Abstract
Background: Transesophageal echocardiography (TEE) has been used for percutaneous atrial septal defect (ASD) closure, with intracardiac echocardiography (ICE) guidance recently being introduced.
Methods and Results: The Japanese Structural Heart Disease Registry was established by the Japanese Association of Cardiovascular Intervention and Therapeutics. This study analyzed data from the Registry for 2,859 consecutive cases undergoing percutaneous ASD closure between January 2015 and December 2020. ASD closure was performed under ICE guidance (n=519; 18.2%), TEE guidance (n=1,428; 49.9%), or TEE plus ICE guidance (“Both”; n=900 cases; 31.5%). The success rates were similar in the TEE, ICE, and both groups (99.0%, 99.2%, vs. 98.0%, respectively; P=0.054), as were complication rates (1.2%, 0.5%, vs. 2.1%, respectively; P=0.24). In the TEE and Both groups, 92.4% and 79.6% of patients required general anesthesia, compared with only 2.9% of patients in the ICE group (P<0.001). Fluoroscopic time was longer in the ICE and Both groups than in the TEE group (median [interquartile range] 19 [14–28] and 21 [13–30] vs. 12 [8–19] min, respectively; P<0.001). Rim deficiency and larger defect diameter were inversely related, whereas hospital volume was positively related to ICE guidance.
Conclusions: Percutaneous transcatheter ASD closure was as feasible under ICE as under TEE guidance. ICE guidance is used for less challenging cases in high-volume centers in Japan.
An atrial septal defect (ASD) is one of the most common congenital cardiovascular anomalies. Percutaneous device closure for ASD was first described in 1974,1,2 and it has now largely replaced surgical interventions because it is safe and less invasive.3 Accurate evaluation of the anatomic features of an ASD is indispensable for diagnosis, case selection, technical planning, and guidance to avoid procedural complications.4 Traditionally, transesophageal echocardiography (TEE) under general anesthesia has been recommended as imaging guidance for percutaneous ASD closure.5 However, TEE-related complications such as pharyngeal injury and aspiration pneumonitis are matters of concern. More recently, intracardiac echocardiography (ICE) guidance without general anesthesia has been introduced.6 ICE guidance can be performed continuously without deep sedation. Studies from experienced centers have suggested that percutaneous ASD closure under ICE guidance is safer and superior to TEE guidance in shortening fluoroscopic and procedural times and reducing the radiation dose.7–11 Alqahtani et al reported that ICE guidance for interatrial procedures increased from 9.7% in 2003 to 50.6% in 2014.12 Singh et al also reported that ICE use has increased exponentially from 3.3% of procedures in 2001 to 55% of procedures in 2010.13 However, these studies from the US did not provide detailed technical information and specifics regarding imaging guidance. Moreover, the trends in the selection of imaging modality have not been clarified in current clinical settings, in which both TEE and ICE are available.
Editorial p 525
The aims of the present study, based on a nationwide database in Japan, were to: (1) elucidate the current status of imaging guidance during percutaneous transcatheter ASD closure and compare the features of ICE vs. TEE guidance; and (2) identify factors associated with the selection of an ICE-guided strategy.
Methods
Study Population and Collection of Clinical Data
The present study analyzed data from the national Japanese Structural Heart Disease (J-SHD) Registry collected between January 2015 and December 2020. The J-SHD Registry is a prospective, nationwide, multicenter registry of structural heart disease (SHD), organized by the Japanese Association of Cardiovascular Intervention and Therapeutics (CVIT) and designed to collect clinical variables and in-hospital outcome data of consecutive patients undergoing various structural heart interventions. The CVIT requires interventionalists and their cardiovascular centers to register all SHD interventional procedures in the J-SHD Registry to apply for board certification and its renewal. Therefore, all hospitals that perform SHD interventions other than specialized pediatric hospitals were mandated to participate in the Registry.14,15 The CVIT holds an annual meeting of data managers to ensure appropriate data collection and performs random audits (20 institutions annually) to check the quality of abstracted data. The definitions of variables in the J-SHD Registry are available online from the CVIT.
The study protocol and analyses of the J-SHD Registry were approved by the Institutional Review Board of the St. Marianna University School of Medicine (Ethics Committee Approval No. 4312.) The requirement for written informed consent was waived because of the retrospective and observational nature of the study. All data were fully anonymized before the present analysis was performed. The J-SHD Registry can be accessed by participating institutions, but the database is not publicly available. However, all relevant data concerning this study are included in the paper.
The present study extracted the data of percutaneous transcatheter ASD closures from the database. The indications for and techniques of percutaneous ASD closure were left entirely to the discretion of the board-certified attending physicians, who were well trained to be familiar with the standard technique, as described previously.16 The closure devices used were selected from the following, which are approved in Japan: Amplatzer®
Septal Occluder, Amplatzer®
Cribriform (Abbott, Abbott Park, IL, USA), and Occlutech®
Figulla Flex II (Occlutech Holding AG, Schaffhausen, Switzerland). No information about the model of the ICE catheter or TEE probe is provided in the Registry data.
