Abstract
Background: Atrial fibrillation (AF) patients often have concomitant coronary artery disease (CAD); however, there are little data on clinical characteristics and outcomes of such patients in daily clinical practice in Japan.
Methods and Results: The Fushimi AF Registry is a community-based prospective survey of AF patients in Fushimi-ku, Kyoto, Japan. Follow-up data were available for 4,464 patients, and the median follow up was 5.1 (interquartile range: 2.3–8.0) years. History of CAD was present in 647 patients (14%); of those patients, 267 (41%) had history of myocardial infarction (MI). Patients with CAD were older and had more comorbidities than those without CAD. The crude incidences (% per patient-year) of cardiovascular events were significantly higher in patients with CAD than those without CAD (cardiac death: 1.8 vs. 0.7, stroke or systemic embolism [SE]: 2.9 vs. 2.1, MI: 0.6 vs. 0.1, composite of those events: 5.1 vs. 2.8, respectively, all log-rank P<0.01). After multivariate adjustment, concomitant CAD was associated with incidence of cardiac events, and history of MI was associated with incidence of MI; however, neither history of CAD nor MI was associated with the incidence of stroke/SE.
Conclusions: In Japanese AF patients, concomitant CAD was associated with higher prevalences of major co-morbidities and higher incidences of cardiovascular events; however, history of CAD was not associated with the incidence of stroke/SE.
Atrial fibrillation (AF) and coronary artery disease (CAD) are common cardiovascular diseases, and share common risk factors. The proportion of concomitant CAD among patients with AF is ~25% in Western countries and ~15% in Japan.1,2 Thus, concomitance of these 2 diseases is common in routine clinical practice.
For AF patients with CAD, it is necessary to select the optimal antithrombotic therapy. A combination of oral anticoagulants (OAC) and antiplatelet drugs (APD) is often prescribed to those patients; however, this is likely to increase the risk of bleeding. The guideline recommendation of antithrombotic therapy for these patients has been revised based on the results of randomized controlled trials;3,4 however, it is unclear whether it can be applied in the real-world clinical settings, which include elderly patients with various clinical backgrounds. In Western countries, previous studies reported the characteristics and outcomes of AF patients with CAD in real-world clinical settings,5–7 demonstrating that AF patients with CAD had higher incidence of cardiovascular events including cardiac death and stroke than those without CAD. However, these data are lacking in Japan. Moreover, reports on 10-year prognosis of AF patients with CAD are rare in the world. The objective of this study was to describe clinical characteristics and long-term prognosis of Japanese AF patients with CAD using data from the Fushimi AF Registry. We believe this study provides important information in understanding the current status of AF patients with CAD in contemporary practice in Japan, and also provides a benchmark against which subsequent data can be compared.
Methods
The Fushimi AF Registry, a community-based prospective survey, was designed to enroll all AF patients who visited the participating medical institutions in Fushimi-ku, Kyoto, Japan. A detailed study design of the Fushimi AF Registry was previously described.1 The inclusion criterion for the registry was the documentation of AF on a 12-lead electrocardiogram or Holter monitoring at any time. There were no exclusion criteria. A total of 81 institutions participated in the registry, which consisted of 2 cardiovascular centers, 10 small- and medium-sized hospitals (<400 beds), and 69 primary care clinics. Patient enrollment started in March 2011 and ended in May 2017. Clinical patient data were registered in the Internet Database System (https://edmsweb16.eps.co.jp/edmsweb/002001/FAF/top.html) by the doctors in charge at each institution. Data were automatically checked for missing or contradictory entries and values out of the normal range. Additional editing checks were performed by clinical research coordinators at the general office of the registry. Follow-up data were collected at least annually by the doctors in charge or via telephone follow up. The study protocol conformed to the ethics guidelines of the 1975 Declaration of Helsinki, and was approved by the ethics committees of the National Hospital Organization Kyoto Medical Center and Ijinkai Takeda General Hospital.
The entire cohort of this study consisted of 4,464 AF patients enrolled in the Fushimi AF Registry whose follow-up data, including prescription data, were available as of August 2021. The median follow-up period was 1,848 days (interquartile range 824–2,927 days). We divided the entire cohort into patients with or without CAD, and then divided those with CAD into those with or without myocardial infarction (MI).
Definition
A history of CAD or MI was registered based on the judgment of the doctors in charge at each institution. Coronary angiography findings were not mandatory. We collected history of percutaneous coronary intervention (PCI) and coronary artery bypass grafting (CABG) data; however, we had no data on when patients underwent PCI or CABG or which stent was placed in each patient.
