Abstract
Background: This study analyzed the safety and performance of the Perceval valve for aortic valve replacement (AVR) in patients at 1 year after undergoing aortic stenosis (AS) treatment, and its effect on significant declines in the platelet count during the immediate postoperative period.
Methods and Results: Data were collected retrospectively for the initial 121 patients (median age 77 years; 47.1% females) who underwent Perceval sutureless AVR between May 2019 and July 2022. Implantation was successful in all (100%), with median cross-clamp and CPB times of 59 and 100 min, respectively. Postoperative thrombocytopenia (platelet count <50×103/μL) was noted in 80 (66.1%) patients. Multivariate analysis showed advanced age (>80 years), preoperative low platelet count (<200×103/μL), and a sternotomy approach as significant risk factors for postoperative thrombocytopenia. One (0.8%) patient died within 30 days after the procedure. The 2-year site-reported event rate was 14% (n=17) for all-cause mortality, 0.8% (n=1) for cardiac mortality, 4.1% (n=5) for stroke, and 1.7% (n=2) for endocarditis and valve-related reoperation; there were no instances of paravalvular leakage or structural valve deterioration.
Conclusions: Thrombocytopenia was common after Perceval sutureless AVR, although its impact was not significant. Although Perceval sutureless AVR was found to be a safe and effective option, preoperative assessment of potential bleeding should be performed and the Perceval valve should not be used for patients with a high bleeding risk.
Aortic valve stenosis (AS) is the most commonly encountered valve disease, with a mortality rate of 30–50% in severe and symptomatic patients at the 1-year follow-up time point without intervention.1 Open surgery for aortic valve replacement (AVR) with cardiopulmonary bypass (CPB) under cardioplegic arrest remains the gold standard for symptomatic patients with severe AS.2 However, older age and concomitant comorbidities significantly increase the risk of both mortality and morbidity.3 Novel sutureless bioprosthesis methods have been introduced that combine the advantages of both conventional AVR and transcatheter aortic valve implantation (TAVI) techniques. Recently, sutureless AVR (SU-AVR) has been proposed as a method for high-risk patients considered to be in the “gray zone” between TAVI and conventional surgery.4,5 Compared with standard AVR, the advantages of SU-AVR include shorter valve implantation, aortic cross-clamp (ACC), and CPB times, as well as beneficial hemodynamic parameters.6,7 In contrast to a TAVI procedure, during SU-AVR the native stenosed valve is totally removed, thus ensuring good valve fixation on the aortic ring. In North America, sutureless valves were introduced more than 10 years ago, and their use is considered an important alternative to conventional valves for surgical aortic valve replacement in the setting of either isolated or concomitant procedures.8 In Japan, the Perceval bioprosthetic valve (Perceval; Corcym Corp., Vancouver, Canada) was introduced in 2019 and its availability is expected to result in an increase in AVR surgeries.
The use of a CPB procedure has always been considered to be the primary cause of perioperative thrombocytopenia after cardiac surgery.9 Conversely, that phenomenon seems to not be confined only to cases with a surgically implanted valve. Indeed, recent studies have also reported transient perioperative thrombocytopenia in patients undergoing TAVI.10 Furthermore, periprocedural thrombocytopenia is related to in-hospital adverse events, with acute kidney injury, major bleeding, need for blood transfusion, and longer intensive care unit (ICU) time known to be associated with post-TAVI thrombocytopenia.11 However, that study noted that results concerning actual differences in thrombocytopenia among sutureless bioprosthesis patients, as well as in relation to periprocedural, 30-day, and 1-year mortality, are controversial, with no conclusive explanation for this phenomenon yet presented.11 Compared with their Western counterparts, elderly Japanese patients tend to have a smaller body and reduced aortic annular diameter.12 Although a few studies have presented findings of early outcomes and thrombocytopenia in relation to Perceval SU-AVR procedures conducted by institutions in Asia,13 the results of a large cohort treated in Japan have yet to be published. The present study was conducted to assess postoperative thrombocytopenia and early clinical outcomes in 121 patients with severe AS who underwent a Perceval SU-AVR procedure via a conventional median sternotomy or mini-sternotomy at a single Japanese institution.
Methods
Study Population
Between May 2019 and July 2022, 121 consecutive patients who presented with symptomatic severe AS or steno-insufficiency underwent SU-AVR with a Perceval bioprosthesis at Chiba-Nishi General Hospital, including 41 who underwent minimally invasive cardiac surgery (MICS) AVR via a right small thoracotomy approach.14 All patients underwent AVR with a Perceval valve and had follow-up results available, which were retrospectively reviewed. Patients with severe aortic ostium deformation, true bicuspid aortic valve (Type 0), pathological change in the ascending aorta (e.g., annular dilatation >28 mm, aneurysm), or systemic connective tissue disease (e.g., Marfan’s syndrome) were excluded from undergoing Perceval aortic implantation. Demographic, intraoperative, and short-term outcome data for all patients were collected and included in the registry. Because this was our standard approach, institutional review board approval was not needed, although standard informed consent regarding the surgical approach and expected outcome was obtained by either the operating surgeon or surgical team.
Thrombocytopenia was defined as a platelet count <50×103/µL.15 The primary endpoints were minimum platelet count during the hospital stay, the time until that minimum count was reached, and platelet count at discharge; the secondary endpoint was platelet transfusion. For patient evaluations, transthoracic echography examinations were performed preoperatively, at implantation, at the time of or within 30 days of hospital discharge, and then postoperatively at 3, 6, 12, and 24 months. All patients were evaluated based on findings of a medical examination or telephone interview to determine clinical status, including New York Heart Association (NYHA) classification, blood analysis, coagulation profile, and the occurrence of bleeding complications.
Surgical Techniques
Anesthetic and surgical techniques were standardized according to institutional guidelines, with a full median sternotomy or less invasive approach chosen according to individual surgeon preference. An aortic valve approach and myocardial protection strategy factors related to a right mini-thoracotomy were followed, as reported previously by Masuda et al.16 For the present patients who needed mitral valve repair, the mitral valve was approached prior to AVR through Sondergaard’s groove, and then replaced or repaired. AVR was then performed after completion of the mitral valve repair procedure.17 Otherwise, retractors used for mitral valve procedures may result in distortion or malpositioning of a sutureless aortic valve, as noted in the expert consensus statement presented by Gersak et al.18
A transverse aortotomy was performed approximately 3.5 cm above the annulus in all patients. After excision of the aortic valve leaflet and decalcification of the annulus, prosthesis size was determined by measuring the aortic annulus using a manufacturer-specific annular valve sizer. According to the manufacturer’s instructions, surgeons must avoid under- or oversizing the prosthesis, because the former can lead to migration, whereas the latter could result in excessive compression or rupture of the aorta, stent invagination, fatal arrhythmia or hemorrhage, regurgitation, or altered hemodynamics. When in doubt, most experienced proctors and Perceval experts prefer a slightly undersized prosthesis.18 Thus, the same size selection criteria were used. To ensure correct positioning, 3 guiding sutures were placed as a reference for accurate alignment of the inflow section of the prosthesis with the insertion plane of the native leaflets. These were positioned at the nadir of each valve sinus as single annular sutures and then passed through the corresponding eyelets in the prosthetic inflow ring. The guiding suture for the right coronary cusp, in a position that could not be visualized by the operator, was used as a control spot for fixing the guiding sutures. Once the prosthesis was accurately placed, the guiding sutures were removed and the valve dilated with a low-pressure balloon catheter for 30 s under 4× atmospheric pressure with warm saline irrigation. Following confirmation of proper implantation, the aortotomy was closed in a traditional manner. Intraoperative transesophageal echocardiography was performed after weaning from CPB to confirm correct positioning of the bioprosthesis and to assess the presence of paravalvular leakage (PVL). Treatment with aspirin was recommended according to the standard protocol for at least 3 months under a condition of platelet count >60×103/μL.
