Abstract
Background:
In the Japanese clinical setting, the prevalence, potential cofounders of gastrointestinal (GI) bleeding from anticoagulant therapy, including direct oral anticoagulants (DOACs) and warfarin, and prognosis after GI bleeding are unclear.
Methods and Results:
We examined about GI bleeding from anticoagulant therapy using data from the SAKURA AF Registry, a prospective multicenter registry in Japan. Among 3,237 enrollees, 48.8% (n=1,561) were warfarin users and 51.2% (n=1,676) DOAC users. GI bleeding was identified in 68 patients (2.1%). No incidental differences in GI bleeding were observed between the DOAC and warfarin users (32 [1.9%] patients [0.67 events per 100 person-years] vs. 36 [2.3%] patients [0.75 events per 100 person-years], respectively; P=0.43). Multivariate Cox proportional hazard model analysis revealed that creatinine (hazard ratio [HR] 1.379, 95% confidence interval [CI] 1.091–1.743 P=0.007) and hemoglobin (HR 0.814, 95% CI 0.705–0.941, P=0.005) remained independent determinants of GI bleeding. Patients experiencing GI bleeding events had a higher risk of all-cause death (18%) than those without GI bleeding (6%) (P=0.045).
Conclusions:
GI bleeding was strongly associated with anemia and renal impairment. Patients experiencing GI bleeding had higher risk for all-cause death than those without GI bleeding.
Atrial fibrillation (AF) is the most common arrhythmia in elderly individuals and currently affects approximately 0.6% of the Japanese population.1
AF is a strong risk factor for stroke and death, but anticoagulant therapy can reduce the risk of stroke among affected patients.2–5
Recently, direct oral anticoagulants (DOACs) have been used worldwide as an alternative therapy to warfarin because of simpler use, fewer drug and food interactions, and better outcomes.2–5
In real-world data from Japan, several registries have also provided evidence to support the benefits of DOACs over warfarin in terms of safety and/or effectiveness among Japanese AF patients.6–10
Despite the effectiveness and safety of DOACs over warfarin, randomized control trials (RCTs) and Western real-world data have shown that the incidence of gastrointestinal (GI) bleeding among AF patients is higher with DOACs than with warfarin treatment.2–5,11
Recent data have suggested that major bleeding events are associated with poor prognosis.12
However, in the Japanese clinical setting, the prevalence, potential cofounders of GI bleeding from anticoagulant therapy, including DOACs and warfarin, and the prognosis after GI bleeding are unclear. To address this issue, we explored the incidence of GI bleeding and subsequent outcomes following anticoagulant therapy using data from a multicenter registry, the SAKURA AF Registry (UMIN Clinical Trials Registry: UMIN000014420), established to support prospective observational research and to determine the current status of anticoagulation therapy in Japan.
Editorial p 1456
Methods
Study Population
The study design, data collection, and baseline characteristics of the study population have been reported previously.8,9
Patient eligibility for enrollment in the registry were as follows: a diagnosis of non-valvular AF based on 12-lead ECG; 24-h Holter ECG recording or event-activated ECG recording; age ≥20 years; and treatment, either recently initiated or ongoing, using any anticoagulant drug for stroke prophylaxis. Recruitment began in September 2013 and ended in December 2015. The registry included 1,578 patients who were treated with warfarin at the time of enrollment, and 1,690 patients treated with 1 of the 4 available DOACs. A total of 3,268 patients were enrolled in the registry by 63 institutions (2 cardiovascular centers, 20 affiliated hospitals or community hospitals and 41 private clinics) in the Tokyo area. Of the 3,268 patients enrolled in the SAKURA AF Registry, 31 were lost to follow-up. Follow-up data at the 1- and 2-year endpoints after enrollment into the Registry for 3,157 (97.5%) and, 2,952 (91.2%), respectively, was available for 3,237 of the remaining patients. This study was approved by Nihon University Itabashi Hospital Certified Clinical Research Review Board and each participating hospital’s institutional review board (IRB), and followed the Declaration of Helsinki and ethical standards of the responsible committee on human experimentation. All patients provided written informed consent for participation in the Registry.
Data Collection, Definition, and Outcomes
A web-based registration system was created for the SAKURA AF Registry and used to collect relevant patient clinical data (comorbidities, medication use and laboratory test results) and to obtain follow-up information, including the prothrombin time-international normalized ratio (PT-INR) in warfarin users, the creatinine clearance (CrCl) rate, and hemoglobin (Hb) levels. The follow-up data were collected through a central registry office, twice yearly (in March and September), for up to 4 years after enrollment. The time in therapeutic range (TTR) was calculated using the Rosendaal method.13
For the administration of DOACs, “appropriate standard-dose” and “appropriate low-dose” regimens were defined as administration according to standard or low-dose administration. The following low-dose regimens were considered to be appropriate: dabigatran, 110 mg (twice daily), for patients with a CrCl of 30–50 mL/min, age ≥70 years and a prior history of bleeding;2
rivaroxaban, 10 mg (once daily), for patients with a CrCl of 15–50 mL/min;3
apixaban, 2.5 mg (twice daily), for patients with any 2 of the following characteristics: age ≥80 years, body weight <60 kg and serum Cr level ≥1.5 mg/dL;4
and edoxaban, 30 mg (once daily), for patients with a CrCl of 15–50 mL/min or body weight <60 kg.5
Under-dosing (i.e., off-label low-dose) therapy was defined as the administration of a low-dose of DOAC despite the standard dosage criteria being met. Overdose (off-label standard-dose) therapy was defined as administration of a standard dose of DOAC despite the low-dose regimen criteria being met. Dabigatran administration was considered to be contraindicated for patients with a CrCl <30 mL/min,2
and for the other DOACs, administration was contraindicated for patients with CrCl <15 mL/min.3–5
Of note, although under-dosing of dabigatran (up to 110 mg, twice daily) was defined according to the criteria indicated above (namely, CrCl 30–50 mL/min; age ≥70 years; and prior bleeding), rather than based on a specific standard, almost all physicians followed the criteria defined above for dabigatran under-dosing.
