2021 Volume 85 Issue 11 Pages 2053-2062
2021 Volume 85 Issue 11 Pages 2053-2062
Background: Optical coherence tomographic (OCT) imaging has enabled identification of lipid, with increasing interest in how it may affect coronary interventions and clinical outcomes. This review summarizes the available evidence around OCT identification of lipid and its effect on interventions, clinical events, and the natural history of coronary disease.
Methods and Results: We conducted a scoping review using the Medline, HealthStar, and Embase databases for articles published between 1996 and 2021. We screened 1,194 articles and identified 51 for inclusion in this study, summarizing the key findings. The literature supports a common OCT definition of lipid as low-signal regions with diffuse borders, validated against histology and other imaging modalities with acceptable intra- and inter-rater reliability. There is evidence that OCT-identified lipid at the site of stent implantation increases the risk of edge dissection, incomplete stent apposition, in-stent tissue protrusion, decreased coronary flow after stenting, side branch occlusion, and post-procedural cardiac biomarker increases. In mostly retrospective studies, lipid indices measured at non-stented sites are associated with plaque progression and the development of recurrent ischemic events.
Conclusions: There is extensive literature supporting the ability of OCT to identify lipid and demonstrating a substantial impact of lipid on percutaneous coronary intervention outcomes. Future work to prospectively evaluate the effect of the characteristics of lipid-rich plaques on long-term clinical outcomes is needed.
Plaque morphology is recognized as an important factor in the outcome of percutaneous coronary intervention (PCI)1,2 and the natural history of coronary disease.3–5 Although the role of calcified plaque in PCI has received much attention, there has been a relatively limited focus on lipidic plaque. Optical coherence tomographic (OCT) imaging has enabled identification of lipid, and a growing literature base has explored how it may affect the procedural outcomes of PCI, as well as longer-term clinical outcomes.6 To assist the practicing interventionalist in applying this evidence base, we conducted a literature review to summarize the available evidence regarding OCT identification of lipid and its consequences. Our objectives were to review: (1) the reliability and validity of lipid identification by OCT compared with histopathology and other detection modalities; (2) the impact of lipid on in-laboratory and post-laboratory PCI outcomes; and (3) the effect of lipid on recurrent ischemic events in natural history studies of coronary disease.
To explore the breadth and depth of the literature around the OCT identification of lipid and its consequences, we selected a scoping review methodology.7 This allowed us to summarize the diversity of the literature available and to identify key evidence and gaps without being limited to samples and datasets that can be easily aggregated using more conventional systematic review approaches.8
Search StrategyWith the aid of a librarian, we conducted an OVID search of the Medline, HealthStar, and Embase databases for article published between 1996 and February 2021 using the following keywords: tomography, optical OR tomography, optical coherence/ AND (lipid OR thin cap fibroatheroma OR TCFA). We excluded ophthalmologic studies, non-human studies, studies not published in English and duplicates.
ScreeningArticles were screened in duplicate using titles and abstracts by 4 reviewers (M.S., M.A., J.C., G.D.) using an online systematic review platform (https://rayyan.qcri.org/). All reviewers trained on a common set of 50 articles to ensure consistency in inclusion and exclusion criteria. We included empirical data (including case series, cross-sectional, cohort, controlled trials, randomized data, systematic reviews, expert consensus statements, and meta-analyses) pertaining to OCT-identified lipid for the purposes of defining or validating lipid pools, correlating lipid pools with intraprocedural, periprocedural, and long-term clinical outcomes. We excluded intravascular imaging studies without OCT comparison, studies with data only relating to differences in lipid pools based on demographics or clinical variables, studies related to the impact of pharmacologic intervention on lipid pools, studies related to neoatherosclerosis, case reports, review articles, expert consensus, commentaries, animal studies, basic science studies including ex vivo models and computational modeling, and studies related to bioabsorbable scaffolds. In all, 274 articles were screened in triplicate because at least 1 reviewer was undecided; 17 conflicts were resolved through discussion.
Eligibility and Data ExtractionThe full text of articles meeting the screening criteria were assessed by 2 reviewers (two of M.S., M.A., J.C., G.D.) for inclusion, with disagreements resolved through consensus. Data were extracted from the full text into 3 predefined tables for reliability and validity evidence and intra- and post-procedural outcomes by a single reviewer (one of M.A., J.C., G.D.) and verified by a second (M.S.).
IRB InformationEthics approval was not applicable for this study because the data collected were from previously published studies.