We defined OAC as warfarin, dabigatran, rivaroxaban, apixaban, and edoxaban. APD included aspirin, ticlopidine, clopidogrel, and cilostazol. CHADS2
score,8 CHA2DS2-VASc score,9 and HAS-BLED score10 were calculated as previously described. The “V” in the CHA2DS2-VASc score was defined as prior MI or peripheral artery disease. The “L” in HAS-BLED score was not incorporated in this analysis, as we checked the international normalized ratio only at enrollment.
The primary outcome of this study was a composite of cardiac death, stroke/systemic embolism (SE) or MI. Stroke was defined as the sudden onset of a focal neurologic deficit in a location consistent with the territory of a major cerebral artery, and it was confirmed on computed tomography or magnetic resonance imaging. SE was defined as an acute vascular occlusion of an extremity or organ. As secondary endpoints, we evaluated the individual components of the primary endpoint (cardiac death, stroke/SE, MI), as well as all-cause death, major bleeding, and hospitalization due to heart failure. Major bleeding was defined as a reduction in hemoglobin level by at least 2 g/dL, transfusion of at least 2 units of blood, or symptomatic bleeding in a critical area or organ, following the definition by the International Society on Thrombosis and Hemostasis.11
Statistical Analysis
Continuous variables were expressed as mean±standard deviation and compared using a Student’s t-test or the Wilcoxon rank-sum test based on their distributions. Categorical variables were presented as numbers and percentages, and compared using the chi-squared test when appropriate; otherwise, we used Fisher’s exact test. The Kaplan-Meier method was used to estimate the cumulative incidence of the clinical events, and the log-rank test was used to compare the differences among the groups. A Cox proportional hazards model was used to compare outcomes among the groups, with the results expressed as a hazard ratio (HR) with a 95% confidence interval (CI). To assess the factors associated with clinical events, we performed multivariate analyses using a Cox proportional hazards model. The covariates were chosen based on traditional risk factors. Two-sided P<0.05 was considered significant. Data analysis was performed with JMP version 16 (SAS Institute, Cary, NC, USA).
Results
Baseline Characteristics
Of 4,464 patients, 647 patients (14%) had CAD at enrollment. Of those 647 patients, 267 patients (41%) had a history of MI at enrollment. Baseline characteristics of those patients are presented in Table 1. Compared with patients without CAD, patients with CAD were more often male (65.7% vs. 58.6%; P<0.01), older (mean age: 76.4 vs. 73.1; P<0.01), and had more comorbidities such as congestive heart failure, hypertension, diabetes mellitus, dyslipidemia, chronic kidney disease, prior major bleeding, and peripheral artery disease. In contrast, history of stroke or transient ischemic attack was comparable. Patients with CAD had higher mean CHADS2, CHA2DS2-VASc, and HAS-BLED scores than those without CAD (2.62 vs. 1.93, P<0.01; 4.41 vs. 3.20, P<0.01; 2.34 vs. 1.64, P<0.01, respectively). Patients with CAD more frequently received antihypertensive agents, antidiabetic agents, and statin. Among patients with CAD, patients with MI were more often male than those without MI (70.8% vs. 62.1%, P=0.02), but mean age was similar between the 2 groups (76.8 vs. 76.2, P=0.54). The prevalence of congestive heart failure and history of stroke were higher, and those of hypertension and peripheral artery disease were lower in the patients with MI. Mean CHADS2, CHA2DS2-VASc, and HAS-BLED scores were higher in patients with MI than those without (2.73 vs. 2.54, P=0.049; 4.99 vs. 4.01, P<0.01; 2.50 vs. 2.23, P<0.01, respectively). In patients with MI, prescriptions of β-blocker, diuretic, and statin were higher, and prescription of Ca blocker was lower.