In this study, the primary endpoint was GI bleeding, which was diagnosed by documentation of witnessed hematemesis, melena, hematochezia, or any combination thereof. This was assessed by medical personnel according to the history and physical examination of the patient, with or without a positive fecal occult blood test result, and with or without a significant drop in Hb levels or hematocrit. GI bleeding was characterized according to source as upper and lower GI tract (GIT), small intestine, or indeterminate on the basis of history and clinical presentation, history, and endoscopic findings. The finding of an endoscopic lesion was documented to be consistent with bleeding in a specific location of the GIT assigned the source of bleeding to that portion of the GIT. With the exception of hematemesis, the source of bleeding was considered to be indeterminate if endoscopy was not performed. Major bleeding in patients with GI bleeding was defined as a reduction in the Hb level ≥2 g/dL, or transfusion of at least 2 units of blood.14
Death from any cause was also considered as a study secondary endpoint. In particular, we detailed deaths of patients who experienced GI bleeding.
Statistical Analysis
Continuous variables are reported as mean±SD. Categorical variables are indicated as percentage and number of patients. Differences between warfarin and DOAC users in continuous variables were analyzed by a 2-sample t-test, and differences in categorical variables were analyzed by the χ2
test. Because of the small number of edoxaban users, we excluded them from comparisons according to DOAC type. Differences in continuous variables between users of the 3 different DOACs were tested by one-way analysis of variance (ANOVA), and differences among these 3 groups in categorical variables were analyzed by χ2
test. Incidence rates are expressed as events per 100 person-years and 95% confidence interval (CI). Comparisons of the incidence rates between groups were analyzed by Exact Poisson test, and among 3 groups by Exact Poisson test with a Bonferroni adjustment. The results of Cox proportional hazards modeling for GI bleeding were expressed as hazard ratios (HRs) and 95% CIs for components of the bleeding risk HAS-BLED score,14
female sex, diabetes mellitus, body weight (kg), Hb (g/dL), and history of vascular disease. Significant variables were moved to the multivariate model. Multivariate Cox proportional hazards modeling for all-cause death was conducted by entering the presence of GI bleeding events, and age ≥75 years, male sex, body weight ≤50 kg, heart failure, and CrCl ≤50 mL/min, which were the significant covariables based on the results from the same cohort as reported previously.9
Receiver-operating characteristic (ROC) analysis was performed to determine the diagnostic value of Cr and Hb levels, and the HAS-BLED, CHADS2
and CHA2DS2-VASc scores to correctly separate patients with GI bleeding from those without it. Overall performance was determined by the total area under the curve (AUC) and cutoff values were assessed based on the best tradeoff between sensitivity and specificity. Kaplan-Meier curves of the cumulative incidence of GI bleeding or all-cause death were constructed to compare patients with and without anemia, or those with and without renal dysfunction, or those with and without GI bleeding using a log-rank test. All statistical analyses were performed using SPSS Statistics 24 (IBM Corp., Armonk, NY, USA) and MedCalc Software Version 16.4.1 (Mariakerke, Belgium). P<0.05 was considered statistically significant.
Results
Baseline Characteristics
Among the 3,237 patients enrolled in the study, 48.8% (n=1,561) were warfarin users and 51.2% (n=1,676) DOAC users (dabigatran, n=456 [14.1%], rivaroxaban, n=761 [23.5%], apixaban, n=428 [13.2%], or edoxaban, n=31 [1.0%]). Clinical characteristics of the warfarin users vs. DOAC users are summarized in
Table 1. Medication history and laboratory test results for these patients have been reported in detail elsewhere.9
There was no difference in age, sex, body weight, body mass index (BMI), or AF type between warfarin and DOAC users. Overall, comorbidities associated with stroke, such as hypertension and heart failure, tended to be less prevalent in DOAC users than in warfarin users. Concomitant use of antiplatelet drugs was less prevalent in DOAC users than in warfarin users. The CHADS2
and CHA2DS2-VASc scores tended to be lower in DOAC users than in warfarin users, and the CrCl rate was significantly higher in DOAC users. Of importance, the percentage of patients who were new users of an anticoagulant (which is defined as anticoagulation therapy initiated within 3 months before the patient’s enrollment in this registry) was significantly higher in DOAC users than in warfarin users. Characteristics of the DOAC users stratified according to the specific DOAC administered are shown in
Table 1. Apixaban users had lower height and weight than dabigatran and rivaroxaban users, and they were more likely to be female and have paroxysmal AF. The CrCl rate was lower in apixaban users than in dabigatran and rivaroxaban users. Patients taking rivaroxaban were older than those taking dabigatran, and those taking apixaban were older than those taking rivaroxaban. The percentage of patients who were new users of an anticoagulant increased similarly, starting with the percentage of those taking rivaroxaban. Apixaban users were at relatively high risk of stroke events.
Table 1.
