2023 Volume 87 Issue 4 Pages 481-486
2023 Volume 87 Issue 4 Pages 481-486
Background: Clinical studies in regenerative medicine remain insufficient in Japan due to ethical concerns regarding the control group and a lack of statistical methodology to evaluate efficacy in a small treatment group. This study evaluated the efficacy of autologous myoblast patch (AMP) treatment for heart failure using restricted mean survival time (RMST) analysis by comparing data from a small single-arm trial to epidemiological data from a registry.
Methods and Results: The clinical trial arm included 55 patients with advanced ischemic cardiomyopathy who received an AMP between 2010 and 2020. The registry-based control group comprised 937 participants with severely impaired left ventricular function who were hospitalized for heart failure during the study period. Due to the limited number of patients, RMST analysis was used to compare survival between the 2 groups. Cox regression analyses revealed non-significant differences in survival between the groups at 3, 3.5, and 4 years. In contrast, RMST analyses revealed significant differences in survival at 3 years (P=0.008) and 3.5 (P=0.024) years, but not at 4 years.
Conclusions: This small single-arm trial using RMST analyses was able to detect the efficacy of AMP transplantation for advanced heart failure (compared with a registry-based control group), with better survival until 3.5 years. This approach may be useful for efficacy analyses in regenerative medicine, where traditional clinical trials are difficult.
Despite advances in medical technology, many rare diseases still lack reliable treatments. Furthermore, the incentive for researchers to develop new medical technologies, such as regenerative medicine, to eradicate intractable diseases has diminished.1–7 In the field of regenerative medicine, this is associated with difficulties in performing traditional clinical trials for intractable diseases and accurately assessing efficacy outcomes in small, often single-arm, clinical trials.8 Further, although we have previously performed single-arm clinical trials to evaluate autologous myoblast patch (AMP) transplantation in patients with intractable heart failure, efficacy analyses were difficult because of the small sample size and lack of control groups.9,10
Editorial p 487
In Japan, a new expedited approval system has been proposed for regenerative medicinal products. This system involves conditional/time-limited authorization based on efficacy data from a single-arm study, and was introduced to mitigate the difficulties associated with conducting traditional randomized clinical trials, such as insufficient efficacy evaluations with small-sample, single-arm data (as mentioned above), diverse patient backgrounds (e.g., variations in disease severity and lifestyle), ethical concerns regarding the creation of a control group, and poor availability of epidemiological data. Under this new regulatory system, AMP transplantation has been conditionally approved as a treatment for ischemic cardiomyopathy; however, existing statistical methods are insufficient to evaluate the efficacy of this treatment.11–13 To overcome these problems, we previously developed a multivariate risk model for predicting survival in Japanese patients with heart failure using epidemiological data and parameters that are readily observable in the clinical setting.14 In addition, recent reports have suggested using restricted mean survival time (RMST) instead of traditional hazard ratio (HR) analysis to evaluate overall survival for robust results.15,16 Therefore, the aim of the present study was to evaluate the efficacy of AMP treatment using RMST analysis, comparing data from a small single-arm trial to epidemiological data from a registry.17
The trial arm included 55 patients with advanced heart failure (left ventricular ejection fraction [LVEF] <35%, New York Heart Association functional class ≥II, and maxima in medical and surgical treatments already reached) who underwent AMP transplantation at Osaka University Hospital between 2010 and 2020. The trial protocol (jRCT1050200090) was approved by the Ethical Review Board of Osaka University Hospital (Osaka, Japan) and followed the principles of the Declaration of Helsinki. Written informed consent was obtained from the all patients before AMP transplantation; data were subsequently anonymized for analysis.
A randomized trial comparing the outcomes of patients who underwent AMP transplantation with those of patients in a matched control group who received open chest surgery would be ideal, but is not ethically feasible because of the disbenefit to those in the control group. Thus, to form a comparison control cohort, we evaluated registry-based medical records of 1,138 patients with severe heart failure who were hospitalized before March 1, 2016. Patients with incomplete data were excluded; the control group ultimately included 937 patients (Figure 1).
