2023 Volume 87 Issue 1 Pages 111-119
2023 Volume 87 Issue 1 Pages 111-119
Background: Idiopathic bradyarrhythmia is considered to be due to pathological degeneration of the cardiac conduction system (CCS) during aging. There appears to have been no comprehensive genetic investigations in patients with idiopathic bradyarrhythmia.
Methods and Results: Ten autopsy cases with advanced bradyarrhythmia (6 men and 4 women; age: 70–94 years, 81.5±6.9 years; 5 cases each of sinus node dysfunction [SND] and complete atrioventricular block [CAVB]) were genetically investigated by using whole-exome sequencing. Morphometric analysis of the CCS was performed with sex-, age- and comorbidity-matched control cases. As a result, severe loss of nodal cells and distal atrioventricular conduction system were found in SND and CAVB, respectively. However, the conduction tissue loss was not significant in either the atrioventricular node or the proximal bundle of His in CAVB cases. A total of 13 heterozygous potential variants were found in 3 CAVB and 2 SND cases. Of these 13 variants, 4 were missense in the known progressive cardiac conduction disease-related genes: GATA4 and RYR2. In the remaining 9 variants, 5 were loss-of-function mutation with highly possible pathogenicity.
Conclusions: In addition to degenerative changes of selectively vulnerable areas in the heart during advancing age, the vulnerability of the CCS, which may be associated with “rare variants of small effect,” may also be a contributing factor to the degeneration of CCS, leading to “idiopathic” bradyarrhythmia.
Bradyarrhythmias are a common clinical condition and comprise various rhythm disorders, including sinus node dysfunction (SND) and atrioventricular conduction blocks.1 The prevalence of SND increases with age, and its rate is approximately 1/1,000 person-years in adults aged >45 years and 1/600 in those aged >65 years.2 The idiopathic degenerative fibrosis of the nodal tissue that might be associated with aging is considered the most common intrinsic cause of SND.1,2 Complete (third-degree) atrioventricular block (CAVB) is the absence of conduction from the atrium to the ventricle, and its incidence is estimated to be 0.4%.3 Idiopathic degeneration of the AV conduction system, histologically reported by Lenegre4 and Lev,5 accounts for approximately 50% of intrinsic AV block cases.1
Editorial p 120
Many previous pathological examinations for advanced bradyarrhythmia cases focused on the amount of collagen fiber in conduction tissue.6 However, other pathological features, including replacement of the conduction tissue by fatty infiltration or neoplastic change, have been reported as pathological substrates for bradyarrhythmia, and advanced fibrosis was not observed in such cases.7 Therefore, objective morphological investigation into the amount of conduction fibers may be important for considering the etiology of idiopathic bradyarrhythmia. Moreover, the etiology of degeneration and/or fibrosis in the conduction system in idiopathic bradyarrhythmia cases has not yet been explored.
Genetic investigations are important for the correct diagnosis and/or examining the etiology of cardiovascular disease, including arrhythmogenic diseases, such as long-QT syndrome, Brugada syndrome, and sudden arrhythmogenic death syndrome.8,9 Our previous comprehensive pathological and genetic investigations showed that sudden unexpected death cases with restricted myocardial necrosis/inflammation in the heart could have had pathogenic mutations in cardiomyopathy-related genes.8,10,11 A comprehensive genetic investigation can examine the pathological effect of a defective gene in familial bradyarrhythmia cases.12 In contrast, there appears to have been no comprehensive genetic investigations with detailed pathological investigations in patients with idiopathic bradyarrhythmia.
In the present study, to explore the pathological substrate and the etiology of older-onset idiopathic bradyarrhythmia, we performed quantitative morphometric analysis of the pathological specimen using the cardiac conduction system (CCS) and genetic investigation by whole-exome sequencing and multiplex ligation-dependent probe amplification to determine whether genetic factors are associated with the development of older-onset idiopathic bradyarrhythmia.
Ten autopsy cases of individuals with permanent pacemaker implantation owing to acquired bradyarrhythmia, who were without a clinical history of cardiovascular disease before pacemaker implantation (6 men and 4 women; age: 70–94 years, 81.5±6.9 years; 5 cases each of SND cases and CAVB), were investigated. Clinical diagnosis of all SND cases was sick sinus syndrome. Family history was not evident in any of the 10 cases. The period of pacemaker implantation was 3 months to 19 years. With regard to the cause of death, 2 patients died of sudden cardiac death and 8 died from an accident (5 from drowning outdoors, 2 from burns, and 1 from hypothermia where the patient had a history of advanced dementia). Comorbidities were found in 8 patients: 5 had hypertension, 3 had diabetes, and 1 had hyperlipidemia. A summary of the patients’ characteristics in the bradyarrhythmia group is shown in Table 1. Only case 10 was found to have a mild reduction of ejection fraction at the implantation of a pacemaker. We also investigated the pathological findings of 2 groups of 10 autopsy cases in whom bradyarrhythmia was not found by an electrocardiogram within 1 month before death; these acted as control groups. Control Group 1 was composed of 2 age- (within 2 years), sex-, and comorbidity-matched individuals for every 5 SND patients (age range, 69–93 years; mean age, 79.5±8.1 years), and Control Group 2 was similarly matched for every 5 CAVB patients (median age, 83.1±3.2 years). A summary of the patients’ characteristics in the control group is shown in Supplementary Table 1. Toxicological screening was negative in all cases.
