2020 Volume 84 Issue 3 Pages 427-435
2020 Volume 84 Issue 3 Pages 427-435
Background: There is little evidence regarding the effect of outpatient cardiac rehabilitation (CR) on exercise capacity or the long-term prognosis in patients after coronary artery bypass graft surgery (CABG). This study aimed to determine whether participation in outpatient CR improves exercise capacity and long-term prognosis in post-CABG Japanese patients in a multicenter cohort.
Methods and Results: We enrolled 346 post-CABG patients who underwent cardiopulmonary exercise testing during early (2–3 weeks) and late (3–6 months) time points after surgery. They formed the Active (n=240) and Non-Active (n=106) CR participation groups and were followed for 3.5 years. Primary endpoint was a major adverse cardiac event (MACE): all-cause death or rehospitalization for acute myocardial infarction/unstable angina/worsening heart failure. Peak oxygen uptake at 3–5 months from baseline was significantly more increased in Active than in Non-Active patients (+26±24% vs. +19±20%, respectively; P<0.05), and the MACE rate was significantly lower in Active than Non-Active patients (3.4% vs. 10.5%, respectively; P=0.02). Multivariate Cox proportional hazard analysis showed that participation in outpatient CR was a significant prognostic determinant of MACE (P=0.03).
Conclusions: This unique study showed that a multicenter cohort of patients who underwent CABG and actively participated in outpatient CR exhibited greater improvement in exercise capacity and better survival without cardiovascular events than their counterparts who did not participate.
Cardiac rehabilitation (CR) improves exercise capacity, quality of life (QOL), and long-term prognosis in patients with coronary artery disease (CAD).1,2 and therefore participation in outpatient CR is recommended by the guidelines for secondary prevention of CAD.3 Beneficial effects of CR have also been documented in patients after coronary artery bypass graft surgery (CABG) with regard to exercise capacity, coronary risk factors, and QOL.4 To date, however, only 3 studies have investigated the long-term prognosis in a cohort of post-CABG patients, and all 3 were retrospective and observational.5–7 Of them, Kutner et al investigated post-CABG patients on chronic haemodialysis.5 The remaining studies6,7 were conducted in single centers and assessed total mortality only. More importantly, they did not correct the baseline exercise capacity between the CR and non-CR groups. Because baseline exercise capacity has been reported to be a powerful prognostic predictor in CAD patients,8,9 and patients participating in CR may have a higher exercise capacity at baseline than non-participants (i.e., healthy cohort bias),10 correction of baseline exercise capacity may be mandatory to avoid healthy cohort bias in observational cohort studies.
Editorial p 378
Accordingly, the present study aimed to assess the effect of active CR participation on exercise capacity and long-term prognosis in a multicenter cohort of post-CABG Japanese patients using multivariate analysis, including correction of baseline exercise capacity.
This Japanese Study of Cardiac Rehabilitation after CABG (the J-REHAB CABG study) was a multicenter retrospective study conducted in 11 Japanese cardiology teaching hospitals, each of which had a cardiovascular surgery division and CR facilities. The study protocol was approved by the independent review board of all institutions, and an opt-out procedure was allowed in each institution. Study patients were recruited retrospectively from medical charts in each hospital. The inclusion criteria were: (1) patients who underwent CABG surgery between January 2003 and December 2007; (2) no contraindications for and able to perform exercise training; (3) presence of available data for coronary arteriography and left ventricular ejection fraction (LVEF); (4) presence of exercise capacity data for both early (2–3 weeks) and late (3–6 months) periods after CABG surgery; and (5) information on attendance at supervised CR exercise sessions and home exercise. Patients were excluded when they could not participate in outpatient CR because of severe myocardial ischemia, cerebrovascular disease, an orthopedic disorder, chronic kidney disease (creatinine >3.0 mg/dL), liver dysfunction (alanine aminotransferase >200 U/L), other severe organ failure, and/or other medical reasons (e.g., walking disability).
