Abstract
Background:
Atrial fibrillation (AF) is a common arrhythmia in the elderly, and causes complications such as cardioembolic stroke. Serum high-sensitivity C-reactive protein (hs-CRP), a marker of systemic inflammation, has been reported to be a risk factor for developing AF in Western countries. However, few community-based studies have examined this issue in general Asian populations.
Methods and Results:
A total of 2,510 community-dwelling Japanese participants aged ≥40 years without a history of AF were divided into 4 groups according to the sex-specific quartiles of serum hs-CRP concentrations (Q1, lowest and Q4, highest) and followed up for 24 years. The hazard ratios and their 95% confidence intervals for the development of AF were estimated using a Cox proportional hazards model. During the follow up, 234 subjects developed AF. The risk of AF increased significantly with elevating serum hs-CRP levels after adjustment for potential confounding factors (hazard ratio [95% confidence interval], Q1, 1.00 [reference]; Q2, 1.26 [0.83–1.92]; Q3, 1.77 [1.18–2.66]; and Q4, 1.89 [1.24–2.86]; P for trend <0.001).
Conclusions:
The study findings suggest that elevated serum hs-CRP levels are an independent risk factor for the development of AF in a general Japanese population.
Atrial fibrillation (AF) is a common arrhythmia in elderly people and its morbidity has been increasing with population aging.1
The major complication of AF is cardioembolic stroke, which is the most severe subtype of cerebral infarction.2
It has also been reported that AF is a risk factor for heart failure;3
therefore, prevention of AF is an important strategy to reduce the risk of AF-related complications such as cerebral infarction and heart failure. Previous epidemiological studies have reported several risk factors for AF, such as aging, male sex, hypertension, diabetes, congestive heart failure, valvular heart disease, obesity, smoking, drinking, chronic obstructive pulmonary disease, obstructive sleep apnea4
and hyperthyroidism.5
Recently, prospective observational studies from Western countries have shown that an increasing level of serum high-sensitivity C-reactive protein (hs-CRP), a marker of systemic inflammation, is also a risk factor for the development of AF.6–8
East Asian people, including Japanese, have been reported to have relatively lower serum concentrations of hs-CRP than other ethnic groups;9,10
therefore, the association of serum hs-CRP levels with the risk of AF should be evaluated in general Asian populations.
The aim of the present study was to examine the association between serum hs-CRP levels and the development of AF using data from a long-term prospective cohort study in a general Japanese population.
Methods
Study Population
The Hisayama Study, a population-based prospective cohort study of cardiovascular and cerebrovascular diseases, was begun in 1961 in the town of Hisayama, a suburb of the Fukuoka metropolitan area on Kyushu Island in Japan. In 1988, a baseline health examination for the present study was performed in this town. A detailed description of this study was published previously.11
In brief, a total of 2,742 residents aged ≥40 years (participation rate, 80.9%) consented to participate in this survey. After the exclusion of 57 subjects who had AF or atrial flutter (AFL) at baseline or in their medical history, 8 subjects without electrocardiogram (ECG) examination at baseline, and 129 subjects without serum hs-CRP measurement, 2,548 subjects were eligible for the follow-up survey. After the additional exclusion of 38 subjects lacking the requisite information to determine the presence or absence of AF during follow up, the remaining 2,510 subjects (1,050 men and 1,460 women) were enrolled in the present study (follow-up rate, 98.5%).
Measurement of Serum hs-CRP Concentrations
Serum specimens collected at the baseline examination were stored at −20℃ until the measurement of hs-CRP in 2002. Serum hs-CRP concentrations were measured using a modification of the Behring latex-enhanced CRP assay on a BN-100 nephelometer (Dade Behring) with a 2% interassay coefficient of variation. The hs-CRP levels were categorized into 4 groups according to the sex-specific quartiles (men: Q1, ≤0.23 mg/L; Q2, 0.24–0.54 mg/L; Q3, 0.55–1.25 mg/L; Q4, ≥1.26 mg/L; women: Q1, ≤0.19 mg/L; Q2, 0.20–0.39 mg/L; Q3, 0.40–0.91 mg/L; Q4, ≥0.92 mg/L).
Follow-up Survey
The study participants were followed up prospectively for 24 years, from December 1988 to November 2012. The method for the ascertainment of incident AF cases was described previously.12
Information on new events of AF was primarily collected at the annual health examinations in Hisayama during the follow-up periods, including a conventional 12-lead ECG and an interview by a study physician. For the participants who did not participate in the health examination or who moved away from the town, a standard questionnaire was performed by mail or telephone interviews. In the questionnaire, we asked the participants whether they had experienced new cardiovascular events, including stroke, coronary artery diseases (CADs), arrhythmia, and other heart diseases. In addition, we collected information on new cardiovascular events and AF through a daily monitoring system established by the study team, local physicians and members of the town’s Health and Welfare Office. When a new cardiovascular event and AF was suspected or a subject died, we reviewed all the available clinical information, including standard 12-lead ECG, ambulatory monitoring ECG, physicians’ diagnosis on medical records, and death certificates, to confirm whether the participants had experienced new-onset AF. During the follow-up period, 1,027 subjects (497 men and 530 women) died from any cause.
