2021 Volume 85 Issue 3 Pages 291-299
2021 Volume 85 Issue 3 Pages 291-299
Background: Implantable cardioverter defibrillator (ICD) therapies, even when appropriate, are associated with increased risk. Therapy-reducing strategies have been shown to reduce the mortality rate.
Methods and Results: In total, 895 patients with ICD and cardiac resynchronization therapy with defibrillation function (CRT-D) were included in the study; of these, 506 (57%) patients undergoing secondary prevention were included. Devices implanted before May 2014 were programmed according to conventional programming (CP), the others according to our novel programming (NP) with high rate cut-off, longer detection intervals and 4–6 anti-tachycardia pacing (ATP) trains in the ventricular tachycardia (VT) zone. Time-to-first-event for mortality, appropriate and inappropriate therapies were analyzed. Follow-up time was 24.0 months (IQR 13.0–24.0 months). There was a significant reduction in mortality rate (11.4% vs. 25.4%, P<0.001) and in the rate of appropriate (18.8% vs. 42.2%, P<0.001) and inappropriate therapies (5.2% vs. 18.0%, P<0.001) with NP according to Kaplan-Meier analyses. In multivariate analysis, NP (hazard ratio [HR]=0.35; P<0.001), chronic kidney disease (HR=1.55), reduced ejection fraction (EF) (HR=1.35), secondary ICD indication (HR=2.35) and age at implantation (HR=1.02) were associated with mortality reduction. NP was also associated with significant reduction in the rate of appropriate and inappropriate therapies. These results were consistent after stratification for primary and secondary prevention.
Conclusions: Novel ICD programming reduced mortality and morbidity due to appropriate or inappropriate ICD therapies in secondary as well as in primary ICD indication.
Implantable cardioverter defibrillator (ICD) therapy and cardiac resynchronization therapy with defibrillation function (CRT-D) have been demonstrated to reduce mortality in patients with heart failure.1–7
Specifically, patients who received an ICD for secondary prevention generally have a higher mortality risk and expectedly a higher need for appropriate ICD therapies compared to patients with primary ICD indication.8 In contrast, patients with ICD may also suffer from inappropriate therapies for supraventricular tachycardia or non-sustained ventricular tachycardias (VT).9 There is an established association between ICD shock therapy and death, regarding appropriate as well as inappropriate shocks.9–11 Furthermore, it had also been shown that those patients who received an ICD shock for a fast VT episode had increased mortality, but this was not the case for those who received anti-tachycardia pacing (ATP) for fast VT without shock.11 Other data questioned the harmlessness of ATP therapies, demonstrating a higher mortality rate in patients who have more ATP therapies and similar appropriate shock therapies.12 An explanation might be the acceleration of “slow” VTs into fast VTs or ventricular fibrillation (VF).13 In summary, there are conflicting data about the effects of various ICD therapies on long-term mortality.
Recently, new programming algorithms with higher rate cut-offs and delayed detection intervals have been introduced, which were shown to reduce mortality and morbidity, mainly in primary prevention patients.12,14–17 Data on the effects of the novel programming in patients requiring secondary prevention is scarce.18,19
After the introduction of these novel programming strategies, we developed an institutional device programming standard combining different aspects of these suggested strategies. Here, we present the long-term effects of this novel programming strategy on the mortality and morbidity rate in a large real-life cohort, which consists of patients with primary and secondary ICD indication.
For this study, we screened all 1,172 patients in whom an ICD or CRT-D had been implanted at the University Hospital Regensburg, Germany, since January 2002, based on the local Registry Regensburg ICD Survival Trial (Res-IST). Inclusion criteria were the documentation of at least 2 device interrogations at our institution. Exclusion criteria were age <18 years and unwillingness to sign the informed consent form.
We included all the device interrogations as well as clinical follow-up data into an electronic database each time we performed a device follow up in our clinic. Follow up was also stopped when a patient had undergone heart transplantation. If the ICD was explanted or deactivated, we only stopped observing the device therapies, but not survival, although we judged this as the end of the observation in this study.
Inclusion of patients was continued until October 2017.
The study was approved by the Health Sciences Research Ethics Board at the University Regensburg, Regensburg, Germany, and all participants gave both verbal and written informed consent.
Device InterrogationEach patient was followed up in our clinic for 4–6 weeks after the implantation; thereafter, every 3–6 months. During each visit, device-related parameters including lead function, appropriate and inappropriate therapies, device-related complications, battery status, device history and device programming were evaluated and documented.
Defining Device TherapiesAppropriate therapy was defined as an episode of VT or VF treated with ATP and/or shock. Inappropriate therapies were ATP or shock therapies in the case of a supraventricular arrhythmia (atrial fibrillation [AF]), atrial flutter, atrioventricular node reentrant tachycardia, atrioventricular reentrant tachycardia, sinus tachycardia, focal atrial tachycardia) or of an oversensing due to lead issues or due to electromagnetic interferences. If an episode was treated with both ATP and shock, shock therapy was selected as the endpoint. An electrical storm was defined as ≥3 separate episodes of sustained VT or VF in 24 h.
