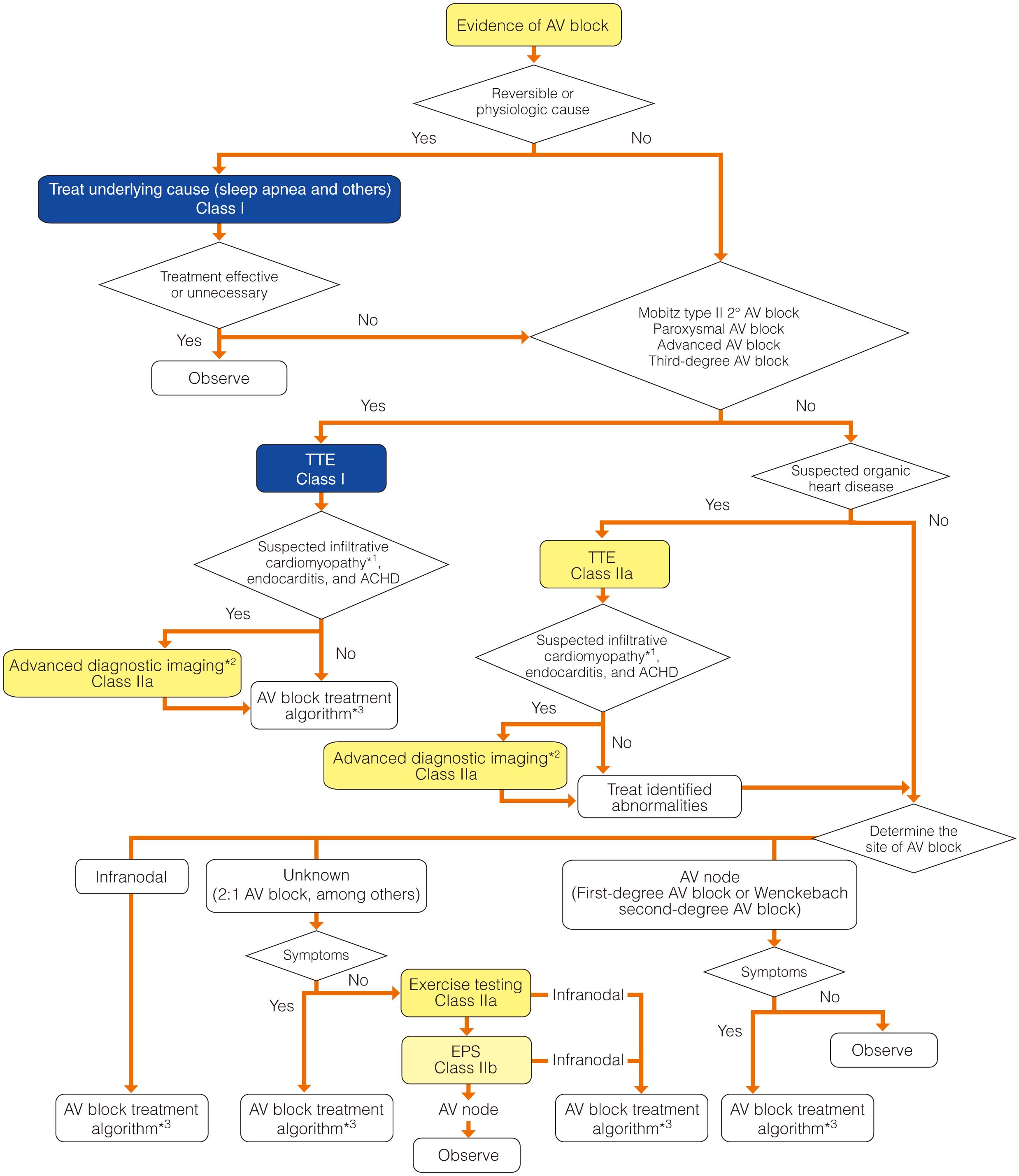
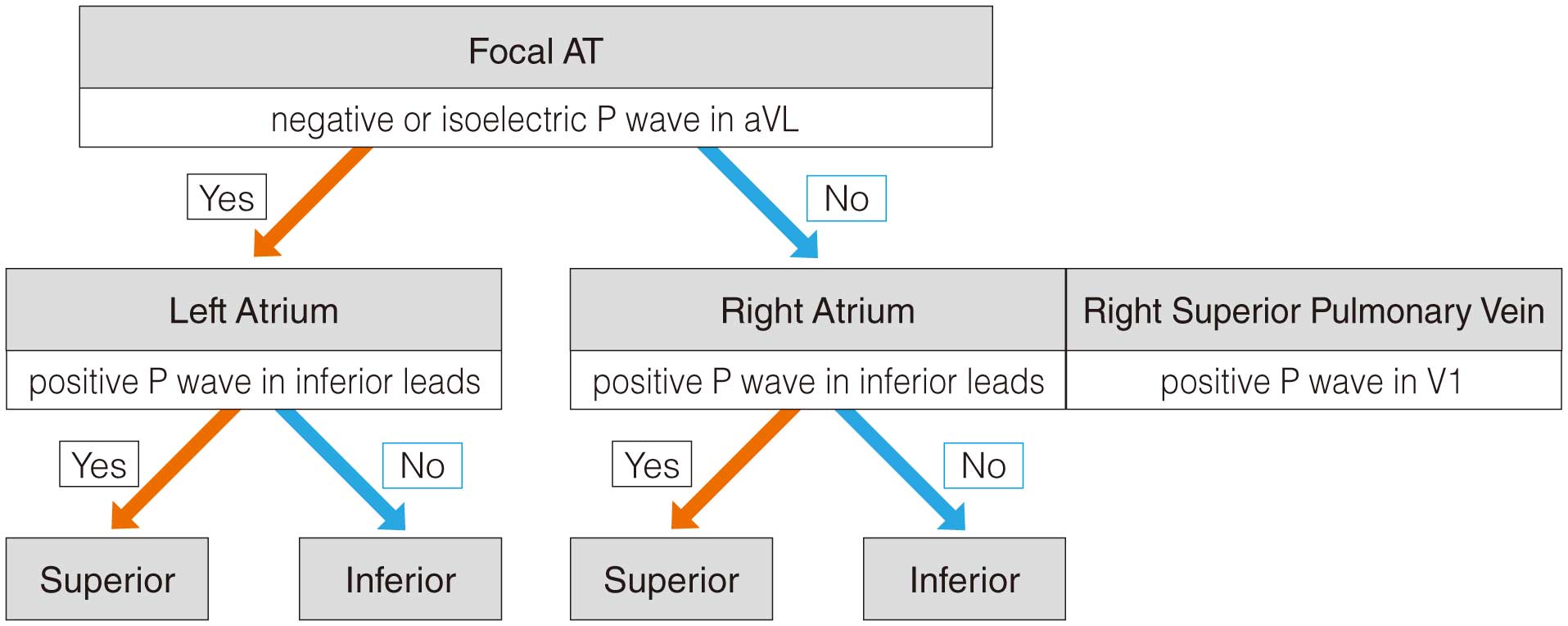
I. Arrhythmia Symptoms and Test Methods
1. Main Symptoms of Arrhythmia
1.1 Symptoms of Bradycardia
Persistent severe bradycardia tends to manifest symptoms such as shortness of breath on exertion and chest discomfort, but symptoms do not appear during sleep, at rest, while the condition is gradually developing, or if activity levels are reduced. Prolonged cardiac arrest can also cause dimmed vision or syncope. Despite fewer subjective symptoms, signs of heart failure can be confirmed based on findings such as lower limb edema, cardiac dilatation on chest X-ray, and blood tests. Furthermore, bradycardia is often asymptomatic, showing no subjective symptoms in sports trainees or people who normally have low heart rates.
1.2 Symptoms of Tachycardia
Heart rate increases as a physiological response caused by increased sympathetic nerve activity, such as during exercise, mental excitation, and fever. However, a sudden increase in heart rate causes symptoms with varying severity depending on the level of cardiac function, the duration of the tachycardia, the cause of arrhythmia (supraventricular, ventricular), and the presence or absence of an underlying heart disease. Although supraventricular arrhythmia can cause symptoms such as palpitations, chest pain, and chest discomfort, it has relatively little effect on hemodynamics. Severe and sustained ventricular arrhythmias affect cardiac output and disrupt hemodynamics. They can also result in cerebral hypoperfusion, palpitations with cold sweat, dizziness, dimmed vision, or syncope.10 Tachyarrhythmias with a high incidence of syncope are caused by fatal ventricular arrhythmias such as sustained ventricular arrhythmia and ventricular fibrillation (VF). However, on rare occasions, supraventricular arrhythmias, such as tachycardia atrial fibrillation (AF), 1 : 1 atrioventricular (AV) conduction of atrial flutter, and paroxysmal supraventricular tachycardia (PSVT), may also result in syncope or near-syncopal symptoms.
1.3 Symptoms of Abnormal Sinus Rhythm
1.3.1 Palpitations, Intermittent Pulse
Irregular pulse can indicate extrasystole or AF, but patients also may be asymptomatic or have few symptoms with isolated or infrequent extrasystoles. Patients may present with palpitations or intermittent pulse deficiency when there are repeated or frequent extrasystoles. In paroxysmal AF, the sudden disappearance of AV synchrony causes rapid and/or irregular pulse-to-beat, which may result in severe symptoms. In a survey of 756 patients with symptomatic AF, the most common symptom was palpitations, occurring in 54% of patients, followed by shortness of breath, malaise, syncope or dizziness, and chest pain, while 11% of patients were asymptomatic.11 Reports on paroxysmal AF indicate that 79%, 23%, and 17% of patients have symptoms of palpitations, shortness of breath, as well as syncope and dizziness, respectively, while 5% are asymptomatic. Conversely, in permanent AF, 45%, 47%, and 8% of patients have symptoms of palpitations, shortness of breath, as well as syncope and dizziness, respectively, while 16% are asymptomatic. Therefore, paroxysmal and permanent AF lead to different frequencies and types of symptoms.
1.3.2 Shortness of Breath, Dizziness
Symptoms such as shortness of breath, fatigue, and dizziness are observed relatively frequently in patients with bradyarrhythmia. For conditions such as sick sinus syndrome (SSS) and atrioventricular block, the heart rate may not increase sufficiently with physiological heart rate response or exertion (chronotropic incompetence), and patients may complain of symptoms such as shortness of breath, fatigue, and dizziness. These symptoms tend to occur when standing or during exertion and are uncommon while supine or at rest. The same arrhythmia may not always manifest the same symptoms in the same patient.
1.3.3 Syncope
The sudden onset of extreme bradycardia, cardiac arrest, or extreme tachycardia causes a temporary reduction or interruption in blood flow to the brain, resulting in whole cerebral hypoperfusion and causing syncope (Stokes-Adams syndrome). Syncope is defined as rapid onset, transient loss of consciousness, and inability to maintain posture, followed by prompt recovery spontaneously.
Symptoms vary depending on the type of arrhythmia, severity of bradycardia, duration of cardiac arrest, and posture. A cardiac arrest with a duration of 2–5 s may cause presyncopal symptoms such as dizziness, lightheadedness, and dimmed vision, whereas if the cardiac arrest lasts >6 s, body posture may not be maintained, and the patient eventually faints.12 However, these symptoms may not appear if the cerebral hypoperfusion is improved when the patient lies down, or if the causative arrhythmia terminates in a short time. Therefore, syncope often involves facial or head injury (see Chapter II.5 for differential diagnosis).
2. Epidemiology and Pathophysiology of Sudden Cardiac Death
2.1 Actual Sudden Cardiac Death and Underlying Diseases
2.1.1 Status of Cardiac Arrest Patients in Japan
Sudden cardiac death is defined as unexpected death within 24 h (or within 1 h) of the onset of acute symptoms assumed to be caused by heart disease. Results of a statistical analysis based on an Utstein-style survey by the Fire and Disaster Management Agency13 showed that 127,718 patients who had a cardiac arrest were transported by ambulance in the fiscal year (FY) 2018, and incidence increased with age stratified by 10-year age groups; patients aged 80–89 years had the highest incidence, accounting for 34% of the total sample. Although there has been no significant change in the number of transported patients or in the sex ratio over the past 10 years, the percentage of old patients has increased.
Among the aforementioned 127,718 patients who had a cardiac arrest, 79,400 (62%; male, 57%; female, 43%) experienced cardiogenic cardiac arrest, and 31,819 (25%) were witnessed at the onset, and 25,756 of them were witnessed by members of the public (Figure 1).13 The cardiac arrest occurred at home (66%), in public places (retirement homes, hospitals/clinics, inns/hotels, restaurants, car parks; 24%), on the road (5%), and other sites (5%).

The 1-month survival rate of the patient group witnessed by members of the public was 36.2% for patients whose initial ECG waveforms were VF and pulseless ventricular tachycardia (VT), and the 1-month social rehabilitation rate was 25.1%. These rates increased by 5.9 points and 4.6 points, respectively, compared with 10 years earlier. Furthermore, the 1-month survival rate of the patient group witnessed by ambulance officers and others was 55.7%, and the 1-month social rehabilitation rate was 46.1%, which increased by 13.0 points and 11.4 points, respectively.
The Japan Society for Holter and Noninvasive Electrocardiology collected Holter ECG records of patients who went into cardiac arrest from 41 medical facilities nationwide and investigated the types of arrhythmias in 132 patients (73% tachyarrhythmia, 27% bradyarrhythmia).14 Tachyarrhythmia was more common than bradyarrhythmia in the younger age group (mean age, 58 years vs. 70 years), with fewer complications such as stroke or heart failure. Tachyarrhythmia halted spontaneously in 38% of cases, which can also be considered near-miss sudden cardiac deaths. These results reiterate the importance of preliminary risk assessment.
2.1.2 Pathology and Underlying Disease of Sudden Cardiac Death
Factors leading to sudden cardiac death are broadly divided into cardiogenic and non-cardiogenic (see Chapter II.3.4 Table 18). Cardiogenic diseases in Japan include coronary artery disease (50–60%) and cardiomyopathy (30–35%)15 (Figure 2A). Hereditary arrhythmias account for up to 10%, and there are also a small number of valvular diseases (<10%). Hereditary arrhythmias include long QT syndrome (LQTS), short QT syndrome (SQTS), Brugada syndrome, early repolarization syndrome (ERS), catecholaminergic polymorphic ventricular tachycardia (CPVT), and progressive cardiac conduction disturbance (PCCD).