Diagnosis of AF
The definition of AF for the present study included paroxysmal, persistent, and permanent AF (Minnesota code of 8-3-1 or 8-3-3) and AFL (Minnesota code of 8-3-2 or 8-3-4). The primary outcome of the present study was a new onset of AF, which was diagnosed at the annual health examinations, clinics, or hospitals. ECG records of the participants with AF were reviewed and adjudicated by the study team, including cardiologists, to confirm the diagnosis of AF. During the follow-up period, a total of 234 first-ever events of AF were observed. Among them, 52 events were first identified by ECG at the annual health examinations and 182 events were first diagnosed by ECG at clinics or hospitals. Among the latter, 46 AF events were detected again by ECG at the subsequent health examinations during the follow up. Therefore, a total of 98 AF events were detected by ECG at the health examinations and these events were used in the sensitivity analysis.
Subtypes of AF
It was difficult to classify AF events using a standard classification (paroxysmal, persistent, and permanent AF or AFL) in our study design. Accordingly, new-onset AF events (234 events) were classified into AF in a narrow sense (222 events) and AFL (12 events), and AF events in a narrow sense were further classified into “definite permanent AF” (69 events) and “other or indefinite subtype of AF” (153 events), as described previously.12
Subjects were defined as having “definite permanent AF” if they had AF on all of the 12-lead ECGs at the subsequent annual health examinations during the follow up. Subjects were defined as having “other or indefinite subtype of AF” if they experienced sinus rhythm on ECG at least once after the onset of AF during the follow up, or if they did not undergo ECG examinations after the AF onset during the follow up.
Other Risk Factor Measurements
We included traditional risk factors for cardiovascular disease and previously reported risk factors for AF in the multivariable-adjusted model.13,14
Sitting blood pressure was measured 3 times at the upper arm using a mercury sphygmomanometer after 5 minutes of rest. The average of 3 measurements was used for the analysis. Hypertension was defined as blood pressure levels of ≥140 mmHg (systolic) or ≥90 mmHg (diastolic) or current treatment with antihypertensive agents. Plasma glucose levels were measured by using the glucose-oxidase method. Diabetes mellitus was defined by a 75-g oral glucose tolerance test (1998 World Health Organization definition15), fasting plasma glucose levels (≥7.0 mmol/L), postprandial glucose levels (≥11.1 mmol/L), or by the use of oral hypoglycemic agents or insulin, as described previously.11,16
Serum levels of total cholesterol and high-density lipoprotein cholesterol were determined enzymatically. Hypercholesterolemia was defined as a serum cholesterol level of ≥5.69 mmol/L. Body height and weight were measured in light clothes without shoes, and body mass index was calculated as body weight (kg) divided by body height (m) squared. Obesity was defined as a body mass index of ≥25 kg/m2. Serum creatinine concentration was measured by using Jaffe methods and converted to an enzymatic method value, as described previously.17
The estimated glomerular filtration rate was determined by the Chronic Kidney Disease Epidemiology Collaboration formula with the Japanese coefficient,18
as described previously.19
Chronic kidney disease (CKD) was defined as the presence of proteinuria (1+ or more using a dipstick test) or decreased estimated glomerular filtration rate (<60 mL/min/1.73 m2).20
No participants were undergoing hemodialysis at baseline. History of CAD was defined as any preexisting event of acute or silent myocardial infarction, percutaneous coronary intervention, or coronary artery bypass surgery. Cardiac murmur was defined as systolic murmur of Levine III to VI and/or any diastolic murmur determined by a study physician.1
The findings of a standard 12-lead ECG at baseline were classified by the Minnesota Code Classification System. Left ventricular hypertrophy was defined as a Minnesota code of 3-1. ST depression was defined as a Minnesota code of 4-1 to 4-3. Atrioventricular block included first, second, and third atrioventricular block (Minnesota code of 6-1 to 6-3). Wide QRS complex was defined as a QRS duration >120 ms (Minnesota code of 7-1, 7-2, or 7-4). Prolonged QT interval was defined as a rate-corrected QT interval (QT interval divided by square root of the RR interval in seconds) >440 ms. Heart rate was recorded by 12-lead ECG at baseline. Information on smoking habits, alcohol intake, and physical activity during leisure time was obtained using a self-administered questionnaire. Smoking habits and alcohol intake were classified as either current use or not. Subjects engaging in sports or other forms of exertion ≥3 times a week during their leisure time were defined as the regular exercise group.
Changes in Serum hs-CRP Levels and the Risk of AF
In this cohort, serum hs-CRP levels were measured again at the health examination in 2002 (approximately 14 years after the baseline examination) for 1,688 subjects. The association between the changes in serum hs-CRP levels during the first 14 years and the risk of AF during the subsequent 10 years was examined as a substudy. In this substudy, 56 subjects who had developed AF during the first 14 years were excluded from the study outcome and 112 events of first-ever AF were confirmed during the subsequent 10 years. The serum hs-CRP levels in 1988 and in 2002 were classified into lower and higher levels, respectively, using sex-specific cut-off values (see the
Statistical Analysis
section). The subjects were divided into 4 subgroups according to the serum hs-CRP levels in 1988 and in 2002: (1) persistently low (lower than the cut-off values both in 1988 and in 2002); (2) increased (lower than the cut-off values in 1988 and higher than the cut-off values in 2002); (3) decreased (higher than the cut-off values in 1988 and lower than the cut-off values in 2002); and (4) persistently high (higher than the cut-off values both in 1988 and in 2002).