ICD ProgrammingIn our institution, we developed a new device programming standard to reduce therapies by using the proposed strategies from the known therapy reduction studies published in the last years.12,14–17 Our novel programming strategy differs from the conventional ICD programming because we have chosen a higher rate cut-off for the VT zone with delayed detection, among other measures. Opposed to the other therapy reduction studies, our novel ICD programming was characterized by up to 4 sequences of ATP in patients with marked left ventricular (LV) dysfunction (EF <40%) and 6 sequences of ATP in patients with slightly reduced and normal LV function (EF >40%). Novel and conventional ICD programming for patients with primary prevention ICD indication is shown in Table 1.
| Conventional programming (CP) | Novel programming (NP) | |||||
|---|---|---|---|---|---|---|
| Heart rate (beats/min) |
Detection time | Therapy | Heart rate (beats/min) |
Detection time | Therapy | |
| VT1 Zone | – | – | – | Boston Sc.: 170 Medtronic: 170 Abbott: 170 Biotronik: 171 |
Boston Sc.: 60 s Medtronic: 20 Abbott: 100 Biotronik: 60 intervals |
– |
| VT2 Zone Medtronic: FVT |
170 | Boston Sc.: 2.5 s Medtronic: NID 20 Abbott: 20 intervals Biotronik: 16 intervals |
3 ATPs Scan 3 ATPs Ramp Shock therapy |
Boston Sc.: 190 Medtronic: 188 Abbott: 187 Biotronik: 187 If AF: 200 |
Boston Sc.: 12 s Medtronic: NID 20 Abbott: 40 intervals Biotronik: 40 intervals |
2 ATPs Scan† 2 ATPs Ramp† Shock therapy |
| VF Zone | 210 | Boston Sc.: 1.0 s Medtronic: NID 12/16 Abbott: 12 intervals Biotronik: 8/12 intervals |
1 ATP during charging (if available) Shock therapy |
Boston Sc.: 250 Medtronic: 250 Abbott: 240 Biotronik: 250 |
Boston Sc.: 5 s Medtronic: NID 30/40 Abbott: 20 intervals Biotronik: 24/30 interval |
1 ATP during charging (if available) Shock therapy |
Details of the device programming in the different settings (†If EF >40%, then 3 ATPs Scan and 3 ATPs Ramp). AF, atrial fibrillation; ATP, antitachycardia pacing; EF, ejection fraction; FVT, fast ventricular tachycardia; NID, number of intervals to detect; VF, ventricular fibrillation; VT, ventricular tachycardia. We cannot provide an evaluated NP strategy for Livanova devices. Therapies using Livanova devices in the CP group were programmed according to CP with a VT zone from 170 to 210 beats/min with 6 ATP sequences (3 Scans and 3 Ramps) followed by shock therapies and a VF zone >210 beats/min with 1 ATP during charging followed by shock therapies. The detection time was 16 intervals in the VT zone and 8/12 intervals in the VF zone.
In patients with primary prevention indication without previously documented VT, a “monitor only zone” was programmed routinely. Sustained rate duration was deactivated in all patients.
In conventional, as well as in novel programming, the first ATP sequence was a scan train (Boston & St. Jude Medical (SJM): 88% coupling interval, 88% burst cycle length, 10 ms decrement; Medtronic: 88% coupling interval, 10 ms decrement; Biotronik: 90% coupling interval, 10 ms decrement) and the second sequence was a ramp train (Boston & SJM: 88% coupling interval, 84% burst cycle length, 10 ms decrement; Medtronic: 91% coupling interval; Biotronik: 85% coupling interval, 10 ms S1-decrement).
In secondary prevention patients with documented VT >200 beats/min, the programming strategy corresponded to the primary prevention ICD programming settings. In those with documented VT <200 beats/min, the VT zone was programmed 10–15 beats/min slower than the slowest documented VT rate. If the VT was hemodynamically tolerable, we programmed only ATP therapies, but if it was not tolerable, both ATP and shock therapy were programmed.
In inherited arrhythmia syndromes like Brugada, long QT and short QT syndrome etc., we programmed a monitor zone from 187 to 220 beats/min, with a detection time of 60 s and a VF zone starting from 220 beats/min with a 5-s detection time, as the expected arrhythmia is a VF.20,21
Study EndpointsThe prespecified primary endpoint was the first occurrence of the following: death, appropriate therapy (with differentiation between ATP and shock therapy) and inappropriate therapy. Additionally, heart transplantation was considered as an endpoint.
