2020 Volume 84 Issue 3 Pages 479-486
2020 Volume 84 Issue 3 Pages 479-486
Background: Aldehyde dehydrogenase 2 (ALDH2) plays a central role in the biotransformation of glyceryl trinitrate (GTN) or nitroglycerin, which is widely used for the treatment of coronary artery disease (CAD). The deficient variant ALDH2 genotype (ALDH2*2) is prevalent among East Asians. This study examined whether there are differences in nitroglycerine-mediated dilation (NMD) and flow-mediated dilation (FMD) response between wild ALDH2*1/*1 and variant ALDH2*2 patients with CAD.
Methods and Results: The study subjects comprised 55 coronary spastic angina (CSA) patients, confirmed by coronary angiography and intracoronary injection of acetylcholine (42 men and 13 women, mean age 68.0±9.0 years). They underwent NMD and FMD tests in the morning before and after continuous transdermal GTN administration for 48 h. NMD was lower at baseline in ALDH2*2 than in the ALDH2*1/*1 group (P=0.0499) and decreased significantly in both groups (P<0.0001 and P<0.0001, respectively) after GTN, with significantly lower levels in the ALDH2*2 group (P=0.0002). FMD decreased significantly in both ALDH2*1/*1 and ALDH2*2 groups (P<0.0001and P=0.0002, respectively) after continuous GTN administration, with no significant differences between the 2 groups both before and after GTN.
Conclusions: Continuous administration of GTN produced endothelial dysfunction as well as nitrate tolerance in both ALDH2*1/1 and ALDH2*2 patients with CSA. ALDH2*2 attenuated GTN response and exacerbated GTN tolerance, but not endothelial dysfunction, as compared to ALDH2*1/*1 in patients with CSA.
Organic nitrates, including nitroglycerin or glyceryl trinitrate (GTN), have been widely used in the treatment of coronary artery disease (CAD) and heart failure.1,2 However, development of tolerance limits their usefulness and precludes continuous administration to patients.1–4 The mechanisms by which nitrate tolerance develops are still not well understood. Nitrates are metabolized to produce bioactive metabolites leading to release of nitric oxide (NO), which causes vasodilatation and inhibition of platelet activation.1–4 Mitochondrial aldehyde dehydrogenase 2 (ALDH2) is an enzyme detoxifying toxic aldehydes, including lipid peroxidation-derived aldehydes such as 4-HNE, as well as ethanol-derived acetaldehyde.5 Recent studies have revealed that ALDH2 also plays a crucial role in the biotransformation of GTN and the development of nitrate tolerance and endothelial dysfunction.2–4,6 There are polymorphisms in the ALDH2 gene, and variant ALDH2*2 genotype-encoding deficient enzyme activity is prevalent in up to 40% among East Asians and is associated with alcohol flushing syndrome.7–9 Coronary spastic angina (CSA) or angina pectoris caused by coronary spasm is a common CAD among East Asians including Japanese,10,11 and we and others have shown that ALDH2*2 is significantly associated with CAD, including CSA and acute myocardial infarction (AMI).9,11–13 In the present study, we examined whether the variant ALDH2*2 genotype is more susceptible to nitrate tolerance and endothelial dysfunction compared to the wild ALDH2*1/*1 genotype in patients with CSA.
Editorial p 384
The study subjects consisted of consecutive 55 Japanese CSA patients (42 men and 13 women, mean age of 68.0±9.0 years) diagnosed by intracoronary injection of acetylcholine (ACh), as well as by coronary angiography, subjective symptoms, and electrocardiographic ischemic changes, from August 2017 to August 2019 at our institution (Kumamoto Kinoh Hospital). Patients with AMI, 3-vessel organic disease, left main trunk lesion, history of coronary artery bypass surgery, uncontrolled arrhythmias, heart failure, resting hypertension >180/110 mmHg, acute systemic illness, and hepatic or renal insufficiency, malignancy or other severe conditions, were excluded from the study. Patients with severe headache or discomfort on continuous GTN administration (4 men with ALDH2*2 and 1 woman with ALDH2*1/*1) also were excluded from the nitrate tolerance study. All vasoactive medications including calcium channel blockers, β-receptor blockers, angiotensin-converting enzyme inhibitors, angiotensin II receptors blockers, statins and aspirin were withdrawn for at least 3 days before the angiography, except for GTN, which was used for attacks.
