2020 Volume 84 Issue 12 Pages 2175-2184
2020 Volume 84 Issue 12 Pages 2175-2184
Background: Extended dual antiplatelet therapy (DAPT) after drug-eluting stent (DES) implantation is frequently used for high-risk patients in real-world practice. However, there are limited data about the long-term efficacy of extended DAPT after percutaneous coronary intervention (PCI).
Methods and Results: This study investigated 1,470 patients who underwent PCI. The study population was divided into 2 groups based on DAPT duration: guideline-based DAPT (G-DAPT; DAPT ≤12 months after PCI; n=747) and extended DAPT (E-DAPT; DAPT >12 months after PCI; n=723). The primary endpoint was major adverse cardiovascular and cerebrovascular events (MACCEs), defined as cardiac death, myocardial infarction (MI), repeat target vessel revascularization, or stroke. The median follow-up duration was 80.8 months (interquartile range 60.6–97.1 months). The incidence of MACCE was similar in the G-DAPT and E-DAPT groups (21.0% vs. 18.3%, respectively; P=0.111). However, the E-DAPT group had a lower incidence of non-fatal MI (hazard ratio [HR] 0.535; 95% confidence interval [CI] 0.329–0.869; P=0.011), and target lesion revascularization (HR 0.490; 95% CI 0.304–0.792; P=0.004), and stent thrombosis (HR 0.291; 95% CI 0.123–0.688; P=0.005). The incidence of bleeding complications, including major bleeding, was similar between the 2 groups (5.2% vs. 6.3%, respectively; P=0.471).
Conclusions: Although E-DAPT after DES implantation was not associated with a reduced rate of MACCE, it was associated with a significantly lower incidence of non-fatal MI, TLR, and stent thrombosis.
Dual antiplatelet therapy (DAPT) with a combination of aspirin and P2Y12 receptor inhibitors is essential in preventing ischemic complications for patients with coronary artery disease who have been implanted with a drug-eluting stent (DES). The optimal duration of DAPT, balancing the risks of ischemic and bleeding events, has been debated. The current guidelines recommend at least 6 months of DAPT for patients with stable ischemic heart disease (SIHD) and at least 12 months for patients with acute coronary syndrome (ACS) after DES implantation.1,2 However, a previous randomized clinical trial, the DAPT study, showed that the continuation of DAPT beyond 12 months after DES implantation was associated with a reduced rate of stent thrombosis and major adverse cardiovascular and cerebrovascular events (MACCEs).3 Based on these results of that study, extended DAPT is frequently used for high-risk patients in real-world practice. However, there are limited data about the long-term safety and efficacy of extended DAPT after DES implantation in such high-risk patients in real-world practice. In addition, the ability of randomized clinical trials to reflect real-world practice, in which high-risk procedures are usually performed, may be limited. Thus, the aim of the present study was to evaluate the major determinants and long-term outcomes of extended DAPT after DES implantation in real-world practice.
We reviewed the clinical data, retrieved from the medical database of Yeungnam University Medical Center percutaneous coronary intervention (PCI) registry, of 1,707 patients with coronary artery disease who underwent DES implantation between January 2010 and December 2013. After excluding 237 patients (died in hospital, n=104; did not adhere to DAPT medication, n=5; lost to follow-up within 6 months, n=101; and those needing anticoagulation immediately after PCI, n=27), data for 1,470 patients were available for analysis.
The study population was divided into 2 groups based on DAPT duration: (1) guideline-based DAPT (G-DAPT; DAPT duration ≤12 months after PCI; n=747); and (2) extended DAPT (E-DAPT; DAPT duration >12 months after PCI; n=723). The DAPT continuation rate of patients in the G-DAPT and E-DAPT groups 1 year after PCI was 4.8% and 100%, respectively. ACS patients who continued with DAPT for 15 months after PCI because of their follow-up schedule were allocated to the G-DAPT group. At 2 years after PCI, the DAPT continuation rate was 0% and 54.1% in the G-DAPT and E-DAPT, respectively; at 3 years, the DAPT continuation rate was 0% and 38.9%, respectively. Figure 1 outlines the study population selection process and the DAPT continuation rate between the G-DAPT and E-DAPT groups.

Study population. (A) Study population selection process. (B) Dual antiplatelet therapy (DAPT) continuation rate between patient groups receiving guideline-based DAPT (G-DAPT) and extended DAPT (E-DAPT). PS, propensity score.
Data on the baseline medical history, medications, revascularization procedure, and immediate and late outcomes were collected from patients’ electronic medical records.
The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki. The Institutional Review Board (IRB) of Yeungnam University Medical Center approved this study (IRB No. 2019-09-007) and waived the requirement for patient informed consent because of the retrospective nature of the study.
Angioplasty Procedure and Clinical Follow-upPCI was decided based on angiographic findings of ≥70% or ≥50% diameter stenosis at the time of the angiogram with evidence of myocardial ischemia, such as ischemic symptoms or a positive stress test. All study patients were administered at least 100 mg aspirin and a total of 300 mg clopidogrel as a loading dose at least 12 h before PCI. In the case of ACS, some patients were administered a loading dose of 180 mg ticagrelor, followed by 90 mg ticagrelor twice daily according to current guidelines.1,2 Whether to maintain clopidogrel or ticagrelor at the time of hospital discharge was at the discretion of the attending physicians. An intra-arterial bolus of 5,000 IU heparin was injected after placement of the sheath, and additional heparin was administered to maintain an activated clotting time of >250 s. The PCI procedures were performed using current conventional techniques: predilation with a plain balloon, followed by DES implantation and adjuvant dilation with a non-compliant balloon if significant residual stenosis was noted. The type of DES used was left up to the attending physicians.