Altogether, 354 patients met the inclusion criteria and were divided into 2 groups according to whether they actively participated in CR after hospital discharge. Patients were considered to have actively participated in CR if (1) they continued exercise training at least 3 times per week including at least once-a-week attendance at a supervised CR session, and (2) the total exercise time (including supervised and home exercise) was ≥150 min/week for 3–5 months. The categorization of patients was performed by 2 investigators who were unaware of patient prognosis. Of the 354 patients, 242 met the criteria of active participation (Active CR participation group) and 107 patients did not (Non-Active group); 5 patients were excluded because of lack of information on exercise training. In addition, follow-up data could not be obtained for 2 patients in the Active CR participation group and 1 patient in the Non-Active group. Thus, 240 patients in the Active CR participation group and 106 patients in the Non-Active group were included in the final analysis. The follow-up rate was 97.7% (Figure 1).

Study flow chart. CR, cardiac rehabilitation.
The outpatient CR program began approximately 2–3 weeks after CABG and continued for 3–5 months. The program included hospital-based supervised exercise sessions (1–3 times weekly, consisting of walking, bicycling with an ergometer, and calisthenics), educational classes and individual counselling, combined with home exercise. The exercise intensity was determined individually at 50–60% of the heart rate reserve, or the heart rate at the anaerobic threshold (AT) level obtained via symptom-limited bicycle ergometry-assessed cardiopulmonary exercise testing (CPX), or with a perceived exercise rating (original Borg’s score) of 12–13 on a scale of 6–20. Home exercise consisted mainly of brisk walking at a prescribed heart rate for 30–60 min 2–4 times each week.
Patients were encouraged to attend educational classes with lectures on CAD, secondary prevention, diet, smoking cessation, medications, and physical activities suggested by physicians, nurses, dieticians, pharmacists, and exercise instructors.
MeasurementsData were collected on clinical background, number of diseased vessels, LVEF, status of CABG (number of bypass grafts, whether it was on- or off-pump, arterial grafts only, elective/emergency surgery, length of hospital stay, prescriptions at discharge, laboratory findings (C-reactive protein, hemoglobin, creatinine, B-type natriuretic peptide), exercise capacity (peak oxygen uptake [peak V̇O2] and ventilator AT), and adherence to CR participation. Peak V̇O2 and AT were determined by symptom-limited bicycle ergometer CPX using the ramp protocol at the early (2–3 weeks) and late (3–6 months) time points after CABG, which corresponded approximately with the beginning and end of the CR program.
Follow-upThe clinical outcome was assessed mainly from hospital records, but if the information was not available, a questionnaire was sent by mail to the patient or the family.
The primary endpoint of this study was a major adverse cardiac event (MACE) defined as all-cause death, acute myocardial infarction (AMI), or unplanned rehospitalization because of unstable angina (UAP) or worsening heart failure (HF). Secondary endpoints were all-cause rehospitalization, rehospitalization for cardiac reasons, and all coronary events that required unplanned rehospitalization because of AMI, UAP, percutaneous coronary intervention (PCI), or repeat CABG (re-CABG). After the baseline assessment, the Active CR patients were followed for 1,281±597 days and Non-Active patients for 1,290±586 days (for combined groups: 1,284±592 days).
Statistical AnalysisBaseline characteristics of the 2 groups were compared using an unpaired t-test and a chi-squared test. Data at baseline and 3 months were compared by paired t-test. The results are presented as mean±standard deviation. Time-to-event data were analyzed using the Kaplan-Meier method, and statistical significance was determined using the log-rank test. The Cox proportional hazards method was used for multivariate analysis. In this analysis, variables that were deemed statistically significant (P<0.05) at baseline were included.
In addition, to address a potential selection bias that Non-Active patients might have had some illness at baseline that kept the patients from active participation in exercise-based CR, we repeated the Kaplan-Meier survival analysis using the follow-up data but excluding the first 6 months. A value of P<0.05 was considered to indicate statistical significance. Statistical analysis was performed using JMP® 10.0.2 (SAS Institute Japan, Tokyo, Japan).