Statistical AnalysisDescriptive data are presented as mean (±standard deviation (SD)), medians (±SD) or percentages. Normally distributed values were evaluated by using a Student’s unpaired 2-sided t-test. The Mann-Whitney U-test was used in the case of continuous non-normally distributed variables. Correlation coefficients were calculated according to Spearman’s rank correlation coefficient.
The following endpoints were defined: mortality, appropriate therapy (with differentiation between ATP and shock therapy) and inappropriate therapy. Therapies were analyzed according to time-to-first-event. Event rate was calculated as event for each patient per 100 person-years and was reported separately for each event. Data regarding therapy frequency for each single patient was not incorporated in the analysis.
Event-free survival analysis was conducted by using Kaplan-Meier survival curves. Mode of ICD programming was used as binary cut-point and the curves were compared by using the log-rank test. Multivariable Cox proportional-hazard analyses were performed as stepwise regressions with backward elimination to evaluate possible independent predictors of each endpoint. Models were adjusted for baseline prognostic factors including age, sex, primary or secondary ICD prevention, diabetes mellitus, renal insufficiency, degree of EF reduction, history of myocardial infarction (MI), transient ischemic attack or stroke, AF and valve surgery. Risk relationships were shown as hazard ratios (HRs) and 95% confidence intervals (CIs), generated with the use of the Multivariable Cox proportional hazard model. As a result, appropriate and inappropriate shocks were classified as 2 independent events.
Data were analyzed using commercially available statistical software packages (SPSS 22.0; SPSS Inc., Chicago, IL, USA).
Out of 1,172 patients, 548 were followed up in our institution and 616 in nearby consulting cardiology clinics; 8 patients were lost to follow up, mainly because of a change of the location. ICDs of 245 patients initially were programmed according to CP, but were reprogrammed according to NP in progress. These 245 patients were excluded from the study to avoid a group of patients who had been treated with both programming strategies. And 24 patients who had a subcutaneous ICD (S-ICD) were also excluded (Figure 1). Thus, data from 895 patients could be analyzed. Information regarding survival was shown in all patients and information regarding all therapies received was available for 586 patients (Table 2). Also, 309 patients with partially missing therapy data were only analyzed in terms of mortality, leading to differing population numbers in the according analyses.

All the screened patients were registered in our ICD Registry since 2002.
| NP (n=233) |
CP (n=662) |
P value | NP with full therapy data (n=202) |
CP with full therapy data (n=384) |
P value | |
|---|---|---|---|---|---|---|
| Age (years) | 66±13.4 | 64±12.7 | <0.05 | 66±13.5 | 64±13.3 | NS |
| Sex | ||||||
| Male | 192 (82.4) | 532 (80.2) | NS | 171 (84.7) | 314 (81.6) | NS |
| First prevention | 103 (44.2) | 286 (43.3) | NS | 93 (46.0) | 186 (48.6) | NS |
| CRT-D | <0.05 | <0.05 | ||||
| Single-Chamber ICD | 123 (52.8) | 390 (58.8) | 107 (53.0) | 234 (60.8) | ||
| Dual-Chamber ICD | 49 (21.0) | 171 (25.8) | 40 (19.8) | 95 (24.7) | ||
| CRT-D | 61 (26.2) | 102 (15.4) | 55 (27.2) | 56 (14.5) | ||
| Biotronik | 10 (4.3) | 25 (3.8) | 7 (3.5) | 15 (3.9) | ||
| Boston Sc./CPI/Guidant | 198 (85.0) | 473 (71.3) | 172 (85.1) | 271 (70.4) | ||
| Medtronic | 20 (8.6) | 137 (20.7) | 19 (9.4) | 84 (21.8) | ||
| Livanova/Ela Medical | 5 (0.8) | 3 (0.8) | ||||
| Abbott/SJM | 5 (2.1) | 23 (3.7) | 4 (2.0) | 12 (3.2) | ||
| Post myocardial infarction | 68 (29.2) | 258 (38.9) | <0.05 | 57 (28.2) | 152 (39.5) | NS |
| Coronary artery disease | 143 (61.4) | 356 (53.7) | <0.05 | 120 (59.4) | 206 (53.5) | NS |
| Cardiomyopathy | 74 (31.8) | 252 (38.0) | NS | 69 (34.2) | 145 (37.7) | NS |
| Inherited cardiac arrhythmia | 17 (7.3) | 26 (3.9) | <0.05 | 17 (8.5) | 16 (4.2) | <0.05 |
| EF ≤35% | 114 (48.9) | 342 (51.6) | NS | 100 (49.5) | 213 (55.3) | NS |
| NYHA – Class ≥II | 153 (65.7) | 527 (79.5) | NS | 136 (67.3) | 310 (80.5) | NS |
