2021 Volume 85 Issue 7 Pages 1035-1041
2021 Volume 85 Issue 7 Pages 1035-1041
Background: The PERIcardial SurGical AOrtic Valve ReplacemeNt (PERIGON) Japan Trial was developed to assess the safety and effectiveness of the 17-mm Avalus bioprosthesis (Medtronic, Minneapolis, MN, USA) in patients undergoing surgical aortic valve replacement.
Methods and Results: The primary endpoint in the trial was the percentage of patients achieving the composite of at least 1 class improvement in New York Heart Association (NYHA) functional class at 1 year compared with baseline and effective orifice area index (EOAI) of 0.6 cm2/m2 or greater at 1-year after implantation, compared with a performance goal of 60%. The present study reports outcomes through 2 years. Eleven patients were implanted (10 [91%] female, median age 78.3 years). From baseline to 1 year, 10 subjects (91%) showed an improvement in NYHA classification. At 1 year, mean (±SD) EOAI was 0.82±0.17 cm2/m2, with 10 patients (91%) having an EOAI ≥0.6 cm2/m2. As such, 9 of 11 patients (82%) successfully met the primary endpoint. One death occurred between the 1- and 2-year follow-up visits, unrelated to the valve. There were no valve reinterventions, explants, or device deficiencies through 2 years.
Conclusions: The PERIGON Japan Trial met its primary endpoint. Surgical implantation of the 17-mm Avalus aortic bioprosthesis can be performed with an acceptable incidence of device-related adverse events, and the valve performs effectively based on echocardiographic findings.
The Avalus aortic valve bioprosthesis (Medtronic, Minneapolis, MN, USA), a stented bovine pericardial tissue valve, is available in 17- to 29-mm sizes. Although the large US and European PERIcardial SurGical AOrtic Valve ReplacemeNt (PERIGON) Trial has reported excellent results up to 3-years follow-up, that study included only 1 patient with a 17-mm valve.1,2 Therefore, the safety and efficacy of the 17-mm valve remains unclear and further data are required. The aortic annulus size of Japanese patients is, on average, smaller than that of patients from other countries.3 One assessment of early results following aortic valve replacement after prior cardiovascular surgery using the Japan Adult Cardiovascular Surgery Database (JACVSD) found that >50% of bioprostheses used were ≤18- or 19-mm valves.4 Therefore, the safety and effectiveness of the 17-mm Avalus bioprosthesis needed to be established, and the PERIGON Japan Trial was designed to provide important clinical data on safety and performance. Here we present the primary endpoint and 2-year results.
The PERIGON Japan Trial for the AvalusTM valve is a prospective, interventional, non-randomized, multicenter trial designed to evaluate the safety and effectiveness of the 17-mm Avalus bioprosthesis in patients undergoing surgical aortic valve replacement. Although the device is available in 17- to 29-mm sizes, for this trial only the 17-mm size was evaluated.
Briefly, patients estimated to require a 17-mm aortic valve replacement of the native or prosthetic valve were eligible for inclusion. In addition, patients were required to have severe aortic stenosis or regurgitation, with a clinical indication for replacement of their native or prosthetic aortic valve with a bioprosthesis. Patients with pre-existing devices in other positions or needing replacement of mitral, pulmonary, or tricuspid valves were excluded, as were those with active endocarditis, myocarditis, or other infection. Other exclusions were for anatomic abnormalities and life expectancy <2 years. A full list of all trial inclusion and exclusion criteria is provided in the Supplementary Appendix.
The trial was conducted according to Japanese GCP Ordinance, the Pharmaceuticals and Medical Devices Act, and the Declaration of Helsinki. The study was sponsored by Medtronic (Minneapolis, MN, USA). An independent core laboratory (Medstar Health Research Institute, Washington, DC, USA) provided oversight for echocardiographic assessments. An independent clinical events committee (CEC) adjudicated safety-related adverse events, and an independent data safety monitoring board provided data review. All patients provided written, informed consent, and approval was provided by the institutional review board at each participating hospital.