After successful PCI, cardiovascular medications, including β-blockers, renin-angiotensin-aldosterone antagonists, and lipid-lowering drugs, were prescribed unless contraindicated. DAPT with a combination of aspirin and a P2Y12 receptor inhibitor was prescribed for at least 6 months for patients with SIHD and for at least 12 months for patients with ACS. In particular, patients at high risk of ischemic events were maintained on an extended period of DAPT, which was left to the discretion of attending physicians. The duration of DAPT was decided by physicians on an individual basis depending on a patient’s ischemic and bleeding risks. All patients were followed up clinically 1 month after the procedure and every 3 months thereafter. Follow-up coronary angiography was conducted if clinically indicated.
Study Objectives and DefinitionsThe aims of the present study were to: (1) evaluate the major determinants of E-DAPT after PCI in real-world practice; and (2) investigate the long-term safety and efficacy of E-DAPT, as well as the clinical outcomes of E-DAPT according to lesion complexity. Lesion morphology was classified using the American College of Cardiology (ACC) and American Heart Association (AHA) system, based on a previous study.4 Multivessel disease was defined as the presence of ≥75% luminal diameter stenosis in ≥2 major epicardial arteries. The definition of complex PCI associated with future ischemic events was based on previously published reports.2,5,6 Specifically, complex PCI was defined as a procedure with at least one of the following angiographic characteristics: 3-vessel disease, chronic total occlusion, total stent length ≥60 mm, or bifurcation 2-stenting. In the case of patients with multiple target vessels, the decision regarding complex PCI was based on the most severely affected vessel.
The DAPT and the Predicting Bleeding Complication in Patients Undergoing Stent Implantation and Subsequent Dual Antiplatelet Therapy (PRECISE-DAPT) scores were calculated for each patient using online calculators (DAPT score: http://tools.acc.org/DAPTriskapp/#!/content/calculator/, PRECISE-DAPT score: http://www.precisedaptscore.com/predapt/).7,8
Study EndpointsThe primary endpoint of this study was MACCEs, defined as cardiac death, myocardial infarction (MI), repeat target vessel revascularization (TVR), or stroke, based on the Academic Research Consortium.9 Deaths without an explainable non-cardiac cause were considered cardiac deaths. MI was defined based on the third universal definition of MI.10 TVR was defined as any repeat PCI of the target vessel or bypass surgery of the target vessel performed for restenosis or other complications of the target vessel. All revascularizations were considered clinically indicated if angiography at follow-up showed a percentage diameter stenosis ≥70% or ≥50%, as assessed by quantitative coronary angiographic analysis, with either ischemic symptoms or a positive stress test. Stroke was defined as a sudden focal neurologic deficit of presumed cerebrovascular etiology that persisted beyond 24 h and was not due to another identifiable cause. Brain imaging (computed tomography or magnetic resonance imaging) was recommended for all suspected strokes.
Secondary endpoints were each component of the primary endpoint, TLR, stent thrombosis, non-cardiac death, and bleeding events. TLR was defined as repeat PCI of the lesion within 5 mm of stent deployment or bypass surgery. Stent thrombosis was defined as definite or confirmed stent thrombosis,9 because we excluded patients who died in hospital and were lost to follow-up early in whom stent thrombosis was probable or possible. Major or minor bleeding was determined according to the Thrombolysis in Myocardial Infarction (TIMI) definition.11 All endpoint events were adjudicated by 3 analysts who were blinded to both clinical and angiographic information.
Statistical AnalysisData are expressed as the number (%), mean±SD, or median with the interquartile range (IQR). Continuous variables were compared using Student’s t-test, whereas categorical data were compared using Chi-squared statistics or Fisher’s exact test. Odds ratios (ORs) were calculated using univariate analysis with the binary logistic regression model to analyze determinants for E-DAPT. Variables with P<0.10 in the univariate analysis were entered into the multivariate analysis model to determine independent determinants of E-DAPT.
Event-free survival was analyzed using Kaplan-Meier survival curves, and differences between event-free survival curves were compared using the log-rank test. Adjusted hazard ratios (HR) were calculated using Cox regression analysis, with age, sex, diabetes, chronic kidney disease, old cerebrovascular accidents, previous PCI, ACS at index procedure, left main (LM) disease, ACC/AHA lesion Type C, bifurcation 2-stenting, and complex PCI, all of which achieved P<0.10 in the univariate analysis (Supplementary Table), included as factors. Landmark analysis was performed at 3 years among patients who were event-free at the 3-year time point.
To reduce the effect of differences in baseline characteristics between the 2 patient groups on study endpoints, we adjusted for confounding factors using propensity score (PS) matching. PSs were estimated using multivariate logistic regression analyses. The estimated PSs were used to match patients in the E-DAPT group with those in the G-DAPT group. In this study, the PSs were calculated for each patient using a logistic regression model that included hypertension, LM disease, and complex PCI. The calibration ability and discrimination of the PS model were assessed using the Hosmer-Lemeshow goodness-of-fit test and Harrell’s C-index.12
Statistical analyses were performed using SPSS version 20.0.0 (IBM, Armonk, NY, USA) and R Statistical Software version 3.0.1 (Foundation for Statistical Computing, Vienna, Austria). Two-sided P<0.05 was considered significant.