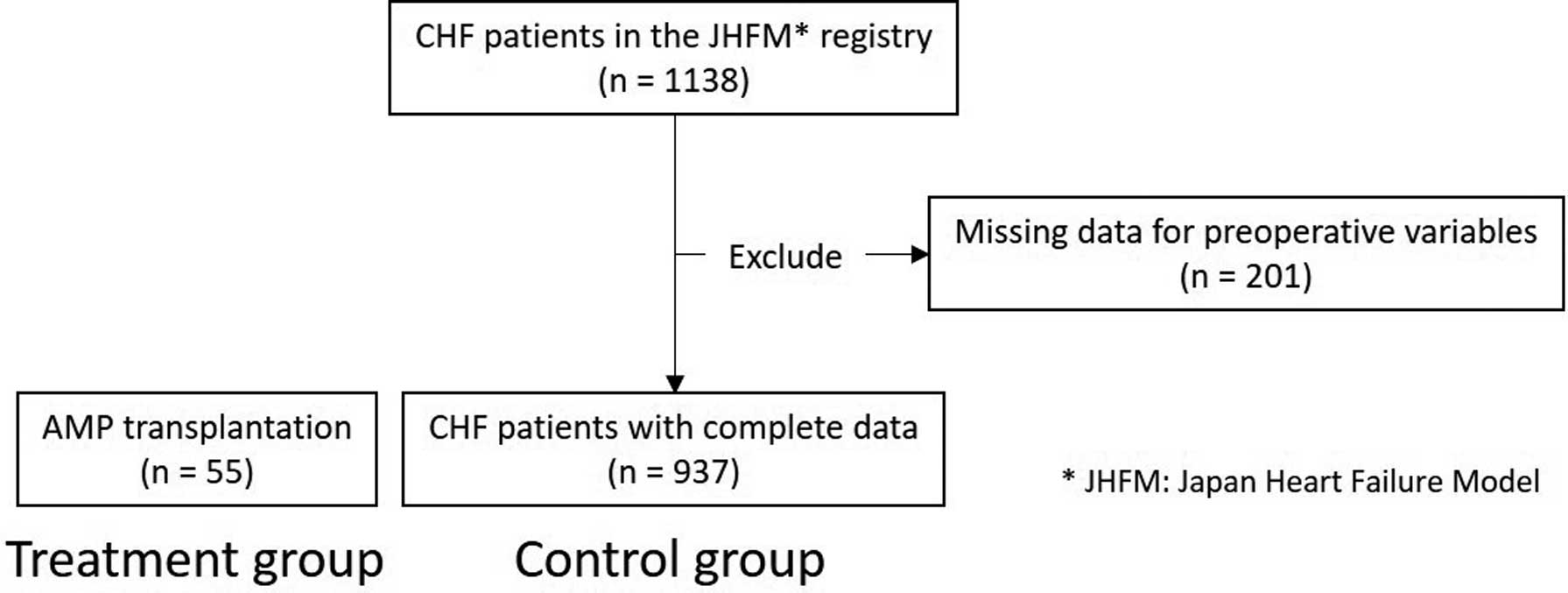
Study flowchart. Fifty-five patients with advanced heart failure received autologous myoblast patches (AMPs; treatment group). As the control group, 937 eligible participants from the Japan Heart Failure Model (JHFM) registry were included. CHF, chronic heart failure.
