2021 Volume 85 Issue 8 Pages 1349-1355
2021 Volume 85 Issue 8 Pages 1349-1355
Background: The number of patients undergoing cardiac resynchronization therapy has increased. Consequently, there is increased frequency in the removal and reimplantation of coronary venous (CV) leads due to infection or malfunction.
Methods and Results: A total of 345 consecutive patients referred for lead(s) extraction were reviewed. Of these, 34 patients who underwent a CV lead removal were investigated. The indications for CV leads removal were device-related infections in 29 patients and lead malfunctions in 5 patients. The average duration of the CV leads was 4.1±3.8 years. All CV leads were successfully removed without any major complications, except for 1 in-hospital death. Successful CV lead removal by simple traction (ST) was achieved in 21 patients (62%), whereas extraction tools were required in 13 patients (38%). Local infection and CV lead dwell time were significantly associated with successful ST (P=0.04 and P=0.014, respectively). CV lead re-implantation was successfully performed in 25 patients; however, a right-side approach was required in 92%, and occlusion/stenosis of the previous CV was observed in 80% of the patients.
Conclusions: CV lead removal is relatively successful and safe. The presence of local infection and a shorter lead duration may enable successful ST of a CV lead. However, the re-implantation procedure should be well prepared for the complexity related to the right-side approach and occlusion/stenosis of the previous CV.
Cardiac resynchronization therapy (CRT) is an established cardiac device therapy used to improve symptoms and reduce mortality in heart failure (HF) patients with left ventricular (LV) dysfunction and QRS prolongation.1–3 As indications for CRT have expanded, the frequency of CRT device removal and reimplantation due to infection or dysfunction have also increased.4,5 Patients with a CRT device are known to be at high risk of device infection;6,7 moreover, these patients could be at high risk for the device removal procedure due to severe HF status. Coronary venous (CV) lead failure is also one of the critical complications leading to CRT suspension; therefore, CV lead removal and reimplantation is necessary for these patients. However, there have been few reports on the technical issues, complications, and the clinical outcomes of percutaneous CV lead removal and reimplantation.
A CV lead could be easily removed by simple manual traction, but some extraction tool(s) for mechanical dissection of adhesive tissue may be required due to an increase in dwell time of the CV lead.8–11 However, predictors to distinguish the technical difficulty of CV lead removal have not been investigated. In addition, a CV lead reimplantation procedure could be complicated due to right-sided reimplantation or former CV stenosis/occlusion.12,13 However, there have been few studies on procedural and clinical outcomes in patients undergoing CV lead reimplantation.14,15
Therefore, this study aimed to: (1) elucidate the predictors for successful simple manual traction for CV lead removal; and (2) evaluate the procedural and clinical outcomes in patients undergoing reimplantation of a CRT device.
A total of 345 patients who underwent CIEDs removal between August 2008 to June 2020 at our hospital were reviewed for study eligibility. Of these, 34 consecutive patients who required CRT device removal were enrolled in this study. Lead removal for transvenous LV pacing leads with active fixation mechanisms such as StarFix® (Medtronic, MN, USA), Attain stability® (Medtronic, MN, USA), or trans-septal endocardial LV leads, were excluded. This study was a retrospective study approved by the Ethics Committee of the Tokyo Women’s Medical University (approval number: 2020-0015) and conducted in accordance with the principles of the Declaration of Helsinki. Written informed consent was obtained from all patients or their guardians.
CV Lead ExtractionThe indications of CV lead removal were decided in accordance with the expert consensus statement on lead management and extraction.16–18 Based on the consensus of all our medical staff, the lead extraction procedure was performed under general anesthesia with transesophageal ultrasound monitoring in the hybrid operation room for high-risk patients, whereas the remaining patients had the procedure performed under local anesthesia in the catheter laboratory. The standard strategy for CV lead removal in our institution was as follows: (1) a standard stylet was utilized and a simple traction was attempted as the first approach for CV lead removal; (2) if the manual traction was unsuccessful, then further manual traction using a locking stylet was attempted; (3) if the dissection of adherent fibrotic tissue was required, a non-powered polypropylene mechanical sheath or a laser sheath was used; and (4) a femoral approach was also considered when the CV lead removal by a superior approach was difficult despite lead mobility within the implanted vein. The lead removal strategy was entrusted to the operator; however, there were differences in the tools approved for use in Japan during the study period.
