Abstract
Background:
Multiple spikes in the QRS complex (fragmented QRS [fQRS]) on 12-lead electrocardiography have been associated with ventricular arrhythmic events (VAEs) in patients with hypertrophic cardiomyopathy (HCM). The aim of this study was to assess the association between new appearances of fQRS and cardiac events in patients with HCM.
Methods and Results:
The association between baseline fQRS and cardiac events, namely VAEs, heart failure-related hospitalization, and all-cause death, was evaluated retrospectively in 146 HCM patients (46 patients with fQRS, 100 without fQRS). The median follow-up was 5.3 years. Cardiac events occurred in 29 patients with baseline fQRS and 32 patients without baseline fQRS (63% vs. 32%; P<0.001). VAEs occurred in a significantly larger percentage of patients with than without baseline fQRS (54% vs. 23%, respectively; P<0.001). Of the 100 patients without baseline fQRS, 33 had a new appearance of fQRS during the 4.6-year follow-up, whereas 67 did not. VAEs occurred more frequently in the 33 patients with the appearance of fQRS than in those without (42% vs. 13%, respectively; P=0.001). Multivariable analysis showed that the new appearance of fQRS documented before VAEs was associated with VAEs (hazard ratio 4.29, 95% confidence interval 1.81–10.2; P=0.001).
Conclusions:
The new appearance of fQRS was associated with an increased risk of VAEs in HCM patients.
Hypertrophic cardiomyopathy (HCM) is caused by numerous mutations of genes encoding sarcomere proteins, with widely different phenotypic expression.1–3
The main causes of death in HCM are left ventricular dysfunction and ventricular arrhythmic events (VAEs). In recent reports, improved diagnosis and treatment resulted in a reduction of the mortality rate to <1% per year, which is comparable to that of the general population. However, some high-risk groups experience progressive deterioration of cardiac function and fatal arrhythmic events.
Among HCM patients, the rate of sudden cardiac death (SCD) from arrhythmic events has been reported to be 0.5% per year, and SCD is preventable with the proper use of implantable cardiac defibrillators (ICDs).3
Conventionally, decisions regarding ICD implantation are guided by many risk factors, including family history, massive left ventricular hypertrophy, a history of syncope, left ventricular outflow tract obstruction (LVOTO), non-sustained ventricular tachycardia, and left atrial dilatation.4–6
For patients with multiple risks, documenting VAEs (i.e., non-sustained [NSVT] and sustained ventricular tachycardia [VT]) leads to ICD implantation in most patients.7
However, VAEs are often asymptomatic and comparatively rare (reported rates are as high as 15–30%), with repeated Holter electrocardiography and stress tests failing to reveal high-risk patients who should have an ICD implanted to avoid SCD.8
Fragmented QRS (fQRS) is defined as multiple fine spikes on normal resting electrocardiograms (ECGs). Reflecting regional myocardial damage, an association between fQRS and the occurrence of VAEs during myocardial infarction and Brugada syndrome has been reported.9
It has also been reported that regional myocardial damage, demonstrated by late gadolinium enhancement (LGE) during cardiac magnetic resonance imaging (CMR), is associated with the presence of fQRS in HCM patients.10
An association between baseline fQRS and the occurrence of VAEs has also been reported in HCM patients,11–15
and a recent meta-analysis showed a significant increase in arrhythmic events in HCM patients with baseline fQRS.16
However, in some HCM patients without baseline fQRS, fQRS newly appears during follow-up, and little is known about the significance of this newly appeared fQRS. In 1 case report, an HCM patient with new appearance of fQRS was reported to have VAEs 7 years after the baseline ECG,17
but an association between cardiac events and the appearance of fQRS in HCM patients has never been reported.
The aim of the present study was to examine the association between baseline fQRS and cardiac events in HCM patients. In addition, we investigated the association between the new appearance of fQRS and VAEs in patients without baseline fQRS.
Methods
Study Population
The study population comprised 165 consecutive patients who were suspected of having HCM and who had been referred to Okayama University Hospital between April 2004 and April 2017. Patients without follow-up after their initial ECG (n=10) or with inadequate clinical data (n=9) were excluded from the study, leaving 146 patients enrolled in this study. Data for 100 patients without baseline fQRS were also used to analyze the new appearance of fQRS (Figure 1).