Table 1. Baseline Patient Characteristics and Medication Use
| |
CAD (−) |
CAD (+) |
P value |
CAD (+) |
P value |
| MI (−) |
MI (+) |
| Number of patients |
3,817 |
647 |
|
380 |
267 |
|
| Male sex |
2,235 (58.6) |
425 (65.7) |
<0.01 |
236 (62.1) |
189 (70.8) |
0.02 |
| Age, years |
73.1±11.1 |
76.4±8.6 |
<0.01 |
76.2±8.5 |
76.7±8.7 |
0.54 |
| ≥75 |
1,865 (48.9) |
398 (61.5) |
<0.01 |
231 (60.8) |
167 (62.5) |
0.85 |
| 65–74 |
1,225 (32.1) |
192 (29.7) |
116 (30.5) |
76 (28.5) |
| ≤65 |
727 (19.0) |
57 (8.8) |
33 (8.7) |
24 (9.0) |
| Height, cm |
160.0±10.2 |
159.7±9.7 |
0.57 |
159.2±9.5 |
160.6±9.9 |
0.07 |
| Body weight, kg |
59.4±13.6 |
59.7±12.6 |
0.48 |
60.1±12.5 |
59.1±12.8 |
0.36 |
| Low body weight (≤50 kg) |
886 (23.2) |
146 (22.6) |
0.72 |
79 (20.8) |
67 (25.1) |
0.2 |
| Body mass index, kg/m2 |
23.1±4.1 |
23.3±3.9 |
0.15 |
23.6±4.0 |
22.8±3.7 |
0.01 |
| SBP, mmHg |
125±19 |
123±19 |
0.01 |
126±18 |
120±21 |
<0.01 |
| DBP, mmHg |
72±13 |
68±14 |
<0.01 |
70±13 |
67±14 |
<0.01 |
| Pulse rate, beats/min |
78±16 |
78±17 |
0.5 |
78±17 |
77±15 |
0.8 |
| Type of AF, % |
| Paroxysmal AF |
48.5 |
55.3 |
<0.01 |
50.0 |
62.9 |
<0.01 |
| Persistent AF |
10.6 |
9.0 |
11.8 |
4.9 |
| Permanent AF |
40.9 |
35.7 |
38.2 |
32.2 |
| Congestive HF |
929 (24.3) |
292 (45.1) |
<0.01 |
152 (40.0) |
140 (52.4) |
<0.01 |
| Valvular heart disease |
644 (16.9) |
125 (19.3) |
0.13 |
77 (20.3) |
48 (18.0) |
0.47 |
| Cardiomyopathy |
110 (2.9) |
15 (2.3) |
0.41 |
14 (3.7) |
1 (0.4) |
<0.01 |
| Hypertension |
2,331 (61.1) |
485 (75.0) |
<0.01 |
301 (79.2) |
184 (68.9) |
<0.01 |
| Diabetes mellitus |
802 (21.0) |
252 (38.9) |
<0.01 |
144 (37.9) |
108 (40.4) |
0.51 |
| Dyslipidemia |
1,555 (40.7) |
421 (65.1) |
<0.01 |
241 (63.4) |
180 (67.4) |
0.29 |
| Chronic kidney disease |
1,264 (33.1) |
337 (52.1) |
<0.01 |
188 (49.5) |
149 (55.8) |
0.11 |
| Hemodialysis |
72 (1.9) |
36 (5.6) |
<0.01 |
20 (5.3) |
16 (6.0) |
0.69 |
| COPD |
198 (5.2) |
38 (5.9) |
0.48 |
23 (6.1) |
15 (5.6) |
0.82 |
| Prior major bleeding |
162 (4.2) |
41 (6.3) |
0.02 |
21 (5.5) |
20 (7.5) |
0.32 |
| CHADS2 score |
1.93±1.32 |
2.62±1.27 |
<0.01 |
2.54±1.20 |
2.73±1.34 |
0.049 |
| CHA2DS2-VASc score |
3.20±1.64 |
4.41±1.62 |
<0.01 |
4.01±1.52 |
4.99±1.59 |
<0.01 |
| HAS-BLED score |
1.64±1.01 |
2.34±0.98 |
<0.01 |
2.23±0.98 |
2.50±0.96 |
<0.01 |
| Stroke |
676 (17.7) |
126 (19.5) |
0.28 |
61 (16.1) |
65 (24.3) |
<0.01 |
| Transient ischemic attack |
65 (1.7) |
16 (2.5) |
0.19 |
13 (3.4) |
