2020 Volume 84 Issue 3 Pages 382-383
2020 Volume 84 Issue 3 Pages 382-383
Despite outstanding technological advances in coronary revascularization therapy, heart failure (HF) caused by coronary artery disease (CAD) is still a global public health problem, particularly when associated with diabetes mellitus (DM). In fact, patients with HF have a 4-fold higher prevalence of type 2 DM than those without HF, and patients with type 2 DM have a 75% higher risk of cardiovascular death or HF hospitalization than those without DM.1
Article p 471
In this issue of the Journal, Takeji et al2 report the effect of DM on the long-term risk for HF hospitalization in patients with ischemic heart disease who underwent coronary revascularization procedures. They show that the adjusted long-term risk for HF hospitalization was significantly higher in DM subjects than in non-DM subjects (hazard ratio 1.47 [95% confidence interval 1.30–1.67], P<0.0001). Furthermore, the DM subjects showed a significantly higher adjusted risk for all-cause and cardiac death than the non-DM subjects. The study population consisted of a large number of patients (n=15,231) based on the CREDO-Kyoto registry cohort-2,3,4 which is convincing evidence. It is noteworthy that neither the revascularization strategy (either percutaneous coronary intervention or coronary artery bypass grafting surgery) nor insulin use affected the increased HF hospitalization risk in DM subjects.
Although the underlying mechanisms were not determined in the present study, various myocardial and vascular disorders are thought to be involved in the pathophysiology of the diabetic heart (Figure). Of note, the mechanisms underlying the progression of DM and HF are closely intertwined,1,5 and subjects with concomitant DM and HF have diverse pathophysiologic, metabolic, and neurohumoral abnormalities.6 Above all, derangement of cardiac energy substrate metabolism (i.e., impaired metabolic flexibility), as represented by insulin resistance, plays a key role in the pathogenesis of the diabetic heart as well as HF per se.7,8 Although fatty acids (FAs) are the predominant fuel of energy metabolism in the normal adult heart, glucose becomes an important preferential substrate under specific pathological conditions, such as ischemia, as it provides greater efficiency for producing high energy products per oxygen molecule consumed than FAs.9 Therefore, targeting the acceleration of myocardial glucose utilization may become of particular importance under insulin resistant conditions, such as DM, in which glucose utilization is impaired in response to various stimuli, including insulin stimulation as well as ischemic insult.10
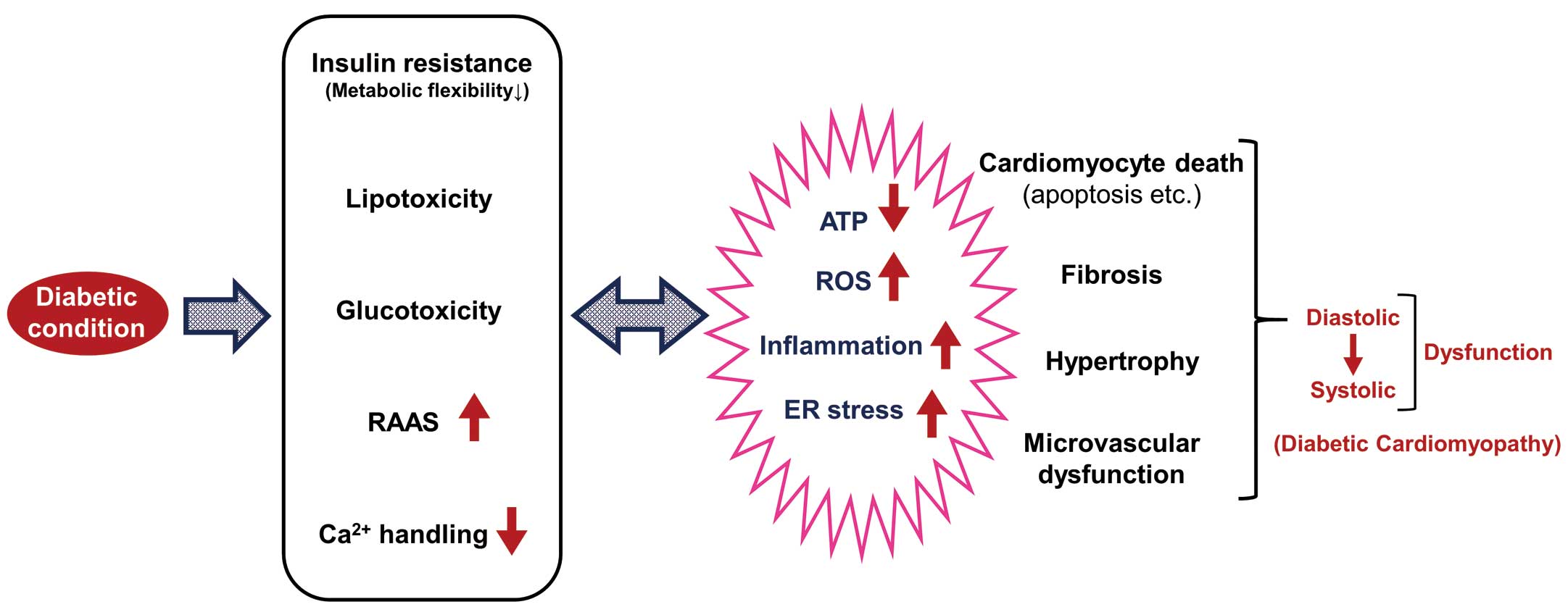
Impact of diabetes on myocardial function. ER, endoplasmic reticulum; RAAS, renin-angiotensin-aldosterone system; ROS, reactive oxygen species.
Together with insulin resistance as well as neurohumoral activation, such as enhanced catecholamine activity, the circulating free FA level and uptake into the myocardium are increased, exceeding the FA oxidation (FAO) capacity of the diabetic heart. The mismatch between FA uptake and FAO results in myocardial triglyceride accumulation, namely, lipotoxicity. In parallel with lipotoxicity, hyperglycemia causes glucotoxicity (including the accumulation of advanced glycation products), activation of the renin-angiotensin-aldosterone system (RAAS), and impairment of mitochondrial Ca2+ handling.11 These metabolic, neurohumoral, and ion homeostasis disorders under diabetic conditions induce oxidative stress, inflammation, and endoplasmic reticulum (ER) stress, as well as reducing ATP synthesis in the heart, all of which account for the impaired metabolic flexibility and Ca2+ handling, drifting into a vicious cycle (Figure). These pathophysiological abnormalities ultimately promote cardiomyocyte death, cardiac fibrosis, and hypertrophy, as well as microvascular dysfunction, leading to cardiac diastolic and systolic dysfunction.
Considering the various pathophysiological mechanisms underlying the diabetic heart, as described above, the present study by Takeji et al2 supports the idea that revascularization is not the only strategy for preventing HF attributable to CAD with concomitant DM. Likewise, of particular interest is that DM was consistently associated with a higher risk of HF hospitalization than non-DM in their analysis, even including the effects of the current gold-standard optimal medical therapy, such as angiotensin-converting enzyme inhibitors/angiotensin-receptor blockers and mineralocorticoid-receptor antagonists.12 Clinicians should thus develop strategies with special consideration of the pathophysiology of the diabetic heart, in addition to the established conventional treatment for HF. Increasing attention has been focused on SGLT2 inhibitors as novel antidiabetic agents that reduce cardiovascular events, as shown in recent large clinical trials.13–15 Further studies will be needed in order to determine whether or not SGLT2 inhibitors reduce the long-term risk for HF hospitalization after coronary revascularization in DM subjects.