2021 Volume 85 Issue 5 Pages 667-668
2021 Volume 85 Issue 5 Pages 667-668
With the advent of an aging society, the number of patients with cardiovascular diseases has increased markedly. For these patients, therapeutic angiogenesis is being established as a new therapeutic strategy. The presence of mesenchymal stem cells in adipose tissues has been reported, and the development of therapeutic angiogenesis using these cells has attracted attention in Japan and overseas.1 Adipose-derived stem cells (ADSCs) are known to have the ability to differentiate into various cells, secrete many cytokines, and exert a proangiogenic action after implantation.2,3
Article p 657
At present, validation studies and clinical trials of treatment with ADSCs for various diseases (e.g., Crohn’s disease-associated fistulas, breast reconstruction after partial mastectomy, and osteogenesis for bone defects) are being conducted in Japan and overseas.4 For example, in the cardiovascular field, European studies include the APOLLO and ADVANCE trials in patients with acute myocardial infarction, as well as the PRECISE trial in patients with chronic myocardial ischemia.5,6 In the US, the Athena trial has been conducted in patients with chronic heart failure.7 In the APOLLO trial, ADSCs were injected into the coronary arteries of patients with ST-elevation acute myocardial infarction who underwent successful percutaneous coronary intervention.5 In the PRECISE trial, ADSCs were injected into the myocardium under the guidance of the NOGA XP Cardiac Navigation System in patients with myocardial ischemia who were determined to be unsuitable for revascularization.6 Both trials demonstrated that treatment with ADSCs is safe and effective. The ADVANCE and Athena trials are currently underway, and their results are awaited.
We are also proceeding with the clinical introduction of therapeutic angiogenesis with ADSC implantation in patients with critical limb ischemia.8 When ADSCs were implanted into the ischemic limbs of 5 patients, pain relief was achieved in all patients, in addition to partial or complete healing of ulcers and prolongation of the 6-min walk distance,6 months after implantation. Amputation of the affected limb was also avoided.8 Based on these results, we are currently conducting a multicenter prospective study at 8 institutions across Japan.9
Although, as described above, various cell therapies with ADSC implantation are used in the cardiovascular field, there are some issues. One issue is how to implant ADSCs. Direct needle injection and intravascular injection are associated with many problems, such as the limited size of the implanted area, loss of implanted cells during the procedure, and the induction of inflammation at the injection site. In this respect, cell sheet technology enables efficient implantation and engraftment. Several attempts have been made to develop cell sheets derived from ADSCs for treatment targeting ischemic heart diseases and heart failure. For example, cell sheet technology using a temperature-responsive culture dish and cell sheet fabrication using magnetic nanoparticles have been developed.10,11 In particular, cell sheet engineering technology using a temperature-responsive culture dish has been converted into commercial products and subjected to clinical studies of the implantation of sheets derived from various cells, such as skeletal muscle cells.12 Watanabe et al have created dual reporter transgenic rats expressing green fluorescence protein and luciferase and fabricated ADSC sheets using a temperature-responsive culture dish.13 When these ADSC sheets were implanted in the rat model of myocardial infarction, tracking of luciferase signals revealed that the implanted ADSC sheets survived for ≥2 weeks after implantation. After ADSC sheet implantation, there was an increase in microvessels in the ischemic area, and cardiac function after myocardial infarction improved.13 Thus, ADSC sheet implantation may allow cells to survive for a relatively long time and for them to perform their functions.
The second issue regarding cell therapy with ADSC implantation is that the detailed mechanism as to why ADSC implantation is effective remains unknown. Based on the results of previous studies, the effects of ADSC implantation on the cardiovascular system are considered to be primarily attributable to the paracrine effect of potent angiogenic growth factors (e.g., vascular endothelial growth factor [VEGF], hepatocyte growth factor [HGF], and basic fibroblast growth factor [bFGF]) secreted from implanted ADSCs, rather than the direct consequence of the differentiation of ADSC into vascular endothelial cells.2,11 In fact, a study in a mouse model of myocardial infarction showed that secretion of potent angiogenic factors and improvement in cardiac function were greater in mice implanted with cell sheets than in mice treated with intramuscular injection of ADSCs to the myocardium.11 Watanabe et al identified endothelial cell-specific molecule 1 (ESM1) and stanniocalcin 1 (Stc1) as new angiogenic growth factors, in addition to previously reported angiogenic growth factors (e.g., VEGF, HGF, and bFGF), secreted from ADSCs.13 Because the addition of recombinant ESM1 and Stc1 proteins to vascular endothelial cells induces tube formation comparable with that induced by VEGF, these factors appear to have a potent proangiogenic action. Some reports indicate that ADSC implantation is more effective in improving cardiac function after myocardial infarction than the use of other stem cells, such as bone marrow-derived stem cells.14 The ability of ADSCs to secrete various angiogenic factors, such as ESM1 and Stc1, may be the reason for the superior outcomes of therapeutic angiogenesis with ADSCs compared with other stem cells. In addition, ADSCs can be prepared in a minimally invasive and simple manner in a relatively short time, and their safety is guaranteed.8,15 With the effects of and mechanisms underlying the therapeutic angiogenesis with ADSCs being revealed one after another, as described by Watanabe et al and others, and with further improvements in administration techniques, we expect that the need for this therapy as a therapeutic tool will increase in the cardiovascular field, and that the therapy will be widely used in the near future (Figure).