3 (1.1) |
0.052 |
| MI |
0 (0) |
267 (41.3) |
NA |
|
|
|
| History of PCI |
0 (0) |
363 (56.1) |
NA |
195 (51.3) |
168 (62.9) |
<0.01 |
| History of CABG |
0 (0) |
100 (15.5) |
NA |
45 (11.8) |
55 (20.6) |
<0.01 |
| Peripheral artery disease |
101 (2.6) |
82 (12.7) |
<0.01 |
57 (15.0) |
25 (9.4) |
0.03 |
| History of EVT |
34 (0.9) |
35 (5.4) |
<0.01 |
26 (6.8) |
9 (3.4) |
0.048 |
| History of bypass surgery |
11 (0.3) |
14 (2.2) |
<0.01 |
9 (2.4) |
5 (1.9) |
0.67 |
| SE |
35 (0.9) |
19 (2.9) |
<0.01 |
6 (1.6) |
13 (4.9) |
0.02 |
| Antihypertensive agents |
2,665 (69.8) |
543 (83.9) |
<0.01 |
316 (83.2) |
227 (85.0) |
0.52 |
| ACEI/ARB |
1,601 (41.9) |
383 (59.2) |
<0.01 |
222 (58.4) |
161 (60.3) |
0.63 |
| β-blocker |
1,123 (29.4) |
247 (38.2) |
<0.01 |
129 (33.9) |
118 (44.2) |
<0.01 |
| Ca blocker |
1,193 (31.3) |
226 (34.9) |
0.07 |
157 (41.3) |
69 (25.8) |
<0.01 |
| Diuretic |
1,014 (26.6) |
261 (40.3) |
<0.01 |
141 (37.1) |
120 (44.9) |
<0.05 |
| Antidiabetic agents |
442 (11.6) |
115 (17.8) |
<0.01 |
62 (16.3) |
53 (19.9) |
0.25 |
| Insulin |
110 (2.9) |
35 (5.4) |
<0.01 |
19 (5.0) |
16 (6.0) |
0.58 |
| Oral hypoglycemic agent |
373 (9.8) |
88 (13.6) |
<0.01 |
47 (12.4) |
41 (15.4) |
0.28 |
| Statin |
780 (20.4) |
315 (48.7) |
<0.01 |
169 (44.5) |
146 (54.7) |
0.01 |
| Digitalis |
428 (11.2) |
65 (10.0) |
0.38 |
39 (10.3) |
26 (9.7) |
0.83 |
| Antiarrthythmic drug |
778 (20.4) |
102 (15.8) |
<0.01 |
61 (16.1) |
41 (15.4) |
0.81 |
Categorical data are presented as n (%). Continuous data are presented as mean±standard deviation (SD). ACEI, angiotensin-converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin receptor blocker; CABG, coronary artery bypass grafting; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; DBP, diastolic blood pressure; EVT, endovascular therapy; HF, heart failure; MI, myocardial infarction; NA, not applicable; PCI, percutaneous coronary intervention; SBP, systolic blood pressure; SE, systemic embolism.
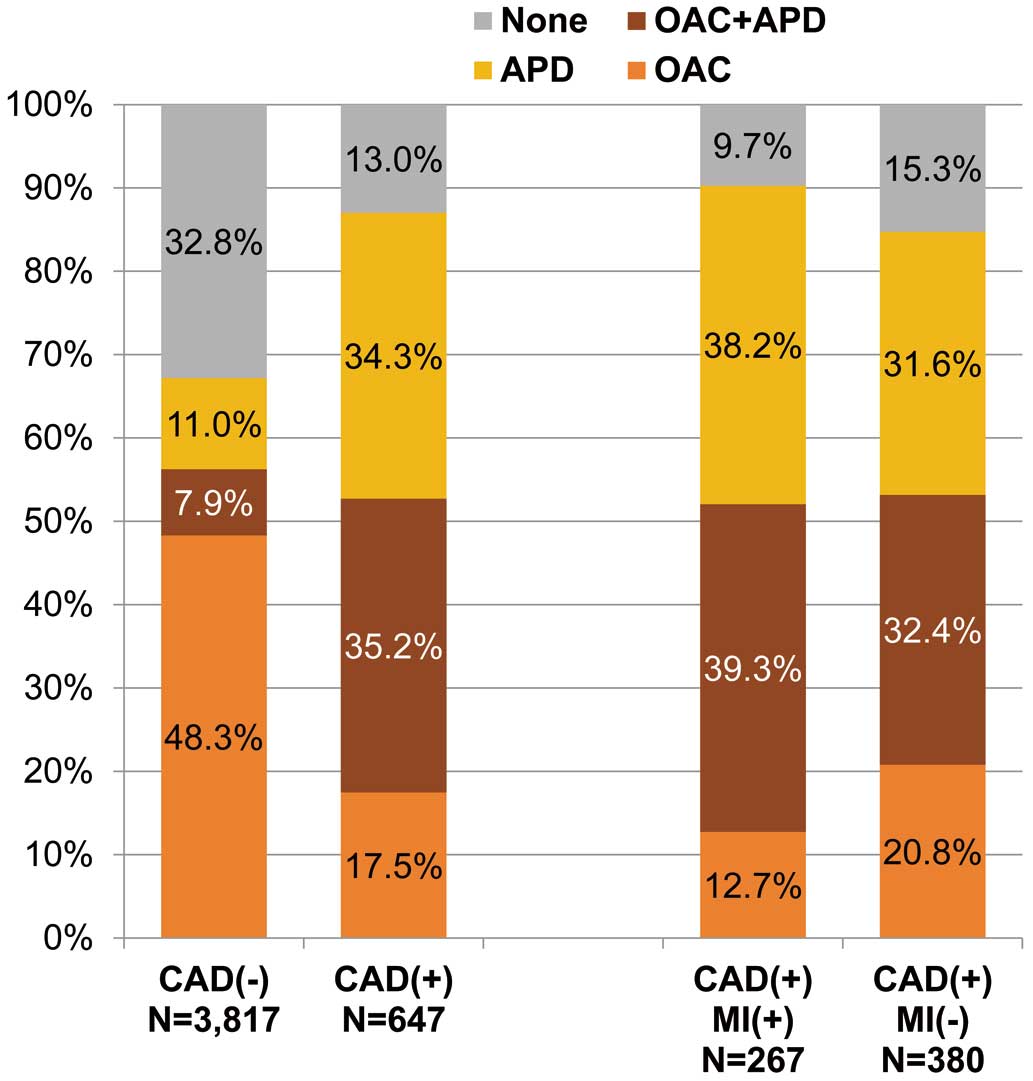
The distributions of antithrombotic therapy are shown in Figure 1. In patients with CAD, patients receiving OAC alone, APD alone, or a combination of OAC and APD were 17.5%, 34.3%, and 35.2%, respectively. Whereas in patients without CAD, they were 48.3%, 11.0%, and 7.9%, respectively. The proportion of OAC prescription was similar between the 2 groups (52.7% vs. 56.2%), but the proportions of APD prescription and combination of OAC and APD were higher in patients with CAD (69.6% vs. 18.9%, 35.2% vs. 7.9%, respectively). Among patients with CAD, the proportion of OAC prescription was similar between patients with and without MI (52.1% vs. 53.2%), and the proportions of APD prescription and combination of OAC and APD were higher in patients with MI than without MI (77.5% vs. 63.9%, 39.3% vs. 32.4%, respectively).
