2020 Volume 84 Issue 5 Pages 799-805
2020 Volume 84 Issue 5 Pages 799-805
Background: The combination of a bioresorbable scaffold and antiproliferative drugs is a promising treatment for peripheral artery disease. The novel paclitaxel-eluting peripheral Igaki-Tamai stent (PTX-ITS) has the same backbone design as the drug-free peripheral Igaki-Tamai stent and a paclitaxel coating. Arterial responses to the PTX-ITS and ITS using optical coherence tomography (OCT) and histological analysis in a porcine iliac artery model were compared.
Methods and Results: In total, 6 PTX-ITSs and 6 ITSs implanted in porcine iliac arteries were evaluated. Quantitative measurements of the scaffold, lumen, neointimal areas, and percent area stenosis were performed using OCT at 1 and 3 months. Histological evaluations (PTX-ITS [n=5], ITS [n=4]) were performed following euthanasia at 3 months. Injury, inflammation, endothelialization, and fibrin scores were measured. Baseline angiographic characteristics were similar in both groups. The ITS group showed significantly smaller scaffold areas than the PTX-ITS group at 1 month (18.50±3.62 mm2 vs. 23.54±3.64 mm2; P=0.037) and 3 months (15.82±2.57 mm2 vs. 21.67±3.57 mm2; P=0.009). Percent area stenosis was significantly lower in the PTX-ITS group at 3 months (28.70±7.24% vs. 40.36±7.07%; P=0.018). Histological evaluations revealed similar low-grade inflammatory reactions for both scaffolds.
Conclusions: PTX-ITSs showed significantly better suppression of late scaffold shrinkage and lower in-scaffold stenosis for up to 3 months. Additionally, PTX-ITSs exhibited high biocompatibility, which is comparable to ITSs.
The fully bioresorbable scaffold (BRS) is a promising option that epitomizes the “leave nothing behind” concept in the endovascular therapy (EVT) for femoro-popliteal (FP) occlusive disease. The drug-free peripheral Igaki-Tamai stent (ITS; Kyoto Medical Planning Co., Ltd., Kyoto, Japan) is the first commercially available peripheral BRS, and it has been used for treating peripheral arteries in Europe since 2009. This scaffold is made of a drug-free bioresorbable poly-l-lactide (PLLA) polymer. Several non-randomized clinical trials have evaluated the efficacy of ITS in non-complex FP lesions.1,2 Despite high technical success rates and acceptable short-term results, low primary patency rates at 12 months (32.1–58%) have prevented this scaffold from being applied routinely in the clinical setting. These results may be partly induced by the non-drug-eluting property of the scaffold. Therefore, an antiproliferative drug-eluting BRS is anticipated to be the genuine solution epitomizing the “leave nothing behind” concept.
As an antiproliferative agent, paclitaxel has been used widely in coronary3,4 and peripheral arteries.5–7 By interfering with microtubule function, paclitaxel inhibits the migration and proliferation of vascular smooth muscle cells, resulting in the suppression of neointimal hyperplasia. We developed the paclitaxel-eluting peripheral Igaki-Tamai stent (PTX-ITS), which has the same backbone design as that of the ITS, with the added property of paclitaxel elution. The aim of this study was to compare arterial responses to the PTX-ITS and ITS in a porcine iliac artery model using optical coherence tomography (OCT) and histological analysis.
An ITS is made of drug-free bioresorbable PLLA polymer fabricated into a zigzag helical coil structure with a strut thickness of 240 μm (Figure 1A). Previous data suggest that the polymer is completely resolved within 3 years after deployment.8 The PTX-ITS is a newly developed BRS with the same backbone design as that of the ITS (Figure 1B), with the backbone strut thickness reduced to 230 μm. The drug-free PLLA polymer backbone is coated with a bioresorbable PLLA polymer matrix loaded with paclitaxel (0.76 μg/mm2) for continuous drug release. A top coat of polymer-free microcrystals of paclitaxel (0.85 μg/mm2) is added through a process of seeding and crystal growth for early drug release (Figure 1C).9 The dose of paclitaxel in PTX-ITS was chosen based on our preliminary dose-escalation study (data not shown). The total strut thickness is 240 μm (comprising the 230-μm PLLA polymer backbone and 10-μm coating layer). The top coating releases ∼80% of the loaded paclitaxel within 24 h, with complete release within 7 days. Furthermore, continuous drug release from the PLLA polymer matrix continues over 90 days.
