Abstract
Background: We investigated the efficacy of left ventricular (LV) myocardial damage by native T1
mapping obtained with cardiac magnetic resonance (CMR) for patients undergoing transcatheter edge-to-edge repair (TEER).
Methods and Results: We studied 40 symptomatic non-ischemic heart failure (HF) patients and ventricular functional mitral regurgitation (VFMR) undergoing TEER. LV myocardial damage was defined as the native T1
Z-score, which was converted from native T1
values obtained with CMR. The primary endpoint was defined as HF rehospitalization or cardiovascular death over 12 months after TEER. Multivariable Cox proportional hazards analysis showed that the native T1
Z-score was the only independent parameter associated with cardiovascular events (hazard ratio 3.40; 95% confidential interval 1.51–7.67), and that patients with native T1
Z-scores <2.41 experienced significantly fewer cardiovascular events than those with native T1
Z-scores ≥2.41 (P=0.001). Moreover, the combination of a native T1
Z-score <2.41 and more severe VFMR (effective regurgitant orifice area [EROA] ≥0.30 cm2) was associated with fewer cardiovascular events than a native T1
Z-score ≥2.41 and less severe VFMR (EROA <0.30 cm2; P=0.002).
Conclusions: Assessment of baseline LV myocardial damage based on native T1
Z-scores obtained with CMR without gadolinium-based contrast media is a valuable additional parameter for better management of HF patients and VFMR following TEER.
Ventricular functional mitral regurgitation (VFMR) is a common complication for heart failure (HF) patients with reduced left ventricular (LV) ejection fraction (LVEF), and a strong association between the severity of VFMR and both all-cause mortality and HF hospitalization has been reported.1–4 It has also been reported that more than moderate-to-severe VFMR occurred in 29.8% of 2,057 HF patients with an LVEF <40% and was an independent predictor of 5-year mortality,2 whereas severe VFMR occurred in 24% of 1,256 patients with non-ischemic cardiomyopathy (NICM) and was an independent predictor of death or HF hospitalization, regardless of LVEF, at a median 2.5 years of follow-up.4 Transcatheter edge-to-edge repair (TEER) is a therapeutic alternative to mitral valve surgery for patients with prohibitive surgical risk,5,6 and is effective for symptomatic HF patients with reduced LVEF and severe VFMR despite the use of optimal medical therapy based on current guidelines.7,8 Two major randomized controlled trials have been conducted, namely MITRA-FR (Percutaneous Repair with the MitraClip Device for Severe Functional/Secondary Mitral Regurgitation)9 and COAPT (Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients with Functional Mitral Regurgitation),10 to assess the efficacy and safety of TEER for symptomatic HF patients with reduced LVEF and severe VFMR. These 2 trials targeted the same patient populations with the same disease using the same devices, but the results of these trials were diametrically opposed. According to these 2 trials, patients with too severe LV dilation and/or dysfunction (i.e., too extensive LV myocardial damage) can be considered less likely to benefit from TEER,9,10 although the evaluation of LV myocardial damage in HF patients remains challenging.
Editorial p 528
CMR is the leading non-invasive imaging modality for the assessment of myocardial structure and function, as well as the characterization of myocardial tissue in HF patients.7,8 In particular, native T1
mapping, a novel CMR technique, can be used to estimate the intrinsic longitudinal relaxation time of myocardial tissue without the use of gadolinium-based contrast media. Moreover, native T1
mapping can quantitatively evaluate myocardial interstitial remodeling at an even earlier stage than CMR with late gadolinium enhancement (LGE), and both techniques feature ideal variables for the risk stratification of patients with NICM for adverse cardiovascular events, particularly patients with negative LGE.11,12 Accordingly, the objective of this study was to investigate the utility of native T1
mapping for symptomatic HF patients with NICM and VFMR undergoing TEER.
Methods
Study Design and Patient Selection
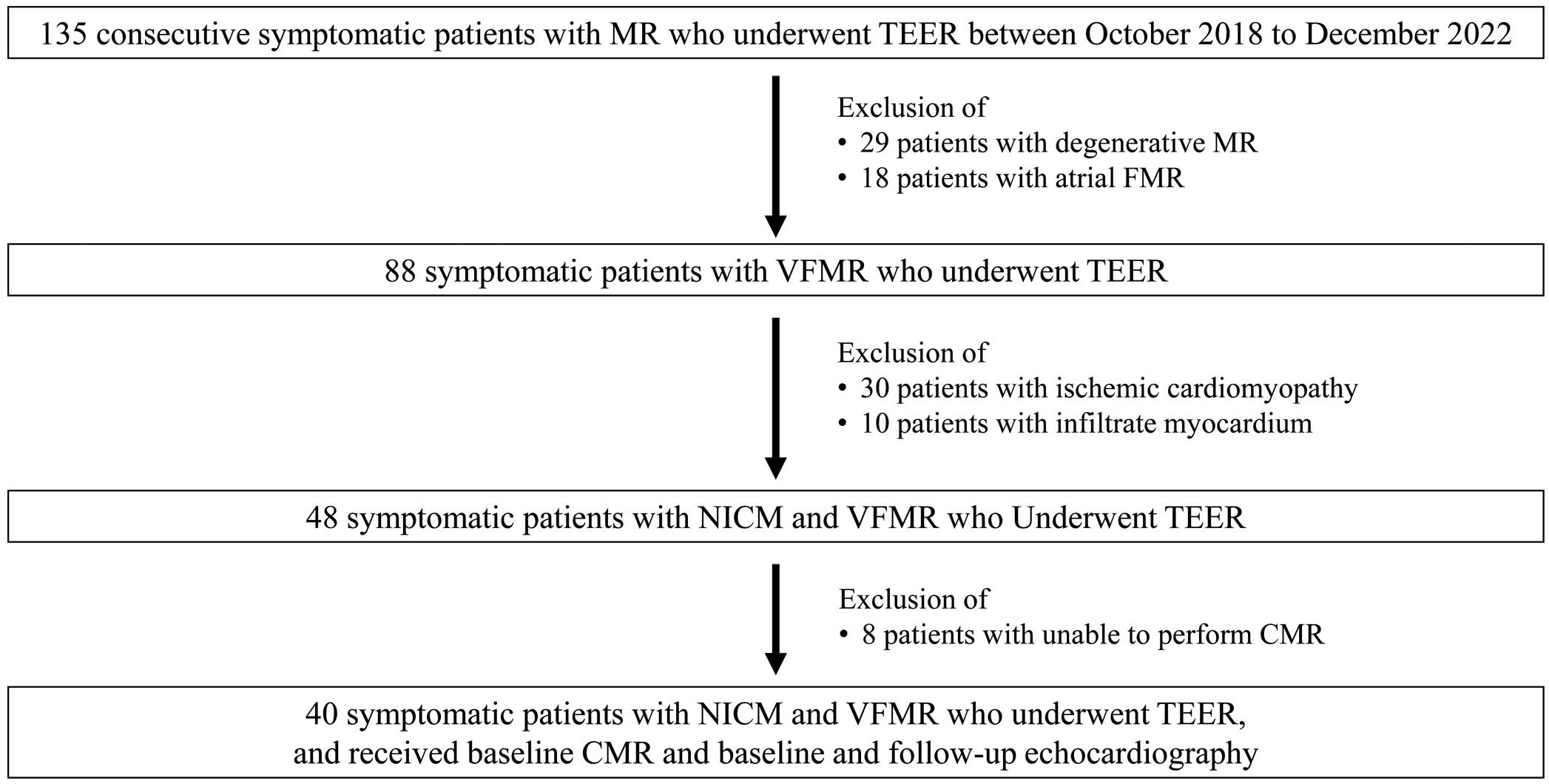
This study involved 135 consecutive symptomatic patients with mitral regurgitation (MR) who had undergone TEER between October 2018 and December 2022 at Hyogo Prefectural Harima-Himeji General Medical Center. We excluded 29 patients with degenerative MR, 18 with atrial functional MR, 30 with ischemic cardiomyopathy, and 10 with secondary cardiomyopathy (5 patients with cardiac sarcoidosis, 4 with cardiac amyloidosis, and 1 with Fabry disease). Of the 48 remaining patients, a further 8 in whom CMR could not be performed were also excluded so that the final study group consisted of 40 patients (Figure 1). The indication for TEER in individual patients was decided upon by consensus of a multidisciplinary heart team at Hyogo Prefectural Harima-Himeji General Medical Center, consisting of clinical cardiologists, interventional cardiologists, cardiac surgeons, imaging specialists with expertise in interventional imaging, cardiovascular anesthesiologists, and physical therapists. The team used current guidelines to reach their consensus.13
