Abstract
Background:
Implantable cardioverter-defibrillator (ICD) therapy is important for the prevention of sudden cardiac death, but data on clinical outcomes of ICD therapy in Asian pediatric patients are scarce. The aim of this Korean multicenter study was to evaluate the current state and elucidate the clinical outcomes of ICD therapy in children.
Methods and Results:
Data from 5 pediatric cardiology centers were retrospectively collected from 2007 to 2019. Altogether, 99 patients were enrolled (mean age 13.9±4.1 years). The most common underlying disease was a primary electrical disease (56%). An ICD was implanted for primary prevention in 19%. Appropriate shock occurred in 44% of patients at a median of 1.6 years after implantation. There was no significant difference in the appropriate shock rate between patients with primary and secondary prevention indications (32% vs. 48%, respectively). A total of 33 patients (33%) experienced inappropriate shock, which was associated with primary electrical disease and follow-up duration on multivariate analysis. 17% of patients had ICD-related complications.
Conclusions:
The utilization rate of ICD for primary prevention was still low in the pediatric population in Korea, but there was a substantial rate of appropriate shock in these patients. Efforts to increase ICD usage to save the lives of high-risk patients and reduce the incidence of inappropriate shock are required.
The implantable cardioverter-defibrillator (ICD) is a widely accepted therapy for adult patients with a high risk of sudden cardiac death (SCD),1,2
and the pediatric indication has increased for both primary and secondary prevention of SCD.3
However, data on ICD outcomes in pediatric patients are limited due to the heterogeneous patient group and the small number of patients.3
A high complication rate (~32%) and inappropriate shocks (19–47%), as well as ICD-related deaths of 0.5–3.5% in children and young patients, make the decision regarding ICD implantation difficult.4–6
Additionally, clinical reports of ICD for pediatric patients in Asian populations are scarce.
This Korean multicenter study aimed to evaluate the current state and clinical outcomes of ICD implantation in pediatric patients.
Methods
We conducted a multicenter retrospective study of pediatric patients, aged younger than 19 years, who underwent ICD implantation between April 2004 and January 2019; 5 tertiary hospitals of the Republic of Korea participated in this study. Medical records, including demographic data, underlying heart disease, ventricular function, ICD analyses, complications, and data on delivered therapies were collected. The indications for ICD implantation were retrospectively analyzed with reference to the medical record at the time of implantation and re-evaluation at the time of this study. Type and size of shock leads, date of shock lead change, causes of lead failure, and cases of removal or turned-off ICD were investigated. The data from ICD interrogation included the date of the first appropriate shock, the number of appropriate shocks, the occurrence of inappropriate shocks, antitachycardia pacing, and ventricular pacing with pacing mode. ICD therapy zones were divided into high- (≥200/min) and low- (<200/min) rate therapy zones according to the heart rate that triggered the shock therapy.
Patients were categorized into 4 groups according to the underlying heart disease: primary electrical disease, congenital heart disease (CHD), cardiomyopathy, and others. Patients with both CHD and long QT syndrome were classified into the primary electrical disease group for group comparison by age at ICD implantation, appropriate and inappropriate shocks, complications, and lead failure. Primary electrical diseases included inherited channelopathy and idiopathic ventricular fibrillation (VF). Acute complications were defined as complications that occurred within 1 month of ICD implantation.
This study was approved by the 5 institutional review boards. The study was conducted in accordance with the Declaration of Helsinki. The institutional review boards waived the need for informed consent from the patients to be included in the analyses and the need for review by a critical event committee because of the retrospective nature of the study, and the absence of patient identification data.
Statistical Analysis
Continuous variables were expressed as mean±standard deviation or as median and range, and the values of the normally distributed parameters were compared between groups using Student’s t-test. Categorical variables are expressed as frequency (percentage). The Chi-square and Fisher›s exact tests were used to compare categorical data between 2 or 3 groups. Kaplan-Meier estimates were used for the analysis of cumulative probability of the freedom from appropriate shock in patients fitted with an ICD for primary and secondary prevention, and for analysis of cumulative lead survival.
Multivariable analysis using a Cox proportional hazards model was conducted for the prediction of risk of appropriate shock and lead failure using the forward stepwise method, with criteria for entry and exit at P<0.05 and P<0.1, respectively. Multivariate logistic regression was used to determine the risk factors for inappropriate shock using the forward stepwise method, with criteria for entry and exit at P<0.05 and P<0.1, respectively.
These statistical analyses were performed using SPSS software (version 23.0, IBM Corporation, Armonk, NY, USA). A P value <0.05 was considered statistically significant.
Results
Patients’ Characteristics
Altogether, 99 pediatric patients (68 males, 69%) were enrolled with a mean follow-up duration of 4.9±3.7 years. The baseline clinical characteristics of patients are shown in
Table 1. The mean age at ICD implantation was 13.9±4.1 years (median 14.7, range 0.2–18.7 years). The median age at ICD implantation was 14.8 years in the primary electrical disease group, 14.7 years in the CHD group, 14.7 years in the cardiomyopathy group, and 14.1 years in the others group. There was no difference in implantation age according to underlying disease (P=0.435). Three patients with cardiac resynchronized therapy with defibrillator were included.
Table 1.
