Abstract
Background:
Transcatheter aortic valve implantation (TAVI) is an established treatment for symptomatic patients with severe aortic stenosis (AS). Sometimes patients with severe AS taking immunosuppressants are encountered. The effect of immunosuppressive therapy on clinical outcomes in patients with AS following TAVI were investigated.
Methods and Results:
In total, 282 consecutive patients with severe AS who underwent transfemoral TAVI from January 2016 to December 2018 at St. Marianna University School of Medicine were reviewed. They were divided into 2 groups: the immunosuppressants group (IM group) in which patients continually used immunosuppressive drugs (n=22) and the non-immunosuppressants group (non-IM group) (n=260). The composite endpoints of a major adverse cardiovascular and cerebrovascular event (MACCE) defined as non-lethal myocardial infarction, unstable angina pectoris, heart failure requiring hospitalization, stroke, and cardiovascular death were evaluated. There were no differences in the incidence of vascular access complications (32% vs. 20%, P=0.143) and the rate of procedure success (100% vs. 93%, P=0.377) between the IM and non-IM groups. During the median follow-up period of 567 (16–1,312) days after the TAVI procedure, there were no significant differences between the IM and non-IM groups in the incidence of infectious complications (14% vs. 9%, P=0.442) or MACCE (18% vs. 20%, respectively; P=0.845).
Conclusions:
The use of IM after TAVI is not associated with increased vascular access complications or mid-term MACCE in patients with severe AS treated with TAVI.
Transcatheter aortic valve implantation (TAVI) is rapidly becoming widespread as a minimally invasive alternative to surgery for aortic stenosis (AS). There are several prohibitive risks that are posed by surgery including cerebrovascular, liver, lung diseases, porcelain aorta, severe frailty, and history of previous cardiac surgery. We have treated patients for their AS during their immunosuppressive therapy. The use of immunosuppressants, including steroids, has been reported to cause surgical complications such as postoperative infection.1–3
The past report showed that systematic steroid treatment inhibits the immune system and increases skin fragility, which can cause such complications.4
Although a case report demonstrated the relationship between steroid use and TAVI for the first time,5
there have been few clinical researches regarding immunosuppressant use by patients with AS. Analyses of the data compiled in Japan’s OCEAN-TAVI (Optimized CatheEter vAlvular iNtervention-Transcatheter aortic valve implantation) registry revealed that chronic steroid use was related to an increased risk of vascular complications at the access site. However, the OCEAN-TAVI registry covered only the use of steroids, not non-steroids. In addition, because the entry period for the registry was from 2013 to 2016, the new-generation equipment currently in use was seldom used for the patients in the registry. We therefore investigated the prognostic effect of immunosuppressive therapy including both steroids and non-steroids on the clinical outcomes for patients with severe AS treated with TAVI in the current era.
Methods
Study Population and Study Design
This study included 297 consecutive patients with AS who underwent TAVI at St. Marianna University Hospital from January 2016 to December 2018. Fifteen patients who underwent TAVI via an alternative approach were excluded from the study. Finally, the cases of the included 282 patients with AS who underwent TAVI with the transfemoral approach were retrospectively analyzed (Figure 1).
Immunosuppressive therapy was defined as taking an oral immunosuppressant drug (including steroids) within 24 h before or after the TAVI procedure. This definition does not include topical steroid applications, one-time systemic therapy, and inhaled steroid therapy. In this study, 20 of the 282 patients (7%) used immunosuppressants. We divided them into 2 groups: the immunosuppressants group (IM group, n=20) and the non-immunosuppressants group (non-IM group, n=262).