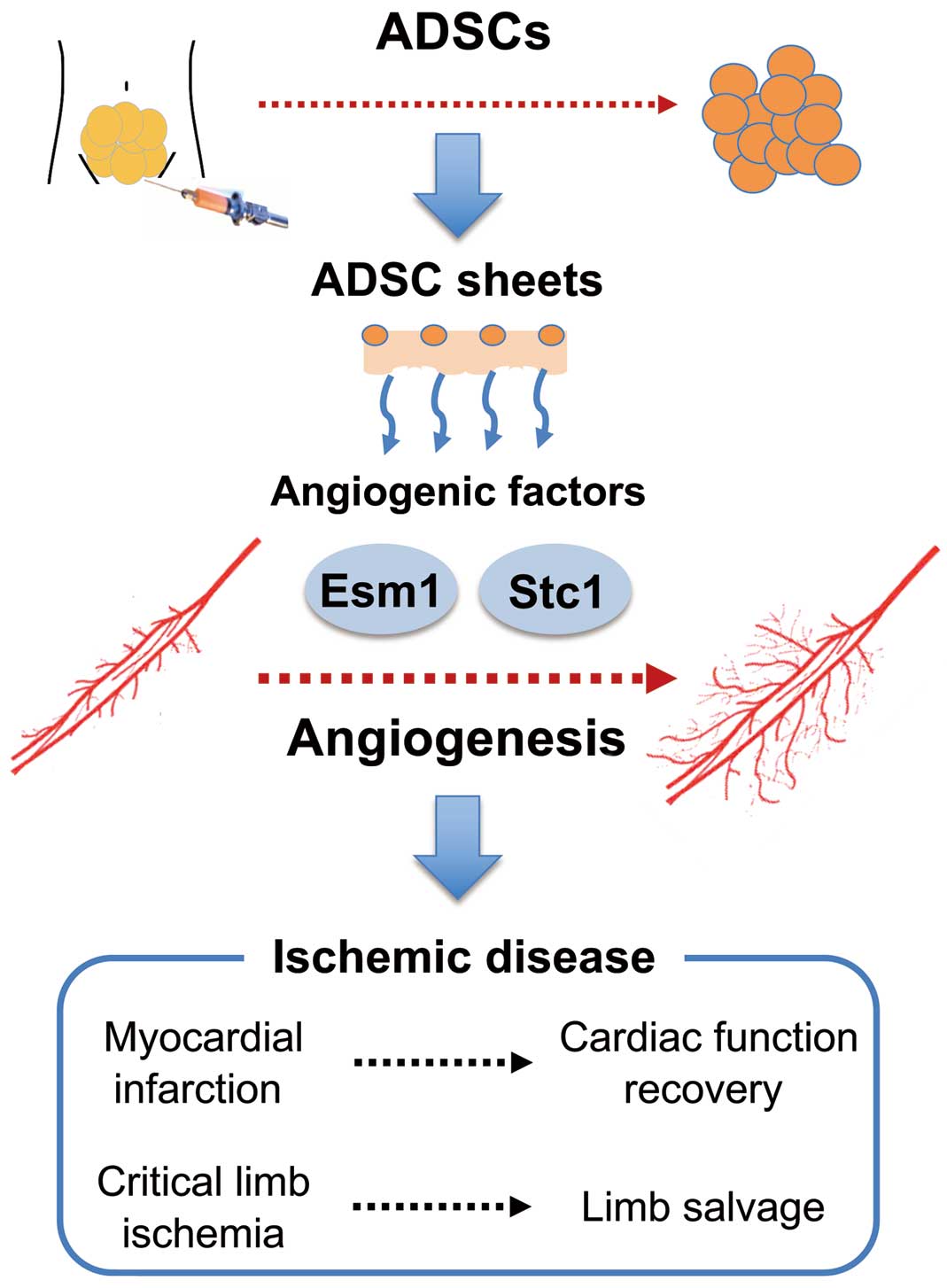
Future of therapeutic angiogenesis using adipose-derived stem cells (ADSCs) in the cardiovascular field. Esm1, endothelial cell-specific molecule 1; Stc1, stanniocalcin 1.
R.S. belongs to a department endowed by Medtronic Japan Co., Ltd. T.M. is a member of Circulation Journal’s Editorial Team.