2020 Volume 84 Issue 4 Pages 650-655
2020 Volume 84 Issue 4 Pages 650-655
Background: Patients with severe Buerger disease, also known as thromboangiitis obliterans (TAO), are at risk of major limb amputation. It has been shown that autologous bone marrow mononuclear cell (BM-MNC) implantation improves the condition of critical limb ischemia in TAO patients. This study was conducted to further clarify the long-term (>10 years) results of autologous BM-MNC implantation in patients with TAO.
Methods and Results: An observational study was conducted of the long-term results of BM-MNC implantation in 47 lower limbs of 27 patients with TAO. The mean (±SD) follow-up period was 12.0±8.6 years. There was no major amputation event up to 10 years of follow-up in patients treated with BM-MNC implantation. The overall amputation-free survival rates were significantly higher in patients who underwent BM-MNC implantation than in internal controls and historical controls. There was no significant difference in amputation-free survival rates between the historical and internal controls. There was also no significant difference in overall survival between patients who underwent BM-MNC implantation and the historical controls.
Conclusions: BM-MNC transplantation successfully prevented major limb amputation over a period of >10 years in patients with severe TAO who had no other therapeutic options.
Thromboangiitis obliterans (TAO) or Buerger disease is an intractable peripheral arterial disease (PAD), defined in Japan as a specified rare disease in 1972. TAO is characterized by segmental lesions in arteries and veins of the extremities and is closely associated with smoking in young and middle-aged males.1 However, due to an increase in smoking among young females, TAO is also affecting this group in significant numbers. Although the cause of TAO is unknown, many studies have shown that the development of TAO is correlated with smoking, nutritional disorder, autoimmunity, activation of endothelial cells, and infection including chronic anaerobic periodontitis.2–6 Diagnostic criteria for TAO include age of onset <50 years, a history of smoking, occlusions observed in the popliteal artery or its distal branches, arterial occlusions or a history of migrating phlebitis, and no hypertension, dyslipidemia, or diabetes.1,5
Editorial p 549
Critical limb ischemia (CLI) in TAO patient leads to a risk of amputation.7 Disruption of blood supply occurs due to the gradual progression of inflammation in mid-sized and small arteries, with thrombosis in the extremities causing pain at rest, with or without tissue loss. This condition may also propagate into larger vessels and can occur in both the upper and lower extremities.
CLI is diagnosed when there is an abnormal ankle-brachial index (<0.90) with pain at rest and tissue loss due to severe PAD. Guidelines for determining the severity of CLI and appropriate treatments have been updated throughout the years.8–10 The treatment options for TAO patients are risk modification techniques, exercises, pain and ulcer management, oral medicines, lumbar or thoracic sympathectomy, and revascularization interventions performed via endovascular or bypass surgical approaches. Patients in whom revascularization is not an option are likely to undergo amputations.1,7 The risk of amputation persists in ongoing smokers for a median period of 14.8 years (mean 15.6 years) after the initial diagnosis of TAO, and the survival rate has been shown to be significantly lower in a TAO cohort than in a matched US population.11
In previous clinical studies, the trend for major amputation in patients with CLI after bone marrow mononuclear cell (BM-MNC) implantation decreased in patients with atherosclerotic PAD and patients with TAO.12–16 Patients with TAO have less remarkable underlying medical complications. Hence, limb salvage and survival rates are significantly higher in patients with TAO after cell therapy. However, there is limited information on long-term clinical outcomes (i.e., >10 years) after cell implantation in severe TAO patients.
This study determined the long-term (>10 years) outcomes in TAO patients with CLI who underwent BM-MNC implantation, with the aim of finding ways to reduce the amputation rate and improve overall survival for patients with no subsequent therapeutic options.