Muscle tissue specimens were harvested from each patient’s vastus medialis muscle. Muscle fibers were collected by removing the connective tissue using collagenase and TrypLE Select Enzyme (Invitrogen, Carlsbad, CA, USA) and suspended in MCDBb131 medium (Invitrogen) with 20% fetal bovine serum. Cultured cells were harvested using TrypLE Select Enzyme and counted using 0.4% Trypan blue (Thermo Fisher Scientific, Waltham, MA, USA). The cell suspensions were then placed in temperature-responsive cell culture dishes (UpCell; CellSeed, Tokyo, Japan), which were coated with a temperature-responsive polymer (poly[N-isopropylacrylamide]). When the temperature was reduced to 32℃, the dish surface became rapidly hydrated, and the adherent cells completely detached, forming an AMP.12,18,19 After detachment from the temperature-responsive dishes, the top surface of each AMP was reinforced using fibrin glue and then transplanted (detailed below; Figure 2). Cytokine secretion (by the AMP) into the supernatant was measured using ELISA (Bio-Rad, Hercules, CA, USA). Tests for endotoxin and mycoplasma were negative, suggesting that the AMPs were sterile.
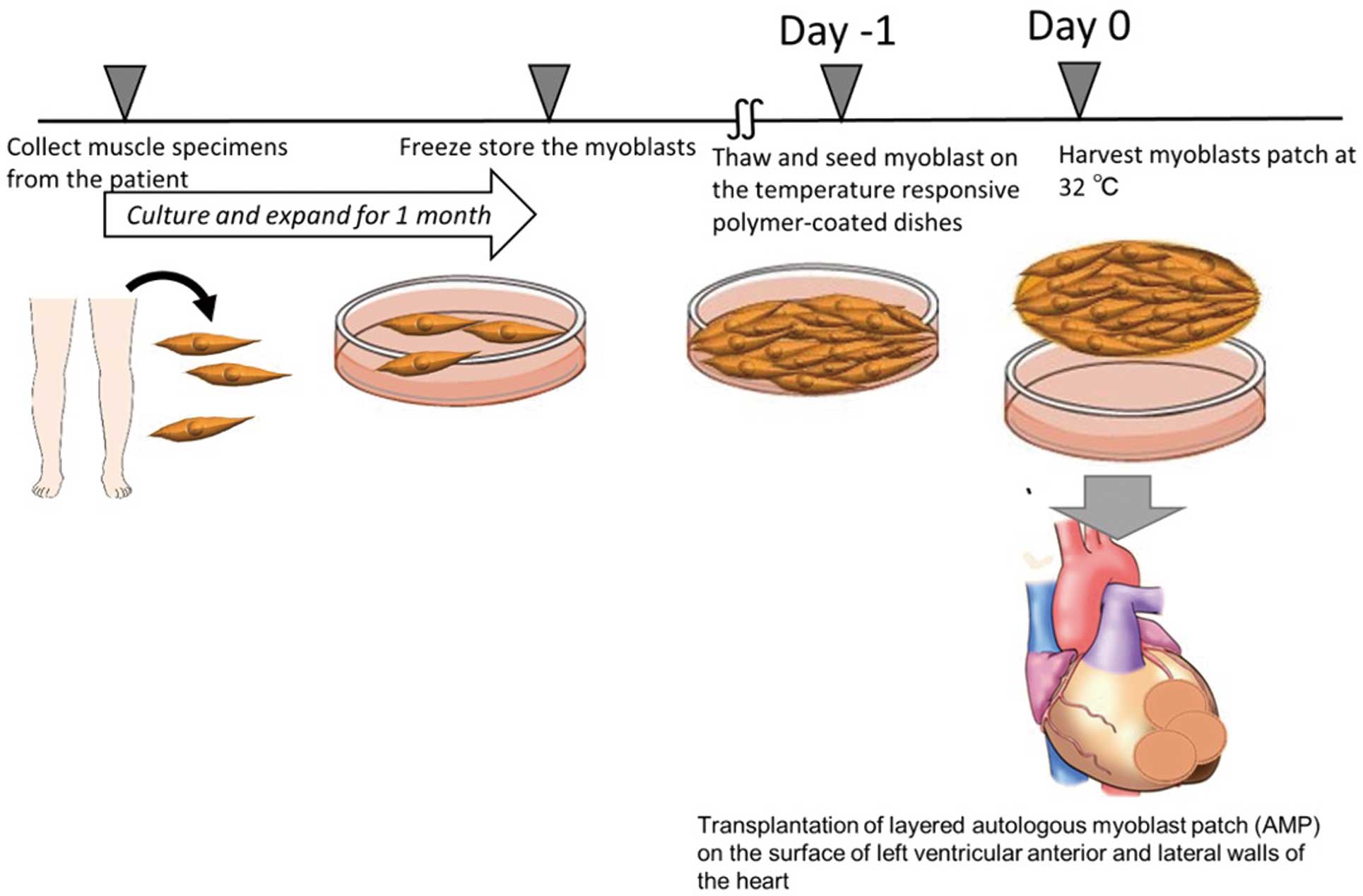
Creating autologous myoblast patches. Skeletal myoblasts are isolated from a patient’s muscle specimen, cultured, expanded for 1 month, and then stored. One day before surgery, the myoblasts are thawed and seeded on temperature-responsive polymer-coated dishes. On the day of surgery, the myoblast cells are harvested as a patch (by lowering the temperature to 32℃) and immediately transplanted onto the left ventricular anterior and lateral walls of the heart.
For AMP transplantation, the patient was placed under general anesthesia and single-lung ventilation before the fifth or sixth intercostal space was opened. The pericardium was then opened parallel to the phrenic nerve. While the left ventricular lateral wall was resected, 3–4 layers of AMPs were produced on a clean bench and transferred to the operating table. The AMPs were then placed onto the left ventricular anterior and lateral walls to ensure as much coverage as possible and fixed with 7-0 Prolene® (Supplementary Figure 1). After placement of the patches, the entire top surface was coated with fibrin glue (i.e., no fibrin was placed below or between the patches) to fix the AMPs to the epicardium, with some additional stitches.