Statistical Analysis
Descriptive statistics are presented for baseline demographic and clinical parameters. Variables are shown as the median with interquartile range (IQR) and nominal variables are presented as frequencies (%). The significance of differences between 2 groups was determined using t-tests or Wilcoxon rank-sum tests, as appropriate, for continuous variables. Two-tailed P<0.05 was considered to indicate statistical significance.
Time-to-event data were demonstrated using Kaplan-Meier curves. Univariate logistic regression analysis was conducted to identify variables associated with postoperative thrombocytopenia, with those with P<0.10 entered into multiple logistic regression analysis. All statistical analyses were performed using IBM SPSS Statistics for Windows version 25.0 (IBM Corp., Armonk, NY, USA).
Results
Patient Characteristics
In all, 121 patients (64 men, 57 women; median age 77 years [IQR 74–80 years]; 31 [25.6%] aged ≥80 years) underwent Perceval SU-AVR between May 2019 and July 2022 and were enrolled in the present study. Detailed baseline characteristics are presented in Table 1. The median EuroSCORE II-predicted mortality rate was 2 (IQR 1.5–3.3) and 4 (3.3%) patients had previously undergone cardiac surgery. Thirteen patients (10.7%) had ≥1 conduction disturbances: left bundle branch block (LBBB) in 3 (2.5%) and right bundle branch block (RBBB) in 10 (8.3%). Atrial fibrillation was noted in 17 patients (14%). Echocardiographic results are presented in Table 1. Before AVR, the median peak aortic gradient was 69.8 mmHg, whereas the median aortic gradient was 44.7 mmHg.
Table 1.
Preoperative Clinical Characteristics in All Patients (n=121)
| Age (years) |
77 [74–80] |
| Age ≥80 years |
31 (25.6) |
| Female sex |
57 (47.1) |
| BSA (m2) |
1.51 [1.39–1.62] |
| NYHA Class III or IV |
26 (21.5) |
| Diabetes (medication) |
39 (32.2) |
| COPD |
11 (9.1) |
| Atrial fibrillation |
17 (14.0) |
| RBBB |
10 (8.3) |
| LBBB |
3 (2.5) |
| Paced rhythm |
1 (0.8) |
| Chronic renal failure |
41 (33.9) |
| Dialysis dependent |
27 (22.3) |
| Prior cardiac operation |
4 (3.3) |
| EuroSCORE II (%) |
2 [1.5–3.3] |
| LVEF (%) |
65.7 [58.8–70.4] |
| LVEF <40% |
4 (3.3) |
| Aortic valve MaxPG (mmHg) |
69.8 [55.5–83.2] |
| Aortic valve MeanPG (mmHg) |
44.7 [34.1–52.2] |
| Pulmonary hypertension (>45 mmHg) |
15 (12.4) |
Data are presented as the median [interquartile range] or n (%). BSA, body surface area; COPD, chronic obstructive pulmonary disease; LBBB, left bundle branch block; LVEF, left ventricular ejection fraction; MaxPG, maximum peak gradient; MeanPG, mean peak gradient; NYHA, New York Heart Association; RBBB, right bundle branch block.
Procedural Outcomes
Forty-one (33.9%) patients underwent minimally invasive surgery (right lateral thoracotomy 24, right anterior thoracotomy 17), whereas 80 (66.1%) underwent a median sternotomy. The success rate for all Perceval SU-AVR cases was 100% and no patient was classified as “failure to implant”. However, 2 patients who underwent a minimally invasive procedure later underwent reimplantation because of a size mismatch after aorta declamping. Size S (annulus range 19–21 mm) was used in 51 (42.2%) patients, Size M (annulus range 21–23 mm) was used in 41 (33.9%) patients, Size L (annulus range 23–25 mm) was used in 26 (21.5%) patients, and Size XL (annulus range 25–27 mm) was used in 3 (2.5%) patients. For all cases, the median CPB and ACC times were 100 and 59 min, respectively; these times were significantly shorter in the MICS-Perceval SU-AVR group (79 and 54 min, respectively). Forty-five (37.2%) patients underwent concomitant procedures, with coronary artery bypass grafting performed in 26 (21.5%) patients and mitral valve surgery performed in 10 (8.2%). Most of the MICS-AVR patients underwent Perceval valve implantation without a concomitant surgical procedure. There were no cases of intraoperative conversion to a sternotomy. Operative data are summarized in Table 2.
Table 2.
Procedure Details in All Patients (n=121)
| Isolated AVR |
76 (62.8) |
| Concomitant procedures |
45 (37.2) |
| CABG |
26 (21.5) |
| Mitral valve plasty |
9 (7.4) |
| Mitral valve replacement |
1 (0.8) |
| Tricuspid valve annuloplasty |
2 (1.6) |
| Ascending aorta replacement |
1 (0.8) |
| Atrial fibrillation surgery |
5 (4.0) |
| Myectomy |
2 (1.6) |
| LAA closure |
11 (9.1) |
| Surgical approach |
| Median sternotomy |
80 (66.1) |
| Minimally invasive approach |
41 (33.9) |
| Right lateral thoracotomy |
24 (19.8) |
| Right anterior thoracotomy |
17 (14.0) |
| Conversion to sternotomy (n) |
0 |
| Valve size |
| Small |
51 (42.2) |
| Medium |
41 (33.9) |
| Large |
26 (21.5) |
| Extra large |
3 (2.5) |
| All operations |
| Total time (min) |
227 [160–292] |
| CPB time (min) |
100 [74–117] |
| Cross-clamp time (min) |
59 [51–77] |
| Isolated AVR (full sternotomy) |
| Total time (min) |
218 [195.5–263.3] |
| CPB time (min) |
89.5 [76–101.8] |
| Cross-clamp time (min) |
59 [51.3–71.8] |
| Isolated AVR (mini-sternotomy) |
| Total time (min) |
149 [134–179] |
| CPB time (min) |
79 [72–89] |
| Cross-clamp time (min) |
54 [49–60] |
Unless indicated otherwise, data are presented as the median [interquartile range] or n (%). AVR, aortic valve replacement; CABG, coronary artery bypass grafting; CPB, cardiopulmonary bypass; LAA, left atrial appendage.