TAVI Parameters and Procedure
The left ventricular ejection fraction (LVEF), aortic valve pressure gradient (AVPG), and aortic valve area (AVA) were assessed with transthoracic echocardiography (TTE). The LVEF was determined by the biplane modified Simpson’s method. The AVPG was measured by continuous-wave Doppler echocardiography using multiple acoustic windows to determine the highest velocity. The AVA was calculated using the continuity equation. All TTE parameters of the severe AS patients were determined according to the guidelines of the American Society of Echocardiography.6,7
Therefore, the diagnosis of AS was made based on findings obtained by using TTE. Before the TAVI procedure, we generally added on antiplatelet therapy (100 mg of aspirin or 75 mg of clopidogrel) or anticoagulant therapy (warfarin or direct oral anticoagulants) for all patients. Procedural anticoagulation was achieved by using heparinization. The target-activated clotting time is 250–300 s.
We used two TAVI systems: the Medtronic CoreValveTM
or Evolut RTM/PROTM
(Medtronic, Minneapolis, MN, USA) and the Edwards SAPIEN XTTM
or SAPIEN 3TM
(Edwards Lifesciences, Irvine, CA, USA). We performed transfemoral TAVI (TF-TAVI) procedures by surgical cut-down or the direct puncture method. We performed the direct puncture method with a Perclose ProGlideTM
Suture-Mediated Closure System (Abbott Vascular Devices, Redwood City, CA, USA). The selection of the method used depended on the conferred decision made by the heart team at our institution. The TAVI procedure time was defined as the time from the puncture (or surgical skin incision) of the access route to the end of the pressure hemostasis after the removal of the femoral sheaths.
Definitions of Comorbidities
Hypertension was defined as office systolic blood pressure ≥140 mmHg, office diastolic blood pressure ≥90 mmHg, or receiving medical treatment for hypertension before admission.8
Dyslipidemia was defined as total cholesterol ≥220 mg/dL, low-density lipoprotein cholesterol ≥140 mg/dL, high-density lipoprotein cholesterol <40 mg/dL, triglyceride ≥150 mg/dL, or receiving medical treatment for dyslipidemia before admission.9
Diabetes mellitus was defined as a fasting plasma glucose level ≥126 mg/dL, a 2-h plasma glucose level after 75 g glucose loading ≥200 mg/dL, casual plasma glucose level ≥200 mg/dL, hemoglobin A1c ≥6.5% (national glycol-hemoglobin standardization program value), or medical treatment for diabetes before admission.10
Arteriosclerosis obliterans was defined as decreased arterial perfusion in the lower extremities (below the iliac arteries) in a patient whose ankle-brachial index was <0.90.11
Outcomes
The primary outcomes were the major adverse cardiovascular and cerebrovascular events (MACCE) defined as non-lethal myocardial infarction, unstable angina pectoris, heart failure requiring hospitalization, stroke, or cardiovascular death. The secondary outcome was all-cause mortality. Endpoints and procedural complications were evaluated by the Valve Academic Research Consortium-2 (VARC-2) criteria.12
Vascular access complications were defined as the complications of the access route indicated by a medical examination which a TAVI sheath actually passed through (iliofemoral arteries and abdominal aorta).
Statistical Analyses
All statistical analyses were carried out by using SPSS/Windows, ver. 19.0 (SPSS, Chicago, IL, USA). Data are presented as the mean (±SD) or frequencies (percentages). We used the unpaired t-test, Mann-Whitney U-test, and the chi-squared test to examine differences between groups, and a Kaplan-Meier analysis to examine changes over time to the endpoints. The multivariate Cox regression analysis assessed whether treatment with immunosuppressants was independently associated with the study endpoints. A 2-tailed P value <0.05 was considered significant.
Results
The underlying diseases and details of the immunosuppressive drugs used in the IM group are described in
Table 1
and
Table 2. The total number of drugs used to treat rheumatoid arthritis is the highest in the IM group. Prednisolone accounted for 80% of the immunosuppressive drugs used.
Table 1.
Underlying Diseases of Patients in the IM Group
| Diseases |
n |
| Rheumatoid arthritis |
7 |
| Sjögren’s syndrome |
4 |
| Polymyalgia rheumatica |
3 |
| Dermatomyositis |
2 |
| Systemic lupus erythematosus |
2 |
| Mixed connective tissue disease |
2 |
| Autoimmune hepatitis |
2 |
| Others |
10 |
Table 2.
