2020 Volume 84 Issue 4 Pages 609-615
2020 Volume 84 Issue 4 Pages 609-615
Background: Recently, an interoperative catheter electrode mapping system, termed ExTRa Mapping (EXT), was developed for precise diagnosis and effective treatment of non-paroxysmal atrial fibrillations (non-PAF). However, the mapping accuracy of EXT is still unclear.
Methods and Results: In this study, the reliability of the EXT in comparison with that of high-resolution optical membrane potential mapping was compared. Spiral wave re-entries (SWRs) were induced in the excised rabbit hearts (n=8, 42 episodes). Electrical signals were measured by electrodes on a transparent silicone plate, with the same arrangement as in the clinical catheter, and fluorescence signals were recorded simultaneously across the plate. Based on the phase maps derived by EXT, activation patterns (one-directed propagations: 26, rotational activities: 16) were identified correctly with 95% accuracy (40/42), and the correlation coefficient of the ratio of the non-passive period was 0.95. In the rotational episodes (15), the mean position error of the centers of gravity of the SWR trajectory (2,000 ms) was 2.0 mm. For the one-directional episodes (25), the correlation coefficient of the directions of one-way propagation was 0.99.
Conclusions: The phase map sequence by EXT is consistent with that by the analyses of high-resolution optical mapping. EXT is reliable for analyzing the activation pattern in the region of interest.
Atrial fibrillation (AF) is one of the most common arrhythmias, particularly in aging societies. Pulmonary vein isolation (PVI), proposed by Haissaguerre et al, is an established ablation strategy currently used for the treatment of AF.1–4 While the success rate of PVI for paroxysmal AF (PAF) is adequate, PVI ablation alone for persistent or long-standing persistent AF; that is, non-paroxysmal AF (non-PAF), which often accompanies remodeling of the atrial tissue, is still limited.5 A reliable ablation strategy has not yet been established for non-PAF patients.
For a more precise diagnosis and effective treatment of non-PAF, a variety of diagnostic techniques have been proposed.6–11 For example, body surface mapping is a non-invasive diagnostic method that estimates the activation pattern of the atrial tissue non-invasively from multiple body surface electrode signals.12 Complex fractionated atrial electrogram (CFAE) mapping and low-voltage area (LVA) mapping are more direct methods that identify the ectopic site of the pathological atrial tissue from the waveform feature of a single intra-cardiac bipolar electrogram.13–16 Recently, for enriched and more intuitive visualization, catheter mapping methods using designated a high-density electrode catheter have been proposed.6,11 Phase mapping using ExTRa Mapping (EXT) is one such high-density mapping method that has been specifically designed for intraoperative high-resolution and online, real-time visualization of atrial activation dynamics. By using the spiral-shaped 20-pole electrode catheter, EXT estimates the phase map sequence inside the region of interest (ROI) under the mapping catheter to judge the complexity of the activation in the region. In a previous clinical study, the effectiveness of the EXT-guided, non-PAF ablation was demonstrated.17 However, similar to other multielectrode mapping methods, the accuracy of the estimated activation patterns has not yet been sufficiently verified.
Here, we investigate the accuracy of EXT through ex vivo experiments of simultaneous measurement using high-resolution optical membrane potential mapping (OPT). SWRs induced in excised rabbit hearts were measured using both mapping methods, and the activation pattern estimated by EXT was compared with that estimated by OPT.
The protocol followed in this study was approved by the Institutional Animal Care and Use Committee of the University of Tokyo. The experimental models have been described in other studies.18–20 Hearts of Japanese white rabbits (weight: 1.7–2.0 kg) of both sexes were isolated and connected to a Langendorff perfusion apparatus with Krebs–Ringer solution. The endocardial wall of the left ventricle was destroyed by performing cryoablation of the cardiac tissues from the endocardial side to create a 2D preparation. The cryoablation agent utilized was a cryoprobe cooled with liquid nitrogen. The heart was stained with the voltage-sensitive dye, di-4-ANEPPS (0.2 μmol/L). Then, 2,3-butanedione monoxime (15 μmol/L) was added to the perfusate to inhibit heart contractions. Rapid pacing stimulations (2–5 V, 120–200 ms interval) were applied from the linear catheter (Livewire; St. Jude Medical Inc. St Paul, MN, USA) inserted in the right ventricular chamber to induce spiral wave re-entries.