According to the Japan Cardiac Device Treatment Registry database16 of the Japanese Heart Rhythm Society, ≈20% of implantable cardioverter defibrillators (ICDs) were used for primary prevention of ischemic heart disease, and ≈80% for secondary prevention. Given that the appropriate activation rate of ICDs for cases of primary prevention is similar to that of secondary prevention, strict adherence to the indication criteria for implantation can be assumed.
On the other hand, there is a risk that sudden cardiac death may not be completely avoided in patients not indicated for ICD. In the Nippon Storm Study,17 1,570 patients fitted with devices, including 68% using ICDs and 32% using implantable cardiac resynchronization therapy devices (CRT-Ds) with biventricular pacing function, were registered by 48 medical facilities over a 2-year period from 2010 (Figure 2B), and a 2-year follow-up was conducted. The mean age of the patients was 62±14 years, and 78% of them were male. The underlying heart diseases were ischemic heart disease (31%), cardiomyopathy (36%), and hereditary arrhythmias (13%). In patients with ischemic heart disease, the devices were used for secondary prevention 3-fold more often than for primary prevention, whereas in those with cardiomyopathy, Brugada syndrome, and others the frequency was almost similar for primary and secondary prevention.
A comparison with several other countries showed that the number of sudden cardiac deaths in the USA ranges from 200,000 to 450,000 people per year,18 while Europe registered 300,000 people per year.19 In Western countries, the major underlying disease is coronary artery disease (80%), but these trends differ significantly when analyzed by age group.15 The cause is often unknown in neonates and infants, while cardiomyopathy and hereditary arrhythmias rank top in the group of children and young adults. In contrast, the incidence of ischemic heart disease increases in adults aged up to 35 years, and the majority of cases are caused by myocardial infarction in adults older than 35 years.20,21 The prospective cohort Rotterdam Study, which investigated 14,628 people aged ≥45 years, found that between 1990 and 2000 the incidence of sudden cardiac death per 1,000 people per year was 4.7, which decreased to 2.1 from 2001 to 2010.22 This might be due to advances in treatment for coronary artery disease,21 and the effect of implantable defibrillators (ICD, CRT-D). Measures such as risk stratification for primary prevention of cardiomyopathy and hereditary arrhythmias, as well as preventative measures among relatives, are considered important to further reduce sudden cardiac deaths.23–25
2.1.3 Risk Factors of Sudden Cardiac Death
The most important risk factors for sudden cardiac death are a history of VT/VF and cardiac arrest, as well as a family history of sudden death.26,27 Familial risk factors are involved not only in hereditary arrhythmias but also in genetic vulnerability to sudden death at the onset of acute coronary syndrome.28 The genetic mechanisms are multifactorial, and environmental interactions further complicate physiological and pathological mechanisms.
Coronary risk factors known to relate to sudden death include hypertension,29 diabetes,26 dyslipidemia,30 and smoking.31 Pathologies associated with sudden death include AF,32 and renal dysfunction.33,34 These are independent risk factors related to sudden death, and large-scale systematic reviews can rule out a causal relationship with myocardial ischemia.35,36 A review of 15 studies (39,908 patients, follow-up period of 4.2 years) on hypertensive patients treated with pharmacotherapy found that although antihypertensive drugs reduced the incidence of fatal myocardial infarction, they did not reduce the incidence of sudden death.35 This finding indicates that acute myocardial infarction may not be the main cause of sudden cardiac death. Instead, VT/VF caused by abnormalities in repolarization and modification in the modulation of the autonomic nervous system may result in sudden cardiac death.
For example, it has been clarified that prolongation of the interval between the peak and end of the T-waves (Tpeak–Tend) may be a predictive factor for VT/VF and death in hypertensive patients.37 A meta-analysis of 30 prospective studies (304,323 people) on diabetic patients conducted a risk assessment adjusting for several confounding factors (obesity index [body mass index (BMI)], waist-to-hip ratio, smoking, physical activity, alcohol intake, and resting heart rate), and potential intermediate risk factors (coronary artery disease, heart failure, AF, hypercholesteremia, hypertension, etc.), and reported that diabetes itself was a relatively high risk factor for sudden cardiac death.36 The reason is assumed to be that diabetes causes autonomic nervous system disorders that prolong QT and reduce heart rate variability (HRV), which may induce VT/VF.
Recently, research has focused on the mechanism of sudden death in obstructive sleep apnea syndrome,38,39 epileptic seizures,40,41 and drug abuse,42 in which each pathology plays an independent role in the sudden death.
3. Types and Characteristics of Electrocardiography
ECG is recognized as the most important test alongside medical history and physical findings, and it is utilized not only to detect arrhythmia but also as part of risk assessment (Table 3).
Table 3.
Class of Recommendation and Level of Evidence for Arrhythmia Diagnosis and Risk Assessment With ECG
| |
COR |
LOE |
| 12-lead ECG |
The 12-lead ECG is used to search for organic heart diseases that can cause arrhythmias, such
as coronary artery disease and ARVC/ACM |
I |
A |
The 12-lead ECG is used to diagnose hereditary arrhythmias, such as LQTS and Brugada
syndrome |
I |
A |
| Holter ECG/Event ECG |
| Holter ECG is used to detect paroxysmal arrhythmias with and without symptoms |
I |
A |
The use of a loop event recorder may be considered to detect paroxysmal arrhythmias with and
without symptoms |
IIa |
B |
The use of a non-loop event recorder may be considered to determine the relationship between
symptoms and arrhythmias |
IIb |
B |
The use of Holter ECG indices may be considered to predict sudden cardiac death or fatal
arrhythmia |
IIb |
B |
| Exercise stress test (ECG) |
The use of an exercise stress test may be considered for the assessment of exercise-induced
ventricular arrhythmia in patients with CPVT |
IIa |
C |
An exercise stress test may be considered for the diagnosis of patients with suspected congenital
LQTS |
IIa |
B |
The use of abnormal blood pressure response in patients with HCM during an exercise stress
test may be considered for risk assessment of sudden cardiac death |
IIa |
B |
| Latest ECG monitors/Automated AI diagnosis |
The use of a built-in smartphone camera and heart rate monitor program may be considered to
detect arrhythmia |
IIb |
B |
| The use of a smartwatch ECG program may be considered to detect arrhythmia |
IIb |
B |
| Auxiliary use of an AF diagnosis function using an AI algorithm may be considered |
IIb |
C |
ACM, arrhythmogenic cardiomyopathy; AF, atrial fibrillation; AI, artificial intelligence; ARVC, arrhythmogenic right ventricular cardiomyopathy; COR, Class of Recommendation; CPVT, catecholaminergic polymorphic ventricular tachycardia; ECG, electrocardiogram; HCM, hypertrophic cardiomyopathy; LOE, Level of Evidence; LQTS, long QT syndrome.
3.1 12-Lead ECG
The 12-lead ECG is the most basic examination of the heart. The 12 leads are depicted from a total of 10 electrodes placed on the body surface, including 4 on the limbs and 6 on the chest. The limb leads (I, II, III, aVR, aVL, aVF) depict sagittal sections in vertical and horizontal directions, while the chest leads (V1–6) depict horizontal sections in the anterior–posterior and horizontal directions. Sometimes, a right anterior chest lead such as V3R
or V4R
and a high intercostal chest lead superior to the 4th intercostal space may be added. The 12-lead ECG is useful not only for exploring possible heart disease but also for detecting findings such as left ventricular hypertrophy, bundle branch block, ST-T changes, T-wave abnormalities, and the extent of disparity between QT intervals (QT dispersion), that are associated with sudden death.43,44
3.1.1 QRS Wave Abnormalities
QRS prolongation has long been known as a predictive factor for the onset of arrhythmia. Fragmentation of the waveform at the end of the QRS is used for risk assessment of arrhythmogenic right ventricular cardiomyopathy (ARVC), arrhythmogenic cardiomyopathy (ACM), and Brugada syndrome.45
3.1.2 T-Wave Abnormalities
Negative conversion of T-waves is indicative of ischemia or hypertrophy. Giant negative T-waves are often seen in hypertrophic cardiomyopathy (HCM), takotsubo (ampulla-shaped) cardiomyopathy, and acute (sub)endocardial myocardial infarction. Analysis of T-waves is useful for the identification of patients with LQTS.46,47 U-waves following T-waves not during an attack (i.e., at rest) are seen in some cases of CPVT.48 The Tpeak–Tend
interval obtained by measuring from the peak to the end of the T-wave indicates non-uniformity in repolarization and is used for risk assessment of various diseases.49 In SQTS, the ST segment is not visible, the Tpeak–Tend
interval is shortened, and sharp T-waves with left–right symmetry are seen.50
3.1.3 ST-T Changes
Changes in ST-T are often found in coronary artery diseases such as angina, myocardial infarction, cardiomyopathy, and Brugada syndrome.44 The incidence of sudden death is twice as high in populations presenting with ST-T changes without any history of heart disease in both men and women, compared with populations without ST-T changes.51 Axial abnormalities in T-waves have also been useful predictive factors for sudden death.52 The diagnosis of disease types of Brugada syndrome is based on changes in the ST segment, and this information is also used for risk assessment.53
3.1.4 QT Abnormalities
The 12-lead ECG is useful for the diagnosis of LQTS. The QT interval is measured on the normal 12-lead ECG II lead or the V5
and V6
leads. It is preferable to take manual measurements using the tangent line technique, or other similar methods. When sinus arrhythmia occurs, the mean measurement of ≥3 consecutive heartbeats should be found.54 Characteristic QT prolongation appears in patients with congenital LQTS. Secondary LQTS is characterized by the appearance of QT prolongation or normal QT intervals with some factors that cause QT prolongation. QT prolongation with bradycardia is useful for the diagnosis of conditions such as SSS, the presence of AV block with a 2nd or higher degree, changes in T-waves with heart rate, and the appearance of R on T-type extrasystoles.55
3.1.5 QT Dispersion
QT dispersion is defined as the difference between the maximum and minimum QT intervals on a 12-lead ECG, which is an index of the non-uniformity of repolarization time of the ventricular muscle.56,57 Post-myocardial infarction,58 aortic stenosis,59,60 and ARVC/ACM61 are associated with the onset of ventricular arrhythmia and sudden cardiac death. The cutoff value is often set at around 70 ms. However, using QT dispersion as a clinical index has declined in recent years with the emergence of other useful ECG indices.
3.1.6 Early Repolarization
Early repolarization is often seen in healthy individuals. However, Rosso et al reported that an elevation of the J-point to ≥0.1 mV is important, and there is little diagnostic value in changing the V4–6
leads. In addition, an elevated J-point on the lower wall leads (I, III, aVF), I, or aVL leads should be noted.62 Recently, this finding has attracted attention in relation to ERS (J-wave syndrome).
3.2 Monitor ECG
The monitor ECG is a bipolar lead ECG with 2 plus–minus electrodes, mainly used in critical care areas and hospital wards. Generally, II leads are used. CM5 leads may be used to diagnose ischemia, but it is difficult to determine the vertical axis of the waveform on a monitor ECG (e.g., whether the ST is increased or decreased). Therefore, ischemic changes cannot be captured. This type of ECG is often used to monitor arrhythmias.
3.3 Holter ECG
The 12-lead ECG takes ≈30 s, whereas the Holter ECG can capture 24-h records, with a detailed examination including the assessment of arrhythmia onset and activity of the autonomic nervous system. The recording media is digitized, and the device is very light, weighing only ≈30 g. The electrodes are often bipolar leads attached to the chest. Generally, the Holter ECG uses a combination of CM5 and NASA leads, with their excellent ability to diagnose ischemia and arrhythmia, respectively. Recently, waterproof models have been released, which can be used to record during bathing. There have also been advances in analysis such that the Holter ECG now is able to measure ventricular late potential (LP), T-wave alternans (TWA), QT measurement indices, HRV, and heart rate turbulence (HRT), all of which are useful for predicting sudden cardiac death.
The ability of the Holter ECG to detect non-sustained ventricular tachycardia (NSVT) is useful for predicting sustained VT.63 A report that examined the cause of sudden cardiac death during Holter ECG recording found the cause of death was VF, torsade de pointes (TdP), VT, and bradyarrhythmia in 8%, 13%, 62%, and 17% of cases, respectively.64 Although less sensitive for detecting CPVT compared with exercise stress testing, the Holter ECG remains useful, particularly for patients who are unable to complete an exercise stress test, such as post-resuscitation and for infants, or for detecting supraventricular or ventricular arrhythmias in patients whose symptoms tend to be triggered by emotions.65
3.4 Event ECG (Non-Implantable)
An electrocardiogram recorded by an event electrocardiograph (recorder) is often indicated when arrhythmia is not detected with a 24-h Holter ECG. There are various types of event ECGs, but they can be broadly divided into (1) non-loop event recorders and (2) loop event recorders (see Chapter I.6 for information on implantable types).
3.4.1 Non-Loop Event Recorder
The portable recorder is placed on the chest during an event and the ECG is recorded by pressing the event button, making this device only indicated for patients with symptoms. Although it is used for patients to manage their own health, it is useful for understanding the causal relationship between symptoms and arrhythmia66 (Appendix 1: Figure 35).
3.4.2 Loop Event Recorder
Electrodes are affixed to the chest to continuously record the ECG. Because it is a loop recorder, ECGs from several seconds to several minutes prior to the event can be recorded. There are also implantable loop ECGs (see Chapter I.6). This device is also useful for detecting arrhythmia in asymptomatic patients.66
3.5 Exercise Stress ECG
Diseases in which arrhythmia events and sudden death can be triggered by exercise include congenital LQTS, CPVT, ARVC/ACM, and HCM. The 12-lead ECG is a basic test for congenital LQTS, which presents with characteristic T-wave abnormalities due to genetic changes.67 However, ≤40% of patients with congenital LQTS with genetic mutations have a normal QTc at rest, and even if QT is prolonged, it often remains undiagnosed due to inaccurate measurements.68–70 On the other hand, the 12-lead ECG is mostly normal in patients with CPVT, and there is no QT prolongation. Therefore, a treadmill exercise stress test is the most useful test for definitive diagnosis.71 Catecholamine has significant involvement in the induction of ventricular arrhythmia in ARVC/ACM.72,73 There is insufficient data on the risk of exercise in patients with Brugada syndrome. However, given that exercise-induced ST elevation and VT are independent predictors for arrhythmia events,74 restricting strenuous exercise should be considered. (Please refer to Chapter II for detailed information on exercise loading in each disease.)
3.5.1 Bradycardia
Bradycardia is rarely induced by exercise. Exercise loading may induce severe conduction disorders in patients with conduction disorders below the bundle of His.75 Bradycardia may be seen during exercise loading in patients with triple-vessel disease and left main coronary artery disease, which are severe forms of ischemic heart disease.
3.5.2 Premature Ventricular Contraction
A large-scale cohort study on 6,101 asymptomatic middle-aged men with a 23-year follow-up clarified that frequent premature ventricular contractions (PVCs) during exercise were associated with a relative risk of cardiovascular death.76 Exercise stress testing of 2,885 participants without cardiovascular disease in the Framingham Heart Study found that age and male sex were strongly associated with PVCs during exercise loading, and the total mortality rate was significantly increased during the 15-year observation period.77 Elevated heart rate during exercise is the result of the balance between suppression of the parasympathetic nervous system and activation of the sympathetic nervous system. However, a lack of compensatory increase in the vagus nerve after exercise reduces the heart rate recovery and response, as well as increasing the incidence of death and coronary artery disease.78 A study compared the onset and increase in PVCs during exercise with those during recovery after exercise in terms of using these indicators to predict the risk of death. A treadmill exercise stress test was completed by 29,244 patients with no history of heart failure, valvular heart disease, or arrhythmia. PVCs during exercise recovery were more useful for predicting the risk of death than PVCs during exercise.79
3.5.3 Pressor Response
A poor or reduced pressor response in patients with HCM under 50 years of age during exercise stress tests is one of the major risk factors for sudden death.80–82 A study that conducted treadmill exercise stress tests on 161 young HCM patients with a 44±20-month follow-up found that sudden death occurred in 12 patients and predicting sudden death based on abnormal pressor response had a sensitivity of 75%, specificity of 66%, positive predictive value of 15%, and negative predictive value of 97%.83 It was also found that abnormal pressor response during ergometer loading in 126 HCM patients was associated with cardiovascular death, with a positive predictive value of 14% and a negative predictive value of 95% during a follow-up period of 3.7–4.7 years.84
3.6 Latest ECG Monitors
3.6.1 Contact Photoplethysmography
Heart rate can be measured from pulse wave vibrations obtained by placing the finger on a smartphone camera (Appendix 1: Figure 36). It is also possible to detect AF by analyzing pulse wave fluctuations85–87 (see Chapter II.2.1.3.c for clinical results).
3.6.2 Non-Contact Photoplethysmography
The development of non-restrained, non-contact heart rate monitors is also in progress. Specific examples include detecting facial photoelectric pulse waves using a web camera or built-in smartphone camera, and diagnosis of AF has been trialed by calculating fluctuations in the pulse waves.88,89
3.6.3 Automated Oscillometric Blood Pressure Monitors
Electronic sphygmomanometers measure blood pressure using pulse waves (oscillometry). Sensors mounted on the cuff of electronic sphygmomanometers determine evidence of arrhythmia based on the regularity of the pulse waves generated by the blood vessel walls during decompression. The sensitivity and specificity of this technique for detecting AF are >90%.90,91
3.6.4 Wearable ECG
Wearable heart rate monitors and wearable ECGs capable of long-term recording are useful for the detection of infrequent arrhythmias. Wearable heart rate monitors include wristband types such as smart watches (Appendix 1: Figure 37), ring types and necklace types (Appendix 1: Figure 38), as well as bracelet and eyeglass types.92–94 The electrodes of wearable ECGs (Appendix 1: Figure 39) are sewn inside a T-shirt, and ECGs are recorded over a long period of time by attaching a miniature ECG to the electrodes. The recordings are then uploaded to a mobile digital terminal or the cloud for diagnosis (Appendix 1: Figure 40).
3.6.5 Smart Speakers and Others
Smart speakers process emitted voices using artificial intelligence (AI) and output the voice as instructed. Heart rate monitoring becomes possible by emitting inaudible sound waves of 18–22 kHz toward a living body from the smart speakers and analyzing the reflected sound signal.95 Furthermore, this technology can also identify heart rate irregularities, suggesting that it may support the diagnosis of arrhythmia. Another device is being developed to measure the heart rate by irradiating the back or thigh with microwaves (10GHz) and processing the reflected waves.96 If this device is mounted on the back of a chair, the heart rate could be measured by simply sitting on the chair. Mounting the device on beds in nursing care facilities is expected to be useful for watching over users.
3.6.6 Smartphones, Smartwatches
Using a smartphone and an external dedicated ECG recording device to record I-lead ECGs makes it possible to check the ECG in PDF format on a paired smartphone application (AliveCor, Appendix 1: Figure 40).97,98 The smartwatch has electrodes on its back, bezel, or strap, and touching these areas enables the I-lead ECG to record data (Appendix 1: Figure 37). It is possible to then immediately check the ECG in PDF format on a paired smartphone application.99
Specifications of “Home heart rate monitoring programs” for smartphones and “Home ECG programs” for smartwatches were approved in January 2021 in Japan as “Home medical devices” that detect and suggest disease symptoms and encourage the individual to seek medical attention, rather than as categories such as general medical devices or controlled medical devices. When an arrhythmia due to AF is suspected, missing the opportunity for a proper medical examination is considered a risk. Thus, these devices have been developed as a risk mitigation measure. However, patients with AF should not change their medication of their own volition based on the frequency of AF attacks measured by these devices. Research on records other than I-leads using smartwatches is aimed at detecting AF and myocardial ischemia, but this is not yet at the practical stage.100,101 The number of patients seen at medical institutions with the chief complaint of “signs of arrhythmia” based on a smartwatch is expected to increase in the future.
It is essential that doctors understand the accuracy and limitations of “suspected arrhythmia” findings using smartphones or smartwatches and explain to the patient that a definitive diagnosis of the disease must be established with the use of appropriate medical devices and treatment should be provided based on those results.102 Meanwhile, the government, as well as manufacturers and sales companies of these medical devices, should monitor the Internet to determine whether baseless information has been uploaded and adopt information security measures to prevent leakage of personal information, including ECGs, uploaded to the cloud. To date, data regarding the usage of these devices are not sufficient. Thus, the risks and benefits of “Home heart rate monitoring programs” and “Home ECG programs” are unknown. The provision of additional information and post-marketing safety measures related to the use of these applications are planned through the collection of information, including usage status.
3.7 Automated Diagnosis Using AI
AI diagnostics has evolved from the ability to process and store vast amounts of information by computers. Machine learning (ML) is the core of AI diagnosis, and deep neural networks (DNN) based on mathematical modeling of neurons in the human central nervous system are often used. ML using DNN extracts features from information on a large number of patients and then increases the accuracy of diagnosis by adjusting each parameter (weight and bias).103,104 Actually, using AI to analyze ECG images enables verification of complex patterns undetectable by the human eye. Most of the automated AI diagnoses in the field of ECG are at the research stage, but this is expected to be expanded broadly to incorporate event prediction.
3.7.1 Age, Prognosis
With AI diagnosis, a patient’s age could be estimated based on a 12-lead ECG.105 If the estimated age is significantly higher than the patient’s actual age, then reduced cardiovascular function or ischemic heart disease is suspected.105,106 This technology can estimate the patient’s prognosis 1 year after the test.107
3.7.2 Cardiac Function, Cardiac Hypertrophy, Ischemic Heart Disease, and Others
AI diagnosis with a 12-lead ECG can estimate left ventricular function,108–110 HCM,111 aortic stenosis,112,113 acute coronary syndrome,114–116 and hyperkalemia.117 There are also research reports on rare hereditary cardiomyopathies.118,119 AI diagnosis is useful for differentiating the major types of LQTS.120 AI can estimate the corrected QT intervals from a single-lead ECG, which may improve the ease of LQTS screening.121
3.7.3 Arrhythmia Diagnosis
Diagnostic AI algorithms are now used to detect AF from single-lead ECGs using wearable heart rate monitors and wearable ECGs.98,122–127 Similarly, a study that diagnosed 12 types of arrhythmias using a 30-s single-lead ECG achieved good results, with an area under the receiver operating characteristic (ROC) curve (AUC) of 0.97 and an F1 score (harmonic mean of precision and recall) of 0.837.128 In another study that simultaneously detected multiple arrhythmias and conduction disorders from 12-lead ECGs, AI outperformed experienced cardiologists.129
3.7.4 Predicting the Onset of AF
It is possible to predict the onset of AF from a sinus rhythm on a 12-lead ECG.130 A recent study analyzing data from 430,000 people reported that the AUC for the prediction of new AF events within 1 year of sinus rhythm on a 12-lead ECG was 0.85.131 Furthermore, the study detected 62% of patients who developed a new AF-related cerebral infarction within 3 years. Therefore, this technology is expected to be applied to the prevention of cardiogenic stroke from normal sinus rhythm before the discovery of paroxysmal AF.
4. Methods and Indications for the Cardiac Electrophysiological Study
The cardiac electrophysiological study (EPS) is a general term for studies that analyze local potential information and positional information of electrodes on catheters inserted transvascularly into the heart chamber by cardiac catheterization procedures. An EPS analyzes the characteristics of arrhythmia induction and intracardiac conduction patterns, including programmed stimulation of various regions of the myocardium, as well as intravenous loading of agonists and antiarrhythmic drugs of the autonomic nervous system.2 It is also used for diagnosis, including identification of the mechanisms underlying arrhythmia, identification of optimal sites for catheter ablation (hereinafter referred to as ablation), determination of therapeutic effect, and risk determination, including sudden cardiac death.2,132 In contrast to static diagnostic methods using long-term observation such as Holter ECG and implantable loop recorder (ILR), the EPS is dynamic and aims to actively reproduce the pathology, similar to exercise stress and standing load tests.2,4,132,133
The role of the EPS in arrhythmia treatment has changed significantly with advances in non-pharmacotherapy. Its main role has been to identify optimal ablation sites, with the rapid advances in ablation for tachyarrhythmia, but it is now also used to assess the risk of fatal VT/VF from the perspective of preventing sudden death.2,4,8,132,133 Table 4 shows the standard assessment methods for assessing the severity of major arrhythmias,2,132 and Table 5 shows the Class of Recommendation and Level of Evidence.
Table 4.
Standard Assessment Methods for Determining the Severity of Arrhythmia With an Electrophysiological Study
| Arrhythmia |
Electrode
arrangment |
Main assessment items |
Stimulation
site |
Programmed stimulation |
Drug loading |
| SSS |
HRA, HBE |
SNRT, SACT |
HRA |
OST, single premature
stimulation*1 |
PAB*2 |
| AV block |
HRA, HBE |
Atrioventricular, His – ventricular
conduction |
HRA |
Basic stimulation + single
premature stimulation, frequent
stimluation |
Atropine,
Procainamide |
| PSVT |
HRA, HBE,
RVA, CS |
Tachycardia induction, abnormal
conduction such as accessory
pathway |
HRA, RVA |
Basic stimulation + single
premature stimulation, rapid
stimluation |
Isoproterenol |
| VT/VF |
HRA, HBE,
RVA*3 |
VT/VF inducibility |
RVA, RVOT,
left ventricle |
Basic stimulation + 1–3 extra
stimulations,*4 rapid stimulation |
Isoproterenol |
*1SACT assessment using the Strauss method. May also use fixed cycle length stimulation (Narulla method).
*2Performed during the assessment of endogenous function with atropine and β-blockers.
*3RVOT and left ventricle may also be used depending on the induction protocol.
*4The short stimulation coupling interval is generally set to ≥180 ms to avoid non-specific VT/VF induction. Up to 2 consecutive short ventricular stimuli with a coupling interval of ≥200 ms are recommended for induction in patients with Brugada syndrome.
AV, atrioventricular; CS, coronary sinus; HBE, His bundle electrocardiogram; HRA, high right atrium; OST, overdrive suppression test; PAB, pharmacologic autonomic blockade; PSVT, paroxysmal supraventricular tachycardia; RVA, right ventricular apex; RVOT, right ventricular outflow tract; SACT, sinoatrial conduction time; SNRT, sinus node recovery time; SSS, sick sinus syndrome; VF, ventricular fibrillation; VT, ventricular tachycardia.
Table 5.
Class of Recommendation and Level of Evidence Related to Arrhythmia Diagnosis and Risk Assessment With an Electrophysiological Study
| |
COR |
LOE |
| Bradycardia |
EPS may be considered for cases of syncope, bundle branch block, or prolonged QRS complex
found on ECG, and when bradycardia is suspected as the cause, but there is no abnormal ECG |
IIb |
C |
EPS may be considered for the assessment of sinus node function in cases of palpations preceding
syncope without abnormalities on non-invasive examination |
IIb |
C |
EPS may be considered for the assessment of sinus node function and atrioventricular conduction
disorders in cases of sinus node dysfunction or AV block |
IIb |
C |
EPS may be considered for the identification of the block site in asymptomatic cases of Mobitz
type II 2nd-degree AV block, 3rd-degree AV block, and a bifascicular or trifascicular block |
IIb |
C |
| Tachyarrhythmia |
EPS may be considered for the assessment of tachycardia inducibility for syncope of unknown
origin or NSVT in patients with organic heart disease with LVEF ≤40% |
IIa |
B |
EPS may be considered for the assessment of tachycardia inducibility when arrhythmia cannot
be ruled out as the cause of cardiac arrest resuscitation cases |
IIa |
B |
EPS may be considered for the assessment of arrhythmogenic activity by antiarrhythmic drugs in
patients with sustained monomorphic VT |
IIb |
A |
EPS may be considered for the assessment of tachycardia inducibility when there is NSVT in
patients with organic heart disease without reduced cardiac function |
IIb |
B |
EPS may be considered for the assessment of tachycardia inducibility in patients with frequent
PVC or NSVT and positive ventricular right potential on SAECG |
IIb |
B |
AV, atrioventricular; COR, Class of Recommendation; ECG, electrocardiogram; EPS, electrophysiological study; LOE, Level of Evidence; LVEF, left ventricular ejection fraction; NSVT, non-sustained ventricular tachycardia; PVC, premature ventricular contraction; SAECG, signal-averaged electrocardiogram; VT, ventricular tachycardia.
4.1 Bradycardia
Bradycardia accounts for 10–20% of all cases of sudden cardiac death.14,64,134–137 In almost 30% of sudden deaths, the direct cause is asystole, but more often it is due to torsade de pointes (TdP) and VF with bradycardia-dependent QT prolongation.14,134 SSS and AV block jointly account for 90% of arrhythmias that cause bradycardia.138
According to guidelines, pacemakers are indicated for bradycardia confirmed from long-term records and with clinical symptoms (cerebral ischemic symptoms such as dizziness, or heart failure symptoms due to bradycardia), but there are no positive indications for an EPS.2,4 However, supplementary information may be obtained from the EPS for patients with suspected syndromes but without an abnormal ECG. For SSS, the EPS is useful for measuring sinoatrial conduction time with atrial stimulation, and for assessment of sinus node automaticity with the overdrive suppression test.139,140 The bundle of His potential is recorded for assessing AV block and intraventricular conduction disturbance. If the His–ventricular (HV) block is confirmed by abnormal prolongation of the HV interval or conduction interruption, a pacemaker is indicated. Cases of HV interval prolongation with bifascicular block transition to 3rd-degree AV block at a rate of 2.3% per year. However, if the HV interval is >100 ms, 3rd-degree AV block appears within 22 months in 25% of cases.141–143 A pacemaker must be considered when an HV block appears with atropine or antiarrhythmic drug (procainamide, etc.) loading.144,145 However, there are no clear indices to determine whether sudden cardiac death occurs as a result of bradycardia.
4.2 Tachyarrhythmia
4.2.1 Secondary Prevention of Ventricular Arrhythmias With Organic Heart Disease
Reentrant-sustained monomorphic VT can be induced at a high rate (>80%) using 1–3 programmed consecutive premature ventricular stimuli, and this technique has a high diagnostic value.132,146 When monomorphic VT is induced, it means there is a relatively stable reentrant substrate within the ventricle,147–151 and treatment such as ablation is expected to be effective. The reproducibility of tachycardia induction with an EPS is reduced in cases of multiple VT morphologies, polymorphic VT, and VF. Currently, the EPS is rarely used to determine preventative therapeutic effects due to the limited reliability of what is known as EPS-guided treatment to determine the preventative effect of treatment based on the inhibitory effect on tachycardia induction.2,152 The arrhythmogenicity (effect of inducing different tachycardias) of antiarrhythmic drugs can be assessed by VT/VF induction with an EPS, but generally, there is little need for this procedure in secondary prevention cases where an ICD are indicated.
4.2.2 Primary Prevention of Ventricular Arrhythmias With Organic Heart Disease
The ICD has been demonstrated to improve prognosis in cases of reduced left ventricle ejection fraction (LVEF) after myocardial infarction (LVEF ≤35–40%).153,154 In the MADIT study, non-sustained VT and procainamide being ineffective for VT induced by an EPS were added as conditions. However, a subanalysis of the MADIT-II study showed that induction of ventricular arrhythmia by EPS was not associated with subsequent proper operation of an ICD, indicating the validity of stratification based on reduced LEVF alone. On the other hand, the MUSTT study showed that prognosis was improved with an ICD in patients with coronary artery disease, reduced LVEF (≤40%), and confirmed NSVT, where VT/VF was induced with an EPS. The utility of stratification with an EPS has also been suggested.155
PVC and non-sustained VT are found at a high rate in idiopathic dilated cardiomyopathy (DCM), but the VT/VF induction rate with an EPS is low, and the utility of this technique is also low.156–158 In the SCD-HeFT study, ICD therapy was superior to placebo and amiodarone in improving prognosis in patients with reduced LVEF (≤35%) and symptoms corresponding to the New York Heart Association (NYHA) Functional Classification II–III.159 Based on these results, it is assumed that VT/VF is involved in the sudden death of patients with reduced LVEF, but there is little significance in inducing VT/VF with an EPS. VT/VF is known as a cause of sudden death in HCM against a background of complicated abnormal myocardial tissue,160 and induction of non-specific VT/VF is highly likely with an EPS. Thus, there are discrepant opinions on the significance of the study for inducibility.156–158 It has been reported that the assessment of VT/VF inducibility by EPS is useful for stratification in ARVC and arrhythmogenic cardiomyopathy (ACM).161,162
4.2.3 WPW Syndrome and Ventricular Pre-Excitation Syndrome
In Wolff-Parkinson-White (WPW) syndrome, there is a risk of sudden death due to VF via tachycardia with frequent ventricular responses at the onset of AF, known as pseudo-VT. Factors making WPW syndrome prone to VF include a short antegrade effective refractory period of the Kent bundle (<250–270 ms) or short minimum RR interval during AF (<250 ms) and the existence of multiple accessory conduction pathways. This condition can be assessed with an EPS. However, currently, when it is generally treated with ablation, there is little significance in independently conducting an EPS only.163–166
4.2.4 Inherited Arrhythmia
There are reports of abnormal prolongation of the intracardiac monophasic action potential through adrenaline loading in LQTS. However, TdP is rarely induced by programmed stimulation, and this technique has little utility for prognosis prediction.167 Short-coupled early ventricular stimulation is likely to induce polymorphic VT, but it is not related to prognosis.168
The reproducibility of tachycardia induction by programmed stimulation is poor, even in CPVT, and assessment with an EPS is not recommended.8
VT/VF is likely to be induced by extraventricular stimulation of the left or right ventricular outflow tract in Brugada syndrome,169 and inducibility is reported to be high in patients with a history of VT/VF or genetic mutation of SCN5A.170–172 Although there are reports of abnormalities such as fractionated potential delayed on the right ventricular epicardium side, biphasic waves, and low voltage,173,174 there are discrepant opinions as to whether VT/VF inducibility by an EPS is useful for risk stratification.8,170,175–178
Early repolarization syndrome (ERS) is the general term for ECG abnormalities showing elevation of the terminal part of the QRS wave and is included in what is known as J-wave syndrome. ERS is also recorded at a high rate in healthy individuals and athletes, which has little clinical significance when the condition is asymptomatic.
5. Types and Indications for Mapping Analysis
5.1 Concept
Each 3D mapping system has its own characteristics. Advances in technology and multielectrode catheters have made it possible to efficiently acquire a large amount of action potential information in a short period of time. It is also possible to identify reentrant tachycardia circuits and the site of earliest activation. Visualizing the tachycardia circuit, highly accurate display of electrode catheter positions, as well as marking (tagging) abnormal action potential and ablation sites shorten the procedure time and contribute to reduced radiation exposure.2,179–183 In particular, as this technology can also be integrated with CT, MRI, and intracardiac echocardiography, the contact force of the catheter tip can be displayed in real time. As a result, the risks of over-cauterization, ineffective energization, and cardiac injury can be reduced, which improves safety and efficacy, making these systems indispensable for diagnosis and treatment.
5.2 Types
The first 3D mapping system (CARTO®) was introduced to Japan in 2000, and there are currently 3 types of mapping system available for use (Table 6).
Table 6.
Main Features of Current Mapping Systems
| |
CARTO® System
(Johnson & Johnson) |
EnSite NavXTM System
(Abbott) |
RHYTHMIATM System
(Boston Scientific) |
| Features |
The vector of the ablation catheter and strength
of contact with the tissue are displayed in 3D in
real time, making the system more efficient.
Integrating the system with intracardiac
echocardiography and fluoroscopic imaging can
further reduce radiation exposure |
Electrode patches are attached to the body
surface. Multiple electrode catheters can
be displayed in real time, and mapping
can be performed quickly with all electrode
catheters. There is no limit to the number
of capture points |
An IntellaMap OrionTM catheter
with multiple electrodes is used
for mapping. A large number of
points can be acquired with 1
heartbeat |
5.2.1 CARTO® System (Johnson & Johnson)
Information on anatomical position is acquired by electrophysiological information and a magnetic sensor in the catheter, depicting highly accurate 3D images. It is mainly be used to diagnose excitation patterns of arrhythmia, visualize areas of low action potential and arrhythmia substrate, as well as displaying information on anatomical position and ablation. 3D geometry can be created with intracardiac echocardiography (CARTOSOUND®
function) and the vector of the ablation catheter is displayed in 3D, so effective energization can be achieved. The CARTOUNIVU®
function captures the required fluoroscopic information on a single screen, enabling efficient operation with further reduction of radiation exposure.
5.2.2 EnSite NavXTM System (Abbott)
Three pairs of electrodes are attached to the surface of the body, and a minute current is generated by the electrodes to create an impedance field around the heart. The spatial position is determined by measuring the voltage attenuation of the catheter electrode in the heart chamber to display the catheter on the screen. Multiple electrode catheters can be displayed in real time, irrespective of the catheter type or manufacturer, and rapid mapping is possible with all electrode catheters. The latest EnSite X EP system was approved in Japan in June 2021. The NavX mode of this system supports all electrode catheters based on conventional impedance, and the Voxel mode visualizes accurate positional information and anatomy based on the magnetic field.
5.2.3 RHYTHMIATM System (Boston Scientific)
This system can automatically acquire multiple points for only those heartbeats that satisfy certain recording conditions, as well as accurately and rapidly obtaining multipoint maps.184 An IntellaMap OrionTM
mapping catheter with a total of 64 electrodes equipped with a magnetic sensor is used for mapping.
5.2.4 ExTRa Mapping System (Nihon Kohden)
ExTRa Mapping is an online real-time arrhythmia imaging system created in Japan.185 It expresses the electrical activity of the myocardium with a 2D color map from excitation waves based on bipolar induction recorded with a spiral electrode catheter (20 electrodes). The system can visualize complex excitatory dynamics such as AF in real time.
5.3 Indications
The Class of Recommendation and Level of Evidence for the diagnosis of arrhythmia with a mapping system are shown in Table 7. In terms of patient background, mapping systems are useful for children and pregnant women,186–190 and are effective for all diseases from the perspective of reducing radiation exposure from fluoroscopy.
Table 7.
Class of Recommendation and Level of Evidence Related to Arrhythmia Diagnosis With Mapping Systems
| |
COR |
LOE |
Mapping systems are used in children or pregnant women with tachyarrhythmia to reduce radiation
exposure due to fluoroscopy |
I |
A |
The use of mapping systems is considered for the diagnosis of arrhythmia after surgery for
congenital heart disease (AT, AF, VT) |
IIa |
B |
The use of mapping systems is considered for the identification of AF not originating from the
pulmonary vein and assessment of abnormal potentials such as areas of low potential and CFAE |
IIa |
B |
The use of mapping systems is considered for mapping the endocardium and epicardial side in
VT with structural heart disease |
IIa |
B |
The use of mapping systems is considered for mapping PVC or VT originating from the outflow
tract, papillary muscle, or close to the left main coronary trunk, as well as verapamil-sensitive
idiopathic VT, bundle branch reentrant tachycardia, and PVC originating from peripheral Purkinje
fibers that trigger VF |
IIa |
B |
The use of mapping systems may be considered for the diagnosis of atrioventricular nodal
reentrant tachycardia and WPW syndrome, with accessory conduction pathways close to the
bundle of His, in the interventricular septum, or in the right ventricular free wall and for AT |
IIb |
C |
AF, atrial fibrillation; AT, atrial tachycardia; CFAE, complex fractionated atrial electrogram; COR, Class of Recommendation; LOE, Level of Evidence; PVC, premature ventricular contraction; VF, ventricular fibrillation; VT, ventricular tachycardia; WPW, Wolff-Parkinson-White.
Mapping systems are indispensable for advances in AF treatment and are indicated for the identification of the origin of AF other than in the pulmonary vein and additional cauterization in procedures such as line ablation and complex fractionated atrial electrogram (CFAE).180,182,191–196 Not only can mapping systems record local abnormal potentials and areas of low potential for tachycardia after surgery for congenital heart disease with complex anatomy,197–203 and VT with scarring,204–208 but these systems can also make a significant difference by visualizing complex reentry circuits. They are also useful for mapping the endocardium and epicardium for VT, which is difficult to map and diagnose during tachycardia,209–211 and mapping abnormal potential, including the epicardial side in diseases with fatal ventricular arrhythmia such as Brugada syndrome.173,212–215 and arrhythmogenic right ventricular cardiomyopathy/arrhythmogenic cardiomyopathy216 In addition, mapping systems are also useful for PVCs or VT wherein ascertaining structural elements such as the coronary arteries, aortic valve, and papillary muscle is useful for ablation,217,218 verapamil-sensitive idiopathic left VT with specific ablation sites,219–223 and bundle branch reentrant tachycardia.224,225
Using the mapping systems for AV nodal reentrant tachycardia (AVNRT) and WPW syndrome with accessory conduction pathways close to the bundle of His226–229 can avoid AV block with ablation. The systems are also useful in cases of unstable catheter fixation and right-sided accessory conduction pathways with a high rate of recurrence.230
6. Indications and Characteristics of Implantable Loop Recorder
6.1 Concept
ILRs, also called implantable cardiac monitors, are ECG devices placed under the skin to detect paroxysmal arrhythmia that is difficult to detect with either a standard ECG or long-term ECG recording. In Japan, ILRs are covered by insurance for 2 uses: detection of AF in syncope of unknown origin and in cryptogenic stroke. At the onset of symptoms, the patient or another person records the event, which stores an ECG covering a few minutes before the event. An ECG is also automatically stored when preset heartbeat abnormalities occur, such as bradycardia, cardiac arrest, tachycardia, and AF. Remote monitoring is also possible, making the ILR a useful medical device for identifying the causative disease. ILRs currently available have 2 electrodes on the main ILR, and the subcutaneous ECG is recorded from the potential between the 2 electrodes. ILRs have a battery life of 3–5 years, and are very compact, with a volume of ≤2 cm.3 ILRs are compatible with MRI and manual recording is also possible. The Class of Recommendation and Level of Evidence for the diagnosis of arrhythmia with ILRs are shown in Table 8.
Table 8.
Class of Recommendations and Level of Evidence Related to Arrhythmia Diagnosis With an Implantable Loop Recorder
| |
COR |
LOE |
ILR is used for the early assessment of patients with irregular or rare episodes of recurrent
syncope of unknown origin with no clinical signs* of suspected cardiogenic syncope, but non-
cardiogenic syncope such as reflex syncope or orthostatic hypotension have been ruled out |
I |
B |
ILR is used in patients with clinical signs* of suspected cardiogenic syncope, but comprehensive
evaluation cannot identify the cause of the syncope or specific treatment cannot be determined |
I |
B |
ILR is used to detect AF as the cause when long-term ECGs, including Holter ECGs, have failed
to identify the cause in patients diagnosed with cryptogenic stroke |
I |
B |
The use of ILRs is considered for the assessment in patients with suspected reflex syncope with
frequent recurrence or a history of syncope associated with trauma when pacemaker treatment is
considered for bradycardia |
IIa |
C |
*Refer to Chapter II.5 Table 37. AF, atrial fibrillation; COR, Class of Recommendation; ECG, electrocardiogram; ILR, implantable loop recorder; LOE, Level of Evidence.
6.2 Syncope of Unknown Origin
6.2.1 Incidence of Syncope of Unknown Origin
There is no disease classification for the diagnosis of “syncope” in Japan, so it is difficult to ascertain the accurate number of patients. However, a report that examined patients transported by ambulance to university hospitals within the Tokyo metropolitan area found that 13% of patients had the main complaint of transient impairment of consciousness, 79% of which were syncope.231 Thus, syncope is encountered frequently in routine medical care.
6.2.2 Significance of Cardiogenic Syncope
The cause of syncope varies but can be broadly divided into 3 categories: (1) orthostatic hypotension, (2) reflex, and (3) cardiogenic. The prognosis of cardiogenic syncope is the worst. People who have had cardiogenic syncope have approximately twice the hazard ratio of death and more than twice the number of cardiovascular events compared with those who have never experienced syncope.232,233 Thus, finding the cause at an early stage is extremely important.
6.2.3 Usefulness of the ILR for the Diagnosis of Syncope
Diagnosis of syncope can be very difficult: it has been reported that 47% of cases remain undiagnosed even after various tests.234 Therefore, an ILR should be considered at an early stage if the cause remains unknown despite various cardiovascular tests and reflex syncope tests being conducted for patients with high-risk findings suspicious of cardiogenic syncope, or even low-risk patients with frequent syncopal episodes.
6.2.4 Usefulness of the ILR in Patients With a Definitive Diagnosis of Reflex Syncope
The efficacy of pacemaker implantation after using the ILR in patients with syncope associated with asystole of ≥3 s, or asystole lasting ≥6 s without syncope has been reported (Figure 3).235 An ILR is useful when considering pacemaker treatment for patients with reflex syncope and has been shown to reduce the recurrence of syncope. The PICTURE study,236 a multicenter, prospective observational study involving 71 medical facilities (11 countries), is the basis of this finding. That study examined 570 patients without a definitive diagnosis despite undergoing an average of 13 different types of tests in 3 different clinical departments prior to ILR implantation. Syncope recurred in 218 patients (36%) within 12 months, and the cause was diagnosed with the ILR in 170 (78%) of the patients. The study conducted the tilt test and cardiac EPS in 66% of cases, and implanted an ILR at an early stage of examination to find the cause in 22% of patients, but there was no difference in the subsequent diagnosis rate of the cause of syncope. The study reported that prioritizing an ILR over various other tests, and implanting it at an early stage would contribute to reducing medical costs.

