Abbreviations
| ACS |
acute coronary syndrome(s) |
| BP |
blood pressure |
| CABG |
coronary artery bypass grafting |
| CAC |
coronary artery calcium |
| CAD |
coronary artery disease |
| CCS |
chronic coronary syndrome(s) |
| CCTA |
coronary computed tomography angiography |
| CKD |
chronic kidney disease |
| CMR |
cardiac magnetic resonance |
| COI |
conflict of interest |
| DFR |
diastolic hyperemia-free ratio |
| dPR |
diastolic pressure ratio |
| eGFR |
estimated glomerular filtration rate |
| ESC |
European Society of Cardiology |
| FFR |
fractional flow reserve |
| FFR-CT |
fractional flow reserve-computed tomography |
| GLP-1 RA |
glucagon-like peptide-1 receptor agonists |
| iFR |
instantaneous wave-free ratio |
| JCS |
Japanese Circulation Society |
| LDL-C |
low-density lipoprotein cholesterol |
| LMCA |
left main coronary artery |
| LV |
left ventricular |
| LVEF |
left ventricular ejection fraction |
| MI |
myocardial infarction |
| MPI |
myocardial perfusion imaging |
| NHPRs |
non-hyperemic pressure ratios |
| OMT |
optimized medical therapy |
| PCI |
percutaneous coronary intervention |
| PCSK-9 |
proprotein convertase subtilisin/kexin type 9 |
| QOL |
quality of life |
| RCT |
randomized controlled trial |
| RFR |
resting full-cycle ratio |
| SAQ |
Seattle Angina Questionnaire |
| SGLT2i |
sodium/glucose cotransporter 2 inhibitor |
| SPECT |
stress single photon emission computed tomography |
Acronyms of Trials or Registries
| BARI 2D |
Bypass Angioplasty Revascularization Investigation 2 Diabetes |
| CONFIRM |
Coronary Computed Tomographic Angiography and Risk of All-Cause Mortality |
| COURAGE |
Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation |
| FAME 2 |
Fractional Flow Reserve Versus Angiography for Multivessel Evaluation 2 |
| ISCHEMIA |
Initial Invasive or Conservative Strategy for Stable Coronary Disease |
| LoDoCo2 |
Low-Dose Colchicine 2 |
| MR-INFORM |
Myocardial Perfusion CMR versus Angiography and FFR to Guide the Management of Patients
with Stable Coronary Artery Disease study |
| ORBITA |
Objective Randomised Blinded Investigation with optimal medical Therapy of Angioplasty in
stable angina |
| PROMISE |
Prospective Multicenter Imaging Study for Evaluation of Chest Pain |
| REDUCE-IT |
Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention |
| SCOT-HEART |
Scottish Computed Tomography of the HEART |
| SHARP |
Study of Heart and Renal Protection |
| SPRINT |
Systolic Blood Pressure Intervention |
| STICH |
Surgical Treatment for Ischemic Heart Failure |
| STRENGTH |
Statin Residual Risk Reduction with Epanova in High CV Risk Patients with Hypertriglyceridemia |
| SYNTAX |
anatomical synergy between percutaneous coronary intervention with taxus and cardiac
surgery |
Recommendations and Levels of Evidence
In this clinical practice guideline, the recommendations and levels of evidence are classified in accordance with the updated JCS statement, encompassing the estimated benefit in proportion to risk (Tables 1,2).
Table 1. Class of Recommendation
| Class I |
There is evidence and/or general agreement that a given procedure or treatment is effective and/or useful |
| Class II |
There is conflicting evidence and/or a divergence of opinion about the efficacy/usefulness of a given
procedure or treatment |
| Class IIa |
There is a high probability of efficacy/usefulness based on evidence and opinion |
| Class IIb |
Effectiveness/usefulness is not well established based on evidence and opinion |
| Class III |
There is evidence and/or general agreement that the procedure or treatment is not effective and/or useful,
or may even be harmful |
Class III
(No benefit) |
There is evidence and/or general agreement that the procedure or treatment is not effective and/or useful |
Class III
(Harm) |
There is evidence and/or general agreement that the procedure or treatment is harmful |
Table 2. Level of Evidence
| Level A |
Demonstrated by multiple randomized clinical trials and/or meta-analyses |
| Level B |
Demonstrated by a single randomized clinical trial or large non-randomized studies |
| Level C |
Consensus from expert opinion and/or small clinical trials (including retrospective studies and case series) |
Introduction
Preamble
Coronary artery disease (CAD) remains the leading cause of mortality and morbidity in developed countries. In patients presenting with acute coronary syndromes (ACS), timely revascularization has become the cornerstone of its management. In contrast, for patients presenting with stable CAD, major randomized controlled trials (RCTs) have not provided clear evidence on whether revascularization improves outcomes, or whether it should be offered, after more than two decades of research.1 Despite this, more than 100,000 percutaneous coronary interventions (PCIs) per year are performed for stable CAD indications in Japan, with approximately one-third of the procedures performed in apparently asymptomatic patients.2
Recently, the Initial Invasive or Conservative Strategy for Stable Coronary Disease (ISCHEMIA) trial,3 published in April 2020 and including 5,179 patients from 320 sites (including 4 sites from Japan) with stable CAD and moderate-to-severe ischemia burden, demonstrated no statistical difference in the incidence of the primary endpoint between patients assigned to initial invasive and conservative strategy; initial invasive strategy: invasive coronary angiography followed by revascularization within one month, with optimized medical therapy (OMT); conservative strategy: invasive coronary angiography reserved for failure of OMT, Appendix Tables 1,2). Furthermore, the incidence of the main secondary endpoints (myocardial infarction [MI], cardiovascular death, and death from all causes) did not differ between the two arms.
The ISCHEMIA trial demonstrated a number of important features, such as a larger study population than in previous trials, the presence of moderate-to-severe ischemia determined by non-invasive stress imaging in the majority of patients (76%), and excellent control of risk factors through OMT including lifestyle modification. The ISCHEMIA trial also highlighted the efficacy of contemporary OMT that includes goal-targeted risk factor modification for prevention of cardiovascular events and symptom relief based on patient-reported outcome. At the same time, it is also important to appropriately recognize the inclusion and exclusion criteria. For instance, patients with ACS and those with progressing to worsening or refractory type of angina were excluded from the ISCHEMIA trial.
Concurrently, revision of strategic algorithms has been suggested, accounting for present-day practice in Japan, and incorporating patient preference and the physician–patient relationship. The Japanese Circulation Society (JCS) Guidelines Committee launched the updating project for 2 previously published clinical practice guidelines; “JCS 2018 guideline on diagnosis of chronic coronary heart diseases” and “JCS 2018 guideline on revascularization of stable coronary artery disease”. This focused update aims to provide practical recommendations for the management of patients with stable CAD, in accordance with the evidence provided by recent major clinical studies. Accordingly, the guideline panel members, in collaboration with a representative committee from The Japanese Society for Cardiovascular Surgery, The Japanese Society of Nuclear Medicine, Japanese Association of Cardiovascular Intervention and Therapeutics, The Japanese Coronary Association, The Japanese Association for Thoracic Surgery, and The Japan Radiological Society, have provided updates on the following topics:
1. Systematic evaluation of pre-test probability incorporating clinical likelihood of stable CAD to guide downstream non-invasive imaging tests.
2. Optimal pathway for selection of non-invasive imaging tests based on pre-test probability/clinical likelihood sequence accounting for institutional availability.
3. Indications and timing of invasive coronary angiography and revascularization according to anatomic/functional risk and symptomatic response to OMT.
4. Early introduction of contemporary, goal-targeted OMT for event prevention and symptom relief.
Addendum: Content Not Covered Within This Focused Update
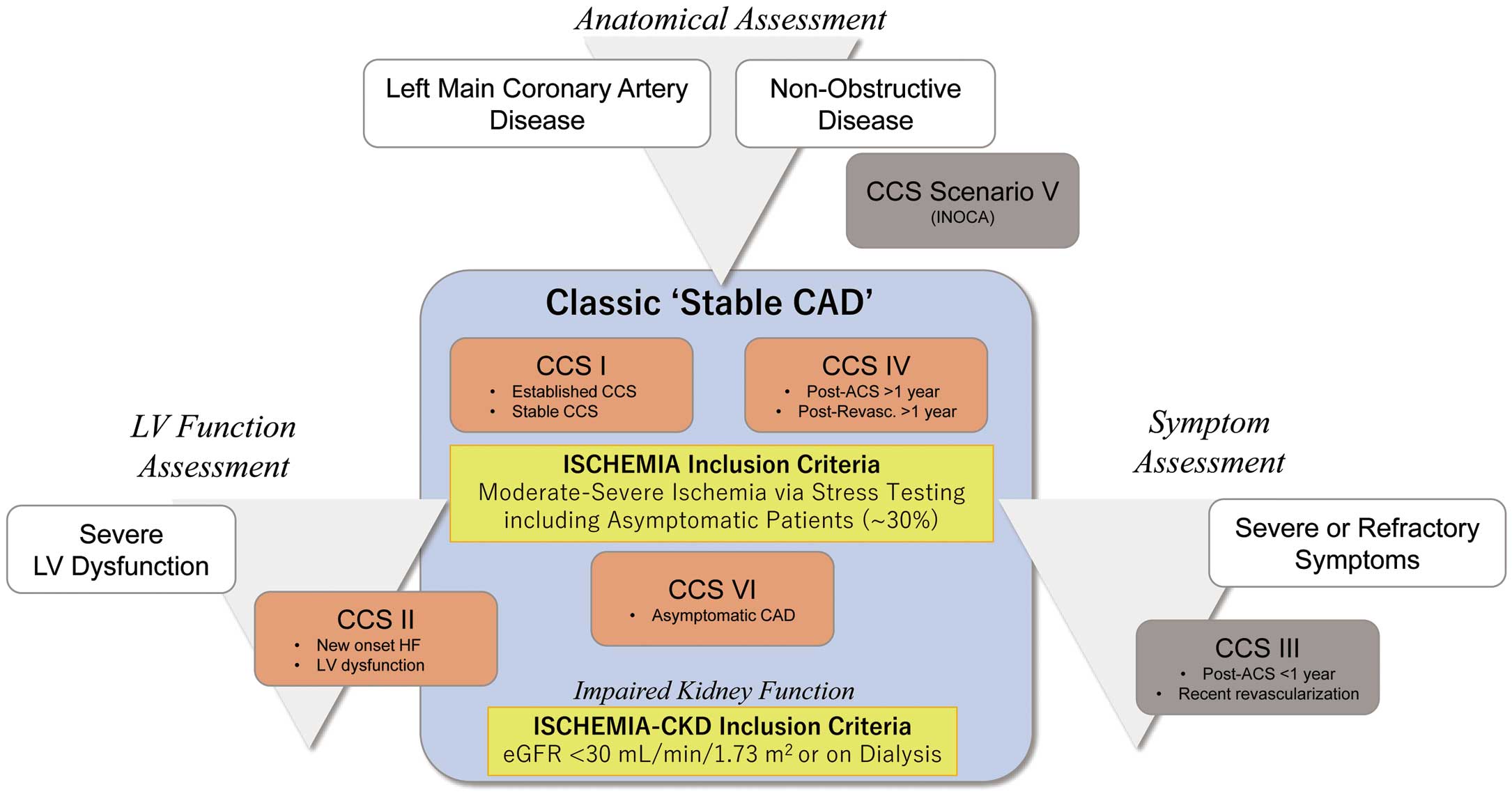
In the updated clinical practice guidelines of the European Society of Cardiology, 6 frequently encountered clinical presentation patterns in patients with suspected or established chronic coronary syndromes (CCS) were presented (Figure 1). Of these, CCS I and IV are typically referred as classic “stable CAD” patients. These 2 presentation patterns were dominant among the participants of the ISCHEMIA trial and are the main target of this update. Patients with CCS II and VI are also covered, as well as stable CAD patients with impaired kidney function (eGFR <30 mL/min/1.73 m2
or on dialysis).
Chronic Coronary Syndrome (CCS) Subtypes Covered in This Update
CCS I: Patients with suspected CAD with ‘stable’ anginal symptoms and/or dyspnea
CCS II: New onset of heart failure or left ventricular dysfunction and suspected CAD
CCS IV: Asymptomatic or symptomatic patients >1 year after initial diagnosis or revascularization
CCS VI: Asymptomatic subjects in whom CAD is detected at screening
Conversely, asymptomatic and symptomatic patients with stabilized symptoms <1 year after an ACS, or patients with recent revascularization (CCS III) and patients with angina and suspected vasospastic or microvascular disease (CCS V) are beyond the scope of this update, and are covered separately in relevant clinical practice guidelines or updates. Notably, in the ISCHEMIA trial, 17% of the patients did not have obstructive CAD; these patients are known to be at higher risk of adverse clinical events6,7 and case-based management is warranted for these patients at the present time.
I. Baseline Systematic Evaluation Including Pre-Test Probability and Clinical Likelihood of Coronary Artery Disease
Summary
Individuals with an intermediate probability of obstructive coronary artery disease (CAD) are recommended to undergo further evaluation with non-invasive cardiovascular imaging; individuals with a low probability for CAD typically do not warrant further investigation. Importantly, these low-probability patients are also excluded from modern clinical trials. Accordingly, disregarding pre-test probability (and its adjustment with clinical likelihood) will lead to improper interpretation of trials, resulting in overuse of both diagnostic imaging tests and therapeutic modalities. Of note, the pre-test probability in CAD-suspected patients has been decreasing in recent years, associated with changes in lifestyle and the advent of modern preventive cardiovascular therapeutics, leading to an increasing proportion of suspected CAD individuals assigned a low pre-test probability. Nevertheless, estimation of pre-test probability and clinical likelihood of CAD is frequently underappreciated in contemporary practice.
In this chapter, we provide a brief overview of the role of updated pre-test probability/clinical likelihood assessment to guide appropriate imaging tests, allowing for risk stratification and subsequent indication of invasive strategy. Overall recommendations for pre-test probability/clinical likelihood sequence and patient-reported outcomes in cases of suspected CAD are presented in Table 3.
Table 3. COR and LOE for PTP/CL Sequence and Patient-Reported Outcomes in Patients With Suspected CAD
| |
COR |
LOE |
Systematic baseline assessment of PTP/CL sequence is recommended as an initial step
to guide downstream testing and improve diagnostic accuracy9–11 |
I |
A |
Baseline and serial PRO evaluation should be considered for risk stratification and
comprehension of change in severity4 |
IIa |
B |
CAD, coronary artery disease; CL, clinical likelihood; COR, class of recommendation; LOE, level of evidence; PTP, pre-test probability; PRO, patient-reported outcomes.
Evaluation of patient demographics, history of present illness, including characterization of anginal symptoms, and risk factors are crucial steps for the diagnosis of stable CAD.
Classic Assessment of Anginal Symptoms
1. Chest symptoms are categorized as typical angina, atypical angina, or non-anginal chest pain, depending on the clinical characteristics. These characteristics include:
(1) constricting discomfort or pain in the substernal chest or in the neck, jaw, shoulder, or arm
(2) precipitated by physical exertion or emotional stress
(3) relieved by rest or nitrates within 5 min13–15
2. Typical angina meets all 3 characteristics, and atypical angina meets only 2. If the pain has none or only 1 of the above characteristics, it is considered non-anginal/non-cardiac chest symptoms
In addition to these classical definitions of anginal characteristics, chest symptom assessment according to its location or nature to determine if it is ischemic in origin is recommended. Chest pain with certain characteristics (e.g., central, squeezing and gripping) represents a higher probability of ischemia origin.16 Of note, the recently published 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR “Guideline for the evaluation and diagnosis of chest pain” has emphasized using the terms “cardiac”, “possibly cardiac”, or “non-cardiac” chest pain instead of “typical” or “atypical” chest pain because the traditional expressions were not helpful in determining the cause of chest symptoms and can be misinterpreted as benign in nature.16
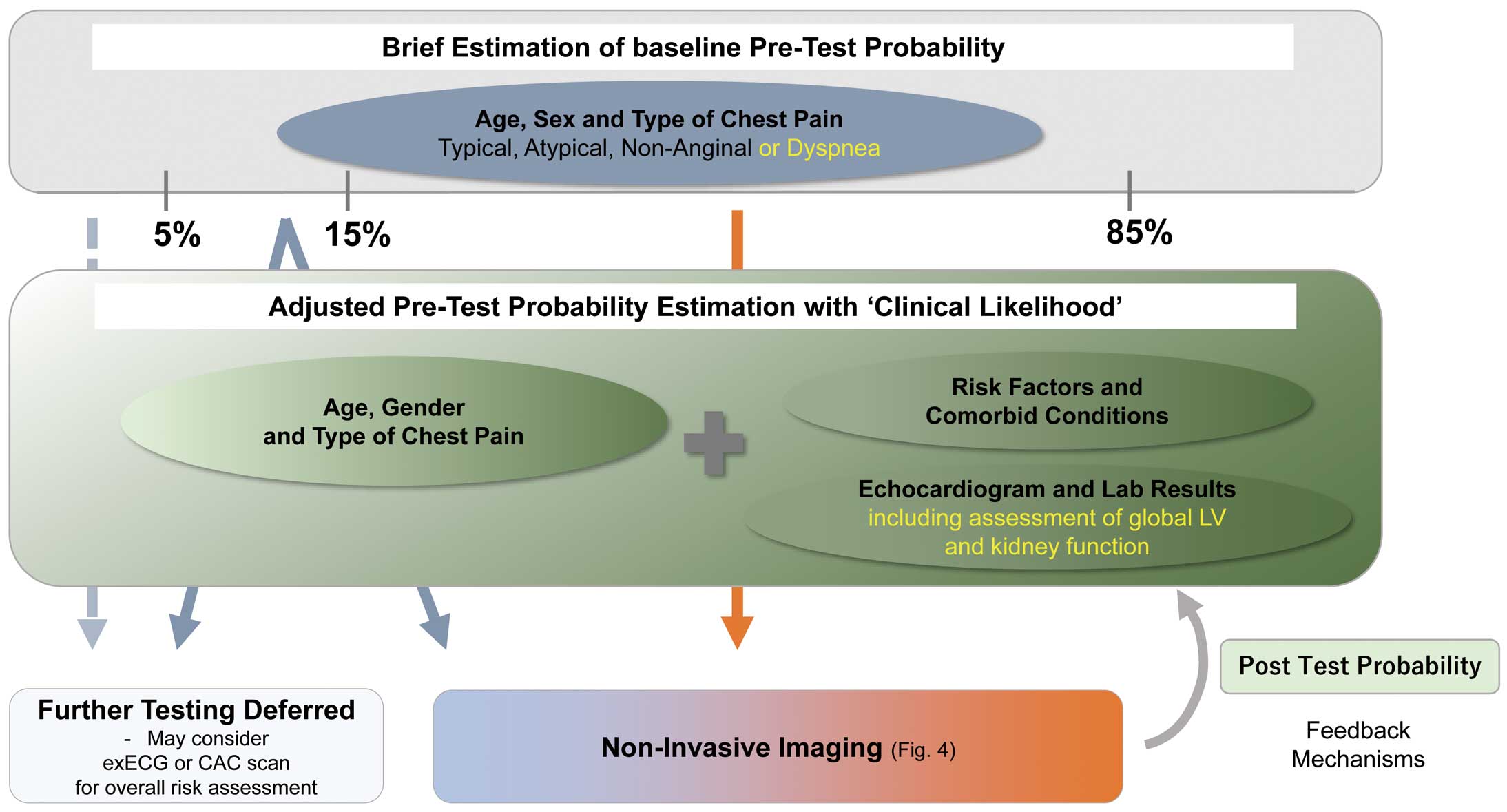
Historically, based on the above listed clinical chest symptom characteristics and patient factors such as age and sex, a pre-test probability was assigned to each patient according to Diamond and Forrester approach.15,17 Further, by incorporating clinical likelihood (i.e., comorbidities or baseline test results) to the already-estimated pre-test probability, each patient was assigned an adjusted (updated) pre-test probability, ranging from low (<5%), intermediate (5–85%), to high (>85%) pre-test probability of CAD. As stated earlier, the non-invasive testing modalities have the highest impact on a patient’s post-test likelihood of CAD in the intermediate probability group. On the other hand, patients with low pre-test probability are typically excluded from further downstream diagnostic tests, as a rare rule-in opportunity exists.17,18 The Duke Clinical Score is an alternative to pre-test probability evaluation that includes factors such as types of chest symptoms, sex, age, previous myocardial infarction (MI), smoking, hypercholesterolemia, diabetes mellitus, and electrocardiographic ST-T changes.19
1.1 Pre-Test Probability
Contemporary large-scale studies have very recently revealed that the above classic Diamond-Forrester approach results in lower diagnostic accuracy by overestimating the likelihood of CAD.20,21 In more than 4,000 suspected CAD patients who were included in the Prospective Multicenter Imaging Study for Evaluation of Chest Pain (PROMISE) trial, the proportion of patients with obstructive CAD (defined as >50% stenosis in major vessels) was only 13.9% among those classified as ‘intermediate risk’ based on the pre-test probability as defined above.10 Given these limitations, the European Society of Cardiology (ESC) guidelines published in 2019 recommend incorporating the updated pre-test probability table in the diagnostic algorithm for stable CAD.15,22 The aforementioned PROMISE trial found that >50% of patients previously defined as having an intermediate probability of CAD (15–85% pre-test probability) were reclassified as having a pre-test probability <15% according to the new guidelines.9
Within this focused update, the use of the updated pre-test probability model shown in the ESC guideline (Table 4) is encouraged for the time being.8 However, the prevalence of CAD is known to differ across regions and countries. The prevalence of disease is an essential determinant of pre-test probability. Further studies to validate the model in East Asian patients are highly anticipated.23
Table 4. Updated Pre-Test Probability of Obstructive Coronary Artery Disease According to Age, Sex and the Nature of Symptoms
| |
Typical |
Atypical |
Non-anginal |
Dyspnea |
| Age |
Men |
Women |
Men |
Women |
Men |
Women |
Men |
Women |
| 30–39 |
3% |
5% |
4% |
3% |
1% |
1% |
0% |
3% |
| 40–49 |
22% |
10% |
10% |
6% |
3% |
2% |
12% |
3% |
| 50–59 |
32% |
13% |
17% |
6% |
11% |
3% |
20% |
9% |
| 60–69 |
44% |
16% |
26% |
11% |
22% |
6% |
27% |
14% |
| 70+ |
52% |
27% |
34% |
19% |
24% |
10% |
32% |
12% |
In addition to the classic Diamond and Forrester approach, dyspnea is included as a primary symptom. Patients with pre-test probability <5% (columns with gray background) may not necessitate further non-invasive testing (see Section I.1.3) (Reprinted from the ESC guidelines. 2020.15 Reproduced by permission of Oxford University Press on behalf of the European Society of Cardiology.)
