Abstract
Background: This study investigated the impact and predictive factors of concomitant significant tricuspid regurgitation (TR) and evaluated the roles of right ventricle (RV) function and the etiology of TR in the clinical outcomes of patients with severe aortic stenosis undergoing transcatheter aortic valve implantation (TAVI).
Methods and Results: We assessed grading of TR severity, TR etiology, and RV function in pre- and post-TAVI transthoracic echocardiograms for 678 patients at Keio University School of Medicine. TR etiology was divided into 3 groups: primary TR, ventricular functional TR (FTR), and atrial FTR. The primary outcomes were all-cause and cardiovascular death. At baseline, moderate or greater TR was found in 55 (8%) patients and, after adjustment for comorbidities, was associated with increased all-cause death (hazard ratio [HR] 2.11; 95% confidence interval [CI] 1.19−3.77; P=0.011) and cardiovascular death (HR 2.29; 95% CI 1.06−4.99; P=0.036). RV dysfunction (RVD) also remained an independent predictor of cardiovascular death (HR 2.06; 95% CI 1.03−4.14; P=0.042). Among the TR etiology groups, patients with ventricular FTR had the lowest survival rate (P<0.001). Patients with persistent RVD after TAVI had a higher risk of cardiovascular death than those with a normal or recovered RV function (P<0.001).
Conclusions: The etiology of TR and RV function play an important role in predicting outcomes in concomitant TR patients undergoing TAVI.
Although the tricuspid valve (TV) has long been regarded as the “forgotten” valve of the heart patients with untreated tricuspid regurgitation (TR) have recently been found to have a poor prognosis.1 In patients with severe aortic stenosis (AS), concomitant significant TR may appear in approximately 10–30% of cases.2–4 Guidelines suggest repairing significant TR when left-sided heart surgery is performed.5 However, with the arrival of TAVI, new problems have arisen because these patients are unable to undergo concomitant tricuspid surgery.
Editorial p 460
Recently, many studies have focused on the role of concomitant TR in patients undergoing TAVI. Lindman et al3 reported that after adjustment for right ventricle (RV) dysfunction (RVD), significant TR was independently associated with increased mortality. In contrast, in other studies, RVD, rather than significant TR, led to adverse outcomes.2,4 As stated by Bolling, the association between RV function and TR is complex and intertwined.6 In considering the management of AS with TR, we thought it important to clarify the relationship between the etiology of TR, RVD, and prognosis. Therefore, the aim of this study was to investigate: (1) the clinical impact of concomitant significant TR on patients undergoing TAVI; (2) the influence of the etiology of TR on outcome, and factors associated with an improvement in or persistence of TR; and (3) the role of the RV and its effects on clinical outcomes following TAVI in patients with concomitant TR.
Methods
Study Population
Between October 2013 and April 2019, 712 consecutive patients with symptomatic severe AS underwent TAVI at Keio University School of Medicine and were included in our ongoing single-center prospective registry of TAVI. All patients were considered at high risk for valvular surgery by the institution’s heart team. We excluded 34 patients who had either incomplete preprocedural echocardiographic data (n=3) or were part of the preclinical trial phase (n=31). Patient characteristics, echocardiographic parameters, and clinical outcomes were analyzed in the remaining 678 TAVI recipients who were stratified according to the presence or absence of significant TR.
The study protocol was approved by the institutional ethics committee at Keio University School of Medicine, and all patients provided written informed consent. The study procedures were performed in accordance with the Declaration of Helsinki.
Echocardiography
Echocardiographic data were recorded at baseline, after TAVI (before discharge), and at 1 year in a standard manner using multiple echo machines (Vivid 7, Vivid E9, and Vivid E95 [GE Medical Systems, Milwaukee, WI, USA]; iE-33 and EOIQ [Philips Healthcare, Inc., Andover, MA, USA]; and Artida [Toshiba Medical Systems, Tokyo, Japan]). Measurements and recordings were obtained according to the American Society of Echocardiography guidelines.7 The severity of AS was defined by the aortic valve area calculated using the standard continuity equation (aortic valve area <1.0 cm2
or <0.6 cm2/m2). Low flow was defined as a stroke volume index <35 mL/m2, a low pressure gradient (PG) was defined as a transaortic pressure gradient <40 mmHg, and low left ventricular ejection fraction (LVEF) was defined as <50%.8 TR severity was assessed using an integrated approach and graded as none, mild, moderate, and severe according to current guidelines.9 We defined patients who had moderate or greater TR as “significant TR” and patients who had mild or less TR as “non-significant TR”. In patients with significant TR, we assessed the change in TR grading within 1 year after TAVI during follow-up and defined persistent TR as the persistence of significant TR after TAVI; improved TR was defined as an improvement to mild or less TR after TAVI. The tricuspid annular diameter was measured from the insertion of the septal leaflet to the insertion of the anterior leaflet and was averaged over 3 consecutive heartbeats in the apical 4-chamber view at end-diastole.10 Tricuspid tenting height was measured as the distance between the linear apical displacement of the tricuspid leaflet coaptation from the line connecting the annulus hinge point towards the RV cavity at end-systole;11 the tenting area was defined as the area between the atrial surface of the leaflets and the annulus plane at end-systole.12
For each patient with significant TR, we assessed the etiology of TR.13 Primary TR was defined as the presence of primary damage to the TV apparatus, including rheumatic, prolapse, and lead-induced causes of TR. Patients with secondary TR were divided into 2 groups: (1) those with ventricular functional TR (FTR), involving cases with significant dilatation of the RV leading to tethering of the TV leaflets and a coaptation gap, with a cut-off value of >8 mm for tenting height14 or 1.6 cm2
for tenting area;10 and (2) atrial FTR, defined as dilatation of the tricuspid annulus due to the dilatation of the right atrium (>40 mm or 21 mm/m2)5 in the presence of atrial fibrillation (AF). RV dimensions were measured at the base, at the middle, and from the base to the apex in the 4-chamber view. RV function was evaluated using fractional area change (FAC; %) and was calculated as follows:

RVD was defined as an RV FAC <35%.7
Statistical Analyses
Continuous normally distributed data are presented as the mean±SD or as the median with interquartile range (IQR). Normally distributed data were compared using Student’s t-test, whereas in the case of non-normal distribution, the Wilcoxon or Mann-Whitney test were used, as appropriate. Categorical variables are presented as counts and percentages and were compared using Fisher’s exact or the χ2
test, as appropriate.