Baseline characteristics of the study population are summarized in Table 1. The mean age was 64.0±10.9 years, and 71.3% of the patients were men. The mean duration of DAPT was significantly shorter in the G-DAPT than E-DAPT group (355.7±241.9 vs. 1,231.9±866.7 days, respectively; P<0.001). Baseline clinical variables were similar between the 2 groups, but patients in the E-DAPT group were more likely to be current smokers (57.6% vs. 62.7%; P=0.046). Patients in the G-DAPT group were more likely to have a history of hypertension than those in the E-DAPT group, but the difference was not statistically significant (55.4% vs. 50.3%; P=0.051). The risk stratification score for bleeding events (i.e., the DAPT and PRECISE-DAPT scores) was similar between the 2 groups.
| Total population | PS-matched population | |||||
|---|---|---|---|---|---|---|
| G-DAPT (n=747) | E-DAPT (n=723) | P value | G-DAPT (n=652) | E-DAPT (n=652) | P value | |
| Age (years) | 64.1±10.7 | 63.9±11.1 | 0.679 | 64.2±10.7 | 64.1±11.1 | 0.782 |
| Female sex | 216 (28.9) | 206 (28.5) | 0.858 | 179 (27.5) | 190 (29.1) | 0.499 |
| Hypertension | 414 (55.4) | 364 (50.3) | 0.051 | 357 (54.8) | 357 (54.8) | 1.0 |
| Diabetes | 256 (34.3) | 263 (36.4) | 0.398 | 224 (34.4) | 238 (36.5) | 0.418 |
| Dyslipidemia | 526 (70.4) | 522 (72.2) | 0.450 | 451 (69.2) | 468 (71.8) | 0.302 |
| CKD | 14 (1.9) | 11 (1.5) | 0.601 | 14 (2.1) | 10 (1.5) | 0.410 |
| Smoking | 430 (57.6) | 453 (62.7) | 0.046 | 375 (57.5) | 408 (62.6) | 0.062 |
| Previous PCI | 61 (8.2) | 68 (9.4) | 0.401 | 53 (8.1) | 63 (9.7) | 0.331 |
| Previous MI | 37 (5.0) | 38 (5.3) | 0.792 | 33 (5.1) | 32 (4.9) | 0.899 |
| Old CVA | 87 (11.6) | 82 (11.3) | 0.855 | 71 (10.9) | 77 (11.8) | 0.600 |
| Clinical presentation | 0.227 | 0.265 | ||||
| Stable angina | 305 (40.8) | 323 (44.7) | 271 (41.6) | 291 (44.6) | ||
| Unstable angina | 94 (12.6) | 95 (13.1) | 81 (12.4) | 90 (13.8) | ||
| Acute MI | 348 (46.6) | 305 (42.2) | 300 (46.0) | 271 (41.6) | ||
| LVEF (%) | 56.4±25.9 | 55.5±10.7 | 0.425 | 56.1±27.5 | 55.8±10.6 | 0.759 |
| Laboratory findings | ||||||
| Hemoglobin (g/dL) | 12.6±3.9 | 12.6±3.8 | 0.950 | 12.6±3.8 | 12.6±3.8 | 0.950 |
| Creatinine (mg/dL) | 1.1±1.0 | 1.2±1.0 | 0.209 | 1.1±1.0 | 1.2±1.0 | 0.690 |
| TC (mg/dL) | 187.1±44.4 | 188.0±87.0 | 0.802 | 186.8±44.1 | 188.4±90.2 | 0.690 |
| LDL-C (mg/dL) | 110.1±41.6 | 111.5±93.4 | 0.705 | 109.7±41.0 | 111.6±97.4 | 0.657 |
| HDL-C (mg/dL) | 44.8±15.4 | 44.9±12.3 | 0.873 | 44.6±15.9 | 44.7±12.2 | 0.930 |
| TG (mg/dL) | 161.1±115.9 | 166.8±135.9 | 0.402 | 161.5±117.2 | 169.4±137.3 | 0.276 |
| DAPT score | 2.02±1.24 | 2.06±1.28 | 0.521 | 2.01±1.24 | 2.03±1.25 | 0.739 |
| PRECISE-DAPT score | 15.19±10.25 | 16.01±9.61 | 0.115 | 15.53±10.42 | 16.12±9.54 | 0.286 |
Unless indicated otherwise, data are given as the mean±SD or as n (%). CKD, chronic kidney disease; CVA, cerebrovascular accident; DAPT, dual antiplatelet therapy; E-DAPT, extended DAPT; G-DAPT, guideline-based DAPT; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; LVEF, left ventricular ejection fraction; MI, myocardial infarction; PCI, percutaneous coronary intervention; PRECISE-DAPT, Predicting Bleeding Complication in Patients Undergoing Stent Implantation and Subsequent Dual Antiplatelet Therapy; PS, propensity score; TC, cholesterol; TG, triglycerides.