Similar to the indication for lead removal, the definitions of procedural success, complications, and all medical terms were used in accordance with the criteria organized by the expert consensus statement.16 Simple traction was defined as successful manual traction without any extraction tools, whereas lead extraction was defined as lead removal with any extraction tool(s). Accordingly, study patients were divided into the simple traction group (ST) and the lead extraction tools group (ET) to compare their baseline characteristics.
Reimplantation of a CV LeadThe need for continuation of CRT in patients undergoing CV lead removal was always reassessed after lead removal. The CRT reimplantation procedures were performed after an appropriate period of antibiotic therapy (median 24 days, interquartile range 14–48 days) in patients that were indicated for extraction due to infection, whereas reimplantation was performed immediately after extraction in patients with a non-infection indication for extraction. The reimplantation was performed contralaterally in patients with infection, whereas the re-implantation site was ipsilateral in the latter non-infected patients. The reimplantation procedures were performed in a standard fashion similar to a new implantation. After occlusive venography of the coronary vein, a transvenous CV lead was implanted in a CV branch with commercially available lead delivery tools. Twelve-lead ECG and intracardiac electrograms were simultaneously recorded using a digital recording system (Axiom Sensis XP; Siemens, Germany).
Follow up was performed by a review of medical records, device interrogation, and discharge summaries for inpatient hospitalizations for all study patients. The endpoint was defined as a composite event of total mortality, heart failure hospitalization, local or systemic reinfection, and ventricular arrhythmic events including appropriate defibrillator therapy.
Statistical AnalysisAll statistical analyses were performed using JMP version 15.0 (SAS Institute, Cary, NC, USA). Continuous variables are expressed as the mean±SD, whereas categorical variables are given as frequency and percentage. Comparisons between continuous variables were made by using the Wilcoxon exact test, whereas categorical variables were analyzed using Fisher’s exact test. Receiver operating characteristic (ROC) curve analysis was performed to determine the optimal cut-off value of the dwell time of CV lead for predicting successful simple traction. A multivariate logistic regression model was constructed to assess the association of clinical variables to predict technical difficulty of CV lead extraction. Variables reaching P<0.10 on univariate analysis were included in multivariate models. Both the number of leads for removal and the removal for only CV lead had a significant statistical correlation with the presence of local infection; therefore, those variables were excluded from the multivariate analysis to avoid bias due to confounding. Consequently, the multivariate analysis was constructed with the presence of local infection, the dwell time of the CV lead, and the use of a quadripolar CV lead. Cumulative event rates were assessed by using the Kaplan-Meier method. A 2-tailed P<0.05 was considered statistically significant.
A total of 345 patients were admitted to our hospital for CV lead extraction. Of these, 34 patients (10%) who were referred for CV lead extraction were enrolled in this study. Mean age was 64.0±10.3 years, 28 patients were male (82%), and 31 patients (91%) had a CRTD device (Table 1). Nine patients were classified as New York Heart Association (NYHA) III and IV, and the mean LVEF was 30.6±11.1% at the extraction procedure. The lead extraction was indicated for systemic or local infection in 29 patients (85%) and for malfunctions in 5 patients (15%). Mean dwell time of the CV leads was 4.1±3.8 years (median 2.4 years, interquartile range [IQR] 0.9–7.0 years). The quadripolar lead was implanted in 9 patients (27%) and mean French size of the target CV leads was 5.4±0.8 Fr (Table 2).