The clinical endpoints were time to all-cause death and cardiovascular death according to the Valve Academic Research Consortium-2 criteria.15 Kaplan-Meier analyses were performed using the log-rank test to evaluate differences between survival curves. All P values are 2-sided, and P<0.05 was considered statistically significant. In univariate and multivariate Cox regression analyses, we selected covariates that were assumed to be the cause of an event based on previous literature and general viewpoints regarding valvular heart disease. Hazard ratios (HRs) were calculated using univariate and multivariate logistic regression models with 95% confidence intervals (CIs). Binary logistic regression with persistence of significant TR or RVD after TAVI as the dependent variable was performed to assess the clinical and echocardiographic factors associated with persistence of significant TR and RVD.
All data were analyzed using the SPSS version 26.0 (SPSS, Chicago, IL, USA).
Results
Study Population
Table 1 presents the clinical baseline characteristics of the 678 patients undergoing TAVI stratified according to the severity of TR. At baseline, significant TR was found in 55 (8%) patients. In the significant and non-significant TR groups, most of the patients were women (82% and 65%, respectively; P=0.013); 56% and 17%, respectively, had a history of AF (P<0.001), 9.1% and 2.9%, respectively, had prior pacemaker implantation (P=0.015), and 89% and 72%, respectively, had chronic kidney disease (P=0.007).
Table 1.
Baseline Characteristics
| |
Non-significant TR
(n=623) |
Significant TR
(n=55) |
P value |
| Age (years) |
84±6 |
86±5 |
0.55 |
| Female sex |
407 (65) |
10 (82) |
0.013 |
| Body surface area (m2) |
1.45±0.18 |
1.39±0.18 |
0.70 |
| NYHA Class III/VI |
259 (42) |
22 (40) |
0.81 |
| Clinical frailty scale |
3.5±1.0 |
3.8±1.2 |
0.2 |
| STS score (%) |
6.5±3.8 |
7.5±4.2 |
0.052 |
| Hypertension |
362 (58) |
23 (42) |
0.019 |
| Diabetes |
136 (22) |
8 (15) |
0.21 |
| Coronary artery disease |
188 (30) |
10 (18) |
0.036 |
| COPD |
129 (21) |
13 (24) |
0.61 |
| Prior pacemaker |
18 (3) |
5 (9) |
0.015 |
| Atrial fibrillation |
103 (17) |
31 (56) |
<0.001 |
| Hemoglobin (g/dL) |
11.3±1.6 |
10.8±1.3 |
0.018 |
| eGFR (mL/min/1.73 m2) |
49.6±16.9 |
45.6±14.4 |
0.11 |
| CKD |
451 (72) |
49 (89) |
0.007 |
| BNP (pg/mL) |
352±418 |
387±311 |
0.44 |
Unless indicated otherwise, values are expressed as the mean±SD or n (%). BNP, B-type natriuretic peptide; CKD, chronic kidney disease (estimated glomerular filtration rate [eGFR] <60 mL/min/1.73 m2); COPD, chronic obstructive pulmonary disease; NYHA, New York Heart Association; STS, Society of Thoracic Surgeons; TR, tricuspid regurgitation.
There was no significant differences between the 2 groups in the prevalence of New York Heart Association functional class III/VI.
Echocardiographic Assessment
Echocardiographic characteristics measured at baseline are presented in Table 2. Patients with significant TR had a significantly larger left ventricle dimension, but there was no difference between the 2 groups in LVEF. A low transaortic PG was more frequently observed in patients with significant than non-significant TR (29.1% vs. 15.1%; P=0.007), as was low flow (30% vs. 16%; P=0.008). There were more patients with concomitant mitral regurgitation in the significant than non-significant TR group (32.7% vs. 6.7%; P<0.001), and pulmonary artery systolic pressure (sPAP) was higher in the former group (43.7±12.6 vs. 32.4±9.4 mmHg; P<0.001). Finally, RV dilation (38.2% vs. 13.3%; P<0.001) and RVD (27.3% vs. 9.6%; P<0.001) were more frequently observed in patients with significant TR than in those with non-significant TR.
Table 2.