Statistical AnalysesThe following endpoints differed for the 2 groups because the 2 groups were not randomized: in the AMP group, the endpoint was defined as the time from treatment to all-cause mortality, whereas in the control group the endpoint was defined as the time from heart failure-related admission to all-cause mortality. Kaplan-Meier survival curves were created for the 55 AMP-treated patients and 937 registry-based controls. To ensure a fair comparison, background characteristics were balanced using the approach reported previously.15–17,20 We also performed an intention-to-treat analysis of all registry data and important covariates. Survival outcomes were compared between the AMP and control groups using RMST analysis. In addition, HRs were determined in traditional Cox regression analyses. Statistical analyses were performed using R version 4.0.4 (R Foundation for Statistical Computing, Vienna, Austria), and 2-sided P<0.05 was considered statistically significant.
Interestingly, according to an enrichment analysis, patients in the AMP group were significantly younger and had higher albumin concentrations, lower LVEF, and a higher proportion of patients in the AMP than control group using loop diuretics (Table 1; Supplementary Table).
| HR | Standard error | z | P>|z| | 95% CI | |
|---|---|---|---|---|---|
| Age | 1.030 | 0.006 | 5.12 | 0.000 | 1.019–1.042 |
| Diabetes | 1.192 | 0.190 | 1.10 | 0.271 | 0.872–1.629 |
| LVEF | 0.980 | 0.009 | −2.21 | 0.027 | 0.962–0.998 |
| Albumin | 0.587 | 0.079 | −3.96 | 0.000 | 0.451–0.764 |
| BMI | 0.953 | 0.020 | −2.27 | 0.023 | 0.915–0.993 |
| Hemoglobin | 0.888 | 0.032 | −3.30 | 0.001 | 0.827–0.953 |
| Sodium | 0.961 | 0.014 | −2.72 | 0.007 | 0.934–0.989 |
| Use of diuretics | 1.070 | 0.157 | 0.46 | 0.642 | 0.803–1.426 |
BMI, body mass index; CI, confidence interval; HR, hazard ratio; LVEF, left ventricular ejection fraction.