This study was conducted in accordance with the Declaration of Helsinki and approved by the ethics committee of our institution, and written informed consent was obtained from each patient.
Angiographic Documentation of Coronary SpasmCoronary spasm was defined as a transient total or subtotal occlusion or severe diffuse vasoconstriction of an epicardial coronary artery associated with ischemic changes on ECG with or without chest discomfort.9–11 Coronary spasm was induced by the intracoronary injection of ACh after diagnostic catheterization in the morning. The details of the method were reported previously.9–11 Significant organic coronary stenosis was defined as >50% luminal diameter.
Assessment of Flow-Mediated Dilation (FMD) and Nitroglycerine-Mediated Dilation (NMD)Vascular responses to reactive hyperemia and sublingual-administrated nitroglycerin in the brachial artery have been used for assessment of endothelial-dependent and nitroglycerin-mediated vasodilation, respectively, and guidelines have been published.14–17 Accordingly, the study subjects fasted the previous night and abstained from alcohol, smoking, caffeine, and exercise for at least 12 h. The study began at 8:00 AM and the subjects were kept in the supine position for 10–15 min in a quiet, dark, air-conditioned room (constant temperature of 22–25℃) throughout the study. The baseline brachial artery diameter was measured for >30 s, and an occlusion cuff was placed around the forearm, with the proximal edge of the cuff at the elbow. The cuff was inflated to a compression pressure of 50 mmHg over the systolic pressure for 5 min. Post-deflation diameter was monitored continuously from deflation for >3 min. Both FMD and NMD were assessed in the left arm to avoid the possible brachial endothelial injury effect by cardiac catheterization test. A high-resolution linear artery transducer was coupled to computer-assisted analysis software (UNEXEF 38G, UNEX Co, Nagoya, Japan) using an automated edge detection system for measurement of brachial artery diameter.15 In this system, the diastolic diameter of the brachial artery is determined semi-automatically and the changes of the diameter are tracked automatically. When the clearest B-mode image of the anterior and posterior intimal interfaces between the lumen and vessel wall was obtained, the transducer was held at the same point throughout the scan by a special probe holder (UNEX Co, Nagoya, Japan) to ensure consistency of the image. FMD was automatically calculated as the percentage change in peak vessel diameter from the baseline value.
After the FMD test, the recovery of the brachial artery diameter to the basement diameter was confirmed after waiting for 20–30 min. Sublingual GTN spray (0.3 mg) was then given to the patient and the brachial artery diameter was automatically and continuously documented from the maximum to the basement diameter during about 10 min after nitrate. The value was calculated as the percentage change (%) in peak vessel diameter from the baseline value. After the completion of the FMD and NMD tests, the study subjects received continuous GTN transdermal patch treatment (Nitroderm TTS 25 mg) for 2 days, and the tests were again performed in the similar conditions in the morning.
The examination was performed by the sole and experienced sonographer (I.K.), who was blinded to the genotype of the patients. The coefficient of variation (CV) for diameter and FMD were 1.2% and 8.7%, respectively.
GenotypingThe details of the method are reported elsewhere.18 Briefly, single-nucleotide polymorphism genotyping of ALDH2 (Glu504Lys; rs671) was performed using the TaqMan assay on an ABI 7300 Real Time polymerase chain reaction (PCR) System (Applied Biosystems, Foster City, CA, USA) without DNA extraction on whole blood. The mixture was 20 μL and consisted of 10 μL of a Thunderbird Probe qPCR Mix (QPS-101, Toyobo, Osaka, Japan), 0.4 μL of a ×50 ROX reference dye (Toyobo), 1 μL of a ×20 ADH1B TaqMan Probe and ADH1B Primer Mix (C_2688467_20; TaqMan Drug Metabolism Genotyping Assays, Applied Biosystems) or a ×20 ALDH2 TaqMan Probe and ALDH2 Primer Mix (C_11703892_10, Applied Biosystems), 2 μL of each polymerase chain reaction product, and 6.6 μL of distilled water. The thermal cycling process was performed according to the Applied Biosystems PCR conditions: 2 min at 50℃, 10 min at 95℃, 40 cycles of denaturation at 95℃ for 15 s and annealing and extension at 60℃ for 1 min. The results were analyzed by ABI Prism 7300 SDS software.