Statistical Analysis
The trends in the frequencies and means (standard deviations) of risk factors across the serum hs-CRP quartiles were tested by logistic and linear regression analysis, respectively, using an ordinal variable (1, 2, 3, and 4 for the first [Q1], second [Q2], third [Q3], and fourth [Q4] quartiles, respectively). The crude cumulative incidence of AF was estimated with the Kaplan-Meier method and compared by using the log-rank test. The incidence rates of AF were calculated by the person-year method with adjustment for age and sex using a direct method. The age- and sex-adjusted or multivariable-adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) for the development of AF across the hs-CRP quartile groups or per every 1 log-transformed hs-CRP concentration were estimated by using a Cox proportional hazard model. The association between the changes in serum hs-CRP levels during the first 14 years and the risk of AF during the subsequent 10 years was examined by using a Cox proportional hazard model. The follow up was censored at the date of the last follow-up survey, the date of death, or 30 November 2012. The proportional hazards assumption was checked graphically with a log cumulative hazard plot. The sub-distribution HRs and 95% CI with adjustment for the effect of the competing risk of death were calculated using the model proposed by Fine and Gray.21
The heterogeneity in the association between the subgroups stratified by major demographic/clinical factors was tested by adding the multiplicative interaction term to the relevant Cox model. The sex-specific optimal cut-off values of serum hs-CRP for the discrimination of AF risk were determined as the serum hs-CRP concentration that minimizes the value of  in the receiver operating characteristic analysis. All statistical analyses were performed with the SAS statistical software program (version 9.4; SAS Institute, Cary, NC, USA). A 2-tailed P value <0.05 was considered to be statistically significant.
in the receiver operating characteristic analysis. All statistical analyses were performed with the SAS statistical software program (version 9.4; SAS Institute, Cary, NC, USA). A 2-tailed P value <0.05 was considered to be statistically significant.
Results
The baseline characteristics of the study population according to the serum hs-CRP levels are shown in
Table 1. The mean values of age, systolic and diastolic blood pressure, fasting plasma glucose, serum total cholesterol, body mass index, and heart rate, and the frequencies of hypertension, use of antihypertensive drugs, diabetes mellitus, hypercholesterolemia, obesity, CKD, proteinuria, prolonged QT interval, and current smoking increased significantly with higher serum hs-CRP levels, whereas the mean values of serum high-density lipoprotein cholesterol and estimated glomerular filtration rate decreased significantly with higher serum hs-CRP levels.
Table 1.
Baseline Characteristics of Study Subjects According to Serum hs-CRP Levels
| Variables |
Serum hs-CRP levels, mg/L |
P for
trend |
Q1 (men: ≤0.23;
women: ≤0.19) |
Q2 (men: 0.24–0.54;
women: 0.20–0.39) |
Q3 (men: 0.55–1.25;
women: 0.40–0.91) |
Q4 (men: ≥1.26;
women: ≥0.92) |
| (n=615) |
(n=642) |
(n=627) |
(n=626) |
| Age, years |
55±11 |
58±11 |
59±11 |
62±12 |
<0.001 |
| Men, % |
41.6 |
41.7 |
42.3 |
41.7 |
0.94 |
| Hypertension, % |
29.4 |
40.5 |
44.8 |
50.8 |
<0.001 |
| Systolic blood pressure, mmHg |
129±20 |
133±21 |
135±22 |
138±21 |
<0.001 |
| Diastolic blood pressure, mmHg |
76±11 |
78±11 |
79±11 |
79±12 |
<0.001 |
| Use of antihypertensive drugs, % |
7.8 |
14.2 |
15.0 |
22.0 |
<0.001 |
| Diabetes mellitus, % |
5.9 |
9.7 |
13.9 |
17.1 |
<0.001 |
| Fasting plasma glucose, mmol/L |
5.59±1.06 |
5.69±1.08 |
5.87±1.40 |
6.07±1.55 |
<0.001 |
| Hypercholesterolemia, % |
28.6 |
37.9 |
38.8 |
41.5 |
<0.001 |
| Serum total cholesterol, mmol/L |
5.16±1.03 |
5.39±1.08 |
5.45±1.10 |
5.45±1.13 |
<0.001 |
| Serum HDL cholesterol, mmol/L |
1.37±0.30 |
1.34±0.30 |
1.27±0.30 |
1.22±0.30 |
<0.001 |
| Obesity, % |
12.9 |
20.4 |
30.6 |
30.0 |
<0.001 |
| Body mass index, kg/m2 |
21.7±2.8 |
22.7±2.8 |
23.4±3.2 |
23.5±3.4 |
<0.001 |
| Chronic kidney disease, % |
6.2 |
7.5 |
10.5 |
13.7 |
<0.001 |
| eGFR, mL/min/1.73 m2 |
83.0±10.4 |
81.3±10.2 |
79.3±11.3 |
77.9±11.0 |
<0.001 |
| Proteinuria, % |
4.1 |
4.8 |
6.2 |
7.8 |
0.002 |
| History of coronary artery disease, % |
0.2 |
0.0 |
0.2 |
0.2 |
0.80 |
| Cardiac murmur, % |
2.0 |
0.6 |
1.9 |
1.8 |
0.73 |
Left ventricular hypertrophy and/or ST
depression, % |
16.3 |
12.9 |
16.1 |
16.5 |
0.55 |
| Atrioventricular block, % |
0.7 |
2.2 |
1.6 |
1.8 |
0.23 |
| Wide QRS complex, % |
2.3 |
2.3 |
1.9 |
3.8 |
0.14 |
| Prolonged QT interval, % |
11.4 |
10.0 |
13.7 |
21.1 |
<0.001 |
| Heart rate, beats/min |
64.6±9.9 |
65.4±10.5 |
65.5±10.8 |
67.8±11.1 |
<0.001 |
| Smoking habits, % |
20.3 |
20.9 |
25.2 |
32.0 |
<0.001 |
| Alcohol intake, % |
30.2 |
30.5 |
31.5 |
29.8 |
0.95 |
| Regular exercise, % |
10.8 |
10.0 |
9.1 |
11.7 |
0.73 |
Values are presented as means±standard deviations or percentages. eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; hs-CRP, high-sensitivity C-reactive protein.