Clinical Characteristics of Warfarin vs. DOAC Users and of Dabigatran vs. Rivaroxaban vs. Apixaban Users
| |
Warfarin users
(n=1,561) |
DOAC users
(n=1,676) |
P value* |
Dabigatran
users (n=456) |
Rivaroxaban
users (n=761) |
Apixaban users
(n=428) |
P value** |
| Age (years) |
72.2±9.3 |
71.8±9.5 |
0.22 |
70.9±9.5 |
71.5±9.2 |
73.2±10.0 |
0.001 |
| Female |
367 (23.5) |
480 (28.6) |
0.001 |
113 (24.8) |
204 (26.8) |
153 (35.7) |
0.001 |
| Height (cm) |
162.8±9.4 |
162.1±9.6 |
0.025 |
163.4±8.9 |
162.2±9.4 |
160.7±10.2 |
<0.001 |
| Weight (kg) |
64.0±12.5 |
63.7±13.4 |
0.60 |
65.1±13.2 |
63.7±13.4 |
62.5±13.4 |
0.015 |
| BMI (kg/m2) |
24.0±3.6 |
24.1±3.9 |
0.53 |
24.2±3.6 |
24.1±4.0 |
24.1±4.0 |
0.22 |
| High alcohol use |
43 (2.8) |
48 (2.9) |
0.85 |
17 (3.7) |
27 (3.6) |
4 (0.9) |
0.018 |
| AF type |
| Paroxysmal |
492 (31.5) |
709 (42.3) |
<0.001 |
195 (42.8) |
306 (40.2) |
194 (45.3) |
0.001 |
| Persistent |
361 (23.1) |
351 (20.9) |
|
97 (21.3) |
136 (18.0) |
108 (25.2) |
|
| LS-AF |
693 (44.4) |
603 (36.0) |
|
163 (35.7) |
311 (40.9) |
122 (28.5) |
|
| Not reported |
15 (1.0) |
13 (0.8) |
|
1 (0.22) |
9 (1.2) |
4 (0.92) |
|
| Medical history |
| Hypertension |
1,145 (73.4) |
1,163 (69.4) |
0.013 |
310 (68.0) |
533 (70.0) |
302 (70.6) |
0.66 |
| Diabetes mellitus |
378 (24.2) |
362 (21.6) |
0.08 |
105 (23.0) |
151 (19.8) |
101 (23.6) |
0.23 |
| Heart failure |
399 (25.6) |
319 (19.0) |
<0.001 |
79 (17.3) |
145 (19.1) |
91 (21.3) |
0.33 |
| Vascular disease |
204 (13.1) |
195 (11.7) |
0.21 |
43 (9.4) |
96 (12.6) |
53 (12.4) |
0.21 |
| Stroke/TIA |
190 (12.2) |
176 (10.5) |
0.13 |
58 (12.7) |
78 (10.2) |
37 (8.6) |
0.14 |
| Major bleeding |
15 (1.0) |
16 (1.0) |
0.98 |
5 (1.1) |
8 (1.1) |
3 (0.7) |
0.80 |
| AF ablation |
130 (8.3) |
158 (9.4) |
0.27 |
50 (11.0) |
64 (8.4) |
44 (10.3) |
0.29 |
| Antiplatelet use |
303 (19.4) |
211 (12.6) |
<0.001 |
50 (11.0) |
105 (13.7) |
53 (12.1) |
0.37 |
| NSAID use |
21 (38.9) |
33 (61.1) |
0.17 |
7 (1.5) |
14 (1.8) |
11 (2.6) |
0.52 |
| CHADS2 score |
1.89±1.16 |
1.72±1.14 |
<0.001 |
1.71±1.17 |
1.67±1.12 |
1.81±1.14 |
0.12 |
| CHA2DS2-VASc score |
3.08±1.51 |
2.92±1.46 |
0.002 |
2.82±1.46 |
2.87±1.45 |
3.11±1.47 |
0.005 |
| HAS-BLED score |
1.61±0.88 |
1.28±0.78 |
<0.001 |
1.07±0.71 |
1.32±0.77 |
1.42±0.81 |
<0.001 |
| New use |
67 (4.3) |
397 (23.7) |
<0.001 |
43 (11.4) |
178 (23.4) |
155 (36.2) |
<0.001 |
| PT-INR |
2.03±1.79 |
|
|
|
1.50±1.46 |
1.25±0.35 |
|
| APTT (s) |
|
|
|
44.0±9.9 |
|
|
|
| TTR |
65.4±31.1 |
|
|
|
|
|
|
| Overdose |
|
66 (4) |
|
35 (7) |
25 (3) |
4 (1) |
<0.001 |
| Appropriate standard dose |
|
746 (45) |
|
88 (19) |
406 (53) |
246 (57) |
<0.001 |
| Appropriate low dose |
|
477 (29) |
|
234 (51) |
138 (18) |
92 (21) |
<0.001 |
| Underdose |
|
369 (22) |
|
92 (20) |
185 (24) |
82 (19) |
<0.001 |
| CrCl (mL/min) |
65.4±25.6 |
70.3±27.3 |
<0.001 |
74.4±30.0 |
70.3±26.1 |
66.2±26.0 |
<0.001 |
Values are mean±SD or n (%). *Comparison between warfarin and DOAC users, by Student’s t-test or χ2 test, as appropriate. **Comparison between DOAC users by ANOVA or χ2 test, as appropriate. Because of the small number of edoxaban users, we excluded them from the analysis. AF, atrial fibrillation; APTT, activated partial thromboplastin time; BMI, body mass index; CHADS2: congestive heart failure, hypertension, age ≥75 years, diabetes and stroke; CHA2DS2-VASc: congestive heart failure, hypertension, age ≥75 years, diabetes, stroke, vascular disease, age 65–74 years and sex category; HAS-BLED, uncontrolled hypertension (baseline systolic blood pressure >160 mmHg), abnormal renal function (serum CrCl ≥2.26 mg/dL) / liver function (chronic hepatic disease [e.g., cirrhosis] or aspartate aminotransferase and/or alanine aminotransferase >3× normal range), stroke, prior major bleeding, elderly (age ≥65 years), drugs (antiplatelet drugs or NSAIDs) / high alcohol use (≥160 g/day), Labile INR (overdosing shown by baseline PT-INR in warfarin users); CrCl, creatinine clearance; DOAC, direct oral anticoagulant; High alcohol use (alcohol ≥160 g/day: Pequignot et al reported that >160 g/day alcohol intake was high risk of liver cirrhosis, therefore we defined it similarly32); LS-AF; long-standing persistent AF (lasting >1 year); NA, not applicable; NSAID, nonsteroidal anti-inflammatory drug; PT-INR, prothrombin time-international normalized ratio; TIA, transient ischemic attack; TTR, time in therapeutic range.
