Abbreviations
| CR |
cardiac rehabilitation |
| 1RM |
1-repetition maximum |
| 6MWD |
6-minute walk distance |
| ACC (F) |
American College of Cardiology (Foundation) |
| ACS |
acute coronary syndrome |
| ADL |
activities of daily living |
| AF |
atrial fibrillation |
| AHA |
American Heart Association |
| AMI |
acute myocardial infarction |
| AT |
anaerobic threshold |
| BMI |
body mass index |
| BNP |
brain natriuretic peptide / B-type natriuretic peptide |
| CABG |
coronary artery bypass grafting |
| CAD |
coronary artery disease |
| CHD |
congenital heart disease |
| CKD |
chronic kidney disease |
| CORE |
cardio-oncology rehabilitation |
| COPD |
chronic obstructive pulmonary disease |
| CONUT |
Controlling Nutritional Status |
| CPX |
cardiopulmonary exercise testing |
| CRT |
cardiac resynchronization therapy |
| CRT-D |
cardiac resynchronization therapy defibrillator |
| CRT-P |
cardiac resynchronization therapy pacemaker |
| CVD |
cardiovascular disease |
| CVRF |
cardiovascular risk factor |
| eGFR |
estimated glomerular filtration rate |
| EOV |
exercise oscillatory ventilation |
| ESC |
European Society of Cardiology |
| GLIM |
Global Leadership Initiative on Malnutrition |
| GNRI |
Geriatric Nutritional Index |
| HFpEF |
heart failure with preserved ejection fraction |
| HFrEF |
heart failure with reduced ejection fraction |
| HIIT |
high-intensity interval training |
| HR |
heart rate |
| HRR |
heart rate reserve |
| HRQOL |
health-related quality of life |
| IADL |
instrumental activities of daily living |
| ICD |
implantable cardioverter defibrillator |
| LVAS/LVAD |
left ventricular assist system/device |
| LVEF |
left ventricular ejection fraction |
| MET |
metabolic equivalent |
| MI |
myocardial infarction |
| MNA® |
Mini Nutritional Assessment |
| MNA®-SF |
Mini Nutritional Assessment-Short Form |
| PAD |
peripheral arterial disease |
| PCI |
percutaneous coronary intervention |
| peak V̇O2 |
peak oxygen uptake |
| PH |
pulmonary hypertension |
| PVCs |
premature ventricular contraction |
| QOL |
quality of life |
| RCT |
randomized controlled trial |
| RPE |
rating of perceived exertion |
| SGA |
Subjective Global Assessment |
| STEMI |
ST elevation myocardial infarction |
| TG |
triglyceride |
| TAVI |
transcatheter aortic valve implantation |
| VAD |
ventricular assist device |
| V̇CO2 |
carbon dioxide output |
| V̇E |
minute ventilation |
| V̇E/V̇CO2 |
ventilatory equivalent for carbon dioxide |
| V̇E vs. V̇CO2 slope |
minute ventilation vs. carbon dioxide output slope |
| V̇O2 |
oxygen uptake |
| V̇O2/HR |
oxygen pulse |
Introduction
Eight years have passed since the publication of the only guideline on cardiac rehabilitation (CR) in Japan, “Guidelines for rehabilitation in patients with cardiovascular disease (JCS 2012)” (Chair: Ryuji Nohara).1 In that time, the situation surrounding CR, including cardiovascular care in Japan, has changed dramatically. Specifically, compared with 2012, the following changes have occurred: (1) new findings on CR and exercise training, including transcatheter aortic valve implantation (TAVI) and pulmonary hypertension (PH), have emerged, (2) CR is becoming highly recognized as a disease management program after discharge from acute care hospitals due to the shortening of hospital stay and the increasing number of older patients with heart failure (HF), (3) several clinical guidelines, such as the “Guideline on diagnosis and treatment of acute and chronic heart failure” have been revised, but descriptions are inconsistent from guideline to guideline, (4) due to revision of the Japanese medical reimbursement system, the criteria that CR facilities must follow have changed, causing a discrepancy between the guidelines and the medical practice.
The main points of this revision are as follows.
(1) From the viewpoint that comprehensive CR is important in daily clinical practice, in addition to exercise training, we have added sections on nutrition and diet therapy, patient education, and disease management as well as psychological interventions.
(2) With a growing number of older patients with cardiovascular disease (CVD), we have listed the targets of basic physical function, in particular muscle strength and walking ability of lower limbs; as important goals; sarcopenia and frailty are also discussed.
(3) With the growing number of HF patients, we have included HF as a target disease, and added sections on rehabilitation after TAVI, device implantation, and endovascular treatment of aortic disease and that for PH.
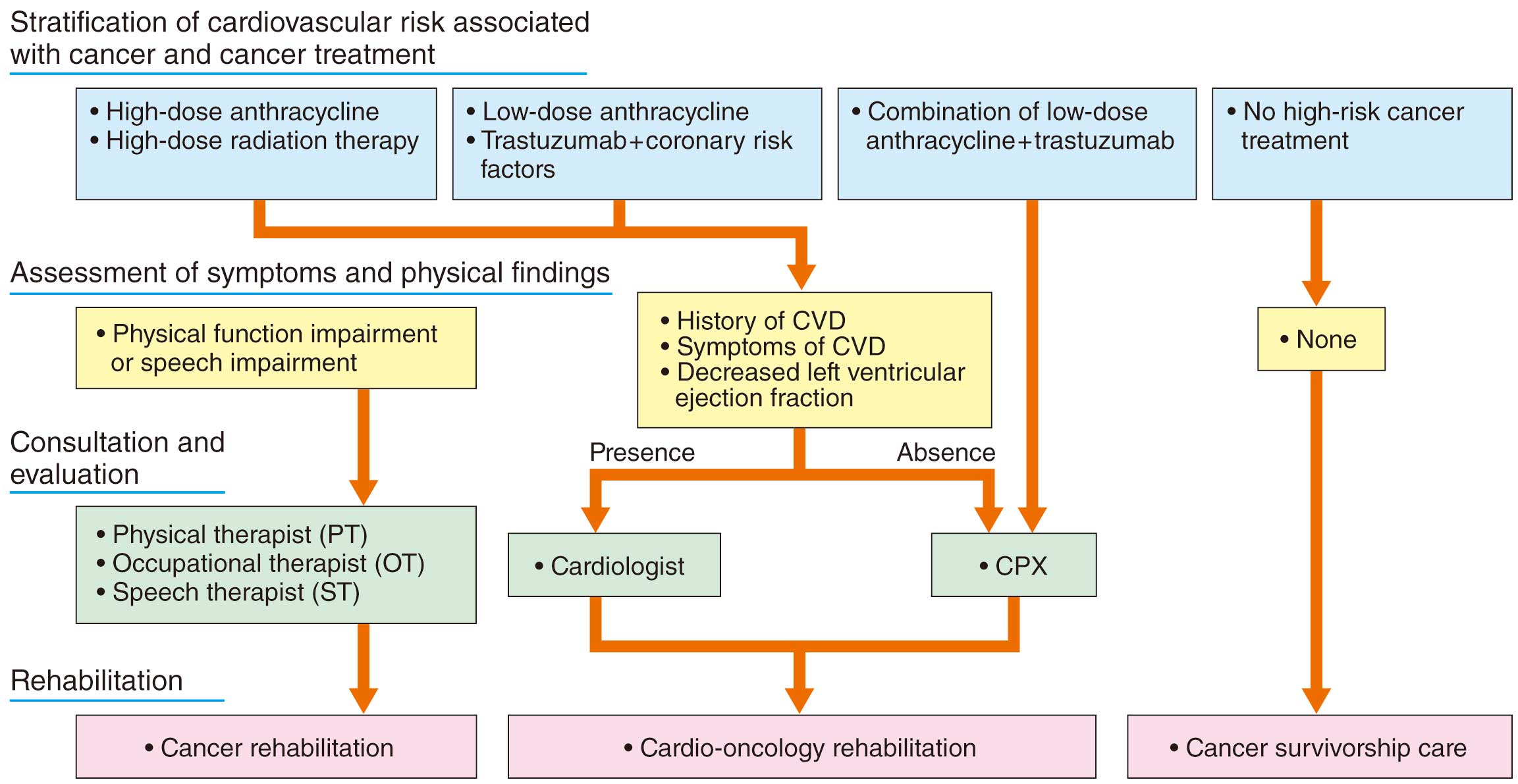
(4) As a special patient group, older cardiac patients (including super-aged patients) are listed as an independent section. Additionally we have completely rewritten the sections on rehabilitation protocol during intravenous inotropic drug administration, rehabilitation after implantation of a ventricular assist device (in particular, the implantable type), and rehabilitation after heart transplantation. We have also explained and incorporated onco-cardiology as a focus, because it is becoming a topic in cardiology practice, and the role of CR is attracting attention in this field.
(5) As for future issues and perspectives, we have included the theoretically ideal CR in the recovery phase, and the relationship with home medical care, community-based comprehensive care, and palliative care. We have also added a section on telemedicine as a new approach to outpatient and maintenance CR, given the new coronavirus epidemic.
After the first meeting of the Guideline Development Group for the JCS/JACR “2021 Guideline on rehabilitation in patients with cardiovascular disease” in March 2019, the initial draft of this guideline was revised three times by the group members and collaborators. In light of the comments from external reviewers, the guideline was finalized after discussions at the group meeting. This guideline was based on the latest guidelines from Europe and the USA, while incorporating evidence and actual clinical experience in Japan, with the aim of becoming a standard guideline for CR. We express our gratitude again to all involved and hope that this guideline will make a significant contribution to CR medicine in Japan and be useful to many medical professionals in their daily clinical practice.
Classes of Recommendations and Levels of Evidence
The classes of recommendations (COR) and levels of evidence (LOE) in this guideline were determined by the authors based on the literature published to date, and finally assessed by peer review of the members and external evaluators; the descriptions are in accordance with the conventional methods of labeling COR and LOE (Tables 1,2). In the cardiovascular field in Japan, the conventional classifications of COR and LOE are widely used, and are consistent with the overseas descriptors. In contrast, the Medical Information Network Distribution Service (MINDS), a medical information service operated by the Japan Council for Quality Health Care, describes the MINDS grades of recommendations and MINDS levels of evidence differently in the “MINDS handbook for clinical practice guideline development 2007” (Tables 3,4).2 Therefore, in this guideline, the MINDS Grades of Recommendations and MINDS Levels of Evidence are provided for reference only.
Table 1. Classes of Recommendations
| Class I |
Evidence and/or general agreement that a given procedure or treatment is effective and useful |
| Class IIa |
Weight of evidence/opinion is in favor of usefulness/efficacy |
| Class IIb |
Usefulness/efficacy is less well established by evidence/opinion |
Class III
(No benefit) |
Evidence or general agreement that the given procedure or treatment is not useful/effective |
Class III
(Harm) |
Evidence or general agreement that the given procedure or treatment is harmful |
Table 2. Levels of Evidence
| Level A |
Data derived from multiple randomized clinical trials or meta-analyses |
| Level B |
Data derived from a single randomized clinical trial or large non-randomized clinical trials |
| Level C |
Consensus of opinion of the experts and/or small clinical trials (including retrospective trials, registries) |
Table 3. MINDS Grades of Recommendations
| Grade A |
Strongly recommended and supported by strong evidence |
| Grade B |
Recommended with moderately strong supporting evidence |
| Grade C1 |
Recommended despite the lack of supporting evidence |
| Grade C2 |
Not recommended because of the absence of supporting evidence |
| Grade D |
Not recommended as evidence indicates that the treatment is ineffective or even harmful |
The grades of recommendations are based on a comprehensive assessment of the level and quantity of evidence, variation of conclusion, level of clinical efficacy, clinical applicability, and evidence of harm and cost. (Adapted from MINDS Handbook for Clinical Practical Guideline Development, 2007, p.16.2)
Table 4. MINDS Levels of Evidence (Levels of Evidence in the Literature on Treatment)
| I |
Systematic review/meta-analysis of randomized controlled trials |
| II |
One or more randomized controlled trial |
| III |
Non-randomized controlled trials |
| IVa |
Analytical epidemiological studies (cohort studies) |
| IVb |
Analytical epidemiological studies (case-control studies, cross-sectional studies) |
| V |
Descriptive research (case reports, case series) |
| VI |
Not based on patient data, or based on opinions from a specialist committee or individual specialists |
(Adapted from MINDS Handbook for Clinical Practice Guideline Development, 2007, p.162 with modifications.)
I. Definition, Components, and Phase-Based Classification
1. Definition
The title of this guideline is “JCS/JACR 2021 guideline on rehabilitation in patients with cardiovascular disease.” The term “cardiovascular rehabilitation” has been used consistently since the revision in 2007, but because the definition of cardiac rehabilitation (CR) clearly states that cardiovascular diseases (CVDs) are the target of CR, and “cardiac rehabilitation (CR)” is widely used in daily clinical practice and related academic societies, the term “cardiac rehabilitation (CR)” is used in this revision.
The Japanese Society of Cardiac Rehabilitation defines CR as follows: “Cardiac rehabilitation refers to a long-term, multifaceted, comprehensive program designed to optimize a cardiac patient’s physical, psychological, social, and vocational status, in addition to stabilizing, slowing, or even reversing the progression of the underlying atherosclerotic or heart failure processes, thereby reducing recurrence, rehospitalization and mortality and enabling patients to live comfortably and actively. Cardiac rehabilitation programs include ‘medical assessment, prescribed exercise training, coronary risk factor modification, patient education, counseling and optimal medical therapy’ for individual patients, which are provided by a multi-disciplinary team in a coordinated manner.”
Recent trends in the treatment of CVDs in Japan include: (1) a decrease in in-hospital mortality rates for acute myocardial infarction (AMI) and acute heart failure (HF),3 (2) an increase in patients with multiple comorbidities such as chronic HF, atrial fibrillation (AF), diabetes mellitus, chronic kidney disease (CKD), and dementia,4 (3) an increase in older cardiac patients with the complication of frailty, (4) progression of disuse syndrome and the need for nursing care due to prolonged bed rest,5 (5) an increase in readmissions due to inadequate management after hospital discharge,4 and (6) an increase in national health care expenditure.3 Considering these current circumstances, we need to focus not only on reducing the acute mortality, but also on improving quality of life (QOL) after discharge and preventing rehospitalization.
2. Components
The components of CR include the following conventional aspects: (1) medical evaluation of the patient’s condition and severity, (2) exercise prescription and exercise training based on medical evaluation, (3) improvement of coronary risk factors and patient education, and (4) counseling on psychosocial factors and on returning to work.6 Also, (5) disease management. “Prevention of hospital readmission, prevention of frailty, and improvement of depression” are also important objectives of CR.7 The disease management program consists of interventions by a multidisciplinary medical team, including (1) provision of standard medical care based on practice guidelines, (2) thorough self-management (self-care) through “patient education, lifestyle intervention, and motivation”, and (3) early detection of disease progression through telephone and home monitoring. These interventions have been reported to reduce rehospitalization for HF and improve life expectancy.8,9 Considering the current situation of the limitations of advanced acute care, outpatient CR is important as a comprehensive disease management program after hospital discharge.
3. Phase-Based Classification
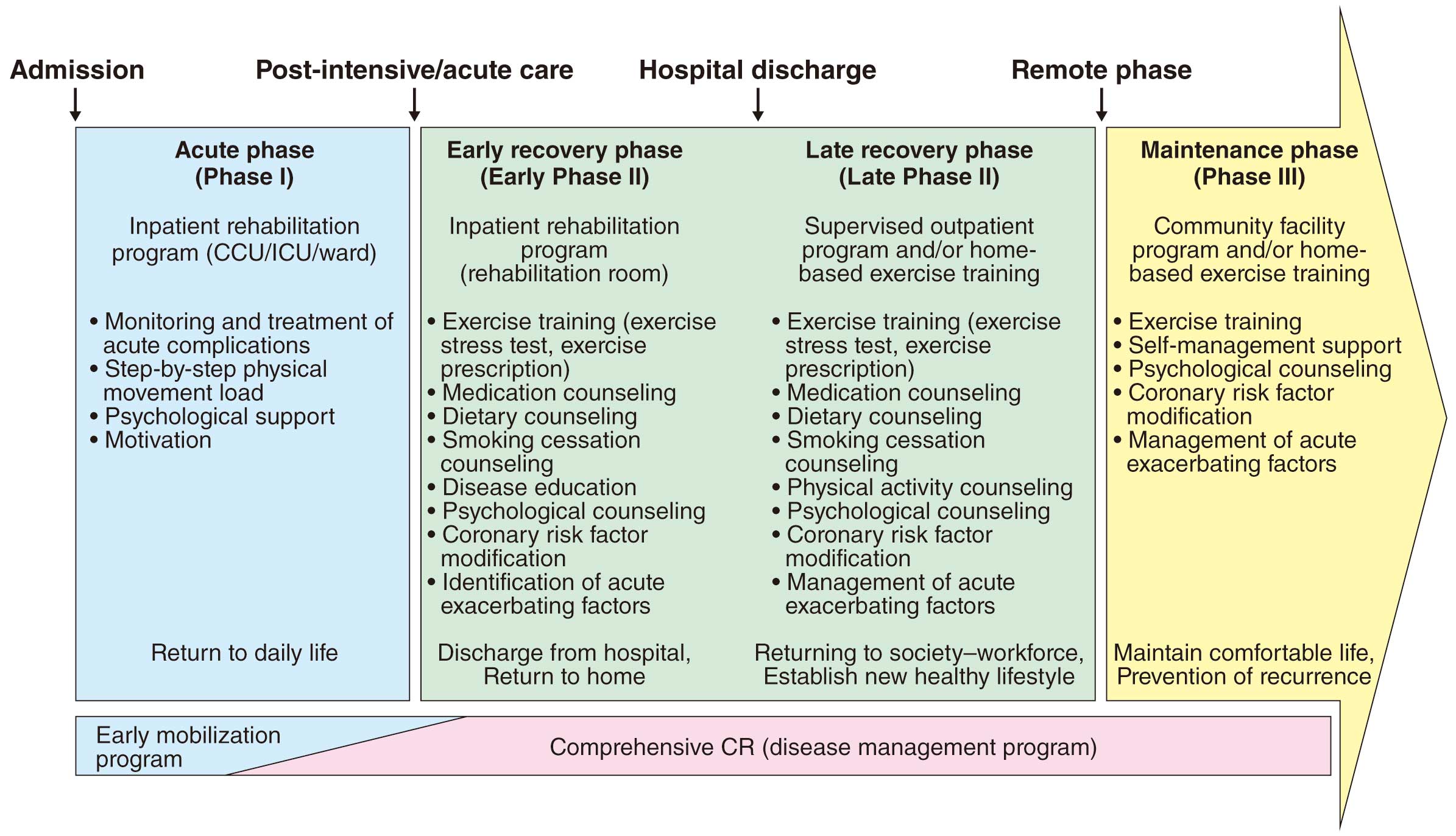
CR is a comprehensive and long-term intervention program. Currently, it is thought that rehabilitation should be classified according to the form (monitoring level) and content, such as ambulation and return to social life, and is classified as “acute phase (phase I)” from the day of onset (surgery) to getting out of bed, “recovery phase (phase II)” after getting out of bed (early recovery phase, late recovery phase), and “maintenance phase (phase III)” after social return (Figure 1).10 It is important to note that the acute phase and early recovery phase were shortened in the 2000s, and the focus changed to lifelong prevention. CR is systematically performed not only as exercise training but also recurrence prevention with lifestyle interventions and correction of coronary risk factors, and it is expected to include a comprehensive disease management program from the acute phase to the chronic phase.
3.1 Acute Phase (Phase I)
CR in the acute phase is performed under supervision in the intensive care unit, cardiac care unit or hospital ward. The goal of CR is to enable the patient to safely perform activities of daily living (ADL), such as eating, toileting, and bathing (independence in ADL), and to start secondary prevention education. In the treatment of AMI, a clinical pathway including acute CR is also used. Immediately after hospitalization for AMI or acute HF, or in the acute phase after cardiovascular surgery, treatment is aimed at achieving hemodynamic stability.
If bed rest is prolonged during this phase, exercise capacity will decline and frailty will progress. Therefore, an early mobilization program should be started from the bedside, in parallel with the acute treatment, leading to early exercise training. At the end of this phase, a 6-minute walk test is performed, and if the patient can walk more than 300 m, the program shifts from an ambulation program to an exercise training program. It is also important to provide patient education in parallel with the ambulation program. The patient’s understanding of his/her own condition will not only be useful for subsequent lifestyle interventions and management of coronary risk factors, but will also motivate the patient to engage in CR.
3.2 Recovery Phase (Phase II): Early and Late Recovery Phases
CR in the recovery phase is defined as the period from becoming ambulatory until the time when the condition stabilizes after social return. CR in the early recovery phase is started under supervision in the CR room during hospitalization, and is followed by supervised exercise training in the outpatient CR room after discharge. In late recovery phase CR, outpatient supervised exercise training and home unsupervised exercise training are combined; in low-risk patients, it is possible to perform only the unsupervised home exercise training. Eventually, patients are instructed to self-manage their exercise program. Exercise capacity is assessed by cardiopulmonary exercise testing (CPX), an exercise prescription is prepared based on the risk in terms of severity of illness, and then a treatment and CR plan is established. If CPX cannot be performed due to complications, low physical fitness, or low left ventricular function, exercise capacity should be confirmed by the 6-minute walk test.
For frail patients, after hospital discharge, their living environment, nursing care certification, and use of nursing care services should be confirmed. In addition, a counseling program for lifestyle modification and adherence to medication, assessment and management of comorbidity, and psychological counseling should be provided. It is not rare for patients with cardiac disease to be depressed after hospital discharge due to anxiety about their physical health, financial problems, and concerns about returning to work or sexual potency.11 Regarding recovery phase CR, a comprehensive disease management program that includes exercise training, smoking cessation, diet therapy, appropriate treatment of coronary risk factors, as well as psychological evaluations, return-to-work counseling, and psychological support, are important. In addition, the importance of self-management to prevent recurrence should be explained to patients and their families, and information on treatment goals and the content of the recovery CR program should be shared within the multidisciplinary CR team and discussed at regular conferences. Counseling will be given on appropriate physical activity based on exercise capacity, and excessive or low activity should be adjusted to the appropriate activity level. If there are signs or findings that suggest exacerbation of medical condition/heart failure or excessive exercise load, the exercise prescription must be reviewed with consideration given to intensify the treatment.
3.3 Maintenance Phase (Phase III)
Maintenance CR should be performed throughout life, after social return. Self-health management, such as maintaining exercise capacity, lifestyle modification, and correction of coronary risk factors, acquired during recovery CR, will be the main focus. Considering the individual’s background, such as age, occupation, and physical activity level, a program tailored to the individual’s lifestyle is created at home or at a private exercise training facility. When referring to local institutions or clinics, a medical information sheet that includes the medical history, current cardiac function, prescriptions, and exercise program should be provided; later, a system that allows for periodic evaluation and review of the exercise program is needed.
In Japan, the numbers of patients with many comorbidities such as chronic HF, AF, diabetes, CKD, and dementia, as well as very old patients with heart disease complicated by frailty, are increasing.4,5 It is a difficult issue to secure disease management and intervention for HF at the level of home care after the end of the CR insurance period (150 days), and close community medical cooperation, including home nursing, day care, and day services, is necessary.
II. Assessment of Physical Activity Capacity
1. Definition
Physical activity capacity is almost synonymous with maximal exercise capacity, exercise capacity, and exercise tolerance,12 and is defined by both the maximal capacity of the cardiovascular system to deliver oxygen to the exercising muscle and the maximal capacity of skeletal muscle to utilize oxygen.13 In the absence of respiratory disease or anemia, the oxygen-carrying capacity to the exercising muscle is determined by the maximal cardiac output, the oxygen content of the arterial blood, and the ratio of blood flow to the exercising muscle to the maximal cardiac output.14 Therefore, physical activity capacity is evaluated by a perceived maximal stress, or a maximal exercise stress test that increases the work rate until the stress discontinuation criteria is met. In general, CPX is performed, and physical activity capacity is expressed as the oxygen uptake (V̇O2) obtained at maximal load.
In patients with heart disease in particular, peak oxygen uptake (peak V̇O2) is widely used as an indicator of exercise capacity and prognosis. Because the peak V̇O2
becomes higher as the muscle mass mobilized for exercise increases (i.e., as body size increases), it is generally expressed by correcting according to body weight (expressed in mL/min/kg). Thus, exercise capacity may be underestimated in overweight subjects, and likewise, may be overestimated in highly lean subjects, so care must be taken when interpreting the results of CPX in these subjects. The peak V̇O2
decreases with age in healthy adults and is lower in women than in men of the same age. Therefore, in CPX reports, peak V̇O2
is usually expressed as mL/min/kg, which is the measured value divided by body weight, and is also expressed as % of the predicted value (standard value) calculated on the basis of age and sex.
2. Evaluation Method, Index (Table 5)
Table 5. Recommendations and Levels of Evidence for Assessment of Physical Activity Capacity and Physical Function in CR
| |
COR |
LOE |
GOR
(MINDS) |
LOE
(MINDS) |
| Assessment of muscle strength |
For patients with predicted muscle weakness, assessment of muscle strength
should be considered |
IIa |
B |
B |
IVb |
| Comprehensive assessment of lower extremity function |
For patients with anticipated frailty, assessment of Short Physical Performance
Battery (SPPB) should be considered |
IIa |
B |
B |
IVa |
| Assessment of exercise capacity |
| If CPX cannot be performed, the 6-minute walk test should be considered |
IIa |
B |
B |
II |
| Shuttle walking test may be considered |
IIb |
B |
C1 |
II |
| Exercise stress tests (other than CPX) may be considered |
IIb |
B |
C1 |
III |
| Assessment of balance function |
In patients with suspected frailty and risk of falling, “Single leg standing time”,
“Functional reach test”, or “Timed up and go test” may be considered |
IIb |
B |
C1 |
III |
COR, class of recommendation; CPX, cardiopulmonary exercise testing; CR, cardiac rehabilitation; GOR, grade of recommendation; LOE, level of evidence.
In CR clinical practice, it is extremely important to measure and evaluate physical function, as improvement of physical function is one of the major goals of CR; the body part to be trained and the function to be improved differ according to which specific part of the body is impaired. There are many methods to measure physical function; here we describe the more commonly used ones in the clinical practice of CR (Table 6).
Table 6. Characteristics of Various Physical Function Evaluation Methods and Indices
Evaluation methods
and indices |
Characteristics, benefits, and disadvantages |
| Classification based on physical activity capacity |
| NYHA functional class |
Strong relationship with exercise capacity and prognosis |
| Widely used in daily clinical practice; simple and highly useful |
| Broad classification, and difficult to reflect detailed changes in symptoms |
| Lack of objectivity |
SAS
(Specific Activity Scale) |
Developed to complement the NYHA functional class, and quantified V̇O2 at symptom onset by MET |
| Suitable for evaluation of NYHA class II |
| Evaluation of muscle strength and muscle mass |
Measurement of knee
extension muscle strength |
Important to evaluate current muscle strength level because it directly affects gait and ADL |
| Also useful for determining the effect of resistance training |
Measurement of lower
extremity muscle mass |
Screening for sarcopenia and frailty is possible. There are many measurement methods such as dual-energy
X-ray absorptiometry (DXA), bioimpedance analysis (BIA), MRI, and CT |
| It is necessary to measure with an understanding of the characteristics of each method |
| Comparison of different measurement methods is difficult |
| Handgrip strength |
Measuring with a relatively simple device is possible |
| Usefulness as a prognostic factor has been reported in large-scale clinical trials |
| Also included in the diagnostic criteria for sarcopenia and frailty in Japan |
| Comprehensive lower extremity functional evaluation |
SPPB
(Short Physical
Performance Battery) |
Especially in older people with frailty or at risk of frailty, it can be used for comprehensive evaluation of lower
limb function |
Good at predicting prognosis and ADL, such as inability to walk in the next few years, and commonly used in
clinical practice |
| Somewhat complicated due to measuring with different 3 methods |
| Walk test |
| Gait speed |
Physiological exercise of walking, not requiring any special equipment |
| In patients with CVD or older people, comfortable gait speed often used |
| Also used as a criterion for sarcopenia and frailty |
| Balance ability (important test to assess stability) |
| Single leg standing time |
Usually performed with the eyes open |
| Used to diagnose “musculoskeletal ambulation disability symptom complex” |
| Easy-to-use and highly useful |
| Functional reach test |
Also useful as a screening tool for fall risk in older people |
| Timed Up & Go test |
Used to evaluate “musculoskeletal ambulation disability symptom complex”, and also used to assess the risk
of falling and as a simple screening for frailty |
| Exercise capacity evaluation |
| 6-minute walk test |
A maximal exercise stress test to measure the 6MWD |
| Methods are issued in detail in the statement of American Thoracic Society |
| Highly correlated with peak V̇O2 obtained from CPX, and also used to estimate prognosis |
Difficult to make interinstitutional comparisons: the results are influenced by the method used, and the results
get better with each test |
| Shuttle walking test |
A multistep incremental maximal exercise stress test as well as the six-minute walk test |
| V̇O2 dynamics are the same as in the 6-minute walk test, and the reliability and reproducibility are excellent |
Exercise stress test
(so-called exercise stress
electrocardiogram) |
An exercise stress test without simultaneous expiratory gas analysis |
| Used to evaluate HR and BP response by exercise, and ECG diagnosis of ischemia |
6MWD, 6-minute walk distance; BP, blood pressure; CPX, cardiopulmonary exercise testing; CT, computed tomography; CVD, cardiovascular disease; HR, heart rate; MET, metabolic equivalent; MRI, magnetic resonance imaging; peak V̇O2, peak oxygen uptake; V̇O2, oxygen uptake.
2.1 Muscle Strength
2.1.1 Knee Extensor Strength (Lower Extremity Muscle Strength)
Lower extremity muscle strength is often assessed before the start of exercise training because it is associated with many of the walking abilities and ADL. Resistance training is recommended for all CVDs, except for contraindications, for which muscle strength evaluation is essential. The most convenient bedside muscle strength evaluation is the manual muscle test. However, there are some problems related to bias of the examiner and the quantification of muscle strength. In order to measure lower extremity muscle strength more accurately, isokinetic muscle strength measurement devices are used.
2.1.2 Grip Strength
Grip strength is relatively simple to measure. In a meta-analysis of 23,480 patients with cardiac disorders, grip strength was an independent predictor of cardiac death, all-cause death and hospital admission for HF.15 In older CVD patients or those with sarcopenia, ADL such as opening the lid of a plastic bottle may be impaired, and it is important to assess upper limb muscle strength represented by grip strength.
2.2 Short Physical Performance Battery
The SPPB16 can comprehensively assess lower extremity function in suspected frail patients, especially in older patients. It consists of a 3-item assessment: (1) “balance test” (standing with the feet together side by side, semi-tandem, and tandem positions), (2) “time to walk 4 m”, and (3) “time to rise 5 times from a chair”. Each item is rated from 0 to 4, with a maximum total score of 12. The total score is interpreted in 3-point increments: “very low motor function,” “low motor function,” “moderate motor function,” and “good motor function.” A SPPB score of ≤8 is adopted by the European Working Group as one of the diagnostic criteria for sarcopenia.17
2.3 6-Minute Walk Test
The 6-minute walk test is a simple test that measures the 6MWD with maximal effort, but not using any special equipment. The purpose of this test is to evaluate capacity of walking as far as possible in 6 min. The 6-minute walk test has gained worldwide consensus according to an ATS (American Thoracic Society) statement.18 It is known to be affected by age, sex, height, weight, etc., and the formulas for estimating the normal ranges for men and women have been reported.19 In patients with heart disease, many associations with exercise capacity or prognosis have been noted, and it is also widely used to judge the effectiveness of CR.20
3. CPX (Table 7)
Table 7. Recommendations and Levels of Evidence for CPX in CR
| |
COR |
LOE |
GOR
(MINDS) |
LOE
(MINDS) |
It is recommended to perform CPX in order to consider the indications for
heart transplantation and other advanced therapies |
I |
B |
B |
II |
In patients with dyspnea on exertion or easy fatigability as a factor exercise
limitation, it is recommended to perform CPX to identify the cause |
I |
B |
B |
IVb |
| It is recommended to measure peak V̇O2 for assessing prognosis |
I |
B |
B |
II |
| It should be considered in order to create an exercise prescription |
IIa |
B |
B |
II |
It should be considered to determine the HR response and an optimal program
for patients with AF or pacemakers, to assess BP, arrhythmia, and physical
activity during exercise, and to evaluate changes in exercise capacity and
treatment |
IIa |
B |
B |
II |
| It is not recommended to perform this as a routine test |
III (No
benefit) |
C |
C2 |
VI |
AF, atrial fibrillation; BP, blood pressure; COR, class of recommendation; CPX, cardiopulmonary exercise testing; CR, cardiac rehabilitation; GOR, grade of recommendation; HR, heart rate; LOE, level of evidence; peak V̇O2, peak oxygen uptake.
3.1 Purpose and Significance
CPX measures V̇O2, carbon dioxide output (V̇CO2), respiratory rate, and tidal volume by expiratory gas analysis. This method can be used to determine the statuses of cardiac function, myocardial ischemia, peripheral circulation, skeletal muscle function, vascular endothelial cell function, anemia, and autonomic nervous system activity.14 The significance of CPX is (1) to identify the cause of dyspnea and exercise limitation during exertion, (2) to determine the indication for surgery, predict prognosis, and determine the effect of treatment, as the most reliable objective indicator of exercise capacity, and (3) to determine the exercise prescription in CR and exercise programs.
3.2 Method and Timing of Implementation
Methods of stress loading include ramp (linear incremental) loading with a bicycle ergometer or treadmill. The peak respiratory exchange ratio (RER) is an indicator of the degree of loading. It is important to have a peak RER ≥1.10 to obtain a reliable peak V̇O2
value. In order to obtain reliable peak V̇O2
values, it is desirable to perform this method a few days after the patient has become accustomed to low-level exercise training (e.g., bicycle ergometer loading or treadmill walking).1
Submaximal loading is usually recommended at 4–6 days after AMI onset, and symptom-limited exercise testing at 14–21 days. CPX is performed 7–10 days after cardiac surgery, after weaning from intravenous infusion in HF, during the first week of the program after heart transplantation, and when the patient is able to walk 300–500 m continuously after the installation of a left ventricular assist system/device (LVAS/LVAD). An exercise prescription using CPX is recommended in postoperative congenital heart disease (CHD), and also in older patients with HF.1 During the outpatient CR period, CPX is performed several times to evaluate the effects and to reset the exercise prescription.1
3.3 How to Determine the Exercise Prescription
The V̇O2
just before the addition of anaerobic metabolism to aerobic metabolism is called the anaerobic threshold (AT).21 In activities above the AT, acidosis progresses and catecholamine secretion is enhanced.22 Therefore, by knowing the AT, we can set an exercise capacity range for HF patients.1 When prescribing aerobic exercise using CPX with ramp loading, prescribe at the work rate 1 min before the AT.1 The exercise intensity of the high-intensity portion of high-intensity interval training (HIIT) should reach at least 80% of the maximum exercise intensity, but it is advisable to check the safety of high-intensity loading with CPX beforehand. Peak V̇O2
is closely related to skeletal muscle function.
3.4 Significance of Key Indices During the Ramp Exercise Protocol
The normal changes in the major indices during a ramp exercise protocol are shown in Figure 2. The Japanese standard values of peak V̇O2, AT,23 minute ventilation vs. carbon dioxide output slope (V̇E vs. V̇CO2
slope), and ventilatory equivalent for carbon dioxide (V̇E/V̇CO2) minimum24 using a bicycle ergometer are listed in Table 8. These are affected by age and sex. V̇E vs. V̇CO2
slope and V̇E/V̇CO2
are affected by the magnitude of ventilation perfusion mismatch and chemoreceptor response.25 Abnormally high values are observed in HF, pulmonary thromboembolism/PH, pulmonary edema, and emphysema.
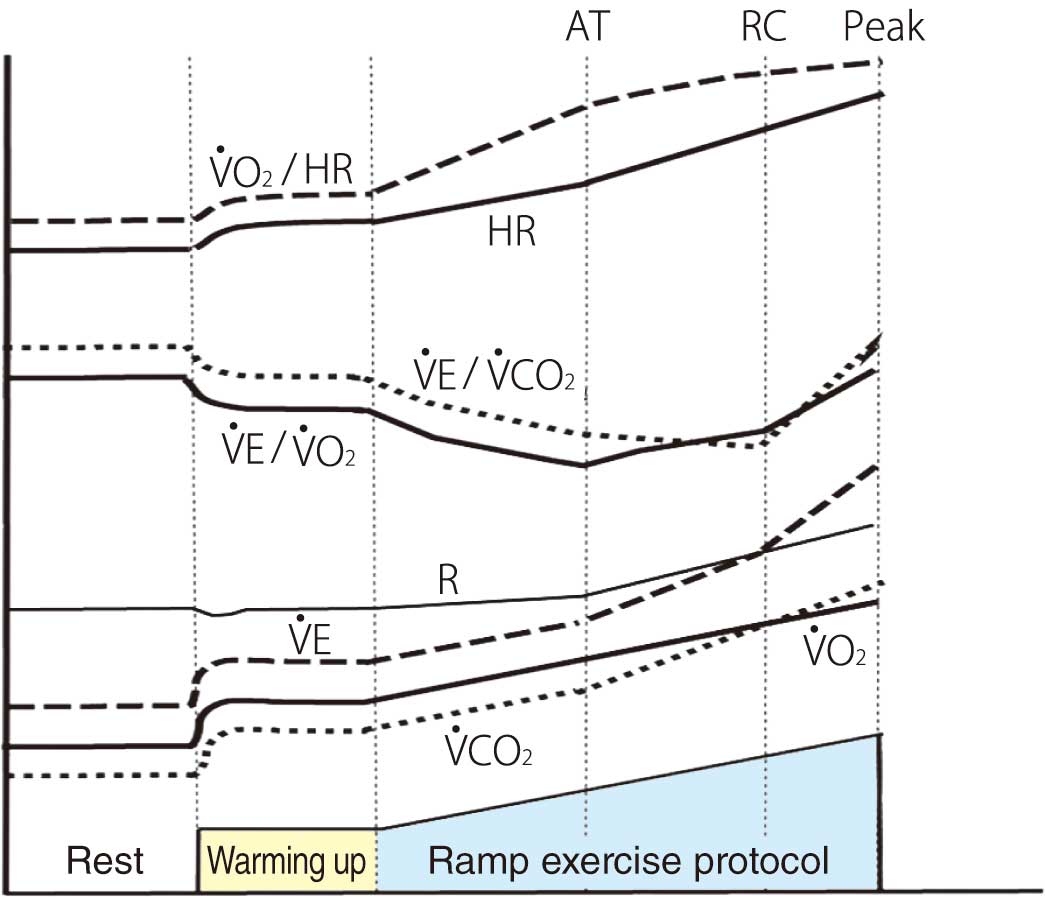
Table 8. Standard Values of Cardiopulmonary Function Indices During Cycle Ergometer Use in Japanese Subjects
| Index |
Men |
Women |
| peak V̇O2 |
−0.272 × age +42.29 |
−0.196 × age +35.38 |
| AT |
−0.100 × age +21.44 |
−0.069 × age +19.35 |
| V̇E vs. V̇CO2 slope |
0.080 × age +22.17 |
0.055 × age +24.02 |
| Minimum value of V̇E/V̇CO2 |
0.118 × age +21.03 |
0.055 × age +25.27 |
AT, anaerobic threshold; peak V̇O2, peak oxygen uptake; V̇E/V̇CO2, ventilatory equivalent for carbon dioxide; V̇E vs. V̇CO2
slope, minute ventilation vs. carbon dioxide output slope.
More advanced HF exhibits lower levels of peak V̇O2
and V̇O2/HR and also ∆V̇O2/∆work rate (WR), and a higher level of the V̇E vs. V̇CO2
slope. In cases of markedly blunted or decreased increases in ∆V̇O2/∆WR26 and V̇O2/HR,27 accompanied by ST-segment depression, and the WR reaches a certain level, then myocardial ischemia is the most likely cause. Thus, the severity of ischemia can be determined by the degree of change.26 The peak V̇O2
of HF patients can be improved by 8–16% through aerobic exercise performed 3–5 times each week for 40–50 min each time for 3 months.28,29 It is reported that the degree of improvement is greater in patients with a lower body mass index (BMI).28 However, it is also reported that frail patients do not show significant improvement.30
A phenomenon in which V̇E fluctuates >15% of the basal value in a cycle of ≈80 seconds during exercise, is called “exercise oscillatory ventilation” (EOV). EOV is an indicator of advanced HF because it is caused by delayed circulation time, hyperchemosensitivity, and decreased PaCO2.31 EOV is improved by CR.32
3.5 Assessment of Exercise Capacity
The most objective indicator of exercise capacity is peak V̇O2.33 The AT is approximately 50–55% of peak V̇O2; because compensatory hyperventilation does not occur below this level,34 it is an indicator of the level of daily activity. Because the standard values of peak V̇O2
differ according to age and sex, it is difficult to assess the severity of disease based on absolute values of peak V̇O2. It is recommended to classify deterioration of exercise capacity and HF severity based on 80%, 60%, and 40% of the standard values of peak V̇O2
and the AT, respectively (Table 9).35 When evaluating peak V̇O2
divided by body weight, it is important to note that it may be underestimated in overweight individuals and overestimated in extremely thin individuals.
Table 9. Classification of Severity of HF by Peak V̇O
2
Predicted rate of peak V̇O2 relative to
age-specific reference values |
Severity of
heart failure |
| ≥80% of standard value |
Normal |
| 60–80% of standard value |
Mild |
| 40–60% of standard value |
Moderate |
| Unable to perform the test, or <40% of the standard value |
Severe |
HF, heart failure; peak V̇O2, peak oxygen uptake. (Source: based on Japan Intractable Diseases Information Center.35)
3.6 Prognosis
The peak V̇O2
is the most powerful prognostic indicator when sufficient load can be applied.33,36,37 The extent to which peak V̇O2
improves after CR is useful for prognosis prediction.38 In recent years, the prognosis of HF has greatly improved due to therapeutic advances, and the prognosis of patients with the same peak V̇O2
has improved.39 The cardiovascular risk at 15 mL/min/kg before the year 2000 has now, after 2006, become the risk at 14 mL/min/kg. The AT is also a prognosis prediction factor.40 V̇E vs. V̇CO2
slope is also a well-established prognosis prediction indicator, with >34 or >35 being considered as a poor prognosis.41,42 The MECKI score, which predicts the incidence of LVAS insertion within 2 years in HF and HF death, incorporates peak V̇O2
and the V̇E vs. V̇CO2
slope.43 A prognostic method that combines peak V̇O2, the V̇E vs. CO2
slope and EOV has also been proposed.44
4. Sarcopenia, Frailty, and Cachexia (Table 10)
Table 10. Recommendations and Levels of Evidence for the Assessment of Sarcopenia and Frailty in CR
| |
COR |
LOE |
GOR
(MINDS) |
LOE
(MINDS) |
Patients with suspected sarcopenia or frailty should be considered for
assessment |
IIa |
B |
B |
IVa |
COR, class of recommendation; CR, cardiac rehabilitation; GOR, grade of recommendation; LOE, level of evidence.
Sarcopenia and frailty are relatively new concepts, and unified definition or diagnostic criteria have not yet been established. For cachexia, though the pathogenesis has been known for a long time, elucidation of the specific details of the pathogenesis and the quest for a treatment are issues that need to be addressed in the future.
4.1 Sarcopenia
The term “sarcopenia” has been translated as “age-related loss of muscle mass” or “age-related muscle weakness”. Sarcopenia is a concept of a condition in which skeletal muscle mass decreases due to a decrease in physical activity associated with aging, poor nutrition (low calorie, low protein intake), and various diseases, resulting in a decline in overall muscle mass and physical function.
The 2010 European Sarcopenia Working Group classified sarcopenia as “primary sarcopenia,” which has no factors other than aging, and “secondary sarcopenia,” which has factors such as disuse, inflammation, underlying disease, and poor nutrition.17,45 Especially in HF, skeletal muscle tends to decrease due to the pathological condition, and skeletal muscle mass is reported to be associated with exercise capacity,46 and prognosis.47 However, the prevalence of sarcopenia in HF patients is not clear.
Criteria combining skeletal muscle mass and function (muscle strength, gait speed, etc.) have been devised to assess sarcopenia. In Japan, the diagnostic criteria proposed by the Asian Working Group for Sarcopenia are often used (Table 11).48
Table 11. Diagnostic Criteria for Sarcopenia of the Asian Working Group for Sarcopenia
| 1. Muscle mass |
| Mass of skeletal muscle index (skeleton appendicular muscle mass/height2, kg/m2) |
| Dual energy X-ray absorptiometry (DXA) |
| Men: <7.0 kg/m2, Women: <5.4 kg/m2 |
| Bioelectrical impedance analysis (BIA) |
| Men: <7.0 kg/m2, Women: <5.7 kg/m2 |
| 2. Muscle strength |
| Grip strength: Men: <28 kg, Women: <18 kg |
| 3. Physical function |
| Gait speed: <1.0 m/s |
(Source: based on Chen LK, et al, 2020.48)
4.2 Frailty
Among the diagnostic criteria for frailty proposed to date,49 the criterion of Fried et al (phenotype model) is the most commonly used in many academic studies.50 This criterion, proposed by the Cardiovascular Health Study (CHS), is determined by the following 5 items that emerge together with the manifestation of frailty: weight loss, muscle weakness, decreased gait speed, poor endurance or exhaustion, and low activity (Table 12).50–53 In a meta-analysis in the Netherlands, the prevalence of frailty in community-dwelling older people based on the CHS criteria was 9.9% (95% confidence interval (CI) 9.6 to 10.2),54 and in Japan it was reported to be 7.4% (95% CI 6.1 to 9.0).55 A meta-analysis of HF patients in Oregon, USA, reported a high prevalence of frailty based on the CHS criteria, with a prevalence of 43% (95% CI 34 to 52).56 However, most of the original papers cited in that meta-analysis did not specify “inability to walk” as an exclusion criterion, and did not take into account the point of “not a disability, but reversible with appropriate intervention”.49 In other words, the complication rate of frailty in HF is likely to be overestimated.
Table 12. Diagnostic Criteria for Frailty by the Cardiovascular Health Study
| • Weight loss: Unintentional annual weight loss of ≥5%, etc. |
| • Decrease in grip strength (weakness): men <26 kg, women <18 kg, etc. |
| • Becomes tired easily (poor endurance or exhaustion): feeling tired, unusual weakness, etc. |
| • Decreased gait speed (slowness): <0.8 m/s, etc. |
| • Decrease in physical activity (low activity): men <383 kcal/week, women <270 kcal/week, etc. |
| *If ≥3 items apply, the patient is considered to be frail. If only 1 or 2 items apply, the patient is diagnosed as pre-frailty |
(Source: based on Fried LP, et al, 200150, Walston J, et al, 200651, Xue QL, et al, 200852, Singh M, et al, 2014.53)
It is necessary to understand frailty as a syndrome that includes undernutrition, mental and cognitive decline, and comorbidities, in addition to the decline in physical function, and to apply exercise training, which is expected to be an effective treatment, and to practice innovative comprehensive CR, such as combining it with new treatment strategies.57
4.3 Cachexia
Cachexia is a pathological status caused by nutritional disorder, inflammation or oxidative stress, hypogonadism, anemia, insulin resistance, and enhanced protein catabolism associated with chronic diseases including malignancy, chronic HF, and chronic respiratory failure; in addition to skeletal muscle loss, a metabolic abnormality characterized by adipose tissue loss is included. This is a syndrome marked by weight loss, muscle weakness, and decreased capacity for physical activity.58 In 2006, the Cachexia Consensus Working Group proposed a definition that combines weight loss with clinical findings.58 Although cachexia is a strong poor prognostic factor in HF,59 it is distinguished from sarcopenia, which is mainly characterized by skeletal muscle mass loss, by the fact that adipose tissue is also reduced in addition to skeletal muscle mass.58 Nutritional disorder is a characteristic finding of cachexia, and its possible causes include anorexia, polypharmacy, digestive and absorptive deficiencies associated with intestinal edema, physical inactivity, and increased resting energy demand.60 In addition, it has been suggested that in patients with HF, intestinal edema from congestion, and ischemia due to circulatory failure, can increase intestinal mucosal permeability; endotoxins and bacteria from among the Gram-negative rods in the intestine can enter the bloodstream, increasing inflammatory cytokines and enhancing catabolism.61–64
III. General Principles of Exercise Prescription
1. Exercise Prescription (Table 13)
Table 13. Recommendations and Levels of Evidence for Aerobic Exercise Training and Resistance Training in CR
| |
COR |
LOE |
GOR
(MINDS) |
LOE
(MINDS) |
| It is recommended to perform moderate-intensity endurance training |
I |
A |
A |
I |
In patients with muscle weakness and frailty, it is recommended to perform
resistance training from a low intensity |
I |
A |
B |
I |
| In addition to endurance training, resistance training should be considered |
IIa |
B |
B |
II |
During the initial phase of the exercise program, or for patients with reduced
exercise capacity, low-intensity endurance training may be considered |
IIb |
B |
B |
II |
Relatively high-intensity endurance training may be considered in the late
recovery period or maintenance period when the disease has stabilized |
IIb |
B |
B |
II |
| High-intensity interval training may be considered for low-risk, stable patients |
IIb |
C |
B |
I |
COR, class of recommendation; CR, cardiac rehabilitation; GOR, grade of recommendation; LOE, level of evidence.
1.1 Concept of Exercise Prescription
A safe and effective exercise training program designed to meet the individual’s health status and physical function is termed an “exercise prescription”.65 Exercise programs in cardiac rehabilitation (CR) focus on aerobic exercise, resistance training, and stretching.66
1.2 Components of the Exercise Training Session
Each exercise session consists of a 5–10-min warm-up, the main exercise prescribed in its duration (aerobic or resistance training), and a 5–10-min cool-down. The warm-up and cool-down consist of stretching and aerobic exercise of lower intensity than the main exercise. As for the main exercise, aerobic exercise and resistance training are generally performed on separate days, but they may be performed on the same day if tolerated.
2. Types of Exercise
2.1 Aerobic Exercise
Aerobic exercise is based on rhythmic motion of large muscle groups (pectoralis major, latissimus dorsi, quadriceps femoris, rectus abdominis, gluteus maximus, and erector spinae) for a certain period of time. The exercise prescription is based on FITT-VP: frequency, intensity, time, type, volume, and progression/revision66 (Table 14).
Table 14. FITT-VP Principles of Exercise Prescription
| F |
Frequency: how often |
| I |
Intensity: how hard |
| T |
Time: duration or how long |
| T |
Type: mode or what kind of exercise |
| V |
Volume: amount |
| P |
Progression/revision |
2.1.1 Frequency
The frequency of aerobic exercise is determined according to exercise capacity, exercise intensity, target health status and physical function. High-intensity exercise is performed at least 3 times per week, a combination of moderate- to high-intensity exercise 3–5 times per week, and low- to moderate-intensity exercise at least 5 times per week. Although exercising once or twice per week has been shown to be beneficial to health and physical function if a high level of physical activity is maintained, engaging in an unfamiliar exercise only once or twice per week is not recommended because it increases the risk of injury. In addition to participation in supervised exercise training, outpatients should be instructed in unsupervised voluntary training, and maintaining the frequency of exercise.
