Abstract
Background:
The aim of this study was to evaluate the efficacy and safety of transdermal β-blocker patches, which offer stable blood concentration and easy availability during operation, for prevention of perioperative myocardial injury (PMI) in high-risk patients.
Methods and Results:
In this randomized controlled trial, patients aged >60 years with hypertension and high revised cardiac risk index (≥2) undergoing non-cardiac surgery were randomly assigned to a bisoprolol patch or control group. Primary efficacy outcome was incidence of PMI, defined as postoperative high-sensitivity cardiac troponin T (hs-cTnT) >0.014ng/mL and relative hs-cTnT change ≥20%. Secondary efficacy outcomes were number of cardiovascular events and 30-day mortality. From November 2014 to February 2019, 240 patients from 5 hospitals were enrolled in this study. The incidence of PMI was 35.7% in the bisoprolol patch group and 44.5% in the control group (P=0.18). Incidence of major adverse cardiac events including non-critical myocardial infarction, strokes, decompensated heart failure and tachyarrhythmia was similar between the 2 groups. Tachyarrhythmia tended to be higher in the control group. There were no significant differences in safety outcomes including significant hypotension and bradycardia requiring any treatment between the 2 groups.
Conclusions:
Bisoprolol patches do not influence the incidence of PMI and cardiovascular events in high-risk patients undergoing non-cardiac surgery, but perioperative use of these patches is safe.
Perioperative myocardial injury (PMI), which is defined as an increase in cardiac troponin after surgery, is increasingly recognized as being associated with a higher risk of morbidity and mortality after non-cardiac surgery. It occurs in 8–52% of patients who undergo non-cardiac surgery and has received considerable attention since it was reported to be associated with an increased risk of morbidity and mortality.1–9
The main pathology of PMI is thought to be myocardial ischemia, possibly attributable to pre-existing coronary artery disease (CAD), plaque rupture, or imbalance between myocardial oxygen supply and demand. Interestingly, the incidence of coronary artery stenosis is poorly correlated with postoperative myocardial infarction; and revascularization before high-risk surgery does not improve the long-term outcome.10
Indeed, it was recently reported that non-ischemic factors, such as sepsis, tachyarrhythmia, and hypotension, play a greater role than does ischemia.11–13
Beta-blocker treatment has been expected to reduce cardiovascular events after surgery, but a meta-analysis of randomized controlled trials (RCT) suggested that β-blockers reduce non-fatal myocardial infarction but increase stroke and hypotension.14
The efficacy of perioperative β-blockers, even in high-risk patients, is therefore controversial and current guidelines do not recommend routine use of perioperative β-blockers. Bisoprolol patches, a newly developed form of β-blocker in Japan, are designed to deliver bisoprolol through the skin to produce stable blood concentration.15
The safety and efficacy of bisoprolol patches in patients with heart failure have recently been demonstrated.16
We therefore designed this RCT to evaluate the safety and efficacy of bisoprolol patches in high-risk patients undergoing non-cardiac surgery.
Methods
Study Design
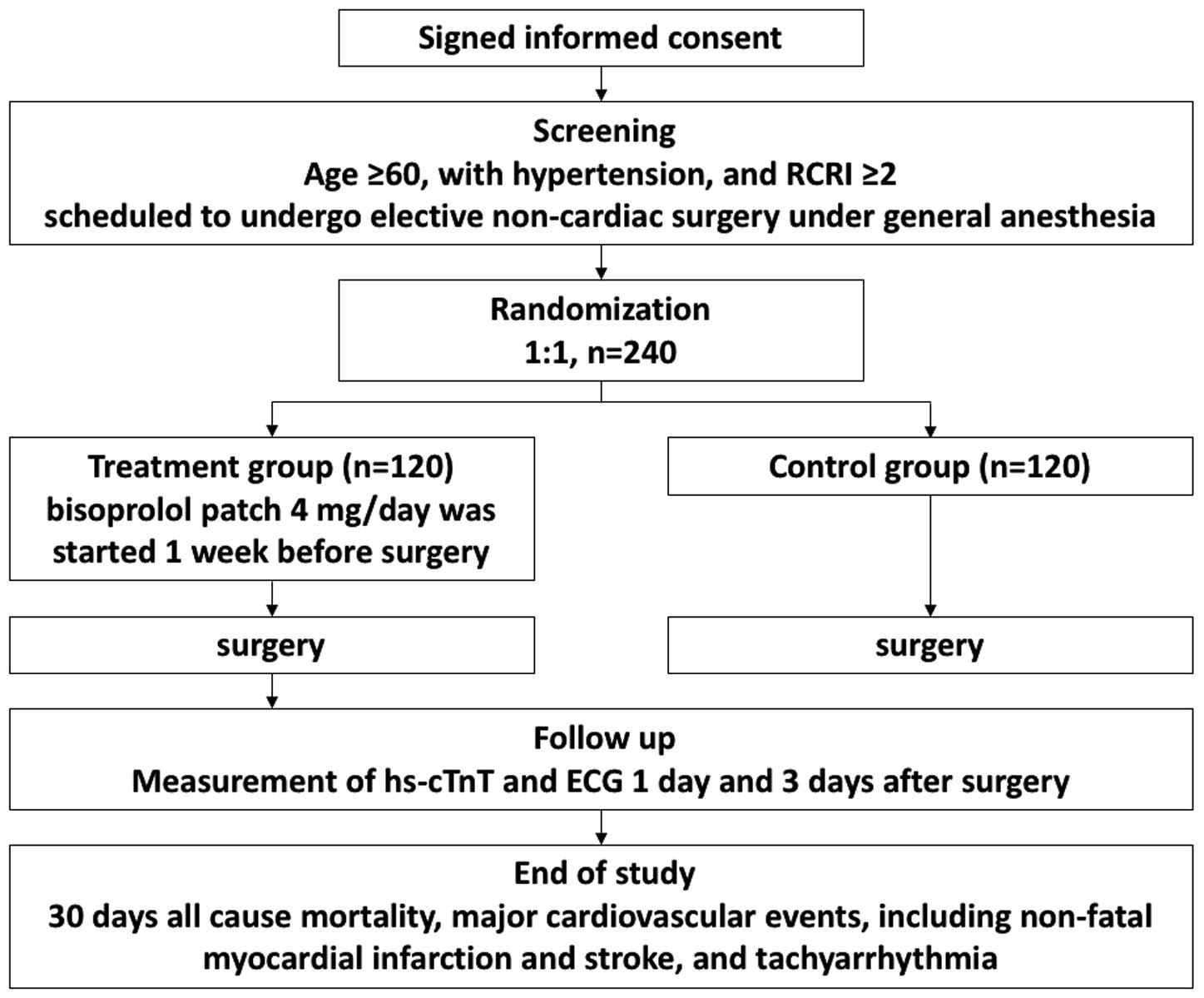
This study (MAMACARI study; UMIN000016908) was a multicenter, prospective randomized controlled, open-label study designed to evaluate the efficacy and safety of β-blocker patches for prevention of PMI in high-risk patients undergoing non-cardiac surgery. An outline of the study protocol is shown in
Figure 1. The study was approved by the local medical ethics committee (application number of Ethics Committee of Okayama University m07015) and all patients provided written informed consent. This trial was conducted in compliance with the Declaration of Helsinki.
