Abstract
Background: Resistance exercise is beneficial in patients with lower extremity arterial disease. Muscle-derived exosomes contain many types of signaling molecules, including microRNAs (miRNAs). Here, we tested the hypothesis that exosomal miRNAs secreted by growing muscles promote an angiogenic response in endothelial cells (ECs).
Methods and Results: Skeletal muscle-specific conditional Akt1 transgenic (Akt1-TG) mice, in which skeletal muscle growth can be induced were used as a model of resistance training. Remarkable skeletal muscle growth was observed in mice 2 weeks after gene activation. The protein amount in exosomes secreted by growing muscles did not differ between Akt1-TG and control mice. Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway frequency analysis of 4,665 target genes, identified using an miRNA array miRNAs, revealed a significant increase in Akt and its downstream signaling pathway genes. Among the upregulated miRNAs, miR1, miR133, and miR206 were significantly upregulated in the serum of Akt1-TG mice. miR206 was also increased in insulin-like growth factor (IGF)-1-stimulated hypertrophied myotubes. Exogenous supplementation of exosomal miR206 to human umbilical vein ECs promoted angiogenesis, as assessed using the spheroid assay, and increased the expression of angiogenesis-related transcripts.
Conclusions: Exosomal miR206 is upregulated in the blood of Akt1-TG mice and in IGF-stimulated cultured myotubes. Exogenous supplementation of miR206 promoted an angiogenic response in ECs. Our data suggest that miR206 secreted from growing muscles acts on ECs and promotes angiogenesis.
The number of patients with lower extremity arterial disease (LEAD) is increasing worldwide.1 In addition to optimal medical therapy and arterial revascularization, exercise therapy is the most fundamental and effective intervention for these patients. Supervised exercise is recommended as a Class I intervention for patients with LEAD in the Japanese Circulation Society (JCS)/Japanese Society for Vascular Surgery (JSVS) 2022 Guideline on the Management of Peripheral Arterial Disease.2 Aerobic exercises, such as walking, running, and cycling, are generally recommended for these patients.2,3 Recently, resistance training, which is aimed at maintaining or increasing muscle mass, was shown to have a beneficial effect on LEAD; thus, this type of exercise is expected to be a complementary intervention for patients with LEAD.4–6 Although hindlimb ischemia is well known as a mouse model of LEAD,7 the mechanisms through which resistance training have beneficial effects on ischemic lower limbs are unclear because of a lack of an appropriate mouse model of resistance training.
Skeletal muscle was recently recognized not only as a motor organ, but also as a secretory organ that can produce and release a variety of circulating factors, referred to as “myokines”, which can influence remote or neighboring cells in an endocrine or paracrine manner.8–10 Activation of Akt signaling in skeletal muscle has been shown to promote muscle hypertrophy and fiber growth with a little effect on fiber specification in regenerating skeletal muscle.11,12 We have previously generated skeletal muscle-specific, conditional Akt1-overexpressing mice as a mouse model of resistance exercise training.13 Using these mice, we previously demonstrated that muscle growth improves metabolic parameters in obese mice,13 attenuates cardiac remodeling after myocardial infarction,14 attenuates renal damage in a kidney disease model,15 and improves blood flow recovery following hindlimb ischemia,16 at least partially through the secretion of muscle-derived factors.
Exosomes contain several proteins, nucleic acids, and microRNAs (miRNAs). Exosome-mediated miRNA transfer is a novel mechanism of genetic exchange between cells.17,18 In the cardiovascular system, exosome-mediated miRNA transfer is one of the mechanisms that regulates atherosclerosis and cardiac hypertrophy.19,20 Myotubes are an important source of exosomal miRNAs.21 In this context, we hypothesized that exosomal miRNAs secreted by growing muscles promote an angiogenic response in endothelial cells. The aim of the present study was to test this hypothesis using skeletal muscle-specific conditional Akt1 transgenic (Akt1-TG) mice and insulin-like growth factor (IGF)-stimulated cultured myotubes.
Methods
Animals
Muscle creatine kinase (MCK)-reverse tetracycline trans-activator transgenic mice (1256 [3Emut] MCK-rtTA TG)22 were crossed with tetracycline responsive element-myristoylated Akt1 transgenic mice (TRE-myrAkt1-TG)23 to generate double-transgenic mice (Akt1-TG mice).13 The MCK promoter construct used in the driver line is mutated and the transgene is expressed in a muscle subset, but not in the heart.22 To express the Akt1 transgene, Akt1-TG mice were administered doxycycline (0.5 mg/mL) through the drinking water. Male 12-week-old mice were used in the present study. As a control group, rtTA single-transgenic mice administered doxycycline were used. Serum exosomal miRNAs from control and Akt1-TG mice were isolated and subjected to a quantitative polymerase chain reaction (PCR) array.