At a median follow-up of 39.3 months (range, 28.5–43.6 months) after enrollment, major bleeding was identified in 124 (3.8%) patients. Among the 3,237 patients, GI bleeding was identified in 68 patients (2.1%): 37 patients (1.1%) had GI bleeding that fulfilled the criteria of major bleeding (a reduction in Hb ≥2 g/dL or transfusion of at least 2 units), and the remaining 31 patients (1.0%) had non-major bleeding. No incidental differences in GI bleeding were observed between the DOAC and warfarin users (32 [1.9%] patients [0.67 events per 100 person-years] vs. 36 [2.3%] patients [0.75 events per 100 person-years], respectively; P=0.43). Among the DOAC users, patients treated with apixaban had a modestly higher incidence of GI bleeding (1.1 events per 100 person-years) than did other DOAC users (dabigatran [0.58 events per 100 person-years] and rivaroxaban [0.54 events per 100 person-years]), although the difference was not statistically significant. Among the warfarin users, 32 patients had available PT-INR data at the time of GI bleeding, with an average PT-INR of 1.83 from a minimum value of 0.9 to a maximum value of 2.85. The site of GI bleeding was characterized as upper in 44%, lower in 33%, small intestine in 3%, and indeterminate in 20% of patients, and there was no difference in the distribution of the source of GI bleeding between the warfarin and DOAC users (upper: warfarin 1.0%, DOAC 0.9%; lower: warfarin 0.6%, DOAC 0.6%; small intestine: warfarin 0.06%, DOAC 0.1%; indeterminate: warfarin 0.5%, DOAC 0.3%) or according to the type of DOAC. Both warfarin and any DOAC users had a higher prevalence of upper GI bleeding than lower GI bleeding, and this tendency was persistent regardless of the type of DOAC used (Table 2).
Table 2.
Bleeding Events in Warfarin vs. DOAC Users, and in Dabigatran vs. Rivaroxaban vs. Apixaban Users
| |
Warfarin
(n=1,561) |
DOAC
(n=1,676) |
P value* |
Dabigatran
(n=456) |
Rivaroxaban
(n=761) |
Apixaban
(n=428) |
P value** |
| Major bleeding |
66 (4.2) |
58 (3.4) |
0.26 |
12 (2.6) |
27 (3.5) |
19 (4.4) |
0.35 |
| Annual rate |
1.37 (1.06–1.74) |
1.22 (0.92–1.57) |
0.50 |
0.87 (0.45–1.52) |
1.20 (0.79–1.75) |
1.73 (1.04–2.70) |
NS |
| GI bleeding |
36 (2.3) |
32 (1.9) |
0.43 |
8 (1.7) |
12 (1.5) |
12 (2.8) |
0.40 |
| Annual rate |
0.75 (0.52–1.03) |
0.67 (0.46–0.95) |
0.65 |
0.58 (0.25–1.15) |
0.54 (0.28–0.93) |
1.10 (0.57–1.91) |
NS |
| Upper |
17 (1.0) |
15 (0.9) |
0.58 |
5 (1.0) |
4 (0.5) |
6 (1.4) |
0.41 |
| Lower |
10 (0.6) |
10 (0.6) |
0.87 |
1 (0.2) |
6 (0.8) |
3 (0.7) |
0.61 |
| Small intestine |
1 (0.06) |
2 (0.1) |
0.61 |
0 (0) |
1 (0.1) |
1 (0.2) |
0.79 |
| Indeterminate |
8 (0.5) |
5 (0.3) |
0.34 |
2 (0.4) |
1 (0.1) |
2 (0.5) |
0.68 |
Values are number (%) or events per 100 person-years (95% confidence interval). *Comparison between warfarin and DOAC users, by χ2 test or Exact Poisson test, as appropriate. **Comparison between DOAC users by χ2 test or Exact Poisson test with a Bonferroni adjustment, as appropriate. NS, not significant for each comparison. DOAC, direct oral anticoagulant; GI, gastrointestinal.
Results of the Cox regression analysis evaluating the relative risk of GI bleeding are summarized in
Table 3. In the univariate Cox proportional hazards model analysis, age, renal dysfunction, and anemia were associated with GI bleeding (Table 3). Multivariate Cox proportional hazard model analysis revealed that Cr (HR 1.379, 95% CI 1.091–1.743, P=0.007) and Hb (HR 0.814, 95% CI 0.705–0.941, P=0.005) remained independent determinants of GI bleeding. The ROC curve revealed that the AUCs of Cr and Hb were 0.57 and 0.60, respectively, and the AUCs of HAS-BLED, CHADS2
and CHA2DS2-VASc scores were 0.55, 0.59, and 0.59, respectively. The best cutoff value of Cr for identification of GI bleeding events was 0.93 mg/dL with a sensitivity of 56.9% and specificity of 64.2%, and that of Hb was 13.2 g/dL with a sensitivity of 52.9% and specificity of 61.2%. Kaplan-Meier curves revealed that patients with Hb <13.2 g/dL and Cr ≥0.93 mg/dL had a higher incidence of GI bleeding than those with Hb ≥13.2 g/dL and Cr <0.93 mg/dL (Figure 1).
Table 3.
Determinants of GI Bleeding
| |
Crude HR
(95% CI) |
P value |
Adjusted HR
(95% CI) |
P value* |
| Age (per year) |
1.041 (1.012–1.071) |
0.004 |
1.027 (0.996–1.059) |
0.86 |
| Female |
1.128 (0.664–1.917) |
0.63 |
|
|
| Hypertension |
1.532 (0.851–2.758) |
0.16 |
|
|
| Diabetes mellitus |
1.147 (0.880–1.495) |
0.32 |
|
|
| Body weight (per kg) |
0.995 (0.976–1.014) |
0.58 |
|
|
| Creatinine (per mg/dL) |
1.471 (1.179–1.833) |
0.005 |
1.379 (1.091–1.743) |
0.007 |
| Hemoglobin (per g/dL) |
0.796 (0.690–0.919) |
0.002 |
0.814 (0.705–0.941) |
0.005 |
| Persistent AF (vs. paroxysmal AF) |
0.868 (0.474–1.590) |
0.64 |
|
|
| History of vascular disease |
1.016 (0.883–1.169) |
0.83 |
|
|
| History of stroke or TIA |
1.023 (0.937–1.117) |
0.61 |
|
|
| History of major bleeding |
1.181 (0.966–1.444) |
0.17 |
|
|
| Antiplatelet use |
1.002 (0.526–1.911) |
0.99 |
|
|
| NSAID use |
0.886 (0.123–6.386) |
0.91 |
|
|
| DOAC use (vs. warfarin) |
0.910 (0.565–1.467) |
0.70 |
|
|
*Adjusted by age, creatinine, and hemoglobin. CI, confidence interval; HR, hazard ratio. Other abbreviations as in Tables 1,2.