Demographic Data of Pediatric Patients
| Variable |
n=99 |
| Age at implantation (years) |
13.9±4.1 |
| Follow-up duration (years) |
4.9±3.7 |
| Height at implantation, median (range), cm |
162 (53–191) |
| Weight at implantation, median (range), kg |
52.3 (3.9–100) |
| Male, n (%) |
68 (69%) |
| Primary prevention, n (%) |
19 (19%) |
| Secondary prevention, n (%) |
80 (81%) |
| Cardiac arrest before ICD, n (%) |
72 (73%) |
| VT before ICD, n (%) |
28 (28%) |
| VF before ICD, n (%) |
60 (61%) |
| Syncope before ICD, n (%) |
40 (40%) |
| Family history of SCD, n (%) |
17 (17%) |
| Age at implantation by underlying disease, median (range), years |
| Primary electrical disease (n=55) |
14.8 (0.2–18.7) |
| Congenital heart disease (n=12) |
14.7 (12.3–17.5) |
| Cardiomyopathy (n=29) |
14.7 (0.6–18.0) |
| Others (n=3) |
14.1 (2.7–15.6) |
| CRT-D, n (%) |
3 (3%) |
| Death, n (%) |
3 (3%) |
| Transplantation, n (%) |
1 (1%) |
| High-rate ICD therapy zone, n (%) |
58 (59%) |
| Ventricular pacing mode |
95 (96%): 74 in VVI/21 in DDD |
CRT-D, cardiac resynchronization therapy-defibrillator; ICD, implantable cardioverter-defibrillator; SCD, sudden cardiac death; VF, ventricular fibrillation; VT, ventricular tachycardia.
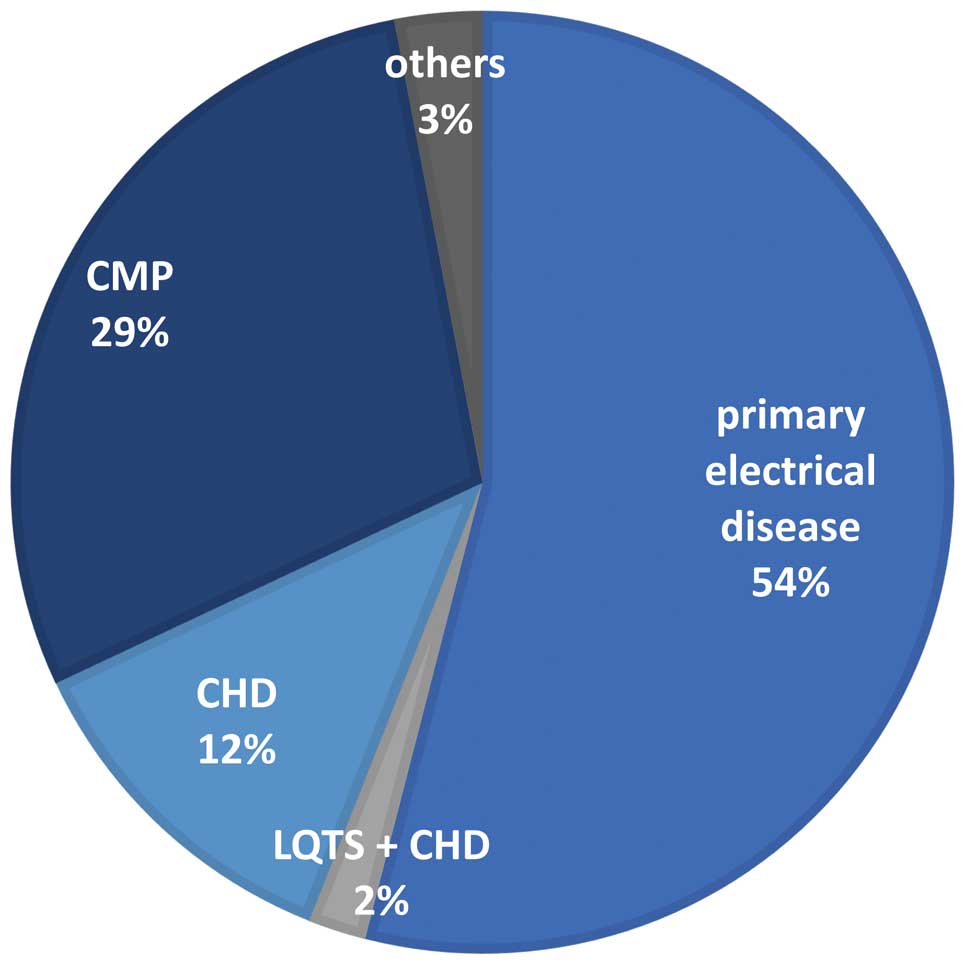
Figure 1
shows the underlying diseases. Primary electrical disease accounted for 56% of patients (n=55), including 2 with coexisting CHD. Of the patients with primary electrical disease, idiopathic VF was discovered in 23, and inherited channelopathies were found in 32 patients. Among patients with channelopathy, 20 with long QT syndrome, 5 with Brugada syndrome, 3 with catecholaminergic polymorphic ventricular tachycardia (VT), 3 with early repolarization syndrome, and 1 with compound heterozygote mutation of RYR2 and ANK2 were identified. Among the patients with long QT syndrome, the diagnosis was genetically confirmed in 13 patients: 1 patient had type 1 long QT syndrome, 3 patients had type 2, 8 patients had type 3, and 1 had type 7. One patient with SCN5A mutation had both dilated cardiomyopathy and QT prolongation.
Among the patients with long QT syndrome, 2 had coexisting CHD: repaired ventricular septal defect (VSD), and large patent ductus arteriosus, respectively.