The baseline characteristics of both groups are summarized in Table 1. Compared with the Non-Active group, the Active CR participants were older, had a higher rate of low-level high-density lipoprotein cholesterol, higher LVEF, less β-blocker use, and higher peak HR (P<0.05). Other baseline characteristics were similar between groups. In general, both groups of patients were relatively young and had preserved LV function, but they had multiple coronary risk factors and moderately decreased exercise capacity. Notably, their rates of off-pump coronary artery bypass (OPCAB) and CABG with arterial grafts only were both ∼70%.
| Characteristic | Active CR participation group (n=240) |
Non-Active group (n=106) |
P value |
|---|---|---|---|
| Age (years) | 66±8 | 63±1 | 0.01 |
| Male sex | 212 (88.3) | 91 (85.7) | 0.50 |
| Hypertension | 165 (68.8) | 79 (74.3) | 0.33 |
| DM/impaired glucose tolerance (%) | 128 (53.4) | 54 (50.5) | 0.67 |
| Hyperlipidemia | 181 (75.4) | 88 (83.0) | 0.13 |
| Low HDL-C (<40 mg/dL) | 126 (52.4) | 71 (66.7) | 0.04 |
| Current smoking (%) | 77 (32.2) | 30 (28.4) | 0.44 |
| Obesitya | 55 (22.7) | 35 (32.7) | 0.05 |
| Prior MI | 54 (22.4) | 26 (24.3) | 0.69 |
| History of PCI | 51 (21.3) | 19 (17.8) | 0.45 |
| 3-vessel disease | 157 (65.3) | 60 (56.2) | 0.20 |
| LVEF (%) | 55.4±12.8 | 51.4±13.3 | 0.02 |
| OPCAB | 167 (69.4) | 72 (68.1) | 0.81 |
| Emergency surgery | 23 (9.8) | 10 (9.5) | 0.76 |
| ≥4 Bypass grafts | 125 (52.3) | 47 (44.8) | 0.35 |
| Arterial graft only | 153 (63.7) | 74 (70.2) | 0.35 |
| Hospital stay (days) | 26.4±13.0 | 28.6±20.9 | 0.24 |
| CRP (mg/dL) | 1.7±1.9 | 2.0±2.1 | 0.28 |
| Hb (g/dL) | 11.8±1.7 | 11.8±1.7 | 0.92 |
| Cr (mg/dL) | 0.91±0.3 | 0.87±0.2 | 0.17 |
| BNP (pg/mL) | 164±147 | 149±139 | 0.42 |
| ACEI/ARB | 51 (21.4) | 28 (26.7) | 0.11 |
| β-blocker use | 143 (59.4) | 79 (74.3) | 0.009 |
| Statin use | 87 (36.1) | 43 (41.0) | 0.37 |
| Resting HR (beats/min) | 80±14 | 77±13 | 0.13 |
| Peak HR (beats/min) | 122±19 | 117±21 | 0.04 |
| Peak RER | 1.16±0.13 | 1.16±0.16 | 0.91 |
| Peak V̇O2 (mL/kg/min) | 17.0±4.3 | 17.2±4.2 | 0.79 |
| V̇O2 at AT (mL/kg/min) | 10.7±2.4 | 10.6±2.2 | 0.81 |
Data are expressed as mean±standard deviation or the number (%). Hyperlipidemia includes statin use. aBMI ≥25 kg/m2. ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; AT, anaerobic threshold; BMI, body mass index; BNP, B-type natriuretic peptide; CR, cardiac rehabilitation; Cr, serum creatinine; CRP, C-reactive protein; DM, diabetes mellitus; EF, ejection fraction; Hb, hemoglobin; HDL-C, high-density lipoprotein cholesterol; HR, heart rate; LVEF, left ventricular ejection fraction; MI, myocardial infarction; OPCAB, off-pump coronary artery bypass; PCI, percutaneous coronary intervention; RER, respiratory exchange ratio; V̇O2, peak oxygen uptake.
Attendance at CR sessions, frequency of exercise training, and total amount of exercise time were, by definition, significantly greater in the Active CR participation group than in the Non-Active group (Table 2).
| Rehabilitation parameter | Active CR participation group (n=240) |
Non-Active group (n=106) |
P value |
|---|---|---|---|
| Supervised session attendance (times/month) | 6.9±4.4 | 3.3±2.5 | <0.001 |
| Frequency home exercise (times/week) | 4.9±2.2 | 1.8±2.1 | <0.001 |
| Total amount of exercise training (min/week) | 360±214 | 116±96 | <0.001 |
Data are expressed as mean±standard deviation. CR, cardiac rehabilitation.