Angiographic characteristics are summarized in Table 2. The incidence of LM, 3-vessel, and multivessel diseases, as well as complex PCI, was higher in the E-DAPT than G-DAPT group. However, findings of pre- or post-procedural quantitative angiography analysis, such as diameter stenosis, lesion length, or acute gain, were similar between the 2 groups.
| Total population | PS-matched population | |||||
|---|---|---|---|---|---|---|
| G-DAPT (n=747) | E-DAPT (n=723) | P value | G-DAPT (n=652) | E-DAPT (n=652) | P value | |
| Target vessel | ||||||
| LM | 29 (3.9) | 54 (7.3) | 0.004 | 29 (4.4) | 29 (4.4) | 1.0 |
| LAD | 415 (55.6) | 426 (58.9) | 0.192 | 365 (56.0) | 385 (59.0) | 0.263 |
| LCX | 217 (29.0) | 223 (30.8) | 0.453 | 190 (29.1) | 196 (30.1) | 0.716 |
| RCA | 281 (37.6) | 257 (35.5) | 0.410 | 249 (38.2) | 228 (35.0) | 0.227 |
| No. vessels involved | 0.005 | 0.435 | ||||
| One | 421 (56.4) | 366 (50.6) | 358 (54.9) | 349 (53.5) | ||
| Two | 251 (33.6) | 246 (34.0) | 225 (34.5) | 219 (33.6) | ||
| Three | 75 (10.0) | 111 (15.4) | 69 (10.6) | 84 (12.9) | ||
| Multivessel disease | 326 (43.6) | 357 (49.4) | 0.027 | 294 (45.1) | 303 (46.5) | 0.617 |
| Stent type | 0.224 | 0.100 | ||||
| 1 st-generation DES | 54 (7.2) | 41 (5.7) | 52 (8.0) | 37 (5.7) | ||
| 2nd-generation DES | 693 (92.8) | 682 (94.3) | 600 (92.0) | 615 (94.3) | ||
| RVD (mm) | 3.01±0.48 | 3.04±0.49 | 0.126 | 3.01±0.48 | 3.04±0.49 | 0.359 |
| Minimal lumen diameter (mm) | 0.22±0.21 | 0.22±0.21 | 0.962 | 0.22±0.21 | 0.22±0.20 | 0.630 |
| Diameter stenosis (%) | 89.2±9.9 | 88.5±10.5 | 0.232 | 89.1±10.0 | 88.6±10.1 | 0.377 |
| Lesion length (mm) | 20.9±11.0 | 21.6±11.3 | 0.411 | 21.0±11.1 | 21.2±11.2 | 0.769 |
| Acute gain (mm) | 2.92±0.46 | 2.93±0.47 | 0.869 | 2.92±0.46 | 2.92±0.47 | 0.896 |
| CTO | 35 (4.7) | 46 (6.4) | 0.159 | 33 (5.1) | 34 (5.2) | 0.900 |
| Bifurcation lesion | ||||||
| Single stent | 149 (19.9) | 173 (23.9) | 0.065 | 137 (21.0) | 149 (22.9) | 0.422 |
| Two stents | 16 (2.1) | 21 (2.9) | 0.351 | 16 (2.5) | 17 (2.6) | 0.860 |
| ACC/AHA lesion description | 0.251 | 0.462 | ||||
| Type A or B | 587 (78.6) | 550 (76.1) | 503 (77.1) | 514 (78.8) | ||
| Type C | 160 (21.4) | 173 (23.9) | 149 (22.9) | 138 (21.2) | ||
| In-stent restenosis | 36 (4.8) | 45 (6.2) | 0.238 | 33 (5.1) | 41 (6.3) | 0.338 |
| Complex PCIA | 206 (27.6) | 244 (33.7) | 0.010 | 192 (29.4) | 192 (29.4) | 1.0 |
| Total no. stents | 1.41±0.74 | 1.46±0.76 | 0.144 | 1.44±0.77 | 1.44±0.74 | 0.958 |
| Stent diameter (mm) | 3.18±0.63 | 3.15±0.44 | 0.419 | 3.18±0.66 | 3.15±0.44 | 0.276 |
| Total stent length (mm) | 31.7±20.2 | 33.5±21.5 | 0.095 | 32.4±20.9 | 32.6±20.4 | 0.850 |
Unless indicated otherwise, data are given as the mean±SD or as n (%). AComplex PCI was defined as a composite of 3-vessel disease, chronic total occlusion (CTO), total stent length ≥60 mm, or bifurcation 2-stenting. ACC, American College of Cardiology; AHA, American Heart Association; DES, drug-eluting stent; LAD, left anterior descending artery; LCX, left circumflex artery; LM, left main; RCA, right coronary artery; RVD, reference vessel diameter. Other abbreviations as in Table 1.
After PS matching, there were no significant differences between the 2 groups with regard to baseline clinical variables or angiographic and procedural findings.
Determinants for E-DAPTThe following clinical and angiographic variables were associated with E-DAPT in the univariate binary logistic regression model (Table 3): hypertension, smoking, LM PCI, bifurcation lesion, and complex PCI. In multivariate analysis, smoking (OR 1.236; 95% CI 1.001–1.527; P=0.049), LM PCI (OR 1.719; 95% CI 1.054–2.805; P=0.030), and complex PCI (OR 1.336; 95% CI 1.065–1.677; P=0.012) were independent determinants for E-DAPT.
