III. Basic Knowledge of Radiation Safety Control
1. Effects of Radiation on the Human Body
1.1 Types and Categories of the Effects
The effects of radiation exposure on the human body are categorized according to the location of the radiation source (i.e., X-ray and nuclear medicine), the magnitude of the dose, the form of exposure, including systemic or local exposure, and manifestation of the effects.
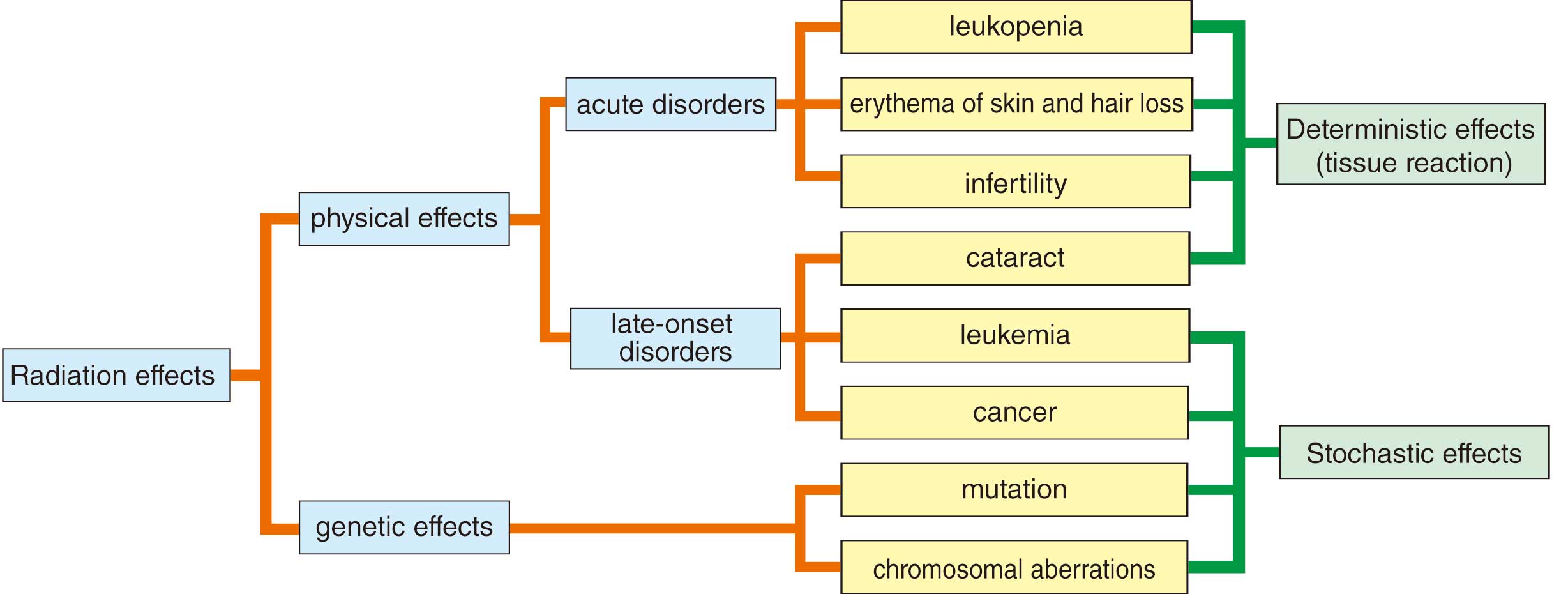
In terms of manifestation, the effects of radiation exposure are divided into 2 forms, physical and genetic effects, and further classified as deterministic effects (tissue reaction) and stochastic effects (Figure 2).19,20
The physical effects appear only in people who have been exposed to radiation, and the genetic effects are the effects on offspring as a result of radiation exposure to parental germinal cells, though genetic effects have not been confirmed in humans. Physical effects are the development of acute symptoms (skin disorders, hair loss, infertility, etc.) within a few weeks of exposure and late symptoms (cancer, cataracts) from months to years after exposure.
1.2 Deterministic Effects (Tissue Response) and Stochastic Effects
A deterministic effect refers to a situation in which there is damage to a certain number of cells in a tissue or organ by radiation exposure and tissue function cannot be maintain, resulting in symptoms developing. In International Commission on Radiological Protection (ICRP) Publication 103 (2007 Recommendations), this is also referred to as a “tissue reaction”.20 The minimum dose at which symptoms are recognized is the “threshold dose for tissue reactions”, defined as the “dose estimated to result in only 1% incidence of tissue reactions”. Above this threshold dose, the incidence of deterministic effects increases rapidly as the dose increases (Figure 3). Skin symptoms and the time from onset according to each threshold dose of radiation exposure on skin are shown in Table 5.17 In the case of the skin absorbed dose exceeding the threshold dose during cardiac catheterization, more careful must be taken to prevent the symptoms by deterministic effects becoming more severe.12,28

Table 5. Manifestations of Radiation Damage to the Skin
| Threshold dose (Gy) |
Effect |
Time of onset |
| 2 |
Early transient erythema |
2–24 h |
| 3 |
Temporary epilation |
≈3 weeks |
| 6 |
Main erythema reaction |
≈1.5 weeks |
| 7 |
Permanent epilation |
≈3 weeks |
| 14 |
Dry desquamation |
≈4 weeks |
| 15 |
Late erythema |
8–10 weeks |
| 18 |
Moist desquamation |
≈4 weeks |
| 18 |
Ischemic dermal necrosis |
>10 weeks |
| 24 |
Secondary ulceration |
>6 weeks |
(Adapted from ICRP, 200017 with modification.)
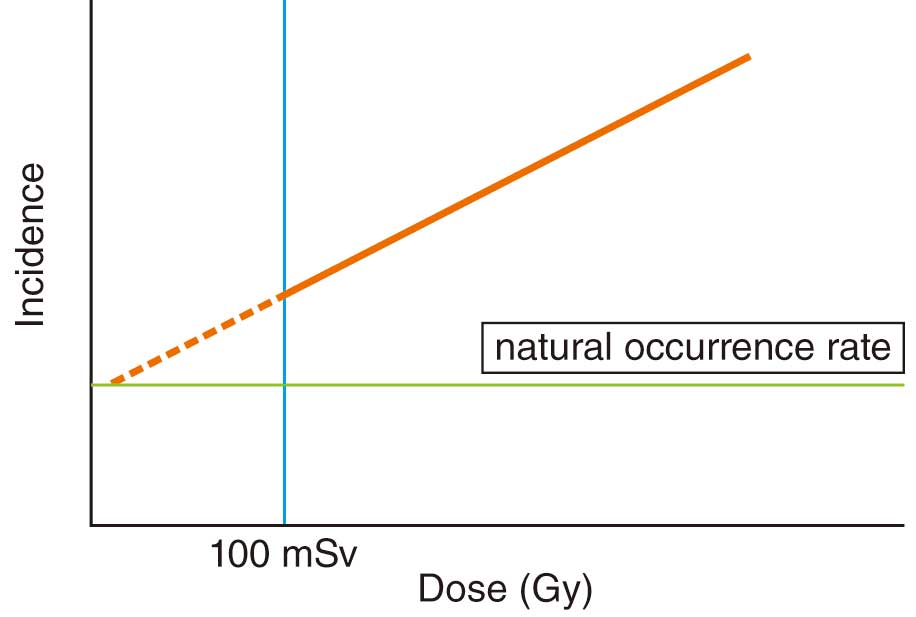
A stochastic effect is the occurrence of cancer and leukemia as a result of malignant transformation of a large number of cells during the process of cell repair after radiation exposure that did not result in killing the cells. Because damaged cells are generated even at low doses, there is no threshold dose for stochastic effects, and the incidence of stochastic effects increases with increasing radiation dose regardless of the severity of stochastic effects (Figure 4). For stochastic effects, carcinogenesis is significantly increased above ≈100 mSv per year, but even below ≈100 mSv, the incidence is assumed to increase with increasing dose.20
1.3 Radiation Doses and Units
To objectively assess the effects of invisible radiation on the human body, it is necessary to understand the difference between the units of radiation dose and the units used to assess the adverse effects by radiation exposure.
1.3.1 Radioactivity
Radioactivity is the unit used in nuclear medicine tests. It is the ability to emit radiation from an atomic nucleus, and is defined as 1 becquerel [Bq] when a nucleus decays in 1 s. The SI unit of becquerel [Bq] is [s−1].
1.3.2 Exposure
This is the total amount of X-ray energies generated by the X-ray device. Using the ionizing effect on air, it is defined as a quantity [C/kg] based on the amount of electron charge generated [C]. The old unit of dose is the roentgen [R].
1 C/kg = 33.97 Gy
1.3.3 Absorbed Dose
This is average energy of radiation imparted to a substance, with Gray [Gy] used as a special unit. In particular, air kerma [AK, Gy] is used for energy given to air, and AK at the reference point is displayed on the X-ray machine for cardiology. The absorbed dose is called the tissue absorbed dose [Gy] because it is the amount of energy imparted to each tissue, and it is the basic dose for calculating the equivalent dose and effective dose and is used in assessing deterministic effects.
1.3.4 Equivalent Dose
This value indicates the extent to which tissues and organs are affected by radiation. The absorbed dose represents the average energy of radiation imparted to tissues and organs, and this is a dose corrected by the type of radiation (e.g., X-rays and neutrons) because, even with the same tissue absorbed dose, the adverse effects change according to the type of radiation. The unit of the equivalent dose is the sievert [Sv] and, based on the exposed organ, it is categorized as the skin equivalent dose or lens equivalent dose. In laws and regulations, equivalent doses are used to assess tissue reactions (deterministic effects) as dose limits, and skin equivalent doses, lens equivalent doses, and abdominal surface equivalent doses for pregnant women are used for radiation workers.
1.3.5 Effective Dose
The effective dose is used for comparing the stochastic risk of non-uniform radiation exposure. In understanding the effective dose, the radiation effect received by tissues and organs is converted into the effect on the whole human body. It is not a simple average of equivalent doses received by tissues and organs, but is weighted by the sensitivity of each tissue or organ to radiation, and is expressed in [Sv]. It should be noted that in medical exposures in cardiology, local equivalent doses are often high even when the effective dose is low. In the law, dose limits are specified for radiation workers in terms of the integrated dose for 5 years, the annual dose and the dose for emergency work.
1.3.6 Personal Dose Equivalent
The personal dose equivalent is used for monitoring of individual radiation workers and is measured in sievert [Sv]. Because the effective dose is assessed in 1-cm dose equivalents and the individual dosimeter is worn on the chest (abdomen for women of childbearing potential) and uneven exposure occurs when radiation protective clothing is worn, the effective dose is calculated from 2 dosimeters: 1 inside the protective clothing and 1 outside (usually on the head and neck). Note that when protective eyewear is worn for measuring equivalent doses to the lens, the dose inside the eyewear must be assessed.
2. Dose Control in the Laboratory
2.1 Patient Exposure
2.1.1 Angiography Room (Table 6)
Table 6. COR and LOE for Patient Dosimetry and Dose Control in the Angiography Room
| |
COR |
LOE |
| It is recommended that dose control is based on the integrated AK value17,29 |
I |
A |
| It is recommended that dose control is based on the AK–area product value17,29 |
I |
A |
| Dose control based on the maximum incident skin dose should be considered17,29 |
IIa |
B |
| Dose control based on fluoroscopy time should be considered17,29 |
IIa |
B |
| Dose control by number of images may be considered17,29 |
IIb |
C |
Dose control by radiation dose rate at the patient’s irradiation reference point dose using
20 cm of acrylic may be considered30 |
IIb |
C |
AK, air kerma; COR, Class of Recommendation; LOE, Level of Evidence.
a. Integrated AK Value and AK–Area Product Value
Article 28 of the Act on Medical Radiology Technicians and Article 16 of the Enforcement Regulations of the Act on Medical Radiology Technicians31 requires radiologists to make a record of the radiation exposure to the human body as part of dose control in the angiography room. Although there are specific instructions for describing the irradiation method in the irradiation record, the laws and regulations do not clearly provide for the proper management of medical exposure of patients.
ICRP Publication 85 (2000 recommendations)17 reports actual cases of radiation skin disorders caused by interventional radiology (IVR) procedures. The document clearly states that the most important aspect of patient exposure is the skin dose at the site of maximum exposure during the IVR procedure, but no system for accurately measuring the intraoperative peak skin dose (PSD) has yet been established.
Publication 85 further states that the clinical protocols for various IVR procedures should include a description of the radiographic procedures (direction, frequency, and imaging conditions), fluoroscopy time, AK rate, and the total skin dose and irradiation site produced by the IVR procedure at each institution. These stated values provide the IVR practitioner with reference levels of patient skin dose, which permit comparison of irradiation conditions and resulting skin doses occurring during the actual procedure.
The most useful patient skin dose information during a procedure is the cumulative AK [mGy or Gy] at the patient entrance reference point (PERP). Because this value is an integrated value for all skin areas exposed to X-rays, the PSD is generally overestimated and is a safe control value. Other recommended control values are the AK rate [mGy/min] and total fluoroscopy time [min] during fluoroscopy at the same site, as described above.
ICRP Publication 118 in 2011 proposed a threshold dose of 0.5 Gy for both cerebral and cardiovascular organ doses,24 and ICRP Publication 120 warned that cardiac organ doses in cardiovascular IVR may reach this level.29 Furthermore, the importance of informed consent regarding radiation risk has been pointed out and it is advocated that patient dose data be recorded and managed in the medical record. In the management of the dose, alert levels are set for patients, and in the event of an excess dose level, early detection and follow-up of skin disorders are performed. The recommended alert levels are 3 Gy for PSD and 5 Gy for AK, and the kerma–area product (KAP) is 500 Gy/cm2.
Two cases of characteristic symptoms are presented.32
Case 1: Patient underwent percutaneous coronary intervention (PCI) for chronic total occlusion in the right coronary artery and was discharged from hospital 1 week later with no symptoms. At the time of outpatient visit 6 weeks after PCI, erythema was observed in an area consistent with the working angle at the time of the procedure. The erythema gradually remitted (Figure 5A). Four years later, when PCI was performed for restenosis in the same area, redness was observed the next day, at a site consistent with the working angle 4 years ago (Figure 5B). The total dose (AK) was 1.8 Gy, which was below the threshold for initial erythema, but careful skin observation is required after PCI.
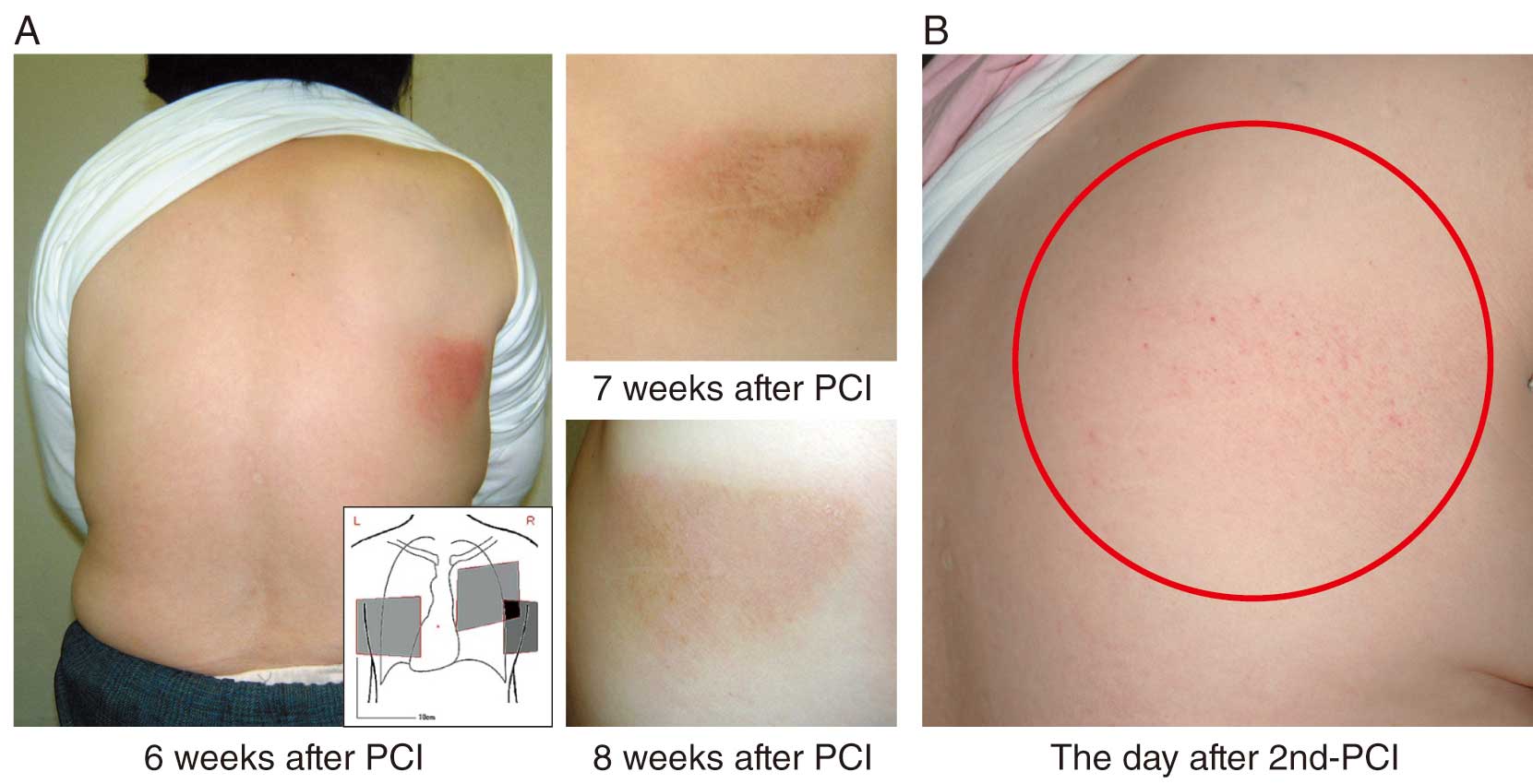
Case 2: Patient underwent PCI for chronic total occlusion in the right coronary artery with a total dose (AK) of 12 Gy. Three days after the procedure, trained staff detected the initial erythema (Figure 6A), whereas untrained staff could not until 10 days after the procedure (Figure 6B). Six months after PCI, there was only mild hyperpigmentation (Figure 6C), but 12 months after the procedure, there was a flare-up of redness (Figure 6D). Considering the recurrence of erythema, it is necessary to continuously monitor the skin.
b. Peak Skin Dose
The PSD of a patient at the time of IVR must be monitored to prevent radiation skin damage. Currently, several systems for monitoring entrance skin doses are used in clinical practice. Figure 7 shows a real-time dosimeter using a scintillator as the sensor element. The reading section of the dosimeter is connected to the sensor by an optical cable. The sensor and the optical cable are highly X-ray transparent and have little effect on X-ray images. A dosimeter capable of measuring up to 4 channels simultaneously is also available, which can be affixed to a presumed PSD site for real-time dose monitoring during IVR.33

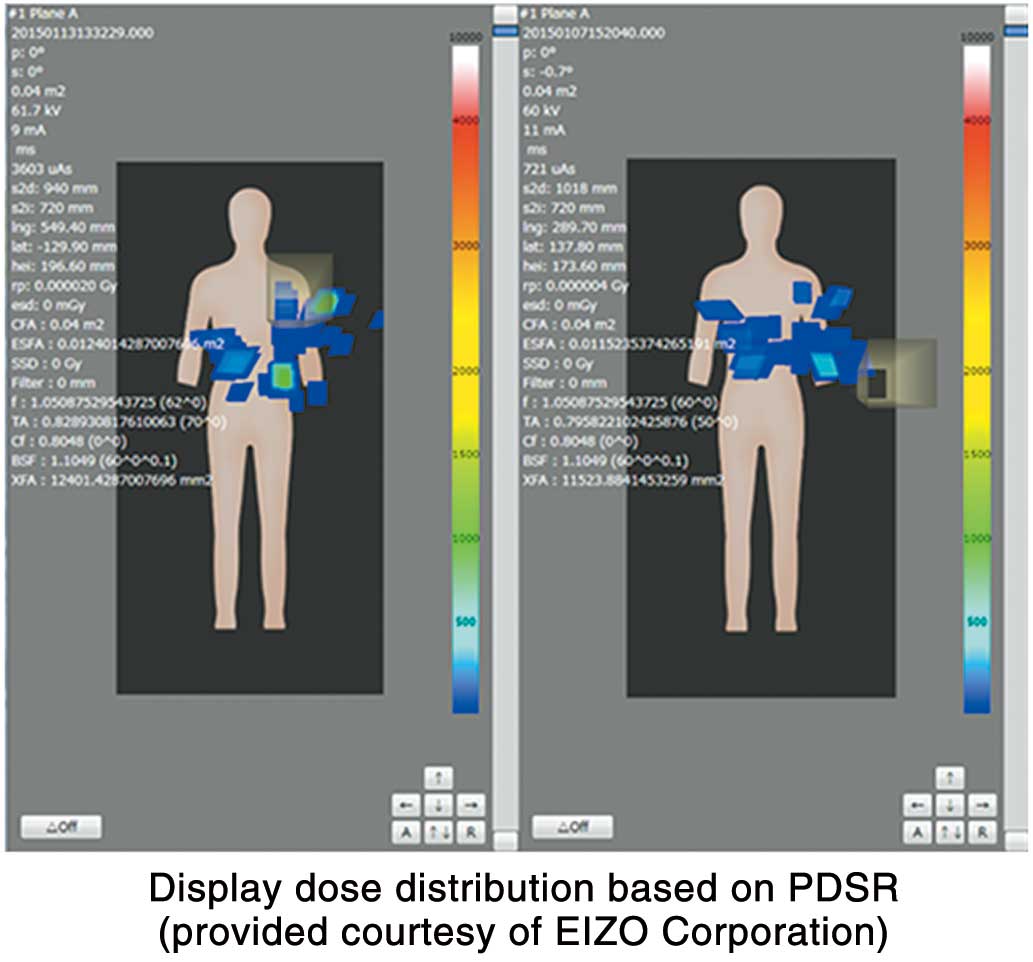
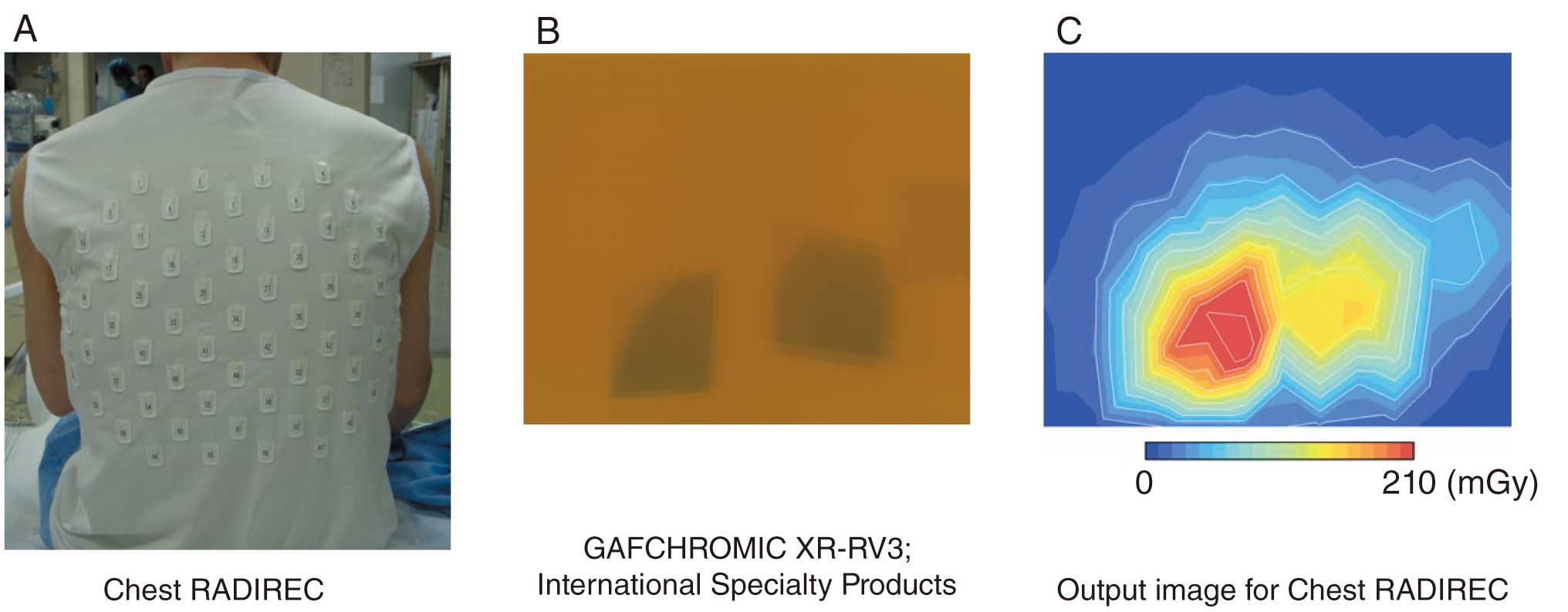
The Chest RADIREC system34 can accurately assess the dose and its distribution on the patient’s skin surface, but real-time monitoring is not possible (Figure 8). The GAFCHROMIC Film for dosimetry depicts the dose as the degree of darkening (Figure 8B), whereas with the Chest RADIREC system the dose is measured by wearing a jacket in which a number of fluorescent glass dosimeters are inserted (Figure 8A), and the fluorescent glass dosimeters are read after IVR to create a distribution map (Figure 8C). Figure 8B,C shows the dose distributions measured simultaneously using GAFCHROMIC Film and Chest RADIREC, and similar dose distributions can be observed for both.