HCM was diagnosed according to the 1995 criteria of the World Health Organization/International Society and Federation of Cardiology Task Force.18
The study protocol was approved by the Institutional Ethics Committee on Human Research of Okayama University (Approval no. 1809-029). Because of the anonymous nature of the data, the requirement for informed consent was waived. The first author takes complete responsibility for the integrity of the data and the accuracy of the data analysis.
ECG Recording and Definition of fQRS
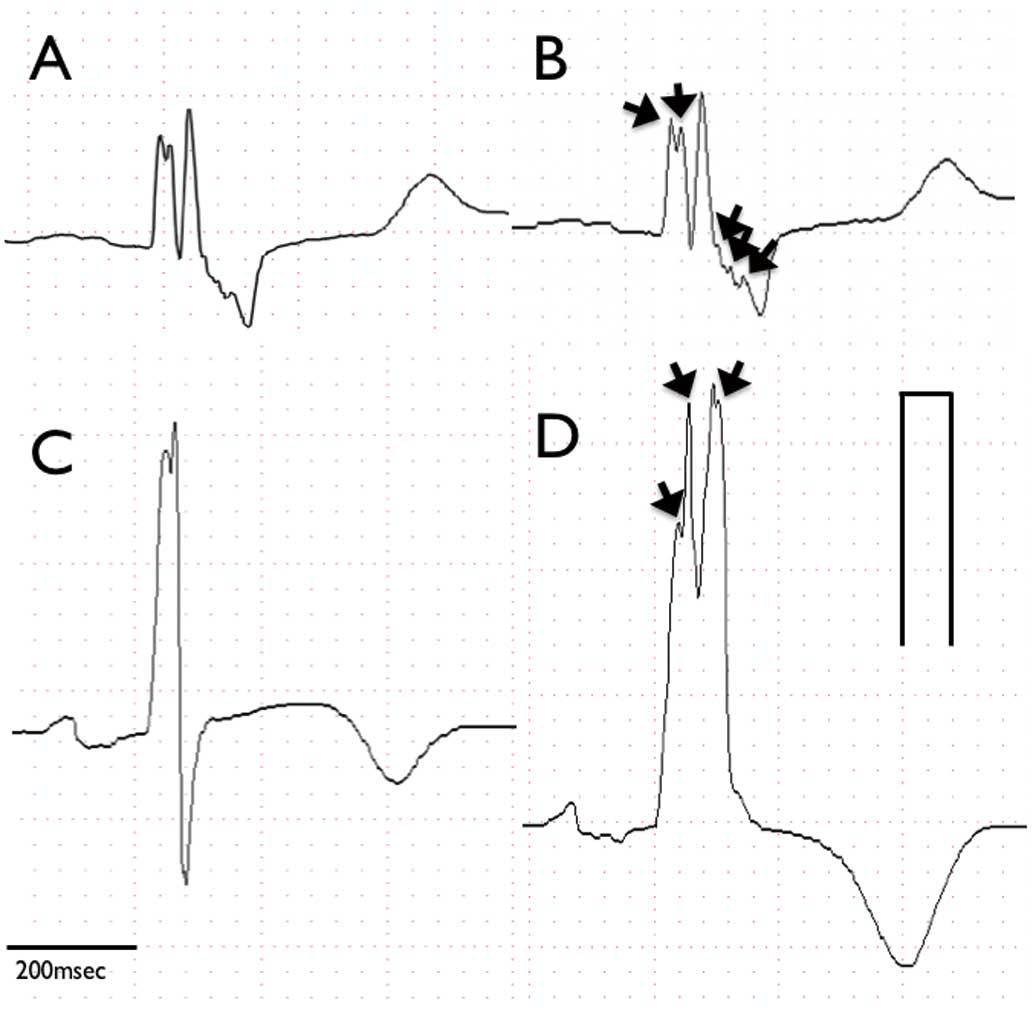
Standard 12-lead electrocardiography with a 0- to 150-Hz filter was used to record ECGs for all patients. ECGs acquired at admission were analyzed as the initial ECGs. The digital ECG without a filter was evaluated at 400% of the initial size on a personal computer monitor; each parameter and fragmentation of the QRS complex were measured as described previously;9
a typical example of the ECGs obtained is shown in
Figure 2. Abnormal fragmentation within the QRS complex was defined as more than 2 positive spikes within the QRS complex in 2 contiguous leads. Analysis of fragmentation needed 3 consecutive beats or more. The minimum size of the QRS fragmentation was as small as 0.02 μV. The ECGs of patients without fQRS were retrospectively analyzed and the new appearance of fQRS was investigated (Figure 2). ECGs were reviewed by 3 cardiologists (S.O., K. Nakamura and H.M.) in a blinded manner.