| Total (N=34) |
Simple traction group (N=21) |
Extraction tool group (N=13) |
P value | |
|---|---|---|---|---|
| Age at removal (years) | 64.0±10.3 | 63.6±11.4 | 64.8±8.7 | |
| Male gender (%) | 28 (82) | 16 (76) | 12 (92) | 0.37 |
| NYHA III/IV (%) | 9 (26) | 4 (19) | 5 (38) | 0.25 |
| Ischemic etiology (%) | 11 (32) | 8 (38) | 3 (23) | 0.47 |
| CRTD device (%) | 31 (91) | 18 (86) | 13 (100) | 0.27 |
| Hypertension (%) | 15 (44) | 9 (43) | 6 (46) | 1 |
| Diabetes mellitus (%) | 13 (38) | 8 (38) | 5 (38) | 1 |
| Renal impairment (eGFR <60 mL/min/1.73 m2) (%) | 23 (68) | 15 (71) | 8 (62) | 0.71 |
| Stroke (%) | 5 (15) | 4 (19) | 1 (8) | 0.63 |
| Atrial fibrillation (%) | 17 (50) | 10 (48) | 7 (54) | 1 |
| LVEF (%) | 30.6±11.1 | 31.8±11.9 | 28.8±9.8 | 0.38 |
| LVEDD (mm) | 64.1±11.5 | 63.3±12.0 | 65.5±11.0 | 0.52 |
| LVESD (mm) | 55.0±12.8 | 53.5±13.1 | 57.5±12.5 | 0.42 |
Values are expressed as mean±SD or number (%). CRTD, cardiac resynchronization therapy with defibrillation device; eGFR, estimated glomerular filtration rate; NYHA, New York Heart Association classification; LVEDD, left ventricular end-diastolic dimension; LVEF, left ventricular ejection fraction; LVESD, left ventricular end-systolic dimension.
| Total (N=34) |
Simple traction group (N=21) |
Extraction tool group (N=13) |
P value | |
|---|---|---|---|---|
| Infection indication (%) | 29 (85) | 20 (95) | 9 (69) | 0.06 |
| Systemic infection (%) | 13 (38) | 7 (33) | 6 (46) | 0.49 |
| Local infection (%) | 21 (62) | 16 (76) | 5 (38) | 0.04 |
| Non-infection indication (%) | 5 (15) | 1 (5) | 4 (31) | 0.06 |
| No. of implanted leads at removal | 3.2±0.4 | 3.2±0.4 | 3.2±0.4 | 0.79 |
| No. of leads for removal | 2.9±0.8 | 3.1±0.5 | 2.4±1.0 | 0.01 |
| Removal for CV lead only (%) | 4 (12) | 0 (0) | 4 (31) | 0.02 |
| Removal for more than one lead (%) | 30 (88) | 21 (100) | 9 (69) | |
| Mean CV lead dwell time (years) | 4.1±3.8 | 5.0±6.3 | 7.6±3.9 | 0.03 |
| Median CV lead dwell time (years) (IQR) | 2.4 (0.9–7.0) | 1.7 (0.8–8.5) | 6.7 (5.6–9.6) | |
| Quadripolar CV lead (%) | 9 (26) | 8 (38) | 1 (8) | 0.11 |
| CV lead size (Fr.) | 5.4±0.8 | 5.3±0.8 | 5.5±1.0 | 0.44 |
Values are expressed as mean±SD or number (%). CV, coronary venous; Fr., French size; IQR, interquartile range; No., number.
According to the technical difficulty of CV lead removal, patients were divided into the ST group (21 patients, 62%) and ET group (13 patients, 38%). Physical background was similar between these 2 groups (Table 1); however, the indication of CV lead extraction due to local infection was significantly higher (76% vs. 38%, P=0.04) and the mean dwell time of the CV lead was significantly shorter (5.0±6.3 years vs. 7.6±3.9 years, P=0.03) in the ST group compared to the ET group (Table 2). After ROC curve analysis for optimal cut-off value of CV lead dwell time to predict simple traction, 4.7 years of dwell time had a sensitivity and specificity of 90.5% and 84.6%, respectively, with the area under the curve of 0.88. A multivariate logistic regression analysis showed that CV lead extraction for local infection (OR 11.2, 95% CI 1.42–180.23, P=0.04) and the dwell time of the CV lead (OR 0.63, 95% CI 0.41–0.86, P=0.014) were independent predictors of successful simple traction (Table 3).