Krahn et al divided 60 patients with loss of consciousness of unknown cause into an ILR and conventional testing (external loop recorder + tilt test + EPS) group and examined the subsequent diagnosis rate after 12 months. It was 52% and 20% for the ILR and conventional testing (P=0.012), respectively, indicating the overwhelming superiority of the ILR, and 90% of the syncope was caused by bradycardia.237 A study with a larger number of patients (n=201) found the diagnostic usefulness of the ILR was significantly high, with a hazard ratio of 6.53.238 A meta-analysis showed similar findings,239 suggesting that an ILR should be considered in the early stage of frequent syncope of unknown origin. Similarly, in Japan, a study reported a high diagnostic rate of 56% using ILRs, despite being a single facility study.240
6.3 Indications for Cryptogenic Stroke
The ILR is also useful for detecting AF in cryptogenic stroke. The CRYSTAL AF study reported that AF was detected in 12.4% of the ILR group after 12 months, but in only 2% in the conventional testing group.241 In response to these findings, using an ILR for the detection of AF in cryptogenic stroke is now covered by insurance in Japan. The usefulness of ILRs has also been reported by multicenter studies in Japan.242,243 Furthermore, the ILR has also been reported as useful in patients with sinus rhythm who are at high risk of cerebral infarction.244 Thus, the ILR is also expected to be useful as a screening tool for detecting AF (see Chapter II.6).
6.4 Indications for a Fatal Arrhythmia
In the CARISMA study, an ILR was implanted in 312 patients with acute myocardial infarction and LVEF ≤40%, and it was reported that 8% of the patients developed VT or VF over a 2-year period.245 Therefore, an ILR may also be useful for risk stratification for patients who are asymptomatic but at high risk of fatal arrhythmias, such as organic heart disease.
7. Types and Characteristics of Predictive Indices for Sudden Cardiac Death
Many cases of sudden cardiac death are caused by fatal arrhythmias such as AF and VT. The presence of negative factors, such as cardiac dysfunction, genetic abnormalities, depolarization or repolarization abnormalities, and abnormalities in autonomic nervous system activity, form the background to the development of these types of arrhythmias (Figure 4). To prevent sudden cardiac death, it is important to detect these abnormal factors beforehand and to treat affected patients in advance. Test markers that detect abnormal factors are called predictive indices. However, no universal index has yet been defined. Not only the characteristics, but also the examination procedures, measurements, and applicable diseases all differ slightly in each individual, and, depending on the patient or disease, some factors cannot be assessed. Therefore, it is essential to understand and utilize these factors.
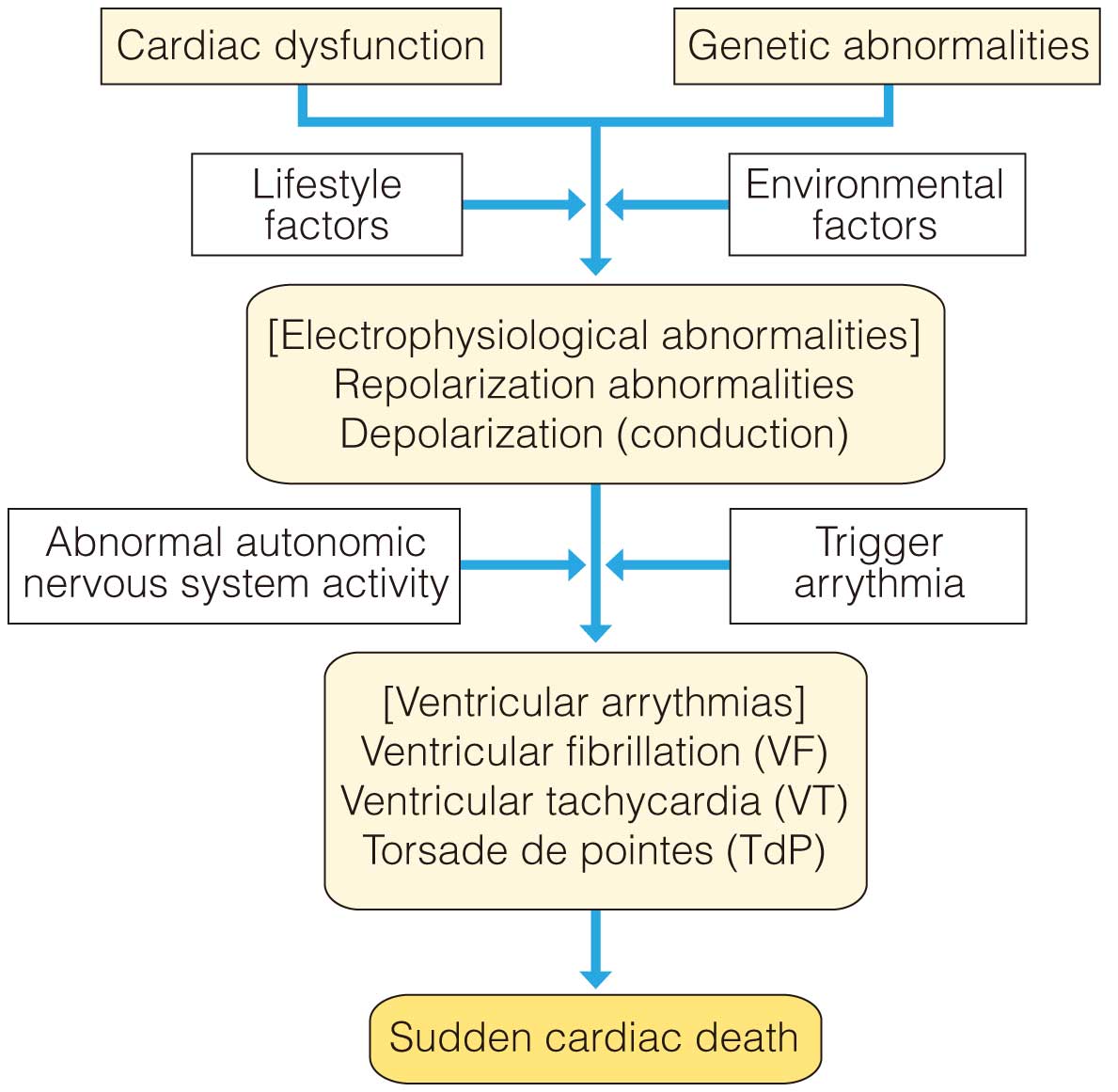
Typical tests to detect abnormal factors include LVEF measured by diagnostic imaging such as echocardiography, and inducibility of ventricular arrhythmia by cardiac EPS. However, although the indices of cardiac electrophysiology tests are measured non-invasively and are also used clinically, they are not as effective as LVEF. Indices include ventricular LP,246,247 TWA,248 T-wave variability (TWV),247,249 QT/RR slope, HRV index, HRT,250 and deceleration capacity (DC)251 (Table 9). All the predictive indices shown in this guideline can be measured using ECG devices sold in Japan. The Class of Recommendation and Level of Evidence for risk assessment of cardiac events are shown in Table 10.
Table 9.
Indices Used to Predict Sudden Cardiac Death
| Functional structure |
Electrophysiology |
Modifying factors |
| Indices of cardiac dysfunction |
Indices of direct triggers |
Indices of abnormal autonomic
nervous system activity |
• LVEF
• NYHA cardiac function
• BNP |
• Serious PVC
• Non-sustained VT (NSVT) |
• HRV
• HRT
• BRS
• DC |
| Indices of repolarization abnormalities |
• TWA
• TWV
• QT interval index |
Indices of depolarization
(conduction) abnormalities |
• Wide QRS complex
• Ventricular LP
• Inducbility with EPS |
BNP, B-type natriuretic peptide; BRS, baroreceptor sensitivity; DC, deceleration capacity; EPS, electrophysiological study; HRT, heart rate turbulence; HRV, heart rate variability; LP, late potential; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; PVC, premature ventricular contraction; TWA, T-wave alternans; TWV, T-wave variability; VT, ventricular tachycardia.
Table 10.
Class of Recommendation and Level of Evidence for Risk Assessment of Cardiac Events
| |
COR |
LOE |
| LVEF |
Reduction in LVEF (35–40% reduction) is used for risk assessment of cardiac events after
myocardial infarction or heart failure patients |
I |
A |
| NSVT |
NSVT is considered for risk assessment of cardiac events after myocardial infarction, as well as
in patients with DCM and HCM |
IIa |
B |
| EPS |
Using EPS in combination with NSVT is considered for risk assessment in patients after myocardial
infarction with a reduction in LVEF (35–40% reduction) |
IIa |
B |
| Indices that reflect depolarization/repolarization abnormalities |
Use for risk assessment in combination with indices of non-invasive cardiac electrophysiology
study, such as ventricular LP and TWA, is considered after myocardial infarction or in patients
with non-ischemic cardiomyopathies |
IIa |
A |
Using any of the indices of non-invasive cardiac EPS, such as ventricular LP and TWA, in
combination with LVEF for risk assessment is considered after myocardial infarction or in patients
with non-ischemic cardiomyopathies |
IIa |
A |
| Detection of ventricular LP is considered for diagnosis in patients with suspected ARVC/ACM |
IIa |
B |
Detection of ventricular LP is considered for diagnosis in patients with suspected Brugada
syndrome |
IIa |
B |
| Indices that reflect abnormal autonomic nervous system activity |
Use of HRT in combination with TWA or NSVT for risk assessment is considered after myocardial
infarction or in patients with non-ischemic cardiomyopathies |
IIa |
B |
Use of HRT, HRV, and indices such as standard deviation of the normal-to-normal interval (SDNN),
BRS, and DC may be considered for risk assessment of patients after myocardial infarction |
IIb |
B |
ACM, arrhythmogenic cardiomyopathy; ARVC, arrhythmogenic right ventricular cardiomyopathy; BRS, baroreceptor sensitivity; COR, Class of Recommendation; DC, deceleration capacity; DCM, dilated cardiomyopathy; EPS, electrophysiological study; HCM, hypertrophic cardiomyopathy; HRT, heart rate turbulence; HRV, heart rate variability; LOE, Level of Evidence; LP, late potential; LVEF, left ventricle ejection fraction; NSVT, non-sustained ventricular tachycardia; TWA, T-wave alternans.
7.1 Left Ventricular Ejection Fraction (LVEF)
Although several indices reflect a decline in cardiac function, a reduction in LVEF measured by various diagnostic imaging devices is the gold standard for prognostic indices. It is currently the index with the highest Level of Evidence in patients with organic heart disease. A reduction in LVEF is often defined as LVEF ≤35% or 40%.
The usefulness of LVEF as a predictive index was established by the MADIT-II study, which evaluated patients with a low cardiac function of LVEF ≤30% after myocardial infarction,154 and by the SCD-HeFT study, which evaluated patients with heart failure and LVEF ≤35%159 in Europe and the USA. These studies evaluated the indication of ICD as primary prevention to prevent arrhythmia death in patients who had never had a dangerous arrhythmia. In Japan, the usefulness of LVEF reduction as an indicator is evaluated in patients after myocardial infarction. It has been shown that there are many fatal ventricular arrhythmia events in patients with reduced LVEF (≤40%), although the cutoff value differs from that used in Europe and the USA.252 Decreasing the cutoff value of LVEF increases the specificity for sudden cardiac death but decreases the sensitivity. A cutoff value of 35–40% for LVEF reduction is considered appropriate based on the results of the clinical trials.
7.2 Non-Sustained Ventricular Tachycardia (NSVT)
PVC and NSVT assessed by Holter ECG are arrhythmias that trigger fatal VF and sustained VT, which have long been used as predictors of fatal arrhythmias or sudden cardiac death. Although PVCs are considered a risk when they are frequent or polymorphic, the Level of Evidence of NSVT as a predictive indicator is higher than that of PVCs.253
NSVT is generally defined as a series of ≥3 consecutive PVCs, with a cycle ≥100 beats/min.4,254,255 However, as evaluated in the MERLIN-TIMI 36 study, it is better to use a stricter definition (4–7 consecutive PVCs, or even ≥8 consecutive PVCs).254 The usefulness of NSVT has been established in patients after myocardial infarction, as well as those with DCM or HCM.4,188,255 NSVT remains the most reliable index for HCM patients.133
7.3 Cardiac Electrophysiological Studies
Programmed stimulation with a cardiac EPS is the only invasive test for the prediction of sudden cardiac death. The EPS is an invasive test; thus, it is a secondary test to pinpoint high-risk patients rather than a screening tool.
The usefulness of the EPS as a predictive index was demonstrated by the MUSTT study,256 which also validated the utility of ICDs as primary prevention in post-myocardial infarction patients with LVEF ≤40%, NSVT, and EPS-induced ventricular arrhythmia (sustained VT or VF). As a result, the study demonstrated the validity of EPS-guided decisions on indicating ICD therapy; that is, EPS was shown to be useful as a predictive index. A subanalysis of this study also showed that patients with EPS-induced ventricular arrhythmias had a higher incidence of fatal arrhythmic events than those without.
However, some clinical trials have shown the limitations of EPS as a predictive index. A subanalysis of the MADIT-II study showed that EPS-induced ventricular arrhythmia was not associated with subsequent proper ICD operation (spontaneous onset of ventricular arrhythmia).257 EPS-induced VF has also been utilized to identify high-risk patients with Brugada syndrome, but the usefulness is inconsistent,170,176,258 because the predictive accuracy changes according to the protocol for the induction test, such as setting the number of early stimulations, the stimulation interval, and so on.259 However, risk assessment is often performed with an EPS in patients who present with a typical ECG waveform (type 1) and have a history of syncope of unknown origin.258
7.4 Signal-Averaged Electrocardiogram (SAECG)
The SAECG detects minute potentials that are buried in noise in normal body surface ECGs by removing noise via adding and averaging the ECG signals. Ventricular LP is a typical micropotential recorded by the SAECG and is found in the terminal part of the QRS complex (Figure 5). Ventricular LP is associated with delay or non-uniformity of excitation conduction due to local ventricular disorders and reflects depolarization (conduction) abnormalities.
SAECG recordings conform to the Simson method,260 in which the leads are a vector magnitude waveform obtained by finding the root mean square of the X, Y, and Z leads, with a noise level ≤0.3 μV and R-wave synchronization; 200–250 heartbeats are used for the calculation, and a 40–250 Hz band pass filter is generally used for the recording.
Three indices are used to determine ventricular LP: (1) fQRS, (2) RMS40, and (3) LAS40. fQRS is the filtered QRS duration, RMS40 is the root mean square of the potential recorded at the terminal 40 ms in the QRS, and LAS40 is the duration of low-amplitude signals <40 μV in the terminal QRS. Ventricular LP is diagnosed as positive when more than 2 of the 3 indices satisfy the positive criteria: (1) fQRS >114 ms, (2) RMS40 <20 μV, and (3) LAS40 >38 ms.261 However, it should be noted that this standard is proposed as a risk assessment for myocardial infarction and the criteria differ depending on the model.
Although the association of ventricular LP with VT and prognosis after myocardial infarction has been indicated previously,262,263 the limited utility of this index has been shown in several clinical trials since the active use of reperfusion therapy for acute myocardial infarction in recent years.264,265 On the other hand, there are reports on its utility for predicting sudden cardiac death and fatal arrhythmia in Brugada syndrome;266,267 however, despite its widespread use, it has not been established as evidence. The conduction delay on the right ventricular epicardial side has been indicated as the mechanism that enables the detection of ventricular LP in Brugada syndrome.174,267 Although ventricular LP is useful for predicting fatal arrhythmia in DCM,268 its usefulness has not been established. Furthermore, as ventricular LP is frequently observed in ARVC/ACM, it is included in the minor diagnostic criteria.269 However, its usefulness in predicting sudden cardiac death and fatal arrhythmia has not been clarified.
In patients with organic heart disease such as myocardial infarction, the use of ventricular LP in combination with other non-invasive ECG indices, rather than ventricular LP alone, improves the predictive accuracy of cardiac death and arrhythmic death. A multicenter prospective study (JANIES) in Japan, and previous studies that added ventricular LP to the evaluation, reported that using ventricular LP in combination with other non-invasive ECG indices increased the usefulness in patients with organic heart disease.270,271 A combination of non-invasive ECG indices and ventricular LP could also be useful for patients with organic heart disease such as myocardial infarction.133
7.5 T-Wave Alternans (TWA)
TWA is the phenomenon of a beat-to-beat alternation of the shape of the T-waves, which reflects abnormal repolarization of the ventricles,272 and is known to be detected in conditions such as acute myocardial ischemia, electrolyte imbalance, severe heart failure, hypothermia, pericardial effusion, LQTS, and Brugada syndrome.
A large number of prospective studies of microvolt TWA (M-TWA) obtained by spectral analysis of ECGs recorded during exercise in patients with ischemic heart disease and non-ischemic cardiomyopathy have been published, and the usefulness of M-TWA has also been shown in a meta-analysis.252,264,271,273–275 The ABCD study reported in 2009 found that M-TWA was useful in predicting arrhythmic events and sudden death in patients with reduced LVEF after myocardial infarction (EF ≤40%).276 However, the MASTER study evaluated M-TWA alone in patients with reduced LVEF after myocardial infarction and found that although it was useful for predicting cardiac death, M-TWA was not useful for predicting arrhythmia events.277 This result ruled out the usefulness of M-TWA alone in predicting fatal arrhythmias.
TWA is characterized by a low positive predictive value and an extremely high negative predictive value in predicting fatal arrhythmia.278 Therefore, TWA is not an index for identifying high-risk patients based on a positive judgment, but rather an index for selecting low-risk patients based on a negative judgment. A meta-analysis of several studies evaluated TWA separately as negative and non-negative (positive or indeterminate), which could be considered an evaluation of the significance of TWA negativity.279 A study in Japan (PREVENT-SCD) was also conducted as a prospective evaluation of the utility of TWA limited to patients with reduced LVEF but showed a high negative predictive value for fatal arrhythmia.280
The utility of TWA for predicting cardiac events (fatal arrhythmia, sudden cardiac death, and cardiac death) increases when used in combination with other non-invasive ECG indices.271 In recent years, combined evaluation of TWA and HRT using the M-TWA or modified moving average (MMA)-TWA, described below, is useful for predicting cardiac death and fatal arrhythmia in patients after myocardial infarction.281,282 A meta-analysis of studies in patients with structural heart disease, including many ischemic heart diseases, evaluated predictive factors for sudden cardiac death and listed LVEF, presence of coronary artery disease, and M-TWA positivity as significant predictors of sudden cardiac death. Evaluation using the combination of these factors has higher prognostic predictive accuracy than using LVEF alone.283 Thus, TWA could be considered a predictive index that can be used not only alone but in combination with other non-invasive indicators in patients with structural heart disease.133
There have been several evaluations of TWA in patients with non-ischemic heart disease, but its utility has been inconsistent. The utility of M-TWA for predicting cardiac death and arrhythmia events was reported in the ALPHA study, which had a relatively large number of cases,284 but that finding was negated in other small-scale studies. The usefulness of TWA has also been evaluated for HCM and hereditary arrhythmia diseases (ARVC/ACM, LQTS, and Brugada syndrome), but the evidence is insufficient because all of the studies were small-scale.
Measurement methods have recently been developed to detect TWA continuously for 24 h using time-domain analysis of Holter ECG waveforms, called the MMA method, or using frequency domain/spectral analysis (FD-TWA) similar to M-TWA. These methods have already been used in clinical practice,285,286 but there is as yet insufficient evidence of their usefulness.
7.6 Other Indices of Repolarization Abnormalities
7.6.1 T-Wave Variability (TWV)
TWV is an index that reflects the variability of T-waves and evaluates the variability of repolarization. The aforementioned TWA evaluates the fluctuation of alternate T-waves, and the TWV/T-wave amplitude variance (TAV) is a method of analyzing changes in the shape, amplitude, and polarity of the T-wave. A substudy of the MADIT-II study found that TAV >59 μV was a predictor of arrhythmia events (hazard ratio 2.01, 95% confidence interval 1.2–3.5) in patients with an ICD for myocardial infarction with LVEF ≤30%.249 There are also reports on the utility of TWV for predicting arrhythmia events in DCM,287 and for risk stratification of all-cause death in Chagas disease.288 TWA (T-var), which considers the vibration of T-waves, is more useful for predicting arrhythmia events.289
7.6.2 QT Measurement Index
As reported in recent guidelines and statements, the utility of QT interval indices, such as QT dispersion, in assessing the risk of sudden cardiac death and fatal arrhythmias in patients with heart disease has been negated.133 However, QT/RR-related indices, such as the QT/RR slope, have been reported as useful for predicting cardiac death. The QT/RR relationship is analyzed using the Holter ECG and calculated using a regression equation from a scatter plot obtained by plotting the RR interval on the horizontal axis and the QT interval on the vertical axis. The larger the value of the QT/RR slope, the greater the risk of sudden death and all-cause death. The cutoff values used for the index are QTe/RR slope >0.18 in patients with myocardial infarction, as well as QTe/RR slope >0.20 and QTe/RR slope >0.22 in patients with heart failure.290,291
7.7 Indices of Autonomic Nervous System Abnormalities
7.7.1 HRV Indices
Periodic fluctuation of the heart rate due to fluctuations in the autonomic nervous system is called heart rate fluctuation, and a reduction in the fluctuation reflects a decline in autonomic nervous system function. In practice, the HRV is evaluated by analyzing the RR interval during sinus rhythm in long-term ECG recordings such as Holter ECG. Analysis is classified into linear and non-linear. Indices of linear analysis include time-domain indices, such as standard deviation of the normal-to-normal interval (SDNN), and frequency analysis indices, such as low frequency (LF), high frequency (HF), LF/HF, and others. Indices of non-linear analysis indices include the Poincaré plot and fractal dimension (α1, β).
The findings of certain time-domain analyses have long been suggested to be associated with sudden death after myocardial infarction, and these findings have been evaluated the most extensively.292 A prospective observational study (ATRAMI) of patients after myocardial infarction showed a higher relative risk of cardiac death in the group with SDNN ≤70 ms.293
In frequency domain analysis, LF, HF, and the LF/HF ratio have been used to assess the balance of the sympathetic nervous system in a variety of disorders, including sleep apnea,294 heart failure,295 and hypertension.296
Non-linear analysis has shown that Poincaré plot analysis is useful for risk assessment of patients with DCM.297 A substudy of the DIAMOND-MI study revealed that a short-term fractal scaling exponent α1 of <0.75, calculated using the detrended fluctuation analysis method, increased the relative risk of death in patients after acute myocardial infarction. It has been shown to be more strongly associated with the outcome than indices of conventional time-domain and frequency-domain analyses.298 Another study of patients with acute myocardial infarction found that both α1<1.025 and β≤−1.507 were associated with the recurrence of non-fatal coronary events.299 However, results that negate the usefulness of HRV indices have recently been published. In particular, there may be limitations to the use of the LF component and LF/HF ratio, which are frequency domain analyses, to evaluate sympathetic nervous system function.300
7.7.2 HRT Indices
HRT assesses the RR interval after compensatory rest using a single PVC as the base point; that is, it is used to determine how the heart rate fluctuates. This is a heart rate response mediated by vagus nerve stimulation due to the carotid baroreceptor reflex in response to a transient reduction in blood pressure after a PVC and is an index that reflects parasympathetic nervous system function. The aforementioned HRV cannot be measured when PVCs occur frequently. Conversely, HRT is an index that is measured using PVCs.
HRT is measured using the Holter ECG and is evaluated based on turbulence onset (TO [%]), which is the amount of shortening of the RR interval after a compensatory rest period, followed by a subsequent RR interval prolongation of the velocity turbulence slope (TS [ms/RR]). The usefulness of HRT as a predictive index for cardiac events and prognosis was shown in the MPIP and EMIAT studies conducted in patients after myocardial infarction.301 Those studies defined TO and TS outliers as TO ≥0% and TS ≤2.5 ms/R-R. Multivariate analysis indicated that HRT was highly useful in predicting HRT-positive cardiac events and demonstrated the same prediction accuracy as LVEF reduction. There are 3 categories of HRT determination, which are defined as Category 0 when both TO and TS are normal, or if there are very few PVCs, Category 1 when one of either TO or TS is abnormal, and Category 2 when both TO and TS are abnormal. Category 2 is often used to determine a positive indication.
Several prospective studies and meta-analyses have reported the usefulness of HRT as a predictive index for cardiac death after myocardial infarction or heart failure.302,303 HRT is the most reliable index among predictive indices that reflect abnormal autonomic nervous system activity. In particular, there are many reports showing the usefulness of using HRT in combination with TWA to predict cardiac death,281,282,304,305 and a clinical study (REFINE-ICD) is currently in progress to verify the usefulness of this combination. There is also a report showing the effectiveness of using HRT in combination with NSVT to predict cardiac death and all-cause death in patients with LV dysfunction with reduced LVEF or relatively maintained LVEF.306 The multicenter prospective study (JANIES) in Japan also demonstrated the usefulness of this combination in predicting cardiac death.250 Therefore, using a combination of non-invasive ECG indices, including HRT, is considered useful in assessing the risk of cardiac death.133
7.7.3 Baroreceptor Sensitivity (BRS)
The baroreceptor reflexes of the aortic arch and carotid sinus activate the reflex of the parasympathetic nervous system in response to elevated blood pressure, resulting in decelerated heart rate. In the BRS test, phenylephrine, an α-receptor stimulant, is intravenously injected, and the degree of RR interval prolongation with respect to the degree of increase in systolic blood pressure is quantified to find the BRS value (ms/mmHg).307 The normal value is said to be within a small range between 8 and 15 ms/mmHg.
The ATRAMI study evaluated the association of BRS, HRV, arrhythmia on Holter ECG, and ventricular LP with prognosis in patients after myocardial infarction.293 As a result, BRS (<3 ms/mmHg), LVEF, and the number of PVCs were found to be predictors. The study showed that prognosis was poor in the group with confirmed NSVT, as well as BRS <3 or SDNN <70 ms, and the mortality rate was high in patients with LVEF ≤35% and BRS <3.308 However, clinical trials conducted in the modern era of optimal drug treatment have not provided any insight into the usefulness of BRS in predicting prognosis.309,310 There are also reports negating its usefulness as a predictive index of sudden cardiac death in patients with relatively preserved cardiac function.311 Thus, the use of this index is on the decline. In addition, as intravenous injection of phenylephrine is invasive, alternative assessment by non-invasive test methods is also being considered.312
7.7.4 Deceleration Capacity (DC)
The cardiac parasympathetic nervous system controls the beat-to-beat HRV and is known to exert a rapid effect. DC is an index used to assess the ability of the cardiac parasympathetic nervous system to control HRV by extracting only RR intervals longer than the immediately preceding RR interval from the fluctuations in RR intervals and averaging them.
A study of patients after myocardial infarction showed that DC ≤2.5 ms had a higher prognostic prediction accuracy than the HRV index (SDNN), and DC >4.5 ms indicated a lower incidence of cardiac events.313
However, a study of heart failure patients with relatively preserved cardiac function (LVEF >30%) found that DC was not useful for predicting sudden death and arrhythmic events.314
8. Methods and Characteristics of Genetic Testing for Arrhythmias
8.1 What Is Genetic Testing?
The clinical significance of genetic testing and diagnosis for arrhythmic diseases is increasing because of recent advances in genetic research and diagnosis. Unlike general clinical laboratory tests, genetic testing includes content that may affect different generations. Therefore, all processes, including pre-testing counseling, the accuracy of testing, and the interpretation of post-testing results, are important. Genetic testing should be conducted under the Guidelines for genetic testing and diagnosis in medical care315 published by the Japanese Association of Medical Sciences in 2011, and is recommended under the condition that genetic counseling and comprehensive clinical genetic medicine can be provided.
Before carrying out genetic testing, it is necessary to explain the advantages and disadvantages and to obtain informed consent from the patient or his/her family. In particular, if a familial inheritance is suspected, it should be considered that the result of genetic testing may affect the diagnosis of not only the patient but also the carrier among the family members. In general, genetic testing for the diagnosis of the carrier of diseases that usually develop after 20 years of age should be performed when he or she has reached adulthood and is able to make independent decisions. Thus, genetic testing for carrier diagnosis in children should only be conducted at the discretion of the patient’s legal representative, such as parents.315 However, this shall not apply to cases of diseases that are actionable by an early genetic diagnosis.
When the results of comprehensive genetic testing using a next-generation sequencer will apply to clinical practice, it is necessary to establish how to handle the secondary or incidental findings from the test and to explain these to the patients.
Approval of the institutional review board is required even if the tests are conducted as part of research.316 Table 11 shows the Class of Recommendation and Level of Evidence for genetic testing.
Table 11.
Class of Recommendation and Level of Evidence for Genetic Testing for Arrhythmia Patients
| |
COR |
LOE |
| LQTS |
Comprehensive or LQT1–3 (KCNQ1, KCNH2, and SCN5A) targeted LQTS genetic testing is
recommended for any patient in whom a cardiologist has established a strong clinical index of
suspicion for LQTS based on examination of the patient’s clinical history, family history, and
expressed electrocardiographic (resting 12-lead ECGs and/or provocative stress testing with
exercise or catecholamine infusion) phenotype |
I |
C |
Comprehensive or LQT1–3 (KCNQ1, KCNH2, and SCN5A) targeted LQTS genetic testing is
recommended for any asymptomatic patient with QT prolongation in the absence of other clinical
conditions that might prolong the QT interval (such as electrolyte abnormalities, hypertrophy,
bundle branch block, etc., i.e., otherwise idiopathic) on serial 12-lead ECGs defined as QTc
≥480 ms (prepuberty) or ≥500 ms (adults) |
I |
C |
Mutation-specific genetic testing is recommended for family members and other appropriate
relatives subsequently following the identification of the LQTS-causative mutation in an index case |
I |
C |
Comprehensive or LQT1–3 (KCNQ1, KCNH2, and SCN5A) targeted LQTS genetic testing may
be considered for any asymptomatic patient with otherwise idiopathic QTc values ≥460 ms
(prepuberty) or ≥480 ms (adults) on serial 12-lead ECGs |
IIb |
C |
| Cardiomyopathy |
Identification of lamin A/C gene mutations is conducted for DCM patients with familial conduction
disorders (not covered by insurance) |
I |
C |
| CPVT |
Genetic testing is conducted for patients with clinically diagnosed or suspected CPVT (not
covered by insurance) |
I |
C |
Screening for the gene mutation identified in the proband is considered for family members of
patients with CPVT (not covered by insurance) |
IIa |
C |
| Brugada syndrome |
Comprehensive or SCN5A-restricted genetic testing is considered for patients with type 1 ECG,
clinical symptoms, and family history associated with Brugada syndrome (not covered by
insurance) |
IIa |
C |
Genetic screening for the causative gene mutation of Brugada syndrome identified in the proband
is considered for family members (not covered by insurance) |
IIa |
C |
COR, Class of Recommendation; CPVT, catecholaminergic polymorphic ventricular tachycardia; DCM, dilated cardiomyopathy; ECG, electrocardiogram; LOE, Level of Evidence; LQTS, long QT syndrome.
8.2 Target Arrhythmias
Many arrhythmic diseases for which genetic testing is useful are fatal arrhythmias that can cause syncope or sudden cardiac death, such as congenital LQTS, CPVT, and Brugada syndrome.
Among the arrhythmic diseases, only genetic testing for congenital LQTS is covered by insurance in Japan. Genetic diagnosis for congenital LQTS is recommended when it is strongly suspected by cardiologists based on clinical findings, or when a 12-lead ECG shows significant QT prolongation (prepuberty: QTc ≥480 ms, adults: QTc ≥500 ms) even if the patient is asymptomatic, and for targeting family members with the causative gene mutation of LQTS identified in the proband.8,317
Genetic testing is recommended for patients diagnosed with or suspected CPVT based on clinical findings to confirm the diagnosis. It is also recommended to test the family members of the patient, targeting genetic abnormalities found in the patient.317 Although the SCN5A mutation is useful for risk stratification in Brugada syndrome,318 there is no evidence of a clinical indication for genetic testing in Brugada syndrome.
Genetic testing can also be useful for other channelopathies and cardiomyopathies, including acquired LQTS,319 PCCD,320 familial sinus bradycardia, SQTS, ARVC and DCM. In particular, lamin A/C cardiomyopathy causes sudden death and heart failure due to arrhythmia after middle age.321 Therefore, genetic testing has great clinical significance in early diagnosis of lamin-cardiomyopathy.322 Genetic testing rarely identifies pathogenic variants in patients with unexplained sudden cardiac death due to VF (idiopathic VF).
8.3 Testing Methods
Blood (whole blood) is generally used as the sample for genetic testing, and DNA is extracted from white blood cells. Blood collection tubes containing ethylenediaminetetraacetic acid (EDTA, 2Na or 2K) as an anticoagulant are used, but not heparin because it inhibits the polymerase chain reaction (PCR). In general, whole blood is stored at 4℃. In addition, DNA can be extracted from not only a blood sample but also cellular components contained in saliva.
The most reliable method of DNA sequence analysis (base sequence determination) is Sanger sequencing, which amplifies the gene of interest with PCR in the DNA region, and the base sequence inside the gene is determined using electrophoresis. However, the multiplex ligation-dependent probe amplification method is useful for the analysis of copy number variant, such as larger numbers of consecutive base pair deletions, and duplicate mutations.323
Panel analysis by next-generation sequencer focusing on the several causative genes of a specific disease group (arrhythmia, cardiomyopathy, etc.) is useful as comprehensive genetic testing, because it can simultaneously analyze several to hundreds of genes associated with inherited primary arrhythmic syndrome or cardiomyopathies. However, specialized knowledge and experience are required to interpret the results of this test. When the clinical laboratory department is unable to interpret the results alone, it is important to collaborate with other professionals, including the genetic medicine department, specialists for the diseases, and bioinformaticians, then compare the genetic results with clinical information to carefully interpret the results. When notifying patients of the test result, it is preferable to confirm the results using another test method (e.g., Sanger sequencing).
8.4 Interpretation of Results
The results of genetic testing need to be explained to patients and their families in an easily understood way. In addition, the disease diagnosis should be made based on a comprehensive evaluation of clinical medical information, not simply based on the results of genetic testing. In particular, to avoid causing anxiety in patients and families when explaining and disclosing the results, much care should be taken of findings such as new variants or variants of uncertain significance (VUS), or if the disorder does not show 100% penetrance, because these variants may not always be associated with the disease.
Even if the variant changes amino acids, it may not always be associated with functional dysfunction or only associated with very minor dysfunction. Therefore, it is necessary to check various databases and literature to confirm whether the variant is pathogenic, likely pathogenic, VUS, likely benign or benign. In particular, ethnic differences can be observed in many single-nucleotide polymorphisms identified in healthy individuals. Therefore, these must be confirmed in Japanese databases. It should also be noted that a negative result does not completely rule out several arrhythmic diseases and cardiomyopathies.
8.5 Handling Personal Information and Personal Genetic Information
Medical personnel who access genetic information are required to fully understand the characteristics of that information and to handle it appropriately. The results of the genetic testing conducted to diagnose a patient who has already developed symptoms must generally be explained in the patient’s medical records because the information is shared with medical personnel involved in the patient’s medical care, as with other clinical information.
9. Conditions and Settings for Exercise Tolerance in Arrhythmia Patients
Please refer to the Japanese Society of Pediatric Cardiology and Cardiac Surgery guidelines regarding the management of school activities for children with arrhythmia but without underlying organic heart disease.324 The present guideline provides information on strategies for adults. The intensity of exercise or physical activity is expressed in metabolic equivalents of task (METs).325,326 The MET standard is defined as 1 MET, which is equivalent to 1.0 kcal (4.184 kJ)/kg/h, based on the ratio of the working metabolic rate to the resting metabolic rate of a person.325,326 The intensity of physical activity is classified light (<3 METs), moderate (<6 METs), and vigorous (≥6 METs). Undertaking ≥30 min of moderate aerobic exercise for ≥2.5 h/week, ≥20 min of vigorous exercise for ≥75 min/week, or a combination of moderate and vigorous exercise corresponding to 500–1,000 METs/min weekly prevents cardiovascular diseases and provides health benefits, which can be expected to inhibit the onset of arrhythmia.327,328 On the other hand, a person who does not exercise regularly and suddenly starts to exercise, especially vigorous exercise, has a risk of experiencing adverse events, including arrhythmia.328,329
When considering exercise for arrhythmias, it must first be considered whether there is any underlying heart disease. There are patients with underlying diseases such as myocardial infarction, cardiomyopathy, heart failure, and a history of surgery for congenital heart disease in whom the arrhythmic substrate (structural and/or histological abnormalities in the myocardium) is the cause of the arrhythmia, and other patients in whom changes in cardiovascular dynamics associated with exercise trigger arrhythmia. When there is an underlying heart disease, exercise therapy can be expected to prevent deterioration or progression of the arrhythmic substrate by improving myocardial ischemia and suppressing the sympathetic nervous system. Table 12 shows the Class of Recommendation and Level of Evidence for exercise tolerance in patients with arrhythmia.
Table 12.
Class of Recommendation and Level of Evidence for Exercise Tolerance in Patients With Arrhythmia
| |
COR |
LOE |
| Exercise is permitted under appropriate monitoring in patients after ICD implantation |
I |
A |
Consider regular moderate or lower intensity exercise to improve exercise tolerance in patients
with AF |
IIa |
C |
Consider not restricting exercise in athletes with asymptomatic bradycardia or Wenckebach
2nd-degree AV block that improves with exercise |
IIa |
C |
Consider not restricting exercise in patients with monomorphic PVCs and in those with only 1 or
2 consecutive PVCs at rest or during exercise and if it can be confirmed that there is no organic
heart disease |
IIa |
C |
Consider risk assessment of cardiovascular death in patients with PVCs who have polymorphic
PVCs, increased polymorphic PVCs, or ≥3 consecutive PVCs during exercise or during recovery |
IIa |
C |
| Consider avoidance of vigorous exercise in patients with CPVT |
IIa |
C |
| Consider avoiding competitive sports and vigorous exercise in patients with ARVC/ACM |
IIa |
C |
| Consider avoiding strenuous exercise and swimming in patients with LQT1 |
IIa |
C |
Exercise may be considered for patients with Brugada syndrome by monitoring their status
immediately after strenuous exercise |
IIb |
C |
ACM, arrhythmogenic cardiomyopathy; AF, atrial fibrillation; ARVC, arrhythmogenic right ventricular cardiomyopathy; AV, atrioventricular; CPVT, catecholaminergic polymorphic ventricular tachycardia; COR, Class of Recommendation; ICD, implantable cardioverter defibrillators; LOE, Level of Evidence; LQT, long QT; PVC, premature ventricular contraction.
9.1 Bradyarrhythmia (Sick Sinus Syndrome and Atrioventricular Block)
A detailed examination is required for athletes with symptomatic bradycardia or Wenckebach 2nd-degree AV block that does not improve with exercise, Mobitz type II 2nd-degree or advanced AV block, or 3rd-degree AV block that appears at rest and during exercise.330
9.2 Bundle Branch Block
It is not necessary to restrict exercise in patients with asymptomatic complete right bundle branch block (CRBBB) when there are no PVCs, and 2nd-degree or higher AV block does not appear during exercise. BBB may progress to AV block when it is associated with a left axis deviation, because of which progression in these patients should be monitored.330
It is not necessary to restrict exercise in patients with left bundle block and no organic heart disease, providing that the condition does not progress to Mobitz type II 2nd-degree or higher AV block.330
9.3 Premature Atrial Contraction and Premature Ventricular Contraction
Premature atrial contractions (PAC) increase with age, although the association with exercise is not constant.331 Even frequent PACs do not cause hemodynamic disruption, but the association with future onset of AF should be noted.
It is not necessary to restrict exercise when PVCs are monomorphic and there are only 1 or 2 consecutive PVCs at rest or during exercise. If PVCs reduce or disappear with exercise, restriction of exercise is not required. A prospective controlled trial with a 23-year follow-up conducted exercise stress tests on 6,101 asymptomatic middle-aged men (aged 42–53 years), with the participants being divided into a high-frequency PVC group (appearance of 2 consecutive PVCs or ≥10% in 30 s), a low-frequency group, as well as a group with no PVCs at rest, during exercise, and during recovery after exercise. The study found that there were a significantly higher number of cardiovascular deaths in the high-frequency PVC group during exercise and during recovery.76 A detailed examination is required for patients with increased PVCs and those with polymorphic or ≥3 consecutive PVCs during exercise and during recovery after exercise.76,332 A detailed examination is particularly essential for right bundle block-type PVCs that increase with exercise and has QRS intervals exceeding 130 ms.332
9.4 Atrial Fibrillation and Atrial Flutter
AF is induced by vigorous exercise and is the most common form of arrhythmia.333 The risk of AF with physical activity and exercise differs depending on the patient’s age and whether the patient is an athlete or not.331
The onset of AF can be suppressed in generally healthy people with active daily lives. The Cardiovascular Health Study of 5,446 American men and women aged ≥65 years found that higher levels of physical activity (kcal/week) significantly reduced the risk of developing AF over a 10-year period.333 The risk of developing AF was significantly lower with moderate-intensity exercise than with low-intensity and high-intensity exercise.333
According to a meta-analysis of 6 studies (n=205,094) investigating the relationship between endurance fitness (cardiopulmonary performance) and AF/atrial flutter (AFL), increased fitness was found to reduce the risk of AF/AFL by 9%.334 Compared with the group with the lowest exercise tolerance, the risk of AF/AFL was reduced by 28% in the moderate group and by 49% in the highest group.334 On the other hand, a prospective cohort study in Sweden that registered 1,126,899 men with a mean age of 18.2 years and conducted follow-up for 26.3 years found that although the risk of developing cardiovascular disease decreased with increased fitness, the incidence of AF and bradyarrhythmia increased in highly fit individuals, suggesting a U-shaped association between the onset of AF and physical fitness.335
A systematic review of randomized control trials and cohort studies evaluated the effect of exercise and the amount of physical activity in patients with AF (paroxysmal, persistent, or permanent) reported before September 2011. The review found that moderate exercise improved QOL and fitness level, although long-term high-intensity exercise increased the risk of AF.336 Increased long-term, high-intensity exercise increased the risk of both AF and AFL, and both conditions can develop concurrently.337
Exercise stress tests are recommended to set exercise intensity. The American College of Cardiology Foundation (ACC)/American Heart Association (AHA) guidelines recommend a heart rate of 60–80 beats/min at rest and 90–115 beats/min during moderate exercise, based on empirical findings rather than evidence.338 If the resting heart rate is <110 beats/min, the suitability of exercise is determined based on factors such as the extent of increase in heart rate during exercise loading, subjective symptoms, duration of exercise, and peak metabolic equivalent.
Patients with AF have a greater degree of increase in heart rate relative to exercise load, and the same patient may also have a different heart rate response depending on their physical condition at the time, which makes it difficult to set exercise intensity based on heart rate. Setting exercise intensity is based on the load at the anaerobic metabolic threshold using a cardiopulmonary exercise stress test. In the treadmill test, exercise is prescribed at an exercise rate of 40–60% of MET at the maximum exercise load for moderate loading and 20–40% for light loading.339 If the resting heart rate exceeds 110 beats/min, exercise for the day ceases, the exercise intensity is lightened, or the duration of exercise is shortened.
The suitability of exercise for patients with AF should be considered after a full evaluation to determine whether they also have heart failure. These patients are often treated with anticoagulants to prevent cerebral infarction, because of which sufficient care is needed for bleeding complications due to trauma such as falls.
9.5 Ventricular Tachycardia
Resuming a competitive sport is possible for athletes without an underlying organic heart disease and those without VT for ≥3 months after ablation therapy for sustained monomorphic VT.330 Suppression of VT can be expected with exercise therapy through mechanisms such as improvement of myocardial ischemia, suppression of the sympathetic nervous system, and increased parasympathetic nervous system activity. Exercise is discontinued if there are ≥3 consecutive VT or polymorphic VT.
9.5.1 Long QT Syndrome
Not only high-intensity exercise, but also regular work, exercise, or emotional excitation may induce arrhythmia.340 According to 2 studies in a cohort of patients with LQTS, there were no cases of death during exercise in patients who continued with competitive or recreational sports, and non-fatal cardiac events were rare at a rate of 1/650 people per year341 and 0/755 people per year.342 The incidence of sport-induced VT decreases with the use of β-blockers.343 Exercise is to be avoided in high-risk patients and patients who have experienced syncope during exercise. In general, competitive-level exercise should be avoided, particularly in patients with LQT1,8 which is characterized by an association with sudden death during swimming;344,345 thus, patients with LQT1 should only swim under supervision.346
9.5.2 Catecholaminergic Polymorphic Ventricular Tachycardia
In this condition, ventricular arrhythmia may appear during moderate to high-intensity exercise or under stress; therefore, it is recommended to strictly restrict exercise and avoid stress.343,347 Generally, patients with CPVT should avoid competitive-level exercise and high-intensity leisure sports. On the other hand, sports may be permitted under the management of adequate pharmacotherapy provided by specialists and patient education.346,348
9.5.3 Arrhythmogenic Right Ventricular Cardiomyopathy/Arrhythmogenic Cardiomyopathy
These patients have a high risk of sudden death during exercise, particularly during high-intensity exercise.349 Endurance athletes engaging in prolonged exercise may experience ARVC or ACM-like changes, even without mutations in genes encoding desmosomal proteins.350 A primary prevention cohort study of patients with ARVC/ACM revealed an association between high-intensity exercise and fatal arrhythmia, which was particularly notable when the duration of exercise exceeded 2.5 h/week.351 A study of 129 patients with ARVC/ACM after fitting an ICD examined the relationship between changes in exercise level during the 5 years after diagnosis and ICD shocks, and found that the group with the lowest amount and duration of exercise had significantly fewer ICD shocks.352 Patients with ARVC/ACM should be refrain from competitive sport and high-intensity exercise, although <2.5 h/week of light to moderate exercise.29 or up to 12.5 METs/h weekly353 is allowed. Moderate-intensity static strength training is also permitted.349
9.5.4 Brugada Syndrome
There is a report of sudden death of a patient with Brugada syndrome during a soccer game,354 and there are also reports of elevated ST and syncope during exercise or during recovery after exercise;74,346,355 thus, vigorous exercise is to be avoided by patients with Brugada syndrome.356
9.6 After Device (Pacemaker, Cardiac Resynchronization Therapy Defibrillator, and Implantable Cardioverter Defibrillator) Implantation
Any sport with high exercise stress is to be avoided after pacemaker implantation, when conduction is completely reliant on the pacemaker.330 A prospective cohort study in Europe and the USA of 372 athletes fitted with ICDs found that there were no ICD shocks during sport, and all patients other than those with ARVC/ACM were able to return to high-intensity competitive sport under proper management.357,358 A meta-analysis of 6 studies of 1,603 individuals comparing the safety and efficacy of supervised exercise therapy in patients with heart failure fitted with an ICD found that exercise tolerance significantly improved in the 12-week exercise group, and the incidence of ICD shocks was significantly reduced (53%).359 A meta-analysis of 16 studies of 2,547 individuals comparing the safety and efficacy of supervised exercise therapy with a non-exercise group of patients after fitting an ICD or a cardiac resynchronization therapy defibrillator found that shocks during exercise were rare (0.9%) over a median of 84 days. Moreover, ICD shocks during the 109-day follow-up period were significantly reduced (32%) and exercise tolerance was significantly improved in the exercise group.360 These findings demonstrate that injury to the ICD implantation site can be prevented and that appropriate exercise prescription after fitting a ICD is safe and effective.
II. Methods for the Diagnosis of Arrhythmias and Related Pathologies
1. Bradyarrhythmia
1.1 Sick Sinus Syndrome (SSS)
1.1.1 Introduction
Sinus node dysfunction may be either transient (reversible/physiological) due to the involvement of the autonomic nervous system or pharmaceuticals, or chronic due to degenerative or organic heart disease. It is a condition caused by a decline in sinus node automaticity or a sinoatrial conduction disorder. Sick sinus syndrome (SSS) is a chronic sinus node dysfunction that causes more severe disorders, namely Stokes-Adams syndrome, heart failure, or fatigue. Generally, patients with these conditions have a good prognosis, and it is very important to determine whether there are any symptoms associated with bradycardia when considering the indication of pacemaker treatment.
1.1.2 Classification
a. Classification Based on Cause and Clinical Course
• Reversible/physiological (transient)
• Organic (chronic)
b. Classification Based on Electrocardiography (Rubenstein Classification361)
• Type I: Sinus bradycardia (<50 beats/min) (Figure 6A)
• Type II: Sinus arrest (Figure 6B) or sinoatrial block (Figure 6C)
• Type III: Bradycardia–tachycardia syndrome (Figure 6D)
1.1.3 Cause
Many cases are idiopathic, although there are also secondary cases that can originate from various causes. Transient (reversible/physiological) cases may be caused by factors such as parasympathicotonia, pharmaceutical agents, electrolyte imbalance, endocrine abnormality, increased intracranial pressure, and hypothermia. Organic (chronic) cases may be caused by factors such as ischemic heart disease, hypertension, cardiomyopathy, amyloidosis, pericarditis, myocarditis, and collagen diseases. Transient factors may exacerbate chronic sinus node dysfunction, and it may be difficult to differentially diagnose the 2 different types.
Increased age is an important factor in idiopathic SSS, with the 70 s to 80 s being the most common ages of onset362,363 and there is no sex-associated difference. The changes are assumed to be associated with age-related degeneration and fibrosis of sinus node cells or surrounding atrial muscle. When SSS occurs in children, it is often associated with congenital heart disease, and although familial onset due to genetic abnormalities is known,364,365 such cases are uncommon. There is significant involvement of the autonomic nervous system, the parasympathetic nervous system in particular, and there is marked bradycardia at night. Reports have indicated that SSS is also associated with sleep apnea syndrome and Brugada syndrome, making it essential to take note of these underlying diseases.366–368
1.1.4 Diagnosis
Table 13 shows the Class of Recommendation and Level of Evidence for the diagnosis of SSS.
Table 13.
Class of Recommendation and Level of Evidence Related to Diagnosis of Sick Sinus Syndrome
| |
COR |
LOE |
Take a detailed case history when SSS is suspected based on symptoms, although the causal
association with symptoms cannot be verified on ECG |
I |
B |
| Conduct screening tests, such as 12-lead ECG and TTE, to rule out underlying heart disease |
I |
B |
| Conduct various ECG tests in line with the frequency of events |
I |
B |
| Consider an exercise stress ECG if the symptoms are thought to be associated with exercise |
IIa |
B |
| Consider conducting a sleep apnea test if there is marked bradycardia at night |
IIa |
B |
EPS may be considered when the events cannot be captured on ECG in patients with symptoms,
such as syncope |
IIb |
C |
COR, Class of Recommendation; ECG, electrocardiogram; EPS, electrophysiological study; LOE, Level of Evidence; SSS, sick sinus syndrome; TTE, transthoracic echocardiography.
a. Subjective Symptoms
Affected patients generally present with generalized malaise, shortness of breath due to decreased cardiac output, dizziness, syncope, and dimmed vision due to transient cerebral ischemia, although various symptoms may appear, ranging from minor to syncope. The type of SSS may be surmised from the subjective symptoms and condition of the patient; thus, taking a detailed case history (whether the patient has had any syncope, presyncope, dizziness, generalized malaise, or shortness of breath; the frequency and duration of symptoms; association with body position and exercise; the status and timing of onset; association with meals; urination and psychological stress; medical history; medication and family history; etc.) is important for differential diagnosis. Generalized malaise and shortness of breath are often caused by sustained bradycardia, while dizziness and syncope are often caused by sinoatrial block or sinus arrest. Bradycardia–tachycardia syndrome is characterized by complaints of dizziness and syncope after cessation of palpitations due to tachycardia.
Embolus can be a complication of SSS, and the symptoms of an embolus may appear first. If there are symptoms associated with exercise (syncope, convulsions, dimmed vision, dizziness, shortness of breath, fatigue, heart failure, and others), exercise stress ECG should be used to confirm whether there is a failure of heart rate response during exercise (Figure 7). In terms of heart rate response, the maximum heart rate is generally considered to be 220-age (beats/min) during an exercise stress test, and heart rate response failure is defined as when the person cannot reach ≥85% of the predicted maximum heart rate by age.369
b. Check for Coexisting Heart Disease
The 12-lead ECG can be used to check for coexisting heart disease, atrioventricular (AV) block, branch block, and bundle branch block, and searching for background diseases by adding echocardiography is necessary for differential diagnosis from other heart diseases that cause sudden death. If there is bradycardia at night, it is necessary to check for sleep apnea syndrome.
Ultimately, SSS is diagnosed by directly verifying the relationship between bradycardia and the symptoms on ECG. The Rubenstein classification has long been used for SSS classification on ECG361 (Figure 6).
• Type I (sinus bradycardia): Sinus bradycardia at ≤50 beats/min of unknown origin is sustained
• Type II (sinus arrest and sinoatrial block): Sinus arrest is when the PP interval is suddenly prolonged to ≥150% of the basic PP interval at sinus rhythm. A sinoatrial block is when the PP interval is suddenly prolonged by an integral multiple of the basic PP interval at sinus rhythm
• Type III (bradycardia–tachycardia syndrome): Tachycardia is often atrial fibrillation (AF), although it may also be atrial flutter (FL) or AT. Sinus arrest often occurs at night, making diagnosis difficult with daytime ECG recording, and long-term ECG recording is useful. Long-term ECGs include Holter ECGs, external event loop recorders, and implantable loop recorders (ILRs), of which an ILR is effective for patients with recurrent syncope. Appropriate ECG tests should be selected to suit the frequency and status of the patient’s symptoms
c. EPS
Electrophysiological study (EPS) is considered when the causal association between sinus dysfunction and syncope symptoms has not been established370 although the advent of event recorders and ILRs has resulted in a decline in the frequency of using EPS to diagnose SSS. EPS is currently used as supplementary testing for other arrhythmias when conducting catheterization and treatment.
EPS is conducted to (1) diagnose sinus dysfunction and assess the severity and type, and (2) determine the optimal pacemaker site and pacing mode. Indices for sinus function assessment with EPS include the (1) sinus node recovery time (SNRT), (2) sinoatrial conduction time, and (3) sinus node effective refractory period. The most commonly used test method nowadays is the assessment of sinus node automaticity conducted by frequent atrial stimulation.139,140 The index for this test is the SNRT, which indicates the time required to regain sinus rhythm after atrial pacing. The normal value is within 1,500 ms. There is also the modified SNRT (SNRT–basic sinus cycle; the normal value is within 550 ms) and SNRT divided by the basic sinus cycle (SNRT/basic sinus cycle; the normal value is within 150%) as SNRTs that consider the effect of self-basic sinus rhythm.
1.2 Atrioventricular Block
Table 14 shows the Class of Recommendation and Level of Evidence for the diagnosis of AV block.
Table 14.
Class of Recommendation and Level of Evidence for the Diagnosis of Atrioventricular Block
| |
COR |
LOE |
| Take a detailed case history and perform a physical examination in patients with AV block |
I |
C |
| Diagnose sleep apnea disorders that may be the cause of the AV block |
I |
B |
Perform TTE in patients with Mobitz type II 2nd-degree AV block, 2 : 1 AV block, paroxysmal AV
block, advanced AV block, or complete AV block |
I |
B |
Consider advanced diagnostic imaging*1 when conditions such as infiltrative cardiomyopathy,*2
endocarditis, or ACHD are suspected after detecting Mobitz type II 2nd-degree AV block, 2 : 1 AV
block, paroxysmal AV block, advanced AV block, or complete AV block |
IIa |
C |
Consider advanced diagnostic imaging*1 when conditions such as infiltrative cardiomyopathy,*2
endocarditis, or ACHD are suspected after detecting 1st-degree AV block or Wenckebach
2nd-degree AV block |
IIa |
C |
Consider TTE when organic heart disease is suspected after detecting 1st-degree AV block or
Wenckebach 2nd-degree AV block |
IIa |
B |
Consider an exercise stress ECG to diagnose the site of the AV block when the block site cannot
be clearly identified in conditions, such as 2 : 1 AV block |
IIa |
C |
EPS may also be considered to diagnose the site of the AV block when the block site cannot be
clearly identified by non-invasive tests |
IIb |
C |
*1MRI, cardiac nuclear medicine imaging, CT, TEE, and others.
*2Cardiac amyloidosis, cardiac sarcoidosis, hemochromatosis, Fabry disease, and others.
ACHD, adult congenital heart disease; AV, atrioventricular; COR, Class of Recommendation; CT, computed tomography; ECG, electrocardiogram; EPS, electrophysiological study; LOE, Level of Evidence; MRI, magnetic resonance imaging; TEE, transesophageal echocardiography; TTE, transthoracic echocardiography.
1.2.1 Definition
AV block is defined as a delay or interruption of electrical conduction at any site in the conduction system (AV node, bundle of His, and His–Purkinje system) when transmitting electrical signals from the atria to the ventricles.6
1.2.2 Classification
The classifications of AV blocks on ECG are shown below.
• 1st-degree AV block (Figure 8A): PR interval >0.2 s (>0.3 s is diagnosed as pathological)
• 2nd-degree AV block
(1) Wenckebach AV block (Mobitz type I) (Figure 8B): Repeated cycles where the PR interval gradually lengthens, AV block appears, and the PR interval after block recovery is shorter than the PR interval immediately before the block
(2) Mobitz type II AV block (Figure 8C): Sudden appearance of AV block without lengthening of the PR interval
(3) 2 : 1 AV block (Figure 8D): Block with an AV conduction ratio of 2 : 1
(4) Paroxysmal AV block (Figure 8E): Sudden drop out of the QRS complex over an extended period of time during normal P-wave conduction371
(5) Advanced AV block (Figure 8F): Block with an AV conduction ratio of ≥3 : 1
• 3rd-degree AV block (complete AV block) (Figure 8G): Complete absence of AV conduction. Complete dissociation of the appearance of P-waves and QRS complex
1.2.3 Diagnosis6
Undiagnosed AV blocks are unlikely to be captured with a 12-lead ECG. Holter ECGs, external event loop recorders, and ILRs are useful for the diagnosis. A diagnosis flowchart when an AV block has been recorded is shown in Figure 9372 to determine whether the AV block is generated by a reversible cause (e.g., drug-induced) or physiological factors and whether there are background diseases that may have caused the AV block (e.g., sleep apnea). 1st-degree AV block and Wenckebach 2nd-degree AV block with parasympathetic nervous system dominance during sleep at night are physiological and harmless.
a. 1st-Degree AV Block and Wenckebach 2nd-Degree AV Block
When 1st-degree AV block or Wenckebach 2nd-degree AV block has been detected, the presence of organic heart disease should first be suspected. Transthoracic echocardiography (TTE) should be considered if organic heart disease is suspected. If infiltrative cardiomyopathy (cardiac amyloidosis, cardiac sarcoidosis, hemochromatosis, Fabry disease, and others), endocarditis, or adult congenital heart disease (ACHD) is suspected, conduct advanced diagnostic imaging (cardiac magnetic resonance imaging [MRI], cardiac nuclear medicine imaging such as 99 mTc pyrophosphate scintigraphy and positron emission tomography, heart computed tomography [CT], and transesophageal echocardiography [TEE]) and treat the identified disease. If an organic heart disease is not suspected, observe the clinical course.
If the PR interval becomes >0.3 s in 1st-degree AV block and AV contraction synchronization has been lost, which can lead to decreased cardiac output and increased pulmonary artery wedge pressure, the condition is diagnosed as pathological.373
b. Mobitz Type II AV Block, 2 : 1 AV Block, Paroxysmal AV Block, Advanced AV Block, and Complete AV Block
First, perform TTE. If a condition such as infiltrative cardiomyopathy, endocarditis, or ACHD is suspected based on the results, conduct advanced imaging diagnostics. Proceed to the treatment algorithm for AV block regardless of the results of TTE and advanced diagnostic imaging (determining the indications for permanent pacemaker implantation).
Paroxysmal AV blocks are AV blocks that can cause syncope and sudden cardiac death, although they have been subclassified into the following subtypes based on recent research: (1) intrinsic (organic): blocks caused by an electrical conduction disorder, (2) extrinsic (mechanical): blocks caused by vagotonia, and (3) blocks that arise due to low blood adenosine concentration (low-adenosine syncope). It has been reported that the severity also differs depending on the subtype.371
c. Identification of the Site of the AV Block
The more distal the site of the block, the greater the severity due to the decline in escaped rhythm. Determine whether the site of the block is within the AV node or distal to the AV node (within the bundle of His or distal to the bundle of His). Infranodal blocks progress slowly and respond well to atropine and isoproterenol. In contrast, blocks within the bundle of His or distal to the bundle of His progress rapidly and respond poorly to atropine.
When it is clinically apparent that the block is distal to the AV node, proceed to the treatment algorithm. It is often difficult to differentiate whether a 2:1 AV block is Wenckebach or Mobitz type II. If a 2 : 1 AV block is symptomatic, proceed to the treatment algorithm. If it is asymptomatic, conduct an exercise stress ECG. If the block appears or worsens with exercise, it can be determined that the block is distal to the AV node; thus, proceed to the treatment algorithm. If the site of the AV block cannot be identified with an exercise stress ECG, consider an EPS. Proceed to the treatment algorithm for blocks distal to the AV node. If the block is asymptomatic and is an infranodal block, conduct follow-up, provided the block is not associated with cardiac arrest of ≥3 s or severe bradycardia of <40 beats/min.
It is acceptable to consider most 1st-degree AV blocks and Wenckebach 2nd-degree AV blocks as infranodal blocks. If the block is symptomatic, proceed to the treatment algorithm. If it is asymptomatic, conduct follow-up.
2. Diagnosis of Supraventricular Tachyarrhythmia
2.1 Atrial Fibrillation (AF)
Clinically, AF is the most commonly encountered arrhythmia; its prevalence in Japan is estimated to be 0.6–1.1% of the population.374,375 It is particularly common in older people aged ≥65 years and is a risk factor for complications such as heart failure, cerebral infarction, dementia, and death. Known factors associated with the onset of AF include male sex, hypertension, heart failure, coronary artery disease, valvular heart disease, diabetes, obesity, sleep apnea disorders, hyperuricemia, smoking, alcohol intake, and genetics.376–380
The electrophysiological mechanism of AF is assumed to be abnormal automaticity triggered predominantly by the pulmonary veins,381 and electrical or structural remodeling of the atrial muscle is thought to play an important role in sustaining AF.382,383
The patient’s hemodynamics are involved in AF treatment strategies, and AF complicated by heart failure or shock requires acute treatment. In reality, many patients with AF have stable hemodynamics; thus, in these cases, assess the risk of cerebral infarction and, if indicated, administer anticoagulants and rate control medication depending on the heart rate. Additionally, consider rhythm control medication and non-pharmaceutical treatment such as ablation depending on the symptoms, type, and duration of AF.384 Table 15 shows the Class of Recommendation and Level of Evidence for the diagnosis of AF.
Table 15.
Class of Recommendation and Level of Evidence Related to the Diagnosis of Atrial Fibrillation
| |
COR |
LOE |
Pulse taking is recommended in older people aged ≥65 years. Conduct an ECG if the pulse is
irregular |
I |
A |
ECG recordings are required for the diagnosis of AF. AF is clinically diagnosed if a clear P-wave
≥30 s is not observed, or absolute irregular RR intervals are observed on a 12-lead ECG or an
ECG with ≥1 leads |
I |
B |
Conduct a short-term ECG recording followed by long-term ECG monitoring (≥72 h, external ECG)
for patients with a history of cerebral infarction or transient ischemic attack |
I |
B |
Perform ILR implantation for patients with subclinical cerebral infarction when the cause cannot
be identified with long-term ECG, including Holter ECG |
I |
B |
Confirm periodic atrial high rate episodes (AHRE) in patients with an implanted device. If AHRE
is detected, confirm the presence of AF on the ECG. If AF is diagnosed, start treatment depending
on the risk of stroke |
I |
B |
| Record ECG during arrhythmia events for the diagnosis of AF |
I |
C |
Conduct a complete cardiovascular assessment for patients with AF (medical history, laboratory
tests, comorbidities, etc.) |
I |
C |
| Conduct TTE to determine AF management and treatment strategy |
I |
C |
Consider ECG screening for older people aged ≥75 years and patients at high risk of cerebral
infarction |
IIa |
B |
| Consider long-term ECG monitoring for accurate assessment of heart rate |
IIa |
C |
AF, atrial fibrillation; COR, Class of Recommendation; ECG, electrocardiogram; ILR, implantable loop recorder; LOE, Level of Evidence; TTE, transthoracic echocardiography.
2.1.1 Diagnosis
The ECG is indispensable for the diagnosis of AF, because it records the absolute irregularity of the RR intervals, the baseline that irregularly fluctuates up and down or sways minutely (fibrillation wave: f-wave) (Figure 10A). In permanent AF, the f-waves become small and may not be clearly recognizable, making it difficult to differentiate from atrial standstill (Figure 10B). If there is a complete AV block and escaped rhythm, the condition is regular bradycardia. Normally, episodes that persist for ≥30 s are clinically diagnosed as AF.385
2.1.2 Classification
The following classifications are involved in determining treatment strategies, including an indication of ablation.
a. Classification Based on Staging
AF is classified into 5 types according to factors such as clinical stage, duration, and whether there is spontaneous termination.384,386
i. First Diagnosed AF
AF is confirmed for the first time on an ECG regardless of the duration of the AF or whether it is actually the first onset. This AF is classified based on the clinical course after diagnosis as follows.
ii. Paroxysmal AF
AF returning to sinus rhythm within 7 days of onset is classified as paroxysmal AF even if defibrillation occurs.
iii. Persistent AF
AF persists for >7 days after onset, including when defibrillation with pharmaceutical agents or direct current electrical shock occurs after 7 days of onset.
iv. Long-Standing Persistent AF
AF that persists for >1 year is defined as long-standing persistent AF.
v. Permanent AF
Permanent AF is defined as when both the patient and physician accept that the condition is AF. When treatment is considered for resuming and maintaining sinus rhythm, it is classified as persistent or long-standing persistent AF instead of permanent.
b. Classification Based on Symptoms
i. Symptomatic AF
Symptoms due to AF include palpitations, shortness of breath, fatigue, and chest discomfort. Approximately half of AF cases are symptomatic. Of the 2,464 patients with AF in the KICS-AF registry who were symptomatic, 41.3% had palpitations, 16.4% had shortness of breath, 4.4% have fatigue, 3% had chest discomfort, 4.1% had reduced exercise tolerance, and 1.7% had syncope.387 Symptoms generally tend to be more severe in paroxysmal AF than in persistent AF.
ii. Asymptomatic AF
Asymptomatic AF is defined as AF without symptoms. A subanalysis of the Fushimi AF registry.388 found that 1,971 (52.6%) of the 3,749 patients with AF (paroxysmal and persistent) and 41.8% of the 2,464 patients in the KICS-AF registry were asymptomatic387 Asymptomatic AF is often incidentally diagnosed during visits to medical institutions for medical checkups or with other diseases. Paroxysmal and asymptomatic AF is often difficult to diagnose.
c. Classification Based on Diagnosis
i. Clinical AF
Clinical AF is defined as AF lasting ≥30 s recorded on a body surface ECG with or without symptoms, and AF with a definitive diagnosis.
ii. AHRE Recorded on an Implanted Device
Atrial high rate episodes (AHRE) refers to frequent atrial excitation ≥175 beats/min lasting ≥5 min recorded on a cardiac implanted device with an atrial lead. It is necessary to review the records and confirm that the findings are not artifacts.389–391
iii. Subclinical AF
Subclinical AF is defined as cases in which AF has never been clinically diagnosed but AHRE is recorded on an implanted device, or AF is recorded on an ILR but not body surface ECG.
2.1.3 Detection Methods
Testing methods for arrhythmia are summarized in another section, so this section describes the methods used to detect AF.
a. Pulse Palpation
Pulse palpation is a simple training method that enables the differentiation of a normal pulse and an irregular pulse, even by older people.392 The usefulness of this method for the early detection of asymptomatic AF has been reported.393 However, it is difficult to continuously perform pulse palpation over a long period of time,394 and it requires motivation to repeat the process. Moreover, although diagnosis with pulse palpation has high sensitivity, it has low specificity;395 thus, diagnosis using an ECG is required. There is also a report showing that AF was newly detected with pulse palpation in 1.4% of patients aged ≥65 years with unknown AF.396
The Japan Stroke Association and Japanese Heart Rhythm Society have designated March 9 as “Pulse Day” and the week between March 9 and 15 as “AF Week”. During this week, these organizations launch a campaign for the prevention of cerebral infarction developing from AF by widely publicizing information to the Japanese public on the symptoms of AF, the danger of cerebral infarction, and the necessity of preventing cerebral infarction through medical management (http://www.shinbousaidou-week.org/).
b. Sphygmomanometer
There are commercially available automatic sphygmomanometers that detect pulse irregularities and are reported to be superior to pulse palpation in detecting pulse irregularities,397 with both sensitivity and specificity being >90%.398 Although confirmation with an ECG is required, using sphygmomanometers is an important method for detecting pulse irregularities.399
c. Wearable and Smart Devices
Recently, many wearable and smart devices have become available for the diagnosis of arrhythmia. Photoplethysmography uses an LED light on the skin to detect pulse waves based on changes in color tone. There are also a large number of applications that can detect AF by placing a finger on a smartphone camera.400,401 Although these applications have high sensitivity and specificity, there are many false positives, and these applications do not have a high positive predictive value.86 Some smartwatches have built-in LED lights and sensors on the back of the watch, and AF detection with these watches has been reported. The Apple Heart Study using the Apple smartwatch reported that AF was confirmed in 34% of people notified of arrhythmia, and the positive predictive value was 84%.123 Similarly, the Huawei Heart Study had a positive predictive value of 91.6% for AF detection using smartwatches. Although there are currently a large number of wearable and smart devices being developed, many of the devices record pulse waves with photoplethysmography; thus, an ECG is required for a definitive diagnosis of AF. There is also a system that can take ECG recordings using 2 small electrodes attached to the back of a smartphone, and when the phone is held in the left and right hands, the ECG is recorded by the smartphone (KardiaBand, AliveCor). The REHEARSE-AF Study followed patients aged ≥65 years with a CHA2DS2-VASc score ≥2 points using this system for 52 weeks and found that AF was detected in 19 of 500 people, 5 people in the control group developed AF, and the system was significantly superior in detecting AF with a hazard ratio of 3.9.402 The latest Apple smartwatch has electrodes on the bezel and back of the watch, and an ECG can be recorded by putting the watch on one arm and touching the bezel with the other hand. This application was also introduced to Japan in January 2021 (see Chapter I.3.6 for details).
d. Non-Loop Event Recorder
Non-loop event recorders are small enough to be portable and are able to record 30-s ECGs from the patient holding the device. It is not necessary to attach electrodes, so there is little burden on the patient. Non-loop event recorders capture signals when a patient is experiencing symptoms, which makes them superior to Holter ECGs in the diagnosis of symptomatic arrhythmia.403 A meta-analysis comparing non-loop event recorders with Holter ECGs reported that a cumulative 19-min recording by a non-loop event recorder was comparable to that by a Holter ECG.404
Recording for 30 s twice daily with non-loop event recorders in patients after AF ablation is superior to a monthly 24-h Holter ECG for detecting AF.405,406 There tends to be a mixture of symptomatic and asymptomatic AF after AF ablation.407 Therefore, if monitoring is conducted with a non-loop event recorder, it is necessary to periodically transfer data not only during a symptomatic event but also during asymptomatic periods.
e. Holter ECGs and Loop Event Recorders
When detecting paroxysmal AF with a Holter ECG, the detection rate increases with longer observation periods. It has been reported that Holter ECGs >24 h are superior in detecting AF recurrence during follow-up after AF ablation.408 The AF detection rate after AF ablation is significantly lower with a 5-day Holter ECG than with a 7-day Holter ECG, and recurrences may be missed in many patients, particularly with a Holter ECG recording <3 days.409 Loop event recorders require the attachment of ECG electrodes. The REAL-AF study, which examined patients not previously diagnosed with AF, found that a 14-day external loop event recorder had a higher AF detection rate than 24-h Holter ECG, with a diagnosis rate of new AF cases of 3.7% each year.410
f. Implantable Loop Recorder
ILRs are able to continuously record events and are considered to have the highest AF detection rate.408 The ABUCUS study compared ILRs with Holter ECGs for patients after AF ablation and found that an ILR detected AF recurrence at a higher rate.411 Detection of AF with an ILR is approved only for the diagnosis of subclinical cerebral infarction. Figure 11 illustrates the characteristics of each device used for the diagnosis of AF.
2.1.4 Screening for Asymptomatic AF
Asymptomatic AF can be detected by ECG screening during medical checkups. According to health screening data from Tama city, the prevalence of AF was 2% in men aged ≥65 years and women aged ≥70 years. Furthermore, AF newly developed at a rate of 2.5/1,000 person-years in an observation of 52,707 person-years.412 There is a higher prevalence of AF in older people. Conducting screening ECG or conducting ECG when a physician detects an abnormality by pulse palpation in people aged ≥65 years is effective for the detection of AF. The latter method is considered to be more cost-effective.413,414 The 72-h monitor ECG screening is particularly recommended for high-risk patients, such as those at the acute stage of cerebral infarction.415 Asymptomatic paroxysmal AF tends to be overlooked,416 and repeated daily ECG increases the detection rate.417,418 Although using the aforementioned wearable and/or smart devices require a definitive diagnosis with ECG, these devices are thought to increase the detection rate of patients with asymptomatic paroxysmal AF.419,420
When screening for AF, the importance of its detection and the significance of treatment should be explained to the patient. It is also necessary to develop a medical care system to ensure patients with positive screening results are able to receive a definitive diagnosis of AF and subsequent optimal treatment.418 Patients with positive screening results receive a definitive diagnosis of AF for the first time once a doctor confirms AF with a single-lead ECG for ≥30 s or a 12-lead ECG.402 Although large-scale screening of the general public may lead to early detection of AF, the clinical effect has not been fully investigated.421
2.1.5 AF Recorded With Cardiac Implantable Devices
Pacemakers with atrial leads and implantable cardioverter defibrillators (ICDs) can monitor atrial rhythm. AHRE is recorded in 10–15% of patients with pacemakers.
The ASSERT study found that AHRE was associated with clinically recorded AF (hazard ratio, 5.56; P=0.001) and cerebral infarction/systemic embolism (hazard ratio, 2.49; P<0.007).422 The results of a meta-analysis of subclinical AF detected with devices reported that the prevalence of stroke in patients with a mean CHADS2
score of 2.1±0.1 was 2.76% per year, and although subclinical AF was strongly associated with clinical AF and stroke, the risk of stroke was lower than that of clinical AF.423 A subanalysis of the ASSERT study analyzed the duration of subclinical AF divided into periods of 6 min–6 h, 6–24 h, and ≥24 h and found that the risk of cerebral infarction/systemic embolism was significantly increased only in the group with a duration ≥24 h (hazard ratio, 3.24; P=0.003).424 Patients implanted with devices should be regularly checked for AHRE,396,425 and if an event is recorded, an ECG screening test or risk assessment for cerebral infarction should be performed.426
Another issue that needs to be addressed in the future is how often AHRE should be detected before the use of anticoagulants is recommended if a definitive diagnosis of AF is not possible on ECG.427
2.1.6 Detection of AF After Cerebral Infarction
ECG monitoring of patients after cerebral infarction detects AF in 11.5–23.7% of patients.428,429 The AF detection rate depends on patient selection, observation period, and monitoring method, although monitoring for several weeks with an ILR has the highest detection power.430 It is necessary to conduct long-term ECG monitoring for patients without a confirmed diagnosis of AF after cerebral infarction (see Chapter II.6).
2.1.7 Diagnosis of AF and Assessment Methods
AF may be complicated by cardiovascular disease; thus, it is important to conduct a comprehensive diagnosis.
A case history is taken, including the current condition and medical history. Factors such as the pattern and duration of AF, comorbid heart disease, risk of cerebral infarction, embolism, and left ventricular dysfunction are assessed. A 12-lead ECG is conducted for a definitive diagnosis of AF, as well as to assess the heart rate and underlying heart disease. Blood sampling is performed to assess thyroid function, kidney function, electrolytes, and anemia. B-type natriuretic peptide is known as a marker for heart failure, although it is also elevated in AF. However, there is currently no recommended cutoff value for the diagnosis of AF.431 Echocardiography is recommended for all patients with AF. TTE is conducted to determine whether there is underlying heart disease, such as valvular disease, cardiomyopathy, or ischemic heart disease, as well as to assess left ventricular function, left atrial function, and right ventricular function.432
Outpatient-based devices, such as Holter ECGs and non-loop event recorders, can be used to assess the effect of rate control, the concordance between paroxysmal AF symptoms and arrhythmia, as well as whether there is post-treatment recurrence.
TEE is useful for the assessment of valvular disease, left auricular thrombus, and in particular, intracardiac thrombus when there is insufficient time for anticoagulation before AF defibrillation. A heart CT scan and stress test are conducted when ischemic heart disease is suspected. Suspected cerebral infarction is assessed with cranial CT and cranial MRI.
2.2 Atrial Flutter (AFL)
The prevalence of AFL is one-tenth that of AF,433 although AF and AFL often mutually transition to each other.434 Additionally, AF transitioning into AFL due to antiarrhythmic drugs, especially Class IC antiarrhythmic drugs, is frequently observed in clinical practice.435 AFL originates from an anatomical isthmus, the cavotricuspid isthmus, and is generated by excitation in the circuit around the tricuspid valve at 250–350 times/min in a counterclockwise direction.436 On ECG, AFL manifests as a negative sawtooth wave (F-wave) on the inferior limb leads (II, III, and aVF leads)437 (Figure 12). V1
appears as a positive wave, and V6
appears as a negative wave. There is often 4 : 1 or 2 : 1 conduction to the ventricles, although 1 : 1 AV conduction may also be observed, particularly under the administration of oral antiarrhythmic drugs.438 It may be difficult to observe sawtooth waves in AFL with stable 2 : 1 conduction, although it becomes apparent by inhibiting AV conduction.