Patient recruitment and BM-MNC implantations were performed between May 2002 and April 2014. Twenty-seven patients with severe TAO were diagnosed after they presented with severe pain at rest and non-healing ulcers. Patients who were not candidates for angioplasty or surgical revascularization underwent BM-MNC implantation. For current and historical control analyses, data were obtained from the Hiroshima University PAD database. In Japan, TAO is diagnosed according to the Guidelines for Management of Vasculitis Syndrome (Japanese Circulation Society [JCS] 2008).11 To rule out other vasculitis and hypercoagulable states, rheumatoid factors and lupus anticoagulants were assessed and serological investigations conducted. The diagnosis of arterial occlusion leading to ischemia was confirmed by angiography. CLI was classified according to the Trans-Atlantic Inter-Society Consensus Document on Management of Peripheral Arterial Disease (TASC) II guidelines.9 Major amputation is defined as an above-the-ankle amputation.
The Ethics Committee of Hiroshima University Graduate School of Biomedical Sciences approved the study protocol. Informed consent was obtained from all subjects as part of the clinical trial. This study is registered with the UMIN Clinical Trials Registry (ID: UMIN00000492).
Data CollectionData were obtained from the Hiroshima University PAD database.12 Statistical analyses were performed for 47 treated limbs in 27 patients with TAO.
In addition, historical and control groups were included in the study. Historical control data were obtained retrospectively from the Hiroshima University Hospital PAD database for 18 TAO patients with 22 affected limbs up to October 2018 who had been treated previously for CLI in Hiroshima University Hospital.15 The internal control consisted of an ischemic leg similar to that in which BM-MNC implantation was performed.
The major amputation-free period was defined as the time from the day on which major amputation was decided on to the day BM-MNC implantation was performed.15
Study DesignThis study was a retrospective observational study to determine the long-term outcomes of BM-MNC implantation in CLI patients. The short-term results for these patients were reported in 2011.12 In the present study, additional data for the patients was obtained to determine overall survival and major amputation-free survival rates. In addition, we report causes of death in patients after more than 10 years of follow-up. Amputation-free survival rates were compared between TAO patients and both the internal controls and historical controls. To identify limb survival projections as an internal control, the same limbs that were diagnosed with severe TAO and had no option for conventional treatments were estimated for amputation at the time of BM-MNC implantations were performed.15
BM-MNC ImplantationBM-MNCs were isolated and implanted in TAO patients as described previously.12,15 Briefly, 500 mL bone marrow was aspirated from the ileum of patient under general anesthesia and BM-MNCs were immediately isolated using a CS3000-Plus blood-cell separator (Baxter, Deerfield, IL, USA) to obtain a final volume of 50 mL. Using a 22-gauge needle, 1-mL BM-MNCs was implanted intramuscularly into each site, 40 sites in total over a 3-cm×3-cm grid, at a depth of 1.5 cm into the gastrocnemius of the ischemic leg. The mean (±SD) number of BM-MNCs implanted per patient was 1.8×109±0.5×109.
Statistical AnalysisResults for categorical variables are presented as frequencies, and for continuous variables as the mean±SD. All reported probability values were 2-tailed, and P<0.05 was considered significant. Continuous variables were compared between 2 groups using t-tests. Categorical variables were compared using Chi-squared or Fisher exact tests. Time-to-event endpoint analyses were performed using the Kaplan-Meier method. A log-rank test was used to compare amputation-free survival and overall survival between groups. Data were processed using JMP version 13.0 (SAS Institute, Cary, NC, USA).