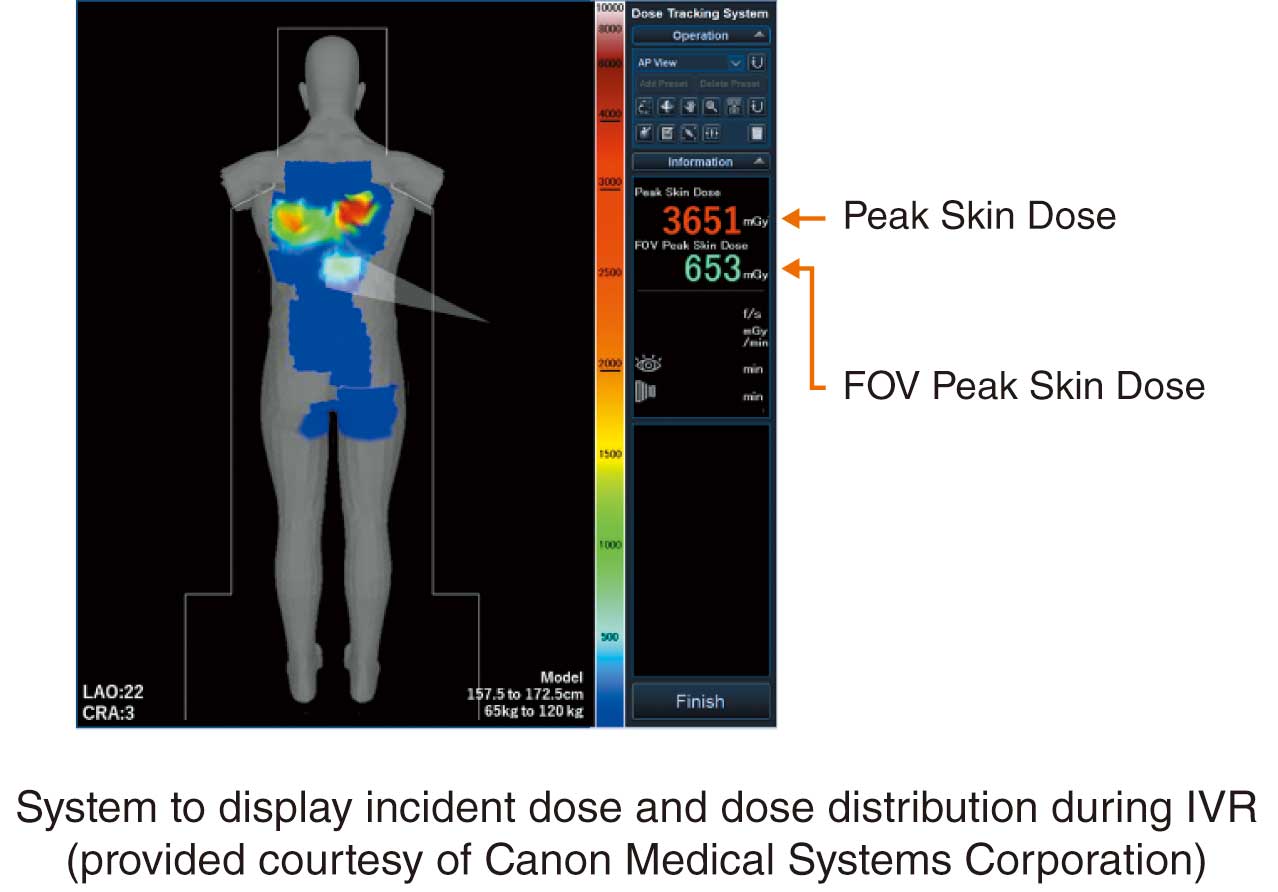
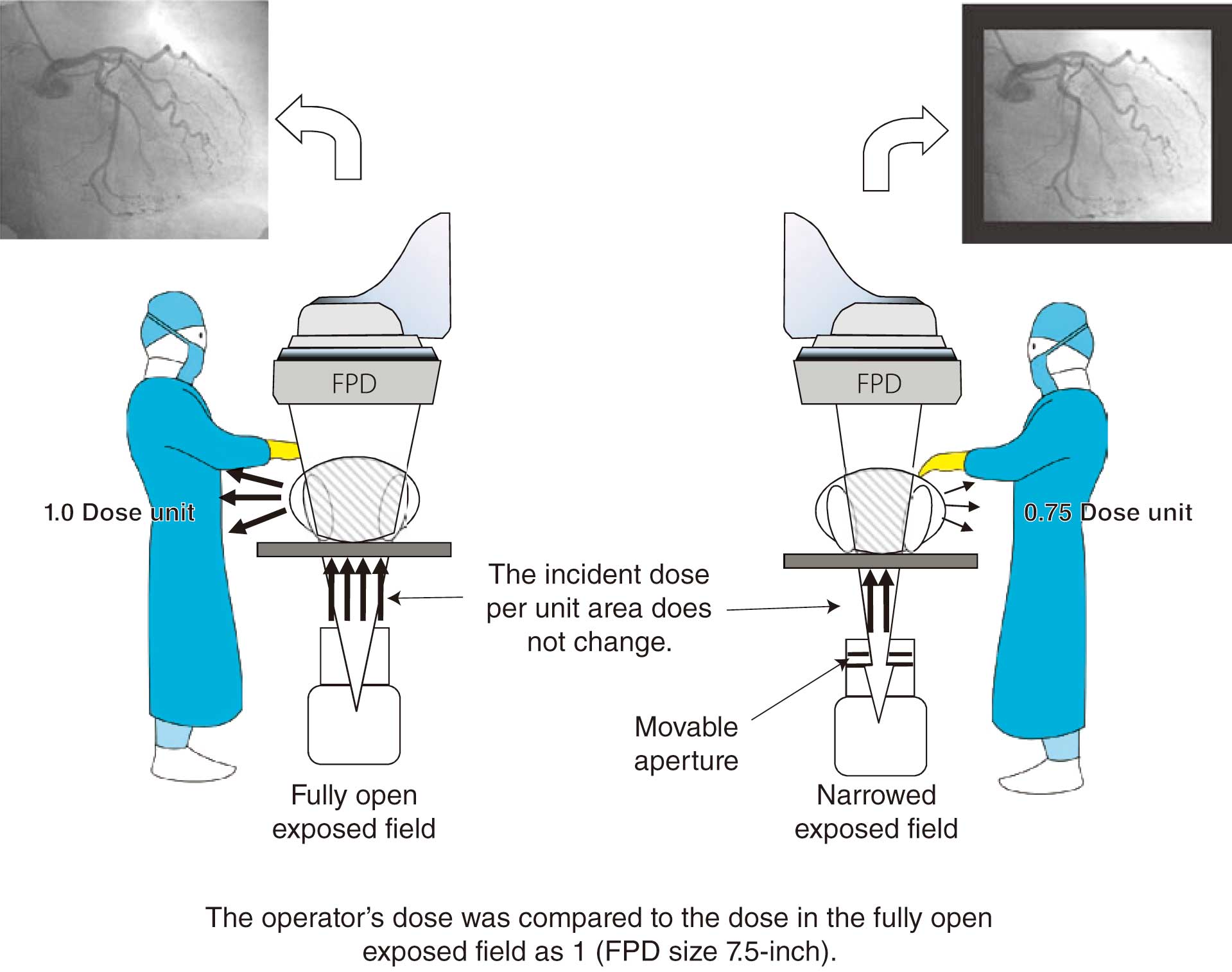
In recent years, along with advancement of dose control systems, tools to calculate and plot the incident skin surface and PSD by receiving a radiation dose structured report (RDSR) from the radiography system have been developed (Figure 9). In this case, the dose distribution map is displayed after the IVR procedure and the RDSR is generated. However, a system that displays the dose distribution and PSDs in real time during IVR procedures has been developed (Figure 10). The geometric relationships of the C-arm and catheter bed and the X-ray conditions are used to visualize patient doses on a patient model. Real-time evaluation of PSD is possible, and when the incident dose approaches the alert level, the operator can reduce the dose during the procedure by changing the irradiation field size, irradiation position, and dose setting.
c. Dose Optimization by DRL
ICRP Publication 73 (1996 Recommendations) recommended the use of DRLs for optimization of comprehensive protection in medical exposure.16 Furthermore, ICRP Publication 105 (2007 Recommendations) stated that, in principle, DRLs should be used in IVR to facilitate patient dose control and avoid the stochastic effects of unnecessary radiation.22 On the other hand, the International Atomic Energy Agency states that the dose used to estimate stochastic effect is KAP.35 It also states that the tissue response (deterministic effects) of radiation skin damage is associated with PSD and can be estimated from the AK of the PERP [mGy or Gy].
In April 2020, the Ordinance for Enforcement of the Medical Care Act was amended to make patient dosimetry and management a mandatory requirement.36 Although the Ministry of Health, Labour and Welfare guidelines for developing guidance do not specifically address the issue of dose recording and management, the Ordinance suggests that management of AK in consideration of tissue response (deterministic effects) and KAP in consideration of stochastic effects may be necessary. Furthermore, the Japan Radiological Society requires the recording of fluoroscopy time in addition to AK and KAP, and recommends recording the number of frames taken if possible.30 It is necessary to compare the median of these measurements for patients with normal body shape at each institution with the DRLs published by the Society for dose optimization.
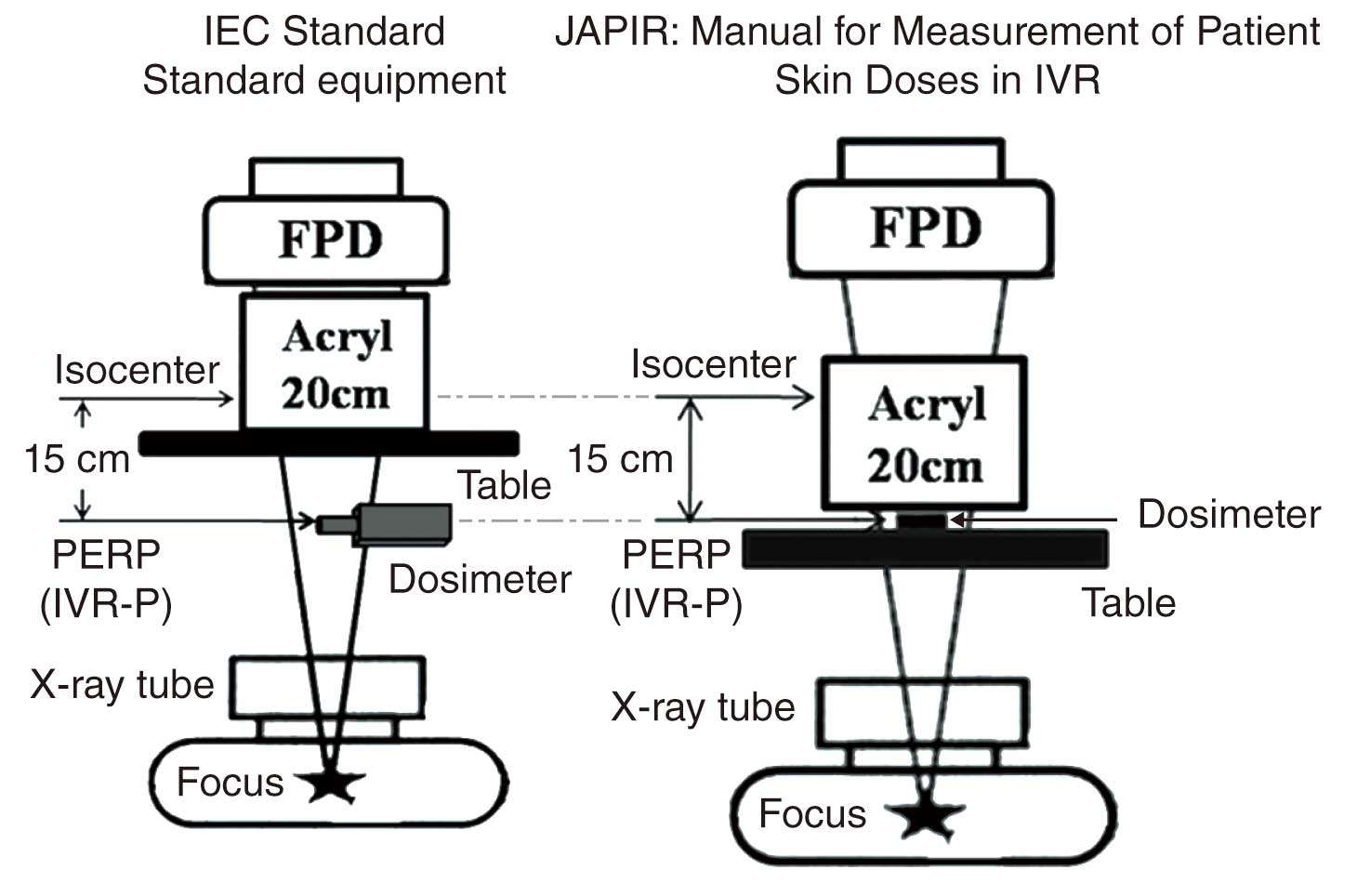
Dosimetry is an important factor for the dose control in IVR because IVR has a high risk of radiation injury. At present, there are 2 methods for dosimetry at the PERP, located 15 cm from the isocenter of the device to the X-ray focal point, as shown in Figure 11.37,38 According to the International Electrotechnical Commission (IEC) standard for device indications, the AK rate should be measured with an acrylic (polymethyl methacrylate: PMMA) phantom placed on a bunk as far away from the dosimeter as possible. In Japan, on the other hand, the AK rate is generally measured by placing an acrylic phantom on top of a dosimeter to measure the AK rate, taking into account the scattered radiation from the subject, in accordance with the “Guidelines for avoidance of radiation-induced skin injuries in IVR” reported by the Japan Association on Radiological Protection in Medicine.39 The “DRLs 2015” of the Japan Network for Research and Information on Medical Exposures also uses measurements with this arrangement, and the Japan professional accreditation board of radiological technologists for angiography and intervention (JAPIR) recommends it as useful for device management. Understanding the difference between the 2 methods and the relationship between the instrumental AK value and the entrance surface dose considering the scattered rays will help us to understand the relationship between AK and PSD in clinical practice.
2.1.2 CT Room (Table 7)
Table 7. COR and LOE for Dose Control in the CT Room
| |
COR |
LOE |
| It is recommended that dose control by CTDI and DLP is performed40–42 |
I |
B |
It is recommended that dosimetry with weighted CTDI100 or CTDIfree air is performed
at least every 6 months 41 |
I |
B |
| Dose assessment by size-specific dose estimates should be considered43–45 |
IIa |
B |
COR, Class of Recommendation; CT, computed tomography; CTDI, CT dose index; DLP, dose–length product; LOE, Level of Evidence.
a. CTDI as a Standard Indicator
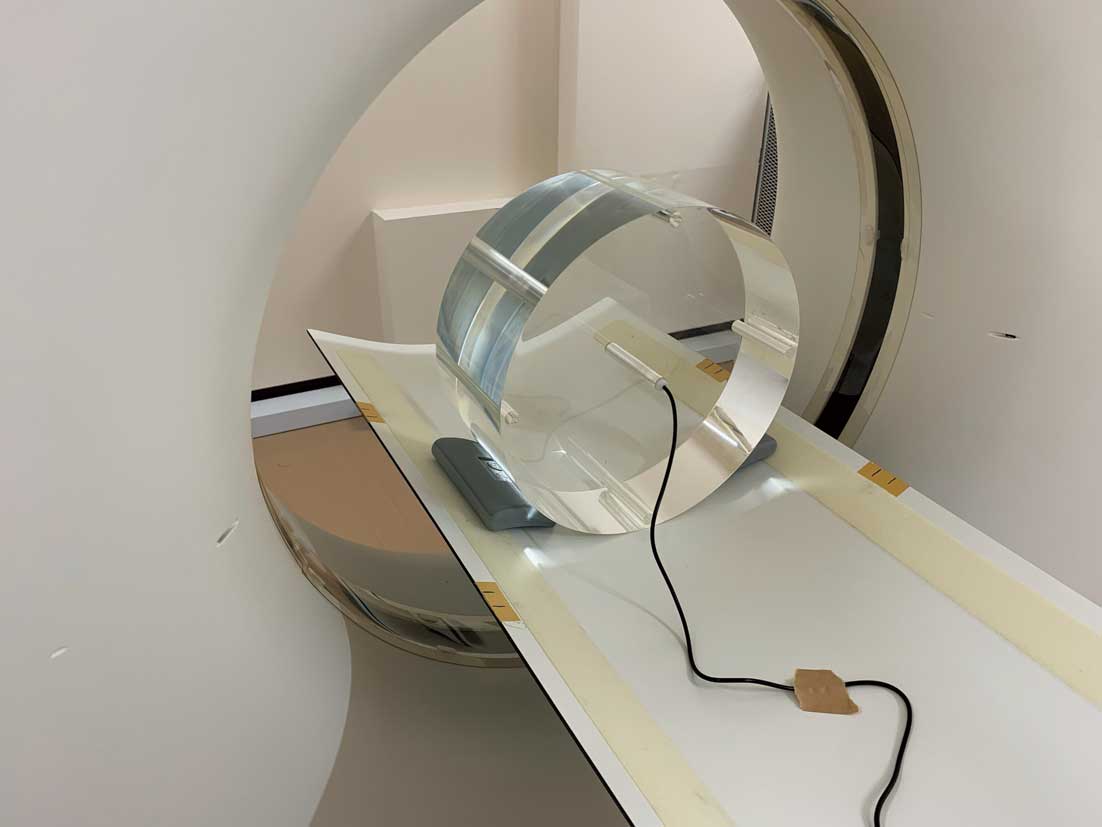
The standard method for dosimetry in CT examinations is measurement of the CT dosimetry index (CTDI) using a CT ionization chamber dosimeter and an acrylic resin (polymethyl methacrylate: PMMA) cylindrical phantom (Figure 12).40 A PMMA cylindrical phantom with a diameter of 160 mm is used for the head and trunk of children and the head of adults, and a phantom with a 320 mm diameter for the trunk of adults.46
The CTDI100
is used as a practical value for CTDI measurement, because a pencil-type CT ionization chamber dosimeter with an ionization length of 10 cm is generally used. For the CTDI100, the method of calculating the integral of the dose profile in the range from −50 mm to +50 mm is used. The CTDI100
is dealt as a weighted CTDI (CTDIw) by calculating weighted averages of measurements at the center and at 4 locations on the periphery of CTDI measurement phantom (12, 3, 6, and 9 o’clock, 1 cm inside the edge of the phantom).47
Furthermore, by dividing the CTDIw
using the helical pitch for helical scans and by multiplying the CTDIw
using “nominal slice thickness × number of slices/transfers between consecutive scans” for axial scans, the mean dose in the central scan area (volume CTDI; CTDIvol) is obtained.48–50 The CTDIvol
is specified to be displayed in mGy units (AK) on the control panel before the start of a series of scans,46 and the displayed CTDIvol
should always be checked before starting the inspection. The accuracy of the displayed values should be checked periodically by comparing both the displayed CTDIvol
and the CTDIvol
obtained by measurement in facilities with CT ionization chamber dosimeters and PMMA cylindrical phantoms.
b. Periodic Inspection of Equipment by CTDI
CT systems are classified as “specially-designated medical devices requiring maintenance” that “require specialized knowledge and skills for maintenance, inspection, repair and other management”, as stipulated in Article 2, paragraph 8 of the Order for Enforcement of the Act on Securing Quality, Efficacy and Safety of Products Including Pharmaceuticals and Medical Devices, and daily maintenance and inspections by the user (starting and end-of-work inspections), as well as periodic inspections at stipulated intervals, are required to ensure proper operation. This work can be outsourced to manufacturers or qualified personnel who are capable of repair and inspection. In general, periodic inspections by the contractor are carried out in accordance with the technical reference manuals supplied with the equipment, etc., but much of the content is based on the constancy tests of the CT system.41 In the constancy tests of CT system, the dose is assessed with CTDIfree air
measured without a CTDIw
or PMMA cylindrical phantom (Figure 13) and should be measured at least once every 6 months.
c. Other Evaluation Indicators
Although the CTDIvol
is different from the absorbed dose received by patient,41 the American Association of Physicists in Medicine Report No. 204 proposed a method for estimating the absorbed dose received by patients from the CTDIvol
by multiplying the long and short diameters (or effective diameters derived from them) of trunk sections by the corresponding conversion factors (size-specific dose estimates: SSDE),43 and also provides a conversion factor for water equivalent diameter, which takes into account the difference in absorption rates of X-rays by organ type.44 The method of calculating SSDE has been summarized as an international standard,32 which will be incorporated into the “Basic safety and basic performance standards for CT systems” as a new dose index, and is expected to be displayed on the control panel before the start of a series of examinations, similar to the CTDIvol.
The dose–length product (DLP) is defined as the integral of the CTDIvol
in the area irradiated by the X-rays, and like the CTDIvol, it is stipulated that the DLP be displayed on the control panel in mGy-cm before the start of a series of inspections.46 Although an increase of the CTDIvol
results in an increase of radiation dose per site in each patient, the total exposure dose of the patient increases with the extent of the scan, even if CTDIvol
is constant, and this is an index that takes into account the effect of the scan extent.
DLP, like CTDIvol, does not directly represent the patient dose,42 but ICRP Publication 102 (2007 Recommendations)51 provides conversion factors for estimating effective doses from the DLP. The effective dose is a value used to express the whole body’s exposure to radiation. However, based on the assumption that the whole body is equally exposed to radiation, it should be pointed out that it may not be possible to properly evaluate the examinee’s exposure dose in the case of local exposure such as CT,52 and care must be taken in its use.
Dose calculation software is a convenient method for calculating absorbed dose and effective dose in the patient’s organs and tissues. These systems have a basic dataset of organ and tissue absorbed doses for a specific CT system and specific scan parameters, and are designed to display the organ and tissue absorbed doses and effective doses, etc., after inputting the CT system and scan parameters to be calculated.
d. Specific Methods of Dose Control and Dose Recording
In accordance with the amendment of the Ordinance for Enforcement of the Medical Care Act in March 2019,36 it has become mandatory from April 2020 to “record the dose due to medical exposure of the person who receives medical treatment using medical devices subject to management and documentation”, and using the CT system for whole-body examination is included in the subjects of dose control and dose recording.
DRL-based dose optimization is the most effective method for dose control in CT examinations. Because the DRLs for CT are set in terms of CTDIvol
and DLP, recording and managing the CTDIvol
and DLP for each test is the most effective tool at present. These values are also included in the RDSR output from the device, which has the advantage of being easy to record and collect.
It is desirable to conduct dosimetry at one’s own facility on a regular basis for periodic comparison with DRLs. At the time of the dosimetry study, it is desirable to collect data on 30 patients with standard body size for each of the testing protocols.21 The dosimetry survey may be done prospectively or retrospectively, but the latter case can be easily implemented by utilizing the dosimetry information management systems, and all the patients in the study period can be included, irrespective of the number of patients.
Some dosimetry management systems are capable of calculating organ doses and effective doses of patients at each examination, which facilitates patient-specific dose control. In addition, the results of the survey and analysis can be used to promote optimization by creating a team to manage CT exposure doses and imaging protocols in the facility.
2.1.3 Nuclear Medicine Room (Table 8)
Table 8. COR and LOE for Patient Dose Control in the Nuclear Medicine Room
| |
COR |
LOE |
| Dose control based on actual doses is recommended27,53,54 |
I |
B |
In PET/CT and SPECT/CT imaging, dose control based on CTDIvol or DLP is
recommended27,53–55 |
I |
B |
| Patient dose assessment should be considered21 |
IIa |
B |
COR, Class of Recommendation; CT, computed tomography; CTDI, CT dose index; DLP, dose–length product; LOE, Level of Evidence; PET, positron emission tomography; SPECT, single-photon emission tomography.
a. Compliance With Revised Regulations
In March 2019, the Ministerial Ordinance Partially Amending the Ordinance for Enforcement of the Medical Care Act was promulgated, and the Enforcement of the Ministerial Ordinance Partially Amending the Ordinance for Enforcement of the Medical Care Act (Notification of Director-General of the Medical Administration Bureau, MHLW, 0312, No. 7),36 which was issued afterwards, states that the administrators of medical institutions with X-ray systems are required to assign a person in charge of safety management of the use of radiological equipment, and the person in charge must establish guidelines for the safe use of radiological equipment. For the safe use of medical radiation, in nuclear medicine, exposure doses for “X-ray CT combined positron emission tomography systems”, “X-ray CT combined SPECT systems”, “radioisotope for positron emission tomography”, and “radioisotopes for medical treatment” are to be appropriately managed and recorded (effective April 1, 2020).
Until now, no specific management methods have been used in nuclear medicine, and each institution has been struggling to cope with the situation, but in March 2020, the “Guidelines for the development of guidance on the safe use of medical radiation in nuclear medicine”53 were published and management should be done accordance with this guideline. However, each facility has already been promoting its own radiation dose control.
b. Dose Recording and Management
Dose records in the field of nuclear medicine should cover radiopharmaceuticals and CT imaging doses in PET/CT and SPECT/CT systems.
For radiopharmaceuticals, the name of radiopharmaceutical, the time of administration, and the actual dose are recorded. Most of medical institutions in Japan use radioactive-labeled pharmaceutical prepared in pharmaceutical companies because they are easy to handle. Because the amount of radioactivity of labeled preparations at the date of verification is specified in accordance with the Minimum Requirements for Radiopharmaceuticals, it is practical to obtain the actual dose from the correction for physical half-life, and the full dose can be treated as an error range without measuring the residual dose.27,54 Radiopharmaceuticals prepared in the hospital are administered after measurement with a dose calibrator or with an automated dispensing and injection system, in which case the measured values are used as the actual dose. Some commercially available dose control systems automatically record the actual dose and the name of the administered drugs from the radiopharmaceutical RDSR (RRDSR) or digital imaging and communications in medicine (DICOM) images, and calculate the organ absorbed dose and the effective dose.