Clinical Outcomes
Clinical outcomes and Kaplan-Meier analyses of cardiovascular events are shown in Table 2, and Figures 2 and 3. Composite of cardiac death, stroke/SE or MI occurred more frequently in patients with CAD than those without (5.1% vs. 2.8% per person-year; HR: 1.82; 95% CI: 1.53–2.18; log rank P<0.01). All-cause death, cardiac death, stroke/SE, MI, hospitalization due to heart failure, and major bleeding also occurred more frequently in patients with CAD. In patients with CAD, the incidence of composite of cardiac death, stroke/SE or MI was similar between patients with and without MI (5.8% vs. 4.6% per person-year; HR: 1.25; 95% CI: 0.91–1.72; log rank P=0.17). Cardiac death and MI occurred more frequently in patients with MI; however, incidences of all-cause death, stroke/SE, hospitalization due to heart failure, and major bleeding were similar between the 2 groups.
Table 2. Frequency of Cardiovascular Events
| |
CAD (−) (N=3,817) |
CAD (+) (N=647) |
HR (vs.
CAD [−]) |
95% CI |
CAD (+) MI (−) (N=380) |
CAD (+) MI (+) (N=267) |
HR (vs.
CAD [+]
MI [−]) |
95% CI |
Number of
events |
Event rate
(per 100
person-years) |
Number of
events |
Event rate
(per 100
person-years) |
Number of
events |
Event rate
(per 100
person-years) |
Number of
events |
Event rate
(per 100
person-years) |
Composite of cardiac death, MI,
stroke, or SE |
541 |
2.8 |
154 |
5.1 |
1.82 |
1.53–2.18 |
85 |
4.6 |
69 |
5.8 |
1.25 |
0.91–1.72 |
| All-cause death |
914 |
4.4 |
244 |
7.4 |
1.65 |
1.44–1.90 |
145 |
7.2 |
99 |
7.7 |
1.06 |
0.82–1.37 |
| Cardiac death |
135 |
0.7 |
60 |
1.8 |
2.75 |
2.03–3.73 |
28 |
1.4 |
32 |
2.5 |
1.78 |
1.07–2.96 |
| Stroke or SE |
406 |
2.1 |
89 |
2.9 |
1.40 |
1.11–1.76 |
58 |
3.1 |
31 |
2.5 |
0.81 |
0.53–1.26 |
| MI |
29 |
0.1 |
18 |
0.6 |
3.87 |
2.15–6.97 |
4 |
0.2 |
14 |
1.1 |
5.53 |
1.82–16.81 |
| Hospitalization due to HF |
516 |
2.7 |
184 |
6.5 |
2.40 |
2.03–2.84 |
101 |
5.9 |
83 |
7.5 |
1.28 |
0.96–1.72 |
| Major bleeding |
347 |
1.8 |
89 |
2.9 |
1.62 |
1.29–2.04 |
62 |
3.3 |
27 |
2.2 |
0.66 |
0.42–1.04 |
CI, confidence interval; HR, hazard ratio. Other abbreviations as in Table 1.