Questionnaire SurveyThe subjects were asked to complete a simple questionnaire concerning alcohol flushing on alcohol intake, alcohol drinking habit, and smoking. A habitual drinker was defined as an alcohol drinker more than 5 days a week. Alcohol flushing was defined as a current or a history of facial flushing immediately after drinking a glass of beer (ethanol 10 g) or the equivalent of alcoholic beverages. Smokers were defined as current and past smokers.
Blood Chemistry MeasurementsBlood samples for measurement of clinical chemistry and other data were collected after an overnight fast with the patients in the supine position. The biochemical and other analyses were conducted using standard laboratory procedures.
Statistical AnalysisAllele frequencies were determined by direct gene counting, and genotype distributions were checked for departure from Hardy-Weinberg equilibrium using the Pearson Chi-squared test. The baseline clinical data were expressed as the mean±SD or median (25th, 75th percentile) for continuous variables, and differences within the group were evaluated with an unpaired t-test or the Mann-Whitney rank sum test. For discrete variables, the data were expressed as counts and percentages and analyzed with the Chi-squared test. A 2-tailed value of P<0.05 was considered to be statistically significant. The analyses were conducted using the STATA software program (STATA 16.0, STATA Corp., College Station, TX, USA).
Genotype distributions did not depart from the Hardy-Weinberg equilibrium for ALDH2 genes in the whole 55 CSA patients (χ2=0.669; P=0.413). Frequencies of ALDH2*1/*1, ALDH2*1/*2 and ALDH2*2/*2 genotype were 56.4% (31/55), 34.5% (19/55) and 9.1% (5/55), respectively. Frequency of the ALDH2*2 allele was thus 26.4% (29/110) in the CSA patients in this study. This is high as compared to that of the general population in Japan (23.5% [247/1,050] in Kumamoto and 24.3% [183/752] in Tokyo, Japan),7 but did not reach the significant level probably because of the small number of the study patients as compared with our previous studies.9,13 This study also indicates that the variant ALDH2*2 genotype exists mainly as the heterozygote (ALDH2*1/*2), and we combined the heterozygote (ALDH2*1/*2) and homozygote of (ALDH2*2/*2) as a single category of variant ALDH2*2 and compared them with the wild homozygote ALDH2*1/*1 in the following analysis.
Comparison of Clinical Characteristics of the Study Patients Between ALDH2*1/*1 and ALDH2*2 GroupTable 1 compares the clinical characteristics between the patients in the ALDH2*2 and ALDH2*1/*1 groups. There were no significant differences in variables including age, gender, BMI, blood pressure, comorbidities, smoking, coronary organic stenosis, biochemical parameters, and pre-admission medications between the 2 groups, with the exception of alcohol flushing and habitual drinking (%), which were significantly lower (P<0.001 and P=0.003, respectively) in the ALDH2*2 group as compared to the ALDH2*1/*1 group. The finding is thus in line with Mendelian randomization implying that the ALDH2*2 allele is randomly assigned at conception independently of the possible confounding factors,19,20 and is associated with alcohol flushing syndrome and lower alcohol intake habit compared to the ALDH2*1/*1 allele.8,9,13