In PET/CT and SPECT/CT examinations, CTDIvol
and DLP are used to record the dose in CT scanning. However, unlike CT examinations in diagnosis, imaging conditions vary depending on the purpose of the examination (e.g., for attenuation correction, for fusion images, etc.), thus CTDIvol
and DLP must be recorded for each purpose.55 The dose control system can be combined with the effective dose measurement function in CT imaging to enable comprehensive management of the patient’s exposure dose including the dosage information.
For children, it is necessary to refer these regulations and management to the “Japanese consensus guidelines for pediatric nuclear medicine”.56
For dose control, optimization of dosage should be attempted by collecting actual doses in at least 20–30 standard patients at each institution and comparing the median dose with the DRL. With respect to PET/CT or SPECT/CT examinations, it is appropriate to set and present DRLs for each of the combined modalities separately.21 It is important to manage and optimize the imaging protocol of nuclear medicine at one’s own institution by referring to various guidelines and considering the image quality.
c. Equipment Maintenance and Inspection and Management System
Proper maintenance and inspection of nuclear medical imaging devices is required by regulations such as the Ordinance for Enforcement of the Medical Care Act.
In 2007, the Medical Care Act was partially amended by the Enforcement of the Act for Partial Revision of the Medical Care Act for the Purpose of Establishing a System to Provide Quality Medical Care and it became necessary to ensure the safety management system for medical devices at each facility. This amendment specifies specially-designated medical devices requiring maintenance, which includes not only nuclear medicine equipment such as PET and SPECT systems but also diagnostic imaging systems such as CT and MRI systems.
Because the specially-designated medical devices require specialized knowledge and skills for maintenance, inspection, repair and other management, they must be properly managed. The standards established by the Japan Medical Imaging and Radiological Systems Industries Association stipulate maintenance and inspection standards for the performance and safety of nuclear medicine diagnostic equipment and its accessories and devices used in routine diagnostics.57–60
2.2 Occupational Exposure of Healthcare Workers
2.2.1 Vascular Imaging Room (Table 9)
Table 9. COR and LOE for Dose Control for Healthcare Workers in the Vascular Imaging Room
| |
COR |
LOE |
| Unequal exposure control is recommended17,61 |
I |
A |
| Wearing radiation protection clothing17 |
I |
A |
| Wearing radiation protection glasses is recommended17 |
I |
A |
It is recommended to use protective equipment such as ceiling-suspended protective boards,
protective screens, and bedside lead curtains17 |
I |
A |
| Wearing thyroid protection should be considered17 |
IIa |
B |
| Equal exposure control is not recommended17,61 |
III
(No benefit) |
C |
COR, Class of Recommendation; LOE, Level of Evidence.
a. Wearing a Personal Dosimeter
Staff wearing protective clothing while working in the vascular imaging room are subject to unequal exposure control under Article 8, Section 3 of the Ordinance on Prevention of Ionizing Radiation Hazards.61 In this case, a personal dosimeter should be worn inside the protective apron (chest for men and abdomen for women of childbearing potential), with an additional dosimeter in the area of the trunk most likely to be exposed to radiation (usually the head and neck), or at the extremities in cases where the extremities are most likely to be exposed (fingers in IVR procedures23). However, if the exposure dose at the extremities is less than that of the trunk, a private dosimeter at the extremities is not required. Thus, there are 3 possible application sites for unequal exposure control: chest (abdomen) + head and neck, chest (abdomen) + extremities, or chest (abdomen) + head and neck + extremities and ≥2 personal dosimeters are required.
b. Dose Limit of the Lens
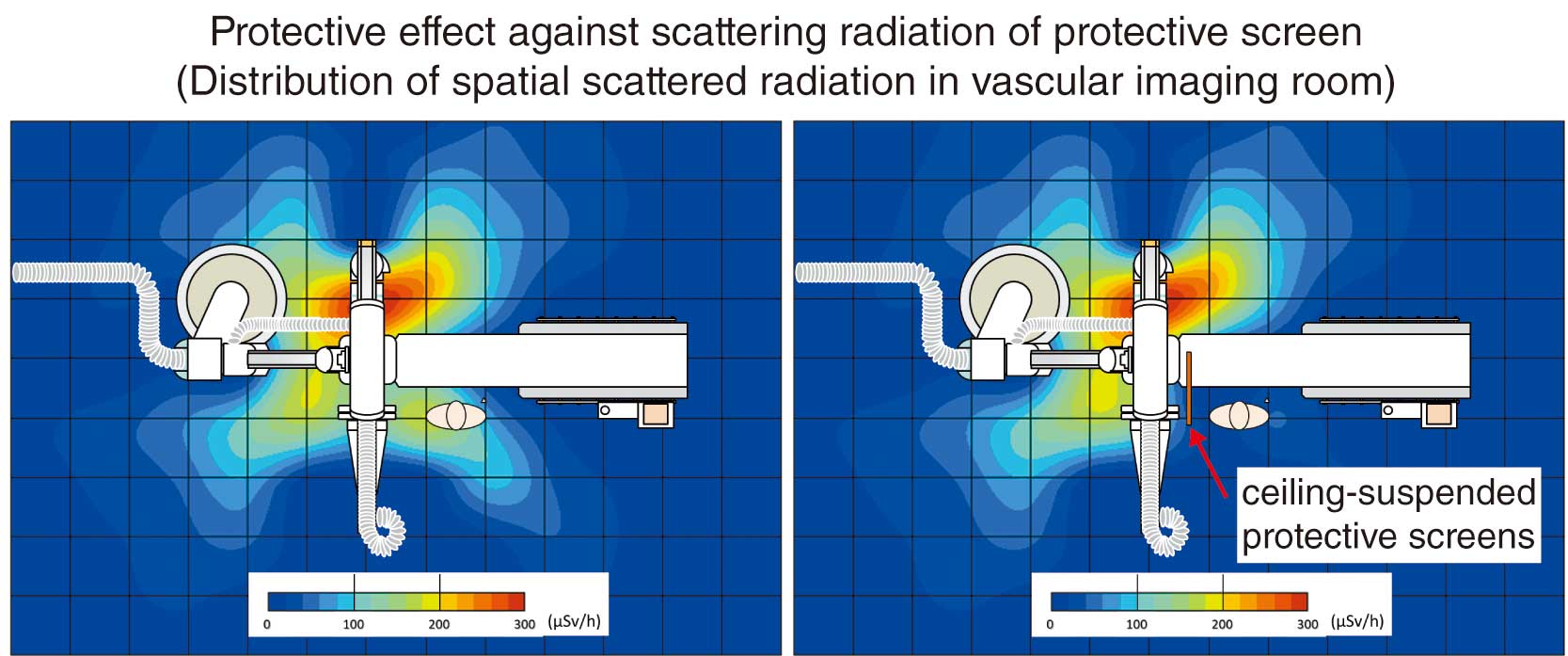
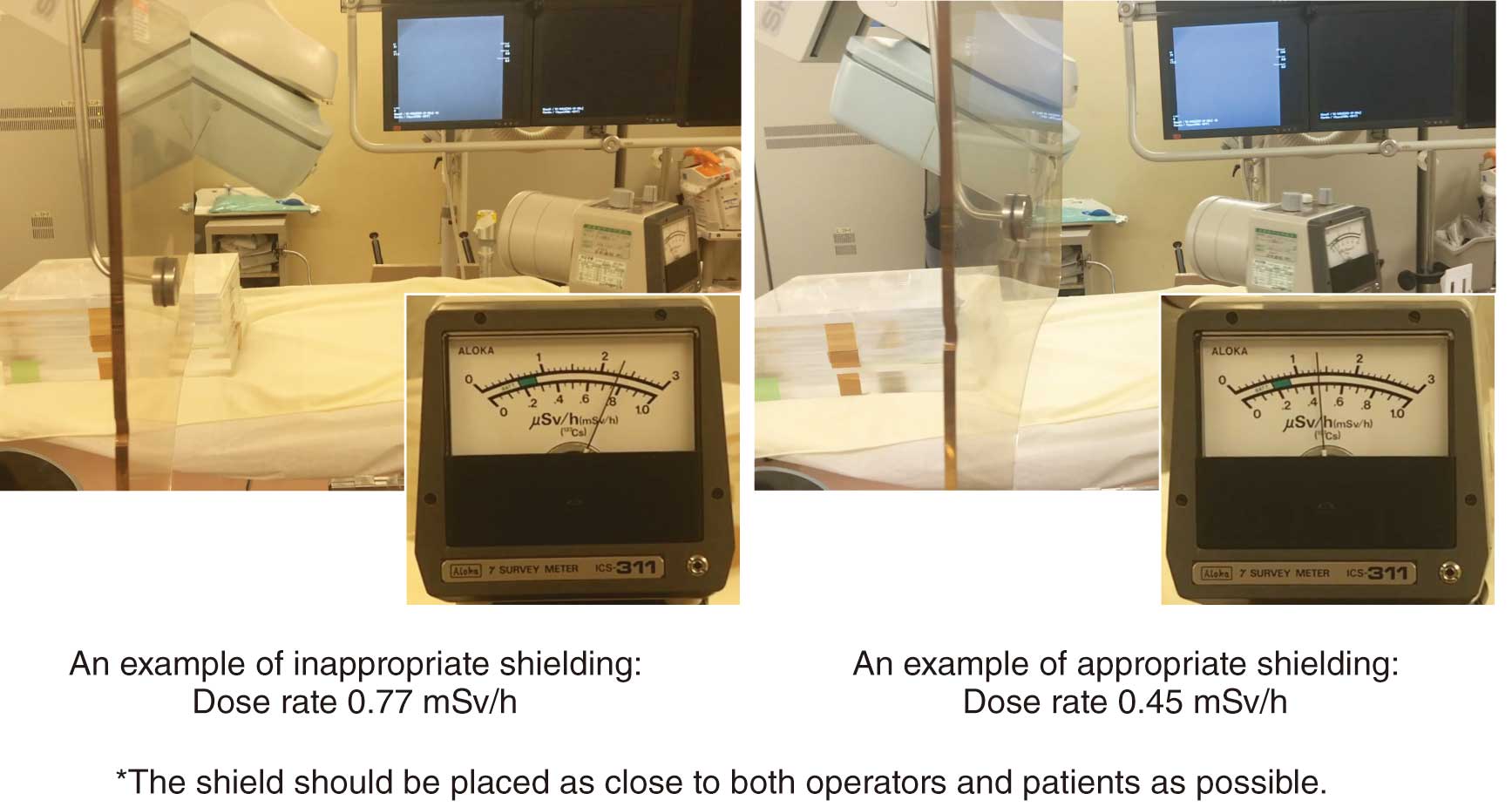
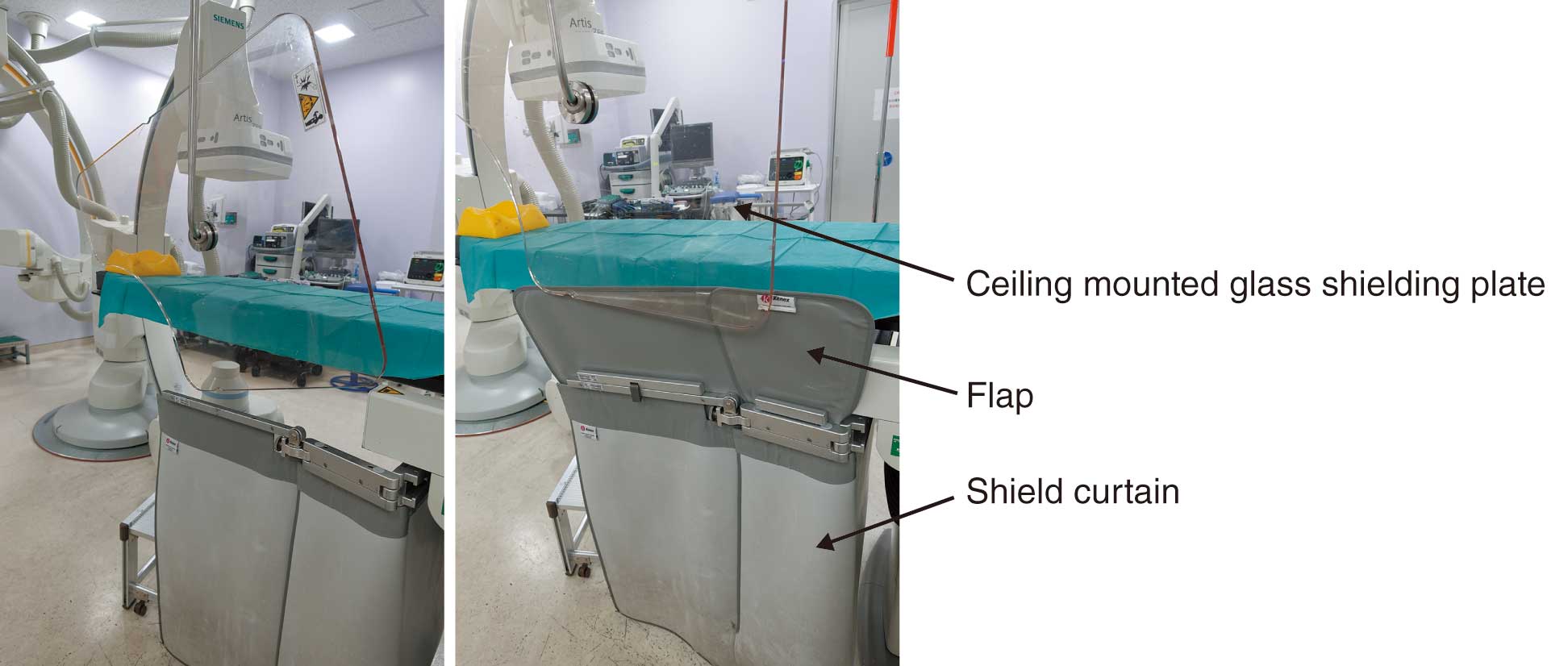
In Japan, the law concerning dose limits for the lens of the eye has been recently revised, and the dose limits for medical personnel are set as shown in Table 10.61,62 The reason for the lowering of the lens dose limit is that ICRP Publication 118 (2012 Recommendations)24 stated that the lens is more radiosensitive than previously thought and radiation cataracts may occur at lower doses, and thus the threshold for radiation cataracts was lowered from 5 Gy to 0.5 Gy. Healthcare workers involved in cardiovascular IVR may be exposed to doses in excess of the dose limit, even if they wear radiation protection eyewear (Figure 14). Therefore, it is necessary to reduce the exposure dose to the lens by correctly using ceiling-suspended protective screens (protective screens) and bedside lead curtains (Figure 15).17,25,63–65
Table 10. Dose Limits for Healthcare Workers
| |
Dose limits (occupational exposure) |
| Effective dose |
Average of 20 mSv/year for a given 5-year period (not to exceed 50 mSv
in any given year) |
Annual equivalent dose
Lens of the eye
|
Average of 20 mSv/year for a given 5-year period (not exceeding 50 mSv
in any given year) |
| Skin |
500 mSv/year |
| Limbs and feet |
500 mSv/year |
(Source: based on Ministry of Labour, 197261 and MHLW, 2020.62)
2.2.2 CT Room (Table 11)
Table 11. COR and LOE for Dose Control for Healthcare Workers in the CT Room
| |
COR |
LOE |
When personnel remain in the laboratory during CT scanning, wearing protective
eyewear is recommended66 |
I |
C |
When healthcare workers remain in the laboratory during CT scanning, they should
wear protective clothing66–68 |
IIa |
C |
During CT fluoroscopy-guided punctures, use of assistive devices such as needle guides
and radiation protection cloths should be considered69 |
IIa |
B |
COR, Class of Recommendation; CT, computed tomography; LOE, Level of Evidence.
a. Wearing a Personal Dosimeter
In CT imaging, workers basically move to the control room side, and therefore, exposure is not a problem. However, because the walls of the examination room do not completely shield the scattered radiation, personal dosimeters must be worn by workers when they are engaged in their work. Protective clothing and eyeglasses are also effective in reducing radiation exposure when workers remain in the examination room during CT scans for the purpose of restraining children or patients with a low level of consciousness, or confirming extravascular leakage during contrast injection.67,68 This will result in unequal exposure, and therefore, 2 personal dosimeters should be attached to the inside of the protective clothing: 1 on the chest (male) or abdomen (female) and 1 on the neck of the outside of the protective clothing. In particular, it is recommended that workers who often remain in the examination room during CT imaging wear protective eyewear and a dedicated lens dosimeter inside the eyewear.
Understanding the distribution of scattered doses in the examination room is also useful to reduce the exposure dose.67,68 Figure 16 shows an example of the distribution of scattered doses in an examination room.
b. Exposure Associated With Contrast Agent Observation
In administering contrast agent intravenously, there are individual differences in circulation rates. Thus, in order to eliminate its effect and tailor the timing of imaging to the individual patient, the bolus tracking method (a method for observing the flow of contrast agent into the artery in real time on a specified slice section and start imaging when the desired concentration is reached)70 and the test bolus method (a method of injecting contrast agent on a trial basis before the actual imaging to determine the optimal timing to start imaging)71 are used. It is necessary to take measures to reduce exposure doses, because, with these methods, X-rays are started at a relatively early stage after the initiation of injection, which exposes the workers beside the patient checking for extravascular leakage, and these methods are applied more frequently in facilities where contrast imaging is performed more frequently. Particularly in the case of cardiac CT, high X-ray tube rotation speed and high tube current are used to improve the temporal resolution, which is the temporal sensitivity distribution of projection data that contributes to an image, so the radiation exposure of workers beside the patient may be higher than during other CT examinations.
Specific measures to reduce exposure doses include not only wearing protective clothing and eyewear, but also placing a transparent protective screen in the examination room. The dose of the workers can be significantly reduced by checking for extravascular leakage from behind the protective screen, because the shielding ability of a protective screen from scattering rays is higher than that of protective clothing and eyewear. It is also important to exit the examination room immediately after administration of the contrast agent and not to stay in the room unnecessarily.
c. CT Fluoroscopy-Guided Procedures
Although the radiation control of workers during CT fluoroscopy-guided procedures is basically the same as in the angiography room, CT fluoroscopy-guided procedures have higher X-ray output than ordinary radioscopy and the fingers of the operator are often directly exposed; thus it is also desirable to wear an upper extremity dosimeter on the fingers that may enter the irradiation field. In order to reduce the exposure dose, measures should be taken to prevent the fingers from entering the irradiation field as much as possible, and aids for CT fluoroscopy-guided puncture (e.g., needle guides) and radiation protection cloths are also effective.69
2.2.3 Nuclear Medicine Room (Table 12)
Table 12. COR and LOE for Dose Control for Healthcare Workers in the Nuclear Medicine Room
| |
COR |
LOE |
Use of automated dispensing and injection system for the administration of PET agents
should be considered44 |
IIa |
C |
Reducing contact time with the patient by having more than 1 worker in the room should
be considered41,44 |
IIa |
C |
| Use of protective clothing or radiation shields should be considered44,50 |
IIa |
C |
COR, Class of Recommendation; LOE, Level of Evidence; PET, positron emission tomography.
a. Widespread Use of Nuclear Medicine
Nuclear cardiology is widely used in the diagnosis, assessment of disease severity, determination of treatment strategy, and prognostic assessment of cardiac diseases. The AHA/ACC/ASNC published guidelines on the usefulness and level of evidence for nuclear medicine in 200372 and in Japan, based on numerous reports, the “Guidelines for Clinical Use of Cardiac Nuclear Medicine (JCS2010)”, were published to propose the effective and efficient use of nuclear medicine testing in cardiac diseases.73
At present, nuclear medicine, including SPECT and PET scans, is the mainstay of nuclear medicine in Japan. Since the 1970s, a wide range of clinical applications of myocardial blood flow scintigraphy with 201Tl in coronary artery disease had been reported, including the diagnosis of the presence and location of ischemia and infarction, evaluation of the severity of the disease, determination of residual myocardium, determination of the indication for revascularization, evaluation of therapeutic response, and prognosis prediction.73 In the 1980s, 99 mTc-labeled myocardial blood flow scintigraphy products, of similar diagnostic value to 201Tl myocardial blood flow scintigraphy, became available, and they are still widely used to date with the advantages of prospective ECG-gated scans.73 In addition, 123I-MIBG scintigraphy, which reflects the state of sympathetic nerve activity in the myocardium and evaluates the hyperactivity of sympathetic nerve function, especially in heart failure, and 123I-BMIPP scintigraphy, which evaluates fatty acid metabolism in the myocardium and has been shown to be useful in ischemic heart disease, cardiomyopathy, and failing heart, have also been widely applied clinically in Japan.73
Since 2002, myocardial viability diagnosis after myocardial infarction using 18F-FDG has been covered by insurance in Japan. The usefulness of the 18F-FDG-PET test in the diagnosis of myocardial viability is well established and has become the gold standard.72,74,75 It can also differentiate viable myocardium and myocardial scarring by combining myocardial metabolism and blood flow to detect blood flow–metabolism mismatch, providing useful information for prognostic evaluation and determination of indications for revascularization surgery.73 In addition, myocardial blood flow can be quantitatively assessed using 82Rb, 13N-ammonia, and 15O-H2O, and the myocardial blood flow reserve is an important indicator in the diagnosis of myocardial ischemia.
Cardiac nuclear medicine in children is indicated for various congenital and acquired coronary artery diseases (mainly Kawasaki disease), cardiomyopathy, myocardial disorders, and right ventricular pressure overload.73 When determining and then performing pediatric myocardial blood flow scintigraphy, its specificity must be kept in mind.73 Adequate consideration should also be given to the exposure of children to radiation. During myocardial perfusion scintigraphy in children, accumulation appears more clearly with pharmacologic stress than with exercise stress.76 Pharmacologic stress is often used to detect unknown coronary artery stenoses and to assess progression of coronary artery stenosis over time.76,77 In contrast, exercise stress is selected when evaluating exercise induced abnormality (e.g., presence of exercise ischemia due to known coronary artery abnormality, etiology of exercise ECG abnormality, evaluation of therapeutic effect on myocardial ischemia, etc.) and the stress method needs be selected appropriately depending on the purpose.
b. Current Status of Exposure and Radiation Protection
A comparison of effective doses of radiopharmaceuticals used in cardiac nuclear medicine examinations for cardiac nuclear medicine workers, based on ICRP Publication 128 (2015 Recommendations), showed that the effective doses differed depending on the examination and the radiopharmaceuticals used, with 0.14 mSv/MBq for 201Tl myocardial blood flow scintigraphy, 0.009 mSv/MBq (at rest) and 0.0079 mSv/MBq (at load) for 99 mTc-MIBI myocardial blood flow scintigraphy, 0.008 mSv/MBq (at rest) and 0.0069 mSv/MBq (at load) for 99 mTc-tetrofosmin, 0.032 mSv/MBq for 123I-MIBG and 123I-BMIPP scintigraphy, and 0.019 mSv/MBq for 18F-FDG-PET.77–79
Kanaya estimated myocardial doses of 0.13±0.10 μSv (201Tl: 110–140 MBq) and 0.47±0.27 μSv (99 mTc: 296–740 MBq) for healthcare workers involved in cardiac nuclear medicine examinations. On the other hand, for intravenous injection, the effective dose at 185 MBq of 99 mTc was 0.10±0.05 μSv and 0.33±0.13 μSv at 740 MBq of 99 mTc, and it is stated that 0.5-mm lead-equivalent of protective clothing can provide 50–70% shielding.80 In particular, it is expected that nuclear cardiologists involved in PET examinations will be exposed to a large amount of radiation in a short period of time due to the high effective dose rate. Therefore, individual radiation exposure control is important and efforts should be made to reduce exposure. It is also recommended that administrators should periodically evaluate the radiation doses of radiation workers and set a target dose of 5 mSv/year.81
Regarding the doses for workers involved in PET examinations, the results of a questionnaire survey reported 25–40 μSv/month for physicians administering and explaining PET examinations, 110 μSv/month for medical radiologists guiding patients and operating PET equipment, 25 μSv/month for pharmacists involved in the synthesis, quality inspection, and dispensing of radiopharmaceuticals, and 100 μSv/month for cyclotron operators.82 Furthermore, Fujibuchi et al found that finger doses among radiation workers were 22.4 μSv/day for physicians involved in the administration of radiopharmaceuticals, 51.3 μSv/person for 18F-FDG-PET examinations (53.9 μSv for the right finger and 47.2 μSv for the left finger), and 29.6 μSv/person for radiologists dispensing 99 mTc preparations, 31.5 μSv/time for pharmacists involved in 18F-FDG examinations and 1.9 µSv/person for radiologists.83 In terms of lens exposure, based on the effective dose of SPECT examinations, the annual equivalent dose to the lens is considered to be a few μSv, which is sufficiently low, but it is possible to reduce the exposure dose sufficiently by considering the introduction of automatic administration equipment for PET examinations, and by examining the working system, shielding, and timing of administration and imaging.84
3. Dose Limits for Healthcare Workers
3.1 Equivalent Dose Limit
Most of the exposure for medical workers is attributable to scattered radiation from the patients. In other words, it is important to control scattered radiation to reduce the dose to the operators.
According to the laws and regulations, radiation workers must avoid exposure in excess of the following dose limits:
(1) 100 mSv/5 years
(2) 50 mSv/year
(3) In addition to the above, 5 mSv/3 months for females
(4) In addition to the above, internal exposure of 1 mSv for pregnant women from the time of self-declaration of pregnancy to the delivery
(5) For the surface of the abdomen of a pregnant woman, 2 mSv per period specified in (4)
(6) For the lens of the eye, 20 mSv/year on average for 5 years, with no single year exceeding 50 mSv
(7) For skin, 500 mSv/year.
