2020 Volume 84 Issue 3 Pages 436-444
2020 Volume 84 Issue 3 Pages 436-444
Background: This analysis compared short-term mortality, sternal wound infection (SWI), and long-term survival outcomes in diabetic patients who underwent coronary artery bypass grafting (CABG) with bilateral (BIMA) vs. single (SIMA) internal mammary artery, as well as in diabetic vs. non-diabetic patients undergoing BIMA grafting.
Methods and Results: Nineteen studies were included in the study, covering 21,143 different patients. Of these patients, 6,464 underwent CABG with BIMA, 10,264 underwent CAGB with SIMA, 11,584 had diabetes, and 6,717 did not. Compared with SIMA, BIMA had a significantly lower risk of in-hospital mortality (odds ratio [OR] 0.73, P=0.02), but a significantly higher risk of SWI (OR 1.30, P=0.04). However, compared with non-diabetic patients who underwent CABG with BIMA, diabetic patients with BIMA grafting did not have significantly higher risks of either mortality (OR 1.22, P=0.53) or SWI (OR 1.10, P=0.72). No significant differences were detected with different harvesting techniques. Longer term, BIMA was associated with a significantly higher rate of survival than SIMA (hazard ratio [HR] 0.76, P<0.001).
Conclusions: Results from the 2 types of comparisons indicate that BIMA is a preferable option for diabetic patients, even though it has a higher risk of infection. CABG with BIMA is also associated with a long-term survival benefit.
In recent decades it was clearly demonstrated that grafting the bilateral internal mammary artery (BIMA) was superior than single internal mammary artery (SIMA) in terms of mortality1,2 and other cardiac-related events.3 It was reported that 60% of venous grafts were occluded 10 years after surgery,4 whereas the left anterior descending artery (LAD) had a higher 10-year patency rate, reaching 95%.5 However, the use of BIMA has not been widely adopted, and it has been a matter of debate over the past decade.
Currently, 40% of patients undergoing coronary artery bypass grafting (CABG) are diabetic,6 and the prevalence of diabetes continues to increase. Diabetes mellitus (DM) is an independent risk factor for mortality and other comorbidities after CABG, and the diffuse nature of diabetic coronary atherosclerosis makes it an especially crucial problem in the long term.7 Numerous clinical trials have demonstrated that the use of arterial grafts is associated with survival benefits after CABG in diabetic patients compared with venous grafts.8 Nevertheless, the better outcomes with the BIMA method have been tempered by concerns over the risk of sternal wound infections (SWI) among diabetic patients.9–11 Taggart et al recently reported that the risk of SWI was significantly higher in the BIMA group,12,13 and Iribarne et al reported similar postoperative outcomes after CABG in BIMA and SIMA groups.14 Moreover, previous studies have considered the harvesting of BIMA as another adjunct risk factor for the SWI. Some studies have demonstrated that a skeletonized harvest is associated with a reduced risk of SWI,15–17 whereas another study reported that pedicled harvest could be equally beneficial to diabetic patients without an increase in the risk of SWI.18
Therefore, in the study we compared postoperative mortality, SWI, and long-term survival associated with BIMA or SIMA grafting. The comparisons were made in 2 different ways: (1) between BIMA or SIMA grafting in diabetic populations; and (2) between diabetic and non-diabetic patients undergoing BIMA grafting. Our hope was that the results from 2 different aspects could lead to a more thorough understanding of the problem and shed more light on surgical choices for diabetic patients in the future.