Echocardiographic Characteristics
| |
Non-significant TR
(n=623) |
Significant TR
(n=55) |
P value |
| LV end-diastolic dimension (mm) |
43.3±5.8 |
44.1±7.5 |
0.005 |
| LV end-systolic dimension (mm) |
27.5±6.2 |
28.8±8.4 |
0.003 |
| Fractional shortening (%) |
37.0±7.9 |
35.8±8.9 |
0.63 |
| LV mass index (g/m2) |
114±29 |
114±27 |
0.20 |
| Relative wall thickness |
0.50±0.10 |
0.47±0.10 |
0.70 |
| LV end-diastolic volume (mL) |
82.8±30.9 |
85.2±41.0 |
0.044 |
| LV end-systolic volume (mL) |
33.2±22.1 |
37.6±31.7 |
0.019 |
| LVEF (%) |
62.9±11.2 |
61.0±13.1 |
0.11 |
| LVEF <50% |
75 (12) |
10 (18) |
0.187 |
| Low flow |
97 (16) |
16 (29) |
0.008 |
| Stroke volume index (mL/m2) |
46.0±10.9 |
43.2±12.1 |
0.59 |
| Left atrial dimension (mm) |
41.3±7.0 |
45.5±9.3 |
<0.001 |
| E wave (cm/s) |
75±26 |
92±28 |
0.66 |
| A wave (cm/s) |
102±27 |
96±42 |
0.14 |
| E/A ratio |
0.76±0.39 |
0.97±0.76 |
<0.001 |
| E′ (cm/s) |
4.0±1.3 |
4.7±1.3 |
0.96 |
| E/E′ average |
19.9±9.5 |
20.7±8.6 |
0.55 |
| AVA (cm2) |
0.63±0.18 |
0.61±0.18 |
0.50 |
| Indexed AVA (cm2/m2) |
0.44±0.11 |
0.45±0.12 |
0.71 |
| Peak velocity (m/s) |
4.60±0.80 |
4.37±0.70 |
0.21 |
| Mean transaortic PG (mmHg) |
50.2±17.8 |
44.2±14.4 |
0.083 |
| Moderate or greater aortic regurgitation |
24 (4) |
6 (11) |
0.066 |
| Moderate or greater mitral regurgitation |
42 (7) |
18 (33) |
<0.001 |
| sPAP (mmHg) |
32.4±9.4 |
43.7±12.6 |
<0.001 |
| RV base dimension (mm) |
34.1±5.8 |
37.8±7.5 |
0.004 |
| RV mid-dimension (mm) |
24.0±4.9 |
26.6±7.7 |
<0.001 |
| RV long dimension (mm) |
67.1±7.7 |
66.0±9.7 |
0.023 |
| RV dilatation |
79 (13) |
20 (36) |
<0.001 |
| RV end-diastolic area (cm2) |
15.0±3.6 |
17.0±6.2 |
<0.001 |
| RV end-systolic area (cm2) |
8.0±2.6 |
10.0±4.8 |
<0.001 |
| RV FAC (%) |
47.3±9.4 |
42.7±9.6 |
0.48 |
| RVD |
60 (10) |
14 (25) |
<0.001 |
Unless indicated otherwise, values are expressed as the mean±SD or n (%). AVA, aortic valve area; FAC, fractional area change; LV, left ventricle; LVEF, left ventricular ejection fraction; PG, pressure gradient; RV, right ventricle; RVD, right ventricle dysfunction; sPAP, pulmonary arterial systolic pressure. Other abbreviations as in Table 1.
Procedural Characteristics
There were significant differences in the procedure or type of valve implanted between the 2 groups (Supplementary Table 1). Moreover, the prevalence of post-TAVI moderate or severe aortic regurgitation did not differ between the 2 groups.
Significant TR and Outcomes
Over a median follow-up period of 748 days (range 403–1,116 days), 125 (18.4%) deaths were observed, of which 48 (38.4%) were of a cardiovascular cause. Figure 1 shows Kaplan-Meier curves for all-cause death and cardiovascular death according to TR severity. Significant TR was significantly associated with worse survival for both endpoints (log-rank P=0.005 and P=0.001, respectively). After adjusting for LVEF <50%, low PG AS, and RV dilation in the multivariate analysis, female sex (HR 2.20; 95% CI 1.49–3.25; P<0.001), clinical frailty scale (HR 1.25; 95% CI 1.03–1.51; P=0.022), Society of Thoracic Surgeons score (HR 1.05; 95% CI 1.00–1.09; P=0.036), low flow (HR 1.73; 95% CI 1.12–2.65; P=0.013), and significant TR (HR 2.11; 95% CI 1.19–3.77; P=0.011) remained as independent predictors of all-cause death (Supplementary Table 2). As for cardiovascular death, after adjustment for AF, low flow, and sPAP >60 mmHg in the multivariate analysis, only significant TR (HR 2.29; 95% CI 1.06–4.99; P=0.036) and RVD at baseline (HR 2.06; 95% CI 1.03–4.14; P=0.042) remained as independent predictors (Table 3).
