2023 Volume 87 Issue 4 Pages 490-497
2023 Volume 87 Issue 4 Pages 490-497
Background: Elderly patients with acute myocardial infarction (AMI) are a high-risk population for heart failure (HF), but the association between physical frailty and worsening prognosis, including HF development, has not been documented extensively.
Methods and Results: As part of the FLAGSHIP study, we enrolled 524 patients aged ≥70 years hospitalized for AMI and capable of walking at discharge. Physical frailty was assessed using the FLAGSHIP frailty score. The primary outcome was a composite outcome of all-cause death and HF rehospitalization within 2 years after discharge. The secondary outcome was all-cause death and HF rehospitalization. After adjusting for confounders, physical frailty showed a significant association with an increased risk of the composite outcome (hazard ratio [HR]=2.09, 95% confidence interval [CI]: 1.03–4.22, P=0.040). The risk of HF rehospitalization increased with physical frailty, but the association was not statistically significant (HR=2.14, 95% CI: 0.84–5.44, P=0.110). Physical frailty was not associated with an increased risk of all-cause death (HR=1.45, 95% CI: 0.49–4.26, P=0.501).
Conclusions: The findings suggest that physical frailty assessment serves as a stratifying tool to identify high-risk populations for post-discharge clinical events among ambulant elderly patients with AMI.
With the rapid aging of the population in Japan, currently patients with acute myocardial infarction (AMI) aged ≥70 years account for approximately 40% of the male population and 70% of the female population in Japan.1 Recent guidelines on heart failure (HF) have defined Stage B HF as a structural heart disease, characterized by increased filling pressure and elevated biomarker expression, but without symptoms of HF according to the ACC/AHA Heart Failure Classification.2 The treatment goal for Stage B HF events, such as AMI, is the prevention of symptomatic HF development (i.e., progression to Stage C HF).2,3 Compared with younger patients, elderly patients with AMI constitute a high-risk population for HF development and death.4,5 Therefore, long-term disease management, including preventing progression to Stage C HF, is a major clinical concern for secondary HF prevention in elderly patients with AMI.
Editorial p 498
The long-term management of the risk factors of HF is crucial for preventing post-AMI HF progression. The risk factors include age, comorbidities such as diabetes and anemia, low left ventricular ejection fraction (LVEF), and a prior history of HF.6,7 In addition to conventional risk factors, frailty, which represents a weakened resistance to stressors and decreased physiological reserve,8 has been recently identified as a prognostic factor of AMI. Although the prevalence of physical frailty increases rapidly with age, its prevalence in patients with AMI is higher than that in the general elderly population for the same age group.9,10 Physical frailty is associated with an increased risk of major bleeding, rehospitalization, and all-cause death;10 however, only a few reports have investigated the association between physical frailty at discharge and prognosis after AMI, including new-onset HF. A study of elderly patients with Stage B HF showed that patients with physical frailty had a 2.83-fold higher risk of developing Stage C HF within 6 months, but the prevalence of MI in those patients was only 15%.11 In addition, few previous studies have not excluded patients incapable of walking, which does not meet the international consensus that frailty is the predisabled stage.12 These findings indicate that limited evidence is available on the association between physical frailty and HF development and/or post-AMI death.
In this study, we examined the association between physical frailty and a composite outcome of death and/or HF rehospitalization in ambulant elderly patients with AMI without a history of HF.
This prospective observational study was performed as part of a multicenter cohort study (FLAGSHIP) designed to develop frailty-based prognostic criteria in patients with HF.13 The inclusion criterion of the FLAGSHIP study was: (1) patient hospitalized due to acute HF or worsening chronic HF and capable of walking 20 m at the time of discharge; or (2) patient aged ≥70 years hospitalized for AMI not complicated by HF and capable of walking 20 m at the time of discharge. The ability to walk 20 m did not depend on whether assistance or walking aids were used. Non-ambulatory patients were not included, in line with the international consensus that frailty is the predisabled stage.12 Complications associated with HF were determined by cardiologists at each participating center. The exclusion criteria included the presence of ≥1 of the following: (1) severe cognitive impairment, characterized by a Mini-Mental State Examination (MMSE)14 score <17 points; (2) severe mental disorder; (3) difficulty in responding to questionnaires; and (4) an assumed short-term lifespan (e.g., presence of severe aortic valve stenosis without surgical indication or terminal-stage cancer). Patients with severe mental disorders or severe cognitive impairment were excluded because part of the frailty assessment included self-administered questionnaires and the study protocol also included a post-discharge questionnaire survey. We enrolled patients from September 2015 to December 2018 and all registered patients were followed up for 2 years after discharge. The enrollment of patients who were readmitted to hospital during the study period was considered to begin at the time of the first hospitalization. The main findings of the primary FLAGSHIP analysis that studied patients hospitalized due to acute HF and worsening chronic HF has been published elsewhere.15
Of the patients enrolled in the FLAGSHIP study, the present subanalysis included the patients aged ≥70 years who were hospitalized for AMI not complicated by HF and was a prespecified subanalysis to investigate physical frailty in elderly patients with AMI.13 In the case of AMI not complicated by HF, only patients aged ≥70 years were included in the FLAGSHIP study due to the high prevalence of frailty and the risk of HF onset in elderly AMI populations.16 We excluded patients with a history of hospitalization for HF treatment and missing data on physical frailty and endpoints.