1.2 Clinical Likelihood of CAD
The ESC guidelines also highlight the use of clinical likelihood measures in the risk stratification of stable CAD. The clinical likelihood is built based on traditional risk factors (e.g., hypertension) and fundamental test results (e.g., resting electrocardiography [ECG], echocardiography, and laboratory tests, with emphasis on global left ventricular [LV] function and chronic kidney disease [CKD] assessment) as its modifiers.24 In this focused update, the suggested factors for clinical likelihood obtainable at baseline evaluation are shown in Table 5. The practical application of clinical likelihood using Fagan’s nomogram is provided as supplementary information at the end of this chapter.
Table 5. Factors Affecting Clinical Likelihood of Coronary Artery Disease
| Interview/tests |
Suggested components of
clinical likelihood |
| History |
• Previous cardiovascular disease/polyvascular disease |
• Comorbidities (e.g., hypertension, dyslipidemia, diabetes, stroke, peripheral
artery disease, CKD) |
| • Family history of premature CAD |
| • Smoking habit |
| Resting ECG |
• Abnormal Q waves |
| • ST-T segment changes |
| Resting echocardiography |
• Left ventricular (segmental/diffuse) wall motion abnormality |
| Blood/urine analysis |
• Abnormal lipid profile |
| • Abnormal blood glucose level/tolerance |
Information on factors affecting clinical likelihood is collected via medical interview and baseline testing. Pre-test probability can be updated and modified in s stepwise manner by incorporating these factors. CAD, coronary artery disease; CKD, chronic kidney disease.
1.3 Pre-Test Probability / Clinical Likelihood Sequence to Guide Downstream Testing
An optimal plot of the pre-test probability-based diagnostic algorithm for CAD is provided in Figure 2.24a After modification of baseline pre-test probability (by 2019 ESC model) in combination with clinical likelihood (i.e., estimation of adjusted pre-test probability), patients that are classified into more than intermediate pre-test probability can undergo further diagnostic tests. Of importance, with the higher testing threshold, invasive coronary angiography cannot be a first-line test for the majority of patients with intermediate pre-test probability. Therefore, non-invasive examinations, particularly non-invasive imaging, are the primary method in the diagnostic flowchart. For example, a young woman with atypical chest pain is estimated to have a baseline pre-test probability ≈3%.15 In the absence of previous CAD (i.e., negative clinical likelihood), her adjusted pre-test probability is further downgraded,27 and may not necessitate further non-invasive testing (or may optionally undergo exercise ECG or coronary artery calcium [CAC] scan for risk assessment). In contrast, for a 65-year-old man with typical angina, his pre-test probability would be estimated ≈44%,15 so proceeding to downstream non-invasive imaging testing is preferable, especially if any factors for a positive clinical likelihood, such as dyslipidemia, are present. Sequential pre-test probability and clinical likelihood assessment facilitates the reclassification of patients from intermediate to either low or high post-test probability of CAD, by allowing for optimal diagnostic performance for each testing tool.11 In summary, essential features of the contemporary initial diagnostic strategy for stable CAD are (1) brief estimation of baseline pre-test probability, (2) establishment of adjusted pre-test probability by assessing individual clinical likelihood of CAD, and (3) optimal selection of non-invasive imaging modalities based on clinical likelihood-adjusted pre-test probability.
Using patient-reported outcomes, coming directly from patients about how they feel or function in relation to a health condition and its therapy, is increasingly becoming pertinent in the initial evaluation of patients with stable CAD. Although clinicians often underestimate the effects of stable CAD on quality of life (QOL), incorporation of patient-reported outcomes has been reported to be associated with improved symptom management, QOL and prognosis.28
The Seattle Angina Questionnaire (SAQ)
The SAQ is one form of patient-reported outcomes, consisting of a self-administered questionnaire that has been validated for use in patients with CAD. The SAQ has several domains, including angina frequency, QOL, treatment satisfaction, and physical limitation.29
Practical Tips for the Use of the SAQ
• For example, scores for angina pectoris (AP) frequency are described as a score on a 100-point scale, with 100 representing no AP and 0 representing AP occurring 4 times/day. Similarly, scores for the physical limitation domain of the SAQ are also calculated on a 100-point scale, with 100 representing no limitation and 0 representing severe physical limitations because of AP
• In the ISCHEMIA trial, sequential assessment of the SAQ demonstrated that a difference in angina symptoms between invasive and non-invasive strategies becomes smaller overtime and this is more likely seen in patients with higher baseline SAQ.4 These findings suggest that the benefits of revascularization are limited to CAD patients with lower (worse) SAQ at baseline4
Initial and Serial Patient-Reported Outcomes Assessment
Assessment of patient-reported outcomes at baseline can guide optimal therapeutics to improve prognosis for patients with CAD and is prerequisite for shared decision making. Further, the SAQ correlates with the degree of effort necessary to induce angina symptoms,30 and initial patient-reported outcomes assessment also aids in ruling out near-acute coronary syndrome (ACS)/unstable angina or futile patients for downstream diagnostic and therapeutic strategies (e.g., those with high frailty). It should be noted that serial patient-reported outcomes assessment can also evaluate the effect of therapeutics chosen and requirement of non-invasive imaging tests or invasive coronary angiography during the follow-up period. When physicians identify deterioration in patient-reported outcomes during follow-up, such as decline in exertional threshold, increase in angina frequency, or more limited activity, adding further testing including non-invasive imaging may be useful for shared decision making, in view of the potential benefit of revascularization.
3. Identification of “Near-ACS” or Unstable Condition
Angina may progress, improve, or remain stable for many years. Progression of symptoms merits attention, particularly when angina occurs more frequently, is unprovoked, is more severe, or lasts longer. This may reflect progression of underlying CAD or microvascular disease. However, follow-up of stable CAD patients, particularly the high-risk patients, and effectively identifying “near-ACS” conditions remain a clinical challenge. A recent outpatient-based observational study (CLARIFY) demonstrated diverse trajectories of clinical course among stable CAD patients:31
Summary of Findings From the CLARIFY Registry
• Data on degree of angina burden, medications, implementation of coronary interventions, and clinical outcomes of 32,691 patients with stable CAD were prospectively observed
• Among 7,212 patients with angina at baseline, symptoms disappeared (without coronary revascularization) in 39.6% between baseline and year 1, with further decreases annually
• Coronary revascularization was required in 4.5% of patients, increases or changes in anti-anginal medications in 11.1%, and regression without new medical intervention in 84.4% of cases
• Angina control with medications was achieved by adding at least 1 anti-anginal drug in 46.9%, switching treatments in 40.0%, and increasing β-blocker dose in 13.0% of patients
• Conversely, in 25,479 patients without angina at baseline, 2.0–4.8% developed angina annually
• At the end of follow-up, only 3.9% of patients had anginal symptoms, 7.2% had died, 3.9% had undergone elective revascularization, and 3.3% had had either a MI or urgent revascularization
Recent studies have also indicated a substantial overlap between stable CAD and low-risk, unstable angina. When new-onset angina spontaneously subsides at rest and remains stable in patients with a low-risk profile, the condition can be reclassified from unstable angina to stable CAD, and thus, the patient can undergo the diagnostic and management flowchart for stable CAD.32 Conversely, acute coronary events can occur abruptly or after a relatively mild degree of pre-infarction angina; therefore, switching from a conservative strategy to an invasive strategy can be a clinical challenge. There remains the possibility that timely alteration of the 2 strategies is not achieved, even in the trial setting, as depicted in the higher incidence of spontaneous acute MI in the initial conservative group compared with the initial invasive group within the ISCHEMIA trial.3 Studies using a more contemporary definition of stable CAD or ACS are required to appropriately guide treatment in gray-zone patients. At present, due to lack of evidence, we do not recommend routine quantification of cardiac biomarkers during the diagnostic work-up or follow-up of stable CAD patients, unless onset or progression to ACS is suspected.
Symptom progression (e.g., worsening angina) remains the foundation of identifying “unstable” patients, albeit physicians may not accurately and reliably capture patients’ symptoms. Recently, a patient-reported outcomes approach (e.g., SAQ) have been introduced and studies have demonstrated that these scores were independently associated with the risks of subsequent death, hospitalization, and MI. For example, when evaluating 2 patients with an otherwise similar estimated risk of experiencing clinical events, a patient with a SAQ physical limitation and angina frequency score <25 points is 4-fold more likely to die and twice as likely to be hospitalized for an ACS than a patient who has similar clinical characteristics and SAQ score >75 points.30,33,35 Yet, implementation of patient-reported outcomes in routine clinical care requires the development of methods to regularly collect, score, and present the scores within the clinical workflow. Web-based or mobile application software would ideally allow the patient to complete the SAQ in the waiting room or immediately before a clinical visit, and the responses could then be integrated with the electronic health record for immediate physician review. Formal testing is needed to examine whether the routine use of patient-reported outcomes measures, such as the SAQ, can improve patient care and outcomes; however, the use of patient-reported outcomes measures has been associated with improvements in treatment and survival among patients with cancer.36
Lastly, “stable, but refractory angina” should be carefully assessed before non-invasive diagnostic tests. Patients showing anginal symptoms with a lower intensity exercise (i.e., 3–4 metabolic equivalents physical activity) or classified as Canadian Cardiovascular Society class III (or IV) have much greater mortality than those with lower angina severity.37 For these patients, when any signs or symptoms of exacerbation are observed in the diagnostic work-up, invasive coronary angiography should be considered without delay, taking their pre-test probability/clinical likelihood and risk of events into account.
In conclusion, the clinical course of stable CAD patients is highly heterogeneous, and establishing a universally applicable follow-up protocol for these patients is challenging. Serial assessment of signs and symptoms of instability using valid patient-reported outcomes measures (that quantitatively measure not only the symptoms but limitations in activities of daily life and the anxiety/concern of the patients themselves) may effectively detect progression of disease and aid in early identification of patients at risk of future adverse events.
Addendum: Adjustment of Pre-Test Probability by Clinical Likelihood
The clinical likelihood can be feasibly calculated in current practice,25,26 and can function as a “modifier ” of the baseline pre-test probability defined by the ESC model. This diagnostic approach is more dynamic and the pre-test probability is updated in a stepwise manner by adding new information.
1. When assessing a patient with suspected CAD, the presence of dyslipidemia, hypertension and abnormal ECG change should raise the clinical likelihood of CAD, resulting in an adjusted pre-test probability higher than the baseline pre-test probability. On the other hand, if a patient with the same baseline pre-test probability is substantially healthy without any traditional risk factors or abnormal findings on basic testing, the adjusted pre-test probability adjusted by clinical likelihood becomes lower than the baseline pre-test probability. This is a diagnostic process for modifying the pre-test probability of individuals by weighting various clinical information.
2. In real-world practice, patients with 5–15% pre-test probability are the predominant population; without modification of the pre-test probability, excessive use of imaging modalities is anticipated, with increasing numbers of false-positive cases and medical cost.
3. The statistical consequences of predicting pre-test probability can be simply described on the Fagan’s nomogram integrating Bayes’ theorem.25,26 For example, when conducting coronary computed tomography angiography (CCTA) with 85% sensitivity and 95% specificity (i.e., 17 of positive likelihood ratio and 0.16 of negative likelihood ratio) to a patient with 15% baseline pre-test probability, the positive post-test probability of having CAD is 75% and 3% for positive (stenosis present) and negative (stenosis absent) CCTA results, respectively. Further, if this patient’s pre-test probability is modified and raised to 35% by adding positive clinical likelihood (e.g., hypertension or diabetes), the positive CCTA result can be estimated as >90% of post-test probability for the presence of CAD (Figure 3).24a
II. Choice of Non-Invasive Imaging Tests Based on Pre-Test Probability / Clinical Likelihood Sequence and Availability
Summary
Following systematic baseline evaluation, functional or anatomical imaging modalities are recommended as the initial test considering the results from contemporary large-scale randomized controlled trials (RCTs). We strongly recommend choosing the appropriate non-invasive imaging modality based on the pre-test probability/clinical likelihood sequence and patient’s characteristics, and alter the decision based on institutional availability or preference for imaging modalities.
Patients with intermediate modified pre-test probability can undergo CCTA to rule out the presence of coronary artery disease (CAD). It should be noted that CCTA is not suitable for the patients with severe calcification (e.g., coronary calcium score >1,000), uncontrolled or irregular heart rate, or renal dysfunction. If obstructive CAD is identified by CCTA, myocardial perfusion imaging (MPI) or fractional flow reserve-computed tomography (FFR-CT, if available and considered suitable) can be applied for further risk stratification.
On the other hand, MPI is preferred as an initial imaging test in patients with a high pre-test probability or known history of CAD.
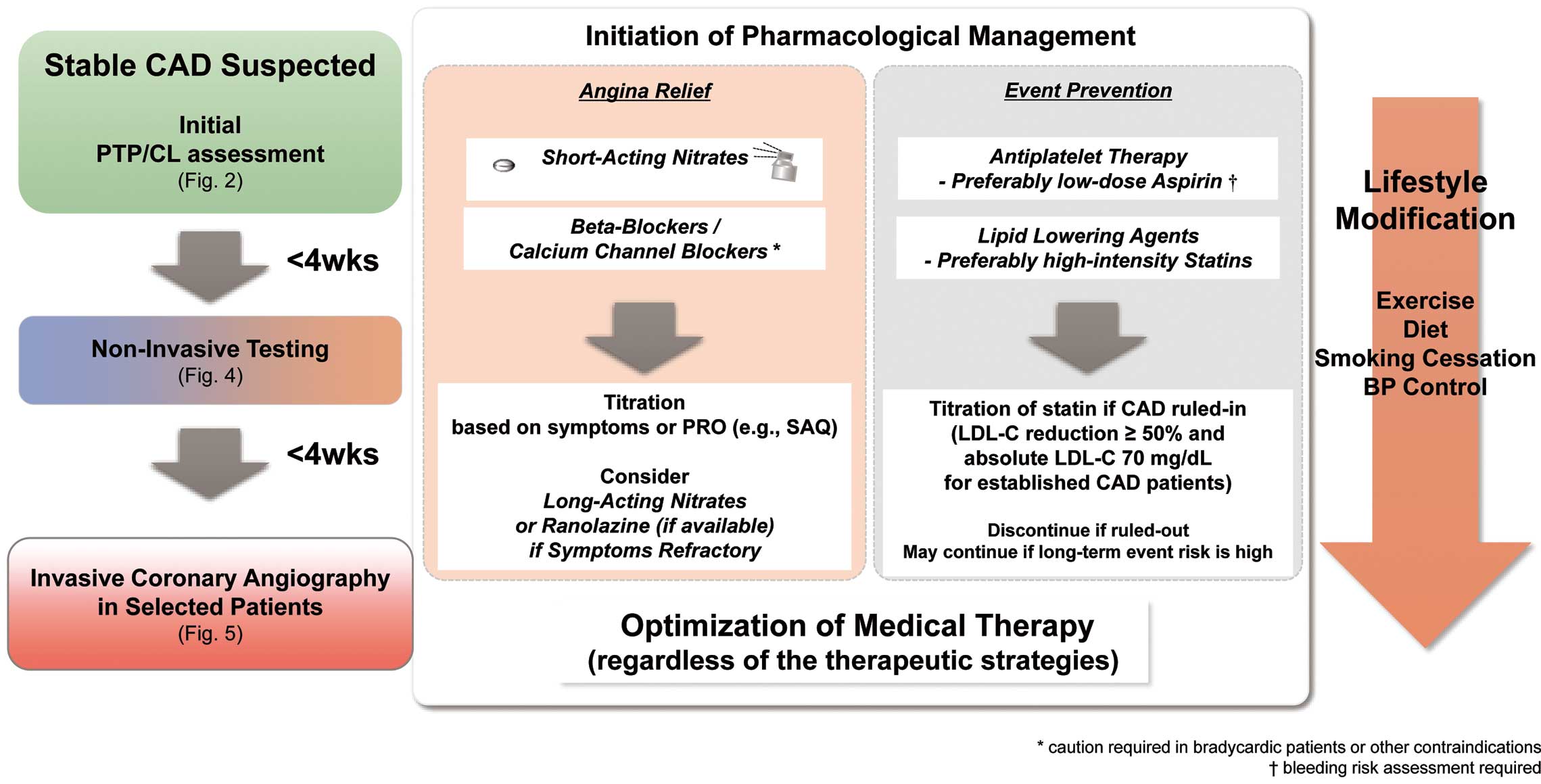
Of note, optimized medical therapy (OMT) should be initiated during the diagnostic work-up process for event prevention and symptom relief unless contraindicated (as indicated in Chapter IV). In patients with findings suggestive of left main coronary artery (LMCA)/LMCA-equivalent disease, proceeding to invasive coronary angiography may be reasonable.
1. Non-Invasive Imaging
The initial evaluation of the patient’s baseline characteristics and basic testing determines the pre-test probability/clinical likelihood of CAD, and patient-reported outcomes delineates the effects of symptoms on the patient’s quality of life (QOL). Non-invasive cardiac imaging methods (e.g., CCTA or MPI) are recommended for further diagnosis and risk stratification in patients with at least intermediate pre-test probability.38–42 In this section, we provide a downstream diagnostic flowchart for evaluating stable CAD patients based on the pre-test probability/clinical likelihood sequence and the availability of each tool, following a brief overview of recent studies on diagnostic accuracy and prognosis.
1.1 Anatomical Non-Invasive Imaging (i.e., CCTA)
CCTA has emerged as a useful diagnostic and prognostic test for patients at low to intermediate risk when presenting with symptoms consistent with angina but without a known history of CAD, as it provides excellent negative predictive value.15,43 The Coronary Computed Tomographic Angiography and Risk of All-Cause Mortality (CONFIRM) registry has reported that the absence of CAD determined by CCTA was associated with significantly improved prognosis compared with >50% coronary artery stenosis in ≥1 proximal segment.44 CCTA also has reasonable predictive value in detecting LMCA stenosis.44a
Updated Clinical Information on CCTA
• The ability to assess the extent of coronary plaque deposition is an important advantage of CCTA, and the relationship between plaque morphology and future acute coronary syndrome has been demonstrated.40,45–47 Use of CCTA has been associated with facilitation in the use of aggressive preventive therapy48
• The Scottish Computed Tomography of the HEART (SCOT-HEART) trial demonstrated that computed tomography (CT)-guided management was associated with improved patient outcomes compared with the traditional approach. This positive finding was driven by the higher implementation of OMT in the CT arm48
• Several other studies have reported that the low-attenuation plaque detected by CCTA could be a therapeutic target to facilitate preventive medicine49–51
The study protocol for the Initial Invasive or Conservative Strategy for Stable Coronary Disease (ISCHEMIA) trial required the use of CCTA prior to randomization to exclude patients with LMCA CAD and non-obstructive CAD.3 Conversely, the positive predictive value for non-LMCA CAD is moderate at best due to the overestimation of stenosis. The European Society of Cardiology (ESC) guidelines have highlighted the use of CCTA as an initial imaging modality for patients with stable CAD (though the benefit of repeated CCTA for those with known CAD is limited).15 However, caution is needed, as a number of studies have shown that substantial overestimation of organic stenosis as assessed by CCTA may increase the use of invasive coronary angiography, especially when the positive finding is not further assessed by non-invasive modalities such as MPI or other functional testing.51 Fractional flow reserve (FFR) assessment from the scanned images (i.e., FFR-CT) is also available in certain institutions (see next section).