| Case no. |
Age (years) |
Sex | Height (cm) |
Weight (kg) |
BMI | Clinical history |
Age at pacemaker implantation (years) |
Echocardiography (2D measurement) |
Heart weight (g) |
Arrhythmia | Cardiac pathology |
Cause of death |
Thickness of the ventricle |
MVR | AVR | MN | OCF (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LVDd | LVDs | EF | L (cm) | R (cm) | SA | AV | His | |||||||||||||||
| 1 | 79 | M | 168 | 44.5 | 15.8 | HT | 60 | 52 | 34 | 60 | 490 | SND | ILVH | Burns | 1.3 | 0.2 | 9.8 | 7.2 | − | 3.2 | 26.0 | 38.8 |
| 2 | 74 | F | 143 | 48.2 | 23.6 | AF | 73 | 48 | 32 | 61 | 385 | SND | – | Drowning | 1.3 | 0.3 | 8.5 | 6.8 | − | 10.7 | 26.1 | 43.9 |
| 3 | 82 | F | 143 | 46.0 | 22.5 | DM | 77 | 45 | 28 | 58 | 339 | SND | – | Drowning | 1.5 | 0.2 | 8.3 | 6.2 | − | 7.5 | 31.0 | 42.4 |
| 4 | 94 | M | 155 | 52.1 | 21.7 | – | 92 | 50 | 34 | 56 | 449 | SND | ILVH | Drowning | 1.5 | 0.2 | 9.3 | 7.3 | − | 16.2 | 33.9 | 47.5 |
| 5 | 70 | M | 166 | 53.1 | 19.3 | DM, HT, HL | 68 | 44 | 30 | 60 | 390 | SND | – | Drowning | 1.5 | 0.2 | 9.4 | 6.5 | − | 9.2 | 25.7 | 43.3 |
| 6 | 79 | F | 163 | 53.0 | 20.0 | HT, AVB | 77 | 46 | 32 | 58 | 400 | CAVB | ILVH | SCD | 1.8 | 0.3 | 8.6 | 6.8 | − | 44.3 | 35.2 | 30.2 |
| 7 | 86 | M | 164 | 51.5 | 19.2 | RBBB | 82 | 42 | 34 | 56 | 374 | CAVB | – | Burns | 1.5 | 0.2 | 9.3 | 8.2 | − | 24.4 | 21.0 | 27.0 |
| 8 | 88 | M | 154 | 53.4 | 22.5 | HT, LBBB | 86 | 54 | 35 | 49 | 605 | CAVB | ILVH | SCD | 1.8 | 0.2 | 9.4 | 7.0 | − | 32.7 | 30.5 | 45.5 |
| 9 | 80 | F | 153 | 47.6 | 20.3 | DM, LBBB | 78 | 42 | 26 | 50 | 417 | CAVB | ILVH | Drowning | 2.0 | 0.2 | 9.2 | 7.5 | + | 42.2 | 34.8 | 48.5 |
| 10 | 83 | M | 164 | 56.6 | 21.0 | HT, LBBB | 75 | 60 | 40 | 49 | 573 | CAVB | ILVH | Hypothermia | 1.7 | 0.2 | 11.8 | 7.8 | + | 30.1 | 32.3 | 45.1 |
2D, 2-dimensional; AF, atrial fibrillation; AV, atrioventricular node; AVB, atrioventricular block (1st degree); AVR, length of the aortic ring (normal range: women 5.7–7.9 cm; men 6.0–8.5 cm); BMI, body mass index; CAVB, complete atrioventricular block; DM, diabetes mellitus; EF, ejection fraction; F, female; His, bundle of His; HL, hyperlipidemia; HT, hypertension; ILVH, idiopathic left ventricular hypertrophy; L, left; LBBB, left bundle branch block; LVDd, left ventricular end-diastolic diameter; LVDs, left ventricular end-systolic diameter; M, male; MN, minimal necrosis; MVR, length of the mitral ring (normal range: women 8.2–9.1 cm, men 9.2–9.9 cm);13 OCF, occupational rate of conduction fibers; R, right; RBBB, right bundle branch block; SA, sinoatrial node; SCD, sudden cardiac death; SND, sinus node dysfunction.
All bradyarrhythmia and control autopsies were performed on behalf of investigating authorities.
Examination of Cardiac PathologyThe methods of pathological investigation of the heart in our department have been described in previous reports.11 Sections at the level of the papillary muscle and at the level just above the apex were subjected to a thorough histological examination. Both atria, major epicardial coronary arteries, the SA node and AV conduction system were histologically examined. The thickness of the anterior left ventricular wall and right ventricular wall were measured. The mitral and aortic valve circumferences were then measured and compared with the previously shown normal size13 and the present study control cases.
The diagnostic criteria of structural heart disease are shown in Supplementary Table 2.