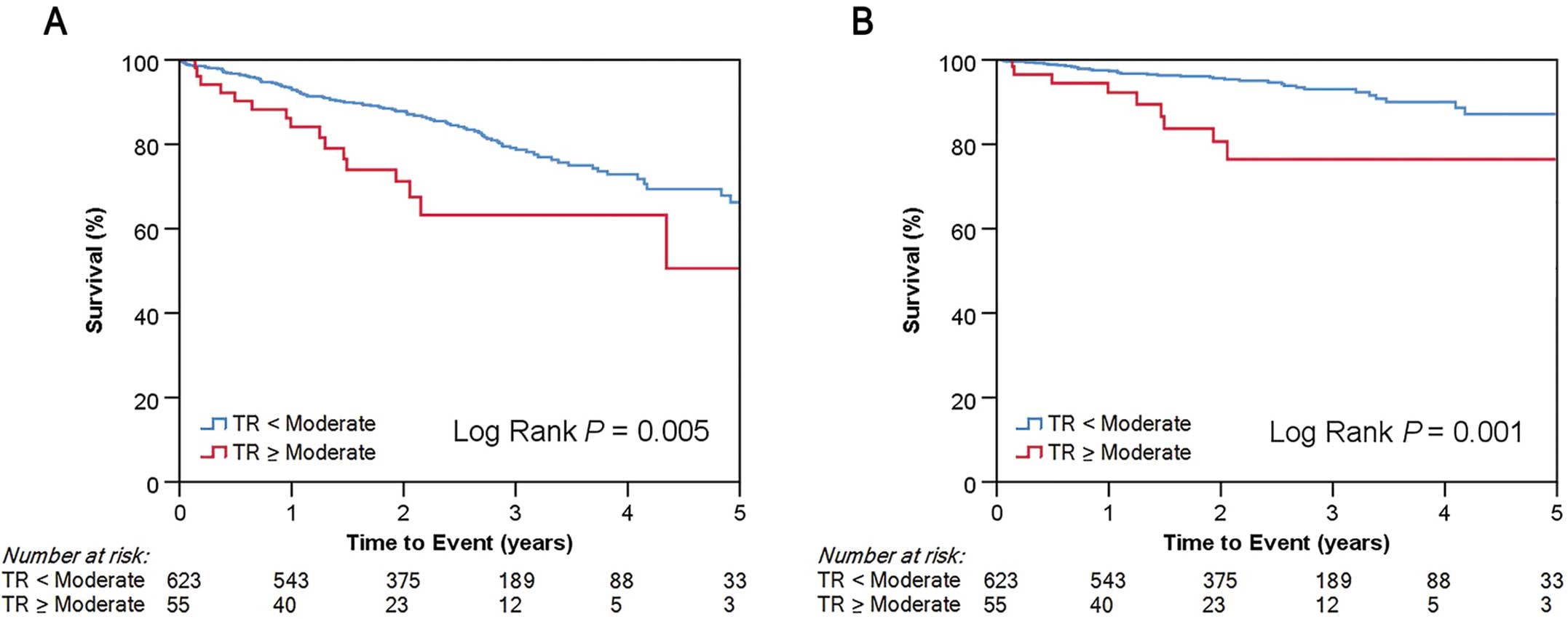
Table 3.
Univariate and Multivariate Cox Regression Analysis for Cardiovascular Death
| |
Univariate analysis |
Multivariate analysis |
| HR (95% CI) |
P value |
HR (95% CI) |
P value |
| Age |
0.97 (0.93–1.01) |
0.19 |
|
|
| Female sex |
1.38 (0.77–2.47) |
0.27 |
|
|
| CKD |
2.02 (0.91–4.50) |
0.086 |
|
|
| Atrial fibrillation |
2.38 (1.33–4.27) |
0.004 |
1.55 (0.81–2.97) |
0.19 |
| LVEF <50% |
1.88 (0.93–3.76) |
0.078 |
|
|
| Low flow |
2.49 (1.33–4.67) |
0.004 |
1.92 (0.99–3.72) |
0.054 |
| Low pressure gradient AS |
1.61 (0.82–3.16) |
0.17 |
|
|
| RVD |
2.98 (1.58–5.64) |
0.001 |
2.06 (1.03–4.14) |
0.042 |
| Baseline moderate or greater MR |
1.56 (0.66–3.67) |
0.31 |
|
|
| Baseline moderate or greater TR |
3.21 (1.55–6.63) |
0.002 |
2.29 (1.06–4.99) |
0.036 |
| sPAP >60 mmHg |
3.26 (1.17–9.07) |
0.024 |
2.10 (0.69–6.32) |
0.19 |
| Post-TAVI AR moderate or severe |
1.55 (0.21–11.23) |
0.67 |
|
|
AR, aortic regurgitation; AS, aortic stenosis; CI, confidence interval; HR, hazard ratio; MR, mitral regurgitation; TAVI, transcatheter aortic valve implantation. Other abbreviations as in Tables 1,2.
Predictors of Persistent TR and Outcomes
We observed an improvement of significant TR to less than moderate TR in 19 patients (34.5%) within 1 year after TAVI (Figure 2). We further analyzed the characteristics of patients with persistent TR and improvement in TR (Table 4). AF, RV dilatation, and RV FAC before and after TAVI were associated with persistent TR after TAVI. However, the severity grade (moderate or severe TR), the etiology of TR, baseline sPAP and post-TAVI sPAP were not associated with the persistence of TR, and an improvement in TR did not lead to better survival (log-rank P=0.988). We performed additional survival analysis according to the presence or absence of persistent significant TR and RVD. We demonstrated that all the patients with persistent RVD showed persistent TR, and patients with persistent TR and persistent RVD had the worst prognosis (P<0.001). In patients without persistent RVD, the prognosis was similar regardless of the presence or absence of persistent TR (Supplementary Figure).

Table 4.