The results of the literature search are presented in Figure 1. In all, 1,194 articles were identified, 920 of which were screened in duplicate and 274 were screened in triplicate because at least 1 reviewer was undecided. Seventeen conflicts were resolved through discussion. Of the 135 articles that underwent full text review, 51 met the criteria for inclusion in the present study.

Flow diagram of the scoping review methodology.
Lipid was consistently defined in OCT imaging as plaque with low signal intensity regions with diffuse borders.6,9–13 Reports suggest good intraobserver (κ=0.75–0.97) and interobserver (κ=0.76–0.93) agreement,12,14–18 with improvement documented in 1 paper after training on artifacts and lipid mimics14 (see Table 1). Interobserver agreement on maximal lipid arc was similarly good (intraclass correlation coefficient [ICC] 0.82–0.90).14,16,19 OCT detection of lipid was sensitive 89–94%, but less specific 70–92%, based on comparisons with near-infrared spectroscopy (NIRS; sensitivity 86–90%, specificity 70–90%),15,20 histologic examination of direct atherectomy (sensitivity 89%, specificity 75%),12 and pathology specimens (sensitivity 90–94%, specificity 90–92%;21 Table 2). Reasons for false positives based on histologic comparisons include macrophages, foam cells, red thrombus, and collagen-poor extracellular matrix.12,21 Detecting lipid in patients presenting with acute coronary syndromes, especially in ST-elevation myocardial infarction, may be limited by red thrombus, which both obscures lipid and may share similar optical characteristics with lipid.4
| Study | Year | No. raters |
Lipid-rich plaque | Thin cap fibroatheroma | ||||
|---|---|---|---|---|---|---|---|---|
| Definition | Intrarater reliability (κ) |
Inter-rater reliability (κ) |
Definition | Intrarater reliability (κ) |
Inter-rater reliability (κ) |
|||
| Brown et al16 | 2015 | Not reported |
Signal-poor region with a minimum lipid arc of ≥90° |
0.85 | 0.66 | Lipid-rich plaque with a fibrous cap thickness <85 μm |
0.64 | 0.66 |
| Di Vito et al15 | 2017 | 2 | Signal-poor region diffusely bordered by overlying signal-rich bands |
0.97 | 0.93 | |||
| Fujii et al25 | 2015 | Not reported |
Lipid content >1 quadrant with fibrous cap measuring <65 μm |
0.92 | 0.81 | |||
| Habara et al12 | 2017 | 2 | Signal-poor regions without sharp borders |
0.83 | 0.76 | |||
| Kini et al14 | 2015 | 3 | Signal-poor region with poorly delineated borders, little or no signal backscattering, and an overlying signal-rich layer spanning >90° in any cross-section |
0.66 (improving with training to 0.75–0.87) |
Lipid-rich plaque with minimal fibrous cap thickness <65 mm |
0.43 (improving with training to 0.83–0.88) |
||
| Lee et al17 | 2011 | 2 | More than 2 quadrants of signal-poor plaque |
0.90 | 0.83 | Lipid-rich plaque with a fibrous cap thickness <70 μm |
0.83 | 0.77 |
| Lee et al18 | 2011 | 2 | More than 2 quadrants of signal-poor plaque |
0.86 | 0.78 | Lipid-rich plaque with a fibrous cap thickness <70 μm |
0.82 | 0.80 |
| Lee et al24 | 2015 | 2 | Low-signal region with a diffuse border >90° |
Lipid-rich plaque with a fibrous cap thickness <80 μm |
0.86 | 0.90 | ||
| Paoletti et al22 | 2016 | Not reported |
Signal-poor region diffusely bordered by overlying signal-rich bands |
Lipid plaque with a cap thickness <80 μm |
R=0.93 | R=0.92 | ||
| Phipps et al26 | 2016 | Not reported |
Fibrous cap <65 μm overlying a lipid core |
0.83 | ||||
| Radu et al23 | 2016 | 2 | Fibroatheroma with minimum fibrous cap thickness<65 μm |
0.50 (improving to 1.0 with semiautomatic assessment) |
0.23 (improving to 1.0 with semiautomatic assessment) |
|||