This study was approved by the local ethics committee of Hyogo Prefectural Harima-Himeji General Medical Center (No. 2-11) and was conducted in accordance with the Declaration of Helsinki.
CMR Protocols and Image Analysis
CMR scans were performed for all patients within 1 month before TEER using a 1.5-T scanner (Ingenia 1.5T CX; Philips Medical Systems, Best, Netherlands). The CMR protocol included cine CMR and native T1
mapping using a Modified Look-Locker inversion recovery (MOLLI) sequence.14,15 The MOLLI sequence was used for T1
quantification acquired in 3 short-axis images (basal, mid, and distal LV) during end-expiration breath-hold.14 All CMR image analyses without native T1
mapping were performed using cvi42 software (Circle Cshort-axisar Imaging, Calgary, Canada). The native T1
value was measured using the conservative septal technique with mid-septal measurements that were analyzed with a post-processing workstation (Ziostation 2; Ziosoft, Tokyo, Japan).16 The mean (±SD) normal native T1
value at Hyogo Prefectural Harima-Himeji General Medical Center was 1,018±46 ms, which was determined using 10 subjects with no clinical evidence of LV cardiomyopathy and normal CMR findings (mean [±SD] age 63±14 years, 5 males). The native T1
values were converted to Z-scores, which were calculated from the population mean and standard deviation derived from normal subjects at Hyogo Prefectural Harima-Himeji General Medical Center, to eliminate differences between our facility and the CMR device because external factors such as the field strength of the CMR system and manufacturer-specific hardware design affect native T1
values.17
Echocardiographic Examination
All patients had undergone transthoracic echocardiography before and a mean (±SD) 6.6±0.6 months after TEER, whereas echocardiographic data were obtained with a commercially available echocardiographic system (GE Vivid E95 Ultra Edition, GE Vingmed Ultrasound, Horten, Norway). Standard echocardiographic measurements were obtained in accordance with the current guidelines of the European Association of Cardiovascular Imaging.18 MR severity was classified as 0 (none or trivial), 1+ (mild), 2+ (moderate), 3+ (moderate-severe), or 4+ (severe) by integrating qualitative, semiquantitative, and quantitative parameters, as recommended elsewhere.18 The effective regurgitant orifice area (EROA) was also measured by means of the proximal isovelocity surface area method.
Endpoint Definition
The primary endpoint was defined as the first rehospitalization for HF or cardiovascular death during a median follow-up period of 12 months after TEER.
Statistical Analysis
Continuous variables are expressed as the mean±SD for normally distributed data and as the median with interquartile range (IQR) for data that were not normally distributed. Categorical variables are expressed as frequencies and percentages. Parameters of 2 subgroups were compared using Student’s t-test or the Mann-Whitney U test, depending on data distribution. The significance of differences in proportions were evaluated using Fisher’s exact test. Survival curves, consisting of freedom from first rehospitalization for HF or cardiovascular death, were determined with the Kaplan-Meier method, and cumulative event rates were compared using the log-rank test. The initial Cox proportional hazards analysis to identify univariate parameters associated with first rehospitalization for HF or cardiovascular death was followed by a multivariable Cox proportional hazards model based on stepwise selection. The entry criteria for individual items into the multivariable Cox proportional hazards model were previous reports of these factors being associated with prognosis after TEER,19–21 and candidate predictors were age, sex (male), LV end-diastolic volume index, LVEF, global longitudinal strain, EROA, native T1
Z-score, and right ventricular end-diastolic volume index. Receiver operating characteristic (ROC) curves were computed to determine the optimal cut-off value of the native T1
Z-score for its association with the primary endpoint.
Statistical significance was defined as P<0.05 for all comparisons. All analyses were performed using SPSS version 29 (IBM Corp., Armonk, NY, USA).
Results
Baseline Patient Characteristics
The baseline clinical characteristics and echocardiographic and hemodynamic parameters of the 40 patients are summarized in Table 1. Their mean age was 77.6±7.4 years and 27 (68%) were male. The mean EROA was 0.31±0.08 cm2
(Grade 2: 6 [15%] patients; Grade 3: 10 [25%] patients; and Grade 4: 24 [60%] patients), and mean LVEF was 33.6±7.8%.
Table 1.