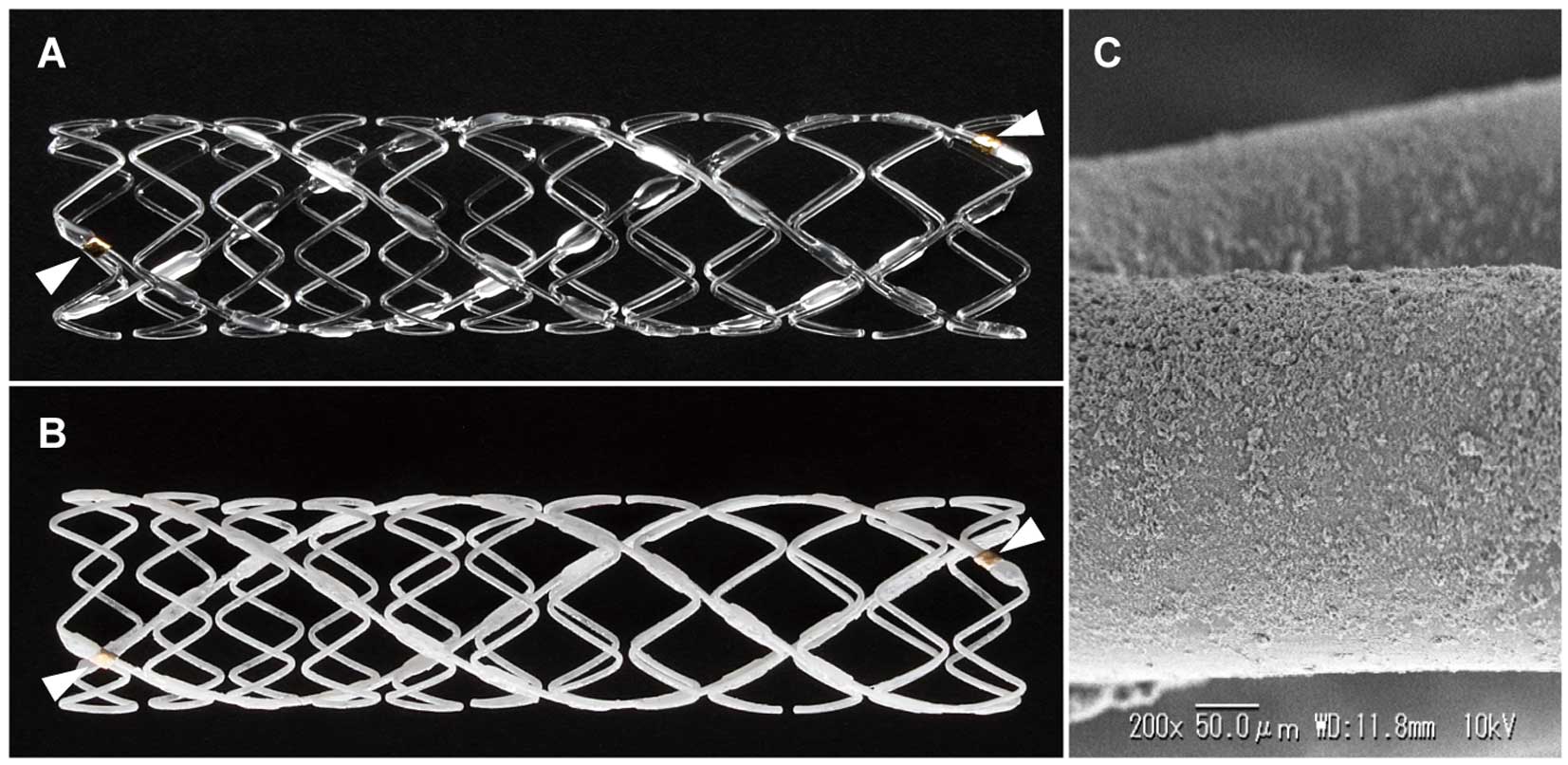
Designs of the 2 fully bioresorbable scaffolds. (A) Drug-free peripheral Igaki-Tamai stent (ITS). (B) Paclitaxel-eluting peripheral Igaki-Tamai stent (PTX-ITS). The PTX-ITS has the same backbone design of the ITS, with the addition of a paclitaxel coating. The arrowheads indicate a radio-opaque gold marker in each end of those scaffolds. (C) The scanning electron microscopy (SEM) image of the paclitaxel coating on the surface of the PTX-ITS.