In addition, there are various obligations of hospital managers, such as the measurement of doses in areas where there is a risk of radiation hazards, the measurement of external exposure of radiation workers, and the calculation and recording of equivalent doses at each site based on the measured values.
For the lens, the threshold doses have been revised and the equivalent dose limits will be lowered based on the findings of epidemiological studies on atomic bomb survivors and Chernobyl accident reconstruction workers. Worker exposure of each organ is described below.
3.2 Lens (Table 13)
Table 13. COR and LOE for Lens Protection for Radiation Workers’ Eyes
| |
COR |
LOE |
It is recommended to manage an equivalent dose limit for the lens of the eye of
20 mSv/year on average for 5 years, with no single year exceeding 50 mSv24 |
I |
A |
It is recommended that any worker who exceeds 20 mSv/year in unequal exposure
should be under control of a radiation administrator for 5 years, and then be managed
not to exceed 100 mSv in 5 years using a dedicated lens dosimeter85 |
I |
A |
It is recommended to prepare protective eyewear for simultaneous use by all workers
in the laboratory, including medical staff86 |
I |
A |
Wearing protective eyewear capable of protecting against radiation from the sides as
well as in front should be considered87,88 |
IIa |
B |
COR, Class of Recommendation; LOE, Level of Evidence.
3.2.1 Equivalent Dose Limit
In April 2011, the International Commission on Radiological Protection, in its “Statement on tissue reactions”, changed the equivalent dose limit of the lens for planned occupational exposure from “not exceeding 150 mSv/year” to “an equivalent dose limit for the lens of 20 mSv/year averaged over 5 years, with no single year exceeding 50 mSv”.24 The criteria for this recommendation have been adopted by the International Atomic Energy Agency in its “Radiation protection and safety of radiation sources: international basic safety standards”, and several European countries and the USA have already incorporated the dose limits for lenses in their legislation. In Japan, equivalent dose limits for lenses are scheduled to be lowered from April 2021.
3.2.2 Function, Structure and Characteristics of the Lens
The lens functions as a convex lens to refract incoming light and focus it on the retina (Figure 17).89 It has a monolayer of epithelial cells anteriorly in contact with the cornea, and is encapsulated in a membrane called the lens capsule. The front half of the capsule is called the anterior capsule, the latter half is the posterior capsule, and the boundary between the anterior and posterior capsule is called the equatorial region.
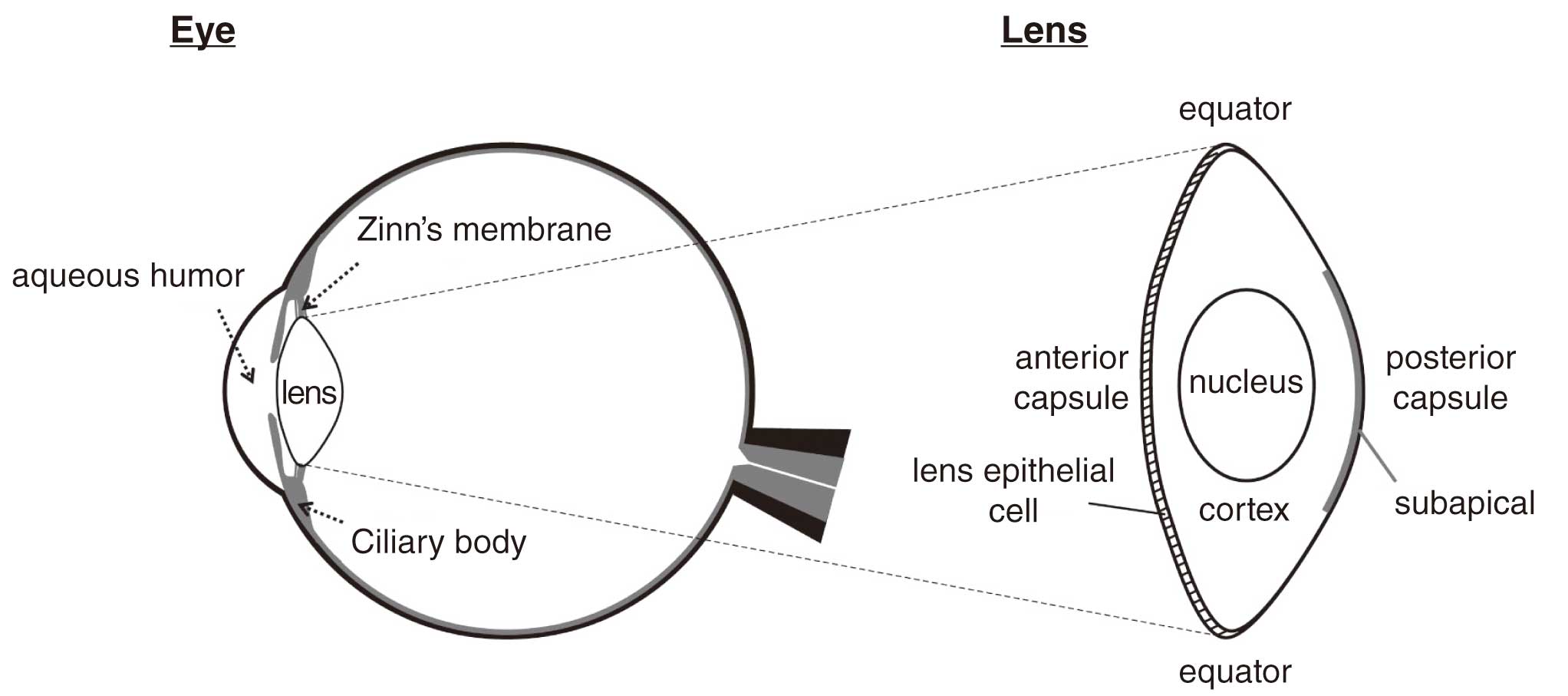
Lens can become opacified, and the symptoms of progressive opacity are called cataracts. Aging is a major cause of cataracts, with more than 96% of the population over 60 years old reported to have lens opacity.90 Lens opacity can be also caused by radiation exposure and may progress to visual impairment that requires cataract surgery (radiation cataract). Radiation induces abnormal differentiation of the lens epithelial cells, resulting in the formation of dysplastic fibrous cells and micro-opacities. Recent reports indicate that micro-opacities may progress to visually disabling cataracts, and thus the threshold (equivalent dose limit) has been lowered.
Patients with artificial or aphakic eyes from cataract surgery do not develop cataracts because there is no lens to be opacified.
3.2.3 Current Status of Exposure
Most radiation workers do not meet the new standards in the general medical field.91 According to the distribution of equivalent doses in each clinical department, those exceeding 20 mSv in 1 year are most commonly cardiology, gastroenterology, and gastrointestinal surgery, and those exceeding 50 mSv are in gastroenterology, orthopedics, neurosurgery, and cardiology, in that order. In the field of cardiology, 15.4% of physicians are >20 mSv and 0.3% are >50 mSv, which means that immediate action is needed because the rate of exceeding the standard will be high when the new standard is introduced.
3.2.4 Protective Eyewear
There are various types of protective eyewear, such as typical eyeglasses with extensions to the sides of the face, goggle-type, and lead-containing own glasses. Protective eyewear can provide up to 90% radiation shielding and can be effective in reducing the radiation dose to the lens with proper use. For devices with biplane X-ray tubes or for procedures in which the position of the X-ray tube is frequently changed, the goggle-type is effective because it gives a wide range of coverage.
Operators and medical staff who stand near the fluoroscopy unit and who remain in the catheterization room must wear protective eyewear. Article 596 of the “Ordinance on Industrial Safety and Health” requires managers of institutions to prepare enough protective equipment equal to or greater than all working personnel at the same time and to keep them effective and clean.85
3.2.5 Issues in Exposure Control
a. Measurement Methods
Although unequal exposure control by wearing personal dosimeters inside and outside of the protective clothing is recommended, it is applicable in only about 30% of cases and the remaining is under equal exposure control. With equal exposure control, lens exposure is underestimated. As the current regulations do not require measurements in the proximity of the eyes in unequal exposure control, measurements in the head and neck area have been used for tentative values, which may be overestimated.87
The Ministry of Health, Labour and Welfare (MHLW) made the following changes in the recent revision of the guidelines. When any worker, engaged in radiological practices, exceeds 20 mSv/year to the lens by conventional methods of personal dosimetry, there should be controlled management of lens exposure for 5 years, which cannot exceed 100 mSv in the 5 years. In addition, workers should wear a specific dosimeter for the lens within the shielding area of their protective eyewear and should be instructed by their managers to strive for adequate radiation safety.85 Cardiologists who are engaged in IVR and who may be exposed to more than 20 mSv/year are recommended to wear a special dosimeter (Figure 18) inside their protective eyewear while perform the procedure.

The use of protective eyewear and the wearing of a dosimeter inside it provides 60–90% protection. Due to the position of the workers, exposure to the lens is more common in the left eye than in the right eye, and the protective effect is also greater in the left eye. Also, the protective effects of protective eyewear vary depending on its shape, and the goggle-type eyewear with lenses extending to the sides is recommended.
Dosimeters currently available for attachment to protective eyewear are not widely used because their fixation is insufficient, and they obstruct the physician’s field of view. Improvement of dosimeters by the manufacturers is desirable.
b. Economic Burden
The purchase of protective goggles and dosimeters would be a significant financial burden if they were to be distributed to all radiation workers. It is necessary to consider whether the administrative agencies or each facility should bear this burden.
c. Exposure of Highly Skilled Workers
Some conditions, such as chronic total occlusion lesions, are difficult to successfully treat without a highly skilled physician. In addition, there are limited numbers of doctors in remote areas, and radiation exposure may be concentrated in selected physicians. In these cases, the exposure dose may exceed the new standard, making it difficult to continue routine clinical practice. The transfer of skills to many doctors and the enrichment of medical personnel in remote areas are desired.
3.3 Skin (Table 14)
Table 14. COR and LOE for Skin Protection for Radiation Workers
| |
COR |
LOE |
| It is recommended to manage skin equivalent doses not to exceed 500 mGy/year92 |
I |
A |
COR, Class of Recommendation; LOE, Level of Evidence.
The dose limit for the skin of healthcare workers is 500 mGy/year.92 Although there are no standards for occupational accident certification for skin cancer, the standards for certification of chronic radiation skin disorders are defined as follows.
(1) Chronic exposure of the skin to ionizing radiation at a dose of approximately 25,000 mSv or higher over a period of ≥3 months.
(2) The disease must have occurred after a period of approximately several years or more after the start of the occupational exposure.
(3) Disease with chronic ulcers that occurred after a period of dry desquamation and atrophic scarring associated with dysfunction.
There are ring-shaped personal dosimeters for measuring finger doses. Although this may be uncomfortable when performing delicate procedures, it is expected that the finger exposure dose is higher than that indicated by a personal dosimeter on the trunk of the body when operating with a finger in the vicinity of the irradiation field. Therefore, it is necessary to wear a ring-shaped personal dosimeter on the fingers, which have the highest exposure dose, and to control the exposure dose to not exceed 500 mSv/year for the skin (fingers). Wrist-worn dosimeters may also be useful when a finger-mounted dosimeter is not available.93,94
In procedures where fingers and other parts of the hand often enter the irradiation field, the operator should use protective gloves to reduce exposure to scattered radiation. Protective gloves designed for clean procedures are effective in reducing exposure to scattered radiation, but they have not been developed to reduce direct radiation exposure, because of its radiolucency. Most fluoroscopy systems have an automatic adjustment function of the image quality according to the size of the subject, so that when the operator’s hand is on top of the patient, the device assumes that the subject is thicker and automatically increases the X-ray dose. In cases where disinfection conditions are not required, such as cardiac massage near the irradiation field, gloves with a shielding capacity of 0.35-mm lead-equivalent to that of typical protective clothing should be used.
3.4 Thyroid Gland
No dose limits have been set for the thyroid gland for medical personnel.
The report of the “Study Group on ionizing radiation hazards outside the workplace” of the MHLW95 reported an increase in the risk of thyroid cancer due to radiation exposure associated with nuclear power plant accidents,96 but there are no reports showing the minimum exposure dose that resulted in a statistically significant increase in the development of thyroid cancer. The findings of the United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) on all solid cancers, including thyroid cancer, demonstrated a statistically significant increase in risk at doses of ≥100–200 mSv, but the epidemiological methods failed to show identifiable cancer risk in the dose range up to approximately 100 mSv. Therefore, it is necessary to provide radiation protection such that the cumulative exposure dose is <100 mSv. The risk of thyroid cancer has been reported to be increased from the 5th to the 9th year after a nuclear accident.
A neck guard is a protective device for the thyroid gland and is made of the same lead-containing sheet as protective clothing. A neck guard made of 0.25-mm lead-equivalent sheets provides ≈90% reduction in radiation exposure as per protective clothing.97–99
3.5 Female Healthcare Workers
For the female breast, the tissue weighting factor for radiation injury was revised from 0.05 in ICRP Publication 60 (1990 recommendations) to 0.12 in ICRP Publication 103 (2007 recommendations), which requires further protection against exposure. There are no dose limits for the breasts of healthcare workers. Preventing patient exposure is the best way to protect healthcare workers from exposure.
“The Japanese Breast Cancer Society Clinical Practice Guidelines for systemic treatment of breast cancer, 2018 edition” provides the following statements on the relationship between exposure and breast cancer.100
(1) High-dose exposure increases the risk of breast cancer, and the risk is highest when exposed at a young age.101
(2) Medical exposure, such as frequent X-rays and radiation therapy to the chest, increases the risk of breast cancer, and the risk is higher when the exposure occurs at a younger age.102
(3) No conclusions can be drawn as to whether low-dose exposure increases the risk of breast cancer.103
Healthcare workers also need to prevent both exposure from a young age and frequent exposure.
Protective clothing may be useful for breast protection. There are 2 types of protective clothing with lead-equivalent values of 0.25 mm and 0.35 mm. Generally, for the intensity of radiation used in IVR and CT fluoroscopy, the ability to shield radiation is 90% for 0.25 mm lead-equivalent and 95% for 0.35 mm lead-equivalent. Compared with protective clothing with a lead-equivalent of 0.25 mm, 0.35-mm protective clothing provides better radiation shielding, but is heavier to wear. Separating the lower body cover from the upper can help reduce the tension on the lumbar spine. In addition, an apron-like design with an open back may be sufficient for protection if the body is fixed in the direction of the patient standing in the same place for assistance. The choice of protective clothing should be tailored to the individual’s practice situation.
3.6 Head
It has been reported that 85% percent of head and neck cancers in interventional physicians occur on the left side,104 and the effect of exposure is a concern because the radiation source is usually located on the left side of the work area. However, there are few studies showing a clear relationship between left side occurrence of cancer and radiation exposure.
A study of using a radiation protection cap on the head did not show a clear reduction in exposure dose, and its usefulness is unclear.105
3.7 Sharing of Procedural Information
Advanced notice of the procedure to the catheter laboratory staff is helpful for the use of correct protection. Most hospitals have introduced a “time out” before surgery and other procedures to share important information among medical staff. As with surgery, clinical practice using radiation involves a variety of professionals, including physicians, nurses, radiology technicians, and clinical engineers. The outline of the procedure should be explained by the operator in order to prepare a shielding plate, clean vinyl, and protective eyewear, and to predict the appropriate positions of the operators.
4. Fetal Exposure of Pregnant Healthcare Workers (Table 15)
Table 15. COR and LOE for Dose Control for Female Radiation Workers During Pregnancy
| |
COR |
LOE |
| It is recommended to recognize the dangers of fetal exposure106 |
I |
B |
It is recommended to understand the amount of exposure of the fetus by various imaging
methods106 |
I |
B |
| It is recommended to implement radiation safety controls for healthcare workers106 |
I |
B |
COR, Class of Recommendation; LOE, Level of Evidence.
4.1 Risk of Fetal Exposure
ICRP Publication 84 (2000 Recommendations) “Pregnancy and medical radiation” states that “Thousands of pregnant patients and radiation workers are exposed to ionizing radiation each year. Lack of knowledge is one of the major reasons for great anxiety and probably unnecessary termination of pregnancies. For many female patients, the exposure is usually appropriate, although some exposure may be inappropriate for the unborn child”.106 This indicates that the risks of radiation exposure to the fetus may have been overestimated. However, ICRP Publication 84 also states that “Compared to routine medical radiation practices, medical exposure of a pregnant patient should be based on ethical considerations”.
The problem with radiation exposure to the fetus is malformations and genetic abnormalities due to exposure in excess of the threshold. The consequences of these effects vary by fetal age, but include fetal death, malformations, and mental retardation. The ICRP states that fetuses are not affected in this respect at <100 mGy. However, exposure >10 mGy may increase the incidence of leukemia and other cancers. The effects of fetal exposure are thought to be 2–3-fold greater than those of adults. It should be kept in mind that it is scientifically unknown whether exposure <100 mGy increases the incidence of cancer in adults.107
Taking the above into account, it is important to fully consider the justification and optimization of radiation exposure, to explain the consequences of the examination firmly to the target women, and to improve the understanding of all parties involved.
4.2 Doses of Fetal Exposure
In cardiovascular practice, both examination and treatment are usually performed in the chest area. Fetal exposure is limited and does not exceed the standard because the exposure site is away from the pelvis. Of course, this is not the case if the fetus is the subject of the examination or treatment. However, the dose at the surface is not a direct dose to the fetus, because absorption by the mother reduces the fetus’ exposure.
“Approximate fetal doses from common diagnostic procedures in the United Kingdom” as described in ICRP publication 84 (2000 recommendations)106,108 are shown in Table 16. Currently, the diagnostic reference level (DRL)27 for plain chest radiography is 0.3 mGy at the entrance.106 This value is derived from a large body data and is considered to have no dissociation from actual clinical values. The fetal dose during plain chest radiography is less than 0.01 mGy, which is less than 1/30 of the DRL, indicating that the exposure dose is low when the examination area is far from the fetus. On the other hand, with abdominal plain X-ray, the DRL dose is 3 mGy at the entrance surface and the fetal dose is 1.4 mGy on average. However, The DRL data were published in 2015, whereas the fetal dose was in 1998, indicating that the fetal dose was assumed to be high considering the progress in imaging systems and equipment, and the ratio of the two may be lower.
Table 16. Approximate Fetal Doses From Common Diagnostic Procedures in the United Kingdom Examination
| |
Mean (mGy) |
Maximum (mGy) |
| Conventional X-ray |
| Abdomen |
1.4 |
4.2 |
| Chest |
<0.01 |
<0.01 |
| Intravenous urogram |
1.7 |
10 |
| Lumbar spine |
1.7 |
10 |
| Pelvis |
1.1 |
4 |
| Skull |
<0.01 |
<0.01 |
| Thoracic spine |
<0.01 |
<0.01 |
| Fluoroscopy |
| Barium meal (UGI) |
1.1 |
5.8 |
| Barium enema |
6.8 |
24 |
| Computed tomography |
| Abdomen |
8.0 |
49 |
| Chest |
0.06 |
0.96 |
| Head |
<0.005 |
<0.005 |
| Lumbar spine |
2.4 |
8.6 |
| Pelvis |
25 |
79 |
UGI, upper gastrointestinal. (Adapted from ICRP, 2000106 and adapted from Sharp et al, 1998108 with modification.)
In general, the fetal dose from CT scans is higher than the dose from plain radiography, especially in the pelvis, where the dose is as high as 25 mGy (Table 16). The DRL value of 20 mGy in the upper abdomen to one phase of the pelvis is lower than the fetal dose prescribed in1998, although it may not be comparable because it represents the CTDI, which is the average dose at a single point on the phantom made by acrylic resin. In addition, a new image processing technique known as iterative reconstruction is becoming widespread as a method of both reducing exposure and ensuring image quality,109 which is expected to further reduce fetal doses.110
4.3 Dose Limits and Threshold Doses
Dose limits for occupational exposure are set by law. The Ordinance on Prevention of Ionizing Radiation Hazards states that “the effective dose to pregnant women from internal exposure shall not exceed 1 mSv” and “the equivalent dose on the surface of the abdomen due to external exposure shall not exceed 2 mSv”. Occupational exposure is usually repeated, and detailed dose limits have been established and controlled by means of personal dosimeters and other instruments, taking into account the inevitability of work-related exposure. Because patient exposure is medical exposure, dose limits are not applied to clinically essential examinations and treatments. Malignant tumors and genetic effects from exposure of fetus are of great concern.
Table 17 and Table 18 show the duration of fetal growth and the threshold dose for the occurrence of damage to the fetus.111 According to the ICRP, if the fetal dose is <100 mGy, there is no need to consider the health effects of the fetus, even in the most sensitive fetal period.107 Moreover, fetal exposure is unlikely to reach 100 mGy with current medical equipment, and fetal exposure should not be a problem in radiological examinations. Nevertheless, it goes without saying that this is only a professional finding and should be fully explained to pregnant women.
Table 17. Period of Occurrence of Major Congenital Abnormalities Due to Fetal Exposure
| |
Pre-implantation |
Organogenesis |
Fetal stage |
| Gestation stage* |
0–9 days |
2–8 weeks |
8–15 weeks |
15–25 weeks |
>25 weeks |
| Abortion |
+++ |
+ |
− |
− |
− |
| Malformation |
− |
+++ |
− |
− |
− |
| Growth retardation |
− |
+ |
+ |
+ |
+ |
| Mental retardation |
− |
− |
+++ |
+ |
− |
| Neoplasms |
− |
+ |
+ |
+ |
+ |
| hereditary effects |
− |
− |
− |
− |
− |
*Days or weeks after conception. (Adapted from Kusama et al, 2002111 John Wiley & Sons. ©Japanese Teratology Society.)
Table 18. Threshold Dose of Deterministic Effects of Radiation in the Fetus
| Effects |
Minimum dose (mGy) |
| Lethality |
100< |
| Gross malformation |
100–200 |
| Mental retardation |
120 |
(Adapted from Kusama et al, 2002111 John Wiley & Sons. ©Japanese Teratology Society.)
4.4 Radiation Protection
Pregnant healthcare workers should take appropriate radiation protections, including those described above. As shown in Figure 19, when separate-type and wrap-around skirt-type protective clothing with a lead-equivalent of 0.25 mm are used, the front is 0.5 mm because of the overlapping sheets and the sides are 0.25 mm, which reduces the exposure from the surroundings (Figure 20). Accurate layering in the front is important to ensure that the protective clothing fits the body shape throughout the pregnancy. If necessary, an additional protective clothing such as large-sized protective clothing for pregnant women that fits the abdomen, or apron-type protective clothing around the abdomen may be provided. However, caution is required because heavy protective clothing can cause back pain and musculoskeletal problems.
4.5 Radiation Safety Management
If a female healthcare worker becomes pregnant, she must immediately report it to her manager. By law, the equivalent dose limit for the surface of the abdomen during the period of pregnancy (from the time of being diagnosed as pregnant to the time of delivery) is stipulated at 2 mSv. The total equivalent dose on the surface of the abdomen must be recorded monthly during the entire pregnancy. For fetuses, the absorbed dose during pregnancy must be limited to 1 mSv.
The law stipulates that the exposure status of pregnant healthcare workers must be fully monitored and controlled. However, this does not prohibit the complete avoidance of work with radiation or radioactive materials, nor does it prohibit access to or work in a radioactive area. On the basis of current evidence, the genetic or developmental risk to the fetus of pregnant healthcare workers is very low under conditions of adequate radiation protection and compliance with dose limits.112 It is important that managers and colleagues are fully aware of these conditions and perform their daily duties. In any case, female healthcare workers should have sufficient discussion with their managers so that they can continue to work safely during the pregnancy.
IV. Practices of Radiation Exposure Control
1. Plain Chest and Abdomen X-ray (Table 19)
Table 19. COR and LOE for Dose Reduction With Plain X-rays
| |
COR |
LOE |
| Healthcare workers |
| It is recommended that caregivers be at least 2 m away from the patient113 |
I |
B |
| Showing a dose distribution diagram of the scattered rays should be considered114 |
IIa |
C |
| Patients |
| Latest imaging modalities should be considered115 |
IIa |
B |
| Risk of exposure |
It is recommended that risk communication be tailored to the individual patient and family
situation116–118 |
I |
C |
COR, Class of Recommendation; LOE, Level of Evidence.
1.1 Methods to Reduce Exposure Dose
1.1.1 Healthcare Workers
The exposure of healthcare workers during plain X-ray mostly depends on the assistance given to patients who have difficulty in maintaining a stationary position.
If protective equipment, such as a screen, is not available, the appropriate irradiation factors and distance from the X-ray source are important.