All procedures were performed in accordance with the Kumamoto University Animal Care Guidelines (Approval reference no. B25-121 R1), which conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health.24
Cell Culture
C2C12 mouse myoblasts (American Type Culture Collection, Manassas, VA, USA) were maintained in a growth medium (Dulbecco’s modified Eagle’s medium [DMEM] supplemented with 10% fetal bovine serum), which was replaced with a differentiation medium (DMEM supplemented with 2% heat-inactivated horse serum); the cells were cultured in this medium for 4 days to induce differentiation. We experimented by culturing in Growth medium and changing to differentiation medium at the timing of inducing differentiation. C2C12 myocytes were stimulated with IGF-1 (5 or 50 mM; 2 days) to induce Akt and subsequent myotube hypertrophy. Exosomal miRNAs were isolated from the culture medium and subjected to droplet digital PCR (ddPCR).
Human umbilical vein endothelial cells (HUVECs) were used to evaluate the angiogenic properties of exogenous miR206.
Isolation of Exosomal miRNAs From Serum and Culture Medium
Exosomes were purified from serum or culture medium using ultracentrifugation. Briefly, samples were centrifuged at 2,000 g for 30 min at 4℃ and subsequently at 10,000 g for 30 min at 4℃ to eliminate debris and cellular components. The supernatant was ultracentrifuged at 10,000 g for 3 h at 4℃. The exosome pellet thus obtained was washed with phosphate-buffered saline (PBS), dissolved in PBS, and stored at −80℃ until use.25 The amount of protein in the exosomes was quantified using the BCA assay (Thermo Scientific, Waltham, MA, USA), western blot analysis, and nanoparticle tracking analysis. The isolation of exosomal miRNAs, quantitative (q) PCR array, and data mining were performed by Takara Bio Inc. (Otsu, Japan).
Western Blot Analysis
Western blotting was performed using a sodium dodecyl sulfate–polyacrylamide gel electrophoresis system as described previously.14 The primary antibodies used were against phosphorylated (p) Akt (Ser473), total Akt (t-Akt), glyceraldehyde-3-phosphate dehydrogenase (GAPDH; all from Cell Signaling Technology), CD9 and CD63 (both from Abcam).
miRNA Transfection
HUVECs were transfected with 5 nM miRNA (mmu-miR-206-3p, miRNA mimic negative control) using the Lipofectamine RNAiMAX reagent, as per the manufacturer’s instructions (ThermoFisher, https://www.thermofisher.com/order/catalog/product/13778150).
Nanoparticle Tracking Analysis
Murine blood collected from the right ventricle was used to prepare serum. A 100-µL sample of serum was used for nanoparticle tracking analysis of exosomes. The serum was centrifuged at 2,000 g and subsequently at 10,000 g for 30 min at 4℃ each, and the supernatant was ultracentrifuged at 100,000 g for 90 min. The exosome pellet was suspended in 2 mL PBS and analyzed using a NANOSIGHT NS 300 (Malvern, Co. Ltd.).
Droplet Digital PCR
Total RNA was prepared using the Qiagen RNeasy Fibrous Mini Kit according to the manufacturer’s instructions, and cDNA was prepared using the PrimeScriptTM
RT-PCR Kit (Takara, Otsu, Japan). The reaction mixture for PCR contained 10 µL of 2× ddPCR supermix for probes without UTP (Bio-Rad), 1 µL of 20× PrimeTime standard qPCR assay (Integrated DNA Technologies), template DNA, and water added to 20 µL. Droplets were generated using a QX200 automated droplet generator (Bio-Rad). Amplification was performed under the following cycling conditions: 95℃ for 10 min; 40 cycles of 94℃ for 30 s, followed by 60℃ for 60 s; 98℃ for 10 min; and hold at 4℃. The droplets were immediately analyzed using a QX200 droplet reader.
Spheroid Assay
Cultured HUVECs (PromoCell) were collected and 2,000 cells were transferred to a low-adhesive U-bottomed 96-well plate. Spheroids were formed after 2 days of incubation with 50 µL culture medium (EGM-2; Lonza). Sixteen aggregated spheroids were mixed with 50µL collagen gel (Nitta gelatin) and mounted on a 3.5-cm glass-bottomed dish.
The gelled spheroids were cocultured with 1.0×106
H9C2 cells in a cell culture insert (Millicell) and incubated with 20 ng/mL medium.
Quantitative Real-Time PCR
Total RNA from HUVEC lysate was prepared using a Qiagen RNeasy Mini Kit according to the manufacturer’s instructions. cDNA synthesis and quantitative real-time PCR were performed using the THUNDERBIRD SYBR qPCR/RT set (Toyobo, Osaka, Japan). Transcript levels were determined as the number of transcripts relative to that of GAPDH. The sequences of the primers used in this study are listed in the Table.
Table.