Simultaneous Mapping SystemFigure 1A shows the schematic diagram of the developed simultaneous mapping system. Electrical signals at the epicardial ventricular surface of rabbit heart samples were measured on the electrodes located on a transparent silicon plate (PDMS, 1-mm thickness) held by a metal frame (Figure 1B, Left). The arrangement of the electrodes was made similar to that of a multi-electrode catheter (REFLEXION HD; St. Jude Medical) clinically used for EXT, with 70% scale, taking into consideration the difference in the conduction velocity of the rabbit ventricle (40 cm/s) and human atrium (63 cm/s) (Figure 1B, Right). Regional electrograms were recorded and analyzed on a polygraph (RMC-5000; Nihon Kohden, Tokyo, Japan). Simultaneously, the optical signal through the transparent electrode plate was recorded by a high-speed camera (SA4; Photoron, Tokyo, Japan) with 1,000 frames/s. The detail of the optical mapping system has been described in previous studies.20–22 Both optical and electrical measurements were synchronized by the external trigger generated by the stimulator (SEC-5104; Nihon Kohden).
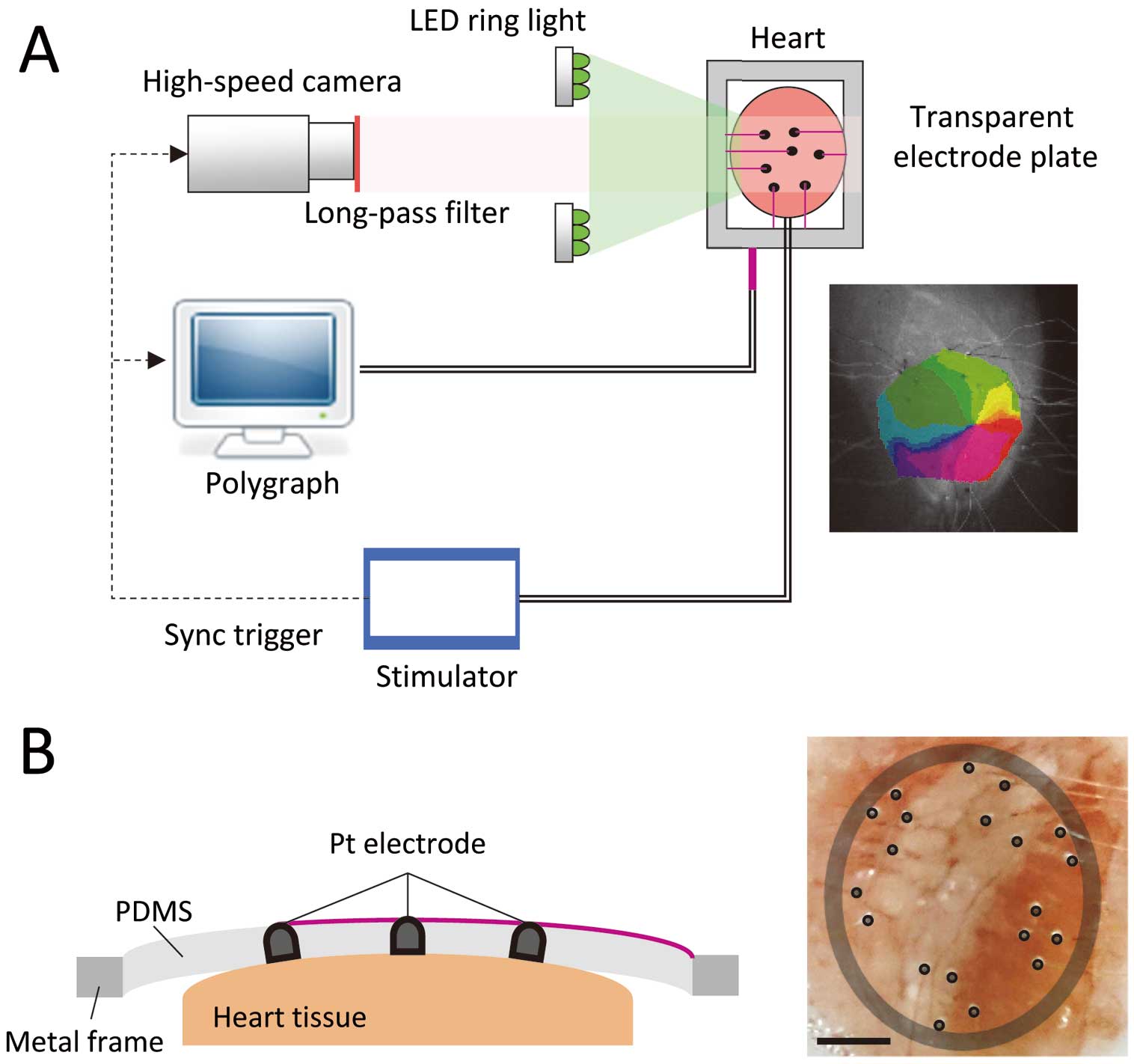
Simultaneous mapping system developed for verification of catheter mapping. (A) Schematic diagram of the simultaneous electrical and optical mapping system. Both measurements are synchronized by an external trigger generated by the stimulator. The right-hand side photograph shows the phase map overlaid on the optical measurement image. (B) Transparent electrode plate. Platinum electrodes are mounted on the surface of the transparent silicone plate (left-hand side). The right-hand side photograph shows the camera side view of the electrodes attached on a posterior surface of a rabbit ventricle.