Details of IM Drugs Taken by Patients in the IM Group
| Drugs |
n |
Daily mean dosage (mg) |
| Steroids |
| Prednisolone |
16 |
6.3 |
| Hydrocortisone |
1 |
20 |
| Non-steroids |
| Methotrexate |
2 |
4† |
| Tacrolimus hydrate |
1 |
1 |
| Combined therapy |
| Prednisolone |
1 |
7 |
| Azathioprine |
75 |
| Prednisolone |
1 |
3 |
| Tacrolimus hydrate |
1 |
†Weekly mean dosage. IM, immunosuppressants.
The baseline characteristics of the patients are summarized in
Table 3. The IM group tended to be younger than the non-IM group, but these differences were not significant. Although the prevalence of hypertension and dyslipidemia in the IM group was significantly lower than in the non-IM group, there was no significant difference in Society of Thoracic Surgeons (STS) score between the 2 groups. The prevalence of peripheral artery diseases below iliac arteries and the severity of AS did not differ significantly between the IM and non-IM groups.
Table 3.
Patient Baseline Characteristics
| |
IM group
(n=22) |
Non-IM group
(n=260) |
P value |
| Age (years) |
81±9 |
84±5 |
0.148 |
| Female, n (%) |
16 (73) |
174 (67) |
0.577 |
| Body mass index (kg/m2) |
22.2±4.5 |
22.4±3.9 |
0.844 |
| Body surface area (m2) |
1.44±0.21 |
1.45±0.18 |
0.766 |
| Smoking, n (%) |
1 (5) |
11 (4) |
0.944 |
| NYHA III or IV, n (%) |
6 (27) |
48 (18) |
0.313 |
| Diabetes, n (%) |
6 (27) |
68 (26) |
0.909 |
| Hypertension, n (%) |
11 (50) |
223 (86) |
<0.001 |
| Dyslipidemia, n (%) |
4 (18) |
139 (53) |
0.001 |
| Arteriosclerosis obliterans,† n (%) |
1 (5) |
25 (10) |
0.430 |
| Estimated GFR (mL/min/1.73 m2) |
36.4 (28.7–48.7) |
40.4 (29.6–51.4) |
0.395 |
| STS score |
5.4 (4.1–9.7) |
5.0 (3.5–7.2) |
0.283 |
| LVEF (%) |
71 (64–77) |
65 (59–71) |
0.009 |
| Aortic valve area index (cm2/m2) |
0.40 (0.33–0.45) |
0.40 (0.33–0.49) |
0.494 |
| Aortic valve peak velocity (m/s) |
4.1 (3.9–5.3) |
4.2 (3.6–4.9) |
0.339 |
| Mean aortic valve pressure gradient (mmHg) |
42 (32–60) |
41 (27–55) |
0.452 |