Cardiomyopathy was diagnosed in 29% of patients (n=29), including hypertrophic cardiomyopathy (HCM) in 22, dilated cardiomyopathy in 6, and uremic cardiomyopathy in 1.
A total of 12 patients (12%) were classified into the CHD group. All patients were under follow-up after corrective surgery. Of them, 4 had tetralogy of Fallot, 3 had VSD, and there was 1 patient each with transposition of great arteries, Taussig-Bing anomaly, Ebstein anomaly, congenital aortic steno-insufficiency, and congenitally corrected transposition of great arteries.
Among the patients with repaired VSD, 1 had pacemaker-induced cardiomyopathy after pacemaker implantation for postoperative complete atrioventricular block, 1 had undergone aortic valve replacement 17 years after VSD repair due to severe steno-insufficiency of the aortic valve, and 1 patient had severe pulmonary hypertension.
Other underlying diseases included cardiac tumor with VF in 2 patients, and ischemic cardiomyopathy after Kawasaki disease with giant aneurysm in 1.
Figure 2
shows the outcomes of ICD implantation according to underlying heart disease.
Primary Prevention vs. Secondary Prevention
ICDs were implanted for primary prevention in 19 patients (19%): 10 of 55 patients (18%) with primary electrical disease, 1 (8%) of 12 CHD patients, 7 (24%) of 29 cardiomyopathy patients, and 1 patient (33%) of the others group (Figure 3A). Among 32 patients with inherited channelopathy, 10 (31%) had ICD for primary prevention. There were no differences in the proportion of primary or secondary prevention cases among the patient groups with underlying diseases (P=0.617).
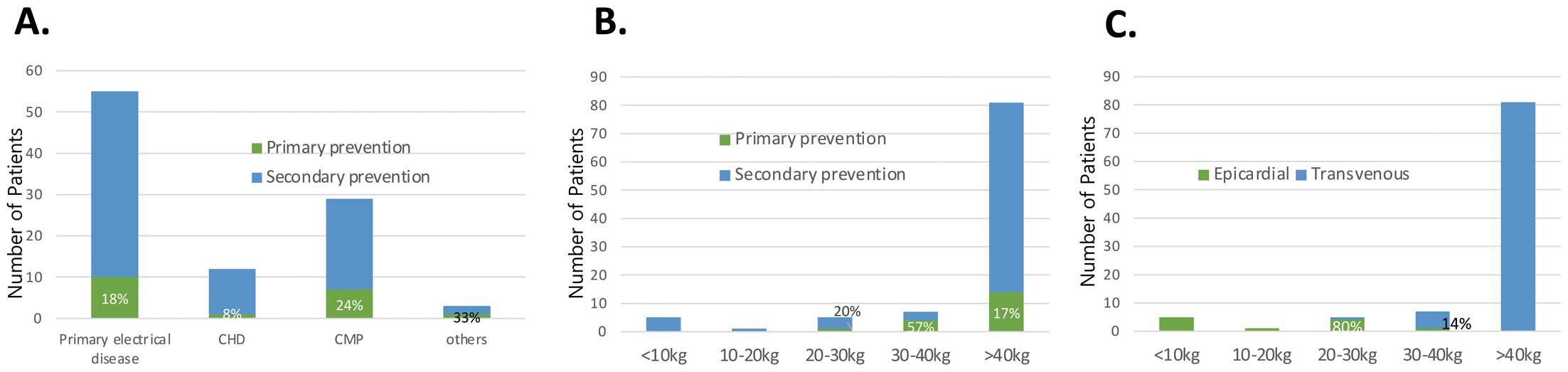
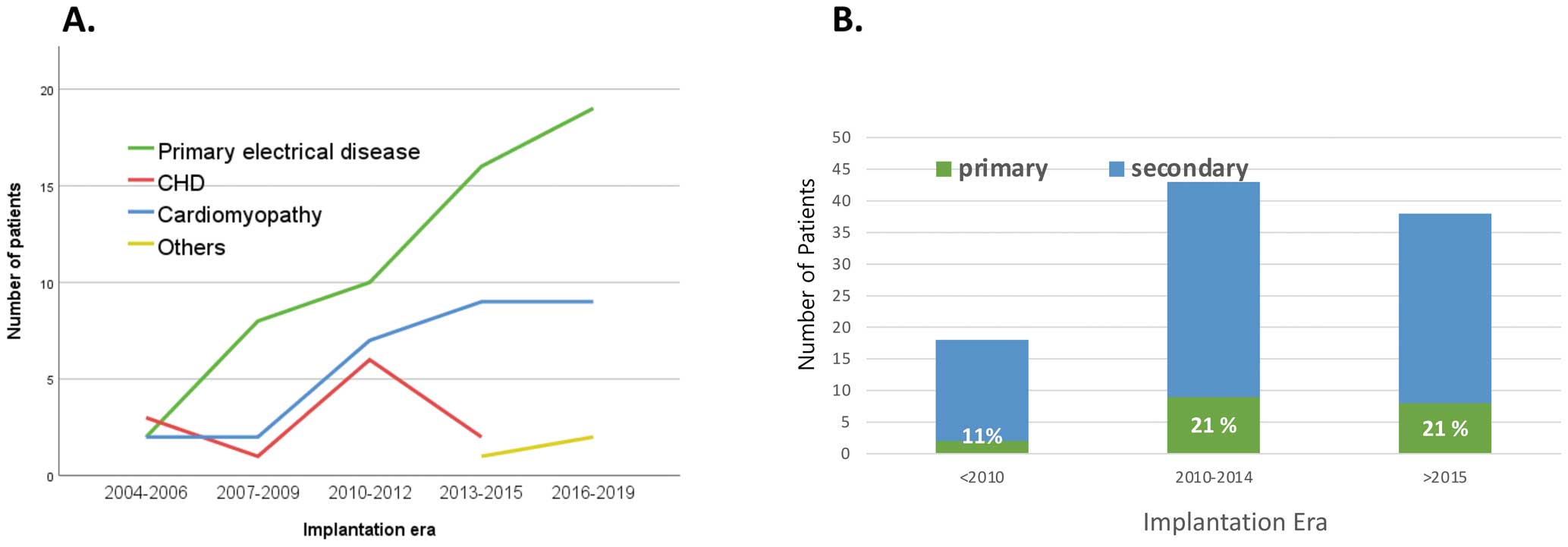
There were 6 patients who weighed <20 kg at ICD implantation, and all of those patients underwent epicardial ICD implantation for secondary prevention (Figure 3B,C). Among them, 4 had primary electrical disease, 1 had HCM, and 1 had a cardiac tumor. ICD placements have increased over time in patients with primary electrical disease and cardiomyopathy, and there was an increase in primary prevention indications by time period; the ratio of primary prevention was 11% and 22% before and after 2010, respectively. However, the primary prevention ratio did not change anymore after 2010 (Figure 4).
