2025 Volume 89 Issue 2 Pages 184-194
2025 Volume 89 Issue 2 Pages 184-194
Background: Sleep apnea (SA), subjective sleep duration (SSD), and objective sleep duration (OSD) were reported as risk factors for atrial fibrillation (AF). However, the association between AF and the combination of SA and OSD has not been clarified. Nor has a mismatch between SSD and OSD been investigated.
Methods and Results: We assessed SA with polysomnography, OSD with actigraphy, and SSD in patients who underwent radiofrequency catheter ablation for persistent AF. We investigated associations among SA, OSD, OSD×3% oxygen desaturation index (3%ODI), and AF recurrence, considering SSD–OSD (i.e., the difference between SSD and OSD) and OSD. Seventy of 94 (74.4%) participants had moderate-to-severe SA (apnea-hypopnea index [AHI] ≥15). Participants were classified into OSD tertiles. Participants in Tertile 3 (mean OSD: 7.3 h) had decreased SSD–OSD (0.0 h) with increased Stage N1 sleep. Over 27.6 months, 10 AF recurrences occurred in 51 participants without treatment for SA. AHI ≥20 and OSD Tertile 3 were associated with AF recurrence (hazard ratios 5.7 [95% confidence interval 1.1–24.7] and 10.3 [95% confidence interval 1.2–88.4], respectively). Participants with AF recurrence had a higher OSD×3%ODI.
Conclusions: SA and long OSD were predictors of recurrent AF through long exposure to intermittent hypoxia during sleep. SSD–OSD was low in patients with long OSD, possibly because of decreased sleep quality.
Atrial fibrillation (AF) is among the most common of the supraventricular arrhythmias and occasionally leads to stroke, heart failure, and death. Hence, management of risk factors for AF is an important issue. In recent years, there has been evidence of associations between sleep apnea (SA), which is characterized by intermittent hypoxia during sleep and includes obstructive SA (OSA) and central SA (CSA), and AF. That OSA is a risk factor for AF has been shown,1–3 as has recurrence of AF after catheter ablation for AF.4–7 CSA has also been reported to be a risk factor for incident AF.8 In addition, continuous positive airway pressure (CPAP) therapy for SA may decrease the recurrence of AF in patients with SA who have undergone catheter ablation for AF,9–12 although recent randomized controlled trials failed to demonstrate the efficacy of CPAP for preventing AF recurrence.13,14 However, in some of these previous studies, questionnaires were used to assess the presence of SA5–7 and a previous diagnosis of SA may not have been made by polysomnography.4,9,11,12 Therefore, an accurate assessment of the prevalence of SA derived from polysomnography data among patients who have undergone catheter ablation for persistent AF is warranted.
It was previously reported that both short and long sleep duration, mainly subjective sleep duration (SSD), based on questionnaires may be a possible risk factor for conditions such as hypertension, diabetes, and metabolic diseases.15,16 However, in a previous study, we found no association between habitual objective sleep duration (OSD) derived from wrist actigraphy (but not SSD) and the prevalence of hypertension or diabetes in a large general cohort, although sleep disordered breathing was associated with hypertension and diabetes.17 Thus, there may be some discrepancies in the associations between sleep duration, including SSD or OSD, and several diseases. Recently, the association between sleep duration and AF has gained attention. It was suggested that both short and long SSD are risk factors for AF,18–21 although only a limited number of studies have investigated the association between OSD and AF.22,23 Furthermore, the above studies did not focus on the risk of AF recurrence in patients who had undergone catheter ablation or who were at high risk of AF recurrence, but rather focused on those at risk of incident AF in general populations. Therefore, the association between AF recurrence after catheter ablation and habitual OSD has not yet been fully investigated.
In addition, recent studies showed some mismatches between the duration of SSD and OSD as indicated by SSD minus OSD (SSD–OSD). Generally, SSD is longer than OSD (SSD>OSD).24,25 However, in patients with insomnia, SSD–OSD results could be negative.26 In addition, we recently reported that SSD–OSD was lower in individuals with OSD ≥7 h, greater in individuals with OSD <7 h, and even greater in patients with severe sleep disordered breathing.27 Nevertheless, the association between SSD–OSD and long OSD in patients with persistent AF, as well as factors affecting the SSD–OSD value in these patients have not been investigated.
As mentioned above, there has been increased interest in associations among SA, sleep duration, including OSD and SSD, the mismatch between SSD and OSD, and AF. In addition, because both SA and long OSD are possibly associated with the risk of AF, these associations may be explained by a combination of the physiological features of SA and long OSD. Therefore, we hypothesized that prolonged exposure to intermittent hypoxia during sleep caused by SA and long sleep duration are associated with the risk of AF. Consequently, in this study, we aimed to accurately survey the prevalence of SA assessed by polysomnography and the association between SSD–OSD and long OSD in consecutive patients who underwent radiofrequency catheter ablation (RFCA) for persistent AF. Furthermore, we also aimed to investigate longitudinal associations among SA, OSD, possible burdensome indices of prolonged exposure to intermittent hypoxia during sleep, and AF recurrence in patients who have undergone RFCA for persistent AF.
This was a prospective observational study to investigate the prevalence of SA and the association between SSD–OSD and long OSD in consecutive patients who underwent RFCA for persistent AF in a high-volume single center. We also assessed longitudinal associations among SA, OSD, prolonged exposure to intermittent hypoxia during sleep, and recurrence of AF after RFCA.
Study Participants and Ethical ConsiderationsPersistent AF was defined as AF lasting more than 7 days, and longstanding persistent AF was defined as persistent AF lasting more than 12 months. We attempted to enroll all consecutive patients who were admitted to the Department of Cardiovascular Medicine of Kyoto University Hospital to undergo an initial session of RFCA for persistent AF. The inclusion criteria were age 20–80 years, duration of persistent AF <10 years, and Class 1 and Class 2a indications in the Japanese guidelines at that time for arrhythmias.28 The exclusion criteria were a treatment history for SA, end-stage renal disease requiring hemodialysis, physical limitations that meant patients were unable to use the wrist actigraph, and dementia.
The study was approved by the Ethics Committee of Kyoto University Graduate School and Faculty of Medicine (R1206) and was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all patients before participation.