Baseline Characteristics of Patients
| Variables |
All patients
(n=40) |
Patients with
CV events
(n=9) |
Patients without
CV events
(n=31) |
P value
(with vs. without
CV events) |
| Clinical data |
| Age (years) |
77.6±7.4 |
73.2±10.3 |
78.9±6.0 |
0.14 |
| Male sex |
27 (68) |
6 (67) |
21 (68) |
0.62 |
| Body surface area (m2) |
1.5±0.2 |
1.50±0.13 |
1.52±0.19 |
0.86 |
| NYHA Class III or IV |
40 (100) |
9 (100) |
31 (100) |
0.78 |
| STS score (%) |
17.0±11.3 |
18.0±9.2 |
16.7±12.0 |
0.76 |
| Comorbidity |
| Hypertension |
9 (23) |
3 (33) |
6 (19) |
0.65 |
| Diabetes |
13 (33) |
3 (33) |
10 (33) |
1.00 |
| Atrial fibrillation |
16 (41) |
4 (44) |
12 (40) |
1.00 |
| Chronic kidney disease |
34 (85) |
8 (89) |
26 (84) |
1.00 |
| Stroke/transient ischemic attack |
7 (18) |
1 (11) |
6 (19) |
0.67 |
| Prior implantation of pacemaker, ICD, or CRT |
6 (15) |
2 (22) |
4 (13) |
0.60 |
| Blood examination |
| Hemoglobin (g/dL) |
11.9±1.8 |
11.2±2.0 |
12.1±1.8 |
0.17 |
| BNP (pg/mL) |
477 [237–1,329] |
515 [242–1,006] |
462 [237–1,086] |
0.52 |
| eGFR (mL/min/1.73 m2) |
32.2±13.5 |
32.3±14.6 |
32.1±9.4 |
0.97 |
| Medications on admission |
| ACEIs/ARBs/ARNI |
22 (55) |
4 (44) |
18 (58) |
0.36 |
| β-blocker |
35 (88) |
8 (89) |
27 (87) |
1.00 |
| SGLT2 inhibitors |
6 (15) |
2 (22) |
4 (13) |
0.60 |
| MRAs |
26 (65) |
6 (67) |
20 (65) |
1.00 |
| Diuretics |
40 (100) |
9 (100) |
31 (100) |
|
| Transthoracic echocardiography |
| LVESDI (mL/m2) |
33.8±7.1 |
34.9±6.7 |
33.4±7.3 |
0.56 |
| LVEDVI (mL/m2) |
123.7±33.0 |
130.6±39.5 |
121.7±31.3 |
0.48 |
| LVESVI (mL/m2) |
82.5±28.3 |
85.9±30.4 |
81.5±28.2 |
0.68 |
| LVEF (%) |
33.6±7.8 |
30.4±8.3 |
34.6±7.5 |
0.16 |
| LVSVI (mL/m2) |
31.9±10.7 |
28.3±13.4 |
32.9±9.7 |
0.26 |
| GLS (%) |
7.8±3.1 |
6.6±4.1 |
8.3±2.6 |
0.15 |
| Left atrial volume index (mL/m2) |
80.2±25.4 |
87.8±26.2 |
78.4±25.3 |
0.39 |
| Grade of mitral regurgitation |
| Moderate, Grade 2+ |
6 (15) |
2 (22) |
4 (13) |
0.22 |
| Moderate-to-severe, Grade 3+ |
10 (25) |
3 (33) |
7 (23) |
|
| Severe, Grade 4+ |
24 (60) |
4 (44) |
20 (65) |
|
| Effective regurgitant orifice area (cm2) |
0.31±0.08 |
0.30±0.09 |
0.32±0.08 |
0.68 |
| TAPSE (mm) |
14.2±3.5 |
13.9±3.6 |
14.2±3.5 |
0.80 |
| Pulmonary vein flow S/D |
0.52±0.37 |
0.63±0.51 |
0.49±0.24 |
0.31 |
| Cardiac catheterization |
| Mean pulmonary artery pressure (mmHg) |
25.6±12.7 |
29.0±11.7 |
24.2±12.8 |
0.23 |
| Pulmonary capillary wedge pressure (mmHg) |
18.7±8.9 |
19.8±7.9 |
18.3±9.3 |
0.71 |
| Cardiac index |
1.9±0.6 |
1.8±0.5 |
1.9±0.7 |
0.60 |
| Cardiac output |
2.8±0.9 |
2.6±0.7 |
2.9±1.0 |
0.42 |
| CMR parameters |
| Native T1 values (ms) |
1,109.9±58.5 |
1,164.4±50.0 |
1,094.0±51.3 |
0.001 |
| Native T1 Z-score |
2.07±1.20 |
3.20±0.91 |
1.74±1.07 |
0.001 |
| LVEDVI (mL/m2) |
137.6±42.4 |
154.4±44.4 |
132.7±41.3 |
0.20 |
| LVESVI (mL/m2) |
103.5±43.6 |
119.6±46.6 |
98.8±42.3 |
0.21 |
| LVEF (%) |
26.6±12.2 |
24.1±11.5 |
27.4±12.5 |
0.48 |
| LVSVI (mL/m2) |
23.4±8.8 |
24.6±11.4 |
23.0±8.0 |
0.63 |
| LV mass index (g/m2) |
88.4±27.2 |
89.9±17.1 |
88.0±29.7 |
0.85 |
| RVEDVI (mL/m2) |
77.1±26.1 |
95.4±28.0 |
71.8±23.4 |
0.02 |
| RVESVI (mL/m2) |
53.0±23.1 |
66.5±26.8 |
49.1±20.8 |
0.05 |
| RVEF (%) |
31.8±13.5 |
32.0±14.4 |
31.7±13.4 |
0.95 |
| RVSVI (mL/m2) |
24.0±11.7 |
28.7±11.3 |
22.7±11.6 |
0.17 |
Data are presented as the mean±SD for normally distributed data, median [interquartile range] for non-normally distributed data, or n (%). ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor antagonist; ARNI, angiotensin receptor-neprilysin inhibitor; BNP, B-type natriuretic peptide; CMR, cardiovascular magnetic resonance; CRT, cardiac resynchronization therapy; eGFR, estimated glomerular filtration rate; GLS, global longitudinal strain; ICD, implantable cardioverter defibrillator; LVEDDI, left ventricular end-diastolic diameter index; LVEDVI, left ventricular end-diastolic volume index; LVEF, left ventricular ejection fraction; LVESDI, left ventricular end-systolic diameter index; LVESVI, left ventricular end-systolic volume index; LVSVI, left ventricular stroke volume index; MRA, mineralocorticoid receptor antagonists; NYHA, New York Heart Association; RVEDVI, right ventricular end-diastolic volume index; RVEF, right ventricular ejection fraction; RVESVI, right ventricular end-systolic volume index; SGLT2, sodium-glucose cotransporter 2; STS, Society of Thoracic Surgeons; TAPSE, tricuspid annular plane systolic excursion.