The literature review was conducted by 2 authors (C.W. and F.Z.) simultaneously and independently. Different databases were searched automatically and manually, including the Cochrane Library, Embase, Medline, and the ISI Web of Science. Initial searches identified studies published from January 2000 to September 2019 using the key words “bilateral internal mammary artery”, “single internal mammary artery”, “diabetes mellitus”, and “survival”. There was no restriction on the language of publication. The references of selected articles and conference proceedings were also screened. Titles and abstracts were filtered and duplicate studies were deleted. This research was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Inclusion and Exclusion CriteriaTo be eligible for inclusion in this review, trials had to meet the following criteria: (1) report on comparisons between BIMA and SIMA in diabetic patients or comparisons of outcomes between diabetic and non-diabetic patients following BIMA grafting; (2) clearly describe CABG surgical techniques; (3) provide preoperative data (including age, sex, hypertension, hyperlipidemia, and other relative risk factors); (4) report 30-day postoperative mortality and SWI rate (including both superficial and deep SWIs); (5) clearly describe long-term results; and (6) provide Kaplan-Meier survival curves.
Trials those without control groups, with irrelevant comparison groups (e.g., BIMA vs. other types of surgeries), without early postoperative results, long-term survival data, or survival curves, and those with other irrelevant characteristics were excluded from the review.
The full text of all potentially eligible trials was reviewed. Disagreements were resolved by discussion with the senior author (J.L.).
Data Collection and Quality AssessmentThe following data were independently collected for all studies by 3 authors (C.W., P.L., Q.K.): authors, year of publication, study type, matching method, sample size, basic demographic and preoperative data, harvest method, 30-day mortality rate, infection rate, long-term survival data, and other important information.
Quality assessment was performed thoroughly and independently using consensus criteria by 3 authors (C.W., P.L., Q.K.). The Cochrane risk of bias tool (http://handbook-5-1.cochrane.org/chapter_8/8_5_the_cochrane_collaborations_tool_for_assessing_risk_of_bias.htm) was used to examine randomized control trials (RCTs) and the Newcastle-Ottawa Scale (http://www.ohri.ca/programs/clinical_epidemiology/nosgen.pdf) was used to examine observational studies. Publication bias was evaluated by visual inspection of funnel plots and an Egger linear regression test.
Data Acquisition and Statistical AnalysisAll data were derived directly from the selected studies. The 30-day mortality rates, infection rates, and long-term survival rates were directly reported in most studies, whereas other survival data were acquired from Kaplan-Meier survival curves or the actuarial survival curves using Engauge Digitizer software (http://markummitchell.github.io/engauge-digitizer/), according to standard approaches for extracting data for time-to-event analyses.19 Data acquired with this software were recalibrated manually with clearly identified data in the studies. Odds ratios (ORs) were used to describe short-term results, and hazard ratios (HRs) were used to describe long-term time-to-event and survival results.
Statistical meta-analyses were performed using STATA version 15.0 (StataCorp, College Station, TX, USA), and survival analyses were performed using SPSS version 19.0 (IBM Corp., Armonk, NY, USA). Dichotomies were measured by ORs with 95% confidence intervals (CIs), and survival events were measured by HRs with 95% CI. Two-tailed P<0.05 was considered significant. The heterogeneity of studies was measured by the I2 statistic: I2 <50% was considered to be low heterogeneity, I2 between 50% and 75% was considered to be moderate heterogeneity and I2 >75% was considered to be high heterogeneity. A fixed effects model was used in the case of low heterogeneity, and a random effects model was used in the case of moderate or high heterogeneity.
In all, 19 studies (Figure 1) were included in this analysis, covering 21,143 different patients. Of these patients, 6,464 underwent CABG with BIMA, 10,264 underwent CAGB with SIMA, 11,584 patients were diabetic, and 6,717 patients were non-diabetic. Eighteen studies were observational (14 were retrospective, with 10 using propensity score matching [PSM]; 3 were prospective), and 1 study was an RCT. Of these 19 studies, 18 compared the BIMA and SIMA techniques in diabetic patients, and 5 compared the outcomes of diabetic vs. non-diabetic patients following BIMA grafting. In general, the patient populations were similar between groups. A summary of the studies is provided in Supplementary Table.
Flow chart of study election.
The funnel plot for publication bias, shown in Supplementary Figure 1, and the Egger linear regression test both indicated low heterogeneity. The Cochrane risk bias scale indicated medium performance bias, and the Newcastle-Ottawa Scale summary table for 18 observational studies indicated a relatively high score (Figure 2), meaning that all studies had a low risk of bias, guaranteeing high quality for the present meta-analysis. The overall risk bias was relatively low.