Participants
The key inclusion and exclusion criteria are detailed in
Table 1. Patients were eligible if they were aged ≥60 years, had hypertension and revised cardiac risk index (RCRI) ≥2, and were scheduled to undergo elective non-cardiac surgery under general anesthesia. RCRI assigns 1 point for each of 6 criteria (high-risk type of surgery; history of ischemic heart disease; history of congestive heart failure; history of cerebrovascular disease; preoperative treatment with insulin; and preoperative serum creatinine >2.0 mg/dL).17
The category of high-risk surgery was defined as including all intrathoracic, intraperitoneal, and supra-inguinal vascular procedures. Ischemic heart disease was defined as a history of myocardial infarction, positive exercise test, current symptom of ischemic chest pain, use of nitrate therapy, or Q waves on electrocardiogram. Patients with prior coronary artery bypass grafting or percutaneous coronary intervention were defined as having ischemic heart disease only if they had current episodes of chest pain that were presumed to be attributable to ischemia. Congestive heart failure was defined as a history of congestive heart failure, pulmonary edema, or paroxysmal nocturnal dyspnea, with bilateral rales or S3 gallop on physical examination, or pulmonary vascular redistribution on chest radiograph.18
Patients already receiving a β-blocker or with contraindications to β blockers (pre-operative systolic blood pressure <90 mmHg and heart rate <50 beats/min), or with severe renal insufficiency (estimated glomerular filtration rate [eGFR] <15 mL/min/1.73 m2, or undergoing hemofiltration or dialysis) were excluded. Participants who met the eligibility criteria were randomly assigned to a bisoprolol patch or control group. Randomization was performed using a computer-generated random sequence Web response system. The patients were stratified by age (<70 years, ≥70 years), sex (male, female), baseline renal function (eGFR ≥60 mL/min/1.73 m2, <60 mL/min/1.73 m2), and RCRI score (2, ≥3). In the bisoprolol patch group, 4 mg bisoprolol in patch form was started 7 days before surgery and continued until 7 days after surgery. A 4-mg bisoprolol patch is equivalent to 2.5 mg oral bisoprolol. If the initiation dose of the bisoprolol patch was not tolerated, it was reduced to 2 mg/day, or stopped if this lower dosage was not tolerated.
Table 1.
Inclusion and Exclusion Criteria
| Inclusion criteria |
| (a) Diagnosis of hypertension |
| (b) Planning for elective non-cardiac surgery with general anesthesia |
| (c) RCRI ≥2 |
| (d) Age ≥60 years |
| (e) Provision of written informed consent prior to participation |
| Exclusion criteria |
| (a) Use of β-blocker before randomization |
| (b) SBP at screening period <90 mmHg |
| (c) Heart rate at screening period <50 beats/min |
| (d) Absolute indication for β-blocker including severe myocardial ischemia and severe tachycardia |
| (e) Planning for emergency surgery |
| (f) Planning for PCI or CABG in study period |
| (g) Severe renal insufficiency (eGFR <15 mL/min/1.73 m2, or treated with hemofiltration or dialysis) |
CABG, coronary artery bypass grafting; eGFR, estimated glomerular filtration rate; PCI, percutaneous coronary intervention; RCRI, revised cardiac risk index; SBP, systolic blood pressure.
Baseline clinical data were obtained in the preoperative period before randomization and consisted of patient characteristics, physical status, comorbidities, all perioperative medication, and type of surgery. American Society of Anesthesiologists (ASA) class and RCRI were also determined for all patients. All patients underwent electrocardiography, chest radiography, transthoracic echocardiography, and pulmonary function test in the preoperative period. The anesthesia route, surgical status, and duration of surgery were recorded.
Serum concentration of fifth-generation high-sensitivity cardiac troponin T (hs-cTnT) was measured before and 24 h (postoperative day 1) and 72 h (postoperative day 3) after non-cardiac surgery. All plasma samples were frozen and stored at −20℃ in the hospital’s laboratory until analysis. hs-cTnT concentration was measured using a commercially available Electrochemiluminescence Immunoassay and a Cobas®
8000 modular analyzer (Roche Diagnostics, Rotkreuz, Switzerland). hs-cTnT concentration >0.014 ng/mL was considered to be high, in accordance with the hospital laboratory’s normal range.