Definitions
Cardiovascular risk factors were defined according to domestic clinical guidelines. Hypertension was considered to be systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or treatment with antihypertensive medications.17 Dyslipidemia was defined as fasting triglyceride levels ≥150 mg/dL, fasting high-density lipoprotein cholesterol (HDL-C) concentrations <40 mg/dL, fasting low-density lipoprotein cholesterol concentrations (calculated using the Friedewald equation) ≥140 mg/dL, non-HDL-C concentrations ≥170 mg/dL, or treatment with antihyperlipidemic medication.18 Diabetes was defined as fasting glucose levels ≥126 mg/dL, non-fasting glucose levels ≥200 mg/dL, HbA1c levels ≥6.5%, or treatment with antidiabetic medications.19 Smoking was defined as any history of smoking in past years. Chronic kidney disease was defined as the presence of proteinuria, serum creatinine concentrations ≥1.3 mg/dL, or estimated glomerular filtration rate ≤60 mL/min/1.73 m2. End-stage renal disease on dialysis included both hemodialysis and peritoneal dialysis. Coronary artery disease was defined as a history of myocardial infarction or revascularization for a stenotic coronary artery lesion, whereas cerebrovascular disease was defined as a history of cerebral infarction or hemorrhage with neurological symptoms sustained for ≥24 h. Rim deficiency was defined as rim length (defined as the distance between the ASD and surrounding structures) <5 mm.
The annual case volume of an institution was defined as the number of percutaneous transcatheter ASD closures conducted during the year prior at the institution. For example, the institutional volume of ASD closure on April 1, 2020 was the number of ASD closures conducted at the institution between April 1, 2019 and March 31, 2020.
Statistical Analysis
Unless indicated otherwise, data are presented as the median with interquartile range (IQR) for continuous variables and as frequencies and percentages for categorical variables. P<0.05 was considered significant; 95% confidence intervals (CIs) are reported where appropriate. The annual change in procedural strategies was examined using the Chi-squared test. The significance of differences in clinical parameters among groups were tested by the Kruskal-Wallis test for continuous variables and by the Chi-squared test for discrete variables. The association of clinical parameters with the selection of ultrasonography was explored using the mixed multinomial logit model in which the interinstitution and interoperator variabilities were treated as random effects. Baseline characteristics that showed a significant intergroup difference during the crude comparison by the Kruskal-Wallis test and the Chi-squared test were entered as fixed effects in the mixed multinomial logit model. During the analysis, missing data were addressed by list-wise deletion.
All statistical analyses were performed using R version 4.1.1 (R Development Core Team, Vienna, Austria).
Results
Study Population
Between 2015 and 2020, 2,859 cases with percutaneous transcatheter ASD closure were registered in the J-SHD Registry. Of the 2,859 cases, 519 were treated under the ICE-guided strategy, 1,428 were treated with TEE, and 900 cases were treated with both ICE and TEE (“Both”); procedural strategy was not identified in the remaining 12 cases.
Temporal Trends in Imaging Guidance for Percutaneous ASD Closure
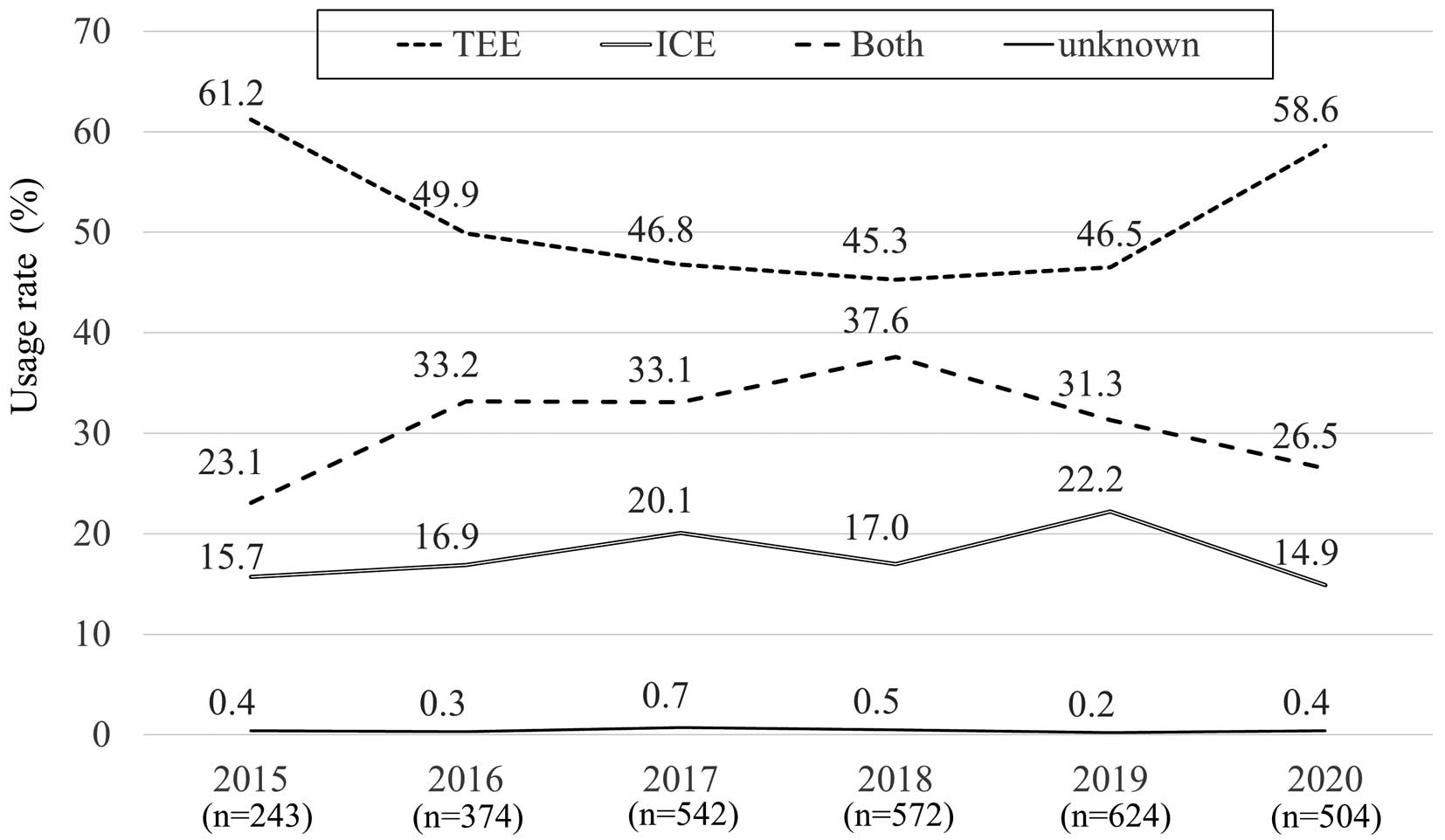
Temporal trends in imaging guidance for percutaneous ASD closure are shown in Figure 1. The proportion of ICE guidance was approximately 20%, which did not show a clear temporal increase or decrease between 2015 and 2019, whereas there was a sudden increase in TEE guidance and a decrease in ICE and Both guidance in 2020.