Postoperative Thrombocytopenia
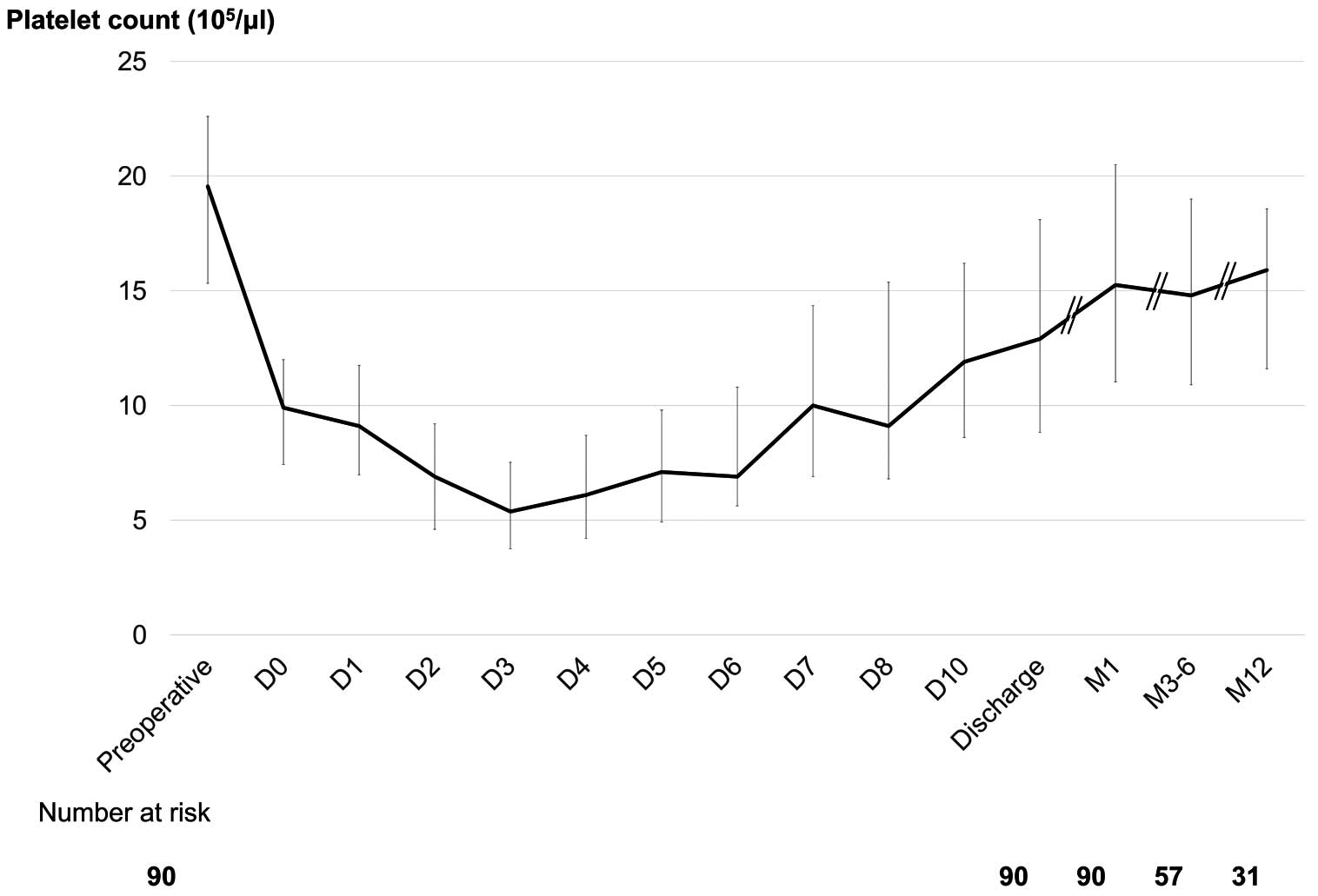
Thrombocytopenia (platelet count <50×103/μL) was observed in 80 (66.1%) patients. Thirty (24.8%) patients required platelet transfusion until Postoperative Day (POD) 3 because of a decreased count, although the count had spontaneously recovered in most patients by the time of discharge. Excluding those who required platelet transfusion, the platelet count details of the remaining 91 patients are presented in Figure 1. The platelet count continued to drop until POD 3 or 4, with a slow recovery until Days 7–10. However, recovery of the platelet count to that noted prior to surgery did not occur until 1, 3–6, or even 12 months later. Compared with the preoperative platelet count, the platelet count at 1 year showed a mean recovery rate of 81.7% (Figure 1).
As for preoperative factors, logistic regression analysis was performed to compare values between patients with thrombocytopenia or who required platelet transfusion (n=80) and those without thrombocytopenia (n=41; Table 3). Those findings for patients with and without thrombocytopenia showed differences related to advanced age (>80 years), dialysis episode, preoperative low platelet count (<200×103/µL), and the use of a median sternotomy approach. In contrast, there was no association between thrombocytopenia and body surface area, concomitant surgery, choice of Perceval Size S, or CPB time. In multivariate analysis, advanced age (>80 years), sternotomy approach, and preoperative low platelet count (<200×103/µL) were shown to be significant risk factors for postoperative thrombocytopenia (P<0.05). Finally, none of the Perceval patients required re-exploration for excessive bleeding, or treatment for gastrointestinal bleeding or another type of bleeding event.
Table 3.
Univariate and Multivariate Logistic Regression Analyses of Variables Associated With Severe Postoperative Thrombocytopenia
| |
Univariate analysis |
Multivariate analysis |
| OR (95% CI) |
P value |
OR (95% CI) |
P value |
| Advanced age (>80 years) |
4.98 (1.61–15.41) |
0.005* |
6.14 (1.72–21.00) |
0.005* |
| Sex (female) |
0.95 (0.45–2.03) |
0.9 |
|
|
| BSA (>1.4 m2) |
0.86 (0.37–1.98) |
0.72 |
|
|
| EuroSCORE II (>2.9) |
2.25 (0.95–5.35) |
0.06* |
1.56 (0.56–4.40) |
0.4 |
| Preoperative platelets (<200×103/μL) |
6.05 (2.65–13.81) |
<0.0001* |
5.29 (2.06–13.58) |
0.0005* |
| Dialysis |
3.73 (1.19–11.67) |
0.02* |
1.89 (0.53–6.77) |
0.33 |
| COPD |
0.89 (0.24–3.22) |
0.86 |
|
|
| PAD |
1.20 (0.40–3.57) |
0.74 |
|
|
| LVEF (>60%) |
1.25 (0.53–2.96) |
0.61 |
|
|
| Pulmonary hypertension |
2.24 (0.59–8.42) |
0.23 |
|
|
| MICS approach |
0.39 (0.18–0.85) |
0.02* |
0.29 (0.10–0.81) |
0.02* |
| Concomitant surgery |
1.22 (0.56–2.68) |
0.62 |
|
|
| CABG |
1.20 (0.47–3.05) |
0.71 |
|
|
| Perceval Size S |
0.96 (0.45–2.06) |
0.91 |
|
|
| CPB time (>80 min) |
1.61 (0.72–3.61) |
0.25 |
|
|
*P<0.05 was considered to indicate statistical significance. CI, confidence interval; MICS, minimally invasive cardiac surgery; OR, odds ratio; PAD, peripheral arterial disease. Other abbreviations as in Tables 1,2.