2.1.2 Intensity of Exercise
Exercise of <3.0 metabolic equivalents (METs) is generally described as “low-intensity”, 3.0 to <6.0 METs as “moderate-intensity”, and >6.0 METs as “high-intensity”, but these general values are not used in exercise training for patients with cardiovascular disease (CVD). As for the methods used for determining exercise prescription based on exercise stress tests, there are exercise prescriptions based on the anaerobic threshold (AT), heart rate reserve (HRR), and rating of perceived exertion (RPE) (Table 15). Using a resting HR of +30 (20) beats/min is not a recommended method and should only be used for convenience in patients who have not yet undergone an exercise stress test until an exercise prescription is determined based on the exercise stress test.67 For patients with chronotropic incompetence, such as those with pacemaker implantation or those receiving β-blockers, and for those with arrhythmias such as atrial fibrillation (AF), cardiopulmonary exercise testing (CPX) is used to directly measure energy metabolism and ventilatory response during exercise; the exercise prescription is made at the AT level, or relative intensity (% maximal V̇O2, % peak V̇O2) to maximal V̇O2
or peak V̇O2
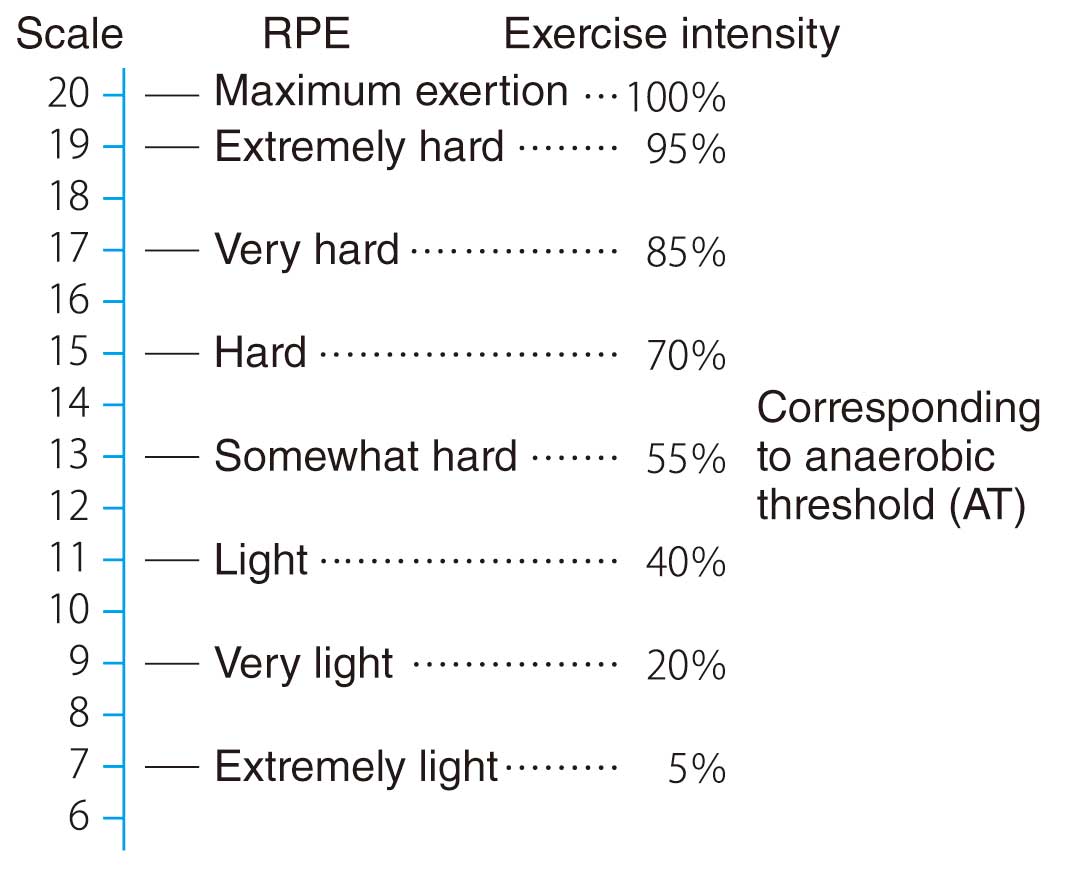
is used.66 The AT can be also assessed from submaximal exercise testing, so it can be determined even in critically ill patients. Exercise intensity at less than the AT is very safe because it is unlikely to cause acidosis or a marked increase in blood levels of catecholamines. When exercise stress testing is not possible due to the patient’s condition or a poorly equipped facility, training intensity should be determined by one of the methods listed in Table 16. If the RPE is used as an evaluation index, the Borg scale is used (Figure 3).68 Talk Test is one of the simple methods for estimating exercise intensity.69
Table 15. How to Determine Exercise Intensity for Patients With AMI After the Recovery Phase
| A. 40–60% of HRR (= maximum HR − resting HR) |
| Karvonen formula: [maximum HR − resting HR] × k + resting HR |
| k: 0.6 for normal patients (e.g., young patients with uncomplicated AMI) |
| 0.4–0.5 for high-risk patients, and 0.3–0.5 for heart failure patients |
| B. HR at the AT level or 40–60% of peak V̇O2 |
| C. RPE: “Somewhat hard” or just below (Borg scale: 12–13) |
| D. Simplified method: resting HR + 30/min (patients receiving beta-blockers: resting HR + 20/min) |
Note that exercise intensity should be low in high-risk patients [(1) left ventricular dysfunction (LVEF <40%), (2) prolonged
occlusion of the left anterior descending artery (patients who failed reperfusion therapy), (3) patients with severe 3-vessel disease,
(4) older patients (≥70 years)] |
AMI, acute myocardial infarction; AT, anaerobic threshold; HR, heart rate; HRR, heart rate reserve; LVEF, left ventricular ejection fraction; peak V̇O2, peak oxygen uptake; RPE, rating of perceived exertion.
Table 16. How to Determine Exercise Intensity When Exercise Stress Test Cannot Be Conducted
| |
Simple HR prescription |
RPE |
Talk Test |
| Method |
Intensity of resting HR + 30/min
(20/min in patients receiving beta
blockers) |
Borg scale 12–13
Borg scale 11–13 for heart
failure patients |
Exercise intensity that can
be done while talking
comfortably |
| Points to note |
The maximum allowable range is
120/min or less |
Need to be interviewed frequently during exercise |
| Not applicable |
Patients with chronotropic
incompetence, AF, pacemaker
implantation |
Patients with few symptoms, such as silent myocardial
ischemia, and patients with communication problems such
as dementia |
Note: See text for simplified HR prescription. AF, atrial fibrillation; HR, heart rate; RPE, rating of perceived exertion.
2.1.3 Time (Duration)
The duration of exercise should be at least 10 min per session, but for patients with severely impaired exercise capacity it should begin with less than 10 min and gradually increase by 1–5 min per session.70 The final goal is 20–60 min.
2.1.4 Type (Mode or What Kind)
Continuation of exercise training is important, so the choice of exercise that the patient is able to continue comfortably for a long time is important. In general, walking is the easiest exercise to perform and its intensity can be easily adjusted. Other forms of exercise, such as cycling, dancing, and water exercise, can provide similar aerobic benefits, so the type of exercise is determined in consideration of comorbidities and patient preference. Combining multiple types of exercise, such as walking and cycling (cross-training), can help reduce the burden on a single joint or bone and may also improve general endurance.66
2.1.5 Exercise Volume (Amount)
Exercise volume is the product of frequency, intensity, and duration of exercise. If a person is prescribed 30 min of exercise per session, he or she should be instructed to perform 30 min of exercise in 1 session, or 10 min of exercise 3 times in 1 session. The “Physical Activity Reference for Health Promotion 2013”71 recommends 8,000 to 10,000 steps/day or 23 exercises per week (exercise = MET × time) as the target amount of exercise for healthy adults.
2.1.6 Progression/Revision
In order to maintain exercise adherence and prevent complications such as exercise-related injuries, start with low-intensity and short-duration exercise, then gradually increase the intensity and duration.66 Especially in frail patients and patients with greater physical deconditioning, it is important to start with low-intensity exercise of less than 10 min per session and gradually increase the intensity by 1–5 min per session to reach the goal.72–75 The target training content should be set according to the activity level before the onset of disease, type of employment, hobbies, living environment, and the patient’s wishes. In patients with heart failure (HF), it is necessary to periodically review (revise) the exercise prescription appropriately; sometimes the intensity and duration of exercise need to be reduced according to changes in the disease state.
2.2 Resistance Training
In addition to improving muscular strength and endurance, resistance training during the recovery phase is prescribed to increase lean body mass, improve insulin sensitivity, prevent falls, improve self-efficacy, and prevent and manage chronic diseases such as low back pain and obesity.76 Exercise prescription for resistance training is mainly done in Phase II (recovery phase), especially for the upper extremities, starting 10–12 weeks after midline incision.65 Absolute contraindications to this training include: (1) unstable angina, (2) uncompensated HF, (3) uncontrolled arrhythmia, (4) severe pulmonary hypertension (PH: mean pulmonary artery pressure >55 mmHg), (5) severe and symptomatic aortic valve stenosis, (6) acute myocarditis, endocarditis, epicarditis, (7) uncontrolled hypertension (>180/110 mmHg), and (8) acute aortic dissection. The relative contraindications that should be discussed with a physician before training include: (1) major risk factors for coronary artery disease (CAD), (2) diabetes mellitus, and (3) uncontrolled hypertension (>160/100 mmHg).76 Resistance training should be prescribed separate from rhythmic low-intensity resistance exercise using rubber bands/balls at the bedside in the acute phase, or squats and calf raises at the bedside. Exercise prescription for resistance training is based on FITT (i.e., frequency, intensity, time, and type), as in the case of aerobic exercise prescription.65,66,76
2.2.1 Frequency
Ideally, the frequency should be with an interval of about 2 days between, 2–3 times per week.76,77
2.2.2 Intensity of Exercise
For the intensity of the exercise, measure the 1-repetition maximum (1RM) and prescribe using 40–60% of 1RM (%1RM method). In addition to this method, there are others such as the progression method, in which the weight is gradually increased from a moderate weight, and the estimated %1RM method, in which a certain intensity is determined and the approximate load is determined by the number of repetitions (as a guide, if 5 repetitions are possible, 90% of 1RM, if 8 repetitions, 80% of 1RM, if 12 repetitions, 70% of 1RM, etc.),76 and also the Borg scale can be used.
At the time of exercise initiation, it is recommended to start with an intensity of 10–15 repetitions without significant fatigue, of rating of perceived exertion (RPE) 11–13,76 or to start with preliminary training with an intensity of 30% 1RM or RPE <12.78
2.2.3 Time (Duration or How Long)
It is recommended to prescribe 1–3 sets of 8–10 different types of exercises, mainly for the large muscle group, for 30–45 min. A 90-s recovery period between sets avoids a cumulative increase in blood pressure.79
2.2.4 Type (Mode or What Kind)
In addition to dumbbells and iron arrays, pneumatic and hydraulic resistance, rubber bands, and body weight can also be used. A well-balanced training of large muscle groups should be prescribed,65,66,76 and older patients who have difficulty using a machine can be instructed to exercise with a rubber band.
2.2.5 Other Methods
In recent years, a training method in which the base of the extremity is moderately pressurized with a special pressure belt and blood flow is restricted has been used (KAATSU training); doing this for a short period of time and with a low-intensity load (20–30% of 1RM) has been attracting attention, but clinical studies in patients with cardiac disease are still limited.80,81
2.3 High-Intensity Interval Training
In recent years, increasing evidence has shown the feasibility and short-term effectiveness of HIIT, which involves alternating high- and moderate-intensity exercise.82–86 Table 17 shows examples of common protocols. In many cases, it is difficult to continue 3–4 min of high-intensity exercise (85–95% HRmax) from the beginning, so it is desirable to prepare individual protocols, such as starting with 70% HRmax intensity at the beginning of training.87 In patients with stable symptoms and no problems with conventional aerobic training, it is recommended to prescribe HIIT that takes into account the patient’s wishes, exercise capacity, severity of underlying disease, and comorbidities.65,88 The long-term effects of HIIT have not yet been established, and further studies are needed.
Table 17. Example of General Protocol for HIIT
| Training frequency |
3 times per week |
| Warming up |
Intensity: 60% of maximum HR, or 20–30% or maximum load (work rate) |
| Time: 5–10 min |
| Exercise intensity |
High intensity: 85–95% of maximum HR |
| Medium intensity: 60–70% of maximum HR |
| Interval |
3–4 min of high-intensity exercise × 4 times |
| 3–4 min of moderate-intensity exercise × 3 times |
| Cool down |
Intensity: 50% of the maximum HR or 20% of the maximum load (work rate) |
| Time: 5 min |
| Duration |
40–50 min |
| Type of exercise |
Bicycle ergometer, treadmill |
HIIT, high intensity interval training; HR, heart rate.
2.4 Respiratory Muscle Training
In patients with chronic HF, respiratory muscle strength is decreased, which is associated with HF severity, exercise capacity, and prognosis.89–91 In addition, inspiratory muscle training for chronic HF improves inspiratory muscle strength, exercise capacity, and quality of life (QOL).92 It is particularly effective in patients with decreased inspiratory muscle strength.93 In the field of cardiovascular surgery, it has been reported to be effective mainly in post-CABG patients. A meta-analysis reported that preoperative training improved inspiratory muscle strength, vital capacity, and expiratory volume in 1 second, and contributed to a reduced risk of postoperative pulmonary complications and a shorter postoperative hospital stay.94 However, the relationship of such training and exercise capacity or prognosis is unclear, and further evidence needs to be collected.
2.5 Neuromuscular Electrical Stimulation
Neuromuscular electrical stimulation (NMES) is a physical therapy that stimulates muscle contraction by percutaneously applying electrical current to the nerves. Although a meta-analysis of chronic HF patients has shown that NMES improved exercise capacity,95 it was inferior to aerobic exercise in improving peak V̇O2.95 Therefore, NMES is positioned as an alternative therapy for patients who are unable to do voluntary exercise sufficiently.
3. Exercise Training in Practice
In Japan, the standard CR program after hospital discharge is a combination of supervised outpatient CR (known as center-based CR or hospital-based CR) and home-based exercise training. The outpatient CR period is the recovery period from hospital discharge to the end of the insurance coverage period (150 days). The first half of this period will be aimed at recovery of physical function and lifestyle management under the supervision and counseling of multidisciplinary team, and the second half will be a transition period aiming at independence. The usefulness of center-based CR has been widely reported in patients after myocardial infarction (MI), after CABG surgery,96,97 and in chronic heart failure.98,99 In patients with CAD, there is a report that the more outpatient CR a patient participates in, the lower the risk of total mortality and MI over the subsequent 4 years.100
3.1 Exercise Type, Time, Intensity, and Frequency
The exercise modalities in outpatient CR are influenced by the size of the facility, but facilities should prepare the equipment to perform many modalities of exercise, such as aerobic exercise (including bicycle ergometers, treadmills, track walking, and aerobic exercises) and resistance training (including weight-bearing, rubber bands, dumbbells, and strength training machines); these methods are combined based on the patient’s risk, comorbidities, and wishes. Aerobic exercise is the mainstay, but in cases of significant muscle weakness, sufficient time should be spent on low-intensity resistance training.
The duration of exercise should be gradually increased according to the patient’s condition, with a daily target of 60 min, which is the upper limit, and the standard duration is 180 min/week. The intensity of exercise is recommended to set based on the HR response and the AT by CPX before and after hospital discharge. The frequency of exercise should be set at the number of times in the exercise prescription (e.g., 5 times/week) in combination with supervised outpatient CR and home-based exercise training. The frequency of outpatient CR is affected by the patient’s motivation, employment/family support, financial status, and access, but it is desirable for patients to participate in outpatient CR 2–3 times each week if possible, or at least once a week.
3.2 Supporting Changes During the Program
a. Support for Changes in Response During Exercise
In outpatient CR, if the Borg scale or HR decreases with the same aerobic exercise, it is considered to be an improvement in exercise capacity, and increasing the volume of exercise (mainly intensity and duration) should be considered. On the other hand, if the Borg scale or HR increases, it is considered to be a deterioration of exercise capacity. Whether the cause is insufficient total physical activity, or worsening chronic HF or myocardial ischemia, the countermeasures to be taken are completely different. Therefore, careful judgment should be made by referring to several indices, such as body weight, edema, resting HR, blood concentration of BNP, oxygen saturation during exercise, and ECG. If it is the former cause, ask whether there are any changes in physical activity in the home exercise environment, type, or time of day as a measure to increase the amount of home exercise (mainly frequency and duration); if the latter cause, take measures according to the medical condition (see “ IV.3.2 Chronic Heart Failure ”).
b. Adjustment of Exercise Prescription
At the time of discharge from the acute care hospital or introduction to the late recovery phase, an exercise stress test should be performed to evaluate the presence of myocardial ischemia, exercise capacity, and the safety zone of exercise training to determine the exercise prescription. The recommended moderate-intensity exercise intensity to be prescribed for patients with CVD is 40–60% of peak V̇O2
and 40–60% of HRR (k=0.4–0.6 in the Karvonen formula). In Japan, exercise prescription of the AT with CPX is widely used, and in this case, it corresponds to ≈40–60% of peak V̇O2. The advantage is that AT prescription can also be obtained from maximal stress testing.
In the early recovery phase, if the patient has a good understanding of CR and is judged to have a wide safety margin for exercise, the exercise stress test can be omitted and the exercise prescription is adjusted based on the HR and blood pressure responses during exercise and the RPE. At the time of transition to the maintenance phase, CPX is recommended for the purpose of exercise capacity evaluation and re-prescription.
The resting HR may gradually decrease during the program due to stabilization of the disease or the effect of β-blockers. In such a case, the intensity and duration of exercise required to reach a certain target HR gradually increase, but at the same time, if the exercise capacity is sufficiently improved by the rehabilitation effect, the program can be continued without difficulty. If it becomes practically difficult to reach the target HR, or as a result of such a gradual increase in exercise volume in chronic HF there is an increase in BNP concentration, probably caused by overload, necessary tests should be performed and the prescription should be revised. At the end of the CR program, CPX should be performed again, and the exercise prescription would be reviewed based on the results and progress during the program period to determine the final exercise prescription.
4. Target Diseases, Indications and Contraindications
4.1 Target Diseases and Indications in CR
In Japan, the target diseases in CR covered by insurance are AMI, angina pectoris, after implantation of arrhythmia devices and assistive devices, HF in general including PH, after heart transplantation, after cardiac surgery including transcatheter aortic valve implantation, after stent graft intervention including great vessel disease, and peripheral vascular diseases that cause intermittent claudication.101
4.2 Contraindications in Exercise Stress Test and Exercise Training
When conducting an exercise stress test, contraindications should be considered when there is a high risk that exercise stress will rapidly worsen the condition, such as severe AMI or unstable valvular disease. Contraindications to exercise testing are listed in Table 18.66,102,103 In the case of relative contraindications, the stress test should be performed when the benefit outweighs the risk.
Table 18. Contraindications of Diseases and Conditions for Exercise Stress Test
| Absolute contraindications |
| 1. AMI developed within 2 days |
| 2. Unstable angina not controlled with medical treatment |
| 3. Uncontrolled arrhythmia that causes symptoms or hemodynamic compromise |
| 4. Symptomatic severe aortic stenosis |
| 5. Uncontrolled symptomatic heart failure |
| 6. Acute pulmonary embolism or pulmonary infarction |
| 7. Acute myocarditis or pericarditis |
| 8. Acute aortic dissection |
| 9. Mental disorders associated with communication difficulties |
| Relative contraindications |
| 1. Left main coronary artery stenosis |
| 2. Moderate stenotic valvular heart disease |
| 3. Electrolyte abnormality |
| 4. Severe hypertension* |
| 5. Tachyarrhythmia or bradyarrhythmia |
| 6. Hypertrophic cardiomyopathy or other outflow tract obstruction |
| 7. Mental or physical impairment leading to inability to exercise adequately |
| 8. Advanced atrioventricular block |
*It is recommended that severe hypertension is considered as systolic blood pressure >200 mmHg and/or a diastolic blood pressure >110 mmHg, in principle. AMI, acute myocardial infarction.
Absolute and relative contraindications to aggressive exercise training are listed in Table 19.66,102,103 Even advanced HF of NYHA classification IV, which is not indicated for systemic exercise training, is an indication for low-intensity physical therapy and exercise training if the patient is stable with no ongoing deterioration. Exercise training is indicated in stable patients, even with severe CAD, who do not have a tendency to deteriorate rapidly. Even if exercise load is contraindicated at some point, it may be indicated later as the condition changes, so re-evaluation should be performed.
Table 19. Contraindications for Aggressive Exercise Training
| Absolute contraindications |
| 1. Unstable angina or low-threshold myocardial ischemia induced by slow walking (2 METs) on a flat surface |
| 2. Exacerbation of perceived heart failure symptoms of (e.g., dyspnea, easy fatigability) during the recent 3 days |
| 3. Uncontrolled arrhythmias causing hemodynamic abnormalities (ventricular fibrillation, sustained ventricular tachycardia) |
| 4. Severe valvular disease indicated for surgery, especially symptomatic aortic stenosis |
| 5. Severe left ventricular outflow tract stenosis due to obstructive hypertrophic cardiomyopathy, etc. |
| 6. Acute pulmonary embolism, pulmonary infarction and deep vein thrombosis |
| 7. Active myocarditis, pericarditis, endocarditis |
| 8. Acute systemic disease or fever |
9. Other diseases in which exercise training is contraindicated (acute aortic dissection, moderate or severe aortic aneurysm,
severe hypertension,*1 thrombophlebitis, embolism within 2 weeks, and serious organ diseases, etc.) |
| 10. Mental or physical impairment that interferes with safe implementation of exercise training |
| Relative contraindications |
| 1. AMI within 2 days of onset with high risk of serious complications*2 |
| 2. Left main coronary artery stenosis |
| 3. Asymptomatic severe aortic stenosis |
| 4. Advanced atrioventricular block |
5. Tachyarrhythmias or bradyarrhythmias with poorly controlled HR that are hemodynamically preserved (e.g., nonsustained
ventricular tachycardia, tachyarrhythmia with AF, tachyarrhythmia with atrial flutter, etc.) |
| 6. Recent stroke*3 |
| 7. Mental or physical impairment leading to inability to exercise adequately |
| 8. Systemic diseases that have not been corrected*4 |
| Not contraindicated |
| 1. Older patients |
| 2. Decreased LVEF |
| 3. Arrhythmia with hemodynamically preserved and well-controlled HR (e.g., AF, atrial flutter) |
| 4. Hemodynamically stable patients on intravenous inotropic drugs |
| 5. Placement of LVAD, cardiac implantable device (e.g., permanent pacemaker, ICD, CRT-D) |
*1As a general rule, defined as systolic blood pressure >200 mmHg or diastolic blood pressure >110 mmHg, or both. *2Transmural extensive anterior infarction, prolonged ST-segment elevation, etc. *3Includes transient ischemic attack. *4Anemia, electrolyte abnormality, thyroid disorder, etc. AF, atrial fibrillation; AMI, acute myocardial infarction; CRT-D, cardiac resynchronization therapy defibrillator; HR, heart rate; ICD, implantable cardioverter defibrillator; LVAD, left ventricular assist device; LVEF, left ventricular ejection fraction; MET, metabolic equivalent. (Source: based on JCS 2017 guideline,102 Fletcher GF, et al, 2013,66 JCS 2018 guideline.103)
5.1 Criteria for Terminating Exercise Training
The criteria for terminating exercise should be based on both patient-perceived factors and objective factors assessed by medical professionals, such as cardiologists and CR instructors.104 The criteria for terminating exercise should be divided into absolute and relative criteria based on the patient’s condition, comorbidities, and medications (Table 20). If a patient wishes to stop during exercising, it is an absolute criterion to terminate exercise immediately, regardless of the reason. Exercise should be stopped immediately, not only when the patient is unaware of dangerous symptoms during exercise, such as when the patient does not respond sufficiently to a call during exercise, but also when the medical staff cannot objectively detect a dangerous situation for any reason, such as when ECG electrodes are removed. Exercise should be discontinued immediately when ≥2 of the objective termination criteria occur simultaneously. If chest symptoms (chest pain, shortness of breath, palpitations) or other perceived symptoms (hypoglycemic attack, arrhythmia, dizziness, headache, leg pain, severe fatigue, mood disorder, arthralgia, muscle pain, etc.) worsen at the same exercise intensity, and if these perceived symptoms continue to worsen even when the exercise intensity is reduced, the exercise should be terminated.
Table 20. Criteria for Terminating Exercise Training
| Absolute termination criteria |
| • Patient’s wish to terminate exercise |
| • Patient is expected to be unable to detect dangerous symptoms during exercise, or patient has deteriorating consciousness |
• Incidence of cardiac arrest, severe bradycardia, fatal arrhythmia (ventricular tachycardia, ventricular fibrillation), or when these
cannot be ruled out |
• Sudden deterioration of vital signs or appearance of perceived symptoms (severe chest, abdominal/back pain, epileptic seizure,
loss of consciousness, hypotension, severe joint/muscle pain, etc.) |
| • On ECG, ST-segment elevation ≥1 mm in the induction without Q wave (other than aVR, aVR, V1 induction) |
| • Accidents (falls, trauma, equipment failure, etc.) |
| Relative termination criteria |
• Worsening of perceived chest symptoms or other symptoms (e.g., hypoglycemic attack, arrhythmia, dizziness, headache,
leg pain, severe fatigue, poor mood, joint or muscle pain) at the same intensity or with a decrease in exercise intensity |
| • Transcutaneous arterial oxygen saturation drops to less than 90%, or decrease of 5% or more from rest |
| • New arrhythmia or ST-segment depression ≥1 mm on ECG |
• Blood pressure decreased (systolic pressure <80 mmHg) or increased (systolic pressure ≥250 mmHg, diastolic pressure
≥115 mmHg) |
| • Appearance of bradycardia (HR ≤40/min) |
• If the patient is judged to be unable to follow instructions during exercise, or it is difficult to continue exercise training due to the
risk of falling |
5.2 Risk of Exercise Training
Regarding the safety reports of CR, events (AMI, cardiac arrest, or death) did not occur in 277,721 patient-hours in exercise training based on the CR program recommended by the Japanese Association of CR, and there was no difference in events between the exercise training group and the non-exercise group for HF.105,106 Therefore, it is considered that supervised exercise training can be safely performed with exercise prescriptions based on the exercise stress tests. However, patients with CAD who have residual ischemia are at high risk of developing acute coronary syndrome (ACS) or fatal arrhythmias during exercise, so caution is required (Table 20). For further information on the risks of exercise training and the evaluation for each CVD, please refer to the individual sections (Chapters IV and V).
The general risks of exercise training are falls and fractures. In particular, older and obese patients, who often have orthopedic diseases of the lumbar spine and lower limb joints, should be aware of the risk of exacerbation by exercise. There is a risk of venous thromboembolism in patients who have been bedridden for more than 3 days, have undergone major surgery within 4 weeks, have been confined to a wheelchair for a long period of time, are obese, or have cancer and are not anticoagulated. Patients with diabetic complications should be carefully evaluated for the various risks of complications. Patients with peripheral neuropathy may be at risk for worsening foot lesions with exercise training, thus it is important to refrain from load exercise and to provide adequate foot care.
5.3 Accident Prevention
From the viewpoint of preventing accidents during exercise, not only patients who are contraindicated for exercise training, but also those who are judged not to be given exercise training due to their physical condition on that day, should not perform such exercises. In patients with CVD, 72% of complications during exercise training have been reported to occur during warm-up and cool-down.107 To prevent accidents during exercise training, sufficient attention should be paid not only during exercise but also after exercise. At the beginning of exercise, start with preparatory exercises such as stretching, and perform a thorough warm-up. At the end of exercise, cool down by running/walking at a reduced intensity and speed, or by performing consolidation exercises such as stretching, and gradually return to resting blood pressure and HR to prevent hypotension and dizziness after exercise. To date, there are few reports on the prevention of accidents during exercise. As a reference for general information, the “Guidelines for safety management and promotion in rehabilitation medicine, 2nd edition”108 by the Japanese Association of Rehabilitation Medicine, may be used, but care should be taken because it does not describe the prevention of accidents during exercise in CR.
In exercise training, it is important to extract and share information about possible cardiovascular events and any complications that may occur in each patient as taken from the standpoint of each team member as an expert. It is important to explain these risks to patients in advance and to take measures to minimize the risks, while always being prepared for the unexpected.
IV. CR by Disease
1. AMI, ACS (Table 21)
Table 21. Recommendations and Levels of Evidence for CR in Patients With ACS
| |
COR |
LOE |
GOR
(MINDS) |
LOE
(MINDS) |
It is recommended to continue the recovery phase of CR to improve exercise
capacity, QOL, and prognosis |
I |
A |
A |
I |
It is recommended to perform acute-phase CR using a clinical pathway in the
acute phase |
I |
A |
B |
II |
It is recommended to perform exercise stress test assessment before or early
after hospital discharge to evaluate the prognosis, physical activity, and
necessity of additional treatment |
I |
A |
B |
II |
It is recommended that the attending physician actively encourage CR to
increase the adoption rate of outpatient CR |
I |
A |
A |
I |
It is recommended that a combined program of supervised outpatient CR with
home-based exercise training is provided for intermediate and high-risk
patients, if in stable condition |
I |
A |
A |
I |
Risk of post-AMI should be considered, and early discharge should be
considered in patients with low risk |
IIa |
A |
B |
II |
For patients who have achieved early reperfusion and have no obvious
complications, CR should be considered in the early acute phase in the CCU
for early ambulation |
IIa |
B |
B |
II |
| For exercise training, combining with resistance training should be considered |
IIa |
B |
B |
II |
ACS, acute coronary syndrome; AMI, acute myocardial infarction; CCU, coronary care unit; COR, class of recommendation; CR, cardiac rehabilitation; GOR, grade of recommendation; LOE, level of evidence; QOL, quality of life.
1.1 Purpose
Acute coronary syndrome (ACS) is classified into ST elevation myocardial infarction (STEMI), non-STEMI, and unstable angina.109 The purpose of CR for ACS is to safely achieve activities of daily living (ADL) and improve prognosis after hospital discharge by comprehensive intervention for patients early in their hospitalization.
1.2 Phase-Based Classification
In Japan, in-hospital deaths from acute myocardial infarction (AMI) remains high at ≈8%, cardiovascular events occur within 1 year in ≈20% of surviving patients, and major coronary events occur in ≈50% of patients with previous MI.110,111 It is known that cardiac rehabilitation (CR) improves exercise capacity, coronary risk factors and quality of life (QOL); in addition, it reduces cardiovascular recurrence/mortality and total mortality.6,112–114 It is important to continue CR seamlessly from the acute phase to the maintenance phase, but although outpatient CR reduces cardiovascular mortality, it does not improve total mortality.96 CR is recommended as Class I in the “2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction”,115 the “2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes”,116 the “2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST elevation”,117 and the “JCS 2018 guideline on diagnosis and treatment of acute coronary syndrome”.109
1.2.1 Acute Phase
In patients with severe circulatory failure, respiratory rehabilitation, prophylactic positional management, and prone therapy in addition to early CR should be performed within 3 days because physical function decline after ICU discharge (ICU-AW) affects QOL after discharge. Subsequently, the patient should be able to safely do basic self-care and education for secondary prevention should be started. Although bedrest does reduce the increase in heart rate and myocardial oxygen consumption, excessive bedrest is detrimental to the body’s condition.118 Rehabilitation is also useful in preventing orthostatic hypotension and thrombus formation associated with bedrest.109
In patients with adequate reperfusion after percutaneous coronary intervention (PCI) and no need for life support, bedrest should be minimized and limited to 24 h without complications.119 CR should be started after confirming the highest creatine kinase or troponin level after PCI. If the patient is able to pass the walk test, he/she should be transferred from the CCU/ICU to the general ward. If there is a high risk of cardiac rupture during the acute phase, exercise training with increased blood pressure should not be performed.109
1.2.2 Early Recovery Phase
The purpose of CR during this period is to return to work and society. It involves comprehensively and systematically performing (1) prognostic risk assessment by exercise stress test, (2) regular exercise training based on an exercise prescription, (3) secondary prevention education including lifestyle improvement, and (4) return to work and psychological counseling, etc. During hospitalization, patients are encouraged to get out of bed according to the program, and when they are able to walk 200 m without myocardial ischemia, they are transferred to endurance training. The exercise stress test is performed before or early after discharge to assess prognosis, prescribe physical activity, and determine the effects of medical therapy and PCI.104 Because the length of hospital stay has been shortened, it is necessary to select a program that suits the patient and facilitates a smooth transition to secondary preventive education and rehabilitation in the late recovery phase.
1.2.3 Examples of Inpatient Programs for AMI
An example of an inpatient rehabilitation program (clinical path) is shown in Table 22.109 Staged progression to the subsequent stage follows the criteria in Table 23. The time periods in the pathway are approximate and may be shortened or extended depending on the status of progression. The use of a clinical pathway can help standardize care and thereby improve the quality of care.109 The ESC guidelines suggest that low-risk patients (age ≤70 years, LVEF >45%, 1- or 2-vessel disease, successful revascularization, no persistent arrhythmia) should be considered for discharge within 48–72 h.120–122 Automatic referral is a program in which patients who require outpatient CR are automatically selected and the necessary procedures for participation in CR are performed before discharge; such an automatic referral may improve the participation rate123 and offer a seamless shift to the outpatient CR program.
Table 22. A Clinical Pathway for CR for AMI (National Cerebral and Cardiovascular Center)
| Day |
10 day
pathway |
Day 1 after PCI |
Day 2 |
Day 3 |
Day 4 |
Day 5 |
Day 6 |
Day 7 |
Day 8 |
Day 9 |
Day 10 |
14 day
pathway |
Days 8–10 |
Days 11–13 |
Day 14 |
| Goals |
|
Prevent complications of AMI and catheter-
related complications |
Prevent
complications
of AMI |
No myocardial
ischemia |
• No myocardial ischemia
• Patient can take the prescribed
drugs
• Patient can access information
about important points for daily
living after discharge |
• No myocardial
ischemia
• Patient can
understand
important points
for daily living
after discharge |
• No ischemia at
submaximal
exercise
• Patient can list
important points
for daily living
after discharge |
Discharge |
Bed rest
level |
|
After tourniquet
removal, allow the
patient to move
freely on the bed |
Allow the patient to
move freely in the
room |
Allow the
patient to walk
to the toilet,
after passing a
stress load test |
Allow the patient to walk around
the ward within a 200-m radius
of the room (encourage walking
200 m × 3 times/day) |
After passing a submaximal stress test, allow bathing and
walking freely in the rehabilitation ward |
| Hygiene |
|
• Face washing with
assistance
• Body cleaning |
• Face washing: using the sink
• Body cleaning, hair washing, foot
bathing |
• Face washing: using the sink
• Cleansing: assistance with the
back only and hair washing |
Shower bathing |
Patient
education |
|
• Explanation based on the AMI brochure
and patient path
- Rest degree, double stress avoidance
- Nurse call when symptoms appear
• Bowel control |
• Explanation of rest degree, double stress
avoidance, bowel control
• Explanation of CR
• Explanation of important points for daily living
• Medication counseling, self-administration |
• How to get
emergency
consultation
• How to manage
when having
heart attack
• Explanation of
medication, diet,
and smoking
cessation |
• Confirm instructional content |
Patient
care/stress
test |
10-day
pathway |
• Blood sampling
(every 3 h until CK
maximum value is
reached)
• ECG (every 6 h)
• Echocardiography
• Continuous
heparin
• Sheath removal
• Tourniquet
removal |
• Blood sampling
• ECG (every 6 h)
• Echocardiography
• Discontinue
heparin
• Remove the
urinary catheter |
• ECG (once
daily)
• 50-m
walking
stress testing |
• ECG (once
daily)
• 200-m
walking
stress testing |
Day 5 |
Day 6 |
Day 7 |
Day 8 |
Day 9 |
• ECG (once
daily)
• Entry testing
for CR |
• ECG (once daily) up to day 7
• Conduct exercise sessions in the CR room (if the patient has
not attended the program, conduct a Master’s single test or
allow having a bath as a trial) |
14-day
pathway |
Day 5 |
Day 6 |
Day 7 |
Days 8–10 |
Days 11–13 |
• ECG (once daily)
• Entry testing for CR (if
the patient has not
attended the program,
conduct a 500-m
walking stress testing
on day 6) |
• ECG (once daily) up to day 7
• Conduct exercise sessions in the CR room (if
the patient has not attended the program,
conduct a Master’s single test or allow having a
bath as a trial) |
AMI, acute myocardial infarction; CK, creatine kinase; CR, cardiac rehabilitation; ECG, electrocardiogram; PCI, percutaneous coronary intervention. (Adapted from Japanese Circulation Society, 2019.109)
Table 23. Criteria for Evaluating the Staging up of CR for Patients With AMI
| 1. No perceived symptoms such as chest pain, dyspnea or palpitation |
| 2. No increase in HR to ≥120 beats/min or by an increase of ≥40 beats/min |
| 3. No development of potentially dangerous cardiac arrhythmias |
| 4. No ischemic ST depression ≥1 mm, or significant ST elevation |
| 5. No change in systolic blood pressure ≥20 mmHg during the period before the patient was allowed to use a bedside commode |
| (the criteria for blood pressure are not used for patients 2 weeks after the onset of AMI) |
Patients who fail the exercise stress test should receive appropriate drug treatment or other measures, and undergo the same test on the following day. AMI, acute myocardial infarction; HR, heart rate.
1.2.4 Late Recovery Phase
The purpose of CR during this period is to reissue the exercise prescription based on the exercise stress test after hospital discharge, to evaluate the effect of treatment, to predict prognosis, and to conduct nutritional and psychological evaluations as well. The final step is self-management, including exercise programs, and re-instruction of not only exercise habits but also lifestyle habits that have been corrected, to ensure a smooth transition to the maintenance phase (Figure 4). After discharge from hospital, the desirable frequency of outpatient clinic is about once every 2 weeks, and a comprehensive program including exercise, smoking cessation, diet, and lifestyle intervention is provided to attain the pre-disease ADL. For exercise training, the duration and frequency of exercise should start at 10 min twice daily, with a gradual increase to 20–30 min twice daily, and an aim of 30–60 min twice daily in the stable phase; finally, a goal of at least 3 times per week, and preferably daily is desirable. In older patients, sufficient time for preparatory exercise should be taken to prevent cardiac events, trauma, and falls during exercise.
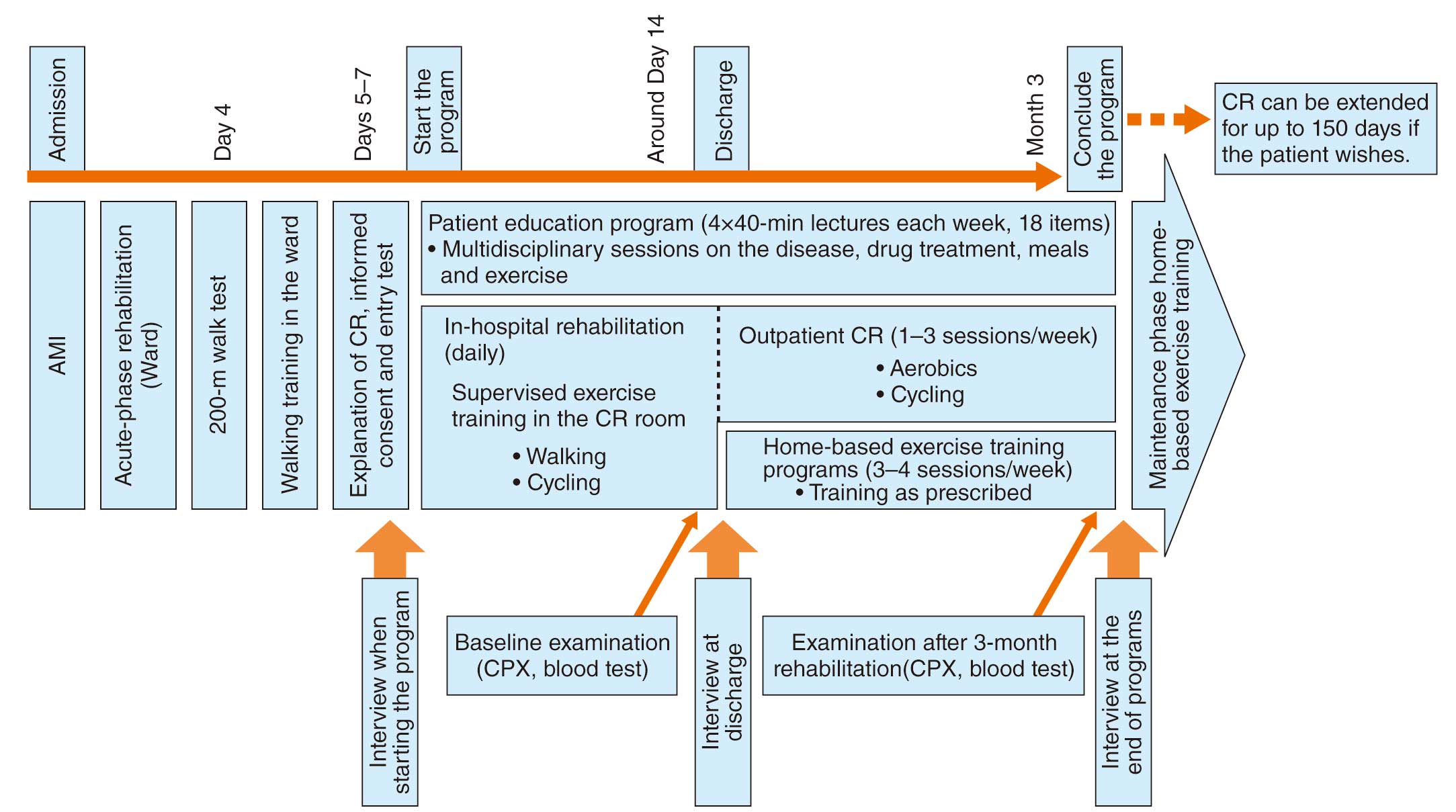
1.2.5 Maintenance Phase
The purpose of CR during this period is to prevent recurrence, to maintain the adequate physical and mental function obtained in the recovery phase, and to continue lifelong rehabilitation. CR is incorporated into daily life, and exercise training is provided at home or at a local exercise facility; secondary prevention through diet and smoking cessation is continued. Because the Japanese insurance coverage period is 150 days, it is necessary to design a program that can provide CR in the maintenance phase.
1.3 Resistance Training
Early resistance training can be safely performed even after STEMI; it improves QOL, exercise capacity, and endothelial function.124,125 The exercise prescription is 30–40% of 1 repetition maximum (RM) for upper extremity exercise, 50–60% for lower extremity exercise, with a load amount that can be repeated 10–15 times per set, with moderate fatigue, and a Borg scale of 11–13 “slightly strenuous” at the upper limit of 2–3 times per week. The resistance training uses rubber bands, ankle and wrist weights, dumbbells, free weights, pulleys, weight machines, and consists of 1–3 of 8–10 different exercises for the upper and lower extremities.
1.4 Current Status and Issues
The participation rate in outpatient CR after AMI is not high, even in Western countries, with 14–35% in the USA, 29% in the UK, and 23% in France, but it is remarkably low in Japan at only 4–8%.126,127 Factors that contribute to participation in exercise training include external factors (safety, transportation, and social support), internal factors (physical function, cognitive function, and emotion), and cultural factors, with cognitive function and social factors being particularly important.128 The percentage of ACS patients who are recommended to have CR by their physicians has been as low as 32%.129 In the future, it is necessary to enhance outpatient CR facilities and to create a simple and easy introduction system for patients to participate.
The American Association of Cardiovascular and Pulmonary Rehabilitation (AACPR)/AHA/ACC has issued a statement on the introduction of Home-Based CR,130 which should be considered for patients who have difficulty visiting a hospital for outpatient CR.
2. Stable Angina Pectoris With/Without PCI (Table 24)
Table 24. Recommendations and Levels of Evidence for CR in Patients With Stable Angina Pectoris
| |
COR |
LOE |
GOR
(MINDS) |
LOE
(MINDS) |
It is recommended to perform comprehensive CR for patients with SAP unless
contraindicated |
I |
B |
B |
II |
It is recommended to perform comprehensive CR for patients with CAD after
elective PCI unless contraindicated |
I |
A |
A |
I |
Exercise training alone or in combination with a disease management program
should be considered to improve anginal symptoms |
IIa |
B |
B |
II |
Performing the exercise stress test 1–3 days after PCI and initiating exercise
training should be considered |
IIa |
B |
C1 |
II |
CAD, coronary artery disease; COR, class of recommendation; GOR, grade of recommendation; LOE, level of evidence; PCI, percutaneous coronary intervention; SAP, stable angina pectoris.
2.1 Pathology and Indications
CR is useful for any coronary artery disease (CAD), not only after AMI, but also after PCI such as after MI, stable/unstable angina, and after coronary artery bypass grafting (CABG).131,132 For stable angina, increased daily activity is closely associated with lower all-cause/cardiovascular death.131,132 The CR program includes comprehensive nutritional counseling, psychological counseling, management of coronary risk factors and exercise training, to reduce cardiovascular events while improving QOL by improving exercise capacity. In addition, CR has been reported to improve residual risk.133,134 In low-risk patients (1- or 2-vessel disease excluding proximal left anterior descending branch disease), the J-SAP study in Japan showed no significant difference in cardiac death or ACS incidence between the PCI group and the medical treatment group during a 3-year follow-up.135 In the COURAGE study in Europe and the USA, there was no significant difference in death or non-fatal MI in 3 years between patients with stable CAD who received optimal medical therapy alone, including lifestyle intervention such as exercise, diet, and smoking cessation, and those who received optimal medical therapy plus PCI.136 The results suggest that PCI alone is insufficient to improve the prognosis of patients with stable angina, and that comprehensive management with exercise training is important.
2.2 Effect on Cardiovascular Events and Prognosis
The level of evidence for the efficacy of exercise training for stable angina is not as high as for AMI. Based on the evidence to date, the ACC/AHA131,137 and ESC132 guidelines for angina pectoris recommend it as Class I, while the UK National Institute for Health and Care Excellence (NICE) guidelines for stable angina state that, although short-term effects of diet and exercise have been studied, there is little research on recurrence and prognosis, and there is no clinical or medical economic evidence for CR in angina.138 A Cochrane review showed that CR improved the rate of revascularization, exercise capacity, and cardiovascular hospitalization in patients with stable angina, but did not reduce all-cause death or the incidence of MI.139 Among patients with angina, those with low exercise capacity, residual symptoms on exertion, and low cardiac function in particular are good candidates for CR.
2.3 Effect on Angina Symptoms
Exercise training improves exercise capacity, raises the ischemic threshold, and improves QOL.131,132 These mechanisms include direct improvement of coronary artery endothelial cell function and indirect improvement of dyslipidemia and antihypertensive effects, which may improve vasodilation, coronary flow reserve, and myocardial perfusion, which is reduced by ischemia. Interventional trials using exercise training and lifestyle modification have shown that exercise training improves angina symptoms and inhibits or improves the progression of coronary artery stenosis.140,141 Interventional trials with exercise training and lifestyle modification have been shown to reduce or improve the progression of coronary artery stenosis as well as improve angina symptoms.142
2.4 Indications After PCI
Although there is some evidence for CR in patients after PCI, there are no clear criteria regarding the timing of CR in the ACCF/AHA Guidelines “Core components of cardiac rehabilitation/secondary prevention programs: 2007 Update”143 or in the European Association of Cardiovascular Prevention and Rehabilitation (EACPR) “Secondary prevention through cardiac rehabilitation: from knowledge to implementation”.144 Balady et al reported that exercise testing to a mean of 71% of the predicted maximum HR within 3 days (mean 38 h) of uncomplicated PCI resulted in no cardiac events within 48 h and a faster return to work compared with the non-exercise group.145 Soga et al reported on 800 patients who underwent submaximal-load (Borg scale 11–13) CPX on the day after stent implantation and early introduction of exercise training, and the acute event rate was similar to that of the control group, suggesting that it can be performed safely.146 CR can be started the day after PCI, and exercise training can be started immediately if the intensity of exercise is less than 6–7 METs.
2.5 Exercise Training in Practice
Exercise training should be based on the results of CPX, and an individualized program should be implemented for each patient. The recommended exercise is moderate- to high-intensity aerobic exercise for 30–60 min/day, at least 5 days per week.143,144 Exercise intensity should be limited to 70–85% of the HR at which ST depresses 1 mm or at 10 beats/min lower HR for asymptomatic myocardial ischemia. The intensity should be aimed at the anaerobic threshold (AT: 40–60% of peak V̇O2), Karvonen’s formula [(maximum HR − resting HR) × (0.4 − 0.6) + resting HR], and a rating of perceived exertion (RPE) Borg scale of 11–13 (Table 25). A supervised exercise program is desirable in patients with low cardiac function, heart failure symptoms, low exercise threshold, and high residual ischemia.
Table 25. Setting of Exercise Intensity in CR for Patients With Angina Pectoris and After PCI
| • If asymptomatic myocardial ischemia, 70–85% of the HR at which ST depresses 1 mm, or 10 beats/min lower HR |
| • AT level (≈40–60% of peak V̇O2) |
| • Karvonen’s formula [(maximum HR − resting HR) × (0.4 – 0.6) + resting HR] |
| • Target of the RPE: Borg scale 11–13 |
AT, anaerobic threshold; HR, heart rate; peak V̇O2, peak oxygen uptake; RPE, rating of perceived exertion.
The main type of exercise should be endurance exercise; careful stretching and warm-up exercises are important to prevent angina attacks during exercise. Combination resistance training with dead weight and cool down is used. Other types of exercise and sports can be included if the intensity is below 60% of the AT level or maximal exercise capacity and does not cause ischemic signs. In a meta-analysis, resistance exercise performed 2–3 times per week in addition to aerobic exercise was shown to be more effective than aerobic exercise alone in reducing body fat and improving upper and lower limb muscle strength in CAD patients. In addition, cardiopulmonary function and QOL are known to improve.147
Before the start of CR, it is important to evaluate the presence or absence of coronary artery stenosis (complete revascularization), which defines the oxygen supply, and the presence or absence of comorbidities or conditions, such as heart failure, that can cause hypoxia. It is also important to watch for excessive increases in blood pressure and HR during exercise. Whenever possible, CPX should be performed, and exercise training should be performed according to the exercise prescription.
3. Acute/Chronic Heart Failure
3.1 Acute Heart Failure (Acute Phase to Early Recovery Phase) (Table 26)
Table 26. Recommendations and Levels of Evidence for CR in Patients With Acute HF
| |
COR |
LOE |
GOR
(MINDS) |
LOE
(MINDS) |
An educational program on relapse prevention and self-management is
recommended for all patients with acute heart failure |
I |
C |
C1 |
VI |
Early ambulation is recommended to shorten hospital stay and prevent ADL
decline with attention to hemodynamic deterioration |
I |
C |
B |
IVa |
For all patients with acute heart failure, CR programs should be considered
after the patient’s condition has stabilized |
IIa |
C |
C1 |
VI |
After the stabilization of hemodynamic condition, exercise training should be
considered |
IIa |
C |
C1 |
IVb |
Rehabilitation, such as low-intensity resistance training under close
supervision, may be considered for hemodynamically stable patients with
heart failure receiving intravenous inotropic drugs |
IIb |
C |
C1 |
V |
ADL, activities of daily living; COR, class of recommendation; CR, cardiac rehabilitation; GOR, grade of recommendation; HF, heart failure; LOE, level of evidence.
3.1.1 Purpose and Effect
The purpose of CR for patients with acute heart failure (HF) is to (1) prevent the adverse effects of prolonged bed rest (e.g., decline in physical function, decline in cognitive function, delirium, pressure ulcers, pulmonary embolism) due to early ambulation, and (2) develop and implement a plan for early and safe discharge and for the prevention of rehospitalization. In particular, early initiation of CR for older HF patients is associated with a shorter hospital stay,148 maintenance of ADL at discharge, and the prevention of rehospitalization after discharge.149 Continuation of exercise training has been proven to improve the long-term prognosis,150,151 so it is extremely important to motivate patients to participate in CR and to continue CR after discharge.
3.1.2 Ambulation Program
The ambulation program should proceed from the early stage of hospitalization, while confirming that hemodynamic values do not deteriorate, to prevent deterioration of physical function and deconditioning due to excessive rest. The specific ambulation program is shown in Table 27. For severe patients who cannot be weaned from catecholamines, the introduction of low-intensity resistance training on the bed to maintain muscle strength and combination of Waon therapy are considered. Patients with low ADL before admission, such as frail patients, are transferred to the rehabilitation room after intravenous infusion management is completed, and strength training and training for independence in ADL are performed. (For details, see Chapter V.1. Older Patients With Heart Disease.)
Table 27. Acute Phase Ambulation Program for Patients With Acute HF
| Stage |
Permissible degree
of rest |
Rehabilitation
venue |
Target sitting period*
(total hours/day) |
Stage up
loading test |
| 1 |
Bed rest |
On bed |
Reclined seating |
Sitting upright |
| 2 |
Sitting upright |
Bedside |
1 h |
Walk test (free speed)
10 m |
| 3 |
Free within room |
Bedside |
2 h |
Walk test (free speed)
40 m |
| 4 |
Walk to toilet |
Ward |
3 h |
Walk test (free speed)
80 m |
| 5 |
Walk freely within ward
(up to 80 m) |
Ward
(rehabilitation room) |
3 h |
Walk test (free speed)
80 m × 2–3 times |
| 6 |
Walk freely within ward |
Ward
(rehabilitation room) |
3 h |
6-minute walk test |
*It is important not to place the patient in bed rest unnecessarily. HF, heart failure. (Adapted from Izawa H, et al, 201910 with modification.)
3.1.3 Exercise Program
After the patient has progressed through the ambulation program and is able to perform the 6-minute walk test, exercise training should be initiated, making sure that there is no exacerbation of HF symptoms and no contraindication to exercise training (Table 28).10 As an exercise program, warm-up and cool-down are set before and after the exercise, which consists of low-intensity aerobic exercise and resistance training.10 The aerobic exercise should be started at a low intensity of 5–10 min of indoor walking or 0–20 W × 5–10 min of work rate on a bicycle ergometer, and the frequency and duration of exercise should be gradually increased according to the progress of perceived symptoms and physical findings. A Borg scale of 11–13 should be used as the initial exercise intensity. Resistance training is performed with rubber bands, ankle and wrist weights, dumbbells, and free weights, and is based on low intensity with a Borg scale of ≤13. The number of repetitions and sets are gradually increased, starting with 5–10 repetitions per set.
Table 28. Contraindications of Conditions and Symptoms for Exercise Training in Patients With HF
| Absolute contraindications |
| 1. Exacerbation of perceived symptoms within the previous 3 days |
| 2. Unstable angina or low-threshold myocardial ischemia |
| 3. Severe valvular heart disease indicated for surgery, especially symptomatic aortic stenosis |
| 4. Severe left ventricular outflow tract obstruction |
5. Poorly controlled arrhythmias causing hemodynamic abnormalities (ventricular fibrillation, sustained ventricular
tachycardia) |
| 6. Active myocarditis, pericarditis, endocarditis |
| 7. Acute systemic disease or fever |
8. Other diseases in which exercise is contraindicated (acute aortic dissection, moderate or severe aortic aneurysm, severe
hypertension, thrombophlebitis, embolism that developed in the recent 2 weeks, and serious multiple organ damage |
| Relative contraindications |
| 1. NYHA Class IV |
| 2. Exacerbation of perceived symptoms or increase in body weight ≥2 kg in the previous week |
| 3. Moderate left ventricular outflow tract obstruction |
4. Tachyarrhythmia or bradyarrhythmia with poorly controlled HR with hemodynamic preservation (nonsustained ventricular
tachycardia, tachycardiac AF, tachycardiac atrial flutter, etc.) |
| 5. Advanced atrioventricular block |
| 6. Exacerbation of exercise-induced perceived symptoms (fatigue, dizziness, excessive sweating, dyspnea, etc.) |
Note: “Exercise training” refers to aerobic exercise and resistance training with sufficient exercise intensity for the purpose of improving exercise capacity and muscle strength. AF, atrial fibrillation; HF, heart failure; HR, heart rate. (Source: based on Izawa H, et al, 2019.10)
3.2 Chronic Heart Failure (Late Recovery Phase to Maintenance Phase)
Comprehensive CR, including exercise training, is one of the most important treatments for HF because it not only improves exercise capacity and QOL in patients with chronic HF,150 but also leads to left ventricular reverse remodeling,152 improved autonomic function,153 improved vascular endothelial function,154 and reduced rates of HF and rehospitalization for all causes.150,151 It is considered one of the most important treatments for HF.