During the follow-up period, 234 subjects (113 men and 121 women) developed AF. The average (standard deviation) age at onset of AF was almost constant across the hs-CRP levels (Q1: 78 [11]; Q2: 78 [10]; Q3: 78 [10]; Q4: 79 [11] years; P for trend=0.70). The crude cumulative incidence of AF using the Kaplan-Meier method (Figure 1) and the age- and sex-adjusted incidence rates of AF using the person-year method (Figure 2) increased with higher serum hs-CRP levels. This association was substantially unchanged after the adjustment for potential confounding factors, and was slightly attenuated but remained significant after further adjustment for competing risk of death (Table 2). A sensitivity analysis in which the study outcome was limited to 98 events of AF that were confirmed at the health examination did not make any material difference in the findings, although some P values failed to reach the level of statistical significance (Table 3). The association of serum hs-CRP levels with the risk of AF subtypes is shown in
Supplementary Table 1. A higher serum hs-CRP level was associated with increased risks of both “definite permanent AF” and “other or indefinite subtype of AF”, but no clear association was observed for AFL, probably because of the lower number of events.

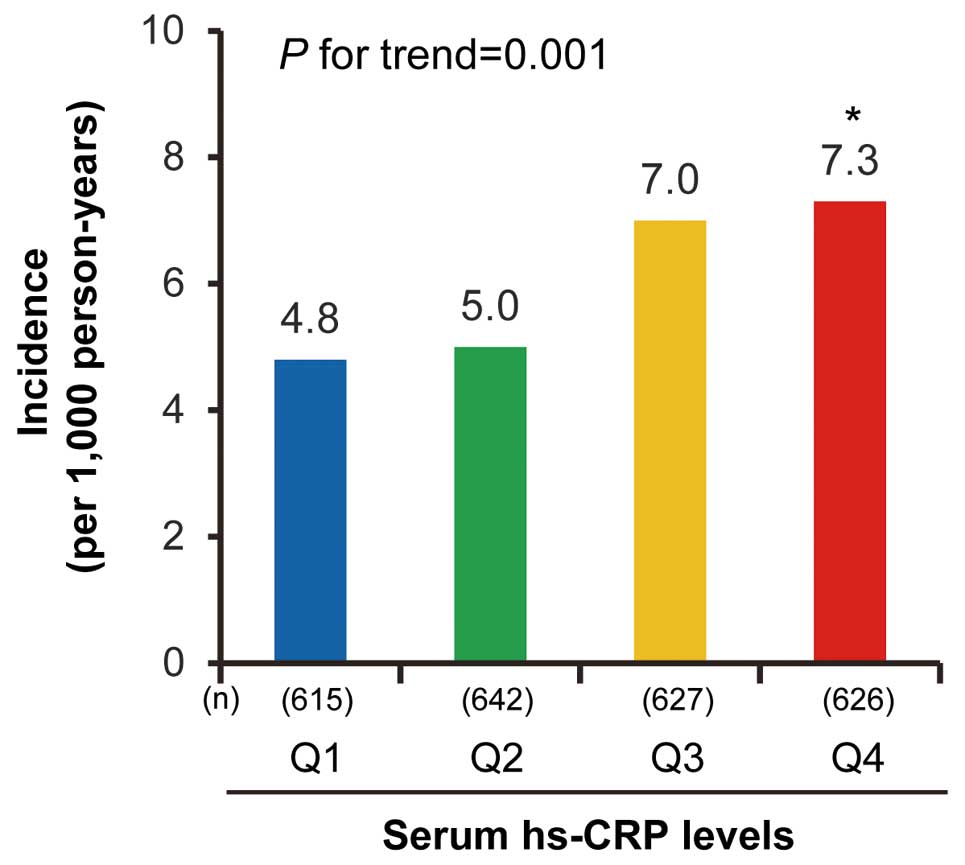
Table 2.