In both groups, peak V̇O2 (Active vs. Non-Active groups) increased from 17.0±4.3 to 20.9±4.6 mL/kg/min (P<0.0001) vs. 17.1±4.8 to 20.1±5.6 mL/kg/min (P<0.0001), and the AT increased from 10.7±2.4 to 12.3±2.4 mL/kg/min (P<0.0001) vs. 10.6±2.2 to 11.6±2.4 mL/kg/min (P<0.0001). The increases were statistically significant from the baseline (2–3 weeks) to late (3–6 months) after CABG. However, the percentage increases of both variables were significantly greater for the Active CR participants than for the Non-Active patients (both P=0.02) (Figure 2).

Changes in exercise capacity from early to late period after coronary artery bypass grafting. Percent change in (A) peak V̇O2 and (B) anaerobic threshold (AT) after 3 months.
The incidences of MACE (all-cause death or rehospitalization because of AMI/UAP/HF worsening), all-cause rehospitalization, rehospitalization for cardiac reasons, and coronary events (AMI/UAP/PCI/re-CABG) requiring unplanned hospitalization during the follow-up period were all significantly lower for the Active CR participants than for the Non-Active group (Table 3).
| Parameter | Active CR participation group (n=240) |
Non-Active group (n=106) |
P value |
|---|---|---|---|
| MACE | 8 (3.4) | 11 (10.5) | 0.02 |
| All-cause death | 3 (1.2) | 2 (1.9) | 0.66 |
| All-cause rehospitalization | 57 (23.8) | 41 (38.7) | 0.003 |
| Rehospitalization for cardiac reasons | 20 (8.3) | 29 (18.9) | 0.006 |
| Coronary eventsa requiring hospitalization | 7 (2.9) | 10 (9.4) | 0.009 |
Data are expressed as number (%). aCoronary events included AMI, UAP, PCI, and re-CABG. MACE included all-cause death or rehospitalization because of AMI/UAP/HF worsening. AMI, Acute myocardial infarction; CR, cardiac rehabilitation; HF, heart failure; MACE, major adverse cardiac event; PCI, percutaneous coronary intervention; re-CABG, repeat coronary artery bypass graft; UAP, unstable angina.