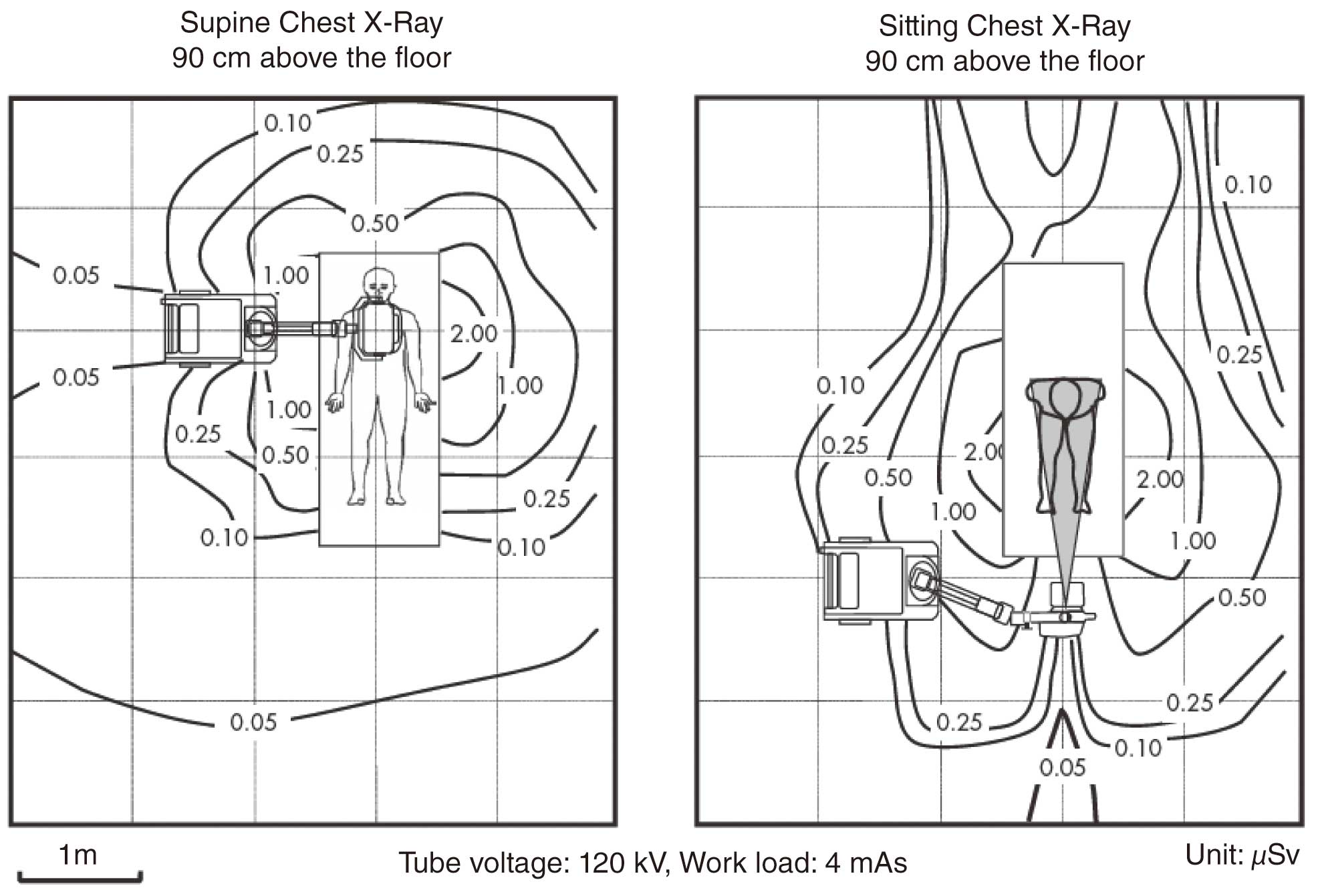
The Medical Care Act stipulates that “portable X-ray equipment and X-ray units used for surgery should be operated at a distance of at least 2 m from the X-ray tube and the patient”.113 It is also recommended that the patient’s family, caregivers and visitors should be at least 2 m away from the X-ray tube and patient. Figure 21 shows the spatial dose distribution of a patient’s chest in the prone and seated positions on a hospital bed.114 At a distance of >2 m from the vicinity of the patient’s bed, the dose is almost undetectable, indicating that it is safe enough for the healthcare worker and others not to wear protective clothing.
1.1.2 Patients
Plain X-ray examinations in cardiology predominantly involve the chest and abdomen, and the doses are lower than those used for interventional radiology (IVR) and computed tomography (CT) examinations. When comparing the diagnostic reference level (DRL) among radioactive modalities, IVR is 20 mGy/min (fluoroscopy dose rate) and the chest CT scan is 15 mGy (CTDIvol) in one phase and 20 mGy (CTDIvol) for one phase of the upper abdomen to pelvis, whereas the chest (frontal) and the abdominal (frontal) X-ray doses are as low as 0.3 mGy and 3.0 mGy, respectively. Furthermore, in recent years, digital imaging such as computed radiography (CR) and flat panel detector (FPD) have replaced analog imaging with film in most cases, and some reports suggest that FPD imaging can further reduce exposure doses compared with film and CR.115
However, it is common for patients treated in the intensive care unit to undergo chest X-ray almost every day. Even though each dose for imaging may be low, it will add up over time. Therefore, physicians should be aware of the justification for the test before ordering imaging.
Although it is scientifically inconclusive as to whether carcinogenicity increases in adults even at exposures of ≤100 mGy, 100 mGy of radiation may not have the same effects in children. Especially in terms of stochastic effects, the younger at the time of the exposure, the higher the probability of carcinogenicity, and it has been assessed that the incidence and the mortality rates of solid cancer and leukemia are 2–3-fold higher for infants exposed between the ages of 0 and 6 years than for adults exposed between the ages of 20 and 60 years.119,120
Although the radiation dose from plain X-ray is low and concern about tissue reactions may be less, it is important to keep stochastic effects in mind when performing a radioactive examination.
1.2 Explanations for Patients
As described in detail in the previous section, clinical practice using radiation always involves the risk of exposure. From the perspective of radiation protection, “justification of practice” and “dose optimization” in medical care must be achieved. The justification of practice is that the benefits of radiological treatment outweigh the radiation damage caused by exposure. It is important to inform patients of the benefits and risks (radiation exposure) of radiation practice.
In March 2019, the Ministerial Order Partially Amending the Ordinance for Enforcement of the Medical Care Act was promulgated, and the regulations regarding the safety management system for radiological treatment came into effect on April 1, 2020. Each medical institution is required to establish guidelines for the safe use of radiation for medical treatment. One of these guidelines has the “Basic policy on information sharing between medical personnel and patients”, which requires a statement of the policy for explaining risks (radiation exposure) and benefits to patients undergoing radiology treatment.
However, a considerable gap has been reported between the understanding of healthcare providers who provide information about radiology and the understanding of patients.116 Because of the wide variety in the background and pathologies of patients, not only in radiology, and risk communication needs to be tailored to the individual patient and family situation.117
Appropriate communication regarding the benefits and risks of radiation is part of good medical practice. The World Health Organization also notes that risk communication is an important element of proper radiological practice,118 which is in line with the rationale of the legal amendment.
1.3 Q&A
Q: Daily Examination to Follow the Patient
Patients with cardiovascular disease admitted to the intensive care unit (ICU) are often subjected to daily plain chest X-rays for follow-up. How should we think about the exposure associated with repeated examinations?
A:
The exposure dose of plain chest and abdominal X-rays is much lower than that of CT scans and other studies. However, it is important not only to determine the amount of radiation exposure of the patient, but also to justify the test as a necessary follow-up examination for medical care. If deemed necessary, dose optimization is essential while obtaining image quality that can achieve the diagnostic objectives.
2. CT Scans (Table 20)
Table 20. COR and LOE for Reducing Patient Exposure With CT Scans
| |
COR |
LOE |
Automatic irradiation control is recommended during the setting of the tube current value to
optimize the radiographic dose according to the patient’s body size and other factors121,122 |
I |
B |
In coronary CT imaging of patients with stable low heart rate (<65 beats/min), the application
of prospective ECG-gated non-helical scanning or prospective ECG-gated helical
scanning using high helical pitch should be considered123–125 |
IIa |
C |
In order to reduce image noise, the application of iterative reconstruction should be considered
as an image reconstruction method126–128 |
IIa |
B |
ECG, echocardiography; COR, Class of Recommendation; CT, computed tomography; LOE, Level of Evidence.
2.1 Increase in Radiation Dose Due to the Progress and Uptake of CT Scanners
In recent years, there have been remarkable improvements in CT scanning technology. The introduction of multirow detectors, increased rotation speed and capacity of X-ray tubes have enabled wide area imaging, high-speed imaging, and evaluation of thin slices, thus expanding the indications of CT scanning.
Cardiology is one of the fields that have significantly benefited from improvements in CT technology, but the repetition and wide area of imaging due to the higher speed of imaging is a factor in increased exposure doses. In addition, image noise increases if thin slices are used to improve the spatial resolution. The dose and exposure increase with reduction of this noise.129 Furthermore, the number of CT examinations in Japan has been increasing in line with the expansion of testing indications, and there is concern about the increase in per capita exposure dose from CT examinations. Accordingly, identification and control of medical exposure is required.
2.2 Methods to Reduce Exposure Dose
Based on the basic principles of radiation control and protection, CT scans are justified only when the benefit outweighs the risk from exposure, and should be performed based on the assumption of optimized equipment and imaging conditions. In recent years, advances in CT equipment have made it possible to obtain stable and highly accurate images, but the influence of CT scanning in the development of malignant tumors has been a concern and must always be kept in mind when justifying and optimizing CT.
According to the “Guidelines for the development of guidance for the safe use of medical radiation”,130 which were developed by the Ministry of Health, Labour and Welfare after revision of the Ordinance for Enforcement of the Medical Care Act in April 2020, “explanation to a person receiving radiological treatment must be performed by an attending physician or dentist” and “explanations based on the Need for Risk-Benefit Testing and Treatment (Justification) are provided prior to the examination”. In other words, the physician ordering a CT scan has to explain the disadvantages of exposure to the patient, comparing it with other modalities to justify the test. With regard to the justification of CT examinations in cardiology, the guidelines for various diseases issued by the Japanese Society of Cardiology should be referred to.114
2.2.1 Optimization of the Dose to Accommodate the Purpose and the Patient’s Body Size
To reduce the patient’s radiation exposure, the dose should be optimized according to the purpose of the examination. With regard to optimization, it is advisable that the facility determines a standard protocol for each test purpose in advance. It is necessary to set the tube current value according to the patient’s physique. As this may be dependent on the sense and experience of the operator, it is recommended to use automatic irradiation control as much as possible.121,122
2.2.2 Automatic Irradiation Control
The automatic control system has 3 functions: (1) adjusting the tube current by recognizing the size of the patient, (2) modulating the tube current by recognizing the X-ray absorption difference in the body axis direction, and (3) modulating the tube current by recognizing the X-ray absorption difference in the cross-sectional direction. By combining these values, the tube current is set, and the imaging dose is determined accordingly. Because CT scanners have their particular functions, it is necessary to check them before application.
Selective reduction of exposure to tissues such as the mammary glands has been achieved by means of recent CT systems with automatic irradiation control. Some scanners still do not have functionality to modify the helical pitches, therefore image noise increases. Attention must be paid to the use of such devices especially for young patients.
Prospective ECG-gated imaging is used in cardiac CT scans. ECG-gated helical scans always require a smaller helical pitch, resulting in an increase in the patient’s exposure dose. To mitigate this problem, ECG-gated automatic irradiation control can be combined. It is possible to reduce the exposure dose by scanning at the maximum tube current for an arbitrary cardiac phase (generally from end-systole to diastole), while scanning at a low tube current for the other cardiac phases. However, when combined with ECG-gated automatic irradiation control, the changes in the RR interval due to arrhythmia and other factors may cause low tube currents to be captured in the expected cardiac phase. It is desirable to determine whether or not to apply automatic irradiation control while assessing the patient’s condition and the characteristics of the device’s ECG-gated automatic irradiation control.
Methods to further reduce the exposure dose in cardiac CT scans include prospective ECG-gated non-helical scanning and prospective ECG-gated helical scanning using high helical pitch. Although these methods can significantly reduce exposure to radiation compared with normal ECG-gated helical scanning, they may not provide enough image quality for diagnosis in patients with a high heart rate or arrhythmia, and therefore, use should be restricted to patients with a stable, low heart rate of <65 beats/min or after β-blockers are administered.123–125
2.2.3 Low Tube Voltage Images and Iterative Reconstruction Method
Low tube voltage imaging and reconstruction using the iterative reconstruction method may also be used to reduce exposure doses. Applying low tube voltage depending on the patient’s body size is effective in reducing exposure doses, but it has been reported that blooming artifacts increase in patients with coronary artery calcification.131 It should be applied with caution, as it may also alter image contrast and lead to an increase in image noise.
Even when imaging noise is increased by reducing the radiographic dose during CT scanning, the noise is reduced by iterative reconstruction processing, resulting in reduction of the exposure dose while maintaining the same level of image noise.126–128 However, it is known that the frequency characteristics of noise in images obtained by iterative reconstruction vary according to the type of processing, and visual discomfort may occur, especially when the intensity of the processing is increased. Therefore, when iterative reconstruction is applied, it should always be confirmed that the image quality necessary for diagnosis is obtained by visual evaluation and the processing intensity should be optimized (see Q&A).
2.3 Risk of Exposure
When considering the risk of exposure, it is important to know the dose. With CT scans, the dose is assessed based on the CTDIvol
and dose–length product (DLP). The exposure dose in CT scanning depends on the scanner used, and even with the same device, dose varies according to the number of scans, the helical pitch, current, and other parameter settings.129 Therefore, it is important to know the standard dose for each device for each protocol. Because most of the current CT examinations of the torso use automatic irradiation control, the exposure dose differs greatly depending on the patient’s body size, even with the same protocol. Thus, it is difficult to determine the exact exposure dose for an individual patient before a CT scan, but the organ and tissue doses are generally less than several 10 s of mGy and the effective dose is less than several 10 s of mSv per examination.
Radiation effects on the human body from CT examinations should be discussed separately as deterministic effects (tissue reaction) and stochastic effects. The deterministic effects are disorders that occur when the threshold dose is exceeded, and it is unlikely that an organ or tissue dose will exceed the threshold dose in a single ordinary CT scan. However, the threshold dose for fetal malformations, etc. is as low as 100 mGy, so the threshold dose may be exceeded when the pelvic region of a pregnant woman is imaged repeatedly. In addition, because the threshold dose for the lens of the eye has recently been reduced to 500 mGy and similar thresholds have been proposed for the heart and brain,24 careful consideration should be given to the possibility of deterministic effects when multiple CT examinations including these tissues and organs in the scope of imaging are performed.
The risk of developing cancer from low-level radiation, such as from diagnostic imaging, has not been clearly defined. There have been a few reports describing a significant increase in cancer after CT scans in young patients.132–134 On the other hand, it has been suggested that some underlying abnormality is related to the development of the cancer, so it does not necessarily mean that the CT scan induces carcinogenesis.134,135 The effective dose of medical exposure from a single CT scan is usually <100 mSv, and the risk of stochastic effects on an individual may not be high, but carcinogenesis is not negligible in a large population of cases.
2.4 Informed Consent by Patients
Informed consent must be obtained for CT scans, and explanations of exposure in CT scans must be given to the patient.118,136 As mentioned above, the Ministry of Health, Labour and Welfare guidelines state that the attending physician or attending dentist who ordered the examination is responsible for giving the explanation to the patient. It is advisable for institutions to prepare an explanatory document as follows.
First, the necessity and usefulness of CT scans should be explained in detail. Alternative examinations, such as ultrasound and MRI, that do not involve exposure to radiation, and the superiority of CT scans over these examinations are also discussed. The benefit of CT scans in the negative study should also be mentioned because some patients may misinterpret it as an unnecessary exposure to radiation if the abnormality is not found.
Next, the exposure dose in CT scans should be explained. However, explanation of exposure dose tends to be difficult for patients to understand. Estimation of the dose for an individual at one’s own facility is possible, but complicated and sometimes uncertain. In many cases, it may be practical to give a rough estimation of the dose of the CT scan relative to natural radiation.
In terms of the effects of exposure, they should be separated into deterministic effects and stochastic effects. As mentioned above, a typical single CT scan is unlikely to have a deterministic effect. Radiation exposure in CT scans is usually <100 mSv, and the risk of carcinogenesis at this dose has not been demonstrated. The risk, if any, is very low compared with the risk of cancer due to other factors such as lifestyle. In light of these points, explanations should be given according to the circumstances. In addition, special attention should be paid to pregnant women at the time of informed consent.
It is desirable to mention the efforts being made at each facility to reduce medical exposure in the explanation.
2.5 Q&A
Q: CT Scans for Pregnant Women
What are the effects of exposure to radiation on CT scans for pregnant patients and how we treat them?
A:
More consideration than usual should be made when performing a CT scan in pregnant patients. First, we should try non-radioactive examinations such as ultrasonography and MRI as an alternative modality for CT scanning. If this is not feasible, caution should be paid to minimize the absorbed dose to the fetus.
The effect of radiation on the fetus depends on the timing of post-conception exposure and the dose absorbed. If exposure >100 mGy occurs during the organogenesis period (3–8 weeks after conception), the incidence of malformations may be increased. However, a fetal dose of <100 mGy should not be the reason for an abortion.106,137 Exposure >100 mGy in the period 8–25 weeks post-conception can cause central nervous system damage.
It is assumed that the risk of inducing cancer in the embryo and fetus during almost the entire gestation period at exposures ≥100 mGy is about the same as in children. On the other hand, there is no consensus on the risk of carcinogenesis or genetic effects at doses <100 mGy, but optimization of protection should be based on the linear non-threshold model, because the incidence of radiation effects increases even at low doses.
Q: CT Scans for Children
What kind of considerations should be made for CT scans in children, who are highly radiosensitive?
A:
In terms of justification, as for pregnant women CT scans should be considered when sufficient information cannot be obtained by ultrasonography or MRI. However, MRI may also include the need for sedation, which may be a different risk for children. The guidelines of the Japanese Society of Cardiology can be used as a reference for the indications for testing in congenital and childhood heart disease.138,139
With regard to optimization, the lowest possible dose for diagnosis should be attempted (see ALARA principles, II.2.2.5 Optimization of Protection), and techniques such as low tube voltage imaging, fine-tuned tube current settings according to the patient’s physique, automatic irradiation control, or iterative reconstruction should be actively used.138 In addition, when the coronary arteries are not the target of evaluation, non-ECG-gated imaging with lower exposure doses is often adequate to provide sufficient information.
Q: Cautions in Reconstruction Using Iterative Reconstruction
I heard that the use of the iterative reconstruction method can reduce the exposure dose. Are there any cautions in using iterative reconstruction?
A:
The iterative reconstruction method has been recently introduced by many manufacturers of CT systems. When the radiation dose used in CT scanning is reduced considering the patient’s exposure, the image quality will be degraded by the increased noise using conventional image reconstruction methods. Iterative reconstruction can reduce noise level without compromising image quality. On the other hand, attention must be paid to the fact that iterative reconstruction adversely affects the spatial resolution and the frequency characteristics of noise.140
Conventional indices for the physical characteristics of image quality, including the standard deviation of CT values (evaluation of noise level), contrast/noise ratio (evaluation of low contrast resolution), and bar pattern visibility or modulation transfer function using the wire method (evaluation of spatial resolution), may not represent the intrinsic properties of images obtained by iterative reconstruction. Therefore, when applying iterative reconstruction, image quality should always be confirmed by visual evaluation.
Q: Difference Between CT Scans and Interventional Procedures
Why can radiation skin lesions be a problem during interventional procedures using angiography, but ordinary CT scans do not cause radiation skin problems?
A:
In CT systems, the X-ray tube is rotated around the patient’s body, so the irradiated area is dispersed. In contrast, angiography systems often irradiate particular region of the patient with the X-ray tube in a certain direction, and most of the irradiated X-rays enter the patient’s skin. In addition, the X-ray tube voltage is lower in angiography than in CT equipment. So, the penetration power with angiography is lower than with CT and more X-rays are absorbed near the skin surface during angiography. Accordingly, radiation skin disorders may occur with vascular imaging systems.
Q: Risk Assessment Based on Effective Dose
I’ve heard that it’s inappropriate to assess a patient’s cancer risk based on effective dose. Why is that?
A:
The International Commission on Radiological Protection (ICRP) states that effective dose should not be used for retrospective assessments of exposure for specific individuals or for epidemiological assessments in human.22 The tissue weighting factors for the calculation of effective dose were originally established for the purpose of assessing stochastic effects in the population of workers and the general public, but the radiosensitivity of organs and tissues actually depends on age and sex. Therefore, it is considered inappropriate to apply these tissue weighting factors to patients or individuals of different sexes and ages. For risk assessment of patients, individual organ doses should be calculated and risk coefficients for each organ should be applied based on the patient’s age and sex.20
Q: Cautions for Frequent CT Scans
CT scans are increasingly used for the diagnosis and follow-up of coronary artery disease and aortic aneurysms. What should be considered when performing repeated and frequent CT scans?
A:
Because repeated and frequent CT examinations increase the accumulated dose, it is necessary to consider the indications, including the interval between examinations. Easygoing CT examinations should be discouraged. Depending on the true nature of the disease, we need to confirm whether sufficient information is obtained even with dose reduction by limiting the extent of imaging, the number of imaging sessions (whether or not a simple CT scan or dynamic study is performed), and the amount of tube current.
3. Nuclear Medicine (Table 21)
Table 21. COR and LOE for Dose Reduction With SPECT Scans
| |
COR |
LOE |
| Healthcare workers |
It is recommended to comply with the 3 principles of radiation dose reduction: time,
shielding, and distance |
I |
A |
| Patients |
| The use of 99 mTc is recommended for Anger-type camera141,142 |
I |
B |
Technological improvements (absorption correction, supine imaging, software
correction, semiconductor SPECT devices) should be incorporated if possible141 |
I |
B |
| It is recommended that the dose be optimized for the patient’s weight143 |
I |
C |
COR, Class of Recommendation; LOE, Level of Evidence; SPECT, single-photon emission tomography.
3.1 SPECT Examinations
3.1.1 Radionuclides
The radionuclides used in SPECT examinations include 201Tl (thallium chloride: Tl-Cl), 99 mTc (tetrofosmin, MIBI), and 123I (BMIPP, MIBG), and these doses are calculated based on ICRP Publications. Although the calculation of the effective dose for 201Tl-labeled products tends to be lower with each revision, from 0.22 mSv/MBq in ICRP Publication 80 (1998) to 0.17 mSv/MBq in Publication 53 Addendum 5 (2001), the working group of the Japanese Society of Nuclear Cardiology (JSNC) adopted 0.14 mSv/MBq as described in ICRP Publication 106 (2008) and Publication 128 (2014) for the calculation of exposure doses.141
The effective doses per unit of radioactivity for each nuclide are shown in Table 22, the age-specific effective doses for 201Tl and 99 mTc-labeled products are shown in Table 23, and the effective doses for 123I-labeled products are shown in Table 24.141 It should be noted that the effective dose of both 201Tl and 99 mTc-labeled products increases at younger ages compared with adults.
Table 22. Effective Dose per Unit of Radioactivity for Each Radionuclide
| |
201Tl-Cl |
99 mTc-tetrofosmin |
99 mTc-MIBI |
| During exercise |
At rest |
During exercise |
At rest |
| Adult effective dose (mSv/MBq) |
0.14 |
0.0069 |
0.0080 |
0.0079 |
0.0090 |
| Redistributed |
| Actual dose at IV injection (MBq) |
111 |
350 |
702 |
350 |
670 |
| Exposure dose per injection (mSv) |
15.54 |
2.415 |
5.616 |
2.765 |
6.021 |
| Exposure dose per examination (mSv) |
15.54 |
8.031 |
8.786 |
| Non-redistributed |
| Actual dose at IV injection (MBq) |
|
418 |
660 |
366 |
660 |
| Exposure dose per injection (mSv) |
|
2.884 |
5.28 |
2.891 |
5.940 |
| Exposure dose per examination (mSv) |
|
8.164 |
8.831 |
As reported by the working group of the Japanese Society of Nuclear Cardiology. Estimates of the actual doses of the 99 mTc-labeled products are given using 296/740 MBq for tetrofosmin and 259/740 MBq syringes for MIBI, redistributed to 350/1,115 MBq for tetrofosmin and 350/1,062 MBq for MIBI at 9:00 a.m. (top row) and measured without redistribution (bottom row) at 9:00 and 13:00 a.m., respectively. MIBI, methoxy-isobutyl-isonitrile. (Adapted from Katabuchi et al, 2019141 with modification.)
Table 23. Age-Specific Effective Dose of
201Tl and
99 mTc-Labeled Products
| |
Adult |
15 years old |
10 years old |
5 years old |
1 year old |
| 201Tl-Cl |
0.14 |
0.2 |
0.56 |
0.79 |
1.3 |
| 99 mTc-tetrofosmin (during exercise) |
0.0069 |
0.0088 |
0.013 |
0.021 |
0.039 |
| 99 mTc-tetrofosmin (at rest) |
0.0080 |
0.01 |
0.015 |
0.024 |
0.046 |
| 99 mTc-MIBI (during exercise) |
0.0079 |
0.01 |
0.016 |
0.023 |
0.045 |
| 99 mTc-MIBI (at rest) |
0.0090 |
0.012 |
0.018 |
0.028 |
0.053 |
MIBI, methoxy-isobutyl-isonitrile. Unit: mSv/MBq. (Adapted from Katabuchi et al, 2019.141)
Table 24. Effective Dose of
123I-Labeled Products
| |
123I-BMIPP |
123I-MIBG |
| Effective dose (mSv/MBq) |
0.016 |
0.013 |
| Dosage (MBq) |
111 |
111 |
| Exposure dose per injection (mSv) |
1.776 |
1.443 |
BMIPP: based on ICRP Publication 128; MIBG: based on ICRP Publication 80.77 (Adapted from Katabuchi et al, 2019.141)
3.1.2 Gamma Cameras
Gamma cameras used for SPECT include the conventional Anger device and semiconductor systems. Because the sensitivity of semiconductor systems to gamma rays is higher than that of Anger-type systems, the image quality of these systems is equivalent or better than that of ordinary Anger-type systems, even at low dosages. Therefore, the JSNC strongly recommends the introduction of technological improvements, including the use of semiconductor systems, especially when using 201Tl-labeled products.141
Because the half-life of 201Tl is about 12-fold longer than that of 99 mTc, the dose per unit of radioactivity for 201Tl is about 15–18-fold greater than that of 99 mTc. If the standard dose for 99 mTc-labeled products is 1,110 MBq, the dose for 201Tl-labeled products to keep the exposure at the same level would be <70 MBq, which reduces the count and image quality in an Anger device using a conventional parallel hole collimator. For this reason, it is recommended to use 99 mTc-labeled products. In the semiconductor system, even if 201Tl-labeled products are used, it is possible to minimize the exposure dose by using the protocol described below
3.1.3 Examination Protocol
a. Stress-First Protocol With 201Tl-Labeled Products
Although the INCAPS study142 recommends avoiding tests with 201Tl-labeled products and using 99 mTc-labeled products for those under 70 years of age, as an example of a protocol using the low-radioactive 201Tl-labeled products in a semiconductor device, a 10-min stress test with 1.0 MBq/kg of 201Tl (exercise stress) or 0.6 MBq/kg of 201Tl (adenosine stress), followed by a 20-min acquisition of late images, has provided satisfactory image quality.143 At this dose, an exposure of 9 mSv/test can be achieved in exercise stress testing up to 64 kg of body weight and adenosine stress testing under 107 kg of body weight.
