2023 Volume 87 Issue 4 Pages 543-550
2023 Volume 87 Issue 4 Pages 543-550
Background: To predict mortality in patients with acute heart failure (AHF), we created and validated an internal clinical risk score, the KICKOFF score, which takes physical and social aspects, in addition to clinical aspects, into account. In this study, we validated the prediction model externally in a different geographic area.
Methods and Results: There were 2 prospective multicenter cohorts (1,117 patients in Osaka Prefecture [KICKOFF registry]; 737 patients in Kochi Prefecture [Kochi YOSACOI study]) that had complete datasets for calculation of the KICKOFF score, which was developed by machine learning incorporating physical and social factors. The outcome measure was all-cause death over a 2-year period. Patients were separated into 3 groups: low risk (scores 0–6), moderate risk (scores 7–11), and high risk (scores 12–19). Kaplan-Meier curves clearly showed the score’s propensity to predict all-cause death, which rose independently in higher-risk groups (P<0.001) in both cohorts. After 2 years, the cumulative incidence of all-cause death was similar in the KICKOFF registry and Kochi YOSACOI study for the low-risk (4.4% vs. 5.3%, respectively), moderate-risk (25.3% vs. 22.3%, respectively), and high-risk (68.1% vs. 58.5%, respectively) groups.
Conclusions: The unique prediction score may be used in different geographic areas in Japan. The score may help doctors estimate the risk of AHF mortality, and provide information for decisions regarding heart failure treatment.
Because of its increased prevalence in the aging population, acute heart failure (AHF) is an important and common cause of hospitalization, morbidity, and mortality in cardiology.1 Some studies have revealed that the median age of patients with AHF is 80 years, and that these patients have various comorbidities, physical disorders, and social problems.2,3 A decline in physical activity is a valuable predictor of adverse outcomes in patients with heart failure (HF),4,5 and social problems are associated with HF.6
Some mortality prediction models and scoring systems for AHF risk stratification have been reported and validated in Japanese patients with AHF.7–10 Most of these tools primarily use clinical and laboratory variables, with physical activity and social problems not considered in these scores. Therefore, using data from the Kitakawachi Clinical Background and Outcome of Heart Failure (KICKOFF) registry and a machine learning model, we developed a clinical score (the KICKOFF score) incorporating physical and social factors, in addition to clinical factors, to predict long-term mortality in patients with AHF.11 The model’s performance was evaluated with temporal validation in a previous study.11 However, the KICKOFF score has not been validated externally in different geographic areas of Japan.
The KICKOFF registry and the Kochi Registry of Subjects with Acute Decompensated Heart Failure (Kochi YOSACOI) study were designed as prospective multicenter cohorts of Japanese patients with AHF in Osaka Prefecture and Kochi Prefecture, respectively. Using these databases from different geographic areas, the aim of this study was to compare the clinical characteristics and social background between the 2 cohorts, and validate the KICKOFF score in a different geographic area in Japan.
This study was a retrospective analysis of the KICKOFF registry and Kochi YOSACOI study databases, which included hospitalized patients with AHF. The institutions participating in the KICKOFF registry were 13 hospitals in the north of Kitakawachi (Hirakata, Neyagawa, and Katano cities) and Yawata areas between April 2015 and August 2017. Kitakawachi and Yawata were typical satellite communities in Japan, and they are located at the eastern end of Osaka Prefecture and the southern end of Kyoto Prefecture, respectively.12 The population of the north of Kitakawachi and Yawata is approximately 798,000, and the proportion of people aged ≥65 years reached 27.2% in 2018. The institutions participating in the Kochi YOSACOI study between May 2017 and December 2019 were 6 hospitals in Kochi Prefecture. Kochi Prefecture is a typical superaging area in Japan that is located in the south of the Shikoku region.13 The population of Kochi Prefecture is approximately 706,000, and the proportion of people aged ≥65 years reached 34.7% in 2018.