Morphometric Investigation of the Cardiac Conduction SystemWe selected histological slides containing the central part of the SA node, AV node, and the proximal bundle of His in the central fibrous body with Elastica-Masson stain. Stained specimens were photographed with a digital camera (DP73; Olympus, Tokyo, Japan; image size: 1,280×960 pixels) under a 40× lens magnification using the BX51 microscope (Olympus). Morphometric analyses of these images were conducted using Fiji, which is an open-source image processing package based on ImageJ®.14 The investigator who was blinded to the diagnosis performed the morphometric measurements. We measured the occupational rate of conduction fibers (OCF) in the prepared specimens containing the largest area of nodal tissue. After measuring the total area of the nodal tissue in the specimen, images were split into 3× (red, blue, and green) 8-bit channels. The image of the red channel, which contained conduction fibers stained by Masson’s Trichrome stain, was extracted under a common threshold level (30–255 pixels) that was determined by preliminary experiments for control cases. Finally, the OCF to the total area in each nodal tissue was calculated (Figure 1). The transitional zones, which were situated between the compact SA node and the surrounding working myocardial cells of the right atrium, were excluded to restrict the investigation to the compact portion of typical SA nodal cells.15
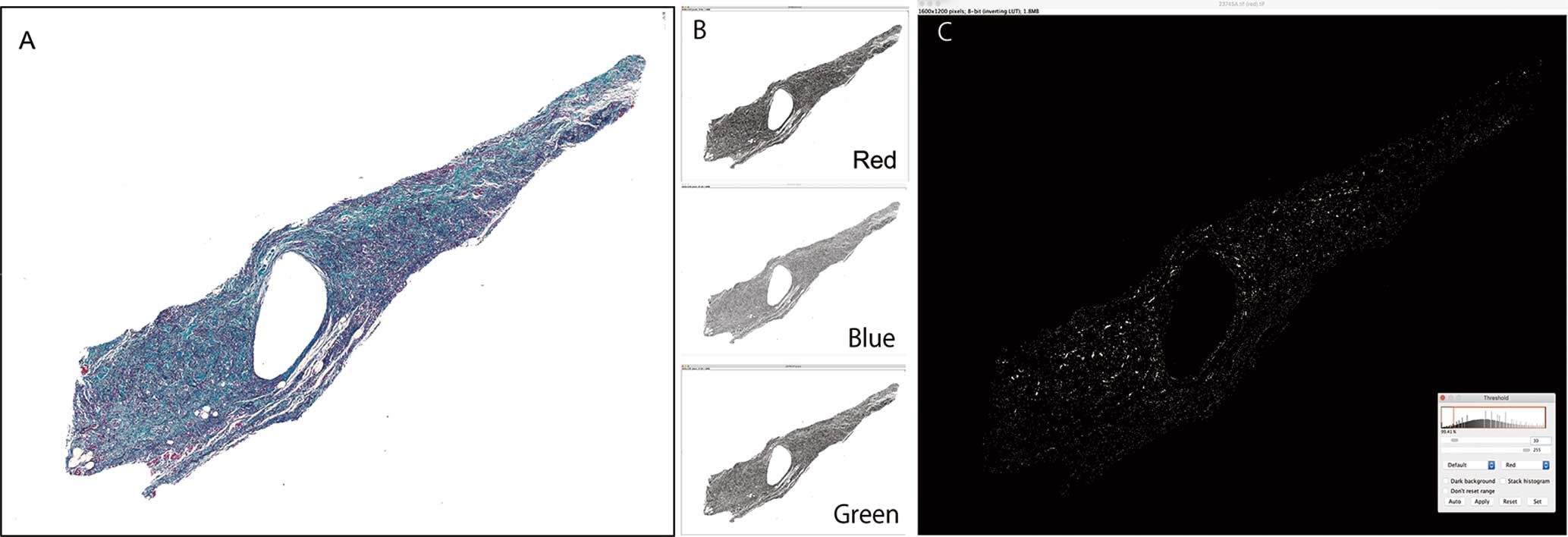
Morphometric analysis of the conduction system. (A) Extraction of the area of the sinoatrial node (Elastica-Masson staining). (B) After measuring the total area of the nodal tissue in the specimen, images were split into 3× (red, blue, and green) 8-bit channels. (C) An image of the red channel, which contains conduction fibers, was extracted under a common threshold level (30–255 pixels).
Methods of genetic testing including the methods of selection of potential variants, functional assessment, classification of pathogenicity, sanger sequencing, gene ontology enrichment analysis, multiplex ligation-dependent probe amplification and minigene splicing assay, are shown in Supplementary Figures 1–3 and Supplementary Tables 3–6.8,10,16
Statistical AnalysisAll statistical analyses were performed using R statistical software (https://www.rstudio.com/products/rstudio/). Data are shown as mean and standard deviation. The Kruskal-Wallis chi-squared test was used to compare the groups. Post-hoc Tukey tests were used to compare the subgroups. The statistical significance level was established at P<0.05.
Idiopathic left ventricular hypertrophy was found in 4 of 5 cases of CAVB and 2 of 5 cases of SND. Inflammatory heart disease or other significant structural heart disease was not observed in the bradyarrhythmia or control groups. There was no significant difference in body mass index, heart weight, and left ventricular thickness between the SND cases (20.6±3.1, 410.6±59.1 g, and 1.59±0.23 cm, respectively) and the control group 1 (19.9±4.6, 389.2±81.5 g, and 1.48±0.27 cm, respectively) and between the CAVB cases (20.6±1.2, 473.8±106.9 g, and 1.77±0.18 cm, respectively) and the control group 2 (22.8±4.1, 452.4±69.8 g, and 1.66±0.22 cm, respectively). Neurodegenerative diseases were observed in 3 patients in the bradyarrhythmia group (Alzheimer’s disease: Case 10, progressive supranuclear palsy: Case 9, Lewy body disease: Case 3).
There was no significant difference in the thickness of both ventricular walls and both valve circumferences between the bradyarrhythmia and control groups. One case (Case 10) showed mild dilatated left ventricular hypertrophy (Figure 2A) and elongation of mitral valve circumference with mild billowing of the valvular leaflet (Figure 2B, Table 1). A few minimal necrotic foci were found in the left ventricle in Cases 9 and 10 (Figure 2C,D).