Early Clinical Outcomes
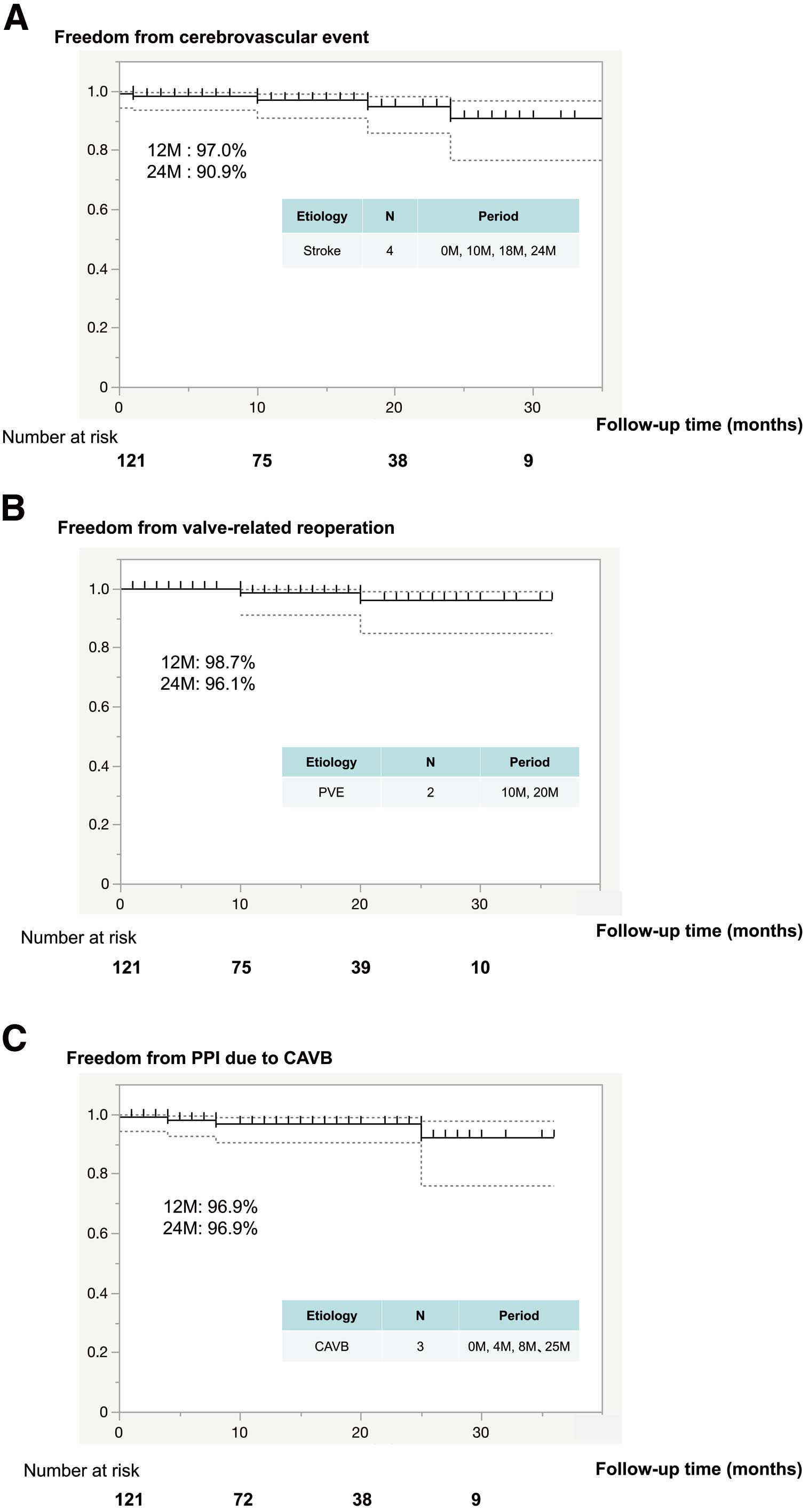
Early outcomes and adverse event rates are presented in Table 4. There was 1 in-hospital death (0.8%), which occurred on POD 28 and was due to cerebral vascular accident. Median hospital and ICU stays were 12 days (IQR 10–15 days) and 2 days (IQR 2–3 days), respectively. Of the 104 patients admitted for sinus rhythm, new-onset atrial fibrillation occurred in 28 (23.1%), whereas 1 had a complete atrioventricular block (CAVB) incident that required permanent pacemaker implantation.
Table 4.
Early Outcomes and Echocardiographic Parameters for Hemodynamics in All Patients (n=121)
| In-hospital mortality |
1 (0.8) |
| Intensive care unit stay (days) |
2 [2–3] |
| Hospital stay (days) |
12 [10–15] |
| Ventilation duration (hours) |
8 [6–12] |
| Blood transfusion (intra- or postoperative) |
75 (62.0) |
| Red blood cell transfusion (intra- or postoperative) |
72 (59.5) |
| Fresh frozen plasma transfusion (intra- or postoperative) |
72 (59.5) |
| Platelet transfusion (intra- or postoperative) |
30 (24.8) |
| Thrombocytopenia (<50×103/μL) |
80 (66.1) |
| Total drain discharged fluid (mL) |
480 [270–770] |
| Low cardiac output syndrome (n) |
0 |
| Re-exploration (n) |
0 |
| Respiratory insufficiency (n) |
0 |
| Gastrointestinal bleeding (n) |
0 |
| Temporary renal replacement therapy (n) |
0 |
| Cerebral vascular accident |
1 (0.8) |
| Complete atrioventricular block |
| Temporary |
3 (2.4) |
| Permanent |
1 (0.8) |
| New onset postoperative LBBB |
1 (0.8) |
| New onset postoperative atrial fibrillation |
28 (23.1) |
Unless indicated otherwise, data are presented as the median [interquartile range] or n (%). LBBB, left bundle branch block.
All patients underwent transthoracic echocardiography prior to discharge and then routine follow-up examinations. Hemodynamic outcomes are listed in Supplementary Table 1. Following the AVR procedure, the median peak/mean aortic gradients were decreased from 69.8/44.7 mmHg at the intraoperative examination, to 23.0/13.0 mmHg at discharge (each P<0.001) and 19.4/11.0 mmHg at the final follow-up examination conducted after a median of 13.5 months (IQR 5–22 months; each P<0.001). The left ventricular mass index also decreased from a preoperative value of 138.9 to 110.8 g/m2
at the latest follow-up (P<0.007).