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age ≥75years | 1.205 (0.924–1.570) | 0.169 | ||
| Male sex | 1.021 (0.814–1.280) | 0.858 | ||
| Diabetes | 1.097 (0.885–1.358) | 0.398 | ||
| Hypertension | 0.816 (0.664–1.001) | 0.051 | 0.820 (0.652–1.031) | 0.089 |
| CKD | 0.809 (0.365–1.794) | 0.601 | ||
| Old CVA | 0.970 (0.704–1.337) | 0.855 | ||
| Smoking | 1.237 (1.003–1.525) | 0.046 | 1.236 (1.001–1.527) | 0.049 |
| LVEF <30% | 1.389 (0.691–2.795) | 0.357 | ||
| Previous PCI | 1.168 (0.813–1.677) | 0.402 | ||
| Previous MI | 1.065 (0.669–1.694) | 0.792 | ||
| ACS at index procedure | 0.855 (0.695–1.051) | 0.136 | ||
| Left main PCI | 1.959 (1.230–3.117) | 0.005 | 1.719 (1.054–2.805) | 0.030 |
| CTO | 1.382 (0.880–2.172) | 0.160 | ||
| Lesion Type C | 1.154 (0.904–1.474) | 0.251 | ||
| In-stent restenosis | 1.311 (0.835–2.057) | 0.239 | ||
| Bifurcation lesion | 1.262 (0.985–1.617) | 0.065 | 1.128 (0.868–1.465) | 0.367 |
| Bifurcation 2-stent | 1.367 (0.707–2.641) | 0.353 | ||
| Complex PCIA | 1.338 (1.071–1.671) | 0.010 | 1.336 (1.065–1.677) | 0.012 |
| 2nd-generation DES | 1.296 (0.852–1.972) | 0.226 | ||
AComplex PCI was defined as a composite of 3-vessel disease, CTO, total stent length ≥60 mm, or bifurcation 2-stenting. ACS, acute coronary syndrome; CI, confidence interval; HR, hazard ratio. Other abbreviations as in Tables 1,2.
The long-term clinical outcomes according to the duration of DAPT are summarized in Table 4. The median follow-up duration of the study population was 80.8 months (IQR 60.6–97.1 months). There were no significant differences in the rate of MACCE during the follow-up period between the 2 groups. However, the incidence of non-fatal MI was significantly higher in the G-DAPT than E-DAPT group (6.3% vs. 3.6%, respectively; P=0.013). After adjustment for covariates with P<0.10 in the univariate analysis, E-DAPT was associated with a reduced rate of MACCE (HR 0.774; 95% CI 0.612–0.979; P=0.032). Although both groups had a comparable incidence of the primary endpoints, the incidence of TLR and stent thrombosis was significantly lower in the E-DAPT group. The incidence of bleeding complications, including major bleeding, was similar between the 2 groups.
| G-DAPT | E-DAPT | Unadjusted HR (95% CI) |
P value | Adjusted HR (95% CI) |
P value | |
|---|---|---|---|---|---|---|
| Total population (n=1,470) | ||||||
| No. patients | 747 | 723 | ||||
| MACCE | 157 (21.0) | 132 (18.3) | 0.828 (0.657–1.044) | 0.111 | 0.774 (0.612–0.979) | 0.032 |
| Cardiac death | 16 (2.1) | 17 (2.4) | 1.054 (0.532–2.088) | 0.880 | 0.946 (0.487–1.957) | 0.976 |
| Non-fatal MI | 47 (6.3) | 26 (3.6) | 0.543 (0.336–0.877) | 0.013 | 0.535 (0.329–0.869) | 0.011 |
| TVR | 64 (8.6) | 52 (7.2) | 0.810 (0.562–1.169) | 0.261 | 0.757 (0.522–1.096) | 0.140 |
| Stroke | 71 (9.5) | 62 (8.6) | 0.858 (0.610–1.207) | 0.380 | 0.817 (0.577–1.157) | 0.256 |
| TLR | 50 (6.7) | 26 (3.6) | 0.520 (0.323–0.835) | 0.007 | 0.490 (0.304–0.792) | 0.004 |
| Stent thrombosis | 23 (3.1) | 7 (1.0) | 0.314 (0.135–0.734) | 0.007 | 0.291 (0.123–0.688) | 0.005 |
| Non-cardiac death | 23 (3.1) | 17 (2.4) | 0.770 (0.410–1.444) | 0.414 | 0.708 (0.372–1.350) | 0.295 |
| Any bleeding | 84 (11.9) | 102 (15.0) | 1.244 (0.931–1.663) | 0.140 | 1.246 (0.928–1.671) | 0.143 |
| Major bleeding | 37 (5.2) | 43 (6.3) | 1.176 (0.757–1.826) | 0.471 | 1.219 (0.779–1.905) | 0.386 |
| PS-matched population (n=1,304) | ||||||
| No. patients | 652 | 652 | ||||
| MACCE | 135 (20.7) | 117 (17.9) | 0.837 (0.653–1.072) | 0.158 | 0.800 (0.623–1.027) | 0.079 |
| Cardiac death | 13 (2.0) | 15 (2.3) | 1.122 (0.533–2.361) | 0.762 | 1.151 (0.539–2.460) | 0.717 |
| Non-fatal MI | 42 (6.4) | 23 (3.5) | 0.523 (0.315–0.870) | 0.013 | 0.507 (0.303–0.848) | 0.010 |
| TVR | 53 (8.1) | 48 (7.4) | 0.883 (0.597–1.306) | 0.534 | 0.821 (0.554–1.218) | 0.328 |
| Stroke | 61 (9.4) | 56 (8.6) | 0.888 (0.617–1.277) | 0.522 | 0.857 (0.593–1.239) | 0.413 |
| TLR | 39 (6.0) | 23 (3.5) | 0.577 (0.344–0.966) | 0.037 | 0.536 (0.319–0.902) | 0.019 |
| Stent thrombosis | 19 (2.9) | 6 (0.9) | 0.315 (0.126–0.790) | 0.014 | 0.285 (0.111–0.734) | 0.009 |
| Non-cardiac death | 23 (3.5) | 17 (2.6) | 0.736 (0.393–1.380) | 0.339 | 0.702 (0.369–1.336) | 0.281 |
| Any bleeding | 74 (12.0) | 93 (15.1) | 1.240 (0.913–1.684) | 0.168 | 1.253 (0.920–1.705) | 0.152 |
| Major bleeding | 34 (5.5) | 40 (6.5) | 1.153 (0.730–1.822) | 0.542 | 1.172 (0.736–1.864) | 0.504 |
Unless indicated otherwise, data are given as n (%). MACCE, major adverse cardiovascular and cerebrovascular events; TLR, target lesion revascularization; TVR, target vessel revascularization. Other abbreviations as in Tables 1,3.