Other Clinical Examinations
All other clinical examinations were performed at the time of diagnosis. All patients underwent echocardiography and the left ventricular ejection fraction was calculated using the disc summation method. Cardiac catheterization, including coronary angiography and myocardial biopsy, was performed using the Seldinger technique, as described previously.19
In all, 64 patients underwent CMR. A high-intensity area in the myocardium appearing as gadolinium enhancement was considered positive for LGE. Plasma B-type natriuretic peptide (BNP) concentrations were measured using a radioimmunoassay specific for human BNP.
Endpoints
Endpoints were defined as cardiac events, namely VAEs, heart failure-related hospitalization, and all-cause deaths, with VAEs defined as NSVT, VT and ventricular fibrillation. NSVT was defined as spontaneous ventricular tachycardia at a rate of >120 beats/min that did not last >30 s. VT was defined as spontaneous VT at a rate of >120 beats/min that lasted >30 s. Patients were followed from the date of the initial ECG until the first documented date of cardiac events or the end of follow-up. Follow-up information was obtained from patients’ medical records, contact with the patients’ physicians, or interrogation of the ICDs. In patients without baseline fQRS, the association between the new appearance of fQRS and cardiac events was also evaluated.
Statistical Analyses
Continuous variables are presented as the mean±SD or as the median with interquartile range (IQR). Categorical variables are presented as frequencies and percentages. The significance of differences was analyzed using Student’s t-test for continuous variables and the χ2
test for categorical variables. The event-free survival rate was estimated using a Kaplan-Meier analysis, with the significance of differences analyzed using a log-rank test. Predictors of cardiac events were analyzed using Cox proportional hazards analysis and logistic regression analysis. Variables for the univariate analysis were family history, syncope history, massive left ventricular hypertrophy, and fQRS. Hazard ratios (HRs) are presented with 95% confidence intervals (CIs). Statistical analyses were performed using SPSS version 24 (IBM Inc., Armonk, NY, USA) and P<0.05 was considered significant.
Results
Baseline Characteristics of Patients With and Without Baseline fQRS
The clinical characteristics of patients with and without baseline fQRS are given in
Table 1. Data for 146 patients (64% men; mean age 60±16 years, range 14–80 years) were analyzed. Patients’ physical characteristics, medical history, and medications were similar between the 2 groups. However, some differences were noted between the 2 groups. First, pacemaker implantation was performed more often in patients with baseline fQRS. Second, the percentage of patients with right bundle branch block (RBBB) and left bundle branch block (LBBB) was much higher in the group with fQRS, therefore the duration of both QRS and QTc was longer in patients with fQRS (QRS: 124 vs. 101 ms [P<0.001]; QTc: 462 vs. 442 ms [P<0.001]). However, the number of fragmentations per QRS duration was higher in patients with than without baseline fQRS (3.2±0.8 vs. 1.8±1.2 n/100 ms, respectively; P<0.001). The ejection fraction determined with echocardiography was lower in patients with than without fQRS, but was within the normal range in both groups (57% and 62%, respectively; P=0.015). There were no significant differences in plasma BNP concentrations between the 2 groups (377 vs. 297 pg/mL in patients with and without fQRS, respectively; P=0.21).
Table 1.