Patient Characteristics
To elucidate the status of imaging guidance for percutaneous ASD closure, comparative analyses were conducted among the 3 groups. There were no differences in sex, age, or the prevalence of diabetes, hypertension, chronic respiratory insufficiency, dyslipidemia, renal insufficiency, end-stage renal disease on dialysis, or cerebrovascular disease, although the proportions of smokers and of coronary artery disease were higher in the Both group (Table 1).
Table 1. Patient Characteristics
| |
All
(n=2,859) |
TEE
(n=1,428) |
ICE
(n=519) |
Both
(n=900) |
P value |
| Male sex |
1,064 (37.2) |
530 (37.1) |
185 (35.6) |
343 (38.1) |
0.65 |
| Age (years) |
58 [40–70] |
59 [40–69] |
59 [41–70] |
57 [41–70] |
0.56 |
| Diabetes |
237 (8.3) |
121 (8.5) |
37 (7.1) |
76 (8.4) |
0.61 |
| Hypertension |
759 (26.5) |
377 (26.4) |
145 (27.9) |
232 (25.8) |
0.67 |
| Chronic respiratory failure |
40 (1.4) |
19 (1.3) |
7 (1.3) |
13 (1.4) |
0.97 |
| Dyslipidemia |
366 (12.8) |
175 (12.3) |
62 (11.9) |
127 (14.1) |
0.35 |
| Smoking |
249 (8.7) |
122 (8.5) |
25 (4.8) |
98 (10.9) |
<0.001 |
| Chronic kidney disease |
155 (5.4) |
74 (5.2) |
23 (4.4) |
57 (6.3) |
0.27 |
| End-stage renal disease |
22 (0.8) |
9 (0.6) |
3 (0.6) |
9 (1.0) |
0.53 |
| Coronary artery disease |
131 (4.6) |
54 (3.8) |
18 (3.5) |
59 (6.6) |
0.003 |
| Cerebrovascular disease |
195 (6.8) |
89 (6.2) |
45 (8.7) |
59 (6.6) |
0.16 |
Unless indicated otherwise, data are given as the median [interquartile range] or n (%). Data were missing on the selection of ultrasound for 12 (0.4%) patients. ICE, intracardiac echocardiography; TEE, transesophageal echocardiography; Both, TEE plus ICE guidance.
The hemodynamic and anatomic characteristics of target ASDs are presented in Table 2. There were significant differences among the TEE, ICE, and Both groups in the pulmonary to systemic blood flow (Qp/Qs) ratio (2.00 [1.60–2.50], 1.80 [1.50–2.30], and 2.00 [1.60–2.50], respectively; P<0.001), as well as in the proportion of patients with rim deficiencies (72.8%, 56.3%, and 69.8%, respectively; P<0.001). In addition, there were significant differences among the TEE, ICE, and Both groups in both the short-axis diameter (12 [9–15], 11 [8–15], and 12 [9–16] mm, respectively; P=0.008) and long-axis diameter (16 [12–21], 15 [11–19], and 17 [12–21] mm, respectively; P<0.001).
Table 2. Characteristics of Target Atrial Septal Defects and Hospital Procedure Volumes
| |
All
(n=2,859) |
TEE
(n=1,428) |
ICE
(n=519) |
Both
(n=900) |
P value |
| Qp/Qs ratio |
2.00 [1.60–2.50] |
2.00 [1.60–2.50] |
1.80 [1.50–2.30] |
2.00 [1.60–2.60] |
<0.001 |
| Size |
| Short-axis diameter (mm) |
12 [8–15] |
12 [9–15] |
11 [8–15] |
12 [9–16] |
0.008 |
| Long-axis diameter (mm) |
16 [12–21] |
16 [12–21] |
15 [11–19] |
17 [12–21] |
<0.001 |
| Rim deficiency |
|
|
|
|
<0.001 |
| Aortic |
1,560 (69.4) |
886 (72.8) |
193 (56.3) |
475 (69.8) |
|
| Coronary sinus |
36 (1.6) |
19 (1.6) |
4 (1.2) |
13 (1.9) |
|
| IVC |
51 (2.3) |
35 (2.9) |
8 (2.3) |
8 (1.2) |
|
| Mitral |
6 (0.3) |
2 (0.2) |
2 (0.6) |
2 (0.3) |
|
| Posterior |
74 (3.3) |
36 (3.0) |
12 (3.5) |
26 (3.8) |
|
| SVC |
51 (2.3) |
24 (2.0) |
11 (3.2) |
15 (2.2) |
|
| No deficiency |
471 (20.9) |
215 (17.7) |
113 (32.9) |
142 (20.9) |
|
| Hospital volume (/year) |
25 [17–37] |
24 [16–38] |
32 [24–40] |
22 [16–30] |
<0.001 |
Unless indicated otherwise, data are given as the median [interquartile range] or n (%). Data were missing on the selection of ultrasound for 12 (0.4%) patients, on the pulmonary to systemic blood flow (Qp/Qs) ratio for 19 (0.7%) patients, on short-axis diameter for 23 (0.8%) patients, on long-axis diameter for 22 (0.8%) patients, on rim deficiency for 610 (21.3%) patients, and on hospital volume for 685 (24.0%) patients. IVC, inferior vena cava; SVC, superior vena cava. Other abbreviations as in Table 1.