From the recorded electrode signals, the phase map sequences, with a speed of 4 ms/frame, were estimated by the ExTRa MappingTM software (RMC-5000; Nihon Kohden). Briefly, this algorithm estimates phase maps of the region of the mapping catheter by estimating the phase value at each point using a numerical simulation model of a cardiac cell23 and spaciotemporal interpolation by specialized artificial intelligence.17
The corresponding phase maps were also derived from the OPT signals. The details of the algorithm are described in other studies.24–26 The phase value of each pixel in the camera image was calculated by applying reverse normalization and Hilbert transform to the OPT signal.
Coordinate TransformationThe derived phase maps were defined on different coordinate systems; that is, the coordinate systems of OPT and EXT. To compare these phase maps, coordinate transformation matrices were derived from the coordinates of 4 corresponding electrodes by using estimation of the perspective transformation matrix.
Detection of SWR CentersThe Center of the SWR is an important feature point for judging the complexity of excitation of the measured region. From the derived phase maps, the phase singularities (PSs) corresponding to the centers of the SWRs were detected automatically by using phase variance analysis.25 Briefly, this algorithm detects the position of PSs as the point around which the local variance of phase values is large.
Similarity of Excitation ComplexityFrom the detection results of PSs, the similarity between the sequences of the EXT phase maps and OPT phase maps were evaluated quantitatively. Optical phase maps were transformed into the EXT image coordinates and down-sampled to 4 ms/frame to allow comparison with EXT results.
The objective of multi-electrode catheter mapping is to identify the region in which the SWR can be observed with high probability. To quantify this probability, we defined an index termed the non-passive ratio (%NP), as follows.
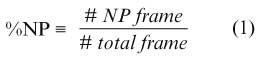
The non-passive (NP) frame was defined as the frame in which at least one PS was detected.