Sequences of Primers Used in This Study
| Gene |
Primer sequence (5’–3’) |
| GAPDH |
F: GTCTCCTCTGACTTCAACAGCG |
| R: ACCACCCTGTTGCTGTAGCCAA |
| HIF1 |
F: CCAGTTAGGTTCCTTCGATCAGT |
| R: TTTGAGGADTTGCGCTTTCA |
| VEGF |
F: TTGCCTTGCTGCTCTACCTCCA |
| R: GATGGCAGTAGCTGCGCTGATA |
| SDF1 |
F: CTCAACACTCCAAACTGTGCCC |
| R: CTCCAGGTACTCCTGAATCCAC |
| PDGF |
F: CAGCGACTCCTGGAGATAGACT |
| R: CGATGCTTCTCTTCCTCCGAATG |
| ANGPT1 |
F: CAACAGTGTCCTTCAGAAGCAGC |
| R: CCAGCTTGATATACATCTGCACAG |
| ANGPT2 |
F: ATTCAGCGACGTGAGGATGGCA |
| R: GCACATAGCGTTGCTGATTAGTC |
ANGPT1, angiopoietin 1; ANGPT2, angiopoietin 2; F, forward; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; HIF1, hypoxia-inducible factor-1; PDGF, platelet-derived growth factor; R, reverse; SDF1, stromal cell-derived factor 1; VEGF, vascular endothelial growth factor.
Statistical Analysis
Continuous variables are presented as the median and interquartile range based on their distributions. Continuous variables were compared using Student’s t-test or Wilcoxon’s rank-sum test based on their distributions. In this study, all tests were two-tailed; values of P<0.05 were considered statistically significant. These analyses were performed using JMP software for Windows (version 13.0.0; SAS, Cary, NC, USA).
Results
Isolation of Exosomal miRNAs From Mouse Serum
Consistent with our previous report,13 Akt1 phosphorylation in skeletal muscle of Akt1-TG mice increased in response to 2 weeks of doxycycline treatment (Figure 1A). Akt1-mediated skeletal muscle growth, as assessed using gastrocnemius muscle weight, was significantly enhanced 2 weeks after doxycycline treatment (Figure 1B).
To examine whether Akt1-mediated muscle growth affects the amount of circulating exosomes, we isolated exosomes from the serum of control and Akt1-TG mice after 2 weeks doxycycline treatment. Western blot analysis using CD9 and CD63 antibodies showed that the amount of exosomes from the serum was comparable between the Akt1-TG and control mice (Figure 1C). We also performed nanoparticle tracking analysis to compare the amount of exosomal protein from the serum between Akt1-TG and control mice, and confirmed that there was no difference between the 2 groups (Figure 1D).
To investigate whether the circulating exosomal miRNA profile from the serum was altered in response to Akt1-mediated muscle growth, miRNAs were extracted from serum exosomes, and a real-time PCR array was performed (Figure 2A). Among the 641 miRNAs, 50 were upregulated and 56 were downregulated in Akt1-TG mice compared with control mice (Figure 2B). Target gene prediction was performed using TargetScan and miRDB for Akt1-TG mice (Figure 2C). There were 6,930 target genes in the upregulated miRNA group and 8,171 in the downregulated miRNA group. Among these target genes, 4,665 target genes in the 2 groups overlapped. Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway frequency analysis was performed for the 4,665 target genes (Figure 2D). Axon guidance, Focal adhesion, Insulin signaling, PI3 kinase-Akt signaling, and downstream signaling pathways, such as the FoxO signaling pathway, were enriched for these target genes.

Next, we focused on individual miRNAs. Myocyte-enriched miRNAs, such as miR1, miR133a, and miR206, were upregulated in Akt1-TG mice compared with control mice. These results were validated using ddPCR, and miR133 and miR206 were confirmed to be significantly upregulated in Akt-TG mice (Figure 2E). These results indicate that Akt1-mediated skeletal muscle growth leads to an increase in the expression of myocyte-enriched miRNAs in serum exosomes.
Upregulation of miR206 in the Culture Medium of IGF-1-Stimulated C2C12 Myotubes
To examine whether upregulated miRNAs were derived from myocytes, we performed in vitro experiments using C2C12 myotubes. The differentiated C2C12 myotubes were stimulated with IGF-1 to activate Akt 1, and subsequently myotube hypertrophy (Figure 3A; Supplementary Figure 1). Western blot analysis revealed that the ratio of p-Akt to t-Akt increased by 2.4-fold in IGF-1-stimulated C2C12 cell lysates (Figure 3B). Under these conditions, exosomal miRNAs were extracted from the culture medium of C2C12 cells and subjected to ddPCR. The expression of miR206, but not of miR-133, was significantly upregulated in the culture medium of IGF-1-stimulated C2C12 myotubes (Figure 3C). In contrast, neither miR-206 nor miR-133 was upregulated in the gastrocnemius muscle harvested from Akt1-TG mice (data not shown).