Comparison of Baseline Patient Characteristics Between Patients With and Without Cardiovascular Events
All patients successfully underwent TEER (MitraClipTM; Abbot Vascular, Inc., Santa Clara, CA, USA) without any major complications, and the residual MR grade was Grade 0–1+ in 38 (95%) patients and Grade 2+ in 2 (5%) patients. During the follow-up period of 10.7±2.9 months, 9 (22.5%) patients reached the primary endpoint: cardiovascular death in 3 patients and hospitalization due to worsening HF in the remaining 6. Baseline clinical characteristics and echocardiographic and hemodynamic parameters of patients with and without cardiovascular events are presented in Table 1. All parameters were similar between the 2 groups, except for patients with the primary endpoint being more likely than those without to have higher native T1
values (1,164.4±50.0 vs. 1,094±51.3 ms; P=0.001), native T1
Z-scores (3.20±0.91 vs. 1.74±1.07; P=0.001), and right ventricular volume (right ventricular end-diastolic volume index 95.4±28.0 vs. 71.8±23.4 mL/m2; P=0.02).
Associations of Native T1
Z-Score With Cardiovascular Events
Hazard ratios (HRs) and 95% confidence intervals (CIs) for each of the variables in the univariable and multivariable Cox proportional hazards analyses for association with cardiovascular events are listed in Table 2. An important finding of the multivariable Cox proportional hazards analysis was that the native T1
Z-score was independently associated with cardiovascular events (HR 3.40; 95% CI 1.51–7.67; P=0.003). In addition, analysis of the ROC curve identified a native T1
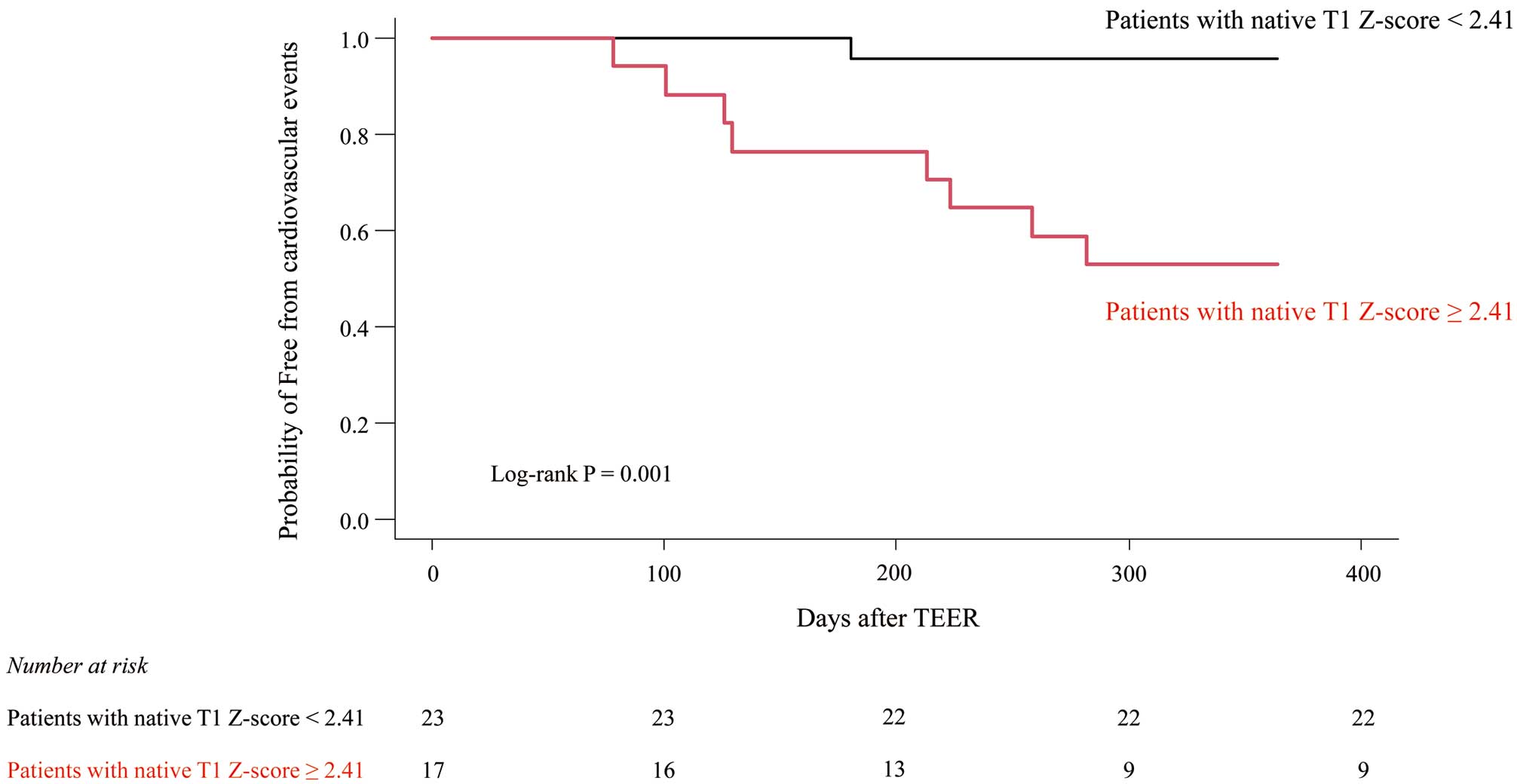