Similarity of the Average Direction of Phase PropagationTo compare the directions of 1-way propagation in the passive regions between EXT and OPT, the average direction of propagation on each phase map was calculated as follows:
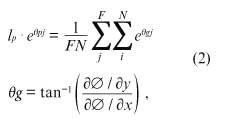
where lp and θp correspond to the average velocity and direction of phase propagation, respectively, ∅ is the phase value on each pixel, F is the total number of the frames in each episode (494 frames, 4 ms/frame), and N is the number of pixels in the measurement region (5,718 pixels).
Similarity of the Average Relative Position of SWRTo evaluate the similarity of the phase maps of NP episodes, the center of gravity (COG) of the SWR trajectory was calculated and compared as follows:
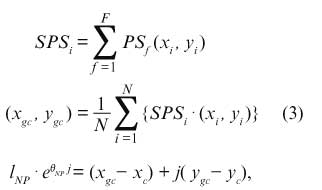
where F is the total number of the frames in each episode (494 frames, 4 ms/frame), and PSf is the binary image of PSs at the f-th frame. N is the total number of the pixels in the measurement region (5,718 pixels), and (xgc, ygc) is the position of the COG. To evaluate the similarity of the relative position, the polar coordinates of the COG (lNP, θNP) from the center of the region (xc, yc) were calculated and compared.
Statistical AnalysisFor quantitative evaluation of the similarities between OPT and EXT, correlation coefficients of the above described 4 indices (% NP, θg, lNP, θNP) were calculated. Moreover, each episode was classified into 2 categories by thresholding %NP. Considering that the objective of EXT is the detection of the NP area, we defined an episode with %NP ≥0.5 as a positive NP episode, and an episode with %NP <0.5 as a negative NP episode. The sensitivity and specificity of NP episodes by EXT were compared with those of OPT.
VTs were induced in 8 hearts by rapid pacing from the right ventricular endocardium. Video images of excitation during long-lasting VTs (more than 30 s) were analyzed. From 8 heart samples, 58 episodes were recorded. Among the episodes from the same heart sample, the electrode position was modified, and VT was re-induced. Sixteen episodes out of 58 episodes were removed from the comparison target due to the bad signal quality of the optical measurement. In the remaining 42 episodes, certain forms of SWR (single-loop or figure-of-eight re-entry) were documented in 16 (38%) episodes. The remaining 26 VT episodes showed 1-way propagation of wave fronts traversing the observation areas.
Activation Patterns During Ventricular TachyarrhythmiaFigure 2 shows representative examples of comparisons between EXT and OPT. OPT phase maps were transformed onto the EXT image coordinate system. In the passive case (Figure 2A), no PS was observed and 1-way propagation of a planar wave from left to right was observed in both phase maps. In contrast, in the NP case (Figure 2B), a clockwise spiral wave propagation with its center meandering around the right side of the region was observed in both measurements. In Figure 2B, the white lines indicate the wave front, and the black dots indicate the PS pixels derived from the phase variance analysis. In another non-passive case with a complex excitation pattern (Figure 2C), a wave-break of 1-way propagation from left-bottom to right-top (white arrow in 0 ms) and subsequent figure-of-eight re-entries: a pair of rotations with the opposite directions (white curved arrows in 40 ms and 80 ms), were observed in OPT results. Also, in EXT results, wave-break and subsequent figure-of-eight re-entries were observed at similar timings and locations.