EndpointsThe primary endpoint was a composite change in New York Heart Association functional class (NYHA-FC) and effective orifice area index (EOAI) from baseline to 1 year compared with a performance goal of 60%. The parameters to be estimated were NYHA-FC improvement of at least 1 class compared with baseline and an EOAI of ≥0.6 cm2/m2 at 1 year. If at least 60% of the subjects achieved the specified improvement in NYHA-FC and EOAI ≥0.6 cm2/m2 or greater, the primary endpoint would be met. Secondary endpoints included NYHA classification, hemodynamic performance, safety endpoints, and quality of life, as assessed by the 36-Item Short Form Health Survey (SF-36; https://www.rand.org/health-care/surveys_tools/mos/36-item-short-form/survey-instrument.html [accessed August 5, 2020]). Specific safety endpoints assessed included thromboembolism, thrombosis, hemorrhage (all and major), paravalvular leak (all and major), endocarditis, hemolysis, structural valve deterioration, non-structural dysfunction, reintervention, explant, and death.
Follow-upAfter the procedure, patients were seen in the office at discharge up to 30 days (≤30 days visit), 3–6 months, 1 year, and annually (2–5 years) thereafter. In addition, patients were contacted by telephone 18 and 30 months after the procedure to review vital status, medications, and adverse events. At all office visits, the following evaluations were completed: NYHA classification, SF-36 questionnaire (not completed at the ≤30 day visit), 12-lead electrocardiogram (ECG), hematology and chemistry data, transthoracic echocardiography examination, relevant medications, and adverse events/device deficiency.
Statistical AnalysisContinuous data are summarized as either the median with interquartile range (IQR) or as the mean±SD. Categorical variables are summarized as frequencies and percentages. All statistical analyses were performed using SAS software, version 9.4 (SAS Institute, Cary, NC, USA).
In all, 25 patients were enrolled in the study; 3 withdrew and 11 were not implanted with the study valve due to the 17-mm valve being too small for their annulus size, resulting in 11 implants in total (Supplementary Figure). In all 11 patients (100%), annulus size was listed as the reason for selecting a 17-mm valve; in addition, in 5 of these patients, patient age was identified as another reason for selecting this valve. Concerns around other risk factors or warfarin complications were also provided for 3 or 11 patients, and body surface area (BSA) was indicated as a reason for 1 patient.
Of the 11 implanted patients, most (82%) presented with aortic stenosis. Preoperative EOAI was 0.44±0.14 cm2/m2. This was a predominantly elderly population (median age 78.3 years), and 10 of the 11 patients were female (91%). Patients had a median weight of 44.4 kg, a body surface area of 1.3 m2, and a mean Society of Thoracic Surgeons Predicted Risk of Mortality (STS-PROM) of 3.9%. Comorbidities were generally infrequent, but most patients (91%) presented with hypertension, and nearly half (46%) had coronary artery disease. One patient had previously undergone both coronary artery bypass surgery (9%) and previous aortic valve implantation (9%), and 1 patient had previously undergone percutaneous coronary intervention (9%). Full patient demographics are provided in Table 1.