The INCAPS study also recommends avoiding the simultaneous use of 201Tl and 99 mTc-labeled products for those under 70 years of age. Therefore, this technique is not recommended for SPECT examinations using the Anger-type device in the non-elderly population. However, with use of the semiconductor type camera in a dual-isotope protocol,144 150 MBq of 99 mTc at rest and 50 MBq of 201Tl at stress test can be used with an exposure of about 8.2 mSv/test.
b. Protocol With 99 mTc-Labeled Products
In the Japanese DRLs (DRLs 2015), the DRL for the 99 mTc test for twice administration is set at 1,200 MBq, and either a rest-first or stress-first protocol can be safely performed with this dosage. It has been reported that the 99 mTc single-day protocol with a 14-min acquisition using a semiconductor device reduced the dose to <1 mSv.145
In stress-only imaging, images are checked after the stress, and if there is no apparent perfusion defect, the resting examination can be omitted. There is an operational difficulty because the diagnosis from stress-imaging is required before the decision to omit the rest imaging. But theoretically there is no reason to take images at rest if there is no abnormality in the images at stress. In myocardial perfusion SPECT examinations with 99 mTc, if the imaging at rest can be omitted in the single-day protocol, the exposure dose can be significantly reduced.142 A report on stress-only imaging in Japan showed that imaging at rest was omitted in about two-thirds of cases,146 contributing to a significant reduction in radiation dose.
The dose at stress-imaging may be increased to improve image quality and diagnostic accuracy. In fact, the JSNC states that exposure in cardiac nuclear medicine is in the range of stochastic effects, and reduction of the total population’s exposure is the primary goal, meaning that it is not problematic even if the doses at stress are slightly higher for each examination. Therefore, the JSNC strongly recommends stress-only imaging.141 In the clinical scenario of sequential imaging, where SPECT is preceded by coronary CT and/or other modalities, this strategy should be considered.
3.1.4 Methods to Reduce Exposure Dose
a. Healthcare Workers
Kanaya et al reported that in Japan physicians in charge of nuclear medicine examinations were exposed to 0.20 μSv for 555 MBq of 99 mTc, and to 0.33 μSv for 740 MBq of 99 mTc during intravenous infusion.80 In a study of a short-term protocol using a 99 mTc-labeled product of 1,200 MBq, the exposure of the leader of the examination was 6.2 μSv and that of the assistant was 2.5 μSv.147 The reasons for the difference in exposure doses between the two roles are considered to be the differences in position relative to the patient and the tasks performed during the examination.
Reduction of exposure doses can be achieved based on the 3 principles of time, shielding, and distance in general. But this is difficult to achieve in the case of healthcare workers when performing ultrasound examinations (especially echocardiography) of the patients with nuclide administration. It has been reported that exposure of the patient’s abdominal surface after 201Tl test was around 0.3 μSv/min, which would be roughly equivalent for echocardiographers.148 The JSNC recommend protective clothing with 0.25 mm lead to reduce the exposure dose by >40% with 99 mTc-labeled products.141 It is desirable to educate physicians who order nuclear medicine to avoid performing echocardiography and nuclear medicine testing on the same day, or echocardiography should be done before nuclear medicine.
b. Patients
In the INCAPS study,142 the following 8 “best practices” were proposed for optimization of protection.
1) Avoid the Use of 201Tl-Labeled Products
Patients under 70 years of age should not be tested with 201Tl; instead 99 mTc-labeled product is recommended using an Anger-type device for such patients.
The 99 mTc-labeled products are strongly recommended for children because of the greater stochastic and deterministic effects in the future, especially for the small hearts of infants, in terms of both exposure dose and image quality. In the myocardial stress–rest protocol in children, the 201Tl-labeled products are expected to provide approximately 8–10-fold higher doses than the 99 mTc-labeled products.
2) Avoid the Use of 2 Radionuclides
It is advisable to avoid the use of simultaneous dual-isotope methods with 201Tl- and 99 mTc-labeled products in Anger-type devices. The 123I-BMIPP or 123I-MIBG and 201Tl combination should be performed in carefully selected subjects.
3) Optimize the Dosage of 99 mTc-Labeled Products
The dosage of 99 mTc-labeled product should be kept below 1,332 MBq and the effective dose should be kept below 15 mSv; however, based on the “ALARA principles”, lower doses are recommended as long as adequate image quality is achieved. In Japan, it is recommended to comply with the DRL of 1,200 MBq for the twice 99 mTc test as specified in the DRLs 2015.
4) Optimize the Dosage of 201Tl-Labeled Products
It is recommended to keep the dose of 201Tl-labeled product below 129.5 MBq. In the DRLs 2015, the DRL for 201Tl myocardial perfusion scintigraphy was above that value, but it will be decreased significantly in the future. The use of 201Tl-labeled products is not recommended, at least in Anger-type devices.
5) Stress-Only Imaging Protocol
As described previously, stress-only imaging can eliminate the need for imaging at rest and can be expected to significantly reduce the exposure dose.
6) Reduction of the Dose by Technical Methods
The INCAPS study142 recommended the introduction of more than 1 of the following 4 technical methods: (1) absorption correction, (2) imaging in multiple postures (e.g., prone position imaging), (3) software correction, and (4) engineering improvement.
The JSNC proposes the following 4 technical methods be used: (1) longer collection time, (2) engineering improvements (e.g., semiconductor systems), (3) imaging in multiple postures (e.g., prone position imaging), and (4) software correction (e.g., aperture correction). The first is the most common technical method, but improvements in equipment performance have enabled maintenance of image quality with an acceptable extension of time, even if the dosage is reduced. Method (2) will increase the sensitivity of the examination, and in particular, when 201Tl-labeled products are used, the introduction of engineering improvements using semiconductor systems is strongly recommended as mentioned previously.
7) Optimize the Dosage According to the Patient’s Body Weight
In Japan, prefilled syringe products are often used, and individual dose adjustment is difficult. From the viewpoint of the exposure dose, there is no reason to administer the same dosage to lower-weight subjects as to higher-weight subjects. The JSNC recommends a lower dosage for patients under 50 kg body weight, and recommends the use of the dosage tables for children.141
8) Adjustment of Stress and Rest Dosages to Avoid Shine-Through
“Shine-through” is a phenomenon in which the count of the isotope administered in the first test (stress in the stress–rest protocol and rest in the rest–stress protocol) overlaps with the image of the second test, which affects image quality. In 1-day methods using 99 mTc-labeled products, the second dose is recommended to be at least 3-fold higher than the first. In Japan, where prefilled syringes are predominantly used, the use of a 1:3 redistribution of 370 MBq and 740 MBq syringes at 9:00 a.m. slightly reduces the actual dose. This distribution improves image quality as well as reduces exposure to radiation, and therefore, it is desirable to adjust the dosage ratio to 1:3 or more to avoid “shine-through”.
3.2 PET Scans
3.2.1 Commercially Available Products
At present, only 18F-FDG products can be delivered to facilities. The absorbed doses for each organ of the patient as listed in the products’ package inserts are shown in Table 25.78
Table 25. Absorbed Doses of
18F-FDG by Organ
| Organ |
Absorbed dose (mGy/MBq) |
| Adrenal |
0.012 |
| Bladder |
0.13 |
| Bone surface |
0.011 |
| Brain |
0.038 |
| Breast |
0.0088 |
| Gall bladder |
0.013 |
| Stomach |
0.011 |
| Small intestine |
0.012 |
| Colon |
0.013 |
| Heart |
0.067 |
| Kidney |
0.017 |
| Liver |
0.021 |
| Lungs |
0.020 |
| Muscles |
0.010 |
| Esophagus |
0.012 |
| Ovary |
0.014 |
| Pancreas |
0.013 |
| Red bone marrow |
0.011 |
| Skin |
0.0078 |
| Spleen |
0.011 |
| Testicle |
0.011 |
| Thymus |
0.012 |
| Thyroid |
0.010 |
| Uterus |
0.018 |
| Others |
0.012 |
| Effective dose |
0.019 mSv/MBq |
(Source: based on ICRP, 2008.78)
The effective dose, which is an indicator of stochastic effects obtained from the absorbed doses to each organ, is shown in Table 26.77 The effective dose for adults administered 200 MBq is 3.8 mSv. For the relationship between effective dose and stochastic effects (carcinogenesis) (see section 3.2.4 below).
Table 26. Effective Dose of
18F-FDG by Age
| Age (years) |
Effective dose (mSv/MBq) |
| Adults |
0.019 |
| 15 |
0.025 |
| 10 |
0.036 |
| 5 |
0.050 |
| 1 |
0.095 |
(Source: based on ICRP, 1998.77)
The DRLs for 18F-FDG as described in “Japan DRLs 2020” are shown in Table 27.149 The DRL value represents the 75th percentile of the doses checked under specified conditions in many hospitals. If the dosage at a given facility is higher than this value, the facility would be included in the highest quarter, which indicates that dose reduction should be considered.
Table 27. DRLs of
18F-FDG Preparation for Cardiac PET
| Test |
Radiopharmaceutical |
DRL (MBq) |
| Myocardial glucose metabolism |
Delivered 18F-FDG |
240 |
| In-house produced 18F-FDG |
240 |
| 18F-FDG (dose/body weight) |
5 |
| Myocardial perfusion |
In-house produced 13N-NH3 |
520 |
DRL, diagnostic reference level; PET, positron emission tomography. (Adapted from Japan Network for Research and Information on Medical Exposure (J-RIME), 2020.149)
As shown in Table 26, the effective dose varies greatly depending on the age of the subject, and the exposure to CT scan in PET/CT is also added. Therefore, attention should be paid when children and young subjects are examined. Table 28 shows the body weight and standard doses based on the classifications of the radiopharmaceuticals and the weight-specific coefficients of each class, which are listed in “Part 1: Pediatric radiopharmaceutical administered doses (Japanese Society of Nuclear Medicine (JSNM) pediatric dosage card)” in the “Japanese consensus guidelines for pediatric nuclear medicine” by the JSNM.56 This is a standard dosage for pediatric patients and may need to be increased or decreased according to circumstances. The “Guidelines for standardization of the whole-body FDG-PET scanning protocol” of the JSNM set the standard dose of radioactivity at 2.0–5.0 MBq/kg,150 and at 3.7 MBq/kg, the dose for a patient with a body weight of 30–70 kg is 111–259 MBq.
Table 28. Typical Doses of
18F-FDG by Body Weight in Pediatric Trunk Examinations
| Body weight (kg) |
Dose (MBq) |
| 3 |
26.0 |
| 6 |
30.8 |
| 10 |
48.8 |
| 20 |
87.5 |
| 30 |
123.5 |
| 40 |
159.5 |
| 50 |
192.8 |
(Source: based on Japanese Society of Nuclear Medicine, 2019.56)
For the delivered products, if the dose of radioactivity is fixed, the interval between the test of radioactivity and time of administration should be adjusted so that the dose is appropriate for each body weight using the decay table (Table 29).151 On the other hand, there are some pharmaceutical companies that set a number of test times and supply formulations containing various amounts of radioactivity (flexible dose). There are 15 different test times per day and 5 different radioactivity doses at each test, and there are many combinations of radioactivity doses and test times, even when the doses are similar, so it is important to be careful when ordering a product.
Table 29. Radioactive Decay of
18F
Time elapsed
(min) |
Residual radioactivity
(%) |
Time elapsed
(min) |
Residual radioactivity
(%) |
| −110 |
200.3 |
20 |
88.1 |
| −100 |
188.0 |
30 |
82.7 |
| −90 |
176.5 |
40 |
77.7 |
| −80 |
165.7 |
50 |
72.9 |
| −70 |
155.6 |
60 |
68.5 |
| −60 |
146.0 |
70 |
64.3 |
| −50 |
137.1 |
80 |
60.3 |
| −40 |
128.7 |
90 |
56.7 |
| −30 |
120.9 |
100 |
53.2 |
| −20 |
113.5 |
110 |
49.9 |
| −10 |
106.5 |
120 |
46.9 |
| 0 |
100.0 |
130 |
44.0 |
| 10 |
93.9 |
140 |
41.3 |
(Adapted from FUJIFILM Toyama Chemical Co., Ltd. 2018.151)
The package insert for the 18F-FDG preparation contains the following information on its use in pregnant women and nursing women:151 “In principle, this product should not be administered to pregnant women or women who may be pregnant. Lactating women should not receive this drug in general and may be administered only when the diagnostic benefits are judged to outweigh the disadvantages of exposure. When administered to lactating women, feeding should be discontinued for 24 h and close contact with infants should be avoided for 12 h after administration.”
For PET/CT, the dose from CT scanning is added to the dose from the radiopharmaceuticals. The former varies depending on the system, with ∼2 mSv under the absorption correction condition and ∼12 mSv under the fusion imaging condition.152 These values tend to decrease as technology advances. With the help of manufacturers, it is advisable to know the doses under PET/CT imaging conditions at one’s own facility and to investigate the risks at those doses.
3.2.2 In-House Produced Radiopharmaceuticals
The DRLs for 18F-FDG (Table 27)149 and the dosage in children are similar to the delivered products. Table 30 shows the effective doses for cyclotron preparation other than 18F-FDG in myocardial perfusion tests using 13N-NH3
and 15O-H2O.153 For the relationship between effective dose and stochastic effects (See section 3.2.4 below).
Table 30. Effective Dose of Cyclotron Preparation for Cardiac PET
| Test |
Radiopharmaceutical |
Dose (MBq) |
Effective dose (mSv) |
| Myocardial perfusion |
13N-NH3 |
550 |
2 |
| Myocardial perfusion |
15O-H2O |
2,000 |
2 |
PET, positron emission tomography. (Source: based on UNSCEAR, 2000.153)
3.2.3 Methods to Reduce Exposure Dose
a. Healthcare Workers
Because 18F emits higher energy gamma rays than single-photon radionuclides, it is important for healthcare workers to reduce their exposure dose during PET scanning. Doses should be measured by personal dosimeter. According to the guidelines, it is advisable to develop procedures and manuals with a target value of ≈5 mSv/year for occupational exposure from PET examinations.77 If new 18F products, such as 18F-flurpiridaz, become available in the future, many facilities will perform dynamic imaging, which may increase the exposure of healthcare workers, as well as examinations with 13N and 15O. New guidelines for the reduction of exposure doses will be necessary at that time.
The following are some specific tips to reduce exposure.77,152,154
(1) Avoid overlapping the patient’s route in the laboratory with those of the staff and other people in the PET laboratory, as far as possible. This should be considered from the stage of designing the facility.
(2) Explanation to the patients should be made prior to the administration of radioactive isotopes (RIs) and contact after administration should be limited as much as possible.
(3) If a patient has difficulty with moving, or requires assistance to move, it is better to ask other staff for assistance in advance to prevent the concentration of exposure to the laboratory staff.
(4) A dedicated protective screen should be prepared for PET examinations. In particular, physicians and nurses tend to be exposed to more radiation during the administration of PET preparations. Introduction of an automated administration device, shortening of the needle extraction time, use of a shielding plate, and installation of a needle disposal box are effective in dose reduction.
(5) The IV route should be secured before the RI is administered. If an injection leak occurs during administration, the exposure dose to the staff will increase due to the need to re-establish the IV route. Be careful of liquid leakage due to lose line connections.
(6) The Personal handy-phone system or intercom can be used to call the camera room.
(7) Because radiologists tend to be exposed to higher radiation doses due to their position during imaging, it is recommended to shorten the stay as much as possible and to adjust the table in the operating room. Because the visibility of the imaging room is limited from operating room, it is desirable to install a monitoring device in the operating room.
(8) Consider job rotation to avoid concentrating exposure to specific medical staff.
(9) The exposure dose for the medical staff from each patient after a certain amount of time is relatively low. However, consideration for reducing exposure doses is necessary for staff that contact many patients, such as the drivers of patient transport vehicles or buses. In facilities where a large number of 18F-FDG tests are performed, appropriate guidance and measures should be provided to workers, including information desk staff, receptionists, accountants, and transportation vehicles, as well as patients, and the effectiveness of these measures should be confirmed through monitoring.
(10) Unlike delivered products that are usually administered using commercially available automatic administration equipment, cyclotron-produced radionuclides require careful consideration for reducing the exposure dose to medical staff because each facility has its own administration system and protocol. When 15O or 13N is administered manually without an automatic administration system, it is necessary to consider a much larger amount of radioactivity during preparation than only that from the actual dose, because the half-life of 15O and 13N is much shorter than that of 18F and thus, careful attention should be paid to radiation exposure. Because of the high energy of the emitted gamma rays, there is a limit to the effectiveness of shielding, and the distance and time (especially the latter) must be considered. If less experienced staff members perform the examination, they should carefully simulate the administration and their standing position so that they can perform the operation smoothly during the actual examination.
The exposure of staff in drug production is beyond the objective of this guideline.
b. Patients, Patient’s Families, and Caregivers
In order to reduce the exposure of patients, caregivers, patient’s families, and the general public, the following procedures should be considered by radiation workers when using 18F-FDG.77,152,154
(1) Patients should be encouraged to drink water before and after administration of the drug. However, this does not apply to patients with heart or kidney failure. Instruct the patient to urinate before imaging and before leaving the controlled area.
(2) Within 1 half-life (≈2 h) after administration of 18F-FDG, patients should stay in a waiting room in the controlled area, shielded from the surroundings, to reduce exposure to the public and others.
(3) After administration of 18F-FDG, instruct the patient to reduce the duration of contact and keep a distance from radiation-sensitive pregnant women and children less than 10 years of age. As mentioned previously, the package inserts for the delivered product state, “If administered to a nursing woman, breastfeeding should be stopped for 24 h and instruct the woman to avoid close contact with infants for 12 h after administration.”
(4) Those who are in the vicinity of patients for a long time should be monitored using personal dosimeters, if necessary.
Measures to reduce exposure doses in examinations using cyclotron-produced radionuclides other than 18F-FDG are basically the same as those for delivered products. However, due to the short physical half-lives of the nuclides, exposure of people around patients is rarely a problem, except for medical workers.
3.2.4 Risk of Exposure and Explanations to Patients
a. Patients
The risk (effect) of exposure includes deterministic effects (tissue response) and stochastic effects. The former effect is usually not a problem because the half-life of the PET radionuclides is short and below the threshold for the doses used in the examination.
The stochastic effects are the risk of carcinogenesis and cancer death. For stochastic effects, the probability of occurrence increases with dose, not the severity of symptoms. The risk of carcinogenesis was not significantly increased at doses <1 Sv in the follow-up data of atomic bomb survivors, but the risk of carcinogenesis is not zero even at low doses at the level of PET examinations.
ICRP Publication 103 (2007 Recommendations) describes that 1 Sv exposure results in ≈5% increase in lifetime mortality. An exposure of 10 mSv corresponds to an increase in mortality of 0.05%. However, the actual risk is considered to be lower than the linear approximation because of the favorable repair mechanisms for radiation damage with decreasing radiation dose. The risk is strictly estimated and linearly approximated for safety management.
Explanations to patients and their families do not necessarily need to refer to these details but should be given so that the people understand the above points easily and that the benefits of the test outweigh the risks of radiation exposure.
b. Patient’s Families and Caregivers
Patients themselves become the source of radiation in nuclear medicine examinations and expose others to radiation. Because exposure dose is inversely proportional to the square of the distance from the source, it is important to keep a distance between the patient and other people, and this should be mentioned in the informed consent process. Care should be taken when infants and toddlers with high sensitivity to radiation come close to the patient, such as while breastfeeding or holding them. As mentioned above, the package inserts for 18F-FDG state, “If administered to a nursing woman, breastfeeding should be stopped for 24 h and instruct the woman to avoid close contact with infants for 12 h after administration.”78
3.3 Q&A
Q: Cardiac Catheterization on the Same Day or the Next Day of Myocardial Perfusion Scintigraphy
What should be considered about contamination of the patient’s blood or catheterization equipment by radiopharmaceuticals when 201Tl or 99 mTc products are used to assess myocardial viability and then cardiac catheterization is performed on the same day or the day after administration?
A:
Because the radiopharmaceuticals administered to patients during nuclear medicine examinations are in small doses, cardiac catheterization can generally be performed on the day of administration or the day afterwards without any problems with exposure of the operator or other staff. However, because the half-lives of 201Tl and 99 mTc are approximately 73 h and 6 h, respectively, 99 mTc is recommended for testing.
Radiopharmaceuticals remain in the patient’s blood, even in very small amounts, and needles and catheters used for cardiac catheterization may be contaminated. Because radiopharmaceuticals are largely excreted in the urine, it should be handled with more caution than blood. Urine bags and used diapers of patients should be treated, as well as catheters and needles. The estimated collection period for diapers etc. is 7 days for 201Tl-labeled product and the day of administration for 99 mTc-labeled product.155 After a certain period of storage, confirm that the radioactive contamination is not detectable by measuring instruments and dispose of it as infectious waste.
4. Cardiovascular and Thoracoabdominal / Cervical Vascular Interventions (Table 31)
Table 31. COR and LOE for Dose Reduction in Cardiovascular and Thoracoabdominal / Cervical Vascular Interventions
| |
COR |
LOE |
| Healthcare workers and patients |
It is recommended to comply with the 3 principles of external dose reduction: time,
shielding, and distance156 |
I |
A |
| It is recommended to set the frame rate lower than 15 f/s17,156 |
I |
B |
| Healthcare workers |
| It is recommended to use proper shielding and radiation protective clothing99,157,158 |
I |
B |
| It is recommended to wear leaded eye protection to reduce lens exposure159,160 |
I |
B |
It is recommended that thorough monitoring of exposure doses using personal dosimeters
should be carried out17,156 |
I |
B |
Wearing a neck thyroid shield should be considered for workers with personal dose
readings of 4 mSv/month or higher at the neck161–163 |
IIa |
B |
COR, Class of Recommendation; LOE, Level of Evidence.
4.1 Methods to Reduce Exposure Dose
4.1.1 Increasing Radiation Exposure and Skin Injury
Over the past 20 years, X-ray fluoroscopic imaging, as represented by catheterization and interventions, has advanced greatly and is widely used for the management of cardiovascular diseases, which has improved greatly, but it has also increased the radiation exposure to the patients. Medical professionals should be aware of the risk of radiation exposure in patients who undergo diagnostic examinations and interventions.
It is important to know the most serious radiation-related hazard is skin injury, and the skin dose is dependent on fluoroscopic time, pulse rate, imaging frame rate and imaging time. In addition, it is important to be familiar with the skin doses for each procedure (Tables 32,33), because the radiation dose varies greatly depending on the type of procedure and the patient’s physique.
Table 32. Patient Skin Doses in Various Interventional Procedures
| Procedure |
Patient’s skin doses (Gy) |
Ref. |
| Embolization of head and neck vessels |
Cerebral arteriovenous malformation:
(median) 3.74
Cerebral aneurysm: (median) 3.78
Tumor: (median) 3.77 |
164 |
| Arterial embolization for hepatocellular carcinoma |
Conventional devices: 1.07±0.44
FPD devices: 0.28±0.13 |
165 |
| Catheter placement for hepatic arterial infusion |
0.89±0.87 |
|
| Biliary drainage |
(median) 1.16 |
164 |
| Vena cava filter placement |
(median) 0.22 |
|
| IVR for gastrointestinal bleeding |
(median) 2.77 |
|
| Uterine artery embolization |
(median) 2.75 |
|
| Percutaneous transluminal coronary angioplasty |
2.2±2.2 |
166 |
| Thoracic stent grafting |
0.87±0.41 |
167 |
| Abdominal stent grafting |
1.11±0.57 |
|
| Transcatheter aortic valve replacement |
Transfemoral: 0.53±0.26
Transapical: 0.33±0.13 |
168 |
FPD, flat panel detector; IVR, interventional radiology.
Table 33. Operator Exposure Doses in Various Interventional Procedures
| Procedure |
Operator exposure dose (μSv) |
Ref. |
| Embolization of head and neck vessels |
Between eyebrows: 170±120
Left hand: 150±180
Chest outside of protective clothing: 90±70 |
|
| Arterial embolization for hepatocellular carcinoma |
Chest outside of protective clothing:
64.5±23.2 |
165 |
| Percutaneous transluminal coronary angioplasty |
Between eyebrows: 290±420
Left hand: 860±2,450
Chest outside of protective clothing: 380±920 |
|
Advancements in fluoroscopic imaging systems have enabled operators to perform more complex cardiovascular interventions with superior image quality. However, in reducing external radiation exposure, it is important to always be aware of and thoroughly implement the 3 principles of “time” (shorten the exposure time), “shielding” (shield the radiation as much as possible), and “distance” (far enough from the source).156
4.1.2 Occupationally Exposed Healthcare Workers
The reason why the radiation exposure of healthcare workers differs from that of patients is that patients can expect to receive benefits from diagnostic testing or interventions, whereas the occupational exposure of healthcare workers can only be detrimental to their health. However, because all medical personnel have a social responsibility to perform medical treatment, it is necessary to fulfill that responsibility while devising ways to reduce exposure doses to the maximum extent. Specific methods of protection are described below.