Baseline clinical characteristics in the BM-MNC implantation and historical control groups are summarized in Table. There were significant differences in sex and the use of antiplatelet medications between the 2 groups. There were no significant differences in the other parameters. The mean follow-up period was 12.0±8.6 years.
| Historical control (n=18) |
BM-MNC implantation (n=27) |
P-value | |
|---|---|---|---|
| Age (years) | 49.3±14.6 | 40.0±10.8 | 0.128 |
| No. men/women | 8/10 | 24/3 | <0.005 |
| Body mass index (kg/m2) | 23.9±3.9 | 22.9±4.0 | 0.499 |
| Fontaine category, n (%) | |||
| III | 2 (11) | 10 (37) | |
| IV | 16 (89) | 17 (63) | |
| Rutherford category, n (%) | |||
| 3 | 0 (0) | 2 (7) | |
| 4 | 2 (11) | 8 (30) | |
| 5 | 15 (83) | 14 (52) | |
| 6 | 1 (5) | 3 (11) | |
| Medical history, n (%) | |||
| Hypertension | 3 (18) | 1 (4) | 0.286 |
| Dyslipidemia | 4 (22) | 10 (37) | 0.343 |
| Diabetes | 0 (0) | 2 (7) | 0.509 |
| Chronic kidney disease | 0 (0) | 0 (0) | NA |
| Smoker (pre) | 17 (94) | 27 (100) | 0.400 |
| Medications, n (%) | |||
| Anticoagulants | 3 (17) | 3 (11) | 0.670 |
| Antiplatelets | 9 (50) | 24 (89) | 0.006 |
| ACE inhibitors | 1 (6) | 0 (0) | 0.400 |
| ARBs | 1 (6) | 1 (4) | >0.99 |
| Calcium channel blockers | 3 (17) | 5 (18) | >0.99 |
| Statins | 1 (6) | 1 (4) | >0.99 |
| Sulfonylurea, metformin, other | 0 (0) | 0 (0) | NA |
| Insulin | 0 (0) | 0 (0) | NA |
| Previous therapies, n (%) | |||
| Bypass operation | 1 (6) | 5 (18) | 0.377 |
| Endovascular treatment | 2 (11) | 0 (0) | 0.154 |
| Sympathectomy or nerve block | 5 (28) | 6 (22) | 0.732 |
Unless indicated otherwise, data are presented as the mean±SD or as n (%). ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; BM-MNC, bone marrow mononuclear cells; NA, not applicable.