In the PS-matched population, the rate of MACCE was also similar between the 2 groups (Figure 2A). However, the incidence of nonfatal MI, TLR, and stent thrombosis was significantly lower in the E-DAPT group, even after adjustment for covariates. The incidence of any bleeding or major bleeding during the study period was similar, even after adjustment for covariates, among the PS-matched population.

Kaplan-Meier survival curves according to the duration of dual antiplatelet therapy (DAPT) in the propensity score-matched population. (A) The rate of major adverse cardiovascular and cerebrovascular events (MACCE) was similar between the patient groups receiving guideline-based DAPT (G-DAPT) and extended DAPT (E-DAPT), but (B) landmark analysis showed that the risk of MACCE between 3 and 8 years after percutaneous coronary intervention appeared to be lower in the E-DAPT than G-DAPT group. CI confidence interval; DES, drug-eluting stent; HR, hazard ratio.
Landmark analysis showed that the risk of MACCE between 3 and 8 years seemed to be lower in the E-DAPT than G-DAPT group (HR 0.645; 95% CI 0.449–0.924; P=0.017; Figure 2B). With regard to bleeding events, landmark analysis showed that although any bleeding between 3 and 8 years seemed to be higher in the E-DAPT than G-DAPT group (HR 1.596; 95% CI 1.056–2.413; P=0.026), the rate of major bleeding was similar between 3 and 8 years (Supplementary Figure).
Subgroup AnalysisFigure 3 shows the results of a subgroups analysis of MACCE according to the duration of DAPT in the total study population. E-DAPT was significantly associated with a reduced rate of MACCE in non-diabetic patients, those with LM disease, and those with bifurcation 2-stenting, whereas the benefit of G-DAPT was not evident in the contrasting subgroups. However, there was a significant interaction only in the subgroup with LM disease and bifurcation 2-stenting. That is, patients with LM disease or bifurcation 2-stenting were more likely to benefit from E-DAPT than G-DAPT.
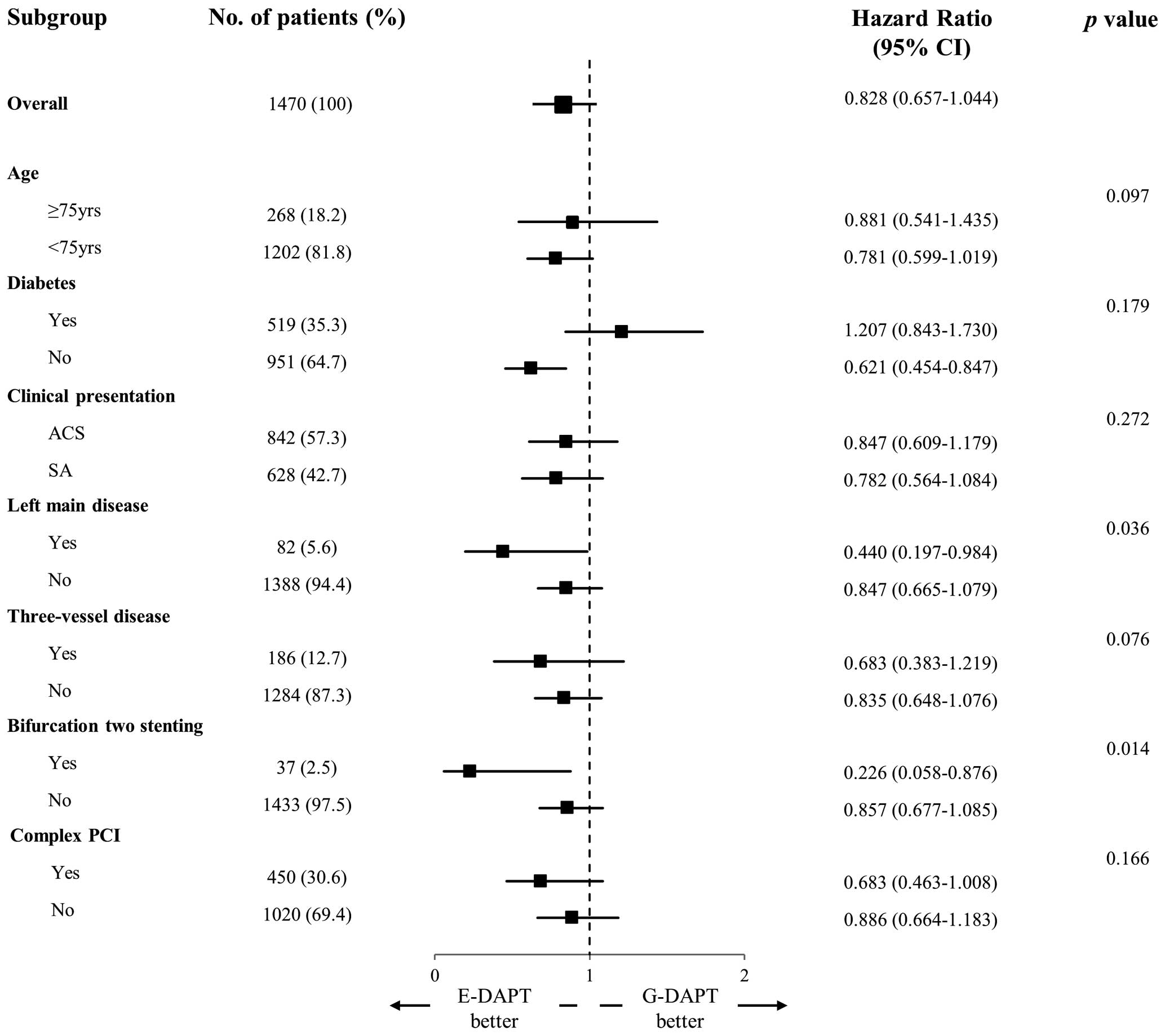
Forest plot of subgroup analyses of major adverse cardiovascular events in the total study population. The squares and horizontal lines indicate hazard ratios (HRs) and 95% confidence intervals (CIs). ACS, acute coronary syndrome; E-DAPT, extended dual antiplatelet therapy; G-DAPT, guideline-based dual antiplatelet therapy; PCI, percutaneous coronary intervention; SA, stable angina.
In this study we evaluated the major determinants for E-DAPT after DES implantation and long-term outcomes of E-DAPT in real-world practice. Even though the guidelines suggest DAPT for up to 12 months after DES implantation, physicians decided to extend DAPT for 49.2% of the study population based on a consideration of patients’ future ischemic events.