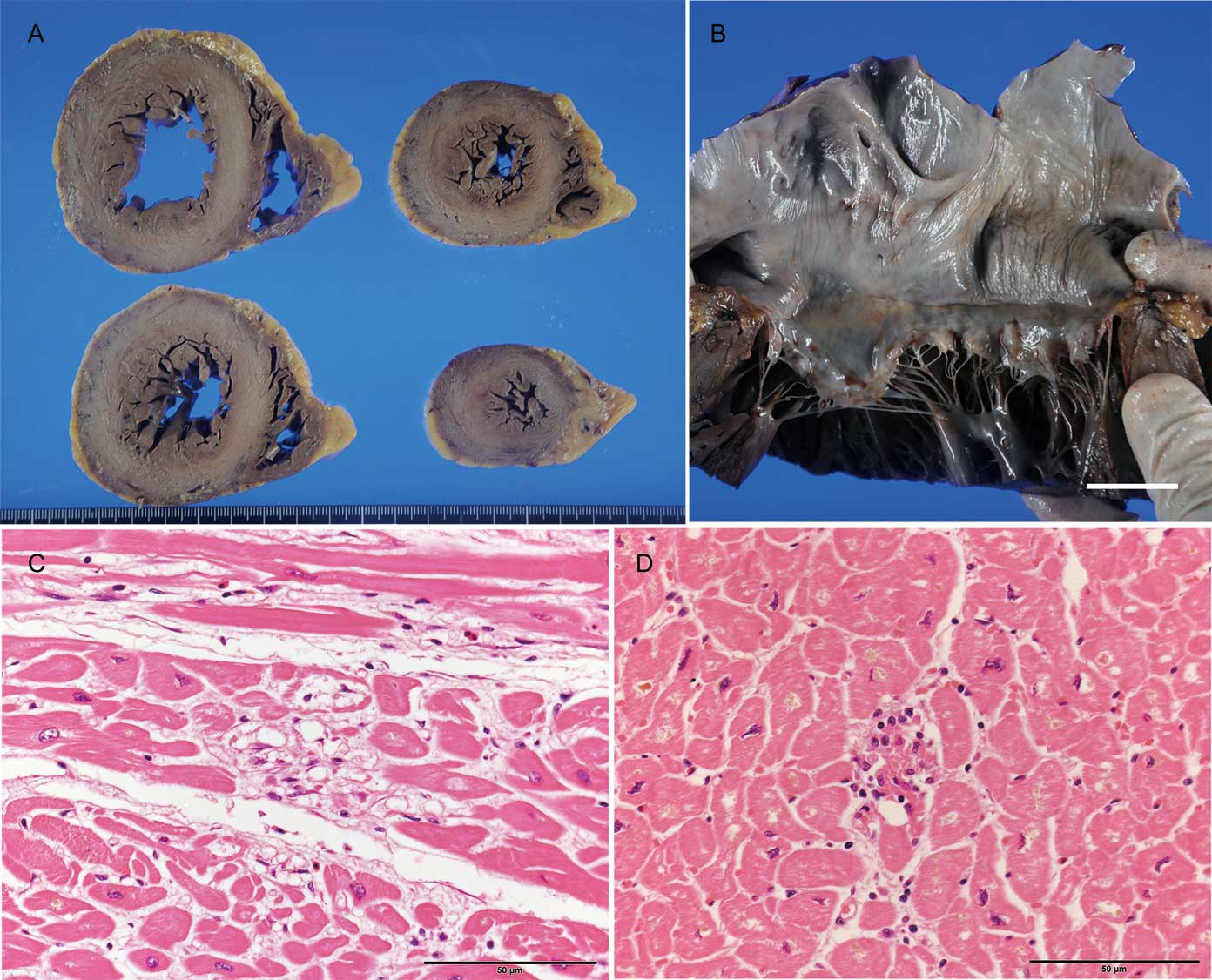
Pathological appearance of Cases 9 and 10. (A) Horizontal section of ventricles showed mildly dilated hypertrophy of the left ventrticle in Case 10. (B) Mild billowing of mitral leaflet and elongation of the chorda tendineae. (C,D) Minimal necrosis of the left ventricular myocardium in Cases 9 (C) and 10 (D). The scale bar = 1 cm in panel B and 50 μm in panels C and D.
The histological appearance of the SA node in SND, and that of the AV node and proximal bundle of His in CAVB cases are shown in Figure 3A–O and Figure 4A–C. Among the 5 SND cases, advanced fatty infiltration was found in the SA node in 1 case (Case 1, Figure 3A and Figure 4A), whereas the other 4 cases showed a marked loss of SA nodal cells with fibrosis (Figure 3B–E, Figure 4B). In patients with CAVB, 1 (Case 6) showed a reduction in conduction tissue with severe fibrosis of the AV node (Figure 3F and Figure 4C), and the other 4 cases showed increased fatty tissue around the AV node (Figures 3G–J). However, such findings were also found in some control cases. Advanced fibrosis and a loss of nodal fibers were not observed in the proximal bundle of His in all 5 CAVB cases. However, a reduction in conduction fibers with an increase in fibrous tissue in the distal bundle of His in the ventricular septal summit and/or the vicinity of the beginning site of the left and right branches were found in all 5 cases (Figures 5A–J).