HRs for the Development of Atrial Fibrillation According to Serum hs-CRP Levels
Serum hs-CRP levels,
mg/L |
Number of
events/subjects |
Age- and sex-adjusted |
Multivariable-adjusted* |
Fine and Gray model† |
| HR (95% CI) |
P value |
HR (95% CI) |
P value |
SHR (95% CI) |
P value |
| Q1 (men: ≤0.23; women: ≤0.19) |
39/615 |
1.00
(reference) |
|
1.00
(reference) |
|
1.00
(reference) |
|
Q2 (men: 0.24–0.54; women:
0.20–0.39) |
54/642 |
1.16
(0.77–1.75) |
0.49 |
1.26
(0.83–1.92) |
0.29 |
1.26
(0.83–1.91) |
0.28 |
Q3 (men: 0.55–1.25; women:
0.40–0.91) |
69/627 |
1.66
(1.12–2.46) |
0.01 |
1.77
(1.18–2.66) |
0.006 |
1.55
(1.03–2.35) |
0.04 |
Q4 (men: ≥1.26; women:
≥0.92) |
72/626 |
1.75
(1.18–2.59) |
0.006 |
1.89
(1.24–2.86) |
0.003 |
1.58
(1.04–2.40) |
0.03 |
| P for trend |
|
|
0.001 |
|
<0.001 |
|
0.02 |
Per 1 log-transformed concentration
of serum hs-CRP, mg/L |
|
1.18
(1.07–1.30) |
<0.001 |
1.18
(1.07–1.31) |
0.001 |
1.14
(1.03–1.26) |
0.009 |
*Adjusted for age, sex, hypertension, diabetes mellitus, serum total cholesterol, serum high-density lipoprotein cholesterol, body mass index, chronic kidney disease, history of coronary artery disease, cardiac murmur, left ventricular hypertrophy and/or ST depression, atrioventricular block, wide QRS complex, prolonged QT interval, heart rate, smoking habits, alcohol intake, and regular exercise. †Adjusted for competing risk of death with adjustment for the covariates included in the multivariable-adjusted model. The numbers of deaths were 190 for Q1, 228 for Q2, 266 for Q3, and 343 for Q4. CI, confidence interval; HR, hazard ratio; hs-CRP, high-sensitivity C-reactive protein; SHR, sub-distribution hazard ratio.
Table 3.
HRs for the Development of Atrial Fibrillation According to Serum hs-CRP Levels Restricted to Atrial Fibrillation Detected at Annual Health Examinations
| Serum hs-CRP levels, mg/L |
Number of
events/subjects |
Age- and sex-adjusted |
Multivariable-adjusted* |
| HR (95% CI) |
P value |
HR (95% CI) |
P value |
| Q1 (men: ≤0.23; women: ≤0.19) |
21/615 |
1.00 (reference) |
|
1.00 (reference) |
|
| Q2 (men: 0.24–0.54; women: 0.20–0.39) |
21/642 |
0.91 (0.50–1.67) |
0.76 |
0.92 (0.49–1.72) |
0.80 |
| Q3 (men: 0.55–1.25; women: 0.40–0.91) |
26/627 |
1.25 (0.70–2.22) |
0.45 |
1.26 (0.69–2.29) |
0.45 |
| Q4 (men: ≥1.26; women: ≥0.92) |
30/626 |
1.54 (0.88–2.71) |
0.13 |
1.64 (0.90–2.98) |
0.11 |
| P for trend |
|
|
0.07 |
|
0.06 |
Per 1 log-transformed concentration of serum
hs-CRP, mg/L |
|
1.21 (1.04–1.40) |
0.01 |
1.23 (1.05–1.44) |
0.01 |
*Adjusted for age, sex, hypertension, diabetes mellitus, serum total cholesterol, serum high-density lipoprotein cholesterol, body mass index, chronic kidney disease, history of coronary artery disease, cardiac murmur, left ventricular hypertrophy and/or ST depression, atrioventricular block, wide QRS complex, prolonged QT interval, heart rate, smoking habits, alcohol intake, and regular exercise. Abbreviations as in Table 2.
Figure 3
shows multivariable-adjusted HRs for the development of AF per 1 increment in log-transformed hs-CRP among the major demographic/clinical subgroups (age, sex, hypertension, diabetes, hypercholesterolemia, obesity, and CKD). There was no evidence of heterogeneity in the associations of hs-CRP level with the development of AF among the subgroups, except for CKD. A higher serum hs-CRP concentration was a significant risk factor for incident AF in the subjects without CKD, but no clear association was observed in those with CKD.
Then, the cut-off value of serum hs-CRP concentration that optimized the discriminating ability (i.e., sensitivity and specificity) for the risk of the development of AF was examined. The optimal cut-off values of serum hs-CRP were 0.88 mg/L for men and 0.83 mg/L for women (Supplementary Figure).
Finally, the association between the changes in serum hs-CRP levels during the first 14 years and the risk of AF during the subsequent 10 years was examined as a substudy (Supplementary Table 2). The “persistently high” subgroup (i.e., subjects in whom serum hs-CRP levels were higher than the cut-off values both in 1988 and in 2002) and the “increased” subgroup (i.e., subjects in whom serum hs-CRP levels were lower than the cut-off values in 1988 but higher in 2002) had a 2.11-fold and a 1.75-fold higher risk of AF than the “persistently low” subgroup (i.e., subjects in whom serum hs-CRP levels were lower than the cut-off values both in 1988 and in 2002). In contrast, the “decreased” subgroup (i.e., subjects in whom serum hs-CRP levels were higher than the cut-off values in 1988 but lower in 2002) was not associated with a risk of AF.