| Peripheral artery disease | 23 (9.9) | 69 (10.4) | NS | 18 (8.9) | 45 (11.7) | NS |
| Carotid artery stenosis | 12 (5.2) | 34 (5.1) | NS | 11 (5.4) | 21 (5.5) | NS |
| Valve surgery | 36 (15.9) | 49 (7.4) | <0.001 | 28 (13.9) | 26 (6.8) | <0.05 |
| AF | 93 (39.9) | 217 (32.7) | <0.05 | 79 (39.1) | 116 (28.6) | <0.05 |
| Obesity | 61 (26.2) | 155 (23.4) | NS | 53 (26.2) | 89 (23.1) | NS |
| Hypertension | 155 (66.5) | 410 (61.8) | NS | 133 (65.8) | 247 (64.2) | NS |
| Diabetes | 64 (27.5) | 222 (33.5) | NS | 54 (26.7) | 114 (29.6) | NS |
| Renal insufficiency | 59 (25.3) | 226 (34.1) | <0.05 | 52 (25.7) | 122 (31.7) | NS |
| TIA/stroke | 37 (15.9) | 74 (11.2) | NS | 32 (15.8) | 42 (10.9) | NS |
| Renin-angiotensin-aldosterone blocker | 191 (82.0) | 555 (83.7) | NS | 161 (79.7) | 333 (86.5) | NS |
| β-locker | 194 (83.3) | 574 (86.6) | NS | 168 (83.2) | 343 (89.1) | NS |
| Aldosterone antagonist | 123 (52.8) | 393 (59.3) | NS | 113 (55.9) | 234 (60.8) | NS |
| Digitalis | 22 (9.4) | 115 (17.3) | <0.05 | 17 (8.4) | 58 (15.1) | <0.05 |
| Diuretics | 157 (67.4) | 518 (78.1) | <0.05 | 137 (67.8) | 297 (77.1) | <0.05 |
| Ivabradin | 7 (3.0) | 4 (0.6) | <0.05 | 7 (3.5) | 2 (0.5) | NS |
| Amiodarone/Sotalol | 14 (6.0) | 64 (9.7) | NS | 11 (5.4) | 32 (8.3) | NS |
Data are presented as mean±SD or n (%). CPI, Cardiac Pacemakers, Inc; CRT-D, cardiac resynchronization therapy with defibrillation function; NYHA, New York Heart Association; NS, not significant; SJM, St. Jude Medical; TIA, transient ischemic attack. Other abbreviations as in Table 1.
Patients were predominantly male, and the majority had ischemic heart disease, and in almost half of the patients, the EF was ≤35%. The majority of patients had a secondary ICD indication. Patients who had NP were significantly older than patients who had CP, had more valve interventions and atrial fibrillation, and less often had a history of MI. Less digitalis was prescribed in patients who had NP (P<0.05). New York Heart Association class ≥2 did not differ between the NP and CP groups.
Mean duration of follow up was 24.0 months (interquartile range [IQR] 13.0–24.0 months); there was a mean duration of 24 months (IQR 15–24 months) for CP and a mean duration of 19 months (IQR 11–24 months) for NP.
Appropriate TherapiesA total of 182 (31.1%) appropriate therapies including both ICD shocks and ATP were detected during the study (131 ATP [22.4%], 99 shocks [16.9%]) (Table 3).
| Events NP | Events CP | Kaplan-Meier estimator NP (%) |
Kaplan-Meier estimator CP (%) |
P value | |
|---|---|---|---|---|---|
| Mortality | 21/233 (9.0) | 160/662 (24.1) | 11.4 | 25.4 | <0.001 |
| Appropriate therapies | 29/202 (14.4) | 153/384 (40.0) | 18.8 | 42.2 | <0.001 |
| Appr. ATPs | 18/202 (9.0) | 113/384 (29.4) | 11.1 | 31.2 | <0.001 |
| Appr. Shocks | 14/202 (6.9) | 85/384 (22.1) | 10.5 | 23.8 | <0.05 |
| Inappropriate therapies | 8/202 (4.0) | 64/384 (16.7) | 5.2 | 18.0 | <0.001 |
| Inappr. ATPs | 4/202 (2.0) | 30/384 (7.8) | 2.6 | 8.5 | <0.05 |
| Inappr. Shocks | 5/202 (2.5) | 36/384 (9.4) | 3.5 | 10.2 | <0.05 |
| First prevention | |||||
| Mortality | 6/103 (5.8) | 56/286 (20.0) | 8.2 | 25.4 | <0.05 |
| Appropriate therapies | 7/93 (7.5) | 69/186 (37.1) | 10.2 | 39.9 | <0.001 |
| Appr. ATPs | 3/93 (3.2) | 55/186 (29.6) | 4.6 | 31.5 | <0.001 |
| Appr. Shocks | 4/93 (4.3) | 30/186 (16.1) | 6.9 | 17.2 | NS |
| Inappropriate therapies | 3/93 (3.2) | 28/186 (15.1) | 4.2 | 16.2 | <0.05 |
| Inappr. ATPs | 1/93 (1.1) | 14/186 (7.6) | 1.1 | 8.1 | NS |
| Inappr. Shocks | 2/93 (2.2) | 15/186 (8.0) | 3.0 | 8.6 | NS |
| Second prevention | |||||
| Mortality | 15/130 (11.5) | 104/376 (27.7) | 14.0 | 28.8 | <0.05 |
| Appropriate therapies | 22/109 (20.2) | 85/198 (42.9) | 26.1 | 45.2 | <0.05 |
| Appr. ATPs | 15/109 (13.8) | 58/198 (29.3) | 16.7 | 30.9 | <0.05 |
| Appr. Shocks | 10/109 (9.2) | 55/198 (27.8) | 13.4 | 30.2 | <0.05 |
| Inappropriate therapies | 5/109 (4.6) | 36/198 (18.2) | 6.1 | 19.8 | <0.05 |
| Inappr. ATPs | 3/109 (2.8) | 16/198 (8.1) | 3.8 | 8.7 | NS |
| Inappr. Shocks | 3/109 (2.8) | 21/198 (10.6) | 3.8 | 11.6 | NS |
Data are presented as n/N (%). Appr., appropriate; ATP, antitachycardia pacing; Inappr., inappropriate. Other abbreviations as in Table 1.
