I. General Remarks for Sleep Disordered Breathing (SDB)
1. Normal Sleep and Sleep Disorders
1.1 Normal Sleep
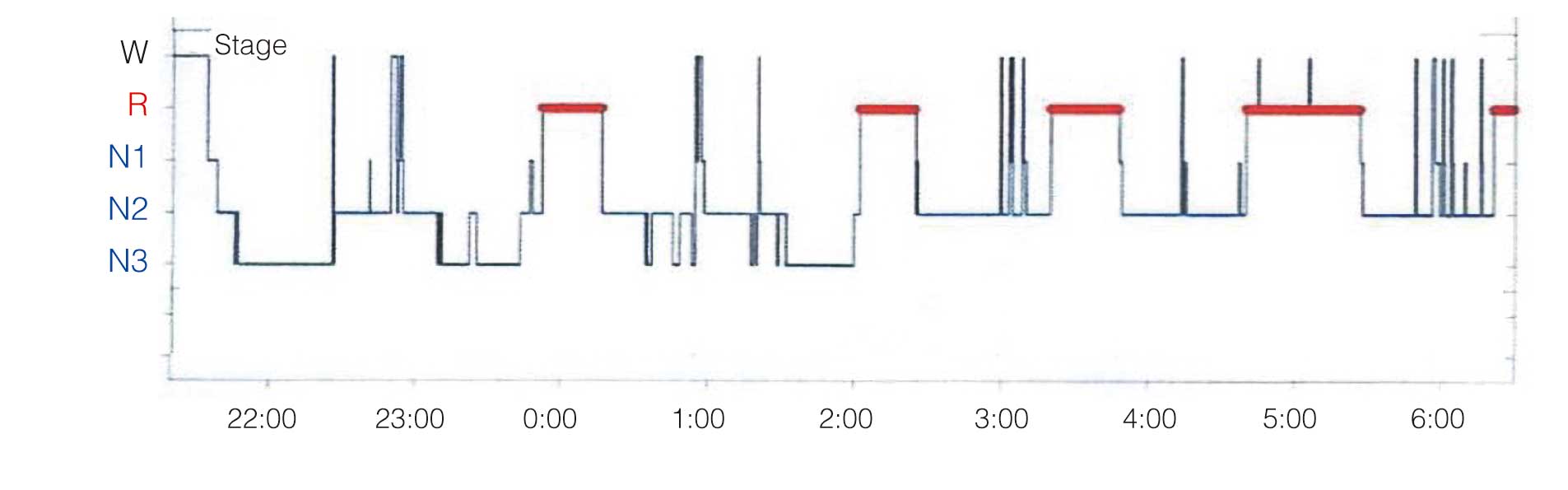
Sleep is essential for maintaining good health and normal body function.1 Both heart rate and blood pressure have a diurnal rhythm that decreases during the night. In the normal 24-h blood pressure pattern, the mean nocturnal blood pressure is ≥10% lower than the mean daytime blood pressure. However, sleep deprivation and sleep disturbance can alter this physiological pattern and induce cardiovascular disease (CVD). Normal human sleep begins with the lightest non-REM sleep (Stage N1), progresses to Stage N2, and then Stage N3 (deep non-REM sleep). Rapid eye movement (REM) sleep (Stage R) appears 80–100 min after sleep onset and alternates with non-REM sleep in a 90-min cycle (Figure 1).2 Normally, deep sleep (Stage N3) is concentrated in the first half of sleep, and the proportion of REM sleep increases in the second half of sleep. Deep sleep decreases with age, and wake time after sleep onset and light sleep increase. On the other hand, REM sleep does not decrease substantially with age and remains at 20–25%.3
1.2 Characteristics and Determination of Sleep Stages
The Scoring Manual published by the American Academy of Sleep Medicine (AASM),5 classifies non-REM sleep into light sleep (Stage N1, Stage N2) and deep sleep (Stage N3), and currently the AASM criteria5 are recommended for determining sleep stages.
1.3 Association of Sleep Disorders With CVD
In the International Classification of Sleep Disorders 3rd edn (ICSD-3), sleep disorders are broadly classified into 7 categories (Table 3).3 Restless legs syndrome (RLS), a group of sleep-related movement disorders, is relatively common in patients with coronary artery disease (CAD: 8.0%)12 and 14% of patients with heart failure (HF) also have RLS.13 Periodic limb movement in sleep (PLMS) is also included in the group of sleep-related movement disorders. PLMS increases sympathetic nervous activity, blood pressure and heart rate, suggesting an association with CVD risk.
Table 3.
International Classification of Sleep Disorders
| I. Insomnia |
| II. Sleep Related Breathing Disorders |
| III. Central Disorders of Hypersomnolence |
| IV. Circadian Rhythm Sleep-Wake Disorders |
| V. Parasomnias |
| VI. Sleep Related Movement Disorders |
| VII. Other Sleep Disorder |
(Source: based on American Academy of Sleep Medicine [AASM], 2014.3)
With regard to sleep duration, a meta-analysis reported that both short (<5–6 h) and long (>8–9 h) sleep were significantly associated with CAD risk and death due to CAD, and long sleep was associated with overall CVD risk.14 A prospective study of 380,055 individuals over a mean 11.1-year follow-up, excluding obstructive sleep apnea (OSA), obesity with body mass index (BMI) ≥40 kg/m2
and history of CVD, found that poor sleep quality was significantly associated with overall death, total cardiovascular death and ischemic stroke death.15 During non-REM sleep, vagal activity is increased and sympathetic activity in the heart and peripheral nervous system is decreased to maintain stable blood pressure and heart rate. In REM sleep, on the other hand, autonomic nervous system activity is unstable, resulting in fluctuations in blood pressure and pulse rate, the average values of which are higher than those in non-REM sleep.16 In REM sleep, muscle activity is significantly suppressed and upper airway collapse is more likely to occur than in non-REM sleep, and OSA is often more severe. It has been reported that SDB during REM sleep is associated with hypertension and cardiovascular events.17,18 Central sleep apnea (CSA) is more common in light non-REM sleep (Stages N1 and N2) than in deep non-REM sleep (Stage N3). REM sleep is also a period of blunted chemosensitivity during which CSA, including with Cheyne-Stokes respiration (CSA-CSR), often improves.19
RLS is associated with worse quality of life (QOL) and more severe insomnia in patients with CAD12 and HF.13 Stratified analysis of RLS symptom frequency in a cohort study showed an increased prevalence of CVD when RLS symptoms were present daily/nightly (Wisconsin Sleep Cohort: WSC)20 or >15 days/month (Sleep Heart Health Study [SHHS]).21 PLMS in patients with RLS is associated with increased heart rate (≈10%) and increased systolic (≈22 mmHg) and diastolic blood pressure (≈11 mmHg).22 The incidence of CVD is approximately 1.8-fold higher when the number of PLMS/h is ≥5.23 The prevalence of CVD is also increased when the number of PLMS/h is ≥5. In hospitalized patients with reduced left ventricular ejection fraction (LVEF) after acute decompensated HF, the presence of severe PLMS was significantly associated with increased clinical events, independent of SDB severity.24 Thus, not only SDB, but also RLS, PLMS, insomnia and sleep deprivation are cardiovascular risks, and many reports have shown an association with insomnia and worsened QOL in patients with CVD.25,26
2. Diagnosis
2.1 Diagnostic Criteria
The diagnosis in Japan is made according to the ICSD-3 diagnostic criteria, which have 2 major changes from ICSD-2: (1) diagnosis by out-of-center sleep testing (OCST) and (2) diagnosis of OSA with ≥5 respiratory events, in the presence of complications. It is important to note that the criteria for treatment eligibility differ between the USA and Japan. In the USA, patients are eligible for treatment if they have ≥5 apnea/hypopnea events with symptoms or even without symptoms but with complications. They are also eligible for treatment without conditions if they have ≥15 respiratory events. In Japan, insurance coverage for continuous positive airway pressure (CPAP) therapy requires that patients have at least 20 apnea/hypopnea events on polysomnography (PSG) or 40 apnea/hypopnea events on portable monitor, as well as symptoms.
Severity is commonly classified according to the number of respiratory events, in accordance with AASM guidelines.3
2.2 Sleep Study Monitoring Device / System
2.2.1 Types of Sleep Study Monitoring Device / System
In the USA, sleep-study monitoring devices/systems are classified as types 1–4.4 A Type 1 study in Japan is synonymous with attended PSG, and Type 2 with unattended PSG. In Japan, the term “portable monitor” refers to Types 3 and 4 devices.
a. Portable Monitors
Portable monitors are used as screening tests when OSA is suspected based on subjective symptoms such as snoring and drowsiness, or when SDB is suspected to be associated with CVD. When SDB is very severe and symptoms suggest typical OSA, diagnosis may be made using a portable monitor other than a pulsoximeter. However, it should be noted that because the sleep EEG is not recorded, sleep quality (sleep depth and sleep fragmentation) cannot be determined, whether the patient is actually sleeping or not cannot be strictly determined, and because the test is performed at home and unattended, the recording status cannot be guaranteed.
Although a test using only a pulse oximeter is sometimes included in portable monitors, Japanese insurance covers pulse oximetry but in a different category of the Japanese reimbursement system from that regarding portable monitoring.
i. Sensors
A sensor that records airway sounds (snoring sounds) is required for Japanese insurance coverage. In addition, few of the portable monitors used in Japan are equipped with respiratory movement sensors, but the international standard for home sleep testing is the Type 3 device equipped with a respiratory movement sensor.
ii. Features of Various Portable Monitors
iii. Pulse Oximeter
In the type classification of the AASM, the pulse oximeter alone is used only to measure the frequency of intermittent hypoxia (i.e., the oxygen desaturation index [ODI]), not the frequency of apneas or hypopneas, although it is used in Japan as a screening test. Although it is difficult to rule out all SDBs even with low ODI values, there are Japanese reports in which ODI ≥5 was associated with poor clinical outcomes in patients with CVD.6,7 The test can be used as a simple test for risk stratification and prediction of poor clinical outcomes in the field of cardiovascular medicine.
iv. Type 4
Type 4 is the most common type of portable monitor in Japan. It has an airflow sensor, an airway sound recorder (or a nasal pressure transducer), and a pulse oximeter, but does not have a respiratory movement sensor. The reliability of the results may vary, depending on the type of sensor used for the airflow sensor and the recording conditions. The lack of a respiratory movement sensor makes it impossible to determine whether the respiratory event is obstructive or central. On the other hand, there is a report from Japan in which SDB based on the respiratory event index (REI) by the Type 4 test in patients with CVD was associated with a poor clinical outcome.8 Only risk stratification may be possible with the Type 4 test rather than PSG.
v. Type 3
Compared with Type 4, the Type 3 monitor has a respiratory movement sensor. The OSCT used in the USA refers to Type 3 and unattended PSG (Type 2 test). As with the Type 4 test, the type of sensor used for the airflow sensor and the recording status must be taken into account to determine the results. In reports from Japan, an association was found between SDB determined based on REI ≥10 by Type 3 and poor clinical outcome in patients with CVD.9,10 Only risk stratification may be possible using REI by Type 3 rather than the apnea–hypopnea index (AHI) by PSG.
vi. Device Using Peripheral Artery Tonometry (PAT)
Respiratory events can be determined by combining the peripheral arterial wave detected by PAT and a pulse oximeter. The number of respiratory events calculated using PAT divided by the estimated sleep duration is reported as the pAHI, which is used as an index to replace the REI of the portable monitor and the AHI of PSG. It is also possible to estimate the percentage of deep sleep and REM sleep from changes in vascular tone and pulse rate using the PAT.
Now that a device that can distinguish between CSA and OSA and evaluate respiratory events, and calculate the pAHI is available, it may be positioned closer to Type 3. In patients with CVD (including HF and atrial fibrillation [AF]) who are taking β-blockers or vasodilators, the correlation and agreement between the pAHI and AHI obtained from PSG are still high.11,12 On the other hand, it has been reported that a difference is more likely to occur in patients with increased arterial stiffness.13 Therefore, it is necessary to be careful.
b. PSG
PSG is used not only for the diagnosis of SDB, but also for the diagnosis of other sleep disorders. PSG is often performed when there are abnormal findings on a portable monitor or Holter ECG, when SDB is suspected, and when SDB is thought to be highly likely to require treatment.
There are 2 types of PSG: attended (Type 1) and unattended (Type 2). In Type 1, interventions can be made during the examination to address poor sensor wear, whereas in Type 2 the only way to interpret the results is to make a comprehensive judgment based on post-examination analysis.
PSG may be performed with positive airway pressure devices or under oxygenation to determine pressure settings and oxygen flows, known as titration studies. In such cases, PSG is usually attended by a laboratory technician, who adjusts the optimal pressure and oxygen flows while observing the occurrence of respiratory events.
2.2.2 Equipment Selection Guidelines
According to the requirements of the Japanese reimbursement system, a portable monitor is to be “used for the diagnosis of sleep apnea syndrome in patients with a strong suspicion of SDB”. However, as emphasized in a statement issued by the Japanese Sleep Society that was based on the history of the adoption of OCST in the USA, PSG is necessary for diagnosis except in very severe typical cases, and the portable monitor is positioned as a screening device.
It is also common to see the values of automatic analysis used as they are in the analysis of portable monitors, but the validity of automatic analysis has not yet been established, so care must be taken in interpreting the results. When diagnosing only with a portable monitor, use a Type 3 device instead of a Type 4 device as often as possible, and make judgments while checking the actual waveform, which will lead to more accurate diagnosis.
Because SDB associated with CVD is often combined with CSA, it is difficult to evaluate SDB by portable monitoring alone, and PSG is recommended. In the case of OSA diagnosed by PSG, reevaluation after the introduction of treatment may be done with a portable monitor, but it is appropriate if the initial PSG is not remarkable for the presence of other sleep disorders or CSA.39 Recommendations and levels of evidence for testing for the diagnosis and treatment of SDB are listed in Table 4.
Table 4.
Recommendations and Levels of Evidence for Tests for Diagnosis and Treatment of SDB in CVD
| |
COR |
LOE |
| PSG is recommended to diagnose SDB, and to assess the efficacy of treatment in patients with CVD |
I |
A |
Portable monitors (except pulse oximeter alone) should be considered to assess the
efficacy of treatment for SDB which has been diagnosed by PSG |
IIa |
C |
Portable monitors (except pulse oximetrr alone) should be considered as a screening
test for SDB |
IIa |
C |
Portable monitors (except pulse oximeter alone) may be considered for the diagnosis
and treatment of SDB in patients with CVD |
IIb |
C |
| Pulse oximetry may be considered as a screening test for SDB |
IIb |
C |
COR, Class of Recommendation; CVD, cardiovascular disease; LOE, Level of Evidence; PSG, polysomnography; SDB, sleep disordered breathing.
2.3 Scoring Rules
2.3.1 AASM Manual
The 2010 edition of this guideline includes rules for scoring based on The AASM Manual for the Scoring of Sleep and Associated Events (AASM Manual 2007) published by the AASM in 2007.9 Subsequent revisions have been made, and as of June 2022, ver. 2.629 is the latest version. There are no scoring rules unique to Japan (Table 5).
Table 5.
Characteristic Findings of Obstructive Hypopnea
| • There is snoring during the event |
• During inspiration, a flattening (flow limitation) of the airflow signal from the nasal pressure sensor or positive airway
pressure device is observed |
| • Paradoxical thoracoabdominal movements during the event that were not seen in pre-event respirations |
| Central hypopnea is defined as an event that meets the criteria for hypopnea in which none of the above findings are present |
3. Epidemiology
3.1 Epidemiology of OSA
Although there are numerous reports on the prevalence of SDB in the general population (Table 6),41–46 many of them do not clearly distinguish between OSA and CSA because many studies used portable monitors in terms of suitability for cohort studies. In the general population, however, the prevalence of CSA is considered to be much lower than that of OSA, and SDB prevalence approximates OSA prevalence. The prevalence of OSA in Japan is estimated to be ≈22 million (32.7%) for an AHI ≥5 and ≈9.4 million (14.0%) for an AHI ≥15, using an original algorithm based on the AASM 2012 criteria.44 The prevalence rate of OSA in Japan was reported in 1995. In an epidemiologic survey of 910 general residents in Japan, 3.3% of men and 0.5% of women (1.7% overall)47,48 had an AHI ≥10.
Table 6.
Prevalence of SDB
| |
Valuation index |
Male prevalence (%) |
Female prevalence (%) |
| WSC41 |
AHI ≥5 |
24 |
9 |
| WSC42 (1988–1994) |
AHI ≥5 |
26.4 |
13.2 |
| WSC42 (2007–2010) |
AHI ≥5 |
33.9 |
17.4 |
| HypnoLaus43 |
AHI ≥5 |
83.8 |
60.8 |
| AHI ≥15 |
49.7 |
23.4 |
| CIRCS45 |
3%ODI ≥5 |
39.7 |
18.6 |
| 3%ODI ≥15 |
8.9 |
2.2 |
| Nagahama46 |
3%ODI ≥5 |
81 |
Premenopausal |
Postmenopausal |
| 25.8 |
60.2 |
| 3%ODI ≥15 |
23.7 |
1.5 |
9.5 |
| 3%ODI ≥30 |
4.4 |
0 |
1.2 |
AHI, apnea–hypopnea index; CIRCS, Circulatory Risk in Communities Study; ODI, oxygen desaturation index; SDB, sleep disordered breathing; WSC, Wisconsin Sleep Cohort.
3.1.1 CVD and OSA (Figure 2)
The association between OSA and CVD is strong, not only because of the high frequency of both complications, but also the modification of the pathogenesis of CVD by OSA.
a. Hypertension
Approximately 50% of patients with OSA (AHI ≥5) have hypertension, and conversely, 59% (AHI ≥5) of hypertensive patients have OSA.50,51 In the baseline data of the SHHS, when AHI ≥30 and AHI <15 were compared after adjusting for body size (BMI, neck circumference, waist-to-hip ratio), alcohol intake, smoking, etc., the incidence of hypertension was significantly higher in the AHI ≥30 group.54 The prevalence of OSA in patients with resistant hypertension is reported to be even higher: 83% (AHI ≥10)55 and 64% (AHI ≥15).56
b. Diabetes Mellitus
A meta-analysis showed that OSA was a risk factor for developing type 2 diabetes (relative risk: 1.4).57 The prevalence of type 2 diabetes in patients with OSA (AHI or 4%ODI ≥5) has been reported to be 15–30%.58–60 The prevalence of type 2 diabetes increases with the severity of OSA (odds ratio: mild 1.3, moderate 1.7, severe 1.9) in comparison with people free of OSA.60 The prevalence of OSA in patients with type 2 diabetes has been reported to be 86% (AHI ≥5) and >30% (AHI ≥15).57,61 On the other hand, the prevalence of OSA (AHI ≥5) in patients with type 1 diabetes was reported to be 46%.62
c. Chronic Kidney Disease (CKD)
The prevalence of SDB in patients with CKD has been reported to be 65% (AHI ≥5),63 41–50% (AHI ≥15)63–65 and 22.5% (AHI ≥30).66 The prevalence of OSA in CKD patients was 32% (mild), 25% (moderate), and 8% (severe), according to the severity of OSA, in a Japanese cross-sectional study.63
d. Ischemic Heart Disease
According to the SHHS, severe OSA (AHI ≥30) increased the risk of developing CAD in men aged 40–70 years by 1.7-fold that in patients with AHI <5.67 OSA (AHI ≥15) was found in 49.6% of patients with acute coronary syndrome (ACS) (2,551 patients) and 45.3% of patients after percutaneous coronary intervention (PCI) (1,311 patients).68,69 A meta-analysis showed that the prevalence of SDB in patients with ACS is 69% (AHI >5), 43% (AHI >15), and 25% (AHI >30).70 The prevalence of ischemic heart disease in patients with OSA is higher in men than in women.71
e. HF
The prevalence of SDB in patients with chronic HF was reported to be 76% (AHI ≥5),72 71% (AHI ≥10),73 47% (AHI ≥15)74 in patients with HF with reduced ejection fraction (HFrEF) and 69.3% (AHI ≥5),75 55% (AHI ≥10),76 and 31.8% (AHI ≥15)77 in patients with HF with preserved ejection fraction (HFpEF). The prevalence of SDB in patients with acute decompensated HF is reported to be even higher: 92%, and 97% (AHI ≥5), and 69%, 76%, and 82% (AHI ≥15).78–80
f. Arrhythmia and Sudden Death
In the SHHS, the prevalence of AF and nonsustained ventricular tachycardia in patients with SDB (AHI ≥30) was 4.8% and 5.3%, respectively.81 When adjusted for age, sex, BMI, and prevalent CAD, the risk of arrhythmia complications in patients with SDB (AHI ≥30) was significantly higher for AF (odds ratio: 4.0), nonsustained ventricular tachycardia (3.4), and ventricular extrasystole (1.7) compared with those without SDB. On the other hand, the prevalence of SDB in patients with AF was also high: 74% (43% OSA, 31% CSA-CSR, AHI >5) or 81.4% (AHI ≥5).82,83 Nocturnal arrhythmias are present in up to 50% of patients with OSA.84 The most common arrhythmias during sleep include AF, nonsustained ventricular tachycardia, sinus arrest, second-degree atrioventricular block, and frequent premature ventricular contractions.84 ECG analysis during PSG showed that patients with severe SDB (AHI ≥30) had a 2–4-fold higher risk of nocturnal complex arrhythmias compared with controls (AHI <5).81 In a report of 112 patients who had undergone PSG and died suddenly from cardiogenic causes, from midnight to 6 a.m., 46% of patients with OSA (AHI ≥5) had sudden death, as compared with 21% of patients without OSA, suggesting that the presence of OSA is associated with a higher risk of sudden cardiac death during sleep (relative risk: 2.6-fold).85
g. Cerebrovascular Disease
According to an observational study of patients aged ≥50 years with an average follow-up of 3.4 years, the risk of stroke and death in OSA patients with AHI ≥5 was significantly higher (hazard ratio: 2.0) than in controls (AHI <5).86 In a meta-analysis of the studies reporting the SDB prevalence following stroke or transient ischemic attack, SDB prevalence was 66.8% (AHI ≥5), 50.3% (AHI ≥15), and 31.6% (AHI ≥30), respectively, within 1 month after stroke or transient ischemic attack.87 Overall, the prevalence of SDB was reported to be 71% (AHI >5), 40% (AHI >20), and 30% (AHI >30), respectively.88
h. Aortic Disease
Regarding the frequency of SDB in thoracic aortic aneurysms, in an observational study of 208 patients, the prevalence of AHI ≥5 was 63%.89 For abdominal aortic aneurysms, the prevalence of OSA was reported to be 63.4% (AHI >5), 41.5% (AHI >10), 27.6% (AHI >15), and 14.6% (AHI >30) in an observational study of 123 patients.90 In Marfan syndrome, the pharyngeal cavity is more likely to collapse due to craniofacial skeletal abnormalities,91,92 and the frequency of OSA is reported to be 32.8–64%.93,94 In a meta-analysis of the risk of aortic dissection (AD) in OSA (5 observational studies, 56,291 subjects analyzed), the incidence of OSA in AD was 45%, and the odds ratio of AD in OSA compared with non-OSA was 1.60.100 The odds ratio for AD was 4.43 in patients with moderate to severe OSA (AHI ≥15).100
i. Pulmonary Hypertension (PH)
OSA and PH frequently coexist, and the involvement of OSA in the pathogenesis of PH has been implicated. Among 220 OSA patients with AHI >20, 17% patients had PH (mean pulmonary artery pressure ≥20 mmHg).101 All 8 (21.6%) OSA patients with PH had severe OSA (AHI ≥30) (33.3% of the severe group).102 On the other hand, the prevalence of OSA (AHI ≥5) in patients with PH was 89%: 22% (AHI 5–14), 39% (AHI 15–29), and 28% (AHI ≥30).103
3.2 Epidemiology of CSA
In a community cohort of 741 men in Pennsylvania, the prevalence of CSA (AHI ≥10) was noted to be 0.4% overall, but 1.1% in those aged ≥65 years, with an age-specific prevalence of a central apnea index (CAI) ≥2.5: 0% (age 20–44 years), 1.7% (45–64 years), and 12.1% (65–100 years).104 In an analysis of the SHHS in subjects aged ≥40 years, the prevalence of CSA (AHI ≥5) was 0.9% and the frequency of CSA-CSR was 0.4%.105 In a cohort of 2,911 men aged ≥65 years, the prevalence of CSA (CAI ≥5) was reported to be 7.5%.106 CSA has been associated with HF,72,107–113 cerebrovascular disease,88,114–116 AF,117,118 CKD119,120 and medications.121
3.2.1 CVD and CSA
a. HF
The prevalence of CSA (AHI ≥15) in HF patients has been reported to be 21–40%.72,74,108 In patients with left ventricular systolic dysfunction, CSA-CSR is one of the major prognostic factors and increases the risk of death by 2.1-fold.110 Patients with HF with CSA-CSR have a significantly higher mortality and heart transplantation rates than patients with HF without CSA-CSR (relative risk: 2.5).111 In a study of 60 patients with severe HF, the mortality rate was 3.8-fold higher in patients with CSR during ≥10% of the daytime compared with patients with CSR during <10% of the daytime, indicating that CSR during ≥10% of the daytime is an independent predictor of death.112 Risk factors for CSA in HF patients are reported to be male sex, AF, age ≥60 years, hypocapnia (≤38 mmHg), and diuretic (loop, thiazide) use.74,109
b. Cerebrovascular Disease
The prevalence of CSA (CAI ≥5) in stroke patients is reported to be 1.4%,114 and that of CSA (AHI ≥5) in stroke and transient ischemic attack patients is 12%.88 The prevalence of CSR in patients with lacunar stroke has been reported to be 20.6%,115 and 26.1% in patients with stroke or transient ischemic attack.116 The CAI in stroke and transient ischemic attack patients decreases from 6.2 in the acute phase (48–72 h after onset) to 3.3 in the stable phase (3 months after onset), while the obstructive apnea index (OAI) remains unchanged.116
c. AF
The prevalence of AF in patients with CSA (AHI ≥10) is 27%,117 and the complication risk of AF is higher in patients with CSA (CAI ≥5) and CSA-CSR (odds ratio, CSA: 2.6, CSA-CSR: 2.3).118
d. CKD
Among CKD patients, CSA is found in patients with endstage renal failure, and a study of chronic renal failure patients on hemodialysis reported a prevalence of CSA (AHI ≥15) of 57% and CSR of 12%.64,119 A systematic review identified 30 CSA patients of a total 313 CKD patients and indicated that the aggregate point prevalence of CSA was 9.6%.120
4. Pathophysiology
4.1 Pathogenesis of OSA
4.1.1 Mechanisms
a. Anatomical Abnormalities of the Upper Airway
In general, patients with OSA have anatomically smaller upper airways than unaffected subjects due to the amount of soft tissue around the upper airway, maxillofacial morphology, tongue volume, and tonsillar hypertrophy.122–124 Fat deposition in the peripharynx due to obesity is the most important factor affecting the size of the upper airway.