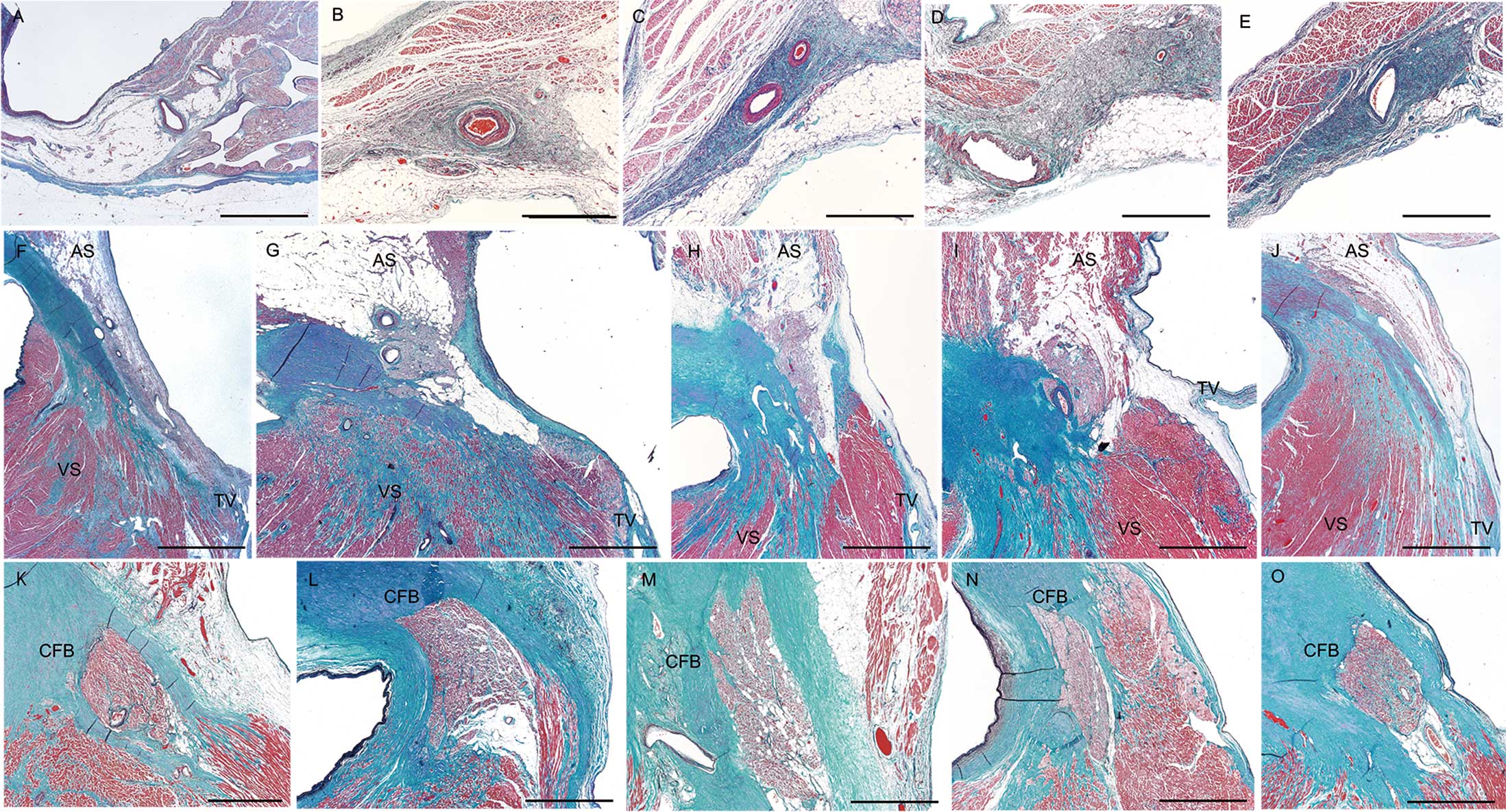
Histological appearance of the sinoatrial node in sinus node dysfunction cases (A–E), and that of the atrioventricular node (F–J) and proximal bundle of His in the central fibrous body (K,L) in complete atrioventricular block cases. Case 1 (A) shows a reduction in nodal cells with advanced fatty infiltration. Cases 2 (B), 3 (C), 4 (D), and 5 (E) show a reduction in nodal cells with fibrosis. Cases 6 (F,K), 7 (G,L), 8 (H,M), 9 (I,N), and 10 (J,O) do not show advanced loss of conduction tissue. Elastica-Masson staining was used. The scale bar=500 µm in panels A–E and K–O, and 2 mm in panels F–J.

High-power view of representative pathological lesions in the cardiac conduction system. (A) Fatty infiltration of the sinoatrial node in Case 1. Asterisk shows the sinoatrial node artery. (B) Severe reduction of nodal cells with advanced fibrosis in the sinoatrial node in Case 3. Asterisk shows the sinoatrial node artery. (C) Fibrosis of the atrioventricular node in Case 6. Elastica-Masson staining was used. Asterisk shows the atrioventricular node artery. The scale bar=100 µm.
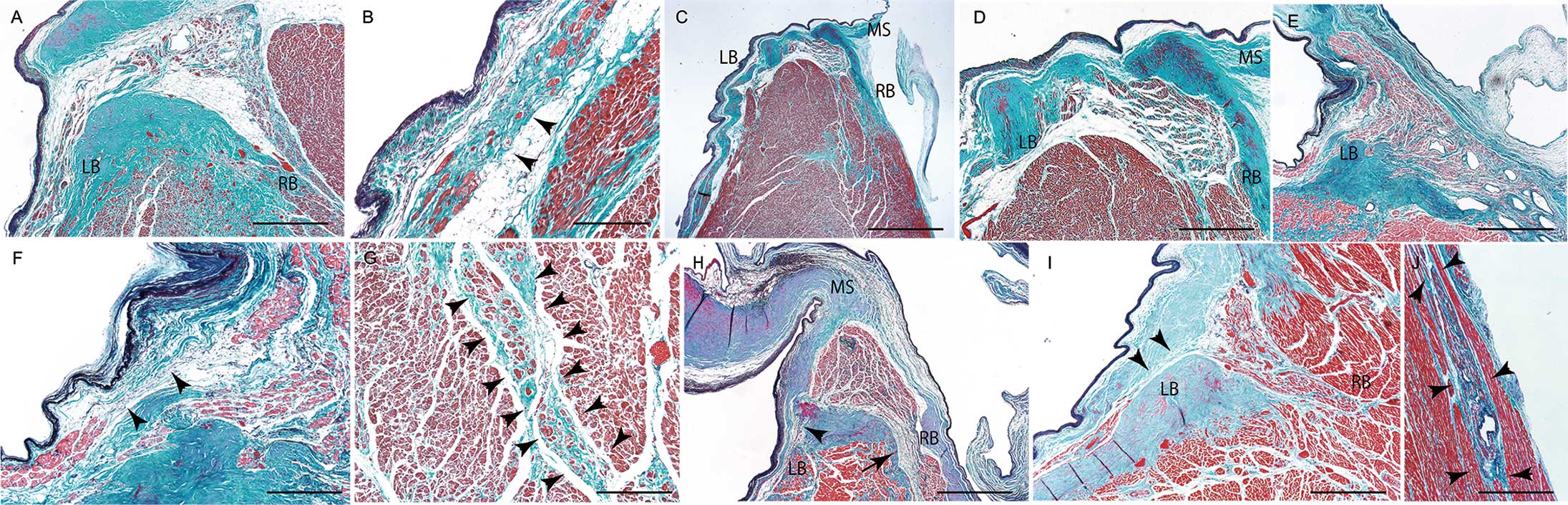
Pathological appearance of the distal atrioventricular conduction system. (A) Reduction of conduction fibers with fibrosis in the LB and RB in Case 6. (B) High-power view of the left branch with disruption of conduction fibers (arrowheads) in Case 6. (C) Low-power view of the distal atrioventricular conduction system in Case 7. (D,E) High-power view of the atrioventricular conduction system shows disruption of the LB (D) and fibrosis of the RB (E) in Case 7. (F) Disruption of the LB with increased fibrous tissue (arrowheads) in the septal summit in Case 8. (G) Reduction of conduction fibers with fibrosis of the RB (arrowheads) in Case 8. (H) Reduction of conduction fibers at the origin of the LB (arrowhead) and RB (arrow) in Case 9. The MS is attached to the summit of the ventricular septum. (I,J) Marked reduction in conduction fibers of the bundle of His, LB (I, arrowheads), and RB (J, arrowheads) in Case 10. The MS attached to the summit of the ventricular septum can be seen. Elastica-Masson staining was used. The scale bar=500 µm for panels A,D,E,G,I, and J, 200 µm for panels B and F, 1 mm for panel H, and 2 mm for panel C. LB, left branch; MS, membranous septum; RB, right branch.
Morphometric analysis of the CCS showed that the OCF in the SA node in SND cases was significantly lower compared with that in Control Groups 1 and 2 and in CAVB cases (all P<0.001). In contrast, the OCF in the AV node and the proximal bundle of His in CAVB cases was not significantly different compared with that in the 2 control groups and SND cases (Figure 6).