| Variables | ALDH2*2 (Variant) (n=24) |
ALDH2*1/*1 (Wild) (n=31) |
P value |
|---|---|---|---|
| Age, years | 69.7±8.7 | 67.5±8.7 | 0.36 |
| Gender (Male), n (%) | 18 (75.0) | 24 (77.4) | 0.83 |
| BMI, kg/m2 | 24.4±2.7 | 24.5±3.7 | 0.90 |
| Systolic BP, mmHg | 127±19 | 136±21 | 0.11 |
| Diastolic BP, mmHg | 78±12 | 83±13 | 0.12 |
| Heart rate, beats/min | 58.5±10.3 | 60.1±7.5 | 0.52 |
| Total protein, mg/dL | 6.9±0.4 | 6.9±0.6 | 0.83 |
| hs-CRP, mg/L | 0.61 (0.2, 1.78) | 0.47 (0.31, 1.75) | 0.82 |
| Glucose, mg/dL | 111 (103, 117) | 109 (99, 127) | 0.99 |
| Total cholesterol, mg/dL | 194 (172, 208) | 195 (172, 225) | 0.60 |
| Triglycerides, mg/dL | 121 (99, 142) | 107 (80, 191) | 0.87 |
| HDL-C, mg/dL | 59 (49, 67) | 56 (42, 69) | 0.39 |
| LDL-C, mg/dL | 103 (92, 123) | 100 (62, 139) | 0.46 |
| Creatinine, mg/dL | 0.96±0.42 | 0.83±0.13 | 0.11 |
| Uric acid, mg/dL | 5.1 (3.9, 6.6) | 5.7 (4.9, 6.5) | 0.56 |
| BNP, pg/mL | 34 (27, 68) | 30 (13, 65) | 0.32 |
| Leukocyte, /μL | 5,250 (4,700, 6,300) | 5,800 (4,700, 6,800) | 0.41 |
| Hemoglobin, g/dL | 14.5±1.6 | 14.3±1.7 | 0.62 |
| Platelets, ×104/μL | 19.7±4.2 | 21.9±5.9 | 0.13 |
| Hypertension, n (%) | 7 (29.2) | 12 (38.7) | 0.46 |
| Hyperlipidemia, n (%) | 8 (33.3) | 13 (43.3) | 0.45 |
| Diabetes mellitus, n (%) | 1 (4.2) | 4 (12.9) | 0.26 |
| CHF, n (%) | 1 (4.2) | 4 (12.9) | 0.26 |
| Smokers, n (%) | 16 (66.7) | 23 (74.2) | 0.54 |
| Alcohol flushing, n (%) | 22 (91.7) | 10 (32.3) | <0.001 |
| Habitual drinking, n (%) | 9 (37.5) | 24 (70.6) | 0.003 |
| Organic vessels, n (%) | 4 (16.7) | 10 (32.3) | 0.19 |
| LAD stenosis, n (%) | 4 (16.7) | 7 (22.6) | 0.73 |
| CX stenosis, n (%) | 0 (0.0) | 2 (6.5) | 0.29 |
| RCA stenosis, n (%) | 2 (8.3) | 4 (12.9) | 1.00 |
| ACEI, n (%) | 3 (12.5) | 6 (19.4) | 0.50 |
| ARB, n (%) | 5 (20.8) | 7 (22.6) | 0.88 |
| Aspirin, n (%) | 4 (16.7) | 8 (25.8) | 0.42 |
| Ca antagonist, n (%) | 6 (25.0) | 6 (19.4) | 0.62 |
| Nitrates, n (%) | 1 (4.2) | 5 (16.1) | 0.16 |
| β-blocker, n (%) | 4 (16.7) | 4 (12.9) | 0.69 |
| Statin, n (%) | 8 (33.3) | 10 (32.3) | 0.93 |
| Diuretics, n (%) | 5 (20.8) | 3 (9.7) | 0.24 |
| Pioglitazone, n (%) | 0 (0.0) | 2 (6.5) | 0.20 |
| SGLT2 inhibitors, n (%) | 1 (4.2) | 3 (9.7) | 0.44 |
ACEI/ARB, angiotensin-converting enzyme inhibitors/angiotensin-II receptor blockers; AFS, alcohol flushing syndrome; ALDH2, aldehyde dehydrogenase type 2; BMI, body mass index; BNP, B-type natriuretic peptide; BP, blood pressure; CHF, congestive heart failure; CKD, chronic kidney disease; CPK, creatine phosphokinase; CX, circumflex; EF, ejection fraction; hs-CRP, high sensitivity C-reactive protein; HDL, high-density lipoprotein; LAD, left coronary artery; LDL, low-density lipoprotein; RCA, right coronary artery; SGLT2 inhibitors, Sodium-glucose co-transporter-2.