Late Clinical Outcomes
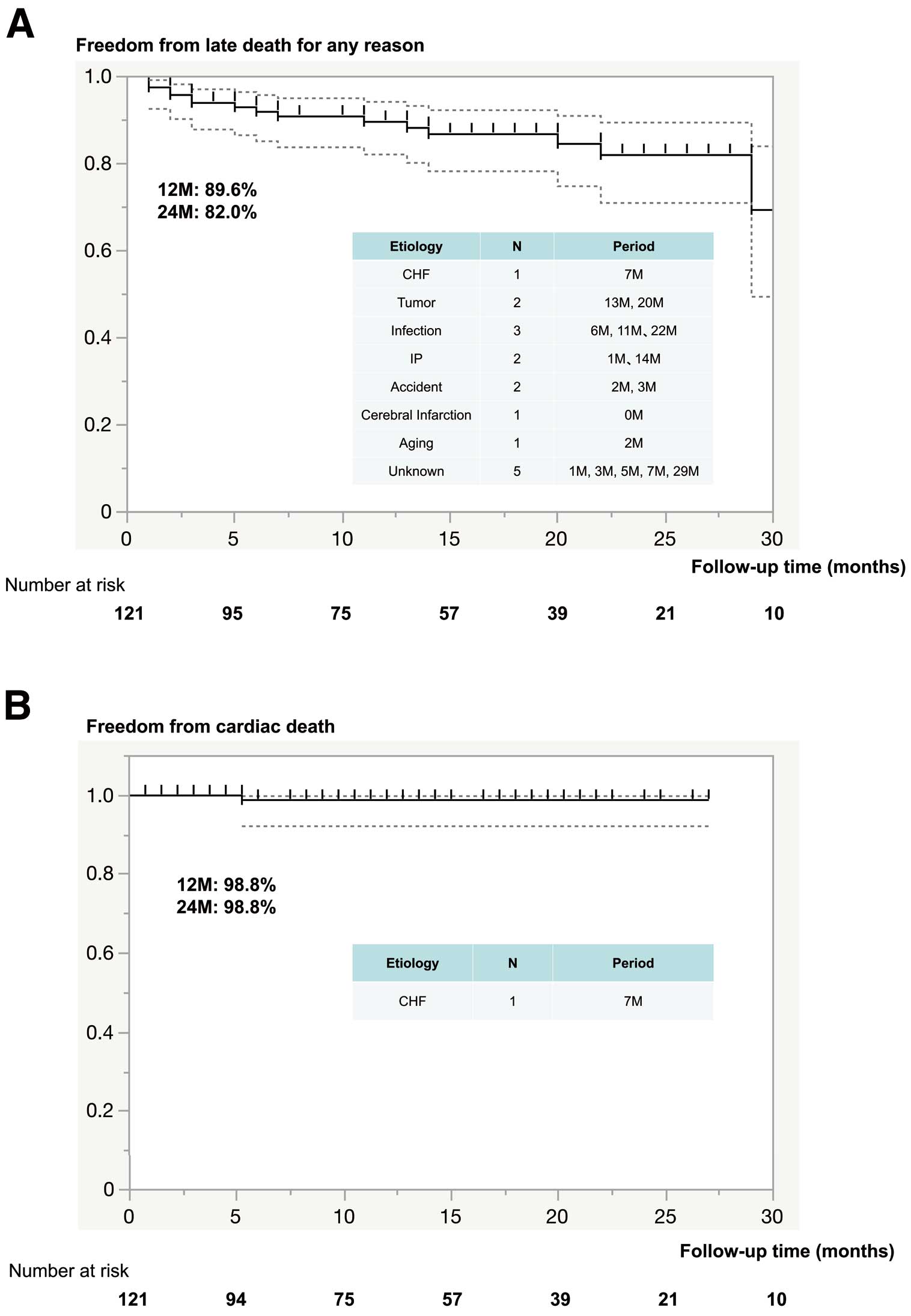
The median follow-up period was 13.5 months (IQR 5–22 months; maximum 36 months, cumulative 1,723 patient-months). Valve-related complications are summarized in Supplementary Table 2. There were 16 (13.2%) cases of late (>30 days) mortality, 15 of which were due to non-cardiac causes; the remaining death was due to a non-valve-related cardiac cause because the patient died from chronic heart failure (Figure 2). No valve-related deaths occurred. Details regarding mortality and morbidity event rates are shown in Figure 2. Stroke occurred in 4 patients (at 0, 10, 18, 24 months), although none of these stroke events was related to cardiac arrhythmia or the prosthesis (Figure 3A). Furthermore, there was no occurrence of endocarditis reported early after the operation. During the follow-up period, 2 (2%) patients presented with prosthetic valve endocarditis, 1 with an aortic prosthesis (10 months) and the other with an aortic root abscess (20 months; Figure 3B). Following a course of antibiotics, both patients underwent successful surgical intervention for aortic root replacement using a graft insertion technique previously described by Nakamura et al.19 In addition, PVL during the follow-up period was not seen in any patients, including a low quantity, nor was valve-related hemolytic anemia noted. Postoperative pacemaker implantation (PPI) was required in 1 patient in the early phase, whereas PPI as a result of CAVB during the later follow-up period was noted in 3 (2.5%) patients (at 4, 8 and 25 months; Figure 3C).
Discussion
There are 2 important findings in the present study to consider. First, thrombocytopenia was noted in patients with Asian ethnicity who had a small body surface area and were treated with a Perceval sutureless valve. Second, this is the first report of a series of cases that received treatment with a Perceval SU-AVR performed at an institution in Japan. Compared with Western patients, the decrease in platelets was remarkable and the postoperative recovery rate clearly lower. Although there were no complications due to thrombocytopenia, Perceval indications should be reconsidered, such as for patients who require antiplatelet medication, have a history of gastrointestinal bleeding, and/or are prone to falling. Conversely, use of a Perceval prosthesis provides the possibility of better AVR with shortened CPB and ACC times, extremely rare in patients with a complete atrioventricular block and PVL, as well as reproducibility, thereby facilitating concomitant cardiac surgery or treatment for poor left ventricular function. When patient selection is appropriate, use of the Perceval AVR provides clinical advantages that take precedence over a possible thrombocytopenia complication.
Although the use of a Perceval valve was found to be associated with a significant drop in platelet count along with a slow recovery phenomenon, there was no association with any other significant clinical consequences. The drop in platelet count generally began on POD 3 or 4, with a very slow recovery until POD 7–10, with the lowest drop in platelet count following Perceval valve implantation found to be 69.5% of the preoperative baseline level. To the best of our knowledge, this phenomenon has been not reported in patients of Asian ethnicity possessing a sutureless valve for more than 1 year. Previous studies have noted thrombocytopenia after undergoing a Perceval valve implant and similar progress results were reported in each (Table 5),20–24 whereas the present results are favorable as compared with those. Nevertheless, important remaining issues include more severe thrombocytopenia and worse recovery noted in the present group of patients with Asian ethnicity compared with prior studies conducted in Europe.