Effects of Exogenous Supplementation of miR206 on Angiogenic Response in HUVECs
To address whether exogenous miRNA was incorporated into another cell, we transfected PKH26-labeled exosome in HUVECs. To visualize the exosome, we stained exosome with PKH26 staining solution (MERCK, co. ltd). We found that PKH26-labeled exosomes were taken up by HUVECs (Supplementary Figure 2). To evaluate the functional role of exogenous miR206 in another cell, we transfected HUVECs with miR-206 mimics. Because cell–cell interaction between myocytes and endothelial cells is also important in the heart, we performed a spheroid assay using H9C2 cells as a different series of experiments from C2C12 cells. As shown in Figure 4A,B, miR-206-3p mimics stimulated spheroid formation in HUVECs, as revealed by sprout length and the number of sprouts per spheroid. Expression of angiogenesis-related transcripts was assessed using real-time PCR. Supplementation with exogenous miRNA206 increased the expression of angiogenesis-related genes, such as vascular endothelial growth factor (VEGF) and stromal cell-derived factor 1 (SDF1), in endothelial cells (Figure 4C). These data suggest that miR206 secreted from growing muscles acts on endothelial cells and promotes angiogenesis.
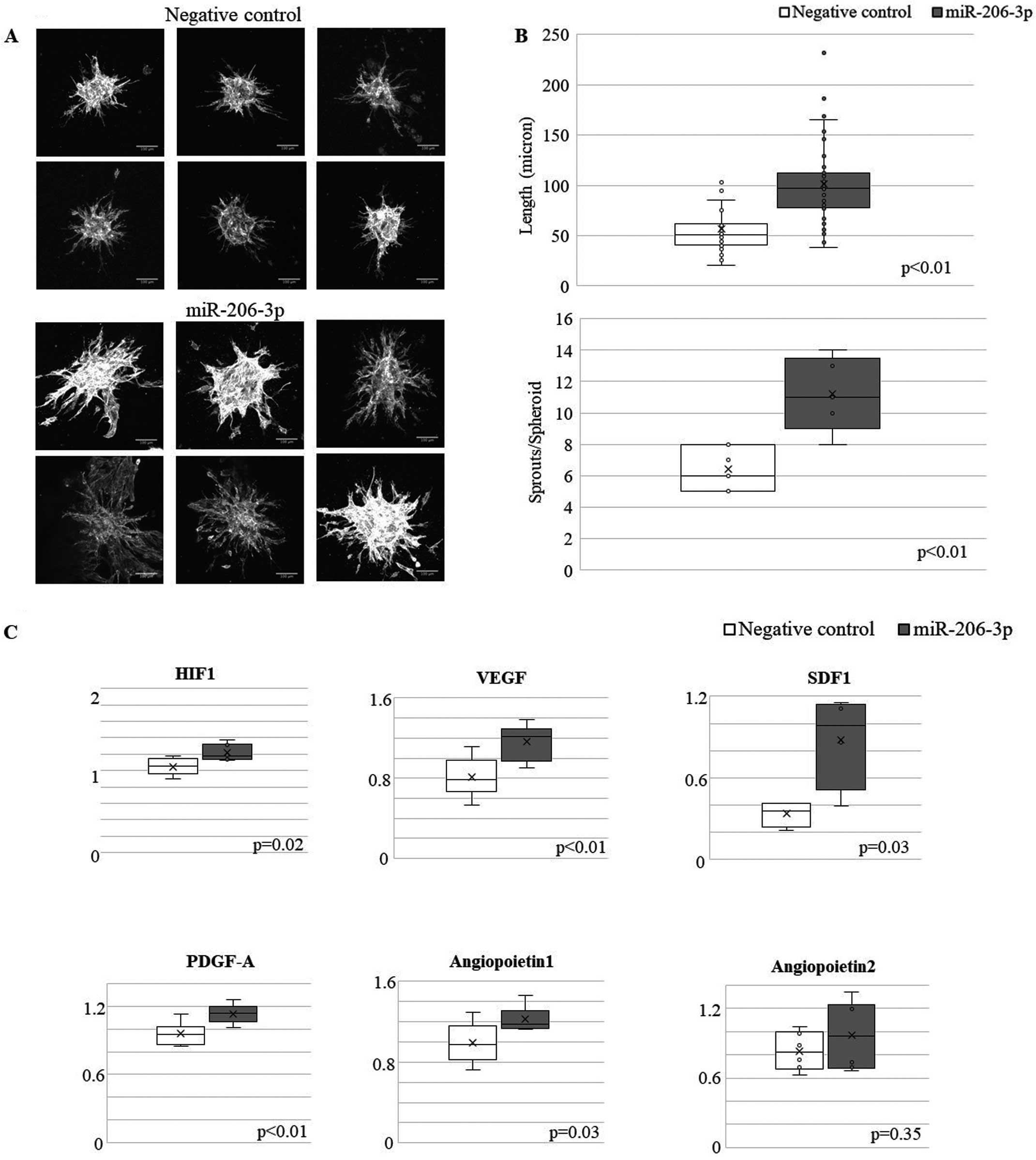
Discussion
Resistance training aimed at maintaining or increasing muscle mass reportedly has beneficial effects in patients with LEAD.2 However, the molecular mechanisms underlying these beneficial effects remain unclear. In the present study, we demonstrated that: (1) miR1, miR133, and miR206 were significantly upregulated among the exosomal miRNAs derived from Akt-TG mouse serum; (2) exosomal miRNA206 was significantly increased in the culture medium of IGF-1-treated C2C12 myotubes; and (3) exogenous supplementation with miRNA206 promotes angiogenic responses in HUVECs. Our data indicate that myocyte-derived exosomal miRNAs could be therapeutic targets for patients with LEAD.