Based on the Framingham criteria, HF was diagnosed in patients when there were at least 2 major criteria or one major and 2 minor criteria.14 Detailed study designs of the KICKOFF registry and Kochi YOSACIO study have been described previously.11,13 The clinical data of all patients were collected through reviews of medical records, as well as interviews with patients or other family members conducted by the investigators at the participating hospitals during the registration period. Data were entered via the Internet Database System, and double-checked by the general office of the registry.
The study protocol followed the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Ethics Committee of Hirakata Kohsai Hospital (Osaka, Japan; Approval no. 2021-012), the Ethics Committee on Medical Research of Kochi Medical School (Kochi, Japan; Approval no. 28-68), and the ethics committees of all participating hospitals. Written informed consent was obtained from all participants prior to enrollment in each study. Direct patient identifiers were not registered to preserve patient confidentiality. The study protocol did not affect any of the treatments or outpatient care methods of patients with AHF.
Patient Data and OutcomesThe KICKOFF registry and Kochi YOSACOI study enrolled 1,253 and 1,051 patients with hospitalized patients with AHF, respectively (Supplementary Tables 1,2). After excluding patients who died during the index hospitalization and for whom data were missing for calculation of the KICKOFF score, the present study enrolled 1,086 patients from the KICKOFF registry and 737 patients from the Kochi YOSACOI study who were discharged alive from the index hospitalization and who had data available data for calculation of the KICKOFF score (Figure 1). Baseline characteristics were compared between the 2 groups (i.e., KICKOFF registry and Kochi YOSACOI study). Patients in each group were divided into 3 risk score groups according to the KICKOFF score, and the survival rate was compared for validation analysis (Figure 2).11
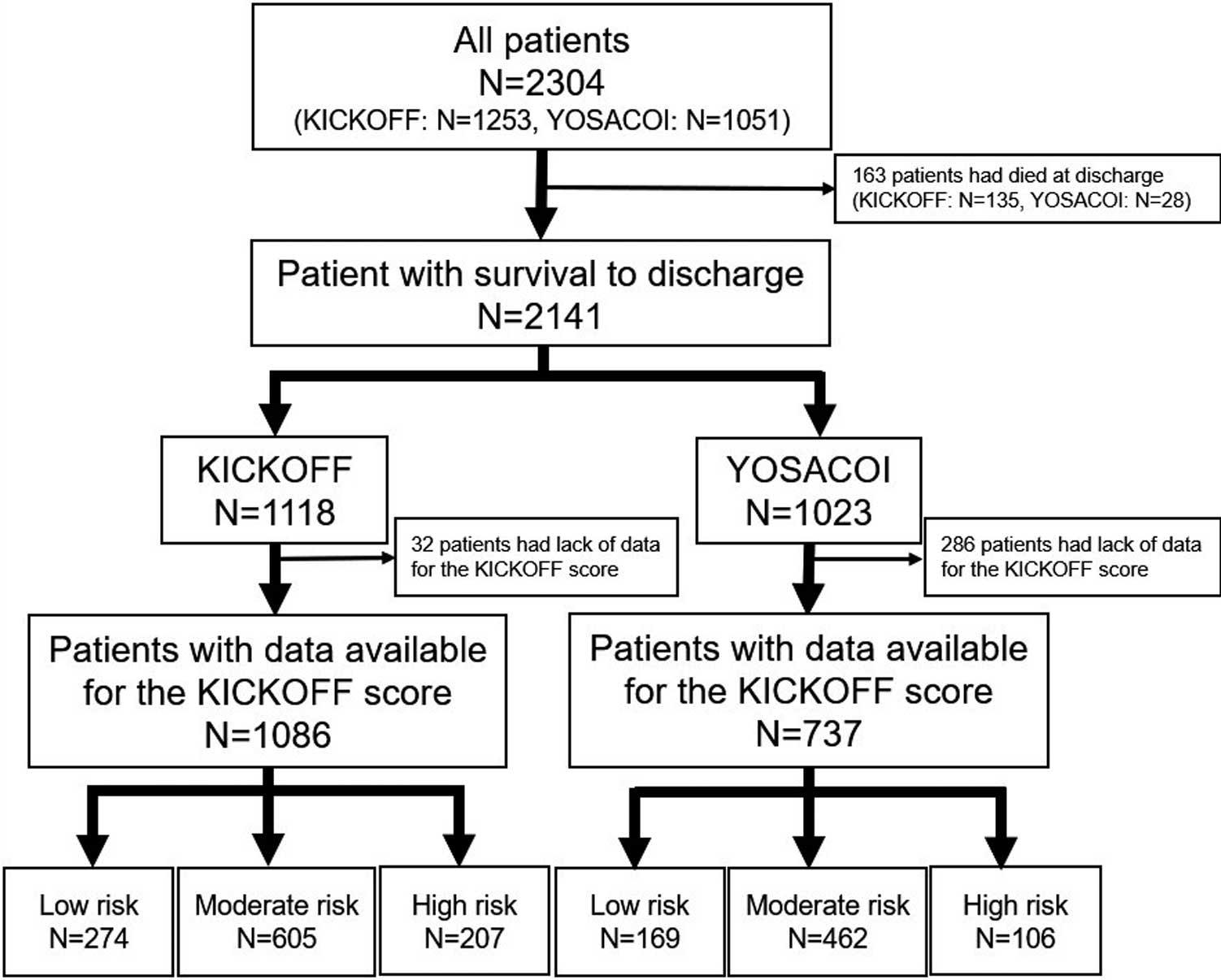
Patient flowchart. KICKOFF, Kitakawachi Clinical Background and Outcome of Heart Failure; YOSACOI, Kochi Registry of Subjects with Acute Decompensated Heart Failure.

Risk stratification according to the KICKOFF score. ADL, activities of daily living; BMI, body mass index; BNP, B-type natriuretic peptide; eGFR, estimated glomerular filtration rate.