Characteristics of Patients With and Without Baseline Fragmented QRS
| |
Baseline fQRS |
P-value |
| Yes (n=46) |
No (n=100) |
| Age (years) |
61±16 |
58±16 |
0.39 |
| Male sex |
31 (67) |
63 (63) |
0.60 |
| Body mass index (kg/m2) |
23±3.8 |
23±4.1 |
0.30 |
| Family history |
13 (28) |
17 (17) |
0.12 |
| Syncope history |
6 (13) |
25 (25) |
0.10 |
| Follow-up duration (years) |
6.1±4.6 |
5.0±4.3 |
0.15 |
| Medications |
| ACEI or ARB |
25 (54) |
42 (42) |
0.16 |
| β-blockers |
28 (61) |
56 (56) |
0.58 |
| MRA |
9 (20) |
8 (8.0) |
0.043 |
| Amiodarone |
6 (13) |
5 (5.0) |
0.088 |
| Diuretics |
16 (35) |
23 (23) |
0.13 |
| Plasma BNP (pg/mL) |
377 [13–1,905] |
297 [5–2,126] |
0.21 |
| Electrocardiogram |
| SR |
31 (67) |
85 (85) |
0.014 |
| AF or AFL |
5 (11) |
11 (11) |
0.98 |
| AVB |
1 (2.2) |
0 (0) |
0.14 |
| RBBB |
15 (33) |
7 (7.0) |
<0.001 |
| LBBB |
6 (13) |
0 (0) |
<0.001 |
| PR (ms) |
187±46 |
171±33 |
0.78 |
| QRS (ms) |
124±36 |
101±18 |
<0.001 |
| QTc (ms) |
462±38 |
442±32 |
<0.001 |
| Fragmentations (n/100 ms) |
3.2±0.78 |
1.8±1.2 |
<0.001 |
| Late-potential positive |
7 (44) |
17 (41) |
0.88 |
| Echocardiography |
| LA diameter (mm) |
44±5.5 |
42±8.1 |
0.091 |
| Ejection fraction (%) |
57 [14–84] |
63 [21–93] |
0.015 |
| LVMI (g/m2) |
135±42 |
140±55 |
0.59 |
| Massive LVH (LVMI >100 g/m2) |
39 (85) |
73 (73) |
0.12 |
| E/e’ |
17±7.8 |
17±7.6 |
0.88 |
| LVOTO (pressure gradient >10 mmHg) |
5 (11) |
10 (10) |
0.89 |
| Apical aneurysm |
6 (14) |
9 (9.0) |
0.47 |
| Previous procedure |
| Pacemaker implantation |
7 (15) |
2 (2.0) |
0.002 |
| ICD implantation |
5 (11) |
7 (7.0) |
0.43 |
| Septal ablation |
1 (2.2) |
0 (0) |
0.14 |
| Myectomy |
2 (4.3) |
0 (0) |
0.036 |
| Risk of SCD at 5 years |
2.15 [0.49–18] |
1.89 [0.69–14] |
0.96 |
Data are expressed as the mean±SD, median [interquartile range], or n (%). ACEI, angiotensin-converting enzyme inhibitor; AF, atrial fibrillation; AFL, atrial flutter; ARB, angiotensin II type 1 receptor antagonist; AVB, atrioventricular block; BNP, B-type natriuretic peptide; E/e’, ratio between early mitral inflow velocity and mitral annular early diastolic velocity; fQRS, fragmented QRS; ICD, implantable cardioverter-defibrillator; LA, left atrial; LBBB, left bundle block; LVH, left ventricular hypertrophy; LVMI, left ventricular mass index; LVOTO, left ventricular outflow tract obstruction; MRA, mineralocorticoid receptor antagonist; RBBB, right bundle branch block; SCD, sudden cardiac death; SR, sinus rhythm.
Cardiac events occurred in 29 patients with fQRS and in 32 without fQRS (63% vs. 32%, respectively; P=0.0001;
Supplementary Table). In terms of cardiac events, VAEs (NSVT, VT, ventricular fibrillation) and all-cause death occurred in a significantly larger percentage of patients with than without fQRS (Supplementary Table). There was no significant difference in the frequency of hospitalization for heart failure between the 2 groups (Supplementary Table).
Kaplan-Meier Analysis
Kaplan-Meier analysis showed a significant difference in the frequency of all events and VAEs between patients with and without fQRS (log-rank P=0.001 and 0.001, respectively;
Figure 3). Kaplan-Meier analysis did not show significant differences in the occurrences of hospitalization for heart failure and all-cause death between the 2 groups (log-rank P=0.52 and 0.14, respectively).
Multivariable Analysis
Multivariable analysis showed that baseline fQRS was associated with VAEs (HR 2.59; 95% CI 1.41–4.75; P=0.002), but not with hospitalization for heart failure (HR 0.95; 95% CI: 0.37–2.41; P=0.92;
Figure 4).
LGE With CMR
LGE with CMR was observed more frequently in patients with than without baseline fQRS (17/19 [90%] vs. 29/45 [64%], respectively; P=0.042).
Baseline Characteristics of Patients With and Without New Appearance of fQRS
The clinical characteristics of patients with and without new appearance of fQRS are given in
Table 2. Data were analyzed for 100 patients (63% men; mean age 61±16 years, range 17–80 years). Patients’ physical characteristics, medical history, medications, and follow-up duration were similar in the 2 groups. The follow-up period was 4.6±4.3 years and did not differ between the 2 groups.