The FLAGSHIP study protocol and this analysis were approved by the Ethics Committee of Nagoya University School of Medicine and complied with the principles of the Declaration of Helsinki. Ethical approval was also obtained from each participating hospital, and all patients provided written informed consent for study participation.
Study Follow-up and EndpointsThe primary endpoint was a composite of all-cause death and HF rehospitalization within 2 years of discharge; the secondary endpoints were all-cause death and HF rehospitalization. A follow-up survey was performed for each patient using the medical records from the hospitals where the patients were previously admitted, and HF rehospitalization was determined by cardiologists at each enrolled institute. If patients did not attend follow-up visits at the respective hospitals, prognostic data were obtained from a survey questionnaire mailed directly to the patients every 4 months. The follow-up period was defined as the time from discharge to the main endpoint or till the last date of event-free survival, as confirmed by medical records or data from the mail survey, or until 2 years after discharge.
Assessment of Physical FrailtyPhysical frailty was assessed at discharge using the frailty score presented in the primary analysis of the FLAGSHIP study.15 This physical frailty assessment was proposed with reference to the frailty domains included in the phenotype model,17 and the score could be calculated using objective measurements collected within 10 min.15
The physical frailty assessment had 4 domains: weakness, slowness, physical inactivity, and exhaustion. Weakness and slowness were assessed by grip strength and usual walking speed, respectively. Grip strength was measured at discharge using a Jamar dynamometer (Digital Hand Dynamometer, DHD-1, SAEHAN Corp., South Korea) set at the second handle position. Two attempts with each hand were allowed, and the maximum value for each hand was recorded. A 10-m walkway was used to measure the usual walking time, and the lowest of the two values was used. Physical inactivity was assessed using the Self-Efficacy for Walking-7 (SEW-7), which has a moderate-to-strong correlation with accelerometer-measured step counts and a moderate correlation with vigorous physical activity.18 SEW-7 is scored from 7 to 35 points, with a lower score corresponding to lower physical activity. Exhaustion was assessed using the Performance Measure for Activity of Daily Living-8 (PMADL-8),19 which exhibits a strong negative correlation with the peak V˙O2.20 PMADL-8 is scored from 8 to 32 points, with higher scores indicating more severe functional limitations.
The cutoff value and assigned score for each physical frailty domain were determined based on the prognosis of HF in the main analysis of the FLAGSHIP study as follows: weakness=grip strength <30 kg for men and <17.5 kg for women, 5 points; slowness=usual walking speed <0.98 m/s, 4 points; physical inactivity=SEW-7 ≤20 points, 3 points; and exhaustion=PMADL-8 ≥21 points, 2 points. The score of each domain was weighted by multivariate analysis for prognostic prediction, and the total score was considered as the frailty score (0–14 points).15 In this study, a score ≥9 was considered to indicate physical frailty.
Data CollectionPatients’ characteristics, including age, sex, body mass index, and clinical characteristics (comorbidities and medications at discharge), were obtained from their medical records. Echocardiographic and biochemical data were collected immediately before discharge. The Simpson’s method was used to calculate the LVEF by 2D echocardiography. LVEF <40% was considered low.2,21 Biochemical data (levels of B-type natriuretic peptide [BNP], N-terminal [NT]-proBNP, hemoglobin, estimated glomerular filtration rate [eGFR], low-density lipoprotein cholesterol, triglyceride [TG], serum albumin, and high-sensitivity C-reactive protein [hs-CRP]) were also collected. BNP ≥200 pg/mL or NT-proBNP ≥900 pg/mL was considered to represent a high BNP level.22 Hemoglobin level <13 g/dL in men or <12 g/dL in women was indicative of anemia.23 Global cognitive function was assessed using the MMSE,14 which is a standard 11-question test with scores from 0 to 30. Depression was assessed using a 5-item Geriatric Depression Scale questionnaire (score: 0–5) and indicated by a score ≥2 points.24
Statistical AnalysisContinuous variables are expressed as median with interquartile range (IQR), and categorical variables are reported as percentages. Patient characteristics were compared using the Mann-Whitney test, and categorical variables were analyzed using the Chi-square test for data obtained from patients with and without physical frailty.