Aerobic and resistance training are effective therapeutic interventions for age-associated cardiovascular and metabolic diseases. Levels of circulating exosomes released from good-quality skeletal muscles were reported to decrease with aging in humans, and resistance training could reverse this.26 In contrast, resistance training has been reported to decrease plasma concentrations of CD63, an exosomal marker, and protein expression in elderly individuals.27 In the present study, the amount of circulating exosomal proteins, evaluated using western blotting and nanoparticle tracking analysis, did not differ between Akt-TG and control mice. These differences in the findings may be explained by the fact that time, duration, and intensity are important determinants of circulating exosome levels because although the amount of circulating exosomes was reported to be altered in response to resistance exercise, the extent of change differed according to exercise time.28
The IGF/Akt signaling pathway is preferentially activated following resistance training in skeletal muscle.29 It has been reported that resistance training increases IGF-1 concentrations at rest and after exercise in humans.30,31 Overexpression of constitutively active Akt has been reported to induce muscle hypertrophy and to promote fiber growth with a little effect on fiber specification in regenerating skeletal muscle.11,12 These reports indicated Akt1 signaling in the control of organ size and cellular hypertrophy. Therefore, we used Akt1-TG mice as a mimic model of resistance exercise training in this study. It has been reported that high-intensity resistance training increases levels of miR-206.32 Although we did not demonstrate whether miR206 is actually increased in the serum of exercise-loaded mice in the present study, these previous reports support our notion that miR206 is increased in the serum following resistance exercise training. Furthermore, Axon guidance and Focal adhesion were enriched in KEGG pathway frequency analysis because these pathways are closely related to the angiogenic response.
Exercise is expected to prevent age-associated organ dysfunction by upregulating the expression of circulating exosomal miRNAs. In fact, circulating exosomes secreted from muscle cells carry some miRNAs that alter the expression of genes in the liver, resulting in improved body metabolism.33 In the cardiovascular system, exosome-mediated miRNA transfer is one of the mechanisms regulating atherosclerosis and cardiac hypertrophy.19,20 Exercise-induced exosomal miR342-5p was reported to protect the heart from ischemia-reperfusion injury.34 In the present study, we observed that exosomal miRNAs, especially miR206, secreted from growing muscles affected endothelial cells and promoted angiogenic responses. Although further in vivo evidence is required, our findings highlight the beneficial effects of resistance exercise on patients with LEAD. However, exosomes isolated from growing muscle are supposed to contain a variety of miRNAs; further studies would be required to identify other miRNAs that could act on endothelial cells.
The mechanism by which activation of Akt signaling enhances the expression of exosomal miR206 could not be deciphered in the present study. However, previous reports indicate that miRNA that is highly expressed in skeletal muscle such as miR1, miR133a, and miR206, are targets of the IGF-1/Akt/mammalian target of rapamycin signaling pathway.35,36 It has been reported that acute resistance exercise training increases the expression of miR-133a and miR206 in skeletal muscle,32 and circulating exosomal miR-206 was significantly increased after iso-inertial resistance training in humans.37 Based on these findings, we speculate that IGF/Akt signaling upregulates miR206 without affecting exosome concentration.
In addition to miR1 and miR133, miR206 is a well-known miRNA involved in the growth and differentiation of skeletal muscle; collectively, these miRNAs are referred to as “MyomiRs”.38 We previously reported that the growth and proliferation of skeletal muscle cells are highly upregulated in Akt-TG mice;13 therefore, we speculated that the activation of Akt-induced growth and proliferation of muscle cells, either directly or indirectly, leads to increased expression of miR206, miR1, and miR133. In the present study, miR133 was upregulated in serum exosome in Akt1-TG mice, but not in the culture media from IGF-1-stimulated C2C12 myotubes. Therefore, we speculated that miR133a was not a myocyte-derived exosomal miRNA in this experimental model. However, further studies are required to determine the downstream signaling pathways that contribute to the upregulation of these miRNAs in skeletal muscle cells.
Although miR-206 and miR-133 were enriched in the culture medium of IGF-1-stimulated C2C12 myotubes, we did not observe upregulation of these miRNAs in the gastrocnemius muscle harvested from Akt1-TG mice. Because in vivo muscle tissues contains not only myocytes, but also other cell types, such as fibroblasts and vascular cells, upregulation of these miRNAs in myocytes was possibly masked. Another possibility is that there was some time lag in the upregulation of these miRNAs between muscle tissue and serum.