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Age at extraction (1-year increase) | 0.99 | 0.92–1.06 | 0.74 | – | – | |
| Male gender | 0.27 | 0.01–1.95 | 0.25 | – | – | |
| Ischemic etiology | 2.05 | 0.43–9.78 | 0.37 | – | – | |
| LVEF (1% increase) | 1.03 | 0.96–1.10 | 0.44 | – | – | |
| Presence of local infection | 5.12 | 1.19–25.07 | 0.03 | 11.2 | 1.42–180.23 | 0.04 |
| Presence of systemic infection | 0.58 | 0.14–2.43 | 0.46 | – | – | |
| Dwell time of CV lead (1-year increase) | 0.64 | 0.46–0.83 | <0.01 | 0.63 | 0.41–0.86 | 0.014 |
| Use of Quadripolar CV lead | 7.38 | 1.11–147.64 | 0.08 | 3.79 | 0.34–93.31 | 0.31 |
| French size of CV lead (1 Fr increase) | 0.71 | 0.29–1.66 | 0.43 | – | – | |
Variables with P<0.05 were included in the multivariate models. CI, confidence interval; OR, odds ratio. Other abbreviations as in Tables 1,2.
A CV lead extraction tool(s) was required in 13 patients, whereas simple traction sufficed in 21 patients. In the ET group, the locking stylet was used in 5 patients and only 1 patient had a successful extraction by manual traction (Table 4). In the remaining 4 patients who required a locking stylet and the other 8 patients that required a standard stylet, a powered/non-powered sheath with a superior approach or a femoral approach using snares were required. The non-powered polypropylene mechanical sheath (Byrd dilator sheath; Cook Medical, PA, USA) and the excimer laser sheath (SLSII; Spectranetics, CO, USA) were required in 7 patients (54%) and 6 patients (46%), respectively. Of these, 8 patients underwent a successful extraction using the superior approach. However, the femoral approach, which required a snare or EP catheters, was needed in the remaining 4 patients. Tight adhesive sites where mechanical dissection was required were observed from the entry site to the superior vena cava in more than half of the ET group patients. Tight adhesion tissue was also observed at the coronary sinus ostium in 5 patients; however, mechanical dissection was not required within the coronary vein (Table 4). All patients who required the femoral approach had an adhesion site at the coronary sinus ostium.
| Extraction tool group (N=13) |
|
|---|---|
| CV lead extraction method | |
| Use of Locking stylet (%) | 5 (38) |
| Locking stylet + traction (%) | 1 (8) |
| Use of non-powered polypropylene mechanical sheath (%) | 7 (54) |
| Use of laser sheath (%) | 6 (46) |
| Inferior approach (%) | 4 (31) |
| Adhesive point requiring mechanical dissection | |
| Entry site at subclavian vein (%) | 8 (62) |
| Innominate vein (%) | 6 (46) |
| Superior vena cava (%) | 6 (46) |
| Coronary sinus ostium (%) | 5 (38) |
| Coronary vein (%) | 0 (0) |
Values are expressed as number (%). CV, coronary vein.