In Kaplan-Meier analysis, NP was associated with a significantly lower occurrence of appropriate therapies (shock and ATP) than in patients with CP (18.8% vs. 42.2%, P<0.001), which was also evident after stratification for each shock and ATP therapy (shock 10.5% vs. 23.8%, P<0.05; ATP 11.1% vs. 31.2%, P<0.001).
A significant reduction in appropriate therapies with NP persisted also after stratifying the study group for primary and secondary prevention indication (primary prevention 10.2% vs. 39.3%, P<0.001; secondary prevention 26.1% vs. 45.2%, P<0.05).
For the patients with NP in the primary prevention group, the probability for ATP therapy was significantly lower (4.6% vs. 31.5%, P<0.001) than for patients with CP. For shock therapies, there was no significant difference (6.9% vs. 17.2%, P=0.058).
In the primary prevention group, patients with NP had received significantly less appropriate therapies compared with patients with CP (7.5 appropriate therapies per 100 patient years vs. 27.3 appropriate therapies per 100 patient years, P<0.001).
The same was also found in patients with secondary prevention (21.6 vs. 35.6 appropriate therapies per 100 patient years, with NP vs. CP, respectively, P<0.05).
There was no difference in the frequency of electrical storms with NP vs. CP (6 in 202 patients vs. 24 in 384 patients, P=0.541).
In a total of 6 patients in NP group, slow VTs occurred in the monitor zone. A comparison with CP was not possible due to a lack of a monitor zone in CP.
In the case of a sustained, symptomatic VT, we usually turned the monitor zone into a therapy zone with ATPs only, or in case of a syncope, to correlate the VT, we also added shocks therapies.
Inappropriate TherapiesA total of 72 (12.2%) inappropriate therapies were observed during the study.
Compared with CP, patients with NP showed significantly less inappropriate therapies (1st prevention: 3.1 vs. 9.1 per 100 patient years, P<0.05; 2nd prevention: 4.3 vs. 11.9 per 100 patient years, P<0.05).
In Kaplan-Meier analysis in patients with NP, the probability for receiving an inappropriate shock was significantly lower than in patients with CP (3.5% vs. 10.2% P<0.05; Table 3).
MortalityOverall, 181 (20.2%) patients died during the study period. Significantly less deaths occurred in patients with NP than with CP (6.5 per 100 patient years vs. 15.2 deaths per 100 patient years, respectively [P=0.001]).
In Kaplan-Meier analysis, NP was associated with a significantly reduced cumulative mortality compared with CP (11.4% vs. 25.4%, P<0.001). The mortality rate was also significantly lower in patients with NP after stratifying for primary and secondary prevention (8.2% vs. 20.9% in primary prevention and 14.0% vs. 28.8% in secondary prevention, each P<0.05; Table 3 and Figures 2–4).

In Kaplan-Meier analysis, novel programming (NP) was associated with a significantly reduced cumulative mortality compared with conventional programming (CP; 11.4% vs. 25.4%, P<0.001).