Angiogenesis was reported to be inhibited by an increase in the expression of miR-206 in colorectal cancer cells.39 Moreover, overexpression of miR-206 in non-small cell lung cancer cells attenuated angiogenesis by inhibiting the hypoxia-inducible factor-1/VEGF pathway.40 In contrast, endothelial progenitor cell homing and angiogenesis were increased upon inhibition of miR-206 in a rheumatoid arthritis model.41 These studies indicate the anti-angiogenic role of miR206 in pathological angiogenesis. In the present study, we observed that exogenous supplementation with miRNA206 promoted an angiogenic response in endothelial cells. The spheroid-based sprouting assay is widely used as an angiogenesis model in endothelial cells.42 Spheroid formation and the panel of angiogenic transcription factors was increased by exogenous miR206 supplementation, suggesting that miR206 acts as a proangiogenic miRNA in our model. Because the Akt-TG mice used in this study showed increased growth of capillary vessels in skeletal muscles after 2 weeks of Akt1 activation without any pathological stimulus,43 the discrepancy regarding the angiogenic property of miR-206 may reflect the difference under physiological and pathological angiogenesis.
In conclusion, exosomal miR206 secreted from growing skeletal muscles may promote an angiogenic response in endothelial cells. Thus, exosome-mediated miRNA transfer may be an additional mechanism underlying the beneficial effects of resistance training in patients with cardiovascular diseases.
Acknowledgments
The authors thank Saeko Tokunaga and Megumi Nagahiro for excellent technical assistance.
Sources of Funding
This work was supported, in part, by a Grant-in-Aid for Scientific Research C from the Japanese Ministry of Education, Culture, Sports, Science, and Technology (19K08546 to M.Y.), Suzuken Memorial Foundation, Mitsui Sumitomo Insurance Welfare Foundation, and the Japanese Association of Cardiac Rehabilitation (to Y.I.).
Disclosures
M.Y., K.T., and D.F. are members of Circulation Journal’s Editorial Team. The remaining authors have no conflicts of interest to disclose.
IRB Information
The present study was granted an exemption from requiring ethics approval by Kumamoto University because it did not involve human subjects.
Supplementary Files
Please find supplementary file(s);
https://doi.org/10.1253/circj.CJ-23-0353
References
- 1.
Criqui MH, Matsushita K, Aboyans V, Hess CN, Hicks CW, Kwan TW, et al. Lower extremity peripheral artery disease: Contemporary epidemiology, management gaps, and future directions: A scientific statement from the American Heart Association. Circulation 2021; 144: e171–e191, doi:10.1161/CIR.0000000000001005.
- 2.
Azuma N. JCS/JSVS 2022 guideline on the management of peripheral arterial disease. https://www.j-circ.or.jp/cms/wp-content/uploads/2022/03/JCS2022_Azuma.pdf (accessed March 18, 2023) (in Japanese).
- 3.
Aboyans V, Ricco JB, Bartelink MEL, Bjorck M, Brodmann M, Cohnert T, et al. 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries endorsed by: The European Stroke Organization (ESO), The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur Heart J 2018; 39: 763–816, doi:101093/eurheartj/ehx095.
- 4.
McDermott MM, Ades P, Guralnik JM, Dyer A, Ferrucci L, Liu K, et al. Treadmill exercise and resistance training in patients with peripheral arterial disease with and without intermittent claudication: A randomized controlled trial. JAMA 2009; 301: 165–174, doi:10.1001/jama.2008.962.
- 5.
Ritti-Dias RM, Wolosker N, de Moraes Forjaz CL, Carvalho CR, Cucato GG, Leao PP, et al. Strength training increases walking tolerance in intermittent claudication patients: Randomized trial. J Vasc Surg 2010; 51: 89–95, doi:10.1016/j.jvs.2009.07.118.
- 6.
Askew CD, Parmenter B, Leicht AS, Walker PJ, Golledge J. Exercise & Sports Science Australia (ESSA) position statement on exercise prescription for patients with peripheral arterial disease and intermittent claudication. J Sci Med Sport 2014; 17: 623–629, doi:10.1016/j.jsams.2013.10.251.
- 7.
Alsaigh T, Di Bartolo BA, Mulangala J, Figtree GA, Leeper NJ. Bench-to-bedside in vascular medicine: Optimizing the translational pipeline for patients with peripheral artery disease. Circ Res 2021; 128: 1927–1943, doi:10.1161/CIRCRESAHA.121.318265.
- 8.
Pedersen BK, Akerstrom TC, Nielsen AR, Fischer CP. Role of myokines in exercise and metabolism. J Appl Physiol (1985) 2007; 103: 1093–1098, doi:10.1152/japplphysiol.00080.2007.
- 9.
Severinsen MCK, Pedersen BK. Muscle–organ crosstalk: The emerging roles of myokines. Endocr Rev 2020; 41: 594–609, doi:10.1210/endrev/bnaa016.