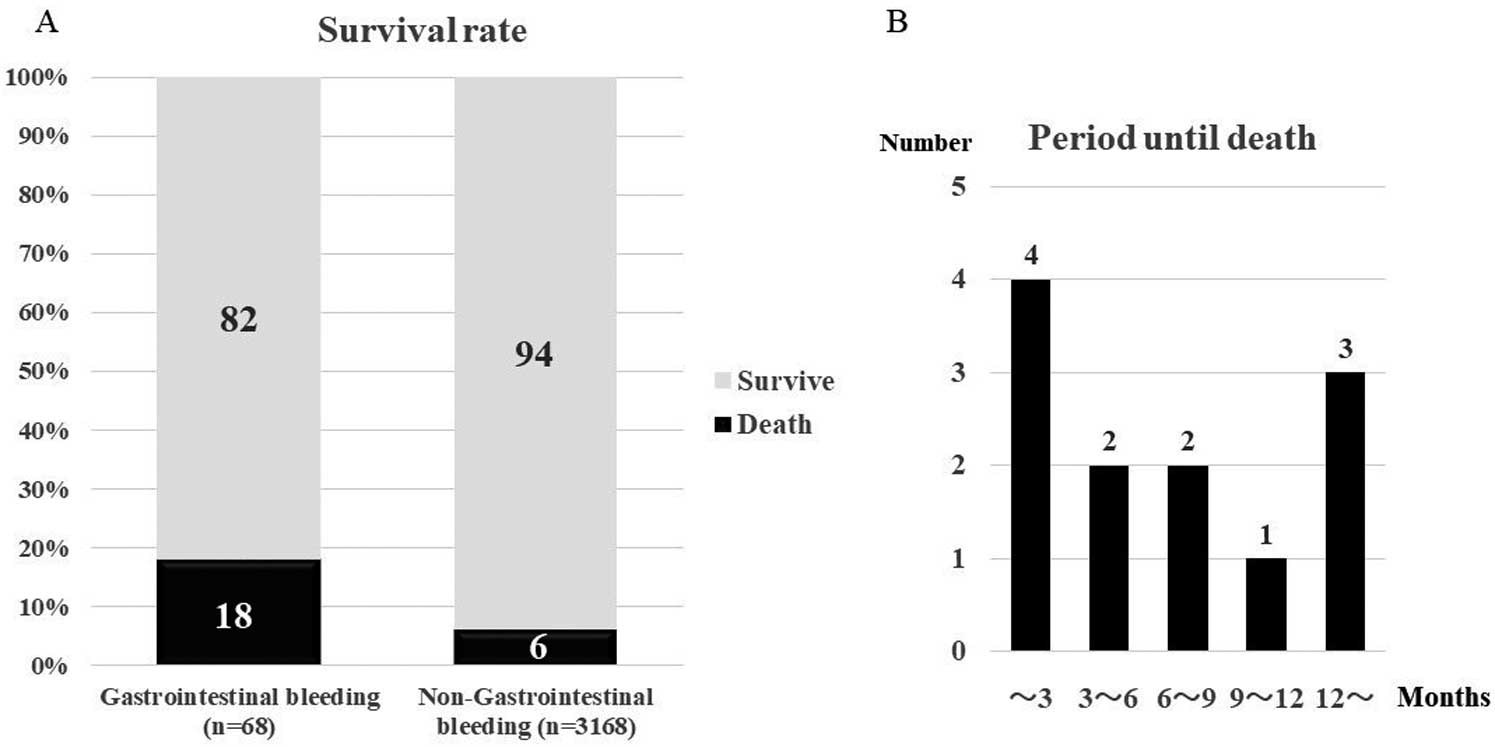
Among the 3,237 patients, 200 (6%) had died. Patients experiencing GI bleeding events had a higher risk of all-cause death (18% [12/68]) than those without GI bleeding (6% [188/3,169]; P=0.045 by log-rank test;
Figure 2). Multivariate Cox regression analysis by covariates relevant for all-cause death revealed that GI bleeding was an independent predictor for all-cause death (HR 2.361, 95% CI 1.315–4.237, P=0.004).
Death of patients with concomitant GI bleeding occurred in the early phase within 3 months in 4 (33%) patients, and in the late phase at >1 year in 3 patients (25%) (Figure 3). The cause of death in patients with GI bleeding (n=12) was GI bleeding (3), pneumonia (3), heart failure (2), cancers of GIT (2), and sudden death (2).
Discontinuation of Anticoagulant Therapy and Thromboembolic Events Following GI Bleeding
After GI bleeding, termination of anticoagulation was required in 48 (71%) of the 68 patients with a GI bleeding event (transient termination: 38 patients [56%], permanent termination: 10 patients [15%]). We did not have data regarding termination of anticoagulant therapy in 20 (29%) of the 68 patients who experienced GI bleeding. One patient died immediately because of GI bleeding. However, during the period of anticoagulation termination, there were no thromboembolic events.
Discussion
The major findings of our study were as follows. First, GI bleeding was identified in 1.9% of DOAC users and in 2.3% of warfarin users. Second, the source of GI bleeding was more prevalent in the upper GIT regardless of DOAC or warfarin use. Third, GI bleeding was statistically associated with anemia and renal impairment. Fourth, patients with a history of GI bleeding had a higher risk for all-cause death than did those without GI bleeding.