The KICKOFF score includes not only basic clinical variables, but also physical and social factors to predict adverse events in patients with AHF. It was constructed using the least absolute shrinkage and selection operator regularization with 10-fold cross-validation and the optimal value of the penalty parameter (lambda.1se) was set using the “glmnet” package.15 The selected predictors were set into a multivariable logistic regression model to generate the β coefficient with a standard error for each predictor. We developed a risk scoring system to predict the outcome using a simple integer based on each variable’s β coefficient. Furthermore, based on the sum of scores, 3 risk groups (low, moderate, and high risk) were set for a rule-in and rule-out approach to help with decision making.
Comorbidities were defined in the previous study.11 Patients were categorized into 4 activities of daily living (ADL) categories by their physicians: (1) independent outdoor walking; (2) independent indoor walking; (3) indoor walking with assistance; or (4) abasia before discharge. In the present study, patients were divided into 2 ADL groups at discharge: (1) independent walking outside or at home (independent outdoor walking and independent indoor walking); and (2) non-independent walking (indoor walking with assistance and abasia).
The “main drug therapy manager” was defined as the person who most frequently managed the patients’ drug therapy on a daily basis (i.e., the patients themselves or their partners, a son or daughter, a caretaker, or a nursing home, or a hospital). In the present study, patients were divided into 2 social background categories based on the main drug therapy manager: (1) the patients themselves; or (2) other managers. Data regarding physical and social factors were collected by physicians and trained medical staff from a review of medical records and through interviews with the patients or other family members.
The primary outcome to be predicted was the incidence of all-cause death over a 2 year follow-up period. Clinical follow-up was performed at 6 months and 1 and 2 years after discharge. Follow-up data were collected primarily by a review of hospital records, with additional follow-up information obtained via telephone or mail contact with the patients or their relatives.
Statistical AnalysisPatient characteristics and predictor candidate variables were described for each cohort. Continuous variables are presented as the mean±SD or as the median with interquartile range (IQR). Categorical variables are presented as numbers and percentages. Categorical variables were compared using Chi-squared tests, whereas continuous variables were compared using Student’s t-test.