Characteristics of Patients With Moderate or Greater Tricuspid Regurgitation
| |
Pre-TAVI |
Post-TAVI |
P value |
| All TR patients |
Persistent TR (n=36) |
Improvement in TR (n=19) |
| TR severity |
| Moderate |
46 (84) |
29 (63) |
17 (37) |
0.40 |
| Severe |
9 (16) |
7 (78) |
2 (22) |
0.40 |
| TR etiology |
| Primary |
10 (18) |
7 (70) |
3 (30) |
0.74 |
| Atrial FTR |
36 (65) |
22 (61) |
14 (39) |
0.35 |
| Ventricular FTR |
9 (17) |
7 (78) |
2 (22) |
0.35 |
| TV morphology |
| TA diameter (mm) |
32.5±5.3 |
33.7±5.7 |
30.3±4.0 |
0.024 |
| TA/BSA (mm/m2) |
23.5±3.5 |
23.9±3.8 |
22.9±2.8 |
0.365 |
| Tenting height (mm) |
2.8±2.8 |
3.0±3.0 |
2.3±2.5 |
0.360 |
| Tenting area (cm2) |
2.3±0.9 |
2.4±0.9 |
1.7±0.3 |
0.349 |
| RV function and size |
| Baseline RV FAC (%) |
46.9±9.5 |
40.5±8.9 |
46.8±9.9 |
0.022 |
| Post RV FAC (%) |
44.5±10.0 |
42.4±10.6 |
48.5±8.1 |
0.035 |
| RV dilatation |
20 (36) |
18 (90) |
2 (10) |
0.004 |
| Pulmonary sPAP |
| Baseline sPAP |
|
42.5±12.6 |
46.0±12.8 |
0.232 |
| Post-TAVI sPAP |
|
43.3±12.9 |
38.5±9.5 |
0.119 |
| Atrial fibrillation |
31 (56) |
24 (67) |
7 (37) |
0.034 |
Unless indicated otherwise, values are expressed as the mean±SD or n (%). BSA, body surface area; FTR, functional tricuspid regurgitation; TA, tricuspid annulus; TV, tricuspid valve. Other abbreviations as in Tables 1–3.
Etiology of TR and Outcomes
As indicated in Table 4, at baseline we found primary TR in 10 (18%) patients, atrial FTR in 36 (65%), and ventricular FTR in 9 (16%). Among patients with significant TR and RVD at baseline, 6 (43%) had ventricular FTR, 6 (43%) had atrial FTR, and 2 (14%) had primary TR. We found a significant relationship between RVD and the etiology of TR in patients with FTR (P=0.006). Kaplan-Meier analysis (Figure 3) showed a significant difference in survival between patients with primary TR, atrial FTR, ventricular FTR, and patients with non-significant TR, demonstrating the lowest survival rate in patients with ventricular FTR (log-rank P<0.001). Patients with atrial FTR had a survival rate close to that of patients with less than moderate TR (log-rank P=0.453). Although there was no significant difference in survival between patients with ventricular FTR and those with primary TR (log-rank P=0.585), we detected a significant difference in survival outcomes between patients with atrial FTR and those with ventricular FTR (log-rank P=0.027).

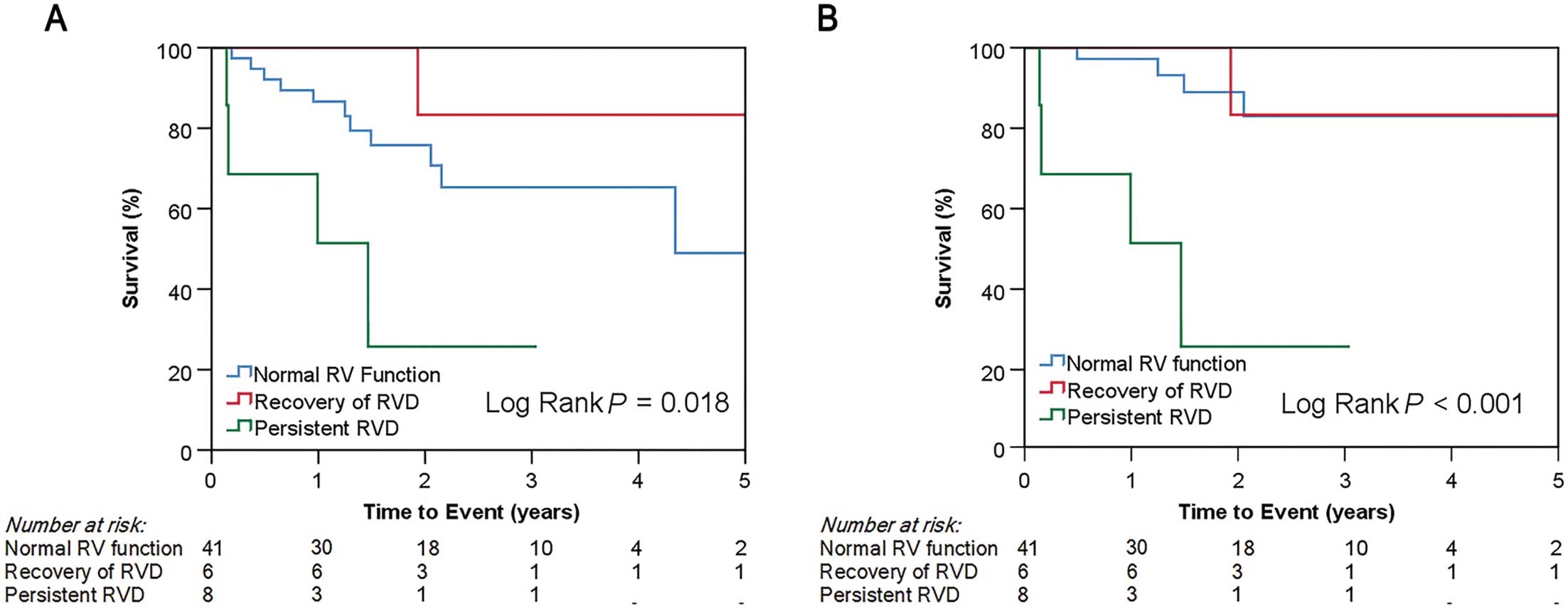
RVD and Outcomes in All Patients and Significant TR Patients
In this cohort, 74 patients had RVD at baseline, and 19 patients (26%) had persistent RVD after TAVI. Survival analysis showed better outcomes for cardiovascular death in patients with recovered RV function than patients with persistent RVD (log-rank P<0.001). In a univariate analysis of all patients with RVD at baseline, RV dilatation, sPAP >60 mmHg, and significant TR at baseline were negative predictors of the improvement in RVD after TAVI (odds ratio [OR] 0.16 [95% CI 0.052–0.51; P=0.002], OR 0.052 [95% CI 0.006–0.48; P=0.009], and OR 0.17 [95% CI, 0.049–0.58; P=0.005], respectively)
To clarify the parameters associated with the clinical outcome in patients with concomitant TR, we performed Cox regression analysis, particularly in the patient group with significant TR at baseline (Supplementary Table 3). In the univariate analysis, ventricular FTR and the RVD at baseline showed a weak and statistically non-significant correlation with cardiovascular death (HR 3.45 [95% CI 0.36–13.83; P=0.081] and HR 3.57 [95% CI 0.96–13.35; P=0.058], respectively). However, RVD post-TAVI was a significant predictor of cardiovascular death (HR 7.09; 95% CI 1.88–26.69; P=0.004). RVD was present in 25.5% of patients with significant TR before TAVI and recovered in 42.9% of patients after TAVI. Figure 4 shows that the persistence of RVD is associated with a worse survival rate compared with patients with normal RV function or with recovery of RV function after TAVI for both all-cause death and cardiovascular death (log-rank P=0.018 and P<0.001, respectively). In the univariate analysis, the factors associated with the persistence of RVD in patients with significant TR were LVEF <50% (OR 5.33; 95% CI 1.11–25.64; P=0.037), RV dilatation (OR 8.89; 95% CI 1.63–48.51; P=0.012), baseline RV FAC (OR 0.78; 95% CI 0.66–0.92; P=0.004), and TV tethering (OR 13.13; 95% Cl 2.48–69.55; P=0.002). In contrast, neither baseline sPAP (OR 1.63; 95% CI 0.28–9.56; P=0.59) nor post-TAVI sPAP >60 mmHg (OR 7.5; 95% CI 0.89–63.6; P=0.065) were significantly associated with the persistence of RVD.