Table 2.
Characteristics of Patients With and Without New Appearance of Fragmented QRS
| |
New appearance of fQRS |
P-value |
| Yes (n=33) |
No (n=67) |
| Age (years) |
60±15 |
58±16 |
0.72 |
| Male sex |
25 (76) |
38 (57) |
0.064 |
| Body mass index (kg/m2) |
24 [16–37] |
24 [16–33] |
0.89 |
| Family history |
8 (24) |
9 (13) |
0.18 |
| Syncope history |
12 (36) |
13 (19) |
0.066 |
| Follow-up duration (years) |
4.8±4.2 |
4.4±4.4 |
0.738 |
| Medications |
| ACEI or ARB |
12 (36) |
30 (45) |
0.42 |
| β-blockers |
19 (58) |
37 (55) |
0.82 |
| MRA |
3 (9.1) |
5 (7.5) |
0.78 |
| Amiodarone |
1 (3.0) |
4 (6.0) |
0.53 |
| Diuretics |
13 (19) |
10 (30) |
0.22 |
| Plasma BNP (pg/mL) |
340±477 |
275±329 |
0.43 |
| Electrocardiogram |
| AF or AFL |
6 (18) |
5 (7.5) |
0.11 |
| RBBB |
6 (18) |
1 (1.5) |
0.002 |
| LBBB |
0 (0) |
0 (0) |
– |
| PR (ms) |
182 [110–316] |
165 [122–262] |
0.081 |
| QRS (ms) |
109 [76–188] |
98 [76–122] |
0.029 |
| QTc (ms) |
445±30 |
441±32 |
0.58 |
| Fragmentations (n/100 ms) |
1.8±0.95 |
1.6±0.99 |
0.61 |
| Late-potential positive |
17 (47) |
8 (36) |
0.48 |
| Echocardiography |
| LA diameter (mm) |
42±7.8 |
43±8.3 |
0.79 |
| LA dilatation |
20 (61) |
38 (58) |
0.77 |
| Ejection fraction (%) |
59±13 |
65±9.5 |
0.019 |
| LVMI (g/m2) |
144±64 |
138±9.5 |
0.63 |
| Massive LVH (LVMI >100 g/m2) |
23 (70) |
49 (75) |
0.55 |
| E/e’ |
16±6.1 |
17±8.4 |
0.50 |
| LVOTO (pressure gradient >10 mmHg) |
5 (15) |
13 (19) |
0.60 |
| Apical aneurysm |
3 (9.1) |
6 (9.1) |
1.00 |
| Delayed enhancement in CMR (%) |
8 (57) |
21 (65) |
0.58 |
| Previous procedure |
| Pacemaker implantation |
2 (6.1) |
0 (0) |
0.042 |
| ICD implantation |
6 (18) |
1 (1.5) |
0.002 |
| Risk of SCD at 5 years |
3.7±3.4 |
2.6±3.1 |
0.12 |
Data are expressed as the mean±SD, median [interquartile range], or n (%). CMR, cardiac magnetic resonance (imaging); E/e’, ratio of transmitral Doppler early filling velocity to tissue Doppler early diastolic mitral annular velocity. Other abbreviations as in Table 1.
Regarding previous procedures, pacemaker and ICD implantation were performed more often in patients with new appearance of fQRS than in those without (pacemaker implantation: 6.1% vs. 0% [P=0.042]; ICD implantation: 18% vs. 1.5% [P=0.002]). No patients in either group underwent septal ablation or myectomy.
Plasma BNP concentrations did not differ between patients with and without new appearance of fQRS (340 vs. 275 pg/mL, respectively; P=0.43). Ejection fraction determined by echocardiography was within the normal range in both groups, but lower in patients with new appearance of fQRS (59% vs. 65%, respectively; P=0.019). The percentage of patients with LVOTO and massive left ventricular hypertrophy did not differ between those with and without new fQRS (LVOTO: 5% vs. 13%, respectively [P=0.60]; massive left ventricular hypertrophy: 70% vs. 75%, respectively [P=0.55]).