3.2.1 Assessment of Exercise Capacity (Table 29)
Table 29. Recommendations and Levels of Evidence for the Methods of Exercise Capacity Assessment in HF Patients Undergoing CR
| |
COR |
LOE |
GOR
(MINDS) |
LOE
(MINDS) |
It is recommended to assess NYHA classification, exercise capacity,
psychological status, comorbidities, cognitive ability, and social environment,
by interview |
I |
B |
B |
IVa |
It is recommended to use CPX to assess the severity and prognosis of HF, to
determine the effectiveness of treatment and to determine the indications for
heart transplantation and other therapies |
I |
B |
B |
II |
It is recommended to use CPX to differentiate between causes of dyspnea
and easy fatigability during exertion |
I |
B |
B |
IVb |
Assessment of sarcopenia and frailty should be considered in patients
suspected of having either |
IIa |
B |
B |
IVa |
If CPX cannot be performed, 6MWD should be considered to evaluate
prognosis and determine the effect of treatment |
IIa |
B |
B |
II |
| It should be considered to use CPX to develop the exercise prescription |
IIa |
B |
B |
II |
COR, class of recommendation; CPX, cardiopulmonary exercise testing; CR, cardiac rehabilitation; GOR, grade of recommendation; HF, heart failure; LOE, level of evidence.
Methods of evaluating exercise capacity in HF patients include the NYHA classification, questionnaires such as the Specific Activity Scale (SAS), the 6-minute walk distance (6MWD), and cardiopulmonary exercise testing (CPX). Evaluation of exercise capacity is used to predict the prognosis, stratify risks associated with exercise training, determine exercise prescription and its effects, and determine indications for and effects of heart transplantation and other advanced therapies. The 6MWD is also a popular method for assessing exercise capacity in HF patients because it does not require special equipment. The 6MWD correlates with peak V̇O2
and it is as prognostic as peak V̇O2
and the V̇E vs. V̇CO2
slope,155 and thus it is used to determine the efficacy of various therapies.156 In older patients, the 6MWD is related to respiratory, circulatory, and metabolic functions, as well as to limiting factors specific to older patients, such as lower limb muscle strength and balance.156–158 In addition, in older patients with heart disease, usual gait speed and 6MWD show a strong positive correlation,159,160 and the prognostic value is also reported to be similar.159
3.2.2 Significance and Effectiveness (Table 30)
Table 30. Recommendations and Levels of Evidence for CR in Patients With Chronic HF
| |
COR |
LOE |
GOR
(MINDS) |
LOE
(MINDS) |
In patients with HFrEF, it is recommended to use exercise training to improve
subjective symptoms and exercise capacity, improve QOL, and reduce
rehospitalizations |
I |
A |
A |
I |
A comprehensive CR program with a multidisciplinary team should be
considered for all patients without contraindications |
I |
A |
A |
I |
Exercise training should be considered to improve the life expectancy of
patients with HFrEF |
IIa |
B |
B |
II |
In patients with HFpEF, exercise training should be considered to improve
subjective symptoms and exercise capacity |
IIa |
B |
A |
I |
In patients with advanced deconditioning or reduced physical function,
resistance training should be considered to improve ADL and QOL |
IIa |
C |
B |
IVb |
Note: For patients receiving intravenous inotropic drugs, see Tables 26 and 51. ADL, activities of daily living; COR, class of recommendation; CR, cardiac rehabilitation; GOR, grade of recommendation; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; LOE, level of evidence; QOL, quality of life.
The major mechanisms of reduced exercise capacity in HF with reduced ejection fraction (HFrEF) are mainly peripheral factors such as skeletal muscle mass loss, metabolic abnormalities, decreased vasodilatation, and increased ergoreceptor reflex161 It has been suggested that skeletal muscle damage is one of the limiting factors for exercise capacity in HF with preserved ejection fraction (HFpEF).162 Improvement in exercise capacity in HF patients is mainly due to improvement in peripheral function.163
CR based on exercise training for HFrEF is effective in improving prognosis,106,151 exercise capacity, and QOL,164 and reducing the risk of all-cause rehospitalization,150 and the risk of rehospitalization for HF.150,151 In HFpEF, exercise training has been shown to improve peak V̇O2
and QOL, and to be associated with better prognosis151,165 A multicenter, retrospective observational study in Japan151 found that CR participation was associated with a higher risk of events in patients with HFpEF and mild to moderate frailty, regardless of age, sex or comorbidities.166 Because the rate of uptake of outpatient CR in patients with HF is extremely low in Japan, widespread use of CR is a major challenge.
3.2.3 Indications, Contraindications, and Safety
Exercise training is indicated for patients with stable, controlled HF without worsening of perceived symptoms or physical findings of HF in at least the recent 3 days, without excessive fluid retention or dehydration, and with NYHA class II–III.
The absolute and relative contraindications to exercise training in HF are listed in Table 28. In some patients with NYHA class IV, low-intensity resistance training, ADL exercises, and local skeletal muscle training such as neuromuscular electrical stimulation are applicable.78,167 Older age, reduced LVEF, using a supplemental cardiac assist device, and after implantation of an implantable cardioverter defibrillator (ICD) are not contraindications for exercise training.
Fatal events directly related to exercise training for HF have not been reported in exercise training for HF in more than 60,000 person-hours.98 The incidence of major cardiac events does not differ between the exercise training group and non-exercise training group.102,106 According to a report from Japan,168 the incidence of cardiac events (worsening HF, hypotension, arrhythmia) that caused patients to drop out of the program was 5% in exercise training for patients with moderate to advanced HF with an average LVEF of 25%, and the incidence of cardiac events that required temporary suspension of exercise training was 8%. Predictors of cardiac events are listed as left ventricular enlargement (end-diastolic diameter ≥65 mm), high BNP, poor exercise capacity, increased ventilation during exercise, and after implantation of a pacemaker or ICD.
3.2.4 Exercise Program (Table 31)
Table 31. Recommendations and Levels of Evidence for Individualized CR Programs for Patients With Chronic HF
| |
COR |
LOE |
GOR
(MINDS) |
LOE
(MINDS) |
It is recommended to provide individualized exercise and physical therapy to
patients with reduced physical function |
I |
B |
A |
II |
Comprehensive CR program from a multidisciplinary team is recommended
for all patients without contraindications |
I |
A |
A |
I |
In patients who are deconditioned and have difficulty with exercise training,
neuromuscular electrical stimulation of lower extremity skeletal muscles should
be considered |
IIa |
B |
B |
I |
Inspiratory muscle training should be considered for patients with decreased
inspiratory muscle strength |
IIa |
B |
B |
I |
High-intensity interval training may be considered to improve exercise capacity
in patients with low-risk, stable HFrEF |
IIb |
B |
C1 |
II |
Note: There are few reports on high-intensity interval training, neuromuscular electrical stimulation, and respiratory muscle training in Japan. COR, class of recommendation; CR, cardiac rehabilitation; GOR, grade of recommendation; HFrEF, heart failure with reduced ejection fraction; LOE, level of evidence.
Exercise training for chronic HF is basically performed according to the exercise prescription (Table 32).10 It should be performed under supervision, especially in older patients, those with markedly reduced left ventricular function, and those with potentially dangerous arrhythmias or ischemia. In low-risk stable patients who have not had an exacerbation of HF for more than 2–3 months and who can safely perform the above-listed intensity exercise, prescriptions for higher intensities, such as 60–80% of peak V̇O2,102,168 or high-intensity interval training (HIIT),82,169,170 it should be considered under supervision. (For more information on HIIT, see Chapter III.2.3 High-Intensity Interval Training.)
Table 32. Exercise Program for Patients With Chronic HF
| Composition |
| An exercise program consisting of aerobic and resistance exercise, including warm-up before exercise and cool-down after exercise |
| Aerobic exercise |
| The frequency, intensity, duration, and modality of aerobic exercise will be prescribed based on the results of CPX |
| • Modality: walking, bicycle ergometer, treadmill, etc. |
| • Frequency: 3–5 times per week (≈3 times per week in severely affected patients) |
| • Intensity: 40–60% of peak V̇O2, 30–50% of HRR, 50–70% of maximal HR, or HR at the AT |
→ In low-risk patients stable for at least 2–3 months without worsening HF and can safely perform the above-intensity exercise program,
consider prescribing higher intensity under supervision (e.g., equivalent to 60–80% of peak V̇O2, or HIIT) |
| • Duration: Start with 5–10 min twice daily and gradually increase to 20–30 min/day. Be careful not to exacerbate HF |
| If CPX cannot be performed |
| • Intensity: Borg scale 11–13, HR at rest +20–30 beats, and HR during exercise is less than 120 beats/min |
| • Modality, frequency, and duration are the same as for exercise prescription based on the results of CPX |
| Resistance training |
| • Modality: rubber bands, ankle and wrist weights, dumbbells, free weights, weight machines, etc. |
| • Frequency: 2–3 times per week |
| • Intensity: Low to moderate intensity |
30–40% of 1RM for upper extremity exercises, 50–60% of 1RM for lower extremity exercises, with a load capacity of 10–15 repetitions per
set, Borg scale ≤13 |
| • Duration: 1–3 sets of 10–15 repetitions |
| Indicators suggesting an excessive exercise load |
| • Suspected fluid retention for weight gain ≥2 kg in 3 days (immediate response) and 7 days (increased surveillance) |
• Decrease in systolic blood pressure ≥20 mmHg despite gradual increase in exercise intensity, with symptoms/signs of poor peripheral
circulation, such as peripheral coldness |
| • Exacerbation of perceived chest symptoms at the same exercise intensity |
| • Increase in HR by ≥10 beats/min, or increase in Borg scale by ≥2 levels at the same exercise intensity |
| • Decreased transcutaneous arterial oxygen saturation to <90% or decreased by ≥5% from rest |
| • Appearance of new arrhythmia or ST depression ≥1 mm on ECG |
| Precautions |
• As a general rule, exercise should be performed under supervision at the beginning, and should be performed with a combination of
supervised and non-supervised (home exercise training) in the stable period |
| • During the course, always pay attention to perceived symptoms, and changes in body weight and blood BNP or NT-proBNP levels |
| • Exercise capacity should be assessed periodically by symptom-limited exercise test, and exercise prescription should be reviewed |
• Check for the presence of new comorbidities (orthopedic disease, PAD, cerebrovascular/neurological disease, pulmonary disease, renal
disease, psychiatric disease, etc.) and changes in treatment that may affect exercise |
AT, anaerobic threshold; CPX, cardiopulmonary exercise testing; HR, heart rate; HRR, heart rate reserve; PAD, peripheral arterial disease; peak V̇O2, peak oxygen uptake; RM, repetition maximum. (Source: based on Izawa H, et al, 2019.10)
For patients with reduced physical activity capacity due to sarcopenia or frailty, individualized exercise and physical therapy based on the results of individual patient assessment may possibly improve exercise capacity and reduce the risk of rehospitalization for HF.151,171 Various types of training are effective for patients who are unable to undertake adequate exercise training.78,167,172 (For information on CR for older people, see Chapter V.)
3.2.5 Periodic Observation and Evaluation
The appropriateness of exercise training for chronic HF is assessed from the perspective of both efficacy and safety (Figure 5).173 Exercise capacity assessments, such as peak V̇O2
and 6MWD, motor function indices such as the Short Physical Performance Battery, gait speed, and overall muscle strength, ADL and instrumental ADL (IADL) indices, and health-related QOL (HRQOL) indices (Kansas Cardiomyopathy Questionnaire, SF-36, etc.), are used depending on the patient’s goals.174,175 It can be said that the effect of exercise training is evident if exercise capacity is maintained.
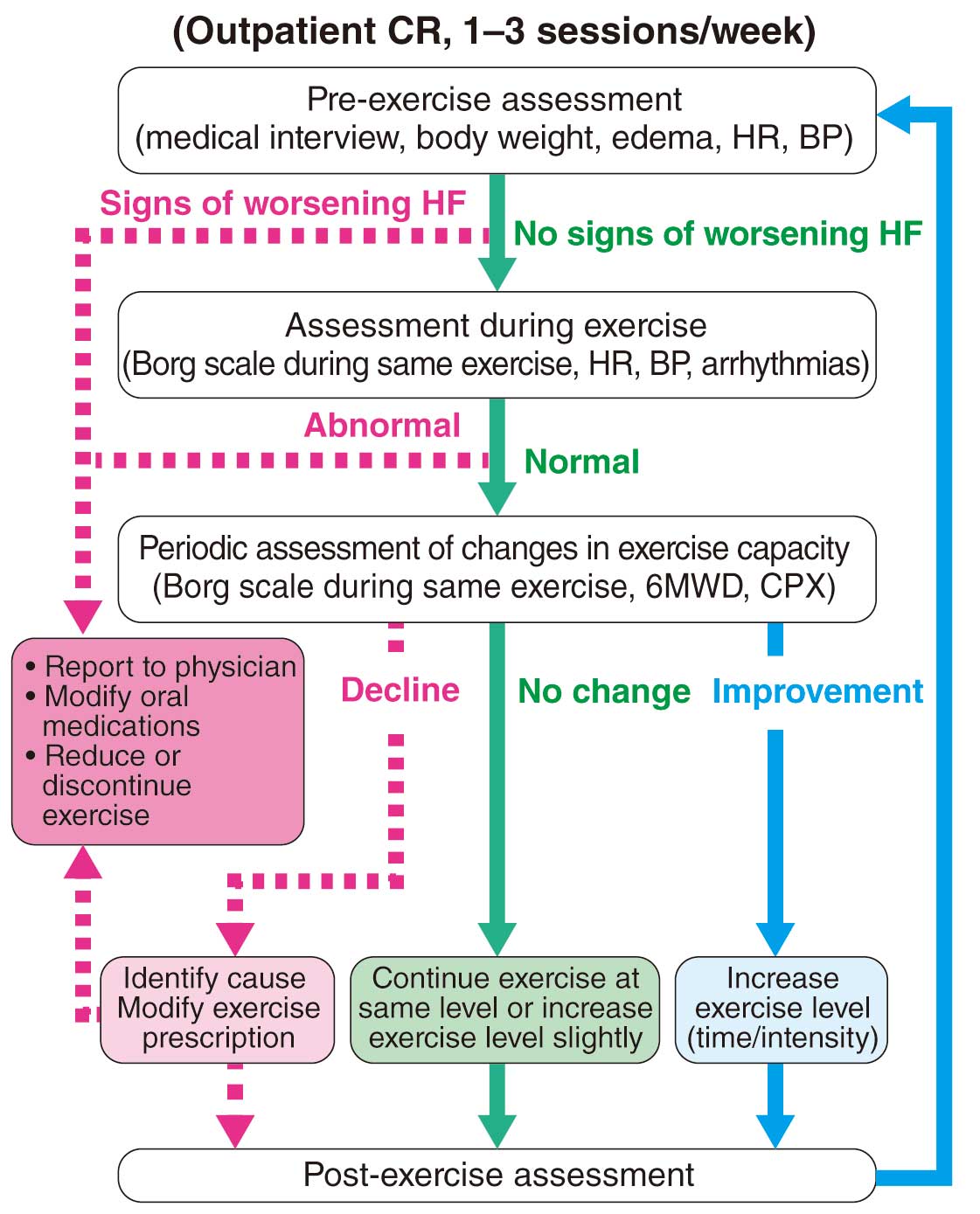
From a safety standpoint, hemodynamic indices and symptoms during and before/after exercise, daily monitoring of HF signs, and periodic BNP (NT-proBNP) measurement should be used to guide the outpatient CR with reference to indicators of overloading (Table 32). In addition, the CR team plays a central role, because it is multidisciplinary and can examine the factors that exacerbate HF and provide patient education on these critical factors from different perspectives.176,177 For details of patient education and management, see Chapters VII and VIII.
4. After Cardiac Surgery (Table 33)
Table 33. Recommendations and Levels of Evidence for CR After Cardiac Surgery
| |
COR |
LOE |
GOR
(MINDS) |
LOE
(MINDS) |
Exercise training is recommended to improve perceived symptoms and
exercise capacity and to correct coronary risk factors after CABG |
I |
A |
A |
I |
Exercise training is recommended to improve perceived symptoms and
exercise capacity in postoperative valvular heart disease |
I |
A |
B |
II |
Exercise training should be considered to improve long-term prognosis after
CABG |
IIa |
B |
B |
I |
| Early ambulation should be considered after cardiac surgery |
IIa |
B |
B |
I |
| A chest belt should not be worn routinely after cardiac surgery |
III
(Harm) |
C |
C1 |
IVb |
CABG, coronary artery bypass grafting; COR, class of recommendation; CR, cardiac rehabilitation; GOR, grade of recommendation; LOE, level of evidence.
4.1 Effectiveness
Exercise training after cardiac surgery has been proven as effective in various aspects, such as exercise capacity, coronary risk factors, autonomic activity, cardiac and peripheral function, QOL, mental health, rehospitalization rates, and healthcare costs, and thus is strongly recommended in the guidelines of Europe and the USA.178,179 As a prognostic study, a meta-analysis of 18 randomized controlled trials (RCTs) of adults after cardiac surgery showed no mortality benefit from rehabilitation vs. usual care. However, a systematic review of 15 prospective observational studies showed that total mortality was significantly lower in the rehabilitation group.180 Results of a Japanese multicenter study on the effectiveness of outpatient CR after CABG showed that major cardiovascular events, total rehospitalizations, rehospitalization for cardiac diseases, and coronary events were significantly lower in the active participation group than in the nonparticipation group.181 To date, there is no evidence of improved long-term outcomes after cardiac surgery other than CABG.
4.2 Rehabilitation in Practice
4.2.1 Acute Phase
Early postoperative rehabilitation is effective in improving physical function, HRQOL, peripheral skeletal muscle, respiratory muscle strength, duration of ventilator use, ICU stay, and hospital stay.182
a. Criteria for Early Mobilization
When starting ambulation, refer to Table 34 and the criteria in the guideline of the Japanese Society of Intensive Care Medicine.183 If the benefits of ambulation are judged to be significant, the patient should be able to get out of bed under close monitoring, even if all criteria are not met.
Table 34. Criteria for Early Mobilization
| The patient can start to get out of bed if the following conditions are ruled out: |
| 1. Condition of low output syndrome (LOS), |
(1) the patient is wearing a life-support system such as a ventilator, intra-aortic balloon pumping device or
percutaneous cardiopulmonary support device |
| (2) the patient is receiving a large amount of catecholamine such as noradrenaline |
| (3) systolic blood pressure is < 80–90 mmHg under catecholamine administration |
| (4) cold extremities and cyanosis |
| (5) metabolic acidosis |
| (6) urine output <0.5–1.0 mL/kg/h for >2 h |
| 2. Swan-Ganz catheter insertion |
| 3. Resting HR ≥120 beats/min |
| 4. Unstable blood pressure (blood pressure decreases only by changing position) |
| 5. Hemodynamically unstable arrhythmia (newly developed AF, ventricular arrhythmia with Lown IVb or greater) |
| 6. Dyspnea or tachypnea at rest (respiratory rate >30/min) |
| 7. Postoperative bleeding tendency continues |
AF, atrial fibrillation; HR, heart rate.
b. Step-up Criteria
After the start of the ambulation process, the patient’s exercise protocol has proceeded step by step while checking the step-up criteria as follows: (1) no chest pain, severe shortness of breath, strong fatigue (Borg scale >13), dizziness, lightheadedness, or leg pain, (2) no cyanosis, pallor or cold sweat, (3) no tachypnea (≥30 breaths/min), (4) no increase in arrhythmia or rhythm change to AF with exercise, (5) no ischemic ECG change with exercise, (6) no excessive blood pressure changes with exercise, (7) no increase in HR by more than 30 beats/min with exercise, and (8) no decrease in arterial oxygen saturation to <90% with exercise.
c. Program Progression
A multicenter survey in Japan reported that the average number of days before independent walking after successful elective cardiac surgery was 3.8.184 Currently, the standard program is to start standing and walking on the day after surgery and to achieve independence on the 4th day after surgery. Excessive progress of the program is prohibited; the program is modified according to the patient’s condition.
d. Causes of Rehabilitation Delay
Rehabilitation steps were delayed in 8.7–25.4% of patients after cardiac surgery,185 because of prolonged HF, the appearance of new arrhythmias, preoperative loss of motor function, acute renal injury, and the development of cerebral complications. Among these, postoperative AF occurred at a high rate of 25–40% and it was associated with postoperative lower limb dysfunction.186
4.2.2 Early Recovery Phase
In the recovery phase of rehabilitation, the goal is independence in walking, expansion of ADL, and social return. Therefore, in addition to improving exercise capacity, support is provided for the acquisition of self-management skills in post-discharge life.
a. When to Start Exercise Training
In general, aerobic exercise training using exercise equipment will be started when the patient is able to walk 200 m. Aerobic exercise can be safely performed from the 7th day after surgery in patients who are progressing well, leading to improvement in graft patency187 and early recovery of exercise capacity.188
b. Points to Consider When Starting
Exercise training should be started when the following conditions are met: (1) no fever, and inflammatory response is steadily improving, (2) no significant pericardial or pleural effusion, (3) no new AF, (4) anemia, but hemoglobin is ≥8 g/dL and has an improving tendency.
c. Aerobic Exercise
Exercise intensity is recommended to be at the aerobic exercise level, and if possible is set based on the AT calculated by CPX. Even at the AT level, if there is excessive blood pressure elevation or signs of myocardial ischemia, the intensity of exercise should be reduced. When CPX cannot be performed, symptom-limited exercise ECG should be performed, and the exercise prescription should be based on the target HR calculated by the Karvonen method. In the acute postoperative period, the patient often presents with chronotropic incompetence, which is defined as the inability to increase the HR adequately during exercise to match cardiac output to metabolic demands,189 and the maximal HR should be measured by an exercise stress test. If none of the above can be performed, exercise training should be performed under close supervision with a Borg scale of 11–13 as a guide.
d. Resistance Training
For postoperative resistance training, it is recommended to combine multiple isotonic exercises rather than isometric training.190 Training of the upper extremities should be avoided, due to possible overload during the first 3 months after surgery until bony fusion of the sternal incision is completed. As excessive rest can lead to soft tissue adhesions around the sternal incision, it is advisable to start some movement exercises to increase the range of motion within 24 h after surgery.190 Resistance training for the lower extremities should be performed 2–3 times/week, with 10–15 repetitions at 30–50% of the maximum load.191
e. Protection After Median Sternotomy
Patients who have had a median sternotomy should be instructed to restrict upper extremity elevation load to no more than 5–8 pounds (2.27–3.63 kg) for 5–8 weeks after surgery.192 As a chest belt may adversely affect respiratory function by restricting thoracic motion, and contribute to pulmonary complications, it should not be worn routinely.193 As an alternative to a chest belt, a sternal support band has been reported to be effective in protecting the sternum during body movements or coughing.194
f. Patient Education
The patient education program at discharge should be given together with the patient’s family, including appropriate exercise training based on the exercise prescription, lifestyle modification, medication, diet, smoking cessation, self-monitoring, wound management, and emergency procedures.
4.2.3 Late Recovery Phase
Outpatient CR should be continued with the goal of social return and acquiring new lifestyle habits after discharge. A Japanese multicenter study reported that active participation in outpatient CR improves exercise capacity and long-term prognosis after CABG.181
4.2.4 Maintenance Phase
To maintain the physical function improvement during the acute and recovery phases of CR, it is essential to continue lifelong exercise training. Home programs conducted under medical control are as effective as those conducted in hospitals and other facilities.195
4.3 Features and Cautions Following Each Surgical Technique
4.3.1 After CABG
It is essential to confirm the success or failure of complete revascularization before starting rehabilitation During rehabilitation, it is important to pay attention to chest symptoms and ECG changes in anticipation of early postoperative graft occlusion. Although off-pump coronary artery bypass (OPCAB) is less invasive and allows for faster postoperative exercise training than CABG with extracorporeal circulation, OPCAB is at times chosen for patients with a high preoperative risk, so a case-specific program should be developed.196
4.3.2 After Surgery for Valvular Disease
As a caution after valve replacement, patients must understand the need for postoperative anticoagulation and the characteristics of the prosthetic valve selected. In recent years, valvuloplasty has been increasingly chosen, and there are a wide variety of methods available. Strict blood pressure control may be necessary to avoid pressure overload at the site of the valve, and information should be shared with the cardiac surgeon before rehabilitation.
4.3.3 After Minimally Invasive Cardiac Surgery
With the advances of devices, minimally invasive cardiac surgery (MICS) with small incisions has become popular. It has been reported that MICS patients gain the ability to stand and walk earlier postoperatively than those who underwent conventional median sternotomy.197
5. After TAVI (Table 35)
Table 35. Recommendations and Levels of Evidence for CR Before and After TAVI
| |
COR |
LOE |
GOR
(MINDS) |
LOE
(MINDS) |
| CR is recommended in the perioperative period |
I |
C |
B |
I |
In patients who are suspected to be frail, assessment of frailty should be
considered |
IIa |
C |
C1 |
V |
COR, class of recommendation; CR, cardiac rehabilitation; GOR, grade of recommendation; LOE, level of evidence; TAVI, transcatheter aortic valve implantation.
Transcatheter aortic valve implantation (TAVI), a new treatment for aortic stenosis, became covered by insurance in Japan in 2018.198 Many patients who are indicated for TAVI are contraindicated for conventional surgical aortic valve replacement (SAVR), such as in the very old and frail patients. Therefore, the role of CR in the perioperative period of TAVI is important to achieve better postoperative outcomes (Table 36).199
Table 36. Role of CR for TAVI Patients
| |
Before TAVI |
Postoperative
acute phase |
Recovery phase |
Maintenance phase |
| Problem |
• High risk older people
• Majority are frail
• Limited activity |
• Perioperative management
(anesthesia, analgesia)
• Post-intensive care
syndrome (delirium,
cognitive impairment, etc.) |
• Discharged while still
recovering (postoperative
complications, physical
function) |
• Continuation of preoperative
lifestyle
• Decline in physical function |
| Goal |
• Maintenance of ADL
• Improvement of
nutritional status |
• Early ambulation
• Reestablishment of
preoperative ADL
• Reestablishment of daily
functions |
No frailty
→ Improvement of exercise
capacity
With frailty
→ improvement of muscle
strength and balance
ability
• Establishment of daily life
functions
• Avoidance of secondary
complications (falls, etc.) |
• Maintenance and improvement
of QOL
• Cooperation with local medical
care and welfare
• Cooperation with multiple
professions |
| Approach |
• Frailty assessment
• Nutrition intervention
• Life function assessment
• Build trusting
relationships
• Discharge and post-
discharge social services
Service review |
• Smooth and early release
from hospital
• ADL expansion
• Improvement of exercise
capacity |
No frailty
• Extend walking distance
• Aerobic exercise
• Strength training
With frailty
• Strength training
• Balance training
• Walking training |
• Home exercise training
• Expansion of activity range
• Enhancement of leisure time
activities
• Periodic assessment of frailty to
deal with deteriorating patients
• Recovery phase rehabilitation
hospitals or home medical care,
nursing care insurance,
cooperation among private
exercise facilities and local
governments, etc. |
CR, cardiac rehabilitation; QOL, quality of life; TAVI, transcatheter aortic valve implantation. (Adapted from Higuchi T, et al, 2019199 with modifications.)
In addition to being frail in general, older patients who undergo TAVI have diverse backgrounds, such as low nutrition, dementia, and depression, and it is recommended that each case be evaluated by a heart team.200,201 The characteristics of CR in post-TAVI patients include the absence of a mid-sternum incision, relative freedom to perform upper extremity and trunk exercises, and compared to SAVR, early walking training is possible. However, post-TAVI patients are often discharged from hospital earlier than those undergoing SAVR, so they do not spend enough time in early recovery phase CR. According to the report from Zanettini et al,202 the average hospital stay for patients after TAVI is 11 days, and the average length of subsequent rehabilitation is 18 days. During a mean follow-up of 540 days after discharge, 20% were rehospitalized, 25% of which were due to CVD.202 The need for comprehensive CR after discharge may also apply to patients after TAVI.
There are reports that CR after TAVI is as effective as CR after SAVR in improving exercise capacity and QOL,203 and that both the 6MWD and Functional Independence Measure (FIM) scores are significantly better than before TAVI at the time of hospital discharge (≈19 days after surgery).204 Although there are few reports of continued CR after TAVI, there is a report of significant improvement in exercise capacity, muscle strength, and QOL in an intervention group that continued exercise training combining aerobic exercise and resistance training 2–3 times/week for 8 weeks after TAVI.205 In the PARTNER study, the mortality rate at 2 years was 42.5%, 31.2%, and 28.8%, respectively, in three groups of “inability to walk,” “slow walk,” and “fast walk,” suggesting that CR intervention may improve prognosis.206
For CR after TAVI, serious complications specific to TAVI have already been resolved at the time of postoperative CR, but there are other complications, such as perivalvular regurgitation, and arrhythmias, such as atrioventricular block, and also injuries to the catheter access route. In the case of the transapical approach, pain at the anterior thoracic incision or drain site, and respiratory failure due to pleural effusion may occur, sometimes making it difficult to proceed with postoperative CR. Perivalvular regurgitation can cause HF if the regurgitant flow is high; even if there is no atrioventricular block immediately after surgery, atrioventricular block may occur several days later, so ECG monitoring during CR is important.
CR after TAVI can be performed with the same program as for post-open heart surgery;207 it generally proceeds sequentially with sitting at a bed side, standing, stepping stairs, and walking exercises. Although there are individual differences, patients start to stand up on the day after surgery (ICU), start walking on the second day (high care unit (HCU) or general ward), and start aerobic exercise using a bicycle ergometer or treadmill in the CR room from 3 to 5 days after surgery. In the older patients, postoperative delirium often interferes with CR. TAVI patients have many preoperative comorbidities, often including frailty, so it is advisable to implement an individualized program depending on physical function and ADL.208,209 With the advancement in devices, the less invasive transfemoral approach is becoming more common, and as a result, earlier CR progression and hospital discharge have become possible. However, TAVI patients are often older and present with decreased physical function and ADL, requiring prolonged CR.210 Therefore, it is important to collaborate with multiple professionals, including the recovery phase rehabilitation hospital staff and community services using care insurance; it is necessary to establish a medical care system in cooperation with home medical care, geriatric healthcare facilities, private exercise facilities, and local government.211
In the USA and Europe, TAVI has been reported to be effective in low-risk patients,212 so the number of TAVI patients is expected to increase in the future, and it will be necessary to establish appropriate CR programs based on individual case risk.
6. Arrhythmia, After Device Implantation
*The device in this section refers to a cardiac implantable electronic device (CIED).
6.1 Arrhythmia (Table 37)
Table 37. Recommendations and Levels of Evidence for CR in Patients With Arrhythmias
| |
COR |
LOE |
GOR
(MINDS) |
LOE
(MINDS) |
In patients with AF who have poor exercise capacity or complicated HF,
exercise training should be considered to improve exercise capacity and QOL |
IIa |
B |
B |
II |
For AF patients complicated with obesity, body weight control and the
management of other risk factors should be considered to reduce the burden
and symptoms of AF213 |
IIa |
B |
A |
II |
Exercise training may be considered to improve exercise capacity after AF
catheter ablation |
IIb |
B |
B |
II |
Exercise training should not be performed for patients with ventricular
arrhythmias contraindicated to exercise training |
III
(Harm) |
C |
B |
IVa |
AF, atrial fibrillation; COR, class of recommendation; CR, cardiac rehabilitation; GOR, grade of recommendation; LOE, level of evidence; QOL, quality of life.
When considering exercise training for arrhythmia, the presence or absence of underlying cardiac disease must first be considered. If there is underlying cardiac disease, CR including exercise training improves myocardial ischemia and suppresses the sympathetic nervous system, and thereby can be expected to prevent worsening and progression of the arrhythmia substrate.
6.1.1 Premature Ventricular Contractions (PVCs)
Regarding PVCs, the following factors should be evaluated for risk stratification: organic heart disease, cardiac function, mode of occurrence of arrhythmia (frequency, timing of occurrence, presence or absence of a series), and family history of hereditary arrhythmia. High risk is indicated by the following: frequency of PVCs >30/h, polymorphism, sustained extrasystoles more than triplet, R-on-T pattern, and a short coupling interval.213 Increased PVCs during the exercise stress test is also a risk factor.214 According to the AHA guidelines, when ≥3 consecutive PVCs or polymorphic ventricular tachycardia occur, discontinuation of exercise should be considered.
6.1.2 Atrial Fibrillation (AF)
The frequency of AF is increasing in Japan year by year, although it depends on age and underlying disease status. AF not only causes cerebral embolization, but also increases the risk of dementia, impaired mobility, HF, MI, and sudden cardiac death.213 Compared with the forward progress in the treatment of AF, the prevention of AF onset has not made much progress. Attention is focused on the importance of managing lifestyle-related factors as modifiable clinical risk factors.215–217 In addition to the evaluation of cardiac diseases, such as HF, CAD, and valvular heart disease, the Japanese Circulation Society guidelines (Class IIa, Evidence level B)213 recommend assessing age, sex, hypertension, diabetes mellitus, obesity, sleep-disordered breathing, uric acid level, smoking, alcohol consumption, and genetic predisposition. It is thought that sustained stress on the atria changes the electrical and structural properties of cells and tissues, and causes the formation of substrates that promote the development and maintenance of AF (i.e., atrial remodeling).213
The relationship between exercise and the risk of developing AF is complex and differs between the general population and athletes. The risk of developing AF is higher in athletes who have frequent endurance exercise events, such as endurance exercise three times a week for >10 years.218 In contrast, in the general population, the level of activity in daily life is related to the onset of AF. A meta-analysis of 205,094 patients from 6 studies found that improved exercise capacity was associated with a 9% reduction in the risk of developing AF, with the lowest exercise capacity group having a higher risk of developing AF.217 This suggests that exercise may reduce the risk of developing AF in the general population.
6.1.3 Efficacy of Exercise Training for AF Patients
a. Paroxysmal and Persistent AF
Malmo et al reported that in patients with paroxysmal or persistent AF, exercise training (including HIIT) contributed to a reduction in AF duration.219 Though there is a report that no difference was found,220 there is also a report in patients with AF after catheter ablation showing that exercise capacity improved in the exercise group compared with the control group.221 Kato et al conducted a RCT in 61 patients who returned to sinus rhythm after ablation of persistent AF, and reported that the exercise group showed significant improvement in 6MWD, grip strength, leg muscle strength, and high-sensitivity C-reactive protein compared with the control group, without an increase in the recurrence of AF.222 Exercise training in patients with paroxysmal AF is safe and can improve exercise capacity, but further study is necessary to determine the evidence for its efficacy in reducing AF occurrence.
b. Chronic AF
In patients with chronic AF, exercise capacity can be significantly improved by exercise performed with an intensity of 60–90% of reserve capacity (Karvonen method k=0.6–0.9),223 or with an intensity of 70–90% of maximal HR three times/week.224 Regarding patients with concomitant HF, in the HF-ACTION study of patients with LVEF ≤35%, of the 382 AF patients, 193 had supervised exercise training 3 times/week for 3 months and then performed home exercise training for 2 years. As a result, the AF group showed similar improvements in exercise capacity and QOL as in the sinus rhythm group, although there was a problem with compliance.225
c. After Cardiac Surgery
AF after cardiac surgery occurs most frequently within 5 days of surgery, and particularly within the first 24–72 h after surgery.226 Postoperative AF is said to occur in 16–40% of patients after CABG, in 33–49% of patients after valvular surgery, and in 36–63.6% of patients after CABG plus valvular surgery.226–228 Risk factors include advanced age, hypertension, history of AF, left atrial enlargement, HF, and chronic obstructive pulmonary disease (COPD), with advanced age being the most significant risk factor.226 Herdy et al randomized patients scheduled for CABG who were able to exercise for at least 5 days prior to surgery to an exercise group or a control group, and prospectively evaluated postoperative complications and hospital stay. Postoperative AF was significantly reduced in the exercise group (10% in the exercise group vs. 37% in the control group, P=0.03).229 Pulmonary complications and hospital stay were also significantly reduced in the exercise group. It is recommended to start CR before surgery and to resume it early after surgery to reduce postoperative AF.
6.1.4 Actual Exercise Training for Patients With AF
A flowchart of exercise training for patients with AF is shown in Figure 6. Because many patients with AF also have HF, it is important to evaluate the HF before considering whether exercise training is appropriate. In addition, because anticoagulation is often administered to prevent cerebral infarction, it is necessary to pay attention to hemorrhagic complications due to trauma, such as falls, adherence to medication, and anticoagulation control.
a. Exercise Stress Test
When an exercise stress test is performed in patients with AF, ST depression occurs at a high rate together with an increase in HR. As for the cause of such ST depression, the effects of digitalis medication, left ventricular hypertrophy, and the possibility of myocardial ischemia should be considered. If the HR is poorly controlled or ventricular arrhythmias that meet the ACCF/AHA discontinuation criteria occur, it is necessary to alter the HR, such as with β-blockers, and to investigate and treat the cause of the ventricular arrhythmias, before introducing exercise training. The optimal HR at rest and during exercise for chronic AF has not been clarified, but the ACCF/AHA guidelines recommend a pulse rate of 60–80 beats/min at rest and 90–115 beats/min during moderate-intensity exercise.230 On the other hand, the RACE II study of HR control in AF found that there was no significant difference in the composite cardiovascular event rate between the strict control group (target resting HR <80 beats/min) and the non-strict control group (target resting HR <110 beats/min).231 Further, there was no significant difference in exercise capacity between the normal HR response group (85–115% of the maximum HR predicted by age) and the excessive HR response group (>115% of the maximum HR).232 Therefore, the optimal HR at rest and during exercise is unclear at this time.
b. Exercise Prescription
Because AF patients have marked HR elevation in response to exercise load, and the response varies from patient to patient and according to physical condition, setting the exercise intensity according to HR is difficult. For setting exercise intensity, it is preferable to prescribe the AT using CPX, but, if the AT is difficult to detect, exercise capacity (peak V̇O2) is assessed and the Borg scale may sometimes be used to set exercise intensity. If there is no decline in cardiac function, moderate-intensity load exercise is started. After the introduction of exercise training, blood pressure, HR, and perceived symptoms should be checked, and if the load is judged to be insufficient, the load intensity should be reviewed (Figure 6). For AF patients with concomitant HF, see the Acute/Chronic Heart Failure section (3.0) in this chapter.
6.2 After Device Implantation (Table 38)
Table 38. Recommendations and Levels of Evidence for CR in HF Patients After Device Implantation
| |
COR |
LOE |
GOR
(MINDS) |
LOE
(MINDS) |
For patients with HF after ICD implantation, it is recommended to provide
exercise training and comprehensive disease counseling to improve exercise
capacity and QOL |
I |
A |
B |
II |
For patients with HF after cardiac resynchronization therapy device (CRT-P/
CRT-D) implantation, it is recommended to provide exercise training and
comprehensive disease counseling in order to improve exercise capacity
and QOL |
I |
B |
B |
II |
Exercise training should be considered for patients with HF after CRT-P/
CRT-D implantation in order to further improve cardiac function |
IIa |
B |
B |
II |
For patients with HF after pacemaker, ICD, or CRT-P/CRT-D implantation,
taking into account the optimal program setting, exercise training should be
considered to improve exercise capacity and QOL |
IIa |
B |
B |
II |
For HF patients with AF or after pacemaker implantation, CPX should be
considered to determine the HR response and optimal program, to assess
blood pressure, arrhythmias, and physical activity during exercise, and to
evaluate changes in exercise capacity and treatment effects |
IIa |
B |
B |
II |
Device: cardiac implantable electronic device. AF, atrial fibrillation; COR, class of recommendation; CPX, cardiopulmonary exercise testing; CR, cardiac rehabilitation; CRT-D, cardiac resynchronization therapy defibrillator; CRT-P, cardiac resynchronization therapy pacemaker; GOR, grade of recommendation; HF, heart failure; HR, heart rate; ICD, implantable cardioverter defibrillator; LOE, level of evidence; QOL, quality of life.
6.2.1 Pacemakers
Patients who are dependent on pacemaker-derived pacing show decreased exercise capacity.233 The possible causes include abnormal contraction due to right ventricular pacing, or a poor HR response during exercise (chronotropic incompetence). In particular, patients who are dependent on pacemakers are prone to exercise intolerance.234 In such cases, the rate-response function is used, but no specific conclusion has been confirmed regarding the improvement of exercise capacity by rate-response alone. The factors determining exercise capacity are considered to be the following: the degree of chronotropic incompetence and its relationship with cardiac function, inadequate rate-response setting during exercise, and skeletal muscle funciton.235 To improve exercise capacity, adjustment of pacemaker settings should be considered in addition to exercise training.
6.2.2 Implantable Cardioverter Defibrillator
ICD is the most effective and well-established therapy for preventing sudden cardiac death due to fatal tachyarrhythmias and improving life expectancy, regardless of the type of cardiac disease.236 In addition to implantation for secondary prevention of fatal ventricular arrhythmias, implantation for even primary prevention purposes, such as for patients with HF due to old MI or nonischemic cardiomyopathy with LVEF <35% and NYHA classification II or higher is recommended as “Class I” if nonsustained ventricular tachycardia is present, and recommended as “Class IIa” even if it is not present.236 Evidence for exercise training in patients with ICD implantation has accumulated, and improvements in exercise capacity,237,238 vascular endothelial function,237 QOL,239 and relief of anxiety and depression240 have been reported. In numerous meta-analyses the exercise training group shows significantly improved exercise capacity compared with the control group.241
6.2.3 Cardiac Resynchronization Therapy
Cardiac resynchronization therapy (CRT) improves cardiac function by resolving dyssynchrony, resulting in improved perceived symptoms and prognosis. CRT is indicated for patients with moderate to advanced HF despite appropriate medical therapy (primarily NYHA class III–IV, but also NYHA class II in patients with left bundle branch block), and with reduced LVEF (<35%), and wide QRS duration.236 Although patients with cardiac resynchronization therapy pacemaker (CRT-P)/cardiac resynchronization therapy defibrillator (CRT-D) implantation are good candidates for CR, to date there are few reports on its effectiveness. Conraads et al conducted a RCT of 17 patients with CRT randomized into usual treatment group or exercise group, and reported that peak V̇O2, maximal workload, LVEF, dyssynchrony, and QOL improved in both groups at 5 months, but the improvement was significantly greater in the exercise group.242 Patwala et al randomly assigned 50 patients to an exercise group or non-exercise group 3 months after CRT-P implantation, with CR for 3 months at 3 times/week, and those with exercise training showed further improvement in NYHA classification and peak V̇O2.243 Smolis-Bąk et al divided 52 patients with CRT-D implantation into 2 groups, and after the initial exercise training in the hospital, the group that continued a remotely monitored training program 5 times/week for 8 weeks at home was compared with a control group. After 3–4 months, exercise capacity was significantly improved in the exercise group compared with the control group.244 In a subanalysis of the HF-ACTION study, 224 of 435 patients with CRT-P implantation were assigned to the exercise group. Although peak VO2
increased in the exercise group, there was no difference in all-cause death, cardiovascular death, or the incidence of rehospitalization.245
6.2.4 Precautions in Exercise Training and Exercise Stress Testing
CPX is also useful for setting up devices and should be performed whenever possible to create an accurate exercise prescription.66
a. Adjustment of the Pacing Settings
(1) Rate-response function: If the HR does not rise above the lower rate even during exercise, set the rate-response function. The sensitivity of the sensor, the slope of the HR response, and the upper rate should be set by observing symptoms, such as palpitations. Various sensors, such as accelerometer V̇E, and intracardiac impedance are used. Because accelerometers often do not respond to bicycle ergometer exercise, a treadmill or walking test is recommended. Patients with HF are more likely to have chronotropic incompetence, which is exacerbated by β-blockers and antiarrhythmic drugs. It is controversial whether or not the rate-response function can improve exercise capacity in HF patients.246 In patients with severe chronotropic incompetence, it is recommended to add the rate-response function.
(2) Upper tracking rate: This is the upper limit rate of ventricular pacing following the autologous atrial wave in patients with atrioventricular (AV) block or CRT, and it is a determinant of exercise capacity. It should be set higher in younger patients.
(3) The AV interval: During exercise, biventricular pacing failure may occur in CRT patients due to enhanced AV conduction, which can often be avoided by adjusting the settings of the pacemaker.
b. Adjusting the Tachycardia Detection and Defibrillation Settings
The detection rates of ventricular tachycardia (VT) and ventricular fibrillation (VF) should be checked in advance; the patient should perform exercise at a level to avoid reaching the VT and VF zones. HR of 10–15 beats/min lower than the VT zone should be the upper limit of exercise intensity.66 AF patients with high-voltage implanted devices should be monitored for possible HR elevation during exercise.
c. Actual Exercise Therapy for Patients With Implanted Devices
After device implantation, it is considered acceptable to start exercise if HF is under control and high-risk ventricular arrhythmias such as VT and VF are under control, while checking the condition of the wound and lead position, including hematoma and bleeding, and proceeding with checking arrhythmias under ECG monitoring for a time (Table 39).247 Upper limb elevation on the side of the implantation should be limited to 90 degrees of abduction (horizontal elevation) until discharge, but the patient should be instructed to avoid excessive rest, which may limit the range of motion of the shoulder joint.
Table 39. CR Program After Device Implantation (ICD, CRT-P/CRT-D)
| |
Rest level/shoulder joint
elevation on implant side |
Device
check |
Examination |
Rehabilitation,
disease counseling |
| Preoperative |
|
|
Echocardiography,
laboratory tests |
Information collection |
| Day of surgery |
Indoor/abduction up to 90° |
✓ |
Chest X-ray |
Wound check, walking in
the room |
| Postoperative day 1 |
Same as above |
|
Chest X-ray |
Walking in the ward |
| Postoperative day 2 |
in the ward/up to 90° abduction |
|
|
Walking in the ward |
| Postoperative day 3 |
Same as above |
|
|
Aerobic exercise in the
rehabilitation room, muscle
strength assessment, etc. |
| Postoperative day 4 |
Same as above |
|
|
Aerobic exercise, Strength
training using body weight |
| Postoperative day 5 |
In-hospital/up to 90° abduction |
|
|
Same as above
Nutritional counseling,
medication counseling |
| Postoperative day 6 |
Same as above |
|
Chest X-ray, laboratory
tests |
Same as above, heart
failure disease counseling |
Postoperative
day 7–10/Discharge |
In-hospital/discharge, shoulder joint restriction
is lifted, but swimming and abduction above
90° should be avoided as much as possible
until the outpatient checkup at 1 month, with
re-evaluation after confirmation |
✓ |
6-minute walk test, CPX,
echocardiography |
Same as above, exercise
prescription and counseling
based on CPX |
CPX, cardiopulmonary exercise testing; CR, cardiac rehabilitation; CRT-D, cardiac resynchronization therapy defibrillator; CRT-P, cardiac resynchronization therapy pacemaker; ICD, implantable cardioverter defibrillator. (Adapted from Goto Y, 2017247 with modifications.)
Table 40. Recommendations and Levels of Evidence for CR in Patients With PH
| |
COR |
LOE |
GOR
(MINDS) |
LOE
(MINDS) |
Exercise training under supervision should be considered at experienced
facilities for pulmonary arterial hypertension and chronic thromboembolic PH
patients with less than moderate disease severity who are stable with
treatment |
IIa |
B |
B |
II |
For chronic thromboembolic PH with improved pulmonary hemodynamics after
balloon pulmonary angioplasty, exercise training should be considered under
supervision at experienced facilities |
IIa |
B |
B |
III |
| Excessive physical activity with accompanying symptoms is not recommended |
III
(Harm) |
C |
C2 |
VI |
COR, class of recommendation; CR, cardiac rehabilitation; GOR, grade of recommendation; LOE, level of evidence; PH, pulmonary hypertension.
Pulmonary hypertension (PH) is defined as a disease that presents with a resting mean pulmonary artery pressure ≥25 mmHg on right heart catheterization248 (>20 mmHg was proposed at the 6th World Symposium on Pulmonary Hypertension in 2018).249 Depending on the pathogenesis, there are 5 groups of PH: Group 1: pulmonary arterial hypertension (PAH), which is mainly caused by vascular remodeling due to overgrowth of endothelial cells and smooth muscle cells in the pulmonary arteries, Group 2: PH due to left heart disease, Group 3: PH due to lung disease and/or hypoxia; Group 4: chronic thromboembolic PH (CTEPH), which is caused by organizing thrombi in the pulmonary arteries; Group 5: PH with unclear and/or multifactorial mechanisms.248 In PAH and CTEPH, as advances in treatment have improved hemodynamics and prognosis;250–252 rehabilitation aimed at improving exercise capacity and QOL has been recognized as an adjunct to standard treatment.
Recently, in RCTs of rehabilitation for PAH and CTEPH, the 6MWD was significantly prolonged in the rehabilitation group that received 3–15 weeks of exercise training.253–258 Peak V̇O2
increased significantly in the rehabilitation group253,257,258 with an increase of 24%,257 which was comparable to that of respiratory and cardiac diseases. In addition, there were significant improvements in physical activity,254 fatigue,254 and HRQOL scores253,255,257 in the rehabilitation group. In the 2015 ESC/ERS guidelines, supervised exercise training for physical deconditioning in PAH under pharmacological treatment is classified as Class IIa.248 From the aforementioned RCTs,253–258 the modality, frequency, intensity, and duration of exercise training have not yet been established. Because residual pulmonary vasculopathy may cause an abnormal pulmonary hemodynamic response during exercise, great care should be taken. As described in Table 40, all exercise training should be performed under close supervision in a facility that is experienced in PH treatment and rehabilitation to avoid excessive exercise. As for Groups 2 and 3 PH, there is little evidence for rehabilitation, and it should be done with caution.
In PAH, chronic hypoperfusion of the skeletal muscles and prolonged physical inactivity can cause morphological changes and dysfunction of the skeletal muscle.259,260 Exercise training has been shown to increase capillary density and oxidative enzyme activity, which is positively correlated with increased muscular endurance.261 The primary mechanism for the improvement of exercise capacity is thought to be improvement of the metabolic capacity of skeletal muscle. In addition, it has been suggested that there may be peripheral endothelial dysfunction in PAH and CTEPH, and thus improvement of peripheral circulation through exercise training is expected to contribute to improved metabolism in skeletal muscle.262 In a prospective study in Japan of patients with CTEPH whose pulmonary hemodynamics had been improved by balloon pulmonary angioplasty, peak V̇O2
and other parameters increased significantly in the 12-week rehabilitation group compared with the non-rehabilitation group, with no adverse events or worsening of clinical condition.263 However, syncope, presyncope, and supraventricular tachycardia have been reported as adverse events of exercise training for PH patients.264 In an animal study, it was reported that exercise caused leucocyte infiltration in the right ventricle and remodeling of the pulmonary arteries in rats with severe PH, resulting in decreased survival;265 therefore, long-term safety may be controversial and further study is necessary.
8. Aortic Aneurysm, Aortic Dissection (Table 41)
Table 41. Recommendations and Levels of Evidence for CR in Patients With Aortic Disease
| |
COR |
LOE |
GOR
(MINDS) |
LOE
(MINDS) |
Preoperative exercise and respiratory rehabilitation with circulatory monitoring
is recommended to strengthen cardiopulmonary function and improve
postoperative outcomes in patients with abdominal aortic aneurysms |
I |
A |
B |
I |
After open repair of the aorta, exercise training and respiratory/
swallowing rehabilitation are recommended in order to improve
ADL and exercise capacity |
Abdominal |
I |
A |
B |
II |
| Thoracic |
I |
C |
C1 |
V |
In cases of acute aortic dissection for which medical treatment is indicated,
ambulation and return to daily life according to a rehabilitation program under
HR and blood pressure control should be considered |
IIa |
C |
C1 |
V |
ADL, activities of daily living; COR, class of recommendation; GOR, grade of recommendation; HR, heart rate; LOE, level of evidence.