| Roleder et al19 | 2014 | Not reported |
Lipid-rich plaque with fibrous cap thickness <65 μm |
0.84 | ||||
| Yabushita et al21 | 2002 | Not reported |
Diffusely bordered signal-poor regions with overlying signal-rich bands |
0.88 | 0.91 | |||
| Study | Year | No. patients |
Comparative standard |
OCT criteria | Sensitivity (%) |
Specificity (%) |
PPV (%) |
NPV (%) |
|---|---|---|---|---|---|---|---|---|
| Presence of lipid | ||||||||
| Di Vito et al15 | 2017 | 43 | NIRS | Signal-poor region diffusely bordered by overlying signal-rich bands |
86 | 70 | 59 | 90 |
| Yonetsu et al20* | 2014 | 17 | NIRS | Diffusely bordered signal-poor region with signal attenuation by the overlying signal-rich layer |
90 | 60 | 90 | 60 |
| Habara et al12 | 2018 | 25 | Directional atherectomy |
Signal-poor regions without sharp borders |
89 | 75 | 67 | 92 |
| Yabushita et al21 | 2002 | 90 | Autopsy | Diffusely bordered signal-poor regions with overlying signal- rich bands |
90–94 | 90–92 | 74–75 | 97–98 |
| Thin cap fibroatheroma | ||||||||
| Fujii et al25 | 2015 | 60 | Autopsy | Lipid content >1 quadrant with fibrous cap measuring < 65 μm |
100 | 97 | 41 | 100 |
| Miyamoto et al28* | 2011 | 81 | Integrated backscatter IVUS >55% lipid pool |
Thin fibrous cap <65 μm overlying a lipid-rich plaque >90° |
95 | 88 | 88 | 94 |
| Phipps et al26 | 2016 | 10 | Autopsy | Fibrous cap <65 μm overlying a lipid core |
87 | 92 | 37 | 99 |
*Data calculated based on numbers provided. IVUS, intravascular ultrasound; NIRS, near-infrared spectroscopy; NPV, negative predictive value; OCT, optical coherence tomography; PPV, positive predictive value.
TCFA was most frequently defined as a lipid-rich plaque with a minimum fibrous cap thickness of <65 µm.6,14 Interobserver agreement of cap thickness (ICC 0.52–0.91) and TCFA (κ=66–0.93)14,16–19,22–24 was fair to good, improving with training to identify macrophages and calcification,14 and with semi-automated detection of fibrous cap.23 Although OCT detection of TCFA was sensitive (87–100%) and specific (92–97%) in histopathology studies, positive predictive values are lower (41–47%), with microcalcifications, macrophages, foam cells, hemosiderin, fibrin, and loose connective tissue contributing to false positives.12,25,26 Macrophages, foam cells, and microcalcifications are hypothesized to result in extensive scattering of light, resulting in signal attenuation that mimics lipid.25,26
Measurements of OCT lipid (greater angle and thinner cap) were associated with higher maximum lipid core burden index by NIRS.19,27 Detection of lipid by OCT was more sensitive than with intravascular ultrasound (IVUS), even when IVUS was performed with enhanced integrated backscatter.28 Lipid and TCFA are more common in plaques with higher grades of stenosis.4,29 Lipid arc and cap are correlated with atheroma volume.10 Lipid is more commonly found in the main branch side of bifurcations, with TCFA more frequently opposite the flow divider.11,30 TCFA is more common in the proximal and mid-left anterior descending artery and proximal circumflex artery,30 particularly around the obtuse marginal bifurcation,31 with broader distribution in the right coronary artery clustered around the mid-vessel and distal bifurcation.30,31
Effect of Lipid on In-Laboratory OutcomesStenting lipid is associated with in-stent plaque prolapse,32,33 intra-stent thrombus,32,34,35 and incomplete stent apposition36 (Table 3). Fibrous cap thickness is inversely correlated with in-stent plaque prolapse.37 Landing a stent in lipid was associated with edge dissections,38 both proximal39 and distal,40 and edge restenosis.41 Lipid opposite a side branch ostium was associated with angiographic stenosis of the side branch origin after provisional stenting.42