Complete procedural success in CV lead extraction was achieved in all patients, whereas complete success in total leads was achieved in 32 out of 34 patients (94%). The remaining 2 patients were partial successes because a small tip of the right atrial lead remained in situ in 1 patient and a tip of a broken right atrial lead had reached the distal portion of the pulmonary artery. During the extraction procedure and/or in the postoperative period, continuous infusion of catecholamine(s) was required in 18 patients (53%), and mechanical hemodynamic support by intra-aortic balloon pumping (IABP) was needed in 4 patients (12%). Temporary pacing was required after lead extraction in 16 patients (47%) for treatment of bradycardia and/or management of hemodynamics. Of these, 7 patients were tolerable to non-physiological VVI pacing; however, physiological pacing such as AAI or DDD pacing was required in 2 and 7 patients, respectively, to avoid the deterioration of hemodynamics due to VVI pacing. Regarding major complications, in-hospital death occurred in 1 patient (3%) after the lead extraction procedure but before reimplantation. This patient was a 59-year-old male treated with a CRT-D device for 6 years due to ischemic cardiomyopathy. During his hospitalization for treatment of acute decompensated HF, he developed local and systemic infection requiring removal of the leads. The procedure was performed under the general anesthesia, supported with continuous catecholamine infusion and IABP. The CV lead and RA lead were extracted using locking stylets and non-powered polypropylene mechanical sheaths, whereas a 14Fr laser sheath was required for the RV lead extraction. The leads extraction procedure was successful without procedure-related complications. Five days after the procedure, he suffered from uncontrollable ventricular fibrillation consequent to deterioration of HF, and died. This major complication was not directly related to the lead extraction procedure, but was due to the postoperative HF deterioration. Any other major complications or minor complications were not observed during the perioperative period in the remaining patients.
Reimplantation of a CV LeadAfter the CV lead extraction procedure, 3 patients were no longer indicated for CRT, 2 patients were deemed to be at too high risk for a reimplantation procedure, and 1 patient died prior to reimplantation. The remaining 3 patients were transferred to the previous hospital for additional treatment. The CRT reimplantation was performed in 25 patients (74%) (Table 5). CV lead reimplantation was successfully performed in all patients; however, several technical issues were encountered. Right-sided reimplantation was required in 92% of the patients and the occlusive coronary venous venography revealed complete occlusion or severe stenosis at the former CV branch in 80% of the patients (Table 6). Therefore, CV lead reimplantation in the same CV branch was achieved only in 6 patients (24%), whereas CV lead reimplantation in the other CV branch was required in 19 patients (76%). No predictive factor for former CV branch occlusion/stenosis was found. Nevertheless, there was no significant difference in QRS duration between pre-extraction and post-reimplantation (162.0±24.2 vs. 157.4±22.0, P=0.16).
| Age at reimplantation (years) | 64.5±10.3 |
| Male gender (%) | 20 (80) |
| Infection indication for lead removal (%) | 21 (84) |
| Left side CRT device implantation before extraction (%) | 24 (96) |
| NYHA III/IV (%) | 6 (24) |
| Ischemic etiology (%) | 5 (20) |
| QRS duration before extraction | 162.0±24.2 |
| Reimplantation device, CRTD (%) | 22 (88) |
| LVEF (%) | 31.6±10.1 |
| LVEDD (mm) | 64.3±11.1 |
| LVESD (mm) | 55.0±12.7 |
Values are expressed as mean±SD or number (%). Abbreviations as in Tables 1,2.
| Successful CV lead reimplantation (%) | 25 (100) |
| Time from extraction to reimplantation (days) | 26.8±19.2 |
| Right-sided reimplantation (%) | 23 (92) |
| Patency of the former coronary vein (%) | |
| Total occlusion | 15 (60) |
| Stenosis | 5 (20) |
| Patent | 5 (20) |
| CV lead reimplantation at the previous position (%) | 3 (12) |
| CV lead reimplantation within the same CV branch (%) | 6 (24) |
| CV lead position (%) | |
| Postero-lateral | 6 (24) |
| Lateral | 11 (44) |
| Antero-Lateral | 8 (32) |
| Apical | 2 (8) |
| Mid | 15 (60) |
| Basal | 8 (32) |
| Biventricular pacing QRSd after reimplantation (ms) | 157.4±22.0 |
| Delta QRSd between pre-extraction and post re-implantation (ms) | −5.6±27.3 |
Values are expressed as number (%). CV, coronary vein; QRSd, QRS duration.
During the median follow-up period of 29.7 months (IQR 7.9–52.4 months), a total of 6 patients (24%) reached the composite endpoint: 2 patients died (1 death from unknown causes and 1 death due to HF). Two patients were referred for HF worsening, whereas 3 patients suffered from an electrical storm with multiple appropriate defibrillations for ventricular arrhythmia. Cumulative event-free rate at 2 years of follow up was 74.5% (Figure).