Polysomnography With a Single-Channel ElectroencephalographAfter admission, all participants underwent overnight polysomnography with a single-channel electroencephalograph to screen for SA before the RFCA. We used the Sleep Profiler PSG2 (Advanced Brain Monitoring, Carlsbad, CA, USA),29,30 which has an electrooculogram, chin electromyogram, air flow sensor, pulse oximeter, chest belt, and abdominal belt. An investigator (T.M.) equipped each participant with the apparatus and conducted the sleep study from 21:00 to 06:00 hours before the day of the RFCA. Some data that failed to be reliably measured by the instrumentation (e.g., by electroencephalography, the air flow sensor, pulse oximeter, chest belt, and abdominal belt) were excluded. The acquired data were anonymized and sent to an analysis center to be analyzed by polysomnographic technologists who did not know the clinical background of the study participants.
Sleep stages were divided into rapid eye movement (REM) and non-REM (NREM; Stage N1, N2, and N3) sleep.29 In general, Stage N1 is light sleep, Stage N2 is deeper sleep than N2, and Stage N3 is deep sleep. We defined apnea as a decrease in airflow by ≥90% of baseline for more than 10 s and hypopnea as a decrease in pressure by ≥30% of baseline for more than 10 s with ≥3% desaturation and/or arousal from sleep. Apnea without respiratory effort was defined as central apnea, and we calculated the central apnea index (CAI) according to episodes of central apnea per hour over total sleep time. We calculated the apnea-hypopnea index (AHI) according to episodes of apnea and hypopnea per hour over total sleep time. The severity of SA was categorized as none/mild (AHI <15), moderate (AHI 15–30), and severe (AHI ≥30). As an index of intermittent hypoxia, we calculated the 3% oxygen desaturation index (3%ODI) as episodes of ≥3% desaturation per hour over total sleep time. In addition, as an index of the hypoxic burden, we calculated the cumulative recording time with SpO2 <90% (CT90).
Assessment of OSD and Other Data Derived From Wrist ActigraphyUpon discharge after RFCA, all participants were asked to wear a wrist actigraph (Actiwatch 2 or Actiwatch Spectrum Plus; Philips Respironics, Murrysville, PA, USA) for 7 days to measure OSD at home and to concurrently keep a sleep diary for the same 7 days. The well-trained investigator (T.M.) set the bed-in and bed-out times derived from participants’ sleep diaries in the actigraph software and determined total sleep duration using the standard factory-default algorithm; we equated total sleep duration to OSD. We calculated the mean OSD over the 7 days for each participant, and divided patients into mean OSD tertile groups. Measurements for ≥4 days were included in the analyses.31 Awakening after sleep onset was calculated as the sum of the duration of awakening from sleep onset to final awakening. Sleep efficiency was calculated as OSD divided by the duration from bed-in to bed-out time. The fragmentation index was calculated by the sum of percent mobile and immobile bouts of <1-min duration compared with the number of immobile bouts for a given interval. Sleep latency was defined as the time from the bed-in time to sleep onset.
Questionnaires, SSD, SSD–OSD, and Sleep Duration–OSDWe asked participants to complete the Pittsburgh Sleep Quality Index (PSQI),32 which assesses sleep quality and disturbances, and the Japanese version of the Epworth Sleepiness Scale (JESS) during admission.33 A component of the PSQI was used to assess SSD. Total PSQI and JESS scores were calculated. Excessive daytime sleepiness was defined as a total JESS score ≥11. The difference between the duration of SSD and OSD was determined as “SSD–OSD”.27 Not all participants completed the questionnaires; therefore missing data on SSD–OSD, which was a focused variable, are shown in each table. The difference between sleep duration according to polysomnography and OSD according to actigraphy was determined as “sleep duration–OSD” to show the discrepancy between sleep duration based on clinical examination and habitual sleep duration.
Wrist Actigraphy×PolysomnographyWrist actigraphy data multiplied by polysomnography data were used to estimate exposure to total respiratory events related to SA in a single night. OSD×AHI (exposure to SA severity during sleep), OSD×3%ODI (exposure to intermittent hypoxia severity during sleep), and OSD×CT90 (exposure to sustained severe hypoxia) were also assessed.
Definition of ComorbiditiesHypertension was defined as a history of hypertension or the use of antihypertensive drugs. Diabetes was defined as a history of diabetes. Chronic heart failure was defined as a history of hospital admission for heart failure, the use of diuretics for heart failure, or an ejection fraction by transthoracic echocardiography ≤50%. Ischemic stroke was defined as a history of ischemic stroke or transient ischemic attack diagnosed by a physician. Cardiovascular diseases were defined as a history of angina, myocardial infarction, or arteriosclerosis obliterans.
RFCA ProtocolWe performed extensive encircling pulmonary vein isolation with the double circular catheter method, placing 2 20-pollar circular-shaped catheters (Lasso [Biosense Webster, Irwindale, CA, USA] or Orbiter PV [C.R. Bard Electrophysiology, Lowell, MA, USA]) in ipsilateral superior and inferior pulmonary veins. A 3.5-mm tip irrigation catheter (NAVISTAER THERMOCOOL; Biosense Webster) was used in all cases.34,35 We also routinely used tricuspid valve isthmus ablation for most cases. Additional ablation procedures such as mitral isthmus line and left atrial roof line ablation for atrial tachycardia were performed when AF converted to sustained atrial tachycardia during the procedure. Additional ablation procedures such as superior vena cava isolation, low voltage ablation, and complex fractionated atrial electrogram ablation were performed whenever necessary.
Follow-up, Recurrence of AF, and CPAP InitiationRoutinely, patients were followed up at approximately 1, 3, 6, and 12 months after the RFCA, and additionally as needed. A 12-lead electrocardiogram was obtained at every visit, and 24-h Holter monitoring was usually performed at 3, 6, and 12 months after the RFCA and yearly in symptomatic participants. AF recurrence was defined as any documented supraventricular arrhythmia lasting ≥30 s (e.g., AF, atrial tachycardia, and atrial flutter) after a 90-day blanking period. We recommended participants with an AHI ≥20, which is an indication in the Japanese guidelines for CPAP therapy,36 to start CPAP therapy from results of the polysomnography. However, many of the participants were admitted to Kyoto University Hospital from distant areas, and we could not ascertain whether they continued with or adhered to CPAP therapy. In addition, the timing of initiation of CPAP therapy was diverse. Therefore, participants who started CPAP therapy after RFCA were excluded from analyses of AF recurrence.