- 10.
Zunner BEM, Wachsmuth NB, Eckstein ML, Scherl L, Schierbauer JR, Haupt S, et al. Myokines and resistance training: A narrative review. Int J Mol Sci 2022; 23: 3501, doi:10.3390/ijms23073501.
- 11.
Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, et al. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol 2001; 3: 1014–1019, doi:10.1038/ncb1101-1014.
- 12.
Takahashi A, Kureishi Y, Yang J, Luo Z, Guo K, Mukhopadhyay D, et al. Myogenic Akt signaling regulates blood vessel recruitment during myofiber growth. Mol Cell Biol 2002; 22: 4803–4814, doi:10.1128/MCB.22.13.4803-4814.2002.
- 13.
Izumiya Y, Hopkins T, Morris C, Sato K, Zeng L, Viereck J, et al. Fast/glycolytic muscle fiber growth reduces fat mass and improves metabolic parameters in obese mice. Cell Metab 2008; 7: 159–172, doi:10.1016/j.cmet.2007.11.003.
- 14.
Araki S, Izumiya Y, Hanatani S, Rokutanda T, Usuku H, Akasaki Y, et al. Akt1-mediated skeletal muscle growth attenuates cardiac dysfunction and remodeling after experimental myocardial infarction. Circ Heart Fail 2012; 5: 116–125, doi:10.1161/CIRCHEARTFAILURE.111.964783.
- 15.
Hanatani S, Izumiya Y, Araki S, Rokutanda T, Kimura Y, Walsh K, et al. Akt1-mediated fast/glycolytic skeletal muscle growth attenuates renal damage in experimental kidney disease. J Am Soc Nephrol 2014; 25: 2800–2811, doi:10.1681/ASN.2013091025.
- 16.
Onoue Y, Izumiya Y, Hanatani S, Ishida T, Arima Y, Yamamura S, et al. Akt1-mediated muscle growth promotes blood flow recovery after hindlimb ischemia by enhancing heme oxygenase-1 in neighboring cells. Circ J 2018; 82: 2905–2912, doi:10.1253/circj.CJ-18-0135.
- 17.
Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 2007; 9: 654–659, doi:10.1038/ncb1596.
- 18.
Yellon DM, Davidson SM. Exosomes: Nanoparticles involved in cardioprotection? Circ Res 2014; 114: 325–332, doi:10.1161/CIRCRESAHA.113.300636.
- 19.
Hergenreider E, Heydt S, Treguer K, Boettger T, Horrevoets AJ, Zeiher AM, et al. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat Cell Biol 2012; 14: 249–256, doi:10.1038/ncb2441.
- 20.
Bang C, Batkai S, Dangwal S, Gupta SK, Foinquinos A, Holzmann A, et al. Cardiac fibroblast-derived microRNA passenger strand-enriched exosomes mediate cardiomyocyte hypertrophy. J Clin Invest 2014; 124: 2136–2146, doi:10.1172/JCI70577.
- 21.
Forterre A, Jalabert A, Chikh K, Pesenti S, Euthine V, Granjon A, et al. Myotube-derived exosomal miRNAs downregulate Sirtuin1 in myoblasts during muscle cell differentiation. Cell Cycle 2014; 13: 78–89, doi:10.4161/cc.26808.
- 22.
Grill MA, Bales MA, Fought AN, Rosburg KC, Munger SJ, Antin PB. Tetracycline-inducible system for regulation of skeletal muscle-specific gene expression in transgenic mice. Transgenic Res 2003; 12: 33–43, doi:10.1023/a:1022119005836.
- 23.
Shiojima I, Sato K, Izumiya Y, Schiekofer S, Ito M, Liao R, et al. Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure. J Clin Invest 2005; 115: 2108–2118, doi:10.1172/JCI24682.
- 24.
National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals. 8th edition. Washington (DC): National Academies Press (US); 2011, doi:10.17226/12910.
- 25.
Sano S, Izumi Y, Yamaguchi T, Yamazaki T, Tanaka M, Shiota M, et al. Lipid synthesis is promoted by hypoxic adipocyte-derived exosomes in 3T3-L1 cells. Biochem Biophys Res Commun 2014; 445: 327–333, doi:10.1016/j.bbrc.2014.01.183.
- 26.
Xhuti D, Nilsson MI, Manta K, Tarnopolsky MA, Nederveen JP. Circulating exosome-like vesicle and skeletal muscle microRNAs are altered with age and resistance training. J Physiol 2023, doi:10.1113/JP282663.
- 27.
Estebanez B, Visavadiya NP, de Paz JA, Whitehurst M, Cuevas MJ, Gonzalez-Gallego J, et al. Resistance training diminishes the expression of exosome CD63 protein without modification of plasma miR-146a-5p and cfDNA in the elderly. Nutrients 2021; 13: 665, doi:10.3390/nu13020665.
- 28.