In both the KICKOFF registry and Kochi YOSACOI study, patients were divided into 3 groups based on the KICKOFF score: low risk (scores 0–6), moderate risk (scores 7–11), and high risk (scores 12–19). For external validation, the Kaplan-Meier method was used to evaluate the cumulative incidence of all-cause death and C-statistics were calculated for the KICKOFF score in each cohort. The estimated differences were compared using a log-rank test. The mortality rate was calculated at 6 months and 1 year and 2 years after discharge for each risk group in both cohorts.
Statistical analyses were performed using JMP version 14 (SAS Institute, Cary, NC, USA). The estimated values were calculated with 95% confidence intervals (CIs).
Patients’ clinical characteristics and social background are presented in the Table. There was no significant difference in the proportion of males between the KICKOFF registry and Kochi YOSACOI study, but mean age was lower in the KICKOFF registry. The proportion of patients with a body mass index <25 kg/m2 was lower, whereas the proportion of patients with previous hospitalizations for HF was higher in the KICKOFF registry. There was no significant difference in estimated glomerular filtration rate, B-type natriuretic peptide (BNP) concentrations, or in the proportion of patients who managed their own medications between the 2 groups. The proportion of patients with serum albumin concentrations <4.0 or 3.0 mg/dL and the proportion of patients unable to walk independently were lower in the KICKOFF registry than in the Kochi YOSACOI study.
| KICKOFF | YOSACOI | P value | |
|---|---|---|---|
| No. patients | 1,086 | 737 | |
| KICKOFF risk score group | |||
| Low | 274 (25.2) | 169 (22.9) | 0.001 |
| Moderate | 605 (55.7) | 426 (62.7) | |
| High | 207 (19.1) | 106 (14.4) | |
| Male sex | 558 (51.4) | 357 (48.4) | 0.218 |
| Age (years) | 77.4±11.4 | 79.1±11.3 | 0.002 |
| Age group | |||
| ≥75 years | 707 (65.1) | 528 (71.6) | 0.003 |
| ≥85 years | 312 (28.7) | 272 (36.9) | <0.001 |
| BMI (kg/m2) | 21.9±4.1 | 21.2±4.2 | 0.002 |
| BMI <25 kg/m2 | 884 (81.4) | 632 (85.8) | 0.014 |
| SBP (mmHg) | 115.8±18.6 | 113.8±17.7 | 0.021 |
| DBP (mmHg) | 65.7±13.3 | 63.6±11.6 | 0.001 |
| Heart rate (beats/min) | 71.9±13.7 | 72.0±12.5 | 0.912 |
| Previous hospitalization for HF | 395 (36.4) | 201 (27.3) | <0.001 |
| Length of hospital stay (days) | 19 [13–31] | 20 [14–32] | 0.136 |
| Comorbidities | |||
| Hypertension | 726 (66.9) | 548 (74.4) | 0.001 |