Kaplan-Meier survival curve analysis showed that the Active CR participation group had a significantly better event-free survival regarding the occurrence of MACE (Figure 3A, P=0.02), all-cause rehospitalization (Figure 3B, P=0.006), rehospitalization for cardiac reasons (Figure 3C, P=0.003), and coronary events requiring hospitalization (Figure 3D, P=0.012).
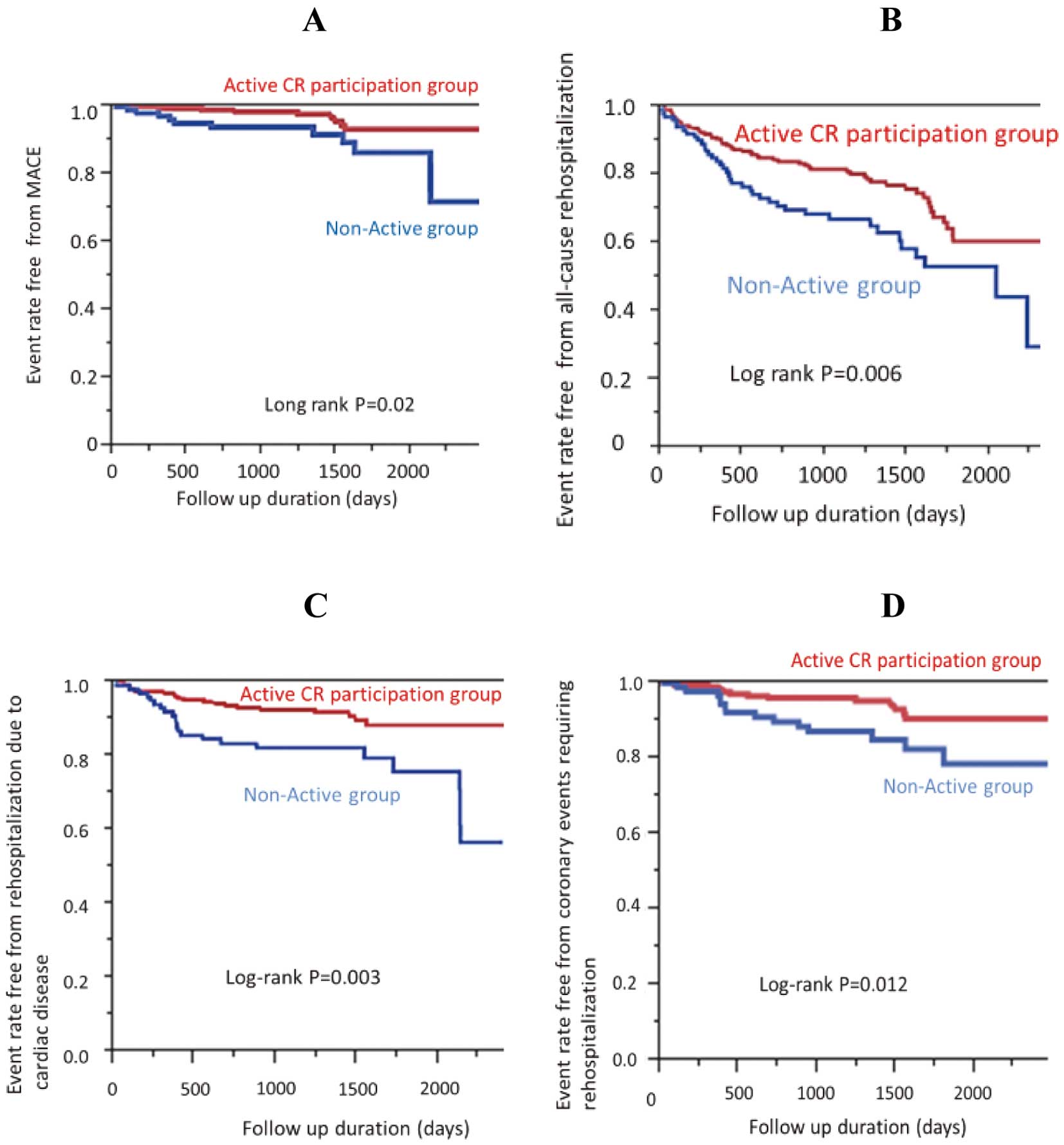
Survival curves free from cardiac events for the 2 study groups. Event rates, free from (A) major adverse cardiac events (MACE), (B) all-cause rehospitalization, (C) rehospitalization because of cardiac disease and (D) coronary events requiring rehospitalization. CR, cardiac rehabilitation.
Furthermore, when we performed the same analysis using data that excluded the first 6 months, the results were unchanged (Supplementary Figure).
Multivariate Cox proportional analysis showed that active participation in CR was an independent prognostic predictor for MACE, all-cause rehospitalization, rehospitalization for cardiac disease, and coronary events (Table 4).
| Factor | MACE | All-cause rehospitalization |
Rehospitalization for cardiac reasons |
Coronary events | ||||
|---|---|---|---|---|---|---|---|---|
| RR (95% CI) | P value | RR (95% CI) | P value | RR (95% CI) | P value | RR (95% CI) | P value | |
| Active CR participation group |
0.09 (0–0.75) |
0.02 | 0.41 (0.14–1.2) |
0.11 | 0.003 (0.003–0.43) |
0.03 | 0.003 (0.002–0.39) |
0.003 |
| Age | 0.93 (0.78–1.07) |
0.32 | 1.02 (0.95–1.09) |
0.54 | 0.97 (0.83–1.10) |
0.63 | 0.75 (0.82–1.10) |
0.52 |
| Low HDL-C (<40 mg/dL) | 0.67 (0.08–5.82) |
0.70 | 0.56 (0.21–1.55) |
0.26 | 0.28 (0.04–1.49) |
0.14 | 0.03 (0.01–0.85) |
0.03 |
| Peak HR | 0.99 (0.93–1.05) |
0.75 | 0.99 (0.96–1.02) |
0.67 | 0.98 (0.93–1.04) |
0.53 | 1.02 (0.92–1.10) |
0.59 |
| LVEF | 0.99 (0.92–1.06) |
0.76 | 0.99 (0.95–1.03) |
0.48 | 0.99 (0.93–1.05) |
0.71 | 1.0 (0.92–1.10) |
0.90 |
| β-blocker use | 0.61 (0.06–5.00) |
0.61 | 1.04 (0.36–3.10) |
0.94 | 0.03 (0.03–1.94) |
0.20 | 0.12 (0.02–1.47) |
0.12 |
CI, confidence interval; RR, relative risk. Other abbreviations as in Tables 1,3.