| Age (years) | 78.3 [76.9–80.4] |
| Female sex | 91 (10/11) |
| Weight (kg) | 44.4 [40.9–49.4] |
| Body surface area (m2) | 1.3 [1.3–1.4] |
| STS-PROM (%) | 3.5 [2.5–4.6] |
| Primary indication for valve replacement | |
| Aortic stenosis | 82 (9/11) |
| Aortic regurgitation | 0 (0/11) |
| Mixed | 9 (1/11) |
| Failed prosthesis | 9 (1/11) |
| Angina | 18 (2/11) |
| Atrial fibrillation/flutter | 0 (0/11) |
| Chronic obstructive lung disease | 0 (0/11) |
| Congestive heart failure | 9 (1/11) |
| Coronary artery disease | 45 (5/11) |
| Diabetes | 27 (3/11) |
| Insulin | 9 (1/11) |
| Dyslipidemia | 64 (7/11) |
| Endocarditis | 0 (0/11) |
| Hypertension | 91 (10/11) |
| Liver disease | 9 (1/11) |
| Myocardial infarction | 0 (0/11) |
| Peripheral vascular disease | 0 (0/11) |
| Renal dysfunction/insufficiency | 0 (0/11) |
| Stroke/CVA | 27 (3/11) |
| Transient ischemic attack | 0 (0/11) |
| Prior coronary artery bypass | 9 (1/11) |
| Prior percutaneous coronary intervention | 9 (1/11) |
| Previous aortic valve implanted | 9 (1/11) |
| No. previous open-heart surgeries | |
| 0 | 91 (10/11) |
| 1 | 0 (0/11) |
| 2+ | 9 (1/11) |
Values are given as the median (interquartile range) or as % (n/N). CVA, cerebrovascular accident; STS, Society of Thoracic Surgeons Predicted Risk of Mortality.
All patients (100%) underwent a median sternotomy. Four patients (36%) underwent concomitant coronary artery bypass grafting. Median bypass time for the procedure was 101 minutes and the aortic cross-clamp time was 68 min (Table 2). No patients underwent annular enlargement or aortic root/sinotubular junction enlargement.
| Surgical approach | |
| Median sternotomy | 100 (11/11) |
| Hemisternotomy | 0 (0/11) |
| Right thoracotomy | 0 (0/11) |
| Aortotomy technique | |
| Hockey stick/J shape | 9 (1/11) |
| Lazy S/oblique | 27 (3/11) |
| Transverse | 64 (7/11) |
| Concomitant surgical procedures | |
| None | 64 (7/11) |
| Coronary artery bypass graft | 36 (4/11) |
| Total bypass time (min) | 101 [88–29] |
| Total aortic cross clamp time (min) | 68 [60–96] |
| Condition of the ascending aorta | |
| Normal | 64 (7/11) |
| Calcified | 27 (3/11) |
| Dilated | 9 (1/11) |
| Etiology of aortic disease | |
| Congenital bicuspid | 18 (2/11) |
| Degenerative | 73 (8/11) |
| Biological valve deterioration | 9 (1/11) |
| Final implanted position of valve | |
| Intra-annular | 18 (2/11) |
| Supra-annular | 82 (9/11) |
Values are given as the median (interquartile range) or as % (n/N).
Echocardiographic findings at 1 year showed a mean EOAI of 0.82±0.17 cm2/m2, with 10 of 11 patients (91%) having an EOAI ≥0.6 cm2/m2. In addition, 10 patients (91%) demonstrated at least 1 class improvement in NYHA-FC from baseline to 1 year. As such, 9 of 11 subjects (82%) successfully met the primary endpoint, a composite of NYHA-FC improvement and EOAI ≥0.6 cm2/m2, meeting the requirements of the study primary objective (Table 3). Two patients did not meet the primary endpoint: 1 with an EOAI of 0.52 cm2/m2 at 1 year and 1 whose NYHA-FC was unchanged from baseline to 1 year.
| Assessment at 1 year | |
| Primary objective met | Yes |
| Composite NYHA and EOAI endpoint | 82 (9/11) |
| At least 1 class improvement in NYHA-FC from baseline | 91 (10/11) |
| EOAI ≥0.6 cm2/m2 | 91 (10/11) |
| NYHA-FC at 1 year compared with baseline | |
| Improved | 91 (10/11) |
| No change | 9 (1/11) |
| Worsened | 0 (0/11) |
| EOAI at 1 year (cm2/m2) | 0.82±0.17 |
Values are given as % (n/N) or as the mean±SD. EOAI, effective orifice area index; NYHA-FC, New York Heart Association functional class.