There were no significant differences in blood pressure, heart rate, FMD, and FMD-related data between the ALDH2*2 and ALDH2*1/*1 groups at baseline except NMD, which was lower in the ALDH2*2 group (P=0.0499) (Table 2 and Figure).
| Variables | ALDH2*2 (Variant) (n=24) |
ALDH2*1/*1 (Wild) (n=31) |
P value |
|---|---|---|---|
| Systolic BP (mmHg) | |||
| Before | 127.1±18.5 | 135.8±21.2 | 0.11 |
| After | 125.2±14.8 | 132.2±19.4 | 0.15 |
| Diastolic BP (mmHg) | |||
| Before | 77.9±11.8 | 83.2±12.7 | 0.24 |
| After | 76.5±9.7 | 84.0±16.0 | 0.0495 |
| Heart rate (beats/min) | |||
| Before | 58.5±10.3 | 60.1±17.5 | 0.89 |
| After | 63.3±10.6* | 63.5±14.8 | 0.94 |
| NMD (%) | |||
| Before | 17.7 (13.7, 21.9) | 21.4 (17.2, 25.3) | 0.0499 |
| After | 6.0 (2.2, 10.4)** | 12.2 (9.0, 17.0)** | 0.0002 |
| Base dimen. of NMD (mm) | |||
| Before | 3.90±0.57 | 3.88±0.43 | 0.86 |
| After | 4.61±0.73** | 4.38±0.56** | 0.19 |
| Max. dimen. of NMD (mm) | |||
| Before | 4.59±0.60 | 4.71±0.43 | 0.41 |
| After | 4.89±0.67** | 4.92±0.55** | 0.83 |
| FMD (%) | |||
| Before | 5.3 (3.5, 7.1) | 4.9 (2.8, 7.5) | 0.72 |
| After | 2.6 (1.2, 4.0)* | 3.0 (1.7, 4.6)** | 0.24 |
| Base dimen. (mm) | |||
| Before | 3.87±0.58 | 3.84±0.45 | 0.83 |
| After | 4.57±0.74** | 4.37±0.54** | 0.25 |
| Max. dimen. (mm) | |||
| Before | 4.08±0.60 | 4.04±0.41 | 0.74 |
| After | 4.68±0.74** | 4.51±0.49** | 0.31 |
BP, blood pressure; dimen., dimension. *P<0.001 vs. before value, *P<0.0001 vs. before value.
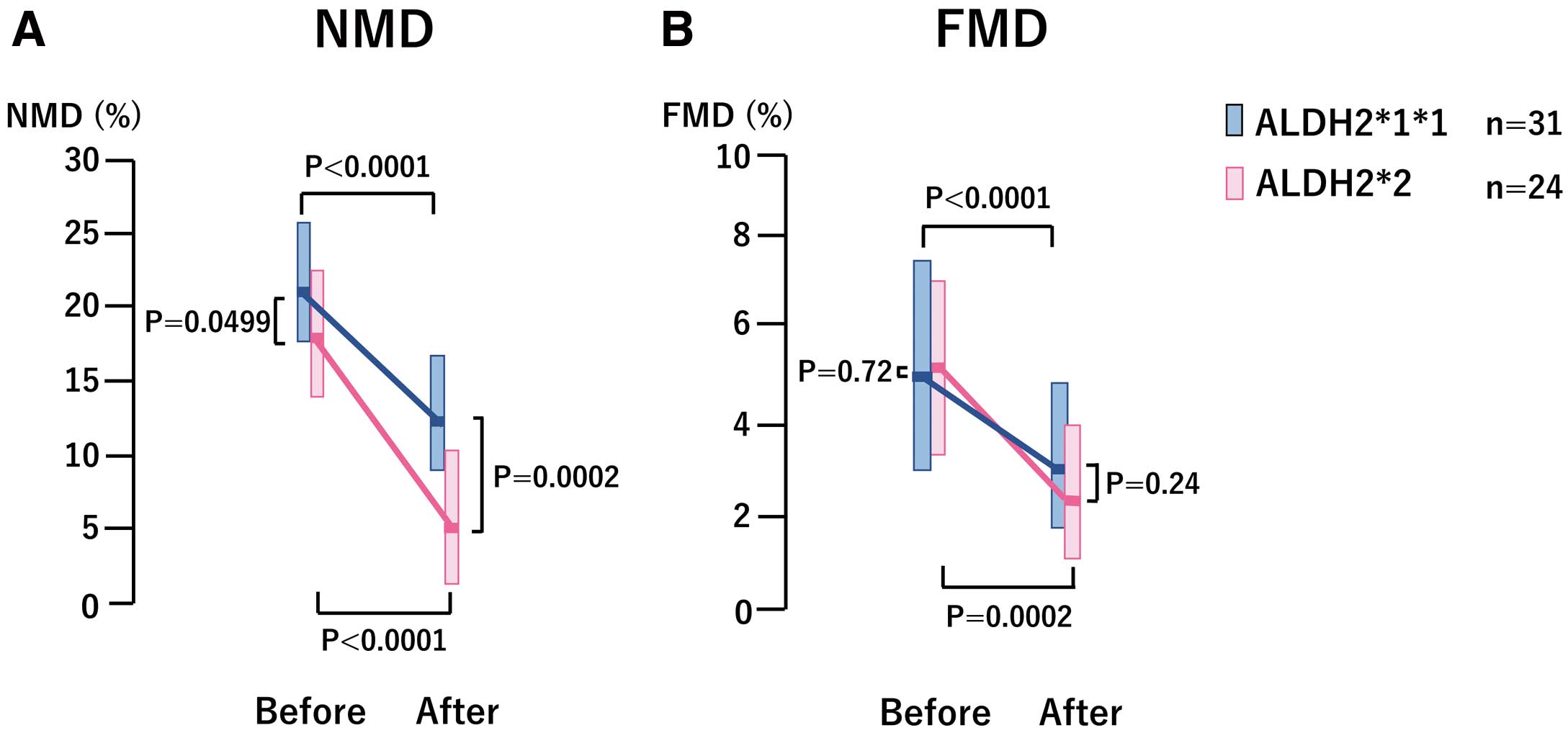