| Study | Year | Population | No. patients | Association |
|---|---|---|---|---|
| Edge dissections | ||||
| Antonsen et al38 | 2016 | NSTEMI | 97 | Edge dissection associated with thick-fibroatheroma (P<0.001) |
| Gonzalo et al40 | 2010 | Stable angina and ACS | 73 | Distal edge dissection associated with plaque type (fibrocalcific 43.8%, lipid rich 37.5%, fibrous 10%; P=0.009) |
| Zeglin-Sawczuk et al39 | 2013 | Stable angina and ACS | 203 | >90° lipid arc associated with proximal edge dissection (OR 23.6, P<0.01) |
| Tissue protrusion | ||||
| Bryniarski et al34 | 2017 | ACS and stable angina | 786 | Maximal lipid arc (187° vs. 162°; P=0.004), mean lipid arc (138° vs. 119°; P=0.001) and lipid index (P=0.003) |
| Sugiyama et al56 | 2017 | Stable angina and ACS | 145 | >180° lipid arc (P<0.001) |
| Feng et al33 | 2011 | Unstable Angina | 68 | Lipid associated with tissue prolapse (P<0.001) and in-stent microthrombosis (P<0.001) |
| Incomplete apposition | ||||
| Bernelli et al32 | 2016 | ACS | 140 | >90° lipid arc (OR 1.3, P=0.04) |
| Reduced post-stenting flow | ||||
| Gamou et al43 | 2014 | Stable angina and ACS | 141 | TCFA <65 μm (HR 12.32, P=0.0005) |
| Ikenaga et al53 | 2012 | STEMI | 39 | Length of lipid pool >9 mm (P=0.002) |
| Ozaki et al54 | 2011 | ACS | 211 | TCFA <70 μm (P=0.012) |
| Soeda et al44 | 2017 | STEMI | 145 | Maximum lipid arc (344° vs. 320°; P=0.032) and lipid index (P<0.001) |
| SB occlusion >50% | ||||
| Kini et al42 | 2017 | Stable angina | 30 | Maximal lipid arc (146° vs. 75°; OR 1.014 per degree, P=0.038), presence of lipid plaque contralateral to SB ostium (OR 8.14, P=0.046) |
| Periprocedural biomarker elevation | ||||
| Imola et al46 | 2013 | Stable angina | 30 | Lipid pool quadrants (P=0.09) and lipid pool arc (P=0.006) with all lipid arc >154° |
| Kini et al51 | 2015 | Stable angina | 110 | Greater cap thickness (OR 0.90, P<0.001) |
| Lee et al18 | 2011 | Stable and unstable angina | 135 | ≥2 quadrants of lipid (OR 3.49, P=0.003) and plaque cavity (OR 2.92, P<0.017) |
| Lee et al24 | 2015 | Stable angina | 206 | Lipid arc (178° vs. 160° degrees; OR 1.01 per degree, P=0.018), lipid length (8 vs. 5 mm; OR 1.08 per mm, P=0.008), and TCFA <80 μm (OR 4.5, P<0.001) |
| Lee et al52 | 2017 | NSTEMI | 167 | TCFA <70 μm (OR 2.88, P=0.011) and lipid length (10 vs. 7 mm; OR 1.12, P=0.02) |
| Porto et al37 | 2012 | NSTEMI and stable angina | 50 | TCFA < 65 μm (OR 29.7, P=0.008) |
| Ueda et al50 | 2014 | Stable angina | 68 | Colocalization of lipid and spotty calcification (OR 8.4, P<0.01) |
| Uzu et al35 | 2017 | Stable angina | 94 | Lipid index (P<0.001) |
| Yamamoto et al47 | 2013 | Stable angina | 79 | Optimal cut-off values: lipid arc >115°, fibrous cap thickness <110 μm, and lipid length >7.7 mm |
ACS, acute coronary syndrome; HR, hazard ratio; NSTEMI, non-ST segment elevation myocardial infarction; OR, odds ratio; SB, side branch; STEMI, ST-elevation myocardial infarction; TCFA, thin cap fibroatheroma. Other abbreviations as in Table 2.
In patients undergoing elective stenting, the presence of lipid plaque was associated with a reduction in Thrombolysis in Myocardial Infarction (TIMI) flow.32,43 In patients undergoing primary PCI, the extent of lipid was associated with no-reflow44 and no-reflow with filter device use.45 One study identified an association between lipid and incomplete stent apposition in a primary PCI cohort, where malapposition was also associated with the presence of thrombus and larger proximal vessel diameters.36 The impact of lipid on stenting is summarized in Figure 2.