Echocardiographic findings were consistent through 2 years, demonstrated by 2-year mean effective orifice area (EOA) of 0.96±0.21 cm2, a mean aortic valve gradient of 20.3±5.7 mmHg (Figure 1A), and an EOAI of 0.70±0.16 cm2/m2 (Figure 1B). Mean left ventricular mass regression change from baseline to discharge or 30 days was −17.55 cm2/m2, but larger decreases were observed at later time points (−27.90 cm2/m2 at 2 years; Figure 1C). In addition, at 2 years, 9 of 10 patients (90%) had no aortic regurgitation; 1 patient (10%) had moderate paravalvular regurgitation (Figure 2).
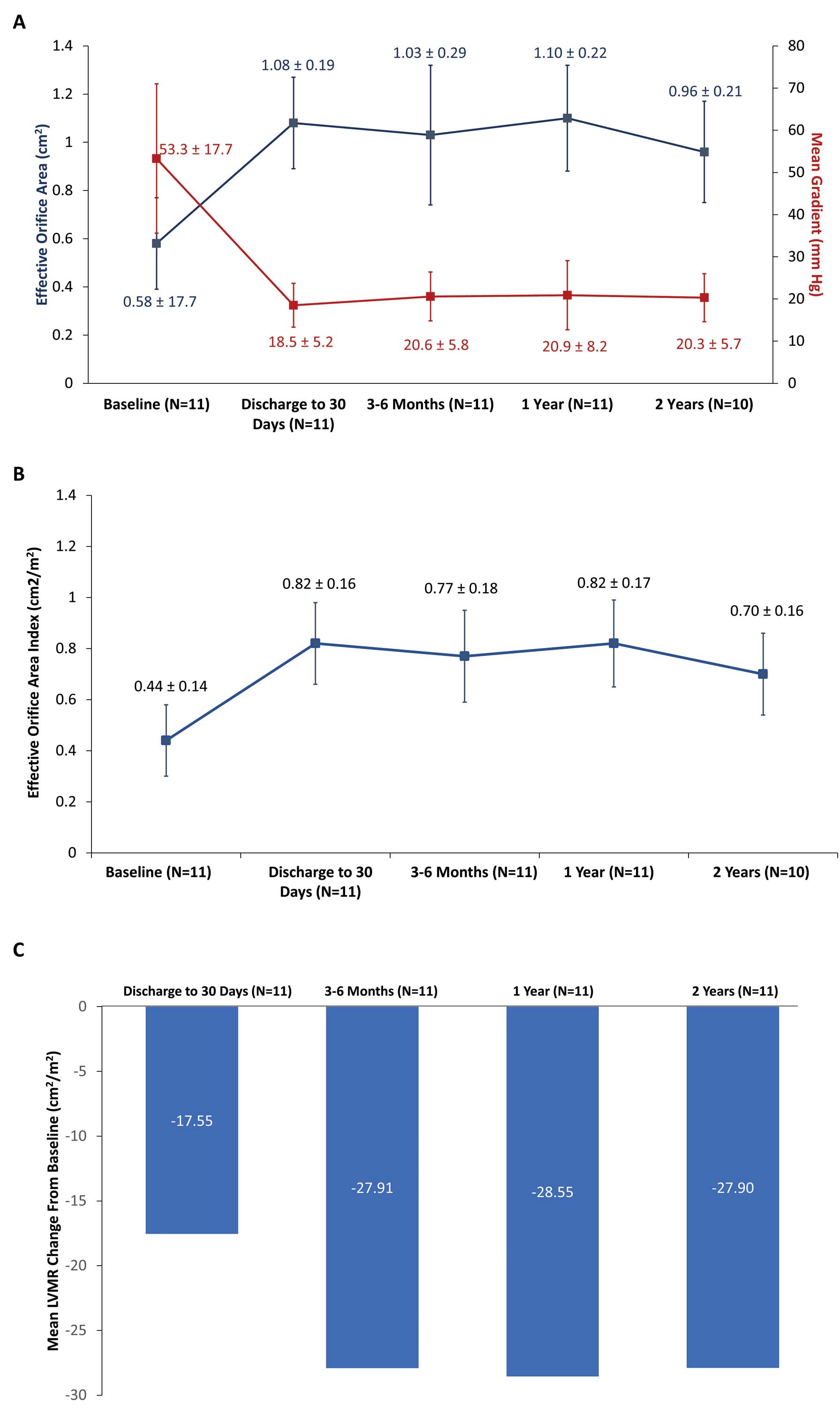
Hemodynamics from baseline through 2 years of follow-up. (A) Effective orifice area and mean aortic gradient and (B) effective orifice area index. Data show the mean±SD. (C) Mean left ventricular mass regression (LVMR) change from baseline.
