2020 Volume 84 Issue 3 Pages 397-403
2020 Volume 84 Issue 3 Pages 397-403
Background: Atrial fibrillation (AF) is an important prognostic determinant in heart failure (HF) with preserved ejection fraction (HFpEF). However, it is unclear which HFpEF phenotypes are affected by AF in terms of long-term clinical outcomes because HFpEF is a heterogeneous syndrome with comorbidities such as coronary artery disease (CAD). In this study we determined the differential prognostic significance of AF in HFpEF patients according to CAD status.
Methods and Results: Data for 408 hospitalized HFpEF patients enrolled in the Japanese Heart Failure Syndrome with Preserved Ejection Fraction Nationwide Multicenter Registry were analyzed. Patients were divided into 4 groups according to the presence of AF and CAD. The primary outcome was the composite of all-cause death and HF rehospitalization. The incidence of adverse events was higher in the AF–non-CAD than non-AF–non-CAD group (P=0.004). On multivariable Cox regression analysis with prespecified confounders, AF–non-CAD was significantly associated with an increased risk of adverse events than non-AF–non-CAD (adjusted HR, 1.91; 95% CI: 1.02–3.92) regardless of the type of AF. In contrast, risk was comparable between the AF–CAD and non-AF–CAD groups (adjusted HR, 1.24; 95% CI: 0.64–2.47).
Conclusions: In HFpEF patients without CAD, AF was independently related to adverse events, indicating that intensive management of AF would have more beneficial effects particularly in HFpEF patients without CAD.
Heart failure (HF) with preserved ejection fraction (HFpEF) and atrial fibrillation (AF) are coexisting conditions. The prevalence of HFpEF in AF is high, at around 20%,1,2 and the prevalence of AF in HFpEF is as high as 32–61.5%.3–5 AF is also an independent prognostic determinant in patients with HF.6,7 Notably, the development of AF may have more unfavorable effects on clinical outcomes in patients with HFpEF than in those with HF with reduced ejection fraction (HFrEF).8,9
Editorial p 374
Other comorbid conditions in HFpEF, such as hypertension, diabetes, obesity, chronic obstructive pulmonary disease, and coronary artery disease (CAD), result in heterogeneity of HFpEF and are very important not only in the development of HFpEF, but also in the development of subsequent adverse events.10,11 Despite HFpEF being a heterogeneous syndrome, most previous studies have dealt with all phenotypes of HFpEF in a similar manner.12 In fact, the prognostic value of AF has been evaluated only in the whole HFpEF cohort, without subgroup analyses in each specific comorbidity.6,13,14 This delays the characterization of HFpEF patients in whom AF may have a greater adverse effect. CAD has been reported to be a major comorbidity in HFpEF, with a prevalence of 27.7–44%,3–5,15 and is also independently associated with increased mortality in HFpEF patients regardless of the presence of AF.11 However, it remains unknown whether AF has a different prognostic impact in patients with HFpEF with underlying CAD. Accordingly, in this study we investigated differences in the prognostic significance of AF in HFpEF patients with and without CAD.
Data from the Japanese Heart Failure Syndrome with Preserved Ejection Fraction (JASPER) Registry, obtained between November 2012 and March 2015, were analyzed retrospectively. Details of the JASPER Registry have been described previously.5 Briefly, the study is a multicenter, observational, prospective cohort that includes consecutive patients aged ≥20 years requiring hospitalization with a diagnosis of acute decompensated HF according to the Framingham criteria16 by at least 2 experienced cardiologists, with preserved left ventricular (LV) systolic function, defined as LV ejection fraction (LVEF) ≥50% by the modified Simpson method or LV fractional shortening ≥25% by echocardiography. Patients with acute coronary syndrome, those on hemodialysis, or those with a history of heart transplantation were excluded. The study was approved by the institutional review board of each participating site, and is registered with the Japanese UMIN Clinical Trials Registry (UMIN000010601).