Prevalence of GI Bleeding
GI bleeding occurred at a frequency of 0.67 events per 100 person-years in DOAC users and 0.75 events per 100 person-years in warfarin users. In our registry, the prevalence of GI bleeding was similar between DOAC and warfarin users. Most RCTs date have reported the inferiority of DOACs to warfarin with regards to GI bleeding.2–5
The RE-LY trial reported more frequent GI bleeding events (1.85% vs. 1.25%; P<0.001) in patients on the 150 mg, twice-daily dose of dabigatran compared with those on dose-adjusted warfarin.2
The ENGAGE AF-TIMI 48 trial also showed a higher frequency of GI bleeding events (1.51% vs. 1.23%; P=0.03) in patients who received 60 mg of edoxaban vs. dose-adjusted warfarin.5
Further, the ROCKET AF trial showed higher frequency of GI bleeding episodes (2.00% vs. 1.24%; P<0.001) in patients who received 15 mg of rivaroxaban vs. dose-adjusted warfarin.3
Accordingly, a meta-analysis suggested that rivaroxaban and high dosages of dabigatran and edoxaban should be avoided in patients at high risk of GI bleeding.15
Alternatively, the ARISTOTLE trial showed similar rates of GI bleeding (0.76% vs. 0.86%; P=0.37) in apixaban- vs. warfarin-treated patients.4
Previous real-world data from the ORBIT-AF2 registry revealed that the incidence rate of GI bleeding was 1.8%/year in DOAC users and 1.3%/year in warfarin users.11
RCT data from global, Asian, and even Japanese data reported an approximately 1% incidence rate of GI bleeding.2–5,16–21
Results from these previous RCTs and meta-analysis, and data from Western real-world registries are slightly inconsistent with our data,9
which may be attributed to the off-label under-dosing of the DOAC user in our registry. As we previously reported, 22.2% of patients who received under-dosing DOAC therapy were identified and they experienced a lower incidence of bleeding events. It is, therefore, not surprising that physicians would favor under-dosing for older Asian patients with renal dysfunction to lower the risk of major bleeding events.22
In fact, under-dosed DOAC users had a lower incidence of GI bleeding than did appropriate standard-dose DOAC users (under-dose 0.3% vs. appropriate standard-dose 2.3%). A further explanation for the observed lower incidence of GI bleeding than in other studies may be the careful selection of the type of DOAC at the physicians’ discretion in clinical practice. In contrast to the results of a RCT for apixaban,4
our registry showed that apixaban users had a non-significant but modestly higher incidence of GI bleeding than other DOAC users. Because of the lower incidence of major and GI bleeding events in the ARISTOTLE trial compared with other DOAC trials, physicians might have hesitated in prescribing rivaroxaban, dabigatran, and edoxaban for patients with a major or high risk of GI bleeding. Indeed, apixaban users were older, had a lower BMI, and impaired renal function compared with other DOAC users in our registry, and thus, apixaban users experienced a non-significant but modestly higher incidence of GI bleeding.
Sources of GI Bleeding
In this registry, the source of GI bleeding was characterized as upper in 44%, lower in 33%, small intestine in 3%, and undefined in 20%. Conversely, a Western observational study reported that the prevalence of lower GI bleeding was higher than upper GI bleeding especially in DOAC users (upper GI: 22.0%, lower GI: 31.1%).23
A higher incidence of lower GI bleeding could be explained by dabigatran metabolism and age-related GIT features. RCT trials24
have reported that lower GI bleeding following dabigatran treatment has a higher prevalence (47%) than upper GI bleeding events, likely because of the lower bioavailability of dabigatran after oral ingestion. It is possible that the metabolism of dabigatran etexilate by esterases leads to progressively higher concentrations of the active drug during transit of the GIT. The prevalence of GIT pathology, such as diverticulosis and angiodysplasia, increases with age, and the risk of bleeding from the affected areas might be increased by direct exposure to dabigatran. Thus, the local effects of dabigatran on diseased mucosa could account for the relative increase in lower GI bleeding. Patients in Japan on anticoagulation therapy have less morbidity associated with colon cancer and diverticulosis than those in Western countries.25.26
In the present registry, dabigatran was used preferably in younger patients in whom diverticulosis and angiodysplasia would be less prevalent. These factors might have led to the lower incidence of the lower GIT bleeding in our study.
Factors Correlating With GI Bleeding
GI bleeding was statistically associated with anemia and renal impairment in this study. For patients with AF, risk stratification is a critical step in management. Current guidelines emphasize the use of thrombotic risk scores and shared decision-making with patients, carefully considering the stroke and bleeding risks.27
Various bleeding risk scores such as the HAS-BLED, ATRIA, ORBIT have been proposed to assess the risk of bleeding in patients with AF taking oral anticoagulants.14,28,29
In our study, the prognostic performance of the bleeding risk score HAS-BLED or thrombotic risk scores CHADS2
and CHA2DS2-VASc for patients with GI bleeding was low, as indicated by the low AUC of 0.55–0.59. Sherwood et al reported on the relationship between GI bleeding with rivaroxaban vs. warfarin therapy and identified several independent risk factors.30
Similar to our study, they found that a decrease in CrCl and anemia at baseline were predictors of GI bleeding. Although the prognostic performance was relatively low, the Cr and Hb levels can be easily assessed by routine laboratory examination. Thus, in patients presenting with Cr ≥0.93 mg/dL or Hb <13.2 g/dL, it would be better to perform a fecal occult blood test before starting oral anticoagulants and to administer proton-pump inhibitors for the prevention of upper GI bleeding. In particular, in the patients who have experienced GI bleeding events, a further intensive monitoring of renal function and Hb is needed to prevent recurrent GI bleeding.