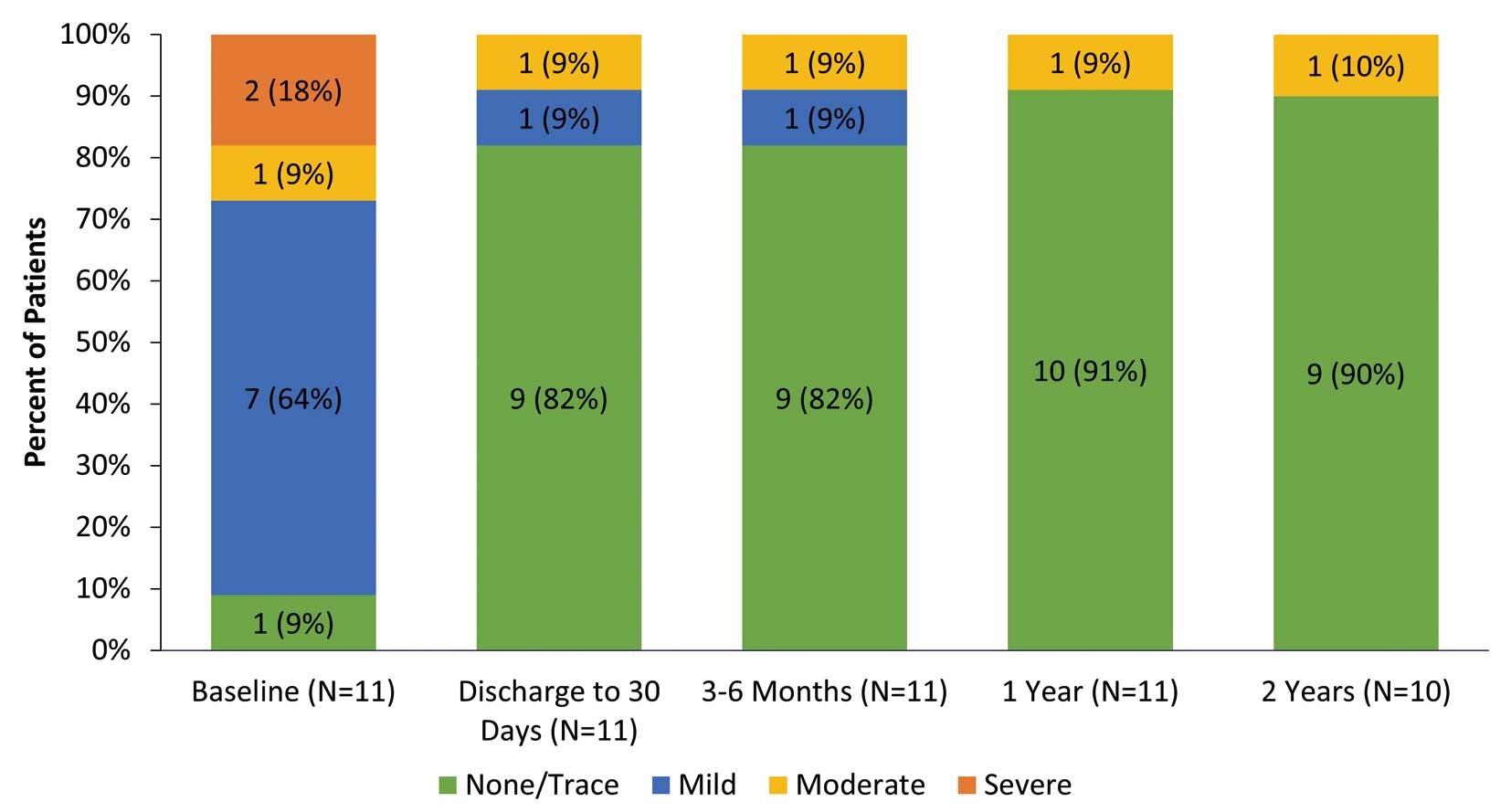
Total aortic regurgitation over time. Values in the bars depict the number of patients (%).