The prevalence of OSA is not significantly different between Asian and Western countries,41,125 even though the percentage of obese population is larger and the degree of obesity is more severe in Western countries than in Asia. Racial differences in anatomical upper airway morphology can be explained using the anatomical balance model (Figure 3).126,127 The size of the upper airway is determined by the balance between the soft tissue and bony enclosure comprising maxilla, mandible, and cervical vertebrae. In Westerners, the diameter of the pharyngeal cavity does not decrease until the subject becomes quite obese (Figure 3B) because of the large size of the airway. In East Asians, cephalometrically, anatomical skeletal factors such as shortening of the anterior cranial base length, maxillary length, and mandible length have been observed.128 In other words, the cavity surrounded by bony structures is relatively small in East Asians, and the pharyngeal cavity diameter shrinks with a small increase of soft tissue (Figure 3C).

b. Neurogenic Dysregulation of the Upper Airway (Figure 4)
The genioglossus, which plays an important role among the upper airway muscles, actively keeps the upper airway open regardless of the respiratory phase (tonic activity) and further increases its activity to prevent collapse of the upper airway against negative pressure in the upper airway produced by the diaphragm during inspiration (phasic activity). In addition, the genioglossus muscle receives input not only from the respiratory rhythm formation area, central chemoreceptor area, and upper airway negative pressure receptors, but also from the brainstem arousal–sleep regulatory center.131 This disruption of the compensatory mechanism of the upper airway opening muscles, combined with anatomic abnormalities of the upper airway, is thought to form the basic pathophysiology of OSA.131,133
c. Instability of the Respiratory Control System
OSA can also cause instability of the respiratory control system (respiratory instability).134,135 Ventilatory drive from the medullary respiratory center also activates the hypoglossal nerve, which innervates the genioglossus muscle, and instability of the medullary respiratory center affects upper airway resistance.
d. Arousal Threshold
Transient arousal on EEG has been considered a necessary defense response to terminate obstructive apnea during sleep. In fact, it is estimated that 10–25% of obstructive apneas are relieved without an arousal response. The increase in ventilation volume induced by the arousal response leads to a decrease in the concentration of carbon dioxide in arterial blood, which induces respiratory instability, and it is possible that the arousal response is a factor in repeated obstructive apneas via respiratory instability.137
4.1.2 Clinical Manifestations
The typical symptoms of OSA are very loud snoring and respiratory arrest, which are often reported by the bed partner. Among middle-aged patients with an AHI ≥5, 22.6% of males and 15.5% of females complain of excessive daytime sleepiness at least twice each week.41 The Epworth sleepiness scale (ESS) is used as a subjective measure of sleepiness, with a score ≥11 indicating abnormal sleepiness and ≥16 indicating severe sleepiness. The ESS has been partially modified for the Japanese, and is available on the Internet.7 It has been reported that patients with HF are objectively more drowsy, although they are less likely to feel drowsy,138 and that patients with AF also show a very weak correlation between the ESS, which indicates drowsiness, and the degree of OSA.139 Therefore, when examining patients with CVD, it is necessary to pay attention to the presence or absence of obesity, facial morphology, and pharyngeal morphology abnormalities, and to actively look for OSA when these abnormalities are suspected. On the other hand, it has been reported that the possibility of developing HF is higher in the group of patients with OSA who complain of severe drowsiness than in other groups, so caution should be taken with such patients.140 In Japan, symptoms including excessive daytime somnolence, lack of sound sleep, general malaise, nocturia, and nocturnal dyspnea have been reported in patients with an AHI ≥5 (Table 7).141 Among these symptoms, nocturnal choking and wheezing are reported to be the most common predictors of OSA with an AHI of 10 or 15.142 Gastroesophageal reflux disease is often associated with OSA, although the detailed mechanism is not yet clear.143 Some reports suggest that CPAP therapy improves the symptoms of gastroesophageal reflex.144 Patients with OSA also breathe through the mouth at night, which causes dryness of the oral cavity and pharynx, resulting in recurrent pharyngitis and tonsillitis, which can often be alleviated by CPAP and other treatments. In a report from Japan, 93% of patients with OSA had snoring, while 33% of patients who snored ≥3 times/week had an AHI of 5–15, and 28% had SDB with an AHI ≥15.141 In addition, many patients have been reported by their bed partner to have apnea or abnormal body movements during sleep.141
Table 7.
Frequency of Appearance of Subjective Symptoms and Other Signs in OSA* (n=195)
| Symptoms/signs |
Frequency (%) |
| Snoring during sleep |
93 |
| Sleep apnea noted |
92 |
| Excessive daytime sleepiness |
83 |
| Abnormal body movements during sleep |
54 |
| General malaise |
51 |
| Night sweats |
51 |
| Lack of sound sleep upon awakening |
51 |
| Urination more than twice during the night |
40 |
| Arousals with choking sensation during sleep |
38 |
| ≥3 awakenings during the night |
35 |
| Headache upon awakening |
35 |
| Decreased ability to concentrate |
28 |
| Insomnia |
19 |
*Apnea–hypopnea index (AHI) ≥5 and subjective symptoms such as habitual snoring, drowsiness, etc. OSA, obstructive sleep apnea. (Adapted from Hiroki Sakakibara et al., 2000.141)
4.1.3 Hemodynamic Effects
a. Effect of Negative Pressure in the Thoracic Cavity
In OSA, because inspiration occurs with the upper airway closed, a negative pressure of −40 to −50 cmH2O on average, exceeding −100 cmH2O in some cases, is repeatedly generated in the thoracic cavity throughout the night.145–147 This has the same effect as intermittent external suctioning of the entire heart (increase in transmural pressure), which directly adversely affects cardiac contraction by increasing afterload due to the force applied to the left ventricular wall during left ventricular contraction.148 On the other hand, when the intrathoracic cavity becomes negatively pressurized, venous return increases rapidly and the volume of the right ventricular system increases rapidly. As a result, the ventricular septum is displaced toward the left ventricle, preventing left ventricular dilation and resulting in a decrease in stroke volume145 (Figure 5). It has been reported that such inspiration under airway obstruction decreases cardiac output by an average of 15% and increases the pulmonary artery wedge pressure by an average of 8 mmHg in severe OSA.149,150

b. Hypoxemia
Hypoxia increases sympathetic nerve activity, which in turn increases heart rate and blood pressure, and concurrent hypercapnia has been shown to further increase heart rate and blood pressure.158 The rapid increase in sympathetic nerve activity associated with nocturnal OSA causes hypertension due to peripheral vasoconstriction, which, together with the increase in transmural pressure due to negative intrathoracic pressure, further increases cardiac afterload.
4.1.4 Sympathetic Nerve Activity and Its Effect on Neurohumoral Factors
a. OSA-Induced Sympathetic Nervous System Hyperactivity
Increased sympathetic nerve activity can be observed in patients with OSA,165,166 not only during sleep apnea, but also during wakefulness without apnea.167
A transient increase in sympathetic activity during apnea is associated with hypoxemia, hypercapnia, loss of the lung stretch reflex, and mid-onset arousal. Sympathetic hyperactivity persists even when apnea is not observed during the day. However, this phenomenon cannot be explained by the mechanism of sympathetic hyperactivity during sleep. It is closely related to exposure to intermittent hypoxia over several hours during the night and consecutive days and to the frequency of arousal responses.174–176
i. Intermittent Hypoxia
In an experimental system that mimics intermittent hypoxia during sleep induced by OSA, increased sympathetic nerve activity, increased blood pressure, and decreased endothelial function were observed after exposure to intermittent hypoxia for 4 weeks at 8 h/day.174 Furthermore, because the blood pressure–sympathetic nerve activity relationship shifted to the right, intermittent hypoxia is thought to cause a baroreflex resetting.175
ii. Arousal Response
In patients with OSA, the arousal index positively correlates with resting sympathetic nerve activity recorded during the day.176
b. Effects of OSA on the Renin–Angiotensin–Aldosterone (RAA) and Natriuretic Peptide Systems
Animal studies have shown that intermittent hypoxia increases plasma renin activity via an increase in renal sympathetic nerve activity.177 In contrast, plasma angiotensin II is elevated in patients with OSA, and the plasma aldosterone concentration is increased in the group with hypertension, but plasma renin activity results are not consistent.178 Atrial natriuretic peptide has also been reported to be elevated in patients with OSA and decreased by CPAP treatment.179,180
4.1.5 Effects on Inflammation, Oxidative Stress, and NO (Vascular Endothelium, Atherosclerosis)
Intermittent hypoxemia and reoxygenation due to OSA cause inflammation and oxidative stress in the body, resulting in vascular endothelial dysfunction and the development of atherosclerosis.182 Inflammatory marker levels in blood are reported to be higher in patients with OSA than in healthy subjects, and the levels become higher as the severity increases.184 In OSA patients, markers of oxidative stress including thioredoxin,192 malondialdehyde,193 and reduced iron194 have been reported to correlate with decreased AHI and oxygen saturation. In patients with OSA, endothelium-dependent vasodilation, evaluated by forearm plethysmography, was impaired compared with healthy subjects.196 Flow-mediated vasodilation and reactive hyperemia have been used to evaluate vascular endothelial function before and after CPAP in patients with OSA, and significant improvements were reported.199,200
4.1.6 Association With Insulin Resistance
An 11-year observational study of nondiabetic men showed that an AHI ≥5 was independently associated with insulin resistance as expressed by HOMA-IR (homeostasis model assessment-insulin resistance).203 Mechanisms by which OSA induces insulin resistance include intermittent hypoxemia,204 restricted sleep duration,205,206 sleep fragmentation,207 increased sympathetic nerve activity and oxidative stress, and systemic inflammation. Prior randomized controlled trials (RCTs) investigating the effects of CPAP on insulin resistance yielded both positive and negative results.212–217 The presence or absence of diabetes, the severity of OSA, duration of diabetes, diabetes medications, and differences in CPAP adherence may have influenced the results of those studies.
4.1.7 Relationship to Thrombosis and Platelet Activity
CVD associated with OSA can result in arterial thrombi, also known as atherosclerotic thrombi. Recently, it has also been reported that OSA is a risk factor for the development of fibrin-rich venous thrombi.221 OSA induces thrombosis through intermittent hypoxemia, increased oxidative stress, sympathetic nerve activity, increased production of inflammatory cytokines, and vascular endothelial damage.222 OSA has also been shown to promote thrombus formation by increasing coagulation factors and platelet activation, enhancing platelet aggregation, and impairing thrombolytic activity.225 In a large cohort study, patients with OSA were reported to have an independent risk of developing deep vein thrombosis that was twice as high after a mean follow-up of 5 years,221 and three times as high after a mean follow-up of 3.6 years226 compared with non-OSA patients.
4.1.8 Obesity Hypoventilation Syndrome (OHS) (Table 8)
Table 8.
Recommendations and Levels of Evidence for Treatment of OHS
| |
COR |
LOE |
| CPAP therapy for stable OHS should be considered |
IIa |
B |
| bi-level PAP may be considered if neither CPAP therapy is effective nor well tolerated |
IIb |
B |
bi-level PAP, bi-level positive airway pressure; COR, Class of Recommendation; CPAP, continuous positive airway pressure; LOE, Level of Evidence; OHS, obesity hypoventilation syndrome.
OHS is a condition characterized by obesity (BMI >30 kg/m2) and alveolar hypoventilation (arterial partial pressure of carbon dioxide [PaCO2] >45 Torr) during the daytime.3,227 Hypoventilation is known to be exacerbated during sleep (especially during REM sleep) and is accompanied by symptoms such as daytime drowsiness and fatigue. The pathogenesis cannot be explained by upper airway obstruction alone, and the mechanical load on breathing associated with obesity, the reduced ventilatory response associated with chronic hypoventilation, and the influence of fluid factors are under investigation.3,227,229 Cardiovascular complications are more frequent than in patients with OSA alone, and untreated OSA is associated with a higher frequency of hospitalization and death.230 Treatment is CPAP via nasal or nasal–mouth mask or bi-level positive airway pressure (bi-level PAP, with or without backup ventilation), together with weight loss.231,232 Because >70% of patients with OHS present with severe OSA, the clinical question of “which should be the first choice, CPAP or bi-level PAP therapy” has been addressed in 4 RCTs,234–237 and a meta-analysis of 3 has been reported.238 The results showed that initial treatment of stable OHS was equally effective in terms of frequency of hospitalization, length of hospitalization, incidence of cardiovascular events, life expectancy, and improvement in sleepiness, blood pressure, and blood gases. Therefore, at present for stable OHS the first line treatment is CPAP, and bi-level PAP should be considered if the therapeutic effect or tolerability of CPAP treatment is insufficient.
4.2 Pathogenesis of CSA
4.2.1 Mechanisms
Although CSA can be caused by cerebrovascular disease, drugs, and high altitude environments, we describe here the mechanism of CSA-CSR caused by HF. The respiratory regulatory system comprises chemical, neural, and behavioral regulatory systems, and their contributions to respiration changes during wakefulness and sleep. During non-REM sleep, respiration is mainly regulated by the chemical regulatory system, so the PaCO2
level plays an important role. In REM sleep, on the other hand, the behavioral regulatory system has a greater influence on respiratory regulation than the chemical regulatory system, and CSA caused by fluctuations in PaCO2
is rarely observed. The neuromodulatory system is a regulatory system in which ventilation stimulates mechanoreceptors in the upper airway, lungs and respiratory muscles to provide the respiratory center with information on ventilation, such as lung distention, via afferent nerve fibers. In HF, the vagal unmyelinated C-fiber endings, described below, are involved in the pathogenesis of CSA-CSR. The PaCO2
level at which apnea occurs is called the PaCO2
apnea threshold, and CSA occurs when PaCO2
falls below the apnea threshold due to transient hyperventilation. These phenomena are explained in Figure 6 using the relationship between alveolar ventilation and PaCO2, which consists of a metabolic hyperbola and a hypercapnic ventilatory response (HCVR) line. Because CO2
production is constant at steady state, the hyperbolic relationship between the alveolar ventilation rate and PaCO2
is the alveolar ventilation equation, where the product of the 2 is constant. Steady-state ventilation is achieved at the point corresponding to the intersection of the metabolic hyperbola and the HCVR line (Figure 6A: point a [normal subjects], point b [HF patients]), and the intersection of the HCVR line and the X axis (i.e., the point at which alveolar ventilation becomes zero. Figure 6A: point c [normal subjects], point d [HF patients]) is the PaCO2
apnea threshold. The difference between the PaCO2
apnea threshold and eupnea PaCO2, the CO2
reserve and HCVR, is an important factor in causing CSA-CSR. In patients with HF, pulmonary congestion stimulates the vagal unmyelinated C-fiber endings (C-fiber endings), so-called J-receptors, in the lung parenchyma near the pulmonary capillaries, resulting in increased ventilation and low PaCO2
(Figure 6A: a→b).240 Because the HCVR has an O2–CO2
interaction, hypoxemia associated with HF further increases chemoreceptor sensitivity, especially the HCVR (Figure 6A: the slope of the line is steeper than that of normal subjects).241 Furthermore, it has been pointed out that increased sympathetic nerve activity in HF patients also increases the HCVR.242 As a result, the PaCO2
apnea threshold is increased in HF patients (Figure 6A: c→d) and the CO2
reserve is decreased. HCVR differs during waking and sleeping and is blunted during sleep (Figure 6B: the slope of the line becomes slower in non-REM sleep). When a HF patient awakens from non-REM sleep, PaCO2
decreases with an increase in alveolar ventilation and shifts from point f to point e in Figure 6B. When the patient re-enters sleep, apnea is present for a short period of time before returning to point f. Such repetitions cause CSA-CSR. The repeated CSA-CSR can also be explained from another point of view. As shown in Figure 7,247 in the case of normal HCVR (Figure 7A) and in the case of elevated HCVR (Figure 7B), a certain decrease in ventilation can cause repeated apneas and hyperventilation, depending on the slope of the HCVR line. Circulation time, which negatively correlates with cardiac output and stroke volume, also plays an important role in the appearance of CSA-CSR in HF patients. It has also been pointed out that fluid stored in the lower extremities of HF patients during the day migrates to the upper body during supine sleep at night, contributing to pulmonary congestion and causing hyperventilation, thereby lowering the PaCO2
and inducing CSA-CSR.249,250 Furthermore, metabolic alkalosis induced by diuretics in HF patients raises the PaCO2
apnea threshold and decreases the CO2
reserve, which is another mechanism for inducing CSA-CSR.

4.2.2 Clinical Manifestations
There are no specific subjective symptoms of patients with CSA, and they are essentially the same as those of patients with OSA. They include daytime sleepiness, insomnia (difficulty falling asleep or staying asleep, frequent awakenings, or nonrestorative sleep), and awakenings due to dyspnea.251 In addition, pure CSA patients do not snore as much as OSA patients. For these reasons, it is difficult to diagnose CSA from subjective symptoms, and an objective test such as PSG is needed. In addition, deep sleep is decreased, the percentage of REM sleep is decreased, and arousal responses are increased in patients with CSA compared with HF patients without CSA.109 Despite this impaired sleep architecture in patients with CSA, the ESS does not change in patients with CSA138 and does not correlate with the AHI.253 Although an association between CSA and nocturnal paroxysmal dyspnea has been reported,254 it is difficult to strictly distinguish between dyspnea derived from HF and symptoms derived from CSA.105 Some patients with CSA have CSR even when awake.112,255 In a study of 574 HF patients, 34% showed CSR in the supine position only and 14% showed CSR in both the upright and supine positions.113 In many cases, CSR is not evident during waking hours, but is clarified by cardiopulmonary stress testing.256
4.2.3 Differences Between CSA and OSA
Compared with HF patients with OSA, HF patients with CSA are more likely to be male, older, have a lower BMI, more frequently have AF and diuretic use, lower arterial carbon dioxide concentrations,74,109 and higher pulmonary artery wedge pressure.257 CSA improves when the pulmonary arterial wedge pressure is reduced by HF treatment.257 Overnight, there is a transition from OSA to CSA in response to increased ventilation and decreased arterial CO2
concentration.258 In addition, when PSG is performed at intervals ≥1 month, there are cases of reciprocal transition between CSA and OSA.259 As with OSA, sympathetic activity is increased in patients with CSA, and patients with HF and CSA have higher levels of norepinephrine in their blood and urine during sleep.260 Therapeutic interventions for CSA may be effective in reducing sympathetic activity261 and ventricular arrhythmias during sleep.262 These findings suggest that CSA increases sympathetic activity during sleep and has a negative effect. The hemodynamic effects of apnea differ between OSA and CSA: the intrathoracic pressure decreases from −50 mmHg to −80 mmHg during apnea in OSA patients, which results in an increase in right ventricular capacitance load due to the increased venous return.145 On the other hand, CSA does not produce the intrathoracic pressure changes seen with OSA, so the effect on cardiac function is different, and changes in cardiac function during the apneic and CSR phases have been verified. The stroke volume decreases during obstructive apneic events, but increases slightly during central events.263 CSR has been hypothesized to be an adaptive response in HF.264
4.2.4 Treatment-Emergent Central Sleep Apnea (TECSA)
a. Definition
TECSA, formerly called complex sleep apnea syndrome, refers to the occurrence of diagnostically problematic central-type breathing events, mainly during CPAP treatment, in patients diagnosed with mainly obstructive-type breathing events (Figure 8). It refers to the occurrence of central respiratory events not only during CPAP titration, but also after otolaryngologic surgical treatment or during oral appliance (OA) therapy. TECSA is defined as a phenomenon occurring during treatment that meets criteria A–C in ICSD-3.3
[A] PSG shows ≥5 obstructive predominant respiratory events (obstructive or mixed apnea/hypopnea or respiratory effort-related arousal) per hour of sleep.