1.2 Functional Non-Invasive Imaging
Functional non-invasive imaging tests such as nuclear stress single photon emission computed tomography (SPECT), stress MR perfusion imaging, stress echocardiography, and positron emission tomography (PET) can delineate areas of ischemic myocardium with high rule-in power in the diagnosis of obstructive CAD, and can evaluate the extent and severity of ischemia to stratify event risk. SPECT is one of the most commonly used functional imaging techniques, and numerous studies have confirmed its diagnostic accuracy and also validated its role in long-term risk prediction. SPECT uses various stress protocols as a functional testing modality.52
Updated Clinical Information on Stress Imaging
• Patients with normal perfusion are known to exhibit an excellent prognosis (<1% per year for adverse cardiac events)53
• Previous registry studies in Japan have demonstrated that the prognosis of stable CAD patients is primarily related to the extent of perfusion defects, along with left ventricular dysfunction, and the presence of diabetes mellitus and chronic kidney disease further increases the risk for cardiovascular events53
• The Myocardial Perfusion CMR vs. Angiography and FFR to Guide the Management of Patients with Stable Coronary Artery Disease (MR-INFORM) study reported that cardiac magnetic resonance (CMR)-based stress assessments were comparable to FFR in terms of prognostic utility, and, importantly, CMR-guided management strategies significantly reduced the need for invasive coronary angiography or revascularization54
• In 1 of the largest meta-analyses to date, which included 4,131 patients from 23 studies, Knuuti et al reported that CCTA and invasive coronary angiography yielded relatively lower diagnostic accuracy compared with invasive functional test in which functionally significant CAD determined by FFR was considered as reference (93% and 53% for CCTA; 68% and 73% for invasive coronary angiogram, in terms of sensitivity and specificity, respectively)11
• Quantitative assessment of myocardial flow reserve using 201thallium MPI yields high diagnostic accuracy for detection of LMCA or triple vessel disease.55 Further, myocardial flow reserve assessment using PET 13N-ammonia should be considered to detect LMCA or severe multivessel disease according to the latest JCS guideline (https://doi.org/10.1253/circj.CJ-19-1131) and the Joint Position Paper from the Society of Nuclear Medicine and American Society of Nuclear Cardiology56,57
1.3 Selection of Non-Invasive Imaging Based on Pre-Test Probability / Clinical Likelihood and Local Institutional Availability of Test Modalities
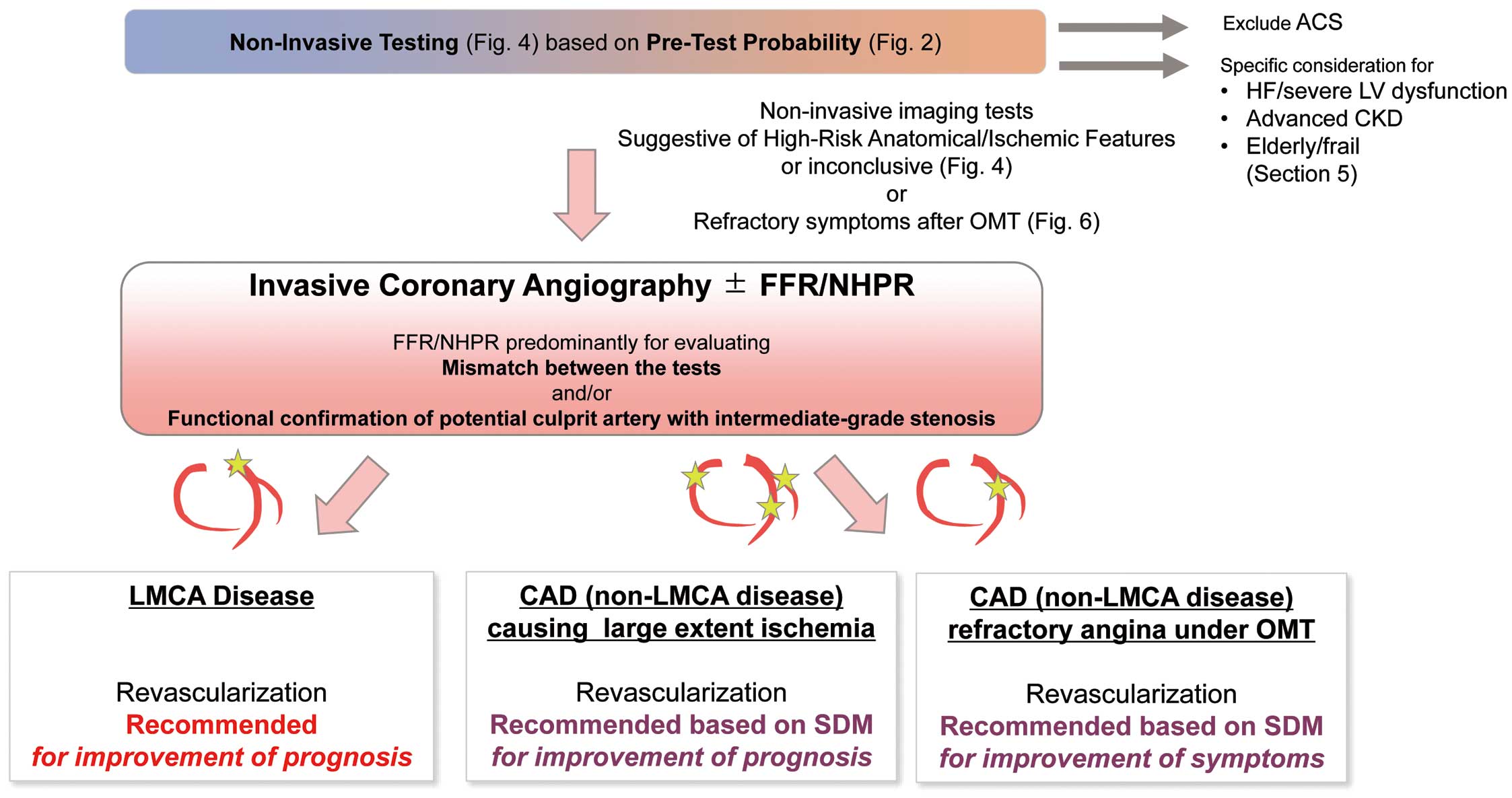
The flowchart of the proposed diagnostic pathway based on the pre-test probability/clinical likelihood sequence and availability of non-invasive testing is demonstrated in Figure 4. In accordance with the results of the ISCHEMIA trial (conservative approach is a reasonable strategy if the patient is trial eligible), a finding of moderate-to-severe ischemia alone does not prompt further invasive evaluation.3 However, proceeding to invasive coronary angiography may be reasonable if a cardiovascular event is highly expected from the overall risk assessment including patient-reported outcomes. Specific considerations for the high-risk conditions besides a large ischemic area are discussed in Chapter V. The flowchart is not applicable to patients with reduced left ventricular ejection fraction (LVEF), as the assessment of extent of inducible ischemia alone cannot stratify risk in patients with LV dysfunction.58

Suspected or established stable CAD patients with intermediate or high pre-test probability should undergo non-invasive imaging for further evaluation and management. Invasive coronary angiography is preferred if a non-invasive imaging test demonstrates findings suggestive of LMCA/LMCA-equivalent disease (e.g., extensive ischemia by functional imaging accompanied by progression of symptoms, Table 6).
Table 6. High-Risk Anatomical (for CCTA) and Ischemic (for Functional Imaging) Features Suggestive of LMCA/LMCA-Equivalent Disease
| Modality |
Findings suggestive of LMCA/LMCA-equivalent disease |
| CCTA |
• ≥50% obstruction in LMCA61 |
| • Significant stenosis in proximal LAD and dominant LCx |
| • Significant stenosis in proximal LAD and dominant RCA |
| SPECT |
• Inducible ischemic area >10% of left ventricular myocardium62 |
| • Post-ischemic myocardial stunning/transient ischemic dilatation63 |
| Stress CMR |
• Stress perfusion deficit (e.g., >4/32 LV segments)62 |
| • Stress-induced dysfunctional motion (e.g., >3/16 LV segments62) |
| Stress echocardiography |
• Stress-induced hypokinesia/akinesia (e.g., ≥3/16 LV segments62) |
Findings suggestive of LMCA/LMCA-equivalent disease include extensive ischemic area greater than the threshold used in the ISCHEMIA trial, which correspond to a median rate of coronary artery disease death or myocardial infarction of >5%/year.62 CCTA, coronary computed tomography angiography; CMR, cardiac magnetic resonance; LAD, left anterior descending artery; LCx, left circumflex artery; LMCA, left main coronary artery; LV, left ventricular; RCA, right coronary artery; SPECT, stress single photon emission computed tomography.
If a CT scanner is the only imaging device available at an institution, we propose that non-obstructive coronary artery should first be ruled out by CCTA (Figure 4 Left-upper panel). Subsequent invasive procedures should be prioritized if high-risk anatomical features, refractory angina (under OMT) or near-acute coronary syndrome (ACS) status are present. When CCTA suggests the presence of obstructive CAD other than LMCA/LMCA-equivalent disease, adding functional testing including stress imaging test and FFR-CT (if available) is ideally recommended for further risk assessment before invasive coronary angiography. Performing invasive coronary angiography in the absence of these functional studies is not recommended for the majority of stable CAD cases, given unfavorable cost-effectiveness despite similar safety margin (compared with providing functional testing in addition to CCTA) in an anatomical assessment-only approach.54 If the institution is experienced with functional stress imaging, it is suitable to mainly apply those imaging techniques for diagnosis and risk stratification (Figure 4 Right-upper panel), although contemporary registry studies have demonstrated that the availability of functional imaging modalities may be limited.59
In institutions capable of performing multimodal imaging (Figure 4 Lower panel), CCTA is the preferred imaging to rule out the presence of CAD. On the other hand, stress imaging is preferred as an initial imaging test in patients with a high pre-test probability or known history of CAD for risk assessment.
Caution When Using CCTA as a First-Line Imaging Modality
• It should be noted that acquiring high-quality CCTA images is less common in patients with inadequate heart rate control, irregular rhythm or ectopy, extensive coronary artery calcification, or large body habitus and in those with placement of intracoronary stents60
• Renal dysfunction, left bundle-branch block, artificial pacemaker, known drug allergy, exercise intolerance, and risk of radiation exposure should be allowed for in the selection of optimal initial imaging modality
Coronary Artery Calcium (CAC) Scanning
CAC scanning can be alternatively performed to rule out the presence of calcified obstructive CAD in patients with low pre-test probability. CAC scanning provides important diagnostic and prognostic information in patients with CAD, even in the presence of a heavily calcified coronary lesion. Also, CAC scanning is widely available at low cost and radiation exposure, making it a sentinel test. Alternatively, exercise ECG can be optionally performed to confirm absence of exercised-induced ischemic change in these patients (see Section II.2).
Updated Clinical Information on CAC
• Patients with CAC score of zero rarely demonstrate obstructive CAD, showing better prognosis in comparison with their counterpart (i.e., “the power of zero” philosophy)64
• However, the question as to whether CAC scan reliably differentiates non-obstructive from obstructive CAD remains controversial. Although there continues to be growing evidence regarding the “de-risking” role of zero CAC in symptomatic low- to intermediate-risk patients, there have been no large-scale studies to reassure that it could be considered a gatekeeper across the range of pre-test probability of CAD64
Fractional Flow Reserve-Computed Tomography
Recently, FFR-CT has emerged as a novel non-invasive method for detecting hemodynamic flow-limiting disease.
Updated Clinical Information on FFR-CT
• FFR-CT is useful to evaluate the functional significance of intermediate stenoses on CCTA, particularly in the setting of multivessel disease, to determine ischemia-causing artery65–67
• Adding FFR-CT to CCTA increases specificity, positive predictive value, and diagnostic accuracy over conventional CCTA68
• The 1-year outcomes from the international multicenter prospective ADVANCE FFRCT
Registry analyzing 5,083 patients showed low rates of events in all patients, with less revascularization and a trend toward lower major adverse cardiovascular events and significantly lower cardiovascular deaths or myocardial infarction in patients with a negative FFR-CT compared with patients with abnormal FFR-CT values69
FFR-CT may be used as an alternative functional assessment tool parallel to other non-invasive functional imaging tests. The use of FFR-CT may aid in avoiding unnecessary invasive coronary angiography,70,71 albeit its true cost-effectiveness remains controversial.72
1.4 Radiation Exposure
Radiation exposure needs to be considered for both CCTA and nuclear cardiology studies. In particular, a cumulative effective dose due to multiple scans remains a pertinent issue. As for the radiation of CCTA, the recent technological development in the latest generations of CT scanners and reconstruction algorithms has minimized the effective dose.43,73 For nuclear MPI studies, several recommendations are proposed to reduce effective dose (e.g., stress-only or low-dose protocol).74
Updated Clinical Information on Radiation Exposure With CAD-Related Imaging Tests
• International Atomic Energy Agency Nuclear Cardiology Protocol Cross-Sectional Study, a worldwide survey of effective doses for nuclear MPI, reported that the median effective dose was 10.4 mSv per scan, and the only 30% had achieved the recommended median effective dose.75 Achievement of a median effective dose ≤9 mSv is recommended by the American Society of Nuclear Cardiology75,75a
• In a country-specific survey in the International Atomic Energy Agency Nuclear Cardiology Protocol Cross-Sectional Study, Otsuka et al reported that the effective dose was significantly higher in Japan (14.0±5.5 mSv), driven mainly by frequent use of 201thallium74
• Danad et al estimated an average effective dose of 5.9 mSv for snapshot CT-MPI and 9.2 mSv for dynamic CT-MPI76
• When using CCTA for purely anatomical assessment, the effective dose can be estimated between 3 and 5 mSv per scan43,77
2. Exercise Treadmill ECG Testing
According to the JCS guidelines and the American College of Cardiology/American Heart Association 2002 guidelines, exercise ECG (exECG) testing, mainly using a treadmill, is recommended for the diagnosis of stable CAD in patients with an intermediate pre-test probability who are able to exercise and who have an interpretable resting ECG, taking cost-effectiveness into account.60,78 Despite these recommendations, in the ISCHEMIA trial the proportion of patients undergoing exECG for initial diagnostic testing was only 24.5%.3 In the ESC guidelines, exECG is recommended as an “alternative approach”, given its limited sensitivity and specificity in diagnosing obstructive CAD;15 the performance of exECG is only adequate when the pre-test probability is extremely high (≥80%) or low (≤19%).11
Risks associated with the performance of exercise testing, such as its potential to induce unstable angina or worsen a patient’s hemodynamic status, should be always considered. Performance of exercise testing requires a physician’s supervision at all institutions in Japan. These disadvantages of performing exercise testing in addition to its low diagnostic accuracy must be weighed against its benefit.79
This focused update has revised that exECG can be performed to confirm the absence of exercised-induced ischemic change in patients with low pre-test probability rather than in those with intermediate pre-test probability. It should also be noted that exercise capacity itself is a potent predictor of clinical risk, even in the setting of non-ischemic (normal) functional imaging.80 ExECG is recommended for the assessment of exercise tolerance, cardiac symptoms, arrhythmias, and blood pressure responses, and for determining event risks in selected patients as in the ESC guideline.15 Thus, in the present era, the primary aim of exECG is to confirm manageable exercise capacity in the absence of an unfavorable hemodynamic response. The overall recommendations and their level of evidence for non-invasive imaging and exECG in patients with suspected CAD are demonstrated in Table 7.
Table 7. COR and LOE for Non-Invasive Imaging in Patients With Suspected Stable CAD
| |
COR |
LOE |
Non-invasive anatomical (CCTA) or functional imaging test (SPECT, stress CMR, or stress
echocardiography) is recommended for the diagnosis of CAD and assessment of event risk in
patients with intermediate or high PTP of CAD3,11,48,81 |
I |
A |
It is recommended to choose appropriate non-invasive imaging modality based on the PTP/CL
sequence and patients’ characteristics (e.g., heart rate/bundle-branch block/artificial pacemaker,
renal dysfunction, drug allergy/exercise intolerance, or risk of radiation exposure) |
I |
C |
Complimentary functional tests (i.e., functional imaging tests and FFR-CT) should be considered
for further risk assessment or in patients whose findings on CCTA are inconclusive68 |
IIa |
B |
Invasive coronary angiography should be considered for the diagnosis of CAD prior to titration of
OMT when findings on non-invasive imaging tests are suggestive of LMCA or LMCA-equivalent
disease, or symptoms deteriorate during diagnostic work-up3 |
IIa |
B |
CAC scan or exECG may be considered as an optional test to help rule out CAD in asymptomatic
or minimally symptomatic patients with low PTP11,24,82 |
IIb |
B |
Invasive coronary angiography should be avoided prior to initiation and titration of OMT unless
non-invasive imaging test suggests evidence of LMCA or LMCA-equivalent disease3 |
III
(Harm) |
B |
CAC, coronary artery calcium; CAD, coronary artery disease; CCTA, coronary computed tomography angiography; CL, clinical likelihood; CMR, cardiac magnetic resonance; COR, class of recommendation; exECG, exercise ECG; FFR-CT, fractional flow reserve-computed tomography; LMCA, left main coronary artery; LOE, level of evidence; OMT, optimized medical therapy; PTP, pre-test probability; SPECT, stress single photon emission computed tomography.
III. Indications and Timing for Invasive Coronary Angiography and Revascularization
Summary
Coronary revascularization via percutaneous coronary intervention (PCI) or CABG has long been believed to improve prognosis of stable coronary artery disease (CAD) patients. The ISCHEMIA trial,3 however, demonstrated that initial invasive treatment strategies, as compared with optimized medical therapy (OMT) alone (i.e., conservative strategy), do not necessarily improve cardiovascular outcomes among stable CAD patients with moderate-to-severe ischemia. Therefore, invasive coronary angiography should be considered only when a patient is a potential candidate for coronary revascularization with a high-risk profile (e.g., near-acute coronary syndrome (ACS) status, refractory angina despite best efforts to initiate and titrate OMT, left main coronary artery (LMCA)/LMCA-equivalent disease, advanced kidney disease, or heart failure (HF)/left ventricular (LV) dysfunction), or for those who cannot be diagnosed or risk-assessed by non-invasive testing. Intracoronary pressure measurement (e.g., fractional flow reserve [FFR], or instantaneous wave-free ratio [iFR]) may be beneficial to identify the flow-limiting coronary artery with intermediate stenotic lesions on angiography when non-invasive cardiac imaging tests are not feasible or findings on non-invasive cardiac imaging are inconclusive.
Finally, the treating physician should proactively engage in shared decision making with their patients, particularly when coronary revascularization is considered.
1. Invasive Coronary Angiography and Intracoronary Pressure Measurement
Invasive coronary angiography has long served as the gold-standard for diagnosing CAD. In the contemporary era, initial invasive coronary angiography is to be considered for CAD patients with a high-risk profile (e.g., anginal symptoms occurring with a low intensity of exercise or near-ACS status) or patients with refractory angina despite best efforts to initiate and titrate OMT, or those who cannot be diagnosed or risk-assessed by non-invasive testing such as coronary computed tomography angiography (CCTA) or nuclear myocardial perfusion imaging (MPI). Further, if there is discrepancy between the results of non-invasive imaging and invasive coronary angiography, the indication for revascularization may be determined with coronary pressure measurements during invasive coronary angiography.
1.1 Invasive Coronary Angiography
In Japan, approximately 500,000 invasive coronary angiograms are obtained annually (https://www.j-circ.or.jp/jittai_chosa/media/jittai_chosa2020web.pdf). The invasive coronary angiogram conveys relevant clinical information regarding the severity of CAD by visualizing the coronary anatomy. It is important to consider the risks and benefits for invasive coronary angiography, because there can be hemorrhagic and thrombotic complications. Previous reports have shown that death, myocardial infarction (MI), and stroke occur in about 0.1–0.2% of cases in association with this invasive diagnostic procedure.83
Updated Clinical Information on Invasive Coronary Angiography
• Lesion complexity represented by the SYNTAX score and its updated formula has been widely used to guide optimal revascularization strategy (https://doi.org/10.1253/circj.CJ-20-1282)15,85
• The functional SYNTAX score incorporates only coronary vessels that demonstrate significant reduction in coronary FFR into the calculation.86 In recent years, SYNTAX score II, which adds patient information, and SYNTAX score II 2020, aimed at predicting long-term prognosis, have also been proposed87,88
In terms of its prognostic significance, greater number of diseased vessels on the invasive coronary angiogram has been reported to be associated with worse outcomes.3,84 However, invasive coronary angiography does not provide direct information on coronary vessel diameter or characteristics of coronary plaque. Of note, the degree of coronary artery stenosis demonstrated by invasive coronary angiography does not necessarily reflect physiological severity and is known to differ from findings on FFR.89
The indication for invasive coronary angiography should account for factors such as age, activities of daily living, presence of comorbidities, and the patient’s willingness to proceed with invasive treatment. Immediate invasive coronary angiography is not indicated in symptomatically stable CAD patients in whom early revascularization is considered not necessary by non-invasive testing. Invasive coronary angiography may be indicated for stable CAD patients when performance of non-invasive imaging tests does not lead to a definitive diagnosis or adequate risk assessment.15 Early invasive coronary angiography should be considered for stable CAD patients with the abovementioned high-risk profile because of the potential benefit from early revascularization.15 In addition, early invasive coronary angiography may be indicated in patients with a history of HF or LV dysfunction (Chapter V).90 Importantly, invasive procedures should not be hesitated in patients undergoing conservative therapy when unstable symptoms suggestive of ACS occur during follow-up.15 In the ISCHEMIA trial, invasive coronary angiography and subsequent revascularization were eventually performed in 26% and 21% in the conservative strategy group, respectively.3 Indications for invasive coronary angiography for the diagnosis of stable CAD are shown in Table 8.
Table 8. COR and LOE for Indication of Invasive Coronary Angiography in Patients With Stable CAD
| |
COR |
LOE |
| Invasive coronary angiography is recommended |
| • In patients with uncontrolled angina despite initiation and titration of OMT3 |
I |
B |
• When high-risk CAD disease (e.g., LMCA or LMCA-equivalent disease) is strongly
suspected based on clinical symptoms and results of non-invasive tests |
I |
C |
| Invasive coronary angiography should be considered |
• In patients with suspected CAD who cannot undergo non-invasive imaging
tests or whose test results are inconclusive |
IIa |
C |
| • In patients with HF and suspected CAD who may benefit from revascularization |
IIa |
C |
| Invasive coronary angiography should not be performed |
• (For risk assessment purposes) in patients with stable CAD who are not candidates
for revascularization because of comorbidities or the patient’s lack of
willingness to proceed with invasive treatment15 |
III
(Harm) |
B |
• In patients with preserved LV function and clinically low-risk profile, who have not
undergone non-invasive testing or revealed any findings suggestive of
myocardial ischemia3,15 |
III
(Harm) |
B |
CAD, coronary artery disease; COR, class of recommendation; HF, heart failure; LV, left ventricular; LOE, level of evidence; OMT, optimized medical therapy.
1.2 Invasive Intracoronary Pressure Measurement
Invasive intracoronary pressure measurement, including FFR, is a diagnostic test to physiologically evaluate the degree of flow limitation caused by coronary artery stenosis. Intracoronary pressure measurement is used to assess whether moderately stenosed coronary lesions on the invasive coronary angiogram are associated with myocardial ischemia and thus, identify the target lesions for coronary revascularization.15,91 Invasive intracoronary pressure measurement may be considered if patients cannot undergo non-invasive tests in advance, or in cases where ischemia assessed by non-invasive tests is discordant with anatomically significant coronary lesions determined by invasive coronary angiography. Of note, the benefit and risk of intracoronary pressure measurement should be carefully weighed in individual cases with severe coronary curvature or calcified lesions, where guidewire insertion is expected to be technically challenging.92
Fractional Flow Reserve
The Fractional Flow Reserve Versus Angiography for Multivessel Evaluation 2 (FAME 2) trial demonstrated a reduction in MI and urgent revascularization in the remote period after FFR-guided determination for performing PCI (positive if FFR value ≤0.80).93 In the Initial Invasive or Conservative Strategy for Stable Coronary Disease (ISCHEMIA) trial, FFR was needed in approximately 20% of cases of patients undergoing PCI where invasive coronary angiographic findings were discordant with those of non-invasive testing.3 A Japanese prospective multicenter registry showed that revascularization for lesions with FFR >0.8 was not associated with improvements in the 1-year event rate that included death, stroke, MI, and revascularization as compared with a defer strategy.94 On the other hand, deferred lesions with lower FFR values were associated with a higher incidence of target vessel-related MI and revascularization.
Updated Clinical Information on Invasive FFR
• FFR has been widely used for nearly a decade, and long-term clinical trial findings other than the FAME-2 trial have also demonstrated the benefit of FFR.95–97 The beneficial effects of FFR have been also demonstrated in a number of clinical scenarios (e.g., previous MI, multivessel CAD, non-culprit lesions of ACS, LMCA lesion, and preoperative assessment for CABG)98–104
• The FFR value is not solely treated as a binary variable divided at the cutoff value, but also as a continuous value. Revascularization of particularly severely affected lesions, generally with an FFR <0.65, is suggested to reduce cardiovascular events such as cardiac death or MI105,106
• Coronary lesions with a FFR value >0.80 are generally not candidates for revascularization,107 but the risk of cardiovascular events has been reported to be high with subtly reduced FFR (e.g., FFR<0.85 or 0.90)105,106,108,109
• The CVIT-DEFER Study conducted in Japan showed that FFR measurements changed the treatment strategy in 39% of patients, decreased PCI implementation by 22%, and increased pharmacotherapy implementation by 41%.94 In particular, there have been reports of the possibility of improving clinical outcomes with intracoronary pressure measurement guidance, especially when a patient is diagnosed with multivessel coronary artery lesions by invasive coronary angiography110,111
• The FAME 2 trial used 0.80 as a cutoff value to pursue or defer coronary revascularization, and demonstrated the validity of FFR-guided PCI.93 Despite a relatively small sample size, the FAME 2 trial demonstrated a reduction in MI and urgent revascularization in the remote period after PCI (similar to the ISCHEMIA trial).3,95 It is also known that PCI using FFR has an economic advantage over PCI based on invasive coronary angiography alone112,113
• Technologies for calculating FFR based on invasive coronary angiography, intravascular ultrasound, and optical coherence tomography have also emerged. Moreover, a FFR calculation technique from the invasive coronary angiogram is currently covered by national health insurance (FFR angio). The implications and practicalities of these methods in daily clinical practice await further investigation114–117
Non-Hyperemic Pressure Ratio
Non-hyperemic pressure ratios (NHPRs) such as iFR, resting full-cycle ratio (RFR), diastolic hyperemia-free ratio (DFR), and diastolic pressure ratio (dPR), are available as alternative intracoronary pressure measurement methods. Although evidence demonstrating their prognostic value are lacking, except for iFR, their diagnostic performance has been reported as comparable to FFR.