Table 5.
Principal Data and Outcomes of Interest From Individual Studies
| Study |
Country |
No.
participants |
Age
(years) |
No. (%)
sternotomy/MICS |
CPB time
(min) |
Maximum drop in
platelet count (×105/μL) |
Day of maximum drop
in platelet count |
Platelet
transfusion |
POD 3 platelet
count (105/μL) |
POD 3 platelet count
degression rate (%) |
Platelet count
follow-up phase |
Follow-up recovery
rate (%) |
Reoperation for
bleeding, tamponade |
Predictors for severe
thrombocytopenia |
| Jiritano et al20 |
Italy |
16 |
75.9±7.1 |
NA |
61.8±5.6 |
71.9±21.2 |
3 |
4 units/16 patients |
71.9±21.2 |
73.2 |
1 year |
98.9 (1 year) |
0 |
NA |
| Sanchez et al21 |
Spain |
27 |
76.0±4.5 |
16 (59.3)/11(40.7) |
68.0±23.6 |
78.4±30.0 |
2 |
NA |
84.6 |
55.6 |
POD 3 |
44.4 (POD 3) |
0 |
Perceval S valve, age >75 years,
preoperative platelet count
<150×103/μL |
| Stanger et al22 |
Switzerland, Austria |
48 |
76.2±5.2 |
48 (100)/0 |
50.4±13.7 |
97.6±37.2 |
2~3 |
2 (4.2%) |
NA |
60±10 |
POD 9 |
93.0 (POD 9) |
0 |
NA |
| Mujtaba et al23 |
UK |
72 |
75.0 [54.0–84.0] |
72 (100)/0 |
56.5 [25–119] |
111.4 |
3 |
0.3 platelet pools/
72 patients |
112.8 |
53.5 |
POD 7 |
85.8 (POD 7) |
2 (2.8%) |
NA |
| Stegmeier et al24 |
Germany |
25 |
79.0 [74.5–82.0] |
20(80)/5 (20) |
75.0 [63–96] |
47.0 [38.0–66.0] |
64.8 h [40.8–76 h] |
0 |
47.0 [38.0–66.0] |
76.5 |
POD 12 |
73.0 (POD 12) |
5 (20%) |
NA |
| Present studyA |
Japan |
121 |
77.0 [74.0–80.0] |
80 (66.1)/41(33.9) |
100 [74–117] |
50.0 [36.0–72.0] |
3 |
30 (24.8%) |
55.0 [38.5–77.8] |
70.3 [60.9–76.9] |
1 year |
31.9 (POD 3), 53.9 (POD 7),
70.6 (POD 12), 81.7 (1 year) |
0 |
Sternotomy approach, age >80
years, preoperative platelet count
<200×103/μL |
Values are expressed as absolute numbers, percentages, median [interquartile range], or the mean±SD. AFollowing the exclusion of 30 patients who underwent a platelet transfusion procedure in the present study, details regarding platelet count and recovery rate are summarized for 91 patients. POD, postoperative day. Other abbreviations as in Tables 2,3.
Jiritano et al20 reported recovery of the platelet count in 1-year postoperative examinations, whereas recovery to the preoperative count was not seen in any of the present cohort and the recovery rate was generally low (Supplementary Table 3A). Furthermore, there were 3 patients whose platelet count remained significantly low for a long period (Supplementary Table 3B; Supplementary Figure). Unfortunately, no specific features of persistent platelet depression were found and further elucidation of this issue is needed. Edelstein et al25 also noted that phosphatidylcholine transfer protein encoding contributes to racial differences in protease-activated receptor 4 (PAR4)-mediated platelet activation, indicating a genomic contribution to platelet function that differs among ethnicities. It is considered possible that these factors could lead to a greater decrease in platelet count. Severe thrombocytopenia can more directly affect patient outcome by increasing bleeding events, and Asians with atrial fibrillation have been found to have a 4-fold greater risk of warfarin-related intracranial hemorrhage than Caucasian patients.26 Another study reported that Asian ethnicity was a risk factor for intracerebral hemorrhage during anticoagulation treatment with warfarin.27 Furthermore, severe thrombocytopenia after transcatheter aortic valve replacement can directly affect patient outcome by increasing bleeding events,28 although that phenomenon was not found to be associated with an adverse clinical event in the present SU-AVR patients. In that regard, several studies have noted excellent clinical outcomes with use of sutureless valves, whereas few have reported thrombocytopenia as a potential complication.29,30 In the present study, 30 (24.8%) patients required platelet transfusion until POD 3 because of a decreased platelet count in the initial stage of SU-AVR use. However, thrombocytopenia was never associated with any adverse clinical outcome during the initial stage. For this reason, we have changed the late-stage platelet count threshold to indicate transfusion to ≤20,000 (Patients 1–49 [before protocol] 41% [n=20] vs. Patients 50–121 [after protocol] 14% [n=10]).
The present analysis revealed 3 independent predictors of severe thrombocytopenia: advanced age (>80 years), the use of a median sternotomy approach, and a preoperative low platelet count (<200×103/µL). Albacker29 reported transfusion requirements in patients older than 70 years, because a greater number of platelet and red blood cell transfusions are required in these patients than in younger patients. Furthermore, Gammie et al31 found no differences regarding re-exploration for bleeding between patients who underwent minimally invasive mitral valve surgery and those who underwent a traditional sternotomy (open), although there was a significantly higher level of perioperative red blood cell use in the open group (52.6% vs. 41%) and a greater number of cases of platelet transfusion (25.3% vs. 15.8%). Sanchez et al21 reported multivariate analysis findings showing that predictors for severe thrombocytopenia were age >75 years, preoperative platelet count (<150×103/µL), and the use of a Perceval S prosthesis. The use of a small-sized valve has been shown to cause some turbulence across the valve, resulting in platelet activation or destruction, and consequently postoperative thrombocytopenia, whereas a long CPB duration has been suggested by other studies to be associated with postoperative thrombocytopenia.32 Extreme caution should be taken before routine use of a Perceval valve in elderly or median sternotomy patients who are already at risk of thrombocytopenia following the operation.
Various centers are undertaking investigations to determine the cause of thrombocytopenia, which remains unknown. Several causes of platelet dysfunction have been speculated, including detoxification performed with homocysteic acid and storage with an aldehyde-free solution,33 the use of a naked alloy stent,29 and mechanical stress and turbulence, especially when using a small-sized valve.34 Although platelet dysfunction has been reported to be potentially more clinically relevant in SU-AVR patients, the issue remains controversial in surgical cases. A better understanding and awareness of the underlying mechanisms, and/or the presence of predisposing factors, such as a periprocedural events, requires further analysis.