Z-score ≥2.41 as the optimal cut-off value for association with cardiovascular events, with a sensitivity of 71%, specificity of 89%, and area under the curve of 0.839 (P<0.001; Figure 2). For this cut-off value, the Kaplan-Meier curve indicated that the occurrence of cardiovascular events was significantly lower for patients with a native T1
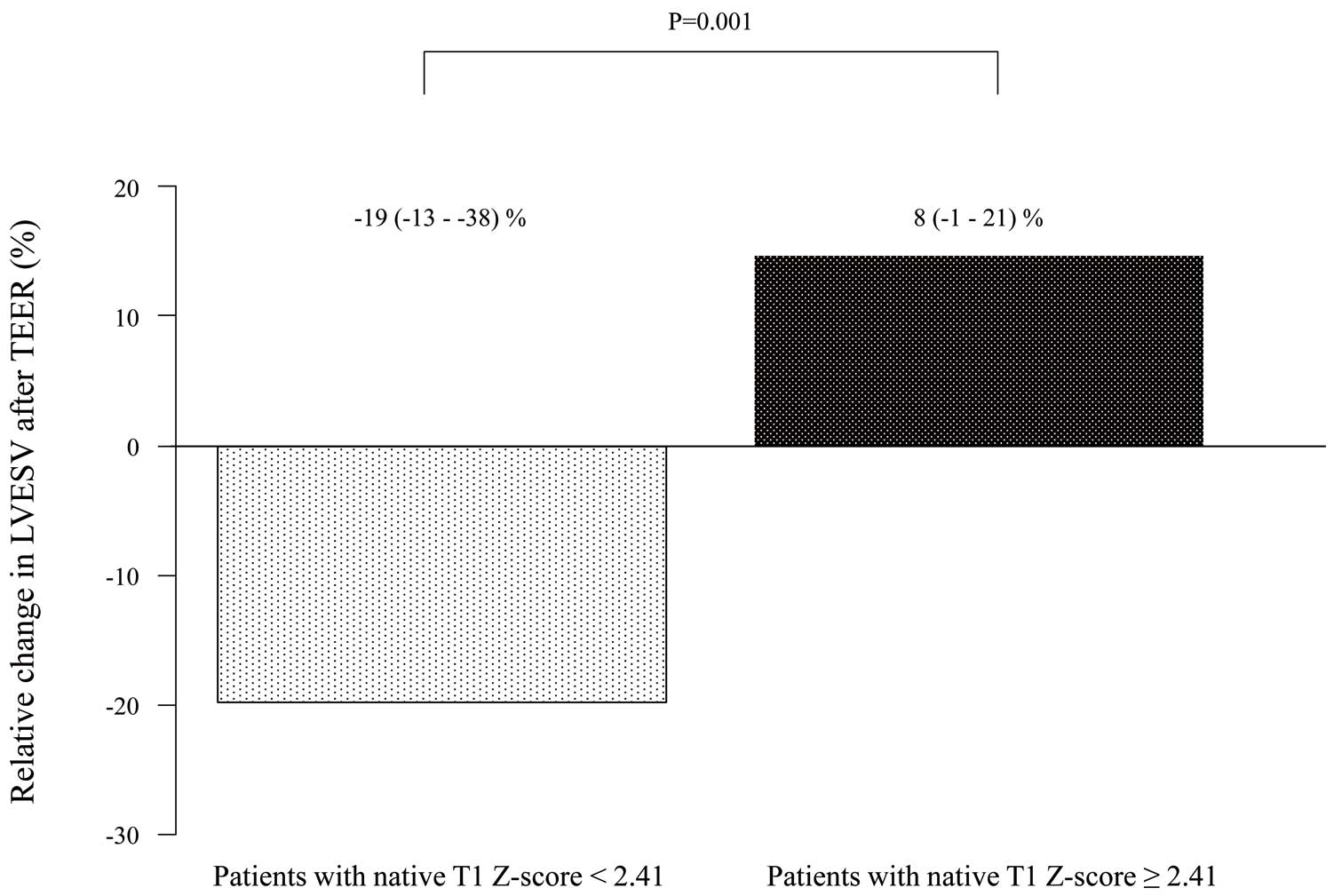
Z-score <2.41 than ≥2.41 (log-rank P=0.001; Figure 3). In addition, the relative change in LV end-systolic volume 6.6±0.6 months after TEER was significantly larger for patients with a native T1
Z-score <2.41 than ≥2.41 (median −19% [IQR −13, −38%] vs. 8% [−1, 21%]; P=0.001; Figure 4).
Table 2.
Univariable and Multivariable Proportional Hazards Analyses for Association With Cardiovascular Events
| Variables |
Univariable |
Multivariable |
| HR (95% CI) |
P value |
HR (95% CI) |
P value |
| Age |
0.92 (0.98–1.03) |
0.047 |
|
|
| Male sex |
0.95 (0.24–3.82) |
0.38 |
|
|
| LVEDVI (echocardiography) |
1.00 (0.99–1.03) |
0.48 |
|
|
| LVEF |
0.94 (0.85–1.03) |
0.15 |
|
|
| GLS |
1.00 (0.62–1.03) |
0.08 |
0.91 (0.71–1.16) |
0.45 |
| Effective regurgitant orifice area |
0.08 (0.00–448.3) |
0.56 |
|
|
| Native T1 Z-score |
3.08 (1.43–6.62) |
0.004 |
3.40 (1.51–7.67) |
0.003 |
| RVEDVI (CMR) |
1.03 (1.00–1.05) |
0.03 |
|
|
CI, confidence interval; HR, hazard ratio. Other abbreviations as in Table 1.

Next, we divided all patients into 4 subgroups by using the optimal native T1
Z-score cut-off value of 2.41 and an EROA of 0.30 cm2. There were 15 patients with a low native T1
Z-score <2.41 and a larger VFMR of EROA (i.e., ≥0.30 cm2). This characteristic was associated with fewer cardiovascular events than for the 8 patients with a high native T1
Z-score (≥2.41) and smaller VFMR of EROA (<0.30 cm2; log-rank P=0.002; Figure 5).