Statistical AnalysisData are presented as the mean±SD, median with interquartile range, or as numbers and percentages. Continuous variables were analyzed using analysis of variance and the Mann-Whitney U test or Kruskal-Wallis test, as appropriate. Categorical variables were analyzed using Chi-squared tests. Post hoc pairwise comparisons among groups were performed using the Tukey-Kramer test or Dunn test, as appropriate. In analyses of AF recurrence, we attempted to determine the AHI value (i.e., AHI ≥10, ≥15, ≥20, ≥25, and ≥30) that was predictive of AF recurrence, as well as associations between OSD tertiles and AF recurrence. AF-free survival curves in each group are shown by Kaplan-Meier plots and were compared using log-rank tests. A Cox proportional hazards model was used to investigate which AHI value or which OSD tertile was associated with AF recurrence. Two-tailed P<0.05 was considered significant in all analyses. Analyses were conducted using SPSS version 25 (SPSS Inc., Chicago, IL, USA).
During the recruitment period from January 2018 to June 2019, 148 patients met the inclusion criteria, 48 of whom declined to participate (Figure 1). All 100 individuals who agreed to participate in the study underwent polysomnography before the RFCA session. First, after excluding 6 participants whose parsable sleep duration was <1 h, we assessed the prevalence of SA and SA severity in 94 participants. Second, after excluding 17 participants without OSD data, we divided the remaining 77 participants into OSD tertiles. Finally, we excluded 26 participants who initiated CPAP therapy after RFCA and, in January 2022, assessed the recurrence of AF in 51 participants.
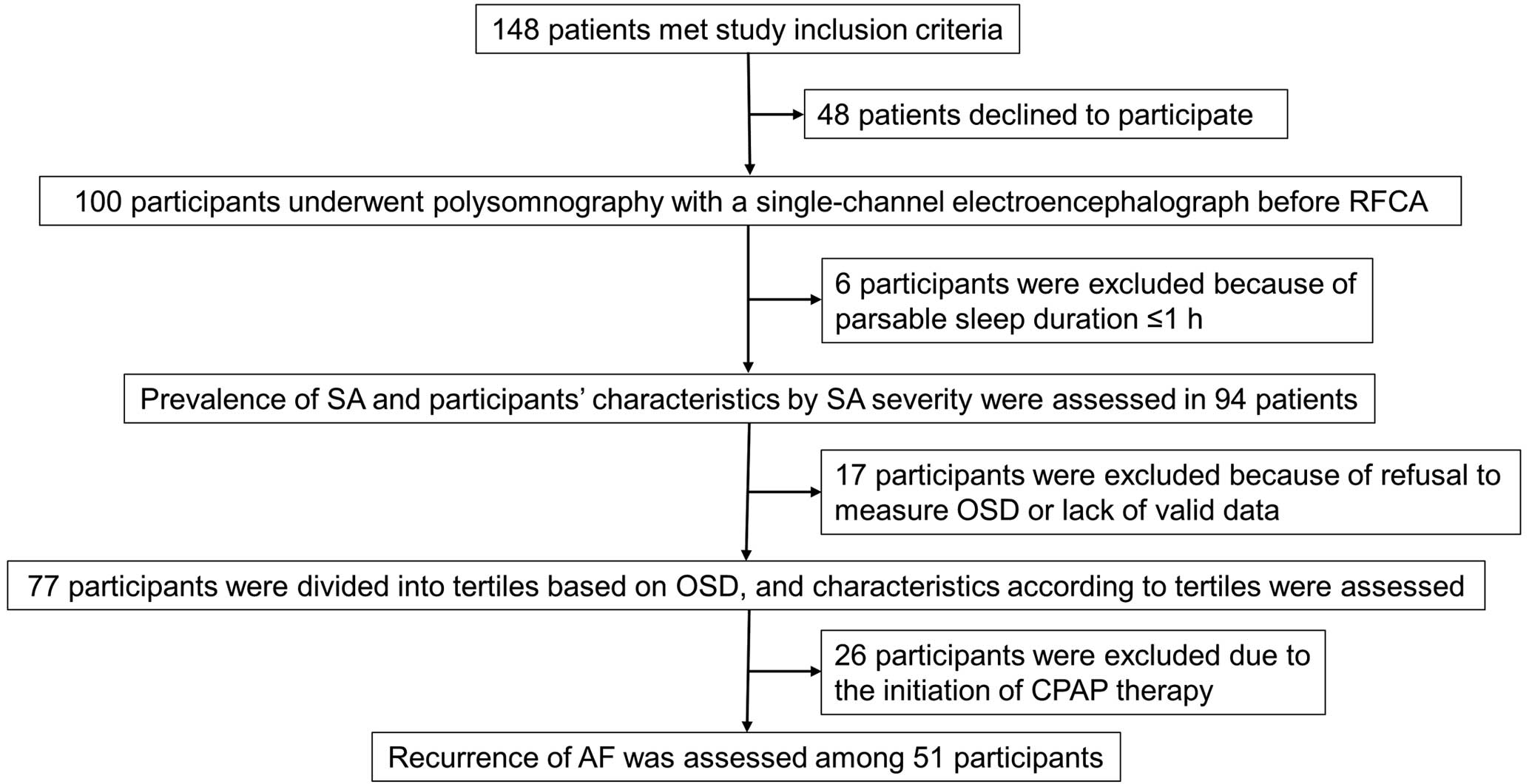
Flowchart of study procedures and participation. AF, atrial fibrillation; CPAP, continuous positive airway pressure; OSD, objective sleep duration; RFCA, radiofrequency catheter ablation; SA, sleep apnea.