The AMPs were white and circular (Supplementary Figure 2). The mean (±SD) concentration of angiogenic cytokines secreted by the AMPs (before transplantation) was as follows: vascular endothelial growth factor, 6.0±1.9 ng/mL; hepatocyte growth factor 1, 6.2±6.3 ng/mL; and stromal cell-derived factor 1, 1.8±0.7 ng/mL (n=9 each). These concentrations were considered adequate given the concentrations that have been reported to have efficacy.21
Mortality in the AMP and Control GroupsIn the registry-based cohort, age and albumin concentrations were strongly associated with survival to 4 years. In addition, survival was associated with a higher hemoglobin concentration, as well as lower LVEF and sodium concentrations (Table 1).
Observed outcomes for the registry-based control and AMP groups are shown in Figure 3. However, given the significant differences in several baseline characteristics between the AMP and control groups, an analysis was performed after adjusting for these baseline characteristics, which resulted in an upward shift of the survival curve in the control group (Figure 3). The adjusted control curve was then compared to the curve for the AMP group. Although the survival curve for the AMP group lay above the adjusted survival curve for the control group, the lines converged at the end of the third year. Thus, traditional HR analyses would have been unable to detect this early separation and subsequent convergence of the curves, even if one group had significantly better early outcomes. Consistent with this, the HR for 3-year survival was not statistically significant (HR 0.57; P=0.14; Table 2).
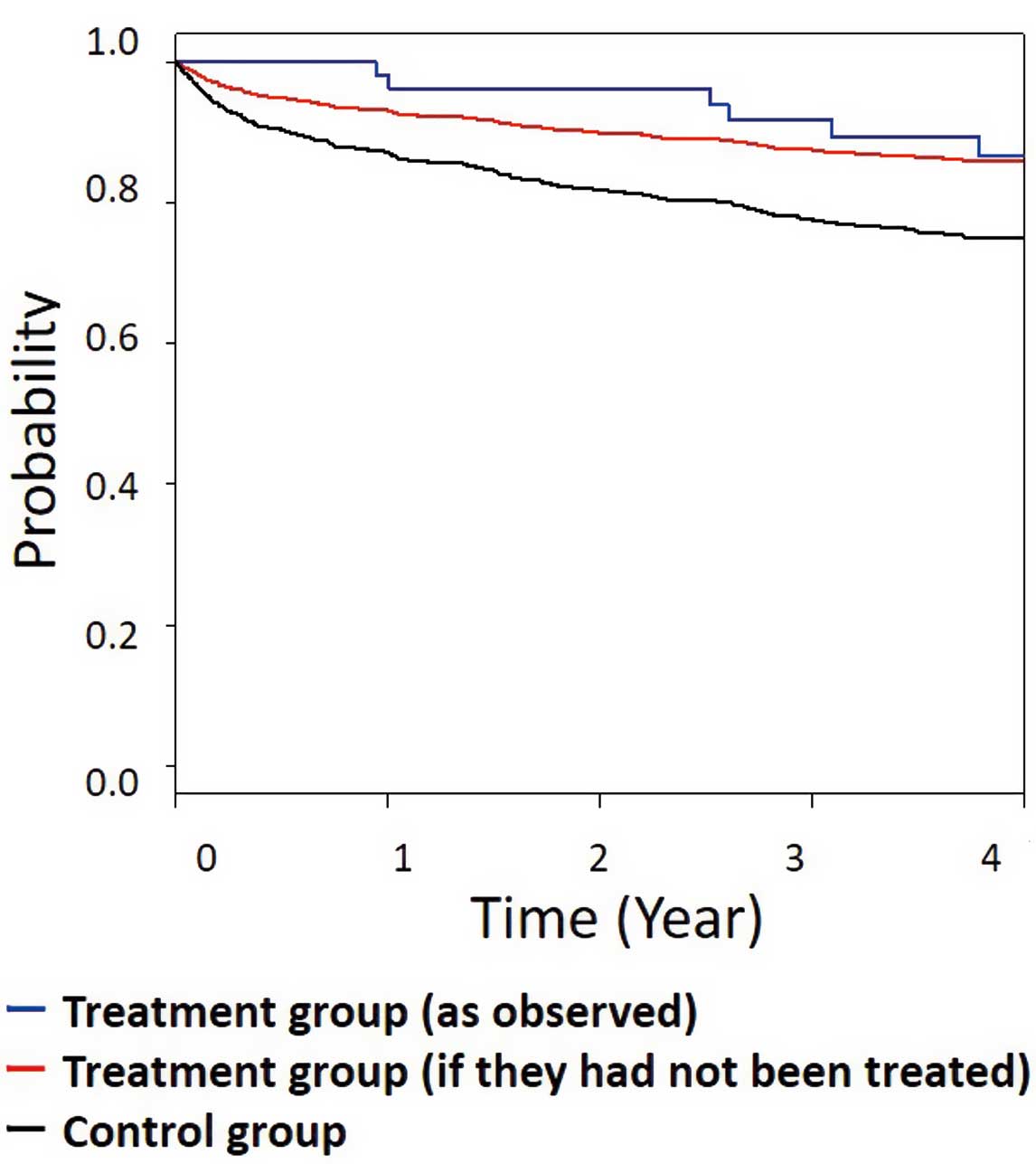
Comparison of the survival curves in the autologous myoblast patch (AMP)-treated and control groups. Black lines indicate observed outcomes in the registry-based control group; red lines indicate adjusted survival curve for the registry-based control group; and blue lines indicate survival curve for the AMP group. Survival within 3 years based on restricted mean survival time is significantly better in the AMP than control group.