Infrequently, AFL may also be generated by excitation in the circuit around the tricuspid valve in a clockwise direction. Unlike in counterclockwise AFL, in clockwise AFL the waves are positive with II, III, and aVF, negative with V1, and positive with V6
on ECG.439
Reentry that rotates around the tricuspid area is called common AFL, and all other intraatrial reentry tachycardias are sometimes called uncommon AFLs, although the latter is synonymous with macroreentry atrial tachycardia (AT).
2.3 Paroxysmal Supraventricular Tachycardia (PSVT)
2.3.1 Introduction
PSVT is suspected when there is continuous narrow QRS (QRS <120 ms) with RR intervals of <600 ms (heart rate ≥100 beats/min) on ECG. A 12-lead ECG is essential for the diagnosis of PSVT. It is important to take ECGs during both tachycardia and normal sinus rhythm. The 3 major causes of supraventricular tachycardia are (1) AV reentrant tachycardia (AVNRT), (2) AV reciprocating tachycardia (AVRT), and (3) AT.
EPS is essential for the detailed diagnosis of PSVT. EPS for PSVT is conducted on the premise of ablation. The purposes of conducting an EPS are (1) induction and diagnosis of tachycardia, (2) identification of the origin and circuits of the tachycardia, which are the targets for ablation, and (3) determination of the effect of ablation. Each type of tachycardia has characteristic findings, although no finding has complete sensitivity and specificity; thus, multiple observed findings should be comprehensively considered to reach an electrophysiological diagnosis. Diagnosis is not difficult in cases of typical findings, whereas for atypical cases, it is necessary to make a diagnosis through the full use of various EPSs. Table 16 shows the Class of Recommendation and Level of Evidence for the Diagnosis of PSVT.
Table 16.
Class of Recommendation and Level of Evidence for the Diagnosis of Paroxysmal Supraventricular Tachycardia
| |
COR |
LOE |
| Record a 12-lead ECG for the diagnosis of PSVT |
I |
C |
| Conduct EPS for the differential diagnosis of AVNRT, AVRT, and AT |
I |
C |
AT, atrial tachycardia; AVNRT, atrioventricular reentrant tachycardia; AVRT, atrioventricular reciprocating tachycardia; COR, Class of Recommendation; ECG, electrocardiogram; EPS, electrophysiological study; LOE, Level of Evidence.
2.3.2 Atrioventricular Nodal Reentry Tachycardia
In the AV node area, there is a fast pathway entering from the anterior (superior) direction and a slow pathway entering from the posterior (inferior) direction to the compact AV node. AVNRT is the general term for a tachycardia that triggers reentry in the same area. It is broadly classified into common and uncommon.440
a. Common Type (Slow–Fast Type) AVNRT
There are 2 conduction pathways in the AV node area: a fast pathway with a fast conduction velocity and a long refractory period, as well as a slow pathway with a slow conduction velocity and a short refractory period. Tachycardia with a reentrant circuit with anterograde conduction in the slow pathway and retrograde conduction in the fast pathway is diagnosed as common AVNRT441–443 (Figure 13). The slow–fast type is the most common type of AVNRT.444 Figure 14 shows a typical example of common AVNRT on a 12-lead ECG. During tachycardia, the end of the QRS complex overlaps with a P’ wave, indicating retrograde atrial excitation.