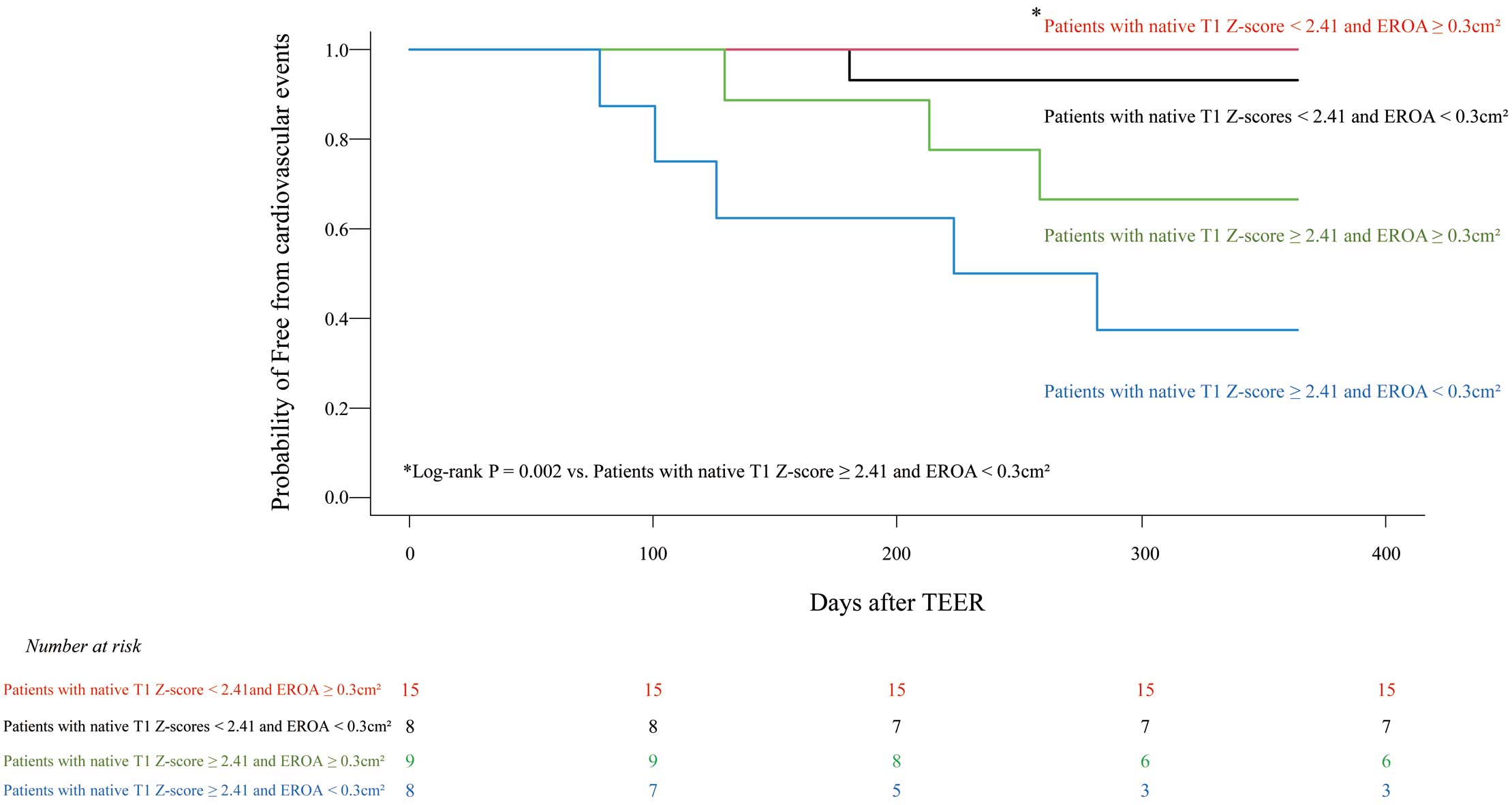
Figure 6 shows representative findings for T1
mapping obtained with CMR before TEER and echocardiography before and 6 months after TEER for patients with and without cardiovascular events. Patient 1 was an 81-year-old man with LV reverse remodeling 6 months after TEER who experienced no cardiovascular events during a follow-up period of 23 months. His lowest baseline native T1
Z-score was 1.54. Patient 2 was a 78-year-old man without LV reverse remodeling 6 months after TEER who experienced cardiovascular events 7 months after TEER. His highest baseline native T1
Z-score was 3.71.
Discussion
This is the first study to evaluate the efficacy of native T1
mapping for symptomatic NICM patients with reduced LVEF and VFMR undergoing TEER. The main findings of this study are that: (1) the baseline native T1
Z-score is independently associated with cardiovascular events after TEER; (2) the occurrence of cardiovascular events is significantly lower for patients with a native T1
Z-score <2.41 than for those with a native T1
Z-score ≥2.41; (3) the relative change in LV end-systolic volume after TEER was significantly larger for patients a with native T1
Z-score <2.41 than for those with a native T1
Z-score ≥2.41; and (4) patients with a native T1
Z-score <2.41 and EROA ≥0.30 cm2 experienced fewer cardiovascular events than those with a native T1
Z-score ≥2.41 and EROA <0.30 cm2.
Appropriate Timing of TEER for HF Patients With VFMR
In the MITRA-FR trial, the 307 HF patients with LVEF 15–40% and severe VFMR assigned to undergo TEER had substantially more LV damage than patients in a control group who did not undergo TEER.9 However, after the 12-month follow-up, patients who were assigned to undergo TEER showed characteristics similar to those of the control group with respect to the risk of death or the risk of hospitalization for HF. Furthermore, the patients who underwent TEER experienced a short-term reduction in the degree of VFMR, but the use of the procedure did not result in reduced LV volumes after the 12- month follow-up.9 Conversely, the COAPT trial, which compared findings between a group of 614 randomly assigned HF patients with LVEF 20–50% and severe VFMR scheduled to undergo TEER and a control group, which did not undergo TEER, showed that patients who were assigned to TEER had a lower risk of death from any cause and a lower risk of hospitalization for HF after a 2-year follow-up.10 Furthermore, TEER led to a significant reduction in LV volume after a 1-year follow-up. The reasons for the conflicting results of the MITRA-FR and COAPT trials have been discussed elsewhere.19,22 Compared with patients in the COAPT trial, those enrolled in the MITRA-FR trial showed substantially more LV damage. The latter patient group had larger LV end-diastolic volumes (135±35 vs. 101±34 mL/m2 in MITRA-FR and COAPT, respectively), suggesting a more advanced stage of LV disease. This difference is likely to be related to the fact that COAPT excluded patients with very severe LV dilation and/or LV dysfunction (LV end-systolic diameter <70 mm), whereas MITRA-FR had no LV dimension limit. Moreover, the inclusion criteria regarding LVEF in MITRA-FR were more advanced than in COAPT (15–40% vs. 20–50%, respectively). Several studies have reported that for HF patients with VFMR, severe LV dilation (LV end-diastolic diameter >65 mm) and LV dysfunction (LVEF <20%, LV end-systolic diameter >55 mm) are associated with high rates of persistent and/or recurrent MR, less reverse LV remodeling, and worse outcomes after surgical correction of VFMR.23,24 Therefore, patients with too extensive LV myocardial damage (i.e., too severe LV dilation and/or dysfunction) may not benefit from TEER. In addition, differences in the degree of baseline VFMR are considered to have contributed to the differences in results between the MITRA-FR and COAPT trials.19,22 MITRA-FR patients had less severe VFMR than COAPT patients (0.31±0.10 vs. 0.41±0.15 cm2, respectively). To summarize, HF patients with reduced LVEF but not too extensive LV myocardial damage and more severe VFMR were more likely to benefit from TEER. In the present study, less LV myocardial damage (native T1
Z-score <2.41) and more severe VFMR (EROA ≥0.30 cm2) were associated with fewer cardiovascular events in patients than severe LV myocardial damage (native T1
Z-score ≥2.41) and a smaller VFMR (EROA <0.30 cm2).