Updated Clinical Information on iFR and Other NHPRs
• iFR has shown comparable efficacy to FFR in large-scale clinical trials118,119
• NHPRs pressure ratios other than iFR, such as RFR, DFR, and dPR, have also been developed, and their values are reported to be consistent with iFR values,120,121 although evidence demonstrating prognostic value is lacking
• An iFR cutoff value of 0.89 is considered valid for considering revascularization.118,119,122,123 Because all available NHRPs have approximately analogous values, it is believed that NHRPs other than iFR can similarly use a cutoff value of 0.89120,124,125
• In recent years, data supporting the use of NHRPs indices have accumulated in individual clinical scenarios such as assessment of LMCA lesions or multivessel disease and preoperative coronary artery bypass assessments126–128
• Discordances between FFR and NHRPs are found in 10–20% of cases when using the abovementioned cutoff value.124,129–133 Nonetheless, the prognosis of discordant cases is generally reported to be good124,130,132,133
Indications for FFR and NHPRs are shown in Table 9. The choice of intracoronary pressure measurement method is left to the discretion of the treating physician.
Table 9. COR and LOE for Invasive Coronary Artery Pressure Measurements in Moderate Coronary Artery Stenosis in Stable Coronary Artery Disease
| |
COR |
LOE |
| FFR/NHPRs* are recommended |
| • To identify coronary stenosis that can cause myocardial ischemia123,134,135 |
I |
A |
| • To determine indications of PCI aimed at reducing cardiovascular events95–97,118,119 |
I |
A |
FFR/NHPRs* should be considered when myocardial ischemia demonstrated on non-
invasive imaging test is inconsistent with anatomical findings assessed by invasive
coronary angiography3 |
IIa |
B |
FFR/NHPRs* may be considered to determine optimal graft anastomotic site prior to
CABG99,102 |
IIb |
B |
FFR/NHPRs* should not be performed when non-invasive imaging studies have proven
myocardial ischemia consistent with anatomical coronary stenosis† |
III (No
benefit) |
C |
*Among NHPRs, including instantaneous wave-free ratio, resting full-cycle ratio, diastolic hyperemia-free ratio, and diastolic pressure ratio, only the instantaneous wave-free ratio has shown comparable efficacy to FFR in large-scale clinical trials.118,119†For tandem coronary disease, FFR/NHPRs may be useful to identify the target lesion for stenting and to optimize stent length.136–138 CABG, coronary artery bypass grafting; COR, class of recommendation; FFR, fractional flow reserve; LOE, level of evidence; NHPR, hyperemic pressure ratios; PCI, percutaneous coronary intervention.
The purpose of coronary revascularization in stable CAD is (1) to improve angina symptoms and (2) to reduce the risk of incident cardiovascular events. The ISCHEMIA trial did not demonstrate prognostic benefit of early revascularization over OMT for cardiovascular events.3 The ISCHEMIA trial results are thought to be applicable to Japanese patients, because the baseline characteristics of the ISCHEMIA participants and the patients enrolled in the Japanese registry were comparable.139
However, initial invasive treatment strategies may provide prognostic benefit in patients that do not meet the ISCHEMIA or ISCHEMIA-CKD inclusion criteria (e.g., near-ACS status, LMCA/LMCA equivalent disease, or HF/LV dysfunction). Stable CAD patients presenting with a higher degree of chest discomfort on the SAQ demonstrated significant improvement from coronary revascularization.3 Thus, physicians should engage in shared decision making with stable CAD patients who exhibit chest symptoms that interfere with their daily lives to consider the risks and benefits of each treatment choice.
2.1 Impact of Coronary Revascularization on Cardiovascular Events
As stated, improvement in clinical outcomes after revascularization for stable CAD patients (compared with OMT) has not been clearly shown, even in patients with severe myocardial ischemia. Coronary revascularization for lesions without evidence of ischemia does not improve cardiovascular outcomes and may even worsen the patient’s condition,107 Therefore, it is important to identify patients who may benefit from revascularization through assessment of patient-reported outcomes and non-invasive imaging, as addressed in the previous chapters.
Pertinent Study Results on Revascularization for Prognosis in Stable CAD
• A single-center retrospective study by Hachamovitch et al suggested that revascularization may be associated with reduced risk of cardiac deaths among patients with large myocardial ischemic areas (>10% of the LV myocardium)38,140
• In contrast, a nuclear substudy of the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial enrolling 314 patients who underwent serial rest or stress SPECT demonstrated a greater reduction in ischemic myocardium in the PCI + OMT arm than in the OMT alone arm. Notably, patients with ≥5% reduction in myocardial ischemia had lower risk for death and MI regardless of the treatment strategy141
• In the COURAGE trial, the addition of PCI-based revascularization to OMT did not demonstrate prognostic benefit.142,143 However, as the COURAGE trial incorporated a large number of patients without significant myocardial ischemia, the results were diluted. Therefore, beneficial prognostic effect of revascularization in patients with proven myocardial ischemia of a certain extent or larger was not determined. Of note, the 2019 ESC guidelines stated that revascularization is indicated in cases of >10% myocardial ischemic area.15,91
• In a further analysis of MI events from the ISCHEMIA trial, initial invasive treatment strategies resulted in increased early periprocedural MI associated with PCI and CABG as compared with OMT alone, but reduced the occurrence of late spontaneous MI, and overall mortality did not differ between groups. There is an ongoing debate on whether these 2 types of MI events can be weighed equally. Spontaneous MI was more greatly associated with subsequent outcome, including overall mortality, compared with periprocedural MI in a subsequent subanalysis of the ISCHEMIA trial144
• The median follow-up period of 3.2 years in the ISCHEMIA trial may have been too short to evaluate the efficacy of revascularization, particularly for mortality assessment. A meta-analysis including the ISCHEMIA trial suggested that elective coronary revascularization reduces cardiac death and spontaneous MI, and that long-term follow-up may reveal greater effectiveness.145 A longer follow-up of the ISCHEMIA trial (ISCHEMIA-EXTENDED, ClinicalTrials.gov Identifier: NCT04894877) is currently ongoing to clarify this point
2.2 Impact of Coronary Revascularization on Angina Symptoms
Coronary revascularization has been shown to significantly improve patients’ angina symptoms and quality of life (QOL), especially in those with a high burden of angina-related symptoms and those refractory to OMT. In contrast, revascularization is unlikely to improve QOL in patients with minimal or no chest symptoms prior to revascularization.4,146
Pertinent Study Results on Revascularization for Symptoms of Stable CAD
• Angina symptoms reduce QOL and physical endurance, and are associated with depression, frequent hospital visits and increased clinical events.147,148 The Objective Randomised Blinded Investigation with optimal medical Therapy of Angioplasty in stable angina (ORBITA) trial compared the efficacy of PCI-based revascularization relative to sham control on improvement in exercise tolerance (exercise time increment) among patients with stable CAD with single-vessel lesions.149 At 6 weeks after randomization, the differences between the 2 groups were not significant, and it was presumed that the placebo effect played a role in the improvement of exercise tolerance after PCI149
• In the ISCHEMIA trial, a QOL analysis using the SAQ was conducted, and the results indicated that revascularization significantly contributed to an improvement of symptoms in patients.4 In the initial invasive strategy group, the angina-free status was maintained at high for more than 3 years. This finding was consistent with those in the ORBITA trial that showed a higher proportion achieving angina-free status in the PCI group150
• The results of a 5-year follow-up of the aforementioned FAME 2 trial revealed that PCI improved QOL and reduced the use of anti-anginal drugs95
• Although the COURAGE trial showed an improvement in QOL (i.e., resolution of angina pectoris) by revascularization, the significant difference from the conservative treatment group diminished at approximately 2 years after revascularization.151 The reason for this is unclear, but could be associated with the fact that the COURAGE trial included patients without myocardial ischemia, and most patients underwent PCI with a bare-metal stent.
Indication and Timing of Revascularization Based on Shared Decision Making
The optimal timing of coronary revascularization for stable CAD patients is not necessarily urgent, because early revascularization is unlikely to alter cardiovascular outcomes if the patient is being treated with OMT including lifestyle modification, although in actual practice, implementation of OMT can be challenging. Revascularization should be considered thoroughly with the patient involved in a discussion of the potential risks and benefits of OMT alone vs. OMT plus revascularization (e.g., the risk of spontaneous/periprocedural MI, dose and number of anti-anginal drugs required, or effect on symptoms/overall mortality, and additionally, on the appropriate revascularization method where applicable).146,152
Left Main Coronary Artery Lesion and Multivessel Disease
No update has been made from the recommendations provided by the “Guidelines for revascularization of stable coronary artery disease (2018 revision)” with regard to revascularization for high-risk lesions such as LMCA lesion or multivessel disease.
For these patients, it is desirable that the Heart Team be involved in advance in discussions on how to revascularize. Performance of FFR-CT (if available) may be helpful for the Heart Team conference by presenting areas of ischemic myocardium in relation to the ischemia-causing artery.
Updated Clinical Information on LMCA and Multivessel Disease
• There have been RCTs directly comparing CABG and medical treatment in the LMCA lesions, but they are all small-scale studies conducted 30–40 years ago.153–155 Subsequent registry studies and meta-analyses have consistently shown improved outcomes with coronary revascularization in patients with high-risk anatomies156–158
• In 2020, a database study from the United Kingdom showed that short- and long-term mortality after PCI to the last remaining patent vessel in patients with multivessel disease were very high, and that prognosis was poor.159 Complete revascularization by means of CABG is likely to improve prognosis for such anatomy, and thus, future clinical evidence accumulation is awaited
Ad hoc PCI
The role of ad hoc revascularization (performing PCI during the same session as diagnostic invasive coronary angiography) remains controversial. Although ad hoc PCI is commonly performed in other countries (>75% in the USA and South Korea),160,161 it is less common in Japan (≤30%).162 It is also known that there are wide interhospital disparities in the implementation of ad hoc PCI, and ad hoc PCI is more commonly performed in centers performing smaller numbers of annual PCIs.162
Ad hoc PCI may be a reasonable choice to reduce cost, shorten length of stay, and ameliorate the patient’s burden by omitting invasive coronary angiography for diagnostic purpose only. However, in order to avoid PCI for inappropriate indications, the following conditions are mandatory.
1. Expected benefit of revascularization and overall risk including periprocedural and post-treatment bleeding risk associated with antithrombotic therapy should be evaluated prior to ad hoc PCI. Anatomical risk and complexity shown by CCTA (or FFR-CT) must be carefully assessed.
2. The abovementioned risks and benefits associated with PCI should be shared in full with the patient and/or caregivers, and the treating physicians must engage in shared decision making prior to ad hoc PCI.
3. Ad hoc PCI should only be performed to coronary lesions demonstrating Class I or IIa indication in the latest JCS guideline (https://doi.org/10.1253/circj.CJ-20-1282). In other cases, appropriate revascularization strategies should be discussed in the Heart Team conferences.
Updated Clinical Information on ad hoc PCI
• Ad hoc PCI may reduce paracentesis complications and bleeding events, as well as healthcare costs, and increase patient satisfaction160,163–165
• Ad hoc PCI may increase the prevalence of rarely appropriate indications for PCI (i.e., PCI in cases of poor indication for revascularization or PCI in cases better suited to CABG)162,166
• Ad hoc PCI to multivessel coronary lesions and chronic total occlusion lesions has been reported as associated with worse outcomes160
Based on the above evidence, the updated indication for coronary revascularization is shown in Table 10 and illustrated in Figure 5.
Table 10. COR and LOE for Indications of Revascularization in Patients With Stable Coronary Artery Disease
| |
COR |
LOE |
| Revascularization is recommended |
| • To reduce risk of cardiovascular events in patients with LMCA lesions155–158 |
I |
B |
• To improve prognosis based on shared decision making in patients with extensive
myocardial ischemia on non-invasive imaging3,95,140,143,145,167 |
I |
B |
• To relive symptoms based on shared decision making in patients with daily life
activity-limiting anginal symptoms despite OMT4,95,149,151 |
I |
A |
Risks and benefits of revascularization should be considered on a case-by-case basis for
patients with HF or LV dysfunction, advanced kidney disease, or physical frailty |
IIa |
C |
For revascularization methods, see “Guidelines for revascularization of stable coronary artery disease (2018 revision)”. COR, class of recommendation; HF, heart failure; LMCA, left main coronary artery; LOE, level of evidence; LV, left ventricular; OMT, optimized medical therapy.
IV. Implementation of Contemporary Optimized Medical Therapy
Summary
Optimized medical therapy (OMT) is the term used for goal-targeted intensification of pharmacologic treatment in combination with lifestyle modification for the purpose of symptom relief and event prevention. Given the results of the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) and Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trials, OMT is regarded as the cornerstone of stable coronary artery disease (CAD) management.142,204 The ISCHEMIA trial also demonstrated that the majority of patients with stable CAD can be managed conservatively with strict adherence to OMT (antiplatelet agents and statins dispensed in >95% of participants).3
Both the initiation and titration of OMT during the process of definitive diagnosis and risk assessment of CAD is recommended (Figure 6).
• For symptom relief, following administration of short-acting nitrates for diagnostic purpose, β-blockers or calcium-channel blockers are recommended as first-line anti-anginal drugs.
• For event prevention, reduction of low-density lipoprotein cholesterol (LDL-C) ≥50% and a goal of <70 mg/dL for stable CAD patients using a high-intensity statin with or without complimentary medication is recommended. The use of antithrombotic agents in patients with CAD is detailed in the recently updated JCS guidelines, including for those with implanted coronary stent.172
• Regular exercise is not contraindicated unless the patient’s condition is unstable.
However, recent reports from real-world settings indicate inadequate implementation of OMT despite clinical practice guideline recommendations.166a,177 Because the efficacy of OMT is mainly proven in patients with obstructive CAD rather than for ischemia with non-obstructive CAD, our recommendations of OMT are in patients with obstructive CAD. The typical definition of obstructive CAD is as follows.
1. Patients with previous revascularization.
2. Imaging studies such as coronary angiography or coronary computed tomography angiography (CCTA) showing ≥1 coronary stenosis of >50% with clinical significance by hemodynamic study (i.e., fractional flow reserve [FFR]) or proven ischemia.
3. Patients with a clinical history of myocardial infarction (MI) by the 4th universal definition171 with established atherosclerotic plaque in the plausible coronary artery lesion.
Whether to extend OMT to ischemia with non-obstructive CAD or MI with non-obstructive coronary arteries remains to be investigated.
1. Pharmacologic Intervention
Pharmacologic intervention aims to relieve symptoms or prevent cardiovascular events.
1.1 Symptom Relief (i.e., Anti-Anginal Drugs)
Anti-anginal drugs can effectively improve quality of life (QOL) for stable CAD patients.151 Beta-blockers or calcium-channel blockers are recommended as the first choice of anti-anginal drugs, and a combination should be considered if angina symptoms are not sufficiently controlled by monotherapy. In the 2019 ESC guideline, a stepwise strategy for long-term anti-anginal drug therapy is proposed based on patients’ vital signs and the presence of heart failure or left ventricular (LV) dysfunction.15 Calcium-channel blockers are widely used in Japan, mainly because of the favorable effects observed in the management of vasospastic angina176,177 (which is known to be related to ≈40% of Japanese stable CAD patients).175 For the management of obstructive CAD, we recommend obtaining detailed characteristics of the angina, because calcium-channel blockers have not shown beneficial effects in patients with a history of MI or reduced LV ejection fraction178 (whereas β-blockers have been shown to decrease the incidence of major adverse cerebral and cardiovascular events [MACCE] in these patients).179
Long-acting nitrates and nicorandil should be considered as second-line treatment because of the paucity of data compared with β-blockers or calcium-channel blockers. They are applied when initial therapy with a β-blocker and/or a calcium-channel blocker is contraindicated, poorly tolerated, or inadequate to control angina symptoms.15 Lastly, although international clinical practice guidelines recommend that trimetazidine, ivabradine, and ranolazine should be considered as a second-line treatment, they are unavailable for use as anti-anginal drugs in Japan.15
1.2 Event Prevention
Drugs for event prevention include antithrombotic agents, lipid-lowering agents, antihypertensive drugs, and glucose-lowering drugs. In this update, we emphasized the importance of antithrombotic agents and strict goal-directed lipid-lowering therapy.
Antithrombotic Agents
Antiplatelet agents improve prognosis in patients with stable CAD by inhibiting platelet activation/aggregation and coagulation. For all stable CAD patients with sinus rhythm, low-dose (75–100 mg daily) aspirin is strongly recommended, and for those with aspirin intolerance, clopidogrel 75 mg daily is recommended. The use of antithrombotic agents in patients with CAD is detailed in the recently updated JCS guidelines, including those with implanted coronary stents.172
Lipid-Lowering Agents
For patients with stable CAD, lipid-lowering therapy with high-intensity statins remains the cornerstone of OMT. A previous patient-level meta-analysis of 170,000 participants in 26 randomized controlled trials (RCTs) showed the benefit of intensive lipid-lowering therapy regardless of the baseline value of LDL-C.180 Especially, a large-scale RCT demonstrated that decreasing the LDL-C to <70 mg/dL using high-intensity statins improved long-term outcomes in patients with stable CAD.181 In line with these results, the 2018 American Heart Association/American College of Cardiology Cholesterol Guideline for patients with clinical atherosclerotic cardiovascular disease recommends maximally tolerated statin (atorvastatin 40–80 mg or rosuvastatin 20–40 mg daily) for a goal of LDL-C reduction of ≥50%.182 That guideline also recommends strong consideration of ezetimibe or proprotein convertase subtilisin/kexin type 9 (PCSK-9) inhibitor to complement high-intensity statin for patients with very high risk (multiple atherosclerotic cardiovascular disease events or 1 major atherosclerotic cardiovascular disease event and multiple high-risk conditions such as older age, heterozygous familial hypercholesterolemia, and diabetes mellitus) if LDL-C level is persistently ≥70 mg/dL.182 These goals for LDL-C (LDL-C reduction ≥50%; LDL-C <70 mg/dL) are also recommended for patients with stable CAD and moderate or severe ischemia according to the ISCHEMIA protocol.1 In this context, the present focused update also recommends LDL-C reduction ≥50% and the goal of LDL-C <70 mg/dL for stable CAD patients (documented ischemia on functional testing, or significant stenosis noted on anatomical testing) by using the maximally tolerated dose of high-intensity statin with or without ezetimibe or PCSK-9 inhibitor. Because the maximum available doses of high-intensity statins are low in Japan (atorvastatin 20 mg, rosuvastatin 20 mg), physicians should prescribe these drugs at the maximum dose and should consider using a combination with ezetimibe or PCSK-9 inhibitor if the patient’s value of LDL-C is >70 mg/dL. Choice of ezetimibe or PCSK-9 inhibitor addition on statins should be discussed with patients regarding efficacy, cost, and the patient’s preference.182–184 If mild adverse muscle symptoms occur with high-intensity statins, which lead to temporary interruption of statin therapy, physicians should consider restarting at a lower dose of the same drugs or switch to an alternative class of statins, rather than discontinue statins indefinitely.182 For an alternative class of statins, a high-dose (4 mg) of pitavastatin might be preferable due to its safely profile for Japanese patients with stable CAD in the REAL-CAD trial.185
Clinical Information on Modification of OMT After CAD Work-up
• After work-up for diagnosis and risk stratification completed, reevaluate the patient’s prescription because the absolute benefit and harm of these agents differ based on patients’ risk profile, especially established macrovascular disease
• Specifically, aspirin for primary prevention does not reduce cardiovascular events but does increase upper gastrointestinal and intracranial bleeding186,187
• In addition, target value of LDL-C differs in primary prevention from that in secondary prevention188
• If tests show (1) borderline stenosis that is not hemodynamically significant, or, (2) stenosis without proven reversible ischemia (on imaging tests), physicians should discuss with patients about the clinical benefit and potential adverse events with titration of OMT because of the lack of evidence of management strategy for such situations
1.3 Emerging Therapies
For event prevention, there is new evidence on lowering blood pressure (BP), as well as novel glucose-lowering agents, marine omega-3 fatty acids, and anti-inflammatory drugs that are available for patients with stable CAD patients.
Tighter control of BP may be beneficial in stable CAD patients, based on the final report from the SPRINT trial (targeting systolic BP <120 mmHg resulted in lower rates of major adverse cardiovascular events and lower all-cause mortality than targeting systolic BP <140 mmHg).189 Although the United States clinical practice guidelines have not set an actual target for BP (antihypertensive initiation is recommended for any patient with BP >140/90 mmHg), the European Society of Cardiology guidelines set a target for systolic BP of <120–130 mmHg (130–140 mmHg in individuals aged ≥65 years) for stable CAD patients.15,168 Therefore, we recommend a BP target of <130/80 mmHg for young patients in this focused update. For elderly patients, although a strict BP goal may be appropriate in older patients, physicians should closely monitor adverse events such as orthostatic hypotension and acute kidney injury, and consider a personalized target value of BP (i.e., <140/90 mmHg).
Recently, novel glucose-lowering agents have been introduced. Sodium/glucose cotransporter 2 inhibitors (SGLT2i) and glucagon-like peptide-1 receptor agonists (GLP1-RA) have reduced MACCE by nearly 15% and overall mortality by 10–20% in diabetic patients with macrovascular disease, with reduction of renal failure and minimum risk of hypoglycemia.190–192 International clinical practice guidelines recommend both SGLT2i and GLP1-RA as part of the medications for comprehensive cardiovascular risk reduction.193,194 These agents may be used preferably in stable CAD patients complicated with type 2 diabetes.