Table 1 shows the characteristics of the study participants according to the severity of SA. Among the 94 patients who underwent polysomnography, 25.5% had none/mild SA (AHI <15), 28.7% had moderate SA (AHI 15–30), and 45.7% had severe SA (AHI ≥30). There was a significant difference in BMI among these groups, but no significant differences in longstanding persistent AF, additional ablation procedures, SSD, OSD, and SSD–OSD. However, wrist actigraphy×polysomnography data (e.g., OSD×AHI, OSD×3%ODI, and OSD×CT90) increased with the severity of SA.
Participants’ Characteristics According to SA Severity
| None/mild SA (n=24) |
Moderate SA (n=27) |
Severe SA (n=43) |
P value | |
|---|---|---|---|---|
| Clinical background | ||||
| Age (years) | 66.7±6.8 | 65.5±10.9 | 68.4±7.4 | 0.37 |
| BMI (kg/m2) | 23.7±2.3 | 24.3±2.9 | 25.8±3.1† | 0.009 |
| Male sex | 19 (79.2) | 21 (77.8) | 39 (90.7) | 0.27 |
| Disease duration of AF (months)* | 24.0 [4.0–60.0] | 24.0 [5.5–45.0] | 18.0 [3.0–35.0] | 0.66 |
| Duration of persistent AF (months)* | 4.0 [3.0–12.0] | 5.0 [3.0–30.5] | 6.0 [1.8–30.0] | 0.98 |
| Longstanding persistent AF | 8 (33.3) | 11 (40.7) | 20 (46.5) | 0.57 |
| Hypertension | 9 (37.5) | 18 (66.7) | 26 (60.5) | 0.085 |
| Diabetes | 0 (0.0) | 2 (7.4) | 7 (16.3) | 0.085 |
| Chronic heart failure | 9 (37.5) | 5 (18.5) | 9 (20.9) | 0.22 |
| Ischemic stroke | 3 (12.5) | 2 (7.4) | 3 (7.0) | 0.72 |
| Cardiovascular diseases | 1 (4.2) | 4 (14.8) | 5 (11.6) | 0.45 |
| CHA2DS2 score | 1.1±1.1 | 1.3±1.0 | 1.4±1.0 | 0.55 |
| CHA2DS2-VASc score | 2.0±1.6 | 2.2±1.3 | 2.3±1.3 | 0.75 |
| Vaughan Williams Class II/IV AAD | 2 (8.3) | 2 (7.4) | 3 (7.0) | 0.98 |
| Vaughan Williams Class I/III AAD | 14 (58.3) | 14 (51.9) | 23 (53.5) | 0.89 |
| Heart rate (beats/min) | 83.5±17.0 | 76.1±15.8 | 80.3±16.1 | 0.28 |
| Left atrial diameter (cm) | 45.7±4.3 | 44.6±6.1 | 45.7±5.6 | 0.69 |
| Ejection fraction (%) | 59.0±9.8 | 64.9±9.8 | 62.3±10.6 | 0.13 |
| eGFR (mL/min/1.73 m2) | 60.5±11.8 | 65.9±13.5 | 63.3±12.6 | 0.32 |
| BNP (pg/mL)* | 152.7 [73.1–207.6] | 104.9 [66.2–156.5] | 126.4 [66.1–210.6] | 0.80 |
| Additional ablation | ||||
| Superior vena cava isolation | 1 (4.2) | 0 (0.0) | 1 (2.3) | 0.58 |
| Low-voltage zone ablation | 3 (12.5) | 4 (14.8) | 7 (16.3) | 0.92 |
| Complex fractionated atrial electrogram ablation | 1 (4.2) | 2 (7.4) | 4 (9.3) | 0.73 |
| Questionnaires, SSD, SSD–OSD, and sleep duration–OSD | ||||
| JESS (points) | 8.4±3.9 | 5.6±4.0 | 8.4±5.1‡ | 0.037 |
| Excessive daytime sleepiness | 5 (25.0) | 3 (12.0) | 12 (29.3) | 0.27 |
| PSQI (points) | 6.3±4.1 | 5.7±2.7 | 5.3±2.6 | 0.55 |
| SSD (h) | 6.5±0.9 | 6.8±1.3 | 6.8±1.3 | 0.59 |
| SSD–OSD (h) | 0.4±1.0 | 0.6±1.0 | 0.7±1.2 | 0.53 |
| Missing data (n) | 5 | 11 | 7 | |
| Sleep duration–OSD (h) | −1.83±1.69 | −1.12±1.65 | −1.73±1.62 | 0.35 |
| Wrist actigraphy | ||||
| OSD (h) | 6.1±0.7 | 6.2±1.2 | 6.0±1.2 | 0.88 |
| Sleep efficiency (%) | 80.1±8.2 | 78.3±7.4 | 76.0±10.5 | 0.25 |
| Wake after sleep onset (min) | 60.7±36.6 | 64.9±36.0 | 74.0±41.3 | 0.41 |
| Fragmentation index | 35.1±14.6 | 38.0±12.2 | 43.3±15.3 | 0.094 |
| Sleep latency (min)* | 63.0 [33.0–89.0] | 58.0 [20.5–105.5] | 58.0 [31.0–93.5] | 0.65 |
| Time of sleep onset (hh:mm) | 23:05 | 23:30 | 22:55 | |
| Time of sleep end (hh:mm) | 06:15 | 06:45 | 06:18 | |
| Polysomnography | ||||
| Sleep duration (h) | 4.3±1.6 | 5.0±1.2 | 4.3±1.4 | 0.11 |
| Stage N1 sleep (%) | 30.6±19.4 | 29.0±14.1 | 42.1±17.8†,‡ | 0.003 |
| Stage N2 sleep (%) | 53.2±17.1 | 52.0±14.0 | 39.8±14.6†,‡ | <0.001 |
| Stage N3 sleep (%)* | 0.2 [0.0–1.1] | 0.1 [0.0–1.1] | 0.0 [0.0–0.7] | 0.55 |
| REM sleep (%) | 14.7±8.8 | 17.9±7.7 | 16.4±9.8 | 0.46 |
| Arousal index (events/h) | 20.7±12.9 | 21.6±8.8 | 31.6±10.5†,‡ | <0.001 |
| AHI (events/h)* | 9.9 [5.5–13.9] | 20.4 [17.8–26.4]† | 46.3 [37.2–60.8]†,‡ | <0.001 |