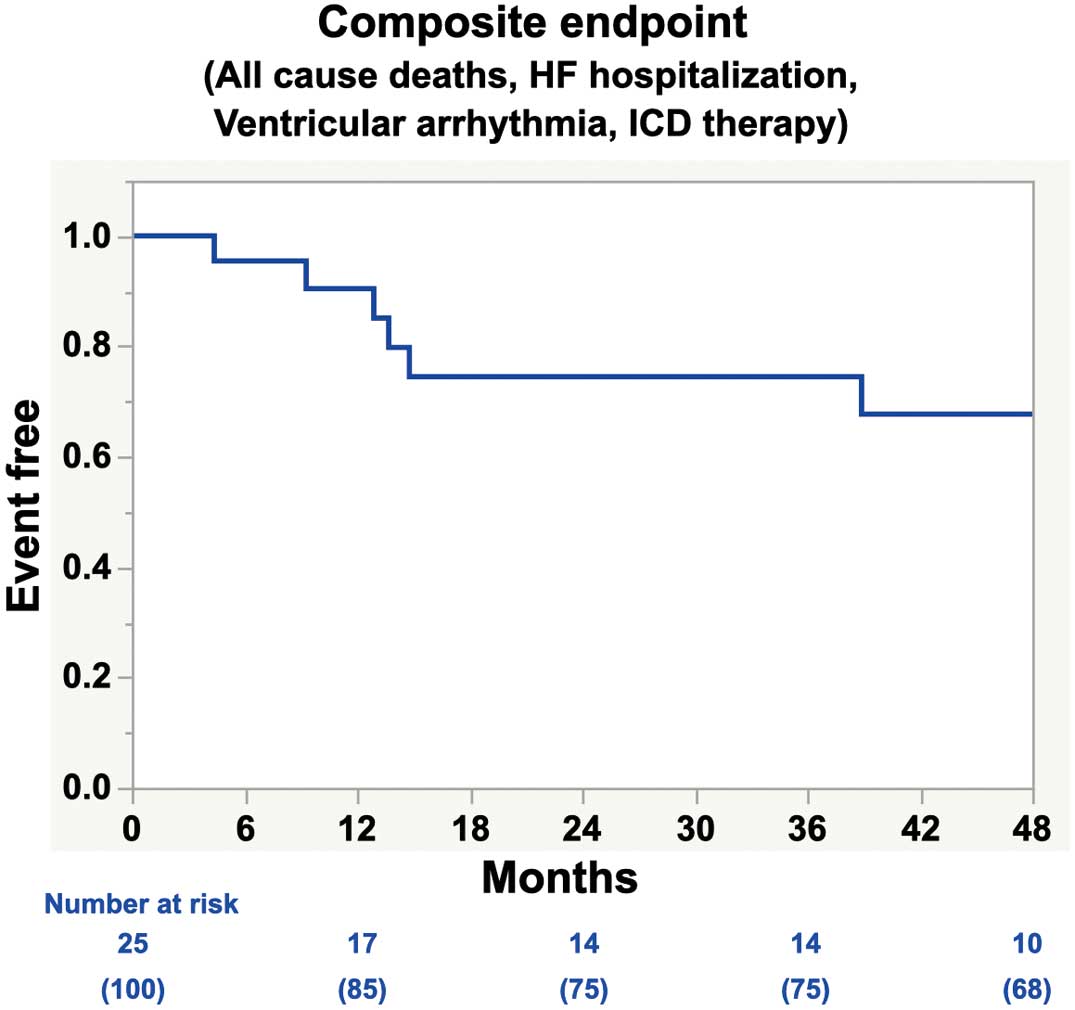
Kaplan-Meier survival curves for the composite endpoints in patients who underwent cardiac resynchronization therapy (CRT) reimplantation. The composite endpoint includes all cause death, heart failure hospitalization, ventricular arrhythmia, and the appropriate defibrillator therapy. HF, in heart failure; ICD, implantable cardioverter defibrillator.