Discussion
The important findings of this are that: (1) patients with concomitant significant TR who undergo TAVI have a higher risk of all-cause and cardiovascular death; (2) among patients with atrial FTR, primary TR, or non-significant TR, those with ventricular FTR have the worst survival outcome; and (3) the persistence of RVD after TAVI leads to a worse survival outcome than normal RV function or recovery of RV function in patients with concomitant significant TR.
Outcomes of Patients With Concomitant TR and AS
In concordance with the findings of Lindman et al,3 significant TR was associated with a 2-fold increased risk of all-cause mortality and cardiovascular death after adjustment for multiple variables. Furthermore, similar results were found in a surgical study by Amano et al,16 demonstrating that the rate of 5-year freedom from aortic valve-related death or hospitalization due to heart failure in patients who need surgical aortic valve replacement (SAVR) was significantly lower for those with significant TR than for those with non-significant TR. We believe that significant TR in patients with AS is associated with an adverse outcome, regardless of the prevalence of other comorbidities and the type of intervention, and should always be assessed thoroughly before any planned intervention.
Etiology of TR
To the best of our knowledge, this study is the first to report a detailed account of the etiology of TR in patients with severe AS. Kaplan-Meier analysis revealed the worst outcome for patients with ventricular FTR. Ventricular FTR, due to significant tethering of the TV leaflets, results from an advanced state of RVD, and, in the present study, we showed a significant relationship between RVD and ventricular FTR. Ventricular FTR due to RVD ultimately causes a low cardiac output and severe venous congestion leading to liver and renal failure.14 This status affects the prognosis of medical and surgical treatment. Interestingly, atrial FTR did not show the same adverse prognosis in our population during the 2-year median follow-up period because the survival rate in that group did not differ significantly from that for patients without significant TR. Hence, atrial FTR with preserved RV function may result in better outcomes than ventricular FTR with TV tethering. Of note, untreated longstanding atrial FTR could develop into ventricular FTR due to continued volume overload of the RV, affecting the long-term prognosis. Our results support the need to assess FTR based not only on its severity, but also on RV dilatation and mode of TV leaflet coaptation, as has been suggested by Dreyfus et al,17 to predict prognosis.
Effects of Changes in TR Severity on Outcomes After TAVI
We did not observe a difference in survival between patients with persistent significant TR and patients with improvement to no TR after TAVI, which is in contrast to previous studies performed by Yoshida et al18 and Schwartz et al,4 who reported that an improvement in the severity of TR was associated with better survival rates. Conversely, Amano et al16 found no survival benefit in patients undergoing SAVR with additional tricuspid annuloplasty compared with isolated SAVR in patients with AS and concomitant significant TR, and our observations are similar. These finding suggests that TR is a surrogate marker of poor prognosis in an advanced stage of AS. Furthermore, our findings demonstrated that RVD stratified prognosis in patients with AS accompanied by TR; in particular, survival was worst for patients with persistent RVD. Progression of LV pressure overload due to AS leads to deterioration of chamber function and changes in RV geometry, and it is suggested that persistent RVD affects prognosis.