Both scaffolds are radiolucent and have a radio-opaque gold marker at each end (Figure 1A,B). A balloon-expandable covered sheath system compatible with a 7-Fr sheath and a 0.018-inch wire was used for deployment. The device sizes used in this study were 6.0×38 mm and 7.0×38 mm for both scaffolds.
Study Design and Animal ExperimentsThis study received protocol approval from the Institutional Animal Care and Use Committee (approval number: 2017-74, 2017-75). Experiments were conducted in accordance with the Guide to Care and Use of Laboratory Animals (National Institutes of Health 1996). The study design is shown in Figure 2. Eight non-atherosclerotic Nippon Institute for Biological Science (NIBS) swine (3 male and 5 female), weighing 35–50 kg, underwent implantation using a general sterile technique via carotid access. Administration of clopidogrel 75 mg and acetylsalicylic acid 200 mg was initiated 3 days and 2 days before the procedure, respectively, and continued until termination. For the scaffold implantation and follow-up procedures, general anesthesia was induced using 2 mg/kg of xylazine, 15 mg/kg of ketamine, and 0.05 mg/kg of atropine by intramuscular injection and maintained with isoflurane inhalation. A 150 U/kg dose of heparin was administered intra-arterially at the beginning of the procedure. Nitroglycerin was administered intra-arterially to prevent and relieve arterial spasms. An angiographic analysis was performed before and immediately after the procedure to ensure proper scaffold implantation. All animals were implanted with a PTX-ITS or ITS in each external iliac artery according to a pre-defined matrix. The implanted scaffold size was matched in accordance with a target balloon-to-artery ratio of 1.2–1.35. In total, 9 PTX-ITSs (7.0×36 mm [n=6], 6.0×36 mm [n=3]), and 7 ITSs (7.0×36 mm [n=5], 6.0×36 mm [n=2]) were implanted, with both types of scaffolds implanted in 7 animals and only PTX-ITSs implanted in 1 animal (for additional data on PTX-ITS). All animals underwent serial follow up at 1 month and 3 months. At the time of each follow up, angiographic and OCT analyses were conducted. All animals were humanely euthanized after the final follow up at 3 months.

Study flow chart. PTX-ITS, paclitaxel-eluting peripheral Igaki-Tamai stent; ITS, drug-free peripheral Igaki-Tamai stent; OCT, optical coherence tomography.
For the histological analysis, 5 iliac arteries implanted with a PTX-ITS and 4 iliac arteries implanted with an ITS were harvested and fixed with neutral 10% buffered formalin solution following perfusion with saline immediately after euthanasia. The tissues were embedded in paraffin and cut at 3 in-scaffold levels (proximal, middle and distal). The sections were stained with hematoxylin-eosin and evaluated with light microscopy. The rest of the iliac arteries (1 implanted with a PTX-ITS and 2 implanted with an ITS) were pre-determined to be used for the assessment of mechanical strength (data not shown).