The cumulative incidence rates for the composite outcome and all-cause death were calculated using the Kaplan-Meier survival method, with comparisons between patients with and without physical frailty being performed using the log-rank test. To account for competing risk, the cumulative incidence of HF rehospitalization was compared between patients with and without physical frailty using Gray’s test. The proportional hazards assumption was assessed using the Schoenfeld residuals test. Hazard ratios (HRs) and 95% confidence intervals (CIs) for the composite outcome and all-cause death were calculated using a Cox proportional hazards model, and the HR for HF rehospitalization was calculated using a Fine and Gray method to account for competing risk. A multivariable model adjusted for age, sex, and variables with a P value <0.2 in the univariate analysis was used to assess the association between the study outcomes and physical frailty. As the secondary analysis, the association between each physical frailty domain and the composite outcome was assessed using a Cox proportional hazards model adjusted for age and sex.
The proportion of missing data was 0–5% for most variables. To avoid bias caused by the exclusion of patients with missing data, the missing values of covariates were imputed using multiple imputation by chained equations.25 Parameter estimates and CIs were obtained by combining 20 imputed datasets.25 Statistical analyses were performed using Stata version 17 (Stata Corporation, College Station, TX, USA). A P value <0.05 indicated statistical significance, and a P value <0.10 indicated marginal significance.
We enrolled 489 elderly patients hospitalized for AMI (Figure 1). Their median age was 76 years (IQR: 72–80 years), and men constituted 72.4% of the population. The characteristics of the patients with and without physical frailty are presented in Table 1. Compared with the patients without physical frailty, patients with physical frailty were older and more often female, with a higher prevalence of hypertension, anemia, use of diuretic drugs, and depression, a lower eGFR, albumin level, and MMSE score, and a higher hs-CRP level.
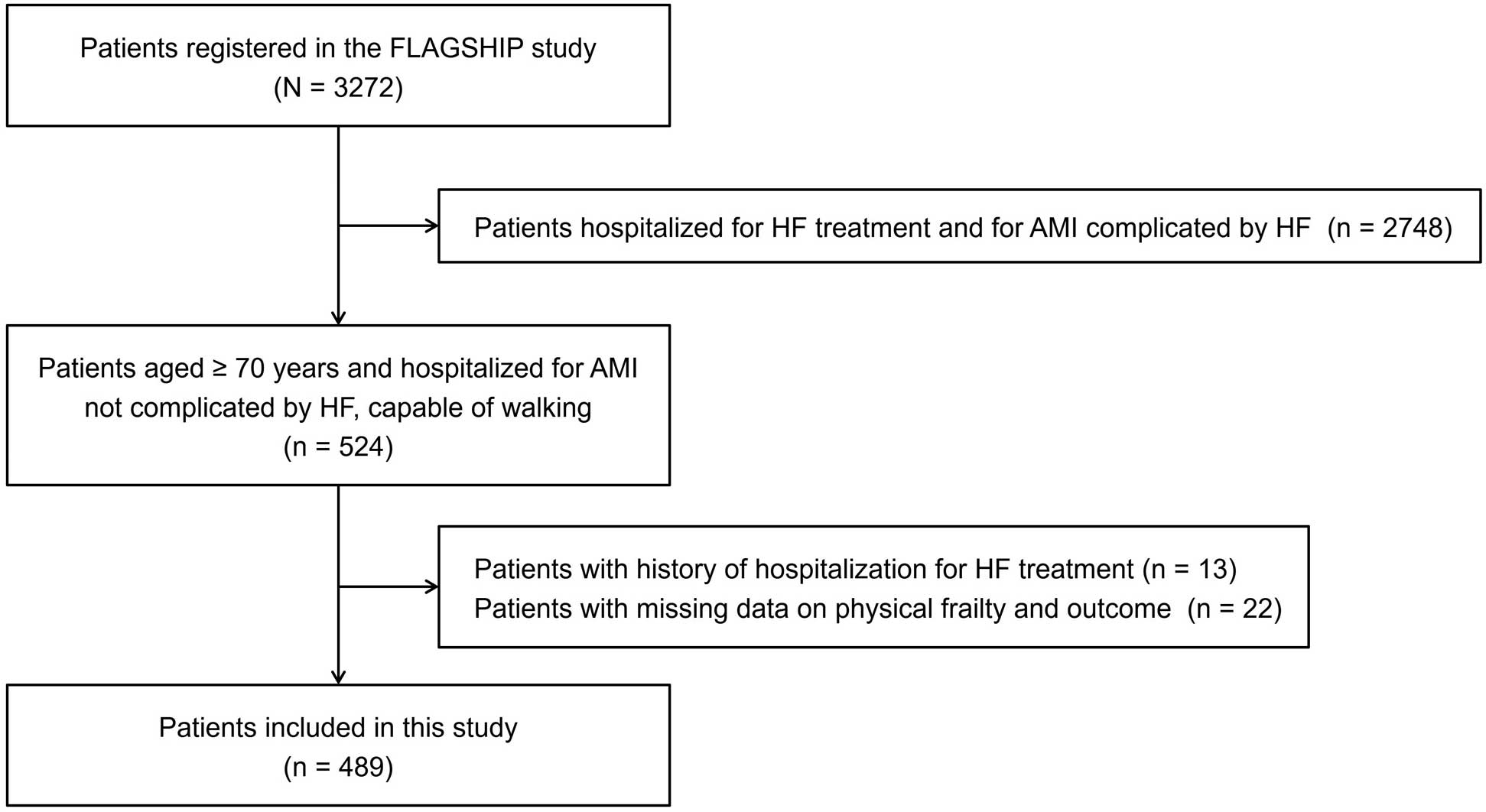
Flowchart of the patient inclusion method. AMI, acute myocardial infarction; HF, heart failure.