RVD as a Predictor of Cardiovascular Death in Patients With Concomitant TR
Recent studies have reported conflicting results with either RVD or TR remaining as independent predictors of outcome in patients undergoing TAVI after multivariable adjustment. In the study of Lindman et al,3 TR was independently associated with an increased risk of death after adjustment for multiple variables, whereas RVD was not. However, in that study, RV function was determined by FAC only when feasible or by visual estimate and categorized as normal or mild, moderate or severe dysfunction, rather than using consecutive data.3 In contrast, a surgical study by Kammerlander et al2 showed that RV FAC remained an independent predictor of mortality after adjustment for multiple variables, whereas significant TR did not. In the present study, we demonstrated that both RVD and significant TR at baseline were independent predictors of cardiovascular death in patients with severe AS. Furthermore, we found that persistent RVD in patients with concomitant significant TR led to much worse survival compared with patients with a normal RV function or with recovery of RV function after TAVI. Similar results were reported by Asami et al,19 who found a 2.8-fold increased risk of cardiovascular death 1 year after TAVI in patients with RVD at baseline, and a gradient of risk of death from patients with normal RV function, to those with RVD recovery and new RVD, to those with persistent RVD. This emphasizes the importance of RV function assessment before and after TAVI, with the intention of predicting prognosis. Moreover, we demonstrated that the factors associated with persistent RVD in patients with concomitant TR are LV dysfunction and low RV FAC at baseline. This suggests that baseline LV dysfunction also influences the course of RV function in cases of TR after TAVI and could be important in predicting outcome. The clinical implication of our study is that we demonstrated prognosis was stratified with TR etiology and RVD in patients with AS and TR. We clarified that, with regard to TR etiology, patients with ventricular FTR had the worst survival and that prognosis was stratified by the presence or absence of RVD improvement, not by TR improvement. Our findings suggest that the etiology of TR and RV function should be assessed when considering the management of AS with TR, and that the survival benefit of TAVI may be low for ventricular FTR with persistent RVD. However, there is a possibility that the symptoms in such patients could be improved by transcatheter intervention for TR, which is now widely used worldwide, and further investigations are required.
Study Limitations
This was a retrospective single-center study, and the number of patients with significant TR was small. Because we were unable to quantify TR severity, we used semiquantitative measurements based on the TR jet area and vena contracta width. However, these methods are widely available and used in clinical practice. Furthermore, due to incomplete echo data of tricuspid annular plane systolic excursion (TAPSE) and tissue Doppler, we only assessed RV function based on RV FAC and RV dimensions. It would be ideal to examine the interrelationships and prognostic associations among all the FAC, TAPSE, and RV-S′ measures of right ventricular function, but those kinds of studies are not currently available because of their difficulty. The present situation is that there is no established consistent view regarding the RV function parameter that stratifies the prognosis of TR. TAPSE is often an overestimated reflection of RV function in the presence of TR because the measurement is only taken in the longitudinal dimension of the RV. In addition, tricuspid annular motion increases with TR regardless of RV function.20 Therefore, we consider RV FAC, representing both the longitudinal and radial components of RV contraction, and which has been shown to correlate better with RV systolic function by cardiovascular magnetic resonance (CMR),21 is a more reasonable parameter for RV function assessment with regard to TR. In a recent study using the combination of CMR-derived RVEF and TAPSE, global RV dysfunction, defined as CMR RVEF <45%, was an independent predictor of all-cause mortality and HF hospitalization.22 This suggests that acknowledging the complex interplay of longitudinal and circumferential function is important. In the present study, although the only RV parameter we used was FAC, which reflects a combined index of longitudinal and circumferential function, we showed that the presence and transition of RVD stratified the prognosis of TR associated with AS. Further studies are needed to define specific groups of patients who would benefit from additional interventional procedures.
However, because we did not assess heart failure admission and symptoms, the effects of TR improvement after TAVI on these outcomes are unknown.
Conclusions
Concomitant TR is an independent predictor of cardiovascular death in patients with severe AS. In particular, ventricular FTR and the persistence of RV dysfunction after TAVI are associated with adverse outcomes. Hence, not only TR severity, but also the etiology of TR and RV function should be assessed to predict the prognosis of patients with severe AS and concomitant significant TR.
Acknowledgments
The authors thank Makiko Dan, Yasuko Hatori, Akemi Okamoto, Kumiko Abe, Kazuko Nakajima, Makiko Kondo, Mai Iwao, Tomoko Okazeri, Yurie Ensaka, Kazuaki Nagatsuka, and Namiki Yoshie for technical assistance.
Sources of Funding
This study did not receive any specific funding.
Disclosures
K.H. is a proctor for Edwards Lifesciences and Medtronic. H.S. is a proctor for Edwards Lifesciences. K.H. and K.F. are members of Circulation Journal’s Editorial Team. All other authors declare no conflicts of interest.
Author Contributions
All authors participated in designing the study, collecting and analyzing the data, and drafting and revising the manuscript.
IRB Information
The study protocol was approved by the institutional ethics committees at Keio University Hospital (IRB No. 2013270) and all patients provided written informed consent.
Supplementary Files
Please find supplementary file(s);
https://doi.org/10.1253/circj.CJ-22-0262
References
- 1.
Nath J, Foster E, Heidenreich PA. Impact of tricuspid regurgitation on long-term survival. J Am Coll Cardiol 2004; 43: 405–409.
- 2.