Kaplan-Meier analysis showed that the 27 TAO patients who underwent BM-MNC implantation had better major amputation-free survival outcomes than did the internal controls and historical controls (Figure 1). There was no major amputation up to 10 years of follow-up after diagnosis of TAO in the BM-MNC implantation group. There was no significant difference in overall major amputation-free survival rates between the internal controls and historical controls (P=0.305).
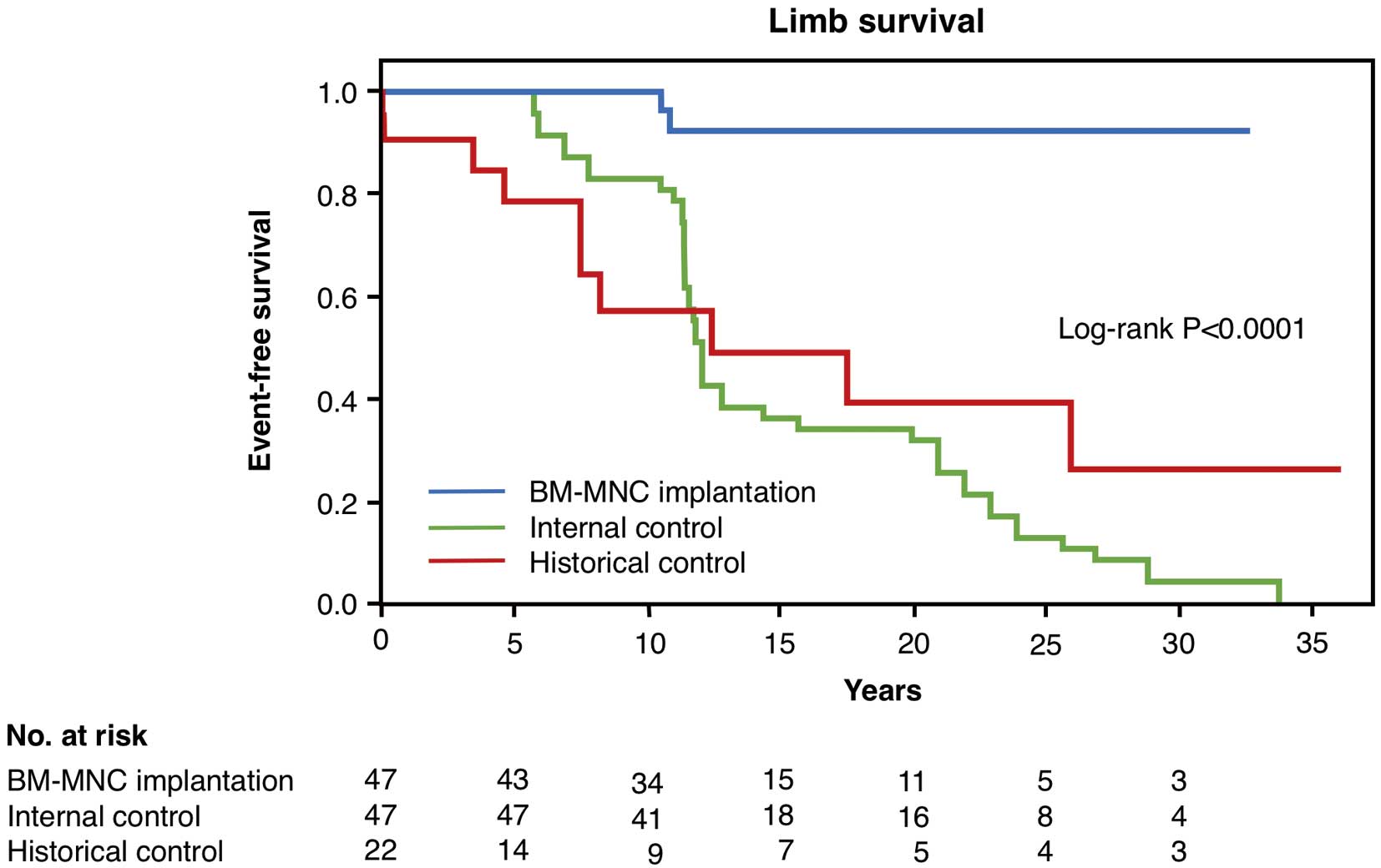
Major amputation-free survival rates in thromboangiitis obliterans patients who underwent bone marrow mononuclear cell (BM-MNC) implantation, internal controls, and historical controls.
There was no significant difference in overall survival rates between patients who underwent BM-MNC implantation and historical controls (Figure 2). After more than 10 years of follow-up data were reviewed, there were 2 deaths in the BM-MNC implantation group due to lung malignancy and abdominal aorta aneurysm leak. In the historical group, there was 1 death due to lung malignancy.

Overall survival rates in thromboangiitis obliterans patients who underwent bone marrow mononuclear cell (BM-MNC) implantation and historical controls.
There were no severe acute adverse effects in patients who underwent autologous BM-MNC implantation.
This study was conducted to determine the long-term outcomes of BM-MNC implantation performed in TAO patients with CLI from 2002 to 2014 at Hiroshima University Hospital in Japan. The length of the follow-up period from TAO diagnosis among the patients, including those in the historical control group, was >30 years.