With regard to ECG parameters, the percentage of patients with RBBB was higher in the group with than without new appearance of fQRS (18% vs. 1.5%, respectively; P=0.068), but LBBB was not seen in either group. Thus, QRS duration was slightly longer in patients with than without new appearance of fQRS (109 vs. 98 ms, respectively; P=0.029), but QTc duration did not differ between the 2 groups (445 vs. 441 ms, respectively; P=0.58). The number of fragmentations per QRS duration in the initial ECG did not differ between the group with and that without new appearance of fQRS (1.8±1.0 vs. 1.6±1.0 n/100 ms, respectively; P=0.61). In addition, we evaluated the variability and reproducibility of fQRS through repeated recording of ECGs. The number of fragmentations was counted in patients without fQRS at baseline and during short-term follow-up. Fifty-one patients without baseline fQRS had repeated follow-up ECGs within 3 months, and the median duration until repeated ECG was 25 days (IQR 2–84 days). The numbers of fragmentations did not differ at the 2 time points (1.8±1.0 vs. 1.8±0.9, respectively; P=0.57).
Except for minor differences in the percentage of patients with RBBB, QTc duration, and ejection fraction, the characteristics in the 2 groups at follow-up were similar to those seen at baseline.
Association Between VAEs and New Appearance of fQRS
Among the 100 patients without baseline fQRS, the new appearance of fQRS was seen in 33. During the 4.6-year follow-up, VAEs and heart failure-related hospitalization were seen more frequently in the 33 patients with new appearance of fQRS (n=14) than in those without (n=9; VAEs: 42% vs. 13%, respectively [P=0.001]; heart failure-related hospitalization: 24% vs. 7.5%, respectively [P=0.019]). However, all-cause death did not differ between the 2 groups (9.1% vs. 4.5% in those with vs. without new fQRS, respectively; P=0.36).
Time-dependent associations were evaluated the between new appearance of fQRS documented before VAEs and the occurrence of VAEs. Kaplan-Meier analysis showed a significant difference in the frequency of VAEs between patients with and without new appearance of fQRS (log-rank P<0.001;
Figure 5). Multivariable analysis showed that new appearance of fQRS documented before VAEs was associated with VAEs (HR 4.29; 95% CI 1.81–10.2; P=0.001;
Table 3).

Table 3.
ORs for the Occurrence of VAEs
| |
Univariate |
Multivariate |
| OR (95% CI) |
P-value |
OR (95% CI) |
P-value |
| Age (over 65 years) |
1.18 (0.59–2.37) |
0.65 |
|
|
| Male sex |
0.89 (0.43–1.81) |
0.74 |
|
|
| Family history |
2.52 (1.11–5.72) |
0.03 |
1.08 (0.40–2.94) |
0.88 |
| Massive LVH |
1.48 (0.63–3.49) |
0.36 |
|
|
| Syncope history |
1.39 (0.61–3.16) |
0.44 |
|
|
| LVOTO |
0.68 (0.25–1.86) |
0.45 |
|
|
| LA dilatation |
2.02 (0.95–4.28) |
0.65 |
|
|
| New appearance of fQRS |
4.75 (1.77–12.7) |
0.001 |
4.29 (1.81–10.2) |
0.001 |
All variables with a significant association in univariate analysis were included in the multivariate analysis. The odds ratio (OR), 95% confidence interval (CI), and P-value are given for each independent variable. VAEs, ventricular arrhythmic events. Other abbreviations as in Table 1.
Discussion
In this study we investigated associations between baseline fQRS and cardiac events, as well as the new appearance of fQRS and cardiac events in HCM patients. The results showed that baseline fQRS was associated with the occurrence of VAEs and all-cause death, but not with heart failure-related hospitalization. Baseline fQRS also corresponded well with CMR findings with LGE. In patients without fQRS, the new appearance of fQRS during the follow-up period was associated with VAEs and heart failure-related hospitalization.
Although previous studies have shown that fQRS in Brugada syndrome and myocardial infarction are associated with VAEs,9
the association between cardiac events and fQRS in HCM patients has not been clarified. A recent meta-analysis showed that baseline fQRS significantly increased the risk of major arrhythmic events in HCM patients.16
An observational study showed that a myocardial scar visualized by CMR with LGE predicted major adverse events in HCM patients,10
but the cost and availability of CMR prevents repetitive testing during long-term follow-up. Thus, more convenient and safer clinical measures to assess cardiac event risks in HCM patients are needed. fQRS is expected to be a useful predictor of VAEs because fQRS can be obtained from a standard normal resting ECG; however, problems remain for patients without baseline fQRS. In some HCM patients without baseline fQRS, we saw the new appearance of fQRS during follow-up. An association between the new appearance of fQRS and adverse events has been reported in adult congenital heart disease,20
and 1 case report discussed an HCM patient without baseline fQRS who developed VAEs with the new appearance of fQRS 7 years after the initial ECG.17
To use fQRS as a risk predictor for HCM patients, the association between the new appearance of fQRS and VAEs must be clarified.