Compared with the cardiac field, there is less evidence for rehabilitation in the aortic field, and there are no established methods of rehabilitation, effectiveness assessment, or evaluation. Since the advent of endovascular treatment of aortic disease, endovascular treatment has been performed in patients with poor general health such as older patients or frail patients, resulting in a diversification of patients. In the future, aortic rehabilitation is an area where improvement in prognosis and QOL can be expected if appropriate methodologies are established.266
8.1 Before Elective Invasive Treatment
The mean age of patients undergoing aortic repair is higher than that of patients undergoing cardiac surgery, and they more frequently have the complication of COPD.267 Therefore, rehabilitation programs must also take these differences into account. There are several RCTs and systematic reviews that support preoperative exercise training before abdominal aortic aneurysm (AAA) repair.268–275 However, there are no definitive answers as to the timing, the duration and the intensity of exercise training. Table 42 lists the exclusion and monitoring criteria for exercise training until the repair for AAAs with diameter <50 mm.272 Exercise training for the prevention of aortic aneurysm enlargement is also attracting attention.275,276 It has been suggested that anti-inflammatory effects and improvement of vascular endothelial cell function as a result of exercise training may inhibit aneurysm expansion.277
Table 42. Example of Exercise Training in Patients With Abdominal Aortic Aneurysm Until the Indication for Invasive Treatment (Aneurysm Diameter <50 mm)
| Target |
2,000 kcal/week: ≈1 h/day, moderate intensity exercise |
| Example: 3 times/week |
Aerobic exercise
Treadmill
Bicycle ergometer
Stepping stairs
Rowing
Cross-training |
45 min of aerobic exercise
+10 min of resistance exercise |
| Duration |
≥1 year to 3 years (reset in 3 years) |
| Objective exercise intensity |
Start at 60% of HRR, and aim for 80% |
| Perceived exercise intensity |
Borg scale 12–14, as a guide |
Exclusion criteria:
(1) Unable or unwilling to complete exercise training for 3 years or a life expectancy <5 years
(2) Unable to participate in an exercise stress test or to exercise consistently because of orthopedic or musculoskeletal problems
(3) Morbid obesity (BMI >39 kg/m2)
(4) Weight gain or loss of 9 kg over the past 3 months
(5) History of severe liver disease (International Normalized Ratio >2, serum albumin <3.0 g/dL, jaundice)
(6) Unstable angina
(7) Uncontrolled AF, defined as mean 24-h HR >85 beats/min, or 24-h maximal ventricular rate >150 beats/min; and uncontrolled ventricular arrhythmias, defined as recurrent ventricular tachycardia >3 PVCs in succession, or 24-h PVC count >20%
(8) Critical aortic stenosis (peak systolic pressure gradient >50 mmHg with an aortic valve orifice area <0.75 cm2 in an average-size adult)
(9) Class III/IV HF and/or EF <20%
(10) Active pericarditis or myocarditis
(11) Any embolism within the past 6 months
(12) Thrombophlebitis
(13) Hospitalized because of an infectious disease within the past 3 months
(14) Pulmonary disease with a drop in oxygen saturation with exercise to 90% without oxygen
AF, atrial fibrillation; EF, ejection fraction; HR, heart rate; HRR, heart rate reserve; PVCs, premature ventricular contractions. (Source: based on Myers JN, et al, 2010.272)
Several RCTs and systematic reviews have shown that preoperative respiratory rehabilitation is effective for COPD, which is the most frequent preoperative complication of aortic repair.94,278–284 Older age, preoperative dementia or mild cognitive impairment, ASA (American Society of Anesthesiologists) score ≥3, concomitant hypertension and heart disease, prolonged extracorporeal circulation, and massive bleeding have been reported to be associated with postoperative cognitive impairment, postoperative delirium, and impossibility of home discharge.285,286 Objective assessment of cognitive function using the Mini Mental State Examination (MMSE) should be performed before aortic repair, and an early ambulation program should be also implemented before aortic repair.
8.2 After Invasive Treatment
In recent years, with the spread in the use of clinical pathways, various rehabilitation programs including early ambulation have been formulated and started in the early postoperative period. It is advisable to proceed with early ambulation while checking the progress of medical management: cerebral and spinal nervous system complications, respiratory status, and circulatory dynamics. There is little evidence for recovery-phase CR, compared with what there is for CR for heart disease.
8.2.1 Notes on Program Formulation
a. Intraoperative and Immediate Postoperative Complications
In the acute phase after invasive treatment, refer to the guidelines for rehabilitation in the respective specialty if the patient suffers a neurological disorder such as stroke or spinal cord injury.287,288
b. Difference Between Endovascular Therapy and Open Repair
Because endovascular treatment is less invasive and requires a shorter period of bed rest than open repair, the postoperative rehabilitation program should take this difference into account.
8.2.2 Early Ambulation After Invasive Treatment
A fast-track rehabilitation and enhanced recovery after surgery (ERAS) is a program for early postoperative ambulation that includes preoperative and intraoperative anesthesia, surgical techniques, and fluid and nutritional management. Once the conditions for ambulation are met (Table 39), ambulation and rehabilitation should begin as early as possible. Early extubation, reduction of respiratory complications, early discharge, and reduction of medical costs can be expected with an early-ambulation program.289–295
8.2.3 Post-Hospital Discharge
In many cases of postoperative aortic dissection, the dissection remains, so it is common to impose certain restrictions on daily life and physical activity. In other words, aerobic exercise of 3–5 METs for ≈30 min/day (150 min/week) is recommended, and physical strain with exertion should be avoided.296 However, in clinical practice, patients are often subjected to even greater restrictions for fear of recurrence or worsening of the dissection, and few patients are able to return to their pre-dissection life.297 Table 43 shows the guidelines for lifestyle and exercise restrictions after aortic dissection.296,298
Table 43. Guidelines for Lifestyle and Exercise Restrictions in Patients After Aortic Dissection (Subacute to Chronic)
| Exercise |
| [Recommendations] |
| • At least 150 min/week of moderate aerobic exercise (3–5 METs for 30 min/day) |
| • Hiking |
| • Snorkeling |
| • Golf |
| • Tennis |
| • Cycling |
| • Weight training 12 RM or lighter |
| [Not recommended] |
| • Weightlifting |
| • Competitive sports |
| • Exercise using maximal muscle strength |
| • Weight training 1–11 RM |
| Daily life |
| • No limitations in lifestyle (but only for those who completed the appropriate rehabilitation after aortic dissection) |
| • Sex |
| • Stepping stairs |
| • Gardening |
| • Shopping |
| • Travel (airplane, carrying a load <20 kg) |
RM (repetition maximum): maximum number of repetitions. MET, metabolic equivalent. (Source: based on Chaddha A, et al, 2014,296 Spanos K, et al, 2018.298)
In the recovery phase after aortic aneurysm repair without residual disease, it is recommended to continue exercise training similar to CR after cardiac surgery.299 It is desirable to provide multifaceted support for behavioral change through comprehensive CR by a multidisciplinary team from the acute phase to the recovery phase so that patients can continue lifestyle habits such as smoking cessation, salt reduction, and regular exercise.266,300–302
8.3 Acute Aortic Dissection
8.3.1 Indication
Patients who undergo invasive treatment should undertake the rehabilitation described above. However, patients with uncomplicated acute aortic dissection who are judged not to require invasive treatment in the acute phase require bed rest within 24 h of onset and subsequent CR. In some cases, uncomplicated patients convert to complicated cases as described below during the acute phase (within 14 days of onset) or subacute phase (within 3 months of onset). The CR technicians often notice the change. The current definition of “complicated” includes the following 5 items:303,304
(1) Rupture, impending rupture
(2) Malperfusion: impaired perfusion to major abdominal branches, lower limbs, spinal nerves, etc.
(3) Persistent or recurrent pain under appropriate medical treatment
(4) Uncontrollable hypertension under appropriate medical treatment
(5) Large aortic diameter (combined with thoracic aortic aneurysm), or rapidly enlarging aortic dissection
The judgment of “uncomplicated” means the patient does not fit the above 5 items; however, cases of (2) to (5) may occur during acute or subacute rehabilitation, and the indications for invasive treatment should be reconsidered.303,305,306
8.3.2 Methods
A typical rehabilitation schedule for Stanford type B uncomplicated patients in the acute phase is shown in Table 44.307 Hemodynamic management during this phase aims for a HR <60 beats/min and systolic blood pressure ≤120 mmHg at rest, and a HR <100 beats/min and systolic blood pressure ≤140 mmHg during exercise training. There are few reports on rehabilitation in the chronic phase, both for uncomplicated Stanford B dissection and for postoperative acute Stanford A dissection. However, recently the conventional instructions on excessive lifestyle and exercise restrictions have been slightly relaxed, and there are more reports supporting active exercise training and return to unrestricted daily life.298 Table 43 shows a guide for lifestyle and exercise restrictions after aortic dissection.296,298
9. Peripheral Arterial Diseases (Table 45)
Table 45. Recommendations and Levels of Evidence for Exercise Training in Patients With Peripheral Arterial Disease and Claudication
| |
COR |
LOE |
GOR
(MINDS) |
LOE
(MINDS) |
| Supervised exercise training (for at least 3 months) is recommended |
I |
A |
A |
I |
Prior to revascularization, supervised treadmill exercise training is
recommended |
I |
B |
B |
I |
| After revascularization, exercise training is recommended |
I |
A |
A |
I |
In patients who have difficulty with supervised exercise training, home-based
exercise training (>3 months) should be considered |
IIa |
A |
A |
II |
Ergometer exercises of the upper or lower extremities under supervision
should be considered |
IIa |
A |
B |
II |
COR, class of recommendation; GOR, grade of recommendation; LOE, level of evidence.
9.1 Comprehensive CR Program Based on Exercise Training
Exercise training is the basic and crucial treatment for peripheral arterial disease (PAD) other than critical limb ischemia (CLI). The majority of deaths in patients with PAD are not due to gangrene of the lower extremities, but rather to MI and stroke,308–311 which may be due to reduced exercise capacity and daily activity (Figure 7).312 The prognosis of asymptomatic PAD without symptoms of claudication is also reported to be poor, suggesting the need for early diagnosis and intervention. In CLI, endovascular treatment or bypass surgery should be performed first to save the limb (Figure 8).313–316 After that, it is reported that the amount of daily activity does not increase much unless exercise training is well supervised.317 In order to complete the treatment of CLI, it is necessary not only to relieve tissue ischemia by revascularization but also to continuously improve exercise capacity and vascular function by exercise training. In addition, a comprehensive CR program includes complete smoking cessation, blood pressure control with antihypertensive medication and salt reduction for patients with hypertension, glycemic control for diabetes mellitus, and appropriate medication such as antiplatelet agents and statins.
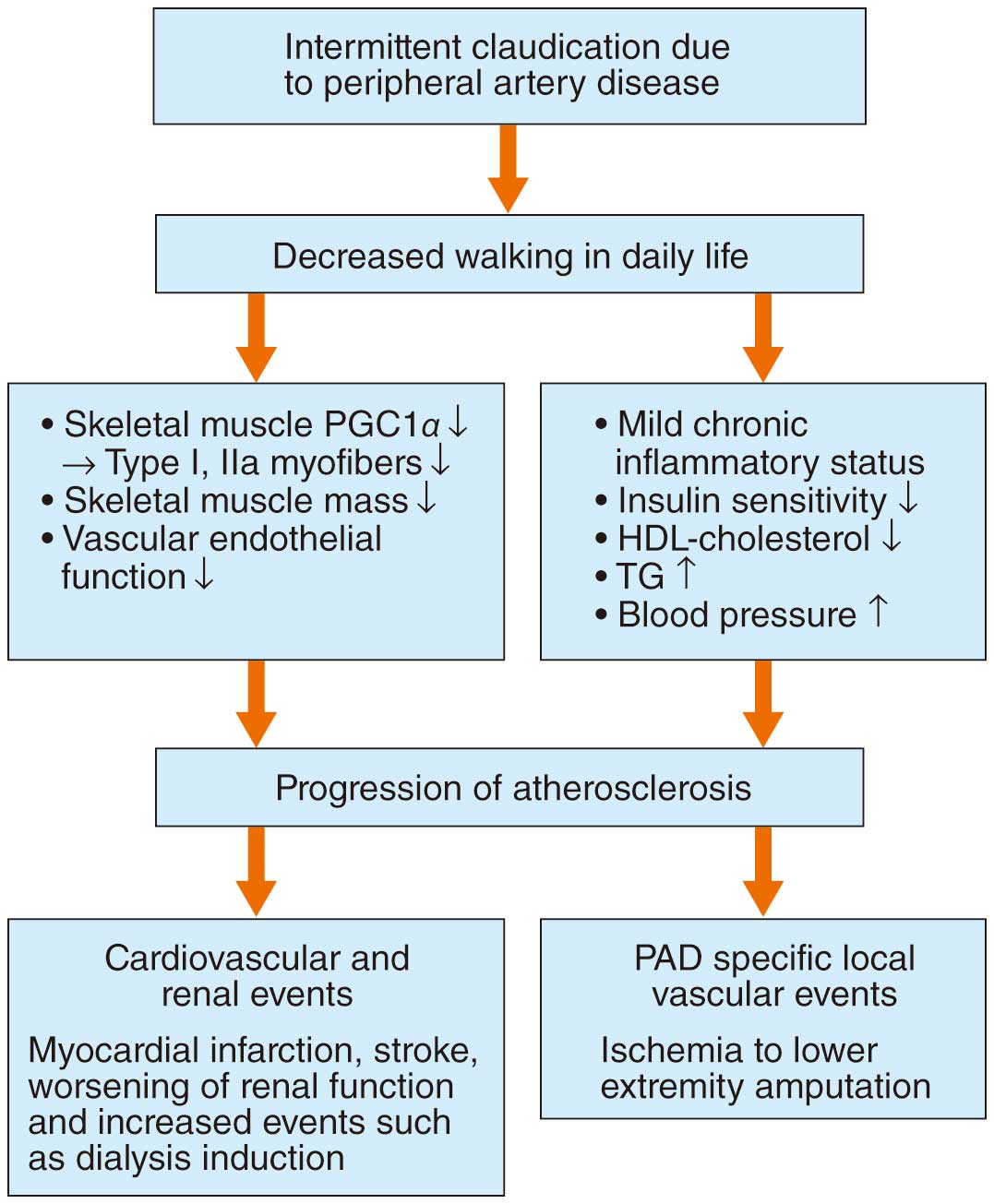
9.2 Indications and Contraindications
Exercise training is indicated in patients with chronic peripheral arterial stenosis, with or without intermittent claudication. The Fontaine and Rutherford classifications are frequently used to classify the severity of the disease based on clinical symptoms (Table 46).317 In cases of severe lower extremity ischemia with ulceration or gangrene, it should be kept to mild exercise with loading in the absence of infection. Exercise training is contraindicated in acute arterial occlusion (embolism or thrombosis) and in CLI with infection.
Table 46. Classification and Treatment of Peripheral Arterial Disease Based on Symptoms
Fontaine
classification |
Rutherford
classification |
Treatment modality |
| I: Asymptomatic |
0: Asymptomatic |
Atherosclerosis risk management, antiplatelet agents,
exercise training, foot care |
| IIa: Mild claudication |
1: Mild claudication |
Atherosclerosis risk management, exercise training,
antiplatelet agents, foot care |
IIb: Moderate to severe
claudication |
2: Moderate claudication |
Exercise training, antiplatelet agents, revascularization,
arteriosclerosis risk management |
| 3: Severe claudication |
| III: Pain at rest |
4: Pain at rest |
Revascularization, antiplatelet agents, exercise training,
foot care, arteriosclerosis risk management |
| IV: Ulceration, gangrene |
5: Minor tissue loss |
Revascularization with wound care, antiplatelet agents,
decompression and exercise training (if there is no
infection after revascularization), arteriosclerosis risk
management |
| 6: Major tissue loss |
(Source: based on Norgren L, et al, 2007.317)
9.3 Preparation
9.3.1 Medical Check
The risk factors for PAD include diabetes mellitus, smoking, hypertension, dyslipidemia, old age, and renal failure. Because of the peripheral neuropathy in diabetic patients, and very older patients and chronic renal failure, patients do not walk, PAD progresses without perceived symptoms, and patients will not come as outpatients until they develop CLI with ulceration and necrosis. There is a possibility that 15% of patients diagnosed with spinal canal stenosis may have PAD, which requires attention. In the examination, palpation of the inguinal femoral artery, popliteal artery, dorsal foot artery, and posterior tibial artery should be performed; skin temperature and the presence of skin lesions should always be checked.
9.3.2 Exercise Stress Test
PAD is often associated with other atherosclerotic diseases; the patient should be monitored for ischemic symptoms in vital organs during the initial exercise stress test. It is necessary to confirm that there are no anginal symptoms, no ECG changes, and no arrhythmias during the exercise stress test while monitoring blood pressure and ECG. To evaluate the severity of the disease, walking distance measurement using the Gardner protocol treadmill and ankle joint blood pressure measurement before and after exercise are useful, and can be used as an index of claudication symptoms before and after treatment.313–316,318
9.4 Protocols
9.4.1 Supervised and Home-Based Exercise Training
Supervision for exercise training is recommended for symptomatic PAD (Class I, Level of Evidence A).312,313,319 In a meta-analysis of exercise training for PAD patients with intermittent claudication, predominantly supervised exercise training (30 min of track or treadmill walking 3 days/week) increased the maximum walking distance by 260 m compared with usual care.317 In other meta-analyses, supervised exercise training was associated with a maximum walking distance of approximately 150 m longer at 3 months than home-based exercise training, suggesting that supervised exercise training is important.312,313,319 In a recent meta-analysis of 25 RCTs,314 supervised exercise training increased the maximum walking distance by 180 m in the treadmill test compared with the control group. In a RCT of patients with only Fontaine II iliac artery lesions, supervised exercise training significantly prolonged the maximum walking distance compared with stenting, suggesting that it is important to prioritize supervised exercise training even in patients with iliac artery lesions.320 However, in reality, supervised exercise training is not feasible for various reasons, including the difficulty of frequent hospital visits. Therefore, it is necessary to start unsupervised walking exercise (home-based exercise training with oral medication: Class IIa Level of Evidence A)312,313,319,321 as early as possible, and to support the establishment of an exercise habit by checking the number of steps with a pedometer and keeping an exercise diary.
9.4.2 Exercise Training in Practice
In order to learn correct exercise training and to educate patients, if possible supervised exercise training should be performed for the first time, followed by a transition to home-based exercise training. Even after the patient is able to walk after revascularization, the same instructions should be provided for exercise training to improve the prognosis (Figure 8). The type of exercise (treadmill, track walking, ergometer), frequency (3–5 days/week), intensity (speed and incline), and duration (30–45 min) are determined by the results of the exercise stress test for each patient. As symptoms improve, the exercise prescription should be changed accordingly. Exercise should be continued at least 3 times/week for at least 12 weeks.
9.4.3 Types of Exercise
Treadmill, track walking, and water walking are recommended for PAD. Exercise training is programmed in the following order: (1) warm-up, (2) walking exercise, and (3) cool-down. Treadmills and bicycle ergometers are easy to use because the intensity of exercise can be adjusted, but walking on a track with a pacemaker can also be used.
9.4.4 Exercise Intensity, Duration, and Interval
The recommended settings for treadmill walking are to start at an incline of 12% and a speed of 2.4 km/h and walk until lower extremity pain occurs at a submaximal load of about “quite painful” (Borg scale 15–17)322–324 (Table 47). If the patient can walk for >10 min at this intensity, then the speed should be increased to 3.2 km/h or the incline should be increased. Stop walking when the pain becomes “very painful” during walking. Take a break (≈1–5 min) until the pain disappears, and perform the same exercise again until moderate claudication appears. This should be repeated for at least 30 min in the first training session, and extended by 5 min for each training session, up to a maximum of 60 min. Exercise is performed once or twice daily, at least 3 times/week (5 days/week or more if possible), for at least 3 months. In addition, “continuous walking training at home” in between supervised exercise training should be actively performed.
Table 47. Supervised Exercise Training Program for Patients With Peripheral Arterial Disease
| 1. 10 min of stretching as a preparatory exercise |
2. Initial exercise intensity should be set based on the maximum walking time of the Gardner protocol treadmill, and the treadmill
walking exercise should be performed at a speed that causes claudication in 3–5 min. The patient should be allowed to walk
continuously until reaching the Borg scale 15–17 of “very hard” |
| 3. After a break of a few minutes, perform treadmill walking exercise according to No. 2 above |
| 4. Continue this interval walking training for ≈30–60 min |
| 5. Finish the exercise with a cool-down exercise |
9.4.5 Combination With Drug Therapy
Cilostazol (phosphodiesterase III inhibitor) significantly prolonged the maximum walking distance in a study of PAD patients with intermittent claudication, and it is recommended as a first-line treatment in the guidelines of TASC II,317 ACC/AHA,313 and the Japanese Circulation Society316 (Class I, Level of Evidence A) (Figure 8). In cases where the use of cilostazol is difficult, such as when complicated with HF, salpogrelate (serotonin receptor antagonist), prostaglandins, and eicosapentaenoic acid are alternatively selected. Antiplatelet agents (aspirin, clopidogrel) are also recommended as Class I because they are expected to inhibit cardiovascular events, although they do not improve claudication symptoms. Statins are now recommended313,325 as Class I, with evidence that they improve prognosis and claudication symptoms. It has been reported that intravenous heparin and continued exercise training program can safely reduce claudication symptoms and increase the maximum walking distance.326
9.4.6 After Revascularization
Exercise training instruction after revascularization is important in terms of secondary prevention and the prevention of cardiovascular events, and it should be started the day after surgery if there are no complications312,313,316 (Figure 8). However, a prospective observational study of PAD patients who underwent Fontaine II-degree endovascular therapy found that although the ankle–brachial index and maximum walking distance improved significantly after endovascular therapy, the average daily step count and physical activity did not improve afterwards.327 Therefore, comprehensive CR with multidisciplinary interventions is necessary to increase the number of participants and ultimately contribute to the reduction of healthcare costs.
V. CR for Special Patient Groups
1. Older Patients With Heart Disease (Table 48)
Table 48. Recommendations and Levels of Evidence for CR in Older Patients With Heart Disease
| |
COR |
LOE |
GOR
(MINDS) |
LOE
(MINDS) |
In older patients with CAD, aerobic exercise and resistance training are
recommended to be performed concomitantly to improve exercise capacity,
muscle strength, and QOL |
I |
A |
A |
II |
In older patients with CAD, aerobic exercise is recommended to correct
coronary risk factors |
I |
A |
A |
II |
| In older patients with HF, exercise training is recommended to improve QOL |
I |
A |
B |
II |
In older patients with HF, a combined exercise regimen consisting of aerobic
exercise, resistance training, balance training, and flexibility exercises should
be considered to improve exercise capacity and physical function |
IIa |
A |
B |
II |
In older heart disease patients with combined frailty and sarcopenia, exercise
and nutritional therapy should be combined to improve exercise capacity and
muscle strength |
IIa |
B |
B |
II |
Patient education may be considered to improve the prognosis of older
patients with HF |
IIb |
B |
C1 |
II |
Resistance training may be considered to improve physical function in very old
patients with heart disease |
IIb |
C |
C1 |
VI |
CAD, coronary artery disease; COR, class of recommendation; CR, cardiac rehabilitation; GOR, grade of recommendation; LOE, level of evidence; QOL, quality of life.
1.1 Characteristics of Older Patients With Cardiac Disease and Precautions During Exercise Training
The ACCF/AHA “guidelines for the secondary prevention of CAD in patients aged 75 years and older” recommend that treatment of coronary risk factors should follow the same principles as in the general adult population.328 On the other hand, the provision of medical care to older people can be difficult because of the wide variation in disease symptoms and response to treatment due to physical changes associated with aging, the fact that they may suffer from multiple chronic diseases, and the fact that medical treatment guidelines for older people are not well established.329 In addition, because the progressive rate of aging varies among individuals and the degree of the effect on physical, mental, and social functioning varies, it is important to consider the effect not only on health problems such as cardiac diseases and complications but also on the psychological and social aspects (biopsychosocial model) in older patients with cardiac diseases.330 Together with the patient, family, and caregivers, it is necessary to determine an individualized treatment plan based on the characteristics of the older patient (Figure 9).330

Although there is disagreement on the effects of cardiac rehabilitation (CR) on the prognosis and exercise capacity in older patients with cardiac disease,331–336 there is growing evidence that CR can improve quality of life (QOL).336,337 Cardiac disease in older patients is characterized by a number of comorbidities, which may define their prognosis and prevent them from participating in CR due to the need to be very careful. There are specific cautions for older patients during exercise training.
1.1.1 Complications of Frailty and Sarcopenia
Complications of frailty and reduced gait speed are factors that worsen the prognosis.338 A complex exercise regimen for older heart failure (HF) patients171 and patients after cardiac surgery,339 consisting of aerobic exercise, resistance training, balance training, and flexibility exercises has been reported to improve frailty and the Short Physical Performance Battery. However, there is insufficient evidence for comprehensive CR for older cardiac patients with frailty.
Although several meta-analyses have shown that resistance training for sarcopenia improves muscle mass, muscle strength, and gait speed,340,341 there are no reports examining the effects of CR in older cardiac patients with sarcopenia, and thus more evidence is needed.
1.1.2 Very Old Patients
There are no reports examining the effects of CR in very old patients with cardiac diseases aged ≥90 years. However, in a study examining the effects of exercise training on older patients with an average age of 87 years in an institution, it was reported that resistance training significantly improved knee extensor strength and gait speed.342 Furthermore, an exercise program combining resistance training with aerobic exercise and balance training has been reported to improve physical function and reduce the risk of falling.343 Although there have been no reports of serious adverse events caused by exercise training in very old patients, there have been cases in which exercise was discontinued due to muscle pain or knee pain. As the number of complications increases with age, it is necessary to pay close attention to physical condition management.
2. Pediatric Patients With Heart Disease
2.1 Congenital Heart Disease (Table 49)
Table 49. Recommendations and Levels of Evidence for Exercise Training in Pediatric Patients With CHD
| |
COR |
LOE |
GOR
(MINDS) |
LOE
(MINDS) |
Exercise training should be considered in order to increase exercise capacity
and improve QOL |
IIa |
B |
B |
II |
Note: When implementing exercise training, it is advisable to take into account disease specificity. CHD, congenital heart disease; COR, class of recommendation; GOR, grade of recommendation; LOE, level of evidence; QOL, quality of life.
2.1.1 Current Status of Survival and Rehabilitation
Over the past few decades, there has been remarkable progress in improving the surgical outcomes and survival rates of patients with congenital heart disease (CHD), and the number of CHD patients reaching adulthood has been increasing.344–347 The survival rate of CHD patients up to age 16 years has increased from 62.4% in 1971–1989 to 86.9% in 1990–2011.347 In Japan, the number of adult CHD (ACHD) patients exceeded 400,000 in 2007, which is already higher than the number of pediatric CHD patients, and the number is reported to increase by 9,000 cases per year.345 In ACHD patients, regular exercise training is important to improve physical fitness and QOL.
At present, there are no clear guidelines on exercise training and other rehabilitation for pediatric CHD patients, so decisions must be made on a case-by-case basis, taking into account the disease, severity, and timing (age). In addition, there is little evidence on the rehabilitation and permission of exercise for CHD patients younger than 10 years, so the exercise prescription should be made with caution, especially for patients younger than 6 years, for whom exercise testing is not possible.
2.1.2 Objectives of Exercise Training
With the exception of those who do not have impaired exercise capacity preoperatively or postoperatively, children with CHD have obvious physical weakness and some limitations in school and social life. Exercise habits and a healthy and active lifestyle during growth are important not only for improving exercise capacity, but also for cardiovascular and musculoskeletal development, mental development, and the prevention of metabolic syndrome.348 The purpose of exercise training for CHD in children with reduced exercise capacity and abnormal cardiovascular responses to exercise is to: (1) improve exercise capacity, safety, and QOL, (2) improve active social participation and productive role, and (3) correct future coronary risk factors such as hypertension, diabetes mellitus, and dyslipidemia by developing an exercise habit.
2.1.3 Assessment of Exercise Capacity and Exercise Prescription
Cardiopulmonary exercise testing (CPX) is commonly used to assess exercise capacity. Exercise intensity is prescribed based on disease and patient specificity, but particular attention should be paid to the risk and history of obvious ventricular dysfunction and arrhythmias. Based on previous reports,349–357 a common exercise protocol for patients with CHD is a 10-min warm-up or stretch, 40 min of aerobic exercise, and a 10-min cool-down. The exercise intensity should be, as a guide, 60–70% of heart rate reserve (HRR), 65–80% of maximum HR, HR of the anaerobic threshold (AT), and <80% of peak V̇O2. However, the intensity of exercise should vary according to the severity of the heart disease. In general, the exercise frequency prescription is 2–3 times/week, for ≈60 min each time, and for ≈12 weeks.
2.1.4 Effectiveness of Exercise Training
Exercise training has also been shown to increase peak V̇O2.349,353,356 In a meta-analysis, the improvement of peak V̇O2
after exercise training was 3.68 mL/min/kg (95% confidence interval 1.58 to 5.78) in weighted mean difference.349 Peripheral effects accounted for a large part of the mechanism of increased exercise capacity,351 but a significant increase in stroke volume has been noted.358 In addition, there are reports of improved HR response,352 improved respiratory function,354,355 and other effects such as increased muscle strength,359 and improved QOL.349,357
3. Heart Failure Patients Under Intravenous Inotropic Drugs (Table 50)
Table 50. Recommendations and Levels of Evidence for CR in Patients With HF Receiving Intravenous Inotropic Drugs
| |
COR |
LOE |
GOR
(MINDS) |
LOE
(MINDS) |
In hemodynamically stable patients, CR may be considered to prevent or
improve the progression of deconditioning |
IIb |
C |
C1 |
V |
COR, class of recommendation; CR, cardiac rehabilitation; GOR, grade of recommendation; HF, heart failure; LOE, level of evidence.
3.1 Evidence
In principle, exercise training is contraindicated in patients with acute or advanced HF who are hemodynamically unstable or have symptoms such as dyspnea even at rest. However, in recent years, many institutions have started to offer exercise training to patients with advanced HF who are receiving intravenous inotropic drugs, as long as they are hemodynamically stable and have no symptoms at rest.
Although there are no randomized controlled trials (RCTs) that have examined the effects of CR in a large number of patients with HF receiving intravenous inotropic drugs, there are many reports that low-intensity rehabilitation can be performed safely.360–367
Arena et al reported that a patient on the waiting list for heart transplantation receiving intravenous inotropic drugs underwent 246 sessions of aerobic exercise for 180 h over a period of a little more than 1 year, and exercise capacity improved by >30%, with only one adverse event being a hypotensive episode.361 Forestieri et al reported that in patients on the waiting list for heart transplantation receiving intravenous inotropic drugs, the 6-minute walk distance (6MWD) was significantly improved in the bicycle ergometer exercise group (7 patients) compared with the control group (11 patients).367
3.2 Adaptation
In patients with HF receiving intravenous inotropic drugs, CR is indicated in the same way as for normal patients with chronic HF: no contraindications to exercise training (Table 28), hemodynamically controlled and stable (i.e., no worsening of subjective symptoms of HF (dyspnea, fatigue, etc.) or physical findings (edema, pulmonary congestion, etc.) in at least the recent 3 days, and no excessive fluid retention or dehydration). In addition, the patient must be independent in terms of activities of daily living (ADL) before admission, and must have an understanding and willingness to undergo CR.
In practice, the main indications are for patients who are expected to have difficulty with early withdrawal of intravenous inotropic drugs and for patients on the waiting list for heart transplantation who are dependent on intravenous inotropic drugs. In order to avoid starting CR before reaching a stable stage and hindering recovery in HF, or rushing to stage up and causing HF to worsen again, it is critical that the timing of starting CR and staging up, as well as the pathology, are thoroughly understood and careful judgment is made by cardiologists.
3.3 Risks and Discontinuation Criteria
Patients with HF receiving intravenous inotropic drugs are considered to be at higher risk than usual of worsening HF and arrhythmia, even during the stable period. Therefore, to avoid overloaded rehabilitation, the criteria for stopping rehabilitation should be set in advance, and rehabilitation should be discontinued when those criteria are met, such as shortness of breath or fatigue with a Borg scale of ≥14. Although it is difficult to apply the same discontinuation criteria to all patients with HF, it is possible to safely perform rehabilitation by making modifications to the basic discontinuation criteria (Table 51) according to the patient’s medical condition.
Table 51. Terminating Criteria for the Rehabilitation Motion in HF Patients Receiving Intravenous Inotropic Drugs (An Example From the National Cerebral and Cardiovascular Center)
1) Perceived symptoms: shortness of breath, fatigue (Borg scale ≥14), consciousness disturbance, fainting, unsteady walking,
cold sweat, etc. |
| 2) Heart rate (in sinus rhythm): <50 beats/min or ≥130 beats/min, or an increase of ≥30 beats/min from rest |
| 3) Systolic blood pressure: <70 mmHg, or decrease of ≥20 mmHg from rest |
| 4) Appearance of new arrhythmia |
| 5) Percutaneous arterial oxygen saturation (SpO2): <90% |
| 6) Occurrence of intravenous line complication |
4.1 After VAD Implantation (Table 52)
Table 52. Recommendations and Levels of Evidence for CR in Patients After VAD Implantation
| |
COR |
LOE |
GOR
(MINDS) |
LOE
(MINDS) |
It is recommended to implement a comprehensive rehabilitation program for
all patients who are eligible to participate |
I |
B |
B |
III |
Exercise training combining aerobic exercise and resistance training is
recommended |
I |
C |
B |
III |
It is recommended to implement a CR program for patients with VAD in
facilities with expertise in managing VAD patients |
I |
C |
C1 |
IVb |
Exercise training based on exercise prescription by RPE or CPX should be
considered |
IIa |
C |
B |
III |
Periodic 6-minute walk test and CPX should be considered to evaluate
exercise capacity and prescribe the appropriate exercise training program |
IIa |
C |
B |
III |
COR, class of recommendation; CR, cardiac rehabilitation; GOR, grade of recommendation; LOE, level of evidence; RPE, rating of perceived exertion; VAD, ventricular assist device.
4.1.1 Objectives of CR
In patients with advanced HF who are eligible for VAD implantation, it is quite common that their muscle strength and nutritional status are impaired, and their exercise capacity is often severely impaired. Although VAD improves patients’ hemodynamic parameters, it takes time before their exercise capacity improves. Even if the purpose of VAD is “bridge to transplantation”, the waiting time before heart transplantation is quite long and more than several years in Japan, so there is a high need for CR to improve ADL, exercise capacity, and QOL during this period.368,369 Regarding the safety and efficacy of CR after VAD implantation, there are many positive reports,370–376 but most include only a small number of patients; a meta-analysis showed improvements in exercise capacity and QOL.377,378 The purpose of CR for patients after VAD implantation is shown in Table 53.
Table 53. Purposes of CR in Patients With a VAD
| Phase |
Purpose and significance |
| Acute phase |
• Improvement of deconditioning due to prolonged rest and muscle disuse |
| • Acquisition of ADL that enable patients to live at home |
| • Establishment of safe daily activities with a VAD |
Early recovery
phase |
• Improvement of exercise capacity, mental health and QOL for social return with a long-term VAD |
| • Education and support for the safe management of the VAD, driveline care, and anticoagulation management |
| • Education and support for VAD-related complications and their prevention, recognition and management |
Late recovery
phase –
maintenance
phase |
In addition to the content of the early recovery phase |
| • Education and support (including caregivers) on safety management of VAD devices |
| • [For patients waiting for heart transplant] Education and support in preparation for heart transplant surgery |
ADL, activities of daily living; CR, cardiac rehabilitation; QOL, quality of life; VAD, ventricular assist device.
4.2 After Cardiac Transplantation (Table 54)
Table 54. Recommendations and Levels of Evidence for CR in Patients After Cardiac Transplantation
| |
COR |
LOE |
GOR
(MINDS) |
LOE
(MINDS) |
A comprehensive rehabilitation program should be implemented for all eligible
patients |
I |
A |
A |
I |
In the management of patients after cardiac transplantation, it is important to
use a facility with appropriate expertise |
IIa |
B |
B |
II |
Exercise training that combines aerobic exercise and resistance training
should be considered |
IIa |
B |
B |
II |
Exercise training based on exercise prescription by RPE or CPX should be
considered |
IIa |
C |
C1 |
III |
6-minute walk test and CPX should be performed periodically to evaluate
exercise capacity and for the appropriate exercise prescription |
IIa |
C |
C1 |
III |
COR, class of recommendation; CR, cardiac rehabilitation; GOR, grade of recommendation; LOE, level of evidence; RPE, rating of perceived exertion.
4.2.1 Physiology of the Transplanted Heart (Denervated Heart)
In the denervated heart,379–381 HR and cardiac contractility before and after exercise are controlled by adrenaline secreted by the adrenal gland, so that there is little increase in HR during exercise, but rather it is increased by adrenal-derived catecholamines after the end of exercise, followed by a slow decrease in HR. In heart transplant patients, the HR at 1 min after the end of exercise is often higher than the HR at the time of maximal exercise loading. Because of denervation, the increase in cardiac output in the early phase of exercise in transplanted hearts is influenced by decreased peripheral vascular resistance and increased venous perfusion by skeletal muscle pumps. However, the increase in cardiac output by this mechanism is up to 20%, and a further increase must wait for an increase in adrenaline in the blood. Furthermore, chest pain during myocardial ischemia is no longer present.
4.2.2 Efficacy of Exercise Training
Reports on the effects of exercise training in heart transplant recipients have appeared since the 1980s,382 and in 1999, an RCT of heart transplant recipients assigned to an exercise group or a control group was reported.383 In that report, early post-transplant exercise training significantly improved exercise capacity beyond spontaneous recovery. In 2005, it was reported that there was no difference in the effect of exercise training between men and women.384 Therefore, exercise training is useful in improving exercise capacity and QOL in patients after heart transplantation, and CR is recommended for all heart transplant recipients who are able to participate.382–384
4.2.3 Pediatric Patients After Heart Transplantation (Table 55)
Table 55. Recommendations and Levels of Evidence for Exercise Training in Pediatric Patients After Heart Transplantation
| |
COR |
LOE |
GOR
(MINDS) |
LOE
(MINDS) |
| Exercise training may be considered |
IIb |
C |
B |
V |
COR, class of recommendation; GOR, grade of recommendation; LOE, level of evidence.
In recent years the number of pediatric heart transplant recipients in Japan has been gradually increasing. Most of the candidates have dilated or restrictive cardiomyopathy. It has been reported that the exercise capacity of children after heart transplantation is severely inferior to that of healthy children.385–387 The cause of the decreased exercise capacity may be multifactorial, but the main cause is chronotropic incompetence due to surgical sympathetic disconnection at the time of heart transplantation. In addition, there are other causes, such as increased pulmonary vascular resistance and increased pulmonary vascular resistance. It has been reported that 16 of 29 patients with sympathetic reinnervation by positron emission tomography during follow-up after heart transplantation showed an elevated HR response during exercise.388
Although the effectiveness of rehabilitation after heart transplantation has been reported in adults,383,387 Hsu et al reported improved QOL after exercise training in 45 heart-transplant children.389 Their protocol consisted of a 10-min warm-up, exercise intensity of 50–80% of peak V̇O2, 25–30 min of walking on a bicycle ergometer or treadmill, and a 10-min cool-down. Exercise training improved peak V̇O2
by an average of 24% in 2–3 months. It has been reported that resistance training increases skeletal muscle mass and strength, and high-intensity interval training has been shown to improve exercise capacity, health awareness, anxiety, and depression.390
5. Cancer Patients With Cardiac Diseases
5.1 Overview of Cardio-Oncology Rehabilitation
“Cardio-oncology rehabilitation” (CORE) is a new concept proposed by the AHA and is being studied with the goal of becoming the standard of care in the future.391,392 Adding CR to cancer rehabilitation is expected to improve cardiopulmonary endurance in cancer survivors393–396 and reduce the risk of cardiovascular disease (CVD).397,398
The so-called “cancer rehab” is medical care that diagnoses and treats physical, cognitive, and psychological disorders in order to improve the independence and QOL of cancer patients,393 whereas CR is comprehensive and a long-term medical care that includes medical examinations, an exercise prescription, cardiovascular risk factor (CVRF) reduction, education and counseling, and lifestyle improvement for the purpose of CVD treatment and recurrence prevention.394
5.2 Practice of CORE
5.2.1 Target Diseases
Based on the American Society of Clinical Oncology Practice Guideline,399 the AHA has identified high-risk cancer treatments (e.g., high-dose radiation therapy, high-dose anticancer drugs, or combination of anticancer drugs and molecularly targeted drugs) and high-risk patients (e.g., history of cardiac disease or multiple CVRFs) as the primary targets for CORE.391,400
(1) High-dose anthracycline (doxorubicin equivalent ≥250 mg/m2), high-dose radiotherapy (≥30Gy and treatment field including the heart), or combination of low-dose anthracycline and low-dose radiotherapy.
(2) Presence of risk factors, such as use of low-dose anthracycline or trastuzumab alone, and CVRF (≥2 of smoking, hypertension, diabetes, obesity, or dyslipidemia), age ≥60 years, or cardiac dysfunction (history of myocardial infarction (MI), reduced left ventricular ejection fraction, moderate valvular disease).
(3) Low-dose anthracycline followed by trastuzumab combination therapy.
This should be referred to as the starting point for introducing CORE, and the final decision should be made by a cardiologist or oncologist, taking into account the type and stage of cancer, physical function, and comorbidities.
5.2.2 Implementation Algorithm
Figure 10 shows an overview of CORE.391 Referral to CORE is based on identification of high-risk treatment, high-risk patient, and comprehensive assessment of clinical symptoms and comorbidities. At the start of CORE, safety is first assessed by CPX and the 6-minute walk test. For safety issues related to cancer treatment (e.g., frailty, neuromusculoskeletal disorders, osteoporosis, cognitive decline), the exercise plan is optimized for each patient as part of “cancer rehab” (e.g., physical therapy, occupational therapy, speech therapy). As for the collaboration between oncologists and cardiologists, it is important to consider the circumstances of each institution and region, such as whether it should be implemented as team medical care at a cancer center or as community medical care collaboration based on family physicians.
VI. Nutrition and Diet Therapy
1. Nutritional Assessment Methods (Table 56)
Table 56. Recommendations and Levels of Evidence for Intervention/Counseling for Nutritional Assessment Methods and Lifestyle-Related Disease in CR
| |
COR |
LOE |
GOR
(MINDS) |
LOE
(MINDS) |
It is recommended to provide nutritional counseling using anthropometry/body
measurements and nutritional assessment tools |
I |
C |
C1 |
III |
A combination of nutrition education, diet modification and exercise training is
recommended for weight loss |
I |
A |
A |
I |
| Instruction of a low-sodium diet of ≈6 g/day should be considered |
IIa |
C |
B |
II |
COR, class of recommendation; CR, cardiac rehabilitation; GOR, grade of recommendation; LOE, level of evidence.
Because each nutritional assessment method has its own characteristics, it is important to implement in combination with multiple assessments.401
1.1 Anthropometry/Body Measurements
Body weight (BW) as a nutritional assessment is among the most convenient of the indices because it is not invasive to measure.401 However, because changes in fluid balance complicate the assessment of BW in patients with heart disease, it should be measured before meals, and the body mass index (BMI), rate of change in BW, and body composition should be measured in the absence of edema. Nutritional assessment using physical measurements includes the following:
(1) BMI = weight (kg) / height (m)2
(2) Standard body weight ratio: %IBW (% ideal body weight) = present weight (kg) / IBW ([height (m)2] × 22) × 100
(3) Weight change rate: % LBW (% loss of body weight)= (normal weight − weight at measurement) / normal weight × 100
(4) Body composition measurement
Bioelectrical impedance analysis is a method of analyzing body components (muscle mass, fat mass, and water content) by applying a weak electric current through the body to obtain resistance values.401 Compared with dual-energy X-ray absorptiometry, there is no radiation exposure and less inter-measurement error. According to the Asian Sarcopenia Working Group (AWGS) sarcopenia diagnostic criteria (2019), sarcopenia should be evaluated by skeletal muscle index (SMI, ≤7.0 kg/m2
for men and ≤5.7 kg/m2
for women), gait speed (<1.0 m/s), and grip strength (<28 kg for men, <18 kg for women).48
(5) Grip strength
Grip strength can be easily measured, and its increase is an indicator of improvement in muscle function. When nutritional status improves, muscle function improves before muscle mass, making it an excellent nutritional assessment method.
1.2 Blood Test Data
Blood albumin, which is the nutritional assessment of visceral protein stores, reflects the severity of disease, such as inflammation and dilution of blood due to increased fluid volume; alone it is not an indicator of nutritional status.401
1.3 Nutritional Assessment Tools
Nutritional assessment tools include the following: (1) Subjective Global Assessment (SGA),402 (2) Controlling Nutritional Status (CONUT),403 (3) Geriatric Nutritional Index (GNRI),404 (4) Mini Nutritional Assessment-Short Form (MNA®-SF) and Mini Nutritional Assessment (MNA®),405–407 and (5) Global Leadership Initiative on Malnutrition (GLIM) criteria.408 Each tool has its own features and should be used with an understanding of the advantages and disadvantages.
1.3.1 CONUT
Albumin, total lymphocyte count, and total cholesterol are scored, and the sum of these scores is the CONUT value (Table 57).403 Because it is frequently used as a general biochemical test, CONUT is suitable for use in many patients, including inpatients and outpatients. However, because there is no information on physical findings, it should be judged in combination with clinical information.
Table 57. Nutritional Assessment by CONUT
| Parameter |
Value |
Score |
| Serum albumin (mg/dL) |
≥3.50 |
0 |
| 3.49–3.00 |
2 |
| 2.99–2.50 |
4 |
| <2.50 |
6 |
| Total lymphocytes (/μL) |
≥1,600 |
0 |
| 1,599–1,200 |
1 |
| 1,199–800 |
2 |
| <800 |
3 |
| Total cholesterol (mg/dL) |
≥180 |
0 |
| 179–140 |
1 |
| 139–100 |
2 |
| <100 |
3 |
| |
Nutritional status |
Total score |
| Degree by CONUT |
Normal |
0–1 |
| Light |
2–4 |
| Moderate |
5–8 |
| Severe |
9–12 |
(Source: based on Ignacio de Ulíbarri J, et al, 2005.403)
1.3.2 GNRI
This index is a predictive index of complications and death in the older people over 65 years old. Like CONUT, it is suitable for screening, but is sensitive to albumin and body weight. Calculate using the formula at screening (Table 58).404 It has been reported that the incidence of cardiovascular events in chronic heart failure increases as the CONUT and GNRI values worsen.
Table 58. Nutritional Assessment by GNRI
| GNRI value (14.89 × albumin level) |
Nutritional status |
| ≥98 |
Good |
| ≥92 to <98 |
Low risk |
| ≥82 to <92 |
Moderate risk |
| <82 |
High risk |
(Source: based on Bouillanne O, et al, 2005.404)
1.3.3 GLIM Criteria
These criteria for diagnosing undernutrition were published in 2018.409 The diagnosis is made in 2 stages: assessment and diagnosis. In the first stage, nutritional screening, such as SGA, is performed, and if there is a problem with the nutritional status, the patient proceeds to the second stage. The diagnosis and severity of the disease are determined using assessment tools for current symptoms (rate of weight loss, low BMI, SMI) and etiology (decreased food intake or malabsorption, disease burden, or degree of inflammation). If ≥1 of the 3 current symptoms (weight loss, low BMI, low muscle mass such as SMI), or ≥1 of the 2 etiological factors are met, the patient is considered to be undernourished. In addition, the severity of the undernutrition will be evaluated based on current symptoms and classified as “moderate undernutrition” or “severe undernutrition”.
The type of undernutrition that should receive intervention depends on the “etiology” associated with the undernutrition and inflammation: (1) undernutrition with chronic disease and inflammation, (2) undernutrition with severe inflammation due to acute disease or trauma, (3) undernutrition due to chronic disease with little/no inflammation, and (4) undernutrition due to starvation without inflammation. Action should be taken on the issues that need to be improved (Figure 11).408
1.4 Use of Nutritional Assessment
If cardiac rehabilitation (CR) is conducted for undernourished patients with insufficient energy, it will lead to further muscle wasting and deterioration of nutritional status. Nutritional intervention may be necessary to supplement energy deficits, then observe changes in nutritional indicators, such as BW and muscle mass, and develop the next nutritional plan.410 If the level of nutritional impairment is mild, it can be improved by adjusting the usual diet and between-meal eating, but in more severe cases, the need for supplemental nutrition and hospitalization increases. In heart failure patients with fluid volume changes, nutritional assessment is important for the early detection of unintentional muscle mass loss.411
2. General Diet Therapy for CVDs
2.1 Objective
Nutritional management in CR has 2 aspects: (1) nutritional management for lifestyle-related diseases that are the result of overnutrition, and (2) nutritional enhancement for undernutrition caused by the progression of heart failure (HF).102,412 Nutritional management in CR is comprehensively determined within the CR team with reference to the severity of HF and the assessment of activities of daily living (ADL) and nutrition. The general diet for cardiovascular diseases (CVDs), including lifestyle-related diseases, is shown in Table 59.413–416
Table 59. Nutritional Management Targeting Weight Control in Patients With CVD
| 1) Target BMI by age |
|
| 18–49 years |
18.5–24.9 kg/m2 |
| 50–64 years |
20.0–24.9 kg/m2 |
| ≥65 years |
21.5–24.9 kg/m2 |
| 2) Energy setting [kcal/day = IBW or ABW × physical activity*] |
| BMI 18.5 to 24.9 kg/m2 |
Use IBW |
| Height m2× age-specific target BMI |
| BMI ≥27.5 kg/m2 |
Use ABW |
| [(current weight in kg – IBW) × 0.25] + IBW |
| 3) Protein target** |
1.0–1.5 g/IBW/day 15–20% of energy |
Avoid eggs and fatty meats, and choose soy/processed soy
products, and fish |
| 4) Fat target |
20–30% of energy |
| (1) Saturated fatty acid |
4.5–7% Avoid fatty meats, animal fats, and milk fat |
| (2) Omega-3 polyunsaturated fatty acids |
Choose blue fish |
| (3) Trans fatty acids |
Avoid confectioneries that use shortening and margarine |
| (4) Cholesterol |
<200 mg/day of cholesterol Avoid eggs and liver |
| 5) Carbohydrate target |
50–60% of energy Increase fiber and avoid sugar |
Choose brown rice, pressed barley, buckwheat, whole grain
bread, and millet as staple foods |
Choose 1–2 mixed vegetables (mushrooms, seaweed, and
legumes) with each meal |
| 6) Alcohol consumption |
≤25 g/day |
| 7) Salt |
<6 g/day |
*Light exertion = 25–30 kcal, moderate exertion = 30–35 kcal, heavy exertion = 35 kcal. **Patients with complications of CKD are set individually. ABW, adjusted body weight; BMI, body mass index; CKD, chronic kidney disease; CVD, cardiovascular disease; IBW, ideal body weight. (Source: based on Atherosclerosis Society of Japan, 2017,413 Ministry of Health, Labour and Welfare, 2019,414 Japan Obesity Society, 2016,415 Krenitsky J, 2005.416)
Cardiac function decline affects nutritional status, and nutritional impairment increases the risk of developing heart failure and accelerates the severity of the disease.102,412 Nutritional management in CR is aimed at preventing rehospitalization and performed to correct the coronary risk factors.102,130,413,417,418 Assessing changes in eating behaviors and making positive and affirmative associations with body size changes and laboratory results can help patients perceive success and enhance self-efficacy.419
3. Intervention and Education for Patients With Heart Failure (Table 60)
Table 60. Recommendations and Levels of Evidence for Nutritional Interventions and Nutritional Counseling of CR in HF Patients
| |
COR |
LOE |
GOR
(MINDS) |
LOE
(MINDS) |
For HF patients, it is recommended to assess for possible undernutrition and
the risk of undernutrition |
I |
C |
B |
IVa |
Adequate energy intake and administration (resting energy expenditure of
22–24 kcal/kg/day × activity factor) should be considered. In older patients, with
the aim of 20–30 kcal/kg/day as a guide, the dose should be increased or
decreased according to the general condition of the patient, taking care not to
inadequately consume energy or administer energy |
IIa |
C |
C1 |
IVb |
For insufficient intake due to decreased appetite, concomitant use of enteral
nutrition (oral intake) should be considered |
IIa |
B |
C1 |
II |
Adequate protein intake and administration (≥1.1 g/kg/day) should be
considered with attention to renal dysfunction. In older patients, the target
should be ≥1.2 g/kg/day |
IIa |
B |
C1 |
II |
Counseling on weight measurement and recording, and periodic target weight
reviews should be considered |
IIa |
C |
C1 |
IVa |
Reviewing the uniform salt restriction (<6 g/day), counseling may be
considered for older patients with HF |
IIb |
C |
C1 |
IVb |
Aggressive nutritional therapy to improve the prognosis of older patients with
endstage HF is not recommended |
III (No
benefit) |
C |
C2 |
VI |
COR, class of recommendation; CR, cardiac rehabilitation; GOR, grade of recommendation; HF, heart failure; LOE, level of evidence.
3.1 Nutritional Status and Prognosis
In patients with undernutrition, which is often observed in HF, exercise training alone is not expected to be effective and must be combined with nutritional counseling.59,420,421 In the CHART-2 study, which is the observational study in Japan, the proportion of patients with mild or moderate undernutrition among patients with HF classification B, was 33.3% in those younger than 70 years and 43% in those older than 70 years.422 In particular, older patients with HF have a higher probability of undernutrition because of nutritional deficits caused by the pathogenesis of HF, such as malabsorption of nutrients due to intestinal edema, decreased appetite, and imbalance of protein catabolism and anabolism, in addition to physiological changes due to aging.