This study elucidated technical and clinical features of CV lead removal and reimplantation in patients with CRT based on our 12-year experience of lead extraction. Our major findings are as follows: (1) among the patients referred for CIEDs removal, 10% of those patients had a CRT device; (2) of these, CV lead was successfully removed by simple traction in 60% of the patients, whereas nearly 40% of the patients required some extraction tool(s) for CV lead extraction; (3) local infection and the dwell time of the CV lead were independent predictors for successful simple traction; (4) the CV lead extraction procedure showed a remarkably high success rate with relatively low complication risk; (5) for CV lead reimplantation, most of the patients required right-sided reimplantation and 80% of the patients showed occlusion/stenosis at the former CV branch; however, CV lead was successfully reimplanted in all patients; and (6) the event-free survival for the composite endpoint at 2 years follow up was 74.5%.
Local Infection and Dwell Time are Associated With the Successful Simple Traction of the CV LeadCV lead removal by simple traction has been successful in 73–90% of patients, and the final clinical success rate of CV lead extraction has been high in recently conducted clinical studies.8,9,19,20 Similarly, the proportion of the ST group patients and the ET group patients were 62% and 38% in this study, respectively, and the final clinical success of CV lead extraction was 100%. A CV lead seems to be easily removed by simple traction; however, some extraction tools are required in some patients for CV lead extraction. Bongiorni et al reported that the locations of tight adherence were observed from entry site to the subclavian vein in 60% of patients and from the innominate vein to the superior vena cava in 20–30% of patients; therefore, nearly 30% of the patients required mechanical dissection of adhesive tissue for CV lead extraction in their study.19 This study similarly demonstrated that tight adhesion was observed from the entry site to the subclavian and innominate vein in 62% and 46% of the ET group patients, respectively.
Regarding the selection of extraction tools, a laser sheath was mainly used in patients with an infection indication; however, Evolution® (Cook medical, IN, USA) was not used so as to avoid venous laceration due to the difference between CV lead diameter and the sheath size. In contrast, in patients with a non-infection indication, a non-powered polypropylene mechanical sheath was used mainly to avoid collateral damage to other leads by laser attempts. We also had successful CV lead extraction by using the femoral approach in 5 patients whose CV lead was tightly adhered to the coronary sinus ostium. When the superior approach was difficult, despite lead mobility, we attempted the femoral approach, because it was possible to change the pulling direction, and it was effective in releasing the adhesion at the coronary sinus ostium or within the CV branches. Mechanical dissection of tight adhesive tissue at the coronary sinus ostium or within the CV branch may be considered to be at high risk of venous laceration; therefore, the femoral approach may be a reasonable solution in these patients.
The presence of local infection and the dwell time of the CV lead were independent predictors for successful simple traction of the CV lead. By logic, a longer in-situ CV lead may have tightly adhered to various venous portions. In fact, recent reports have shown that patients requiring extraction tools for CV lead removal had longer dwell time of the CV lead. Sheldon et al demonstrated that longer duration since implantation and larger lead diameter were associated with the complexity of CV lead removal.10 However, the optimal cut-off value of dwell time for successful simple manual traction has not been evaluated. The present study found that 4.7 years of dwell time of the CV lead can predict successful simple traction regardless of the indication for extraction. Williams et al reported that CV leads requiring laser sheath for mechanical dissection had an average 4.1 years of dwell time.11 Hamid et al and Rickard et al similarly demonstrated 5.2 years8 and 4.8 years9 of dwell time, respectively, for CV lead extraction requiring laser sheath; therefore, our proposed cut-off value of 4.7 years of dwell time for CV lead removal by simple traction is reasonable.
The dissolution of adhesive tissue may enable lead mobility which is an important factor for successful simple traction. This study demonstrated that local pocket infection rather than systemic infection was an independent predictor of simple traction; therefore, a bacterial infection that may lead to the dissolution of adhesive tissue from the entry site to the subclavian vein may have contributed to lead mobility and successful simple traction. Our findings could be useful for preparation and strategy for CV lead removal.