Outcomes
The primary efficacy outcome was the incidence of PMI, for which there is currently no generally accepted definition. In previous studies, hs-cTnT has been shown to be a more reliable marker for predicting adverse short-term and long-term outcomes in a perioperative setting.19,20
Therefore in our study we used fifth-generation hs-cTnT to improve the accuracy of diagnosis of PMI. In accordance with our previous study, PMI was defined as postoperative hs-cTnT >0.014ng/mL and relative hs-cTnT change ≥20%.21
Secondary efficacy outcomes were 30-day all-cause mortality and major cardiovascular events, including non-fatal myocardial infarction and stroke, decompensated heart failure and tachyarrhythmia including new-onset atrial fibrillation (AF), atrial tachycardia, paroxysmal supraventricular tachycardia, inappropriate sinus tachycardia and ventricular tachycardia ≤30 days after surgery. Myocardial infarction was defined according to a universally accepted definition.22
Safety outcomes included significant hypotension and bradycardia requiring discontinuation of bisoprolol patch or any treatment with continuous infusion of catecholamines.
Power Calculation
It was assumed on the basis of our preliminary trial21
that 34% of the control group would have PMI. It was further assumed that 17% of the bisoprolol patch group would have PMI, the latter estimate being based on the largest published retrospective cohort trial, which reported an approximately 50% risk reduction with β-blockers in patients with ≥2 RCRI.23
Thus, a minimum of 222 patients (111 patients per group) was required to provide 80% power with a 2-sided α of 0.05 on Pearson chi-squared test, and to identify a significant difference in incidence of PMI between the bisoprolol patch and control groups. Estimating that 10% of the patients would withdraw from participation during the study period, the final enrolment target was set at 240 patients (120 patients per group).
Statistical Analysis
Comparison of primary and secondary efficacy outcomes was based on intention-to-treat (ITT) analysis; that is, all participants who had baseline measurements and underwent non-cardiac surgery were analyzed as part of the group to which they were randomized. The primary and secondary efficacy outcomes were analyzed using Pearson’s chi-squared test. Additionally, a logistic regression model was used to calculate odds ratios (OR) between study groups with adjustment for age (<70 years, ≥70 years), sex (male, female), RCRI (2, ≥3), and baseline renal function (eGFR ≥60 mL/min/1.73 m2, <60 mL/min/1.73 m2). The primary cohort for the safety analysis was all patients who received at least 1 dose of study drug. Safety outcomes (significant hypotension and bradycardia, requiring discontinuation of bisoprolol patch, or any treatment with continuous infusion of catecholamines) were analyzed using the Pearson’s chi-squared test with the same stratification factors as for primary outcome. Subgroup analysis for primary efficacy outcome, including age, sex, previous heart disease, coronary risk factors, risk of operation, and preoperative hemodynamic variables, was also performed. P<0.05 was considered to indicate significance. Statistical analysis was performed using IBM SPSS 24.0 for Windows (IBM, Armonk, NY, USA).
Results
Patient Characteristics
This study started on 1st
November 2014 and ended on 28th
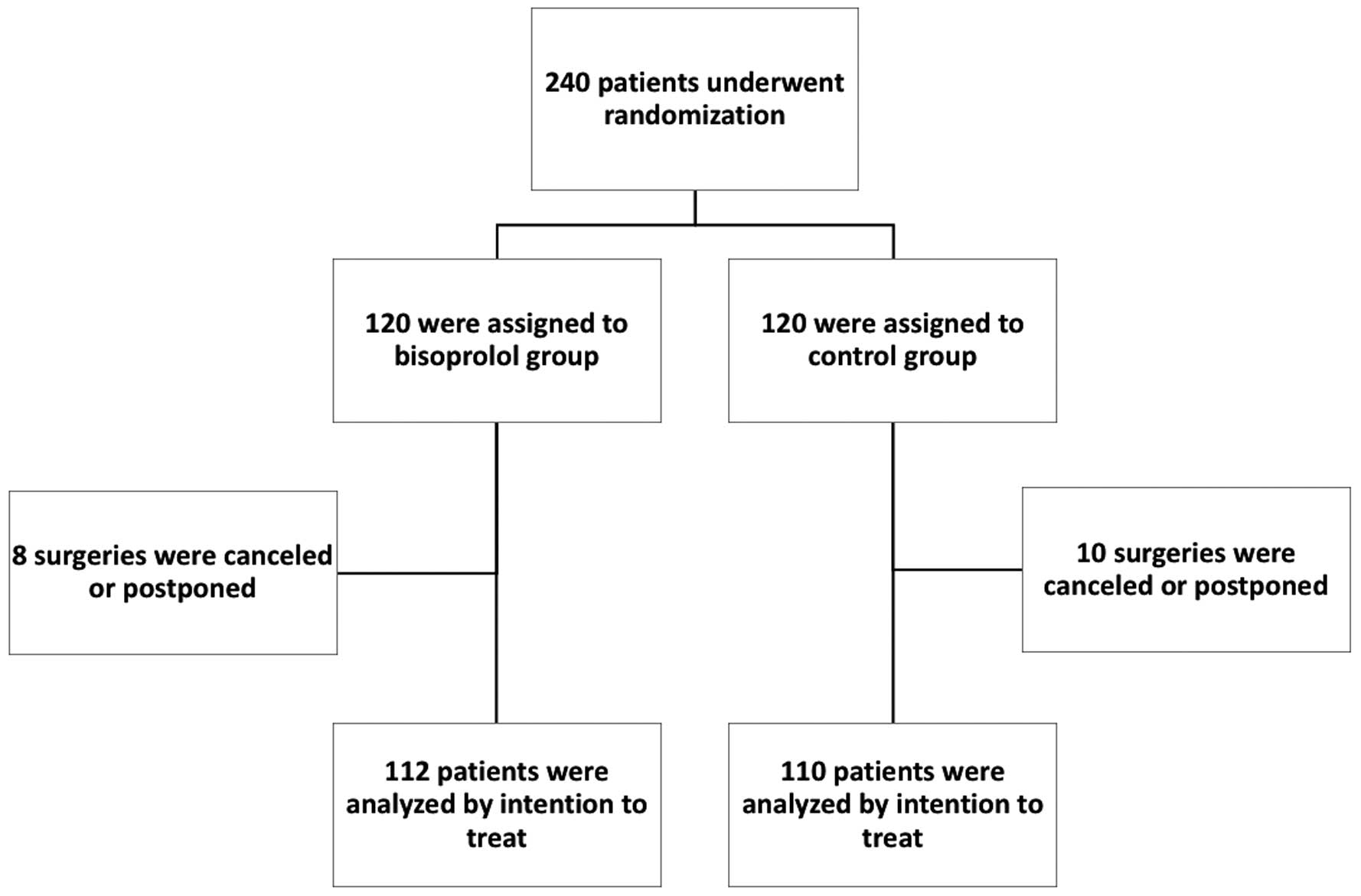
February 2019 and involved 5 institutions in Japan. A total of 240 patients undergoing non-cardiac surgery were randomly assigned to a bisoprolol patch or control group. Some patients were excluded because of cancellation or postponement of surgery. ITT analysis was performed on 112 patients in the bisoprolol patch group and 110 in the control group. The trial protocol is shown in
Figure 2.