The Newcastle-Ottawa Scale summary of scores.
The meta-analysis of diabetic patients undergoing coronary revascularization showed that BIMA grafting was associated with a significantly lower risk of postoperative hospital all-cause mortality than was SIMA (OR 0.73, 95% CI 0.56–0.96, P=0.021, I2=0%; Figure 3A). Because of the dominance of observational studies, another meta-analysis was conducted with PSM studies and the RCT; this analysis also showed that BIMA grafting was associated with a significantly lower risk of postoperative hospital all-cause mortality (OR 0.75, 95% CI 0.56–0.98, P=0.038, I2=0%; Figure 3B). In addition, we ran meta-analyses comparing the outcomes of diabetic and non-diabetic patients within the BIMA group to investigate the relationship between DM and BIMA grafting. In this analysis, there was no significant difference in mortality between BIMA and SIMA grafting (OR 1.22, 95% CI 0.66–2.29, P=0.53, I2=0%; Figure 3C). Sensitivity analysis did not show any particular study that could largely influence the result.
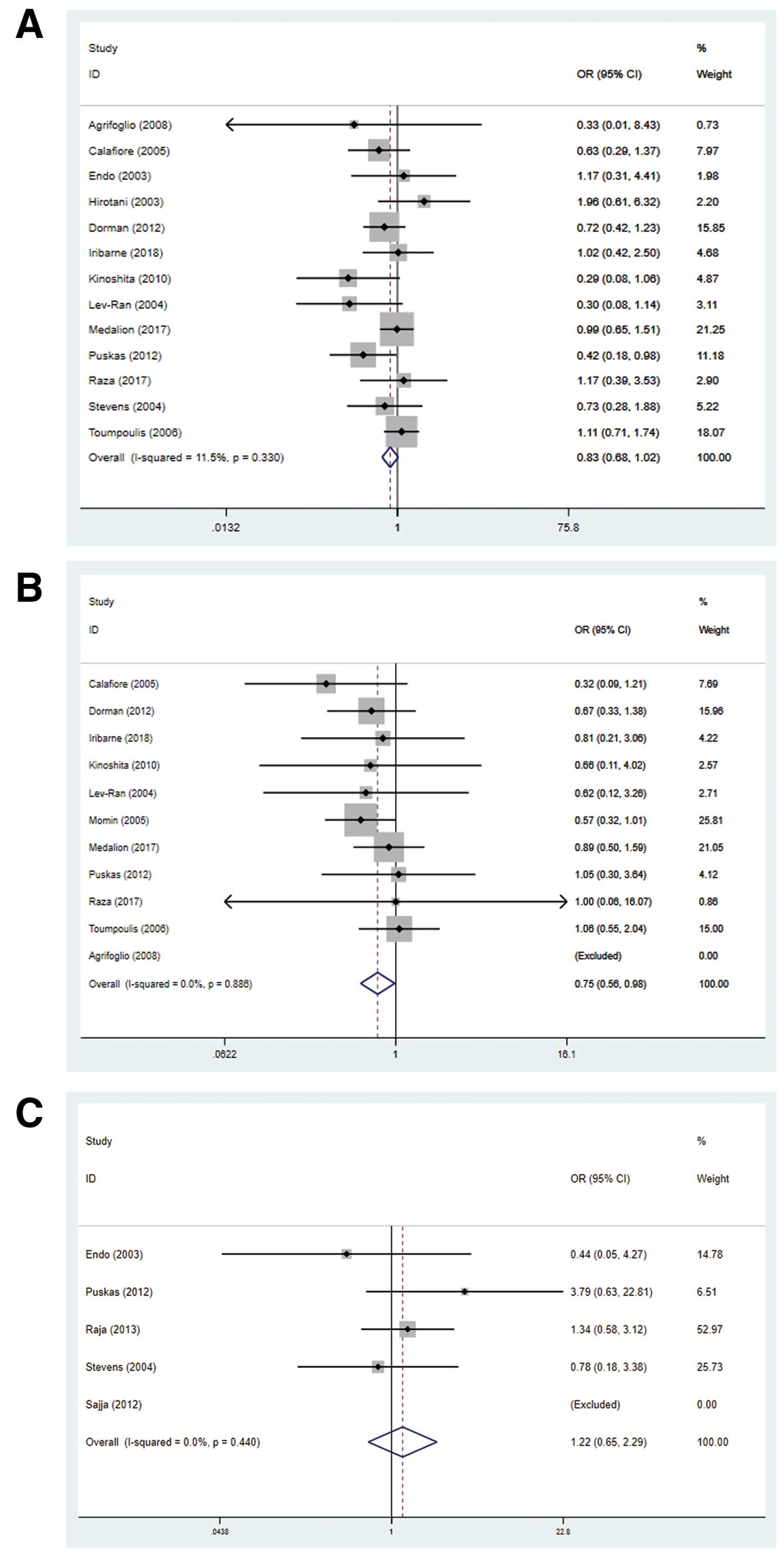
Forest plots showing postoperative mortality comparisons for (A) diabetics undergoing coronary artery bypass grafting with bilateral (BIMA) vs. single (SIMA) internal mammary artery grafts, (B) propensity score-matched diabetics with BIMA vs. SIMA grafts, and (C) diabetic vs. non-diabetic patients with BIMA grafts. CI, confidence interval; OR, odds ratio.