ICD Shock Therapy
Appropriate shock was delivered in 44 patients (44%) after a mean follow-up interval of 1.6±1.9 years, with a median of 3 shocks (range, 1–38) per patient. One patient with laminopathy and dilated cardiomyopathy with apical aneurysm and myocardial fibrosis on MRI had frequent appropriate ICD shock for VT after ICD implantation for secondary prevention. Antitachycardia pacing was turned on in 76 patients, with 15 (20%) experiencing VT termination by antitachycardia pacing. Patients with primary and secondary prevention indications received their first appropriate shock 1.3±1.7 years (median 1.2, range 6 days to 3.5 years) and 1.6±2.0 years (median 0.7 years, range 2 days to 8.1 years) after ICD implantation, respectively. Appropriate shock rates did not differ between patients with primary and secondary prevention indications (32% vs. 48%, P=0.209;
Figure 5A). Among 19 patients with an indication for primary prevention, 5 of 10 patients (50%) with channelopathy and 1 of 7 patients (14%) with cardiomyopathy experienced appropriate shock therapy. There were no significant differences in underlying heart disease (Figure 5B), ventricular dysfunction, unexplained syncope, family history of SCD, nonsustained VT, or sex for appropriate shock delivery (Table 2). In the multivariate analysis, pre-ICD cardiac arrest, pre-ICD sustained VT, and channelopathy were independently associated with appropriate shocks. A total of 49 patients had a successful VT termination by antitachycardia pacing or appropriate shock therapy; among them, 7 and 42 patients had an ICD for primary and secondary prevention, respectively.
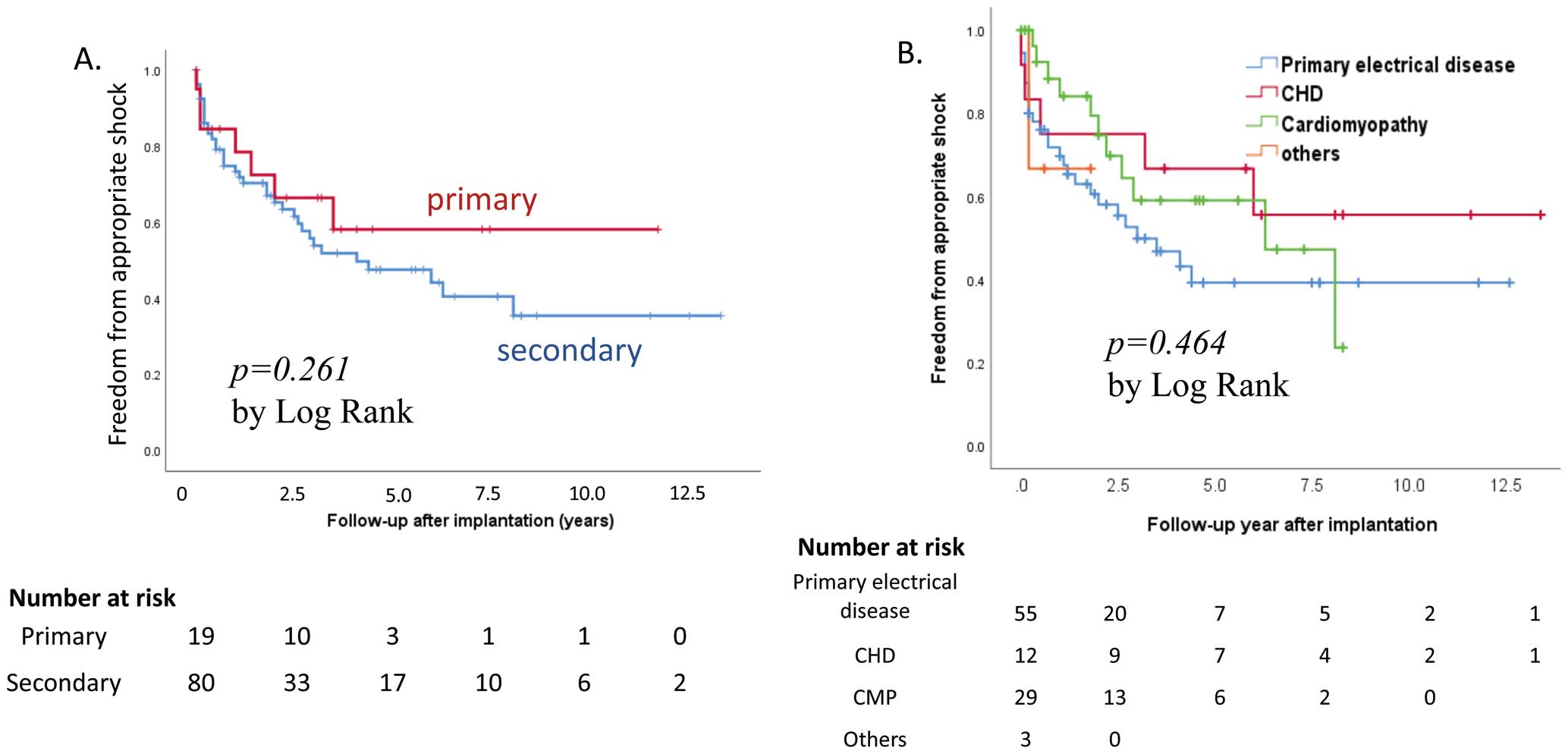
Table 2.