Discussion
The present study clearly demonstrated that increased levels of serum hs-CRP, a marker of systemic inflammation, were significantly associated with a higher risk of the development of AF, even after adjustment for major cardiovascular risk factors. Further, there was no evidence of heterogeneity in the associations of serum hs-CRP level with the development of AF among the subgroups defined by major cardiovascular risk factors other than CKD (age, sex, hypertension, diabetes, hypercholesterolemia, and obesity). These results suggest that systemic inflammation is an independent risk factor for the development of AF regardless of the absence or presence of major cardiovascular risk factors other than CKD, and that serum hs-CRP may be a useful biomarker for detecting people at a higher risk of developing AF.
Several prospective epidemiological studies have examined the association between serum hs-CRP levels and the risk of AF mainly in Western populations, but the findings have been inconsistent.6–8,22
The Copenhagen City Heart Study6
and the Cohorts for Heart and Aging Research in Genomic Epidemiology AF consortium of community-based cohort studies7
reported a significant positive association between serum hs-CRP levels and the development of AF, whereas the Tromso Study8
found a significant association only in men. In contrast, the Study of Atrial Fibrillation in High Risk Elderly22
failed to show a significant association between serum hs-CRP levels and the risk of AF, probably because of the relatively short follow-up period and insufficient statistical power. Moreover, it would be valuable to address the influence of low-grade systemic inflammation on the risk of AF among Asian populations with different genetic and lifestyle backgrounds. Indeed, East Asian people have been reported to have relatively lower serum concentrations of hs-CRP than other ethnic groups.9,10
A hospital-based observational study in South Korea23
reported a significant association between serum hs-CRP levels and the risk of AF, but ~60% of the participants in this study were working employees or their spouses, likely biasing to individuals with easier access to health care (a healthy-screenee bias). The present population-based study showed a significant association between serum hs-CRP levels and the risk of AF independent of other cardiovascular risk factors using prospective cohort data from Japan, which had a relatively high participation rate and a lower risk of selection bias. A similar association was also reported by another population-based cohort study in Japan, recently.24
In addition, the present study showed that the subjects with a persistently high serum hs-CRP level showed an approximately 2-fold higher risk of AF than those with a persistently low level. These findings suggest that long-term exposure to low-grade systemic inflammation is a risk factor for the development of AF. In contrast, the risk of AF did not increase among the “decreased” subgroup (i.e., subjects in whom serum hs-CRP levels were high in 1988 but low in 2002) in the present study, suggesting that the risk of AF could be reduced by suppressing systemic inflammation in some way.
Elevation of serum hs-CRP concentration reflects systemic inflammation. As a possible mechanism underlying the positive association between hs-CRP levels and the risk of AF, systematic inflammation has been reported to lead to structural and electrical remodeling in the heart. A cross-sectional study by Canpolat et al25
reported that hs-CRP is a predictor of both structural and electrical remodeling. Structural remodeling leads to myocyte hypertrophy and fibrosis and electrical remodeling leads to alterations in the expression of ion channels and shortening of the wavelength of the atrial impulse.26,27
These remodelings cause cardiac conduction disturbance and generate reentry, resulting in the development of AF.28,29
In the present study, no clear association between serum hs-CRP levels and the risk of AF was observed in the subgroup with CKD, probably because CKD increased the risk of AF regardless of the level of systemic inflammation. CKD is known to confer a risk for AF30,31
through some mechanisms other than inflammation, including chronic volume overload,32
activation of the renin-angiotensin-aldosterone system,33
increased sympathetic activity,34
and abnormalities of electrolytes,35
which lead to cardiac structural and electronic remodeling.
The strengths of the present study were its prospective community-based design, high participation rate in the baseline examination, and comprehensive follow up of participants based on repeated health examinations, questionnaires, and information from clinics or hospitals, and the accuracy of AF diagnosis. Furthermore, serum hs-CRP data were available not only at baseline (in 1988) but also during the follow up (in 2002), and we were able to examine the association between the changes in serum hs-CRP levels during the first 14 years and the risk of AF during the subsequent 10 years. However, some limitations should be discussed. First, AF events were likely to be underdiagnosed because the diagnosis was mainly based on a conventional 12-lead ECG at the annual health examinations, clinics, and hospitals. For example, asymptomatic or paroxysmal AF without evidence of ECG (covert AF) was not included in the study outcome. In addition, participants with other cardiovascular risk factors or other cardiovascular events during the follow-up period were likely to have a higher hs-CRP level at baseline and likely to visit clinics or hospitals more frequently than those without. Such people were likely to experience ECG examinations more frequently and have a higher chance of AF detection. However, the association between serum hs-CRP levels and AF risk was substantially unchanged even in the sensitivity analysis in which the study outcome was limited to AF events confirmed at the annual health examinations. Second, the serum samples were measured after being stored at −20℃ for a long period. However, a previous study confirmed the stability of the CRP concentration of frozen serum samples stored for a long term.36
Third, although we considered comprehensive confounding factors in the multivariable analysis, some residual confounding factors, such as anti-inflammatory treatment, might exist. Unfortunately, information for anti-inflammatory treatment was not available in this cohort. Fourth, the present study was performed in a single Japanese population and our conclusions may not be generalizable to other populations with different lifestyles and genetic backgrounds.