| Annulus area (mm2) |
369 (342–413) |
394 (347–447) |
0.289 |
| Minimum lumen diameter of access (mm) |
6.1±1.3 |
6.1±1.1 |
0.853 |
| Balloon expandable valves (%) |
20 (91) |
216 (83) |
0.340 |
| Procedure time (min) |
68 (59–140) |
86 (60–120) |
0.688 |
| Direct puncture (%) |
16 (73) |
171 (66) |
0.507 |
| Valve type |
| SAPIEN XTTM or SAPIEN 3TM, n |
20 (91) |
220 (85) |
0.340 |
| CoreValveTM or Evolut RTM/PROTM, n |
2 (9) |
40 (15) |
| Sheath size |
|
|
– |
| SAPIEN XTTM or SAPIEN 3TM |
|
|
| 14 F, n |
17 (85) |
193 (88) |
| 16 F, n |
2 (10) |
20 (9) |
| 18 F, n |
1 (5) |
6 (3) |
| 20 F, n |
0 (0) |
1 (0) |
| CoreValveTM or Evolut RTM/PROTM |
|
|
| 14 F, n |
1 (50) |
4 (10) |
| 18 F, n |
1 (50) |
30 (75) |
| 20 F, n |
0 (0) |
6 (15) |
| Valve size |
|
|
– |
| SAPIEN XTTM or SAPIEN 3TM |
|
|
| 20 mm, n |
3 (15) |
21 (10) |
| 23 mm, n |
12 (60) |
124 (56) |
| 26 mm, n |
5 (25) |
66 (30) |
| 29 mm, n |
0 (0) |
9 (4) |
| CoreValveTM or Evolut RTM/PROTM |
|
|
| 23 mm, n |
0 (0) |
4 (10) |
| 26 mm, n |
1 (50) |
20 (50) |
| 29 mm, n |
1 (50) |
16 (40) |
Values are presented as mean±SD, median (Q1–Q3), or n (%). IM, immunosuppressants; NYHA, New York Heart Association; GFR, glomerular filtration rate; STS, Society of Thoracic Surgeons; LVEF, left ventricular ejection fraction. †Arteriosclerosis obliterans showed arterial perfusion decreasing to the lower extremities (below iliac arteries).
Regarding the MDCT parameters, the aortic annulus area (369 [342–413] mm2
vs. 394 [347–447] mm2, P=0.289) and the minimum lumen diameter of the femoral access (6.1±1.3 mm vs. 6.1±1.1 mm, respectively; P=0.678) were not significantly different between the IM and non-IM groups. LVEF was preserved in both groups, but was significantly higher in the IM group than in the non-IM group. The rate of the direct puncture was not significantly different between the IM and non-IM groups (73% vs. 66%, P=0.507). The rate of the use of new-generation devices (SAPIEN 3TM