| Total n=489 |
Without frailty n=359 (73.4%) |
With frailty n=130 (26.6%) |
P value | |
|---|---|---|---|---|
| Age (years) | 76 [72–80] | 75 [72–79] | 80 [74–85] | <0.001 |
| Men (%) | 72.4 | 76.3 | 61.5 | 0.001 |
| BMI (kg/m2) | 22.6 [20.7–24.8] | 22.6 [21.0–24.8] | 22.8 [20.1–24.7] | 0.436 |
| Comorbidities | ||||
| Hypertension (%) | 63.4 | 61.0 | 70.0 | 0.068 |
| Dyslipidemia (%) | 52.8 | 52.1 | 54.6 | 0.621 |
| Diabetes mellitus (%) | 33.3 | 31.8 | 37.7 | 0.219 |
| Smoking | ||||
| Non (%) | 43.1 | 42.3 | 45.4 | 0.759 |
| Past (%) | 36.4 | 36.5 | 36.1 | |
| Current (%) | 20.5 | 21.2 | 18.5 | |
| Atrial fibrillation (%) | 6.1 | 5.6 | 7.7 | 0.388 |
| COPD (%) | 2.5 | 2.8 | 1.5 | 0.431 |
| Anemia (%) | 61.3 | 58.1 | 70.0 | 0.017 |
| LVEF (%) | ||||
| <40 | 7.4 | 7.2 | 8.2 | 0.888 |
| 40–49 | 20.0 | 20.4 | 18.8 | |
| ≥50 | 72.6 | 72.4 | 72.0 | |
| High BNP level (%) | 41.1 | 39.8 | 44.4 | 0.367 |
| eGFR (mL/min/1.73 m2) | 57.8 [47.2–68.0] | 59.7 [49.0–68.5] | 53.5 [42.5–67.4] | 0.006 |
| LDL-C (mg/dL) | 89 [70–112] | 91 [71–113] | 84 [67–111] | 0.196 |
| TG (mg/dL) | 101 [76–135] | 101 [78–134] | 99 [70–136] | 0.545 |
| Albumin (g/dL) | 3.6 [3.2–3.9] | 3.6 [3.3–3.9] | 3.5 [3.1–3.8] | <0.001 |
| hs-CRP (mg/dL) | 0.51 [0.18–1.51] | 0.42 [0.17–1.45] | 0.76 [0.23–1.72] | 0.010 |
| Medications | ||||
| Antiplatelet agent (%) | 91.0 | 90.5 | 92.3 | 0.544 |
| Anticoagulant agent (%) | 28.2 | 27.6 | 30.0 | 0.599 |
| Statin (%) | 82.8 | 83.8 | 80.0 | 0.319 |
| β-blocker (%) | 77.7 | 77.4 | 78.5 | 0.810 |
| ACEi/ARB (%) | 74.2 | 74.4 | 73.9 | 0.906 |
| Calcium-channel blocker (%) | 16.6 | 16.2 | 17.7 | 0.686 |
| Diuretic (%) | 21.5 | 18.9 | 28.5 | 0.024 |
| MMSE (points) | 28 [26–30] | 28 [26–30] | 27 [24–29] | <0.001 |
| Depression (%) | 27.2 | 22.7 | 38.5 | <0.001 |
| Usual walking speed (m/s) | 1.08 [0.92–1.23] | 1.15 [1.04–1.26] | 0.85 [0.70–0.95] | <0.001 |
| Grip strength (kg) | ||||
| All | 28.3 [21.4–34.1] | 31.1 [24.1–36.0] | 22.7 [15.4–27.0] | <0.001 |
| Men | 31.4 [27.1–36.2] | 32.9 [29.8–38.2] | 26.0 [23.3–28.5] | <0.001 |
| Women | 18.5 [15.3–21.2] | 19.8 [18.3–22.5] | 14.7 [12.4–16.8] | <0.001 |
| PMADL-8 (points) | 17 [12–20] | 16 [11–19] | 22 [19–25] | <0.001 |
| SEW-7 (points) | 22 [17–27] | 24 [20–28] | 16 [13–20] | <0.001 |
| Frailty score (points) | 5 [0–9] | 3 [0–5] | 11 [9–14] | <0.001 |
Continuous variables are expressed as median and interquartile range. Anemia was defined as hemoglobin <13 g/dL in men or <12 g/dL in women, and a high BNP level was defined as BNP ≥200 pg/mL or NT-proBNP ≥900 pg/mL. ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BMI, body mass index; BNP, B-type natriuretic peptide; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; hs-CRP, high-sensitivity C-reactive protein; LDL-C, low-density lipoprotein cholesterol; LVEF, left ventricular ejection fraction; MMSE, Mini-Mental State Examination; PMADL-8, Performance Measure for Activity of Daily Living-8; SEW-7, Self-Efficacy for Walking-7; TG, triglycerides.