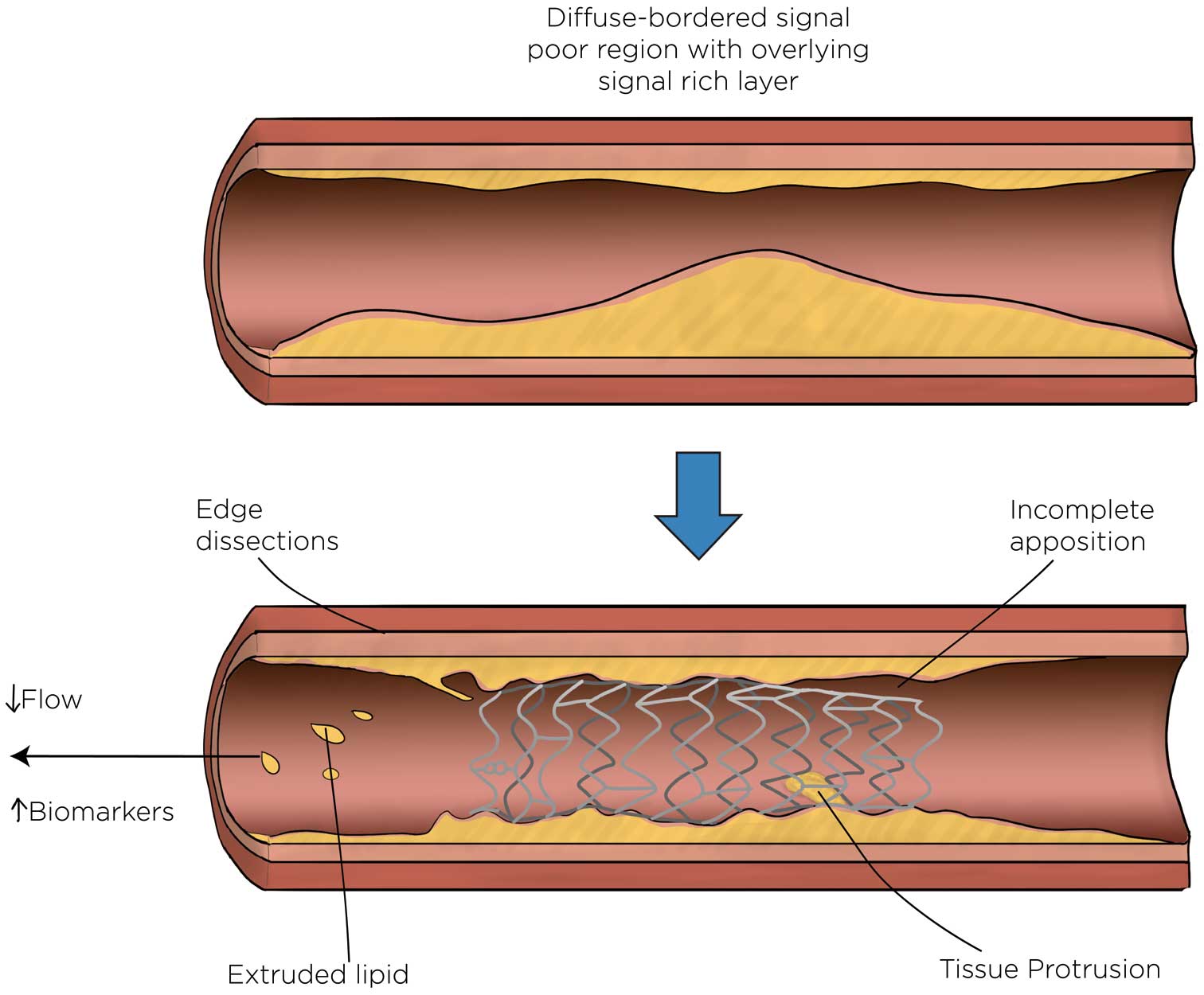
Effect of optical coherence tomography-identified lipid on stenting.