The nutritional status of HF patients affects not only their physical function and body composition, but also their prognosis.422–427 Moreover, undernutrition in patients over 70 years of age increases the risk of HF hospitalization,422,425 and undernutrition in older patients with HF under outpatient treatment is associated with skeletal muscle loss,423 as well as decreased quality of life.428
Therefore, in the implementation of CR for HF patients, it is recommended that nutritional status is assessed individually, taking into account the high risk of undernutrition, and that counseling be given regarding appropriate energy intake according to age, basal metabolic rate, and activity level, as well as the intake of nutrients such as protein and salt.412,429
3.2 Energy Requirements
Obesity is a risk factor for the development of HF, and energy restriction to prevent or reduce obesity is important for secondary prevention (see above Section 2. General Diet Therapy for CVDs). On the other hand, inadequate energy intake relative to energy requirements can lead to undernutrition. In an observational study of adult patients with stage B and C HF, the resting energy expenditure was 22–24 kcal/kg/day.421 In an observational study of patients with advanced HF on a left ventricular assist device, the resting energy expenditure was 18kcal/kg/day.430 The resting energy expenditure plus the activity level is the energy requirement431 (Table 61).432
Table 61. Calculation of Energy and Protein Intake Requirements for HF Patients
Measurement of resting energy
metabolism by indirect
calorimetry |
Heart failure stage |
Total energy requirement |
| If available |
NYHA Classification I–IV
AHA Stage B–D |
Resting energy metabolism × activity factor* |
| If not available |
NYHA Classification I–IV
AHA Stage B–D |
22–24 kcal/kg/day × activity factor |
| Advanced heart failure |
18 kcal/kg/day × activity factor |
*Sitting most of the time = 1.0–1.4, low activity = 1.4–1.6, active = 1.6–1.9, very active = 1.9–2.5. Protein intake is calculated at 1.1–1.4 g/kg. (In patients with CKD, each patient should be considered individually.) If the actual body weight is ≥125% of the standard body weight (22 × height (m)2), the formula is: Corrected weight (kg) = (actual weight – standard weight) × 0.25 + standard body weight. HF, heart failure. (Adapted from Kuehneman T, et al, 2018432 with modifications.)
Although there is a lack of evidence on the energy requirements of older patients with HF, the European Society for Clinical Nutrition and Metabolism (ESPEN) guideline for energy requirements for all older patients, including those with HF, is 30 kcal/kg/day,433 and the Japanese Society for Clinical Nutrition and Metabolism (formerly, the Japanese Society for Parenteral and Enteral Nutrition) guideline suggests 20–30 kcal/kg/day.434 Even for the older patients with HF, the target energy dose should be 20–30 kcal/kg/day as well, but weight loss and thinness are associated with a poor prognosis in older patients with HF; thus it is recommended that the dosage be increased or decreased according to the patient’s general condition, always taking care to administer sufficient energy.
3.3 Decreased Appetite
Older patients are more prone to decreased energy intake due to decreased appetite compared with younger patients.435 In addition to decreased taste and digestive function,436 the severity of HF,437 inflammation, use of loop diuretics, and cachexia also contribute to decreased appetite.438 In patients with HF, decreased appetite has been shown to be associated with a poor prognosis, and also with decreased physical function.438
Oral nutritional supplements (ONS) for appetite loss in older people increase energy intake,433 but they do not improve skeletal muscle mass, exercise function, or indices related to HF.439 More evidence is needed on the effectiveness of ONS for decreased appetite in older patients with HF. In addition, salt restriction for the prevention of worsening HF may contribute to decreased appetite, and if the adverse effects of low nutrition are judged to be significant, relaxation of salt restriction and provision of meals tailored to preferences should be considered.412
3.4 Protein Intake
In addition to the imbalance in protein catabolism and anabolism caused by HF,421,440 the decline in muscle protein synthesis with age places older heart HF at high risk for developing sarcopenia and frailty. In addition to exercise, protein intake is necessary for skeletal muscle maintenance and muscle protein synthesis. In order to induce muscle protein synthesis, a certain level of amino acid concentration in the blood is required, but this threshold increases with age.441 Therefore, it is necessary to consume sufficient protein at each meal, and even when the total daily protein intake is the same, the rate of muscle protein synthesis increases significantly when protein is consumed equally at all three meals. Therefore, dietary patterns should also be considered.442
The ESPEN guidelines recommend a protein intake of 1.2–1.5 g/kg/day for older adults with any acute or chronic disease, including HF.433,443,444 The goal of protein intake for older patients with HF should be at least 1.2 g/kg/day, and in the case of chronic kidney disease, the need for protein restriction should be considered on a case-by-case basis.
3.5 Weight Management
Patients with HF should measure and record BW every day, and it is important to support self-monitoring for early detection by self-examination of early symptoms and physical findings of acute exacerbation of HF. The target weight should be determined comprehensively based on the physical findings and examination results of each patient. The target weight should be reviewed periodically because weight varies not only with fluid volume but also with nutritional status. Although there is no single index for determining weight fluctuations due to fluid volume or nutritional status, it should be noted that the amount of stored energy required to gain 1 kg of BW is approximately 7,000 kcal, and weight fluctuations due to nutritional status take a relatively long time.
Studies documenting the optimal BMI for HF patients are lacking, and the relationship between intentional weight gain and prognosis in HF patients is unclear. European guidelines and the Heart Failure Society of America do not recommend weight loss for HF patients with a BMI of <35 kg/m2, but more evidence is needed in Japan to account for differences in body size.445
3.6 Salt Intake
The Japanese guidelines suggest a salt intake of <6 g/day for HF patients,102 but there is a lack of evidence that salt restriction improves life expectancy and QOL in patients with symptomatic HF. Three recently published randomized controlled trials in patients with HF with reduced ejection fraction and an observational study including patients with HF with preserved ejection fraction all showed a poor prognosis in the salt-restricted group.446–448 In addition, salt restriction may decrease appetite and worsen nutritional status in older patients. Therefore, it is advisable to examine whether the effect of salt restriction outweighs the risk of undernutrition in each individual case, and to revise strict salt restriction as appropriate depending on dietary intake.102,412
VII. QOL and Psychological Assessment and Intervention
1. Methods and Indicators for Assessing Quality of Life (Table 62)
Table 62. Recommendations and Levels of Evidence for Improvement of QOL With CR
| |
COR |
LOE |
GOR
(MINDS) |
LOE
(MINDS) |
| In patients with CAD, CR is recommended to improve QOL |
I |
A |
A |
I |
| In patients with HF, CR is recommended to improve QOL |
I |
A |
A |
I |
| Use of the SF-36 should be considered to assess QOL in patients with CAD |
IIa |
A |
B |
I |
The Minnesota Living with Heart Failure Questionnaire (MLHFQ) should be
considered to use as a measure of QOL in patients with HF |
IIa |
A |
B |
I |
CAD, coronary artery disease; COR, class of recommendation; CR, cardiac rehabilitation; GOR, grade of recommendation; HF, heart failure; LOE, level of evidence; QOL, quality of life.
1.1 QOL With a Focus on Health
In the field of health and medical care, such as medical treatment, rehabilitation mental health care, nursing, and care, the quality of life (QOL) scale has been used to a large extent as a standard to evaluate the effectiveness of interventions. In this case, health-related QOL (HRQOL) is used in the health and medical fields to focus on factors directly related to health, excluding QOL related to social environmental factors such as economic status, living environment, and sanitary conditions.449 HRQOL was used in a study of the effects of treatment in inoperable cancer patients, which led to its current wide use for chronic disease.
1.2 Evaluation Scale (Evaluation Method)
There are many different measurements of QOL, and Table 63 lists the primary measurements developed to date.
Table 63. Primary Measurements of Quality of Life
| Name |
Features |
Time required |
SF-36 v2® (Japanese version)
(Japanese version of MOS 36-Item
Short-Form Health Survey) |
36 items with the following subscales: (a) physical function, (b) daily role function
(physical), (c) body pain, (d) overall health, (e) vitality, (f) social function, (g) daily
role function (mental), (h) 8 subscales and 3 components of mental health: QOL
Physical Component Summary (PCS), Mental Component Summary (MCS), and
Role/social Component Summary (RCS) |
10 min |
SF-12® (Japanese version)
(Japanese version of MOS 12-Item
Short-Form Health Survey) |
12 items; subscales are the same as the SF-36 v2 |
2–3 min |
SF-8TM (Japanese version)
(Japanese version of MOS 8-Item
Short-Form Health Survey) |
8 items, subscales are the same as the Japanese version of SF-36 v2 |
1–2 min |
WHOQOL-26
(World Health Organization Quality of
Life-26) |
26 items with 5 levels of response, including “not at all”, “a little”, “somewhat”,
“much”, and “very much”
4 domains (physical, psychological, social relationships, and environmental) and
a subscale for overall QOL |
10 min |
EQ-5D-5L (Japanese version)
(Japanese version of EuroQOL 5
dimensions 5-level) |
15 items and includes subscales for mobility, personal care, daily activities, pain/
discomfort, and anxiety/depression |
5 min |
| Heart QOL |
14 items, with subscales for physical, emotional, and overall QOL, which were
developed by the European Association of Preventive Cardiology (EAPC) |
5 min |
MLHFQ (Japanese version)
(Japanese version of Minnesota Living
with Heart Failure Questionnaire) |
21 items and includes subscales for shortness of breath, sleep, fatigue, and
appetite |
5 min |
1.3 Evaluation of Improvement Effects
Many reports document that cardiac rehabilitation (CR) is effective in improving QOL.450 There are reports that QOL can be improved by general exercise training programs,451 but there are also comprehensive programs that include psychological counseling, stress management, and patient education. A comparison of the 2 programs showed that the comprehensive program had a greater effect on improvement.
1.3.1 CAD
In a study comparing exercise training alone and exercise training plus counseling in patients with coronary artery disease (CAD), QOL improved only in the exercise training plus counseling group.452 Furthermore, a meta-analysis of randomized controlled trials (RCTs) examined the effect of psychological intervention on QOL in addition to exercise training, and the result showed that not only were anxiety and depression scores significantly lower in the intervention group (2,024 patients) than in the control group (1,156 patients), but also mortality and morbidity were lower, pointing to the importance of psychological intervention in conjunction with exercise training; the improvement in QOL was higher in women than in men.453
1.3.2 Chronic Heart Failure
Psychological support and intervention programs have been reported to be effective in reducing anxiety and depression, improving QOL, and reducing the number of rehospitalizations in patients with HF.454 The causes of hospitalization for exacerbations in older patients with chronic HF are more likely to be due to poor medication compliance, inability to restrict fluids and salt, and social isolation, such as living alone, than to the deterioration of the condition itself; therefore, a comprehensive response is needed in addition to direct therapeutic management of the disease.455 In a recent meta-analysis of four RCTs (299 patients), significant improvement in the Minnesota Living with Heart Failure Questionnaire (MLHFQ) was reported in patients with chronic HF after a brief exercise intervention.456 In addition, 13 studies (3,990 patients) using the HRQOL showed a significant improvement in exercise capacity in the CR group based on exercise training, compared with the control group.457 There is sufficient evidence for the effectiveness of QOL assessment in patients with chronic HF, and QOL assessment using the MLHFQ is recommended in Japan.
2. Psychological Evaluation (Table 64)
Table 64. Recommendations and Levels of Evidence for Psychological Assessment (Methods and Indicators of Anxiety and Depression) in CR
| |
COR |
LOE |
GOR
(MINDS) |
LOE
(MINDS) |
| In patients with CAD, screening for depression is recommended |
I |
A |
A |
I |
It is recommended to use the PHQ-9 and other scales to assess depressive
symptoms in patients with CAD |
I |
A |
A |
I |
In patients with CAD, evaluation of cognitive function may be considered,
keeping in mind the possibility of depression or delirium |
IIb |
A |
A |
I |
DS-14 may be considered for assessment of personality traits in patients with
CVD |
IIb |
B |
B |
II |
CAD, coronary artery disease; COR, class of recommendation; CR, cardiac rehabilitation; CVD, cardiovascular disease; GOR, grade of recommendation; LOE, level of evidence.
2.1 CVD and Depressive/Anxiety Symptoms
Depressive symptoms are symptoms that accompany various diseases, but their prevalence is higher in patients with cardiac diseases, ranging from 17% to 27% which is nearly 3-fold higher than in the general population (10.3%).458 In HF, in particular, the prevalence of depression increases with the severity of the disease, and it is said to be as high as 42% in NYHA Classification IV.459 Depressive symptoms and various other mental and psychological symptoms have a negative effect on the condition, prognosis, and QOL in cardiovascular diseases (CVDs). The AHA has recommended screening for depression and intervention for depression in patients with CVDs.460 Anxiety has also received attention as a factor affecting CAD as well as depressive symptoms.461
2.2 Mental and Psychological Features
As for the behavioral characteristics of CVD patients, starting with Type A behavioral patterns defined in 1959, the effects of anger and hostility have been studied, and now Type D (personality tendency), negative and repressive coping behaviors, is attracting attention as a factor in the onset of CVDs.462 This type of personality is characterized by a tendency to perceive things negatively, but also by perseverance and difficulty in expressing one’s own intentions, and it has been reported that cardiac patients with Type D have poor perceived health.463
In a meta-analysis based on major adverse cardiovascular events for CAD, anger/hostility, depression, and distress were reported as psychological factors associated with major disease events in women, and anxiety, depression, and distress were reported in men.464
2.3 Questionnaire-Based Screening
In CR, questionnaire-based screening tests for psychiatric symptoms and cognitive functions are also used to assess the mental and psychological status of patients. These tests are simple and useful auxiliary diagnostic tools that can quantify the presence and severity of symptoms even in the absence of a professional, but unfortunately, the fact that the examinee can easily manipulate the results renders these tests to be possibly biased.
Table 65 summarizes psychological tests and assessment scales for mental and psychological aspects that are frequently used in the field of CR. The diagnosis of depression should not be made on the basis of screening results, but should be based on diagnostic criteria such as DSM-V (American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders, 5th edition).465 In order to diagnose dementia, it is necessary to exclude secondary deterioration of brain function by blood tests and CT/MRI of the brain, and it is difficult to diagnose dementia or mild cognitive impairment based only on the results of screening tests.
Table 65. Psychological Tests Commonly Used in CR
| PHQ-9 |
| Formal name |
Patient Health Questionnaire for Depression Screening-9 |
| Features |
9-question screening test for depression based on the diagnostic criteria for depression, widely used in medical institutions
internationally |
A score of 0–4 indicates no symptoms, 5–9 indicates mild depression, 10–14 indicates moderate depression, 15–19 indicates
moderate to severe depression, and 20–27 indicates severe depression |
| Time required |
5–7 min |
| PHQ-2 |
| Formal name |
Patient Health Questionnaire for Depression Screening-2 |
| Features |
Screening is possible with 2 questions (the first 2 questions of PHQ-9) for depression |
Symptoms are rated as follows: “Never” =0 points, “A few days” =1 point, “More than half” =2 points, and “Almost every
day” =3 points. If the score is ≥1 for any of the 2 questions, reevaluation by PHQ-9 is recommended. A score of ≥3 out of 6
is sometimes considered “symptomatic” |
| Time required |
1–2 min |
| CES-D |
| Formal name |
Center for Epidemiologic Studies Depression Scale |
| Features |
Screening test for depression developed by the U.S. National Institute of Mental Health (NIMH) based on existing scales
such as SDS and MMPI |
| The number of questions is as small as 20, and simple. A score of ≥16 indicates depressive symptoms |
| Time required |
10–15 min |
| HAM-D/HRSD |
| Formal name |
Hamilton Depression Rating Scale/Hamilton Rating Scale for Depression |
| Features |
Rating scale for severity of depression |
It is not a self-rating system, but an interview conducted by a trained physician or other professional. 0–7 points: normal,
8–13 points: mild, 14–18 points: moderate, 19–22 points: severe, ≥23 points: most severe |
| Time required |
10–20 min |
| BDI/BDI-II |
| Formal name |
Beck Depression Inventory |
| Features |
Depressive symptoms and their severity are assessed by a total score of 63 points, calculated from 0 to 3 points for each of
the 21 items. 0–13 points: very mild, 14–19 points: mild, 20–28 points: moderate, and 29–63 points: severe |
| *Version II was revised after DSM-IV |
| Time required |
5–10 min |
| SDS |
| Formal name |
Self-rating Depression Scale |
| Features |
Rating scale for depression |
20-item questionnaire with 4-point self-assessment. Consists of questions about emotional, physiological, and psychological
symptoms. Half of the items are reversal items. A total score of <40 points: slight depression, 40-point range: mild depression,
≥50 points: moderate depression |
| Time required |
10–15 min |
| HADS |
| Formal name |
Hospital Anxiety and Depression Scale |
| Features |
Test for depressive and anxiety symptoms in patients with physical illness |
The test consists of 7 questions for anxiety and depression, respectively. The total score is calculated from 0 to 3 points for
each question: 0–7 points: no symptoms, 8–10 points: suspicious, ≥11 points: symptoms |
| Time required |
5–10 min |
| GAD-7 |
| Formal name |
Generalized Anxiety Disorder-7 |
| Features |
Screening test for generalized anxiety disorder (GAD) |
Consists of 7 items, each with a score of “never”: 0 point, “a few days” (out of 2 weeks): 1 point, “more than half of the time”:
2 points, and “almost every day”: 3 points. The total symptom score is 0–4 points: normal, 5–9 points: mild, 10–14 points:
severe |
| Time required |
5–7 min |
| DS-14 |
| Formal name |
Type D Scale-14 |
| Features |
Measure of Type D (distress) personality, which is common in patients with heart disease |
Consists of 2 items, Negative Affect (NA) and Social Inhibition (SI). Each of the 7 items has a score of 0–4: score of ≥10 on
both NA and SI indicates Type D |
| Duration |
5–7 min |
| MMSE |
| Formal name |
Mini-Mental State Examination |
| Features |
Screening test for dementia consists of 11 items: time and place awareness, immediate and delayed reproduction of 3 words,
calculation, object calling, sentence recitation, 3-step verbal commands, writing commands, writing sentences, and graphic
copying. On a 30-point scale, ≤23 points: suspected dementia, ≤27 points: suspected mild cognitive impairment |
| Duration |
6–10 min |
| Mini-Cog |
| Formal name |
Mini-Cognitive Assessment Instrument |
| Features |
Simple screening test for dementia consists of 3 tests: immediate and delayed reproduction and clock drawing of 3 words;
score of ≤2 indicates suspicion of dementia |
| Duration |
2 min |
| MoCA-J |
| Formal name |
Japanese version of Montreal Cognitive Assessment |
| Features |
Screening test for mild cognitive impairment includes visuospatial and executive functions, naming, memory, attention,
recitation, word recall, abstract concepts, delayed replay, and disorientation. Score of ≤25 points indicates suspected mild
cognitive impairment |
| Duration |
10 min |
| TMT |
| Formal name |
Trail Making Test |
| Features |
Frontal lobe dysfunction: a measure of executive function (e.g., attention/processing speed). It assesses many functions
such as attention, number and letter recognition qualification search, eye/hand coordination, speed of information processing,
flexibility of mental activity, and exercise capacity |
| Duration |
15 min |
CR, cardiac rehabilitation.
Table 66. Recommendations and Levels of Evidence for Psychological Interventions and Counseling in CR for Patients With CAD
| |
COR |
LOE |
GOR
(MINDS) |
LOE
(MINDS) |
| It is recommended to perform CR to improve depressive symptoms |
I |
A |
A |
I |
It should be considered to administer antidepressants and to conduct
psychotherapy, such as cognitive behavioral therapy, to improve depressive
symptoms |
IIa |
B |
B |
II |
COR, class of recommendation; CR, cardiac rehabilitation; GOR, grade of recommendation; LOE, level of evidence.
3.1 Communicating With the Patient
It has been reported that many patients with CVD are in an ambivalent state of mind, having a variety of concerns such as “How can I get back to normal?” But at the same time, they are ambivalent about discussing these concerns with their health care providers.466 Therefore, it is expected that they are unlikely to voluntarily consult a healthcare professional and take action for improvement. It is desirable to understand and support such a psychological state by utilizing screening tests.
3.2 Dealing With Depression and Other Psychological Symptoms
In general, psychotherapy, including psychoeducation and cognitive-behavioral therapy, and pharmacotherapy as needed are useful as basic interventions for depression and anxiety symptoms. It has been reported that exercise, which is practiced in CR, has a moderate effect on depressive symptoms, in addition to reducing the risk of CVD, stroke, and diabetes.467
Regarding psychological interventions for patients with CAD, a meta-analysis of 35 studies (total of 10,703 patients) reported that traditional psychotherapeutic interventions did not affect the overall mortality and recurrence rates of CAD, but did reduce cardiac mortality and depressive, anxiety, and stress symptoms.468
3.3 Integrated Support (Collaborative Care)
It has been reported that the combination of exercise and pharmacotherapy can improve depression, but a recent meta-analysis reported that exercise-based CR was effective in improving depression and anxiety in myocardial infarction patients.468 CR can provide a variety of mental and psychological supports from multiple professions, rather than single support from psychological professionals. Collaborative care (integrated support) can improve depression, anxiety, and QOL in CVD patients even when psychiatrists and other medical specialists are not available to treat the psychiatric and psychological symptoms, and studies on the effects of this support method have been conducted in recent years.469 Taking advantage of the characteristics of CR as team medical care, it is necessary to create an environment in which multiple professionals can utilize the essence of psychotherapy and other methods in their respective patient education and support.
VIII. Patient Education and Disease Management
1. Methods of Patient Education, Evaluation Methods and Evidence (Table 67)
Table 67. Recommendations and Levels of Evidence for Patient Education in CR
| |
COR |
LOE |
GOR
(MINDS) |
LOE
(MINDS) |
It is recommended to educate patients with CAD in order to improve HRQOL
and prevent cardiovascular death and rehospitalization |
I |
B |
B |
II |
It is recommended to educate patients with chronic heart failure in order to
improve their HRQOL and prevent rehospitalization |
I |
B |
B |
II |
CAD, coronary artery disease; COR, class of recommendation; CR, cardiac rehabilitation; GOR, grade of recommendation; HRQOL, health-related quality of life; LOE, level of evidence.
1.1 Methods
In randomized controlled trials that have evaluated the effectiveness of patient education in cardiac rehabilitation (CR), a variety of educational methods were reported.221,470–473 A 1-year outpatient psycho-educational consultations follow-up that included 12 weeks of physical activity and 4 psycho-educational consultations,221 a 3-week self-management program that included bidirectional interactive sessions (60–75 min) with patients by a multidisciplinary team,470 and a 36-month regular telephone intervention follow-up aimed at secondary prevention473 have been reported. However, it is difficult to manage the necessary time to provide patient education in addition to exercise training in conventional CR, which lasts ≈1 h/session, and there are limitations to multidisciplinary intervention. In the USA, “Intensive CR” began reimbursement in January 2011 for patients with angina pectoris, after coronary artery bypass grafting, heart failure, left ventricular assist device, and after heart transplantation. During each 4-h session, patients receive 1 h of supervised exercise. In addition, they receive 1 h of stress management, a 1-h group support session, and a 1-h group meal with a lecture.474 Although the standard CR program is an exercise-based program that consists of 1 h of exercise 3 times/week for 12 weeks (total of 36 h), the Intensive CR program consists of 2 sessions/week for 9 weeks (total of 72 h), and becomes a more systematic and intensive educational CR program (Table 68).474
Table 68. Intensive Educational and Conventional CR Programs in the USA
| |
Intensive and Educational CR |
Normal CR |
Program structure
(Time required) |
Physician-supervised exercise (1 h)
Nutritional education (1 h)*
Stress management (1 h)
Group support (1 h) |
Mainly supervised exercise training, with
some patient education (1 h) |
Insurance coverage in
the USA |
4 h per session, twice each week, for 9
weeks (72 h in total) |
1 h per session, 3 times/week, for 12 weeks
(36 h in total) |
*Balanced diet with a focus on vegetarian food (limited intake of processed foods, refined carbohydrates, trans-fatty acids, saturated fatty acids, and animal proteins). CR, cardiac rehabilitation. (Source: based on Freeman AM, et al, 2019.474)
Patient education in CR covers a wide range of topics, including exercise, nutrition, stress management, and returning to work/social life. In patient education, it is important to increase the patient’s adherence to CR. To achieve this, the following approaches have been proposed: strengthening motivation, continuous and professional education by healthcare providers, and social support from key persons.475,476 Improvement of the patient’s adherence to exercise training and lifestyle changes can be achieved if perceived benefits in quality of life (QOL) are better after intensive intervention.
It has also been reported that education in CR can improve the lifestyle of patients.9,477,478 In recent years, the importance of enhancing individual health literacy has been reported.479 Health literacy refers to the knowledge, motivation, and ability to obtain, understand, evaluate, and utilize health information. As for the evaluation indicators for health literacy, objective evaluation of skills, group-level evaluation, and self-reported evaluation have been reported.480 In CR, it is necessary to check the health literacy of patients and provide them with individualized educational content.
1.2 Evaluation of Effectiveness
The effectiveness of patient education in CR has been reported in terms of the following: (1) physical function, (2) coronary risk factors, (3) participation rate in CR programs, (4) exercise rate, (5) self-management ability, (6) satisfaction, (7) self-efficacy, (8) knowledge, and (9) QOL.221,470–473,481,482 Assessment methods include exercise testing, blood tests, and psychosocial measures (depression, anxiety, social support, and adherence).
2. Management of Coronary Risk Factors, Smoking Cessation Counseling (Table 69)
Table 69. Recommendations and Levels of Evidence for Coronary Risk Factor Management and Smoking Cessation Counseling in CR
| |
COR |
LOE |
GOR
(MINDS) |
LOE
(MINDS) |
It is recommended to perform comprehensive CR for smoking cessation,
weight control, blood pressure (including home blood pressure measurement),
lipids, and blood glucose |
I |
A |
A |
I |
COR, class of recommendation; CR, cardiac rehabilitation; GOR, grade of recommendation; LOE, level of evidence.
2.1 Evidence
Correcting coronary risk factors is one of the goals of CR. Table 70 shows the definition of each coronary risk factor and the target values for control.483–485
Table 70. Diagnosis of Coronary Risk Factors and Management Targets in CR
Coronary risk
factors |
Diagnostic criteria |
Management targets |
| High blood pressure |
(1) Office blood pressure ≥140/90 mmHg
(2) Out-of-office blood pressure
Home blood pressure ≥135/85 mmHg
24-h blood pressure ≥130/80 mmHg
Nocturnal blood pressure ≥120/70 mmHg
(1) or (2) |
Office blood pressure
CAD: <130/80 mmHg
Systolic blood pressure in heart failure:
<130 mmHg in patients with preserved left ventricular
contractility, 110–130 mmHg in patients with reduced
left ventricular contractility |
| Dyslipidemia |
LDL-C ≥140 mg/dL
HDL-C <40 mg/dL
TG ≥150 mg/dL
Non-HDL-C ≥170 mg/dL
Any of the above |
CAD:
LDL-C <100 mg/dL, non-HDL-C <130 mg/dL
HDL-C ≥40 mg/dL, TG <150 mg/dL
In patients with prior ACS, familial hypercholesterolemia,
diabetes mellitus, and/or atherothrombotic stroke,
LDL-C <70 mg/dL, non-HDL-C <100 mg/dL |
Diabetes mellitus/
impaired glucose
tolerance |
(1) Fasting blood glucose ≥126 mg/dL
(2) Casual blood glucose ≥200 mg/dL
(1) or (2) and HbA1c ≥6.5%
Impaired glucose tolerance: fasting glucose ≥110 mg/dL or
≥140 mg/dL at 2 h after loading |
HbA1c <7.0%
In individuals older than 65 years old, less than
8.0–8.5%, taking into account for cognitive function,
ADL decline, and hypoglycemic risk |
Obesity/metabolic
syndrome |
Waist circumference: men ≥85 cm, women ≥90 cm
In addition to the above, at least 2 of the following items
(1) Fasting TG ≥150 mg/dL or HDL-C ≤40 mg/dL
(2) Systolic blood pressure ≥130 mmHg or diastolic blood
pressure ≥85 mmHg
(3) Fasting glucose ≥110 mg/dL |
Correction of the left column
BMI <25 kg/m2 |
| CKD |
(1) Urinalysis, imaging, hematology, and pathology show the
presence of renal damage, especially proteinuria
≥0.15 g/gCr (albuminuria ≥30 mg/gCr)
(2) eGFR <60 mL/min/1.73 m2
Either or both (1) and (2) persists for more than 3 months |
eGFR ≥60 mL/min/1.73 m2 |
| Physical inactivity |
All awake actions with energy expenditure ≤1.5 METs |
|
| Smoking |
Cigarettes, cigars, electrically heated cigarettes, electronic
cigarettes |
No smoking and avoidance of passive smoking |
eGFR = 194 × Cr−1.094 × age−0.287 × 1 (× 0.739 for women). Fasting is defined as blood collection during fasting for ≥10 h. ACS, acute coronary syndrome; BMI, body mass index; CAD, coronary artery disease; CKD, chronic kidney disease; CR, cardiac rehabilitation; eGFR, estimated glomerular filtration rate; HDL-C, HDL cholesterol; LDL-C, LDL cholesterol; MET, metabolic equivalent; non-HDL-C, total cholesterol-HDL-C, TG, triglyceride.
A meta-analysis of 48 randomized controlled trials (RCTs) reported through 2003 examined the effects of CR in patients with coronary artery disease (CAD) and found significant improvements in total mortality (−20%), cardiovascular disease mortality (−26%), total cholesterol (−14.3 mg/dL), triglyceride (TG) (−20.4 mg/dL), systolic blood pressure (−3.2 mmHg), and smoking rate (−36%) in the CR group compared with the no-CR group.486 In a meta-analysis of 19 RCTs of exercise-based CR, approximately half of the reduction in cardiac death (−28%) was attributed to correction of coronary risk factors such as smoking cessation, lowering of cholesterol, and lowering of systolic blood pressure, with smoking cessation having a particularly large effect.487 In a study of 7,998 patients younger than 80 years who experienced acute coronary syndrome or underwent revascularization (CABG or PCI) in Europe, the rate of smoking cessation was significantly higher in the CR group, and the proportion of obese patients was lower.488
As already stated, a comprehensive CR program is useful for reducing smoking rates, controlling body weight, and improving blood pressure, lipid metabolism, and glucose tolerance.486,489
2.2 Hypertension and Blood Pressure Control
Hypertension is the greatest risk factor for cardiovascular disease (CVD).483 It is strongly associated with the development of CAD and heart failure in particular. Adequate and sustained lowering of blood pressure reduces the incidence of CVD and death from CVD, regardless of age, sex or comorbidity.483 The antihypertensive effects of moderate- to vigorous-intensity aerobic exercise and handgrip exercise have been established in a number of meta-analyses.483
2.3 Dyslipidemia and Lipid Management
The efficacy of lowering low-density lipoprotein cholesterol (LDL-C) for primary and secondary prevention of CAD has been established according to meta-analyses of large-scale RCTs. The absolute reduction in cardiovascular events correlated with LDL-C lowering, and the absolute reduction in events was particularly greater in high-risk patients, indicating the significance of aggressive lowering of LDL-C.490 In cohort studies, large-scale RCTs, and meta-analyses, high levels of non-high-density lipoprotein cholesterol (HDL-C) and triglyceride (TG), and a low level of HDL-C have been indicated as residual risks on statin therapy.491–493
2.4 Diabetes and Blood Glucose Control
Diabetes mellitus (DM) promotes atherosclerosis and is a cause of myocardial infarction (MI), stroke, aortic aneurysm and peripheral atherosclerotic disease, as well as heart failure (HF) and atrial fibrillation.494 DM is also a prognostic factor for MI and HF.495 The risk of CVD increases from the time of glucose intolerance and postprandial hyperglycemia before the development of DM. Therefore, it is important to evaluate postprandial hyperglycemia, decreased insulin secretion, and insulin resistance by oral glucose tolerance tests.494
It is well established that exercise training and increased daily physical activity are effective in lowering blood glucose levels in type 2 DM.485,496
2.5 Obesity, Metabolic Syndrome
Metabolic syndrome (MS) is a condition characterized by a combination of coronary risk factors based on insulin resistance associated with visceral fat accumulation. In a meta-analysis of 15 RCTs examining the effects of CR on MS and its components, CR significantly improved the incidence of MS by −22%, HDL-C by +2.13 mg/dL, waist circumference by −2.25 cm, systolic blood pressure by −6.2 mmHg, diastolic blood pressure by −2.53 mmHg, TG by −27.45 mg/dL, and fasting glucose by −6.42 mg/dL.497
2.6 CKD
Chronic kidney disease (CKD) is a risk factor not only for endstage renal failure but also for CVD.484 In patients with heart disease who participated in outpatient CR for 3–5 months, eGFR was significantly improved in patients with CKD.498,499
2.7 Physical Inactivity
Prolonged sedentary time is a risk factor for the development of CVD and DM and for total mortality, independent of physical activity.484,500
There is no consensus on whether CR decreases sedentary time. In a meta-analysis of 9 RCTs comparing patients using physical activity monitoring devices (wearing group) with those who did not (not wearing group) during maintenance CR, there was a significant improvement in exercise capacity and an increase in the number of steps taken in the wearing group.501 Wearable physical activity monitoring devices are useful for increasing daily physical activity.
2.8 Smoking and Smoking Cessation Counseling
Smoking (including cigarettes and cigars) is an independent risk factor for all CVDs and significantly increases the risk of CVD death and total mortality. Exposure to even small amounts of tobacco smoke significantly increases the risk of CVD.502 The risk of developing CAD due to smoking is 25% higher in women than in men.503
Passive smoking significantly increases the risk of developing CAD by 1.3-fold. Enactment of antismoking laws in communities and workplaces has significantly reduced the incidence of acute myocardial infarction (AMI) by 20% after 12 months, and the longer the period after enactment, the more pronounced the effect.504
Smoking cessation reduces the risk of disease progression, morbidity, and mortality in patients, irrespective of the presence of CVD, and this effect is seen in patients of any age and sex.484 A meta-analysis of cohort studies of patients with CAD showed that smoking cessation reduced the relative risk of death by 36%.505 Another multicenter observational study that enrolled patients with AMI in Japan showed that the multivariate-adjusted hazard ratio for mortality was 2.27-fold higher in continuing smokers than in nonsmokers.506
The 5A approach (Ask, Advice, Assess, Assist, Arrange) is recommended as a method of teaching smoking cessation.507 Newer forms of cigarettes (such as electrically heated cigarettes and e-cigarettes), which are different from traditional cigarettes, have been reported to be harmful to health due to the aerosols they produce508 and thus should be avoided. Although exercise training itself is not directly linked to smoking cessation, comprehensive CR can motivate patients to continue exercise and smoking cessation.
3. Self-Management and Lifestyle Interventions for Heart Failure (Table 71)
Table 71. Recommendations and Levels of Evidence for Self-Management and Lifestyle Interventions in CR of HF Patients
| |
COR |
LOE |
GOR
(MINDS) |
LOE
(MINDS) |
| It is recommended to provide education and support to improve self-care |
I |
A |
A |
I |
| It is recommended to utilize social resources |
I |
A |
A |
I |
| It is recommended to provide smoking cessation education |
I |
C |
B |
IVb |
| It is recommended to provide exercise instruction |
I |
B |
B |
II |
| It should be considered to counsel about their alcohol intake |
IIa |
C |
C1 |
VI |
| It should be considered to provide salt reduction instruction |
IIa |
C |
C1 |
VI |
| It should be considered to provide education on appropriate calorie intake |
IIa |
C |
C1 |
VI |
It should be considered to provide education on infection prevention and
vaccination |
IIa |
C |
B |
IVb |
| Sleep instruction may be considered |
IIb |
B |
C1 |
II |
COR, class of recommendation; CR, cardiac rehabilitation; GOR, grade of recommendation; HF, heart failure; LOE, level of evidence.
Self-management of heart failure (HF self-care) is the process of making decisions about all behaviors in daily life that are necessary to maintain a stable state without worsening symptoms.509 Self-care is considered essential in the management of HF to avoid acute worsening and increasing the risk of death.510 Patient education on how to recognize and appropriately manage HF symptoms is important.102,445,511
Self-care involves adherence to treatment and healthy lifestyle activities (e.g., adherence to medication, exercise, proper diet) (Process 1: Self-Care Maintenance), as well as being aware of HF symptoms and being able to understand their meaning (e.g., being aware of shortness of breath and edema and being able to determine that fluid retention is occurring). Self-care monitoring, such as weight measurement, home blood pressure measurement, and self-monitoring of pulse, is useful for understanding HF symptoms (Process 2: Awareness and Understanding of Heart Failure Symptoms). Finally, the goal is to be able to take appropriate action when HF symptoms are recognized (e.g., see a doctor promptly when symptoms worsen) (Process 3: Self-Care Management). These three processes include elements that can be resolved by the patient’s own decisions and elements that require consultation with medical personnel, such as medication.509
Self-care behaviors vary depending on disease-specific factors (e.g., degree of HF symptoms, complication status), personal factors (e.g., cognitive function, culture, faith), and environmental factors (e.g., social isolation, availability of social resources, living environment, economic environment). In order to improve and sustain self-care, it is important to gain a sense of self-efficacy not only through knowledge but also through one’s own successes and the experiences of others.512
4. Disease Management in Outpatient CR (Table 72)
Table 72. Recommendations and Levels of Evidence for Disease Management in Outpatient CR
| |
COR |
LOE |
GOR
(MINDS) |
LOE
(MINDS) |
For patients with chronic HF, it is recommended to provide disease
management by a multidisciplinary team |
I |
B |
A |
II |
For patients with CAD, it should be considered to provide disease
management by a multidisciplinary team |
IIa |
B |
B |
III |
CAD, coronary artery disease; COR, class of recommendation; GOR, grade of recommendation; HF, heart failure; LOE, level of evidence.
4.1 Significance and Challenges of Disease Management
A disease management program is a systematic program that aims to improve prognosis, including reduction in rehospitalizations, through standardized medical care and patient education based on medical guidelines. Outpatient CR is also a disease management program that focuses on exercise training, medication counseling, dietary counseling, lifestyle intervention, counseling, correction of coronary risk factors, and management of acute exacerbation.10 The outcomes of disease management in outpatient CR are improvement of life expectancy, prevention of rehospitalization, and prevention of physical deterioration. For this purpose, it is important to support patients and their families to continue appropriate self-management behaviors in their daily lives.
Outpatient CR is a comprehensive program developed by a multidisciplinary team (physicians, nurses, physical therapists, health and exercise instructors, pharmacists, dietitians, clinical psychologists/licensed psychologists, etc.) and is useful as a system for effective disease management. In addition, outpatient CR is an ideal setting for patients to receive necessary lifestyle intervention because it is monitored and patients can receive appropriate observation and counseling on their physical condition during and after exercise training from a multidisciplinary team.478
Davidson et al reported that for patients hospitalized with moderate HF, after 3 months of outpatient CR intervention (weekly supervised exercise training, HF assessment by a nurse specialist, and multidisciplinary educational counseling, home exercise training counseling, and telephone counseling), QOL, 6MWD, and HF severity improved, and readmission rates were significantly lower by 1 year.9 These results indicate that outpatient CR programs not only improve exercise capacity, but also improve QOL, prevent rehospitalization, and are useful as disease management programs. A systematic review of disease management in HF showed that multidisciplinary interventions reduced all-cause death and that case management by nurses with specialized training may reduce the rate of rehospitalization for HF or any cause.514
A report from Europe showed that outpatient CR, including a disease management program after acute myocardial infarction, significantly improved all-cause death by 38% at 1 year in a backward-looking observational study using propensity score matching.515 In another observational study, a significant improvement in exercise capacity and coronary risk factors was observed with active participation in outpatient CR, even in the low-risk group.113
Recently, a survey on multidisciplinary collaborative care and CR for HF patients reported that although the implementation rate of outpatient CR was 56.5%, only 7% of patients hospitalized for HF received outpatient CR after discharge.166 For patients hospitalized for CAD or HF, comprehensive educational support is provided during hospitalization based on acute rehabilitation but after discharge, systematic disease management and lifestyle intervention are discontinued in the outpatient clinic, despite significant changes in the living environment, such as diet and physical activity.516 Therefore, increasing the prevalence of outpatient CR, especially after hospital discharge, is a major challenge.
4.2 Disease Management in Practice
At the introduction of outpatient CR, the CR staff evaluates coronary risk factors, cardiac function, and exercise capacity, confirms the purpose of CR with the patient, collects and evaluate information on lifestyle and provides consultation, support, and counseling. At each outpatient visit for CR, self-monitoring of physical symptoms before and during exercise and educational support for daily life are provided (see Figure 5). After that, periodic evaluations (after 1 month, 3 months, 6 months, 1 year, and every year thereafter) should be performed, and disease management be based on the evaluation results. After outpatient CR is completed, it is expected that a system will be established that will lead to evaluation and disease management in the maintenance CR setting.
4.3 Inter-Professional Collaboration (Table 73)
Table 73. Recommendations and Levels of Evidence for Inter-Professional Collaboration in CR
| |
COR |
LOE |
GOR
(MINDS) |
LOE
(MINDS) |
It is recommended to perform CR by a multidisciplinary team including doctors,
nurses, physical therapists, occupational therapists, and others |
I |
C |
A |
III |
COR, class of recommendation; CR, cardiac rehabilitation; GOR, grade of recommendation; LOE, level of evidence.
4.3.1 Occupations Comprising Team Medicine
Table 74 shows the components of CR and the occupations, roles, and responsibilities of the staff involved. CR is a comprehensive intervention that can improve ADL and QOL by restoring systemic functions. CR can also help patients recover from cardiac dysfunction and prevent recurrence by creating a good systemic environment for the heart. In other words, CR is the setting in which patients are evaluated, monitored, and trained to educate themselves in self-management to prevent recurrence. For this purpose, it is ideal to form a multidisciplinary team that includes the following professionals: (1) cardiologist, (2) CR nurse with specialized training, (3) physical or occupational therapist, (4) specialist who prescribes exercise and instructs exercise training, (5) clinical laboratory technician who is in charge of exercise stress tests, (6) dietitian, and (7) others such as a clinical or licensed psychologist, pharmacist, and social worker.
Table 74. Division of Roles of Staff Involved in CR
| Role |
Profession |
| Facility director |
Management and operation of the facility
Management responsibility |
Cardiologist |
| Exercise training |
Create exercise program
Provide counseling to exercise instructors |
Physical therapists, occupational therapists,
nurses, health fitness programmer, etc.
Exercise instructors |
| Exercise program implementation |
Physical therapist, occupational therapist, nurse,
health and exercise instructor, etc.
Exercise instructors |
| Diet therapy |
Dietary counseling |
Managerial dietitian, nurse |
| Medication |
Medication education |
Pharmacist, nurse |
| Consultation |
Smoking cessation education
Instruction on stress management, etc. |
Nurses
Clinical psychologist/licensed psychologist, etc. |
| Utilization of social resources |
Social worker |
| Examination |
Examination of coronary risk factors
CPX |
Clinical examination techniques |
CPX, cardiopulmonary exercise testing; CR, cardiac rehabilitation.
Exercise training should be instructed by physical therapists or occupational therapists from the acute phase (phase I) to the late recovery phase (phase II), but health exercise instructors are also acceptable for primary prevention and maintenance phase (phase III). However, for primary prevention and the maintenance phase (phase III), a health exercise instructor could serve this role. For patients with HF, a precise exercise prescription is particularly necessary, and the presence of a laboratory technician skilled in exercise stress testing is essential.
The Japanese Society for Cardiac Rehabilitation has established and operated a CR instructor system since 2000 to standardize the knowledge of those involved in CR, such that CR can be performed without being restricted to a specific profession. There are already more than 5,800 certified CR instructors (as of March 2021).
4.3.2 Multidisciplinary Conferences
Regular multidisciplinary conferences are essential for team medicine to function as a high-quality cooperative body that is different from simply a group of professionals. It is desirable that as many professionals as possible, including physicians, nurses, physical therapists, dietitians, pharmacists, clinical psychologists/licensed psychologists, medical social workers, and exercise instructors, participate in the conference. Such a conference should include the following: introduction of new patients, progress reports of patients who have completed the program, information sharing and planning of countermeasures for problematic cases, and consultation on social rehabilitation and mental aspects. Counseling on social rehabilitation and mental health is included, in addition to counseling on cooperation with social welfare services.
As patients become part of the super-aging group, multidisciplinary conferences are becoming more and more important in establishing the future direction. In addition, it is necessary to hold multidisciplinary conferences to discuss the future treatment policy itself, such as considering the presence of multiple diseases, frailty, sarcopenia, and dementia in critically ill patients, and the extent to which they should be treated, and the transition to palliative medicine and home care.
IX. Tele-CR (Table 75)
Table 75. Recommendations and Levels of Evidence for Targeted Tele-CR
| |
COR |
LOE |
GOR
(MINDS) |
LOE
(MINDS) |
It should be considered to introduce tele-CR to improve the prognosis of
cardiac disease |
IIa |
B |
B |
II |
| It should be considered to introduce tele-CR to improve exercise capacity |
IIa |
B |
B |
II |
| It should be considered to introduce tele-CR to improve risk factors for CAD |
IIa |
B |
B |
II |
CAD, coronary artery disease; COR, class of recommendation; CR, cardiac rehabilitation; GOR, grade of recommendation; LOE, level of evidence.
The rate of participation in outpatient cardiac rehabilitation (CR) is low worldwide, ranging from 10.3% to 16.3% in patients with coronary artery disease CAD in the USA,517 ≈50% in patients with CAD in Europe,488 and 7% in both inpatient and outpatient CR according to the AMED-CHF study in Japan.166 A major factor for poor implementation of outpatient CR is access and time barriers to CR facilities.518–521 In the USA and Europe, home-based CR, in which patients exercise alone at home, has been proposed as an alternative to outpatient CR.195,418 Furthermore, since 2000, tele-CR based on information and communication technology (ICT), including interactive communication using the Internet and biometric information management, has become widespread.522
2. Evidence
2.1 Usefulness of Outpatient CR and Tele-CR
In a meta-analysis of 880 patients in 6 randomized controlled trials (RCTs) reported through 2003 comparing home-based CR with center-based supervised CR in patients with CAD, improvements in exercise capacity, systolic blood pressure, and total cholesterol were similar between groups, but quality of life (QOL) was better with home-based CR.523 In a meta-analysis of 1,938 patients in 12 RCTs reported through 2008 in patients with CAD or heart failure, there were no significant differences in the management of death, reinfarction, revascularization, cardiac readmission, exercise capacity, or risk factors between the home-based and outpatient CR groups.524 A meta-analysis of 17 RCTs including 2,172 patients with CAD or heart failure reported by 2014 showed no significant differences in mortality, cardiac events, exercise capacity, serum lipids, blood pressure, QOL, or medical costs between the home-based and outpatient CR groups, and a slight improvement in adherence in the home-based CR group.525 A meta-analysis of 2,890 patients in 23 RCTs reported through 2016 found no significant differences in mortality, exercise capacity, or QOL between the 2 groups.526
These results suggest that the efficacy and safety of home-based CR is comparable to that of outpatient CR, as long as the cardiac condition and response to exercise stress testing are confirmed and exercise is performed as prescribed.
2.2 Use of Monitoring Devices and Image Communication
Since around the year 2000, the effectiveness of weight control, smoking cessation, and diet and exercise training using ICT for continuing CR has been reported,527,528 and has been applied to tele-CR.522 RCTs from Europe and the USA reported on comparing home-based CR with outpatient CR using an internet-based ECG waveform monitoring device.522,529–531 Although each of the studies included small numbers of patients, there was comparable safety and improvement in physical function between the two groups, and the home-based CR group showed comparable or greater improvement in QOL. In an RCT from Australia, Varnfield et al reported that home-based tele-CR using a smartphone provided blood pressure and weight monitoring, pedometer data, interviews with a supervisor, and educational content, and that the home-based tele-CR group showed a better understanding of CR, better adherence, weight loss, and improved QOL than the outpatient CR group.532
X. Medical Economic Evaluation
1. Impact on Medical Costs
Two review articles compared the medical costs of cardiac rehabilitation (CR), which adds education and other services to exercise training, with those of usual care, and found that the development of institutional interventions and ancillary programs can reduce costs such as readmissions.533,534 Seven other papers reported that the implementation of CR or a similar intervention program can reduce healthcare costs in the long term compared with no intervention, although it is not possible to make a general comparison because of differences in program content, evaluation period, and cost calculation items.535–541 In addition, reports of tele-rehabilitation have increased in recent years, and 1 randomized controlled trial (RCT) reported a reduction in healthcare costs.542 Another RCT reported that it is an economical procedure if evaluated over the long term.543
These results suggest that the initial cost of the management program can be recovered in the long run by actively recruiting patients to receive comprehensive CR, and also suggest that long-term CR is effective in preventing recurrence of cardiac disease and may have a significant impact on health insurance finances by optimizing healthcare costs compared with noncompliant patients.
Although there are currently few reports with a high level of evidence in chronic heart failure, there are 2 RCTs including heart failure patients. A recent report of an institutional intervention showed that a 12-week CR (exercise training plus education and counseling) for patients with heart failure, including those with coronary artery disease (CAD) or valve replacement, was not superior in improving health-related quality of life (HRQOL) at 6 months (−0.000 quality-adjusted life years (QALYs), 95% confidence interval (CI) −0.021 to 0.020). However, it tended to reduce social costs (including indirect medical costs) (−1,609 €/person, 95% CI −6,162 to 2,942).
2. Evaluation by Cost-Effectiveness
According to 10 major reports of cost utility analysis (CUA),540–549 the sample size of the CUAs using QALYs as the outcome measure ranged from 109 to 825 patients. The target diseases were acute coronary syndrome, myocardial infarction, and chronic heart failure, and the CR program was based on exercise training such as treadmill, bicycle ergometer, and walking. In 1 paper,540 only exercise training was included, but in the others, education and counseling on risk factors and lifestyle modification were also included in the program to evaluate the effect of comprehensive CR. All control subjects received usual care without CR. All reports were based on RCTs with a high level of evidence, and CR was expected to have a high economic performance if continued for more than 1 year.
Indeed, the systematic review (15 papers) of Papadakis et al533 and the meta-analysis of Takura et al (5 papers)550 also concluded that the procedure was cost effective (cost of US$ 668–16,118 per QALY gained and significant “cost savings and a significant increase in QALYs”). There are an increasing number of reports on tele-intervention as a health economics evaluation of CR. According to 3 CUAs based on RCTs,542,543,551 comprehensive programs are superior in deploying a combination with institutional interventions on a large scale (and with increased productivity).
Next is cost-effectiveness analysis (CEA), of which there are 6 major reports.475,537,552–555 Of these, the 2 with the highest level of evidence475,554 had total sample sizes of 100–296 patients (NYHA classifications II, III, and IV), including those with chronic heart failure, and the measures of effectiveness were 3-year mortality, survival, and coronary artery stenosis score. They reported that the CR group had a survival advantage over the non-CR group in relation to the required cost.
In addition, a review article556 that included educational and counseling interventions and exercise found that the outcomes of HRQOL and prolonged survival relative to cumulative medical costs were superior with CR in terms of long-term prognosis. Improvement in HRQOL was also reported in a meta-analysis of articles.557 In addition, 2 recent reports on tele-interventions,558,559 found that improvements in peak V̇O2
and cardiovascular risk were cost-effective in combination with institutional interventions and under long-term observation.
Appendix 1. Details of Members
Chairs:
• Shigeru Makita, Department of Cardiac Rehabilitation, Saitama Medical University International Medical Center
• Takanori Yasu (Vice-Chair), Department of Cardiovascular Medicine and Nephrology, Dokkyo Medical University Nikko Medical Center
Members:
• Hitoshi Adachi, Department of Cardiology, Gunma Prefectural Cardiovascular Center
• Yoshihiro J Akashi, Division of Cardiology, Department of Internal Medicine, St. Marianna University School of Medicine
• Yoshihiro Fukumoto, Division of Cardiovascular Medicine, Department of Internal Medicine, Kurume University School of Medicine
• Yutaka Furukawa, Department of Cardiovascular Medicine, Kobe City Medical Center General Hospital
• Emiko Hasegawa, Faculty of Psychology and Social Welfare, Seigakuin University
• Shunichi Ishihara, Department of Psychology, Bunkyo University Faculty of Human Sciences
• Yoshitaka Iso, Division of Cardiology, Showa University Fujigaoka Hospital
• Hideo Izawa, Department of Cardiology, Fujita Health University of Medicine
• Yutaka Kimura, Department of Health Sciences, Kansai Medical University Hospital
• Shinji Koba, Division of Cardiology, Department of Medicine, Showa University School of Medicine
• Masahiro Kohzuki, Department of Internal Medicine and Rehabilitation Science, Tohoku University Graduate School of Medicine
• Akira Koike, Department of Cardiology, Faculty of Medicine, University of Tsukuba
• Shin-ichiro Miura, Department of Cardiology, Fukuoka University School of Medicine
• Masatoshi Nagayama, Department of Cardiovascular Medicine, Sakakibara Heart Institute
• Hideo Ohuchi, Department of Pediatrics, National Cerebral and Cardiovascular Center
• Yusuke Ohya, Department of Cardiovascular Medicine, Nephrology and Neurology, Graduate School of Medicine, University of the Ryukyus
• Koichi Okita, Graduate School of Lifelong Sport, Hokusho University
• Kazuto Omiya, Shimazu Medical Clinic
• Masataka Sata, Department of Cardiovascular Medicine, Tokushima University Graduate School of Biomedical Sciences
• Kazunori Shimada, Department of Cardiology, Juntendo University School of Medicine
• Tomoki Shimokawa, Department of Cardiovascular Surgery, Teikyo University
• Hirokazu Shiraishi, Department of Cardiovascular Medicine, Kyoto Prefectural University of Medicine
• Naokata Sumitomo, Department of Pediatric Cardiology, Saitama Medical University International Medical Center
• Tetsuya Takahashi, Department of Physical Therapy, Faculty of Health Science, Juntendo University
• Tomoyuki Takura, Department of Healthcare Economics and Health Policy, Graduate School of Medicine, The University of Tokyo
• Hiroyuki Tsutsui, Department of Cardiovascular Medicine, Graduate School of Medical Sciences, Kyushu University
• Sumio Yamada, Department of Integrated Health Sciences, Nagoya University Graduate School of Medicine
• Yuichiro Yamada, Center for Diabetes, Endocrinology and Metabolism, Kansai Electric Power Hospital
• Satoshi Yasuda, Department of Cardiovascular Medicine, Tohoku University Graduate School of Medicine
• Toshiko Yoshida, College of Nursing, St. Luke’s International University
• Dai Yumino, Yumino Heart Clinic
Collaborators:
• Takuji Adachi, Department of Physical Therapy, Nagoya University Graduate School of Medicine
• Kanta Fujimi, Department of Rehabilitation, Fukuoka University Hospital
• Taiki Higo, Department of Cardiovascular Medicine, Graduate School of Medical Sciences, Kyushu University
• Tasuku Honda, Department of Cardiovascular Surgery, Hyogo Brain and Heart Center
• Toshimi Ikegame, Department of Nursing, Sakakibara Heart Institute
• Takeshi Ishida, Department of Medicine, Saitama Citizens Medical Center
• Kazuhiro P Izawa, Kobe University Graduate School of Health Science
• Naoya Kakutani, Health Link Co., Ltd.