Finally, pharmacologic interventions for hypertriglyceridemia and inflammation have shown possible effects on reducing events in stable CAD patients, although the routine use of these emerging medications as standard care needs further evidence.
Updated Clinical Information on Evolving Medical Treatments
• Hypertriglyceridemia is a risk factor for stable CAD, and its treatment has been expected to improve prognosis. In the Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention (REDUCE-IT) trial, adding highly purified EPA omega-3 fatty acids to the standard treatment reduced MACCE for patients with stable CAD presenting hypertriglyceridemia.195 Contrarily, the recent Statin Residual Risk Reduction with Epanova in High CV Risk Patients with Hypertriglyceridemia (STRENGTH) trial has shown that addition of omega-3 fatty acids on standard OMT did not reduce the incidence of MACCE.196 Differences in the form of omega-3 fatty acid and placebos would have partially contributed to the inconsistencies in outcomes.197
• Anti-inflammatory agents are expected to be novel secondary prevention drugs. Colchicine, an anti-inflammatory drug that inhibits the polymerization of β-tubulin, is known to reduce MACCE in post-MI patients.198 The recent Low-Dose Colchicine 2 (LoDoCo2) trial found that adding 0.5 mg of colchicine to the standard treatment for relatively high-risk patients with stable CAD (>80% had prior acute coronary syndrome) reduced cardiovascular death or MI by 30% (hazard ratio 0.71, 95% confidence interval 0.55–0.92).199 However, the LoDoCo2 trial also showed a non-statistically significant increase in non-cardiovascular deaths in the colchicine arm (0.7 events/100 person-years in the colchicine group vs. 0.5 events/100 person-years in the placebo group, hazard ratio 1.51, 95% confidence interval 0.99–2.31).199
2. Updates on Lifestyle Modification
Adherence to lifestyle modifications, including smoking cessation, healthy diet, and exercise, can have important preventive effects on cardiovascular risk.168 Maintaining a physically active lifestyle, even during the diagnostic work-up or initiation of OMT, may be particularly important for stable CAD patients. Exercise-based cardiac rehabilitation is encouraged. Regular exercise is not contraindicated unless unstable conditions associated with unstable ischemia, severe and symptomatic valvular disease, decompensated heart failure, or uncontrolled arrhythmia are present.200 Moderate-intensity aerobic exercise training ≥3 times per week for 30 min/session is recommended for all stable CAD patients.15,168,201
Recommendations of OMT in patients with stable CAD are shown in Table 11.
Table 11. COR and LOE for Optimal Medical Therapy in Patients With Stable Coronary Artery Disease
| |
COR |
LOE |
It is recommended to provide necessary symptom-relieving agents before considering
invasive modalities and continue conservative management if high-risk features are not
present3 |
I |
B |
It is recommended to provide antithrombotic therapy with low-dose aspirin and goal-targeted
lipid-lowering therapy independent of symptom status to reduce adverse cardiovascular
events3,180,183,202,203 |
I |
A |
COR, class of recommendation; LOE, level of evidence.
V. High-Risk Populations Requiring Specific Consideration
Summary
High-risk factors such as aging, frailty, advanced chronic kidney disease (CKD), and heart failure (HF) predispose patients to a high prevalence of coronary artery disease (CAD) and increased risk of cardiovascular events, albeit these patients have been underrepresented in clinical trials. In previous large-scale clinical trials, the proportion of patients aged ≥80 years, those with CKD stage ≥4 (except in the ISCHEMIA-CKD trial), and those with a low left ventricular ejection fraction (LVEF) <30% (except in the Surgical Treatment for Ischemic Heart Failure [STICH]trial) remains low (∼5% or less).3,8,142,149,204–210 Stable CAD patients with LV dysfunction should be managed according to conventional HF guidelines. Revascularization is to be chosen with deliberation on whether reversal of LV function or long-term survival benefit is expected. For patients with advanced CKD, the use of coronary computed tomography angiography (CCTA) should be restricted because of the risk of deterioration of kidney function (except for anuric patients) and the potential of excessive coronary calcification, leading to low image quality. Statin-based lipid-lowering therapy should be prioritized only in predialysis CKD patients, and conservative treatment strategies would be reasonable in a substantial proportion of CKD patients due to high risk of complication and limited proven benefit of revascularization according to the findings of recent large randomized controlled trials (RCTs). For elderly patients, assessment of symptoms is often challenging and performing exercise stress testing may be impractical. Consideration of invasive therapy should be highly personalized, incorporating relevant risk, frailty, or life expectancy.
1. Patients With HF/LV Dysfunction
Stable CAD frequently leads to systolic dysfunction due to myocardial injury and persistent ischemia.15 Generally, a low LVEF due to ischemic etiology portends high risk for sudden cardiac death.211 However, LV dysfunction involving CAD is heterogeneous. Patients may suffer directly from multivessel disease, but may have CAD as a bystander for cardiomyopathy of any other etiology.
Clinical Information on Treating Patients With HF/LV Dysfunction
• LV dysfunction is evaluated using resting echocardiography in conjugation with ischemic change on resting ECG (see Chapter I), and once identified, downstream non-invasive testing and OMT should be modified accordingly
• In addition to aforementioned non-invasive imaging (see Chapter II), assessment of myocardial viability may help predict the benefit of revascularization58,212
Therapeutic Challenge in Patients With HF/LV Dysfunction
Together with the delineation of the coronary arterial anatomy, either by CCTA or invasive coronary angiography, patients with symptomatic HF should be managed based on practice guideline recommendations for the corresponding HF phenotype.213–216 An implanted device (cardiac resynchronization therapy/implantable cardioverter-defibrillator) is indicated in selected patients.217 Beneficial effects of revascularization on LV dysfunction theoretically include reversal of function in the hibernating myocardium or presumed prevention for life-threatening arrhythmias.218
Updated Clinical Information on Stable CAD Patients With HF/LV Dysfunction
• Patients with a low LVEF (≤35%) were not included in the Initial Invasive or Conservative Strategy for Stable Coronary Disease (ISCHEMIA) trial; however, in a subanalysis of patients with moderately reduced LVEF (35–45%) and HF history, there was a trend toward better event-free survival in the invasive treatment group compared with the conservative treatment group90
• For surgical revascularization, the Surgical Treatment for Ischemic Heart Failure (STICH), enrolling patients with LVEF ≤35% and CAD, demonstrated no benefit of revascularization via CABG during the approximately 5-year observation period.205 However, a subanalysis of the trial revealed that patients with LVEF ≤27% benefited from revascularization over optimized medical therapy (OMT) alone. In a study with an extended observation period of 10 years, the addition of CABG to OMT was associated with lower all-cause mortality and cardiovascular hospitalization rates compared with OMT alone219
2. Patients With CKD
CKD is a common comorbidity in patients with CAD. As the estimated glomerular filtration rate (eGFR) declines below ∼60–75 mL/min/1.73 m2, the probability of developing CAD increases in a linear fashion.220 Risk stratification for predicting cardiovascular events in patients with CKD can be improved by using albuminuria assessments in combination with conventional eGFR-based models.221
Diagnostic Challenge in Patients With CKD
Although exercise and pharmacological stress perfusion imaging may be less accurate for detecting CAD in CKD patients than in those without CKD, functional stress testing and non-invasive imaging have been widely used in these patients.222–225 Due to contraindication and possible complications associated with contrast medium, the use of CCTA and enhanced cardiac magnetic resonance imaging (CMR) is substantially limited. Instead, nuclear study is generally used for patients with CKD in daily practice.226,227 Notably, high prognostic value of myocardial perfusion imaging (myocardial ischemia on stress single photon emission computed tomography [SPECT]-myocardial perfusion imaging [MPI]228 or coronary flow reserve on stress/rest positive emission tomography229) in patients with CKD has been reported.
Therapeutic Challenge in Patients With CKD
The prognostic benefit of statin-based treatment decreases as the eGFR declines, with no evidence of beneficial outcomes among patients receiving dialysis. For patients with advanced CKD (including hemodialysis patients) and CAD, conservative therapy is reasonable given the risk of periprocedural complications associated with revascularization, and no proven survival benefit from recent large-scale RCTs.8 If managed conservatively, careful monitoring of changes in symptoms and LV function is needed to avoid clinical deterioration, as the incidence of cardiovascular events in these patients is high. For hemodialysis patients, the benefit of invasive therapy requires further investigation.
Updated Clinical Information on Stable CAD Patients With CKD
• The risks of acute kidney injury and nephrogenic systemic fibrosis must be considered when performing CCTA and enhanced CMR, respectively230
• In a prospective study of 138 patients referred for pre-renal transplant cardiac evaluation (43% on dialysis) and who underwent coronary artery calcium scan, CCTA, and SPECT, SPECT had low sensitivity (53%) and modest specificity (82%) for detecting obstructive CAD (≥50% stenosis) determined by invasive coronary angiography.224 Among patients with a normal SPECT, 14% demonstrated obstructive CAD on invasive coronary angiography
• In the Study of Heart and Renal Protection (SHARP) trial, combination therapy with simvastatin and ezetimibe reduced cardiovascular events in patients with CKD (but not in those receiving dialysis)231
• For those undergoing hemodialysis, 2 RCTs demonstrated no benefit of statin therapy for primary prevention, and the SHARP trial showed equivocal results231–233
• The ISCHEMIA-CKD trial randomized 777 CKD patients with stable CAD and greater than moderate ischemia (CCTA was not mandated as part of the work-up). The use of an initial invasive strategy failed to reduce the risk of death or non-fatal MI, instead increasing the incidence of stroke compared with conservative strategy8
At this point in time, the sole expected benefit of revascularization in patients with CKD is symptom relief, according to the results of the ISCHEMIA-CKD trial; however, more RCTs are warranted to further evaluate the prognostic effect of revascularization among CKD patients
3. Elderly Patients
Elderly CAD patients, often with accompanying frailty, are at risk for high mortality rates.234
Clinical Information on Evaluating Elderly Patients
• Elderly patients frequently present with atypical symptoms, and accurate estimation of the pre-test probability or patient-reported outcomes can be limited234,235
• The incidence of elevated cardiac troponin, likely reflecting subclinical myocardial injury, is higher in older than in younger adults. Therefore, the upper limit of normal for troponin levels used to define MI is typically set at a higher value in the older patients236
• Although assessing exercise capacity can provide incremental information for respective risk stratification, it is frequently impractical due to decreased muscle strength and cognitive ability in older patients237
Therapeutic Challenge in Elderly Patients
Specific considerations (as listed below) are required for both medical and invasive treatments.
Clinical Information on Treating Elderly Patients
• Side effects of drugs, as well as drug intolerance and overdosing, are frequent in the elderly, and the 2019 ESC guidelines recommend careful monitoring of these patients15
• Hypotension and cardiac conduction disturbances are often observed in elderly patients receiving anti-anginal medications such as nitrates, β-blockers, or dihydropyridine/non-dihydropyridine calcium-channel blockers
• Similarly, antithrombotic therapy for the elderly should only be administered following evaluation of bleeding as well as thrombosis risks172
• Although lipid-lowering agents, including statins, are generally recommended for patients with CAD (i.e., as secondary prevention), the expected benefit of lipid-lowering therapy remains undetermined, as most of the large RCTs evaluating the effect of statins on prognosis have only included patients aged 45–75 years238
• The treatment of stable CAD in the elderly is also complicated by their increased vulnerability to complications with invasive strategies, and revascularization decisions should be highly personalized based on multifactorial modifiers such as life expectancy239
• The only definite recommendation in the 2019 ESC guidelines for treating elderly patients is the use of a drug-eluting stent if percutaneous coronary intervention is applicable,15 based on the results of 2 recent trials.240,241 Both trials demonstrated the superiority of a drug-eluting stent in combination with short dual-antiplatelet therapy over bare-metal stents
Appendix 1.
Appendix Table 1. Summary of the ISCHEMIA Trial: Purpose, Design, Eligibility and Endpoints
1,3
| Purpose |
To determine whether an initial invasive approach with ICA and optimal revascularization (if feasible) plus OMT (i.e.,
INV) offers incremental value over a conservative approach with catheterization reserved for failure of OMT (i.e., CONS)
in stable CAD patients with moderate or severe ischemia |
| Design |
International, multicenter, RCT |
Key inclusion
criteria |
• Moderate or severe ischemia on a stress test (determined by non-invasive stress imaging or exercise ECG) |
| • Age ≥21 years |
Key exclusion
criteria |
LV dysfunction or HF |
| • LVEF <35% |
| • NYHA class III–IV HF or hospitalization for HF within the previous 6 months |
| Severe angina/ACS status |
| • Canadian Cardiovascular Society class III of recent onset |
| • Angina of any class with rapid progression or acceleration pattern |
| • CCS class IV |
| • Unacceptable level of anginal pain/symptoms despite maximal medical therapy |
| • ACS within the previous 2 months |
| Kidney function |
| • Advanced kidney disease with eGFR <30 mL/min/1.73 m2 or on dialysis |
| Coronary anatomy |
| • Known unprotected LMCA stenosis ≥50% (also screened on a blinded CCTA) |
| • Known non-obstructive coronary artery (also screened on a blinded CCTA) |
| • Known unsuitable anatomy for PCI or CABG |
| Previous coronary intervention |
| • PCI or CABG within the previous 12 months |
| Primary endpoint |
• Composite of death from cardiovascular causes, MI, hospitalization for unstable angina or HF, or resuscitated cardiac arrest |
Key secondary
endpoints |
• Composite of death from cardiovascular causes or MI |
| • All-cause death |
| • Angina QOL |
The ISCHEMIA trial included patients with stable CAD who were assigned to initial invasive (invasive coronary angiography followed by revascularization within 1 month plus OMT) and initial conservative strategy (invasive coronary angiography reserved for failure of OMT). There was no statistical difference in the primary or main secondary endpoints. The initial invasive strategy showed greater improvement of QOL. ACS, acute coronary syndrome; CABG, coronary artery bypass grafting; CAD, coronary artery disease; CCS, chronic coronary syndrome(s); CCTA, coronary computed tomography angiography; CONS, conservative strategy; eGFR, estimated glomerular filtration ratio; HF, heart failure; ICA, invasive coronary angiography; INV, invasive strategy; LMCA, left main coronary artery; LV, left ventricular; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NYHA, New York Heart Association; OMT, optimized medical therapy; PCI percutaneous coronary intervention; QOL, quality of life; RCT, randomized controlled trial.
Appendix Table 2. Summary of the ISCHEMIA Trial: Results
3,4
| No. of patients randomized |
5,179 (INV n=2,588 and CONS n=2,591) |
| Median follow-up period |
3.2 years |
| Median age (interquartile range) |
64 years (58–70) |
| Asian race or ethnic group |
29.0% |
| Proportion of ICA performed |
96% in INV and 26% in CONS |
Proportion of revascularization
performed |
74% in INV (74% PCI and 26% CABG) and 21% in CONS |
| Primary endpoint |
• Incidence of composite endpoint |
| 12.9% (12.3% in INV and 13.9% in CONS, P=0.34) |
| • Cumulative composite outcome event rate at 5 years |
| 16.4% in INV and 18.2% in CONS (95% CI, −4.7 to 1.0) |
| Key secondary endpoints |
• Incidence of composite of deaths from cardiovascular causes or MI |
| 11.4 % (10.7% in INV and 12.1% in CONS, P=0.21) |
| • Cumulative composite outcome event rate at 5 years: |
| INV 14.2% and CONS 16.5% (95% CI, −5.0 to 0.4) |
| • Incidence of death from any cause: |
| 5.6% (5.6% in INV and 5.6% in CONS, P=0.67) |
| • Incidence of MI |
| 8.6% (8.1% in INV and 9.0% in CONS, P=0.33) |
| • Angina QOL |
| INV had greater improvement in angina-related health status than CONS in patients with frequent angina |
CI, confidence interval. Other abbreviations as in Appendix Table 1.
Appendix 2. Details of Members
Chair:
• Shintaro Nakano, Cardiology, Saitama Medical University International Medical Center
Vice Chair:
• Shun Kohsaka, Cardiology, Keio University School of Medicine
Members:
• Taishiro Chikamori, Cardiology, Tokyo Medical University
• Kenji Fukushima, Department of Radiology and Nuclear Medicine, Fukushima Medical University
• Yoshio Kobayashi, Cardiovascular Medicine, Chiba University School of Medicine
• Ken Kozuma, Cardiology, Teikyo University School of Medicine
• Susumu Manabe, Cardiac Surgery, International University of Health and Welfare Mita Hospital
• Hitoshi Matsuo, Cardiovascular Medicine, Gifu Heart Center
• Masato Nakamura, Cardiovascular Medicine, Toho University Ohashi Medical Center
• Takayuki Ohno, Cardiovascular Surgery, Mitsui Memorial Hospital
• Mitsuaki Sawano, Cardiology, Tokyo Dental College, Ichikawa General Hospital
• Koichi Toda, Cardiovascular Surgery, Osaka University Graduate School of Medicine
• Yasunori Ueda, Cardiovascular Division, National Hospital Organization Osaka National Hospital
• Hiroyoshi Yokoi, Cardiovascular Center, International University of Health and Welfare Fukuoka Sanno Hospital
Collaborators:
• Yodo Gatate, Cardiology, Self-Defense Forces Central Hospital
• Tokuo Kasai, Cardiology, Uonuma Kikan Hospital
• Yoshiaki Kawase, Cardiovascular Medicine, Gifu Heart Center
• Naoya Matsumoto, Cardiology, Nihon University Hospital
• Hitoshi Mori, Cardiology, Saitama Medical University International Medical Center
• Ryo Nakazato, Nakazato Clinic
• Nozomi Niimi, Cardiology, Keio University School of Medicine
• Yuichi Saito, Cardiovascular Medicine, Chiba University School of Medicine
• Ayumi Shintani, Medical Statistics, Osaka City University Graduate School of Medicine
• Ippei Watanabe, Cardiovascular Medicine, Toho University School of Medicine
• Yusuke Watanabe, Cardiology, Teikyo University School of Medicine
Independent Assessment Committee:
• Yuji Ikari, Cardiology, Tokai University School of Medicine
• Masahiro Jinzaki, Radiology, Keio University School of Medicine
• Takeshi Kimura, Cardiovascular Medicine, Kyoto University Graduate School of Medicine
• Masami Kosuge, Cardiology, Yokohama City University Medical Center
• Kenichi Nakajima, Functional Imaging and Artificial Intelligence, Kanazawa University
(Listed in alphabetical order; affiliations as of January 2022)
Appendix 3. Disclosure of Potential Conflicts of Interest (COI): JCS 2022 Guideline Focused Update on Diagnosis and Treatment in Patients With Stable Coronary Artery Disease (2019/1/1–2021/12/31)
| Author |
Member’s own declaration items |
COI of the marital partner,
first-degree family members,
or those who share income
and property |
COI of the head of the organization/
department to which the member
belongs (if the member is in a
position to collaborate with the head
of the organization/department) |
Employer/
leadership
position
(private
company) |
Stakeholder |
Patent
royalty |
Honorarium |
Payment for
manuscripts |
Research grant |
Scholarship
(educational) grant |
Endowed chair |
Other
rewards |
Employer/
leadership
position
(private
company) |
Stakeholder |
Patent
royalty |
Research grant |
Scholarship
(educational)
grant |
Chair:
Shintaro Nakano |
|
|
|
Pfizer Japan Inc.
Otsuka
Pharmaceutical
Co., Ltd. |
|
|
|
|
|
|
|
|
|
|
Vice Chair:
Shun Kohsaka |
|
|
|
Bayer Yakuhin, Ltd.
Otsuka
Pharmaceutical
Co., Ltd.
AstraZeneca K.K.
Bristol-Myers Squibb |
|
Daiichi Sankyo
Company, Limited
Novartis Pharma
K.K. |
|
|
|
|
|
|
|
|
Members:
Taishiro
Chikamori |
|
|
|
|
|
CMIC Co., Ltd |
FUJIFILM Toyama
Chemical Co., Ltd.
Daiichi Sankyo
Company, Limited
Bayer Yakuhin, Ltd.
WIN
INTERNATIONAL
CO., LTD. |
Abbott Medical Japan
L.L.C
Abbott Vascular Japan
Co., Ltd. |
|
|
|
|
|
|
Members:
Yoshio Kobayashi |
|
|
|
AstraZeneca K.K.
Abbott Medical
Japan L.L.C
Otsuka
Pharmaceutical
Co., Ltd.
Ono Pharmaceutical
Co., Ltd.
Nippon Boehringer
Ingelheim Co.,
Ltd.
Takeda
Pharmaceutical
Company Limited
Novartis Pharma
K.K.
Bayer Yakuhin, Ltd.
Bristol-Myers Squibb
Daiichi Sankyo
Company, Limited |
|
|
Takeda
Pharmaceutical
Company Limited
TERUMO
CORPORATION
Nipro Corporation
WIN
INTERNATIONAL
CO., LTD.
Otsuka
Pharmaceutical Co.,
Ltd.
Nippon Boehringer
Ingelheim Co ., Ltd.
Daiichi Sankyo
Company, Limited
Astellas Pharma Inc.
Japan Lifeline Co.,
Ltd.
JAPANESE
SOCIETY OF
CLINICAL
PHYSIOLOGY
Abbott Medical Japan
L.L.C |
Abbott Medical Japan
L.L.C
BIOTRONIK Japan,
Inc.
Fukuda Denshi Co.,
Ltd |
|
|
|
|
|
|
Members:
Ken Kozuma |
|
|
|
Abbott Vascular
Japan Co., Ltd.
Abbott Medical
Japan L.L.C
Amgen K.K.
Sanofi K.K.
ZEON MEDICAL
INC.
Novartis Pharma
K.K.
Bayer Yakuhin, Ltd.
Bristol-Myers Squibb
Boston Scientific
Japan K.K.
Life Science
Institute, Inc.
Otsuka
Pharmaceutical
Co., Ltd.
Daiichi Sankyo
Company, Limited
Abiomed Japan
K.K.
Nippon Boehringer
Ingelheim Co.,
Ltd.
Medtronic Japan
Co., Ltd.
Takeda
Pharmaceutical
Company Limited |
|
Boston Scientific
Japan K.K.
Daiichi Sankyo
Company, Limited
Orbusneich Medical
K.K. |
Abbott Medical Japan
L.L.C
Boston Scientific
Japan K.K. |
|
|
|
|
|
|
|
Members:
Hitoshi Matsuo |
|
|
|
Abbott Vascular
Japan Co., Ltd.
Abbott Medical
Japan L.L.C
Amgen K.K.
KANEKA MEDIX
CORP.
ZEON MEDICAL
INC.
HeartFlow Japan
G.K.
Philips Japan, Ltd.