| Years | No. AMP events |
HR | 95% CI | Two-sided P value |
RMST | RMLT ratio (AMP/control) |
95% CI | Two-sided P value |
|
|---|---|---|---|---|---|---|---|---|---|
| AMP | Control | ||||||||
| 3 | 4 | 0.57 | 0.14–1.20 | 0.14 | 2.90 | 2.73 | 0.36 | 0.04–0.78 | 0.008 |
| 3.5 | 5 | 0.67 | 0.16–1.29 | 0.26 | 3.35 | 3.18 | 0.46 | 0.08–0.93 | 0.024 |
| 4 | 6 | 0.80 | 0.27–1.43 | 0.58 | 3.79 | 3.62 | 0.54 | 0.14–1.01 | 0.058 |
AMP, autologous myoblast patches; RMLT, relative mean lost time; RMST, restricted mean survival time. Other abbreviations as in Table 1.
Different follow-up times were used to compare the survival outcomes of patients in the 2 groups; this revealed a promising effect size for AMP treatment at each time point, especially the earlier time points. Only 2 events were observed in the AMP group during the first 2.5 years; thus, the earliest evaluable time point was 3 years (Table 2). RMST analyses using the area under the Kaplan-Meier curve also confirmed the AMP group as more responsive early after treatment compared with survival in the control group. Although the HRs obtained were not significant, the time lost due to mortality (based on the RMST value) was significantly lower at 3, 3.5, and 4 years. At 4 years, the average AMP-treated patient had spent 3.79 years alive (RMST) and 0.21 years dead (relative mean lost time [RMLT]), whereas the average control patient had spent 3.62 years alive (RMST) and 0.38 years dead (RMLT). A comparison of the RMLT values revealed a ratio of 0.55 (0.21/0.38), which implies that the total burden of mortality in the AMP group was 45% lower than that in the control group (95% confidence interval 0.14–1.01).