• Kentaro Kamiya, Department of Rehabilitation, School of Allied Health Sciences, Kitasato University
• Yusuke Kasahara, Department of Rehabilitation, St. Marianna University Yokohama Seibu Hospital
• Masaaki Kato, Department of Cardiovascular Surgery, Morinomiya Hospital
• Shintaro Kinugawa, Department of Cardiovascular Medicine, Graduate School of Medical Sciences, Kyushu University
• Yasuyuki Kobayashi, Department of Medical Technology, Gunma Prefectural Cardiovascular Center
• Yuji Kono, Department of Rehabilitation, Fujita Health University Hospital
• Teruyuki Koyama, Department of Rehabilitation, Kameda Medical Center
• Noriko Matsumoto, Department of Nutrition, St. Luke’s International Hospital
• Yasuharu Matsumoto, Department of Cardiovascular Medicine, Shioya Hospital, International University of Health and Welfare
• Ikuko Miyawaki, Department of Nursing, Kobe University Graduate School of Health Sciences
• Makoto Murata, Department of Cardiology, Gunma Prefectural Cardiovascular Center
• Eisaku Nakane, Cardiovascular Center, Kitano Hospital
• Michio Nakanishi, Department of Cardiovascular Medicine, National Cerebral and Cardiovascular Center
• Mari Nishizaki, Department of Rehabilitation, National Hospital Organization Okayama Medical Center
• Hiroaki Obata, Division of Internal Medicine, Division of Rehabilitation, Niigata Minami Hospital
• Naohiko Osada, Department of Physical Checking, St. Marianna University Toyoko Hospital
• Neiko Ozasa, Cardiovascular Medicine, Kyoto University Hospital
• Kazuhiro Sase, Clinical Pharmacology and Regulatory Science, Graduate School of Medicine, Juntendo University
• Shinji Sato, Department of Physical Therapy, Teikyo Heisei University
• Tatsuhiro Shibata, Division of Cardiovascular Medicine, Department of Internal Medicine, Kurume University School of Medicine
• Norio Suzuki, Division of Cardiology, Department of Internal Medicine, St. Marianna University School of Medicine
• Daisuke Tamaki, Department of Nutrition, Showa University Fujigaoka Hospital
• Noboru Watanabe, Department of Cardiology, Hokushin General Hospital
• Shusuke Yagi, Department of Cardiovascular Medicine, Tokushima University Graduate School of Biomedical Sciences
• Midori Yamada, Faculty of Nursing, Kyoritsu Women’s University
• Minako Yamaoka-Tojo, Department of Rehabilitation, School of Allied Health Sciences, Kitasato University
• Masanobu Yanase, Department of Transplantation, National Cerebral and Cardiovascular Center
• Miho Yokoyama, Department of Cardiovascular Medicine, Juntendo University Graduate School of Medicine
Independent Assessment Committee:
• Yoichi Goto, Yoka Municipal Hospital
• Ken-Ichi Hirata, Department of Internal Medicine, Kobe University Graduate School of Medicine
• Haruki Ito, Sakakibara Heart Institute
• Takeshi Kimura, Department of Cardiovascular Medicine, Kyoto University Graduate School of Medicine
• Syunei Kyo, Tokyo Metropolitan Geriatric Medical Center
• Ryuji Nohara, Hirakata Kohsai Hospital
(Listed in alphabetical order; affiliations as of March 2021)
Appendix 2. Disclosure of Potential Conflicts of Interest (COI): JCS/JACR 2021 Guideline on Rehabilitation in Patients With Cardiovascular Disease (2018/1/1–2020/12/31)
| Author |
Member’s own declaration items |
COI of the marital partner,
first-degree family members,
or those who share income
and property |
COI of the head of the
organization/department to
which the member belongs
(if the member is in a position
to collaborate with the head of
the organization/department) |
Employer/
leadership
position
(private
company) |
Stakeholder |
Patent
royalty |
Honorarium |
Payment for
manuscripts |
Research grant |
Scholarship
(educational) grant |
Endowed chair |
Other
rewards |
Employer/
leadership
position
(private
company) |
Stakeholder |
Patent
royalty |
Research
grant |
Scholarship
(educational)
grant |
Chairs:
Shigeru Makita |
|
|
|
Otsuka
Pharmaceutical
Co., Ltd.
Daiichi Sankyo
Company,
Limited
Fukuda Denshi
Co., Ltd |
|
|
|
|
|
|
|
|
|
|
Chairs:
Takanori Yasu |
|
|
|
|
|
MTG Co., Ltd.
Kowa Company,
Ltd.
AstraZeneca K.K. |
Abbott Medical
Japan L.L.C |
|
|
|
|
|
|
|
Members:
Yoshihiro J
Akashi |
|
|
|
Daiichi Sankyo
Company,
Limited |
|
Amgen Astellas
BioPharma K.K.
Hubit Genomix
Inc.
National Cerebral
and
Cardiovascular
Center
Otsuka
Pharmaceutical
Co., Ltd.
Daiichi Sankyo
Company,
Limited
Mitsubishi Tanabe
Pharma
Corporation
Nippon Boehringer
Ingelheim Co.,
Ltd.
Nihon Medi-Physics
Co., Ltd. |
MC, Inc.
Abbott Vascular
Japan Co., Ltd.
Abbott Medical
Japan L.L.C
Edwards
Lifesciences
Corporation
Nippon Boehringer
Ingelheim Co.,
Ltd.
Nihon Medi-Physics
Co., Ltd.
Medtronic Japan
Co., Ltd.
Nihon Kohden
Corp.
FUJIFILM RI
Pharma Co., Ltd.
FUJIFILM
Toyama Chemical
Co., Ltd.
Takeda
Pharmaceutical
Company Limited |
|
|
|
|
|
|
|
Members:
Yoshihiro
Fukumoto |
|
|
|
Actelion
Pharmaceuticals
Japan Ltd.
AstraZeneca K.K.
Novartis Pharma
K.K.
Bayer Yakuhin,
Ltd.
Janssen
Pharmaceutical
K.K.
Ono
Pharmaceutical
Co., Ltd.
Otsuka
Pharmaceutical
Co., Ltd.
Daiichi Sankyo
Company,
Limited |
|
Alnylam
Pharmaceuticals
Inc.
Actelion
Pharmaceuticals
Japan Ltd.
Daiichi Sankyo
Company,
Limited
Nippon Shinyaku
Co., Ltd. |
MSD K.K.
Astellas Pharma Inc.
Abbott Medical
Japan L.L.C
Eisai Co., Ltd.
Novartis Pharma
K.K.
Bayer Yakuhin, Ltd.
Shionogi & Co., Ltd.
Kowa
Pharmaceutical
Co., Ltd.
Mochida
Pharmaceutical
Co., Ltd.
Teijin Pharma
Limited
Japan Lifeline Co.,
Ltd.
Takeda
Pharmaceutical
Company Limited |
|
|
|
|
|
|
|
Members:
Yutaka Furukawa |
|
|
|
Daiichi Sankyo
Company,
Limited
Ono
Pharmaceutical
Co., Ltd.
Bayer Yakuhin,
Ltd.
Novartis Pharma
K.K. |
|
|
|
|
|
|
|
|
|
|
Members:
Hideo Izawa |
|
|
|
Daiichi Sankyo
Company,
Limited
Otsuka
Pharmaceutical
Co., Ltd.
Bristol-Myers
Squibb |
|
|
Takeda
Pharmaceutical
Company Limited
FUJIFILM
Toyama Chemical
Co., Ltd.
Kowa Company,
Ltd.
Bayer Yakuhin, Ltd.
Boston Scientific
Japan K.K.
Abbott Medical
Japan L.L.C
BIOTRONIK
Japan, Inc. |
|
|
|
|
|
|
|
Members:
Yutaka Kimura |
|
|
|
Japan Broadcasting
Corporation
Osaka Medical
Association
Japan Organization
of Occupational
Health and Safety
AstraZeneca K.K.
Daiichi Sankyo
Company,
Limited
Pfizer Japan Inc.
Mochida
Pharmaceutical
Co., Ltd.
Novartis Pharma
K.K.
Astellas Pharma
Inc.
Mitsubishi Tanabe
Pharma
Corporation
Takeda
Pharmaceutical
Company
Limited |
Sekaibunka
Holdings |
|
|
|
|
|
|
|
Koga Software
Company
Total Brain
Care Co.,
Ltd
Family Inada
Co., Ltd. |
Takeda
Pharmaceutical
Company
Limited
Astellas Pharma
Inc.
Daiichi Sankyo
Company,
Limited
Mochida
Pharmaceutical
Co., Ltd. |
Members:
Shinji Koba |
|
|
|
Pfizer Japan Inc.
Kowa Company,
Ltd.
Takeda
Pharmaceutical
Company
Limited
MSD K.K. |
|
|
|
|
|
|
|
|
|
|
Members:
Masahiro
Kohzuki |
|
|
|
Bayer Yakuhin,
Ltd.
Kyowa Kirin Co.,
Ltd.
Daiichi Sankyo
Company,
Limited
Chugai
Pharmaceutical
Co., Ltd. |
Makino
Shuppan
IGAKUSHOIN
Ltd.
Ishiyaku
Publishers,
Inc. |
|
|
|
|
|
|
|
|
|
Members:
Shin-ichiro Miura |
|
|
|
Bayer Yakuhin,
Ltd.
Daiichi Sankyo
Company,
Limited
Nippon Boehringer
Ingelheim Co.,
Ltd.
Takeda
Pharmaceutical
Company
Limited |
|
|
Astellas Pharma Inc.
Abbott Vascular
Japan Co., Ltd.
OMRON
HEALTHCARE
Co., Ltd.
HeartFlow Japan
G.K.
Bayer Yakuhin, Ltd.
Sompo Japan
Insurance Inc.
Daiichi Sankyo
Company,
Limited
Takeda
Pharmaceutical
Company Limited |
|
|
|
|
|
|
|
Members:
Yusuke Ohya |
|
|
|
Daiichi Sankyo
Company,
Limited
Takeda
Pharmaceutical
Company
Limited
Bayer Yakuhin,
Ltd. |
|
KANEKA
CORPORATION |
Edwards
Lifesciences
Corporation
KANEKA
CORPORATION
KANEKA MEDIX
CORP.
Baxter Limited
Medtronic Japan
Co., Ltd.
Japan Lifeline Co.,
Ltd.
Daiichi Sankyo
Company,
Limited
Takeda
Pharmaceutical
Company Limited |
|
|
|
|
|
|
|
Members:
Masataka Sata |
|
|
|
Takeda
Pharmaceutical
Company
Limited
Bayer Yakuhin,
Ltd.
Mitsubishi Tanabe
Pharma
Corporation
Daiichi Sankyo
Company,
Limited
Astellas Pharma
Inc. |
|
Bayer Yakuhin, Ltd.
Daiichi Sankyo
Company,
Limited
Nippon Boehringer
Ingelheim Co.,
Ltd. |
Takeda
Pharmaceutical
Company Limited
Mitsubishi Tanabe
Pharma
Corporation
Astellas Pharma Inc.
MSD K.K. |
Nippon Boehringer
Ingelheim Co.,
Ltd. |
|
|
|
|
|
|
Members:
Kazunori Shimada |
|
|
|
Takeda
Pharmaceutical
Company
Limited
Daiichi Sankyo
Company,
Limited |
|
|
Daiichi Sankyo
Company,
Limited
Takeda
Pharmaceutical
Company Limited
Roche Diagnostics
K.K. |
|
|
|
|
|
|
|
Members:
Tomoki
Shimokawa |
|
|
|
|
|
|
TERUMO
CORPORATION
Edwards
Lifesciences
Corporation
Abbott Medical
Japan L.L.C
Japan Lifeline Co.,
Ltd. |
|
|
|
|
|
|
|
Members:
Hirokazu Shiraishi |
|
|
|
Medtronic Japan
Co., Ltd.
Abbott Medical
Japan L.L.C
Japan Lifeline Co.,
Ltd. |
|
|
Medtronic Japan
Co., Ltd.
Abbott Medical
Japan L.L.C |
|
|
|
|
|
|
|
Members:
Tetsuya Takahashi |
|
|
|
|
|
|
|
|
|
|
|
|
Philips Japan,
Ltd.
TOHO
HOLDINGS
CO., LTD. |
|
Members:
Tomoyuki Takura |
|
|
|
|
|
|
|
Chugai
Pharmaceutical
Co., Ltd. |
|
|
|
|
|
|
Members:
Hiroyuki Tsutsui |
|
|
|
AstraZeneca K.K.
Novartis Pharma
K.K.
Bayer Yakuhin,
Ltd.
Pfizer Japan Inc.
Kowa Company,
Ltd.
Ono
Pharmaceutical
Co., Ltd.
Otsuka
Pharmaceutical
Co., Ltd.
Daiichi Sankyo
Company,
Limited
Teijin Pharma
Limited
Mitsubishi Tanabe
Pharma
Corporation
Nippon Boehringer
Ingelheim Co.,
Ltd. |
nippon rinsho
Co., Ltd. |
IQVIA Services
Japan K.K.
OMRON
HEALTHCARE
Co., Ltd.
Medical Innovation
Kyushu
MEDINET Co.,
Ltd.
Daiichi Sankyo
Company,
Limited
Mitsubishi Tanabe
Pharma
Corporation
Japan Tobacco Inc.
Nippon Boehringer
Ingelheim Co.,
Ltd. |
St.Mary's Hospital
Daiichi Sankyo
Company,
Limited
Teijin Pharma
Limited
Teijin Healthcare
Limited
Mitsubishi Tanabe
Pharma
Corporation
Nippon Boehringer
Ingelheim Co.,
Ltd. |
Actelion
Pharmaceuticals
Japan Ltd. |
|
|
|
|
|
|
Members:
Sumio Yamada |
PREVENT
inc. |
|
MINATO
MEDICAL
SCIENCE
CO., LTD. |
|
|
MINATO
MEDICAL
SCIENCE CO.,
LTD |
|
|
|
|
|
|
|
|
Members:
Satoshi Yasuda |
|
|
|
CSL Behring K.K.
Bayer Yakuhin,
Ltd.
Bristol-Myers
Squibb
Kowa Company,
Ltd.
Daiichi Sankyo
Company,
Limited
Takeda
Pharmaceutical
Company
Limited |
|
IQVIA Services
Japan K.K.
JSR Corporation
NEC Solution
Innovators, Ltd.
Actelion
Pharmaceuticals
Japan Ltd.
Abbott Vascular
Japan Co., Ltd.
Bayer Yakuhin, Ltd.
Kowa Company,
Ltd.
Daiichi Sankyo
Company,
Limited
Takeda
Pharmaceutical
Company Limited |
Abbott Medical
Japan L.L.C
Amicus
Therapeutics, Inc.
Bayer Yakuhin, Ltd.
Roche Diagnostics
K.K.
Kowa Company,
Ltd.
Otsuka
Pharmaceutical
Co., Ltd.
Sumitomo
Dainippon
Pharma Co., Ltd.
Takeda
Pharmaceutical
Company Limited |
Abbott Medical
Japan L.L.C
Sound Wave
Innovation CO.,
LTD.
ZEON MEDICAL
INC.
Tesco
TERUMO
CORPORATION
Shionogi & Co., Ltd.
Kowa Company,
Ltd.
Mochida
Pharmaceutical
Co., Ltd.
Ono Pharmaceutical
Co., Ltd.
Otsuka
Pharmaceutical
Co., Ltd.
Nippon Boehringer
Ingelheim Co.,
Ltd.
Medtronic Japan
Co., Ltd.
Japan Lifeline Co.,
Ltd.
Nihon Kohden
Corp.
Nippon Shinyaku
Co., Ltd.
Takeda
Pharmaceutical
Company Limited |
|
|
|
|
|
|
Members:
Dai Yumino |
|
|
|
Daiichi Sankyo
Company,
Limited
Novartis Pharma
K.K. |
|
|
|
|
|
|
|
|
|
|
Collaborators:
Kentaro Kamiya |
|
|
|
Otsuka
Pharmaceutical
Factory, Inc.
Otsuka
Pharmaceutical
Co., Ltd.
EIKEN Chemical
Co., Ltd. |
|
EIKEN Chemical
Co., Ltd.
SoftBank Corp. |
EIKEN Chemical
Co., Ltd. |
|
|
|
|
|
|
|
Collaborators:
Shintaro
Kinugawa |
|
|
|
|
|
|
|
Actelion
Pharmaceuticals
Japan Ltd. |
|
|
|
|
|
|
Collaborators:
Eisaku Nakane |
|
|
|
Nippon Boehringer
Ingelheim Co.,
Ltd. |
|
|
|
|
|
|
|
|
Bayer
Yakuhin,
Ltd. |
|
Collaborators:
Tatsuhiro Shibata |
|
|
|
Otsuka
Pharmaceutical
Co., Ltd.
Daiichi Sankyo
Company, |
|
|
|
|
|
|
|
|
|
|
Collaborators:
Minako
Yamaoka-Tojo |
|
|
|
Bayer Yakuhin,
Ltd. |
Bayer
Yakuhin,
Ltd.
Mitsubishi
Tanabe
Pharma
Corporation |
|
|
|
|
|
|
|
|
|
Independent
Assessment
Committee:
Ken-Ichi Hirata |
|
|
|
Kowa
Pharmaceutical
Co., Ltd.
Takeda
Pharmaceutical
Company
Limited |
|
Actelion
Pharmaceuticals
Japan Ltd.
Sysmex Corporation
TERUMO
CORPORATION
Daiichi Sankyo
Company,
Limited |
MSD K.K.
Actelion
Pharmaceuticals
Japan Ltd.
Abbott Vascular
Japan Co., Ltd.
Abbott Medical
Japan L.L.C
Sanofi K.K.
Novartis Pharma
K.K.
Bayer Yakuhin, Ltd.
BIOTRONIK
Japan, Inc.
Kowa Company,
Ltd.
Kowa
Pharmaceutical
Co., Ltd.
Otsuka
Pharmaceutical
Co., Ltd.
Nippon Boehringer
Ingelheim Co.,
Ltd.
Nihon Medi-Physics
Co., Ltd.
Nippon Shinyaku
Co., Ltd.
FUJIFILM RI
Pharma Co., Ltd.
FUJIFILM
Toyama Chemical
Co., Ltd.
Takeda
Pharmaceutical
Company Limited |
Abbott Medical
Japan L.L.C
Sysmex Corporation
Medtronic Japan
Co., Ltd. |
|
|
|
|
|
|
Independent
Assessment
Committee:
Takeshi Kimura |
|
|
|
Abbott Vascular
Japan Co., Ltd.
Sanofi K.K.
Bristol-Myers
Squibb
Boston Scientific
Japan K.K.
Kowa Company,
Ltd.
Nippon Boehringer
Ingelheim Co.,
Ltd. |
|
Edwards
Lifesciences
Corporation
EP-CRSU Co., Ltd.
Pfizer Japan Inc.
Kowa Company,
Ltd.
Daiichi Sankyo
Company,
Limited |
Astellas Pharma Inc.
MID,Inc.
Otsuka
Pharmaceutical
Co., Ltd.
Daiichi Sankyo
Company,
Limited
Mitsubishi Tanabe
Pharma
Corporation
Nippon Boehringer
Ingelheim Co.,
Ltd.
Takeda
Pharmaceutical
Company Limited |
|
|
|
|
|
|
|
Independent
Assessment
Committee:
Syunei Kyo |
|
TOBU
RAILWAY
CO., LTD. |
|
|
|
|
|
|
|
Daiko
Jitsugyo,
Ltd |
TOBU
RAILWAY
CO., LTD. |
|
|
|
Independent
Assessment
Committee:
Ryuji Nohara |
|
|
|
|
|
|
NAKAJIMA
STEEL PIPE Co.,
Ltd. |
|
|
|
|
|
|
|
*Notation of corporation is omitted.
*The following persons have no conflict of interest to declare:
Members: Hitoshi Adachi
Members: Emiko Hasegawa
Members: Shunichi Ishihara
Members: Yoshitaka Iso
Members: Akira Koike
Members: Masatoshi Nagayama
Members: Hideo Ohuchi
Members: Koichi Okita
Members: Kazuto Omiya
Members: Naokata Sumitomo
Members: Yuichiro Yamada
Members: Toshiko Yoshida
Collaborators: Takuji Adachi
Collaborators: Kanta Fujimi
Collaborators: Taiki Higo
Collaborators: Tasuku Honda
Collaborators: Toshimi Ikegame
Collaborators: Takeshi Ishida
Collaborators: Kazuhiro P Izawa
Collaborators: Naoya Kakutani
Collaborators: Yusuke Kasahara
Collaborators: Masaaki Kato
Collaborators: Yasuyuki Kobayashi
Collaborators: Yuji Kono
Collaborators: Teruyuki Koyama
Collaborators: Noriko Matsumoto
Collaborators: Yasuharu Matsumoto
Collaborators: Ikuko Miyawaki
Collaborators: Makoto Murata
Collaborators: Michio Nakanishi
Collaborators: Mari Nishizaki
Collaborators: Hiroaki Obata
Collaborators: Naohiko Osada
Collaborators: Neiko Ozasa
Collaborators: Kazuhiro Sase
Collaborators: Shinji Sato
Collaborators: Norio Suzuki
Collaborators: Daisuke Tamaki
Collaborators: Noboru Watanabe
Collaborators: Shusuke Yagi
Collaborators: Midori Yamada
Collaborators: Masanobu Yanase
Collaborators: Miho Yokoyama
Independent Assessment Committee: Yoichi Goto
Independent Assessment Committee: Haruki Ito
References
- 1.
Nohara R, Adachi H, Goto Y, Hasegawa E, Ishihara S, Haruki Itoh H, et al; JCS Joint Working Group. Guidelines for rehabilitation in patients with cardiovascular disease (JCS 2012). Circ J 2014; 78: 2022–2093.
- 2.
Minds Guideline Center, Japan Council for Quality Health Care. Minds handbook for clinical practice guideline development 2007 [in Japanese]. Igaku-Shoin, 2007.
- 3.
Japanese Circulation Society. 循環器疾患診療実態調査報告書 (2018年度実施・公表) [in Japanese]. https://www.j-circ.or.jp/jittai_chosa/media/jittai_chosa2017web.pdf [accessed December, 2022].
- 4.
Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics: 2019 update. A report from the American Heart Association. Circulation 2019; 139: e56–e528.
- 5.
Takabayashi K, Ikuta A, Okazaki Y, Ogami M, Iwatsu K, Matsumura K, et al. Clinical characteristics and social frailty of super-elderly patients with heart failure: The Kitakawachi Clinical Background and Outcome of Heart Failure Registry. Circ J 2016; 81: 69–76.
- 6.
Leon AS, Franklin BA, Costa F, Balady GJ, Berra KA, Stewart KJ, et al. Cardiac rehabilitation and secondary prevention of coronary heart disease: An American Heart Association scientific statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Cardiac Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity), in collaboration with the American association of Cardiovascular and Pulmonary Rehabilitation. Circulation 2005; 111: 369–376.
- 7.
Goto Y. Optimization of coronary secondary prevention by cardiac rehabilitation. J Jpn Coron Assoc 2017; 23: 174–181 [in Japanese].
- 8.
McAlister FA, Stewart S, Ferrua S, McMurray JJ. Multidisciplinary strategies for the management of heart failure patients at high risk for admission: A systematic review of randomized trials. J Am Coll Cardiol 2004; 44: 810–819.
- 9.
Davidson PM, Cockburn J, Newton PJ, Webster JK, Betihavas V, Howes L, et al. Can a heart failure-specific cardiac rehabilitation program decrease hospitalizations and improve outcomes in high-risk patients? Eur J Cardiovasc Prev Rehabil 2010; 17: 393–402.
- 10.
Izawa H, Yoshida T, Ikegame T, Izawa KP, Ito Y, Okamura H, et al; Japanese Association of Cardiac Rehabilitation Standard Cardiac Rehabilitation Program Planning Committee. Standard cardiac rehabilitation program for heart failure. Circ J 2019; 83: 2394–2398.
- 11.
Hare DL, Toukhsati SR, Johansson P, Jaarsma T. Depression and cardiovascular disease: A clinical review. Eur Heart J 2014; 35: 1365–1372.
- 12.
Balady GJ, Arena R, Sietsema K, Myers J, Coke L, Fletcher GF, et al; American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee of the Council on Clinical Cardiology. Clinician’s guide to cardiopulmonary exercise testing in adults: A scientific statement from the American Heart Association. Circulation 2010; 122: 191–225.
- 13.
Braunwald E. Heart disease: A textbook of cardiovascular medicine, 5th edn. WB Saunders, 1997.
- 14.
Wasserman K, Hansen JE, Sue DY, Stringer WW, Sietsema KE, Sun XG, et al. Principles of exercise testing and interpretation: including pathophysiology and clinical applications, 5th edn. Lippincott Williams & Wilkins, 2012.
- 15.
Pavasini R, Serenelli M, Celis-Morales CA, Gray SR, Izawa KP, Watanabe S, et al. Grip strength predicts cardiac adverse events in patients with cardiac disorders: An individual patient pooled meta-analysis. Heart 2019; 105: 834–841.
- 16.
Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 1994; 49: M85–M94.
- 17.
Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010; 39: 412–423.
- 18.
ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: Guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002; 166: 111–117.
- 19.
Enright PL, Sherrill DL. Reference equations for the six-minute walk in healthy adults. Am J Respir Crit Care Med 1998; 158: 1384–1387.
- 20.
Rasekaba T, Lee AL, Naughton MT, Williams TJ, Holland AE. The six-minute walk test: A useful metric for the cardiopulmonary patient. Intern Med J 2009; 39: 495–501.
- 21.
Wassermank K, McIlroy MB. Detecting the threshold of anaerobic metabolism in cardiac patients during exercise. Am J Cardiol 1964; 14: 844–852.
- 22.
Hughson RL, Green HJ, Sharratt MT. Gas exchange, blood lactate, and plasma catecholamines during incremental exercise in hypoxia and normoxia. J Appl Physiol (1985) 1995; 79: 1134–1141.
- 23.
Itoh H, Ajisaka R, Koike A, Makita S, Omiya K, Kato Y, et al. Committee on Exercise Prescription for Patients (CEPP) Members. Heart rate and blood pressure response to ramp exercise and exercise capacity in relation to age, gender, and mode of exercise in a healthy population. J Cardiol 2013; 61: 71–78.
- 24.
Ashikaga K, Itoh H, Maeda T, Itoh H, Ichikawa Y, Tanaka S, et al. Committee on Exercise Prescription for Patients (CEPP) Members. Ventilatory efficiency during ramp exercise in relation to age and sex in a healthy Japanese population. J Cardiol 2021; 77: 57–64.
- 25.
Apostolo A, Laveneziana P, Palange P, Agalbato C, Molle R, Popovic D, et al. Impact of chronic obstructive pulmonary disease on exercise ventilatory efficiency in heart failure. Int J Cardiol 2015; 189: 134–140.
- 26.
Tajima A, Itoh H, Osada N, Omiya K, Maeda T, Ohkoshi N, et al. Oxygen uptake kinetics during and after exercise are useful markers of coronary artery disease in patients with exercise electrocardiography suggesting myocardial ischemia. Circ J 2009; 73: 1864–1870.
- 27.
Munhoz EC, Hollanda R, Vargas JP, Silveira CW, Lemos AL, Hollanda RM, et al. Flattening of oxygen pulse during exercise may detect extensive myocardial ischemia. Med Sci Sports Exerc 2007; 39: 1221–1226.
- 28.
Marume K, Takashio S, Nakanishi M, Kumasaka L, Fukui S, Nakao K, et al. Efficacy of cardiac rehabilitation in heart failure patients with low body mass index. Circ J 2019; 83: 334–341.
- 29.
Murphy RM, Shah RV, Malhotra R, Pappagianopoulos PP, Hough SS, Systrom DM, et al. Exercise oscillatory ventilation in systolic heart failure: An indicator of impaired hemodynamic response to exercise. Circulation 2011; 124: 1442–1451.
- 30.
Ushijima A, Morita N, Hama T, Yamamoto A, Yoshimachi F, Ikari Y, et al. Effects of cardiac rehabilitation on physical function and exercise capacity in elderly cardiovascular patients with frailty. J Cardiol 2021; 77: 424–431.
- 31.
Kinoshita H, Sairaku A, Morishima N, Dohi Y, Sada Y, Higashi A, et al. Prognostic significance of oscillatory ventilation at rest in patients with advanced heart failure undergoing cardiopulmonary exercise testing. Int J Cardiol 2020; 301: 142–146.
- 32.
Yamauchi F, Adachi H, Tomono J, Toyoda S, Iwamatsu K, Sakuma M, et al. Effect of a cardiac rehabilitation program on exercise oscillatory ventilation in Japanese patients with heart failure. Heart Vessels 2016; 31: 1659–1668.
- 33.
Mancini DM, Eisen H, Kussmaul W, Mull R, Edmunds LH Jr, Wilson JR. Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation 1991; 83: 778–786.
- 34.
Wasserman K, Van Kessel AL, Burton GG. Interaction of physiological mechanisms during exercise. J Appl Physiol 1967; 22: 71–85.
- 35.
Japan Intractable Diseases Information Center. Idiopathic dilated cardiomyopathy (incurable disease #57) [in Japanese]. https://www.nanbyou.or.jp/entry/3986 [accessed March, 2021].
- 36.
Keteyian SJ, Patel M, Kraus WE, Brawner CA, McConnell TR, Piña IL, et al; HF-ACTION Investigators. Variables measured during cardiopulmonary exercise testing as predictors of mortality in chronic systolic heart failure. J Am Coll Cardiol 2016; 67: 780–789.
- 37.
Nakanishi M, Takaki H, Kumasaka R, Arakawa T, Noguchi T, Sugimachi M, et al. Targeting of high peak respiratory exchange ratio is safe and enhances the prognostic power of peak oxygen uptake for heart failure patients. Circ J 2014; 78: 2268–2275.
- 38.
Nakanishi M, Nakao K, Kumasaka L, Arakawa T, Fukui S, Ohara T, et al. Improvement in exercise capacity by exercise training associated with favorable clinical outcomes in advanced heart failure with high B-type natriuretic peptide level. Circ J 2017; 81: 1307–1314.
- 39.
Paolillo S, Veglia F, Salvioni E, Corrà U, Piepoli M, Lagioia R, et al; MECKI Score Research Group. Heart failure prognosis over time: How the prognostic role of oxygen consumption and ventilatory efficiency during exercise has changed in the last 20 years. Eur J Heart Fail 2019; 21: 208–217.
- 40.
Gitt AK, Wasserman K, Kilkowski C, Kleemann T, Kilkowski A, Bangert M, et al. Exercise anaerobic threshold and ventilatory efficiency identify heart failure patients for high risk of early death. Circulation 2002; 106: 3079–3084.
- 41.
Arena R, Myers J, Aslam SS, Varughese EB, Peberdy MA. Peak VO2 and VE/VCO2 slope in patients with heart failure: A prognostic comparison. Am Heart J 2004; 147: 354–360.
- 42.
Tsurugaya H, Adachi H, Kurabayashi M, Ohshima S, Taniguchi K. Prognostic impact of ventilatory efficiency in heart disease patients with preserved exercise tolerance. Circ J 2006; 70: 1332–1336.
- 43.
Agostoni P, Corrà U, Cattadori G, Veglia F, La Gioia R, Scardovi AB, et al; MECKI Score Research Group. Metabolic exercise test data combined with cardiac and kidney indexes, the MECKI score: A multiparametric approach to heart failure prognosis. Int J Cardiol 2013; 167: 2710–2718.
- 44.
Malhotra R, Bakken K, D’Elia E, Lewis GD. Cardiopulmonary exercise testing in heart failure. JACC Heart Fail 2016; 4: 607–616.
- 45.
Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al; Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019; 48: 16–31.
- 46.
Fülster S, Tacke M, Sandek A, Ebner N, Tschöpe C, Doehner W, et al. Muscle wasting in patients with chronic heart failure: Results from the studies investigating co-morbidities aggravating heart failure (SICA-HF). Eur Heart J 2013; 34: 512–519.
- 47.
Narumi T, Watanabe T, Kadowaki S, Takahashi T, Yokoyama M, Kinoshita D, et al. Sarcopenia evaluated by fat-free mass index is an important prognostic factor in patients with chronic heart failure. Eur J Intern Med 2015; 26: 118–122.
- 48.
Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, et al. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc 2020; 21: 300–307.
- 49.
Morley JE, Vellas B, van Kan GA, Anker SD, Bauer JM, Bernabei R, et al. Frailty consensus: A call to action. J Am Med Dir Assoc 2013; 14: 392–397.
- 50.
Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56: M146–M156.
- 51.
Walston J, Hadley EC, Ferrucci L, Guralnik JM, Newman AB, Studenski SA, et al. Research agenda for frailty in older adults: Toward a better understanding of physiology and etiology: Summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc 2006; 54: 991–1001.
- 52.
Xue QL, Bandeen-Roche K, Varadhan R, Zhou J, Fried LP. Initial manifestations of frailty criteria and the development of frailty phenotype in the Women’s Health and Aging Study II. J Gerontol A Biol Sci Med Sci 2008; 63: 984–990.
- 53.
Singh M, Stewart R, White H. Importance of frailty in patients with cardiovascular disease. Eur Heart J 2014; 35: 1726–1731.
- 54.
Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: A systematic review. J Am Geriatr Soc 2012; 60: 1487–1492.
- 55.
Kojima G, Iliffe S, Taniguchi Y, Shimada H, Rakugi H, Walters K. Prevalence of frailty in Japan: A systematic review and meta-analysis. J Epidemiol 2017; 27: 347–353.
- 56.
Denfeld QE, Winters-Stone K, Mudd JO, Gelow JM, Kurdi S, Lee CS. The prevalence of frailty in heart failure: A systematic review and meta-analysis. Int J Cardiol 2017; 236: 283–289.
- 57.
Bendayan M, Bibas L, Levi M, Mullie L, Forman DE, Afilalo J. Therapeutic interventions for frail elderly patients. Part II: Ongoing and unpublished randomized trials. Prog Cardiovasc Dis 2014; 57: 144–151.
- 58.
Evans WJ, Morley JE, Argilés J, Bales C, Baracos V, Guttridge D, et al. Cachexia: A new definition. Clin Nutr 2008; 27: 793–799.
- 59.
Anker SD, Ponikowski P, Varney S, Chua TP, Clark AL, Webb-Peploe KM, et al. Wasting as independent risk factor for mortality in chronic heart failure. Lancet 1997; 349: 1050–1053.
- 60.
Collamati A, Marzetti E, Calvani R, Tosato M, D’Angelo E, Sisto AN, et al. Sarcopenia in heart failure: Mechanisms and therapeutic strategies. J Geriatr Cardiol 2016; 13: 615–624.
- 61.
Anker SD, von Haehling S. Inflammatory mediators in chronic heart failure: An overview. Heart 2004; 90: 464–470.
- 62.
Sandek A, Bauditz J, Swidsinski A, Buhner S, Weber-Eibel J, von Haehling S, et al. Altered intestinal function in patients with chronic heart failure. J Am Coll Cardiol 2007; 50: 1561–1569.
- 63.
Pasini E, Aquilani R, Testa C, Baiardi P, Angioletti S, Boschi F, et al. Pathogenic gut flora in patients with chronic heart failure. JACC Heart Fail 2016; 4: 220–227.
- 64.
Valentova M, von Haehling S, Bauditz J, Doehner W, Ebner N, Bekfani T, et al. Intestinal congestion and right ventricular dysfunction: A link with appetite loss, inflammation, and cachexia in chronic heart failure. Eur Heart J 2016; 37: 1684–1691.
- 65.
American College of Sports Medicine. ACSM’s guidelines for exercise testing and prescription, 10th edn. Williams & Wilkins, 2017.
- 66.
Fletcher GF, Ades PA, Kligfield P, Arena R, Balady GJ, Bittner VA, et al; American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee of the Council on Clinical Cardiology, Council on Nutrition, Physical Activity and Metabolism, Council on Cardiovascular and Stroke Nursing, and Council on Epidemiology and Prevention. Exercise standards for testing and training: A scientific statement from the American Heart Association. Circulation 2013; 128: 873–934.
- 67.
Mytinger M, Nelson RK, Zuhl M. Exercise prescription guidelines for cardiovascular disease patients in the absence of a baseline stress test. J Cardiovasc Dev Dis 2020; 7: 15.
- 68.
Borg GA. Perceived exertion. Exerc Sport Sci Rev 1974; 2: 131–153.
- 69.
Persinger R, Foster C, Gibson M, Fater DC, Porcari JP. Consistency of the talk test for exercise prescription. Med Sci Sports Exerc 2004; 36: 1632–1636.
- 70.
Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, et al; American College of Sports Medicine. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med Sci Sports Exerc 2011; 43: 1334–1359.
- 71.
Ministry of Health, Labour and Welfare. 健康づくりのための身体活動基準2013 [in Japanese]. https://www.mhlw.go.jp/stf/houdou/2r9852000002xple.html [accessed November 5, 2020].
- 72.
Demopoulos L, Bijou R, Fergus I, Jones M, Strom J, LeJemtel TH. Exercise training in patients with severe congestive heart failure: Enhancing peak aerobic capacity while minimizing the increase in ventricular wall stress. J Am Coll Cardiol 1997; 29: 597–603.
- 73.
Ades PA. Cardiac rehabilitation and secondary prevention of coronary heart disease. N Engl J Med 2001; 345: 892–902.
- 74.
Ozasa N, Morimoto T, Bao B, Shioi T, Kimura T. Effects of machine-assisted cycling on exercise capacity and endothelial function in elderly patients with heart failure. Circ J 2012; 76: 1889–1894.
- 75.
Motoki H, Nishimura M, Kanai M, Kimura K, Minamisawa M, Yamamoto S, et al. Impact of inpatient cardiac rehabilitation on Barthel Index score and prognosis in patients with acute decompensated heart failure. Int J Cardiol 2019; 293: 125–130.
- 76.
Williams MA, Haskell WL, Ades PA, Amsterdam EA, Bittner V, Franklin BA, et al. Resistance exercise in individuals with and without cardiovascular disease: 2007 update: A scientific statement from the American Heart Association Council on Clinical Cardiology and Council on Nutrition, Physical Activity, and Metabolism. Circulation 2007; 116: 572–584.
- 77.
American Association of Cardiovascular and Pulmonary Rehabilitation. Guidelines for cardiac rehabilitation and secondary prevention programs, 5th edn. Human Kinetics, Inc., 2013.
- 78.
Piepoli MF, Conraads V, Corrà U, Dickstein K, Francis DP, Jaarsma T, et al. Exercise training in heart failure: From theory to practice. A consensus document of the Heart Failure Association and the European Association for Cardiovascular Prevention and Rehabilitation. Eur J Heart Fail 2011; 13: 347–357.
- 79.
Lamotte M, Fleury F, Pirard M, Jamon A, van de Borne P. Acute cardiovascular response to resistance training during cardiac rehabilitation: Effect of repetition speed and rest periods. Eur J Cardiovasc Prev Rehabil 2010; 17: 329–336.
- 80.
Nakajima T, Kurano M, Sakagami F, Iida H, Fukumura K, Fukuda T, et al. Effects of low-intensity KAATSU resistance training on skeletal muscle size/strength and endurance capacity in patients with ischemic heart disease. Int J Kaatsu Training Res 2000; 6: 1–7.
- 81.
Ishizaka H, Uematsu A, Mizushima Y, Nozawa N, Katayanagi S, Matsumoto K, et al. Blood flow restriction increases the neural activation of the knee extensors during very low-intensity leg extension exercise in cardiovascular patients: A pilot study. J Clin Med 2019; 8: 1252.
- 82.
Wisløff U, Støylen A, Loennechen JP, Bruvold M, Rognmo Ø, Haram PM, et al. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: A randomized study. Circulation 2007; 115: 3086–3094.
- 83.
Moholdt TT, Amundsen BH, Rustad LA, Wahba A, Løvø KT, Gullikstad LR, et al. Aerobic interval training versus continuous moderate exercise after coronary artery bypass surgery: A randomized study of cardiovascular effects and quality of life. Am Heart J 2009; 158: 1031–1037.
- 84.
Wewege MA, Ahn D, Yu J, Liou K, Keech A. High-intensity interval training for patients with cardiovascular disease-is it safe? A systematic review. J Am Heart Assoc 2018; 7: e009305.
- 85.
Hannan AL, Hing W, Simas V, Climstein M, Coombes JS, Jayasinghe R, et al. High-intensity interval training versus moderate-intensity continuous training within cardiac rehabilitation: A systematic review and meta-analysis. Open Access J Sports Med 2018; 9: 1–17.
- 86.
Taylor JL, Holland DJ, Keating SE, Leveritt MD, Gomersall SR, Rowlands AV, et al. Short-term and long-term feasibility, safety, and efficacy of high-intensity interval training in cardiac rehabilitation: The FITR Heart Study randomized clinical trial. JAMA Cardiol 2020; 5: 1382–1389.
- 87.
Iso Y, Suzuki H, Kyuno E, Maeda A, Tsunoda F, Miyazawa R, et al. Therapeutic potential of cycling high-intensity interval training in patients with peripheral artery disease: A pilot study. Int J Cardiol Heart Vasc 2018; 18: 30–32.
- 88.
Pelliccia A, Sharma S, Gati S, Bäck M, Börjesson M, Caselli S, et al. 2020 ESC guidelines on sports cardiology and exercise in patients with cardiovascular disease. Task Force on sports cardiology and exercise in patients with cardiovascular disease of the European Society of Cardiology (ESC). Eur Heart J 2021; 42: 17–96.
- 89.
Meyer FJ, Borst MM, Zugck C, Kirschke A, Schellberg D, Kübler W, et al. Respiratory muscle dysfunction in congestive heart failure: Clinical correlation and prognostic significance. Circulation 2001; 103: 2153–2158.
- 90.
Yamada K, Kinugasa Y, Sota T, Miyagi M, Sugihara S, Kato M, et al. Inspiratory muscle weakness is associated with exercise intolerance in patients with heart failure with preserved ejection fraction: A preliminary study. J Card Fail 2016; 22: 38–47.
- 91.
Palau P, Domínguez E, Núñez E, Ramón JM, López L, Melero J, et al. Inspiratory muscle function and exercise capacity in patients with heart failure with preserved ejection fraction. J Card Fail 2017; 23: 480–484.
- 92.
Wu J, Kuang L, Fu L. Effects of inspiratory muscle training in chronic heart failure patients: A systematic review and meta-analysis. Congenit Heart Dis 2018; 13: 194–202.
- 93.
Montemezzo D, Fregonezi GA, Pereira DA, Britto RR, Reid WD. Influence of inspiratory muscle weakness on inspiratory muscle training responses in chronic heart failure patients: A systematic review and meta-analysis. Arch Phys Med Rehabil 2014; 95: 1398–1407.
- 94.
Gomes Neto M, Martinez BP, Reis HF, Carvalho VO. Pre- and postoperative inspiratory muscle training in patients undergoing cardiac surgery: Systematic review and meta-analysis. Clin Rehabil 2017; 31: 454–464.
- 95.
Smart NA, Dieberg G, Giallauria F. Functional electrical stimulation for chronic heart failure: A meta-analysis. Int J Cardiol 2013; 167: 80–86.
- 96.
Anderson L, Oldridge N, Thompson DR, Zwisler AD, Rees K, Martin N, et al. Exercise-based cardiac rehabilitation for coronary heart disease: Cochrane systematic review and meta-analysis. J Am Coll Cardiol 2016; 67: 1–12.
- 97.
Dunlay SM, Pack QR, Thomas RJ, Killian JM, Roger VL. Participation in cardiac rehabilitation, readmissions, and death after acute myocardial infarction. Am J Med 2014; 127: 538–546.
- 98.
Smart N, Marwick TH. Exercise training for patients with heart failure: A systematic review of factors that improve mortality and morbidity. Am J Med 2004; 116: 693–706.
- 99.
RS, Long L, Mordi IR, Madsen MT, Davies EJ, Dalal H, et al. Exercise-based rehabilitation for heart failure: Cochrane systematic review, meta-analysis, and trial sequential analysis. JACC Heart Fail 2019; 7: 691–705.
- 100.
Hammill BG, Curtis LH, Schulman KA, Whellan DJ. Relationship between cardiac rehabilitation and long-term risks of death and myocardial infarction among elderly Medicare beneficiaries. Circulation 2010; 121: 63–70.
- 101.
診療点数早見表 2020年4月版 [in Japanese]. Igakutushinsya, 2020.
- 102.
Tsutsui H, Isobe M, Ito H, Ito H, Okumura K, Ono M, et al; Japanese Circulation Society and Japanese Heart Failure Society Joint Working Group. JCS 2017/JHFS 2017 guideline on diagnosis and treatment of acute and chronic heart failure: Digest version. Circ J 2019; 83: 2084–2184.
- 103.
Yamagishi M, Tamaki N, Akasaka T, Ikeda T, Ueshima K, Uemura S, et al; Japanese Circulation Society Working Group. JCS 2018 guideline on diagnosis of chronic coronary heart diseases. Circ J 2021; 85: 402–572.
- 104.
Gibbons RJ, Balady GJ, Bricker JT, Chaitman BR, Fletcher GF, Froelicher VF, et al. ACC/AHA 2002 guideline update for exercise testing: Summary article: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines). Circulation 2002; 106: 1883–1892.
- 105.
Saito M, Ueshima K, Saito M, Iwasaka T, Daida H, Kohzuki M, et al; Japanese Cardiac Rehabilitation Survey Investigators. Safety of exercise-based cardiac rehabilitation and exercise testing for cardiac patients in Japan: A nationwide survey. Circ J 2014; 78: 1646–1653.
- 106.
O’Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, et al; HF-ACTION Investigators. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 2009; 301: 1439–1450.
- 107.
Haskell WL. Cardiovascular complications during exercise training of cardiac patients. Circulation 1978; 57: 920–924.
- 108.
日本リハビリテーション医学会. リハビリテーション医療における安全管理・推進のためのガイドライン 第2版 [in Japanese]. Shindan To Chiryo Sha, Inc., 2018.
- 109.
Kimura K, Kimura T, Ishihara M, Nakagawa Y, Nakao K, Miyauchi K, et al; Japanese Circulation Society Joint Working Group. JCS 2018 guideline on diagnosis and treatment of acute coronary syndrome. Circ J 2019; 83: 1085–1196.
- 110.
Japanese Circulation Society. 循環器疾患診療実態調査報告書 (2017年度実施・公表) [in Japanese]. https://www.j-circ.or.jp/jittai_chosa/media/jittai_chosa2016web.pdf [accessed December, 2022].
- 111.
Ishihara M, Fujino M, Ogawa H, Yasuda S, Noguchi T, Nakao K, et al; J-MINUET investigators. Clinical presentation, management and outcome of Japanese patients with acute myocardial infarction in the troponin era: Japanese Registry of Acute Myocardial Infarction Diagnosed by Universal Definition (J-MINUET). Circ J 2015; 79: 1255–1262.
- 112.
Uchida I, Takaki H, Kobayashi Y, Okano Y, Satoh T, Matsubara T, et al. O2 extraction during exercise determines training effect after cardiac rehabilitation in myocardial infarction. Circ J 2002; 66: 891–896.
- 113.
Kamakura T, Kawakami R, Nakanishi M, Ibuki M, Ohara T, Yanase M, et al. Efficacy of out-patient cardiac rehabilitation in low prognostic risk patients after acute myocardial infarction in primary intervention era. Circ J 2011; 75: 315–321.
- 114.
Izawa K, Hirano Y, Yamada S, Oka K, Omiya K, Iijima S. Improvement in physiological outcomes and health-related quality of life following cardiac rehabilitation in patients with acute myocardial infarction. Circ J 2004; 68: 315–320.
- 115.
O’Gara PT, Kushner FG, Ascheim DD, Casey DE Jr, Chung MK, de Lemos JA, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 61: e78–e140.
- 116.
Amsterdam EA, Wenger NK, Brindis RG, Casey DE Jr, Ganiats TG, Holmes DR Jr, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 64: e139–e228.
- 117.
Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2018; 39: 119–177.
- 118.
Chobanian AV, Lille RD, Tercyak A, Blevins P. The metabolic and hemodynamic effects of prolonged bed rest in normal subjects. Circulation 1974; 49: 551–559.
- 119.
Antman EM, Hand M, Armstrong PW, Bates ER, Green LA, Halasyamani LK, et al. 2007 Focused Update of the ACC/AHA 2004 guidelines for the management of patients with ST-elevation myocardial infarction: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines: Developed in collaboration With the Canadian Cardiovascular Society endorsed by the American Academy of Family Physicians: 2007 Writing Group to Review New Evidence and Update the ACC/AHA 2004 Guidelines for the Management of Patients With ST-Elevation Myocardial Infarction, Writing on Behalf of the 2004 Writing Committee. Circulation 2008; 117: 296–329.
- 120.
Grines CL, Marsalese DL, Brodie B, Griffin J, Donohue B, Costantini CR, et al; PAMI-II Investigators. Safety and cost-effectiveness of early discharge after primary angioplasty in low risk patients with acute myocardial infarction. J Am Coll Cardiol 1998; 31: 967–972.
- 121.
De Luca G, Suryapranata H, van’t Hof AW, de Boer MJ, Hoorntje JC, Dambrink JH, et al. Prognostic assessment of patients with acute myocardial infarction treated with primary angioplasty: Implications for early discharge. Circulation 2004; 109: 2737–2743.
- 122.
Noman A, Zaman AG, Schechter C, Balasubramaniam K, Das R. Early discharge after primary percutaneous coronary intervention for ST-elevation myocardial infarction. Eur Heart J Acute Cardiovasc Care 2013; 2: 262–269.
- 123.
Grace SL, Scholey P, Suskin N, Arthur HM, Brooks D, Jaglal S, et al. A prospective comparison of cardiac rehabilitation enrollment following automatic vs usual referral. J Rehabil Med 2007; 39: 239–245.
- 124.
Arthur HM, Gunn E, Thorpe KE, Ginis KM, Mataseje L, McCartney N, et al. Effect of aerobic vs combined aerobic-strength training on 1-year, post-cardiac rehabilitation outcomes in women after a cardiac event. J Rehabil Med 2007; 39: 730–735.
- 125.
Vona M, Codeluppi GM, Iannino T, Ferrari E, Bogousslavsky J, von Segesser LK. Effects of different types of exercise training followed by detraining on endothelium-dependent dilation in patients with recent myocardial infarction. Circulation 2009; 119: 1601–1608.
- 126.
Abreu A, Pesah E, Supervia M, Turk-Adawi K, Bjarnason-Wehrens B, Lopez-Jimenez F, et al. Cardiac rehabilitation availability and delivery in Europe: How does it differ by region and compare with other high-income countries? Eur J Prev Cardiol 2019; 26: 1131–1146.
- 127.
Goto Y. 国循 心臓リハビリテーション実践マニュアル [in Japanese]. Medicus Shuppan, 2017.
- 128.
Campkin LM, Boyd JM, Campbell DJT. Coronary artery disease patient perspectives on exercise participation. J Cardiopulm Rehabil Prev 2017; 37: 305–314.
- 129.
Ohtera S, Kanazawa N, Ozasa N, Ueshima K, Nakayama T. Proposal of quality indicators for cardiac rehabilitation after acute coronary syndrome in Japan: A modified Delphi method and practice test. BMJ Open 2017; 7: e013036.
- 130.
Thomas RJ, Beatty AL, Beckie TM, Brewer LC, Brown TM, Forman DE, et al. Home-based cardiac rehabilitation: A Scientific Statement From the American Association of Cardiovascular and Pulmonary Rehabilitation, the American Heart Association, and the American College of Cardiology. Circulation 2019; 140: e69–e89.
- 131.
Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: Executive Summary: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 2012; 60: 2564–2603.
- 132.
Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, et al. 2013 ESC guidelines on the management of stable coronary artery disease: The Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J 2013; 34: 2949–3003.
- 133.
Takashima A, Ise T, Yagi S, Iwase T, Kimura S, Ueda Y, et al. Cardiac rehabilitation reduces serum levels of oxidized low-density lipoprotein. Circ J 2014; 78: 2682–2687.
- 134.
Kimura S, Ueda Y, Ise T, Yagi S, Iwase T, Nishikawa K, et al. Impact of supervised cardiac rehabilitation on urinary albumin excretion in patients with cardiovascular disease. Int Heart J 2015; 56: 105–109.
- 135.
Tanihata S, Nishigaki K, Kawasaki M, Takemura G, Minatoguchi S, Fujiwara H. Outcomes of patients with stable low-risk coronary artery disease receiving medical- and PCI-preceding therapies in Japan: J-SAP study 1–1. Circ J 2006; 70: 365–369.
- 136.