[B] PSG under positive-pressure breathing without backup ventilation shows a significant improvement in obstructive events and the appearance or persistence of central apnea or central hypopnea, and both of the following are met:
(1) Central AHI (CAHI) ≥5 times per hour of sleep
(2) Central apnea and central hypopnea counts >50% of apneas and hypopneas
[C] Central apnea cannot be explained by other CSA disorders (such as CSA-CSR or drug- or substance-induced CSA).
b. Frequency
Initially, TECSA was reported to occur in 15–25% of patients with OSA in studies conducted in other countries, and thought to be a very frequent phenomenon.270–272 Subsequent systematic reviews have reported a range of 2.5–20%.270–280 In 3 studies from Japan, 194 of 4,582 patients (4.2%),281 66 of 1,312 patients (5.0%),277 and 17 of 297 patients (5.7%)279 were reported to have TECSA during CPAP titration.
c. Triggers and Pathogenesis
With regard to the risk of developing TECSA, it has been reported that patients with split night titration,280 higher AHI or arousal index or higher optimal CPAP pressure,272 males with smaller BMI,271,283 or a CAI greater during the entire diagnostic PSG275 or greater during the supine/non-REM sleep phase were significantly more likely to develop TECSA.279 The association between TECSA and CVD has been reported to be high in patients with HF,272 but either no or a weak association has been found in other studies.273–277,279,281 Although the mechanism of TECSA is unknown, judging from the fact that CSA is reduced or eliminated in many patients with TECSA, who are retested several months after initiation of CPAP,276,283,285 it is speculated that a reflexive respiratory suppression in response to rapid lung expansion associated with initiation of CPAP (Hering-Breuer reflex), or elimination of OSA by CPAP in patients with high HCVR and low arousal threshold might induce CSA, resulting in TECSA.286–288
d. Treatment
Because, in many cases, the initial CSA resolves after several months of CPAP alone,276,278,283,289 if CPAP therapy is acceptable, treatment of TECSA should begin after a few months of observation. As for pharmacological therapy, improvement of CSA has been reported with the use of acetazolamide,292 but it is unclear if it can be used to treat patients with TECSA. It has been reported that CSA can be controlled by passing a small amount of CO2
through a positive-pressure breathing circuit to maintain a constant concentration in the circuit, but this requires special facilities and equipment and currently is not recommended as a general treatment.289 For TECSA patients in whom CPAP is ineffective, adaptive servo-ventilation (ASV) has the best outcomes.293–296 However, considering that ASV is extremely expensive and not available under the medical insurance system in Japan, it is currently recommended to use ASV only after first performing CPAP treatment, and after careful consideration of whether residual CSA is unacceptable from the viewpoint of sleep disturbance and hypoxia.
5. Treatment
5.1 Treatment of SDB Complicated by CVD
Patients with SDB associated with CVD require periodic evaluation and appropriate adjustment of treatment to manage the changes in the patient’s general condition and circulatory dynamics that occur with disease progression.
5.2 Treatment of OSA
5.2.1 Weight Loss
A prospective observational study of the Wisconsin Sleep Cohort in the USA reported a 32% increase in the AHI with a 10% increase in body weight over a 4-year period, and conversely, a 26% decrease in the AHI with a 10% decrease in body weight.298 However, there are no data showing such a relationship in Japanese patients who have different body sizes and maxillofacial morphology. Meta-analyses have consistently shown that the AHI improves with weight loss.299–304 Therefore, weight loss is recommended for obese patients with OSA.305,306 Lifestyle interventions include programs that include diet therapy, increased physical activity, aerobic exercise, cognitive behavioral therapy, and sleep hygiene advice.307 A meta-analysis that did not include Japanese data on the effects of lifestyle interventions for mild to moderate OSA showed that a 14-kg weight loss was associated with a 16% reduction in the AHI and a 14% increase in the minimum SpO2.302 However, it is difficult to achieve weight loss >10% by lifestyle intervention alone, and it is unlikely to be effective enough to improve the AHI to the target level of treatment.308 Therefore, weight loss therapy is not a stand-alone treatment, but is recommended in combination with other treatments such as CPAP and OA.215
Bariatric surgery is an effective treatment for severe obesity (BMI >35), resulting in sustained weight loss and reduction of obesity-related comorbidities and mortality.309 The bariatric surgery performed in Japan is laparoscopic sleeve gastrectomy, which is covered by health insurance (Tables 9,10).310,311 An RCT comparing bariatric surgery and lifestyle intervention showed that bariatric surgery significantly reduced weight and improved the AHI more than the lifestyle intervention. A meta-analysis also reported that bariatric surgery reduced both BMI and AHI, but many patients remain obese after surgery, suggesting long-term monitoring for residual OSA as well as appropriate treatment, taking into account symptoms and comorbidities, can be performed.316–319 Because many Japanese patients with OSA are less obese than patients in other countries, the results of these studies should be applied with caution to Japanese patients with OSA.
Table 9.
Bariatric Surgery Indication Criteria
| Covered by insurance (laparoscopic sleeve gastrectomy, revised 2022) |
| Obese patients who do not respond adequately to medical therapy for >6 months |
| • BMI ≥35 and ≥1 of the following complications: diabetes mellitus, hypertension, dyslipidemia, sleep apnea syndrome |
• Poorly controlled diabetes (HbA1c ≥8.0%) with BMI between 32 and 34.9, hypertension (systolic blood pressure
≥160 mmHg), dyslipidemia (LDL-C ≥140 mg/dL or non-HDL-C ≥170 mg/dL), or sleep apnea (AHI ≥30) Inadequate treatment
of ≥1 complications of hypertension (systolic blood pressure >160 mmHg), dyslipidemia (LDL-C >140 mg/dL or non-HDL-C
>170 mg/dL), or sleep apnea (severe AHI >30) |
AHI, apnea–hypopnea index; BMI, body mass index; HbA1c, hemoglobin A1c; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol. (Source: Ministry of Health, Labor and Welfare.310)
Table 10.
Patient Indications for Bariatric Surgery
The indication for surgery is, in principle, primary obesity in patients between the ages of 18 and 65 years, who do not
show significant weight loss or improvement in obesity-related complications despite medical treatment for ≥6 months, and
who meet 1 of the following conditions. (ELIa 2a) |
| (1) Bariatric surgery for weight loss is indicated for patients with a BMI ≥35 kg/m2 (EL2a) |
(2) Indication for metabolic surgery for the treatment of complications (diabetes, hypertension, dyslipidemia, liver
dysfunction, sleep apnea, etc.) is diabetes or BMI ≥32 kg/m2 if the patient has ≥2 complications other than diabetes. (EL2b) |
(3) The indication for BMI <35 kg/m2 should be treated as a clinical study, requiring strict informed consent, follow-up,
and clinical registration (EL6) |
BMI, body mass index. (Source: Japanese Society for Treatment of Obesity.311)
5.2.2 Lifestyle Modification and Exercise (Table 11)
Table 11.
Recommendations and Levels of Evidence for Lifestyle Modifications in OSA
| |
COR |
LOE |
| It is recommended to exercise in combination with weight loss |
I |
B |
| It is recommended to quit smoking |
I |
B |
| Consider avoiding benzodiazepine use should be considered |
IIa |
B |
| Use of nonbenzodiazepines or orexin-receptor antagonists may be considered |
IIb |
B |
| Prohibition of alcohol consumption before bedtime may be considered |
IIb |
B |
COR, Class of Recommendation; LOE, Level of Evidence; OSA, obstructive sleep apnea.
Drinking alcohol affects the respiratory center,320 relaxes the upper airway patency muscle, and increases upper airway resistance.321 In a meta-analysis, alcohol consumption was a risk factor for the development of OSA even after adjustment for BMI,322 and prohibition of alcohol consumption before bedtime is recommended for the treatment of OSA. However, whether restricting alcohol consumption is effective in reducing or preventing the onset of OSA remains unresolved.322 In a large cohort study in the USA, current smokers developed OSA at a rate 4.4-fold higher than that of nonsmokers, but past smokers showed no significant difference compared with nonsmokers, suggesting that the risk of developing OSA due to smoking may be limited to during the duration of smoking.323 Therefore, more aggressive instruction for smoking cessation is recommended for patients at high risk of developing OSA. The evidence regarding the effects of smoking on relaxation of the airway opening muscles, induction of upper airway inflammation, and lowering of the arousal threshold is less than conclusive.324
The use of benzodiazepines is associated with relaxation of the upper airway opening muscles and decreased ventilatory response to hypoxia, which may lead to more severe OSA. In a small RCT, long-acting benzodiazepine sleep medication (flurazepam 30 mg) in subjects without a diagnosis of OSA increased the number of apneic events and prolonged the apnea duration compared with subjects not receiving the medication.325 However, in patients with moderate to severe OSA, administration of a long-acting benzodiazepine (temazepam 10 mg, not available in Japan) worsened hypoxemia during sleep in patients with increased ventilatory response upon awakening, but did not worsen the AHI.326 Nonbenzodiazepines are less potent muscle relaxants, and in patients with moderate or severe OSA, 3 mg of eszopiclone327 or 10 mg of zolpidem328 did not worsen the AHI, but rather improved sleep efficiency. The AHI and mean SpO2
were not affected by administration of suvorexant and lemborexant.329,330 Therefore, benzodiazepines should be used with caution in patients at risk for OSA, and use of nonbenzodiazepine orexin-receptor antagonists may be considered.
A RCT showed that exercise therapy combining aerobic endurance and resistance exercise improved the AHI in patients with OSA, even in the absence of weight loss.331 Long-term exercise rehabilitation after treatment for CAD showed improvement in the AHI.332 However, in a large long-term follow-up study, patients with OSA who exercised for several hours per week had a reduced risk of developing or exacerbation of OSA after adjustment for sex and age, but there was no significant difference after adjustment for BMI.333 Therefore, exercise is recommended in conjunction with weight loss to prevent exacerbation of OSA.
5.2.3 Positional Therapy
It is known that more than half of patients with OSA are prone to apnea due to pharyngeal airway narrowing associated with certain positions (mainly supine) during sleep, but which is reduced in other positions.334 Such patients are referred to as having position-dependent OSA (positional OSA). The well-known diagnostic criterion for positional OSA proposed by Cartwright is “OSA in which the AHI doubles when the patient changes from the side-lying to the supine position during sleep”.335 However, there is no internationally accepted definition of positional OSA.334 Positional therapy is a treatment method to reduce the AHI in patients with positional OSA by preventing them from unconsciously lying supine. In recent years, devices in the form of a band worn around the neck or chest (when a sensor of sleep position detects supine position, vibration continues and prevents supine positioning) have been developed mainly overseas.337,338 Several randomized crossover studies have been conducted on the efficacy of positional therapy using various devices,339–341 and it is recommended as an alternative treatment for patients with mild positional OSA who are not eligible for CPAP therapy and for those who have difficulty adjusting to CPAP.
5.2.4 Drug Treatment
a. Drug Treatment for Anatomical Factors
AHI reduction after improvement of obesity has been reported with serotonin and norepinephrine reabsorption inhibitor (not approved in Japan),348,349 liraglutide, a GLP-1 receptor agonist (covered by insurance for type 2 diabetes, not for obesity),350 and phentermine topiramate (not approved in Japan),351 which combines sympathomimetic and GABAergic sedation. In patients with HF and fluid retention such as edema, a combination of spironolactone, an aldosterone antagonist, and other diuretics has been shown to improve the AHI to some extent.352–354 For patients with nasal obstruction, the combination of nasal vasoconstrictors and atomized steroids to improve nasal ventilation has been reported to improve the AHI.355,356
b. Drug Treatment for Collapse of the Upper Airway Opening Muscle Group (Especially the Genioglossus Muscle)
Serotonin agonists,357 cholinergic agonists,358 and α 1-receptor agonists359 have been studied in the past, but their efficacy in clinical practice has not been established.360,361 However, a recent report reported that the combination of atomoxetine, a selective noradrenaline reuptake inhibitor (covered by insurance for attention-deficit/hyperactivity disorder) and oxybutynin, a muscarinic receptor antagonist (covered by insurance for overactive or unstable bladder) improved the AHI in patients with OSA.
c. Drug Therapy for Instability of the Respiratory Regulatory System
Acetazolamide, a carbonic anhydrase inhibitor, has long been reported to reduce the AHI through improvement of respiratory instability.364,365 A meta-analysis of 13 previous studies has shown that acetazolamide treatment has some effect on reducing the AHI in patients with OSA as well as central apnea.366 In a RCT of sultiam (a carbonic anhydrase inhibitor) for OSA, an improvement in AHI was also reported.367
d. Drug Therapy for Low Arousal Threshold
A low arousal threshold causes frequent awakenings, which may exacerbate OSA due to sleep disruption and ventilatory response variability. Sleep medications may be effective in treating this mechanism of OSA aggravation via frequent awakenings, but the efficacy of benzodiazepines,368 nonbenzodiazepines,369 GABA-reuptake inhibitors,370 and melatonin receptor agonists371 has not been established (see Section 5.2.2 Lifestyle Modification and Exercise).
e. Other Drug Therapy
OSA associated with hypothyroidism or acromegaly can be treated with hormonal therapy for the underlying disease, which can be expected to reduce the AHI.372–376 Although limited, there is a possibility that oral treatment with spironolactone, an aldosterone antagonist, may reduce the AHI in patients with OSA associated with resistant hypertension.352 Modafinil has also been reported to improve sleepiness symptoms in patients with OSA during CPAP treatment.377,378
5.2.5 CPAP (Tables 12–14)
Table 12.
Recommendations and Levels of Evidence for Improving Symptoms and QOL With CPAP Therapy in OSA
| |
COR |
LOE |
| It is recommended to use CPAP therapy to improve daytime sleepiness |
I |
A |
| CPAP therapy to improve QOL should be considered |
IIa |
A |
COR, Class of Recommendation; CPAP, continuous positive airway pressure; LOE, Level of Evidence; OSA, obstructive sleep apnea; QOL, quality of life.
Table 13.
Recommendations and Levels of Evidence for the Effectiveness of Humidifiers in CPAP Therapy
| |
COR |
LOE |
| Using a humidifier to improve nasal obstruction should be considered |
IIa |
C |
| Using a humidifier to improve inflammation of the nasal mucosa should be considered |
IIa |
C |
| Using a humidifier to improve adherence may be considered |
IIb |
C |
COR, Class of Recommendation; CPAP, continuous positive airway pressure; LOE, Level of Evidence.
Table 14.
Recommendations and Levels of Evidence for the Effectiveness of EPR/FLEX in CPAP Therapy
| |
COR |
LOE |
| Using EPR/FLEX to improve adherence in patients with high nasal resistance should be considered |
IIa |
C |
| Using EPR/FLEX to improve adherence may be considered |
IIb |
C |
COR, Class of Recommendation; CPAP, continuous positive airway pressure; EPR/FLEX, effectiveness of the expiratory pressure release; LOE, Level of Evidence.
In Japan, CPAP treatment has been covered by insurance since 1998, and is indicated for patients with subjective symptoms such as daytime sleepiness and fatigue, and an AHI ≥20 on PSG or ≥40 on portable monitoring. However, there are still some problems, such as the inability to initiate CPAP for patients with an AHI of 15–20. For an algorithm for the diagnosis and treatment of sleep apnea that takes into account the current indications for insurance reimbursement, please refer to the Sleep Apnea Syndrome (SAS) Guidelines 2020 (Figure 9)2 and consider the treatment goals of each patient.

It is essential to provide guidance and management of environmental factors such as mask fitting, pressure settings, and room temperature, as well as the use of a humidifier, to maintain adherence with CPAP. In particular, patients in the early stages of CPAP use may complain of difficulty exhaling when wearing the mask. There is a report that there was no significant difference in the effectiveness of the pressure relief function (expiratory pressure release [EPR/FLEX]) between the time of use and the time of non-use,399 but adherence was improved in patients with high nasal resistance.400 Adherence to CPAP is significantly impaired in patients with nasal obstruction, in which case the use of a humidifier may be suggested. There are reports that humidifiers improve nasal obstruction and inflammation of the nasal mucosa,401 but to date there are no reports of significant improvement in adherence.402 There are reports of little difference in adherence between auto-CPAP and fixed-CPAP.403 A meta-analysis also found that although auto-CPAP was used for a longer time, the minimum oxygen saturation was improved more with fixed-CPAP, and the difference in treatment effect was unclear.404
Telemonitoring guidance is expected to improve CPAP adherence, reduce the burden on providers, and increase convenience for patients. In a RCT in Japan, 3 groups were compared: monthly telemonitoring at 3-month intervals, no telemonitoring at 3-month intervals, and no telemonitoring at monthly visits, with monthly telemonitoring at 3-month intervals being non-inferior to the other 2 groups.405 Although CPAP therapy is considered effective in improving apnea in patients with OSA, there are a certain number of patients who are intolerant to this therapy. Therefore, the possibility of treatment other than CPAP should always be kept in mind.
5.2.6 Other Positive-Pressure Treatments (Table 15)
Table 15.
Recommendations and Levels of Evidence for Positive-Pressure Therapy (Other Than CPAP) in OSA
| |
COR |
LOE |
It is recommended to use bi-level PAP (with backup ventilation) for OSA with hypoventilation
due to low pulmonary function |
I |
A |
| Performing HFNC for older patients with up to moderate OSA may be considered* |
IIb |
B |
*In Japan, HFNC for OSA is not covered by insurance. bi-level PAP, bi-level positive airway pressure; COR, Class of Recommendation; CPAP, continuous positive airway pressure; HFNC, high-flow nasal cannula; LOE, Level of Evidence; OSA, obstructive sleep apnea.
Positive-pressure treatments other than CPAP for OSA include ASV, bi-level PAP (without backup ventilation), and bi-level PAP (with backup ventilation), etc. ASV is mainly used for the treatment of CSA (see Section 5.3.3 CPAP). CPAP and bi-level PAP have different effects: CPAP has an airway patency effect, whereas bi-level PAP has an expiratory positive airway pressure (EPAP) and inspiratory positive airway pressure (IPAP) effect. bi-level PAP (without backup ventilation) and bi-level PAP (with backup ventilation) also differ in the way inspiratory pulmonary arterial pressure (PAP) is applied to compensate for hypopnea. In Japan, the former is covered by insurance for OSA, but the latter is not covered by insurance for OSA alone; a comparison of the effects of CPAP and bi-level PAP on OSA has been reported. For OHS, the use of bi-level PAP (without backup ventilation) has been shown to reduce respiratory events and improve utilization in patients with OSA who cannot tolerate CPAP or CPAP pressures.407,408 Adherence to bi-level PAP (with backup ventilation) has also been shown to improve adherence in OSA patients with poor CPAP adherence.409 (For OHS, see Section 4.1.8 Obesity Hypoventilation Syndrome (OHS).) Patients with OSA associated with sleep hypoventilation due to low pulmonary function or central alveolar hypoventilation during sleep are indicated for bi-level PAP (with backup ventilation). High-flow nasal cannula (HFNC), which was introduced in the 2010s for acute respiratory failure type I and some cases of acute respiratory failure type II and has become widely used, provides heated and humidified high-flow air continuously through a thick cannula that covers the nasal cavity. Unlike the positive end-expiratory pressure (PEEP) set by CPAP or bi-level PAP, the airway pressure easily fluctuates with the flow rate set and with opening and closing of the mouth.410 Overseas, its efficacy in the treatment of OSA in older patients with up to moderate disease has also been demonstrated.411 In Japan, HFNC for OSA is currently not covered by insurance and is not positioned as an alternative to CPAP. Future studies are needed to determine the appropriate flow rate of titration and consider the cost of the large amount of sterile purified water required for introducing HFNC for OSA in Japan.
5.2.7 OA (Table 16)
Table 16.
Recommendation and Level of Evidence for OA Treatment to Improve AHI in OSA
| |
COR |
LOE |
OA treatment to improve AHI in patients with mild to moderate OSA and those who cannot
continue CPAP therapy should be considered |
IIa |
B |
AHI, apnea–hypopnea index; COR, Class of Recommendation; CPAP, continuous positive airway pressure; LOE, Level of Evidence; OA, oral appliance; OSA, obstructive sleep apnea.
In Japan, OA has been covered by insurance since 2004 and is widely used as a treatment for OSA. It is a device that moves the mandible or tongue forward to improve the obstruction or narrowing of the upper airway, and is custom-made from the individual patient’s dental model. According to the clinical practice guideline jointly developed by the AASM and the American Academy of Dental Sleep Medicine in 2015,412 a meta-analysis of post-replacement changes in the AHI (for mild to severe OSA), showed a decrease of 13.5. In addition, another meta-analysis showed that the reduction in the AHI was −10.9 compared with placebo.413 Although CPAP is superior to OA in terms of short-term efficacy (i.e., AHI reduction), OA is superior in terms of long-term compliance and is therefore equivalent when regarding efficiency of use vs. efficacy.414 In this respect, an OA is indicated for patient with mild to moderate OSA and for those in whom CPAP cannot be used continuously, but in recent years there has been an opinion that the indication should include severe cases. A systematic review of the treatment effects of OA on CVD was reported by de Vries et al. in 2018, in which they found an antihypertensive effect but no significant reduction in heart rate.415 A meta-analysis of 2 RCTs on heart rate showed no significant difference, with a post-treatment reduction of −1.1.415 Although an observational study demonstrated that OA reduced cardiovascular events,416 more careful clinical studies are desirable to evaluate the effect of OA on CVD in the future. Although an OA is a simpler treatment than CPAP, there are many cases in which an OA is not suitable due to the condition of the patient’s remaining teeth. Therefore, the dentist in charge must have sufficient experience in dental sleep medicine,412 and the physician in charge must also have a good understanding of the appropriate use of an OA. On the other hand, it has been reported that long-term use of OA causes tooth movement but less exacerbation of periodontitis.417 The key to the fabrication of OA is the positioning of the mandible. OA titration is a specialized procedure that must be performed in consideration of treatment efficacy and side effects. It is also necessary to determine the effectiveness of OA and to follow the patient’s progress after the introduction of the OA, preferably by an experienced dentist.
5.2.8 Surgical Treatment (Upper Airway Surgery) (Table 17)
Table 17.
Recommendations and Levels of Evidence for Surgical Treatment of OSA
| |
COR |
LOE |
| Surgery to improve nasal obstruction may be considered as a support for CPAP therapy |
IIb |
C |
| Pharyngoplasty may be considered to improve AHI |
IIb |
C |
AHI, apnea–hypopnea index; COR, Class of Recommendation; CPAP, continuous positive airway pressure; LOE, Level of Evidence; OSA, obstructive sleep apnea.