Quantitative Angiographic AnalysisQuantitative angiographic analysis was performed offline using QAngio XA software version 7.3 (Medis Medical Imaging Systems, Leiden, The Netherlands). The marker wire placed in the iliac artery was used for calibration. The mean lumen diameter and minimal lumen diameter (MLD) were measured before and after scaffold implantation and at the 1-month and 3-month follow ups. Late lumen loss (LLL) was defined as the difference between post-implantation MLD and the MLD at each follow up.
OCT AcquisitionIntravascular OCT imaging using a commercially available frequency-domain OCT system (ILUMIEN; St. Jude Medical, St. Paul, MN, USA) was performed at the 1-month and 3-month follow ups. Before acquiring the OCT image, flow arrest was obtained by temporary balloon occlusion using a 7-Fr balloon-tipped occlusion catheter (Optimo; Tokai Medical Products, Aichi, Japan) in the proximal external iliac artery. Automated pullback (20 mm/s and 100 frames/s) was performed during the continuous injection of contrast medium.
OCT AnalysisOCT measurements were performed using commercial software for off-line analysis (LightLab Imaging, Westford, MA, USA). Cross-sectional OCT images were analyzed at 1-mm longitudinal intervals according to previously reported methods.10 The following quantitative measurements were performed after calibration: scaffold area (SA), lumen area (LA), neointimal area (NIA=SA–LA), and percent area stenosis ([NIA/SA]×100).
Histological AnalysisThe histological analysis was conducted by an independent pathology laboratory (CMIC Pharma Science Co., Ltd., Yamanashi, Japan). The injury score was graded for each strut, as previously described.11 The injury score for each cross-section was calculated by dividing the total injury score by the total number of struts at that cross-section. The inflammation score for each strut was semi-quantitatively scored as follows: 0, no inflammatory cells surrounding the strut; 1, slight, non-circumferential inflammatory cells infiltrating and surrounding the strut; 2, localized, moderate-to-dense cellular aggregate surrounding the strut non-circumferentially; and 3, circumferential dense inflammatory cells infiltrating the strut.12 The inflammation score for each cross-section was calculated by dividing the total inflammation score by the total number of struts at that cross-section. The endothelialization score was defined as follows: 1, endothelial cells cover <25% of the circumference of the artery; 2, endothelial cells cover 25–75% of the circumference of the artery; and 3, endothelial cells cover >75% of the circumference of the artery.13 As a marker of an active antiproliferative drug effect, fibrin deposition (0–3) was semi-quantitatively scored for each cross-section, as previously described.13
Statistical AnalysisContinuous variables with normal distribution were presented as the mean±standard deviation. Data normality was assessed using the Shapiro-Wilk test. Categorical valuables are expressed as the median with interquartile range. Intergroup comparisons for continuous variables with normal distribution were performed using the 2-tailed Student’s t-test. The Mann-Whitney U-test was used to compare categorical valuables. P-values <0.05 were considered significant. All statistical analyses were performed using JMP 12.0 (SAS Institute, Cary, NC, USA).
All animals remained healthy until the final follow up. One PTX-ITS was excluded because of a high balloon-to-artery ratio of 1:1.4 at the index procedure, as this deviated from the study protocol. During follow up, 2 PTX-ITSs and 1 ITS were shown to be disrupted and an OCT analysis was not feasible. These scaffolds were also excluded from the final evaluation. In total, 6 PTX-ITSs (7.0×36 mm [n=4], 6.0×36 mm [n=2]) and 6 ITSs (7.0×36 mm [n=4], 6.0×36 mm [n=2]) were included in the final evaluation.