Comparison of nitroglycerin-mediated dilation (NMD) and flow-mediated dilation (FMD) between the ALDH2*2 and ALDH2*1/*1 groups before and after continuous administration of glyceryl trinitrate (GTN). (A) Both the ALDH2*2 and ALDH2*1/*1 groups developed attenuated NMD response or tolerance to continuous GTN administration. ALDH2*2 patients had lower NMD at baseline and developed more attenuated NMD response to continuous GTN administration compared to those with ALDH2*1/*1. (B) FMD or endothelium-dependent dilation decreased significantly in both the ALDH2*1/*1 and ALDH2*2 groups after the continuous GTN administration, indicating that endothelial dysfunction developed by continuous GTN administration. There were no significant differences in FMD or endothelial dysfunction between the ALDH2*1/*1 and ALDH2*2 groups both before and after continuous GTN administration.
After continuous GTN administration, NMD decreased significantly in both groups (from 17.7 [13.4, 21.9] to 6.0 [2.2, 10.4], P<0.0001 for the ALDH2*2 group and from 21.4 [17.2, 25.3] to 12.2 [9.0, 17.0], P<0.0001 for the ALDH2*1/*1 group, respectively), but the values were significantly lower after continuous GTN administration in the ALDH2*2 group compared to the ALDH2*1/*1 group (6.0 [2.2, 10.4] vs. 12.2 [9.0, 17.0], P=0.0002) (Table 2 and Figure).
The NMD change rate (%) after continuous GTN was significantly decreased in the ALDH2*2 group compared with the ALDH2*1/*1 group (−67.9 [−38.7, −90.1] vs. 39.9 [−17.6, −60.9], P=0.0081), but the FMD change rate (%) did not change significantly (−42.9 [−23.5, −78.3) vs. −42.3 [−17.1, −56.8], P=0.32). These findings indicated that nitrate tolerance developed in both groups after continuous GTN administration, and that the tolerance was higher in the ALDH2*2 group.