PatientsOf the 535 patients enrolled in the JASPER Registry, 408 patients were ultimately included in the present study after applying further exclusion criteria shown in Figure 1. Patients were divided into 4 groups: (1) patients without either AF or CAD (non-AF–non-CAD); (2) patients with AF and without CAD (AF–non-CAD); (3) patients without AF and with CAD (non-AF–CAD); and (4) patients with both AF and CAD (AF–CAD). CAD was defined as the presence of any history of myocardial infarction, angina pectoris, or coronary revascularization (percutaneous coronary intervention or coronary artery bypass grafting). AF was also divided into 2 types: paroxysmal or persistent. Persistent AF was defined as AF rhythm both on admission and at discharge, and paroxysmal AF was defined as any other history of AF.

Study flow diagram. AF, atrial fibrillation; CAD, coronary artery disease; CIED, cardiac implantable electronic device; HF, heart failure; HFpEF, heart failure with preserved ejection fraction.
The present study was approved by the National Cerebral and Cardiovascular Center Institutional Review Board (24-093).
Clinical OutcomeThe primary outcome of interest was the composite of all-cause death and HF rehospitalization.
Statistical AnalysisCategorical variables are presented as numbers and percentages. Continuous variables are expressed as median (IQR). Comparisons of differences among groups, classified across CAD and AF status, were performed using the Chi-squared or Fisher’s exact tests for categorical variables when appropriate, and by the Kruskal-Wallis test for continuous variables. Survival distribution in each group was calculated using the Kaplan-Meier method, and differences were evaluated using log-rank test. A multivariable Cox proportional hazards model was used to estimate the relationship between the groups and the composite of all-cause death and HF rehospitalization. Prespecified covariates (albumin on admission, New York Heart Association [NYHA] Class III or IV at discharge, log concentration of B-type natriuretic peptide [BNP] at discharge, systolic blood pressure on admission, and sodium on admission) were entered into the model according to our previous study.5 All tests were 2-tailed, and P<0.05 was considered significant. All analyses were performed with JMP Pro version 14 (SAS Institute, Cary, NC, USA).
Baseline characteristics of the patients are given in Table 1. Patients with a history of AF had a higher rate of oral anticoagulant administration than those without a history of AF. Patients without CAD had a lower prevalence of diabetes mellitus, hypertension, dyslipidemia, and chronic kidney disease, and a lower rate of β-blocker use than those with CAD. Patients without either CAD or AF were the youngest, had the lowest BNP concentrations and prevalence of prior HF admission, and had the smallest left atrial size among all groups.
| Overall (N=408) |
Non-CAD | CAD | P-value | |||
|---|---|---|---|---|---|---|
| Non-AF (n=81) | AF (n=207) | Non-AF (n=52) | AF (n=68) | |||
| Age (years) | 80 [73–84] | 73 [62–81] | 80 [74–85] | 80 [74–83] | 81 [77–85] | <0.001 |
| Female | 203 (50) | 42 (52) | 112 (54) | 25 (48) | 24 (35) | 0.056 |
| BMI (kg/m2) | 23.4 [21.1–26.2] | 23.7 [20.9–26.2] | 23.1 [20.9–26.1] | 25.0 [22.1–28.2] | 23.0 [21.3–25.3] | 0.082 |
| NYHA Class III or IV | 26 (7) | 4 (5) | 16 (8) | 3 (6) | 3 (5) | 0.80 |
| Heart rate (beats/min) | 66 [58–74] | 66 [59–75] | 68 [60–76] | 61 [57–69] | 64 [58–74] | 0.076 |