Kammerlander AA, Marzluf BA, Graf A, Bachmann A, Kocher A, Bonderman D, et al. Right ventricular dysfunction, but not tricuspid regurgitation, is associated with outcome late after left heart valve procedure. J Am Coll Cardiol 2014; 64: 2633–2642.
- 3.
Lindman BR, Maniar HS, Jaber WA, Lerakis S, Mack MJ, Suri RM, et al. Effect of tricuspid regurgitation and the right heart on survival after transcatheter aortic valve replacement: Insights from the Placement of Aortic Transcatheter Valves II inoperable cohort. Circ Cardiovasc Interv 2015; 8: e002073, doi:10.1161/circinterventions.114.002073.
- 4.
Schwartz LA, Rozenbaum Z, Ghantous E, Kramarz J, Biner S, Ghermezi M, et al. Impact of right ventricular dysfunction and tricuspid regurgitation on outcomes in patients undergoing transcatheter aortic valve replacement. J Am Soc Echocardiogr 2017; 30: 36–46.
- 5.
Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Guyton RA, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129: e521–e643.
- 6.
Bolling SF. Tricuspid regurgitation after left heart surgery: Does it matter? J Am Coll Cardiol 2014; 64: 2643–2644.
- 7.
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015; 16: 233–270.
- 8.
Baumgartner H, Hung J, Bermejo J, Chambers JB, Edvardsen T, Goldstein S, et al. Recommendations on the echocardiographic assessment of aortic valve stenosis: A focused update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J Am Soc Echocardiogr 2017; 30: 372–392.
- 9.
Zoghbi WA, Adams D, Bonow RO, Enriquez-Sarano M, Foster E, Grayburn PA, et al. Recommendations for noninvasive evaluation of native valvular regurgitation: A report from the American Society of Echocardiography developed in collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr 2017; 30: 303–371.
- 10.
Fukuda S, Song JM, Gillinov AM, McCarthy PM, Daimon M, Kongsaerepong V, et al. Tricuspid valve tethering predicts residual tricuspid regurgitation after tricuspid annuloplasty. Circulation 2005; 111: 975–979.
- 11.
Kim HK, Kim YJ, Park JS, Kim KH, Kim KB, Ahn H, et al. Determinants of the severity of functional tricuspid regurgitation. Am J Cardiol 2006; 98: 236–242.
- 12.
Sagie A, Schwammenthal E, Padial LR, Vazquez de Prada JA, Weyman AE, Levine RA. Determinants of functional tricuspid regurgitation in incomplete tricuspid valve closure: Doppler color flow study of 109 patients. J Am Coll Cardiol 1994; 24: 446–453.
- 13.
Muraru D, Guta AC, Ochoa-Jimenez RC, Bartos D, Aruta P, Mihaila S, et al. Functional regurgitation of atrioventricular valves and atrial fibrillation: An elusive pathophysiological link deserving further attention. J Am Soc Echocardiogr 2020; 33: 42–53.
- 14.
Taramasso M, Gavazzoni M, Pozzoli A, Dreyfus GD, Bolling SF, George I, et al. Tricuspid regurgitation: Predicting the need for intervention, procedural success, and recurrence of disease. JACC Cardiovasc Imaging 2019; 12: 605–621.
- 15.
Kappetein AP, Head SJ, Genereux P, Piazza N, van Mieghem NM, Blackstone EH, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: The Valve Academic Research Consortium-2 consensus document. Eur J Cardiothorac Surg 2012; 42: S45–S60, doi:10.1093/ejcts/ezs533.
- 16.
Amano M, Izumi C, Taniguchi T, Morimoto T, Miyake M, Nishimura S, et al. Impact of concomitant tricuspid regurgitation on long-term outcomes in severe aortic stenosis. Eur Heart J Cardiovasc Imaging 2019; 20: 353–360.
- 17.
Dreyfus GD, Martin RP, Chan KM, Dulguerov F, Alexandrescu C. Functional tricuspid regurgitation: A need to revise our understanding. J Am Coll Cardiol 2015; 65: 2331–2336.
- 18.
Yoshida J, Ikenaga H, Hayashi A, Yamaguchi S, Nagaura T, Rader F, et al. Predictors and outcomes of persistent tricuspid regurgitation after transcatheter aortic valve implantation. Am J Cardiol 2019; 124: 772–780.
- 19.
Asami M, Stortecky S, Praz F, Lanz J, Raber L, Franzone A, et al. Prognostic value of right ventricular dysfunction on clinical outcomes after transcatheter aortic valve replacement. JACC Cardiovasc Imaging 2019; 12: 577–587.
- 20.
Hsiao SH, Lin SK, Wang WC, Yang SH, Gin PL, Liu CP. Severe tricuspid regurgitation shows significant impact in the relationship among peak systolic tricuspid annular velocity, tricuspid annular plane systolic excursion, and right ventricular ejection fraction. J Am Soc Echocardiogr 2006; 19: 902–910.
- 21.
Anavekar NS, Gerson D, Skali H, Kwong RY, Yucel EK, Solomon SD. Two-dimensional assessment of right ventricular function: An echocardiographic-MRI correlative study. Echocardiography 2007; 24: 452–456.
- 22.
Kresoja KP, Rommel KP, Lücke C, Unterhuber M, Besler C, Roeder M, et al. Right ventricular contraction patterns in patients undergoing transcatheter tricuspid valve repair for severe tricuspid regurgitation. JACC Cardiovasc Intervention 2021; 14: 1551–1561.