Baseline patient characteristics are presented in
Table 2; none differed significantly between the 2 groups. The mean age was 76 years and 28% were women. Median RCRI was 2 points and the rate of high-risk procedures was >80%. The incidence of concomitant diseases did not differ significantly between the 2 groups. There were no significant differences in blood pressure, heart rate, ejection fraction, or medication during the preoperative period between the 2 groups. Lung function, surgical specialty, and duration of surgery also did not differ significantly between the 2 groups.
Table 2.
Baseline Characteristics of All Randomized Patients
| |
Bisoprolol patch (n=120) |
Control (n=120) |
| Age (years) |
76 (67–85) |
76 (66–86) |
| Female sex |
28 (23) |
28 (23) |
| BMI (kg/m2) |
22.8 (20.6–24.7) |
22.8 (21.2–25.3) |
| RCRI score |
2 (2–2) |
2 (2–2) |
| RCRI factors |
| High-risk procedure |
98 (82) |
103 (86) |
| History of IHD |
73 (61) |
65 (54) |
| History of heart failure |
34 (28) |
29 (24) |
| History of CVD |
37 (31) |
36 (30) |
| Renal failure (creatinine ≥2.0 mg/dL) |
5 (4) |
6 (5) |
| Preoperative insulin use |
19 (16) |
22 (18) |
| ASA class |
| 2 |
82 (68) |
82 (68) |
| 3 |
38 (32) |
38 (32) |
| History |
| Hypertension |
120 (100) |
120 (100) |
| Diabetes mellitus |
53 (44) |
62 (52) |
| Dyslipidemia |
62 (52) |
62 (52) |
| Smoker |
77 (64) |
72 (60) |
| PAF |
5 (4.2) |
9 (8) |
| Peripheral artery disease |
11 (9) |
9 (8) |
| Obstructive lung disease |
30 (25) |
35 (29) |
| Asthma |
2 (2) |
3 (3) |
| Previous treatment |
| PCI |
16 (13) |
19 (16) |
| CABG |
1 (1) |
5 (4) |
| Pacemaker |
4 (3) |
2 (2) |
| Bisoprolol patch dose (mg) |
2 (2–4) |
|
| SBP (mmHg) |
140 (128–149) |
140 (127–151) |
| DBP (mmHg) |
74 (66–83) |
76 (65–83) |
| Pulse (beats/min) |
71 (64–80) |
72 (62–82) |
| LVEF (%) |
66 (61–69) |
66 (62–69) |
| LAD (mm) |
37 (34–40) |
36 (32–40) |
| E/e’ |
11.4 (8.6–14.8) |
12.3 (9.3–14.9) |
| eGFR (mL/min/1.73 m2) |
58.4 (47–70) |
57.2 (47.4–71.2) |
| Medication |
| Calcium blocker |
71 (59) |
70 (58) |
| ACEI |
9 (8) |
12 (10) |
| ARB |
62 (52) |
53 (44) |
| Diuretics |
18 (15) |
11 (9) |
| Antiplatelet therapy |
37 (31) |
49 (41) |
| OAC |
16 (13) |
16 (13) |
| Statin |
44 (37) |
49 (41) |
| Vital capacity (L) |
2.96 (2.3–3.41) |
2.78 (2.47–3.29) |
| FEV1.0 (%) |
75 (68–79) |
75 (68–80) |
| Surgical specialty |
| General |
31 (26) |
34 (28) |
| Thoracic |
45 (38) |
57 (48) |
| Vascular |
26 (22) |
20 (17) |
| Neurosurgery |
5 (4) |
0 (0) |
| Orthopedic |
3 (3) |
2 (2) |
| Otolaryngology |
2 (2) |
2 (2) |
| Urology |
3 (3) |
4 (3) |
| Others |
5 (4) |
1 (1) |
| Anesthesia |
| Inhalation anesthesia |
78 (65) |
73 (61) |
| Duration of surgery (min) |
249 (187–374) |
250 (180–385) |
Data given as n (%) or median (IQR). ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; ASA class, American Society of Anesthesiologists; BMI, body mass index; CVD, cerebrovascular disease; DBP, diastolic blood pressure; E/e’, ratio of transmitral early filling velocity to early diastolic tissue velocity; FEV1.0, forced expiratory volume in 1 s; IHD, ischemic heart disease; LAD, left atrial dimension; LVEF, left ventricular ejection fraction; OAC, oral anticoagulants; PAF, paroxysmal atrial fibrillation; RCRI, Revised Cardiac Risk Index. Other abbreviations as in Table 1.
The initial dose was set to 4 mg, and the dose could be reduced to 2 mg or use of the patch could be stopped (at the discretion of the attending physician) if this low dose was not tolerated. Bisoprolol patch dose at the time of surgery was 4 mg for 34 patients, 2 mg for 65 patients, 1 mg for 6 patients, and 0 mg for 7 patients.