There was a significant difference in the hospital volume of transcatheter ASD closure among the groups, showing that the hospital volume was higher for the ICE than the TEE and Both groups (32 [24–40] vs. 24 [16–38] and 22 [16–30], respectively; P<0.001; Table 2).
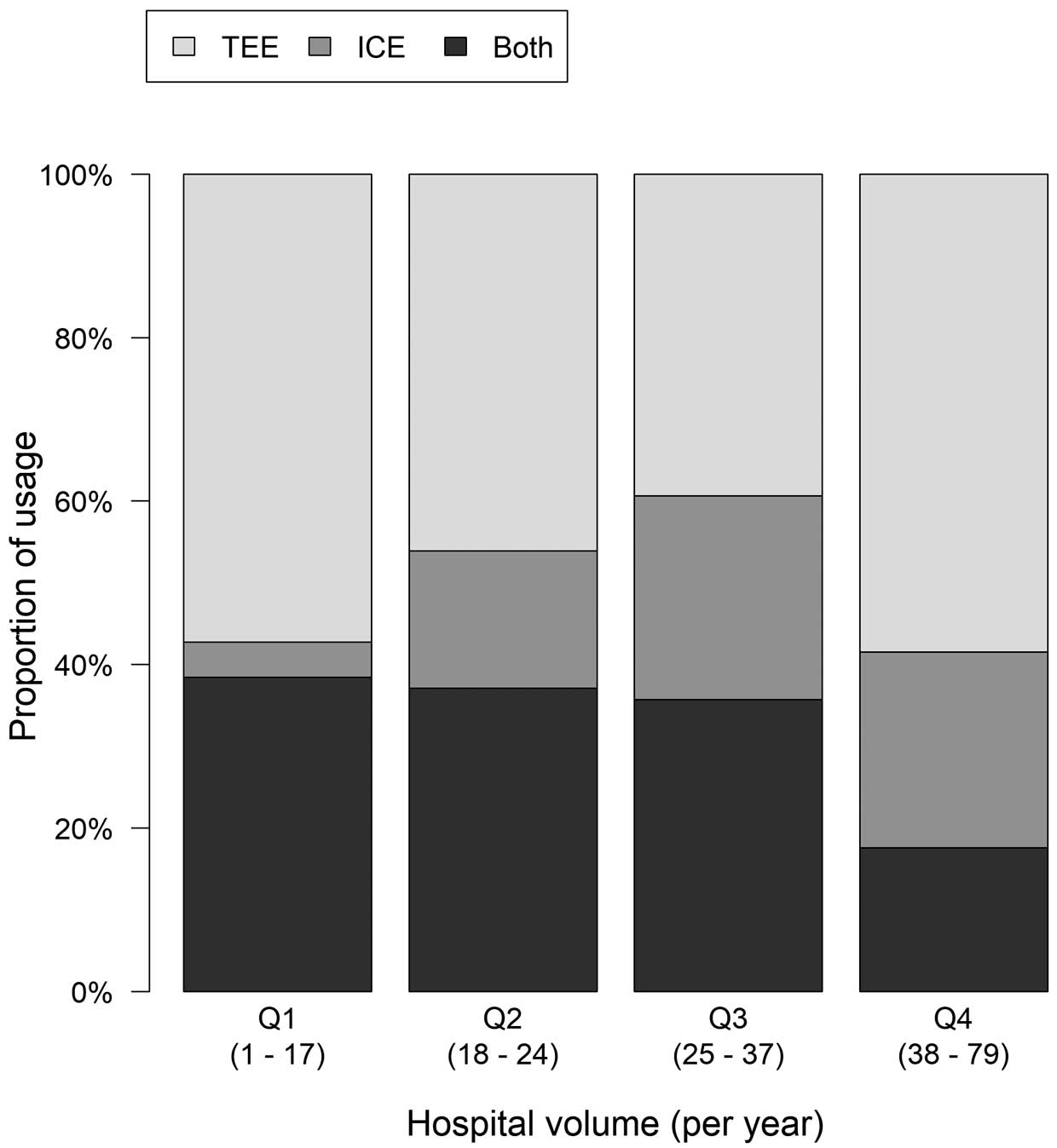
The proportions of imaging guidance by quartiles of hospital volume are shown in Figure 2. There was a significant difference among the quartiles (P<0.001). ICE guidance was less likely in low-volume hospitals (Q1, 4.3%; Q2, 16.8%; Q3, 24.9%; Q4, 24.0%; P<0.001).