or Evolut RTM/PROTM) reached 92%.
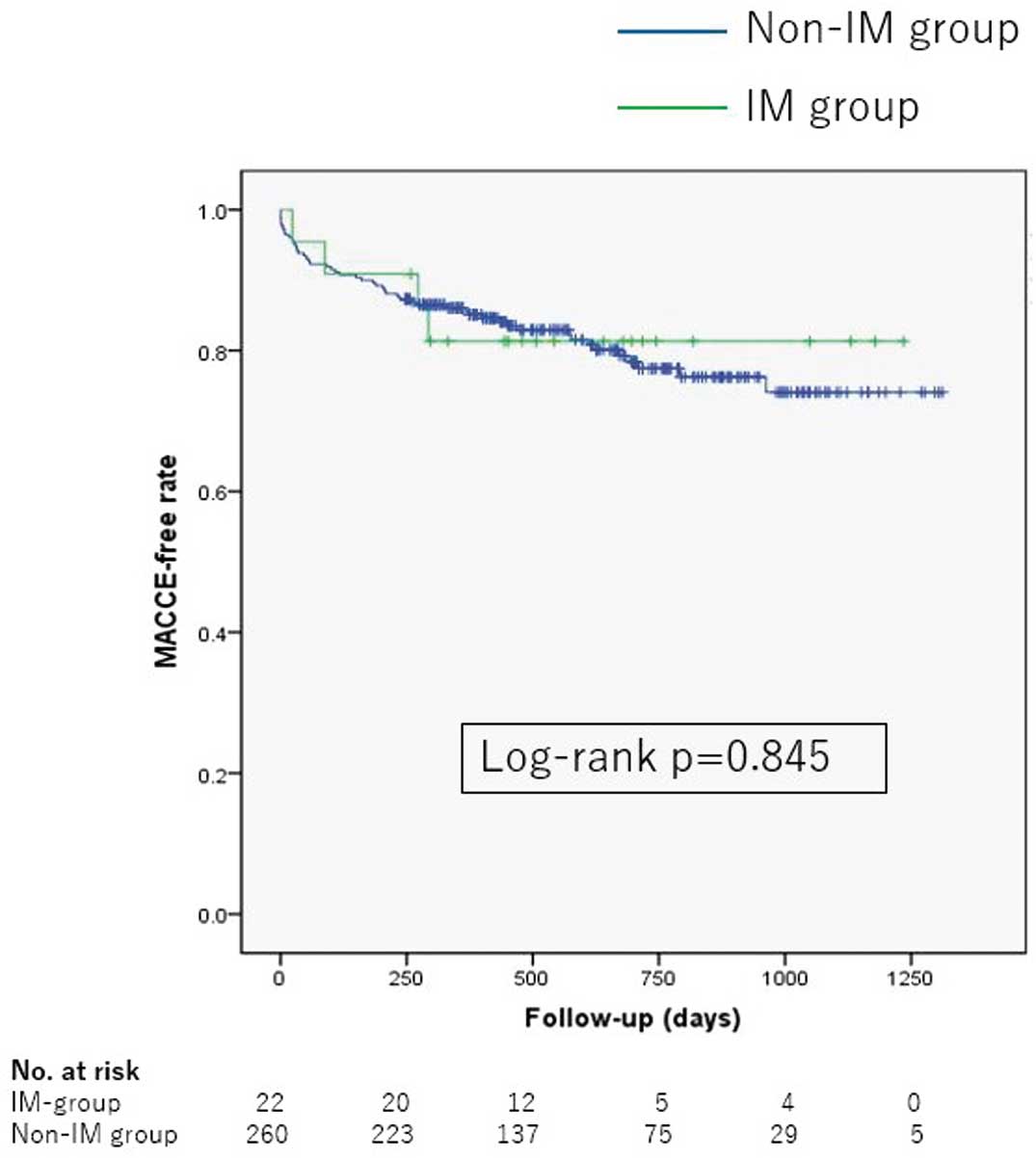
The procedure success rate of TAVI based on the VARC-2 criteria, defined as correct positioning of the prosthetic heart valve with the absence of MACCE during hospitalization in the IM and non-IM groups, was 100% and 93%, respectively, without significant difference (P=0.377). During the median follow-up duration of 567 (16–1,312) days, 57 patients had a vascular access complication, 55 patients had a MACCE, and 26 patients had an infectious complication requiring hospitalization, the details of which are shown as
Table 4. Although the rate of all infectious complications requiring hospitalization tended to be greater in the IM group than the non-IM group, the difference was not significant (14% vs. 9%, P=0.442,
Figure 2). The incidence of vascular access complications (32% vs. 20%, P=0.143,
Figure 3) and that of MACCE (18% vs. 20%, P=0.845, respectively;
Figure 4) did not differ between the IM and non-IM groups. All-cause mortality did not differ between the IM and non-IM groups, either (P=0.879).
Table 4.
Total Numbers of Infectious Complications
| Infectious complications |
n |
| Pneumonia |
13 |
| Puncture or cut-down site infection |
6 |
| Infectious endocarditis |
2 |
| Urinary tract infection |
2 |
| Pancreatitis |
2 |
| Cholangitis |
1 |
| Pyelonephritis |
1 |
| Enteritis |
1 |
| Mesenteric panniculitis |
1 |
| Sepsis (origin unknown) |
1 |
We performed a multivariate Cox regression analysis and included the STS score, the indexed AVA, and the minimum lumen diameter of the access route as covariates affecting the outcomes. The results of the analysis showed that immunosuppressant treatment was not an independent predictor of all infectious complications requiring hospitalization (HR 2.73, 95% CI: 0.92–8.06, P=0.069), vascular access complications (HR 0.80, 95% CI: 0.10–6.20, P=0.834), MACCE (HR 0.72, 95% CI: 0.25–2.08, P=0.547), or all-cause mortality (HR 0.76, 95% CI: 0.21–2.68, P=0.664).