Results of nodal fiber index analysis. Scatter plots show the nodal fiber index in the CAVB group (n=5), SND group (n=5), and 2 control groups (n=10). There was a significant difference in the nodal fiber index between the control and SND groups, and between the CAVB and SND groups, but not between the control and CAVB groups. CAVB, complete atrioventricular block; SND, sinus node dysfunction. Data are shown as the mean±standard deviation. *P<0.001 by the Kruskal-Wallis chi-squared test with post-hoc Tukey tests.
The results of the genetic investigation for all 10 cases are shown in Table 2 and Supplementary Tables 4 and 5. Four known missenses variants in the PCCD-related genes (3 in RYR2 and one in GATA4) were found in 3 of the 10 cases (Table 2). Based on the ACMG score, every variant was classified as an uncertain significance (Supplementary Table 4). Conversely, identified variants with HPO terms were categorized into functional impact groups, including 5 high-impact variants (including 2 variants not previously reported; frameshift, n=1; splice acceptor, n=1; stop gain, n=3) and 4 moderate-impact variants (including 2 variants not previously reported; missense, n=4) (Table 2). We determined the outcome of variations in TWNK splicing found in Case 6 using a mini-gene assay. The c.1485-1 G>A mutation of TWNK induced in-frame skipping of exon 3 to produce p.T496_R531del (Table 2, Supplementary Table 1, Supplementary Figure 3). TWNK exon 3 (c.1485_1592) encodes for amino acids 496–531, which are part of the SF4 helicase domain (SF4 helicase domain; residues 384–635). According to the ACMG score, the high-impact variants were considered pathogenic or likely pathogenic, and moderate variants were classified as an uncertain significance (Supplementary Table 5). We only detected 2 variants in AV block-related genes (SYNE1 and TTN); however, 5 of the 9 variant genes were found in genes encoding cellular components of the sarcomere (GO: 0030017; FLNB, KCNA5, LDB3, SYNE1, and TTN), and 2 were found in genes encoding cellular components of the mitochondrial nucleoid (GO: 0042645; TWNK and ATAD3A) (Supplementary Table 6). In genes examined with multiplex ligation-dependent probe amplification kits, no relevant copy number variations were detected in the DNA in all cases.
| Case no. |
Etiology | Known PCCD-related variants |
Gene variants with HPO terms | |
|---|---|---|---|---|
| (1) High-impact variants |
(2) Moderate-impact variants |
|||
| 1 | SND | – | – | – |
| 2 | SND | – | – | – |
| 3 | SND | RYR2_p.G7S (U) | – | GLI2_p.A896V (U) |
| 4 | SND | GATA4_p.V432G (U) | – | – |
| 5 | SND | – | – | – |
| 6 | CAVB | – | TWNK_c.1485–1G>A (P) | – |
| 7 | CAVB | – | – | – |
| 8 | CAVB | – | – | – |
| 9 | CAVB | RYR2_p.V1241I (U) RYR2_p.K4392R (U) |
KCNA5_p.F369fs*87 (P) | FLNB_p.K1379E (U) |
| 10 | CAVB | – | TTN_p.R27414Ter (P) SYNE1_p.E3695Ter (LP) TET2_p.K1188Ter (P) |
ATAD3A_p.P290C (U) LDB3_ p.C582Y (U) |
CAVB, complete advanced atrioventricular block; HPO, Human Phenotype Ontology; (LP), ACMG classification ‘likely pathogenic’; (P), ACMG classification ‘pathogenic’; PCCD, progressive cardiac conduction disease; SND, sinus node dysfunction; (U), ACMG classification ‘uncertain significance’.