Procedural Features and Clinical Outcomes
Procedural features and clinical outcomes are summarized in Table 3. In the TEE and Both groups, 92.4% and 79.6% of patients, respectively, required general anesthesia, compared with only 2.9% of patients in the ICE group (P<0.001). Fluoroscopic time was longer in the ICE and Both groups than in the TEE-group (19 [14–28] and 21 [13–30] vs. 12 [8–19] min, respectively; P<0.001). The total procedure time was longer in the Both group than in the TEE and ICE groups (90 [66–136] vs. 57 [38–100] and 62 [42–79] min, respectively; P<0.001). Concerning device selection, approximately half the patients were treated with an Amplatzer Septal Occluder, most of the remaining patients were treated with an Occlutech®
Figulla Flex II, and only a small number were treated with an Amplatzer Cribriform. The use of the Occlutech®
Figulla Flex II was slightly lower in the ICE than TEE group (45.9% vs. 58.4%, respectively). The success rate was similar in the TEE, ICE, and Both groups (99.0%, 99.2%, and 98.0%, respectively; P=0.054), as was the complication rate (1.2%, 0.5%, and 2.1%, respectively; P=0.24). The procedural mortality was 0% in all groups.
Table 3. Clinical Parameters by Choice of Ultrasound
| |
All
(n=2,859) |
TEE
(n=1,428) |
ICE
(n=519) |
Both
(n=900) |
P value |
| General anesthesia |
2,052 (72.1) |
1,320 (92.4) |
15 (2.9) |
716 (79.6) |
<0.001 |
| Procedural time (min) |
70 [47–114] |
57 [38–100] |
62 [42–79] |
90 [66–136] |
<0.001 |
| Fluoroscopic time (min) |
15 [10–25] |
12 [8–19] |
19 [14–28] |
21 [13–30] |
<0.001 |
| Implanted device |
|
|
|
|
<0.001 |
| Amplatzer Septal Occluder |
1,189 (45.8) |
502 (39.4) |
242 (50.5) |
444 (52.9) |
|
| Amplatzer Cribriform |
59 (2.3) |
28 (2.2) |
17 (3.5) |
14 (1.7) |
|
| Occlutech Figulla Flex II |
1,347 (51.9) |
745 (58.4) |
220 (45.9) |
382 (45.5) |
|
| No. devicesA |
|
|
|
|
0.43 |
| 0 |
7 (0.6) |
3 (0.5) |
0 (0.0) |
4 (1.2) |
|
| 1 |
1,105 (98.2) |
572 (97.9) |
213 (100.0) |
320 (97.6) |
|
| 2 |
13 (1.2) |
9 (1.5) |
0 (0.0) |
4 (1.2) |
|
| Procedural success |
2,812 (98.7) |
1,414 (99.0) |
515 (99.2) |
882 (98.0) |
0.054 |
| Periprocedural complications |
15 (1.3) |
7 (1.2) |
1 (0.5) |
7 (2.1) |
0.24 |
| In-hospital death |
2 (0.2) |
2 (0.3) |
0 (0.0) |
0 (0.0) |
0.40 |
| Procedural death |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
|
| Major bleeding |
1 (0.1) |
1 (0.2) |
0 (0.0) |
0 (0.0) |
0.63 |
| Emergency cardiovascular surgery |
1 (0.1) |
0 (0.0) |
0 (0.0) |
1 (0.3) |
0.30 |
| Device malapposition |
9 (0.8) |
2 (0.3) |
1 (0.5) |
6 (1.8) |
0.045 |
| OthersB |
2 (0.2) |
2 (0.3) |
0 (0.0) |
0 (0.0) |
0.40 |
Unless indicated otherwise, data are given as the median [interquartile range] or n (%). Data were missing on the selection of ultrasonography for 12 (0.4%) patients, on general anesthesia for 11 (0.4%) patients, on procedural time for 1,594 (55.8%) patients, on fluoroscopic time for 546 (19.1%) patients, on implanted device for 264 (9.2%) patients, on the number of devices for 1,734 (60.7%) patients, on procedural success for 11 (0.4%) patients, and on procedural complications for 1,734 (60.7%) patients. AData on the number of devices implanted has been available since 2019. BOther perioperative complications include cerebral infarction (n=1) and hematoma at the puncture site (n=1). Abbreviations as in Table 1.
Table 4 presents associations between clinical parameters and the choice of ultrasonography using the mixed multinomial logit model. Rim deficiency and greater ASD diameter were inversely associated with the selection of ICE guidance, especially relative to TEE guidance, whereas hospital volume was positively associated with ICE guidance, especially relative to Both guidance. Hospital volume had a negative association with Both guidance relative to TEE guidance.