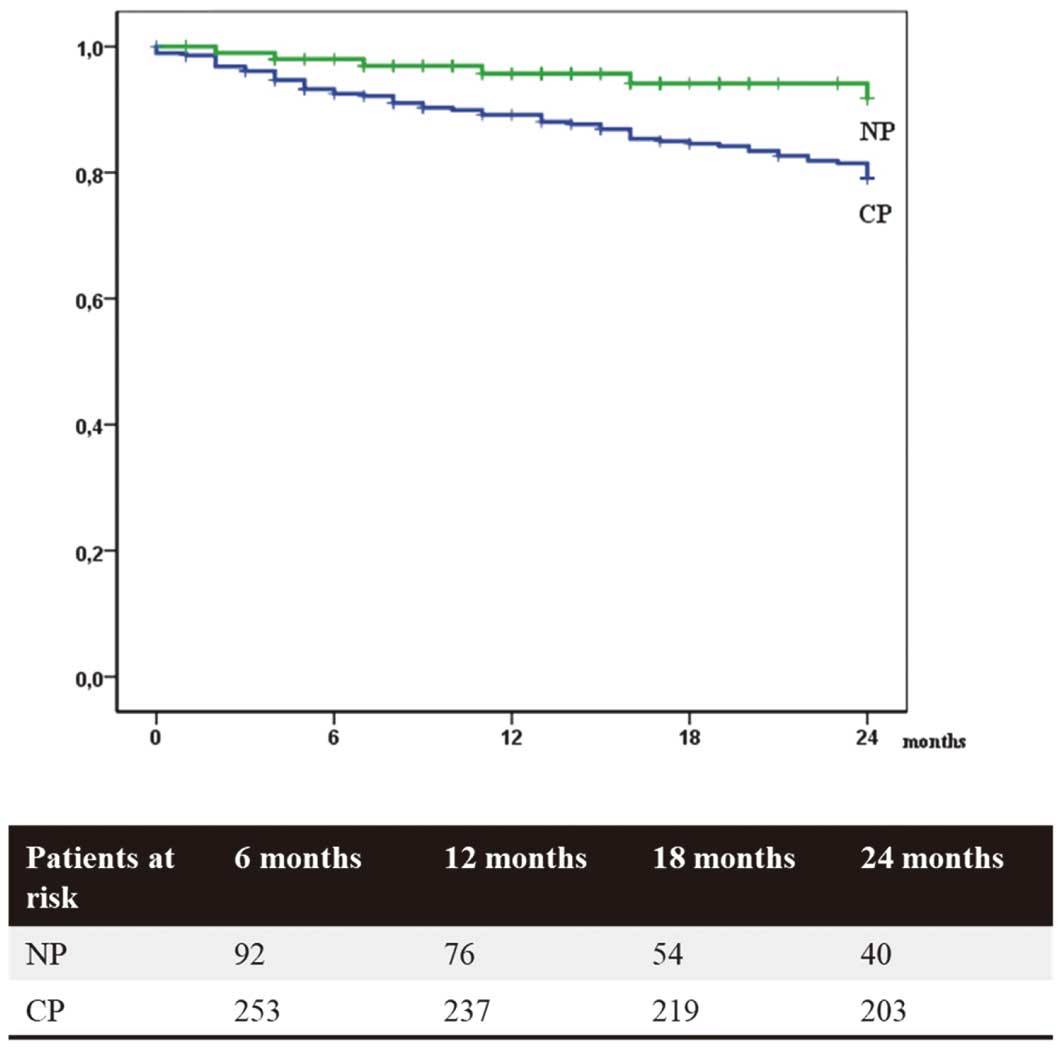
Mortality rate in patients with primary prevention was significantly lower with novel programming (NP) than with conventional programming (CP; 8.2% vs. 20.9%, P<0.05).

In the secondary prevention group, novel programming (NP) resulted in significantly less deaths than conventional programming (CP; 14.0% vs. 28.8%, P<0.05).
According to Cox regression analysis, NP was associated with a 66% relative reduction in mortality (HR=0.34, 95% CI 0.20–0.60, P<0.001). A similar relative risk reduction was observed in primary and secondary prevention (HR=0.21, 95% CI 0.06–0.67 vs. HR=0.42, 95% CI 0.22–0.80, each P<0.05).
Furthermore, a 59% relative reduction (HR=0.41 95% CI 0.27–0.62; P<0.001) in the risk of appropriate ICD therapies with NP vs. CP was observed. Regarding inappropriate therapies, a 77% relative reduction was evident favoring novel ICD programming (HR=0.23, 95% CI 0.10–0.54; P<0.001; Table 4).