Subgroup analyses stratified by age, OPCAB, arterial graft only, and β-blocker use were performed using the Cox proportional hazard analysis for MACE (Figure 4A), all-cause rehospitalization (Figure 4B), hospitalization for cardiac reasons (Figure 4C), and coronary events (Figure 4D). In most subgroups, risk ratios for Active CR participation were significantly, or tended to be, lower than those of the Non-Active patients, and the results were consistent across all subgroups and all outcomes except for MACE in elderly patients.
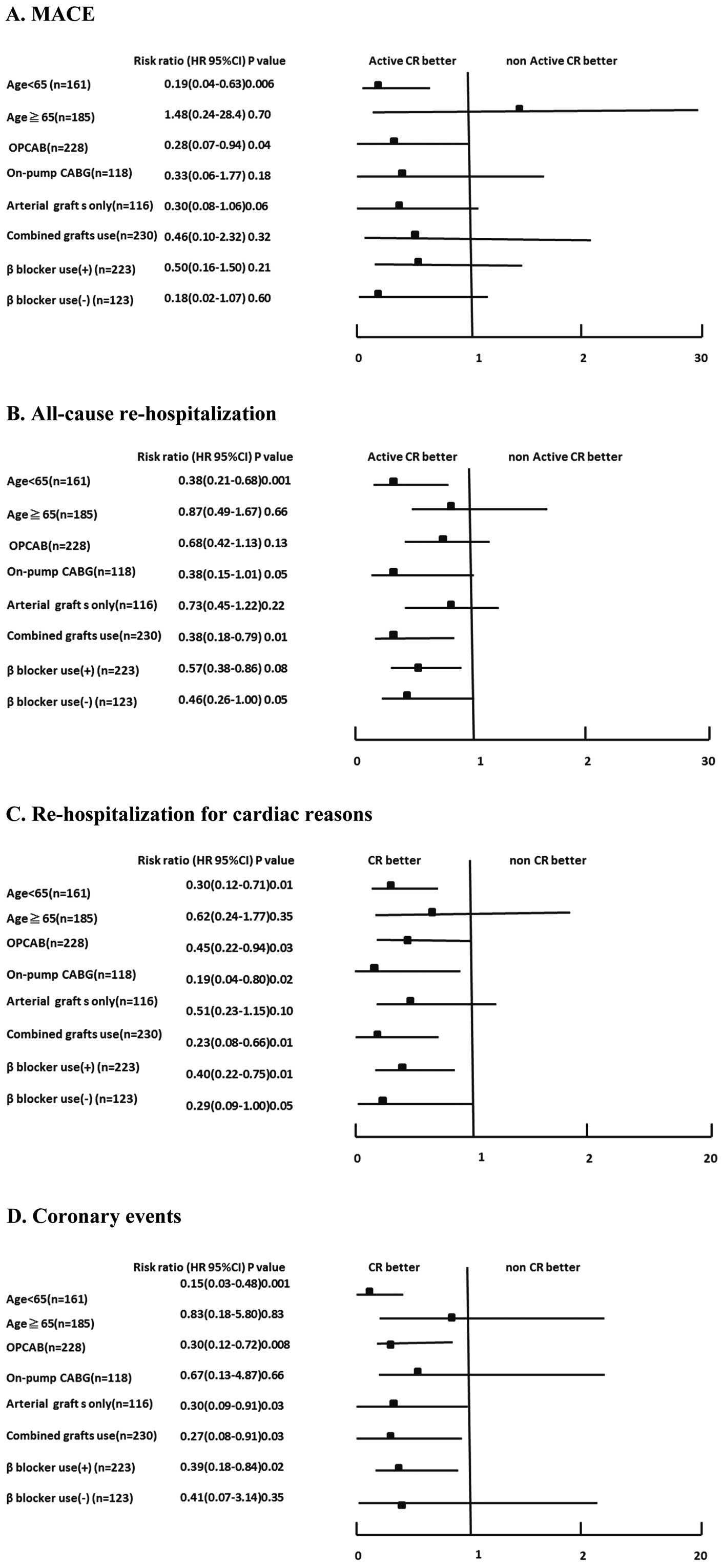
Risk ratio by subgroup analysis for (A) MACE, (B) all-cause rehospitalization, (C) rehospitalization for cardiac reasons and (D) coronary events. CABG, coronary artery bypass grafting; CI, confidence interval; CR, cardiac rehabilitation; HR, hazard ratio; OPCAB, off-pump coronary artery bypass.