Replacement with fibrotic tissue and degenerative loss of SA nodal cells are frequently observed in pathological specimens in patients with SND.17 The amount of fibrosis within the SA node is inversely correlated with heart rate, whereas age is considered to be positively correlated with fibrosis.14 de Melo et al suggested that a loss of cells within the node throughout life, together with associated fibrosis, could be responsible for dysfunction of older SA nodes.18 Other studies have shown that nodal tissue fibrosis is idiopathic and associated with aging.6,19 In contrast, Alings et al showed that the size and collagen content in the SA node remains stable at an older age.20 Opthof et al also found little association between collagen and fibroblast content and the conduction time of the cardiac action potential across the SA node or spontaneous activity of the SA node.21 These studies suggest that progressive structural remodeling in older people is not associated with an increase in the incidence of SND. Additionally, a previous study showed that a few cases of SND had extensive cardiac lipomatosis as a disease-causing lesion.7 Our Case 1 showed that reduction in functional nodal cells may be a more common pathological substrate of advanced SND rather than fibrosis and/or fatty infiltration in many cases.
In idiopathic CAVB, Lev disease frequently occurs in older people, and the origin of the left bundle branch and adjacent bifurcating bundle degenerates with the preservation of the bundle in the peripheral region in typical cases.4 Lenegre disease occurs in younger people and is characterized by the primary progressive development of a cardiac conduction defect in the His–Purkinje system. This defect leads to a wide QRS complex and bradyarrhythmia that may trigger syncope. Pathologically, the selective degeneration in left and right branch conduction fibers is found in typical cases.4 The 5 older cases of CAVB in this study showed markedly reduced conduction fibers with fibrosis in the origin of the left bundle branch and adjacent bifurcating bundle. Therefore, these cases were consistent with Lev disease. Morphometric analysis in this study objectively showed that the amount OCF in the AV node and proximal bundle of His was preserved in cases with Lev disease compared with that in control cases.
Localized degeneration of the SA node in SND and the distal AV conduction system in Lev disease suggests that a specific anatomical condition may be associated with the development of idiopathic bradyarrhythmia. We previously investigated the CCS in 80 hearts without gross injury in patients who had received cardiopulmonary resuscitation owing to non-traumatic causes.22 We found fresh localized hemorrhage in 7 of these patients. And 6 of the 7 patients showed fresh hemorrhage in conduction fibers, 3 showed hemorrhage in the SA node, and the other 3 showed hemorrhage in the AV conduction system.22 Generally, the distal portion of the bundle of His, including the branching point of the left and right bundle branches, follows the course of the lower margin of the membranous septum. Continuous, hinge-like movement of the thin membranous septum at the junction of the ventricular septal summit by cardiac movement might cause fairly weak irritation to the ventricular summit. This irritation might then cause degeneration and/or fibrosis in the summit of the ventricular septum, including the origin of the left bundle branch and the adjacent bifurcating bundle, which are common sites of pathological lesions in Lev disease.5,22Supplementary Figure 4A showed fresh hemorrhage in the distal bundle of His found in the patient who received cardiac massage.
In patients with injury in and around the SA node after cardiac massage, stretching, and rotation of the area beneath the penetrating part of the superior vena cava, which is located near the SA node, may be the cause of injury to the SA node (Supplementary Figure 4B). This injury occurs because the superior vena cava is fixed at the penetrating area into the cardiac sac. An increase in tension at the region between the fixed superior vena cava and the right atrium near the SA node might occur in some individuals because of its anatomical orientation and acquired structural remodeling of the heart.22
Some studies have identified inherited bradyarrhythmia cases due to genetic defects, despite being found in younger patients with few structural heart diseases present.23 Conversely, the genetic investigation of idiopathic bradyarrhythmia cases has been extremely limited, possibly because genetic diseases are commonly thought to have an early onset in life; however, this concept is not always correct – the physiological age-dependent molecular and structural remodeling of an organ may magnify the pathological effects of a defective gene in an adult or older person.12 Manolio et al showed that variants with few pathogenic effects are rare, even if heritability is not evident by a genome-wide association study,24 and Takata et al subsequently showed the presence of such variants by comprehensive genetic analysis targeting developmental and epileptic encephalopathy.25 Considering these results, 4 rare variants of known cardiac conduction disease-related genes (three RYR2, one GATA4) may have few pathogenic effects that influence the slow progressive degeneration of the conduction system,23,26 leading to the onset of advanced bradyarrhythmia during aging.
In the present study, all 5 high-impact variants determined by the sequencing-based approach with strict screening conditions, and all variants were limited to the cases with CAVB. This study’s emerging evidence indicates that, even when a primarily acquired etiology is aging, genetic factors can contribute to the phenotypic expression of certain forms of idiopathic bradyarrhythmia. KCNA5, TTN, and SYNE1 are related to the sarcomere (GO:0030017), and detected truncated variants have a high possibility of pathogenicity, even if it is weak. KV1.5 is encoded by KCNA5, and is a voltage-gated potassium ion channel, and is the molecular basis for the atrial-specific potassium current, IKur.27 Titin, which is encoded by TTN, plays essential roles in muscle assembly, force transmission at the Z-line, and maintenance of resting tension in the I-band region. The A-band of TTN interacts with β-myosin heavy-chain tails, where it may regulate the filament length and assembly, and it is thought to be critical for biomechanical sensing and signaling.28 Dominant TTN truncating variants in the A-band have recently been reported as the most common genetic cause of dilated cardiomyopathy, present in up to 25% of cases.29 TTN_R27414Ter, which was found in Case 10, is in the A-band. Nesprin-1, which is encoded by SYNE1, is part of the linker of the nucleus to the cytoskeleton (LINC) complex. Nesprin-1 is a ubiquitous protein, composed of 3 major domains: a variable N-terminal domain, a C-terminal transmembrane KASH (Klarsicht, ANC1, and Syne homology) domain, and a rod domain composed of at least 74 spectrin repeats (SR).30 Alternative transcription and splicing of SYNE1 generates multiple isoforms that vary greatly in size.31 A full-length isoform localizes at the outer nuclear membrane (ONM), and a shorter isoform such as nesprin-1α localizes at the inside of the nuclear membrane (INM).32SYNE1 gene variants in the C-terminus part of the SR have been potentially associated with the pathogenesis of dilated cardiomyopathy via its connections at both the INM and ONM.33 SYNE1_E3695Ter, which was found in Case 10, is in the SR32 and is located almost in the middle of the SR domain. Even though the heterozygous variant of SYNE1 is predicted to be truncating the full-length nesprin-1 isoform, it would not affect a shorter isoform such as nesprin-1α. However, the loss of the largest giant nesprin-1, which localizes at the ONM, could lead to defects in nuclear morphology and nucleocytoskeletal coupling.