CEC-adjudicated adverse events were extremely rare. One death, due to heart failure, occurred between the 1- and 2-year follow-up visits and was not related to the valve. There were no valve reinterventions, explants, or device deficiencies through 2 years. Event rates compared to 2× the objective performance criteria (OPC) rate are provided in Table 4.
| PERIGON Japan (n=11) |
Rate (2× OPC; % per patient-years) |
|
|---|---|---|
| Endocarditis | 0 (0/11) | 2.4 |
| Hemorrhage | 0 (0/11) | 2.8 |
| Paravalvular leak | 0 (0/11) | 2.4 |
| Thromboembolism | 0 (0/11) | 5.0 |
| Valve thrombosis | 0 (0/11) | 0.4 |
Unless indicated otherwise, values are given as % (n/N). OPC, objective performance criteria.
All patients were NYHA Class II at baseline. This improved through follow-up, with most patients in NYHA Class I (Figure 3). When SF-36 quality of life assessment summary scores were evaluated as the median change from baseline to each follow-up visit, significant improvements were noted in mental health at the 2-year follow-up visit (Figure 4).

New York Heart Association (NYHA) classification over time. Values in the bars depict the number of patients (%).

36-Item Short Form Health Survey (SF-36) quality of life assessment. Values show median changes from baseline summary scores for physical and mental components summary scores at each follow-up visit.
The PERIGON Japan Trial has demonstrated high levels of safety and durable hemodynamics through 2 years of follow-up in 11 patients who received the 17-mm aortic bioprosthesis. Although the Avalus bioprosthesis is available in 17- to 29-mm sizes, the US and European PERIGON Trial1,2 only reported data on 1 patient with a 17-mm valve, making the present study important for the assessment of outcomes in patients with a smaller annular size. Patients in the present trial were predominantly female, elderly, and of small body size, requiring the 17-mm valve size. Outcomes up to 2 years were excellent, with no valve-related safety events, consistent hemodynamics, and improved functional status.
The primary endpoint in this trial comprised 2 components assessed at the 12-month time point: (1) an EOAI threshold; and (2) a 1-level improvement in NYHA symptom status. Because the valve size evaluated was small, the achievement criterion for the EOAI was defined as ≥0.6 cm2/m2. This criterion was developed based on the definition of severe aortic stenosis in the American College of Cardiology (ACC)/American Heart Association (AHA) guidelines for the management of valvular heart disease.5 Because clinical improvement may not necessarily be reflected by safety and hemodynamics with prosthesis and mechanical valves, an improvement in NYHA status was included in the endpoint. Regardless of valve type, clinical improvement after aortic valve replacement can be expected, and was confirmed in the present study. A small valve may only increase the EOA a small extent with limited functional impact, particularly if prosthesis patient mismatch (PPM) is present, which is why this component was included.