Predictors of Appropriate Shocks by Univariate and Multivariate Analyses
| Univariate analysis |
Total
(n=99) |
Appropriate shock |
P value |
| Yes (n=44) |
No (n=55) |
| Male |
68 |
31 (46%) |
37 |
0.734 |
| Follow-up duration (years) |
|
6.0±3.7 |
4.0±3.5 |
0.009 |
| Age at implantation (years) |
|
14.1±3.9 |
13.8±4.3 |
0.725 |
| Primary prevention |
19 |
6 (32%) |
13 |
0.209 |
| Secondary prevention |
80 |
38 (48%) |
42 |
| Primary electrical disease |
55 |
27 (49%) |
28 |
0.761 |
| Congenital heart disease |
12 |
5 (42%) |
7 |
| Cardiomyopathy |
29 |
11 (38%) |
18 |
| Others |
3 |
1 (33%) |
2 |
| Channelopathy |
32 |
19 (59%) |
13 |
0.039 |
| Idiopathic VF |
23 |
8 (35%) |
15 |
0.287 |
| Cardiac arrest |
72 |
35 (49%) |
37 |
0.173 |
| Pre-implant VT |
28 |
15 (54%) |
13 |
0.251 |
| Pre-implant VF |
60 |
28 (47%) |
32 |
0.581 |
| Nonsustained VT |
14 |
7 (50%) |
7 |
0.652 |
| SVT |
8 |
1 (13%) |
7 |
0.075 |
| Syncope |
40 |
20 (50%) |
20 |
0.36 |
| Family history for SCD |
17 |
7 (41%) |
10 |
0.766 |
| LV dysfunction (<35%) |
9 |
3 (33%) |
6 |
0.727 |
| High-rate ICD therapy zone, n (%) |
58 |
23 (40%) |
35 |
0.254 |
Multivariate analysis
(Cox hazard model) |
Hazard ratio |
95% CI |
P value |
| Cardiac arrest |
2.579 |
1.195–5.536 |
0.015 |
| Pre-ICD VT |
2.037 |
1.108–4.092 |
0.031 |
| Channelopathy |
2.344 |
1.333–4.554 |
0.006 |
CI, confidence interval; LV, left ventricle; SVT, supraventricular tachycardia. Other abbreviations as in Table 1.
Inappropriate shocks occurred in 33 patients within a mean follow-up period of 5.8 years. Inappropriate shock therapy most frequently occurred in patients with primary electrical disease in the underlying disease group (P=0.031,
Figure 2B). In the multivariate analysis, the risk factors for inappropriate shock were primary electrical disease and long follow-up duration (Table 3). The causes of inappropriate shock therapy were sinus tachycardia in 16 patients, supraventricular tachycardia in 9, T wave oversensing in 5, lead failure in 2, nonsustained VT in 1, and noise oversensing in 1 patient.
Table 3.
Risk Factors for Inappropriate Shocks by Univariate and Multivariate Analyses
| Univariate analysis |
Total |
Inappropriate shock |
P value |
| Yes (n=33) |
No (n=66) |
| Age at implantation (years) |
|
14.4±4.2 |
13.7±4.1 |
0.449 |
| Follow-up duration (years) |
|
5.8±3.9 |
4.5±3.5 |
0.005 |
| Male |
68 |
24 (35%) |
44 |
0.54 |
| Primary prevention |
19 |
5 (26%) |
14 |
0.47 |
| Secondary prevention |
80 |
28 (35%) |
52 |
|
| Primary electrical disease |
55 |
25 (46%) |
30 |
0.031 |
| Congenital heart disease |
12 |
3 (25%) |
9 |
| Cardiomyopathy |
29 |
5 (17%) |
24 |
| Others |
3 |
0 |
3 |
| Channelopathy |
32 |
12 (38%) |
20 |
0.543 |
| Idiopathic VF |
23 |
13 (57%) |
10 |
0.007 |
| ICD insertion era |
| <2010 |
18 |
10 (56%) |
8 |
0.086 |
| 2010–2014 |
43 |
12 (28%) |
31 |
| ≥2015 |
38 |
11 (29%) |
27 |
| LV dysfunction (<35%) |
9 |
2 (22%) |
7 |
0.458 |
| Cardiac arrest |
72 |
25 (35%) |
47 |
0.632 |
| Lead failure |
10 |
6 (60%) |
4 |
0.079 |
| Pre-implant VF |
60 |
24 (40%) |
36 |
0.081 |
| Pre-implant VT |
28 |
8 (29%) |
20 |
0.528 |
| Nonsustained VT |
14 |
4 (29%) |
10 |
0.769 |
| SVT |
8 |
4 (50%) |
4 |
0.432 |
| Family history of SCD |
17 |
6 (35%) |
11 |
0.851 |
| Syncope |
40 |
12 (30%) |
28 |
0.562 |
| High-rate ICD therapy zone, n (%) |
58 |
19 (33%) |
39 |
0.885 |
| Multivariate analysis (log regression) |
Odds ratio |
95% CI |
P value |
| Primary electrical disease |
4.915 |
1.787–13.518 |
0.002 |
| Follow-up duration (years) |
1.160 |
1.022–1.319 |
0.023 |
Abbreviations as in Tables 1,2.