Boston Scientific
Japan K.K. |
|
|
|
|
|
|
|
|
|
|
Members:
Masato
Nakamura |
|
|
|
Bayer Yakuhin, Ltd.
Boston Scientific
Japan K.K. |
|
Boston Scientific
Japan K.K.
TERUMO
CORPORATION |
|
|
|
|
|
|
|
|
Members:
Yasunori Ueda |
|
|
|
Daiichi Sankyo
Company, Limited |
|
Amgen Astellas
BioPharma K.K. |
Abbott Vascular Japan
Co., Ltd.
Nihon Kohden Corp. |
|
|
|
|
|
|
|
Members:
Hiroyoshi Yokoi |
|
|
|
Amgen Astellas
BioPharma K.K.
Abbott Medical
Japan L.L.C
Amgen K.K.
KANEKA MEDIX
CORP.
Sanofi K.K.
TERUMO
CORPORATION
HeartFlow Japan
G.K.
Bayer Yakuhin, Ltd.
Philips Japan, Ltd.
Boston Scientific
Japan K.K.
Otsuka
Pharmaceutical
Co., Ltd.
Daiichi Sankyo
Company, Limited
Mitsubishi Tanabe
Pharma
Corporation
Medtronic Japan
Co., Ltd.
Takeda
Pharmaceutical
Company Limited |
|
|
Daiichi Sankyo
Company, Limited |
|
|
|
|
|
|
|
Collaborators:
Tokuo Kasai |
|
|
|
Nihon Medi-Physics
Co., Ltd. |
Nihon Medi-
Physics Co.,
Ltd. |
|
|
|
|
|
|
|
|
|
Collaborators:
Yoshiaki Kawase |
|
|
|
Abbott Vascular
Japan Co., Ltd.
Boston Scientific
Japan K.K.
ZEON MEDICAL
INC. |
|
|
|
|
|
|
|
|
|
|
Collaborators:
Naoya
Matsumoto |
|
|
|
Nihon Medi-Physics
Co.,Ltd.
FUJIFILM Toyama
Chemical Co., Ltd. |
|
|
FUJIFILM Toyama
Chemical Co., Ltd. |
|
|
|
|
|
|
|
Collaborators:
Hitoshi Mori |
|
|
|
Johnson & Johnson
K.K.
Boston Scientific
Japan K.K. |
|
|
|
|
|
|
|
|
|
|
Collaborators:
Ayumi Shintani |
|
|
|
Kyowa Kirin Co.,
Ltd.
Daiichi Sankyo
Company, Limited
Chugai
Pharmaceutical
Co., Ltd.
Janssen
Pharmaceutical
K.K.
Shionogi & Co., Ltd. |
|
Takeda
Pharmaceutical
Company Limited
Kyowa Kirin Co.,
Ltd. |
|
|
|
|
|
|
|
|
Collaborators:
Yusuke Watanabe |
|
|
|
Edwards
Lifesciences
Corporation
Medtronic Japan
Co., Ltd.
Abbott Japan LLC |
|
|
|
|
|
|
|
|
|
|
Independent
Assessment
Committee:
Yuji Ikari |
|
|
|
Amgen Astellas
BioPharma K.K.
AstraZeneca K.K.
Abbott Medical
Japan L.L.C
Amgen K.K.
Sanofi K.K.
TERUMO
CORPORATION
Novartis Pharma
K.K.
HeartFlow Japan
G.K.
Bayer Yakuhin, Ltd.
Bristol-Myers Squibb
Boston Scientific
Japan K.K.
Ono Pharmaceutical
Co., Ltd.
Otsuka
Pharmaceutical
Co., Ltd.
Daiichi Sankyo
Company, Limited
Medtronic Japan
Co., Ltd.
Takeda
Pharmaceutical
Company Limited |
|
Daiichi Sankyo
Company, Limited
Research Institute
for Production
Development
CMIC Co., Ltd
Toho University
Ohashi Medical
Center
Otsuka
Pharmaceutical
Co., Ltd.
TERUMO
CORPORATION
ASAHI INTECC
J-sales, INC.
Meditrix
Corporation
Bayer Yakuhin, Ltd.
Nihon Medi-Physics
Co., Ltd.
Abbott Vascular
Japan Co., Ltd. |
Boston Scientific
Japan K.K.
FARMEL CO., Ltd.
TERUMO
CORPORATION
Abbott Medical Japan
L.L.C
Daiichi Sankyo
Company, Limited |
|
|
|
|
|
|
|
Independent
Assessment
Committee:
Masahiro Jinzaki |
|
|
|
|
|
CANON
MEDICAL
SYSTEMS
CORPORATION
GE Healthcare
Japan Corporation
Bayer Yakuhin, Ltd.
FUJIFILM
Corporation |
Fuji Pharma Co., Ltd.
Daiichi Sankyo
Company, Limited
Eisai Co., Ltd.
FUJIFILM Toyama
Chemical Co., Ltd.
Guerbet Japan K.K.
GE Healthcare
Pharma Corporation |
Nihon Medi-Physics
Co., Ltd. |
|
|
|
|
|
|
Independent
Assessment
Committee:
Takeshi Kimura |
|
|
|
Abbott Medical
Japan L.L.C
Abbott Vascular
Japan Co., Ltd.
Kowa Company,
Ltd.
Bristol-Myers Squibb
Boston Scientific
Japan K.K. |
|
Bayer Yakuhin, Ltd.
Edwards
Lifesciences
Corporation
Daiichi Sankyo
Company, Limited
EP-CRSU Co., Ltd.
Kowa Company,
Ltd.
Pfizer Japan Inc. |
Nippon Boehringer
Ingelheim Co ., Ltd.
Otsuka
Pharmaceutical Co.,
Ltd.
Daiichi Sankyo
Company, Limited
Mitsubishi Tanabe
Pharma Corporation
Bayer Yakuhin, Ltd.
Takeda
Pharmaceutical
Company Limited
Astellas Pharma Inc. |
|
|
|
|
|
|
|
Independent
Assessment
Committee:
Masami Kosuge |
|
|
|
Daiichi Sankyo
Company, Limited |
|
|
|
|
|
|
|
|
|
|
Independent
Assessment
Committee:
Kenichi Nakajima |
|
|
|
FUJIFILM Toyama
Chemical Co., Ltd. |
|
FUJIFILM Toyama
Chemical Co., Ltd.
Spectrum Dynamics
Medical Ltd. |
|
FUJIFILM Toyama
Chemical Co., Ltd.
Nihon Medi-Physics
Co., Ltd.
Siemens Healthcare
K.K. |
|
|
|
|
|
|
*The following persons have no conflict of interest to declare:
Members: Kenji Fukushima, none
Members: Susumu Manabe, none
Members: Takayuki Ohno, none
Members: Mitsuaki Sawano, none
Members: Koichi Toda, none
Collaborators: Yodo Gatate, none
Collaborators: Ryo Nakazato, none
Collaborators: Nozomi Niimi, none
Collaborators: Yuichi Saito, none
Collaborators: Ippei Watanabe, none
References
- 1.
Maron DJ, Hochman JS, O’Brien SM, Reynolds HR, Boden WE, Stone GW, et al. International Study of Comparative Health Effectiveness with Medical and Invasive Approaches (ISCHEMIA) trial: Rationale and design. Am Heart J 2018; 201: 124–135.
- 2.
Inohara T, Kohsaka S, Spertus JA, Masoudi FA, Rumsfeld JS, Kennedy KF, et al. Comparative trends in percutaneous coronary intervention in Japan and the United States, 2013 to 2017. J Am Coll Cardiol 2020; 76: 1328–1340.
- 3.
Maron DJ, Hochman JS, Reynolds HR, Bangalore S, O’Brien SM, Boden WE, et al; ISCHEMIA Research Group. Initial invasive or conservative strategy for stable coronary disease. N Engl J Med 2020; 382: 1395–1407.
- 4.
Spertus JA, Jones PG, Maron DJ, O’Brien SM, Reynolds HR, Rosenberg Y, et al; ISCHEMIA Research Group. Health-status outcomes with invasive or conservative care in coronary disease. N Engl J Med 2020; 382: 1408–1419.
- 5. Deleted in proof.
- 6.
Tavella R, Cutri N, Tucker G, Adams R, Spertus J, Beltrame JF. Natural history of patients with insignificant coronary artery disease. Eur Heart J Qual Care Clin Outcomes 2016; 2: 117–124.
- 7.
Jespersen L, Hvelplund A, Abildstrøm SZ, Pedersen F, Galatius S, Madsen JK, et al. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur Heart J 2012; 33: 734–744.
- 8.
Bangalore S, Maron DJ, O’Brien SM, Fleg JL, Kretov EI, Briguori C, et al. Management of coronary disease in patients with advanced kidney disease. N Engl J Med 2020; 382: 1608–1618.
- 9.
Hoffmann U, Ferencik M, Udelson JE, Picard MH, Truong QA, Patel MR, et al. Prognostic value of noninvasive cardiovascular testing in patients with stable chest pain: Insights from the PROMISE Trial (Prospective Multicenter Imaging Study for Evaluation of Chest Pain). Circulation 2017; 135: 2320–2332.
- 10.
Foldyna B, Udelson JE, Karády J, Banerji D, Lu MT, Mayrhofer T, et al. Pre-test probability for patients with suspected obstructive coronary artery disease: Re-evaluating Diamond-Forrester for the contemporary era and clinical implications: Insights from the PROMISE trial. Eur Heart J Cardiovasc Imaging 2019; 20: 574–581.
- 11.
Knuuti J, Ballo H, Juarez-Orozco LE, Saraste A, Kolh P, Rutjes AWS, et al. The performance of non-invasive tests to rule-in and rule-out significant coronary artery stenosis in patients with stable angina: A meta-analysis focused on post-test disease probability. Eur Heart J 2018; 39: 3322–3330.
- 12. Deleted in proof.
- 13.
Diamond GA. A clinically relevant classification of chest discomfort. J Am Coll Cardiol 1983; 1: 574–575.
- 14.
Genders TS, Steyerberg EW, Alkadhi H, Leschka S, Desbiolles L, Nieman K, et al. A clinical prediction rule for the diagnosis of coronary artery disease: Validation, updating, and extension. Eur Heart J 2011; 32: 1316–1330.
- 15.
Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J 2020; 41: 407–477.
- 16.
Gulati M, Levy PD, Mukherjee D, Amsterdam E, Bhatt DL, Birtcher KK, et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guideline for the evaluation and diagnosis of chest pain: A report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021; 144: e368–e454.
- 17.
Diamond GA, Forrester JS. Analysis of probability as an aid in the clinical diagnosis of coronary-artery disease. N Engl J Med 1979; 300: 1350–1358.
- 18.
Pryor DB, Shaw L, Harrell FE Jr, Lee KL, Hlatky MA, Mark DB, et al. Estimating the likelihood of severe coronary artery disease. Am J Med 1991; 90: 553–562.
- 19.
Pryor DB, Harrell FE Jr, Lee KL, Califf RM, Rosati RA. Estimating the likelihood of significant coronary artery disease. Am J Med 1983; 75: 771–780.
- 20.
Cheng VY, Berman DS, Rozanski A, Dunning AM, Achenbach S, Al-Mallah M, et al. Performance of the traditional age, sex, and angina typicality-based approach for estimating pre-test probability of angiographically significant coronary artery disease in patients undergoing coronary computed tomographic angiography: Results from the multinational coronary CT angiography evaluation for clinical outcomes: An international multicenter registry (CONFIRM). Circulation 2011; 124: 2423–2432, 1–8.
- 21.
Reeh J, Therming CB, Heitmann M, Højberg S, Sørum C, Bech J, et al. Prediction of obstructive coronary artery disease and prognosis in patients with suspected stable angina. Eur Heart J 2019; 40: 1426–1435.
- 22.
Saraste A, Knuuti J. ESC 2019 guidelines for the diagnosis and management of chronic coronary syndromes: Recommendations for cardiovascular imaging. Herz 2020; 45: 409–420.
- 23.
Nakata T, Takura T, Yokoi H, Nakajima K, Kohsaka S, Hashimoto A, et al. Design of the Japanese Comprehensive Health-Economic Assessment for Appropriate Cardiac Imaging Strategy Including Outcome and Cost-Effectiveness in Stable Coronary Artery Disease Study (J-CONCIOUS). Circ Rep 2020; 2: 759–763.
- 24.
Winther S, Schmidt SE, Mayrhofer T, Bøtker HE, Hoffmann U, Douglas PS, et al. Incorporating coronary calcification into pre-test assessment of the likelihood of coronary artery disease. J Am Coll Cardiol 2020; 76: 2421–2432.
- 24a.
Watanabe I. HEART’s Selection, A Paradigm shift of stable CAD: Seeking the strategies after ISCHEMIA. Ch2. PTP: An essential technique for diagnosing CAD. Heart 2021; 9: 894–896 [in Japanese].
- 25.
Caraguel CG, Vanderstichel R. The two-step Fagan’s nomogram: Ad hoc interpretation of a diagnostic test result without calculation. Evid Based Med 2013; 18: 125–128.
- 26.
Quintana M, Viele K, Lewis RJ. Bayesian analysis: Using prior information to interpret the results of clinical trials. JAMA 2017; 318: 1605–1606.
- 27.
Joshi PH, de Lemos JA. Diagnosis and management of stable angina: A review. JAMA 2021; 325: 1765–1778.
- 28.
Rotenstein LS, Huckman RS, Wagle NW. Making patients and doctors happier: The potential of patient-reported outcomes. N Engl J Med 2017; 377: 1309–1312.
- 29.
Kimble LP, Dunbar SB, Weintraub WS, McGuire DB, Fazio S, De AK, et al. The Seattle Angina Questionnaire: Reliability and validity in women with chronic stable angina. Heart Dis 2002; 4: 206–211.
- 30.
Thomas M, Jones PG, Arnold SV, Spertus JA. Interpretation of the Seattle Angina Questionnaire as an outcome measure in clinical trials and clinical care: A review. JAMA Cardiol 2021; 6: 593–599.
- 31.
Mesnier J, Ducrocq G, Danchin N, Ferrari R, Ford I, Tardif JC, et al. International observational analysis of evolution and outcomes of chronic stable angina: The multinational CLARIFY study. Circulation 2021; 144: 512–523.
- 32.
Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, et al. [2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC)]. G Ital Cardiol (Rome) 2016; 17: 831–872 (in Italian).
- 33.
Spertus JA, Jones P, McDonell M, Fan V, Fihn SD. Health status predicts long-term outcome in outpatients with coronary disease. Circulation 2002; 106: 43–49.
- 34. Deleted in proof.
- 35.
Arnold SV, Morrow DA, Lei Y, Cohen DJ, Mahoney EM, Braunwald E, et al. Economic impact of angina after an acute coronary syndrome: Insights from the MERLIN-TIMI 36 trial. Circ Cardiovasc Qual Outcomes 2009; 2: 344–353.
- 36.
Basch E, Deal AM, Dueck AC, Scher HI, Kris MG, Hudis C, et al. Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA 2017; 318: 197–198.
- 37.
Owlia M, Dodson JA, King JB, Derington CG, Herrick JS, Sedlis SP, et al. Angina severity, mortality, and healthcare utilization among veterans with stable angina. JAHA 2019; 8: e012811.
- 38.
Hachamovitch R, Hayes SW, Friedman JD, Cohen I, Berman DS. Comparison of the short-term survival benefit associated with revascularization compared with medical therapy in patients with no prior coronary artery disease undergoing stress myocardial perfusion single photon emission computed tomography. Circulation 2003; 107: 2900–2907.
- 39.
Shaw LJ, Iskandrian AE. Prognostic value of gated myocardial perfusion SPECT. J Nucl Cardiol 2004; 11: 171–185.
- 40.
Cheruvu C, Precious B, Naoum C, Blanke P, Ahmadi A, Soon J, et al. Long term prognostic utility of coronary CT angiography in patients with no modifiable coronary artery disease risk factors: Results from the 5 year follow-up of the CONFIRM International Multicenter Registry. J Cardiovasc Comp Tomogr 2016; 10: 22–27.
- 41.
Danad I, Szymonifka J, Twisk JWR, Norgaard BL, Zarins CK, Knaapen P, et al. Diagnostic performance of cardiac imaging methods to diagnose ischaemia-causing coronary artery disease when directly compared with fractional flow reserve as a reference standard: A meta-analysis. Eur Heart J 2017; 38: 991–998.
- 42.
Sand NPR, Veien KT, Nielsen SS, Nørgaard BL, Larsen P, Johansen A, et al. Prospective comparison of FFR derived from coronary CT angiography with SPECT perfusion imaging in stable coronary artery disease: The ReASSESS study. JACC Cardiovascr Imaging 2018; 11: 1640–1650.
- 43.
Stocker TJ, Deseive S, Leipsic J, Hadamitzky M, Chen MY, Rubinshtein R, et al. Reduction in radiation exposure in cardiovascular computed tomography imaging: Results from the PROspective multicenter registry on radiaTion dose Estimates of cardiac CT angIOgraphy iN daily practice in 2017 (PROTECTION VI). Eur Heart J 2018; 39: 3715–3723.
- 44.
Hadamitzky M, Achenbach S, Al-Mallah M, Berman D, Budoff M, Cademartiri F, et al. Optimized prognostic score for coronary computed tomographic angiography: Results from the CONFIRM registry (COronary CT Angiography EvaluatioN For Clinical Outcomes: An InteRnational Multicenter Registry). J Am Coll Cardiol 2013; 62: 468–476.
- 44a.
Neglia D, Rovai D, Caselli C, Pietila M, Teresinska A, Aguadé-Bruix S, et al. Detection of significant coronary artery disease by noninvasive anatomical and functional imaging. Circ Cardiovasc Imaging 2015; 8: e002179.
- 45.
Motoyama S, Sarai M, Narula J, Ozaki Y. Coronary CT angiography and high-risk plaque morphology. Cardiovascular Interv Ther 2013; 28: 1–8.
- 46.
Hulten E, Bittencourt MS, Singh A, O’Leary D, Christman MP, Osmani W, et al. Coronary artery disease detected by coronary computed tomographic angiography is associated with intensification of preventive medical therapy and lower low-density lipoprotein cholesterol. Circ Cardiovasc Imaging 2014; 7: 629–638.
- 47.
Feuchtner G, Kerber J, Burghard P, Dichtl W, Friedrich G, Bonaros N, et al. The high-risk criteria low-attenuation plaque <60 HU and the napkin-ring sign are the most powerful predictors of MACE: A long-term follow-up study. Eur Heart J Cardiovasc Imaging 2017; 18: 772–779.
- 48.
Newby DE, Adamson PD, Berry C, Boon NA, Dweck MR, Flather M, et al. Coronary CT angiography and 5-year risk of myocardial infarction. N Engl J Med 2018; 379: 924–933.
- 49.
van Rosendael AR, Bax JJ. Improved risk stratification with computed tomographic coronary angiography in patients with suspected coronary artery disease. Eur Heart J Cardiovasc Imaging 2017; 18: 849–850.
- 50.
van Rosendael AR, Shaw LJ, Xie JX, Dimitriu-Leen AC, Smit JM, Scholte AJ, et al. Superior risk stratification with coronary computed tomography angiography using a comprehensive atherosclerotic risk score. JACC Cardiovascr Imaging 2019; 12: 1987–1997.
- 51.
Bittencourt MS, Hulten EA, Murthy VL, Cheezum M, Rochitte CE, Di Carli MF, et al. Clinical outcomes after evaluation of stable chest pain by coronary computed tomographic angiography versus usual care: A meta-analysis. Circ Cardiovasc Imaging 2016; 9: e004419.
- 52.
de Jong MC, Genders TS, van Geuns RJ, Moelker A, Hunink MG. Diagnostic performance of stress myocardial perfusion imaging for coronary artery disease: A systematic review and meta-analysis. Eur Radiol 2012; 22: 1881–1895.
- 53.
Nishimura T, Nakajima K, Kusuoka H, Yamashina A, Nishimura S. Prognostic study of risk stratification among Japanese patients with ischemic heart disease using gated myocardial perfusion SPECT: J-ACCESS study. Eur J Nucl Med Mol Imaging 2008; 35: 319–328.
- 54.
Nagel E, Greenwood JP, McCann GP, Bettencourt N, Shah AM, Hussain ST, et al. Magnetic resonance perfusion or fractional flow reserve in coronary disease. N Engl J Med 2019; 380: 2418–2428.
- 55.
Shiraishi S, Sakamoto F, Tsuda N, Yoshida M, Tomiguchi S, Utsunomiya D, et al. Prediction of left main or 3-vessel disease using myocardial perfusion reserve on dynamic thallium-201 single-photon emission computed tomography with a semiconductor gamma camera. Circ J 2015; 79: 623–631.
- 56.
Fiechter M, Ghadri JR, Gebhard C, Fuchs TA, Pazhenkottil AP, Nkoulou RN, et al. Diagnostic value of 13N-ammonia myocardial perfusion PET: Added value of myocardial flow reserve. J Nucl Med 2012; 53: 1230–1234.
- 57.
Murthy VL, Bateman TM, Beanlands RS, Berman DS, Borges-Neto S, Chareonthaitawee P, et al. Clinical quantification of myocardial blood flow using PET: Joint Position Paper of the SNMMI Cardiovascular Council and the ASNC. J Nucl Med 2018; 59: 273–293.
- 58.
Shaw LJ, Cerqueira MD, Brooks MM, Althouse AD, Sansing VV, Beller GA, et al. Impact of left ventricular function and the extent of ischemia and scar by stress myocardial perfusion imaging on prognosis and therapeutic risk reduction in diabetic patients with coronary artery disease: Results from the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial. J Nucl Cardiol 2012; 19: 658–669.
- 59.
Inohara T, Kohsaka S, Miyata H, Ueda I, Ishikawa S, Ohki T, et al. Appropriateness ratings of percutaneous coronary intervention in Japan and its association with the trend of noninvasive testing. JACC Cardiovasc Interv 2014; 7: 1000–1009.
- 60.
Yamagishi M, Tamaki N, Akasaka T, Ikeda T, Ueshima K, Uemura S, et al. JCS 2018 guideline on diagnosis of chronic coronary heart diseases. Circ J 2021; 85: 402–572.
- 61.
Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: Executive summary: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation 2011; 124: 2574–2609.
- 62.
Shaw LJ, Berman DS, Picard MH, Friedrich MG, Kwong RY, Stone GW, et al. Comparative definitions for moderate-severe ischemia in stress nuclear, echocardiography, and magnetic resonance imaging. JACC Cardiovasc Imaging 2014; 7: 593–604.
- 63.
Xu Y, Arsanjani R, Clond M, Hyun M, Lemley M Jr, Fish M, et al. Transient ischemic dilation for coronary artery disease in quantitative analysis of same-day sestamibi myocardial perfusion SPECT. J Nucl Cardiol 2012; 19: 465–473.