Angiographic AnalysisBalloon-to-artery ratios were similar in both groups (PTX-ITS 1.31±0.03 vs. ITS 1.29±0.06; P=0.583). Baseline and follow-up angiographic parameters are shown in Table 1. Angiographic characteristics at pre- and post-implantation were not significantly different between the groups. The mean lumen diameter was significantly larger in the PTX-ITS group than in the ITS group at 1 month and 3 months. The MLD was significantly larger in the PTX-ITS group than in the ITS group at 1 month (P=0.008). Although numerically appreciable, the difference in MLD only approached statistical significance at 3 months (P=0.050). The LLL was significantly smaller in the PTX-ITS group than in the ITS group at 1 month and 3 months. Representative angiographic images at baseline and follow up are presented in Figure 3.
| Pre-implantation | Post-implantation | 1-month FU | 3-month FU | |
|---|---|---|---|---|
| Mean lumen diameter (mm) | ||||
| PTX-ITS | 4.97±0.38 | 4.68±0.62 | 4.76±0.52 | 4.26±0.41 |
| ITS | 4.98±0.57 | 5.06±0.50 | 3.69±0.61 | 3.41±0.45 |
| P-value | 0.968 | 0.271 | 0.008* | 0.007* |
| MLD (mm) | ||||
| PTX-ITS | 4.69±0.39 | 4.08±0.35 | 4.30±0.52 | 3.46±0.50 |
| ITS | 4.62±0.59 | 4.23±0.35 | 3.02±0.79 | 2.92±0.32 |
| P-value | 0.808 | 0.488 | 0.008* | 0.050 |
| LLL (mm) | ||||
| PTX-ITS | NA | NA | −0.22±0.35 | 0.62±0.41 |
| ITS | NA | NA | 1.21±1.06 | 1.31±0.57 |
| P-value | NA | NA | 0.010* | 0.037* |
Values are expressed as mean±standard deviation. *Statistically significant difference. FU, follow-up; PTX-ITS, paclitaxel-eluting peripheral Igaki-Tamai stent; ITS, drug-free peripheral Igaki-Tamai stent; MLD, minimal lumen diameter; LLL, late lumen loss; NA, not applicable.

Representative angiographic images of the same iliac artery for each scaffold at baseline and at the 1-month and 3-month follow ups. The arrows indicate radio-opaque markers at the proximal and distal ends of the scaffolds. ITS, drug-free peripheral Igaki-Tamai stent; PTX-ITS, paclitaxel-eluting peripheral Igaki-Tamai stent.
Overall, 791 OCT cross-sections were analyzed. The analysis was performed at the device level. The SA was significantly smaller in the ITS group than in the PTX-ITS group at 1 month (18.50±3.62 mm2 vs. 23.54±3.64 mm2; P=0.037). This difference remained significant at 3 months (15.82±2.57 mm2 vs. 21.67±3.57 mm2; P=0.009) (Figure 4A). The LA was also significantly smaller in the ITS group than in the PTX-ITS group throughout the follow-up period (P=0.002 at 1 month and P=0.003 at 3 months) (Figure 4B). The PTX-ITS group showed a significantly smaller NIA than did the ITS group at 1 month (P=0.005), but this difference vanished at 3 months (P=0.819) due to a subsequent increase of neointima in the PTX-ITS group (Figure 4C). The percent area stenosis was significantly lower in the PTX-ITS group than in the ITS group at 1 month (16.56±3.53% vs. 33.83±4.63%; P<0.001) and 3 months (28.70±7.24% vs. 40.36±7.07%; P=0.018) (Figure 4D). Representative OCT images at 1 month and 3 months, demonstrating a larger SA and lower in-scaffold stenosis in the PTX-ITS group than in the ITS group for up to 3 months, are presented in Figure 5.

Optical coherence tomography (OCT) results at the 1-month and 3-month follow ups. Results at the 1-month and 3-month follow ups demonstrate a greater scaffold area (A) and lumen area (B) in the paclitaxel-eluting peripheral Igaki-Tamai stent (PTX-ITS) compared to that in the drug-free peripheral Igaki-Tamai stent (ITS) for up to 3 months. The PTX-ITS shows a significantly smaller neointimal area (C) than the ITS at 1 month, but the difference vanishes at 3 months. The percent area stenosis (D) is lower in the PTX-ITS group compared to the ITS group at the 1-month and 3-month follow ups. Box plots indicate the median value (within boxes), interquartile range (upper and lower limits of boxes), and maximal and minimal values (upper and lower bars outside of the boxes), respectively. ITS, drug-free peripheral Igaki-Tamai stent; PTX-ITS, paclitaxel-eluting peripheral Igaki-Tamai stent.