In contrast with previous studies,35 with PVL rates as high as 12.5%, the present study had a PVL rate of 0% during the follow-up period. Rare PVL is important and the patients remained stable from discharge to the 1-year checkup. This is likely due to precise annular debridement, the main difference between a Perceval and transcatheter AVR, which allows for good apposition of the stent. The design of the prosthesis, which features a conformable stent and pericardial sealing collar, is also key for preventing PVL. Nevertheless, the frequency of PPI after SU-AVR remains high. The self-expanding nitinol frame of a Perceval valve compresses the left ventricle outflow tract, which can potentially damage atrioventricular conduction tissue. Pooled estimates of PPI for Perceval SU-AVR were satisfactory, with 1 (0.8%) incident noted perioperatively and 2 (1.7%) incidents noted at a postoperative follow-up examination. In other studies, Moscarelli et al performed a meta-analysis of 394 articles and 26 studies including 9,492 patients to estimate the rate of pacemaker implantation after use of Perceval for AVR; these authors found a pooled event rate for PPI of 7% and that PPI rates tended to decrease over time.36 In the study of van Boxtel et al,37 SU-AVR with the Perceval bioprosthesis was frequently complicated by a new LBBB conduction disturbance, although that phenomenon remains to be clearly elucidated. Although we did not observe a higher incidence of LBBB (0.8%), cardiac surgeons should be aware of this possible perioperative complication. In particular, 3 (2.4%) of our patients developed pacemaker implantation complications in the late postoperative phase. Of these patients, 2 (8 months and 25 months after PPI) experienced an episode of advanced atrioventricular block and complete RBBB preoperatively, whereas the other (4 months after PPI) had no preoperative conduction disorders but developed LBBB immediately after Perceval S implantation, which shifted from LBBB to complete atrioventricular block at 4 months postoperatively. The incidence rate of valve deterioration and dislocation was 0% at the perioperative and postoperative follow-up examinations. However, 2 (1.7%) patients had a valve-related reoperation during the follow-up period, with both these patients having prosthetic valve endocarditis. Fortunately, these patients recovered after undergoing a novel graft insertion technique for damaged aortic root reconstruction.19 In their follow-up study (mean 3 years; range 1–11 years after the procedure), Szecel et al38 reported that 5 (1.1%) patients underwent procedures for endocarditis and none of the procedures were for structural valve degeneration. A bare metal part is used in the Perceval SU-AVR, so attention regarding prosthetic valve endocarditis is needed.
The present study has several limitations. First, the postoperative effective orifice area of the prosthetic valves was not assessed in these patients. In addition, this was an observational non-randomized single-center retrospective analysis, which may be the most significant factor. Furthermore, because the patient cohort was relatively small, certain confounding factors associated with postoperative morbidity or mortality may have been overlooked. The results could also have been affected by unmeasured or hidden covariates.
Conclusions
The present findings indicate that Perceval SU-AVR is a safe and effective option. Although thrombocytopenia was commonly observed in the present cohort after the procedure, no significant impact on clinical outcome was noted. Nevertheless, preoperative assessment of potential bleeding is necessary and patients with a high risk of bleeding should excluded from treatment with Perceval SU-AVR.
Sources of Funding
This study did not receive any specific funding.
Disclosures
Y.N. is a local master proctor for the Perceval valve in Japan. The remaining authors have no conflicts of interest to disclose.
IRB Information
This study was approved by the Tokushukai Group Ethics Committee Review Board (TGE02016-025). The procedures reported in this study were performed in accordance with the Declaration of Helsinki, as well as ethical standards for human experimentation established by responsible institutional committees.
Supplementary Files
Please find supplementary file(s);
https://doi.org/10.1253/circj.CJ-22-0587
References
- 1.
Vahanian A, Otto CM. Risk stratification of patients with aortic stenosis. Eur Heart J 2010; 31: 416–423.
- 2.
Schwarz F, Baumann P, Manthey J, Hoffmann M, Mehmel HC, Schmitz W, et al. The effect of aortic valve replacement on survival. Circulation 1982; 66: 1105–1110.
- 3.
Litwinowicz R, Bartus K, Drwila R, Kapelak B, Konstanty-Kalandyk J, Sobczynski R, et al. In-hospital mortality in cardiac surgery patients after readmission to the intensive care unit: A single-center experience with 10,992. patients. J Cardiothorac Vasc Anesth 2015; 29: 570–575.
- 4.
D’Onofrio A, Messina A, Lorusso R, Alfieri OR, Fusari M, Rubino P, et al. Sutureless aortic 24 valve replacement as an alternative treatment for patients belonging to the “gray zone” between transcatheter aortic valve implantation and conventional surgery: A propensity-matched, multicenter analysis. J Thorac Cardiovasc Surg 2012; 144: 1010–1018.
- 5.
Miceli A, Santarpino G, Pfeiffer S, Murzi M, Gilmanov D, Concistre G, et al. Less invasive aortic valve replacement with Perceval S sutureless valve: Early outcomes and one-year survival from two European centers. J Thorac Cardiovasc Surg 2014; 148: 2838–2843.
- 6.
Mazine A, Teoh K, Bouhout I, Bhatnagar G, Pelletier M, Voisine P, et al. Sutureless aortic valve replacement: A Canadian multicentre study. Can J Cardiol 2015; 31: 63–68.
- 7.
Wendt D, Thielmann M, Buck T, Janosi RA, Bossert T, Pizanis N, et al. First clinical experience and 1-year follow-up with the sutureless 3F-Enable aortic valve prosthesis. Eur J Cardiothorac Surg 2008; 33: 542–547.
- 8.
Szecel D, Eurlings R, Rega F. Perceval sutureless aortic valve implantation. Ann Thorac Surg 2021; 111: 1331–1337.
- 9.
Kerendi F, Thourani VH, Puskas JD, Hilgo PD, Osgood M, Guyton RA, et al. Impact of heparin-induced thrombocytopenia on postoperative outcomes after cardiac surgery. Ann Thorac Surg 2007; 84: 1548–1553.
- 10.
Gul M, Uyarel H, Akgul O, Uslu N, Yildirim A, Eksik A, et al. Hematologic and clinical parameters after transcatheter aortic valve implantation (TAVI) in patients with severe aortic stenosis. Clin Appl Thromb Hemost 2014; 20: E304–E310.
- 11.
Hernández-Enríquez M, Regueiro A, Romaguera R, Andrea R, GÓmez-Hospital JA, Pujol-LÓpez M, et al. Thrombocytopenia after transcatheter aortic valve implantation: A comparison between balloon-expandable and self-expanding valves. Catheter Cardiovasc Interv 2019; 93: 1344–1351.
- 12.
Domoto S, Jujo K, Yamaguchi J, Otsuki H, Isomura S, Tanaka K, et al. Utility of the minimum-incision transsubclavian approach for transcatheter aortic valve replacement on clinical outcomes in patients with small vessel anatomy. J Cardiol 2021; 78: 31–36.