- 64.
Nasir K, Narula J, Mortensen MB. Message for upcoming chest pain management guidelines: Time to acknowledge the power of zero. J Am Coll Cardiol 2020; 76: 2433–2435.
- 65.
Min JK, Koo BK, Erglis A, Doh JH, Daniels DV, Jegere S, et al. Effect of image quality on diagnostic accuracy of noninvasive fractional flow reserve: Results from the prospective multicenter international DISCOVER-FLOW study. J Cardiovasc Comp Tomogr 2012; 6: 191–199.
- 66.
Min JK, Leipsic J, Pencina MJ, Berman DS, Koo BK, van Mieghem C, et al. Diagnostic accuracy of fractional flow reserve from anatomic CT angiography. JAMA 2012; 308: 1237–1245.
- 67.
Nørgaard BL, Leipsic J, Gaur S, Seneviratne S, Ko BS, Ito H, et al. Diagnostic performance of noninvasive fractional flow reserve derived from coronary computed tomography angiography in suspected coronary artery disease: The NXT trial (Analysis of Coronary Blood Flow Using CT Angiography: Next Steps). J Am Coll Cardiol 2014; 63: 1145–1155.
- 68.
Nakazato R, Park HB, Berman DS, Gransar H, Koo BK, Erglis A, et al. Noninvasive fractional flow reserve derived from computed tomography angiography for coronary lesions of intermediate stenosis severity: Results from the DeFACTO study. Circ Cardiovasc Imaging 2013; 6: 881–889.
- 69.
Patel MR, Nørgaard BL, Fairbairn TA, Nieman K, Akasaka T, Berman DS, et al. 1-year impact on medical practice and clinical outcomes of FFR(CT): The ADVANCE registry. JACC Cardiovasc Imaging 2020; 13: 97–105.
- 70.
Graby J, Metters R, Kandan SR, McKenzie D, Lowe R, Carson K, et al. Real-world clinical and cost analysis of CT coronary angiography and CT coronary angiography-derived fractional flow reserve (FFR(CT))-guided care in the National Health Service. Clin Radiol 2021; 76: 862.e19–862.e28.
- 71.
Kimura T, Shiomi H, Kuribayashi S, Isshiki T, Kanazawa S, Ito H, et al. Cost analysis of non-invasive fractional flow reserve derived from coronary computed tomographic angiography in Japan. Cardiovasc Interv Ther 2015; 30: 38–44.
- 72.
Curzen N, Nicholas Z, Stuart B, Wilding S, Hill K, Shambrook J, et al. Fractional flow reserve derived from computed tomography coronary angiography in the assessment and management of stable chest pain: The FORECAST randomized trial. Eur Heart J 2021; 42: 3844–3852.
- 73.
Tanabe Y, Kido T, Kimura F, Kobayashi Y, Matsunaga N, Yoshioka K, et al. Japanese survey of radiation dose associated with coronary computed tomography angiography: 2013 data from a multicenter registry in daily practice. Circ J 2020; 84: 601–608.
- 74.
Otsuka R, Miyazaki Y, Kubo N, Kawahara M, Takaesu J, Fukuchi K. The status of stress myocardial perfusion imaging using 99 mTc pharmaceuticals in Japan: Results from a nationwide survey. Asia Ocean J Nucl Med Biol 2018; 6: 90–96.
- 75.
Einstein AJ, Pascual TN, Mercuri M, Karthikeyan G, Vitola JV, Mahmarian JJ, et al. Current worldwide nuclear cardiology practices and radiation exposure: Results from the 65 country IAEA Nuclear Cardiology Protocols Cross-Sectional Study (INCAPS). Eur Heart J 2015; 36: 1689–1696.
- 75a.
Cerqueira MD. Recommendations for reducing radiation exposure in myocardial perfusion imaging. Journal of Nuclear Cardiology volume 17, pages 709–718 (2010).
- 76.
Danad I, Szymonifka J, Schulman-Marcus J, Min JK. Static and dynamic assessment of myocardial perfusion by computed tomography. Eur Heart J Cardiovasc Imaging 2016; 17: 836–844.
- 77.
Vonder M, van der Werf NR, Leiner T, Greuter MJW, Fleischmann D, Vliegenthart R, et al. The impact of dose reduction on the quantification of coronary artery calcifications and risk categorization: A systematic review. J Cardiovasc Comp Tomogr 2018; 12: 352–363.
- 78.
Gibbons RJ, Balady GJ, Bricker JT, Chaitman BR, Fletcher GF, Froelicher VF, et al. ACC/AHA 2002 guideline update for exercise testing: Summary article. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines). J Am Coll Cardiol 2002; 40: 1531–1540.
- 79.
Fletcher GF, Ades PA, Kligfield P, Arena R, Balady GJ, Bittner VA, et al. Exercise standards for testing and training: A Scientific Statement from the American Heart Association. Circulation 2013; 128: 873–934.
- 80.
Rozanski A, Gransar H, Min JK, Hayes SW, Friedman JD, Thomson LE, et al. Long-term mortality following normal exercise myocardial perfusion SPECT according to coronary disease risk factors. J Nucl Cardiol 2014; 21: 341–350.
- 81.
Douglas PS, Hoffmann U, Patel MR, Mark DB, Al-Khalidi HR, Cavanaugh B, et al. Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med 2015; 372: 1291–1300.
- 82.
Budoff MJ, Mayrhofer T, Ferencik M, Bittner D, Lee KL, Lu MT, et al. Prognostic value of coronary artery calcium in the PROMISE study (Prospective Multicenter Imaging Study for Evaluation of Chest Pain). Circulation 2017; 136: 1993–2005.
- 83.
Noto TJ Jr, Johnson LW, Krone R, Weaver WF, Clark DA, Kramer JR Jr, et al. Cardiac catheterization 1990: A report of the Registry of the Society for Cardiac Angiography and Interventions (SCA&I). Cathet Cardiovasc Diagn 1991; 24: 75–83.
- 84.
Coronary Artery Surgery Study (CASS): A randomized trial of coronary artery bypass surgery. Survival data. Circulation 1983; 68: 939–950.
- 85.
Serruys PW, Morice MC, Kappetein AP, Colombo A, Holmes DR, Mack MJ, et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med 2009; 360: 961–972.
- 86.
Nam CW, Mangiacapra F, Entjes R, Chung IS, Sels JW, Tonino PA, et al. Functional SYNTAX score for risk assessment in multivessel coronary artery disease. J Am Coll Cardiol 2011; 58: 1211–1218.
- 87.
Farooq V, van Klaveren D, Steyerberg EW, Meliga E, Vergouwe Y, Chieffo A, et al. Anatomical and clinical characteristics to guide decision making between coronary artery bypass surgery and percutaneous coronary intervention for individual patients: Development and validation of SYNTAX score II. Lancet 2013; 381: 639–650.
- 88.
Takahashi K, Serruys PW, Fuster V, Farkouh ME, Spertus JA, Cohen DJ, et al. Redevelopment and validation of the SYNTAX score II to individualise decision making between percutaneous and surgical revascularisation in patients with complex coronary artery disease: Secondary analysis of the multicentre randomised controlled SYNTAXES trial with external cohort validation. Lancet 2020; 396: 1399–1412.
- 89.
Meijboom WB, Van Mieghem CA, van Pelt N, Weustink A, Pugliese F, Mollet NR, et al. Comprehensive assessment of coronary artery stenoses: Computed tomography coronary angiography versus conventional coronary angiography and correlation with fractional flow reserve in patients with stable angina. J Am Coll Cardiol 2008; 52: 636–643.
- 90.
Lopes RD, Alexander KP, Stevens SR, Reynolds HR, Stone GW, Piña IL, et al. Initial invasive versus conservative management of stable ischemic heart disease in patients with a history of heart failure or left ventricular dysfunction: Insights from the ISCHEMIA trial. Circulation 2020; 142: 1725–1735.
- 91.
Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J 2019; 40: 87–165.
- 92.
Kawase Y, Matsuo H, Akasaka T, Shiono Y, Tanaka N, Amano T, et al. Clinical use of physiological lesion assessment using pressure guidewires: An expert consensus document of the Japanese Association of Cardiovascular Intervention and Therapeutics. Cardiovasc Interv Ther 2019; 34: 85–96.
- 93.
De Bruyne B, Fearon WF, Pijls NH, Barbato E, Tonino P, Piroth Z, et al. Fractional flow reserve-guided PCI for stable coronary artery disease. N Engl J Med 2014; 371: 1208–1217.
- 94.
Tanaka N, Nakamura M, Akasaka T, Kadota K, Uemura S, Amano T, et al. One-year outcome of fractional flow reserve-based coronary intervention in Japanese daily practice: CVIT-DEFER registry. Circ J 2017; 81: 1301–1306.
- 95.
Xaplanteris P, Fournier S, Pijls NHJ, Fearon WF, Barbato E, Tonino PAL, et al. Five-year outcomes with PCI guided by fractional flow reserve. N Engl J Med 2018; 379: 250–259.
- 96.
Zimmermann FM, Ferrara A, Johnson NP, van Nunen LX, Escaned J, Albertsson P, et al. Deferral vs. performance of percutaneous coronary intervention of functionally non-significant coronary stenosis: 15-year follow-up of the DEFER trial. Eur Heart J 2015; 36: 3182–3188.
- 97.
van Nunen LX, Zimmermann FM, Tonino PA, Barbato E, Baumbach A, Engstrøm T, et al. Fractional flow reserve versus angiography for guidance of PCI in patients with multivessel coronary artery disease (FAME): 5-year follow-up of a randomised controlled trial. Lancet 2015; 386: 1853–1860.
- 98.
Usui Y, Chikamori T, Yanagisawa H, Morishima T, Hida S, Tanaka N, et al. Reliability of pressure-derived myocardial fractional flow reserve in assessing coronary artery stenosis in patients with previous myocardial infarction. Am J Cardiol 2003; 92: 699–702.
- 99.
Fournier S, Toth GG, De Bruyne B, Johnson NP, Ciccarelli G, Xaplanteris P, et al. Six-year follow-up of fractional flow reserve-guided versus angiography-guided coronary artery bypass graft surgery. Circ Cardiovasc Interv 2018; 11: e006368.
- 100.
De Bruyne B, Pijls NH, Bartunek J, Kulecki K, Bech JW, De Winter H, et al. Fractional flow reserve in patients with prior myocardial infarction. Circulation 2001; 104: 157–162.
- 101.
Hamilos M, Muller O, Cuisset T, Ntalianis A, Chlouverakis G, Sarno G, et al. Long-term clinical outcome after fractional flow reserve-guided treatment in patients with angiographically equivocal left main coronary artery stenosis. Circulation 2009; 120: 1505–1512.
- 102.
Glineur D, Grau JB, Etienne PY, Benedetto U, Fortier JH, Papadatos S, et al. Impact of preoperative fractional flow reserve on arterial bypass graft anastomotic function: The IMPAG trial. Eur Heart J 2019; 40: 2421–2428.
- 103.
Ntalianis A, Sels JW, Davidavicius G, Tanaka N, Muller O, Trana C, et al. Fractional flow reserve for the assessment of nonculprit coronary artery stenoses in patients with acute myocardial infarction. JACC Cardiovasc Interv 2010; 3: 1274–1281.
- 104.
Van Belle E, Gil R, Klauss V, Balghith M, Meuwissen M, Clerc J, et al. Impact of routine invasive physiology at time of angiography in patients with multivessel coronary artery disease on reclassification of revascularization strategy: Results from the DEFINE REAL study. JACC Cardiovasc Interv 2018; 11: 354–365.
- 105.
Ahn JM, Park DW, Shin ES, Koo BK, Nam CW, Doh JH, et al. Fractional flow reserve and cardiac events in coronary artery disease: Data from a prospective IRIS-FFR registry (Interventional Cardiology Research Incooperation Society Fractional Flow Reserve). Circulation 2017; 135: 2241–2251.
- 106.
Johnson NP, Tóth GG, Lai D, Zhu H, Açar G, Agostoni P, et al. Prognostic value of fractional flow reserve: Linking physiologic severity to clinical outcomes. J Am Coll Cardiol 2014; 64: 1641–1654.
- 107.
Sud M, Han L, Koh M, Austin PC, Farkouh ME, Ly HQ, et al. Association between adherence to fractional flow reserve treatment thresholds and major adverse cardiac events in patients with coronary artery disease. JAMA 2020; 324: 2406–2414.
- 108.
Weerts J, Pustjens T, Amin E, Ilhan M, Veenstra LF, Theunissen R, et al. Long-term outcome after deferred revascularization due to negative fractional flow reserve in intermediate coronary lesions. Cathet Cardiovasc Interv 2021; 97: 247–256.
- 109.
Depta JP, Patel JS, Novak E, Gage BF, Masrani SK, Raymer D, et al. Risk model for estimating the 1-year risk of deferred lesion intervention following deferred revascularization after fractional flow reserve assessment. Eur Heart J 2015; 36: 509–515.
- 110.
Van Belle E, Rioufol G, Pouillot C, Cuisset T, Bougrini K, Teiger E, et al. Outcome impact of coronary revascularization strategy reclassification with fractional flow reserve at time of diagnostic angiography: Insights from a large French multicenter fractional flow reserve registry. Circulation 2014; 129: 173–185.
- 111.
Escaned J, Collet C, Ryan N, De Maria GL, Walsh S, Sabate M, et al. Clinical outcomes of state-of-the-art percutaneous coronary revascularization in patients with de novo three vessel disease: 1-year results of the SYNTAX II study. Eur Heart J 2017; 38: 3124–3134.
- 112.
Fearon WF, Bornschein B, Tonino PA, Gothe RM, Bruyne BD, Pijls NH, et al. Economic evaluation of fractional flow reserve-guided percutaneous coronary intervention in patients with multivessel disease. Circulation 2010; 122: 2545–2550.
- 113.
Fearon WF, Nishi T, De Bruyne B, Boothroyd DB, Barbato E, Tonino P, et al. Clinical outcomes and cost-effectiveness of fractional flow reserve-guided percutaneous coronary intervention in patients with stable coronary artery disease: Three-year follow-up of the FAME 2 trial (Fractional Flow Reserve Versus Angiography for Multivessel Evaluation). Circulation 2018; 137: 480–487.
- 114.
Yu W, Tanigaki T, Ding D, Wu P, Du H, Ling L, et al. Accuracy of intravascular ultrasound-based fractional flow reserve in identifying hemodynamic significance of coronary stenosis. Circ Cardiovasc Interv 2021; 14: e009840.
- 115.
Collet C, Onuma Y, Sonck J, Asano T, Vandeloo B, Kornowski R, et al. Diagnostic performance of angiography-derived fractional flow reserve: A systematic review and Bayesian meta-analysis. Eur Heart J 2018; 39: 3314–3321.
- 116.
Tu S, Westra J, Adjedj J, Ding D, Liang F, Xu B, et al. Fractional flow reserve in clinical practice: From wire-based invasive measurement to image-based computation. Eur Heart J 2020; 41: 3271–3279.
- 117.
Huang J, Emori H, Ding D, Kubo T, Yu W, Huang P, et al. Diagnostic performance of intracoronary optical coherence tomography-based versus angiography-based fractional flow reserve for the evaluation of coronary lesions. EuroIntervention 2020; 16: 568–576.
- 118.
Davies JE, Sen S, Dehbi HM, Al-Lamee R, Petraco R, Nijjer SS, et al. Use of the instantaneous wave-free ratio or fractional flow reserve in PCI. N Engl J Med 2017; 376: 1824–1834.
- 119.
Götberg M, Christiansen EH, Gudmundsdottir IJ, Sandhall L, Danielewicz M, Jakobsen L, et al. Instantaneous wave-free ratio versus fractional flow reserve to guide PCI. N Engl J Med 2017; 376: 1813–1823.
- 120.
Lee JM, Choi KH, Park J, Hwang D, Rhee TM, Kim J, et al. Physiological and clinical assessment of resting physiological indexes. Circulation 2019; 139: 889–900.
- 121.
Van’t Veer M, Pijls NHJ, Hennigan B, Watkins S, Ali ZA, De Bruyne B, et al. Comparison of different diastolic resting indexes to iFR: Are they all equal? J Am Coll Cardiol 2017; 70: 3088–3096.
- 122.
Petraco R, Escaned J, Sen S, Nijjer S, Asrress KN, Echavarria-Pinto M, et al. Classification performance of instantaneous wave-free ratio (iFR) and fractional flow reserve in a clinical population of intermediate coronary stenoses: Results of the ADVISE registry. EuroIntervention 2013; 9: 91–101.
- 123.
Berry C, van’t Veer M, Witt N, Kala P, Bocek O, Pyxaras SA, et al. VERIFY (VERification of Instantaneous Wave-Free Ratio and Fractional Flow Reserve for the Assessment of Coronary Artery Stenosis Severity in EverydaY Practice): A multicenter study in consecutive patients. J Am Coll Cardiol 2013; 61: 1421–1427.
- 124.
Lee JM, Rhee TM, Choi KH, Park J, Hwang D, Kim J, et al. Clinical outcome of lesions with discordant results among different invasive physiologic indices: Resting distal coronary to aortic pressure ratio, resting full-cycle ratio, diastolic pressure ratio, instantaneous wave-free ratio, and fractional flow reserve. Circ J 2019; 83: 2210–2221.
- 125.
Lee JM, Lee SH, Hwang D, Rhee TM, Choi KH, Kim J, et al. Long-term clinical outcomes of nonhyperemic pressure ratios: Resting full-cycle ratio, diastolic pressure ratio, and instantaneous wave-free ratio. JAHA 2020; 9: e016818.
- 126.
Wada T, Shiono Y, Kubo T, Honda K, Takahata M, Shimamura K, et al. Impact of instantaneous wave-free ratio on graft failure after coronary artery bypass graft surgery. Int J Cardiol 2021; 324: 23–29.
- 127.
Andell P, Berntorp K, Christiansen EH, Gudmundsdottir IJ, Sandhall L, Venetsanos D, et al. Reclassification of treatment strategy with instantaneous wave-free ratio and fractional flow reserve: A substudy from the iFR-SWEDEHEART trial. JACC Cardiovasc Interv 2018; 11: 2084–2094.
- 128.
Warisawa T, Cook CM, Rajkumar C, Howard JP, Seligman H, Ahmad Y, et al. Safety of revascularization deferral of left main stenosis based on instantaneous wave-free ratio evaluation. JACC Cardiovasc Interv 2020; 13: 1655–1664.
- 129.
Goto R, Takashima H, Ohashi H, Ando H, Suzuki A, Sakurai S, et al. Independent predictors of discordance between the resting full-cycle ratio and fractional flow reserve. Heart Vessels 2021; 36: 790–798.
- 130.
Lee JM, Shin ES, Nam CW, Doh JH, Hwang D, Park J, et al. Discrepancy between fractional flow reserve and instantaneous wave-free ratio: Clinical and angiographic characteristics. Int J Cardiol 2017; 245: 63–68.
- 131.
Kato Y, Dohi T, Chikata Y, Fukase T, Takeuchi M, Takahashi N, et al. Predictors of discordance between fractional flow reserve and resting full-cycle ratio in patients with coronary artery disease: Evidence from clinical practice. J Cardiol 2021; 77: 313–319.
- 132.
Lee JM, Shin ES, Nam CW, Doh JH, Hwang D, Park J, et al. Clinical outcomes according to fractional flow reserve or instantaneous wave-free ratio in deferred lesions. JACC Cardiovasc Interv 2017; 10: 2502–2510.
- 133.
Lee SH, Choi KH, Lee JM, Hwang D, Rhee TM, Park J, et al. Physiologic characteristics and clinical outcomes of patients with discordance between FFR and iFR. JACC Cardiovasc Interv 2019; 12: 2018–2031.
- 134.
Hwang D, Jeon KH, Lee JM, Park J, Kim CH, Tong Y, et al. Diagnostic performance of resting and hyperemic invasive physiological indices to define myocardial ischemia: validation with (13)N-ammonia positron emission tomography. JACC Cardiovasc Interv 2017; 10: 751–760.
- 135.
Pijls NH, De Bruyne B, Peels K, Van Der Voort PH, Bonnier HJ, Bartunek J, et al. Measurement of fractional flow reserve to assess the functional severity of coronary-artery stenoses. N Engl J Med 1996; 334: 1703–1708.
- 136.
Kikuta Y, Cook CM, Sharp ASP, Salinas P, Kawase Y, Shiono Y, et al. Pre-angioplasty instantaneous wave-free ratio pullback predicts hemodynamic outcome in humans with coronary artery disease: Primary results of the international multicenter iFR GRADIENT registry. JACC Cardiovasc Interv 2018; 11: 757–767.
- 137.
Collet C, Sonck J, Vandeloo B, Mizukami T, Roosens B, Lochy S, et al. Measurement of hyperemic pullback pressure gradients to characterize patterns of coronary atherosclerosis. J Am Coll Cardiol 2019; 74: 1772–1784.
- 138.
Jeremias A, Davies JE, Maehara A, Matsumura M, Schneider J, Tang K, et al. Blinded physiological assessment of residual ischemia after successful angiographic percutaneous coronary intervention: The DEFINE PCI study. JACC Cardiovasc Interv 2019; 12: 1991–2001.
- 139.
Niimi N, Sawano M, Ikemura N, Nagai T, Nakano S, Shoji S, et al. Applicability and eligibility of the International Study of Comparative Health Effectiveness with Medical and Invasive Approaches (ISCHEMIA) for patients who underwent revascularization with percutaneous coronary intervention. J Clin Med 2020; 9: 2889.
- 140.
Hachamovitch R, Rozanski A, Shaw LJ, Stone GW, Thomson LE, Friedman JD, et al. Impact of ischaemia and scar on the therapeutic benefit derived from myocardial revascularization vs. medical therapy among patients undergoing stress-rest myocardial perfusion scintigraphy. Eur Heart J 2011; 32: 1012–1024.
- 141.
Shaw LJ, Berman DS, Maron DJ, Mancini GB, Hayes SW, Hartigan PM, et al. Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: Results from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial nuclear substudy. Circulation 2008; 117: 1283–1291.
- 142.
Boden WE, O’Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med 2007; 356: 1503–1516.
- 143.
Sedlis SP, Hartigan PM, Teo KK, Maron DJ, Spertus JA, Mancini GB, et al. Effect of PCI on long-term survival in patients with stable ischemic heart disease. N Engl J Med 2015; 373: 1937–1946.
- 144.
Chaitman BR, Alexander KP, Cyr DD, Berger JS, Reynolds HR, Bangalore S, et al. Myocardial infarction in the ISCHEMIA trial: Impact of different definitions on incidence, prognosis, and treatment comparisons. Circulation 2021; 143: 790–804.