In conclusion, the present study clearly demonstrated that increased serum hs-CRP levels were an independent risk factor for developing AF in a general Japanese population. Serum hs-CRP may be a useful biomarker for detecting people at higher risk of AF, regardless of the presence or absence of additional cardiovascular risk factors other than CKD.
Acknowledgment
We thank the staff of the Division of Health and Welfare of Hisayama for their cooperation in this study. The statistical analyses were carried out using the computer resources offered under the category of General Projects by the Research Institute for Information Technology, Kyushu University.
Sources of Funding
This study was supported, in part, by a Grant-in-Aid for Scientific Research (A) (JP16H02692), (B) (JP17H04126, JP18H02737 and JP19H03863) and (C) (JP18K07565, JP18K09412, JP19K07890, JP20K10503, and JP20K11020), Grant-in-Aid for Early-Career Scientists (JP18K17925), and a Grant-in-Aid for Research Activity Start-up (JP19K23971) from the Ministry of Education, Culture, Sports, Science and Technology of Japan; by a Health and Labour Sciences Research Grant from the Ministry of Health, Labour and Welfare of Japan (20FA1002); and by the Japan Agency for Medical Research and Development (JP19ek0210082, JP19ek0210083, JP20dk0207025, JP20 km0405202, JP20fk0108075).
Disclosures
H.T. and T.K. are members of
Circulation Journal’s Editorial Team. The other authors have no conflicts of interest to declare.
IRB Information
This study was conducted in accordance with the “Declaration of Helsinki” and approved by the Kyushu University Institutional Review Board for Clinical Research (reference number: 2019-499), and written or oral informed consent was obtained from the participants.
Data Availability
The deidentified participant data will not be shared.
Supplementary Files
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-20-0751
References
- 1.
Schnabel RB, Yin X, Gona P, Larson MG, Beiser AS, McManus DD, et al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: A cohort study. Lancet 2015; 386: 154–162.
- 2.
Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: The Framingham Study. Stroke 1991; 22: 983–988.
- 3.
Maisel WH, Stevenson LW. Atrial fibrillation in heart failure: Epidemiology, pathophysiology, and rationale for therapy. Am J Cardiol 2003; 91(suppl): 2D–8D.
- 4.
Lau DH, Nattel S, Kalman JM, Sanders P. Modifiable risk factors and atrial fibrillation. Circulation 2017; 136: 583–596.
- 5.
Selmer C, Olesen JB, Hansen ML, Lindhardsen J, Olsen AM, Madsen JC, et al. The spectrum of thyroid disease and risk of new onset atrial fibrillation: A large population cohort study. BMJ 2012; 345: e7895.
- 6.
Marott SC, Nordestgaard BG, Zacho J, Friberg J, Jensen GB, Tybjaerg-Hansen A, et al. Does elevated C-reactive protein increase atrial fibrillation risk?: A mendelian randomization of 47,000 individuals from the general population. J Am Coll Cardiol 2010; 56: 789–795.
- 7.
Sinner MF, Stepas KA, Moser CB, Krijthe BP, Aspelund T, Sotoodehnia N, et al. B-type natriuretic peptide and C-reactive protein in the prediction of atrial fibrillation risk: The CHARGE-AF Consortium of community-based cohort studies. Europace 2014; 16: 1426–1433.
- 8.
Nyrnes A, Njolstad I, Mathiesen EB, Wilsgaard T, Hansen JB, Skjelbakken T, et al. Inflammatory biomarkers as risk factors for future atrial fibrillation. An eleven-year follow-up of 6315 men and women: The Tromso Study. Gend Med 2012; 9: 536–547.
- 9.
Shah T, Newcombe P, Smeeth L, Addo J, Casas JP, Whittaker J, et al. Ancestry as a determinant of mean population C-reactive protein values: Implications for cardiovascular risk prediction. Circ Cardiovasc Genet 2010; 3: 436–444.
- 10.
Kelley-Hedgepeth A, Lloyd-Jones DM, Colvin A, Matthews KA, Johnston J, Sowers MR, et al. Ethnic differences in C-reactive protein concentrations. Clin Chem 2008; 54: 1027–1037.
- 11.
Ohmura T, Ueda K, Kiyohara Y, Kato I, Iwamoto H, Nakayama K, et al. Prevalence of type 2 (non-insulin-dependent) diabetes mellitus and impaired glucose tolerance in the Japanese general population: The Hisayama Study. Diabetologia 1993; 36: 1198–1203.
- 12.
Nagata T, Hata J, Sakata S, Oishi E, Honda T, Furuta Y, et al. Serum N-terminal pro-B-type natriuretic peptide as a predictor for future development of atrial fibrillation in a general population: The Hisayama Study. Int J Cardiol 2020; 320: 90–96.
- 13.
Li Y, Pastori D, Guo Y, Wang Y, Lip GYH. Risk factors for new-onset atrial fibrillation: A focus on Asian populations. Int J Cardiol 2018; 261: 92–98.
- 14.
Kokubo Y, Matsumoto C. Traditional cardiovascular risk factors for incident atrial fibrillation. Circ J 2016; 80: 2415–2422.
- 15.
Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 1998; 15: 539–553.
- 16.