Therefore, the variants may cause vulnerability of specialized myocytes, and this vulnerability may be associated with a reduction in conduction tissue. We previously found microscopic minimal necrosis in the macroscopic normal heart in sudden unexpected death cases, and some cases had a cardiomyopathy-related sarcomere gene defect.8,10,11 In the present Cases 9 and 10 with these high-impact cardiomyopathy-associated genes and minimal necrosis, cellular vulnerability associated with a weakly pathogenic sarcomere gene defect might have been a contributing factor for the degeneration of conduction tissue in some patients with Lev disease, even if the pathogenicity of these variants was not so high.
Defects in nuclear-encoded proteins involved in mitochondrial DNA maintenance have recently been shown to be a frequent cause of inherited metabolic disorders34 and are also important in neurodegeneration and aging.35,36TWNK encodes the mitochondrial helicase, Twinkle, which is required for mitochondrial DNA replication and maintenance.35,36 The predicted in-frame deletion of TWNK is likely to exhibit some type of functional deficits associated with domain-specific deletion based on the results of the mini-gene, even if an association between TWNK and heart disease has not yet been fully established.
TET2 encodes methylcytosine dioxygenase, which removes methyl groups from cytosine nucleotides in DNA. Defects in this gene are associated with several myeloproliferative disorders (Immunodeficiency_OMIM:619126). TET2_K1188Ter, which was found in Case 10, was evaluated as pathogenic in accordance with the ACMG guidelines. Somatic mutations in TET2 are associated with coronary artery disease and early-onset myocardial infarction,37 but there are few known germline TET2 variants. Therefore, TWNK_c.1485-1G>A and TET2_p.K1188Ter variants may be recorded as disease-causing genetic variants.
An association between bradyarrhythmia and all 4 moderate impact variants has not been shown. Two variants of sarcomere-associated genes (FLNB and LDB3) and ATAD3A, a ubiquitously expressed nuclear DNA-encoded mitochondrial membrane protein for nucleoid maintenance,38 were found in the present study. Glioma-associated factor 2 encodes a vertebral transcription factor involved in sonic hedgehog (600275) signal transduction, and myocyte enhancer factor 2C plays important roles in the development of embryonic heart muscle and enhances cardiomyogenesis in stem cells.39 GLI2_A896V that was found in Case 3 is located in a well-established functional domain, which comprises a carboxy-terminal domain required for transcriptional activity and a zinc finger domain that binds DNA in a sequence-specific manner.40
Study LimitationsThe main limitation to this study is the low number of investigated cases. In addition, we could not obtain more clinical details, such as serological, electrophysiological, and radiological information in some patients before pacemaker implantation because all of the patients died outside of hospital. Another limitation is that whole-exome sequencing sometimes cannot detect insertion, deletion, and copy number variants. Because we used autopsy material, immunostaining data corresponding to the causing mutation could not be shown. Finally, genetic investigation of family members could not be performed in all cases.
This study showed a significant reduction in conduction tissue in the SA node in cases with idiopathic SND. Severe loss of the distal AV conduction system, which was consistent with the appearance of Lev disease, was uniformly found in all 5 CAVB cases. Both the SA node and distal AV conduction system were consistent with the most common site of injury by cardiac massage. Genetic investigation under relatively strict screening conditions showed 4 rare cardiac conduction disease-related genetic defects, 5 rare high-impact heart disease-related genetic defects, 4 moderate impact variants in 3 CAVB cases, and 2 SND cases, even the small effect size of the variants. In addition to the subtle anatomical variation of the CCS and/or pathological remodeling of the heart, genetic factors may be associated with the degenerative process of conduction tissue, leading to the development of idiopathic bradyarrhythmia.
The authors thank Ms. Syuko Matsumori, Ms. Miyuki Maekawa, Ms. Misa Kusaba, and Mr. Osamu Yamamoto for their technical assistance. We thank Enago for editing a draft of this manuscript.
This work was supported, in part, by JSPS KAKENHI Grant Number JP21H03211 to Y.H.
The authors declare that there are no conflicts of interest.
Written consent was obtained from the next of kin or guardian for the genetic investigation and for the publication of anonymized data obtained through the clinical characterization and the scientific research carried out. This study was performed in accordance with the ethical standards established in the 1964 Declaration of Helsinki. All experimental protocols of present study, including the use of the pathological specimens obtained at autopsies for research upon anonymization, were approved by the official ethical institutional review board of the University of Toyama (I2020006).
The main data supporting the findings of our study are available within the article and its supplementary material. Most materials and reagents used in the present study are commercially available. Additional data and/or materials are available upon reasonable request to the corresponding author, with some restrictions to protect research participants’ privacy.
Please find supplementary file(s);
https://doi.org/10.1253/circj.CJ-22-0397