| Coronary artery disease | 312 (28.7) | 246 (33.4) | 0.035 |
| Valvular disease | 334 (30.8) | 153 (20.8) | <0.001 |
| Diabetes | 376 (34.6) | 224 (30.4) | 0.059 |
| Dyslipidemia | 416 (38.3) | 332 (45.1) | 0.004 |
| Atrial fibrillation | 459 (42.3) | 348 (47.2) | 0.037 |
| Chronic kidney disease | 587 (54.1) | 549 (74.5) | <0.001 |
| COPD | 154 (14.2) | 59 (8.0) | <0.001 |
| Device implantation | 96 (8.8) | 24 (3.3) | <0.001 |
| Cognitive dysfunction | 335 (30.9) | 127 (17.2) | <0.001 |
| Active malignancy | 45 (4.1) | 37 (5.0) | 0.378 |
| Blood tests | |||
| Total protein (mg/dL) | 6.6 [6.1–7.1] | 6.5 [6.1–6.9] | 0.129 |
| Albumin (mg/dL) | 3.5 [3.0–3.7] | 3.5 [3.1–3.7] | 0.039 |
| Albumin <3.0 mg/dL | 233 (21.5) | 104 (14.1) | <0.001 |
| Albumin <4.0 mg/dL | 938 (86.4) | 657 (89.3) | <0.001 |
| Hemoglobin (g/dL) | 11.6 [10.3–13.2] | 11.3 [9.9–12.8] | <0.001 |
| Creatinine (mg/dL) | 1.03 [0.81–1.46] | 1.00 [0.80–1.46] | 0.444 |
| BUN (mg/dL) | 22.1 [15.9–32.1] | 23.4 [17.6–31.3] | 0.584 |
| eGFR (mL/min/1.73 m2) | 46.1 [31.9–62.1] | 44.7 [32.2–60.2] | 0.755 |
| eGFR <30 mL/min/1.73 m2 | 235 (21.6) | 155 (21.0) | 0.756 |
| BNP (pg/dL) | 222 [94–482] | 266 [137–501] | 0.263 |
| BNP ≥400 pg/dL | 333 (30.7) | 261 (35.4) | 0.05 |
| BNP ≥600 pg/dL | 190 (17.5) | 122 (16.6) | |
| HbA1c (JDS) (%) | 6.2 [5.8–6.9] | 6.0 [5.6–6.4] | <0.001 |
| Echocardiography | |||
| LV diastolic diameter (mm) | 48.5±9.2 | 50.0±8.8 | <0.001 |
| LV systolic diameter (mm) | 35.5±10.9 | 37.5±10.9 | <0.001 |
| LVEF (%) | 52.1±17.5 | 48.5±16.2 | <0.001 |
| Medication | |||
| RAS inhibitors (ARB or ACEI) | 670 (61.7) | 337 (45.7) | <0.001 |
| β-blocker | 474 (43.7) | 234 (31.8) | <0.001 |
| Diuretic | 850 (78.3) | 663 (90.0) | <0.001 |
| Mineralocorticoid receptor antagonist | 179 (16.5) | 256 (34.7) | <0.001 |
| Calcium channel blocker | 228 (21.0) | 236 (32.0) | <0.001 |
| Oral inotropic agent | 72 (6.6) | 36 (4.9) | 0.117 |
| Digitalis | 45 (4.1) | 2 (0.3) | <0.001 |
| Living style | |||
| Living alone | 324 (29.8) | 162 (22.5) | 0.001 |
| Living only with partner | 208 (19.2) | 183 (25.4) | |
| Living with son or daughter | 433 (39.9) | 292 (40.5) | |
| Living in an institution for the aged or in hospital | 121 (11.1) | 84 (11.7) | |
| Main drug management | |||
| Self | 783 (72.1) | 547 (74.2) | 0.487 |
| Partner | 65 (6.0) | 31 (4.2) | |
| Son or daughter | 110 (10.1) | 77 (10.5) | |
| Caretaker | 17 (1.6) | 9 (1.2) | |
| Institution for aged or hospital | 111 (10.2) | 73 (9.9) | |