Figure 4 shows Kaplan-Meier analyses according to antithrombotic therapy at enrollment in patients with CAD. Compared with patients with combination therapy of OAC and APD as a reference, there were no significant differences in the incidence of composite of cardiac death, stroke/SE or MI for other 3 antithrombotic prescriptions (OAC alone: HR: 1.31; 95% CI: 0.86–2.00, APD alone: HR: 0.80; 95% CI: 0.55–1.18, no antithrombotic drug: HR: 0.63; 95% CI: 0.34–1.16) (Figure 4A). Moreover, similar results were seen in other cardiovascular events (Figure 4B–D). Kaplan-Meier analyses in patients without CAD are shown in Supplementary Figure.
Association of CAD or MI With Cardiovascular Events
Multivariate analyses for factors associated with cardiovascular events in the entire cohort are shown in Figure 5. Presence of CAD was significantly associated with composite of cardiac death, stroke/SE or MI (HR: 1.33; 95% CI: 1.08–1.66). Other factors associated with composite of cardiac death, stroke/SE or MI were presence of cerebrovascular disease, age (≥75 years), low body weight (≤50 kg), heart failure, diabetes mellitus, dyslipidemia, chronic kidney disease, and APD prescription. Presence of CAD was significantly associated with cardiac death, but it was not associated with onset of stroke/SE. Figure 6 shows factors associated with cardiovascular events in patients with CAD. History of MI was not associated with composite of cardiac death, stroke/SE or MI (HR: 1.06; 95% CI: 0.74–1.51). History of MI was not associated with cardiac death or stroke/SE, but was associated with onset of MI.
Discussion
There were 3 main findings of the present study. First, patients with CAD had distinct clinical backgrounds and had higher incidences of cardiovascular events. Second, after multivariate adjustment, concomitant CAD was associated with cardiac events, and history of MI was associated with the incidence of MI, but neither CAD nor MI was associated with the incidence of stroke/SE. Third, in AF patients with CAD, there was no difference in the incidence of cardiovascular events between OAC monotherapy and a combination of OAC and APD.
Clinical Backgrounds and Outcomes of AF Patients With CAD
In the present study, we found that the incidences of cardiovascular events, all-cause death, and major bleeding were higher in AF patients with CAD than those without CAD. AF and CAD share common risk factors such as age, hypertension, diabetes mellitus, obesity, sleep apnea syndrome, and smoking. AF patients with CAD were older and had more comorbidities than those without CAD; thus, they showed higher incidences of cardiovascular events.
Event incidences in this study were generally higher than those in the AFIRE (Atrial Fibrillation and Ischemic Events with Rivaroxaban in Patients with Stable Coronary Artery Disease) trial conducted in Japan for AF patients with CAD. The incidence of all-cause death was 1.85% per person-year in an OAC monotherapy group and 3.37% per person-year in a combination therapy group in the AFIRE trial;12 however, it was 7.4% per person-year in this study. This is presumably due to differences in patient characteristics, as patients enrolled in such trials were relatively young and had stable medical conditions, but the patients enrolled in observational studies, such as the Fushimi AF Registry, included elderly and ill patients.
Previously, we reported that heart failure was an important cause of cardiac death in patients with AF.13 In this study, patients with CAD were more than twice as frequently hospitalized due to heart failure as those without CAD, highlighting the importance of preventing the exacerbation of heart failure.
Association of Concomitant CAD or MI With Cardiovascular Events in AF Patients
Several studies conducted in Western countries demonstrated that prior MI, but not CAD, was associated with the incidence of stroke in AF patients.6,14 Therefore, prior MI was included as factor “V” in the CHA2DS2-VASc score. In the present study, neither prior MI nor CAD was identified as a risk factor for thromboembolism. The J-RHYTHM (Japanese Rhythm Management Trial for Atrial Fibrillation) Registry, which enrolled Japanese AF patients, also demonstrated that onset of stroke or SE had no association with prior MI or presence of CAD.15 We do not know the exact reason for this apparent discrepancy, but it may be due to the differences in patient backgrounds including age, co-morbidities and ethnicity.