Lipid was associated with post-procedural increases in cardiac biomarkers in elective stenting,17,18,24,46–52 which was related to landing the stent in lipid proximally,46 the lipid arc,23,24 lipid length,18,24,47 and the presence of TCFA.18,24,37,47,49 In patients undergoing primary PCI, the extent of lipid was related to the lack of prompt resolution of stent thrombosis53 and higher increases in enzymes.53 In patients with acute coronary syndromes, fibrous cap thickness was inversely correlated with microvascular obstruction identified by magnetic resonance imaging.54
The impact of lipid on stent healing is less clear, with reports of greater neointimal hyperplasia of struts overlying lipid at OCT imaging follow-up at 3 and 9 months,55,56 and greater persistence of malapposition of stented lipid plaques at 8 months.57 The presence of fibrous cap disruption and a necrotic core with macrophages in an acute culprit before stenting were independent predictors of the composite of cardiovascular death, non-fatal myocardial infarction and clinically driven revascularization at 1 year in a retrospective cohort of 209 patients.58
Effect of Lipid on Clinical OutcomesIn a natural history study of 1,000 patients, half of whom had an left anterior descending (LAD) culprit, lipid arc >180° and TCFA were both associated with the composite of cardiac death and target vessel LAD segment myocardial infarction at 1 year.5
In non-culprit disease, unstented lipid and TCFA were associated with rapid plaque progression based on imaging at 6-month intervals59 (Table 4). In a cohort of 1,378 patients followed prospectively for 6 years, lipid arc >180° and fibrous cap thickness <65 µm were each independently associated with the development of acute coronary syndrome at the plaque level.60 The hazard ratio was increased when both these variables were present but fewer lesions possessed both characteristics. In receiver operating characteristic curve analysis, fibrous cap thickness <150 µm had the greatest sensitivity (88%) and negative predictive value (99%) to rule out incident acute coronary syndrome; the presence of lipid plaque with a lipid arc >180° in combination with TCFA <65 µm had the greatest specificity (97%) and positive predictive value (33%).60 Lipid length >5.9 mm, lipid arc >193°, and area stenosis >69% were predictive of the composite of cardiac death, myocardial infarction, and ischemia-drive revascularization among 1,474 patients followed for a median of 2 years.61
| Study | Year | No. patients |
Study design |
Mean follow-up (months) |
Outcomes | Stenosis severity |
Lipid extent | Macrophages | Fibrous cap thickness |
Plaque rupture |
|---|---|---|---|---|---|---|---|---|---|---|
| Iannaccone et al58 | 2018 | 209 | Retrospective, multicenter |
12.6 | Patient level: composite of death from cardiac causes, non-fatal MI, and clinically driven target vessel revascularization |
Presence of necrotic core (signal-poor region with poorly delineated borders) with macrophages (HR 3.3, 95% CI 1.6–6.6, P<0.01) |
Fibrous cap thickness <65 μm (HR 2.3, 95% CI 1.6–5.4, P=0.04) |
Fibrous cap discontinuity (HR 3.7, 95% CI 1.4–9.8, P<0.01) |
||
| Xing et al61 | 2017 | 1,474 | Retrospective multicenter |
48 | Patient level: composite of cardiac death, acute MI, and ischemia-driven revascularization in non-culprit segment |
Area stenosis >69% (HR 6.1, P<0.001; AUC 0.66, P=0.005) |
Lipid arc >193° (HR 2.49, P=0.024; AUC 0.64, P=0.012) Lipid length >5.9 mm (HR 5.69, P=0.001; AUC 0.66, P=0.005) |
|||
| Araki et al59 | 2020 | 248 | Retrospective, single center |
7.1 | Angiographic: rapid progression (decrease in mean luminal diameter ≥0.4 mm) |
Lipid arc >90° (OR 3.1, P<0.001) | Present (OR 2.22, P=0.036) |
Fibrous cap thickness <65 μm and lipid arc >90° (OR 4.08, P<0.001) |
Fibrous cap discontinuity with cavity formation (OR 2.76, P=0.029) |
|
| Prati et al5 | 2020 | 1,003 | Prospective, multicenter |
12 | Patient level: composite of cardiac death and target MI in imaged LAD territory |
MLA <3.5 mm2 (HR 2.07, P=0.032) |
Lipid arc >180° (HR 2.40, P=0.017) | Present (HR 2.66, P=0.027) |
Fibrous cap thickness <75 μm (HR 4.65, P<0.001) |
|
| Kubo et al60 | 2021 | 1,378 | Retrospective single center |
72 | Plaque level: ACS in non-culprit imaged segment |
MLA <2.9 mm2 (HR 4.56, P<0.001) |
Lipid arc >180° (HR 12.6, P<0.001) | Present (HR 4.83, P<0.001) |
Fibrous cap thickness <65 μm (HR 10.4, P<0.001) |
|
AUC, area under the curve; MI, myocardial infarction; MLA, minimum lumen area. Other abbreviations as in Table 3.
This literature has some limitations. Multivessel imaging was not performed in any of the studies identified. A prospective study design was only used in 1 study (CLIMA: Coronary plaque morphology of the left anterior descending artery and twelve months clinical outcome5), whereas the remaining studies were retrospective evaluations of OCT images acquired during image-guided PCI and were confined to the non-stented segment of the PCI target vessel. Only 1 study had exclusively plaque-level endpoints in the study analysis.57 The imaging parameters associated with events in the current literature are summarized in Figure 3.