Discussion
The use of immunosuppressants was not associated with increased vascular access complications or mid-term MACCE in patients with AS who were treated with TAVI.
Steroids are the representative immunosuppressant drugs, and they are known to cause delayed wound healing in surgery.3,13
Several studies have shown that chronic steroid use is also related to a higher rate of vascular complications in TF-TAVI patients.5,14,15
However, in the present study, there was no significant difference between the IM and non-IM groups in terms of vascular access complications or mid-term MACCE. This discrepancy in findings warrants further discussion.
First, the rate of the use of surgical cut-down was lower in our present patient series compared to the above-cited studies. For example, vascular complications involving the access route occurred more often with surgical cut-down than with the puncture method under steroid therapy in the Japanese OCEAN registry, which had a 56% direct puncture rate.14
That cohort study reported that surgical cut-down should be avoided for severe AS patients with chronic steroid use if possible. In this study, the patients with severe AS who underwent a surgical cut-down also tended to have a higher incidence of infectious complications compared to those treated with the direct puncture method (13% vs. 6%, P=0.161,
Supplementary Figure). Moreover, the recent evolution of the TAVI device has enabled a reduction of the sheath diameter and the use of the direct puncture method for femoral arteries. A past report mentioned the sheath size of 18F for the CoreValveTM
(Medtronic) prosthesis and 16–20F for the Edward Sapien XTTM
valve.15
In the present study patient population, the Sapien series was used for 84% of the patients and a 14F sheath is now used for 20–26 mm Edward Sapien 3TM
valves. As a result, the relatively low rate of surgical cut-down may have contributed to the low rate of vascular access complications.
Second, we analyzed the cases of patients who used not only steroids but also immunosuppressants including methotrexate, tacrolimus hydrate, azathioprine in this study. Although both steroids and immunosuppressants can cause a compromised situation, their precise advantages and disadvantages remain to be established. A previous study about TAVI showed the data for immunosuppressants except steroids, but it just compared the prognosis between TAVI and surgical aortic valve replacement.16
There have been no previous studies that consider the vascular complications of TF-TAVI patients with comorbid disorders treated by using immunosuppressants other than steroids. This difference may have influenced our findings.
Third, the steroid regimens, dosages, and administration routes were not integrated in the past studies. We excluded inhaled steroids in this study, and the average steroid dose was 6.1 mg/day of prednisolone, which is not very high. It may have affected the results of this study.
Technological innovations for TAVI continue to advance. The latest generation of the balloon-expandable SAPIEN 3 UltraTM
system is said to achieve a lower profile of a sheath.17
Our present findings indicate that the use of immunosuppressants (including steroids) may be relatively safe for patients with AS, as the TAVI procedure is non-invasive.
Study Limitations
This study has some limitations. It was a single-center study, and the sample size was relatively small (n=282); however, the unification of the TAVI procedure and a reliable follow up of the patients could help counteract these limits. In addition, this was a retrospective analysis, and there thus may have been selection bias. Although we minimized the risk of selection bias by using multivariate analysis, there is a possibility of inconclusive results. Finally, we excluded the patients with severe AS who underwent TAVI by using an alternative study approach; however, their prognosis is also important and presents a future topic of discussion.
Conclusions
The use of immunosuppressants was not associated with increased vascular access complications or mid-term MACCE in patients with AS who underwent TAVI.
Acknowledgments
We gratefully acknowledge Ms. J. Ueno, Ms. M. Suzuki, Ms. T. Takeda, and Ms. A. Fujitani for their coordination and data management in this study and editorial assistance.
Disclosures
Y.J.A. is a member of the
Circulation Journal
’ Editorial Team.
IRB Information
This study was granted an exemption from obtaining ethics approval from the St. Marianna University School of Medicine Ethics Committee because this study was a retrospective observational study.