In 2021, the AASM published clinical guidelines for consultation with sleep surgeons regarding (1) sleep surgery as an alternative treatment for patients who are intolerant or unaccepting of positive-pressure therapy (strong recommendation), (2) weight loss surgery as an alternative treatment for obese patients (strong recommendation), (3) sleep surgery as supportive care for patients with poor CPAP adherence (conditional recommendation), and (4) positive-pressure treatment as initial treatment (as a perioperative risk management) for patients scheduled for surgery with anatomical abnormalities of the upper airway (conditional recommendation). The Japanese guidelines2,419 recommend consultation for the presence of upper airway disease prior to the initiation of OSA treatment, and the management depends on the type of upper airway disease and the purpose and technique of treatment. First, if upper airway disease such as tumor or sinusitis is present, treatment should be given in parallel with or as a priority over treatment of OSA. However, positive-pressure therapy should be initiated prior to surgery for perioperative management according to (4) above. Second, for nasal disease that affects acceptance and continuation of CPAP, surgery should be performed as supportive to standard treatment such as CPAP according to (3) above. A few RCTs have reported the effects of nasal surgery in lowering the optimal CPAP pressure and prolonging the duration of CPAP use (CPAP pressure decreased from 11.6 cmH2O to 9.5 cmH2O, and duration of CPAP use increased from 3.0 h to 5.5 h).420 On the other hand, nasal surgery does not improve the AHI, but does significantly improve the ESS, subjective sleep quality, and QOL,421 which is consistent with the objectives of OSA treatment as stated in the AASM clinical guidelines.27 Therefore, nasal surgery should coexist with CPAP therapy as a supportive measure, and it is effective when performed for appropriate indications and at appropriate timings during the overall treatment. The third is salvage surgery, as described in (1) above, which is performed when standard therapies such as CPAP do not work, and includes soft tissue surgery around the upper airway, maxillofacial surgery, and hypoglossal nerve stimulation therapy. Pharyngoplasty, including uvulopalatopharyngoplasty (UPPP), is performed for patients with Freidman Classification I422 (hypertrophy of the palatine tonsils, no abnormal findings of the soft palate, and no obesity). (2) Soft palate findings are normal. A meta-analysis of UPPP reported an improvement in the AHI from 35.6 to 13.9 and in the ESS from 11.6 to 5.0 at 8 months postoperatively.423 A RCT of patients who dropped out of CPAP reported a significant improvement in the AHI from 47.9 to 20.8 and in ESS from 12.4 to 5.3 at 6 months after UPPP.424 On the other hand, laser-assisted uvuloplasty, a snoring treatment, is not recommended because its therapeutic effect has not been confirmed and there are reports of airway narrowing due to scar contracture.425 Trans-oral robotic surgery, which is not performed in Japan, has been reported to improve the AHI from 48.1 to 19.0 and the ESS from 11.4 to 5.6.426 The effectiveness of upper airway soft-tissue surgery depends on the indication, and long-term postoperative adverse symptoms such as inadequate closure of the soft palate after UPPP, pharyngeal discomfort, effect on swallowing, and abnormal taste have been reported.427 Sleep surgery should be performed under safe management, with the indication being accurately diagnosis, side effects fully explained, and consent obtained.
5.2.9 Surgical Treatment (Orthognathic Surgery)
Originally, orthognathic surgery treated abnormal jaw alignment caused by abnormal development and morphology of the jawbone. Maxillomandibular advancement (MMA) aimed to improve the obstruction and narrowing of the upper airway in patients with OSA by moving the maxilla and jaw forward. The relationship between maxillofacial morphology and pharyngeal airway has been suggested as a cause of OSA, and a report on the relationship between facial morphology and pharyngeal airway stated that the upper airway is significantly narrower while opening the mouth in patients with a smaller mandible.428 A systematic review of pre- and postoperative changes in the upper airway found that the size of the pharyngeal airway was increased by MMA.429–431 In a systematic review of the effects of MMA on OSA by Zhou et al., the AHI decreased from 57.3 preoperatively to 10.4 postoperatively.431 There is also a report that MMA improves sleep efficiency.432 In the studies analyzed in the systematic reviews, treatment was evaluated at 6–12 months postoperatively, but a meta-analysis of changes in the AHI was conducted in a systematic review of longer-term treatment evaluations:433 the AHI decrease from 4 to 8 years postoperatively was −53.4, and the AHI decrease at ≥8 years postoperatively was −29.9. These results suggest that although there is a significant short-term improvement in the AHI, long-term individual aging and weight changes should be kept in mind. In the systematic review by Zhou et al.431 there were no deaths, but major complications such as reoperation, failure of bone junction healing (pseudarthrosis), and sudden respiratory distress occurred in 3.2% of patients. In addition, transient dysesthesia of the face (branches II and III of the trigeminal nerve) was seen in 76.9% of patients, and 18.5% had persistent symptoms even after 6 years of follow-up.429
5.2.10 Implantable Hypoglossal Nerve Stimulation Therapy (Table 18, Figure 10)
Table 18.
Recommendation and Level of Evidence for Implantable Hypoglossal Nerve Stimulation Therapy for OSA
| |
COR |
LOE |
| Implantable hypoglossal nerve stimulation therapy to improve AHI should be considered |
IIa |
B |
AHI, apnea–hypopnea index; COR, Class of Recommendation; LOE, Level of Evidence; OSA, obstructive sleep apnea.

Hypoglossal nerve stimulation therapy focuses on the responsiveness of the pharyngeal dilator muscle group, a functional factor in OSA. Its development began in Japan434 and was subsequently commercialized by some companies in the Western countries. In 2014, a multicenter prospective clinical trial (the STAR Trial) was conducted, and 929 participants were enrolled, of whom 124 were eventually followed; the median AHI at 12 months decreased by 68%, from 29 to 9,435 and efficacy was subsequently reported at 3- and 5-year follow-up.436 In 2019, a total of 584 patients from the STAR trial, a German cohort, a US cohort, and the ADHERER registry were included, and a 77.1% efficacy was reported according to surgical treatment criteria.439 In Japan, the implantable hypoglossal nerve stimulator has been covered by insurance since June 2021 and is indicated for the criteria shown in Table 19.
Table 19.
Indication Criteria for Implantable Hypoglossal Nerve Stimulation Therapy
| (1) The patient must have OSA syndrome with an AHI ≥20 |
| (2) Unsuitable or intolerant to CPAP therapy |
| (3) Absence of severe anatomical abnormalities such as enlarged tonsils |
| (4) The applicant must be ≥18 years of age |
| (5) BMI <30 kg/m2
|
| (6) No concentric collapse of the soft palate on drug sleep endoscopy |
| (7) Central apnea rate must be <25% |
AHI, apnea–hypopnea index; BMI, body mass index; CPAP, continuous positive airway pressure; OSA, obstructive sleep apnea.
5.3 Treatment of CSA
5.3.1 Effects of HF Treatment on CSA
a. Drug Therapy (Table 20)
Table 20.
Recommendations and Levels of Evidence for Drug Therapy to Reduce AHI in CSA Complicated by HF
| |
COR |
LOE |
| It is recommended to optimize guideline-based drug treatment of HF |
I |
B |
| Administration of carvedilol and ARNI in HFrEF should be considered |
IIa |
B |
AHI, apnea–hypopnea index; ARNI, angiotensin receptor–neprilysin inhibitors; COR, Class of Recommendation; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; LOE, Level of Evidence.
Because CSA complicated by HF is caused by the HF itself, regardless of the LVEF, optimization of HF treatment based on HF guidelines is fundamental in the treatment of this type of CSA.443 The efficacy of several drugs for HF on CSA has been demonstrated. A small observational study reported that captopril, an angiotensin-converting enzyme (ACE) inhibitor, reduced the AHI assessed by PSG in patients with chronic HF (NYHA functional class II/III).444 Diuretics can improve CSA in HF patients by improving pulmonary congestion.257 Small observational studies reported that in patients with chronic HF complicated by CSA (NYHA functional class II/III, LVEF <50%), carvedilol significantly reduced AHI and CAI assessed by PSG, and that its effect was dose-dependent.226,447 Several studies have investigated the effect of angiotensin receptor–neprilysin inhibitors (ARNI) on sleep apnea in patients with HFrEF. However, a RCT compared the AHI before and 8 weeks after treatment with an ARNI or enalapril in HFrEF patients (NYHA functional class II/III) with SDB (mostly obstructive; AHI ≥15 assessed by portable monitoring) and reported that both the ARNI and enalapril showed no significant reduction in the AHI.451
It should be noted that when HF improves with optimized therapy, thereby resulting in reducing the severity of CSA, OSA sometimes become apparent.246,446,449
b. Device and Surgical Treatment (Table 21)
Table 21.
Recommendations and Levels of Evidence for HF Treatment With Devices and Surgery as Treatment for CSA
| |
COR |
LOE |
It is recommended to perform device and surgical treatment for HF that meets
indication criteria in patients with HF complicated by CSA |
I |
C |
Reevaluation of CSA and consideration of treatment for CSA after device or surgical
treatment for HF should be considered |
IIa |
C |
| Device and surgical treatment for HF solely to reduce CSA should not be performed |
III
(Harm) |
C |
COR, Class of Recommendation; CSA, central sleep apnea; HF, heart failure; LOE, Level of Evidence.
Cardiac resynchronization therapy (CRT) has been reported to reduce CSA in patients with chronic HF.291,456–458 In their first report, Sinha et al. found that CRT in 14 HF patients with CSA significantly reduced the AHI (from 19.2 to 4.6) and increased the minimum SpO2
after 17±7 weeks, and improved sleep quality; however, sleep quality did not improve after CRT in 10 patients without CSA.458 Oldenburg et al. showed that the AHI did not change in the predominantly OSA group, but decreased significantly in the predominantly CSA group, and that the AHI decreased only in patients who responded well to CRT.460 The degree of improvement in CSA after CRT in HF patients with CSA may be useful in determining the efficacy of CRT.461 However, the extent to which the reduction in the AHI with CRT contributes to the efficacy of CRT in HF patients is unknown and requires further investigation.
Improvements in CSA have been reported after catheter inatervention462 and bypass surgery463,464 in patients with ischemic cardiomyopathy, and after mitral valvuloplasty465–467 in patients with mitral regurgitation.465–468 Recently, transcatheter aortic valve implantation (TAVI) has been performed for aortic stenosis, and a report evaluated the relationship among severe aortic stenosis, SDB and CSA before and after TAVI.468 In 29 patients with severe aortic stenosis, a portable monitoring system was used before and 3 months after TAVI: 41% had CSA and 31% had OSA before TAVI. CSA strongly correlated with the left ventricular end-diastolic pressure before TAVI, but not with the LVEF, systolic pulmonary artery pressure or NT-pro B-type natriuretic peptide (BNP) level. After TAVI, the AHI improved significantly (from 43.5 to 19.4), especially in the CSA group, and the prevalence and severity of SDB decreased from 72% to 59%.
In a report evaluating the change in CSA before and after left ventricular assist device (LVAD) implantation in patients with severe HF, CSA remained after LVAD implantation despite improvements in hemodynamics and major organ function.469 Recently, a study of the short-term effects of varying pump speed in patients after LVAD implantation revealed that increasing the pump speed of the device decreased CSA in a cardiac output-dependent manner, but increased OSA, albeit to a lesser degree, due to worsened alveolar–capillary gas diffusion.470 Nevertheless, the authors reported a marked improvement in total AHI.470 In a report examining the effect of heart transplantation on CSA, CSA often disappeared once cardiac function normalized, but may persist.471 Of 13 patients with CSA before heart transplantation who maintained normal cardiac function and normalized sympathetic hyperactivity 6 months after transplantation, CSA resolved in 7 patients after transplantation, but remained in 3 patients, and 4 patients progressed to OSA.290 It is possible that in patients with severe HF, even if cardiac function is normalized by nonpharmacological treatment, the central respiratory system may be irreversibly impaired, and there may be cases of residual CSA. The use of steroid hormones may lead to weight gain after heart transplantation and exacerbation of OSA, which should be carefully monitored.472
c. Other Treatments (e.g., Exercise Therapy)
Exercise therapy is an important nonpharmacologic treatment for HF.473,474 The pathogenesis of CSA is associated with hyperventilation during exertion253,477 and exercise has been reported to improve hyperventilation during exertion and CO2
chemoreceptor hyperactivity.478 Eexercise oscillatory ventilation (EOV) and CSA have similar mechanisms of occurrence,480–482 and because exercise therapy has been shown to improve EOV,483 it is expected to also improve CSA. However, in a study that examined prognosis by dividing patients into 4 groups according to the presence or absence of CSA and EOV, EOV alone, CSA alone, and the combination of CSA and EOV were associated with poorer prognosis in that order, and the combination showed differences in prognosis, suggesting that CSA and EOV are of independent significance.256 Exercise therapy may also improve CSA by improving excessive ergoreceptor reflexes within skeletal muscle and regulating excessive ventilation during exercise.484 Although exercise therapy may improve CSA in HF patients, studies examining the effect of exercise therapy alone on CSA are scarce,485–487 and future validation from large-scale studies is expected.
Comprehensive management is important to prevent exacerbation of HF. Lifestyle modification, nutritional guidance, assessment and management of medication adherence and comorbidities, and psychological counseling should be implemented along with exercise therapy.
5.3.2 Home Oxygen Therapy (HOT)
a. Indications and Mechanisms
Nocturnal HOT has been available in Japan since 2004 as a treatment for CSA. Insurance coverage is limited to “patients with chronic HF of NYHA functional class III or higher, who are observed to have CSR during sleep, and whose AHI is 20 or higher as confirmed by PSG”. Because the sensitivity of CO2
chemoreceptors increases as the partial pressure of oxygen (PaO2) in the blood decreases489 (Figure 11), HOT should be administered to patients with CSR to increase PaO2. Oxygen therapy may correct the increased sensitivity of CO2
chemoreceptors and reduce CSA by increasing the PaO2.
b. Efficacy of HOT for CSA
The effect of HOT on CSA has been confirmed as a reduction in both CSA-CSR and sleep time with SaO2
<90%.490 The following secondary effects have been demonstrated: (1) improved sleep quality (increased total sleep time [TST], decreased apnea frequency and nocturnal awakening frequency),491 (2) correction of sympathetic hyperactivity,261 (3) improvement of LVEF,492,493 and (4) improvement of exercise tolerance.494,495 As stated earlier, nocturnal HOT can be expected to reduce or improve CSA to some extent. On the other hand, it did not improve cardiovascular death in HF, which is a background disease of CSA. However, physical activity capacity increased by approximately 1 MET, indicating a therapeutic effect.492,493
c. Problems and Issues
Although the efficacy of HOT in CSA has been demonstrated, it is unclear whether its efficacy is consistent regardless of the cause of HF. Future studies are needed to determine the difference in benefit of HOT in patients with ischemic or nonischemic HF, as well as in those with or without AF. In previous clinical trials of oxygen therapy, the oxygen flow ranged from 1 to 5 L/min, but titration of oxygen levels was not performed to individual CSA. Excessive oxygen supply to tissues may induce increased oxidative stress caused by supersaturation of oxygenation.496 On the other hand, repeated cycles of hypoxia and rapid oxygenation (intermittent hypoxia) induced in association with SDB have been shown in vitro to increase the production of oxidative stress more than sustained hypoxia (SH).188 Based on this, the challenge is to administer an optimal amount of oxygen to CSA patients without excess or deficiency.
5.3.3 CPAP
Positive-pressure breathing includes CPAP with flat PEEP to maintain airway patency, bi-level PAP, and ASV with pressure support adapted to spontaneous breathing in addition to constant PEEP. ASV stabilizes ventilation and respiratory rate by varying the degree of pressure support according to respiratory status (Figure 12).497 In some cases, CSA-CSR remains even after optimal HF treatment, and respiratory support therapies such as CPAP, bi-level PAP, and ASV are considered.2,498 As the mechanism by which positive-pressure therapy including CPAP is effective in reducing CSA-CSR, positive pressure in the thoracic cavity reduces cardiac workload through reduced preload and afterload, and reduces right and left cardiac workload, resulting in improved cardiac function. Additional possible mechanisms include lung dilation to reduce sympathetic nerve activity and sensitivity to CO2, increased residual air volume to ameliorate hypoxemia, and increased PaCO2
with CPAP use.

5.3.4 Other Positive-Pressure Treatments
Although bi-level PAP has been reported to improve LVEF in the short term,501 in practice it is rarely used due to the difficulty of setting up and the lack of data on long-term prognostic improvement.1 ASV improves CSA-CSR more effectively than CPAP, bi-level PAP or oxygen therapy,502 because it monitors patient respiration, adjusts pressure support and backup ventilation, and stabilizes ventilatory volume and respiratory rate by varying the degree of ventilatory support (Figure 13).503 Similar to auto-CPAP, ASV can set the PEEP to keep the upper airway open, making it possible to deal with both OSA and CSA complicated by HF. In Japan, ASV is used to improve pulmonary congestion in HF with or without SDB.473 In HF with residual pulmonary congestion, the pulmonary venous and pulmonary capillary pressures increase, resulting in water extravasation into the alveoli. The addition of PEEP to ASV reduces water extravasation from the pulmonary capillaries, re-expands atelectactic and collapsed alveoli, increases the functional residual air volume, improves pulmonary compliance and airway resistance, and reduces respiratory muscle workload (Figure 14).497 Furthermore, positive pressure in the thoracic cavity, which is physiologically negative, is expected to decrease venous return, reduce preload, and decrease the force (transmural pressure) applied to the left ventricular wall during left ventricular contraction, thereby decreasing cardiac afterload, increasing cardiac output, decreasing left ventricular end-diastolic pressure, and reducing functional mitral valve regurgitation.

5.3.5 Phrenic Nerve Stimulation (PNS)
Electrical stimulation of the phrenic nerve during sleep causes the diaphragmatic muscles to contract and breathe during CSA episodes. As a treatment, PNS is unaffected by patient adherence and is safe and effective for adult patients with moderate to severe CSA. The US Food and Drug Administration approved an implantable device in 2017 (the RemedēSystem),504,505 which consists of a pulse generator, stimulation lead, and sensing lead. A pocket is created under the skin in the upper left or right anterior thoracic region, the pulse generator is implanted, and a sensor in the generator detects the patient’s body position and movement. The stimulation lead is introduced through the axillary or subclavian vein and placed transvenously in the left pericardiophrenic vein or the right brachiocephalic vein (the sensing lead is placed in an azygos vein if necessary), resulting in nerve stimulation of the adjacent phrenic nerve, which causes contraction of the diaphragm, negative intrathoracic pressure, and restoration of normal breathing. In a prospective, multicenter, nonrandomized study of HF patients, the treatment group showed a 48% reduction in the AHI and a 90% reduction in the CAI, as well as a reduction in the arousal index and improvement in hypoxemia.509 In the Remedē System Pivotal Trial, a prospective multicenter RCT, patients with an AHI >20 who underwent PSG were randomized to treatment or control and compared. Results showed that the proportion of patients in the treatment group (51%, 35 of 68 patients) with AHI >50% reduction at 6 months was significantly higher than that in the control group (11%, 8 of 73 patients),505 and this effect was maintained at 12 months.504 Secondary endpoints showed a significant reduction in 4%ODI (P<0.0001) and significant improvement in the Patient Global Assessment (PGA) health-related QOL instrument and ESS in the treatment group compared with the control group.505 In an exploratory post-hoc comparison of the HF and non-HF patients in The Remedē System Pivotal Trial, 22 of 35 (63%) HF patients in the treatment group had ≥50% reduction in AHI at 6 months compared with 2 of 45 (4%) HF patients in the control group. Among the non-HF patients, 13 of 23 (57%) patients in the treatment group had ≥50% reduction in AHI at 6 months compared with 6 of 28(21%) patients in the control group.505 There have been no deaths related to device implantation, and the frequency of complications such as lead displacement, wound infection, and hematoma is reported to be similar to that of other transvenous implantation devices, making this a safe procedure.505,506,514
5.3.6 Drug Therapy (Table 22)
Table 22.
Recommendations and Levels of Evidence for Drug Therapy of CSA
| |
COR |
LOE |
| Acetazolamide and theophylline administration may be considered |
IIb |
B |
| Benzodiazepines and nonbenzodiazepines administration may be considered |
IIb |
C |
COR, Class of Recommendation; CSA, central sleep apnea; LOE, Level of Evidence.
The efficacy of several drug therapies has been investigated for CSA-CSR itself, which is complicated in chronic HF.
a. Acetazolamide
In a double-blind RCT of HF with left ventricular systolic dysfunction, acetazolamide significantly reduced both the AHI from 55 to 34 and the CAI from 44 to 23 in the acetazolamide group.292 In a meta-analysis including 15 clinical studies (256 patients in total), acetazolamide significantly reduced the AHI (mean difference −15.82).515 Metabolic acidosis caused by urinary excretion of bicarbonate ions shifts the CO2/ventilatory response to the left and increases the difference between PaCO2
and the apnea threshold,445 suppresses the sensitivity of peripheral chemoreceptors to O2
and CO2,516 and improves lung congestion through diuresis.516 In Japan, insurance coverage is available for SDB treatment, but the efficacy of long-term use for CSA-CSR associated with chronic HF has not been established due to concerns about side effects such as metabolic acidosis, electrolyte abnormalities, and sensory abnormalities.
b. Theophylline
In a double-blind RCT, theophylline clearly decreased the AHI from 47 to 18 and the CAI from 26 to 6 in the treatment group,517 and in a non-RCT, theophylline decreased AHI from 42.6 to 20.8 and CAHI from 31.5 to 10.1.518 It is thought that theophylline competes with adenosine, which has a respiratory depressant effect, to increase ventilation,519–522 and also decreases CSA via an increase in cardiac output due to a positive effect on phosphodiesterase III inhibition.523,524 Theophylline has proarrhythmic effects, and the efficacy of its long-term use has not been established.
c. Sleeping Medication
Triazolam, a benzodiazepine sleep medication, reduced the CAI by approximately 50% compared with placebo without affecting the OAI in a randomized, double-blind, crossover study in generally healthy patients with CSA assigned to placebo or 0.125 mg or 0.2 mg dose of triazolam.525 It was also reported that temazepam may decrease the arousal response during sleep in patients with HF.526 It is widely believed that arousals from sleep that occur during the hypercapnic phase of CSA-CSR cause a ventilatory overshoot, which then leads to greater hypocapnia and new, longer central apneas, so it has been suggested that preventing arousals may be a treatment option to reduce or suppress CSA-CSR.527 However, benzodiazepines may decrease upper airway muscle tone and contribute to airway obstruction, so caution should be exercised when administering them to patients with obesity, snoring, or other elements of OSA.526 Zolpidem, a nonbenzodiazepine sleep medication, significantly reduced the AHI and CAHI (30.0 to 13.5 and 26.0 to 7.1, respectively) without worsening oxygenation or obstructive events in a non-RCT of 20 patients with idiopathic CSA, which improved wakefulness, sleep quality and daytime sleepiness.528 A randomized, double-blind, placebo-controlled crossover study in HF patients demonstrated that zolpidem did not decrease AHI, but increased TST (324.7 to 383.2 min) and deep sleep (stage N3, 20.4% to 27.1%) but decreased minimum SpO2
(83.6% to 80.7%).529 Although zolpidem reduces arousals and stabilizes sleep, it may exacerbate hypoxia, and further investigation is needed.
d. Other Drugs
Buspirone, a serotonin 5-HT1A receptor agonist, reduced CO2
chemosensitivity as well as both the daytime and nighttime AHI and CAI in chronic HF patients with CSA in a single-center, placebo-controlled, randomized, double-blind crossover study.530 Although buspirone is not approved in Japan, further investigation in a large-scale study is warranted.
5.4 Other SDB Treatments
5.4.1 Atrial Overdrive Pacing (AOP) (Table 23)
Table 23.
Recommendations and Levels of Evidence for AOP for SDB
| |
COR |
LOE |
| AOP for patients with OSA is not recommended |
III (No
benefit) |
B |
| AOP for patients with CSA may be considered |
IIb |
B |
AOP, atrial overdrive pacing; COR, Class of Recommendation; CSA, central sleep apnea; LOE, Level of Evidence; OSA, obstructive sleep apnea; SDB, sleep disordered breathing.
Garrigue et al. reported that AOP at 15 beats/min earlier than the mean nocturnal heart rate decreased the AHI in 15 patients with mixed CSA and OSA who underwent pacemaker implantation for a diagnosis of bradycardia.531 Lüthje et al.532 reported that AOP at 15 beats/min earlier than the mean nocturnal heart rate reduced the AHI in 20 subjects in sinus rhythm without HF and with a mean AHI of 20.9, who had pacemakers or electrical cardioverter-defibrillators implanted. They reported that AOP was performed at 7 beats/min or 15 beats/min faster than the nocturnal heart rate for consecutive three nights, but neither heart rate significantly decreased the AHI. No other studies have reported significant AHI improvement with AOP in patients with OSA.533–535 On the other hand, a study of the acute effect of CRT followed by overnight AOP in HF patients with CSA showed a significant reduction in the AHI (from 23.8 to 21.5).536
A meta-analysis reported that the usefulness of AOP for CSA remains open for further investigation;537,538 the most recent meta-analysis reported that the effect of AOP on OSA was negative, although the AHI was significantly reduced, the degree of reduction was small, and AOP could be used as an adjunct to other treatments.539
5.4.2 Dialysis (Table 24)
Table 24.