Table 4. Association Between Clinical Parameters and Choice of Ultrasound
| |
TEE |
ICE |
Both |
| aOR (95% CI) |
P value |
aOR (95% CI) |
P value |
aOR (95% CI) |
P value |
| Multinomial logit model with TEE as the reference |
| Smoking |
|
|
0.40 (0.11–1.54) |
0.18 |
0.89 (0.44–1.79) |
0.74 |
| Coronary artery disease |
|
|
1.45 (0.44–4.71) |
0.54 |
0.84 (0.38–1.85) |
0.67 |
| Qp/Qs ratio (per 1-unit increase) |
|
|
0.79 (0.55–1.13) |
0.20 |
1.09 (0.95–1.25) |
0.24 |
Long-axis diameter (per 1-mm
increase) |
|
|
0.94 (0.91–0.98) |
0.002 |
0.98 (0.96–1.01) |
0.25 |
| Rim deficiency |
|
|
0.54 (0.33–0.88) |
0.014 |
0.79 (0.53–1.19) |
0.27 |
| Hospital volume (vs. Q1) |
| Q2 |
|
|
4.93 (1.55–15.72) |
0.007 |
1.28 (0.68–2.40) |
0.45 |
| Q3 |
|
|
3.51 (1.01–12.14) |
0.048 |
0.39 (0.18–0.82) |
0.014 |
| Q4 |
|
|
2.20 (0.53–9.04) |
0.28 |
0.21 (0.08–0.56) |
0.002 |
| Multinomial logit model with ICE as the reference |
| Smoking |
2.58 (0.69–9.62) |
0.16 |
|
|
2.29 (0.67–7.83) |
0.19 |
| Coronary artery disease |
0.71 (0.22–2.28) |
0.57 |
|
|
0.60 (0.19–1.85) |
0.37 |
| Qp/Qs ratio (per 1-unit increase) |
1.26 (0.88–1.79) |
0.21 |
|
|
1.37 (0.96–1.94) |
0.080 |
Long-axis diameter (per 1-mm
increase) |
1.06 (1.02–1.10) |
0.002 |
|
|
1.04 (1.00–1.09) |
0.030 |
| Rim deficiency |
1.86 (1.14–3.04) |
0.013 |
|
|
1.47 (0.89–2.43) |
0.13 |
| Hospital volume (vs. Q1) |
| Q2 |
0.19 (0.06–0.57) |
0.003 |
|
|
0.24 (0.08–0.70) |
0.009 |
| Q3 |
0.26 (0.08–0.87) |
0.028 |
|
|
0.10 (0.03–0.32) |
0.001 |
| Q4 |
0.42 (0.11–1.65) |
0.22 |
|
|
0.09 (0.02–0.33) |
0.001 |
| Multinomial logit model with Both as the reference |
| Smoking |
1.13 (0.56–2.28) |
0.72 |
0.46 (0.13–1.61) |
0.22 |
|
|
| Coronary artery disease |
1.16 (0.53–2.54) |
0.71 |
1.69 (0.54–5.33) |
0.37 |
|
|
| Qp/Qs ratio (per 1-unit increase) |
0.92 (0.80–1.06) |
0.24 |
0.73 (0.51–1.04) |
0.077 |
|
|
Long-axis diameter (per 1-mm
increase) |
1.02 (0.99–1.04) |
0.25 |
0.96 (0.92–0.99) |
0.025 |
|
|
| Rim deficiency |
1.25 (0.83–1.88) |
0.28 |
0.67 (0.40–1.12) |
0.13 |
|
|
| Hospital volume (vs. Q1) |
| Q2 |
0.77 (0.42–1.45) |
0.42 |
3.78 (1.25–11.42) |
0.019 |
|
|
| Q3 |
2.50 (1.19–5.24) |
0.015 |
8.67 (2.65–28.34) |
<0.001 |
|
|
| Q4 |
4.64 (1.74–12.37) |
0.002 |
10.11 (2.61–39.11) |
0.001 |
|
|
Adjusted odds ratios (aORs), 95% confidence interval (CIs), and P values are derived from the multinomial logit models with random effects. Qp/Qs ratio, pulmonary to systemic blood flow ratio; Q1–Q4, first to fourth quartiles. Other abbreviations as in Table 1.
Discussion
This is the first study to evaluate the status of intraprocedural imaging guidance for percutaneous transcatheter ASD closure in a large cohort that analyzed not only patient characteristics, but also the anatomic and hemodynamic features of ASDs. The results can be summarized as follows. The proportion of ICE guidance was approximately 20% in Japan. In the less complex cases with a smaller shunt and less rim deficiencies, ICE guidance was preferred, whereas 67.1% of patients treated with ICE guidance had some kind of rim deficiency. The clinical outcomes were similar among the groups. In most cases of ICE guidance, general anesthesia was not required. Fluoroscopic time was longer in the ICE and Both groups than in the TEE group. Moreover, total procedure time was longer in the Both group. Finally, ICE-guided procedures were performed primarily in experienced hospitals.
Traditionally, percutaneous ASD closure has been performed under TEE. ICE was first used for imaging guidance of percutaneous interatrial closure in 2001,6 and it has now spread widely to other interventions, such as catheter ablation, transcatheter aortic valve implantation, mitral valve repair, and left atrial appendage closure.20 The advantage of ICE guidance is that it is less invasive than TEE guidance because it can be performed continuously without general anesthesia or deep sedation. Several previous studies have suggested that percutaneous ASD closure under ICE guidance is safer and may be superior to TEE guidance in terms of shortening the fluoroscopic and procedure times, as well as reducing the radiation dose7,9–11 without increasing ICE-related complications, such as access site bleeding and cardiac injury. The present study showed that the procedure time with ICE guidance was similar, but fluoroscopic time was longer, compared with TEE guidance, findings that are similar to those reported by Shimizu et al of their experience at their institution in Japan.8 This may suggest that Japanese physicians perform careful maneuvers using the ICE catheter to prevent cardiovascular injury, leading to longer fluoroscopy time.