Efficacy
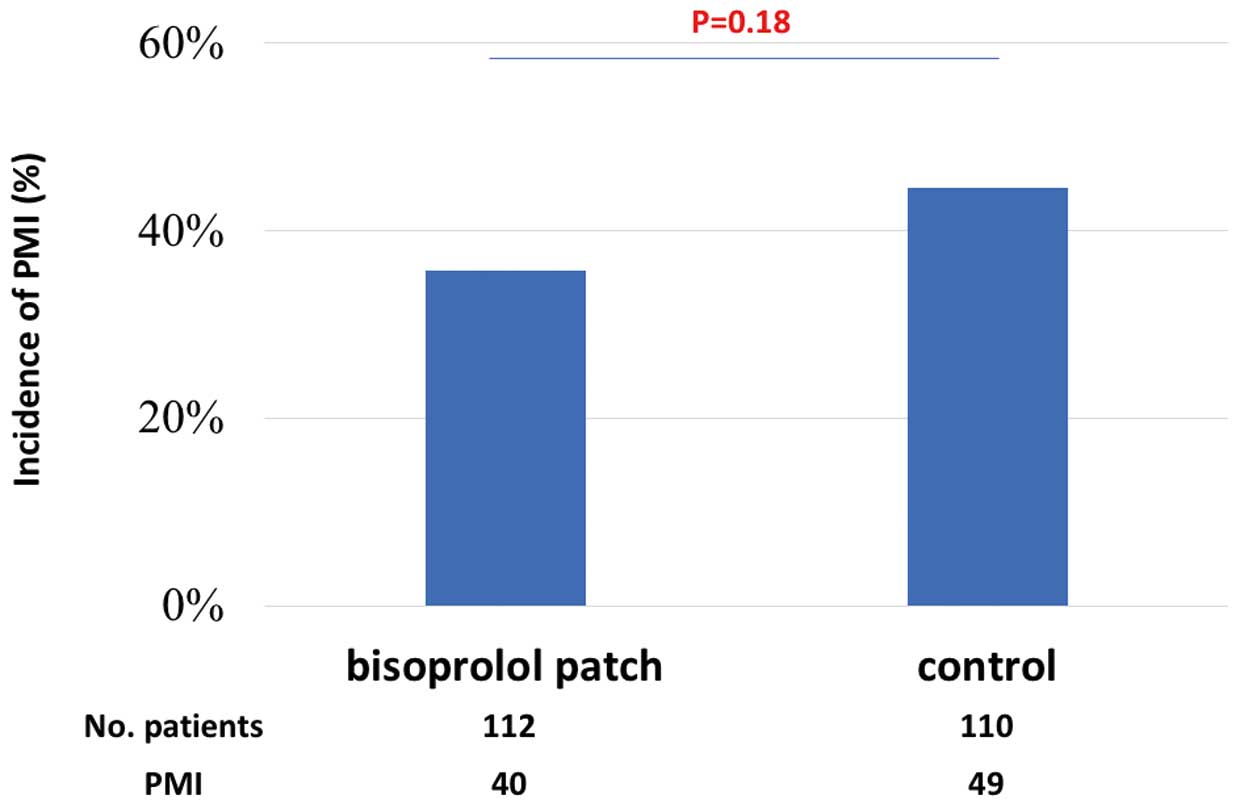
In the whole study cohort, 37% of patients were diagnosed with PMI. The primary efficacy outcome, the incidence of PMI, was 35.7% in the bisoprolol patch group and 44.5% in the control group (P=0.18;
Figure 3); thus, the difference was not statistically significant. The incidence of major adverse cardiac events including non-critical myocardial infarction, strokes, decompensated heart failure and tachyarrhythmia was similar between the 2 groups.
Tachyarrhythmia including new-onset AF, atrial tachycardia, inappropriate sinus tachycardia and ventricular tachycardia requiring any treatment ≤30 days after surgery occurred more often in the control group (Table 3), but the difference between the 2 groups was not statistically significant. The incidence of AF, however, was significantly lower in the bisoprolol group than in the control group (P<0.035).
Table 3.
Secondary Efficacy and Safety Outcomes
| Events |
Bisoprolol group |
Control group |
P-value |
| 30-day mortality |
0 (0) |
0 (0) |
n.s. |
| Major cardiovascular events |
12 (10.7) |
19 (17.2) |
0.16 |
| Non-fatal myocardial infarction |
2 (1.8) |
1 (0.9) |
0.57 |
| Non-fatal stroke |
1 (0.9) |
2 (1.8) |
0.55 |
| Decompensated heart failure |
2 (1.8) |
1 (0.9) |
0.57 |
| Tachyarrhythmia |
7 (6.3) |
15 (13.6) |
0.07 |
| Atrial fibrillation |
4 (3.6) |
12 (10.9) |
0.035 |
| Atrial tachycardia |
2 (1.8) |
1 (0.9) |
0.57 |
| Inappropriate sinus tachycardia |
1 (0.9) |
1 (0.9) |
0.99 |
| Ventricular tachycardia |
0 (0) |
1 (0.9) |
0.31 |
| Bradycardia |
8 (7.1) |
2 (1.8) |
0.06 |
| Hypotension |
13 (11.6) |
7 (6.4) |
0.17 |
The incidence of decompensated heart failure was also similar between the 2 groups. In this study, there were 3 cases of decompensated heart failure after surgery: 2 patients were in the bisoprolol patch group and 1 was in the control group. In 1 of those 2 patients assigned to the bisoprolol patch group, bradycardia occurred before surgery and use of the bisoprolol patch was discontinued. That patient, however, had a tachycardia attack that led to decompensated heart failure 1 day after surgery, and continuous i.v. infusion of landiolol, a β-blocker, had to be started. The other patient with bisoprolol patch and the 1 patient without bisoprolol patch had decompensated heart failure because of volume overload and increasing blood pressure after surgery.