(1) Exposure monitoring.
Healthcare workers should wear 2 personal dosimeters: 1 inside the radiation protective clothing (chest for men and abdomen for women) and 1 outside of the clothing, for uneven exposure, in the area of the trunk most likely to be exposed to radiation maximally (left side of the head and neck for IVR).17
(2) Wear a radiation protective coat.
Compared with the radiation protective coat, the radiation protective apron without back protection increases the exposure when working with the back facing to the patient. If the dorsal side is covered by protective clothing with a 0.25 mm lead-equivalent, the dorsal tissue absorption dose can be reduced by half. There are 2 lead-equivalent standards for radiation protection clothing (e.g., 0.25 mm and 0.35 mm) and the higher the value, the greater the radiation shielding ability but also the greater the physical burden on the individual because of the heavier weight. Because there is no significant difference in radiation shielding effect between 0.25 mm and 0.35 mm, selecting lighter 0.25 mm protective clothing may be considered to reduce back strain issues.157
(3) Always wear a thyroid protective collar.
A thyroid protective collar with 0.25 mm lead-equivalent has ≈90% protective effect as well as protective clothing. When a thyroid protective collar is used in combination with a radiation protective apron, the effective dose can be reduced by 50% compared with radiation protective clothing alone.161 The risk of radiation-related cancer due to thyroid exposure depends greatly on the age at the time of exposure, and the risk is extremely low in men 30 years and older and women 40 years and older.162 Therefore, although not all operators should use a thyroid collar, but those who have a personal monitor reading of ≥4 mSv/month near the collar should wear a thyroid protective collar.163
(4) Use protective eyewear to reduce lens exposure.
Even relatively thin protective eyewear with 0.07 mm lead-equivalent can reduce exposure by 60%. Appropriate protective eyewear, which fits securely on the face with side protection, should be used to reduce lens exposure and development of cataracts.159,160
(5) Keep as much distance as possible from the patient during fluoroscopy and imaging.
(6) Use mobile lead-containing acrylic sheets and lead curtains.
Scattered radiation that leaves the patient’s body is the principal source of exposure to nearby medical personnel. By placing an acrylic shield between the patient and the operator as close as possible to the target of protection (the operator), the operator’s exposure to scattered radiation can be reduced. Particularly in the cranial and caudal views, the position of the acrylic plate must be adjusted, otherwise the operator will be exposed to more scattered radiation (Figure 22). Also, to prevent the gonads and other parts of the body from being exposed to scattered radiation from below, which cannot be protected by radiation protective clothing alone, a lead-curtain rubber shield should be attached to the catheter table.99,158
(7) Keep the hands out of the irradiation field.
4.1.3 Patients
Specific methods to reduce patient exposure are listed.
(1) Do not perform unnecessary fluoroscopy or cine acquisition.
(2) Lower the frame rate and minimize the shooting time.
(3) Reduce the pulse rate as much as possible.
(4) Use an additional filter.
Low-energy photons in continuous X-rays used in procedures such as percutaneous coronary intervention (PCI) do not contribute to image formation and are absorbed by the patient’s skin, leading to an increase in the patient’s radiation exposure. Additional filters are usually installed in fluoroscopy systems, but older system may not be equipped with a filter. The availability of the filter should be confirmed at each facility.170
(5) Keep the distance between the patient’s body and the X-ray tube.
When the patient moves away from the X-ray tube, the distance between the X-ray tube focus and the X-ray receiver increases, which increases the X-ray output, but the dose to the skin decreases because it attenuates before reaching the patient’s skin. As a low position of the catheter table narrows the distance between the X-ray tube and the patient and increases the exposure dose,157 raise the catheter table as high as possible to keep the patient away from the X-ray tube but without affecting the examination and treatment procedures.
(6) Position the X-ray detector as close to the patient as practicable.
When the X-ray detector (image intensifier (I.I.), FPD) is moved away from the patient, the distance from the X-ray tube to the X-ray detector is increased. In this situation, the X-ray fluoroscopy system automatically adjusts the X-ray dosage to obtain a consistent image, which leads to an increase in the patient’s exposure dose. Therefore, the X-ray detector should be placed as close to the patient as possible. This is an operation in conjunction with 5) above, where the distance between the patient and the X-ray tube should be as far as possible, and the distance between the patient and the X-ray detector should be as small as possible.157
(7) Minimize the magnified fluoroscopy and cine acquisition.
Expanding the field of view of fluoroscopy and cine acquisition increases the radiation dose, and thus the patient’s exposure dose increases. In the I.I. system, where the brightness of the output phosphor surface depends on the size of the input phosphor surface, narrowing the input field of view leads to lack of light intensity in the output image, which requires a higher incident dose to maintain the brightness of the output image (Figure 23). In contrast, in FPD systems, which are increasingly being used, the output image brightness is maintained even when the field of view is magnified, because luminance is not amplified by the focusing electrodes, as is the case with I.I. However, because the image will be noisy as it is, the input dose is controlled to increase with the magnification of the field of view to prevent noise degradation (Figure 24). Magnified field of view, which is essential for some procedures to perform PCI safely, should be kept to a minimum to prevent skin damage.171

(8) Avoid prolonged fluoroscopy and photography in the same direction.
X-ray systems are controlled so that the dose to the radiographic detector is constant in order to obtain a uniform image. The left anterior oblique view, in which the heart is viewed from the patient’s right back through the spine and mediastinum, involves a high dose. Moving the fluoroscopy system further cranial or caudal in the left anterior oblique position increases the thickness of the body through which the X-rays pass, resulting in a further increase in dose. Therefore, the pulse rate should be reduced in patients who require prolonged left anterior cranial or left anterior oblique caudal views.29
(9) Reduce the exposed field size by collimation to the smallest size necessary.
Figure 25 shows a comparison between the irradiation field being fully opened and being narrowed down to 70%. By narrowing down the irradiation field, the dose per unit area does not change, but the area that could cause skin damage does.17,172
4.2 Risk of Radiation Exposure and Explanations to Patients
The effects of radiation on the human body can be divided into acute and late-onset effects as both physical effects and genetic effects due to exposure to germinal cells (see Figure 2). There is also a distinction between “deterministic effects” (tissue reaction) where the severity of damage increases with radiation dose and “stochastic effects” where the incidence is proportional to the radiation dose.
The effects on the skin, lens and hematopoietic organs are generally recognized as deterministic effects, which can be prevented by controlling the exposure to radiation so that threshold values are not exceeded. As mentioned above, interventions in the trunk are of greatest concern for skin injury, but other risks, such as cataracts and hair loss, may occur with head and neck interventions, and therefore, informed consent is required for each site of intervention.
The substantial radiation dose level (SRDL) refers to a radiation dose at the level of alert that may cause clinically relevant damage and requires medical follow-up. A SRDL for patients with a skin dose of 3 Gy has been proposed.157 Patients should be advised to observe the skin at the exposure site after 2–4 weeks, because exceeding the SRDL is likely to result in early skin injury.173
ICRP Publication 118 (2012) recommended lowering the threshold dose for cataracts caused by lens exposure from 5 Gy to 0.5 Gy, emphasizing the importance of optimizing lens exposure protection.24 The lens equivalent dose limit for occupational exposure, which is 20 mSv/year on average over a 5-year period and does not exceed 50 mSv in any 1 year, is also specified.
On the other hand, there is no threshold dose for prevention of carcinogenesis and genetic effects, because they are stochastic effects. It is necessary to control the radiation dose within an acceptable level of risk.
4.3 Issues Related to Novel Interventional Procedures
The hybrid operating room, which many facilities have been equipped with in recent years, allows simultaneous endovascular and surgical procedures, and has been used in cardiovascular interventions such as aortic stent grafting and transcatheter aortic valve implantation.
There are 2 types of C-arms for angiography in general: the ceiling-mounted type and floor-mounted type. As the ceiling-mounted C-arm requires rails on the ceiling to move the C-arm, the location for installation is limited. It is also necessary to install high-efficiency particulate air (HEPA) filters on the ceiling surface in the operating room to maintain room cleanliness, and the location of these filters must be considered in relation to interference with the rails. In addition, because surgical lights are required in operating rooms, many facilities may not be able to install a ceiling-suspended protective shield in the correct position due to limited space.
In addition, the standing position of the operators varies for each hybrid procedure, and a ceiling-mounted protective plate may not be able to cover all positions. Surgical beds often may not allow a lead-curtain rubber shield to hang from the table for protection against exposure from downward scattering radiation due to product specifications. In this case, it is important to use a self-standing floor-mounted protective shield. A floor-mounted protective shield allows all cables to be stored in the equipment or on the floor, thus leaving the ceiling free. This allows for flexible selection of the location of the HEPA filter and ceiling arms, and often allows for the installation of a ceiling-mounted protective shield in a suitable place. Radiation protection should be individualized according to the particular hybrid system and procedures performed in each institution.
4.4 Special Considerations for Children
With advances in non-invasive diagnostic methods such as echocardiography, CT and MRI, routine cardiac catheterization is no longer performed in children.174 However, pediatric patients with significant congenital heart disease may require cardiac catheterization and intervention as early as a neonate. As a result of recent advances in medical devices, more pediatric patients with congenital heart disease, which used to be treated only by surgery, are being treated with transcatheter procedures. It is estimated that approximately 7% of all cardiac angiographic procedures are performed in children aged 0–15 years.153
The susceptibility of children to the stochastic effects of radiation is ≈2–3-fold higher than that of adults,19 and the relationship between the effective dose and the kerma–area product (KAP) varies greatly depending on age, with a 10-fold difference in effective dose for the same KAP value.175 Children have a longer potential life expectancy than adults and therefore have a longer potential time to develop radiation-related sequelae. The probability of developing a fatal cancer per fluoroscopy-guided interventional procedure in children is estimated to be approximately 0.07–0.08%, but this risk can vary greatly depending on the patient’s age, basic life expectancy, and the type of procedure.176,177
The commonly performed procedures in pediatric cardiac patients include balloon valvuloplasty, closure of atrial septal defects, patent foramen ovale and patent ductus arteriosus, implantation of a prosthetic valve for pulmonary artery stenosis and aortic stenosis, and electrophysiological studies.178 Compared with diagnostic catheterization, interventional procedures require longer fluoroscopy time and more cine imaging, resulting in a higher radiation dose.179 Therefore, it is extremely important to optimize equipment based on the “ALARA principles” (see II.2.2.5 Optimization of Protection).180
Although measures to reduce exposure doses in children are the same as those used for adults, because of the low incidence of scattered radiation from subjects of small stature, it is possible to reduce exposure doses in children up to 8 years of age or under 20 kg in weight by removing the scattered radiation removal grid.180–183 Diagnostic catheterization and interventions using a biplane system is recommended in children,184 with the upper extremity raised to avoid projection of the upper extremity on the lateral image. Because the elbow is reflected in the lateral screen in the natural limb position, the upper limb must be raised firmly, but it should be cautioned that prolonged lifting of the upper limb may cause peripheral nerve damage.185
Due to the small size of children, the puncture site is close to the imaging site, and the operator often performs the procedure from a position close to the X-ray tube. Because the procedure is performed under general anesthesia and sedation, the exposure of staff, including the anesthetist as well as the operator, must be thoroughly protected using suspended protective boards, protective skirts, protective shields, protective eyewear, etc.186
4.5 Q&A
Q: Dose Limits for Healthcare Workers
As a healthcare worker working in the catheter lab, radiation injury is a concern. How much of the work per year may affect one’s health?
A:
Current X-ray systems are highly sophisticated, and leakage from them is not at a level of concern. It is important to control the scattered radiation from the patient to reduce the operator’s exposure. In other words, reducing the patient’s exposure dose will reduce the operator’s exposure dose.
Because PCI involves prolonged high-dose-rate fluoroscopy, the radiation exposure of PCI operators who are positioned near the patients is higher than that of other staff, and development of lens cataracts has been reported. According to ICRP Publication 118 (2012), from April 2021, the effective dose limit for health care workers will be changed to 100 mSv for a total of 5 years and 50 mSv/year for the lens and 500 mSv/year for skin. The laws and regulations require that radiation workers engaged in cardiac catheterization, etc. not exceed the dose limits indicated in III.2.3 “Dose Limits for Healthcare Workers”.
For this purpose, it is important to wear protective equipment such as protective clothing and eyewear to reduce exposure doses, and to wear personal dosimeters such as glass badges® and to work in a controlled environment so as not to exceed the dose limits.97–99
Q: Protective Effects of the Types of Protective Clothing
Protective clothing is available as a means of preventing radiation injury to workers. What is the difference in the dose reduction effect among the lead-equivalent dose and the types of clothing (i.e., coat type, apron type and separate type)?
A:
Protective clothing with greater coverage is better, but in general, the greater the protective effect, the heavier the clothing. Heavy protective clothing may disturb the operator’s concentration and may also cause back pain.
Currently, the Japanese Industrial Standards specify sheets with lead-equivalent values of 0.25 mm, 0.35 mm, and 0.50 mm as the materials for protective clothing. In general, the protective capacity increases with the increase in the lead-equivalent, but it becomes heavier. Based on the actual measured thickness of the shielding material of the protective clothing, the shielding capacity of 0.25 mm lead-equivalent is sufficient and there is no significant difference in shielding capacity between 0.25 mm and 0.35 mm.97,99,187 Instead of covering the entire body with a heavy material, the operator is advised to use a relatively light weight (≈0.25 mm) covering in conjunction with other protective equipment.
Unless the operator has the back facing the patient, the dose of incident X-rays from the dorsal side is not very high and therefore, rather than covering the body with a heavily weighted and less operative coat, a lightweight protective apron should be worn, and care should be taken not to expose the uncovered back to the patient during the procedure. In addition, although the weight of the protective apron is mainly on the shoulders, the weight of the separate type of protective clothing is distributed to the shoulders and waist, improving operator fatigue and workability.97–99,188
Q: Reducing the Exposure Dose for Patients Undergoing PCI
A:
The principles of dose reduction must be well understood. The 3 principles for dose reduction from sources outside the body, such as X-rays used during PCI, are: (1) “time” (to shorten the exposure time), (2) “shielding” (to shield the radiation), and (3) “distance” (to move away from the source). It is recommended that each principle should be discussed and implemented at each facility to reduce patient doses as much as feasible. It should also be noted that even with same frame rate and fluoroscopy time, the maximum skin dose absorbed by patients varies greatly depending on various factors, such as body size, position of the X-ray tube, location of the X-ray irradiation site, angle of X-ray incidence, presence of the upper arm in the irradiation field, and fluoroscopy mode.17,172,189
Q: Accumulated Effects of Exposure Due to Repeated Procedures
Do the effects of radiation exposure accumulate when repeated PCI is performed on multivessel lesions and restenosis? If so, how long should the intervals be? In addition, in patients who have been exposed to higher cumulative radiation doses, does a longer time between repeated procedure prevent skin injury in the future?
A:
Even at the same dose, the risk is different when exposed to a single dose and when exposed in divided doses. It is generally believed that the risk of radiation hazard is lower with divided exposure, because the repair function of damaged cells occurs prior to the repeated procedure. The repair function of damaged cells differs according to the cell type and normal cells are considered to have better repair function than cancer cells. This is based on the dose–rate effect and is also the reason why fractional irradiation is generally used in radiotherapy. In recent years, attempts have been made to quantitatively evaluate the dose-rate effect in experiments using cells.
However, the effectiveness of extending the time between procedures in order to reduce radiation hazard is unclear. The effects of radiation can be divided into 2 types: deterministic effects (tissue reaction) and stochastic effects, and it is necessary to develop a treatment plan tailored to each patient’s medical condition.
(1) Deterministic effects
In terms of the radiation effect in PCI, the prevention of skin injury, as a deterministic effect, is the most important. In patients undergoing frequent PCI, the risk of radiation skin injury should be reduced to the greatest extent possible.
The relationship between the dose and the chance of developing moist dermatitis with fractionated radiotherapy was investigated using radiotherapy data (ICRP Publication 141 [2019]). It was found that the dose that caused moist dermatitis was increased by decreasing the area of irradiation and increasing the number of fractions. In human skin, the threshold dose of X-ray that causes erythema over an area of 10 cm2
is 6–8 Gy for a single short exposure and >30 Gy for multiple fractions. Because the time scale of the radiobiological repair process is 1,000–10,000 s (≈16–160 min), it is estimated that the risk reduction effect is small even if the interval is longer than that. In other words, the effect on the skin is less likely to occur when the dose and the area of irradiation are divided into smaller portions, even if the dose is the same.
From the perspective of avoiding severe radiation skin injury, it is useful to allow a sufficient postoperative observation period. After a high-dose procedure, another procedure should be postponed for the period of time during which symptoms are likely to occur. In any case, reducing the irradiation field size is more effective for reducing the risk of skin injury. If the previously irradiated skin area is identified, the risk of skin injury with the next irradiation can be reduced by changing the irradiation area. In this way, the cumulative dose does not increase significantly.
(2) Stochastic effects
The radiation hazardous risk of accumulated doses is not well understood. However, even with dose accumulation, the risk is considered much smaller than the risk of coronary artery disease being untreated. In the absence of special circumstances, such as young age of the patient, the significance of risk control of stochastic effects is relatively small, and there seems to be no clear benefit in focusing solely on the irradiation interval.190,191
Q: Informed Consent Before PCI
How should radiation hazards be explained to patients to ensure their safety during and after the procedure?
A:
Patients should be informed that the prolonged radiation exposure during PCI may cause skin injury. The development of a manual for informed consent and uniformity of explanations at institutions is important. In explaining the effects of radiation, try to understand the patient’s concerns and reassure them of the effects of radiation, rather than enumerating technical terms and figures. Specifically, the following explanation should be given.
(1) There are thresholds for radiation doses that cause radiation skin injury (i.e., doses that affect 1–5% of the population exposed to radiation).
(2) In some cases, the skin incident dose may exceed the threshold, in which case we will ask for permission to continue the procedure.
(3) The equipment is well maintained and the procedure is always carried out at the optimum dose.
(4) Monitoring of exposure and other methods are used to determine the incident dose to the skin.
(5) Management of radiation skin injury has been developed in the institution.
Patients should be given these information even in the case of emergency procedures.171,192
Q: Determining Whether to Continue the Procedure When Approaching the Threshold Dose
When the procedure is prolonged because of complex lesions, resulting in the threshold dose for skin injury being approached, how should the decision on whether or not to continue the procedure be made?
A:
The threshold dose for early transient erythema is 2 Gy. Because there are significant differences in the exposure dose per unit of time depending on the facility and the imaging setting (patient’s size, imaging angle, frame rate, etc.), it is necessary to determine the fluoroscopy and imaging time corresponding to 2 Gy and the guideline for the air kerma (AK) value (Gy) to be displayed on the device at each facility. Whether to continue or discontinue the procedure is determined when it becomes clear that the fluoroscopy and imaging times and the cumulative AK values displayed on the device are likely to reach the guideline values. The interests of the patient, whether the procedure is elective or urgent, are basically the priority, but it is advisable to reconfirm the wishes of the patient and the patient’s family at that time.
If the procedure is continued despite a high probability of reaching the threshold dose, further efforts will be made to reduce the additional exposure dose. It is also possible to reduce the pulse rate of fluoroscopy and the frame rate of imaging, and to move the patient’s skin irradiation field to a different position by changing the angle of fluoroscopy.
Q: Responsibilities of the Radiation Safety Manager
What procedure should be taken if it is discovered that the patient’s dose might have exceeded the threshold dose for skin injury?
A:
The Radiation Safety Manager, who must be established by the Medical Care Act from April 1, 2020, is informed of the exposure dose and the expected risk of skin injury by physicians in charge of the procedure. In addition, the Radiation Safety Manager takes the following actions:
(1) Obtain repeated informed consent from the patient and the patient’s family.
(2) Prepare a report regarding patient skin doses so that proper follow-up can be taken.
(3) If transient erythema is observed immediately after the procedure, the exposed area needs to be examined by the physician who performed the procedure. If necessary, the physicians or nurses who care for the patient during the hospitalization should be informed.
(4) Depending on the degree of expected skin injury, a dermatologist should be consulted with information of the exposure site, dose, and the degree of expected skin injury. The radiation dose report of the patient with the related reference should be provided.
(5) Documents that a dose exceeding the threshold for skin injury has been irradiated.
Because repeated PCI may cause skin damage at doses lower than the thresholds, it is important to keep a record of the irradiation sites and doses to prevent excessive exposure.
As for the patients, the Radiation Safety Manager informs them of the areas where radiation skin injury may occur, explains the institution’s policy for dealing with radiation skin injury, and informs them of the following.
(1) Exposure may have reached the threshold dose, but diagnosis and treatment were essential.
(2) Periodic follow-up visits are required.
(3) Do not scratch the irradiated area, avoid using irritating bath salts and soaps, and do not apply medications other than those prescribed by the physician.
(4) Because the effects of radiation may be delayed, see a physician if there is any change in skin condition.
It is desirable to prepare a manual at each institution that includes the above.171,192
Q: The Need for Close Follow-up of Healed Acute Dermatitis
If minor acute dermatitis occurred and rapidly healed by itself, is dermatological follow-up necessary?
A:
Minor skin lesions that resolve spontaneously are thought to be early transient erythema (the threshold dose is 2 Gy) or major erythema (the threshold dose is 6 Gy). In the case of major erythema, hyperpigmentation (or depigmentation) often remains after healing. If the skin is cured without any residual pigmentation, the likelihood of developing delayed skin injury is very small, and no follow-up is necessary.
If the early skin rash has healed, but some abnormalities of coloration remain in the area, even though the changes are initially minor, the risk of delayed skin lesions cannot be ruled out and periodic follow-up, every 3 months up to 1 year, by a dermatologist is necessary. However, residual pigmentary abnormalities usually do not require treatment other than observation.171
5. Peripheral Vascular Interventions (Table 34)
Table 34. COR and LOE for Dose Reduction in Peripheral Endovascular Treatments
| |
COR |
LOE |
| Healthcare workers and patients |
It is recommended to shorten the fluoroscopy time as much as practicable, select an
appropriate fluoroscopy pulse rate, and image acquisition rate (or, if using the same device
as the coronary intervention, change the settings to those appropriate for the peripheral
interventions)193,194 |
I |
B |
It is recommended to place the patient as far away from the X-ray tube as possible and as
close to the X-ray detector as possible to properly narrow the field of radiation193,194 |
I |
B |
Use of supplemental imaging techniques, such as vascular and intravascular ultrasound, may
be considered to reduce the dose of radiation195 |
IIb |
C |
| Healthcare workers |
It is recommended that the appropriate puncture site is selected so that the operator can keep
the distance from the radiation field193,194 |
I |
B |
Use of appropriate protective devices (protective clothing, eyewear, etc.) is
recommended193,194 |
I |
B |
It is recommended that the operator’s hand not to be close to or reflected in the irradiation
field193,194 |
I |
B |
Use injectors for contrast injection during cine acquisition (especially with digital subtraction
angiography) so that physicians and medical personnel are able to keep away from the
radiation source193,194 |
IIa |
B |
COR, Class of Recommendation; LOE, Level of Evidence.
5.1 Methods to Reduce Exposure Dose
The prevalence of peripheral arterial diseases (PAD) is increasing due to the aging of society and westernization of the diet in Japan. With the recent rapid advances in medical technology and devices, the indications for endovascular therapy (EVT) are expanding.196 Traditionally, EVT for PAD has been performed by vascular surgeons and radiologists, but due to the high incidence of concomitant coronary artery disease and expansion of the indications, cardiologists are increasingly treating these patients. Although most cardiologists are familiar with PCI, there is a large disparity in experience of peripheral vascular interventions among physicians and institutions. In order to effectively reduce the risk of radiation exposure, it is essential to know the specific exposure risks related to EVT.193,194
5.1.1 Imaging Methods
Digital subtraction angiography (DSA), which is frequently used in EVT, collects precise information of vessels by digitally removing unnecessary imaging elements for angiography, such as bone, and displays only the vessels. This technique reduces the amount of contrast, but the exposure dose is higher than that of conventional digital angiography (DA). Limiting the imaging to the minimum necessary and using an injector to administer contrast while operators and medical personnel are leaving the room will reduce exposure to staff. The imaging mode should be chosen based on the fact that the small FPDs used in PCI have a narrower imaging range, requiring more image acquisitions.
5.1.2 Vascular Approach Routes
Although the fluoroscopy dose used during EVT tends to be slightly lower than that during PCI,197 and the exposure risk may generally be lower, some vascular approaches may put the operator and assistants close to the irradiated area, and require measures to reduce the exposure dose that are different from those used during PCI.
The crossover approach via a contralateral femoral artery is a common technique in the treatment of lower extremity arteries, but the positioning of the operator and the FPD differs greatly between right femoral puncture followed by left leg treatment and left femoral puncture followed by right leg treatment. In the treatment of the left lower extremity via right femoral puncture, the radiation exposure risk to the operator is not increased because the tube is moved away from the operator, but in the treatment of the right lower extremity via left femoral puncture, the tube is moved closer to the operator and the radiation exposure risk to the operator is increased. In this situation, some operators perform the procedure while holding the FPD in their arms, but this practice should be avoided because of the excessive radiation exposure risk. As an alternative, an anterograde approach via ipsilateral femoral puncture is preferable. The puncture site should be carefully selected based on the treatment strategy and exposure risk.