TAO is a non-atherosclerotic segmental inflammatory disease that most commonly affects small and medium-sized arteries and veins in the upper and lower extremities in relatively young and middle-aged patients. The characteristic acute phase lesions associated with occlusive cellular thrombosis and acute inflammation involving all layers of the vessel wall led TAO to be classified as a vasculitis.2 Leo Buerger took 18 years to reach a conclusion regarding his findings in circulatory disturbances of the extremities, including gangrene and vasomotor and trophic disorders. Buerger correlated the features of TAO to possible etiological factors, including smoking, that had been suggested by several investigators.17 Since then, repeated reviews over the years have highlighted the incidence of TAO in young and middle-aged men. Despite the rarity of TAO diagnoses in women, the proportion of women diagnosed with TAO has been reported to range between 10% and 23%.1,18–22 In the present study, there was difference in the proportion of males and females in the BM-MNC implantation and historical control groups. Data for these patients was obtained from the Hiroshima University Hospital database. As a tertiary center receiving severe and uncommon PAD referrals, there was a slightly higher number of female patients with TAO arriving for treatment prior to the BM-MNC implantation study. Because the recruitment of PAD patients was intensified during the period of the BM-MNC implantation clinical trial, there were more male patients with severe TAO referred to the center.
The principal treatments for TAO include encouraging smoking cessation, avoiding passive smoke, walking and exercise strategies, as well as keeping the affected limbs warm and avoiding exposure to low temperatures. The treatment options include pharmaceutical treatment, exercise therapies, conventional revascularization for suitable patients, sympathectomy, and pain control.11 Therapeutic angiogenesis by means of gene therapy and cell therapy, including autologous BM-MNC transplantation, peripheral mononuclear cell transplantation, and endothelial progenitor cell transplantation, is being developed to provide options for effective revascularization.12–16
Cell therapy for CLI patients was first reported in Japan in 2002 by Tateishi-Yuyama et al, who investigated the efficacy of intramuscular implantation of autologous BM-MNCs.14 The Therapeutic Angiogenesis by Cell Transplantation (TACT) trial demonstrated that cell therapy using intramuscular implantation of BM-MNCs leads to extension of the amputation-free interval and improvements in ischemic pain, ulcer size, and pain-free walking distance.14 The safety and efficacy of cell therapy are not inferior to conventional revascularization therapies, and cell therapy is more effective in patients with TAO than in patients with PAD.13 Kondo et al investigated the long-term clinical outcomes in patients in the TACT trial with a median follow-up period of 31.7 months and concluded that therapeutic angiogenesis using autologous BM-MNC implantation is feasible and safe in patients with no-option CLI, particularly patients with CLI caused by TAO or collagen disease-associated vasculitis.16
The mononuclear cell population in bone marrow includes hematopoietic progenitor cells, lymphoid cells, monocytes, endothelial progenitor cells, and cells of non-hematopoietic lineage. These cells are recruited to injured tissue throughout the circulatory system in response to large amounts of cytokines and growth factors from locations of affected tissue to prevent apoptosis, protect viable cells, elicit anti-inflammatory effects, and reduce fibrosis. Recruitment of specific stem cells leads to stimulation of angiogenesis. Local implantation of autologous BM-MNCs augmented angiogenesis and collateral vessel formation in an ischemic limb model.23 Paracrine effects on resident endothelial cells by secretion of angiogenic growth factors and cytokines to increase neovessel formation at the capillary level were made possible by direct implantation of BM-MNCs, leading to improved blood supply to the ischemic tissue. Ischemic tissue is unable to recruit cells required for repair and to remain viable due to disrupted vessels. As the ischemic tissue regresses, an inadequate cellular response causes further deterioration of the affected limb. Implantation of BM-MNCs into ischemic limbs promotes the acute phase of paracrine-mediated stimulation because of the direct availability of the mononuclear cell population. Promotion of postnatal neovascularization by sheer stress and the supply of angiogenic cytokines after BM-MNC implantation lead to increases in collateral blood vessel formation, which is of utmost importance at the microcirculation level. These cells do not remain in the tissue in the long term. As the collaterals become mature and stable, blood supply to the affected tissue is improved. With the improvement in collateral blood vessels and adequate inflow of blood, the threatened limbs are no longer deemed critical for amputation. The availability of collaterals, established after the acute phase of BM-MNC implantation, would continue to help with microcirculation homeostasis and for the tissue to remain viable. We speculate that avoiding major amputation of the threatened limb during the critical phase and optimized medical care would enable major amputation to be avoided in the long term.15
The analyses in the present study of the outcomes of patients who underwent BM-MNC implantation and optimal medical care with risk modifications over a median follow-up period of 12.0±8.6 years revealed that the patients with severe TAO who underwent cell therapy had significantly better limb survival than did the internal controls and historical controls (Figure 1). There were no significant differences in limb survival between the internal controls and historical controls. It was thought that the patients who underwent BM-MNC implantation would have to undergo amputation if they did not receive cell therapy.