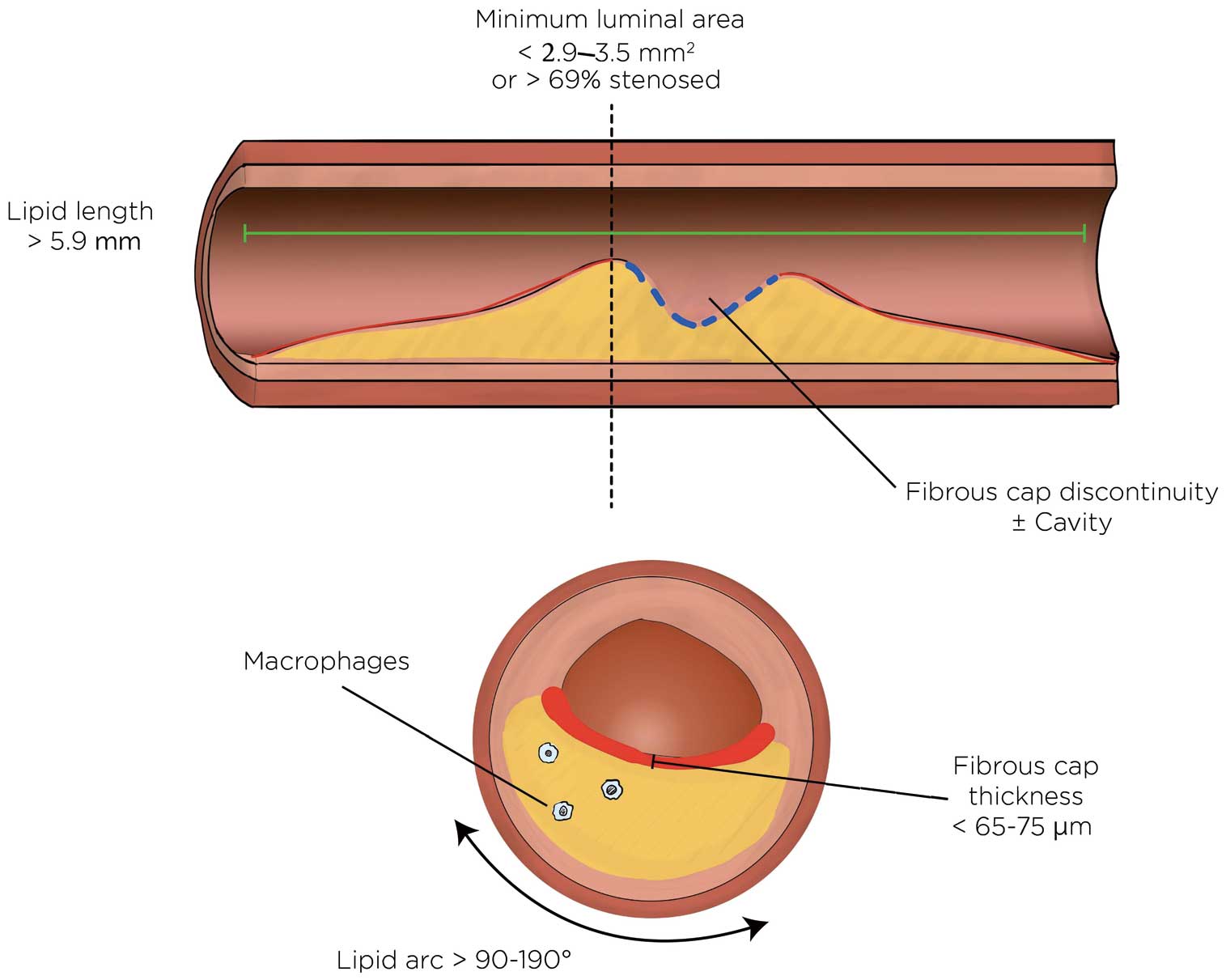
Lipid-related optical coherence tomography predictors of adverse outcomes.
This review identified several key findings: (1) OCT identification of lipid had acceptable reliability, with the potential for improvements with training and automated detection; (2) stenting lipid was associated with plaque prolapse, incomplete apposition, and a reduction in post-intervention coronary flow; (3) stenting lipid was associated with post-procedural increases in cardiac biomarkers and other markers of microvascular obstruction; and (4) unstented lipid has an unfavorable natural history.