Nevertheless, BIMA grafting was associated with a significantly higher risk of postoperative SWI in diabetic patients (OR 1.30, 95% CI 1.02–1.67, P=0.037, I2=10%; Figure 4A). This results was not changed if the retrospective study without PSM was excluded from the analysis (OR 1.50, 95% CI 1.12–2.00, P=0.006, I2=15%; Figure 4B). Similarly, no significant differences were detected in SWI outcomes between diabetic and non-diabetic patients in the BIMA group (OR 1.10, 95% CI 0.66–1.82, P=0.72, I2=0%; Figure 4C). Sensitivity analysis did not show any particular study that could largely influence the result.
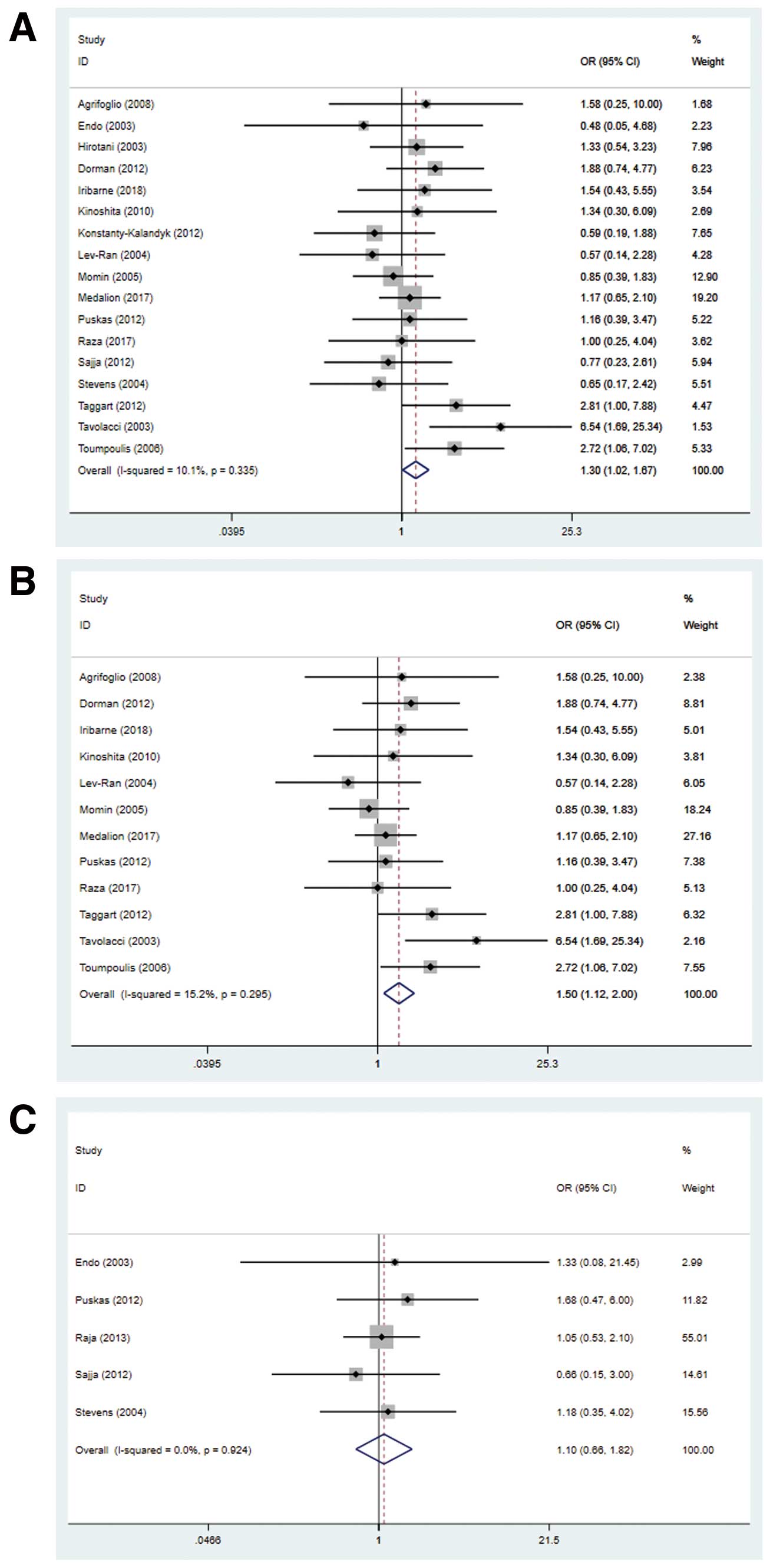
Forest plots showing postoperative wound infection rates for (A) diabetics undergoing coronary artery bypass grafting with bilateral (BIMA) vs. single (SIMA) internal mammary artery grafts, (B) propensity score-matched diabetics with BIMA vs. SIMA grafts, and (C) diabetic vs. non-diabetic patients with BIMA grafts. CI, confidence interval; OR, odds ratio.