Representative optical coherence tomography (OCT) images at the 1-month and 3-month follow ups. The longitudinal OCT assessments of the drug-free peripheral Igaki-Tamai stent (ITS, 6.0×38 mm) and paclitaxel-eluting peripheral Igaki-Tamai stent (PTX-ITS, 6.0×38 mm) are shown.
Overall, 27 cross-sections and 355 struts were analyzed. The analysis was performed at the cross-section level. The results are summarized in Table 2. The injury scores were low and comparable between groups (P=0.180). The inflammation scores were minimal to mild in both groups, with no significant difference between the groups (P=0.520). The scaffold struts in both groups were well endothelialized at 3 months. There was no fibrin deposition in the ITS group, while mild deposits of fibrin were observed in the PTX-ITS group (ITS 0.00 [0.00–0.00] vs. PTX-ITS 1.00 [1.00–1.00]; P<0.001). Representative histological cross-sections for each scaffold are shown in Figure 6. The depletion of medial smooth muscle cells was evident only in the PTX-ITS group (Figure 6D).
| PTX-ITS (n=15) |
ITS (n=12) |
P-value | |
|---|---|---|---|
| Injury score | 0.46 (0.31–0.75) | 0.69 (0.39–0.84) | 0.180 |
| Inflammation score | 0.00 (0.00–0.08) | 0.04 (0.00–0.28) | 0.520 |
| Endothelialization score | 3.00 (3.00–3.00) | 3.00 (3.00–3.00) | 1.000 |
| Fibrin score | 1.00 (1.00–1.00) | 0.00 (0.00–0.00) | <0.001* |
Values are expressed as median (interquartile range). *Statistically significant difference. Abbreviations as in Table 1.

Representative images of hematoxylin-eosin stained cross-sections 3 months after scaffold deployment. (A) Drug-free peripheral Igaki-Tamai stent (ITS). (B) High-power photomicrograph of an ITS showing minimal inflammatory response around the scaffold struts. (C) Paclitaxel-eluting peripheral Igaki-Tamai stent (PTX-ITS). (D) High-power photomicrograph of a PTX-ITS, showing a low inflammatory response around the scaffold struts, similar to that in the ITS. Note: *Indicates the marked depletion of smooth muscle cells in the tunica media.
In the present study, we compared arterial responses to a novel paclitaxel-eluting peripheral BRS (PTX-ITS) and a commercially available drug-free peripheral BRS (ITS) in a porcine iliac artery model. The main findings of the present analysis were as follows: (1) scaffold shrinkage was observed in ITSs at 1 month, whereas PTX-ITSs showed less shrinkage for up to 3 months; (2) in-scaffold stenosis on OCT and angiographic late loss were significantly lower for PTX-ITSs than for ITSs at both 1 month and 3 months; and (3) the histological analysis showed high biocompatibility for both the PTX-ITS and ITS.
Late Scaffold Shrinkage in the ITSHigh rates of restenosis after ITS implantation in peripheral arteries have been reported in clinical trials,1,2 but so far, the mechanism of restenosis has not been evaluated clinically using imaging modalities, especially OCT. In the present study, a significantly smaller SA was detected with ITSs than with PTX-ITSs, both at 1 and 3 months, using OCT. Considering the similar angiographic post-implantation lumen diameter in both scaffolds, the smaller SA at follow up in ITSs may be ascribed to late scaffold shrinkage. This result is congruent with that of a recent comparative analysis using intravascular ultrasound after ITS and self-expanding nitinol bare-metal stent (BMS) implantation in porcine iliac arteries, whereby a significant decrease in the external elastic membrane area was detected at 6 weeks only for ITSs.14 Metallic stents conquer negative remodeling due to their strong radial force; however, this is not the case for ITSs because of their relatively weak mechanical performance during the degradation process, resulting in late scaffold shrinkage. Using OCT, our study demonstrated that late scaffold shrinkage is a major cause of restenosis with ITSs.