| Systolic BP (mmHg) | 113 [102–124] | 120 [104–132] | 112 [101–121] | 120 [107–133] | 112 [100–122] | <0.001 |
| Diastolic BP (mmHg) | 60 [52–68] | 60 [51–71] | 60 [53–68] | 60 [53–66] | 59 [51–67] | 0.65 |
| Past history | ||||||
| Smoking | 185 (47) | 36 (45) | 91 (45) | 23 (47) | 35 (55) | 0.60 |
| Prior HF admission | 144 (37) | 11 (14) | 78 (39) | 23 (47) | 32 (48) | <0.001 |
| Diabetes mellitus | 157 (39) | 26 (32) | 60 (29) | 29 (56) | 42 (62) | <0.001 |
| Hypertension | 316 (78) | 64 (79) | 147 (72) | 45 (87) | 60 (88) | 0.008 |
| Dyslipidemia | 172 (42) | 30 (38) | 68 (33) | 39 (75) | 35 (51) | <0.001 |
| CVA | 98 (24) | 15 (19) | 48 (23) | 10 (20) | 25 (37) | 0.057 |
| CKD | 195 (48) | 30 (37) | 85 (41) | 38 (73) | 42 (62) | <0.001 |
| COPD | 34 (8) | 5 (6) | 15 (7) | 9 (17) | 5 (8) | 0.160 |
| SAS | 35 (10) | 7 (9) | 15 (8) | 4 (9) | 9 (15) | 0.54 |
| Laboratory data | ||||||
| Sodium (mEq/L) | 140 (137–141) | 139 (136–141) | 140 (137–142) | 140 (139–142) | 139 (136–141) | 0.038 |
| BUN (mg/dL) | 25.0 (19.0–36.0) | 23.0 (16.8–32.6) | 24.9 (18.3–36) | 28.1 (20.8–40.0) | 26.5 (20.0–37.3) | 0.110 |
| Creatinine (mg/dL) | 1.05 (0.84–1.50) | 0.95 (0.74–1.72) | 1.02 (0.84–1.31) | 1.38 (1.00–1.99) | 1.15 (0.98–1.71) | <0.001 |
| BNP (pg/mL) | 160 (81–304) | 104 (44–235) | 162 (82–293) | 148 (88–283) | 185 (120–347) | 0.017 |
| Albumin (g/dL) | 3.6 (3.4–4.0) | 3.7 (3.2–4.0) | 3.6 (3.4–4.0) | 3.7 (3.3–4.0) | 3.6 (3.4–4.0) | 0.99 |
| Echocardiography data | ||||||
| LVEF (%) | 60 (54–65) | 62 (55–69) | 60 (55–65) | 56 (48–63) | 60 (54–65) | 0.065 |
| LAD (mm) | 45 (40–50) | 41 (35–47) | 45 (41–51) | 45 (42–50) | 46 (42–52) | 0.001 |
| Medication | ||||||
| ACEI/ARB | 295 (72) | 56 (69) | 145 (70) | 39 (75) | 55 (81) | 0.28 |
| β-blockers | 266 (65) | 42 (52) | 130 (63) | 45 (87) | 49 (72) | <0.001 |
| MRAs | 135 (33) | 21 (26) | 74 (36) | 15 (29) | 25 (37) | 0.33 |
| OACs | 248 (61) | 8 (10) | 179 (86) | 7 (13) | 54 (79) | <0.001 |
| Warfarin | 191 (47) | 8 (10) | 133 (64) | 6 (12) | 44 (65) | |
| Thrombin inhibitor | 14 (3) | 0 (0) | 11 (5) | 0 (0) | 3 (4) | |
| Factor Xa inhibitors | 43 (11) | 0 (0) | 35 (17) | 1 (2) | 7 (11) | |
Data given as n (%) or median (IQR). ACEI, angiotensin-converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin II receptor blocker; BMI, body mass index; BNP, B-type natriuretic peptide; BP, blood pressure; BUN, blood urea nitrogen; CAD, coronary artery disease; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident; HF, heart failure; LAD, left atrial dimension; LVEF, left ventricular ejection fraction; MRA, mineral corticoid-receptor antagonist; NYHA, New York Heart Association; OAC, oral anticoagulant; SAS, sleep apnea syndrome.
Of the 408 patients in the study, 139 (34.1%) experienced the primary outcome: 37 patients died and 102 were rehospitalized for HF during a median follow-up period of 714 days (IQR 326–801 days).
Patients with AF had a higher cumulative rate of the primary outcome than those without AF (Supplementary Figure 1). Moreover, Kaplan-Meier analysis demonstrated that the primary outcome occurred more frequently in AF–non-CAD than non-AF–non-CAD patients, whereas there was a comparable incidence of the primary outcome in the AF–CAD and non-AF–CAD groups (Figure 2). In multivariable analysis, patients categorized as AF–non-CAD also had higher risk of adverse events than those categorized as non-AF–non-CAD (Table 2). In contrast, there was no significant difference in risk between the AF–CAD and non-AF–CAD groups (Table 2).