Weintraub WS, Spertus JA, Kolm P, Maron DJ, Zhang Z, Jurkovitz C, et al; COURAGE Trial Research Group. Effect of PCI on quality of life in patients with stable coronary disease. N Engl J Med 2008; 359: 677–687.
- 137.
Smith SC Jr, Benjamin EJ, Bonow RO, Braun LT, Creager MA, Franklin BA, et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: A guideline from the American Heart Association and American College of Cardiology Foundation endorsed by the World Heart Federation and the Preventive Cardiovascular Nurses Association. J Am Coll Cardiol 2011; 58: 2432–2446.
- 138.
Archbold RA. Comparison between National Institute for Health and Care Excellence (NICE) and European Society of Cardiology (ESC) guidelines for the diagnosis and management of stable angina: Implications for clinical practice. Open Heart 2016; 3: e000406.
- 139.
Long L, Anderson L, Dewhirst AM, He J, Bridges C, Gandhi M, et al. Exercise-based cardiac rehabilitation for adults with stable angina. Cochrane Database Syst Rev 2018; 2: CD012786.
- 140.
Bernstein RD, Ochoa FY, Xu X, Forfia P, Shen W, Thompson CI, et al. Function and production of nitric oxide in the coronary circulation of the conscious dog during exercise. Circ Res 1996; 79: 840–848.
- 141.
Austin GE, Ratliff NB, Hollman J, Tabei S, Phillips DF. Intimal proliferation of smooth muscle cells as an explanation for recurrent coronary artery stenosis after percutaneous transluminal coronary angioplasty. J Am Coll Cardiol 1985; 6: 369–375.
- 142.
Lee JY, Yun SC, Ahn JM, Park DW, Kang SJ, Lee SW, et al. Impact of cardiac rehabilitation on angiographic outcomes after drug-eluting stents in patients with de novo long coronary artery lesions. Am J Cardiol 2014; 113: 1977–1985.
- 143.
Balady GJ, Williams MA, Ades PA, Bittner V, Comoss P, Foody JA, et al. Core components of cardiac rehabilitation/secondary prevention programs: 2007 update: A scientific statement from the American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee, the Council on Clinical Cardiology; the Councils on Cardiovascular Nursing, Epidemiology and Prevention, and Nutrition, Physical Activity, and Metabolism; and the American Association of Cardiovascular and Pulmonary Rehabilitation. J Cardiopulm Rehabil Prev 2007; 27: 121–129.
- 144.
Piepoli MF, Corrà U, Benzer W, Bjarnason-Wehrens B, Dendale P, Gaita D, et al. Secondary prevention through cardiac rehabilitation: From knowledge to implementation. A position paper from the Cardiac Rehabilitation Section of the European Association of Cardiovascular Prevention and Rehabilitation. Eur J Cardiovasc Prev Rehabil 2010; 17: 1–17.
- 145.
Balady GJ, Leitschuh ML, Jacobs AK, Merrell D, Weiner DA, Ryan TJ. Safety and clinical use of exercise testing one to three days after percutaneous transluminal coronary angioplasty. Am J Cardiol 1992; 69: 1259–1264.
- 146.
Soga Y, Yokoi H, Ando K, Shirai S, Sakai K, Kondo K, et al. Safety of early exercise training after elective coronary stenting in patients with stable coronary artery disease. Eur J Cardiovasc Prev Rehabil 2010; 17: 230–234.
- 147.
Hollings M, Mavros Y, Freeston J, Fiatarone Singh M. The effect of progressive resistance training on aerobic fitness and strength in adults with coronary heart disease: A systematic review and meta-analysis of randomised controlled trials. Eur J Prev Cardiol 2017; 24: 1242–1259.
- 148.
Kakutani N, Fukushima A, Kinugawa S, Yokota T, Oikawa T, Nishikawa M, et al. Progressive mobilization program for patients with acute heart failure reduces hospital stay and improves clinical outcome. Circ Rep 2019; 1: 123–130.
- 149.
Kono Y, Izawa H, Aoyagi Y, Ishikawa A, Sugiura T, Mori E, et al. Predictive impact of early mobilization on rehospitalization for elderly Japanese heart failure patients. Heart Vessels 2020; 35: 531–536.
- 150.
Long L, Mordi IR, Bridges C, Sagar VA, Davies EJ, Coats AJ, et al. Exercise-based cardiac rehabilitation for adults with heart failure. Cochrane Database Syst Rev 2019: CD003331.
- 151.
Kamiya K, Sato Y, Takahashi T, Tsuchihashi-Makaya M, Kotooka N, Ikegame T, et al. Multidisciplinary cardiac rehabilitation and long-term prognosis in patients with heart failure. Circ Heart Fail 2020; 13: e006798.
- 152.
Haykowsky MJ, Liang Y, Pechter D, Jones LW, McAlister FA, Clark AM. A meta-analysis of the effect of exercise training on left ventricular remodeling in heart failure patients: The benefit depends on the type of training performed. J Am Coll Cardiol 2007; 49: 2329–2336.
- 153.
Pearson MJ, Smart NA. Exercise therapy and autonomic function in heart failure patients: A systematic review and meta-analysis. Heart Fail Rev 2018; 23: 91–108.
- 154.
Pearson MJ, Smart NA. Effect of exercise training on endothelial function in heart failure patients: A systematic review meta-analysis. Int J Cardiol 2017; 231: 234–243.
- 155.
Forman DE, Fleg JL, Kitzman DW, Brawner CA, Swank AM, McKelvie RS, et al. 6-min walk test provides prognostic utility comparable to cardiopulmonary exercise testing in ambulatory outpatients with systolic heart failure. J Am Coll Cardiol 2012; 60: 2653–2661.
- 156.
Ferreira JP, Duarte K, Graves TL, Zile MR, Abraham WT, Weaver FA, et al. Natriuretic peptides, 6-min walk test, and quality-of-life questionnaires as clinically meaningful endpoints in HF trials. J Am Coll Cardiol 2016; 68: 2690–2707.
- 157.
Nozaki K, Kimura M, Kamiya K, Noda C, Yamaoka-Tojo M, Masuda T, et al. 慢性心不全患者の退院時における6分間歩行距離を規定する因子の検討. J Clin Phys Ther Res 2014; 31: 25–29 [in Japanese].
- 158.
Kono Y, Izawa H, Aoyagi Y, Ishikawa A, Sugiura T, Mori E, et al. The difference in determinant factor of six-minute walking distance between sarcopenic and non-sarcopenic elderly patients with heart failure. J Cardiol 2020; 75: 42–46.
- 159.
Kamiya K, Hamazaki N, Matsue Y, Mezzani A, Corrà U, Matsuzawa R, et al. Gait speed has comparable prognostic capability to six-minute walk distance in older patients with cardiovascular disease. Eur J Prev Cardiol 2018; 25: 212–219.
- 160.
Uehara A, Obata H, Watanabe H, Izumi Y, Suzuki Y, Izumi T. The baseline speed of 10-m gait predicts ambulatory discharge for hospitalized frail elderly after DOPPO rehabilitation. Int J Rehabil Res 2018; 41: 331–336.
- 161.
Okita K, Yonezawa K, Nishijima H, Hanada A, Ohtsubo M, Kohya T, et al. Skeletal muscle metabolism limits exercise capacity in patients with chronic heart failure. Circulation 1998; 98: 1886–1891.
- 162.
Kitzman DW, Nicklas B, Kraus WE, Lyles MF, Eggebeen J, Morgan TM, et al. Skeletal muscle abnormalities and exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Physiol Heart Circ Physiol 2014; 306: H1364–H1370.
- 163.
Del Buono MG, Arena R, Borlaug BA, Carbone S, Canada JM, Kirkman DL, et al. Exercise intolerance in patients with heart failure: JACC State-of-the-Art Review. J Am Coll Cardiol 2019; 73: 2209–2225.
- 164.
Rees K, Taylor RS, Singh S, Coats AJ, Ebrahim S. Exercise based rehabilitation for heart failure. Cochrane Database Syst Rev 2004: CD003331.
- 165.
Pandey A, Parashar A, Kumbhani D, Agarwal S, Garg J, Kitzman D, et al. Exercise training in patients with heart failure and preserved ejection fraction: Meta-analysis of randomized control trials. Circ Heart Fail 2015; 8: 33–40.
- 166.
Kamiya K, Yamamoto T, Tsuchihashi-Makaya M, Ikegame T, Takahashi T, Sato Y, et al. Nationwide survey of multidisciplinary care and cardiac rehabilitation for patients with heart failure in Japan: An analysis of the AMED-CHF Study. Circ J 2019; 83: 1546–1552.
- 167.
Groehs RV, Antunes-Correa LM, Nobre TS, Alves MJ, Rondon MU, Barreto AC, et al. Muscle electrical stimulation improves neurovascular control and exercise tolerance in hospitalised advanced heart failure patients. Eur J Prev Cardiol 2016; 23: 1599–1608.
- 168.
Nishi I, Noguchi T, Furuichi S, Iwanaga Y, Kim J, Ohya H, et al. Are cardiac events during exercise therapy for heart failure predictable from the baseline variables? Circ J 2007; 71: 1035–1039.
- 169.
Ellingsen Ø, Halle M, Conraads V, Støylen A, Dalen H, Delagardelle C, et al; SMARTEX Heart Failure Study (Study of Myocardial Recovery After Exercise Training in Heart Failure) Group. High-intensity interval training in patients with heart failure with reduced ejection fraction. Circulation 2017; 135: 839–849.
- 170.
Pattyn N, Beulque R, Cornelissen V. Aerobic interval vs. continuous training in patients with coronary artery disease or heart failure: An updated systematic review and meta-analysis with a focus on secondary outcomes. Sports Med 2018; 48: 1189–1205.
- 171.
Reeves GR, Whellan DJ, O’Connor CM, Duncan P, Eggebeen JD, Morgan TM, et al. A novel rehabilitation intervention for older patients with acute decompensated heart failure: The REHAB-HF pilot study. JACC Heart Fail 2017; 5: 359–366.
- 172.
Adamopoulos S, Schmid JP, Dendale P, Poerschke D, Hansen D, Dritsas A, et al. Combined aerobic/inspiratory muscle training vs. aerobic training in patients with chronic heart failure: The Vent-HeFT trial: A European prospective multicentre randomized trial. Eur J Heart Fail 2014; 16: 574–582.
- 173.
Goto Y. How to operate out-patient cardiac rehabilitation as a heart failure management program. Heart View 2014; 18: 520–527 [in Japanese].
- 174.
Forman DE, Arena R, Boxer R, Dolansky MA, Eng JJ, Fleg JL, et al; American Heart Association Council on Clinical Cardiology, Council on Cardiovascular and Stroke Nursing, Council on Quality of Care and Outcomes Research, Stroke Council. Prioritizing functional capacity as a principal end point for therapies oriented to older adults with cardiovascular disease: A Scientific Statement for Healthcare Professionals from the American Heart Association. Circulation 2017; 135: e894–e918.
- 175.
Anker SD, Schroeder S, Atar D, Bax JJ, Ceconi C, Cowie MR, et al. Traditional and new composite endpoints in heart failure clinical trials: Facilitating comprehensive efficacy assessments and improving trial efficiency. Eur J Heart Fail 2016; 18: 482–489.
- 176.
Roccaforte R, Demers C, Baldassarre F, Teo KK, Yusuf S. Effectiveness of comprehensive disease management programmes in improving clinical outcomes in heart failure patients: A meta-analysis. Eur J Heart Fail 2005; 7: 1133–1144.
- 177.
Tsuchihashi-Makaya M, Matsuo H, Kakinoki S, Takechi S, Kinugawa S, Tsutsui H; J-HOMECARE Investigators. Home-based disease management program to improve psychological status in patients with heart failure in Japan. Circ J 2013; 77: 926–933.
- 178.
Hillis LD, Smith PK, Anderson JL, Bittl JA, Bridges CR, Byrne JG, et al. 2011 ACCF/AHA guideline for coronary artery bypass graft surgery: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Developed in collaboration with the American Association for Thoracic Surgery, Society of Cardiovascular Anesthesiologists, and Society of Thoracic Surgeons. J Am Coll Cardiol 2011; 58: e123–e210.
- 179.
Butchart EG, Gohlke-Bärwolf C, Antunes MJ, Tornos P, De Caterina R, Cormier B, et al; Working Groups on Valvular Heart Disease, Thrombosis, and Cardiac Rehabilitation and Exercise Physiology, European Society of Cardiology. Recommendations for the management of patients after heart valve surgery. Eur Heart J 2005; 26: 2463–2471.
- 180.
Blokzijl F, Dieperink W, Keus F, Reneman MF, Mariani MA, van der Horst IC. Cardiac rehabilitation for patients having cardiac surgery: A systematic review. J Cardiovasc Surg (Torino) 2018; 59: 817–829.
- 181.
Origuchi H, Itoh H, Momomura SI, Nohara R, Daida H, Masuda T, et al. Active participation in outpatient cardiac rehabilitation is associated with better prognosis after coronary artery bypass graft surgery: J-REHAB CABG study. Circ J 2020; 84: 427–435.
- 182.
Kayambu G, Boots R, Paratz J. Physical therapy for the critically ill in the ICU: A systematic review and meta-analysis. Crit Care Med 2013; 41: 1543–1554.
- 183.
Ad Hoc Committee for Early Rehabilitation, The Japanese Society of Intensive Care Medicine. Evidence based expert consensus for early rehabilitation in the intensive care unit. J Jpn Soc Intensive Care Med 2017; 24: 255–303 [in Japanese].
- 184.
Morisawa T, Yuguchi S, Oura K, Kamisaka K, Kato M, Saitou M, et al. Characteristics of delayed rehabilitation following cardiac surgery: Multicenter study. General Rehabilitation 2015; 43: 459–464 [in Japanese].
- 185.
Sakurada K, Takahashi T. 心臓血管外科術後リハビリテーション–患者の特徴や疾患特異性, 術式別特徴を把握したプログラムで介入. INTENSIVIST 2016; 8: 105–116 [in Japanese].
- 186.
Kato M, Saitoh M, Kawamura T, Iwata K, Sakurada K, Okamura D, et al. Postoperative atrial fibrillation is associated with delayed early rehabilitation after heart valve surgery: A multicenter study. Phys Ther Res 2019; 22: 1–8.
- 187.
Dubach P, Myers J, Wagner D. Optimal timing of phase II rehabilitation after cardiac surgery: The cardiologist’s view. Eur Heart J 1998; 19(Suppl): O35–O37.
- 188.
Sato S, Kamata J, Ueshima K, Saitoh K, Saito M, Kobayashi N, et al. Changes in exercise tolerance before and after coronary artery bypass graft surgery. J Jpn Phys Ther Assoc 1999; 26: 249–253 [in Japanese].
- 189.
Omiya K, Itoh H, Osada N, Kato M, Koike A, Sagara K, et al. Impaired heart rate response during incremental exercise in patients with acute myocardial infarction and after coronary artery bypass grafting: Evaluation of coefficients with Karvonen’s formula. Jpn Circ J 2000; 64: 851–855.
- 190.
Pollock ML, Franklin BA, Balady GJ, Chaitman BL, Fleg JL, Fletcher B, et al. AHA Science Advisory. Resistance exercise in individuals with and without cardiovascular disease: Benefits, rationale, safety, and prescription: An advisory from the Committee on Exercise, Rehabilitation, and Prevention, Council on Clinical Cardiology, American Heart Association; Position paper endorsed by the American College of Sports Medicine. Circulation 2000; 101: 828–833.
- 191.
Pollock ML, Gaesser GA, Butcher JD, Després JP, Dishman RK, Franklin BA, et al. ACSM Position Stand: The recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness, and flexibility in healthy adults. Med Sci Sports Exerc 1998; 30: 975–991.
- 192.
日本体力医学会体力科学編集委員会 監訳. 心疾患患者の運動処方. 運動処方の指針–運動負荷試験と運動プログラム (原著第8版). 南江堂 2011: 214–231 [in Japanese].
- 193.
Yabe Y, Maruyama H, Kiyama H, Kaki N, Uchida R, Makita S. Impact of chest band after open heart surgery on respiratory function and cardiopulmonary exercise test. J Jpn Assoc Card Rehabil 2009; 14: 193–195 [in Japanese].
- 194.
Gorlitzer M, Wagner F, Pfeiffer S, Folkmann S, Meinhart J, Fischlein T, et al. A prospective randomized multicenter trial shows improvement of sternum related complications in cardiac surgery with the Posthorax® support vest. Interact Cardiovasc Thorac Surg 2010; 10: 714–718.
- 195.
Thomas RJ, Beatty AL, Beckie TM, Brewer LC, Brown TM, Forman DE, et al. Home-based cardiac rehabilitation: A Scientific Statement From the American Association of Cardiovascular and Pulmonary Rehabilitation, the American Heart Association, and the American College of Cardiology. J Am Coll Cardiol 2019; 74: 133–153.
- 196.
Honda T, Mukohara N, Yoshida M. Early cardiac rehabilitation after coronary artery bypass grafting provides faster recovery of walking capacity. J Jpn Coron Assoc 2014; 20: 102–106 [in Japanese].
- 197.
Iribarne A, Easterwood R, Russo MJ, Chan EY, Smith CR, Argenziano M. Comparative effectiveness of minimally invasive versus traditional sternotomy mitral valve surgery in elderly patients. J Thorac Cardiovasc Surg 2012; 143(Suppl): S86–S90.
- 198.
Kawamura A, Sakamoto K, Akagi T, Izumi C, Otsuki S, Ohno Y, et al; Japanese Circulation Society. JCS/JCC/JSCS/JSVS/JATS 2021 guideline on catheter intervention for congenital heart disease and structural heart disease [in Japanese]. https://www.j-circ.or.jp/cms/wp-content/uploads/2021/03/JCS2021_Sakamoto_Kawamura.pdf [accessed December, 2022].
- 199.
Higuchi T, Higo T, Sonoda H, Shiose A, Tsutsui H. Cardiac rehabilitation for patients after transcatheter aortic valve implantation. Jpn J Clin Med 2019; 77(Suppl 1): 555–560 [in Japanese].
- 200.
Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Guyton RA, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: Executive Summary: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129: 2440–2492.
- 201.
Sola M, Ramm CJ, Kolarczyk LM, Teeter EG, Yeung M, Caranasos TG, et al. Application of a multidisciplinary enhanced recovery after surgery pathway to improve patient outcomes after transcatheter aortic valve implantation. Am J Cardiol 2016; 118: 418–423.
- 202.
Zanettini R, Gatto G, Mori I, Pozzoni MB, Pelenghi S, Martinelli L, et al. Cardiac rehabilitation and mid-term follow-up after transcatheter aortic valve implantation. J Geriatr Cardiol 2014; 11: 279–285.
- 203.
Russo N, Compostella L, Tarantini G, Setzu T, Napodano M, Bottio T, et al. Cardiac rehabilitation after transcatheter versus surgical prosthetic valve implantation for aortic stenosis in the elderly. Eur J Prev Cardiol 2014; 21: 1341–1348.
- 204.
Fauchère I, Weber D, Maier W, Altwegg L, Lüscher TF, Grünenfelder J, et al. Rehabilitation after TAVI compared to surgical aortic valve replacement. Int J Cardiol 2014; 173: 564–566.
- 205.
Pressler A, Christle JW, Lechner B, Grabs V, Haller B, Hettich I, et al. Exercise training improves exercise capacity and quality of life after transcatheter aortic valve implantation: A randomized pilot trial. Am Heart J 2016; 182: 44–53.
- 206.
Green P, Cohen DJ, Généreux P, McAndrew T, Arnold SV, Alu M, et al. Relation between six-minute walk test performance and outcomes after transcatheter aortic valve implantation (from the PARTNER trial). Am J Cardiol 2013; 112: 700–706.
- 207.
Hansen D. Exercise intervention after transcatheter aortic valve implantation: Current evidence and issues to be resolved. Eur J Prev Cardiol 2018; 25: 791–793.
- 208.
Iwanami Y, Uchi M, Fukuda H, Kuroda H, Sugisawa T, Ebihara S. Transcatheter aortic valve implantation and cardiac rehabilitation. Jpn J Rehabil Med 2019; 56: 1002–1008 [in Japanese].
- 209.
Hori K. TAVI後患者に対する心臓リハビリテーション. MED REHABIL 2019; 231: 7–13 [in Japanese].
- 210.
Pressler A, Förschner L, Hummel J, Haller B, Christle JW, Halle M. Long-term effect of exercise training in patients after transcatheter aortic valve implantation: Follow-up of the SPORT:TAVI randomised pilot study. Eur J Prev Cardiol 2018; 25: 794–801.
- 211.
GotoY. わが国の循環器医療提供体制の現状と今後のあり方–退院後疾病管理における運動・栄養介入の重要性. J JCS Cardiol 2019; 28: 57–66 [in Japanese].
- 212.
Popma JJ, Deeb GM, Yakubov SJ, Mumtaz M, Gada H, O’Hair D, et al; Evolut Low Risk Trial Investigators. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med 2019; 380: 1706–1715.
- 213.
Ono K, Iwasaki Y, Shimizu W, Akao M, Ikeda T, Ishii K, et al. JCS/JHRS 2020 guideline on pharmacotherapy of cardiac arrhythmias. 2020: 42–43, 75 [in Japanese]. https://www.j-circ.or.jp/cms/wp-content/uploads/2020/01/JCS2020_Ono.pdf [accessed March, 2021].
- 214.
Jouven X, Zureik M, Desnos M, Courbon D, Ducimetière P. Long-term outcome in asymptomatic men with exercise-induced premature ventricular depolarizations. N Engl J Med 2000; 343: 826–833.
- 215.
Garg PK, O’Neal WT, Ogunsua A, Thacker EL, Howard G, Soliman EZ, et al. Usefulness of the American Heart Association’s Life Simple 7 to predict the risk of atrial fibrillation (from the REasons for Geographic And Racial Differences in Stroke [REGARDS] study). Am J Cardiol 2018; 121: 199–204.
- 216.
Huxley RR, Lopez FL, Folsom AR, Agarwal SK, Loehr LR, Soliman EZ, et al. Absolute and attributable risks of atrial fibrillation in relation to optimal and borderline risk factors: The Atherosclerosis Risk in Communities (ARIC) study. Circulation 2011; 123: 1501–1508.
- 217.
Zhu W, Shen Y, Zhou Q, Xu Z, Huang L, Chen Q, et al. Association of physical fitness with the risk of atrial fibrillation: A systematic review and meta-analysis. Clin Cardiol 2016; 39: 421–428.
- 218.
Karjalainen J, Kujala UM, Kaprio J, Sarna S, Viitasalo M. Lone atrial fibrillation in vigorously exercising middle aged men: Case-control study. BMJ 1998; 316: 1784–1785.
- 219.
Malmo V, Nes BM, Amundsen BH, Tjonna AE, Stoylen A, Rossvoll O, et al. Aerobic interval training reduces the burden of atrial fibrillation in the short term: A randomized trial. Circulation 2016; 133: 466–473.
- 220.
Skielboe AK, Bandholm TQ, Hakmann S, Mourier M, Kallemose T, Dixen U. Cardiovascular exercise and burden of arrhythmia in patients with atrial fibrillation: A randomized controlled trial. PLoS One 2017; 12: e0170060.
- 221.
Risom SS, Zwisler AD, Rasmussen TB, Sibilitz KL, Madsen TL, Svendsen JH, et al. Cardiac rehabilitation versus usual care for patients treated with catheter ablation for atrial fibrillation: Results of the randomized CopenHeartRFA trial. Am Heart J 2016; 181: 120–129.
- 222.
Kato M, Ogano M, Mori Y, Kochi K, Morimoto D, Kito K, et al. Exercise-based cardiac rehabilitation for patients with catheter ablation for persistent atrial fibrillation: A randomized controlled clinical trial. Eur J Prev Cardiol 2019; 26: 1931–1940.
- 223.
Vanhees L, Schepers D, Defoor J, Brusselle S, Tchursh N, Fagard R. Exercise performance and training in cardiac patients with atrial fibrillation. J Cardiopulm Rehabil 2000; 20: 346–352.
- 224.
Hegbom F, Sire S, Heldal M, Orning OM, Stavem K, Gjesdal K. Short-term exercise training in patients with chronic atrial fibrillation: Effects on exercise capacity, AV conduction, and quality of life. J Cardiopulm Rehabil 2006; 26: 24–29.
- 225.
Luo N, Merrill P, Parikh KS, Whellan DJ, Piña IL, Fiuzat M, et al. Exercise training in patients with chronic heart failure and atrial fibrillation. J Am Coll Cardiol 2017; 69: 1683–1691.
- 226.
Mathew JP, Fontes ML, Tudor IC, Ramsay J, Duke P, Mazer CD, et al; Investigators of the Ischemia Research and Education Foundation. A multicenter risk index for atrial fibrillation after cardiac surgery. JAMA 2004; 291: 1720–1729.
- 227.
Almassi GH, Schowalter T, Nicolosi AC, Aggarwal A, Moritz TE, Henderson WG, et al. Atrial fibrillation after cardiac surgery: A major morbid event? Ann Surg 1997; 226: 501–513.
- 228.
Creswell LL, Schuessler RB, Rosenbloom M, Cox JL. Hazards of postoperative atrial arrhythmias. Ann Thorac Surg 1993; 56: 539–549.
- 229.
Herdy AH, Marcchi PL, Vila A, Tavares C, Collaço J, Niebauer J, et al. Pre- and postoperative cardiopulmonary rehabilitation in hospitalized patients undergoing coronary artery bypass surgery: A randomized controlled trial. Am J Phys Med Rehabil 2008; 87: 714–719.
- 230.
Fuster V, Rydén LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): Developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation 2006; 114: e257–e354.
- 231.
Van Gelder IC, Groenveld HF, Crijns HJ, Tuininga YS, Tijssen JG, Alings AM, et al; RACE II Investigators. Lenient versus strict rate control in patients with atrial fibrillation. N Engl J Med 2010; 362: 1363–1373.
- 232.
Jaber J, Cirenza C, Amaral A, Jaber J, Oliveira Filho JA, de Paola AA. Correlation between heart rate control during exercise and exercise capacity in patients with chronic atrial fibrillation. Clin Cardiol 2011; 34: 533–536.
- 233.
Ujeyl A, Stevenson LW, West EK, Kato M, Liszkowski M, Campbell P, et al. Impaired heart rate responses and exercise capacity in heart failure patients with paced baseline rhythms. J Card Fail 2011; 17: 188–195.
- 234.
Sharp CT, Busse EF, Burgess JJ, Haennel RG. Exercise prescription for patients with pacemakers. J Cardiopulm Rehabil 1998; 18: 421–431.
- 235.
Kindermann M, Schwaab B, Finkler N, Schaller S, Böhm M, Fröhlig G. Defining the optimum upper heart rate limit during exercise: A study in pacemaker patients with heart failure. Eur Heart J 2002; 23: 1301–1308.
- 236.
Kurita T, Nogami A, Abe H, Andou K, Ishikawa T, Imai K, et al; Japanese Circulation Society, Japanese Heart Rhythm Society. 2018 JCS/JHRS guideline on non-pharmacotherapy of cardiac arrhythmias. https://www.j-circ.or.jp/cms/wp-content/uploads/2018/07/JCS2018_kurita_nogami.pdf [accessed December, 2022].
- 237.
Belardinelli R, Capestro F, Misiani A, Scipione P, Georgiou D. Moderate exercise training improves functional capacity, quality of life, and endothelium-dependent vasodilation in chronic heart failure patients with implantable cardioverter defibrillators and cardiac resynchronization therapy. Eur J Cardiovasc Prev Rehabil 2006; 13: 818–825.
- 238.
Fan S, Lyon CE, Savage PD, Ozonoff A, Ades PA, Balady GJ. Outcomes and adverse events among patients with implantable cardiac defibrillators in cardiac rehabilitation: A case-controlled study. J Cardiopulm Rehabil Prev 2009; 29: 40–43.
- 239.
Berg SK, Pedersen PU, Zwisler AD, Winkel P, Gluud C, Pedersen BD, et al. Comprehensive cardiac rehabilitation improves outcome for patients with implantable cardioverter defibrillator: Findings from the COPE-ICD randomised clinical trial. Eur J Cardiovasc Nurs 2015; 14: 34–44.
- 240.
Fitchet A, Doherty PJ, Bundy C, Bell W, Fitzpatrick AP, Garratt CJ. Comprehensive cardiac rehabilitation programme for implantable cardioverter-defibrillator patients: A randomised controlled trial. Heart 2003; 89: 155–160.
- 241.
Pandey A, Parashar A, Moore C, Ngo C, Salahuddin U, Bhargava M, et al. Safety and efficacy of exercise training in patients with an implantable cardioverter-defibrillator: A meta-analysis. JACC Clin Electrophysiol 2017; 3: 117–126.
- 242.
Conraads VM, Vanderheyden M, Paelinck B, Verstreken S, Blankoff I, Miljoen H, et al. The effect of endurance training on exercise capacity following cardiac resynchronization therapy in chronic heart failure patients: A pilot trial. Eur J Cardiovasc Prev Rehabil 2007; 14: 99–106.
- 243.
Patwala AY, Woods PR, Sharp L, Goldspink DF, Tan LB, Wright DJ. Maximizing patient benefit from cardiac resynchronization therapy with the addition of structured exercise training: A randomized controlled study. J Am Coll Cardiol 2009; 53: 2332–2339.
- 244.
Smolis-Bąk E, Dąbrowski R, Piotrowicz E, Chwyczko T, Dobraszkiewicz-Wasilewska B, Kowalik I, et al. Hospital-based and telemonitoring guided home-based training programs: Effects on exercise tolerance and quality of life in patients with heart failure (NYHA class III) and cardiac resynchronization therapy. A randomized, prospective observation. Int J Cardiol 2015; 199: 442–447.
- 245.
Zeitler EP, Piccini JP, Hellkamp AS, Whellan DJ, Jackson KP, Ellis SJ, et al; HF-ACTION Investigators. Exercise training and pacing status in patients with heart failure: Results from HF-ACTION. J Card Fail 2015; 21: 60–67.
- 246.
Atwater BD, Friedman DJ. Are we approaching chronotropy (in)competently? JACC Heart Fail 2018; 6: 114–116.
- 247.
Goto Y. 国循 心臓リハビリテーション実践マニュアル. Medicus Shuppan, 2017: 178 表2 [in Japanese].
- 248.
Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016; 37: 67–119.
- 249.
Galiè N, McLaughlin VV, Rubin LJ, Simonneau G. An overview of the 6th World Symposium on Pulmonary Hypertension. Eur Respir J 2019; 53: 1802148.
- 250.
Galiè N, Palazzini M, Manes A. Pulmonary arterial hypertension: From the kingdom of the near-dead to multiple clinical trial meta-analyses. Eur Heart J 2010; 31: 2080–2086.
- 251.
Jenkins D, Madani M, Fadel E, D’Armini AM, Mayer E. Pulmonary endarterectomy in the management of chronic thromboembolic pulmonary hypertension. Eur Respir Rev 2017; 26: 160111.
- 252.
Lang I, Meyer BC, Ogo T, Matsubara H, Kurzyna M, Ghofrani HA, et al. Balloon pulmonary angioplasty in chronic thromboembolic pulmonary hypertension. Eur Respir Rev 2017; 26: 160119.
- 253.
Mereles D, Ehlken N, Kreuscher S, Ghofrani S, Hoeper MM, Halank M, et al. Exercise and respiratory training improve exercise capacity and quality of life in patients with severe chronic pulmonary hypertension. Circulation 2006; 114: 1482–1489.
- 254.
Weinstein AA, Chin LM, Keyser RE, Kennedy M, Nathan SD, Woolstenhulme JG, et al. Effect of aerobic exercise training on fatigue and physical activity in patients with pulmonary arterial hypertension. Respir Med 2013; 107: 778–784.
- 255.
Chan L, Chin LMK, Kennedy M, Woolstenhulme JG, Nathan SD, Weinstein AA, et al. Benefits of intensive treadmill exercise training on cardiorespiratory function and quality of life in patients with pulmonary hypertension. Chest 2013; 143: 333–343.
- 256.
Ley S, Fink C, Risse F, Ehlken N, Fischer C, Ley-Zaporozhan J, et al. Magnetic resonance imaging to assess the effect of exercise training on pulmonary perfusion and blood flow in patients with pulmonary hypertension. Eur Radiol 2013; 23: 324–331.
- 257.
Ehlken N, Lichtblau M, Klose H, Weidenhammer J, Fischer C, Nechwatal R, et al. Exercise training improves peak oxygen consumption and haemodynamics in patients with severe pulmonary arterial hypertension and inoperable chronic thrombo-embolic pulmonary hypertension: A prospective, randomized, controlled trial. Eur Heart J 2016; 37: 35–44.
- 258.
González-Saiz L, Fiuza-Luces C, Sanchis-Gomar F, Santos-Lozano A, Quezada-Loaiza CA, Flox-Camacho A, et al. Benefits of skeletal-muscle exercise training in pulmonary arterial hypertension: The WHOLEi+12 trial. Int J Cardiol 2017; 231: 277–283.
- 259.
Mainguy V, Maltais F, Saey D, Gagnon P, Martel S, Simon M, et al. Peripheral muscle dysfunction in idiopathic pulmonary arterial hypertension. Thorax 2010; 65: 113–117.
- 260.
Batt J, Ahmed SS, Correa J, Bain A, Granton J. Skeletal muscle dysfunction in idiopathic pulmonary arterial hypertension. Am J Respir Cell Mol Biol 2014; 50: 74–86.
- 261.
de Man FS, Handoko ML, Groepenhoff H, van’t Hul AJ, Abbink J, Koppers RJ, et al. Effects of exercise training in patients with idiopathic pulmonary arterial hypertension. Eur Respir J 2009; 34: 669–675.
- 262.
Peled N, Bendayan D, Shitrit D, Fox B, Yehoshua L, Kramer MR. Peripheral endothelial dysfunction in patients with pulmonary arterial hypertension. Respir Med 2008; 102: 1791–1796.
- 263.
Fukui S, Ogo T, Takaki H, Ueda J, Tsuji A, Morita Y, et al. Efficacy of cardiac rehabilitation after balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. Heart 2016; 102: 1403–1409.
- 264.
Grünig E, Lichtblau M, Ehlken N, Ghofrani HA, Reichenberger F, Staehler G, et al. Safety and efficacy of exercise training in various forms of pulmonary hypertension. Eur Respir J 2012; 40: 84–92.
- 265.
Handoko ML, de Man FS, Happé CM, Schalij I, Musters RJ, Westerhof N, et al. Opposite effects of training in rats with stable and progressive pulmonary hypertension. Circulation 2009; 120: 42–49.
- 266.
Bahia SS, Holt PJ, Ray KK, Ussher M, Poloniecki JD, Sharma R, et al. Cardiac rehabilitation versus standard care after aortic aneurysm repair (Aneurysm CaRe): Study protocol for a randomised controlled trial. Trials 2015; 16: 162.
- 267.
Pasin L, Nardelli P, Belletti A, Greco M, Landoni G, Cabrini L, et al. Pulmonary complications after open abdominal aortic surgery: A systematic review and meta-analysis. J Cardiothorac Vasc Anesth 2017; 31: 562–568.
- 268.
Lima RM, Vainshelboim B, Ganatra R, Dalman R, Chan K, Myers J. Exercise training improves ventilatory efficiency in patients with a small abdominal aortic aneurysm: A randomized controlled study. J Cardiopulm Rehabil Prev 2018; 38: 239–245.
- 269.
Kato M, Kubo A, Green FN, Takagi H. Meta-analysis of randomized controlled trials on safety and efficacy of exercise training in patients with abdominal aortic aneurysm. J Vasc Surg 2019; 69: 933–943.
- 270.
Pouwels S, Willigendael EM, van Sambeek MR, Nienhuijs SW, Cuypers PW, Teijink JA. Beneficial effects of pre-operative exercise therapy in patients with an abdominal aortic aneurysm: A systematic review. Eur J Vasc Endovasc Surg 2015; 49: 66–76.
- 271.
Wee IJY, Choong AMTL. A systematic review of the impact of preoperative exercise for patients with abdominal aortic aneurysm. J Vasc Surg 2020; 71: 2123–2131.
- 272.
Myers JN, White JJ, Narasimhan B, Dalman RL. Effects of exercise training in patients with abdominal aortic aneurysm: Preliminary results from a randomized trial. J Cardiopulm Rehabil Prev 2010; 30: 374–383.
- 273.
Tew GA, Batterham AM, Colling K, Gray J, Kerr K, Kothmann E, et al. Randomized feasibility trial of high-intensity interval training before elective abdominal aortic aneurysm repair. Br J Surg 2017; 104: 1791–1801.
- 274.
Tew GA, Weston M, Kothmann E, Batterham AM, Gray J, Kerr K, et al. High-intensity interval exercise training before abdominal aortic aneurysm repair (HIT-AAA): Protocol for a randomised controlled feasibility trial. BMJ Open 2014; 4: e004094.
- 275.
Myers J, McElrath M, Jaffe A, Smith K, Fonda H, Vu A, et al. A randomized trial of exercise training in abdominal aortic aneurysm disease. Med Sci Sports Exerc 2014; 46: 2–9.
- 276.
Nakayama A, Morita H, Nagayama M, Hoshina K, Uemura Y, Tomoike H, et al. Cardiac rehabilitation protects against the expansion of abdominal aortic aneurysm. J Am Heart Assoc 2018; 7: e007959.
- 277.
Bailey TG, Perissiou M, Windsor MT, Schulze K, Nam M, Magee R, et al. Effects of acute exercise on endothelial function in patients with abdominal aortic aneurysm. Am J Physiol Heart Circ Physiol 2018; 314: H19–H30.
- 278.
Ge X, Wang W, Hou L, Yang K, Fa X. Inspiratory muscle training is associated with decreased postoperative pulmonary complications: Evidence from randomized trials. J Thorac Cardiovasc Surg 2018; 156: 1290–1300.
- 279.
Kendall F, Oliveira J, Peleteiro B, Pinho P, Bastos PT. Inspiratory muscle training is effective to reduce postoperative pulmonary complications and length of hospital stay: A systematic review and meta-analysis. Disabil Rehabil 2018; 40: 864–882.
- 280.
Katsura M, Kuriyama A, Takeshima T, Fukuhara S, Furukawa TA. Preoperative inspiratory muscle training for postoperative pulmonary complications in adults undergoing cardiac and major abdominal surgery. Cochrane Database Syst Rev 2015; CD010356.
- 281.
Mans CM, Reeve JC, Elkins MR. Postoperative outcomes following preoperative inspiratory muscle training in patients undergoing cardiothoracic or upper abdominal surgery: A systematic review and meta analysis. Clin Rehabil 2015; 29: 426–438.
- 282.
Vagvolgyi A, Rozgonyi Z, Kerti M, Agathou G, Vadasz P, Varga J. Effectiveness of pulmonary rehabilitation and correlations in between functional parameters, extent of thoracic surgery and severity of post-operative complications: Randomized clinical trial. J Thorac Dis 2018; 10: 3519–3531.
- 283.
Boden I, Skinner EH, Browning L, Reeve J, Anderson L, Hill C, et al. Preoperative physiotherapy for the prevention of respiratory complications after upper abdominal surgery: Pragmatic, double blinded, multicentre randomised controlled trial. BMJ 2018; 360: j5916.
- 284.
do Nascimento Junior P, Módolo NS, Andrade S, Guimarães MM, Braz LG, El Dib R. Incentive spirometry for prevention of postoperative pulmonary complications in upper abdominal surgery. Cochrane Database Syst Rev 2014: CD006058.
- 285.
Raats JW, van Eijsden WA, Crolla RM, Steyerberg EW, van der Laan L. Risk factors and outcomes for postoperative delirium after major surgery in elderly patients. PLoS One 2015; 10: e0136071.
- 286.
Galyfos GC, Geropapas GE, Sianou A, Sigala F, Filis K. Risk factors for postoperative delirium in patients undergoing vascular surgery. J Vasc Surg 2017; 66: 937–946.
- 287.
日本脳卒中学会 脳卒中ガイドライン委員会. VII. リハビリテーション. 脳卒中治療ガイドライン2015. 協和企画 2015: 269–318 [in Japanese].
- 288.
日本理学療法士協会. 7. 脊髄損傷 理学療法ガイドライン. 理学療法ガイドライン第1版. 2011 [in Japanese]. http://www.japanpt.or.jp/upload/jspt/obj/files/guideline/13_cord_injury.pdf [accessed March, 2021].
- 289.
Brustia P, Renghi A, Aronici M, Gramaglia L, Porta C, Musiani A, et al. Fast-track in abdominal aortic surgery: Experience in over 1,000 patients. Ann Vasc Surg 2015; 29: 1151–1159.
- 290.
Feo CV, Portinari M, Tsolaki E, Romagnoni G, Verri M, Camerani S, et al. The effect of an Enhanced Recovery Program in elective retroperitoneal abdominal aortic aneurysm repair. J Vasc Surg 2016; 63: 888–894.
- 291.
Pasin L, Nardelli P, Landoni G, Beretta L, Piras D, Baccellieri D, et al. Enhanced recovery after surgery program in elective infrarenal abdominal aortic aneurysm repair. J Cardiovasc Surg (Torino) 2019; 60: 369–374.
- 292.
Preece R, Stenson K, Shaw S, Budge J, Patterson B, Holt P, et al. Recent developments and current controversies in short-stay endovascular aneurysm repair. J Cardiovasc Surg (Torino) 2019; 60: 460–467.
- 293.
Krajcer Z, Ramaiah VG, Henao EA, Metzger DC, Nelson WK, Moursi MM, et al; LIFE Registry Investigators. Perioperative outcomes from the prospective multicenter least invasive fast-track EVAR (LIFE) registry. J Endovasc Ther 2018; 25: 6–13.
- 294.
Krajcer Z, Ramaiah VG, Huetter M, Miller LE. Fast-track endovascular aortic repair: Interim report from the prospective LIFE registry. Catheter Cardiovasc Interv 2016; 88: 1118–1123.
- 295.
Gurgel SJ, El Dib R, do Nascimento P Jr. Enhanced recovery after elective open surgical repair of abdominal aortic aneurysm: A complementary overview through a pooled analysis of proportions from case series studies. PLoS One 2014; 9: e98006.
- 296.
Chaddha A, Kline-Rogers E, Woznicki EM, Brook R, Housholder-Hughes S, Braverman AC, et al. Cardiology patient page: Activity recommendations for postaortic dissection patients. Circulation 2014; 130: e140–e142.
- 297.
Delsart P, Maldonado-Kauffmann P, Bic M, Boudghene-Stambouli F, Sobocinski J, Juthier F, et al. Post aortic dissection: Gap between activity recommendation and real life patients aerobic capacities. Int J Cardiol 2016; 219: 271–276.
- 298.
Spanos K, Tsilimparis N, Kölbel T. Exercise after aortic dissection: To run or not to run. Eur J Vasc Endovasc Surg 2018; 55: 755–756.
- 299.
Hayashi K, Kobayashi K, Shimizu M, Tsuchikawa Y, Kodama A, Komori K, et al. Self-efficacy is an independent predictor for postoperative six-minute walk distance after elective open repair of abdominal aortic aneurysm. Disabil Rehabil 2018; 40: 1114–1118.
- 300.
Flink BJ, Long CA, Duwayri Y, Brewster LP, Veeraswamy R, Gallagher K, et al. Women undergoing aortic surgery are at higher risk for unplanned readmissions compared with men especially when discharged home. J Vasc Surg 2016; 63: 1496–1504.e1.
- 301.
Sacks GD, Lawson EH, Dawes AJ, Gibbons MM, Zingmond DS, Ko CY. Which patients require more care after hospital discharge? An analysis of post-acute care use among elderly patients undergoing elective surgery. J Am Coll Surg 2015; 220: 1113–1121.
- 302.
Karthikesalingam A, Bahia SS, Patterson BO, Peach G, Vidal-Diez A, Ray KK, et al. The shortfall in long-term survival of patients with repaired thoracic or abdominal aortic aneurysms: Retrospective case – control analysis of hospital episode statistics. Eur J Vasc Endovasc Surg 2013; 46: 533–541.
- 303.
Trimarchi S, Eagle KA, Nienaber CA, Pyeritz RE, Jonker FH, Suzuki T, et al; International Registry of Acute Aortic Dissection (IRAD) Investigators. Importance of refractory pain and hypertension in acute type B aortic dissection: Insights from the International Registry of Acute Aortic Dissection (IRAD). Circulation 2010; 122: 1283–1289.
- 304.
Patel AY, Eagle KA, Vaishnava P. Acute type B aortic dissection: Insights from the International Registry of Acute Aortic Dissection. Ann Cardiothorac Surg 2014; 3: 368–374.
- 305.
Reutersberg B, Trenner M, Haller B, Geisbüsch S, Reeps C, Eckstein HH. The incidence of delayed complications in acute type B aortic dissections is underestimated. J Vasc Surg 2018; 68: 356–363.
- 306.
Afifi RO, Sandhu HK, Leake SS, Boutrous ML, Kumar V 3rd, Azizzadeh A, et al. Outcomes of patients with acute type B (DeBakey III) aortic dissection: A 13-year, single-center experience. Circulation 2015; 132: 748–754.
- 307.
Niino T, Hata M, Sezai A, Yoshitake I, Unosawa S, Shimura K, et al. Optimal clinical pathway for the patient with type B acute aortic dissection. Circ J 2009; 73: 264–268.
- 308.
Fowkes FG, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: A systematic review and analysis. Lancet 2013; 382: 1329–1340.
- 309.
Alahdab F, Wang AT, Elraiyah TA, Malgor RD, Rizvi AZ, Lane MA, et al. A systematic review for the screening for peripheral arterial disease in asymptomatic patients. J Vasc Surg 2015; 61(Suppl): 42S–53S.
- 310.
Steg PG, Bhatt DL, Wilson PW, D’Agostino R Sr, Ohman EM, Röther J, et al; REACH Registry Investigators. One-year cardiovascular event rates in outpatients with atherothrombosis. JAMA 2007; 297: 1197–1206.
- 311.
Alberts MJ, Bhatt DL, Mas JL, Ohman EM, Hirsch AT, Röther J, et al; REACH Registry Investigators. Three-year follow-up and event rates in the international REduction of Atherothrombosis for Continued Health Registry. Eur Heart J 2009; 30: 2318–2326.
- 312.
Yasu T. Rehabilitation for patients with peripheral arterial disease. J Jpn Soc Limb Salvage Podiatric Med 2012; 4: 113–116 [in Japanese].
- 313.
Gerhard-Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: Executive Summary: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2017; 135: e686–e725.
- 314.
McDermott MM. Exercise training for intermittent claudication. J Vasc Surg 2017; 66: 1612–1620.
- 315.
Treat-Jacobson D, McDermott MM, Bronas UG, Campia U, Collins TC, Criqui MH, et al; American Heart Association Council on Peripheral Vascular Disease; Council on Quality of Care and Outcomes Research; and Council on Cardiovascular and Stroke Nursing. Optimal exercise programs for patients with peripheral artery disease: A Scientific Statement from the American Heart Association. Circulation 2019; 139: e10–e33.
- 316.
Azuma N, Iida O, Soga Y, Oura N, Ohki T, Ozaki M, et al; Japanese Circulation Society. JCS/JSVS 2022 guideline on the management of peripheral arterial disease [in Japanese]. https://www.j-circ.or.jp/cms/wp-content/uploads/2022/03/JCS2022_Azuma.pdf [accessed December, 2022].
- 317.
Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG, et al; TASC II Working Group. Inter-society consensus for the management of peripheral arterial disease (TASC II). Eur J Vasc Endovasc Surg 2007; 33(Suppl): S1–S75.
- 318.
Watson L, Ellis B, Leng GC. Exercise for intermittent claudication. Cochrane Database Syst Rev 2008: CD000990.
- 319.
Leng GC, Lee AJ, Fowkes FG, Whiteman M, Dunbar J, Housley E, et al. Incidence, natural history and cardiovascular events in symptomatic and asymptomatic peripheral arterial disease in the general population. Int J Epidemiol 1996; 25: 1172–1181.
- 320.
Murphy TP, Cutlip DE, Regensteiner JG, Mohler ER 3rd, Cohen DJ, Reynolds MR, et al. Supervised exercise, stent revascularization, or medical therapy for claudication due to aortoiliac peripheral artery disease: The CLEVER study. J Am Coll Cardiol 2015; 65: 999–1009.
- 321.
McDermott MM, Liu K, Guralnik JM, Criqui MH, Spring B, Tian L, et al. Home-based walking exercise intervention in peripheral artery disease: A randomized clinical trial. JAMA 2013; 310: 57–65.
- 322.
Pymer S, Palmer J, Harwood AE, Ingle L, Smith GE, Chetter IC. A systematic review of high-intensity interval training as an exercise intervention for intermittent claudication. J Vasc Surg 2019; 70: 2076–2087.
- 323.
Cornelis N, Nassen J, Buys R, Fourneau I, Cornelissen V. The impact of supervised exercise training on traditional cardiovascular risk factors in patients with intermittent claudication: A systematic review and meta-analysis. Eur J Vasc Endovasc Surg 2019; 58: 75–87.
- 324.
Lundgren F, Dahllöf AG, Lundholm K, Scherstén T, Volkmann R. Intermittent claudication--surgical reconstruction or physical training? A prospective randomized trial of treatment efficiency. Ann Surg 1989; 209: 346–355.
- 325.
Arao K, Yasu T, Endo Y, Funazaki T, Ota Y, Shimada K, et al; Investigators of the Anti-Arteriosclerosis and Lipid Lowering with Pitavastatin Evaluation Study in Nippon (ALPEN). Effects of pitavastatin on walking capacity and CD34+/133+ cell number in patients with peripheral artery disease. Heart Vessels 2017; 32: 1186–1194.
- 326.
Maejima Y, Yasu T, Ueba H, Kobayashi N, Hashimoto S, Kubo N, et al. Exercise after heparin administration: New therapeutic program for patients with-option arteriosclerosis obliterans. Circ J 2005; 69: 1099–1104.
- 327.
Otsuka S, Morisawa T, Yuguchi S, Hojo Y, Matsuo T, Nakajima M, et al. Clinical importance of change in physical activity after endovascular treatment combined with exercise training in patients with peripheral arterial disease. Heart Vessels 2017; 32: 143–148.
- 328.
Williams MA, Fleg JL, Ades PA, Chaitman BR, Miller NH, Mohiuddin SM, et al. Secondary prevention of coronary heart disease in the elderly (with emphasis on patients > or =75 years of age): An American Heart Association Scientific statement from the Council on Clinical Cardiology Subcommittee on Exercise, Cardiac Rehabilitation, and Prevention. Circulation 2002; 105: 1735–1743.
- 329.
Ishii S, Kojima T, Yamaguchi K, Akishita M. Guidance statement on appropriate medical services for the elderly. Geriatr Gerontol Int 2014; 14: 518–525.
- 330.
Gorodeski EZ, Goyal P, Hummel SL, Krishnaswami A, Goodlin SJ, Hart LL, et al; Geriatric Cardiology Section Leadership Council, American College of Cardiology. Domain management approach to heart failure in the geriatric patient: Present and future. J Am Coll Cardiol 2018; 71: 1921–1936.
- 331.
Hung C, Daub B, Black B, Welsh R, Quinney A, Haykowsky M. Exercise training improves overall physical fitness and quality of life in older women with coronary artery disease. Chest 2004; 126: 1026–1031.
- 332.
Antonicelli R, Spazzafumo L, Scalvini S, Olivieri F, Matassini MV, Parati G, et al. Exercise: A “new drug” for elderly patients with chronic heart failure. Aging (Albany NY) 2016; 8: 860–872.
- 333.
Dalal HM, Taylor RS, Jolly K, Davis RC, Doherty P, Miles J, et al. The effects and costs of home-based rehabilitation for heart failure with reduced ejection fraction: The REACH-HF multicentre randomized controlled trial. Eur J Prev Cardiol 2019; 26: 262–272.
- 334.
Chen YM, Li Y. Safety and efficacy of exercise training in elderly heart failure patients: A systematic review and meta-analysis. Int J Clin Pract 2013; 67: 1192–1198.
- 335.
Kitzman DW, Brubaker PH, Herrington DM, Morgan TM, Stewart KP, Hundley WG, et al. Effect of endurance exercise training on endothelial function and arterial stiffness in older patients with heart failure and preserved ejection fraction: A randomized, controlled, single-blind trial. J Am Coll Cardiol 2013; 62: 584–592.
- 336.
Witham MD, Fulton RL, Greig CA, Johnston DW, Lang CC, van der Pol M, et al. Efficacy and cost of an exercise program for functionally impaired older patients with heart failure: A randomized controlled trial. Circ Heart Fail 2012; 5: 209–216.
- 337.
Eder B, Hofmann P, von Duvillard SP, Brandt D, Schmid JP, Pokan R, et al. Early 4-week cardiac rehabilitation exercise training in elderly patients after heart surgery. J Cardiopulm Rehabil Prev 2010; 30: 85–92.
- 338.
Tanaka S, Kamiya K, Hamazaki N, Matsuzawa R, Nozaki K, Maekawa E, et al. Incremental value of objective frailty assessment to predict mortality in elderly patients hospitalized for heart failure. J Card Fail 2018; 24: 723–732.
- 339.
Molino-Lova R, Pasquini G, Vannetti F, Paperini A, Forconi T, Polcaro P, et al. Effects of a structured physical activity intervention on measures of physical performance in frail elderly patients after cardiac rehabilitation: A pilot study with 1-year follow-up. Intern Emerg Med 2013; 8: 581–589.
- 340.