T1 Mapping in Primary MR
T1
mapping can evaluate diffuse myocardial fibrosis that could not be simply identified by LGE. T1
mapping-based extracellular volume (ECV) and native T1
mapping are predictors of HF and arrhythmic events in NICM patients.25 In addition to patients with NICM, T1
mapping has demonstrated a good correlation with histologic findings of interstitial myocardial fibrosis in several conditions, including valvular heart disease.26 In patients with primary MR, chronic volume overload due to MR leads to progressive LV remodeling with diffuse interstitial fibrosis and increased T1
mapping-based ECV, and increased ECV is associated with adverse outcomes.27,28 Although there are very few reports of T1
mapping associated with VFMR, myocardial fibrosis in VFMR is thought to be primarily due to pre-existing cardiomyopathy rather than volume loading caused by MR. Because VFMR can be improved by LV reverse remodeling, it is important to evaluate the extent of progressive myocardial fibrosis in patients with VFMR using T1
mapping-based ECV or native T1
mapping.
Clinical Implications
Myocardial fibrosis is a pathological symptom found in NICM that can result in malignant ventricular arrhythmias and sudden cardiac death.29 Although endocardial biopsy is considered the gold standard for the detection of myocardial fibrosis, it is seldom performed because of its invasiveness. Previous studies have shown that patients with NICM combined with regional fibrosis, identified by CMR with LGE using gadolinium-based contrast media, have adverse outcomes, including HF hospitalization and death.30,31 However, detection of LGE requires at least a portion of the myocardium to be normal to identify an area of fibrosis, which has increased signal intensity on conventional LGE images relative to the normal area of the myocardium. Moreover, CMR with gadolinium-based contrast media is contraindicated for patients with severe kidney dysfunction because of the risk of nephrogenic systemic fibrosis.32,33 Conversely, native T1
mapping can be performed without the use of gadolinium-based contrast media and, unlike LGE, allows for quantitative evaluation.11,12 Li et al recently reported that native T1
mapping was associated with cardiac-related death and heart transplantation in 659 consecutive NICM patients, and native T1
mapping had the strongest associations for NICM patients with negative LGE.12 In the present study, native T1
mapping (native T1
Z-score) was independently associated with cardiovascular events, and cardiovascular events occurred significantly less frequently among patients with a native T1
Z-score <2.41 than among those with a native T1
Z-score ≥2.41. In addition, relative changes in LV end-systolic volume after TEER were significantly greater for patients with a native T1
Z-score <2.41 than for those with a native T1
Z-score ≥2.41. Because a high rate of kidney dysfunction is characteristic of symptomatic HF patients with reduced LVEF and severe VFMR (the mean estimated glomerular filtration rate was 32.2±13.5 mL/min/1.73 m2
in this study), CMR-derived native T1
mapping would be useful for non-invasive evaluation of LV myocardial damage for such patients.
Study Limitations
This was a retrospective, single-center study with a small number of patients, so further studies with larger patient populations and involving more institutions will be needed to validate our findings.
Conclusions
Baseline native T1
mapping was independently associated with cardiovascular events for symptomatic NICM patients with reduced LVEF and VFMR following TEER. Assessment of native T1
mapping is thus a valuable additional parameter for the better management of such patients.
Sources of Funding
This study did not receive any specific funding.
Disclosures
H. Tanaka has received remuneration from AstraZeneca plc, Otsuka Pharmaceutical Company, Limited, Ono Pharmaceutical Company, Limited, Pfizer Inc., Daiichi Sankyo Company, Limited, and Novartis International AG. K.H. has received research funding from Daiichi Sankyo Company, Limited, Actelion Pharmaceuticals Japan, Terumo Corporation, Abbott Vascular Japan, Otsuka Pharmaceutical Company, Limited, Kowa Company, Limited, Takeda Pharmaceutical Company Limited, Nihon Medi-Physics Company Limited, Novartis Pharma Company Limited, Bayer Company Limited, Biotronic Japan Company Limited, FUJIFILM Toyama Chemical Company Limited, Medtronic Japan Company Limited, Sysmex Company Limited. K.H. is a member of Circulation Journal’s Editorial Team. The remaining authors have no conflicts of interest to declare.
IRB Information
This study was approved by the local ethics committee of Hyogo Prefectural Harima-Himeji General Medical Center Clinical and Translational Research Center (Reference no. 2-11).
References
- 1.
Grigioni F, Enriquez-Sarano M, Zehr KJ, Bailey KR, Tajik AJ. Ischemic mitral regurgitation: Long-term outcome and prognostic implications with quantitative Doppler assessment. Circulation 2001; 103: 1759–1764.
- 2.
Trichon BH, Felker GM, Shaw LK, Cabell CH, O’Connor CM. Relation of frequency and severity of mitral regurgitation to survival among patients with left ventricular systolic dysfunction and heart failure. Am J Cardiol 2003; 91: 538–543.
- 3.
Bursi F, Barbieri A, Grigioni F, Reggianini L, Zanasi V, Leuzzi C, et al. Prognostic implications of functional mitral regurgitation according to the severity of the underlying chronic heart failure: A long-term outcome study. Eur J Heart Fail 2010; 12: 382–388.
- 4.
Rossi A, Dini FL, Faggiano P, Agricola E, Cicoira M, Frattini S, et al. Independent prognostic value of functional mitral regurgitation in patients with heart failure: A quantitative analysis of 1256 patients with ischaemic and non-ischaemic dilated cardiomyopathy. Heart 2011; 97: 1675–1680.
- 5.
Feldman T, Kar S, Elmariah S, Smart SC, Trento A, Siegel RJ, et al. Randomized comparison of percutaneous repair and surgery for mitral regurgitation: 5-year results of EVEREST II. J Am Coll Cardiol 2015; 66: 2844–2854.
- 6.
Grover FL, Vemulapalli S, Carroll JD, Edwards FH, Mack MJ, Thourani VH, et al. 2016 Annual report of the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry. J Am Coll Cardiol 2017; 69: 1215–1230.