The OCT recognition of lipid as a low signal intensity area with diffuse borders is supported by correlative studies using histology and other imaging modalities. However, there is a false positive rate because a minority of plaques with these characteristics are not lipid based on histologic assessment. Operators are encouraged to examine adjacent cross-sections to improve their specificity in identifying lipid. Further, lipid plaque frequently contains micro- and macrocalcifications, and vice versa, making the identification of lipid deep to calcified plaque difficult. Automated approaches hold promise to improve the detection of lipid and to reduce the operator training required to achieve reliable lipid detection. Focal bands of attenuation not found in adjacent cross-sections are characteristic of macrophage infiltration, intraluminal extension with irregular borders is more consistent with red thrombus, and sharply delineated borders in contiguous cross-sections favor calcium. Using a threshold of <65 µm, TCFA is accurately diagnosed by OCT in correlative studies. Thresholds for evaluating intra- and interobserver identification of TCFA vary from 65 to 85 µm with similar results. Semiautomated, machine-based approaches show early promise for improving the consistency of image interpretation of TCFA, and further evaluation of automated detection of lipid plaque is warranted.
Of all plaque types, lipid appears to be the most easily deformed and disrupted. A paucity of supportive fibrous and connective tissues likely contributes to lipid exuding through metallic struts, resulting in intra-stent plaque prolapse and, in some cases, overt mechanical displacement of lipid downstream of the stented segment. Downstream displacement of micro- and macro-lipid plaque components could lead to microvascular obstruction, no-reflow phenomenon, post-procedural increases in enzymes, and leave tissue voids contributing to malapposition. Image guidance during intervention has the advantage of anticipating these potential complications when stenting lipidic lesions. Observation of extensive lipid warrants a focus on appropriate evidence-based systemic lipid therapy.62
Despite a diversity of a priori thresholds defining lipid plaque, lipid arc, and TCFA, the associations with stent-elated complications are consistent, with a narrow range of thresholds found. Potential management implications of identifying lipid have not been defined in clinical trials, and there are no established interventional approaches specific to OCT-identified lipid. However, given the observational literature, avoiding ending stents in lipid plaques seems justified. Moderating the approach to post-dilation that takes into account the extent of lipid plaque burden would be reasonable. In contrast with the existing literature, which problematizes stent underexpansion with calcified plaque as the principal determinant of restenosis,63,64 the absence of literature around underexpansion related to lipid is notable.
The presence of lipid-rich plaque is associated with recurrent ischemic events in non-stented segments. However, there were no OCT studies of plaque natural history conducted with a prospective multivessel imaging protocol. In contrast, IVUS plaque characteristics were assessed in 3 large prospective, multivessel imaging studies with adjudication of events at the patient and plaque level.3,65,66 Patients who have a significant lipid burden frequently have other risk factors for recurrent events, making it important to have enough outcome events in observational studies to allow clinical variables to be adjusted for. When imaging findings are evaluated for predictive value at the patient level, they serve as an overall measure of risk rather than providing insight into plaque-level events. Therefore, the development of a treatment target for lipid-rich plaque will require robust plaque-level data. Adequately powered natural history studies are needed to provide further evidence for the effects of lipid plaque on future events at both the patient and plaque levels. Ideally, these studies would be conducted with reproducible quantification of lipid by automated and computerized approaches, laying the groundwork for a definition of clinically relevant lipid based on predictive ability for future events.
Study LimitationsSeveral limitations of this study are worth highlighting. The scoping review methodology provides an overview of the available evidence, without formally assessing study quality or aggregating outcomes across studies. Studies that compare OCT with other imaging modalities or histopathologic specimens rely on manual coregistration, which can introduce the possibility of bias, especially because lipid is frequently found colocated and may be mistaken for calcium, and vice versa. Given the observational nature of the data, the results are linked to the specific populations studied and include heterogeneity in the a priori imaging thresholds applied to assess for associations.
Lipid and TCFA are readily identifiable on OCT with acceptable reliability. Stenting lipid is associated with plaque prolapse, incomplete apposition, a reduction in post-stenting coronary flow, and post-procedural increases in cardiac biomarkers. Characteristics of unstented lipid are linked to rapid plaque progression and recurrent ischemic events and should be investigated further.
Impact on Daily PracticeLipid is identified as a low signal intensity area with diffuse borders on OCT. Stenting lipid is associated with edge dissections, reduced vessel flow and periprocedural increases in enzymes. It is reasonable to avoid ending stents in lipid and to moderate the approach to post-dilation.
None.
Not applicable.
This study did not receive any specific funding.
M.S., N.P.-E., T.S. have received speakers’ honoraria from Abbott. The remaining authors have no conflicts of interest to declare.