In this study, presence of CAD was independently associated with composite of cardiovascular events and cardiac death, and prior MI was strongly associated with new onset of MI in AF patients with CAD. Sub-analysis of the international GARFIELD-AF (Global Anticoagulant Registry in the Field-Atrial Fibrillation) Registry demonstrated that history of acute coronary syndrome was associated with worse 2-year outcomes including cardiac death and new onset of acute coronary syndrome.6
Antithrombotic Therapy in AF Patients With CAD
In the present study, ~50% of AF patients with CAD received OAC regardless of their high CHADS2
scores, whereas ~70% received APD. Furthermore, 35% received a combination of OAC and APD, and 13% received none of them. Incidences of cardiovascular events were statistically similar between patients with a combination of OAC and APD and those with other antithrombotic strategies. Patients treated with APD monotherapy and those treated without antithrombotic drugs showed a non-significantly lower risk of cardiovascular events. In patients treated with OAC, there were no differences in incidence of cardiovascular events with concomitant use of APD. This result was consistent with previous observational studies.7,16 In the AFIRE trial,12 however, cardiovascular events occurred more frequently in patients treated with a combination of OAC and APD than with OAC monotherapy. Unlike randomized controlled trials, patients’ backgrounds such as age and comorbidities are not balanced in observational studies, and this may be the reason for the inconsistency.
Around 2011, when the Fushimi AF Registry started, physicians frequently prescribed APD rather than OAC to AF patients with CAD due to the difficulty of controlling warfarin and the high risk of bleeding. However, several randomized controlled trials have demonstrated that reduction or discontinuation of APD under OAC prescription increased safety while maintaining efficacy for AF patients with CAD.12,17–21 Current guidelines in Japan and Western countries recommend that only patients at extremely high risk of thromboembolism receive triple therapy just after coronary stenting and combination of OAC and APD >1 year after coronary stenting.3,4,22
Study Limitations
There were several limitations in this study. First, this was a prospective observational study; therefore, it showed only associations, but not causalities. Second, history of CAD or MI was registered based on the judgement of the doctors in charge at each institution and we did not confirm anatomical data of the coronary artery or basis for the diagnosis of MI. Unlike randomized controlled trials, in which the diagnosis criteria of concomitant diseases are strictly pre-defined, this is one of the limitations of a registry survey collecting data from daily clinical practice. As our daily practice is based on such a clinical diagnosis, we believe that the present study results are of clinical relevance. Third, we did not have information on the date of PCI or CABG and type of stent. This information was associated with the antithrombotic regimen of patients with CAD. Fourth, we did not know when OAC or APD were discontinued or initiated because we collected prescription data only once a year; therefore, we have no data on the antithrombotic therapy that was administered when cardiovascular events occurred in these patients. Finally, current antithrombotic therapies have changed significantly since 2011, when the registry started. Around 2011, warfarin was mainly prescribed as OAC, but there has been a major transition from warfarin to direct OAC in the last decade.
Conclusions
In Japanese AF patients, concomitant CAD was associated with higher prevalences of major co-morbidities and higher incidences of cardiovascular events; however, history of CAD was not associated with the incidence of stroke/SE.
Acknowledgments
We sincerely appreciate the help of all the institutions participating in the registry and the clinical research coordinators (Shinagawa T, Mitamura M, Fukahori M, Kimura M, Fukuyama M, Kamata C, Nishiyama N).
Sources of Funding
This research was partially supported by the Practical Research Project for Life-Style related Diseases including Cardiovascular Diseases and Diabetes Mellitus from the Japan Agency for Medical Research and Development, AMED (19ek0210082 h0003, 18ek0210056 h0003).
The Fushimi AF Registry was supported by research funding from Boehringer Ingelheim, Bayer Healthcare, Pfizer, Bristol-Myers Squibb, Astellas Pharma, AstraZeneca, Daiichi-Sankyo, Novartis Pharma, MSD, Sanofi-Aventis, and Takeda Pharmaceutical. The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Disclosures
Dr. Akao received research funding from Bayer Yakuhin, scholarship funds from Bayer Yakuhin and Daiichi-Sankyo, and lecture fees from Pfizer, Bristol-Myers Squibb, Boehringer Ingelheim, Bayer Yakuhin, and Daiichi-Sankyo. All other authors have no relationships relevant to the content of this paper to disclose.
IRB Information
The study protocol was approved by the ethical committees of the National Hospital Organization Kyoto Medical Center and Ijinkai Takeda General Hospital (Reference numbers were 10-058 and 14-033).
Data Availability
Deidentified participant data will not be shared.
Supplementary Files
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-22-0180
References
- 1.
Akao M, Chun YH, Wada H, Esato M, Hashimoto T, Abe M, et al. Current status of clinical background of patients with atrial fibrillation in a community-based survey: The Fushimi AF Registry. J Cardiol 2013; 61: 260–266.
- 2.