Limb amputation is an important risk that could worsen the overall outcome of patients with CLI.24 Nevertheless, in the present study, despite better limb survival in the cell therapy group, there were no significant differences in survival rates between the BM-MNC and historical control groups. In patients with atherosclerotic PAD, a history of amputation is a predictor of overall outcome, including mortality.7,24 However, it is known that there is no significant difference in the overall survival rate between patients with TAO and age- and sex-matched healthy subjects, despite patients with TAO having ulcers, with or without amputations. Only older age at diagnosis and a decade since diagnosis were associated with an increased risk of death.21 Indeed, although Le Joncour et al. reported that 10-year vascular event-free and amputation-free rates were 23% and 74%, respectively, in patients with TAO, there were only 3 unknown deaths reported (1.3%) and there was no cardiovascular death during long follow-up periods.18 In addition, Guo et al reported that cell therapy improved the amputation-free survival rate over a 10-year follow-up period in Chinese patients with TAO who underwent BM-MNC implantations compared with the control group and that there were no deaths, major adverse cardiac events, or malignancy in the 2 groups during the follow-up period.25 These findings suggest that increases in cardiovascular events, including limb amputation, are not related to the increase in mortality rate in TAO patients with and without cell therapy. We should consider various factors (e.g., age, race, sex, and other traditional cardiovascular risk factors) influencing cardiovascular events in TAO patients. Further studies are needed to establish the long-term overall survival rate of TAO patients who have undergone cell therapy in a large trial.
Study LimitationsThis study was not a prospective randomized trial and was a single-center trial. The number of patients recruited was small. BM-MNC implantation in patients with TAO was performed according to inclusion and exclusion criteria and a treatment strategy using an ideal protocol of the TACT trial.13,14 The retrospectively data for the historical control were collected with no inclusion and exclusion criteria. The patients were referred to the University Hospital Vascular Unit due to the severity of the disease, which rendered them unsuitable for conventional treatments. There were significant differences in sex and the use of antiplatelet medications between the groups. We cannot rule out the possibility that these factors influenced the outcome of this study. Assessments of factors and functional parameters (e.g., post-treatment smoking habit, ulcer size, incidence of infection and necrosis, pain score, ulcer healing, pre-post 6-minute walk distance, and maximum walking distance) and perfusion indices (e.g., pre-post ankle-brachial index, transcutaneous oxygen pressure, skin perfusion pressure, and angiographic improvements) would enable more specific conclusions to be drawn regarding the role of BM-MNC implantation in the prevention of major amputation in patients with CLI. Evaluation of cardiovascular outcomes and the onset of cancer using a prospective randomized controlled study design is needed. In addition, we were unfortunately not able to obtain more accurate information on comorbidities other than death and major amputation during the long-term follow-up period.
Autologous BM-MNC transplantation successfully prevented major limb amputation in TAO patients with CLI over a long-term (>10 years) period and improved the amputation-free survival rate in patients who had no other options for therapy.
The authors thank Megumi Wakisaka and Satoko Michiyama for their excellent secretarial assistance.
This study was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science and Culture of Japan (18590815 and 21590898 to Y.H.) and a Grant-in-Aid of Japanese Arteriosclerosis Prevention Fund (to Y.H.). In addition, funding support was received from the Tsuchiya Foundation (to M.K.). The authors received no specific funding for this work.
The authors declare no conflicts of interest associated with this manuscript.