Suppression of Late Scaffold Shrinkage by PaclitaxelCompared to ITSs, suppression of late scaffold shrinkage was observed for PTX-ITSs in the present study. This phenomenon may be attributed to paclitaxel, as both scaffolds are expected to have a similar radial force due to the use of the same polymer and backbone structure. To the best of our knowledge, no other report has assessed the effect of paclitaxel on the suppression of late scaffold shrinkage after BRS implantation. One hypothesis is that paclitaxel inhibits negative remodeling by interfering with smooth muscle cell function and proliferation in the tunica media. Medial smooth muscle cells are involved in the maintenance of vascular tonus; thus, their impediment probably results in reduced negative remodeling. Our histological analysis revealed marked depletion of medial smooth muscle cells only for PTX-ITSs (Figure 6D). The same phenomenon has been reported in other animal studies using paclitaxel-coated stents.15,16
As in previous studies showing an effect of paclitaxel on the inhibition of neointimal proliferation after EVT in peripheral arteries,5,6,17 neointimal proliferation was significantly inhibited for PTX-ITSs at 1 month and 3 months compared to that for ITSs, as demonstrated by a lower percent area stenosis on OCT. Although the neointimal area on OCT was not statistically different in the groups at 3 months, when considering that a marked scaffold shrinkage only occurred for ITSs, the percent area stenosis might reflect the neointimal reaction more exactly than the neointimal area in our model.
Histological Responses After Scaffold ImplantationOne concern associated with BRS implantation is the arterial inflammatory reaction to the polymer and its degradation products. The ITS and PTX-ITS use the same polymer as the coronary Igaki-Tamai stent, which was the first fully bioresorbable coronary scaffold implanted in humans. The high biocompatibility of the coronary Igaki-Tamai stent has been shown in animals18 and humans.8,19,20 Consistent with these previous reports, the inflammatory reaction to ITSs was minimal, and the struts were well endothelialized at 3 months in the present study. Similarly, the PTX-ITS showed favorable arterial responses, comparable to those of the ITS, indicating high biocompatibility for both scaffolds.
It is important to mention that a recent meta-analysis21 reported concerns about the long-term safety of paclitaxel-based devices; however, these concerns are yet to be elucidated, and this does not dispute our finding that paclitaxel suppresses late scaffold shrinkage and in-scaffold stenosis, and our findings remain highly meaningful for the future development of peripheral BRSs.
Study LimitationsThe present study had several limitations. First, this study used a healthy porcine iliac artery model. This may limit the translation of our findings to clinical practice in humans. Second, the number of scaffolds examined in this study was small (12 scaffolds). Third, we used a follow-up period of 3 months for the evaluation of durability. A longer follow-up period may reveal important differences not shown in the present study. Fourth, we did not perform baseline OCT because of the risk of arterial spasm, which can cause scaffold migration or malapposition. Finally, we excluded 3 scaffolds from the final evaluation due to scaffold disruptions. This may be the result of the harsh environment for scaffold implantation in the swine iliac artery, as greater mechanical forces (e.g., bending, torsion, and compression) are exerted in swine than in humans.
Compared to the ITS, the PTX-ITS showed significant suppression of late scaffold shrinkage and significantly lower in-scaffold stenosis on OCT and angiographic late loss for up to 3 months. Additionally, the PTX-ITS exhibited high biocompatibility, comparable to that of ITS, on histological analysis.
None.
The authors have no conflicts of interest to declare.
This study was funded by Kyoto Medical Planning Co., Ltd.