Kirchhof P, Ammentorp B, Darius H, De Caterina R, Le Heuzey JY, Schilling RJ, et al. Management of atrial fibrillation in seven European countries after the publication of the 2010 ESC guidelines on atrial fibrillation: Primary results of the PREvention oF thromboemolic events: European Registry in Atrial Fibrillation (PREFER in AF). Europace 2014; 16: 6–14.
- 3.
Nakamura M, Kimura K, Kimura T, Ishihara M, Otsuka F, Kozuma K, et al. JCS 2020 guideline focused update on antithrombotic therapy in patients with coronary artery disease. Circ J 2020; 84: 831–865.
- 4.
Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom-Lundqvist C, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021; 42: 373–498.
- 5.
Goto S, Bhatt DL, Rother J, Alberts M, Hill MD, Ikeda Y, et al. Prevalence, clinical profile, and cardiovascular outcomes of atrial fibrillation patients with atherothrombosis. Am Heart J 2008; 156: 855–863, 863.e2.
- 6.
Verheugt FWA, Ambrosio G, Atar D, Bassand JP, Camm AJ, Costabel JP, et al. Outcomes in newly diagnosed atrial fibrillation and history of acute coronary syndromes: Insights from GARFIELD-AF. Am J Med 2019; 132: 1431–1440.e7.
- 7.
Lamberts M, Gislason GH, Lip GY, Lassen JF, Olesen JB, Mikkelsen AP, et al. Antiplatelet therapy for stable coronary artery disease in atrial fibrillation patients taking an oral anticoagulant: A nationwide cohort study. Circulation 2014; 129: 1577–1585.
- 8.
Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: Results from the National Registry of Atrial Fibrillation. JAMA 2001; 285: 2864–2870.
- 9.
Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: The euro heart survey on atrial fibrillation. Chest 2010; 137: 263–272.
- 10.
Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: The Euro Heart Survey. Chest 2010; 138: 1093–1100.
- 11.
Schulman S, Kearon C, Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 2005; 3: 692–694.
- 12.
Yasuda S, Kaikita K, Akao M, Ako J, Matoba T, Nakamura M, et al. Antithrombotic therapy for atrial fibrillation with stable coronary disease. N Engl J Med 2019; 381: 1103–1113.
- 13.
An Y, Ogawa H, Yamashita Y, Ishii M, Iguchi M, Masunaga N, et al. Causes of death in Japanese patients with atrial fibrillation: The Fushimi Atrial Fibrillation Registry. Eur Heart J Qual Care Clin Outcomes 2019; 5: 35–42.
- 14.
Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation: Analysis of pooled data from five randomized controlled trials. Arch Intern Med 1994; 154: 1449–1457.
- 15.
Kodani E, Atarashi H, Inoue H, Okumura K, Yamashita T, Otsuka T, et al. Impact of blood pressure control on thromboembolism and major hemorrhage in patients with nonvalvular atrial fibrillation: A subanalysis of the J-RHYTHM Registry. J Am Heart Assoc 2016; 5: e004075. doi:10.1161/JAHA.116.004075.
- 16.
Lemesle G, Ducrocq G, Elbez Y, Van Belle E, Goto S, Cannon CP, et al. Vitamin K antagonists with or without long-term antiplatelet therapy in outpatients with stable coronary artery disease and atrial fibrillation: Association with ischemic and bleeding events. Clin Cardiol 2017; 40: 932–939.
- 17.
Dewilde WJ, Oirbans T, Verheugt FW, Kelder JC, De Smet BJ, Herrman JP, et al. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: An open-label, randomised, controlled trial. Lancet 2013; 381: 1107–1115.
- 18.
Vranckx P, Valgimigli M, Eckardt L, Tijssen J, Lewalter T, Gargiulo G, et al. Edoxaban-based versus vitamin K antagonist-based antithrombotic regimen after successful coronary stenting in patients with atrial fibrillation (ENTRUST-AF PCI): A randomised, open-label, phase 3b trial. Lancet 2019; 394: 1335–1343.
- 19.
Cannon CP, Bhatt DL, Oldgren J, Lip GYH, Ellis SG, Kimura T, et al. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N Engl J Med 2017; 377: 1513–1524.
- 20.
Gibson CM, Mehran R, Bode C, Halperin J, Verheugt FW, Wildgoose P, et al. Prevention of bleeding in patients with atrial fibrillation undergoing PCI. N Engl J Med 2016; 375: 2423–2434.
- 21.
Lopes RD, Heizer G, Aronson R, Vora AN, Massaro T, Mehran R, et al. Antithrombotic therapy after acute coronary syndrome or PCI in atrial fibrillation. N Engl J Med 2019; 380: 1509–1524.
- 22.
January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jr, et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the management of patients with atrial fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation 2019; 140: e125–e151.