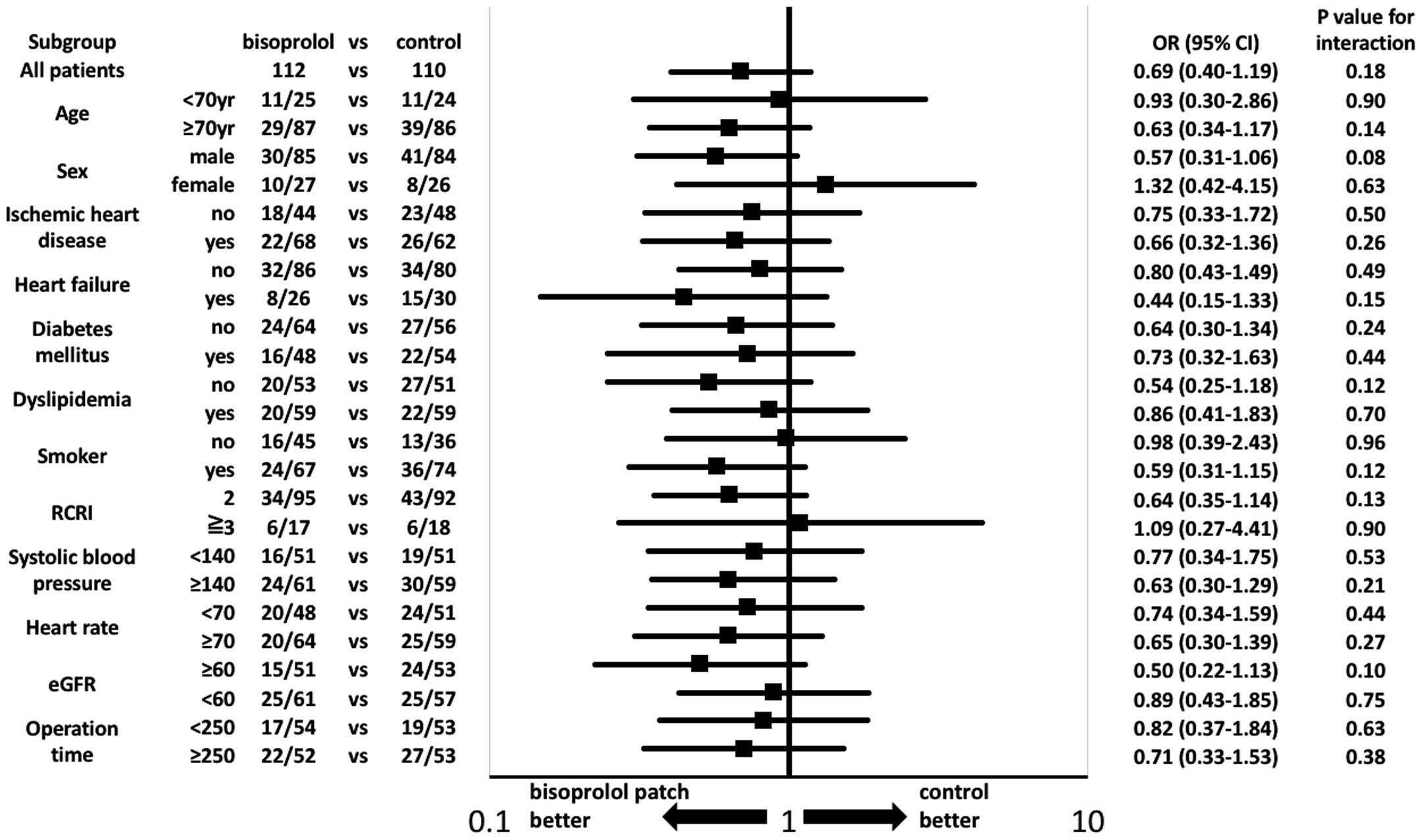
On subgroup analysis, age, sex, previous heart disease, coronary risk factors, risk of operation, and preoperative hemodynamic variables were not significantly different between the 2 groups with regard to the primary efficacy outcome (Figure 4).
Safety
Safety outcomes, including hypotension and bradycardia, did not differ significantly between the bisoprolol patch and control groups (hypotension, 11.6% vs. 6.4%, P=0.17; bradycardia, 7.1% vs. 1.8%, P=0.06;
Table 3). Use of the bisoprolol patch was discontinued in 9 patients due to bradycardia (n=3), hypotension (n=3), and bradycardia and hypotension (n=3).
Discussion
The safety and efficacy of bisoprolol patches in high-risk patients undergoing non-cardiac surgery were evaluated in this RCT, and it was found that bisoprolol patches do not influence the occurrence of PMI as defined by cTnT elevation and major cardiovascular events, including non-critical myocardial infarction and stroke. There were no significant differences in safety outcomes, such as in hypotension and bradycardia, between the bisoprolol patch and control groups. Therefore, the safety of bisoprolol patches in the perioperative period has been demonstrated in this trial.
PMI is recognized as an important surrogate for short- and long-term mortality after non-cardiac surgery.1–9
The main pathology of PMI is myocardial ischemia, possibly caused by pre-existing CAD, plaque rupture, or imbalance between myocardial oxygen supply and demand. Anemia and hemodynamic instability caused by major bleeding during surgery may accelerate myocardial ischemia. Non-ischemic causes, such as hypotension, sepsis, and AF, have also been shown to be more common than ischemic causes.11,13
We previously reported that left ventricular diastolic dysfunction and intraoperative tachycardia are predictors of PMI, and that AF is a common complication after surgery.21
Therefore, the use of prophylactic β-blockers before surgery was expected to prevent PMI and associated adverse events.