Recommendations and Levels of Evidence for Dialysis Therapy to Improve SDB in End-Stage Renal Failure
| |
COR |
LOE |
Optimization of ESRD treatment itself, focusing on fluid management through hemodialysis
and ultrafiltration should be considered |
IIa |
B |
| Nocturnal hemodialysis may be considered |
IIb |
C |
COR, Class of Recommendation; ESRD, end stage renal disease; LOE, Level of Evidence; SDB, sleep disordered breathing.
It has been reported that treatment of endstage renal disease (ESRD) leads to improvement of SDB. Nocturnal dialysis therapy in patients with ESRD may be effective in the treatment of complicated SDB. A meta-analysis of 3 crossover studies showed an improvement in the AHI from 24.6 to 12.6 and 1.3% improvement in the mean SpO2.542,543 On the other hand, TST was rather decreased, and subjective symptoms related to sleep quality were not improved.543 Changing from nocturnal to continuous peritoneal dialysis was associated with an increase in the AHI, resulting from an increase in soft tissue volume of the glossopharynx as shown on magnetic resonance imaging. Nocturnal peritoneal dialysis resulted in greater water removal and urea excretion, which may have affected both OSA and CSA. In a clinical trial examining changes in SDB immediately before and after hemodialysis in ESRD patients with moderate SDB on maintenance hemodialysis, no significant decrease in the AHI was observed after hemodialysis, but significant decreases in both OSA and the hypopnea index (HI) were observed.545 The amount of fluid volume change before and after hemodialysis positively correlated with the number of changes in OSA and was an independent factor predicting improvement in OSA. Furthermore, in a clinical trial examining the effect of additional fluid removal by ultrafiltration (mean 2.1L) on SDB in ESRD patients, the AHI improved from 43.8 to 28.0, which was a comparable decrease in OSA and CSA.546 This decrease in the AHI positively correlated with a decrease in the systemic extracellular fluid volume, including cervical soft tissue, suggesting that dehydration improved OSA. At the same time, the removal of water by ultrafiltration increased the transcutaneous PCO2, suggesting an ameliorative effect on CSA by improving heightened CO2
chemoreceptor activity. Ultrafiltration did not affect uremia or metabolism,546 suggesting that in ESRD patients, effective dehydration during dialysis therapy to relieve congestion may be therapeutic for both OSA and CSA. Although improvement in the AHI has been observed in maintenance dialysis patients undergoing renal transplantation,547 complete suppression of SDB has not been achieved, which may be due to the fact that ESRD patients often have a background of SDB such as obesity.547 Nocturnal hemodialysis, strict fluid management with dialysis therapy, or renal transplantation may be effective in treating SDB associated with ESRD. However, because ESRD patients often have multiple comorbidities, mainly CVD, CPAP therapy should be considered for on an individual case basis.
5.4.3 Compression Stockings
Compression stockings assist in the treatment of OSA by improving the fluid shift through increased hydrostatic pressure in the lower extremities, preventing leakage of fluid from the vasculature into the interstitium and reducing fluid retention in the lower extremities.548–550 Indeed, in the first nonrandomized, noncontrolled study of 6 non-obese men with moderately to severely OSA, 1 day of wearing compression stockings up to the thighs reduced the AHI by 37% and the fluid shift by 40%.551 In a randomized crossover study by the same group, wearing compression stockings up to the thighs for 1 week by 12 moderately to severely nonobese OSA patients with lower extremity venous insufficiency reduced the fluid shift by 62% and the AHI by 36%.552 A RCT of 45 OSA patients, including obese patients, showed that wearing compression stockings below the knee for 2 weeks was associated with a 25% decrease in the AHI in association with decreased fluid shift and increased morning upper airway inner diameter.553 However, in a randomized crossover comparative study of 14 patients with mild to severe OSA on hemodialysis, a comparison of the effects of wearing compression stockings for 1 week vs. 1 night of CPAP showed that wearing compression stockings did not improve the AHI, decrease the accumulation of lower extremity fluid during the day or total body fluid compared to wearing CPAPA for 1 night.554 This may be due to the inability of dialysis patients wearing compression stockings to drain excess fluid. A meta-analysis including these 4 studies showed the usefulness of compression stockings in improving the AHI.555
II. Relationships With Each Cardiovascular Disease (CVD)
1. Hypertension
Obstructive sleep apnea (OSA) and hypertension are frequently associated with each other, and OSA is a contributing factor to hypertension. OSA is the most frequent cause of secondary hypertension and the underlying disease of uncontrolled hypertension, including treatment-resistant hypertension. The importance of OSA is emphasized in the Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2019),556 and OSA should be constantly kept in mind when treating hypertension.557–559
1.1 Hypertension Risk and OSA
It is important to note that OSA and hypertension are not just comorbid, but OSA itself is a contributing factor to hypertension. In the prospective, population-based Wisconsin Sleep Cohort (WSC) study, it was shown that an increased apnea–hypopnea index (AHI) is a risk for future development of hypertension independent of age and body mass index (BMI).560 The impact of OSA as a risk for hypertension is greater in younger people and decreases in older people. The impact on systolic hypertension in the elderly is less.561
In a study using actigraphy to estimate sleep duration in adolescents, shorter sleep duration (<6.5 h) and lower sleep efficiency (<85th percentile) were more likely to increase the risk of prehypertension (2.5-fold, 3.5-fold, respectively) (defined as blood pressure >90th percentile for age, sex, and height) independent of other factors, which suggests that the quantity and quality of sleep are important as a risk of hypertension.562 In Japan, not only hypertension but also elevated blood pressure (130–139/85–89 mmHg) is a risk factor for future CVD, especially stroke.563 Obesity is a determinant of hypertension and its impact as a determinant of hypertension and elevated blood pressure is greater at younger ages.564 A study of weight gain or loss and incidence of OSA over a 4-year period in a 45-year-old population showed that a 10% increase in body weight was associated with a 6-fold increased risk of developing moderate to severe OSA, and that weight loss reduced the incidence of this disease.298 Therefore, efforts to maintain an appropriate body weight from a younger age may also be associated with a reduction in the incidence of hypertension related to OSA. The large prospective cohort study, the Sleep Heart Health Study (SHHS), has shown that lower percentage of deep sleep (Stage N3) assessed by polysomnography (PSG) at baseline is a future risk for new-onset hypertension, independent of OSA and sleep fragmentation.565
1.2 Characteristics of Blood Pressure Under Free Action in OSA (Table 25)
Table 25.
Characteristics of Hypertension in OSA
| • Treatment-resistant hypertension |
| • Masked hypertension |
| • Nocturnal hypertension (non-dipper/riser type, nocturnal surge blood pressure) |
| • Early morning hypertension (augmentation of morning blood pressure surge) |
| • Hypertension with increased heart rate |
| • Diastolic (predominant) hypertension in young people |
OSA, obstructive sleep apnea.
Hypertension occurring in OSA is also called neurogenic hypertension,566 and is characterized by nocturnal hypertension with marked blood pressure variability (Table 25, Figure 15).566 It is known that patients with nocturnal hypertension of the non-dipper type with abnormal diurnal blood pressure variation and decreased nighttime blood pressure fall, and the riser type with increased nighttime blood pressure are at high risk of developing hypertensive organ damage and future heart failure (HF) and arteriosclerotic CVD.567–571 Shortened sleep duration and nocturnal hypertension are independently associated with the risk of CVD.572 The pathogenesis of nocturnal hypertension (non-dipper/riser type) is known to include increased circulating blood volume (e.g., HF and chronic kidney disease [CKD]), autonomic nervous system disorders (e.g., diabetes mellitus), and poor sleep quality (e.g., sleep disordered breathing [SDB] and depression).557,571 The frequency of OSA complications is high.557,571 Furthermore, during nocturnal apneic attacks of OSA, in addition to the maximal negative pressure load in the thoracic cavity, a marked increase in blood pressure (sleep surge blood pressure) occurs coincident with the time phase from the late apnea to the release of the apnea.571,573,574 Although such an increase in blood pressure has long been known to occur, the clinically important point is that this surge blood pressure varies widely from ≈20 mmHg to >100 mmHg among individuals, despite similar reductions in the partial pressure of oxygen. This increase in the nocturnal surge blood pressure may be a trigger for the nocturnal onset of cardiovascular events seen in OSA.

The sleep surge blood pressure can be detected with a trigger nighttime blood pressure monitoring device, which measures blood pressure using an oscillometric method by detecting a decrease in partial pressure of oxygen, or with a tonometry-type wearable surge blood pressure monitoring device that can measure the beat-by-beat blood pressure variability.571,573–575 Pulse wave transmission time (PTT) sphygmomanometers that estimate from the PTT are also used.576,577 Both types of device can detect peak blood pressure during sleep, which is higher than nighttime blood pressure measured at regular intervals.
The pressor response is enhanced in patients with OSA, and possible mechanisms include vasoreactivity due to nocturnal hypoxemia and increased vasoconstriction in response to sympathetic hyperactivity and sympathetic stimulation due to increased chemoreceptor sensitivity.557 Even in pediatric OSA patients without arteriosclerosis, not only nighttime blood pressure increases, but also morning blood pressure surges are enhanced.578
1.3 Treatment-Resistant Hypertension (Table 26)
Table 26.
Recommendations and Levels of Evidence for Evaluation of OSA in Hypertension
| |
COR |
LOE |
| It is recommended to screen for OSA in patients with treatment-resistant hypertension |
I |
A |
Screening for OSA in hypertensive patients with nocturia, nocturnal dyspnea or a history
of nocturnal onset of cardiovascular events should be considered |
IIa |
C |
Screening for OSA in patients with left ventricular hypertrophy despite normotension
should be considered |
IIa |
B |
COR, Class of Recommendation; LOE, Level of Evidence; OSA, obstructive sleep apnea.
OSA can also cause treatment-resistant hypertension, which is usually defined as an inability to control office blood pressure ≥140/90 mmHg despite administration of ≥3 antihypertensive therapies, including diuretics.556–558 It has been reported that >80% of patients with treatment-resistant hypertension have OSA with an AHI ≥10,55 and that OSA is an independent determinant of poor blood pressure control in hypertensive patients aged younger than 50 years.579 In particular, OSA is suspected in patients with treatment-resistant early morning hypertension whose early morning blood pressure level measured at home remains persistently high (>135/85 mmHg) despite specific treatment for nocturnal and early morning hypertension, such as bedtime administration of antihypertensive medications. In patients with OSA, elevated plasma aldosterone levels are associated with treatment-resistant hypertension.580 The difference between morning and evening blood pressure at home also should be referred.581–584
1.4 Mechanisms of Hypertension and Organ Damage
OSA is a risk factor for all hypertension-related CVD, including ischemic heart disease, HF, arrhythmias, large-vessel disease and cerebrovascular disease.585,586 The most upstream of these risks is increased sympathetic activity. The mechanisms by which OSA increases the risk of hypertension and its organ damage are diverse.587 OSA may cause negative intrathoracic pressure, decreased pulmonary stretch receptor stimulation, chemoreceptor stimulation, hypoxemia, microcarbia, and microarousal. High-sensitivity C-reactive protein (CRP), a marker of inflammatory response, is increased in OSA patients, and this increase is greater in OSA patients with non-dipper type diurnal blood pressure variation than in dipper-type patients.588 This finding indicates that cardiovascular risk is increased in OSA patients with non-dipper and riser-type nocturnal hypertension. Therefore, it is recommended that patients with OSA perform 24-hour ambulatory blood pressure monitoring (ABPM) to assess nighttime blood pressure. The nighttime blood pressure surge caused by OSA is suppressed by renal denervation.589
1.5 Hypertension Treatment Process Considering OSA
Hypertensive patients often have no subjective symptoms related to OSA, so in clinical practice, it is important to suspect OSA even in the absence of subjective symptoms and to conduct a detailed interview. The process of clinical evaluation for masked hypertension considering OSA is shown in Figure 16.557 First, early morning blood pressure is measured by a home blood pressure monitoring device, and if the level is ≥135/85 mmHg, the patient is considered to have early morning hypertension, and antihypertensive therapy targeting early morning blood pressure is administered. When the early morning blood pressure level is <135/85 mmHg, ABPM is performed, and if the 24-hour blood pressure level averages ≥130/80 mmHg, the patient is considered to have stress hypertension if the daytime blood pressure is high and nocturnal hypertension if the nighttime blood pressure is high, and antihypertensive treatment targeting these conditions is administered. If the patient has treatment-resistant nocturnal and early morning hypertension in which the nocturnal and early morning blood pressure is not controlled by foregoing therapy, OSA is suspected.557 In addition, OSA is actively suspected in patients with nocturia, nocturnal dyspnea, a history of nocturnal cardiovascular events or left ventricular hypertrophy despite normotension, even if the 24-hour blood pressure is normal, including nighttime blood pressure. Even if the 24-hour blood pressure is normal (<130/80 mmHg), OSA should be suspected in cases with advanced organ damage, especially left ventricular hypertrophy, which is easily affected by pressure overload.557 In OSA patients, even if the 24-hour blood pressure including nighttime blood pressure assessed by ABPM is completely normal, the periodic negative intrathoracic pressure causes a strong pressure load on the left ventricular wall, resulting in the development of hypertensive heart disease.

1.6 Treatment of Hypertension Complicated by OSA (Table 27)
Table 27.
Recommendations and Levels of Evidence for Treatment of OSA in Hypertension
| |
COR |
LOE |
| CPAP therapy for hypertensive patients with moderate or severe OSA should be considered* |
IIa |
A |
CPAP therapy for treatment-resistant hypertensive patients with moderate or severe OSA should
be considered* |
IIa |
A |
| OA treatment for hypertensive patients with mild or moderate OSA should be considered |
IIa |
A |
Strict antihypertensive therapy with a target nighttime blood pressure <120/70 mmHg for
hypertensive patients with mild or moderate OSA should be considered |
IIa |
C |
Strict antihypertensive therapy with the goal of achieving a nocturnal blood pressure
<120/70 mmHg for hypertensive patients with moderate or severe OSA who refuse or self-disrupt
CPAP therapy should be considered |
IIa |
C |
*Health coverage for CPAP in Japan is for AHI ≥20 by PSG or AHI ≥40 by portable monitoring. AHI, apnea–hypopnea index; COR, Class of Recommendation; CPAP, continuous positive airway pressure; LOE, Level of Evidence; OA, oral appliance; OSA, obstructive sleep apnea; PSG, polysomnography.
1.6.1 Non-Drug Treatment
Obesity and hypertension are closely related590 and weight loss and exercise are most effective in obese OSA patients. Alcohol and SDB are clear risk factors for hypertension,591 so strongly advise sobriety. Smokers should be instructed to quit smoking. Digital therapies that support these lifestyle modifications are expected to be effective for patients with OSA, as they show significant reductions in blood pressure.592
1.6.2 Continuous Positive Airway Pressure (CPAP)
In hypertensive patients with moderate or severe OSA, CPAP therapy should be performed (Figure 16).557 CPAP treatment produces a blood pressure lowering effect in many patients and reduces blood pressure surges at night. In a clinical trial comparing the blood pressure lowering effects of CPAP and supplemental oxygenation therapy, blood pressure reduction was seen only in the CPAP-treated group.593 A meta-analysis of 31 randomized controlled trials (RCTs) to date found a significant reduction in systolic and diastolic blood pressures of 2.6 mmHg and 2.0 mmHg, respectively, in the CPAP group compared with controls.594 The effect of CPAP varies among individuals, and in hypertensive patients with characteristics such as higher blood pressure levels, untreated hypertension, nocturnal hypertension, and treatment-resistant hypertension, CPAP has a greater antihypertensive effect.386,595–597 Especially in the non-dipper/riser type of nocturnal hypertension, CPAP selectively lowers blood pressure during sleep and often restores normal dipper-type blood pressure.598 In addition, the degree of blood pressure reduction by CPAP is greater in patients with more severe OSA (AHI ≥30) and high BMI.386,599 The presence or absence of daytime sleepiness also influences the antihypertensive effect of CPAP. Patients with OSA may not benefit from CPAP for daytime blood pressure reduction599–601 and the retention rate of CPAP treatment is low. The antihypertensive effect of CPAP treatment can be expected when adherence to CPAP is good, and it is important to use it for at least 3 h/night595 and for longer periods of time.602 Use of CPAP therapy for >4 h/night, compared with <4 h, reduces early morning home blood pressure the day after CPAP use, with the reduction being more pronounced in winter months.603 The antihypertensive effect of CPAP therapy is particularly significant in patients with OSA complicated by treatment-resistant hypertension.566 A meta-analysis of 4 RCTs found a 6.7 mmHg systolic and 5.9 mmHg diastolic blood pressure reduction in the CPAP group compared with the control group.604 In the HIPARCO RCT study of OSA patients with resistant hypertension, 24-hour blood pressure was reduced by ≈3 mmHg after 3 months of CPAP treatment compared with the CPAP-naïve group. The degree of reduction was proportional to the duration of CPAP use. In addition, the CPAP group was 2.4-fold more likely to have normal dipper pattern in the analysis of nighttime blood pressure patterns.396 In the analysis of the study participants who presented good adherence to CPAP, increased hypoxia (partial pressure of oxygen <90%) time, severe OSA (AHI 30) or greater, and higher blood pressure at baseline had greater blood pressure reduction with CPAP therapy.605 In patients with OSA complicated by hypertension who were being treated with ≥3 antihypertensive drugs, CPAP therapy also reduced 24-hour blood pressure by 4.4 mmHg systolic and 2.9 mmHg diastolic compared with the control group, and also significantly reduced blood troponin I and B-type natriuretic peptide (BNP) levels.606
1.6.3 Antihypertensive Medications
Cardiovascular risk remains in patients with mild or moderate OSA hypertension and in patients with moderate/severe OSA hypertension who refuse or self-disrupt CPAP. Such patients should be considered high-risk hypertensive cases and should be treated with more rigorous 24-hour antihypertensive therapy.557 Although there is no evidence yet on the target antihypertensive level, it is important to keep blood pressure below the threshold of 120/70 mmHg, especially at night, taking into account the increased negative intrathoracic pressure load during apneic attacks on the thoracic aorta and heart.587 There is also no clear evidence regarding the type of antihypertensive medication. Antihypertensive drugs of all classes, including centrally acting α-methyldopa, β-blockers, calcium-channel blockers, and angiotensin-converting enzyme (ACE) inhibitor, do not alter SDB itself.607,608 In a study of a small number of patients, β-blockers significantly reduced office diastolic blood pressure compared with calcium-channel blockers, ACE inhibitors, angiotensin II receptor blockers (ARBs), and diuretics. There is also a report that β-blockers significantly reduced nocturnal systolic and diastolic blood pressures compared with calcium-channel blockers, ACE inhibitors, and ARBs (no difference with diuretics), although there was no difference in the degree of reduction in daytime and waking blood pressures.609 However, there is a report that β-blocker monotherapy reduced daytime blood pressure but there was difficulty controlling nocturnal sleep blood pressure,610 and there is no certainty regarding the specific efficacy of β-blockers for OSA. Plasma aldosterone levels are increased in treatment-resistant hypertension in OSA.580 In a study that screened 203 patients with OSA for primary aldosteronism, it was detected in 11.8% of Caucasians and 5.9% of Chinese, and there was a correlation between serum aldosterone levels and the AHI in Caucasians.611 Spironolactone, an aldosterone antagonist, significantly reduces both the severity of OSA and blood pressure.612
In terms of suppressing organ damage, the renin–angiotensin–aldosterone (RAA) system inhibitors may be useful in patients with OSA, especially in obese patients, because the RAA system is hyperactive and left ventricular hypertrophy is a common complication. In hypertensive patients with OSA complicated by HF, diuretics can improve fluid retention in the peripharyngeal mucosa and thus OSA.613 On the other hand, in OSA patients with dry cough induced by ACE inhibitors, it has been pointed out that coughing may cause inflammation of the upper airway, which may worsen OSA itself.614 Sodium glucose cotransporter 2 (SGLT2) inhibitors615–617 and angiotensin receptor–neprilysin inhibitors (ARNI),618–620 novel drugs for HF, reduce 24-hour blood pressure in patients with nocturnal hypertension, but may also be effective in lowering blood pressure in patients with OSA.557,568,621 SGLT2 inhibitors also reduce cardiovascular events in patients with OSA,622 and ARNI also lowered AHI in a prospective cohort study of sleep apnea patients with HF.443
1.6.4 Other Treatments
In a RCT of CPAP and oral appliance (OA) in patients with OSA and a mean AHI of 25.6, CPAP was superior in lowering AHI, but adherence was better with the OA, and the 24-hour mean blood pressure reduction was not different between groups. A meta-analysis of RCTs showed that an OA reduced both systolic and diastolic blood pressures by 2.7 mmHg.623 A RCT of uvulopalatopharyngoplasty (UPPP) showed a 9.4 mmHg reduction in systolic blood pressure and a 6.4 mmHg reduction in diastolic blood pressure.624 In a RCT of patients with moderate to severe OSA complicated by treatment-resistant hypertension, renal sympathetic denervation reduced the AHI and office and 24-hour blood pressures, and global longitudinal strain on echocardiography was significantly improved.625 Hypoglossal nerve stimulation improved the AHI and OSA severity,435 but there was insufficient evidence for a 24-hour blood pressure-lowering effect.
2. Diabetes Mellitus (Table 28)
Table 28.
Recommendation and Level of Evidence for Evaluation of SDB in Diabetes Mellitus
| |
COR |
LOE |
| It is recommended to ask patients about clinical symptoms of SDB and screen for SDB |
I |
B |
COR, Class of Recommendation; LOE, Level of Evidence; SDB, sleep disordered breathing.
The risk of developing type 2 diabetes is 1.2–2.1-fold higher, depending on the severity of OSA. A meta-analysis of 9 studies showed a 1.4-fold risk of developing type 2 diabetes in OSA.57
The mechanism by which OSA causes type 2 diabetes is thought to be that sympathetic hyperactivity due to intermittent hypoxia and sleep fragmentation during the night increases hypothalamic–pituitary–adrenal (HPA axis) function, leading to abnormal glucose metabolism and insulin resistance (Figure 17). Only a few small studies have reported the impact of OSA on the cardiovascular outcomes in patients with type 2 diabetes. In a large 14-year retrospective cohort study from the UK of 3,667 patients, OSA in patients with type 2 diabetes was significantly associated with the development of cardiovascular events,628 which indicates that patients with type 2 diabetes complicated by OSA are a high-risk population for cardiovascular events and demonstrates the importance of assessing the presence of OSA in the management of patients with type 2 diabetes.