In the present study, ICE guidance was used in only 20% of cases, with the remaining procedures performed under TEE guidance or TEE in addition to ICE. Two large-scale studies in the US recently reported that 37.7–50.6% cases of interatrial communication were closed under ICE guidance.12,13 The reasons for the lower rate of ICE guidance in the present study compared with earlier studies are assumed to be as follows. First, Japanese clinicians are not familiar with ICE guidance. In addition, Japanese medical insurance usually covers the costs of both ICE and TEE in a procedure. Therefore, Japanese physicians may not have hesitated to use TEE in addition to ICE when they felt uncertain with ICE guidance alone, resulting in a decrease in the rate of ICE guidance alone and an increase in the use of TEE plus ICE (“Both”) guidance procedures. However, this is not ideal, because the procedure time and fluoroscopic time were longer in the Both group in the present study. Moreover, using both is more expensive and invasive than using ICE alone or TEE alone. Second, earlier studies from Western countries included not only ASD, but also patent foramen ovale, which is simpler and easier to treat with ICE than ASD, leading to an increase in the usage rate in those studies.
The present study showed that more complex cases tended to be treated with TEE guidance, whereas two-thirds of cases treated with ICE guidance had some kind of rim deficiency. The present study also demonstrated that ICE guidance was mostly performed in experienced hospitals, which may suggest that such facilities take up the challenge to treat relatively complex cases with ICE guidance. To effectively use ICE for interventional procedures, a learning curve is needed.7,20,21 Similar to the results of the present study, ICE guidance was used more frequently in teaching hospitals in the US.12 Indeed, the present study demonstrated that ICE guidance alone was more frequent and that guidance with both TEE and ICE was used less frequently in the experienced hospitals with the highest procedural volume. Because guidance with both TEE and ICE has the disadvantages of longer fluoroscopic and total procedure times and higher cost, it should be performed for limited cases, such as those with large defects with a small left atrium or posterior rim deficiency, which cannot be evaluated well using TEE or ICE alone. The present study suggests the importance of experience and education for physicians to increase the effective use of ICE in clinical practice.
Finally, concerning temporal trends, the usage rate of each type of guidance did not change between 2015 and 2019. However, there was a sudden increase in TEE guidance in 2020. This may have been provoked by the COVID-19 pandemic. Due to the COVID-19 pandemic, it is possible that there was an increase in cases in which preoperative TEE was omitted and, instead, ASD closure was performed with TEE guidance. In fact, the COVID-19 pandemic affected other catheter procedures in Japan; the Japanese Percutaneous Coronary Intervention (J-PCI) Registry organized by the CVIT demonstrated that elective percutaneous coronary intervention decreased substantially, and that more patients presented with high-risk characteristics and had higher in-hospital mortality at the start of the COVID-19 pandemic.22
Study Limitations
The present study has limitations inherent to any large registry study. The fact that the diagnoses and treatment were at the discretion of each institution and individual clinicians may have given rise to various biases, although the clinicians were trained and familiar with the standard procedures of the CVIT. The rates of procedural complications were extremely low, which suggests that mild cases of complications that the physicians did not need to report may have been overlooked. Moreover, no information was available about arrhythmic complications in the J-SHD Registry. A propensity score-matched comparison among groups was not performed due to the small number of cases in the ICE group. Preoperative evaluations such as TEE or cardiac computed tomography may have an effect on the selection of the imaging modality during ASD closure. However, no information about preoperative imaging evaluations was available. Concerning ASD morphology, septal malalignment and a transverse sinus are also risk factors for cardiac erosion after ASD closure, in addition to rim deficiencies, and may influence the selection of imaging guidance.23–25 Unfortunately, the J-SHD Registry did not collect such information. Acknowledging these limitations, the present study has important strengths, including its large sample size, indicating the current status of ICE guidance for percutaneous ASD closure.
Conclusions
Percutaneous transcatheter ASD closure was as feasible under ICE guidance as under TEE guidance. ICE guidance has a major advantage over TEE guidance because it can be performed without general anesthesia. However, ICE guidance is primarily used in experienced hospitals, such as those with a large number of cases, in Japan. It has been suggested that the experience and education of physicians are important in increasing the effective use of ICE in clinical practice.
Acknowledgments
The authors extend their appreciation to all investigators, clinical coordinators, and institutions involved in the J-SHD Registry for their contribution to the study.
Sources of Funding
This work was supported by the Japanese Association of Cardiovascular Intervention and Therapeutics (CVIT) and Grants in-Aid for Scientific Research from the Japan Society for the Promotion of Science (KAKENHI; No. 16KK0186, 16H05215, and 17K09493).
Disclosures
T.A. is an Associate Editor of Circulation Journal. Y.J.A. and Y. Ikari are members of Circulation Journal’s Editorial Board. The remaining authors have no conflicts of interest to declare.
IRB Information
The study protocol and analyses of the J-SHD Registry were approved by the Institutional Review Board of the St. Marianna University School of Medicine (Kawasaki-city, Kanagawa, Japan; Ethics Committee Approval No. 4312).
References
- 1.
King TD, Mills NL. Nonoperative closure of atrial septal defects. Surgery 1974; 75: 383–388.
- 2.
King TD, Thompson SL, Steiner C, Mills NL. Secundum atrial septal defect. Nonoperative closure during cardiac catheterization. JAMA 1976; 235: 2506–2509.
- 3.
Singh V, Badheka AO, Patel NJ, Chothani A, Mehta K, Arora S, et al. Influence of hospital volume on outcomes of percutaneous atrial septal defect and patent foramen ovale closure: A 10-years US perspective. Catheter Cardiovasc Interv 2015; 85: 1073–1081.
- 4.
Bartel T, Müller S. Device closure of interatrial communications: Peri-interventional echocardiographic assessment. Eur Heart J Cardiovasc Imaging 2013; 14: 618–624.
- 5.