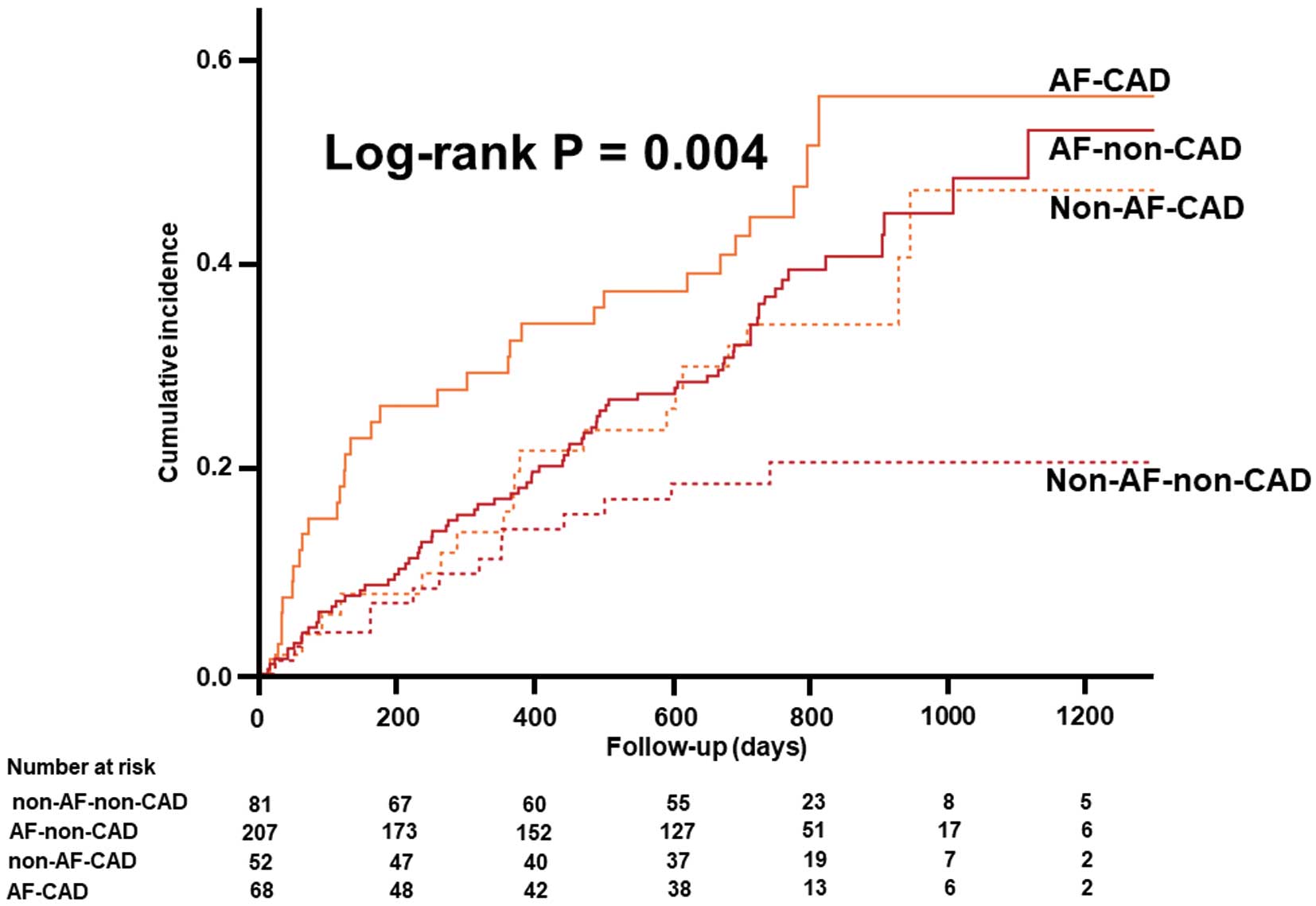
Kaplan-Meier analysis for the composite of all-cause death and heart failure (HF) rehospitalization categorized by coronary artery disease (CAD) and atrial fibrillation (AF) status.