Mukai N, Hata J, Hirakawa Y, Ohara T, Yoshida D, Nakamura U, et al. Trends in the prevalence of type 2 diabetes and prediabetes in a Japanese community, 1988–2012: The Hisayama Study. Diabetol Int 2019; 10: 198–205.
- 17.
Nagata M, Ninomiya T, Doi Y, Yonemoto K, Kubo M, Hata J, et al. Trends in the prevalence of chronic kidney disease and its risk factors in a general Japanese population: The Hisayama Study. Nephrol Dial Transplant 2010; 25: 2557–2564.
- 18.
Horio M, Imai E, Yasuda Y, Watanabe T, Matsuo S. Modification of the CKD Epidemiology Collaboration (CKD-EPI) equation for Japanese: Accuracy and use for population estimates. Am J Kidney Dis 2010; 56: 32–38.
- 19.
Takae K, Hata J, Ohara T, Yoshida D, Shibata M, Mukai N, et al. Albuminuria increases the risks for both Alzheimer disease and vascular dementia in community-dwelling Japanese elderly: The Hisayama Study. J Am Heart Assoc 2018; 7: e006693.
- 20.
Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int 2013; 3(suppl): 1–150.
- 21.
Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999; 94: 496–509.
- 22.
Casaclang-Verzosa G, Barnes ME, Blume G, Seward JB, Gersh BJ, Cha SS, et al. C-reactive protein, left atrial volume, and atrial fibrillation: A prospective study in high-risk elderly. Echocardiography 2010; 27: 394–399.
- 23.
Kwon CH, Kang JG, Lee HJ, Kim NH, Sung JW, Cheong E, et al. C-reactive protein and risk of atrial fibrillation in East Asians. Europace 2017; 19: 1643–1649.
- 24.
Tanaka M, Imano H, Kubota Y, Yamagishi K, Umesawa M, Muraki I, et al. Serum high-sensitivity C-reactive protein levels and the risk of atrial fibrillation in Japanese population: The Circulatory Risk in Communities Study. J Atheroscler Thromb 2021; 28: 194–202.
- 25.
Canpolat U, Oto A, Yorgun H, Sunman H, Sahiner L, Kaya EB, et al. Association of plasma fibronectin level with left atrial electrical and structural remodelling in lone paroxysmal atrial fibrillation: A cross-sectional study. Turk Kardiyol Dern Ars 2015; 43: 259–268.
- 26.
Nattel S, Li D. Ionic remodeling in the heart: Pathophysiological significance and new therapeutic opportunities for atrial fibrillation. Circ Res 2000; 87: 440–447.
- 27.
Allessie M, Ausma J, Schotten U. Electrical, contractile and structural remodeling during atrial fibrillation. Cardiovasc Res 2002; 54: 230–246.
- 28.
Schotten U, Ausma J, Stellbrink C, Sabatschus I, Vogel M, Frechen D, et al. Cellular mechanisms of depressed atrial contractility in patients with chronic atrial fibrillation. Circulation 2001; 103: 691–698.
- 29.
Allessie MA, de Groot NM, Houben RP, Schotten U, Boersma E, Smeets JL, et al. Electropathological substrate of long-standing persistent atrial fibrillation in patients with structural heart disease: Longitudinal dissociation. Circ Arrhythm Electrophysiol 2010; 3: 606–615.
- 30.
Alonso A, Lopez FL, Matsushita K, Loehr LR, Agarwal SK, Chen LY, et al. Chronic kidney disease is associated with the incidence of atrial fibrillation: The Atherosclerosis Risk in Communities (ARIC) Study. Circulation 2011; 123: 2946–2953.
- 31.
Watanabe H, Watanabe T, Sasaki S, Nagai K, Roden DM, Aizawa Y. Close bidirectional relationship between chronic kidney disease and atrial fibrillation: The Niigata Preventive Medicine Study. Am Heart J 2009; 158: 629–636.
- 32.
Hung SC, Lai YS, Kuo KL, Tarng DC. Volume overload and adverse outcomes in chronic kidney disease: Clinical observational and animal studies. J Am Heart Assoc 2015; 4: e001918.
- 33.
Iravanian S, Dudley SC Jr. The renin-angiotensin-aldosterone system (RAAS) and cardiac arrhythmias. Heart Rhythm 2008; 5: S12–S17.
- 34.
Schlaich MP, Socratous F, Hennebry S, Eikelis N, Lambert EA, Straznicky N, et al. Sympathetic activation in chronic renal failure. J Am Soc Nephrol 2009; 20: 933–939.
- 35.
Fisher H, Hsu CY, Vittinghoff E, Lin F, Bansal N. Comparison of associations of urine protein-creatinine ratio versus albumin-creatinine ratio with complications of CKD: A cross-sectional analysis. Am J Kidney Dis 2013; 62: 1102–1108.
- 36.
Danesh J, Wheeler JG, Hirschfield GM, Eda S, Eiriksdottir G, Rumley A, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med 2004; 350: 1387–1397.


 in the receiver operating characteristic analysis. All statistical analyses were performed with the SAS statistical software program (version 9.4; SAS Institute, Cary, NC, USA). A 2-tailed P value <0.05 was considered to be statistically significant.
in the receiver operating characteristic analysis. All statistical analyses were performed with the SAS statistical software program (version 9.4; SAS Institute, Cary, NC, USA). A 2-tailed P value <0.05 was considered to be statistically significant.