The new appearance of fQRS occurred in 33% of patients without fQRS during the 4.6-year follow-up. The appearance of fQRS was associated with VAEs and hospitalization for heart failure. The incidence of VAEs in patients with new appearance of fQRS was as high as that in patients with baseline fQRS (i.e., on the initial ECG; 42% vs. 52%, respectively). If the appearance of fQRS indicates progression of myocardial injury and increased risk of VAE and SCD, it may be possible to determine whether ICD implantation is required by evaluating normal follow-up ECGs.
Regarding heart failure, the new appearance of fQRS was associated with heart failure-related hospitalization (24% vs. 7.5% in those with and without fQRS, respectively; P=0.019), although baseline fQRS was not associated with heart failure-related hospitalization (20% vs. 13%, respectively; P=0.30) despite the lower ejection fraction. This may be explained by the size, distribution, and rate of progression of regional scars relative to the entire ventricular myocardium. Chronic scars representing baseline fQRS may be too small and too widely distributed to affect global left ventricular function to result in heart failure-related hospitalization. However, secondary to progression, the emergence of minor regional scars representing the new appearance of fQRS could have caused the heart failure events.
Study Limitations
Several limitations of this study should be considered. First, this was a single-center retrospective study that involved only 146 Japanese patients with HCM. Further investigation in a larger population is needed to definitively determine the association between VAEs and fQRS in HCM patients. Second, confounding factors may have existed between patients with and without fQRS in our cohort. There were differences in physiological functional test results and medications between the 2 groups, especially regarding ejection fraction and ECG parameters. Third, variability in the interpretation of fQRS is possible, but we used special precautions in this study to ensure that the ECG interpretations by the 3 reviewers were consistent with the fQRS criteria. Fourth, we did not show a clear mechanism for the relationship between fQRS and SCD risk. This study showed that VAEs in HCM patients were associated with both the presence and appearance of fQRS, but not with SCD. Nevertheless, we believe that identifying fQRS in a single normal ECG could be a useful clue to preventing SCD in HCM patients. Fifth, we did not analyze QRS fragmentations with differential or double differential values in this study. Because QRS fragmentations were smaller changes in the ECG, analysis with differential or double differential values could have helped us evaluate fQRS more scientifically. Further studies are needed to clarify this point.
Conclusions
The new appearance of fQRS in patients without baseline fQRS was a predictor of VAEs and myocardial injury in HCM patients.
Acknowledgements
The authors thank Miyuki Fujiwara and Masayo Ohmori for their excellent technical assistance and Nancy Schatken and Jane Charbonneau from the Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Sources of Funding
This study did not receive any specific funding.
Conflict of Interests
None of the authors has any financial disclosures that are relevant to this article. H.I. is a member of the Editorial Board of
Circulation Journal.
Supplementary Files
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-19-0968
References
- 1.
Miura K, Nakagawa H, Morikawa Y, Sasayama S, Matsumori A, Hasegawa K, et al. Epidemiology of idiopathic cardiomyopathy in Japan: Results from a nationwide survey. Heart 2002; 87: 126–130.
- 2.
McKenna W, Deanfield J, Faruqui A, England D, Oakley C, Goodwin J. Prognosis in hypertrophic cardiomyopathy: Role of age and clinical, electrocardiographic and hemodynamic features. Am J Cardiol 1981; 47: 532–538.
- 3.
Elliott PM, Gimeno JR, Thaman R, Shah J, Ward D, Dickie S, et al. Historical trends in reported survival rates in patients with hypertrophic cardiomyopathy. Heart 2006; 92: 785–791.
- 4.
O’Mahony C, Jichi F, Ommen SR, Christiaans I, Arbustini E, Garcia-Pavia P, et al. International external validation study of the 2014 European Society of Cardiology Guidelines on Sudden Cardiac Death Prevention in Hypertrophic Cardiomyopathy (EVIDENCE-HCM). Circulation 2018; 137: 1015–1023.
- 5.