The major findings of the present study: (1) patients in the E-DAPT group had a higher prevalence of LM, 3-vessel, and multivessel diseases and complex PCI; (2) major determinants for E-DAPT were smoking, LM PCI, and complex PCI; (3) although the incidence of MACCE was similar between the G-DAPT and E-DAPT groups, the incidence of nonfatal MI, TLR, and stent thrombosis was significantly lower in the E-DAPT group; and (4) LM disease or bifurcation 2-stenting was associated with a greater benefit from E-DAPT than G-DAPT.
The DAPT study, designed to investigate the efficacy of E-DAPT beyond 1 year after DES implantation, showed that E-DAPT up to 30 months reduced the risk of ischemic events, especially stent thrombosis.3 The DAPT study defined MACCE as a composite of death, MI, or stroke, whereas the primary endpoint of MACCE in the present study included TVR. The difference in the rate of MACCE in the DAPT study was driven primarily by MI (HR 0.47; 95% CI 0.37–0.61; P<0.001).3 The present study also demonstrated that E-DAPT was associated with a lower incidence of MI and stent thrombosis among the total and PS-matched populations, consistent with results of previous randomized clinical trials.3 The DAPT study only included selected patients who were event free for 12 months of DAPT after the index procedure.3 Therefore, the results of the DAPT study are applicable only to low-risk patients who tolerated DAPT for 1 year after PCI. In the present, those patients who were considered to be at a low bleeding risk and tolerated DAPT during an early phase after PCI were categorized as E-DAPT. Thus, the results of the present study are only applicable to patients with a low bleeding risk who tolerate DAPT during the early phase after PCI.
The DAPT study included selected patients who were at lower ischemic risk for late adverse events. However, the present study population had more complicated lesion anatomy, such as LM disease (5.6% in the present study vs. 1.1% in the DAPT study3). Thus, E-DAPT may reduce the future risk of MI, stent thrombosis, and TLR for real-world patients with a more complex lesion anatomy. The present study found a similar rate of MACCE between the 2 groups during the first 3 years after DES implantation, but the rate of MACCE between 3 and 8 years was significantly lower in the E-DAPT than G-DAPT group. The differences in ischemic events between the 2 groups were mostly driven by non-fatal MI, TLR, and stent thrombosis. Most of the TLR in the G-DAPT group was related to very late stent thrombosis (46% of the total population; 48.7% in the PS-matched population). It is possible that E-DAPT tends to reduce the rate of ischemic events, especially those related to very late stent thrombosis or new plaque rupture. However, a mortality benefit was not proven by either the DAPT study or the present study. Previous meta-analyses also suggested that an extended duration of DAPT was not associated with cardiovascular or non-cardiovascular mortality.13,14 Thus, the optimal balance between ischemic and bleeding risks should always be considered before extended administration of DAPT.