| CAI (events/h)* | 0.1 [0.0–0.5] | 0.0 [0.0–1.6] | 3.9 [0.7–12.9]† | <0.001 |
| 3%ODI (events/h)* | 7.7 [5.5–10.0] | 17.1 [12.6–21.8]† | 41.2 [30.7–56.5]†,‡ | <0.001 |
| Minimum SpO2 (%) | 89.3±3.6 | 86.7±4.1 | 82.4±6.2†,‡ | <0.001 |
| Median SpO2 (%) | 96.6±1.3 | 96.1±1.4 | 95.3±1.8†,‡ | 0.007 |
| CT90 (min)* | 0.0 [0.0–1.0] | 2.0 [0.0–5.5] | 10.0 [1.5–43.0]†,‡ | <0.001 |
| Wrist actigraphy×polysomnography | ||||
| OSD×AHI* | 60.3 [37.5–81.1] | 122.6 [94.8–153.6]† | 308.9 [218.3–359.9]†,‡ | <0.001 |
| OSD×3%ODI* | 43.7 [33.5–70.2] | 94.9 [75–135.3]† | 249.4 [168.7–364.8]†,‡ | <0.001 |
| OSD×CT90* | 0.0 [0.0–6.1] | 10.4 [0.0–31.1] | 65.2 [8.5–241.3]†,‡ | <0.001 |
Data are expressed as the mean±SD, median [interquartile range], or n (%). *Kruskal-Wallis test was used to compare differences between groups; analysis of variance was used otherwise. †P<0.05 vs. None/mild SA, ‡P<0.05 vs. Moderate SA. AAD, antiarrhythmic drug; AF, atrial fibrillation; AHI, apnea-hypopnea index; BMI, body mass index; BNP, B-type natriuretic peptide; CAI, central apnea index; CT90, cumulative percentage of recording time with SpO2 <90%; eGFR, estimated glomerular filtration rate; JESS, Japanese version of the Epworth Sleepiness Scale; ODI, oxygen desaturation index; OSD, objective sleep duration; PSQI, Pittsburgh Sleep Quality Index; SA, sleep apnea; SSD, subjective sleep duration.
Seventy-seven participants were stratified according to OSD tertiles based on mean OSD length (Table 2). The mean OSDs determined by wrist actigraphy were 5.0±0.6 h for Tertile 1, 6.0±0.2 h for Tertile 2, and 7.3±0.5 h for Tertile 3. There were no differences in clinical background among these 3 groups. Although Tertiles 2 and 3 were linked to longer SSD, the SSD–OSD for Tertile 3 (0.0 h) was smaller than that for Tertile 1 and Tertile 2. Although there was no significant difference in sleep duration determined by polysomnography among the 3 groups, sleep duration–OSD increased with increasing OSD. Compared with Tertile 1, Tertile 3 had higher rates of Stage N1 sleep and lower rates of Stage N2 sleep on polysomnography, as well as greater sleep efficiency and shorter sleep latency according to wrist actigraphy. In this group of 77 participants, there were no differences in clinical and sleep parameters between those with and without longstanding persistent AF (Supplementary Table).
Participants’ Characteristics According to OSD Tertile
| Tertile 1 (n=26) |
Tertile 2 (n=26) |
Tertile 3 (n=25) |
P value | |
|---|---|---|---|---|
| Clinical background | ||||
| Age (years) | 64.6±10.5 | 66.3±6.8 | 69.1±7.2 | 0.17 |
| BMI (kg/m2) | 25.8±3.3 | 25.1±2.9 | 23.9±2.4 | 0.076 |
| Male sex | 23 (88.5) | 23 (88.5) | 20 (80.0) | 0.61 |
| Disease duration of AF (months)* | 20.0 [5.0–51.0] | 25.0 [4.8–56.3] | 7.0 [2.8–41.0] | 0.29 |
| Duration of persistent AF (months)* | 9.0 [3.0–26.3] | 4.5 [3.0–32.0] | 4.0 [2.0–22.5] | 0.59 |
| Longstanding persistent AF | 12 (46.2) | 11 (42.3) | 8 (32.0) | 0.56 |
| Hypertension | 12 (46.2) | 13 (50.0) | 13 (52.0) | 0.91 |
| Diabetes | 3 (11.5) | 2 (7.7) | 2 (8.0) | 0.87 |
| Chronic heart failure | 5 (19.2) | 5 (19.2) | 8 (32.0) | 0.46 |
| Ischemic stroke | 3 (11.5) | 1 (3.8) | 1 (4.0) | 0.44 |
| Cardiovascular diseases | 3 (11.5) | 2 (7.7) | 3 (12.0) | 0.86 |
| CHA2DS2 score | 1.2±1.2 | 0.9±0.8 | 1.2±0.8 | 0.43 |
| CHA2DS2-VASc score | 2.0±1.6 | 1.7±0.9 | 2.4±1.2 | 0.18 |
| Vaughan Williams Class II/IV AAD | 15 (57.7) | 11 (42.3) | 16 (64.0) | 0.28 |
| Vaughan Williams Class I/III AAD | 4 (15.4) | 2 (7.7) | 1 (4.0) | 0.35 |
| Heart rate (beats/min) | 78.1±13.6 | 79.3±16.6 | 82.7±17.9 | 0.58 |
| Left atrial diameter (cm) | 46.7±5.8 | 46.5±5.1 | 43.9±4.8 | 0.12 |
| Ejection fraction (%) | 65.0±9.1 | 59.3±11.2 | 61.5±10.4 | 0.14 |
| eGFR (mL/min/1.73 m2) | 66.1±14.4 | 64.1±13.5 | 60.6±10.7 | 0.33 |
| BNP (pg/mL)* | 105.2 [59.4–207.9] | 98.7 [57.4–156.3] | 155.2 [86.3–229.1] | 0.080 |