- 7.
McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021; 42: 3599–3726.
- 8.
Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: A report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022; 145: e895–e1032.
- 9.
Obadia JF, Messika-Zeitoun D, Leurent G, Iung B, Bonnet G, Piriou N, et al. Percutaneous repair or medical treatment for secondary mitral regurgitation. N Engl J Med 2018; 379: 2297–2306.
- 10.
Stone GW, Lindenfeld J, Abraham WT, Kar S, Lim DS, Mishell JM, et al. Transcatheter mitral-valve repair in patients with heart failure. N Engl J Med 2018; 379: 2307–2318.
- 11.
Shah RV, Kato S, Roujol S, Murthy V, Bellm S, Kashem A, et al. Native myocardial T1 as a biomarker of cardiac structure in non-ischemic cardiomyopathy. Am J Cardiol 2016; 117: 282–288.
- 12.
Li S, Zhou D, Sirajuddin A, He J, Xu J, Zhuang B, et al. T1 mapping and extracellular volume fraction in dilated cardiomyopathy: A prognosis study. JACC Cardiovasc Imaging 2022; 15: 578–590.
- 13.
Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, et al. 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J 2022; 43: 561–632.
- 14.
Messroghli DR, Moon JC, Ferreira VM, Grosse-Wortmann L, He T, Kellman P, et al. Correction to: Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: A consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J Cardiovasc Magn Reson 2018; 20: 9.
- 15.
Petersen SE, Khanji MY, Plein S, Lancellotti P, Bucciarelli-Ducci C. European Association of Cardiovascular Imaging expert consensus paper: A comprehensive review of cardiovascular magnetic resonance normal values of cardiac chamber size and aortic root in adults and recommendations for grading severity. Eur Heart J Cardiovasc Imaging 2019; 20: 1321–1331.
- 16.
Rogers T, Dabir D, Mahmoud I, Voigt T, Schaeffter T, Nagel E, et al. Standardization of T1 measurements with MOLLI in differentiation between health and disease--The ConSept study. J Cardiovasc Magn Reson 2013; 15: 78.
- 17.
Kellman P, Hansen MS. T1-mapping in the heart: Accuracy and precision. J Cardiovasc Magn Reson 2014; 16: 2.
- 18.
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015; 16: 233–270.
- 19.
Grayburn PA, Sannino A, Packer M. Proportionate and disproportionate functional mitral regurgitation: A new conceptual framework that reconciles the results of the MITRA-FR and COAPT Trials. JACC Cardiovasc Imaging 2019; 12: 353–362.
- 20.
Medvedofsky D, Milhorini Pio S, Weissman NJ, Namazi F, Delgado V, Grayburn PA, et al. Left ventricular global longitudinal strain as a predictor of outcomes in patients with heart failure with secondary mitral regurgitation: The COAPT trial. J Am Soc Echocardiogr 2021; 34: 955–965.
- 21.
Mizote I, Nakamura D. Who benefits from the MitraClip? Circ J 2022; 86: 412–414.
- 22.
Pibarot P, Delgado V, Bax JJ. MITRA-FR vs. COAPT: Lessons from two trials with diametrically opposed results. Eur Heart J Cardiovasc Imaging 2019; 20: 620–624.
- 23.
Braun J, van de Veire NR, Klautz RJ, Versteegh MI, Holman ER, Westenberg JJ, et al. Restrictive mitral annuloplasty cures ischemic mitral regurgitation and heart failure. Ann Thorac Surg 2008; 85: 430–436; discussion 436–437.
- 24.
Braun J, Bax JJ, Versteegh MI, Voigt PG, Holman ER, Klautz RJ, et al. Preoperative left ventricular dimensions predict reverse remodeling following restrictive mitral annuloplasty in ischemic mitral regurgitation. Eur J Cardiothorac Surg 2005; 27: 847–853.
- 25.
Cadour F, Quemeneur M, Biere L, Donal E, Bentatou Z, Eicher JC, et al. Prognostic value of cardiovascular magnetic resonance T1 mapping and extracellular volume fraction in nonischemic dilated cardiomyopathy. J Cardiovasc Magn Reson 2023; 25: 7.
- 26.
de Meester de Ravenstein C, Bouzin C, Lazam S, Boulif J, Amzulescu M, Melchior J, et al. Histological validation of measurement of diffuse interstitial myocardial fibrosis by myocardial extravascular volume fraction from Modified Look-Locker Imaging (MOLLI) T1 mapping at 3 T. J Cardiovasc Magn Reson 2015; 17: 48.
- 27.
Kitkungvan D, Yang EY, El Tallawi KC, Nagueh SF, Nabi F, Khan MA, et al. Extracellular volume in primary mitral regurgitation. JACC Cardiovasc Imaging 2021; 14: 1146–1160.
- 28.
Liu B, Neil DAH, Premchand M, Bhabra M, Patel R, Barker T, et al. Myocardial fibrosis in asymptomatic and symptomatic chronic severe primary mitral regurgitation and relationship to tissue characterisation and left ventricular function on cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2020; 22: 86.
- 29.
Elming MB, Hammer-Hansen S, Voges I, Nyktari E, Raja AA, Svendsen JH, et al. Myocardial fibrosis and the effect of primary prophylactic defibrillator implantation in patients with non-ischemic systolic heart failure-DANISH-MRI. Am Heart J 2020; 221: 165–176.
- 30.
Halliday BP, Gulati A, Ali A, Guha K, Newsome S, Arzanauskaite M, et al. Association between midwall late gadolinium enhancement and sudden cardiac death in patients with dilated cardiomyopathy and mild and moderate left ventricular systolic dysfunction. Circulation 2017; 135: 2106–2115.
- 31.
Assomull RG, Prasad SK, Lyne J, Smith G, Burman ED, Khan M, et al. Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy. J Am Coll Cardiol 2006; 48: 1977–1985.
- 32.
Takahashi EA, Kallmes DF, Mara KC, Harmsen WS, Misra S. Nephrotoxicity of gadolinium-based contrast in the setting of renal artery intervention: Retrospective analysis with 10-year follow-up. Diagn Interv Radiol 2018; 24: 378–384.
- 33.
Weinreb JC, Rodby RA, Yee J, Wang CL, Fine D, McDonald RJ, et al. Use of intravenous gadolinium-based contrast media in patients with kidney disease: Consensus statements from the American College of Radiology and the National Kidney Foundation. Radiology 2021; 298: 28–35.