When anterograde conduction changes from the fast pathway to the slow pathway due to extra stimulation from the high right atrium, the fast pathway that escapes the refractory period undergoes retrograde conduction, inducing tachycardia. The earliest excitatory site of the retrograde fast pathway conduction is anterior (superior) to the AV junction. During tachycardia, the extra stimulation from the right ventricle that enters during the refractory period of the bundle of His does not capture the atrium early. Retrograde conduction shows decremental conduction characteristics, and in para-Hisian pacing, there is a prolongation of the Spike-A time with prolongation of the QRS complex.445
Maruyama et al reported their attempt to differentiate AVRT or AT from AVNRT based on the electrophysiological response to overdrive pacing from the right ventricular apex, which is 10–40 ms shorter than the tachycardia cycle during tachycardia.443 The final diagnosis of common AVNRT is a diagnosis by exclusion of AVRT and AT.
b. Atypical Type: Uncommon Type AVNRT
Atypical types have previously been classified as conventional fast–slow AVNRT and slow–slow AVNRT, although leftward inferior extension slow–fast type, left atrial slow–fast type, and superior fast–slow type have been also reported. In atypical AVNRT, the P’ wave during tachycardia can appear in the latter half of the RR interval (RP’ interval > P’R interval; long RP’ tachycardia). Differential diagnoses include AVRT and AT with slow Kent’s bundle as the retrograde pathway.446
i. Fast–Slow Type AVNRT
Fast–slow type AVNRT is thought to be AVNRT formed by AV node rightward inferior extension and leftward inferior extension. This type of tachycardia involves the anterograde conduction pathway having a leftward inferior extension and the retrograde conduction pathway having a rightward inferior extension, with the earliest atrial excitation site during tachycardia being close to the coronary sinus ostium.440 Kaneko et al reported a subtype of fast–slow AVNRT with a superior slow pathway as the retrograde conduction pathway, where the earliest atrial excitation site is superior to Koch’s triangle.447
ii. Slow–Slow Type AVNRT
Slow–slow type AVNRT is thought to be AVNRT formed by AV node rightward inferior extension and leftward inferior extension. This type of tachycardia involves the anterograde conduction pathway having a rightward inferior extension and the retrograde conduction pathway a having leftward inferior extension, with the earliest atrial excitation site during tachycardia being close to the coronary sinus ostium.441
2.3.3 Atrioventricular Reciprocating Tachycardia
a. Wolff-Parkinson-White Syndrome
WPW syndrome is a disease with an accessory pathway (Kent’s bundle) linking the atria and ventricles outside the AV node, and it presents with various types of tachycardia in addition to specific ECG findings during sinus rhythm. The syndrome is called WPW syndrome because it was reported by Wolff, Parkinson, and White in 1930, although it is also called pre-excitation syndrome.448
A reentry circuit not found in healthy people is formed by the accessory pathway, causing various supraventricular tachycardia events. Generally, this disease has a good prognosis, although if WPW syndrome is complicated by AF, it may transition to fatal ventricular arrhythmia. Thus, caution is needed even if the condition is asymptomatic.449
WPW syndrome is classified by Rosenbaum et al as Type A, which has a high R on the V1
lead and an accessory pathway on the left ventricle mitral annular ring, and Type B, which has an rS pattern and an accessory pathway on the right ventricle tricuspid annular ring.450 In Japan, WPW syndrome is further classified into Type C, where the V1
lead presents with a QS pattern and the accessory pathway is estimated to be in the septum.451 Arruda et al reported that it is possible to estimate the localization of the accessory pathway by examining the polarity of the delta wave on a 12-lead ECG in detail.452
When an accessory pathway (delta wave) is observed on a 12-lead ECG, it is termed “manifest WPW syndrome”. In almost all cases, the accessory pathways in manifest WPW syndrome have anterograde conduction from the atrium to the ventricle and retrograde conduction from the ventricle to the atrium. In cases of anterograde accessory pathways with a short effective refractory period, patients have a high risk of sudden cardiac death.163 On the other hand, there are also cases in which the accessory pathway conducts only from the ventricle to the atrium, and the accessory pathway is first discovered by an EPS. When delta waves are not found with body surface ECGs, but a retrograde conduction pathway is suspected at the onset of tachycardia, it is termed “concealed WPW syndrome”. There is another type called “intermittent WPW syndrome”, in which the delta waves temporarily disappear or appear.453 Figure 15 shows a typical example of AVRT on 12-lead ECG. A P’ wave showing retrograde excitation is confirmed behind the QRS complex during tachycardia.

The existence of an accessory pathway is suspected when the EPS induces supraventricular tachycardia, maps the earliest retrograde atrial wave, and the earliest excitation site of tachycardia is not the annular septum. The attachment site of the anterograde accessory pathway identifies the earliest ventricular excitation site during sinus rhythm or atrial stimulation. The accessory pathway may also be oblique, because of which the earliest phase at the ventricular end may not always correlate with the earliest phase at the atrial end.441
Even if the existence of an accessory pathway is shown, it is essential to induce supraventricular tachycardia to prove that the accessory pathway is involved in tachycardia. If extra stimulation is applied from the right ventricle during the His bundle refractory period during supraventricular tachycardia, the atrial wave of the retrograde pathway is captured early, and reset of the tachycardia cycle can be confirmed; then it can be determined that the accessory pathway is involved in the tachycardia. Diagnosis of accessory pathways on the septal side is difficult. Hirao et al reported a method for differentiating septal accessory pathways from AV node retrograde pathways with para-Hisian pacing.445 Maruyama et al also reported a method for differentiating AVRT from AVNRT with right ventricular overdrive pacing at short intervals of 10–40 ms during tachycardia.443
Many accessory pathways are attached to the endocardial side of the annulus between the atrium and ventricle, although very occasionally, accessory pathways traversing the epicardial side and attaching to the atrial appendage, coronary sinus ostium, and coronary diverticulum have also been reported.454–456
b. Special Pre-Excitation Syndromes
Mahaim pathways short circuit the atrial bundle branch and are characterized by anterograde decremental conduction but lack retrograde conduction. The presence of Maheim’s bundle can result in AVRT that rotates Maheim’s bundle in the antegrade direction and the atrioventricular node in the retrograde direction.457
2.3.4 Atrial Tachycardia (Other Than Atrial Tachycardia After Heart Surgery and Ablation)
AT is a type of supraventricular tachycardia that presents as long RP’ tachycardia on the 12-lead ECG. The P’ wave differs from that occurring during sinus rhythm. Adenosine triphosphate (ATP) has the effect of stopping tachycardia for narrow QRS tachycardia and is useful for differentiating between AT and sinus tachycardia. Diagnosis of AT and sinus tachycardia can be confirmed by the absence of change in the P-waves before and after the suppression of AV node conduction with ATP. Tachycardia that is stopped with a small dose of ATP is called ATP-sensitive AT.458–460
AT is suspected when the earliest excitation site is other than the annular ring or the AV node area with atrial mapping during tachycardia in the EPS, although AT also occurs close to the AV node. The mechanism of AT includes abnormal automaticity (ectopic automaticity and triggered activity), AT showing focal excitation with microreentry (hereinafter referred to as focal AT), and AT caused by macroreentry. Yamabe et al reported that the mechanism of verapamil-sensitive AT is reentry.461 They conducted atrial pacing with a cycle length shorter than the tachycardic cycle length from multiple points within the atrium while recording the potential at the earliest excitation site during tachycardia for verapamil-sensitive AT close to the AV node and at the atrioventricular annulus. They reported that the manifest entrainment phenomenon was observed.
a. Focal AT
The origin of focal AT varies and can include the sinus node area, AV node area, the area close to the bundle of His, annulus, crista terminalis, pulmonary vein, superior vena cava, coronary sinus ostium, atrial septum, and appendage. Thus, a 3D mapping system is essential for AT mapping.462–468 Tang et al reported that the origin of focal AT can be predicted if P-waves can be confirmed on the 12-lead ECG.469 Endocardial mapping can be performed efficiently by predicting the origin, although puncture of the atrial septum is required to approach the left atrium (Figure 16).469
b. Macroreentrant AT
Macroreentrant AT can be diagnosed by electrophysiological mapping during tachycardia.470 The terms macroreentrant AT and atypical AFL not reliant on the cavotricuspid isthmus are often used for tachycardias with similar mechanisms, and these cannot be clearly defined or differentiated from electrophysiological findings.
2.3.5 Sinus Nodal Reentrant Tachycardia (SNRT)
SNRT includes part of the sinus node area in the reentrant circuit. The P’ waves during tachycardia resemble P-waves during sinus rhythm. SNRT is suspected from 12-lead ECG waveforms,471 although a definitive diagnosis is made with an EPS.
3. Diagnosis of Tachycardiac Ventricular Arrhythmias
3.1 Premature Ventricular Contractions and Non-Sustained Ventricular Tachycardia
Depending on the number of origins and whether there is a compensatory pause after the event, premature ventricular contraction (PVC) can be classified as unifocal, which is a single QRS complex generated from a single origin, or multifocal, which is composed of multiple QRS complexes generated from multiple origins. The PVC that is interpolated between basic waveforms is called interpolated, and that with a pause after a PVC where the basic wave interval before and after is twice that of the basic cycle is called full compensatory. If it occurs in 1 heart beat, it is called a single. If it occurs in 2 successive heart beats, it is called a couplet, and if it occurs in ≥3 successive heart beats, it is termed non-sustained ventricular tachycardia (NSVT) (Figure 17).

PVCs are recorded at a certain frequency, even in healthy people, on the Holter ECG, although in young people with no cardiovascular risk factors, PVC is not an immediate risk factor for sudden cardiac death.472–474 PVCs that increase during exercise stress test loading and during recovery have been reported as a risk factor.76,78 Patients with frequent PVCs, such as those ≥10,000 beats or ≥10% per day, should be monitored for arrhythmia-induced decreased cardiac function.475 Treatment is indicated for PVCs that trigger ventricular fibrillation (VF).476,477
The involvement of PVCs and NSVT in sudden cardiac death differs according to the underlying disease and severity of the decreased cardiac function. Reports have shown that NSVT ≥13 h after myocardial infarction is a risk factor for arrhythmic death.478,479 The GISSI-2 study found that NSVT in patients treated with thrombolytic therapy was not a predictor for all-cause death or sudden cardiac death 6 months after infarction, although PVCs (≥10 events/h) were a predictor.480 A study has demonstrated that an ICD is effective for the primary prevention of arrhythmic death in patients with dilated cardiomyopathy (DCM) and decreased cardiac function complicated by NSVT or PVCs (≥10 events/h).481
The incidence of PVCs and NSVT increases with the progression of reduced cardiac function in patients with chronic heart failure, regardless of the underlying disease. In the Val-HeFT study (left ventricular ejection fraction [LVEF] ≈30%),482 and CHF-STAT study (LVEF ≈25%),483 PVCs and NSVT were not predictors of sudden cardiac death. However, NSVT in the GESICA study (LVEF ≤20%),484 as well as PVCs (≥30 events/h) and NSVT in the PROMISE study,485 were predictors of sudden cardiac death independent of cardiac function.
In addition to the 12-lead ECG, investigations such as Holter ECG, stress exercise ECG, signal-averaged ECG (SAECG), T-wave alternans (TWA), cardiac MRI,486,487 and an EPS are conducted for risk assessment of sudden cardiac death.256,488 Various autonomic nervous system activity tests are also reported to be useful for risk assessment of sudden cardiac death (see Chapter I.7).
The Class of Recommendation and Level of Evidence for the diagnosis and risk assessment of sudden cardiac death for PVCs and ventricular tachycardia (VT) are shown in Table 17.63,64,154,159 Refer to Chapter I.4.2 for information on the EPS.
Table 17.
Class of Recommendation and Level of Evidence for the Diagnosis and Risk Assessment of Premature Ventricular Contractions and Ventricular Tachycardia63,64,154,159
| |
COR |
LOE |
Measure LVEF in cases of ischemic cardiomyopathy or non-ischemic cardiomyopathy to detect
cases of low cardiac function (≤30–35%) |
I |
A |
Conduct NSVT assessment tests in cases of ischemic cardiomyopathy or non-ischemic
cardiomyopathy and with low cardiac function (≤30–35%) |
I |
A |
Consider cardiac MRI or CT in VT cases of suspected underlying heart disease to detect underlying
heart disease and analyze the characteristics |
IIa |
C |
Consider EPS for risk assessment of sustained VT in cases of ischemic cardiomyopathy, non-
ischemic cardiomyopathy, or adult cases after surgery for congenital heart disease with syncope
or symptoms indicative of VT, although primary prevention with ICD therapy is not applicable |
IIa |
B |
EPS may be considered for risk assessment of sudden cardiac death in adult cases after surgery
for tetralogy of Fallot with ≥1 risk factors (reduced left ventricular function, NSVT, and QRS
interval >180 ms) |
IIb |
B |
| EPS is not recommended for risk assessment of sudden cardiac death in HCM cases |
III (No
benefit) |
B |
EPS is not recommended for risk assessment of sudden cardiac death in adult cases after
surgery for congenital heart diseases that are asymptomatic or have no risk factors |
III (No
benefit) |
B |
COR, Class of Recommendation; CT, computed tomography; EPS, electrophysiological study; HCM, hypertrophic cardiomyopathy; ICD, implantable cardioverter defibrillator; LOE, Level of Evidence; LVEF, left ventricle ejection fraction; MRI, magnetic resonance imaging; NSVT, non-sustained ventricular tachycardia; VT, ventricular tachycardia.
3.2 Sustained Ventricular Tachycardia (VT)
Sustained VT is diagnosed with a 12-lead ECG,489,490 although an EPS is useful for the differentiation of supraventricular tachycardia with a wide QRS complex.256,488 In Japan, the percentage of old myocardial infarction as the underlying disease of sustained VT is low (≈35%), whereas non-ischemic heart disease accounts for a high percentage (45–50%).2 Sustained VT may also develop due to the proarrhythmic effect of antiarrhythmic drugs or electrolyte imbalance. Idiopathic VT without organic heart disease is diagnosed from physical findings, ECG, and various imaging diagnostics. Right ventricular outflow tract VT491 and verapamil-sensitive reentrant VT492 are common forms and prognosis is generally good with these conditions, although cardiac function may decrease due to tachycardia (tachycardia-induced cardiomyopathy).493 Event pattern analysis with a Holter ECG and exercise stress ECG is useful when considering treatment methods.
Organic heart diseases that can cause VT are listed below. Refer to Chapter II.4.6 for information on arrhythmogenic right ventricular cardiomyopathy (ARVC)/arrhythmogenic cardiomyopathy (ACM).
3.2.1 Acute Myocardial Infarction
Most episodes of VT/VF occur soon after myocardial infarction (within 48 h), and it remains a leading cause of out-of-hospital deaths. Acute-onset VT/VF is not necessarily a predictor of poor prognosis, although events that occur later (>48 h) require strict measures.494–496
A meta-analysis of cases of ST-elevation acute myocardial infarction (n=57,158) reported that the risk factors for the onset of VT/VF were ST elevation, early onset after infarction, smoking, male sex, lack of pre-infarction angina, low heart rate, AV block, and hypokalemia.497 Another report indicated that advanced age, high systolic blood pressure, history of myocardial infarction, severity of Killip classification, anterior wall infarction, and decreased LVEF were also risk factors.498 The appearance of J-waves on the 12-lead ECG has also been reported to be associated with the incidence and prognosis of VT/VF.499,500
3.2.2 Chronic Coronary Artery Disease
The main mechanism of VT/VF with chronic coronary artery disease is reentry. Conduction delays and/or interruptions of wave propagation in the damaged myocardium create arrhythmic substrates. Patients with a history of arrhythmic events and those resuscitated from cardiac arrest are at high risk of sudden cardiac death (secondary prevention).152,501,502 For primary prevention, reduced LVEF (≤30–35%) is listed as an independent risk factor.153,154,159 There are a number of studies that support the assessment of the arrhythmic substrate with cardiac MRI.503
It has been reported that using a combination of SAECG and TWA for risk assessment enables highly accurate assessment.271 The utility of body surface potential mapping and magnetocardiography has also been reported.504–506 However, it is not recommended to determine the indication of ICD implantation with these non-invasive tests alone.
The JCS/JHRS Guideline on non-pharmacotherapy of cardiac arrhythmias (2018 revision)2 recommends EPS when NSVT is present in cases of underlying heart disease and positive ventricular late potential (LP) on SAECG. Thus, ICD therapy is considered, in accordance with the results of the MUSTT study,256 when (1) the patient has coronary artery disease (≥40 days since myocardial infarction or ≥90 days after coronary revascularization); (2) sufficient pharmacotherapy has been provided; (3) there is a ≤40% reduction in LVEF; (4) the patient has NSVT; and (5) VT/VF is induced on EPS.
3.2.3 Dilated Cardiomyopathy
The main cause of death in patients with DCM is heart failure and sudden cardiac death, and the main cause of sudden cardiac death is VT/VF. Conduction disorders due to factors such as myocardial fibrosis form the arrhythmic substrate.
A history of VT/VF and reduced LVEF (≤30–35%) are known risk factors for sudden cardiac death.159,507 NSVT in patients with reduced cardiac function (LVEF <30%) may be a risk factor for sudden cardiac death.508 There are also reports showing that fragmented QRS509 recorded on ECG and TWA-positive are useful for the selection of high-risk cases. There are many negative opinions on assessment using the EPS.82 On the other hand, there are several studies supporting the assessment of the arrhythmic substrate with cardiac MRI.510
3.2.4 Hypertrophic Cardiomyopathy
An epidemiological study in Japan reported the annual mortality rate of hypertrophic cardiomyopathy (HCM) to be 2.8%.511 The majority of deaths are attributed to sudden cardiac death. HCM is an important cause of sudden death in young people. Although an association between sustained VT and sudden cardiac death has been shown, there is insufficient knowledge of the role of NSVT.
NSVT is listed as a risk factor for sudden cardiac death. The negative predictive value is high (≥95%), but the positive predictive value is low (20–25%).512–514 Abnormal blood pressure response on exercise stress test (elevation <25 mmHg, persistent reduction in blood pressure, and transient reduction in blood pressure during early recovery) is also listed as a risk factor for sudden cardiac death.80,81 The results of a meta-analysis showed that the presence and spread of delayed contrast on cardiac MRI are related to sudden death (3.41 times), cardiac death (2.93 times), and all-cause death (1.8 times).515 The induction rate of sustained VT in the EPS is not high, and polymorphic VT/VF is often induced, although these findings do not necessarily suggest a poor prognosis.516,517 Therefore, risk assessment by EPS is not recommended. There are reports showing that LP recorded on SAECG is associated with arrhythmic events,518,519 although no consensus has been reached.
The 2017 American Heart Association (AHA)/American College of Cardiology (ACC)/Heart Rhythm Society (HRS) Guidelines520 list the following as established risk factors: (1) resuscitation from cardiac arrest due to sustained VT/VF; (2) history of spontaneous sustained VT causing syncope or hemodynamic compromise; (3) family history of sudden death associated with HCM; (4) left ventricular wall thickness ≥30 mm; (5) unexplained syncope within previous 6 months; (6) NSVT; and (7) abnormal blood pressure response during exercise. The 2015 European Society of Cardiology (ESC) guideline188 proposes estimating the 5-year sudden cardiac death rate using a risk factor formula (HCM Risk-SCD Calculator).
Japan’s Cardiomyopathy Treatment Guidelines (2018 revised edition)82 place recent cardiogenic or unexplained syncope, marked left ventricular wall thickness (≥30 mm), and HCM Risk-SCD Calculator (event prediction ≥6%) as particularly important risk factors and recommend risk stratification using a combination of other risk factors (family history of sudden death, NSVT, and abnormal blood pressure response during exercise), and modifying factors (left ventricular outflow tract obstruction, extensive gadolinium delayed contrast findings on cardiac MRI, dilated phase of HCM, and ventricular aneurysm).
3.2.5 After Surgery for Congenital Heart Disease
Sustained VT associated with congenital heart disease is often reported in patients after surgery for tetralogy of Fallot and complete transposition of great arteries. A high risk of VT and sudden death has been reported in patients in Europe and the USA in whom a transannular patch was used for surgery,521 although similar results have not been obtained in Japanese studies.522 Characteristically, these are macroreentrant VT with the surgical incision wound and medical material (patches, valves) forming the arrhythmic substrate,203,523 although microreentry and non-reentrant VT can also occur.
Risk factors to be considered for patients after surgery for tetralogy of Fallot include: (1) moderate to severe pulmonary valve regurgitation; (2) history of sustained VT; (3) prolonged QRS complex (≥180 ms); and (4) decreased LVEF (<40%).524 Persistent postoperative hemodynamic abnormalities and reduced ventricular function are of particular importance.525,526 Although the ESC guideline states that an EPS may be considered for the assessment of patients with ≥1 of the following risk factors: left ventricular dysfunction, NSVT, or prolonged QRS complex (≥180 ms) (Class of Recommendation IIb),527 an EPS is not recommended in patients with no risk factors or symptoms (Class of Recommendation III).188,527 Similar recommendations are included in the AHA guideline,520 which states that an EPS is useful for the assessment of sustained VT/VF in patients after surgery for tetralogy of Fallot with risk factors and arrhythmia (Class of Recommendation IIa).
3.3 Torsades de Pointes (TdP)
TdP is a polymorphic VT characterized on ECG by oscillatory changes in the amplitude of the QRS complexes around the isoelectric line from moment to moment (twisting of the points)71 (Figure 18). Typical diseases with TdP include long QT syndrome (LQTS) and short-coupled idiopathic VF (SCIVF). (Refer to Chapter II.4.1 for information on LQTS.) TdP may also be generated after a short–long–short sequence QRS coupling interval arising from bradycardia such as advanced AV block or electrolyte imbalance such as hypokalemia (secondary LQTS). However, in non-sustained episodes and when the number of available recording leads is limited, this type of waveform and twisting around the isoelectric line may not always be observed.
3.3.1 Short-Coupled Idiopathic Ventricular Fibrillation (SCIVF)
PVCs with a short coupling period have been known to trigger TdP and VF.528 SCIVF is recognized as an idiopathic paroxysmal VF and manifests TdP from short coupling periods of ≤300 ms.529 QT prolongation is not observed on ECG, and TdP does not form the shape of polymorphic VT. A study by Leenhardt et al reported that 30% of 14 patients with SCIVF had a family history of sudden death, and the mean coupling period was 245 ms.529 A number of genetic variations have been reported in SCIVF.530–533 The mechanism remains unknown, and the cause is also unclear (possible causes include emotional stress, catecholaminergic factors, and bradycardia). Therefore, diagnosis is only possible with telemetry or ICD recording at the onset of VF. The CASPER study mentioned that SCIVF might be an underestimated cause of cardiac arrest.534 Identifying short-coupled PVCs with repeat monitoring using a Holter ECG or event ECG may assist diagnosis.
3.4 Ventricular Fibrillation (VF)
VF is characterized by irregular electrical activity with marked fluctuations in the QRS complex and very fast QRS conduction velocity, with the ventricular velocity exceeding 300 beats/min (cycle length <200 ms)71 (Figure 19). VF has the worst prognosis among fatal tachyarrhythmias that cause cardiac arrest. Although various pathologies are involved in the onset of VF, the causes in adults are broadly classified as cardiogenic and non-cardiogenic (Table 18).