A number of cross-sectional studies have examined the relationship between OSA and diabetic microvascular complications, and it has been speculated that OSA and type 2 diabetes may share an increased susceptibility to oxidative stress,629,630 affecting retinal endothelial cells, mesangial cells in the renal glomerulus, neurons and Schwann cells in peripheral nerves. In a longitudinal study looking at associations over time, a retrospective cohort study found a 1.18-fold increase in new onset of CKD severity class G3–G5 in patients with type 2 diabetes mellitus.628 There are conflicting results for the effect of CPAP on glucose metabolic abnormalities in patients with OSA complicated by type 2 diabetes. The effect of CPAP on glycemic variability has not been conclusively demonstrated.212,213,633–635 Some have reported that overnight CPAP use improved glycemic variability,636 while others have reported that 12 weeks of CPAP use did not improve glycemic variability.637 From these studies, it seems unlikely that CPAP alone, at least, has a clinical benefit sufficient to outweigh the effects of other factors. In contrast, interventions with both CPAP and weight loss have been shown to significantly improve HbA1c,215,638 suggesting the need for lifestyle modification as well as CPAP. A subanalysis of the EMPA-REGOUTCOME trial reported that empagliflozin, an SGLT2 inhibitor for the treatment of type 2 diabetes, reduced the risk of new OSA in patients with type 2 diabetes.622 Future prospective RCTs are needed to evaluate the efficacy of SGLT2 inhibitors on the risk of residual cardiovascular events associated with OSA.
3. CKD / Endstage Renal Disease (ESRD) (Table 29)
Table 29.
Recommendations and Levels of Evidence for Evaluation of SDB in CKD
| |
COR |
LOE |
| It is recommended to ask patients about clinical symptoms of SDB and screen for SDB |
I |
B |
| It is recommended to optimize the treatment of CKD itself, including fluid management |
I |
A |
CPAP therapy to improve renal prognosis in CKD patients with moderate to severe OSA
should be considered |
IIa |
B |
CKD, chronic kidney disease; COR, Class of Recommendation; CPAP, continuous positive airway pressure; LOE, Level of Evidence; OSA, obstructive sleep apnea; SDB, sleep disordered breathing.
The diagnosis and treatment of SDB in patients with CKD are considered important, given that SDB is a poor prognostic factor in such patients.642
3.1 Prevalence and Characteristics of SDB
Even after adjusting for variables such as age and BMI, the presence of CKD is an independent risk factor for SDB, of which the prevalence and severity increase as CKD progresses.64 Although OSA is the predominant form of SDB associated with CKD, CSA is especially present in patients with ESRD.64 ESRD has features of the pathogenesis of both CSA and OSA, which are characterized by altered sleep architecture and respiratory muscle fatigability due to uremic toxins, instability of the central respiratory drive, and airway narrowing with edema of the upper airway due to fluid shifts and fluid retention during the night.643,644
CKD patients are less likely to develop SDB symptoms, although SDB symptoms such as easy fatigue and lack of sound sleep during the daytime often overlap with symptoms of CKD and other comorbidities. Furthermore, insomnia, midnight awakenings, daytime somnolence, and restless legs syndrome (RLS) are common symptoms of ESRD. Therefore, screening for SDB is important. Aggressive suspicion and close examination are recommended in CKD patients with sleep-related symptoms and treatment-resistant hypertension.
3.2 Impact of SDB on the Onset and Progression of CKD
The presence of OSA in the general population is an independent risk factor for the development of CKD and for decline in the glomerular filtration rate (GFR) over time.645,646 In patients with CKD stages G3 and G4, moderate or greater SDB is associated with a significantly faster decline in GFR than with less severe SDB, and nocturnal hypoxia is an independent risk factor for GFR decline.648 Furthermore, the more severe the SDB, the greater the proportion of patients with albuminuria and the AHI correlates positively with urinary protein.649
It is considered that the hypoxic activation of the RAA system, and increased sympathetic nerve activity resulting from SDB are causally associated with CKD. The renal medulla is particularly sensitive to hypoxia, and chronic nocturnal hypoxemia leads to tubulointerstitial damage. Intermittent hypoxia also increases reactive oxygen species (ROS) and the inflammatory response in renal tissues and induces myofibroblast differentiation and extracellular matrix accumulation. In addition, the complex systemic effects of SDB, such as oxidative stress, vascular endothelial dysfunction, atherosclerosis, and elevated blood pressure, may contribute to the progression of CKD (Figure 18).577 Very short-term blood pressure variability (standard deviation) of diastolic blood pressure during the night is reported to be associated with CKD.
3.3 Prognostic Impact of SDB in CKD Patients
The presence of SDB is associated with adverse prognosis in CKD patients. A meta-analysis examining the association between CKD patients and SDB prognosis revealed that the presence of SDB was related to increased mortality in CKD patients (risk ratio 1.47),642 as well as increased risk of cardiovascular events in ESRD patients on maintenance dialysis (risk ratio 2.45). In CKD patients with stages G4 and G5, the risk of all-cause death increased with the severity of SDB, and multivariate analysis showed that low mean oxygen saturation and the percentage of sleep time with oxygen saturation <90% were independent prognostic factors for all-cause death.650
3.4 Treatment of SDB in CKD Patients
CPAP treatment for SDB may improve renal hemodynamics, decrease RAA system activity, and reduce the progression of renal injury. In a clinical trial examining the effects of short-term CPAP treatment on renal function in patients with OSA, 1 month of CPAP increased renal blood flow, decreased plasma aldosterone and urinary protein levels, and improved renal plasma flow responsiveness to angiotensin II.651 In addition, CPAP treatment for 3 months reduced albuminuria in patients with OSA,649 and a meta-analysis showed an improvement in GFR in OSA patients using CPAP for >3 months.652 A study examining the effect of long-term CPAP treatment on renal function in patients with OSA showed that fixed-CPAP was more effective than auto-CPAP in reducing GFR decline over time during a 541-day observation period. In patients with CKD stages G3–G5 with OSA, use of CPAP with good adherence (>70%) for at least 4 h/day was found to be effective in reducing GFR decline and the level of urinary protein.653 However, in a subanalysis of the SAVE trial, a RCT of CPAP in patients with OSA, CPAP did not improve renal outcomes such as GFR decline or urinary protein appearance compared with the non-CPAP group.655 Another RCT examining the effect of CPAP on renal function in patients with CKD stage G3 or G4 with OSA showed no difference in GFR and albuminuria between the CPAP and non-CPAP groups during the 12-month study,656 but the possibility of an improvement in GFR by CPAP has been suggested in patients at low risk of CKD progression. Because CKD patients often have CVD, their comorbidities should be taken into account and the indications for CPAP should be considered on an individual basis. It has been reported that 6 months of adaptive servo-ventilation (ASV) therapy led to improvements in GFR and cystatin C levels in CKD patients with HF.657 Nocturnal dialysis therapy and renal transplantation may improve SDB in patients with ESRD, but future studies are needed (see Chapter I, Section 5.4.2 for details).
4. Hyperuricemia
A report from Taiwan found that hyperuricemia is a complication in approximately 25% of patients with SDB.658 In a report from Brazil, patients with OSA with an AHI between 5 and 14.9 had significantly higher uric acid (UA) levels than controls, even after adjusting for age, sex, BMI, and cardiovascular risk factors.659 This finding was also been shown in a meta-analysis. Mechanisms associated with elevated UA levels in SDB include the following: nocturnal tissue hypoxia reduces ATP synthesis by oxidative phosphorylation of the mitochondrial electron transfer system, which increases ATP, ADP, and AMP degradation and hypoxanthine production. Next, reoxygenation following hypoxia may promote xanthine oxidase (XO)-mediated hypoxanthine and xanthine metabolism, and excessive production of UA as an end product. In fact, a study examining UA excretion as an indicator of nocturnal tissue hypoxia due to OSA reported that UA excretion is enhanced in OSA with pronounced hypoxemia,661 and that CPAP reduces UA excretion in OSA patients to a level not different from controls.662 Therefore, there is a possibility that suppression of OSA by CPAP may reduce blood UA levels; however, in a RCT examining the UA-lowering effects of CPAP treatment on OSA patients with diabetes, there was no significant difference in the change in UA levels before and after the intervention,663 which suggests that in OSA patients with other lifestyle-related diseases the cause of hyperuricemia is not only the effect of hypoxia due to OSA, but also from multiple factors, including decreased UA excretion. On the other hand, it has also been shown that reduction of UA levels was influenced by CPAP usage. UA levels in patients with OSA may be associated with the risk of vascular damage and CVD, with increased UA levels being associated with increased P-wave width on ECG, which is a predisposing factor of atrial fibrillation (AF),665 increased incident AF666 increased arterial stiffness,667 and incident CVD.668 In addition, a RCT evaluating allopurinol in patients with OSA found that the drug improved vascular endothelial function.669
5. HF (HF With Reduced Ejection Fraction [HFrEF])
5.1 Characteristics of SDB
SDB in HF patients is characterized by a high rate of central sleep apnea with Cheyne-Stokes respiration (CSA-CSR) in addition to OSA. Factors that determine the presence of SDB in patients with HFrEF include male sex, age, BMI, and AF.670 In patients with HF, OSA alone or CSA-CSR alone is rather rare, and the combination is more common.
5.2 Related Mechanisms
5.2.1 Mechanisms by Which OSA Develops and Progresses to HF
Intermittent hypoxemia and arousals due to upper airway obstruction during sleep increase sympathetic nerve activity, blood pressure, and heart rate during the night and day. In addition, exertional breathing during airway obstruction produces excessive negative pressure in the thoracic cavity as low as −80 cmH2O, which is repeated with each respiratory event throughout the night. The result is high transmural pressure in the left ventricle, increased afterload, left ventricular hypertrophy, and adverse effects on left ventricular function,148 leading to an imbalance between oxygen supply and demand to the myocardium, increasing the risk of myocardial ischemia, myocardial contractile dysfunction, and arrhythmias.84,244,673 The concomitant increase in pulmonary vascular resistance due to hypoxic pulmonary vasoconstriction and the increase in right ventricular filling due to increased venous return induce right HF, as well as compression of the ventricular septum during diastole, impair left ventricular filling and reduce cardiac output.145,674 In addition to hemodynamic stress, other mechanisms through which OSA induces cardiovascular injury include endothelial dysfunction, oxidative stress, inflammation, increased coagulation, and metabolic dysfunction such as obesity and insulin resistance.675 These induce coronary plaque disruption, myocardial damage, and arrhythmias, which in turn lead to the development and progression of underlying cardiac diseases that cause HF.243,676 Although HF causes systemic fluid retention, excessive fluid is stored mainly in the lower extremities during daytime activities when the patient is standing upright. A vicious cycle develops in which the supine position during sleep causes fluid to shift from the lower extremities, resulting in upper airway edema and exacerbation of OSA.244,250,677
In a large cohort study in the USA, men with untreated severe OSA were shown to be at higher risk of developing HF,49,67 and a recent study reported that sleep apnea-specific hypoxic burden (SASHB) is more strongly associated with the development of HF than the AHI. Because the prognosis of HFrEF patients with OSA is poor,107 and some reports suggest that treatment of OSA in HFrEF patients improves cardiac function and prognosis,154,679 OSA is a risk factor for the onset and progression of HF and may influence each stage of chronic HF.
5.2.2 Mechanisms of CSA-CSR
Whereas OSA increases the risk of the onset or exacerbation of HF, CSA-CSR is considered a consequence of HF. Patients with HF and CSA-CSR tend to have higher pulmonary artery wedge pressure,257 larger left ventricular end-diastolic volume,240 higher urinary noradrenaline concentration,680 increased urinary noradrenaline,681 and increased ventilatory response to exercise.253 The major predictors of CSA-CSR in patients with HFrEF include older age, male sex, AF, and hypocapnia (arterial partial pressure of carbon dioxide [PaCO2] ≤38 mmHg).109 CSA-CSR is rare in women. The pathogenesis of CSA-CSR is discussed in detail in Chapter I, Section 4.2.1.
5.3 Impact of SDB on HFrEF Prognosis
Few studies have examined the impact of comorbid OSA on the long-term prognosis in HF patients. In an observational study of HF patients with left ventricular ejection fraction (LVEF) <45%, the group with an AHI ≥15 and untreated OSA had a worse prognosis than the group with AHI <15, and multivariate analysis showed that untreated OSA with an AHI ≥15 was a prognostic factor. In a multivariate analysis, untreated OSA with AHI ≥15 was a prognostic factor in HFrEF patients.107 CSA-CSR is a consequence of HF, but in patients with severely reduced cardiac function, CSA-CSR may further adversely affect cardiac function, and indeed, there are reports showing an association with poor prognosis. CSA-CSR with AHI ≥30 is an independent determinant of cardiac death in patients with low left ventricular function (LVEF ≤35%).110,684 Furthermore, in HFrEF patients, CSA-CSR with AHI ≥30 is associated with a poor prognosis.111 In addition, CSA with an AHI ≥15 on tests performed during hospitalization of patients with HFrEF is a predictor of rehospitalization for a cardiac event within 6 months.685
Many reports suggest that SDB, regardless of its type, has a negative impact on the prognosis of patients with HFrEF; the incidence of fatal arrhythmias causing sudden death is higher in patients with SDB, whether OSA or CSA, and the need for implantable cardioverter-defibrillator (ICD) therapy is higher.686,687 In a 3-year study of post-discharge survival in patients with acute uncompensated HF (LVEF ≤45%), OSA and CSA with an AHI ≥15 detected by sleep testing during hospitalization were prognostic factors, and the prognosis was poor when these were not treated.688 In a recent report, patients with acute HF with an AHI ≥15, regardless of SDB type, who did not receive positive-pressure therapy had a high incidence of death or rehospitalization for HF after discharge.689 On the other hand, it has been reported that hypoxamic burden (SpO2
<90% of total time) is a stronger determinant of all-cause death in patients with HFrEF than AHI,671 and further research is warranted on indices to assess SDB in HF patients.
5.4 Evaluation of SDB Associated With HFrEF
It has been reported that in patients with HFrEF complicated by SDB, those without excessive daytime sleepiness have a higher mortality rate than those with sleepiness.691 Therefore, in patients with chronic HF, the presence of SDB should always be kept in mind, regardless of the presence or absence of symptoms such as daytime sleepiness. In patients hospitalized for acute decompensated HF, a 4%ODI ≥5 assessed by pulse oximeter before discharge has been reported as a marker of poor prognosis, including rehospitalization for HF,30 so screening with pulse oximetry may also have some value. On the other hand, although there are reports that assessment of HF patients with portable monitors shows good correlation with PSG for both severity and type of SDB,2 even systems capable of detecting respiratory motion generally have low accuracy in determining CSA and are not recommended as diagnostic.692 PSG is important to assess sleep quality in HF patients when moderate or severe SDB is suspected and to evaluate for complications of periodic limb movements,24,693 which are associated with poor prognosis.
5.5 Treatment of SDB Associated With HFrEF
5.5.1 Treatment of HF Itself
For OSA, lifestyle modifications such as treatment of obesity, smoking cessation, alcohol moderation, and appropriate exercise, which are risk factors or aggravating factors for OSA, are useful for HF itself, and should be applied aggressively first. It has been reported that diuretics in the treatment of HF reduce OSA itself, which may be due to the reduction in nocturnal fluid shift in addition to the improvement the upper airway edema by fluid optimization. On the other hand, because CSA-CSR is caused by HF itself, it is most important to optimize the treatment of HF. For details, please refer to Chapter I, Section 5.3.6. If SDB persists even after optimizing HF treatment, direct intervention for SDB should be considered.
5.5.2 Positive-Pressure Treatment for OSA Complicated by HFrEF (Table 30)
Table 30.
Recommendations and Levels of Evidence for the Treatment of OSA in HFrEF
| |
COR |
LOE |
It is recommended to provide guideline-recommended CPAP therapy for symptomatic
OSA patients |
I |
A |
CPAP therapy to improve left ventricular function in HFrEF patients with moderate* or
severe OSA should be considered |
IIa |
A |
CPAP therapy to improve prognosis in HFrEF patients with moderate* or severe OSA may
be considered |
IIb |
C |
*Moderate level is generally defined as AHI ≥15, but the insurance coverage level in Japan is AHI ≥20. AHI, apnea–hypopnea index; COR, Class of Recommendation; CPAP, continuous positive airway pressure; HFrEF, heart failure with reduced ejection fraction; LOE, Level of Evidence; OSA, obstructive sleep apnea.
For OSA with or without HF, the efficacy of CPAP treatment is largely established. In patients with HFrEF, RCTs have reported that CPAP improved LVEF in patients with severe OSA and even mild to moderate OSA,695 and meta-analyses have confirmed similar effects.695 Observational studies have shown that CPAP treatment for patients with moderate or severe OSA improves the prognosis of HF.107,679,696 The results of RCTs evaluating the primary and secondary prevention of CVD with CPAP in a large number of patients, regardless of whether they had HF or not, were not significantly different between the CPAP and control groups.393,394,397 One of the most important reasons for this is low adherence to CPAP, which supports the idea that CPAP should be used for at least 4 h to reduce cardiovascular events. There have been no RCTs of the prognostic value of CPAP in HF patients with OSA. A multicenter RCT of ASV in HF patients with moderate or severe OSA or CSA-CSR and LVEF <45% (ADVENT-HF), was presented at the European Cardiology Society Congress in August 2022.699 The overall analysis did not confirm a significant effect of SDB treatment with ASV on cardiovascular events, and the same was true in the subgroup analysis of OSA-predominant patients, so a report with more detailed information is awaited. At present, patients with OSA-related symptoms such as daytime sleepiness should be treated whether or not they have HF, and CPAP therapy should be considered for HFrEF patients with moderate or severe OSA to improve LVEF (Table 30).473
5.5.3 Positive-Pressure Treatment for CSA-CSR Complicated by HFrEF (Table 31)
Table 31.
Recommendations and Levels of Evidence for Treatment of CSR-CSA in HFrEF
| |
COR |
LOE |
It is recommended to optimize HF treatment itself in accordance with HF guidelines for patients
with CSA-CSR and HFrEF |
I |
A |
CPAP therapy to improve subjective symptoms, exercise tolerance, and left ventricular function in
HFrEF patients with moderate or severe CSA-CSR should be considered |
IIa |
B |
ASV therapy to improve subjective symptoms, exercise tolerance, and left ventricular function for
HFrEF patients (LVEF ≤45%) with moderate or severe CSA-CSR who are intolerant to or unable
to tolerate CPAP therapy may be considered |
IIb |
B |
Patients with HFrEF with CSA-CSR (LVEF ≤45%) should not be allowed to continue ASV
therapy* indefinitely after improvement or stabilization of HF |
III
(Harm) |
B |
*Particular care must be taken when continuing to use the device under the same conditions (ASV model, pressure settings, etc.) as in the SERVE study for HFrEF patients with similar backgrounds to those enrolled in the SERVE study. ASV, adaptive servo-ventilation; CSA-CSR, central sleep apnea with Cheyne-Stokes respiration; COR, Class of Recommendation; CPAP, continuous positive airway pressure; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; LOE, Level of Evidence; LVEF, left ventricular ejection fraction.
a. CPAP
CPAP has been shown to have a short-term effect not only in suppressing CSA-CSR associated with HFrEF, but also in reducing sympathetic nerve activity, improving LVEF, and improving exercise tolerance.243 However, in the CANPAP study,499 a RCT examining the effect of CPAP on the prognosis of HF patients with CSA-CSR, although the AHI was halved, LVEF increased, and short-term improvements in 6-minute walking distance were obtained in the CPAP group compared with the control group, no improvement of prognosis was demonstrated. However, a post-hoc analysis of the CANPAP study showed that the group of patients who improved their AHI <15 under CPAP at 3 months after starting CPAP (CPAP responder) had a better prognosis than those who did not.500
b. ASV
ASV can treat CSA-CSR more effectively than CPAP, and RCTs and meta-analyses have indicated that ASV improves LVEF and exercise tolerance and lowers BNP levels in HFrEF with CSA-CSR.700,701 In HF patients with comorbid CSA-CSR and OSA, ASV was associated with better adherence and improved LVEF and BNP levels at 3 months compared with CPAP alone.702 In addition, the use of ASV in patients with residual CSA-CSR on CPAP showed improvement in both CSA-CSR and LVEF.703 Other effects on respiratory and hemodynamic status from ASV, in addition to lowering BNP,657,704 improving LVEF,657,704,705 suppressing sympathetic nerve activity,706–710 reducing inflammation,657 improving renal function,657,711 and suppressing AF and ventricular arrhythmia,712,713 have been reported from Japan and other countries. In addition, observational studies have demonstrated reductions in cardiac mortality and HF rehospitalization rates.657,704 For patients with residual SDB after cardiac resynchronization therapy (CRT), ASV lowered BNP levels and reduced rehospitalization rates for HF in these patients.714 Although these results were obtained in a relatively small number of patients, a subsequent meta-analysis showed that CPAP tended to improve life expectancy and ASV improved life expectancy in patients with chronic HF complicated by SDB.715 However, the SERVE-HF study,682 a large RCT examining the prognostic value of ASV in 1,325 chronic HF patients with LVEF ≤45% and CSA-dominant SDB with AHI ≥15, found that the primary endpoints of total deaths, life-saving cardiovascular interventions (cardiac transplantation, left ventricular assist device [LVAD] implantation, resuscitation from cardiac arrest, and ICD-appropriate cardiac arrest) and the secondary endpoints of all-cause death and cardiovascular death were rather increased in the ASV group. Based on this, the 2016 ESC guidelines and the 2017 ACC/AHA/HFSA guideline revisions position ASV use as not recommended (Class III “not recommended”) for CSA-driven chronic HF patients with LVEF ≤45%.716,717 The CAT-HF trial, in which SDB (AHI ≥15) was detected in patients hospitalized with acute decompensated HF and then assignment to the ASV or control group, was stopped early due to the results of SERVE-HF.718 However, the SERVE-HF study has also raised a number of issues, including the large number of crossover patients between the 2 groups, problems with the intention-to-treat analysis, and differences with the Japanese patients’ backgrounds and the results of previous studies. A subanalysis of the SERVE-HF trial showed an interaction between ASV use and hospitalization for HF, with more cases of HF in the ASV group among patients with LVEF <30%, but fewer cases in the ASV group amongpatients with LVEF >36%.723 The results also suggest that long-term ASV use in patients with severe HFrEF may carry risks such as low cardiac output.682 Similarly, an interaction was observed for the CSA-CSR ratio in SDB, with a trend toward fewer HF exacerbations in the ASV group among patients with a CSA-CSR ratio <20%, while HF exacerbations were significantly higher in the ASV group among patients with a CSA-CSR ratio ≥50%.682,723 These results suggest that CSA-CSR is a compensatory mechanism in HF and that hyperventilation due to CSA-CSR itself is cardioprotective by decreasing CO2, increasing the endogenous positive end-expiratory pressure (PEEP), and increasing cardiac output.724 Even now, however, the evidence on the possibility of treatment of CSA-CSR itself is not been fully established. On the other hand, the phenotype of CSA-CSR may differ depending on the degree of pulmonary congestion, circulatory delay, and hyperventilation involved in the pathogenesis of CSA-CSR,265,266,712 suggesting that a detailed classification of CSA-CSR phenotypes may reveal the types of CSA-CSR that should be treated with positive-airway pressure therapy.265,266,712 Furthermore, the SERVE-HF study did not include cases of HF with preserved ejection fraction (HFpEF), OSA-predominant SDB, or acute and subacute HF, suggesting that the results should not be generalized to all HF cases.682 The results of another RCT of ASV for SDB in HFrEF (ADVENT-HF trial699) did not find that ASV was associated with worse prognosis in the CSA-predominant subgroup, and reported that there was a non-significant but modest trend for cardiovascular events in the ASV intervention group in the CSA subgroup. It is noteworthy that the trend was different to the results of the SERVE-HF trial.
c. ASV for HFrEF Patients in Japan
In Japan, the SAVIOR-C trial, a multicenter RCT conducted in patients with HFrEF, showed a significant improvement in the ASV group in the clinical response, a composite of symptoms and HF exacerbation at 6 months, although this was a secondary endpoint,725 and even after the SERVE-HF trial, based on statements by the Japanese Circulation Society and the Japanese Heart Failure Society (2nd report) (Appendix) and the recommendations in the “Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure” (JCS 2017/JHFS 2017), the use of ASV is not contraindicated for HFrEF complicated by CSA-dominant SDB. In particular, the clinical composite response in the SAVIOR-C trial was evaluated at 6 months, and the impact of ASV therapy over a longer period is unknown. Therefore, the switch to CPAP should be considered as soon as possible after the introduction of ASV. In light of the results of the ADVENT-HF trial, a revision of this statement may be considered.73 Considering the secondary endpoint of the SAVIOR-C study (i.e., improvement in the clinical composite response) and considering that ASV has been used in real clinical practice to improve not only symptoms but also stabilize cardiac function in patients with severe HF whose congestive symptoms have not improved despite standard treatment and that ASV has shown a certain level of efficacy in real world in Japan,473 it is possible to use ASV to relieve congestion and continue it after discharge from the hospital under insurance reimbursement. However, it should be noted that it is necessary to reexamine the possibility of withdrawal from ASV or switching to a treatment other than ASV when HF is clinically judged to have stabilized or 6 months have passed since the introduction of ASV.