Yoshimura Y, Wakabayashi H, Yamada M, Kim H, Harada A, Arai H. Interventions for treating sarcopenia: A systematic review and meta-analysis of randomized controlled studies. J Am Med Dir Assoc 2017; 18: 553.e1–553.e16.
- 341.
Antoniak AE, Greig CA. The effect of combined resistance exercise training and vitamin D3 supplementation on musculoskeletal health and function in older adults: A systematic review and meta-analysis. BMJ Open 2017; 7: e014619.
- 342.
Fiatarone MA, O’Neill EF, Ryan ND, Clements KM, Solares GR, Nelson ME, et al. Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med 1994; 330: 1769–1775.
- 343.
Ansai JH, Aurichio TR, Gonçalves R, Rebelatto JR. Effects of two physical exercise protocols on physical performance related to falls in the oldest old: A randomized controlled trial. Geriatr Gerontol Int 2016; 16: 492–499.
- 344.
Nieminen HP, Jokinen EV, Sairanen HI. Late results of pediatric cardiac surgery in Finland: A population-based study with 96% follow-up. Circulation 2001; 104: 570–575.
- 345.
Shiina Y, Toyoda T, Kawasoe Y, Tateno S, Shirai T, Wakisaka Y, et al. Prevalence of adult patients with congenital heart disease in Japan. Int J Cardiol 2011; 146: 13–16.
- 346.
Larsen SH, Emmertsen K, Johnsen SP, Pedersen J, Hjortholm K, Hjortdal VE. Survival and morbidity following congenital heart surgery in a population-based cohort of children: Up to 12 years of follow-up. Congenit Heart Dis 2011; 6: 322–329.
- 347.
Erikssen G, Liestøl K, Seem E, Birkeland S, Saatvedt KJ, Hoel TN, et al. Achievements in congenital heart defect surgery: A prospective, 40-year study of 7038 patients. Circulation 2015; 131: 337–346.
- 348.
Thaulow E, Fredriksen PM. Exercise and training in adults with congenital heart disease. Int J Cardiol 2004; 97(Suppl): 35–38.
- 349.
Gomes-Neto M, Saquetto MB, da Silva e Silva CM, Conceição CS, Carvalho VO. Impact of exercise training in aerobic capacity and pulmonary function in children and adolescents after congenital heart disease surgery: A systematic review with meta-analysis. Pediatr Cardiol 2016; 37: 217–224.
- 350.
Dulfer K, Duppen N, Blom NA, Van Domburg RT, Helbing WA, Verhulst FC, et al. Effects of exercise training on behavioral and emotional problems in adolescents with tetralogy of Fallot or a Fontan circulation: A randomized controlled trial. Int J Cardiol 2014; 172: e425–e427.
- 351.
Moalla W, Elloumi M, Chamari K, Dupont G, Maingourd Y, Tabka Z, et al. Training effects on peripheral muscle oxygenation and performance in children with congenital heart diseases. Appl Physiol Nutr Metab 2012; 37: 621–630.
- 352.
Singh TP, Curran TJ, Rhodes J. Cardiac rehabilitation improves heart rate recovery following peak exercise in children with repaired congenital heart disease. Pediatr Cardiol 2007; 28: 276–279.
- 353.
Rhodes J, Ubeda Tikkanen A, Jenkins KJ. Exercise testing and training in children with congenital heart disease. Circulation 2010; 122: 1957–1967.
- 354.
Moalla W, Maingourd Y, Gauthier R, Cahalin LP, Tabka Z, Ahmaidi S. Effect of exercise training on respiratory muscle oxygenation in children with congenital heart disease. Eur J Cardiovasc Prev Rehabil 2006; 13: 604–611.
- 355.
Amiard V, Jullien H, Nassif D, Bach V, Maingourd Y, Ahmaidi S. Effects of home-based training at dyspnea threshold in children surgically repaired for congenital heart disease. Congenit Heart Dis 2008; 3: 191–199.
- 356.
Fredriksen PM, Kahrs N, Blaasvaer S, Sigurdsen E, Gundersen O, Roeksund O, et al. Effect of physical training in children and adolescents with congenital heart disease. Cardiol Young 2000; 10: 107–114.
- 357.
Dulfer K, Duppen N, Kuipers IM, Schokking M, van Domburg RT, Verhulst FC, et al. Aerobic exercise influences quality of life of children and youngsters with congenital heart disease: A randomized controlled trial. J Adolesc Health 2014; 55: 65–72.
- 358.
Rhodes J, Curran TJ, Camil L, Rabideau N, Fulton DR, Gauthier NS, et al. Impact of cardiac rehabilitation on the exercise function of children with serious congenital heart disease. Pediatrics 2005; 116: 1339–1345.
- 359.
Buckenmeyer PJ, Vaccaro P, Vaccaro J, et al. Effect of a pediatric cardiac rehabilitation program on isokinetic strength and power measures in children. J Cardiopulm Rehabil 1986; 6: 437.
- 360.
Kataoka T, Keteyian SJ, Marks CR, Fedel FJ, Levine AB, Levine TB. Exercise training in a patient with congestive heart failure on continuous dobutamine. Med Sci Sports Exerc 1994; 26: 678–681.
- 361.
Arena R, Humphrey R, Peberdy MA. Safety and efficacy of exercise training in a patient awaiting heart transplantation while on positive intravenous inotropic support. J Cardiopulm Rehabil 2000; 20: 259–261.
- 362.
Yamada T, Saitoj M, Yanagihori A, Uewaki R, Kuwabara A, Ishii N, et al. 重症心不全患者に対する入院期心臓リハビリテーションの検討. J Jpn Assoc Card Rehabil 2009; 14: 157–161 [in Japanese].
- 363.
Dean AS, Libonati JR, Madonna D, Ratcliffe SJ, Margulies KB. Resistance training improves vasoreactivity in end-stage heart failure patients on inotropic support. J Cardiovasc Nurs 2011; 26: 218–223.
- 364.
Paul EHR, Camarda R, Foley LL, Givertz MM, Cahalin LP. Case report: Exercise in a patient with acute decompensated heart failure receiving positive inotropic therapy. Cardiopulm Phys Ther J 2011; 22: 13–18.
- 365.
Macauley K. Physical therapy management of two patients with Stage D heart failure in the cardiac medical intensive care unit. Cardiopulm Phys Ther J 2012; 23: 37–45.
- 366.
玉城雄也, 小西治美, 堀池聖子, 烏脇麻希子, 山本壱弥, 福井教之, 他. 重症心不全患者に対する心臓集中治療室での早期心臓リハビリテーションプログラムの導入. J Jpn Assoc Card Rehabil 2016; 22: 71–76 [in Japanese].
- 367.
Forestieri P, Guizilini S, Peres M, Bublitz C, Bolzan DW, Rocco IS, et al. A cycle ergometer exercise program improves exercise capacity and inspiratory muscle function in hospitalized patients awaiting heart transplantation: A pilot study. Braz J Cardiovasc Surg 2016; 31: 389–395.
- 368.
Feldman D, Pamboukian SV, Teuteberg JJ, Birks E, Lietz K, Moore SA, et al. The 2013 International Society for Heart and Lung Transplantation Guidelines for mechanical circulatory support: Executive Summary. J Heart Lung Transplant 2013; 32: 157–187.
- 369.
Jung MH, Gustafsson F. Exercise in heart failure patients supported with a left ventricular assist device. J Heart Lung Transplant 2015; 34: 489–496.
- 370.
Laoutaris ID, Dritsas A, Adamopoulos S, Manginas A, Gouziouta A, Kallistratos MS, et al. Benefits of physical training on exercise capacity, inspiratory muscle function, and quality of life in patients with ventricular assist devices long-term postimplantation. Eur J Cardiovasc Prev Rehabil 2011; 18: 33–40.
- 371.
Adamopoulos S, Gouziouta A, Mantzouratou P, Laoutaris ID, Dritsas A, Cokkinos DV, et al. Thyroid hormone signalling is altered in response to physical training in patients with end-stage heart failure and mechanical assist devices: Potential physiological consequences? Interact Cardiovasc Thorac Surg 2013; 17: 664–668.
- 372.
Kugler C, Malehsa D, Schrader E, Tegtbur U, Guetzlaff E, Haverich A, et al. A multi-modal intervention in management of left ventricular assist device outpatients: Dietary counselling, controlled exercise and psychosocial support. Eur J Cardiothorac Surg 2012; 42: 1026–1032.
- 373.
Hayes K, Leet AS, Bradley SJ, Holland AE. Effects of exercise training on exercise capacity and quality of life in patients with a left ventricular assist device: A preliminary randomized controlled trial. J Heart Lung Transplant 2012; 31: 729–734.
- 374.
Karapolat H, Engin C, Eroglu M, Yagdi T, Zoghi M, Nalbantgil S, et al. Efficacy of the cardiac rehabilitation program in patients with end-stage heart failure, heart transplant patients, and left ventricular assist device recipients. Transplant Proc 2013; 45: 3381–3385.
- 375.
Kerrigan DJ, Williams CT, Ehrman JK, Saval MA, Bronsteen K, Schairer JR, et al. Cardiac rehabilitation improves functional capacity and patient-reported health status in patients with continuous-flow left ventricular assist devices: The Rehab-VAD randomized controlled trial. JACC Heart Fail 2014; 2: 653–659.
- 376.
Marko C, Danzinger G, Käferbäck M, Lackner T, Müller R, Zimpfer D, et al. Safety and efficacy of cardiac rehabilitation for patients with continuous flow left ventricular assist devices. Eur J Prev Cardiol 2015; 22: 1378–1384.
- 377.
Mahfood Haddad T, Saurav A, Smer A, Azzouz MS, Akinapelli A, Williams MA, et al. Cardiac rehabilitation in patients with left ventricular assist device: A systematic review and meta-analysis. J Cardiopulm Rehabil Prev 2017; 37: 390–396.
- 378.
Grosman-Rimon L, Lalonde SD, Sieh N, Pakosh M, Rao V, Oh P, et al. Exercise rehabilitation in ventricular assist device recipients: A meta-analysis of effects on physiological and clinical outcomes. Heart Fail Rev 2019; 24: 55–67.
- 379.
Pope SE, Stinson EB, Daughters GT 2nd, Schroeder JS, Ingels NB Jr, Alderman EL. Exercise response of the denervated heart in long-term cardiac transplant recipients. Am J Cardiol 1980; 46: 213–218.
- 380.
Quigg RJ, Rocco MB, Gauthier DF, Creager MA, Hartley LH, Colucci WS. Mechanism of the attenuated peak heart rate response to exercise after orthotopic cardiac transplantation. J Am Coll Cardiol 1989; 14: 338–344.
- 381.
von Scheidt W, Neudert J, Erdmann E, Kemkes BM, Gokel JM, Autenrieth G. Contractility of the transplanted, denervated human heart. Am Heart J 1991; 121: 1480–1488.
- 382.
Kavanagh T, Yacoub MH, Mertens DJ, Kennedy J, Campbell RB, Sawyer P. Cardiorespiratory responses to exercise training after orthotopic cardiac transplantation. Circulation 1988; 77: 162–171.
- 383.
Kobashigawa JA, Leaf DA, Lee N, Gleeson MP, Liu H, Hamilton MA, et al. A controlled trial of exercise rehabilitation after heart transplantation. N Engl J Med 1999; 340: 272–277.
- 384.
Haykowsky M, Riess K, Figgures L, Kim D, Warburton D, Jones L, et al. Exercise training improves aerobic endurance and musculoskeletal fitness in female cardiac transplant recipients. Curr Control Trials Cardiovasc Med 2005; 6: 10.
- 385.
Pahl E, Sundararaghavan S, Strasburger JF, Mitchell BM, Rodgers S, Crowley D, et al. Impaired exercise parameters in pediatric heart transplant recipients: Comparison of biatrial and bicaval techniques. Pediatr Transplant 2000; 4: 268–272.
- 386.
Davis JA, McBride MG, Chrisant MR, Patil SM, Hanna BD, Paridon SM. Longitudinal assessment of cardiovascular exercise performance after pediatric heart transplantation. J Heart Lung Transplant 2006; 25: 626–633.
- 387.
Kavanagh T, Mertens DJ, Shephard RJ, Beyene J, Kennedy J, Campbell R, et al. Long-term cardiorespiratory results of exercise training following cardiac transplantation. Am J Cardiol 2003; 91: 190–194.
- 388.
Bengel FM, Ueberfuhr P, Schiepel N, Nekolla SG, Reichart B, Schwaiger M. Effect of sympathetic reinnervation on cardiac performance after heart transplantation. N Engl J Med 2001; 345: 731–738.
- 389.
Hsu DT, Garofano RP, Douglas JM, Michler RE, Quaegebeur JM, Gersony WM, et al. Exercise performance after pediatric heart transplantation. Circulation 1993; 88: II238–II242.
- 390.
Christensen SB, Dall CH, Prescott E, Pedersen SS, Gustafsson F. A high-intensity exercise program improves exercise capacity, self-perceived health, anxiety and depression in heart transplant recipients: A randomized, controlled trial. J Heart Lung Transplant 2012; 31: 106–107.
- 391.
Gilchrist SC, Barac A, Ades PA, Alfano CM, Franklin BA, Jones LW, et al; American Heart Association Exercise, Cardiac Rehabilitation, and Secondary Prevention Committee of the Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; and Council on Peripheral Vascular Disease. Cardio-oncology rehabilitation to manage cardiovascular outcomes in cancer patients and survivors: A Scientific Statement from the American Heart Association. Circulation 2019; 139: e997–e1012.
- 392.
Sase K, Kida K, Furukawa Y. Cardio-Oncology rehabilitation: Challenges and opportunities to improve cardiovascular outcomes in cancer patients and survivors. J Cardiol 2020; 76: 559–567.
- 393.
日本リハビリテーション医学会. がんのリハビリテーション診療ガイドライン第2版 [in Japanese]. Kanehara & Co., Ltd., 2019.
- 394.
Scott JM, Nilsen TS, Gupta D, Jones LW. Exercise therapy and cardiovascular toxicity in cancer. Circulation 2018; 137: 1176–1191.
- 395.
Jones LW, Eves ND, Haykowsky M, Joy AA, Douglas PS. Cardiorespiratory exercise testing in clinical oncology research: Systematic review and practice recommendations. Lancet Oncol 2008; 9: 757–765.
- 396.
Schmid D, Leitzmann MF. Cardiorespiratory fitness as predictor of cancer mortality: A systematic review and meta-analysis. Ann Oncol 2015; 26: 272–278.
- 397.
Lakoski SG, Eves ND, Douglas PS, Jones LW. Exercise rehabilitation in patients with cancer. Nat Rev Clin Oncol 2012; 9: 288–296.
- 398.
Peterson LL, Ligibel JA. Exercise and cardiovascular outcomes in older women with breast cancer: The Heart of the Matter. JACC CardioOncol 2019; 1: 51–53.
- 399.
Armenian SH, Lacchetti C, Barac A, Carver J, Constine LS, Denduluri N, et al. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 2017; 35: 893–911.
- 400.
Mehta LS, Watson KE, Barac A, Beckie TM, Bittner V, Cruz-Flores S, et al; American Heart Association Cardiovascular Disease in Women and Special Populations Committee of the Council on Clinical Cardiology, Council on Cardiovascular and Stroke Nursing, Council on Quality of Care and Outcomes Research. Cardiovascular disease and breast cancer: Where these entities intersect: A Scientific Statement from the American Heart Association. Circulation 2018; 137: e30–e66.
- 401.
日本静脈経腸栄養学会. 静脈経腸栄養テキストブック. 南江堂 2018; 127–137, 174–178 [in Japanese].
- 402.
Detsky AS, McLaughlin JR, Baker JP, Johnston N, Whittaker S, Mendelson RA, et al. What is subjective global assessment of nutritional status? J Parenter Enteral Nutr 1987; 11: 8–13.
- 403.
Ignacio de Ulíbarri J, González-Madroño A, de Villar NG, González P, González B, Mancha A, et al. CONUT: A tool for controlling nutritional status. First validation in a hospital population. Nutr Hosp 2005; 20: 38–45.
- 404.
Bouillanne O, Morineau G, Dupont C, Coulombel I, Vincent JP, Nicolis I, et al. Geriatric Nutritional Risk Index: A new index for evaluating at-risk elderly medical patients. Am J Clin Nutr 2005; 82: 777–783.
- 405.
Vellas B, Villars H, Abellan G, Soto ME, Rolland Y, Guigoz Y, et al. Overview of the MNA®: Its history and challenges. J Nutr Health Aging 2006; 10: 456–463.
- 406.
Rubenstein LZ, Harker JO, Salvà A, Guigoz Y, Vellas B. Screening for undernutrition in geriatric practice: Developing the short-form Mini-Nutritional Assessment (MNA-SF). J Gerontol A Biol Sci Med Sci 2001; 56: M366–M372.
- 407.
Guigoz Y. The Mini Nutritional Assessment (MNA®) review of the literature: What does it tell us? J Nutr Health Aging 2006; 10: 466–487.
- 408.
Cederholm T, Jensen GL, Correia MITD, Gonzalez MC, Fukushima R, Higashiguchi T, et al; GLIM Core Leadership Committee. GLIM criteria for the diagnosis of malnutrition: A consensus report from the global clinical nutrition community. Clin Nutr 2019; 38: 1–9.
- 409.
de van der Schueren MAE, Keller H, Cederholm T, Barazzoni R, Compher C, Correia MITD, et al; GLIM Consortium. Global Leadership Initiative on Malnutrition (GLIM): Guidance on validation of the operational criteria for the diagnosis of protein-energy malnutrition in adults. Clin Nutr 2020; 39: 2872–2880.
- 410.
日本栄養士会 監訳. 国際標準化のための栄養ケアプロセス用語マニュアル. 第一出版 2012: 2–9 [in Japanese].
- 411.
Nakaya Y. 心不全患者の栄養評価. J Jpn Soc Limb Salvage Podiatric Med 2019; 25: 254–257 [in Japanese].
- 412.
日本心不全学会ガイドライン委員会. 心不全患者における栄養評価・管理に関するステートメント. 2018 [in Japanese]. http://www.asas.or.jp/jhfs/pdf/statement20181012.pdf [accessed July, 2019].
- 413.
日本動脈硬化学会. 動脈硬化性疾患予防ガイドライン2017年版. ナナオ企画 2017: 61–77 [in Japanese].
- 414.
厚生労働省. 「日本人の食事摂取基準 (2020年版)」策定検討会 資料 [in Japanese]. https://www.mhlw.go.jp/content/10904750/000586553.pdf [accessed December, 2020].
- 415.
日本肥満学会. 肥満治療ガイドライン2016 [in Japanese]. ライフサイエンス出版 2016.
- 416.
Krenitsky J. Adjusted body weight, pro: Evidence to support the use of adjusted body weight in calculating calorie requirements. Nutr Clin Pract 2005; 20: 468–473.
- 417.
Scottish Intercollegiate Guidelines Network. SIGN 150. Cardiac rehabilitation: A national clinical guideline. 2017. https://www.sign.ac.uk/sign-150-cardiac-rehabilitation [accessed July, 2019].
- 418.
Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts). Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J 2016; 37: 2315–2381.
- 419.
Bandura A. Self-efficacy: The exercise of control. W.H. Freeman and Company, 1997.
- 420.
Takiguchi M, Yoshihisa A, Miura S, Shimizu T, Nakamura Y, Yamauchi H, et al. Impact of body mass index on mortality in heart failure patients. Eur J Clin Invest 2014; 44: 1197–1205.
- 421.
Aquilani R, Opasich C, Verri M, Boschi F, Febo O, Pasini E, et al. Is nutritional intake adequate in chronic heart failure patients? J Am Coll Cardiol 2003; 42: 1218–1223.
- 422.
Nochioka K, Sakata Y, Takahashi J, Miyata S, Miura M, Takada T, et al; CHART-2 Investigators. Prognostic impact of nutritional status in asymptomatic patients with cardiac diseases: A report from the CHART-2 Study. Circ J 2013; 77: 2318–2326.
- 423.
Saitoh M, Dos Santos MR, Ebner N, Emami A, Konishi M, Ishida J, et al. Nutritional status and its effects on muscle wasting in patients with chronic heart failure: Insights from Studies Investigating Co-morbidities Aggravating Heart Failure. Wien Klin Wochenschr 2016; 128: 497–504.
- 424.
Suzuki N, Kida K, Suzuki K, Harada T, Akashi YJ. Assessment of transthyretin combined with mini nutritional assessment on admission provides useful prognostic information in patients with acute decompensated heart failure. Int Heart J 2015; 56: 226–233.
- 425.
Joaquín C, Alonso N, Lupón J, de Antonio M, Domingo M, Moliner P, et al. Mini Nutritional Assessment Short Form is a morbi-mortality predictor in outpatients with heart failure and mid-range left ventricular ejection fraction. Clin Nutr 2020; 39: 3395–3401.
- 426.
Kinugasa Y, Kato M, Sugihara S, Hirai M, Yamada K, Yanagihara K, et al. Geriatric nutritional risk index predicts functional dependency and mortality in patients with heart failure with preserved ejection fraction. Circ J 2013; 77: 705–711.
- 427.
Kootaka Y, Kamiya K, Hamazaki N, Nozaki K, Ichikawa T, Nakamura T, et al. The GLIM criteria for defining malnutrition can predict physical function and prognosis in patients with cardiovascular disease. Clin Nutr 2021; 40: 146–152.
- 428.
Sargento L, Satendra M, Almeida I, Sousa C, Gomes S, Salazar F, et al. Nutritional status of geriatric outpatients with systolic heart failure and its prognostic value regarding death or hospitalization, biomarkers and quality of life. J Nutr Health Aging 2013; 17: 300–304.
- 429.
Habaybeh D, de Moraes MB, Slee A, Avgerinou C. Nutritional interventions for heart failure patients who are malnourished or at risk of malnutrition or cachexia: A systematic review and meta-analysis. Heart Fail Rev 2020; 26: 1103–1118.
- 430.
Yost G, Gregory M, Bhat G. Nutrition assessment with indirect calorimetry in patients evaluated for left ventricular assist device implantation. Nutr Clin Pract 2015; 30: 690–697.
- 431.
U.S. Department of Health and Human Services. Physical Activity Guidelines Advisory Committee Report, 2008. https://health.gov/our-work/physical-activity/previous-guidelines/2008-physical-activity-guidelines/advisory-report [accessed March, 2021].
- 432.
Kuehneman T, Gregory M, de Waal D, Davidson P, Frickel R, King C, et al. Academy of Nutrition and Dietetics evidence-based practice guideline for the management of heart failure in adults. J Acad Nutr Diet 2018; 118: 2331–2345.
- 433.
Volkert D, Beck AM, Cederholm T, Cruz-Jentoft A, Goisser S, Hooper L, et al. ESPEN guideline on clinical nutrition and hydration in geriatrics. Clin Nutr 2019; 38: 10–47.
- 434.
日本静脈経腸栄養学会. 静脈経腸栄養ガイドライン 第3版 [in Japanese]. 照林社 2013.
- 435.
Giezenaar C, Chapman I, Luscombe-Marsh N, Feinle-Bisset C, Horowitz M, Soenen S. Ageing is associated with decreases in appetite and energy intake: A meta-analysis in healthy adults. Nutrients 2016; 8: 28.
- 436.
Landi F, Calvani R, Tosato M, Martone AM, Ortolani E, Savera G, et al. Anorexia of aging: Risk factors, consequences, and potential treatments. Nutrients 2016; 8: 69.
- 437.
Xia T, Chai X, Shen J. Pancreatic exocrine insufficiency in patients with chronic heart failure and its possible association with appetite loss. PLoS One 2017; 12: e0187804.
- 438.
Saitoh M, Dos Santos MR, Emami A, Ishida J, Ebner N, Valentova M, et al. Anorexia, functional capacity, and clinical outcome in patients with chronic heart failure: Results from the Studies Investigating Co-morbidities Aggravating Heart Failure (SICA-HF). ESC Heart Fail 2017; 4: 448–457.
- 439.
Broqvist M, Arnqvist H, Dahlström U, Larsson J, Nylander E, Permert J. Nutritional assessment and muscle energy metabolism in severe chronic congestive heart failure: Effects of long-term dietary supplementation. Eur Heart J 1994; 15: 1641–1650.
- 440.
von Haehling S, Doehner W, Anker SD. Nutrition, metabolism, and the complex pathophysiology of cachexia in chronic heart failure. Cardiovasc Res 2007; 73: 298–309.
- 441.
Nicastro H, Artioli GG, Costa Ados S, Solis MY, da Luz CR, Blachier F, et al. An overview of the therapeutic effects of leucine supplementation on skeletal muscle under atrophic conditions. Amino Acids 2011; 40: 287–300.
- 442.
Mamerow MM, Mettler JA, English KL, Casperson SL, Arentson-Lantz E, Sheffield-Moore M, et al. Dietary protein distribution positively influences 24-h muscle protein synthesis in healthy adults. J Nutr 2014; 144: 876–880.
- 443.
Deutz NE, Bauer JM, Barazzoni R, Biolo G, Boirie Y, Bosy-Westphal A, et al. Protein intake and exercise for optimal muscle function with aging: Recommendations from the ESPEN Expert Group. Clin Nutr 2014; 33: 929–936.
- 444.
Bauer J, Biolo G, Cederholm T, Cesari M, Cruz-Jentoft AJ, Morley JE, et al. Evidence-based recommendations for optimal dietary protein intake in older people: A Position Paper from the PROT-AGE Study Group. J Am Med Dir Assoc 2013; 14: 542–559.
- 445.
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016; 18: 891–975.
- 446.
Paterna S, Parrinello G, Cannizzaro S, Fasullo S, Torres D, Sarullo FM, et al. Medium term effects of different dosage of diuretic, sodium, and fluid administration on neurohormonal and clinical outcome in patients with recently compensated heart failure. Am J Cardiol 2009; 103: 93–102.
- 447.
Paterna S, Gaspare P, Fasullo S, Sarullo FM, Di Pasquale P. Normal-sodium diet compared with low-sodium diet in compensated congestive heart failure: Is sodium an old enemy or a new friend? Clin Sci (Lond) 2008; 114: 221–230.
- 448.
Doukky R, Avery E, Mangla A, Collado FM, Ibrahim Z, Poulin MF, et al. Impact of dietary sodium restriction on heart failure outcomes. JACC Heart Fail 2016; 4: 24–35.
- 449.
アンドレア・ストレイト・シュライナー 監修, 守本とも子, 星野政明 編. 新・QOLを高める専門看護, 介護を考える [in Japanese]. 中央法規 2009.
- 450.
Michalsen A, Grossman P, Lehmann N, Knoblauch NT, Paul A, Moebus S, et al. Psychological and quality-of-life outcomes from a comprehensive stress reduction and lifestyle program in patients with coronary artery disease: Results of a randomized trial. Psychother Psychosom 2005; 74: 344–352.
- 451.
Toda G, Shibata S, Nakamizo R, Seto S, Yano K. Effect of physical exercise training on health-related quality of life and exercise tolerance in patients with left ventricular dysfunction. J Cardiol 2004; 44: 179–187.
- 452.
Ott CR, Sivarajan ES, Newton KM, Almes MJ, Bruce RA, Bergner M, et al. A controlled randomized study of early cardiac rehabilitation: The Sickness Impact Profile as an assessment tool. Heart Lung 1983; 12: 162–170.
- 453.
Linden W, Stossel C, Maurice J. Psychosocial interventions for patients with coronary artery disease: A meta-analysis. Arch Intern Med 1996; 156: 745–752.
- 454.
Molloy GJ, Johnston DW, Gao C, Witham MD, Gray JM, Argo IS, et al. Effects of an exercise intervention for older heart failure patients on caregiver burden and emotional distress. Eur J Cardiovasc Prev Rehabil 2006; 13: 381–387.
- 455.
後藤秀世, 長井祐介, 長沼文雄, 他. 慢性心不全患者における在宅療養上の問題点の抽出および患者教育に関する検討. J Jpn Assoc Card Rehabil 2005; 10: 130–135 [in Japanese].
- 456.
Zhang Y, Xu L, Yao Y, Guo X, Sun Y, Zhang J, et al. Effect of short-term exercise intervention on cardiovascular functions and quality of life of chronic heart failure patients: A meta-analysis. J Exerc Sci Fit 2016; 14: 67–75.
- 457.
Taylor RS, Walker S, Smart NA, Piepoli MF, Warren FC, Ciani O, et al. ExTraMATCH II Collaboration. Impact of exercise rehabilitation on exercise capacity and quality-of-life in heart failure: Individual participant meta-analysis. J Am Coll Cardiol 2019; 73: 1430–1443.
- 458.
Evans DL, Charney DS, Lewis L, Golden RN, Gorman JM, Krishnan KR, et al. Mood disorders in the medically ill: Scientific review and recommendations. Biol Psychiatry 2005; 58: 175–189.
- 459.
Rutledge T, Reis VA, Linke SE, Greenberg BH, Mills PJ. Depression in heart failure: A meta-analytic review of prevalence, intervention effects, and associations with clinical outcomes. J Am Coll Cardiol 2006; 48: 1527–1537.
- 460.
Lichtman JH, Froelicher ES, Blumenthal JA, Carney RM, Doering LV, Frasure-Smith N, et al; American Heart Association Statistics Committee of the Council on Epidemiology and Prevention and the Council on Cardiovascular and Stroke Nursing. Depression as a risk factor for poor prognosis among patients with acute coronary syndrome: Systematic review and recommendations: A Scientific Statement from the American Heart Association. Circulation 2014; 129: 1350–1369.
- 461.
Celano CM, Millstein RA, Bedoya CA, Healy BC, Roest AM, Huffman JC. Association between anxiety and mortality in patients with coronary artery disease: A meta-analysis. Am Heart J 2015; 170: 1105–1115.
- 462.
Denollet J, Sys SU, Stroobant N, Rombouts H, Gillebert TC, Brutsaert DL. Personality as independent predictor of long-term mortality in patients with coronary heart disease. Lancet 1996; 347: 417–421.
- 463.
Versteeg H, Spek V, Pedersen SS, Denollet J. Type D personality and health status in cardiovascular disease populations: A meta-analysis of prospective studies. Eur J Prev Cardiol 2012; 19: 1373–1380.
- 464.
Smaardijk VR, Maas AHEM, Lodder P, Kop WJ, Mommersteeg PMC. Sex and gender-stratified risks of psychological factors for adverse clinical outcomes in patients with ischemic heart disease: A systematic review and meta-analysis. Int J Cardiol 2020; 302: 21–29.
- 465.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM–5). American Psychiatric Publishing, 2014.
- 466.
McPhillips R, Salmon P, Wells A, Fisher P. Cardiac rehabilitation patients’ accounts of their emotional distress and psychological needs: A qualitative study. J Am Heart Assoc 2019; 8: e011117.
- 467.
Silveira H, Moraes H, Oliveira N, Coutinho ES, Laks J, Deslandes A. Physical exercise and clinically depressed patients: A systematic review and meta-analysis. Neuropsychobiology 2013; 67: 61–68.
- 468.
Zheng X, Zheng Y, Ma J, Zhang M, Zhang Y, Liu X, et al. Effect of exercise-based cardiac rehabilitation on anxiety and depression in patients with myocardial infarction: A systematic review and meta-analysis. Heart Lung 2019; 48: 1–7.
- 469.
Thota AB, Sipe TA, Byard GJ, Zometa CS, Hahn RA, McKnight-Eily LR, et al; Community Preventive Services Task Force. Collaborative care to improve the management of depressive disorders: A community guide systematic review and meta-analysis. Am J Prev Med 2012; 42: 525–538.
- 470.
Meng K, Musekamp G, Schuler M, Seekatz B, Glatz J, Karger G, et al. The impact of a self-management patient education program for patients with chronic heart failure undergoing inpatient cardiac rehabilitation. Patient Educ Couns 2016; 99: 1190–1197.
- 471.
Lynggaard V, Nielsen CV, Zwisler AD, Taylor RS, May O. The patient education - Learning and Coping Strategies - improves adherence in cardiac rehabilitation (LC-REHAB): A randomised controlled trial. Int J Cardiol 2017; 236: 65–70.
- 472.
Dankner R, Drory Y, Geulayov G, Ziv A, Novikov I, Zlotnick AY, et al. A controlled intervention to increase participation in cardiac rehabilitation. Eur J Prev Cardiol 2015; 22: 1121–1128.
- 473.
Mayer-Berger W, Simic D, Mahmoodzad J, Burtscher R, Kohlmeyer M, Schwitalla B, et al. Efficacy of a long-term secondary prevention programme following inpatient cardiovascular rehabilitation on risk and health-related quality of life in a low-education cohort: A randomized controlled study. Eur J Prev Cardiol 2014; 21: 145–152.
- 474.
Freeman AM, Taub PR, Lo HC, Ornish D. Intensive cardiac rehabilitation: An underutilized resource. Curr Cardiol Rep 2019; 21: 19.
- 475.
Inglis SC, Pearson S, Treen S, Gallasch T, Horowitz JD, Stewart S. Extending the horizon in chronic heart failure: Effects of multidisciplinary, home-based intervention relative to usual care. Circulation 2006; 114: 2466–2473.
- 476.
Mittag O, China C, Hoberg E, Juers E, Kolenda KD, Richardt G, et al. Outcomes of cardiac rehabilitation with versus without a follow-up intervention rendered by telephone (Luebeck follow-up trial): Overall and gender-specific effects. Int J Rehabil Res 2006; 29: 295–302.
- 477.
Ghisi GL, Britto R, Motamedi N, Grace SL. Disease-related knowledge in cardiac rehabilitation enrollees: Correlates and changes. Patient Educ Couns 2015; 98: 533–539.
- 478.
Ades PA, Keteyian SJ, Balady GJ, Houston-Miller N, Kitzman DW, Mancini DM, et al. Cardiac rehabilitation exercise and self-care for chronic heart failure. JACC Heart Fail 2013; 1: 540–547.
- 479.
Weiss BD, AMA Foundation. Health literacy: A manual for clinicians: Part of an educational program about health literacy. American Medical Association, 2003.
- 480.
Health Literacy Tool Shed: A database of health literacy measures. https://healthliteracy.bu.edu/ [accessed August 30, 2019].
- 481.
Zhang KM, Prior PL, Swartzman LC, Suskin N, Unsworth KL, Minda JP. Can causal explanations about endothelial pathophysiology benefit patient education? A cluster randomized controlled trial in cardiac rehabilitation. Patient Educ Couns 2019; 102: 1672–1679.
- 482.
Molan N, Emmanuel S, Langley T, Holloway CJ. Evaluating the effectiveness of an online cardiac rehabilitation resource (www.svhhearthealth.com.au) in improving knowledge and confidence for patients with newly diagnosed cardiac conditions: A pre-experimental pilot study. Heart Lung Circ 2019; 28: 761–770.
- 483.
Umemura S, Arima H, Arima S, Asayama K, Dohi Y, Hirooka Y, et al. The Japanese Society of Hypertension guidelines for the management of hypertension (JSH 2019). Hypertens Res 2019; 42: 1235–1481.
- 484.
Kinoshita M, Yokote K, Arai H, Iida M, Ishigaki Y, Ishibashi S, et al; Committee for Epidemiology and Clinical Management of Atherosclerosis. Japan Atherosclerosis Society (JAS) guidelines for prevention of atherosclerotic cardiovascular diseases 2017. J Atheroscler Thromb 2018; 25: 846–984.
- 485.
Araki E, Goto A, Kondo T, Noda M, Noto H, Origasa H, et al. Japanese clinical practice guideline for diabetes 2019. Diabetol Int 2020; 11: 165–223.
- 486.
Taylor RS, Brown A, Ebrahim S, Jolliffe J, Noorani H, Rees K, et al. Exercise-based rehabilitation for patients with coronary heart disease: Systematic review and meta-analysis of randomized controlled trials. Am J Med 2004; 116: 682–692.
- 487.
Taylor RS, Unal B, Critchley JA, Capewell S. Mortality reductions in patients receiving exercise-based cardiac rehabilitation: How much can be attributed to cardiovascular risk factor improvements? Eur J Cardiovasc Prev Rehabil 2006; 13: 369–374.
- 488.
Kotseva K, Wood D, De Bacquer D, EUROASPIRE investigators. Determinants of participation and risk factor control according to attendance in cardiac rehabilitation programmes in coronary patients in Europe: EUROASPIRE IV survey. Eur J Prev Cardiol 2018; 25: 1242–1251.
- 489.
Balady GJ, Williams MA, Ades PA, Bittner V, Comoss P, Foody JM, et al. Core components of cardiac rehabilitation/secondary prevention programs: 2007 update: A Scientific Statement from the American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee, the Council on Clinical Cardiology; the Councils on Cardiovascular Nursing, Epidemiology and Prevention, and Nutrition, Physical Activity, and Metabolism; and the American Association of Cardiovascular and Pulmonary Rehabilitation. Circulation 2007; 115: 2675–2682.
- 490.
Silverman MG, Ference BA, Im K, Wiviott SD, Giugliano RP, Grundy SM, et al. Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: A systematic review and meta-analysis. JAMA 2016; 316: 1289–1297.
- 491.
Boekholdt SM, Arsenault BJ, Mora S, Pedersen TR, LaRosa JC, Nestel PJ, et al. Association of LDL cholesterol, non-HDL cholesterol, and apolipoprotein B levels with risk of cardiovascular events among patients treated with statins: A meta-analysis. JAMA 2012; 307: 1302–1309.
- 492.
Schwartz GG, Abt M, Bao W, DeMicco D, Kallend D, Miller M, et al. Fasting triglycerides predict recurrent ischemic events in patients with acute coronary syndrome treated with statins. J Am Coll Cardiol 2015; 65: 2267–2275.
- 493.
Boekholdt SM, Arsenault BJ, Hovingh GK, Mora S, Pedersen TR, Larosa JC, et al. Levels and changes of HDL cholesterol and apolipoprotein A-I in relation to risk of cardiovascular events among statin-treated patients: A meta-analysis. Circulation 2013; 128: 1504–1512.
- 494.
Araki E, Tanaka A, Inagaki N, Ito H, Ueki K, Murohara T, et al; Directors of the JCS and JDS. Diagnosis, prevention, and treatment of cardiovascular diseases in people with Type 2 diabetes and prediabetes: A Consensus Statement Jointly from the Japanese Circulation Society and the Japan Diabetes Society. Circ J 2020; 85: 82–125.
- 495.
Kavanagh T, Mertens DJ, Hamm LF, Beyene J, Kennedy J, Corey P, et al. Prediction of long-term prognosis in 12 169 men referred for cardiac rehabilitation. Circulation 2002; 106: 666–671.
- 496.
Colberg SR, Sigal RJ, Yardley JE, Riddell MC, Dunstan DW, Dempsey PC, et al. Physical activity/exercise and diabetes: A Position Statement of the American Diabetes Association. Diabetes Care 2016; 39: 2065–2079.
- 497.
Sadeghi M, Salehi-Abargouei A, Kasaei Z, Sajjadieh-Khajooie H, Heidari R, Roohafza H. Effect of cardiac rehabilitation on metabolic syndrome and its components: A systematic review and meta-analysis. J Res Med Sci 2016; 21: 18.
- 498.
Takaya Y, Kumasaka R, Arakawa T, Ohara T, Nakanishi M, Noguchi T, et al. Impact of cardiac rehabilitation on renal function in patients with and without chronic kidney disease after acute myocardial infarction. Circ J 2014; 78: 377–384.
- 499.
Iso Y, Kitai H, Kowaita H, Kyuno E, Maezawa H, Hashimoto T, et al. Association of aging with glomerular filtration changes in cardiac rehabilitation participants with chronic kidney disease. Int J Cardiol 2015; 187: 283–285.
- 500.
Biswas A, Oh PI, Faulkner GE, Bonsignore A, Pakosh MT, Alter DA. The energy expenditure benefits of reallocating sedentary time with physical activity: A systematic review and meta-analysis. J Public Health (Oxf) 2018; 40: 295–303.
- 501.
Hannan AL, Harders MP, Hing W, Climstein M, Coombes JS, Furness J. Impact of wearable physical activity monitoring devices with exercise prescription or advice in the maintenance phase of cardiac rehabilitation: Systematic review and meta-analysis. BMC Sports Sci Med Rehabil 2019; 11: 14.
- 502.
Hackshaw A, Morris JK, Boniface S, Tang JL, Milenković D. Low cigarette consumption and risk of coronary heart disease and stroke: Meta-analysis of 141 cohort studies in 55 study reports. BMJ 2018; 360: j5855.
- 503.
Huxley RR, Woodward M. Cigarette smoking as a risk factor for coronary heart disease in women compared with men: A systematic review and meta-analysis of prospective cohort studies. Lancet 2011; 378: 1297–1305.
- 504.
Lightwood JM, Glantz SA. Declines in acute myocardial infarction after smoke-free laws and individual risk attributable to secondhand smoke. Circulation 2009; 120: 1373–1379.
- 505.
Critchley JA, Capewell S. Mortality risk reduction associated with smoking cessation in patients with coronary heart disease: A systematic review. JAMA 2003; 290: 86–97.
- 506.
Kinjo K, Sato H, Sakata Y, Nakatani D, Mizuno H, Shimizu M, et al; Osaka Acute Coronary Insufficiency Study (OACIS) Group. Impact of smoking status on long-term mortality in patients with acute myocardial infarction. Circ J 2005; 69: 7–12.
- 507.
日本循環器学会, 日本肺癌学会, 日本癌学会, 他. 禁煙治療のための標準手順書 第6版. 2014: 1–52 [in Japanese].
- 508.
Buchanan ND, Grimmer JA, Tanwar V, Schwieterman N, Mohler PJ, Wold LE. Cardiovascular risk of electronic cigarettes: A review of preclinical and clinical studies. Cardiovasc Res 2020; 116: 40–50.
- 509.
Riegel B, Dickson VV, Faulkner KM. The situation-specific theory of heart failure self-care: Revised and updated. J Cardiovasc Nurs 2016; 31: 226–235.
- 510.
Sethares KA, Sosa ME, Fisher P, Riegel B. Factors associated with delay in seeking care for acute decompensated heart failure. J Cardiovasc Nurs 2014; 29: 429–438.
- 511.
Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, et al. 2013 ACCF/AHA guideline for the management of heart failure: Executive Summary: A report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013; 128: 1810–1852.
- 512.
Joekes K, Van Elderen T, Schreurs K. Self-efficacy and overprotection are related to quality of life, psychological well-being and self-management in cardiac patients. J Health Psychol 2007; 12: 4–16.
- 513.
Deleted in proof.
- 514.
Takeda A, Martin N, Taylor RS, Taylor SJ. Disease management interventions for heart failure. Cochrane Database Syst Rev
2019: CD002752.
- 515.
Wita K, Kułach A, Sikora J, Fluder J, Nowalany-Kozielska E, Milewski K, et al. Managed care after acute myocardial infarction (MC-AMI) reduces total mortality in 12-month follow-up-results from a Poland’s National Health Fund program of comprehensive post-MI care: A population-wide analysis. J Clin Med 2020; 9: 3178.
- 516.
Goto Y. Current status and future perspective of cardiac rehabilitation in Japan. J Jpn Coron Assoc 2015; 21: 58–66 [in Japanese].
- 517.
Beatty AL, Truong M, Schopfer DW, Shen H, Bachmann JM, Whooley MA. Geographic variation in cardiac rehabilitation participation in medicare and veterans affairs populations: Opportunity for improvement. Circulation 2018; 137: 1899–1908.
- 518.
Kusunoki S, Maruji A, Kobayashi K, Hirao N, Konishi H, Fukui N, et al. Subjective barriers to adherence to cardiac rehabilitation program after hospital discharge in patients with acute myocardial infarction. J Jpn Coron Assoc 2008; 14: 206–210 [in Japanese].
- 519.
Endo N, Goto A, Suzuki T, Matsuda S, Yasumura S. Factors associated with enrollment and adherence in outpatient cardiac rehabilitation in Japan. J Cardiopulm Rehabil Prev 2015; 35: 186–192.
- 520.
Im HW, Baek S, Jee S, Ahn JM, Park MW, Kim WS. Barriers to outpatient hospital-based cardiac rehabilitation in Korean patients with acute coronary syndrome. Ann Rehabil Med 2018; 42: 154–165.
- 521.
Leung YW, Brual J, Macpherson A, Grace SL. Geographic issues in cardiac rehabilitation utilization: A narrative review. Health Place 2010; 16: 1196–1205.
- 522.
Clark RA, Conway A, Poulsen V, Keech W, Tirimacco R, Tideman P. Alternative models of cardiac rehabilitation: A systematic review. Eur J Prev Cardiol 2015; 22: 35–74.
- 523.
Jolly K, Taylor RS, Lip GY, Stevens A. Home-based cardiac rehabilitation compared with centre-based rehabilitation and usual care: A systematic review and meta-analysis. Int J Cardiol 2006; 111: 343–351.
- 524.
Taylor RS, Dalal H, Jolly K, Moxham T, Zawada A. Home-based versus centre-based cardiac rehabilitation. Cochrane Database Syst Rev
2010: CD007130.
- 525.
Taylor RS, Dalal H, Jolly K, Zawada A, Dean SG, Cowie A, et al. Home-based versus centre-based cardiac rehabilitation. Cochrane Database Syst Rev
2015: CD007130.
- 526.
Anderson L, Sharp GA, Norton RJ, Dalal H, Dean SG, Jolly K, et al. Home-based versus centre-based cardiac rehabilitation. Cochrane Database Syst Rev
2017: CD007130.
- 527.
Conroy MB, Yang K, Elci OU, Gabriel KP, Styn MA, Wang J, et al. Physical activity self-monitoring and weight loss: 6-month results of the SMART trial. Med Sci Sports Exerc 2011; 43: 1568–1574.
- 528.
Dellifraine JL, Dansky KH. Home-based telehealth: A review and meta-analysis. J Telemed Telecare 2008; 14: 62–66.
- 529.
Bravo-Escobar R, González-Represas A, Gómez-González AM, Montiel-Trujillo A, Aguilar-Jimenez R, Carrasco-Ruíz R, et al. Effectiveness and safety of a home-based cardiac rehabilitation programme of mixed surveillance in patients with ischemic heart disease at moderate cardiovascular risk: A randomised, controlled clinical trial. BMC Cardiovasc Disord 2017; 17: 66.
- 530.
Kraal JJ, Van den Akker-Van Marle ME, Abu-Hanna A, Stut W, Peek N, Kemps HM. Clinical and cost-effectiveness of home-based cardiac rehabilitation compared to conventional, centre-based cardiac rehabilitation: Results of the FIT@Home study. Eur J Prev Cardiol 2017; 24: 1260–1273.
- 531.
Piotrowicz E, Zieliński T, Bodalski R, Rywik T, Dobraszkiewicz-Wasilewska B, Sobieszczańska-Małek M, et al. Home-based telemonitored Nordic walking training is well accepted, safe, effective and has high adherence among heart failure patients, including those with cardiovascular implantable electronic devices: A randomised controlled study. Eur J Prev Cardiol 2015; 22: 1368–1377.
- 532.
Varnfield M, Karunanithi M, Lee CK, Honeyman E, Arnold D, Ding H, et al. Smartphone-based home care model improved use of cardiac rehabilitation in postmyocardial infarction patients: Results from a randomised controlled trial. Heart 2014; 100: 1770–1779.
- 533.
Papadakis S, Oldridge NB, Coyle D, Mayhew A, Reid RD, Beaton L, et al. Economic evaluation of cardiac rehabilitation: A systematic review. Eur J Cardiovasc Prev Rehabil 2005; 12: 513–520.
- 534.
Shields GE, Wells A, Doherty P, Heagerty A, Buck D, Davies LM. Cost-effectiveness of cardiac rehabilitation: A systematic review. Heart 2018; 104: 1403–1410.
- 535.
Southard BH, Southard DR, Nuckolls J. Clinical trial of an Internet-based case management system for secondary prevention of heart disease. J Cardiopulm Rehabil 2003; 23: 341–348.
- 536.
Wheeler JR, Janz NK, Dodge JA. Can a disease self-management program reduce health care costs? The case of older women with heart disease. Med Care 2003; 41: 706–715.
- 537.
Ades PA, Huang D, Weaver SO. Cardiac rehabilitation participation predicts lower rehospitalization costs. Am Heart J 1992; 123: 916–921.
- 538.
Levin LA, Perk J, Hedbäck B. Cardiac rehabilitation: A cost analysis. J Intern Med 1991; 230: 427–434.
- 539.
Stewart S, Marley JE, Horowitz JD. Effects of a multidisciplinary, home-based intervention on unplanned readmissions and survival among patients with chronic congestive heart failure: A randomised controlled study. Lancet 1999; 354: 1077–1083.
- 540.
Hautala AJ, Kiviniemi AM, Mäkikallio T, Koistinen P, Ryynänen OP, Martikainen JA, et al. Economic evaluation of exercise-based cardiac rehabilitation in patients with a recent acute coronary syndrome. Scand J Med Sci Sports 2017; 27: 1395–1403.
- 541.
Yu CM, Lau CP, Chau J, McGhee S, Kong SL, Cheung BM, et al. A short course of cardiac rehabilitation program is highly cost effective in improving long-term quality of life in patients with recent myocardial infarction or percutaneous coronary intervention. Arch Phys Med Rehabil 2004; 85: 1915–1922.
- 542.
Frederix I, Hansen D, Coninx K, Vandervoort P, Vandijck D, Hens N, et al. Effect of comprehensive cardiac telerehabilitation on one-year cardiovascular rehospitalization rate, medical costs and quality of life: A cost-effectiveness analysis. Eur J Prev Cardiol 2016; 23: 674–682.
- 543.
Kidholm K, Rasmussen MK, Andreasen JJ, Hansen J, Nielsen G, Spindler H, et al. Cost-utility analysis of a cardiac telerehabilitation program: The Teledialog Project. Telemed J E Health 2016; 22: 553–563.
- 544.
Hansen TB, Zwisler AD, Berg SK, Sibilitz KL, Thygesen LC, Kjellberg J, et al. Cost-utility analysis of cardiac rehabilitation after conventional heart valve surgery versus usual care. Eur J Prev Cardiol 2017; 24: 698–707.
- 545.
Oldridge N, Furlong W, Feeny D, Torrance G, Guyatt G, Crowe J, et al. Economic evaluation of cardiac rehabilitation soon after acute myocardial infarction. Am J Cardiol 1993; 72: 154–161.
- 546.
Briffa TG, Eckermann SD, Griffiths AD, Harris PJ, Heath MR, Freedman SB, et al. Cost-effectiveness of rehabilitation after an acute coronary event: A randomised controlled trial. Med J Aust 2005; 183: 450–455.
- 547.
Martin AJ, Glasziou PP, Simes RJ. A cardiovascular extension of the Health Measurement Questionnaire. J Epidemiol Community Health 1999; 53: 548–557.
- 548.
Dehbarez NT, Lynggaard V, May O, Søgaard R. Learning and coping strategies versus standard education in cardiac rehabilitation: A cost-utility analysis alongside a randomised controlled trial. BMC Health Serv Res 2015; 15: 422.
- 549.
Eriksson MK, Hagberg L, Lindholm L, Malmgren-Olsson EB, Osterlind J, Eliasson M. Quality of life and cost-effectiveness of a 3-year trial of lifestyle intervention in primary health care. Arch Intern Med 2010; 170: 1470–1479.
- 550.
Takura T, Ebata-Kogure N, Goto Y, Kohzuki M, Nagayama M, Oikawa K, et al. Cost-effectiveness of cardiac rehabilitation in patients with coronary artery disease: A meta-analysis. Cardiol Res Pract 2019; 2019: 1840894.
- 551.
Hwang R, Morris NR, Mandrusiak A, Bruning J, Peters R, Korczyk D, et al. Cost-utility analysis of home-based telerehabilitation compared with centre-based rehabilitation in patients with heart failure. Heart Lung Circ 2019; 28: 1795–1803.
- 552.
Georgiou D, Chen Y, Appadoo S, Belardinelli R, Greene R, Parides MK, et al. Cost-effectiveness analysis of long-term moderate exercise training in chronic heart failure. Am J Cardiol 2001; 87: 984–988.
- 553.
Hambrecht R, Walther C, Möbius-Winkler S, Gielen S, Linke A, Conradi K, et al. Percutaneous coronary angioplasty compared with exercise training in patients with stable coronary artery disease: A randomized trial. Circulation 2004; 109: 1371–1378.
- 554.
Jacobs N, Evers S, Ament A, Claes N. Cost-utility of a cardiovascular prevention program in highly educated adults: Intermediate results of a randomized controlled trial. Int J Technol Assess Health Care 2010; 26: 11–19.
- 555.
Ades PA, Pashkow FJ, Nestor JR. Cost-effectiveness of cardiac rehabilitation after myocardial infarction. J Cardiopulm Rehabil 1997; 17: 222–231.
- 556.
Edwards K, Jones N, Newton J, Foster C, Judge A, Jackson K, et al. The cost-effectiveness of exercise-based cardiac rehabilitation: A systematic review of the characteristics and methodological quality of published literature. Health Econ Rev 2017; 7: 37.
- 557.
Francis T, Kabboul N, Rac V, Mitsakakis N, Pechlivanoglou P, Bielecki J, et al. The effect of cardiac rehabilitation on health-related quality of life in patients with coronary artery disease: A meta-analysis. Can J Cardiol 2019; 35: 352–364.
- 558.
Maddison R, Rawstorn JC, Stewart RAH, Benatar J, Whittaker R, Rolleston A, et al. Effects and costs of real-time cardiac telerehabilitation: Randomised controlled non-inferiority trial. Heart 2019; 105: 122–129.
- 559.
Frederix I, Solmi F, Piepoli MF, Dendale P. Cardiac telerehabilitation: A novel cost-efficient care delivery strategy that can induce long-term health benefits. Eur J Prev Cardiol 2017; 24: 1708–1717.