| Questionnaires, SSD, SSD–OSD, and sleep duration–OSD | ||||
| JESS (points) | 8.5±4.3 | 7.5±4.9 | 6.8±4.8 | 0.45 |
| Excessive daytime sleepiness | 7 (28.0) | 5 (20.8) | 4 (16.7) | 0.62 |
| PSQI (points) | 6.3±2.6 | 4.9±3.3 | 5.5±3.4 | 0.32 |
| SSD (h) | 5.8±0.9 | 6.9±0.9† | 7.3±0.9† | <0.001 |
| SSD–OSD (h) | 0.9±1.0 | 0.9±1.0 | 0.0±1.0†,‡ | 0.005 |
| Missing data (n) | 2 | 3 | 1 | |
| Sleep duration–OSD (h) | −0.42±1.45 | −1.89±1.54† | −2.61±1.14† | <0.001 |
| Wrist actigraphy | ||||
| OSD (h) | 5.0±0.6 | 6.0±0.2† | 7.3±0.5†,‡ | <0.001 |
| Sleep efficiency (%) | 73.8±9.0 | 77.0±9.7 | 82.5±7.1† | 0.003 |
| Wake after sleep onset (min) | 61.8±32.8 | 77.7±44.5 | 64.4±37.7 | 0.29 |
| Fragmentation index | 41.7±14.0 | 42.6±16.9 | 34.5±12.2 | 0.10 |
| Sleep latency (min)* | 18.7 [14.6–33.2] | 9.5 [5.4–26.9] | 11.1 [3.9–17.3]† | 0.015 |
| Time of sleep onset (hh:mm) | 23:56 | 23:10 | 22:10 | – |
| Time of sleep end (hh:mm) | 06:08 | 06:30 | 06:32 | – |
| Polysomnography | ||||
| Sleep duration (h) | 4.5±1.4 | 4.1±1.6 | 4.7±1.4 | 0.33 |
| Stage N1 sleep (%) | 27.1±10.3 | 37.5±17.6 | 42.0±20.9† | 0.007 |
| Stage N2 sleep (%) | 54.5±12.7 | 44.7±15.1 | 39.9±17.3† | 0.003 |
| Stage N3 sleep (%)* | 0.2 [0.0–0.8] | 0.0 [0.0–0.8] | 0.0 [0.0–0.4] | 0.61 |
| REM sleep (%) | 16.3±8.4 | 16.6±11.4 | 17.0±8.5 | 0.96 |
| Arousal index (events/h) | 24.3±11 | 27.6±13.4 | 26.8±11.7 | 0.59 |
| AHI (events/h)* | 31.2 [15.7–45.7] | 17.8 [11.4–44.7] | 32.9 [16.8–47.8] | 0.38 |
| CAI (events/h)* | 1.5 [0.0–6.6] | 0.3 [0–3.1.0] | 0.6 [0.0–5.4] | 0.49 |
| 3%ODI (events/h)* | 19.7 [12.6–37.4] | 16.0 [7.6–46.7] | 21.1 [12.1–42.5] | 0.58 |
| Minimum SpO2 (%) | 84.2±7.1 | 85.9±5.1 | 85.3±5.4 | 0.60 |
| Median SpO2 (%) | 95.6±1.8 | 96.0±1.5 | 96.0±1.6 | 0.54 |
| CT90 (min)* | 1.5 [0.0–28.3] | 3.5 [0.0–9.3] | 3.0 [0.0–9.0] | 0.94 |
| Wrist actigraphy×polysomnography | ||||
| OSD×AHI* | 130.6 [79.3–247.3] | 104.4 [69.5–262.8] | 223.1 [125.0–364.1] | 0.082 |
| OSD×3%ODI* | 91.8 [61.9–195.6] | 93.4 [45.0–272.9] | 157.1 [90.5–328.9] | 0.21 |
| OSD×CT90* | 7.7 [0.0–153.8] | 20.4 [0.0–58.8] | 22.5 [0.0–69.4] | 0.996 |
Data are expressed as the mean±SD, median [interquartile range], or n (%). *Kruskal-Wallis test was used to compare differences between groups; analysis of variance was used otherwise. †P<0.05 vs. Tertile 1, ‡P<0.05 vs. Tertile 2. Abbreviations as in Table 1.
After excluding participants who initiated CPAP therapy after RFCA, AF recurrence was assessed in 51 participants. Among these 51 individuals, AF recurrence was observed in 10 over a mean observation period of 27.6±11.7 months. First, we investigated the AHI cut-off value to predict recurrence of AF in Kaplan-Meier curves. In these analyses, only AHI ≥20 was significantly associated with AF recurrence (log-rank P=0.028; crude hazard ratio [HR] 5.66; 95% confidence interval [CI] 1.11–24.73; P=0.036; Supplementary Figures 1–4; Figure 2). We also investigated associations between OSD tertiles and AF recurrence using Kaplan-Meier curves for the different OSD tertiles. Compared with Tertile 1, Tertile 3 was significantly associated with AF recurrence (log-rank P=0.029), with a crude HR of 10.34 (95% CI 1.21–88.36; P=0.033; Figure 3). Table 3 presents data for participants without and with AF recurrence. Regarding wrist actigraphy×polysomnography, participants with recurrent AF had significantly higher OSD×3%ODI (P=0.049; Table 3). Information obtained from the questionnaires showed that participants with AF recurrence had significantly higher SSD, and lower PSQI and JESS scores, than those without AF recurrence (Table 3).

Kaplan-Meier curves showing atrial fibrillation (AF)-free survival according to apnea-hypopnea index (AHI) <20 and ≥20.

Kaplan-Meier curves showing atrial fibrillation (AF)-free survival according to objective sleep duration (OSD) tertile.