Table 18.
Diseases That May Cause Cardiac Arrest
| Cardiogenic |
| I. Structural heart disease |
Ischemic heart disease
• Acute coronary syndrome
• Vasospastic angina
• Old myocardial infarction
Non-ischemic heart disease
• HCM
• DCM
• Secondary cardiomyopathy (cardiac sarcoidosis and amyloidosis etc. )
• Arrhythmogenic cardiomyopathy
• Myocarditis
• Arrhythmogenic mitral valve prolapse
• Congenital heart disease after surgery
• Others |
| II. Non-structural heart disease |
• LQTS
• SQTS
• Brugada syndrome
• ERS
• CPVT
• IVF
• SCIVF
• AF with WPW syndrome
• AFL manifesting 1 : 1 AV conduction
• Bradyarrhythmia (SSS, AV block, and progressive cardiac conduction defect)
• Others |
| Non-cardiogenic |
| I. Intrinsic |
• Acute pulmonary thromboembolism
• Acute aortic dissection
• Acute aortic aneurysm rupture
• Bronchial asthma
• Subarachnoid hemorrhage
• Epilepsy
• Sudden infant death syndrome (definitive diagnosis with autopsy)
• Others |
| II. Extrinsic |
• Trauma (sharp and blunt force injuries), including commotio cordis
• Electric shock
• Burns
• Accidental hypothermia
• Drowning
• Choking
• Toxins (alkaloids such as caffeine and those of aconite and narcissus)
• Others |
AF, atrial fibrillation; AFL, atrial flutter; AV, atrioventricular; CPVT, catecholaminergic polymorphic ventricular tachycardia; DCM, dilated cardiomyopathy; ERS, early repolarization syndrome; HCM, hypertrophic cardiomyopathy; IVF, idiopathic ventricular fibrillation; LQTS, long QT syndrome; SCIVF, short-coupled idiopathic ventricular fibrillation; SQTS, short QT syndrome; SSS, sick sinus syndrome; WPW, Wolff-Parkinson-White.
The pattern in organic heart disease often depicts the onset of VT, which then transitions to VF; however, the pattern of the onset is often from VF in Brugada syndrome and at induction during EPS. In LQTS, short QT syndrome (SQTS), and catecholaminergic polymorphic ventricular tachycardia (CPVT), there may be repeated recurrence of polymorphic VT and/or VF in a short period of time, resulting in an electrical storm.
This section describes the narrow definition of idiopathic VF. Refer to Chapter II.4.1–4.5 for information on LQTS, SQTS, Brugada syndrome, early repolarization syndrome (ERS), and CPVT.
3.4.1 Narrow Definition of Idiopathic VF
The CASPER study examined 200 patients with unexplained cardiac arrest without apparent heart disease at initial assessment and reported that among 81 people for whom a definitive diagnosis was reached, 28 (35%) had organic heart disease (ARVC/ACM, coronary artery vasospasm, DCM, myocarditis, and others) and 53 (65%) had diseases such as hereditary arrhythmia without organic heart disease.534 The breakdown of diseases without organic heart disease was LQTS (22%), ERS (16%), CPVT (12%), Brugada syndrome (8%), and SCIVF (6%).
If all the factors leading to cardiac arrest are ruled out and the cause is still unknown, the condition is defined as idiopathic VF (IVF) in the narrow sense.9 Patients who have had return of spontaneous circulation through cardiopulmonary resuscitation require a strategic approach to diagnosis based on a comprehensive algorithm.71,178,535–538 However, the IVF diagnostic criteria can sometimes be arbitrary because there are no clear guidelines even at the global level.535 Elucidating the mechanism of channelopathies has increased the diagnostic confirmation rate; therefore, the frequency of IVF diagnosis is declining. On the other hand, the definition of IVF changes every year.534 Recent reports have indicated that the frequency of IVF diagnosis ranges between 5% and 10% in resuscitated patients after unexpected cardiac arrest.539,540 The implementation rate of ajmaline (sodium-channel blocker) challenge to induce coronary artery vasospasm and for the differential diagnosis of Brugada syndrome was <20% in the Paris-SDEC registry.540 A series of comprehensive clinical assessments must be implemented as required to differentially diagnose arrhythmias, including ECG monitoring, advanced imaging diagnostics (coronary angiography, coronary CT, cardiac MRI, and cardiac nuclear medicine imaging), load testing, and genetic testing.71,178,535–538,541
It should also be noted that structural changes and progression of pathology occur over time during follow-up after resuscitation.535,539 The frequency of changes to diagnostic terms against the background of genetic abnormalities in arrhythmias has escalated to 20%, suggesting duplication, transformation, and diversification of phenotypes.541 The diagnostic algorithm for the diagnosis of cardiac arrest is shown in Figure 20.

Figure 20.
Diagnostic algorithm for ventricular fibrillation, pulseless ventricular tachycardia, PEA, and asystole. ACEI, angiotensin-converting enzyme inhibitor; ACh, acetylcholine; AF, atrial fibrillation; AFL, atrial flutter; ARB, angiotensin II receptor blocker; ARNI, angiotensin receptor neprilysin inhibitor; ARVC, arrhythmogenic right ventricular cardiomyopathy; AVB, atrioventricular block; CAG, coronary angiography; CLBBB, complete left bundle branch block; CPR, cardiopulmonary resuscitation; CPVT, catecholaminergic polymorphic ventricular tachycardia; CT, computed tomography; DCM, dilated cardiomyopathy; DVT, deep vein thrombosis; ECG, electrocardiogram; ERS, early repolarization syndrome; HCM, hypertrophic cardiomyopathy; ICD, implantable cardioverter defibrillator; IHD, ischemic heart disease; ILR, implantable loop recorder; LQTS, long QT syndrome; MRB, mineralocorticoid receptor blocker; MRI, magnetic resonance imaging; OMI, old myocardial infarction; PEA, pulseless electrical activity; PE, pulmonary embolism; PMK, pacemaker; RCM, restrictive cardiomyopathy; ROSC, return of spontaneous circulation; SCIVF, short-coupled idiopathic ventricular fibrillation; SGLT-2, sodium-glucose transport protein 2; SQTS, short QT syndrome; SSS, sick sinus syndrome; TdP, torsade de pointes; VF, ventricular fibrillation; VT, ventricular tachycardia; WPW, Wolff-Parkinson-White.
4. Diagnosis of Genetic Arrhythmias
4.1 Long QT Syndrome (LQTS)
4.1.1 Overview
Congenital LQTS is a group of diseases that generate a characteristic polymorphic VT known as TdP due to prolongation of the QT interval, which may result in syncope and sudden cardiac death from VF. This section focuses on the diagnosis and prediction of sudden death. Detailed clinical features and treatment are provided in the Guidelines for diagnosis and management of inherited arrhythmias (JCS 2017),8 and the JCS/JHRS 2020 Guideline on pharmacotherapy of cardiac arrhythmias.1 Sudden death is often the first symptom of this disease, although with accumulation of evidence on the efficacy of β-blockers,542–548 diagnostic criteria with high detection sensitivity and risk assessment methods have been created, taking into account the importance of early diagnosis and treatment (Table 19).
Table 19.
Class of Recommendation and Level of Evidence for the Diagnosis of Congenital Long QT Syndrome
| |
COR |
LOE |
Clinical diagnosis of congenital LQTS is made with an LQTS risk score ≥3.5 after excluding
secondary LQTS |
I |
C |
Clinical diagnosis of congenital LQTS is made with a QTc ≥500 ms on repeated ECG recordings a
fter excluding secondary LQTS |
I |
C |
Genetic diagnosis of congenital LQTS is made when pathogenic mutations of LQTS-related genes
are found, regardless of the QT interval |
I |
C |
Clinical diagnosis of congenital LQTS is considered with QTc of 480–499 ms on repeated ECG
recordings and unexplained syncope after excluding secondary LQTS |
IIa |
C |
Clinical diagnosis of congenital LQTS is considered with QTc ≥480 ms on repeated ECG
recordings, after excluding secondary LQTS |
IIb |
C |
COR, Class of Recommendation; ECG, electrocardiogram; LOE, Level of Evidence; LQTS, long QT syndrome; QTc, corrected QT interval.
4.1.2 Clinical Diagnostic Criteria
The Schwartz diagnostic criteria549–552 are widely used for the clinical diagnosis of congenital LQTS, and the current version is the 2011 Revised Edition, which has added exercise stress test findings (Table 20).552 ECG findings (QT interval, TdP, TWA, notched T-wave, and low heart rate for age), clinical symptoms (syncope and congenital deafness), and family history are scored, and a diagnosis is possible with a score of ≥3.5.551 The joint statement issued in 2013 by the HRS/European Heart Rhythm Association (EHRA)/Asia Pacific Heart Rhythm Society (APHRS) made some adjustments to enable early diagnosis even if the case does not satisfy the 2011 Schwartz diagnostic criteria (LQTS risk score ≥3.5). It is now possible to clinically diagnose congenital LQTS for cases with a corrected QT interval (QTc) of ≥500 ms repeatedly recorded on an ECG and those with a history of syncope that cannot be explained by other factors, even if the QTc is 480–499 ms.43 Furthermore, the ESC guideline defines a QTc of ≥500 ms itself as a high-risk factor for sudden death and recommends that it should be possible to clinically diagnose congenital LQTS based on ECG findings of a QTc of ≥480 ms alone.188
Table 20.
Risk Score and Diagnostic Criteria for Congenital Long QT Syndrome
| Criteria items |
Score |
| ECG findings |
Prolonged QT (QTc)*1 |
≥480 ms |
3 |
| 460–479 ms |
2 |
| 450–459 ms (in male individuals) |
1 |
QTc 4th minute of recovery from exercise
stress test |
≥480 ms |
1 |
| TdP*2 |
2 |
| Visible T-wave alternans |
1 |
| Notched T-wave (in ≥3 leads) |
1 |
| Low heart rate for age*3 |
0.5 |
| Clinical history |
Syncope*2 |
With stress |
2 |
| Without stress |
1 |
| Congenital deafness |
0.5 |
| Family history*4 |
Family members with definite LQTS*5 |
1 |
| Unexplained sudden cardiac death at <30 years old among immediate family members |
0.5 |
Classified based on total score: ≥3.5 points: high probability of LQTS, 1.5–3 points: intermediate probability of LQTS, ≤1 point: Low probability of LQTS.
*1Recorded before treatment or under conditions without factors that would cause QT prolongation; QTc calculated by Bazett’s formula.
*2Two points if the patient has both TdP and syncope.
*3Refer to the Guideline on diagnosis and treatment of inherited arrhythmia (2017 edition):8 when the resting heart rate is below the second percentile for age.
*4One point if the patient has both.
*5Family history of congenital LQTS and a risk score ≥3.5 points (from Schwartz PJ, et al. 2011.552 ©2011 American Heart Association, Inc.).
ECG, electrocardiogram; LQTS, long QT syndrome; QTc, corrected QT interval; TdP, torsade de pointes.
QT prolongation referred to as secondary or acquired LQTS based on acquired factors (bradycardia, drug-induced, electrolyte imbalance, and others) is often problematic in routine clinical practice. Therefore, it is necessary to diagnose congenital LQTS after excluding these acquired factors (Table 21).
Table 21.
Main Causes of Secondary Long QT Syndrome
| • Drug-induced* |
Antiarrhythmic drugs, antibacterial drugs, antifungal drugs, antihistamines, antipsychotic drugs, lipid-lowering drugs, and
anticancer drugs |
| • Bradycardia |
| AV block or SSS |
| • Electrolyte imbalance |
| Hypokalemia, hypocalcemia, and hypomagnesemia |
| • Heart disease |
| Acute myocardial infarction, cardiomyopathy, myocarditis, and ampulla cardiomyopathy |
| • Cerebrovascular accident |
| Brain hemorrhage, subarachnoid hemorrhage, cerebral infarction, and other brain or nervous system disorders |
| • Endocrine metabolic disease |
| Hypothyroidism, anorexia nervosa, and female hormones |
*Refer to the CredibleMeds website for information on drugs associated with prolonged QT (https://www.crediblemeds.org/). AV, atrioventricular; SSS, sick sinus syndrome.
a. ECG Diagnosis
The QT interval changes depend on the heart rate (preceding RR interval); thus, it is recommended to use the QTc corrected with Bazett’s correction formula (QT interval/RR interval1/2), which divides the QT interval by the square root of the preceding RR interval. However, QTc is overestimated in individuals with heart rates >75 beats/min, such as children. Accordingly, the Japanese Society of Pediatric Cardiology and Cardiac Surgery recommends the Fridericia correction formula (QT interval/RR interval1/3), which uses the cube root for QT interval correction, or the correction formula to the power of 0.31 (exponential correction) for school physical examinations.553 The normal QTc value for healthy adults is <440 ms, and QTc ≥440 ms is defined as QT prolongation. QTc between 440 and 460 ms is borderline, and QTc ≥480 ms suggests a strong possibility of LQTS.
Together with QT interval prolongation, the T-wave morphology and TWA are also important findings. The T-wave morphology differs by genotype. LQT1 has broad-based T-waves, LQT2 has low-amplitude and notched T-waves, and LQT3 is characterized by late-appearing T-waves with long ST segments (Figure 21). TWA is a phenomenon in which the T-wave changes with each heartbeat, and in LQTS, visually recognizable T-wave changes can be observed.
Patients without clear QT prolongation at rest make up 36% of LQT1 cases and 19% of LQT2 cases.68 Further detailed examination is considered for borderline patients with suspected LQTS based on family or syncope history. Holter ECG can detect diurnal variations in QT intervals, and because TWA is detected at a high rate with left chest leads,554 it is recommended to take ECG recordings using the CC5 and CM5 leads.
b. Exercise Stress ECG
The exercise stress ECG was added to the Schwartz diagnostic criteria revised in 2011 due to its effectiveness for diagnosing QT prolongation 4 min after the recovery period following exercise loading.552 Patients with congenital LQTS have insufficient QT shortening relative to the increased heart rate during exercise. QTc prolongation is observed during the recovery process after the end of loading.555–557 The QT interval during the recovery period varies with genotype.558,559 Although QTc prolongation is observed throughout the recovery period in LQT1,560,561 there is little QTc prolongation during the early recovery period in LQT2 and LQT3; instead, QTc prolongation is observed during the late recovery period.559,562
When exercise stress tests were conducted in 14 patients with congenital LQTS, 9 family members, and 40 control subjects, the criteria using the interval from the R-wave to the T-wave peak at 1 min after the recovery period (delta RT >25 ms) had a diagnostic sensitivity of 73% and specificity of 92%.563 A study that investigated the relatives of 69 patients with LQTS (LQT1, n=28; LQT2, n=20; non-carrier, n=21) using a combination of QTc at rest and QTc 4 min after the recovery period started found that the criteria had a sensitivity of 94% and specificity of 90% for detecting LQTS carriers.559 A study investigating 115 patients with the KCNQ1/ KCNH2 gene in 30 families with congenital LQTS found that the diagnostic sensitivity of the exercise stress test was 92%, and the specificity was 93%.564 The adrenaline test is useful for patients for whom exercise loading is difficult or those who require genotype estimation.565
It is essential to be well prepared when conducting exercise stress or adrenaline tests, such as preparing an emergency cart and defibrillator, due to the associated risk of TdP and VF.
c. Syncope and TdP
Syncope in patients with LQTS is thought to be TdP that spontaneously terminates. On the other hand, when TdP persists and transitions to VF, it triggers sudden cardiac death. Triggers for cardiac events (syncope and sudden cardiac death) in congenital LQTS are characterized by genotype.566,567 LQT1 is triggered by stimulation of the sympathetic nervous system, for instance during exercise (especially swimming), whereas LQT2 involves emotional stress (fear and startle) and is triggered by noise stimuli such as an alarm clock during sleep. Compared with LQT1, in which cardiac events are rare after 20 years of age, LQT2 is characterized by cardiac events in adolescence, and these events also often occur in pre- and postpartum women.568,569 On the other hand, cardiac events in LQT3 occur during sleep and at rest.
d. Family History
Family history, emphasized in the Schwartz diagnostic criteria, allocates 2 points for having a relative (1st-degree relative) who has been diagnosed with congenital LQTS and a close relative (1st- or 2nd-degree relative) who died suddenly at an age <30 years.
4.1.3 Genetic Tests
The first LQTS case reported in 1957 was of Jervell and Lange-Nielsen syndrome, an autosomal latent inherited (recessive) disorder with congenital deafness.555 Although the incidence is relatively rare, it is important in the field of pediatrics because it is a particularly severe form of LQTS. There have been reports of homozygous mutation of the causative genes of LQTS, KCNQ1 and KCNE1, described below.
On the other hand, in the 1960s, Romano and Ward separately reported LQTS without deafness (Romano-Ward syndrome),570–572 an autosomal dominant inheritance disorder, which was subsequently reported in many families. The genetic mutations were named in order of discovery: LQT1 (KCNQ1), LQT2 (KCNH2), and LQT3 (SCN5A). Moreover, 17–18 genotypes have been reported over the subsequent few decades, and the number of candidate causative genes continues to increase.566,573–576 (Table 22). Gene mutations are identified at a relatively high frequency (50–80%) of clinically diagnosed LQTS cases. The characteristics and treatment for each genotype are being investigated in detail, and genetic testing was approved for Japanese insurance coverage on April 1, 2008. Indications for genetic diagnosis comply with the HRS/EHRA joint expert statement317 (see Chapter I.8).
Table 22.
Causative Genes of Congenital Long QT Syndrome
| Type |
Gene locus |
Causative gene |
Ion current |
Characteristics |
| Romano-Ward syndrome |
| LQT1 |
11p15.5 |
KCNQ1 |
I Ks (α) |
High sympathetic sensitivity |
| LQT2 |
7q35-q36 |
KCNH2 |
I Kr (α) |
Sudden death due to sound stimulus |
| LQT3 |
3p21 |
SCN5A |
I Na (α) |
Sudden death during sleep |
| LQT4 |
4q25-q27 |
ANK2 |
I Na/K ATPase |
Associated with severe bradycardia |
| LQT5 |
21q22.12 |
KCNE1 |
I Ks (β) |
Clinical profile resembles LQT1 |
| LQT6 |
21q22.12 |
KCNE2 |
I Kr (β) |
Clinical profile resembles LQT2 |
| LQT7 |
17q23.1-q24.2 |
KCNJ2 |
I K1 |
Anderson Tawill syndrome |
| LQT8 |
12p13.3 |
CACNA1C |
I Ca-L |
Timothy syndrome |
| LQT9 |
3p25 |
CAV3 |
I Na |
|
| LQT10 |
11q23.3 |
SCN4B |
I Na |
|
| LQT11 |
7q21-q22 |
AKAP-9 |
I Ks |
|
| LQT12 |
20q11.2 |
SNTA1 |
I Na |
|
| LQT13 |
11q23.3-24.3 |
KCNJ5 |
I K ACh |
|
| LQT14 |
14q32.11 |
CALM1 |
I Ca-L |
Overlap with CPVT4 |
| LQT15 |
2p21 |
CALM2 |
I Ca-L |
|
| LQT16 |
19q13.2-13.3 |
CALM3 |
I Ca-L |
Overlap with CPVT6 |
| LQT17 |
20q11.2 |
TRDN |
I Ca-L |
Overlap with CPVT5 |
| N/A |
4q13.1 |
TECRL |
Ca handling? |
Overlap with CPVT3 |
| Jervell-Lange-Nielsen syndrome |
| JLN1 |
11p15.5 |
KCNQ1
(homozygous) |
I Ks (α) |
QT prolongation more severe than
LQT1 with congenital deafness |
| JLN2 |
21q22.12 |
KCNE1
(homozygous) |
I Ks (β) |
QT prolongation more severe than
LQT5 with congenital deafness |
CPVT, catecholaminergic polymorphic ventricular tachycardia; LQT, long QT; N/A, not available.
The detection frequency of each gene is 40% for LQT1, 40% for LQT2, and 10% for LQT3, with LQT1–LQT3 accounting for 80–90% of all LQTS cases. Recently, LQT1–LQT3 is being termed as “major LQTS”, while other LQTS variants discovered later and with a low incidence (<2–3%) are termed “minor LQTS”, and gene names are allocated without numbering.577 For example, describing LQT4 as ANK2-LQTS and LQT17 as TRDN-LQTS or TRDN-mediated LQTS is under consideration.575
4.1.4 Risk Assessment
Unlike the risk assessment of organic heart disease that uses cardiac function as a criterion, that of congenital LQTS attempts to be based on age, sex, and genotype8 (Table 23).
Table 23.
Class of Recommendation and Level of Evidence for the Risk Assessment of Sudden Cardiac Death in Congenital Long QT Syndrome
| |
COR |
LOE |
| QTc ≥500 ms depicts high risk, regardless of age, sex, and genotype |
I |
C |
History of cardiac events (syncope and cardiac arrest) is associated with high risk, regardless
of QTc |
I |
C |
Patients with repeated syncope despite treatment with beta-blockers are considered to be at
high risk |
IIa |
C |
Mutations in the transmembrane region in LQT1 and missense mutations in the transmembrane
pore region in LQT2 are considered to be associated with high risk |
IIa |
C |
| LQT1 with QTc <500 ms in male individuals aged <13 years* is considered to depict moderate risk |
IIa |
C |
| LQT1 with QTc <500 ms in male individuals aged ≥13 years is considered to depict low risk |
IIa |
C |
| LQT2 with QTc <500 ms in female individuals aged ≥13 years is considered to depict moderate risk |
IIa |
C |
*The Fridericia correction formula is recommended for QT interval correction in children who have high heart rates. COR, Class of Recommendation; LOE, Level of Evidence; LQT, long QT; QTc, corrected QT interval.
When assessed by genotype, the total cardiac event rate, including that of syncope, has been reported to be lower in LQT3 than in LQT1 and LQT2.544,546 However, LQT3 is reported to have a higher ratio of fatal events to total events.544,546 When considering sex variations, women with LQT1 and LQT2 tend to have a higher rate of cardiac events than men after adolescence.68,544,546 However, boys younger than 13 years of age with LQT1 and LQT2 are reported to have a significantly higher rate of cardiac events than girls in the same age group.544,546 Priori et al reported that of the patients with LQT3, only men with a QTc ≥500 were at high risk of cardiac events.68 This finding has now been negated, and many studies have reported that there is no sex difference.
4.2 Short QT Syndrome (SQTS)
SQTS is a rare disease that causes syncope and sudden cardiac death due to VF against a background of short QT. It is a relatively new disease concept that was first reported in 2000578 and has not yet been fully investigated at the clinical and genetic levels. Typical ECG waveforms of SQTS are shown in Figure 22. In addition to a short QT interval, the diagnosis is based on a comprehensive assessment, including clinical symptoms, family history, and genetic mutations. In 2011, Gollob et al proposed a risk score similar to that for LQTS.579 Although there is a report showing that the risk score is useful for detecting symptomatic SQTS,580 the scoring system is somewhat complex and is not used generally.581,582 The HRS/EHRA/APHRS joint statement recommends the diagnosis of SQTS in cases of QTc ≤330 ms, without consideration of clinical findings.43 On the other hand, the 2015 ESC guideline considers SQTS to have a high mortality rate, with 40% of untreated patients dying before the age of 40 years,583 and accordingly, recommends diagnosis in cases of QTc ≤340 ms to increase the detection sensitivity in asymptomatic patients.188

There is still some debate over setting the QTc cutoff value for asymptomatic SQTS to ≤330 ms or ≤340 ms, although in this guideline, the recommendation table has been created based on previous studies and in accordance with the diagnostic criteria and treatment strategies set out in the HRS/EHRA/APHRS joint statement (Tables 24,25).
Table 24.
Class of Recommendation and Level of Evidence for the Diagnosis of Short QT Syndrome
| |
COR |
LOE |
| QTc ≤330 ms is clinically diagnosed as SQTS |
I |
C |
A diagnosis of SQTS may be considered in cases of QTc between 331 and 360 ms and
satisfying ≥1 of the following conditions:
• Causative gene for SQTS being identified
• Family history of SQTS
• Family history of sudden death before the age of 40 years
• History of VT or VF with no organic heart disease |
IIa |
C |
COR, Class of Recommendation; LOE, Level of Evidence; QTc, corrected QT interval; SQTS, short QT syndrome; VF, ventricular fibrillation; VT, ventricular tachycardia.
Table 25.
Class of Recommendation and Level of Evidence for Risk Assessment of Sudden Cardiac Death Caused by Short QT Syndrome
| |
COR |
LOE |
Symptomatic patients diagnosed with SQTS accompanied by either or both of the following
conditions are at high risk:
• History of cardiopulmonary arrest
• Spontaneous sustained VT (with or without syncope) |
I |
C |
Asymptomatic patients diagnosed with SQTS with a family history of sudden cardiac death
are considered at moderate risk |
IIa |
C |
COR, Class of Recommendation; LOE, Level of Evidence; SQTS, short QT syndrome; VT, ventricular tachycardia.
SQTS is an inherited autosomal dominant disease. To date, 8 causative genes have been reported, encoding for K+
channels, L-type Ca2+
channels, Cl/HCO3–
exchangers, and others (Table 26).43,584 As of January 2022, genetic testing for SQTS is not covered by insurance in Japan.
Table 26.
Causative Genes for Short QT Syndrome
| Type |
Gene locus |
Causative gene |
Ion current |
Characteristics |
| SQT 1 |
7q35-q36 |
KCNH2 |
I Kr (α) |
|
| SQT 2 |
11p15.5 |
KCNQ1 |
I Ks (α) |
|
| SQT 3 |
17q23.1-q24.2 |
KCNJ2 |
I K1 |
|
| SQT 4 |
12p13.3 |
CACNA1C |
I Ca-L |
Overlap with Brugada syndrome |
| SQT 5 |
10p12.33-p12.31 |
CACNB2B |
I Ca-L |
Overlap with Brugada syndrome |
| SQT 6 |
7q21.11 |
CACNA2D1 |
I Ca-L |
Overlap with Brugada syndrome |
| SQT 7 |
3p21 |
SCN5A |
I Na (α) |
Overlap with Brugada syndrome |
| SQT 8 |
2q35 |
SLC4A3 |
Cl–/HCO3– |
|
4.3 Brugada Syndrome
Brugada syndrome presents with a characteristic ST elevation (greatly amplified J-point) in the right precordial lead of the 12-lead ECG. It is a disease that can cause nocturnal ventricular fibrillation (VF), mainly in young to middle-aged men, which may lead to sudden death,585 and has a relatively high prevalence in East Asia, including Japan. Although the coved-type (type 1) ST elevation, a characteristic ECG finding described later, is reported to have a prevalence of 0.02–0.15% in the Western population,586–588 the prevalence is reported to range from 0.1% to 0.3% in the Japanese population.589–591
4.3.1 Diagnosis
Brugada syndrome is diagnosed based on 12-lead ECG findings, with or without symptoms. ST elevation ≥2 mm (0.2 mV) on the 12-lead ECG followed by an inverted T-wave is defined as the coved-type ST elevation (type 1), whereas an ST elevation without an inverted T-wave is called a saddleback ST elevation. If the end of the ST (trough) is ≥1 mm (0.1 mV), it is defined as a type 2 ECG, but if the trough is <1 mm (0.1 mV), it is called a type 3 ECG (Figure 23).592