| Model 1 (crude) | Model 2 (covariate-adjusted) | |||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| AF–non-CAD vs. non-AF–non-CAD | 2.13 (1.25–3.93) | 0.005 | 1.91 (1.02–3.92) | 0.044 |
| AF–CAD vs. non-AF–CAD | 1.57 (0.89–2.82) | 0.119 | 1.24 (0.64–2.47) | 0.52 |
| Non-AF–CAD vs. non-AF–non-CAD | 1.95 (0.98–3.96) | 0.057 | 2.47 (1.13–5.55) | 0.023 |
| AF–CAD vs. AF–non-CAD | 1.43 (0.93–2.15) | 0.104 | 1.61 (0.96–2.61) | 0.068 |
The covariates for multivariable analyses included albumin on admission, NYHA Class III or IV at discharge, log BNP concentration at discharge, systolic BP on admission, and sodium on admission. CI, confidence interval; HR, hazard ratio. Other abbreviations as in Table 1.
Regarding AF status, the rate of different types of AF in patients with and without CAD were comparable (Supplementary Figure 2). In patients without CAD, the incidence and risk of adverse events were significantly increased in both paroxysmal and persistent AF compared with non-AF (Table 3; Figure 3A). Among patients without CAD, those with paroxysmal AF had the highest frequency of HF rehospitalization during the follow-up period, even though there were only a few differences in baseline characteristics among the different groups without CAD (Supplementary Figure 3; Supplementary Table 1). Conversely, there were no significant differences in the incidence and risk of adverse events according to AF in patients with CAD (Table 3; Figure 3B).
| Model 1 (crude) | Model 2 (covariate-adjusted) | |||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Non-CAD | ||||
| Paroxysmal AF vs. non-AF | 2.45 (1.35–4.70) | 0.003 | 2.29 (1.12–4.99) | 0.022 |
| Persistent AF vs. non-AF | 1.91 (1.06–3.63) | 0.031 | 2.22 (1.09–4.88) | 0.028 |
| Paroxysmal AF vs. persistent AF | 1.29 (0.81–2.03) | 0.28 | 1.03 (0.60–1.76) | 0.91 |
| CAD | ||||
| Paroxysmal AF vs. non-AF | 1.67 (0.79–3.40) | 0.177 | 1.22 (0.47–2.93) | 0.66 |
| Persistent AF vs. non-AF | 1.50 (0.79–2.85) | 0.21 | 1.09 (0.51–2.32) | 0.82 |
| Paroxysmal AF vs. persistent AF | 1.11 (0.53–2.27) | 0.77 | 1.12 (0.44–2.65) | 0.80 |
The covariates for multivariable analyses included albumin on admission, NYHA Class III or IV at discharge, log BNP concentration at discharge, systolic BP on admission, and sodium on admission. Abbreviations as in Tables 1,2.