| Overall cohort | 1st prevention | 2nd prevention | |||||||
|---|---|---|---|---|---|---|---|---|---|
| All-cause mortality |
Appropriate therapies |
Inappropriate therapies |
All-cause mortality |
Appropriate therapies |
Inappropriate therapies |
All-cause mortality |
Appropriate therapies |
Inappropriate therapies |
|
| NP | 0.34 (0.20–0.60) P<0.001 |
0.41 (0.27–0.62) P<0.001 |
0.23 (0.10–0.54) P<0.001 |
0.21 (0.06–0.67) P<0.05 |
0.25 (0.11–0.58) P=0.001 |
0.22 (0.05–0.94) P<0.05 |
0.42 (0.22–0.80) P<0.05 |
0.48 (0.29–0.80) P<0.05 |
0.26 (0.09–0.74) P<0.05 |
| Diabetes | NS | NS | 0.41 (0.21–0.80) P<0.05 |
NS | NS | NS | NS | NS | NS |
| Renal insufficiency |
1.55 (1.13–2.11) P<0.05 |
NS | NS | NS | NS | NS | 1.73 (1.16–2.58) P<0.05 |
NS | NS |
| EF | 1.35 (1.12–1.62) P=0.001 |
NS | NS | NS | NS | NS | 1.32 (1.10–1.60) P<0.05 |
NS | NS |
| TIA/ stroke | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Age (years) | 1.02 (1.00–1.04) P<0.05 |
1.02 (1.00–1.03) P<0.05 |
NS | 1.03 (1.00–1.06) P<0.05 |
NS | NS | 1.02 (1.00–1.04) P<0.05 |
NS | 0.98 (0.96–1.00) P<0.05 |
| Prevention | 2.35 (1.63–3.39) P<0.001 |
NS | NS | NS | NS | NS | NS | NS | NS |
| Device | NS | 0.78 (0.63–0.97) P<0.05 |
NS | NS | NS | NS | NS | NS | NS |
| AF | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| MI | NS | NS | 1.88 (1.10–3.24) P<0.05 |
NS | NS | NS | NS | NS | NS |
| Valve surgery | NS | NS | NS | NS | NS | NS | NS | NS | NS |
MI, myocardial infarction before implantation. Other abbreviations as in Tables 1,2. All significant variables are shown with hazard ratio, 95% confidence interval and P value.
The current study showed for the first time in a large real-life cohort with 895 patients a beneficial effect of a novel ICD programming strategy. NP outperformed the conventional ICD programming, with significantly lower rates of appropriate and inappropriate therapies, as well as a significantly lower mortality rate. One of the strengths of our study is the remarkably high number of patients with secondary ICD indication (57%) and a patient collective that has not been sufficiently studied yet with a novel programming strategy. Interestingly, the beneficial effects of novel ICD programming were more pronounced in patients with secondary than with primary prevention ICD indication.
Novel ICD ProgrammingThe proposed novel ICD programming was compounded by several other trials.12,14–17 It is unique because it combines high rate cut-offs with delayed detection times and ATP therapies.12,22,23 The VT zone, starting at 190 beats/min in patients with sinus rhythm and 200 beats/min in patients with a history of atrial fibrillation, was programmed up to 250 beats/min (240 in SJM ICDs), with 4 ATPs in patients with marked LV dysfunction and 6 ATPs in patients with slightly reduced and normal LV function. To the best of our knowledge, such a programming has not yet been investigated in a clinical trial. Moreover, the ATP trains in our novel programming strategy were less aggressive than in the therapy reduction trials like the Multicenter automatic defibrillator implantation trial: reduce inappropriate therapy (MADIT-RIT) trial (the cycle length of the first ATP train in the NP is 88% of the cycle length of the VT vs. 81% in MADIT-RIT trial).13 Even though the ATP therapies in our study were more conservative, they still resulted in significantly lowered mortality rates.
After adding new or increasing the existing antiarrhythmic medication; for example, by amiodarone or ß-blockers, depending on the VT indicating the ICD in patients with secondary prevention indication, we used lower heart rate cut-offs, usually 20–30 beats/min below the frequency of the initial VT.
The cut-off rates and detection times we used were examined in several big trials and have shown to be secure as well as effective, at least regarding patients with primary prevention ICD indication.12,14,16,24
Comparison of Novel and Conventional ProgrammingBoth appropriate and inappropriate ICD therapies are associated with a higher risk of death in primary as well as in secondary prevention studies.2,3–5 Therefore, to minimize the risk of causing inappropriate therapies, new device programming strategies had been developed to bring the ICD to intervene only when a sustained life-threatening ventricular arrhythmia had occurred. Indeed, such a strategy led to significantly lower mortality rates and inappropriate shock density – measured with events per 100 patient-years of follow up – in patients with therapy-reduction programming compared to conventional programming.12,14,15,24 Interestingly, in the MADIT-RIT trial, appropriate ICD shock rates were not different in both groups, showing that the inappropriate shock, as well as ATP therapies, were responsible for the increased mortality in the conventional group.12
The OBSERVational registry from Italy showed a reduced frequency of electrical storms in patients with less aggressive ICD programming.25 Interestingly, our study showed no difference in the occurrence of electrical storm between both programming strategies. One explanation may be that the conventional programming of the current study was not as aggressive as that in the Italian registry; for example, the sustained rate duration was never programmed and ATP during charging was programmed in all patients.