| Not the patients themselves | 303 (27.9) | 190 (25.8) | 0.316 |
| ADL at discharge | |||
| Independent outdoor walking | 620 (57.1) | 412 (55.9) | <0.001 |
| Independent indoor walking | 247 (22.7) | 223 (30.3) | |
| Indoor walking with assistance | 121 (11.1) | 63 (8.6) | |
| Abasia | 98 (9.0) | 39 (5.3) | |
| Unable to walk independently | 219 (20.2) | 102 (13.8) | <0.001 |
| Long-term care insurance certification | 419 (38.6) | 279 (37.8) | 0.708 |
| Support required 1 (e.g., standing on one foot) | 53 (4.9) | 44 (5.9) | |
| Support required 2 (e.g., walking; possibly improved) | 69 (6.4) | 46 (6.2) | |
| Care level 1 (e.g., walking; maintained) | 56 (5.2) | 63 (8.6) | |
| Care level 2 (e.g., moving, wear/pull off trousers) | 115 (10.6) | 50 (6.8) | |
| Care level 3 (e.g., washing face, oral care) | 64 (5.9) | 33 (4.5) | |
| Care level 4 (e.g., dietary intake, communication) | 41 (3.8) | 25 (3.4) | |
| Care level 5 (e.g., swallowing, memorization) | 21 (1.9) | 9 (1.2) | |
| Unknown | 0 (0.0) | 9 (1.2) | |
| Home-visit medical service | 66 (6.1) | 33 (4.5) | 0.138 |
| Day service or day care | 164 (15.1) | 123 (16.7) | 0.349 |
Unless indicated otherwise, continuous data are presented as the mean±SD or median [interquartile range]; categorical data are presented as n (%). ACEI, angiotensin-converting enzyme inhibitor; ADL, activities of daily living; ARB, angiotensin receptor blocker; BMI, body mass index; BNP, B-type natriuretic peptide; BUN, blood urea nitrogen; COPD, chronic obstructive pulmonary disease; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HF, heart failure; JDS, Japan Diabetes Society; LV, left ventricular; LVEF, left ventricular ejection fraction; RAS, renin-angiotensin system; SBP, systolic blood pressure.
The observed and predicted mortality rates were described by Kaplan-Meier curves according to risk group stratification of patients in the KICKOFF registry and Kochi YOSACOI study (Figure 3). The Kaplan-Meier curves in both cohorts clearly demonstrated the score’s predictive ability. The C-statistic of the prediction model was 0.799 and 0.762 in the KICKOFF registry and Kochi YOSACOI study, respectively.
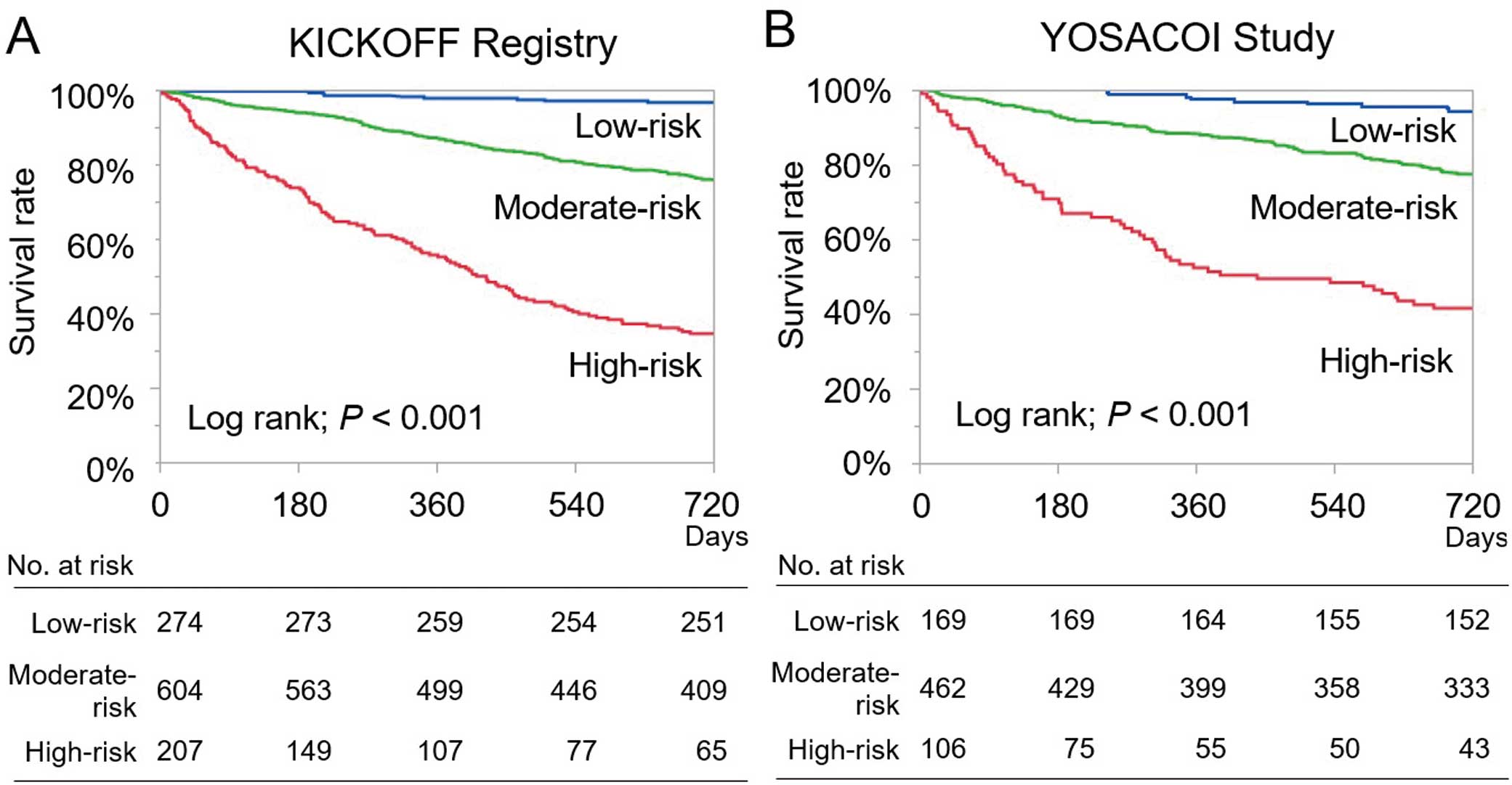
Kaplan-Meier curves for mortality in the low-, moderate-, and high-risk groups according to the KICKOFF score for patients in the (A) KICKOFF (Kitakawachi Clinical Background and Outcome of Heart Failure) and (B) YOSACOI (Kochi Registry of Subjects with Acute Decompensated Heart Failure) registries.