A total of 37 composite outcomes occurred during 905.7 person-years of follow-up (24 rehospitalization events owing to HF, 6 cardiac deaths [including the deaths of 3 patients after HF readmission], and 10 non-cardiac deaths). The median duration from discharge to the time of HF rehospitalization was 208 days (IQR: 66–545 days), and the median duration to the time of all-cause death was 484 days (IQR: 184–586 days). Figure 2 shows the cumulative incidence rates for the study endpoints according to physical frailty. Patients with physical frailty showed significantly higher cumulative incidence rates of the composite outcome and all-cause death than patients without physical frailty. Patients with physical frailty also showed a marginally higher cumulative incidence rate of HF rehospitalization.
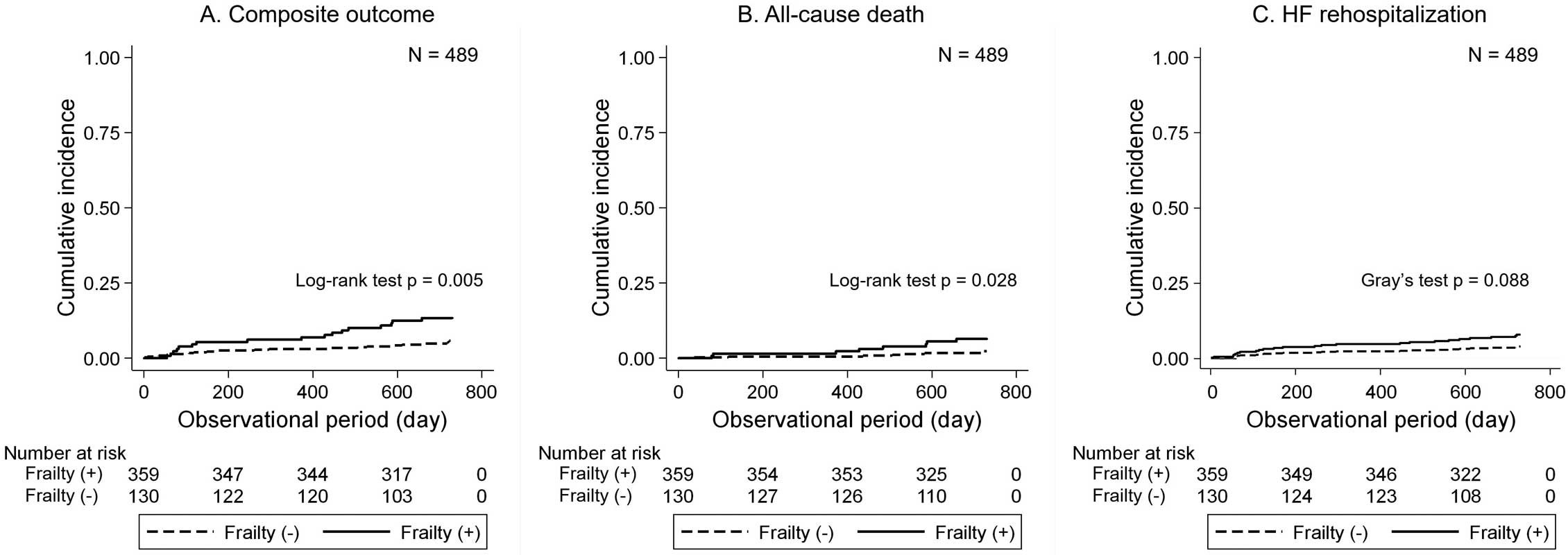
Cumulative incidence rates for the composite outcome, all-cause death, and heart failure (HF) rehospitalization within 2 years based on physical frailty.