Prior studies have evaluated the use of small valve sizes in Japan, demonstrating favorable clinical outcomes.4,6–9 Most of the labeled valve sizes used in these series were 19–23 mm, with limited data available on 17-mm devices. However, it should be noted that labeled sizes from manufacturers are often inconsistent in terms of which diameter is being measured, so direct comparisons between valve sizes are difficult. Small aortic annuli are often defined in the surgical literature as an aortic annulus ≤23 mm, either measured by echocardiography prior to surgery or by direct intraoperative sizing.10 The prevalence of small annuli ranges from 22% to 44% in the US and Northern Europe,10–13 and Asian populations reportedly have significantly smaller aortic annular diameters than European patients.14
Transcatheter aortic valve replacement (TAVR) has emerged as a less-invasive option for the treatment of aortic stenosis. It has been argued that especially in patients with a small annulus, the self-expandable, supra-annular Evolut platform is of benefit due to the larger EOAs than small surgical valves. Patients with small annuli have often been excluded from TAVR trials. In an analysis of the Surgical Replacement and Transcatheter Aortic Valve Implantation (SURTAVI) trial, patients undergoing TAVR with a self-expandable device had lower mean gradients, higher EOAI, and lower rates of PPM, regardless of multidector computed tomography-based aortic annular size, than those undergoing surgery.15 Similarly, a balloon-expandable TAVR device was evaluated according to annular size tertiles in the Placement of Aortic Transcatheter Valve (PARTNER) trial; patients with small aortic annuli had similar mortality whether undergoing TAVR or surgical aortic valve replacement, but TAVR yielded better hemodynamics and less PPM.16 The CoreValve US Pivotal High Risk Trial reported on outcomes according to annular size, but among 347 surgical valves in the trial, which was conducted only in the US, 74 (21%) were categorized as “small”, defined as a perimeter-derived diameter <23 mm,17 which is still relatively large. Although access is generally achieved through the femoral arteries, in patients with very small annuli, as included in the present study, this may not be feasible due to sizing issues. Alternative access sites, such as subclavian, transapical, or direct aortic, will need to be considered in patients with very small annuli. Despite a lack of comparative data, it has been reported that some of these routes are prone to complications, and some patients may not qualify for those access routes either.18–20 Sheathless transfemoral TAVR has been used in some patients with small access diameters who were not suitable for direct aortic or transapical access.21 Moreover, the dimensions of even the smallest TAVR valves are too large for patients who receive a 17-mm bioprosthetic valve.
One alternative for patients with small aortic annuli is surgical enlargement of the annulus. A group of investigators in Japan compared outcomes between those requiring aortic annular enlargement and those undergoing standard aortic valve replacement, finding that aortic annular enlargement in these patients was safe, with no difference in survival or PPM.22 A second study from Japan comparing annular enlargement to standard surgical aortic valve replacement demonstrated a slight survival benefit with enlargement alone (P<0.10) and fewer valve-related events (P<0.05), but 10-year freedom from reoperation was similar in the 2 groups.23
In conclusion, the PERIGON Japan Trial has demonstrated the safety and effectiveness of the 17-mm Avalus bioprosthesis. This small device size broadens the range of patients that can be treated surgically for aortic stenosis or regurgitation. The findings should be viewed in light of the relatively small sample size, and long-term outcomes should be assessed to continue following durability of the device.
Medical writing support under the direction of the lead author was provided by Jessica Dries-Devlin, PhD, whereas study management support was provided by Hiroko Ookubo and Jessica Halverson (all are employees of Medtronic). The authors thank Yukikatsu Okada (Midori Hospital) for providing a medical review of Pharmaceuticals and Medical Devices Agency (PMDA) safety reporting.
The PERIGON Japan Study was funded by Medtronic.
E.G. is an employee and shareholder of Medtronic. The remaining authors have no conflicts of interest to declare.
This study was approved by the Saitama Medical University International Medical Center: Device Clinical Research Institutional Review Board (IRB; Reference no. 3030005011020), Jichi Medical University Saitama Medical Center IRB, Sakakibara Heart Institute IRB (Reference no. 5011105004756), National Cerebral and Cardiovascular Center IRB (Reference no. 3120905003033), Kobe University IRB, Hyogo Brain and Heart Center IRB, Kurashiki Central Hospital IRB (Reference no. 7260005003230), and the Tokushima Red Cross Hospital IRB.
The data, analytic methods, and study materials are owned by the sponsor (Medtronic) and will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-20-1024