The major findings of this study were as follows: (1) active CR participation was associated with significantly greater increases in peak V̇O2 and AT during the period from early (2–3 weeks) to late (3–6 months) after CABG compared with non-active participation; (2) active CR participation was an independent determinant of better long-term survival free from MACE, all-cause rehospitalization, rehospitalization for cardiac reasons, and recurrent coronary events; and (3) better event-free survival with active CR participation were seen in most subgroups including patients with OPCAB, arterial grafts only, and β-blocker use. To date, we believe that this is the first multicenter study that has assessed the effectiveness of outpatient CR after CABG regarding both exercise capacity and long-term prognosis in current post-CABG patients.
Previous StudiesWe found only 2 reports6,7 that studied the effect of CR on long-term prognosis in a cohort of only post-CABG patients (except for one other study of patients on hemodialysis5). In their community-based study, Pack et al reported that CR attendance was associated with a significant (46%) relative risk reduction in 10-year all-cause death of patients after CABG.6 Lee et al also reported that participation in phase I and phase II CR was associated with better long-term survival after CABG (20% risk reduction during phase I and 40% during phase II).7 Both of those studies were performed at a single center, however, and did not assess the effects of CR on exercise capacity or long-term outcomes other than total mortality. In addition, both studies defined the CR group as patients who participated in at least 1 CR session during the 3–6 months after CABG, which might not necessarily translate into clinical effects of CR.
More importantly, neither study assessed the patients’ baseline exercise capacity, despite the general notion that baseline exercise capacity is a powerful prognostic predictor in CAD patients.8,9 Because patients participating in CR are likely to have a lower risk profile and a higher exercise capacity at baseline than non-participants (i.e., healthy cohort bias),10 assessment and correction of baseline exercise capacity are necessary to avoid the healthy cohort bias in an observational cohort study.
Although both the Pack et al and Lee et al studies used a propensity score-matching analysis to adjust for background differences between the CR participants and non-participants, it would be impossible to guarantee that there was not a healthy cohort bias10 (i.e., a bias that the CR group might have consisted of healthier patients with higher baseline exercise capacity, resulting in a better long-term prognosis).
Present StudyThis study is the first multicenter study to show beneficial effects of outpatient CR on both exercise capacity and long-term prognosis in a cohort of post-CABG patients only. Although the patients in the present study were relatively young and predominantly male, approximately two-thirds of them had 3-vessel disease, underwent OPCAB, and received arterial grafts only. Their average exercise capacity (peak V̇O2) was moderately decreased at baseline, indicating that they represented current post-CABG patients.
There are strengths of the present study when compared with previous studies. (1) The baseline peak V̇O2 was assessed and confirmed to be equivalent in the Active and Non-Active CR participants, so the healthy cohort bias was avoided. (2) Active CR participation was rigorously defined according to the frequency of CR session attendance and total exercise time, including home exercise. (3) Major cardiac events during follow-up were assessed so that the beneficial effects of CR on long-term event-free survival (i.e., MACE, rehospitalization) were apparent. (4) The concomitantly shown improvements in exercise capacity and long-term prognosis were directionally concordant with each other. (5) The results of the subgroup analysis were consistent across almost all subgroups.
Additionally, the results of the improved long-term prognosis with active CR participation remained unchanged even when the first 6 months of data were excluded from the follow-up analysis. This finding negates the possibility that the Non-Active patients might have abstained from CR participation because of the presence of some latent illness at baseline that might have resulted in the worse prognosis (i.e., a reverse form of the healthy cohort bias).
Subgroup AnalysisThe subgroup analysis indicated that the beneficial effects of active CR participation on long-term prognosis were directionally consistent regardless of age and use of OPCAB, arterial grafts only, or β-blockers (Figure 4). Although the reason for the insignificant increase in MACE in the elderly Active CR group is unclear, considering the definition of MACE (all-cause death or rehospitalization because of AMI/UAP/HF worsening) and the small numbers of MACE (4 events in patients aged ≥65 and 12 events in patients aged <65), it might be explained by a small increase in noncardiac deaths in the elderly Active CR group. To clarify this point, further studies with a greater number of patients are necessary.