The results of the hazards regression model are presented in Table 2. In the crude model and the age- and sex-adjusted model, physical frailty was significantly associated with a high risk of the post-discharge composite outcome. The variables with a P value <0.20 in the univariate analysis included diabetes mellitus, low levels of LVEF, eGFR, TG, the use of antiplatelet agents, and anticoagulant agents. After adjusting for age, sex, and the above variables, the association between physical frailty and the composite outcome remained statistically significant (HR=2.09, 95% CI: 1.03–4.22, P=0.040). The association between physical frailty and HF rehospitalization was not statistically significant after adjustment for the confounding factors (HR=2.14, 95% CI: 0.84–5.44, P=0.110). This result was unchanged following further adjustment for the use of diuretic drugs (HR=2.17, 95% CI: 0.83–5.69, P=0.116). The correlation between physical frailty and all-cause death was not statistically significant after adjustment for the confounding factors (HR=1.45, 95% CI: 0.49–4.26, P=0.501).
| Without frailty (n=359) |
With frailty (n=130) |
P value | |
|---|---|---|---|
| Composite outcome | |||
| Person-years of follow-up | 673.1 | 232.6 | |
| No. of composite outcome | 20 | 17 | |
| Incidence rate/1,000 person-years | 29.7 | 73.1 | |
| HR in the crude model | 1 | 2.45 (1.28–4.68) | 0.007 |
| HR in the age- and sex-adjusted model | 1 | 2.53 (1.27–5.02) | 0.008 |
| HR in the adjusted modela | 1 | 2.09 (1.03–4.22) | 0.040 |
| All-cause death | |||
| Person-years of follow-up | 688.2 | 243.1 | |
| No. of all-cause deaths | 8 | 8 | |
| Incidence rate/1,000 person-years | 11.6 | 32.9 | |
| HR in the crude model | 1 | 2.53 (0.98–6.57) | 0.056 |
| HR in the age- and sex-adjusted model | 1 | 2.46 (0.89–6.77) | 0.082 |
| HR in the adjusted modelb | 1 | 1.45 (0.49–4.26) | 0.501 |
| HF rehospitalization | |||
| Person-years of follow-up | 678.2 | 234.2 | |
| No. of HF rehospitalizations | 14 | 10 | |
| Incidence rate/1,000 person-years | 20.6 | 42.7 | |
| HR in the crude model | 1 | 2.02 (0.90–4.53) | 0.088 |
| HR in the age- and sex-adjusted model | 1 | 2.14 (0.87–5.26) | 0.098 |
| HR in the adjusted modelc | 1 | 2.14 (0.84–5.44) | 0.110 |
High BNP level was defined as BNP ≥200 pg/mL or NT-proBNP ≥900 pg/mL. aAdjusted for age, sex, diabetes mellitus, low LVEF, eGFR, TG, antiplatelet and anticoagulant agents. bAdjusted for age, sex, diabetes mellitus, low LVEF, eGFR, TG, anticoagulant agent, statin, calcium-channel blocker, diuretic, and depression. cAdjusted for age, sex, dyslipidemia, diabetes mellitus, and albumin. HF, heart failure; HR, hazard ratio.
Figure 3 shows the association between each physical frailty domain and the study outcome. Weakness and exhaustion exhibited marginal associations with an increased risk of the endpoint (weakness: HR=1.95, 95% CI: 0.99–3.83, P=0.052; exhaustion: HR=1.86, 95% CI: 0.93–3.71, P=0.077).