Participants’ Characteristics According to Recurrence of AF
| Participants without AF recurrence (n=41) |
Participants with AF recurrence (n=10) |
P value | |
|---|---|---|---|
| Clinical background | |||
| Age (years) | 65.7±9.6 | 65.9±6.5 | 0.96 |
| BMI (kg/m2) | 24.4±2.7 | 24.9±2.4 | 0.54 |
| Male sex | 35 (85.4) | 2 (20.0) | 0.68 |
| Disease duration of AF (months)* | 21.0 [4.0–52.5] | 32.5 [2.9–60.0] | 0.95 |
| Duration of persistent AF (months)* | 4.5 [3.0–24.8] | 21.0 [2.1–51.0] | 0.36 |
| Longstanding persistent AF | 16 (39.0) | 10 (60.0) | 0.23 |
| Hypertension | 15 (36.6) | 8 (80.0) | 0.013 |
| Diabetes | 2 (4.9) | 2 (20.0) | 0.11 |
| Chronic heart failure | 11 (26.8) | 0 (0.0) | 0.064 |
| Ischemic stroke | 5 (12.2) | 0 (0.0) | 0.25 |
| Cardiovascular diseases | 4 (9.8) | 1 (10.0) | 0.98 |
| CHA2DS2 score | 1.1±1.1 | 1.0±0.5 | 0.84 |
| CHA2DS2-VASc score | 1.9±1.5 | 2.0±0.8 | 0.88 |
| Vaughan Williams Class II/IV AAD | 22 (53.7) | 7 (70.0) | 0.35 |
| Vaughan Williams Class I/III AAD | 5 (12.2) | 0 (0.0) | 0.35 |
| Heart rate (beats/min) | 80.6±16.7 | 82.9±16.3 | 0.70 |
| Left atrial diameter (cm) | 46.4±5.9 | 44.2±4.4 | 0.29 |
| Ejection fraction (%) | 60.8±11.8 | 63.0±7.6 | 0.58 |
| eGFR (mL/min/1.73 m2) | 61.3±12.3 | 65.6±11.8 | 0.33 |
| BNP (pg/mL)* | 154.0 [78.2–251.2] | 108.5 [65.8–183.5] | 0.36 |
| Questionnaires, SSD, SSD–OSD, and sleep duration–OSD | |||
| JESS, (points) | 8.3 (4.4) | 3.3 (3.4) | 0.003 |
| Excessive daytime sleepiness | 10 (26.3) | 0 (0.0) | 0.083 |
| PSQI (points) | 6.3±3.4 | 2.7±1.6 | 0.010 |
| SSD (h) | 6.4±1.0 | 7.3±1.0 | 0.0498 |
| SSD–OSD (h) | 0.5±1.1 | 0.6±0.9 | 0.74 |
| Missing | 3 | 3 | |
| Sleep duration–OSD (h) | −1.34±1.63 | −1.60±1.44 | 0.66 |
| Wrist actigraphy | |||
| OSD (h) | 5.9±1.1 | 6.5±0.7 | 0.13 |
| Sleep efficiency (%) | 77.6±8.9 | 80.1±9.2 | 0.44 |
| Wake after sleep onset (min) | 65.1±37.1 | 68.9±33.8 | 0.77 |
| Fragmentation index | 37.7±13.2 | 39.0±14.0 | 0.78 |
| Sleep latency (min)* | 15.4 [7.0–26.4] | 10.3 [3.1–14.6] | 0.15 |
| Time of sleep onset (hh:mm) | 23:34 | 22:11 | – |
| Time of sleep end (hh:mm) | 06:37 | 05:49 | – |
| Polysomnography | |||
| Sleep duration (h) | 4.6±1.5 | 4.9±1.0 | 0.58 |
| Stage N1 sleep (%) | 28.9±13.4 | 39.1±26.0 | 0.084 |
| Stage N2 sleep (%) | 52.6±13.9 | 42.3±17.9 | 0.051 |
| Stage N3 sleep (%)* | 0.2 [0.0–1.0] | 0.3 [0.0–2.6] | 0.62 |
| REM sleep (%) | 17.3±7.8 | 16.0±9.1 | 0.66 |
| Arousal index (events/h) | 23.3±11.3 | 25.2±14.7 | 0.64 |
| AHI (events/h)* | 15.7 [10.3–30.4] | 31.9 [16.7–59.2] | 0.099 |
| CAI (events/h)* | 0.4 [0.0–2.9] | 0.0 [0.0–4.7] | 0.31 |
| 3%ODI (events/h)* | 13.4 [7.5–22.2] | 22.7 [13.4–50.6] | 0.094 |
| Minimum SpO2 (%) | 86.7±5.5 | 83.9±3.3 | 0.13 |
| Median SpO2 (%) | 96.0±1.8 | 95.7±1.3 | 0.69 |
| CT90 (min)* | 1.0 [0.0–8.0] | 3.0 [0.0–8.8] | 0.57 |
| Wrist actigraphy×polysomnography | |||
| OSD×AHI* | 88.7 [63.2–169.7] | 212.7 [98.0–365.5] | 0.083 |
| OSD×3%ODI* | 75.0 [44.1–137.8] | 151.2 [80.4–314.2] | 0.049 |
| OSD×CT90* | 6.8 [0.0–44.1] | 17.5 [0.0–57.0] | 0.60 |
Data are expressed as the mean±SD, median [interquartile range], or n (%). *Mann-Whitney test was used to compare differences between groups. Abbreviations as in Table 1.
In this prospective study of individuals who underwent RFCA for persistent AF, we accurately evaluated the prevalence of SA (moderate SA: 28.7%; severe SA: 45.7%) by polysomnography with a single-channel electroencephalograph (Table 1). Polysomnography also showed that participants with long OSD had decreased SSD–OSD, increased Stage N1 sleep, and decreased Stage N2 sleep (Table 2). Furthermore, SA (AHI ≥20) and long OSD were predictors of the recurrence of AF (Figures 2, 3). Compared with participants without AF recurrence, those with recurrent AF were exposed to prolonged periods of intermittent hypoxia during sleep (Table 3).
Previous studies have investigated the prevalence of SA among patients with AF. However, various methods were used to screen for SA. For example, some studies used questionnaires to screen for SA,6,7,37 whereas others used objective methods other than polysomnography.38–41 According to some reports, questionnaires may have underestimated the prevalence of SA (24.2–58.4%) in patients with AF.5–7 Using polysomnography with a single-channel electroencephalograph, we showed that 74.4% of patients who underwent RFCA for persistent AF had moderate to severe SA.