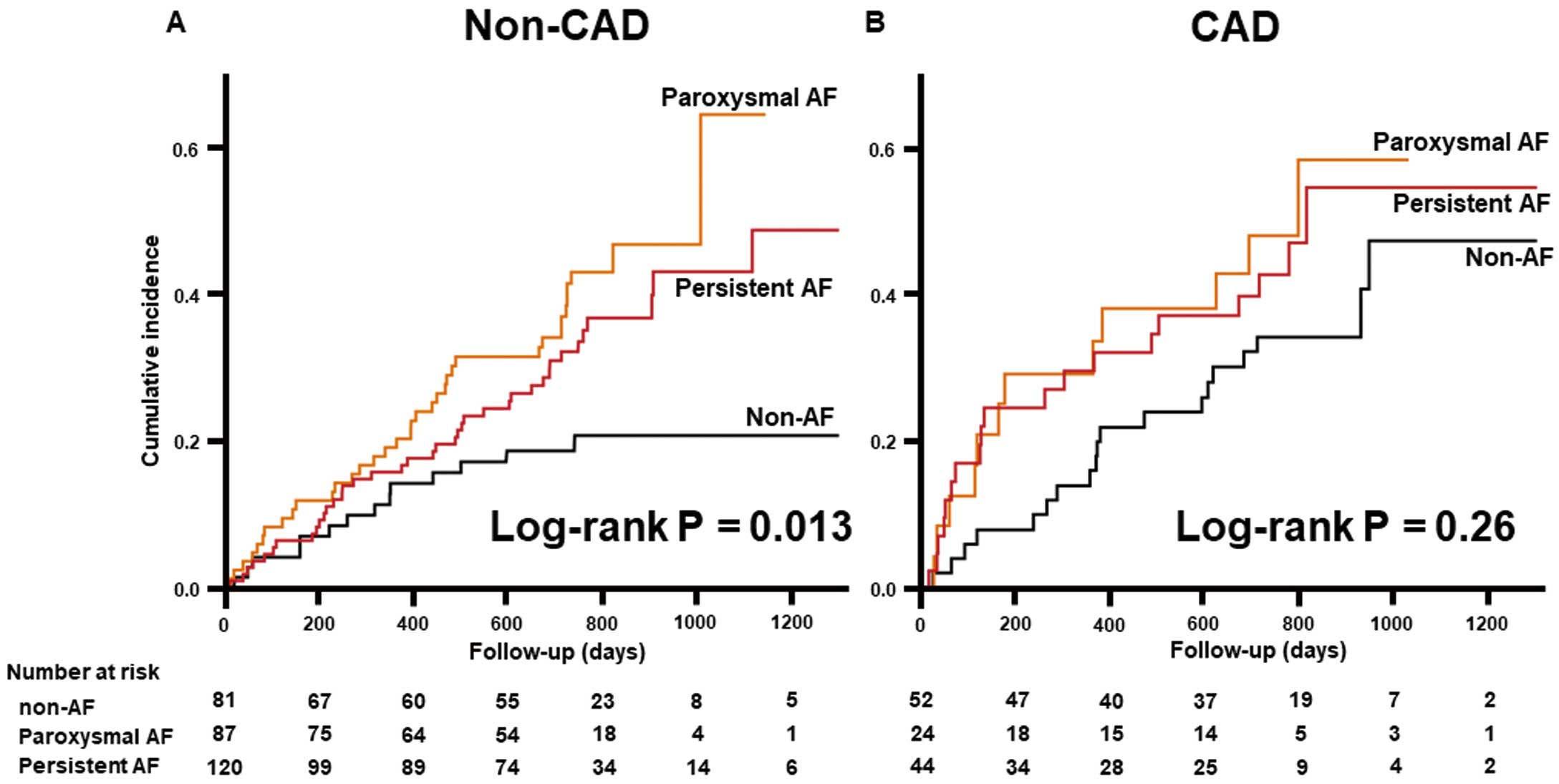
Kaplan-Meier analysis for the composite of all-cause death and heart failure (HF) rehospitalization categorized by atrial fibrillation (AF) status in patients (A) without or (B) with coronary artery disease (CAD).
The present study highlighted a differential prognostic impact of AF in HFpEF patients with and without CAD in a Japanese nationwide multicenter prospective registry. The presence of AF affected subsequent adverse events of all-cause death and HF rehospitalization in HFpEF patients without CAD, but not in those with CAD, regardless of AF type.
Patients with HF have a higher risk of developing AF than those without HF.17,18 Previous large cohort studies and meta-analyses showed that the presence of AF in HF was associated with adverse clinical outcomes, and that the adverse effect was present in patients who had not only HFrEF, but also HFpEF.8,9,13,14 Thus, it is suggested that AF is not only a driver of HFpEF, but also a potent prognostic indicator in HFpEF. However, in spite of being shown in HFrEF,19 there are few prospective studies showing that interventions for AF in HFpEF have a prognostic advantage.20 Furthermore, phenotypic heterogeneity in HFpEF is probably much greater than in HFrEF;12 therefore, identifying specific HFpEF phenotypes in which AF has greater unfavorable effects would lead to further understanding of AF as a therapeutic target in HFpEF. In the present study, AF had a different prognostic impacts on HFpEF patients with and without CAD. In the subanalysis, we found that the difference remained regardless of the type of AF. However, it is difficult to confirm that the prognostic effects between paroxysmal and persistent AF are similar because of the small sample size in the present study. A previous study, focusing on HFrEF, showed that paroxysmal AF had greater risk of subsequent HF hospitalization than persistent AF.21 Notably, the findings of the present study may indicate that “sudden onset of AF”, which was called paroxysmal, itself could be a trigger for HF rehospitalization (Figure 3; Supplementary Figure 3). Further studies with larger sample sizes are warranted.