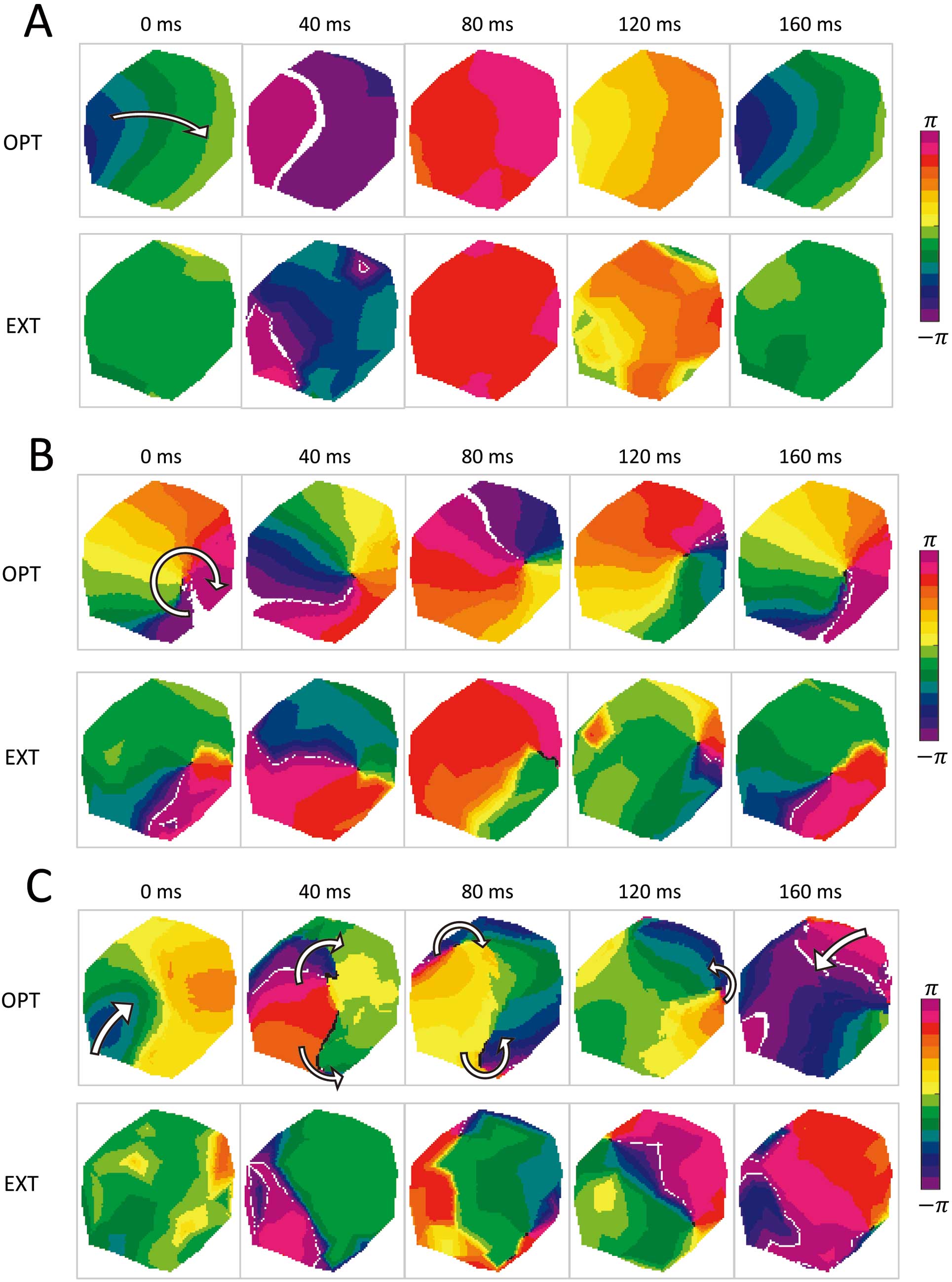
Representative examples of comparison results between optical membrane potential mapping (OPT) and ExTRa (EXT) mapping. The white lines represent the wave front. The white arrows in the 0 ms OPT phase maps indicate the observed activation patterns. (A) Passive case. One-way propagation can be observed from left to right in both phase map sequences. (B) Non-passive case with a stable rotor. Clockwise spiral wave propagation with its center meandering around the right region of the measurement region can be observed in both measurements. (C) Non-passive case with complex excitation pattern. In both OPT and EXT results, a wave-break (~40 ms) of 1-way propagation (from left-bottom to right-top) and subsequent figure-of-eight re-entries were observed.