In this study, we first reported that participants with persistent AF who had long OSD (mean 7.3 h) also had a small SSD–OSD (almost 0 h). Although SSD is usually longer than OSD,24,25 we recently showed that the value of SSD–OSD was lower when OSD exceeded approximately 7 h, as in the present study,27 and that a low SSD–OSD value was a significant factor for non-restorative sleep (subjective experience of unrefreshing sleep).42 The findings of the present study indicate that habitual sleep duration was adequate in participants with long OSD. However, sleep quality derived from polysomnography data of participants with long OSD may actually be low (increased Stage N1), and OSD could be increased, without an increased in SSD, resulting in low SSD–OSD values in participants with long OSD. This suggests that objective sleep quality (not sleep duration per se, but rather the duration of different sleep stages) is also associated with SSD–OSD, as for subjective sleep quality (non-restorative sleep). Indeed, a longer duration of Stage N1 sleep has been shown in patients with hypertension.43
The novel finding of the present study is that in addition to SA or long OSD, long periods of exposure to intermittent hypoxia during sleep, representing a combination of SA and long OSD, may be a risk factor for AF. First, the prevalence of moderate to severe SA was quite high (74.4%) in our study cohort, and we found that SA (especially AHI ≥20) was a predictor of AF recurrence after RFCA (Figure 2), as did previous studies.4,6,7,37 In Japan, AHI ≥20 is an indication for CPAP therapy.36 Therefore, in clinical practice, we recommend that physicians routinely screen for SA using an objective measurement, such as polysomnography, in all patients with persistent AF who are to undergo RFCA and consider initiating CPAP therapy in such patients. Second, we showed that long habitual OSD was a predictor of AF recurrence (Figure 3). Although previous studies reported an association between sleep duration and the risk of AF,18–21 studies using OSD are limited.22,23 One population-based study assessed habitual OSD by actigraphy, as in the present study, and cross-sectionally surveyed the association between habitual OSD and the prevalence of AF; however, no significant association was found.22 Another study using OSD derived from diagnostic polysomnography reported that short sleep duration was a predictor of incident AF.23 However, the OSD determined by diagnostic polysomnography may not be identical to habitual OSD. Our results showed that sleep duration measured by polysomnography did not match habitual OSD (Table 2). Therefore, the strength of the present study is that it is the first to show that habitual long OSD is a predictor of AF recurrence in patients who are to undergo RFCA for persistent AF. We also showed that a combination of SA and OSD may indicate a risk of AF. As indicated in Table 3, participants with recurrent AF had higher OSD×3%ODI than those without recurrent AF. This demonstrates that participants with recurrent AF have been exposed to intermittent hypoxia during sleep for long periods during their life through a combination of SA and habitual long OSD. Future studies should investigate appropriate interventions to simultaneously treat both SA and long OSD.
We acknowledge this study has some limitations. First, the present study was small. In particular, the number of those with AF recurrence was very small, and the results associated with AF recurrence might be insufficient for analysis. Second, participants who initiated CPAP therapy were excluded from the analysis of AF recurrence because continuation with or the adherence to CPAP could not be fully determined. This may result in a potential bias in analyses of recurrent AF. Third, this study was an observational study and did not reveal causality between SA, OSD, a combination of SA and OSD, and AF recurrence. Fourth, we used polysomnography with a single-channel electroencephalograph. Although this system has not been well validated, the usefulness of nearly the same system has been demonstrated previously.29,30
In conclusion, the prevalence of moderate to severe SA determined by polysomnography with a single-channel electroencephalograph was 74.4% and long OSD was associated with a small SSD–OSD value, which could be explained by possible decreased sleep quality, in patients undergoing RFCA for persistent AF. In addition, SA (particularly AHI ≥20) and long OSD were identified as predictors of AF recurrence, and prolonged exposure to intermittent hypoxia during sleep may induce AF recurrence in these patients.
The authors sincerely thank Keiko Kado, Mami Anraku, Taisuke Nakanoue, Hiroyasu Kubo, Kazuyuki Ueda, Rika Sugiura, Tomoko Toki, and all the staff on the wards in the Department of Cardiology and in the Catheterization Laboratory at Kyoto University Hospital for their cooperation in conducting this study.
This study was funded by a university grant, Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology in Japan (26293198, 17H04182, 20K17860, 20H03690, 23K07638), and grants from the Center of Innovation Program and the Global University Project from the Japan Science and Technology Agency, Japan Agency for Medical Research and Development (AMED; grant numbers ek0210096, ek0210116, ek0210150, and wm0425018). This research was also supported by grants from the Japanese Ministry of Education, Culture, Sports, Science and Technology; the Intractable Respiratory Diseases and Pulmonary Hypertension Research Group from the Ministry of Health, Labour and Welfare of Japan (H29-intractable diseases-general-027, JPMH20FC1027, and JPMH23FC1031); the Special Health Check-Up Research Group from the Ministry of Health, Labor, and Welfare of Japan (R3-ComprehensiveResearch on Life-Style Related Disease including Cardiovascular Diseases and Diabetes Mellitus-21FA1004); the Research Foundation for Healthy Ageing; Health, Labour and Welfare Sciences Research Grants, and Research on Regional Medical Care (H30-iryo-ippan-009); and the Fukuda Foundation for Medical Technology. This study was supported by Teijin Pharma; equipment for the examination of SA was borrowed from, and corresponding records were analyzed by, Teijin Pharma based on a joint research agreement.
The department of Advanced Medicine for Respiratory Failure of Kyoto University Graduate School of Medicine is funded by grants from Teijin Pharma to Kyoto University. The Department of Respiratory Care and Sleep Control Medicine of Kyoto University is funded by grants from Philips Japan, Fukuda Denshi, Fukuda Lifetec Keiji, and ResMed Japan to Kyoto University. The Department of Sleep Medicine and Respiratory Care, Division of Respiratory Medicine, Department of Internal Medicine of Nihon University is funded by grants from Philips Japan, Fukuda Denshi, Fukuda Lifetec Tokyo, and ResMed Japan to Nihon University. K.O. is a member of Circulation Journal’s Editorial Team.
This study was approved by the Ethics Committee of Kyoto University Graduate School and Faculty of Medicine (Reference no. #R1206).
The deidentified participant data will not be shared.
Please find supplementary file(s);
https://doi.org/10.1253/circj.CJ-24-0537