Subgroup analyses were conducted to determine whether different harvest methods could lead to different infection rates for diabetic patients undergoing CABG with BIMA or SIMA. Meta-analysis showed that in the skeletonized subgroup, patients undergoing CABG with BIMA were 16% less likely to have SWIs than those undergoing CABG with SIMA (OR 0.84, 95% CI 0.39–1.82, P=0.65, I2=0%; Supplementary Figure 2A), whereas in the pedicled subgroup patients undergoing CABG with BIMA were 5% more likely to have SWIs than those undergoing CABG with SIMA (OR 1.05, 95% CI 0.70–1.58, P=0.81, I2=0%; Supplementary Figure 2B). However, in both cases, the differences did not reach statistical significance.
Actuarial Survival Rates and Long-Term OutcomesLong-term results followed the same pattern as short-term results. CABG with BIMA was associated with a significantly higher rate of survival than CABG with SIMA (HR=0.76, 95% CI 0.68–0.85, P<0.001, I2=58%; Figure 5). Sensitivity analysis did not show any particular study that could appreciably influence the result. An actuarial estimate of the Kaplan-Meier survival curve based on the patient data just described is shown in Figure 6. This graph shows a more intuitive result of the cumulative survival rate at each time point. CABG with BIMA continued to provide diabetic patients with better survival outcomes.

Forest plot for long-term outcomes of bilateral (BIMA) vs. single (SIMA) internal mammary artery grafts. CI, confidence interval.