The FMD decreased significantly in both the ALDH2*2 and ALDH2*1/*1 groups (from 5.3 [3.5, 7.1] to 2.6 [1.2, 4.0], P=0.0002, and from 4.9 [2.8, 7.5] to 3.0 [1.7, 4.6], P<0.0001, respectively), and there were no significant differences in the values between the 2 groups both before and after continuous GTN administration (P=0.72 and P=0.24, respectively) (Table 2 and Figure).The findings thus indicated that endothelial dysfunction developed in both groups after continuous GTN administration with no significant differences between the 2 groups.
Adverse Effects of GTNHeadache in the continuous GTN administration group precluded 5 CSA patients (4 men with ALDH2*2 and 1 woman with ALDH2*1/*1) from participating in the study.
Organic nitrates such as GTN have been widely used in the treatment of CAD, but development of side-effects such as tolerance and endothelial dysfunction limits their usefulness and precludes continuous administration to patients.1–4 GTN causes vasodilation through formation of NO, with subsequent activation of soluble guanylyl cyclase (sGC), resulting in accumulation of cGMP in vascular smooth muscle.1–4 ALDH2 is an enzyme detoxifying toxic aldehydes, including lipid peroxidation-derived aldehydes such as 4-HNE, as well as ethanol-derived acetaldehyde.5 There are polymorphisms in the ALDH2 genes and the variant mitochondrial ALDH2 genotype, with a substitution of glutamate to lysine at position 504 (Glu504Lys) or ALDH2*2 is present in up to 40% of East Asians, exerting a dominant negative effect over wild-type homozygote ALDH2*1/*1 and a severely reduced ALDH2 activity.5,7,8,21 The carriers of ALDH2*2 thus manifest the characteristic alcohol flushing syndrome caused by accumulation of acetaldehyde and are intolerant of alcohol.5,7,8 ALDH2*2 genotype is a risk factor for CAD in East Asians including Japanese, Koreans and Chinese.9,11–13 CSA is a common CAD among East Asians including Japanese,10,11 and we have shown that ALDH2*2 is causally associated with both CSA and AMI.9,11–13
ALDH2 also is shown to play a key role in the biotransformation of GTN to NO, activating sGC and leading to vascular relaxation at clinically relevant concentrations or a high-potency pathway.1–6 Recent experimental studies have revealed that ALDH2 also participates in the development of nitrate tolerance and endothelial dysfunction.1–4 The mechanism of the development of nitrate tolerance is complex, involving neurohormonal counter-regulation (pseudo-tolerance), such as activation of the renin-angiotensin-aldosterone system and sympathetic nervous system, as well as intrinsic vascular processes (true vascular tolerance) involving impaired GTN biotransformation, increased superoxide production, desensitization of sGC, increase in phosphodiesterase activity, increased sensitivity to vasoconstrictors, and increased endothelin expression.1–4 Münzel et al have shown that nitrate tolerance and endothelial dysfunction are linked to increased reactive oxygen species (ROS) derived from mitochondria, NADPH oxidase, and uncoupled NO synthase, and that ALDH2 is involved in the development of tolerance and endothelial dysfunction through ROS generation in both in vivo and in vitro animal and human experiments.2,3 However, it is not well known whether genetic polymorphisms of ALDH2 affect nitrate tolerance and endothelial dysfunction in humans. A previous study reported that sensitivity to GTN was decreased in CAD patients with ALDH2*2 compared to those with ALDH2*1/*1, based on patients’ subjective symptoms.21 However, Sakata et al reported that there were no differences in both NMD and FMD between ALDH2*1/*1 and ALDH2*2 subjects.22