Figure 3 shows the quantitative evaluation results of similarities between EXT and OPT, and Table shows its summary. Figure 3A shows the scatter plot of %NP of EXT and OPT in each episode. The correlation coefficient of %NP of EXT and OPT was 0.95 (n=42). The sensitivity and specificity of the NP episode with a threshold of %NP=0.5 were 0.94 (15/16) and 0.94 (15/16), respectively. These results indicate that EXT mapping can categorize the excitation complexity in the measurement region with some accuracy.
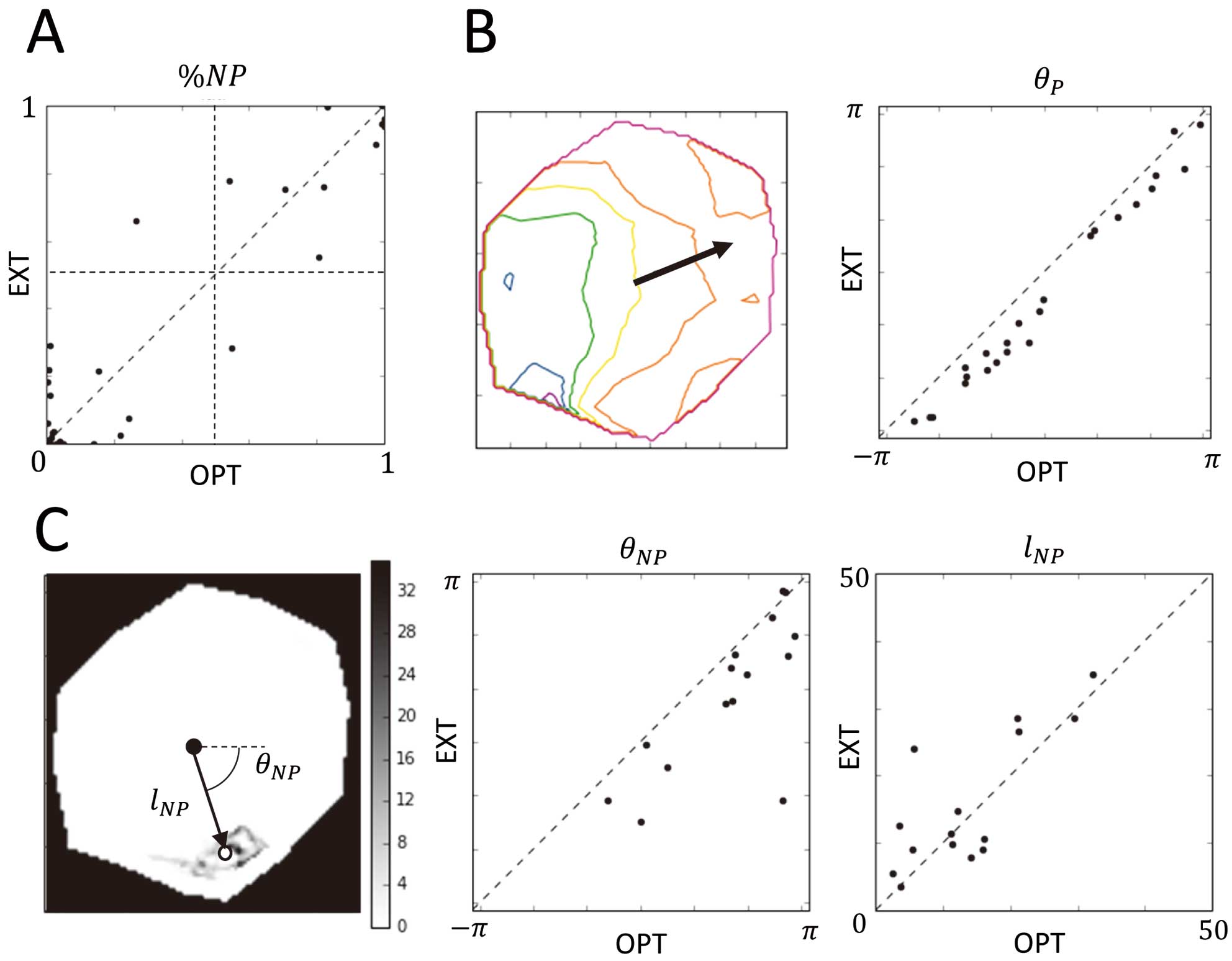
Evaluation of similarities between optical membrane potential mapping (OPT) and ExTRa (EXT) mapping. (A) Similarity of non-passive ratio (%NP). The vertical and horizontal dotted lines show the threshold (%NP=0.5) of passive and non-passive episodes. (B) Similarity of the average direction of propagation in passive cases (n=25). The left-hand side image shows an example of phase contour on a frame, and the black arrow represents the average direction of its gradient. The right-hand side graph shows the similarities of the direction. (C) Similarity of the average position of the spiral wave re-entry (SWR) in non-passive cases (n=15). The left-hand side image shows an example of the SWR trajectory (dark area), its center of gravity (white dot), and the center of region (black dot). The right-hand side graphs show the similarity of the average relative position of the SWR from the center of region.