Actuarial survival curve of bilateral (BIMA) and single (SIMA) internal mammary artery grafts.
It has been clearly documented by numerous studies that BIMA grafting is associated with a significant reduction in mortality20 and cardiac-related comorbidities,21,22 as well as significant long-term survival benefits23 compared with SIMA grafting. In addition to the well-known “gold standard” of CABG with LAD to the left internal mammary artery (LIMA), right internal mammary artery (RIMA) grafting has been proven to have a long-term patency rate similar to that of LIMA grafts.20 Magee et al24 showed that, compared with an artery graft, venous grafts had a 2.6-fold greater tendency to dysfunction, whereas the RIMA grafts had high patency (96% at 5 years, 81% after 10 years), comparable to that of the LIMA grafts (98% and 95% at 5 and 10 years, respectively).25
Despite consistently favorable outcomes regarding the survival benefits of BIMA grafting,23 CABG in diabetic patients is associated with a higher risk of perioperative mortality26 and a higher risk of SWI.27 Moreover, insulin therapy could be an independent risk factor for the lower survival rate.28 Surgeons are reluctant to use BIMA grafting in diabetic populations due to these concerns, and the use of BIMA has become an increasingly important matter of debate due to the prevalence of diabetes and the diffuse nature of diabetic coronary atherosclerosis.22
In the present analysis, we found that BIMA grafting was associated with a significantly lower risk of postoperative hospital all-cause mortality than SIMA grafting in diabetic populations, which is consistent with previous studies.29,30 The use of BIMA appeared to be advantageous in diabetic patients because of the diffuse nature of diabetic coronary lesions and the small caliber of the coronary arteries.31 The internal mammary arteries were proved to enhance the production of endothelium-derived nitric oxide,32 which may ameliorate DM-induced endothelial dysfunction and susceptibility to progressive atherosclerosis and adverse cardiac-related events.33 Dorman et al revealed that, compared with SIMA grafting, diabetic patients undergoing BIMA grafting had a 23% improvement in long-term survival without differences in postoperative complications.33 Lytle et al demonstrated improved survival and a reduced need for repeat revascularization in diabetics with BIMA grafting;1 however, these authors also noted that this procedure should be avoided in patients with higher operative risks due to the prolonged cross-clamping time. Endo et al reported that the survival benefit of BIMA grafting was evident only in patients with preserved cardiac function (ejection fraction [EF] >40%), and that the benefit may be reduced with a lower EF.31 Toumpoulis et al demonstrated that the survival benefit of BIMA grafting was only evident in diabetic patients between 60 and 69 years of age, and that there was no survival benefit for diabetic patients aged >79 years.34 Similarly, Konstanty-Kalandyk et al reported higher mortality following BIMA grafting in older patients with lower EF.35 Conversely, Hirotani et al reported no survival benefits in diabetic patients undergoing BIMA vs. SIMA grafting,36 and Puskas et al did not support the routine use of BIMA in all non-insulin-dependent diabetic patients;30 these authors believed that survival benefits of BIMA grafting only existed in lower-risk non-insulin-dependent diabetic patients.