The present study showed that ALDH2*2 patients had reduced response to GTN at baseline and developed more severe tolerance as compared to those with ALDH2*1/*1, and that the tolerance developed in both the ALDH2*2 and ALDH2*1/*1 patient groups to continuous GTN administration. Continuous use of GTN for 48 h has been shown to develop tolerance in previous human studies.23,24 The study further revealed that FMD decreased significantly after the continuous GTN administration in both groups with no significant differences in the levels between the 2 groups both at baseline and after GTN administration. The findings thus indicated that endothelial dysfunction developed in both groups on continuous GTN administration with no significant differences between the groups. The discrepancy between the Sakata study and our study may be explained by the differences of the study subjects. Sakata et al examined young and healthy subjects, whereas we examined older and CSA patients. We have shown that both aging and CSA are associated with endothelial dysfunction and increased ROS.11,25–28 Indeed, most of the FMD levels were lower than the reference cut-off value for coronary events,29 with no differences in the degrees of endothelial dysfunction between ALDH2*2 and ALDH2*1/*1 CSA patients in the present study, thereby indicating that endothelial dysfunction plays a crucial role in the pathogenesis of CSA irrespective of ALDH2 genotypes.10,11,25–28
Daiber et al have demonstrated in animal models that prolonged GTN treatment caused endothelial dysfunction as well as tolerance due to mitochondrial ROS production and that there is crosstalk between mitochondrial and cytosolic NADPH oxidase-derived ROS in endothelial dysfunction triggered by GTN.3,23,24,30 It seems likely that ALDH2*2 does not further exacerbate endothelial dysfunction on GTN administration in the presence of already increased ROS and endothelial dysfunction, as in the case of CSA.11,22–26,30,31 It is also possible that factors other than ALDH2 are involved in the development of endothelial dysfunction on continuous GTN.3,32–34 Further studies are required to solve this problem.
Clinical ImplicationsNitrate, including GTN, are widely used for the treatment of CAD including CSA,1–3,10 and ALDH2 plays a key role in the biotransformation of GTN to NO with resultant vascular relaxation.1–6 The present study shows that continuous administration of GTN leads to tolerance and endothelial dysfunction in CSA patients, and that those with deficient variant ALDH2*2 have an attenuated response to GTN and develop more severe tolerance than those with wild ALDH2*1/*1. Reduced FMD and GTN tolerance were reported to be significant predictors of cardiovascular events.29,35,36 The results of the present study thus suggest that continuous administration of organic nitrates, including GTN, should be avoided as much as possible, particularly in those with ALDH2*2. Indeed, recent studies reported that adverse cardiac events occurred on chronic nitrate treatment in patients with CAD including CSA.37–41 It is to be noted, however, that other organic nitrates such as isosorbide-5-mononitrate (ISMN) and isosorbide dinitrate (ISDN) used in the chronic treatment of CAD also develop tolerance and endothelial dysfunction through ROS, although the mitochondrial ALDH2 is not critical in the bioactivation of these nitrates.42 It is also interesting to note in this connection that angiotensin-converting enzyme (ACE) inhibitors and angiotensin II type 1 receptor blockers (ARBs), as well as hydralazine, prevent the development of nitrate tolerance and endothelial dysfunction by suppressing ROS production in patients with CAD.43–45
Study LimitationsThe number of study patients, particularly of ALDH2*2/*2, was small because of the strict study protocol, and the dose effect of mutant ALDH2 allele among 3 groups of ALDH 2*1/*1/, *1/*2, and *2/*2 could not be assessed in this study. However, the study patients were randomized according to the Mendelian randomization and there were no differences in clinical variables except alcohol flushing syndrome and alcohol intake habit between the ALDH2*1/*1 and ALDH2*2 groups.
Continuous administration of GTN produced endothelial dysfunction as well as nitrate tolerance in patients with CAD including CSA. The response to GTN was attenuated and nitrate tolerance exacerbated in patients with variant ALDH2*2 as compared to those with wild ALDH2*1/*1. Continuous administration of organic nitrate including GTN should be avoided in the treatment of CSA, particularly in those patients with ALDH2*2.
This study was supported, in part, by the Japan Heart Foundation, Tokyo, and the Japan Vascular Disease Research Foundation, Kyoto, Japan.
None.
We thank Ms. Yoshimi Tokunaga et al at the clinical laboratory of our institution for measuring laboratory data and Ms. Akiko Oda for secretarial assistance.