- 145.
Navarese EP, Lansky AJ, Kereiakes DJ, Kubica J, Gurbel PA, Gorog DA, et al. Cardiac mortality in patients randomised to elective coronary revascularisation plus medical therapy or medical therapy alone: A systematic review and meta-analysis. Eur Heart J 2021; 42: 4638–4651.
- 146.
Whitney SN, McGuire AL, McCullough LB. A typology of shared decision making, informed consent, and simple consent. Ann Intern Med 2004; 140: 54–59.
- 147.
Spertus JA, Salisbury AC, Jones PG, Conaway DG, Thompson RC. Predictors of quality-of-life benefit after percutaneous coronary intervention. Circulation 2004; 110: 3789–3794.
- 148.
Steg PG, Greenlaw N, Tendera M, Tardif JC, Ferrari R, Al-Zaibag M, et al. Prevalence of anginal symptoms and myocardial ischemia and their effect on clinical outcomes in outpatients with stable coronary artery disease: Data from the International Observational CLARIFY Registry. JAMA Intern Med 2014; 174: 1651–1659.
- 149.
Al-Lamee R, Thompson D, Dehbi HM, Sen S, Tang K, Davies J, et al. Percutaneous coronary intervention in stable angina (ORBITA): A double-blind, randomised controlled trial. Lancet 2018; 391: 31–40.
- 150.
Al-Lamee R, Howard JP, Shun-Shin MJ, Thompson D, Dehbi HM, Sen S, et al. Fractional flow reserve and instantaneous wave-free ratio as predictors of the placebo-controlled response to percutaneous coronary intervention in stable single-vessel coronary artery disease. Circulation 2018; 138: 1780–1792.
- 151.
Weintraub WS, Spertus JA, Kolm P, Maron DJ, Zhang Z, Jurkovitz C, et al. Effect of PCI on quality of life in patients with stable coronary disease. N Engl J Med 2008; 359: 677–687.
- 152.
Hoffmann TC, Montori VM, Del Mar C. The connection between evidence-based medicine and shared decision making. JAMA 2014; 312: 1295–1296.
- 153.
European Coronary Surgery Study Group. Long-term results of prospective randomised study of coronary artery bypass surgery in stable angina pectoris. Lancet 1982; 2: 1173–1180.
- 154.
Takaro T, Peduzzi P, Detre KM, Hultgren HN, Murphy ML, van der Bel-Kahn J, et al. Survival in subgroups of patients with left main coronary artery disease: Veterans Administration Cooperative Study of Surgery for Coronary Arterial Occlusive Disease. Circulation 1982; 66: 14–22.
- 155.
Yusuf S, Zucker D, Peduzzi P, Fisher LD, Takaro T, Kennedy JW, et al. Effect of coronary artery bypass graft surgery on survival: Overview of 10-year results from randomised trials by the Coronary Artery Bypass Graft Surgery Trialists Collaboration. Lancet 1994; 344: 563–570.
- 156.
Bittl JA, He Y, Jacobs AK, Yancy CW, Normand SL. Bayesian methods affirm the use of percutaneous coronary intervention to improve survival in patients with unprotected left main coronary artery disease. Circulation 2013; 127: 2177–2185.
- 157.
Lee PH, Ahn JM, Chang M, Baek S, Yoon SH, Kang SJ, et al. Left main coronary artery disease: Secular trends in patient characteristics, treatments, and outcomes. J Am Coll Cardiol 2016; 68: 1233–1246.
- 158.
Bainey KR, Alemayehu W, Welsh RC, Kumar A, King SB 3rd, Kirtane AJ. Long-term clinical outcomes following revascularization in high-risk coronary anatomy patients with stable ischemic heart disease. JAHA 2021; 10: e018104.
- 159.
Shoaib A, Rashid M, Kontopantelis E, Sharp A, Fahy EF, Nolan J, et al. Clinical characteristics and outcomes from percutaneous coronary intervention of last remaining coronary artery: An analysis from the British Cardiovascular Intervention Society database. Circ Cardiovasc Interv 2020; 13: e009049.
- 160.
Faridi KF, Rymer JA, Rao SV, Dai D, Wojdyla D, Yeh RW, et al. Ad hoc percutaneous coronary intervention in patients with stable coronary artery disease: A report from the National Cardiovascular Data Registry CathPCI Registry. Am Heart J 2019; 216: 53–61.
- 161.
Kim YH, Park DW, Ahn JM, Park GM, Cho YR, Lee JY, et al. Impact of ad hoc percutaneous coronary intervention with drug-eluting stents in angina patients. EuroIntervention 2013; 9: 110–117.
- 162.
Toyota T, Morimoto T, Shiomi H, Ando K, Ono K, Shizuta S, et al. Ad hoc vs. non-ad hoc percutaneous coronary intervention strategies in patients with stable coronary artery disease. Circ J 2017; 81: 458–467.
- 163.
Blankenship JC, Gigliotti OS, Feldman DN, Mixon TA, Patel RA, Sorajja P, et al. Ad hoc percutaneous coronary intervention: A Consensus Statement from the Society for Cardiovascular Angiography and Interventions. Cathet Cardiovasc Interv 2013; 81: 748–758.
- 164.
Adele C, Vaitkus PT, Wells SK, Zehnacker JB. Cost advantages of an ad hoc angioplasty strategy. J Am Coll Cardiol 1998; 31: 321–325.
- 165.
Le Feuvre C, Helft G, Beygui F, Zerah T, Fonseca E, Catuli D, et al. Safety, efficacy, and cost advantages of combined coronary angiography and angioplasty. J Interv Cardiol 2003; 16: 195–199.
- 166.
Nallamothu BK, Krumholz HM. Putting ad hoc PCI on pause. JAMA 2010; 304: 2059–2060.
- 166a.
Maddox TM, Chan PS, Spertus JA, Tang F, Jones P, Ho PM, et al. Variations in coronary artery disease secondary prevention prescriptions among outpatient cardiology practices: Insights from the NCDR (National Cardiovascular Data Registry). J Am Coll Cardiol 2014; 63: 539–546.
- 167.
Bangalore S, Maron DJ, Stone GW, Hochman JS. Routine revascularization versus initial medical therapy for stable ischemic heart disease: A systematic review and meta-analysis of randomized trials. Circulation 2020; 142: 841–857.
- 168.
Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the diagnosis and management of patients with stable ischemic heart disease: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 2012; 60: e44–e164.
- 169.
Chen HY, Saczynski JS, Lapane KL, Kiefe CI, Goldberg RJ. Adherence to evidence-based secondary prevention pharmacotherapy in patients after an acute coronary syndrome: A systematic review. Heart Lung 2015; 44: 299–308.
- 170.
Patterson T, McConkey HZR, Ahmed-Jushuf F, Moschonas K, Nguyen H, Karamasis GV, et al. Long-term outcomes following Heart Team revascularization recommendations in complex coronary artery disease. JAHA 2019; 8: e011279.
- 171.
Domienik-Karłowicz J, Kupczyńska K, Michalski B, Kapłon-Cieślicka A, Darocha S, Dobrowolski P, et al. Fourth universal definition of myocardial infarction: Selected messages from the European Society of Cardiology document and lessons learned from the new guidelines on ST-segment elevation myocardial infarction and non-ST-segment elevation-acute coronary syndrome. Cardiol J 2021; 28: 195–201.
- 172.
Nakamura M, Kimura K, Kimura T, Ishihara M, Otsuka F, Kozuma K, et al. JCS 2020 guideline focused update on antithrombotic therapy in patients with coronary artery disease. Circ J 2020; 84: 831–865.
- 173.
Takagi Y, Takahashi J, Yasuda S, Miyata S, Tsunoda R, Ogata Y, et al. Prognostic stratification of patients with vasospastic angina: A comprehensive clinical risk score developed by the Japanese Coronary Spasm Association. J Am Coll Cardiol 2013; 62: 1144–1153.
- 174.
Robertson RM, Wood AJ, Vaughn WK, Robertson D. Exacerbation of vasotonic angina pectoris by propranolol. Circulation 1982; 65: 281–285.
- 175.
Pristipino C, Beltrame JF, Finocchiaro ML, Hattori R, Fujita M, Mongiardo R, et al. Major racial differences in coronary constrictor response between Japanese and Caucasians with recent myocardial infarction. Circulation 2000; 101: 1102–1108.
- 176.
Endo A, Kohsaka S, Miyata H, Kawamura A, Noma S, Suzuki M, et al. Disparity in the application of guideline-based medical therapy after percutaneous coronary intervention: Analysis from the Japanese prospective multicenter registry. Am J Cardiovasc Drugs 2013; 13: 103–112.
- 177.
Nakao K, Yasuda S, Nishimura K, Noguchi T, Nakai M, Miyamoto Y, et al. Prescription rates of guideline-directed medications are associated with in-hospital mortality among Japanese patients with acute myocardial infarction: A report from JROAD-DPC study. JAHA 2019; 8: e009692.
- 178.
Bangalore S, Parkar S, Messerli FH. Long-acting calcium antagonists in patients with coronary artery disease: A meta-analysis. Am J Med 2009; 122: 356–365.
- 179.
Bangalore S, Steg G, Deedwania P, Crowley K, Eagle KA, Goto S, et al. β-Blocker use and clinical outcomes in stable outpatients with and without coronary artery disease. JAMA 2012; 308: 1340–1349.
- 180.
Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: A meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010; 376: 1670–1681.
- 181.
Cannon CP, Braunwald E, McCabe CH, Rader DJ, Rouleau JL, Belder R, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004; 350: 1495–1504.
- 182.
Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019; 139: e1082–e1143.
- 183.
Zhan S, Tang M, Liu F, Xia P, Shu M, Wu X. Ezetimibe for the prevention of cardiovascular disease and all-cause mortality events. Cochrane Database Syst Rev 2018; 11: CD012502.
- 184.
Schmidt AF, Carter JP, Pearce LS, Wilkins JT, Overington JP, Hingorani AD, et al. PCSK9 monoclonal antibodies for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst Rev 2020; 10: CD011748.
- 185.
Taguchi I, Iimuro S, Iwata H, Takashima H, Abe M, Amiya E, et al. High-Dose Versus Low-Dose Pitavastatin in Japanese Patients With Stable Coronary Artery Disease (REAL-CAD): A randomized superiority trial. Circulation 2018; 137: 1997–2009.
- 186.
Saito Y, Okada S, Ogawa H, Soejima H, Sakuma M, Nakayama M, et al. Low-dose aspirin for primary prevention of cardiovascular events in patients with type 2 diabetes mellitus: 10-year follow-up of a randomized controlled trial. Circulation 2017; 135: 659–670.
- 187.
Uchiyama S, Ishizuka N, Shimada K, Teramoto T, Yamazaki T, Oikawa S, et al. Aspirin for stroke prevention in elderly patients with vascular risk factors: Japanese Primary Prevention Project. Stroke 2016; 47: 1605–1611.
- 188.
Kinoshita M, Yokote K, Arai H, Iida M, Ishigaki Y, Ishibashi S, et al. Japan Atherosclerosis Society (JAS) guidelines for prevention of atherosclerotic cardiovascular diseases 2017. J Atheroscler Thromb 2018; 25: 846–984.
- 189.
Lewis CE, Fine LJ, Beddhu S, Cheung AK, Cushman WC, Cutler JA, et al. Final report of a trial of intensive versus standard blood-pressure control. N Engl J Med 2021; 384: 1921–1930.
- 190.
Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: A systematic review and meta-analysis of cardiovascular outcome trials. Lancet 2019; 393: 31–39.
- 191.
Kristensen SL, Rørth R, Jhund PS, Docherty KF, Sattar N, Preiss D, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: A systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabet Endocrinol 2019; 7: 776–785.
- 192.
Palmer SC, Tendal B, Mustafa RA, Vandvik PO, Li S, Hao Q, et al. Sodium-glucose cotransporter protein-2 (SGLT-2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists for type 2 diabetes: Systematic review and network meta-analysis of randomised controlled trials. BMJ (Clin Res Ed) 2021; 372: m4573.
- 193.
Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J 2020; 41: 255–323.
- 194.
[American Diabetes Association. ] 10. Cardiovascular disease and risk management: Standards of medical care in diabetes-2021. Diabetes Care 2021; 44: S125–S150.
- 195.
Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med 2019; 380: 11–22.
- 196.
Nicholls SJ, Lincoff AM, Garcia M, Bash D, Ballantyne CM, Barter PJ, et al. Effect of high-dose omega-3 fatty acids vs corn oil on major adverse cardiovascular events in patients at high cardiovascular risk: The STRENGTH randomized clinical trial. JAMA 2020; 324: 2268–2280.
- 197.
Virani SS, Nambi V, Ballantyne CM. Has the ‘strength’ of fish oil therapy been ‘reduced’? Reconciling the results of REDUCE-IT and STRENGTH. Eur Heart J Cardiovasc Pharmacother 2021; 7: e7–e8.
- 198.
Bouabdallaoui N, Tardif JC, Waters DD, Pinto FJ, Maggioni AP, Diaz R, et al. Time-to-treatment initiation of colchicine and cardiovascular outcomes after myocardial infarction in the Colchicine Cardiovascular Outcomes Trial (COLCOT). Eur Heart J 2020; 41: 4092–4099.
- 199.
Nidorf SM, Fiolet ATL, Mosterd A, Eikelboom JW, Schut A, Opstal TSJ, et al. Colchicine in patients with chronic coronary disease. N Engl J Med 2020; 383: 1838–1847.
- 200.
Fletcher GF, Balady GJ, Amsterdam EA, Chaitman B, Eckel R, Fleg J, et al. Exercise standards for testing and training: A statement for healthcare professionals from the American Heart Association. Circulation 2001; 104: 1694–1740.
- 201.
Anderson L, Thompson DR, Oldridge N, Zwisler AD, Rees K, Martin N, et al. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database System Rev 2016; 2016: CD001800.
- 202.
Fulcher J, O’Connell R, Voysey M, Emberson J, Blackwell L, Mihaylova B, et al. Efficacy and safety of LDL-lowering therapy among men and women: Meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet 2015; 385: 1397–1405.
- 203.
Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, Peto R, et al. Aspirin in the primary and secondary prevention of vascular disease: Collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009; 373: 1849–1860.
- 204.
Frye RL, August P, Brooks MM, Hardison RM, Kelsey SF, MacGregor JM, et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med 2009; 360: 2503–2515.
- 205.
Velazquez EJ, Lee KL, Deja MA, Jain A, Sopko G, Marchenko A, et al. Coronary-artery bypass surgery in patients with left ventricular dysfunction. N Engl J Med 2011; 364: 1607–1616.
- 206.
De Bruyne B, Pijls NH, Kalesan B, Barbato E, Tonino PA, Piroth Z, et al. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med 2012; 367: 991–1001.
- 207.
Dumaine RL, Montalescot G, Steg PG, Ohman EM, Eagle K, Bhatt DL. Renal function, atherothrombosis extent, and outcomes in high-risk patients. Am Heart J 2009; 158: 141–148.e1.
- 208.
Ducrocq G, Wallace JS, Baron G, Ravaud P, Alberts MJ, Wilson PW, et al. Risk score to predict serious bleeding in stable outpatients with or at risk of atherothrombosis. Eur Heart J 2010; 31: 1257–1265.
- 209.
Vidal-Petiot E, Ford I, Greenlaw N, Ferrari R, Fox KM, Tardif JC, et al. Cardiovascular event rates and mortality according to achieved systolic and diastolic blood pressure in patients with stable coronary artery disease: An international cohort study. Lancet 2016; 388: 2142–2152.
- 210.
Kohsaka S, Fukushima K, Watanabe I, Manabe S, Niimi N, Gatate Y, et al. Contemporary management of stable coronary artery disease: Implications of the ISCHEMIA trial. Circ J 2021; 85: 1919–1927.
- 211.
Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002; 346: 877–883.
- 212.
Garcia MJ, Kwong RY, Scherrer-Crosbie M, Taub CC, Blankstein R, Lima J, et al. State of the Art: Imaging for myocardial viability: A Scientific Statement from the American Heart Association. Circ Cardiovasc Imaging 2020; 13: e000053.
- 213.
Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2016; 17: 1321–1360.
- 214.
Steeds RP, Garbi M, Cardim N, Kasprzak JD, Sade E, Nihoyannopoulos P, et al. EACVI appropriateness criteria for the use of transthoracic echocardiography in adults: A report of literature and current practice review. Eur Heart J Cardiovasc Imaging 2017; 18: 1191–1204.
- 215.
Tsutsui H, Isobe M, Ito H, Ito H, Okumura K, Ono M, et al. JCS 2017/JHFS 2017 guideline on diagnosis and treatment of acute and chronic heart failure: Digest version. Circ J 2019; 83: 2084–2184.
- 216.
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37: 2129–2200.
- 217.
Daubert C, Gold MR, Abraham WT, Ghio S, Hassager C, Goode G, et al. Prevention of disease progression by cardiac resynchronization therapy in patients with asymptomatic or mildly symptomatic left ventricular dysfunction: Insights from the European cohort of the REVERSE (Resynchronization Reverses Remodeling in Systolic Left Ventricular Dysfunction) trial. J Am Coll Cardiol 2009; 54: 1837–1846.
- 218.
Ryan M, Morgan H, Petrie MC, Perera D. Coronary revascularisation in patients with ischaemic cardiomyopathy. Heart (Br Card Soc) 2021; 107: 612–618.
- 219.
Velazquez EJ, Lee KL, Jones RH, Al-Khalidi HR, Hill JA, Panza JA, et al. Coronary-artery bypass surgery in patients with ischemic cardiomyopathy. N Engl J Med 2016; 374: 1511–1520.
- 220.
Fox CS, Matsushita K, Woodward M, Bilo HJ, Chalmers J, Heerspink HJ, et al. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: A meta-analysis. Lancet 2012; 380: 1662–1673.
- 221.
Matsushita K, Coresh J, Sang Y, Chalmers J, Fox C, Guallar E, et al. Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: A collaborative meta-analysis of individual participant data. Lancet Diabet Endocrinol 2015; 3: 514–525.
- 222.
Herzog CA, Natwick T, Li S, Charytan DM. Comparative utilization and temporal trends in cardiac stress testing in U.S. Medicare beneficiaries with and without chronic kidney disease. JACC Cardiovasc Imaging 2019; 12: 1420–1426.
- 223.
Bangalore S. Stress testing in patients with chronic kidney disease: The need for ancillary markers for effective risk stratification and prognosis. J Nucl Cardiol 2016; 23: 570–574.
- 224.
Winther S, Svensson M, Jørgensen HS, Bouchelouche K, Gormsen LC, Pedersen BB, et al. Diagnostic performance of coronary CT angiography and myocardial perfusion imaging in kidney transplantation candidates. JACC Cardiovasc Imaging 2015; 8: 553–562.
- 225.
Wang LW, Fahim MA, Hayen A, Mitchell RL, Baines L, Lord S, et al. Cardiac testing for coronary artery disease in potential kidney transplant recipients. Cochrane Database System Rev 2011; 2011: CD008691.
- 226.
Hatta T, Nishimura S, Nishimura T. Prognostic risk stratification of myocardial ischaemia evaluated by gated myocardial perfusion SPECT in patients with chronic kidney disease. Eur J Nucl Med Mol Imaging 2009; 36: 1835–1841.
- 227.
Wang LW, Masson P, Turner RM, Lord SW, Baines LA, Craig JC, et al. Prognostic value of cardiac tests in potential kidney transplant recipients: A systematic review. Transplantation 2015; 99: 731–745.
- 228.
Al-Mallah MH, Hachamovitch R, Dorbala S, Di Carli MF. Incremental prognostic value of myocardial perfusion imaging in patients referred to stress single-photon emission computed tomography with renal dysfunction. Circ Cardiovasc Imaging 2009; 2: 429–436.
- 229.
Murthy VL, Naya M, Foster CR, Hainer J, Gaber M, Dorbala S, et al. Coronary vascular dysfunction and prognosis in patients with chronic kidney disease. JACC Cardiovasc Imaging 2012; 5: 1025–1034.
- 230.
Kott J, Reichek N, Butler J, Arbeit L, Mallipattu SK. Cardiac imaging in dialysis patients. Kidney Med 2020; 2: 629–638.
- 231.
Baigent C, Landray MJ, Reith C, Emberson J, Wheeler DC, Tomson C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): A randomised placebo-controlled trial. Lancet 2011; 377: 2181–2192.
- 232.
Wanner C, Krane V, März W, Olschewski M, Mann JF, Ruf G, et al. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med 2005; 353: 238–248.
- 233.
Fellström BC, Jardine AG, Schmieder RE, Holdaas H, Bannister K, Beutler J, et al. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med 2009; 360: 1395–1407.
- 234.
Tse G, Gong M, Nunez J, Sanchis J, Li G, Ali-Hasan-Al-Saegh S, et al. Frailty and mortality outcomes after percutaneous coronary intervention: A systematic review and meta-analysis. J Am Med Dir Assoc 2017; 18: 1097.e1–1097.e10.
- 235.
Kojima G, Iliffe S, Taniguchi Y, Shimada H, Rakugi H, Walters K. Prevalence of frailty in Japan: A systematic review and meta-analysis. J Epidemiol 2017; 27: 347–353.
- 236.
Gore MO, Seliger SL, Defilippi CR, Nambi V, Christenson RH, Hashim IA, et al. Age- and sex-dependent upper reference limits for the high-sensitivity cardiac troponin T assay. J Am Coll Cardiol 2014; 63: 1441–1448.
- 237.
Rozanski A, Berman D. Optimizing the assessment of patient clinical risk at the time of cardiac stress testing. JACC Cardiovasc Imaging 2020; 13: 616–623.
- 238.
Goldberg RB, Stone NJ, Grundy SM. The 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guidelines on the management of blood cholesterol in diabetes. Diabetes Care 2020; 43: 1673–1678.
- 239.
Numasawa Y, Inohara T, Ishii H, Yamaji K, Kohsaka S, Sawano M, et al. Comparison of outcomes after percutaneous coronary intervention in elderly patients, including 10 628 nonagenarians: Insights from a Japanese Nationwide Registry (J-PCI Registry). JAHA 2019; 8: e011183.
- 240.
Varenne O, Cook S, Sideris G, Kedev S, Cuisset T, Carrié D, et al. Drug-eluting stents in elderly patients with coronary artery disease (SENIOR): A randomised single-blind trial. Lancet 2018; 391: 41–50.
- 241.
Urban P, Meredith IT, Abizaid A, Pocock SJ, Carrié D, Naber C, et al. Polymer-free drug-coated coronary stents in patients at high bleeding risk. N Engl J Med 2015; 373: 2038–2047.