The success rate of the CV lead removal was relatively high without procedure-related complications; however, one in-hospital death was observed in this study. This major complication occurred due to worsening of HF after the CV lead extraction, and was not directly related to the procedure. The HF condition could be deteriorated in patients treated with CRT, especially associated with systemic infection or septic shock. Therefore, HF management throughout the perioperative period involving procedures such as catecholamine infusion, IABP support or utilization of temporary physiological pacing such as AAI, DDD or CRT pacing rather than VVI pacing, after lead extraction may be needed. Careful hemodynamics management is critical throughout the perioperative period.
Technical Features of a CV Lead Reimplantation and Clinical OutcomeCV lead reimplantation procedures may encounter technical difficulties such as requiring a right-side approach, or venous occlusion/stenosis in the former CV branch. In general, the initial CRT device implantation is preferably performed from the left side unless there is a specific reason not to do so; however, the reimplantation of a new device from the opposite site has been recommended in patients who had undergone lead extraction due to infection. Consequently, the rate of right-sided reimplantation of a CRT device would be inevitably high. Zucchelli et al studied CV lead reimplantation procedures in a relatively large population, and right-side reimplantation was required in 70% of the patients and CV venoplasty was needed in 2 patients; however, the success rate was 95.6% and reimplantation at the postero-lateral area was feasible in 62% of the patients.13 Similarly, this study demonstrated that a right-side reimplantation was required in most patients and CV occlusion/stenosis was commonly observed in 80% of the patients. The complication risks of CV venoplasty may be avoided by replacing the CV lead into the other CV branch as a first approach. Unfortunately, any predictor or tendencies for former CV occlusion/stenosis was not found in the present study; therefore, further large studies are warranted. However, CV lead reimplantation was successful in all patients and there was no significant difference in biventricular pacing QRS duration between pre-extraction and after reimplantation.
There have been few reports regarding prognosis after CV lead reimplantation. The 1-year and 2-year mortality after CRT device reimplantation has been reported to be approximately 5–10%13,15 and approximately 20%,14,15 respectively. The cumulative composite event-free rate in this study was acceptable at 74.5% at 2-years and is comparable to recent reports. In addition, reinfection was not observed during the follow-up period in our population. The timings of reimplantation and contralateral reimplantation in infected patients have strictly adhered to the expert consensus statement,17,18 and these might have reduced the reinfection rate in this study.
Study LimitationsThis study has several limitations. First, this was a single center retrospective cohort study and was not sufficiently sized to evaluate clinical outcomes; therefore, further large prospective studies are warranted. Second, patients who required CRT device removal since 2008 were included in this study; however, the laser sheath has been approved since 2009 in our country. The extraction strategy might be affected due to differences in extraction tools approved in Japan. Third, CV leads with active fixation mechanisms and trans-septal LV endocardial pacing leads were excluded in this study because those leads have not been approved and StarFix® was less frequently used in Japan during the study period. Finally, the first CRT device implantation was not always performed at our hospital; therefore, the analyses regarding CV anatomy at the first implantation was not possible in this study.
The removal of CV leads with passive fixation mechanisms could be relatively successful, and the presence of local infection and the dwell time of the CV lead were associated with successful simple manual traction. However, careful hemodynamics management throughout the perioperative period is essential to avoid serious deterioration of HF. In addition, the reimplantation procedure should be well prepared for the complexity related to a right-side approach and CV occlusion/stenosis that can be encountered in a significant number of patients.
None.
N.H. is a member of Circulation Journal’s Editorial Team. M.S. and K.E. belong to an endowed department established by contributions from Medtronic Japan, Boston-Scientific, Biotronik Japan, and Abbott Medical. The other authors do not have grants, conflicts, or relationships with industry to declare.
This study was approved by the Ethics Committee of the Tokyo Women’s Medical University (approval number: 2020-0015).