The mean predicted probabilities of 2-year mortality in each of the 3 risk groups were comparable between the KICKOFF registry and Kochi YOSACOI study: low risk, 4.4% (95% CI 0.0–9.1%) vs. 5.3% (95% CI 0.0–11.3%), respectively; moderate risk, 25.3% (95% CI 22.1–28.5%) vs. 22.3% (95% CI 18.7–25.9%), respectively; and high risk, 68.1% (95% CI 62.7–73.5%) vs. 58.5% (95% CI 51.0–66.0%), respectively (Figure 4). Those trends were demonstrated not only for cardiovascular deaths, but also non-cardiovascular deaths (Supplementary Table 3).

Mortality at 6 months, 1 year and 2 years among patients in the KICKOFF (Kitakawachi Clinical Background and Outcome of Heart Failure) and YOSACOI (Kochi Registry of Subjects with Acute Decompensated Heart Failure) registries according to risk stratification using the KICKOFF score.
In 2 prospective registries in Japan, the KICKOFF risk score accurately predicted the incidence of all-cause death after discharge among patients with AHF, and was thus validated externally. Using the KICKOFF score, adverse events in the high-, moderate-, and low-risk groups in different geographic areas of Japan could be predicted.
External validation is a preliminary step in the implementation of newly developed models, and the general procedure should be performed in a separate patient population to that used for internal development and validation.16 The KICKOFF registry was registered in the north of Kitakawachi and Yawata, which are typical suburban communities in Osaka, Japan. Meanwhile, the Kochi YOSACOI study was registered in Kochi Prefecture, which is a typical rural community in Japan. Using the same inclusion criteria for this study, patient characteristics were found to differ between the 2 cohorts in mean age, the proportion of those with previous HF, and comorbidities. The social background also differed: the proportion of patients living alone was higher and walking ability at discharge was worse for those in the KICKOFF registry than in the Kochi YOSACOI study. Despite these differences in background between the 2 cohorts, the Kaplan-Meier curves show good risk stratification into the 3 risk groups for the 2 cohorts. The KICKOFF score, which includes physical and social factors, has an acceptable number of variables and comprises information collected simply at discharge. This study revealed that assessing the physical activity and social background of patients is critical to preventing adverse events for AHF.