Many studies on the means of preventing PMI have recently been performed. Coronary artery revascularization before elective surgery does not reduce the incidence of cardiac events after surgery.10
Clinical studies on the ability of various drugs to reduce perioperative cardiovascular events have been reported,24
but none has shown positive effects. Of these, β-blockers were the only drugs expected to reduce cardiovascular events. The POISE trial was a large and well-known RCT on the efficacy of β-blockers.25
Fewer patients in the metoprolol group than in the placebo group had myocardial infarction, but the rates of stroke and death were higher in the metoprolol than in the placebo group.25
The problem with that trial was that the use of a relatively high dose of the long-acting metoprolol just before surgery, without titration, caused serious hypotension and stroke. The advantages and disadvantages of β-blockers in the perioperative period need to be recognized.
To overcome the limitations of previous studies of perioperative β-blockers, several points, including trial design and dosage and route, require consideration. Bisoprolol transdermal patch, a newly developed form of β-blocker in Japan, is designed to deliver the drug through the skin. Using bisoprolol transdermal patches has several benefits. First, it enables stabilization of bisoprolol blood concentration, making hemodynamic instability less likely. Second, bisoprolol patch can be used in patients with gastrointestinal disease undergoing abdominal surgery, for whom perioperative oral treatment would be difficult. Third, bisoprolol patches can be safely and effectively used in patients with AF and heart failure.16,26,27
In the perioperative setting, it is important to introduce a β-blocker over several days before surgery. In this study, the use of bisoprolol patches from 7 days before surgery resulted in lower frequencies of intraoperative and postoperative hypotension and bradycardia than in previous studies.11,28
This does not prove that using bisoprolol patches in high-risk patients prevents PMI. We think that these negative results may be attributable to heterogeneity in factors such as patient background, surgical procedures, intraoperative and postoperative hemodynamics, and postoperative inflammation. We did, however, show that bisoprolol patches have the potential to prevent ischemia- and tachycardia-related events without causing hemodynamic instability.
In the whole study cohort, 37% of patients were diagnosed as having PMI; this is similar to that in other trials.1–4,9,20
In the present study, approximately one-third of patients diagnosed with PMI had some type of event after surgery; and tachyarrhythmia-related events occurred in half of those who had any events.
We have previously reported that intraoperative tachycardia is an independent predictive factor for cardiovascular events.21
In the present study, however, baseline heart rate at rest was not associated with the incidence of PMI or with cardiovascular events. Distribution of PMI by type of surgical procedure is an important consideration, but the incidence of PMI did not differ significantly by surgical procedure in this trial. Further studies are recommended to determine the indications for bisoprolol patch, to achieve reduction in the incidence of PMI and of cardiovascular events.
Study Limitations
This study had several limitations. First, there is no universally accepted definition of PMI. Second, we calculated that we required a sample of 240 patients for this study, this calculation being based on a previous cohort trial in which use of β-blockers was associated with approximately 50% risk reduction, but a 50% reduction may have been too high for this study. A third limitation is patient selection. We enrolled patients with relatively low risk in this study and excluded those with absolute or relative indications for bisoprolol. Fourth, the bisoprolol patch dose may not have been sufficient to prevent PMI. The median bisoprolol dose used was 2 mg in the bisoprolol patch group, and this dose may have been too low for prevention of PMI and cardiovascular events. If, however, the dose is increased further, the incidence of side-effects such as bradycardia and hypotension may increase. Based on the present results, we consider that 2 mg bisoprolol in the patch is the optimal dose for Japanese high-risk patients undergoing non-cardiac surgery. A further large-scale study is needed.
Conclusions
In this prospective, multicenter randomized controlled, open-label trial the use of bisoprolol patches moderately reduced the incidence of PMI in high-risk patients undergoing non-cardiac surgery, but this was not statistically significant, possibly because the study was underpowered. Conversely, this trial did establish the safety of bisoprolol patches in the perioperative period in such patients.
Acknowledgments
This trial was supported by MAMACARI Investigators. We would like to acknowledge the people who worked with us on this project. We thank Kaoru Akazawa, Megumi Kondo and Masayo Ohmori for their excellent technical assistance and Dr. Trish Reynolds, MBBS, FRACP, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Funding
This study was funded in part by TOA EIYO LTD. (JP).
Disclosures
H.I. received a trust research/joint research fund from TOA EIYO LTD. (JP). The other authors declare no conflicts of interest.
Appendix
MAMACARI Investigators, in addition to the authors, are Kunihisa Kohno, Takuro Masuda, Hideki Fujio, Kunihiko Hatanaka, Toshiaki Kurasako, Naoki Mukohara, Hisatoshi Mori, Masatoshi Sugiyama, Takefumi Oka, Yoichiro Naito, Hidekuni Hidaka, Masaki Yoshikawa, Mitsuru Munemasa, Koji Kabutan and Hiromi Matsubara.
References
- 1.
van Waes JA, Nathoe HM, de Graaff JC, Kemperman H, de Borst GJ, Peelen LM, et al. Myocardial injury after noncardiac surgery and its association with short-term mortality. Circulation 2013; 127: 2264–2271.
- 2.
Landesberg G, Shatz V, Akopnik I, Wolf YG, Mayer M, Berlatzky Y, et al. Association of cardiac troponin, CK-MB, and postoperative myocardial ischemia with long-term survival after major vascular surgery. J Am Coll Cardiol 2003; 42: 1547–1554.
- 3.
Kim LJ, Martinez EA, Faraday N, Dorman T, Fleisher LA, Perler BA, et al. Cardiac troponin I predicts short-term mortality in vascular surgery patients. Circulation 2002; 106: 2366–2371.
- 4.
Oscarsson A, Eintrei C, Anskar S, Engdahl O, Fagerstrom L, Blomqvist P, et al. Troponin T-values provide long-term prognosis in elderly patients undergoing non-cardiac surgery. Acta Anaesthesiol Scand 2004; 48: 1071–1079.
- 5.
Kertai MD, Boersma E, Westerhout CM, Klein J, Van Urk H, Bax JJ, et al. A combination of statins and beta-blockers is independently associated with a reduction in the incidence of perioperative mortality and nonfatal myocardial infarction in patients undergoing abdominal aortic aneurysm surgery. Eur J Vasc Endovasc Surg 2004; 28: 343–352.