- 13.
Kim DJ, Lee S, Joo HC, Youn YN, Yoo KJ, Lee SH, et al. Clinical and hemodynamic outcomes in 121 patients who underwent Perceval sutureless aortic valve implantation: Early results from a single Korean institution. Circ J 2021; 85: 1011–1017.
- 14.
Nakamura Y, Narita T, Kuroda M, Nakayama T, Tsuruta R, Yoshiyama D, et al. Sutureless aortic valve replacement through lateral mini-thoracotomy: Feasibility and effectiveness. Circ J 2022; 86: 1733–1739.
- 15.
Stasi R. How to approach thrombocytopenia. Hematology Am Soc Hematol Educ Program 2012; 1: 191–197.
- 16.
Masuda T, Nakamura Y, Ito Y, Kuroda M, Nishijima S, Okuzono Y, et al. The learning curve of minimally invasive aortic valve replacement for aortic valve stenosis. Gen Thorac Cardiovasc Surg 2020; 68: 565–570.
- 17.
Nakayama T, Nakamura Y, Kanamori K, Hirano T, Kuroda M, Nishijima S, et al. Early and midterm results of minimally invasive aortic and mitral valve surgery via right mini-thoracotomy. J Card Surg 2020; 35: 35–39.
- 18.
Gersak B, Fischlein T, Folliguet TA, Meuris B, Teoh KHT, Moten SC, et al. Sutureless, rapid deployment valves and stented bioprosthesis in aortic valve replacement: Recommendations of an international expert consensus panel. Eur J Cardiothorac Surg 2016; 49: 709–718.
- 19.
Nakamura Y, Tagusari O, Kobayashi J, Nakajima H. Secure anastomosis for damaged aortic root reconstruction: Graft insertion technique. J Thorac Cardiovasc Surg 2011; 142: 948–950.
- 20.
Jiritano F, Cristodoro L, Malta E, Mastroroberto P. Thrombocytopenia after sutureless aortic valve implantation: Comparison between Intuity and Perceval bioprosthesis. J Thorac Cardiovasc Surg 2016; 152: 1631–1633.
- 21.
Sanchez E, Corrales JA, Fantidis P, Tarhini IS, Khan I, Pineda T, et al. Thrombocytopenia after aortic valve replacement with Perceval S sutureless bioprosthesis. J Heart Valve Dis 2015; 24: 75–81.
- 22.
Stanger O, Grabherr M, Gahl B, Longnus S, Meinitzer A, Fiedler M, et al. Thrombocytopenia after aortic valve replacement with stented, stentless and sutureless bioprostheses. Eur J Cardiothorac Surg 2017; 51: 340–346.
- 23.
Mujtaba SS, Ledingham S, Shah AR, Schueler S, Clark S, Pillay T. Thrombocytopenia after aortic valve replacement: Comparison between sutureless Perceval S valve and Perimount Magna Ease bioprosthesis. Braz J Cardiovasc Surg 2008; 33: 169–175.
- 24.
Stegmeier P, Schlomicher M, Stiegler H, Strauch JT, Bechtel M. Thrombocytopenia after implantation of the Perceval S aortic bioprosthesis. J Thorac Cardiovasc Surg 2020; 160: 61–68.e8.
- 25.
Edelstein LC, Simon LM, Montoya TR, Holinstat M, Chen ES, Bergeron A, et al. Radical differences in human platelet PAR4 reactivity reflect expression of PCTP and miR-376c. Nat Med 2013; 19: 1609–1616.
- 26.
Shen AY, Yao JF, Brar SS, Jorgensen MB, Chen W. Racial/ethnic differences in the risk of intracranial hemorrhage among patients with atrial fibrillation. J Am Coll Cardiol 2007; 50: 309–315.
- 27.
Hart RG, Tonarelli SB, Pearce LA. Avoiding central nervous system bleeding during antithrombotic therapy: Recent data and ideas. Stroke 2005; 36: 1588–1593.
- 28.
Dvis D, Genereux P, Barbash IM, Kodali S, Benn-Dor I, Williams M, et al. Acquired thrombocytopenia after transcatheter aortic valve replacement: Clinical correlates and association with outcomes. Eur Heart J 2014; 35: 2663–2671.
- 29.
Albacker TB. Thrombocytopenia associated with Perceval sutureless aortic valve replacement in elderly patients: A word of caution. Heart Surg Forum 2015; 18: E093–E097.
- 30.
Flameng W, Herregods MC, Hermans H, Mieren GVd, Vercalsteren M, Toortmans G, et al. Effect of sutureless implantation of the Perceval S aortic valve bioprosthesis on intraoperative and early postoperative outcomes. J Thorac Cardiovasc Surg 2011; 142: 1453–1457.
- 31.
Gammie JS, Zhao Y, Peterson ED, O’Brien SM, Rankin S, Griffith BP. Less-invasive mitral valve operations: Trends and outcomes from the Society of Thoracic Surgeons adult cardiac surgery database. Ann Thorac Surg 2010; 90: 1401–1410.
- 32.
van Straten AH, Hamad MA, Berreklouw E, Woorst JF, Martens EJ, Tan ES. Thrombocytopenia after aortic valve replacement: Comparison between mechanical and biological valves. J Heart Valve 2010; 19: 394–399.
- 33.
Piccardo A, Rusinaru D, Petitprez B, Marticho P, Vaida I, Tribouilloy C, et al. Thrombocytopenia after aortic valve replacement with Freedom Solo bioprosthesis: A propensity study. Ann Thorac Surg 2010; 89: 1425–1430.
- 34.
Yerebakan C, Kaminski A, Westphal B, Kundt G, Ugurlucan M, Steinhoff G, et al. Thrombocytopenia after aortic valve replacement with Freedom Solo stentless bioprosthesis. Interact Cardiovasc Thorac Surg 2008; 7: 616–620.
- 35.
Concistrè G, Santarpino G, Pfeiffer S, Farneti P, Miceli A, Chiaramonti F, et al. Two alternative sutureless strategies for aortic valve replacement: A two-center experience. Innovations (Phila) 2013; 8: 253–257.
- 36.
Moscarelli M, Santarpino G, Athanasiou T, Mastroroberto P, Fattouch K, Nasso G, et al. A pooled analysis of pacemaker implantation after Perceval sutureless aortic valve replacement. Interact Cardiovasc Thorac Surg 2021; 33: 501–509.
- 37.
van Boxtel AG, Houthuizen P, Hamad MA, Sjatskig J, Tan E, Prinzen FW, et al. Postoperative conduction disorders after implantation of the self-expandable sutureless Perceval S bioprosthesis. J Heart Valve Dis 2014; 23: 319–324.
- 38.
Szecel D, Eurlings R, Rega F, Verbrugghe P, Meuris B. Perceval sutureless aortic valve implantation: Midterm outcomes. Ann Thorac Surg 2021; 111: 1331–1337.