From the DAPT study, the DAPT score and the PRECISE-DAPT scores were proposed.7,8 Several previous reports assessed the external validation of the DAPT score in the real-world population, but this scoring system could not be generalized to real-world practice.15,16 In the present study, patients’ clinical variables were not associated with E-DAPT, but lesion complexity, such as LM PCI or complex PCI, was a major independent determinant of E-DAPT. Furthermore, the DAPT and PRECISE-DAPT scores were similar between the 2 groups. That is, based on our data, most of the physicians more likely decided on E-DAPT based on patients’ lesion complexity. A practical scoring system that reflects real-world practice needs to be established to guide decision making on the duration of DAPT after DES implantation.
A previous substudy of the duration of DAPT and complex lesions from the DAPT study revealed that the benefits of E-DAPT were similar in patients with and without complex lesions.17 However, the relative difference in ischemic events was numerically higher in patients with more than 2 complex lesions on E-DAPT.17 Other reports from several randomized trials of the duration of DAPT after PCI suggest that the ischemic benefit with long-term DAPT is higher in patients who undergo complex PCI.5,6 The present study also showed that patients with complex lesions, such as LM disease or bifurcation 2-stenting, benefitted more from E-DAPT than G-DAPT. Previous registry data about the duration of DAPT after LM PCI also showed that prolonged DAPT reduced ischemic events, especially in the 2-stenting group.18,19 On the basis of these results, physicians can determine the duration of DAPT after DES implantation considering complex lesion anatomy, such as LM bifurcation.
A previous meta-analysis of 7 randomized trials showed that bleeding events with prolonged DAPT were higher for East Asian than Western populations.20 This finding is based on randomized control trials that do reflect real-world patients. From these data, the annual bleeding risk of prolonged DAPT was approximately 1.0%.20 Recent published data about the efficacy of extremely short-term DAPT after PCI showed that the annual bleeding risk was approximately 1.5% for a 12-month course of DAPT.21 From our real-world PCI data, the annual bleeding risks for patients in the G-DAPT and E-DAPT groups were 1.3% and 1.6%, respectively, which are similar to the previously reported data. The incidence of any bleeding event was numerically higher in the E-DAPT than G-DAPT group (15.0% vs. 11.9%), but the rate of major bleeding was similar between the 2 groups (5.2% vs. 6.3%). In real-world practice, E-DAPT may be a safe treatment option for patients with high ischemic risk. For patients with ACS, current guidelines recommended the use of potent P2Y12 receptor inhibitors, preferably combined with aspirin for at least 12 months. However, East Asian patients with ACS undergoing PCI may be more likely to have bleeding events than Western patients with potent P2Y12 receptor inhibitors.22–24 For this reason, more medical attention is required to maintain an extended duration of potent P2Y12 receptor inhibitor for East Asian patients with ACS who undergo PCI.
Study LimitationsThis study has several limitations. First, the study was based on single-center PCI registry data, and has intrinsic limitations related to its retrospective design. However, the center in this study is one of the high-volume centers in the Korean Society of Interventional Cardiology.25 Given that our data were based on patients’ electronic medical records, it is difficult to acquire data about adverse clinical events in other hospitals.
Because of the significant differences in baseline characteristics and procedural variables between the 2 groups, PS matching was performed. The results after PS matching were similar to those obtained for the unmatched study population. Furthermore, the purpose of this study was to demonstrate the long-term outcomes of E-DAPT after PCI in real-world practice based on retrospective data.
In addition, we could not validate the short-term duration of DAPT after PCI from the data. Some patients with high bleeding risk were maintained on short-term DAPT based on the discretion of the attending physician. However, the mean duration of G-DAPT was 356 days (IQR 290–385 days), which is in accordance with current guidelines. Furthermore, the duration of DAPT could have been affected by ischemic events within the 3-year period after PCI. For this reason, we performed landmark analysis and evaluated long-term clinical efficacy after 3 years. Finally, the study included some patients with first-generation DES, but these patients accounted for a relatively small proportion of the study population (6.5%), and there was no significant difference in the stent generations between the 2 groups.
In conclusion, the long-term incidence of MACCE was not significantly different between the G-DAPT and E-DAPT groups in real-world practice. However, E-DAPT could be associated with a lower incidence of non-fatal MI, TLR, and stent thrombosis without an increase in bleeding events. Particularly for patients with LM disease and bifurcation 2-stenting, E-DAPT could be effective in reducing MACCE.
The authors thank Jaekyung Bae and Ji-Hyung Yoon for help with data collection.
This study did not receive any specific grants from any funding agency in the public, commercial, or not-for-profit sectors.
The authors have no conflicts of interest to disclose.
This study was approved by the Institutional Review Board of Yeungnam University Medical Center (Reference no. 2019-09-007).
The deidentified participant data will not be shared.
H.-S.S., M.-G.S., S.-I.K.: Data curation, Formal analysis, Original draft preparation. J.-I.P.: Data Curation, Formal analysis. J.-H.L.: Conceptualization, Data Curation, Investigation, Methodology, Software, Writing, Reviewing and Editing. U.K.: Investigation, Reviewing and Editing. J.-S.P.: Investigation, Reviewing and Editing.
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-20-0668