A high-rate ICD therapy zone was set in 58 patients (59%). Mortality was not increased in patients with a high-rate therapy zone compared to those with a low-rate therapy zone (0% vs. 7.3%, P=0.068). A high-rate ICD therapy zone was associated with neither appropriate nor inappropriate shock. However, in patients with idiopathic VF, patients with a high-rate ICD therapy zone had less frequent incidences of appropriate and inappropriate shock (P=0.001 and P=0.027, respectively) than with the other underlying heart diseases.
A total of 95 patients were in ventricular pacing mode; 74 and 21 patients were in VVI and DDD pacing mode, respectively. Ventricular pacing was <1% in 68 patients, 1–5% in 12, 5–20% in 4, 20–40% in 5, 40–80 in 1, 80–98% in 2, and 98–100% in 3 patients. Ventricular pacing rate was not associated with appropriate or inappropriate shock (P=0.215 and P=0.492, respectively).
Complications
A total of 17 patients (17%) had 22 complications related to ICD; 3 patients had acute complications: 2 had lead dislodgement, while 1 had hemothorax. Chronic complications were lead failure in 10 patients, vein occlusion in 2, skin erosion in 3, infection in 2, and psychological problems, including anxiety and depression, in 2. Complications occurred more frequently in patients who underwent ICD implantation before 2010 than after 2010 (33% vs. 12%, P=0.027). However, age at implantation, sex, body weight, height, somatic growth, or use of the epicardial or transvenous approach was not associated with complications. Complications did not differ according to the underlying disease (Figure 2C).
Shock Coil Lead Failure
A total of 108 shock coil leads were placed in 99 patients. Of these 108 leads placed, 96 were transvenous shock leads, 11 were epicardial shock leads, and 1 was a subcutaneous shock lead. Epicardial ICD implantation was performed in patients with a small body size. The median body weight of patients with epicardial implantation was 16.8 kg (range, 3.9–33.2 kg).
A total of 10 shock leads failed (2.3% per year) during a mean follow-up of 4.4±3.4 years. Among them, there was only 1 epicardial shock lead; all the others were transvenous leads. Actual survival rates for all leads were 99%, 93%, and 89% at 1, 3, and 5 years after ICD implantation, respectively (Supplementary Figure). Of the 10 failed shock leads, 9 were replaced: 4 leads were extracted, and 5 remained. Abnormal rise in impedance in 2 patients (including 1 with ICD shock storm), noise/T wave oversensing in 2 patients, ICD pocket infection with skin erosion in 1, and lead fracture in 3 patients were identified as causes of lead change. Fidelis (Medtronic, Inc., Minneapolis, MN, USA) or Riata (St. Jude Medical, Sylmar, CA, USA), lead size, age at implantation, number of chambers (dual chamber vs. single chamber), number of coils (single coil vs. dual coil), type (screw type vs. tinned type), lead location (epicardial vs. transvenous), and underlying disease were not associated with lead failure. Generator change was performed in 25 patients, with a mean interval of 5.6±0.6 years from initial ICD implantation.
Mortality
In total, 3 patients died and 1 underwent heart transplantation during follow-up. The causes of death were heart failure in 1 patient with SCN5A mutation with QT prolongation and dilated cardiomyopathy, and SCD in 2 patients (1 with ischemic cardiomyopathy after Kawasaki disease with giant coronary aneurysm, and 1 with HCM). One patient with laminopathy and dilated cardiomyopathy received a heart transplant because of frequent ICD shock therapy due to medically intractable VT and progressive heart dysfunction. There were no ICD-related deaths.
Discussion
This is the first clinical analysis of the outcomes of shock therapies for pediatric patients with ICD in an Asian population. There are increasing numbers of pediatric patients with ICD for primary or secondary prevention, with a high appropriate shock rate of 44%. However, the rate of primary prevention is still low compared with data from Western countries (15–19% vs. 42–60%).3,7–11
Asakai et al reported a trend in pediatric ICD therapy using data from a Japanese nationwide registry, which showed a relatively low utilization of the treatment for primary prevention (15%),7
as was also shown in our series. However, data from both Japan and Korea showed a relatively high appropriate shock therapy rate (44%) compared with Western studies, which reported appropriate shock rate of 19–31%.8,9
Studies in children and young adults have reported that appropriate shocks are more common in patients with ICD for secondary prevention than in those with a primary prevention indication.10–12
However, there was no difference in appropriate shock therapy between primary and secondary prevention in this study; the appropriate shock rate was also substantial (32%) in patients with a primary prevention indication. A total of 7 of 19 patients (37%) with a primary prevention indication experienced life-saving interventions such as appropriate ICD shock and successful VT termination by antitachycardia pacing. Among the underlying diseases, channelopathy was significantly associated with appropriate shock therapy, which differed from other studies reporting no significant difference in appropriate shock therapy among the underlying diseases.10,11
For the patients with inherited arrhythmia syndrome, we implanted ICDs with reference to the consensus statements.13
Although based on nonrandomized studies, 50% of patients with channelopathy who had an ICD for primary prevention experienced appropriate shock.