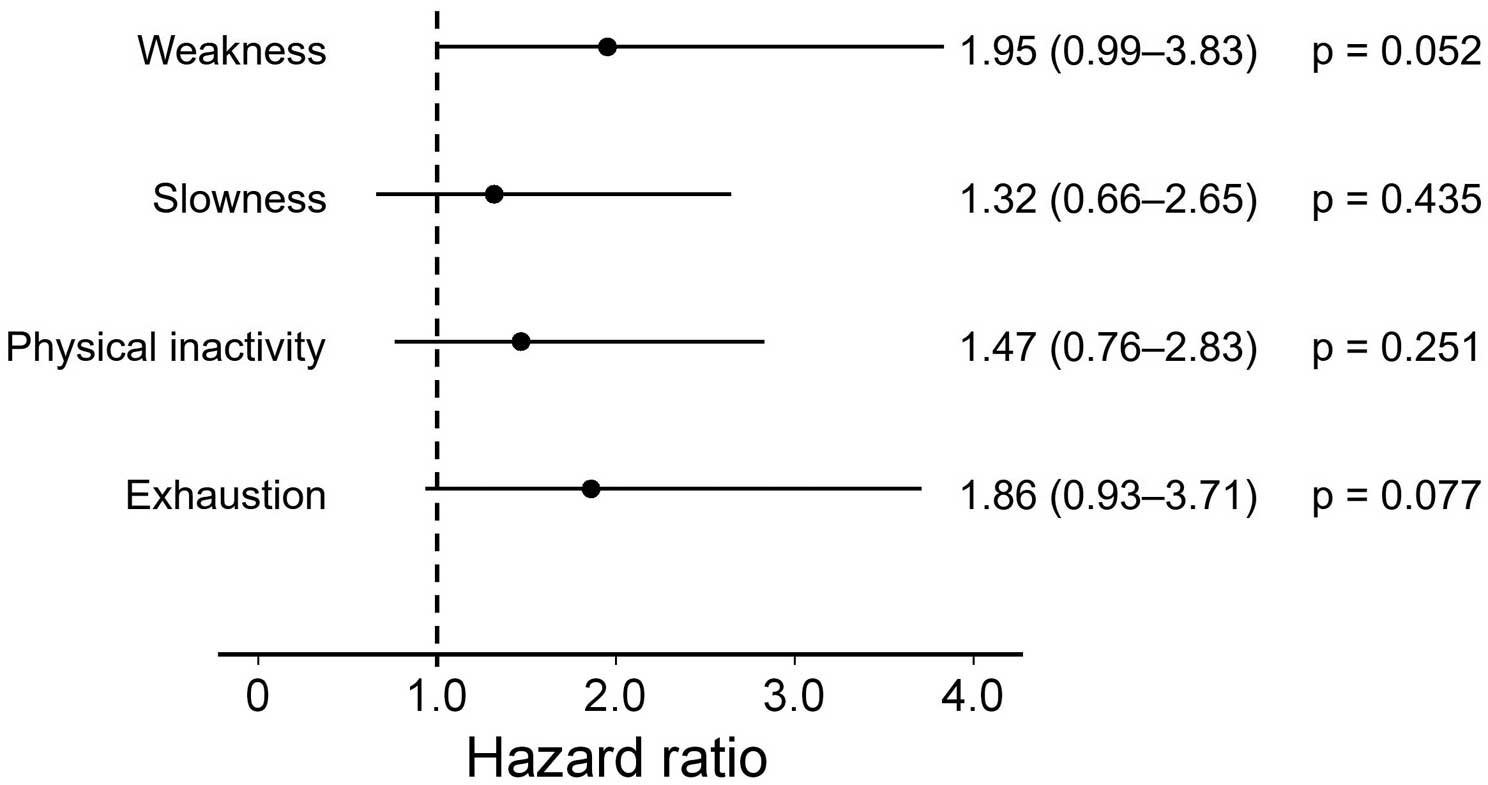
Adjusted hazard ratios of the composite outcome within 2 years associated with the different domains of physical frailty. The hazard ratios were adjusted for age and sex.
We investigated the association between physical frailty and the composite outcome of all-cause death and HF rehospitalization within 2 years of discharge in elderly patients with AMI without a history of HF and capable of walking. We found that physical frailty was associated with a poor prognosis in elderly patients hospitalized for AMI. To our knowledge, this is the first study to report that physical frailty provides information on prognosis, including the likelihood of new-onset HF, in elderly patients with AMI not complicated by symptomatic HF. The results of this study suggest the clinical importance of physical frailty assessment for long-term disease management in ambulant elderly patients with AMI not complicated by HF.
In this study, patients with physical frailty had a 2.09-fold higher risk of the composite outcome within 2 years of discharge. The study results also suggested a possible association of frailty with HF rehospitalization, although it was not statistically significant, possibly because of the limited sample size. This result was consistent with that reported in a previous study that examined the association between physical frailty and the risk of symptomatic HF development in patients with Stage B HF.11 In the previous study, however, approximately 85% of patients had an etiology other than MI. Thus, our results add to the evidence of the association between physical frailty and disease progression in elderly patients with AMI not complicated by symptomatic HF. Moreover, patients with severe physical impairment, including difficulty with walking, were not excluded in previous studies.10,26 However, there is a consensus on frailty based on the premise that frailty is the predisabled stage;12 elderly participants with severe functional impairment beyond frailty should thus be excluded from studies of physical frailty. From this perspective, the findings presented here provide additional evidence of the clinical significance of physical frailty, even among patients capable of walking, which is another strength of the study.