In complex EVT cases, such as those using a retrograde approach, fluoroscopic time and dose tend to increase.198 Furthermore, because the puncture site and the operator’s hand are very close to each other with this approach and direct radiation may be unavoidable, the risk of exposure is higher than in cases where revascularization can be achieved with only an antegrade approach. Because health hazards due to radiation exposure have been reported in physicians who performs procedures near the X-ray tube,199 it is important to avoid direct radiation exposure by keeping enough distance from the X-ray tube, wearing goggles and gloves, using a ceiling-mounted protective shield, selecting an appropriate fluoroscopic pulse rate and image acquisition rate, applying an aperture in the irradiation field, and avoiding unnecessary imaging and to substitute a fluoroscopic collection function.200
Manipulating guidewires using the body surface and intravascular ultrasound as a guide, which is often performed in Japan, may reduce radiation doses for both patients and healthcare workers, but there are only a few studies directly comparing the exposure doses of both methods. The success rate of the ultrasound-guided strategy may not be as high as that of the angio-guided strategy and further evaluation is warranted.195
5.1.3 Other General Cautions
As mentioned above, the patient’s radiation exposure risk during EVT may be lower than during PCI, but with the recent expansion of the indications for EVT, the opportunities for EVT on complex lesions have increased and the exposure risk may increase as well. From the perspective of reducing exposure risk, unnecessarily prolonged procedures should be avoided to take advantage of the minimally invasive nature of EVT. If a complex and prolonged procedure is expected, or if the duration of the procedure is longer than expected, the patient’s benefits must be maximized by reconsidering the treatment strategy and taking into account the need for referral to more specialized facilities.
In terms of the exposure risk for healthcare workers, especially for the operators, it is important to understand that the exposure risk differs depending on the imaging method and the combination of the approach and treatment sites, as described above. The following procedures should be taken to properly reduce the exposure risk for EVT.
(1) Choose an appropriate fluoroscopic pulse rate and image acquisition rate (do not use the same settings as coronary angiography/PCI).193,194
(2) Select a puncture site (i.e., the operator’s position) away from the treatment site, if the therapeutic strategy allows.193,194
(3) Especially in DSA imaging, an injector should be used to infuse contrast media, and the operator/assistant should be as far from the irradiated area as possible.
Because of the recent rapid development of EVT and the expansion of its indications, basic data on the exposure risks of each imaging method have not been accumulated compared with PCI. The accumulation and evaluation of data at each facility should be considered.
5.2 Q&A
Q: Risks of Exposure During EVT
What is the difference in exposure risk for healthcare workers in EVT compared with PCI and what should we be aware of?
A:
Although the fluoroscopy dose of EVT is lower than that of PCI, it is necessary to know that the risk of operator exposure varies greatly depending on the imaging method and the relationship between the approach site and the treatment site. For example, DSA imaging has a higher radiation dose than DA imaging, so it is preferable for the operator and medical staff to leave the room (using injectors for contrast injection). Approaches that allow as much distance between the operator and the X-ray tube as possible should also be chosen when selecting a treatment strategy.193,194 Unnecessary magnification of fluoroscopy and direct exposure of the operator’s hands in the radiation field should be also avoided during the procedure.193,194
Q: Exposure Risk Under Ultrasound-Guided Procedures
Can body surface and intravascular ultrasound-guided procedures reduce the risk of exposure?
A:
Treatment of the femoral–popliteal artery with body surface ultrasound (US) guidance may reduce fluoroscopy time and exposure risks, but few well-designed studies have examined the efficacy of the US-guided strategy. It has been reported that the US-guided technique alone does not produce good quality of the images and did not achieve a sufficient success rate for the procedure,195 so case-specific selection is necessary. When fluoroscopy is used concurrently, the exposure of the echocardiographer’s hand in the irradiation field must be strictly avoided. As for intravascular echocardiography, there are no high-quality studies on fluoroscopy time, and concurrent use might lengthen the procedure and fluoroscopy time, so its use should be tailored for each case.
6. Electrophysiological Studies and Catheter Ablation (Table 35)
Table 35. COR and LOE for Dose Reduction in Electrophysiological Studies and Catheter Ablation
| |
COR |
LOE |
| Healthcare workers |
| It is recommended to use a 3D mapping system during catheter ablation201 |
I |
A |
It is recommended to lower the frame rate and pulse rate during ablation as much as
possible201,202 |
I |
A |
| Patients |
| It is recommended to keep the skin dose to ≤3 Gy201 |
I |
B |
COR, Class of Recommendation; LOE, Level of Evidence.
6.1 Electrophysiological Study, Therapeutic Procedures, and Exposure
In recent years, the volume of catheter ablations for atrial fibrillation has increased rapidly. In particular, the number of ablations for more complex arrhythmias has been increasing, requiring longer procedures and more than 1 procedure in some cases. Contrast-enhanced cardiac CT and rotational angiography, in which a FPD is rotated, are also performed preoperatively to obtain accurate 3D information. It is important to be aware of the patient’s exposure risks in these procedures.
Fluoroscopy is mainly used in electrophysiological studies and catheter ablations, and cine acquisition is rarely used. Because the electrode catheter is less X-ray permeable than the guidewire, the pulse rate of fluoroscopy can be reduced. These factors provide a protective advantage compared with PCI, but catheter ablation is more likely to use fluoroscopy with a fixed radiation angle, in which the same area is exposed continuously. In particular, the right subscapularis and the right upper limb may be intensively exposed to radiation for a long time in the left anterior oblique position.
If the patient’s upper arm is placed around the irradiation field, which is not involved in determining the irradiation setting, the distance between the X-ray tube and the skin is shortened by the thickness of the arm, and the dose at the skin incidence plane is increased. When the arm is located in the center of the image detector, the skin dose to the upper arm in the irradiation field is very high because the distance between the X-ray tube and the skin is shortened and the irradiation conditions are higher for the arm. Therefore, the upper arm should be positioned as far from the irradiation field as possible by changing the angle of X-ray incidence and isolating the trunk from the upper arm.
Fluoroscopy is often used in the treatment of arrhythmias to determine the exact location in the heart and the intensity and direction of contact of catheter with myocardium. A 3D mapping system has been developed to obtain this information while minimizing fluoroscopy. In fact, advances in 3D mapping systems have made the use of fluoroscopy much less frequent.
6.2 Exposure Dose
Although there is wide variability in exposure doses associated with electrophysiological studies, catheter ablation, and device implantation procedures, doses tend to be higher in cases such as atrial fibrillation, which requires a septal puncture to the left atrium, and ventricular tachycardia, which requires extensive mapping. Ablation procedures that require epicardial puncture also require coronary angiography prior to puncture, and frequent fluoroscopy for confirmation of the puncture needle. Doses for major arrhythmia treatment procedures reported in Europe in 2014 are shown in Table 36.202,203
Table 36. Radiation Doses for Catheterization and Treatment of Arrhythmias
| |
Patient radiation dose (mSv)
Median (min–max) |
ESD of RAO (mGy)
Mean (min–max) |
ESD of LAO (mGy)
Mean (min–max) |
| Electrophysiological study |
3.2 (1.3–23.9) |
|
|
| Ablation |
15.2 (1.6–59.6) |
|
|
| Atrial fibrillation |
16.6 (6.6–59.6) |
262 (14–2,049) |
568 (24–2,599) |
| Supraventricular tachycardia |
4.4 (1.6–25) |
147 (28–536) |
277 (6–1,104) |
| Ventricular tachycardia |
12.5 (3–>45) |
129 (38–271) |
229 (31–688) |
ESD, entrance skin dose; LAO, left anterior oblique; RAO, right anterior oblique. (Source: based on Heidbuchel et al, 2014202 and Seguchi et al, 2016.203)
Although patient factors (e.g., body size, anatomy, etc.), the equipment used, and operator’s experience affect the variability of reported doses, the operator’s perception of exposure is the most important factor. Experienced physicians performing the procedure with optimal fluoroscopy settings will reduce the exposure dose. It is important that all personnel involved in the procedure, such as surgeons, nurses, radiology technicians, and clinical engineers, are aware of the importance of reducing exposure doses, and it is necessary to share information and report the doses for each case.204,205
6.3 3D Mapping System
The 3D mapping systems began to be used in Europe and the USA in the 1990s and were introduced to Japan in 2000. They have been actively used for catheterization of atrial fibrillation, which started around the same time,206 and has greatly contributed to the improvement of treatment outcomes and safety.
6.3.1 Display of Multiple Catheters
Although the first CARTO Unix® system was able to display only the ablation catheters, the EnSite® system introduced in 2006 can display diagnostic catheters, such as ring catheters, in addition to the ablation catheters, and it has been reported that the system contributes to a shorter procedure time, fluoroscopy time, and a higher success rate compared with fluoroscopic pulmonary vein isolation and linear ablation.206,207 Accurate anatomy can be reconstructed by placing a catheter in contact with the myocardium and recording its 3D information. The distance from the catheter to the myocardial wall, the stability of the catheter, and its position in relation to the diagnostic catheter can be seen on a 3D map under non-fluoroscopy, reducing the frequency of fluoroscopy use.
6.3.2 Integration With CT/MRI
Since 2008, preoperative images from CT or magnetic resonance imaging (MRI) can be reconstructed in 3D using 3D mapping system software and integrated with intraoperative maps, allowing for more accurate mapping (CARTO XP, EnSite Version 8). These systems have been reported to improve treatment outcomes and reduce fluoroscopy time.208,209 The CARTO Sound® system was developed to integrate the images delineated by tracing the myocardium using intracardiac echocardiography intraoperatively with 3D maps, even when preoperative imaging is not possible, and it has been reported to reduce fluoroscopy time even more than integration with MRI.210
6.3.3 Display of Intracardiac Echocardiography Images
Intracardiac echocardiography is a highly versatile system, especially used in atrial fibrillation ablations in which atrial septal punctures may also be performed. The CARTO Sound system is able to provide intraoperative echocardiographic images of arterial valves, coronary arteries, and papillary muscles, as well as myocardial scarring using echo luminance. The images can be integrated with 3D maps and provide useful information for ventricular tachycardia ablation as well as atrial fibrillation without increasing radiation exposure.211
6.3.4 Mapping With Multipolar Electrodes
Initially, mapping was possible only with bipolar electrodes at the tip of the ablation catheter, but since the late 2000s, mapping with multipolar electrodes has become possible, enabling more detailed mapping in a shorter time. In 2016, a new ultra-high-density mapping system (Rhythmia®) from Boston Scientific was introduced in Japan. The basket-shaped catheters allow rapid and safe mapping, but their contribution to dose reduction is not yet well established.
6.3.5 Contact Force Technology
In the 2010s, it became possible to measure and display the contact force at the tip of an ablation catheter in real time. It has been reported that the catheter position, contact strength, and stability can be accurately visualized under non-fluoroscopy, as well as by tactile feedback, and significant reductions of dose and fluoroscopy time have been achieved.212–214
Thus, advances in 3D mapping systems have contributed to improvements in treatment efficacy and safety, as well as reduction of radiation doses (Table 37). In a study of approximately 1,900 patients who underwent atrial fibrillation ablation at a single center over a 12-year period, the mean fluoroscopy time and dose were 61 min and 1,365 mGy in the early years of 2004–2006, which decreased to 17 min and 304 mGy, respectively, between 2013 and 2015, and to 11 min and 200 mGy in procedures using a contact force catheter.214 The development of a sheath that can be displayed on a 3D mapping system is currently underway, and further reduction of exposure doses is expected.
Table 37. Advances in 3D Mapping Systems for Catheterization of Arrhythmias
| 2000 |
First 3D mapping system introduced in Japan |
| 2005 |
Multiple catheter display (EnSite NavX®, CARTO3®) |
| 2010 |
Integration with CT/MRI |
| Intracardiac echocardiography image display (CARTO Sound® module) |
| 2015 |
Mapping with multipolar electrodes |
| Contact force-sensing ablation catheter |
CT, computed tomography; MRI, magnetic resonance imaging.
6.4 Catheter Navigation System
A navigation system that assists in catheter manipulation in combination with a 3D mapping system has been developed and contributes to reducing radiation exposure.
6.4.1 Remote Magnetic Navigation System
A catheter with a magnetic sensor is used within a special system that creates a weak magnetic field, and is remotely operated using a joystick in a separate room (Niobe® ES).215 The operator can see the catheter on a 3D mapping system (CARTO RMT), which avoids intraoperative exposure, and only limited fluoroscopy use is needed due to the very soft tip of the catheter. This system can be expected to reduce exposure dose compared with conventional systems, especially in cases of special anatomy (e.g., postoperative congenital cardiac disease, severe thoracic anomalies).216 A recent meta-analysis also reported a significant reduction in fluoroscopy time in atrial fibrillation ablation using this system.217
6.4.2 Non-Fluoroscopic Catheter Navigation System
This is a system in which catheters and guidewire tips with built-in sensors are displayed on a pre-recorded fluoroscopic image (MediGuide®). The biological reference patch attached to the anterior chest wall reproduces the fluoroscopic images synchronized with ECG and respiration, which provides similar feedback to the operating catheters and guidewires under fluoroscopy. The use of this system has been reported to reduce radiation exposure in ablation of atrial fibrillation, supraventricular tachycardia and outflow premature ventricular contraction. In 1,000 prospectively registered atrial fibrillation ablations using this system, a fluoroscopy time of 0.9 min and a dose of 345.1 cGy-cm2
were reported.218
Dedicated guidewires are mainly used for coronary sinus lead insertion during cardiac resynchronization therapy, with reported dose reductions of as much as 95% in combination with low-dose fluoroscopy settings.219 It has been reported that low-dose septal punctures can be performed during ablation by using a dedicated guide wire inserted into the Brockenbrough needle, allowing the needle tip to be seen and operated under non-fluoroscopy conditions.220 Alternatively, a low-dose epidural puncture with a low radiation dose can be performed by inserting the needle into the tip of an epidural puncture needle and viewing the needle tip on a pre-saved coronary angiography image.221
6.4.3 Integrating Fluoroscopy and 3D Mapping
The CARTO Univu® system integrates fluoroscopy and 3D mapping. Once fluoroscopic images in typical views (e.g., left and right anterior oblique), coronary angiography, and the contrast image of the chamber to be mapped are stored, the system integrates the 3D map on its images.222 It is not a substitute for the previously mentioned navigation system, because it is only displays at a predetermined angle. However, non-permeable catheter navigation systems and remote magnetic navigation systems are expensive and require the equipment to be integrated in the design of the catheterization laboratory, which limits the facilities equipped with such systems, but CARTO Univu is a module that can be added to a conventional mapping system, which seems more versatile.
The 3D mapping system and catheter navigation system described above can greatly contribute to reducing the radiation dose when used by operators who are familiar with these systems. Recently, it has been reported that “zero fluoroscopy” ablation is possible using these systems,223,224 which may be particularly beneficial for children and pregnant women. However, there are situations in which fluoroscopy is necessary for all procedures from puncture through to wire manipulation. Insistence on zero fluoroscopy should not put patients at risk by avoiding necessary procedures.
6.5 Conditions and Diseases Requiring Special Attention
Children and adults with congenital heart disease associated with complex cardiac malformations often undergo imaging studies that include repeated catheterization and radiation exposure even in infancy. Frequent fluoroscopy is often required during ablation because of the unusual cardiac anatomy. The anatomy must be fully understood prior to the procedure. The catheter navigation system is useful in terms of dose reduction and catheter manipulation, especially in complex malformations.
In the case of atrial fibrillation ablation, which is the most common type of ablation, many institutions perform preoperative cardiac CT scanning to evaluate the morphology of the pulmonary veins, left atrial appendage thrombi, and epicardial fat, etc. Enabling integration into a 3D mapping system has contributed to a reduction of intraoperative doses, but the dose increases due to the ECG synchronization required to obtain good CT images.225
Multiple ablations are often required due to the recurrence of atrial fibrillation, atrial tachycardia, and atrial flutter, leading to increased radiation exposure due to repeated procedures and preoperative examinations. Switching the preoperative examination from CT to MRI may be a good alternative to reduce radiation exposure.
6.6 Methods to Reduce Exposure Dose
6.6.1 General Considerations
In catheterization, recent advances in 3D mapping have allowed for accurate understanding of the cardiac anatomy and determination of the contact force between the catheter and the myocardium, leading to significant reduction in fluoroscopy time. Electrode catheters can be seen even with minimum settings of pulse and frame rate of fluoroscopy.
The 3 principles of dose reduction are time, shielding and distance. Because reduction of the irradiation time is the most effective, it should be achieved through minimizing the fluoroscopy time by avoiding unnecessary irradiation as much as possible and avoiding unnecessary imaging.
Because the scattered radiation dose is inversely proportional to the square of the distance from the source, it is necessary to keep as much distance from the irradiation field as possible. Because the dose of direct X-rays is markedly higher than that of scattered X-rays, hands should not be placed in the irradiation field. Medical workers are mainly exposed to scattered X-rays from the patients. Scattered rays are often generated on the side of the X-ray tube, so when using a lateral tube, it should be on the opposite side of the operator. It is important to realize that a reduction of the patient’s radiation dose leads to a reduction of the operator’s radiation dose. In the treatment of arrhythmias, the left anterior oblique position is generally used, which results in a large amount of exposure to a specific area. Extreme angles for frontal views of the atria and ventricular septum should be avoided because they lead to an increase in dose.
An esophageal thermometer is often used in the atrial fibrillation ablation process, which may be handled by a technician. This procedure performed under fluoroscopy without a shielding plate leads to excessive exposure, and the operator must refrain from using fluoroscopy during this process. Nurses working close to the patient during the ablation procedure should be encouraged to avoid unnecessary exposure. The exposure of the operator should not be neglected, and the use of an X-ray protective shield (RADPAD®) should be considered to protect the operator from scattered radiation. The shield has a mixture of bismuth and antimony, and attenuates >60% of radiation during ablation and device implantation, and it is useful because it attenuates scattered radiation to not only the operator but also to other persons in the room.226
With regard to patients, exposure from multidetector CT performed preoperatively, especially during atrial fibrillation ablation, should not be underestimated. Routine preoperative and postoperative imaging should be avoided.
6.6.2 Fluoroscopy Settings
It is necessary to lower the pulse rate in fluoroscopy and the frame rate in cine images within a range that does not significantly affect image quality. These days, the frame rate is often reduced from 3 f/s to 1 f/s. To minimize fluoroscopy time, elevate the catheter table to extend the distance from the tube, and move the FPD and image intensifier closer to the patient. Familiarize yourself with catheter manipulation on the map using a 3D mapping system. Although it is important to avoid unnecessary cine image acquisition, it is also important not to hesitate to use fluoroscopy as needed.
Because unnecessary magnification of the field of view increases the dose to the patient, the field of view should be kept to a minimum to prevent skin damage. Although narrowing down the irradiation field does not change the dose per unit area received by the patient, it is important to avoid irradiating unnecessary areas. The overlapping area can be minimized when the angle is changed, which is useful for avoiding skin disorders. Narrowing the irradiation field reduces the operator’s radiation dose. It is important for the radiology technician to frequently adjust the filters.
In terms of shielding, 2 types should be used, ceiling-mounted shield and under-table shielding curtain, and it is important to overlap them so that there is no gap between them (Figure 26).
6.6.3 Use of a Protective Cabin
As ablation procedures that require prolonged exposure, such as atrial fibrillation, became more common, radiation protection cabins have been developed to reduce the operator’s exposure dose (Figure 27). The cabin is a box-shaped device with 3 faces made of leaded transparent plastic with a thickness of 2 mm, and the operator passes the arm through the box to perform the procedure. Since its early days of use, radiation protection equivalent to or better than that of protective clothing has been reported for both ablation227 and device implantation,228 and it is particularly superior because the whole body is covered.
6.7 Explanations to Patients
Physicians explaining the possibility of radiation-related skin injury to patients prior to the procedure should be careful not to unnecessarily increase the patient’s anxiety. The physician should explain that there are thresholds for radiation doses that can cause skin injury, and if radiation doses exceeding these thresholds are reached over the course of time, the patient can give permission to continue treatment or not, that the equipment is controlled and the examinations are always performed at appropriate doses, and that there is a plan for dealing with radiation skin disorders. It is important to provide a consistent explanation using a uniform format.
6.8 Catheter Ablation for Children
The number of catheter ablations performed in children is increasing. Although children are more radiosensitive than adults, the radiation dose per unit time is low due to their thin physique. Low-rate pulsed fluoroscopy is useful for reducing patient doses, although some facilities do not use it because of the high heart rate in children, but it is important to try the low-rate first to see if it is feasible. Off-grid methods are also useful. The grid, which is mounted in front of the FPD or image intensifier, is used to remove scattered rays from the subject and maintain image quality. Scattered rays can be removed by placing the FPD and image intensifier 10–20 cm away from the subject. When the FPD or image intensifier is used, the target area may not fall into the field of view in adult patients because of the magnification of the image, but it is not a problem for children because of their small target areas.
6.9 Q&A
Q: Characteristics of Exposure During Catheter Ablation
What are the characteristics of radiation exposure in catheter ablation?
A:
In electrophysiological studies and catheter ablations, cine acquisition is rarely used, and fluoroscopy is mainly used. Because electrode catheters and guidewires used in ablation procedures are more radiopaque than the guidewires used in PCI, they can be seen with reduced pulse rate fluoroscopy. Compared with PCI, a fixed fluoroscopy angle is often used, especially in the left anterior oblique position. In the past, the exposure to the right scapula and the right upper arm was high, but recent advances in 3D mapping systems have reduced the exposure remarkably.
7. Cardiac Device Implantation (Table 38)
Table 38. COR and LOE for Dose Reduction for Healthcare Workers and Patients During Device Implantation for Arrhythmias
| |
COR |
LOE |
| It is recommended to lower the pulse rate as much as possible during device implantation202 |
I |
A |
It is recommended to use a portable C-arm system with the radiation source under the
table202 |
I |
A |
It is recommended to use a standard angiography system for device
implantation202,229 |
I |
B |
COR, Class of Recommendation; LOE, Level of Evidence.
7.1 Methods to Reduce Exposure Dose
Reduction of the patient’s exposure dose also leads reduction of the exposure of medical personnel. Because clinical engineers, nurses, and other medical personnel are often present during device implantation, it should be confirmed that no medical personnel are near the tube or patient when fluoroscopy is used.
7.2 Explanations to Patients
Doses associated with device implantation are generally very low, but implantation of CRT-P/CRT-D devices that require coronary sinus lead placement can increase the dose. Complications of the procedure, including possible skin injury, should be explained in advance.
It should be explained that there are thresholds for radiation doses that can cause radiation skin disorders, and that radiation doses exceeding these thresholds may be reached over the course of treatment, in which case the patient can give permission to continue treatment or not, that the equipment is controlled and the examinations are always performed at appropriate doses, and that there is a plan for dealing with radiation skin disorders. It is important to provide a consistent explanation using a uniform format. Doses for major arrhythmia treatment procedures as reported in Europe in 2014 are shown in Table 39.202
Table 39. Radiation Dose During Device Treatment of Arrhythmias
| |
Patient radiation dose (mSv)
Median (min–max) |
| VVI/DDD pacemaker or ICD implantation |
4 (1.4–17) |
| CRT-P/CRT-D implantation |
22 (2.0–16) |
CRT-D, cardiac resynchronization therapy-defibrillator; CRT-P, cardiac resynchronization therapy-pacemaker; ICD, implantable cardioverter defibrillator. (Source: based on Heidbuchel et al, 2014.202)
7.3 Main Factors in Increased Exposure
In cardiac resynchronization therapy (CRT), which involves the insertion of a lead into the coronary sinus, leads are placed in more anatomically challenging locations compared with conventional devices (i.e., single- or dual-chamber pacemakers, or implantable defibrillators). Because coronary sinus angiography is performed (especially in cine mode) to examine the implantation site, the exposure dose is higher than with usual device implantation.230 It should also be noted that the radiation dose in CRT procedures is high due to the use of the left anterior oblique (LAO) position, and the operator’s head is exposed to a higher dose of scattered radiation.
In the LAO position, the focal point of the X-ray tube is closer to the patient’s skin surface than in the right anterior oblique (RAO) position, so the incident dose to the patient is about twice as high as that to the RAO position if the patient is irradiated for the same time. The heart is viewed from the patient’s left back through the lungs in the RAO position, whereas in the LAO position, it is viewed from the patient’s right back through the spinal column or mediastinum. The lungs contain a large amount of air that is easily penetrated by X-rays, but the spine and mediastinum are dense and require a higher dose of X-rays. For this reason, patients who undergo prolonged fluoroscopy and imaging in the LAO position have higher radiation doses and more areas of skin damage in the right paraspinal region.230
Angiographic system of the catheterization suite is more commonly used than the C-arm for device implantation, especially for CRT. The scattered radiation shielding curtains under the table are usually placed on the right side of the patient and must be moved for implantation on the left side. RADPAD® has been reported to reduce radiation exposure by more than 80% during device implantation.229
7.4 Use of the C-Arm in the Operating Room
The image quality of the portable C-arm fluoroscopy is not as good as with the standard angiographic system because of its low power output. As in the case of the stationary type, the pulse rate can be lowered to reduce the exposure dose, but it is difficult to use it constantly because the heating of the X-ray tube is higher than that of continuous fluoroscopy. It is necessary to avoid excessive magnification of the field of view because the skin incident dose rate increases with zooming. It is preferable to use the portable device with the radiation tube under the bed to reduce the exposure of the patient and the operator’s upper body.
7.5 Q&A
Q: Characteristics of Exposure During Device Implantation
What are the characteristics of exposure during device implantation (e.g., pacemakers, implantable cardioverter-defibrillators, cardiac resynchronization therapy/biventricular pacemakers)?
A:
The use of 3D mapping has increased in catheter ablation, and the intraoperative radiation dose has been reduced accordingly. On the other hand, device implantation rarely uses 3D mapping, and because C-arms may be used in the operating room, the operator must be careful not to perform unnecessary fluoroscopy or imaging. Because left anterior oblique fluoroscopy and venography are often used for cardiac resynchronization therapy, the lowest possible frame rate and pulse rate should be used.