Long-Term Outcomes After GI Bleeding
In our study, patients with GI bleeding had a higher risk of death than did those without GI bleeding. GI bleeding has been reported to be associated with a high mortality rate, especially among elderly patients with AF and multiple comorbidities who take antithrombotic agents.31
A Danish study12
reported that 39.9% of AF patients died within 2 years after GI bleeding. Stroke prevention among these high-risk patients is a clinical challenge and a multidisciplinary task.12
Higher mortality after GI bleeding is associated with both thromboembolism and recurrent bleeding events. In our study, approximately 18% deaths occurred after GI bleeding, which was slightly lower than in the Danish study.12
In our study, approximately 80% of 12 patients died, not due to thromboembolic events, but rather from pneumonia or GI bleeding, etc. The Danish study suggested that a higher rate of resuming antithrombotic therapy was associated with lower thromboembolic mortality.12
In our study, the permanent termination rate of anticoagulant drugs occurred in only 15% of 68 GI bleeding patients (the Danish study12
reported a permanent termination rate of anticoagulant drugs of 27%). This might have lowered the rates of mortality and thromboembolic events after GI bleeding in our cohort.
Restarting treatment with a single oral anticoagulant agent was associated with the lowest risk of all-cause mortality and thromboembolism and relatively safe use in terms of lower risk of recurrent bleeding events, compared with non-resumption of antithrombotic treatment or restarting other antithrombotic treatment regimens.12
A higher resumption rate of anticoagulant treatment after GI bleeding might have affected the lower overall mortality observed in our study.
Study Limitations
There are several limitations that should be acknowledged. First, as an observational retrospective study, we could not establish any causal relationships but only report associations. Second, there is an unavoidable possibility of a patient selection bias due to the prospective nature of observational studies, despite our use of Cox proportional hazards models to minimize the influence of the patients’ background factors. Third, the registry included patients only from selected institutions located in the capital city of Tokyo, or its suburbs. Therefore, the data, and our findings, may not be reflective of all areas of Japan. We note, however, that patient selection and regional enrollment biases are limitations of all prospective observational studies. Fourth, whether GI agents such as proton-pump inhibitors were being taken was an important consideration for this study. However, no data were available regarding this.
Conclusions
Our registry characterized GI bleeding events in Japanese AF patients treated with DOACs or warfarin. Different from the results of other Western registries and RCTs, the overall GI bleeding event rate observed was lower, and the prevalence and distribution of the origin of GI bleeding was comparable between DOAC and warfarin users. This result may derive from careful management of oral anticoagulant therapy based on physicians’ discretion in Japan. GI bleeding was strongly associated with anemia and renal impairment. Patients experiencing GI bleeding had a higher risk for all-cause death than those without GI bleeding. These data will help in the management of GI bleeding in Japanese AF patients.
Acknowledgments
The authors thank all centers that participated in the study and all patients who consented to participate.
Disclosures
Dr. Okumura accepted remuneration from Daiichi-Sankyo. Dr. Hirayama received research funding from Bayer Healthcare, Daiichi-Sankyo, Otsuka Pharmaceutical, Astellas Pharma, Eisai, Sumitomo Dainippon Pharma, MSD, Nihon Medi-Physics, Bristol-Meyers Squibb, Boehringer Ingelheim, Pfizer, Boston Scientific Corporation, Hokushin Medical, and has accepted remuneration from Bayer Healthcare, Daiichi-Sankyo, Eisai, Bristol-Meyers Squibb, Astellas Pharma, Sanofi, and Takeda Pharmaceutical. Dr. Matsumoto received research funding from Daiichi-Sankyo, Otsuka Pharmaceutical, and Sumitomo Dainippon Pharma, and accepted remuneration from Nihon Medi-Physics, FUJIFILM RI Pharma, and Biosensors Interventional Technologies Japan.
Dr. Hirayama is a member of
Circulation Journal
’ Editorial Team.
Funding Sources
The study was financially supported by Bayer Yakuhin Ltd., and conducted as an investigator-initiated research based on a contract with Bayer Yakuhin Ltd. The study was also supported by scholarship funds from Daiichi-Sankyo, Astellas Pharma, Eisai, Sumitomo Dainippon Pharma, MSD, Nihon Medi-Physics, Bristol-Meyers Squibb, Boehringer Ingelheim, and Pfizer.
IRB Information
Nihon University Itabashi Hospital Certified Clinical Research Review Board (CRB3180013)
References
- 1.
JCS Joint Working Group. Guidelines for pharmacotherapy of atrial fibrillation (JCS 2013): Digest version. Circ J 2014; 78: 1997–2021.
- 2.
Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361: 1139–1151.
- 3.
Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, et al; ROCKET AF Investigators. Comment regarding: Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365: 883–891.
- 4.
Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, et al; ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011; 365: 981–992.
- 5.
Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, et al; ENGAGE AF-TIMI 48 Investigators. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013; 369: 2093–2104.
- 6.
Kodani E, Atarashi H, Inoue H, Okumura K, Yamashita T, Origasa H; J-RHYTHM Registry Investigators. Beneficial effect of non-vitamin K antagonist oral anticoagulants in patients with nonvalvular atrial fibrillation: Results of the J-RHYTHM Registry 2. Circ J 2016; 80: 843–851.
- 7.
Yamashita Y, Uozumi R, Hamatani Y, Esato M, Chun YH, Tsuji H, et al. Current status and outcomes of direct oral anticoagulant use in real-world atrial fibrillation patients: Fushimi AF Registry. Circ J 2017; 81: 1278–1285.
- 8.
Okumura Y, Yokoyama K, Matsumoto N, Tachibana E, Kuronuma K, Oiwa K, et al. Current use of direct oral anticoagulants for atrial fibrillation in Japan: Findings from the SAKURA AF Registry. J Arrhythmia 2017; 33: 289–296.
- 9.
Okumura Y, Yokoyama K, Matsumoto N, Tachibana E, Kuronuma K, Oiwa K et al; SAKURA AF Registry Investigators. Three-year clinical outcomes associated with warfarin vs. direct oral anticoagulant use among Japanese patients with atrial fibrillation: Findings from the SAKURA AF Registry. Circ J 2018; 82: 2500–2509.
- 10.
Hayashi K, Tsuda T, Nomura A, Fujino N, Nohara A, Sakata K, et al; Hokuriku-Plus AF Registry Investigators. Impact of B-type natriuretic peptide level on risk stratification of thromboembolism and death in patients with nonvalvular atrial fibrillation: The Hokuriku-Plus AF Registry. Circ J 2018; 82: 1271–1278.