Vaidyanathan B, Simpson JM, Kumar RK. Transesophageal echocardiography for device closure of atrial septal defects: Case selection, planning, and procedural guidance. JACC Cardiovasc Imaging 2009; 2: 1238–1242.
- 6.
Hijazi Z, Wang Z, Cao Q, Koenig P, Waight D, Lang R. Transcatheter closure of atrial septal defects and patent foramen ovale under intracardiac echocardiographic guidance: Feasibility and comparison with transesophageal echocardiography. Catheter Cardiovasc Interv 2001; 52: 194–199.
- 7.
Bartel T, Konorza T, Arjumand J, Ebradlidze T, Eggebrecht H, Caspari G, et al. Intracardiac echocardiography is superior to conventional monitoring for guiding device closure of interatrial communications. Circulation 2003; 107: 795–797.
- 8.
Shimizu S, Kawamura A, Arai T, Ohno Y, Mogi S, Kodaira M, et al. Intracardiac echocardiography for percutaneous closure of atrial septal defects: Initial experiences in Japan. Cardiovasc Interv Ther 2013; 28: 368–373.
- 9.
Mullen MJ, Dias BF, Walker F, Siu SC, Benson LN, McLaughlin PR. Intracardiac echocardiography guided device closure of atrial septal defects. J Am Coll Cardiol 2003; 41: 285–292.
- 10.
Moon J, Park Y, Park SJ, Oh PC, Jang AY, Chung WJ, et al. Comparison of intracardiac echocardiography and transesophageal echocardiography for image guidance in percutaneous patent foramen ovale closure. Medicina (Kaunas) 2020; 56: 401.
- 11.
Bartel T, Konorza T, Neudorf U, Ebralize T, Eggebrecht H, Gutersohn A, et al. Intracardiac echocardiography: An ideal guiding tool for device closure of interatrial communications. Eur J Echocardiogr 2005; 6: 92–96.
- 12.
Alqahtani F, Bhirud A, Aljohani S, Mills J, Kawsara A, Runkana A, et al. Intracardiac versus transesophageal echocardiography to guide transcatheter closure of interatrial communications: Nationwide trend and comparative analysis. J Interv Cardiol 2017; 30: 234–241.
- 13.
Singh V, Badheka AO, Patel NJ, Chothani A, Mehta K, Arora S, et al. Influence of hospital volume on outcomes of percutaneous atrial septal defect and patent foramen ovale closure: A 10-years US perspective. Catheter Cardiovasc Interv 2015; 85: 1073–1081.
- 14.
Minatsuki S, Takahara M, Kiyosue A, Kodera S, Hatano M, Ando J, et al. Characteristics and in-hospital outcomes of patients undergoing balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension: A time-trend analysis from the Japanese nationwide registry. Open Heart 2021; 8: e001721, doi:10.1136/openhrt-2021-001721.
- 15.
Sawano M, Yamaji K, Kohsaka S, Inohara T, Numasawa Y, Ando H, et al. Contemporary use and trends in percutaneous coronary intervention in Japan: An outline of the J-PCI registry. Cardiovasc Interv Ther 2020; 35: 218–226.
- 16.
Oho S, Ishizawa A, Akagi T, Dodo H, Kato H. Transcatheter closure of atrial septal defects with the Amplatzer septal occluder: A Japanese clinical trial. Circ J 2002; 66: 791–794.
- 17.
Shimamoto K, Ando K, Fujita T, Hasebe N, Higaki J, Horiuchi M, et al. The Japanese Society of Hypertension guidelines for the management of hypertension (JSH 2014). Hypertens Res 2014; 37: 253–390.
- 18.
Teramoto T, Sasaki J, Ishibashi S, Birou S, Daida H, Dohi S, et al. Executive summary of the Japan Atherosclerosis Society (JAS) guidelines for the diagnosis and prevention of atherosclerotic cardiovascular diseases in Japan: 2012 version. J Atheroscler Thromb 2013; 20: 517–523.
- 19.
Araki E, Goto A, Kondo T, Noda M, Noto H, Origasa H, et al. Japanese clinical practice guideline for diabetes 2019. Diabetol Int 2020; 11: 165–223.
- 20.
Bartel T, Müller S, Biviano A, Hahn RT. Why is intracardiac echocardiography helpful?: Benefits, costs, and how to learn. Eur Heart J 2014; 35: 69–76.
- 21.
Enriquez A, Saenz LC, Rosso R, Silvestry FE, Callans D, Marchlinski FE, et al. Use of intracardiac echocardiography in interventional cardiology: Working with the anatomy rather than fighting it. Circulation 2018; 137: 2278–2294.
- 22.
Yamaji K, Kohsaka S, Inohara T, Numasawa Y, Ando H, Wada H, et al. Percutaneous coronary intervention during the COVID-19 pandemic in Japan: Insights from the nationwide registration data. Lancet Reg Health West Pac 2022; 22: 100434.
- 23.
Kijima Y, Akagi T, Nakagawa K, Promphan W, Toh N, Nakamura K, et al. Cardiac erosion after catheter closure of atrial septal defect: Septal malalignment may be a novel risk factor for erosion. J Cardiol Cases 2014; 9: 134–137.
- 24.
Takaya Y, Akagi T, Nakagawa K, Nakayama R, Miki T, Watanabe N, et al. Clinical significance of septal malalignment for transcatheter closure of atrial septal defect. J Interv Cardiol 2020; 2020: 6090612.
- 25.
Amin Z. Echocardiographic predictors of cardiac erosion after Amplatzer septal occluder placement. Catheter Cardiovasc Interv 2014; 83: 84–92.