The present study demonstrated that AF worsened clinical outcomes in HFpEF patients without CAD rather than in those with CAD. There are 2 possible mechanisms underlying this finding: one involves the autonomic nervous system (ANS) and the other is ventricular fibrosis. Both AF and CAD are associated with ANS imbalance, which results in ANS dysfunction. Previous studies showed that myocardial infarction causes ANS imbalance in animal models and human subjects.22,23 Moreover, ANS imbalance extends not only to myocardial infarction, but also to CAD associated with chronic ischemia and hypoxia because of the susceptibility of autonomic nerve tissue to ischemia.24 ANS imbalance is also prevalent in AF,25 and plays a significant adverse role in AF to enhance automaticity and triggered activity, development of substrate, and promote the maintenance of AF.25,26 Indeed, ANS imbalance is related to mortality in patients with HF.27 Therefore, in HFpEF patients without CAD, AF would predominantly add to the ANS imbalance, which would lead to worse outcomes. In contrast, in patients with CAD, the worsening effects of AF on prognosis are weakened because CAD already affects the ANS. AF was reported to be associated with the development of ventricular fibrosis, resulting from both tachycardia-mediated cardiomyopathy and inflammation, which caused ventricular remodeling through enhanced renin–angiotensin–aldosterone system and transforming growth factor-β1 signaling.28,29 In the present study, HFpEF patients without CAD had a higher LVEF and lower BNP concentrations than those with CAD (Supplementary Table 2). These data suggest that those without CAD had the less progressed fibrosis than those with CAD. Thus, AF in patients without CAD would play a primary role in driving ventricular fibrosis and worsening outcome. In contrast, in patients with CAD, AF may have little additive adverse effects on clinical outcomes because CAD also causes ventricular fibrosis.
The findings of this study indicate that HFpEF patients without CAD may receive larger benefit from intensive management of AF than those with CAD. Among the techniques used in the management of AF in patients with HFrEF, catheter ablation is associated with improvement of not only functional capacity, but also clinical outcomes compared with medical therapy.19,30 In addition, in those with HFpEF, maintenance of sinus rhythm with catheter ablation and/or antiarrhythmic drugs improved LV diastolic function.31 Therefore, even in those with CAD, the aggressive management of AF may have a certain effect. However, this effect could be less in those with than without CAD. Accordingly, the findings of the present study may help identify the specific HFpEF phenotype receiving greater prognostic benefit by intensive management of AF (e.g., catheter ablation). Future prospective studies are warranted to determine whether intensive management of AF in HFpEF patients without CAD leads to better clinical outcomes than in those with CAD.
Study LimitationsThis study has several limitations. First, the sample size was relatively small, thereby limiting the ability to generalize findings and the statistical power for detecting differences in negative data, especially in the subanalysis across AF status. Therefore, a larger-scale study is warranted to confirm our findings. Second, we excluded 127 (23.7%) patients from the JASPER registry; therefore, selection bias may be present in this study, despite there being little difference in baseline characteristics and outcomes between the studied and excluded populations (Supplementary Table 3). Third, there was no detailed information regarding the management of AF, which could affect its prognostic significance. Fourth, the definition of AF status in this study was different from the standard definition because rhythm status was available only at the time of admission and discharge. Therefore, some patients with persistent AF may have been classified as paroxysmal AF despite the left atrial dimension in persistent AF, which reflects the duration of AF, being larger than that in paroxysmal AF (46 [42–53.5] vs. 44.5 [39.8–48] mm, respectively; P=0.002).
Concomitant AF had a greater adverse impact on clinical outcomes in HFpEF patients without CAD than in those with CAD. Intensive management for AF would have more beneficial prognostic effects in HFpEF patients without CAD.
The authors are grateful for the contributions of all the investigators, clinical research coordinators, and data managers involved in the JASPER registry.
This work was supported by a grant from the Japan Cardiovascular Research Foundation to T.A. (24-4-2).
The authors declare no conflicts of interest.
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-19-0963