| Index | Value |
|---|---|
| Correlation coefficient of %NP (N=42) | 0.95 |
| Sensitivity of NP episodes (%NP >0.5) | 0.94 (15/16) |
| Specificity of NP episodes (%NP >0.5) | 0.94 (15/16) |
| Correlation coefficient of average propagation direction θg (N=25) | 0.99 |
| Correlation coefficient of average SWR position relative to the center of region (N=15) | |
| θNP | 0.74 |
| lNP | 0.76 |
| Mean distance of COGs of SWR (N=15) | 2 mm |
COG, center of gravity; NP, non-passive; SWR, spiral wave re-entry.
Figure 3B shows the results of evaluation of the similarity of the average propagation direction in the true-negative (passive) cases (n=25). In the left-hand side image, the black arrow with its origin on the center of the region indicates the calculated average direction of propagation on a frame. The correlation coefficients of lP and θP were 0.77 and 0.99, respectively. The right-hand side image of Figure 3B show scatter plots of θP of OPT and EXT. The high correlation coefficient of the θP indicates that EXT mapping can visualize 1-way propagation with proper direction.
Figure 3C shows the results of evaluation of the similarity of the average SWR position in the true-positive NP cases (n=15). The left-hand side image shows an example of a PS trajectory image derived by accumulating detection results of PS on each frame along the time axis (SPS in Equation (3)). The dark area in the region (bottom) denotes that PSs were detected frequently around it. The white dot indicates the center of gravity of the SWR during the episode, and the black dot represents the center of the measurement region. The right-hand side graphs show the similarity of the relative position of the SWR from the center of the measurement region between EXT and OPT. Correlation coefficients of the polar coordinates θNP and lNP were 0.74 and 0.76, respectively. The mean distance of the COGs of EXT and OPT was 2.0 mm. These results indicate that EXT can detect the average SWR position in the measurement region with some accuracy.
The aim of this study was to validate phase mapping by using EXT, a diagnosis system developed by Ashihara’s group,17 for intraoperative visualization of the activation pattern for better ablation therapies. We developed an experimental system of simultaneous optical and electrical measurements using a transparent silicone electrode plate. Using this system, we performed the first experiment comparing EXT with OPT. The results of the measurement of those mapping methods of VTs on extracted rabbit hearts have revealed that EXT is able to visualize the activation pattern of both planar and spiral wave propagation in a manner consistent with OPT (Figure 2).
Reliability of EXTQuantitative analysis of the similarity between OPT and EXT have revealed that EXT is able to judge the complexity of the excitation pattern with high accuracy (95%, 40/42) by calculating the index %NP in Equation (1). Moreover, the features of the activation pattern visualized by EXT, the propagation direction for passive cases, and the average SWR position for NP cases were also similar to those of the OPT measurement results (Table and Figure 3). These results suggest that EXT has good reliability to diagnose the excitation pattern and its complexity in the measurement region.