Elliott P, McKenna WJ. Hypertrophic cardiomyopathy. Lancet 2004; 363: 1881–1891.
- 6.
Elliott PM, Poloniecki J, Dickie S, Sharma S, Monserrat L, Varnava A, et al. Sudden death in hypertrophic cardiomyopathy: Identification of high risk patients. J Am Coll Cardiol 2000; 36: 2212–2218.
- 7.
Wang W, Lian Z, Rowin EJ, Maron BJ, Maron MS, Link MS. Prognostic implications of nonsustained ventricular tachycardia in high-risk patients with hypertrophic cardiomyopathy. Circ Arrhythm Electrophysiol 2017; 10: e004604.
- 8.
Adabag AS, Casey SA, Kuskowski MA, Zenovich AG, Maron BJ. Spectrum and prognostic significance of arrhythmias on ambulatory Holter electrocardiogram in hypertrophic cardiomyopathy. J Am Coll Cardiol 2005; 45: 697–704.
- 9.
Morita H, Watanabe A, Morimoto Y, Kawada S, Tachibana M, Nakagawa K, et al. Distribution and prognostic significance of fragmented QRS in patients with Brugada syndrome. Circ Arrhythm Electrophysiol 2017; 10: e004765.
- 10.
Ratheendran AC, Subramanian M, Bhanu DK, Prabhu MA, Kannan R, Natarajan KU, et al. Fragmented QRS on electrocardiography as a predictor of myocardial scar in patients with hypertrophic cardiomyopathy. Acta Cardiol, doi:10.1080/00015385.2018.1547355.
- 11.
Debonnaire P, Katsanos S, Joyce E, Van Den Brink OV, Atsma DE, Schalij MJ, et al. QRS fragmentation and QTc duration relate to malignant ventricular tachyarrhythmias and sudden cardiac death in patients with hypertrophic cardiomyopathy. J Cardiovasc Electrophysiol 2015; 26: 547–555.
- 12.
Femenia F, Arce M, Van Grieken J, Trucco E, Mont L, Abello M, et al. Fragmented QRS as a predictor of arrhythmic events in patients with hypertrophic obstructive cardiomyopathy. J Interv Card Electrophysiol 2013; 38: 159–165.
- 13.
Kang KW, Janardhan AH, Jung KT, Lee HS, Lee MH, Hwang HJ. Fragmented QRS as a candidate marker for high-risk assessment in hypertrophic cardiomyopathy. Heart Rhythm 2014; 11: 1433–1440.
- 14.
Nomura A, Konno T, Fujita T, Tanaka Y, Nagata Y, Tsuda T, et al. Fragmented QRS predicts heart failure progression in patients with hypertrophic cardiomyopathy. Circ J 2015; 79: 136–143.
- 15.
Ozyilmaz S, Akgul O, Uyarel H, Pusuroglu H, Karayakali M, Gul M, et al. Assessment of the association between the presence of fragmented QRS and the predicted risk score of sudden cardiac death at 5 years in patients with hypertrophic cardiomyopathy. Anatol J Cardiol 2017; 18: 54–61.
- 16.
Rattanawong P, Riangwiwat T, Kanitsoraphan C, Chongsathidkiet P, Kanjanahattakij N, Vutthikraivit W, et al. Baseline fragmented QRS increases the risk of major arrhythmic events in hypertrophic cardiomyopathy: Systematic review and meta-analysis. Ann Noninvasive Electrocardiol 2018; 23: e12533.
- 17.
Femenia F, Arce M, Arrieta M, Baranchuk A. Surface fragmented QRS in a patient with hypertrophic cardiomyopathy and malignant arrhythmias: Is there an association? J Cardiovasc Dis Res 2012; 3: 32–35.
- 18.
Richardson P, McKenna W, Bristow M, Maisch B, Mautner B, O’Connell J, et al. Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the Definition and Classification of cardiomyopathies. Circulation 1996; 93: 841–842.
- 19.
Nakamura K, Kusano KF, Matsubara H, Nakamura Y, Miura A, Nishii N, et al. Relationship between oxidative stress and systolic dysfunction in patients with hypertrophic cardiomyopathy. J Card Fail 2005; 11: 117–123.
- 20.
Helsen F, Vandenberk B, De Meester P, Van De Bruaene A, Gabriels C, Troost E, et al. Appearance of QRS fragmentation late after Mustard/Senning repair is associated with adverse outcome. Heart 2017; 103: 1036–1042.