Conkright WR, Beckner ME, Sterczala AJ, Mi Q, Lovalekar M, Sahu A, et al. Resistance exercise differentially alters extracellular vesicle size and subpopulation characteristics in healthy men and women: An observational cohort study. Physiol Genomics 2022; 54: 350–359, doi:10.1152/physiolgenomics.00171.2021.
- 29.
Nader GA, Esser KA. Intracellular signaling specificity in skeletal muscle in response to different modes of exercise. J Appl Physiol (1985) 2001; 90: 1936–1942, doi:10.1152/jappl.2001.90.5.1936.
- 30.
Rubin MR, Kraemer WJ, Maresh CM, Volek JS, Ratamess NA, Vanheest JL, et al. High-affinity growth hormone binding protein and acute heavy resistance exercise. Med Sci Sports Exerc 2005; 37: 395–403, doi:10.1249/01.mss.0000155402.93987.c0.
- 31.
Borst SE, De Hoyos DV, Garzarella L, Vincent K, Pollock BH, Lowenthal DT, et al. Effects of resistance training on insulin-like growth factor-I and IGF binding proteins. Med Sci Sports Exerc 2001; 33: 648–653, doi:10.1097/00005768-200104000-00021.
- 32.
D’Souza RF, Markworth JF, Aasen KMM, Zeng N, Cameron-Smith D, Mitchell CJ. Acute resistance exercise modulates microRNA expression profiles: Combined tissue and circulatory targeted analyses. PLoS One 2017; 12: e0181594, doi:10.1371/journal.pone.0181594.
- 33.
Castano C, Mirasierra M, Vallejo M, Novials A, Parrizas M. Delivery of muscle-derived exosomal miRNAs induced by HIIT improves insulin sensitivity through down-regulation of hepatic FoxO1 in mice. Proc Natl Acad Sci U S A 2020; 117: 30335–30343, doi:10.1073/pnas.2016112117.
- 34.
Hou Z, Qin X, Hu Y, Zhang X, Li G, Wu J, et al. Longterm exercise-derived exosomal miR-342-5p: A novel exerkine for cardioprotection. Circ Res 2019; 124: 1386–1400, doi:10.1161/CIRCRESAHA.118.314635.
- 35.
Wang XH. MicroRNA in myogenesis and muscle atrophy. Curr Opin Clin Nutr Metab Care 2013; 16: 258–266, doi:10.1097/MCO.0b013e32835f81b9.
- 36.
Yan B, Zhu CD, Guo JT, Zhao LH, Zhao JL. miR-206 regulates the growth of the teleost tilapia (Oreochromis niloticus) through the modulation of IGF-1 gene expression. J Exp Biol 2013; 216: 1265–1269, doi:10.1242/jeb.079590.
- 37.
Annibalini G, Contarelli S, Lucertini F, Guescini M, Maggio S, Ceccaroli P, et al. Muscle and systemic molecular responses to a single flywheel based iso-inertial training session in resistance-trained men. Front Physiol 2019; 10: 554, doi:10.3389/fphys.2019.00554.
- 38.
Luo W, Nie Q, Zhang X. MicroRNAs involved in skeletal muscle differentiation. J Genet Genomics 2013; 40: 107–116, doi:10.1016/j.jgg.2013.02.002.
- 39.
Xu Z, Zhu C, Chen C, Zong Y, Feng H, Liu D, et al. CCL19 suppresses angiogenesis through promoting miR-206 and inhibiting Met/ERK/Elk-1/HIF-1alpha/VEGF-A pathway in colorectal cancer. Cell Death Dis 2018; 9: 974, doi:10.1038/s41419-018-1010-2.
- 40.
Xue D, Yang Y, Liu Y, Wang P, Dai Y, Liu Q, et al. MicroRNA-206 attenuates the growth and angiogenesis in non-small cell lung cancer cells by blocking the 14-3-3zeta/STAT3/HIF-1alpha/VEGF signaling. Oncotarget 2016; 7: 79805–79813, doi:10.18632/oncotarget.12972.
- 41.
Su CM, Hsu CJ, Tsai CH, Huang CY, Wang SW, Tang CH. Resistin Promotes Angiogenesis in endothelial progenitor cells through inhibition of microRNA206: Potential implications for rheumatoid arthritis. Stem Cells 2015; 33: 2243–2255, doi:10.1002/stem.2024.
- 42.
Tetzlaff F, Fischer A. Human endothelial cell spheroid-based sprouting angiogenesis assay in collagen. Bio Protoc 2018; 8: e2995, doi:10.21769/BioProtoc.2995.
- 43.
Ouchi N, Oshima Y, Ohashi K, Higuchi A, Ikegami C, Izumiya Y, et al. Follistatin-like 1, a secreted muscle protein, promotes endothelial cell function and revascularization in ischemic tissue through a nitric-oxide synthase-dependent mechanism. J Biol Chem 2008; 283: 32802–32811, doi:10.1074/jbc.M803440200.