In the present study we evaluated the cumulative incidence of all-cause death at 6 months and 1 and 2 years after discharge. In line with the previously reported risk scores, the KICKOFF score has revealed both short- and long-term prediction of adverse events.17,18 The cumulative incidence of all-cause death was also comparable between risk groups in the KICKOFF registry and Kochi YOSACOI study at 6 months (low risk, 0.7% vs. 0.0%; moderate risk, 7.3% vs. 6.9%; high risk, 33.8% vs. 29.3%) and 1 year (low risk, 2.2% vs. 2.4%; moderate risk, 16.0% vs. 11.7%; high risk, 51.7% vs. 47.2% at 1 year). Among patients in the high-risk group, one-third and half the patients had adverse events at 6 months and 1 year, respectively. Due to the high likelihood of death within 1 year, the KICKOFF score may assist physicians with decision making regarding advance care planning (ACP)19 and to choose palliative care for the management of patients with AHF at discharge. Most physicians, medical staff, patients, and their families have more time and chances to discuss ACP during hospitalization than during outpatient care. We should know the prognosis of AHF in each risk group and provide proper perspectives regarding ACP and palliative care to patients with AHF. The KICKOFF score may help physicians optimize available social resources and to implement cardiac rehabilitation programs, allowing patients to be offered solutions with potential benefits in terms of quality of life and prognostic expectations. We believe that the KICKOFF score is well suited for use to provide better medical services in the present clinical setting outside Osaka and Kochi Prefecture in Japan, although further external validation is needed.
This study has several limitations. First, the diagnosis of AHF was left to the judgment of physicians using the Framingham criteria; therefore, there may be selection or referral bias. Second, the model may increase the risk of overfitting, especially in the case of high-risk scores, which is a modeling error that fits the statistical model with too many degrees of freedom in the modeling process. Third, we did not have detailed information to evaluate patients’ ADL using quantitative indicators, such as the Barthel index or the Functional Independence Measure score. Fourth, social and physical status changes over time. Unfortunately, data regarding any changes in the status of patients in these registries were not available. However, the KICKOFF risk score could be simply calculated again in patients with HF at rehospitalization for HF and revised. Fifth, we only had BNP data and no data on N-terminal pro BNP (NT-proBNP). Some studies have developed a formula to convert between BNP and NT-proBNP.20,21 However, the formulas are still unclear. Finally, the utility of this prediction model in other regions of Japan and under present clinical settings is unclear. In the present study, we performed an external validation model only in Kochi Prefecture before 2020.22 Therefore, we expect to conduct evaluations of score fitting in patients with AHF in different regions or countries and in present clinical settings in the future.
The KICKOFF score, a unique prediction score, may be applicable in different geographic areas of Japan. This score may be useful in predicting long- and short-term mortality in patients with AHF, providing appropriate guidance for decision making in HF care.
The authors extend their sincere appreciation to all the institutions participating in the registries and the clinical research coordinators. The authors also thank all the physicians and medical staff who made this study possible.
This research was supported by a Kochi Prefecture sponsorship project, as well as by research funding from Nakajima Steel Pipe Company Limited.
H.K. is a member of Circulation Journal’s Editorial Team. All remaining authors have no relationships relevant to the contents of this paper to disclose.
The study protocol followed the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Ethics Committee of Hirakata Kohsai Hospital (Osaka, Japan; Approval no. 2021-012), the Ethics Committee on Medical Research of Kochi Medical School (Kochi, Japan; Approval no. 28-68), and the ethics committees of all participating hospitals.
Please find supplementary file(s);
https://doi.org/10.1253/circj.CJ-22-0652