Brugada syndrome is diagnosed when a type 1 ECG occurs spontaneously after fever or sodium-channel blocker provocation in ≥1 of the V1–V2
leads placed on the 2nd–4th intercostal space, including higher intercostal space positions.43 Brugada syndrome with unexplained cardiac arrest, VF or polymorphic VT, nocturnal agonal respiration, and/or unexplained syncope is called symptomatic Brugada syndrome, and the condition without symptoms is called asymptomatic Brugada syndrome.8 Non-type 1 (types 2 and 3) ECGs are not diagnosed as Brugada syndrome, although a type 1 ECG may appear over time. Therefore, follow-up is essential, especially when a patient is seen at the onset of syncope.
Precordial lead ECGs with higher intercostal space positions and drug provocation with sodium-channel blockers are considered to diagnose Brugada syndrome in patients resuscitated after unexplained cardiac arrest (Table 27).593
Table 27.
Class of Recommendation and Level of Evidence for the Diagnosis of Brugada Syndrome in Resuscitation Cases After Unexplained Cardiac Arrest
| |
COR |
LOE |
Precordial lead ECGs with higher intercostal space positions are conducted for patients
resuscitated after unexplained cardiac arrest |
I |
C |
Drug provocation with sodium-channel blockers is conducted for patients resuscitated
after unexplained cardiac arrest with non-type 1 ECG findings or with clinical characteristics
of Brugada syndrome |
I |
C |
Drug provocation with sodium-channel blockers is considered for patients resuscitated
after unexplained cardiac arrest |
IIa |
C |
COR, Class of Recommendation; ECG, electrocardiogram; LOE, Level of Evidence.
4.3.2 Risk Assessment
There are circadian and daily variations in the ECG waveforms of Brugada syndrome, and reports from Japan have found that Brugada ECGs can even vary with meals.594 and exercise.74 Therefore, risk assessment has been attempted using a combination of multiple clinical and ECG indices.177,259,595 Figure 24 shows the testing flowchart when Brugada syndrome is diagnosed based on ECG data.
a. Presence/Absence of Symptoms
Assessment of the presence or absence of symptoms associated with ventricular tachyarrhythmia is extremely important in patients with Brugada syndrome. Patients with Brugada syndrome with a history of VF and cardiopulmonary resuscitation, as well as a history of unexplained syncope, are prone to cardiac events, such as sudden cardiac death and VF. In Japan, the prevalence of cardiac events with Brugada syndrome is reported to be 8–10% and 0.5–2% per year in patients with a history of VF and syncope, respectively, and ≈0–0.5% per year in asymptomatic patients.170,596 It is important to differentiate syncopal symptoms from those of reflex syncope (vasovagal syncope). If reflex syncope is diagnosed, it is handled as asymptomatic Brugada syndrome, even with a type 1 ECG.
b. 12-Lead ECG Indices
The following types of 12-lead ECG indices have been reported to be useful for risk stratification:43,44
(1) Spontaneous type 1 ECG177,178,597,598
(2) Fragmented QRS findings on right precordial leads and inferolateral ECG leads43,599–602
(3) Early repolarization (ER) (J-waves) findings on inferolateral ECG leads170,596,600,603,604
(4) S waves on I leads (≥0.1 mV and/or ≥40 ms)605
(5) Significant R waves on aVR leads (wave height ≥0.3 mV or R/q ratio ≥0.75)606
(6) RJ interval (S wave width) prolongation on V1
leads (≥90 ms)607,608
(7) Greatly amplified inverted T-wave (≥−105 µV) on V1
leads609
(8) Time from the peak to the end of T-wave on precordial leads [Tpeak–Tend
interval (Tp-e) >100 ms] and its dispersion (Tp-e
dispersion >20 ms)608,610,611
(9) Wide QRS complex (≥90 ms) on V2
leads596 or QTc prolongation (>460 ms)610
(10) Wide QRS complex (≥90 ms) on V6
leads608,612
(11) Wide QRS complex (≥120 ms)600
(12) Wide PQ interval (≥170 ms)590,609
c. Exercise Stress ECG
A systematic review of patients with Brugada syndrome from 1990 to 2013 (18 papers, 166 patients)356 found that 2 patients developed VT and 1 developed multiple premature ventricular contractions (PVCs) during exercise stress tests. ST elevation was observed in 57% of the patients during the early recovery period after exercise, and 3 patients transitioned from a saddleback to a coved-shaped ST elevation. Most of the data in this systematic review relied on the results of 2 large-scale studies;74,355 thus, there are still insufficient data on the risk of exercise for patients with Brugada syndrome. However, exercise-induced ST elevation and VT are independent predictors of arrhythmia events,74 which suggests that limiting strenuous exercise should be considered.
d. Special Indices of ECG Analysis
The utility of the following special indices of ECG analysis has been proposed for predicting a high risk for Brugada syndrome.
i. SAECG
Detection of ventricular LP on the SAECG indicates the presence of an area of conduction delay in the ventricular muscle (depolarization abnormality). There are a large number of reports, mainly from Japan, showing the association between ventricular LP and VF, and the utility of the SAECG has been suggested in prospective studies,613–615 although there is as yet insufficient evidence. Ventricular LP presents with circadian variations,616 and is often detected when ECG waveforms of Brugada syndrome appear.617 Abe et al reported that evaluation of ventricular LP using 24-h Holter ECG was useful for predicting the onset of VF and polymorphic VT246 (see Chapter I.7.4).
ii. HRV
Indices of heart rate variability (HRV) allow assessment of autonomic nervous system activity, and the power value of the high-frequency (HF) component measured with the analysis of the frequency domain sharply reflects vagal nerve activity in particular. Nakazawa et al reported that the HF component was significantly higher in patients with Brugada syndrome with a history of VF than in healthy controls and asymptomatic patients with Brugada syndrome.618 Cardiac events in patients with Brugada syndrome are known to commonly occur at night when going to sleep, in the early morning, or after a meal,74,618–622 suggesting that enhanced vagal nerve activity is involved in the onset of VF.
iii. TWA
TWA is an index that reflects abnormal repolarization, although many reports from Japan have stated that macroscopically distinguishable TWA tends to occur in patients with Brugada syndrome.247,594,623,624 There are also several reports on the utility of time-domain TWA analysis using 24-h Holter ECG.625–627
e. Electrophysiology Study
An EPS is indicated for unexplained syncope with a spontaneous type 1 ECG or when there are factors considered to indicate a risk of sudden death, such as a spontaneous type 1 ECG with clinical history, family history, and other abnormal ECG findings, as well as genetic mutations (Table 28). The protocol for premature ventricular stimulation differs widely, and no method has been established to date.
Table 28.
Class of Recommendation and Level of Evidence for the Diagnosis and Risk Assessment of Brugada Syndrome by Electrophysiology Study
| |
COR |
LOE |
| An EPS is considered for cases of unexplained syncope and a spontaneous type 1 ECG |
IIa |
C |
An EPS may be considered even for asymptomatic patients with a spontaneous
type 1 ECG and other clinical findings that should be considered (age, sex, family history,
and others), other abnormal ECG findings (fragmented QRS and J-waves), and/or
SCN5A mutations |
IIb |
C |
COR, Class of Recommendation; ECG, electrocardiogram; EPS, electrophysiology study; LOE, Level of Evidence.
Generally, the basic cycle is conducted at the right ventricular apex with 600 ms and 400 ms (or 500 ms), as well as at the right ventricular outflow tract with an output of 2-fold of the threshold. Up to double or triple ventricular extra-stimuli are conducted from the right ventricular apex and right ventricular outflow tract, and generally, the shortest stimulation interval is 200 ms. However, Asada et al reported that extra-stimuli with a stimulation interval up to 180 ms were useful as a predictor of VF in asymptomatic Brugada syndrome.602
The positive criteria for induction are generally defined as VT and VF sustained for ≥30 s or requiring electrical cardioversion due to hemodynamic failure. VT/VF inducibility was not a predictor of cardiac events in either the FINGER registry178 or the PRELUDE registry,258 although Brugada et al, reported the importance of consistently confirming inducibility by EPS.175,176 Opinions on whether VT/VF inducibility by EPS is a predictor of cardiac events remain divided. However, a subsequent meta-analysis mainly conducted in Europe reported that induction with single or double ventricular extra-stimuli was useful as a predictor.628,629
The Japanese Guidelines for diagnosis and management of inherited arrhythmias (JCS 2017) recommend referencing risk stratification for differentiation by assessing inducibility with up to double ventricular extra-stimuli by EPS for unexplained syncope with a type 1 Brugada ECG.8 On the other hand, although there are no clear recommendations established for risk stratification by EPS for patients with asymptomatic Brugada syndrome, an EPS may be considered for cases of spontaneous type 1 Brugada ECG presenting with other additional abnormalities, such as family history, SCN5A genetic mutations, fragmented QRS, and J waves8 (Table 28).
f. Genetic Testing
A Japanese report found that patients positive for SCN5A mutation, particularly those with mutations in the pore region, experienced cardiac events more frequently than those without this mutation.318 A report from Italy also found SCN5A mutation to be a useful predictor of prognosis.630 Genetic testing should be considered for suspected symptomatic Brugada syndrome and for relatives of probands with the genetic mutation (see Chapter I.8). On the other hand, there is no evidence of the utility of genetic testing for patients with Brugada syndrome with non-type 1 (type 2 and type 3) ECG and no family history; therefore, the test should be performed with caution.318
4.4 Early Repolarization Syndrome (ERS) and J-Wave Syndrome
ER findings on an ECG were previously assumed to have no pathological significance, but recently, an association between ER and the onset of VF has been clarified. In 1953, Osborn reported that a camel-hump wave (Osborn wave) immediately following the QRS complex was associated with the onset of VF in hypothermic dogs with acidosis.631 In 1993, Aizawa et al reported on a patient with ER who developed VF.632 In 2008, Haïssaguerre et al compiled and reported cases of ERS associated with VF, and proposed the disease concept of ERS.633 The rate of positive findings of ER ranges from 3% to 24% in healthy individuals,62,633–640 and from 23% to 44% in patients with VF without organic heart disease,633,641–647 demonstrating the higher rate of ER in patients with VF compared with healthy individuals. ER is reported to be more common in young men, athletes, African Americans, and people from South-East Asia, including Japan.640,648 Although ERS and Brugada syndrome have many points in common, including epidemiology, clinical symptoms, and ECG findings, there are also differences.44
4.4.1 Diagnosis
ERS is diagnosed in patients who satisfy both (1) ER findings on 12-lead ECG and (2) clinical findings (Table 29).8,43
Table 29.
Diagnostic Criteria for ER and ERS
| Diagnosis of ER |
Slur or notch type ER pattern with a J-point elevation ≥0.1 mV found in ≥2 contiguous inferior and/or lateral leads on
12-lead ECG |
| Diagnosis of ERS |
| The following patient with ER pattern: |
| • VF or polymorphic VT without organic heart disease |
| • Unexplained cardiopulmonary resuscitation or sudden cardiac death |
ECG, electrocardiogram; ER, early repolarization; ERS, early repolarization syndrome; VF, ventricular fibrillation; VT, ventricular tachycardia. (From Japanese Circulation Society 2018.8)
a. ER Findings on 12-Lead ECG
An ER pattern is defined as a J-wave elevation ≥0.1 mV on ≥2 inferior leads and/or ≥2 lateral leads. J-waves are classified as notch type or slur type based on the shape of the wave (Figure 25). Differentiation of a bundle branch block is defined as a QRS complex <120 ms on a lead without notch or slur waveforms.649 It is necessary to differentiate this condition from ER findings associated with the diseases shown in Table 30.
Table 30.
Main Diseases That Should Be Differentiated From Early Repolarization Syndrome
| Heart diseases |
Brugada syndrome, ARVC/ACM, ischemic heart disease, myocarditis, epicarditis, heart tumor |
| Non-heart diseases |
Hypothermia, electrolyte imbalance (hypocalcemia, hypokalemia) |
ACM, arrhythmogenic cardiomyopathy; ARVC, arrhythmogenic right ventricular cardiomyopathy.
b. Clinical Findings
Clinical findings include VF or polymorphic VT without apparent organic heart disease and cases of unexpected cardiopulmonary resuscitation or sudden cardiac deaths. However, it is necessary to exclude VF or polymorphic VT induced by coronary spasm.650
4.4.2 Risk Assessment
The testing flowchart when an ER pattern is found on ECG is shown in Figure 26.
a. Clinical Symptoms
It has been reported that patients with a history of syncope are relatively common among ERS patients with a history of VF.44 On the other hand, it is extremely difficult to predict sudden death in cases of ER findings other than in patients with a history of VF or of resuscitation after cardiac arrest. For example, even if ER findings are discovered through medical checkups or screening, the risk of developing VF in the future is said to be very low.62
b. 12-Lead ECG Indices
The following findings on the 12-lead ECG suggest a high risk of cardiac events: (1) extensive J-point elevation in the inferior and lateral ECG leads, (2) J-point elevation >0.2 mV, (3) horizontal or descending ST segment, and (4) J-wave findings with large circadian or daily variations.651 Kamakura et al reported that among 31 ERS patients with a history of VF, VF recurrence was significantly more common in the 12 with non-type 1 (type 2 or 3) ST elevation in the right precordial leads (non-type 1 findings also on higher intercostal space positions and after drug provocation with sodium-channel blockers) than in the 19 without these findings.652 On the other hand, in Brugada syndrome, ER in the inferior leads is known to increase the risk of VF.170,603 These findings suggest that ER findings in extensive leads are high risk for VF in both Brugada syndrome and ERS.
Assessment of ECG precordial lead waveforms when using higher intercostal space positions and drug provocation with sodium-channel blockers is useful for evaluating VF risk in patients with ERS.
c. Special ECG Indices
Abe et al analyzed ventricular LP, an indicator of abnormal depolarization, by time period based on 24-h ECG recordings from 22 patients with a history of VF with ERS (n=7) and without ERS (non-ERS, n=15). The ERS group showed circadian variations in ventricular LP whereby it was highly positive at night, suggesting that evaluation of circadian variation in ventricular LP may be useful for risk stratification.644 On the other hand, Soliman et al reported no association between ER findings on ECG and ventricular LP, as well as results of the analysis of HRV in 687 people who underwent Holter ECG recording.653
d. EPS
Mahida et al conducted an EPS in 81 patients (mean age 36 years, men n=60) who had ERS with a history of cardiac arrest from VF in multiple facilities, including medical facilities, in Japan.651 VF was induced in 18 of 81 patients (22%), and follow-up was conducted for an average of 7 years. VF recurrence was observed in 6 of 18 patients (33%) in the VF induction group and in 21 of 63 patients (33%) in the VF non-induction group, with no difference between the 2 groups. Although this report only examined patients with a history of VF and did not include asymptomatic cases, the utility of the EPS as risk stratification in patients with ERS is assumed to be low.133
4.5 Catecholaminergic Polymorphic Ventricular Tachycardia (CPVT)
CPVT is a relatively rare arrhythmia,654–656 and patients usually develop VT or VF with exercise or adrenaline administration at around 10 years of age; thus it is an important cause of syncope or sudden death in young people.657 Patients with CPVT do not show structural or functional abnormalities on imaging diagnostics such as echocardiography, CT, and MRI, as well as notable abnormal findings on a resting ECG. Therefore, it is difficult to screen patients with asymptomatic CPVT in routine clinical examinations. The Class of Recommendation and Level of Evidence for the diagnosis of CPVT are shown in Table 31.
Table 31.
Class of Recommendation and Level of Evidence for the Diagnosis of Catecholaminergic Polymorphic Ventricular Tachycardia
| |
COR |
LOE |
Structural/functional heart disease is excluded through diagnostic imaging modalities, such as a
resting ECG, TTE, CT, and MRI |
I |
C |
Genetic testing is conducted in patients with CPVT who have been resuscitated after cardiac
arrest or those with clinically confirmed VF when exercise stress tests and drug provocation tests
are difficult to perform after resuscitation |
I |
C |
Exercise stress tests or adrenaline provocation tests are considered for induction of polymorphic
VT or bidirectional VT |
IIa |
C |
| Holter ECG is considered for the detection of polymorphic VT or bidirectional VT |
IIa |
C |
| EPS to induce VT/VF should not be performed |
III
(Harm) |
C |
COR, Class of Recommendation; CPVT, catecholaminergic polymorphic ventricular tachycardia; CT, computed tomography; ECG, electrocardiogram; EPS, electrophysiology study; LOE, Level of Evidence; MRI, magnetic resonance imaging; TTE, transthoracic echocardiography; VF, ventricular fibrillation; VT, ventricular tachycardia.
4.5.1 Clinical Findings and Tests
a. Symptoms (Syncope, VF)
The primary symptom of CPVT is syncope, usually associated with exercise or emotional stress.654,655 Sumitomo et al reported that CPVT was discovered due to syncope in 23 (79%) and cardiac arrest in 2 (7%) of 29 patients diagnosed with CPVT.655 The earliest syncope event often occurs before the age of 20 years (mainly between the ages of 7 and 12 years).658 Cardiac arrest may also be the first symptom, and sudden infant death syndrome659 and idiopathic VF may also be caused by CPVT.660 CPVT mainly presents as loss of consciousness or ventricular arrhythmia during exercise, tension, or emotional stress in young people. Therefore, it is important to differentiate CPVT from type 1 congenital LQTS (LQT1).661 Some patients previously diagnosed as clinical LQT1 may actually be diagnosed as CPVT.
b. Resting ECG
The resting ECG of CPVT is generally within the normal range, although sinus bradycardia is found in ≈20% of cases,662,663 and U-waves may also be found.48 Supraventricular arrhythmias such as AF and SSS are also found in 16–26% of cases.656,664,665
c. Exercise Stress ECG and Provocative Drug ECG
The exercise stress test is the most powerful test for the diagnosis of CPVT,656 because it can reproducibly induce ventricular arrhythmia.654,655 A PVC appears when the heart rate exceeds 120–130 beats/min during exercise, although continued exercise can cause polymorphic VT and bidirectional VT (tachycardia where the QRS polarity changes 180 degrees with each heartbeat, and the coupling period between VT waveforms is largely constant). In addition, the induction of extremely fast VT/VF can cause syncope and sudden death65,655,666 (Figure 27).65

Bidirectional VT is an important clinical characteristic of CPVT, although the incidence is not necessarily high, and mild cases may only have a single PVC or bigeminy.656 PVC bigeminy or trigeminy with left bundle branch block (LBBB) and inferior axis is considered specific to CPVT, with a positive and negative predictive value of 100% and 92%, respectively,667 although there are also reports of cases presenting with right bundle branch block (RBBB) and superior axis.65 CPVT may also present as irregular polymorphic VT without constant QRS alternans.668,669 Diagnosis of CPVT with the appearance of bidirectional VT by conducting exercise stress tests in asymptomatic CPVT family members has a specificity of 97% and sensitivity of 50%.670 Andersen-Tawil syndrome (ATS and LQT7) must be differentially diagnosed from CPVT. ATS patients have a frequent RBBB-type PVC or VT at rest before exercise, and VT is suppressed during peak exercise. In contrast, CPVT patients usually have no arrhythmia at rest and exercise mainly induces LBBB-type ventricular arrhythmia.671
The adrenaline provocation test is considered to be useful for diagnosing patients who cannot perform exercise stress tests.43,65 Although its specificity was 98%, the sensitivity was only 28% from the results of adrenaline provocation in 36 patients with CPVT and 45 of their family members.672
d. Holter ECG
The Holter ECG656 is especially useful for patients unable to perform exercise stress tests (post-resuscitation patients, infants, and others) and for the detection of arrhythmia when symptoms appear during emotional stress. However, the sensitivity of arrhythmia detection is lower than that of the exercise stress test.65
e. Electrophysiology Study
The bidirectional VT, polymorphic VT, and PVCs observed in CPVT are reported to originate from the Purkinje network, and ablation therapy is considered a treatment option for drug-resistant cases and cases of frequent cardioversion of an ICD. However, there is little usefulness in diagnosing CPVT by EPS, and the ESC guidelines contraindicate VT/VF induction by EPS as a risk for sudden death.188
f. Genetic Tests
Genetic abnormalities are found in 60–70% of patients with CPVT. Although several types of causative genes have been reported, the ryanodine receptor gene RYR2 is the most common (Table 32).8 The ESC guidelines188 indicate that only RYR2 and CASQ2 are causative genes of CPVT.
Table 32.
Catecholaminergic Polymorphic Ventricular Tachycardia Subtypes
| |
CPVT1 |
CPVT2 |
CPVT3 |
CPVT4 |
CPVT5 |
CPVT-associated diseases |
| ATS |
LQT4 |
| Percentage (%) |
50~60 |
1 |
≪ 1 |
≪ 1 |
≪ 1 |
≪ 1 |
≪ 1 |
| Mode of inheritance |
AD |
AR |
AR |
AD |
Sporadic |
AD |
AD |
Onset of first symptoms
(age, in years) |
10 |
7 |
22, 18, 4 |
4 |
2, 26 |
14. 9, 17 |
? |
| Sex difference (M:F) |
1 : 1 |
1 : 1 |
1 : 1 |
1 : 1 |
M=3 |
F>M? |
? |
| Chromosome locus |
1q43 |
1p13.1 |
4p13.1 |
14q32.11 |
6q22.31 |
17q24.3 |
4q25-26 |
| Causative gene |
RYR2 |
CASQ2 |
TECRL |
CALM1 |
TRDN |
KCNJ2 |
ANK2 |
| Protein |
Ryanodine
receptor |
Calsequestrin |
TECRL |
Calmodulin |
Triazine |
Kir2.1α |
Ankyrin B |
Incidence of sudden
cardiac death (%) |
≈10 |
≈42 |
≈57 |
≈18 |
≈25 |
? |
? |
AD, autosomal dominant; AR, autosomal recessive; ATS, Andersen-Tawil syndrome; CPVT, catecholaminergic polymorphic ventricular tachycardia; F, female; LQT, long QT; M, male. ANK2, ankyrin 2; CALM1, calmodulin 1; CASQ2, calsequestrin 2; RYR2, ryanodine 2; TECRL, trans-2;3-enoyl-CoA reductase like protein; TRDN, triazine; KCNJ2, inward-rectifier potassium channel subfamily J member 2. (From Japanese Circulation Society 2018.8)
To date, more than 150 RYR2 pathogenic variants have been reported as the cause of CPVT, many of which are concentrated in specific regions.673 Furthermore, although this variant displays autosomal dominant inheritance, familial cases are rare, but many are sporadic. CASQ2674 and TRDN675 exhibit autosomal recessive inheritance, which is considered a more severe phenotype than those of RYR2 mutations. A report investigating 36 CPVT patients (probands) with CASQ2 homozygous or compound heterozygous abnormalities and 76 family members with only single genetic abnormalities found that the risk of sudden death was extremely high in the probands (76–97%). On the other hand, 33% of the family members with heterozygous abnormalities were clinically diagnosed with CPVT.676
Therefore, genetic testing is recommended for patients with clinically diagnosed or suspected CPVT based on factors such as medical history, family history, and ECG findings, to confirm the diagnosis (not covered by health insurance in Japan, 2022). Genetic testing for family members of the proband is also recommended to determine if they also have the genetic abnormality317 (see Chapter I.8).
4.5.2 Diagnostic Criteria
CPVT is diagnosed in the presence of a structurally normal heart, normal ECG, as well as unexplained exercise or adrenaline-induced bidirectional VT and polymorphic PVCs or VT, in individuals younger than 40 years. CPVT is also diagnosed in patients (index case or family member) who have a pathogenic mutation in CPVT-related genes. If a polymorphic PVC, bidirectional VT, or polymorphic VT is induced by exercise in the proband’s family members, it is diagnosed as CPVT, regardless of an absence of heart disease in the proband’s family.43
4.6 Arrhythmogenic Right Ventricular Cardiomyopathy (ARVC), Arrhythmogenic Cardiomyopathy (ACM), and Arrhythmogenic Left Ventricular Cardiomyopathy (ALVC)
ARVC is characterized by changes in fibrofatty tissue, with progression of fatty degeneration and fibrosis mainly in the right ventricle. ARVC has been reported as a disease that causes ventricular arrhythmia originating from the right ventricle, resulting in sudden death, and the prevalence is reported to be 1 in 1,000–5,000 people.677,678 ARVC is categorized as an inherited cardiomyopathy, and heterozygous mutation of genes related to intercellular desmosomes or the involvement of complex genetic mutations has been suggested.72 However, recently there have been reports of non-desmosome cases and cases unrelated to genetic mutations.679 Lesions may also be found not only in the right ventricle but also in both ventricles or in the left ventricle alone, which is called ACM in the broad sense.680
ACM has diverse genetic backgrounds and clinical features, although the basic diagnostic criteria are set out in the Task Force Criteria published in 2010 (2010-TFC).269 Subsequent advances in diagnostic imaging and genetic diagnosis were incorporated into the HRS expert consensus statement,680 published in 2019 and the Padua criteria,681 as well as assessment of the ESC diagnostic criteria and differential diagnosis,682 both published in 2020.
ARVC/ACM may cause fatal ventricular arrhythmia leading to sudden death, even when there are only mild structural changes in the ventricle or at a stage when there is no apparent cardiac dysfunction. Therefore, early diagnosis is important from the perspective of patient prognosis. The Class of Recommendation and Level of Evidence for the diagnosis and risk assessment of ARVC/ACM are shown in Table 33. The indication for an ICD must also be considered for treatment, and diagnostic criteria that emphasize specificity in addition to diagnostic sensitivity are needed. The current guideline was created based on the 2010-TFC and incorporated the content of the 2019 HRS expert consensus statement (Table 34).680,681 The 2010-TFC has 6 categories, each of which has major and minor criteria. The major criteria are calculated as 2 points, and the minor criteria are calculated as 1 point. Diagnosis is classified as definite, borderline, and possible with a score of ≥4 points, 3 points, and 2 points, respectively.
Table 33.
Class of Recommendation and Level of Evidence for the Diagnosis and Risk Assessment of ARVC/ACM
| |
COR |
LOE |
| The 2010 Task Force Criteria269 are used for the diagnosis of ARVC/ACM | I |
C |
| Echocardiography/cardiac MRI is performed to assess ventricular dysfunction and structural changes |
I |
B |
Inverted T-waves, epsilon waves, and QRS complex TAD in precordial leads on 12-lead ECG are
evaluated for the diagnosis of ARVC/ACM |
I |
B |
| Holter ECG is used for patients with suspected ARVC/ACM |
I |
B |
| Detailed family history and exercise history are obtained for patients with suspected ARVC/ACM |
I |
C |
| SAECG is considered for the diagnosis of patients with suspected ARVC/ACM |
IIa |
B |
Gadolinium-enhanced cardiac MRI is considered for evaluating the histological characteristics of
the ventricle |
IIa |
C |
ACM, arrhythmogenic cardiomyopathy; ARVC, arrhythmogenic right ventricular cardiomyopathy; COR, Class of Recommendation; ECG, electrocardiogram; LOE, Level of Evidence; MRI, magnetic resonance imaging; SAECG, signal-averaged electrocardiogram; TAD, terminal activation duration.
Table 34.
Modified Task Force Criteria for ARVC – Diagnostic Categories Major and Minor Criteria
Definite: 2 major OR 1 major and 2 minor, OR 4 minor criteria from different categories
Borderline: 1 major and 1 minor, OR 3 minor criteria from different categories
Possible: 1 major, OR 2 minor criteria from different categories |
| |
Major |
Minor |
| Global or regional dysfunction and structural alterations determined by echo, MRI, or RV angiography: |
| Echo |
Regional RV akinesia, dyskinesia, or aneurysm
and 1 of the following (end diastole):
a) PLAX RVOT ≥32 mm
(PLAX/BSA ≥19 mm/m2)
b) PSAX RVOT ≥36 mm
(PSAX/BSA ≥21 mm/m2)
c) Fractional area change ≤33% |
Regional RV akinesia, dyskinesia, or aneurysm
and 1 of the following (end diastole):
a) PLAX RVOT ≥29 mm to <32 mm
(PLAX/BSA ≥16 to <19 mm/m2)
b) PSAX RVOT ≥32 to <36 mm
(PSAX/BSA ≥18 to <21 mm/m2)
c) Fractional area change >33 to ≤40% |
| MRI |
Regional RV akinesia or dyskinesia or
dyssynchronous RV contraction and 1 of the
following:
a) Ratio RVEDV/BSA ≥110 mL/m2 (male),
≥100 mL/m2 (female)
b) RVEF ≤40% |
Regional RV akinesia or dyskinesia or
dyssynchronous RV contraction and 1 of the
following:
a) Ratio RVEDV/BSA ≥100 to <110 mL/m2
(male), ≥90 to 100 mL/m2 (female)
b) RVEF >40 to ≤45% |
| RV angiography |
Regional RV akinesia, dyskinesia, or aneurysm |
|
| Tissue characterization of wall |
Endomyocardial biopsy showing
fibrous replacement of the RV
free wall myocardium in ≥1
sample, with or without fatty
replacement and with: |
Residual myocytes <60% by morphometric analysis
(or <50% if estimated) |
Residual myocytes 60% to 75% by morphometric
analysis (or 50% to 65% if estimated) |
| Repolarization abnormalities |
| ECG |
Inverted T waves in right precordial leads (V1, V2,
and V3) or beyond in individuals >14 years of age
(in the absence of complete RBBB QRS ≥120 ms) |
I. Inverted T waves in leads V1 and V2 in individuals
>14 years of age (in the absence of complete
RBBB) or in V4, V5, or V6
II. Inverted T waves in leads V1, V2, V3, and V4 in
individuals >14 years of age in the presence of
complete RBBB |
| Depolarization/conduction abnormalities |
| ECG |
Epsilon wave (reproducible low-amplitude signals
between end of QRS complex to onset of the T
wave) in the right precordial leads (V1 to V3) |
I. Late potentials by SAECG in ≥1 of 3 parameters
in the absence of QRS duration of ≥110 ms
on the standard ECG:
a) Filtered QRS duration (fQRS) ≥114 ms
b) Duration of terminal QRS <40 μV (low-
amplitude signal duration) ≥38 ms
c) Root-mean-square voltage of terminal 40 ms
≤20 μV
II. Terminal activation duration of QRS ≥55 ms
measured from the nadir of the S wave to the
end of the QRS, including R’ in V1, V2, or V3 in
the absence of complete RBBB |
| Arrhythmias |
| |
Nonsustained or sustained VT of LBBB with
superior axis (negative or indeterminate QRS in
leads II, III, and aVF and positive in lead aVL) |
I. Nonsustained or sustained VT or RV outflow
configuration, LBBB morphology with inferior
axis (positive QRS in II, III and aVF and
negative in lead aVL) or of unknown axis
II. >500 ventricular extrasystoles per 24 hours
(Holter) |
| Family history |
| |
I. ARVC confirmed in a first-degree relative who
meets current Task Force Criteria
II. ARVC confirmed pathologically at autopsy or
surgery in a first-degree relative
III. Identification of a pathogenetic mutation
categorized as associated or probably
associated with ARVC in the patient under
evaluation |
I. History of ARVC in a first-degree relative in
whom it is not possible or practical to determine
whether the family member meets current
Task Force Criteria
II. Premature sudden death (<35 years of age) due
to suspected ARVC in a first-degree relative
III. ARVC confirmed pathologically or by current
Task Force Criteria in second-degree relative |
BSA, body surface area; ECG, electrocardiogram; echo, echocardiogram; MRI, magnetic resonance imaging; PLAX, parasternal long-axis; PSAX, parasternal short-axis; RBBB, right bundle branch block; RV, right ventricular; RVEDV, right ventricular end-diastolic volume; RVEF, right ventricular ejection fraction; RVOT, right ventricular outflow tract; SAECG, signal-averaged electrocardiogram; VT, ventricular tachycardia. (From Towbin et al 2019.680 ©2019 Heart Rhythm Society, with permission from Elsevier.)
4.6.1 Global or Regional Dysfunction and Structural Alterations
Global or regional dysfunction and structural alterations in the ventricles are evaluated on echocardiography or cardiac MRI. Cardiac MRI is particularly useful for the diagnosis of ARVC/ACM, which manifests varied phenotypes. Right ventriculography should only be considered when performing an endomyocardial biopsy. Gadolinium-enhanced MRI has a high negative predictive value,682 and therefore may be useful for exclusion of ARVC/ACM. However, follow-up over time is important for relatives diagnosed as ARVC/ACM.
4.6.2 Tissue Characterization of the Right Ventricular Wall
The 2010-TFC assessment is based on myocardial tissue samples collected via myocardial biopsy or surgery. Finding fibrous replacement of the right ventricular free wall myocardium is classified as a major or minor criterion depending on the number of residual myocytes. Whether or not there is fat replacement is not important. Histological assessment using cardiac MRI has not been adopted as a diagnostic criterion, although recently, combining evaluation of regional wall motion dysfunction with late gadolinium enhancement (LGE) using MRI as diagnostic imaging has been reported to be useful for the diagnosis of ARVC/ACM.682 Currently, it is reasonable to use this method as an adjunctive diagnosis.
4.6.3 ECG (Repolarization Abnormalities)
Repolarization abnormalities are classified as major criteria when there are inverted T-waves in the right precordial leads V1–V3
(and V4
or beyond) with QRS ≥120 ms and in the absence of complete RBBB. Inverted T-waves in leads V1
and V2
or V4–V6
with the absence of complete RBBB (Figure 28), or inverted T-waves in leads V1–V4
with the presence of complete RBBB are classified as minor criteria. Inverted T-waves in leads V1–V4
may be observed in healthy individuals aged ≤14 years, although it does not correspond to the diagnostic criteria.
A recent report from Japan suggested that inverted T-waves in the inferior and precordial leads, as well as T-wave discontinuity in the precordial leads, on pediatric ECG are characteristic of ARVC/ACM.683
4.6.4 ECG (Depolarization Abnormalities)
The epsilon wave, a signal of low amplitude, from the end of the QRS complex to the onset of the T-wave in the right precordial leads is a characteristic ECG finding of ARVC/ACM (Figure 28). However, careful evaluation is needed because other diseases may also present with ECG findings similar to the epsilon wave. Particular caution is needed when there are no other findings suggesting ARVC/ACM.
Assessment with ventricular LP observed on SAECG is only used when the QRS duration on the 12-lead ECG is <110 ms. Finding 1 of the 3 ventricular LPs is classified as a minor criterion. On the other hand, a terminal activation duration of QRS ≥55 ms measured from the nadir of the S wave to the end of the QRS (including R’) in any of the V1–V3
leads on 12-lead ECG in the absence of complete RBBB satisfies the minor criteria.
4.6.5 Arrhythmias
Non-sustained or sustained VT of LBBB with superior axis is highly likely to be associated with ARVC/ACM and is classified as a major criterion (Figure 29). On the other hand, in non-sustained VT, sustained VT, or right ventricular outflow configuration, LBBB morphology with inferior axis or of unknown axis has low specificity, which corresponds to the minor criteria. Ventricular arrhythmia inducibility by EPS for suggested ARVC/ACM is not included in the diagnostic criteria. PVCs >500 per 24 h on Holter ECG are often found in ARVC/ACM, which corresponds to the minor criteria. Premature ventricular contraction from the inferior wall of the right ventricle is more specific than that originating from the right ventricular outflow tract. Thus, evaluating the QRS complex of PVCs with treadmill stress tests and 12-lead Holter ECGs is useful for the diagnosis.

The development of arrhythmia in ARVC/ACM significantly affects prognosis. Therefore, it is important to evaluate arrhythmia with Holter ECG regularly even if a definitive diagnosis is not made.
4.6.6 Family History
ARVC/ACM confirmed in a 1st-degree relative (parent or sibling with 50% shared genes) who meets the 2010-TFC, and ARVC/ACM confirmed pathologically with autopsy or surgery in a 1st-degree relative are classified as major criteria. Identification of a pathogenetic mutation categorized as associated or probably associated with ARVC/ACM in patients under evaluation is also classified as a major criterion. Desmosome-associated gene mutations account for approximately 50% of all ARVC/ACM cases, and PKP2 mutation is the most common.682 Reports from Japan indicate that DSG2 mutation is the most common, accounting for 48% of cases.684 However, it is unclear whether these differences in genetic mutations affect the development of arrhythmia and/or prognosis.
4.6.7 Exercise and ARVC/ACM
ARVC/ACM is known to be common in athletes, and a retrospective study has shown that ARVC/ACM progresses with exercise intensity.685 Patients diagnosed with ARVC/ACM in adolescence are more likely to have engaged in endurance sports, such as marathons, cross-country running, and so on, than those diagnosed in adulthood.686 Therefore, it is very important to obtain a detailed exercise history. Increased catecholamine levels in ARVC/ACM significantly affect the induction of ventricular arrhythmias,72,73 and exercise increases the risk of sudden cardiac death.685,688,689 Thus, it is necessary to restrict patients from doing competitive sports and high-intensity recreational activities.690 There are very few reports on exercise stress tests and exercise prescriptions. A report compared drug provocation tests with exercise stress tests in 37 patients with ARVC/ACM and found that the incidence of polymorphic ventricular arrhythmia was significantly higher with isoproterenol testing (89.2% vs. 43.2%).691 A cardiopulmonary exercise stress test in 29 patients with ARVC/ACM found that patients whose ventilation efficiency indices (V˙E/V˙CO2
slope) exceeded 34 during exercise were more likely to develop moderate/severe right ventricular enlargement and heart failure.692
4.6.8 Diagnosis of ALVC
The etiology of some hereditary diseases, such as HCM, LQTS, Brugada syndrome, and CPVT, is assumed to be associated with abnormal proteins due to mutation of the causative gene. The mechanism of the final common pathway in ARVC is thought to be dysfunction of the desmosome or intercalated disk. On the other hand, confirmation of genetic mutations associated with ARVC/ACM (mutations of genes encoding desmoplakin, phospholamban, lamin A/C, filamin C, and others) with ventricular arrhythmia originating from the left ventricle or left ventricular structural alterations that do not satisfy the ARVC/ACM diagnostic criteria is diagnosed as ALVC or left-dominant variant of ARVC. ECG findings are characterized by low potentials (<0.5 mV) in the limb leads and inverted T-waves in the inferior leads, as well as VT presenting as RBBB type (Figure 30). In fact, patients diagnosed with ARVC present with a variety of clinical features, including left ventricular myocardial damage and possibly the biventricular type with ventricular arrhythmia originating from the left ventricle. Therefore, a comprehensive diagnosis along with genetic testing is essential.