ICD implantation for primary prevention in children has been reported to account for 21–52% before 2010,5,9,10,14
but 60% of pediatric or CHD patients and 80% of the adult cohort from 2010 to 2016, according to data from the American National Cardiovascular Data Registry of the American College of Cardiology Foundation.3
However, this is quite different from Asian data. As survival benefits of ICD for primary prevention in patients at high risk of SCD have been reported in large-scale prospective randomized trials and registry data in adults, ICD implants in children also increased dramatically.2,3,15–17
In Korea, reimbursement guidelines of ICD for primary prevention for pediatric and CHD patients have been expanded in line with global guidelines, with subsequent increase in the application of ICD. Subcutaneous ICD has been available since March 2019 with national insurance coverage in Korea. Long-term experience in teenagers showed that 26% of appropriate shock events and 16% of inappropriate shock events happened without major complications, which is comparable to transvenous ICD.18
In children who need life-long device therapy, subcutaneous ICD may be good option if antitachycardia pacing is not needed. However, the perception of device implantation among primary care physicians and pediatricians, as well as patients and their families, is still conservative, which might serve as a barrier to the regularization of ICD implantation in Korea. It might be important to inform and educate people about the risk of SCD and the effectiveness of these devices as prevention.
Inappropriate shock occurred in 33% of the study patients, which is not low. The most common cause of inappropriate shock therapy in this study was sinus tachycardia, followed by supraventricular tachycardia, T wave oversensing, lead failure, and nonsustained VT. Additionally, the risk factors for inappropriate shock were primary electrical disease and follow-up duration. As most of the patients with primary electrical disease had anatomically normal hearts, they may have more active lifestyles than patients with CHD or cardiomyopathy, and sinus tachycardia from increased activity could result in inappropriate shock. This can be reduced by programming, including higher rate cut-off (>200/min) for younger patients and the use of supraventricular tachycardia discrimination enhancements and longer detection duration.19,20
Our study also showed that a high-rate therapy zone was associated with both low inappropriate shock and low appropriate shock, without increased mortality, in patients with idiopathic VF.
The MADIT II and the SCD in Heart Failure trials showed that patients receiving inappropriate shocks, as well as appropriate shocks, had an increased risk of death.21,22
Appropriate shock is clearly associated with ventricular arrhythmia, indicating that the patients have an arrhythmogenic substrate and poor prognosis, but the mechanism of increased risk of death in patients receiving inappropriate shocks remains unclear. ICD implantation can be psychologically distressing, especially for young patients.23
Dewitt et al reported that primary prevention ICD implantation had a 36% risk of adverse events in a pediatric cohort, and the risk-benefit ratio of ICD therapy increased with time.8
In light of this, ICD implantation in pediatric and CHD patients, especially in cases of primary prevention, needs to be carefully considered on a case-by-case basis with patients and their families fully informed of the risks and benefits of therapy. There have been no randomized studies of ICD in pediatric and CHD patients, and all data are retrospective due to small sample sizes and heterogeneous groups. Therefore, ICD indications in pediatric patients are usually based on adult studies and have a level of evidence of B or C.13,24
Further, multicenter prospective studies of pediatric patients with a high risk of SCD are required.
Study Limitations
This study was limited by its retrospective nature. The comparison between the patient groups was limited by heterogeneity in underlying disease, age, and body size, as well as in small sample size. Programming data related to ICD shock were incomplete; the association between programming and shock therapies could not be analyzed. As we did not investigate the timing of inappropriate shock after ICD implantation, a time-dependent risk analysis for inappropriate shock therapy could not be conducted.
Conclusions
The rate of ICD implantation for primary prevention is still low in Korean pediatric patients. However, appropriate shocks and antitachycardia pacing can be successfully delivered to prevent SCD, although complications and inappropriate shock are not uncommon. It is necessary to improve the utilization of ICD as well as reducing complications and selecting the patients with a high risk of SCD.
Disclosure
The authors declare nothing to disclose.
IRB Information
Institutional Review Board of Seoul National University Hospital (no. 1806-028-949); Institutional Review Board of Severance Hospital (no. 4-2018-0879); Samsung Medical Center Institutional Review Board (no. 2018-10-017); Ulsan University Hospital Institutional Review Board (no. S2019-0314); Institutional Review Board of Sejong General Hospital (no. 1840).
Data Availability
The individual identified participant data will be shared immediately after publication, ending 10 years after publication. The data will be shared on a request basis for anyone via e-mail.
Supplementary Files
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-20-0468
References
- 1.
Moss AJ, Multicenter Automatic Defibrillator Implantation Trial. MADIT-II and its implications. Eur Heart J 2003; 24: 16–18.
- 2.
El Moheb M, Nicolas J, Khamis AM, Iskandarani G, Akl EA, Refaat M. Implantable cardiac defibrillators for people with non-ischaemic cardiomyopathy. Cochrane Database Syst Rev 2018; 12: CD012738.
- 3.