5.5.4 Oxygen Therapy for CSA-CSR (Table 32)
Table 32.
Recommendation and Level of Evidence for HOT for Patients With SDB and HF
| |
COR |
LOE |
HOT to improve cardiac function and subjective symptoms in patients with NYHA
functional class III or higher HFrEF (LVEF ≤45%) with moderate* or severe CSA-CSR
should be considered |
IIa |
B |
*Moderate disease is generally defined as AHI ≥15, but the insurance coverage level in Japan is AHI ≥20. AHI, apnea–hypopnea index; COR, Class of Recommendation; CSA-CSR, central sleep apnea with Cheyne-Stokes respiration; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; HOT, home oxygen therapy; LOE, Level of Evidence; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; SDB, sleep disordered breathing.
Short-term studies of nocturnal oxygen therapy have reported disappearance of CSA-CSR, suppression of sympathetic nerve activity, improvement in exercise tolerance, and reduction in plasma BNP concentration in patients with chronic HF.495,706,727 A multicenter study (Chronic Heart Failure Nocturnal Home Oxygen Therapy [CHF-HOT] study) conducted in Japan to evaluate the effect of nocturnal oxygen therapy (3 L/min) in chronic HF patients with CSA-CSR and LVEF ≤45%, found that the oxygen group did not show a reduction in the composite cardiovascular events of HF hospitalization and cardiovascular death. On the other hand, in a recent RCT conducted in Japan, 3 months of nocturnal oxygen therapy resulted in a mild decrease in AHI and a non-significant improvement in LVEF compared with ASV.728 Nocturnal oxygen therapy is a simple, easy-to-use therapy, with low patient burden and generally good compliance. However, because the PaCO2
rarely increases in patients with chronic lung disease or severe obesity, causing impaired consciousness, careful judgment and understanding of the pathophysiology are necessary when adjusting the flow rate. A large RCT of oxygen therapy in chronic HF patients with LVEF <50% complicated by CSA is underway,729 and further studies on efficacy, including cardiovascular death, and safety, including adverse events, are warranted.
5.5.5 Transverse Phrenic Nerve Stimulation (PNS) Therapy for CSA
The Remedē System Pivotal Trial, a RCT of PNS conducted in 151 HFrEF patients with CSA, showed >50% reduction in the AHI at 6 months and a 36-month safety and tolerability profile.505,514 A post-hoc analysis of The Remedē System Pivotal Trial reported a significant improvement in LVEF in HF patients at 12 months post-PNS compared with baseline,508 and a significant improvement in LVEF at 12 months post-PNS compared with baseline.509 PNS may improve outcomes such as death and HF hospitalizations in HF patients through improvement of CSA without affecting patient adherence.508 Future studies should examine cardiac function and prognosis in large RCTs of CSA associated with HF or CVD.
6. HF (HFpEF) (Table 33)
Table 33.
Recommendations and Levels of Evidence for Treatment of SDB in HFpEF
| |
COR |
LOE |
It is recommended to perform CPAP therapy for HFpEF patients with symptomatic OSA to
improve OSA symptoms |
I |
A |
CPAP therapy for HFpEF patients with moderate or severe OSA to improve subjective symptoms,
exercise tolerance, cardiac function, and comorbidities which can be related to the pathophysiology
of HFpEF should be considered |
IIa |
A |
CPAP therapy to improve prognosis in HFpEF patients with moderate or severe OSA may be
considered |
IIb |
C |
ASV therapy to improve subjective symptoms, exercise tolerance, and cardiac function in
patients with HFpEF who have moderate or severe CSA-CSR and who do not tolerate or do not
respond to CPAP therapy should be considered |
IIa |
A |
ASV therapy to improve prognosis in HFpEF patients with moderate or severe CSA-CSR who are
intolerant to CPAP therapy or who do not respond to CPAP therapy should be considered |
IIb |
B |
ASV, adaptive servo-ventilation; CSA-CSR, central sleep apnea with Cheyne-Stokes respiration; COR, Class of Recommendation; CPAP, continuous positive airway pressure; HFpEF, heart failure with preserved ejection fraction; LOE, Level of Evidence; OSA, obstructive sleep apnea; SDB, sleep disordered breathing.
HFpEF is more affected by multiple comorbidities, pathologies, and other organ failures, including SDB.730–733 Effective pharmacotherapy to improve the prognosis of HFpEF is not well established, and there are few treatment options,473,734 suggesting the importance of managing the comorbidities.473,735 The pathogenesis of HFpEF is complexly related to left ventricular hypertrophy, diastolic dysfunction, and arterial stiffness, as well as the presence of hypertension, AF, and coronary artery disease (CAD), all of which are closely associated with SDB.243,740,741 With regard to the effect of SDB on left ventricular diastolic dysfunction, left atrial enlargement, left ventricular hypertrophy, and left ventricular diastolic dysfunction have been observed with increasing severity of OSA.742 In addition, the thoracic pressure fluctuations associated with OSA, as well as the increase in heart rate and blood pressure associated with sympathetic nerve activity, are involved in the pathogenesis of HFpEF,15,18 and their management may be important. There have been several reports of reductions in left ventricular myocardial mass and in right atrial and left atrial volumes with CPAP therapy in patients with OSA.25 In addition, improvement of HFpEF-related factors such as obesity, hypertension, diabetes, AF, CAD, and renal dysfunction with OSA management may prevent the development of HFpEF and improve the patient’s prognosis.18 With respect to OSA management in HFpEF, CPAP should be considered as well as treatment for OSA regardless of HF severity, especially if the patient presents symptoms associated with OSA.5,26–28 The management of body weight, alcohol intake, sleep position, and choice of hypnotic, as well as the use of an OA, may also be helpful. OSA with related symptoms such as daytime sleepiness should be treated in accordance with existing guidelines for the treatment of SDB, regardless of whether the patient has HFrEF or HFpEF. CPAP therapy to improve OSA symptoms is recommended for HFpEF patients with symptomatic OSA (Table 1). Although CPAP is likely to be useful for OSA associated with HFpEF, no evidence has yet been established to indicate the improvement of the prognosis of HFpEF with OSA (Table 1).
On the other hand, CSA-CSR management in HFpEF has not been further established. In HFpEF patients with SDB including CSA-CSR, ASV treatment improved left ventricular diastolic function, arterial stiffness, and endothelial function after 6 months, and reduced cardiac death and HF rehospitalization rates. Similarly, in CSA-predominant HFpEF patients, ASV reduced estimated pulmonary artery pressure and BNP level, and improved right heart function.35,36 The CAT-HF study of ASV therapy showed improvements in the outcomes of patients with HFpEF.37
7. Bradyarrhythmia (Table 34)
Table 34.
Recommendations and Levels of Evidence for Evaluation and Treatment of SDB in Bradyarrhythmia
| |
COR |
LOE |
| It is recommended to ask patients about clinical symptoms of SDB and screen for SDB |
I |
C |
It is recommended to treat SDB (CPAP therapy or weight loss) in patients with sleep-
related bradycardia or conduction disorders and OSA |
I |
C |
Screening for SDB in patients scheduled for pacemaker implantation or who already have
a pacemaker for bradycardia or conduction disturbances should be considered |
IIa |
C |
COR, Class of Recommendation; CPAP, continuous positive airway pressure; LOE, Level of Evidence; OSA, obstructive sleep apnea; SDB, sleep disordered breathing.
7.1 Bradyarrhythmia in Normal Sleep
It is not uncommon to detect bradyarrhythmias in normal sleep, even in the absence of organic cardiac disease. Sinus bradycardia is the most frequent bradyarrhythmia occurring during sleep, but sinus arrest, sino-atrial block, atrioventricular block, and junctional rhythm are also present during sleep. Weak sympathetic activity and increased parasympathetic activity during non-REM sleep have been proposed as mechanisms of bradycardia during sleep.760 In most cases of bradycardia that occurs during sleep, it is a physiologic response and asymptomatic, and does not require therapeutic intervention; guidelines suggest that pacemaker implantation should not be performed (Class III).
7.2 Bradyarrhythmias Associated With SDB
In patients with SDB, bradycardia and conduction disturbances occur more frequently, mainly during apneic events.762,763 In a study of 400 patients with SDB (mean age, 49 years) who underwent all-night PSG and Holter ECG, 43 patients had sinus arrest of 2.5–13 s and 31 patients had 2nd-degree atrioventricular block.763 The typical pattern of bradycardia–tachycardia response is bradycardia during an apneic attack and tachycardia or hypertension at the end of the apneic attack.81 The mechanism of the bradycardia–tachycardia response is thought to be that apneic attacks cause hypoxia, which is followed by repeated arousal responses that cause rapid autonomic nervous system tone changes and conduction disturbances; oxygen administration is reported to improve bradycardia.173 In the SHHS, the group with SDB had more ventricular extrasystoles than those without SDB. No significant difference between patients with and without SDB was reported for bradyarrhythmia. However, the mean age of the patients was 71 years, suggesting that the fluctuations in autonomic nervous system activity may have been attenuated. Patients with nocturnal bradyarrhythmias associated with sleep apnea attacks are often asymptomatic, and bradycardia rarely appears during the day. Treatment of SDB is preferred over pacemaker implantation in patients with bradyarrhythmias associated with SDB.764 The frequency of nocturnal sleep-related bradyarrhythmias in patients with SDB was dramatically reduced by 72–89% with CPAP therapy.765–768 A study of 17 SDB patients with nocturnal bradyarrhythmia treated with CPAP and followed up for 54±10 months reported that no symptomatic bradyarrhythmia occurred in any of the patients.767 Based on these findings, we recommend screening for SDB in patients with nocturnal bradycardia (symptom monitoring and close examination of patients with suspected SDB) and treatment of OSA (CPAP and weight reduction) in cases of bradyarrhythmia during sleep with concomitant OSA.
7.3 SDB in Patients With Pacemaker Implantation Due to Symptomatic Bradyarrhythmia
Screening of 98 pacemaker-implanted patients for sleep apnea by Epworth sleepiness scale (ESS) and PSG revealed that only 25% had symptoms of SDB (ESS ≥11), but 59% of patients were diagnosed with SDB by PSG, and 29% had severe SDB (AHI ≥30). In other words, patients under consideration for pacemaker implantation are a high-risk population for SDB, and screening for SDB is recommended even if the patient is asymptomatic. A meta-analysis of 4 articles reported that SDB detection by implanted devices correlated well with PSG when AHI ≥30 was used as the gold standard. The RESPIRE study reported that 172 (31%) of 553 patients with pacemaker implantation had severe SDB detected by pacemaker monitoring, and those with severe SDB had AF more frequently than those without SDB.772
8. Supraventricular Tachyarrhythmia
8.1 Mechanisms of Tachyarrhythmia in SDB
In SDB, fluctuating negative intrathoracic pressure, periodic hypoxia, and altered autonomic nervous system activity are closely related to arrhythmogenesis (Figure 19). Arrhythmias associated with SDB range from asymptomatic atrial and ventricular premature contractions that do not require therapeutic intervention to lethal arrhythmias that lead to sudden cardiac death (Figure 19). In addition, AF that is not an immediate urgency arrhythmia in itself can increase the risk of developing HF or stroke. Accurate diagnosis and appropriate treatment are needed in some cases. SDB is associated with a number of comorbidities, including lifestyle-related diseases, which are additively and synergistically related to the occurrence of arrhythmias, so treatment of the arrhythmia alone is often insufficient and comprehensive management is required. Age-related cardiac fibrosis, comorbidities such as hypertension, and underlying heart disease cause an arrhythmogenic substrate, but these structural changes are mild in the early stages and are not associated with arrhythmia occurrence. However, repeated apnea episodes each night in addition to these changes, further deteriorate structural and electrical remodeling. In addition, there is a risk of development of arrhythmia with each apnea episode, which triggers atrial and ventricular arrhythmias, although the mechanisms are different. In the absence of appropriate intervention for SDB, comorbidities and underlying heart disease, an arrhythmogenic substrate will subclinically develop, but at some point will exceed the threshold for arrhythmogenesis, resulting in the development of AF and lethal ventricular arrhythmias. In the case of AF, SDB causes progression from paroxysmal to persistent over time, eventually becoming the permanent form (Figure 20). Thus, SDB is characterized by both acute and chronic changes in sleep apnea that are involved in the development of tachyarrhythmia.

8.2 AF (Table 35)
Table 35.
Recommendations and Levels of Evidence for Evaluation and Treatment of SDB in AF
| |
COR |
LOE |
| It is recommended to ask patients about clinical symptoms of SDB and screen for SDB |
I |
A |
It is recommended to comprehensively treat risk factors for comorbidities and underlying
disease of AF along with treatment of SDB |
I |
B |
Treatment of SDB to prevent recurrence of AF and to improve the effectiveness of
treatment (including catheter ablation) for AF should be considered |
IIa |
B |
AF, atrial fibrillation; COR, Class of Recommendation; LOE, Level of Evidence; SDB, sleep disordered breathing.
AF is one of the most common supraventricular arrhythmias associated with SDB. Its prevalence increases with age, and is expected to continue to increase in aging societies such as Japan’s.773 A cohort study in Japan showed that in a population undergoing catheter ablation for AF, a high percentage (53.3%) of patients had coexisting SDB with an AHI ≥15.775 On the other hand, the prevalence of AF complicating SDB is 4.8%.774 However, the development of AF increases over time in the patients with SDB.776 Therefore, SDB increases the cumulative incidence of AF and it is important to evaluate the risk of AF in patients with SDB and to consider screening for SDB in patients with AF.
8.2.1 Hemodynamic Changes Due to SDB
With an obstructed upper airway, negative intrathoracic pressure becomes less than −50 mmHg due to respiratory motion.147 In this situation, transmural pressure becomes 170 mmHg when considering a left ventricular systolic pressure of 120 mmHg. Under conditions where intrathoracic pressure is −10 mmHg, equivalent to the normal range, the left ventricular systolic pressure becomes a load similar to that in a hypertensive patient with a systolic pressure of 160 mmHg. This sustained load, occurring every night during sleep, promotes cardiac remodeling leading to the development of arrhythmogenic substrate. Meta-analysis has indicated a significant correlation between left ventricular hypertrophy and severity of OSA.777 The deep negative intrathoracic pressure increases venous return while cardiac output decreases, altering the blood distribution pattern during obstructive apnea (Figure 19). Additionally, it has been reported that the fluid shift due to supine position during sleep is involved in upper airway obstruction. This is attributed not only to increased venous return resulting from the redistribution of fluid from the lower limbs to the upper body, but also to augmentation of the distribution to the pharyngeal and neck tissues around the upper airway, thereby promoting upper airway obstruction.778 The dynamic changes in circulatory hemodynamics, involving fluid shifts during sleep and episodes of sleep apnea, are considered significant factors contributing to structural remodeling.
8.2.2 Electrophysiological Changes in Atrial Muscle by SDB
Electrophysiological changes in the acute phase during OSA include a shortening of the atrial effective refractory period, which is associated with increased parasympathetic nervous activity due to negative intrathoracic pressure.155,779 In addition to atrial refractory period shortening, atrial stretch and hypoxia may contribute to the electrophysiological substrate for the development of AF.155,779–781 Although these changes are transient, their arrhythmogenic probability increases with repeated episodes of OSA. Indeed, arrhythmias are more common during periods of OSA, and AF in particular has been reported to have a 17.9-fold higher risk of occurring during or immediately after apnea compared with nocturnal state of normal breathing.782 Although paroxysmal AF is more common during the night in younger individuals than in older adults,783 it is speculated that even in the absence of risk factors and structural changes in the atria, AF can develop with OSA a. For cases of AF with a relatively young onset and no apparent risk factors such as hypertension or diabetes mellitus, screening for SDB becomes important not only for diagnosis but also for determining the treatment strategy. Experimental study has also shown that an OSA promotes fibrosis of the atria and decreases the expression of connexin, a cell-to-cell connection protein, and changes its distribution pattern as an arrhythmogenic substrate of AF.784,785 This results in reduced atrial conduction velocity, development of a reentrant circuit and facilitation of AF perpetuation. Voltage mapping during catheter ablation shows low voltage areas in the left atrium in patients with OSA, suggesting progressive structural remodeling. In addition, after pulmonary vein isolation, atrial premature contraction, which originates from non-pulmonary veins and initiates AF, was identified in 11.6% of patients without OSA, but was significantly higher in OSA patients (41.8%), suggesting the development of an extensive arrhythmic substrate in the atria of patients with OSA.786 In addition, it has been reported that P-wave duration (SAPWD) on the signal-averaged ECG of patients with OSA is prolonged with OSA severity, and treatment with CPAP shortens the SAPWD,665,787 suggesting electrical and structural changes in the atria associated with OSA.
8.2.3 Treatment of AF Complicated by SDB
It has been reported that it is more difficult to maintain sinus rhythm by antiarrhythmic drugs in the patients with AF associated with OSA, and that catheter ablation is one of the treatment for symptomatic patients with AF, both paroxysmal and persistent.788 However, even if pulmonary vein isolation is successful, the recurrence rate is high without appropriate treatment for OSA.791 It has been reported that the recurrence rate of AF is reduced by approximately 50–60% in patients who receive CPAP therapy after catheter ablation compared with those who do not receive CPAP therapy.774,791 A meta-analysis reported that CPAP therapy reduced recurrence by 37% in patients with SDB-associated AF.792 OSA by itself is not an indication of requiring anticoagulation therapy, but it has been reported as an independent risk factor for cerebral infarction.797
9. Ventricular Tachyarrhythmia and Sudden Death
9.1 Ventricular Arrhythmias, Sudden Cardiac Death and SDB
Few reports have examined the mechanism of ventricular arrhythmias and sudden death in patients with SDB. Repeated systemic hypoxemia associated with SDB induces ventricular myocardial ischemia on the endocardial side, contributing to the formation of structural and electrical remodeling that leads to sudden death.798 Intermittent hypoxia causes increased sympathetic activation, which contributes to the development of ventricular arrhythmias (Figure 19). Indeed, QT interval prolongation has been reported in patients with SDB, depending on the severity of the disease. It has been suggested that Tp–Te (peak-to-end interval of the T wave), which is used as a predictor of ventricular arrhythmias, and Tp–Te/QTc, corrected for QTc, are also increased in patients with OSA and associated with prognosis.800 The occurrence of non-sustained ventricular tachycardia is significantly higher during or immediately after obstructive apnea episodes, being 17.4-fold more frequent compared with states without obstructive apnea, suggesting a role for hypoxia, altered autonomic nervous system activity, and negative intrathoracic pressure changes782 (Figure 19).
Data on the incidence of sudden cardiac death in patients with OSA are limited, but a review of 10,701 patients who underwent PSG identified 142 cases of sudden cardiac death, including those who were resuscitated, after a mean observation period of 5.3 years.801 The risk of sudden cardiac death in that cohort study was 0.27%/year. Multivariate analysis indicated that minimum oxygen saturation during sleep is an independent risk factor for sudden cardiac death.801 Although the AHI was not an independent risk factor in this cohort study, the important finding was that hypoxia during sleep is associated with sudden cardiac death. It is speculated that the concomitant presence of HF and CAD plays a role in sudden cardiac death in patients with OSA. Previous reports have shown that sudden cardiac death shows a circadian variation, with an increase in deaths beginning at 6:00 a.m. and peaking at 12:00 a.m.802 The risk of sudden cardiac death in patients with OSA during the period between midnight and 6:00 a.m. is 2.57-fold higher than in the general population.85 Of 472 HF patients implanted with a CRT-defibrillator (CRT-D), 283 were followed for 2 years without CPAP or ASV therapy, and 140 patients (55.9%) were found to have ventricular arrhythmias.35 Although the findings are restricted to patients with CRT-D implants, arrhythmic events were significantly higher in the group with SDB. Detection of the interval of the fatal ventricular arrhythmias was significantly shorter in the group of patients with OSA or CSA, indicating that SDB itself constitutes a risk factor for fatal ventricular arrhythmias.803 The AHI ≥10 group was reported to have a significantly higher rate of appropriate ICD therapy during the nighttime hours (midnight to 6 a.m.) compared with the AHI <10 group.803
9.2 Prevention and Treatment of Fatal Ventricular Arrhythmias
The preventive effect of CPAP treatment on sudden cardiac death is unclear. It has been reported that iASV therapy significantly reduced ICD therapy in ICD-implanted patients with HF complicated by CSR.804 On the other hand, the SERVE-HF trial, which examined whether ASV improves long-term prognosis in 1,325 patients with HF and CSA, showed significantly higher rates of all-cause and cardiovascular death in the ASV-treated group. Further study with a large study population is needed to elucidate the efficacy of CPAP treatment as a primary prevention of sudden cardiac death.
10. Ischemic Heart Disease (Table 36)
Table 36.
Recommendations and Levels of Evidence for Evaluation and Treatment of SDB in Ischemic Heart Disease
| |
COR |
LOE |
| It is recommended to ask patients about clinical symptoms of SDB and screen for SDB |
I |
B |
CPAP therapy for severe OSA to prevent the development of ischemic heart disease should
be considered |
IIa |
B |
CPAP therapy (CPAP use for >4 h) with maintained adherence to prevent
cardiovascular events for OSA associated with ischemic heart disease should be considered |
IIa |
B |
| CPAP therapy for OSA to reduce the development of atherosclerosis may be considered |
IIb |
C |
CPAP therapy for severe OSA complicated by ischemic heart disease with nocturnal attacks
may be considered |
IIb |
C |
COR, Class of Recommendation; CPAP, continuous positive airway pressure; LOE, Level of Evidence; OSA, obstructive sleep apnea; SDB, sleep disordered breathing.
10.1 SDB in CAD
CAD is known to be associated with a high rate of SDB,805–807 which is believed to be increased in acute coronary syndrome (ACS) due to effects such as hemodynamic instability and sympathetic nervous system activity. Moruzzi et al. reported that the frequency of AHI ≥10 was almost twice that of chronic ischemic heart disease in acute myocardial infarction (22%) and nearly 3-fold that of unstable angina (36%),809 compared with chronic ischemic heart disease in acute myocardial infarction (AHI ≥5)8 and severe SDB (AHI ≥30), respectively.6 Tsukamoto et al. reported that OSA and CSA were temporarily worse in acute phase PSG compared with chronic phase PSG.810 Hayashi et al. found that 95.3% of patients had an AHI ≥5 and 54.3% had an AHI ≥15 at 14 days and 2 months after the onset of acute myocardial infarction, respectively. However, the mean AHI improved from 22.0 to 18.5 at 2 months after onset, due to a significant improvement in CSA and a trend toward prolonged OSA.811 This suggests that CSA may be a consequence of the onset of ACS and may improve over time.