4.6.9 Differential Diagnosis of Similar Diseases
It is necessary to make a differential diagnosis between ARVC/ACM and idiopathic outflow tract VT because both conditions present with ventricular arrhythmia originating from the right ventricle.682 ARVC/ACM is also morphologically similar to athlete’s heart, intracardiac shunts, and pulmonary hypertension; thus, a differential diagnosis is needed.682,693 Diseases that require a differential diagnosis from cases of left ventricular dominant lesions include DCMneuromuscular disease, cardiac sarcoidosis, and myocarditis. The 2010-TFC has high diagnostic sensitivity. However, diseases such as cardiac sarcoidosis may satisfy the diagnostic criteria, so caution is needed.
4.7 Other Inherited Arrhythmias [Progressive Cardiac Conduction Defect (PCCD), Among Others]
PCCD and familial AF/atrial standstill are included in other inherited arrhythmias. This section will mainly focus on PCCD.
PCCD is an inherited arrhythmia characterized by progressive and familial AVblock, as well as bundle branch block. PCCD is a syndrome characterized by various pathological findings, and several arrhythmias, cardiomyopathies, and musculoskeletal myopathies are thought to clinically and genetically overlap with PCCD. The phenotype of PCCD mainly presents in people up to 50 years of age without any underlying heart disease.43 However, there is as yet no consensus on how the age criterion should be set, whether PCCD should be ruled out if there is underlying heart disease, or whether musculoskeletal myopathies should be excluded. The Class of Recommendation and Level of Evidence for the diagnosis of PCCD are shown in Table 35.
Table 35.
Class of Recommendation and Level of Evidence for the Diagnosis of Progressive Cardiac Conduction Defect
| |
COR |
LOE |
PCCD is diagnosed in the presence of unexplained progressive conduction
abnormalities in young (≤50 years) individuals with structurally normal hearts in the absence
of skeletal myopathies, especially if there is a family history of PCCD |
I |
C |
TTE, cardiac CT, cardiac MRI, and PET are useful to diagnose PCCD combined with DCM
and left ventricular non-compaction, or to exclude secondary cardiomyopathies such as
cardiac sarcoidosis and amyloidosis |
I |
C |
COR, Class of Recommendation; CT, computed tomography; DCM, dilated cardiomyopathy; LOE, Level of Evidence; MRI, magnetic resonance imaging; PCCD, progressive cardiac conduction defect; PET, positron emission tomography; TTE, transthoracic echocardiography.
4.7.1 Clinical Findings and Tests
It may be difficult to differentiate PCCD from age-related conduction disorders, such as SSS and bundle branch block, although differential diagnosis may be possible to a certain extent based on ECG findings, family history, and age at onset.
a. Symptoms
Symptoms of PCCD vary depending on the severity of the conduction disorder, ranging from asymptomatic to lightheadedness, dizziness, syncope, convulsions, cardiac arrest, and sudden death. Cerebral ischemic symptoms with bradycardia (Adams–Stokes seizures) may also occur. PCCD is not a single disease concept, and the symptoms vary significantly depending on the overlapping cardiomyopathies and musculoskeletal myopathies. In particular, PCCD patients with the LMNA mutation mainly develop conduction disorders and atrial arrhythmias generally before the age of 30 years, complicated by decreased cardiac function and severe ventricular arrhythmias after the age of 40 years, which often progresses to sudden death or DCM.321,322,694
b. Family History
Taking a family history is extremely important for the diagnosis of PCCD. Patients with a family history of pacemaker implantation or sudden death before 50 years of age are highly likely to have genetic mutations in causal genes of PCCD, such as SCN5A or LMNA.
c. Resting ECG
ECG findings, such as bifascicular block and Mobitz type II or higher AV block, indicate progressive conduction disorders in PCCD. However, age-related conduction disorders often present with similar ECG findings. Therefore, ECG findings with only RBBB, isolated hemiblocks, 1st-degree or Wenckebach 2nd-degree AV block, and sporadic cases of individuals aged ≥70 years at diagnosis are excluded from PCCD.
ECG features in patients with SCN5A mutation often overlap with those in PCCD patients with Brugada syndrome. Conduction disorders associated with the LMNA mutation may show various phenotypes including P-wave abnormalities, PQ prolongation, atrial standstill, SSS, bundle branch block, and AV block, and often induce sudden death due to ventricular arrhythmias.
d. Holter ECG
The Holter ECG is effective for detecting bradyarrhythmia or tachyarrhythmia. First-degree AV block with bifascicular block and symptomatic high-grade AV block are considered risk factors for sudden death.43
e. Cardiac EPS
Measuring sinus node recovery time, the AH and HV intervals with an EPS is useful for determining whether pacemaker implantation is indicated;2 however, there is no evidence to indicate that induction of VT/VF with programmed electrical stimulation is useful for assessing the risk of sudden death.
f. Imaging Tests
Generally, PCCD is mainly associated with cardiac conduction disorders, and cardiac function is often preserved. However, some patients with PCCD, such as those with genetic mutations in SCN5A or LMNA, may have complications related to cardiomyopathy, including DCM or left ventricular non-compaction. Therefore, diagnostic imaging such as echocardiography, as well as cardiac CT and MRI, is useful. Cardiac sarcoidosis, amyloidosis, and other cardiomyopathies need to be excluded because they can also cause severe arrhythmias, heart failure, and cardiac conduction disorders.
g. Genetic Tests
Genetic testing is useful for the differential diagnosis of the causative disease of PCCD. SCN5A, LMNA, and a non-specific cation channel gene (TRPM4)695 are considered the main genes associated with PCCD. Makita et al reported that SCN5A mutation was identified in 19% of familial PCCD cases, and LMNA mutation was identified in 15% of cases.696
The clinical utility of genetic testing for PCCD is now limited and is not covered by insurance in Japan. However, if a relative has PCCD or there is a family history of sudden death, genetic testing is recommended unless the proband is of advanced age. Moreover, if a genetic mutation has already been identified in a relative, conducting a genetic analysis of family members is important for predicting prognosis.
In particular, the LMNA mutation carriers often have sudden death or heart failure in middle age. Because not all cases are symptomatic, genetic testing is recommended for not only the patient but also family members to predict a risk stratification even if subjects have only mild ECG abnormalities (1st-degree AV block, atrial arrhythmia, and others).
4.7.2 Diagnostic Criteria
Patients who have the following 4 clinical findings are diagnosed with PCCD.8
(1) ECG findings indicating progressive conduction disorder, with bifascicular block or Mobitz type II or higher degree AV block
(2) Previous symptom or family history of syncope due to bradycardia or pacemaker implantation
(3) Age <70 years at diagnosis
(4) The following diseases need to be excluded at diagnosis: organic heart disease, musculoskeletal myopathies, sarcoidosis, autoimmune diseases, and arteriosclerotic disease; however, patients whose symptoms and pathologies caused (or suspected) by bradycardia are preceded by phenotypes of cardiomyopathies or musculoskeletal myopathies are not excluded
5. Diagnosis of Unexplained Syncope
5.1 How to Proceed With Diagnosis
Transient loss of consciousness is defined as “a sudden and brief period of transient unconsciousness which recovers spontaneously”. It is necessary to exclude falls or comas at this time5,697–699 (see ESC Guideline 2009;699 Figure 1). The etiologies of transient loss of consciousness include syncope, epilepsy, and psychogenic disorders. Therefore, the main purpose of the initial assessment is to differentiate syncope from non-syncope, which is a transient loss of consciousness, by taking a detailed medical history, conducting a physical examination, and performing ECG tests (Figure 31).5 Information from witnesses is also useful and important for diagnosis, in addition to that from the patient.

Some basic knowledge of epilepsy is required for physicians to differentiate between syncope and non-syncope (especially epilepsy). For example, the eyes are often open during both syncope and epileptic seizures, whereas they are often closed during psychogenic events. In terms of the duration of the event, loss of consciousness is short during syncope (<1 min), but slightly longer for non-syncope. If the person does not recover consciously and immediately after the loss of consciousness, causes other than syncope should be considered. Syncope should be suspected when palpitations are followed by a brief loss of consciousness and the person shows pallor of the face.
Although syncope is defined as “transient loss of consciousness and inability to maintain posture”, it is a symptom of a condition, not a disease. Pathophysiologically, syncope occurs due to transient global cerebral hypoperfusion and is divided into 3 types depending on etiology: reflex syncope, orthostatic hypotension, and cardiogenic syncope (Figure 32). If syncope is determined with the initial assessment, it is important to find cardiogenic syncope. It is essential to be aware of the characteristic prodromal symptoms and accompanying symptoms of syncopal etiologies. For example, vasovagal syncope should be suspected when prolonged standing without moving is associated with prodromal symptoms. Knowing whether a patient is taking antihypertensive drugs is essential information when orthostatic hypotension is suspected with repeated syncope immediately after standing (Table 36A). Cardiogenic syncope should be suspected if there is a family history of syncope or sudden death while lying down, organic heart disease, reduced cardiac function, and/or bifascicular block on ECG, and when syncope occurs during exercise or is preceded by palpitations or chest symptoms (Table 36B).

Table 36.
Class of Recommendation and Level of Evidence for the Diagnosis of Syncope
| |
COR |
LOE |
| A. Reflex syncope and orthostatic hypotension |
Syncope that occurs in healthy individuals with no underlying disease and is associated with
typical prodromal symptoms (aura) as well as caused by standing load, pain, or fear is diagnosed
as vasovagal syncope |
I |
C |
Syncope that occurs during or immediately after a specific trigger (coughing, urination, defecation,
swallowing, after eating, or after exercise) is diagnosed as situational syncope |
I |
C |
Syncope that occurs while standing with a significant fall in blood pressure confirmed by an
orthostatic test (a reduction in systolic blood pressure ≥20 mmHg or diastolic blood pressure
≥10 mmHg) is diagnosed as orthostatic hypotension |
I |
C |
Vasovagal syncope or orthostatic hypotension is considered if there are no typical symptoms that
satisfy the abovementioned criteria and in the absence of findings indicative of cardiogenic syncope |
IIa |
C |
| B. Cardiogenic syncope |
Differential diagnosis is conducted when the following findings are observed on an ECG, bearing
arrhythmogenic syncope in mind:
• Sustained sinus bradycardia <40 beats/min or sinus arrest ≥3 s while awake despite an
absence of physical training
• 2nd-degree Mobitz type II or 3rd-degree AV block
• Alternating right and left bundle branch blocks
• VT or paroxysmal supraventricular tachycardia with very rapid heartbeat
• Non-sustained polymorphic VT and QT prolongation/shortening
• Pacemaker or ICD dysfunction associated with cardiac arrest |
I |
C |
Syncope associated with acute myocardial ischemia is diagnosed as cardiogenic syncope
caused by myocardial ischemia |
I |
C |
Syncope symptoms in patients with conditions, such as atrial myxoma, left atrial ball thrombus,
severe aortic stenosis, pulmonary embolism, or acute aortic dissection, are suspected to be
syncope due to structural cardiopulmonary abnormalities |
I |
C |
AV, atrioventricular; COR, Class of Recommendation; ECG, electrocardiogram; ICD, implantable cardioverter defibrillator; LOE, Level of Evidence; VT, ventricular tachycardia.
Bradycardia–tachycardia syndrome and paroxysmal AV block are bradyarrhythmias that often cause syncope. Here, syncope is classified into arrhythmogenic (loss of consciousness due to arrhythmia) and non-arrhythmogenic (mainly due to a fall in blood pressure). General characteristic symptoms of each type are listed in Table 37.2,699 Arrhythmogenic syncope also includes syncope triggered by secondary arrhythmia.
Table 37.
Differential Diagnosis Between Arrhythmogenic Syncope and Non-Arrhythmogenic Syncope That Causes Transient Loss of Consciousness
| |
Arrhythmogenic syncope |
Non-arrhythmogenic syncope |
Type of causative
disease |
Cardiogenic syncope due to bradyarrhythmia/
tachyarrhythmia, reflex syncope, and others
[including mainly cardiodepressive, epileptic syncope
(ictal bradycardia/asystole)] |
Orthostatic hypotension, reflex syncope (mainly
hypotensive type), and others |
Aura (prodrome)
characteristics |
Awareness of palpitations is a relative characteristic of
cardiogenic syncope (supraventricular, ventricular,
bradycardia–tachycardia syndrome, and others)
Characteristics of reflex syncope include heavy head,
nausea, vomiting, stomach pain, epigastric discomfort,
dimmed vision, and others |
Reflex syncope is characterized by a heavy head,
nausea, vomiting, stomach pain, epigastric
discomfort, dim sight, and others |
Induction (emotions,
body position, etc.) |
Cardiogenic syncope is associated with exertion. Reflex
syncope occurs without body movement when a person is
standing or sitting, as well as is affected by environmental
factors and stress |
Orthostatic hypotension occurs when a person stands
up. Reflex syncope occurs without body movement
when a person is standing or sitting, as well as is
affected by environmental factors and stress |
| Facial pallor |
Yes |
Yes |
Duration of loss of
consciousness |
Loss of consciousness in cardiogenic syncope and reflex
syncope is short, often <1 min |
Often <1 min |
| Convulsions |
Occurs frequently (from a few seconds to ≈15 s) |
Rare |
| Open eyes |
Open eyes |
Open eyes |
| Automatism |
Often in arrhythmogenic syncope due to epilepsy |
Rare |
Postictal state/
disorientation |
Often in arrhythmogenic syncope due to epilepsy |
No |
| Tongue biting |
Rare, although the front of the tongue may be bitten during
syncope |
Rare, although the side of the tongue may be bitten
during an epileptic seizure |
| Falling/injured site |
Head, face |
Head, face |
5.2 Risk Assessment
Risk assessment is conducted if syncope is strongly suspected, although there is no definitive diagnosis of the causative disease (Table 38).5 Cardiogenic syncope should be suspected if there is 1 high-risk finding. The underlying heart disease and cardiac function should be carefully examined, and exercise ECG test, Holter ECG, as well as long-term ECG monitoring should be considered depending on the frequency of syncope symptoms. A Holter ECG is useful for symptoms that occur ≥1 times a week. External loop recorders for the duration of 1–2 weeks are effective for syncope with symptoms that occur ≥1 times a month. An ILR should be considered for syncope that only occurs very infrequently or irregularly. The cause should also be confirmed using methods such as an EPS and cardiac catheterization, as needed.
Table 38.
Findings of the Risks of Cardiogenic Syncope
| 1. Severe organic heart disease or coronary artery disease |
| Heart failure, reduced LVEF, history of myocardial infarction |
| 2. Clinical or ECG findings suggestive of arrhythmogenic syncope |
| (1) Syncope during exertion or when lying down |
| (2) Palpitations during or before syncope |
| (3) Family history of sudden cardiac death |
| (4) Non-sustained ventricular tachycardia |
(5) Bifascicular block (left bundle block, right bundle block plus left anterior fascicle, or left posterior fascicle block),
other intraventricular conduction abnormalities with QRS ≥120 ms |
(6) Inappropriate sinus bradycardia without negative chronotropic drugs or physical training (<50 beats/min),
sinoatrial block |
| (7) Pre-excitation syndrome |
| (8) QT prolongation or shortening |
| (9) Brugada ECG pattern |
| (10) Inverted T-wave, epsilon wave, ventricular LP on right precordial lead suggestive of ARVC/ACM |
| 3. Other |
| Severe anemia, electrolyte imbalance, among others |
ACM, arrhythmogenic cardiomyopathy; ARVC, arrhythmogenic right ventricular cardiomyopathy; ECG, electrocardiogram; LP, late potential; LVEF, left ventricle ejection fraction.
On the other hand, it is sufficient to simply follow up with patients if there are no high-risk findings, if it is the first syncopal event, or if the events are very infrequent. However, external loop recorders are highly useful for frequently repeated syncopal events, even without high-risk findings. An ILR is also useful for determining the involvement of arrhythmia after the exclusion of non-cardiogenic syncope such as reflex syncope and orthostatic hypotension. Refer to Chapter I.6 and the 2018 JCS/HHRS Guideline on non-pharmacotherapy of cardiac arrhythmias for information on the criteria for the indication of an ILR.2
5.3 Unexplained Syncope in Patients With Bifascicular Block
Patients with unexplained syncope with bifascicular block on ECG (QRS width >120 ms with complete LBBB or complete RBBB associated with left anterior fascicle or left posterior fascicle block) often develop paroxysmal AV block, which is known to be associated with a high mortality rate (especially sudden death). Therefore, the necessity of pacemaker therapy has been investigated.700,701 The Class of Recommendation for pacemaker therapy is Class I associated with an HV interval ≥70 ms on EPS or when 2nd to 3rd-degree AV block is induced with pacing or drug provocation (group I antiarrhythmic drugs).698 However, if AV block is not confirmed on ECG and the HV interval is <70 ms, the Class of Recommendation for pacemaker therapy is IIb. Thus, it is recommended to perform follow-up observations with an ILR before pacemaker treatment.698,702–704
5.4 Unexplained Syncope With a Normal ECG and Without Underlying Heart Disease
Reports have indicated a group of patients with syncope that occurs without prodromes due to paroxysmal AV block, despite normal cardiac function, a normal ECG, and no underlying heart disease.705,706 This condition is highly sensitive to adenosine, because of which it is also called adenosine triphosphate (ATP)-sensitive AV block or low-adenosine AV block.706 No AV conduction abnormalities are found on EPS, and the condition is clinically diagnosed by the onset of an AV block ≥10 s after intravenous injection of 20 mg ATP. The adenosine concentration in the blood is low, AV block is not triggered by premature atrial or ventricular contractions, and the PP interval during the AV block is almost the same as that during AV conduction. Paroxysmal AV block due to disorders in the AV conduction system generally later transition to permanent AV block, although there have been no reports on ATP-sensitive paroxysmal AV block progressing to permanent AV block. Reports suggest the efficacy of oral theophylline, although there is still insufficient evidence to date.707 No recommendations on indications for pacemakers have been established to date.
5.5 Reflex Syncope
The terminology for reflex syncope has been standardized in the Guidelines for diagnosis and management of syncope (JCS 2012),5 and terms such as neurally mediated syncope and neurally mediated syncopal syndrome are no longer used. Reflex syncope now includes vasovagal syncope, carotid sinus syncope, situational syncope, and epileptic syncope. Vasovagal syncope accounts for most of the cases of reflex syncope, with carotid sinus syncope and situational syncope accounting for only a small percentage. Reflex syncope is caused by overstimulation of the parasympathetic nervous system, resulting in a transient reduction in heart rate and blood pressure. This may lead to global cerebral hypoperfusion that results in syncope. This type of syncope is caused not only by sinus bradycardia or sinus arrest but maybe also by paroxysmal AV block.
Vasovagal syncope is classified as (1) cardioinhibitory type, (2) vasodepressor type, and (3) mixed type with both cardioinhibitory and vasodepressor components. However, in the cardioinhibitory type, a long duration of cardiac arrest of 10–20 s is not uncommon and is often associated with convulsion-like reactions. Therefore, caution should be taken, as it may be misdiagnosed as epilepsy.708 The primary diagnoses based on symptoms of reflex syncope and orthostatic hypotension are shown in Table 36A.
The tilt table test is useful for diagnosis. Although the reproducibility and sensitivity of the tilt table test are low, the specificity is high.709 Therefore, a definitive diagnosis is made if the tilt table test induces syncope or symptoms similar to the clinical symptoms, although reflex syncope is not ruled out if the symptoms are not induced. Therefore, a clinical diagnosis can be achieved by taking a detailed history (Table 36A). On the other hand, the tilt table test is useful for predicting therapeutic efficacy when considering pacing treatment.710 Vasovagal syncope may be triggered by psychological or physical stress such as lack of sleep, fatigue, and fear, as well as by environmental factors such as crowding or enclosed spaces, and prolonged standing or sitting. It is accompanied by prodromal symptoms such as nausea, cold sweats, headache, dimmed vision, and abdominal pain, especially tending to occur in young people with no underlying disease. Reflex syncope in older people commonly has little prodromal symptoms and is often associated with underlying disease. Thus, it is important to differentiate reflex syncope from cardiogenic syncope (Table 36).
5.6 Epileptic Syncope
Epileptic seizures are often accompanied by muscle stiffness and convulsions due to abnormal electrical excitation in the brain. However, complex partial seizures are accompanied by prodromes such as upper abdominal discomfort and nausea, no convulsions and muscle stiffness during the attack, as well as automatism, impaired consciousness, postictal memory confusion, and clouded consciousness. Patients commonly present with ictal tachycardia (mainly sinus tachycardia) due to sympathetic hyperactivity during an epileptic seizure. Complex partial seizures due to temporal lobe epilepsy sometimes induce vagotonia caused by epileptic stimulation or vasovagal reflex caused by the release of catecholamine, which in turn causes ictal bradycardia and asystole. It is classified as reflex syncope because the syncope is a secondary complication and is called “epileptic syncope”. Asystole triggered by an epileptic attack stops cerebral blood flow, resulting in the termination of the epileptic attack. Therefore, it is also considered a type of biological defense mechanism.711
ECG findings of epileptic syncope mimic the heart rate fluctuations observed in vasovagal syncope, typically presenting with transiently elevated heart rate followed by sinus bradycardia progressing to a sinus arrest (asystole).711 The duration of asystole is relatively long, and asystole ≥10 s is not uncommon. Therefore, caution is needed because pacemaker therapy is sometimes performed based on a misdiagnosis of primary SSS. There is no evidence of the efficacy of pacemaker therapy for epileptic syncope, and there are reports showing that antiepileptic drug therapy eliminated the effects of pacemaker operation.712,713 There are also reports showing that pacemaker therapy exacerbates epileptic seizures.714
6. Diagnosis of Cryptogenic Stroke
6.1 Concept
Cryptogenic stroke is defined as a cerebral infarction of undetermined etiology.715 The 3 main types of cerebral infarction are atherothrombotic brain infarction, cardiogenic brain embolism, and lacunar stroke.716 Other causes of cerebral infarction include paradoxical embolism from a patent foramen ovale, atrial septal aneurysm, and aortic atheroembolism. However, if the cause remains unclear even after conducting various tests, these cases are generally called cryptogenic strokes.711 Therefore, cryptogenic stroke is diagnosed by excluding strokes with identifiable causes. Many cryptogenic strokes are thought to be caused by undetectable paroxysmal AF.717
There is a term known as the embolic stroke of undetermined source (ESUS), which refers to cases that are diagnosed as a cerebral embolism on diagnostic imaging, although the source of the embolism is undetermined.718 That is, ESUS is a pathology that is included under the umbrella of cryptogenic stroke. The term ESUS has become widely used with the advent of direct-acting oral anticoagulants (DOACs), drugs that prevent cerebral embolism caused by AF.
6.2 Diagnostic Methods
6.2.1 Imaging Diagnostics
When a stroke is suspected based on medical interview, physical examination, and general tests, it is recommended to first conduct brain imaging. Unless contraindicated, diffusion-weighted MRI should be prioritized over MRI sequencing and CT.717,719 If diffusion-weighted MRI cannot be performed for some reason, CT should be conducted instead. Most cryptogenic strokes are considered cardiogenic, mainly cardiogenic brain embolism caused by AF; therefore, the next step is the attempt to detect AF using Holter ECG or event ECG. Meanwhile, the presence of an intracardiac thrombus should also be evaluated with TTE. Left atrial appendage thrombosis is usually difficult to detect on TTE, although thrombi, such as relatively large left atrial thrombi and thrombi inside a left ventricular aneurysm, can be detected on TTE.720
If no abnormalities are detected, the presence of carotid artery stenosis is evaluated using techniques such as carotid Doppler ultrasonography. If abnormalities are still not detected, left atrial appendage thrombosis is assessed using TEE.721 TEE detects thrombi within the left atrial appendage at a higher rate than TTE. Although uncommon as a cause, paradoxical embolus should also be considered, which involves evaluation of deep vein thrombosis with deep venous ultrasonography of the lower extremities, evaluation of the presence of shunt diseases such as patent foramen ovale and atrial septal defect with EE, as well as checking for mural thrombus in the ascending aorta with aortic CT. Strokes with a cause that cannot be identified after performing the aforementioned processes are diagnosed as cryptogenic133 (Table 39, Figure 33).
Table 39.
Class of Recommendation and Level of Evidence Related to the Diagnosis of Cryptogenic Stroke
| |
COR |
LOE |
Diffusion-weighted MRI is conducted as the first brain imaging test for patients with suspected
brain embolism |
I |
A |
| TTE monitoring is conducted when the cause of the brain embolism is suspected to be cardiogenic |
I |
A |
Long-term ECG monitoring using Holter ECG and event ECG are conducted when AF is
suspected to be the cause of the brain embolism |
I |
B |
ILR implantation is performed when AF is suspected to be the cause of the brain embolism,
although no evidence is found on long-term ECG monitoring |
I |
B |
| TEE is considered when AF is suspected to be the cause of brain embolism |
IIa |
B |
Carotid Doppler ultrasonography, deep venous ultrasonography of the lower extremities, and
aortic CT are considered to find the cause of brain embolism |
IIa |
B |
| Detection of paroxysmal AF using a smartphone or smartwatch may be considered |
IIb |
C |
ILR implantation as a means of finding the cause of brain embolism, without first conducting non-
invasive tests such as long-term ECG monitoring, is not recommended |
III (No
benefit) |
B |
AF, atrial fibrillation; COR, Class of Recommendation; CT, computed tomography; ECG, electrocardiogram; ILR, implantable loop recorder; LOE, Level of Evidence; MRI, magnetic resonance imaging; TEE, transesophageal echocardiography; TTE, transthoracic echocardiography.

6.2.2 Detecting AF With ECG Monitoring
Permanent or sustained AF can be easily detected by routine ECG, although paroxysmal AF is often difficult to detect. Current US and European guidelines recommend first conducting ECG monitoring in patients with ischemic stroke, including cryptogenic stroke, in whom transient (paroxysmal) AF is suspected but other causes of cerebral infarction have not been identified.66,717 Holter ECG (24–48 h) or event ECG (2–4 weeks) is used. A meta-analysis reported that the longer the continuous ECG monitoring period, the higher the detection rate of new AF, with new AF detected in 5.1%, 15%, and 29.1% of patients being monitored for <72 h, ≥7 days, and over a 3-month period, respectively.722 Recently, systems have been developed to record ECGs using smartphones and smartwatches (Appendix 1: Figures 37–40), and the use of these devices may further improve the AF detection rate. However, there are still many limitations and issues with detecting AF using these new types of ECGs; thus, they are not yet usable in a medical setting.
6.2.3 Detecting AF With an Implantable ECG
ILRs are being used to detect transient (paroxysmal) AF in patients with cryptogenic stroke. The ILR has been shown to have a significantly higher AF detection rate than non-invasive ECG and is able to conduct ECG monitoring for 2–3 years. It is a particularly useful tool in asymptomatic patients.
The CRYSTAL AF study found that new AF was detected in 8.9% of patients with an ILR for 6 months, compared with 1.4% using conventional non-invasive ECG monitoring. These data increased to 12.4% and 2.0%, respectively, by 12 months, demonstrating the utility of the ILR.241 Similar results were reported in the EMBRACE study, with new AF detected in 16.1% of patients with a 30-day ILR, compared with 3.2% of patients using 24-h monitoring.428 A systematic review found that sequentially stepping up the ECG monitoring period and technique increased the detection rate of new AF in patients with ischemic cerebral infarction. The review showed that AF was detected in 7.7% of patients in Phase 1 (emergency room), 5.1% in Phase 2 (during hospitalization), 10.7% in Phase 3 (at first outpatient visit), 16.9% in Phase 4 (at second outpatient visit with the use of event ECG or ILR), and 23.7% with continued ECG monitoring thereafter with an ILR or other means.429
Therefore, use of an ILR further increases the detection rate of AF. Targeted ECG monitoring using risk stratification scores for the onset of embolism due to AF (such as the CHADS2
score) further enhances the AF detection rate.723
6.3 Treatment Strategies
If AF is not detected, antiplatelet drugs are the first-line therapy for cryptogenic stroke. However, if AF is detected, indications for anticoagulant therapy using drugs, such as DOACs or warfarin, are considered to prevent cerebral infarction (embolism). If necessary, sinus rhythm is maintained with antiarrhythmic drugs or ablation, or the heart rate is modulated with β-blockers.
7. Imaging Diagnostics for Organic Heart Disease That May Cause Sudden Cardiac Death (Cardiac Infarction, Cardiomyopathy, and Others)
The Class of Recommendation and Level of Evidence for risk assessment of imaging tests for sudden cardiac death are shown in Table 40.
Table 40.
Class of Recommendation and Level of Evidence for Risk Assessment Using Imaging Tests for Sudden Cardiac Death
| |
COR |
LOE |
| TTE monitoring is performed to assess cardiac function and structure |
I |
A |
Delayed myocardial enhancement MRI is considered to assess the risk of sudden cardiac death
in HCM |
IIa |
A |
Delayed myocardial enhancement MRI may be considered to assess the risk of sudden cardiac
death in DCM |
IIb |
C |
MIBG myocardial scintigraphy may be considered to predict prognosis in ischemic and non-
ischemic heart failure |
IIb |
C |
COR, Class of Recommendation; DCM, dilated cardiomyopathy; HCM, hypertrophic cardiomyopathy; LOE, Level of Evidence; MIBG, metaiodobenzylguanidine; MRI, magnetic resonance imaging; TTE, transthoracic echocardiography.
7.1 Echocardiography
TTE is one of the simplest and most rapid imaging tools available to non-invasively assess cardiac function in patients with myocardial infarction and non-ischemic cardiomyopathy. It has been reported that the lower the LVEF, an index of left ventricular contractility, the higher the risk of sudden death.724
The MADIT-I study showed that an ICD improved survival rate by 54% in patients with myocardial infarction, asymptomatic NSVT, left ventricular dysfunction (LVEF ≤35%), and induced sustained VT or VF non-responsive to procainamide in an EPS.153 The subsequent MADIT-II study showed that prophylactic ICD implantation improved survival rates (31% reduction in mortality rate) in patients with severe left ventricular dysfunction (LVEF ≤30%) after myocardial infarction, with or without ventricular arrhythmia or inducibility.154
The SCD-HeFT study is the largest primary prevention randomized controlled trial enrolling patients with both ischemic and non-ischemic heart failure.159 The main registration criteria were (1) history of heart failure for ≥3 months, (2) receiving heart failure treatment with angiotensin-converting enzyme inhibitors or β-blockers, (3) LVEF ≤35%, and (4) New York Heart Association (NYHA) cardiac function classification II–III, and coronary artery disease, accounting for 52% of all participants. The results indicated the mortality rate fell by ≈20% in the ICD group compared with the placebo and amiodarone groups. These results recommend ICD indications for patients with organic heart disease associated with reduced LVEF.
In patients with ischemic/non-ischemic cardiomyopathy, LVEF ≤35%, and heart failure symptoms of NYHA Class ≥II who have NSVT, ICD implantation is a Class I indication according to the 2018 JCS/HHRS Guideline on non-pharmacotherapy of cardiac arrhythmias. In patients without NSVT, ICD implantation is a Class IIa indication.2
It is important to measure the left ventricular wall thickness on TTE for HCM. The severity of left ventricular hypertrophy is associated with the risk of sudden death and marked hypertrophy with a left ventricular wall thickness ≥30 mm is considered a major risk factor for sudden cardiac death.82
7.2 Cardiac MRI
LGE on cardiac MRI is indicative of fibrosis and edema, which is found in organic heart diseases such as myocardial infarction and cardiomyopathy (Figure 34).
7.2.1 Acute and Old Myocardial Infarction
LGE occurs in both acute and old myocardial infarcts, and the size of the area is known to correlate with the extent of the infarct.725 It has also been reported that the infarct size measured as the LGE area is a superior prognostic indicator to the conventional prognostic factors of left ventricular volume and LVEF.726
7.2.2 HCM
Recent studies have found that the larger the LGE area on MRI, the greater the risk of sudden death in HCM. A study using enhanced MRI in 1,293 patients with HCM found that the presence of extensive LGE was an independent prognostic indicator for sudden death and reported that the risk of sudden death doubled when the LGE volume exceeded 15% of the left ventricular volume even if HCM was considered low risk.727 A meta-analysis of 2,993 patients found that the presence of LGE was associated with an increased risk of sudden death, with an odds ratio of 3.41 (P<0.0001). The extent of LGE was also significantly associated with increased sudden cardiac death, with a hazard ratio of 1.56 (P<0.0001) in a 10% LGE range. The spread of LGE was significantly associated with the risk of sudden cardiac death even after adjusting for baseline reference values (hazard ratio 1.36, P=0.005).515
7.2.3 DCM
An association between LGE and sudden cardiac death has been reported. Gulati et al reported that 132 of 472 patients with DCM were positive for LGE, and their risk of sudden cardiac death increased 4.61-fold.728 Assomull et al also found LG in the myocardial mid-wall in 35% of patients with DCM and reported that the incidence of sudden cardiac death or VT was 5.2-fold higher in these patients.729
7.2.4 ARVC/ACM
ARVC/ACM and HCM are common causes of sudden death in young people, making diagnosis and risk assessment of arrhythmia important.730 Aquaro et al recently conducted a multicenter prospective study using cardiac MRI in 140 patients with ARVC/ACM. Abnormalities were found on cardiac MRI, including ≥1 wall motion abnormalities, ventricular enlargement, fatty infiltration, and LGE in 126 patients (90%); right ventricular lesion in 58 (41%); lesions in both ventricles in 52 (37%); and left ventricular lesion in 16 (12%). Cardiac events (sudden death, ICD activation, and cardiac arrest) occurred in 48 patients (34%) during a mean follow-up period of 5 years, but did not occur in those with no abnormalities on cardiac MRI. Results of multivariate analysis showed that left ventricular lesion found on MRI was an independent risk factor for cardiac events (hazard ratio 4.2, P=0.0001).731
7.2.5 Cardiac Sarcoidosis
Cardiac sarcoidosis often develops due to conduction disturbances such as AV block and bundle branch block, which leads to fatal arrhythmia and sudden death if it progresses. Greulich et al conducted prospective observation of 155 patients with sarcoidosis and suspected cardiac involvement after performing cardiac MRI. LGE was present in 39 patients (25.5%), and a cardiac event (death, ICD activation, or cardiac arrest) occurred in 12 (25.5%) during a mean follow-up of 2.6 years. Multivariate analysis showed that LGE was an independent and best predictor for cardiac events (hazard ratio 31.6, P=0.0014).732
7.3 MIBG Myocardial Scintigraphy
I-123 metaiodobenzylguanidine (MIBG) myocardial scintigraphy is a radiopharmaceutical with similar kinetic properties to that of noradrenaline and can be used to evaluate cardiac sympathetic nervous system dysfunction. Previous studies have reported that MIBG myocardial scintigraphy is useful for prognostic evaluation in heart failure with quantitative indices, such as the heart-mediastinum ratio (H/M ratio) and washout rate (WR).733–735
The usefulness of MIBG myocardial scintigraphy in predicting sudden death has been indicated in recent years. Tamaki et al reported that an increased WR was an independent risk factor of sudden death in heart failure patients with an LVEF <40%.736 Akutsu et al reported that sudden death and fatal arrhythmias were common in patients with a low H/M ratio (<2.8).737 In addition, Kasama et al reported that multivariate analysis indicated that ∆WR, the difference between the WR at discharge and that at 6 months after treatment, was useful for predicting sudden death in patients hospitalized with heart failure.738