feasibility study. Echocardiography 2008; 25: 632–638. PMID: 18652009
- 288.
Dalal A, Asirvatham SJ, Chandrasekaran K, et al. Intracardiac echocardiography in the detection of pacemaker lead endocarditis. J Am Soc Echocardiogr 2002; 15: 1027–1028. PMID: 12221430
- 289.
Erba PA, Sollini M, Conti U, et al. Radiolabeled WBC scintigraphy in the diagnostic workup of patients with suspected device-related infections. JACC Cardiovasc Imaging 2013; 6: 1075–1086. PMID: 24011775
- 290.
Sarrazin JF, Philippon F, Tessier M, et al. Usefulness of fluorine-18 positron emission tomography/computed tomography for identification of cardiovascular implantable electronic device infections. J Am Coll Cardiol 2012; 59: 1616–1625. PMID: 22538331
- 291.
Ahmed FZ, James J, Cunnington C, et al. Early diagnosis of cardiac implantable electronic device generator pocket infection using 18F-FDG-PET/CT. Eur Heart J Cardiovasc Imaging 2015; 16: 521–530. PMID: 25651856
- 292.
Sohail MR, Uslan DZ, Khan AH, et al. Infective endocarditis complicating permanent pacemaker and implantable cardioverter-defibrillator infection. Mayo Clin Proc 2008; 83: 46–53. PMID: 18174000
- 293.
Rundström H, Kennergren C, Andersson R, et al. Pacemaker endocarditis during 18 years in Göteborg. Scand J Infect Dis 2004; 36: 674–679. PMID: 15370655
- 294.
Baddour LM, Bettmann MA, Bolger AF, et al. Nonvalvular cardiovascular device-related infections. Circulation 2003; 108: 2015–2031. PMID: 14568887
- 295.
Wilkoff BL, Love CJ, Byrd CL, et al. Heart Rhythm Society. Transvenous lead extraction: Heart Rhythm Society expert consensus on facilities, training, indications, and patient management: This document was endorsed by the American Heart Association (AHA). Heart Rhythm 2009; 6: 1085–1104. PMID: 19560098
- 296.
Grammes JA, Schulze CM, Al-Bataineh M, et al. Percutaneous pacemaker and implantable cardioverter-defibrillator lead extraction in 100 patients with intracardiac vegetations defined by transesophageal echocardiogram. J Am Coll Cardiol 2010; 55: 886–894. PMID: 20185039
- 297.
Goya M, Nagashima M, Hiroshima K, et al. Lead extractions in patients with cardiac implantable electronic device infections: Single center experience. J Arrhythm 2016; 32: 308–312. PMID: 27588155
- 298.
Okamura H, Yasuda S, Sato S, et al. Initial experience using Excimer laser for the extraction of chronically implanted pacemaker and implantable cardioverter defibrillator leads in Japanese patients. J Cardiol 2013; 62: 195–200. PMID: 23727152
- 299.
Tanawuttiwat T, Gallego D, Carrillo RG. Lead extraction experience with high frequency excimer laser. Pacing Clin Electrophysiol 2014; 37: 1120–1128. PMID: 24766604
- 300.
Pecha S, Linder M, Gosau N, et al. Lead extraction with high frequency laser sheaths: A single-centre experience. Eur J Cardiothorac Surg 2017; 51: 902–905. PMID: 28137751
- 301.
Reynolds D, Duray GZ, Omar R, et al. Micra Transcatheter Pacing Study Group. A leadless intracardiac transcatheter pacing system. N Engl J Med 2016; 374: 533–541. PMID: 26551877
- 302.
Roberts PR, Clementy N, Al Samadi F, et al. A leadless pacemaker in the real-world setting: The Micra Transcatheter Pacing System Post-Approval Registry. Heart Rhythm 2017; 14: 1375–1379. PMID: 28502871
- 303.
Klug D, Balde M, Pavin D, et al. PEOPLE Study Group. Risk factors related to infections of implanted pacemakers and cardioverter-defibrillators: Results of a large prospective study. Circulation 2007; 116: 1349–1355. PMID: 17724263
- 304.
Kebed KY, Bishu K, Al Adham RI, et al. Pregnancy and postpartum infective endocarditis: A systematic review. Mayo Clin Proc 2014; 89: 1143–1152. PMID: 24997091
- 305.
Warnes CA, Williams RG, Bashore TM, et al. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines on the Management of Adults With Congenital Heart Disease). Developed in Collaboration With the American Society of Echocardiography, Heart Rhythm Society, International Society for Adult Congenital Heart Disease, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 2008; 52: e143–e263. PMID: 19038677
- 306.
Guidelines for Diagnosis and Treatment of Cardiovascular Diseases (2009 Joint Working Groups Report). Guidelines for Indication and Management of Pregnancy and Delivery in Women with Heart Disease (JCS 2010). http://www.j-circ.or.jp/guideline/pdf/JCS2010niwa.h.pdf (in Japanese)
- 307.
Cresti A, Chiavarelli M, Scalese M, et al. Epidemiological and mortality trends in infective endocarditis, a 17-year population-based prospective study. Cardiovasc Diagn Ther 2017; 7: 27–35. PMID: 28164010
- 308.
Durante-Mangoni E, Bradley S, Selton-Suty C, et al. International Collaboration on Endocarditis Prospective Cohort Study Group. Current features of infective endocarditis in elderly patients: Results of the International Collaboration on Endocarditis Prospective Cohort Study. Arch Intern Med 2008; 168: 2095–2103. PMID: 18955638
- 309.
Oliver L, Lavoute C, Giorgi R, et al. Infective endocarditis in octogenarians. Heart 2017; 103: 1602–1609. PMID: 28432160
- 310.
Chirillo F. It is not how old you are, it is how you are old: Need for changes in the management of infective endocarditis in the elderly. Heart 2017; 103: 1562–1564. PMID: 28455298
- 311.
Remadi JP, Nadji G, Goissen T, et al. Infective endocarditis in elderly patients: Clinical characteristics and outcome. Eur J Cardiothorac Surg 2009; 35: 123–129. PMID: 19062301