Several potential mechanisms affect the prognostic impact of physical frailty in AMI. First, the pathological mechanism underlying physical frailty involves chronic inflammation and high insulin resistance, which are also common risk factors for the development of HF.27,28 A previous study reported that physical frailty was a risk factor for HF onset even in the general elderly population.29 The presence of physical frailty may indicate susceptibility to HF via these pathological mechanisms. Another possible explanation is the relationship of physical frailty with endothelial dysfunction,30 and activated inflammatory responses,31 which are 2 known molecular mechanisms underlying atherosclerosis development. In a recent meta-analysis on elderly individuals, physical frailty was shown to be an independent risk factor for the development of coronary artery disease.32 Thus, physical frailty may accelerate the progression of atherosclerosis, leading to a poor prognosis of AMI. Finally, sarcopenia, a core component of physical frailty, is a potential a risk factor for HF with preserved EF (HFpEF). In addition to muscle loss, impaired energy metabolism in skeletal muscles, metabolic/endocrine abnormalities, and hormonal changes have been documented in cases of sarcopenia.33 These factors are considered to be underlying causes of HFpEF development and have emerged as potential therapeutic targets.34 In a cross-sectional study, diastolic dysfunction was observed more frequently in community-dwelling older adults with sarcopenia than in older adults without sarcopenia.35 In the present study, 72.6% of patients had a preserved LVEF ≥50% at discharge. Although the LVEF was not recorded during HF rehospitalization, the subset of individuals requiring HF rehospitalization may have included a certain proportion of individuals with HFpEF. However, supporting data for the above hypotheses were not obtained in the present study; further studies are warranted to examine the suitability of physical frailty as a therapeutic target for secondary prevention after AMI.
In the secondary analysis, each physical frailty domain was not individually associated with the study outcome, indicating the importance of a multidimensional assessment of frailty. Our results are consistent with those of a previous study in which a comprehensive frailty assessment based on Fried’s phenotype model was found to be more predictive of hospitalization risk than a single assessment of each domain in community-dwelling elderly individuals.36 Thus, a frailty assessment with multiple domains is likely to reflect a declining physiological reserve, and, in turn, yield favorable risk stratification. However, the cutoff for each domain in this study was validated by the primary FLAGSHIP outcome-based analysis in patients with HF,15 which possibly attenuated the prognostic accuracy for AMI not complicated by HF. Therefore, there is scope for the development of an optimal frailty-based risk prediction system in a population with AMI. This can be a topic for future investigations, as the sample size of this study was small and did not allow the identification of an AMI-specific cutoff value for each frailty domain.
Study LimitationsFirst, selection bias may have been introduced because only (consecutive) ambulant patients who provided informed consent were included in the FLAGSHIP study. Second, the association between frailty and prognosis was not analyzed in patients aged <70 years, because the FLAGSHIP study did not include this younger population. Third, there may exist potential confounders not measured in this study, including malignancy, history of previous AMI, and the classification of AMI. Fourth, the follow-up period was insufficient for observing the study outcomes in patients with AMI not complicated by HF, which may have reduced the statistical power of the results. The predictive value of frailty for specific clinical events needs to be further evaluated in future studies with a larger sample size and longer follow-up period. Finally, the causal relationship between physical frailty and prognosis, including the development of HF and all-cause death, will need to be established through intervention studies.
Physical frailty was associated with a poor prognosis in elderly patients hospitalized for AMI without complications of symptomatic HF or severe physical dysfunction. For secondary prevention in elderly ambulant patients with AMI, the assessment of physical frailty may serve as a stratification tool to identify populations at a high risk of post-discharge clinical events.
We are grateful to all patients for their cooperation during the study and the collaborating investigators of FLAGSHIP for their contributions. This study was supported by a Grant-in-Aid for Scientific Research (A) from the Japan Society for the Promotion of Science [16H01862].
The authors have no conflicts of interest to declare.
The FLAGSHIP study protocol was approved by the Ethics Committee of Nagoya University School of Medicine (approval no. 2014-0421). Ethical approval was also obtained from each participating hospital. This analysis was approved by the Ethics Committee of Nagoya University School of Health Sciences (approval no. 22-508).
The de-identified participant data will not be shared.
H.A. contributed to the investigation, analysis, and interpretation of data and drafted the manuscript. T.A. contributed to the design, investigation, analysis, and interpretation of data and drafted the manuscript. K.I. and K. Kamisaka contributed to the investigation and interpretation of data. K. Kamiya and Y.U. contributed to the interpretation of data. S.Y. contributed to the conception, design, interpretation of data, and project supervision. All authors critically revised the manuscript, approved the final manuscript, and agreed to be held accountable for all aspects of the work, ensuring integrity and accuracy.