- 11.
Steinberg BA, Simon DN, Thomas L, Ansell J, Fonarow GC, Gersh BJ, et al; Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) Investigators and Patients. Management of major bleeding in patients with atrial fibrillation treated with non-vitamin K Antagonist oral anticoagulants compared with warfarin in clinical practice (from Phase II of the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation [ORBIT-AF II]). Am J Cardiol 2017; 119: 1590–1595.
- 12.
Staerk L, Lip GY, Olesen JB, Fosbøl EL, Pallisgaard JL, Bonde AN, et al. Stroke and recurrent haemorrhage associated with antithrombotic treatment after gastrointestinal bleeding in patients with atrial fibrillation: Nationwide cohort study. BMJ 2015; 351: h5876.
- 13.
Rosendaal FR, Cannegieter SC, van der Meer FJ, Briët E. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost 1993; 69: 236–239.
- 14.
Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in atrial fibrillation patients: The Euro Heart Survey. Chest 2010; 138: 1093–1100.
- 15.
Loffredo L, Perri L, Violi F. Impact of new oral anticoagulants on gastrointestinal bleeding in atrial fibrillation: A meta-analysis of interventional trials. Dig Liver Dis 2015; 47: 429–431.
- 16.
Hori M, Matsumoto M, Tanahashi N, Momomura S, Uchiyama S, Goto S, et al. Rivaroxaban vs. warfarin in Japanese patients with atrial fibrillation: The J-ROCKET AF study. Circ J 2012; 76: 2104–2111.
- 17.
Ogawa S, Shinohara Y, Kanmuri K. Safety and efficacy of the oral direct factor Xa inhibitor apixaban in Japanese patients with non-valvular atrial fibrillation: The ARISTOTLE-J study. Circ J 2011; 75: 1852–1859.
- 18.
Hori M, Connolly SJ, Zhu J, Liu LS, Lau CP, Pais P, et al. Dabigatran versus warfarin: Effects on ischemic and hemorrhagic strokes and bleeding in Asians and non-Asians with atrial fibrillation. Stroke 2013; 44: 1891–1896.
- 19.
Hori M, Matsumoto M, Tanahashi N, Momomura SI, Uchiyama S, Goto S, et al. Predictive factors for bleeding during treatment with rivaroxaban and warfarin in Japanese patients with atrial fibrillation: Subgroup analysis of J-ROCKET AF. J Cardiol 2016; 68: 523–528.
- 20.
Goto S, Zhu J, Liu L, Oh BH, Wojdyla DM, Aylward P. Efficacy and safety of apixaban compared with warfarin for stroke prevention in patients with atrial fibrillation from East Asia: A subanalysis of the Apixaban for reduction in stroke and other thromboembolic events in atrial fibrillation (ARISTOTLE) trial. Am Heart J 2014; 168: 303–309.
- 21.
Yamashita T, Koretsune Y, Yang Y, Chen SA, Chung N, Shimada YJ. Edoxaban vs. warfarin in East Asian patients with atrial fibrillation: An ENGAGE AF-TIMI 48 subanalysis. Circ J 2016; 80: 860–869.
- 22.
Murata N, Okumura Y, Yokoyama K, Matsumoto N, Tachibana E, Kuronuma K, et al. Clinical outcomes of off-label dosing of direct oral anticoagulant therapy among Japanese patients with atrial fibrillation identified from the SAKURA AF Registry. Circ J 2019; 83: 727–735.
- 23.
Xu Y, Schulman S, Dowlatshahi D, Holbrook AM, Simpson CS, Shepherd LE, et al. Direct oral anticoagulant- or warfarin-related major bleeding: Characteristics, reversal strategies, and outcomes from a multicenter observational study. Chest 2017; 152: 81–91.
- 24.
Eikelboom JW, Wallentin L, Connolly SJ, Ezekowitz M, Healey JS, Oldgren J, et al. Risk of bleeding with 2 doses of Dabigatran compared with warfarin in older and younger patients with atrial fibrillation: An analysis of the randomized evaluation of long-term anticoagulant therapy (RE-LY) trial. Circulation 2011; 123: 2363–2372.
- 25.
Nagata N, Niikura R, Aoki T, Shimbo T, Itoh T, Goda Y, et al. Increase in colonic diverticulosis and diverticular hemorrhage in an aging society: Lessons from a 9-year colonoscopic study of 28,192 patients in Japan. Int J Colorectal Dis 2014; 29: 379–385.
- 26.
Watanabe T, Muro K, Ajioka Y, Hashiguchi Y, Ito Y, Saito Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2016 for the treatment of colorectal cancer. Int J Clin Oncol 2018; 23: 1–34.
- 27.
January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014; 64: e1–e76.
- 28.
O’Brien EC, Simon DN, Thomas LE, Hylek EM, Gersh BJ, Ansell JE, et al. The ORBIT bleeding score: A simple bedside score to assess bleeding risk in atrial fibrillation. Eur Heart J 2015; 36: 3258–3264.
- 29.
Singer DE, Chang Y, Borowsky LH, Fang MC, Pomernacki NK, Udaltsova N, et al. A new risk scheme to predict ischemic stroke and other thromboembolism in atrial fibrillation: The ATRIA study stroke risk score. J Am Heart Assoc 2013; 2: e000250.
- 30.
Sherwood MW, Nessel CC, Hellkamp AS, Mahaffey KW, Piccini JP, Suh EY, et al. Gastrointestinal bleeding in patients with atrial fibrillation treated with rivaroxaban or warfarin: ROCKET AF Trial. J Am Coll Cardiol 2015; 66: 2271–2281.
- 31.
Lau JY, Barkun A, Fan DM, Kuipers EJ, Yang YS, Chan FK. Challenges in the management of acute peptic ulcer bleeding. Lancet 2013; 381: 2033–2043.
- 32.
Pequignot G, Chabert C, Eydoux H. Augumentation du risque de cirrhose en fonction de la ration d’alcohol. Rveue Alcoholism
20: 191–202, 1974.