10.2 SDB and the Development of Coronary Atherosclerosis
In recent years, many reports using coronary computed tomography and intravascular ultrasound have demonstrated a relationship between OSA and coronary atherosclerosis; the more severe the OSA, the stronger the degree of coronary atherosclerosis, and a meta-analysis showed that OSA severity was significantly associated with the degree of coronary artery calcification.812 An intravascular ultrasound study reported that patients with OSA and AHI ≥15 had greater atheroma volume in the responsible vessel, and that as such OSA is an independent determinant of atheroma volume.813 Non-calcified lesions and coronary plaques are also common in patients with severe OSA.814–816 There are numerous reports that the degree of OSA is associated with that of coronary artery calcification and that there is a correlation between severity and coronary artery calcium levels.817–819
10.3 Impact of SDB on ACS
SDB patients are prone to progression of atherosclerosis,49 and it is thought that the hypoxia caused by SDB not only induces ischemia, but also leads to plaque instability and coagulation abnormalities, which in turn lead to ACS.820–826 Polycythemia secondary to hypoxia may predispose to the thrombus formation seen in ACS. Other factors such as nocturnal platelet aggregation and hypercoagulability may also play a role in thrombus formation.822 Hypertension, dyslipidemia, and obesity are also causes of atherosclerosis and vascular endothelial damage, hypoxia and increased sympathetic nerve activity may affect the stability of atherosclerotic plaques in the coronary arteries.823,824 Improvement in cardiac function after acute myocardial infarction was reported to be more impaired in the group with SDB, leading to a decline in cardiac function after the onset; Nakashima et al. reported that changes in LVEF before discharge and immediately after percutaneous coronary intervention (PCI) were associated with the severity of OSA, and that LVEF improved less as the AHI increased.827 Buchner et al. also reported that patients with SDB have a lower myocardial salvage index on cardiac MRI than those without SDB.828 On the other hand, it has been reported that OSA patients experience myocardial preconditioning due to intermittent hypoxia compared with non-OSA patients, resulting in less frequent transmural myocardial infarctions.829 When tissue perfusion was assessed using Doppler guidewires in cases of ST-elevation acute myocardial infarction, the group with AHI ≥15 was more likely to have poor tissue perfusion than the control group.830 OSA may cause an increase in the extent of stunned myocardium, preventing recovery of myocardial contractility after myocardial infarction.
10.4 ACS Onset Time and SDB
The time of onset of acute myocardial infarction with OSA is significantly more common between midnight and 6 a.m. compared with patients without OSA, and 91% of acute myocardial infarction cases that occur during this time period are reported to be in patients with OSA.831 This is thought to be caused by the nocturnal increase in sympathetic activity, platelet aggregation capacity, and coagulability associated with SDB. However, others have reported a peak onset in the morning, but this is not a consistent finding.831 Nakashima et al. found no consistent trend in patients with mild OSA, but an increased incidence of acute myocardial infarction in the morning in with moderate to severe OSA, and multivariate analysis showed that moderate or severe OSA is an independent factor for morning onset.832 An association between sudden death at night, including myocardial infarction, and OSA has also been suggested, with an association with the degree of SpO2
reduction rather than the degree of AHI reported as a possible mechanism.801
10.5 Chronic Coronary Disease, Coronary Angina and SDB
Ischemic heart disease patients with OSA are reported to have ST depression during nighttime sleep that is improved with CPAP treatment.389,834 In addition to the hypoxia caused by OSA, several factors have been reported to contribute to myocardial ischemia. OSA causes an increase in transmural pressure due to negative intrathoracic pressure,152 which in turn causes a decrease in stroke volume and a disproportionate oxygen supply between the myocardium and coronary arteries, exacerbating myocardial ischemia. Hamilton et al.835 reported a relationship between OSA and coronary blood flow and myocardial workload. Myocardial workload increased when the airway was released following OSA, but coronary artery blood flow did not, suggesting that the imbalance between myocardial oxygen demand and supply during airway release following OSA may result in myocardial ischemia.835 It is known that attacks of coronary angina tend to appear during sleep from nighttime to early morning, especially coinciding with REM sleep, a period of intense fluctuations in sympathetic, parasympathetic, and serotonin activities. It is thought that the release of vasoconstrictors such as acetylcholine, noradrenaline, and serotonin during this period makes the coronary arteries more prone to spasm. However, there have been very few reports on the relationship between coronary spastic angina and SDB, and the mechanism by which this occurs is still unclear.836,837
10.6 Progression From SDB to Ischemic Heart Disease
The WSC reported that the incidence of CAD or HF was 2.6-fold higher in the severe OSA group than in the untreated group after 24 years of follow-up.838 The 2010 SHHS follow-up study reported that patients with an AHI ≥30 were 1.68-fold more likely to develop CAD than controls.67 However, OSA was an independent factor for cardiovascular events only in men aged ≤70 years, with no association in older men or women. Marin et al. reported 2.87-fold more fatal and 3.1-fold more nonfatal cardiovascular events.676 There have been several reports on the association between positive-pressure treatment, such as CPAP, for SDB and prevention of ischemic heart disease. Marin et al. reported that the frequency of cardiovascular events in patients with OSA with AHI ≥30 and CPAP treatment was comparable to that in controls without OSA.676 Barbé et al. also examined the effect of CPAP on the prevention of cardiovascular events in their RCT, finding a trend toward fewer events in the CPAP-treated group, but not significantly.397 However, a significant reduction in cardiovascular events was observed in patients who used CPAP for >4 h, suggesting that CPAP therapy may improve the prognosis of ischemic heart disease and other CVD when used properly and for an extended period of time. However, this was not a prespecified analysis,397 and a large RCT with prospective adherence is awaited.
10.7 Prognosis of Ischemic Heart Disease Patients With SDB
There are numerous reports that the presence of SDB in patients with CAD is associated with cardiovascular events such as death, myocardial infarction, and cerebrovascular disease.31,33,69,839–842 Lee et al. reported that cardiovascular events were significantly higher when CAD was complicated by OSA.69 Nakashima et al. found that ACS recurrence was significantly higher in the OSA group than in the control group, and the number of major adverse cardiac events (MACE) was significantly higher in the OSA group at approximately 4 years of follow-up. The rate of revascularization at the site of the PCI lesion was similar in the OSA and control groups. In contrast, the percentage of new lesions treated with PCI was significantly higher in the OSA group than in the control group.841 A recent study reported that the combination of OSA and diabetes mellitus increased cardiovascular events after ACS, and further investigation is needed.843–854 The prognostic value of positive-pressure therapy such as CPAP for SDB in patients with CAD has also been investigated.844–857 The SAVE study randomized patients with a history of CAD or CVD to CPAP or non-CPAP groups with endpoints of cardiovascular death, myocardial infarction, stroke or hospitalization for unstable angina, HF, or transient ischemic attack. In the results, CPAP use did not improve outcomes such as cardiovascular events.59 One problem with the SAVE study was that the average duration of CPAP use was ≈3.3 h, and poor CPAP adherence may have attenuated any beneficial effect. A meta-analysis and the RICCADSA trial reported in 2016 that patients with good CPAP adherence had a lower incidence of cardiovascular events.394,847,848 The SAVE trial may have also shown a weakened CPAP effect because of the selection of study subjects: subjects with ESS ≥15 severe drowsiness and severe hypoxia (SpO2
≤80% for >10% of the recording time) were excluded from the study. This is a limitation of RCTs on OSA from an ethical point of view. The study also excluded patients with severe OSA, and it is possible that the CPAP effect was weakened because of the large number of patients with mild to moderate OSA. The RICCADSA study, in which postoperative coronary revascularization patients with OSA were randomized to CPAP or no treatment, found no significant benefit from CPAP and no difference in composite events including cardiovascular death, myocardial infarction, stroke, or revascularization procedure between the 2 groups.848 However, the study also found that average CPAP use of ≥4 h/night was associated with a lower risk of the primary endpoint. Furthermore, the results of the ISAACC trial, in which ACS patients with OSA were randomized to CPAP or no treatment, showed similar improvements in daytime sleepiness as in the SAVE trial, but the occurrence of the composite events, including cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, and hospitalization for unstable angina, HF, or transient ischemic attack, did not differ between the 2 groups.68 The mean duration of CPAP use in the CPAP group was also shorter, 2.8 h/night, which may explain why there was no significant difference in the occurrence of events. Further studies are needed to determine the effects of ischemic heart disease with severe hypoxia and CPAP use for >4 h.
11. Stroke (Table 37)
Table 37.
Recommendations and Levels of Evidence for Evaluation and Treatment of SDB in Stroke
| |
COR |
LOE |
| It is recommended to ask patients about clinical symptoms of SDB and screen for SDB |
I |
B |
| CPAP therapy to improve functional prognosis in stroke patients should be considered |
IIa |
B |
COR, Class of Recommendation; CPAP, continuous positive airway pressure; LOE, Level of Evidence; SDB, sleep disordered breathing.
A history of stroke or transient ischemic attack is associated with a high rate of SDB.850 Because SDB may increase the risk of vascular disease. including stroke, and that SDB has been reported to be an independent risk factor for stroke,851,852 it is necessary to diagnose and treat SDB appropriately to prevent strokes.
11.1 Related Mechanisms
SDB increases the risk of stroke through the following possible mechanism: increased airway resistance and hypoxia/high carbon dioxide state due to SDB leads to repetitive arousal responses, which increases sympathetic hyperactivity and oxidative stress. The incidence of atherothrombotic cerebral infarction, lacunar infarction, and cerebral hemorrhage increases with metabolic syndrome, including elevated blood pressure, glucose intolerance, and lipid abnormalities.
11.2 Primary Stroke Prevention
Many cases of SDB are complicated by metabolic syndrome, such as in obese patients, which may ultimately promote the development of stroke through the development of atherosclerosis. The high prevalence of concomitant AF may also be a risk factor for stroke. The etiology of stroke is reported as small artery occlusion (39%), large artery (22%), hemorrhagic (20%), and cardiogenic (15%), with lacunar infarction due to small arteries more common in patients with OSA than in those without OSA (44% vs. 26%).857 SDB also increases the risk of vascular dementia, as well as the risk of stroke.858 In a study by time of stroke onset, 29 (72.5%) of 40 patients in the group with stroke symptoms upon awakening had OSA, and 30 (45%) of 67 patients in the group with stroke on awakening during the day had OSA.859 This suggests that OSA is one of the factors that make patients more likely to suffer a stroke during sleep. Results from RCTs showed no overall difference between the treated and non-treated groups regarding the effectiveness of CPAP treatment in preventing stroke.393,860 A systematic review of CPAP treatment (9 RCTs and 4 cohort studies) also found a significant reduction in stroke risk with CPAP therapy in the cohort studies.861 However, subgroup analyses of the RCTs reported a reduction in stroke when CPAP was used only in moderate to severe SDB (AHI >15) and with adequate adherence.
11.3 Secondary Stroke Prevention
Although SDB complications have been reported to be common in post-stroke patients, it is important to note that only stroke survivors were included in the study. The prevalence of SDB in patients with transient ischemic attacks was 62% for SDB with AHI ≥10, which was significantly more than the 12% in the control group.865 Similarly, in another report, testing in patients with acute stroke or transient ischemic attack indicated a high prevalence of SDB with AHI ≥5 (62.8%).866 In the acute phase of stroke, damage to the respiratory center due to stroke itself may cause not only OSA but also CSA. In a study of 132 patients admitted for rehabilitation after a cerebrovascular accident, the frequency of OSA, CSA, and mixed OSA was reported to be 17%, 21%, and 1.5%, respectively.867 Although SDB treatment such as CPAP is considered necessary for secondary prevention of stroke, it was reported that only 22% of SDB patients were treated after stroke.868
11.4 SDB Treatment of Stroke Patients
In stroke patients with mild OSA, weight loss, positional therapy, etc. are performed as in the case of non-stroke patients, and an OA may be prescribed in the case of mild to moderate OSA or in relatively thin patients. For moderate to severe OSA, CPAP and other therapies should be considered as in the case of non-stroke patients, but stroke patients, especially the elderly and those with paraplegia and hand disabilities, often need the help of a caregiver such as a family member to help with putting on a mask. If it is difficult to wear a mask, an OA may be selected even in severe cases. In a study examining the rate of CPAP use after cerebral infarction, the rates of CPAP use at 3, 6, 12, 24, and 60 months were 58%, 53%, 48%, 45%, and 39%, respectively, in 191 patients with SDB requiring CPAP,857 and a gradual decreasing trend was observed after the onset of cerebral infarction. The dropout rate in the first 3 months was particularly high, indicating the importance of monitoring for continuous use of CPAP during the introduction period. Clinical studies examining the use and prognosis of CPAP in patients with stroke complicated by SDB are often small, involving only a few dozen patients, and there are few reports of adequate efficacy; studies with short-term CPAP use of around 5 h have shown improved neurologic function in the CPAP group.869,870 A study of 22 patients each in a CPAP group vs. a control group, with an average CPAP use of ≈5 h, showed improved neurologic function in the CPAP group.871 A comparison of 20 CPAP patients with 16 controls reported improved cognitive function after stroke, although the average duration of CPAP use was 2.5 h/night.872 In a study that followed patients for 1 year after initiation of CPAP use, the mean duration of CPAP use was 4.2 h, with an average daily use rate of 76%. The 34 patients in the CPAP group compared with 36 controls showed no difference in vascular events, although there were a few: 1 (3.33%) and 6 (15%), respectively.873 In addition, a study of 40 patients diagnosed with OSA after stroke, divided into 2 groups of 20 patients each, one with CPAP and the other without, showed significant improvement in cognitive function in the CPAP group.874 Similarly, in an observational study of basal ganglia stroke patients with OSA, the National Institutes of Health Stroke Scale (NIHSS), Fugl-Meyer Assessment Scale (FMA), and Barthel Index (BI) were significantly improved after 6 months of CPAP treatment, along with improvement in the SDB index, BI, Mini-Mental State Examination (MMSE), Hamilton Anxiety Scale (HAMA), and Hamilton Rating Scale for Depression (HRSD) scores compared with controls.875 Although some of these small studies have demonstrated neurologic improvement with CPAP, many have failed to show significant differences, and because poor CPAP adherence may be a contributing factor to the lack of CPAP benefit, future large-scale clinical studies that maintain adequate CPAP use are awaited.
12. Aortic Disease / Peripheral Arterial Disease (PAD) (Table 38)
Table 38.
Recommendations and Levels of Evidence for Evaluation and Treatment of SDB in Aortic Disease/PAD
| |
COR |
LOE |
It is recommended to ask patients with AD about clinical symptoms of SDB and screen
for SDB |
I |
C |
Asking patients with aortic aneurysm about clinical symptoms of SDB and screening for
SDB may be considered |
IIb |
C |
CPAP therapy to prevent aortic enlargement in Marfan syndrome complicated by OSA may
be considered |
IIb |
C |
It is recommended to ask patients with PAD about clinical symptoms of SDB and screen
for SDB |
I |
C |
AD, aortic dissection; COR, Class of Recommendation; CPAP, continuous positive airway pressure; LOE, Level of Evidence; OSA, obstructive sleep apnea; PAD, peripheral arterial disease; SDB, sleep disordered breathing.
AD, aortic aneurysm (AA), and PAD may occur in association with SDB. Thoracic AD and thoracic AA may be caused not only by arteriosclerosis, but also through direct stimulation of the aorta by the negative intrathoracic pressure due to OSA.
12.1 Aortic Dissection (AD)
Pathophysiological conditions associated with the development of AD in OSA include negative intrathoracic pressure due to inspiratory effort during obstructive apnea, increased blood pressure via sympathetic hyperactivity during arousal from apnea, and oxidative stress via intermittent hypoxemia and reoxygenation due to OSA. The negative intrathoracic pressure has been reported to range from −50 to −80 cmH2O,878,879 and may cause vascular wall stress in the aorta.880,881 The ESS is not as high in AD patients with OSA as in other atherosclerotic disease.97
Regarding the relationship between AD and SDB severity, the AHI in thoracic AD patients is significantly higher than in hypertensive patients.3 A Japanese study reported a higher rate of severe SDB in AD cases.2 Although there is no evidence regarding the prognostic impact of SDB in patients with AD, the rate of false lumen enlargement was significantly higher in patients with severe OSA (AHI ≥30) than in those with AHI 5–30 (7.5 mm/year vs. 1–3 mm/year).882 There is no evidence for an effect of CPAP for OSA in reducing AD onset and recurrence.
12.2 Aortic Aneurysm
The pathogenesis of AA in OSA may include increased blood pressure via sympathetic hyperactivity, and oxidative stress via intermittent hypoxia and reoxygenation. Negative intrathoracic pressure could theoretically dilate the thoracic aorta via increased stress in the aortic wall,880,881 but would have little effect on the abdominal aorta. In a Japanese report analyzing the relationship between OSA and thoracic aortic dilation, the mean ascending aortic diameter was 5.3 mm larger in the population with OSA compared with non-OSA cases.881 In abdominal AA patients, the rate of aortic diameter enlargement was significantly higher by 2.2 mm/year in the population with an AHI ≥30 compared with an AHI 0–5.90 A larger aortic diameter has also been reported in patients with Marfan syndrome complicated by OSA compared with those without OSA, and OSA is considered a risk factor for aortic disease in Marfan syndrome.91–94,884
Regarding the prognostic value of CPAP therapy for SDB in patients with AA, case studies report that the introduction of CPAP therapy for Marfan’s syndrome complicated by OSA attenuated the increase in aortic diameter,14,15 suggesting that positive-pressure therapy in patients with AA may improve prognosis.15
12.3 PAD
Intermittent hypoxia may cause atherosclerotic disease due to inflammation, vascular endothelial damage via oxidative stress, vasoconstriction via endothelin, and hypertension via sympathetic hyperactivity.850 The ESS is not high in patients with SDB and PAD.885 Regarding the association between severity of PAD and severity of SDB, lower extremity PAD with Fontaine Classification IV (severe PAD with skin ulceration and necrosis) has a higher AHI than mild PAD.886 The prognostic effects of OSA on cardiovascular events in patients with PAD are not well known, nor are the effects of CPAP on SDB in preventing the onset and progression of PAD.
13. Pulmonary Hypertension (PH) (Table 39)
Table 39.
Recommendations and Levels of Evidence for Evaluation and Treatment of SDB in PH
| |
COR |
LOE |
| It is recommended to ask patients about clinical symptoms of SDB and screen for SDB |
I |
B |
CPAP therapy to reduce PAP in patients with SDB who meet the indication criteria should
be considered |
IIa |
B |
| NPPV treatment for PH patients with severe nocturnal hypoventilation should be considered |
IIa |
B |
HOT for PH patients with severe respiratory failure who are difficult to treat with CPAP/NPPV
may be considered |
IIb |
C |
COR, Class of Recommendation; CPAP, continuous positive airway pressure; HOT, home oxygen therapy; LOE, Level of Evidence; NPPV, noninvasive positive-pressure ventilation; PAP, pulmonary arterial pressure; PH, pulmonary hypertension; SDB, sleep disordered breathing.
13.1 Concept of SDB in PH
It has been reported that OSA and sleep-related hypoventilation/hypoxia syndrome are frequently associated with PH.101,888–891 In sleep-related hypoventilation/hypoxia syndrome, treatment of SDB is expected to improve PH.
13.2 Related Mechanisms
Pulmonary artery remodeling from pulmonary vasoconstriction occurs due to frequent and severe intermittent hypoxia and persistent hypoxia in obesity hypoventilation syndrome (OHS).892–894 In general, the pulmonary artery pressure elevation in PH associated with SDB is mild, with mean pulmonary arterial pressure (PAP) ranging from 25 to 30 mmHg;893 however, others have reported it to be 25–30 mmHg, or 40 mmHg.895 SDB can also increase left ventricular end-diastolic pressure,896 which is associated with left HF, and also chronic obstructive pulmonary disease (COPD). OHS patients have a poorer prognosis than non-OHS patients with the same degree of obesity.897 In a cohort study of patients with PH due to pulmonary disease and/or hypoxemia, the 3-year survival rate of PH with OHS was 90%, which was the best among other cases of PH due to pulmonary diseases and/or hypoxia.898 SDB is a risk factor for venous thrombosis, which might be associated with chronic thromboembolic PH in group 4 (see Table 40).899,900
Table 40.
Clinical Classification of Pulmonary Hypertension (PH) (Revised)
| 1. Pulmonary arterial hypertension (PAH) |
| 1.1 Idiopathic pulmonary arterial hypertension (IPAH) |
| 1.2 Hereditary pulmonary arterial hypertension (HPAH) |
| 1.3 Drug- and toxin-induced pulmonary arterial hypertension |
| 1.4 Pulmonary arterial hypertension associated with various diseases |
| 1.4.1 Connective tissue disease |
| 1.4.2 HIV infection |
| 1.4.3 Portal PH |
| 1.4.4 Congenital heart disease |
| 1.4.5 Hematosomiasis |
| 1.5 PH in long-term response to calcium channel blockers |
| 1.6 Pulmonary veno-occlusive disease (PVOD) and/or pulmonary capillary hemangiomatosis (PCH) |
| 1.7 Prolonged pulmonary hypertension of the newborn (PPHN) |
| 2. PH associated with left heart disease |
| 2.1 PH associated with HFpEF |
| 2.2 PH associated with HFrEF |
| 2.3 Valvular heart disease |
| 2.4 Congenital/acquired cardiovascular disease (CVD) leading to postcapillary PH |
| 3. PH due to pulmonary disease and/or hypoxia |
| 3.1 Obstructive lung disease |
| 3.2 Restrictive lung disease |
| 3.3 Other lung diseases with mixed restrictive and obstructive disorders |
| 3.4 Hypoxia without lung disease |
| 3.5 Congenital lung disease |
| 4. PH associated with pulmonary artery obstruction |
| 4.1 Chronic thromboembolic pulmonary hypertension (CTEPH) |
| 4.2 Other pulmonary artery occlusive diseases |
| 5. PH of unknown details |
| 5.1 Hematologic diseases |
| 5.2 Systemic diseases |
| 5.3 Other |
| 5.4 Complex congenital heart disease |
(Adapted from Simonneau et al., 2019.887 Reproduced with permission of the © ERS 2023.)
13.3 Diagnosis of PH Complication in OSA
PH is first suspected from dyspnea on exertion or general fatigue.901 It is investigated by ECG and chest radiography, and then semiquantitative evaluation is performed by echocardiography.901 The search for the cause of PH should include the presence of left heart disease and pulmonary disease (including hypoxia), and if these are ruled out, then pulmonary arterial PH (group 1; Table 40) or chronic thromboembolic PH (group 4; Table 40) is considered the most likely cause of PH.901 PH has been defined as a resting mean PAP ≥25 mmHg,887 but the ESC/ERS guideline in 2022 defined as a mean PAP >20 mmHg and pulmonary vascular resistance >2 Wood units. The diagnosis of SDB is made by screening as described in other sections.
13.4 Therapeutic Effectiveness for PH Complicated by OSA
Weight loss is encouraged in obese or OHS patients as a curative treatment.902 However, weight loss is often difficult, and CPAP, a symptomatic but most effective treatment for SDB, is used (Table 40).903 Although there have been several RCTs with a small number of subjects, which showed PAP reduction by CPAP treatment,904,905 adherence was a problem.906 There are also some reports of reduction of PAP after 3 months of bi-level positive airway pressure (bi-level PAP) for OHS.895





