In this study we also compared mortality between diabetic and non-diabetic patients after BIMA grafting. No significant differences were found between groups, which is in line with some previous studies. For example, Hirotani et al did not detect any significant difference in mortality between groups, and no adverse effects of diabetes were apparent regarding survival and cardiac event-free rates.36 DM may not be a crucial factor when patients have poor cardiac function, with the predominant cardiac risks nullifying the survival benefits of BIMA grafts.31
The major concern prohibiting the wide adoption of BIMA grafting in diabetic patients is the significantly higher risk of SWI. SIMA harvesting could result in a loss of 90% of the hemisternum blood supply, whereas BIMA harvesting could devascularize the entire sternum and interfere with healing of the sternal wound,37 especially in diabetics with microvascular changes. DM could jeopardize the immunological response against infection;18 thus, the harvesting of BIMA could lead to reduced perfusion of the hemisternum during the perioperative period, resulting in decreased sternal circulation and an increased risk of SWI and dehiscence.38 The Arterial Revascularisation Trial (ART) demonstrated that the incidence of SWI was significantly higher in the BIMA than SIMA group (3.5% vs. 1.9%, respectively),39 and Savage et al reported an SWI rate of 2.8% and 1.7% in BIMA and SIMA groups, respectively.27 Further, Hirotani et al reported significant differences in the incidence of SWI between diabetic patients treated with insulin (11%) or not (3.9%).36 In the present study, we found similar outcomes, namely that BIMA was associated with a significantly higher risk of postoperative SWI in diabetic patients, but no significant difference was detected between diabetics and non-diabetics. Previously, Stevens et al reported a similar ratio of hypoperfused to total sternal area between diabetics and non-diabetics, and similar rates of SWI and reoperation for bleeding.40
Some recent studies have blunted the increased risk of SWI with BIMA grafting with skeletonized harvesting.9 Skeletonized harvesting is defined as dissection leaving the muscle attached to the sternal wall and minimizing sternal devascularization,27 in contrast with pedicled harvesting, in which the accompanying endothoracic fascia, parietal pleura, and the transversus thoracis muscle are also harvested.41 A skeletonized conduit could lead to improved sternal perfusion compared with pedicled harvesting.17 Stevens et al demonstrated that skeletonized harvesting may lower the risk of deep SWI in BIMA grafting by preserving sternal blood flow.40 Nevertheless, in the present study, in the skeletonized subgroup, patients undergoing BIMA grafting were less likely to have SWI than those undergoing SIMA grafting, whereas in the pedicled subgroup patients with BIMA grafts were more likely to have SWI than those with SIMA grafts. However, in both cases, the differences did not reach statistical significance. We believe the non-significant results could be due to a meticulous pedicled technique. Agrifoglio et al suggested that meticulous pedicled harvesting, careful wound closure, and keeping the sternal would dry postoperatively may have helped them achieve similar SWI rates between pedicled and skeletonized harvesting.18 Further, both Momin et al7 and Sajja et al41 used electrocautery in pedicle harvesting to reduce sternal ischemia after dissection, believing that this technique would be most beneficial in patients with poor tissue healing power. Further, Puskas et al30 and Raja et al29 reported no significant difference in sternal wound complications between diabetic and non-diabetic patients with skeletonized and pedicled BIMA harvesting respectively. Nishi et al showed that sternal microcirculation damage after pedicled and skeletonized internal mammary artery harvesting was similar, indicating that the latter technique may not be advantageous.29 However, we believe that preoperative selection bias remains a confounding factor in the interpretation of results associated with skeletonized harvesting and the use of BIMA grafts, and that more randomized studies are needed in the future. In addition, many previous studies also showed that perioperative control of blood glucose could lower the risk of SWI.29,42 Calafiore et al reported that insulin-treated patients had prolonged survival than diabetic patients on oral-treatment.22 Lev-Ran et al recommended a 48-h postoperative glucose level of ≤200 mg/dL.43
The present study is the first meta-analysis with a large sample size and using 2 different ways to explore BIMA harvesting in the diabetic population. In addition to mortality and morbidity, we investigated whether the effects of harvesting techniques. However, the results still require confirmation from randomized trials. This study also has other limitations. First, risk profiles and outcomes of CABG are changing dramatically with the rapid development of technology, and so the basic preoperative profile may be different in each study included in the analysis. Second, there may be other confounders affecting the results. Third, some of the survival data were derived from survival curves in previous studies, which may lead to problems associated with rounding.
CABG with BIMA grafting may be a feasible option for diabetic patients. The higher risk of SWI can be reduced by a meticulous harvesting technique and tight control of glucose concentration perioperatively. Further, CABG with BIMA harvesting is associated with a long-term survival benefit in diabetic patients. However, further randomized trials are still needed.
This work was supported by the National Natural Science Foundation of China (Grant no. 81870339).
None declared.
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-19-1050