Although the long-term benefits of OPCAB remain controversial,11–13 it is widely used in Japan.14 The present subgroup analysis indicated that active CR participation was associated with a better long-term prognosis in post-OPCAB patients in terms of MACE, rehospitalization for cardiac reasons, and coronary events. Because previous CR studies in post-CABG patients6,7 did not report the rates of OPCAB use, the present study is the first to show the effects of outpatient CR in such patients.
With regard to the use of arterial grafts only, the beneficial effects of active CR participation on long-term prognosis were consistently apparent regardless of the exclusive use of arterial grafts or combined use of arterial and vein grafts. The finding that statistical significance was observed in more event categories in the subgroup of combined-graft use (i.e., in all-cause rehospitalization, rehospitalization for cardiac reasons, coronary events) might be attributable to higher event rates in the combined-graft subgroup, as recent studies15,16 have shown higher event rates after combined-graft use than with arterial grafts only. Again, because previous CR studies in post-CABG patients did not indicate the rates of arterial grafts only,6,7 the present study is the first to show the effects of outpatient CR in post-CABG patients with arterial grafts only.
Mechanisms of Better Prognosis With CR ParticipationAlthough exploring the mechanisms of the effectiveness of outpatient CR after CABG was not within the main scope of this study, there were 2 notable findings. First, the Kaplan-Meier curves started to diverge at 6–9 months of follow-up (Figure 3), suggesting that mechanisms beyond a short-term process may be involved. Second, although not explicitly shown in Table 3, the prevalence of noncoronary cardiac events was also significantly lower in the Active CR group than in the Non-Active group (13 vs. 19 events, respectively; P=0.001), suggesting that the mechanism may not be limited to amelioration of ischemic events alone.
Exercise training is known to improve vascular endothelial function and autonomic nerve function as well as suppressing systemic inflammation and oxidative stress in CAD patients.17 In addition, the multidisciplinary disease management program in outpatient CR may contribute to patients’ adherence to both self-care and guideline-directed optimal medical therapy.18 Although the present results did not identify any specific mechanism to be most responsible, we suggest that all these potential mechanisms combined contributed to the improved long-term prognosis.
Clinical ImplicationsTraditionally, CR in post-CABG patients has been mainly performed to facilitate recovery from surgery and to improve exercise capacity and QOL rather than to improve the long-term prognosis, partly because of the lack of the evidence on long-term prognosis. Based on the present results, together with those of previous studies,6,7 we believe it is time to acknowledge that CR in post-CABG patients can improve the long-term prognosis “beyond recovery from surgery”.19
Study LimitationsFirst, this was a retrospective study and therefore patients were not randomized. However, because all practice guidelines nowadays strongly recommend that post-CABG patients should participate in outpatient CR,3,4 conducting a randomized study with a non-CR group may be unethical. Second, because the patients in this study consisted mainly of relatively young and predominantly male patients who had preserved LV function and were able to perform CPX, the results may not be applicable to elderly, female patients or to those with impaired LV function or who are unable to perform CPX. Third, there were a certain number of patients hospitalized for unknown reasons during the follow-up (Supplementary Table), which might have partly affected the present results. However, most of those patients were admitted to hospitals other than the present study hospitals (which were the major cardiology centers in each community area), suggesting that the reasons for hospitalization were not cardiac in origin in most cases. In addition, even if the patients hospitalized for unknown reasons were excluded from the analysis, the main results of the present study are unaltered. Further studies are necessary to confirm the long-term benefits of outpatient CR in these specific subgroups of post-CABG patients.
Despite the accumulated evidence of the long-term benefits of outpatient CR, it has been reported that the number of CR facilities in Japan is insufficient, the implementation rate of outpatient CR in cardiology hospitals is low, and the participation rate in outpatient CR is low.20,21 Therefore, to improve the long-term prognosis of patients, urgent efforts should be made to greatly increase the number of CR facilities and the implementation rate of outpatient CR as well as increasing patients’ participation rate in outpatient CR after hospital discharge.
Active participation in outpatient CR was associated with greater improvement in exercise capacity and better survival free from cardiovascular events in patients after CABG surgery.
The authors thank each of the participants for the data collection. We thank Nancy Schatken, BS, for English proofreading of a draft of this manuscript.
All authors have no conflicts of interest in this study.
This study was supported by a Health and Labour Science Research Grant (H22-007) from the Ministry of Health, Labour, and Welfare, Japan.
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-19-0650