Baskar S, Bao H, Minges KE, Spar D, Czosek RJ. Characteristics and outcomes of pediatric patients who undergo placement of implantable cardioverter defibrillators. Circ Arrhythm Electrophysiol 2018; 11: e006542.
- 4.
Korte T, Koditz H, Niehaus M, Paul T, Tebbenjohanns J. High incidence of appropriate and inappropriate ICD therapies in children and adolescents with implantable cardioverter defibrillator. Pacing Clin Electrophysiol 2004; 27: 924–932.
- 5.
Celiker A, Olgun H, Karagoz T, Ozer S, Ozkutlu S, Alehan D. Midterm experience with implantable cardioverter-defibrillators in children and young adults. Europace 2010; 12: 1732–1738.
- 6.
Olde Nordkamp LR, Postema PG, Knops RE, van Dijk N, Limpens J, Wilde AA, et al. Implantable cardioverter-defibrillator harm in young patients with inherited arrhythmia syndromes: A systematic review and meta-analysis of inappropriate shocks and complications. Heart Rhythm 2016; 13: 443–454.
- 7.
Asakai H, Shimizu A, Mitsuhashi T, Ueyama T, Yokoshiki H, Nishii N, et al. Current trends in implantable cardioverter-defibrillator therapy in children: Results from the JCDTR database. Circ J 2018; 83: 52–55.
- 8.
DeWitt ES, Triedman JK, Cecchin F, Mah DY, Abrams DJ, Walsh EP, et al. Time dependence of risks and benefits in pediatric primary prevention implantable cardioverter-defibrillator therapy. Circ Arrhythm Electrophysiol 2014; 7: 1057–1063.
- 9.
Heersche JH, Blom NA, van de Heuvel F, Blank C, Reimer AG, Clur SA, et al. Implantable cardioverter defibrillator therapy for prevention of sudden cardiac death in children in the Netherlands. Pacing Clin Electrophysiol 2010; 33: 179–185.
- 10.
Berul CI, Van Hare GF, Kertesz NJ, Dubin AM, Cecchin F, Collins KK, et al. Results of a multicenter retrospective implantable cardioverter-defibrillator registry of pediatric and congenital heart disease patients. J Am Coll Cardiol 2008; 51: 1685–1691.
- 11.
Von Bergen NH, Atkins DL, Dick M 2nd, Bradley DJ, Etheridge SP, Saarel EV, et al. Multicenter study of the effectiveness of implantable cardioverter defibrillators in children and young adults with heart disease. Pediatr Cardiol 2011; 32: 399–405.
- 12.
Dechert BE, Bradley DJ, Serwer GA, Dick Ii M, Lapage MJ. Implantable cardioverter defibrillator outcomes in pediatric and congenital heart disease: Time to system revision. Pacing Clin Electrophysiol 2016; 39: 703–708.
- 13.
Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation 2018; 138: e272–e391.
- 14.
Lawrence D, Von Bergen N, Law IH, Bradley DJ, Dick M 2nd, Frias PA, et al. Inappropriate ICD discharges in single-chamber versus dual-chamber devices in the pediatric and young adult population. J Cardiovasc Electrophysiol 2009; 20: 287–290.
- 15.
Moss AJ, Madit-II. MADIT-II: Substudies and their implications. Card Electrophysiol Rev 2003; 7: 430–433.
- 16.
Wilkoff BL, Williamson BD, Stern RS, Moore SL, Lu F, Lee SW, et al. Strategic programming of detection and therapy parameters in implantable cardioverter-defibrillators reduces shocks in primary prevention patients: Results from the PREPARE (Primary Prevention Parameters Evaluation) study. J Am Coll Cardiol 2008; 52: 541–550.
- 17.
Sabbag A, Suleiman M, Laish-Farkash A, Samania N, Kazatsker M, Goldenberg I, et al. Contemporary rates of appropriate shock therapy in patients who receive implantable device therapy in a real-world setting: From the Israeli ICD Registry. Heart Rhythm 2015; 12: 2426–2433.
- 18.
Bettin M, Larbig R, Rath B, Fischer A, Frommeyer G, Reinke F, et al. Long-term experience with the subcutaneous implantable cardioverter-defibrillator in teenagers and young adults. JACC Clin Electrophysiol 2017; 3: 1499–1506.
- 19.
Upadhyay GA, Mela T, Singh JP. The burden of inappropriate shocks in young people and how to avoid them. Heart 2012; 98: 1457–1466.
- 20.
Uhm JS, Kim TH, Kim IC, Park YA, Shin DG, Lim YM, et al. Long-term prognosis of patients with an implantable cardioverter-defibrillator in Korea. Yonsei Med J 2017; 58: 514–520.
- 21.
Daubert JP, Zareba W, Cannom DS, McNitt S, Rosero SZ, Wang P, et al. Inappropriate implantable cardioverter-defibrillator shocks in MADIT II: Frequency, mechanisms, predictors, and survival impact. J Am Coll Cardiol 2008; 51: 1357–1365.
- 22.
Poole JE, Johnson GW, Hellkamp AS, Anderson J, Callans DJ, Raitt MH, et al. Prognostic importance of defibrillator shocks in patients with heart failure. N Engl J Med 2008; 359: 1009–1017.
- 23.
Thylen I, Dekker RL, Jaarsma T, Stromberg A, Moser DK. Characteristics associated with anxiety, depressive symptoms, and quality-of-life in a large cohort of implantable cardioverter defibrillator recipients. J Psychosom Res 2014; 77: 122–127.
- 24.
Priori SG, Blomstrom-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J 2015; 36: 2793–2867.