In this study, we used an alternative statistical approach based on the methodology as established by Torbicki et al17 and used by others15,16,20 to demonstrate the efficacy of AMP transplantation for advanced heart failure using data from a small single-arm clinical trial and a patient registry. Although heart failure is common worldwide, only a few patients with advanced heart failure receive treatment because of various ethical and practical issues. Although traditional statistical analyses in this setting are based on HR calculations, they are not adequate for use with a small sample of patients who only experience a few events. Therefore, evaluating RMST and RMLT may provide results that are more pertinent, and may overcome the limitations of traditional HR analyses.
In the present study, because the main statistical challenge was the lack of a comparison group, epidemiological data were used to create a control group. In addition, given the limited number of events, traditional HR analyses were inappropriate and we therefore used RMST to evaluate outcomes at 3, 3.5, and 4 years. RMST analysis revealed that AMP transplantation provided early benefits. The HRs were not significant for survival within 4 years; however, RMST analysis revealed that the AMP group had significantly less time lost due to mortality. The early significant effect of AMP treatment on survival can be explained by differences in the endpoint definitions between the 2 groups, namely time from treatment to all-cause mortality in the AMP group and time from heart failure-related admission to all-cause mortality in the control group. That is, patient background differed between the groups in terms of the urgency of treatment, and the unstable follow-up period immediately after hospitalization for heart failure may have been evaluated in the control group.
Randomized controlled trials are the gold standard for efficacy evaluation; however, they require a control arm. It is unethical and nearly impossible to recruit many patients with a rare disease to create an appropriate control group for a randomized controlled trial. For example, patients may refuse to enroll in a study in which they may not receive the treatment of interest; this results in poor enrollment and ultimate failure of the study. Single-arm trials may help address these issues by collecting data regarding efficacy; however, the conclusions of single-arm studies are limited by the lack of a suitable control group. Therefore, to ensure that new medical treatments are effectively delivered to patients with rare diseases, a suitable method for evaluating efficacy data generated by small, single-arm studies is required.
In the field of regenerative medicine, a control group could conceivably be created as part of a multiple-arm randomized controlled trial; however, ethical concerns and the substantial time and costs required are limiting factors. In contrast, epidemiological data are widely available and highly versatile, although they can be influenced by the development of new medical technologies. Nevertheless, given the potential benefits of new regenerative therapies, rapid analysis using highly versatile epidemiological “control” data is a medically feasible and ethically appropriate strategy that can be used to enhance the reliability of the evidence generated by small, single-arm trials.
In the present study, we could only conclude that AMP transplantation prolonged survival in patients with heart failure for up to 4 years after treatment because of the limited number of treated patients. Further studies with more patients and a longer observation period are warranted to prove the good long-term survival outcomes after AMP transplantation.
We evaluated the efficacy of AMP transplantation for advanced heart failure using data from a single-arm trial and epidemiological data from a registry-based control group. This novel RMST-based method appears to be useful for evaluating the efficacy of new therapeutic technologies that can only be ethically or realistically evaluated in small, single-arm trials. Therefore, using this approach may help advance clinical studies on new therapeutic technologies and improve the delivery of these technologies to patients with serious diseases.
The authors thank Lee-Jen Wei, Brian Claggett, Madoka Takeuchi, and Ai Ushiwata for helpful discussions.
This study was supported by a research grant from the Ministry of Economy, Trade, and Industry of Japan (Project Focused on Developing Key Evaluation Technology: Evaluation for Industrialization in the Field of Regenerative Medicine).
Y. Sawa is a member of Circulation Journal’s Editorial Team and Y. Sakata is an Associate Editor of Circulation Journal. Y. Sawa has received research grants, lecture fees, and scholarship donations from Terumo Corporation. The remaining authors do not have any conflicts of interest to disclose.
The trial protocol (jRCT1050200090) was approved by the Ethical Review Board of Osaka University Hospital (Osaka, Japan).
The deidentified participant data will not be shared.
Please find supplementary file(s);
https://doi.org/10.1253/circj.CJ-22-0319