- 6.
Bursi F, Babuin L, Barbieri A, Politi L, Zennaro M, Grimaldi T, et al. Vascular surgery patients: Perioperative and long-term risk according to the ACC/AHA guidelines, the additive role of post-operative troponin elevation. Eur Heart J 2005; 26: 2448–2456.
- 7.
Blecha MJ, Clark ET, Worley TA, Salazar MR, Podbielski FJ. Predictors of electrocardiographic change, cardiac troponin elevation, and survival after major vascular surgery: A community hospital experience. Am Surg 2007; 73: 697–702.
- 8.
Bolliger D, Seeberger MD, Lurati Buse GA, Christen P, Rupinski B, Gurke L, et al. A preliminary report on the prognostic significance of preoperative brain natriuretic peptide and postoperative cardiac troponin in patients undergoing major vascular surgery. Anesth Analg 2009; 108: 1069–1075.
- 9.
Nagele P, Rao LK, Penta M, Kallogjeri D, Spitznagel EL, Cavallone LF, et al. Postoperative myocardial injury after major head and neck cancer surgery. Head Neck 2011; 33: 1085–1091.
- 10.
McFalls EO, Ward HB, Moritz TE, Goldman S, Krupski WC, Littooy F, et al. Coronary-artery revascularization before elective major vascular surgery. N Engl J Med 2004; 351: 2795–2804.
- 11.
Sessler DI, Meyhoff CS, Zimmerman NM, Mao G, Leslie K, Vasquez SM, et al. Period-dependent associations between hypotension during and for four days after noncardiac surgery and a composite of myocardial infarction and death: A substudy of the POISE-2 Trial. Anesthesiology 2018; 128: 317–327.
- 12.
Sessler DI, Khanna AK. Perioperative myocardial injury and the contribution of hypotension. Intensive Care Med 2018; 44: 811–822.
- 13.
Ackland GL, Abbott TEF, Cain D, Edwards MR, Sultan P, Karmali SN, et al. Preoperative systemic inflammation and perioperative myocardial injury: Prospective observational multicentre cohort study of patients undergoing non-cardiac surgery. Br J Anaesth 2019; 122: 180–187.
- 14.
Bouri S, Shun-Shin MJ, Cole GD, Mayet J, Francis DP. Meta-analysis of secure randomised controlled trials of beta-blockade to prevent perioperative death in non-cardiac surgery. Heart 2014; 100: 456–464.
- 15.
Matsuoka H, Kuwajima I, Shimada K, Mitamura H, Saruta T. Comparison of efficacy and safety between bisoprolol transdermal patch (TY-0201) and bisoprolol fumarate oral formulation in Japanese patients with grade I or II essential hypertension: Randomized, double-blind, placebo-controlled study. J Clin Hypertens (Greenwich) 2013; 15: 806–814.
- 16.
Momomura SI, Saito Y, Yasumura Y, Yamamoto K, Sakata Y, Daimon M, et al. Efficacy and safety of switching from oral bisoprolol to transdermal patch in Japanese patients with chronic heart failure. Circ J 2017; 82: 141–147.
- 17.
Lee TH, Marcantonio ER, Mangione CM, Thomas EJ, Polanczyk CA, Cook EF, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100: 1043–1049.
- 18.
McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: The Framingham study. N Engl J Med 1971; 285: 1441–1446.
- 19.
Noordzij PG, van Geffen O, Dijkstra IM, Boerma D, Meinders AJ, Rettig TC, et al. High-sensitive cardiac troponin T measurements in prediction of non-cardiac complications after major abdominal surgery. Br J Anaesth 2015; 114: 909–918.
- 20.
Nagele P, Brown F, Gage BF, Gibson DW, Miller JP, Jaffe AS, et al. High-sensitivity cardiac troponin T in prediction and diagnosis of myocardial infarction and long-term mortality after noncardiac surgery. Am Heart J 2013; 166: 325–332.e321.
- 21.
Toda H, Nakamura K, Nakagawa K, Watanabe A, Miyoshi T, Nishii N, et al. Diastolic dysfunction is a risk of perioperative myocardial injury assessed by high-sensitivity cardiac troponin T in elderly patients undergoing non-cardiac surgery. Circ J 2018; 82: 775–782.
- 22.
Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, et al. Third universal definition of myocardial infarction. Circulation 2012; 126: 2020–2035.
- 23.
London MJ, Hur K, Schwartz GG, Henderson WG. Association of perioperative beta-blockade with mortality and cardiovascular morbidity following major noncardiac surgery. JAMA 2013; 309: 1704–1713.
- 24.
Landesberg G, Beattie WS, Mosseri M, Jaffe AS, Alpert JS. Perioperative myocardial infarction. Circulation 2009; 119: 2936–2944.
- 25.
POISE Study Group, Devereaux PJ, Yang H, Yusuf S, Guyatt G, Leslie K, Villar JC, et al. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): A randomised controlled trial. Lancet 2008; 371: 1839–1847.
- 26.
Okamura H, Arakawa M, Miyagawa A, Adachi H. Incidence of postoperative atrial fibrillation in transdermal beta-blocker patch users is lower than that in oral beta-blocker users after cardiac and/or thoracic aortic surgery. Gen Thorac Cardiovasc Surg 2019; 67: 1007–1013.
- 27.
Yamashita T, Ikeda T, Akita Y. Comparison of heart rate reduction effect and safety between bisoprolol transdermal patch and bisoprolol fumarate oral formulation in Japanese patients with persistent/permanent atrial fibrillation (BISONO-AF study). J Cardiol 2019; 73: 386–393.
- 28.
Yang H, Raymer K, Butler R, Parlow J, Roberts R. The effects of perioperative beta-blockade: Results of the Metoprolol after Vascular Surgery (MaVS) study, a randomized controlled trial. Am Heart J 2006; 152: 983–990.