Supplementary Files
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-20-0600
References
- 1.
den Broeder AA, Creemers MC, Fransen J, de Jong E, de Rooij DJ, Wymenga A, et al. Risk factors for surgical site infections and other complications in elective surgery in patients with rheumatoid arthritis with special attention for anti-tumor necrosis factor: A large retrospective study. J Rheumatol 2007; 34: 689–695.
- 2.
Giles JT, Bartlett SJ, Gelber AC, Nanda S, Fontaine K, Ruffing V, et al. Tumor necrosis factor inhibitor therapy and risk of serious postoperative orthopedic infection in rheumatoid arthritis. Arthritis Rheum 2006; 55: 333–337.
- 3.
Baxter JD, Forsham PH. Tissue effects of glucocorticoids. Am J Med 1972; 53: 573–589.
- 4.
Stanbury R, Graham E. Systemic corticosteroid therapy--side effects and their management. Br J Ophthalmol 1998; 82: 704–708.
- 5.
Fudim M, Green KD, Fredi JL, Robbins MA, Zhao D. Peripheral vascular complications during transcatheter aortic valve replacement: Management and potential role of chronic steroid use. Perspect Vasc Surg Endovasc Ther 2012; 24: 206–209.
- 6.
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015; 28: 1–39.
- 7.
Baumgartner H, Hung J, Bermejo J, Chambers JB, Edvardsen T, Goldstein S, et al. Recommendations on the echocardiographic assessment of aortic valve stenosis: A focused update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J Am Soc Echocardiogr 2017; 30: 372–392.
- 8.
Umemura S, Arima H, Arima S, Asayama K, Dohi Y, Hirooka Y, et al. The Japanese Society of hypertension guidelines for the management of hypertension (JSH 2019). Hypertens Res 2019; 42: 1235–1481.
- 9.
Kinoshita M, Yokote K, Arai H, Iida M, Ishigaki Y, Ishibashi S, et al. Japan Atherosclerosis Society (JAS) guidelines for prevention of atherosclerotic cardiovascular diseases 2017. J Atheroscler Thromb 2018; 25: 846–984.
- 10.
Haneda M, Noda M, Origasa H, Noto H, Yabe D, Fujita Y, et al. Japanese clinical practice guideline for diabetes 2016. Diabetol Int 2018; 9: 1–45.
- 11.
Aboyans V, Ricco JB, Bartelink MEL, Björck M, Brodmann M, Cohnert T, et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in Collaboration With the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries. Endorsed by: The European Stroke Organization (ESO), The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur Heart J 2018; 39: 763–816.
- 12.
Kappetein AP, Head SJ, Généreux P, Piazza N, van Mieghem NM, Blackstone EH, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: The Valve Academic Research Consortium-2 consensus document. Eur Heart J 2012; 33: 2403–2418.
- 13.
Diethelm AG. Surgical management of complications of steroid therapy. Ann Surg 1977; 185: 251–263.
- 14.
Koyama Y, Yamamoto M, Kagase A, Tsujimoto S, Kano S, Shimura T, et al. Prognostic impact and periprocedural complications of chronic steroid therapy in patients following transcatheter aortic valve replacement: Propensity-matched analysis from the Japanese OCEAN registry. Catheter Cardiovasc Interv 2020; 95: 793–802.
- 15.
Fink N, Segev A, Barbash I, Bogdan A, Hamdan A, Mazin I, et al. Vascular complications in steroid treated patients undergoing transfemoral aortic valve implantation. Catheter Cardiovasc Interv 2016; 87: 341–346.
- 16.
Higuchi R, Tobaru T, Hagiya K, Saji M, Mahara K, Takamisawa I, et al. Transcatheter aortic valve implantation in patients on corticosteroid therapy. Heart and Vessels 2017; 32: 1236–1243.
- 17.
Parma R, Hudziak D, Smolka G, Gocoł R, Ochała A, Wojakowski W. SAPIEN 3 Ultra: Design and procedural features of a new balloon-expandable valve. Cardiol J 2020; 27: 194–196.