In contrast, by thresholding the %NP of EXT and OPT results with %NP=0.5, one false-positive and one false-negative erroneous detection of NP (Figure 3A) were observed in 42 episodes. In the false-negative NP case (the dot in the right bottom quadrant in Figure 3A), a PS meandering around the boundary of the measurement region and iteratively entering and exiting the measurement region was observed in OPT. This error case suggests that care should be taken when interpreting %NP in cases where PSs are meandering around the boundary of the measurement region. In the false-positive NP case (the dot in the left top quadrant in Figure 3A), the signal of the electrode around the center of the measurement region was not measured properly (Supplementary Figure). A possible reason for this issue was that the electrode was not properly attached to the heart surface due to the complex curvature of this surface. This error case suggests that attachment of the catheter electrodes is important to ensure reliable EXT results.
Simultaneous Measurement SystemNot limited to the EXT, simultaneous measurement experiments are essential for validating electrode mapping methods. In a previous study, clinically applicable catheters were adopted for simultaneous measurement, but occlusion due to the shaft of the catheter may make the comparison difficult.27 In another study, a plaque with multiple electrode array was used for multiple stimulations,28 but the pressure caused by the planar plate pushing on the round heart surface may cause undesirable effects on the activation pattern in the region. To avoid these problems, we developed a transparent and flexible silicone electrode plate for simultaneous measurement (Figure 1), which enabled stable simultaneous measurement of electrical and optical measurements in most episodes. The developed system may also be valuable for validation of other electrode mapping systems.
Study LimitationsThe aim of this study is to validate the ability of EXT to analyze the excitation pattern in the measurement region from the catheter electrode signals. There are several differences between the performed animal experiments and clinical measurements. Although EXT is an algorithm designed for visualization of excitation using a human non-PAF atrial myocyte model17,23 and the endocardial mapping catheter, we measured the rabbit ventricular epicardial surface in this validation study. In the developed simultaneous mapping system, the arrangement of the electrodes was at a 70% scale of the original catheter, taking into consideration the difference of the conduction velocity. These variations, due to the differences in the animal species, may affect the results. Although blood exists around the electrodes in real EXT, the electrodes in this study were attached to the surface of the sample perfused in the air. The epicardial surface of the rabbit ventricle wall is smoother than the endocardial surface of the human atrial wall; these differences in measurement conditions may also affect the electrode signal.
In contrast, even with the above differences, the observed excitation patterns in the measurement region had no significant difference from clinical EXT measurements in terms of its complexity, as shown in Figure 3. The measured electrogram waveforms were also similar to clinical measurements. Thus, we believe that the experiment results support our conclusion that ExTRa mapping has reliability for analyzing the activation pattern from electrode signals.
In the future, simultaneous measurement experiments using whole heart samples from large animals and clinical endocardial mapping catheters are necessary for more reliable validation. Furthermore, ablation experiments using developed simultaneous OPT and EXT mapping systems are necessary to evaluate and improve the efficacy of the ablation outcomes based on EXT diagnosis.
In this study, we evaluated the reliability of phase mapping by using EXT in comparison with simultaneous measurement of high-resolution OPT. The evaluation results revealed that the phase map sequence of EXT is consistent with the analysis of high-resolution optical mapping. EXT has some reliability for analyzing the activation pattern in the ROI.
N.T., T.A., I.S., and M.Y. received a research grant from Nihon Kohden Corporation. N.T., T.A., I.S., and M.Y. received research grant from Nihon Kohden Corporation. N.T., I.S., and M.Y. received research grants from Biosense Webster Inc.
This study was supported by Grants-in Aid for Scientific Research (18H02802) to M.Y., (18K18357) N.T., and (18H04161) I.S., from the Japanese Society for Promotion of Science in Japan; Nihon Kohden collaborative grants supported M.Y., N.T., I.S., and T.A.
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-19-1020