In summary, several trials studied different methods to limit ICD therapies to life-threatening ventricular arrhythmias and to avoid therapies for supraventricular arrhythmias or non-sustained ventricular arrhythmias. The results of the current study seem interchangeable between and consistent among the other studies. Our study not only confirms the effectiveness of the published therapy-reduction strategies, but it also shows that incorporating different aspects of such programming strategies can help to reduce therapy rates as well as mortality.26
Secondary Prevention ICD IndicationOne important aspect of the current study is the inclusion of a high number of patients with secondary ICD indication (57%). In the literature, there is a subgroup analysis of the ADVANCE III trial and a small study by Hayashi et al dealing with secondary prevention patients and new programming strategies.18,19 Regarding the ADVANCE III trial, of the 1,902 patients enrolled, 477 were ICD recipients due to secondary prevention, with a median follow-up period of 12 months. There were significantly fewer appropriate shocks in the long detection arm (30/40 NID) compared to standard arm (18/24 NID), but the rate of appropriate ATP therapies was not different in both groups. Unexpectedly, inappropriate shocks were not reduced in the long-detection arm.18 The significant reduction in the inappropriate therapies overall was due to the reduced rate of the inappropriate ATP.18 The mortality rate was not different between the 2 therapy arms, indicating the safety of the long-detection arm.18
Hayashi et al showed data of 65 patients with secondary prevention ICD indication and change of ICD programming during their study; they programmed higher detection rates and longer detection time in the VF zone. Follow-up time was 5 years in conventional and 2.5 years in new programming strategies. Appropriate shocks and ATP therapies, as well as inappropriate therapy, were significantly reduced in the so-called “strategic settings” compared to conventional programming. No data regarding mortality was presented.19
The current study confirmed and expanded the available information regarding ICD programming in patients with secondary prevention ICD indication. Of the whole study population, 74% were programmed with conventional and 26% with novel programming. NP reduced ATP and shock therapies, as well as inappropriate therapies and mortality in these patients. To the best of our knowledge, this is the first study showing that the novel programming reduces appropriate and inappropriate therapies leading to mortality reduction in patients with secondary prevention ICD indication.
In contrast to the other studies, our patients were programmed with several ATP therapies, which might be the reason for increased probability of successful ICD therapy. Our data were retrospectively collected and therefore a randomized trial in patients with secondary prevention ICD indication is needed to confirm our results. Nevertheless, therapy-reduction programming is safe in patients with primary as well as secondary prevention ICD indication, and should replace out-of-the-box programming in all patients.
Study LimitationsA significant limitation of our study is the lack of randomization. Further, the NP was introduced after 2014, whereas the CP existed long before. In the meantime, there were several profound alterations in the treatment of heart failure patients regarding drug therapy and device therapy, which resulted in a better prognosis for the patients.25,27–32 However, in our study, there was no significant difference regarding the use of medications for heart failure in both groups.
The occurrence of syncope was not documented consequently, so we cannot provide a statement about any change in syncope rates.
Furthermore, our experience regarding Livanova ICDs is limited. There are only 5 patients with Livanova devices with CP (respectively Sorin devices when they were implanted) and no patients with NP and Livanova devices.
We made huge efforts to get as much information as possible from all patients. The first ICDs in the study were implanted back in 2002; therefore, electrograms of all events from these patients were not available, thus we used the medical reports of those patients. Data from our own patients are well documented. But there is no guarantee that we received all ICD-detected event information from the outpatient clinics. To minimize bias, we excluded patients with missing follow-up data, leading to differing population numbers in analyses of mortality on the one hand, and appropriate and inappropriate therapies on the other hand. In our opinion, the analyses of appropriate and inappropriate is of such importance, therefore we wanted to present them in this study.
In conclusion, in patients with indication for ICD implantation, novel ICD programming helps to reduce mortality and morbidity due to appropriate and inappropriate ICD therapies. For the first time, a novel ICD programming with a high rate cut-off and longer detection intervals is associated with a benefit in reducing mortality independently from other predictors in patients with secondary prevention ICD indication. Nevertheless, further randomized trials are still warranted to optimize ICD programming. Randomized trials are especially necessary to compare different types of therapy-reduction programming to find the best programming for each patient.
Competency in Medical Knowledge: Programming the ICDs using the new recommendations reduces the rate of appropriate and inappropriate therapies as well mortality.
Competency in Patient Care: Therapy-reducing strategies, as suggested in the MADIT-RIT trial, should be used in all patients with ICDs, no matter if the patient has a primary or secondary ICD indication.
Translational Outlook: Randomized studies including patients with secondary ICD indication are still needed, but so far, our data provides safety information about using strategies to program the ICD with a high-rate cut-off, multiple ATPs and long detection intervals.
The authors declare no conflicts of interest.
The present study was approved by Regensburger Ethikkommission (Reference number: 14-101-0003).
The deidentified participant data will not be shared.